









The SEB Magazine is published biannually — Spring and Autumn (online) — by the Society for Experimental Biology and is distributed to all SEB members.
Advertising
Advertising in the SEB magazine is a great opportunity to reach a large community of biologists. For more details contact b.danois@sebiology.org
Design and artwork: Robert Wood, Time Design Studio robertwood@timedesignstudio.co.uk
Contribute with an article!
Interested in writing an article for the SEB magazine? Get in touch: b.danois@sebiology.org
Deadline for copy:
Issue: Autumn 2025
Deadline: 1st June 2025
SEB Executive Team:
SEB Main Office
The Society for Experimental Biology County Main, A012/A013 Lancaster University, Bailrigg LA1 4YW, UK admin@sebiology.org
Chief Executive Officer
Pamela Mortimer (p.mortimer@sebiology.org)
Governance Officer
Sarah Ellerington (s.ellerington@sebiology.org)
Conference and Events Managers
Keji Aofiyebi (k.aofiyebi@sebiology.org)
Jennifer Symons (j.symons@sebiology.org)
Membership Manager
Jordy Turl (j.turl@sebiology.org)
Administrator Officer
Olubunmi Oduah (b.oduah@sebiology.org)
Membership & Administration Officer
Julius Kelly (j.kelly@sebiology.org)
Education, Outreach and Diversity Manager Dr Rebecca Ellerington (r.ellerington@sebiology.org)
Education, Outreach and Diversity
Ana Caroline Colombo (a.colombo@sebiology.org)
Communications Manager
Benjamin Danois (b.danois@sebiology.org)
SEB Honorary Officers:
President
Tracey Lawson (tlawson@essex.ac.uk)
Vice President
Gudrun De Boeck (gudrun.deboeck@uantwerpen.be)
Treasurer
John Love (J.Love@exeter.ac.uk)
Publications Officer
Diana Santelia (diana.santelia@usys.ethz.ch)
Plant Section Chair
George Littlejohn (george.littlejohn@plymouth.ac.uk)
Cell Section Chair
Ross Sozzani (ross_sozzani@ncsu.edu)
Animal Section Chair
Felix Mark (Felix.Christopher.Mark@awi.de)
Outreach, Education and Diversity Trustee
Sheila Amici-Dargan (anzsld@bristol.ac.uk)
SEB Journal Editors:
Journal of Experimental Botany
John Lunn (Lunn@mpimp-golm.mpg.de)
The Plant Journal
Katherine Denby (katherine.denby@york.ac.uk
Plant Biotechnology Journal
Johnathan Napier (johnathan.napier@rothamsted.ac.uk)
Conservation Physiology
Andrea Fuller (Andrea.Fuller@wits.ac.za)
Plant Direct
New editor announcement coming soon
Disclaimer
The views expressed in this magazine are not necessarily those of the Editorial Board or the Society for Experimental Biology. The Society for Experimental Biology is a registered charity No. 273795


As we look ahead to the SEB Annual Conference in Antwerp this July, we are proud to present the Spring 2025 issue of the SEB Magazine, celebrating the remarkable ways in which experimental biology is rising to meet the global challenges of our time.
From biodiversity loss to climate change, and food security to scientific communication, SEB members are at the forefront of research, innovation and outreach.
This issue offers a preview of the exceptional science and community spirit you can expect at our flagship conference—and highlights the collective power of our disciplines to drive real-world change.
In the animal realm, ‘Long Live the Kingdom’ by Alex Evans showcases the evolving strategies zoologists are using to address global change. The article highlights the importance of co-produced conservation research, built on inclusive partnerships. It also explores the role of microbes in enhancing the climate resilience of reptiles and amphibians, as well as investigating whether fish can evolve quickly enough to survive rising water temperatures through artificial selection. These stories reveal that innovation and collaboration are essential to preserving biodiversity in a warming world. (Page 16)
Concerning the cell feature, in ‘Tomorrow’s Toolbox’, Alex Evans explores a range of cutting-edge techniques that are revolutionising cellular and molecular biology. From high-resolution microscopy in extreme environments to the application of single-cell omics and innovative culture systems, these tools are enabling scientists to investigate biological processes with unprecedented precision. The article highlights how such techniques are accelerating discovery across disciplines (from plant pathology to marine biology) and how their adaptability is opening new avenues for understanding life at the cellular level. (Page 20)
For the plant article, Caroline Wood’s feature ‘Pushing Plant Biology to Address Global Challenges’ examines how experimental plant science addresses major global issues such as food insecurity and climate
BY BENJAMIN DANOIS
change. Caroline highlights cutting-edge research into cytoplasmic genetics, stomatal function and photosynthesis, offering promising routes to enhance crop resilience. This article also emphasises the vital role of interdisciplinary collaboration and the translation of lab discoveries into practical solutions for sustainable agriculture. (Page 24)
In this issue’s spotlight on our members, we proudly showcase the outstanding achievements of our community. Discover the impactful work of our members, each a shining star in the SEB constellation. (Page 12)
This edition includes exclusive interviews with Robyn Emmerson, SEB Early Career Trustee, and George Littlejohn, Chair of the Plant Section. (Page 40)
We also shine a light on two inspirational figures in the community: Frank Van Breusegem and Amanda Wiesenthal. These conversations offer personal perspectives on leadership, research and the future of experimental biology. (Page 44 and 46)
Caroline Wood’s ‘Top Tips for Giving Media Interviews’ offers practical advice to scientists preparing to share their work with the public. From crafting clear messages to managing nerves and staying on-topic, her insights empower researchers to become confident science communicators. (Page 50)
In ‘Empowering Scientists’, Ana Colombo highlights the SEB’s dedicated workshops on communication and research impact. These events help scientists to engage diverse audiences, improve their presentations and develop strategies to amplify their work’s societal relevance. (Page 52)
We are thrilled to host three major events in 2025: SEB Annual Conference Antwerp 2025: Join us for cutting-edge sessions across Animal, Cell, Plant and OED Biology. Don’t miss the inspiring profiles of our President’s Medallists and Plenary Lecturers, featured in this magazine.

JXB 75th Anniversary Symposium: A special celebration of the Journal of Experimental Botany, recognising decades of plant science excellence, taking place in Edinburgh this September.

PEPG Field Techniques Workshop 2025: Also in September, hands-on training in plant environmental physiology, focused on field-based approaches. As experimental biology continues to evolve, so too does its capacity to address the urgent problems of our era. We hope this issue will inspire, inform and energise you—and we look forward to seeing many of you in Antwerp this summer.

Before closing this editorial, we would like to take a moment to honour the memory of Alessandro Coatti, a biologist with whom we had the privilege to collaborate. His recent tragic passing is a great loss to the scientific community, and we send our deepest condolences to his colleagues, friends and family.

2018 ANTWERP, BELGIUM
08 JULY - 11 JULY 2025
SEBIOLOGY.ORG #SEBCONFERENCE

Welcome to the first SEB Newsletter of 2025!

As we embark on a new year, we also introduce a new theme for SEB: ‘The Impact of Experimental Biology in Tackling Global Challenges’. This theme will not only shape the focus of this issue but will also be the foundation of our 2025 Annual Conference. Numerous reports outline the global challenges facing our world, with rankings of most concern according to who compiled the list. However, a common thread among these challenges is their strong connection to biological sciences. Issues such as disease, climate change and its associated extreme weather events, pollution, environmental degradation, water scarcity, biodiversity loss and ecosystem disruption all have biological dimensions. Emerging technology can provide some solutions as well as associated challenges, such as cybersecurity threats and how we use AI to support science and learning. Another concern, frequently highlighted in global assessments, is the growing spread of misinformation and disinformation, particularly when they influence critical societal decisions. These concerns highlight not only the importance of biological research but also the need to increase outreach and engagement with diverse audiences. These challenges demand coordinated efforts and it is clear that experimental biology, spanning our three core themes, plays a crucial role in addressing many of these global challenges, as will be highlighted at this year’s conference.
This year’s annual conference will be held in Antwerp, Belgium, from 8 to 11 July at the amazing A Room with a Zoo, which is the only convention centre in the world with its own zoo. As registered participants at the SEB Annual Conference, you will be able to visit the zoo and sign up for walks
and tours organised by the SEB and Conservation Physiology. We have put together an exciting four-day programme featuring a diverse range of sessions across our three core scientific themes: Animal, Cell and Plant Biology, with several sessions excitingly crossing these disciplines. The science sessions are accompanied by a full week of outreach, education and diversity (OED) sessions covering creativity in higher education, getting educational research published and enabling learning in a digital world, to name a few. As is tradition at our Annual Conference, awards will be presented to President’s Medallists and Plenary speakers (including the Bidder and Woolhouse awards). In the past these awards have been given in the three scientific sessions; however, I am delighted to announce that this year’s awards in both categories will also be awarded in our OED theme. Registration is now open for the Annual Conference with the early bird deadline of 16 May 2025, and final deadline 13 June 2025. We look forward to seeing many of you there.
The Annual Conference will see the end of my term as SEB president, and this will be my final presidential letter for the SEB magazine. I want to take this opportunity to express what an incredible honour and privilege it has been to serve in this role. Reflecting on the past 4 years, two as VicePresident and two as President, I am proud of what we have accomplished together. In my first year as Vice-President and Chair of the Events Committee, we were emerging from the lockdowns of the COVID-19 pandemic. Our 2021 Annual Conference made history as the first fully online SEB conference, held in December instead of our usual summer schedule, and welcomed around 900 registered delegates.
By 2022, we were able to return to an in-person format in Montpellier. While the transition back to organizing a large-scale, complex event came with its challenges, it was truly rewarding to reconnect with so many familiar faces. The following year, in 2023, I had the distinct privilege of serving as President during the SEB’s centenary celebrations in historic Edinburgh. That meeting and year was fantastic, combining cutting-edge scientific sessions with special events that celebrated 100 years of the SEB. It also highlighted the value of in-person meetings, not only for professional networking but also for fostering creative thinking and scientific progress. This momentum carried into our 2024 Annual Conference in Prague, which was widely praised for its excellent organisation and impact.
Amongst the many highlights of my tenure, I am particularly proud of efforts to strengthen the SEB members’ support for our society journals. I have hopefully emphasised the crucial role our journals play, not only in science dissemination but also the significant financial contribution they make to key SEB activities, including the Annual Conference. This is particularly key, because these conferences run at large financial cost to the society and I hope members will continue to recognise the importance of and support our journals moving forward.
One of the most significant challenges during my presidency has been developing a strategy to support the transition of the Journal of Experimental Botany to an open-access model. As I have highlighted in previous letters, SEB’s society journals provide critical financial support for the organisation. The shift to open access represents a substantial financial challenge, and we have implemented a strategic plan to secure funds over the coming years to mitigate these losses. In light of this, alongside rising operational costs, this year we have introduced a modest increase in the Annual Conference registration fees that is necessary to help ensure SEB’s financial stability.
I have also had the privilege to be part of the restructure of our 5-year strategy and build on the gains made in the first 5 years of the programme. As part of the strategy we have put mechanisms in place to financially support some of our ongoing events, alongside the Annual Conference. One such event is the Field Techniques Workshop run by the Plant Environmental Physiology Group (PEPG). The workshop includes hands-on practical sessions every morning, featuring both an academic researcher and a manufacturer representative. Although open to everyone, I would like to specifically encourage students and early career researchers to attend. The PEPG workshops hold a special place in my SEB journey. I attended the second workshop in Rapolano, Italy, as a second-year PhD student, and it was a truly amazing experience. Not only did it provide a unique opportunity to gain both theoretical knowledge and hands-on experience with ecophysiology techniques, but it also allowed me to engage with leading experts in the field, academics and industry professionals alike, in an informal and collaborative setting. Many of these connections later evolved into valuable research collaborations. Beyond professional development, the workshop provided a strong sense of community. I met peers with whom I would cross paths at numerous conferences, including the SEB Annual Conferences, for many years, truly becoming part of the SEB family. Recognising the value of this experience for early career researchers, I led the re-establishment of these workshops in 2012, ensuring that the next generation of plant scientists could benefit from the same inspiration, training and networking opportunities that had such a great impact on my own career. This year the mantle is being carried by two excellent leaders, Amanda Cavanagh and Lorna McAusland. Registration is now open for the 2025 PEPG Field Techniques Workshop, which will take place from 7 to 12 September 2025 in Portugal. Places are limited so don’t miss out, and register as soon as possible.
LOOKING AHEAD, 2025 PROMISES TO BE AN EXCITING YEAR, WITH OUR ANNUAL CONFERENCE ON THE HORIZON. AS I PASS THE PRESIDENCY TO THE EXCEPTIONAL GUDRUN DE BOECK, I AM CONFIDENT THAT THE SEB IS IN EXCELLENT HANDS.
As part of my tenure, I was committed to enhancing scientific impact, ensuring that we recognise how our research translates into societal benefits. I believe we have made significant advances in this area through expanded outreach and engagement initiatives, as well as scientific sessions within the Annual Conference that emphasise translational research and industry collaboration. These efforts have also helped to support growth and diversification in our SEB members. A key SEB focus during my presidency has been promoting inclusivity within the SEB community, an effort championed by Rebecca Ellerington. Additionally, we have expanded our collaborations and partnerships with other societies and institutions, including a newly signed agreement with the Brazilian Society of Plant Physiology (SBFV) and the Federation of Experimental Biology Societies (FeSBE) in Brazil. I hope that, in some small way, I have contributed to advancing these initiatives and laid a strong foundation for their continued growth.
However, none of this would have been possible without the dedication and commitment of the SEB staff and trustees. This has been a collective effort, and I want to extend my deepest gratitude to the entire SEB team and the trustees for their hard work, support and commitment. Their dedication has been essential in delivering the array of SEB activities, and I will greatly miss working alongside them and their friendship.
Looking ahead, 2025 promises to be an exciting year, with our Annual Conference on the horizon. As I pass the presidency to the exceptional Gudrun De Boeck, I am confident that the SEB is in excellent hands. Though my time as President is coming to an end, I remain a dedicated SEB member and look forward to reconnecting with many of you at this year’s Annual Meeting.
Goodbye for now, and until we meet again –safe travels!

Professor
Tracy Lawson
President, Society for Experimental Biology

BY JULIUS KELLY

THANK YOU
To Tracy Lawson, President of the Society, steps down later this year after a 2-year tenure. Tracy has brought much enthusiasm, devotion and passion to the role and has been a strong advocate for the Society, leading it forward in many ways.
The Society wishes her well and we hope to see her at many future Society events.

To Gudrun De Boeck, as the incoming President of the Society later this year. Gudrun is a Professor of Animal Physiology at the Department of Biology, University of Antwerp. Her research focuses on the effects of environmental factors (e.g. pollution, climate change) on the performance of fish. Studies in her lab are comparative in nature and include freshwater and marine teleosts and elasmobranchs. The Society very much looks forward to welcoming Gudrun at the 2025 SEB Annual Conference in Atwerp.

