

Ovarian Germ Cell Tumors
Robert Neff MD

Associate Professor, Gynecologic Oncology
Ohio State University, James Cancer Center
“Ovarian Germ Cell Tumors 101”

Agenda
What are germ cell tumors?
Benign vs malignant
Work-up and diagnosis
Treatment
Surgery/preserving fertility/chemotherapy
Follow up/recurrence
Future treatment directions


What are germ cell tumors and how do we diagnose?

Ovarian germ cell tumors

Abnormal development of early germinal cells (eggs)
One of the most common benign tumors encountered [mature teratoma]
Malignant tumors are rare, 2-3% of all ovary cancers
Commonly found in children/adolescent up through early adulthood (peak age of incidence: early 20’s)
More frequent in women of Asian or African descent
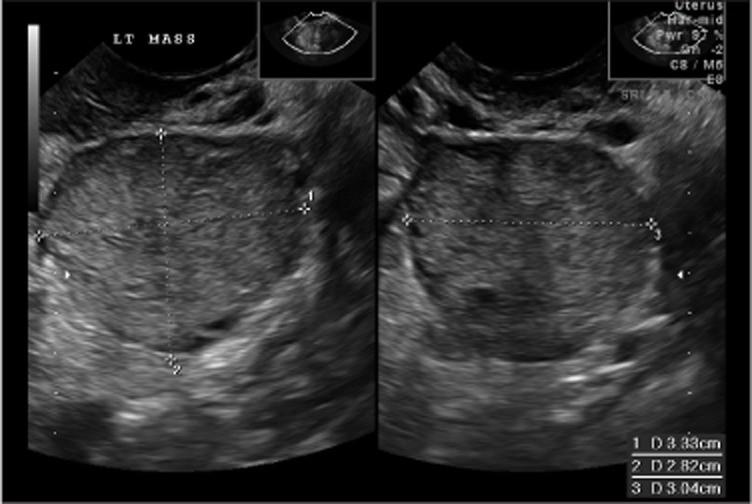
How do women present with germ cell tumors?

“A pelvic mass found in a young female patient”
Like other ovary cancers, symptoms are vague
Pressure, pelvic pain, urinary or bowel dysfunction
Due to age of diagnosis, abnormal menstrual cycles may be a symptom
Mass may grow rapidly, change to abdomen may be noted

Work up and diagnosis

Symptoms or exam findings should lead to imaging study
Pelvic ultrasound is usually first step, CT scan acceptable
No defining ultrasound characteristics for malignant germ cell tumors
Most commonly these are unilateral tumors (1 ovary affected)
Mature benign teratomas (10-15% bilateral)
Dysgerminoma [malignant] (10-12% bilateral)
Surgical resection usually required for diagnosis

Work up and diagnosis: “Why not biopsy the mass?”

Many diagnosed cancers are completed through biopsy
Ovaries reside without a covering in the abdominal cavity
Cancers of the ovary spread like a dandelion: cells released from the surface can attach elsewhere
Puncturing the mass increases the risk of spread and increases the stage.
Work up and diagnosis

Tumor markers are blood tests that look for proteins in body which may be released by malignant tumors

Very useful in germ cell tumors (both for diagnosis and monitoring treatment)

• Alpha fetal protein (AFP)
• hCG (pregnancy hormone)
• LDH (lactic dehydrogenase)

Types of Ovarian Germ Cell Tumors

Benign:
• Mature cystic/solid teratoma

Malignant:

• Dysgerminoma
• Yolk sac tumor (endodermal sinus tumor)
• Immature teratoma
• Non-gestational choriocarcinoma
• Mixed germ cell tumors
Dysgerminoma

Most common of all malignant germ cell tumors

Can be bilateral tumors, however usually visibly abnormal and not microscopic involvement Stage IA can be observed without chemotherapy
LDH (or hCG) most common tumor markers
Immature Teratoma
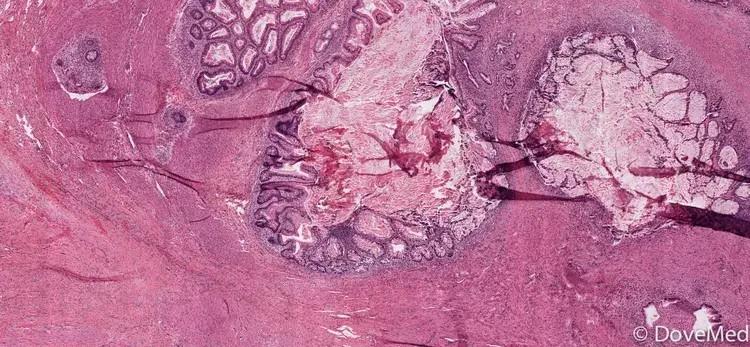
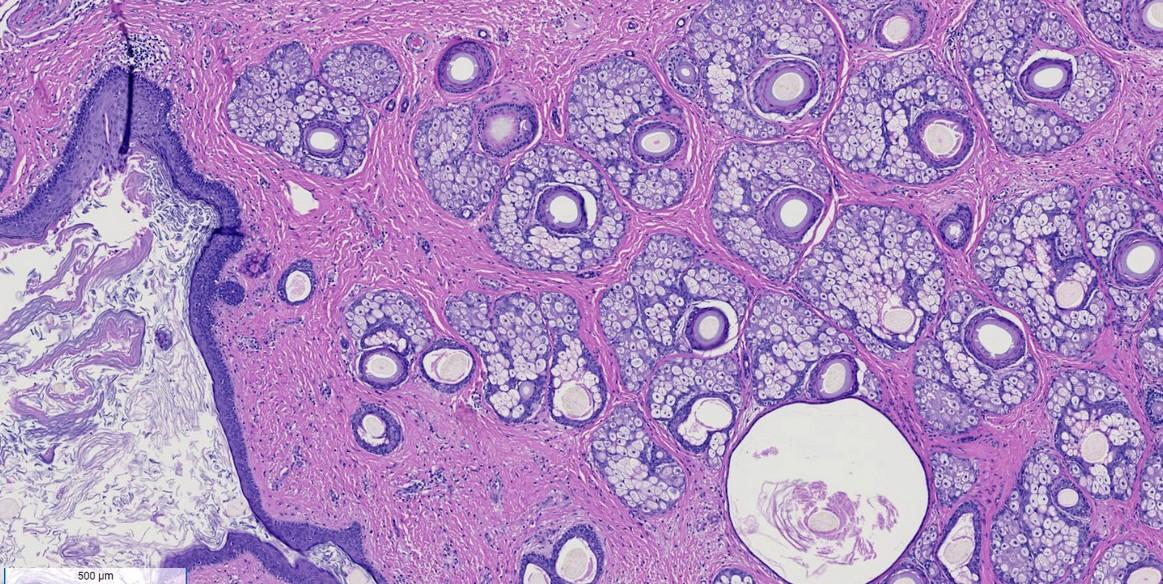
Mature benign teratoma vs immature teratoma (malignant)
Mature tumors often have hair/teeth in them
Grade of tumor can impact treatment
Relies on pathologic assessment of immature neural tissue
IT when treated with chemotherapy, can reemerge as mature teratoma (growing teratoma syndrome)
Important reason for close follow up


