
10 minute read
Organo-functionalized Inorganic Nanofiltration Membranes through Engineering at the Molecular Level
from KGK20231
by sjpuitgevers
Nikos Kyriakou performed his PhD research at the University of Twente (UT) within the project ‘Solvent Tolerant Nanofiltration and Reverse Osmosis membranes (STNF) for the purification of industrial aqueous streams’ funded by the Institute for Sustainable Process Technology (ISPT), supervised by Louis Winnubst, Marie-Alix Pizzoccaro-Zilamy and Arian Nijmeijer of the UT Inorganic Membranes group. The project expands the boundaries of inorganic membrane separations by developing new organo-functionalized ceramic membranes. Applications of these nanofiltration membranes are in challenging conditions such as water-solvent mixtures. Nikos Kyriakou defended his PhD thesis on October 14, 2022. This paper gives a summary of the thesis.*
Separations are involved wherever mixtures are present, including gas, solvent/solute, and reactants/ products/by-products mixtures. An astonishing 10-15 % of the world’s total energy consumption is used for industrial separations.1 This large amount of energy is partially ascribed to the scale of separation processes but is mainly due to the use of high-energy demanding techniques, such as distillation, which the industry is heavily relying on [1]. On the other hand, pressure-driven membrane-based technologies can separate liquid mixtures (even at the molecular level) without phase transition and thus decrease the energy required for separations [2].
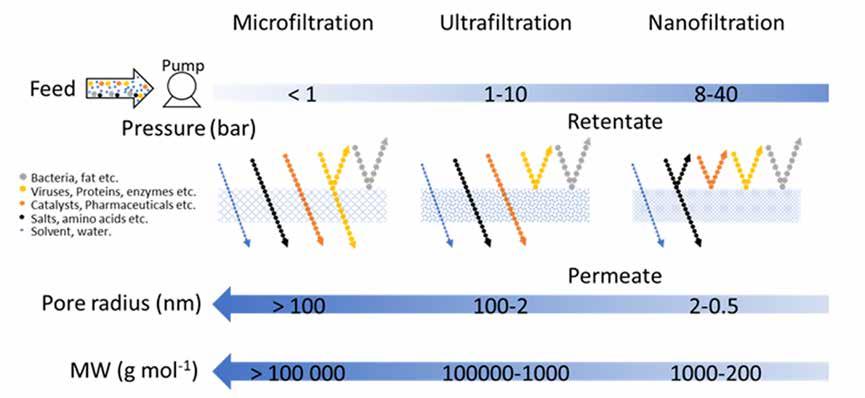
Amongst different membrane technologies, nanofiltration (NF) is used for the separation of liquid mixtures containing solutes with a molecular size between 0.5 and 2 nm (Figure 1). These mixtures include mixtures of solvents, homogeneous catalysts, antibiotics, peptides, and reaction intermediates. Therefore, NF technology can potentially reduce industrial separation costs and mitigate waste generation by purifying and reusing solvents.
NF membranes were first mentioned in the early 1980s to distinguish from reverse osmosis (RO) membranes, which only allow for the permeation of water molecu- les [3]. Since the RO technology was already established, NF membranes were first used for wastewater treatment and water hardness reduction. The potential use of membranes for the separation of organic mixtures down to molecular levels attracted the attention of the petrochemical industry, which resulted in the first application of the NF technology in nonaqueous mixtures, such as in the separation of hydrocarbons [3]. This resulted in the expansion of NF to organic solvents, which was dabbed as Organic Solvent Nanofiltration (OSN). The potential applications of chemically (or solvent) resistant NF membranes (CRNF or SRNF) extend through different industries, including chemical, petrochemical, pharmaceutical, food, and textile. This new technology is regarded by specialists as a possible way for the intensification of industrial processes involving organic solvents, to eliminate the need for energy-intensive separation technologies [3, 4], reduce environmental emissions and materials consumption [3], and potentially allow a continuous process from raw materials to products [5, 6].