THANK YOU
…to Ana Colombo, of the SEB Executive team. Ana has been a key part of and fantastic contributor to the Society’s Outreach, Education and Diversity team. Ana—thank you for being such a great team player right across the executive branch, your cheerfulness will be greatly missed. The team wish you all the very best for the future and we all very much hope to keep in touch and see you at future Society events.
SEB ANNUAL CONFERENCE 2025: THE IMPACT OF EXPERIMENTAL BIOLOGY IN TACKLING GLOBAL CHALLENGES
8–11 JULY 2025 ANTWERP, BELGIUM
SEB’s Annual Conference is celebrated for creating the perfect composition of research, new discoveries and collaborative connections. Be creative and innovative by exchanging ideas and knowledge with masters of biology from all over the world.
ANTWERP 2025


CELEBRATING THE CONTRIBUTIONS OF DAVID RANDALL TO COMPARATIVE PHYSIOLOGY 5–6 JULY 2025
Dave Randall, who passed away in 2024, essentially established the modern field of fish physiology. Over a six-decade career, he made fundamental contributions in the three inter-related areas which are the focus of our 2-day meeting. He inspired and mentored multiple generations of comparative physiologists. His trainees, as well as their own subsequent generations of trainees, continue to push the boundaries using the open-minded, ideadriven and technologically advanced approaches that Dave advocated. During this symposium, we will celebrate his contributions and learn about the latest advances in these fields.
Early career researchers are especially welcome. Thanks to a grant from the SEB, the organisers are able to significantly reduce the registration fee for the first 30 early career researchers, from €300 to €50 to attend the symposium.
17–19 SEPTEMBER 2025
EDINBURGH INTERNATIONAL CONFERENCE CENTRE
This symposium celebrates the 75th anniversary of the founding of the Journal of Experimental Botany in 1950. The history of the journal can be likened to the lifetime of a tree, germinating from the seed of an idea and growing into a mature form that supports a diverse community. The meeting will not only reflect these life stages —Beginnings, Growing and Maturity—but also look to the Future, as we face the challenges of the next 25 years. The meeting will bring together established and upcoming plant researchers to build links between people and research communities, share our knowledge and inspire ideas to help the next generation meet these challenges.
THE PLANT ENVIRONMENTAL PHYSIOLOGY GROUP (PEPG)’S FIELD TECHNIQUES WWORKSHOP
7–12 SEPTEMBER 2025
NATURASOLTA - QUINTA DE SÃO PEDRO, PORTUGAL
REGISTRATION AND ABSTRACT SUBMISSION ARE NOW OPEN!
This event will invite 100 plant biologists to come together for a series of practical workshops, alongside presenting their work in the form of a poster. Selected candidates will be given the opportunity to present a flash-talk alongside their poster.

BECOME A MEMBER FIRST AND SAVE ON FEES!
Join SEB today and unlock exclusive benefits and resources. Membership provides access to free online webinars, training sessions,

EDITED BY
In each issue of the member magazine, we like to highlight some of the fantastic achievements and research from our members. Here are some of the people we would like to congratulate this time around.
The SEB would like to congratulate Nicholas on an impressive series of recent publications spanning top-tier journals including Nature, Conservation Biology, Conservation Physiology and Proceedings B. His research addresses urgent ecological challenges, from amphibian vulnerability to climate change, to disease risk in Southern Hemisphere bats, and the broader impact of preprints in ecology.

A remarkable achievement that showcases both scientific rigour and interdisciplinary reach :
- Wu, N. C., Welbergen, J. A., Villada‐Cadavid, T., Lumsden, L. F., & Turbill, C. (2025). Vulnerability of Southern Hemisphere bats to white‐nose syndrome based on global analysis of fungal host specificity and cave temperatures. Conservation Biology, 39(2), e14390.
-Pottier, P., Kearney, M. R., Wu, N. C., Gunderson, A. R., Rej, J. E., Rivera-Villanueva, A. N., ... & Nakagawa, S. (2025). Vulnerability of amphibians to global warming. Nature, 1-8.
- Wu, N. C., Fuh, N. T., Borzée, A., Wu, C. S., Kam, Y. C., & Chuang, M. F. (2025). Developmental plasticity to pond drying has carryover costs on metamorph performance. Conservation Physiology, 13(1), coaf008.
- Noble, D. W., Xirocostas, Z. A., Wu, N. C., Martinig, A. R., Almeida, R. A., Bairos-Novak, K. R., ... & Lagisz, M. (2025). The promise of community-driven preprints in ecology and evolution. Proceedings B, 292(2039), 20241487.
Durham University
The SEB is thrilled to feature Dipan, whose latest research has been accepted for publication in Science and will be available soon (manuscript ID: Adu1470), though it is currently under embargo.
The study, titled “Redox regulated Aux/IAA multimerization modules auxin responses”, sheds new light on the molecular regulation of auxin signalling.

Remarkably, the project began with an unexpected result while repeating experiments from a previous Science paper on hydropatterning. This serendipitous observation led to a breakthrough, illustrating the importance of following up on surprising data. We look forward to sharing more once the article is released.
Université de Namur
The SEB celebrates Eli, who has just become co-author on their first Science paper entitled “Pharmaceutical pollution influences river-to-sea migration in Atlantic salmon (Salmo salar)”. This landmark publication is already attracting major media coverage, including from The New York Times and Vox — a thrilling first for Eli!

https://www.science.org/doi/10.1126/science. adp7174
The Islamia university of Bahawalpur
The SEB extends its congratulations to Umer for his first Report on Clinical Feasibility of Dried Blood Spot Technique for Hemoglobin Estimation.
This work explores a promising low-cost technique with realworld applications in healthcare diagnostics.
https://als-journal.com/ submission/index.php/ ALS/article/view/2993

JOURNAL OF EXPERIMENTAL BOTANY (JXB)
75TH ANNIVERSARY SYMPOSIUM
SEBIOLOGY.ORG #JXB75

PLENARY TALK
• JANE LANGDALE (UNIVERSITY OF OXFORD)
1: BEGINNINGS – THE ORIGINS OF PLANTS AND THE START OF THE PLANT LIFE CYCLE
• KIN PAN CHUNG (WAGENINGEN UNIVERSITY)
• STEVE PENFIELD (JOHN INNES CENTRE)
2: GROWING – PHOTOSYNTHESIS, RESPIRATION, NUTRIENT ACQUISITION, GROWTHCYCLE
• DIANE BECKLES (UC RIVERSIDE)
• PROFESSOR CHRISTINE RAINES (UNIVERSITY OF ESSEX)
• BERKLEY WALKER (MICHIGAN STATE UNIVERSITY)
3: MATURITY – FLOWERING, REPRODUCTION AND COPING WITH STRESS
• MADELAINE BARTLETT (SAINSBURY LABORATORY CAMBRIDGE)
• CRISTÓBAL UAUY (JOHN INNES CENTRE)
• TEVA VERNOUX (ENS LYON)
4: FUTURE – NEW PLAN(T)S
• DR. FRANZISKA FICHTNER (HEINRICH HEINE UNIVERSIT Y DÜSSELDORF)
• DEVANG META (K U LEUVEN)
• NICOLA PATRON (UNIVERSITY OF CAMBRIDGE)

BY ALEX EVANS

Zoology is a rich and diverse field of study, but one united by a common truth—that the world is rapidly changing. Not only are animals having to adapt to meet new environmental challenges, but researchers are also having to adapt their ways of working to better understand Earth’s ever‑changing status quo. Let’s meet just a few of those researchers at the forefront of global change and conservation research.

Animals may be the subject of zoological research and conservation projects, but humans are the ones that design, deliver, dissect and disseminate those projects, so is it any wonder that the specific humans involved can influence the research’s impact?
This is a question that Steven Cooke, a professor of fish ecology and conservation physiology at Carleton University in Ottawa, Canada, has been keen to investigate. While most of Steve’s research is focused on the conservation of wild fishes and other aquatic animals, he is also interested in the human dimensions of research into the natural world.
Steve’s interests have a clear goal: how do we make it impossible for decision-makers to ignore science? As the founding Editor-in-Chief of the SEB journal Conservation Physiology, he is all too familiar with the global challenges that face wildlife today and is keen to explore contributions both behind the scenes and at the frontline of conservation research. One such contribution is co-production, a collaborative practice involving a wide range of stakeholders in the research to better inform the project design and maximise the impact of the research findings—both locally

for those who are most affected, and globally for the wider scientific community. ‘It is clear that co-producing research with partners from government, industry, Indigenous communities and other actors is the best way to generate new knowledge that is relevant to and embraced by decision-makers,’ explains Steve.
‘Our laboratory was doing co-production for many years before we really recognised that we were doing so,’ he says. ‘We would develop relationships with commercial fishers or fisheries managers, and they would tell us about the issues they faced. Those conversations led to ideas on how we could help to address their issues and we worked with them to make that happen.’ More recently, Steve and his team continue to approach co-production in a similar way but can now act with experience to avoid previous pitfalls and ensure better outcomes.
The consequences of failing to embrace coproduction in field studies can lead to research methodologies that are poorly planned, may have negative implications for local communities or may simply be unactionable. ‘In some cases, even when the science itself is robust, it is still ignored by decision-makers because there can be issues around trust,’ he explains. ‘In that sense, co-production is very much about relationships, which are important given that humans are social creatures.’
In the context of Steve’s work, co-production is critical to his team’s field studies in tracking wild fish with telemetry to better understand challenges to their conservation. ‘Co-production is often essential or simply comes naturally given the need to secure permits and to collaborate with different groups to collect animals,’ he says. ‘Co-producing our telemetry research has revealed some of the potential issues that different end users have with the method.’ Some of these issues have included concerns about tagged animals not being representative of untagged animals given the tagging stress and burden involved, leading the team to do more validation studies and involve those partners in the tagging process so they could see for themselves what was involved.
For researchers interested in incorporating more co-production into their projects, Steve has the following advice: ‘There are lots of good resources on how to do co-production well and what to avoid,’ he says. ‘However, be prepared to make mistakes and be humble enough to own them and move forward.’ Because co-production is based on the foundations of strong relationships, it can take time to incorporate the right voices, but when done properly, the benefits can be huge. ‘I have personally learned so much from interacting with Indigenous elders, recreational anglers, hydropower industry staff, fisheries managers and policymakers,’ he concludes. ‘My career would not be nearly as rewarding or impactful if I did my work in isolation from those folks.’
OUR LABORATORY WAS DOING CO-PRODUCTION FOR MANY YEARS
When people think of animal conservation in the face of global change, their first thoughts probably don’t jump to microscopic bacteria, viruses and fungi—but these all play a critical role in the microbiome that sustains and regulates animal life. Samantha Fontaine, a postdoctoral research fellow at Kent State University in Ohio, USA, is exploring the way that these microbial communities can influence the responses of amphibians and reptiles to global anthropogenic changes, such as land use and climate change.
‘I’ve always loved animals and after learning about biodiversity loss as a high-school student, I decided to major in biology as a university undergraduate because I was passionate about species conservation,’ she says. ‘During my undergraduate studies, I took courses on microbiology and developed an interest in this subject because I thought it was fascinating how such tiny organisms can have such large impacts on the environment and their hosts.’ While it is true that many microbes can be detrimental to animal health, there are plenty that also provide benefits to their survival. ‘A big focus in the field has been on how microbes can protect amphibians and reptiles
WHILE IT IS TRUE THAT MANY MICROBES CAN BE DETRIMENTAL TO ANIMAL HEALTH, THERE ARE PLENTY THAT ALSO PROVIDE BENEFITS TO THEIR SURVIVAL
Ganges with various stakeholders.
Photo credit: Steven Cooke.
Right:

from fungal diseases,’ explains Samantha. ‘Chytrid fungus is a huge threat to amphibians and snake fungal disease is threatening reptiles; studies have shown that microbes on the skin of these animals could help them resist disease.’
Because reptiles and amphibians are ectotherms, they are unable to regulate their own body temperature through physiological means, which led Samantha and her team to wonder about the role that microbes can play in resisting the effects of extreme temperatures. ‘New research, including my own,1.2 has demonstrated that the function of microbes could help these animals better survive both hot and cold extremes,’ she says. ‘I am trying to discover how the function of microbes impacts, and potentially benefits, amphibians and reptiles when they face changes in their environments.’
This is a topic that Samantha first started investigating during her PhD, where she focused on the influence of the microbiome on resisting heat stress in green frog (Lithobates clamitans) tadpoles. ‘We found that raising animals under depleted microbial conditions reduces their acute tolerance to heat and cold and reduces their ability to survive under longer term heat stress,’ she explains. ‘This work has important implications for global change because environmental microbial communities are losing diversity due to anthropogenic impacts and if this loss of diversity impacts wild amphibian
microbiomes, they may be more vulnerable to declines under climate change.’
Surprisingly, while Samantha and her team expected tadpoles with depleted microbiomes to have reduced growth and development, they found the opposite to be true, with those tadpoles growing faster and larger than those with colonised microbiomes. ‘This could represent a trade-off in which animals that are more tolerant to temperature are also smaller, which could have negative consequences in the wild,’ she suggests. ‘An important next step would be to perform these experiments in more natural settings, rather than the laboratory , to get the full picture of how the microbiome impacts animals in the wild.’
Now, in her position as a postdoctoral researcher, she is looking at how the microbiome may be helping invasive common wall lizards (Podarcis muralis) exposed to new habitats. ‘The common wall lizard is a small lizard species native to central Europe that has become introduced and well-established in several locations around the world outside of its native range,’ she explains. ‘I am interested in how the microbiome may facilitate success when the species is introduced to a novel environment.’ For this project, Samantha has been developing a system to manipulate the microbiome of this species to create germ-free lizards, raised completely devoid of microbes. However this technique is
rarely used for non-model species because it can be laboratory and difficult to maintain. ‘I recently completed a pilot study to rear germ-free lizards in the laboratory in a feasible, tractable way,’ Samantha says. ‘If this experiment is successful in maintaining lizards germ-free, I hope to inoculate them with microbes from native versus invasive populations to understand how the microbiome influences physiology and could impact invasion.’
As Samantha explains, there is still plenty to explore within this area of research, with exciting potential applications to aid in the conservation of vulnerable species. ‘Bioaugmentation, or the manipulation of animal microbiomes to achieve a specific outcome, has become a popular topic in the field,’ she concludes.
‘If we can identify the specific microbes that increase amphibian heat tolerance, they could be used as probiotic treatments to protect animals from climate change; however, more research will need to be done before we get to this point.’
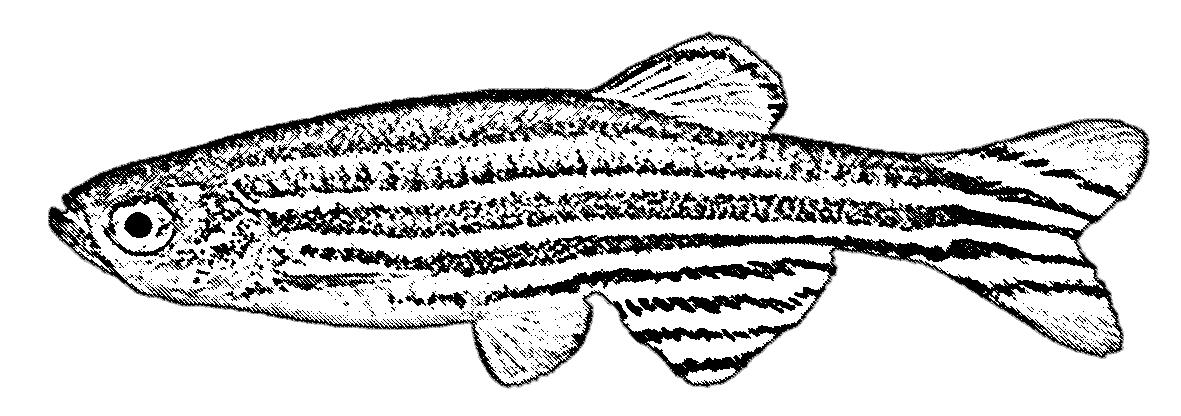
The greatest defence against global change is adaptation, but natural selection and evolution are slow processes, so what hope do animals have in the face of such rapid environmental change? Robin Leeuwis, a postdoctoral research fellow in animal physiology at the Norwegian University of Science and Technology (NTNU) Department of Biology in Trondheim, Norway, is investigating the use of artificial selection as a driver for fasttrack evolution. ‘My research focuses on how the environment affects and shapes the physiology and evolution of animals in aquatic ecosystems,’ says Robin. ‘I primarily study fishes, and I am especially interested in the impacts of climate change on this important group of animals.’
While some animals may be able to overcome warming environments by migrating or adjusting their behaviour, many rely more on the mechanisms of phenotypic plasticity or evolutionary adaptation. ‘My current work focuses on the latter, given it is such a powerful process in nature that is especially important when other coping mechanisms fail,’ she explains. ‘There is also still little known about the adaptation of animals to contemporary climate change, because it can be challenging to study.’