Yolk sac tumor (Endodermal sinus tumor)
Account for about 10-20% of malignant germ cell tumors
AFP is tumor marker
Rapid progression can be noted
Commonly diagnosed at stage I but due to poorer prognosis all patients are offered chemotherapy


Surgical management and chemotherapy
Surgical management of ovarian germ cell tumors

Surgical management is determined based on findings and patient desires for fertility (see next slide)
Due to age of typical patient population: reasonable to leave non-affected ovary
Surgery:
If primary tumor is larger, laparotomy incision required, otherwise reasonable to attempt minimally invasive surgery
Removal of affected ovary/tumor
Staging procedure: removing omentum, peritoneal biopsies, assessment of remaining ovary, lymph node biopsies

Fertility preservation
Due to high prevalence in younger women, fertility preservation must be accounted for
These tumors are most commonly stage I, without both ovary involvement
Attempting to preserve uterus and one ovary is ideal, in rare cases preservation of uterus only
A biopsy of a normal ovary is not needed / may increase risk of ovary dysfunction
Staging still recommended to help with chemotherapy decision
Referral to reproductive endocrinology specialist is advised
What if metastatic disease is found at time of surgery?

For all ovarian cancers, consideration of tumor debulking or removing all visible tumor should be made
Important to have surgical input from a gynecologic oncologist
Data is limited due to the rarity of this finding in germ cell tumors, but may favor debulking (removing all visible tumor) if feasible
Germ cell tumors are very chemo-sensitive
May limit the need for aggressive surgery upfront
1

Stage I: limited to the ovaries
Staging for germ cell tumors
2
3
4
Stage II: disease involves ovary and pelvic structures
Stage III: Involves ovary, surface outside of pelvis, +lymph nodes
Stage IV: distant metastatic disease (outside abdomen or liver metastatic disease)
Who needs chemotherapy?

Chemotherapy not recommended:

Chemotherapy recommended:
• Stage IA grade I immature teratoma
• Stage IA pure dysgerminoma

• All other stages of IT and dysgerminoma
• All stages of other germ cell tumors
What to expect from chemotherapy?

Multiple clinical trials led to a pivotal trial in GCT (GOG 78)
Set standard of care for front line chemo as BEP
Bleomycin
Etoposide
Cisplatin
High cure rates in complete resected disease (90+%) and advanced disease (~60+%)
Chemotherapy given for three cycles, with additional cycles added based on stage and response
What about side effects?

Immediate/acute side effects:
• Hair loss
• Nausea/vomiting/diarrhea/constipation
• Nephrotoxic
• Pulmonary fibrosis
• Specific to bleomycin, requires regular pulmonary testing before and during
• Ototoxicity
• Tinnitus or hearing loss
Late side effects
• Infertility/menstrual dysfunction/early menopause
• Secondary leukemia/lymphoma (etoposide dose dependent)


Disease surveillance/recurrence
What happens next? Surveillance

90% of recurrences develop in the 1st two years.
Dysgerminomas may recur later (>10yrs)
Generally: clinical, serological, and radiological surveillance every 3mo x 2yrs, then every 6 mo x 3 yrs.
Will get CT scans with surveillance even if tumor markers are followed
Dysgerminomas should be followed for at least 10 years.
Recurrent germ cell tumors

Prognosis depends on response to initial cisplatin therapy
Excellent if prior observation only
~20% of early-stage dysgerminoma can recur, but respond well to chemo
High dose chemotherapy with stem cell rescue is most effective option
Should seek a high-volume tertiary care center
Clinical trial participation is encouraged if available
Clinical trials for germ cell tumors

Accelerated vs standard schedule for BEP for high-risk/metastatic germ cell tumors Clincaltrials.gov iD# NCT02582697
De-escalation trial looking at observation vs chemotherapy in specific low-risk GCT
Clinicaltrials.gov iD# NCT03067181 Additional phase I trials available for relapsed/recurrent GCT