Even though NF technology has made enormous progress, there are major challenges that hinder their wider ‘spread’ in industry. First, the chemical, thermal and mechanical stability requirements of the membrane, which affect the long-term performance, differ between aqueous and non-aqueous applications. Second, the lack of control over the microstructure of the selective layer during membrane fabrication, as well as their behaviour under operating conditions, has slowed down the development of NF technology. Third, the unavailability of tools to understand and predict the performance of NF membranes restricts further developments towards industrial applications. Finally, the unavailability of multifunctional membranes weakens commercial interest in this technology. Only if NF technology answers the above questions, it will force the industry to ‘retire’ old energy- and resource-intensive methods and focus on developing and implementing membranes in industrial processes.
It is expected that the organo-functionalized ceramic membranes, as described in the thesis, will have ‘the best of both’, meaning high chemical, thermal and mechanical stability, and tuned selectivity by selecting the proper organic functional group. This PhD research can be divided in: (1) novel fabrication methods, (2) in-depth characterization, and (3) application of these materials as membrane.
Novel methods for the fabrication of organofunctionalized ceramic membranes
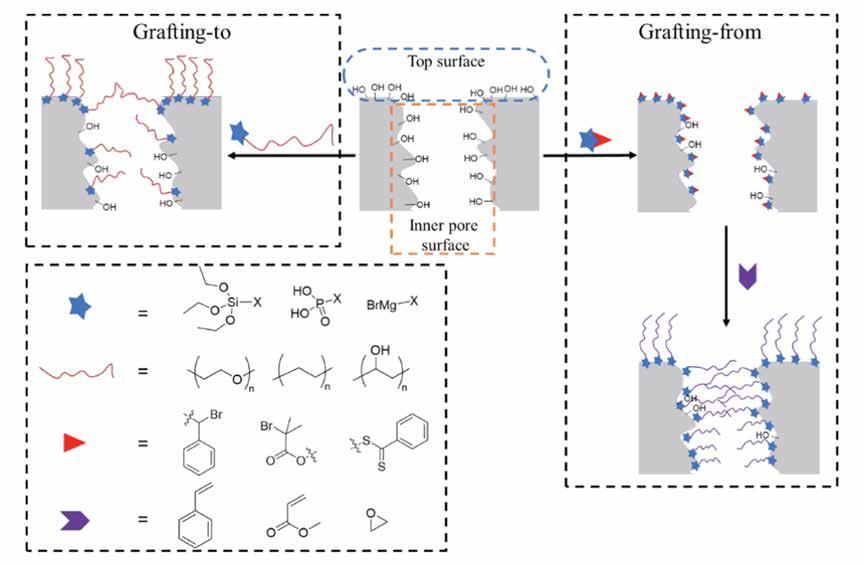
Each chapter of the thesis describes methods for the modification (also called ‘grafting’) at the nanoscale level of either the top or pore surface of a ceramic support. For grafting ceramic surfaces two main approaches can be used, grafting-to and grafting-from (Figure 2). Grafting-to is a simple methodology that typically involves polymers already functionalized with a variety of linking groups. On the other hand, the grafting-from approach, is a stepwise method that starts with the surface modification, followed by a surface-initiated in-situ polymerization to form the final grafted polymer. The two methods result in different grafting densities, due to starting from reagents that significantly differ in size, with the grafting-from typically showing higher densities. The different organically functionalized ceramic membranes studied in this work are categorized into three main groups: polyethylene glycol (PEG), polythioether, and polyimide (PI) grafted alumina membranes.
Chapter 2 and 3 of the thesis are focused on PEG on γ-alumina supports by a grafting-to mechanism. First the influence of the linking group on the hydrolytic stability of the membrane is studied, using PEG-phosphonic acids or PEG-alkoxysilanes. The phosphonic acid graft, in contrast to the alkoxysilane graft, showed stable hydrolytic behaviour even after treating for 216 hours in water [7]. Besides, the PEG phosphonic acid grafting was performed in water, a green solvent, or in the solid state with minimal amounts of solvents. In all cases FTIR analysis was used to show grafting on the surface, however, this was not conclusive. Other techniques (including pore size measurements, water permeability and retention test with a small organic dye) indirectly showed that grafting was successful and that the performance of the membrane was stable. Comparison between the two preparation methods indicate that the solid-state approach is sensitive to reactive groups that are not involved in the grafting reaction, such as the hydroxyl end-group of PEG. By eliminating any side reactions, the solid-state approach led to higher grafting densities as shown by TGA analysis on PEG-grafted γ-alumina powders.
