ignite
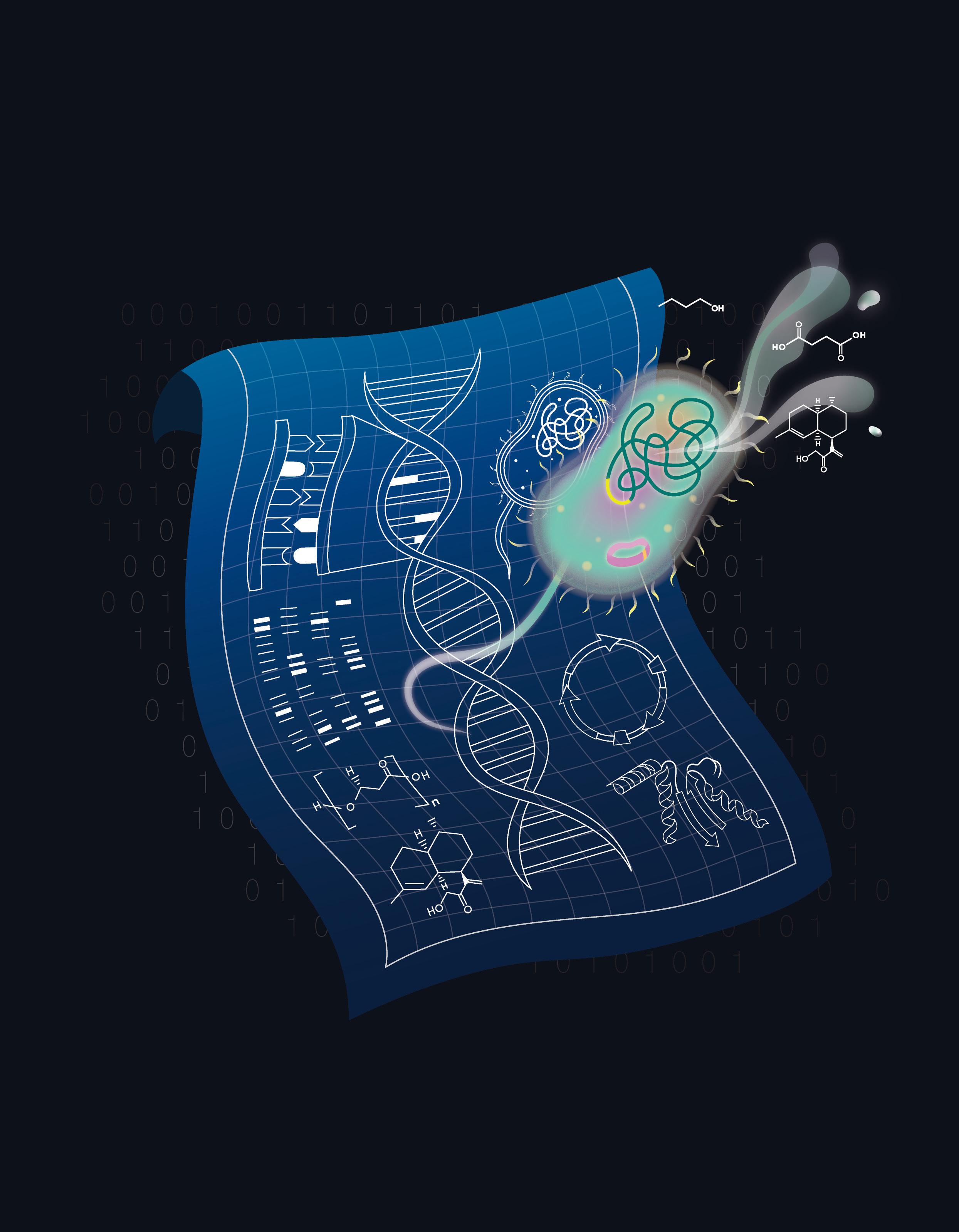
The bioeconomy, reengineered
Micro factories for fine chemical production
Clean and crystal clear: Water as nature intended
Engineering protein of the future




Micro factories for fine chemical production
Clean and crystal clear: Water as nature intended
Engineering protein of the future


Be part of a community dedicated to driving innovation and change in education, research, and service.
15
Colleges, faculties, and schools
4.2k >50
Research staff Research centres and institutes, and Research Centres of Excellence
Our culture of inclusivity and entrepreneurship gives you the freedom to explore your ideas and make a meaningful impact.
For more information, visit us at https://www.nus.edu.sg/careers
Nature exhibits inherent ingenuity in problem-solving. Honed by billions of years of evolution, living organisms across scales, from individual cells to biodiverse communities, have developed intricate strategies to adapt to changing environments, resource scarcity, and other existential threats. These biological solutions are often elegantly efficient, using minimal energy and materials yet achieving remarkable outcomes. With a clearer understanding of the finite details and interconnectedness of different natural processes, researchers are increasingly inspired to design innovative technologies and systems that emulate, exploit and enhance nature’s time-tested problem-solving strategies.
In this issue of Ignite, we explore how researchers at NUS are turning to nature for both inspiration and innovation. We take a look at how genetically engineered bacteria can recover metals from electronic waste more sustainably compared to traditional chemical methods. We explore how modern medicine is being transformed through the use of engineered microbes to produce and deliver new therapeutic interventions. We also look at the governance challenges limiting the effectiveness of carbon sinks such as forests and mangroves in Southeast Asia, and examine innovations in the design of urban environments from cooling systems to coastal protection strategies.
NUS is proud to be at the forefront of nature-inspired and nature-based research. NUS Synthetic Biology for Clinical and Technological Innovation (SynCTI) recently marked its 10th anniversary, celebrating a decade of cuttingedge research in engineering biology. The Centre for Nature-based Climate Solutions, under NUS Science, produces policy-relevant science on naturebased climate solutions to protect and better manage natural ecosystems in the face of a changing climate. Over at the Bezos Centre for Sustainable Protein at NUS, the aim is to advance sustainable protein research. One priority area will be biomass fermentation where researchers look to utilise tofu waste to feed algae, in turn creating high-quality protein. NUS is also host to the Coastal Protection and Flood Resilience Institute (CFI) Singapore under NUS Engineering. This is the country’s first Centre of Excellence focused on integrating naturebased solutions with engineering to protect coastlines.
Nature-based and nature-inspired solutions hold significant potential in addressing some of the worlds most pressing challenges. Whether it involves coastal protection or hybrid meat production, bioremediation or medical interventions derived from engineered microorganisms, by working with and leveraging natural, biological systems, we can rapidly mitigate climate risks, protect our ecosystems, and improve our way of living in ways that are sustainable, equitable, and socially inclusive.

Professor Liu Bin Deputy President (Research & Technology)
Editor-in-Chief
Professor Liu Bin
Editorial Advisory Board
Professor Aaron Thean
Associate Professor Benjamin Tee
Professor Chng Wee Joo
Professor Elaine Ho
Associate Professor Leong Ching
Professor Mohan Kankanhalli
Managing Editor
Dr. Steven John Wolf
Editors
Lim Guan Yu
Low Yuan Lin
Wong Oi Shan
Editorial Designer
Belina Chong
Monica Tan
Illustrators
Belina Chong
Koh Pei Wen
Monica Tan
Dr. Naoki Ichiryu
Published by
NUS Office of the Deputy President (Research & Technology)
© 2025 National University of Singapore. All rights reserved. This magazine and its contents are the property of the National University of Singapore (NUS) and are protected by copyright law. Unauthorised use, reproduction, or distribution of the material contained in this magazine, including but not limited to text, images, and other media, is strictly prohibited without the prior written consent of NUS.
The views and opinions expressed in the articles are those of the authors and do not necessarily reflect the official policy or position of NUS or the Office of the Deputy President (Research & Technology). While every effort has been made to ensure the accuracy of the information contained in this magazine, NUS assumes no responsibility for errors or omissions, or any consequences arising from the use of the information.
For permissions and other inquiries, please contact the Office of the Deputy President (Research & Technology) at NUS.
research@nus.edu.sg



Revolutionising heat transfer with hydrodynamic moiré fluidic superlattices
Green chemistry breakthrough: Iron catalysis unlocks new pathway to Z-alkenes

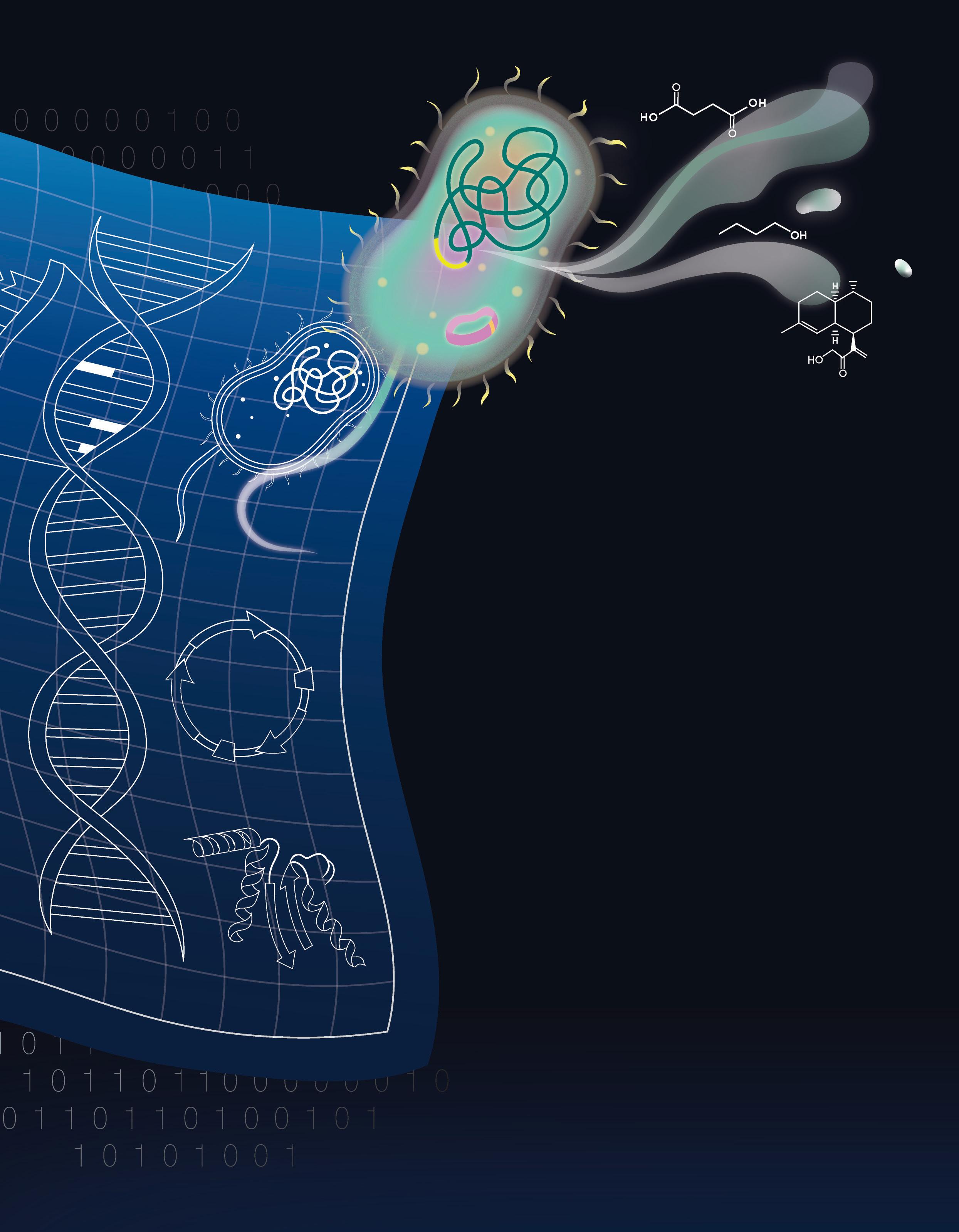
by
Prof Matthew Chang with Asst Prof Lee Jungjoon, Assoc Prof Poh Chueh Loo, Assoc Prof Li Zhi, and Dr. Ang Hui Ying
I t has been 30 years since the first bacterial genome was sequenced. A decade later came a complete map of the approximately 25,000 genes, non-coding regions, regulatory regions and structural sequences of DNA that make up the human genome. Today, tens of thousands of bacterial strains and thousands of animal species have had their genomes sequenced.
These genetic blueprints tell highly complex stories. When learning a language, knowing how to spell a word can only get you so far in communicating information. It is also crucial to understand how words relate to each other in various contexts. Likewise, it is crucial to comprehend the relationships between various genome elements within the context of genome regulation and protein function.
Understanding this genetic code unlocks the ability, with the right tools, to manipulate it in ways that instil new functionalities in cells or mitigate the consequences of genomic abnormalities. This is the fundamental premise of engineering biology, or synthetic biology, an emerging field that aims to design and engineer novel biological systems and is poised to revolutionise how fine chemicals are manufactured, how metals are recovered from electronic waste, and how personalised therapeutics are administered.

One tool that propelled the field forward over the past decade is CRISPR-Cas9, a gene editing method that allows researchers to efficiently cut DNA in living organisms at specific sites, to remove or disrupt genes, or even insert new genes. It is one of the most revolutionary gene editing methods currently in use. Today, the technology continues to evolve. Scientists can now make DNA substitutions using ‘base-editors’, and DNA insertion, deletion and substitutions using ‘prime-editors’. Mitochondrial and chloroplast DNAs can also be targeted for editing.
Assistant Professor Jungjoon Lee leads the Genome Re-Innovation Lab under the NUS Synthetic Biology for Clinical and Technological Innovation (SynCTI). His work focuses on developing and optimising gene editing tools and assays to ensure CRISPR safety. His team developed a directed evolution system called Sniper-screen, an Escherichia coli (E. coli)-based screening system designed to select optimised Cas9 variants with high activity and specificity. They demonstrated that Sniper2L, their latest product, possesses these features through high-throughput screens and small molecule assays. Additionally, his team developed Extru-seq and TAPE-seq, genome-wide off-target detection methods for Cas9 and Prime Editor.
"The field has reached a tipping point, and we will continue to hear news about the approval of new drugs and foods produced from edited genomes. In both fields, people are very sensitive to safety issues. Our lab is committed to developing optimised versions of CRISPRs and safety assays to address these concerns," he added.
While many new technologies are changing how genomes are designed and deployed, there is one that stands above the rest for its potential to be the next revolution in the field —artificial intelligence.
Identifying the elements required to produce a viable, bioengineered system, becomes a seemingly insurmountable task when considering the vast number of biomolecular interactions that are at play to maintain genome regulation and cellular function. Traditionally, a ‘trial-and-error’ approach was the go-to method, however today, AI-based tools are opening new avenues to better design, grow and utilise bioengineered genomes.
They can, for example, analyse complex datasets, identifying functional elements, and predicting outcomes with greater accuracy. AI-driven platforms like DeepCRISPR
enhance genome editing efficiency, while AlphaFold accelerates protein structure prediction. Computer-aided design and modelling tools like Cello, Cameo, OptKnock accelerate the design and engineering of microbes by optimising gene circuits, metabolic pathways and strain performance. Tools like these promise increased capabilities and efficiency in guiding synthetic genome design and construction.
Just as electrical engineers use software to design integrated circuits, synthetic biologists use computeraided design and modelling tools, as well as AI models, to design genetic circuits—combinations of genes that control specific cellular behaviours, and predict how genetic circuits will behave in living cells.
At the Engineering Biology Lab within NUS SynCTI, for example, Associate Professor Chueh Loo Poh, and his team apply engineering principles to design and construct microbes with specific capabilities. To accelerate the process, they developed foundational modelling tools such as BioModel Selection System (BMSS2)—a unified platform to streamline and automate gene circuit model selection, enhancing efficiency in gene circuit design. To harness the powerful capability of AI, the team integrates machine learning with ultra-high throughput sorting technology for large-scale sequence-function data generation and screening. This synergy enables the discovery and design of novel high-performance enzyme sequences, revolutionising enzyme engineering by coupling experimental precision with AI-driven predictive design.
Tools are also now being developed to predict promoter strength and protein structure from genome sequences, predict function from molecular structure information, aid the directed evolution of proteins, forecast base editing outcomes, accelerate metabolic pathway design and optimisation, and streamline analytical chemistry data processing during the test step of strain engineering.

