TheEmergenceofCovalentOn-Surface Polymerization
ChristopheNacci,StefanHechtandLeonhardGrill
Abstract Thecovalentlinkingofmolecularbuildingblocksdirectlyinthe two-dimensionalconfinementofasurface,theso-calledon-surfacepolymerization, hasdevelopedrapidlyinthelastyearssinceitrepresentsareliablestrategytogrow functionalmolecularnanostructuresinacontrolledfashion.Here,wereviewthe growthofsuchstructuresviaon-surfaceUllmanncouplingandhighlightthemajor chemicalandphysicalaspects.Thesesystemsaretypicallystudiedbyscanning tunnelingmicroscopythatallowsexplorationoftheinitialmonomerspecies, intermediateproductsand fi nalnanostructureswithsub-molecularspatialresolution.Inthisway,thechemicalstructuresoftheexsitusynthesizedmolecular buildingblocksaredirectlycorrelatedwiththeoutcomeofthechemicalreaction. Wealsopresentexampleswithdifferentmonomerspeciesinviewofgrowing heterogeneousmolecularstructuresaswellastheimportanceofthemolecular interactionwiththetemplatesurfaceasafurtherkeyparametertocontrolthe moleculardiffusionandtunethe finalmoleculararchitecture.
1Introduction
Assemblingfunctionalmolecularbuildingblocksonasurfaceisapromisingroute towardcentralobjectivesofnanotechnologyandinparticularmolecularelectronics sinceitmightallowthegrowthofelectroniccircuitsbasedonthefunctionalitiesof individualmolecularspecies[1, 2].Otherbottom-upstrategiesleadtothegrowthof
C.Nacci L.Grill(&) DepartmentofPhysicalChemistry,Fritz-Haber-InstituteoftheMax-Planck-Society, 14195Berlin,Germany e-mail:leonhard.grill@uni-graz.at
C.Nacci L.Grill DepartmentofPhysicalChemistry,UniversityofGraz,8010Graz,Austria
S.Hecht(&) DepartmentofChemistry,Humboldt-Universit ätzuBerlin,12489Berlin,Germany e-mail:sh@chemie.hu-berlin.de
© SpringerInternationalPublishingSwitzerland2016
A.Gourdon(ed.), On-SurfaceSynthesis,AdvancesinAtomandSingle MoleculeMachines,DOI10.1007/978-3-319-26600-8_1
extendedsurfacesupportedtwo-dimensionalnetworkswithoutstandingtechnologicalrelevance[3, 4].Thus,althoughtheprecursormoleculesdonotcontaina functioninthesecases,theassemblyofmoleculesinthetwo-dimensionalconfinementofasurfacecanbeveryefficient.Inthe fi eldofweakerintermolecular interactions,manysuccessfulattemptsofgrowingsupramolecularpatternsatsurfaces[5–8]havebeenachieved.However,theuseofcovalentlinkingtostabilize moleculararrangementsatsurfacesattractedconsiderableattentioninthelastyears [9–27],becomingnowadaysawell-establishedtechnique.Thisapproachresultsin thepresenceofmolecularpolymersonsurfacesthatcouldhardlybedepositedonto thesurfaceundercleanconditionsbyusingconventionaltechniquesandpreventing anydefragmentationprocess[28].Thenatureofthecovalentbondprovideshigh stabilityandrobustnesstotheresultingnanostructuresandallowsforefficient “throughbond” chargetransport[29–31].
Inthischapter,wereviewthedevelopmentandconceptualfoundationofthe covalenton-surfacepolymerizationtechnique.Asourandmanyothers’ workis basedontheUllmannreaction[32],wefocusonthearyl–arylhomocouplingof halogenatedmonomerbuildingblockstypicallyperformedoncoinagemetalsurfaces.We firstprovidechemicalconsiderationsregardingthereactionmechanism andderivecriticalparametersforsuccessfullycarryingouton-surfacepolymerizations.Usingthisapproachcovalentlyboundmolecularassemblieswithapredefinedshapeandsizeareproducedunderultrahighvacuum(UHV)conditions.We showhowthe finaltopologyofthedesiredmolecularaggregatesisintimately connectedtothedesignofthesingle-moleculebuildingconstituents.Different growthstrategies,e.g.,one-stepversustwo-step(hierarchical)processes,can eventuallyleadtothesame finalmoleculararchitecture:themajordifferences betweenthetwocasesarehighlighted.Thesubstratesurfacecorrugationcanbe furthermoreexploitedtodriveon-surfacesynthesisprocessesalongcertaindirectionsandpromotethegrowthofnanostructureswithprede finedorientations.Inthis regard,theimportanceofthesurfaceanisotropyisdiscussed.
2ResultsandDiscussions
2.1On-SurfacePolymerizationTechnique
Ingeneral,theon-surfaceassemblyofmolecularbuildingblocksintolargeand extendedstructuresaccordingtoabottom-upschemecanbeachievedbydifferent strategies.Ifstabilizedbyratherweaknon-covalentintermolecularinteractions[5–8, 33],thesenanostructuresbelongtothe fieldofsupramolecularchemistry[34].For instancedipole–dipoleinteractionshavebeenusedtogovernthemolecularaggregationofporphyrinderivatives,carryingtwo trans-positionedcyanophenylgroups, intolonglinearchainsonaAu(111)surface(Fig. 1a)[6].Twoopposingcyanophenyl groupscanengageinaself-complementarydipolarinteraction(hydrogenbond) therebydrivinganddirectingtheself-assemblyintoelongatedporphyrinchains.

Fig.1 Supramolecularself-assembledmolecularstructures. a STMimageat63Kof trans-BCTBPPwiresholdtogetherviadipole–dipoleinteractionsonAu(111)(Reproducedfrom [6],withpermission). b Two-dimensionalnetworksofPTCDIandmelaminemoleculesstabilized viaH-bonding(modelstructuresofthemoleculesandnetworkintheupperpanel).Inthelower panel,anSTMimageofthenetwork( 2V,0.1nA).Inthe inset,ahigh-resolutionviewofthe Ag/Si(111)-√3 × √3R30° isshown(ReprintedbypermissionfromMacmillanPublishersLtd: Nature[7],copyright2003). c STMtopographicimageofanextendedandhighlyregularnetworks formedbyCodirectedassemblyofNC–Ph3–CNlinkers.Inthe inset,thestructureofthemolecule includingitslengthandSTMtopographyofthethreefold Co–carbonitrilecoordinationmotifwith modelstructureisshown(AdaptedwithpermissionfromSchlickumetal.[8].Copyright2007 AmericanChemicalSociety)
Amoreconventionalandstrongermultiplehydrogenbondingmotifwasusedto stabilizeatwo-componentmixtureof3,4,9,10-perylenetetracarboxylicdiimide (PTCDI)andmelaminemoleculesintoahoneycombpatternonametalsurface (Fig. 1b)[7].Thethreefoldsymmetricalmelaminemoleculesrepresentthebranch pointsofthehexagonalnetwork,whilethePTCDImoleculesserveasstraight connectors(Fig. 1b).Theassemblygeometryallowsforthelocalformationofthree hydrogenbondsforeachcomplementarymelamine–PTCDIconnectionandthis ratherstrongnon-covalentinteractionplaysthekeyroleinguidingthementioned speciesintolargelyextendedsupramolecularnetworks.Moreover,manyexamples oftwo-dimensionalmolecularassemblieshavebeenreportedinthe fieldof metallo-supramolecularchemistrywheremetalatomsareusedtobridgesuitably functionalizedmolecularunits(ligands).Themetal–ligandbondistypicallystronger ascomparedtohydrogenbondingandthisallowstheformationofmorerobust networks[33].Suchmetal–ligandinteractionshave,forexample,beenexploitedto fabricatetwo-dimensionalarchitecturesbasedonthecoordinationofrod-likedinitrilemolecules(NC–Phn–CN)tocobaltcenters(Fig. 1c)[8].
