1 0 Y E A R I M P A C T R E P O R T 2 0 2 4





1 0 Y E A R I M P A C T R E P O R T 2 0 2 4




The Pediatric Neuro-Oncology Consortium (PNOC) and the PNOC Foundation work in tandem to combat pediatric brain cancer through research and funding. PNOC, a consortium of leading hospitals and research institutions, spearheads the development of innovative treatments and clinical trials for pediatric brain cancer. The PNOC Foundation supports this mission by raising funds, increasing awareness, and mobilizing resources to finance these cutting-edge trials Together, we form a powerful alliance that has made great strides over the last 10 years Looking ahead, we are committed to building on this success, with the goal of making the next decade one of unprecedented progress in the fight against pediatric brain cancer, bringing hope and healing to more families worldwide.
When it comes to saving lives, speed and smart collaboration are essential Unlike most research entities, the vast majority of PNOC's funding comes from private philanthropy, not federal sources This financial independence grants us a significant advantage: speed Our agility in securing funds enables our scientists to rapidly transform cutting-edge ideas into life-saving clinical trials, drastically shortening the journey from concept to execution. This efficiency is not just a matter of logistical prowess but a response to the urgent needs of children facing brain cancer, a demographic tragically underserved and overlooked by conventional funding models.

5,000 children in the U.S are diagnosed each year with brain tumors, more than 500 of those will die.
Over 40,000 children are estimated to be living with a brain tumor in the U.S.
PNOC’s approach, catalyzed by private philanthropy, has enabled the launch of over 30 clinical trials, offering new hope and possibilities to children who have exhausted standard options. PNOC Foundation is proud to be the biggest philanthropic contributor to PNOC.
47,000 years of life are lost each year to pediatric brain cancer. Sources:1
Among all childhood cancers, brain tumors are the leading cause of death.
PNOC stands out in pediatric oncology research with its agile, philanthropy-driven approach, reminiscent of a startup's ethos, yet backed by a decade of consortium experience. Reflecting the innovative and entrepreneurial spirit of its Bay Area roots, the consortium's success is a testament to the power of collaborative, accelerated research driven by a philanthropic model. With plans for continued global expansion and a relentless pursuit of cures, PNOC continues to redefine the landscape of pediatric cancer research. This unique blend of startup-like agility, deep-rooted expertise, and a philanthropy-driven mission positions PNOC not just as a leading consortium but as a trailblazer, paving the way for a future where we lose no child to brain cancer
Our philanthropic donors and partnerships support this vital work, knowing there has never been a more impactful time to give to pediatric brain cancer research.

42
oftheworld’stop children’shospitals areparticipatingsites
10 countrieshavehad PNOCclinicaltrials availableforchildren inneed
275+
leadingpediatricbrain tumorspecialists workingtogether
1000+
childrenandyoungadults havebeenenrolledin PNOCstudies


13 years ago when our own son George was diagnosed with brain cancer, treatment options were limited – there was no precision medicine, no genetic sequencing or subtyping, there weren’t any new promising therapies, very few clinical trials and treatment centers were working largely in isolation from each other
We were shocked to learn that scientific collaboration was not the norm, far from it Academic advancement, competition for the limited available research grants and the rare nature of this disease and corresponding lack of economic incentive for Big Pharma were all forces conspiring against cooperation and collaboration to defeat this monster. Treatments and outcomes had not progressed in over 50 years.
When Dr’s Michael Prados and Sabine Mueller at UCSF asked us to form a non-profit to support a transformative initiative of true collaboration, institutional partnership and information sharing to accelerate discovery, progress, better treatments and ultimately a cure, we didn’t hesitate. PNOC Foundation was formed to exclusively support the work of the PNOC Scientific Consortia.
This past year we celebrated the end of the first remarkable decade in which the idea of PNOC was conceived and has now grown into the world’s leading international pediatric brain cancer consortia with over 42 sites across 10 countries and five continents, enrolling over 1000 children with life threatening CNS cancers into 31 clinical trials giving hope and access to kids and their families fighting the deadliest of childhood cancers
As we stand at the gates of this next decade we are redoubling and re committing our efforts to create a world in which we lose no child to brain cancer as we harness the scientific breakthroughs and discoveries of this past decade and amplify them with the powerful new discoveries and technologies that will define the next.
None of this would be possible without the incredible support of you our loyal donors. Whether you have given through the PNOC Foundation directly or to the PNOC Scientific Consortia we are incredibly grateful for your support over these last 10 years and we are proud to share this report with you that showcases PNOC’s progress and tireless efforts to change the landscape of pediatric brain cancer treatment.
In Mission,
Allyn & Bruce Campbell

