The Value of Precis: A Process-First Planning Approach to Successful GMP Facility Design
Precis projects are built on a foundation of collaboration, technical rigor, and early alignment. By embedding process architecture at the center and surrounding it with in-house engineering expertise, our team helps clients define their vision, protect their investment, and move from blank page to build-out with confidence.
Introduction
For many organizations, planning a new Good Manufacturing Practice (GMP) facility starts with a blank page—an exciting yet often overwhelming challenge. Whether it’s a fast-growing pharmaceutical company developing its first manufacturing space or a life sciences firm expanding its infrastructure, the process is complex. The start of this endeavor involves navigating regulatory standards, defining operational needs, and making hundreds of critical decisions.
Importantly, this early stage is also when the organization must consider, not only the functional and technical requirements of the facility, but the broader vision it represents. In some cases, the focus is solving immediate project needs within an existing footprint. In others, particularly when building a new facility or expanding significantly, the project carries greater aspirations, serving as a physical embodiment of the company’s values, culture, and strategic ambitions. From the outset, planning must align with both the core operational goals and the long-term vision for how the space will be used and perceived, projecting the company’s identity and supporting its evolution.


When approached with the right framework, however, GMP facility planning can be efficient, collaborative, and strategically invaluable. GMP projects vary widely in scope and complexity, ranging from API and oral solid dose (OSD) manufacturing to sterile and parenteral production, clinical-scale operations to full commercial manufacturing, and a diverse array of specialized support functions. Each modality presents its own regulatory, technical, and operational considerations. At Precis, our team brings deep, cross-functional expertise to these challenges, having partnered with clients across the industry—from global pharmaceutical leaders to fast-growing innovators—to guide complex projects from concept to completion.
The following overview outlines the key phases of planning a GMP facility and highlights how Precis’ multidisciplinary approach helps organizations move forward with clarity and confidence.
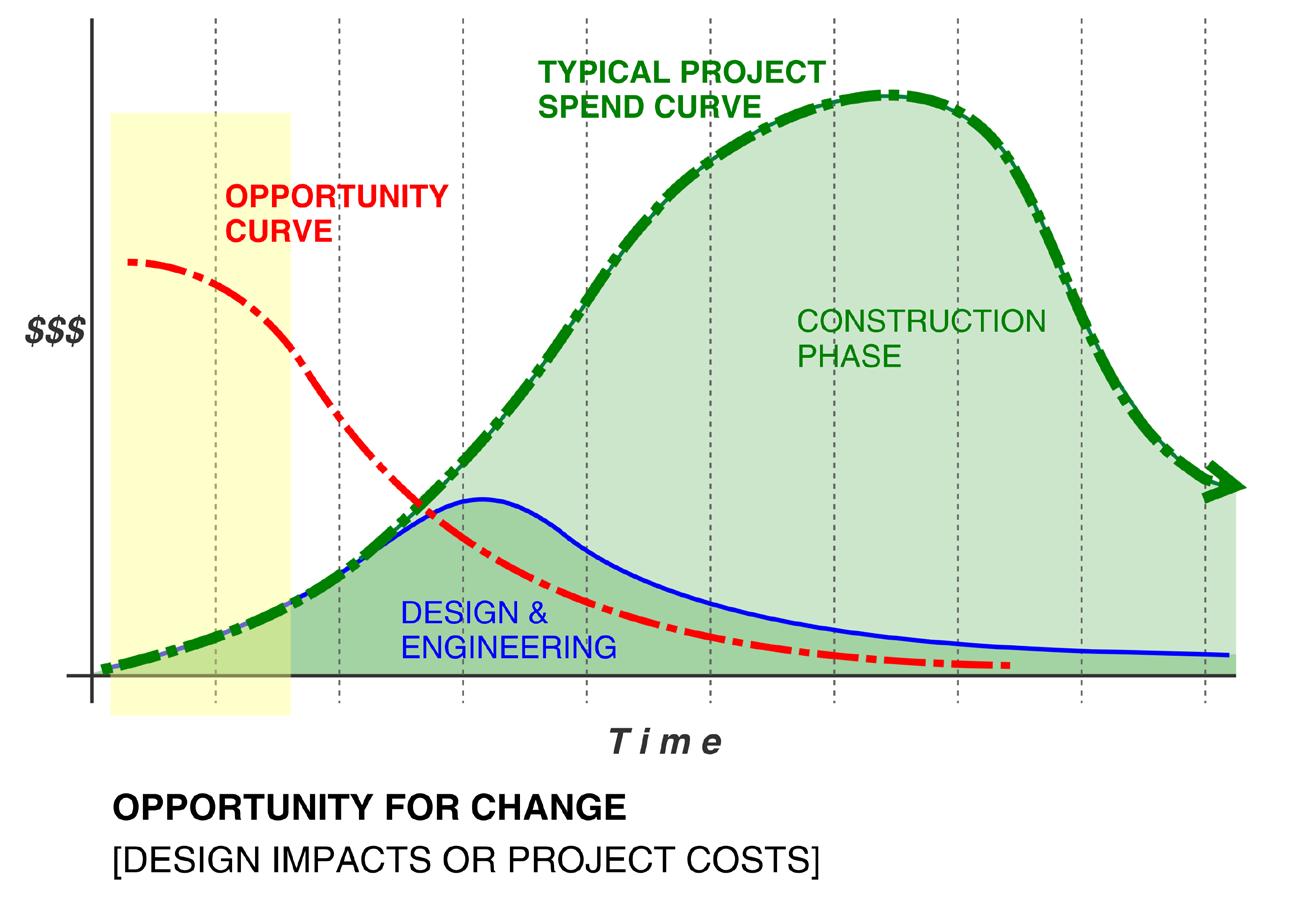
Phase 1: Incubation - From Questions to Concept
Every successful facility begins by understanding what the organization is trying to achieve.
Leading Life Sciences
The Value of Precis: A Process-First Planning Approach to Successful GMP Facility Design