To evaluate the evolution of resilience to chronic warming, Robin is leading a laboratory experiment using wild-caught zebrafish, with a specific interest in the growth performance of the juvenile fish as a measurement of the fish’s ability to adapt to warmer temperatures. As a model species known for its relatively short generation time, zebrafish were a natural choice for this study, and Robin settled on artificially selecting and breeding the fish over six generations. ‘An artificial selection experiment is an excellent research tool that allows us to test whether thermal performance is an evolvable trait in zebrafish, and if so, to assess the rate and extent of evolution,’ she says. ‘This will ultimately help us to answer the central question whether “evolutionary recovery” is possible—whether animals can evolve fast enough to regain fitness and keep pace with global warming.’
While six generations may not sound like a lot in the context of evolutionary timescales, this will be the result of over 2 years of breeding involving the measurement of growth rates from over 10,000 individual animals. ‘As an early career researcher, it was daunting to commit to such a project because the pressure is high in academia to publish as many papers as quickly as possible,’ explains Robin. ‘However, I firmly believe that this “slow science” is essential to advance our understanding of evolution under global warming, and I am excited to be spearheading research that is absolutely ambitious and cutting-edge.’
While Robin has not yet reached the final generation, her preliminary findings are already starting to show promise. While the first generation of fish grew less successfully in warm water conditions compared to control conditions, the next generations did show signs of improving, having almost reached the baseline growth rates set by the fifth generation. ‘Overall, these results suggest that there is potential for evolutionary recovery of growth performance in zebrafish under chronic warming,’ she says. However, Robin acknowledges that these are not the final results and is eager to re-examine the situation with the sixth and final generation of fish. ‘In particular, we are curious to see if
the thermal performance curves of the selected lines have ultimately changed,’ she says. ‘For instance, if the warm-adapted line eventually outperforms the control line in terms of growth at warm temperatures, then this would be further compelling evidence that zebrafish can to some extent adapt to these conditions.’
Not only does Robin hope that these findings provide fundamental knowledge about growth performance through evolution and may be useful for informing conservation efforts, but she also points out that the techniques developed through this project will have tremendous value to other researchers. ‘Due to its large scale, this project also inspired us to develop a new user-friendly machine-learning tool to automate and simplify length measurements from photos,’ she says. ‘The tool will be freely available for anyone in the scientific community who wishes to extract lengths from photos, including researchers pursuing questions related to wildlife conservation.’3
Robin is keen to acknowledge that this research project was by no means a solo effort, and it wouldn’t have been possible without important collaborations. ‘I lead the selection experiment together with Lorena Silva, a doctoral student at our department and a fantastic colleague, but we also get help from an army of undergraduate students to measure and rear all zebrafish in the experiment,’ she concludes. ‘I am really glad to have the support of my postdoc collaborators; everyone on the research team has been instrumental in making this project a success!’
DUE TO ITS LARGE SCALE, THIS PROJECT ALSO INSPIRED US TO DEVELOP A NEW USERFRIENDLY MACHINELEARNING TOOL TO AUTOMATE AND SIMPLIFY LENGTH MEASUREMENTS FROM PHOTOS
References:
1. Fontaine SS, Mineo PM, Kohl KD. Experimental manipulation of microbiota reduces host thermal tolerance and fitness under heat stress in a vertebrate ectotherm. Nat Ecol Evol 2022; 6: 405–417.
2. Fontaine SS, Kohl KD. The microbiome buffers tadpole hosts from heat stress: a hologenomic approach to understand host–microbe interactions under warming. J Exp Biol 2023; 226: jeb245191.
3. Kristiansen HH, Metz M, Silva-Garay L, et al. HusMorph: A simple machine learning app for automated morphometric landmarking. ecoevorxiv 2025; preprint. Machine learning tool available from https://github.com/HenHus/Husmorph
Above:

BY ALEX EVANS
The world of cellular and molecular biology is synonymous with fancy gadgets and delicate devices that allow us to examine a wide range of life critical mechanics and dynamics at exquisitely minute detail. Here is just a glimpse into the frontier of modern microscopy and cutting edge experimental techniques that are driving forwards new breakthroughs in our understanding of cellular science.

Our capacity to understand cellular biology is only as good as our ability to study it in realistic physiological conditions. For many species on Earth, these conditions overlap fairly well with traditional laboratory setups, but for animals living in extreme environments, studying their cells live under realistic circumstances can be a lot more difficult without the appropriate technology. This is a challenge that faced Anne-Pia Marty, a PhD student in the Department of Chemical Engineering and Biotechnology at the University of Cambridge, UK, who has developed a technique for high-resolution microscopy of live cells under the harsh Antarctic conditions.
When Anne-Pia first started looking for a PhD, she was especially interested in exploring the interface between biology and physics that may also serve her interest in technology. Eventually, she found a project that was looking to address the large gaps in our understanding of cold-adapted species. ‘I was looking for something that had more of an impact in environmental issues than health issues, so when I heard about this project, it seemed like a perfect fit,’ she says. ‘The field of polar ecology is quite a small one because of the access to the material and the low number of people specialising in it, so the barrier to entry in that field is quite high.’
One major barrier to research in this field is the extraction of primary cell lines from Antarctic fish, because traditional cell biology techniques were not at all optimised for these cold-adapted species. However, as Anne-Pia points out, this made every new development and discovery throughout her PhD an exciting step forward in this field. While not a lot is known about the cells of Antarctic fish, the limited research does suggest that these fish are quite bad at producing and maintaining proteins, which raises many questions about their ability to survive in such an extreme environment. ‘Antarctic marine fauna is surprisingly diverse, and so despite all the challenges of staying alive, they actually do thrive—but how they do it is a bit of a mystery,’ explains Anne-Pia. ‘That’s what I was hoping to investigate, but first we needed to develop all the tools and the methods to help us answer those questions.’
At temperatures below 0°C, molecular and cellular processes such as protein folding, diffusion and metabolism are slowed and energy becomes more scarce. Anne-Pia was interested to see how the cells from her species of choice, Harpagifer antarcticus, the Antarctic spiny plunderfish, would behave under their physiological conditions between −2°C and 2°C, once she had developed the techniques that would make it possible. ‘Traditional highresolution microscopy isn’t fit to image anything at 0°C,’ says Anne-Pia. ‘There has been a whole 2 years of instrument development to create the tools for microscopy so that we could do highresolution microscopy on those cells and keep them in physiologically relevant conditions.’

Now that this technique has been successfully implemented, it has already provided key cellular and molecular insights into the extreme physiology of these animals, shedding light on how they have adapted to life in the freezing Antarctic waters. ‘We’ve found that some organelles move surprisingly fast given their cold temperature and slow metabolic rate,’ she says. ‘For example, the active processes in the mitochondria under physiological conditions seem to be quite temperature sensitive. When heated up, this type of movement doesn’t speed up, despite the increased thermal energy and decreased viscosity.’ To create useful comparisons with cells from fish species adapted to less extreme environments, Anne-Pia also conducted proteomic experiments with temperate homologues, Lipophrys pholis, known as smooth blennies. Comparing muscle and liver samples for both species highlighted some interesting metabolic dynamics between the two species and tissue types.
More recently, Anne-Pia’s team have brought in postdoctoral researchers to further explore the potential outputs of these methods and build on Anne-Pia’s initial findings. ‘Now that this technique has been published, hopefully it opens up avenues for other groups looking at temperature-controlled microscopy experiments,’ says Anne-Pia. ‘There are a lot of fields that could benefit from this new technique, which was part of the motivation for developing it in the first place, but this is such an interesting and discovery-rich field, and the list of what we could do is essentially endless.’
While single cell omics may not be an especially new tool for some cellular and molecular biologists, it’s still relatively fresh in the context of research in many animal species. One enthusiastic proponent of this technique is Dan Macqueen, Professor of Fish Genomics at The Roslin Institute, University of Edinburgh, UK, who is regularly using single cell omics to explore the molecular mysteries of non-model fish species. ‘My group addresses both fundamental and applied research questions at the intersection of genomics, physiology and evolutionary biology,’ says Dan. ‘Many of our projects are on salmon and related fish species, with several in collaboration with companies in the aquaculture sector.’
Dan describes his introduction to single cell omics and his laboratory’s development of the technique as partly strategic and partly serendipitous. ‘About 5 years ago, single cell sequencing was making a
big splash in the human and model organism world, with some really important knowledge advances being made across most biological disciplines,’ he explains. ‘A postdoctoral researcher I hired called Richard Taylor came from a lab in Edinburgh that was leading the field in using single cell omics to understand human liver physiology and pathology. Working closely with another researcher hired at the same time (Rose Ruiz Daniels, now a lecturer at Stirling University), we saw a great opportunity to transfer key skills into the group and our forays into single cell sequencing started there.’
Dan and Roslin colleagues have since developed several approaches for single cell omics and made them available to other researchers. ‘We have established an in-house genomics facility at the Roslin Institute specialising in single cell methods and their applications in a range of species, which is being used widely by researchers locally and beyond,’ he says. ‘This sort of data was unheard of a decade ago for all but a very small number of researchers involved in generating the first single cell technologies, but is quickly becoming a new gold standard.’
One of the major benefits of single cell omics is the ease of generating large amounts of genetic data, which is fundamentally important for nonmodel species in which very little is known about cell diversity and function. ‘In the past, this was so expensive and hard to do, meaning we had to focus on a small number of samples—while enough to publish a paper, this limited the questions we could answer,’ says Dan. ‘Excitingly, we can currently analyse hundreds of samples in single studies, with various methods becoming more cost-effective and robust practises increasingly established, with the biological insights that follow an order of magnitude greater.’
It’s not all smooth sailing, however, and there can be significant challenges that come with such fine-scale research in non-model species.
‘When working with a non-model species, like a farmed fish, our knowledge of cell biology is typically very limited and we have a much less developed understanding of the genes and genomes of these species,’ Dan explains. ‘This lack of understanding and comparatively poor characterisation of non-model species genetics makes it challenging to interpret and get the most out of single cell omics data. However, at the same time, the same approaches are providing many new insights into what sits “under the hood” of diverse animal species, providing a new toolbox to study cell diversity across the tree of life.’
Jumping forwards to 2025, and this is now a method that Dan’s team are using routinely to explore a wide variety of topics,1,2 leading his team to advance our understanding of fish immunology, species variation and evolution. ‘One of our collaborative single cell studies on a shark species, done with colleagues in the USA (led by Helen Dooley, University of Maryland), helped to clarify the early evolution of a key aspect of adaptive immunity in the vertebrate lineage,’ he explains, adding that he hopes such research demonstrates the value of this technique for other researchers in the field. ‘My group has also published a review about single cell omics in aquatic species of commercial value, which we hope is a useful resource for the community.’

Controlling the spread and pathogenicity of disease is one of the most important biological arms races that humanity is currently engaged in, especially when involving diseases that threaten global food production. To keep pace with rapidly evolving bacterial and viral pathogens, we need revolutionary new tools and techniques. Thankfully, a team led by Kranthi Mandadi, Professor of Pathology and Microbiology at the Texas A&M AgriLife Research & Extension Centre in Weslaco, USA, have developed an innovative method to help screen disease control agents, proving that solutions can often be found in surprising places.
During his time in graduate school, Kranthi became fascinated by the roles that genes and proteins played in organismal biology. ‘That fascination led to my first foray into understanding plant disease resistance against viruses during my postdoctoral work with my mentor, Emeritus Professor Karen Scholthof, at Texas A&M University,’ he says. ‘During that time, I started researching the ways that crop genetics could be harnessed to combat viral diseases’.3 One key area of Kranthi’s research has been identifying candidates for controlling Candidatus Liberibacter spp., a bacteria associated with several devastating plant diseases, such as citrus greening and potato zebra chip.
One of the challenges of researching these bacteria is that they are ‘fastidious’ or ‘unculturable’, making it difficult to study their pathogenicity or the efficacy of potential control measures. ‘Conventional approaches to evaluate the effects of an antimicrobial gene can take over 2 years, are not high throughput and are quite laborious,’ explains Kranthi. ‘This slows the pace of research and the evaluation of potential disease-resistance genes.’
To overcome these barriers, Kranthi and his team looked for innovative ways to grow their bacteria outside of the traditional hosts and in vitro cultures. ‘After some brainstorming, our team came up with the idea of using hairy roots, which are common in plant biology and phytochemistry and used to study gene function or produce high-value plant metabolites,’ explains Kranthi. ‘The nice thing about the hairy roots is that they are connected to the vascular tissues of the ex-plant used for induction, so when using Candidatus Liberibacter spp.-infected plant leaves or stem segments to induce hairy roots, due to source–sink relationships, the bacteria naturally migrate into the hairy roots.’ Once established, these hairy root cultures can serve as a substrate for co-culturing fastidious pathogens for several months to years.
The result of this method has been the successful development of high-throughput antimicrobial efficacy screening. ‘The system is particularly advantageous for rapidly testing various chemical and genetic therapies, offering a speed-up in the screening process of four to six times that of conventional genetic engineering or whole-plant methods,”4 he says. ‘As such, we consider the hairy root-based efficacy assays valuable pre-screening before progressing to the whole plant assays.’
Now that this method has become more established, Kranthi and his colleagues have demonstrated how it can be used for efficacy testing of a variety of candidate therapies. ‘With the aid of the microbial hairy root cultures and assay system, we’ve successfully screened hundreds of potential candidates in just a few years,’ says Kranthi. ‘We have now identified multiple immune-related genes, antimicrobial peptides, CRISPR targets and small molecule-based treatments that can suppress Candidatus Liberibacter spp.5 While it

does not replace the need for developing pure axenic cultures, it helped us and others fast-track the efficacy testing processes toward developing treatments for these devastating pathogens—and we are very proud of that.’
Ultimately, Kranthi wishes to use this story as an example to early-career researchers that may find themselves in tricky situations: ‘Don’t shy away from taking on high-risk research topics if the topic is critical to solving stakeholder problems or advancing science; innovation is key to overcoming

challenges,’ he concludes. ‘Be curious, explore out-of-the-box thinking, and find those innovative solutions, and you will be rewarded amply.’
They say that seeing is believing, but in the world of cell biology, that is often easier said than done. Thankfully, there are constant innovations in microscopy technology and techniques that help to illuminate the hidden world inside of cells. More importantly, there are researchers that help to proliferate these advances throughout the field. Rajdeep Ghosh, a PhD student at Charles University in Prague, Czechia, under the supervision of Fatima Cvrčková, is one such researcher who has been an eager adopter of an exciting microscopy technique that is aiding his exploration of plant cell biology.
The primary question at the heart of Rajdeep’s research is the extent to which actin and formins can influence intercellular transport. The actin cytoskeleton is a fundamental component of cellular architecture, orchestrating a wide range of processes. Formins are key actin nucleators that play a crucial role in these functions. ‘Actin is known to drive cytoplasmic streaming, which is assumed to impact intercellular transport, but there is a gap in knowledge linking actin-related proteins to intercellular movement,’ says Rajdeep. ‘I aim to provide an understanding of the role actin and its regulators play in transport, bridging the gap between cytoskeletal dynamics and cell–cell communication.’
To achieve this goal, Rajdeep needed to observe the movement of specific molecules throughout the cell, and initially employed a widely used technique called fluorescence recovery after photobleaching (FRAP). However, owing to issues with precision and data variability, he started to look for alternatives and found a much more promising option. ‘The DRONPA system is a photo-switchable protein that can be reversibly activated and deactivated using specific laser wavelengths,’ he says. ‘This system, described by Gerlitz et al.,6 enables precise control over fluorescence recovery, making it a powerful tool
for studying plant cell–cell transport.’ Rajdeep and a colleague set about introducing the system into their experimental setup, allowing them to compare between control plants and those in which the function of the actin cytoskeleton was altered either by mutations or pharmacologically. ‘This method has significantly advanced our ability to assess the role of the actin cytoskeleton in intercellular transport with greater accuracy and reproducibility,’ he explains.
Rajdeep’s research is still ongoing, but his preliminary findings show that certain actin-associated protein mutants exhibit a significant difference in intercellular transport compared to wild-type plants, providing evidence for the role of selected actin-associated proteins in symplastic transport. He hopes that these results will help to further our understanding of plant intercellular dynamics, with possible applications for improving crop resilience and productivity. ‘If we can manipulate cytoskeletal components to modulate intercellular transport under adverse conditions, we may be able to improve plant survival, optimise nutrient distribution and ultimately enhance crop yield,’ he says. ‘This knowledge could contribute to developing stress-resistant crops through targeted genetic modifications or agronomic practices that support cytoskeletal stability, paving the way for more resilient agricultural systems in the face of climate change.’
Left Page Bottom: Bioassays of the
THE PRIMARY QUESTION AT THE HEART OF RAJDEEP’S RESEARCH IS THE EXTENT TO WHICH ACTIN AND FORMINS CAN INFLUENCE INTERCELLULAR TRANSPORT.
Rajdeep is proud of his successful establishment and standardisation of the DRONPA system in their laboratory and recognises the critical importance of accurate optimisation when adapting new methodologies, as well as acknowledging the input of his collaborators in this project. ‘Transferring microscopic techniques between labs and adapting them to specific organs or tissues can be challenging due to differences in equipment setup and experimental conditions,’ he concludes. ‘Standardising new techniques like DRONPA in plant research requires teamwork, perseverance and attention to detail—values that I deeply appreciate. The advancements in my research would not have been possible without the contributions of my colleagues, previous lab members and the broader scientific community.’