Inadditiontotheseinteractions,theformationofevenstrongercovalentcarbon–carbonbondsbetweenmoleculesonthesurfacegainedlargeattentioninthelast years[9–18, 30, 31, 35].Thenatureofthecovalentbondallowstoconferhigh stabilityanddurabilitytothemolecularstructures,incontrasttonon-covalent intermolecularbonds-basedstructures.Thispropertyisakeywhenthinkingof potentialuseinfutureapplications[2].Inanalogytotheapproachhere,the
Fig.2 Covalentlylinkedmoleculararchitecturesbyon-surfacepolymerization.Singlebuilding blocksaresynthesizedexsituwithhalogensubstituents.Afterbeingthermallyactivated,the speciesdiffuseacrossasurface,interacttoeachotherandtheformationofnewcarbon–carbon covalentbondstakeplaceattheactivatedsitespositions[9]
bottom-upgrowthoflargenetworksasgraphene[3]andboronnitride[4]sheets alsoledtohighlystablestructures,becauseofthecovalentnatureoftheirlinks.
Theconceptoftheon-surfacepolymerizationtechniqueisillustratedinFig. 2. Eachsinglebuildingblockisbasedonachemicallystablemolecularunitcarryinga certainnumberofpotentiallyreactivesitesatspeci ficpositions.Thesesitesare representedbyacarbon–halogenbondthathasabonddissociationenergylower thanallotherbondsinthemolecularframework.Afterdepositingthemoleculeson asurface,thehalogensubstituentsareactivated(i.e.,halogen–carbonbonddissociation)thermally,leavingthechemicalstructureofthemolecularbuildingblocks intact.Atthesametime,thenewspeciesthermallydiffuseoverthesurfaceand formnewcovalentC–Cbondsattheactivatedsitepositionswhentheygetcloseto eachother.
Thedesignandexsitusynthesisofmoleculeswithdifferentnumbersand arrangementsofinterconnectionpointsopensupthepossibilitytopreciselytunethe topologyofthe finalmoleculararchitecture.Beforedetailingthearchitectural controlachievableusingtheon-surfacepolymerizationapproach,afewaspects regardingthechemistryofboththemonomersaswellasthesurfacesneedtobe considered.Duetoitsdominantuseinthe fieldanditsimportanceforourown work,welimitthefollowingdiscussiontotheUllmannreaction.
3ChemicalConsiderations
WhenconsideringanUllmanncouplingreaction[32]astheconnectionsequence foranon-surfacepolymerization,severalkeycriteriahavetobemet.First,one needstodesignmonomers,whichontheonehandhavetobereactiveatthedesired connectionsitestoallowforregioselectiveactivation,forexample,bycarrying labilehalogensubstituents,yetotherwiseneedtobestableatthedepositionand reactionconditions.Inaddition,theactivatedmonomersalsohavetobemobileon thesurfacetodiffusetoothermonomersandthegrowingpolymer.Thelatterpoint inevitablyalsodependsonthesurface,whichneedstostabilizetheformedaryl
Fig.3 Possibleactivationmechanismsforarylhalidestoinitiatecovalenton-surface polymerization
radicalintermediates,yetalsohastoprovidemobilityandideallyfacilitateboththe activationandconnectionsteps,i.e.,actastemplateandcatalyst.
Whiletheseaspectsgenerallyapplytomoston-surfacepolymerizationreactions, therearesomespecificaspectswhenfocussingontheUllmannreaction.The reactioncanbeinitiatedbyseveraldifferentdissociationmechanismscausedsimply byheat(inabsenceorpresenceofametalcatalyst),electrons(fromthetipofan STM,andelectrodeorareducingagent)orphotons(Fig. 3).
Whileinallcasesthearyl–halogensinglebondisbroken,thetechnique/stimulus usedforactivationpotentiallyprovidescontroloverwheretheUllmannreaction andhencepolymerizationistakingplace.Incontrasttothepioneeringworkofthe RiedergrouponthedimerizationofiodobenzeneinducedwiththeSTMtipatthe stepedgeofaCu(111)surface[36]themajorityofthereportedworkhasbeen exploringthermalactivationmostlyinconjunctionwithcoinagemetalsubstrates. Hereby,thetemperaturerequiredfordissociationofthehalogensubstituentcruciallydependsonthetypeofhalogen(andpotentiallyalsoonthetypeof(het)aryl moiety)aswellasthetypeofsubstrate.The firstaspecthasbeenexploitedbyusfor thehierarchicalgrowthoftwo-dimensionalpolyporphyrinnetworks(seebelow), whereweutilizesequentialactivationof firstiodineandthenbrominesubstituents toseparatethetwoorthogonalgrowthdirections[35].WhiletheC–Ibondsare cleavedat120 °C,theC–Brbondscleaveat250 °ContheemployedAu(111) surface.Ofcourse,thelatterisimportantaswellsincesimilarC–Ibondscleaveat muchhighertemperaturesintheabsenceofacoinagemetalasshownbythework ofGourdon,Kühnle,andcoworkersoncalcite(CaCO3),wheretemperatureabove 300 °Carenecessaryforactivation[37].
ClearlyandnotsurprisinglyinthecontextoftheclassicUllmannworkusing copperspecies[32],coinagemetalsfacilitateactivationandaryl–arylcoupling[38]. However,therearetwoopposingeffectswhencomparingthecoinagemetalswith regardtotheirabilitytoaidon-surfaceUllmanntypepolymerization:Ontheone handthehigherreactivityoflessnoblecoppersurfacesaidsboththeinitialhalide dissociationaswellasthecouplingoftheactivatedarylmonomersbutalsosignificantlylowersthemobilityandhencediffusionofthemonomersandgrowing
polymers,therebyinhibitinggrowth.Faselandcoworkershaveactuallyengagedin adetailedcomparativestudyshowingtheseopposingeffectsfortheCu(111),Ag (111),andAu(111)surfaces[39].Theauthorsfoundtheonsetofnetworkformation fromhexa(meta-phenylene)macrocyclichexaiodidemonomerstooccurat200 °C forCu(111),whileonAu(111)250 °CandonAg(111)300 °Cwererequired. However,themorphologyoftheobtainedpoly(1,3,5-phenylene)sdifferssignificantlyastheCu(111)grownstructuresarehighlybranchedfractal-likewhileinthe caseofAg(111)extendedhigh-quality2Dnetworkswereformed.Basedontheir experimental findingsaswellastheoreticalinvestigations,theyconcludethatthe loweractivityofAg(111)inthearyl–arylcouplingcombinedwiththehigher monomermobility(diffusion)onthissurface,bothcomparedtoCu(111),leadto betternetworkformation.Inourworkwehavebeenmostlyfocussingongold surfacesthatprovideagoodcompromisebetweenthesefeatures.Notethateven withoneandthesamemetalitssurfacereconstructionplaysanimportantroleas shownbyourownwork(seebelow)aswellasothers[40].
Inaddition,defects,stepedges,andadatomsareofutmostimportanceasthey canfacilitateactivation(seebelow)[41],stabilizeintermediates,andeveninhibit theircoupling.Thisisnicelyillustratedbythefactthatactivatedarylmonomers cannotbeconsideredastruly “freeradicals” butarestronglystabilizedbethemetal surface[39].Thisalsopreventsskeletalrearrangementstotakeplaceandthereby assuresregioselectivecouplingattheinitiallyhalide-substitutedpositions(Fig. 4). Dependingonthepresenceofadatoms,analternativecouplingmechanism involvestheformationofanaryl–metal–arylintermediate,whichcanreductively eliminatetoformthedesiredaryl–arylconnection(Fig. 4).Whilethissequencehas infactsuccessfullybeenobservedbyLinandcoworkerstotakeplaceinthe polymerizationof4,4″-dibromoterphenylonaCu(111)surface[42],inmanycases theintermediatelyformedcoppercomplexesareratherstableandcannotbeforced toeliminatethedesiredproducts[43, 44].Forexample,usinghexabenzo-coronene (HBC)dibromidemonomersonCu(111)gaveCu-bridgedHBCchains;however, onaAu(111)surfacethecorrespondinggoldcomplexeswerenotobserved andhencecovalentaryl–arylconnectionscouldsuccessfullybeobtained(Fig. 5)

Fig.4 Possiblecouplingmechanismsforarylhalidestoinitiatecovalenton-surfacepolymerization:regioselectivecoupling(a)andaryl–arylconnectionviaanintermediateformationofan aryl–metal–arylintermediate(b)
Fig.5a ChemicalstructureofBr2–HBC. b Cu-bridgedHBCchainonCu(111)(5.5 × 2.0nm2, 300mV,0.3nA). c HBCchainonAu(111)(5.5 × 2.0nm2, 300mV,0.1nA). d Heightprofiles inSTMimagesalongaHBCtrimeronCu(111)andAu(111)[43]
[43].Therefore,notonlythetypeofsurfacebutalsotheavailabilityofadatoms seemstohaveamarkedeffectonthepolymerizationoutcome.