As we reflect on the past decade since the inception of the Pediatric Neuro-oncology Consortium (PNOC), our hearts are filled with a sense of profound gratitude and a renewed sense of purpose. 10 years ago, driven by the unacceptable reality that children with brain cancer were facing bleak prospects, we embarked on a mission to change the narrative With a vision that was bold, innovative, and collaborative, we aimed to challenge the status quo and bring hope where there was none
Thanks to your unwavering support, PNOC has not only flourished but has become a beacon of hope and a global leader in the fight against pediatric brain cancer. Through the dedication of nearly 300 researchers across more than 40 institutions worldwide, we have launched groundbreaking clinical trials, offering new treatments tailored to the unique biology of each child's tumor. This patient-centric approach has provided new options for those who previously had none. It has given families precious time together.
Our ability to bypass bureaucratic hurdles and rapidly translate innovative ideas into actionable trials has been nothing short of revolutionary. By leveraging private philanthropy and making collaboration a cornerstone of our work, we've achieved unprecedented speed and flexibility in our research efforts, proving that the only way forward is together – no one researcher or institution will solve this problem alone.
As we stand at the threshold of our next decade, we know our work has just begun We are proud of the progress we’ve made over the last 10 years but know there is no victory without a cure With more families facing these awful diseases each day, our resolve to push the boundaries of what is possible in pediatric neuro-oncology research has only strengthened. We are committed to expanding our global reach, enhancing our research capabilities, and ultimately, ensuring that no child is lost to brain cancer.
In closing, we extend our deepest thanks to you, our community of supporters, for believing in our vision and joining our cause. Together, we have built a strong platform, but the road ahead is long and requires our collective effort, innovation, and compassion. We invite you to continue this journey with us, to dream bigger, and to help us bring about a future where every child facing brain cancer has a fighting chance.
With heartfelt appreciation,
Dr. Sabine Mueller and Dr. Mike Prados

“Three years ago we watched him disappear down a long hall for a seven hour brain surgery, today we get to watch him thrive.”
-- Whitney, Carter’s mom

Carter’s first Spring baseball practice of the season started off like any other. 1520 minutes into the training session it took a very alarming turn. Playing catch with the other kids, he began to experience double vision. He sought out his father, “Dad, I’m seeing two baseballs and it’s making it very difficult to catch.” he explained His mom Whitney, a trained nurse, looked at both of his eyes and noticed his right eye appeared to be drifting inwards.
A scan confirmed a mass on Carter’s brain. His parents tried not to think about the possibility that it might be cancer. Two and a half days after his seven hour surgery to remove the mass, they r ng them of a very grave diagnosis. Car th mismatch repair (Lynch Syndrome)
“I think the one shining moment was the possibility that he would be eligible for a clinical trial. That I think when we walked out of there, was the thing that gave us a glimmer of hope.” says Carter’s father Dave.

Knowing PNOC’s treatment was s r’s tumor was very reassuring. His mo We were very fortunate to have a a gr ce with this trial has been very, very p art of this trial is the collaboration and hip with our physician and really talk through what s happening in Carter s body and what they're seeing and come to a plan that we both were in support of.“
Today meeting Carter for the first time, you would never guess what he has been through His eyes light up as he enthusiastically chats about his love and detailed knowledge of super cars - declaring himself “ a McLaren man” over Lamborghini, Ferrari and Bugatti He expertly plays video games and zooms up and down his quiet street on a scooter reaching admirable speeds.