References:
1. Andresen AMS, Taylor RS, Grimholt U, et al. Mapping the cellular landscape of Atlantic salmon head kidney by single cell and single nucleus transcriptomics. Fish Shellfish Immunol 2024; 146: 109357.
2. Daniels RR, Taylor RS, Robledo D, et al. Single cell genomics as a transformative approach for aquaculture research and innovation. Rev Aquac 2023; 15: 1618–1637.
3. Mandadi KK, Scholthof KBG. Plant immune responses against viruses: how does a virus cause disease? Plant Cell 2013; 25: 1489–1505.
4. Irigoyen S, Ramasamy M, Pant S, et al. Plant hairy roots enable high throughput identification of antimicrobials against Candidatus Liberibacter spp. Nat Commun 2020; 11: 5802.
5. Bedre R, Kavuri NR, Ramasamy M, et al. Long intergenic non-coding RNAs modulate proximal protein-coding gene expression and tolerance to Candidatus Liberibacter spp. in potatoes. Commun Biol 2024; 7: 1095.
6. Gerlitz N, Gerum R, Sauer N, et al. Photoinducible DRONPA-s: a new tool for investigating cell–cell connectivity. Plant J 2018; 94: 751–766.
BY CAROLINE WOOD

Experimental plant science will underpin our ability to meet many global grand challenges, from sustainable agriculture and food security, to combatting climate change and restoring nature.
The 2025 SEB Annual Conference in Antwerp will showcase how SEB plant researchers are leading the way—pioneering new methods, collaborating across disciplines and translating insights into real world actions. Caroline Wood takes a look at what’s in store.
Abovet:
Previously, plant genetics has been dominated by studies on nuclear genes, which makes sense given that nuclear-encoded genes make up the vast majority of cellular proteins. However, plastids (photosynthetic organelles, which includes chloroplasts) and mitochondria also contain their own genetic material, known collectively as cytoplasmic genomes. At the SEB Antwerp conference, the session ‘Cytoplasmic genetics: from shaping plant traits to driving speciation’ will shine a spotlight on the significance of cytoplasmic genetics, highlighting its influence on plant physiology, environmental interactions, speciation and plant breeding.
‘While nuclear genetics has been extensively explored, cytoplasmic genomes, which are equally essential for plant survival, have not received as much attention due to technological limitations,’ says session co-organiser Kin Pan Chung from Wageningen University, the Netherlands. ‘Now, with the advent of new tools, we are entering an era where we can manipulate and study cytoplasmic genetics in ways that were previously unimaginable.’
Ultimately, this could enable new approaches to develop climate-resilient crops capable of thriving in increasingly variable and stressful environments. Because plastids and mitochondria play essential roles not only in photosynthesis and respiration but also in stress responses, better understanding of these organelles could reveal novel plant adaptation strategies.
‘Research suggests that cytoplasmic genomes undergo adaptive evolution to help plants survive and grow in diverse habitats,’ adds Kin Pan. ‘By uncovering the underlying mechanisms and exploring the interplay between environmental factors and cytonuclear interactions, we may unlock new ways to engineer cytoplasmic genetics to enhance crop resilience.’
WHILE NUCLEAR GENETICS HAS BEEN EXTENSIVELY EXPLORED, CYTOPLASMIC GENOMES, WHICH ARE EQUALLY ESSENTIAL
As the session will explore, cytoplasmic genetics can influence plant physiology in various different ways. These effects can be direct, through their impact on chloroplast and mitochondrial functions, or indirect, from interactions between the cytoplasm and the nucleus. ‘A particularly interesting phenomenon is cytonuclear incompatibility, where mismatches between nuclear and cytoplasmic genomes can lead to reproductive barriers, driving the evolution of new plant species,’ says Kin Pan. ‘I find all these aspects equally important and exciting, because they demonstrate how these ancient endosymbiotic genomes continue to play a vital role in modern plant life.’
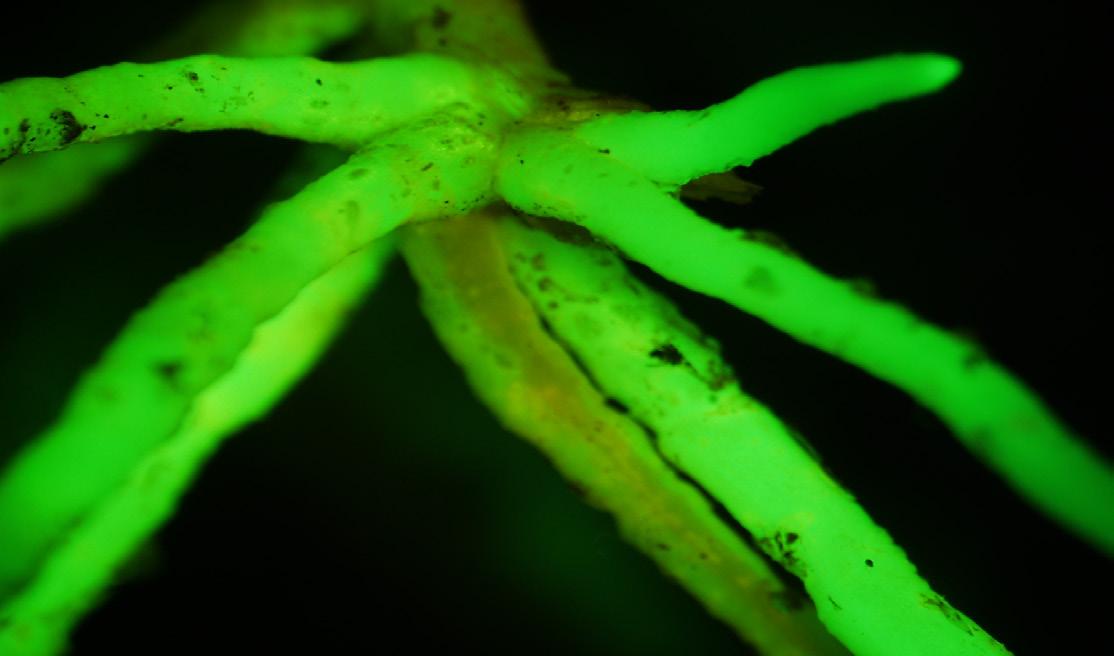
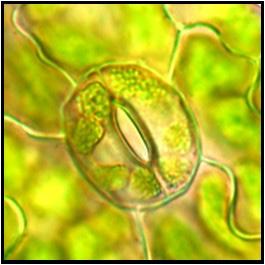
Leaf cells from a transplastomic tobacco plant captured under a confocal microscope, where green fluorescent protein is expressed in the plastids.
Photo credit: Kin Pan Chung.
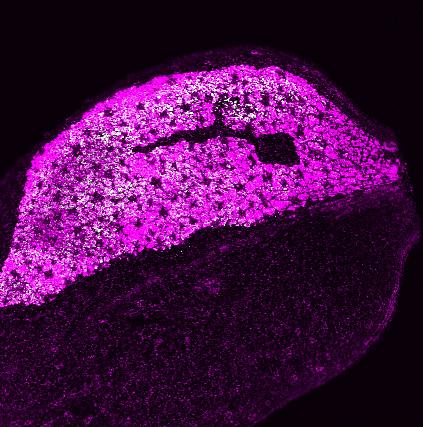
Top Left:
Tobacco leaf captured under a confocal microscope, with paternal plastids tagged with green fluorescence and maternal plastids showing only chlorophyll autofluorescence (represented in magenta). This leaf comes from a plant exhibiting biparental plastid inheritance, generated as part of Dr Kin Pan Chung’s plastid inheritance studies.
Photo credit: Kin Pan Chung.
However, an ongoing mystery is the precise mechanism responsible for the unique, non-Mendelian inheritance pattern of cytoplasmic genes, which (in most species) are transmitted exclusively through the mother. This makes it incredibly difficult for plant breeders to distinguish between the effects caused by the plastid and by mitochondrial genomes on key phenotypic traits such as yield and stress tolerance. Nevertheless, the pieces of the puzzle are slowly coming into place. For instance, Kin Pan and his colleagues recently identified two independently acting mechanisms that ensure maternal inheritance of chloroplast genomes.1
‘We discovered that maternal inheritance breaks down when male gametogenesis occurs at low temperatures,’ he says. ‘Specifically, chilling stress during male gametogenesis leads to increased entry of paternal plastids into sperm cells. The second mechanism we found was an exonuclease which degrades organellar genomes in maturing pollen. By manipulating these factors, we successfully shifted inheritance from strict maternal transmission to biparental inheritance, enabling the passage of paternal plastid genomes to the next generation.’ In time, this could enable plant breeders to induce biparental transmission of cytoplasmic genomes, leading to random segregation that could help separate effects caused by plastid and mitochondrial genomes.
‘These exciting developments are enabling unprecedented exploration of cytoplasmic genetics. I am looking forward to using the session at the Annual Conference to raise awareness and spark discussions in this rapidly emerging field,’ Kin Pan concludes.
A key aim in developing climate-resilient crops is to engineer plants that use less water yet still deliver high yields. Stomata, the tiny pores on the underside of leaves that regulate water and gas exchange, will be fundamental to achieving these aims. Although research on stomata goes back centuries, according to Robert Caine (University of Sheffield, UK) and Caspar Chater (Royal Botanic Gardens, Kew, UK), we now need a paradigm shift if we are to apply this knowledge for global benefit.
‘We have learnt a lot about how stomata impact crop performance, but often at smaller scales—leaflevel or whole plant, for example—and typically the transition from transformational to real-world applications is lacking,’ says Caspar. ‘This inspired us to propose a session for the 2025 SEB Annual Conference on the theme ‘Fine tuning stomata to maximise global crop resilience’. The vision is to conceptualise the ‘bigger picture’ of stomatal research, so that researchers and academics have more confidence to progress from labs to field studies.’
It is no exaggeration, Robert adds, to say that stomata act as microscopic gatekeepers: crucial interaction points between plants and their environment. ‘By adjusting pore area in response to a multitude of stimuli, stomata enable vast and rapid decision-making capacity, which helps optimise plant growth and resilience. Over longer timescales, plants can also adjust stomatal size and/or density to adapt to their environment.’
Indeed, the evolution of a wide diversity of stomatal form and function helped enable plants to colonise

markedly different habitats. Having started their careers exploring stomatal evolutionary biology, both Robert and Caspar have moved progressively closer to applied research. A current focus for Caspar is harnessing stomatal trait diversity in crops (particularly legumes) and wild relatives to uncover novel mechanisms against drought and heat stress. This includes work which uncovered two different water-use strategies in drought-resistant bean genotypes.2 ‘Common bean (Phaseolus vulgaris), the main food legume globally, conserves soil water by closing stomata early, decreasing stomatal density and limiting growth,’ he says. ‘Whereas tepary bean (Phaseolus acutifolius), a species domesticated in arid conditions, shows a “water spender” strategy, with prolonged stomatal opening and high stomatal density. Teasing apart these differential mechanisms could provide unique tools for crop improvement under climate change.’
Robert, meanwhile, is investigating how crops respond to higher temperatures, drier air (vapour pressure deficit) and elevated CO2, with a longterm aim of developing varieties that thrive under future climate extremes. ‘Recently, we found that for wheat (Triticum aestivum), elevated CO2 does not reduce stomatal conductance to water vapour and transpiration during heatwaves; instead, plant
water usage increases,’3 he says. ‘In addition, extreme heat and dryness also affect how plants respond to nitrogen fertiliser, leading to a significant increase in gas exchange from the undersides of leaves. Taken together, this indicates that future wheat crops will use significantly more water during heatwaves than might be expected, which has substantial implications for future global food security.’
At the session during the Antwerp conference, Caspar and Robert hope to combine these findings within a broad range of stomatal research, including molecular and developmental biology, stomatal pathogen responses and stomatal gas exchange modelling.
‘We are very excited to have the opportunity to discuss with a diverse community of stomatal researchers how we can upscale our collective research to achieve greater global impacts,’ says Caspar. ‘Alongside this, a key discussion point will be how we can best make stomatal research accessible to farmers and policy-makers so humanity can benefit from the rapid progress that is occurring in research institutes around the world.’
WE HAVE LEARNT A LOT ABOUT HOW STOMATA IMPACT CROP PERFORMANCE, BUT OFTEN AT SMALLER SCALES—LEAFLEVEL OR WHOLE PLANT, FOR EXAMPLE—AND TYPICALLY THE TRANSITION FROM TRANSFORMATIONAL TO REALWORLD APPLICATIONS IS LACKING,’ SAYS CASPAR.

