Cosmetic Dermatology: 2023 Update

see page 4



see page 4
n
There is significant variation in the recommendations for patient follow-up after initial treatment for keratinocyte carcinomas (KCs), according to new findings

Published in JAMA Dermatology (Jan. 1, 2023; 159(1):87-94), the systematic review from the Skin Investigation Network of Canada was conducted to highlight evidence gaps in the current recommendations.
n Real-world efficacy and safety results similar to those observed in clinical trials
by JOHN EVANS, Senior Editor, The ChronicleOne-year real-world data from the PURE registry show that secukinumab has similar safety and efficacy for treating Latin American patients with moderate to severe plaque psoriasis than was seen in clinical trials.

 by LOUISE GAGNON, Correspondent, The Chronicle
by LOUISE GAGNON, Correspondent, The Chronicle
Canadian dermatologists have strengthened their plaque psoriasis armamentarium with therapies like the biologic agent bimekizumab, the oral treatment deucravacitinib, and various topical therapies.
PURE is an ongoing, prospective, multinational, observational, two-cohort registry of
“Clinicians vary in how often they follow patients after treatment for keratinocyte carcinoma,” said the study’s lead author Sara Mirali, PhD, in an email interview with THE CHRONICLE OF SKIN & ALLERGY. “Follow-up visits aim to detect recurrence, metastasis, and new primary tumours. Unfortunately, the optimal follow-up frequency is unclear, which has Please
→
implications for patient outcomes and healthcare resources.”

Dr. Mirali is an MD/PhD student in the Integrated Physician Scientist Training Program, at Temerty Faculty of Medicine, University of Toronto.
Guidelines from around the world compared in study The researchers searched PubMed, MEDLINE, and Embase for relevant articles related to squamous cell carcinoma (SCC) and basal cell carcinoma (BCC) published from Jan. 2010 to Mar. 2022.
“Canadian dermatologists have become very accustomed to innovations in biologic therapy that are becoming more and more efficacious,” said Dr. Andrei Metelitsa, Medical Director at Beacon Dermatology in Calgary, Alberta, and Clinical Associate Professor at the University of Calgary. “When it comes to our patients, sometimes they ask about oral therapies.”
A recently approved oral therapy for plaque psoriasis is deucravacitinib. An inhibitor of tyrosine kinase, deucravacitinib is available for treating moderate-to-severe plaque psoriasis.
“What is special about this agent is that it comes from the JAK [Janus kinase] family of agents, which are somewhat familiar to dermatologists,” said Dr. Metelitsa. “Unlike the traditional JAK, deu-

Advances in cosmetic dermatology
Canadian physicians discuss new innovations in cosmetic dermatology, including a potential new filler that provides an instantaneous effect patients can observe. . . . 4
Vender on Psoriasis: Risankizumab
Tx for moderate-to-severe psoriasis—A multi-centre, longterm, real-life study from Poland
Also, a study evaluated the effectiveness of brodalumab on anxiety and depressive symptoms in patients with psoriasis 8
Systemic treatment of PsO reduces all-cause mortality
Canadian research was recognized at this year AAD as the “Game Changer” of the year. Lead author
Dr. Richard Langley spoke with THE CHRONICLE about his research. . . 18
Chronicle Postgraduate Educational Supplement
In this issue’s postgraduate educational supplement, researchers investigate metabolic and dietary factors in women with acne vulgaris. Participants were split into two groups, patients with acne vulgaris and the control group. Researchers found the LDL levels and consumption of sweets correlated with acne severity and that contraceptive treatment remained the most effective 21
From the News Resources of The Chronicle
Changes in the order, structure, and number of copies of tumour DNA could be the reason some skin cancers ultimately resist treatment, according to research from the Francis Crick Institute, University College London and The Royal Marsden in London.
The research was part of the PEACE study, funded by Cancer Research UK, which analyzed tumour samples from people who died from melanoma.
Published in Cancer Discovery, the study evaluated 573 samples of 387 tumours from 14 patients with advanced melanoma. All the patients had been previously treated
with immune checkpoint inhibitor therapies (ICI), but by the time of their deaths, the ICI therapies were no longer effective.
The researchers analyzed the genetic code of individual cells within the tumour samples, looking for patterns that might explain metastasis and treatment resistance. They determined that 11 of the 14 people in the study had lost the genes that enable ICI drugs to help the immune system recognize and attack the cancer. They say this occurs because the cancer can either make multiple copies of defective versions of the genes, or use extrachromosomal DNA to override normal copies of the genes.
Medical Editor Wayne Gulliver, MD, FRCPC
John P. Arlette, MD, FRCPC

In this issue of THE CHRONICLE OF SKIN & ALLERGY, we update our readers on important information related to medical, cosmetic, social and cultural aspects of dermatology. For all readers attending the annual sessions of the Canadian Dermatology Association in Toronto, this issue and previous editions will provide important insights into the information being presented during the meeting.
I’d like to bring your attention to two must-reads in this issue. First, Dr. Shakira Brathwaite’s essay on "We must see colour" helps us understand the needs of our patients and demonstrates that diversity in dermatology is good for the patient but especially good for our specialty (see page 20). Dr. Brathwaite is one of the winners of the essay contest for Canadian dermatology residents, conducted by the Dermatology Industry Task Force on Inclusion, Diversity and Equity (DiTiDE).
The second article, Systemic treatment of psoriasis reduces allcause mortality (see page 18), reports on a Canadian paper that was
Please turn to Message page 16→
Editor, Cosmetic Dermatology Sheldon V. Pollack, MD, FRCPC
H. Eileen Murray, MD, FRCPC
June
Published eight times per year by the proprietor, Chronicle Information Resources Ltd., with offices at 1460 The Queensway, Suite 212, Etobicoke, Ont. M8Z
1S4 Canada. Telephone: (416) 916-2476; Facs. (416) 352-6199.
E-mail: health@chronicle.org
ISSN No. 1209-0581
Contents © Chronicle Information Resources Ltd., 2023 except where noted. All rights reserved worldwide.
The Publisher prohibits repro-

duction in any form, including print, broadcast, and electronic, without written permissions.
Printed in Canada. The Chronicle of Skin & Allergy is a Canadian publication. The Publisher certifies that advertising placed in this publication meets Revenue Canada requirements for tax deductibility.
Subscriptions: $85.60 per year in Canada, $129.95 per year in all other countries. Single copies: $10.00 per issue (plus
13% HST).
Canada Post Canadian Publications Mail Sales Product Agreement Number 40016917. Please forward all correspondence on circulation matters to: The Chronicle of Skin & Allergy, 1460 The Queensway, Suite 212, Etobicoke, Ont. M8Z 1S4
Ideas in the Service of Medicinesm
Affiliated journals of the Chronicle Companies include The Chronicle of Cosmetic Medicine + Surgery, The Chronicle of Neurology + Psychiatry, Pediatric Chronicle, and Linacre’s Books/ Les Editions Linacre

Benjamin Barankin, MD, FRCPC
Marc Bourcier, MD, FRCPC
Eric Goldstein, MD, FRCPC
Peter Hull, MD, FRCPC
Richard Langley, MD, FRCPC
Danielle Marcoux, MD, FRCPC
R.A.W. Miller, MD, FRCPC
Kim Papp, MD, FRCPC
Yves Poulin, MD, FRCPC
Melanie D. Pratt, MD, FRCPC
Denis Sasseville, MD, FRCPC
Jerry Tan, MD, FRCPC
Ronald B. Vender, MD, FRCPC
Founding Editor Colin A. Ramsay, MD, FRCPC (1936-2003)
Publisher Mitchell Shannon
Editorial Director R. Allan Ryan
Associate Publisher
Kylie Rebernik
Senior Editor John Evans
Assistant Editor Jeremy Visser
Client Engagment Cristela Tello RuizManager,Operations Cathy Dusome
Comptroller Rose Arciero
n READER SERVICE: To change your address, or for questions about your receipt of the journal, send an e-mail to health@chronicle.org with subject line “Circulation,” or call during business hours at 416.916.CHROn (2476), or toll-free at 866.63.CHRON (24766).

n LETTERS: We welcome your correspondence by mail, fax (416.352.6199), or e-mail. Kindly use the co-ordinates listed above.
Dr. Monica Li, dermatologist, Vancouver (see page 4)
n ADVERTISING: For current rates and data, please contact the publisher.
n REPRINTS: The content of this journal is copyrighted. Please contact Mitchell Shannon for reprint information.
“The average consumer is seeing so much on social media, there is a focus on overall well-being and self-care starting at a younger age.”Image from adobe.stock.com


New and emerging neuromodulators, a filler that combines hyaluronic acid and calcium hydroxylapatite, preventive interventions that enhance natural appearance, and an investigational technique for body contouring, represent some of the latest in cosmetic innovations in dermatology.
More options in neuromodulators expected Patients can look forward to longer lasting neuromodulators becoming available in Canada in the future, according to Dr. Andrei Metelitsa, Co-Director of Beacon Dermatology and Clinical Associate Professor, Section of Dermatology, University of Calgary.
“Relabotulinumtoxin A, a novel form of neuromodulator, has shown duration up to six months in phase 3
clinical trials, while still having a very rapid onset of action,” said Dr. Metelitsa. “Relabotulinumtoxin A is a highly-active, complex-free, and ready-to-use toxin. One of its major advantages is its liquid formulation, which will eliminate the need of reconstitution, a step that is both timeconsuming, usually delegated to staff, and subject to human error.”
Another emerging neuromodulator that is not yet approved in Canada but is available in the U.S. is the peptide-formulated daxibotulinumtoxin A, which has shown prolonged efficacy as well as quick onset of action.
“The cost of these formulations will determine whether they will in fact become first-line therapies once available,” said Dr. Metelitsa.
Dr. Monica K. Li, a dermatologist who practises in Vancouver and Sur-


rey, B.C., echoed that daxibotulinumtoxin A demonstrated an extended duration of effect in clinical trials.
Preventive trends and social media
The proliferation of information and videos on platforms like TikTok and Instagram has produced a demand from younger patient for preventative interventions that enhance appearance, according to Dr. Li.
“The average consumer is seeing so much on social media,” said Dr. Li. “There is a focus on overall well-being and self-care starting at a younger age.
Younger patients having access to information on social media means that they are embracing self-care, preventative care, and preventative management at an earlier stage in life. The use of preventative botulinum toxin
We’re commi琀ed to making a di昀erence in this area of unmet need