PNOC aims to rapidly advance therapies that are globally accessible and transformative for children and young adults with brain tumors. The key goal is to develop the best trials possible through extensive preclinical testing as well as investigations using imaging, body fluids, tumor tissue and functional studies including quality of life and cognitive outcomes that are collected as part of ongoing PNOC trials.
In 2013 PNOC launched their first ever clinical trial. At the time the PNOC collaboration was just a handful of West Coast member institutions Every year has seen more clinical trials developed and launched - 31 studies in the years 2013-2023, an extraordinary accomplishment By the end of 2024, four new trials will have opened, bringing more treatment options to children and young adults in need around the world


PNOC001: Phase II study of everolimus for recurrent or progressive low-grade gliomas in children.
PNOC002: Pilot efficacy study of vemurafenib, an oral inhibitor of BRAFV600E, in children with recurrent/refractory BRAFV600E-mutant gliomas.
PNOC003: Pilot trial testing the clinical benefit of using molecular profiling to determine an individualized treatment plan in children and young adults with newly diagnosed DIPG.
PNOC005: Phase I study of modified measles virus for the treatment of children and young adults with recurrent medulloblastoma or recurrent atypical teratoid rhabdoid tumors (ATRT)
PNOC007: H3 3K27M specific peptide vaccine combined with poly-ICLC and nivolumab for the treatment of newly diagnosed HLA-A2 (02:01)+ H3 3K27M positive diffuse intrinsic pontine glioma (DIPG) and newly diagnosed HLA-A2 (02:01)+ H3 3K27M positive midline gliomas
PNOC008: Pilot trial testing the clinical benefit of using molecular profiling to determine an individualized treatment plan in children and young adults with high grade glioma (excluding diffuse intrinsic pontine glioma).
PNOC009: Phase I and early efficacy study of convection enhanced delivery of irinotecan liposome injection using real time imaging with gadolinium in children with diffuse intrinsic pontine glioma.
PNOC010/NF108: Phase II study of binimetinib in children and adults with NF1 associated plexiform neurofibromas.
PNOC011: Pilot study of the safety and toxicity of acquiring hyperpolarized carbon-13 imaging in children with brain tumors.
PNOC012: Study of tozuleristide and the Canvas imaging system in pediatric subjects with CNS tumors undergoing surgery.
PNOC013: Safety and pharmacokinetic study of single agent REGN2810 in pediatric patients with relapsed or refractory solid or central nervous system (CNS) tumors and a safety and efficacy trial of REGN2810 in combination with radiotherapy in pediatric patients
PNOC014: Phase I/II and target validation study of TAK-580 (MLN2480) for children with lowgrade gliomas and other RAS/RAF/MEK/ERK pathway activated tumors.
PNOC015: Open label single arm Phase I/II study of MTX110 delivered by convection-enhanced delivery (CED) in patients with diffuse intrinsic pontine glioma (DIPG) previously treated with external beam radiation therapy.
PNOC016: Target validation study of fimepinostat in children and young adults with newly diagnosed diffuse intrinsic pontine glioma (DIPG), recurrent medulloblastoma, or recurrent highgrade glioma (HGG).
PNOC017: Target validation/Phase I study of BGB-290 in combination with temozolomide in adolescent and young adult IDH1/2 newly diagnosed and recurrent mutant gliomas.
PNOC018: Phase I clinical trial of autologous T cells expressing TCR specific for H3.3K27M with inhibition of endogenous TCR (KIND T Cells) in HLA-A*0201-positive participants with newly diagnosed H3.3K27M-positive diffuse midline gliomas.