As the engineering biology toolkit expands—with new genome engineering technologies, synthetic biology platforms, and advanced computational methods—and as the network of domain experts grows, innovation and discovery will naturally accelerate. This convergence of expertise and cutting-edge tools is driving a transformation across industries, introducing new methods for developing and deploying products and processes. Healthcare, agriculture, biomanufacturing, and sustainability are just some areas already benefitting from these advances.
Collectively, these changes are laying the foundation for a robust future bioeconomy, where biology is harnessed to address global challenges and drive economic growth.
To date, the pace of translating engineering biology research into market-ready products has been relatively fast. Typically, bio-based startups must navigate lengthy preclinical testing, extended clinical trials, and a complex regulatory approval process. These ventures also demand substantial, highrisk financial investments, placing investors in a challenging position due to the inherent uncertainty of scientific research. Furthermore, many technologies that could eventually address critical issues such as stability, scalability, and efficient production are still in development and may not yet exist.
Nevertheless, the field has achieved notable milestones. Today, several Nasdaq-listed companies are focused on genome editing techniques, and the FDA has approved the first CRISPRbased human therapeutics.
In 2024, CRISPR-edited food products began to enter the US market, marking a milestone in agricultural biotechnology. Additionally, laboratory advances have enabled microbes to sustainably produce key components for lab-grown meats, bio-based plastics, and advanced biofuels. As one example, NUS SynCTI has successfully developed specialty chemicals in collaboration with industry partners, including Wilmar International and HH Chemical, highlighting the rapid progress and broad impact of engineering biology.
Despite early progress, the full potential of engineering biology still faces important challenges before it can be fully realised. One key consideration is how products developed through biologically engineered systems are perceived by the public. Building trust through transparency and clear communication will be essential throughout the development process. Creating opportunities for open dialogue and increasing public familiarity with these innovations can help foster broader acceptance and confidence in both the products and the technologies behind them.
Consistent regulatory standards are equally critical. Fragmented regulatory frameworks can hinder the scalability and international trade of bio-based products. Globally recognised standards and metrics are essential to support the safe, efficient, and timely adoption of biologically engineered systems and their applications.
In response to this need, the Task Force on Engineering Biology Metrics and Technical Standards for the Global Bioeconomy1 was established in 2024. Professor Matthew Chang, Director of NUS SynCTI, is a member of this international task force.
“We have identified 10 critical areas six technical and four non-technical where the development of standards and metrics is essential to unlock the full potential of the bioeconomy,” he said.
“The rapid pace of advancement in biotechnology often outpaces regulatory and policy updates, creating uncertainty for innovators. That’s why ongoing communication and transparency between researchers, regulators, and the public are critical. These conversations help ensure that policy decisions are informed by the latest scientific understanding.”
To further accelerate bioeconomic growth, sustained public investment in research and innovation remains essential. NUS recently launched a S$120 million national research initiative to harness engineering biology for the sustainable production of chemicals vital to energy, materials, consumer products, and nutrition. This initiative focuses on developing next-generation engineering biology platforms for sustainable biomanufacturing from renewable feedstocks, including carbon dioxide.
At the national level, Singapore’s Green Plan 2030 is pursuing a steadfast commitment to sustainability and fostering a supportive environment for industries to pursue bio-based solutions.
Engineering biology stands at the forefront of a new era and is poised to revolutionise how we produce, consume, and sustain. From addressing climate change and food security to advancing resource efficiency, the field offers transformative solutions to some of humanity’s most urgent challenges. Realising this potential will require bold investment, adaptive regulatory frameworks, and crossborder collaboration.
With its integrated approach to innovation, policy, and public engagement, Singapore is rapidly emerging as a global leader in engineering biology. As part of this national effort, NUS is advancing cutting-edge research, shaping global standards, and developing next-generation technologies in engineering biology, contributing to a more resilient, inclusive, and sustainable future.
Director, Singapore Consortium for Synthetic Biology (SINERGY)
Director, NUS Synthetic Biology for Clinical and Technological Innovation (SynCTI)
Director, WIL@NUS Corporate Laboratory
NUS Medicine

1. Freemont, P.S., Ni, C., Aurand, E., Chang, M.W., Hook-Barnard, I., Malley, J., Romantseva, E., Strychalski, E., Vavitsas, K. 2024. Engineering Biology Metrics and Technical Standards for the Global Bioeconomy. London, UK. https://doi.org/10.25561/110822

NUS Biochemistry
NUS Synthetic Biology for Clinical and Technological
Innovation (SynCTI)
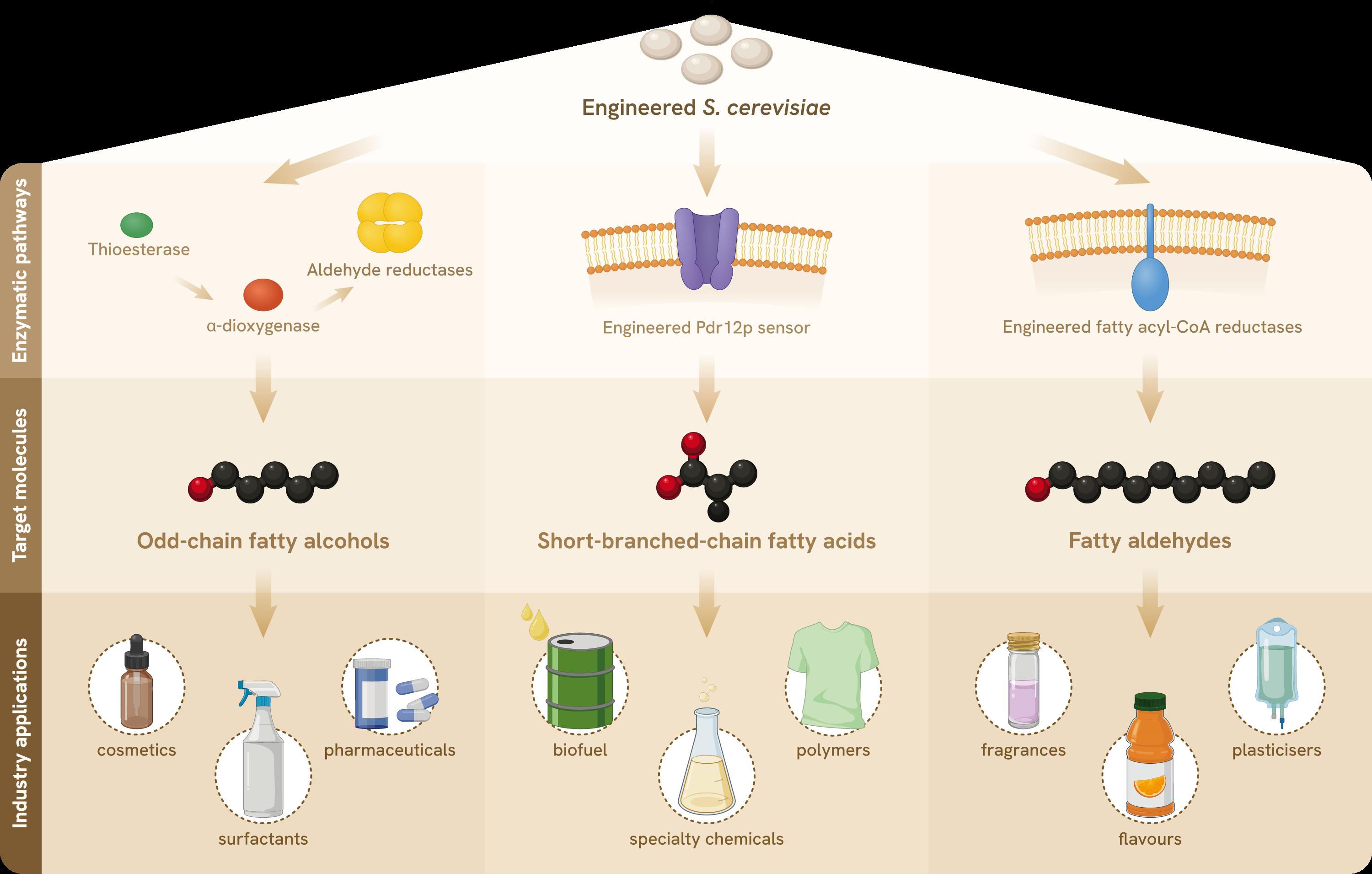
I n the world of sustainable biomanufacturing, Saccharomyces cerevisiae—commonly known as baker’s yeast—has evolved from its traditional role in fermentation to a cutting-edge platform for producing biofuels, specialty chemicals, and highvalue compounds. In fact, it is now at the heart of innovative research aimed at engineering complex metabolic pathways for the production of fatty acids, alcohols, and their derivatives. These advancements are pushing the boundaries of biochemical manufacturing and offering exciting new possibilities for more sustainable, economically viable bio-based production systems.
Fatty acids and their derivatives, such as fatty alcohols and aldehydes, are crucial components in the production of various products, including cosmetics, pharmaceuticals, and biofuels. Their widespread use can be attributed to unique chemical properties like chain length, saturation, and branching.
Traditionally, fatty acids and their derivatives are extracted from plant and animal oils or synthesised chemically. While scalable and efficient, these methods rely on agriculture, cause deforestation, and generate chemical waste. By engineering S. cerevisiae to efficiently produce fatty acid derivatives, researchers are laying the foundation for greener, more efficient production systems that can support a circular bioeconomy.
Producing odd-chain fatty alcohols has long been a challenge due to the natural predominance of even-chain fatty acids in most organisms. However, recent breakthroughs in metabolic engineering have enabled S. cerevisiae to produce odd-chain fatty compounds directly. This has been achieved in our lab at NUS SynCTI (Synthetic Biology for Clinical Technological Innovation) with impressive efficiency.

By introducing enzymes such as thioesterase, α-dioxygenase, and the yeast’s endogenous aldehyde reductases, we produced approximately 20 mg/L of fatty aldehydes and 20 mg/L of fatty alcohols by fermentation of the engineered yeast, all without the need for extensive fine-tuning of metabolic flux in the system. Building upon this pathway, there is great potential for scaling the system to produce oddchain fatty alcohols sustainably.
S. cerevisiae has also been engineered to produce short-branched-chain fatty acids (SBCFAs). Due to their branched structure, SBCFAs often exhibit distinct chemical properties that make them suitable intermediates for a variety of industrial applications. Branching also alters physical properties like the melting point and volatility, meaning SBCFAs can be tailored to specific applications such as biofuels or specialty chemicals.
To facilitate the efficient production of SBCFAs, our team has developed a whole-cell biosensor using the weak organic acid transporter Pdr12p. By optimising the expression of the PDR12 gene, we can now fine-tune the biosensor’s sensitivity and operational range, enabling real-time monitoring of SBCFA production in S. cerevisiae. This system will allow for more efficient screening of SBCFA-producing strains to improve biosynthesis efforts.
Fatty aldehydes and hydrocarbons have also been synthesised by genetically engineered S. cerevisiae. Naturally, an alcohol-forming fatty acyl-CoA reductase (FAR) enzyme consumes aldehyde intermediates derived from fatty acyl-CoAs without accumulating them. To overcome this, we have engineered the FAR to catalyse the direct production of
fatty aldehydes in S. cerevisiae. After modifying the enzyme’s catalytic domains, we achieved a breakthrough production of 2,005 μg/L of fatty aldehyde—the highest reported concentration from fatty acyl-CoA in S. cerevisiae to date.
Engineered yeast strains have the potential for scalable production of a wide range of aldehyde-derived chemicals. Already, biodiesel candidates, alkanes and alkenes, have been produced using this system.
As these technologies continue to mature, engineered S. cerevisiae is poised to become a cornerstone of sustainable biomanufacturing. However, several scientific challenges remain to be addressed.
Strain optimisation is essential to enhance chemical production from yeast for economically viable largescale fermentation. To achieve this, extensive genetic modifications are needed. These modifications would increase the efficiency of the biosynthesis pathway, minimise metabolic burden and enhance cellular tolerance against the toxic chemicals produced, thereby ensuring healthy cell growth and maintaining fermentation efficiency.
With our advancements in novel strain engineering approaches assisted by synthetic yeast and whole-cell biosensors, we aim to accelerate the development of S. cerevisiae into microbial cell factories for efficient chemical production, paving the way for sustainable and commercially viable yeast-based biomanufacturing processes.
products, enabling sustainable real-world
Electronic waste, or e-waste, is becoming one of the fastest-growing solid waste streams worldwide. Consisting of electrical devices such as computers, televisions, phones, and their components, the amount of e-waste generated globally is projected to reach 82 million tonnes by 20301.
Currently, less than one-quarter of the world’s e-waste is recycled, leaving an estimated US$62 billion in recoverable natural resources unaccounted for and increasing pollution risks worldwide1. E-waste is a rich source of valuable metals such as gold, silver, platinum, and rare earth elements. However, most e-waste is disposed of in landfills or incinerated, which prevents the recovery of valuable materials. A small proportion of e-waste is recycled.

NUS Biochemistry
NUS Synthetic Biology for Clinical and Technological Innovation (SynCTI)