Ingeneral,wenotethatusingtheUllmannreactionposestwoinherentlimitationstotheon-surfacepolymerizationprocess.Firstandforemost,thereactionis irreversibleundertheemployedconditions,i.e.,formeddefectscannotbehealed. Therefore,theoutcomeofthereactionsolelyreliesonkineticcontrolandequilibrationtotheglobalthermodynamicminimumstructurescannotbeusedasoften thecasefornon-covalentself-assemblyordynamiccovalentchemistry[45].Using otherconnectionssuchasboronicestersoriminesthisdrawbackcanbeovercome, however,atthecostofstability(towardhydrolysis)andfunctionality(inan optoelectroniccontext).Second,theemployedpolymerizationapproachisthatofa stepgrowth,morepreciselyapolycondensation,andthereforeintrinsiclimitation withregardtopolymerizationefficiencyandcontroloverthepolymerizationoutcomeexist.Aftersketchingthechemicalbasisformakingaryl–arylconnections, wewillnowdetailthemethodofcovalenton-surfacepolymerizationandhighlight themeansofcontrollingtheformedpolymerstructures.
4On-SurfaceSynthesisofCovalentlyBound Nanostructures
Twoalternativemethodscanbeusedfortheactivationofmolecularbuildingblocks (methodsIandII)andthegrowthofcovalentlyboundnanoarchitectures,leadingto similarresults[9].InmethodI,intactmoleculesare firstdepositedontoasurface andsubsequentlythermallyactivated.Conversely,inmethodII,theactivationof molecularspeciestakesplacealreadyintotheevaporatorcellandtheyaredeposited ontothesurface.
Inbothcases,thecovalentlinkingtakesplaceonthesupportingsurfaceupon thermaldiffusion.Asa firstcandidateforon-surfacesynthesis,aporphyrinbuilding blockwithfourbrominesubstituents(Br4TPP)hasbeenused(insetofFig. 6a).If theevaporatortemperaturewas550Korlowerduringdeposition,largeandordered islandsofintactBr4TPPwerefoundasaresultofmoleculardiffusionatthesurface

Fig.6 Molecularnanostructuresformedbydifferentapproaches(methodsIandII). a STMimage (20 × 20nm2)ofaBr4TPPmolecularislandonAu(111)afterdepositionatlowevaporator temperatureof550Kontothesubstratesurfacekeptatroomtemperature.Moleculesaredeposited intactontothesurface.The inset showsthechemicalstructureofBr4TPP. b STMimage (41nm × 41nm2)fordepositionatelevatedevaporatortemperatureof610K.Thiscausesthe activationofthemolecularspeciesintotheevaporatorandsubsequentlytheformationof covalentlyboundstructuresontothesurface.TheAu(111)samplewascleanedbyrepeatedNeion sputtering(E =1.5keV)andsubsequentannealingupto720K.Measurementswereperformed underUHVconditionswithalow-temperatureSTMoperatedatatemperatureof10K.STM imageswererecordedinconstantcurrentmodewiththebiasvoltagereferringtothesamplewith respecttotheSTMtip[9]
(methodI,Fig. 6a).Acarefulanalysisoftheouterborderofthemolecularisland revealsthatmanymoleculeshaveonlythreeBratomsconnectedwhilethereare fourontheintactmolecules.Thissuggeststhattheusedevaporatortemperatureis enoughtoinitiatetheBrdissociationofasmallamountofmolecules(morethan 90%ofthemoleculesremainintact).
Athigherevaporatortemperatures(Fig. 6b)mostofthemoleculesareactivated withthelossofseveralBrsubstituentsintheevaporator(methodII).Theactivated speciescanreactwitheachotheronthesurfaceandformnewintermolecularbonds uponthermaldiffusion,leadingtotheformationofcovalentlyboundstructureswith differentsizesandshapes(Fig. 6b).
Toinvestigatetheabilitytocontrolthearchitectureofthe finalmolecular nanostructures,differentTPP-basedmonomerbuildingblockshavebeensynthesizedwithone,two,andfourBrsubstituents(Fig. 7a–c).Intactmoleculeshave beenidentifi edbyusinglowevaporatortemperatures:theSTMimagesafterthe preparationshowclearlytheexpecteddifferentstructures(Fig. 7d–f).Allspecies havebeendepositedontoaAu(111)surfacekeptatlowtemperature(tosuppress anycarbon–halogenbonddissociation)andafterwardsannealedtothermally activatetheBrdissociation.Thus,thetopologyofthemoleculararchitecturesis intrinsicallyencodedinthedesignofthesinglemonomerbuildingblock(cf. fi rst andthirdrowsofFig. 7).
Ifthemonomerbuildingblockprovidesjustonereactiveside(BrTPP,Fig. 7a),the onlypossibleresultisadimer.Porphyrinbuildingblockscarryingtworeactivesides
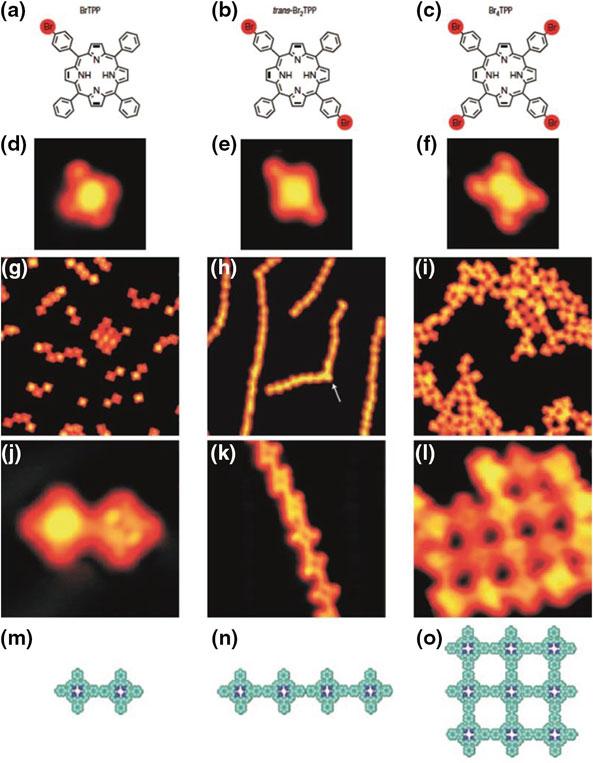
Fig.7 Buildingnanoarchitecturesusingdifferentmonomerbuildingblockscarryingone(left column,preparedbymethodI),two(middlecolumn,preparedbymethodII)andfour(right column,preparedbymethodI)Brsubstituents(a–c).STMimages(3.5 × 3.5nm2)ofthesingle intactmolecules(d–f).OverviewSTMimages(30 × 30nm2)ofthenanostructuresafteractivation andconnection(g–i).DetailedSTMimagesoftheresultingnanoarchitectures(j 5 × 5nm2; k 10 × 10nm2; l 8.5 × 8.5nm2).Correspondingchemicalstructuresofthenanostructures(m–o). Measurementswereperformedunderultrahighvacuum(UHV)conditionswithalow-temperature scanningtunnelingmicroscope(STM)operatedatatemperatureof10K.Covalentlylinked molecularstructureswereproducedincaseofmethodIfrommolecularbuildingblocksvia on-surfacepolymerization[9],i.e.,dehalogenationatatypicaltemperatureof523K(bromine dissociation)for10minandsubsequentcovalentlinkingofthemolecules[9]
astrans-Br2TPP(Fig. 7b)allowsaccordinglytheformationoflongandlinearchainsas showninFig. 7h,k.WhenallfourporphyrinunitlegscarryBrsubstituents(Fig. 7c), theconstructionoftwo-dimensionalmolecularnetworkisenabled(Fig. 7i,l).This provesthatacarefulchoiceofthemoleculardesign,i.e.,thearrangementoftheactive endgroupswithinthemolecularframeworkofthesinglebuildingblock,andasuccessfulexsituorganicsynthesisoftheinitialbuildingblocksgivehighcontroloverthe finalarchitectureofthemolecularstructures.