With photosynthesis being the origin behind almost every calorie we consume, improving this process could have significant impacts on food security. But there remains a common stumbling block in translating our knowledge into practical solutions, as Liana Acevedo-Siaca (Wageningen University) explains: ‘Most of our understanding of plant photosynthesis comes from controlled environments, focusing on how it responds to singular environmental factors, such as a change in temperature, light or CO2,’ she says. ‘But this is unrepresentative of field conditions, where photosynthesis is affected by many different factors at once in highly fluctuating environments.’
This inspired Liana and her Wageningen colleague Silvere Vialet-Chabrand to propose the session ‘Bridging the gap: connecting photosynthesis research from controlled environments to the field’ at the 2025 SEB Annual Conference. A key focus for this will be how different abiotic factors
Left Page
Left to Right:
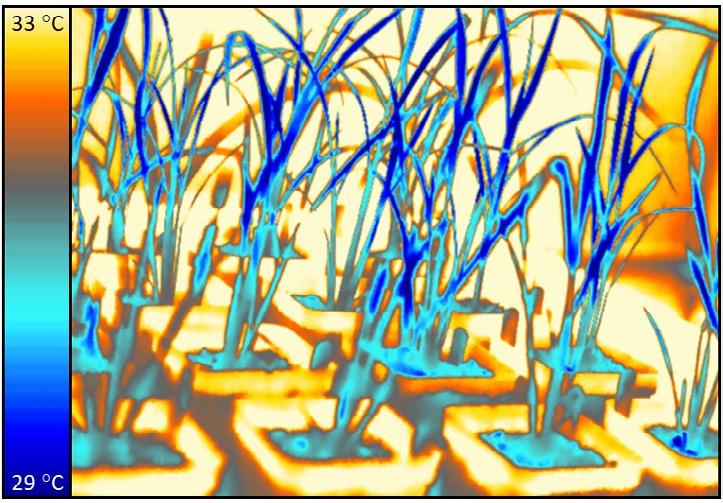
A thermal image of rice (with the darker blue colour being cooler) showing the cooling effect of stomata.
Photo credit: Robert Caine and Muhammad Khan. Plants, Photosynthesis and Soil cluster, University of Sheffield.
Rice stomata. Photo credit: Robert Caine.
Lycophyte stomata. Photo credit: Robert Caine.
interact to affect photosynthesis, for instance, the shift in temperature that occurs when a leaf or plant experiences a change in light intensity.
‘Typically, abiotic factors are examined individually, but by taking into consideration these interactions— which are difficult to separate under field conditions, but possible to examine under controlled environments—we advance our understanding of how plants respond to their dynamic environments,’


says Silvere. His own research has investigated this using sorghum, a crop which often simultaneously experiences limited water availability and rapidly fluctuating light intensities, which influences both photosynthesis and stomatal behaviour.
‘Using a series of controlled environment experiments across 43 sorghum accessions, we found that photosynthesis and water regulation (stomatal conductance) are tightly coupled even when light levels change,’4 he says. ‘Most sorghum plants adjusted their stomata within 5 minutes in response to changing environmental conditions (such as light intensity), maintaining a high water-use efficiency. This dynamic regulation ensures that CO supply meets the photosynthetic demand while minimising water loss.’ Consequently, sorghum has become a prime candidate for uncovering mechanisms that could help design crops capable of retaining high rates of photosynthesis without incurring significant water losses.
Another important consideration, adds Liana, is the need to integrate research on photosynthesis across different timescales and spatial levels. ‘For instance, global nocturnal temperatures are rising more rapidly than daytime temperatures but the performance of many photosynthesis-related traits at night remains poorly understood,’ she says. To help address this, she recently co-led a 3-year field experiment in Mexico to test how the stomatal conductance of 12 wheat types responded to a 2°C increase in night-time temperatures.5
‘We found that higher nocturnal temperatures reduced wheat grain yield by an average of 1.9% per 1°C, even though the plants showed no major changes in their daytime functions. This appears to have been driven by a decrease in nocturnal stomatal conductance on warmer nights, limiting CO entry,’ she says. ‘Surprisingly, some wheat types considered “heat tolerant” during the day had
the biggest drops in yield at night. This indicates that the essential components of nocturnal heat tolerance in wheat are uncoupled from resilience to daytime temperatures, raising fundamental questions for physiological breeding.’
‘As our work demonstrates, controlled environment experiments enable us to recreate stressful or future environments to understand the mechanisms and limitations to improve yield,’ says Silvere. ‘Yet, to fully reproduce the fluctuations encountered in the field in a controlled environment remains quite challenging. We hope our session will facilitate more cross-talk between researchers working at different scales and show that both controlled and field environments are crucial components of plant physiology research.’
WE HOPE OUR SESSION WILL FACILITATE MORE CROSS-TALK BETWEEN RESEARCHERS WORKING AT DIFFERENT SCALES AND SHOW THAT BOTH CONTROLLED AND FIELD ENVIRONMENTS ARE CRUCIAL COMPONENTS OF PLANT PHYSIOLOGY RESEARCH.
Engineering plants with novel functions could help address several key global challenges, making this a key topic for discussion at the SEB conference, as Megan L. Matthews (University of Illinois, USA) explains: ‘Plant engineering could help us decrease the land area required to feed populations, decrease the needed agricultural inputs like fertilisers and irrigation for crop productivity, and mitigate climate change by engineering plants to increase their ability to capture and store carbon longer-term.’ This inspired Megan to join forces with Johnathan Napier (Rothamsted Research, UK) to organise a dedicated session: ‘Plant engineering strategies for achieving sustainability goals: from models to lab to field’.
‘Our goal is to provide a common session for researchers from a broad range of plant subdisciplines to explore synergistic engineering opportunities,’ Johnathan says. ‘We have intentionally included talks covering modelling, lab, greenhouse and/or field experimental work to spark discussions on how we can work together using different approaches and at different scales.’
He adds that both Megan and he are keen to emphasise the importance of translational efforts (such as field trials and industry research) to take potential solutions from the lab to being used ‘in real life’. This is particularly pertinent to Johnathan’s research focus: engineering plants to accumulate nonnative fatty acids such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), the so-called omega-3 fish oils that are vital for human health. These oils are originally produced by microalgae; farmed fish gain them by being fed smaller fish further down
the food chain. But the growing human demand for farmed fish is putting unsustainable pressure on wild fish stocks for feed.
‘Over 10 years ago, we and others demonstrated the possibility of using transgenic Camelina sativa oilseed crops to make a de novo terrestrial source of omega-3 by introducing the enzymes responsible for the metabolic pathway,’ says Johnathan. ‘But generating the plants is only the first step of a long journey towards translation, which includes field trials, feed studies and nutrient profiling.6 Followon work has included verifying that omega-3-rich camelina can replace fish oil in aquaculture feed, confirming the trait is stable under field conditions, checking that non-native omega-3s are confined to the seed, and evaluating crop performance in different geographical regions. We are now carrying out human trials to confirm that camelina EPA + DHA is incorporated into blood lipids as effectively as commercial blended fish oil.’ Having forged this long pathway right from proof-of-concept, he hopes this work can provide a blueprint for future metabolic engineers.
Megan’s group, meanwhile, works on developing multiscale plant models to capture and predict system-level characteristics as a means to identify strategies to engineer plants for the future. A recent milestone was developing a new modelling framework that integrates a metabolic model of photosynthesis at the molecular level with another that predicts how crops grow over an entire season.7 ‘This is the first crop growth model that scales from the individual enzymatic reaction steps in photosynthesis to canopy-level assimilation and crop yield,’ Megan says. ‘It provides a framework for capturing emergent properties that can arise from engineering crops at a whole-field level and exploring how different engineering strategies may respond to different environmental factors.’


‘We are looking forward to sharing the insights we have gained from our research at the 2025 SEB Annual Conference, and learning from the plant engineering journeys others have undertaken,’ she adds. ‘My hope is that this session will inspire more synergy and collaboration between experimental and modelling efforts in plant engineering.’
OUR GOAL IS TO PROVIDE A COMMON SESSION FOR RESEARCHERS FROM A BROAD RANGE OF PLANT SUBDISCIPLINES TO EXPLORE SYNERGISTIC ENGINEERING OPPORTUNITIES
Hungry for more? Details of the other Plant Sessions taking place in Antwerp can be found on the SEB Annual Conference website, including poisonous plants, applying AI approaches to plant science, gravitropism and plant architecture, and plant phenomics.
Above Maize growing at the Energy Farm at University of Illinois Urbana-Champaign.
Photo credit: Ximin Piao.
References:
1. Chung KP, Gonzalez-Duran E, Ruf S, et al. Control of plastid inheritance by environmental and genetic factors. Nat Plants 2023; 9: 68–80.
2. Polania JA, Salazar-Chavarría V, Gonzalez-Lemes I, et al. Contrasting Phaseolus crop water use patterns and stomatal dynamics in response to terminal drought. Front Plant Sci 2022; 13: 894657.
3. Caine RS, Khan MS, Shan Y, et al. Future heatwave conditions inhibit CO2-induced stomatal closure in wheat. bioRxiv 2025, preprint.
4. Battle MW, Vialet-Chabrand S, Kasznicki P, et al. Fast stomatal kinetics in sorghum enable tight coordination with photosynthetic responses to dynamic light intensity and safeguard high water use efficiency. J Exp Bot 2024; 75: 6796–6809.
5. McAusland L, Acevedo-Siaca LG, Suzuky Pinto R, et al. Night‐time warming in the field reduces nocturnal stomatal conductance and grain yield but does not alter daytime physiological responses. New Phytol 2023; 239: 1622–1636.
6. Haslam RP, Michaelson LV, Eastmond PJ, et al. Born of frustration: the emergence of Camelina sativa as a platform for lipid biotechnology. Plant Physiol 2025; 197: kiaf009.
7. He Y, Wang Y, Friedal D, et al. Connecting detailed photosynthetic kinetics to crop growth and yield: a coupled modeling framework. in silico Plants 2024; 6: diae009.
Left Page Dwarf tomato plants in a growth chamber.
Photo credit: Silvere VialetChabrand
Left : Soybean growing at the SoyFACE facility at University of Illinois Urbana-Champaign.
Photo credit: Yufeng He.

Sandra is originally from Montréal, Canada, where she completed a BSc Honours degree at McGill University studying seagrass community dynamics in Barbados. She remained at McGill for her master’s degree, where she discovered her love of fishes. Her master’s research explored environmental drivers of intraspecific morphological variation in the jaws and gills of East African cichlids. During this time, she was also lucky enough to collaborate on several projects with the Smithsonian Tropical Research Institute in Panama, which provided Sandra with the opportunity to study the spread of an invasive crab in the Panama Canal as well as the ecology of coral reef fishes throughout the Caribbean and Eastern Pacific. This experience led her to Australia, where she completed her PhD at the Australian National University in 2014 on morphological and physiological variation in coral reef fishes across gradients of waves and water motion.
The SEB President’s Medals are awarded annually to young scientists of outstanding merit.
This year’s awards will be presented at the 2025 SEB Annual Conference, which takes place in Antwerp.
Many congratulations to our very worthy winners of the 2025 SEB President’s Medal Award.
While diving the Great Barrier Reef and observing cleaning stations, Sandra became interested in host–parasite interactions. She moved to the University of Neuchâtel in Switzerland, where she continued to work on the Great Barrier Reef as a postdoc studying cleaning mutualisms and the role of parasitic infections on fish behaviour and cognition. In 2018, she began her position at the University of Montreal, where she is currently an Associate Professor and the Canada Research Chair in the Eco-Evolution of Host–Parasite Interactions. Her and her students now work mostly on freshwater fishes and their parasites in lakes around the Laurentian region in Québec.
In addition to these diverse interests, Sandra also collaborates with theoreticians on the role of parasites in driving the evolution of host migratory behaviour as well as with the FIN club on topics as diverse as thermal acclimation in ectotherms, premature hatching in aquatic embryos, and ways to enhance transparency and collaboration in science.
Raphael Souza Pavani National Institutes of

Raphael Pavani is a molecular biologist passionate about unravelling the intricacies of genome integrity. He has dedicated his career to advancing our understanding of DNA replication and repair, and the molecular processes underlying diseases. Raphael earned his master’s and PhD degrees from the University of Sao Paulo in Brazil, where he studied DNA replication and repair, and cell cycle control in the pathogenic protozoan parasites Trypanosoma cruzi and T. brucei, causative agents of Chagas disease and sleeping sickness, respectively.
During his PhD studies, Raphael also embarked on an enriching internship at the University of Dundee in the UK, further broadening his expertise in biological chemistry and drug discovery. His PhD work resulted in numerous publications and the prestigious “Outstanding PhD Thesis Award” from the University of Sao Paulo.
In 2019, Raphael joined the Laboratory of Genome Integrity at the National Cancer Institute within the National Institutes of Health in the USA, under the mentorship of André Nussenzweig. Among his significant contributions, he uncovered that the loss of EXO1 is synthetic lethal with BRCA1 deficiency, providing a promising avenue for targeted cancer therapies. Additionally, he developed a cuttingedge system to induce collapsed replication forks at specific genomic loci, revealing fundamental insights into replication-associated DNA damage. His research, recently published in Science, challenged the prevailing view that replication-associated breaks are exclusively one-ended breaks and demonstrated that homologous recombination is the primary repair pathway at broken forks. Moreover, he revealed that the mechanisms involved in repairing these lesions differ from those used in the repair of canonical, replication-independent double-strand breaks, advancing our understanding of DNA repair pathways and their therapeutic implications.

Bipin Pandey is a plant biologist exploring the dynamic interface between roots and soils to uncover how plant roots sense and adapt to soil challenges. His research focuses on the mechanisms by which roots navigate complex soil environments, contributing to our understanding of plant nutrition and root biology.
Bipin completed his early education in Basti, a small town in eastern Uttar Pradesh, India, and later pursued his PhD at the National Institute of Plant Genome Research in New Delhi, where he earned his PhD specialising in plant nutrition and root biology. During the final year of his PhD, Bipin was awarded the Newton-Bhabha Fellowship, which enabled him to collaborate with Malcolm Bennett at the University of Nottingham. Here, he made significant contributions to understanding how root hair elongation and shallow root angles enhance phosphate uptake from the topsoil. This research sparked his enduring fascination with the mechanisms by which roots perceive and respond to the physical and chemical properties of soil.
In 2021, Bipin was awarded a Biotechnology and Biological Sciences Research Council Discovery Fellowship to investigate how plant roots sense and respond to soil compaction. His interdisciplinary research revealed that the gaseous hormone ethylene plays a critical role in sensing soil compaction and orchestrates downstream signalling pathways involving abscisic acid and auxin to mediate root responses.
Building on this groundbreaking work, Bipin was awarded a European Research Council Starting Grant in 2023 to establish his independent research group. His team is now developing innovative tools and techniques to study how roots sense, respond, adapt and shape their responses at cellular levels.
Plant Section
Emma Yhnell Cardiff University

Dr Emma Yhnell is a passionate and multi awardwinning educator and science communicator, she won the Society for Neuroscience Science Educator Award in 2024 and a National Teaching Fellowship in 2023, and is currently Associate Dean of Equality, Diversity and Inclusion and a Reader in Neuroscience at Cardiff University.
Emma obtained a BSc degree in Biochemistry before completing a PhD in Huntington’s disease. She then began an independent research fellowship to translate her findings on cognitive training into the patient clinic. Emma’s clinical research inspired her and demonstrated the need to provide accessible and engaging information to diverse public audiences.
Emma now teaches the next generation of budding scientists as a teaching and scholarship focused academic. Emma delivers engaging and interactive sessions with passion and infectious enthusiasm, and she has built a reputation for her ability to untangle the academically technical and translate it into entertaining, relevant and engaging content. In 2022 she was elected to the Learned Society of Wales as the youngest ever Fellow. As a first generation academic with expertise in equity, diversity and inclusion, and a passion and desire to embrace change, Emma is changing the typical image of academia by making science more open, honest and fun.


Each year at the Annual Conference of the SEB, the work of George Parker Bidder III and Harold Woolhouse are honoured with plenary lectures alongside the Cell Biology plenary lecture and a new category dedicated to Outreach, Education and Diversity.
The lecturers are prominent scientists in their fields of research and are nominated by the committees of their respective sections.
Lucia Strader Duke University

Lucia Strader is a professor of Biology at Duke University and will soon be the Newman Chair of Plant Biology at the Salk Institute. Her lab studies plant growth regulation by gaining a molecular understanding of the actions of the plant hormone auxin, using transdisciplinary tools from cell and molecular biology to biochemistry and biophysics.

Shane Austin is a lecturer in Biochemistry and Deputy Dean of Graduate Research and Outreach in the Faculty of Science and Technology at The University of the West Indies, Cave Hill Campus. He completed his PhD studies at the Medical University of Vienna on a collaborative project with the Center for Molecular Medicine of the Austrian Academy of Sciences. Shane is a member of the American Society for Biochemistry and Molecular Biology, where he serves on the Maximizing Access Committee (MAC). The MAC seeks to improve opportunities for biochemistry and molecular biology scientists from marginalised backgrounds. Shane chairs the awards task force on that committee, which is responsible for awarding scholarships, travel awards and society prizes. Shane is also actively involved with the SEB Outreach Education and Diversity group, where he chairs the Awards nomination taskforce, an initiative to improve diversity in the SEB awards scheme. Shane supervises five research students on topics from snail metabolism to circular bioeconomies using anaerobic digestion. He also explores research topics of biochemical education and uterine fibroids in Afro-Caribbean women.
Andrew

Dr. Leakey is the Michael Aiken Professor at the University of Illinois, Urbana-Champaign, where he has worked since 2002. He is Director of the Center for Advanced Bioenergy and Bioproducts Innovation and was Head of the Department of Plant Biology from 2019 to 2024. CABBI is a nation-wide collaborative research enterprise involving >300 scientists and students. They work together to develop the scientific understanding and technological innovations necessary to support development of a bioeconomy that is both economically profitable and environmentally beneficial. These aims are being met through development of next-generation C4 grass crop feedstocks and non-model yeasts to produce sustainable oils and high-value platform chemicals. Dr. Leakey’s research group focuses on studying the integrative physiology, genetics and genomics of plant water use efficiency, with the aim of developing crops that can produce large amounts of feedstock and food in locations where water supply is currently insufficient. Dr. Leakey has >110 research publications. For this work he received a Fulbright Scholarship, the Calvin-Benson Prize for excellence in early-career research on photosynthesis, and the Kettering Award from the American Society of Plant Biologists. He was elected as a Fellow of the American Association for the Advancement of Science in 2019.

