





Introducing Pharma-Sterile Water — the final touch in your cleanroom cleaning routine. Formulated for use in USP <797> environments, this sterile, 0.1 micron filtered, non-pyrogenic water is the ideal follow-up rinse after cleaning agents or disinfectants have done their job. It’s not just water — it’s your last line of defense against residue, contamination, and compliance gaps.
Pharma-Sterile Water can help you remove residual disinfectants and particles that could compromise your air quality or drug prep surface.
Contact us today for pricing or samples! info@acutecareonline.com 1-888-909-7700 www.pharma-choice.com





















































As the healthcare landscape grows more complex, health system pharmacies are being called upon to do more; serve more patients, manage more data, ensure compliance with evolving regulations, and deliver more value across the care continuum.
At RXinsider, we understand the dynamic challenges and opportunities shaping your work. That’s why, in this edition of 20Ways, we’re proud to present 20 distinct product and service profiles, insightful case studies, and timely editorials, all thoughtfully curated to support the priorities of health system pharmacy professionals.
Our central theme for this issue focuses on 340B Solutions and 340B Management Systems, an essential topic for hospitals committed to maximizing program benefits while ensuring compliance and transparency. As 340B continues to evolve under increased scrutiny and shifting policy landscapes, the need for integrated tools and smart systems has never been more urgent. We also dive deeper into two critical conversations reshaping the pharmacy space. One editorial examines the growing role of automation in the hospital setting and how innovations in robotics, AI, and software platforms are helping pharmacies increase efficiency, reduce error rates, and free up staff for more clinical functions. It’s a powerful look at what’s next — and what’s already here for pharmacy operations. The second explores an equally urgent and critical issue: drug diversion. With rising incidents and increasing awareness, this piece offers a clear-eyed view into the tools and strategies used to prevent diversion, protect staff and patients, and ensure the integrity of pharmaceutical care in our institutions.
At the heart of it all, 20Ways is built to inform, inspire, and equip. Whether you’re refining a 340B strategy, researching new technologies, or simply looking for a better way to deliver care, we hope this issue offers value and maybe a few ideas you haven’t yet considered.

Thank you for your commitment to excellence in pharmacy.
FOUNDER & CEO
Gregory Cianfarani, RPh
DESIGN & PRODUCTION
Design & Layout, Lora Bourque
Multimedia, Eric Simmons
MARKETING & OPERATIONS
Director of Marketing & Operations, Samantha Roy
Marketing & Operations Coordinator, Amanda D’Amico
Credit Analyst & Bookkeeper, Kristin Fennessey
SALES & BUSINESS DEVELOPMENT
EVP, Sales & Marketing, Mike Rahme
VP of Strategic Accounts, Chris Kolkhorst
Executive Sales Director, Shaun Russell
Senior Account Executive, Savannah DaSilva Account Executive, Jake Studley
PHARMACY MARKET INTELLIGENCE
Marketing Manager, Alexa DiLuca
Pharmacy Market Analyst, Lexi Cianfarani
Pharmacy Market Analyst, Michael McEwen
Pharmacy Market Analyst, Emma Cidade
Pharmacy Market Analyst, Jeff Rackliff
EDITORIAL ADVISORY BOARD
Miriam Cho, PharmD
President & Chief Pharmacy Officer
MacRx
Brenda Denson, Pharm.D., BCPPS, FASHP, FALSHP
Pharmacy Educator
Children’s of Alabama
Lindsey Dymowski Constantino
President & Cofounder
Centennial Pharmacy Services & LTC@Home Pharmacy Network
Mark Garofoli, PharmD, MBA, BCGP, CPE, CTTS
Director of Experiential Learning & Clinical Assistant Professor West Virginia University of Pharmacy
Steve Postal, JD
Senior Director, Policy and Regulatory Affairs
National Community Pharmacists Association
Abby Roth Owner/Microbiologist Pure Microbiology
FOLLOW US
LinkedIn @RXinsider
Facebook /RXinsiderB2BSolutions X @RXinsider
Instagram @rxinsider
Vimeo /rxinsider

Greg Cianfarani, RPh Founder & CEO
End-to-end consulting & technology solutions for hospital systems designed to reduce staff burden, improve multi-site visibility, and support an exceptional patient experience beyond hospital walls.

Let’s deliver better, together www.d2rx.com | connect@d2rx.com
Backed by centuries of experience from the clinic floor to the board room, D2 offers solutions designed specifically for the needs of hospitals and hospital systems. As the demand for home infusion grows, D2 is the trusted partner offering support for:
• Program Development
• Payer Contacting & Pharma Access
• Ambulatory Infusion Center Migration
• Specialty & Infusion Contracting Support (Prescription and Medical Benefit)
• Achieving Accreditation(s)
• Navigating State-Specific Home Health & Home Infusion Pharmacy Licenses
• Multi-Site Compliance, Regulatory and Legislation Management
• Patient Services, Engagement & Onboarding
• Custom, System-Specific Needs

340B SOLUTIONS, 340B MANAGEMENT SYSTEMS
340B solutions, 340B management systems industry expert Q&A, Market’s Leaders Buyer’s Guide, and more. page 28
DRUG DIVERSION IN HOSPITAL PHARMACIES
An overview of how controlled substances are illegally redirected in hospital settings, why these incidents are more common than many realize, and the risks they pose to patient safety, staff integrity, and institutional trust. page 22
AVOIDING COSTLY MEDICATION ERRORS
How automation is helping improve care at Texas Children’s Hospital. page 38
DATA INTEGRATION LEADS TO EFFICIENT PHARMACY INVENTORY MANAGEMENT AT NEWYORK-PRESBYTERIAN BROOKLYN METHODIST HOSPITAL
A case study from Swisslog Healthcare. page 56
INDIANA UNIVERSITY HEALTH: CHALLENGES & SOLUTIONS TO CATEGORY 3 COMPOUNDED MEDICATIONS
A case study from AIS Healthcare. page 74

MOHAWK VALLEY HEALTH SYSTEM: DELIVERING A SEAMLESS CARE EXPERIENCE THROUGH SPECIALTY PHARMACY
A Case Study from CPS with Christopher Houle, PharmD, BCPS, BCGP, CDCES, Director of Pharmacy, and Nicolina Castilla, PharmD, Director of Onsite Operations, General Manager page 44
USP <800> RESOURCES
Leading pharmacy suppliers of USP <800> compliant solutions. page 14
TRADE SHOW & MEETING EVENT CALENDAR
Detailing key industry events throughout the year. page 66-69

• ULPA filter, creating ISO 3 workzone, cleaner than standard ISO 5
• ECM blower, creating stable airflow at energy-efficient 150 Watts
• Comfortable low noise at 55 dBA, full legroom, dished work tray



• 7” Touchscreen Controller With built-in user guide, and 21 CFR Part 11 compliance
• ULPA filter, creating ISO 3 workzone, cleaner than standard ISO 5
• Comfortable low noise at 58 dBA, dished work tray, builtin armrest
• Lowest height in the market at 55”, suitable for cleanroom

Class I Ventilated Enclosure
• Two HEPA filter in series, allowing exhaust to room instead of ducting
• Comfortable low noise at 57 dBA and full-width front access opening
• Dished phenolic resin worktop, preventing spill, scratch, and corrosion







• Customizable lead thickness to your application
• Integrated Dose Calibrator, Generator, and Waste compartments
• Sliding Lead Lined Chest Shield
• Suitable for Nuclear Medicine and Theragnostics


VERITY SOLUTIONS — THE FUTURE OF HIGH-PERFORMING HEALTHCARE IS HERE
We serve all healthcare facilities and 340B covered entities by combining innovative technology and best-in-class partnership to optimize pharmacy supply chain outcomes for better financial results and patient care. page 19
LEITERS HEALTH — ELEVATING THE STANDARDS IN 503B PHARMACEUTICAL OUTSOURCING
Leiters Health is a trusted FDA-registered 503B outsourcing provider of ready-to-administer compounded sterile preparations committed to providing healthcare professionals and their patients with the highest-quality medications. page 21
CUSTOM-ENGINEERED WALK-IN REFRIGERATORS AND FREEZERS FROM HELMER SCIENTIFIC
Custom walk-in cold storage provides precision temperature control for large-scale and highly specialized solutions for pharmacy applications like consolidated pharmacy service centers (CPSCs) and specialty pharmacy distribution centers. page 25
REPLENISHMENT HASSLES ARE HISTORY WITH STAT STOCK ® FROM HEALTH CARE LOGISTICS ®
Take the guesswork out of drug processing with this exclusive RFID-based inventory replenishment system that allows users to instantly identify and locate expired or recalled medication, then remove or replenish items accordingly. page 27
INDEPENDENT 340B AUDITS BY VIRTUE 340B — CLARITY, COMPLIANCE, CONFIDENCE
Virtue 340B provides independent 340B audits and expert consulting to help pharmacies strengthen compliance, reduce risk, and maximize program integrity with confidence and clarity. page 33
VISANTE – HIGH-PERFORMING PHARMACY SOLUTIONS FOR HOSPITALS AND HEALTH SYSTEMS
Our mission is to transform healthcare through pharmacy. We help hospitals and health systems optimize pharmacy operations, improve financial performance, and deliver better patient care. page 35
WITH VACCINE STORAGE, THERE IS NO ROOM FOR ERROR
Follett high-performance NSF 456-Certified refrigerators provide precise temperature control for safely storing vaccines and medications. page 37
SIMPLIFI+ ® PHARMACY COMPLIANCE AND SENTRI7 ® CLINICAL SURVEILLANCE — WOLTERS KLUWER
Simplifi+ and Sentri7 applications on one powerful SoleSource® platform help standardize clinical, pharmacy, and compliance programs. Customers access evidence-based content, tailored workflows, and robust analytics to improve patient care, quality, and safety. page 41
DRIVE FINANCIAL GROWTH WITH SCRIPTPRO’S END-TO-END PHARMACY SOLUTIONS
Make pharmacy the strongest link in your health system with ScriptPro — advancing care, accuracy, and operational success. page 43
NOVUM IQ INFUSION PLATFORM FROM BAXTER IS A PUMP PLATFORM LIKE NO OTHER
At every step, the Novum IQ Infusion Platform with Dose IQ Safety Software helps you build and deploy one of the safest and most effective drug libraries possible for large volume, syringe, and enteral infusions. page 51
























ISO-MED — YOUR ASSURANCE FOR TOP-QUALITY PRODUCTS
As one of the top leading suppliers for pharmacy cleanroom supplies, we provide an unparalleled selection of specialized medical products, globally trusted quality and service, and delivery convenience for meeting USP <797> and USP <800> standards. page 53
CUSTOMIZABLE PRIMARY ENGINEERING CONTROLS FROM NUAIRE
Prepare compounded preparations comfortably and efficiently by configuring your laminar airflow workbench, containment ventilated enclosure, or biosafety cabinet around your pharmacy’s specific workflow. page 55
MEDI-DOSE, INC./EPS, INC. DELIVERS BAR CODING, PACKAGING, AND LABELING PHARMACY SOLUTIONS
Improve your Solid Oral Unit Dose needs with our comprehensive Bar Coding, Packaging, and Labeling Solutions — designed by healthcare professionals, for healthcare professionals. page 61
SURECOST – SMARTER PURCHASING & INVENTORY MANAGEMENT FOR PHARMACIES
SureCost is The Smarter Purchasing Solution™ for health system pharmacies — automating purchasing and inventory workflows across departments and facilities in a single platform. Pharmacies reduce purchasing time by 50% and lower cost of goods by 2-5% without price shopping. page 63
EAGLE ANALYTICAL — YOUR LABORATORY PARTNER FOR SCIENCE-BASED SOLUTIONS
Eagle provides expert scientific solutions, including analytical chemistry, microbiology testing, and R&D services. We specialize in validations, regulatory consulting, facility design, and cleanroom certifications — ensuring precision, compliance, and efficiency for your success. page 65
Q.I. MEDICAL, INC.™ YOUR PARTNER IN USP <797> PHARMACY COMPLIANCE
Providing quality assurance products to hospitals and pharmacies practicing sterile compounding, with a singular focus on pharmacy compliance. Our long-standing regional distribution partners are able to provide exceptional in-person service and training. page 71

17 WAY 18 WAY 19 WAY 20 WAY
STERI-TAMP ® TAMPER-EVIDENT STERILE SEALS BY ALLIED PHARMACY PRODUCTS, INC.
The only single-use, tamper-evident seal that provides and maintains a 100% sterile barrier. page 73
TRUDELIVERY ® — READY-TO-USE (RTU) PRODUCTS BY ENDO
The TruDelivery RTU portfolio helps meet the real-world challenges of hospital pharmacies — and is always ready when you are. It’s about time. page 79
CHARLES RIVER ENDOSAFE ® — BACTERIAL ENDOTOXIN TESTING (BET) WITH CARTRIDGE TECHNOLOGY
With Endosafe cartridge technology, any user can generate rapid, automated BET results in about 15 minutes, minimizing manual effort while aligning with regulatory standards and corporate sustainability goals. page 81
SMARTPAK ® BY SAMSON MEDICAL TECHNOLOGIES, L.L.C
The innovative, easy-to-use packaging system ideal for large volume sterile product preparation. page 83

20Ways MISSION
To educate pharmacy management on products and services that serve to improve patient care or improve a pharmacy’s financial bottom line, while distilling and presenting this relevant information via 20 product profiles.
VIEW 20Ways ONLINE
RXinsider.com/20Ways
GENERAL SUBSCRIPTION INQUIRIES
RXinsider LTD
c/o 20Ways
1300 Division Road, Suite 103 West Warwick, RI 02893
General Information: 20Ways@RXinsider.com
20Ways is an official publication of RXinsider.
NOTICE OF POLICY
20Ways is published quarterly by RXinsider LTD, 1300 Division Road, Suite 103, West Warwick, RI 02893. Postmaster: Send address changes to 20Ways/RXinsider, 1300 Division Road, Suite 103, West Warwick, RI 02893. Notification of address change must be made six weeks in advance, including old and new address with zip code.
Editorial — views expressed in articles or profiles in the 20Ways are those of the author(s) and do not necessarily reflect the policies and opinions of RXinsider, our editorial board(s), our advisory board(s), or staff. Advertising – products, services, and educational institutions advertised in 20Ways do not imply endorsement by RXinsider.
Copyright © 2025 RXinsider LTD. All rights reserved. Reproduction without permission is prohibited.

The Corepoint® Scientific Blood Bank Refrigerators deliver consistent, validated performance— because your patients depend on it.

Temperature fluctuations can mean the difference between safe, viable blood and wasted inventory. Many refrigeration systems struggle with uniformity.
Blood banks require unwavering temperature control, regulatory compliance, and reliable performance to ensure the integrity of lifesaving blood products. We understand the pressure of maintaining precise conditions in a demanding environment. That’s why our refrigerators aren’t just built to store blood; they’re engineered to protect it.
Not every refrigerator meets blood bank requirements. If our solution isn’t the best fit, we’ll help guide you to one that is. Long-term trust matters more than a single sale.
Blood Bank Refrigerators from Corepoint® Scientific deliver consistent, validated performance. Your blood supply is too important to risk- let’s get it right.
; Maintain Tight Temperature Uniformity –±0.7°C precision ensures stable storage
; Meet Regulatory Compliance – AABB, FDA, 21CFR Part 820, ETL, cETL, and EPA SNAP compliant
; Enhance Sample Security – Alarm systems, advanced monitoring, and battery backup
; Maximize Storage Efficiency – Stainless steel sliding drawers hold up to 800+ blood bags


















Dexcom G7 is clinically proven to improve glycemic outcomes1-5
>1% A1C reduction after 12 weeks1,2,5
>1 hour increase in time in range for T1D and T2D after 12 weeks1,2

Features to reduce rebound hyper- and hypoglycemia6-8
35% (~$358 PPPM) reduced diabetesrelated inpatient admission costs9 ~50% reduced diabetesrelated inpatient visits10
To learn more about the Dexcom hospital discharge program, scan the QR code
CGM = continuous glucose monitoring; PPPM = per patient per month; T1D = type 1 diabetes; T2D = type 2 diabetes.

BRIEF SAFETY STATEMENT: Failure to use the Dexcom G7 Continuous Glucose Monitoring System (G7) and its components according to the instructions for use provided with your device and available at https://www.dexcom.com/safety-information and to properly consider all indications, contraindications, warnings, precautions, and cautions in those instructions for use may result in you missing a severe hypoglycemia (low blood glucose) or hyperglycemia (high blood glucose) occurrence and/or making a treatment decision that may result in injury. If your glucose alerts and readings from the G7 do not match symptoms, use a blood glucose meter to make diabetes treatment decisions. Seek medical advice and attention when appropriate, including for any medical emergency. 1 Beck RW, et al. JAMA. 2017;317(4):371-378. 2 Beck RW, et al. Ann Intern Med. 2017;167(6):365-374. 3 Martens T, et al. JAMA. 2021;325(22):2262-2272. 4 Laffel LM, et al. JAMA. 2020;323(23):2388-2396. 5 Welsh JB, et al. J Diabetes Sci Technol. 2024;18(1):143-147. 6 Dexcom G7 User Guide, 2023. 7 Acciaroli G, et al. J Diabetes Sci Technol. 2022;16(3):677-682. 8 Wilmot E. Dexcom G7: Unique Features and Correlational Improvements in Glycemic Control. Presented at the 16th International ATTD Conference on February 23, 2023. 9 Norman GJ, et al. Diabetes Technol Ther. 2022;24(7):520-524. 10 Hannah K, et al. Diabetes. 2023;72(Suppl 1):991-P. Dexcom and any related logos and design marks are either registered trademarks or trademarks of Dexcom, Inc. in the United States and/or other countries. ©2024 Dexcom, Inc. All rights reserved. MAT-4717


REUSABLE MOP SYSTEMS




REUSABLE CLEANROOM HOOD MOPS & FRAMES




• HOODS
• COVERALLS
• BOOTS
• PROCESS & PACKAGED MASKS
• FROCKS
• GOGGLES
• INNERWEAR
• SLEEVE COVERS
• HOOD MOPS













CEO: George Puckett
Founded: 2015
Employees: 160
Toll-Free Phone: (800) 581-1378
Phone: (425) 947-1922
We serve all healthcare facilities and 340B covered entities by combining innovative technology and best-in-class partnership to optimize pharmacy supply chain outcomes for better financial results and patient care.

