


Ce nter for Medical INNO VA TION





Ce nter for Medical INNO VA TION


PENN STATE COLLEGE OF MEDICINE is a key component of Penn State University’s interdisciplinary research enterprise, driving transformative discoveries from bench to bedside with the aim of improving human health today and for generations to come.
The Center for Medical Innovation is dedicated to "Empowering Innovation at Penn State College of Medicine for a Healthier Future." Our vision is to foster an environment that accelerates the development of innovative solutions for real-world health care challenges. This involves ensuring access to critical resources and capital, as well as actively engaging with internal collaborators and external partners.
As we continue to implement this strategic plan, the following report highlights the progress, achievements, and milestones of the Center for Medical Innovation, reflecting our unwavering commitment to translational research, innovation, collaboration, and advancing health care solutions that impact lives. This report serves not only as a record of our accomplishments but as a testament to the foundation we are building together for an even more impactful future.
THANK YOU for your continued commitment and support of medical innovation at Penn State College of Medicine.
Karen E. Kim, MD, MS
Dean
Dorothy Foehr Huck and J. Lloyd Huck Chair in Rural Health Research
Penn State College of Medicine
Erika Swift, MHA, MBA
Executive Director, Center for Medical Innovation
Professor of Practice
Penn State College of Medicine




Develop the OPTIMAL ENVIRONMENT with SUFFICIENT ACCESS to RESOURCES and CAPITAL
to enable RESEARCHERS, CLINICIANS, POSTDOCS and STUDENTS
to engage with COLLABORATORS and EXTERNAL PARTNERS
creating INNOVATIVE SOLUTIONS for REAL-WORLD PROBLEMS.
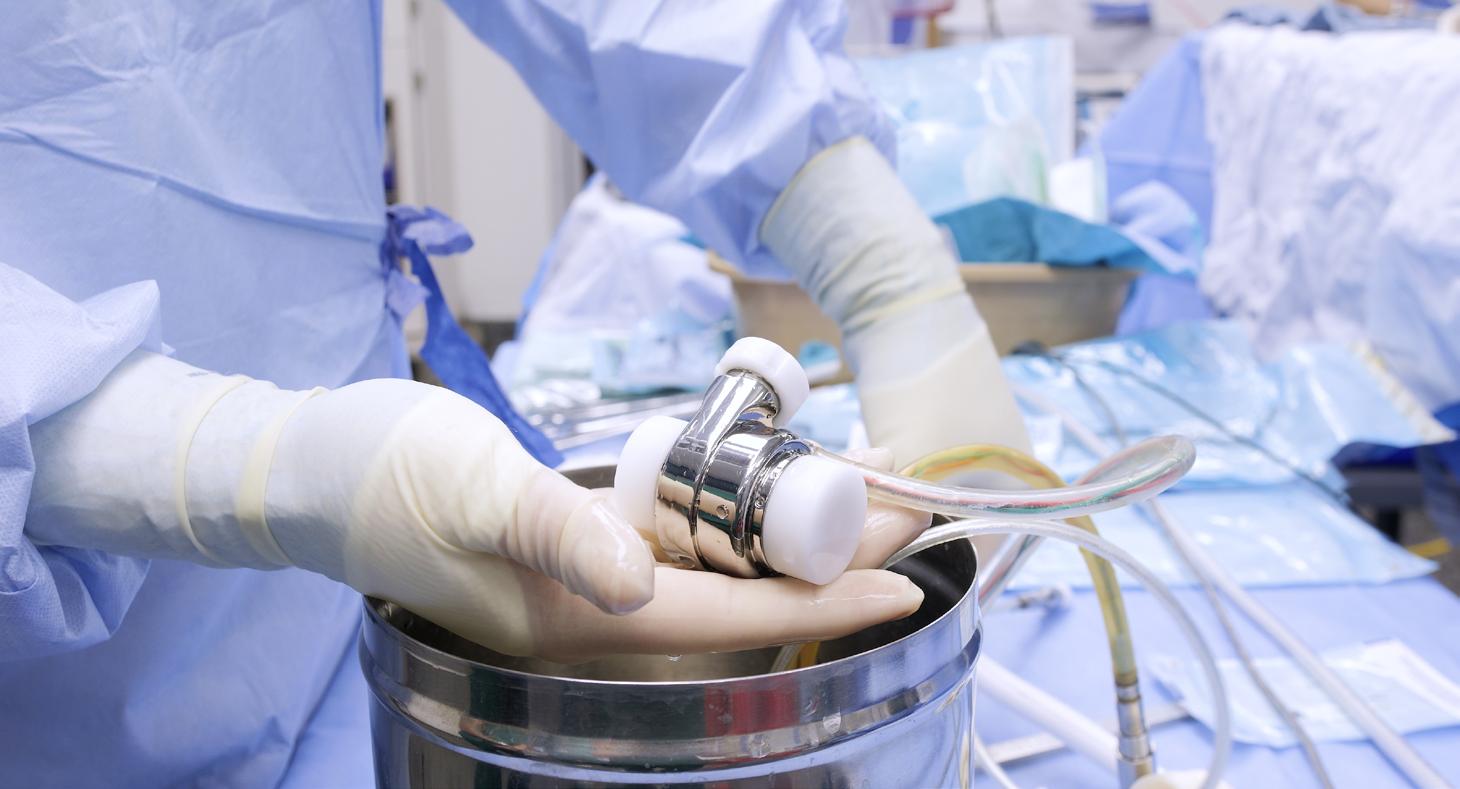
Penn State College of Medicine is pioneering a groundbreaking project to develop a wirelessly powered, fully implantable artificial heart designed to last 10 years.
Led by Penn State faculty Drs. Gerson Rosenberg, Joshua Cysyk and William Weiss, C. McCollister Evarts, MD Professor in Artificial Organs, the project aims to create a permanent total artificial heart (TAH) with dual pumps that mimic the natural Heart's function, adapting to patients of different sizes and adjusting to physical activity. The heart will operate wirelessly, reducing infection and mechanical failure risks, and will be made from materials that minimize clotting or bleeding.
Twenty-five years ago, Dr. Neil Christensen discovered neutralizing monoclonal antibodies for the human papillomavirus (HPV), which led to the development of the original HPV vaccine.
Dr. Neil Christensen and Matthew Smith, Penn State’s senior technology licensing officer, share their multi-decade relationship that led to the licensing of monoclonal antibodies to transform vaccine development worldwide.

