1.1 Dilated Cardiomyopathies: The Classification Pathway
Cardiomyopathies (CMPs) are myocardial disorders in which the heart muscle has structural and functional abnormalities in the absence of other causes sufficient to cause the disease. Until a few decades ago in medical literature, there was uncertainty and confusion about this entity. In the last years, advances in pathophysiology, pathology, biomarkers, genetics and molecular medicine, echocardiography, and cardiac magnetic resonance have brought light in the darkness.
Since 1956 several definitions of CMPs have been adopted using terms as “inflammatory,” “non-coronary,” “myocardial disorders of unknown etiology” [1]. Classifications tried to make order in the complexity and, historically, were mainly based on phenotype [2, 3] missing multiple other aspects. In 2006 the American Heart Association proposed the definition of CMPs as follows: “cardiomyopathies are a heterogeneous group of diseases of the myocardium associated with mechanical and/or electrical dysfunction that usually (but not invariably) exhibit inappropriate ventricular hypertrophy or dilatation and are due to a variety of causes that frequently are genetic. Cardiomyopathies either are confined to the heart or are part of generalized systemic disorders, often leading to cardiovascular death or progressive heart failure (HF) related disability” [4]. This classification is based on etiology, distinguishing CMP in genetic, acquired, and mixed, and splits CMPs into two groups, primary or secondary, as they involve predominately the heart or as a part of systemic disease. Brugada syndrome, long QT syndromes, short QT syndromes, catecholaminergic ventricular polymorphic tachycardia, and Asian sudden unexplained nocturnal deaths are put separately, but for the first time, channelopathies were mentioned in the classification of genetic cardiomyopathies.
Two years later the European Society of Cardiology (ESC) chose a clinical and morphological classification (Fig. 1.1), reporting CMPs as “myocardial disorders in which structure and function of the myocardium are abnormal, in the absence of coronary artery disease, hypertension, valvular heart disease and congenital heart disease sufficient to cause the observed abnormality”. Dilated CMP, hypertrophic CMP, restrictive CMP, and arrhythmogenic right ventricular CMP are the four main specific phenotypes that have to be subsequently subclassified in familial and nonfamilial. Actually the picture is not so simple, with heterogeneity and overlapping forms [5].
FAMILIAL / GENETIC
HCM
DCM
CARDIOMYOPATHIES
ARVC
RCM
Unclassified NON FAMILIAL / NON GENETIC
UNIDENTIFIED GENE DEFECT
DISEASE SUBTYPE
IDIOPATHIC
DISEASE SUBTYPE
Fig. 1.1 The 2008 ESC classification of cardiomyopathies. From Elliott P. et al. European Heart Journal 2008
M.
The need to integrate the above multiple aspects of CMPs prompts last classification available, proposed by Arbustini et al. in 2013 and endorsed by the World Heart Federation, the MOGE(S), a morphofunctional classification, enriched with extracardiac involvement, mode of inheritance with effect of mutation on gene function, and functional status. In details MOGE(S) acronym stands for morphofunctional characteristics (M), organ involvement (O), genetic or familial inheritance pattern (G), etiological information (E), and functional status (S). This system resembles the TNM classification of tumors and provides a genotype-phenotype correlation [6]. It seems to be a challenging way to describe CMPs in everyday life; however, it pushes clinicians to clarify etiology and familiar history and to have a comprehensive approach to the patients, not focusing only on the heart. Actually, even if it represents a translation link between basic science and clinical medicine, However, its use in clinical practice is rare [7].
Although major advances in knowledge as reported above, DCM is the cardiomyopathy that, between all others, still lacks of complete characterization and understanding. The term DCM encloses multiple entities, and, so far, no classification has been able to portrait it adequately.
Anyway, continuous efforts are made by researchers, and in 2016, a new statement has been published. Pinto et al. proposed a revised definition of DCM (Fig. 1.2), which tries to encompass the broad clinical features of the disease and its changes during time. They emphasize the progression of the disease from a preclinical state with no cardiac dilation through isolated ventricular dilation or arrhythmic cardiomyopathy, characterized by arrhythmogenic features as supraventricular/ventricular arrhythmias and/or conduction defects observed in myocarditis, genetic defects, and neuromuscular diseases. Furthermore, they introduce a new entity called “the hypokinetic non-dilated cardiomyopathy (HNDC)” which is the overt phase of systolic dysfunction not associated with ventricular dilation, as it happens in DCM caused by Lamin A/C defects. The final landing remains DCM [8].
Another new concept comes from the recent awareness that DCM overlaps with arrhythmogenic right ventricular cardiomyopathy (ARVC). They may share diseasecausing mutations; desmosomal gene defects are known to be mutated in DCM and
1.2 The DCM clinical spectrum. From Pinto Y.M. et al., European Heart Journal 2016
Fig.
M. Merlo et al.
ARVC. Moreover, patients with ARVC can show a left ventricular involvement, and the other way around a DCM relative may demonstrate ventricular ectopy coming from the right ventricle [8].
Maybe in the next classification there will be room for this overlap form with specific gene defects.
Despite major scientific progresses in the last decades, DCM still remains the third cause of HF and the first cause of cardiac transplant worldwide, with high clinical relevance given its mortality-morbidity risk in such a young population with long life expectancy (mean age at diagnosis is 45 years) (Fig. 1.3).
Major advances have been made in DCM since the 1980s when it was considered an end-stage condition, as a cancer, with 50% of mortality at 2 years. Nowadays, the estimated free survival from death and heart transplant is approximately of 85% at 10 years [9]. This is the result of earlier diagnosis with consequent earlier beginning of evidence-based therapy, which has dramatically improved in the last 30 years with introduction of neurohormonal agent (most recent sacubitril-valsartan) and non-pharmacological therapy (implantable cardioverter defibrillator (ICD) and resynchronization therapy). Unfortunately, we are not always able to adequately

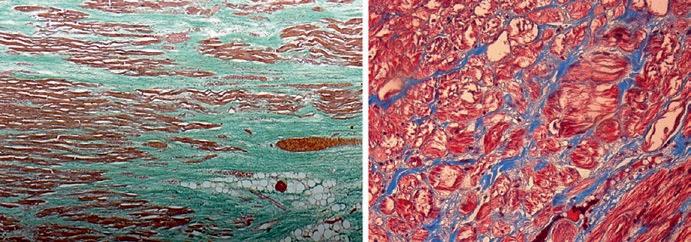
Fig. 1.3 Gross anatomy and histological specimen representative of DCM. (a, b) Gross anatomy of an explanted heart from a 26-year-old patient with DCM. (c) Azan-Mallory staining of a female patient with DCM and severe LV dysfunction; (d) histology from a patient with Duchenne’s dystrophy; (e) Azan-Mallory staining from an explanted heart from a patient affected by genetically determined DCM (double mutation in desmin and potassium channels). Courtesy of Prof. Bussani, University of Trieste
1 Historical Terminology, Classifications, and Present Definition of DCM
stratify the risk in this population, especially at the beginning of the disease when the adverse left ventricular remodeling is not the only adverse predictor and major arrhythmic events can happen in patients not satisfying criteria for ICD implantation. Anyway, severe mitral regurgitation, right ventricular dysfunction, and restrictive filling pattern have been recognized as predictors of adverse events as expression of advanced disease [10–12]. On the other hand, caution has to be taken to avoid early useless ICD implantation motivated only by low ejection fraction: studies have demonstrated that left ventricular reverse remodeling is a process that lasts 3–9 months after the diagnosis (to be completed in 24 months) [13]. A global evaluation comprehensive of late gadolinium enhancement and peak circumferential strain assessed by cardiac magnetic resonance (CMR) performs better than clinicalechocardiographic evaluation alone in the prediction of left ventricular reverse remodeling (LVRR) in patient recently diagnosed with DCM receiving evidencebased therapy [14].
DCM carries important ethical issues as the identification of asymptomatic carriers of gene mutations in a family, potential risk of pregnancy, and sport participation. These are common situations that the clinical cardiologist has to face with, often without specific guidelines.
Some help in the management of DCM comes from registries enrolling clinical, instrumental, and prognostic data of large cohorts of patients affected and strictly followed in the long term. In our Institution this is a common behavior, since we can extrapolate thousands of data from the Heart Muscle Disease Registry, active from 1978 [15].
1.2
Genetic Dilated Cardiomyopathy and Etiological Classification
Familial forms account for the at least 40% of cases, and thanks to the recent discoveries in the genetic field, clinicians have the opportunity, but also the responsibility, to provide an etiological diagnosis, stratify the risk and treat patients with the best strategy available. So, when acquired causes (e.g. hypertension, coronary artery disease, valvular heart disease, arrhythmias, etc.) have been excluded, there is a family history of DCM and there are clinical clues suggesting the diagnosis (what we used to call “red flags”: deafness, blindness, muscular disorders, etc.), we recommend to perform the genetic screening [13]. Anyway, it has to be stressed that de novo mutations exist, so a negative family history doesn’t rule out a genetic DCM, and that is mandatory for an appropriate patient selection in order to avoid noise, as will be explained below.
Guidelines and position papers recommend, with level C of evidence, genetic testing in the proband (the first or the most affected in the family, as this gives a high positive predictive value) in order to provide diagnostic/prognostic information, aid therapeutic choices, and prompt cascade screening in relatives [16]. Family screening allows an early diagnosis in a consistent number of patients, facilitating the diagnosis in non-proband DCM patients at an early stage of the disease, giving the chance to start optimal medical therapy earlier [17].
Genetic background of DCM is a wide and complex issue. So far, more than 50 genes encoding for cytoskeleton, sarcomeric proteins, sarcolemma, nuclear envelope ion channels, and intercellular junctions have been found to be implicated in DCM, and several other genes remain to be discovered. There is variable clinical presentation (also in the same family), incomplete penetrance, age-related penetrance, and lack of specific phenotype (meaning that the same gene mutation can cause different cardiomyopathies) [13].
