LIGHT STRESS


SOCIETY FOR EXPERIMENTAL BIOLOGY SPRING 2023
The SEB Magazine is published biannually — Spring and Autumn (online) — by the Society for Experimental Biology and is distributed to all SEB members.
Advertising
Advertising in the SEB magazine is a great opportunity to reach a large community of biologists. For more details contact b.danois@sebiology.org
Design and artwork:
Robert Wood, Time Design Studio rob@timedesignstudio.co.uk
Contribute with an article!
Interested in writing an article for the SEB magazine? Get in touch: b.danois@sebiology.org
Deadline for copy:
Issue: Autumn 2023
Deadline: 1 July 2023
SEB Executive Team:
SEB Main Office
The Society for Experimental Biology County Main, A012/A013
Lancaster University, Bailrigg LA1 4YW, UK admin@sebiology.org
Chief Executive Officer
Pamela Mortimer (p.mortimer@sebiology.org)
Events Manager
Eniola Alalade (e.alalade@sebiology.org)
Events and Grants Assistant
Keji Aofiyebi (k.aofiyebi@sebiology.org)
Events officer
Jennifer Symons (j.symons@sebiology.org)
Membership Manager
Jordy Turl (j.turl@sebiology.org)
Office Administrator
Julius Kelly (j.kelly@sebiology.org)
Education, Outreach and Diversity Manager
Dr Rebecca Ellerington (r.ellerington@sebiology.org)
Education, Outreach and Diversity
Ana Caroline Colombo (a.colombo@sebiology.org)
Communications Manager
Benjamin Danois (b.danois@sebiology.org)
SEB Honorary Officers:
President
Jim Murray (murrayja1@cardiff.ac.uk)
Vice President Tracey Lawson (tlawson@essex.ac.uk)
Treasurer John Love (J.Love@exeter.ac.uk)
Publications Officer
Martin Parry (martin.parry@bbsrc.ac.uk)
Plant Section Chair
Stefan Kepinski (S.Kepinski@leeds.ac.uk)
Cell Section Chair
David Evans (deevans@brookes.ac.uk)
Animal Section Chair
Felix Mark (Felix.Christopher.Mark@awi.de)
Outreach, Education and Diversity Trustee
Sheila Amici-Dargan (anzsld@bristol.ac.uk)
SEB Journal Editors:
Journal of Experimental Botany
John Lunn (Lunn@mpimp-golm.mpg.de)
The Plant Journal
Lee Sweetlove (lee.sweetlove@plants.ox.ac.uk)
Plant Biotechnology Journal
Henry Daniell (henry.daniell@ucf.edu)
Conservation Physiology
Steven Cooke (steven_cooke@carleton.ca)
Plant Direct
Ivan Baxter (ibaxter@danforthcenter.org)
In association with ASPB
Disclaimer
The views expressed in this magazine are not necessarily those of the Editorial Board or the Society for Experimental Biology. The Society for Experimental Biology is a registered charity No. 273795
1 NEWS & VIEWS EDITORIAL - SHINING A LIGHT ......... 6 PRESIDENT’S LETTER 7 SEB NEWS 8 MEMBERS IN THE NEWS 10
02 LIGHT STRESS | SPRING 2023
FEATURES SPOTLIGHT
PLENARY LECTURERS 2023 WINNERS ..... 28 MEET OUR PRESIDENT MEDALISTS 2023 30 JOURNALS - CONSERVATION PHYSIOLOGY –REFLECTIONS AND A VISION FOR THE FUTURE ....................... 32 JOURNALS - THE PLANT JOURNAL: A HUNDRED YEARS OF METABOLISM RESEARCH ....... 33 JOURNALS - JXB - UNRAVELING THE RNA REVOLUTION: A TIMELINE OF RNA BIOLOGY RESEARCH 34 JOURNALS - PLANT DIRECTROOT BIOLOGY: FROM CURIOSITY TO SAVING THE PLANET OVER 100 YEARS 35 JOURNALS - PLANT BIOTECHNOLOGY –PAST, PRESENT AND FUTURE 36 IN CONVERSATION WITH LIANA ACEVEDO-SIACA 38 IN CONVERSATION WITH MICHAEL SLEIGH 40 SPOTLIGHT ON SHANE AUSTIN ......... 42 SPOTLIGHT ON GEORGES HRAOUI 44 ANIMAL FEATURE: LIGHT OF THEIR LIVES 14 CELL FEATURE: LOOKING ON THE BRIGHT SIDE 18 PLANT FEATURE: LIGHT STRESSTOO MUCH OF A GOOD THING 22 WOMEN WHO HAVE SHAPED THE SEB ...... 48 A VISIT TO THE SEB ARCHIVES 50 MIND THE GAP: NAVIGATING THE ACADEMIC HIRING PROCESS AND CAREER TRANSITIONS ............... 52 GOING GREEN – WHAT DOES A SUSTAINABLE LAB LOOK LIKE ..................... 54
34 03 LIGHT STRESS | SPRING 2023
OUTREACH EDUCATION AND DIVERSITY 2
04 NEWS & VIEWS
NEWS & VIEWS

EDITORIAL - SHINING A LIGHT 6 PRESIDENT’S LETTER 7 SEB NEWS 8 MEMBERS IN THE NEWS 10 05 NEWS & VIEWS
SHINING A LIGHT ON THE IMPACT OF LIGHT STRESS: EXPLORING ITS EFFECTS ON ANIMALS, CELLS AND PLANTS
BY BENJAMIN DANOIS

As researchers and plant enthusiasts, we tend to think of the various forms of abiotic stress that can affect plants, such as cold or heat, drought and nutrient imbalances. However, in recent years, more attention has been given to the concept of light stress and its impact on plant health.
When plants are exposed to excessive light, they can generate reactive oxidation species (ROS) that can harm the function of photosystems. This can disrupt the process of photochemistry, which is crucial for the plant’s survival. While light stress can damage plants at the cellular level, it can also accumulate to affect productivity at the whole plant level.
As we delve deeper into the study of light stress, it’s essential to understand its impact on animals, cells and plants. In animal studies, light stress has been linked to the development of various diseases, including cancer, as well as disruptions in sleep patterns. Research has shown that exposure to excessive light can damage the retina and impair vision, leading to blindness in severe cases.
In cellular studies, researchers have discovered that light stress can have significant impacts on cellular metabolism, leading to changes in gene expression and the activation of stress-related signalling pathways. These changes can ultimately affect cellular health and lead to diseases such as cancer.
In plant studies, researchers have focused on understanding how plants dissipate excessive energy and mitigate the effects of light stress. Studies have shown that plants have a remarkable ability to dissipate excess energy through mechanisms such as non-photochemical quenching (NPQ) and the xanthophyll cycle. These mechanisms help plants to protect their photosystems from the harmful effects of ROS and maintain efficient photosynthesis.
While we tend to focus on other forms of abiotic stress, light stress is an essential area of research that is gaining increasing attention. Understanding the impacts of light stress within the three main sections of animal, cell and plant studies will allow us to develop strategies to mitigate its effects and promote better health and productivity.
Here is a glimpse of what we can have in store in this issue of the SEB Magazine...
FEATURES
In “Light of their lives”, Alex Evans will shine a light on some recent research into the world of animal–light interactions (page 14).
Alex also delves into the Cell realm by exploring the important cell–environment relationships, as highlighted by some researchers in “Looking on the bright side” (page 18).
And to conclude, in “Light stress–too much of a good thing”, Caroline Woods will take a look at fascinating new insights into the exquisite photoprotective mechanisms that help plants to stay safe from light stress (page 22).
OUTREACH, EDUCATION AND DIVERSITY

Our brilliant OED team have spoiled us with a very interesting series of articles we invite you to read. To begin, discover a paper about the unique women who have shaped the SEB (page 48). Then, read about a very special visit to the SEB archives (page 50). On page 52, you can find a very useful resource about navigating the academic hiring process and career transitions. And finally, Caroline Wood showcases “What does a sustainable lab look like?” (page 54).
MEMBERS HIGHLIGHTS
This issue’s spotlight section features brilliant achievements realised by our members! Discover them and their fantastic work (page 08).
SPOTLIGHT
In honour of our Society’s momentous centenary, we are thrilled to present a fantastic opportunity to
explore our esteemed journals in greater depth. To commemorate this milestone, we proudly feature brilliant papers contributed by each of the Editors-inChief of our five prestigious journals: Conservation Physiology, The Plant Journal, Biotechnology Journal, Plant Direct and the Journal of Experimental Botany (page 32-36).
Because this issue serves as a prelude to the highly anticipated Centenary Conference, we are delighted to present the profiles of all our esteemed President’s Medalists and highlight our exceptional Plenary Lecturers of 2023.
Additionally, we are privileged to share captivating interviews with notable biologists from our vibrant community. Prepare to be inspired by their insightful perspectives and groundbreaking research!
CENTENARY MEETING
Countdown to the Society’s Most Important Conference Begins
With just a few weeks remaining, the anticipation builds for the Society’s most significant conference of the year. We kindly remind you that registration is still open, and we encourage you to secure your spot promptly. Don’t miss out on this extraordinary event!
For more details and to register, please visit: www.sebiology.org/events/seb-centenaryconference-2023/registration.html
The entire SEB staff is eagerly awaiting your presence at the SEB Centenary Conference. We look forward to welcoming you there!
06 NEWS & VIEWS
PRESIDENT’S LETTER
 PROFESSOR JIM MURRAY PRESIDENT,
PROFESSOR JIM MURRAY PRESIDENT,
SOCIETY FOR
EXPERIMENTAL
BIOLOGY
Welcome to the Spring 2023 newsletter of the SEB in our Centenary Year.
There are many exciting events planned in celebration, which have already kicked off with a new series of Careers and Coffee events. A big thank you to Rebecca Ellerington who has taken the lead on our Centenary events, and to all others who have been involved in helping out. You can catch up with all the events at www.sebiology.org/centenary.html, including the excellent Careers and Coffee series aimed at providing an insight into different careers and career routes in experimental biology, and the Leaders of the Future webinar series which aims to celebrate past SEB awardees and showcase their scientific discoveries and progression. If you are interested in finding out a bit more about the history of the Society for the past 100 years, head to www.sebiology.org/centenary/seb-history/ history-articles-series.html, where you can read a growing series of short articles, including about some of the women who have shaped the SEB.
It’s not long now until our Centenary Conference in Edinburgh on 4–7 July. Check it out on the website now (www.sebiology.org/events/seb-centenaryconference-2023.html). The early bird deadline is 12 May, and the final registration deadline is 9 June—don’t miss it! Paul Nurse, winner of the Nobel Prize for Physiology or Medicine in 2001,and Director of the Crick Institute and a leading voice in science today, is a special guest as the President’s Centenary Lecturer. Paul will be speaking on the Thursday evening shortly before the conference dinner. I would particularly like to thank Eniola Alalade and the Events Team for all of their hard work in getting the conference organisation in place and on course. Travel funds are still available for students and early career researchers, including top-up funds for those who need to travel farther, and the closing date is 30 May (www.sebiology. org/grants/apply-for-funding.html).
The theme of this magazine issue is light stress, an often-neglected area of abiotic stress studies but, as you will read, of great importance. How plants protect themselves from the effects of excessive light and the consequential reactive oxygen species is becoming an area of increasing study from both the biological and theoretical standpoints. This provides an excellent example of how the SEB is well placed to support and provide a voice to these kinds of interdisciplinary studies.
With this my last letter as President, I would like to extend my thanks to all of you as members who have continued to support your Society, and of course to the whole SEB office team who work tirelessly to keep the Society running and provide the best possible support to members. We are now emerging from the other side of the difficult process of updating all of the IT systems and supporting structures, and hopefully you will be starting to see the improvements on the website through increased functionality and information. None of this happens without a lot of work behind the scenes, for which I am very grateful.
I am convinced that as we move into our second century, the SEB remains as relevant as when first founded. The broad reach of the SEB across the whole of biology is in tune with the increasingly integrated approaches to biology. Science faces unprecedented challenges today, and the need for a Society which supports and promotes biological science founded in experimental approaches— whether field, laboratory or theoretically based—is an important counterbalance to dogmatic and conspiracy-based pseudo-theories that seem to threaten increasing prevalence over empirical and evidence-based science.
However, another type of threat that faces us today, in many ways more insidious because it threatens the very basis of the evidence-based approach that we champion, is scientific fraud, particularly in publications. From examples that we see being detected in papers submitted to, and occasionally published by, our Society journals, this seems to be an increasing problem. I think this really emphasises the importance of society-based journals run from effective and well-managed editorial offices such as we operate. These are much better placed to invest the resources needed to detect and deal with fraudulent content than the high-throughput
commercial operations that are gaining increasing volume and foothold today. It has made me reflect that our journals and our editorial office is an important resource, providing a curated and trusted home for high-quality research, and we should see these as a key asset and not simply a method for the Society to generate revenue.
I hope to see as many of you as possible in Edinburgh to celebrate the centenary in style and to welcome in our new President, Professor Tracy Lawson. Until then, thank you and farewell!
Professor Jim Murray President, Society for Experimental Biology

07 NEWS & VIEWS
SEB NEWS
this cost of living crisis have dramatically increased from previous years. As such, the SEB “top-up fund” is for successful applicants of the SEB Travel Grant whose costs significantly exceed the monetary value of the SEB Travel Grant they were awarded.
In this instance, we are defining “significantly exceed” as travel costs coming to more than twice that of the travel grant funding. For example, if your cheapest flight and hotel options totalled £1600, and you were successful in receiving £800 for the SEB Travel Grant, you could apply for some additional financial aid via the top-up fund.
BY JULIUS KELLY
This year marks 100 years since the foundation of our Society in 1923, and the highlight of our centenary celebrations will be our Centenary Conference to be held 4–7 July in Edinburgh, the historic capital city of Scotland, where we will be celebrating the best that experimental biology has to offer and looking forward to the next 100 years.
Since its inception, the Society has expanded to over 1000 researchers from across the globe who are involved in 23 interest groups helping to advance cell, plant and animal research.
Sir Paul Nurse will be giving the President’s Centenary Lecture, and we are also delighted to welcome the distinguished Plenary Award Speakers Elizabeth Brainerd (Brown University; Animal Section), Lisa Ainsworth (University of Illinois; Plant Section) and Wendy Bickmore (University of Edinburgh; Cell Section).
The SEB executive team are very much looking forward to welcoming members and prospective members to what will most certainly be a hive of informed activity with awesome extracurricular activities in one of the UK’s most vibrant cities.
ASSISTANCE TO ATTEND THE ANNUAL CONFERENCE
As part of our centenary celebrations, the SEB is providing up to £240 of additional funding to successful applicants through the SEB Travel Grant 2023 if their travel costs significantly exceed their travel grant allocation.
The SEB travel grant is designed to help students and early career researchers attend the Centenary Conference with a financial contribution towards travel and accommodation. Although this contribution was never intended to cover the full costs of travel, we are aware that travel costs (particularly flights) post-COVID and in the middle of

Applications for the top-up fund will be assessed on a case-by-case basis. Applicants will be scored on a judging system devised to prioritise attendees with reduced income, from low-income countries, or who have no access to other funding.
GOODBYE…FROM THE SEB EXECUTIVE TEAM
It is with great sadness we see the departure of Eniola Alalade (Events Manager) at the end of May from the executive team. For over 4 years Eniola has been instrumental in managing successful annual conferences and symposiums across the world. Her hard work, dedication and expertise will be sorely missed by the team. We all wish her well in the future and thank her for being an invaluable part of the team.
Also, this year we have seen Jo Barclay (Society Secretariat) move on. Jo was an exceptional team player whose talent and engagement stretched right across the Society. Jo juggled many aspects of Society administration at all levels and in her short time with the Society became an integral member of our close-knit team. We wish her all the best in her future endeavours and she will be greatly missed.

08 NEWS & VIEWS SEB ANNUAL CONFERENCE
2023, EDINBURGH, UK
SEB MEMBERSHIP
70% OF MEMBERS LEARN ABOUT SEB THROUGH WORD OF MOUTH
SEBIOLOGY.ORG #SEBPACK
JOIN THE PACK

MAKE EXTRAORDINARY CONNECTIONS
NETWORK AND BUILD RELATIONSHIPS WITH EXPERIMENTAL BIOLOGISTS FROM AROUND THE WORLD
THINK BIG TAKE PART IN CROSSDISCIPLINARY CONFERENCES AND SHARE INNOVATIVE AND INSPIRING DATA, IDEAS AND RESULTS
DO MORE APPLY FOR GRANTS AND SPONSORSHIP TO INCREASE YOUR OPPORTUNITIES
FAST TRACK YOUR CAREER
ACCESS JOURNALS, EDUCATION AND TRAINING SUPPORT

SOCIETY FOR EXPERIMENTAL BIOLOGY
MEMBER NEWS
EDITED BY BENJAMIN DANOIS
IIn each issue of the member magazine, we like to highlight some of the fantastic achievements and research from our members. Here are some of the people we would like to congratulate this time around.
IVAN MEZA CANALES (INSTITUTE FOR TRANSDISCIPLINARY RESEARCH AND SERVICES, UNIVERSITY OF GUADALAJARA)
FÉLIX LEIVA (RADBOUD UNIVERSITY, NETHERLANDS)
Congratulations to Félix for publishing his thesis entitled “Thermal biology and cell size: An oxygen limitation perspective in ectotherms”: https://www.researchgate.net/ publication/366702411_Thermal_biology_ and_cell_size_An_oxygen_limitation_ perspective_in_ectotherms
We also invite you to read few chapters published inked to this thesis:
Chapter 2: https://royalsocietypublishing.org/doi/ full/10.1098/rstb.2019.0035
Chapter 3:
https://pubmed.ncbi.nlm.nih.gov/31829515/
Chapter 4:
The SEB would like to congratulate Ivan for being awarded a Frontier Science grant by The National Council of Science and Technology (CONACyT) of Mexico, to start a project on the “Regulation of defense responses in plants against herbivores by signals derived from tRNAs”, at the University of Guadalajara, México.