“An estimated 10,000 people could benefit from a total artificial heart or donor heart each year, and only about 2,000 donor organs become available annually.”
Dr. Gerson Rosenberg

Meet Dr. Neil Christensen, director of the Gittlen Laboratories for Cancer Research, supported by the Jake Gittlen Memorial Golf Tournament, and professor in the Department of Pathology and Laboratory Medicine at Penn State College of Medicine. Learn more about his impact on vaccine development worldwide > >
Imagine a world where recovering from broken ribs no longer involves excruciating pain and long hospital stays. Dr. Peter Dillon, former chief of surgery and chief medical officer at Penn State Health, envisioned a minimally invasive solution to transform rib fracture treatment. Retired Penn State surgeon Dr. Don Mackay, along with active surgeon Dr. Randy Haluck and biomedical engineer Barry Fell, joined him on this journey. The team developed a cutting-edge technique that allows for the stabilization of broken ribs from the inside, utilizing a thoracoscope to provide surgeons with a magnified view of the injury— significantly reducing surgical time and trauma to patients.
This innovation, the AdvantageRib™ system, promises faster recovery and fewer complications.
The company SIG Medical was formed to bring the technology to market. Now FDA-cleared and successfully tested, AdvantageRib™ is revolutionizing rib fracture care nationwide.


Since joining Penn State in 2015, Dr. Steven Hicks has become a trailblazer in transforming autism care for children and their families. He enhanced diagnostic assessments by introducing developmental evaluations at Penn State Health’s general pediatric clinic, cutting patient wait times drastically. His groundbreaking research, exploring the use of saliva biomarkers for autism diagnosis, has led to international recognition, including an NIH-funded study, a patent, and a CLIA-certified lab test.
Dr. Hicks also spearheaded the creation of As You Are, an innovative telehealth platform transforming autism evaluations nationwide. This platform now handles a significant amount of all autism assessments in the U.S. and reduced wait times from months to days.
Dr. Hicks’s expertise spans clinical care, research, and health care delivery, making him a true pioneer in autism care and shaping the future of pediatric medicine. He was named Penn State College of Medicine’s Innovator of the Year in 2024.