Dr. Anne Todgham is Professor and Department Chair in the Department of Animal Science at the University of California Davis. Anne earned in B.Sc. at the University of Guelph, Canada and earned her Ph.D. in Zoology from the University of British Columbia, Canada in 2005 studying the role of heat shock proteins in the inducible stress tolerance of intertidal fishes with Profs. Patricia Schulte and George Iwama. Anne held a National Science and Engineering Research Council of Canada postdoctoral fellowship at UC Santa Barbara before joining the faculty at San Francisco State University in 2009. She moved to UC Davis in Fall 2013. Anne is an environmental physiologist with an interest in understanding the biochemical, physiological and behavioral strategies animals use to cope with environmental change. Her research program has an eye towards global climate change and addresses the general question of whether aquatic animals have the physiological flexibility necessary to withstand the unprecedented rates of environmental change. Her research includes conservation objectives as well as stress management in sustainable aquaculture of fishes and invertebrates. She, her students and her postdocs are trying to understand how these animals meet the energetic demands placed by different stressors.
As animal biologists, scientific achievements and breakthroughs are an integral part of our careers. However, Dr. Todgham’s true sense of achievement comes from building capacity of those around her by opening doors, helping others realize their personal goals. She believes to her core that universities should be a place where everyone has the opportunity to be their very best.
BY
THE PLANT JOURNAL - Fan J, Ma X, Zou J, Li S, Liu Y, Guo D, Jiang W, Li X, Liu F, Tan L. LATA1, a RING E3 ligase, modulates the tiller angle by affecting auxin asymmetric distribution and content in rice. The Plant Journal 2024; 120(2): 429–444. https://doi.org/10.1111/tpj.16948
Rice (Oryza sativa) is the staple crop for more than half the world’s population, making yield improvements a critical goal for current breeding efforts. One approach focuses on improving plant architecture, and the tiller angle is a promising trait in this context: a large angle results in a spread-out growth habit that enhances light capture and helps outcompete weeds, while an erect growth habit with a smaller tiller angle allows for dense planting and efficient harvesting, and reduces competition between individual plants.
Domestication of wild rice has favoured an erect growth habit, which correlated with altered gravitropic responses in the shoot. Gravity perception activates a signal transduction cascade that results in the asymmetric distribution of the plant hormone auxin. High auxin concentrations on the lower side lead to increased cell elongation, causing asymmetric growth and a bending of the shoot. Many genes involved in gravitropism signalling have been identified in Arabidopsis thaliana, but very few are known in rice.
Fan et al. identified a rice tiller angle gene, LARGE TILLER ANGLE 1 (LATA1), in which mutation causes a spread-out architecture. Throughout development, the mutant plants consistently exhibited larger tiller angles than the wild type, with the outermost tiller reaching a 25° angle at the heading stage, compared to an 11° angle in the wild type (figure 1A). This phenotype was caused by reduced asymmetric growth at the tiller base. The change in angle was associated with altered shoot gravitropism: after rotating seedlings 90°, gravitropic bending was visibly
delayed in mutant lata1 seedlings (figure 1B). The authors mapped LATA1 to a region on chromosome 5, and further identified a single nucleotide polymorphism (SNP) that results in a lysine to proline substitution (L139P) in the mutant lata1. Complementation studies confirmed that this SNP indeed caused the mutant’s spread-out phenotype. In line with these findings, knock-out of the LATA1 gene by CRISPR-Cas9 also resulted in increased tiller angles and impaired gravitropism in both japonica and indica rice cultivars, while LATA1 overexpression did not affect tiller angle but enhanced the gravitropic response.
Auxin-induced genes showed reduced responsiveness to gravistimulation in the mutant lata1, prompting Fan et al. to further investigate a link between LATA1 and auxin. The auxin content ratio between the lower and upper sides of the shoot increased under gravistimulation in the wild type, but not in lata1 seedlings. Transcript levels of the YUCCA auxin biosynthesis genes were lower in lata1, while indole-3-acetic acid treatment partially restored the mutant’s gravitropic response. In addition, lata1 was hypersensitive to treatment with the polar auxin transport inhibitor N-1-naphthylphthalamic acid. Taken together, these results suggest that lata1 is impaired in both auxin biosynthesis and lateral auxin transport.
The LATA1 protein is predicted to contain a C3H2C3type RING zinc finger domain typically found in E3 ubiquitin ligases. LATA1 is homologous to the Arabidopsis E3 ligase SHOOT GRAVITROPISM 9 (SGR9), which is also involved in gravity sensing. In vitro ubiquitination assays using recombinantly expressed glutathione S-transferase (GST)-LATA1 demonstrated that LATA1 has autoubiquitination activity, which was abolished when conserved
cysteine residues in the RING domain were mutated. Autoubiquitination activity was also reduced in the GSTlata1 mutant protein, suggesting that the L139P substitution, although located outside the RING domain, impairs E3 ligase activity.
The exact mechanism by which LATA1 affects auxin accumulation and auxin distribution remains unclear. Identifying ubiquitination substrates of LATA1 represents a next logical step in further uncovering its function. Given that lata1 mutants did not differ from wild type in
important agronomic traits such as plant height, tiller number, grain number and size, the authors hope that LATA1 can be leveraged to optimise rice architecture further, using natural variation and precise gene editing to fine-tune tiller angle during development.
BY MAREIKE JEZEK
JXB (Journal of Experimental Botany)
Goeckeritz CZ, Grabb C, Grumet R, Iezzoni AF, Hollender CA. Genetic factors acting prior to dormancy in sour cherry influence bloom time the following spring. Journal of Experimental Botany 2024; 75(14): 4428–4452. https://doi.org/10.1093/jxb/erae157
Cherry trees are beloved for their beautiful white blooms in spring and juicy red fruits in summer. Their productivity is, however, threatened by global warming. Goeckeritz et al. (2024) analysed floral development in sour cherry and their results published in the Journal of Experimental Botany can help to breed cultivars that are better adapted to future temperature regimes.
Springtime in Japan is the time of Hanami, the century-old tradition of viewing the majestic white and pink blooms of ornamental cherry trees. Hundreds of thousands of people, among them many visitors from all over the world, fill the parks to celebrate this annual festival of huge cultural importance. The enjoyment of nature’s beauty is, however, short-lived, because cherry blossoms only last for a few days. The delicate flowers’ brief lifetime reminds spectators to fully embrace the
THE EXACT MECHANISM BY WHICH LATA1 AFFECTS AUXIN ACCUMULATION AND AUXIN DISTRIBUTION REMAINS UNCLEAR.
moment, to make peace with the impermanence of all things, and to accept the inevitability of change. Hanami is an invitation to see cherry blossoms as a metaphor for life itself—beautiful, yet ephemeral.
Cherry blossoms, however, have also become alarming signifiers of concerning environmental changes that cannot be stoically accepted. Historical records of the cherry blossom festivals in Kyoto reaching back over 1200 years show progressively earlier blooms since the beginning of the 19th century, most likely caused by global warming as a result of increased industrialisation, and most pronounced in urban areas. Whilst cherry trees used to flower on average in mid-April in Kyoto, peak blooms are nowadays often already reached in late March, with the 26th of March 2021 being the earliest since record tracking began. Early flowering not only affects the timing of Hanami but can also have drastic consequences for fruiting and productivity. Young flowers are vulnerable to cold temperatures, and early blooms increase the risk of damage from spring frost. If the current warming and early flowering trends continue, trees may even no longer synchronise with peak pollinator activity, ultimately disturbing the whole ecosystem dynamics.
Spring frost is also a major challenge in the global production of other fruit trees, such as apple, plum and peach, all of which show high sensitivity of flowers to cold after budbreak. Significant yield losses have been recorded in recent years due to frost-induced fruit abortions. Changes in cultural practices and genetic improvements will be required to adapt to the changing climate. For cherry trees, bloom time is a highly heritable trait that can vary widely between genotypes, making breeding for late bloom a promising approach. A prerequisite for this is a profound understanding of the underlying genetics and physiology. Floral initiation in fruit trees occurs from late summer to autumn. The buds remain dormant during winter and require a cold period to enable budbreak in spring. Once the chilling needs are fulfilled, warm temperatures elicit blooming and promote growth until final flower anthesis.
The detailed study by Goeckeritz et al. (2024) now extends our knowledge of flower development in sour cherries (Prunus cerasus) and shows, for the first time, that bloom time is already determined long before the onset of bud dormancy in the previous year. The authors examined a population of genetically closely related sour cherry trees with blooming time differences of up to 10 days. For 2 years, they regularly sampled shoot apices in floral position to compare early- and later-bloomers. Painstaking microscopic analysis revealed the first morphological differences as early as July, when early-bloomers transitioned from vegetative growth to floral initiation.
Figure 1: Based on detailed histological analysis, Goeckeritz et al. (2024) have established a floral development staging system for sour cherry describing the development prior to floral initiation to just before anthesis. The staging system will be applicable to other Prunus species, such as almond, plum and peach, to aid understanding of early processes in the reproductive growth of these fruit trees. For details see figure 2 in Goeckeritz et al. (2024).
They remained developmentally ahead of latebloomers throughout dormancy until flowering, with differences between the groups becoming more pronounced in spring. Early-bloomers also had lower chilling requirements and responded more quickly to warm temperatures, indicating a shallower state of dormancy. Plant hormones are important components of the temperaturesensing mechanism, and genetic analysis revealed several enzymes involved in hormone synthesis among the key candidate genes underlying the blooming time differences. The data also suggest that the gene versions responsible for a late bloom have been inherited from the very late blooming ground cherry (Prunus fruticose), one of the progenitors of the sour cherry.
The shift in bloom time observed in recent years should increase public awareness of the impact
of anthropogenic climate change and provoke behavioural changes to stop overheating the planet so that future generations will still be able to indulge in the many riches of the cherry tree. Meanwhile, combined efforts of researchers and breeders are required to develop trees that are better adapted to future temperature conditions. The study presented by Goeckeritz et al. (2024) paves the way for marker-assisted breeding strategies. Based on their detailed tissue analysis, the authors have also developed a new staging system (figure 1) to describe the transition from vegetative to reproductive growth in fruit trees, which will enable future studies of early developmental processes in other species.

SEBIOLOGY.ORG
#SEBCONFERENCE
AIMS:
Highlight the lack of diversity in the profile of past award winners
Increase both nominations and nominators from historically marginalised groups for SEB awards
Expand the action of the task force to include other Societies and bioscience awards
Everyone (members and non-members) can get involved. No previous experience is needed.
1. Join the SEB Awards Nomination Task Force
2. Suggest the name of someone deserving of a bioscience award for the SEB Task Force
3. Nominate someone in your own right
‘The SEB are particularly interested in supporting early career researchers, so I feel like I can make suggestions that are really heard,’ says Robyn Emmerson, a postdoctoral researcher of plant epigenetics at the University of Birmingham and the SEB Early Career Researcher Trustee.
What first sparked your interest in plant research?
I went to university wanting to be a microbiologist or an immunologist, but then went along to my lectures and didn’t enjoy microbiology the way I thought I was going to enjoy it. I ended up taking a plant developmental biology module and was like, ‘wow, this is so cool, I can’t believe I’m missing out on this’. And in that time, I’d also become really interested in genetics. I’d never put two-and-two together that plants also have ‘genetics’. At the end of that year, I went to the undergraduate Gatsby Plant Science Summer School; it was so inspiring, especially as a woman in science, to see so many high-achieving women all in one place. After that, I tailored my degree to really focus on plants and genetics. When I came to looking for PhDs, I wanted something that wasn’t just molecular. I wanted to see my plants and feel like they were physical beings. Sometimes you just need to look at a plant and see what’s happening.
I noticed that you also spent some time in the Netherlands—what was that like?

Yeah, I did a summer school over in the Wageningen, Netherlands, and that was very industry focused. They took us around what they called Seed Valley, which is where we could see the industrial scale of plant breeding, which was insane. You’re used to walking into an academic greenhouse and then you go to one of these industrial ones and it’s 20 times the size—it’s incredible.
What was the main focus of your PhD research?
I was interested in how naturally fluctuating light differed from the way that we grow plants traditionally in the lab, which is under a very regimented square light pattern that doesn’t really reflect the way that light actually behaves outside. Plants can’t put on a jacket or sunglasses to adapt to the changing sunlight; they just have to sit there and deal with it. We already knew that this has a big impact in terms of photosynthetic efficiency and how much carbon the plants can assimilate. But we weren’t really sure how that was regulated. We were interested to see whether this could be
regulated on an epigenetic level, so we looked at the DNA methylation of Arabidopsis plants under these square light conditions, where the lights are simply on and off, and under realistic fluctuating conditions, which were directly recorded from outside and then programmed into these lights.
Following on from your PhD, what are you researching now?
At the end of my PhD, I felt like I had a really good understanding of photosynthesis but I wanted to become a bit stronger in my molecular biology, so I took up a position at the University of Birmingham in Marco Cattoni’s lab, looking at transgenerational fitness and DNA methylation mutants. There is one mutant without CGM methylation that is much hardier in bad environmental stimuli, but it can’t make seeds for more than two generations and no one has really worked out why. So, we want to breed plants with low CGM methylation because they are better at surviving, but we also need the plants to start producing seeds!
As well as a researcher, you’re also the SEB’s Early Career Trustee. Can you tell us a little bit about how that came about?
I attended a SEB Conference in the first year of my PhD upon the recommendation of my supervisor, Tracy Lawson; this was pre-presidency, of course. It was the first and only conference I went to because I did my PhD during COVID, but everyone was so lovely that I remained a member throughout my PhD. In the first year of my postdoc, there came an email that said they were looking for a new Early Career Trustee, so I reached out to find a little bit more about what it was and applied and got it. I’ve been doing that for nearly 2 years now.
What does the role of Early Career Trustee entail?
At every council meeting, I can voice my opinion on matters from the kind of events we run for early career researchers, all the way to the budget matters, to make sure that we’re representing the interests of people who are early in their research career.

