Visit to download the full and correct content document: https://textbookfull.com/product/actin-cytoskeleton-in-cancer-progression-and-metast asis-part-c-1st-edition-clement-thomas/

More products digital (pdf, epub, mobi) instant download maybe you interests ...

Breast Cancer Metastasis and Drug Resistance Progress and Prospects 2013th Edition Springer
https://textbookfull.com/product/breast-cancer-metastasis-anddrug-resistance-progress-and-prospects-2013th-edition-springer/

Biota Grow 2C gather 2C cook Loucas
https://textbookfull.com/product/biota-grow-2c-gather-2c-cookloucas/

Cancer and Vitamin C 1st Edition Qi Chen (Editor)
https://textbookfull.com/product/cancer-and-vitamin-c-1stedition-qi-chen-editor/

Cancer and the New Biology of Water Thomas Cowan
https://textbookfull.com/product/cancer-and-the-new-biology-ofwater-thomas-cowan/

String Algorithms in C: Efficient Text Representation and Search 1st Edition Thomas Mailund
https://textbookfull.com/product/string-algorithms-in-cefficient-text-representation-and-search-1st-edition-thomasmailund/




The neuronal cytoskeleton, motor proteins, and organelle trafficking in the axon First Edition Pfister
https://textbookfull.com/product/the-neuronal-cytoskeleton-motorproteins-and-organelle-trafficking-in-the-axon-first-editionpfister/
Theme and Thematic Progression in Chinese College Students English Essays 1st Edition Jing Wei (Auth.)
https://textbookfull.com/product/theme-and-thematic-progressionin-chinese-college-students-english-essays-1st-edition-jing-weiauth/
Soulbound 3 A Progression Fantasy Adventure 1st Edition
Cassius
Lange
https://textbookfull.com/product/soulbound-3-a-progressionfantasy-adventure-1st-edition-cassius-lange/
Notch Signaling in Embryology and Cancer: Notch Signaling in Cancer Jörg Reichrath
https://textbookfull.com/product/notch-signaling-in-embryologyand-cancer-notch-signaling-in-cancer-jorg-reichrath/

ActinCytoskeletoninCancer ProgressionandMetastasis – PartC
INTERNATIONALREVIEWOFCELLAND MOLECULARBIOLOGY
SeriesEditors
GEOFFREYH.BOURNE 1949–1988
JAMESF.DANIELLI 1949–1984
KWANGW.JEON 1967–2016
MARTINFRIEDLANDER 1984–1992
JONATHANJARVIK 1993–1995
LORENZOGALLUZZI 2016–
EditorialAdvisoryBoard
AARONCIECHANOVERWALLACEMARSHALL SANDRADEMARIASHIGEKAZUNAGATA SILVIAFINNEMANNMOSHEOREN
KWANGJEONANNESIMONSEN
CARLOSLOPEZ-OTIN
INTERNATIONAL REVIEWOF CELLANDMOLECULAR BIOLOGY
ActinCytoskeletoninCancer ProgressionandMetastasis – PartC
Editedby CLEMENTTHOMAS
LuxembourgInstituteofHealth, LuxembourgCity,Luxembourg
LORENZOGALLUZZI
WeillCornellMedicalCollege, NewYork,NY,UnitedStates
AcademicPressisanimprintofElsevier
50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates 525BStreet,Suite1650,SanDiego,CA92101,UnitedStates TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 125LondonWall,London,EC2Y5AS,UnitedKingdom
Firstedition2021
Copyright©2021ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronic ormechanical,includingphotocopying,recording,oranyinformationstorageandretrievalsystem, withoutpermissioninwritingfromthepublisher.Detailsonhowtoseekpermission,further informationaboutthePublisher'spermissionspoliciesandourarrangementswithorganizationssuch astheCopyrightClearanceCenterandtheCopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions.
Thisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythe Publisher(otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperience broadenourunderstanding,changesinresearchmethods,professionalpractices,ormedical treatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluating andusinganyinformation,methods,compounds,orexperimentsdescribedherein.Inusingsuch informationormethodstheyshouldbemindfuloftheirownsafetyandthesafetyofothers,including partiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assume anyliabilityforanyinjuryand/ordamagetopersonsorpropertyasamatterofproductsliability, negligenceorotherwise,orfromanyuseoroperationofanymethods,products,instructions,orideas containedinthematerialherein.
ISBN:978-0-12-824138-7
ISSN:1937-6448
ForinformationonallAcademicPresspublications visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: ZoeKruze
AcquisitionsEditor: AshlieM.Jackman
DevelopmentalEditor: TaraNadera
ProductionProjectManager: DennyMansingh
CoverDesigner: AlanStudholme
TypesetbySPiGlobal,India
1.ForcebalancingACT-INthetumormicroenvironment: Cytoskeletalmodificationsincancerandstromalcellstopromote malignancy1
MichelleR.Dawson,BotaiXuan,JeffreyHsu,andDeeprajGhosh
1. Overview2
2. Actincytoskeleton4
3. Measuringintracellularandextracellularforces5
4. Utilizingforcemeasurementstodistinguishnon-invasiveandinvasive cancercells11
5. Utilizingforcemeasurementstostudytumorandstromalcellcrosstalk19
6. Conclusions23
7. Experimentalchallengesandfutureresearchefforts24 References24
2.Novelfacetsofgliomainvasion33
CarinaFabian,MingzhiHan,RolfBjerkvig,andSimoneP.Niclou
1. Introduction34
2. Routesofgliomacellinvasion36
3. Modesofgliomacellinvasion38
4. Theimpactoftumormetabolismoncellinvasion40
5. Theextracellularmatrixofthegliomamicroenvironment41
6. Involvementofproteasesandthetumormicroenvironmentinglioma cellinvasion42
7. Theactincytoskeletonanditsrelatedmembraneprotrusionsinglioma cellinvasion43
8. Cangliomacellinvasionandtheactincytoskeletonbetargeted?53
9. Conclusionsandfutureprospects56 Acknowledgments56 References56
3.Actindynamicsduringtumorcelldissemination65
ChandraniMondal,JulieS.DiMartino,andJoseJavierBravo-Cordero
1. Introduction66
2. Tumordisseminationandmetastasis68
3. Cancercellmigration71
4. Actinstructuresincancercellmigration77
5. Thecellcycleandcancercellinvasion83
6. Imagingadvancesandfuturedirectionsinstudyingtumorcellinvasion83
7. Conclusion86
Acknowledgments87 References87
4.Themultiplerolesofactin-bindingproteinsatinvadopodia99
TakouhieMgrditchian,GabrieleSakalauskaite,TanjaMuller, CelineHoffmann,andClementThomas
1. Introduction100
2. Invadopodialactinassemblies103
3. Actinmachineriesatthecellleadingedge105
4. Actin-bindingproteinsininvadopodiamorphogenesis110
5. Actinpolymerization-basedprotrusionatinvadopodia116
6. Concludingremarks118 Acknowledgments119 References119
5.Cancertype-specificalterationsinactingenes: Worthacloserlook?133
ChristopheAmpe,LauraWitjes,andMarleenVanTroys
1. Introduction134
2. Thehumanactingene/proteinfamily:Ataleoffunctionalredundancy anddistinction136
3. Actin:Basicstructure-functionrelationshipsasacontextforinterpreting actinmutationsincancer139
4. Actingenealterationsandactinmutants:Dotheyoccurincancer?144
5. Alterationsinactingenesinpatientcancergenomes:Anuntappedresource148
6. Summary,conclusions,andperspectives170 References173
Contributors
ChristopheAmpe
DepartmentofBiomolecularMedicine,GhentUniversity,Gent,Belgium
RolfBjerkvig
NORLUXNeuro-OncologyLaboratory,DepartmentofOncology,LuxembourgInstitute ofHealth,Luxembourg,Luxembourg;DepartmentofBiomedicine,UniversityofBergen, Bergen,Norway
JoseJavierBravo-Cordero
DepartmentofMedicine,DivisionofHematologyandOncology,TheTischCancer Institute,IcahnSchoolofMedicineatMountSinai,NewYork,NY,UnitedStates
MichelleR.Dawson
DepartmentofMolecularPharmacology,Physiology,andBiotechnology;Departmentof MolecularBiology,CellBiologyandBiochemistry,BrownUniversity;BrownUniversity, CenterforBiomedicalEngineering,Providence,RI,UnitedStates
CarinaFabian
NORLUXNeuro-OncologyLaboratory,DepartmentofOncology,LuxembourgInstitute ofHealth,Luxembourg,Luxembourg;DepartmentofBiomedicine,UniversityofBergen, Bergen,Norway
DeeprajGhosh
DepartmentofMolecularPharmacology,Physiology,andBiotechnology,Brown University,Providence,RI,UnitedStates
MingzhiHan
DepartmentofBiomedicine,UniversityofBergen,Bergen,Norway;Departmentof Neurosurgery,QiluHospitalofShandongUniversityandInstituteofBrainand Brain-InspiredScience,ShandongUniversity;ShandongKeyLaboratoryofBrainFunction Remodeling,Jinan,China
CelineHoffmann
CytoskeletonandCancerProgression,DepartmentofOncology,LuxembourgInstituteof Health,LuxembourgCity,Luxembourg