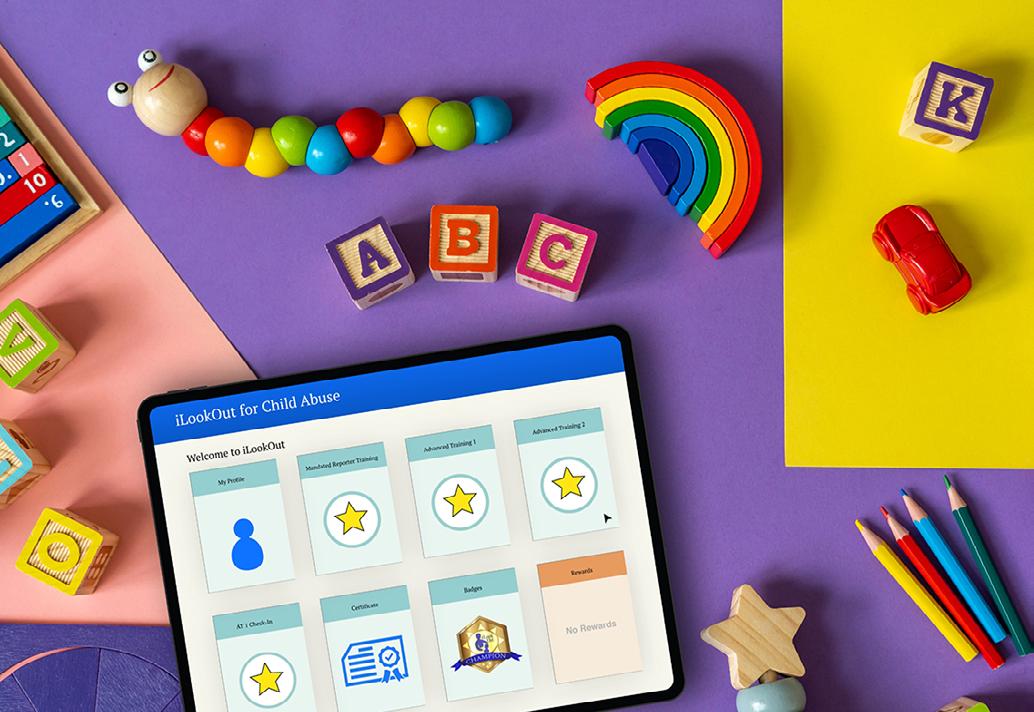
As part of the Penn State Department of Humanities and the Center for the Protection of Children, Dr. Benjamin Levi created an online interactive curriculum for mandated reporters of child abuse. Called iLookOut for Child Abuse, the program helps users better recognize and protect children at risk for abuse. iLookOut teaches people to identify red flags for child maltreatment, distinguish poverty from neglect, and know when and how to take appropriate action. iLookOut is the recommended mandated reporter training for HeadStart programs
in all 50 states. It is available at no cost to anyone in Pennsylvania.
Learn more at ilookoutpa.org
Dr. Levi is University Professor in Humanities at Penn State College of Medicine and a pediatrician at Penn State Health Milton S. Hershey Medical Center. In addition to his innovative work with iLookOut, Dr. Levi also studies advanced care planning for adults facing end-of-life medical decisions, developing innovative solutions to facilitate end-of-life discussions.
The Center for Medical Innovation's Innovation Fellows Program offers research and medical trainees the opportunity to transform translational research into a solution that addresses a critical unmet health need.
Fellows receive training to advance their technology and develop their careers while receiving multi-perspective mentoring from three key mentors: an internal subject matter expert (PI), an internal innovation mentor, and an external industry mentor. This comprehensive support cultivates experiential learning in both innovation and commercialization.
The program is designed to foster the next generation of health care innovators by equipping fellows with the knowledge, skills, and networks they need to bring transformative solutions to market.
FIRST TWO YEARS BY THE NUMBERS:
7 postdoctoral scholars 3 graduate students 2 medical students
The Innovation Fellows Program is supported by generous donations from the Peter and Ann Cullen Tombros Endowment for the Penn State Center for Medical Innovation.
· Intellectual Property: Navigating patents, licensing, and invention protection
· Customer Discovery & Competition: Understanding market needs and challenges
· Regulatory Processes: Mastering FDA approval pathways
· Partnerships: Building relationships and networking
· Fundraising & Private Equity: Securing financial support for tech development
· Additional Skills: Crafting pitch decks, honing presentation abilities, and more!
1 new technology license College of Medicine College of Engineering
Focus Areas: ophthalmology, microbiology & immunology, cancer research, chemical engineering, orthopedics, industrial & manufacturing engineering, mechanical engineering