The SEB are particularly interested in supporting early career researchers, so I feel like I can make suggestions that are really heard. And it’s very humbling to be involved in this sort of thing, I really enjoy it. We’ve talked about how we can put things on to help early career researchers at these events, because going to your first conference can be so scary and we want to make it easy for people to meet people. That’s why we started putting on the Early Career Networking event on day zero of the conference last year. I was so pleased that it went so well, and now we’re running it again. So, hopefully everyone will come to that one and it’ll be even bigger and better—I’m very excited!
If you had one piece of advice for someone attending their first SEB conference, what would it be?
Don’t be afraid to approach people. Of all the conferences I go to, the SEB is by far the friendliest. The vast majority of people are interested to talk to you and want to know about what you do. And then you end up with these friends who do all sorts of random things that you never thought you would know about. It shouldn’t be a scary experience, it’s an exciting experience where people will usually nurture you if you ask for it.
What are you passionate about outside of your research?
Oh, that’s a big question. I think it’s people that keep me going. My family has supported me a lot through this, particularly my mum, but also my friends and my chosen family, the people that actively go out there every day and choose to support me. I don’t know how I got so lucky. I met a lot of people through my PhD as well—it may be some sort of COVID trauma bond or something because we were the only people that each other saw and we became very close. To this day, I’m still very close to a lot of them. And then obviously my partner, he puts up with a lot. I’m very lucky in that he has a job that he can go anywhere with, so with me potentially having to move a lot, it means that he can still have the career that he loves, which I’m very grateful for because I know not everyone is that lucky.
Putting Arabidopsis aside for a second, what are your favourite plants?
Well, I think ferns are really neat. They’re just little living fossils. I think it’s really cool that you can find a fern fossil from however many millions of years ago, and then you can go look at a fern today and it is exactly the same. And I think it’s really neat as well that if you manage to catch their spores, that they look like little sperm. You can actually see them swim and I think that’s really funny!
Well, I’ve learned something new about ferns— thank you, Robyn!
I CAN MAKE SUGGESTIONS THAT ARE REALLY HEARD
I THINK FERNS ARE REALLY NEAT

How would you introduce yourself and your research?
I am an Associate Professor of Plant and Fungal Biology at the University of Plymouth, UK. My work focuses on understanding the cellular basis of rice blast disease (caused by Magnaporthe oryzae) and minimally invasive imaging of plants. What are the key questions you are investigating at the moment?
One of the projects in my lab is investigating the role of the circadian clock in rice blast disease. Using CRISPR-Cas9 gene knockouts, my previous PhD student Ciaran Griffin and I have demonstrated that disruption of the circadian clock in Magnaporthe massively impairs the ability of the fungus to make spores and infect the host plant. Our current work is investigating the molecular and cell biological basis for these differences, through the PhD project of Rehema Mwaipopo.
Another area of interest is the role of the chloroplast in plant immunity, where I have been collaborating for many years with Murray Grant. We have just started, with my PhD student, Kokila Wickramanayake, a series of proteomics studies to elucidate the mechanism behind the physical changes that occur in rice chloroplasts during Magnaporthe infection.
When did you decide to study plant sciences?
I chose to study Genetics for my undergraduate degree at the University of Glasgow because I was totally in love with the subject. I was struck by its elegance, how it worked according to predictable rules, and the interesting problems that remained to be solved. I wasn’t thinking about plant sciences specifically until I did a work placement year at Unilever, working on GM tomatoes. I realised that plants offered highly tractable genetics systems and you could answer questions in ways that would be much more difficult to do in animals. I ended up doing a PhD at the University of Glasgow with Gareth Jenkins, working on flavonoid biosynthesis genes.
During my second PhD year, there was a catastrophic fire in the Bower Building where the lab was and the building was closed for 18 months. All my research was put on pause. I started to do a lot of teaching and demonstrating, and outreach for local schools. It confirmed my ambition to finish the PhD and have an academic career, but my confidence had taken a great hit.
One day, I happened to be in Anna Amtmann’s office, waiting to collect a speaker for a departmental seminar. As we chatted, she mentioned that she had funding for a year-long postdoc and she invited me to ‘come and play’ in her lab for a year. It was exactly what I needed, and gave me time to build up my confidence and reinforce what I wanted to do. Even so, for a long time I felt that I was playing career catch up. I only really shook that off about 20 years later when I became an Associate Professor. That experience helped me empathise with those PhD students who went through the COVID years, and had to build everything back up from scratch.
How has your career progressed since then?
From Glasgow, I went to the University of Exeter, joining John Love’s Lab in 2007 to work on fluorescent biosensors and calcium signalling in Arabidopsis. Here we had a lot of fun pioneering new microscopy techniques in plants, which helped to broaden my outlook beyond genetics. For instance, we published with Julian Moger the first demonstrations of stimulated Raman spectroscopy (SRS) and coherent anti-Stokes Raman spectroscopy (CARS) in plants, which enable you to conduct label-free imaging in plants. We also developed protocols to carry out live cell imaging at deeper levels within plant tissues using perfluorocarbons.
In 2012, I moved to Nick Talbot’s group, also at Exeter, which is where I began working on Magnaporthe and rice, setting the foundation for my current work. It was a very stimulating environment, and a good place to test out new ideas. It was a couple of those ‘Friday afternoon’ experiments that first indicated the influence of the
circadian rhythm on rice blast disease outcomes, and Nick and his lab have since helped us establish CRISPR-Cas9 gene editing in Plymouth.
In 2016, I won a Lectureship at Plymouth, where I have been ever since. At Plymouth we have been actively building agriculture-based research and teaching over the last few years and have recently established the Centre for Research Excellence in Intelligent and Sustainable Productive Systems (CRISPS).
Between 2015 and 2018, you were Chair of the SEB+ Section (now Outreach, Education and Diversity, OED). What were your key actions in that role?
I was particularly keen to promote the SEB grants for teaching-focused academics to attend the Annual Conference. For those on teaching-only contracts, it can be difficult otherwise to find funding to attend scientific conferences. I also supported the work led by Teresa Valencak and Mary Williams to clarify the society’s Equality, Diversity and Inclusion agenda, and introduce coherent policies in this area. To me, these activities don’t just ‘complement’ the SEB’s scientific sections, they are absolutely essential.
In May 2024, you were nominated to be Chair of the SEB Plant Section. What are you hoping to achieve in this role?
A big priority for me and the other Section Chairs is to promote more interaction between the Plant, Animal and Cell Sections, including through sessions on crossover themes, such as mitochondrial biology. I am also hoping to rejuvenate the special interest groups, and consult the SEB membership on what new groups could be created. I’m extremely lucky to have the support of the SEB staff, Plant Section Committee and especially the Plant Section Deputy Chair, Liana Acevedo Siaca.
What advice would you give to early career researchers?
Do what motivates you, keep an open mind, and learn how to communicate with those outside your discipline. I am not saying that specialisation is a bad thing, but researchers can be ‘pigeon-holed’ at times. Increasingly, being able to talk across boundaries—not just to other biologists, but to, for instance, mathematicians and physicists—can open up valuable opportunities for your research.
Do you think it is important to engage the public with science?
Definitely. However, science public engagement tends to reach self-selecting audiences: those already interested in science. For the past few years, I have helped organise the Sidmouth Science Festival in a small town in Devon and we aim to attract those who are engaged already and to bring in those would not normally engage with science. Besides the local community, we get many holiday-makers coming to our events,

who just happened to be in the area. I think it is very important that we have a public who are scientifically literate and appreciate the benefits that research brings—particularly in this age of misinformation and ‘anti-science’ sentiment.
How do you relax from work?
I REALISED THAT PLANTS OFFERED HIGHLY TRACTABLE GENETICS SYSTEMS AND YOU COULD ANSWER QUESTIONS IN WAYS THAT WOULD BE MUCH MORE DIFFICULT TO DO IN ANIMALS
My wife is an artist so the visual arts have a strong presence in our family life. We will often go out to art galleries and shows. It does annoy me that our society distinguishes between science and ‘the creative industries’. To my mind, both are methods we use to try and understand the world around us. My approach to my research and discussion of science has always been to embrace my creative side, and I believe it is much better as a result.
‘Follow your own intellect and your own intuition, try to become an independent thinker as soon as possible.’ Alex Evans
talks with Frank Van Breusegem, Professor of Plant Biotechnology at Ghent University and leader of the Oxidative Stress Signalling group at the VIB Centre, to find out what fascinates him about the world of plant science.
BY ALEX EVANS
t the very start of Frank’s university education, his intellectual passion was split between biology and history, which he says both scratched the itch of discovery-driven learning. However, it was biology that won out, and it was during his Master’s in Zoology at Ghent University that he became especially interested in biotechnology, which had just started to be taught at the university. ‘I did my master’s thesis with Marc van Montagu, who was the inventor of the Agrobacterium-based transformation of plants—kind of the founding father of GMOs in plants,’ says Frank. ‘I was intrigued by all the fascinating inventions that you could do with DNA and RNA, and trying to understand how plants respond to the environment.’
As well as completing both his undergraduate and postgraduate degrees at Ghent University, Frank has remained there for most of his research career; a relatively rare situation in modern academia. While he says that he may slightly regret not having taken up an international postdoctoral position earlier in his career, he acknowledges that Ghent was the best place to be because it was leading the way his chosen field. ‘I was in the cradle of plant biotechnology, working in the labs of people like Jeff Shell and Marc Van Montagu,’ he says. ‘They were heroes at the time and still are today.’

Since the start of his foray into plant cell research, a major theme of Frank’s work has been oxidative stress in plants and the gene and protein networks involved. ‘During my PhD, we tried to engineer stress tolerance in maize by introducing antioxidant enzymes like superoxide dismutase and ascorbate peroxidase,’ he explains. ‘The naive idea was that if we overproduce those enzymes, we might better protect plants against oxidative stress.’ While this technique showed promise in the lab, unfortunately it proved disappointing in the field, but still laid the foundation for developing his interest in plant cell signalling.
A lot of Frank’s subsequent work has continued to deal with the impacts of reactive oxygen species on plant cells. ‘It’s often seen as an enemy of the cell because it damages macromolecules of all kinds, but then it was found that hydrogen
peroxide can also act as a signalling molecule, like a kind of stress hormone,’ he says. ‘In this way, the plant perceives that something is wrong and uses hydrogen peroxide to launch a defence response.’
While Frank’s research is primarily grounded in uncovering the fundamental mysteries at work in the cell, there are applications for real-world problem-solving, such as improving crop resilience. ‘It’s always grounded in curiosity-driven research and trying to understand how things work, but it will be very cool to contribute significantly to one part of that complex and gigantic puzzle, and funders often want economic impact as a return on their investment,’ he says.
Looking back on the highlights of his career so far, Frank shares how his research played a pivotal role in identifying the transcription factors related to nuclear events associated with mitochondrial stress. ‘We identified two transcription factors that specifically activate a subset of genes, which we call the mitochondrial stress response,’ he explains. ‘Nowadays, we mainly focus on redox proteomics, where we try to understand which proteins are affected directly by hydrogen peroxide using a multi-omics approach.’
In more recent years, Frank’s contributions to the research have become more focused on the big picture, meaning that he unfortunately has less time to spend in the lab. ‘The days are now filled with meetings, teaching, organising things— for bench work, you need to dedicate a lot of your time and thoughts,’ he says. ‘But I do have almost daily contact with my PhD students and postdocs, so I can still follow their research and give practical advice to them as well.’ As Frank explains, you can’t overestimate the importance of having good role models and mentors that not only teach and lead the next generation, but also provide them with the freedom to explore their own path. ‘Follow your own intellect and your own intuition, try to become an independent thinker as soon as possible,’ he advises. ‘It’s something I try to promote in my lab with my postdocs, so that people drive their own projects forwards.’ This encouraging approach seems to be working, given that two of Frank’s postdocs have received

early career research grants and are now running their own labs.
One area of research that Frank would still like to explore in more detail is plant proteases. ‘There’s still a lot to discover with plant proteases, with approximately 500 genes encoding for proteases in Arabidopsis,’ he says. ‘I think that’s a huge field to explore in plant biology still, and while we are touching on it, I would like to invest more.’
With his name on over 150 published papers and generating over 3000 citations per year, Frank’s research has reached far and wide, but he remains modest about this achievement and is keen to emphasise that while citations are great for funding, the quality of the research should speak for itself. ‘Reactive oxygen species are such a universal thing, which makes it quite an attractive topic to get cited,’ he says. ‘Other research will be just as fantastic and as valuable, but citations will be lower because fewer people are interested or active in the topic, so I think we need to use some caution in taking citations as a criterion for success.’
Finally, outside of the world of research, Frank values spending time with his family and friends—especially with his wife, who is a fellow scientist. ‘We love going to
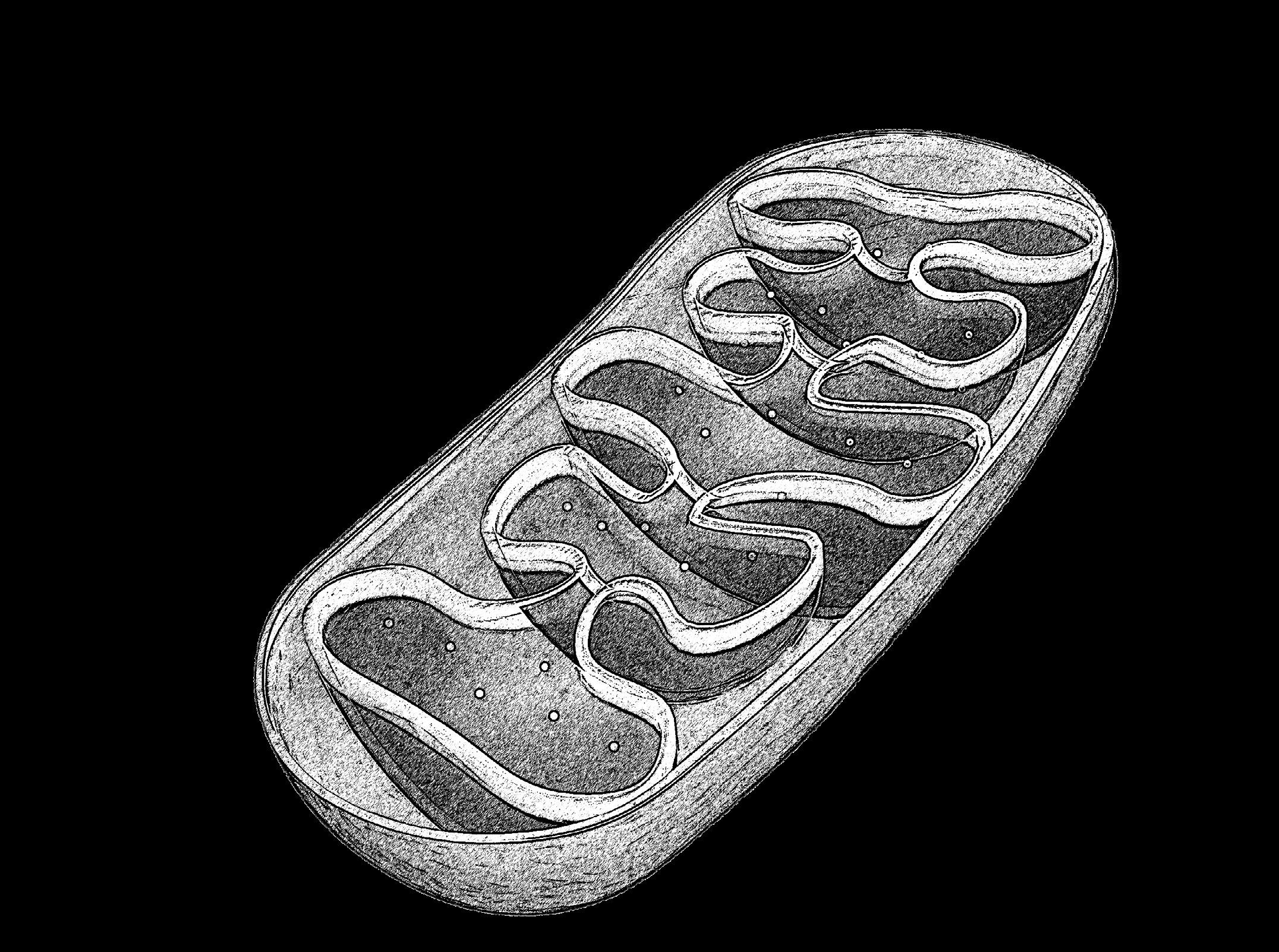
There can’t be many people who would say that one of their highlights from a trip to Hawaii was finding a sea urchin. But even on vacation, Amanda Wiesenthal brings her deep fascination with marine creatures.
BY CAROLINE WOOD
had been looking out for this species Colobocentrotus (Podophora) atratus, known as hā uke uke kaupali in Hawaiian, for a few weeks because it is only found on shores in the Indo-Pacific, including the Hawaiian coast,’ she says. ‘It is unusual for urchins to have short, shingleshaped spines rather than spikey ones. To finally spot one was an amazing moment—I couldn’t understand why the other tourists weren’t as excited as I was.’
A natural reaction, though, for someone who has been captivated by living creatures since childhood. ‘As soon as we started biology classes at school, I became hooked,’ Amanda says. ‘I was so intrigued at how diverse nature is, and wanted to know how everything worked.’ Consequently, she now feels she has her ‘dream job’ studying osmoregulation in aquatic invertebrates, as part of Inna Sokolova’s group at the University of Rostock, Germany.

