ASCO 2025 Highlights
Evelyn Taiwo MD
Associate Professor of Clinical Medicine (PAR)
Weill Cornell Medicine
New York Presbyterian-Brooklyn Methodist Hospital


Evelyn Taiwo MD
Associate Professor of Clinical Medicine (PAR)
Weill Cornell Medicine
New York Presbyterian-Brooklyn Methodist Hospital

▪ EA1181/neoCARHP(Her2+)
▪ DESTINY-Breast09 (Her2+)
▪ SERENA-6 (HR+)
▪ SOFT/TEXT (HR+)
▪ ASCENT-04/KEYNOTE19 (TNBC)
▪ SOUND/INSEMATRIALS (Surgery)
▪ OASIS-4 (Survivorship)
▪ TOPICALESTROGEN (Survivorship)
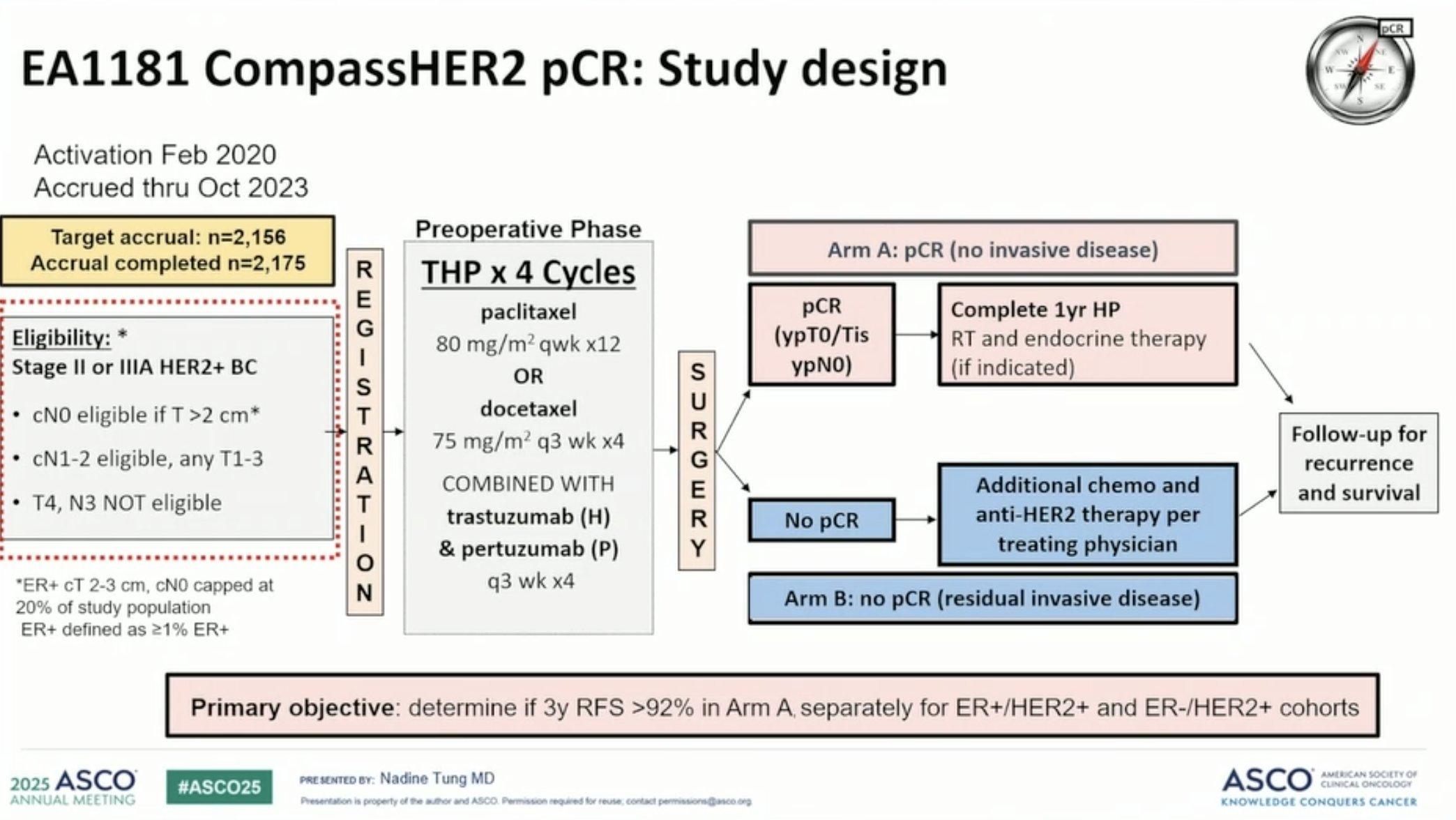
▪ SOC stages II-III Her2 positive BC
– Neoadjuvant chemotherapy;Taxane/Carboplatin/Trastuzumab/Pertuzumab followed by surgery
– 2175 patients were enrolled. Median age was 55 years (range 22-88 years)
– EA1181; Patients received 4 cycles of trastuzumab and pertuzumab (HP) with weekly paclitaxel (12 weeks) or docetaxel (q3w x 4), followed by surgery.