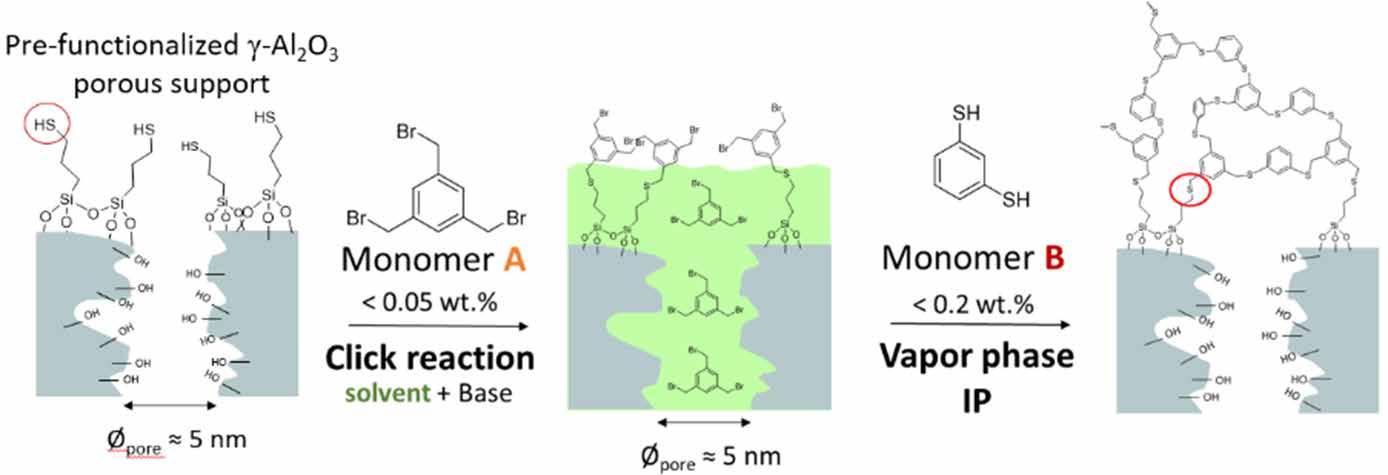
Chapter 4 describes the formation of a crosslinked thioether-based polymeric layer on top of an alumina support [8]. A grafting-from approach is applied, employing a ‘click’ reaction along with a vapor phase polymerization, occurring at the interface between liquid and gas (Figure 3). The use of a ‘click’ reaction and vapor phase polymerization allowed for lower monomer use and hence less formation of waste. This preparation method is one of the few methods reported in membrane fabrication that uses vaporliquid reactions and is the only one done on a porous support.
The aim of the work described in chapters 5 and 6 is to form a well-ordered crystalline polyimide (PI) network via a grafting-from approach. In chapter 5, a crystalline polyimide (PI) crosslinked polymer was formed via an in situ polycondensation method in solution, applying two different routes (Figure 4) [9]. Route A starts with modification of the surface with a small functional molecule (4-aminopropyl triethoxysilane; APTES) followed by the polycondensation reaction. In route B the surface functionalization reaction is followed by intermediate attachment of one of the monomers, and finally by the polycondensation reaction. The polycondensation reaction was performed for 1 or 5 days at 200 °C. The results showed that in all cases the PI was formed on the support. Membranes, made via route A result in higher water fluxes compared to route B, which was attributed to formation of the network near the top surface of the support for route A and formation of the network over the whole γ-alumina inner pore surface via route B.