SARA KOPHAMEL (JAMES COOK UNIVERSITY)
The SEB would like to congratulate Sara Kophamel regarding her last two PhD manuscripts that have been recently promoted in the media.
See here :
https://www.jcu.edu.au/news/releases/2023/ february/new-way-to-check-if-turtles-arefat-and-happy
In preparation, but code and data are already here:
https://github.com/felixpleiva/TDT_DGRP_lines
Chapter 5: https://onlinelibrary.wiley.com/doi/full/10.1111/ gcb.16319
Chapter 6: https://link.springer.com/article/10.1007/s00227018-3406-z
Chapter 7
https://besjournals.onlinelibrary.wiley.com/ doi/10.1111/1365-2435.14294
10 NEWS & VIEWS
2023 marks the 100th anniversary of Journal of Experimental Biology






We are celebrating this historical milestone throughout the year, with activities including:
• publishing a series of Centenary Articles documenting the past, present and future of comparative physiology and biomechanics
• interviewing journal editors about the next 100 years of experimental biology
• promoting early-career researchers and their research
• digitising the full archive of JEB content back to 1923
• celebrating with our community at society meetings
• providing greater funding opportunities to support researchers
Find out more at journals.biologists.com/jeb/pages/100 or scan the QR code below



ANIMAL FEATURE: LIGHT OF THEIR LIVES .............. 14 CELL FEATURE: LOOKING ON THE BRIGHT SIDE ......... 18 PLANT FEATURE: LIGHT STRESSTOO MUCH OF A GOOD THING 22 FEATURES 12 FEATURES

13 FEATURES
LIGHT OF THEIR LIVES
BY ALEX EVANS

The evolution of animal life on Earth has been fundamentally driven by the Sun – not only as our greatest source of heat, but also of natural light. Physiology, biochemistry, community ecology and behaviour are just some of the key aspects of life that are affected by the presence or absence of illumination, so let’s shine a light on some recent research into the world of animal–light interactions.
BUMBLING BEES
Our world is lit by sunlight that animals rely on to find their way around—but the levels of sunlight available vary dramatically depending on when and where an animal finds itself. Performing essential tasks such as looking for food can become quite challenging in low-light environments, especially for animals that rely on flight. Lana de Vries, a PhD candidate at the Experimental Zoology Group of Wageningen University & Research, is currently conducting research on the foraging behaviour of bumblebees and the role that their visual system plays during landing.1 ‘I am interested in understanding the way different animals think, and a good way to study that is looking at their behaviour,’ says Lana. ‘I think insects are especially fascinating because they are so different from us, and by studying the landing behaviour of bumblebees, I’m trying to learn something about the way their brain works.’
As Lana says, understanding the visual systems of animals requires more than just looking at the eyes, you need to explore the secrets of the brain too. ‘Lately, several studies have shown that bumblebees are more intelligent than we thought previously, which makes them a fascinating study species,’ she explains. ‘I also use their intelligence by training the bumblebees to do the things I need them to do in my experiments.’
14 FEATURES
Recently, Lana’s research has focused on the role that light plays in the landing behaviour of bumblebees. ‘I look at the effect of the light spectrum on their landing performance and at how well bumblebees land on moving flowers in different light intensity levels.’ When bumblebees forage, they fly from flower to flower and often have to land on moving surfaces. Their ability to do this successfully and using as little energy as possible depends on their ability to see their target, which itself depends on the light that is available. ‘They need to be able to do this in a large range of light conditions, since the light intensity and spectrum can vary a lot during the day,’ she says. ‘In addition, bumblebees are nowadays often used in greenhouses for the pollination of plants and, inside greenhouses, light intensity levels are often lower than outside and the light spectrum is sometimes manipulated to increase plant growth.’2

To determine how the bumblebee’s flight performance is affected by the light, Lana and her team developed an experimental setup that allowed them to film landing behaviours on moving artificial flowers. To convince the bumblebees to land on them required a bit thought. ‘We came up with the idea of a moving artificial flower with a small hole in the middle, through which the bumblebees would be able to crawl.’ After training the bumblebees to fly towards the flower, land on it and crawl through the hole, they filmed the experimental landings and tracked the movement of the bumblebees using five cameras.
While analysis of her experiments is still ongoing, Lana has a few ideas on the insights that might be in store for her. ‘I would expect that bumblebees will be better able to land on a moving flower if the light intensity is high rather than when the light intensity is low,’ she says. ‘It will, however, be interesting to see how much their ability to land will decline in low-light conditions, given that they generally deal remarkably well with a decrease in light intensity.’ She adds that she is also interested to see if the bumblebees slow down or alter their landing approach flights in low-light conditions to maximise their chances of success with restricted vision.
Expanding on this topic, Lana would like to see how light pollution may affect their behaviour. ‘Knowledge about the behaviour and ecology of night-active insects is still more limited than the knowledge on day-active insects, and light pollution can have large effects on their behaviour, and therefore their fitness,’ she concludes. ‘I would be interested to find out more about the effects of light pollution on insect behaviour and ecology, and to maybe contribute to solutions to decrease these negative effects.’
SYMBIOTIC IN THE AQUATIC
Above
The artificial flower with a hole for the bumblebees to enter Photo credit: Lana de Vries
Photosynthesis is not traditionally a phenomenon associated with animals, but there are a number of animal species that have formed relationships over evolutionary time with organisms capable of harvesting sunlight. One such group of animals is the giant clams (genus Tridacna), who have developed an interesting symbiosis with unicellular algae (zooxanthellae) that can generate food for their hosts in nutrient-poor waters’. In some cases, the algae provide the majority of a clam’s nutrition. However, this useful relationship can also be an unstable one, as Eric Armstrong, an environmental physiologist at the Université de Perpignan Via Domitia in France, explains.
‘I’m working with a large group of international scientists as part of the Tara Pacific Expedition to investigate how reef-building corals and clams across the Pacific Ocean are adapting and responding to climate change,’ explains Eric. ‘My primary area of research is environmental physiology and I’m particularly interested in understanding how ocean warming and acidification affect tropical photosymbionts (giant clams and corals) and intertidal species.’3
Below Tridacna maxima giant clam in Moorea (French Polynesia)

LIGHT POLLUTION CAN HAVE LARGE EFFECTS ON BUMBLEBEE BEHAVIOUR AND THEREFORE THEIR FITNESS
I FELL IN LOVE WITH THE MARINE REALM AND ITS PROSPECTS AS A STUDY SYSTEM
Photo credit: Emily Lam (UC Berkeley)
15 FEATURES
Eric’s interest in giant clams started as a result of a research internship he participated in with Richard Hill at the Michigan State University. ‘Our goal was to examine the role that certain secondary metabolites played in maintaining the symbiosis between reef-building corals and giant clams,’ he says. ‘From there, I fell in love with the marine realm and its prospects as a study system.’ Now, Eric’s research is helping us to understand how clams maintain this symbiosis in the face of fluctuations in their natural environment. ‘In the marine environment, bicarbonate is the primary form of carbon that is available for photosynthesis by the algae within the clams; however, they actually prefer CO2 as a carbon source,’ he says.
One area of Eric’s research has focused on a hostderived protein, vacuolar-type H+-ATPase (VHA), which plays an important role in the processes that convert bicarbonate into the preferred CO2 to increase the productivity of the microalgae, which in turn maximises their photosynthetic production. To determine the full extent that these VHAs play in this symbiotic relationship, Eric and his team have used both molecular and whole organismal methods in their research.4 ‘At the cellular level, I use immunohistochemical staining to determine where certain proteins are located within a cell, which can give clues as to what functional role they play,’ he explains. ‘At the whole organism level, I rely on respirometry to examine the balance between photosynthetic production and respiration within a given host.’ Using this combined approach has allowed Eric to get a more complete picture on the role of VHAs within marine photosymbionts, from their placement within the cell to their activity within the whole animal.
The results of Eric’s study show that VHAs are actually present in all tissues they sampled throughout the clams, but were much more abundant in the mantle where the algae were situated. By inhibiting VHAs, they found that photosynthetic production dropped by almost half—further highlighting the importance they play for both clams and symbionts. ‘I was surprised by the extent to which VHA has been convergently exapted across marine species,’ says Eric. ‘In reef-building corals and giant clams, VHA has been recruited as a carbon pump to promote photosynthesis, but it has also been discovered to play roles both in skeletal formation and in carbonate dissolution—VHA is truly a multipurpose protein!’
Eric’s next steps include investigating whether photoprotective secondary metabolites actually help to mitigate light stress, and if their expressions can be modified by the clams in response to changing environmental conditions. ‘I’d also like to perform a more quantitative examination of the contribution of giant clam acid secretion to reef bioerosion and examine whether liberated carbonate from acid etching is reutilised by the giant clam host or its microalgal symbionts,’ he concludes.
HAWKESTRAL MANOEUVRES IN THE DARK
Animal vision is a fascinating sensory adaptation, without which many animals would be unable to navigate their environment to find food, shelter or mates for reproduction. Processing light into a rich tapestry of information may be something we take for granted, but light doesn’t simply exist in an on-or-off state and different animals are accustomed to different levels of light, with some capable of performing impressive feats of survival at the lower limits of this continuum.
Anna Stöckl, a vision researcher at the University of Konstanz and Fellow of the Zukunftskolleg, tells us more about how hawkmoths function in low-light landscapes.

‘In my last year of school we learned about the mechanisms by which the human retina extracts information from the visual world,’ she remembers. ‘There is a network of neurons that extract certain types of information, discard others, and thus lead to a selective perception of the world.’ From here, Anna became interested in not just human vision, but the diverse spectrum of sight for other animals, including some of the smaller animals on the planet. ‘I research fundamental principles of vision in insects,’ she says. ‘Understanding how insects successfully master many of the same challenges that animals with larger brains do provides very insightful comparisons with larger brains.’
One of Anna’s focuses within this field has been an exploration of the neural processing that enables nocturnal moths to see (and see well enough to fly) in low-light conditions, including those produced solely by starlight. By analysing the behaviour of the nocturnal elephant hawkmoth (Deilephila elpenor) when attempting to suckle nectar from moving flowers, Anna and her team were able to evaluate their flower-tracking performance at different light levels. ‘It was very noticeable that the animals were worse at tracking the flowers at the brightest light intensities such as sunset, compared to the dimmest light such as starlight,’ explains Anna. ‘This was unlike other species of moths, even crepuscular-nocturnal ones, which tracked flowers with higher precision in brighter light intensities.’
Intrigued by this result, the team delved into the neurophysiology of the elephant hawkmoth but found that their visual neurons actually responded similarly to both the higher and lower light intensities, leading the team to suggest that this may be linked to taking the moths out of their flying comfort zone. ‘The impaired flower tracking at higher intensities might be a result of the moths’ internal state, much like when you wake us in the middle of the night,’ she says. ‘Making moths fly outside of their nocturnal phase might be leading to more sluggish behavioural control.’
Anna’s research not only demonstrates the complex interactions between an animal’s anatomy, neurobiology and their internal state, but also raises new questions about the effects that anthropogenic light sources at night may have on low-light
16 FEATURES
specialists such as the elephant hawkmoth. ‘In the near future, we want to understand how flexible the visual system of insects can be to changes in light,’ she says. ‘While the overall light level differs drastically from day to night, this change is very gradual, but light levels can also change rapidly when nocturnal animals fly close to an artificial light source at night.’ Unlike humans and other vertebrates, insects don’t have the rapid pupil mechanisms that regulate light influx to the eye and instead have to rely on neural mechanisms, leaving much that we still don’t know about how insect vision will fare in an increasingly well-lit world.
LOOKING ON THE BRIGHT SIDE
As the famous adage tells us, too much of a good thing can be a bad thing, and light is no exception. Much of the animal kingdom has developed some type of biological mechanism for sensing and interpreting light in the visible or near-visible spectrum, but with continued exposure to light at high intensities, these finely tuned mechanisms can become damaged or even permanently disabled. In a world with a changing environment and increasing pollution from artificial light sources, what impacts can we expect changes in light intensity to have on organisms? Thankfully, Martta Viljanen, a researcher at the Norwegian University of Science and Technology in Trondheim, Norway, is on the case.
‘My main research area is visual ecophysiology and I am specifically interested in how aquatic animals are adapted to different light environments,’ she explains. ‘I also have a soft spot for weird taxa and non-model organisms.’ When she was looking for a topic for her master’s thesis, she was presented with an opportunity to study the visual physiology of a glacial relict opossum shrimp (Mysis relicta) in different light environments, and she eventually continued this research through into her PhD.5 ‘Visual systems of aquatic animals provide a great approach for studying adaptation and acclimation, because underwater light as an environmental factor and artificial light as experimental stimulus are easily measurable and their effects on physiology and behaviour quantifiable,’ she adds.
Animals become acclimated to performing certain actions, including foraging and sleeping, at certain light levels, but interference from humans can affect the timing and intensity of these levels and disrupt the natural stimuli that these animals rely on, exposing them to light-induced damage. ‘Our results show that not only is the light exposure history crucial for the emergence of light-induced morphological and functional changes in the crustacean eyes, but also the timeframe they
are presented in,’ says Maarta. ‘It seems that the adaptive mechanisms are functioning at time scales present in nature, which should be taken into account when, for example, planning laboratory studies on animals caught in dim light environments.’
To fully examine how light is absorbed in these crustaceans, Martta and her team have adopted a full-spectrum approach from molecular methods up to animal behaviour analysis, while also considering the evolutionary and ecological context of these changes. ‘The molecular level includes studying the genes coding opsins (proteins in visual pigments) and their expression in the eyes,’ she says. ‘These are the molecules actually catching the photons and thus responsible for the first steps in vision.’ Scaling up to the cellular and organ levels, Martta also studies the spectral sensitivity of eyes with a microspectrophotometer, which allows them to measure light absorption for single cells, and electroretinography, which allows her to record electrical responses to an array of light stimuli at the across the whole eye. Together, these techniques provide invaluable insights into how light is processed by the eyes, especially when there is too much to absorb safely.
From the results of her work with collaborators at the Moscow State University,5 Martta was surprised to see that light-induced damage in the eyes of these crustaceans can actually take several weeks to recover from. ‘This is again something to consider when planning and conducting experiments,’ she explains. ‘For many marine invertebrates it has been quite common practice to let them dark acclimate 24 hours after a light exposure before any experiments related to visual functions, but if some damage has taken place this might be way too short a time.’ More recently, Martta has been involved in a project called LightLife,6 where she is studying the photobiology of plankton in the Arctic and Northern waters to assess the dynamic range of vision on zooplankton species and the role played by light-induced damage. ‘Part of this work is carried out during the polar night when the zooplankton has been shown to react to extremely small changes in the ambient light levels,’ she concludes.
Reference:
1. https://www.wur.nl/en/project/Visual-system-ofbumblebees-1.html
2. De Vries LJ, Van Langevelde F, Van Dooremalen C, et al. Bumblebees land remarkably well in red–blue greenhouse LED light conditions. Biol Open 2020; 9: bio046730.
3. https://www.armstrongecophys.com/giant-clam-physiology
4 Armstrong EJ, Roa JN, Stillman JH, et al. Symbiont photosynthesis in giant clams is promoted by V-type H+ATPase from host cells. J Exp Biol 2018; 221: jeb177220.
5. Feldman T, Yakovleva M, Viljanen M, et al. Dark-adaptation in the eyes of a lake and a sea population of opossum shrimp (Mysis relicta): retinoid isomer dynamics, rhodopsin regeneration, and recovery of light sensitivity. J Comp Physiol A 2020; 206: 871–889.
6. https://www.instagram.com/lightlife_project/
VISUAL SYSTEMS OF AQUATIC ANIMALS PROVIDE A GREAT APPROACH FOR STUDYING ADAPTATION AND ACCLIMATION’
MARTTA WAS SURPRISED TO SEE THAT LIGHTINDUCED DAMAGE IN THE EYES OF THESE CRUSTACEANS CAN ACTUALLY TAKE SEVERAL WEEKS TO RECOVER FROM.
17 FEATURES
Opposite Page Elephant hawkmoth Photo credit: Anna Stockl
LOOKING ON THE BRIGHT SIDE
BY ALEX EVANS
Light is one of the most abundant natural resources on our planet, one that plays an important role in all manner of biological processes down at the cellular and molecular levels. Naturally, these processes are most commonly found in photosynthetic plant cells, where tiny changes in external light can have huge consequences for the organism as a whole. Let’s hear from some of the researchers highlighting these important cell–environment relationships.
The weird and wonderful relationships that light forms with living things means that we are still finding out how light influences biology right down at the cellular and molecular levels. Marie Hronkova is a plant physiologist at the Faculty of Science, South Bohemia University in České Budějovice, who is exploring the surprising role that light plays in the development of stomatal cells. “The main area of my research is how plants cope with often unpleasant environmental conditions and how these conditions modify them”, explains Marie. “I am fascinated by the mechanisms that nature employs for survival.”
“During my PhD, I focused on the role of abscisic acid (ABA) in photosynthesis,” says Marie. “ABA is the main signal for stomatal opening and closing, but also for development of leaves and stomata cells in epidermis of new leaves.” It was around this time that Marie also became interested in the effects of light intensity on stomata development. “Light is not only used as energy for photosynthesis but also provides important information for cells and for their behaviour,” she adds. “For this work, I closely followed the work of prestigious labs dealing with stomata development in the UK, Spain and especially in the United States.”
18 FEATURES
In 2010, two teams of researchers published research on a small protein called STOMAGEN, which is produced in the mesophyll of leaves, that was shown to help stomatal development,1,2 and Marie was keen to investigate the role it might be playing in light signalling. Thanks to Professor Ikuko Hara-Nishimura, who kindly supplied the seeds of their experimental mutants, Marie was able to further explore STOMAGEN. “The main message of our work was that elevated photosynthetic irradiation stimulates STOMAGEN expression, which positively regulates stomatal formation on both sides of the leaf,” she explains.3 “We were the first to show the important connection between light and STOMAGEN, but unfortunately, we didn’t know the mechanism at work.” Thankfully, more recent research has now revealed that the light-regulated transcription factor ELONGATED HYPOCOTYL 5 (HY5) directly binds to and activates STOMAGEN.4
To carry out her research, Marie uses a range of techniques that allow her to measure different functions of plant tissues. “I can measure physiological functions of the plants, photosynthesis and transpiration using gas exchange and fluorescence methods, and I have also used infrared cameras in my research,” she says. “Simultaneously, I can’t imagine my research without molecular biology methods like transcriptomic techniques using real-time reverse transcriptase PCR.” Marie and her team are continuing with their research on the interactions between light and stomatal development, and would like to focus on the possible consequences for plant immunity. “I really would like to thank the SEB, which celebrates its centenary this year, for the opportunity to present my research at the annual meetings (especially Gothenburg 2017 and Seville 2019), for a stimulating and friendly atmosphere there,” she concludes. “I am a proud and long-time member of this community!”
WHEN PLANTS SEE RED
We often think of light as a combination of all the colours of the rainbow, but under certain conditions, parts of this rainbow are more plentiful than others. For photosynthetic organisms, the available wavelengths of light that they are exposed to can have interesting effects right down to the cellular level. Kasper van Gelderen is an independent group leader at the Centre for Organismal Studies at Heidelberg University in Germany, and he is working to improve our understanding of the role that the ratio of red to far red (R:FR) light plays in root development.
Kaspar was first intrigued by the role of light in root development during his PhD when he worked on the regulation of polar auxin transport, including the light-regulating transcription factor HY5. “I got the opportunity to work in Utrecht in the group of Ronald Pierik on the problem of how
root systems in competing plants get smaller,” he explains. “We knew that somehow there must be a shoot-to-root signal that relays the light quality information from the shoot to the root, and during our research this turned out to be HY5, bringing me back to my PhD work.”