– pCR 44%, 63.7% in HER2+/ER
– Conclusions: NeoadjuvantTHPresulted in pCR in nearly two-thirds of pts with stage II/III HER2+/ER- and in one-third with HER2+/ER+ breast cancer. Lower ER expression and higher grade, and higher HER2Dx score were associated with higher pCR rates.


▪ Current 1st line treatmentTaxane/Herceptin/Perjeta (THP)
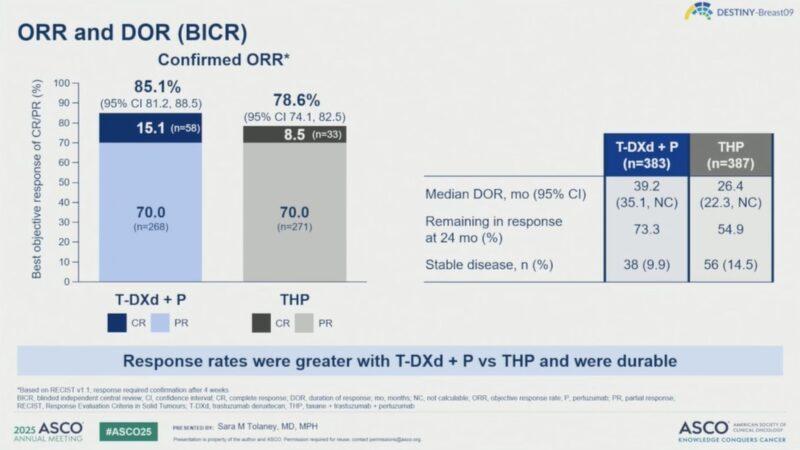
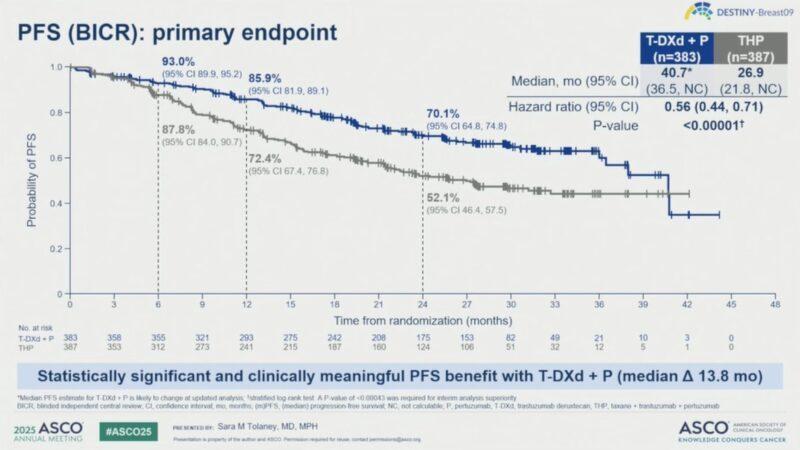
▪ Trastuzumab-Deruxtecan/Pertuzumab vs.Taxane/Trastuzumab/Pertuzumab
▪ Median PFS by BICR: 40.7 months withT-DXd + Pvs 26.9 months withTHP
▪ 24-month PFS rate: 70.1% forT-DXd + Pvs 52.1% forTHP
▪ These results will likely shift current standard of care for the last decade

▪ Long term follow of ovarian suppression withTamoxifen orAI in premenopausal women with ER+ breast cancer