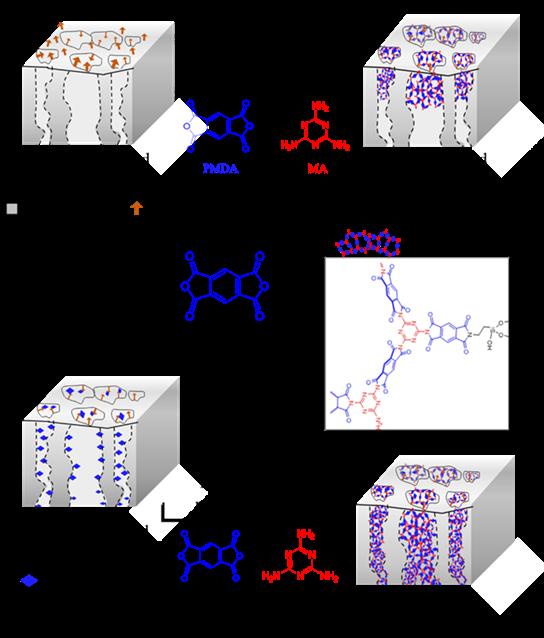
In Chapter 6 a more detailed study is given for the formation of a well-ordered crystalline polyimide (PI) network utilizing only green solvents, a method which can be potentially applied in membrane technology.
Here, a crystalline polyimide was formed in water through pre-organization of the two monomers (pyromellitic acid and melamine) and subsequent precipitation and thermal treatment. Most importantly, it was shown that by employing this method the reaction temperature needed to form a pure polyimide network was below the melting temperature of both monomers used.
In-depth characterization to understand the chemistry involved in the synthesis of organo-functionalized ceramic membranes
Through understanding the chemical make-up of the grafted polymer on the inorganic support, as well as its location, microstructure, crystallinity and reaction mechanisms during membrane synthesis (see e.g., Figure 5), a better understanding of the membrane performance and the stability of the membrane under filtration conditions are obtained. FTIR, due to its simplistic and non-destructive method of analysis, was used throughout the thesis. In some cases, NMR was also used to study the chemical structures of the polymeric layer. Cyclohexane permporometry, again a non-invasive method, was used to investigate the pore size of the inorganic hybrid membranes after various chemical treatments. Even though the method is limited to the molecular size of cyclohexane (0.9 nm), this analysis still provides insights into the pore diameter range of the synthesized membrane samples and is used extensively in this work. Other techniques, such as atomic force microscopy (AFM) and thermogravimetric analysis (TGA), are used as complementary techniques to understand better the materials produced through this work. All these methods together facilitated accurate descriptions of the membranes fabricated and could be used for potentially up scaling the membrane fabrication, and/or improving the materials through adjusting the methods, and finally to develop materials for other research areas.
For example, liquid 1H NMR and water permeability tests were employed as in situ methods to understand the hydrolytic stability of the PEG-grafted samples [7]. Electron microscopy (SEM) was used to visualize the effect of the chemical treatment, such as polymerization, on our ceramic supports. SEM was used to localize the polymer on the inorganic support. Finally, due to the ultrathin thioether-based cross-linked NF layers formed on the support, spectroscopic ellipsometry was used to corroborate the results obtained with SEM analysis to further support the formation of a homogeneous and ultrathin (< 50 nm) thioether-based layer [8].
Elemental analysis, such as energy-dispersive X-ray spectroscopy (EDS) or X-ray fluorescence (XRF) were used as complementary methods for qualitative elemental analysis. For instance, XRF was used to follow the thio-bromo click reaction completion by measuring the relative amounts of bromide on the thioetherbased surface of the support after each reaction step [8]. Finally, EDS was used to study the formation of the PI layer and to understand the influence of the different pre-functionalization steps (resp. route A and B) on the grafting-from method, applied to synthesize the final PI-grafted membranes [9].