PNOC019: Randomized, double-blinded, pilot trial of neoadjuvant checkpoint inhibition followed by combination adjuvant checkpoint inhibition in children and young adults with recurrent or progressive high grade glioma (HGG).
PNOC020: Phase I/II study of RNA-lipid particle (RNA-LP) vaccines for newly diagnosed pediatric high-grade gliomas (pHGG) and adult glioblastoma (GBM).
PNOC021: Phase I trial evaluating the combination of trametinib and everolimus in pediatric and young adult patients with recurrent low grade gliomas
PNOC022: Combination therapy trial using an adaptive platform design for children and young adults with diffuse midline gliomas (DMG) including diffuse intrinsic pontine gliomas (DIPG) at initial diagnosis, post-radiation therapy and at time of progression
PNOC023: Open label Phase I and target validation study of ONC206 in children and young adults with newly diagnosed or recurrent DMG and other primary brain tumors.
PNOC025: Phase I study of Hu5F9-G4 (anti-CD47) in children and adults with recurrent or refractory malignant brain tumors.
PNOC026: Phase II, open-label, multicenter study to evaluate the safety and efficacy of the oral pan-RAF inhibitor DAY101 in pediatric patients with BRAF-altered, recurrent or progressive lowgrade glioma.
PNOC027: Pilot trial testing the clinical benefit of using real time drug screening to determine an individualized treatment plan in children and young adults with relapsed or refractory medulloblastoma.
PNOC028: Phase I study of intra-tumoral injections of ex vivo expanded natural killer cells in children and young adults with recurrent or progressive supratentorial malignant brain tumors.
PNOC029: Nivolumab and DAY101 for the treatment of newly diagnosed or recurrent craniopharyngioma in children and young adults
PNOC030: Registry study for children with atypical teratoid rhabdoid tumors (ATRT)
PNOC031: ETMR – still in early development
PNOC032: Germinoma study
PNOC COMP: Protocol for children and young adults diagnosed with a central nervous system (CNS) tumor to assess cognitive quality of life (QOL) and functional long-term effects of therapies.
PNOC033: Choroid plexus study.
PNOC034: Phase I study and expansion of pegylated arginine deiminase (ADI-PEG 20) plus radiotherapy and temozolomide in children and young adults with newly diagnosed high-grade glioma (HGG).
PNOC035: A Phase I/II adaptive platform, multi-arm study of novel therapies for children with progressive/recurrent ATRT.
All PNOC trials use patient’s tumor tissue to study the biologic underpinnings of disease and treatment response and resistance.
PNOC020 represents a pioneering effort within the Pe Consortium to adapt mRNA vaccine technology from adult Initially focused on adults with glioblastoma (GBM), this trial is pediatric patients with high-grade gliomas (pHGG). The study safety and immunologic activity of RNA-LP vaccines, marking successful adult immunotherapy treatments for children This t dedication to leveraging cutting-edge scientific advancements treatment options for young cancer patients.
Atypical teratoid rhabdoid tumor (ATRT) is a rare, malignant brain and spinal cord of young children. The condition usually Occasionally, it occurs in older children. PNOC is at the forefro the development and launch of the ATRT registry. The regi resource for the innovative PNOC035 trial. This trial utilizes re control group, a method showing significant promise for enha effectiveness By streamlining the incorporation of new ther PNOC aims to accelerate the delivery of groundbreaking potentially setting new standards in pediatric neuro-oncolog research.
PNOC027 exemplifies personalized medicine in pediatric on relapsed medulloblastoma and now expanding to relapsed epe time drug screening and genomic testing, the trial customiz tumor response to various drugs, enhancing efficacy while approach not only advances targeted cancer care but also pa applications in pediatric brain cancer research, embodying precision medicine.



Building on the foundation laid by our extensive list of clinical trials, PNOC has achieved remarkable scientific advancements that underscore our commitment to pioneering pediatric brain cancer research. These accomplishments not only highlight the efficacy of our collaborative research model but also demonstrate tangible progress in the fight against these devastating cancers. PNOC’s global expansion recognized that accelerating success requires collaboration and engagement of researchers across the globe, and the development of the best clinical trials possible.
A key milestone in our journey was the development of the first-ever FDA-approved drug for the most common subtype of pediatric brain tumor, achieved in collaboration with Day One Biopharmaceuticals. This groundbreaking achievement represents a major advancement in treatment options for young patients. More details on this breakthrough will be provided later in this document, highlighting the significant impact of our collaborative efforts.
Our consortium's work on RNA fusion breakpoints, which predict outcomes in pediatric low-grade gliomas, exemplifies the successful translation of clinical trials into actionable scientific insights This significant finding has opened new avenues for targeted therapies, emphasizing the importance of molecular precision in treatment strategies.
Further exemplifying our innovative approach, we conducted the first-ever convectionenhanced delivery trial with repeated treatments in children with diffuse intrinsic pontine glioma (DIPG). Additionally, the initiation of the first pediatric trial of tovorafenib, leading to a Phase II registration trial, marks a pivotal advancement in the treatment of pediatric high-grade gliomas. This trial showcases our relentless pursuit of precision medicine solutions tailored to the unique needs of our young patients
Moreover, we conducted the first assessment of intrathecal measles virus application in children with recurrent medulloblastoma and spearheaded the first precision medicine trial using up to four FDA-approved agents for pediatric high-grade gliomas. These innovative trials are vital steps in advancing our understanding of treatment responses and refining therapeutic approaches in pediatric neuro-oncology.
Through these trials and subsequent scientific endeavors, PNOC continues to lead the way in developing novel therapeutic strategies and enhancing treatment protocols for pediatric brain cancer, bringing hope to families and redefining the future of pediatric oncology.