▪ SOFTandTEXTenrolled premenopausal women with HR+ early BC from November 2003 toApril 2011 (2660 inTEXT, 3047 in SOFTintention-to-treat populations)
▪ 20-year data collection to assess DFS, BCFI, and OS
▪ DFS, BCFI and DRFI continued as significantly improved for E+OFS overT+OFS
▪ The 15y results of the SOFTandTEXTconfirm a role for OFS- and aromatase inhibitor-containing adjuvant endocrine therapy for premenopausal women

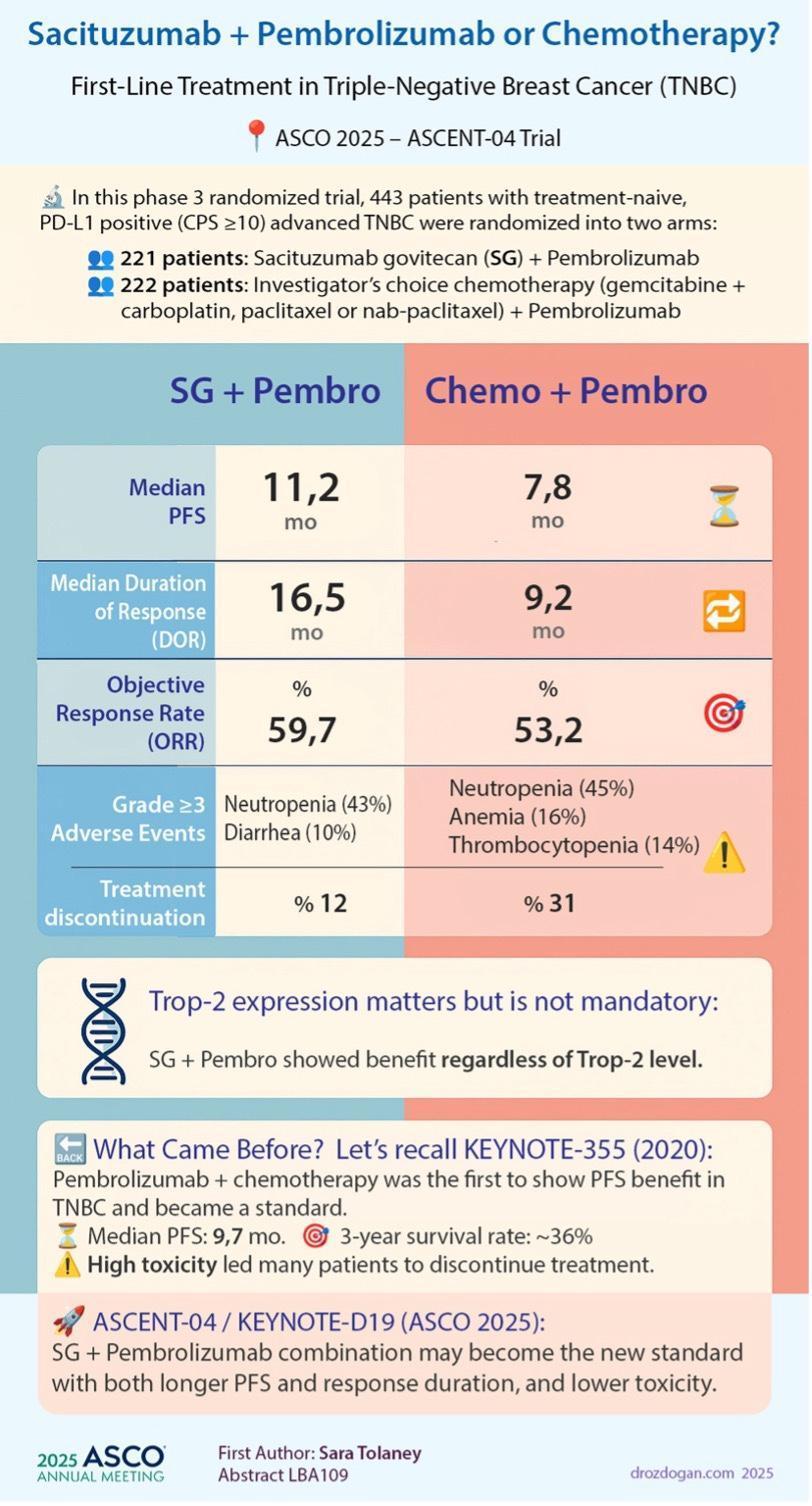
▪ The phase 3 SERENA-6 trial results show the benefit of using circulating tumor DNA(ctDNA) monitoring to guide treatment changes ahead of radiographic or clinical disease progression in patients with hormone receptor (HR)–positive/HER2-negative advanced breast cancer.