Application and performance of organo-functionalized inorganic membranes
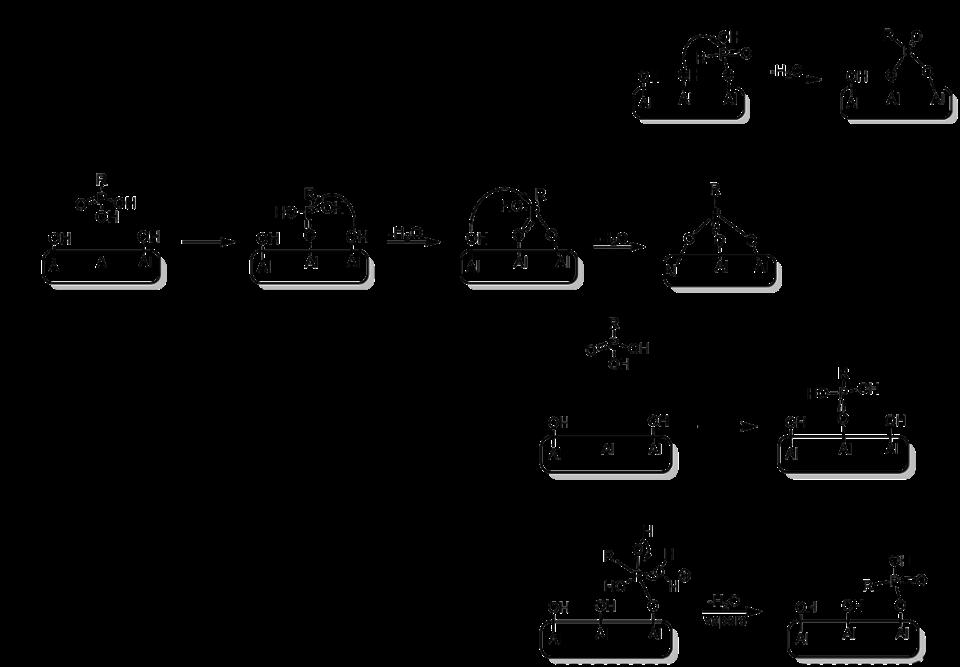
Some results on water permeability (L m-2 h-1 bar-1) and dye retention for these membranes are given in Table 1. In general, the hybrid ceramic membranes prepared in this work resulted in membranes that were stable in water (except when using PEG-alkoxysilane as graft [7]). The higher PEG grafting density obtained with the solid-state reaction, compared with the solutionphase (in water) grafted membranes, was demonstrated with the higher retention of a small organic dye (479 Da) in water compared to both the PEG grafting reaction in water and the pristine γ-alumina membrane.
Figure of the two reaction pathways between organophosphonic acids and γ-alumina: (a) without and with (b) coordination of the phosphoryl group (P=O) on a Lewis acid site (O-Al-O). As water attaches on Lewis acid sites, the proposed reaction mechanism occurring in (c) water should differ significantly from the reaction mechanism occurring in the (d) solid state. The hypothesized mechanisms given in this scheme can be indirectly correlated with the results accumulated from the extensive characterization of the grafted materials
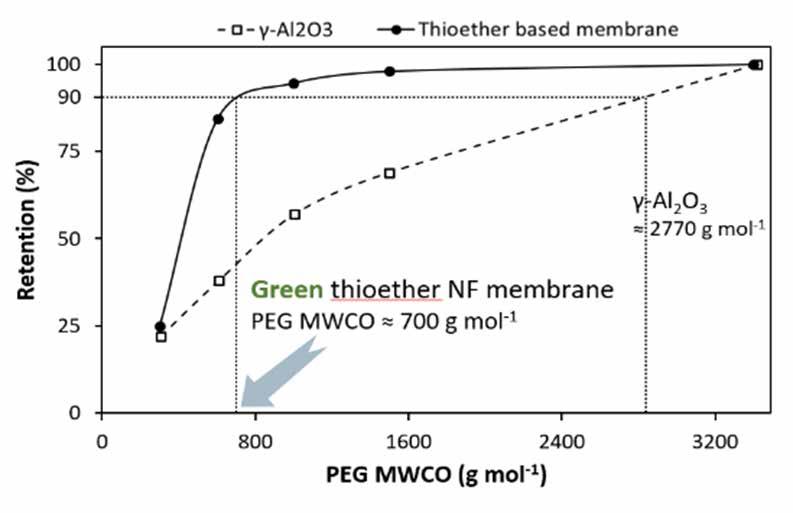
It was demonstrated that the thioether layer exhibits a high chemical stability under a range of conditions, including organic solvents (ethanol, hexane, toluene), extreme pH values (0 or 14), and in sodium hypochlorite solution (10% aqueous) [8]. In addition, the performance (permeability) was not affected by thermal treatment (150 °C for two days). NF tests show permeabilities of 0.6 and 0.5 L m–2 h–1 bar–1 in water and ethanol, respectively. The membrane was found to be impermeable, yet stable, to the apolar solvents toluene and hexane. The potential as a nanofiltration membrane was confirmed through PEG-in-water molecular weight cut-off (≈ 700 g mol–1; Figure 6), and dye retention measurements with two small organic dyes (629 and 479 Da) with the thioether-grafted membranes showing retentions in excess of 90% (Table 1).