Address: 12131 113th Avenue NE, #200 Kirkland, WA 98034
Website: www.verity340b.com
Verity Solutions has been the leader in 340B program administration and now offers pharmacy supply chain optimization solutions for all healthcare entities. Recognized as Best in KLAS: 340B Management Systems for six years, and in the 2024 KLAS Emerging Solutions Top 20 Report, we are on a mission to create pharmacy excellence through predictable automation and outstanding support so that every customer can maximize their savings. We partner with integrated healthcare systems, acute-care hospitals, community health centers, federally qualified health centers, pharmacies, and 340B-eligible covered entities throughout the U.S. who rely on Verity software and services to successfully manage their pharmacy purchasing and 340B program.
Our powerful cloud-based software platform provides comprehensive 340B program management solutions, including split billing, contract pharmacy, and specialty contract pharmacy. Plus, a contract pharmacy gateway solution called VHUB®
Our pharmacy purchase optimization products offer real-time, intelligent solutions that automatically verify contract pricing and select the best priced NDC available for your complete order. We enable pharmacy buyers to dramatically decrease drug spend and save time while maintaining supply.
Our depth of in-house technical and software development resources and our highly skilled account management team are closely aligned to swiftly adapt as changes arise. We offer:
• Agile Software Platform: Built and deployed with security, performance, scalability, and agility as primary goals.
The Verity 340B platform is HITRUST® certified, demonstrating robust HIPAA compliance.
• Intuitive Application: Designed with our users in mind, we maintain ongoing feedback and collaboration with our clients. This collaboration steers our continual software and services development
• Responsive Support: Designated account managers provide focused support, training, audit readiness, and regular business reviews to maximize your 340B program success and help you maintain compliance.
We continually invest in our technology and people to ensure success for our clients. With increasing pharmacy costs and complexity, it is more important than ever to have the right solutions — and the right partner.
• Industry-leading core functionality of our platform with optional, patent-pending add-on modules to enhance all aspects of 340B operations in challenging and unique environments.
• Access to your own unique program test environment — before and after implementation. Test environment runs continually in parallel to your live system.
• Easy and exportable reporting functionality, including detailed data for manufacturer audits, HRSA audits, and UDS reporting.
• Multiple vendor support with controlled substances ordering system (CSOS) — efficiently place orders with both EDI (electronic data interchange) and non-EDI vendors.
• Two-week sprint release cycles ensure timely software updates driven by customer feedback, regulatory changes, and user needs.
• Verity Care Card Program — directly pass 340B savings to uninsured and underinsured patients.
• Referral capture opt-in functionality to compliantly add meaningful savings lift to your 340B program.
• Responsive customer service provided by our in-house staff — 80% of our customer support cases are resolved within two hours; 95% resolved within 24 hours.
With increasing drug shortages and supply chain disruptions, access to quality medicine is more important than ever.
Leiters Health is an FDA-registered 503B outsourcing provider of high-quality compounded sterile preparations and pharmacy services including:


Pre-filled syringes and IV bags; pre-labeled and ready to administer. ON-Q* Pain Relief System Pharmacy Fill Service. Opioid-free surgical pain services medications.
Ophthalmic medications including FDAcompliant repackaged Avastin
Contact us to learn how we can support your hospital pharmacy and your patients.

Leiters.com | 800.292.6772 | info@leiters.com
Leiters Health is a trusted FDA-registered 503B outsourcing provider of ready-toadminister compounded sterile preparations committed to providing healthcare professionals and their patients with the highest-quality medications.
President & CEO: Paul Granberry
Founded: 1926
Employees: 375
Toll-Free Phone: (800) 292-6772
Phone: (720) 697-5140
Fax: (408) 288-8252
Address: 13796 Compark Boulevard Englewood, CO 80112
Website: www.leiters.com
Leiters Health, founded in 1926, is an FDA-registered 503B outsourcing provider of high-quality compounded sterile preparations and pharmacy services. We have a long history of evolving and innovating to meet the latest regulatory requirements and market needs.
All sterile preparations are produced under Section 503B of the FD&C Act (503B Guidance), follow Current Good Manufacturing Practices (cGMP) and USP <797>. Our facility consistently upholds all standards based on the audits conducted by the FDA, states of California and Florida Boards of Pharmacy, multiple health systems, group purchasing organizations, and other independent accreditation organizations.
Our team of experts in sterile pharmaceutical manufacturing, repackaging, and compounding provide a sophisticated understanding of what it takes to elevate the quality and consistency of supply in pharmaceutical outsourcing. We combine a highly experienced team, with robust processes, in state-of-the-art outsourcing facilities to ensure delivery of the highest quality compounded medications and services.
As part of our ongoing commitment to quality, compliance, and sterile manufacturing excellence, we continue to invest in our state-of-the-art 503B facilities, including two existing facilities in Denver, Colorado. We stand ready to support the needs of the healthcare community now and in the future.
Leiters Health provides ready-to-use compounded sterile preparations and pharmacy services across the continuum of healthcare, including hospitals, surgery centers, physician offices, and clinics. Hospital and Surgery Center Products and Services
• Prefilled syringes, vials, and bags.
• Ready-to-administer pain services portfolio to support ERAS and opioid reduction strategies.
• Pharmacy fill service for the Avanos Medical opioid sparing ON-Q* Pain Relief System.
• Prefilled syringes, vials, and dropper bottles including injections, antibiotics and dilating agents.
• FDA-compliant repackaged Avastin® service.
Leiters Health is partnered with many market leading innovative healthcare companies that complement what we do. The products and services offered by these companies may provide additional value to your organization. Our key business partners include Angels for Change, Avanos Medical, Besse Medical, Bluesight, Cardinal Health, End Drug Shortages Alliance, Eye Connect International, Prodigy Health, and The Academy.
Leiters Health provides our products and services to many leading health systems, community hospitals, clinics, and physician offices. In addition, we currently have national contracts with: Cardinal Health Acuity, EyeProGPO, HealthTrust Performance Group, International Physicians Network (IPN), Kaiser Foundation Health Plan, Premier Healthcare Alliance, The Resource Group, Vizient Supply, Vizient Captis Health, and Vizient Pharmacy Aggregation Group. Please contact us for additional information regarding our contracts.
Leiters Health works closely with our 10 strategic health system investors to evaluate, create, and deploy solutions that enable pharmacy efficiencies. Pharmacy leaders from each of our investor health systems hold a seat on the Leiters Health Strategic Pharmacy Advisory Board. We meet with this group regularly to understand and nimbly respond to pharmacy challenges with real-world, innovative solutions. This allows us to offer a meaningful portfolio across the continuum of care to all of our customers and ultimately ensure that healthcare providers and their patients have a continuous supply of critically needed compounded medicines.
We don’t want to simply tell you about what we do, we want to show you! We invite you to visit our facility to better understand the cGMP regulations, sterile manufacturing processes, and automation we use to elevate the quality of our products and services. To schedule a site visit please visit www.leiters.com.

What is drug diversion in hospital pharmacies, and why is it a serious problem?
Drug diversion is the unlawful channeling of regulated medications from legal sources to the illicit market and its prevention is a serious consideration in any setting. When one building (a hospital) has a rather impressive continual supply of controlled substances (mind you, medications that enable surgeries, and manage the intense pain of so very many patients), and humans are involved (whether patients, strangers, or even employees), there will be concerns for diversion prevention.
How can drug diversion monitoring software help detect and prevent incidents?
Drug diversion monitoring software plays a crucial role in detecting and preventing the unauthorized use or even theft of controlled substances in healthcare settings. It is multi-faceted in that the software will typically track and analyze data to identify anomalies; provide real-time alerts; provide audit trails and compliance reporting conduct behavioral analyses; and sometimes even provide education and resources for the team. In essence, drug diversion monitoring software acts as a safeguard, using data-
driven insights to minimize risks and ensure medications are used appropriately, protecting both patients and healthcare institutions.
What role do opioid education platforms play in preventing diversion and misuse?
The prominent pain management interprofessional conference, PAINWeek, has long utilized the mantra “Education is the Best Analgesic”, and upon reflection, one can really digest that concept into the realization that without administration, clinician, and patient education, any one treatment may not have the most positive outcomes possible, let alone when factoring in drug diversion prevention.
Opioid education platforms play a crucial role in preventing diversion and misuse by raising awareness, enhancing knowledge, and promoting responsible practices among patients, healthcare providers, and the community alike. Impact is observed across key opportunities in opioid stewardship including safe prescribing practices (including a diverse treatment plan), proper medication handling, recognizing and responding to misuse, and even public awareness.
Ultimately, by providing accessible and accurate information and fostering a culture of accountability, opioid education platforms
serve as an accomplice to clinicians as frontline defenses against the misuse and diversion of opioids.
How can community outreach technology help combat drug diversion and opioid misuse outside the hospital?
It takes a village! Community outreach technology plays an important role in combating drug diversion and opioid misuse outside the hospital by enhancing education, prevention, early intervention, and support. Mobile apps, websites, and social media campaigns can disseminate information about opioid risks, proper medication disposal, and recognizing signs of misuse. Telehealth platforms can connect at-risk individuals with counselors, addiction specialists, and support groups, reducing barriers to seeking help. Community data collection tools and predictive analytics can help identify geographical pockets of opioid misuse, directly guiding targeted interventions and resource allocation in a timely manner. Systems can provide automated alerts to warn communities about local overdose trends and other drug-related public health concerns.
What are the latest controlled substance diversion prevention technologies used in hospitals?
Artificial intelligence powers analytics on closed-loop tracking systems including automated dispensing cabinets (ADCs) with access control provide continual surveillance and monitoring tools that are very valuable to have specialized compliance and drug diversion prevention teams continually assess. It’s one thing to discover a theft from three months ago, it’s another to discover the same while the thief is still walking in the hospital hallway. These systems can even analyze human (employee) behavior over time to identify any potential concerning patterns associated with diversion such as frequent access to controlled substances at irregular working hours, which may not be a problem, yet should be mitigated.
What best practices can hospitals implement to prevent drug diversion in addition to technology?
The technology we’ve discussed can be great but is nothing without us humans steering the ship. Specialized experts should
(or any setting), rather when. Time is of the essence in reporting drug diversion cases, minimally to prevent patient harm and continued diversion. Most humans, however, do not like conflict, or anything resembling it. Clinicians are well served to remember that there is more than a criminal side to drug diversion mitigation, as there

be identified (and hired) to lead these efforts across entire hospital systems, or even in local rural small hospital. Compliance and drug diversion prevention leaders can help to mold the culture within an institution in respect to diversion prevention through means including policy and procedure development, staff screening and support, and diversion prevention committees, to ultimately create a culture of accountability and awareness.
Why is reporting critical in drug diversion cases, and how should hospitals approach it?
It’s not a question of whether drug diversion will happen in a hospital setting
will undoubtedly, and hopefully, be eventual compassionate help for someone including substance use disorder (addiction) treatment and the providing of resources when the situation involves those needs. It’s all about preventing continued harm, whether perceived or actual.
After all, an ounce of prevention is worth a pound of treatment, and perhaps a kilo of inevitable diversion.
Contributed
by
Mark Garofoli, Clinical Associate Professor, Director of Experiential Learning, & Clinical Pain/Addiction Pharmacist at
WVU School of Pharmacy

Large-scale and highly-specialized solutions for pharmacy applications.


• Precision temperature control for central pharmacies and CPSCs, specialty pharmacy distribution centers and pharmacy automation systems
• Completely flexible: use in retrofits and new construction
• Fit specific use cases and overcome physical constraints of modular units
• Meet tight environmental specifications
Custom walk-in cold storage provides precision temperature control for largescale and highly specialized solutions for pharmacy applications like consolidated pharmacy service centers (CPSCs) and specialty pharmacy distribution centers.
President & CEO: Matt Barga
Employees: 425
Stock Symbol: TT
Toll-Free Phone: (800) 743-5637
Phone: (317) 773-9073
Address: 14400 Bergen Boulevard Noblesville, IN 46060
Website: www.helmerinc.com
Helmer Scientific, now part of Life Science Solutions, a business unit of Trane Technologies, is a U.S.-based manufacturer and worldwide distributor of medical-grade cold storage and laboratory processing equipment. We have over 45 years of experience providing high-quality temperature-controlled environments, with our products being used in over 125 countries. Precise temperature performance and control are essential to the successful storage of pharmaceuticals, and Helmer cold storage products are designed and developed with these considerations.
Integration into the Life Science Solutions business has expanded our portfolio of innovative products and services to include large-scale reachin cold storage for biopharmaceutical manufacturing, custom-engineered walk-in and drive-in refrigerators and freezers for healthcare and life science applications, and flexible service programs for maintenance and upkeep of equipment.
Helmer remains committed to developing innovative, reliable, and sustainable cold storage solutions from 5'3" undercounter refrigerators and freezers to football field-sized walk-in and drive-in units for clinical applications including central pharmacies, specialty pharmacy distribution centers, consolidated pharmacy service centers, retail pharmacies, pharmacy logistics, and more.
Custom-engineered walk-in refrigerators and freezers provide precision temperature control for large-scale pharmacy applications like consolidated pharmacy service centers (CPSCs) and specialty pharmacy distribution centers, and unique or highly specialized applications like encapsulation of pharmacy dispensing robots and integration into pharmacy automation systems.
Custom-engineered walk-ins are completely flexible and can be used in both retrofits and new construction projects to fit extremely specific use cases and to overcome physical constraints that modular units cannot.
With temperature ranges from -80°C to +40°C, custom-engineered walk-in solutions can be designed for almost any use case. Walk-in refrigerators and freezers are commonly used for bulk storage of vaccines and medications
and integrating cold-storage into pharmacy automation systems in central pharmacies and CPSCs, storage of high-value medications in specialty pharmacy distribution centers, and bulk storage of phase change material (PCM) or gel packs for shipment and distribution of medications.
Our team leverages deep-application expertise to design and deliver walk-in systems engineered to meet tight environmental specifications required to maintain cold-chain integrity. We partner throughout the project process for planning the solution, installation and setup, validation services, and training and technical support.
Whether building a new facility or upgrading an existing one, we partner to provide professional engineering, design, and drafting, and end-to-end planning and installation capabilities (engineering and project management). After installation, we provide scalable nationwide maintenance plans and remote preventative and predictive monitoring to ensure that your walk-in continues to operate at peak performance.
If walk-ins are not feasible for your pharmacy’s footprint, Life Science Solutions also manufactures premium medical-grade reach-in cold storage. Pharmacy GX Solutions refrigerators and freezers are designed and tested to deliver world-class temperature performance Helmer customers have come to trust from the leading manufacturer of pharmacy cold storage equipment.
n Standard NSF/ANSI 456 Certification
All Pharmacy GX Solutions refrigerators include NSF/ANSI certification at no additional charge, ensuring that valuable vaccines are safely and properly stored.
n Efficiency and Sustainability
In addition to pharmacy-specific design, GX Solutions refrigerators and freezers support sustainability initiatives while meeting the performance demands of a busy pharmacy.
• ENERGY STAR® certified.
• Utilize low-GWP natural hydrocarbon refrigerants with zero ozone depleting potential.

• Meet many energy standards including the EPA SNAP program.
n Sizes for Any Footprint
From 5'3" models that fit under ADA compliant countertops to 58'3" pass-thru units perfect for USP <797> sterile compounding environments, there’s a GX Solutions pharmacy refrigerator for any application and footprint.






