Above, from left to right:
Jung Hwan Kim, PhD candidate – translational research focuses on developing drug delivery systems for osteoarthritis treatment, aiming to provide sustained, targeted relief with minimal side effects.
Zachary Koroneos, PhD candidate – translational research centers on the biomechanics of fracture fixation and bone defect repair.
Sai Kurapati, MD candidate and multiple scholarship awardee –translational research involves a novel intracanalicular implant to treat cataract surgery-induced inflammation.
”“As an aspiring physician with a love for innovation, this experience has been both eye-opening and inspiring, especially as I’ve seen how ideas move from the lab to real-world applications, with the potential to make tangible impacts on patient lives. Last month, I had the opportunity to present at my first major pitch competition— a unique and rewarding learning experience as a medical student and an empowering moment to advocate for women’s representation in this field.” – Sai Kurapati
Kevin Mekulu, PhD candidate – translational research aims to revolutionize cognitive health via AI-driven, noninvasive diagnostic and therapeutic solutions.
Tarek Haj Shehadeh, MD, postdoctoral scholar – translational research focuses on knee osteoarthritis.
Amandeep Singh, PhD, postdoctoral scholar – translational research focuses on the synthesis and biological evaluation of bioactive compounds.
Sean Thomas, PhD, postdoctoral scholar – translational research involves rehabilitation robotics, specifically the development and application of artificial muscles.
“The innovation fellowship program was a genuinely enriching experience for me. It helped place my research into a clear, practical context, making it more impactful and meaningful. Engaging directly with the people who would be affected by my work was not only deeply motivating but also instrumental in shaping and improving my research direction. Additionally, the mentors I worked with were both highly supportive and inspiring, which added great value to my journey.” – Sean Thomas, PhD

Learn more about the Innovation Fellowship
> > >
Moving medical innovations from the bench to the patient’s bedside is a complex, costly, and lengthy journey requiring diverse expertise and disciplined execution. The Center for Medical Innovation’s Pathways Innovation Accelerator implements a team-based approach by embedding a project manager within the research teams to coordinate milestone-driven technology development, holistically addressing downstream requirements, including intellectual property development, regulatory and reimbursement considerations, and customer discovery and business development.
The CMI Pathways Program offers a comprehensive suite of support, including seed funding, innovation education, technology development, business strategy resources, and access to a robust network. This integrated approach ensures that promising technologies are primed for licensing, facilitating their transition to the private sector for further development and, ultimately, delivery to patients.

Early-Stage: Proof of Concept Award Advanced Stage: Innovation Accelerator Award
Identify & Stimulate Medical Innovation
Devices, Therapeutics, Diagnostics, Digital Health
Pathway, Milestones, Funding, Endpoint
Project Manager
Level 1: Culture Change – educational seminars
Level 2: Innovation Training – experiential learning
Level 3: Specialized Knowledge – PI/project speci c
Subject Matter Experts
Market Analysis
Regulatory Strategy/Reimbursement
Network of Advisors and Partners
Industry Engagement