PNOC’s pivotal role in the multistakeholder collaboration that was dedicated to bringing tovorafenib (DAY101) to a patient cohort without treatment options.

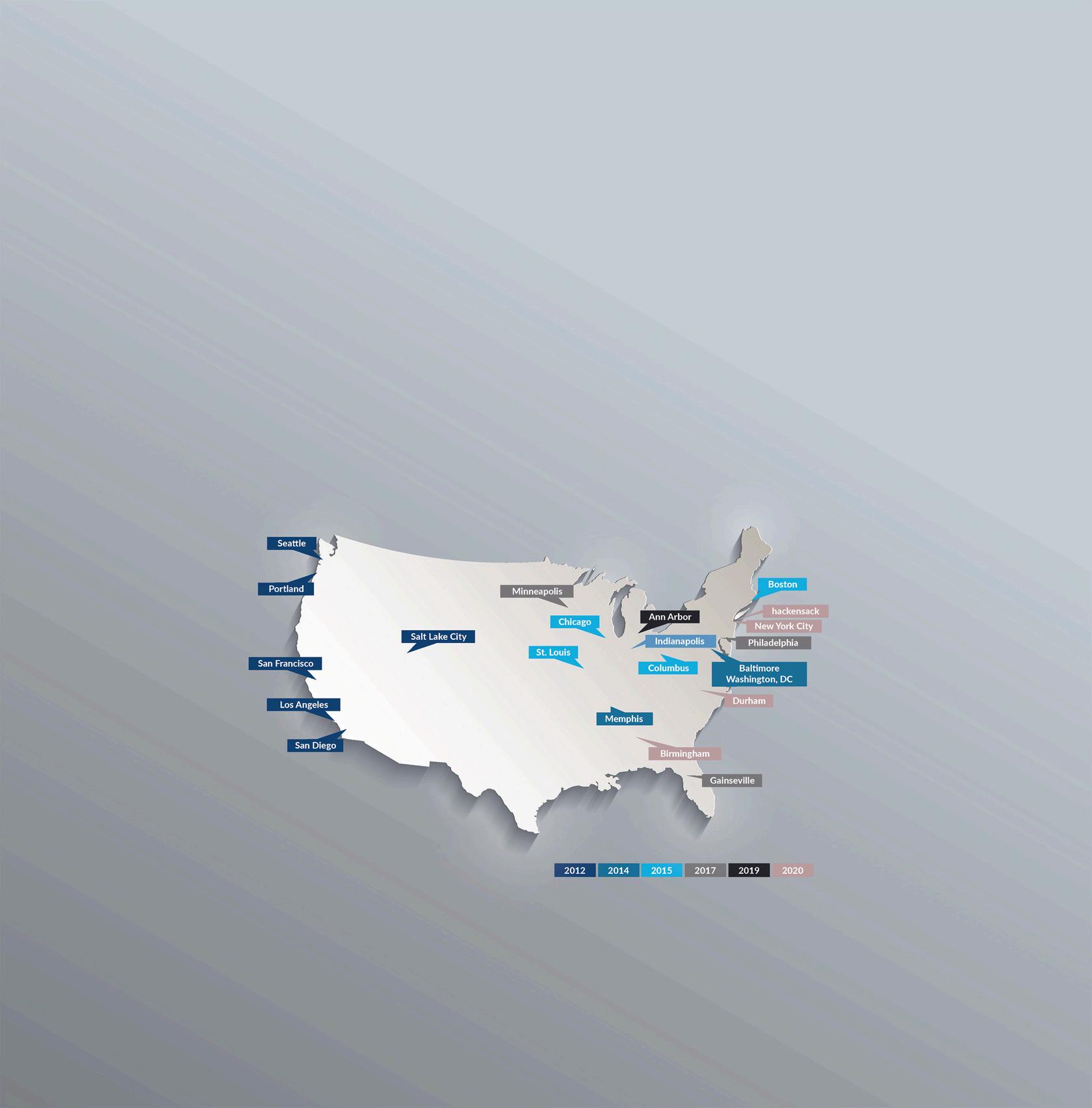
Every child should be afforded the right to have a clinical trial as far as we are concerned. From humble beginnings at USCF with seven initial collaborating sites on the West Coast, PNOC has rapidly grown to include 21 participating sites across the U.S.. Expanding clinical trial access to be available at more sites ensures more children in need have access to PNOC’s breakthrough treatment options. Receiving treatment closer to home not only makes treatment accessible, it keeps patient families closer to vital support networks of family, friends and community. The teams at each of PNOC’s member hospitals are made up of specialists in different areas of pediatric brain tumor treatment – such as oncology, neurosurgery, neuropsychology and radiation therapy – who combine their expertise to optimize care for each patient