As well as improving our understanding of the molecular mechanisms that drive wild plant growth, for those involved with agriculture and horticulture, finding the best way to grow plants has huge economic benefits. “Increased FR wavelengths, or a decreased R:FR ratio is usually indicative of plant–plant competition, given that FR light is reflected by chloroplasts,” Kaspar explains. “FR light is sensed by the plant via phytochrome photoreceptors, and they relay this light quality signal to downstream transcription factors.” Part of the value of Kaspar and his team’s research comes from their ability to create physiologically relevant situations in the laboratory, which allows them to reconstruct real-life phenotypes that they can study at the molecular and genetic level. “For example, when you do vegetable gardening, you have to thin out some of the young plants because they grow too small otherwise,” he explains. “This is due to R:FR signalling, and with the research we have done, we now understand the molecular basis of the limits of planting density.”
In order to create the right environmental conditions for this study, Kaspar and his team had to make sure that they could prevent light reaching the root while allowing extra FR light to reach the shoot. “For that, we covered our square petri dishes with black paper and added an extra insert into the plate in order to create a small opening for the plants to grow through but minimise the light going to
Root growth of Aradopsis seedlings in the white light (WL) and white light plus far-red light (WL+FR), adapted from van Gelderen et al., 20187
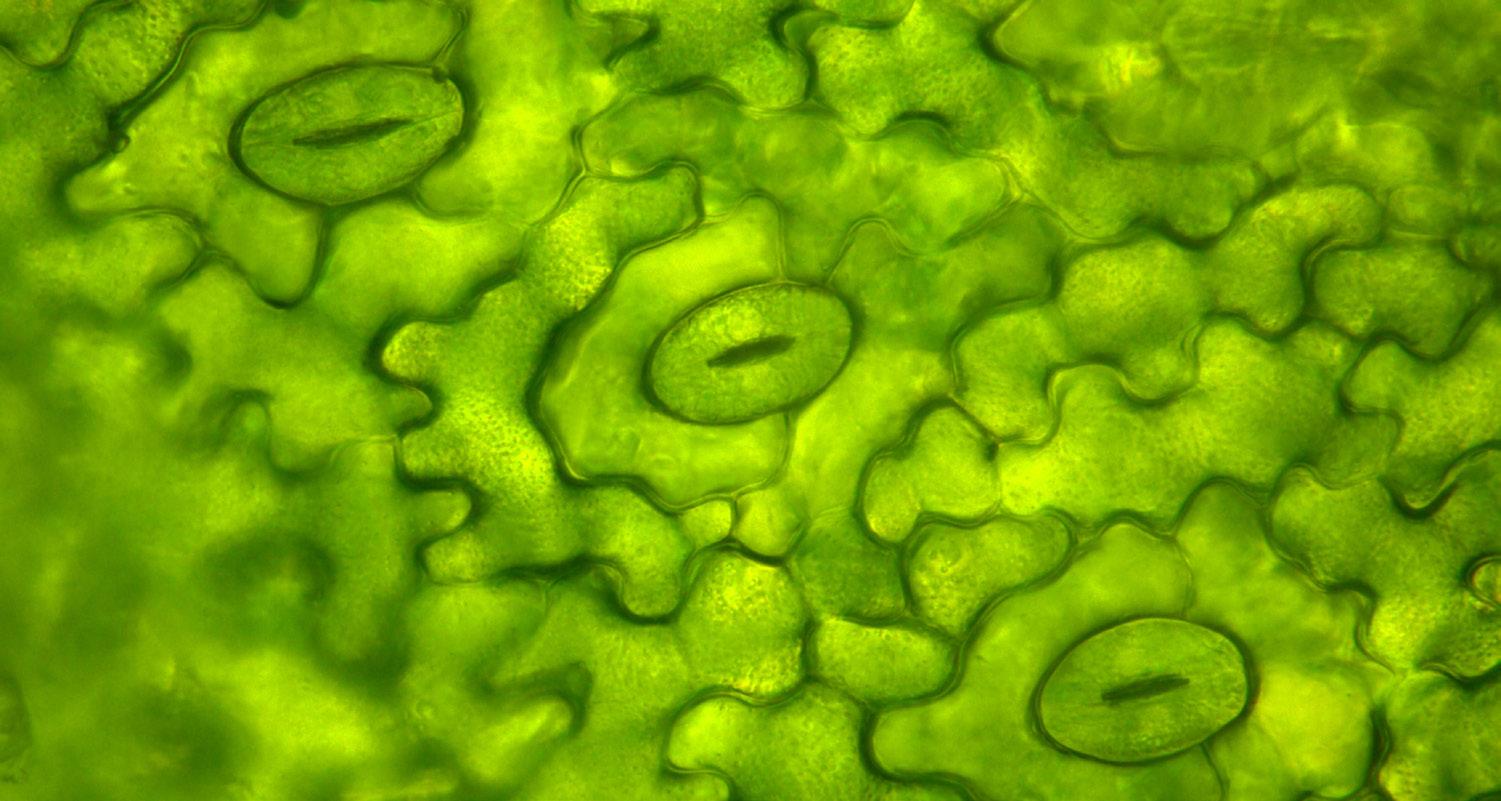
Opposite Page Leaf stomata under the microscope
Photo credit: Shutterstock.com
I AM A PROUD AND LONGTIME MEMBER OF THIS COMMUNITY!
FR LIGHT IS SENSED BY THE PLANT VIA PHYTOCHROME PHOTORECEPTORS, AND THEY RELAY THIS LIGHT QUALITY SIGNAL TO DOWNSTREAM TRANSCRIPTION FACTORS.
Left
19 FEATURES
Photo credit: Kaspar van Gelderen
the root compartment,” he says. They then used a program called SmartRoot to analyse their developing root systems semi-automatically and find out how their experimental conditions were affecting cellular growth. “Finally, we did expression analyses with micro-array and quantitative PCR to look at gene expression differences due to extra FR light,” he adds.
Kaspar’s research has shown experimentally how plants are able to change their root systems based on light cues picked up by their shoots. “A lot of people have asked me… why do plants do that?” he says. “Our best answer today is that they do this to conserve resources for shoot growth, because making lateral roots is very resource intensive.” In a follow-up study, they also combine the FR stimulus with nitrogen stress and show that with low nitrogen abundance, the plants will no longer reduce their root growth in response to FR ligth.
“I am now looking further into the mechanics and cell biology of HY5 transport,” says Kaspar. “There are several papers that have shown that HY5 transport is functionally relevant and HY5 is a small transcription factor, so it should be easier to move around—but how is that regulated, and how does it work in space and time?”
SIMPLIFYING THE COMPLEX
Light is a fundamental requirement for plants because it supplies the energy they need for growth and reproduction through photosynthesis. However, exposure to too much light can be problematic, and many organisms have developed mechanisms to modulate their photosynthetic activity to strike a balance between harvesting enough photons to live and dissipating excess
light to avoid photodamage. One such group of mechanisms involves the light-harvesting complexes (LHCs), which are pigment-protein systems involved in absorbing photons. Roberta Croce, head of the Biophysics of Photosynthesis group at Vrije Universiteit in Amsterdam, has been researching the underlying molecular processes at work to help paint a clearer picture of the role that LHCs play in photosynthesis.
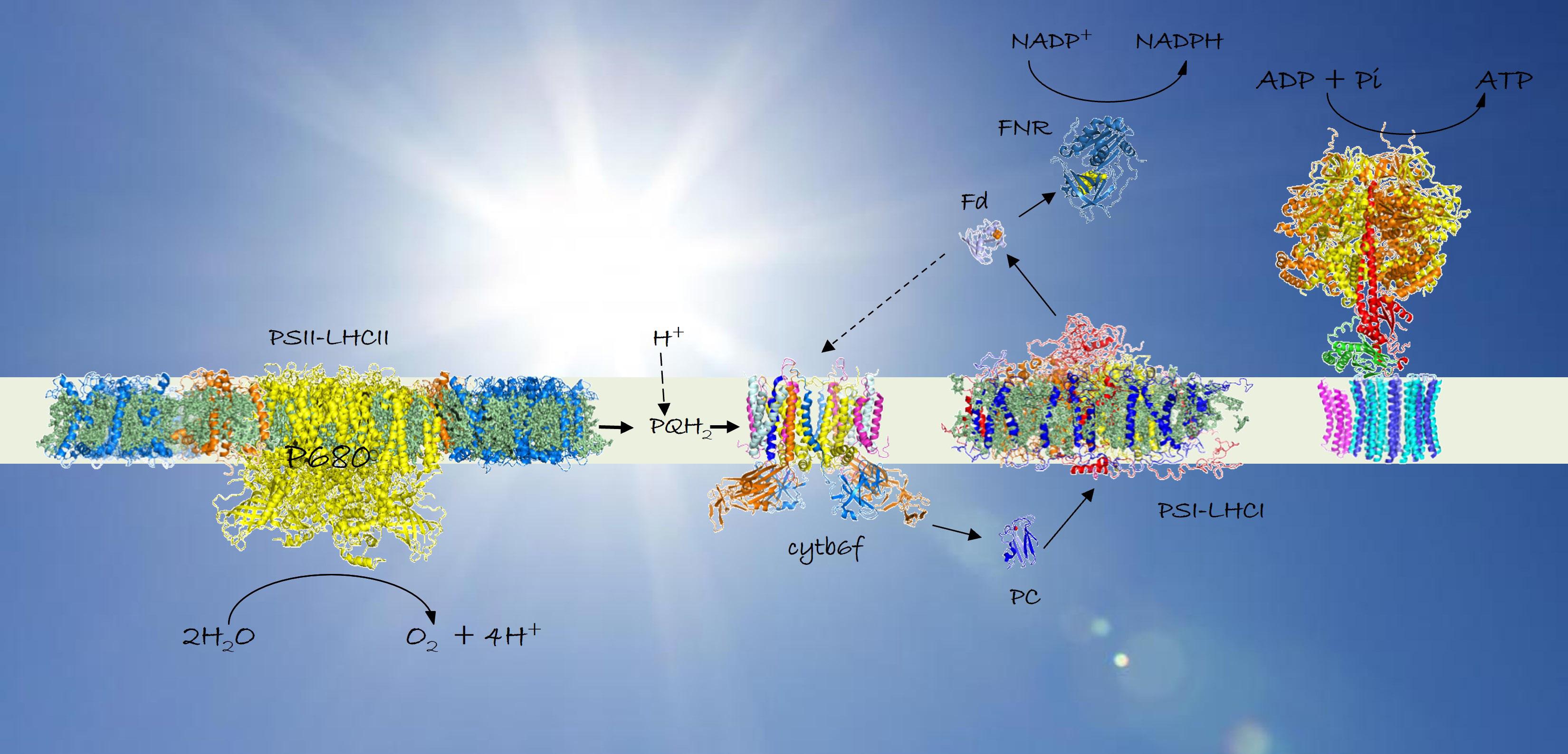
“I find it fascinating to understand the chemistry and physics behind biological processes,” says Roberta. “And, of course, it is hard to overestimate the importance that photosynthetic light harvesting had in shaping and supporting life on Earth.” As part of this research, Roberta and her team have recently demonstrated how LHCs can switch between different roles. “It is very intriguing that, in plants, the same pigment-protein complexes, the LHCs, can play opposite roles depending on the light conditions,” she says. “They absorb as many photons as possible in light-limiting conditions and dissipate as heat the excess energy absorbed in high light.”
As Roberta explains, the ability of organisms to protect themselves from over-exposure to light is a topic of much recent research, and a lot of this work is being conducted at the molecular and cellular level. “It is important to understand how photosynthetic organisms protect themselves; to harvest light and concomitantly produce oxygen is in principle very dangerous so sometimes they overdo it a bit to play safe,” she says. “It was shown that speeding up the quenching relaxation when light intensity decreases can lead to a substantial increase in plant productivity in the field.”
To fully investigate these LHCs, Roberta and her team utilise a suite of techniques that incorporate molecular biology, biochemistry, plant physiology
Above Diagram of the phyosynthetic membrane containing the light-harvesting complexes, adapted from Croce and van Amerongen, 20178
Photo credit: Roberta Croce
20 FEATURES
A LOT OF PEOPLE HAVE ASKED ME: WHY DO PLANTS DO THAT?
and ultrafast laser spectroscopy. “This integrated approach allows us to analyse the problem from different angles and at different levels of complexity, from individual proteins to the leaf,” she explains. “For example, we can measure excitation energy transfer in the photosystems in the leaves with picosecond resolution and we can combine those with mutants that have modified pigment-binding sites or that lack individual complexes.” Alongside this, they are also able to analyse the composition and properties of the photosynthetic apparatus of the leaves, which helps them to understand what is going on in different conditions.
Recently, Roberta and her team discovered that the presence of an antenna increases the photonto-electron conversion quantum efficiency of the photosystems, which is the opposite to what was anticipated.5 “The antenna is there to increase the absorption cross section of the photosystems, but usually this lowers the quantum efficiency,” she explains. “Normally, there is always a trade-off between absorbing more photons and efficiency, but instead, in those cyanobacteria, the antenna increases the absorption AND the efficiency—this was extremely surprising.”
Roberta’s interest in light harvesting certainly isn’t quenched and she has now begun work on lines of enquiry that she is keen to keep exploring. “In the last few years, we have started studying cyanobacteria that can use FR light for growth,” says Roberta. “FR light is outside the photosynthetic active radiation and was considered unable to drive oxygenic photosynthesis, but it is clearly used by many cyanobacteria strains and we want to know how they do it and see if we can also ‘convince’ plants to do it as well.”
HOW TO STAY SHADY
It’s not surprising that plant growth tends to be driven in favour of light over shade, given that light is required for healthy development, but this doesn’t always produce the best outcome for human-cultivated plants. In fact, this biological need to reach out towards the light can actually be a major issue for agriculture, but thankfully Sara Bunti, a Research and Education Assistant at Utrecht University in the Netherlands, is using genetic and molecular techniques to curb these instinctual patterns of growth known as shade avoidance syndrome (SAS).
“SAS consists of the elongation of petioles and internodes together with the upward movement of the leaves, called hyponasty, to escape from shade,” she explains. “From an agronomic point of view, this syndrome is mostly undesirable because the investments in the elongation process can go at the expense of the harvestable
organs.” While several attempts have been made to understand the molecular mechanisms that control SAS, Sara hopes that her research contributes to applications that can suppress this syndrome and improve crop yields.
After studying SAS as part of her PhD project in the group of Ronald Pierik at Utrecht University, Sara continued investigating this topic because the combination of physiology and biotechnology captured her interest, especially in the context of modern agriculture. “The increasing human population coupled to the increasing scarcity of arable land has demanded an intensification of plant cultures,” she explains. “This ultimately leads them to compete for light, which induces the undesirable trait known as SAS, and my research aims to show how the response of plants to different stimuli is highly regulated by a complex network of interactions by transcription factors.”
To achieve this, Sara and her team assessed how the typical traits of SAS responded to red light treatment, which is used to mimic vegetative shade. “I perform these studies in mutants in which candidate genes are knocked out or overexpressed using common techniques of gene cloning and gene editing,” she explains. “Moreover, I investigate this complex network of interactions through protein–protein and DNA–protein interaction techniques such as yeast two-hybrid, yeast one-hybrid, bimolecular fluorescence complementation, protein localisation and co-localisation studies.” Through this multi-technique approach, Sara is able to analyse how the plants can modulate the strength of their shade responses and apply this to strategies for possible crop improvement.
One of the surprising findings of this research was that plants actually respond to shade by expressing genes involved in both elongation and the suppression of elongation simultaneously.
“The reason for this lies in the proposed gasand-brake mechanism composed by the different layers of interaction between proteins with opposite functions,”6 she says. “This describes a fine-tuned mechanism necessary to control the magnitude of the shade avoidance response in order to avoid an exaggerated elongation.”
Sara’s interest in this area is still growing and she is keen to explore the other ways in which light influences plant growth at various levels. “These days, we are very interested in understanding how heterogeneous light or shade signals can be integrated and how light perception by one leaf section can regulate responses elsewhere in the plant,” she says.
“We are also very interested in understanding how expression of specific regulators, such as the helix-loop-helix protein genes, is being controlled.”
Reference:
1. Sugano SS, Shimada T, Imai Y, et al. Stomagen positively regulates stomatal density in Arabidopsis. Nature 2010; 463: 241–244.
2. Kondo T, Kajita R, Miyazaki A, et al. Stomatal density is controlled by a mesophyllderived signaling molecule. Plant Cell Physiol 2010; 51: 1–8.
3. Hronková M, Wiesnerová D, Šimková M, et al. Light-induced STOMAGEN-mediated stomatal development in Arabidopsis leaves. J Exp Bot 2015; 66: 4621–4630.
4. Wang S, Zhou Z, Rahiman R, et al. Light regulates stomatal development by modulating paracrine signaling from inner tissues. Nat Commun 2021; 12: 3403.
5. Mascoli V, Bhatti AF, Bersanini L, et al. The antenna of far-red absorbing cyanobacteria increases both absorption and quantum efficiency of Photosystem II. Nat Commun 2022; 13: 3562.
6. Buti S, Pantazopoulou CK, van Gelderen K, et al. A gas-and-brake mechanism of bHLH proteins modulates shade avoidance. Plant Physiol 2020; 184: 2137–153.
7. van Gelderen K, Kang C, Pierik R. Light signaling, root development, and plasticity. Plant Physiol 2018; 176: 1049-1060.
8. Croce R, Van Amerongen H. Natural strategies for photosynthetic light harvesting. Nature Chem Biol 2014; 10: 492–501.
I AM FASCINATED BY THE SEEMINGLY ABSTRACT WORKINGS OF THE INNER CELL
21 FEATURES
LIGHT STRESS — TOO MUCH OF A GOOD THING
BY CAROLINE WOOD
Light is the fundamental energy that drives plant growth and productivity, but outside the controlled conditions of our research laboratories, its intensity can fluctuate between extremes in a matter of seconds. This can present plants with the constant challenge of striking a delicate balance between maximising gains whilst keeping the delicate photosynthetic machinery safe from damage. Here, we take a look at fascinating new insights into the exquisite photoprotective mechanisms that help plants to stay safe from light stress.
GETTING THE GRADIENT RIGHT
Intense spikes in luminescence—a sudden break in cloud cover, for instance—expose plants to potentially damaging amounts of excess light energy, capable of causing irreparable damage. Photoprotective mechanisms counteract this by dissipating excess light energy and downregulating photosynthesis during these events. Often, this involves adjusting the proton gradient across the thylakoid membrane that transiently stores the energy that powers photosynthesis. “Ongoing energy dissipation in shade periods, however, would decrease the efficiency of photosynthesis under natural, dynamically changing light conditions,” says Ute Armbruster, from the Max Planck Institute of Molecular Plant Physiology. “Consequently, plants rapidly turn off photoprotective mechanisms as soon as light intensity drops again. Our research has demonstrated that these switches in response to changes in light intensity are heavily accelerated by ion transporters in the thylakoid membrane.”
An intriguing question was whether the activity of these photoprotective mechanisms varied depending on the environment of the plant during its early development. To investigate this, Ute and her colleagues grew Arabidopsis thaliana plants under a variety of different light regimes, with some that fluctuated according to regular or random patterns and non-fluctuating control regimes.1 Plants from these different light environments were then exposed to a sudden, ten-fold increase in light intensity. By measuring changes in chlorophyll fluorescence and absorptive processes related to photosynthesis, the team assessed the activity