However, the methods required for this are typically energy-intensive and environmentally harmful. This means that innovative and sustainable solutions to better manage e-waste could not only mitigate the associated environmental and health risks, but also enable urban mining strategies for resource recovery of valuable metals.
The use of biological systems that employ microorganisms to carry out processes like bioleaching and bio-oxidation provide sustainable alternatives for extracting valuable materials from e-waste. They consume less energy and produce fewer pollutants than traditional extraction techniques. However, several hurdles must be overcome if these systems are to become an industrially relevant solution.
First, lower metal recovery rates compared to conventional methods, such as pyrometallurgy or hydrometallurgy, currently prevent large-scale industrial success.
Biological extraction methods are also inherently slower than chemical methods. This reduces their overall efficiency and hinders industrial-scale operations. Microbial viability and activity are also sensitive to various environmental factors, potentially leading to inconsistent performance.
The complex composition of e-waste also further complicates biomining efforts. Electronics are often manufactured with various materials, including metals, plastics, and glass, which makes it difficult for a single process to effectively recover the metals.
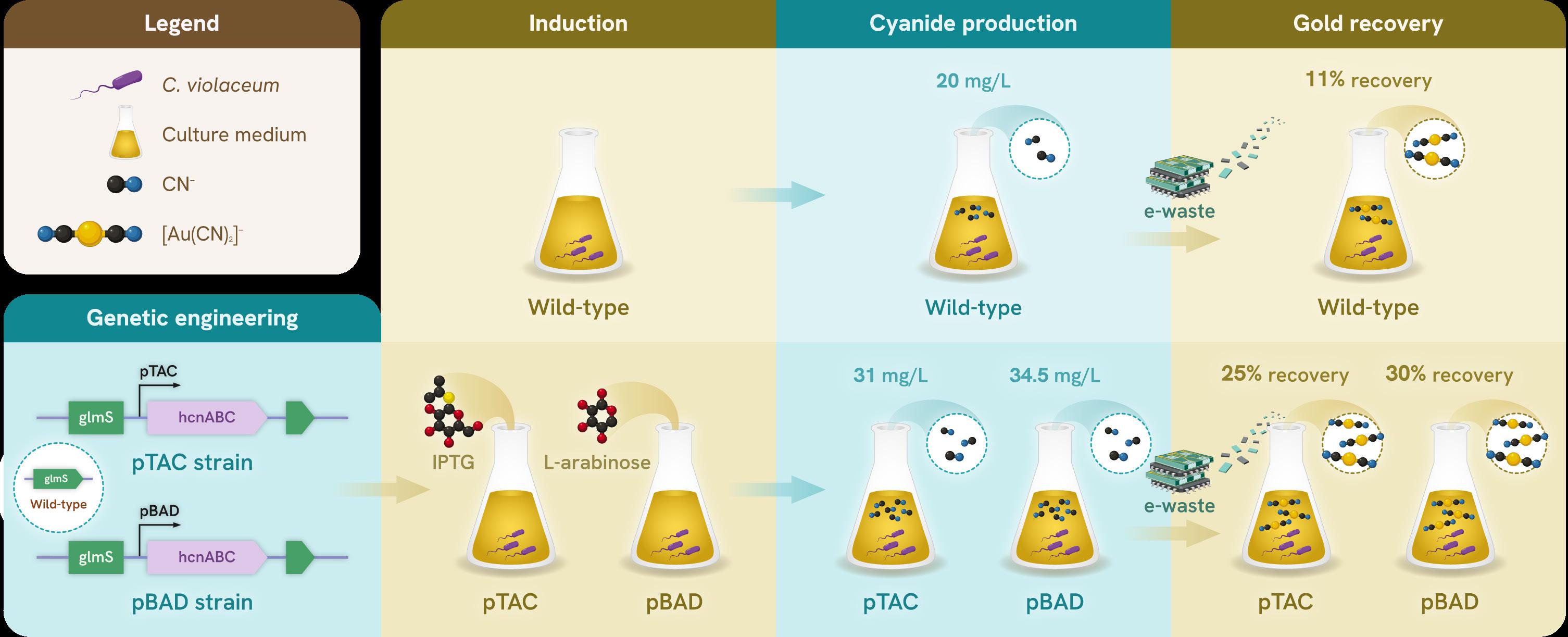
To overcome these limitations, Associate Professor Yew Wen Shan from NUS Biochemistry is engineering microbes to improve their ability to recover metals from e-waste.
In one study2, the team sought to increase the effectiveness of bioleaching using the bacteria Chromobacterium violaceum to extract gold from e-waste. Cyanide is required for this process, and although C. violaceum produces this compound naturally, it does so only in small quantities. In the study, a genetically engineered strain of C. violaceum (pBAD) successfully produced 70% more cyanide, and recovered more than twice as much gold from e-waste as compared to wild-type bacteria. In subsequent studies that optimised cyanide production, the pBAD strain achieved 30% gold recovery, compared to 11% recovery by the wild-type bacteria3.
Of course, there are various metals within e-waste, and an ideal solution would see enzymes working to recover multiple metals at once. To this end, Assoc Prof Yew’s team engineered the MerA enzyme (G415I) in Escherichia coli, and found it to be 15 times more
efficient at converting gold ions into solid gold, and 200 times more efficient at doing the same for silver4
Importantly, industry players are already catching on to the potential of engineering biology for metal recovery, with companies from New Zealand and the UK having taken inceptive steps to deploy microbes in their e-waste recycling workflow.
Establishing supportive policies or regulations to integrate biomining processes with broader recycling and sustainability efforts can foster an ecosystem that encourages sustainable resource recovery.
The value of e-waste is clearly apparent. The UK Royal Mint has, for example, launched the world’s first plant5 to extract gold from circuit boards. The facility uses a patented, low-temperature chemical process, capable of recovering over 99% of the precious metals in e-waste, without the need for sending these overseas for high-temperature smelting.

However, these methods for resource recovery still rely on traditional chemical-based processes. For largescale biomining to be successfully implemented, many factors, from growth conditions to enzyme efficiency must be optimised, with costs and sustainability also considered.
Until this occurs, a hybrid approach that combines biomining with traditional methods may serve as a bridge to improve resource recovery efficiency, whilst allowing novel biomining based technology to be developed further.
1. Cornelis P. Baldé, Ruediger Kuehr, Tales Yamamoto, et al. (2024). International Telecommunication Union (ITU) and United Nations Institute for Training and Research (UNITAR). Global E-waste Monitor 2024 [Accessed 25 Feb 2024]. https://ewastemonitor.info/ the-global-e-waste-monitor-2024/
2. Tay, S. B., Natarajan, G., Rahim, M. N. B. A., Tan, H. T., Chung, M. C. M., Ting, Y. P., & Yew, W. S. (2013). Enhancing gold recovery from electronic waste via lixiviant metabolic engineering in Chromobacterium violaceum. Scientific reports, 3(1), 2236.
3. Natarajan, G., Tay, S. B., Yew, W. S., & Ting, Y. P. (2015). Engineered strains enhance gold biorecovery from electronic scrap. Minerals Engineering, 75, 32-37.
4. Chua, J. P. S., Rajasabhai, R., Teo, W. Z., Xue, B., & Yew, W. S. (2024). Engineering a Metal Reductase for the Bioremediation of Anthropogenic Electronic Wastes: From Hg (II) to Au (III) and Ag (I) Enzymatic Reduction. JACS Au, 4(6), 2335-2342.
5. The Royal Mint. (n.d.). The Royal Mint to build ‘world first’ plant to turn UK’s electronic waste into gold [Accessed 25 Feb 2024]. https://www.royalmint. com/aboutus/press-centre/the-royal-mint-to-buildworld-first-plant-to-turn-uks-electronic-waste-intogold/
Professor Matthew Chang
NUS Medicine
NUS Synthetic Biology for Clinical and Technological Innovation (SynCTI)

F or decades the backbone of modern medicine has been small-molecule drug- and biologic-based interventions. These conventional approaches have saved countless lives, but they come with significant trade-offs: limited precision, systemic side effects, and low efficacy for some medical conditions.
This is where nature offers both inspiration and instruction. Across millions of years, biological systems have evolved exquisitely targeted mechanisms to interact with cells, tissues, and environments in ways modern medicine is only beginning to replicate.
Today, researchers are borrowing from nature’s playbook and emulating nature’s targeting strategies. To do this researchers are reengineering biology and unlocking transformative therapies.
At NUS, scientists are developing programmable microbes to produce valuable compounds, engineered probiotics for brain-targeted delivery, and non-viral platforms for precise gene editing in immune cells.
Ergot fungus has long been used to produce D-lysergic acid (DLA), a key precursor in medications for dementia, Parkinson’s disease, migraines, and other neurological conditions. However, traditional methods of cultivation are slow, resource-intensive, and difficult to scale.
In a collaborative project between NUS Medicine and Imperial College London, Associate Professor Yew Wen Shan and his team programmed baker’s yeast (Saccharomyces cerevisiae) with synthetic genes from the ergot fungus to produce DLA sustainably. Within just a day or two of fermentation in a bioreactor, the engineered yeast generated small but scalable amounts of DLA, approximately 1 to 5 mg per litre, with the potential for multi-tonne annual production. Unlike conventional ergot cultivation, which requires large areas of land and careful fungal management to produce DLA, this approach offers a more sustainable and scalable microbial platform.
Beyond fungus, commensal bacteria, microorganisms that naturally reside in the human body without causing harm, are also being reprogrammed to act as therapeutic allies to challenge the blood-brain barrier. One of medicine’s most formidable challenges, this barrier blocks the vast majority of drugs from entering the central nervous system, excluding nearly 98% of potential drug candidates for
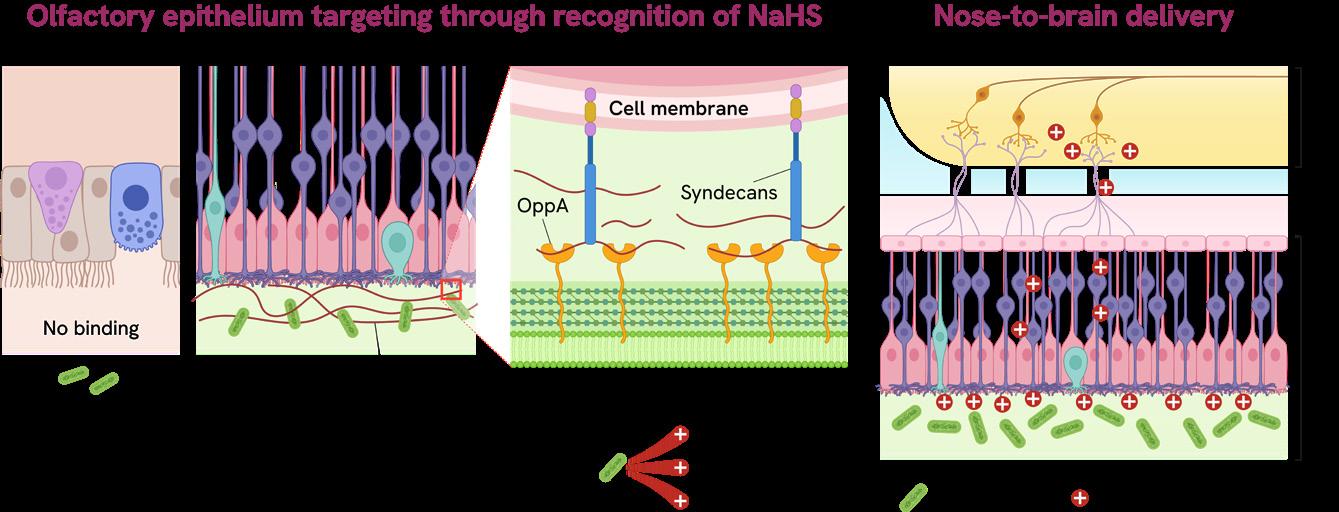
Intranasally delivered Lactobacillus plantarum passes through the respiratory epithelium (RE), binds to the olfactory epithelium (OE), and crosses into the olfactory bulb (OB), providing a direct route for brain-targeted delivery of therapeutic payloads.
neurological disease treatments. To overcome this, NUS scientists, led by Dr Haosheng Shen from the Synthetic Biology Translational Research Programme, engineered Lactobacillus plantarum. The team enabled targeted, efficient transport of drugs, potentially reducing systemic side effects while increasing treatment precision.
Even our own cells are providing a powerful template to revolutionise the human immune system. T cells, a type of lymphocyte, or white blood cell, already know how to recognise and eliminate diseased cells. CAR-T cell therapy builds on this natural intelligence by genetically programming a patient’s T cells with synthetic receptors that precisely target cancer cells—turning them into personalised, living therapies capable of evolving alongside disease.
However, current CAR-T cell therapy faces major challenges. Delivering genetic material into immune cells without any compromise is inherently difficult. Current industry-standard methods include viral vectors and bulk electroporation. Viral vectors are effective but pose safety risks, including immunogenicity and
random gene integration. Bulk electroporation, on the other hand, uses high-voltage pulses that can damage cells and reduce therapeutic quality. In Singapore, a single CAR-T treatment can cost around S$670,000, an astronomical amount for general patients, even with subsidies.
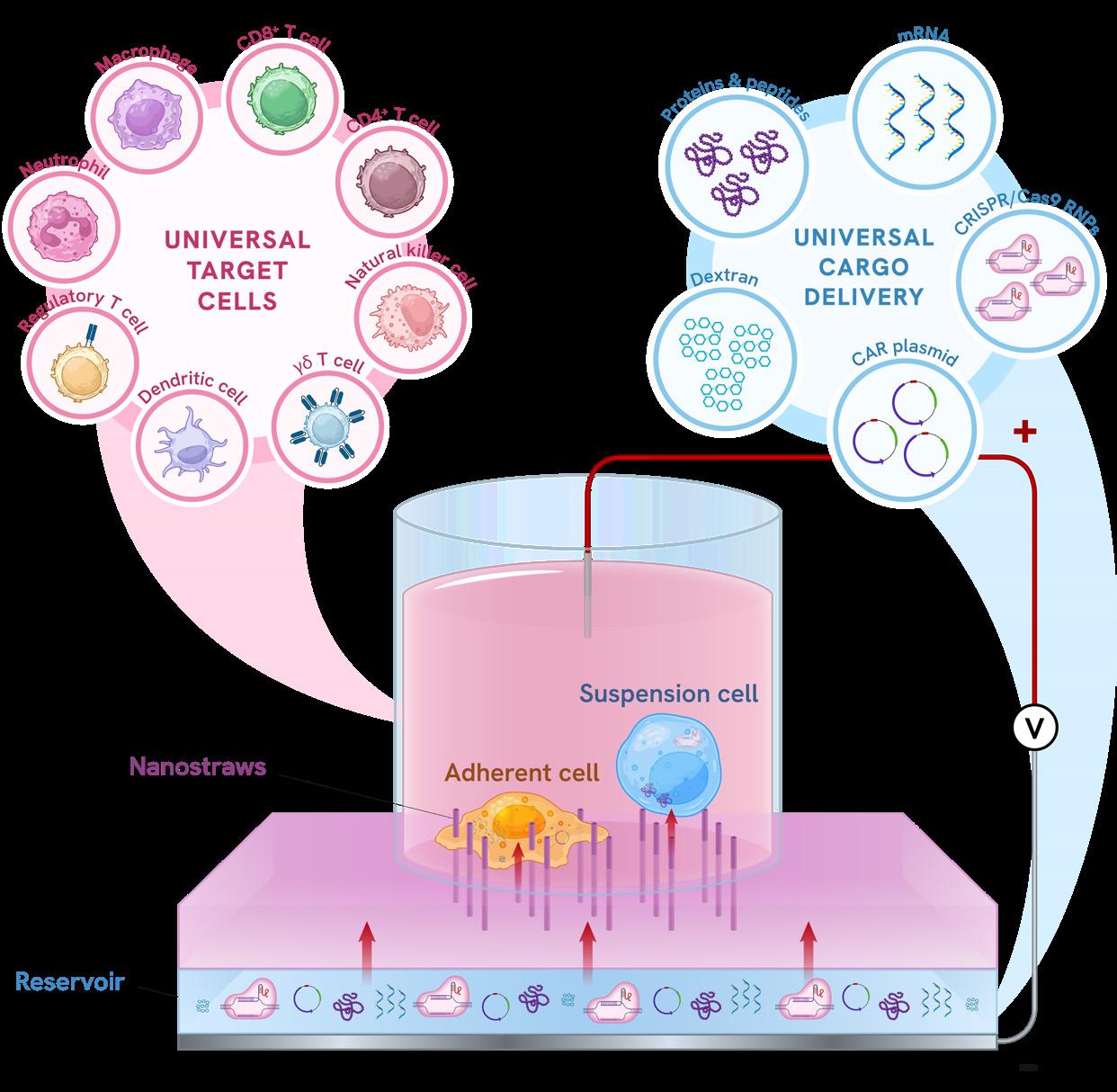
NUS researchers, led by Assistant Professor Andy Tay, tackled this
challenge with the Nanostraw Electroactuated Transfection (NExT) platform, a non-viral system that enables rapid, precise gene transfer into even the hardest-to-engineer immune cells, from macrophages to gamma-delta T cells. By creating tiny “nanostraw” conduits that gently introduce genetic material into cells while minimising stress and damage, NExT preserves cell viability, improves efficiency, and allows researchers to fine-tune cellular functions with unprecedented control. The team is now validating the technology in preclinical studies while partnering with industry to integrate it into existing manufacturing workflows and explore real-world applications.
Today, medicine is no longer just a response to disease—it is a collaboration between human ingenuity in engineered biological systems and the wisdom embedded in nature over the course of evolution.
N ature-based Solutions (NbS) are increasingly recognised worldwide as effective responses to the climate crisis. These approaches work with nature to reduce greenhouse gas emissions, conserve biodiversity, and help communities adapt to climate impacts through more sustainable livelihoods. Examples such as mangrove restoration, peatland protection, and agroforestry demonstrate the promise of NbS, which—in theory—offer benefits for both people and the planet. However, their practical success relies heavily on how they are governed.
Southeast Asia holds some of the world’s most significant carbon sinks, including tropical forests, wetlands, seagrass meadows, and agricultural soils. These ecosystems play a vital role in capturing carbon dioxide, supporting biodiversity, and sustaining local economies. Yet, they face mounting pressure from development and changing land use, making it challenging to balance environmental protection with economic and social needs.
The region’s tropical ecosystems are uniquely powerful carbon sinks because rapid plant growth, dense biomass, and waterlogged soils slow decomposition and preserve carbon for long periods. Safeguarding these ecosystems is crucial for global climate change mitigation efforts.
Environmental governance encompasses the institutions and processes that guide decisions on environmental protection. It involves governments, communities, businesses, and other stakeholders in setting rules, making plans, taking action, and monitoring outcomes. Effective governance is essential for NbS to deliver sustained benefits; however, research reveals persistent weaknesses across Southeast Asia. Challenges such as institutional constraints, lack of capacity, and inadequate funding or technology often limit the potential of NbS.