JeffreyHsu
DepartmentofMolecularPharmacology,Physiology,andBiotechnology,Brown University,Providence,RI,UnitedStates
TakouhieMgrditchian
CytoskeletonandCancerProgression,DepartmentofOncology,LuxembourgInstituteof Health,LuxembourgCity,Luxembourg
ChandraniMondal
DepartmentofMedicine,DivisionofHematologyandOncology,TheTischCancer Institute,IcahnSchoolofMedicineatMountSinai,NewYork,NY,UnitedStates
TanjaMuller
DepartmentofOncology,LuxembourgCentreofNeuropathology,LuxembourgInstitute ofHealth,LuxembourgCity,Luxembourg
SimoneP.Niclou
NORLUXNeuro-OncologyLaboratory,DepartmentofOncology,LuxembourgInstitute ofHealth,Luxembourg,Luxembourg;DepartmentofBiomedicine,UniversityofBergen, Bergen,Norway
JulieS.DiMartino
DepartmentofMedicine,DivisionofHematologyandOncology,TheTischCancer Institute,IcahnSchoolofMedicineatMountSinai,NewYork,NY,UnitedStates
GabrieleSakalauskaite
CytoskeletonandCancerProgression,DepartmentofOncology,LuxembourgInstituteof Health,LuxembourgCity,Luxembourg
ClementThomas
CytoskeletonandCancerProgression,DepartmentofOncology,LuxembourgInstituteof Health,LuxembourgCity,Luxembourg
MarleenVanTroys
DepartmentofBiomolecularMedicine,GhentUniversity,Gent,Belgium
LauraWitjes
DepartmentofBiomolecularMedicine,GhentUniversity,Gent,Belgium
BotaiXuan
DepartmentofMolecularPharmacology,Physiology,andBiotechnology,Brown University,Providence,RI,UnitedStates
ForcebalancingACT-INthetumor microenvironment:Cytoskeletal modificationsincancerand stromalcellstopromote malignancy
MichelleR.Dawsona,b,c,∗,BotaiXuana,JeffreyHsua, andDeeprajGhosha
aDepartmentofMolecularPharmacology,Physiology,andBiotechnology,BrownUniversity,Providence,RI, UnitedStates
bDepartmentofMolecularBiology,CellBiologyandBiochemistry,BrownUniversity,Providence,RI, UnitedStates
cBrownUniversity,CenterforBiomedicalEngineering,Providence,RI,UnitedStates
∗Correspondingauthor:e-mailaddress:michelle_dawson@brown.edu
Contents
1. Overview2
2. Actincytoskeleton4
3. Measuringintracellularandextracellularforces5
3.1 Intracellularparticletrackingmicrorheology6
3.2 Tractionforcemicroscopy9
4. Utilizingforcemeasurementstodistinguishnon-invasiveandinvasive cancercells11
4.1 GeneticallyinducedEMTmakescancercellsmoredeformable11
4.2 Invasivecancercellsexertincreasedandpolarizedtractionforcesina contextdependentmanner14
4.3 Rho-ROCKsignalingregulatesdistinctmechanicalresponseofdiffering cancercelltypes16
4.4 Utilizingforceprofilestocharacterizechemoresistantsubpopulations18
5. Utilizingforcemeasurementstostudytumorandstromalcellcrosstalk19
5.1 Cancercellinvasivenessdeterminedirectintercellularinteractionwith stromalcells20
5.2 MSCsandcancercellsundergodramaticchangesincellmechanicsin responsetoSFcrosstalk22
6. Conclusions23
7. Experimentalchallengesandfutureresearchefforts24 References24 InternationalReviewofCellandMolecularBiology,Volume360Copyright # 2021ElsevierInc. ISSN1937-6448Allrightsreserved. https://doi.org/10.1016/bs.ircmb.2020.09.005
Abstract
Thetumormicroenvironmentisacomplexmilieuthatdictatesthegrowth,invasion, andmetastasisofcancercells.Bothcancerandstromalcellsinthetumortissueencounterandadapttoavarietyofextracellularfactors,andsubsequentlycontributeanddrive theprogressionofthediseasetomoreadvancedstages.Asthediseaseprogresses,a smallpopulationofcancercellsbecomesmoreinvasivethroughacomplexprocess knownasepithelial-mesenchymaltransition,andnearbystromalcellsassumeacarcinomaassociatedfibroblastphenotypecharacterizedbyenhancedmigration,cellcontractility,andmatrixsecretionwiththeabilitytoreorganizeextracellularmatrices.As cellstransitionintomoremalignantphenotypestheirbiophysicalproperties,controlled bytheorganizationofcytoskeletalproteins,arealtered.Actinanditsassociatedproteins areessentialmodulatorsandfacilitatorsofthesechanges.Asthecellsrespondtothe cuesinthemicroenvironment,actindrivenmechanicalforcesinsideandoutsidethe cellsalsoevolve.Recentadvancesinbiophysicaltechniqueshaveenabledustoprobe theseactindrivenchangesincancerandstromalcellsanddemarcatetheirroleindrivingchangesinthemicroenvironment.Understandingtheunderlyingbiophysical mechanismsthatdrivecancerprogressioncouldprovidecriticalinsightonnoveltherapeuticapproachesinthefightagainstcancer.
1.Overview
Thedynamicprogressionofthetumormicroenvironment(TME) requirestheparticipationofawidevarietyofcelltypes,facilitatingacomplex networkofchemicalandphysicalcrosstalk(Balkwilletal.,2012; Fukumura andJain,2007; QuailandJoyce,2013; StrokaandKonstantopoulos,2014; Whiteside,2008).Amultitudeofcelltypesarerecruitedtothetumorunder theinfluenceoftumorsecretedgrowthfactorsandchemokines;thisincludes immunecells,endothelialcells,mesenchymalstemcells(MSCs),andfibroblaststhatplayimportantrolesintumorgrowthbymodulatingtheimmune response,promotingangiogenesis,andformingthestroma.Thesecells crosstalkwithcancercellsthroughdirectcellcontactsandparacrinesignaling, inordertorestructuretheTMEtoonethatispermissiblefortumorgrowth andmetastasis.IncreasedunderstandingofthemoleculesintheTMEandtheir interactionswithcancercellsmaybecriticalinidentifyingnoveltargetsfor therapeuticintervention.
AlongwithselectiveinfluenceswithintheTME,suchasextracellular matrix(ECM)remodelingandabnormalvascularization,cancercellswithin thetumordeveloptranscriptionallyandphenotypicallyheterogeneoussubcloneswithdifferentlevelsofmalignancy(Marusyketal.,2012; Meacham andMorrison,2013).Althoughrecenttechnologicaladvancementsin
deep-sequencingatthesingle-celllevelhaveallowedresearcherstoobtain preliminaryinsightsintocancerheterogeneity(Pateletal.,2014; Tirosh etal.,2016),morestudiesareneededtoprobehowthisintratumorheterogeneityaffectsthedevelopmentofchemoresistantsubpopulations,cancer recurrenceaftertherapy,andcancermetastasis(MeachamandMorrison, 2013; Sharmaetal.,2010).Heterogeneityincancercellphenotypesis thoughttorelyontheinherentvariationintherateofstochasticmutations (Lawsonetal.,2018).Asgenomicinstabilityincreases,thecellcycle becomesmoreabnormalandcancercellswithdiversemalignantcharacteristicsbegintoform,includingcellswithhighmetastaticpotential(Burrell etal.,2013; CifoneandFidler,1981; Joungetal.,2017).However,cancer therapyalsocontributestointratumorheterogeneity.Single-cellsequencing analysisdemonstratedthatthereweredistinctsubpopulationsofcancercells withdifferentgenomicandtranscriptomicprofilesinPaclitaxeltreatedcells/ tumorscomparedtocontrol(D’Alterioetal.,2020; Leeetal.,2014).TME itselfcouldalsoconferselectivepressurestocancercells.Forinstance, stromalcell-secretedgrowthfactorsandcytokineshavebeenfoundtoprofoundlyinfluencephenotypicdevelopmentsincancercellsbypromoting epithelialtomesenchymaltransition(EMT)(PolyakandWeinberg, 2009).InconjunctionwithECMremodeling(changesinmatrixrigidity), environmentalpressurescouldlargelyaltertranscriptomicandproteomic properties,andsubsequently,thephenotypicandbiophysicalproperties ofcancercells(Emonetal.,2018; Hanahanetal.,2011; Luetal.,2012; Spilletal.,2016).Lastly,histologystudieshaveconsistentlyreportedspatial heterogeneityinthetumorarchitecture,whichcouldhaveprofound implicationsoncancerbiophysics(Ramo ´ nyCajaletal.,2020).Thisheterogeneityincludesregionaldifferencesinthecollagenarchitecture,levelof vascularization,stromalcellincorporation,andcancercellphenotype (Malandrinoetal.,2018; Yamauchietal.,2020).Forinstance,someregions ofthetumormayhavecollagen-richbasementmembrane;whereas,other regionshaveverylittlecollagen(Caseetal.,2017; Conklinetal.,2011). Also,someregionsofthetumorarehighlyvascularized;whereas,other regionsarehypoxic(Fukumuraetal.,2010; Petrovaetal.,2018).Cellsnear thecollagen-richbasementmembranemaybemoremigratory;whereas, cellsatthetumorcoremaybeundersolidstress,whichdrivesthecollective migrationofsurroundingcells(Tseetal.,2012).Takentogether,canceris ahighlyheterogeneousdiseasethatrequiresadditionalcharacterization, particularlythroughthelensofcancerbiophysics,tobetterelucidatedrivers ofphenotypicheterogeneityinthetumor.