‘My work focuses on Mytilus edulis mussels, a species that is distributed across a steep salinity gradient from the North Sea into the Baltic Sea,’ she says. ‘As osmoconformers, they adjust their internal osmolarity to match that of the environment, which poses a major challenge to their metabolic machinery. We are currently carrying out comparative physiology studies to explore how different populations have adapted to the local salinity level.’
Up to now, her investigations have concentrated on isolating mitochondria and carrying out high-resolution respirometry experiments. This revealed a shift across populations in the optimal osmolarity for maximum mitochondrial respiration, matching local salinity levels.1 ‘This means that mitochondria from North Sea mussels reach their peak capacity at higher salinity levels than those from Baltic Sea mussels,’ Amanda says. ‘However, it appears that for the Baltic Sea populations, adapting to lower salinities came with a trade-off: a more specialised mitochondrial phenotype with a narrower osmolarity range for optimal mitochondrial capacity. Whereas the North Sea populations have more “general functioning” mitochondria.’
At the moment, the mechanisms causing these differences remain unknown. ‘If I had the resources to do any experiment I wanted to, I would initiate a comparative approach to the response of as many different species inhabiting salinity-relevant habitats as possible under various salinity conditions combined with other stressors like temperature or hypoxia,’ Amanda says. ‘After exposing the organisms, I would like to look at different levels of responses from the whole animal down to the genetic level, and find out how the interaction of stressors affects the physiological response, how the different organisms cope with the conditions, and how the mechanisms work.’
But it was jellyfish, not mussels that originally set the direction of Amanda’s career. After achieving an undergraduate degree in BioGeoAnalysis at the University of Trier, Germany, she applied to the University of St Andrews in Scotland to study a Master’s in Environmental Biology. ‘During my Bachelor’s degree, I did an Erasmus year at the University of Glasgow and fell in love with the country and its people,’ she says. ‘So, I was very keen to return to Scotland for a postgraduate degree, and the topic of the course really appealed to me.’
Whilst there, she became captivated by scyphozoan jellyfish, the subject of her dissertation. ‘I just absolutely loved them, there was something about jellyfish that really caught my attention. Despite being simply structured organisms that consist mostly of water, they are super successful, and have been for a very long time. So, there is certainly more to them than meets the eye. At the same time, I also decided that research was incredibly fun and fascinating—particularly because there is still so much that we don’t understand about nature.’
With no positions involving jellyfish being available, for her PhD Amanda switched to working on Theodoxus fluviatilis snails at the University of Greifswald, Germany, investigating mechanisms underlying osmotolerance. ‘T. fluviatilis occurs in both brackish and freshwater, making it an ideal model to study osmoregulation,’ she says. ‘I have a lot of happy
memories of going out to collect snails from different locations, including some beautiful lakes in Germany and places along the Baltic coast.’
Her first postdoctoral position was a lateral move, working on the effects of ageing on macrophages at Saarland University, Germany. ‘I learnt a great deal, because I didn’t have a strong background in immunology,’ she says. ‘But it confirmed that my heart belongs to aquatic organisms. Also, I realised that I am not very comfortable with mice! So, I was very happy when I took up my current position and transitioned back to marine biology.’
Although she feels her topic could easily sustain an entire academic career, Amanda admits that she finds the precarious nature of academia unsettling. ‘Right now, I don’t know if it will be possible to stay in the system. Academia is a bit of a paradox because you can undergo so much training and become highly qualified, but still not have job security. This makes it really important to be involved with networks such as the SEB. The more people you are connected with, the more likely you are to find out about job opportunities, and meet potential collaborators for grant proposals.’
Indeed, Amanda’s association with the SEB has a long history, starting with her first Annual Conference at SEB Brighton during the second year of her PhD, in 2016. Since then, she has attended every Annual Conference, drawn by the ‘exciting and inspiring’ atmosphere. ‘I really like how the SEB meetings bring together a broad range of expertise, not just people from your own small bubble. It is a great way to stimulate ideas, and to see how people tackle similar problems on other species, using different techniques. The meetings have also become a reunion, where I look forward to catching up in person with the colleagues I see there year after year.’
At the 2024 Annual Conference in Prague, Amanda was invited to co-organise a session on mitochondrial plasticity, an experience she found immensely valuable. ‘It is really good how the SEB gives early career researchers opportunities to be directly involved in organising events. I met a lot more people within the mitochondria community, many of whom I am still in touch with. I’m excited that another mitochondria-focused session will be held at SEB Antwerp this year. It will be great to catch up with everyone and learn about what they have been working on.’
Outside work, Amanda enjoys activities that let her ‘unwind her mind’, including badminton, Irish dancing and archery. But even at home, there are reminders of her research life.
‘The jellyfish found a way back into my life,’ she admits. ‘I have a beaker full of jellyfish polyps in the kitchen. I ended up with them after some

RESEARCH IS INCREDIBLY FUN AND FASCINATINGPARTICULARLY BECAUSE THERE IS STILL SO MUCH THAT WE DON’T UNDERSTAND ABOUT NATURE
preliminary experiments at the University of Greifswald and I couldn’t bear for them to be disposed of. So, they have stayed with me, even moving locations twice! But it is a nice reminder of how happy I am with the job I have. I really can’t imagine doing anything else.’
References:
1. Wiesenthal AA, Timm S, Sokolova IM. Osmotolerance reflected in mitochondrial respiration of Mytilus populations from three different habitat salinities. Mar Environ Res 2025; 205: 106968.
I WANTED TO PLAY MY PART IN HELPING US FIND OUT MORE ABOUT ALL OF THESE REALLY COOL CREATURES AND THEIR INTERCONNECTION WITH THE ENVIRONMENT’


BY CAROLINE WOOD

Surveys consistently show that scientists are among the most trusted people in society. Consequently, researchers who engage with the media and give expert comments on topical issues really help the public to distinguish between fact and fiction. Engaging with the press can also be an excellent opportunity to disseminate your research to a wider audience.

But giving a media interview can be a daunting prospect—especially if it’s going to be broadcast on TV or radio. As a Research Communications Manager at the University of Oxford, Caroline Wood has set up countless media interviews for researchers on a wide range of topics, from exoplanets and endangered species, to artificial intelligence and Anglo-Saxon burials. Here are her top tips for researchers on speaking to the press.
Here are some initial questions to ask when you receive an interview request:
● What is the outlet? If you are receiving a lot of media requests at the same time, you may have to prioritise. Is it a well-known outlet? Is the audience regional, national or international? Will your interview be made available afterwards, so you can use it as evidence of media engagement?
● What are you being asked to speak about and what is the context? Do they want you to talk about your own research, or to comment on a topical issue (which could lead to questions outside your specific area of expertise)?
● What is the outlet’s angle? Why do they want to cover this now?
● Will you be interviewed alone, or as part of a head-to-head debate or panel discussion? If there will be others, who else will be interviewed and what are their views?
● What is the urgency and when do they want you to speak? If you are being asked to comment on a topical news story, the outlet may want to record the interview as soon as possible. Bear in mind that speaking live on air during a morning news show could involve an early start! Whereas an interview for a documentary or podcast can typically be carried out over a much longer timeframe, at a time to suit you.
● Will the interview be live or pre-recorded? Being recorded live on air can be daunting, but at least you know that your words won’t be ‘cut’ before the broadcast.
● Can you see the questions in advance?
Keep in mind that journalists typically work on many stories at once, to short deadlines. So, they appreciate it when academics respond swiftly and have ready availability.
If you accept the interview request, how then should you prepare?
● Find out if you are expected to go to a studio, or if the outlet can record the interview over the phone. If you are asked to go to a studio, they may be able to provide transport.
● If you are being interviewed over the phone, find a quiet location where you won’t be disturbed by family, students, pets, deliveries, etc. Put a sign on the door if necessary! Make sure you have a good headset, phone line and/or internet connection.
● Make sure you know what time to dial in. If you are giving a live interview, they are likely to want to test the connection first.
● If you are being filmed, find a smart outfit and avoid wearing anything distracting, such as dangling earrings or a particularly colourful tie. Besides being less distracting for the viewer, this will help give you confidence.
● Make sure you exchange contact details for the journalist/producer you are dealing with, including backup mobile numbers in case there are technical issues.
● You can use the correspondence with the journalist ahead of the interview to clarify your perspective, and what you are/are not prepared to speak about. For example: ‘I am not a medical doctor, so I wouldn’t be able to answer questions about what patients should do’ or ‘That topic isn’t my expertise but what I do know is…’
● Write out your three ‘take-home messages’. It is very important to focus on just a few points, especially when you only have a handful of minutes to speak.
KEEP IN MIND THAT JOURNALISTS TYPICALLY WORK ON MANY STORIES AT ONCE, TO SHORT DEADLINES. SO, THEY APPRECIATE IT WHEN ACADEMICS RESPOND SWIFTLY AND HAVE READY AVAILABILITY.
● Consider things from your audience’s perspective. Think about why your results are relevant to the public and how you can capture their interest. How could this have an impact on their daily lives? This is ultimately what people will be most concerned about.
● See if you can illustrate your key points using statistics—but make sure any numbers are up to date and accurate!
● Ensure your language is suitable for a public audience. Assume the scientific knowledge of a 14-year-old. Avoid technical terms and jargon, and try to use analogies to communicate complex concepts and scientific units more accessibly—for instance, ‘the width of a human hair’, ‘the size of a football field’.
● Practice speaking aloud to a friend or family member who doesn’t have a research background to make sure your key messages come across clearly.
● Tell your institute’s press office. This helps them to monitor media engagements and they may be able to offer you media training and/or promote your interview, for instance on social media.
● Have your key points to hand so you can easily glance at them, but make sure you don’t noisily rustle any papers.
● Pay attention to your body language, especially for TV broadcasts. Smile, don’t slouch or cross your arms, and try to keep still (moving around or waving your hands is distracting to the viewer).
● Speak slowly and clearly, without rushing.
● If the questions start to stray from your three key messages, try to pull the conversation back to them by using phrases such as ‘what we must remember is...’ or ‘the really important point is...’
● Don’t speak outside your own area of expertise. If you are asked to comment on something you are not confident about, you can state that you are not an expert in this area and say phrases such as ‘I can’t talk about that, but what we do know is that…’ or ‘We are not saying that….’
● Don’t be afraid to state areas of uncertainty, but explain why they are uncertain; for instance, ‘there has been little research in this area up to now’ or ‘it is a difficult thing to measure and quantify’.
● Don’t ramble! Stop as soon as you have answered the question. It is up to the interviewer to keep the conversation flowing.
● If you are presented with a controversial argument or conspiracy theory, acknowledge it, disprove it briefly, then move the discussion back to your key messages.
● More than anything, stay calm and try to enjoy the experience!
● Make a note of what went well, and anything you would like to improve next time.
● Follow-up with the interviewer. Thank them for the opportunity and check if they need any additional information. You can also ask that they keep you in mind for any future requests.
● Look out for the resulting coverage and share the links with your peer network (for instance, on LinkedIn), your department and your institute.
Once you have a few interviews under your belt, you may well be surprised by how quickly your confidence grows. The news landscape may seem intimidating at times, but it is nearly always improved when experts engage with it.

BY REBECCA ELLERINGTON
Among the benefits for SEB members, the Career Development Workshops aim to create a platform for further professional development for scientists beyond the traditional degree }or lab/field experience. Every year the SEB hosts around five workshops that are free for members and open to non‑members under a fee. The topics vary according to the interests of our members collated in feedback forms, our aims as an organisation and trending topics in the field.
In line with this year’s Annual Conference, whose theme is ‘The Impact of Experimental Biology to Tackle Global Challenges’, we have scheduled three workshops to springboard discussions and opportunities:
● Impact Strategies for Fundamental Research
● Confident Scientific Presentations
● How to Successfully Engage with the Media
This workshop happened in January led by Tamika Heiden from Research Impact Academy. It opened the year by building a foundation for understanding research impact, from definitions to developing an impact plan for a research project.
According to the Research Excellence Framework (REF) 2002, research impact is ‘an effect on, change or benefit to the economy, society, culture, public policy or services, health, the environment or quality of life, beyond academia’. There are numerous ways that research can cause an effect after it has been put into action. When thinking about impact, it is important to consider whether you are:
planning for the impact, known as prospective impact. This involves considering the potential effect research could have and is done before the project is started or completed, for example when writing a grant and foreseeing potential effects. assessing the impact, known as retrospective impact. This requires collating information on the effect of research already realised, for example when writing a report to identify successes. There are many tools to gather these data, such as Altmetric, Lens.org and Dimensions.
Tamika focused on prospective impact and provided frameworks to help define the research process and elements to achieve impact. An important message was to consider not only the people who would benefit, but also the people who would use the research to help with decision-making or as a base for their own project. Communication was the key term used during the workshop because impact is about people. Explaining what and why research is done, and articulating its (potential) effects clearly and in tailored way to each audience can be limiting factors to research impact. For example, using a scientific paper to explain research on the risks of water pollution in a community centre probably won’t have any/the same effect as using a poster or video using common words and relatable examples.
With this in mind, the other two workshops will focus on communication with different audiences and formats: presenting to other scientists, and engaging with the media as a means to most effectively spread your research message.
Suzanne Whitby from SciComm Success will lead this interactive and practical workshop in May to teach scientists how to plan, design and deliver a clear scientific presentation. This is an essential workshop for every scientist because presentation is key in the scientific field. Although many have hands-on experience, getting the message and design right for the audience can make a huge difference to a presentation.
Even when your audience encompasses scientists, there are many factors to consider. Are they experts in your field? What is the format of the presentation—a talk, a poster, a short presentation? What is the context, is it for an award, a keynote talk? These different factors mean that each presentation might be different and the message may change. Suzanne will make this message a key point of discussion, because it varies with context and audience, and it is what people will take away from your presentation.
This workshop will help those attending the Annual Conference to raise awareness of their research findings, leading to potential collaborations, insights and networking opportunities.
This ad hoc workshop created for the SEB by media communication experts, Nadine Dereza and Ross Edmonds from Babaco Media, will focus on media communication and engagement. Navigating the media landscape can be challenging for researchers for a myriad of reasons: a lack of confidence; uncertainty about delivering a clear message amid the noise of journalists; discussions of sensitive topics, such as the use of animals in research; or lack of awareness of the process and timelines. This workshop will discuss all these barriers and provide techniques on how to handle the media, what (not) to say and how to come across during interviews.
This is an exciting opportunity in the context of research impact to tackle global challenges because
the media can be a key communication medium for reaching public interest. It complements the work the SEB has been doing over the years with the Press Officer, Alex Evans. Alex creates press releases based on research submitted to and presented at the Conference to garner journalists’ interest and advises scientists on media engagement.
Media engagement can accelerate impact and create opportunities usually not foreseen. It is a great opportunity to develop new skills and contribute to better coverage combatting misinformation and disinformation.
In preparation for the Annual Conference and to support members’ professional development, the SEB has been hosting workshops focusing on communication, engagement and impact during the first semester of 2025. These workshops align with our key aim of enhancing the influence of experimental biology within the scientific community and demonstrating to the public its relevance and impact. The SEB invites members and the community to register for current workshops and keep an eye on future opportunities.
The next semester will also bring exciting topics, such as the already confirmed Interdisciplinary Collaboration Workshop, which links to the SEB’s broad experimental biology community and the importance of collaboration to solve pressing issues. The SEB is also keen to hear from members about their interest in further career-developing workshops. If you have a suggestion, please email Rebecca Ellerington at r.ellerington@sebiology.org.

SEB MEMBERSHIP
70% OF MEMBERS LEARN ABOUT SEB THROUGH WORD OF MOUTH
SEBIOLOGY.ORG #SEBPACK

MAKE EXTRAORDINARY CONNECTIONS NETWORK AND BUILD RELATIONSHIPS WITH EXPERIMENTAL BIOLOGISTS FROM AROUND THE WORLD
THINK BIG TAKE PART IN CROSSDISCIPLINARY CONFERENCES AND SHARE INNOVATIVE AND INSPIRING DATA, IDEAS AND RESULTS

DO MORE APPLY FOR GRANTS AND SPONSORSHIP TO INCREASE YOUR OPPORTUNITIES
FAST TRACK YOUR CAREER
ACCESS JOURNALS, EDUCATION AND TRAINING SUPPORT