▪ An early switch to camizestrant plus a CDK4/6 inhibitor upon ctDNAdetection of ESR1 mutations during first-line therapy prolonged (PFS) versus the conventional approach of switching therapy at clinical progression (median PFS 16.0 vs 9.2 months at first interim analysis).
▪ OS data pending but will help determine advantage of ctDNA-guided treatment in the advanced breast cancer.

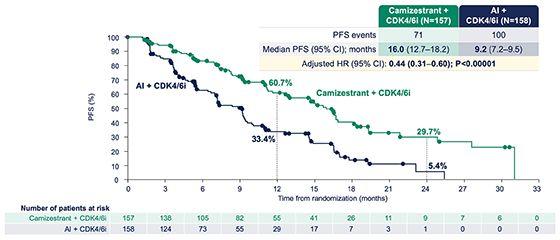
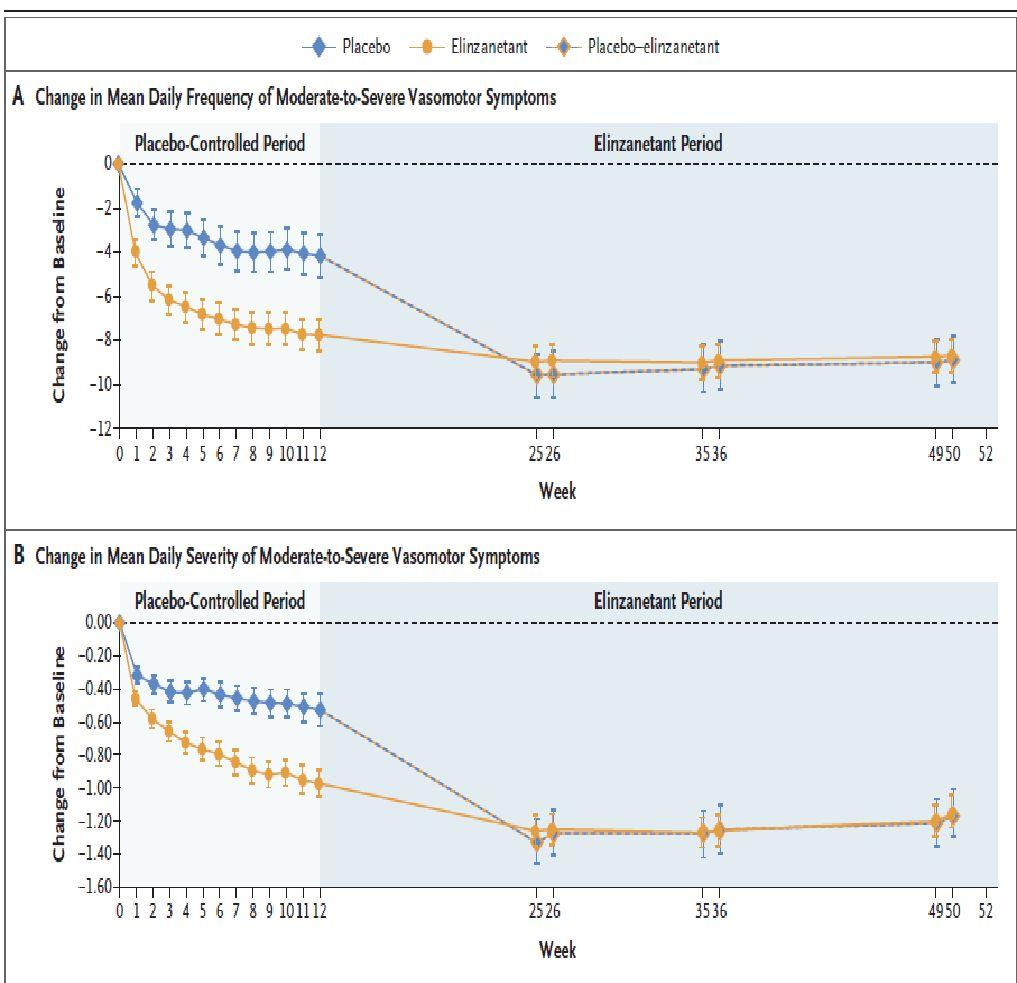
▪ Evaluated the safety and efficacy of elinzanetant in women aged 18–70 years being treated for (HR+) breast cancer and experiencing ≥35 moderate-to-severe VMS/week associated withAET.