Thisreviewwillhighlightrecentworkfromourlabandotherlabsdemonstratingtheimportanceofbiophysicalpropertiesinidentifyingaggressive cellpopulations.Additionally,wewillshowhowchemicalandphysicalcues fromtheTMEaltercellshapeandcytoskeletalorganizationtodynamically affectcellfunctionandcell-cellinteractions.ThecomplexityoftheTMEis amajorbarrierinunderstandingthemolecularandmechanicalinteractions ofcellsinthetumor.Quantitativebiophysicalanalysisallowsustoprobethe biomechanicalpropertiesofcellswithanunprecedentedlevelofdetailto enhanceourunderstandingofcancer.
2.Actincytoskeleton
Cytoskeletalproteinsmechanicallysupportthecellstructureandspatiallyorganizethecontentsofthecell(FletcherandMullins,2010).This groupoffilamentousproteinsiscategorizedintothreemainfamilies:actin microfilaments,microtubules,andintermediatefilaments.Whilemicrotubulesandintermediatefilamentscontributesignificantlytotheorganizationalintegrityofcells,actinanditsassociatedproteinsenablecellsto respondandadapttodynamicchangesinthemicroenvironment.ThehierarchicalstructureoftheactinnetworkiscontrolledbysmallRhoGTPases, myosinmotorproteins,andalargegroupofcytoplasmicmediatorsknown asactinbindingproteins(ABPs)(illustratedin Fig.1)(Hall,1998; Parsons etal.,2010; WinderandAyscough,2005).Dynamicchangesintheorganizationofthecytoskeletontransformcellshapeandgeneratemechanical forcesrequiredfornumerouscellularprocesses,includingadhesion,migration,division,moleculartransport,anddifferentiation(DuFortetal.,2011; Eyckmansetal.,2011; Humphreyetal.,2014; Iskratschetal.,2014).The cytoskeletalnetworkrespondsdynamicallytosolubleormechanicalcues fromthetumorECMandisconnecteddirectlytocanonicalsignaltransductionpathwaysimportantincancer(Chinetal.,2016; HuangandIngber, 2005; Shieh,2011; StrokaandKonstantopoulos,2014).Thefamilyof RhoGTPasesandABPshavebeenstronglyimplicatedinmultiplestages ofcancerprogressiontometastasis(SahaiandMarshall,2002; Stevenson etal.,2012; SUNetal.,2015; VegaandRidley,2008).Forexample, Arp2/3—aproteinfacilitatingactinbranchformation,isoverexpressedin malignanttumors,suchasbreastcarcinomas(MolinieandGautreau, 2018).AnotheractinbindingproteinFilaminthatcrosslinksactinbundles andprovidemechanicalstrengthhasbeendetectedinthebloodfrommetastaticbreastcancerpatients(Yueetal.,2013).
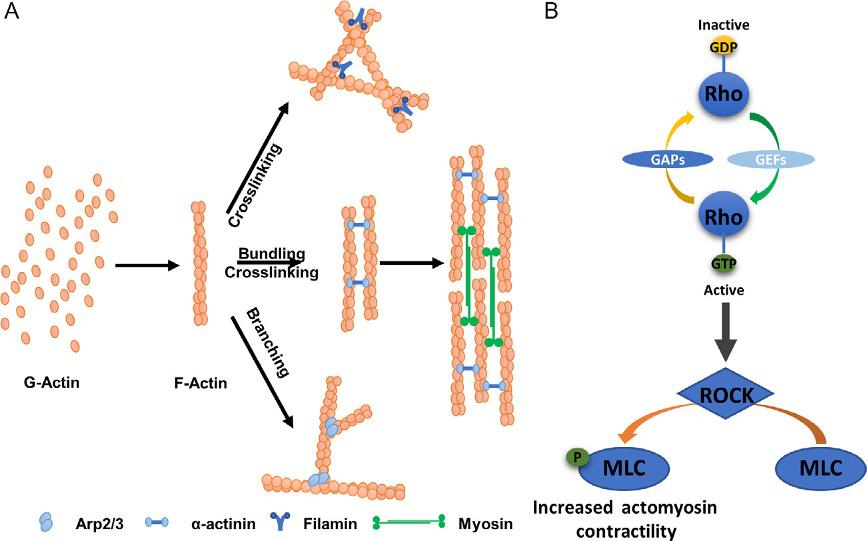
Fig.1 (A)Globularactin(G-actin)subunitspolymerizetoformfilamentousactinstructure(F-Actin),whichcanbeorganizedintomultitudeofnetworkstosupportlocation specificfunctionincells.Actinbindingproteins(ABPs)facilitatetheassemblyofF-Actin intovariousformsincludingbundling,crosslinking,andbranchingexamplesshown here.Additionally,myosinmotorproteinscanbindbetweentwoadjacentbundlesof crosslinkedactinstructurestogeneratecontractileactinstressfibers.(B)Thefamily ofsmallRho-GTPasessuchasRhoAisconvertedfromaGDP-boundinactiveformto GTP-boundactiveformbyguaninenucleotideexchangefactors(GEFs)andthereverse processofinactivationismediatedbyGTPase-activatingproteins(GAPs).Activationof RhoGTPasesleadstoactivationofROCKwhichcantriggermultitudeofdownstream cytoskeletalreorganizationprocessesincludingblockingmyosinlightchain(MLC) phosphataseactivityandfacilitateMLCphosphorylationleadingtoincreasedactomyosincontractility.
3.Measuringintracellularandextracellularforces
Agrowingbodyofevidencehasemergedhighlightingtheimportance ofmechanicalcuesinbothnormaltissuedevelopmentandcancer(DuFort etal.,2011; KumarandWeaver,2009).Despitehighlydivergentchemical signalingcascades,ahighlyconservedfeatureofmechanicalsignalingisthat itrequirestransmissionofforcefromtheECMtotheinternalcytoskeleton, whichformsthestructureofthecell.Forcesfromtheexternalenvironment activateRho/Rhoassociatedproteinkinases(ROCK)signalingpathways thatregulatetheactincytoskeletonandcytoskeletaltension.Upregulation ofROCK,whichincreasesactomyosincontractility,resultsintissuestiffening
andmalignanttransformation.Actinbundling(e.g., α-actinin,fascin)and crosslinking(e.g.,filamin)proteinsgiverisetoactinstressfibersthatlink thecytoskeletontofocaladhesionsandactinnetworksthatmodulateintracellularstiffness(Hall,1998; WinderandAyscough,2005).Sincecytoskeletal alterationsdependonthemechanicalenvironmentsandviceversa,itisnecessarytousebiophysicaltoolstoprobeessentialforcesatboththeintracellular andextracellularlevels.