However, unlike few decades ago, when cardiomyopathies were a confused matter, now we are living an historical breakthrough: from a pure phenotype classification, we are moving toward a best understanding of DCM and a more “personal” characterization of the disease, thanks to genetics [18]. In particular, there is growing evidence in the field of genotype-phenotype correlation with remarkable implications in the management of patients.
Although a strong genotype-phenotype relationship is currently accepted only for LMNA/C, recently a body of data is emerging in this field. Some rare sarcomeric variants carry poor prognosis after the age of 50, supporting the role of genetic testing in further risk stratification [19]. Furthermore, cytoskeleton Z-disk mutations are demonstrated as inversely related with LVRR. Moreover, since these proteins are not involved in beta-adrenergic activity, they are not targeted by antineurohormonal drugs limiting the therapeutic effect of the widespread molecules used in HFβ management [20].
Thus, the updated approach to DCM is now comprehensive of genetic evaluation with identification of genes and their corresponding phenotypic expression, accepting that most genotype-phenotype correlation remains unknown and, to date, globally, the genetic background is not able to predict disease evolution and response to therapy.
1.3 Future Perspectives
As frequently happens in medicine, there are unresolved issues, which are outlined below and which will be further explained in the focused chapters of the book.
Our efforts must focus on identifying the underlying DCM cause, in order to further reduce the number of “idiopathic DCM.” Progresses have been made in this field; we know that in the 1980s, almost 50% of DCM didn’t have a specific cause. Nowadays the etiologic characterization has dramatically improved so that it is possible to understand the etiologic basis of many so-called idiopathic heart muscle disease [3].
Thanks to etiology-directed management, the DCM prognosis has considerably improved and clinicians must persist in this task [21].
In patient with clinically suspected myocarditis as a possible explanation for ventricular dysfunction, there is the need to proceed with endomyocardial biopsy (EMB), with histopathology, immunohistochemistry, and molecular analysis. It has a fundamental role in identifying the underlying etiology (e.g., giant cell, eosinophilic myocarditis, sarcoidosis) which imply different treatments and prognosis. It is also the basis for safe immunosuppressive therapy, after the exclusion of viral infection [22].
M. Merlo et al.
Valuable aids in the etiologic characterization of DCM come from the recent advances in echocardiography.
An interesting tool is speckle-tracking strain analysis for assessing cardiac mechanics and segmental and global LV function. This technique allows the evaluation of myocardial deformation in all its components (i.e., longitudinal and circumferential shortening and radial thickening). All parameters may be reduced in DCM, beginning in the preclinical phase and allowing an early identification of disease [ 23 ].
Another essential tool is cardiovascular magnetic resonance (CMR). It provides additional prognostic information as it is the gold standard technique for biventricular morphological and functional evaluation and tissue characterization [24].
It is frequently adopted in the setting of myocarditis in stable patients or after EMB in life-threatening presentations, according to Lake Louise criteria [22].
A step toward a comprehensive DCM classification and an attempt to reconcile clinic with genetic in the complexity of the disease is genotype-phenotype correlation, with its prognostic implication in clinical practice. A clear example of this relation is the LMNA/C, but other gene defects are emerging, such as Filamin C [25]. It is possible that in the future genetic cluster classification will be completed studying every gene mutation, thanks to whole-genome sequencing, taking care of the patient instead of the disease.
Our efforts are focused on a personalized medicine approach including technologies at the services of each patient maybe with genic therapy or specific antiinflammatory therapy targeted to the specific etiology.
References
1. Arbustini E, Narula N, Tavazzi L, et al. The MOGE(S) classification of cardiomyopathy for clinicians. J Am Coll Cardiol. 2014;64:304–18. https://doi.org/10.1016/j.jacc.2014.05.027.
2. Heartjf B. Report of the WHO/ISFC task force on the definition and classification of cardiomyopathies. Br Heart J. 1980;44:672–3. https://doi.org/10.1136/hrt.44.6.672.
3. Richardson P, McKenna W, Bristow M, et al. Report of the 1995 World Health Organization/ International Society and Federation of Cardiology Task Force on the definition and classification of cardiomyopathies. Circulation. 1996;93:841–2. https://doi.org/10.1161/01. CIR.93.5.841.
4. Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee. Circulation. 2006;113:1807–16. https://doi.org/10.1161/CIRCULATIONAHA.106.174287.
5. Elliott P, Andersson B, Arbustini E, et al. Classification of the cardiomyopathies: a position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008;29:270–6. https://doi.org/10.1093/eurheartj/ehm342.
6. Şahan E, Şahan S, Karamanlıoğlu M, et al. The MOGE(S) classification. Herz. 2016;41:503–6. https://doi.org/10.1007/s00059-015-4394-0.
7. Fedele F, Severino P, Calcagno S, Mancone M. Heart failure: TNM-like classification. J Am Coll Cardiol. 2014;63:1959–60.
8. Pinto YM, Elliott PM, Arbustini E, et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice:
a position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2016;37:1850–8. https://doi.org/10.1093/eurheartj/ehv727.
9. Merlo M, Gigli M, Poli S, et al. La cardiomiopatia dilatativa come malattia dinamica: storia naturale, rimodellamento inverso e stratificazione prognostica. G Ital Cardiol. 2016;17:15–23.
10. Merlo M, Pyxaras SA, Pinamonti B, et al. Prevalence and prognostic significance of left ventricular reverse remodeling in dilated cardiomyopathy receiving tailored medical treatment. J Am Coll Cardiol. 2011;57:1468–76. https://doi.org/10.1016/j.jacc.2010.11.030.
11. Stolfo D, Merlo M, Pinamonti B, et al. Early improvement of functional mitral regurgitation in patients with idiopathic dilated cardiomyopathy. Am J Cardiol. 2015;115:1137–43. https://doi. org/10.1016/j.amjcard.2015.01.549.
12. Merlo M, Stolfo D, Anzini M, et al. Persistent recovery of normal left ventricular function and dimension in idiopathic dilated cardiomyopathy during long-term follow-up: does real healing exist? J Am Heart Assoc. 2015;4:e001504. https://doi.org/10.1161/JAHA.114.000570.
13. Merlo M, Cannatà A, Gobbo M, et al. Evolving concepts in dilated cardiomyopathy. Eur J Heart Fail. 2017;20(2):228–39. https://doi.org/10.1002/ejhf.1103.
14. Merlo M, Masè M, Vitrella G, et al. Comprehensive structural and functional assessment for prediction of left ventricular reverse remodeling in non-ischemic cardiomyopathy. J Am Coll Cardiol. 2018;71:A877. https://doi.org/10.1016/S0735-1097(18)31418-9.
15. Mestroni L, Rocco C, Gregori D, et al. Familial dilated cardiomyopathy: evidence for genetic and phenotypic heterogeneity. Heart Muscle Disease Study Group. J Am Coll Cardiol. 1999;34:181–90.
16. Hershberger RE, Lindenfeld J, Mestroni L, et al. Genetic evaluation of cardiomyopathy—a Heart Failure Society of America practice guideline. J Card Fail. 2009;15:83–97. https://doi. org/10.1016/j.cardfail.2009.01.006.
17. Moretti M, Merlo M, Barbati G, et al. Prognostic impact of familial screening in dilated cardiomyopathy. Eur J Heart Fail. 2010;12:922–7. https://doi.org/10.1093/eurjhf/hfq093.
18. Sinagra G, Mestroni L, Camerini F, editors. Genetic cardiomyopathies: a clinical approach. Milan: Springer; 2013.
19. Merlo M, Sinagra G, Carniel E, et al. Poor prognosis of rare sarcomeric gene variants in patients with dilated cardiomyopathy. Clin Transl Sci. 2013;6:424–8. https://doi.org/10.1111/ cts.12116.
20. Dal Ferro M, Stolfo D, Altinier A, et al. Association between mutation status and left ventricular reverse remodelling in dilated cardiomyopathy. Heart. 2017;103:1704–10. https://doi. org/10.1136/heartjnl-2016-311017
21. Merlo M, Gentile P, Naso P, Sinagra G. The natural history of dilated cardiomyopathy: how has it changed? J Cardiovasc Med. 2017;18:e161–5. https://doi.org/10.2459/ JCM.0000000000000459
22. Caforio ALP, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–48. https://doi.org/10.1093/eurheartj/eht210
23. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–71. https://doi.org/10.1093/ehjci/jev014
24. Hombach V, Merkle N, Torzewski J, et al. Electrocardiographic and cardiac magnetic resonance imaging parameters as predictors of a worse outcome in patients with idiopathic dilated cardiomyopathy. Eur Heart J. 2009;30:2011–8. https://doi.org/10.1093/eurheartj/ehp293
25. Corrado D, Zorzi A. Filamin C: a new arrhythmogenic cardiomyopathy–causing gene? JACC Clin Electrophysiol. 2018;4:515–7.
M. Merlo et al.
1 Historical Terminology, Classifications, and Present Definition of DCM
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Epidemiology
Paola Naso, Luca Falco, Aldostefano Porcari, Andrea Di Lenarda, and Gerardina Lardieri
Abbreviations and Acronyms
CAD Coronary artery disease
DCM Dilated cardiomyopathy
ECG Electrocardiography
EMB Endomyocardial biopsy
HCM Hypertrophic cardiomyopathy
HF Heart failure
ICD Implantable cardioverter device
LV Left ventricular
LVEDD Left ventricular end-diastolic diameter
LVEF Left ventricular ejection fraction
NGS Next-generation sequencing
SCN5A Sodium channel protein type 5 subunit alpha
WGS Whole-genome sequencing
Dilated cardiomyopathy (DCM) is a cardiac disease characterized by LV dilatation and impaired systolic function. An acquired dilated phenotype may result from a variety of factors including coronary artery disease (CAD), hypertension, myocarditis, valvular and congenital heart disease, drug toxicity, alcohol abuse and metabolic disease. Indeed, the diagnosis of “primary” DCM is often of exclusion. Among the forms of primitive DCM, familiar forms and idiopathic forms are identified [1–4].
P. Naso (*) · L. Falco · A. Porcari · A. Di Lenarda · G. Lardieri
Cardiovascular Department, Azienda Sanitaria Universitaria Integrata, University of Trieste (ASUITS), Trieste, Italy
e-mail: andrea.dilenarda@asuits.sanita.fvg.it; gerardina.lardieri@aas2.sanita.fvg.it
© The Author(s) 2019
G. Sinagra et al. (eds.), Dilated Cardiomyopathy, https://doi.org/10.1007/978-3-030-13864-6_2
Table 2.1 Major epidemiologic studies in dilated cardiomyopathy
P. Naso et al.
Study Incidence/prevalence
Torp et al. 1978 3/100,000/year [20]
Bagger et al. 1984 0.73/100,000/year [21]
Williams et al. 1985 8.3/100,000 [22]
Codd et al. 1989 36.5/100,000 [6]
Dolara et al. 1989 1.8/100,000/year [23]
Rakar et al. 1997 6.95/100,000/year [5]
The epidemiology of this condition is quite complex, due to misdiagnosis, continuous reclassification and changing definitions. Furthermore, since investigations were performed on small populations in specific geographic areas and were not representative of the general population, epidemiological studies on DCM are affected by many limitations. Another, but substantial, limitation of epidemiological studies conducted on this pathology depends upon the lack of standardized diagnostic criteria [5].
Initial estimations of prevalence data for DCM came from a population-based study by Codd et al. conducted on the Olmsted Country population (Minnesota, USA) between 1975 and 1984. According to this study, the prevalence rates were higher for men, with a male/female ratio of 3:1 [6]. Age- and sex-adjusted prevalence rates reached 36.5/100,000 subjects, and incidence rates were found 6/100,000 person years. Younger patients (<55 years) were more frequently affected (incidence up to 17.9/100,000). Data related to the epidemiology in different ethnicities suggest a 2.7-fold increased risk associated with black race [7]. Death certificates from the National Center for Health Statistics’ confirmed a 2.5-fold increased risk in blacks more than in whites, with black men having the highest prevalence (27/100,000 in black men versus 11/100,000 in white men) [8]. In Italy, the first data on the incidence of DCM go back to a prospective post-mortem study on consecutive necropsies performed during a 2-year period (November 1987–November 1989) in the Department of Pathology at Trieste University. Incidence of DCM at autopsy was estimated at 4.5/100,000/year, while clinical incidence in the same period was 2.45/100,000/year. The total incidence was 6.95/100,000/year in accordance with the study by Codd et al. [5, 6]. Table 2.1 shows a summary of major epidemiologic studies.
2.1 Towards Contemporary Clinical Epidemiology in Dilated Cardiomyopathy
The 2008 position statement from the European Working Group on Myocardial and Pericardial Diseases was a definitive turning point and shed new light upon the dark side of cardiomyopathies [9]. Cardiomyopathies were defined as “myocardial disorders in which the heart muscle is structurally and functionally abnormal, in the absence of coronary artery disease, hypertension, valvular disease and congenital heart disease sufficient to cause the observed myocardial abnormality” [10].
They were grouped into specific morphological and functional phenotypes and further divided into familial and nonfamilial forms. Diagnostic criteria have two main objectives: to support and facilitate the recognition of the disease and to allow the early diagnosis in affected asymptomatic family members. The consensus paper combined a clinical mind-set with first- and second-level diagnostic tools (i.e. ECG and echocardiography), placing the emphasis on family history of cardiac and neuromuscular diseases. The diagnostic paradigm shifted from a pathophysiological mechanism to a morphological and functional point of view, and the new awareness of a familial pattern in this disease built the basis of relatives screening [11].
In recent years, the diagnosis of DCM became reliable even in centres of different countries, thus allowing multicentre studies with more numerous and representative populations of well-studied patients. Furthermore, female sex gained attention in scientific literature and gender differences became an important topic to address.
Diagnostic criteria only partially overcame the difficulties faced in epidemiologic studies because of the challenging diagnosis and clinical presentation of the disease. Hershberger and colleagues estimated DCM prevalence on the basis of the known DCM to HCM ratio of ≈2:1. Therefore the surrogate DCM was found to be about 1–250 subjects [12], resulting from the early diagnosis, more effective treatments and a reduced mortality of patient partially linked to the identification of DCM in asymptomatic subjects. Current guidelines report a prevalence of familial DCM ranging from ≈30 to 50% of cases, with 40% having an identifiable genetic cause [13–15].
Table 2.2 shows the frequency of DCM in special categories.
DCM was originally considered a rare disease, and the possibility of a familiar substrate was ignored. Over time, DCM was found to be a major cause of HF affecting especially young patients, with absent or nonsignificant comorbidity and a long life expectancy, thus emerging as a major indication to heart transplantation [1]. The need to improve diagnostic accuracy for this population gave new life to scientific literature. DCM started to be considered a systemic condition rather than an isolated disease, and ventricular dilatation was found a common pathway of several cardiac diseases [3].
The studies carried out more recently were not built upon the solely basis of the phenotype, thus reflecting the epidemiology of the disease with higher accuracy. However, despite major efforts, the true incidence and prevalence of DCM still remains to be determined.
Table 2.2 Frequency of dilated cardiomyopathy in special groups
Categories
Female to male
Prevalence ratios
Between 1:1.3 and 1:1.5 [7, 23]
White (W) to African-Americans (AA) 1:2.45
Familial forms
W 11/100,000 AA 27/100,000 [7]
30–50% [14]
2.2 Genetics and Future Perspectives
As previously discussed, it has been known for decades that familial clinical screening in idiopathic DCM would reveal a significant amount of first-degree affected subjects (20–48%). However, only in the last few years, the role of genetics has become predominant in the approach of DCM patients, and the complexity of genetic mechanisms, genotype and environment interactions and genotype- phenotype correlations have become clearer. A fundamental role for these achievements has been played in recent years by the technological progress with the so-called next-generation sequencing (NGS) techniques, also used to sequence the entire human genome (coding and noncoding regions of DNA), referred to as whole-genome sequencing (WGS), with panels of dozens of genes at reduced cost [ 16 ].
In the most recent reports, approximately 40% of DCM cases have an identifiable genetic pathogenic variant. An important issue in this setting is the vast genetic as well as phenotypic heterogeneity in familial DCM, meaning that more than one mutation could be found and sometimes different morphological forms are showed in a single family: this is a major obstacle in clinical practice and in genetic report interpretations, because unreported pathogenic mutations must be validated, a process that needs time and delays the screening of other family members [17].
Thanks to the efforts in this field, a growing number of genes involved in DCM have been identified, and currently most panels cover 30–40 genes. Recently, many European centres have put their data together to create the first “Atlas of the clinical genetics of human Dilated Cardiomyopathy” [18].
Nowadays, the role of genetics is becoming more and more important in clinical practice. In fact, there is an increasing evidence that identifying a diseasecausing variant may have important patient management implications in terms of severity of the disease, prognosis and survival rates. For example, McNair et al. reported that 1.7% of DCM families have SCN5A gene mutations linked to a strong arrhythmic pattern [19] and that Lamin A/C mutation carriers have a wellknown risk of major ventricular arrhythmias/sudden death and conduction system abnormalities: this evidence may lead clinical cardiologist to consider ICD implantations in a cluster of patients that do not match the usual criteria indicated by the HF guidelines [20].
Epidemiology of DCM is rapidly changing. Furthermore, genetic testing may identify asymptomatic carriers, which lead to redefine prevention strategies, sport recommendations and ICD implantation. Nevertheless, it may guide reproductive decision-making, which could further modify the incidence and prevalence of DCM in the future decades [21].
References
1. Aleksova A, Sabbadini G, Merlo M, Pinamonti B, Barbati G, Zecchin M, et al. Natural history of dilated cardiomyopathy: from asymptomatic left ventricular dysfunction to heart failure—a subgroup analysis from the Trieste Cardiomyopathy Registry. J Cardiovasc Med. 2009;10:699–705.
P. Naso et al.
2. Rapezzi C, Arbustini E, Caforio ALP, Charron P, Gimeno-Blanes J, Helio T, et al. Diagnostic work-up in cardiomyopathies: bridging the gap between clinical phenotypes and final diagnosis. A position statement from the ESC Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:1448–58.
3. Pinto YM, Elliott PM, Arbustini E, Adler Y, Anastasakis A, Bohm M, et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: a position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2016;37:1850–8.
4. Caforio ALP, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–48, 2648a–2648d.
5. Rakar S, Sinagra G, Di Lenarda A, Poletti A, Bussani R, Silvestri F, et al. Epidemiology of dilated cardiomyopathy. A prospective post-mortem study of 5252 necropsies. The Heart Muscle Disease Study Group. Eur Heart J. 1997;18:117–23.
6. Codd MB, Sugrue DD, Gersh BJ, Melton LJ 3rd. Epidemiology of idiopathic dilated and hypertrophic cardiomyopathy. A population-based study in Olmsted County, Minnesota, 1975–1984. Circulation. 1989;80:564–72.
7. Coughlin SS, Szklo M, Baughman K, Pearson TA. The epidemiology of idiopathic dilated cardiomyopathy in a biracial community. Am J Epidemiol. 1990;131:48–56.
8. Gillum RF. Idiopathic cardiomyopathy in the United States, 1970–1982. Am Heart J. 1986;111:752–5.
9. Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, et al. Classification of the cardiomyopathies: a position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008;29:270–6.
10. Grunig E, Tasman JA, Kucherer H, Franz W, Kubler W, Katus HA. Frequency and phenotypes of familial dilated cardiomyopathy. J Am Coll Cardiol. 1998;31:186–94.
11. Hershberger RE, Hedges DJ, Morales A. Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nat Rev Cardiol. 2013;10:531–47.
12. Ganesh SK, Arnett DK, Assimes TL, Basson CT, Chakravarti A, Ellinor PT, et al. Genetics and genomics for the prevention and treatment of cardiovascular disease: update: a scientific statement from the American Heart Association. Circulation. 2013;128:2813–51.
13. Mestroni L, Rocco C, Gregori D, Sinagra G, Di Lenarda A, Miocic S, et al. Familial dilated cardiomyopathy: evidence for genetic and phenotypic heterogeneity. Heart Muscle Disease Study Group. J Am Coll Cardiol. 1999;34:181–90.
14. Hershberger RE, Siegfried JD. Update 2011: clinical and genetic issues in familial dilated cardiomyopathy. J Am Coll Cardiol. 2011;57:1641–9.
15. Sweet M, Taylor MRG, Mestroni L. Diagnosis, prevalence, and screening of familial dilated cardiomyopathy. Expert Opin Orphan Drugs. 2015;3:869–76.
16. Haas J, Frese KS, Peil B, Kloos W, Keller A, Nietsch R, et al. Atlas of the clinical genetics of human dilated cardiomyopathy. Eur Heart J. 2015;36:1123–35a.
17. McNair WP, Sinagra G, Taylor MRG, Di Lenarda A, Ferguson DA, Salcedo EE, et al. SCN5A mutations associate with arrhythmic dilated cardiomyopathy and commonly localize to the voltage-sensing mechanism. J Am Coll Cardiol. 2011;57:2160–8.
18. van Rijsingen IAW, Arbustini E, Elliott PM, Mogensen J, Hermans-van Ast JF, van der Kooi AJ, et al. Risk factors for malignant ventricular arrhythmias in Lamin a/c mutation carriers a European cohort study. J Am Coll Cardiol. 2012;59:493–500.
19. Mestroni L, Taylor MRG. Genetics and genetic testing of dilated cardiomyopathy: a new perspective. Discov Med. 2013;15:43–9.
20. Torp A. Incidence of congestive cardiomyopathy. Postgrad Med J. 1978;54:435–9.
21. Bagger JP, Baandrup U, Rasmussen K, Moller M, Vesterlund T. Cardiomyopathy in western Denmark. Br Heart J. 1984;52:327–31.
22. Williams DG, Olsen EG. Prevalence of overt dilated cardiomyopathy in two regions of England. Br Heart J. 1985;54:153–5.
23. Dolara A, Cecchi F, Ciaccheri M. Cardiomyopathy in Italy today: extent of the problem. G Ital Cardiol. 1989;19:1074–9.
P. Naso et
al.
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Pathophysiology
Valerio De Paris, Federico Biondi, Davide Stolfo, Marco Merlo, and Gianfranco Sinagra
Abbreviations and Acronyms
AD Alzheimer’s disease
ANP Atrial natriuretic peptide
ARVC Arrhythmogenic right ventricular Cardiomyopathy
AT1/2R Angiotensin type 1/2 receptor
ATII Angiotensin II
BNP Brain natriuretic peptide
DCM Dilated cardiomyopathy
ECM Extracellular matrix
HF Heart failure
IL1β Interleukin 1 β
LV Left ventricular
MCP-1 Monocyte chemoattractant protein-1
MIP 1α Macrophagic inflammatory protein 1 α
MMP-9 Matrix metalloproteinase-9
PIIINP N-terminal type III collagen peptide
RAAS Renin-angiotensin-aldosterone system
RyR2 Ryanodine receptor 2
SERCA Sarco-/endoplasmic reticulum Ca2+-ATPase
SR Sarcoplasmic reticulum
TIMP-1 Tissue inhibitor of metalloproteinase-1
TNFα Tumor necrosis factor
V. De Paris · F. Biondi · D. Stolfo (*)
Cardiovascular Department, Azienda Sanitaria Universitaria Integrata, University of Trieste (ASUITS), Trieste, Italy
e-mail: Davide.stolfo@asuits.sanita.fvg.it
M. Merlo · G. Sinagra
Cardiovascular Department, Azienda Sanitaria Universitaria Integrata, Trieste, Italy
e-mail: gianfranco.sinagra@asuits.sanita.fvg.it
© The Author(s) 2019
G. Sinagra et al. (eds.), Dilated Cardiomyopathy, https://doi.org/10.1007/978-3-030-13864-6_3
Dilated cardiomyopathy (DCM) is characterized by dilated left ventricle with systolic dysfunction that is not caused by ischemic or valvular heart disease.
The hallmark pathophysiologic feature of DCM is systolic dysfunction of the left or both ventricles. Reduced sarcomere contractility increases ventricular volumes to maintain cardiac output through the Frank-Starling mechanism, producing the thinwalled dilated LV appearance that is observed in overt DCM.
Frank and Starling demonstrated that increased ventricular preload augments contractility, but excessive pressure and volume induces a plateau and then a reduction in myocardial contraction [1]. Abnormal hemodynamics leads further to left ventricular (LV) remodeling.
Cardiac remodeling in response to an inciting myocardial insult or an underlying genetic abnormality has been classically considered the pathognomonic aspect of DCM.
3.1 Ventricular Remodeling in DCM
The term ventricular remodeling refers to alteration in ventricular architecture, with associated increased volume and altered chamber configuration, driven on a histologic level by a combination of pathologic myocyte hypertrophy, myocyte apoptosis, myofibroblast proliferation, and interstitial fibrosis.
Pathologic LV remodeling is closely linked to activation of a series of neuroendocrine, paracrine, and autocrine factors, which are upregulated after myocardial injury and in the setting of increased LV wall stress and hemodynamic derangement. Contributing factors include the renin-angiotensin-aldosterone (RAA) axis, the adrenergic nervous system, increased oxidative stress, pro-inflammatory cytokines, and endothelin. Both RAA system inhibition and beta-adrenergic blockade have shown to markedly attenuate or reverse LV remodeling in patients with heart failure and LV dilation.
Left ventricular remodeling results in characteristic alterations of left ventricular function that can be described in terms of altered left ventricular pressure-volume relationship. Left ventricular dilatation and reduced systolic function induce a rightward displacement of the pressure-volume curve with increased left ventricular enddiastolic volumes and pressures. Despite increased preload, stroke volume may be reduced, and end-systolic pressure to volume ratio (index of contractility) is depressed. In addition to this, diastolic dysfunction due to incomplete relaxation after disturbed excitation-contraction coupling processes and increased stiffness due to altered extracellular matrix composition cause an additional upward shift of the pressure-volume relation.
When the preload reserve is exhausted, the stroke volume becomes sensitive to alterations in the afterload. It depends on blood viscosity, vascular resistance, vascular distensibility, and mainly myocardial wall tension.
Calculations of myocardial wall tension are defined by the Laplace equation and are expressed in terms of tension, T, per unit of cross-sectional area (dynes per centimeter [dyn/cm]).
V. De Paris et al.
3 Pathophysiology
Within a cylinder, the law of Laplace states that wall tension is equal to the pressure within a thick-walled cylinder times the radius of curvature of the wall:
TP Rh =´ / where T is wall tension (dyn/cm), P is pressure (dyn/cm2), R is the radius (cm), and h is wall thickness.
Two fundamental principles stem from the relationship between the geometry of the ventricular cavity and the tension on its muscular walls: (1) dilatation of the ventricles leads directly to an increase in tension and (2) an increase in wall thickness reduces the tension on any individual muscle fiber. Therefore, ventricular hypertrophy reduces afterload by distributing tension among more muscle fibers.
Dilatation of the heart decreases cardiac efficiency as measured by myocardial oxygen consumption unless hypertrophy is sufficient to normalize wall stress. In HF, wall tension (or stress) is high, and thus, afterload is increased. The energetic consequences of the law of Laplace can have some role in progressive deterioration of energy-starved cardiac myocytes in the failing heart.
3.2 Genetic Pathophysiology and New Possible Proteins Involved in DCM
[2]
A great diversity of pathogenetic pathways has been hypothesized to explain the development of DCM, depending on the affected genes and the dislodged intracellular structures or pathways.
The wide variety of genes involved in the pathophysiology of DCM gives an insight to think of DCM as a group of diseases, instead of a single form of cardiomyopathy (Fig. 3.1).
Genetic mutations suggest several mechanisms of ventricular dysfunction in DCM as follows:
• Deficit in force generation (sarcomere DCM): Mutations within genes encoding titin, myosin, actin, troponin, and tropomyosin result in the expression of abnormally functioning proteins, thus leading to myocardial dysfunction and DCM. Sarcomere gene mutations are the most frequent causes of DCM with truncating mutations in titin (TTNtvs) occur in 25% of end-stage disease and in 15% of ambulatory DCM patients [3, 4].
• Defects in nuclear envelope (laminopathies): These diseases are characterized by variable degrees of heart and skeletal muscle involvement. Mutations involve LaminA/C and emerin coding genes. Dominant Lamin A/C mutations occur in approximately 6% of DCM cases and are far more common in DCM with conduction system disease [5]. Electrophysiological abnormalities (conduction system block and atrial fibrillation) often precede DCM that relentlessly progresses to HF [6, 7]. The severity of the associated skeletal myopathy is variable. Most Lamin A/C mutations cause haploinsufficiency, and mouse models of these mutations demonstrate inadequate response to mechanical strain, which may promote premature cardiomyocyte death.
Cytoskeletal Network
Fig. 3.1 Cardiomyocyte compartments contributing to genetically mediated dilated cardiomyopathy. See Legend for abbreviations and acronyms. (Adapted from McNally EM, Mestroni L, Dilated Cardiomyopathy Genetic Determinants and Mechanisms CircRes. 2017;121:731–748. DOI: https://doi.org/10.1161/CIRCRESAHA.116.309396)
• Deficit in force transmission (cytoskeletal cardiomyopathies): Mutations involving protein members of the cytoskeletal apparatus, like filamins, dystrophin, desmin, d-sarcoglycan, and vinculin, are responsible for muscular dystrophies, which are often associated with DCM.
• Filamins are large cytoskeletal actin cross-linking proteins that stabilize the actin filament networks and link them to the cell membrane by binding transmembrane proteins and ion channels [8]. Filamin C encodes a large protein (2725 amino acids) primarily expressed in the cardiac and skeletal muscle that interacts with sarcomeric proteins in the Z-disc and the sarcolemma. Filamin C truncation variants are associated with a severe arrhythmogenic DCM phenotype in the absence of overt skeletal muscle disease.
• Deficit in protein post-translational modifications (glycosylation processescardiomyopathies): An example comes from dolichol kinase gene mutations, resulting in impairment of protein glycosylation processes inside the cell organelles, thus manifesting as syndromic conditions with hypertrophic phenotype and as non-syndromic DCM phenotype [9].
• Impaired cell-to-cell adhesion (desmosomal cardiomyopathies): Mutations in genes encoding desmosomal proteins are responsible for arrhythmogenic right Extracellular
FLNC
PLN
LMNA
RBM20
Nuclear Lamina
Mitochondria
Nucleus
Sarcomeres
TTN, TNNT2, TPM1, MYH7, MyBPC3
Dystrophin Complex
V. De Paris et al.
ventricular Cardiomyopahty (ARVC) and also for DCM, with a prevalence of up to 13% in a DCM cohort [10].
• Deficit in energy production (mitochondrial cardiomyopathies): They are characterized by defects in the oxidative phosphorylation that result in deficient energy production in the form of ATP. They include hypertrophic, dilated, and LV non-compaction phenotypes.
• Calcium-cycling abnormalities: A DCM mutation has been described in the phospholamban gene. Phospholamban is responsible for inhibition of sarco-/ endoplasmic reticulum Ca2+ –ATPase (SERCA) function. Mutations in the gene result in increased SERCA inhibition with defective calcium reuptake, with consequent reduction in contractility and heart dilation.
• Ion channel abnormalities: Mutations in ion channel genes (SCN5A, ABCC9) are typically associated with a variety of arrhythmic disorders. The ventricular dilation and DCM pattern is less common and almost always preceded by arrhythmias and/or conduction system defects [11, 12]. The pathogenetic mechanisms are poorly understood.
• Spliceosomal defects: RBM20 is an RNA binding protein involved in alternative splicing process. DCM associated with RBM20 mutations is frequently associated with early onset, severe heart failure, and high arrhythmic potential.
• Epigenetic perturbation: Missense mutation in GATAD1 gene is associated with DCM. GATAD1 encodes for a protein that is thought to bind to a histone modification site that regulates gene expression.
• Protein misfolding disease: Mutations in presenilin genes have been recently identified in patients with DCM [13]. Presenilins are also expressed in the heart and play a role in heart development. Aβ amyloid is a possible novel cause of myocardial dysfunction. Echocardiographic measurements of myocardial function suggest that patients with Alzheimer’s disease (AD) present with an anticipated diastolic dysfunction. As in the brain, A β40 and A β42 are present in the heart, and their expression is increased in AD [14].
• RAS-MAPK pathway disruption: Mutations in RAF-1 gene are responsible for rare variants of childhood-onset, non-syndromic DCM.
3.3 Molecular Mechanisms of Cardiac Remodeling in HF [15]
DCM is histologically characterized by diffuse fibrosis, compensatory hypertrophy of the other myocytes, and myocyte dropout. Myocyte hypertrophy is promoted by catecholaminergic stimulation, stretch activation of integrins by myocyte and fibroblast, G protein-mediated intracellular signaling, and micro-RNA networks. A new gene expression toward a fetal pattern results in profound morphological rearrangements. The rate of myocyte apoptosis and consequently progressive cells lost is increased in DCM. This process is partly favored by the elevated expression of fetal genes. Neurohormonal systems. Acutely reduced cardiac output or vascular underfilling leads to baroreceptor-mediated sympathetic nervous activity with elevation of heart rate, blood pressure, and vasoconstriction. Although these changes maintain an
Another random document with no related content on Scribd:
tumblerful of home-brewed ale or a glass or two of wine daily; but, as I before remarked, in the generality of cases, either toast and water, or barley-water and milk, for the first week after a confinement, is the best beverage.
607. After a week, either a tumblerful of mild home-brewed ale or of London or Dublin porter, where it agrees, should be taken at dinner; but if ale or porter be given, wine ought not to be allowed. It would be well to keep either to ale or to porter, as may best agree with the patient, and not to mix them, nor to take porter at one meal and ale at another.
608. Barreled, in this case, is superior to bottled porter, as it contains less fixed air. On the whole, however, I should prefer homebrewed ale to porter. Either old, or very new, or very strong ale, ought not, at this time, to be given.
609. Great care is required in the summer, as the warm weather is apt to turn the beer acid. Such beer would not only disagree with the mother, but would disorder the milk, and thus the infant. A nursing mother sometimes endeavors to correct sour porter or beer by putting soda in it. This plan is objectionable, as the constant taking of soda is weakening to the stomach and impoverishing to the blood. Moreover, it is impossible, by any artificial expedient, to make either tart beer or porter sound and wholesome, and fit for a nursing mother. If beer or porter be sour, it is not fit to drink, and ought either to be thrown away or should be given to the pigs.
610. Sometimes neither wine nor malt liquor agrees; then, either new milk and water, or equal parts of fresh milk and barley-water, will generally be found the best beverage. If milk should also disagree, either barley-water, or toast and water, ought to be substituted.
CHANGE OF ROOM.
611. The period at which a lying-in woman should leave her room will, of course, depend upon the season, and upon the state of her health. She may, after the first fourteen days, usually change the chamber for the drawing-room, provided it be close at hand; if it be not, she ought, during the day, to remove—be either wheeled or
carried in a chair—from one bedroom to another, as change of apartment will then be desirable. The windows, during her absence from the room, ought to be thrown wide open; and the bedclothes, in order that they may be well ventilated, should be thrown back. She should, at the end of three weeks, take her meals with the family; but even then she ought occasionally, during the day, to lie on the sofa to rest her back.
EXERCISE IN THE OPEN AIR.
612. The period at which a lady, after her confinement, ought to take exercise in the open air, will, of course, depend upon the season, and upon the state of the wind and weather. In the winter, not until the expiration of a month, and not even then unless the weather be fine for the season. Carriage exercise will at first be the most suitable. In the summer she may, at the end of three weeks, take an airing in a carriage, provided the weather be fine, and the wind be neither in an easterly nor in a northeasterly direction. At the expiration of the month she may, provided the season and weather will allow, go out of doors regularly, and gradually resume her household duties and employments.
PART IV. SUCKLING.
THE DUTIES OF A NURSING MOTHER
613. A mother ought not, unless she intend to devote herself to her baby, to undertake to suckle him. She must make up her mind to forego the so-called pleasures of fashionable life. There ought, in a case of this kind, to be no half-and-half measures; she should either give up her helpless babe to the tender mercies of a wet-nurse, or she must devote her whole time and energy to his welfare—to the greatest treasure that God hath given her.
614. If a mother be blessed with health and strength, it is most unnatural and very cruel for her not to suckle her child—
“Connubial fair! whom no fond transport warms
To lull your infant in maternal arms; Who, blessed in vain with tumid bosoms, hear
His tender wailings with unfeeling ear;
The soothing kiss and milky rill deny
To the sweet pouting lip and glistening eye!
Ah! what avails the cradle’s damask roof,
The eider bolster, and embroidered woof!
Oft hears the gilded couch unpitied plains, And many a tear the tasseled cushion stains!
No voice so sweet attunes his cares to rest, So soft no pillow as his mother’s breast!
Thus charmed to sweet repose, when twilight hours Shed their soft influence on celestial bowers,
The cherub Innocence, with smile divine,
Shuts his white wings, and sleeps on beauty’s shrine.”[111]
615. Oh, if a mother did but know the joy that suckling her infant imparts, she would never for one moment contemplate having a wetnurse to rob her of that joy—
“The starting beverage meets the thirsty lip; ’Tis joy to yield it, and ’tis joy to sip.”[112]
616. Lamentable, indeed, must it be, if any unavoidable obstacle should prevent a mother from nursing her own child!
THE BREAST.
617. As soon as the patient has recovered from the fatigue of the labor—that is to say, in about four or six hours—attention ought to be paid, more especially in a first confinement, to the bosoms.
618. In a first confinement, there is, until the third day, but very little milk; although there is usually on that day, and for two or three days afterward, a great deal of swelling, of hardness, of distention, and of uneasiness of the breasts, in consequence of which, in a first confinement, both care and attention are needed.
619. If there be milk in the breast, which may be readily ascertained by squeezing the nipple between the finger and the thumb, the infant should, at first, be applied—not frequently, as some do, but at considerable intervals, say until the milk be properly secreted—every four hours; when the milk flows, the child ought to be applied more frequently, but still at stated times.
620. To wash away any viscid mucus from the nipple, or any stale perspiration from the bosom, let the breasts and the nipples, before applying the baby, be first sponged with a little warm water, and then be dried with a warm, dry, soft napkin; for some infants are so particular that unless the breasts and the nipples be perfectly free from mucus and from perspiration, they will not suck. If after the above cleansing process there be any difficulty in making him take the bosom, smear a little cream on the nipple, and then immediately apply him to it.
621. If the breasts be full, hard, knotty, and painful, which they generally are two or three days after a first confinement, let them be well but tenderly rubbed every four hours, either with the best olive oil (a little of which should, before using it, be previously warmed, by putting a little of the oil in a teacup on the hob by the fire) or with
equal parts of olive oil and of eau de Cologne, which should be well shaken up in a bottle every time before it be used.
622. On the third day, more especially after a first confinement, the breasts are apt to become very much swollen, painful, and distended. If such be the case, it might be necessary, for a few days, to have them drawn, once or twice daily, by a woman who makes it her business, and who is usually called either a breast-drawer, or, in vulgar parlance, a suck-pap. A clean, sober, healthy, respectable woman ought to be selected. There is, in nearly every large town, one generally to be found who is at the head of her profession. Such a one should be chosen.
623. If the bosoms be more than usually large and painful, in addition to assiduously using the above liniment, apply to the breasts, in the intervals, young cabbage-leaves, which should be renewed after each rubbing. Before applying them, the “veins” of the leaves, with a sharp knife, must be cut smooth, level with the leaf. It will require several, as the whole of the breast ought to be covered. The cabbage-leaves will be found both cooling and comfortable. Each bosom should then be nicely supported with a soft folded silk handkerchief, going under each breast and suspending it; each handkerchief should then be tied at the back of the neck, thus acting as a kind of sling.
624. The patient ought not, while the breasts are full and uncomfortable, to drink much fluid, as it would only encourage a larger secretion of milk.
625. When the milk is at “its height,” as it is called, she ought every morning, for a couple of mornings, to take a little cooling medicine— a Seidlitz powder—and, every four hours, the following effervescing mixture:
Take of Bicarbonate of Potash, one drachm and a half; Distilled Water, eight ounces:
To make a mixture. Two tablespoonfuls to be taken, with two tablespoonfuls of the Acid Mixture, every four hours, while effervescing.
Take of Citric Acid, three drachms; Distilled Water, eight ounces: Mix. The Acid Mixture.
The best way of taking the above effervescing medicine is to put two tablespoonfuls of the first mixture into a tumbler, and two tablespoonfuls of the acid mixture into a wineglass, then to add the latter to the former, and it will bubble up like soda-water; she should instantly drink it off while effervescing.
626. In two or three days, under the above management, the size of the bosoms will decrease, all pain will cease, and the infant will take the breast with ease and comfort.
627. Second and succeeding confinements.—If the breasts are tolerably comfortable (which in the second and in succeeding confinements they probably will be), let nothing be done to them except, as soon as the milk comes, at regular intervals applying the child alternately to each of them. Many a bosom has been made uncomfortable, irritable, swollen, and even sometimes gathered, by the nurse’s interference and meddling. Meddlesome midwifery is bad, and I am quite sure that meddlesome breast-tending is equally so. A nurse, in her wisdom, fancies that by rubbing, by pressing, by squeezing, by fingering, by liniment, and by drawing, she does great good, while in reality, in the majority of cases, by such interference she does great harm.
628. The child will, in second and in succeeding confinements, as a rule, be the best and the only doctor the bosoms require. I am quite convinced that, in a general way, nurses interfere too much, and that the bosoms in consequence suffer. It is, of course, the doctor’s and not the nurse’s province in such matters, to direct the treatment; while it is the nurse’s duty to fully carry out the doctor’s instructions.
629. There is nothing, in my opinion, that so truly tells whether a nurse be a good one or otherwise, as the way she manages the breasts; a good nurse is judicious, and obeys the medical man’s orders to the very letter; while, on the other hand, a bad nurse acts on her own judgment, and is always quacking, interfering, and fussing with the breasts, and doing on the sly what she dare not do openly: such conceited, meddlesome nurses are to be studiously avoided; they often cause, from their meddlesome ways, the breasts to gather.
630. Let the above advice be borne in mind, and much trouble, misery, and annoyance might be averted. Nature, in the majority of cases, manages these things much better than any nurse possibly can
do, and does not, as a rule, require helping. The breasts are sadly too much interfered and messed with by nurses, and by nurses who are, in other respects, tolerably good ones.
STATED TIMES FOR SUCKLING.
631. A mother ought to suckle her baby at stated times. It is a bad habit to give him the bosom every time he cries, regardless of the cause; for be it what it may—over-feeding, griping, “wind,” or acidity —she is apt to consider the breast a panacea for all his sufferings. “A mother generally suckles her infant too often—having him almost constantly at the bosom. This practice is injurious both to parent and to child. For the first month he should be suckled about every hour and a half; for the second month, every two hours; gradually, as he becomes older, increasing the distance of time between, until at length he has the breast about every four hours. If he were suckled at stated periods he would only look for it at those times, and be satisfied.”[113]
632. A mother frequently allows her babe to be at the bosom a great part of every night. Now, this plan is hurtful both to her and to him; it weakens her, and thus enfeebles him; it robs them both of their sleep; and generates bad habits, which it will be difficult to break through; it often gives the mother a sore nipple and the child a sore mouth.
633. It is surprising how soon an infant, at a very early age, may, by judicious management, be brought into good habits; it only requires, at first, a little determination and perseverance: therefore a nursing mother ought at once to commence by giving the child the breast at stated periods, and she should rigidly adhere to the times above recommended.
634. A mother should not, directly after taking a long walk, and while her skin is in a state of violent perspiration, give her baby the bosom; the milk being at that time in a heated state, will disorder the child’s bowels, or it may originate in him some skin disease, and one which it might be difficult to cure. She ought, therefore, before she gives him the breast, to wait until the surface of her body be moderately cool. Let her be careful the while not to sit in draughts.
CLOTHING.
635. A nursing mother ought to have her dress, more especially her stays, made loose and comfortable.
636. A gathered breast sometimes arises from the bones of the stays pressing into the bosom; I should, therefore, recommend her to have the bones removed.
637. If a lady be not in the habit of wearing a flannel waistcoat, she ought at least to have her bosoms covered with flannel, taking care that there be a piece of soft linen over the nipples.
638. I should advise a nursing mother to provide herself with a waterproof nursing apron, which may be procured either at any baby-linen establishment or at an india-rubber warehouse.
DIETARY.
639. A nursing mother ought to live plainly; her diet should be both light and nourishing. It is a mistaken notion that at these times she requires extra good living. She ought never to be forced to eat more than her appetite demands; if she be, either indigestion, or heartburn, or sickness, or costiveness, or a bowel complaint will ensue. It is a folly at any time to force the appetite. If she be not hungry, compelling her to eat will do her more harm than good. A medical man, in such a case, ought to be consulted.
640. The best meats are mutton and beef; veal and pork may, for a change, be eaten. Salted meats are hard of digestion; if boiled beef, therefore, be eaten, it ought to be only slightly salted. It is better, in winter, to have the boiled beef unsalted; it is then, especially if it be the rump, deliciously tender. Salt, of course, must be eaten with the unsalted meat. High-seasoned dishes are injurious; they inflame the blood, and thus they disorder the milk.
641. Some persons consider that there is no care required in the selection of the food, and that a nursing mother may eat anything, be it ever so gross and unwholesome; but, if we appeal to reason and to facts, we shall be borne out in saying that great care is required. It is well known that cow’s milk very much partakes of the properties of
the food on which the animal lives. Thus, if a cow feed on swedes, the milk and the butter will have a turnipy flavor. This, beyond a doubt, decides that the milk does partake of the qualities of the food on which she feeds. The same reasoning holds good in the human species, and proves the absurdity of a nursing mother being allowed to eat anything, be it ever so gross, indigestible, or unwholesome. Again, either a dose of purgative medicine given to her, or greens taken by her at dinner, will sometimes purge the baby as violently, or even more so, than it will herself.
642. Even the milk of a healthy wet-nurse acts differently, and less beneficially, upon the child than the mother’s own milk. The ages of the mother and of the wet-nurse, the ages of her own and the latter’s infant, the constitutions of the one and of the other, the adaptability of a mother’s milk for her own particular child—all tend to make a foster-mother not so desirable a nurse as the mother herself. Again, a mother cannot at all times get to the antecedents of a wet-nurse; and, if she can, they will not always bear investigation.
643. With regard to the ages of the mother and of the wet-nurse— for instance, as a wet-nurse’s milk is generally a few weeks older than the mother’s own milk, the wet-nurse’s milk may, and frequently does, produce costiveness of the bowels of her foster-child; while, on the other hand, the mother’s own milk, being in age just adapted to her babe’s, may and generally does keep her own infant’s bowels regular. The milk, according to the age of the child, alters in properties and qualities to suit the age, constitution, and acquirements of her baby—adapting itself, so to speak, to his progressive development. Hence the importance of a mother, if possible, suckling her own child.
644. A babe who is suckled by a mother who lives grossly is more prone to disease, particularly to skin and to inflammatory complaints, and to disease which is more difficult to subdue.
645. Do not let me be misunderstood: I am not advocating that a mother should be fussily particular—by no means. Let her take a variety of food, both animal and vegetable; let her from day to day vary her diet; let her ring the changes on boiled and stewed, on grilled and roast meats; on mutton and lamb and beef; on chicken and game and fish; on vegetables, potatoes, and turnips; on broccoli and cauliflower, on asparagus and peas (provided they are young and
well boiled), and French beans. “The maxim of the greatest importance in reference to the materials of human food is, mixture and variety—a maxim founded, as has been stated, upon man’s omnivorous nature. Animal and vegetable substances, soups and solid meat, fish, flesh, and fowl, in combination or succession, ought, if due advantage is to be taken of the health-sustaining element in food, to form the dietary of every household.”[114]
646. But what I object to a nursing mother taking are—gross meats, such as goose and duck; highly-salted beef; shell-fish, such as lobster and crab; rich dishes; highly-seasoned soup; pastry, unless it be plain; and cabbages and greens and pickles, if found to disagree with the baby; and any other article of food which is either rich, or gross, or indigestible, and which, from experience, she has found to disagree either with herself or with her child. It will be seen, therefore, from the above catalogue, that my restrictions as to diet are limited, and are, I hope, founded both on reason and on common sense.
647. A moderate quantity—say a tumblerful—either of fresh mild ale or of porter will generally be found the best beverage both for dinner and for supper. There is much more nourishment in either ale —home-brewed—or in porter, than in wine; therefore, for a nursing mother, either ale or porter is far preferable to wine. Wine, if taken at all, ought to be used very sparingly, and then not at the same meal with the porter or ale. In the higher ranks of life, where a lady is in the habit of drinking wine, it is necessary to continue it, although the quantity should not be increased, and ought never to exceed a couple of glasses—dry sherry being the best wine for the purpose.
648. A nursing mother is subject to thirst. When such be the case, she ought not to fly either to beer or to wine to quench it; this will only add fuel to the fire. The best beverages will be either toast and water, milk and water, barley-water, barley-water and new milk (in equal proportions), or black tea, either hot or cold. Cold black tea is a good quencher of thirst.
649. A lady who is nursing is at times liable to fits of depression. Let me strongly urge the importance of her abstaining from wine and from all other stimulants as a remedy; they would only raise for a time her spirits, and then would depress them in an increased ratio. Either a drive in the country, or a short walk, or a cup of tea, or a
chat with a friend, would be the best medicine. The diet should be good and nourishing; plenty of bread and plenty of meat should be her staple food, in addition to which Du Barry’s Arabica Revalenta, made either with fresh milk or with cream and water, is, in these cases, most useful and sustaining. The best time for taking it is either for luncheon or for supper. A lady subject to depression should bear in mind that she requires nourishment, not stimulants,—that much wine and spirits might cheer her for the moment, but will assuredly depress her afterward. Depression always follows overstimulation; wine and spirits therefore, in such a case, if taken largely, are false and hollow friends. It is necessary to bear the above facts in mind, as there are many advocates who strongly recommend, in a case of this kind, a large consumption of wine and brandy. Such men are, at the present moment, doing an immense deal of mischief; they are, in point of fact, inducing and encouraging drunkenness.
650. Spirits—brandy, rum, gin, and whisky—are, during suckling, injurious; I may even say that they are insidious poisons to the parent, and, indirectly, to the child.
651. When an infant is laboring under an inflammatory complaint, a nursing mother ought not to take stimulants, such as either ale or wine. In a case of this kind, toast and water will, for her dinner, be the best beverage, gruel for her supper, and black tea—not coffee, as it would be too stimulating—both for her breakfast and tea.
FRESH AIR AND EXERCISE.
652. Out-door exercise during suckling cannot be too strongly insisted upon; it is the finest medicine both for babe and mother. Whenever the weather will admit, it must be taken. It is utterly impossible for a nursing mother to make good milk, unless she do take an abundance of exercise and breathe plenty of fresh air.
653. Whatever improves the health of the mother, of course at the same time benefits the child: there is nothing more conducive to health than an abundance of out-door exercise. It often happens that a mother who is nursing seldom leaves her house; she is a regular fixture; the consequence is, both she and her babe are usually delicate and prone to sickness.
654. A mother ought not, immediately after taking exercise, to nurse her infant, but wait for half an hour. Nor should she take violent exercise, as it would be likely to disorder the milk.
655. Carriage exercise, if the weather be hot and sultry, is preferable to walking; if that be not practicable, she ought to have the windows thrown wide open, and should walk about the hall, the landings, and the rooms, as she would by such means avoid the intense heat of the sun. Although carriage exercise during intensely hot weather is preferable to walking exercise, yet, notwithstanding, walking must, during some portion of the day, be practiced. There is no substitute, as far as health is concerned, for walking. Many ailments that ladies now labor under could be walked away; and really it would be a pleasant physic—far more agreeable than pills and potions!
THE POSITION OF A MOTHER DURING SUCKLING.
656. Good habits are as easily formed as bad ones. A mother, when in bed, ought always to suckle her child while she is lying down. The sitting up in bed, during such times, is a fruitful source of inflammation and of gathering of the breasts. Of course, during the day the sitting-up position is the best. Let me caution her not to nurse her baby in a half-sitting and half-lying posture; it will spoil her figure, disturb her repose, and weaken her back.
THE TEMPER.
657. Passion is injurious to the mother’s milk, and consequently to the child. Sudden joy and grief frequently disorder the infant’s bowels, producing griping, looseness, etc.; hence, a mother who has a mild, placid temper generally makes an excellent nurse, on which account it is a fortunate circumstance that she is frequently bettertempered during suckling than at any other period of her life; indeed, she usually, at such times, experiences great joy and gladness.
658. The happiest period of a woman’s existence is, as a rule, when she first becomes a mother. “The pleasure of the young mother in her
babe is said to be more exquisite than any other earthly bliss.”[115]
659. It is an old and, I believe, a true saying, that the child inherits the temper of his mother or of his wet-nurse. This may be owing to the following reasons: If the mother or the wet-nurse be goodtempered, the milk will be more likely to be wholesome, which will, of course, make him more healthy, and consequently bettertempered.
660. While, on the other hand, if the mother or the nurse be of an irritable, cross temper, the milk will suffer, and will thus cause disarrangement to his system; and hence ill health and ill temper will be likely to ensue. We all know the influence that good or bad health has on the temper.
661. An important reason, then, why a nursing mother is often better-tempered than she is at other times is, she is in better health, her stomach is in a healthier state:
“A good digestion turneth all to health.”[116]
There is an old and a true saying, “that it is the stomach that makes the man,” and if the man, the woman:
“Your stomach makes your fabric roll, Just as the bias rules the bowl.”[117]
662. Hear what Shakspeare says of the functions of the stomach. The stomach is supposed to speak (and does it not frequently speak, and in very unmistakable language, if we will but only listen to its voice?):
“True is it, my incorporate friends, quoth he, That I receive the general food at first Which you do live upon: and fit it is; Because I am the store-house and the shop Of the whole body: But if you do remember, I send it through the rivers of your blood, Even to the court, the heart, to the seat o ’ the brain; And, through the cranks and offices of man, The strongest nerves, and small inferior veins, From me receive that natural competency
Whereby they live: And though that all at once, You, my good friends, though all at once cannot
See what I do deliver out to each; Yet I can make my audit up, that all From me do back receive the flour of all, And leave me but the bran.”
Coriolanus, act i. sc. 1.
OCCUPATION.
663. I strongly recommend a nursing mother to attend to her household duties. She is never so happy, nor so well, as when her mind is moderately occupied at something useful. She never looks so charming as when she is attending to her household duties—
“For nothing lovelier can be found In woman, than to study household good.”[118]
664. I do not mean by occupation the frequenting of balls, of routs, or of parties: a nursing mother has no business to be at such places; she ought to devote herself to her infant and to her household, and she will then experience the greatest happiness this world can afford.
665. One reason why the poor make so much better nursing mothers than the rich is the former having so much occupation; while the latter, having no real work to do, the health becomes injured, and in consequence the functions of the breasts suffer; indeed, many a fashionable lady has no milk at all, and is therefore compelled to delegate to a wet-nurse one of her greatest privileges and enjoyments.
666. What would not some rich mother give for the splendid supply of milk—of healthy, nourishing, life-giving milk—of the poor woman who has to labor for her daily bread!
667. What is the reason that wealthy ladies so frequently require wet-nurses? The want of occupation! And from whom do they obtain the supply of wet-nurses? From the poor women who have no lack of occupation, as they have to labor for their daily food, and have in consequence the riches of health, though poor in this world’s goods—
“For health is riches to the poor. ”[119]
Bear this in mind, ye wealthy, and indolent, and pampered ladies, and alter your plans of life, or take the consequences, and still let the poor women have the healthy, the chubby, the rosy, the laughing children; and you, ye rich ones, have the unhealthy, the skinny, the sallow, the dismal little old men and women who are constantly under the doctor’s care, and who have to struggle for their very existence! “Employment, which Galen calls ‘nature’s physician,’ is so essential to human happiness, that Indolence is justly considered as the mother of Misery.”[120]
668. Occupation, then—bustling occupation, real downright work, either in the form of out-door exercise, or of attending to her household duties—a lady, if she desire to have a good breast of milk, ought to take; if, in point of fact, she wishes to have healthy children. For the Almighty is no respecter of persons. And he has ordained that work shall be the lot of man and of woman too! It is a blessed thing to be obliged to work. If we do not work, we have all to pay a heavy penalty, in the form of loss of both health and happiness. “For work is the grand cure of all the maladies and miseries that ever beset mankind—honest work, which you intend getting done.”[121]
669. A mother who is listless and idle, lolling either the greater part of every day in an easy-chair, or reclining on a sofa in a room where a breath of air is not allowed to enter, usually makes a miserable and a wretched nurse. She is nervous, dyspeptic, and emaciated; having but little milk, and that little of a bad quality, her baby is puny, pallid, and unhealthy, and frequently drops into an untimely grave. Occupation, then, with fresh air and exercise, is indispensable to a mother who is suckling.
AILMENTS, Etc.
670. The Nipple.—A good nipple is important both to the comfort of the mother and to the well-doing of the child.
671. One, among many, of the ill effects of stays and of corsets is the pushing-in of the nipples; sore nipples, and consequent suffering, are the result. Moreover, a mother thus circumstanced may be quite unable to suckle her infant; and then she will be severely punished for her ignorance and folly; she will be compelled
to forego the pleasure of nursing her own children, and she will be obliged to delegate to hirelings her greatest privilege! Ladies who never wear stays have much better nipples, and more fully-developed bosoms; hence such mothers are more likely to make better nurses to their babies. There is no doubt that the pressure of the stays on the bosom tends both to waste away the gland of the breast (where the milk is secreted), and to cause the nipple either to dwindle or to be pushed in, and thus to sadly interfere with its functions. I should strongly advise every mother who has daughters old enough to profit by it, to bear this fact in mind, and thus to prevent mischief when mischief might be prevented, by not allowing them, when young, to wear stays.
672. Treatment of very small and drawn-in nipples.—The baby ought to suck through the intervention of an india-rubber teat fastened on a boxwood shield, or through an india-rubber teat and shield, made entirely of india-rubber.[122] The india-rubber teat must, before it is used, be softened by dipping it in warm but not in hot water. I have known many mothers able to suckle their children with this contrivance who otherwise would have been obliged either to have weaned them, or to have procured the assistance of a wet-nurse. The above aid, in the generality of instances, will enable the infant to suck with ease. After this has for a time been used, the nipples will be so improved as to render the continuance of it unnecessary. Of course I do not advise the use of an india-rubber teat until a fair trial has been given by applying the babe at once to the nipple; but if he cannot draw out the nipple, then, rather than wean him, or than employ a wet-nurse, let the teat be tried.
673. Remember, as soon as the nipple be sufficiently drawn out, which in all probability it will in a few days, the teat ought to be dispensed with. In such a case, when the infant is not at the breast, Dr. Wansbrough’s Metallic Nipple Shields should be worn. Small and bad nipples have, by the wearing of these shields, frequently been drawn out and made good ones; the dress will suffice to keep them in their places.
674. Sore nipples.—If a lady, during the latter few months of her pregnancy, were to adopt the plan recommended at page 162, paragraph 353, sore nipples, during the period of suckling, would not be so prevalent as they now are.
675. A sore nipple is frequently produced by the injudicious custom of allowing the child to have the nipple almost constantly in his mouth. Stated periods for suckling, as recommended at paragraph 631, ought to be strictly adopted. Another frequent cause of a sore nipple is from the babe having the thrush. It is a folly to attempt to cure the nipple without at the same time curing the mouth of the infant.
676. Treatment.—One of the best remedies for a sore nipple is the following powder:
Take of Biborate of Soda, one drachm; Powdered Starch, seven drachms:
Mix, A pinch of the powder to be frequently applied to the nipple.
677. Dr. A. Todd Thomson’s—my old preceptor—remedy for sore nipple is a very good one; it is as follows:
Take of Finely-powdered Gum Arabic, half an ounce; Powdered Alum, five grains:
To be well mixed together in a mortar to make a powder, of which a pinch should either be sprinkled over the nipple, or it may be applied to the part by means of a camel’s-hair brush every time directly after the child has done sucking. Let the brush, covered with the dry powder, gently sweep over the sore nipple.
As there is nothing in either of the above powders injurious to the infant, the powder, before applying him to the breast, ought not to be wiped off; indeed, either the one or the other of the powders (the former one especially, as it contains borax) is likely to be of service in preventing or in curing the sore mouth of the child.
678. If the above powders should not have the desired effect (efficacious though they usually are), “a liniment composed of equal parts of glycerin and of brandy” (say a vial containing two drachms of each) ought to be tried, which must be shaken up just before using. It should, by means of a camel’s-hair brush, every time directly after the baby has been suckled, be painted on the nipple. A piece of either old soft cambric or lawn, about the size of the palm of the hand, snipped around to make it fit, ought then to be moistened in the glycerin and the brandy, and should be applied to each of the sore nipples, and worn (until they are cured) whenever the child is not at
the breast. These applications will be found of much service and of great comfort, and will act as nipple shields, protecting and healing the nipples. A soft sponge of warm water may be gently applied to the nipple just before putting the child to the bosom.
679. If the above remedies should not succeed in curing the sore nipple, then she ought to try Dr. Wansbrough’s Metallic Nipple Shield (as recommended previously for small and drawn-in nipples), and should, whenever the babe is not sucking, constantly wear it on the nipple. It is very cooling and healing, and keeps off all pressure from the clothes. It will frequently cure a sore nipple when other remedies have failed. The shield may be procured of any respectable surgical-instrument-maker.
680. Cracked and fissured nipples.—Sometimes the nipple is sore from having either cracks or fissures upon it. These cracks or fissures may attack any part of the nipple, but are very apt to form where the nipple joins the breast; and, when very severe, an ignorant nurse, who is always fond of dealing in the marvelous, declares that the child has nearly bitten the nipple off! Treatment.—Now, the best remedy for a cracked and fissured nipple is for the infant, until the cracks and fissures are cured, to suck through the intervention of an india-rubber teat and shield; and every time, directly after the babe has been put to the nipple, to apply to the parts affected either neat brandy or the glycerin and brandy liniment, as I have before recommended. When the child is not at the breast, Dr. Wansbrough’s Nipple Shields should be worn; the dress will keep them in their places.
681. Another cause of a sore nipple is from the mother, after the babe has been sucking, putting up the nipple wet. She, therefore, ought always to dry the nipple—not by rubbing it, but by dabbing it with either a soft cambric or lawn handkerchief, or with a piece of soft linen rag (one or other of which ought always to be at hand), every time directly after the infant has done sucking, and just before applying either of the above powders or liniment to the nipple.
682. When the nipple is very sore, a mother, whenever the child is put to the bosom, suffers intense pain. This being the case, she had better suckle him through the intervention of an india-rubber teat, properly fastened on a shield, as before recommended. See page 276, paragraph 672. But she ought never to use an india-rubber teat
unless it be absolutely necessary—that is to say, if the nipple be only slightly sore, she should not, on any account, apply it; but there are cases where the nipple is so very sore that a mother would have to give up nursing if the shield and teat were not used; these, and very small and drawn-in-nipples, are the only cases in which an indiarubber teat and shield is admissible.
683. A nursing mother is sometimes annoyed by the milk flowing constantly away, making her wet and uncomfortable. All she can do under such circumstances is to wear nipple-glasses, and to apply a piece of flannel to the bosom, which will prevent the milk from chilling her, and will thus do away with the danger of her catching cold, etc.
684. The breast.—A mother ought, before applying the infant to the bosom, to carefully ascertain if there be milk. This may readily be done by squeezing the nipple between the finger and the thumb. If there be no milk, she must wait until the milk be secreted, or serious consequences both to her and to him might ensue: to the former, inflammation and gathering of the bosom, and sore nipples; to the latter, thrush, diarrhœa, and eruptions on the skin.[123]
685. If there be a supply of milk in the breast, and if still the child will not suck, the medical man’s attention ought to be drawn to the fact, in order that he may ascertain whether the child be tongue-tied; if he be, the mystery is explained, and a trifling, painless operation will soon make all right.
686. If the bosoms be full and uneasy, they ought, three or four times a day, to be well although tenderly rubbed with olive oil and eau de Cologne (equal parts of each, mixed in a vial). Some nurses rub with their fingers only. Now, such rubbing does harm. The proper way to apply friction is to pour a small quantity of the oil and eau de Cologne—first shaking the bottle—into the palm of the hand, the hand being warm, and then to well rub the breasts, taking care to use the whole of the inside of the hand.
687. After the bosoms have been well rubbed, each ought to be nicely supported with a large, soft, folded silk handkerchief; each handkerchief must pass under each breast and over the shoulders, and should be tied at the back of the neck, thus acting as a sling.
688. If the bosoms be very uncomfortable, young cabbage-leaves (with the “veins” of each leaf cut level to the leaf) ought, after each application of the oil and eau de Cologne, to be applied; or a large warm white-bread-and-milk and olive oil poultice ought to be used, which must be renewed three or four times a day. The way to make the poultice is as follows: A thick round of bread should be cut from a white loaf; the crust should be removed, the crumb ought to be cut into pieces about an inch square, upon which boiling-hot new milk should be poured; it ought to be covered over for ten minutes; then the milk should be drained off, and the olive oil—previously warmed by placing a little in a teacup on the hob—should be beaten up by means of a fork with the moistened bread until it be of the consistence of a soft poultice. It ought to be applied to the bosom as hot as it can comfortably be borne.
689. Gathered breast.—A gathered bosom, or “bad breast” as it is sometimes called, is more likely to occur after a first confinement and during the first month. Great care, therefore, ought to be taken to avoid such a misfortune. A gathered breast is frequently owing to the carelessness of a mother in not covering her bosoms during the time she is suckling. Too much attention cannot be paid to keeping the breasts comfortably warm. This, during the act of nursing, should be done by throwing either a shawl or a square of flannel over the neck, shoulders, and bosoms.
690. Another cause of a gathered breast arises from a mother sitting up in bed to suckle her baby. An infant ought to be accustomed to take the bosom while he is lying down; if this habit be not at first instituted, it will be difficult to adopt it afterward. Good habits may be taught a child from the very earliest period of his existence.
691. A sore nipple is another fruitful cause of a gathered breast. A mother, in consequence of the suffering it produces, dreads putting the baby to it; she therefore keeps him almost entirely to the other bosom. The result is, the breast with the sore nipple becomes distended with milk, which, being unrelieved, ends in inflammation, and subsequently in gathering.
692. The fruitless attempt of an infant, to procure milk when there is very little or none secreted is another and a frequent cause of a gathered bosom. Dr. Ballard, in his valuable little work before




