▪ Non hormonal drug that regulates a group of estrogen-sensitive neurons in the hypothalamus, which become hyperactive with the decrease of estrogen during menopause, leading to VMS
▪ Women were randomized 2:1 to receive once-daily EZN 120 mg for 52 weeks or placebo (P) for 12 weeks followed by EZN for 40 weeks.
▪ Primary endpoints were mean change in moderate-to-severe VMS frequency from baseline to weeks 4 and 12. Secondary endpoints were mean changes from baseline in moderate-to-severe VMS frequency to week 1 and moderate-to-severe VMS severity to weeks 4 and 12.
▪ Somnolence, fatigue, and diarrhea were more frequently reported with EZN. FewerTEAEs were reported in both groups during weeks 13–52
▪ De-escalation study to evaluate the omission of SLNBx in early-stage breast cancer patients with negative axillary ultrasound.The trial's findings suggest that in some cases, axillary surgery can be safely omitted, potentially impacting future treatment guidelines
▪ Rationale for study is to eliminateAE of SLNBx. Compares to patients to no SLNBx, patients experienced – Pain – wound infection – axillary cording/webbing – Lymphedema (rare)


Olivia Mitchel, Paul Hsu, Jennifer Erdrich
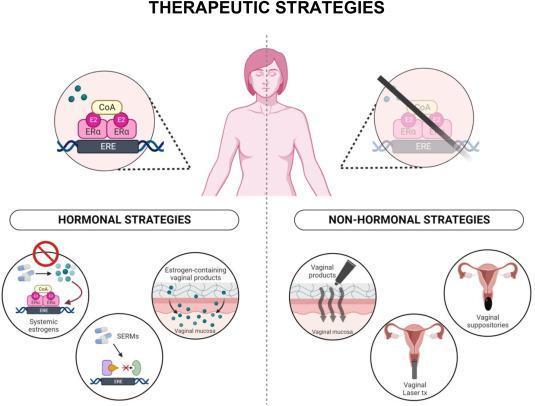
▪ It remains unclear if the use of local vaginal estrogen carries any increased risk of recurrence or mortality in patients with a history of breast cancer.
▪ Previous study show no increase in breast cancer specific mortality
▪ Patients continue to receive conflicting advice and potentially unnecessary avoid of hormone-based products that could possibly provide women symptomatic relief.
▪ Retrospective study of ~ 19,000 women, ≥ 65 years of age diagnosed between 2010-2017
▪ SEER-MHOS registry was performed, comparing breast cancer patients who used local vaginal estrogen (n=800) to those who did not (n=17,820)
▪ Among breast cancer patients who used vaginal estrogen compared to those who did not.
– increase in overall survival (HR=0.56, p<0.0001)
– Increase in breast cancer-specific survival (HR=0.53, p=0.014)
▪ Among those who used vaginal estrogen
– in overall survival for those with a duration of use >7 years
– compared to those with a duration of use <7 years (HR=0.01, p<0.0001)
▪ Subset analysis restricted to patients with hormone positive breast cancer showed a statistically significant increase in overall survival for those who used vaginal estrogen compared to those who did not (HR=0.62, p=0.0007),
▪ nonsignificant increase in breast cancer specific survival
▪ There is a role for deescalated neoadjuvant chemotherapy regimen in patient with early stage Her2 positive breast cancer.
▫ Discuss the possibility of omitting carboplatin.
▪ Elinzanetant is an FDAapproved medication to reduce the common side effects of Vasomotor symptoms (hot flashes)
▪ In women with a previous history of breast cancer, use of local estrogen vaginal estrogen to reduce genitourinary symptoms associated with antiestrogen therapy does increase the risk of breast cancer recurrence.
▫ Discuss use if conservative measures aren’t effective (non hormonal moisturizers)
▪ Post menopausal women with small tumors, low grade, HR+, and no suspicious LN on ultrasound may be able to avoid SLNBx