3.1Intracellularparticletrackingmicrorheology
Cytoskeletalactinformsamesh-likestructureinthecellcytoplasmthatregulatestheintracellulartension(FletcherandMullins,2010; Haleetal., 2009).Parallelactinbundlesprovidetensilestrengthandstrongcontractile activity,whereascrosslinkedbundlesofactinfilamentsincreaseintracellular elasticity.Dependingonthelocationinthecell,actinarchitecturecanvary drasticallyandmanifestheterogeneouslocalmechanicalproperties(Tseng etal.,2002).Tomeasurecellmechanicalproperties,multipletechniques havebeendevelopedovertheyears,including,atomicforcemicroscopy (AFM),magneticbeadtwist,opticaltweezers,micropipetteaspiration,hydrodynamicstretchingandparticle-trackingmicrorheology(Kollmannsberger andFabry,2011; MoeendarbaryandHarris,2014).Intracellularparticletrackingmicrorheology(IPTM)allowsdirectandrapidmeasurementofthelocal microrheologicalpropertiesthroughoutthecell(CrockerandHoffman, 2007; Dawsonetal.,2014; Lietal.,2009; Wirtz,2009).Briefly,fluorescent particlesareballisticallyinjectedintothecellandtheirthermalenergydriven movementscapturedatahighmagnificationwithahigh-speedcamerato obtaininformationaboutthelocalpolymericnetwork.The2DBrownian motionofthesesubmicronprobeparticlesisthenusedtocalculateparticle meansquaredisplacements(MSDs).MSDsofparticlesmovinginaviscous liquidvarylinearly(slope 1)withtimescale.However,forviscoelastic fluids,themotionoftheembeddedparticlesbecomesmorerestricteddue tothepresenceofmesh-likestructures.Becauseofthesub-diffusiverestricted motionofparticles,thetime-dependentMSDcurvesflatten(slope ≪ 1).Ina viscousliquid,diffusivityduetothermalenergydrivenmotioncanbe describedusingtheStokes-EinsteinEq. (1),where D isthediffusioncoefficient, kB isBoltzmann’sconstant, T istemperature, a isparticleradius,and η is thefluidviscosity.
Todescribeviscoelasticpropertiesofcomplexfluids,Masonetal.derived complexshearmodulusoftheviscoelasticfluidusingamodifiedStokesEinsteinequationinthefrequencydomain(Eq. 2),where G∗ isthe frequency-dependentcomplexshearmodulusand Γ isthegammafunction. Thein-phasecomponentofthecomplexshearmodulus(G*)isknownas theelasticmodulus(G0 ),andout-of-phasecomponentisknownastheviscousmodulus(G00 )(Eq. 3).
TheIPTMapproachisillustratedforanalyzingMDA-MB-231breastcancer cellsin Fig.2.Atlowertimescales,particletransportinthecytosolremains restrictedandMSDvariesalmostindependentoftimescale(α ≪ 1);whereas atlongertimescales,asthestructuresaroundtheparticlesbegintorelax, particlesareabletomovelongerdistances,andMSDsvarymorelinearly (α 1).Forthemorerestrictedtransportregimeofembeddedparticles,cells typicallyresembleaviscoelasticfluidwithcomparablemagnitudesofboth viscousandelasticmoduli.Atlinearregimeofparticlemotion,cellsproperties areverysimilartoaviscousliquidwithahighlydominantviscousmodulus. IndividuallocationspecificparticleMSDscanbeusedtocalculatelocalviscoelasticproperties;whereas,allMSDsfromacellcanbeensemble-averagedto evaluateoverallviscoelasticbehavior.Particletrackingmicrorheology(PTM) hasbeensuccessfullyadaptedtocharacterizeagreatrangeofcomplexbiological fluids,includingmucus,reconstitutedactinsolutions,andthecellcytoplasm (Dawsonetal.,2014; Masonetal.,1997; Wirtz,2009).
AlthoughIPTMisprimarilyconductedon2-Dcultures,theapplication ofthismethodin3Dandinvivohasbeeninvestigated(Bakeretal.,2010; Danielsetal.,2006; Panorchanetal.,2006; Zhouetal.,2008).IPTMdoes notrequireanexternalprobeunlikeothertechniqueslikeAFM,thusprovidesanadvantageintrackingcellmechanicsin3D.Panorchanetal. embeddedhumanumbilicalveinendothelialcellsballisticallyinjectedwith 100nmfluorescentparticlesin3Dpeptidehydrogelsandmonitoredchanges
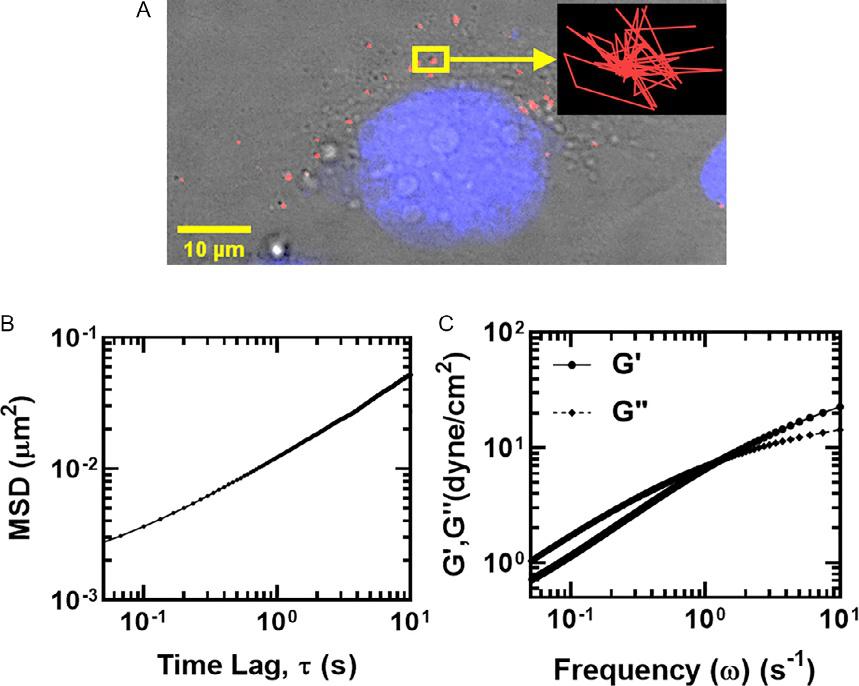
Fig.2 Illustrationofintracellularparticletrackingmicrorheology(IPTM).(A)Fluorescent submicron(200nm)probeparticleswereinjectedintoMDA-MB-231breastcancercells usingPDS-100ballisticparticleinjectionsystem.TherepresentativetraceofasingleparticleundergoingBrownianmotioninthecellcytoplasmisshownintheinset.(B)Thex-y displacementsofparticlesareusedtocalculateensembleaverageMSDs.(C)Frequencydependentviscous(G0 )andelastic(G00 )moduliarethencalculatedfromtheMSDsas describedby Masonetal.(1997) AdaptedfromDawson,M.R.,Tseng,Y.,Lee,J.S.H., McAndrews,K.M.,2014.Intracellularparticletrackingmicrorheology,In:Handbook ofImaginginBiologicalMechanics.CRCPress,pp.381–388.https://doi.org/10.1201/ b17566-40.
incellmechanicsafterstimulationwithvascularendothelialgrowthfactor (VEGF).ExposuretoVEGFledtosofteningofthecytoplasmhighlighted bysignificantreductionintheelasticmodulus(Panorchanetal.,2006). Otherstudiesin3Dhaveusedmicrobeadsandnanotubesorendogenous organellestotrackchangesincellmechanicsinmorephysiologicallyrelevantmicroenvironment.Wuetal.recentlydemonstratedthecombination ofIPTMandintravitalimagingtomeasurebiophysicalparametersoflive cellsinmice(Wuetal.,2020).Inshort,200nmfluorescentparticleswere ballisticallyinjectedintoEGFP-labeledMDA-MB-231breastcancercells.
Thecellswerethenimplantedinmiceusingthedorsalskinfoldchamber windowandexaminedusingintravitalfluorescentmicroscopy.Similarto 2-DIPTM,thethermalmotionofparticlesthatwereembeddedinthe GFP-labeledcellswerecapturedandusedtodeterminecellmicrorheology. Usingthismethod,invivocellbiophysicalpropertiescanbemoreaccuratelycapturedalongwitheffectsofthesurroundingtissuemicroenvironment.However,therewereseverallimitationstothismethod.First,a stringentcorrectionwasneededforanimalmovement,astherhythmic breathingmotionwasseveralmagnitudeshigherthanthetrackedparticle motion.Secondly,invivoimagingrequiresincreasedworkingdistances toseedeepintothetissue,limitingspatialandtemporalresolution,which arecriticalinIPTM.Similarresolutionlimitsmayapplywhenusing IPTMtodeterminecellmicrorheologyin3-Dgels.