Animportantissueistheprecisechemicalnatureofthenewlyformedintermolecularbonds(orintramolecularbondsinthe finalpolymer,respectively).The firstevidencecomesfromthedistancesbetweenthebuildingblocks,whichis characteristicforsuchabond.Thereisagoodagreementbetweentheexperimentallymeasuredneighboringporphyrincoresinterdistance(17.2 ± 0.3 Å)andthe DFT-calculateddistance(17.1 Å)calculatedforacovalentlyboundporphyrins dimer(Fig. 8d).Furthermore,thecovalentnatureoftheintermolecularbondscan beinvestigatedbySTMsingle-moleculemanipulation.Molecularislandsmadeof intactBr4TPP(Fig. 6a)areeasilydisassembledbySTM-basedlateralmanipulation [9].Incontrast,dimers,chains,andmolecularnetworks(Fig. 7)canfollowthe STMtippathwayduringapullingexperiment[30, 31]withoutundergoingfragmentationprocesses.Thisisaclearsignaturefortherobustnessoftheintermolecularbondswithinthemolecularstructuresaftertheend-grouplegsactivation. Consequently,theinterpretationasacovalentbondseemsreasonable.Other

Fig.8 Thecovalentnatureofintermolecularbonds.STMimages(5 × 5nm2)ofaTPPdimerat 0.5V(a)and3.0V(b).Thebrightprotrusioninthemiddleofthedimer(b)isasignaturerelatedto anelectronicfeatureclearlyvisibleinthedI/dV curvemarkedbya cross inpanel(c).ThelowerdI/ dV curve(markedbya circle)takenontopofaporphyrinlegisfeatureless.DFTcalculationsreveal theformationofacovalentbondbetweenthetwoneighboringphenyllegs,withcorrespondingC–C bonding(s)andantibonding(s*)orbitals. d Calculatedgeometricstructureoftheisolateddimer. e Calculatedcontributiontothelocaldensityofstatesduetothestateatabout2.8eVabovethe HOMO(at7 Å fromtheporphyrinplane). f Sideviewofathree-dimensionalcontourplotofthe orbitaldensityofthisstateatamuchhigherdensity.Scanningtunnelingspectroscopy(STS)was performedat10Kwithalock-inamplifierwith20mVpeak-to-peakmodulationamplitudeat 640Hz(frequency)(seecaptionFig. 7 forfurtherexperimentaldetails)[9]
options,i.e.,chemicalbondsasHormetal-ligandbondingand π–π stacking,canbe ruledoutbecauseofthemolecularstructureandadsorptiongeometry,andadditionallytheycouldhardlyexplainwhythenanostructureremainsstablewhenbeing pulledbyanSTMtip.
Aclearsignatureforthecovalentnatureoftheintramolecularbondwithinthe dimerisprovidedbyspectroscopyofsinglemolecules(byscanningtunneling spectroscopy,STS).Theinterconnectionsitewithinthedimerappearshomogenously atlowbiasvoltages,whileitappearsasabrightprotrusionwhenimagedat+3.0V (Fig. 8a,b).Thisprotrusionisindeedrelatedtoanelectronicbroadfeatureslocalized ataround+3.0V(upperSTScurveinFig. 8c)suggestingthepresenceofalocalized orbital[9].DFTcalculationsprovethelocalformationofacovalentC–Cintermolecularbondinfullagreementwiththeexperimentallymeasuredporphyrincores interdistance.Speci fically,thecalculationsrevealedtheformationofC–Cbonding (σ)andantibonding(σ*)orbitalsthatgiverisetothesignalinthedI/dV spectra. Hence,thepeakataround3eVisadirect fingerprintofthechemicalnatureofthe covalentbond.Itiscausedbythestronginteractionwiththetwonon-occupied antibonding π orbitalsassociatedtothetwolegs,resultinginanin-phaseandan out-of-phasecombination,whicharesplitby1.3eV.Thein-phasecombinationis responsiblefortheincreaseofthecalculatedlocaldensityofstatespreciselylocated inbetweentheporphyrincoresatabout2.8eV(Fig. 8e,f)abovethehighestoccupied molecularorbital(HOMO).Thiscalculatedelectronicfeatureisassociatedtothe experimentallyprobedelectronicfeatureatabout3.0eVshowninFig. 8 [9].
AnotherclearexampleofUllmanndehalogenationreactionthatresultsinpolymerizationonthesurfacewasreportedbythegroupofRosei[19].Theydeposited diiodobenzenemolecularspecies(1 and 2 inFig. 9a)onCu(110)andfoundat fi rst theformationofCuboundphenyleneintermediates,i.e.,notyetlinkedbyC–C

Fig.9 Formationofpolyphenylene-basedpolymersbyon-surfacepolymerization. a Ullmann couplingofdiiodobenzenemolecules. b STMimage(T =115K,19 × 19nm2, V = 1.93V, I=1.06nA)ofPPP-basedolygomers.1,3-diodobenzene(1 inpanel a)weredosedonCu(110) keptatroomtemperatureandafterwardsannealedto500K. c 0.2Lof1,3-diiodobenzenedosed ontoCu(110)heldat500K.STMtopography(11.3 × 11.3nm2, Vs = 0.57V, It =0.82nA)of oligomerbranches.AmodelofPMPisoverlaidononeoftheoligomers. Top-rightinset aforce fieldrelaxedmodelofPMPchainintheiodinematrixonaslabofCucorrespondingtothemarked regionintheSTMimage. Bottomrightinset ascaleportionoftheRTdepositedsurface,showinga protopolymerofmolecule 2 inpanel a.Reproducedfrom[19]. © 2009Wiley-VCHVerlagGmbH &Co.KGaA,Weinheim.doi:10.1002/smll.200801943
covalentbondswhendepositingmoleculesonthesurfacekeptatroomtemperature. Heatingthesampleto500Kfor5–10minisneededtoinducetheformationof straightconjugatedPPPolygomers(Fig. 9b).TheformationofzigzagPMPwires andmacrocyclesaswellwerepromotedandobservedwhenusing 1,3-diiodobenzene(Fig. 9c,kinksareascribedtothemolecularsymmetry)[19].
5ControllingNanostructuresbyHierarchicalGrowth
Theresultspresentedsofararerelatedtothegrowthofsimplehomogeneous moleculararchitectures,becausetheyarebasedonaone-stepprocess.Growing complexnanostructures,e.g.,morecomplexmolecularaggregates,requiresa fine andaccuratecontrolofthereactionpathwaythatleadstothe finalmolecular architecture.Thiscanbeachievedsplittingthereactionpathwayintoindividual connectionstepsandcontrollingtheiractivationsequence,thusrealizinga “programmedreactivity” ofthemoleculesthatallows selective activationoftheir reactivityatdifferentsites.Asequentialgrowthfashioncanbeimplementedby designingsinglemolecularbuildingblocksthatcarrydifferenttypesofhalogen substituents.Thesampletemperaturecanbeusedasan “externalknob” thatallows toenableorsuppressspecifi edhalogendissociations,i.e.,on-surfacepolymerizationprocessescanbeinitiatedandsystematicallycontrolledviathesampletemperature.ThetemperatureneededtobreakC–halogenbondsismainlydefinedby thehalogenspeciesandthecatalyticactivityofthesurface.Thecarbon–halogen bonddissociationisactivatedattemperaturesthatdecreasewiththehalogenatomic number.Inotherwords,thebindingenergytothecarbonatomcanbetunedviathe typeofhalogenatom.Iodinedissociationfrommoleculescanbeinitiatedalreadyat roomtemperatureandcompletedataround120 °C,whilethistemperaturerange goesfrom100to250 °CforBrsubstituents[35, 46].Aproperchoiceofthesurface iscrucialasithasbeenshownthattheon-surfacecovalentlinkingoccursat differenttemperaturesfordifferentnoblemetalsurfaces[39],orcanevenbesuppressedforothersurfaces.Gutzleretal.[16]reportedonthegrowthof two-dimensionalcovalentboundnetworksbyusingpolyaromaticmoleculescarryinghalogensubstituents.TheydepositedthesemolecularspeciesonCu(111)and Ag(110)andindeedveri fiedthepresenceofactivatedspeciesalreadyatroom temperature,i.e.,withouttheneedofadditionalactivationenergy.Thesameprocedurerepeatedongraphite(001)resultedintheformationofwell-ordered non-covalentlyboundnetworksstabilizedbyhalogen–hydrogenbonding.This provestheimportanceofthesurfaceinpromotingthecarbon–halogendissociation atroomtemperatureandthesubsequentmolecularassembly.
Asmentionedabove,thearchitectureofthe finalstructuresisencodedinthe singlemonomerbuildingblockbyincorporatingdistinctcarbon–halogenbonds thatdissociateandcreateactivesitesatthehalogensites.Withthispurpose,a porphyrin trans-Br2I2TPPunithasbeendesignedandsynthesizedinordertocarry twodifferenttypesofhalogen–phenylsidegroups(Fig. 10a). Trans-Br2I2TPP
Fig.10 SinglemonomerbuildingblockscarryingBrandIsubstituentsforsequentialactivation. a Chemicalstructureofthe trans-Br2I2TPP.BrandIchemicalgroupshavedifferentchemical activationtemperatures. b STMimage(0.5V,0.1nA)ofasingleintact trans-Br2I2TPPonAu (111).IsubstituentsappearbrighterthanBronesbecauseoftheirdifferentchemicalstructure. MeasurementswereperformedunderUHVconditionswithalow-temperatureSTMoperatedata temperatureof10K.Moleculesweresublimatedat593KontoAu(111)keptatroom temperature[35]
moleculeshavetwopairsofhalogensubstituents(BrandI)eachofthemina trans configurationonoppositesidesoftheporphyrinunit(seeFig. 10a).Thischemical structureintrinsicallyencodestwodifferentgrowthdirections.Asthetwosubstituentshaveapronounceddifferenceintermsofbonddissociationenergy(the bindingenergyofiodine–carbonislowerthanthatofbromine–carbon)[35],this allowstocreateactivesitesinthemoleculestep-by-step.Inthisway,newcovalent intermolecularC–Cbondsareformedwithgeometriccontrol(viathetemperature) andconsequentlysequentialgrowthofnanostructuresisachieved(seegrowth schemeinFig. 10a).Low-temperatureSTMimagingallowstoresolvewith sub-molecularresolutionthefeaturesofintact trans-Br2I2TPPmoleculesdeposited ontopofAu(111):thetypicalfour-legsstructureoftheporphyrinunitisrecognized andsubstituenthalogenscanbechemicallydistinguishedbecauseoftheirdifferent appearanceinSTM(Fig. 10b):IandBrsubstituentshavespecifi capparentheights andtheformerlookbrighterindependentofthebiasvoltageovertheinvestigated range( 1V,+1V)[35].Thus,bycomparisonwithotherporphyrinderivativesthat containeitheronlyBroronlyIsubstituents,itispossibletoassignthecharacteristic apparentheightstoiodineandbrominesubstituents.Thispreciseknowledgeofthe chemicalcompositioninanSTMimage(Fig. 10b)isimportantinthenextstepto identifywhichsubstituentsremainafteraheatingstepandwhichonesare dissociated.
Trans-Br2I2TPPmoleculeshavebeendepositedontoAu(111)whilekeepingthe substrateatatemperatureof80Ktosuppresscatalyticallydriveniodinedissociationfromthemoleculesthatoccursathighertemperatures,thustokeepthe moleculesintactwithallfourhalogensubstituents[35].Undertheseconditions, molecularunitsarepreferentiallyfoundinclose-packedarrangements(Fig. 11b).

Fig.11 Hierarchicalgrowthofhomogeneousmolecularstructures. a Schemeofthesequential activationmechanism(from left to right).Inthe firstactivationstep,Isubstituentsaredissociated andactivesitesina trans geometry(firstgrowthdirection)arecreatedenablingtheformationof linearstructures(from b to c).Inthesecondstep,Braredissociatedbyannealingathigher temperatures.Thisfurtherstepallowstocreatelateralactivesitesthatenablethegrowthalongthe secondgrowthdirection,i.e.,theformationof2Dnetworks(from c to d).STMimages(8 × 8nm2, b)of trans-Br2I2TPPmoleculesonAu(111),afterheatingupto120K(step1,8 × 8nm2, c),and afterfurtherannealingupto250K(step2,10 × 10nm2, d). e STMimage(10 × 10nm2)of close-packedporphyrinchainsafterthe firstactivationstep.Furtherexperimentaldetailsarein captionFig. 9 andRef.[35]
AnnealingofthesampleuptoroomtemperatureinducesapartialIdissociation, whileannealingupto120 °Cenablesanefficientpolymerizationacrossthe transiodinedirection(firstgrowthdirectioninpanelFig. 11a)duringthe fi rst step.Accordingtothe trans-arrangementofhalogensubstituentswithinthesingle monomer,linearchainsofporphyrinunitsaregrown(Fig. 11c).Thisisinanalogy tothe trans-Br2TPPmolecules(Fig. 7h)butatlowertemperaturesbecauseiodineis involvedhere.Therearetwoimportantcharacteristicsoftheseintermediateproducts(showninFig. 11c):(1)Thesechainsalwayshaveabrightlovesattheirend, whichreflectsaniodineatom(asinFig. 10b).Hence,allnewlyformedbondsare locatedatformeriodinesites,whichconfi rmsthesuccessfulselectiveactivationin this firststep.(2)TheBrsubstituents,whichappeardarkerthantheiodines,canbe clearlyseensidewaysatthepolymerchainandarethereforestillpresent.However, theyhavenotbeenactivatedyetandarethereforedormant,waitingtobeactivated atasuitabletemperature.
Furthermore,covalentlylinkedporphyrinchainsarrangethemselvesparallelto eachotherintoclose-packedislands(Fig. 11e).Inthenextgrowthstep,Brsubstituentsareeffi cientlydissociatedbythermalannealingupto250 °Cenablingthe polymerizationprocessalongthesecondgrowthdirection(asindicatedinFig. 8a) andtriggeringtheformationofTPP-basedtwo-dimensionalnetworks(Fig. 11d). Thisrepresentsanelegantwaytogrowtwo-dimensionalnetworksinasequential manner,anditisworthtocompareitwiththesamestructureobtainedbythe one-stepgrowthprocess(TPP-basednetworksinFig. 7i,l).Ananalysisofthe
regularityoftheTPP-basednetworksgrownfollowingbothmethodssuggeststhat thehierarchicalgrowthallowstoprepare2Darchitectureswithlessincorrectly connectedbuildingblocks,i.e.defects,andlargerspatiallyextentregularnetworks (adetailedanalysisispresentin[35]).
Heterogeneousmoleculararchitecturesmightbegrownaccordingtoahierarchicalgrowthscheme.Covalentlylinkedtwo-componentstructuresonmetalsurfacesunderUHVconditionhavealreadybeenachieved[10],althoughinaone-step growthprocessandthuslimitedcontrol.Thecapabilitytoactivatedifferentreaction pathwaysstep-by-stepallowsabettertuningofthegrowthprocess.Whilethe formationoftwo-dimensionalTPPnetworkscouldalsobeachievedinaone-step process(Fig. 7l),themixtureoftwomolecularspeciesinadditiontotheselective activationmechanismleadstomolecularnanostructuresthatcannotbeformedin onestep.Whencombining trans-Br2I2TPPandDBTFmolecules(Fig. 12a)onaAu (111)surfacethetwogrowthstepsaresequentiallyactivatedwhenheatingthe sampleat250 °C.First,iodinesof trans-Br2I2TPPmoleculesaredissociatedand linearporphyrinchainsarecreatedwhileBr-phenylgroupsremainintact(Fig. 11c). Second,BrsitesaredissociatedandDBTFmoleculesformlinearchainsthat connecttotheformerBrsiteofporphyrinbuildingblocks(Fig. 12b).Inthiswaya ladder-typestructureisformedthatcouldnotbeachievedinonestep.
Adetailedanalysisofthecovalentlinksattheactivatedphenylgroupsof porphyrinbuildingblocks(showninFig. 12c)revealsthehighselectivityofthe process:98%oftheformerIsitesof trans-Br2I2TPPmoleculesareconnection pointsforfurtherporphyrinunitsasdesiredfromthemoleculardesign.Only2%of
Fig.12 Hierarchicalgrowthofheterogenousarchitectures. a ChemicalstructureofDBTF molecules. b STMimage(T =10K,18 × 13nm2)ofheterogenousnetworksbasedonDBTFand trans-Br2I2TPPonAu(111)byhierarchicalgrowthafterheatingupto250 °C. c Statisticalanalysis ofporphyrinand fluorineattachmenttotheporphyrin trans-Br2I2TPPmonomeratformerbromine andiodinesites(numberofevaluatedsites: nI =489, nBr =269).Measurementswereperformed underUHVconditionswithalow-temperatureSTMoperatedatatemperatureof10K.AKnudsen cellwasusedtoevaporateBr4TPPmoleculesat550KandDBTFmoleculesat503KontoAu (111).Theon-surfacesynthesiswasachievedraisingthesampletemperatureto250 °C[35]
thesesitesareincorrectlyusedfor fluorineconnections.Thesecondgrowthstep determinesapronouncedoccupationoftheremainingtwoBrsitesby fluorene molecules(70%,seeFig. 11c).Thenumbersarelessimpressiveinthissecond case,becauseattheformerBrsitesalsotwoporphyrinchainscouldbelinkedside toside,whichrepresentacompetitionprocessfortheplannedladderstructure.This provesthatthehierarchicalgrowthleadstotheformationofcopolymersassistedby aremarkabledegreeofselectivityofthechemicalspeciesinvolvedintheprocess.
6Substrate-DirectedGrowthbyOn-SurfaceSynthesis
Theon-surfacesynthesisconsistsoftwoprocessesatwork:activationanddiffusion ofthesinglemonomerbuildingblocksacrossthesurface.Elevatedtemperaturesare requiredtoenabletheseprocesses,butthisalsofavorsdisorderintothemolecular assemblyandcanthereforereducetheefficiencyofthepolymerizationprocess.It shouldbenoted,however,thatthesubstratesurfaceisnotapassivesupportfor chemicalspecies[16]butcanplayanactiveroleintermsofactivationofthe molecularspeciesinvirtueofitscatalyticproperties[16, 41].
Anycrystallinesurfaceexhibitsacertaincorrugation,dependingonthecrystal structureandthesurfaceorientation,whichplaysacrucialroleformoleculardiffusion.Thisfeaturecanbeusedinordertointroduceafurtherdegreeoffreedom, thusimprovingthecovalentlinkingandvaryingthe finalorientationofananostructurecomparedtotheunderlyingsubstratesurface.Bychoosingproperlythe surfaceitispossibletorestrictthemoleculardiffusionalongthelowestcorrugation directionsandfavortheformationofspeci ficmoleculararchitectureswithapredefinedorientation.Forinstance,theAu(110)-1 × 3surfacehasbeenusedto constrainthediffusionandsubsequentpolymerizationofalkylchainsalongits missingrows[40].Theconfinementofmoleculardiffusiontoonedimension (Fig. 13)leadstointermolecularinteractionsbetweenneighboringmoleculesthat resultintheformationoflinearmolecularchains[40].
Theeffectofsurfaceanisotropyonthegrowthoftwo-dimensionalnetworkshas beenstudiedwithanAu(100)single-crystalsurface.Thereconstructedsurface showsaquasi-hexagonal(5 × 20)superstructurewithstraightrowsofvertically displacedatoms[47],asshowninFig. 14a[35]. Trans-Br2I2TPPmoleculeshave beendepositedonAu(100)atlowtemperatureinordertokeepallhalogen–phenyl groupsintact.Afterwardsthesamplewasannealedto120 °C.Afterthisprocedure, covalentlyboundporphyrinchainswithapreferentialorientationarefoundas illustratedinFig. 14a.Hence,thesurfacereconstructiondeterminestheorientation ofthe finalnanostructure.

Fig.13 Polymerizationofhydrocarbonsonananisotropicgoldsurfaces. a STMtopographic imageofDEBmolecules(12 × 12nm2, 0.5V,0.5nA)onAu(110)-1 × 2at300K. b STM topographicimage(17.5 × 6nm2, 1V,2nA)polymerizedDEBchainslocatedinthemissing rowsofAu(110)-1 × 3afterheatingat420Kfor10h. Circles denotethephenylenegroups; arrows denotethemethylsidegroups. c AsectionofDEBpolymerchainandsuperimposedthe molecularstructure(14.4 × 1.6nm2, 1V,2nA).ThenewlyformedC–Cbondsareshownin red From[40].ReprintedwithpermissionfromAmericanAssociationfortheAdvancementofScience
Ananalysisofthechainsangulardistributionrevealsapreferredangleat51° betweenchainsandatomicrows(Fig. 14b).This findingcanbeeasilyrationalized bygeometricargumentssinceallporphyrinunitsareadsorbedonequivalentsites (Fig. 14c),thusreducingthetotalenergybythisparticularangle[35].Thisisin contrasttotherather flatAu(111)surfacewheretheangulardistributionofchainsis lessdefi ned(Fig. 7 andRef.[1])andunderlinestheimportanceofthesurface corrugation.Afterheatingto250 °C(thesecondactivationstep),formationof rectangularnetworksisagainfound(Fig. 14d)withaclearorientationofftheAu (100)atomicrowsorientation[35].Smallnetworksrevealdeviationsfromthe rectangularshape(angle β =101 ± 3° insteadof90°)asshowninFig. 14d.This effectismostlikelyascribedtothereducedrelativecontributionofintermolecular bondenergycomparedtotheinteractionofthemolecularassemblywiththesurface [35]forsmallnetworks.Furthermore,alargeraveragesizeofnetworksisachieved ascomparedtotheAu(111)surface[35],whichcandirectlybeassignedtothe surfaceanisotropy.Thecorrugationrowsleadtoaparallelarrangementofthe intermediateproducts(asillustratedinFig. 14a)thatresultsinasortofzipping mechanismforthetwo-dimensionallinkinginthesecondstep:Ifthe firstlink betweentwochainsisestablished,allotherporphyrinunitsareinaperfect arrangementwithrespecttoeachotherandaratherefficientlinkingoflongchain segmentscanoccur.Asaconsequence,the finalnanostructuresarelargerfor hierarchicalgrowthonacorrugatedsurfacethaninasingle-stepprocess.

Fig.14 Substrate-directedgrowthofnetworks. a STMimage(42 × 42nm2)of trans-Br2I2TPP chainsgrownonAu(100)afterthe firstactivationprocess. b Angulardistributionforchainsshown inpanel a c AdsorptiongeometryschemeofpolymericchainonAu(100)surfacewithanangleof 55° forequivalentadsorptionsitesforallporphyrins(a0 =1.44nmand d0 =1.76nm). d STM image(20 × 20nm2)ofanapproximatelysquaredcovalentlylinkedmolecularnetworkafterthe secondactivationprocess.MeasurementswereperformedunderUHVconditionswitha low-temperaturescanningtunnelingmicroscope(STM)operatedatatemperatureof10K. AKnudsencellwasusedtoevaporateBr4TPPmoleculesat550KontoAu(100).The firstand secondactivationstepswereinducedbyheatingto120and250 °C,respectively[35]
7Summary
ThegrowthofmolecularnanostructuresonsurfacesviaUllmanncouplingcanbe controlledbyboththechemicalstructureoftheinitialbuildingblocks,whichis preciselyreflectedinthe finalproducts,aswellasthesurfaceunderneath,inparticularthepresenceofdefects,stepedges,andadatoms.Diffusionoftheactivated monomersandintermediateoligomersisanotherkeyissuesinceitdefinestherate ofpolymerizationandthepossibilityofsubstrate-directedgrowththatallows improvedlinkingreactions.Variousmoleculeshavebeenusedinthelastyearsand itturnsoutthaton-surfacepolymerizationrepresentsaveryfeasiblemethodto createstablecovalent1Dand2Dpolymersonasurfaceandtoimagethemby scanningprobemicroscopyinrealspaceassuccessfullydemonstratedinmany cases.Thecovalentnatureofthenewlycreatedbondisnotonlyevidentfromthe realspacedistancesandorientations,butcouldadditionallybeprovenbyspectroscopicdetectionofcharacteristicelectronicstates.Whenusingdifferenthalogen substituents,ahierarchicalgrowthschemecouldberealizedsinceselectiveand sequentialactivationofthedifferentsubstituentsresultsinaprogrammedreactivity ofthemolecules.Basedonthegatheredmechanisticinsightandwiththeabilityto directreactivitybydesigningpropermonomerbuildingblocksaswellasusingthe surfaceasatemplate,1Dand2Dpolymersofincreasingstructuralandcompositionalcomplexitywillemerge.Besidesthiscontinuedexplorationofon-surface polymerizationasanewmethodforgeneratingdefinednanostructures,their resultingpropertiesandfunctionswillbecomeincreasinglyimportantinthefuture.
References
1.Joachim,C.,Gimzewski,J.K.,Aviram,A.:Electronicsusinghybrid-molecularand mono-moleculardevices.Nature 408,541–548(2000)
2.Heath,J.R.,Ratner,M.A.:MolecularElectronics.Phys.Today 56,43–49(2003)
3.Wintterlin,J.,Bocquet,M.-L.:Grapheneonmetalsurfaces.Surf.Sci. 603,1841–1852(2009)
4.Nagashima,A.,Tejima,N.,Gamou,Y.,Kawai,T.,Oshima,C.:Electronicdispersionrelations ofmonolayerhexagonalboronnitrideformedontheNi(111)surface.Phys.Rev.B 51,4606–4613(1995)
5.Rabe,J.P.,Buchholz,S.:Commensurabilityandmobilityintwo-dimensionalmolecular patternsongraphite.Science 253,424–427(1991)
6.Yokoyama,T.,Yokoyama,S.,Kamikado,T.,Okuno,Y.,Mashiko,S.:Selectiveassemblyon asurfaceofsupramolecularaggregateswithcontrolledsizeandshape.Nature 413,619–621 (2001)
7.Theobald,J.A.,Oxtoby,N.S.,Phillips,M.A.,Champness,N.R.,Beton,P.H.:Controlling moleculardepositionandlayerstructurewithsupramolecularsurfaceassemblies.Nature 424, 1029–1031(2003)
8.Schlickum,U.,Decker,R.,Klappenberger,F.,Zoppellaro,G.,Klyatskaya,S.,Ruben,M., Silanes,I.,Arnau,A.,Brune,H.,Barth,J.V.:Metal-organichoneycombnanomesheswith tunablecavitysize.NanoLett. 7,3813–3817(2007)
9.Grill,L.,Dyer,M.,Lafferentz,L.,Persson,M.,Peters,M.V.,Hecht,S.:Nano-architecturesby covalentassemblyofmolecularbuildingblocks.NatureNanotech. 2,687–691(2007)
10.Weigelt,S.,Busse,C.,Bombis,C.,Knudsen,M.M.,Gothelf,K.V.,Strunskus,T.,Wöll,C., Dahlbom,M.,Hammer,B.,Lægsgaard,E.,Besenbacher,F.,Linderoth,T.R.:Covalent interlinkingofanaldehydeandanamineonanAu(111)surfaceinultrahighvacuum.Angew. Chem.Int.Ed. 46,9227–9230(2007)
11.Champness,N.R.:Surfacechemistry:buildingwithmolecules.NatureNanotech. 2,671–672 (2007)
12.Matena,M.,Riehm,T.,Stöhr,M.,Jung,T.A.,Gade,L.H.:Transformingsurfacecoordination polymersintocovalentsurfacepolymers:linkedpolycondensedaromaticsthrough oligomerizationofN-heterocycliccarbeneintermediates.Angew.Chem.Int.Ed. 47,2414–2417(2008)
13.VeldM.I.,IavicoliP.,HaqS.,AmabilinoD.B.,RavalR.:Uniqueintermolecularreactionof simpleporphyrinsatametalsurfacegivescovalentnanostructures.Chem.Commun.1536–1538(2008)
14.Zwaneveld,N.A.A.,Pawlak,R.,Abel,M.,Catalin,D.,Gigmes,D.,Bertin,D.,Porte,L.: Organizedformationof2Dextendedcovalentorganicframeworksatsurfaces.J.Am.Chem. Soc. 130,6678–6679(2008)
15.Gourdon,A.:On-surfacecovalentcouplinginultrahighvacuum.Angew.Chem.Int.Ed. 47, 6950–6953(2008)
16.Gutzler,R.,Walch,H.,Eder,G.,Kloft,S.,Heckl,W.M.,Lackinger,M.:Surfacemediated synthesisof2Dcovalentorganicframeworks:1,3,5-tris(4-bromophenyl)benzeneongraphite (001),Cu(111),andAg(110).Chem.Commun.4456–4458(2009)
17.Perepichka,D.F.,Rosei,F.:Extendingpolymerconjugationintotheseconddimension. Science 323,216–217(2009)
18.Sakamoto,J.,VanHeijst,J.,Lukin,O.,Schlüter,A.D.:Two-dimensionalpolymers:Justa dreamofsyntheticchemists?Angew.Chem.Int.Ed. 48,1030–1069(2009)
19.Lipton-Duffin,J.A.,Ivasenko,O.,Perepichka,D.F.,Rosei,F.:Synthesisofpolyphenylene molecularwiresbysurface-confinedpolymerization.Small 5,592–597(2009)
20.Bartels,L.:Tailoringmolecularlayersatmetalsurfaces.NatureChem. 2,87–95(2010)
21.Lipton-Duffin,J.A.,Miwa,J.A.,Kondratenko,M.,Cicoira,F.,Sumpter,B.G.,Meunier,V., Perepichka,D.F.,Rosei,F.:Step-by-stepgrowthofepitaxiallyalignedpolythiopheneby surface-con finedreaction.Proc.Natl.Acad.Sci. 107,11200–11204(2010)
22.Lackinger,M.,Heckl,W.M.:ASTMperspectiveoncovalentintermolecularcoupling reactionsonsurfaces.J.Phys.DAppl.Phys. 44,464011(2011)
23.Mendez,J.,Lopez,M.F.,Martin-Gago,J.A.:On-surfacesynthesisofcyclicorganic molecules.Chem.Sov.Rev. 40,4578–4590(2011)
24.Ouchi,M.,Badi,N.,Lutz,J.-F.,Sawamoto,M.:Single-chaintechnologyusingdiscrete syntheticmacromolecules.NatureChem. 3,917–924(2011)
25.Palma,C.-A.,Samorì,P.:Blueprintingmacromolecularelectronics.NatureChem. 3,431–436 (2011)
26.Colson,J.W.,Dichtel,W.R.:Rationallysynthesizedtwo-dimensionalpolymers.Nat.Chem. 5 (6),453–465(2013)
27.ElGarah,M.,MacLeod,J.M.,Rosei,F.:Covalentlybondednetworksthrough surface-con finedpolymerization.Surf.Sci. 613,6–14(2013)
28.Grill,L.:FunctionalizedmoleculesstudiedbySTM:motion,switchingandreactivity. J.Phys.:Condens.Matter 20,053001(2008)
29.Nitzan,A.,Ratner,M.A.:Electrontransportinmolecularwirejunctions.Science 300,1384–1389(2003)