Each PNOC member institution contributes to the consortium’s goals by carrying out laboratory research, taking part in protocol design and development and treating patients on PNOC trials. Working collaboratively, sharing ideas and information in real time through an innovative platform design is rapidly accelerating research. In fact, in the past 10 years since PNOC was established, more progress has been made towards the understanding of the biology of the most malignant brain tumors, than over the past 40 years combined. After successfully building a collaborative model across the U S for seven years, in 2020 PNOC began to expand internationally PNOC now has member sites in United States, Europe, Asia the Middle East and Australia, bringing together over 275 of the world’s pediatric brain tumor specialists The intent is to be collaborative, forward thinking, strategic, and efficient in developing clinical trials for the highest unmet need patients
In May 2023, PNOC celebrated a decade of groundbreaking work in pediatric brain cancer research with a three-day scientific symposium at UCSF Benioff Children’s Hospital, in San Francisco
This milestone event brought together over one hundred of the world’s leading pediatric neurooncologists, scientists and researchers for a three day scientific summit. Patient families, foundations, philanthropists, and industry leaders also gathered to reflect on 10 years of achievements and to chart the course for the future. The symposium featured a diverse program, including working group meetings, scientific meetings, tours of UCSF, hands-on scientific breakout sessions, expert panels, and a cocktail reception.
The event concluded with a keynote address by the 2009 Nobel Laureate, Dr. Elizabeth Blackburn, whose research on telomeres has inspired the world and continues to impact oncology research today
Attendees and award recipients, listed in alphabetical order, included Cathi Ahearn (Bristol
























What began as a holiday gathering to unite patient families, friends, and the community for childhood brain cancer awareness and fundraising has blossomed into an annual tradition. Inspired by holiday themed movies, we held a private screening and a family friendly reception complete with characters in elaborate themed costumes, holiday activities and even a hot chocolate station with bountiful toppings for the young and young at heart
From those humble yet magical beginnings, the holiday fundraiser has transformed into the year's feel-good event: the Gratitude Gala An unforgettable festive night to come together, joyously raise funds, embrace patient journeys, share PNOC’s medical advancements, and honor those that have contributed extraordinary support towards progressing childhood brain cancer treatments.
Don’t miss this year’s Gratitude Gala on November 2, 2024! Be part of this memorable evening, where joy, gratitude and commitment to a noble cause converge.































In May 2021, we launched a series specific webinars to connect patient families with the world’s pediatric brain cancer experts, and hear the latest news about research and clinical trials At the time there was no resource out there to fulfill this vital need for disease specific, current information from esteemed leading experts
A key mechanism to support our translational research is through our PNOC Working Groups Working group focus areas include: low grade glioma, diffuse midline glioma/high grade glioma, medulloblastoma, atypical teratoid rhabdoid tumors (ATRT), craniopharyngioma, ependymoma, germ cell tumors, immunotherapy, imaging, and DEI (diversity, equity, and inclusion). Our webinars have provided a much needed conduit between the working group researchers and disease specific patient family communities around the world. Hundreds of patient families have tuned in live from 26 countries and our webinars have since accumulated over 7,000 views on Youtube.
“I want to express my gratitude to the organization and the participants for this webinar session. I really appreciated the explanations and all the participant’s interventions Thank you for being there for us, the parents ” -- Christina, webinar attendee












Make a Donation Sponsor an Event Fundraise on our Behalf
Support our cause instantly with a secure online credit card donation, bank transfer or check
Donate goods or services to support our operational or event needs
Sponsor our 2024 Gratitude Gala happening in San Francisco on Nov 2
Help us raise funds through your community or personal network.