3.2Tractionforcemicroscopy
PhysicalinteractionsbetweencellsandthesurroundingECMregulatethe reciprocalforcesviaadhesionmoleculeslinkingthecellcytoskeletontothe ECM.Themagnitudeoftractionforcesgeneratedattheseadhesionsites, alongwiththestrengthofadhesions,arecriticalinregulatingcellprocesses. Tractionforceshavebeenquantifiedinboth2Dand3Denvironments (Kochetal.,2012; Kraning-Rushetal.,2011; McGrailetal.,2015b; Munevaretal.,2001; Sabassetal.,2008).Studieson2Delasticsubstrates formedfromsyntheticmaterials,includingsilicon,polyacrylamide,andpolydimethylsiloxaneallowforcell-generatedforcemeasurementsonawide rangeofstiffnesses.Tractionforcemicroscopy(TFM)hasbeencombined withothertechniquesforsimultaneouscharacterizationofcelladhesion machineryusingtotalinternalreflectionmicroscopy(TIRF)orintracellular rheologyusingIPTM(Gutierrezetal.,2011; McAndrewsetal.,2014).To mimicmorephysiologicallyrelevantmicroenvironments,TFMhasbeen performedin3Dhydrogelsandcollagenmatrices(Legantetal.,2010, 2013; Steinwachsetal.,2016).Duetonon-linearelasticpropertiesof collagenquantitativeanalysisoftractionforcesislimited/notpossible,but particledisplacementsarestillusefulinunderstandingcollagenmatrixdeformations.Inelastichydrogels,thealgorithmtoderivecell-generatedforcesis extremelycomplex,limitingtheusefulnessofthisapproach(Legantetal., 2013).Amoredetailedreviewonthecurrent2Dand3DTFMtechniques andtheirlimitationscanbefoundhere(Choetal.,2016; Huretal.,2020).
2Dtractionforcesarecharacterizedforcellsculturedonpolyacrylamide substrateswithrigiditiestunedtomimicspecificbiologicaltissues(Kim etal.,2009; Nergeretal.,2017; Plotnikovetal.,2014).Fluorescent nanoparticlesembeddedinthesubstratesaredisplacedundercell-exerted stress.Whencellsareremoved,theparticlesreverttotheirunstressed locations.Thus,cell-induceddisplacementsfromstressedandunstressed particleimagescanbeusedintractionforcecalculations(illustratedin Fig.3).InBoussinesqtheory,thedisplacementfieldu * ofanelastic substrateiscorrelatedtothetractionfieldTðÞ * (Eq. 4),whereGisthe Green’sfunction(Munevaretal.,2001; Sabassetal.,2008).
TheestimationoftheGreen’sfunctioniscriticalforinversecalculationof thetractionfield(Eq. 5).TheGreen’sfunctionincludesdisplacementvector r ¼ x * x *0 components(rx, ry),theYoung’smodulusE,andthePoisson ratio υ
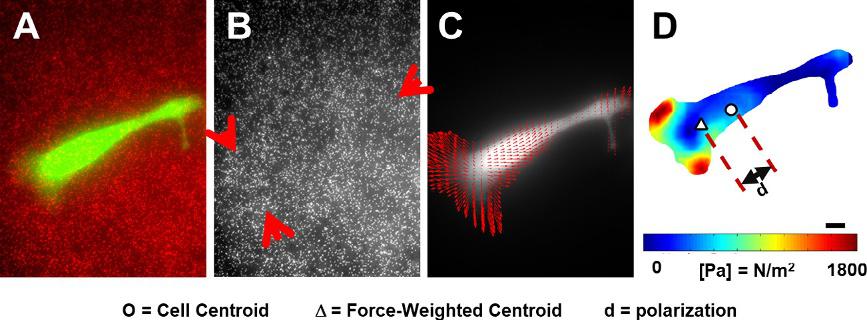
Fig.3 Tractionforcemicroscopy(TFM).(A–B)Fluorescentlylabeledcells(SKOV-3epithelialovariancancercellsshowningreen)wereculturedoncollagen-coatedpolyacrylamidesubstratesembeddedwithfluorescentredparticles(200nm).Imagesofthe embeddednanoparticlesweretakenbefore(A)andafter(B)detachingthecells.Red arrowspointtothezoneswithhighdisplacements.(C–D)Previousimageswereused tocalculatedisplacementvectorsforeachparticleandfollowedbytheestimationof tractionforcefieldandpolarization.(D)Heatmapoftractionforcesisoverlaidwithsymbolsindicatingthecell’scenterofmass( )andforce-weightedcenterofmass(Δ). AdaptedfromMcgrail,D.J.,2015.Mechanics&Malignancy:PhysicalCuesAndChanges ThatDriveTumorProgression.
Furthermore,polarizationiscalculatedasthedifferenceinun-weighted centerofmassofthecellandthetractionforce-weightedcenterofmass ofthecell(Eq. 6),where Miunwt istheun-weightedcenterofmassand Miwt istheweightedcenterofmass.
Theaforementionedtechniquesaretwoofthemostcommonlyused methodstomeasureforcesinsideandoutsideoflivingcells.However,other techniquesexistandcouldbeusefulincollectingsimilarmeasurements.Our chapterfocusesonhowourlabhascombinedthetwomethodswepreviouslydescribedwithcellfateanalysistounderstandcellbehaviorintumor andtissuemicroenvironments.
4.Utilizingforcemeasurementstodistinguish
non-invasiveandinvasivecancercells
Thetransformationofcancercellstohighlyinvasivephenotypes allowscellstodistorttheirshapeandgenerateforcestonavigatethrough densestroma.EpithelialcancercellsundergoingEMTlosesomeepithelial characteristics,includingreducedexpressionofcadherinsresponsiblefor cell-celljunctionsandincreasedexpressionofECMbindingintegrins importantincell-ECMadhesion(KalluriandWeinberg,2009).Invasive andmigratorypropertiesacquiredthroughEMTareprerequisitesformetastasis;thus,itisimperativetoidentifyorevenpredictwhichcancercells undergoEMT.Usingbiophysicalapproachestointerrogateactincytoskeletalmodificationsincancercells,wepreviouslyexaminedthephenotypeof cancercellsundergoingEMT.
4.1GeneticallyinducedEMTmakescancercellsmore deformable
ThebreastcancercelllineMCF7wasmodifiedtoconstitutivelyexpressSnail, azinc-fingertranscriptionfactorthattriggersEMTbysuppressing E-Cadherin expression.Whilecellstransformedwithanemptyvector(MCF7-NEO)
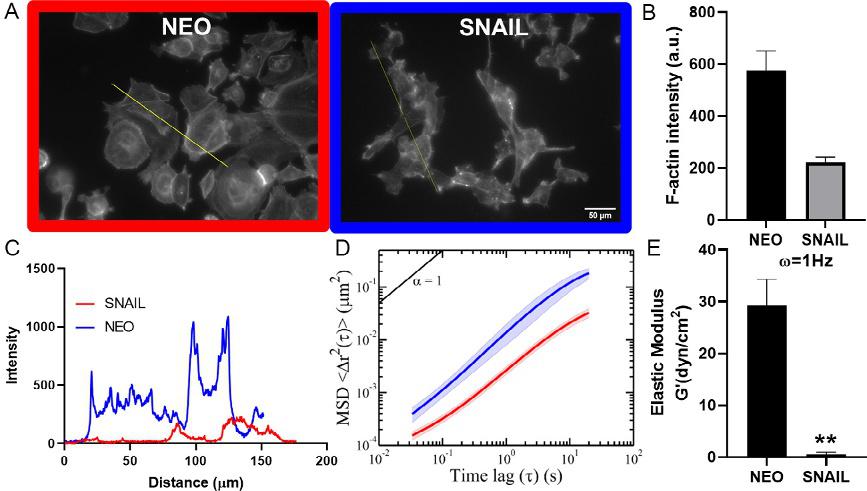
Fig.4 SNAIL-inducedEMTalterscytoskeletalandmechanicalpropertiesofMCF-7cells. (A–C)Immunostainingforactin,showedchangeincytoskeletalorganizationofMCF7SNAILcellscomparedcontrol(Scalebar ¼ 50 μm).Analysisofactinintensityrevealed thatoverexpressionofSNAILreducedactinpolymerization(B).Grayintensitydistributionalongtheline(A)acrosstheimageswerequantifiedinimageJtolookatdifferences inactin(C).(D–E)MSDsof200nmparticlesinjectedintothecytoplasmwereincreased forMCF7-SNAILcellsacrossalltimelagssuggestingrelativelysoftercytosol.ElasticmodulusofMCF7-SNAILcellswasreducedsignificantlyat ω ¼ 1Hz.Tocalculatestatistical significancestudent’s t-testwasusedand P-valuesoflessthan0.05wasconsideredsignificant(*P < 0.05, **P < 0.01, ***P < 0.001)(McGrailetal.,2015b).