The PI nanoconfined membranes were tested in a series of solvents to study their performance as NF membranes [9] In general these membranes showed good performance in water and solvents however in water/solvent mixtures their performance significantly dropped which was attributed to degradation of the polymeric network. It is assumed that the attachment with the surface and between the polymeric particles played a major role in the degradation of the membrane layer. Preliminary results on thermally post-treated PI-grafted membranes showed that the adhesion between the polymeric particles and perhaps the ceramic surface can be enhanced as the retention performance of the thermally treated membranes was significantly improved in different water/solvent mixtures.
*A full copy can be downloaded via: https://doi.org/10.3990/1.9789464690538.
References
[1] Lively, R. P.; Sholl, D. S. ‘From Water to Organics in Membrane Separations’, 2017. https://doi.org/10.1038/ nmat4860.
[2] Drioli, E.; Romano, M. ‘Progress and New Perspectives on Integrated Membrane Operations for Sustainable Industrial Growth’, Industrial and Engineering Chemistry Research. American Chemical Society March 7, 2001, pp 1277–1300. https://doi.org/10.1021/ie0006209.
[3] Marchetti, P.; Solomon, M. F. J.; Szekely, G.; Livingston, A. G. ‘Molecular Separation with Organic Solvent Nanofiltration: A Critical Review’, Chem. Rev. 2014, 114, 10735–10806. https://doi.org/10.1021/cr500006j.
[4] Van der Bruggen, B.; Curcio, E.; Drioli, E. ‘Process Intensification in the Textile Industry: The Role of Membrane Technology’, J. Environ. Manage. 2004, 73 (3), 267–274. https://doi. org/10.1016/j.jenvman.2004.07.007.
[5] Buekenhoudt, A.; Beckers, H.; Ormerod, D.; Bulut, M.; Vandezande, P.; Vleeschouwers, R. ‘Solvent Based Membrane Nanofiltration for Process Intensification’, Chemie Ing. Tech. 2013, 85 (8), 1243–1247. https://doi.org/10.1002/ cite.201200247.
[6] Peeva, L.; Burgal, J. da S.; Valtcheva, I.; Livingston, A. G. ‘Continuous Purification of Active Pharmaceutical Ingredients Using Multistage Organic Solvent Nanofiltration Membrane Cascade’, Chem. Eng. Sci. 2014, 116, 183–194. https://doi. org/10.1016/j.ces.2014.04.022.
[7] Kyriakou, Nikos; Pizzoccaro-Zilamy, Marie-Alix; Nijmeijer, Arian; Luiten-Olieman, Mieke; Winnubst, Louis ‘Hydrolytic Stability of PEG-grafted γ-Alumina Membranes: Alkoxysilane vs Phosphonic Acid Linking Groups’, Microporous and Mesoporous Materials, 2020, 307, 110516. https://doi. org/10.1016/j.micromeso.2020.110516. (Thesis: Chapter 2).
[8] Kyriakou, Nikos; Merlet, Renaud B.; Willott, Joshua D.; Nijmeijer, Arian; Winnubst, Louis; Pizzoccaro-Zilamy, MarieAlix ‘New Method toward a Robust Covalently Attached Cross-Linked Nanofiltration Membrane’, ACS Applied Materials & Interfaces, 2020, 12, 47948-47956. DOI: 10.1021/ acsami.0c13339. (Thesis: Chapter 4).
[9] Kyriakou, Nikos; Winnubst, Louis; Drobek, Martin; De Beer, Sissi; Nijmeijer, Arian; Pizzoccaro-Zilamy, Marie-Alix ‘Controlled Nanoconfinement of Polyimide Networks in Mesoporous γ-Alumina Membranes for the Molecular Separation of Organic Dyes’, ACS Applied Nano Materials 2020, 4, 14035-14046. DOI: 10.1021/acsanm.1c03322. (Thesis: Chapter 5).