Research from the Carbon Governance in Southeast Asia (CGSEA) programme points to several issues that restrict NbS effectiveness. NbS projects are often managed by separate ministries—such as agriculture, environment, forestry, and finance—that rarely coordinate their actions. This lack of alignment results in competing agendas, overlapping legislation, and inconsistent policy enforcement, which undermine restoration and protection efforts.
Funding and investment, particularly through carbon markets, are also problematic. While supports from private sectors can help scale up NbS beyond small pilots, projects must be integrated with national climate strategies and involve local communities. Measurement and verification challenges also exist, especially in diverse forests where different tree species absorb carbon at different rates. This makes it hard to quantify total carbon storage for monetisation in offset markets.
Fairness and equity also remain significant concerns. Communities that have traditionally managed and depended on carbon-rich ecosystems are frequently excluded from decision-making. In many cases, their land and resource rights are restricted or removed without consent or compensation, leading to loss of livelihoods, poverty, or economic migration that can disrupt ecosystems elsewhere.
If these governance challenges are not addressed, NbS actually harm both the ecosystems and communities that rely on them. This emphasises the need for actionable insights to optimise NbS, ensuring Southeast Asian countries meet climate goals in effective and inclusive ways.
Despite these obstacles, there are promising signs of progress. Awareness is growing that NbS must also address broader issues like food and water security, disaster risk reduction, biodiversity conservation, and equitable resource distribution and rural livelihoods. For example, community-driven mangrove restoration in Krabi, Thailand, has improved flood resilience while supporting fisheries and ecotourism. Regionally, ASEAN is taking a more active role in aligning national climate strategies with international

agreements, such as the 2015 Paris Agreement. The ASEAN Climate Change Strategic Action Plan and Peatland Management Strategy, for example, both aim for a resilient and low-carbon Southeast Asia by 2050.
Financing mechanisms are also advancing, with blended finance combining public, private, and community resources to spread risk and rewards more fairly. New tools such as biodiversity credits and debt-for-nature swaps hold promise for directing funds to local conservation, boosting community participation, and supporting NbS that address ecological and socioeconomic needs.
Nested governance is needed for NbS to succeed at scale. Local, national, and regional initiatives must complement each other and reflect real-world contexts. This requires embedding principles such as Free, Prior and Informed Consent into NbS planning, aligning national climate commitments with local policies, and fostering dialogue across sectors and administrative levels. Recognising Indigenous knowledge and customary land rights, and empowering communities to manage their ecosystems, have been shown to improve outcomes.
A well-coordinated and inclusive approach is essential for Southeast Asian countries to meet climate targets, strengthen resilience, and build societal cohesion. NbS hold genuine potential to tackle climate change in sustainable, fair, and inclusive ways. With extensive carbon sinks and a rich tradition of environmental stewardship, Southeast Asia is well-placed to lead. However, unlocking the full benefits of NbS in the long term will require changes in governance, financing, and implementation, with careful attention to who makes decisions, who gains, and who shoulders the costs.
By investing in comprehensive, multi-level NbS, Southeast Asia can lead the way in managing its vital carbon sinks for a more just and resilient climate future.
Professor David Taylor and Dr Michelle Miller are members of the CGSEA project, which is funded by the Social Science Research Thematic Grant (SSRTG) from the Ministry of Education, Singapore. For more information, visit: https://cgsea.org/

Optimising soil blends, growing microalgae, and developing COF membranes towards a more sustainable water future
Water treatment is not just about making water drinkable. It is also about ensuring that treated water can be returned to the environment without harming ecosystems or public health. Conventional water treatment typically involves physical, chemical, and biological methods to remove pollutants. While modern water treatment technologies can target emerging pollutants like pharmaceuticals, microplastics, and heavy metals, the systems are expensive and typically require substantial land area to operate. They also consume significant energy and produce methane and sludge.
Seeking more sustainable and affordable alternatives, researchers at NUS are now turning to natural water treatment processes. Natural ecosystems rely on soil, plants, and microbes to filter, absorb, and break down pollutants with minimal chemical or energy input. In wetlands, for example, sediments and heavy metals are removed as water flows through vegetation.
Improved bioretention systems using engineered soil mixes and moisture conservation techniques for tropical and arid
Hu Jiangyong
NUS Civil and Environmental Engineering
Centre for Water Research
NUS Environmental Research Institute

regions is one idea being explored. Bioretention systems, such as rain gardens and bioswales, slow down stormwater run-off to reduce flooding while filtering pollutants like nitrogen and phosphorus before water enters drains or reservoirs. While essential for plants, nitrogen and phosphorus can cause environmental issues when discharged in excess, such as algae blooms.
Typically, the filter media used in bioretention systems comprise sand, soil, and organic matter like compost. In Singapore, a study1 showed that an optimised blend of sand, compost, and water treatment residues could remove over 90% of sediments and phosphorus, and almost 60% of nitrogen from stormwater. Coconut fibre proved to be an effective filter medium.
Other nature-based wastewater treatment solutions being explored include the use of microalgae2. A collaborative study involving NUS and universities in China and the United States used Chlorella pyrenoidosa algae biofilms grown under mixotrophic conditions to remove 97% of phosphorus and 90% of ammonia from wastewater. The process also produced algae biomass that can be converted into biofuel, fertiliser, or animal feed. Compared to conventional algae cultivation methods, this approach was faster, more efficient, and created more opportunities for resource recovery.
While nature-based solutions utilise living organisms and ecosystems, nature-inspired solutions mimic structures or functions found in nature without using living elements.
For example, advanced membranes based on covalent organic frameworks3 (COFs) replicate biological ion channels in cells, which selectively allow only certain ions to pass through.
This approach has been used to extract lithium from salt lakes. Current adsorption or chemical-based extraction methods are energy-intensive and ineffective at separating lithium (Li+) from magnesium (Mg²+) in mixed ion environments. Using COF membranes, however, pore sizes and functional groups can be aligned, thereby creating a
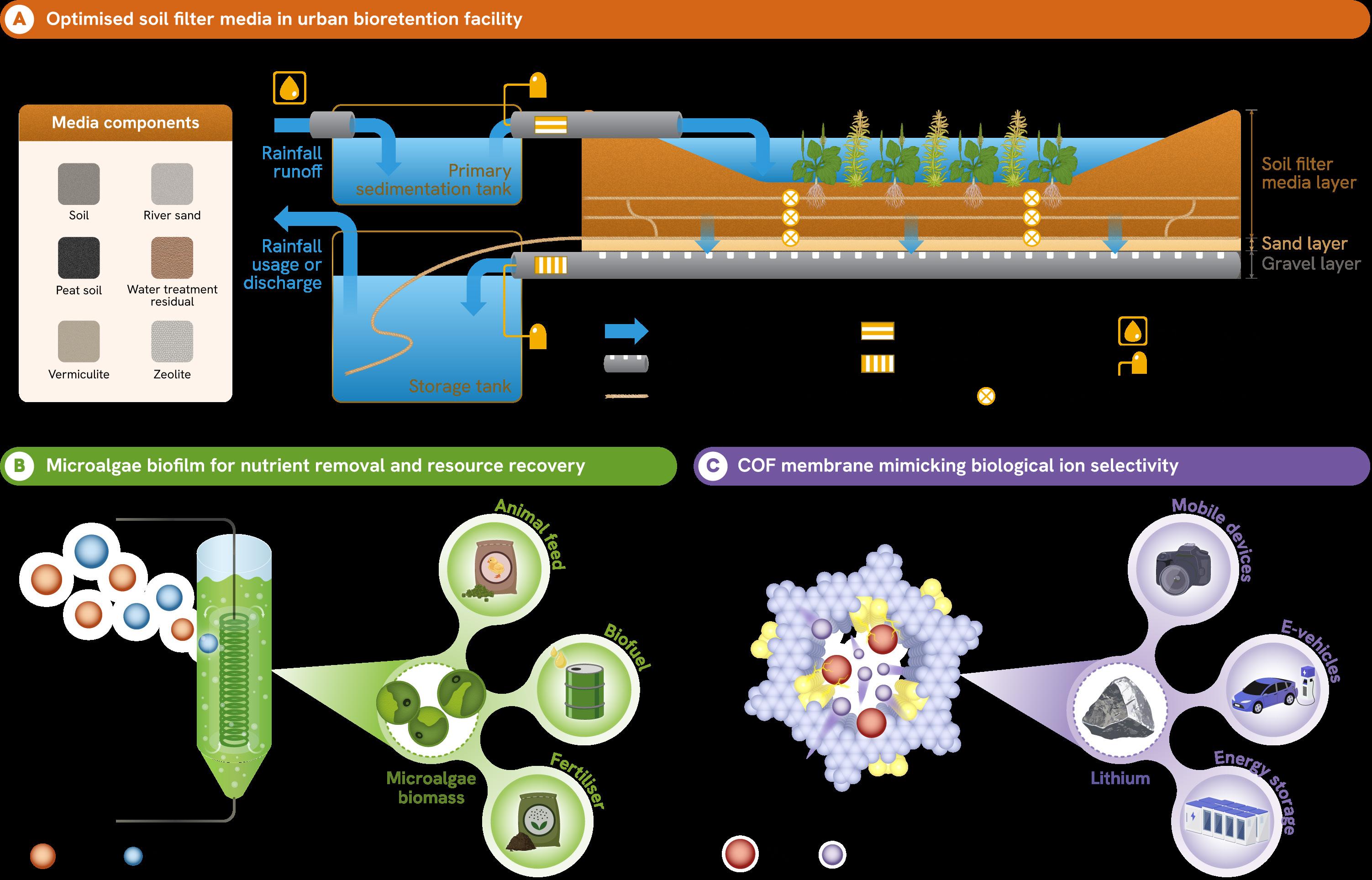
(A) Bioretention facilities enhance stormwater pollutant removal for urban water reuse. (B) Microalgae biofilm reactors accelerate the removal of nutrients from wastewater while enabling the recovery of microalgae biomass for various industry applications. (C) COF membranes inspired by biological ion channels achieve highly selective lithium extraction from mixed ion solutions, advancing energy-efficient water treatment technologies.
dual nanoconfinement effect. Here, the Mg²+ ions interact more with the membrane’s functional groups, while Li+ selectively passes through. This design offers greater selectivity than conventional polymer membranes and could be used for other separations involving monovalent and divalent ions.
In general, nature-based and natureinspired solutions provide sustainable, energy-efficient alternatives that use fewer chemicals and produce fewer harmful by-products.
“They can also be visually appealing, contributing to community acceptance and urban greening. Additionally, many of these systems can capture carbon, supporting climate mitigation goals,” said Professor Hu.
However, challenges remain. Naturebased solutions often require more land and their performance can vary with environmental conditions. To overcome these limitations, hybrid systems have been proposed that combine the sustainability of naturebased solutions with the reliability of conventional technologies.
“Innovative integration strategies with high efficiency and small footprint technologies could play a key role in scaling up these solutions,” said Prof Hu. “In addition, more targeted research and development is needed to tailor these approaches to local climates, ecosystems, and community needs.”
Investing in adaptable, inclusive, and informed solutions is crucial as we face a future with increasingly complex
water security issues. Nature-based and nature-inspired approaches are not silver bullets, but they can complement traditional treatment systems. With the right integration, they can become central to a more resilient and sustainable water future.
1. Guo, H., Lim, F. Y., Zhang, Y., Lee, L. Y., Hu, J. Y., Ong, S. L., ... & Ong, G. S. (2015). Soil column studies on the performance evaluation of engineered soil mixes for bioretention systems. Desalination and Water Treatment, 54(13), 3661-3667.
2. Wu, X., Jin, C., Zhang, C., Li, P., Huang, J. J., Wu, J., ... & Hu, Z. (2024). Mixotrophic Chlorella pyrenoidosa biofilm with enhanced biomass production, microalgal activity, and nutrient removal from nutrient-rich wastewater. Journal of Environmental Sciences.
3. Wang, G., Shao, L., & Zhang, S. (2025). Membrane-Ion Interactions Creating Dual-Nanoconfined Channels for Superior Mixed Ion Separations. Advanced Materials, 2414898.
With climate change making extreme heat events more common across Southeast Asia, urban residents are feeling the effects more acutely than ever. Cities, already prone to the urban heat island effect, routinely record temperatures higher than nearby rural areas—a trend that increases health risks, contributes to heat-related illnesses, and impairs work performance. Meeting this challenge goes beyond adopting new technologies; it requires us to fundamentally rethink how we plan and experience city life.
One surprisingly powerful—and sometimes overlooked— solution lies in harnessing nature itself. Urban greenery, from trees and parks to green roofs and water features, can significantly reduce ambient heat. Research has shown that in Singapore, vertical greenery can lower local temperatures by up to 4°C, while large parks such as the Botanic Gardens can cool adjacent neighbourhoods by as much as 5°C. Under the city’s Landscaping for Urban Spaces and High-Rises (LUSH) programme, rooftop greenery has been shown to reduce surface temperatures by as much as 17°C, with cooling effects extending over a 10 km radius.
Yet, effective cooling isn’t just about planting more trees. For green infrastructure to truly make a difference, it must be deliberately planned—integrated thoughtfully
into the urban fabric, tailored to local microclimates, and distributed equitably across communities. Achieving this level of strategic design requires robust data and advanced planning tools.
At the NUS College of Design and Engineering, researchers led by Associate Professor Yuan Chao from NUS Cities and the Urban Climate Design Lab, and Professor Wong Nyuk Hien from NUS Built Environment, are studying the impacts of the urban heat island effect in Singapore and exploring potential mitigations. The research teams harness GIS-based climate modelling and planning tools to identify urban heat hotspots and estimate the outcomes of different design interventions on heat dispersion. In a separate study, Prof Wong explored the possibility of naturally ventilated spaces, such as semi-outdoor spaces, for shortduration work activities as a means to reduce the use of air conditioners.
By bringing green infrastructure into the core of climatesensitive urban planning, these tools empower city planners to map, quantify, and visualise how nature-based solutions can reduce temperatures, improve airflow, and strengthen urban resilience.
Of course, green infrastructure addresses only part of the problem. While parks, trees, and green roofs help reduce heat in shared spaces, many people—such as outdoor workers, delivery riders, and military personnel—still face challenges associated with high temperatures in their daily work. This is where the Heat Resilience & Performance Centre (HRPC) and the Human Potential Translational Research Programme (HPTRP) at NUS Medicine step in. Their research focuses on fundamental and forward-looking approaches to address challenges associated with living and working in rising ambient heat.

Director, Heat Resilience & Performance Centre (HRPC)
Deputy Chair, Human Potential Translational Research Programme (HPTRP), NUS Medicine
Co-Director, Heat Resilience & Performance Centre (HRPC)

Insights from the research are essential for creating complementary strategies, where human-centred interventions support passive cooling from nature-based solutions. A key part of the teams’ research is focused on understanding the physiological demands of different occupations working in the heat, and quantifying the impacts on health, productivity and performance. The teams then develop targeted and tailored interventions, which can then be translated into policy and practice. The recently completed Project HeatSafe, which investigated the impact of heat stress on outdoor workers in Southeast Asia, demonstrates this. Its findings of reduced productivity and performance in workers resulted in the establishment of new policies that protect outdoor workers in Singapore.
Such work complements urban nature-based solutions by identifying where and for whom cooling matters most. By combining microclimate data with physiological risk modelling, planners can pinpoint hotspots where green infrastructure could have the biggest impact. Workplace design, such as shaded rest areas and shift scheduling to avoid peak heat, can also be improved to protect vulnerable workers.
There are, however, challenges. Green infrastructure can bring unintended consequences, including increased maintenance or “green gentrification,” where rising property values displace lower-income residents. Making these interventions just and inclusive requires long-term cooperation among government, academia, and communities. One promising example is the Less-maintained Campus Greenery pilot at NUS, which explores whether wilder, low-maintenance green spaces can provide sustainable cooling without intensive upkeep.
Ultimately, urban resilience cannot rely on a single solution. It must blend the biological with the built, recognising that nature-based cooling and human adaptation are complementary, not competing, strategies. Singapore's approach— combining human-centred physiological research at HRPC and HPTRP with climate-responsive design and planning—provides a potential model for cities to adopt across the region.
The climate is changing. Our cities must too, evolving not only in appearance but in how they help people live, work, and stay safe in a hotter world.