In the incubation phase, the focus is on discovery: gaining a clear picture of core process needs, what challenges exist, and what future growth or operational models should be considered. For clients unfamiliar with GMP planning, this stage is critical to establishing direction and priorities.
Precis’ process architecture and process engineering teams lead this phase, engaging with stakeholders to uncover technical requirements, regulatory drivers, operational workflows, and business goals. Rather than starting with space planning, the team begins by exploring the process itself.
“Our approach to project initiation emphasizes active listening and immersive collaboration. We believe in fostering a deep, integrated connection between Precis, client leadership, key stakeholders, and end users. This early, immersive stage is essential to capturing the client’s goals and vision for the project, and laying the foundation for effective, tailored solutions.”
- Tim Lisle, AIA, Senior Director - Process Architecture, Precis
By facilitating early alignment and drawing insight directly from those closest to the process, the team ensures that planning is grounded in both technical requirements and operational reality.
Some of the foundational questions addressed include:
• What type of manufacturing and critical processes will take place in the facility?

• Who are the end users and how will they interact with the space?
• What regulations apply, and what level of segregation and environmental controls are required?
• How should the design anticipate growth and change?
This early planning ensures the facility is purpose-built to support both current and future operational demands.
Phase 2: Interpretation - Organizing the Vision
Once process and business needs are understood, the next step is translating that information into a technically sound, compliant, and operationally effective plan.
During the interpretation phase, Precis' process architects and engineers work collaboratively with architecture and engineering teams to map workflows into physical layouts. The goal is to define how the facility fits into the broader site context or organizational ecosystem and how spatial relationships support operational priorities.
Leading Life Sciences
The Value of Precis: A Process-First Planning Approach to Successful GMP Facility Design
This phase addresses considerations such as:
• Facility Program
• Departmental adjacencies and functional zoning
• Process flow and space utilization
• Regulatory compliance strategies
• Scalability and future adaptability
With an integrated team in place, Precis ensures that planning decisions are not only aligned across disciplines but also grounded in regulatory reality and business intent.