Continued from page 4
or laser toning is part of that focus.”
Indeed, young patients in their 20s are seeking interventions designed to relax the muscles in their face so that lines do not appear etched on the skin, explained Dr. Li.
Dr. Jessica Asgarpour, a Toronto dermatologist, agreed that social media is growing the demand for preventative interventions in younger patients.
Dr. Asgarpour is a dermatologist at the Canadian Dermatology Centre and a clinical associate at Women's College Hospital, Department of Dermatology, University of Toronto.
“There is an emphasis toward achieving a more natural appearance,” said Dr. Asgarpour. “That is where ‘Baby Botox’ and preventive Botox come in. Baby Botox is essentially using lower volumes of Botox to prevent fine lines to create a smooth and rested appearance. It does not give you that frozen face that has been associated with traditional Botox.”
Dr. Asgarpour advised that consumers of social media have a healthy skepticism about the benefits of skin care products touted on platforms such as TikTok and others.
“There are many products [on these platforms] that may not have evidence behind them,” said Dr. Asgarpour. “As dermatologists, we advise the use of evidence-based products.”
Advances in body contouring and cellulite management
An investigational approach to body contouring and reducing adipose tissue is the local injection of normal saline and glycerol, noted Dr. Jason Rivers, Medical Director at Pacific Derm in Vancouver.
He noted it is referred to as injection cryolipolysis or injection of an “ice slurry” into subcutaneous fat, with the slurry consisting of 20% to 40% ice content to induce cryolipolysis.
To date, preclinical studies have been performed. Small studies with patients have been performed to demonstrate safety, with erythema and bruising occurring (Plast Reconstr Surg Glob Open 2021; 9:e3818). “There is preliminary work on using this slurry to dissolve fat,” explained Dr. Rivers. “Further research will determine its utility in clinical practice.”
Cellulite management is another area that has experienced an advance, with technologies that use rapid acoustic pulse (RAP) being employed to lessen the visual impact of cellulite, said Dr. Rivers,
“There are devices that use sound
waves. One is called Resonic,” he said. “The device apparently breaks up fibrous septae that cause the dimpling seen with cellulite.”
Poly-l-lactic acid (PLLA) is another treatment that clinicians are selecting to try to diminish the appearance of cellulite, which typically affects body sites such as the thighs and buttocks, said Dr. Rivers. Evolving filler options and considerations in deep skin tones
It is not yet available in Canada, but an emerging filler option is the combination of hyaluronic acid (HA) and calcium hydroxylapatite (CaHA), noted Dr. Li.
“If a product combines both calcium hydroxlyapatite and hyaluronic acid, there is an instantaneous effect of treatment where patients can see results with volumization. At the same time there is more longevity in terms of what is being put into the skin through injection because of the bio-stimulatory, neocollagenesis characteristic,” said Dr. Li. “You are basically getting the best of both worlds because of the advantages of those two products (HA and CaHA) to lift tissue and tighten skin of the cheeks and jawline in one treatment.”
There is potential for hypopigmentation or hyperpigmentation in patients with higher Fitzpatrick skin classifications (IV, V, and VI), so clinicians and patients need to be mindful of this potential side effect, according to Dr. Asgarpour.
“Essentially, anything that can cause inflammation on their skin can cause hypopigmentation or hyperpigmentation,” said Dr. Asgarpour. “If you do not safely and cautiously perform a chemical peel or laser on a patient with a higher Fitzpatrick classification, that patient might develop more inflammation and subsequent hyper- or hypopigmentation.
“We would guide them to specific peels and less aggressive laser settings for treatment to prevent increased inflammation,” said Dr. Asgarpour. “With lasers, you want to avoid a lot of heat on the face in patients with a higher Fitzpatrick classification because this can lead to inflammation resulting in scarring, hypopigmentation or hyperpigmentation. Treatment may then involve more sessions as a result.”
Non-proprietary and brand names of therapies: relabotulinumtoxin A (not approved in Canada); daxibotulinumtoxin A (not approved in Canada); botulinum toxin type A (Botox Cosmetic, Allergan Aesthetics Canada, An AbbVie Company); poly-l-lactic acid (PLLA) (Sculptra, Galderma).
DUOBRII is indicated for improving the signs and symptoms of plaque psoriasis in adult patients with moderate to severe plaque psoriasis.
DUOBRII is not indicated for patients under the age of 18 years.
Clinical trials with DUOBRII did not include sufficient patients aged 65 and older to establish efficacy and safety in geriatric patients.
CONTRAINDICATIONS:
Hypersensitivity to the drug, any medicinal or non-medicinal ingredient in the formulation, any component of the container, or other corticosteroids or retinoic compounds.
Viral lesions of the skin, bacterial or fungal skin infections, parasitic infections, skin manifestations relating to tuberculosis or syphilis, or eruptions following vaccinations.
Seborrheic dermatitis.
Women who are pregnant or may become pregnant.
RELEVANT WARNINGS AND PRECAUTIONS:
• Patients with skin diseases with impaired circulation
Patients with chronic leg ulcers
• HPA axis suppression
• Patients with hepatic impairment
• Patients with impaired immune system function
Patients with concomitant skin infection
• Patients with renal impairment
• Allergic contact dermatitis
• Patients with glaucoma
Striae, telangiectasias, folliculitis, or skin atrophy
Conditions where the skin barrier may be impaired
• Wind or cold weather
• Exposure to excessive sunlight or sunlamps, or to photosensitizing drugs
• Breastfeeding women
• DUOBRII should be used with caution as topical corticosteroid use may lead to rebound relapses, development of tolerance, risk of generalized pustular psoriasis and development of local or systemic toxicity
• Conditions that augment systemic absorption
FOR MORE INFORMATION:
Please see the Product Monograph at https://healthproducts.canada.ca/dpd-bdpp/index-eng.jsp for important information on adverse reactions, drug interactions, and dosing not discussed in this piece. The Product Monograph is also available by calling 1-800-361-4261.
† Based on a prospective, multicentre, randomized, double-blind, phase III clinical trial, comparing DUOBRII lotion to the vehicle lotion, in 215 patients 18 years and older with moderate to severe plaque psoriasis.
REFERENCE:
1. Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: Results of 2 phase 3 randomized controlled trials. Journal of the American Academy of Dermatology. 2018;79(2):287–93.
In moderate to severe plaque psoriasis, the first and only retinoid-steroid.*














• At Week 8 of treatment, 45.3% of DUOBRII patients (n=141) achieved treatment success‡ vs vehicle (12.5% (n=74); 1° endpoint; p<0.001).1
• 4 weeks after stopping treatment, at Week 12, 33.4% of DUOBRII patients (n=141) maintained treatment success rates vs vehicle (8.8% (n=74); 2° endpoint; p<0.001).1
*


This multi-centre, long-term, real-life study assessed the efficacy of risankizumab in moderate-to-severe plaque psoriasis. The study, published in the Journal of Clinical Medicine (2023; 12:1675), enrolled 185 patients from 10 dermatologic departments in Poland who received risankizumab treatment. Disease severity was measured using the Psoriasis Area and Severity Index (PASI) at baseline and at defined timepoints of 4, 16, 28, 40, 52, and 96 weeks of treatment. The study evaluated the percentage of patients achieving PASI 90 and PASI 100 responses, as well as the PASI percentage decrease at these timepoints, and analyzed correlations with clinical characteristics and therapeutic effect.
The number of patients evaluated at each defined timepoint was 136, 145, 100, 93, 62, and 22 at 4, 16, 28, 40, 52, and 96 weeks of treatment, respectively. The results showed that at 4, 16, 28, 40, 52, and 96 weeks, PASI 90 response was achieved in 13.2%, 81.4%, 87.0%, 86.0%, 88.7%, and 81.8% of patients, respectively. Similarly, PASI 100 response was achieved in 2.9%, 53.1%, 67.0%, 68.8%, 71.0%, and 68.2% of patients, respectively.
The study revealed a significant negative correlation between the decrease in PASI and the presence of psoriatic arthritis, as well as the patient's age and duration of psoriasis at several timepoints throughout the observation period. These findings suggest that risankizumab may be an effective treatment option for moderate-to-severe plaque psoriasis, with higher PASI 90 and PASI 100 responses observed over time. However, further research is warranted to better understand the long-term efficacy and safety profile of risankizumab.
The study confirms that a very high PASI 90 is achievable with risankizumab, an extremely effective anti-psoriatic biologic that inhibits IL23. Its efficacy for patients achieving PASI 90 is extremely high and quite often exceeds the average of 70% PASI with ANTI-IL17s. The study confirms that a very high PASI 90 is achievable for patients on risankizumab. In addition to this, in patients that are clear, PASI 100 occurs in over 50% of those treated with risankizumab. My only concern with this study is that its conclusion shows that it “may” be an effective treatment option for patients that have moderate to severe psoriasis. I would suggest that the authors should have made a correction, saying that it “is” an effective treatment for psoriatic patients. That has been proven in many long-term studies as well as real world experience that has been extensively published.
A prospective study called ProLOGUE was conducted in Japan to evaluate the effectiveness of brodalumab on anxiety and depressive symptoms in Japanese patients with psoriasis. The study included 73 patients, predominantly male with a median age of 54 years with plaque psoriasis and had not responded adequately to other therapies.
The results showed the proportion of patients without anxiety symptoms significantly increased from baseline
(72.6%) to weeks 12 (88.9%, p=0.008) and 48 (87.7%, p=0.02). However, the proportion of patients without depressive symptoms did not change significantly. The scores on the Generalized Anxiety Disorder-7 and Patient Health Questionnaire-8 significantly decreased after treatment, indicating a reduction in anxiety and depressive symptoms.
Regardless of the presence of baseline anxiety or depressive symptoms, the Psoriasis Area and Severity Index (PASI), were less than 1 after treatment, indicating improvement in psoriasis symptoms.
At week 12, the researchers observed that patients with baseline depressive symptoms had more impaired health-related quality of life compared to those without depressive symptoms. However, this impairment generally resolved by week 48.
The researchers note that treatment with brodalumab led to a reduction in self-assessed anxiety and depressive symptoms in Japanese patients with psoriasis. While anxiety symptoms improved significantly, depressive symptoms did not completely resolve with brodalumab treatment. They conclude that patients with psoriasis and depressive symptoms may require longterm treatment to effectively address their mental health needs.
Psychiatric comorbidities are common with psoriatic disease. Depression and anxiety compounds other comorbidities by isolating the patient and reducing their chances of socializing, exercising, and eating well. This may compound obesity, hyperlipidemia and could lead to cardiovascular comorbidities. Clearing the skin with safe and effective biologics such as brodalumab improves a patient’s quality of life. The sooner in the psoriatic journey the patient is treated the more likely improvement in depression and anxiety will occur. However, some patients do need continuous psychiatric monitoring as the burden of psoriasis still remains in their mind despite disappearing from the skin.
We invite your comments and questions about this feature at www.derm.city. Dr. Ron Vender is a certified dermatologist with more than 30 years of clinical practice experience and over 100 clinical trials in psoriasis. He is founder and director of Venderm Innovations in Psoriasis, a center of excellence for Psoriasis offering a comprehensive management solution for individuals with psoriasis. www.psoriasis.vip
Dermburo is available across Canada with McKesson code : 77428 and can also be ordered through your local pharmacist.


Modified Burow’s Solution for the temporary relief of minor skin irritations caused by eczema, poison ivy, insect bites and athlete’s foot, as well as rashes (contact dermatitis) caused by soaps, detergents, cosmetics or jewelry.
Can be used as a soak or as a compress. Available in boxes of 12 sachets of 3 g.
Available ay Shoppers Drug Mart and most drug stores.
They included any national or international guidelines containing recommendations for followup frequency after a diagnosis of localized cutaneous KC.
There were challenges to comparing the different guidelines, Dr. Mirali said.
“Some guidelines recommended different follow-up frequencies based on recurrence risk. Unfortunately, there were differences between the guidelines on what characterized a low-risk or high-risk patient,” she said.
“This is partly because there was a lack of universally accepted risk criteria for BCC or SCC at the time of guideline development,” Dr. Mirali said. However, she noted that newly introduced staging systems for these two types of KC may help standardize risk group definitions for future guidelines.
Overall, 14 guidelines met the eligibility criteria. Dr. Mirali and her colleagues found little consensus on the appropriate followup frequency after initial KC treatment.
Duration of follow-up ranged from a single post treatment visit to lifelong surveillance. For low-risk BCC and guidelines that did not stratify by risk, follow-up recommendations ranged from every six to 12 months. For high-risk BCC, one guideline suggested followup every three months and four recommended every six months.
For low-risk SCC, five guidelines recommended annual follow-up, three guidelines recommended every six months, and one guideline said every three months. For high-risk SCC, recommendations included a range of follow-up frequencies, spanning every three months (five guidelines), four months (one), six months (six), or annually (four). One guideline did not use risk stratification and recommended annual screening.
The investigators assessed the quality of the 14 guidelines using the Appraisal of Guidelines Research and Evaluation II (AGREE
II) tool. They found the highest scoring AGREE II domain was "scope and purpose," which assessed the guideline's overall objectives, and the lowest scoring was "applicability," which assessed barriers and facilitators to implementation.
Dr. Mirali said the degree of inconsistency between the different guidelines was unexpected.
“We found it surprising that some guidelines recommended frequent follow-up over extended periods while others suggest minimal or no follow-up. This variation highlights the lack of robust evidence to inform clinical practice,” she said.
The recommendations that exist are based on indirect evidence from observational studies and systematic reviews, Dr. Mirali said
For now, much of the decisionmaking process on follow-up will remain up to the individual physician’s judgement and available resources.
“In the absence of evidence supporting a specific follow-up frequency or duration, clinicians can choose from a range of follow-up options depending on patient factors and available healthcare resources,” said Dr. Mirali. “Higher risk tumours and patients would likely benefit from more frequent follow-up.”
The main takeaway from this study is that more data from randomized trials are needed to define an optimal follow-up schedule to improve long-term outcomes for patients with KCs, said Dr. Mirali.
She noted that with support from the Skin Investigation Network of Canada, researchers and patient partners are planning to address this important clinical question through further research.
“Unfortunately, many guidelines did not consider patient feedback when formulating their recommendations,” Dr. Mirali said. “Future guidelines can benefit from incorporating patient concerns to improve guideline feasibility.”
Sun
Program for ILUMYA® – designed to help you and your patients every step of the way
Pr ILUMYA® (tildrakizumab injection) is indicated for the treatment of adult patients with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy.
For more information:
Please consult the Product Monograph at: info.ilumya.ca/Product_Monograph for important information relating to contraindications, warnings, precautions, adverse reactions, interactions, dosing and conditions of clinical use.
The Product Monograph is also available by calling our medical information department at: 1-844-924-0656.
REFERENCE:
Current ILUMYA® Product Monograph, Sun Pharmaceutical Industries Limited.
© 2023 Sun Pharma, or its subsidiaries and a�liates. All rights reserved.
ILUMYA is a registered trademark of Sun Pharmaceutical Industries Limited. Used under license.
All other trademarks are the property of their respective owners.
Now publicly covered in Ontario, Alberta, Manitoba, Saskatchewan, and the Atlantic provinces (restrictions may apply)
For patients with moderate-to-severe plaque psoriasis


In 2 pooled Phase III clinical trials, common topical adverse events for ARAZLO (n=779) were: application site pain (5.3%), dryness (3.9%), exfoliation (2.1%), erythema (1.9%), and pruritus (1.3%). Overall, 2.8% of subjects discontinued ARAZLO due to TEAEs.1
Scan

ARAZLO (tazarotene lotion, 0.045%) is indicated for the topical treatment of acne vulgaris in patients 10 years of age and older.
Clinical use:
• Geriatrics (>65 years of age): The safety and ef昀cacy of ARAZLO have not been established in this patient population.
• Patients 10 to <12 years of age should limit application of ARAZLO to the face.
Contraindications:
• Hypersensitivity to retinoic compounds
• Pregnant women or women who may become pregnant
• Should not be used in the presence of seborrheic dermatitis
Relevant warnings and precautions:
• For external topical use only
• Use of topical tazarotene may produce contact dermatitis
adverse
bauschhealth.ca
• Avoid concomitant use of medications and cosmetics that have a strong drying effect
• Avoid application to eczematous or sunburned skin
• Photosensitivity
• Caution with coadministration of drugs known to be photosens itizers
• Use adequate birth-control measures in women of childbearing potential

• Discontinue treatment if patient becomes pregnant
• Breastfeeding
For more information:
Please see the Product Monograph at https://bauschhealth.ca/wp-content/ uploads/2021/07/Arazlo-PM-E-2021-07-08.pdf for important information on adverse reactions, drug interactions and dosing not discussed in this piece. The Product Monograph is also available by calling 1-800-361-4261.

Continued from page 1
adult patients with moderate to severe chronic plaque psoriasis in Latin America and Canada. Patients were enrolled in the registry 1:1 having been treated with secukinumab or other approved therapies for moderate to severe plaque psoriasis.
Published in Dermatology and Therapy (Jan. 2023; 13(1):269283), the paper reports on findings from 21 hospital-based specialty sites in Latin America.
Overall, 197 patients were included in this analysis, 89 of whom were initiated on secukinumab and 98 of whom received other treatments.
Collection of data challenging
Collecting strong data from Latin America was somewhat challenging compared to Canadian data, said study author Dr. Melinda Gooderham in an interview with THE CHRONICLE OF SKIN & ALLERGY
Working with multiple national healthcare systems in the participating Latin American countries, with frequently less patient access to advanced medications and a later approval of secukinumab compared to Canada, meant that only one year of real-world data was available, com-
pared to three years of data from the Canadian sites, she said.
Peterborough, Ont.-based Dr. Gooderham is an Assistant Professor at Queen’s University in Kingston, Ont., and also works as a Consultant Physician at the Peterborough Regional Health Centre.
“At the beginning, it was a little slower to get Latin America on board, just finding the sites that would have patients on the medication,” she said. “That is why we are publishing 12month results, whereas, in some of the posters that are being presented for Canada, or the PURE registry in general, we have a lot of Canadian data that is 36 months.”
“It is nice to have a good 187 patients enrolled from Latin American countries that have one year of data to present on them. We are pretty proud of that.”
The data shows at month 12, 84.4%, 71.1% and 53.3% of patients treated with secukinumab achieved Psoriasis Area and Severity Index (PASI) 75/90/100, respectively. This is compared with 66.7%, 47.9% and 29.2% of patients who received other treatments.
Response rates, measured as Investigator Global Assessment (IGA) scores of zero or one (clear or almost clear) in secukinumab versus other Tx were 78.3% versus 36.7% at month three and 81.8% ver-
Important safety information for CIBINQO
Clinical use
Can be used with or without medicated topical therapies for atopic dermatitis.
Limitations of use: use in combination with other JAK inhibitors, biologic immunomodulators, or potent immunosuppressants, such as methotrexate and cyclosporine, has not been studied and is not recommended.
Most serious warnings and precautions
Serious infections: patients may be at increased risk for developing serious bacterial, fungal, viral and opportunistic infections that may lead to hospitalization or death; more frequently reported serious infections were predominately viral. If a serious infection develops, interrupt treatment until the infection is controlled. Risks and benefits of treatment should be carefully considered prior to initiating therapy in patients with chronic or recurrent infection. Monitor for signs and symptoms of infection during and after treatment, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy.
Malignancies: lymphoma and other malignancies were observed in patients taking JAK inhibitors to treat inflammatory conditions and were more frequently observed in patients with rheumatoid arthritis (RA) during a clinical trial with another JAK inhibitor versus TNF inhibitors. Thrombosis: including deep venous thrombosis, pulmonary embolism, and arterial thrombosis have occurred in patients taking JAK inhibitors to treat inflammatory conditions. Many of these events were serious; some resulted in death. Consider risks and benefits prior to treating
sus 66.7% at month 12, respectively. No unexpected AEs seen Overall, the proportion of patients achieving Dermatology Life Quality Index (DLQI) scores of zero or one improved to 76.5% at month 12 from 6.9% at baseline in patients treated with secukinumab versus 54.5% at month 12 compared to 5.6% at baseline in patients on other treatments. No unexpected adverse events were reported during the 12-month observation period.
It was reassuring to see that the findings with this subgroup analysis were consistent with the expected safety and efficacy of secukinumab, Dr. Gooderham said, “because there are disparities between the healthcare systems.”
Since it is often more difficult for patients in Latin American countries to gain access to advanced medications, “one of the things we might worry about is are we getting the worst of the worst [cases of psoriasis] when we are getting fewer patients on the medication? Is it because the patients are more severe or are more difficult to treat?” she asked.
“But we did not see that. We see similar responses with respect to PASI 75, 90, and 100 as we are seeing in other regions. So it nice to see the responses are the same in that population that has some differ-
ences [in medial access] in the real world setting [compared to a clinical trial setting].”
A key takeaway from this analysis is that physicians should be confident the response to secukinumab is very predictable, Dr. Gooderham said. “The responses are consistent with what we have seen in other registries and the clinical trials.”
“There are no curveballs that have been thrown, there are no new safety concerns. So regardless of a physician’s patients, if the patients have a Latin American origin and they have immigrated, the medication will likely work as one would predict.”
“The more populations in which we see the consistency, the more confident physicians can be in the responses and the safety that they can expect.”
The PURE registry is planned to collect five years of data from all sites, so comparative analyses can be conducted in the future, Dr. Gooderham said.
“We will do several sub-analyses, comparing within populations, comparing severities and that sort of thing. Even after the database lock, after five years, there will still be sub-analyses. So this [registry] will be alive for probably another decade.”
Non-proprietary and brand names of therapy: secukinumab (Cosentyx, Novartis).
patients who may be at increased risk. In a clinical trial in patients ≥50 years of age with RA, a higher rate of all-cause mortality and thrombosis occurred in patients treated with another JAK inhibitor versus TNF inhibitors. Patients with symptoms of thrombosis should be promptly evaluated and treated appropriately.
Major adverse cardiovascular events (MACE): including non-fatal myocardial infarction, were observed more frequently in patients ≥50 years of age with RA during a clinical trial comparing another JAK inhibitor versus TNF inhibitors.
Other relevant warnings and precautions
• Driving or operating machinery
• Dose-dependent increase in blood lipid parameters, lipid monitoring and management
• Hematological abnormalities
• Use with potent immunosuppressants
• Vaccination
• Monitoring and laboratory tests
• Fertility
• Women of childbearing potential
• Pregnancy and breastfeeding
• Geriatrics
For more information
Consult the Product Monograph at http://pfizer.ca/pm/en/CIBINQO.pdf for important information regarding adverse reactions, drug interactions and dosing, which have not been discussed in this piece. The Product Monograph is also available by calling 1-800-463-6001.
References: 1. CIBINQO Product Monograph, Pfizer Canada ULC. 2. Bieber T, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med 2021;384:1101–12. † Results from a phase 3 randomized, double-blind, placebo-controlled, double-dummy, parallel group, multicentre study of CIBINQO in combination with background medicated topical therapies in patients aged ≥18 years with moderate-to-severe atopic dermatitis who had an inadequate response to topical therapy or had received systemic therapy, excluding dupilumab. 2:2:2:1 randomization to CIBINQO 200 mg (n=226), CIBINQO 100 mg (n=238), dupilumab (n=243) or placebo (n=131) for 12 weeks. CIBINQO dose: 200 mg or 100 mg taken orally once daily. Dupilumab dose: 300 mg administered subcutaneously every other week after a loading dose of 600 mg at baseline. Matching placebo was dosed accordingly
CIBINQO is indicated for the treatment of patients 12 years and older with refractory moderate-to-severe atopic dermatitis, including the relief of pruritus, who have had an inadequate response to other systemic drugs (e.g., steroid or biologic), or for whom these treatments are not advisable.


A new, highly selective oral JAK1 inhibitor for moderate-to-severe AD*
Once-daily dosing
Flexibility to start patients 12–64 years of age with a 100 or 200 mg dose based on individual goal of therapy and potential risk for adverse reactions. Exceeding 200 mg per day is not recommended.
Contact the P昀zerFlex™ Support Program for dermatology for enrolment forms and information about program services
1-855-935-FLEX (3539)
Monday to Friday, 8 am–8 pm EST
Refer to the page in the bottom-right for additional safety information and for a web link to the Product Monograph discussing:
• The most serious warnings and precautions regarding: serious infections; malignancies; thrombosis; major adverse cardiovascular events
• Other relevant warnings and precautions regarding: driving and operating machinery; dose-dependent increase in blood lipid parameters, lipid monitoring and management; hematological abnormalities; use with other potent
AD=atopic dermatitis; JAK1=Janus kinase 1.
* Clinical significance unknown.
CIBINQO@p昀zer昀ex.com
immunosuppressants; vaccination; monitoring and laboratory tests; fertility; special populations, including women of childbearing potential, pregnancy, breastfeeding, and geriatrics
• Conditions of clinical use, adverse reactions, drug interactions and dosing instructions
In addition, the page contains the reference list relating to this advertisement.
Significantly more CIBINQO patients achieved ≥75% improvement in the EASI score from baseline (defined as EASI-75) at Week 12 vs. placebo1,2†
Rapid and significant itch relief was seen as early as Week 2 vs. placebo as measured by PP-NRS4 (2º endpoint)
Patients achieving a PP-NRS response with ≥4-point improvement from baseline
31.8% § CIBINQO 100 mg
49.1%¶ ||
200 mg
vs.vs.
26.4% dupilumab 300 mg
13.8% placebo
Significantly more CIBINQO 200 mg patients achieved PP-NRS4 vs. dupilumab as early as Day 4 and remained higher through Week 2
Proportion of PP-NRS4 responders with CIBINQO 100 mg was similar to dupilumab at Week 2 and over time.
§ p<0.001 vs. placebo (multiplicity-controlled).
¶ p<0.0001 vs. placebo (multiplicity-controlled).
|| p<0.0001 vs. dupilumab (multiplicity-controlled); statistical comparison between either CIBINQO dose and dupilumab was only performed on the proportion of patients achieving PP-NRS4 at Week 2.
CIBINQO is only indicated in patients who have had an inadequate response to other systemic drugs or for whom these treatments are not advisable. Over 50% of patients in these studies did not have prior exposure to systemic therapy.
Adapted from Product Monograph and Bieber, et al.Continued from page 1
cravacitinib has tremendous safety associated with it because of what is known as allosteric inhibition, which refers to the way it works.”
The efficacy that deucravacitinib achieves is comparable to well-established biologics, according to Dr. Metelitsa. “What it means to dermatologists is that many of us still have patients who have done very well for years on some of the original biologics, including adalimumab and ustekinumab. And we now have an oral molecule at 6 mg once a day, that has overall efficacy that mirrors those traditional molecules without the need for injections,” said Dr. Metelitsa.
Dr. Charles Lynde, a dermatologist based in Markham, Ont. and Associate Professor, Department of Medicine, University of Toronto, agrees that deucravacitinib will find a niche in the psoriasis therapeutic space.
“It’s a new oral and it’s quite effective,” said Dr. Lynde. “It’s not a biologic, but it is at the low end of being a biologic. The side effect profile seems to be quite good. There are a number of
patients who want to use an oral therapy.”
Biologic options evaluated
The latest biologic therapy available to prescribe in Canada is bimekizumab, noted Dr. Lynde. “It is highly effective,” said Dr. Lynde. “You are achieving PASI [Psoriasis Area Severity Index] 90 to 100. There are great numbers to support its use in clinical practice.”
Bimekizumab was studied in three different clinical trials. It achieved superior levels of skin clearance at Week 16, compared to those treated with ustekinumab, placebo and adalimumab, as measured by PASI 90 and an Investigator's Global Assessment (IGA) response of clear or almost clear skin. Clinical responses achieved with bimekizumab at Week 16 were maintained up to one year in all studies.
The side effect of concern in the use of bimekizumab is candidiasis. Oral and, less commonly, vaginal candidiasis may be a side effect of special interest in patients who are taking bimekizumab. “It has a higher rate of candidiasis than secukinumab or ixekizumab, but has even higher efficacy than either of those biologics,” said Dr. Lynde, noting that when candidiasis occurs, dermatologists are skilled in managing this condition
Still another fairly new addition to the plaque psoriasis arsenal is the IL-23 inhibitor tildrakizumab, joining the family of other IL-23 inhibitors such as guselkumab and risankizumab. “It expands our choices of IL-23 inhibitors,” said Dr. Lynde, referring to the addition of tildrakizumab.
Data from the reSURFACE 1 and reSURFACE2 trials demonstrated the efficacy of tildrakizumab. Most recently, pooled analysis of data from those trials suggests that a higher dose of the biologic can be administered to patients who weigh more. Specifically, patients weighing 120 kg or more had better efficacy outcomes at the 200 mg
Continued from page 3
selected as ‘Game Changer’ of the year at the 2023 AAD sessions in New Orleans. This Canadian paper by Professor Richard Langley and the PSOLAR team is deja vu all over again. (Full disclosure, I am Co-chair of the Canadian PSOLAR Team).
PSOLAR is a large, international, long-term, prospective, disease-based registry enrolling patients with psoriasis who are receiving, or are candidates for, treatment with systemic therapies. In the mid-2000s we published many abstracts and papers on similar topics which predated Drs. Joel Gelfand and Langley. In April, 2006, we presented ‘Comorbidities associated with psoriasis in the Newfoundland and Labrador founder population’ at the BIO Chicago conference.
We followed that with additional papers and presentations in 2015-2016. I gave an oral presentation at the 2015 73rd Annual Meeting of the
American Academy of Dermatology in San Francisco titled ‘Cardiovascular disease and moderate to severe psoriasis: Early age of onset linked to 9-fold increased risk of MI: Biologic therapy linked to 83% decreased risk of MI.’
We also published two papers in 2016. The first was titled ‘Psoriasis patients treated with biologics and methotrexate have a reduced rate of myocardial infarction: A collaborative analysis using international cohorts’ (Gulliver WP, et al: J Cutan Med Surg 2016 Nov; 20(6):550-554) and ‘Do biologics protect patients with psoriasis from myocardial infarction? A retrospective cohort’ (J Cutan Med Surg 2016 Nov; 20(6):536-554).
So, game changers all. It’s wonderful to see this new data confirms our data from 17 and seven years ago, respectively.
As always, THE CHRONICLE team invites and welcomes your comments on this issue, or any other topic in dermatology, at www.derm.city.
 —Wayne P. Gulliver, MD, FRCPC, Medical Editor
—Wayne P. Gulliver, MD, FRCPC, Medical Editor

dose compared to the 100 mg dose, with safety being maintained at the higher dose in patients of greater weight (Dermatol Ther (Heidelb) Oct 2022;12(10):2325-2341).
Robust data support the long-term use of the IL-23 inhibitor guselkumab to treat plaque psoriasis. Nearly three-quarters (73%) of patients who achieved PASI 100 responses at Week 24 in the VOYAGE 1 study maintained their response through five years of treatment.
Topical choices in psoriasis
Increasingly, topical options that are not steroids are becoming available to treat mild-to-moderate psoriasis.
One topical that combines halobetasol propionate and tazarotene can be an effective option for some specific sites, according to Dr. Irina Turchin, Assistant Professor in the Division of Clinical Dermatology & Cutaneous Science, Department of Medicine, Dalhousie University, Halifax, and Memorial University in St. John’s, NL, and a dermatology consultant for Horizon Health Network in Fredericton, N.B.. “It can work really well for those thicker plaques on sites like the elbows and knees,” she said.
Still another topical is tapinarof, a therapeutic aryl hydrocarbon receptor modulating agent, which was studied in PSOARING 1 and PSOARING 2, and which met its endpoint in those trials, noted Dr. Turchin.
In PSOARING 1, the Physician’s Global Assessment (PGA) score of clear or almost clear at Week 12 was 37.8% in the tapinarof arm vs. 9.9% in the vehicle arm. In PSOARING 2, the PGA of clear or almost clear was 43.6% in the active arm vs. 8.1% in the vehicle arm.
Roflumilast is also a novel topical agent aimed at effective management of psoriasis, according to Dr. Turchin, one of many Canadian investigators who were involved in clinical trials examining the efficacy of this therapy. “The improvements were seen as quickly as Week 2, and the patients continued to improve up to Week 8,” said Dr. Turchin.
Itch is recognized as the most bothersome symptom of psoriasis, and roflumilast tackles this symptom, pointed out Dr. Turchin. In the clinical trial program, as measured by Worst Itch Number Rating Scale, patients on roflumilast had much bigger improvement in itch compared to patients on vehicle.
Topicals represent a good entry option for psoriasis management, according to Dr. Lynde. “Patients can try a cream, and if it does not work, then they can try something more aggressive,” said Dr. Lynde. Non-proprietary and brand names of therapies: bimekizumab (Bimzelx, UCB Canada); deucravacitinib (Sotyktu, Bristol Myers Squibb); adalimumab (Humira, AbbVie); ustekinumab (Stelara, Janssen); secukinumab (Cosentyx, Novartis); ixekizumab (Taltz, Lilly); Tildrakizumab (Ilumya, Sun Pharma); guselkumab (Tremfya, Janssen); risankizumab (Skyrizi, AbbVie); halobetasol propionate/tazarotene (Duobrii, Bausch Health); tapinarof (not approved in Canada); roflumilast (not approved in Canada).
For moderate to severe plaque psoriasis patients
B IMZELX® IS AVAILABLE FOR THEM
PrBIMZELX® (bimekizumab injection) is indicated for the treatment of moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy.

Please consult the Product Monograph at https://ucb-canada.ca/sites/default/files/2022-03/bimekizumab-pm-ennon-nds_14%20Feb%202022.pdf for important information relating to contraindications, warnings and precautions, adverse reactions, drug interactions, dosing information, and conditions of clinical use which have not been discussed in this piece. The Product Monograph is also available by calling 1-866-709-8444.
Reference: 1. BIMZELX Product Monograph. UCB Canada Inc. February 14, 2022.

At the American Academy of Dermatology (AAD) annual meeting in March 2023, research findings that showed systemic treatment of psoriasis reduced overall mortality risk were recognized by the AAD as the “Game Changer” of the year—work that will have a profound impact on the future of dermatology.
Originally published in the Journal of the American Academy of Dermatology in 2021 (Jan. 2021; 84(1):60-69), the findings came from the Psoriasis Longitudinal Assessment and Registry (PSOLAR) study.
“It was an incredible honour for our research team to have the work recognized by the American Academy of Dermatology as one of the top 10 research papers over the last three years, and then to have your peers vote on it at the American Academy and say it was the game changer of the year,” said the study’s lead author Dr. Richard Langley in an interview with THE CHRONICLE OF SKIN & ALLERGY. “It is a great honour for all the Canadian researchers involved in [the research], as a testament to the quality of the research, and also to the patients who have participated for almost eight years and continue to participate in these studies.”
Dr. Langley is a dermatologist, and professor of medicine in the Division of Clinical Dermatology and Cutaneous Science at Dalhousie University in Halifax.
PSOLAR is an international, prospective, longitudinal registry of patients with psoriasis being
treated with systemic therapies. The registry evaluates patient and disease characteristics, including patient-reported assessment of psoriatic arthritis (PsA); and clinical and quality of life outcomes.
Launched in 2007, PSOLAR gathered data on roughly 12,000 patients initially, and there are plans to recruit approximately 3,500 more, said Dr. Langley.
In the analysis that was recognized by the AAD, Dr. Langley and his colleagues found that in patients with moderate to severe psoriasis, short and long-term treatments with biologics were associated with lower risks of all-cause and cardiovascular mortality compared with no exposure to biologics.
This reduced risk was seen both when they looked at pooled data from all biologic therapies and for sub-analyses considering TNF inhibitors and ustekinumab separately.
Apart from biologics, long-term, but not short-term, treatment with methotrexate was associated with a lower risk of mortality compared to no exposure to methotrexate.
“When I started [practicing medicine] psoriasis was considered by many to be just a skin disease that impaired only the quality of life of patients and their families,” said Dr. Langley. “Many people—sometimes those in the lay public or even other physicians—may have looked at psoriasis and other skin diseases as really just cosmetic conditions.”
Over time, psoriasis was revealed to be a systemic illness associated with increased mortality when it is more severe, he said.
“We have shown that long-term treatment can reduce inflammation and can reduce mortality rates. So [physicians treating psoriasis] have not only improved quality of life but have improved quantity of life,” Dr. Langley said.
He said that the findings emphasize “the importance of early treatment of these patients not only to improve their quality of life but to reduce the cumulative life impact that psoriasis can have. Many patients who develop psoriasis and do not get treated may choose not to get married. Psoriasis can impact how patients choose the jobs they have. We have children who are teased in school and will not go swimming in pools.”
“Early treatment is not aggressive, it is appropriate,” he said.
The idea to include an investigation into the impact of systemic psoriasis treatment on mortality in the PSOLAR study arose from earlier work linking psoriasis with an elevated risk of cardiovascular problems, and other work that showed treatment of systemic inflammation could lower the risk of death from some causes.
Dr. Langley noted that a study by Joel M. Gelfand and colleagues (Archives of Dermatology
2007; 143(12):1493-1499) reported an increased risk of myocardial infarctions in patients with severe moderate to severe psoriasis.
“That was a landmark study because it showed that the inflammation in the skin could potentially result in systemic side effects,” Dr. Langley said. “The logical question after this paper was: Could treatment of psoriasis then reduce mortality?”
A 2002 paper from the rheumatology literature had shown a survival benefit with methotrexate in a small cohort of 1,200 patients with psoriatic arthritis, he said. Other work by Danish investigators had shown systemic anti-inflammatory effects and reduced cardiovascular events in psoriasis patients treated with methotrexate, said Dr. Langley.
“There was a critical knowledge gap that existed around 2007 [when the PSOLAR study was being planned],” he said. “We knew that the pathogenesis of psoriasis is driven by elevated cytokines and those [cytokines] are present in lesional skin. They are also present systemically. The question was, could there be a systemic treatment effect, on cardiovascular and also overall mortality by inhibiting cytokines, not only in the skin but peripherally.”
That was the thinking that spurred the study of mortality using the PSOLAR cohort, Dr. Langley said.
“We wanted to follow our patients prospec-
Please turn to PSOLAR page 28→
When your patient presents with moderate-to-severe plaque psoriasis, SAY TREMFYA®











TREMFYA®/TREMFYA ONE-PRESS® (guselkumab injection) is indicated for the treatment of adult patients with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy.

TREMFYA®/TREMFYA ONE-PRESS® is also indicated for the treatment of adult patients with active psoriatic arthritis. TREMFYA®/TREMFYA ONE-PRESS® can be used alone or in combination with a conventional disease-modifying antirheumatic drug (cDMARD) (e.g., methotrexate).
Please consult the Product Monograph at https://www.janssen.com/canada/our-medicines for important information relating to contraindications, warnings, precautions, adverse reactions, interactions, dosing, and conditions of clinical use that has not been discussed in this piece.



The Product Monograph is also available by calling 1-800-567-3331.
Reference: TREMFYA®/TREMFYA ONE-PRESS® (guselkumab injection) Product Monograph. Janssen Inc. April 13, 2022.











The Chronicle of Skin & Allergy, in partnership with the Dermatology Industry Taskforce on Inclusion, Diversity and Equity (DiTiDE), sponsored a short essay competition for Canadian dermatology residents in 2022.

This contest was open to any resident enrolled in a dermatology training program at a Canadian medical school, with enrolment confirmed by their Program Director. Entrants submitted a 350- to 500-word composition reflecting on matters relating to inclusion, diversity, and equity. Eight winners were announced.
The selected essays will be published in each issue of The Chronicle during 2023.
Dr. Shakira Brathwaite of the University of Toronto was recognized for her essay “We must see colour”.
DiTiDE is a volunteer working group composed of Canadian life sciences managers and executives, dermatologists, patient association leaders, and allied parties committed to improving the patient experience and outcomes of under-represented skin types and underserved ethnicities through developing physician education and resources.
The 2nd annual short-essay competition for Canadian dermatology residents is now open for entries. Details are available at http://derm.city/for-residents.
IN MY CHILDHOOD, THE PHRASE “I DON’T SEE COLOUR” was often taught to school-age children in a well-meaning attempt to assert that all people deserve the same kindness, respect, and dignity, regardless of the colour of their skin. However, as both a Black woman and a dermatology trainee, this statement reminds me of how missing variations of colour and experience can be directly and indirectly harmful to people with darker skin.
Colour is heavily relied on when assessing skin conditions. However, transmitted light can appear differently in different skin tones. From personal experience, I recognize that conditions that can appear strikingly on pale skin can be much more subdued on a deeply pigmented base.
When my brother was an infant, he was covered in itchy, flaky plaques that worsened with certain foods—but he was not red. Our mother took him to multiple specialists and he underwent numerous assessments before finally being diagnosed with atopic dermatitis at a pediatric specialist centre. Despite the relief of knowing, my mother was frustrated with the arduous journey it took to receive a relatively common diagnosis.
Fast-forward to my clerkship training, I recall struggling with applying a “standardized” scoring system to a Black patient whose skin did not appear the same red denoted on the colour scaling tools. Upon reviewing with my staff, I had significantly underscored this patient’s severe flare. It was an incredibly humbling experience. As I progress through my dermatology training, I appreciate the many teachers and patients who help me see that erythema can be red, mauve, violet, or almost grey depending on who is in front of me, and who remind me to consolidate the colours I see with the other morphological findings and history. At the same time, I am reminded that many of our non-dermatology colleagues never get to have these experiences, which can lead to significant gaps in care in terms of who gets referred to dermatology and how they are triaged.
Colour often impacts the patient’s disease course even after correct diagnosis. Despite an ever-increasing arsenal of treatments for inflammatory and auto-immune skin diseases, options are limited for post-inflammatory pigmentary changes—which disproportionately affect people with richly pigmented skin and can be just as distressing as the disease itself. It may also impact how we categorize conditions as pathological or cosmetic. For example, rosacea for most patients is a benign cosmetic condition, and yet it has insured treatment options. Melasma does not. Consequently, I have seen patients who have inappropriately used topical steroids, home remedies, unproven Amazon products, and banned skin bleaching creams with devastating side effects.
Thankfully, interest in skin of colour dermatology has exploded in recent years, and training in this area is becoming more accessible. Moreover, there has been increasing awareness of the lack of diversity in research and clinical trials, which is now being addressed in those spheres. I am hopeful that many of the diagnostic and treatment gaps of the past will resolve as we continue to work toward equitable care.
Shakira Brathwaite is a second-year dermatology resident at the University of Toronto, and a graduate of the Queen’s University School of Medicine. She is a passionate advocate for equity, diversity and inclusion, and participates in several related committees and mentorship groups.


Mateusz Kozłowski,1,* Mirela Niedzielska,2 Anna Lorenz,1 Agnieszka Brodowska,3 Ewelina Malanowska,3 Adam Przepiera,4 Aneta Cymbaluk-Płoska,1 and Elżbieta Sowińska-Przepiera2,5
1Department of Reconstructive Surgery and Gynecological Oncology, Pomeranian Medical University in Szczecin, Al. Powstańców Wielkopolskich 72, 70-111 Szczecin, Poland
2Department of Endocrinology, Metabolic and Internal Diseases, Pomeranian Medical University in Szczecin, Unii Lubelskiej 1, 71-252 Szczecin, Poland
3Department of Gynecology, Endocrinology and Gynecological Oncology, Pomeranian Medical University in Szczecin, Unii Lubelskiej 1, 71-252 Szczecin, Poland
4Department of Urology and Urologic Oncology, Pomeranian Medical University in Szczecin, Al. Powstańców Wielkopolskich 72, 70-111 Szczecin, Poland
5Pediatric, Adolescent Gynecology Clinic, Department of Gynecology, Endocrinology and Gynecological Oncology, Pomeranian Medical University in Szczecin, Unii Lubelskiej 1, 71252 Szczecin, Poland
* Corresponding author
The etiopathogenesis of acne is complex, as several endo- and exogenous factors that affect the sebaceous-hair unit are involved in the development of acne lesions. The main aim of the study was to evaluate selected metabolic parameters before treatment. Another goal of the study was to determine the correlation between selected metabolic and dietary parameters and the severity of acne before treatment. The third objective was to assess the severity of acne before and after treatment, considering the type of treatment used. The final objective was to assess the relationship between the difference in acne severity before and after treatment, considering the type of treatment used and factors of dairy or sweets intake.
A total of 168 women participated in the study. The patients belonged to two groups: the study group (99 patients with acne vulgaris) and the control group (69 patients without skin lesions). The study group was divided into subgroups according to the treatment used: contraceptive preparation, contraceptive preparation and cyproterone acetate, and contraceptive preparation and isotretinoin preparation. We found that LDL levels and consumption of sweets correlated with acne severity. The mainstay of acne treatment is contraceptive treatment (ethinylestradiol and drospirenone). The effectiveness of the three contraceptive-based treatments was confirmed by observing the severity of acne. There were no significant correlations between the difference in acne severity before and after treatment with the three treatments and factors of dairy or sweet consumption.
Keywords: acne; acne vulgaris; treatment; contraception; sweets; dairy; glucose; insulin; cholesterol; triglycerides
Acne vulgaris (AV) is a chronic inflammatory skin disease. It is considered one of the diseases of civilization due to the significant influence of environmental factors on the severity and incidence of these lesions.1 The condition affects about 9.4% of the global population. It is usually associated with
adolescence but can occur between the ages of 11 and 30. It is believed to affect 80% of people in this age group or even 100% of young people.2,3,4 The dermatosis usually appears in the second decade of life, becomes less severe with age, and resolves at the end of that decade or the beginning of the third. However, there are cases of persistence of the disease until the 30th or even beyond the 40th year of life.5 Acne lesions are 95% localized on the face and upper
torso, but also occasionally in other parts of the body. Due to the localization of the lesions and the chronic nature of the condition, it is often a serious psychological problem for teenage girls and young women.6,7,8 For this reason, girls and young women with acne are frequent patients of dermatologists, gynecologists, endocrinologists, psychologists, and clients of cosmetology offices.
The etiopathogenesis of acne is complex, as several endo- and exogenous factors that affect the sebaceoushair unit are involved in the development of acne lesions. The most relevant and documented initiators that influence the formation of acne are genetic conditions, androgen-induced sebum production, colonization of the hair follicle by Cutibacterium acnes, and release of mediators during inflammation and keratinization of the hair follicle outlet; dietary habits and lifestyle factors also influence the severity of acne.6,9,10 It has been shown that environmental factors do not trigger the onset of acne, but rather cause exacerbation of acne lesions. Climatic conditions are documented evidence, as skin lesions in acne patients improve most often during the summer and spring months. Acne vulgaris is more common in urban areas, which may be related to environmental pollution. The potential role of the modern diet in the development of acne lesions has also been studied. So-called insulinotropic foods, especially milk and carbohydrates with a high glycemic index, affect changes in
the concentrations of cytokines and insulin-like growth factors; this can activate receptors for androgens and genes that stimulate keratinocyte proliferation and sebocyte lipogenesis. One environmentally, metabolically, and hormonally determined factor is obesity, which, in addition to severe complications in the form of diabetes and cardiovascular disease, causes a higher risk of severe forms of acne in those with a BMI>27.11,12 In turn, low GL (glycemic load) ± low GI (glycemic index) diets reduce free androgens, increase IGFBP-3 (insulin-like growth factor-binding protein 3), and decrease IGF-1 (insulin-like growth factor-1) levels. In this instance, a reduction in the quantity of lesions and the severity of lesions was confirmed.13
Currently, acne treatment is the domain not only of dermatologists but also of endocrinologists and gynecologists. Hormonal treatment of acne includes anti-androgens (flutamide, spironolactone, anti-androgenic progestogens) and drugs that block ovarian and/or adrenal androgen production (estrogens, cyproterone acetate, GnRH agonists, low-dose glucocorticosteroids, oral contraceptives).14 The American Academy of Dermatology (AAD) working group on the treatment of acne vulgaris focuses on topical and systemic therapies (antibiotics and isotretinoin). The authors emphasize limiting the duration of treatment with systemic antibiotics in adults with acne and prescribing concurrent and/or maintenance topical
Reprinted with permission ©2023 by the authors. Licensee MDPI, Basel, Switzerland.. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Content has been edited to conform with the Canadian Press Publication Style Guide Chronicle.Academy develops bespoke structured learning programs for clinicians providing in-depth education to professionals in the healthcare industry.
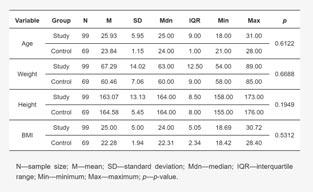
Table 1: Characteristics of study participants including age, weight, height, and BMIthe Pomeranian Medical University in Szczecin and dermatology outpatient clinics. The patients belonged to two groups: the study group (99 patients with acne vulgaris) and the control group (69 patients without skin lesions). The study group was divided into subgroups according to the treatment used:
• OC subgroup—oral contraceptive
• OC+A subgroup—oral contraceptive and cyproterone acetate
• OC+R subgroup—oral contraceptive and isotretinoin
medication permanently, and patient consent to participate in the study.
ligibility criteria for the study group included: the presence of acne in young women not previously treated for it. The control group included healthy young women without acne who attended the outpatient clinic for preventive examination and consented to biochemical tests.
treatments.15 Isotretinoin, when used orally, reduces sebum secretion, inhibits the production of comedones, reduces skin colonization by C. acnes, and exhibits anti-inflammatory effects. Retinoids, derivatives of vitamin A applied to the skin, accelerate the emptying of blackheads and inhibit the formation of new micro-blackheads, weaken the inflammatory reaction, and enhance the penetration of other topical anti-acne preparations. Recommended locally applied retinoids are tretinoin, isotretinoin, and adapalene.16 Oxybrasion17 and hydrogen purification18 have also been used in the treatment of acne vulgaris.
The aim of the study was to evaluate selected metabolic parameters before treatment. Another objective of the study was to determine the correlation between selected metabolic and di-
etary parameters and the severity of acne before treatment. The third objective was to assess the severity of acne before and after treatment, considering the type of treatment used. The final aim was to evaluate the relationship between the difference in acne severity before and after treatment, considering the type of treatment used and factors of dairy or sweet consumption.
The study included 168 women aged between 18 and 31 years who were patients of the Gynecological Endocrinology Outpatient Clinic at the Department of Endocrinology, Metabolic Diseases and Internal Medicine at
All patients in the study group received a contraceptive formulation of 0.03 μg ethinylestradiol and 3 mg drospirenone. The patients were then classified into one of the 3 subgroups described above: OC; OC+A; OC+R. In the OC+A group, an additional 50 mg of cyproterone acetate 1 × 1 for 10 days per treatment cycle was used for three months; as a reduction in acne severity was achieved, the cyproterone acetate dose was halved and used for another three months. In the OC+R group, isotretinoin was used at an initial dose of 10 mg for three months; as a partial reduction in the severity of acne was obtained, this treatment was continued with a dose of 20 mg for another three months.
Patients before treatment (T1) are defined as patients before the administration of acne therapy. Post-treatment (T2) patients are defined as patients after six months of follow-up.
The general inclusion criteria for the study included: age 18 to 31 years, Caucasian women, normal history of puberty with no significant abnormalities on physical examination, not taking
Exclusion criteria from the study group were: disorders of growth and weight gain, endocrine diseases (e.g., thyroid disease, diabetes mellitus, polycystic ovary syndrome—PCOS, congenital adrenal hyperplasia—CAH, premature expiration of ovarian function—POF), which were diagnosed on the basis of history, gynecological examination and laboratory tests. Other exclusion criteria included participation in competitive sports, long-term use of stimulants, incomplete follow-up period, and use of another acne treatment regimen. Patients did not opt for cosmetic procedures or taking supplements to reduce sebum secretion (yeast pills, sulfur pills) and mattifying cosmetics were forbidden.
All patients underwent a subjective and physical examination. Anthropometric measurements (height in cm, weight in kg) were taken and body mass index (BMI (kg/m2)) was calculated based on them.
The following parameters were determined from fasting venous blood taken in the morning: fasting glucose and insulin levels, total cholesterol (CHOL), LDL and HDL cholesterol, and triglycerides (TG). The tests were performed using electrochemiluminescence (ECLIA), enzyme-linked immunosorbent assay (ELISA), chemiluminescence-immunoassay (CLIA), and calorimetry.
The HOMA-IR insulin resistance index was calculated using HOMA2 Calculator software ©The University of Oxford (Oxford, U.K.) 2004–2021.
Visual assessment based on the Leeds scale was used to evaluate acne.19
Statistical calculations were performed through the statistical computing environment (R v.4.1.1 (IDE RStudio v. 1.4.1717)). The significance level of statistical tests in this analysis was α=0.05.
For variables on an interval scale, a description of the study set and drawing some basic conclusions and generalizations about the samples were conducted using grouped descriptive statistics. In addition, a normality test was carried out based on the Shapiro–Wilk test, including the W test statistic along with an indication of p significance.
Variables on a nominal, ordinal
Table 2: Characteristics of selected metabolic parameters in women in the study group (before treatment) compared to the control group. Table 3: Correlation coefficients between metabolic factors and diet and acne severity on the Leeds scale before treatment.scale were analyzed in pairs in the form of contingency tables with an indication of frequency. The relationship of variables was examined using Fisher’s exact test; in addition, Cramer’s V measures of relationship strength were calculated.
For independent variables with a normal distribution with the number of independent groups above two, Welch’s one-way analysis of variance (ANOVA) was used to test the significance of differences. For two groups of independent samples with a normal distribution, the Welch’s t-test with Hedges’ g effect size calculation was used. For two groups of independent samples with a non-normal distribution, the Mann–Whitney U-test was used with a twopoint correlation calculation based on ranks r (rank biserial).
For two dependent variables with a normal distribution, the Student’s t
test with Hedges’ g effect size calculation was used to test the significance of differences. For two dependent variables with a normal distribution, the non-parametric Wilcoxon paired ranksum test was used. The value of the association between the variables was calculated using rank-based two-point correlation r (rank biserial).
In the case of examining the correlation between variables on the ordinal scale and the quotient scale, the Kendall rank correlation coefficient τb was calculated in the form of an appropriate measure of the relationship.
In the case of calculating the correlation between a variable on an ordinal scale and a variable on a nominal (dichotomous) scale in the form of a correlation coefficient, a two-point correlation based on ranks was estimated, r (rank biserial). When the ordi-
nal variable has only two levels, Yule’s phi linkage measure (φc) was used.
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Pomeranian Medical University in Szczecin, Poland (protocol code KB012/78/18 on 18 June 2018).

The study participants (study group and control group) were not statistically significantly different in terms of the study variables shown in Table 1.
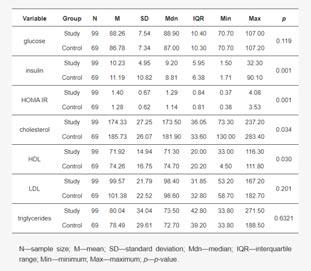
Significant differences were found in insulin (p=0.001), cholesterol (p=0.034), HDL (p=0.030), and HOMA IR (p=0.001) concentrations between the pre-treatment study group and the control group. The remaining concentration differences were not significant. Detailed characteristics of concentrations in the pre-treatment study group and the control group are shown in Table 2.
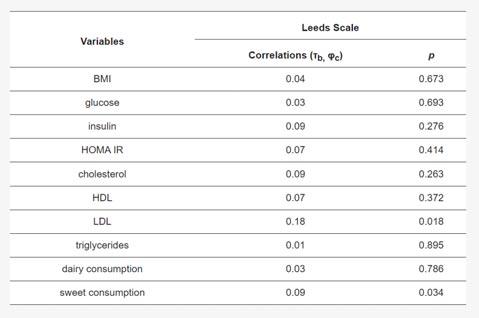
There was a significant correlation between LDL concentration and the Leeds scale (p=0.018) and between sweet consumption and the Leeds scale (p=0.034). The remaining correlations were not significant. Detailed correlations of the study variables with the Leeds scale are shown in Table 3.
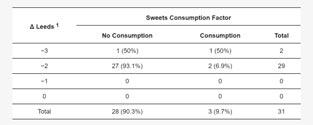
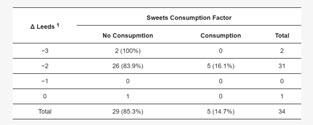
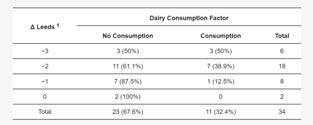
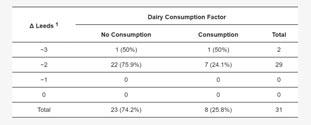
It was found that the severity of acne in
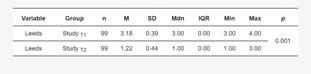
the study group without division by treatment type was significantly lower after treatment when compared to before treatment (p=0.001). The data are shown in Table 4.
The severity of acne on the Leeds scale before and after treatment was also compared, taking into account the type of treatment used:
• Treatment regimen: oral contraception (OC)
A Wilcoxon paired rank-sum test of the dependent variables showed a significant reduction in the severity of acne on the Leeds scale from Mdn=3.00, IQR=0.00 before treatment to Mdn=1.00, IQR=0.00 after treatment with the contraceptive, VWilcoxon=561.00, p<0.001. The measure of association was estimated as “large”, r (rank biserial)=1.0.
• Treatment regimen: oral contraception (OC) + cyproterone acetate (A)
The Wilcoxon paired rank-sum test for the dependent variables showed a significant reduction in the free androgen index (FAI) from Mdn=1.45, IQR=1.01 before treatment to Mdn=0.23, IQR=0.11 after treatment with the contraceptive along with cyproterone acetate, VWilcoxon=496.00, p<0.001. The measure of association was estimated as “large”, r (rank biserial)=1.0.
• Treatment regimen: contraception (OC)+ isotretinoin (R)
The Wilcoxon paired rank-sum test for the dependent variables showed a significant reduction in the free androgen index (FAI) from Mdn=3.00, IQR=1.00 before treatment to Mdn=1.00, IQR=2.00 after treatment with the contraceptive formulation
Table 4: Acne severity scores of the study group before treatment (T1) and after treatment (T2) based on the Leeds scale. Table 5: Frequencies (%) of the distribution of the difference in acne severity on the Leeds scale after and before treatment vs. dairy intake factor in the treatment group with contraception (n=34). Table 6: Frequencies (%) of the distribution of the difference in acne severity on the Leeds scale before and after treatment vs. dairy intake factor in the group treated with the contraceptive formulation along with cyproterone acetate formulation (n=31). Table 7: Frequencies (%) of the distribution of the difference in acne severity on the Leeds scale before and after treatment vs. dairy intake factor in the group treated with the contraceptive along with isotretinoin (n=34). Table 8: Frequencies (%) of the distribution of the difference in acne severity on the Leeds scale before and after treatment vs. sweets intake factor in the treatment group with contraception (n=34). Table 9: Frequencies (%) of the distribution of the difference in acne severity on the Leeds scale before and after treatment vs. the sweets intake factor in the group treated with the contraceptive along with cyproterone acetate (n=31).along with the isotretinoin formulation, VWilcoxon=528.00, p<0.001. The measure of association was estimated as “large”, r (rank biserial)=1.0.
A graphical representation of the comparison of median measures of acne severity on the Leeds scale along with expanded statistical test reporting by treatment type is shown in Figure 1.
Nutrients 15 01488 g001 550Figure 1. Comparison of median measures of acne severity on the Leeds scale before and after treatment by type of treatment performed.
The relationship between nominal factors of dairy and sweets intake, and treatment outcome was examined using contingency tables. Treatment outcome was estimated as the difference in Leeds scores after treatment and before treatment. In addition, the relationship between the variables was examined separately based on the type of treatment.
Subgroup Treated with Oral Contraception
The distribution of the Leeds acne treatment outcome variable after and before treatment with the contraceptive vs. dairy intake factor is shown in Table 5.
Fisher’s test of independence conducted showed no significant relationship between the difference in acne severity levels on the Leeds scale before and after treatment with contraception and the factor of dairy consumption (df=3, p=0.361). The association between the variables was estimated as “moderate,” V=0.33.
Subgroup Treated with Oral Contraception and Cyproterone Acetate
The distribution of the variable acne treatment score on the Leeds scale before and after treatment with a contraceptive along with cyproterone acetate vs. dairy intake factor is shown in Table 6.
Fisher’s test of independence showed no significant relationship between the difference in acne severity levels on the Leeds scale before and after treatment with a contraceptive for-
mulation along with cyproterone acetate and the factor of dairy consumption (df=3, p=0.462). The association between the variables was estimated as “moderate,” V=0.27.
Subgroup Treated with Oral Contraception and Isotretinoin
The distribution of the variable Leeds acne treatment score before and after treatment with the contraceptive along with isotretinoin vs. dairy intake factor is shown in Table 7.
Fisher’s test of independence showed no significant relationship between the difference in acne severity levels on the Leeds scale before and after treatment with a contraceptive formulation along with an isotretinoin formulation and the factor of dairy consumption (df=3, p=0.362). The association between the variables was estimated as “faint,” V=0.33.
Subgroup Treated with Oral Contraception
The distribution of the Leeds acne treatment outcome variable before and after treatment with the contraceptive vs. sweets intake factor is shown in Table 8.
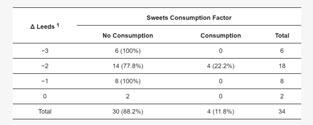
Fisher’s test of independence showed no relationship between the difference in acne severity levels on the Leeds scale before and after treatment with the contraceptive and the factor of sweets consumption (df=3, p=1.000).
The association between the variables was estimated as “faint,” V=0.05.
Subgroup Treated with Oral Contraception and Cyproterone Acetate
The distribution of the variable Leeds acne treatment score before and after treatment with a contraceptive along with cyproterone acetate vs. a factor of sweets consumption is shown in Table 9.
Fisher’s test of independence showed no significant relationship between the difference in acne severity on the Leeds scale before and after treatment with the contraceptive along with cyproterone acetate and the factor of sweets consumption (df=3, p=0.191).
The association between the variables was estimated as “moderate,” V=0.34.
Subgroup Treated with Oral Contraception and Isotretinoin
The distribution of the variable Leeds
acne treatment score before and after treatment with a contraceptive formulation along with an isotretinoin formulation vs. the sweets intake factor is shown in Table 10.
Fisher’s test of independence showed no significant relationship between the difference in acne severity levels on the Leeds scale before and after treatment with a contraceptive along with isotretinoin and the factor of sweet consumption (df=3, p=0.510). The association between the variables was estimated as “moderate,” V=0.34.
The results obtained in this article regarding metabolic factors showed statistical significance in terms of insulin concentrations and HOMA index, which were higher in the study group with acne compared to the control group. Recently, there have been reports on the possible influence of excess insulin on androgen metabolism in the adrenal glands and gonads primarily during adolescence; this can exacerbate acne lesions. Confirmation of the presented results is provided by Emiroglu, et al, who compared glucose and insulin concentrations in 243 female patients with
severe acne to 156 healthy controls. They found that while fasting glucose levels were not significantly different between the groups, fasting insulin levels were significantly higher in the diseased group; in addition, the HOMA index was higher in those with acne.20 In another study, Kartal, et al found elevated levels of insulin, glucose, and HOMA index in 46 female subjects with acne when compared to a group of healthy women. Elevated serum glucose and insulin levels correlated with sebum overproduction and the severity of acne lesions.21 Similarly, Cappel, et al showed that elevated levels of insulin in adult women and men is more likely to accompany acne lesions.22 A study by Vora, et al showed a positive correlation between the amount of sebum secreted on the face and insulin levels in people with acne.23 Another study describing this issue showed that the frequency of IGF-1 genotype (CA) 19 was significantly different between control and acne patients (p=0.0002). This study found a significant association between IGF-I (CA) genotypes and acne severity (p=0.015). There was no significant difference between male and female patients (p>0.05). The results suggest that the
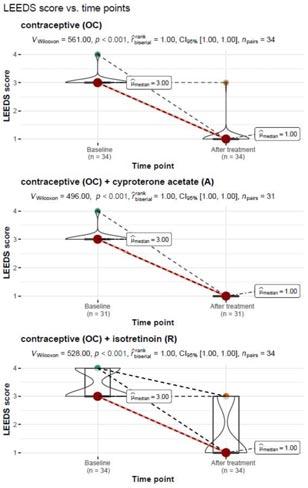 Table 10: Frequencies (%) of the distribution of the difference in the severity of acne on the Leeds scale after and before treatment vs. the sweets intake factor in the group treated with the contraceptive along with isotretinoin (n=34).
Figure 1: Comparison of median measures of acne severity on the Leeds scale before and after treatment by type of treatment performed.
Table 10: Frequencies (%) of the distribution of the difference in the severity of acne on the Leeds scale after and before treatment vs. the sweets intake factor in the group treated with the contraceptive along with isotretinoin (n=34).
Figure 1: Comparison of median measures of acne severity on the Leeds scale before and after treatment by type of treatment performed.
IGF-I (CA) 19 polymorphism may contribute to a predisposition to acne in Turkish patients.24 Conflicting reports were cited by Balta, et al, who evaluated a group of 35 patients with post-adolescent acne. The authors compared the results of glucose metabolism to 35 healthy control subjects; they showed no differences in glucose, insulin, and HOMA index values between the groups.25 Facts worth mentioning concern the analysis of the causes of androgen excess in women with obesity. It seems that the mechanism described in polycystic ovary syndrome associated with excess visceral fat and reduced insulin sensitivity leading to insulin resistance and high blood insulin concentrations comes to the fore. Insulin resistance in peripheral tissues and hyperinsulinemia leads not only to the activation of glucose transport pathways in adipose and muscle tissue but also, selectively, to the activation of testosterone production in the ovaries and an increase in its concentration in the blood. Some of the androgens also come from the increased peripheral conversion of estradiol to testosterone in adipose tissue in obesity.
Hyperinsulinemia also affects the inhibition of hepatic SHBG synthesis, thereby increasing free testosterone concentrations. Evidence for the key role of insulin in androgen production in obesity and hyperinsulinemia is the significant reduction in testosterone and androstendione concentrations, following weight reduction, decreased insulin levels, and improved insulin sensitivity in patients treated with diet and pharmacological therapy.26,27,28 These observations provide a clinical indicator for promoting normal body weight and lifestyle changes in female patients with acne. The so-called “Western” diet, characterized by increased dairy consumption and a high glycemic index (GI), has been shown to affect the levels of hormones involved in the pathogenesis of acne.29 In contrast, this study shows that consumption of sweets correlates with acne severity. In ongoing studies, high-GI diets have been associated with impaired carbohydrate tolerance and higher postprandial insulin concentrations and elevated levels of insulin-like growth factor 1 (IGF1), while low-GI diets have been shown to reduce fasting IGF-1 concentrations.29,30 Based on these studies, many research groups have wondered whether consuming dairy or high-GI foods has an effect on modulating the development and severity of acne. Moreover, patients often ask whether dietary changes can improve their skin condition. As a result, interest in the topic has increased in recent years and the literature is constantly supplemented with new studies.30,31
Akpinar Kara Y, et al conducted a study involving 53 patients with acne vulgaris and 53 age-, gender- and eth-

nicity-matched controls. They showed that cheese and carbohydrate intake was higher in the acne group compared to the control group (p<0.05). In addition, they observed that cheese consumption increased acne formation and carbohydrate consumption increased severity, while fat consumption was not significant.32 In a cross-sectional analysis of a large cohort of 24,452 participants in the French NutriNet-Santé study, showed that the consumption of fatty and sugary foods, sweet drinks, and milk was associated with acne in adults. These results were adjusted for potential confounding variables (age, gender, physical activity, smoking, education level, daily energy intake, number of completed dietary records, and depressive symptoms). The results of this study support the hypothesis that the socalled Western diet (rich in animal products and fatty and sugary foods) is associated with the incidence of acne in adulthood.33 A high glycemic load diet causes an increase in circulating IGF-1 and insulin levels. IGF-1 levels also stimulate the production of androgens, which are associated with sebum production and thus the development of acne.34 Consumption of milk also increases hepatic IGF-1 production and circulating insulin levels; moreover, neither IGF-1 nor insulin are fully inactivated by pasteurization, homogenization, and digestion.35 Hence, consuming milk has similar consequences to a high glycemic load meal.36 Other diverse randomized studies that do or do not confirm the effect of diet on acne severity are less reliable because they did not examine multiple food exposures during the same period and are limited by a small population sample. In addition, observational studies often focus on adolescent acne and rely on frequency questionnaires on previous food exposures, leading to both a lack of precision in food records and recall bias without considering various potential confounders like depression or smoking.37,38,39 On this basis, this article assumes that diet can influence acne in selected populations. Increased carbohydrate intake and a high-GI diet promote and exacerbate acne symptoms, while increased dairy intake promoted acne formation only in Western populations.33
Another analysis in the present article looked at the severity of acne after treatment in the study group and subgroups (OC; OC+A; OC+R). In all study groups with and without treatment, the degree of acne severity after treatment was significantly lower, i.e., there was clinical improvement (p<0.001). Thus, it can be assumed that each treatment option had a beneficial effect on the skin in the study group of female patients. It can be assumed that this was due to the contraceptive preparation. None of the treatment options used in the short six months of use was the
most beneficial. According to the recommendations of dermatologists, treatment is applied depending on the severity of the lesions and the effectiveness of the preparations used. Therefore, a thorough evaluation of acne before treating patients is essential.40,41
The literature shows that elevated androgen levels are present in about 50% of patients; in addition, about 50% to 60% of adult women with acne and normal androgen levels show elevated levels of androgen metabolites, suggesting some form of androgen excess. Therefore, most acne treatment recommendations involve the use of anti-androgen drugs.42,43 According to the recommendations of the American Academy of Dermatology, oral estroprogestogen or anti-androgen therapy is used as second-line treatment for women with moderate to severe adult acne who do not respond to local therapy and antibiotic therapy, regardless of androgen levels (there are no recommendations for measuring androgens in women with adult acne15). European dermatologists use hormonal treatment in patients with mild acne if hyperandrogenism is found, and for moderate acne without signs of androgen excess. In all patients, treatment with estroprogestogens or antiandrogens is used along with local acne treatment.44 From the point of view of the AE-PCOS Task Force, it is important to treat hyperandrogenism regardless of acne severity if there is clinical or biological evidence for it.33 In addition, the team, after careful evaluation of all available data, made recommendations that indicated that the diagnosis of adult female acne is primarily clinical, and that serum androgen levels (total testosterone, free testosterone, and DHEAS) should be determined in all women with adult acne after excluding other diseases. All second- and third generation estroprogestagens can be used, regardless of the dose of estrogen and progestogen component. Importantly, the authors noted that estroprogestagens can be used in non-hyperandrogenemic patients with adult acne as second-line therapy when local treatment has failed.33
Understanding the skin pathology associated with androgenization is important for gynecologists, dermatologists, endocrinologists, family physicians, pediatricians, and cosmetologists. Early diagnosis and treatment will help prevent complications of acne vulgaris.
Our study also had limitations. The main limitation was the six-month observation period which, from the perspective of the preparations used, did not allow us to assess the long-term effect of acne treatment in the subgroups. However, we are continuing the study to identify these long-term effects. Another limitation may be related to deficiencies in the obtained results, which is
the reason for the different number of variables in some correlations. Some of the patients did not complete all the recommended test results. However, the missing values were for side parameters that did not form the core of this work, so, despite this, we decided to exclude patients from the analysis. The patients’ use of several local potential determinants affecting acne severity and the Leeds scale scores cannot be excluded. Nevertheless, due to the appropriate choice of statistical methodology and the large sample size, our findings are reliable, and the assumptions are supported by their substantial consistency with published evidence. There were also concerns about the existing measures of acne severity and the inadequacy of existing acne severity scales. To address this concern, we conducted a critical review of the originally published acne scales to formally assess their quality based on a set of predetermined criteria. The lack of an internationally accepted measure of acne severity hinders high-quality clinical trials and the adoption of best practices with potential consequences for the acne sufferer. We conclude, as others have, that a robust scoring system for assessing acne severity is required. Future development of acne scales or further study of currently available scales is needed to correct these problems and move closer to developing a valid and reliable “gold standard” tool.
LDL levels and consumption of sweets correlate with the severity of acne. The mainstay of acne treatment is contraceptive treatment (ethinylestradiol and drospirenone). The effectiveness of the three contraceptive-based treatments was confirmed by observing the severity of acne. There were no significant relationships between the difference in acne severity before and after treatment with the three treatment regimens and factors of dairy or sweets consumption.
Conceptualization, M.N. and E.S.-P.; methodology, A.P. and E.M.; validation, A.B.; formal analysis, M.K.; investigation, A.L. and A.C.-P.; data curation, A.P. and E.M.; writing—original draft preparation, M.K., M.N. and A.L.; writing—review and editing, A.B.; visualization, A.C.-P.; supervision, E.S.-P. All authors have read and agreed to the published version of the manuscript.
This research received no external funding.
The study was conducted in accordance with the Declaration of Helsinki, and ap-
proved by the Bioethics Committee of the Pomeranian Medical University in Szczecin (protocol code KB-012/78/18 on 18 June 2018).
Informed consent was obtained from all subjects involved in the study.
The data presented in this study are available on request from the author E.S.-P. The data are not publicly available due to ethical restrictions.
The authors declare no conflict of interest.
1. Chen H, Zhang T, Yin X, Man J, Yang X, Lu M: Magnitude and temporal trend of acne vulgaris burden in 204 countries and territories from 1990 to 2019: An analysis from the Global Burden of Disease Study 2019. Br J Dermatol 2021; 186:673–683.
2. Tan J, Zhang X, Jones E, Bulger L: Correlation of photographic images from the Leeds revised acne grading system with a six-category global acne severity scale. J Eur Acad Dermatol Venereol 2012; 27:e414–e419.
3. Tuchayi SM, Makrantonaki E, Ganceviciene R, Dessinioti C, Feldman SR, Zouboulis CC: Acne vulgaris. Nat Rev Dis Prim 2015; 1:15029.
4. Rao A, Douglas S, Hall J: Endocrine disrupting chemicals, hormone receptors, and acne vulgaris: A connecting hypothesis. Cells 2021; 10:1439.

5. Dréno B: What is new in the pathophysiology of acne, an overview. J Eur Acad Dermatol Venereol 2017; 31:8–12.
6. Heng AHS, Chew FT: Systematic review of the epidemiology of acne vulgaris. Sci Rep 2020; 10:5754.
7. Kantor J: This month in JAAD International: March 2022: The psychological impact of acne scarring. J Am Acad Dermatol 2022; 86:532.
8. Koo JYM, Smith LL: Psychologic aspects of acne. Pediatr Dermatol 1991; 8:185–188.
9. Heng AHS, Say Y-H, Sio YY, Ng YT, Chew FT: Gene variants associated with acne vulgaris presentation and severity: A systematic review and meta-analysis. BMC Med Genom 2021; 14:103.
10. Azziz R, Sanchez LA, Knochenhauer ES, Moran C, Lazenby J, Stephens KC, Taylor K, Boots LR: Androgen Excess in Women: Experience with over 1000 consecutive patients. J Clin Endocrinol Metab 2004; 89:453–462.
11. Melnik B, John S, Plewig G: Acne: Risk indicator for increased body mass index and insulin resistance. Acta Dermato-Venereol. 2013; 93:644–649.
12. Mahmood SN, Bowe WP: Diet and acne update: Carbohydrates emerge as the main culprit. J Drugs Dermatol 2014; 13:428–435.
13. Baldwin H, Tan J: Effects of diet on acne and its response to treatment. Am J Clin Dermatol 2020; 22:55–65.
14. Poreba R, Debski R, Kotarski J, Paszkowski T, Pertyn T, Stachowiak G: Complex hormonal therapy in women with acne—Recommendations of the polish gynecologic society expert panel—2011. Ginekol Pol 2012; 83:229–232.
15. Zaenglein AL, Pathy AL, Schlosser BJ, Alikhan A, Baldwin HE, Berson DS, Bowe WP, Graber EM, Harper JC, Kang S, et al: Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol 2016; 74:945–973.e33.
16. Costa CS, Bagatin E, Martimbianco ALC, da Silva EM, Lúcio MM, Magin P, Riera R: Oral isotretinoin for acne. Cochrane Database Syst Rev 2018, 2019, CD009435.
17. Chilicka K, Rogowska AM, Szyguła R, Rusztowicz M, Nowicka D: Efficacy of oxybrasion in the treatment of acne vulgaris: A preliminary report. J Clin Med 2022; 11:3824.
18. Chilicka K, Rusztowicz M, Rogowska AM, Szyguła R, Asanova B, Nowicka D: Efficacy of hydrogen purification and cosmetic acids in the treatment of acne vulgaris: A preliminary report. J Clin Med 2022; 11:6269.
19. Burke BM, Cunliffe WJ: The assessment of acne vulgaris—The Leeds technique. Br J Dermatol 1984; 111:83–92.
20. Emiroğlu N, Cengiz FP, Kemeriz F: Original paper Insulin resistance in severe acne vulgaris. Adv Dermatol Allergol 2015; 4:281–285.
21. Kartal D, Yıldız H, Ertaş R, Borlu M, Utaş S: Association between isolated female acne and insulin resistance: A prospective study. G Ita. Dermatol Venereol 2016; 151:353–357.
22. Cappel M: Correlation between
serum levels of insulin-like growth factor 1, dehydroepiandrosterone sulfate, and dihydrotestosterone and acne lesion counts in adult women. Arch Dermatol 2005; 141:333–338.
23. Vora S, Ovhal A, Jerajani H, Nair N, Chakrabortty A: Correlation of facial sebum to serum insulin-like growth factor-1 in patients with acne. Br J Dermatol 2008; 159:990–991.
24. Tasli L, Turgut S, Kacar N, Ayada C, Coban M, Akcilar R, Ergin S: Insulinlike growth factor-I gene polymorphism in acne vulgaris. J Eur Acad Dermatol Venereol 2011; 27:254–257.
25. Balta I, Ekiz O, Ozuguz P, Ustün I, Karaca S, Kaçar SD, Ekşioğlu M: Insulin resistance in patients with post-adolescent acne. Int J Dermatol 2014; 54:662–666.
26. Aljefri YE, Alahmadi RA, Alajmi RS, Alkhamisi TA, Maaddawi HA, Alraddadi AA, Alamri AM: Cutaneous manifestations and hormonal changes among polycystic ovary syndrome patients at a tertiary care center. Cureus 2021; 13:e20593.
27. Lizneva D, Gavrilova-Jordan L, Walker W, Azziz R: Androgen excess: Investigations and management. Best Pract Res Clin Obstet Gynaecol 2016; 37:98–118.
28. Glintborg D, Andersen M: Management of endocrine disease: Morbidity in polycystic ovary syndrome. Eur J Endocrinol 2017; 176:R53–R65.
29. Cordain L, Lindeberg S, Hurtado M, Hill K, Eaton SB, Brand-Miller J: Acne vulgaris: A disease of western civilization. Arch Dermatol 2022; 138:1584–1590.
30. Dall’Oglio F, Nasca MR, Fiorentini F, Micali G: Diet and acne: Review of the evidence from 2009 to 2020. Int J Dermatol 2021; 60:672–685.
31. Conforti C, Agozzino M, Emendato G, Fai A, Fichera F, Marangi GF, Neagu N, Pellacani G, Persichetti P, Segreto F, et al: Acne and diet: A review. Int J Dermatol 2021; 61:930–934.
32. Kara YA, Ozdemir D: Evaluation of food consumption in patients with acne vulgaris and its relationship with acne severity. J Cosmet Dermato. 2019; 19:2109–2113.
33. Penso L, Touvier M, Deschasaux M, de Edelenyi FS, Hercberg S, Ezzedine K, Sbidian E: Association between adult acne and dietary
behaviors. JAMA Dermatol 2020; 156:854–862.
34. Çerman AA, Aktaş E, Altunay IK, Arıcı JE, Tulunay A, Ozturk FY: Dietary glycemic factors, insulin resistance, and adiponectin levels in acne vulgaris. J Am Acad Dermatol. 2016; 75:155–162.
35. Francis GL, Upton Z, Ballard FJ, A McNeil K, Wallace JC: Insulin-like growth factors 1 and 2 in bovine colostrum. Sequences and biological activities compared with those of a potent truncated form. Biochem J 1988; 251:95–103.
36. Melnik BC: Evidence for acne-promoting effects of milk and other insulinotropic dairy products. Nestle Nutr Workshop Ser Pediatr Program 2011; 67:131–145.
37. Adebamowo CA, Spiegelman D, Danby FW, Frazier AL, Willett WC, Holmes MD: High school dietary dairy intake and teenage acne. J Am Acad Dermatol 2005; 52:207–214.
38. Ulvestad M, Bjertness E, Dalgard F, Halvorsen J: Acne and dairy products in adolescence: Results from a Norwegian longitudinal study. J Eur Acad Dermatol Venereol 2016; 31:530–535.
39. Wolkenstein P, Misery L, Amici J-M, Maghia R, Branchoux S, Cazeau C, Voisard J-J, Taïeb C: Smoking and dietary factors associated with moderate-to-severe acne in French adolescents and young adults: Results of a survey using a representative sample. Dermatology 2014; 230:34–39.
40. Oge’ LK, Broussard A, Marshall MD: Acne vulgaris: Diagnosis and treatment. Am Fam Physician 2019; 100:475–484.
41. Hauk L: Acne Vulgaris: Treatment Guidelines from the AAD. Am Fam Physician 2017; 95:740–741.
42. Carmina E, A Lobo R: Evidence for increased androsterone metabolism in some normoandrogenic women with acne. J Clin Endocrinol Metab 1993; 76:1111–1114.
43. Da Cunha MG, Fonseca FLA, Machado CDAS: Androgenic hormone profile of adult women with acne. Dermatology 2013; 226:167–171.
44. Arowojolu AO, Gallo MF, Lopez LM, A Grimes D: Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012, CD004425.
FOR THE NINTH ANNUAL
The Canadian Conference on Ethnodermatology
OCTOBER 21, 2023
VISIT WWW.SKINSPECTRUM.CA TO REGISTER
USE THE CODE S+A2023 FOR 50% OFF THE REGISTRATION FEE
tively and systematically, which had not been done previously. Patients were followed every six months, and were monitoring for many of the confounding factors that we know are associated with mortality.”
Risk from treatment vs. risk from non-treatment
Dr. Langley noted that when biologic therapies were first being introduced for psoriasis, a focus of physician concern was the risk presented by treatment.
This concern was higher with the non-biologic systemic agents such as methotrexate and cyclosporine.
Those treatments can cause end-organ toxicity in the kidneys and can cause an

increased risk of certain skin cancers such as squamous cell cancer, Dr. Langley said. As a result, during the ‘80s and ‘90s there was discussion in the literature about rotating therapies, he said.
“So the concept of going on a single medication longterm has always been a concern because of the safety,” said Dr. Langley. “With biologic therapies, theoretically, they had better safety because of their very directed and specific nature. They are often blocking a single cytokine.”
However, Dr. Langley said that concern about side effects—by patients or physicians—may contribute to the under-treatment of psoriasis.
“I think [the PSOLAR
COMING
study findings] reassures people that we are now getting long-term data [on safety],” he said. “We are also getting data that is supportive of treatment in terms of survival.”
“I think that is probably why [this paper] grabbed a lot of people's attention. It is emphasizing the changes [in understanding psoriasis], telling us that we need to think about psoriasis as a systemic illness. We need to think about early treatment and appropriate treatment, rather than aggressive treatment,” he said. “The fact is that we can have reduced mortality risk with systemic therapy.”
Next steps
To build on these findings, Dr. Langley said there needs
INDIGENOUS SKIN SPECTRUM SUMMIT SEPTEMBER 2023 INDIGENOUS INDIGENOUS
to be a discussion on how these findings of reduced mortality with systemic treatment should inform clinical practice and prescribing.
The research should also be confirmed and expanded, he said.
“That is happening now with PSOLAR,” Dr. Langley said. The registry has included patients treated with methotrexate, TNF antagonists and ustekinumab.
“But we now have even more effective therapies: the interleukin 17 antagonists and the interleukin 23 antagonists. The question is: in the same way we saw a difference between methotrexate and the biologics [in terms of mortality reduction] will we see a dif-
ference between the older generation of biologics and the newer, more effective therapies?”
Another step to confirm the relationship between systemic psoriasis treatment and mortality would be to conduct a randomized controlled trial, he said.
“Any epidemiological study will have some limitations,” Dr. Langley said. “And certainly PSOLAR had some strengths, because of the size and systematic nature of it. But an events-based prospective, randomized controlled trial would be the next step to really take this and then change the paradigm of treatment, to change our algorithms to put a bit more impetus toward early treatment.”
Chronicle Companies is pleased to support Camp Liberté with monetary and inkind donations through Sandi’s Fund, established to honour our late friend and colleague, Sandra Gail Leckie, RN. Sandi was a nurse, pharmaceutical industry executive and health educator who had a life long affinity for children and children’s charities.

Camp Liberté was created by a group of dermatologists dedicated to offering children with moderate to severe skin conditions an opportunity to enjoy a summer camp experience. With locations in eastern and western Canada, Camp Liberté hosts more than 40 children per summer at no cost to parents, thanks to the support of generous donors.
Our camps are fully equipped with volunteer dermatologists, residents and nurses to care for children with a wide range of skin conditions, including atopic dermatitis, epidermolysis bullosa, and alopecia areata.

A gift to Camp Liberté provides Canadian children with skin conditions an opportunity to grow in confidence and self-esteem through a multi-cultural outdoor camping experience in a fun, safe, bilingual, environment.















Donate Now! / Faites un don dès maintenant!

Le Camp Liberté a été créé par un groupe de dermatologues déterminés à offrir à des enfants qui ont des problèmes de peau variant de modérés à graves la possibilité de vivre l’expérience d’un camp d’été. Avec ses emplacements dans l’est et l’ouest du Canada, le Camp Liberté accueille plus de 40 enfants par été sans qu’il en coûte quoi que ce soit aux parents, grâce à l’appui de généreux donateurs. Nos camps bénéficient des services complets de dermatologues, de médecins résidents et d’infirmières bénévoles qui s’occupent d’enfants aux prises avec un vaste éventail de problèmes de peau, y compris la dermatite atopique, l’épidermolyse bulleuse et la pelade.
Un don au Camp Liberté permet à des enfants canadiens qui ont des problèmes de peau d’accroître leur confiance en soi et leur estime de soi en vivant une expérience multiculturelle de camping en plein air offerte dans un environnement bilingue, sécuritaire et amusant.



ALLERGENS IN VARIOUS BRANDS OF TOPIC AL CLOBETASOL PROPIONATE
Researchers conducted this study to assess the frequency of allergenic ingredients in various brands and manufacturers of clobetasol propionate.Investigators identified common brands of clobetasol propionate using the GoodRx website, then obtained ingredient lists for those products from the U.S. Food & Drug Administration’s Online Label Repository. They then conducted a systematic literature review of the Medline (PubMed) database to find reports of allergic contact dermatitis confirmed by patch testing.
Researchers identified 49 different ingredients among 18 products, with an average of 8.4 ingredients per product. They write that 19 of the ingredients have allergenic potential, while one has protective effects. Two branded foam formulations contained the greatest number of potential allergens (5), while a shampoo formulation contained no potential allergens.
L Ramani, W Haidari, S Hall, S Amin, S Chisolm, S Feldman: Allergens in common brands of clobetasol, in Journal of Drugs in Dermatology (May 1, 2023; 22(5):491-495).
The authors of this paper conducted a retrospective review of case notes from a multidisciplinary service to better characterise the symptomatology of topical steroid withdrawal (TSW). They also detail their experience with management of the condition and assess the proportion of patients who pursue nonconventional management.
They identified 19 cases of TSW were identified between Jan. 2019 and June 2021: 15 females and four males. Most were under 35 years old, and 18 had atopic dermatitis. The most frequently reported features were redness, skin pain (typically 'burning'), skin sensitivity, excessive skin flaking, insomnia, and severe itching. There was a high burden of anxiety and depression, with three patients expressing suicidal thoughts. Roughly half of the patients pursued nonconventional treatments. Some sought private consultation with international dermatologists. The researchers noted improvements in the context of open psychodermatology consultations with an earlier introduction of conventional management options.
TS-R Brookes, R Barlow, P Mohandas, A Bewley: Topical steroid withdrawal: An emerging clinical problem, in Clinical and Experimental Dermatology (April 29, 2023; llad161).
A . Psoriasis
B. Atopic dermatitis
C. Tinea corporis
D. Pityriasis rosea
THE EDITORS invite your participation in this regular feature of
A recent article in Allure (May. 19, 2023) highlighted the dangers associated with skin-lightening creams and their potential to cause vision loss. Some of these skin-lightening creams even have dangerous levels of toxic ingredients such as mercury. A woman from Minnesota experienced vision loss, and high levels of mercury were detected in her and her child’s urine, likely caused by the mercury in skin-lightening creams found in her home. Drs. Ranella Hirsch, a dermatologist in Boston, and Shereene Idriss, a dermatologist in New York, said that people should avoid any exposure to mercury in beauty products. They both agreed that it is important to only purchase products from reputable brands and retailers. They also emphasize the importance of embracing natural skin tones and promoting healthy and inclusive beauty practices.
An article published in The Times of India (May 20, 2023) reported on a woman from Liverpool who, at the age of 41, was diagnosed with an aggressive form of skin cancer. The woman noticed a warning sign of cancer on her forehead after taking a selfie with her smartphone. She observed that the spot didn't resemble her typical acne scars and seemed to appear overnight, prompting her to monitor it closely as it grew over a few days. Seeking medical attention, she consulted a cancer specialist who confirmed squamous cell carcinoma through a biopsy. She underwent a 30-minute surgical procedure, and the cancerous growth was removed. The woman attributed her cancer to exposure to tanning beds and strongly encouraged caution among other users, even advocating for the banning of UV tanning.
According to an article published on MedPage Today (May 16, 2023), a multicentre study showed that artificial intelligence (AI) in clinical decision-making significantly enhanced dermatologists' diagnostic accuracy for melanocytic lesions. The study revealed that AI increased the sensitivity for distinguishing nevi and melanomas to 100% from 84%, and the specificity improved to 83.7% from 72.1%. For most lesions, dermatologists found support from a convolutional neural network (CNN) to be helpful. Patients were open-minded about CNN support, but they rejected the idea of replacing clinician judgment with neural networks. The study suggests that the collaboration of human expertise with AI could benefit clinicians and patients. The successful application of the clinician-and-machine approach in dermatology has potential for broad use in diagnostic AI systems.
A reporter writing an article published in Cosmopolitan (May 19, 2023) consulted Dr. Dendy Engelman, a dermatologist in New York, about the best sunscreens for tattooed skin. According to the publication, it is important to use proper sun care on body art to protect against sunburns and skin cancer but also fading of the ink. Dr. Engelman emphasized that the sun's UV radiation can accelerate the fading process, especially for recently tattooed skin, which is more sensitive and prone to sun damage. She recommends using SPF 50 daily after a new tattoo is fully healed and using at least SPF 30 for older tattoos. Some of the brands she recommends are Mad Rabbit Defend Tattoo Sunscreen, Vanicream’s facial moisturizer SPF 30, Sunnie Skin’s “The Protector” SPF 40, Black Girl Sunscreen Moisturizing Sunscreen Lotion and Aveeno’s Positively Mineral sunscreen stick.
An antipruritic and anti-inflammatory lotion and cream containing hydrocortisone 1% (in acetate form), and pramoxine hydrochloride 1%, Pramox HC is the perfect treatment for minor skin irritations including:


• Rashes
• Itching and redness due to eczema
• Poison ivy, poison oak, and poison sumac
• Contact dermatitis

• Seborrheic dermatitis
• Psoriasis
• Insect bites
Available as a cream, ideal for pruritus, and as a lotion with menthol for a cooling, soothing effect.
Available at Shoppers Drug Mart and most local drugstores.




The 1st topical retinoid indicated to treat facial and truncal acne.
• Trifarotene molecule precisely binds to the most relevant RAR in acne (RAR-ɣ)1
• Patients may see significant results in facial acne in four weeks 3

• Patients reported substantial improvements in self-confidence, social life, and emotional well-being4 with improvement of their acne
AKLIEF.ca
Indication and clinical use:
AKLIEF® (trifarotene 50 mcg/g) cream is indicated for the topical treatment of acne vulgaris of the face and/or trunk in patients 12 years of age and older.
Safety and effectiveness have not been established in geriatric patients (≥65 years).
Contraindications:
• Eczema or seborrheic dermatitis
• Pregnancy or women planning a pregnancy
Most serious warnings and precautions:
• For external use only, not for ophthalmic use
• Pregnancy or planning a pregnancy: Rare reports of birth defects associated with topical retinoids during pregnancy. Women of child-bearing potential should be informed of potential risks and use effective birth-control measures
Other relevant warnings and precautions:
• Discontinue use if allergic/hypersensitivity reactions occur
• Avoid contact with eyes, lips, angles of the nose, mucous membranes, abraded skin, open wounds, cuts, and eczematous and sunburned skin
• Avoid use of other dermatologic medications and potentially irritating topical products that have a strong skin-drying effect and products with high concentrations of alcohol, astringents, spices, or limes
• Non-comedogenic cosmetics should be used
• Treatment area should not be covered with dressings or bandages
• Weather extremes, such as wind or cold, may be more irritating
• Exposure to excessive sunlight, including sunlamps, should be avoided or an effective sunscreen and protective clothing are recommended
• Certain cutaneous signs and symptoms can be expected with use
• Use of electrolysis, “waxing,” and chemical depilatories for hair removal should be avoided
• Caution when taking drugs with known photosensitizers
• Avoid use on chest during breastfeeding
For more information:
Please consult the AKLIEF® Product Monograph at https://pdf.hres.ca/dpd_pm/00054047.PDF for important information relating to adverse reactions, interactions, and dosing information, which have not been discussed in this advertisement.
The Product Monograph is also available by calling us at 1-800-467-2081.