22 FEATURES
of two thylakoid ion transport proteins, VCCN1 and KEA3. As a Cl− channel, VCCN1 lowered the membrane potential and promotes the induction of nonphotochemical quenching to dissipate excess light energy. Conversely, the K+/H+ exchanger KEA3 activated once light levels dropped again, to accelerate the relaxation of quenching mechanisms.
For both transporters, activity during high light stress strongly depended on the growth environment, with VCCN1 activity during high light stress correlating negatively and KEA3 activity positively with the average growth light intensity. “Together, this results in increased and faster activation of photoprotection in plants coming from growth conditions with low light intensities, and decreased activation in plants from growth conditions with higher average light intensities,” says Thekla von Bismarck, lead author of the study.
Their investigations also revealed that KEA3 suppresses the accumulation of zeaxanthin, a pigment which delays the relaxation of energydependent quenching mechanisms. The effect was particularly evident in plants grown under high or strongly fluctuating light intensities. “In fluctuating growth light conditions, this function of KEA3 would contribute to a more rapid response of a major photoprotective mechanism to changes in light intensity,” adds Thekla. “This discovery mandates future crop improvement strategies, which seek to accelerate photosynthetic responses, to consider acclimation effects on target regulator functions.”
According to Ute, another key finding from the study is that the photoprotective activities of thylakoid ion transport proteins only activate when the capacity for photosynthetic assimilation is saturated. This suggests that it may be possible to enhance the light reactions by manipulating carbon assimilation to lift downstream metabolic limitations. “At the same time, it is also plausible that the activities of thylakoid ion transport proteins have a tendency to ‘overprotect’ the system, ultimately restricting photosynthesis,” she adds.
“We are still in the infancy of understanding how thylakoid ion transport proteins sense and respond to metabolic changes, and the next step for our work is to resolve this. Additionally, we are interested to understand how the function and regulation of thylakoid ion transport proteins differ between photosynthetic organisms with variable morphological and physiological characteristics as well as environmental requirements.”
A RAPID READJUSTMENT
The thylakoid membrane may also be implicated in another intriguing photoprotective mechanism through a physical link with plastoglobules. These may be one of the more underappreciated plant organelles, but recent studies indicate that plastoglobules have important roles in mediating stress perception and adaptative responses in plants. Found within chloroplasts, these lipid droplets are continuous with the photosynthetically active thylakoid membrane and, according to Peter Lundquist (Michigan State University), “represent an underexplored component of plant stress tolerance, with clear potential for enhancing crop resilience”. The evidence for this includes the fact that loss-of-function mutations in plastoglobulerelated genes demonstrate greater sensitivity to many abiotic stresses (including light stress), and the observation that virtually any stressful event causes plastoglobules to swell dramatically: often a more than 400-fold increase in volume.
But the exact role that plastoglobules may play in stress responses, besides the mechanisms they use, remain largely unknown. “My theory was that one key process may be a rapid adjustment of the lipid and protein composition in thylakoid membranes,” says Peter. “Despite the physical connectivity between plastoglobules and thylakoids, the plastoglobules maintain a distinct population of proteins and lipids, suggesting the existence of a proactive selection mechanism. Furthermore, the dramatic changes in plastoglobule size during
Chlorophyll fluorescence is captured from Arabidopsis thaliana to determine the activity of photoprotective mechanisms that protect against the damaging effects of excess light energy
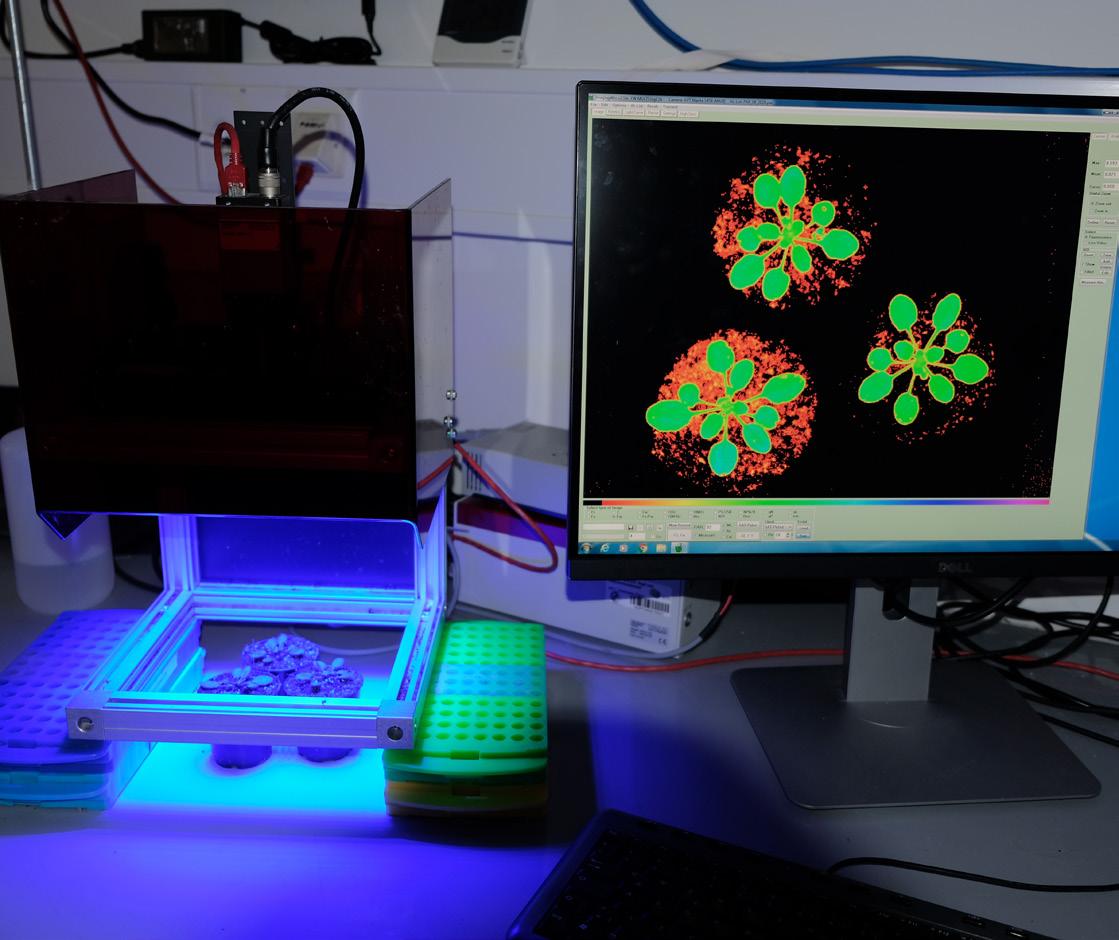
Photo credit: Ute Armbruster
Below Peter with his highperformance liquid chromatography instruments used for measuring carotenoids and other prenyl-lipid compounds
Photo credit: Peter Lundquist

Left
OUR RESEARCH HAS DEMONSTRATED THAT THESE SWITCHES IN RESPONSE TO CHANGES IN LIGHT INTENSITY ARE HEAVILY ACCELERATED BY ION TRANSPORTERS IN THE THYLAKOID MEMBRANE
23 FEATURES
stresses imply a substantial influx of additional lipids, whether shuttled from the thylakoid membrane or synthesized de novo on the plastoglobule surface.”
To investigate this, Peter and his team compared unstressed plastoglobules from Arabidopsis with those that had been subjected to 5 days of light stress.2 Their results demonstrated that light stress induced highly specific, tailored changes to the lipid and protein composition of plastoglobules. In particular, specific carotenoid lipids used in light harvesting, such as zeaxanthin, over-accumulated within the light-stressed plastoglobules. “This indicates that turnover of light-harvesting complexes is occurring and the released cofactor lipids are subsequently shifted to the plastoglobule for storage,” Peter says.
Consistent with this, the plastoglobules also accumulated proteins involved in leaf senescence processes, including a lipase and protease, and an enzyme of the jasmonic acid biosynthetic pathway. “Although the light stress treatment that was applied does not induce visible senescence, our results suggest that senescent processes related to turnover of light harvesting machinery are employed by the plant as an adaptive response to the light stress treatment,” adds Peter. “Early senescent processes, including turnover of thylakoid lipids and protein, do not represent a commitment to cell death and are in fact important to protect the plant from overproduction of harmful reactive oxygen species.”
Potentially, these responses may be driven by changes in kinase activity. The group found that isolated plastoglobules demonstrated kinase activity towards multiple target proteins, and that this was more pronounced in plastoglobules of unstressed than light-stressed leaf tissue. “Our current theory is that kinase activity is the master regulator of the adaptive molecular changes that take place in plastoglobules during stress events,” says Peter. “Thus, the transition from low to high light intensities tailors the activity of plastoglobule proteins through phosphorylation to accumulate compounds derived from thylakoid membrane remodelling, such as carotenes. The next stage of our experiments will be to elucidate the effect that phosphorylation may have on plastoglobule-localised proteins. Possibilities include regulating sub-plastid localisation, activating or inactivating enzymes, or altering the oligomeric state of the proteins.”
A VERSATILE HORMONE
Many of the cellular adaptations that plants use to combat abiotic stresses are coordinated by hormones, which can act as chemical messengers at the whole-plant level. Strigolactones (SLs) are phytohormones derived from carotenoids and are important for root/shoot growth and for promoting the symbiotic interaction with arbuscular mycorrhizal fungi. Recent evidence indicates that they may also have a role in adaptations to high light stress, as Sibu Simon (formerly Mar Athanasios College for Advanced Studies, India, now Mahatma Gandhi University) explains. “We carried out a SL-based transcriptome study in Arabidopsis, and conducted a meta-analysis comparing existing transcriptome data available with a wide range of different plant hormones. We identified that SLs uniquely regulated photosynthesis-related genes, in comparison to other plant hormones. Specifically, they upregulated genes in the light reaction pathway related to photosystem I and photosystem II. This made us ask whether SLs have any direct role in regulating photosynthesis and resulting high light stress tolerance in plants.”3
To investigate this, Sibu and his colleagues measured the photosynthesis rate of Arabidopsis plants after exogenous application of SL and compared their tolerance to high light stress (a jump from 60 to 1000 µmol/m2/s). The SL signalling mutant d14 exhibited a lower photosynthesis rate and was hypersensitive to high light stress, showing a more severe phenotype and a poorer recovery. Wild-type seedlings treated with SL, however, showed less damage and a stronger recovery in high light treatment compared with nontreated plants. Furthermore, SL treatment was ineffective on d14, adding further evidence that the hormonal pathway was responsible for the phenotype.

Additional investigations revealed that the SL treatment significantly reduced both chlorophyll and carotenoid content in the wild-type seedlings. “Because chlorophyll and carotenoids act to protect light harvesting complexes from photoinhibition, this reduction could serve to increase the rate of the light-dependent photosynthesis reactions,” says Sibu. In support of this, chlorophyll fluorescence experiments revealed that SL treatment increased the quantum yield of photochemistry (the fraction of absorbed photons used for photochemical reactions) and decreased nonphotochemical quenching. “This confirms that SL treatment led to better utilisation of light via the photochemical electron transport pathway, and consequently a higher light reaction activity of photosynthesis,” says Sibu.
Metabolite profiling indicated that this had a downstream effect on the carbon fixation reactions. In Arabidopsis seedlings exposed to high light stress and treated with SL, there was a significant upregulation of intermediate compounds in both glycolysis (e.g. glucose-6-phosphate, fructose-6phosphate and pyruvate) and the tricarboxylic acid cycle (e.g. citric acid, succinic acid and-malic acid).
“Our results suggest there is a dynamic change in the metabolite levels as the plants respond to high light stress following SL treatment, suggesting that light acclimation involves modulation in the rate of carbon fixation,” says Sibu.
“This is the first study to have demonstrated a role of SLs in high light stress tolerance in plants. Furthermore, this appears to be through a direct effect of SLs in regulating photosynthesis rate,” he adds. “Upregulating photosynthesis would be an effective means to deal with the excess light energy. Whilst this study was limited to the initial phase of SL treatment during stress conditions, further time series could provide more insights into the dynamic changes throughout the recovery of the seedlings.”
24 FEATURES
THE TRADE-OFFS TO INDOOR LIVING
Of course, one way to protect our crops from light stress would be to grow them indoors under controlled conditions. But providing enough artificial light can incur prohibitively expensive energy costs, prompting plant researchers to investigate how the light spectrum could be optimised to most effectively promote plant photosynthesis and growth. Although red and blue light are considered to be the dominant photosynthetically active radiation, Steven Driever (Wageningen University & Research) is interested in the evidence that wavelengths outside these ranges also affect light harvesting reactions. “For instance, far-red light in the region of 700–800 nm can enhance photosynthesis when given in addition to light in the range of photosynthetically active radiation, known as the Emerson enhancement effect,” he says. “But using this as a means to boost growth is far from straightforward. We have to remember that light is not only used for photosynthesis, but that light is also perceived as a signal by plants to induce developmental changes.”
Alterations in the ratio of red to far-red light (R:FR), for instance, are sensed by phytochromes that trigger morphological changes collectively known as the shade avoidance response. In many “shade-avoider” species, this results in thinner leaves, with a lower mass per unit area, lower chlorophyll and rubisco content, and an increased resistance to CO2 diffusion—all of which may lead to a reduced photosynthetic capacity per unit area. “So, in protected cultivation systems, addition of far-red light could be beneficial by driving photosynthesis on the one hand, but could also be counterproductive by triggering the shade avoidance response on the other hand,” says Steven. “However, in a controlled light environment, light intensity and R:FR can be altered independently, which could potentially give an opportunity to overcome such trade-offs.”
To investigate this, Steven and his group grew tomato plants under white light emitting diodes (LEDs) with and without additional far-red light at two light intensities, and assessed the changes in photosynthetic responses, besides leaf mass, thickness, nitrogen content and CO2 diffusion resistance.4 “Our particular focus was on low, limiting light intensities that occur often when white LED lighting is used as the sole light source,” he says.
For both light intensities, the addition of far-red light to white LED light induced a shade avoidance type response and increased the resistance to CO2 diffusion, as expected. But addition of far-red light to white LED light had a significantly greater negative effect on photosynthesis for the plants that were grown at a low light intensity. This was
WE HAVE TO REMEMBER THAT LIGHT IS NOT ONLY USED FOR PHOTOSYNTHESIS, BUT THAT LIGHT IS ALSO PERCEIVED AS A SIGNAL BY PLANTS TO INDUCE DEVELOPMENTAL CHANGES
due to a stronger shade avoidance response that reduced leaf mass, nitrogen content and, most strongly, increased diffusion resistance to CO2. A less negative effect of additional far-red was found at high light intensity, which suggests that the negative effect on photosynthesis can be partially overcome by increasing light intensity. “This raises the question of whether photosynthesis could be improved if the shade avoidance response is systemically reduced, for instance, in plants that cannot sense far-red light,” says Simon.
Nevertheless, the findings potentially indicate ways in which CO2 diffusion through the leaf mesophyll could be improved for herbaceous crops such as tomato. “The diffusion resistance was lower when leaves had a high mass per unit area and thickness. Because these leaf properties are relatively low for tomato, compared to other crops, there is room to improve CO2 diffusion to enhance photosynthesis when light becomes non-limiting,” says Simon. “We intend to use plants with phytochrome sensing deficiency to investigate the impact of a lack of far-red light sensing on leaf anatomy and CO2 diffusion in the mesophyll.”
Don’t forget! Taking place 4–7 July in Edinburgh, UK, the SEB Centenary Conference 2023 will showcase a wealth of cutting-edge research on plant adaptations to many different types of environmental stresses. The full programme of sessions and booking information can be found on the SEB website.
Above Measurement of a tomato plant with a set-up for gas exchange and cryotrapping of CO2 in the laboratory

Photo credit: Maarten Wassenaar
Top Left
Scanning electron micrograph of isolated Arabidopsis thaliana plastoglobules
Photo credit: Peter Lundquist
References:
1. von Bismarck T, Korkmaz K, Ruß, et al. Light acclimation interacts with thylakoid ion transport to govern the dynamics of photosynthesis. New Phytol 2023; 237: 160–176.
2. Espinoza‐Corral R, Schwenkert S, Lundquist PK. Molecular changes of Arabidopsis thaliana plastoglobules facilitate thylakoid membrane remodeling under high light stress. Plant J 2021; 106: 1571–1587.
3. Thula S, Moturu TR, Salava H, et al. Strigolactones stimulate high light stress adaptation by modulating photosynthesis rate in Arabidopsis. Journal of Plant Growth Regulation 2022; doi: 10.1007/s00344-022-10764-5.
4. Wassenaar MLJ, van Leperen W, Driever SM. Low red to farred ratio increases resistance to CO2 diffusion and reduces photosynthetic efficiency in low light grown tomato plants. Environmental and Experimental Botany 2022; 200: 104918.
Bottom Left Custom growth cabinet “Solinator” with tomato plants, grown in white LED and additional far-red light
Photo credit: Maarten Wassenaar
25 FEATURES

PLENARY LECTURERS 2023 WINNERS 28 MEET OUR PRESIDENT MEDALISTS 2023 .. 30 JOURNALS - CONSERVATION PHYSIOLOGY –REFLECTIONS AND A VISION FOR THE FUTURE 32 JOURNALS - THE PLANT JOURNAL: A HUNDRED YEARS OF METABOLISM R ESEARCH 33 JOURNALS - JXB - UNRAVELING THE RNA REVOLUTION: A TIMELINE OF RNA BIOLOGY RESEARCH ................. 34 JOURNALS - PLANT DIRECTROOT BIOLOGY: FROM CURIOSITY TO SAVING THE PLANET OVER 100 YEARS 35 JOURNALS - PLANT BIOTECHNOLOGY –PAST, PRESENT AND FUTURE 36 IN CONVERSATION WITH LIANA ACEVEDO-SIACA 38 IN CONVERSATION WITH MICHAEL SLEIGH 40 SPOTLIGHT ON SHANE AUSTIN ......... 42 SPOTLIGHT ON GEORGES HRAOUI 44 26 SPOTLIGHT
SPOTLIGHT

27 SPOTLIGHT
MEET OUR PLENARY LECTURERS 2023
Each year at our Annual Conference, we honour George Parker Bidder and Harold Woolhouse with two key Plenary Lectures. These lectures, along with the Cell Biology Plenary Lecture, are given by scientists prominent in their field and are nominated by the committees of their respective sections.


Here we introduce our winners and we hope you can join us in celebrating them at our upcoming SEBCentenary Conference!

28 SPOTLIGHT
CELL WINNER Professor Wendy Bickmore




BIDDER WINNER Professor Elizabeth Brainerd
WOOLHOUSE WINNER Dr Lisa Ainsworth
Wendy Bickmore is Director of the MRC Human Genetics Unit at the University of Edinburgh. Her undergraduate degree is in Biochemistry from the University of Oxford and she then completed a PhD in Molecular Biology at the University of Edinburgh. Following a postdoc in human genetics, Wendy started her independent research group as a fellow of the Lister Institute for Preventive Medicine. She is fascinated by the three-dimensional organisation of the human genome in cells and how that influences genome function in health and disease. Her current research explores how the noncoding genome regulates gene expression, including how distant enhancers communicate with their target gene promoters. Wendy is a Fellow of the Royal Society, the Royal Society of Edinburgh and the Academy
Elizabeth L. Brainerd is the Robert P. Brown Professor of Biology at Brown University. She received her doctorate from Harvard University in 1991 studying the functional morphology of fishes with Professor Karel Liem, and then was a Junior Fellow in the Harvard Society of Fellows for postdoctoral work in biomechanics with Professor Tom McMahon. She was Assistant and Associate Professor at the University of Massachusetts Amherst from 1994 to 2005 and then Full Professor at Brown University. She has broad research interests in the biomechanics and evolution of movement in vertebrate animals, including work on all major groups of extant vertebrates and feeding, breathing and locomotor behaviours. At Brown she has worked with Professor Stephen Gatesy and other colleagues and students to develop X-ray Reconstruction of Moving Morphology (XROMM), which is a set of methods for visualising and quantifying the movements of 3D bones in 3D space. XROMM has been adopted by many research groups around the world and is yielding new insights into musculoskeletal structure, function and evolution.
Thursday 6th July 2023 13:30 - 14:30
Lisa Ainsworth is the Research Leader of the United States Department of Agriculture Agricultural Research Service Global Change and Photosynthesis Research Unit and Professor of Plant Biology at the University of Illinois Urbana-Champaign (UIUC). She received her BS in Biology at University of California, Los Angeles, and her PhD in Crop Sciences from UIUC. Her research addresses crop responses to global atmospheric change and potential solutions to mitigate climate change through agriculture. She has long studied photosynthetic responses of plants to climate change, and her research is broadly integrative, from genetic to agronomic scales. In 2011, she was awarded the President’s Medal from the SEB. In 2019, she was awarded the National Academy of Sciences Prize in Food and Agricultural Sciences and was an elected member of the National Academy of Sciences in 2020.
Talk Date: Wednesday 5th July 2023
Talk Time: 14:00 - 15:00
29 SPOTLIGHT
MEET OUR 2023 PRESIDENT’S MEDALISTS
The SEB President’s Medals are awarded annually to young scientists of outstanding merit.
This year’s awards will be presented at the SEB Centenary Conference 2023, which takes place in Edinburgh on 4–7 July 2023.
Many congratulations to our very worthy winners of the 2023 SEB President’s Medal awards.
30 SPOTLIGHT
ANIMAL SECTION Cosima Porteus Assistant Professor, University of Toronto Scarborough

CELL SECTION

Dr Yangnan Gu Assistant Professor, University of California, Berkeley
PLANT SECTION Dr Yasin Dagdas
Group leader at Gregor Mendel Institute of Molecular Plant Biology
Cosima Porteus received her BSc from the University of Guelph, Canada, majoring in Marine and Freshwater Biology. Early during her undergraduate degree, Cosima became involved in research on freshwater mussels, fish and salamanders and became fascinated by how aquatic animals cope with various environmental challenges. She stayed at the University of Guelph, where she earned her MSc in Integrative Biology under the supervision of Pat Wright, studying the parameters affecting the boundary layers and oxygen supply to developing fish. She then moved to the University of British Columbia, to start her PhD under the supervision of Bill Milsom in the Department of Zoology. Her PhD thesis characterised the timedependent changes that take place in response to low oxygen in fish, highlighting the importance of peripheral oxygen chemoreceptors in establishing these time domains.
As a postdoctoral fellow at the University of Exeter under the supervision of Rod Wilson, Cosima determined why fish exposed to ocean acidification lose their ability to respond appropriately to olfactory cues that are vital for their survival in the wild. The results of this research combined behaviour, electrophysiology and transcriptomics to show a novel mechanism for how ocean acidification affects the behaviour of fish through the olfactory system.
Cosima is currently an Assistant Professor at the University of Toronto Scarborough, where her laboratory is continuing to study the effects and physiological mechanisms of anthropogenic stressors on aquatic animals. Her group have recently shown that ocean acidification negatively affects olfaction in Dungeness crabs, an economically important crustacean, in a similar way as in fish. These findings have wide implications for issues ranging from global food security and marine conservation to recreational and commercially important fisheries.
Yangnan Gu is an Assistant Professor of the Department of Plant & Microbial Biology at the University of California, Berkeley. He received his BS degree in Bioengineering from Xi’an Jiaotong University, China, and earned a PhD degree in Molecular, Cellular, and Developmental Biology and an MS degree in Applied Statistics from Indiana University Bloomington.