Phase 3: IntegrationConnecting the Bigger Picture
With the concept and process layout in place, the next step is evaluating how the new space integrates with the broader facility.
This phase involves assessing utility capacities, infrastructure constraints, and site-specific limitations that could impact feasibility, scope, or budget. The team also identifies high-level cost drivers, such as required utility upgrades or system tie-ins, before design progresses too far.
By addressing these factors early, Precis helps clients make informed decisions, avoid surprises, and ensure the project aligns with overall facility capabilities and long-term operational goals.
The Advantage of Early, Integrated Planning
Thoughtful early-phase planning consistently leads to smoother execution later in design and construction. At Precis, the value of this approach is magnified by having all critical
disciplines—process architecture, process engineering, mechanical, electrical, plumbing, fire protection, structural, sustainability, and architectural design—under one roof.
Proper planning on the front end also has a measurable impact on both timeline and budget. By defining scope early and aligning all disciplines from the beginning, technical conflicts can be identified and resolved before concept design documents are finalized. This ensures the project can avoid the costly rework, change orders, and schedule delays that often plague complex projects. An integrated approach helps protect capital investment and keeps projects on track from day one.
“One of the greatest benefits of our integrated model is the continuity of involvement from our subject matter leaders. The same team that helps define the client’s vision is still engaged as that vision becomes reality. That level of alignment, across disciplines and across phases, is what allows us to deliver facilities that work—not just on paper, but in practice.” - Tim Lisle, AIA, Senior Director - Process Architecture, Precis
Leading Life Sciences
The Value of Precis: A Process-First Planning Approach to Successful GMP Facility Design
Additionally, early alignment enables identification and procurement of long-lead equipment, such as cleanroom HVAC systems, process utilities, or specialized manufacturing equipment, at the right time.
This proactive planning helps protect the project’s critical path and reduces the risk of equipment-related delays during construction and startup.
For clients, this model has several additional advantages:
• A more clearly defined project scope from the outset
• Smarter allocation of time, capital, and resources
• Greater design cohesion across technical systems
• Facilities that function as intended from initial start-up
In regulated environments like GMP facilities, where coordination errors can lead to compliance challenges or costly retrofits, early integration across disciplines is essential.
The Client's Role in the Process
While technical expertise drives much of the planning and design, the most successful projects involve engaged and informed clients. The internal knowledge that organizations bring—about their processes, goals, and challenges—is vital in shaping the right solution.

At Precis, client input is not only welcomed but essential. Strong collaboration between client stakeholders and the design team leads to better decisions, more efficient workflows, and spaces that reflect the real-time needs of those who use them.
Clients are viewed not as spectators, but as strategic partners in the design process.
Final Thoughts: Building with Purpose
Constructing a GMP facility is never a simple undertaking. It demands technical depth, regulatory insight, and a collaborative mindset. But with the right guidance, it can be a clear and well-managed process—one that results in a facility that works as well in practice as it does on paper.
At Precis, our integrated architecture and engineering model, combined with deep pharmaceutical and life sciences expertise, enables us to guide clients through each phase of planning and execution with precision and care.
Leading Life Sciences
The Value of Precis: A Process-First Planning Approach
to Successful GMP Facility Design
From the initial vision to facility commissioning, the focus remains on building intelligently around the process and delivering spaces that meet regulatory demands, support operations, and enable long-term success.
What sets Precis apart is not just what we do, but how we do it. Our approach is personal, flexible, and streamlined—rooted in true collaboration and built around the unique goals of each client.
Our subject matter leaders stay involved throughout the entire project lifecycle, ensuring continuity, responsiveness, and technical precision from start to finish.
This consistency is one of our core strengths at Precis—the deep relationships we form, the hands-on involvement of our senior technical team, and our ability to align quickly and adapt thoughtfully to each client’s evolving needs.
Precis is proud to serve as a trusted partner to organizations seeking a team that doesn’t just deliver facilities, but delivers confidence, clarity, and lasting value.
Meet Our Process Architecture Team