weremoreepithelialwithmostlyroundmorphology,cellstransformedwith Snail(MCF7-SNAIL)exhibitedamoremesenchymalphenotype.MCF7SNAILcellsdisplayedanelongatedmorphology,downregulationof E-Cadherin,andupregulationofN-CadherinandVimentin,characteristics ofamesenchymalphenotype(McGrailetal.,2015b).Tounderstandthe underlyingchangesinthecytoskeletalorganization,weanalyzedtheactin structureusingimmunostaining(Fig.4A).Theintegratedfluorescence intensityofactininMCF7-SNAILwas3-foldlowerincomparisonto MCF7-NEOcells(Fig.4B).Analysisofactindistributionacrossindividual MCF7-NEOcellsshowedhighintensityincorticalregionsindicating presenceofpolymerizedactinstressfibers.Incontrast,MCF7-SNAILcells displayedsignificantlyloweractinintensitysuggestingdissolutionofpolymerizedactinstructure(Fig.4C).UsingIPTM,weconfirmedthatthe intracellularmechanicalpropertiesweremarkedlyaltered.Embedded
nanoparticlesinMCF7-SNAILcellcytosoldisplayedhigherMSDsatall timescalesandsubsequentlyrevealedasignificantreductioninelasticmodulus(Fig.4D–E).Together,theseresultsconfirmedthatMCF7-SNAILcells possessamoredeformablecytosolincomparisontoMCF7-NEO.Results fromourstudycorroboratedwiththeresultsreportedbyCraeneetal.in coloncancercells(DeCraeneetal.,2005).ExpressionofSnailinthesecells ledtosignificantlossincytoskeletalproteins,includingABPs.Additionally, highlyinvasivecancercellsfromdifferenttissuesincludingbreastandovariancancerhavebeenshowntodisplaylessactinstressfiberscomparedtothe normalcells(Alibertetal.,2017).However,otherEMTstudieswithcancer andnormalepithelialcellstreatedwithtransforminggrowthfactor β (TGFβ)—aknowninducerofEMT,haveshownincreasedactinstressfiberformation(Haynesetal.,2011; Nallurietal.,2015; Sousa-Squiavinatoetal., 2019; Zhitnyaketal.,2020).ThisEMTresponsemayvaryindifferentcell types,perhapsduetointrinsicdifferencesinthecellsundergoingEMTor differencesinthesurroundingenvironment.Similarly,whilemoststudies havereportedthatmoreinvasivecancercellsareoftensofterthanlessinvasivecells,afewstudieshavereportedstiffeningofinvasivecancercells (Alibertetal.,2017).Differentprobingtechniquescancontributetothe reporteddifferencesincancercellmechanics.Measurementwithtechniques thatuseexternalprobeatlocalregionsofthecellcanbeinfluencedbycorticalactinstructure,whichismorepolymerizedininvasivecellsthatexhibit hightractionforces.Forexample,studiesusingAFMcanbemeasuringa specificregionofthecell,nottheintracellularmechanics(Alibertetal., 2017).Togethertheseresultshighlighttheneedforamorecomprehensive biophysicalanalysisofcancercellsundergoingmalignanttransformation.
Inadditiontotheintracellularchanges,MCF7-SNAILalsodisplayed significantlydifferenttractionforceprofileonapolyacrylamidesubstrate (Fig.5A–B).Asthecellsassumedamoreelongatedphenotype,itgenerated highertractionforceslocalizedatcellperiphery.MCF7-SNAIL-generated peaktractionforceswerethreefoldhigherthanthoseexertedbyNEOcells. Consequently,MCF7-SNAILdemonstratedsignificantlyhighermigratory behaviorwithmorethan2-foldincreaseincellvelocity(Fig.5C). Coefficientofvariation,calculatedbydividingthestandarddeviationwith themean,providesameasureofheterogeneityinthepopulationandwas significantlyincreasedinSNAILcellsforbothtractionforceandmigration (Fig.5D).
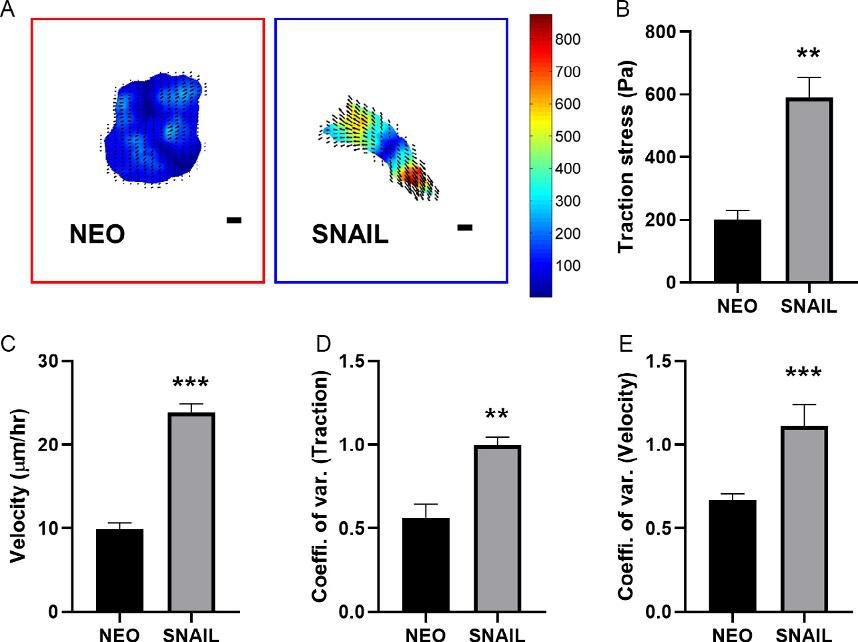
Fig.5 (A)TractionheatmapsofMCF7-NEOandMCF7-SNAILcellsareoverlaidwith matrixdisplacementswithforcerangeisspecifiedinPascals(Scalebars ¼ 10 μm). (B)PeaktractionstressesinSNAILcellsweresignificantlyhigherthanNEOcells. (C)MeanvelocityofMCF7-SNAILcellswassignificantlyincreased.(D–E)Coefficient ofvariationcalculatedforbothtractionforce(D)andcellvelocity(E)wassignificantly higherinMCF7-SNAILcells.Tocalculatestatisticalsignificancestudent’s t-testwasused and P-valuesoflessthan0.05wasconsideredsignificant(*P < 0.05, **P < 0.01, ***P < 0.001)(McGrailetal.,2015b).
4.2Invasivecancercellsexertincreasedandpolarizedtraction forcesinacontextdependentmanner
Inadditiontothebiochemicalsignals,thebiomechanicalpropertiesofthe ECMcanalsodictatethetractionforceprofileofthecancercells.Solid tumorsaregenerallystiffercomparedtotheirsurroundingtissues(Chang etal.,2011; Egebladetal.,2010; Youketal.,2014).Thisincreasedrigidity hasbeenshowntopromoteaninvasivebehaviorincancercellsfrommultipletissues,includingbreast,liver,andprostate(Acerbietal.,2015; Kostic etal.,2009; Leightetal.,2017; Pickupetal.,2014; Tilghmanetal.,2010; Ulrichetal.,2009).Inversely,invasivetumorcellsoftenexhibitsomeform ofdurotaxisorresponsetoincreasedsubstraterigidity(Acerbietal.,2015; Lachowskietal.,2017; Samueletal.,2011).Thisobservationiscertainly
trueinhighlymetastaticMDA-MB-231breastcancercells.Wehaveshown thatwhenMDA-MB-231cellswereculturedonhardpolyacrylamidecollagen-coatedsubstrates( 35kPa),theyexhibitedcharacteristicofmalignancyincluding,significantlyincreasedproliferationrateandresistanceto thechemotherapeutic,comparedtothoseofsoft( 3kPa)substrates (Fig.6A–B)(McGrailetal.,2015a).Othercharacteristicpropertiesofinvasivenessalsofollowedthesametrend.MDA-MB-231cellsshowedasignificantlyhighermigrationvelocity(Fig.6C)andincreasedcellspreading whenculturedonhardsubstratesrelativetosoftsubstrates.However,metastaticSKOV-3ovariancancercellsdisplayedanoppositemechanical responsetosubstratestiffness.WhenSKOV-3cellswereculturedonsoft
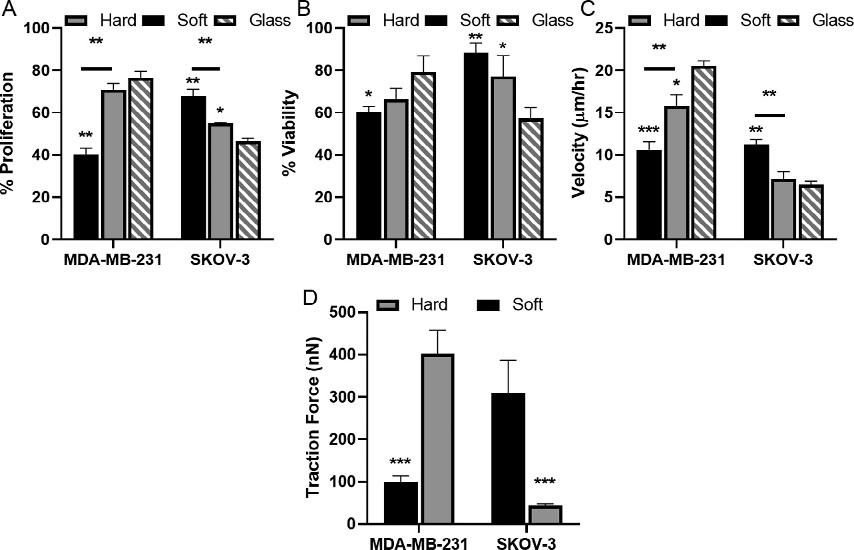
Fig.6 Contextdependentresponseofcancercells.MDA-MB-231andSKOV-3cellswere culturedonsoft( 3kPa)andhard( 35kPa)collagen-coatedpolyacrylamidesubstrates orcollagen-coatedglass.(A)PercentproliferationwasdeterminedbyBrdUincorporation.(B)ViabilitywasdeterminedbyMTTassayafter2 μM(MDA-MB-231)or0.1 μM (SKOV3)Doxorubicin treatment.(C)Theaveragecellvelocitywasdeterminedby trackingcellnucleiat5-minintervalsoveran8-hperiod.(D)Averagetractionforce wasquantifiedfromdisplacementoffluorescentnanoparticlesembeddedinsubstrates showedIncreasedtractionforceswerecorrelatedwithincreasedmigrationvelocity, increasedproliferation,andtreatmentresistanceforSKOV-3cellsonsoftsubstrates andMDA-MB-231cellsculturedonhardsubstratesorglass.Tocalculatestatisticalsignificancestudent’s t-testwasusedand P-valuesoflessthan0.05wasconsideredsignificant(*P < 0.05, **P < 0.01, ***P < 0.001)(McGrailetal.,2015a).