The CMI Pathways Program funding is supported by generous donations from the Anthony Pascotti Cancer Innovation Fund to support cancer research and the Lane, Pascotti, Sourbeer Medical Device Innovation Endowment Fund to support advancing novel medical devices.
Business Development Program
Resource Network Metrics/ Reporting Governance Deliver
Technology Development Program
Pre-clinical Study Design
IND-enabling Studies
Manufacturing Design Considerations
Engaging CDMO, CROs, Design Firms GLP & GMP Considerations
Respana is developing a novel drug to combat excessive immune response. Initially focusing on respiratory infections, the company’s therapeutic monoclonal antibody prevents excessive inflammation, avoiding the development of Acute Respiratory Distress Syndrome (ARDS) resulting from infections including influenza, COVID-19, and subsequent infections (viral or bacterial) – the antibody targets the complications caused by the immune response, not the infectious agent. With millions of people dying worldwide from respiratory infections each year, Respana addresses a significant unmet medical need.
Respana recently secured four C-suite members to their management team: Kevin Harter, CEO – serial entrepreneur; Scott Willett, CTO – 30+ years of experience in recombinant protein manufacturing; Jeremy Middleton, CBO – 35+ years of biopharmaceutical executive experience and Mike Lark, Senior Scientific Advisor. The company is raising a $20 million Series A round to generate significant clinical value in its pipeline.
Of the 3.7 million cataract surgeries performed annually in the U.S., >95% of patients experience postoperative inflammation. Many patients struggle with the standard of postoperative care, which requires patients to administer eye drops multiple times daily over four weeks. Patient non-compliance is 50%, while misapplication is a staggering 93%.
Drs. Seth Pantanelli, Su Yan, and Ethan Gerhard have developed a prototype of a small, biodegradable tear-duct implant that gradually releases Bromfenac, an anti-inflammatory drug, over 30 days. The copolymer used also has antimicrobial properties. Rabbit trials to investigate in vivo efficacy and safety are in progress. The inventors are in discussions with C-suite executives with broad-based experience in bringing medical technology to market and networks to form a startup.

Drs. Fadia Kamal and Amir Sheikhi have developed a paroxetine-based sustained-release injectable platform for the treatment of osteoarthritis. The paroxetine-based biomaterial injection stops the development and progression of osteoarthritis, allowing regeneration of structure and function.
Osteoarthritis affects more than 30 million Americans and is a leading cause of disability in the U.S., according to the NIH. Currently there are no curative solutions, only pain management or surgical interventions. Drs. Kamal and Sheikhi have shown evidence that their treatment prevents degeneration and promotes healing through direct injection into the affected joint. This novel therapeutic has the potential to decrease the disability and improve the lifestyle of osteoarthritis patients.
Dr. Arun Sharma has developed a small-molecule treatment for triple-negative breast cancer (TNBC). Patients with TNBC have poorer outcomes than those diagnosed with other subtypes. Chemotherapy treatments are available but produce systemic toxicity that can have long-term effects on quality of life.
Dr. Sharma’s molecule, ASR490, is orally bioavailable, inhibits tumor growth in TNBC, and does so with a high therapeutic index and no toxicity concerns. It also interacts synergistically with a common chemotherapy agent, doxorubicin, to inhibit tumor growth.
The AMT program identifies, develops and integrates cutting-edge technologies into clinical practice, medical education and research—providing advanced tools directly to clinicians, educators, and researchers within Penn State.
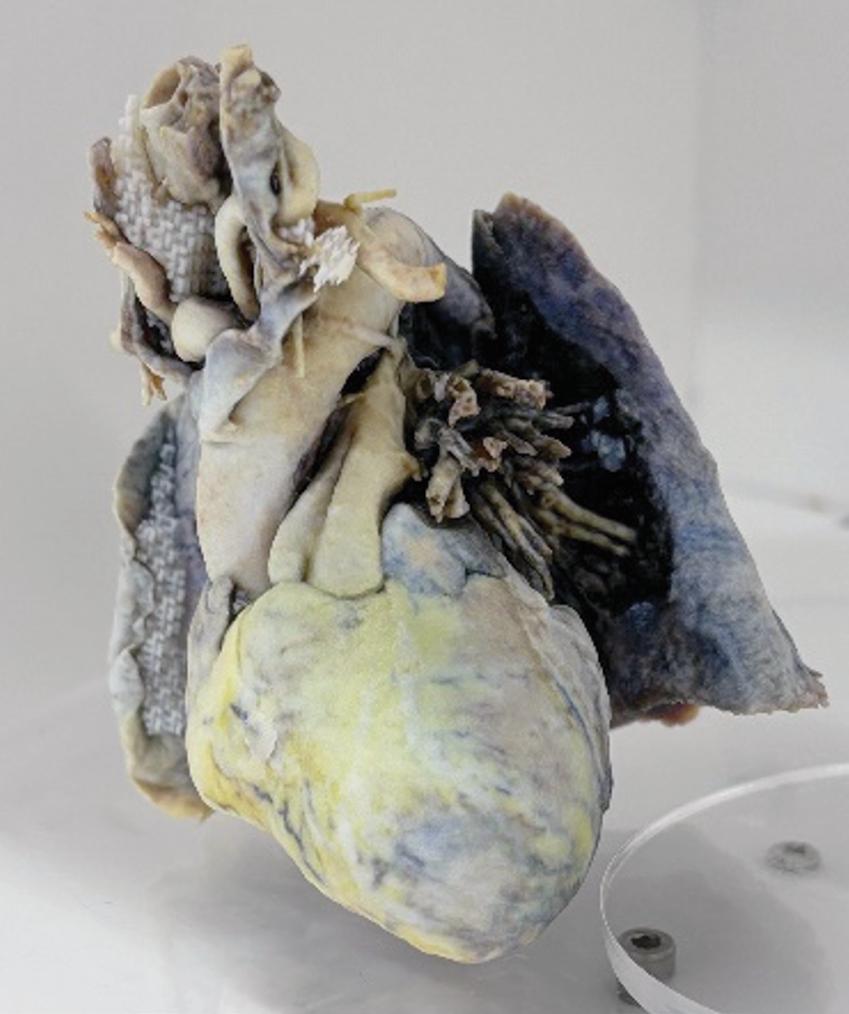
AMT projects have seen significant progress to date. Drs. Evan Goldman and Nicholas Meisel are developing 3D-printed cadaver models for medical education. Gross anatomy dissection is considered the ideal standard for anatomy education. However, gaining access to cadavers can be challenging, expensive, and require a range of resources not available widely at
institutions across the world. While digital visualizations may address certain aspects of anatomy training, complementary physical models can enhance educational outcomes.
To address this gap, supported by CMI's Advanced Medical Technologies Program, the team is developing a pipeline for creating full-color physical facsimiles through a combination of photogrammetry, mesh and texture editing, and material jetting. Through this method of full-color reproduction, true-to-life specimens for anatomy training can be generated and deployed in education. The team presented their results at the Solid Freeform Fabrication Symposium in August 2024.