Yangnan undertook postdoctoral training at Duke University/Howard Hughes Medical Institute, where he explored the role of a novel nuclear pore complex component in plant immune activation. His laboratory currently emphasises the discovery of novel nuclear membrane proteins and investigating their functional importance in different aspects of plant physiology and cell biology. His group is also interested in exploring the specialisation of nuclear transport receptors in mediating nuclear shuttling of signalling cargos across the nuclear envelope under various environmental conditions, and its significance in determining distinct stress responses in plant cells. He is the winner of Molecular Plant Rising Stars in Plant Sciences and currently serving as Reviewing Editor for The Plant Cell.
Yasin Dagdas is from Turkey, where he completed bachelor’s and master’s degrees in Molecular Biology and Genetics at the Middle East Technical University in Ankara. He then pursued his PhD at the University of Exeter, where he studied plant–microbe interactions with Nick Talbot. His research focused on the development of the rice blast fungus during infection. After earning his PhD, he moved to the Sainsbury Laboratory in Norwich, UK, to work in Sophien Kamoun’s laboratory as a postdoc. There, he studied how effectors from the Irish potato famine pathogen subvert plant membrane trafficking pathways, including autophagy. In 2017, he became a group leader at the Gregor Mendel Institute in Vienna, where he has been exploring autophagy-mediated cellular quality control mechanisms in plants.

31 SPOTLIGHT
Physiology is fascinating in its own right, revealing the mechanisms that underpin animal migration, identifying the environmental tolerances of plants, characterising cellular signalling pathways and so on. It is clear that over the last century experimental biology has revolutionised our understanding of organismal function to a level that would have been unfathomable to the physiologists of the 1800s. Experimental biology has rewritten text books, overturned paradigms and unravelled complex mysteries.
Yet, in parallel to that knowledge renaissance, the last century has also seen unprecedented changes in the environment that are now having manifold consequences for biodiversity. Pollution, invasive species, exploitation, habitat alteration, climate change, disease, emerging stressors and complex multi-stressor interactions make life increasingly challenging for wild organisms. Life is also challenging for the policymakers and practitioners responsible for the sustainable management of biological resources, the protection and restoration of imperilled species and ecosystems, and ensuring we have a planet that is liveable for all.
Enter Conservation Physiology: a discipline that applies physiological knowledge, concepts and tools to understand and solve conservation problems. In 2013, the SEB and Oxford University Press launched the journal Conservation Physiology in recognition of the fact that experimental biology and physiology were relevant to biodiversity conservation. A decade later this fully open access journal has steady copy flow, a respectable impact factor and an increasingly diverse body of contributors and contributions, proving that the journal is coming into its own and delivering on its promise. The early days were characterised by more fundamental research of potential interest to
environmental managers. More recently, increasingly collaborative projects have led to more actionable science. Today we can point to numerous success stories where conservation physiology has had a direct impact on policy and practice—from Pacific salmon management to wetland restoration efforts. Yet, there is still much work to do to help reverse declines in biodiversity and restore ecosystems.
I am confident that Conservation Physiology can rise to this challenge. In a series of recent articles spearheaded by the entire editorial team, we have explored topics that we believe will help to position Conservation Physiology (the journal and the discipline) to be more impactful. For example, we have crafted a list of 100 research questions that, if answered, would meaningfully improve our ability to manage biodiversity.1 We have also considered how conservation physiology is relevant to the sustainable development goals.2 And importantly, we have considered what is needed to make the conservation science community a more inclusive, integrative and forward-looking discipline.2

REFLECTIONS AND A VISION FOR THE FUTURE JOURNALS J
For those of us working in the conservation physiology space, there is little more rewarding than to see the development of university courses, symposia, textbooks and faculty positions that demonstrate conservation physiology as an emerging discipline. However, even more rewarding is when a practitioner asks to discuss strategies for unravelling the threat matrix facing an imperilled species in order to better target limited resources, or to provide physiological data to inform decision support tools. I am proud to say that we ARE relevant and I expect that relevance to grow given that conservation physiology research provides mechanistic insights that are foundational to both environmental regulation and restoring populations and ecosystems. While there is much more to do, our conservation physiology community is surely up to the task.
References:
32 SPOTLIGHT
Conservation Physiology
by Steven J. Cooke. Professor, Carleton University, Ottawa, Canada. Editor in Chief.
1. Cooke SJ, Bergman JN, Madliger CL, et al. One hundred research questions in conservation physiology for generating actionable evidence to inform conservation policy and practice. Conserv Physiol 2021; 9: coab009.
2. Cooke SJ, Hultine KR, Rummer JL, et al. Elevating the impact of conservation physiology by building a community devoted to excellence, transparency, ethics, integrity and mutual respect. Conserv Physiol 2022; 10: coac015.
A HUNDRED YEARS OF METABOLISM RESEARCH
The plant Journal
 By Lee Sweetlove. Professor of Plant Sciences, University of Oxford, UK. Editor in Chief.
By Lee Sweetlove. Professor of Plant Sciences, University of Oxford, UK. Editor in Chief.
One hundred years ago, the nature of metabolism was largely a mystery. It was known that the metabolic process of respiration kept organisms alive by consuming food molecules, but exactly what respiration was doing with those food molecules was not known. It was not until 1926 that the first enzyme was isolated and shown to be a protein, sparking a golden era of metabolism research. The race was on to understand the reactions catalysed by enzymes and how those reactions interacted in metabolic pathways.

It’s hard to convey how great a challenge this was. Many of the experimental techniques that we’d turn to now were simply not available. Progress had to made just from chemical analysis of the various ”salts” (metabolic intermediates) isolated from respiring tissues. As the number of salts identified grew ever larger, solving the puzzle of how they all fitted together must have seemed impossible. Why, and how, was inorganic phosphate involved? What did hexose diphosphate do? How did the newly discovered ATP molecule fit in?
There is no doubt that the achievements of Otto Fritz Meyerhoff, Gustav Embden and Hans Krebs in establishing the metabolic pathways that now bear their names were impressive. Not to mention Peter Mitchell’s radical concept of ATP synthesis driven by proton gradients. But arguably, it was a pioneering woman, Maud Menten, working some 20 years earlier, who has had the greatest influence on metabolic science today.
She qualified in 1911 with a medical doctorate (one of the first women in Canada to do so) but was forced forced overseas to pursue her research interests. It was while working in America on the control of pH during anaesthesia that she became acquainted with Leonor Michaelis, an expert on pH and buffers. She subsequently moved to his laboratory in Berlin and there became interested in his earlier work on enzymes. In 1913, they successfully expressed mathematically the relationship between substrate concentration and velocity of the reaction catalysed by the enzyme invertase.
Given that it was not known what enzymes were at that time, their work probably seemed like an obscure quantitative curiosity. But the field of enzyme kinetics that it led to has become one of major significance. The Michaelis¬–Menten kinetic parameters not only capture the unique catalytic properties of an enzyme, but they also allow metabolic pathways to be mathematically simulated. In the modern era, such mathematical models have become the only way of characterising
the increasingly complex reality of the known metabolic network. Metabolic models are now the de facto starting point for redesigning metabolic networks for synthetic biology.
Who knows what Maud Menten would have thought of recent attempts to replace her careful (and laborious) experimental measurements of enzyme kinetics with a machine learning algorithm.1 But she would surely have been pleased to know that her quantitative concept of enzyme kinetics now plays such an important role in biology in the twenty-first century and likely beyond.
33 SPOTLIGHT
References: 1. Li F, Yuan L, Lu H, et al. Deep learning-based kcat prediction enables improved enzyme-constrained model reconstruction. Nat Catal 2022; 5: 662–672.
UNRAVELLING THE RNA REVOLUTION: A TIMELINE OF RNA BIOLOGY RESEARCH
Journal of Experimental Botany
By Pablo A. Manavella Instituto de Agrobiotecnología del Litoral, Santa Fe, Argentina, Editor.
Back in 1869, Friedrich Miescher discovered nucleic acids, which he called nuclein, in the kitchen of a monastery in Tübingen. Fifteen years later, Albrecht Kossel isolated the five nucleobases that shape DNA and RNA.

However, the nature and functions of RNA within living organisms were a complete mystery a century ago. Then, nearly 90 years ago, the role of RNA in protein synthesis was first suggested, a concept that was finally established in the 1950s. These discoveries boosted interest in these molecules, and many laboratories started paying attention to RNA. An avalanche of discoveries followed, including the discovery of the RNA synthesis process by Severo Ochoa; the deciphering of the genetic code and identification of tRNA by Marshall Nirenberg, Robert Holley and Har Gobind Khorana; and the discovery of introns and RNA splicing by Philip Sharp and Richard Roberts.
In the late 1960s and early 1970s, researchers began to explore the role of RNA in plants. As in humans, RNA molecules were shown to regulate gene expression in plants, either by directly binding to DNA or by interacting with other proteins or RNA molecules. Plant RNA research was behind that in animals, until the 1980s when Richard Jorgensen’s laboratory provided the first evidence of gene silencing. They showed that overexpressing the chalcone synthase gene in petunia resulted in flowers that were entirely or partially decoloured because both the transgene and the endogenous copy of the gene were silenced. However, the mechanism behind this result was unknown until studies in the late 1990s forged many concepts and mechanisms foundational to RNA interference. In particular, the work from David Baulcombe was seminal in our understanding of the principles behind RNAi and the identification of small interfering RNAs. Unfortunately, the Nobel committee ignored his fundamental contribution when the 2006 Nobel Prize in Physiology or Medicine was awarded to Andrew Fire and Craig Mello “for their discovery of RNA interference—gene silencing by doublestranded RNA”. This is perhaps one of the most notorious examples of “plant blindness”.
In any case, the discovery of RNAi opened up new avenues for studying gene function in plants, because researchers could now selectively “knock down” the expression of specific genes and study the effects on plant growth and development. It was a powerful tool that would transform the field of RNA biology in plants and many other organisms.
It was also the gateway to studying the molecular basis of epigenetics, especially in plants, where small RNAs are central actors.
Within the last decades, and assisted by the advance of sequencing technologies, we have identified many different RNA molecules with essential roles in plants and other organisms. Groundbreaking discoveries in the RNA biology field have included the development of RNA-mediated gene editing, the therapeutic usages of mRNAs and our current advances in understanding the mechanisms behind epigenetics and the importance of non-coding genomic regions. Plant biologists have made tremendous contributions in this work. This year, the Journal of Experimental Botany and The Plant Cell published RNA biology special issues reviewing many aspects of this exciting field and future perspectives in this area.1,2 As RNA biologists, we are aware of many compelling open questions to tackle in the near future.3 These will only scratch the surface of what is hidden behind, and there is still much to learn about RNA biology’s complex and fascinating world and how RNA biology can help people, plants and our planet.
References:
1. Marquardt S, Manavella PA. A ribose world: current status and future challenges of plant RNA biology. J Exp Bot 2023; 74: 2203–2207.
2. Eckardt NA, Axtell MJ, Barta A, et al. Focus on RNA biology. Plant Cell 2023; in press.
3. Manavella PA, Herz MAG, Kornblihtt AR, et al. Beyond transcription: compelling open questions in plant RNA biology. Plant Cell 2022; in press.
34 SPOTLIGHT
[RNA INTERFERENCE] WAS A POWERFUL TOOL THAT WOULD TRANSFORM THE FIELD OF RNA BIOLOGY IN PLANTS AND MANY OTHER ORGANISMS.
ROOT BIOLOGY: FROM CURIOSITY TO SAVING THE PLANET OVER 100 YEARS
By Larry York
Charles Darwin was fascinated by plants and, in 1880, wrote a book The Power of Movement in Plants based on experiments he conducted with his son, Francis. On the topic of roots, they concluded: It is hardly an exaggeration to say that the tip of the radicle thus endowed, and having the power of directing the movements of the adjoining parts, acts like the brain of one of the lower animals; the brain being seated within the anterior end of the body, receiving impressions from the sense-organs, and directing the several movements.
Were the esteemed father and son correct? Indeed, research since that time has shown how the root cap, and especially the quiescent centre, respond to several stimuli to cause differential bending and even lateral root branching. This type of plant behaviour even gave rise to the controversial field of plant neurobiology. Beyond semantics and mechanisms, nobody can dispute that plants are able to sense the environment and rely on complex embodied cognition to optimise their architecture. Modular growth is needed, because, as plant biologists are simultaneously happy and woe to say, “Plants are sessile organisms”.
Another common trope is that roots are the “hidden half” of the plant, which leads to the common misconception that roots are too difficult to study. However, many tools exist to study roots as a normal activity for plant biologists, so the challenge should not deter the exercise. Free and open-source software like RhizoVision Explorer are lowering barriers to quantify important root traits such as length. In fact, now is the time for more biologists than ever to study roots, because root biology is central to both food security and climate change mitigation. Crop domestication and breeding have selected primarily on yield in agroecosystems that have tended to increase inputs over the years, which may have acted with some level of neutral selection on roots. An important area of research over the past 50 years has been on which specific root traits influence crop water use and nutrient use efficiency, and subsequent identification of specific genes and alleles that are involved.
Ecosystem ecologists have long understood that roots and root exudation are the primary avenues through which carbon arrives in stable, long-term soil pools. As atmospheric carbon dioxide increased and the Earth responded with increased average temperatures (global warming), these potential soil sinks have been highlighted as part of carbon dioxide reduction strategies. Agricultural systems offer the opportunity to
modify millions of hectares around the world with practices and seed that increase root inputs of recalcitrant molecules. However, at the same time researchers need to understand that soil carbon ecology is nuanced, and that poking and prodding carbon pools and fluxes can have unintended consequences. For example, rhizosphere priming can actually lead to the “burning off” of previously stored carbon when new carbon enters the system. Only interdisciplinary teams of crop scientists, geneticists, soil scientists and ecologists can overcome these challenges.
Over the next century, hopefully all types of root biologists, from development researchers to forest ecologists, will come together. Luckily, organisations like the International Society of Root Research and the SEB are well positioned to achieve a more diverse and inclusive root biology community.

NOW IS THE TIME FOR MORE BIOLOGISTS THAN EVER TO STUDY ROOTS, BECAUSE ROOT BIOLOGY IS CENTRAL TO BOTH FOOD SECURITY AND CLIMATE CHANGE MITIGATION.
35 SPOTLIGHT
Plant Direct Journal
Oak Ridge National Laboratory, Tennessee, USA. Editor-in-Chief.
PLANT BIOTECHNOLOGY –PAST, PRESENT AND FUTURE
Plant Biotechnology Journal
By Johnathan Napier Rothamsted Research, Harpenden, Hertfordshire, UK. Editor in Chief.
It is a chastening fact that my university education started before the dawn of Plant Biotechnology, generally accepted as 1983, but I still recall the excitement of reading a paper in Nature from the Ghent group on making transgenic plants.1 This was the first in a wave of groundbreaking studies that demonstrated the feasibility of transferring foreign genes into plants and delivered a paradigm-shift in both research and development. The following decade saw rapid technological advances, and a collective realisation that plant genetic modification (soon to be universally known simply as GM) was already on a path to transforming agriculture (literally and metaphorically) and delivering societal benefits.
My own career journey included a PhD at Kings College, London, and a postdoc at Cambridge. I then went to the Institute for Arable Crops Research Long Ashton Research Station (part of University of Bristol) as a junior project leader, focused on engineering plant seeds. Under the benevolent guidance of my Director, Peter Shewry, a collaboration was established with Keith Stobart (University of Bristol) looking at the assembly of the electron transport chain for fatty acid desaturases. This work introduced me to the world of plant lipids and led to an industry-funded project in 1996 that aimed to clone genes involved in the synthesis of polyunsaturated fatty acids (PUFAs). Thanks to the heroic efforts of my colleague Olga Sayanova, we discovered the first example of the b5 fusion front-end desaturases that are central to PUFA synthesis in all eukaryotes. Our studies paved the way for the next 25 years of work on metabolic engineering and attempts to generate GM plants accumulating non-native omega 3 PUFAs in their seed oils.