30.Lafferentz,L.,Ample,F.,Yu,H.,Hecht,S.,Joachim,C.,Grill,L.:Conductanceofasingle conjugatedpolymerasacontinuousfunctionofitslength.Science 323,1193–1197(2009)
31.Koch,M.,Ample,F.,Joachim,C.,Grill,L.:Voltage-dependentconductanceofasingle graphenenanoribbon.NatureNanotech. 7,713–717(2012)
32.Ullmann,F.,Bielecki,J.:UebersyntheseninderBiphenylreihe.Chem.Ber. 34,2174(1901)
33.Barth,J.V.,Costantini,G.,Kern,K.:Engineeringatomicandmolecularnanostructuresat surfaces.Nature 437,671–679(2005)
34.DeGreef,T.F.A.,Smulders,M.M.J.,Wollfs,M.,Schenning,A.P.H.J.,Sijbesma,R.P.,Meijer, E.W.:Supramolecularpolymerization.Chem.Rev. 109,5687–5754(2009)
35.Lafferentz,L.,Eberhardt,V.,Dri,C.,Africh,C.,Comelli,G.,Esch,F.,Hecht,S.,Grill,L.: Controllingon-surfacepolymerizationbyhierarchicalandsubstrate-directedgrowth.Nature Chem. 4,215–220(2012)
36.Hla,S.-W.,Bartels,L.,Meyer,G.,Rieder,K.H.:Inducingallstepsofachemicalreactionwith thescanningtunnelingmicroscopetip:towardssinglemoleculeengineering.Phys.Rev.Lett. 85(13),2777–2780(2000)
37.Kittelmann,M.,Rahe,P.,Nimmrich,M.,Hauke,C.M.,Gourdon,A.,Kühnle,A.:On-surface covalentlinkingoforganicbuildingblocksonabulkinsulator.ACSNano 5,8420–8425 (2011)
38.Hassan,J.,Sévignon,M.,Gozzi,C.,Schulz,E.,Lemaire,M.:Aryl-arylbondformationone centuryafterthediscoveryoftheUllmannreaction.Chem.Rev. 102,1359–1470(2002)
39.Bieri,M.,Nguyen,M.-T.,Gröning,O.,Cai,J.,Treier,M.,Aїt-Mansour,K.,Ruffieux,P., Pignedoli,C.A.,Passerone,D.,Kastler,M.,Müllen,K.,Fasel,R.:Two-dimensionalpolymer formationonsurfaces:insightintotherolesofprecursormobilityandreactivity.J.Am.Chem. Soc. 132,16669–16676(2010)
40.Zhong,D.,Franke,J.H.,Podiyanachari,S.K.,Blömker,T.,Zhang,H.,Kehr,G.,Erker,G., Fuchs,H.,Chi,L.:Linearalkanepolymerizationonagoldsurface.Science 334,213–216 (2011)
41.Saywell,A.,Schwarz,J.,Hecht,S.,Grill,L.:Polymerizationonsteppedsurfaces:alignmentof polymersandidentificationofcatalyticsites.Angew.Chem.Int.Ed. 51,5096–5100(2012)
42.Wang,W.,Shi,X.,Wang,S.,VanHove,M.A.,Lin,N.:Single-moleculeresolutionofan organometallicintermediateinasurface-supportedUllmanncouplingreaction.J.Am.Chem. Soc. 133,13264–13267(2011)
43.Koch,M.,Gille,M.,Viertel,A.,Hecht,S.,Grill,L.:Substrate-controlledlinkingofmolecular buildingblocks:Au(111)vs.Cu(111).Surf.Sci. 627,70–74(2014)
44.Villagomez,C.J.,Sasaki,T.,Tour,J.M.,Grill,L.:Bottom-upassemblyofmolecularwagons onasurface.J.Am.Chem.Soc. 132,16848(2010)
45.Rowan,S.J.,Cantrill,S.J.,Cousins,G.R.,Stoddart,J.K.,Sanders,J.F.:Dynamiccovalent chemistry.Angew.Chem.Int.Ed. 41,898–952(2002)
Another random document with no related content on Scribd:
CAPITULO XX
Como fue convertido en rana y lo que le sucedio de allí.
G���� —Yo ahogado á la verdad no me pesó, por dejar tanto trabajo y mala compañia que me llevaba. Plugo á Dios que me dieron por complida la penitencia por las deudas de Epulon é fuí convertido allí en rana.
M������.—Cuentame ¡oh Pitágoras! qué vida hacias cuando eras rana.
G����.—Muy buena, porque luego hice amistad con todos los géneros de peces que alli andaban é todos me trataban bien; mi comer era de las ovas del rio, é salida á la orilla saltando y holgando con mis compañeras pasciamos unas yerbecitas delicadas é tiernas que eran buenas para nuestro comer; no teníamos fortuna, ni fuego ni
tempestad ni otro género de acaescimiento que nos perjudicase. Pasado ansi algun tiempo...
CAPITULO XXI
Como fue convertido en ramera mujer llamada Clarichea.
Pasado así algun tiempo en aquel rio fue convertido en Clarichea, ramera famosa.
M������.—¡Oh! qué admirable transformacion; de asno en rana; de rana en ramera galana.
G����.—Pues quién bastara á te contar lo que siendo rana me acontecio y siendo ramera la solicitud que tenía, si no fuera por sernos ya el dia tan cercano para te lo contar muy por extenso, lo qual no me da lugar; y aquel cuidado que tenía de en adquerir los enamorados y el trabajo que sufria en conservar los servidores y el astucia con que los robaba su moneda; aquella manera de los despedir y aquella industria de los volver y el contino hastío que tenia de mis afeites y
composturas de atavíos y el martirio que pasaba mi rostro y manos con las mudas; aquel sufrir de pelar las cejas, que con cada pelo que sacaba se me arrancaba el alma de dolor, y con los afeites y adobos, pues todo mi cuerpo con los baños y ungüentos y otras muchas cosas que aplaciese á todos los que me querian; y aquel sufrir de malas noches y malos días, no tengo ya fuerza para te lo contar por extenso. Despues...
CAPITULO XXII
Como fue convertido en gañan del campo como servio á un avariento y despues fue tornado pavon é otras muchas cosas.
Después desto fue convertido en gañan del campo, adonde de contino con mucho trabajo sin reposo ninguno ni nunca entrar en poblado pasaba muy triste vida. Vine á servir y ser criado de un mísero avariento que me mataba de hambre, de lo cual no te doy entera cuenta lo que en este caso me sucedio, y fue transformado en pavon y agora gallo. ¡Oh! Micillo, si particularmente te hobiese de decir la vida y trabajos que he pasado en cada uno destos míseros estados no bastarían cien mill años que no hiciese sino contártelo. Por eso ya viene la mañana, por lo qual
quiero concluir porque vayas al trabajo, porque en esperanza de tu sueño no moramos de hambre, que creo que desde las diez encomenzamos la prática sin nada nos estorbar y son dadas cinco horas.
M������.—Admirado me tienen los trabajos desta vida, ¡oh Gallo! Pues dime ahora lo que me prometiste, que deseo mucho saber: ¿cual estado te paresció mejor?
G����.—Entre los brutos cuando era rana; entre los hombres siendo un pobre hombre como tú, porque tú no tienes que temer próspera ni adversa fortuna, ni te pueden perjudicar, no estás á la luz del mundo porque nadie te calunie; solo vives sin perjuicio de otro, comiendo de tu sudor ganado á tu placer, sin usuras ni daño de tu ánima; duermes sueño seguro, sin temer que por tu hacienda te hayan de matar ni robar; si hay guerra no hacen cuenta de tí; si préstamos ó censuras no temes que te ha de caber nada. En conclusion que bienaventurado el que vive en pobleza si es prudente en la saber sollevar.
M������.—¡Oh! mi buen Gallo, yo conozco que tienes mucha razon y pues es venido el día quiero ir al
trabajo y por el buen consuelo que me has dado en tu comer te lo agradeceré, como por la obra lo verás. Quédate con Dios, que yo me voy á trabajar.
FIN DEL DIALOGO DE LAS TRANSFORMACIONES




