The Advanced Medical Technology (AMT) Program is supported by generous donations from the Lawrence L. and Julia Z. Hoverter Charitable Foundation Endowment for Advanced Medical Technologies.

The Smart Medical Technology Hub (SMTH), set to be located at Penn State Harrisburg in partnership with Penn State College of Medicine, will be a physical incubator space where clinical providers, engineers, and team members can work together to address health disparities through research to solve clinical problems and develop novel technologies to address realworld health challenges.

Central to these efforts is the Center for Medical Innovation at Penn State College of Medicine, which is dedicated to advancing health care innovation. A major focus is the development of medical devices and technologies aimed at addressing unmet health care needs, particularly those faced by underserved rural populations. This commitment highlights a shared goal of improving health outcomes and driving economic development in the region.
Flexible space for project assembly and finishing
RAPID PROTOTYPING AND DESIGN LAB
Multiple digital fabrication tools, data acquisition systems, and soldering workbenches
3D-printing technologies for the creation of complex geometries in a broad palette of materials
ELECTRONICS & ASSORTED EQUIPMENT LAB
Computer stations, monitors, whiteboards, bench power supply
Team meeting and project design, CAD drawing
SUPPORT THE FUTURE OF INNOVATION
Build the Tech Hub with Us! Your support will help provide cutting-edge resources, collaborative spaces, and expert guidance to turn bold ideas into real-world solutions. Join us in shaping the future—every contribution makes a difference.
Make a gift online at engage.pennstatehealth.org by adding "The Center for Medical Innovation – SMTH" in comments or for more information, please contact Marissa Hoover, DEd, at marissa@psu.edu.