The late 1990s were a boom-time for Plant Biotechnology (herbicide-tolerant crops had just been commercialised and adopted by farmers, an amazing achievement given that the first transgenics were made only 15 years earlier!) and many companies were investing in research on GM traits; unfortunately, that trend did not persist, despite the obvious success of GM agriculture. In the last decade, much of my time and effort has been focused on carrying out field trials of our GM plants and trying to navigate a way through
the complex regulatory landscapes that now seem synonymous with our sector.
So, from a research perspective, what’s different now? Probably the most striking change is the almost trivial availability of DNA sequences, either from databases or de novo. At the start of our project in 1996, only one eukaryotic genome was completed, and the sequence complexity of target organisms was effectively a black box. Gene discovery via whole genome sequencing is now a routine process, whereas in the past approaches such as degenerate PCR or hybridisation required a fair degree of both skill and luck. And, of course, the recent arrival of tools such as CRISPR to allow precise changes to genes in situ represents another level of technological capability.
It gives me an immense sense of pride to work in a field that addresses global challenges. As exemplified by the long-awaited arrival of Golden Rice2 and the more recent Big Purple Tomato,3 we are now witnessing the new dawn of Plant Biotechnology and GM crops. I think it has been worth the wait and truly delivers on the promise that was first apparent in 1983.
References:
1. Herrera-Estrella L, Depicker A, Van Montagu M, et al. Expression of chimaeric genes transferred into plant cells using a Ti-plasmid-derived vector. Nature 1983; 303: 209–213.
2. Burkhardt PK, Beyer P, Wünn J, et al. Transgenic rice (Oryza sativa) endosperm expressing daffodil (Narcissus pseudonarcissus) phytoene synthase accumulates phytoene, a key intermediate of provitamin A biosynthesis. Plant J 1997; 11: 1071–1078.
3. Butelli E, Titta L, Giorgiom M, et al. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat Biotechnol 2008; 26: 1301– 1308.
36 SPOTLIGHT
I STILL RECALL THE EXCITEMENT OF READING A [1983] PAPER IN NATURE FROM THE GHENT GROUP ON MAKING TRANSGENIC PLANTS.
STARTED SETTING UP THE
SEBIOLOGY.ORG #SEBCONFERENCE
SEB AWARDS NOMINATION TASK FORCE TASK



AIMS: GET INVOLVED:
Highlight the lack of diversity in the profile of past award winners
Increase both nominations and nominators from historically marginalised groups for SEB awards
Expand the action of the task force to include other Societies and bioscience awards
Everyone (members and non-members) can get involved. No previous experience is needed.
1. Join the SEB Awards Nomination Task Force















2. Suggest the name of someone deserving of a bioscience award for the SEB Task Force







3. Nominate someone in your own right

SOCIETY FOR EXPERIMENTAL BIOLOGY PRESENTS:
Below Liana
Photo credit:
Kara Headley
ALEX EVANS, IN CONVERSATION WITH...
LIANA ACEVEDO-SIACA

Have you always been interested in plants?
My dad is a botanist, so plants have always been part of my life. When I was little, I would often accompany him on field expeditions around Puerto Rico and apparently there are even some plant specimens in the Smithsonian Natural History Museum that were collected by me! Even so, I aspired to do something different and originally applied to study Environmental Science at Cornell University. But I had the great luck of signing up for a course in plant breeding and genetics that changed my entire trajectory. I was so inspired by how fundamental plant research in the lab and field could ultimately be leveraged to improve crops, give people better livelihoods and help build a better world that literally, within a week, I switched to study Plant Sciences.
Tell us about your PhD
I undertook my Masters and PhD at the University of Illinois at Urbana-Champaign, supervised by Stephen Long. Working on rice, my key focus was to understand how photosynthesis responds to fluctuating light conditions and the factors—whether physiological, biochemical or diffusional— that limit this. As part of this, I assessed the natural variation in photosynthetic responses o fluctuating light conditions across a range of commercial varieties, landraces and wild rice relatives.1,2
Most photosynthesis research has historically been conducted under steady-state light conditions, despite the fact this is rarely the case in “real-world” conditions. Identifying heritable traits that enable plants to maintain a high photosynthetic efficiency under fluctuating light could therefore be an untapped opportunity for crop improvement programmes. In rice, for instance, the strongest limiting factor at the beginning of the process of photosynthetic induction appears to be biochemistry, which offers a focal point for improvement in the future.3,4
What happened next?
I continued at the University of Illinois as a postdoctoral researcher with the Realizing Increased Photosynthetic Efficiency (RIPE) Project, where I worked on a few projects examining water use efficiency in tobacco transformants.5,6 Then in January 2021, I took up a position as Associate Scientist at the International Maize and Wheat Improvement Center (CIMMYT) in Mexico. I was working as part of the Global Wheat Program, with a focus on identifying ways to improve wheat productivity under heat and drought conditions by studying photosynthesis.7
During this time, I became very interested in how wheat spikes contribute to overall wheat productivity, and worked on a few projects examining how different photosynthetic properties of leaves and wheat spikes in diverse wheat germplasm responded to long-term heat stress.
Did you enjoy living in Mexico?
Absolutely—I completely fell in love with the country. Everything from the food to the music, to the art and literature, to the history and landscapes. I particularly enjoyed the pace of life, being able to grab a coffee and sit in a plaza or park, watching the world pass by and ending up talking to complete strangers and learning about their lives. It doesn’t happen so much here in the States! The only thing that wasn’t great was the traffic in Mexico City, but everything else made up for it.
In October 2022, you moved to your current position as a postdoctoral researcher at Michigan State University DOE Plant Research Laboratory. What are you researching now?
The aim of this project is to quantify the cost of photoprotection to photosynthesis in plants grown under “real-world” conditions. I am using chlorophyll fluorescence techniques to better understand plant responses to fluctuating light
I am a plant scientist passionate about improving international food security through plant physiology and photosynthesis research. My work focuses on understanding how plants respond to excessive light stress and how that may interact with other abiotic stresses, such as heat and drought.
Acevedo-Siaca
38 SPOTLIGHT
Far Left
Liana Acevedo-Siaca in a wheat field at the CIMMYT Sanjaya
Rajaram Experimental Station in Toluca, Mexico
Photo credit: Carolina Rivera Left
Liana Acevedo-Siaca in a wheat field at the CIMMYT Sanjaya

Rajaram Experimental Station in Toluca, Mexico
Photo credit: Claire Benjamin
conditions, particularly regarding energy transfer and balance in C3 photosynthesis. In addition, I’m also starting up a new project to assess the feasibility of incorporating photosynthetic traits into breeding programmes to improve crop resilience and yields.
Why do you enjoy being part of the SEB? What is really unique and powerful about the SEB is how it brings together plant, animal and cell sciences. So often we can feel segmented into just one of these areas, but at the SEB conferences you have an opportunity to really see how they intersect. I’ve found myself very inspired after attending a completely unrelated session, for instance on animal biomechanics or cellular regulation. I am looking forward to the 2023 Annual Conference in Edinburgh, where I am helping to organise a session on Plant Biology for Sustainability and another on 100 Years of Plant Biology in SEB.
You say that mentorship is very important for you. Could you explain more?
I know that part of the reason that I am here today was because others took the time to offer their guidance, advocate for me and connect me to opportunities. It has made a big difference to my career, and I hope I can be that person for others. More widely, I strongly believe that the way we treat our colleagues has a big impact on creating a working environment that is deeply rewarding, in both a professional and personal manner. We may not be able to control many external things, such as funding and regulation, but the way we relate to others is always within our control.
What has been the best advice ever given to you?
I think the best piece of advice I received was from a Professor at the National Autonomous University of Mexico who told me that I should always focus on doing what makes me happy. In a world where we are constantly being measured
by the number of publications we have, the funding we receive and how many citations we have, it’s important to remember that there is a life outside of work as well and that our personal happiness is inherently valuable.
Career is important, but you have to put your personal happiness and wellbeing first or you won’t be able to succeed anywhere.
Another important lesson I have learnt is that we mustn’t be afraid of taking a risk or failing. Even if something doesn’t work out, you can always refocus and change direction, and the experience can be a powerful opportunity to learn and develop.
What do you do to relax?
I have a big love for Latin American literature, from classics to contemporary, and just books in general. Recently, I’ve especially enjoyed books by Eduardo Lalo, Ana Teresa Toro and Alvaro Uribe. In terms of non-fiction books, Maria Ressa’s “How to Stand up to a Dictator” was deeply impactful and something I recommend everyone to read, especially those of us who work in science and with how facts are generated and presented.
What would your younger self think of what you are doing now?
She would be very surprised, I think. Growing up, I hardly had any exposure to agriculture, so working in this kind of field was not something that I could have ever imagined. However, I feel very thankful that I have the opportunity to try and work towards more efficient and sustainable agricultural systems through my research. It’s something that I find deeply rewarding and that I hope that I can work towards for many more years into the future.
You can learn more about Liana’s work by following her on Twitter: @Liana_Ace
References:
1. Acevedo-Siaca LG, Coe R, Quick WP, et al. Evaluating natural variation, heritability, and genetic advance of photosynthetic traits in rice (Oryza sativa). Plant Breeding 2021; 140: 745–757.
2. Acevedo-Siaca LG, Dionora J, Laza R, et al. Dynamics of photosynthetic induction and relaxation within the canopy of rice and two wild relatives. Food and Energy Security 2021; 10: e286.
3. Acevedo-Siaca LG, Coe R, Quick WP, et al. Variation between rice accessions in photosynthetic induction in flag leaves and underlying mechanisms. J Exp Bot 2021; 72: 1282–1294.
4. Acevedo-Siaca LG, Coe R, Wang Y, et al. Variation in photosynthetic induction between rice accessions and its potential for improving productivity. New Phytol 2020; 227: 1097–1108.
5. Ruiz-Vera UM, AcevedoSiaca LG, Brown KL, et al. Field-grown ictB tobacco transformants show no difference in photosynthetic efficiency for biomass relative to the wild type. J Exp Bot 2022; 73: 4897–4907.

6. Acevedo-Siaca LG, Głowacka K, Driever SM, et al. Guard-celltargeted overexpression of Arabidopsis Hexokinase 1 can improve water use efficiency in field-grown tobacco plants. J Exp Bot 2022; 73: 5745–5757.
7. Murchie EH, Reynolds M, Slafer GA, et al. A ‘wiring diagram’ for source strength traits impacting wheat yield potential. J Exp Bot 2023; 74: 72–90.
I REALISED THAT ALL THE FUNDAMENTAL PLANT RESEARCH IN THE LAB AND FIELD COULD ULTIMATELY BE LEVERAGED TO IMPROVE CROPS, GIVE PEOPLE BETTER LIVELIHOODS AND HELP BUILD A BETTER WORLD
39 SPOTLIGHT
ALEX EVANS, IN CONVERSATION WITH...
MICHAEL SLEIGH
As the SEB celebrates its 100th anniversary this year, our science writer Alex Evans had the pleasure of talking with someone that first became involved with the SEB almost 70 years ago, biologist and former council member, Michael Sleigh.
Hello Michael! The major focus of your research career has been on protists, how did you become interested in this field of study?
I have indeed worked with protists through much of my career. The reason is that they provide excellent, accessible and often easily cultured examples for research on cilia and flagella. These organelles have been my central interest from my first publication in 1956 to my latest collaborative work published in 2022, and in a majority of the 180 publications between. My attention has been focused on how these organelles achieve their functions in movement of fluids for such purposes as locomotion, feeding or cleansing of surfaces. The subjects of my research have been animals of many invertebrate groups and vertebrates, including humans. Human diseases which result from defects in ciliary function include cystic fibrosis and a family of genetic abnormalities that result in male infertility and impaired clearance of mucus from the lungs, and I have made research contributions in studies on both of these types of disease.
What have been some of your proudest accomplishments in your research career?
Looking back, I am proud of my first book The Biology of Cilia and Flagella, published in 1962 and summarising what was known then. This was timely, for in the next 10 years, electron microscopy revealed many new details and research on biochemistry and physiology of cilia flourished, so that in 1974 I was able to call on many experts, including Irene Manton, to contribute chapters to an update entitled Cilia and Flagella. I am also proud of another monograph The Biology of Protozoa (1973), which was widened and updated as Protozoa and other Protists in 1980, both of which have been used in teaching in Europe as well as across the English-speaking world. In another area, I am proud of an article that I coordinated in The Lancet (1981), publicising the name primary ciliary dyskinesia (PCD) for the family of genetic diseases affecting human ciliary function; there are now PCD units in a number of hospitals in the UK,
including Southampton, concerned with diagnosis of these diseases. This article arose from a meeting in the USA where several different names were being used in discussions about these diseases, first described under the name Kartagener syndrome. My request for agreement about a single name led to me being commissioned to canvas opinion about the name that commanded the largest support from relevant scientists and clinicians.
What are your earliest memories of the SEB, and which was the first event you remember attending?
My first memory of the SEB dates from a conference in Bristol in April 1954. A number of foreign biologists, mostly from Denmark, had been invited to a local symposium on cell physiology in Bristol in the previous week with funding from the Colston Research Society, founded in memory of the Bristol educational and social philanthropist (yes, the one whose statue was toppled into the harbour). Some of these visiting biologists stayed on in Bristol to join in an international meeting of the SEB and, along with other PhD students, I was recruited to help with local arrangements and made friendships that lasted many years. That was a memorable year, for in September 1954, I attended a SEB symposium in Leeds entitled ‘Fibrous Proteins and their Biological Significance’, which provided valuable background for understanding cilia.
Since first joining, which positions and responsibilities have you held in the SEB Council?
I was elected to the SEB Council in 1964 and was persuaded to stand for election as Zoological Secretary in the following year alongside the Botanical Secretary. In those days, the joint secretaries were the principal organisers of SEB activities, supported by a Treasurer who had some secretarial help from the Institute of Biology to deal with membership matters, and a Council of 18 members each serving 3 years. All three of the principal officers were full-time academics running SEB affairs in their spare time, arranging three ordinary conferences and one symposium
Below
40 SPOTLIGHT
This drawing was produced by a member of a committee that Michael chaired
meeting each year. From 1970 to 1973, I served as a Symposium Convenor and arranged and edited two symposia on subjects chosen by a Symposium Committee. The SEB Council organised a dinner for the first Honorary Members in November, 1964. At this dinner, Lancelot Hogben gave a speech about the origins and early history of the Society. The Council felt that the contents of this speech should be recorded for posterity and James Sutcliffe, the Botanical Secretary, persuaded Hogben to write it up for publication in a Society booklet. To accompany this account of the origin of the SEB, I wrote an account of the history of the SEB up to that time, and the two were published together in 1966 in the first SEB history booklet. This was reprinted in 1973 to celebrate the 50th anniversary of the Society with additions to bring it up to date.
Are you still active within the SEB or any other scientific societies?
After my 10 years as an officer of the SEB, I moved on to take a more active role in protistological societies at the national, European and international level, at the expense of being active in the SEB, apart from presenting papers at SEB symposia. In a related area, I served for a term as a director of the Company of Biologists and I am still a member of the Company. I was also elected to the councils of first the Marine Biological Association and then the Freshwater Biological Association in recognition of contributions to research in those areas. After retiring from the staff of the University of Southampton, I was invited to be the Managing Editor of the European Journal of Protistology and held that post from 2000 to 2010. I was awarded the Reichenow Medal of the Deutsche Gesellschaft für Protozoologie in 2001.
Do you have a favourite or particularly memorable SEB conference or symposium?
I think that my favourite SEB conference was a symposium on the effects of pressure on organisms because it brought together scientists from many disciplines to explain how increased pressure, such as that experienced at depth in aquatic habitats, affects living organisms because the pressure doubles for every 10 m of depth beneath the water surface.
Outside of your research career, what else do you enjoy doing?
I have always been a field naturalist and days on the beach and studying stream life during my first field course with an expert and enthusiastic teacher convinced me to study Biology at university. I am still involved in conservation work with a local group, providing expertise in identifying plants and animals found in freshwater samples, but not able to provide manual support any more. After retiring from the university I moved to live in a village at the fringe of the New Forest and a former colleague who also lived in the village persuaded me to join the village History Society. This proved to be unexpectedly interesting and has led me to research in a different direction. I am now the acknowledged expert on the history of our agricultural village from its mention in the will of King Alfred in 870 through to it providing the home for Florence Nightingale up to the time she went to the Crimea. Since our retirements, my wife and I have enjoyed travelling to a large number of countries that had not featured in earlier research and conference visits.

Thank you, Michael
For you, what are
some of the biggest changes that the SEB has undergone over the years?
The SEB has totally changed in the 50 years since I retired from an active role in SEB administration. To a large extent this reflects the changes that have taken place in universities during that time. In 1945, there were about 12 universities in England that were able to award their own degrees and a handful of university colleges that prepared students for degrees of London or another university. In the post-war years, these university colleges became independent and were joined by a selection of new universities such as Sussex, East Anglia and York. The Robbins expansion of higher education enormously increased the numbers of staff and postgraduates in universities and research institutes who might be interested in membership of the SEB, and these have increased further more recently. This has required a transformation from the small, amateur, part-time administration with no salaried staff during my active service in the SEB to what appears to me to be a much larger and more professional organisation today.
THE SEB HAS TOTALLY CHANGED IN THE 50 YEARS SINCE I RETIRED FROM AN ACTIVE ROLE
I HAVE ALWAYS BEEN A FIELD NATURALIST AND DAYS ON THE BEACH AND STUDYING STREAM LIFE DURING MY FIRST FIELD COURSE WITH AN EXPERT AND ENTHUSIASTIC TEACHER CONVINCED ME TO STUDY BIOLOGY AT UNIVERSITY.
Below Michael and his wife at the summit of Mount Vesuvius in 2016Mexico
41 SPOTLIGHT
Photo credit: Michael Sleigh
SHANE AUSTIN SPOTLIGHT ON...
BY ALEX EVANS S
hane currently teaches students at all levels of the biochemistry programme at University of the West Indies in Cave Hill, Barbados, as well as coordinating their graduate studies programmes, but Shane didn’t always see himself as a scientist. In fact, Shane actually considered a career in medicine for a while, but, following his undergraduate degree, Shane moved to Montreal for a master’s in one the topics that he found most fascinating— mitochondria. “For my PhD, I wanted a change of geography while still studying mitochondria so I ended up in a lab in Vienna,” he says. “And I have thoroughly enjoyed every minute of being a research scientist.”

Building on this early work, Shane’s research career has primarily focused on mitochondria, investigating its role in metabolism and how it factors into diseases such as cancer. “For cancer biology, I have worked on a number of projects over my career and all have involved mitochondria, which have been demonstrated to have an ever-evolving role in cancer,” he says. “The first project focused on one of the most commonly prescribed diabetic drugs, metformin, which was shown to reduce oxygen consumption in cancer cells, and some of my very early work on this paved the way for further studies to explore this metformin axis of cancer treatment depending on the cell’s metabolic preferences.”
In addition to his contributions to cancer research, Shane has worked extensively on a single transmembrane mitochondrial protein named LETM1. “The gene is essential in humans and mice, but its exact function was still unknown at the time,” he explains. “It was clear based on my experimental evidence and others that it plays a key role in maintaining osmotic balance in mitochondria but there was an open debate as to if it transported calcium out of mitochondria or not.” From the work started during Shane’s PhD, he recently found that LETM1 interacts with a protein responsible for calcium exchange for protons in mitochondria.1 “This finding was a major conceptual leap in mitochondrial biology because this transporter was the focus of much research by scientists in the 1970s,” he says. “The finding really put to rest a long-held debate about LETM1 function because there was a lot of speculation as to the identity of this calcium transporter.”
Shane’s work has benefited our understanding of not only cancer biology, but also other diseases such as the ZIKA virus. While between PhD studies and his current post, Shane got involved with a project to sample mosquitoes in his home country of Barbados to analyse them for ZIKA virus variants and other viruses. This work eventually identified novel ZIKA variants and over 30 new strains of arbovirus, demonstrating how little we still know about the number of viruses that have the potential to infect humans.2 “After the height of the ZIKA outbreak in the Caribbean region, the importance of this work became clear to most people in the region,” Shane explains. “Recently, I have restarted work similar to this, assessing with an undergraduate research student the local mosquito population in both urban and rural habitats and assessing their bloodmeal preferences, which, to date, has not been done locally.”
From his experiences as both a researcher and educator, Shane is an avid believer that improvement and innovation of experimental techniques is crucial to progressing our understanding of diseases such as cancer. “Without the development of new technologies, we won’t have advancement in these areas, our knowledge becomes limited and worst yet, we start to become unaware of some of the ways it is limited,” he says. “A lot of the findings we see in the big journals and hear about in the media simply weren’t possible at this scale two decades ago; the technology and computing power didn’t exist at this scale.”
As well as contributing to contemporary biochemistry research, Shane is also interested in finding new ways that biochemistry can be taught in the classroom. One such way has involved getting students to engage with LEGO. “I started using LEGO bricks in my classroom to explain concepts to my students and I found that they were inexpensive and easy to use. Importantly, they are also automatically associated with fun,” he explains. “However, initially I was the person doing the building and explaining, so to change that, I started adopting LEGO brick use such that students were the ones who did the construction and explanation.” Shane’s new approach encourages the students to build models and describe them in the context of biochemistry, resulting in a more engaging and creative learning process that he recommends
“Few people realise that scientific prizes are unrepresentative of the whole of science and it is concerning because these prizes can propel the careers of those who win them,” says Shane Austin, a biochemistry lecturer at the University of the West Indies and founding member of the SEB Awards Nomination Task Force.
Above
Shane Austin at the University of the West Indies, Cave Hill, Barbados Photo credit:
Shane Austin
42 SPOTLIGHT
other educators give a try. “Students are often engaged, active and talking about content in class, searching for creative ways to demonstrate what would otherwise be abstract and hard-to-visualise biological and thermodynamic concepts,” he says.3 “I encourage anyone willing to try new things to have a go with it in their classroom.”

As a Barbadian, Shane reflects on how fortunate he feels to be able to come home to his country of origin to educate the next generation of biochemists. “Returning to Cave Hill and teaching and doing research with some of the same people that got me into biochemistry and taught me has been a definite highlight,” says Shane. “Coming to that realisation took me some time to fully appreciate the moment for what it is, but it is a unique privilege, and the more I read about various scientists and their career paths, I have been uniquely privileged in this regard.”
When he’s not teaching, Shane’s interests include gardening and reading—especially novels from the postcolonial world such as Nigeria, India and South Africa. “They all have such diverse cultures, and I always end up learning something new about their cultures and countries with each novel,” he says. “And travelling is one aspect of my job that I especially love, because it opens up chances for me to visit some very cool and unique places to communicate science and interface with likeminded scientists.”
When discussing experimental biology, the range of topics involved are incredibly diverse, but can the same be said for the researchers that we recognise and celebrate? “Most scientific awards currently reinforce traditional stereotypes of scientists and, importantly, exclude historically marginalised groups,” explains Shane. “The SEB realised this was also true of its prizes and committed itself to make a difference in this regard.” Recently, Shane
Above Left Shane working in the lab

Above Right Shane using LEGO bricks to demonstrate biochemistry concepts

Photo credit: Shane Austin
and other advocates for inclusion and equality established the SEB Awards Nomination Task Force with the goal of improving the representation of award nominations for people belonging to these historically marginalised groups.
“Currently, we are in preparation for our first round of nominations for the SEB’s 2024 awards,” he says. While the selection of prize winners remains independent of the task force, Shane and his fellow members hope that by identifying more nominees from marginalised groups, they can positively impact a wider group of people that are deserving of recognition. “We are still looking for members to join us, so if you are interested, please reach out to one of the SEB Outreach, Education and Diversity staff members or me,” he concludes. For more information about the SEB Awards Nomination Task Force, including how to apply to become a member or to nominate someone for an award, please visit centenary/awards-nominationtask-force.html.
References:
1. Austin S, Tavakoli M, Pfeiffer C, et al. LETM1-mediated K+ and Na+ homeostasis regulates mitochondrial Ca2+ efflux. Front Physiol 2017; 8: 839.
2.
Thannesberger J, Rascovan N, Eisenmann A, et al. Viral metagenomics reveals the presence of novel Zika virus variants in Aedes mosquitoes from Barbados. Parasit Vectors 2021; 14: 343.
3.
Austin S, Millar CA, Christmas S. Case study: Perspectives on the use of LEGO® bricks in the biochemistry classroom. Essays Biochem 2022; 66: 53–63.
I HAVE THOROUGHLY ENJOYED EVERY MINUTE OF BEING A RESEARCH SCIENTIST.
I STARTED USING LEGO BRICKS IN MY CLASSROOM TO EXPLAIN CONCEPTS TO MY STUDENTS.
43 SPOTLIGHT
Caroline Wood caught up with him about his journey so far.
GEORGES HRAOUI SPOTLIGHT
BY CAROLINE WOOD
EVen as a child, I had this fierce drive to understand how everything worked,” Georges says. “For instance, I was fascinated by dinosaurs and all their different shapes and sizes—what was the point of the sail on the back of Spinosaurus or the long necks of sauropods?”
In his first year as a student at Université de Montréal, this innate curiosity led Georges to study a diverse range of courses across biology, chemistry and the arts, even dabbling in criminology, before he decided to focus on biology. But the pivotal moment that turned him towards research came during a summer internship at the Institut de recherche en Biologie Végétale (IRBV) with Jacques Brodeur. “Until that point, my education had predominantly been concerned with learning facts to repeat back on exam papers. But on this placement, I was told to search the literature and design a new protocol to achieve our objective. My project was on aphid parasitoids of the genus Aphidius, with the goal to investigate what factors could modulate induction of summer diapause in these species.1 I was suddenly having to think for myself and apply what I had learnt in the classroom to solve a real-world problem—and I was hooked!”

This first taste of “proper research” persuaded Georges to pursue a Master’s in Biological Sciences at Université de Montréal, supervised by Sophie Breton and co-supervised by Daniel Boisclair. His thesis subject was a comparative study of the mitochondrial thermotolerance of invasive and native freshwater mussels. “Quagga mussels (Dreissena bugensis) are a highly problematic invasive species and are rapidly spreading worldwide,” he says. “We hypothesised, based on previous literature, that high thermotolerance may be a key factor for the Quagga mussel’s success, which could be conferred by more heattolerant mitochondria.”
To investigate this, Georges used high-resolution respirometry to compare the mitochondrial respiration of Quagga mussels against a native freshwater species, Elliptio complanate, when exposed to a broad range of temperatures. Unexpectedly, the results showed unequivocally that Quagga mussels possessed less thermotolerant
mitochondria compared with the endemic species, with a much more pronounced aerobic metabolic depression at elevated temperatures.2 “It was completely counterintuitive to our expectations and taught me a very important lesson: even if a theory sounds sensible, you cannot presume it to be true until you have tested it thoroughly,” Georges says.
So, what might be behind the Quagga mussel’s success? “We concluded that life history traits may have a more important role,” says Georges. “Quagga mussels colonised freshwater habitats relatively recently and retain some of the primitive characteristics of their marine ancestors, including high fecundity, fast growth, early maturity and short lifespans. This reduces the selection pressure for resistance mechanisms to environmental stresses, because the population can rapidly bounce back.”
Despite enjoying getting to grips with aquatic physiology, Georges decided it was time for a change for his PhD. “I knew that I loved working with mitochondrial physiology but I was also keen to do something more closely related to human health research. At the same time, Sophie was encouraging me to look at other institutes to broaden my horizons and skill set.” This led to his current position in Diana Averill-Bates’s group at the Université du Québec à Montréal, to characterise reactive oxygen species (ROS) generation in HeLa cells. “Fortunately, the lab has a very knowledgeable research associate, Melanie Grondin, who helped me transition from someone who had never handled a cell culture before to doing experiments independently within a month,” he says.
Georges’ thesis focuses on understanding the role of ROS during adaptive responses to heat stress. As he explains: “When we expose cells to a mild heat stress for an extended period, for instance, 40°C, this can activate a cellular adaptive response that can protect the cell against subsequent exposures to lethal heat shocks, that is 42°C and above.” This adaptive response involves increased expression of heat shock proteins and antioxidants, but the initiating factors are poorly understood. So far, Georges’ work has demonstrated a critical role for Nrf2, a redox-sensitive transcription factor that is
“I love the feeling that my work allows me to leave a positive mark on society by contributing to the advancement of knowledge,” says Georges Hraoui, a 29-year-old PhD student at Université du Québec à Montréal. For him, this means uncovering the intricate mechanisms that regulate mitochondrial metabolism and reactive oxygen species generation.
ON...
Photo credit:
Georges Hraoui
44 SPOTLIGHT
activated by ROS to upregulate antioxidant genes. “Our results show that cellular ROS levels increase during mild heat stress and originate from both mitochondria and NADPH oxidase.3 But if Nrf2 activity is modified via overexpressor and knockdown cell lines, this significantly impacts ROS generation during mild heat stress,” he says. “My challenge now is to clarify the link amongst the mitochondria, ROS and Nrf2 activity during the cellular adaptive response. Understanding this could lead to new therapeutic treatments for a variety of human diseases that implicate increased oxidative stress. Some of our leads in elucidating this cellular cascade could include studying the expression of specific proteins such as DJ-1, a multirole protein that is known to regulate Nrf2, and PGAM5, a protein that binds Nrf2 to mitochondria and allows Nrf2 to become a mitochondrial redox sensor.”
Georges first became involved with the SEB when he was invited to present some of the results from his PhD at the 2022 Annual Conference in Montpelier. “It was my first attendance at an international science conference and it was an amazing experience,” he says. “The SEB has such a strong community of researchers who work on thermal stress and mitochondria, covering many different but overlapping fields. It was incredible to meet some of the biggest names in my area and I received valuable feedback on my presentation, suggesting ways I could take my research forward. Similarly, I even found that I was able to inspire others—for instance, a researcher on plant antioxidant mechanisms who told me I had convinced him to investigate whether antioxidant regulators in potatoes during thermal stress could explain the increased thermal resistance of crops previously exposed to sublethal temperatures.”
Georges believes the Society plays an important role beyond sharing and discussing results. “When you know of researchers just through their papers, it can feel like they are just machines that churn out work. It is so important to have these opportunities to meet people and remember that they are just like you, and go through the same struggles that we all experience at times,” he explains.
OUR RESULTS
Above Georges Hraoui during a short intermission between presentations at the 2022 SEB Conference in Montpellier
Photo credit: Society of Experimental Biology
Left Sophie Breton’s group during a laboratory meeting in 2019

Photo credit: Georges Hraoui
When asked about his future plans, Georges says he is open to opportunities in both academia and industry, but prefers to focus on the present for now. When he’s not in the lab, this tends to involve “seeing friends, playing electronic music and hanging out with my three cats.”

“I am very happy with where I am right now,” Georges concludes. “One of the aspects I enjoy most about my work is the collaborative nature of research. In our lab group for instance, we work on different projects but we are all trying to paint the same big picture, like artists working through different canvases. It is very gratifying to work together in this way to push the boundaries of what we know and understand.”
References:
1. Tougeron, K.,, Hraoui, G.,, Le Lann, C., van Baaren, J. and Brodeur, J., 2018. Intraspecific maternal competition induces summer diapause in insect parasitoids., et al. Intraspecific maternal competition induces summer diapause in insect parasitoids. Insect science,Sci 2018; 25(6), pp.: 1080-–1088.
2. Hraoui, G.,, Bettinazzi, S.,, Gendron, A. D., Boisclair, D., & Breton, S. ( AD, et al. Mitochondrial thermo-sensitivity in invasive and native freshwater mussels. J Exp Biol 2020). Mitochondrial thermo-sensitivity in invasive and native freshwater mussels. Journal of Experimental Biology,; 223(2),: jeb215921.
3. Averill-Bates, D.,, Hraoui, G., M, Grondin, M., Breton, S. 2022. The role of ROS and Nrf2 in the induction of a hormetic, adaptive stress response during mild heat shock at 40 degrees C. M, et al. The role of ROS and Nrf2 in the induction of a hormetic, adaptive stress response during mild heat shock at 40°C. Free Radical Biology and Medicine 2022, 192, pp.: 97.
I WAS SUDDENLY HAVING TO THINK FOR MYSELF AND APPLY WHAT I HAD LEARNT IN THE CLASSROOM TO SOLVE A REAL-WORLD PROBLEM— AND I WAS HOOKED!
45 SPOTLIGHT
SHOW THAT CELLULAR ROS LEVELS INCREASE DURING MILD HEAT STRESS AND ORIGINATE FROM BOTH MITOCHONDRIA AND NADPH OXIDASE
OUTREACH EDUCATION AND DIVERSITY
WOMEN WHO HAVE SHAPED THE SEB 48 A VISIT TO THE SEB ARCHIVES 50 MIND THE GAP: NAVIGATING THE ACADEMIC HIRING PROCESS AND CAREER TRANSITIONS 52 GOING GREEN – WHAT DOES A SUSTAINABLE LAB LOOK LIKE 54
46 OUTREACH EDUCATION AND DIVERSITY


4 47 OUTREACH EDUCATION AND DIVERSITY
WOMEN WHO HAVE SHAPED THE SEB
BY REBECCA ELLERINGTON
The SEB has played a significant role in shaping the field of experimental biology over the past century. While the contributions of many notable male scientists to the SEB are well documented, the pivotal role played by women in the Society’s development and success are sometimes overlooked. In this article, we will explore the lives, research and contributions of some of the pioneering women who have helped to shape the SEB, from the early days of the Society’s formation to the present day.
Marie Victorie Lebour, a British marine biologist born in 1876, made significant contributions to the study of marine life in the early 20th century. Her pioneering work in the field of marine biology helped to lay the groundwork for modern oceanography. Born in Northumberland, England, Marie was the youngest of three daughters in a family with an appreciation for nature. Her father, George Lebour, was an accomplished artist and prominent geologist. Growing up, Marie cultivated an interest for the natural world while accompanying her father on his geological excursions, ultimately inspiring her own career.
After initially studying art, Marie began working at Durham University as a demonstrator in the Department of Zoology from 1906 to 1909. During this time, she pursued her BSc and MSc in Zoology and later became a lecturer at the University of Leeds while earning her doctorate. Even before receiving her formal education, Marie had already begun her research career, publishing her first paper in 1900 on land and freshwater molluscs.
Following her doctorate, Marie worked at the Marine Biological Association in Plymouth, England, where she remained for her entire career studying

the ecology and physiology of marine animals. She focused primarily on marine invertebrates, microplankton, crustaceans and molluscs and made several significant discoveries, identifying 28 new species of microplankton and helping to establish the importance of plankton in the marine ecosystem. Her background in art proved useful, and she created illustrations and watercolours for her research publications.
In addition to her research, Marie was also an active member of the scientific community. She was amongst the first cohort of scientists who joined the SEB in 1923 to collaborate and exchange ideas. Her involvement in the SEB helped to further establish her reputation as a leading figure in the field of marine biology.
Marie passed away on 4 March 1974, yet her legacy in the field of marine biology continues to be felt today. Her contributions have helped shape our understanding of the oceans and the delicate balance of life that exists within them. She was a trailblazer in a field dominated by men and is an inspiration to aspiring marine biologists around the world.
IRENE MANTON (1904–1988)

Irene Manton was a British cytologist who was born in London. Her father, a dental surgeon, introduced her to a microscope, and her mother encouraged her and her sister to take an active interest in natural history. At school, Irene developed a passion for and, despite the staff considering her idle and encouraging her parents to remove her from school to pursue music, she passed the school-leaving examination with ease, winning a Clothworkers Scholarship to Cambridge to study Botany.
After reading the work of Edmund Beecher Wilson, Irene decided she wanted to pursue a career in plant chromosome cytology. In order to achieve this goal, Irene sought advice from cytologist Dr Kathleen
MARIE LEBOUR (1876–1971)
48 OUTREACH EDUCATION AND DIVERSITY
Blackburn and palaeobotanist Dr Hamshaw Thomas, who recommended Professor Otto Rosenberg’s laboratory in Stockholm, Sweden. There, Irene began her early work using chromosome number to elucidate interrelationships between plants and their phylogeny.

In 1929, Irene joined the Botany Department at the University of Manchester as Assistant Lecturer. She worked under the supervision of Professor W.H. Lang, who instilled in her the importance of recording microscopic observations through photography and using fresh plant material. Lang encouraged her to switch her research focus to ferns, a subject she would continue to study for the rest of her career. She later developed the “squash method”, which allowed for more accurate chromosome counting in ferns, and helped to establish that accurate chromosome counting is vital for characterising species and for phylogenetic classification.
Irene was not only a brilliant scientist, but also a generous contributor to the scientific community. She was deeply involved with the SEB, serving on the Council during the 1950s and presenting at numerous SEB conferences. Beyond her research and service to the SEB, Irene was a gifted mentor and educator, inspiring and training young plant biologists. Her enthusiasm, meticulous attention to detail and contagious energy left a lasting impact on generations of postgraduate students.
Apart from her scientific career, Irene had a wide range of artistic interests. She was passionate about music and played the violin to a high standard and collected Chinese and modern art. Irene continued to pursue both her leisure and scientific pursuits into her retirement before passing away on 13 May 1988.
Irene’s legacy in the field of plant biology and as an advocate for young biologists is honoured by The Irene Manton Poster Prize, awarded annually to the best poster presentation by a young scientist at the SEB’s Annual Conference.
CHRISTINE RAINES (–PRESENT)
in our sections and in our council, and therefore it seems only appropriate that we would have a female President”. Her leadership encouraged SEB’s tradition of providing opportunities for young scientists to present their research and network with their more experienced peers, ensuring there was no separation between professors, students and postdocs within the Society and its meetings. Reflecting on her belief she stated “we are all one big community”. In addition to her leadership roles in the SEB Council, Christine also contributed to the history of the SEB journals, serving as the first female Editor-in-Chief of the Journal of Experimental Botany. Under her editorship, the journal published novel research on topics such as the genetic engineering of plants and the regulation of photosynthesis.
Christine continues to be an influential figure in the field of plant science and her contributions to the field and the SEB have been invaluable.
Christine Raines, a British plant biologist who has made significant contributions to the field of plant science, is best known for her research on the molecular mechanisms of photosynthesis and has been widely recognised for her work.

Christine studied for her undergraduate degree in Plant Biology at the University of Glasgow, where she also received her PhD with a thesis on photosynthesis. She then joined the molecular laboratory at the Plant Breeding Institute in Cambridge as a postdoctoral researcher and later joined the faculty at the University of Essex, where she continues to work as Professor of Plant Molecular Biology. Christine has established herself as a leader in her field of research, which has focused primarily on the molecular mechanisms of photosynthesis, with an emphasis on understanding how plants can be optimised for greater efficiency and productivity. She has also investigated how plants respond to environmental stress and how they can be genetically engineered to produce more biomass.
One of Christine’s most notable scientific contributions has been her work on the Calvin cycle. She and her team discovered that the enzyme SBPase plays a critical role in controlling CO2 assimilation in tobacco plants. By increasing the expression of this enzyme, they were able to increase the photosynthetic capacity and yield of transgenic tobacco plants. This finding has important implications for the agricultural industry because it has the potential to significantly increase crop yields, a vital aspect in combating food insecurity.
Christine’s contributions to the scientific community go beyond her research work. In 2017 she became the first female president of the SEB, during which time she worked to increase the participation of women in science and to promote diversity and inclusion in the field. On her appointment, Christine commented “women have played a significant role
The SEB owes much of its success to the pioneering women who have contributed to the Society and the field of experimental biology. Marie Lebour, Irene Manton and Christine Raines are just a few of the inspiring women who dedicated their careers to advancing this field, paving the way for future generations of scientists. Through their groundbreaking work, these women have left an indelible mark on the SEB and the wider scientific community. If you’re interested in learning more about the other women who have shaped the SEB over the past century, you can find an in-depth article on the SEB website under the centenary section.
BIBLIOGRAPHY
Marie Lebour
• Ogilvie MB, Harvey JD. The Biographical Dictionary of Women in Science: Pioneering Lives from Ancient Times to the Mid-20th Century. Routledge, 2000, p745–746.
• Shannen. Half-hidden Histories: Marie Lebour. 21 May 2020. https://storieswithshannen.wordpress. com/2020/05/21/half-hidden-histories-marie-lebour/
• Watts M. Marie V. Lebour 1904-1971. J Mar Biol Assoc UK 1975; 55: 403–410.
Irene Manton
• Leadbeater B. Irene Manton: a biography (1904–1988). The Linnean 2004; Special Issue 5.
• Preston RD. Irene Manton. 17 April 1904–13 May 1988. Biographical Memoirs of Fellows of the Royal Society 1990; 35: 247–261.
Christine Raines
• Professor Christine Raines becomes SEB President. SEB News. 6 January 2017. https://www.sebiology. org/resource/professor-christine-raines-becomesseb-president.html
• Raines C. Letter from the President. SEB Magazine 2017, Autumn, p4–5. https://www.sebiology. org/news-publications/magazine/autumnmagazine-2017.html
• University of Essex Faculty of Science and Health. Professor Christine Raines [faculty page]. https://www. essex.ac.uk/people/RAINE84508/christine-raines
49 OUTREACH EDUCATION AND DIVERSITY
BY ANA CAROLINE COLOMBO AND REBECCA ELLERINGTON
A VISIT TO THE SEB ARCHIVES A
Celebrating 100 years of existence, the SEB is taking a trip down memory lane by diving into its archives. The Outreach, Education and Diversity (OED) team have been sifting through 14 boxes of historical material to organise data for the SEB’s Centenary celebrations


s technology advances, fewer and fewer printed copies are produced, making the archives even more valuable. The team had the privilege of exploring handwritten documents and other fascinating resources that paint a vivid picture of the past. Although not all the material was analysed, we did uncover some interesting information.
How incredible it was to look through live evidence of the past through handwritten minutes and letters! It wasn’t an easy task to go through 14 boxes of history, but one exciting outcome was being able to fill out some gaps in our knowledge, such as the full list of SEB’s presidents. In the early days of the Society, there were no president or section chairs; instead, officers and secretaries were responsible for executive decisions. In 1977, the Society was incorporated as a company and appointed its first president, John William Sutton Pringle (F.R.S.). Professor Christine Raines made history in 2017 when she became the first female president of SEB.
We also found the handwritten minutes of the first Society meeting minutes ever figure 2), which described the formation of the Society, its first resolutions, the list of invited members of the Council, who would be appointed secretaries and treasurer, and considerations about membership, subscriptions and conferences. The official typed letters sent by the secretaries R. Ruggles Gates and Lancelot Hogben to the members about the first few meetings of the Society (figure 3) were also found, as was the handwritten list of members of 1924.
 Figure 2
Figure 1
Figure 2
Figure 1
50 OUTREACH EDUCATION AND DIVERSITY
Figure 3
Printed booking forms and letter exchanges were the main ways that people communicated in the past. Could you imagine organising an entire annual conference through letters? A printed booking form for the Edinburgh meeting in 1989 (figure 4) was among the materials found. We’re very grateful that booking for this year’s Centenary Conference in Edinburgh is all online!
HOW INCREDIBLE IT WAS TO LOOK THROUGH LIVE EVIDENCE OF
decided to continue the series after seeking CoB approvement (figure 5). Since then, the Bidder Lectures have expanded to include three separate awards for outstanding contributions to animal, plant and cell biology. The Woolhouse Lecture is awarded for plant biology and the Cell Plenary Lecture is awarded for cell biology.
MINUTES AND LETTERS!
SEB’s archive also contains materials that shed light on the Society’s progress. A subcommittee set up in 1973 created a report on the future development of the Society. The report included a summary of members’ feedback and recommendations, which included the creation of “Special Interest Groups” (figure 6). Currently, the SEB has one OED group and 22 scientific special interest groups covering the Society’s three sections: animal, plant and cell biology.
An extract of the handwritten minutes of the first Society’s meeting, stating its formation
An official letter from the Honorary Secretaries, Ruggles Gates and Lancelot Hogben, inviting members to one of the first meetings of the SEB in 1924
Booking form for the Edinburgh meeting in 1989