matrices,theyexhibitedgreaterproliferationrates,migrationvelocity,and cellspreadingcomparedtothoseculturedonhardmatrices(Fig.6A–C).This discrepantbehaviorbetweenMDA-MB-231andSKOV-3cellsdemonstratedthatmechanicalresponsestosubstratestiffnessarecellspecific,most likelyduetodifferencesintheirphysiologicalenvironmentintheprimary tumorormetastaticniche(Kosticetal.,2009; Kraning-Rushetal.,2012). Moreover,MDA-MB-231andSKOV-3cellsbecamehighlypolarized andexertedgreatertractionforceonhardandsoftsubstrates,respectively (Fig.6).Themechanismofthisprogressionhasbeenwell-studiedinbreast cancer,whereincreasedECMstiffnessleadstointegrinactivationfollowed byfocaladhesionformationandincreasedactomyosincontractility (Leventaletal.,2009).However,theincreasedforcesexertedbySKOV-3 onsoftmatriceshadnotbeencharacterizedbeforeourstudies.Rigidity dependentbehaviorofcancercellshasbeencorrelatedtotheirabilityto metastasizetospecificlocationsinvivo(Kosticetal.,2009).Indeed,studies inmurinemodelsfoundthatMDA-MB-231cellsyieldedsignificantlymore bone(stiff)metastasescomparedtolung(soft)metastases(Kangetal.,2003; Kosticetal.,2009).Thoughtheideaofdifferentcancercelltypesinvading siteswithcontrastingmechanicalpropertiesmayseemcounterintuitive,our studiesdemonstratedthatcellsadaptedtheirresponseusingRho-ROCK mediatedactomyosincontractilityand intracellularcytoskeletaltension (McGrailetal.,2014,2015a,b).
4.3Rho-ROCKsignalingregulatesdistinctmechanical responseofdifferingcancercelltypes
Mechanotransductionsignalingpathwayscancreateamechanicallyinducedpositivefeedbackloop,wherebyincreasedECMdepositionand rigidityenhancesmalignantpropertiesincancercells(Chinetal.,2016; Luetal.,2012).Forcancercellstofindbalancebetweenintracellularcytoskeletaltensionandextracellularadhesion,optimallevelsofRho-ROCK pathwayactivationmustbemaintained(McGrailetal.,2015a).WedemonstratedthesalienceofoptimalRho-ROCKsignalingactivationinMDAMB-231andSKOV-3cellsculturedonmatricesofdifferentrigiditiesby measuringtractionforcesbeforeandafterintroducingRho-ROCKpathwayinhibitor(Y27632).UsingTFM,wefirstfoundthatbothMDAMB-231andSKOV-3cellsexertedsignificantlylarger(23-foldincrease) cell-substratetractionforceswhenculturedontheirrespectivepreferential substrates,hardandsoft(Fig.7).Inthispaperpreferredsubstratereferredtothe
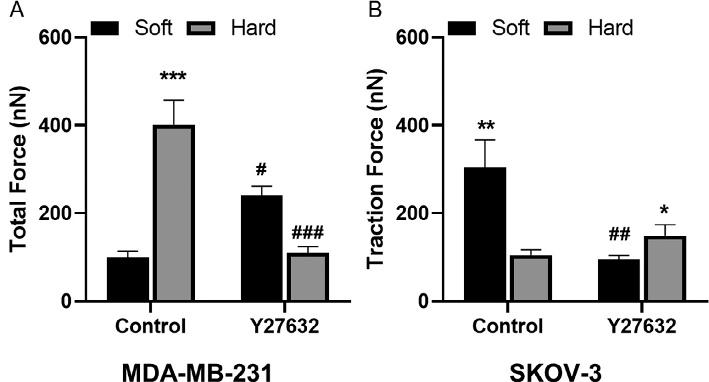
Fig.7 Rho-ROCKpathwaycontrolscancercellresponse.(A–B)SKOV-3andMDA-MB231cellsculturedonsoft( 3kPa)andhard( 35kPa)polyacrylamidesubstrateswere treatedwithROCKinhibitor(Y27632).QuantificationoftractionforcesforMDA-MB-231 (A)andSKOV-3(B),respectively.Tocalculatestatisticalsignificancestudent’s t-testwas usedand P-valuesoflessthan0.05wasconsideredsignificant(*P < 0.05, **P < 0.01, ***P < 0.001)(McGrailetal.,2015a).
substraterigiditythatelicitedmoremalignantpropertiesinmechanosensitive cancercells.SKOV-3ovariancancercellsweremoreproliferative,migrated morerapidly,andexhibitedreducedsensitivitytochemotherapeuticdrugs onsoftsubstrates,whichwereconsideredtheirpreferredrigidity.Generally, increasedRho-ROCKsignalingcorrelateswithincreasedtractionforce,so wesoughttofurtherunderstandhowthispathwaywaslinkedtocontractilityinthecontextofECMrigidity.Whenthecellsweretreatedwith ROCKinhibitorY27632ontheirpreferredsubstrates,wesawnoincrease intractionforceforbothMDA-MB-231andSKOV-3lines.Interestingly, whenMDA-MB-231tumorcellswereculturedontheirnonpreferredsoft substrates,weobservedagain-of-function(slightlyincreasedtractionforce), comparedtothosewithoutY27632treatment.Thisphenomenoncanbe largelyexplainedbytheideathatcancercells’inherentcontractilityneeds tobematchedwithsubstratesofoptimalrigiditytogeneratemaximumtractionforces—largercell-intrinsiccontractilitymatchedwithastifferECM orlowercell-intrinsiccontractilitymatchedwithasofterECMresults inoptimalcell-ECMtraction.Sinceourpaststudieshaveshownthat MDA-MB-231cellsareinherentlymorecontractilethanSKOV-3cells, areductioninMDA-MB-231cells’inherentcontractilitywithY27632 rescueditsfunctiononitsnonpreferred,softsubstrates.Therefore,itis evidentthatoptimalRho-ROCKsignalinginherentinindividualcancer celltypesgovernstheiractomyosincontractility,whichineffectdefines
theirpredisposedmatrixcompliances.Takentogether,thesefindingshighlightthecomplexityofcancerprogression,anddrivetheneedtotakea nuancedapproachinexaminingthebiophysicallandscapesofdifferent tumors.