Transfer stock to us as a tax-efficient way to give.

Direct your philanthropy through recommended DAF grants to us.
Align your business with us to create mutual value.

Leave a legacy by including us in your estate planning.
Designate us as a beneficiary to your policy for future support
Donate your time and skills to make a direct impact
Honor loved ones with gifts that make a lasting impact.

Ann & Robert H Lurie
Children’s Hospital of Chicago CHICAGO, IL
Children’s Cancer Hospital, Egypt CAIRO, EGYPT
Children’s Hospital Los Angeles LOS ANGELES, CA
Children’s Minnesota MINNEAPOLIS, MN
Children’s National Hospital WASHINGTON, DC
Dana-Farber/Boston Children’s Cancer and Blood Disorders Center BOSTON, MA
Doernbecher Children’s Hospital Oregon Health & Science University (OHSU) PORTLAND, OR
Duke University Medical Center DURHAM, NC
John Hunter Hospital NEWCASTLE, AUSTRALIA
Johns Hopkins Hospital BALTIMORE, MD
Joseph M Sanzari Children’s Hospital at Hackensack University Medical Center HACKENSACK, NJ
Monash Children’s Hospital CLAYTON, AUSTRALIA
Nationwide Children’s Hospital COLUMBUS, OH
NYU Langone Health NEW YORK CITY, NY
Perth Children’s Hospital PERTH, AUSTRALIA
Princess Maxima Center UTRECHT, THE NETHERLANDS
Queensland Children’s Hospital BRISBANE, AUSTRALIA
Riley Hospital for Children at Indiana University Health INDIANAPOLIS, IN
Royal Children’s Hospital Melbourne MELBOURNE, AUSTRALIA
St Jude Children’s Research Hospital MEMPHIS, TN
St. Louis Children’s Hospital ST. LOUIS, MO
Seattle Children’s Hospital SEATTLE, WA
Shaare Zedek Medical Center JERUSALEM, ISRAEL
Sydney Children’s Hospital SYDNEY, AUSTRALIA
Sheba Medical Center RAMAT GAN, ISRAEL
Starship Children’s Hospital AUCKLAND, NEW ZEALAND
Tata Memorial Centre, Mumbai MUMBAI, INDIA
The Children’s Hospital of Philadelphia PHILADELPHIA, PA
The Children’s Hospital at Westmead WESTMEAD, AUSTRALIA
University Children’s Hospital Zürich ZURICH, SWITZERLAND
University of Alabama at Birmingham, Children’s of Alabama BIRMINGHAM, ALABAMA
University of California, San Diego Rady Children’s Hospital SAN DIEGO, CA
UCSF Benioff Children’s Hospitals SAN FRANCISCO, CA
University of Michigan – C.S. Mott Children’s Hospital ANN ARBOR, MI
University of Florida Shands Children’s Hospital GAINESVILLE, FL
University of Utah SALT LAKE CITY, UT
Women’s and Children’s Hospital ADELAIDE, AUSTRALIA
Children’s Hospital of Michigan DETROIT, MI
Huntsman Cancer Center SALT LAKE CITY, UTAH
Texas Children’s Hospital HOUSTON, TEXAS
University of California, Los Angeles LOS ANGELES, CA
Yale University, Yale Cancer Center NEW HAVEN, CT

Prof. Dr. Sabine Mueller, Project Lead (UCSF)
Prof Dr Michael Prados, Project Co-Lead (UCSF)
Asst Prof Dr Cassie Kline, Project Co-Lead (CHOP)
Assoc Prof Dr Ashley Margol, Director of Study Development (CHLA)
Prof Dr Annette Molinaro, Statistical Lead (UCSF)
Dr. Adam Resnick, Scientific Director (CHOP)
Dr. Stephanie Mathes, Director of Strategic Alliances (ZURICH)
Bruce Campbell, President
Allyn Campbell, Secretary,
Linda Hartig, Treasurer
Michael Burton
Angela Caltagirone
Amy Cunha
Chris Gallagher
Richie Goldman
Kesley Grady
Barbara Karski
Dr. Sung Poblete
Jason Schlesinger
Heather Davis (Emeritus)
Ana Johnson (Emeritus)
Darren Tucker (Emeritus)