How
Alternative proteins have come a long way—but for many, they still carry an undeniable “ick” factor. They are unfamiliar, expensive, and sometimes they just don’t taste quite right.
By 2050, the world will need to produce enough food to feed 10 billion people. But with shrinking agricultural land, unpredictable climate conditions, and unstable global supply chains, meeting this demand is an immense challenge. As the gap between rising demand and limited supply widens, it becomes clear that the world urgently needs a fundamental rethink of food production.
That’s where the Bezos Centre for Sustainable Protein at NUS comes in. Backed by US$30 million in funding from the Bezos Earth Fund, the Centre serves as a catalyst for innovation in sustainable food systems across Asia Pacific. It brings together scientists, investors, entrepreneurs, and policymakers to drive a bold new vision of food production—one that is resilient, equitable, and grounded in cutting-edge science.

Professor Zhou Weibiao
Head of Department, NUS Food Science & Technology
Acting Director, Bezos Centre of Sustainable Proteins at NUS

Developing high-quality hybrid meats is one area of focus. Unlike traditional cultivated meat or plant-based substitutes, hybrid meat combines cultured meat (real animal cells grown in a lab) with microalgae, a highly efficient, sustainable biomass.
With hybrid meat incorporating real animal cells, the flavour and texture of traditional meat can be replicated much more effectively than pure plant-based alternatives. Microalgae then brings a rich nutritional profile, natural umami flavours, and an environmentally friendly footprint without the need for arable land, fertilisers, or large-scale animal farming.
To produce high-quality, cost-effective, and sustainable microalgae, the Centre adopts a circular bioeconomy framework. In a recently published study, researchers repurposed industrial and agricultural by-products, such as tofu whey and brewer’s spent grains, as nutrient feedstock. This reduces dependence on expensive inputs like glucose and synthetic minerals, lowering production costs and minimising waste.
One of the biggest hurdles in cultured meat production is its reliance on fetal bovine serum (FBS), a costly and ethically problematic ingredient used to grow animal cells. Seeking alternatives, Professor Zhou Weibiao, Acting Director of the Centre, explored the use of bioactive plant protein
hydrolysates (PPHs) from soy and wheat. These innovations have cut FBS use by up to 70%, significantly reducing costs and bringing sustainable proteins a step closer to commercial viability.
Beyond taste and cost, new food products will inevitably be required to meet stringent safety standards, as well as navigate cultural preferences and dietary norms.
To better understand how consumers perceive novel proteins, researchers at the Centre are leading behavioural studies that explore attitudes toward taste, food safety, health, naturalness,
and price. These studies involve educating households about the rapidly changing protein market and tracking their choices between new and traditional protein sources.
They are also active on the regulatory front, with the Centre contributing subject-matter experts to the Singapore Food Agency’s (SFA) Novel Food Regulatory Framework. Their involvement supports the development of robust regulatory standards to ensure the safety and market readiness of novel protein products.
None of these innovations happen in isolation. Behind every breakthrough is a strong ecosystem—one that provides not just funding, but also credibility, stability, and cross-sectoral collaboration.
Singapore, in particular, offers fertile ground for innovation. The country’s integrated ecosystem brings together skilled researchers, responsive regulators, and proactive industry players. Agencies like the SFA, MUIS (Majlis Ugama Islam Singapura), and Enterprise Singapore are working closely with researchers at Bezos Centre @ NUS to ensure that alternative proteins can be safe, trusted, culturally appropriate, and commercially viable. From food safety certification to halal compliance and business incubation, this holistic approach allows expertise to flow across disciplines, rather than stay remain siloed.
Food has always been central to identity in Asia Pacific. With the right innovations and ecosystems, it can remain so: nutritious, accessible, and deeply familiar, even in an unfamiliar future.

Coastlines are dynamic, ever-shifting landscapes, shaped by wind, waves, and tides. For centuries, human settlements along vulnerable shorelines have faced the challenge of protecting their livelihoods and infrastructure from erosion, flooding, and storm surges.
Traditionally, this meant turning to engineered or “grey” coastal infrastructure—seawalls, levees, breakwaters, and other hard structures designed to hold back the sea. However, many of these barriers disrupt natural flows of water and sediment, and are often detrimental to local ecosystems.
The relationship between land and sea is complex but, for millions of years, diverse ecosystems have flourished at its interface. Many of these ecosystems help protect the shore, while supporting biodiversity contained within them as well as further out to sea. Dunes, wetlands, mangroves, coral reefs, and oyster beds, are prime examples of natural, or “green” infrastructure that reduce risks while delivering a host of ecological benefits.
For instance, mangrove forests feature dense root systems that reduce wave energy and trap sediment, thereby protecting shorelines from erosion. Similarly, coastal
wetlands and salt marshes act as natural buffers that absorb floodwaters and store carbon. These systems support biodiversity, improve water quality, and sustain fisheries.
With natural ecosystems serving similar functions to grey infrastructure, researchers and engineers are increasingly inspired to integrate elements of these natural ecosystems into coastal protection strategies.
The US Army Corps of Engineers, for example, has created many successful “Living Shorelines” that are protective, stabilised coastal edges made of natural materials such as
Associate Professor Peter Todd NUS Biological Sciences

Structurally complex seawall tiles, attached to traditional seawalls, support native marine species that benefit surrounding habitats and enhance ecosystem resilience.

plants, sand, or rock. Unlike concrete seawalls or other hard structures, which impede the growth of plants and animals, living shorelines grow over time. There are also ongoing efforts around the world to restore coastal systems such as mangroves, oyster reefs, and salt marshes for their coastal protective function.
However, when it comes to mitigating the effects of extreme weather events like typhoons, storm surges, or rising sea levels, nature-based solutions alone may not provide sufficient protection. These events generate forces and water volumes that can
exceed the buffering capacity of natural systems, particularly when ecosystems are still in the early stages of restoration or experiencing ongoing environmental stress.
Therefore, rather than seek to replace traditional grey infrastructure with nature-based systems entirely, the current focus is on understanding the benefits of integrating the two. Hybrid “green-grey infrastructure” approaches provide the reliability of grey structures with the adaptability and co-benefits of green systems.
Such approaches are being pursued by Associate Professor Peter Todd with funding from Public Utilities Board

through their Coastal Protection and Flood Management Research Programme. Through the “SPINS-Eco” project, his team is developing hybrid coastal protection solutions tailored to Singapore’s coast. Submerged breakwaters that support coral growth, for example, are being tested to see if they can reduce more wave energy than breakwaters without corals, while also supporting greater marine biodiversity.
“Designing and building a hybrid shoreline is a substantial undertaking that requires inputs from engineers, ecologists, architects, planners, and even social scientists. It is critical to understand the local conditions, especially hydrodynamics and ecological processes, as well as human use and needs.”
Assoc Prof Todd likes to think big. For many years he has been adding habitat complexity to Singapore’s shorelines to enhance biodiversity. In his “MaxBETH” project, he collaborates with partners across industry and academia to incorporate complex structures at multiple scales in the design of future coastal defences. This “meandering shorelines” concept features artificial bays, headlands, reefs and estuaries, and has the potential to provide both coastal protection and biodiversity benefits over many kilometres of shoreline.
Recognising the dynamic, organic, nature of shorelines, coastal protection should be about more than just fortifying with walls or barriers. By integrating ecology with engineering, it will be possible to create resilient hybrid defences that are able to respond to changing conditions far into the future.
NUS recently secured significant funding through Singapore's Low-Carbon Energy Research (LCER) Programme that will go towards research aimed at reducing Singapore's carbon footprint.
The funding will be distributed through the Direct Hydrogen Programme (DHP) and Emerging Technology Grant Call (ETGC), with NUS leading one DHP project and six ETGC projects. In total, S$55 million has been awarded through the scheme nationwide.
One project funded under this scheme is led by Assistant Professor Zhang Huangwei from NUS Mechanical Engineering. It will aim to develop an efficient ammonia cracking system to bolster Singapore's hydrogen infrastructure. Other projects focus on energy-efficient carbon capture technologies and electricity-powered catalysts for hydrogen extraction from ammonia. Each project aligns with NUS's goal of advancing sustainable energy solutions and underscore our commitment to enhancing Singapore's capabilities in low-carbon technologies.


The NUS Sustainable Futures is a university-wide initiative fostering interdisciplinary collaboration and cross-sector partnerships with a focus on finding innovative solutions to pressing environmental challenges in Asia. Launched in May 2025, it integrates various academic disciplines and engages with industry, government, and community stakeholders, with research structured around naturebased solutions to combat climate change and biodiversity loss, energy research and innovation, and integrative urban solutions. Led by NUS Chief Sustainability Scientist Professor Koh Lian Pin, its research and training programmes are supported by a S$10 million NUS investment and S$5 million in external funding.
To improve the agility, resilience, and sustainability of regional and global supply chains, the PSA-NUS Supply Chain Living Lab was launched in November 2024. A collaboration between NUS and PSA International, it strengthens industry-academia collaboration, and serves as a sandbox to develop innovative solutions for efficient and practical supply chains. The initiative will receive funding of up to S$10 million from PSA International.
Four outstanding professors from NUS received the nation’s highest honours for scientists and engineers as a recognition of their outstanding achievements in their respective fields.
Organised by the National Research Foundation, Singapore (NRF), the President’s Science & Technology Awards (PSTA) honours the exceptional contributions of researchers based in Singapore who have also helped to advance the country’s strategic research priorities.

President's Science Award



NUS researchers honoured internationally for work in anthropology and history
Assistant Professor Elliott Prasse-Freeman (NUS Sociology and Anthropology) won the 2025 Public Anthropologist Award for Rights Refused: Grassroots Activism and State Violence in Myanmar, a vivid ethnography of community resistance under authoritarian rule. Dr Faizah Zakaria (NUS Southeast Asian and Malay Studies) received the 2025 Harry J. Benda Prize for The Camphor Tree and the Elephant, which shows how religious conversion reshaped human–environment relations. Their work highlights the essential role of social science in understanding political and ecological transformations across Southeast Asia.
Read more here


Asst Prof Iris Yu receives L’Oréal-UNESCO For Women in Science Singapore Award 2024
Assistant Professor Iris Yu from NUS Civil and Environmental Engineering was awarded the L’Oréal UNESCO For Women in Science Singapore Award 2024, for her innovative work in converting biomass and organic waste into sustainable, carbon-neutral materials via thermochemical and biorefinery techniques. The prize includes a S$10,000 endowment to support her research in turning food and biomass waste into energy, chemicals, and useful materials, advancing Singapore’s circular bioeconomy.


NUS Presidential Young Professor (2024)
NUS Chemical & Biomolecular Engineering

NUS Presidential Young Professor (2024)
NUS Chemical & Biomolecular Engineering
Current medical treatments like invasive surgeries pose significant risks and discomfort, highlighting the urgent need for safer, more effective alternatives.
A rising star in biomedical innovation, Assistant Professor Zhang Yamin is revolutionising next-generation therapeutic platforms with smart, patient-friendly, and affordable medical technologies. Her latest breakthrough a millimetre-scale cardiac pacemaker stands as the world’s smallest of its kind. With its tiny size, the pacemaker can be minimally invasively injected into the body, eliminating the need for open-heart surgery and significantly reducing the risks and complications associated with invasive procedures. The device is also bioresorbable, meaning the body safely absorbs it over time, eliminating the need for removal surgery. Its unique capabilities are especially impactful for paediatric patients and offer a much-needed alternative for adults underserved by conventional solutions.
For her contributions, she was named one of MIT Technology Review's "Innovators Under 35".
Synthesis of valuable chemicals from carbon dioxide (CO2) is a cornerstone of future sustainable chemical industries. Yet, producing essential multi-carbon compounds like amino acids remains a major challenge.
Assistant Professor Lei Wang and his team at NUS have developed a novel system that converts CO2 into L-tyrosine, a high-value amino acid widely used in pharmaceuticals and food industries. The team combined electrochemical CO2 reduction with synthetic biology in a two-stage process. First, CO2 is electrochemically converted into a mixture of ethanol and acetic acid, namely the "blended nexus molecules." These are directly fed into genetically engineered E. coli, which can metabolise both molecules to produce L-tyrosine. This dual-pathway strategy led to a fivefold increase in tyrosine yield compared to conventional strains.
The work offers a scalable, energy-efficient route for upgrading waste CO2 into valuable biochemicals, highlighting a promising path toward greener chemical manufacturing.


NUS Presidential Young Professor (2021)
NUS Medicine
Using engineered microbes to biologically break down and convert plastic waste like PET into valuable products is a promising alternative to develop a sustainable circular economy for plastic waste management. However, this process requires multiple enzymes and pathways to work together, encoded by large gene clusters. Traditional directed evolution techniques can only optimise one gene at a time, making it difficult to improve the performance of complex, multi-gene systems.
To overcome this, Assistant Professor Julius Fredens developed LySE (Lytic Selection and Evolution) a phagebased platform that enables rapid, targeted evolution of large gene clusters in E. coli. Using LySE, the team evolved a bacterial strain that can efficiently use ethylene glycol (EG) a PET monomer as its sole carbon source. This work highlights LySE as a powerful tool for accelerating metabolic pathway evolution and advancing biological upcycling of plastic waste into valuable bioproducts.

We aim to attract, support, and empower talented young academics to work alongside a diverse and dynamic community of experts and students, shaping the future through cutting-edge research and innovative teaching.
Ϗ Presidential Young Professorship Ϗ Presidential Fellowship

Professor Qiu Cheng Wei
NUS Electrical and Computer Engineering
DOI: 10.1126/science.adq2329
N US researchers have created hydrodynamic moiré superlattices using vortex lattices in fluid systems, paving the way for innovative applications in energy transport, thermal management, and quantum-inspired physics.
Controlling energy transfer in fluids has been a longstanding challenge in physics and engineering. Unlike in solids, where structural periodicity allows for the precise control of energy transfer, the control of energy in fluids is uniquely complex, as they lack rigidity and experience constantly changing dynamics. Energy also dissipates unpredictably within fluid systems, often leading to inefficiencies in processes like cooling, heating, or energy harvesting. Imposing order and harnessing the fluid’s natural motion for energy transfer requires innovative approaches.
Moiré patterns, known for their ability to enhance energy localisation and transfer in solid-state systems, offer a promising solution for introducing ordered periodicity into fluid systems. Moiré patterns are visual effects that arise from the overlay of two single-layered periodic structures. Their formation results in larger-scale periodicities and unique optical or electronic properties.
When introduced into fluids, moiré patterns could impose structure on what is otherwise chaotic motion, to enable efficient energy transfer and minimises dissipation. Introducing such ordered periodicity into fluids is however, no small feat.
Now, a team of researchers from NUS College of Design and Engineering, led by Professor Qiu Cheng-Wei, along with collaborators from Chongqing Technology and Business University, and Nanyang Technological University, have unveiled the creation of hydrodynamic moiré superlattices. This was achieved by leveraging hydrodynamic vortices, a novel approach to manipulate energy transfer and mass transport in fluid systems.
Hydrodynamic vortices are localised areas of rotational motion within a fluid, creating well-defined points of energy and momentum concentration. These vortices are inherently stable and can be arranged in periodic patterns when controlled by external forces, such as electromagnetic fields.
In the study, each vortex acted as an individual lattice site, analogous to atoms in a crystal lattice. Two layers of these vortex fluids, consisting of sodium chloride solution, were carefully stacked and twisted. Heat was applied
along the z direction to visualise the profile of the moiré superlattice and the propagations of energy across the lattice. The resulting bilayer moiré superlattice was capable of localising and delocalising energy. This occurred when the angles between the two fluid layers was altered.
“The ability to control energy transport and localisation through geometry alone is a game-changer,” said Prof Qiu. “This discovery offers a new framework for designing more efficient systems to manage energy in fluids.”
For instance, in cooling systems or heat exchangers, these superlattices can help optimise thermal performance by directing energy flow with precision, reducing waste and improving overall efficiency.
The researchers found that angles corresponding to Pythagorean triples—sets of integers that satisfy the Pythagoras theorem—resulted in ordered, periodic structures with translational symmetry. Conversely, non-Pythagorean twist angles led to aperiodic, quasicrystalline patterns.
For example, at Pythagorean angles such as 36.87°, the system exhibited periodic distributions of pressure and
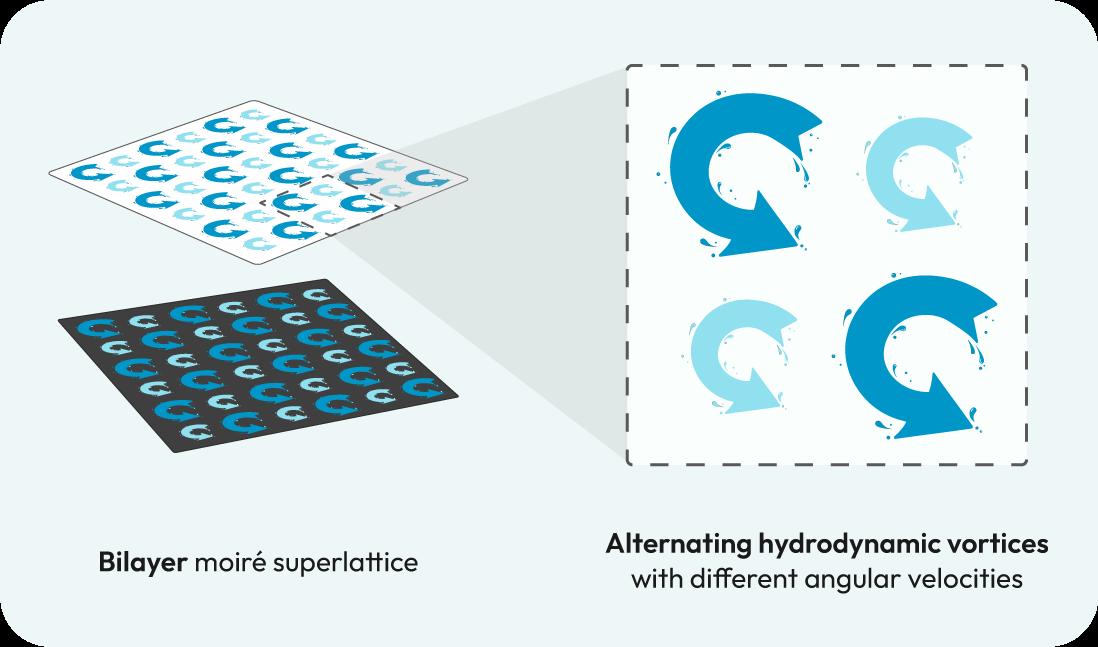
General configuration of the four-unit structure within a hydrodynamic layer with alternating vortices and heat application in the z-direction.
velocity fields, enabling efficient energy transport. In contrast, non-Pythagorean angles disrupted this periodicity, resulting in localised energy states and quasicrystalline behaviours, such as the inhomogeneous energy distributions and localised “hot spot”. These findings highlight the versatility of moiré physics in designing fluidic systems with tailored properties.
“Hydrodynamic moiré superlattices open an unexpected door to control energy transfer, mass transport, and particle navigation,” adds Prof Qiu.
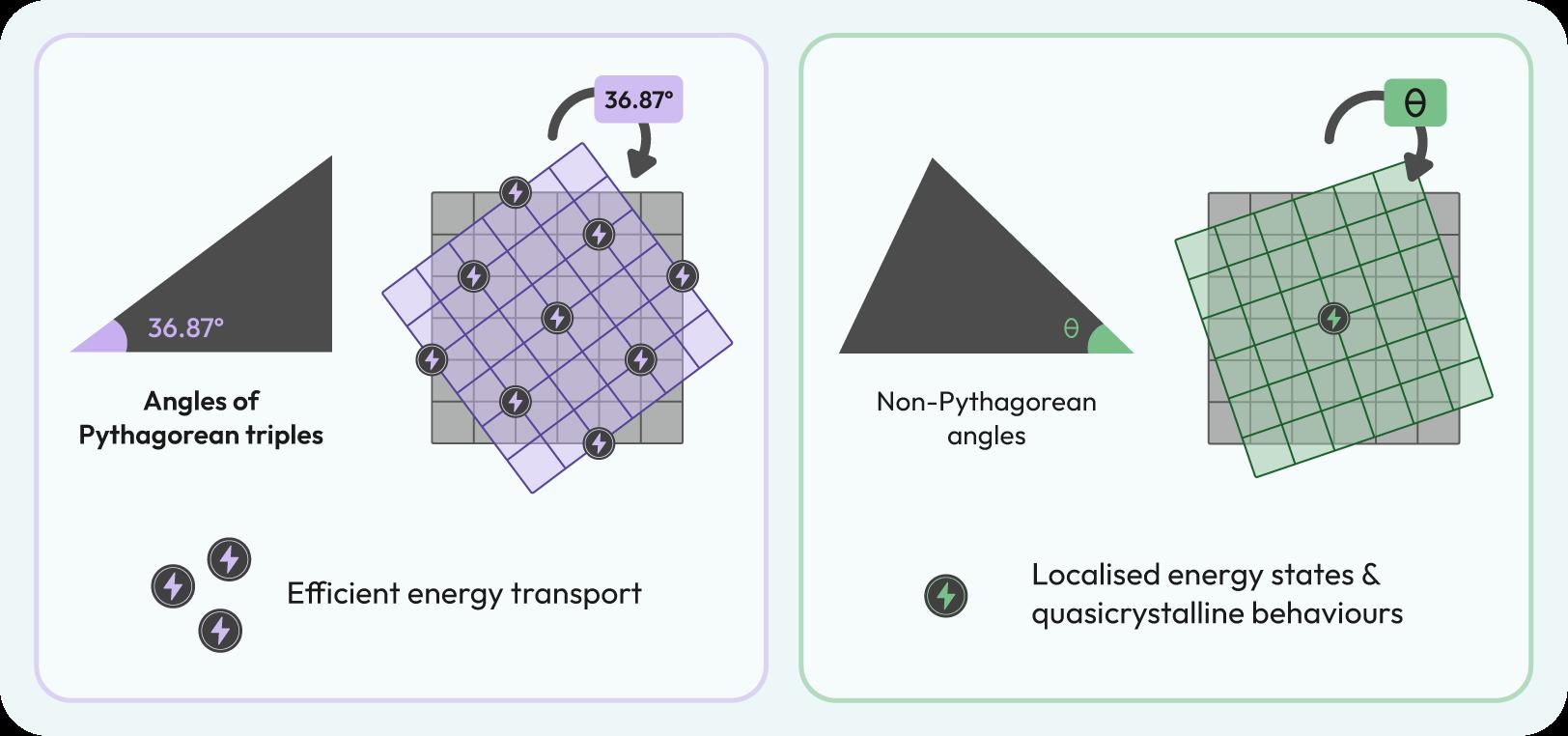
Illustration to determine if the twist angle corresponds to the Pythagorean triples
“They provide a blueprint for exploring how geometric configurations influence physical properties in fluids and beyond.”
In industrial processes, hydrodynamic moiré superlattices could be used to improve fluid mixing or design advanced microfluidic devices, besides optimising heat exchange systems.
Beyond practical applications, the discovery bridges the gap between fluid dynamics and quantum physics. By mimicking quantum phenomena such as flat bands and topological states through various geometric configurations, hydrodynamic moiré superlattices enable the ability to explore, test, and visualise quantum behaviours in a classical, easily controllable setting. Unlike quantum systems, which often require ultra-low temperatures, precise instrumentation, and challenging setups to probe their properties, hydrodynamic systems operate under more accessible laboratory conditions, such as room temperature and macroscopic scales. These systems could inspire new research in wave dynamics, energy transfer, and other interdisciplinary fields.
Associate Professor Koh Ming Joo
NUS Chemistry
DOI: 10.1038/s44160-024-00658-7
S tereochemistry, or the spatial arrangement of atoms within a molecule, greatly influences the function of many chemicals and therapeutics used today. This is true for alkenes, which are fundamental hydrocarbons that contain carboncarbon double bonds (C=C), and which are integral to many applications, from the manufacturing of plastics to therapeutics.
In Z-alkenes, the functional groups are located on the same side of the carbon-carbon double bond (C=C), while in the E-isomer, the same groups are positioned on opposite sides of
the C=C. Often, Z-alkenes are desired due to their higher polarity and lower melting points compared to E-alkenes, making them versatile precursors or intermediates in the manufacture of other valuable molecules.
Tamoxifen, for example, is a therapeutic alkene used in the treatment of breast cancer, and which exhibits different functions depending on the stereoisomer produced. While both isomers bind the target estrogen receptor, the E-isomer activates the receptor, whereas the Z-isomer inhibits it. In dietary supplements, betacarotene, a precursor to vitamin A,
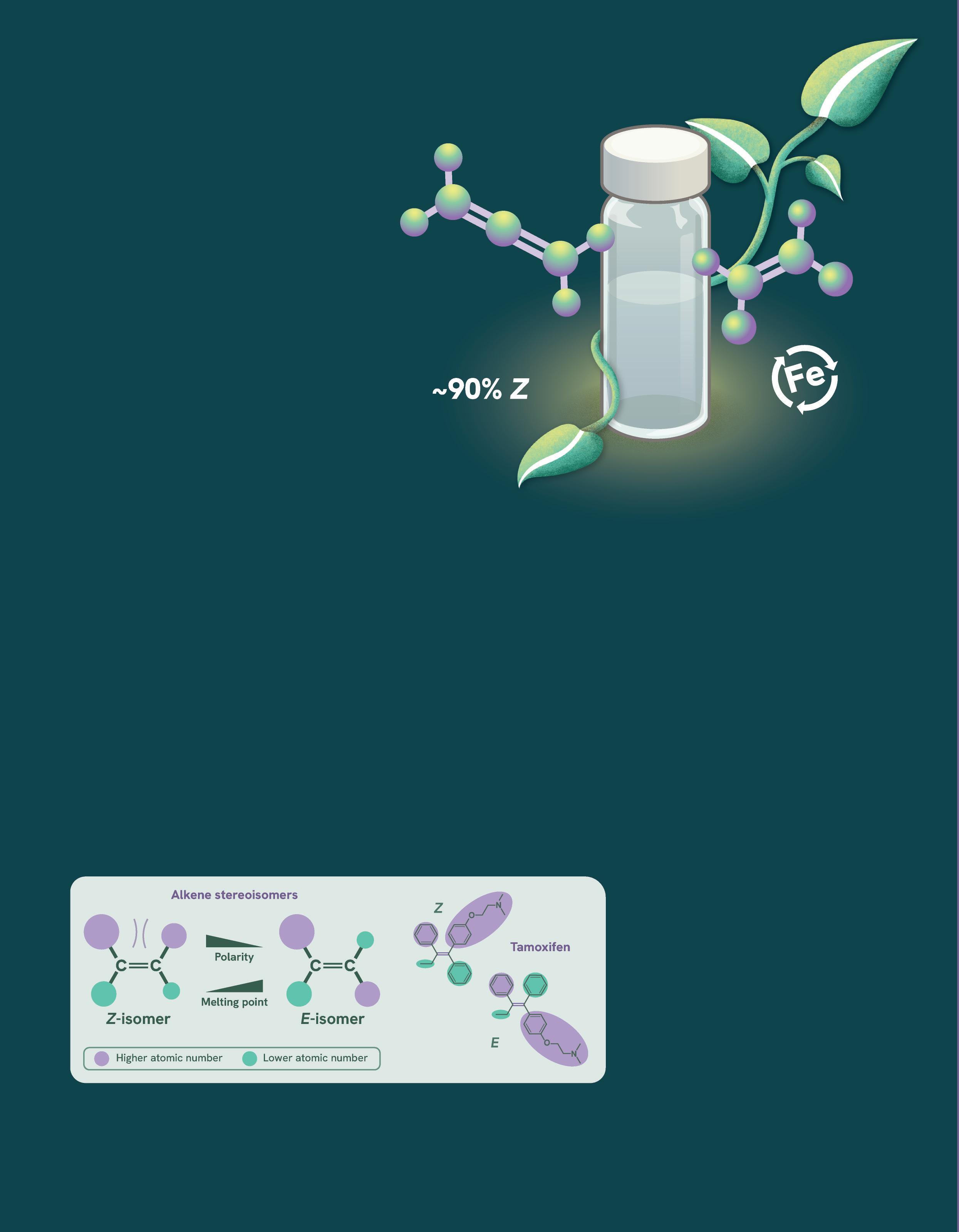
Z-isomers have higher priority groups on the same side of the double bond, while E-isomers have them on opposite sides. This difference in arrangement affects properties like polarity and melting points, and is particularly important in molecules like Tamoxifen, where Z and E isomers can exhibit distinct biological activities.
naturally exists in both Z and E forms, with the Z-isomer being more bioavailable and better absorbed in the body. Another example, fusidic acid, a common antibiotic for treating bacterial skin infections, relies on the Z-alkene configuration to inhibit bacterial protein synthesis, giving it its anti-bacterial properties.
Despite the high demand for Z-alkenes, current production methods remain expensive and environmentally unfriendly. Carbonyl olefination, for example, produces triphenylphosphine oxide as a byproduct which is challenging to remove. Alkyne functionalisation on the other hand relies on expensive palladium catalysts, while cross-metathesis uses precious metal catalysts like ruthenium.
While alternative metal catalysts are available, it is often challenging to recover and recycle them, especially in large-scale production processes. As a result, their use may lead to contamination of the product, and pose environmental risks, limiting the sustainability and scalability of Z-alkene production.
Now, a research team led by Associate Professor Koh Ming Joo from NUS Chemistry, has devised a method for the sustainable and efficient production of Z-alkenes. Their strategy incorporates allenes as starting materials, an iron catalyst, and a straightforward dialkylation reaction, where two alkyl groups are precisely installed onto the allenes. Allenes are hydrocarbons characterised by two adjacent carbon-carbon double bonds (C=C=C).
The research, which was described in Nature Synthesis, was shown to produce trisubstituted Z-alkenes which are important intermediates in applications such as therapeutics.
The highlight of the method is the use of an iron-based catalyst. Traditionally, iron has posed challenges in catalysis due to its ability to adopt multiple oxidation and spin states, which complicates the control of reactivity and selectivity.
According to Assoc Prof Koh, the work represents a significant breakthrough in ‘green chemistry’ as it not only accelerates the production of Z-alkenes and generates less waste, but its abundance as a less toxic and inexpensive transition metal makes their production more sustainable and cost-effective.
As a proof of concept, the team applied their approach to the synthesis of a glucosylceramide synthase (GCS) inhibitor containing a trisubstituted Z-alkene. Inhibiting the GCS enzyme can help reduce the accumulation of glycosphingolipids, which is associated with diseases such as Gaucher disease, cancer, and certain neurodegenerative disorders.
“Previous methods could yield Z-alkenes with only about 10% efficiency, in addition, the ratio of Z-alkenes to E-alkenes is nearly equal, resulting in poor selectivity, necessitating additional separation steps,” he elaborated.
In contrast, the described method achieves a higher yield and selectivity, producing up to 91% of Z-alkene with only 9% E-alkenes.
The method successfully transformed various allenes, including those with functional groups like amines, halogens, and esters, into the desired products with good yields and high selectivity. It also proved effective in producing even more complex structures, such as tetrasubstituted alkenes.
This research was conducted in collaboration with Dr Xinglong Zhang from The Chinese University of Hong Kong. Computational studies from Dr Zhang provided insights into the reaction mechanisms, showing that π-π interactions between the groups on the allene and the ligand on the catalyst enhanced the reaction's efficiency by lowering the activation energy barrier.
Overall, this approach advances the synthesis of trisubstituted Z-alkenes and contributes to the field of sustainable iron catalysis.
"We foresee the widespread adoption of our iron-catalysed method as a practical and eco-friendly tool for creating valuable alkenes, which can support applications like drug discovery," Assoc Prof Koh added.
Moving forward, the team plans to apply similar approaches to the production of industry-relevant chemicals from other common raw materials.
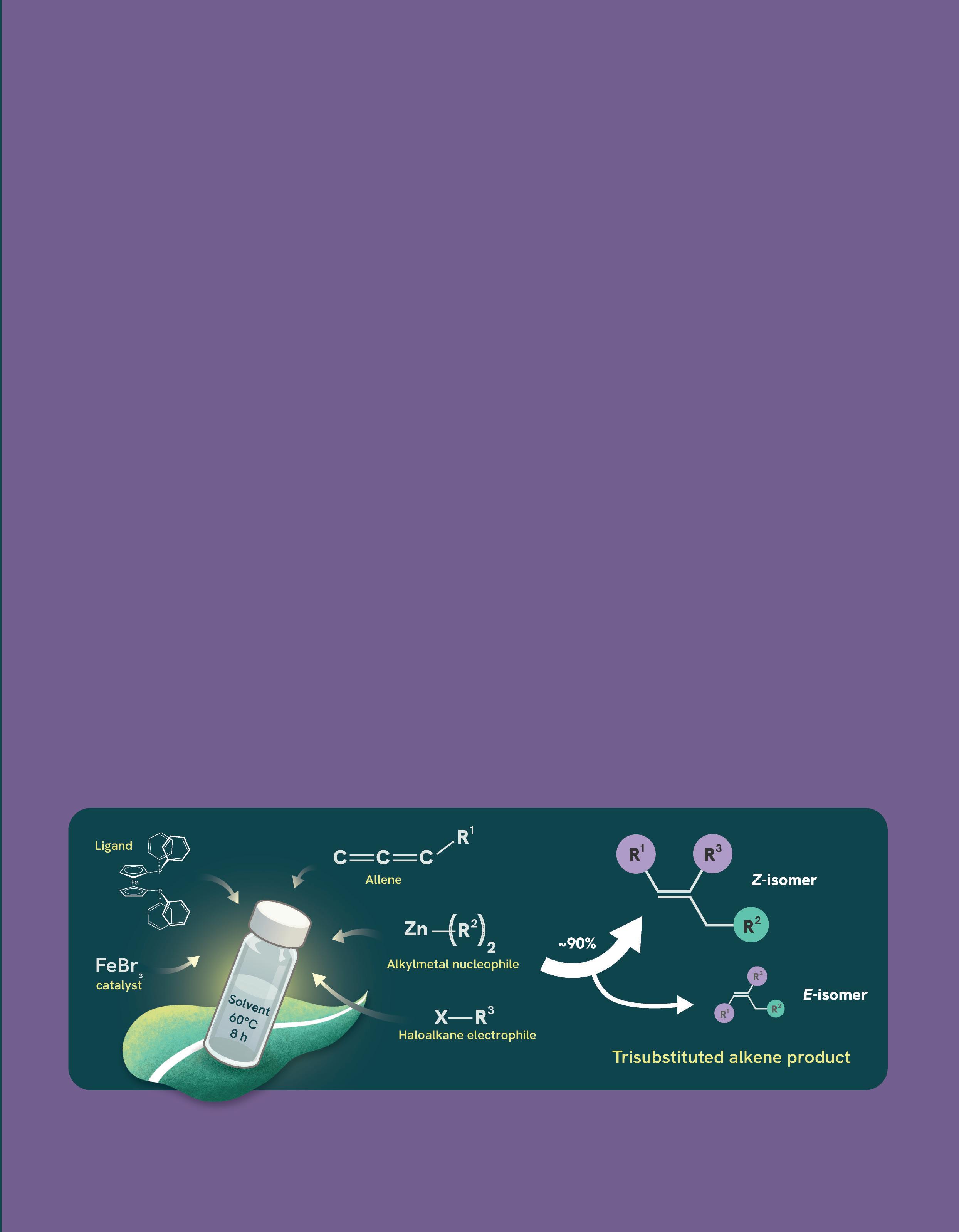
This method uses allene, FeBr3 as an iron catalyst, an alkylmetal nucleophile, a haloalkane electrophile, and the ligand S,S-BDPP to selectively produce trisubstituted alkenes. The reaction, performed at 60°C for 8 hours, yields about 91% Z-alkene and 9% E-alkene, demonstrating high efficiency and selectivity for the Z-isomer.

Professor Liu Bin
NUS Chemical and Biomolecular Engineering
DOI: 10.1126/sciadv.adp9448
Come 2050, the world may see as many as 10 million fatalities a year due to antibiotic-resistant bacterial infections. Contributing to this worrying projection are major challenges in developing effective treatment strategies, including ever-rising resistance to existing antibiotics and a dearth in the discovery and design of nextgeneration antibiotics.
Antibiotic resistance is acquired in bacteria as an evolutionary pressure to survive. With most of the existing antibiotics targeting a single bacterial gene or functional component, bacteria readily adapt to and evade antibiotic effects over time. The growing urgency of this global health issue has spurred scientists to redesign antibiotics with complex mechanisms of action that can fight pathogens on multiple fronts.
While innovative therapeutic solutions may partly address antibiotic resistance, there is yet another fundamental hurdle to overcome—discerning bacterial infections from
other conditions like inflammation and cancers, which can cause similar symptoms. In the absence of tools that can precisely pinpoint bacterial infections, antibiotics will continue to be used as a cautionary measure. Over time, bacteria will adapt to antibiotic effects and patients will stop responding to medications.
Recently, a team of scientists from Singapore and China, led by Professor Bin Liu from NUS Chemical and Biomolecular Engineering, addressed these glaring gaps in the antibiotic development pipeline by synthesizing an antibiotic compound with unique properties that offer it an edge over existing antibiotics. This work was published in Science Advances.
The compound, a fluorescent molecule named TPA2PyBu, can both target and destroy bacteria, including several multidrug-resistant strains, with remarkable precision. In the study, the agent prevented the growth of a wide range of gram-positive and gram-negative bacterial strains, including
TPA2PyBu is able to bind and disrupt the bacterial membrane while also interacts with bacterial DNA, causing it to be inactive
methicillin-resistant Staphyloccus aureus (MRSA), multidrug-resistant Escherichia coli (MDR E. coli), Acinetobacter baumannii and Enterococcus faecium. Importantly, it achieved this at concentrations of 1-10 μM, a dose low enough to minimise collateral damage to other healthy cells. Similar antibiotic candidates tested were effective against gram-negative bacteria only at doses greater than 50 μM.
The high bactericidal activity of TPA2PyBu can be linked to its dual mechanism of action: where a long alkyl chain strongly binds to the bacterial membrane and disrupts its structure and function, before the drug binds to bacterial DNA, causing it to aggregate into a dysfunctional state. This multi-pronged attack not only neutralises the bacteria but also creates substantial challenges for the bacteria to adapt and acquire resistance.
“Due to the dual mechanism of action of TPA2PyBu, we believe that the development of resistance would be significantly delayed compared to conventional antibiotics. Therefore, TPA2PyBu and similar agents serve not only as powerful therapeutics,
but also as time-buying innovations that slow the arms race between drugs and pathogens,” commented Prof Liu.
Its ability to bind selectively to the bacterial membrane also opens up the potential for TPA2PyBu to be used as a diagnostic tool, addressing a critical issue in the treatment of bacterial infections. For this, the researchers tapped a key property of the drug—it fluoresces when in an aggregated state or when bound to a target molecule. Whereas traditional fluorophores are
limited by their inability to penetrate gram-negative bacteria, TPA2PyBu exhibited a multi-fold increase in fluorescence in S. aureus-infected in vivo models. This sharp rise was noted solely at the site of infection, even when the models also included inflammation and cancer conditions. The ability of the drug to precisely differentiate bacterial infections from cancer and inflammation showcases its potential as a reliable diagnostic probe.
Although TPA2PyBu is a long way from being used in the clinic, the innovative strategy adopted by the team is expected to set new trends not just in the antibiotic development pipeline, but also in designing potent antifungal and antiviral therapies. As deadlier and seemingly invincible superbugs continue to emerge, millions of patients worldwide will benefit from tools that can enable real-time imaging-based and diagnosis-guided treatment of pathogens.
“This integration of targeted therapy with diagnostic imaging, known as theranostics, marks a major step toward intelligent and traceable antibiotics,” summed up Prof Liu.

TPA2PyBu is able to fluoresce selectively at sites of bacterial infection, even in the presence of inflammation and cancer.
Assistant Professor Lum Yanwei
NUS Chemical and Biomolecular Engineering
DOI: 10.1126/sciadv.ado5000
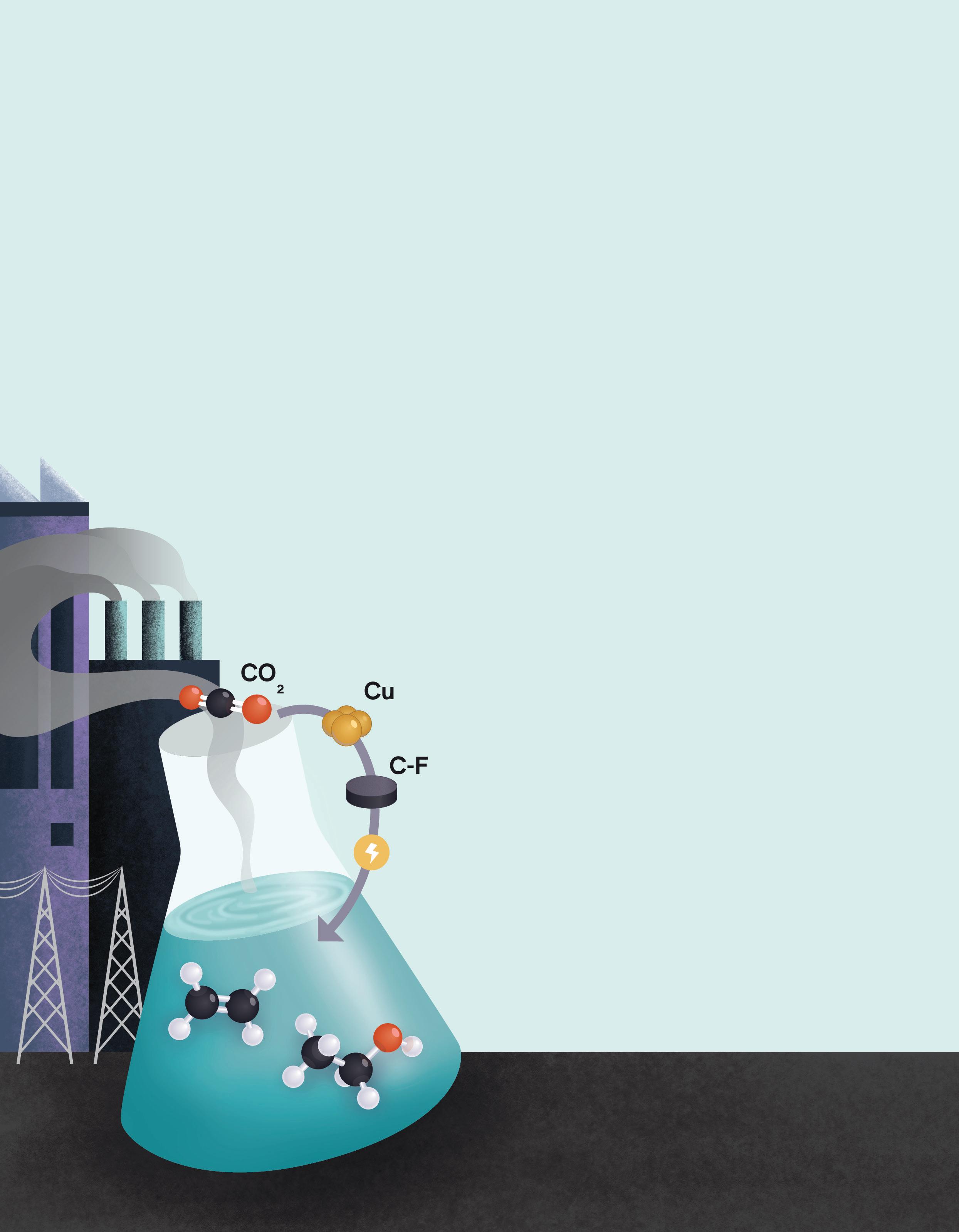
G lobal carbon emissions from fossil fuels reached a record high in 2024. As the urgency to tackle climate change intensifies, scientists are racing to develop ways to turn carbon dioxide from a pollutant into a resource.
One approach with enormous potential is CO2R, or the electrochemical reduction of carbon dioxide. CO2R centres on converting carbon dioxide into high value chemicals and fuels, using electricity from renewable sources. This method mitigates carbon emissions, while closing the loop on industrial systems.
However, CO2R currently faces significant technical challenges in its quest for widespread industrial adoption, such as high operating costs, low energy efficiency and low yields for desired products. For CO2R to be commercially viable, academics need to urgently improve the performance metrics of its electrochemical cell.
A recent study published in Science Advances, led by Assistant Professor Lum Yanwei from NUS Chemical and Biomolecular Engineering, offers a simple but potentially powerful strategy: add fluorine to the carbon catalyst supports.
Catalyst supports are usually seen as inert materials upon which catalysts can be attached. However, introducing impurities to the catalyst support, in a process known as doping, may influence the catalytic performance of the material. This was shown in a previous study where carbon supports were doped with small amounts of nitrogen.
Likening the support material to a dinner table and the catalyst to the food on top, Prof Lum quipped: “You can think of it as the table is actually changing the taste of the food. It makes your food taste better.”
Asst Prof Lum’s study compared carbon supports doped with atoms of low electronegativity, like boron, to high electronegativity, such as fluorine. The most electronegative supports, the researchers discovered, outperformed the undoped supports, while the less electronegative did poorer.
The catalyst with the fluorine-doped carbon support, they were surprised to find, displayed the highest Faradic efficiency (FE) value towards the desired multicarbon products. This meant that based on the total charge passed, the reaction produced the greatest amount of multicarbons relative to its theoretical potential.
It wasn’t obvious to them at the beginning that fluorine would work the best, Lum commented, as nitrogen is the more common choice in the literature.
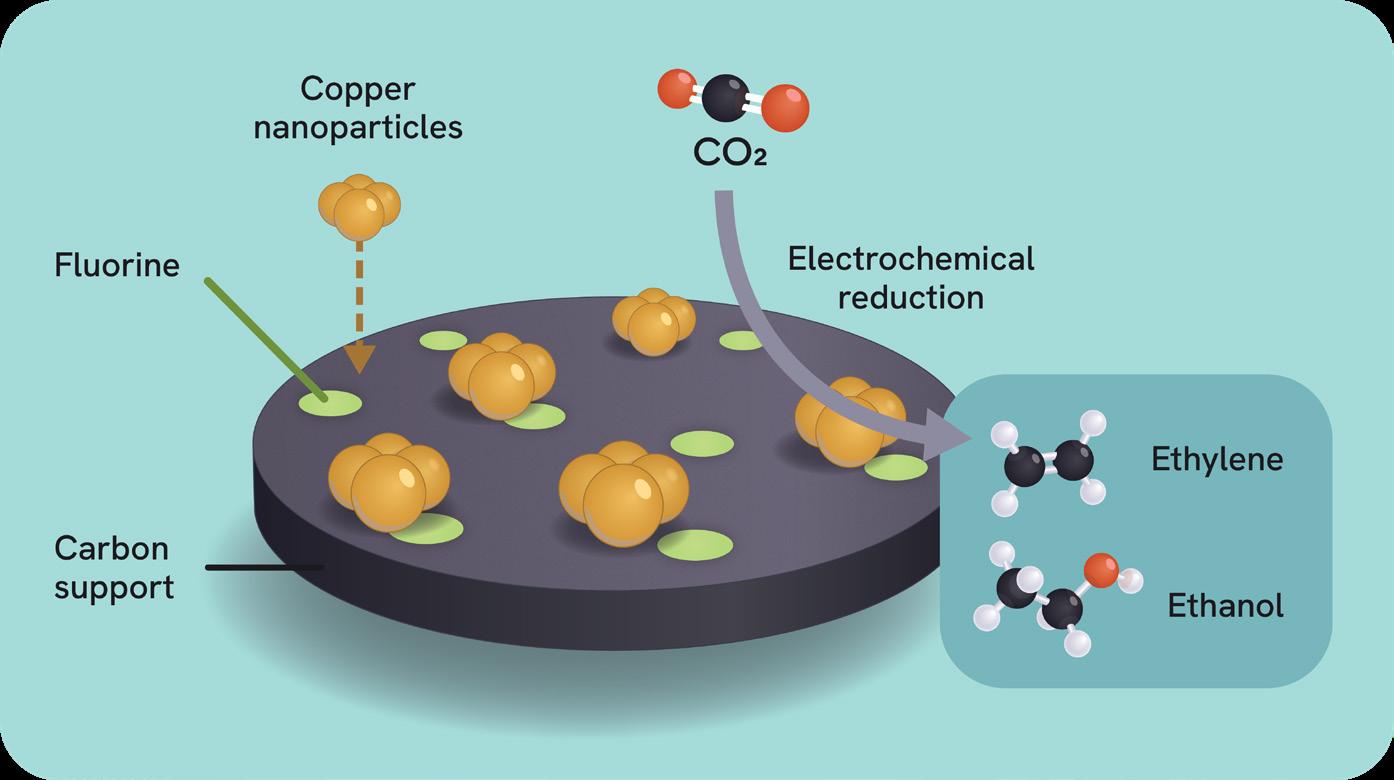
Based on their observations, the team designed a catalyst composite made with copper and fluorinedoped carbon, to steer CO2R pathways towards forming high-value multicarbon products, like ethylene and ethanol.
The researchers tested the composite in a gas diffusion electrode (GDE) flow cell, to mimic current densities found in industrial conditions. For CO2R to be commercially viable, higher current densities are needed to make the system cost-effective enough for large-scale production.
Compared to the control, the team’s composite consistently demonstrated greater FE in producing multicarbons across different current densities. It peaked at an impressive 82.5% at an industrially relevant current density of

400 milliampere per square centimetre, maintaining a stable performance over a 44-hour period.
Finally, the researchers tested the composite against simulated flue gas, mimicking the dirty exhaust produced by real world factories. Results showed that it was more efficient at avoiding the production of water, facilitating more direct conversion to valuable chemicals.
Real world flue gas contains oxygen, explained Lum. “[Oxygen] is like a parasitic species. It likes to steal electrons because the oxygen likes to turn into water,” he said. “But you don’t want to make water, because water is very cheap.”
In their experiments with simulated flue gas, the composite was over five times more efficient at producing multicarbons than a standard copper catalyst.
CO2R may still be a long way from being a practical reality, but this study lights the way towards a scalable electrochemical cell with commercial viability.
Unmodified carbon supports are already commonly used in CO2R, noted Lum. Just by tweaking this cheap, often overlooked material, researchers can move one step closer to realising sustainable, largescale carbon capture and utilisation.
NUS Chemistry
DOI: 10.1038/s44160-025-00797-5

I n the ever-evolving field of nanotechnology, carbon continues to astonish. From diamond to graphene, this element has a remarkable ability to reinvent itself at the atomic scale. Now, scientists are uncovering a new carbonbased marvel: single-walled carbon nanobelts (CNBs).
Structurally, CNBs are tiny loops of curved graphene. More precisely, they are short belt-like slices of single-walled carbon nanotubes (SWCNTs) with unique shapes that confer exceptional electronic properties. Already they have been identified as being ideal candidates for applications in nanoscale electronics and flexible optoelectronic devices.
Despite their promise however, synthesising CNBs is a formidable challenge.
Most synthesised nanobelts have an all-benzenoid structure, with benzene rings fused along the zigzag edge of the backbone. In such structures, electrons do not flow freely across the entire molecule but are instead confined to individual benzene rings. This limits their electronic properties and reduces their usefulness in practical devices.
More ambitious targets, such as fully conjugated cyclacenes suffer from extreme strain and instability. Their curved geometry introduces high reactivity, often resulting in partial or degraded forms during synthesis.
To overcome these limitations, Associate Professor Chi Chunyan from NUS Chemistry developed a new class of fully π-conjugated, non-alternant pentagon-embedded CNBs that allows better electronic properties through deoxygenative aromatisation approach, a chemical process that forms aromatic rings by removing oxygen atoms from oxygenated precursors. In this strategy, oxo-bridged belt precursors are synthesised via a Diels–Alder reaction between benzyne and furan derivatives. These intermediates are then subjected to reductive aromatisation using tin(II) chloride (SnCl2) and hydrobromic acid (HBr), affording two fully π-conjugated carbon nanobelts of different sizes. The stepwise buildup of strain energy, together with the moderate overall strain in the final structures, accounts for the successful formation of both nanobelts—long-standing targets in synthetic chemistry.
These CNBs showed improved electronic properties, including stronger red-light emission and smaller energy gaps. These features are particularly important for advancing technologies that rely on efficient light-matter interactions, such as organic photovoltaic (solar cell) and organic light-emitting diodes (OLEDs). Moreover, the smaller carbon nanobelt can be oxidised to its dicationic form, which possesses an open-shell singlet ground state and exhibits intriguing global aromaticity.
Theoretical studies suggest that the dication adopts a structure consisting of two weakly coupled [32]annulenes with Baird-type aromaticity along its edges. As charge separation plays a pivotal role in organic semiconducting devices, this insight offers a deeper understanding of the electronic delocalisation behaviour of CNBs in their charged states.

Previously reported benzenoid CNBs feature localized π-electrons within individual benzene rings, while the proposed [12]cyclacene remains too unstable for successful synthesis.
This study presents a new strategy for the design and synthesis of carbon nanobelts and related complex carbon nanostructures with enhanced π-conjugation and electron delocalisation. No longer appreciated solely for their topological novelty, these all-carbon systems are now emerging as functional materials with promising applications as topological organic semiconductors. Their ability to support spin-state manipulation suggests potential use in devices that are faster and more energyefficient than traditional chargebased electronics. Additionally, their lightweight, chemically robust, and synthetically tunable nature offers a compelling alternative to conventional magnetic materials, positioning CNBs as attractive candidates for the development of next-generation
quantum materials and spintronic technologies.
“Our discoveries provide a new platform for exploring the behaviour of correlated electrons in a highly controlled, tunable carbon-based system,” Chi explained.
As carbon nanobelts transition from synthetic challenge to functional platform, their future looks increasingly dynamic. With an all-carbon modular design, CNBs offer a versatile foundation for engineering nextgeneration materials. As fabrication techniques advance and theoretical models deepen, CNBs are poised to become powerful tools not just in nanotechnology, but in reimagining the very architecture of future electronics and quantum systems.
A fully π-conjugated, pentagon-embedded non-alternant CNB that can oxidise to a dication, where the unpaired electrons have anti-aligned spins and exhibit rare global delocalisation of π electrons along its two edges.

Professor Zhang Yang
NUS Computing
NUS Biochemistry
Cancer Science Institute (CSI) of Singapore
DOI: 10.1038/s41587-025-02654-4
D-I-TASSER is a hybrid deep learning and physics-based tool that can accurately model human protein structures, especially complex, multi-domain proteins
Proteins are fundamental to life. They catalyse chemical reactions, facilitate cell signalling, and provide structural support throughout cells. The human body contains around 20,000 different proteins, and while scientists have identified their amino acid sequences, the three-dimensional (3D) structures of most proteins remain unknown. This is a problem, because it is only through the precise folding of these amino acids that functionality is conferred.
In recent years, the field of structural biology has been revolutionised by computational tools that can accurately predict protein structure from amino acid sequences. The gold standard is AlphaFold2, a deep learning-based tool developed by Google DeepMind. It analyses patterns in related protein sequences from different organisms and predicts how the protein’s amino acids should be arranged in a 3D space. Since its release in 2020, AlphaFold2 has remained the benchmark for large-scale protein structure prediction. DeepMind released AlphaFold3 in 2024 with moderate improvements over AlphaFold2 in certain tasks.
At present, most computational methods perform best when there is abundant evolutionary information available for a protein and when the protein consists of only a single structural unit, or domain. However, the majority of proteins are made up of two or more domains.
Seeking a tool that can predict the 3D shape of human, multi-domain proteins, a team led by Professor Zhang Yang developed D-I-TASSER (Deep learningbased Iterative Threading ASSEmbly Refinement). This hybrid tool combines deep learning and traditional physicsbased modelling techniques. Their work was published in Nature Biotechnology.
Specifically, D-I-TASSER models the structure of a protein by performing folding simulations guided by AI-derived restraints, which provide information

about which parts of the protein are likely to be close together and the distances between them. For multidomain proteins, it breaks the protein into individual domains, models them separately, and then reassembles them into a 3D structure using advanced physics-based simulation techniques.
Tests on single-domain proteins showed that D-I-TASSER not only outperformed earlier versions of itself (I-TASSER1 and C-I-TASSER2), but also surpassed AlphaFold2 in accuracy. For multi-domain proteins, D-I-TASSER outperformed AlphaFold2 with 13% better accuracy in predicting whole protein structures, and 3% better on domains.
“D-I-TASSER’s strengths lies in its ability to combine multiple sources of structural restraints from both diverse AI models and template-based threading alignments. This multi-source strategy is further strengthened by the use of physics-based Monte Carlo
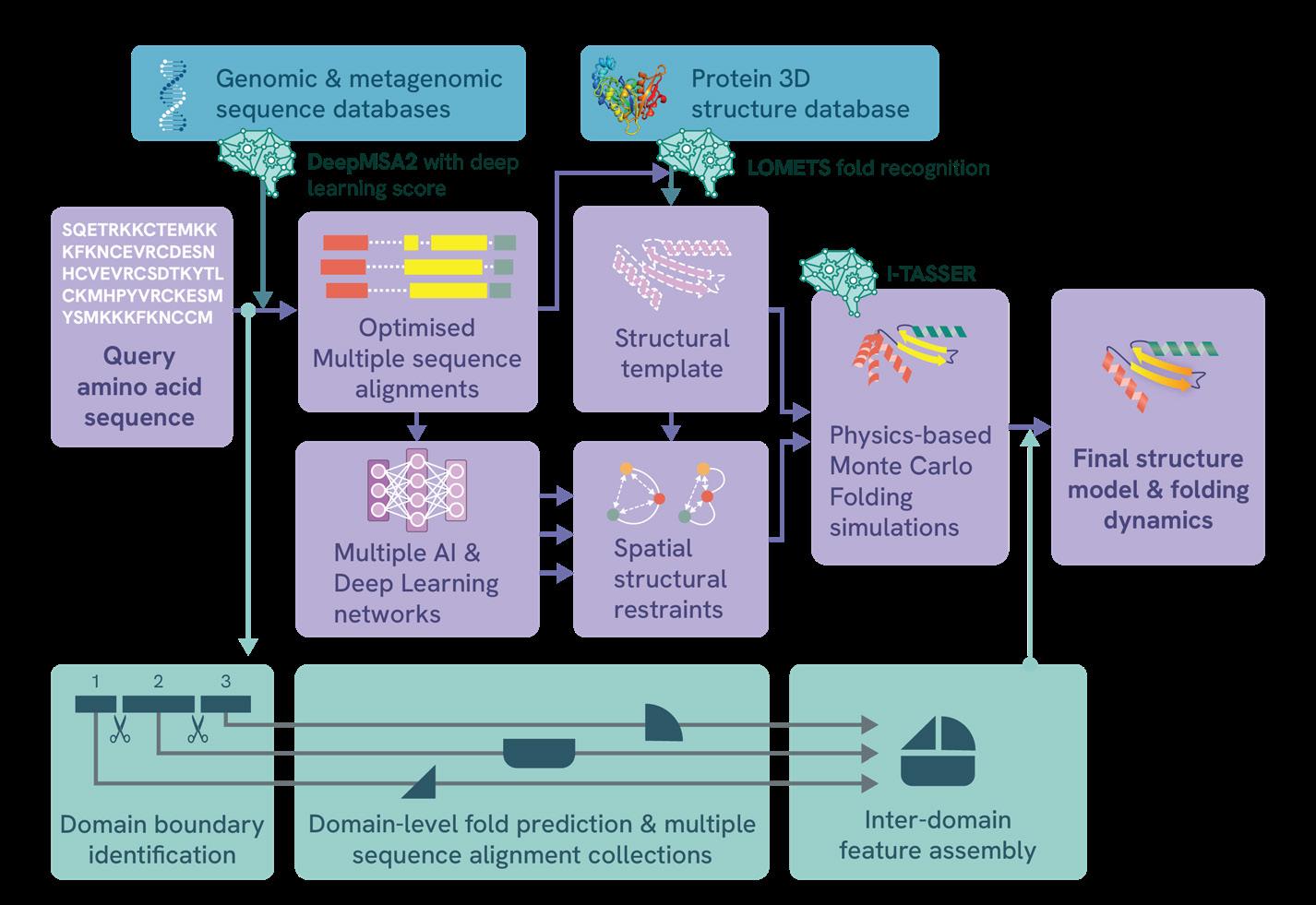
simulations, which fine-tune the AIgenerated structures to achieve greater atomic-level precision,” explained Prof Zhang.
The researchers also compared the coverage of D-I-TASSER to AlphaFold2 on the human proteome. AlphaFold2 could model nearly all human proteins (98.5%) as single chains, even those up to 2,700 amino acids long. D-ITASSER focused on proteins up to 1,500 amino acids but modelled them in finer detail, covering about 95% of the proteome. While AlphaFold2 offered slightly broader proteome coverage, D-I-TASSER delivered higher overall structural accuracy, especially in multidomain proteins.
In the CASP153 blind challenge, D-ITASSER was ranked the most accurate protein structure prediction method outperforming 44 entries, including AlphaFold2.
These results can be attributed to D-ITASSER’s domain-splitting approach, which allows for more effective modelling of complex, multi-domain proteins. D-I-TASSER also addresses gaps left by AlphaFold2, such as limited sequence information or high structural complexity.
With an ability to better predict the 3D structures of human multi-domain proteins, the work of D-I-TASSER is expected to help scientists better understand human biology and advance medical research, from disease studies to novel drug development.
1 I-TASSER is a template-based modeling method that uses threading to identify structural fragments from known proteins, followed by iterative Monte Carlo assembly and refinement. It does not include deep learning.
2 C-I-TASSER improves upon I-TASSER by incorporating residue–residue contact predictions from deep learning, enhancing performance for proteins with weak templates.
3 The Critical Assessment of protein Structure Prediction (CASP) blind test is widely considered the gold standard for benchmarking protein structure prediction methods.

The Office of the Deputy President (Research & Technology) administers research fellowships funded under various schemes.
Lee Kuan Yew
Postdoctoral Fellowship
For young and outstanding academics in science, medicine, and engineering
6 to 12-month fellowship for young faculty members and researchers from ASEAN universities and research institutes

Eric and Wendy Schmidt
AI in Science
Postdoctoral Fellowship
Postdoctoral talents incorporating AI techniques into the natural sciences, engineering, and mathematical science
A premier research opportunity for scholars driving original inquiry in Asian social sciences and humanities
*Scan the QR codes to learn more about each fellowship.