Other materials found in the archives included financial statements, symposium publication books and member handbooks. While there are plans to digitise and organise all these resources in the future, the OED team’s trip to the archive was an opportunity to glimpse into the past and reflect on the Society’s remarkable journey over the last century.
The Bidder Lectures is an ongoing SEB award given to prominent scientists who have demonstrated excellence in the animal field of research. Originally, the Bidder Lectures were a series of six lectures organised in collaboration with the Company of Biologists (CoB), but in a meeting in 1975, it was
Booking form attachment describing booking conditions, conference fees and form for the SEB Grants to Graduate Students
There is still much to uncover, given that the archive contains a total of 68 boxes. The SEB is excited to continue exploring the archive’s resources and will publish articles on its most interesting finds throughout the year. Additionally, the SEB plans to present some of the discovered documents for viewing at the Centenary Conference, allowing attendees to connect with the Society’s rich past and exciting future.
An extract of a meeting minutes of 1975 discussing the future of the Bidder Lectures

Figure 6
An extract of the 1973 report on the future development of the Society regarding the formation of groups (i.e. Special Interest Groups)
 Figure 4a
Figure 5
Figure 6
Figure 4b
Figure 5
Figure 1
The OED team during their visit to the SEB archives
Figure 2
Figure 3
Figure 4a
Figure 4b
Figure 4a
Figure 5
Figure 6
Figure 4b
Figure 5
Figure 1
The OED team during their visit to the SEB archives
Figure 2
Figure 3
Figure 4a
Figure 4b
51 OUTREACH EDUCATION AND DIVERSITY
THE PAST THROUGH HANDWRITTEN
MIND THE GAP: NAVIGATING THE ACADEMIC HIRING PROCESS AND CAREER TRANSITIONS
BY ANA CAROLINE COLOMBO
As a PhD student in Brazil trying to pursue a career in academia, it took me a while to realise that I had to mind the gap between the training I had been undertaking and the requirements to become an academic. And I only started thinking about it when I saw those around me applying for academic positions. For some reason, I’d never asked my supervisor what his journey was. My goal with this article isn’t to go into much detail about the roles and daily activities that each position—PhD student, postdoc, early career researcher, lecturer—entails but to highlight the academic hiring process and the differences amongst some countries. At the end of this article, I will discuss repositioning to a non-academic position.

THE ACADEMIC HIRING PROCESS IN BRAZIL
In Brazil, most of the best universities are public and free to attend. The government subsidises them, and the staff are hired through public tender. The process varies slightly from place to place and field to field, but, in general, the candidate needs to write a long academic autobiography instead of a CV to apply for a position. Yes, this means they need to write, in a storytelling format, their journey and achievements in academia. Writing this can be useful to reflect on their career journey. The issue is time and support to write a good one. This academic autobiography is usually used in the interview stage, during which the candidate needs to do a viva of their content to the judging panel. Some application processes also require a research project that would be similarly used during the interview process. And this is just the application phase. Candidates are required to undertake a written exam about their study area, and this phase is usually eliminatory. This means that you need to study broad topics, and they draw one or a few topics for you to write about. Once the candidate is successful, they would discuss their academic autobiography and research project with the judging panel. But that isn’t all. There is also a teaching exam in which a topic is drawn, and the candidate has 24 h to prepare a lesson about the topic and deliver it to the judging panel. This process is long and requires preparation before and during the tender (around a week).
But you might be asking, why are you telling me about the academic system in Brazil? Because I thought, at the time, that academic hiring processes were similar everywhere. The reality couldn’t be more different. Although you might think you
52 OUTREACH EDUCATION AND DIVERSITY
won’t move countries, it is good to know how to prepare for a career transition and understanding the processes can help you in this preparation. An academic career is commonly international, and people move places a lot. This may not be for you, but it might appeal to the students you are or will be supervising.
MOVING TO THE USA AND THE UK
I moved to the USA and learned about a different transition process there. A book called The Professor Is In1 was one of the reading recommendations at the time. If you have time, it is still worth reading, but pay attention to the differences amongst places and regions. In the USA, becoming a professor/ lecturer (name varies depending on the location, too!) is quite a similar process as in the UK, where I currently live. In these countries, candidates usually apply by submitting an academic CV, which is longer than a traditional CV and focuses primarily on research and academic contributions; a cover letter; names and contacts of references; a list of publications or examples of publication; a research statement to cover your research experience and future proposals; and a teaching statement. Again, what is requested varies according to institution and field, and the documents listed above may vary slightly, but this list gives you a general idea of the application process. The application is considered the first phase of the hiring process, and only a few candidates (around five) are invited for a usually in-person interview. Universities typically cover the travel costs of the candidates. During the interview, different dynamics can be in place, including but not limited to: a talk in which candidates usually include some information about themselves, their career, how they will insert into the department/university context and their 5-year strategy for research and teaching; a social event with other lectures and/or students, e.g. a dinner in which they assess your soft skills (e.g. communication, teamwork, leadership, interpersonal skills); and a human resources (HR) interview, which is a more formal HR-focused questionnaire type of assessment.
REPOSITIONING TO A NON-ACADEMIC JOB
The transition to an academic role isn’t easy and requires much preparation. But imagine that halfway through pursuing an academic career, you realise it is not for you, or you may not find a good match for your goals based on the available positions. You will need to reposition yourself to a non-academic job, which is scary, and there are many discussions about this that I won’t cover in this article. But, again, the
hiring process in a non-academic job varies from country to country and varies a lot compared to everything you might have gone through or thought of going through in the academic journey. The skills learned could be and should be transferable, but candidates must now adapt their mindset to a new perspective (further reading: SEB transferable skills guide2), because in a non-academic job, a candidate’s CV usually has two pages maximum, in which they need to focus much more on skills, projects and successes than their research. Your research will be part of it, but it isn’t the main focus. Usually, the design of the CV and the main subsections matter a lot. Again, it is an entirely new type of preparation. Although candidates can use the same format in different countries, tailoring to that specific place’s requirements and examples give them the best chance in the first screening process. It can take recruiters less than a minute to skim a CV and decide whether an applicant is worth pursuing. In some countries, such as the USA and the UK, a cover letter is also mandatory, while in Brazil, this isn’t that common. Candidates might also need to fill out an application form or take basic exams. The other hiring phases can vary in number and type, including interviews, presentations, project completion and HR assessment, amongst others. This information can commonly be found in the job description or is sent after applying.
It is important to say that I have gone through none of these academic processes myself but was interested in learning about them given that an academic career was on my radar, and I had someone close to me going through these processes more than once. However, I did reposition myself to a non-academic job, and that’s why I’m here writing this article today.
NEXT STEPS FOR YOU
I encourage you to do not only your science research but also career research. Look at what things are asked for in your next career move. How to prepare for this? Sometimes, it may take a year or so to build a relationship to get a good reference contact, for example. As a learned society, the SEB nurtures connections and provides many resources and webinars to develop your career whether you choose to be in academia or not. We currently have the Leaders of the Future and Careers and Coffee webinars in which the speakers talk about their career journeys in academic and non-academic settings, respectively. Keep an eye on the following events and contacts us at r.ellerington@sebiology.org for any support with your career development.
References: 1. Kelsky K. The Professor Is In. New York City, Three Rivers Press, 2015. 2. SEB. Transferable Skills Guide. www.sebiology.org/careers/ transferable-skills-guide.html
I MOVED TO THE USA AND LEARNED ABOUT A DIFFERENT TRANSITION PROCESS THERE. A BOOK CALLED THE PROFESSOR IS IN1 IF YOU HAVE TIME, IT IS STILL WORTH READING
53 OUTREACH EDUCATION AND DIVERSITY
IN SOME COUNTRIES, SUCH AS THE USA AND THE UK, A COVER LETTER IS ALSO MANDATORY, WHILE IN BRAZIL, THIS ISN’T THAT COMMON.
GOING GREEN–WHAT DOES A SUSTAINABLE LAB LOOK LIKE?
BY CAROLINE WOOD
From gloves and gels to pipette tips and petri dishes: labs are notorious for the amount of waste they generate as well as the energy they consume. But can researchers reduce this without affecting the quality and reproducibility of their work? To find out, Caroline Wood took a tour of the Sir William Dunn School of Pathology at the University of Oxford. Here, the Department’s dedicated Green Group have shown that even small actions can add up to make a big difference on the environmental impact of lab research.
January 2020, the group’s 20–30 members are diverse—ranging from PhD students and professors to admin staff to facility managers—but they are united in their commitment to explore all possible ways of reducing the Department’s environmental impact.
“For a long time, we have had a drive to reduce the resource and energy footprint of our research,” says Saroj. “So, during the period when we started returning to the labs after the COVID-19 lockdowns, we took the opportunity to completely reassess our working practices.”
My visit starts in the foyer of the Dunn School, where I meet Saroj Saurya, who leads the Department’s Green Group. Formed in
54 OUTREACH EDUCATION AND DIVERSITY
LOW-IMPACT LABS

Green Group member Emile Roberts takes me to one of the labs to show me the sustainability measures introduced here:
● Recycling points for plastic and paper, and separate streams for brown and clear glass. Prominent posters make sure everyone knows what can and can’t go in.
● Every fume hood has a “Shut the Sash” sticker (one US study found this reduced energy costs by around $1300 per fume hood each year).
● 2Switch off when not in use” stickers are everywhere, for instance on Thermocyclers and Thermoblocks.
● Reusable wooden toothpicks are used for picking bacterial colonies from the plates, rather than single-use plastic pipette tips.
● Reusable glass spreaders or beads are used to spread bacteria on agar plates rather than single-use plastic spreaders.
● Plastic serological pipettes have been switched to glass and are washed in a laboratory dishwasher with a special pipette holder.
● 15/50 mL Greiner tubes are decontaminated, washed and autoclaved to be reused.
● Freezers are scraped down regularly, defrosted as and when required, and kept as full as possible to increase efficiency (freezer blocks fill any empty spaces).
● Plans on fridge and freezer doors show where everything is, allowing people to locate items as quickly as possible.
● As −80°C freezers wear out, they are upgraded to more efficient models that can operate at −70°C, and more than halve energy costs.
● Uncontaminated nitrile and latex gloves and masks are sent for recycling by TerraCycle and appCYCLE to be made into plastic pellets that can be used to make different plastic products.
“Some of these procedures do require a little extra effort, for instance to separate items that can be reused from those that can’t, but people have adopted them into their working practices surprisingly quickly,” says Emile. “We have received really positive feedback, and many people have said how glad they are to take a more active role to reduce our environmental impact,” he adds.
STOCKS AND STORES
We then move to the prep labs and stores where there is a similarly comprehensive approach:
● All brands of pipette tip boxes and tip box inserts are collected for recycling.
● Boxes of materials to be autoclaved are sent only when completely full.
● Reagents are bought in bulk to reduce both costs and transport emissions. Where possible, solutions are made up from powders rather than being purchased in liquid form, again reducing transport emissions.
● Instead of individual labs having their own supplies of reagents, many reagents are provided communally from a bulk stock. Researchers sign out what they use with a grant code and the stores team handle the financial admin.
● All printers use only 100% recycled paper and empty printer cartridges are refilled with ink on site then reused.
● Lab coats are washed by Oxwash, a company who use environmentally friendly detergents and collect/deliver by bicycle.
● Brown glass bottles and 2.5 L bottles are returned to suppliers for reuse.
Those who might worry about contamination from reusing tubes have little to fear, Emile says. “We even analysed tubes that had been cleaned for reuse using mass spectrometry and found virtually no residues.”
But “reuse” goes beyond lab consumables, as Emile explains: “The workshop team are really proactive when it comes to repairing equipment and they only throw things away as a last resort. Even if something is broken beyond repair, they will use as much as possible for parts and spares.”
He then shows me what becomes my favourite part of the tour, what is affectionately called “The Green Room”. This storeroom acts as a central repository where anyone can bring equipment, reagents and lab consumables that are no longer needed (e.g. when a lab group leaves the Department), making them available to anyone who can use them. I’m impressed by the sheer range of items on offer, from near-full RNA preparation kits to desk chairs, and Emile tells me that much of it will soon be claimed. “The rate of turnover here is incredible, and it has really reduced the amount we have to buy in. We also have an email mailing list where people can request to borrow a reagent they only need a small amount of, such as a restriction enzyme or primer. There is a strong culture of sharing and helping each other out.”
A WIDER CULTURE OF SUSTAINABILITY
In the communal areas, the green ethos extends beyond the many plants that have a home here. The café has a dedicated food waste bin and offers discounts to those who bring their own containers (a stack of communal mugs makes this very easy). A bring-and-share table allows people to drop off any unwanted or surplus items, and the departmental bike hire scheme OxBikes allows any member to rent a bicycle for just £2 a day. Emile adds that the Green Group have hosted various sustainability-themed events, including plant and clothes swaps, a sustainable suppliers exhibition and a refill station for toiletries and cleaning products.
According to Saroj, both top-down and bottom-up support within the Department have been critical factors in the success of the green measures. “For instance, the Heads of Department allocated funding specifically for green measures. Amongst other things, this funds the recycling collections for the latex gloves. Meanwhile, many of the PhD and early-career researchers have been very active in proposing new ideas.”
Whilst it is impossible to completely eliminate the environmental impact of lab work, as Saroj says: “When everybody is on board, our collective efforts can make a huge difference. And I’ve found that ‘greener’ labs also tend to be happier and more collaborative places to work as well.”
FIND OUT MORE
The Laboratory Efficiency Assessment Framework (LEAF) is a recognised accreditation scheme that helps research groups to identify and adopt strategies to reduce their environmental impact, and to be recognised for doing so. It covers equipment, space use, ventilation, procurement, waste, samples and chemicals. The programme runs yearly, enabling groups to progress through bronze, silver and gold levels. To find out more and get started, visit https://www.ucl.ac.uk/sustainable/ case-studies/2020/aug/take-part-leaf.
55 OUTREACH EDUCATION AND DIVERSITY
FOR A LONG TIME, WE HAVE HAD A DRIVE TO REDUCE THE RESOURCE AND ENERGY FOOTPRINT OF OUR RESEARCH
SEB CENTENARY CONFERENCE
EDINBURGH 4-7 JULY 2023
SEBIOLOGY.ORG

#SEBCONFERENCE
SEB CENTENARY CONFERENCE 1923-2023
CELEBRATING SUCCESS & SHAPING THE FUTURE
ANIMAL BIOLOGY | PLANT BIOLOGY | CELL BIOLOGY
SESSION TOPICS WILL INCLUDE:
ANIMAL BIOLOGY SESSIONS
• A1 - Senescence in a changing world: ageingrelated mechanisms in ectotherms
• A2 - Evolutionary ecology in extreme environments
• A3 - Masters of none: the impacts of multiple stressors on performance in aquatic organisms
• A4 - Mechanics of mechanoreception across scales and kingdoms
• A5 - Insect osmoregulation
• A6 - Bright nights with dark consequences: the evolutionary ecology of coping with light pollution
• A7 - Rapid evolution of invasive populations due to anthropogenic changes
• A8 - Keeping the pace: integrating mitochondrial and cellular bioenergetics to whole-animal fitness in a changing environment
• A9 - Species interactions and their role in experimental physiology: bringing back the BIO
• A10 - From bacteria to whales: using size spectra to measure global Change
• A11 - Plasticity and resilience of developmental stages to climate change
• A12 - Open biomechanics
• A13 - Open animal
• A14 - Experimental Palaeobiology - Bringing Fossils “Back to Life”
PLANT BIOLOGY SESSIONS
• P1 - Improving Crop Nutrient Status: Discovery, Innovation & Translation
• P2 - Sugar metabolism, transport and signalling in plants
• P3 - Life at the interface - plant membraneprotein dynamics and interactions during responses to environmental change
• P4 - Stomata - a model system for fundamental scientific discovery and a target for crop improvement to meet development goals
• P5 - Exocytosis in plant cells
• P6 - Plant biology for sustainability
• P7 - Engineering Earth’s Carbon Cycle using Plants and their Environments
• P8 - PEPG: Plant physiological responses across scales
• P9 - Cereal biology in 4D : gene expression across space and time
• P10 - Unlocking medicinal plants’ full potential through rigorous application of plant science principles
CELL BIOLOGY SESSIONS
• C1 - Local and Higher Order Nuclear Structural Organization and Dynamics
• C2 - Big Data Biology
• C3 - Cell cycle control in non-mammalian systems
OUTREACH, EDUCATION AND DIVERSITY SESSIONS
• OED1 - Communicating and teaching ethical issues in Bioscience: Why, How, What
• OED2 - Outreach and PE
• OED3 - Teaching innovation and student engagement
• OED4 - Getting started on educational research, getting published and output
• OED5 - Transversal Skills in Experimental Biology: employability in industry and project management
• OED6 - Narrative C.V’s
• OED7 - Building for the future: fostering a diverse and inclusive biosciences community
SCIENCE ACROSS BOUNDRIES
• AP1 - Looking backwards and forwards a er a decade of Conservation Physiology
• ACPO1 - 100 years of SEB
• AC1 - Thermoregulatory and metabolic adaptations in a changing world
• CP1 - General cell and plant biology
WORKSHOPS
• Peer Review Workshop
• Image Integrity Workshop – Good scientific practice of preparing and assessing imagebased data
• How to work with your Data, an interactive workshop
• Science and Social Media. Are they really compatible? Developing E ective Science Communication Strategies.
SOCIETY FOR EXPERIMENTAL
BIOLOGY PRESENTS:





 PROFESSOR JIM MURRAY PRESIDENT,
PROFESSOR JIM MURRAY PRESIDENT,


















































 By Lee Sweetlove. Professor of Plant Sciences, University of Oxford, UK. Editor in Chief.
By Lee Sweetlove. Professor of Plant Sciences, University of Oxford, UK. Editor in Chief.






















































 Figure 2
Figure 1
Figure 2
Figure 1



 Figure 4a
Figure 5
Figure 6
Figure 4b
Figure 5
Figure 1
The OED team during their visit to the SEB archives
Figure 2
Figure 3
Figure 4a
Figure 4b
Figure 4a
Figure 5
Figure 6
Figure 4b
Figure 5
Figure 1
The OED team during their visit to the SEB archives
Figure 2
Figure 3
Figure 4a
Figure 4b