Take the guesswork out of drug processing with this exclusive RFID-based inventory replenishment system that allows users to instantly identify and locate expired or recalled medication, then remove or replenish items accordingly.
President & CEO: Gary Sharpe
Founded: 1978
Employees: 300+
Toll-Free Phone: (800) 848-1633
Phone: (740) 477-3755
Address: P.O. Box 25
Circleville, OH 43113
Website: GoHCL.com
What began in 1978 as a garage-based business at the home of Health Care Logistics Owner Gary Sharpe now encompasses five well-equipped facilities in central Ohio and reaches customers around the globe. Employment has grown too, with more than 300 employees now dedicated to the company’s mission of providing value with every interaction.
Sharpe discovered early in his career the need for healthcare products in sizes, quantities, and materials not readily available and was determined to deliver. And has he ever.
Today HCL®’s inventory includes more than 9,000 different products all designed to provide “Special Answers to Special Problems.” Most of these items are maintained locally in more than 320,000 square feet of warehouse space and meet the supply needs of hospitals, pharmacies, chain drug stores, pharmaceuticals, and many other facets of healthcare.
Drug expirations, shortages, and recalls happen. When they do, the challenge isn’t always finding replacements, it’s updating every crash cart with new supplies. Stat Stock, the RFID-based inventory management system from HCL, identifies drug locations and records each changeout in real time for greater patient safety. Scan tagged medication and Stat Stock automatically adds it to inventory. Then find, remove, and verify drugs that need attention are out of circulation with just a few clicks. Purchase RFID Tags — or use pre-tagged medication — and receive unlimited access to the cloud-based software and HCL’s customer support team. There are no purchase minimums or lengthy contracts and full implementation takes less than one week.
• Central Supply/Purchasing/MM
• Emergency Department
• Facilities/Engineering/Maintenance
• Infection Control/Sterile Processing
• Labs/Clinical
• Nursing
• Oncology/Hematology/Infusion
• O.R.
• Pharmacy
• Surgery Center
HCL offers small package quantities, no order minimums, free samples, and ships most orders the same day. Its hassle-free return policy allows you to return any product at any time for any reason. Connect with the company’s live chat team from 8 a.m. to 8 p.m. EST, Monday through Friday.
Q. How would changing the 340B drug pricing program to a rebate model affect covered entities?
At least five pharmaceutical companies are seeking to change 340B from its current model of upfront discounts to one that would use backend rebates. Such models would require affected covered entities to purchase their drugs at “commercial” prices such as wholesale acquisition cost (WAC), submit claims information to the drugmakers, and wait for the companies to go through validation processes that they control and have not fully defined before they will approve rebates under uncertain timelines.
Rebates would impose massive financial and administrative burdens on hospitals that would be expected to carry the full cost of their 340B drugs while waiting for drug companies to decide whether and when to issue rebates. The models effectively would allow drug companies to establish their own rules for 340B patient eligibility, raising the prospect that they will deny rebates for legitimate claims. Hospitals expect they would need to devote significant staff time and other resources on top of the carrying costs just to process rebate claims and challenge denials. This possibility raises concerns that hospitals would have fewer resources to devote to care and support for patients in need.
The federal government so far has rejected the drug companies’ rebate models, stating they violate 340B law because the drugmakers have not received the administration’s approval to implement them. A federal court in Washington, D.C., is considering lawsuits from the companies seeking to overturn those rejections and prevent the government from imposing any sanctions on the companies for proceeding.
Q. Where do state actions stand on the 340B contract pharmacy issue?
States continue enacting legislation to protect covered entities’ access to 340B pricing on drugs they dispense to their patients through community and specialty contract pharmacies. Prior to 2025, eight states (Arkansas, Kansas, Louisiana, Maryland, Minnesota, Mississippi, Missouri, and West Virginia) added such laws to their books. States that have enacted contract pharmacy protections for all covered entities since the beginning of this year include Nebraska, North Dakota, South Dakota, and Utah.
The pharmaceutical industry continues to challenge all the state contract pharmacy laws in federal courts, with varying results. The first federal appeals court to consider such lawsuits upheld the constitutionality of the first-inthe-nation contract pharmacy protection law in Arkansas, and the U.S. Supreme Court declined to take up the drug industry’s petition for the justices to reconsider that decision.
One lower court in West Virginia has ruled in favor of drugmakers on some of their constitutional challenges, and the state has appealed that decision to a different appellate court. Such appeals courts will be tasked with reconciling lower court decisions that conflict with each other. If several higher courts issue decisions that conflict with each other, it raises the possibility that the U.S. Supreme Court eventually could take up the issue of whether states can enact their own contract pharmacy protections.
In the meantime, numerous drug companies have suspended their restrictive contract pharmacy policies in some or all the states that have enacted bans against such
restrictions. This has had the effect of restoring unlimited access to 340B pricing on drugs to covered entities and their pharmacy partners in those states.
Q. Minnesota recently became the first state to release an annual 340B report. What did the report say?
Minnesota was the first state to enact requirements that 340B covered entities submit certain data to state health officials related to their participation in 340B. The state’s health department released its first annual report based on data in November 2024. It contained aggregated figures, broken down by covered entity type. The figures included:
• Acquisition costs for 340B drugs.
• Payments received for those drugs, by payer type.
• Payments made to 340B contract pharmacies and third-party administrators (TPAs).
The report concludes that Minnesota covered entities received at least $630 million more for 340B drugs in 2023 than it cost to acquire them. This figure included $87 million through net payments from Minnesota’s Medicaid program and health assistance program for low-income patients who do not qualify for Medicaid. The report also found that the entities reporting data paid more than $120 million to contract pharmacies and TPAs in 2023.
However, the report does not measure 340B savings by comparing those figures to what providers would have paid to acquire the drugs at non-340B pricing. It also does not include any information about how they used the 340B savings they obtain through their in-house and contract pharmacies to provide more services and treat more patients.
The $630 million figure that the report details for 2023 is a fraction of the total amounts that Minnesota covered entities spent on delivering care. 340B Health analysis of cost reports from 2022 found that hospitals in the state incurred more than $15 billion in patient care costs. They also provided more than $770 million worth of uncompensated and unreimbursed care that year, a figure that was closer to a billion dollars when such care for Medicare enrollees was included.
Q. What is in the recent White House executive order on drug pricing that might affect 340B?
On April 15, President Trump released an executive order aimed at lowering drug prices. As part of the order, the president ordered his administration to conduct a survey of hospitals’ outpatient drug acquisition costs and propose a plan to set Medicare payments based on those costs. That order could lead to the return of Medicare pay cuts to 340B hospitals, which the U.S. Supreme Court deemed unlawful because the administration had not conducted such a survey beforehand.
The order gave the Health and Human Services (HHS) secretary six months to publish a plan for the acquisition cost survey. Following the conclusion of that survey, the secretary will
consider and propose adjustments to align Medicare payments with the costs of obtaining those outpatient drugs. This could result in Medicare paying rates for 340B drugs that are as low as acquisition cost.
However, under federal “budget neutrality” rules, Medicare must use any savings on 340B drugs to raise rates for nondrug services at 340B and non-340B hospitals. That was the process the agency followed for the now-invalidated Medicare Part B cuts that were in effect from 2018 through most of 2022, when the high court ruled them to be illegal. The new executive order states that any future pay adjustments based on the acquisition cost survey would need to follow the same budget neutrality rules.
At least two other provisions in the executive order would touch on 340B. One would condition federal grants to health centers on those providers offering insulin and injectable epinephrine at the 340B price to certain patients with low incomes. Another would provide recommendations that could lead to changes in the administration of the Medicaid drug rebate program (MDRP), which is closely tied to 340B.
Q. Have there been any new 340B audit trends that you have noticed recently?
The Health Resources & Services Administration (HRSA) recently issued several final 340B audit reports that confirm hospitals risk audit findings if they fail to update their records with information from their most recent Medicare cost reports (MCRs). Several hospitals received audit findings because their information in the Office of Pharmacy Affairs Information System (OPAIS) did not match their most recently filed MCRs at the time of their audits.
The audit findings indicate that HRSA expects hospitals filing MCRs not to wait until the annual 340B recertification process to make necessary updates in OPAIS. Whenever a hospital files a new MCR, its filing date and cost reporting period — at a minimum — will change and should be updated. Other OPAIS fields that must match the most recently filed MCR include the disproportionate share (DSH) adjustment percentage for all 340B hospital types except critical access hospitals (CAHs), control type, and ownership.
Although audit report findings for OPAIS records violations do not involve repayments to drug companies, HRSA has made it clear it considers these record keeping expectations to be an important 340B data integrity issue. 340B Health recommends hospitals ensure compliance by making it a practice to update the relevant fields in OPAIS immediately after filing a new MCR.

Steven Miller is the vice president of pharmacy services for 340B Health, a nonprofit organization of more than 1,600 hospitals and health systems participating in the federal 340B drug pricing program.
340B Health (202) 552-5850 www.340bhealth.org
340BDirect | Procuity (877) 540-4748 340bdirect.com
Apexus LLC (888) 340-2787 www.apexus.com
Aventi Health (414) 792-9673 www.aventihealth.com
CaptureRx (888) 345-6424 capturerx.com
Cencora (610) 727-7000 www.cencora.com
Cervey (800) 710-9348 cervey.com
Clearway Health (833) 966-0506 clearwayhealth.com
Codonics (800) 444-1198 www.codonics.com
CompleteRx (888) 388-1196 www.completerx.com
CPS Solutions, LLC (800) 968-6962 cps.com
Liberty Software (800) 480-9603 libertysoftware.com
Maxor National Pharmacy Services, LLC (800) 658-6146 maxor.com
McKesson (972) 446-4800 www.mckesson.com
Morris & Dickson (800) 388-3833 www.morrisdickson.com
Nuvem (888) 356-6225 nuvem.com
Omnicell www.omnicell.com
PharmaForce (814) 788-2463 thepharmaforce.com
Pillr Health (954) 602-0046 www.pillrhealth.com
Plenful www.plenful.com
PrimeRx (866) 495-3999 micromerchantsystems.com
ProxsysRx (205) 533-9119 proxsysrx.com
R&S Northeast LLC (800) 262-7770 www.rsnortheast.com
RxPreferred (888) 666-7271 rxpreferred.com
ScriptPro (800) 851-2364 www.scriptpro.com
SpendMend (616) 257-6300 www.spendmend.com
SUNRx (866) 958-3402 www.sunrx.com
TailorMed tailormed.co
The Craneware Group (404) 364-2032 www.craneware.com
Verity Solutions (800) 581-1378 verity-solutions.com
Virtue 340B (585) 329-0280 www.virtue340b.com
Visante (866) 388-7583 visanteinc.com
Vizient, Inc. (800) 842-5146 www.vizientinc.com
Wellpartner (877) 805-9483 www.wellpartner.com

The 340B Drug Pricing Program, established in 1992 under Section 340B of the Public Health Service Act, is a vital federal initiative that supports safety-net healthcare providers by requiring pharmaceutical manufacturers participating in the Medicaid Drug Rebate Program to offer outpatient medications at significantly reduced prices. This program enables eligible healthcare organizations — referred to as covered entities, including federally qualified health centers (FQHCs), Ryan White HIV/AIDS Program grantees, critical access hospitals (CAHs), disproportionate share hospitals (DSHs), and rural referral centers (RRCs) — to stretch limited resources, enhance medication access for uninsured or underinsured populations, and reinvest savings into essential health services and community programs.
To maximize program effectiveness and compliance, pharmacies and covered entities may engage outsourced 340B management providers and utilize specialized resources such as third-party administrators (TPAs), contract pharmacy networks, and compliance software platforms. These outsourced solutions help streamline operations, navigate complex regulatory requirements, manage inventory and claims processing, and ensure that medications are dispensed only to eligible patients. The use of contract pharmacies is also common, allowing external pharmacy partners to dispense 340B medications on behalf of covered entities, further extending patient access to affordable treatments. Oversight of the program is administered by the Health Resources and Services Administration (HRSA), which conducts audits to uphold compliance standards and safeguard program integrity. As the healthcare landscape evolves, the use of outsourced 340B management continues to play a crucial role in ensuring that program benefits are fully realized and sustainably administered.













President & CEO: Edward Vargas
Founded: 2022
Employees: 2-10
Phone: (585) 329-0280
Address: 7713 Misty Way Ontario, NY 14519
Virtue 340B provides independent 340B audits and expert consulting to help pharmacies strengthen compliance, reduce risk, and maximize program integrity with confidence and clarity.

Website: www.virtue340b.com
Virtue 340B was founded with a singular mission — to bring clarity, integrity, and expert guidance to the complexities of the 340B Drug Pricing Program. As an independent audit and consulting firm, Virtue 340B partners with health centers, hospitals, and pharmacies to safeguard compliance and strengthen program operations. Our team is led by experienced 340B professionals who understand the regulatory landscape and the operational challenges covered entities face. Since our inception, we have built a reputation for delivering risk-focused audits, practical insights, and educational support that empower pharmacies to navigate the 340B program confidently. Virtue 340B remains committed to advancing transparency and compliance across the healthcare industry.
Virtue 340B offers comprehensive, independent audit and consulting services tailored to 340B covered entities and their pharmacy partners’ unique needs. Our core service — independent 340B audits ensures program compliance by identifying risks, validating eligibility, and offering actionable recommendations. We also provide strategic consulting to support policy development, operational optimization, and HRSA audit readiness. Every engagement is delivered with a focus on education and collaboration, helping pharmacy leaders build confidence in their compliance efforts and enhance the long-term sustainability of their 340B programs.
Our team brings decades of combined experience in 340B program oversight, audit execution, and regulatory compliance. With backgrounds that span federal grantee operations, hospital systems, and national third-party audit engagements, we possess a deep understanding of the practical and policy-driven
challenges faced by covered entities. Every audit is conducted with a disciplined approach grounded in the Standards for the Professional Practice of Internal Auditing, as issued by the Association of Internal Auditors. This commitment ensures each engagement is thorough, objective, and aligned with the highest industry standards. Our expertise is rooted in years of hands-on 340B leadership, enabling us to offer meaningful insights, clear recommendations, and a trusted voice in strengthening program accountability.
Virtue 340B’s independent audit services are designed with flexibility to meet the evolving needs of covered entities and their pharmacy programs. We offer discrete annual audits and ongoing continuous monitoring solutions, allowing organizations to choose the level of oversight that best aligns with their operational goals. Our discrete audits provide a comprehensive, point-intime assessment of 340B program compliance, which is ideal for meeting internal governance or HRSA preparedness needs. For clients seeking more frequent engagement, our continuous monitoring services deliver quarterly claim and purchase reviews, timely risk identification, and proactive guidance throughout the year. Whether through a single audit or ongoing support, Virtue 340B equips pharmacies with clear, data-driven insights and expert recommendations to maintain compliance and program integrity.






CEO: Steve Rough
Founded: 1999
Toll-Free Phone: (866) 388-7583
Our mission is to transform healthcare through pharmacy. We help hospitals and health systems optimize pharmacy operations, improve financial performance, and deliver better patient care.
Address: 101 East 5th Street, #2220 St. Paul, MN 55101
Website: www.visante.com

Visante’s innovative healthcare solutions help health systems improve patient care, operational performance, and financial results. Our approach fully integrates infusion and specialty pharmacy solutions to put the patient first. We maximize client value through deep expertise in highly specialized services including 340B optimization and compliance, medication access services, pharmacy revenue cycle, and pharmacy supply chain optimization.
Visante’s healthcare solution integrates specialty pharmacy and infusion services to offer extraordinary value for our clients. We bring a transformative solution to health systems by delivering an unmatched patient and provider experience and accelerate the system’s ability to achieve its goals and fully leverage its opportunity.
Our innovative, comprehensive approach to specialty pharmacy and infusion services unlocks the value of an integrated program: timely medication access; reduced administrative burden; operational efficiency; improved financial performance; and enhanced clinical outcomes. Visante helps improve the lives of patients while supporting the financial objectives of the health system.
Featured Services
n Specialty Pharmacy Services
A clearly defined specialty pharmacy strategy helps improve patient care and the bottom line. Developing a specialty pharmacy strategy or expanding your existing services can be a complex process, but our team guides you through each phase and delivers lasting ROI. Our specialty pharmacy solution provides operational assessment and pro forma models; multi-year business and strategic plans; facility design including workflow and automation options; implementation and project management; accreditation support; contract pharmacy strategy; wraparound strategies to include consideration for site of care challenges (infusion); and home infusion and business planning.
n
A comprehensive infusion services strategy can help lower total cost of care, maximize patient outcomes and access to quality care, reduce the administrative burden for patients and providers, and optimize organizational financial performance. Visante’s infusion solution provides business planning, design, and implementation of comprehensive infusion
care strategies using our deep and specialized expertise in all aspects of infusion, including home infusion therapy, specialty pharmacy, 340B program management, supply chain strategies, and innovative solutions to drug delivery and therapy administration.
n
Visante’s independent, external audit support provides transparency to your 340B processes, allowing you to recognize compliance gaps while focusing on new opportunities within the program. Our 340B team offers unique expertise in supporting 340B ESP™ data submission and price restoration analysis. Our services include: internal and external audit support, on-site HRSA audit support, and corrective action plan guidance in the event of HRSA audit findings; gap analysis and targeted recommendations, focusing on long-term strategy in the mixed-use and contract pharmacy space to ensure program optimization; program implementation and development of internal oversight structure and maintenance; and split billing RFP guidance, implementation, and program re-designs.
n
Sustained financial growth is one of the top challenges for even the most successful organizations in the healthcare industry. Effective pharmacy programs can increase revenue by maximizing existing opportunities, creating new programs and services, and reducing costs. Our team of experts are here to help maximize financial performance within your organization through our mastery of both pharmacy and revenue cycle operations. Our consultants bring a wide range of experience to the table to assist each of our clients in a way that best fits them and their financial goals.
Visante’s centralized medication access services help hospitals and health systems overcome operational, financial, and administrative barriers. Our team manages the intricacies of medication insurance authorizations, coordinates financial assistance, and supports streamlined access to treatment. Our team provides support through all sites of care, helping patients navigate the journey of accessing and affording their medications. This approach alleviates the administrative burden from the care team, improving provider and patient satisfaction.
n
Supply chain and utilization management are complex, evolving aspects of a pharmacy enterprise. To find success in these areas, disciplined focus and alignment must be prioritized. Visante’s supply chain experts help hospitals and health systems achieve reliable, safe, and efficient drug supply chain performance while also realizing significant financial returns.


Follett pharmacy refrigeration delivers temperature consistency with patented infinite speed control technology.

Infinite speed control technology continuously adjusts cooling output for exact, uniform temperatures, eliminating fluctuations that compromise medication and vaccine integrity.
Configurable interior layouts maximize storage efficiency, adapting to pharmacy requirements.
in temperature



General Manager: Mike Raycher
Founded: 1948
Employees: 500+
Toll-Free Phone: (800) 523-9361
Phone: (610) 252-7301
Fax: (610) 250-0169
Follett high-performance NSF 456-Certified refrigerators provide precise temperature control for safely storing vaccines and medications.

Address: 801 Church Lane Easton, PA 18040
Website: www.follettpharmacy.com
Follett Products, LLC is a leading manufacturer of innovative equipment for the healthcare market, including medical-grade refrigerators and freezers, ice and water dispensing equipment, and ice machines. Our focus on 100% customer satisfaction results in equipment that provides outstanding innovation and design excellence to meet the specific needs of each healthcare facility.
Follett medical-grade pharmacy refrigerators and freezers maintain precise and consistent temperatures for your valuable medications and vaccines. Powerful forced air refrigeration enables quick recovery after door openings, ensuring your critical products are stored at specified temperatures and reducing the risk of lost product.
n Follett Infinity Series™ upright refrigerators and freezers feature a powerful top-mounted refrigeration module and innovative plenum air distribution system for superior temperature consistency.
• Patented “infinite” variable speed control algorithm delivers only the precise amount of refrigeration needed at any given time, providing a ±1 degree C temperature consistency throughout the storage cabinet while minimizing noise and energy consumption.
• Industry-exclusive ducted air distribution delivers cold air at multiple levels for top-to-bottom temperature uniformity, allowing full utilization of the storage space.
• All models are NSF 456-Certified for safe vaccine storage.
• Intuitive full-color touchscreen user interface features enhanced data management with data downloading capability, providing the ultimate in user convenience.
n Full stainless steel cabinet and high-quality components designed with the user in mind.
• Heavy-duty, self-closing doors provide important energy savings and lock open at 90 degrees to allow easy product loading.
• Interchangeable storage options.
• Choose from epoxy-coated shelves, full extension epoxycoated “floating” baskets, or full extension stainless steel drawers.
• Interior can be reconfigured at any time to accommodate changing storage needs.
n Follett’s innovative modular refrigeration system provides outstanding serviceability.
• Top-mounted modular design allows the entire refrigeration system to be removed as one unit without cutting refrigerant lines. If needed, a spare system can slide into place, virtually eliminating downtime or the need to relocate product.
n High-performance countertop refrigerators and freezers offer superior temperature performance in space-saving configurations.
• 1 and 2 cubic feet models fit on standard 24" deep counters.
• Forced-air cooling provides cabinet-wide temperature consistency and quick recovery after door openings.
• External digital temperature display in user-selectable F or C with integral high/low alarming and display sleep mode.
• Keyless entry via keypad and electronic lock option.
n Powerful undercounter refrigerators and freezers are designed for use below standard and lower ADA-compatible counters.
• Heavy-duty forced air cooling for quick recovery after door openings.
• External digital temperature display in user-selectable F or C with integral high/low alarming and display sleep mode.
• Keyless entry via keypad and electronic lock option.
Contact Information
For more information, contact Follett at (800) 523-9361 or visit www.follettpharmacy.com.
By Gee Mathen, Director of Pharmacy Clinical Applications at Texas Children’s Hospital
Hospitals are under pressure to address one of the leading preventable causes of death in the U.S. — medication errors, which are estimated to result in an average of 250,000 preventable deaths each year. While any error involving medication is cause for concern, the consequences can be particularly severe in highstakes environments like operating rooms (ORs), where even the smallest mistake can impact life or death.
Fortunately, technology enhancements in recent years have led to the advent of fit-for-purpose solutions for the OR, and automation is proving to be a viable solution to optimize medication management. By introducing automation into the OR, organizations can gain better visibility into medication inventory and eliminate routine manual tasks, helping to reduce opportunities for human error while ensuring the right medications are always on hand when needed. This ultimately helps improve clinical efficiency and patient safety. At Texas Children’s Hospital (TCH), the incorporation of automation is quickly helping to improve patient outcomes and the overall safety of the OR, serving as a blueprint for other hospitals looking to improve their medication processes.
Modern operating rooms are high-pressure environments where patient care is of the utmost importance, yet there is often little visibility into medication dispensing within the OR. Anesthesiologists typically own the medication distribution process in these settings and demand unfettered access to life-saving drugs during surgical procedures. This requirement has hindered adoption of automated

dispensing cabinets (ADCs) and other automation technology in the OR because of the built-in safeguards that can often limit or delay medication dispensing. Instead, medications in surgical settings are still largely under human control and exposed to human error. There is often little enterprise insight into how much medication is used, wasted, or potentially diverted in the OR. Furthermore, human error in drug administration can increase the risk of patient harm.
While historically viewed by many anesthesiologists as a hindrance, TCH believes that automation — when thoughtfully incorporated — can help maintain autonomy and emergency access to medications while supporting improved patient and staff safety. Even with anesthesiologist oversight of drugs, the pharmacy is ultimately accountable for the safe, secure management of the medications in the OR. Integrating automation and barcode technology in operating room environments helps to reduce manual and administrative tasks. This allows anesthesia providers to focus on their patients, while giving pharmacy visibility into medication usage, better supporting inventory control, and regulatory compliance. Leveraging automation in the OR also improves accountability around controlled substances, which in turn helps reduce the time spent resolving stock discrepancies and investigating potential diversion.
At TCH, we’ve taken steps to integrate medication management automation into the OR, and have streamlined processes, improved accountability, and ultimately, prevented harmful errors as a result.
When we started the process of upgrading our medication management technology, our priorities were clear — efficiency, visibility, and, above all, safety. We recognized the importance of engaging our anesthesia providers early in this process. Their frontline perspective was invaluable, and we collaborated closely to address any concerns. Throughout the decision-making and implementation phases, we had multiple training and testing sessions to ensure smooth integration and the selection of the optimal tool for our specific needs.
Working hand-in-hand with our anesthesia providers, we developed a technology strategy focused on medication management in the perioperative setting. This strategy had to work for everyone — it needed to support the pharmacy’s needs while also being easily adopted by our anesthesia staff. If they weren’t comfortable using the technology and processes, it wouldn’t matter how great it looked on paper.
Adopting new technology is never easy. You must prove that it’s reliable, that it actually solves the problem you’re trying to fix, and that it doesn’t create new problems. Additionally, everyone who interacts with these automated solutions needs to feel confident and equipped to use them successfully. For us, this meant building on our existing relationship with Omnicell. We were already using their XT Automated Dispensing Cabinets (ADCs) to dispense 1.3 million doses annually and leveraged the company’s XR2 robots to automate several aspects of our central pharmacy operation. Through this partnership, we were also introduced to Omnicell’s XT Anesthesia Workstation (AWS) — a medication dispensing cabinet designed to support the unique needs of surgical and perioperative settings designed to provide medication tracking and safeguards while ensuring a convenient medication workflow for anesthesiologists.
The AWS runs on the same server as our XT Automated Dispensing Cabinets and XR2 robots, ensuring that surgical settings are no longer a black hole when it comes to medication dispensing information. Data from these cabinets is captured and consolidated within an enterprise pharmacy intelligence solution, providing pharmacy leadership visibility and insight into medication utilization in the OR.
The intuitive anesthesia workflows provided through the workstation’s user interface reduces administrative tasks during surgical procedures while embedded barcode scanning automatically tracks medication utilization. Real-time dashboards help monitor and reconcile waste and an embedded syringe barcode label printer helps facilitate regulatory compliance.
Our use of the AWS has not only improved pharmacy’s visibility into how medications are being utilized in our ORs, it has also enabled our pharmacists to better serve our anesthesiologists. With improved inventory insight, our pharmacists can see the quantity of any drug in an AWS at any given time. Our technicians can see if a workstation is running low on certain medications and proactively restock these cabinets, ensuring critical medications are always readily available when needed. The built-in safeguards
on the AWS are also helping us to improve patient safety by reducing manual tasks that increase the risk for human error.
In an already strained labor force, incorporating tools that reduce administrative work while improving patient safety is key for sustainable success. Hospitals and health systems looking to streamline their operations with new technologies should consider adopting a strategy that supports patient safety throughout the medication use journey, including perioperative settings. Automating medication management processes gives clinicians valuable time back in their workday, allowing them to focus on clinical care and top of license practice, leading to improved job satisfaction and enhanced patient outcomes.


Gee Mathen Director of Pharmacy Clinical Applications & Technical Services
Gee Mathen is the director of clinical applications and technical services in the pharmacy department at TCH in Houston, Texas, where he manages a team of Epic Willow pharmacy experts that recently completed a three-campus implementation of Epic and Omnicell. He received a bachelor’s degree in industrial engineering at the University of Houston and holds Epic Willow, EpicCare Inpatient, and EpicCare Inpatient Decision Support master’s certifications. Mr. Mathen’s areas of expertise include decision support, best practice alerts, alternative alerts, and patient scoring.
Simplifi 797® IV Workflow Management
MedTrays
MedStorage

Pharmacy Surveillance
Drug Diversion
Infection Prevention
Designed by pharmacists for pharmacists.
Introducing a comprehensive suite of pharmacy compliance and clinical surveillance solutions designed by pharmacy experts, on a single platform, with tailored workflows and reporting.

With Simplifi+ and Sentri7, expect the innovation and expert support of Wolters Kluwer every step of the way.
Access our turnkey applications on the SoleSource platform with Simplifi+ and Sentri7 to:
• Use AI to detect drug diversion quickly to keep patients and staff safe
• Drive proactive medication intervention to reduce costs and risks
• Streamline USP compliance
• Enhance IV workflow management
• Automate drug inventory oversight
• Keep medication storage compliant
President & CEO: Nancy McKinstry
Founded: 1836
Employees: 21,400+
Stock Symbol: WTKWY
Phone: (608) 829-7300, Ext. 2
Simplifi+ and Sentri7 applications on one powerful SoleSource® platform help standardize clinical, pharmacy, and compliance programs. Customers access evidence-based content, tailored workflows, and robust analytics to improve patient care, quality, and safety.

Address: 525 Junction Road, Suite 5000, Madison, WI 53717
Website: www.wolterskluwer.com/en/solutions/solesource
Wolters Kluwer provides trusted clinical technology and evidencebased solutions that engage clinicians, patients, researchers, and the next generation of healthcare providers. More than 6.5 million clinicians use Wolters Kluwer’s expert solutions to reduce the variability that stands in the way of effective care around the world. Solutions include Sentri7 Clinical Surveillance and Simplifi+ Pharmacy Compliance to deliver the best knowledge and evidence into the hands of the people who need it most.
Product Overview
Wolters Kluwer’s clinical surveillance and compliance applications are deployed on a single platform for easy integration with EHRs or into existing workflows, minimizing the impact on resource-strapped IT and clinical teams. Customers can scale and standardize programs for maximum impact on patient safety, quality care measures, and financial performance.
Simplifi+ Pharmacy Compliance
n Simplifi 797®: Ensure USP Chapters 795, 797, and 800 compliance with confidence.
n Simplifi+ IV Workflow: Streamline USP compliance and meet all ISMP and THRIV guidelines.
n Simplifi+ MedTrays: Standardize the configuration of medication trays and improve inventory oversight.
n Simplifi+ MedStorage: Improve medication storage compliance outside the pharmacy.
“Using Simplifi 797 and IV Workflow Management with Assure-Trak helps streamline our compliance efforts and aids our team with both competency instructions and documentation. It also gives pharmacy more control, with less reliance on IT. It just makes sense.”
— Ryan Brown, PharmD, Pharmacy Manager at Conway Regional Health System
Sentri7 Clinical Surveillance
n Sentri7 Drug Diversion: Leverage advanced technology using artificial intelligence to uncover patterns of potential diversion from purchase to the patient.
“Using the Sentri7 Drug Diversion solution, my team is in a better position to be proactive in our drug diversion surveillance, ultimately supporting heightened safety across our organization.”
— Tammy Burns, PharmD, Director of Pharmacy Services, Mary Lanning Healthcare
n Sentri7 Infection Prevention: Reduce time spent identifying patients at risk for HAIs and streamline reporting with one-click submissions to NHSN. Best in KLAS ranks Wolters Kluwer Sentri7 #1 for infection control and monitoring for the fourth time in three years.
n Sentri7 Pharmacy With Antimicrobial Stewardship, Opioid Stewardship, and Bayesian Dosing: Improve pharmacy workflows and reporting to optimize medication therapy, patient outcomes, and cost savings.
n Help Ensure Compliance
• Simplifi+ IV Workflow, together with Simplifi 797, delivers a complete compounding solution that helps ensure USP compliance, meets all ISMP and THRIV guidelines, and includes policies, procedures, tasks, training, continuing education, and competencies.
• Ensure compliance with CDC, The Joint Commission AMS best practices, The Joint Commission’s Pain Management Standards, and pharmacy reporting requirements with Sentri7 Pharmacy.
n Save Time in Pharmacy-Tailored Workflows
• Streamline detection and investigation of potential diversion with Sentri7 Drug Diversion.
• Access workflows that drive standardization and improve productivity with Sentri7 Pharmacy and Simplifi 797 with IV Workflow.
• Delivering policies into pharmacists’ workflows saves time and provides quick access to the latest evidence-based guidance with Simplifi+ MedStorage and Simplifi+ MedTrays.
n Improve Patient Safety
• Avoid adverse drug events, limit medication errors, and manage opioids and antimicrobials with Sentri7 Pharmacy.
• Uncover patterns of potential diversion to intervene earlier with Sentri7 Drug Diversion.
• Elevate patient and staff safety by using streamlined real-world workflows, risk management, and quality assurance practices, reporting, policies, procedures, and training with the Simplifi+ compliance suite.
n Optimize Medication Management
• Optimize the use of high-cost drugs, antimicrobials, and opioids to improve clinical outcomes and reduce costs with Sentri7 Pharmacy.
• Improve drug inventory oversight and ease inspections by documenting from anywhere with Simplifi+ MedTrays and Simplifi+ MedStorage.
“With Sentri7, we’re in control. We’re able to quickly adopt evidence-based guidance into practice yet can easily make changes to our rules in a matter of minutes. This allows us to be nimble, make adjustments quickly to meet our protocols, and not wait on other teams to help facilitate these changes.”
— Katie Schipper, PharmD, Clinical Pharmacist, St. Dominic Hospital















For more than 30 years, ScriptPro has been more than a vendor—we’re your partner in pharmacy excellence. Our comprehensive suite of operational, financial and clinical solutions empowers pharmacies nationwide to achieve measurable success.
Join the ranks of America’s most prestigious healthcare institutions—including 50% of U.S. News & World Report’s Honor Roll Hospitals—who trust ScriptPro to power their pharmacy operations.
Ready to maximize your pharmacy’s potential? Connect with our technology experts today!
| scriptpro.com
Make pharmacy the strongest link in your health system with ScriptPro — advancing care, accuracy, and operational success.
Chairman, CEO, & Founder: Mike Coughlin
Founded: 1994
Toll-Free Phone: (800) 606-7628
Fax: (913) 432-4735
Address: 5828 Reeds Road Mission, KS 66202
Website: scriptpro.com

ScriptPro delivers operational, financial, and clinical solutions backed by 30+ years of pharmacy management expertise. Trusted by hospital and health systems nationwide — including half of U.S. News & World Report’s Honor Roll health systems — we help streamline operations, reduce wait times, and lighten staff workload with advanced robotics and integrated software. Our technology automates vial filling, inventory, payments, eligibility, reimbursement, 340B and more — freeing pharmacists to focus on patient care. Whether you’re looking to reduce costs, improve efficiency, boost revenue, or improve patient adherence, ScriptPro’s experienced team and scalable solutions can help you achieve your goals — today and into the future.
n Operational: ScriptPro’s robotics bring speed, safety, and simplicity to pharmacy operations. Our automated vial-filling, storage, and adherence packaging solutions help you fill more scripts, serve more patients, and free up staff to focus on care.
• Vial-Filling Robots: Our full-size and compact robots are pharmacy workhorses, delivering reliable, direct-to-vial dispensing that eliminates cross contamination. With six robot sizes to fit various volumes and spaces, they process up to 75% of prescriptions and operate quietly with our gravity-fed dispensing.
• Adherence Packaging: Create personalized, single- and multidose pouch packaging in under two minutes with 99.8% accuracy. Improve medication adherence, reduce hospital readmissions, and open new revenue streams — all while ensuring safe, trackable administration.
• Will Call: Securely manage up to 260 patient batches with automated storage and retrieval. Eliminate time spent searching for bags, minimize handout errors, and maintain a complete audit trail with barcode tracking.
From intake to pick up, our robotics create a seamless, end-to-end automation system that elevates performance and patient care.
n Financial: Elevate your pharmacy’s financial oversight with our advanced claims reconciliation platform. Turn accounting processes into
clear insights with automated reconciliation that matches every penny, daily. Our solution locks down your contract terms, ensuring PBMs honor agreed rates while giving you complete visibility of all claims and payments. Dedicated account managers become extensions of your team, preventing revenue leakage through detailed transaction tracking. Custom dashboards deliver instant financial clarity, while seamless integration with ScriptPro’s Pharmacy Management System and other vendor platforms creates a unified workflow. Experience complete confidence in your pharmacy’s financial health, backed by real-time data and expert support that ensures you’re paid fairly, accurately, and on time.
n Clinical: Our cloud-based technology platform empowers specialty pharmacies to meet both URAC and ACHC accreditation standards while enhancing clinical and financial outcomes. The solution streamlines workflows and documentation across 29 disease states with integrated clinical content, automated task management, and clinical decision support. You can build tailored and customized assessments that guide users through patient care, helping keep patients on therapy, reduce hospital readmissions, and improve satisfaction. Deep integration with your pharmacy system or EHR supports efficient operations, enabling pharmacies to close gaps in care, maintain accreditation, and unlock new revenue streams.
“Scriptpro has unlocked our potential. Before, we were constantly battling inefficiencies and compliance issues. Now, we are growing our services, and ultimately, reaching more patients who need our care. ScriptPro doesn’t just help us manage our pharmacy; it enables us to reach our full potential.”
— Judd Ramsey, Director of Pharmacy Services, Waco Family Medicine, TX
Meet us at future trade shows:

See how Waco Family Medicine tripled their revenue and enhanced patient care – Scan Now!
• ASHP Pharmacy Futures 2025, June 8-10
• 340B Coalition Summer Conference, July 8-10
Ready to maximize your pharmacy’s potential? Connect with our technology experts today!
(800) 606-7628 | scriptpro.com


Dr. Gary Zimmer, MD, FACEP Chief Medical Officer
~ Mohawk Valley Health System (MVHS)

Christopher Houle, PharmD, BCPS, BCGP, CDCES Director of Pharmacy
~ Mohawk Valley Health System (MVHS)

Nicolina Castilla, PharmD Director of Onsite Operations, General Manager
~ CPS Solutions, LLC/Mohawk Valley Health System (MVHS)
Based in Utica, New York, Mohawk Valley Health System (MVHS) serves approximately 600,000 people across Oneida, Herkimer, and Madison counties. The system has more than 30 medical offices and specialty clinics throughout the region and also includes Wynn Hospital, located in downtown Utica. The full-service, 373-bed facility and Level III trauma center opened in 2023 to replace two older area hospitals. It offers patients a range of medical and surgical specialties and has dedicated floors for critical care, intermediate care, and women’s health.
“For a medium-sized community hospital with no real ties to any large systems, Wynn Hospital provides a pretty impressive suite of services,” says Dr. Gary Zimmer, MD, FACEP, chief medical officer for MVHS and practicing emergency physician. The new hospital, he explains, is part of the health system’s overarching goal to expand its portfolio of valuebased care initiatives aimed at lowering costs while delivering better patient outcomes and higher-quality services to the surrounding communities.
As MVHS began its value-based journey, they realized being able to have specialty medications readily available to patients was a real gap, says Zimmer. “We had a lot of limitations and concerns about our ability to provide medical treatment to patients who needed it.”
Christopher Houle, PharmD, BCPS, BCGP, CDCES, director of pharmacy for MVHS, agrees. “Originally, we had a small employee-only pharmacy that only served our insured staff,” he says. This left providers and patients on their own to get access to treatment — which can be complex when dealing with specialty medications. “These therapies are often expensive, even with insurance coverage. They just aren’t always accessible to patients.” Adherence to protocols can also be an issue, adding even more of an administrative burden on providers to follow up with patients to ensure they take medications correctly.

“
The promise of real at-the-elbow support and having CPS pharmacy staff in our offices — not just remote support — was key for us. It’s just a much more personalized approach.”
Dr. Gary Zimmer, MD, FACEP Chief Medical Officer
~ Mohawk Valley Health System (MVHS)
“
If the cost of a medication isn’t covered or the co-pay is too high, we’re going to hit the ground running for patients to fix it.”
Nicolina
Castilla,
PharmD Director of Onsite Operations, General Manager
~ CPS Solutions, LLC/Mohawk Valley Health System (MVHS)
“That’s when we started asking ourselves — how do we get medications to patients who need them with the least friction? How do we ensure we give them every opportunity to comply with medication regimens?” Zimmer says. The answer was clear. They needed to add specialty pharmacy services to their initiatives. The next question was: should they do it on their own or find an external partner?
For MVHS, building a specialty pharmacy centered on improving patient outcomes. “We felt bringing an expert on board would help us get the program up and running faster,” Houle explains. After exploring several options, MVHS ultimately chose CPS Solutions, LLC (CPS), one of the country’s largest pharmacy and hospital solution providers. According to Zimmer, what they liked best about CPS’ offering was their local care model. “The promise of real at-the-elbow support and having CPS pharmacy staff in our offices — not just remote support — was key for us. It’s just a much more personalized approach.”
Nicolina Castilla, PharmD, who was born and raised in Utica and now works for CPS as MVHS’ director of onsite operations, general manager, describes how the program works. “The CPS pharmacy team supports both physicians and patients from MVHS’ 30 medical groups and Wynn Hospital. We help patients access specialty prescriptions in a timely way,” Castilla says. “That includes getting prior authorizations and financial assistance for patients in need, so medications are available immediately.”
When prior authorization is required, the CPS team will start the process immediately so patients can receive their prescriptions as soon as possible. On average, the CPS team has achieved a 98% approval rate for submitted prior authorizations. “If the cost of the medication isn’t covered or the co-pay is too high, we’re going to hit the ground running for patients to fix it,” says Castilla. She and her team do whatever they can to help patients overcome financial barriers. As a result, they have secured over $6 million in financial assistance for their patients. According to Houle, taking the administrative burden of prior authorizations and financial assistance away from providers gives frontline clinical staff more time for quality patient visits.
Another way the CPS’ team is freeing up providers’ time is by helping patients better manage their care. They follow up with patients regularly to talk to them about how they are feeling, if they have missed any doses, and if they are having side effects.
“This ongoing communication not only helps improve adherence rates but also reduces the number of sick calls coming into the office. We’ve found that many patient concerns are medication-related, which we’re now addressing before they even think to call the doctor,” explains Castilla. Monitoring adherence and helping patients make a plan to comply with medication protocols also reduces longer-term hospitalization and readmissions.

“It’s the concierge level of service that CPS delivers that makes the quality so good.”
Christopher
Houle, PharmD, BCPS, BCGP, CDCES
Director of
Pharmacy
~ Mohawk Valley Health System (MVHS)
“It’s the concierge level of service that CPS delivers that makes the quality so good,” Houle believes. Patients seem to agree. MVHS achieved a remarkable Net Promoter Score (NPS) of 100 — earning them a spot as a finalist in the annual Managed Markets Insights & Technology (MMIT) specialty pharmacy patient choice awards for the second consecutive year.
“In just three years, we’ve gone from a small employee-only pharmacy to a full-blown specialty pharmacy with a front-facing retail end right in downtown Utica so that we’re able to serve every member of the community,” Houle says.
The specialty pharmacy also earned its Accreditation Commission for Health Care (ACHC) and Utilization Review Accreditation (URAC) accreditations in the same time frame.
“We found that dual accreditation opened doors to new payor and limited distribution networks for us,” says Castilla. “And that just means more access for our patients.”
“Achieving that expanded network access requires certain specialized knowledge and expertise,” adds Houle. “I never had that before working with CPS.” As a result, MVHS continues to serve more patients while demonstrating that they’ve achieved great clinical results. “The high-quality care and expanded access is a self-fulfilling process — it’s a huge win for us and our patients.”
“I think the results we’ve achieved are the easiest way of describing our success — which is based on our choice of partner,” says Zimmer. By leveraging CPS’ expertise and fully customizable solution, “if we’d tried to achieve that on our own, I think it would have probably been a five- to eight-year journey.” Plus, he adds that based on the quick turnaround time — getting medications for patients, addressing payor issues, and providing financial assistance in a timely manner — the specialty pharmacy can now expand its reach. “With the success of the program, we are in a position to start conversations with other private practice specialists in the communities we support and bring our services to their patients to continue building on our value-based care initiatives.”






A US-based company with a solid foundation in generic pharmaceuticals, Amneal is growing its injectables portfolio to include ready-to-use bags, pre-filled syringes, and other presentations to streamline and simplify your workflow.
We’re committed to building our line of ready-to-administer products, now including the first and only FDA-approved Potassium Phosphates in a premixed bag, ensuring quality, convenience and availability for years to come. This is how we make healthy possible.


Scan the code to see Amneal’s extensive catolog of injectable products










According to ISMP guidance for Optimizing Safe Implementation and Use of Smart Infusion Pumps, the goal for drug library compliance should be 95% or greater. Even a single percentage point increase in drug library compliance can significantly reduce the number of unprotected infusions.2 Baxter infusion platforms with Dose IQ Safety Software deliver 97% compliance within one month of implementation.1 AVAILABLE
President & CEO: Jose Almeida
Founded: 1931
Employees: 50,000 globally
Stock Symbol: BAX
Toll-Free Phone: (800) 422-9837
Phone: (224) 948-1812
Address: One Baxter Parkway Deerfield, IL 60015
At every step, the Novum IQ Infusion Platform with Dose IQ Safety Software helps you build and deploy one of the safest and most effective drug libraries possible for large volume, syringe, and enteral infusions.

Website: baxter.com and novumiq.com
Company Background
For over 90 years, Baxter has introduced significant medical innovations, including the first commercially produced intravenous (IV) solutions and the first home-based dialysis system as an alternative to hemodialysis in a hospital or clinic. Baxter has been highly innovative in providing healthcare solutions to unmet medical needs through scientific advancement as well as strategic acquisitions and partnerships
Every day, millions of patients, caregivers, and healthcare providers rely on Baxter’s leading portfolio of diagnostic, critical care, kidney care, nutrition, hospital, and surgical products used across patient homes, hospitals, physician offices, and other sites of care. We’ve been operating at the critical intersection where innovations that save and sustain lives meet the healthcare providers who make it happen. With products, digital health solutions, and therapies available in more than 100 countries, Baxter’s employees worldwide are now building upon the company’s rich heritage of medical breakthroughs to advance the next generation of transformative healthcare innovations. To learn more, visit baxter.com and follow us on X, LinkedIn, and Facebook.
n Novum IQ Infusion Platform — Large Volume and Syringe Pump Models
Benefit from an elevated infusion experience. The Novum IQ Large Volume Pump and the Novum IQ Syringe Pump share advanced technology and enhancements to help protect patients and standardize infusion administration.
• Streamlined workflows and intuitive operation to drive compliance in the use of pump safety software, helping to ensure a safe continuum of care for patients.
• An easy-to-use common interface across large volume and syringe pumps, supported by 350+ hours of human factors testing, helps to simplify pump operation for clinicians and streamline training.
• On-screen barcodes with scan prompts ensure that pumps are correctly identified, helping to provide safety and efficiency during auto-programming and drive auto-programming compliance.
• A visual beacon so clinicians can see from a distance that a pump needs attention.
• A backlit keypad and an ambient light sensor to improve visibility.
• Baxter single-set technology accommodates both large volume pump and gravity infusions to deliver efficiency, safety, and savings.
• A broad range of compatible syringes have been qualified for use with the Novum IQ Syringe Pump.
n Dose IQ Safety Software
Rely on Dose IQ Safety Software to help you protect more infusions and reduce the risk of preventable errors. Accurate and up-to-date drug libraries are important to help ensure patient protective measures are maintained throughout infusion therapy, especially for patients receiving critical medications. However, even when drug libraries are used, current implementation practices can limit their effectiveness. At every step, web-based Dose IQ Safety Software helps you build and deploy one of the safest and most effective drug libraries possible. Dose IQ Safety Software offers unique and best practice capabilities.
• Titration error prevention technology allows Novum IQ pumps to intercept dose and rate changes that could be potentially harmful.
• Unique drug-linking feature that provides more library capacity and flexibility with 32 care areas and 10,000+ drug entries across large volume and syringe pumps.
• Comprehensive units of measure, including MillionUnit dosing.
• Color coding for enteral infusions.
• Configurable anesthesia care area settings with optional passcode protection.
• Standardized drug library creation in partnership with First Databank (FDB) and incorporation of its FDB Infusion Knowledge database.
Count on rigorous cybersecurity to provide advanced protection for patients’ infusion data. The Novum IQ Infusion Platform is built to meet stringent cybersecurity protocols.
• UL 2900 certification, which attests to independent cybersecurity testing and assessment for the IQ Enterprise and Dose IQ2.
• Secure remote updates to IQ Enterprise Connectivity Suite on both servers and network.
• Encrypted software and firmware to pumps.
• Collaboration with Health-ISAC and ICS-CERT organizations.
• 95% field serviceability to help get pumps back in use, potentially reducing cost and downtime.
• Modular design with cost-effective field-replaceable units to streamline service and maintenance.
• Remote troubleshooting and diagnostics combined with parts ordering, to help reduce maintenance burden.

When accuracy matters most, I-SO trust KwikDose™ Oral Dispensers—now proudly distributed by ISO-MED.
These are the same high-quality oral syringes hospitals and pharmacies have trusted for nearly 50 years under the ExactaMed name—just with a new look and the same uncompromising performance.
KwikDose™ delivers:
• ISO-compliant dosing accuracy
• Six sizes: 0.5, 1, 3, 5, 10, and 20 mL
• Clear and amber options
• Oral tip design helps prevent wrong-route delivery
• BPA-, DEHP-, and latex-free
• Made in Denmark | Packaged in the USA

Whether for pharmacy compounding or patient care, KwikDose™ supports your cleanroom and medication delivery needs with the precision and reliability you expect. Contact us for more information or for pricing! sales@iso-med.com
President: David Lowrie
Founded: 2010
Toll-Free Phone: (800) 797-1405
Fax: (951) 547-1681
Address: 1220 Graphite Drive Corona, CA 92881
Website: www.iso-med.com
As one of the top leading suppliers for pharmacy cleanroom supplies, we provide an unparalleled selection of specialized medical products, globally trusted quality and service, and delivery convenience for meeting USP <797> and USP <800> standards.

Since 2011, ISO-MED, Inc., a medical supply distributor, has been a trusted hospital supplier with a solid combination of quality, selection, service, and convenience. We supply quality products for pharmacy cleanrooms and other medical industries in the U.S.
Providing high-grade supplies for cleanrooms, laboratories, homecare, and more, we are dedicated to achieving and maintaining compliant sterile compounding environments.
ISO-MED maintains the largest and broadest portfolio of on-theshelf warehouse of supplies.
We offer customized supply plans, a seamless e-commerce customer experience, easy ordering with matching manufacturer, and dependable purchasing/supplier aggregation services.
With one-on-one scheduled training services, we increase the efficiency of your purchasing processes, consolidate your portfolio, and link your on-site supply demand to your inventory management processes.
Offering value-added services, we provide consistent customer service and on-time delivery with a streamlined transaction process. We are driven to help healthcare-oriented organizations save money.
Features & Options
ISO-MED not only has products that help a hospital conform to cleanroom standards, but we also offer the following products for testing cleanliness.
n ICR Plus TSA LT Contact Plates
Environmental monitoring for isolators and cleanrooms (surface and air monitoring).
n Tryptic Soy Agar
Added measure to assure sterility in your environmental monitoring program! Hardy Diagnostics contact plates are recommended for use in the cultivation of microorganisms from environmental surfaces.
Additional Product Modules
n Premier Product Offerings
• PPE Product Selection
• HD Spill Kit
• Chemotherapy Gowns and Supplies
• Cleaning and Disinfection Solutions
For every cleanroom or critical environment cleaning and disinfection program, ISO-MED offers several brands and ISO-MED, Inc. formularies.
Markets Served
• Hospital
• Compounding
• Infusion
• Retail
Ordering Information
Contact us by phone at (800) 797-1405 or email sales@iso-med.com to gain access to our exclusive online ordering platform.

Containment Primary Engineering Control (C-PEC)
Protection: Product, Personnel, & Environmental
Containment Primary Engineering Control (C-PEC)
LabGard ES AIR NU-543 Series
Optimized for Sterile Hazardous Drug Compounding Systems
Telescoping or Mechanical Auto-Rising Base Stand Options
Optional IV Bar with 3 Height Locations and 6 SST Hooks
Interior Duplex Outlets and Cord Pass-Through Ports
Custom Back-Wall Viewing Window for PC Monitor
LabGard ES NU-813 Series
Optimized for Non-Sterile Hazardous Drug Compounding
Telescoping or Mechanical Auto-Rising Base Stand Options
Optional Ergonomic Mount for PC Monitor, Keyboard, and Mouse
Double Exhaust Filter with Bag-In/Bag-Out Options
Polycarbonate Viewing Window and Sidewalls
Sidewall Waste Pass-Through Ports
Containment Ventilated Enclosure
Protection: Personnel & Environmental

President & CEO: Bill Peters
Founded: 1971
Employees: 251
Prepare compounded preparations comfortably and efficiently by configuring your laminar airflow workbench, containment ventilated enclosure, or biosafety cabinet around your pharmacy’s specific workflow.

Stock Symbol: KEQU (Kewaunee Scientific Corp.)
Toll-Free Phone: (800) 328-3352
Phone: (763) 553-1270
Address: 2100 Fernbrook Lane, Plymouth, MN 55447
Website: www.nuaire.com
NuAire, Inc. has provided primary engineering controls to pharmacies for many years. As a small company with a big impact, NuAire supplies laminar airflow workbenches, biosafety cabinets, containment ventilated enclosures, restricted access barrier systems, and even customized solutions for drug compounding. Learn why NuAire is the safer choice for your pharmacy.
The NU-543 LabGard® provides the pharmacy with a Containment Primary Engineering Control (C-PEC) whose core design and configuration options enable one to optimize it for their sterile, hazardous drug compounding workflows. This model features an airflow sensor and alphanumeric display for pharmacies requiring a clear measurement of their cabinet’s performance and ergonomic access to the work zone. Built standard with two outlets and a cord pass through for needed devices, the cabinet can optionally be made with a smooth interior instead and always includes a prop up work tray to ease regular cleaning under the work surface. Optional base stands are available with motorized height adjustability and support arms for keyboards and monitors to further support an ergonomic workflow. A separately available exhaust canopy with configurable side air gaps provides the installer with flexibility when connecting the cabinet to an external exhaust system.
n NU-240 Laminar Airflow
The AireGard™ NU-240 creates HEPA-filtered horizontal laminar airflow to provide product protection for sterile but non-hazardous drug compounding in pharmacies. The DC ECM motor draws air through a top pre-filter and propels it through a backwall HEPA filter to create an ISO Class 5 level of air cleanliness inside a work zone surrounded by stainless steel walls and a work surface made
of stainless steel with a PVC core. One easily monitors workbench performance through the Aeromax™ control system that visualizes pressure in the internal airflow plenum. The work zone comes standard with cord-pass throughs and LED lighting but can be optionally configured with polycarbonate walls and an IV bar to fit your workflow needs. Optional base stands are available with motorized height adjustability and support arms for keyboards and monitors to further support an ergonomic workflow.
n NU-LXXX Custom Biosafety Cabinets
The ultimate form of configurability is customization of a solution for your specific needs. Whether it is an oversized work zone for automation equipment, a reinforced work surface, support for a camera, or a back wall cutout for a monitor, NuAire can go to the drawing board to offer you a tailor-made solution.
Additional Product Lines
• Class II Type B1 Biosafety Cabinets
• Class II Type B2 Biosafety Cabinets
• Containment Ventilated Enclosures
• Horizontal Laminar Airflow Workbench (Console Style)
• Vertical Laminar Airflow Workbench
• Restricted Access Barrier Systems (RABS)
• CO2 Incubators
• Ultralow Freezers
• Animal Transfer Stations
• Lab Design, May 11-14, 2025, Denver, CO
• FELASA, June 2-5, 2025, Athens, Greece
• ASHP Pharmacy Futures, June 7-11, 2025, Charlotte, NC
• ADLM, July 29-31, 2025, Chicago, IL
• CABS/ACSB, September 23-25, Ottawa, ON
• TRADELINE, October 20-21, 2025, Scottsdale, AZ
• Labs & Research Facilities Summit, TBD
• ABSA, October 24-29, 2025, Raleigh, NC
• ASHP Midyear, December 7-11, Las Vegas, NV
Vizient, HealthTrust, Premier, Ascension
Ordering Information
Visit our website, www.nuaire.com.


NewYork-Presbyterian (NYP) Brooklyn Methodist Hospital is a 650-bed acute care teaching hospital. As part of one of the nation’s most comprehensive academic healthcare systems, the hospital is affiliated with the prestigious medical school Weill Cornell Medicine.

~ NYP Brooklyn Methodist
According to Pharmacy Manager Yang Fan at NYP Brooklyn Methodist, the hospital has a long history of embracing innovation. “From the pharmacy’s perspective, we started our journey toward automation more than 20 years ago with the McKesson robotic system,” he says. It worked well, but as the robot was reaching its end of life, NYP Brooklyn Methodist was also embarking on a major renovation project. “It was just the right time to explore other options since we would be able to build out around whatever solution we chose,” explains Pharmacy Site Director Fabienne Vastey at NYP Brooklyn Methodist.
The pharmacy ultimately decided on the Swisslog Healthcare BoxPicker® and PillPick® systems because of their fully integrated, end-to-end functionality.
“One of the things we liked best about the Swisslog Healthcare robotic systems is that they are all-in-one systems,” says Vastey. When considering the PillPick, “It can not only store and dispense medications — it can repackage them, eliminating the need for additional resources or outside personnel to manage that process.”
The BoxPicker from Swisslog Healthcare is a secure system that automates medication storage and retrieval with intelligent robotic picking to help optimize pharmacy workflows. Its modular design allows for ambient, refrigerated and dual-temperature storage, and multiple workstations, which means the system can grow as pharmacy needs change. The PillPick system is unique in its ability to automate unit-dose packaging and dispensing, so it handles the entire process from manufacturer packaging to patient-specific dispensing.
“One of the things we liked best about the Swisslog Healthcare robot is that it is an all-in-one system. It can not only store and dispense medications — it can repackage them.”
Fabienne Vastey Pharmacy Site Director ~ NYP Brooklyn Methodist
“We package about 300,000 unit-dose tablets a month using the Swisslog Healthcare robotic system. We’re currently running at about 97% efficiency.”
Yang Fan Pharmacy Manager ~ NYP Brooklyn Methodist
By implementing these Swisslog Healthcare systems, NYP Brooklyn Methodist’s goal was to streamline pharmacy operations, reduce errors, and improve patient outcomes. Getting to that point proved to be something of a challenge, however.
Hardware issues impacted accuracy and created a lot of manual work, with staff having to review rejections and load medications back into the robot. “These issues really needed to be addressed in person by an experienced Swisslog Healthcare field technician,” explains Fan. “But we were about three months into the COVID-19 pandemic. It was difficult just to get clearance for people to even travel to us since we were right in the middle of one of the country’s hotspots at the time.”
After months of collaboration and working through pandemic challenges, the pharmacy was able to get the help they needed onsite to begin working through obstacles. Also, weekly meetings were instituted with Swisslog Healthcare service engineers to continually work on optimizing the robot further. As a result, the pharmacy operation has achieved the efficiency rate they were striving for from the outset.
“We package about 300,000 unit-dose tablets a month using the Swisslog Healthcare robotic systems,” says Fan. “We’re currently running at about 97% efficiency — meaning only about 3% of tablets are rejected for whatever reason.” The efficiency is the same for the Swisslog Healthcare dispensing functionality, he says, with 97% to 98% accuracy, which is critical for ensuring the right medications get to the right patients.
Once the Swisslog Healthcare system was up and running, Fan says they faced another challenge — syncing inventory data across multiple systems.
The hospital uses Epic Willow as its overarching pharmacy inventory management system, the Swisslog Healthcare systems for the central pharmacy, and Omnicell automated dispensing cabinets on hospital floors. “Drugs come in through Epic,” Fan begins as he describes the process. “They are then physically loaded into the Swisslog Healthcare BoxPicker and PillPick systems. From there, medications are distributed to patient care units — whether as floor stock or in Omnicell cabinets.” The problem they were having, he says, is that the three systems weren’t talking to one another. “The Epic system was not getting updated with accurate inventory levels.”
This breakdown in data sharing led to significantly inaccurate counts on the roughly 5,000 drugs the hospital keeps in inventory — leading to issues with over- and under-ordering. “When inventory counts are off, we can’t order accurately,” says Fan. “We’re also faced with questions about why we have certain drugs on the shelf we’re not using and why we’re running out of medications we actually need,” adds Vastey. This is compounded, she says, when there are industry-wide drug shortages. “We have a central pharmacy, but we have other locations too. Knowing how much is where — having an accurate count is critical for everyday decision making on what medications need to be ordered and prioritized.”
While there is a lot of messaging and communication standardization in the industry, like Health Level 7 (HL7) for streamlining the transfer of clinical and administrative data between various applications — there’s nothing for pharmacy inventory systems.
“ I think at the end of the day, we want to help other central pharmacies like ours function more efficiently with the best practices we’ve established.”
Yang Fan Pharmacy Manager
~
NYP Brooklyn Methodist
“
The dream for all of us is to have accurate data as a foundation – and then build smarter and smarter automation from there. I think the work Fan and his team have done is an amazing first step.”
Fabienne Vastey
Pharmacy
Site Director
~ NYP Brooklyn Methodist
“The automation is there, but they are all standalone systems from individual companies with unique communication platforms,” says Fan. “All these different inventory areas really need to talk to each other for the pharmacy to run efficiently.”
After consulting with peers and colleagues and conducting extensive research — Fan concluded that there was no existing solution. So, he and his team set out to establish their own standards.
To address their data sharing challenges, NYP Brooklyn Methodist’s pharmacy embarked on a project to build a three-way interface between the systems utilized from Epic, Swisslog Healthcare, and Omnicell. Led by Fan, this involved working with software engineers from each of the providers to develop a robust interface to simplify data synchronization. The goal was to ensure that inventory data from the BoxPicker and PillPick systems, as well as Omnicell cabinets, were accurately reflected in Epic — ultimately helping optimize efficiency and empower decision-making.
Inventory counts went from being accurate only about 5% of the time — to 99% of the time. “Now we have trust in all of the systems,” says Fan. “We know inventory data is correct and can make well-informed decisions to fulfill our mission of delivering the highest-quality patient care.”
“Fixing data synchronization between the systems was major for us in so many different ways,” says Vastey. “We were spending a lot of resources checking the accuracy of drug counts every day.” Fan agrees, saying there are also a lot of downstream benefits to having accuracy in the inventory data. One positive outcome is tied to another quality improvement project he’s been working on to reduce medication requests from doctors and nurses coming into the pharmacy. “In December, we successfully decreased the number of messages we received about missing medications by 26% — and we expect that downward trend to continue because of our optimization interface.”
Fan is currently working on a case study, which will be published this year, that maps out the inventory management interface project in more detail. “I think at the end of the day, we want to help other central pharmacies like ours function more efficiently with the best practices we’ve established,” says Fan. “The dream for all of us is to have accurate data as a foundation — and then build smarter and smarter automation from there. I think the work Fan and his team have done is an amazing first step,” Vastey adds.
The new PillPick® Octave manifests robotics to:
— Package and label the largest variety of drug forms into unit doses
— Optimize pouch design to reduce plastic material used
— Store more than 50K packaged unit doses
— Dispense packaged meds on one patient-specific PickRingTM for bedside verification
— Deliver comprehensive reporting on-demand
swisslog-healthcare.com/pillpick

• Cold Seal
• Tamper-Evident
• Moisture Resistant
• Ultraviolet Inhibitant
Unit Dose, Bar PharmacyCoding, & NursingExperts!Supply Unit Dose, Bar PharmacyCoding, & NursingExperts!Supply
• Reduces Cross Contamination

• Ideal for Meds Covered by USP 800
• 6 and 12-month Beyond-Use Dating
• 1-D and 2-D Bar Coding
• Flexible Label and Report Formatting
• Multiple Sizes and Shapes to Fit Your Meds & Storage Needs


Improve your Solid Oral Unit Dose needs with our comprehensive Bar Coding, Packaging, and Labeling Solutions — designed by healthcare professionals, for healthcare professionals.
President: Robert Braverman
Founded: 1971
Employees: Private
Toll-Free Phone: (800) 523-8966
Phone: (215) 396-8600
Toll-Free Fax: (800) 323-8966
Address: 70 Industrial Drive Ivyland, PA 18974
Website: www.medidose.com
Company Background
Medi-Dose/EPS was founded in 1971 when Milton Braverman, a former pharmaceutical company territory manager, saw the need for inexpensive, manual unit dose packaging allowing a hospital to convert from traditional dispensing. He developed the Medi-Dose System to package, handle, and dispense predetermined amounts of medication so they would be accessible for one regular dose. Comprehensive bar coding and labeling identification is accomplished using our innovative MILT 4 software. Because of the continued success of the MediDose System, Medi-Dose/EPS expanded its product line to include the TampAlerT System, offering tamper-evident liquid packaging without the need for heat tunnels and accessory sealing equipment, as well as a full line of supplies and disposable products designed specifically for the pharmacy and health care professional.
Product Overview
Medi-Dose provides Tamper-Evidence as well as UV and Moisture Resistance for one-year beyond-use dating. Our MILT® 4 software maintains packaging logs and lets you design and print your Lid-Label® Covers with color, bar codes, tall man lettering, and graphics. You can format the labels any way you want, directly from your own computer and printer!
Medi-Dose works well in any pharmacy operation and can be used with all classifications of drugs (meds covered by USP 800, chemo meds, compounded drugs, controlled substances, etc.). It’s affordably priced for all pharmacy budgets. For the best in manual unit dose packaging, check out Medi-Dose!
Features & Options
n Packaging and Labeling Solutions
• Simple to use — no extensive training needed.
• 1-D and 2-D bar coding — including NDC, lot numbers, and expiration dating.
• Ideal for hazardous medications and USP 800 drugs.
• Tall Man Lettering and dynamic formatting options.
• Built-in NDC lookup database and extensive image library.
• Packaging logs and error reporting.
• Six-month and one-year beyond-use dating.
• UV and moisture resistance.
• Tamper-Evidence
• 15 styles of blisters to accommodate virtually all meds.
• MPB® — Multi-purpose blisters to easily package large medications, compounded drugs, double and triple “0” capsules, unit-of-use packaging, repackaged medications, and suppositories.
• New Ointment Lid-Label Covers to package ointments in all Medi-Cup Blisters.
• No machinery or space requirements.
• Inexpensive — no capital outlay required.
Product Specifications
n Accompanying Labeling Software
• MILT 4
• MILT 3.0
• MILT 2.6
• Medi-Dose 2000
Trade Shows/Meetings Attended
ASHP Midyear, ASHP Summer, HCP Pharmacy & Pharmacy Purchasing Meeting, Joint Forces Pharmacy Seminar, National Pharmacy Purchasing Association, EAHP, IACP, and various state, regional, and pharmacy compounding conferences.
Ordering Information

For additional information, please contact us at (800) 523-8966, visit our website at www.medidose.com, or email us at info@medidose.com.

SureCost is The Smarter Purchasing Solution™ for health system pharmacies — automating purchasing and inventory workflows across departments and facilities in a single platform. Pharmacies reduce purchasing time by 50% and lower cost of goods by 2-5% without price shopping.
President & CEO: David Wagner, CEO
Founder & Chief
Product Officer: Calvin Hunsicker
Founded: 2002
Employees: 50+
Toll-Free Phone: (888) 363-7596
Address: 360 Central Avenue, Suite 800 St. Petersburg, FL 33701
Website: www.surecost.com
SureCost was founded by Calvin Hunsicker, a pharmacist who ran his own pharmacy business and saw firsthand the challenges of rising drug costs, complex vendor agreements, and inefficient purchasing processes. Determined to create a smarter solution, he built SureCost — a solution designed to help pharmacies regain control of their costs and maximize savings.
Backed by a team with over 100 years of combined pharmacy experience, SureCost now empowers more than 1,600 pharmacies nationwide, helping them save 2-5% annually on their cost of goods while cutting purchasing time in half. More than 10,000 pharmacy professionals rely on SureCost daily to streamline operations, improve efficiency, and boost profitability.
SureCost is more than a purchasing platform — it’s a strategic advantage. By centralizing purchasing across all primary vendors and over 350 distributors and manufacturers, SureCost gives pharmacies the power to make smarter, faster, and more cost-effective decisions.
With built-in inventory management, compliance tracking, and real-time vendor insights, SureCost helps pharmacies reduce costs, prevent stock shortages, and navigate complex contracts with confidence. Whether you manage a single hospital pharmacy or a complex health system, SureCost ensures every dollar works harder — supporting formulary compliance, 340B programs, and DSCSA readiness.
• Smarter Purchasing: Compare GPO, WAC, and private contract pricing across all vendors in one place to save 2-5% on drug spend systemwide.
• Optimized Inventory Management: Reduce out-of-stocks, manage formulary adherence, and eliminate wasted spend across departments and clinics with real-time visibility.
• Seamless Compliance: Stay ahead of vendor contract and DSCSA compliance with built-in compliance tools and automated tracking.
• System Integrations: Integrate seamlessly with core health system platforms — including pharmacy management systems, accounts
payable solutions, and other operational systems — to streamline purchasing and inventory workflows across the enterprise.
• Customizable and Scalable: Adapt SureCost to hospital purchasing workflows, approval chains, and compliance requirements.
• Time-Saving Automation: Reduce time spent on purchasing by 50% by cutting administrative burden with automated workflows, so teams can focus on patient care and strategic decisions.
The Bottom Line: SureCost helps health system pharmacies buy smarter, manage inventory better, and save more — because every healthcare dollar counts.
SureCost is a web-based platform designed to integrate with most hospital pharmacy systems and works seamlessly with the big three wholesalers. Health systems can also use the free SureCost mobile app to receive inventory across locations, ensuring flexibility, and efficiency.
With customizable user roles and permissions, SureCost allows pharmacies to tailor access based on responsibilities. Executives can view financial overviews and set purchasing guidelines, while frontline staff can manage daily purchasing and inventory tasks — ensuring the right people have the right level of control.
In addition to its core capabilities, SureCost offers DSCSA compliance packages tailored to health system needs — tracking the full supply chain journey of every pharmaceutical product to avoid errors, penalties, and disruptions.
SureCost’s Purchase Manager Compliance Insights™ is included, helping pharmacies maximize savings, improve GPO and wholesaler compliance, and optimize vendor relationships — all without adding extra work. Pharmacies can:
• Gain clear visibility into contract adherence, vendor agreements, and formulary compliance.
• Identify opportunities to increase rebate earnings and maximize cost-saving incentives by aligning purchases with vendor agreements and GPO and wholesaler contracts.
• Get real-time alerts when purchases fall outside preferred vendors or contracts, helping you prevent overspending and unintentional compliance errors.
• Access detailed reporting on purchasing trends, vendor performance, and compliance gaps to make data-driven decisions that boost profitability.
• Ensure staff follows purchasing best practices with automated buying recommendations that steer them toward the best product every time.



President & CEO: Dr. Ross Caputo
Founded: 2003
Employees: 100+
Toll-Free Phone: (800) 745-8916
Eagle provides expert scientific solutions, including analytical chemistry, microbiology testing, and R&D services. We specialize in validations, regulatory consulting, facility design, and cleanroom certifications — ensuring precision, compliance, and efficiency for your success.

Address: 11111 Wilcrest Drive, Suite 1000 Houston, TX 77099
Website: eagleanalytical.com
Background
Founded in 2003, Eagle began as a trusted partner in regulatory support for the compounding industry. Today, we are a premier scientific solutions provider with an FDA-registered CGMP testing and calibration lab spanning 80,000+ square feet. With 200+ years of combined expertise in the FDA-regulated pharmaceutical industry, 300+ services, and state-of-the-art technology, we help facilities mitigate risk with science-backed compliance and safety solutions.
Product Overview
n Laboratory Testing
Our segregated laboratory equipped with advanced technologies provides analytical chemistry and microbiological testing services. From raw materials to finished drug products, we support a wide range of pharmaceutical and compounding testing needs.
Our patented method for testing oil-based compounded products using ScanRDI® sets us apart — delivering fully compliant, rapid sterility results with a one-day turnaround. Additional services include microbial enumeration, endotoxin testing, method verification and validation, preservative efficacy, and stability testing, all designed to meet CGMP and USP requirements.
n Validation Services
Eagle offers a comprehensive suite of validation services to support your regulatory compliance and product quality goals. Our expertise ensures that your processes, methods, and cleaning procedures meet industry standards and regulatory expectations. We provide cleaning validation, disinfectant efficacy studies, stability-indicating method development and validation, USP monograph method verification, and microbiology method validations and suitability testing.
n Consulting
Our consulting team delivers customized GAP audits, due diligence audits, regulatory response support, SOP development, and environmental monitoring solutions. We also manage projects involving cleaning validations, disinfectant efficacy testing, stability studies, sterilization and depyrogenation cycle validations, aseptic process validations, facility design, and other specialized projects upon request.
Our engineers understand the vital role environmental control systems play in ensuring product quality and employee safety. We perform cleanroom certifications (including primary and secondary engineering controls, and fume hoods), equipment calibration, and equipment qualification (IQ/OQ/PQ). Following IQ/OQ, our team completes performance qualification (PQ) to confirm the equipment operates as intended, ensuring consistency and reliability.
“Our experience with Eagle has gone beyond getting reliable and accurate data on our daily testing needs. Eagle’s highly trained team provides quick solutions to our problems through their quality consultative services and courteous care. Being a customer of Eagle is a privilege. It’s a confident decision to add value to our businesses.”
—
Sherry Eghtesadi, Rancho Park
Eagle is uniquely positioned to serve a diverse range of clients, including pharmaceutical drug and device manufacturers, 503B outsourcing facilities, 503A compounding pharmacies, hospitals, and clinical research organizations. Our services reach clients across North America and globally.
RXinsider’s Pharmacy500 Marketplace 24/7/365
200+ Company Profiles, 80+ Aisles www.rxinsider.com
ASHP Pharmacy Futures 2025
June 7-11, 2025 | Charlotte, NC www.ashp.org/meetings-and-conferences/ pharmacy-futures
Cardinal Health RBC
July 9-12, 2025 | Denver, CO www.rbc.cardinalhealth.com
McKesson ideaShare
July 10-13, 2025 | Nashville, TN mckessonideashare.com
340B Coalition Summer Conference
July 21-23, 2025 | National Harbor, MD www.340bsummerconference.org
NACDS Total Store Expo
August 23-25, 2025 | San Diego, CA tse.nacds.org
NASP Annual Meeting & Expo
September 14-17, 2025 | Denver, CO naspnet.org/annual-meeting
Compounders on Capital Hill
September 16-17, 2025 | Washington, DC
a4pc.org/event/cch-2025
ACA Annual Conference & Expo
September 25-27, 2025 | Memphis, TN acainfo.org/ace
NCPA Annual Convention
October 18-21, 2025 | New Orleans, LA ncpa.org/annual-convention








ASCP 2025 Annual Meeting & Exhibition
October 23-26, 2025 | San Diego, CA annual.ascp.com
The Specialty Pharmacy/Distribution Session
November 16-18, 2025 | Bonita Springs, FL ecrm.marketgate.com/Sessions/2025/11/ SpecialtyPharmacyDistribution
The Pharmacy Technology, Services, Supplies, & Automation Session
November 17-19, 2025 | Bonita Springs, FL ecrm.marketgate.com/Sessions/2025/11 PharmacyTechnologyServicesandAutomationEPPS
ASHP Midyear Clinical Meeting and Exhibition
December 7-11, 2025 | Las Vegas, NV midyear.ashp.org
For listing and advertising details, please contact sales@RXinsider.com or call (800) 972-2083.











Left: GM7030 PATT2®, Personal Aseptic Technique test kit, 3mL ampules, 20mL vials, 100mL partially lled minibag. Below: ET1000 EnviroTest® TSA with Lecithin & Tween 80 growth media paddles for surface, air, or glove ngertip sampling.













President & CEO: Brady K. Schwarz
Founded: 1992
Toll-Free Phone: (800) 837-8361
Phone: (530) 272-8700
Fax: (530) 272-8702
Providing quality assurance products to hospitals and pharmacies practicing sterile compounding, with a singular focus on pharmacy compliance. Our long-standing regional distribution partners are able to provide exceptional in-person service and training.

Address: 1415 Whispering Pines Lane, Suite 150 Grass Valley, CA 95945
Website: www.qimedical.com
Q.I. Medical, Inc. was founded in 1992 with a focus on providing the sterile compounding industry with testing kits and products to assure proper technique and quality is maintained in their facility. We have a broad network of distribution partners throughout North America, Canada, and other international markets. These distribution partners provide in-person servicing and education to accounts to comply with USP guidelines.
Our cost-effective, disposable products are available for various needs in the compounding pharmacy. Aseptic technique validation kits are available for low, medium, and high-risk settings. We also sell multiple à la carte sizes of vials, bags, tubes, and syringes of growth media for facilities looking to create a custom media fill test that mimics their day-to-day practice. In regard to environmental monitoring/gloved fingertip sampling, our EnviroTest™ paddle is ideal for both. The rectangle shape and built-in hinge allow for testing critical areas such as the edges and corners of a hood and behind latch handles. Sterility testing products are available in both USP <71> approved methods of testing. For the full filtration method, the QT Micro™ and QT Junior™ systems work for both small and large volume solutions. For the direct inoculation methods, our TuffTest 2™ product is ideal.
All Q.I. Medical growth media is challenged with a battery of USP specified organisms and lot specific Certificate of Analysis (CofA) are available for download. All products are sold through regional stocking distributors in order to provide fast local service and support.
Additional products are available for validation of hazardous drug handling, automated compounder manipulation validation, filter integrity testing, and vial adaptors.
n Support Equipment Includes:
• Incubators
• Sterile Filters
• Vial Blocks
• UV Lights
To learn more about how Q.I. Medical can help your facility, please contact us at (800) 837-8361 or email info@qimedical.com.


• Ensures USP <797> compliance by maintaining the integrity of CSPs.
• Paper-free seals and packaging for enhanced safety.
• Sterile seals provide a 100% sterile barrier* with 3X greater adhesion.
*Tested in Nelson Labs, Salt Lake City, UT
• Patented dual-layer design delivers true tamper evidence with a clear “OPENED” warning.
• Helps prevent drug contamination and offers added protection for pharmacists.
• Tested for cold storage, withstanding temperatures as low as -20ºC.
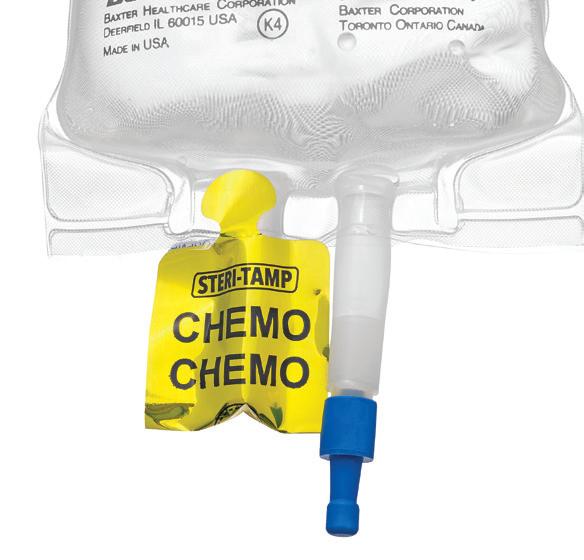









National Sales
Manager: Alex Meadow
Phone: (516) 374-8862
The only single-use, tamper-evident seal that provides and maintains a 100% sterile barrier.
Address: 2905 South Congress Avenue, Suite A Delray Beach, FL 33445
Website: steri-tamp.com
Email: info@steri-tamp.com
Established in 2010, Allied Pharmacy Products, Inc. was created from a commitment to enhance the preparation and distribution of intravenous medication within hospital pharmacies and compounding environments. The development of Steri-Tamp® stems from the insights of a pharmacist who recognized the imperative for improved tamper-evident measures and sterility in IV preparation. Throughout our journey, Allied Pharmacy Products has remained dedicated to listening and responding to the needs of pharmacists. This ongoing responsiveness has been instrumental in our continuous product line expansion.
The Steri-Tamp product line features seven cutting-edge tamper-evident seals. Our IV bag port seals come in blue, red, and yellow (labeled CHEMO) variants. For vial seals, we offer options in 13 mm (red), 20 mm (silver), and 28 mm (blue). Among these, our belly button bag seals (green) stand out for their ability to adhere to both the ICU medical “belly button” bag port and the top of a 13 mm vial.
Steri-Tamp has introduced specialized IV bag port seals tailored to meet the labeling requirements of USP 800 and ISMP. These seals offer a streamlined solution for handling paralytic agents and hazardous drugs in a unified workflow.
What sets Steri-Tamp seals apart is their true tamper-evident design. Utilizing dual-layer technology, these seals serve as a clear indicator to hospital staff if a bag or vial has been tampered with or used. Removing the top foil layer reveals an “opened” layer, after which it cannot be reapplied. Thanks to a 3X stronger adhesive, Steri-Tamp ensures a 100% sterile barrier.
In addition to our sterile options, we also offer two non-sterile syringe seals. These seals boast increased tensile strength, making them easy to remove from the liner and apply to a wide range of containers, including syringes, inhalers, EpiPens, insulin pens, and more. Our latest innovation, the Tamper-Clear Syringe Seal®, enables visibility of syringe markings and barcode scanning on syringes, inhalers, insulin pens, and other related items.
n Steri-Tamp IV Bag Port Seals
• Deliver a foolproof and potentially life-saving method to ascertain medication dispensing.
• Safeguard the IV admixture bag’s point of entry from contamination and accidental double dosing.
• Securely attach to the port through a simple “twist” mechanism, ensuring an airtight seal.
• The green belly button bag seal is purpose-built for the unique “belly button” port on ICU medical bags and can also be used on 13 mm vial tops.
n Steri-Tamp Vial Seals
• Designed to lie flat on the vial top, effectively preventing bacteria from entering the vial, without overlapping the edges.
• Preserve the medication’s integrity.
• Specially sized for 28 mm, 20 mm, and 13 mm vial tops, ensuring the right seal for the corresponding vial size.
n Steri-Tamp Syringe Seals
• Offer non-sterile solutions to provide tamper-evidence for syringes and other medical containers.
• The innovative Tamper-Clear Syringe Seal provides clear visibility to the syringe barrel and facilitates barcode scanning, making it ideal for sealing small oral, pediatric, and NICU syringes.
n Available in rolls of 1,000 seals each.
n All our seals contribute to reducing waste, streamlining workflow, and ultimately saving you time and money.
“We love your product!” — Pharmacy Coordinator, Lakewood, WA
“The only seal worth buying.” — Supervising Pharmacist, Bath, NY
“The 20 mm vial seals work perfectly.” — Pharmacy Buyer, Milton, MA
“Works great and they don’t fall off like the other ones.”
— Clinical Pharmacist, Murray, KY
Additional Product Lines
The Next Generation of Steri-Tamp seals is coming soon — offering enhanced adhesion and flexibility to meet evolving pharmacy needs.
Look for the new packaging with the “Next Gen” label!
Trade Shows/Meetings Attended
ASHP Midyear, EAHP, NPPA Conference, and various ASHP state affiliate pharmacy conferences.
Ordering Information
Available through major wholesalers and distributors. Visit our “order info” page at steri-tamp.com to find Steri-Tamp order numbers for your preferred vendor.
With its multiple, large-scale health facilities, Indiana University Health (IU Health) needed assistance a few years back managing their demand for intrathecal pain pump–compounded medications. Increasing demand for pain-pump refills, and the accompanying federal compliance regulations posed significant operational challenges. So they sought a trusted outsourcing partner that could reduce overhead, streamline access, and maintain the highest possible standards.



IU Health’s pain clinic was tasked with delivering roughly 100 intrathecal pain pump refills per week. Between significant compliance hurdles, and the escalating risk exposure associated with in-house compounding, it became clear that trying to do everything in-house was unsustainable.
Tate Trujillo, PharmD, BCPS, FCCM, FASHP, CPEL, Executive Director of Pharmacy, Clinical Nutrition & Indiana Poison Center at IU Health, explained, “We were trying to manage this out of our satellite pharmacy and were not meeting the need, particularly as the clinic was adding more and more providers.” This led the pharmacy leadership team to explore outsourcing solutions that could provide both regulatory, compliance, and workflow improvements.
IU Health needed to identify a pharmacy specializing in Category 3 medications that could meet USP <797> standards. Ideally, this pharmacy could integrate with IU Health’s existing workflows and streamline administrative burdens. After a thorough evaluation, IU Health chose AIS Healthcare, an outsourcing partner with advanced infrastructure, providing the highest standards in sterile compounding.
AIS immediately began providing:
• Quality control measures, from the industry-leading cleaning, disinfection, and sanitization of its facilities to the latest testing equipment
• Terminal sterilization for enhanced safety and compliance
• Redundant pharmacy locations for reliability and continuity of care, including comprehensive environmental monitoring and staff competency training
• Seamless ordering system that reduced administrative complexity
• Financial support services, including multiple billing options and the AIS patient assistance program
• Requisite regulatory documentation, with detailed quality reports outlining:
oSterility testing
oStaff competency training
oStock solution validation
oEnvironmental assessments
oOngoing compliance measures
Accurate billing was another hurdle. “Trying to actually bill effectively and get reimbursed appropriately is a challenge, and since AIS does a direct bill for us, those solutions have been solved,” said Tate Trujillo.
The transition, which took place more than 6 years ago, was designed for minimal disruption. AIS facilitated staff training and pharmacy onboarding. They also implemented a streamlined ordering process. This groundwork has allowed IU Health to integrate outsourced compounding services with remarkable efficiency to this day.


“Our top priority is ensuring patient safety while maintaining compliance with evolving regulations,” said Tate Trujillo. “AIS Healthcare’s robust processes, including doublegloving, redundant checks, and terminal sterilization, gave us confidence that they could deliver the quality and compliance we require.” Josie Klink, PharmD, Pharmacy Manager of Business Operations & Procurement, reaffirms that, “AIS has been an outstanding partner for us regarding the intrathecal pain pumps and this critical patient population.”
IU Health’s experience with AIS serves as a model for other health systems facing similar challenges. By prioritizing compliance, patient safety, and financial sustainability,
IU Health has successfully navigated the complexities of Category 3 compounding and created a framework for continued excellence in patient care.
See the case study video on AIS Healthcare’s partnership with IU Health at: aiscaregroup.com/videos/#case-studies
To learn more about how AIS Healthcare can help health systems navigate Category 3 compounding challenges, visit aiscaregroup.com or contact Chad Jeske, PharmD, Vice President, Hospital and Health Systems TDD Sales at cjeske@aiscaregroup.com

Lease a modular USP 797/800 cleanroom in days.

Pharmacy demands don’t wait — neither should your operations.
• Eliminate Downtime: Instantly expand your compounding space, indoor or outdoor, without construction delays
• No Build-Out Required: Prefabricated, validated, and delivered ready to use
• Regulatory Ready: Built to USP 797/800 standards for total compliance
• Fully Equipped: Delivered with laminar flow hoods, biosafety cabinets, controls and monitoring, and refrigeration
• Lease with Ease: Flexible rental terms to fit your needs

Behind Every Good Pharmacist ... is a Great Resource
Subscribe to RXinsider’s 411 Email Alerts. Receive our weekly newsletter with up-to-date pharmacy news, products/ services announcements, trends, and more. Join thousands of pharmacy professionals who depend on 411 Email Alerts to stay informed on the market.


® The TruDelivery® RTU portfolio helps meet the real-world challenges of hospital pharmacies—and is ready when you need it. It’s about time.




Reference: 1. Billstein-Leber M, Carrillo CJD, Cassano AT, Moline K, Robertson JJ. ASHP guidelines on preventing medication errors in hospitals. Am J Health-Syst Pharm. 2018;75(19):1493–1517.
Employees: 3,000
The TruDelivery® RTU portfolio helps meet the real-world challenges of hospital pharmacies — and is always ready when you are. It’s about time.
Phone: (800) 462-ENDO (3636)

Endo Website: www.endo.com
TruDelivery® Website: www.TruDelivery.com
Endo focuses on developing and delivering specialty pharmaceutical products — many of which are complex in nature and require unique expertise, technology, and equipment. We focus on medical therapeutics, specifically in the areas of urology, orthopedics, and endocrinology, and on sterile injectables for hospitals and health systems, along with generics. Our Endo Injectable Solutions business unit works with hospitals and healthcare systems to reduce complexity through operational efficiencies, innovating together to add value where it matters — from manufacturing to treatment — so that healthcare providers can focus on patient care. Our aseptic product portfolio spans various therapeutic categories and includes both branded and generic injectables.
Our RTU products, available through our TruDelivery® platform (TruDelivery.com), streamline hospital operations by eliminating the need for preparation time or transfer before patient administration. This can reduce preparation time, enhance convenience and workflow, and minimize potential preparation errors. All these advantages support quality care for patients in hospitals, clinics, and home health and long-term care systems. TruDelivery® RTU products require no compounding, diluting, or mixing1-7 and provide extended dating compared with compounded products.8-15* Most hospital pharmacy buyers can purchase TruDelivery® RTU products with their routine daily wholesale orders. These TruDelivery® RTU products are already available through your health system’s full-line wholesaler and on your group purchasing organization (GPO) contract.
TruDelivery® RTU products are designed to help address some of the challenges facing hospital pharmacies. Products that require the use of a separately packaged diluent, or that require mixing, compounding, or transferring to another container for administration, all require multiple steps before the medication can be administered to a waiting patient. Even under the best circumstances, extra steps may introduce risk of error.16-20 In the Food and Drug Administration’s (FDA) guidance to medication manufacturers, the agency encourages the manufacture of products specifically designed to minimize medication errors. Reducing the steps required for preparation and administration may help reduce risk of medication errors.19
Contact your Endo key account manager for more information on the below TruDelivery® products:
• Adrenalin® (epinephrine in 0.9% sodium chloride injection)
RTU Premixed Bags
• Bivalirudin Injection RTU Bottles
• Dexmedetomidine Hydrochloride Injection RTU Bottles
• Ephedrine Sulfate Injection RTU Vials
• Naloxone Hydrochloride Injection, USP RTU Prefilled Syringes
• PREVDUO® (Neostigmine Methylsulfate and Glycopyrrolate) Injection
RTU Prefilled Syringes
• Vasostrict® (Vasopressin Injection, USP) RTU Bottles
*For Category 3 compounded sterile preparations (CSP), the maximum beyond-use date (BUD) is 90 days at controlled room temperature (CRT), 120 days in refrigerator, and 180 days in freezer15 versus shelf life of 2 years at CRT for Adrenalin®, Dexmedetomidine, Ephedrine, Naloxone, and PREVDUO®8-12; 12 months at CRT and 2 years in refrigerator for Vasostrict®14; 72 hours at CRT and 18 months in refrigerator for Bivalirudin.2,13
References:
1. Adrenalin® (epinephrine in sodium chloride injection). Prescribing Information. Endo USA, Inc. 2. Bivalirudin Injection. Prescribing Information. Endo USA, Inc. 3. Dexmedetomidine Hydrochloride Injection. Prescribing Information. Endo USA, Inc. 4. Ephedrine Sulfate Injection. Prescribing Information. Endo USA, Inc. 5. Naloxone Hydrochloride Injection. Prescribing Information. Endo USA, Inc. 6. PREVDUO® Prescribing Information. Endo USA, Inc. 7. Vasostrict®. Prescribing Information. Endo USA, Inc. 8. U.S. Food & Drug Administration. New Drug Approval for Epinephrine: NDA 215875. April 21, 2023. 9. Data on file. DOF-DX-01. Endo USA, Inc.; July 26, 2023. 10. Data on file. DOF-EH-01. Endo USA, Inc.; July 31, 2023. 11. Data on file. DOF-NHC-01. Endo USA, Inc.; November 13, 2024. 12. Data on file. DOF-PRVD-01. Endo USA, Inc.; July 20, 2023. 13. Healthcare Distribution Alliance. Standard pharmaceutical product and medical device information: Bivalirudin Injection. July 10, 2023. 14. Data on file. DOF-VS-02. Endo USA, Inc.; September 20, 2023. 15. The United States Pharmacopeial Convention. USP compounding standards and beyond-use-dates. https://go.usp.org/2022_ Revisions_795_797. Accessed April 29, 2024. 16. Fanikos J, Burger M, Canada T, et al. An assessment of currently available i.v. push medication delivery systems. Am J Health-Syst Pharm. 2017;74(9):e230-235. 17. Institute for Safe Medication Practices. ISMP safe practice guidelines for adult IV push medications. https://www.ismp.org/sites/default/files/ attachments/2017-11/ISMP97-Guidelines-071415-3.%20FINAL.pdf. Accessed April 29, 2024. 18. Hertig JB, Degnan DD, Scott CR, Lenz JR, Li X, Anderson CM. A comparison of error rates between intravenous push methods: a prospective, multisite, observational study. J Patient Saf. 2018;14(1):60-65. 19. U.S. Food & Drug Administration, Department of Health and Human Services, Center for Drug Evaluation and Research. Safety considerations for product design to minimize medication errors: guidance for industry. https://www.fda.gov/media/84903/ download. Published April 2016. Accessed April 29, 2024. 20. American Society of Health-System Pharmacists. ASHP guidelines on preventing medication errors in hospitals. Am J Health-Syst Pharm. 2018;75(19):1493-1517.

Now, with Endosafe® cartridges, any user can generate rapid, automated BET results in about 15 minutes by loading a cartridge into our instruments and pipetting 25 μL of sample into four wells.
President & CEO: James C. Foster
Founded: 1947
Employees: 21,400
Stock Symbol: CRL
With Endosafe cartridge technology, any user can generate rapid, automated BET results in about 15 minutes, minimizing manual effort while aligning with regulatory standards and corporate sustainability goals.

Toll-Free Phone: (877) CRIVER-1 (274-8371)
Phone: (781) 222-6000
Address: 251 Ballardvale Street Wilmington, MA 01887
Website: www.criver.com
Charles River supports pharmaceutical manufacturers with tools that reduce contamination risk, simplify quality control, and protect patient safety. For over 30 years, Charles River Microbial Solutions has enabled QC labs to modernize through innovations in bacterial endotoxin testing, rapid microbial detection, and microbial identification.
Trusted by organizations worldwide, our platforms support regulatory compliance, streamline workflows, and cut waste. Backed by decades of experience in drug development and manufacturing, Charles River helps laboratories meet today’s expectations — and prepare for tomorrow’s challenges.
Endosafe cartridge technology streamlines bacterial endotoxin testing by automating critical steps — mixing, incubation, and detection — within a self-contained, single-use format. Each cartridge uses just 25 μL of sample and delivers results in approximately 15 minutes using kinetic chromogenic methods. Built-in wells for duplicate samples and positive product controls (PPCs), combined with an archived standard curve, eliminate the need for manual curve preparation, reduce analyst variability, and simplify training. Cartridges are available in a range of sensitivity formats from 0.005 to 10 EU/mL and meet global regulatory standards, including USP <85>, EP 2.6.14, and JP 4.01. They support traditional LAL and animal-free recombinant cascade reagent (rCR) methods — helping labs reduce false positives from β-glucans and transition to more sustainable testing practices. Fully compatible with the Endosafe instrument suite — including the portable nexgen-PTS™, benchtop nexgen-MCS™, and high-throughput Nexus 200™ — these cartridges allow QC labs to scale their testing efficiently while reducing waste, streamlining operations, and supporting compliance across diverse manufacturing environments.
Endosafe cartridges are supported by a suite of instruments designed to scale with laboratory needs.
n Endosafe nexgen-PTS is a portable system optimized for point-ofsample testing, raw material screening, and in-the-field QC. With a touchscreen interface, real-time result display, and secure user access, it’s ideal for fast, decentralized decisions.
n Endosafe nexgen-MCS brings cartridge testing to the benchtop with moderate throughput and independent sample processing. It supports multiple simultaneous tests and integrates with EndoScan-V™ for streamlined data transfer and audit readiness.
n Endosafe Nexus 200 automates high-throughput testing for up to 120 samples per run. Barcode tracking, walkaway operation, and LIMS integration minimize manual handling while supporting 21 CFR Part 11 compliance.
All systems are fully compatible with the complete Endosafe cartridge lineup, helping labs scale intelligently while meeting regulatory demands.
Endosafe cartridge technology is used by quality control labs across pharmaceutical, biotech, cell and gene therapy, compounding pharmacy, medical device, and nuclear medicine sectors. Labs performing raw material, in-process, and final release testing rely on Endosafe to meet global regulatory requirements while reducing test time and operational burden. Its flexibility and compliance-ready design make it suitable for regulated environments requiring consistent, validated results — highthroughput or low-volume products.
Charles River regularly shares insights and solutions at leading conferences, including Interphex, ISPE Annual Meeting, PDA Pharmaceutical Microbiology, Pharma Microbiology Congress, PharmaLab, Pharmig, A3P Microbiology, and the Kilmer Conference. These events focus on regulatory trends, environmental monitoring, and practical solutions for QC labs adapting to modern testing demands.
Access our catalog and implement any of our sustainable offerings from our industry-leading Endosafe portfolio.
Learn why over 5,000 organizations have adopted this rapid and compendial technology to streamline their manufacturing processes. Get more information at criver.com/endosafe.

The innovative, easy-to-use packaging system ideal for large volume sterile product preparation.
CEO: Marvin Samson
Vice President: Scott Samson
Founded: 1999
Toll-Free Phone: (877) 418-3600
Phone: (856) 751-5051
Fax: (856) 751-5044
Address: 1815 Garden Avenue P.O. Box 2730 Cherry Hill, NJ 08034
Website: www.samsonmt.com
Samson Medical Technologies, L.L.C. is a privately-held company founded in 1999 by Marvin Samson, a world-renowned leader in injectable manufacturing and delivery systems, to provide hospital and alternate site pharmacists with safe, convenient, sterile dosage preparation systems and products. The company was established in response to requests by leading healthsystem pharmacists to develop an alternative to cumbersome pharmacy bulk package vials. Samson Medical Technologies has worked with these leaders and a major supplier of anti-infective active ingredients to develop the SmartPak system that enables pharmacy professionals to prepare sterile dosage forms with a great degree of safety, accuracy, and ease.
Available as a sealed, additive-free, latex-free, polymer bag, each SmartPak bag contains up to 300 grams of a sterile powder protected from light, moisture, and oxidation by a foil overwrap. SmartPak is an easy-to-use, space-saving, patented packaging system for injectable drugs, ideal for sterile product preparation. It is a totally closed system, and its polymer construction avoids breakage and potential injury. Its design eliminates the usual time-intensive steps associated with reconstituting multiple vials of drugs, resulting in fewer manipulations, which, in turn, reduces the risk of contamination of product that is administered to a patient. SmartPak is compatible with commercially-available pumps, tubing sets, cassettes, and syringes
• SmartPak is composed of two bags.
• An inner bag is in direct contact with sterile drug product and is made of an additive-free polymer to ensure maximum stability and shelf life.
• Sealed double port on the bottom allows for introduction of diluent and subsequent withdrawal of the bag contents.
• A labeled, filled bag is placed into an outer foil bag.
• This outer bag protects the inner bag from light, moisture, and oxidation, maximizing stability of the drug product.
• Elimination of the usual steps associated with reconstituting multiple vials of drug.
• Totally closed system.
• Fewer manipulations.
• Greater assurance of sterility.
• No glass means no breakage.

• Reduced storage space requirements.
• Reduced waste disposal.
“Having bulk antibiotics in sterile form via SmartPak, enables us to maintain inventory of critical products at times of short supply. It has also reduced our batch preparation time and storage space required.”
— Rich Paoletti, MBA, RPh
“Our Staff reconstitutes over 800,000 doses of IV sterile products annually. SmartPak reduces time, manipulation, and potential for contamination.”
— Kevin Kyle, Pharmacy Buyer
Ensure temperature-sensitive medications stay within range, from storage to delivery, with Rees automated environmental monitoring.

Stay audit-ready with 24/7 monitoring, automated reporting, and adherence to FDA, cGMP, USP standards and more.
Receive real-time alerts for temperature excursions, humidity fluctuations, or any environmental risks, helping you act before issues escalate.
Scalable solutions tailored to fit your facility’s unique needs, from cleanrooms to warehouse environments.
Trusted by industry leaders for over 40 years to maintain operational excellence.
+Environmental Monitoring
+Preventative Maintenance
+CQV Compliance
+CTU Calibrations
+Temperature Mapping


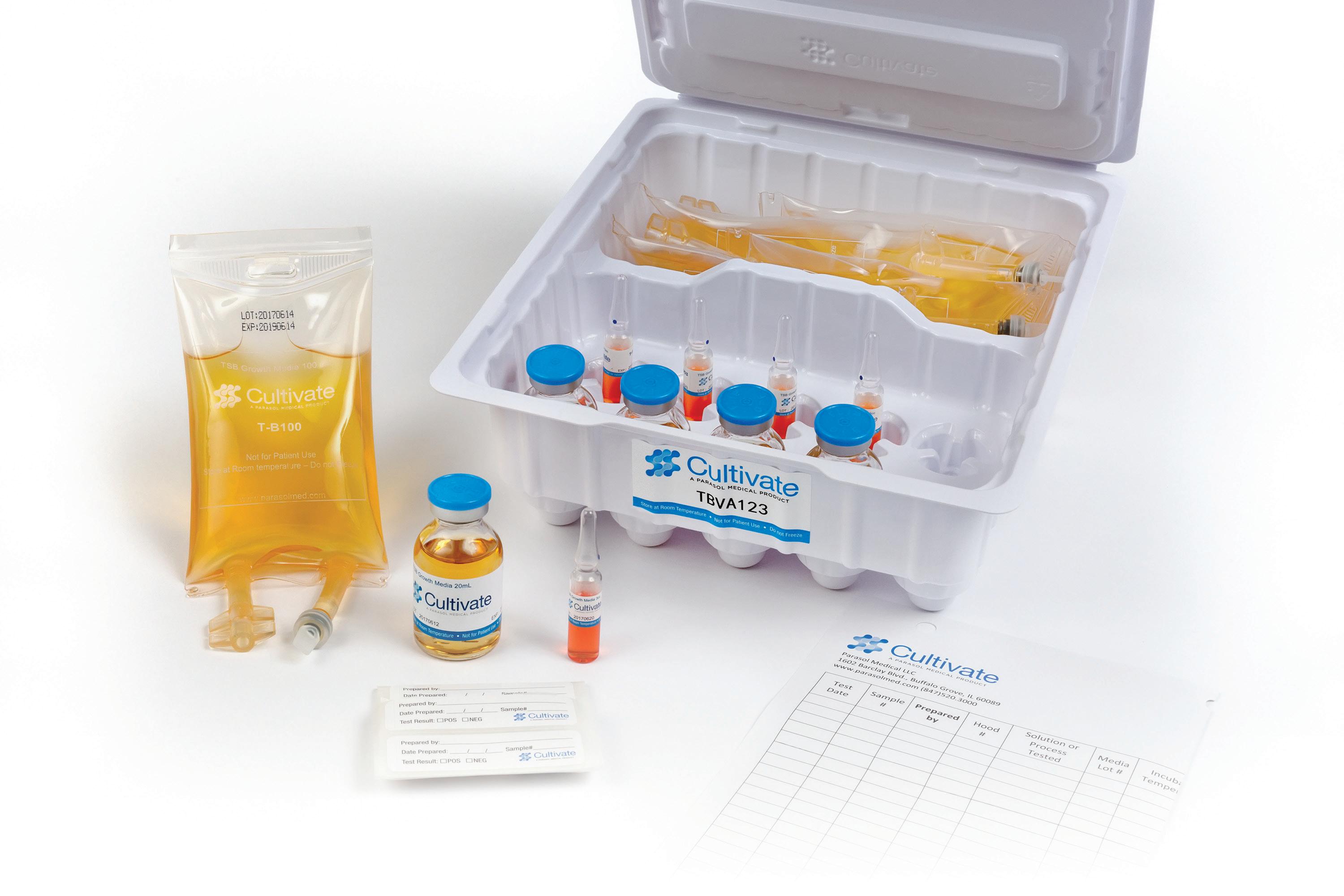


Dr. Reddy’s generic medications have been making life more affordable for patients for over 40 years. Today, our commitment to providing access to high quality, more affordable medications that patients and their doctors can count on remains unchanged. Why? Just ask the more than 23,000 committed employees at Dr. Reddy’s who know that Good Health Can’t Wait.
www.DrReddys.com