BioStrategy Partners, Inc. (BioSP) is a 501(c)(3) nonprofit consortium of academic medical centers and research institutes committed to the development and transfer of academic research into the marketplace.
Life Sciences Greenhouse Investments is an evergreen venture fund focused on helping early-stage companies to grow and flourish through infusions of capital at pivotal points of development.
Ben Franklin Technology Partners offers essential funding and resources for earlystage technology companies and helps established manufacturers innovate. As a statewide network of four regional centers, it also leverages Pennsylvania’s universities and fosters collaboration among companies, investors, and government to advance technology enterprise everywhere in the Commonwealth.
The Center for Medical Innovation is dedicated to fostering a dynamic and diverse ecosystem aimed at advancing health care innovation and entrepreneurship.
Become a Reviewer, Mentor or Supporter in Advancing Medical Innovation
CUSTOMER DISCOVERY INTERVIEWS
INNOVATION FELLOW MENTOR PROJECT REVIEWER
15–30 minutes to talk with faculty, postdocs and students about a problem they are trying to solve
1hr./month from Sept.–June to provide external guidance on innovation project, support career development
1x/year, review 1–2 proof-of-concept grant applications, providing industry or scientific feedback
If you are interested in engaging with us in the above capacities, please contact Saima Farooq at CMI@pennstatehealth.psu.edu
SUPPORT OUR PROGRAMS
Make a gift online at engage.pennstatehealth.org, scan the QR code or contact Marissa Hoover, DEd, executive director of development, at marissa@psu.edu or 717-531-1315 to explore other giving opportunities to support the Center for Medical Innovation.
Data represents Fiscal Year 2024 July 1, 2023, to June 30, 2024
42 NEW INVENTION DISCLOSURES
$3.9M
$97.5k TECHNOLOGY FUNDING
18 PATENT APPLICATIONS
32 TECHNOLOGIES SUPPORTED
10 LICENSES/ OPTIONS
446 AGREEMENTS HANDLED
3 U.S. PATENTS ISSUED
9 I-CORPS PARTICIPANTS



7 INNOVATION FELLOWS



28
STUDENTS ENGAGED IN PROJECTS
7 CAPSTONE DESIGN PROJECTS




Erika Swift, MHA, MBA (center)

Executive Director, Professor of Practice
Nuwan Sella Kapu, PhD, MBA (second from right)
Associate Director
Anne DeChant, MS, MBA (second from left)
Associate Director


Saima Farooq, MBA (right) Associate Innovation Project Manager
Ali Jabbarli, LLM (left) Grants and Contracts Administrator
Sheila Rundle, BS (not pictured) Senior Grants and Contracts Administrator
CMI@pennstatehealth.psu.edu med.psu.edu/innovation

Inspired by our legacy of academic excellence, groundbreaking research and life-changing patient care, we continue to change lives for the better. We continue to honor the dream of Milton S. Hershey to care for those around us.
The Center for Medical Innovation is dedicated to advancing educational excellence and strengthening technology development to achieve our vision.
Importantly, we extend our deep appreciation to our External Advisory Board for their invaluable guidance in shaping initiatives, evaluating technologies and providing mentorship. Their expertise and support are vital to our mission’s success.
As we continue to build momentum, we are expanding our efforts to include robust support for startup ventures, equipping innovators with the resources and infrastructure they need for entrepreneurial success.
Our strategy focuses on enhancing industry engagement and establishing new funding mechanisms at Penn State College of Medicine, paving the way for sustainable innovation.
Bruce Shook, MS, MBA
President & CEO, Intact Vascular, Inc. and Vesper Medical
Vice Chair, Penn State Research Foundation
Drew Kugler, MBA, MS
Principal, AJK Strategic Consulting, LLC
Former VP, Innovation & Product Development, AES Clean Technology
Gregg Gilbert
Chief Financial Officer, Mirador Therapeutics
Fred Pritchard, PhD
Senior Advisor, former VP, Global Drug Development, Celerion
Jacquelyn Fetrow, PhD
Co-founder, Principal, President, Keltrow Solutions LLC
Lynn Pritchard, PhD
Regulatory Affairs (ret), Pfizer
Mark Capone, MS, MBA
Former CEO, Myriad Genetics
CEO, Precision Medicine Advisors
Michael Golden, MBA
Head of Software, Glucotrack
Digital Health Research & Partnerships, Samsung Research America
Michele Washko, MA, MBA
President & CEO, Life Sciences Greenhouse Investments
Molly Fenningham
Director of Operations for Penn State Health, Value Partnership, Siemens Healthineers
Neil Kline, DO, MD
Entrepreneur in Resident, Physician Executive, Ben Franklin Technology Partners
Nishit Trivedi, PhD, MBBS, MBA
Portfolio Manager & Director of Life Sciences Research, Emerald Advisors
Peter Tombros, MBA
Senior Executive (ret), Enzon, Pfizer
Stella Vnook, PhD, RPh, MBA
CEO, Likarda; Founder & President, ACG Accelerator
Stephanie Reisinger, MBA
Senior Vice President & General Manager, Flatiron Health
Timothy Ritter
President & CEO, Universal Protective Packaging, Inc

We invite you to join us in shaping the future of medical innovation.
we can transform ideas into groundbreaking solutions that improve lives.