4.4Utilizingforceprofilestocharacterizechemoresistant subpopulations
Often,asmallsubpopulationofcellscansurviveinitialtreatment,through efficientdrugeffluxorquiescence.Polyploidalgiantcancercells(PGCCs) arethoughttobeabletosurvivechemotherapyviaquiescence(Zhangetal., 2014).Despitetheirapparentdormancyandmorphologicalsimilarities, PGCCsaredistinctfromsenescentcells,astheycangiverisetodaughter cellstroughamitoticbudding(Lvetal.,2014; White-Gilbertsonetal., 2020).Thesemorphologicallyenlargedandoftenmultinucleatedcellsare oftenseenintumorsthathaveundergonetreatment,orinlatestageand aggressivedisease(Feietal.,2015; Lopez-Sa ´ nchezetal.,2014; Zhang etal.,2014).Furthermore,injectionofPGCCsintomousexenograft modelshaveledtothegrowthofnewtumors,highlightingthetumorigenic potentialofthisuniquesubpopulation(Niuetal.,2017).Previousstudies conductedinourlabhaveshownthatMDA-MB-231PGCCshave increasedmigratorypersistenceandmigratemorereadilyintothescratch wound(Xuanetal.,2018).InordertounderstandexactlyhowPGCCs maintaintheirenlargedmorphologyandsustainhighmigratorypersistence despitetheirincreasedsize,weperformedsinglecellIPTMandTFM (Fig.8).WefoundthatPGCCsonaveragehadincreasedcytoplasmicstiffness,andastifferbutmoredeformablenuclei.ThisisevidencedintheMSD plotsofparticlemotionembeddedwithinthecytoplasmofthecellandheterochromaticfociwithinthenucleus,wherePGCCshadalowerMSD, indicatinghigherlevelsofconstraint.Furthermore,weexaminedthe MSDtracesofindividualcellsandnoticedahigherspreadinPGCC populations.ThisindicatesthatPGCCsaremoreheterogeneousthan non-PGCCs;thisincreasedheterogeneityhasbeenobservedin chemoresistantandhighlyinvasivecancercells.Whenwestainedforand quantifiedtheactincytoskeletonofourPGCCs,wefoundthatPGCCs expressedboththickerandlongeractinstressbundles(Fig.8A–B),which isassociatedwithhighertractionforcesandincreasedmigration.Indeed, whenweperformedTFMonourMDA-MB-231cells,wefoundthat PGCCsonaveragehadovertwicetheexertedtractionforcecompared toournon-PGCCs(Fig.8C).Inordertoseeiftheuniqueorganization oftheiractinstructurewasresponsibleforPGCCscytoplasmicstiffness,
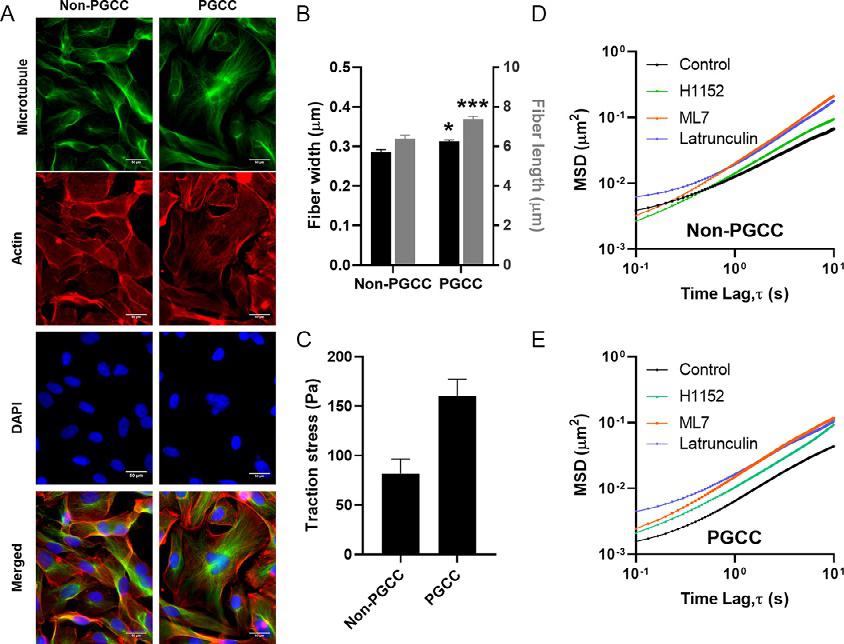
Fig.8 (A)Fluorescentimagesofnon-PGCCsandPGCCsstainedformicrotubule(green), actin(red),andDNA(blue)(Scalebar ¼ 50 μm).(B)Quantificationofaveragestressfiber widthandlength.(C)Meantractionforcesexertedbynon-PGCCandPGCCcancercellson a10kPastiffnesspolyacrylamidesubstrate.(D–E)EnsembleaveragedMSDsoftracked particlesofNon-PGCCs(D)andPGCCs(E)incontrolandinhibitortreatedconditions. Tocalculatestatisticalsignificancestudent’s t-testwasusedand P-valuesoflessthan 0.05wasconsideredsignificant(*P < 0.05, **P < 0.01, ***P < 0.001)(Xuanetal.,2018).
weinhibitedpartsoftheRhoA/ROCKpathway,whichisresponsiblefor controllingactincytoskeletalorganization.UsinginhibitorsML7(MLCK inhibitor),H1152(ROCKinhibitor),andlatrunculinA(actinpolymerizationinhibitor),weobservedconsistentreductionsincytoplasmicstiffnesses inourpolyploidcells(Fig.8D–E).Takentogether,theseresultsdemonstrate thebiophysicalcharacterizationofauniqueandhighlychemoresistantsubpopulation,whichismoreinvasiveaswellashighlytumorigenic.
5.Utilizingforcemeasurementstostudytumor andstromalcellcrosstalk
Interactionsbetweencancerandstromalcellsandthesurrounding TME,withitsdiversityincelltypesandmatrixmechanics,playacriticalrole indirectingcancerprogression.Carcinomaassociatedfibroblasts(CAFs)are
onemajorstromalcontributortoTMEmalignancy(Karagiannisetal.,2012; Luoetal.,2015; Taoetal.,2017).Theymediatehallmarkcancercellbehaviorsbysecretingparacrinefactorsthataltertumorgrowthandcellsurvival, ECMproteinsformatrixstiffening,andpro-inflammatorysignalsimportant incancerprogression.ACAF-likephenotypeischaracterizedbychangesin cytoskeletalarchitecture,motility,andadhesion,alongwithincreasedexpressionof α-smoothmuscleactin(αSMA)andfibroblastactivatedprotein(FAP) (Mishraetal.,2008).Thesemyofibroblast-likecellsarisefromnormalfibroblastsandMSCsthathavebeenactivatedbytumor-secretedfactorstoform CAFs.MSCsthatspontaneouslyhometotumorsfromthebonemarrowmay persistasstemcellsinthetumorordifferentiateintostromalcells(Bergfeldand DeClerck,2010; Spaethetal.,2009; TorsvikandBjerkvig,2013).Thus, MSCsserveasimportanttoolsinthestudyofthestroma-cancercrosstalk andareutilizedextensivelyinourstudies.
5.1Cancercellinvasivenessdeterminedirectintercellular interactionwithstromalcells
Recruitmentandengraftmentofstromalcells,includingfibroblastsand MSCsinTMEarecriticalforcancerprogressiontomalignancyandareassociatedwithpoorprognosis(OudinandWeaver,2016).Asthedisease becomemoreinvasive,thecelladhesionmoleculesontumorcellsurface aresignificantlyalteredandthesealteredinteractionscansubsequentlymodifybothinitialengraftmentandlong-termfateofstromalcells( Janiszewska etal.,2020).Weelucidatedtheroleofalteredadhesionmoleculerepertoire onstromalcellengraftmentwithmonolayersofcancercellswithvarying levelsofaggressiveness(McAndrewsetal.,2015).Stromalcellsweremore adherentandspreadmorereadilyonmoreaggressivebreast,ovarian,prostate,andtaxolresistantcelllines.TheaggressivecelllinesexpressedEMT associatedcell-celladhesionmarkerscadherin2(N-cadherin)and/or cadherin11(OB-cadherin)toadifferentdegree.Bothoftheseproteins andespeciallytheOB-cadherinwerealsoexpressedbystromalcells. Subsequentlyblockingcadherin11onstromalcellsreversedtheenhanced adhesiontoinvasivecancercellsevenwiththecancercellswithlowlevelof OB-cadherinexpression.ThissuggeststhatOB-cadherinonstromalcells enabledthemtobindtothecancercellsviahomotypic(OB-cadherin)or heterotypic(N-cadherin)interactionandcanbeusedasatherapeutictarget toabrogatecancercell-stromalcellinteraction.Throughtheextensivebidirectionalcrosstalkbetweencancercellsandstromalcells,thereisafeedback loopwhereinstromalcellrecruitmentincreasescancercellinvasionand malignancy,whichinturnincreasesstromalcellrecruitment.
Another random document with no related content on Scribd:



