
50 mg, 100 mg 150 mg, 200 mg





50 mg, 100 mg 150 mg, 200 mg








YULAREB is indicated in patients with HR+, HER2- breast cancer at high risk of recurrence as combination therapy in EBC, or monotherapy or combination therapy in MBC1
CDK4 & 6: cyclin-dependent kinase 4 & 6; EBC: early breast cancer; HER2: human epidermal growth factor receptor 2; HR+: hormone receptor positive; MBC: metastatic breast cancer. TAKE HOPE FURTHER

References: 1. YULAREB Approved Professional Information Eli Lilly (S.A.) (Pty) Limited 15 June 2023. 2. Palbociclib 75 mg, 100 mg, 125 mg capsules professional information. Pfizer. 23 October 2023. 3. Ribociclib 200 mg professional information. Novartis. 21 July 2022.
S4 YULAREB 50 mg Film-coated tablet. Reg. No.: 53/26/0438.434. S4 YULAREB 100 mg Film-coated tablet. Reg. No.: 53/26/0439.435. S4 YULAREB 150 mg Film-coated tablet. Reg. No.: 53/26/0440.436. S4 YULAREB 200 mg Film-coated tablet. Reg. No.: 53/26/0441.437. Each film-coated tablet contains 50 mg, 100 mg, 150 mg or 200 mg abemaciclib respectively. For full prescribing information refer to the professional information approved by the Medicines Regulatory Authority (06/2023).
YULAREB is a registered trademark owned or licensed by Eli Lilly and Company, its subsidiaries, or affiliates. © 2024 Aspen Group of Companies or its licensor. All rights reserved. For adverse events reporting, contact: ade_za@lilly.com. For product or medical related queries, contact: za_medinfo@lilly. HCR: Eli Lilly (S.A.) (Pty) Ltd. Reg. No.: 1957/000371. 35 Ballyclare Drive, Bryanston, 2191. Private Bag X119, Bryanston, 2021. Telephone: (011) 510 9300. Marketed and distributed by Pharmacare Limited t/a Aspen Pharmacare. Co. Reg. No. 1898/000252/06 Healthcare Park, Woodlands Drive, Woodmead, 2191. PP-AL-ZA-0430 08/2024



32 OPHTHALMOLOGY
Addiction: Stats, risks and
Addressing the dual impact of
EDITORIAL
EDITOR: René Bosman
René.Bosman@newmedia.co.za
SUB EDITOR: Gill Abrahams
LAYOUT & DESIGN: Allison McCallum
ADVERTISING
ADVERTISING EXECUTIVE
Charissa Piek | 063 281 1205
Charissa.Piek@newmedia.co.za
DISTRIBUTION & SUBSCRIPTIONS
Felicity Garbers
Felicity.Garbers@newmedia.co.za
PUBLISHING TEAM
GENERAL MANAGER: Dev Naidoo
GROUP ACCOUNT DIRECTOR B2B: Johann Gerber
Johann.Gerber@newmedia.co.za
PRODUCTION MANAGER: Angela Silver
ART DIRECTOR: David Kyslinger
CONTACT
Johannesburg Office: Woodlands Office Park, Building 13, Ground Floor, Woodlands Drive, Woodmead, Sandton 2191
CPD: Targeted antihypertensives: Individualising treatment by patient profile
Online CPD: Switching from warfarin to rivaroxaban: A safer, modern approach
CPD: Optimising anticoagulation: Apixaban vs rivaroxaban selection
Saving lives and costs with generic dabigatran
House of Stroke: Thrombectomy and thrombolysis in the management of stroke
Postal Address: PO Box 784698, Sandton, Johannesburg, 2146 T +27(0)11 877 6111 F +27(0)11 713 9024
www.medicalacademic.co.za
PRINTING Printed by Novus Print
COVER PRICE Specialist Forum per issue R80,00 VAT Incl. ISSN: 2218-8282
Published by New Media, a division of Media24 (Pty) Ltd
MANAGEMENT TEAM
CEO: NEW MEDIA: Aileen Lamb
Mixed DED? PG-HPG nanoemulsion to the rescue
35 DERMATOLOGY
The global scoop on skin conditions and their impact
Strategies for tackling recurrent AK
WEBINAR REPORTS
Guiding choices: Practical management of BPH and use of PPIs
The role of DOACs in the management of VTE
A practical approach to managing GORD
COMMERCIAL DIRECTOR: Maria Tiganis
STRATEGY DIRECTOR: Andrew Nunneley
CHIEF FINANCIAL OFFICER: Venette Malone
Interim CEO: MEDIA24: Raj Lalbahadur
HEAD OFFICE 11 Shelley Road, Salt River, Cape Town 7925 PO Box 440, Green Point, Cape Town 8051
Tel: +27 (0)21 406 2002
www.newmedia.co.za
Disclaimer: Please take note that the products featured in this journal are available

Unless previously agreed in writing, Specialist Forum owns all rights to all contributions, whether image or text.
SOURCES: Shutterstock, supplied images, editorial staff.
While precautions have been taken to ensure the accuracy of its contents and information given to readers, neither the editor, publisher, or its agents can accept responsibility for damages or injury which may arise therefrom. All rights reserved. © Specialist Forum. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, photocopying, electronic, mechanical or otherwise without the prior written permission of the copyright owners.

October is World Mental Health Awareness Month, a time to highlight the importance of mental health and advocate for those affected by mental health issues. This year’s theme, set by the World Federation of Mental Health, focuses on workplace mental health, underscoring the significance of fostering a supportive environment in our professional spaces.
Mental health in the workplace is increasingly recognised as a critical issue affecting employees’ well-being and overall productivity. Stress, anxiety, and burnout can stem from various workplace factors, including excessive workloads, lack of support, and poor work-life balance.
These issues not only impact individual employees but can also lead to reduced organisational effectiveness and higher turnover rates. As such, prioritising mental health in the workplace is essential for creating a thriving work culture.
This year’s theme encourages employers, employees, and policymakers to engage in conversations about mental health at work. By raising awareness and reducing stigma, we can create an atmosphere where individuals feel comfortable discussing their mental health challenges and seeking help when needed. Implementing initiatives such as mental health days, counseling services, and flexible work arrangements can significantly improve employees’ mental well-being.
Moreover, promoting mental health in the workplace requires a commitment to training managers and leaders to recognise the signs of mental health issues and respond appropriately.
Training can empower leaders to create open lines of communication, fostering trust and ensuring employees feel valued and heard. Encouraging regular check-ins and feedback can help identify potential stressors and address them before they escalate into more significant problems.
Employers should also consider implementing wellness

programmes that promote mental well-being, including mindfulness sessions, stress management workshops, and team-building activities. These initiatives can enhance team cohesion, boost morale, and promote a sense of belonging among employees.
By taking these steps, we can ensure that workplaces become safe spaces for open dialogue and support, ultimately contributing to healthier, happier, and more productive employees.
Let us advocate for mental health awareness in the workplace and beyond, recognising that the well-being of employees is a shared responsibility that benefits everyone.
In the September issue, we featured an illustration on page 17 depicting a woman living with obesity and the impact on her cardiovascular system. If this image caused any offense, we sincerely apologise. Please note that our editorial images are independently sourced.
Hope you enjoy the read!
Regards
René Bosman



Healthcare solutions provider Altron HealthTech will soon be launching the modernised HealthONE Enterprise. A multi-discipline, platform and cloud-based solution, it has been designed for use in, amongst others, primary healthcare management in hospitals and clinics.
HealthONE Enterprise offers numerous exciting features such as the ability to book an appointment, maintain electronic health records with surgical and medical histories, allow clinicians to manage scripts and medications, electronic scripting and referrals, manage chronic and acute conditions, and better management of overall patient health. It also provides process efficiencies for medical professionals with a simple click.
Electronic health records allow healthcare practitioners instant access to a patient’s healthcare journey including the ability to upload a patient’s x-ray images and pathology results, allowing them to make more informed decisions based on all the available information and alleviating the need to run duplicate tests.
As healthcare providers increasingly look to provide personalised healthcare solutions tailored to an individual’s unique needs, HealthONE Enterprise provides a patient-centred approach to managing the entire patient healthcare journey. Its platform architecture offers the capability to collect vast amounts of data, providing valuable insights, preferences and trends which can be leveraged by clinicians.
The system facilitates informed
decision-making and automates processes to save costs, boost productivity and free medical staff up to provide quality healthcare rather than spending time on administrative tasks.
Built on the latest technology and security protocols, HealthONE Enterprise is POPIA compliant and secures healthcare records to ensure patient privacy and to protect personal information.
The solution has the benefit of being scalable and interoperable with several third-party systems and devices including HR systems, pathology systems and medical devices such as e-vitals, audiometry, spirometry and vision devices. It is also customisable according to healthcare provider’s unique requirements.
Altron HealthTech’s proven, trusted healthcare solutions, including practice management applications, electronic health records solutions, claims management systems and data insight capabilities, run seamlessly in the background of HealthONE Enterprise.
We take care of our clients’ administrative, financial and clinical support needs, allowing them to focus on their patients, in the process improving patient outcomes, explains Leslie Moodley, MD of Altron HealthTech.
Altron HealthTech has a 30-year tradition of providing innovative technology solutions. Its strategy is focused on solving real problems through best-in-class technology. The company’s core strategy is innovation.
The business boasts an impressive array of in-house skills and expertise allowing it to service the entire value chain. Using global best practice and project governance standards, Altron HealthTech offers proactive management and monitoring of the HealthONE Enterprise system, allowing the team to spot issues before they become problems.
Our commitment to enhancing patient lives through technology has resulted in robust collaborations with clinicians and industry leaders to create a platform ecosystem that enables and leverages the partner network effect, says Moodley.
“The business’ goal is to continue to provide a differentiated service and customer support offering with high touch designs focusing on clinical knowledge and medical expertise to meet individual needs, by ensuring a deep understanding of clinical and operational workflows and how technology can be integrated to enhance efficiency and patient outcomes.” SF





















Global impact of substance use
• Alcohol consumption: Responsible for 2.6 million deaths annually, accounting for 4.7% of all fatalities. Of these deaths, ~2 million are men
• Psychoactive drug use: Contributes to 0.6 million deaths, with ~0.4 million deaths among men.4
Prevalence of alcohol use disorders
• 400 million people globally live with alcohol use disorders
• 209 million individuals suffer from alcohol dependence.4
Global drug use statistics
• 64 million people were living with drug use disorders.
• 292 million people used drugs, including:
⚬ 228 million cannabis users
⚬ 13.9 million people injected drugs.
• Drug use has risen by 20% over the past decade, with opioids, amphetamines, cocaine and ecstasy being prevalent.5


Addiction is a primary, chronic disease affecting the brain’s reward, motivation, memory, and related circuits. Dysfunction in these areas leads to distinct biological, psychological, social, and spiritual symptoms. It involves psychological and/ or physical dependence on substances like drugs (including tobacco) or alcohol, or on activities and behaviours such as sex, exercise, and gambling.1,2,3

• 8 million deaths annually due to tobacco use
• 1.3 million deaths occur among non-users exposed to secondhand smoke.6
• Traditionally seen as a failure of willpower
• Modern research reveals addiction as a complex condition rooted in brain mechanisms.7
• Nearly 50% of individuals with substance use disorders also suffer from chronic health conditions or mental disorders
• Increased risks include psychiatric hospitalisations, suicide, premature death, and worse treatment outcomes.8
• Key goals:
⚬ Reduce or stop substance use
⚬ Improve overall health and social functioning
⚬ Prevent future harm.5
• Available treatments:
⚬ Pharmacological therapies can improve recovery rates by up to 40% and reduce relapse rates significantly
⚬ Psychological treatments include cognitive-behavioural therapy, motivational enhancement therapy.5
• Crucial functions:
⚬ Screening and brief interventions
⚬ Referrals to specialised treatments
⚬ Delivering pharmacological and psychosocial treatments
⚬ Reducing stigma associated with seeking help.5
References are available on request. SF

Obesity, often referred to as a epidemic, affects one in eight people globally. In South Africa, an estimated 50% of the population are overweight or obese, according to a recent study by the Human Sciences Research Council. KwaZuluNatal reported the highest prevalence of obesity (39.4%).1,2
The growing prevalence of childhood obesity is particularly concerning, note Segal and Gunturu. Around 37 million children <5-year are overweight and >390 million older children and adolescents are overweight of which ~160 million are living with obesity.1
In 2019, 16% of South African children aged five- to nine-years (>91 100) and 22% of those aged 10- to 14-years (>120 300) were classified as overweight or obese. Overall, 18% of children aged 15- and 17-years (>91 800) were found to be overweight or obese. The rate was significantly higher for girls (22%) than boys (15%). 3,4
Stigmatisation leads to psychological challenges
Individuals living with obesity often face societal stigma and discrimination, leading to psychological challenges. Psychological challenges stem from a combination of biological, psychological, and social factors such as stigmatisation and discrimination. Biologically, genetic predispositions, hormonal imbalances, chronic inflammation, insulin resistance, and changes in brain structure linked to obesity can exacerbate psychological conditions like depression and anxiety. Depression and anxiety negatively impact overall quality of life (QoL) and can lead to maladaptive eating (eg binge or emotional eating) behaviours.1
Individuals living with obesity face an 18%-55% increased risk of developing depression, and those with depression are 37%-58% more likely to develop obesity. Global data indicates a growing burden of both obesity and mood disorders, with a stronger link between obesity and depression observed in women and younger individuals.1
Obesity-related psychological issues can cause complications
Psychological challenges linked to obesity can lead to significant complications affecting mental and physical health and overall QoL. Some key complication include:1


Body mass index 30
Posology and method of administration: The starting dose is 0,6 mg once daily. The dose should be increased to 3,0 mg once daily in increments of 0,6 mg with at least one week intervals to improve gastro-intestinal tolerability. If escalation to the next dose step is not tolerated for two consecutive weeks, consider discontinuing treatment. Daily doses higher than 3,0 mg are not recommended. Saxenda® is for subcutaneous use only. It must not be administered intravenously or intramuscularly. Saxenda® is administered once daily at any time, independent of meals. However, it is preferable that Saxenda® is injected around the same time of the day. It should be injected in the abdomen, thigh or upper arm. Patients must be instructed to perform continuous rotation of the injection site to reduce the risk of developing cutaneous amyloidosis. There may be a potential risk of change in Saxenda® absorption or effect following Saxenda® injections at sites with cutaneous amyloidosis. Saxenda® should not be used in combination with another GLP-1 receptor agonist. When initiating Saxenda®, consider reducing the dose of concomitantly administered insulin or insulin secretagogues (such as sulfonylureas) to reduce the risk of hypoglycaemia. Saxenda® is not recommended for use in children below 12 years of age or in adolescents with a body weight below or equal to 60 kg due to lack of data. Contraindication: • Hypersensitivity to liraglutide or to any of the excipients listed under Composition. • Pregnancy and lactation. Special warnings and precautions for use: Saxenda® must not be used as a substitute for insulin. There is no clinical experience in patients with congestive heart failure New York Heart Association (NYHA) class IV and Saxenda® is therefore not recommended for use in these patients. The safety and efficacy of Saxenda® have not been established in the following patients, viz: • Treated with other products for weight management, • With obesity secondary to endocrinological or eating disorders or to treatment with medicinal products that may cause weight gain, • With severe renal impairment, • With severe hepatic impairment, • With inflammatory bowel disease and diabetic gastroparesis. Use in these patients is not recommended. Acute pancreatitis has been observed with the use of GLP-1 receptor agonists. Patients should be informed of the characteristic symptoms of acute pancreatitis. If pancreatitis is suspected, Saxenda® should be discontinued; if acute pancreatitis is confirmed, Saxenda® should not be restarted. In the absence of other signs and symptoms of acute pancreatitis, elevations in pancreatic enzymes alone are not predictive of acute pancreatitis. In clinical trials, a higher rate of cholelithiasis and cholecystitis was observed in patients treated with Saxenda® than in patients on placebo. Patients should be informed of the characteristic symptoms of cholelithiasis and cholecystitis. In clinical trials in type 2 diabetes, thyroid adverse events, such as goitre have been reported in patients with pre-existing thyroid disease. Saxenda® should therefore be used with caution in patients with thyroid disease. An increase in heart rate was observed in clinical trials. Heart rate should be monitored at regular intervals consistent with usual clinical practice. Patients should be informed of the symptoms of increased heart rate (palpitations or feelings of a racing heartbeat while at rest). For patients who experience a clinically relevant sustained increase in resting heart rate, treatment with Saxenda® should be discontinued. Patients treated with Saxenda® should be advised of the potential risk of dehydration in relation to gastrointestinal side effects and take precautions to avoid fluid depletion. Patients with type 2 diabetes receiving Saxenda® in combination with insulin and/or sulphonylurea have an increased risk of hypoglycaemia. Fertility, pregnancy and lactation: Saxenda® should not be used during pregnancy and lactation. Undesirable effects: Gastrointestinal reactions were the most frequently reported adverse reactions during treatment with Saxenda®. Very common side effects are nausea, vomiting diarrhoea, constipation, headache. Common side effects include: Hypoglycaemia, insomnia, dizziness, dysgeusia, dry mouth, dyspepsia, gastritis, gastro-oesophageal reflux disease, abdominal pain upper, flatulence, eructation, abdominal distension, cholelithiasis, injection site reactions, cutaneous amyloidosis, asthenia & fatigue, increased lipase/increased amylase. Uncommon side effects include: dehydration, tachycardia, pancreatitis, cholecystitis, urticaria & malaise. Rare side effects include: anaphylactic reaction, acute renal failure & renal impairment. Overdose: With overdose, the patients reported severe nausea, vomiting and diarrhoea, but recovered without complications. Severe hypoglycaemia has also been observed. In the event of overdosage, appropriate supportive treatment should be initiated according to the patient’s clinical signs and symptoms. The patient should be observed for clinical signs of dehydration and blood glucose should be monitored. Reg. No.: 50/21.13/1091. For full prescribing information, refer to the Professional Information approved by the Regulatory Authority. Ver. 12/09/2022.

A recent review of key interventions in the management of comorbid obesity and psychological challenges in individuals living with obesity recommend the following:5
_ Integrate cognitive behavioural therapy (CBT) with lifestyle modifications to address both psychological and weightrelated issues effectively.
_ Implement interventions like the Integrated Coaching for Better Mood and Weight programmes, which synergises obesity and depression treatment strategies.
_ Tailor interventions to individual needs, emphasising personalised CBT rather than one-size-fits-all group therapy to enhance outcomes.
_ Provide comprehensive nutritional education, including information on gut microbiota, to support weight loss and improve mental health.
_ Promote at least 150-minutes of moderate to vigorous physical activity per week as part of lifestyle modification programmes.
_ Regularly assess both weight and mental
health outcomes to evaluate intervention effectiveness and adjust treatment plans accordingly.
_ Use stepped-care approaches that allow for gradual intensification of treatment, such as introducing acceptance-based behavioural treatment when initial weight loss goals are not met.
_ Incorporate eHealth resources and self-guided interventions to engage participants and facilitate weight loss while addressing mental health challenges.
_ Conduct secondary analyses to identify factors that mediate outcomes, which can inform the development of future interventions.
_ Recognise and mitigate barriers that may prevent individuals, especially men, from seeking help for both obesity and depression, promoting accessible and appealing intervention options.
In terms of pharmacotherapy, it is important to note that many psychotropic medications such as antidepressants used to treat mood disorders such as depression
and anxiety have obesogenic effects, while several weight-loss medications can have psychological effects.
6
When considering pharmacotherapy for patients with obesity and a mood disorder, it is crucial to understand the weightrelated impacts of psychotropic drugs, and the psychological effects of anti-obesity medications (AOMs). 6
Antidepressants generally cause more modest weight gain compared to other psychotropic drugs such as antipsychotics, with medications such as amitriptyline, mirtazapine, and paroxetine being associated with gains of up to 2.7kg after long-term use. On the other hand, bupropion is more likely to cause weight loss.7
A 2020 study by Tham et al assessed the effectiveness, tolerability, impact on eating behaviours, and psychological safety of AOMs in individuals living with obesity, metabolic syndrome and psychological challenges. Follow-up was 12-months. 8 At the end of the study period, results showed an average weight loss of 11.79kg (12.1%), a body mass index (BMI) reduction of 3.90kg/m², and a 12.6cm decrease in waist circumference. The proportion of participants with good glycaemic control increased from 28.6% to 80.7%, hypercholesterolaemia dropped from 85.2% to 29.9%, and hypertension rates fell from 88.9% to 52%. Depression, anxiety, and stress levels significantly decreased, while eating behaviours improved.
8 AOMs were generally well tolerated, but topiramate was associated with increased psychiatric side effects, including worsened mood (28.2%) and suicidality (30.8%). Overall, AOMs can contribute to significant weight loss and improved metabolic health when combined with lifestyle interventions, though caution is needed with certain medications, concluded the authors. 8
Obesity has significant psychological effects, with individuals often facing societal stigma and discrimination, leading to worsened mental health. This association between obesity and depression highlights the need for a holistic approach to treatment. AOMs can be effective in promoting weight loss, but some may have negative psychiatric side effects. When managing patients with both obesity and mood disorders such as depression and anxiety, it is essential to carefully consider the weight-related impacts of antidepressants and the psychological effects of AOMs. A combination of lifestyle interventions, CBT, and pharmacotherapy tailored to individual needs offers the best outcomes.
References are available on request. SF

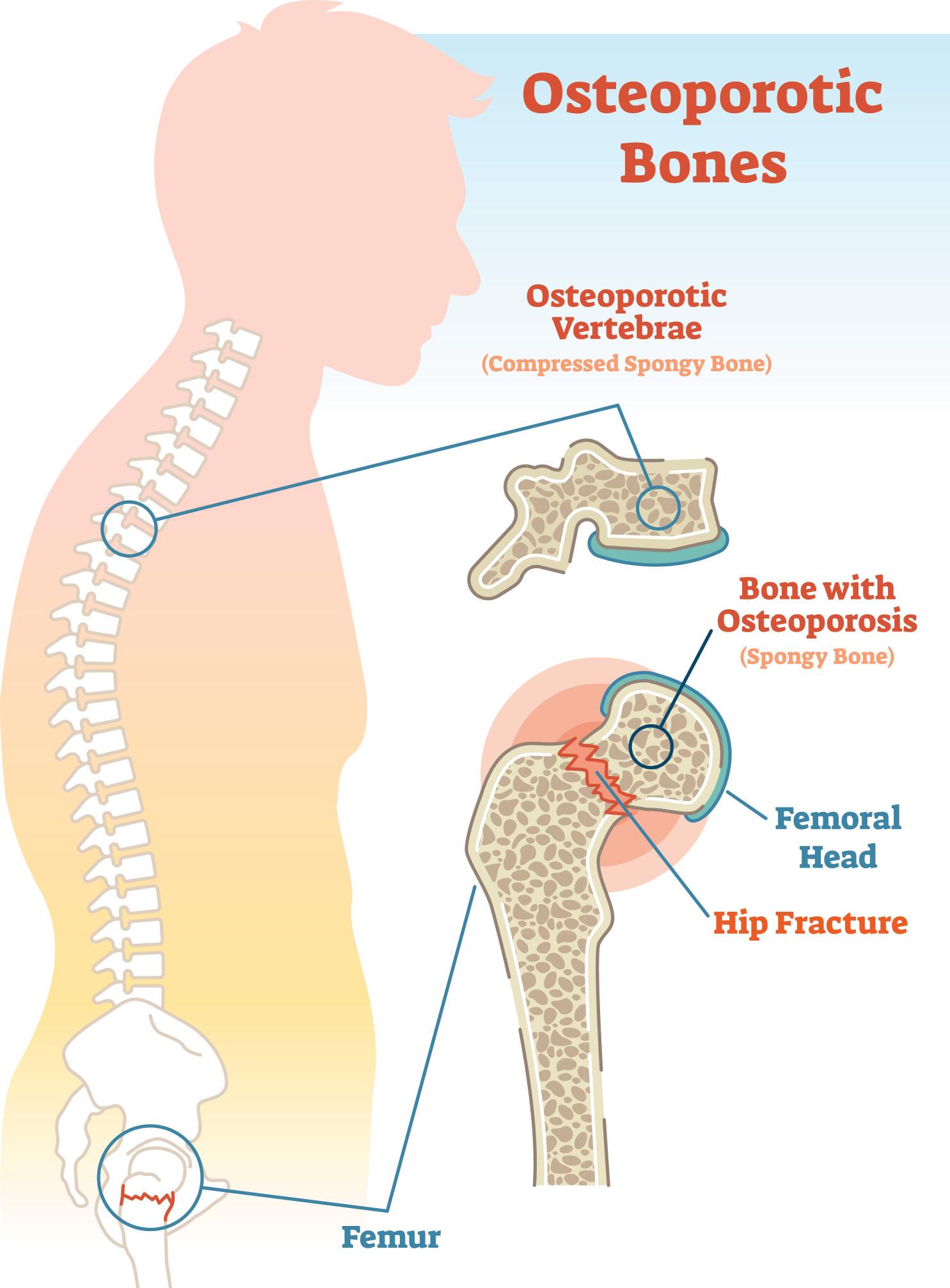
Osteoporosis, characterised by low bone mass and a microarchitectural deterioration of bone tissue, is a silent epidemic that is steadily growing. Startling projections reveal that by 2050, the global incidence of hip fractures in men will soar by 310%, and by 240% in women. Even more concerning, by 2040, the number of people at high risk for fractures will double. Currently one in three women and one in five men, are at risk of fracture.1,2
The most common fractures associated with osteoporosis affect the hip, spine, or wrist. These fractures lead to significant health issues, including increased morbidity, mortality, diminished quality of life, higher rates of institutionalisation, and substantial economic burden. 3
The World Health Organization (WHO) defines osteoporosis as having a bone mineral density (BMD) that is 2.5 standard deviations or more below the average for young, healthy women (a T-score of <−2.5). 3
Apart from ageing (>65-years), other risk factors for the development of osteoporosis include oestrogen deficiency, extended periods of immobilisation, inflammation, bone and hormone metabolism disorders, and stress associated with transcriptional changes in osteogenic genes. These factors can disrupt the delicate balance between bone formation, driven by osteoblasts, and bone resorption, controlled by osteoclasts.4
Maintaining strong bone health throughout the lifespan is key to preventing osteoporosis, however, even after a diagnosis, a bonefriendly lifestyle can reduce the risk of fracture. Research shows that moderate exercise, nutrition, smoking cessation and limiting alcohol consumption can boost bone health.1,4
Exercise interventions
As people age, physical activity decreases
and they tend to adopt more sedentary lifestyles, leading to so-called skeletal unloading, decreasing bone formation and bone mass. 4,5
Exercising regularly plays a crucial role in maintaining and improving bone health by stimulating bone formation and strength through mechanical loading, according to Zhang et al 4
Weight-bearing and resistance exercises, such as whole-body vibration training, have been shown to maintain or even improve bone mass and BMD in post-menopausal women, enhancing overall QoL. 4
Aerobic exercise, too, supports bone health by promoting osteocyte survival and muscle-bone interactions, which are regulated by mechanical load. Additionally, endurance exercise can help slow agerelated mitochondrial decline. 4
Beyond bone health, exercises like Tai Chi and yoga improve balance and postural stability, reducing the risk of falls and fractures. Studies show that Tai Chi can lower fall-related injury risks by up to 50% in older adults. 4
In their study, Pinheiro et al observed a dose-response relationship in exercise programmes that typically last 60 minutes, two to three times per week, and bone health.3
Apart from regular exercise, other key lifestyle
factors that impact bone health include nutrition, smoking, and alcohol consumption. Calcium and vitamin D are essential for bone metabolism. Calcium-rich foods such as dairy products, green vegetables, and mineral water are important, while vitamin D is found in fatty fish and eggs.1
Sun exposure is vital for the body’s production of vitamin D, and the International Osteoporosis Foundation recommends at least 15 minutes of daily outdoor time.1
Research suggests that tobacco smoking disrupts bone turnover, leading to reduced bone mass and BMD, which increases the risk of osteoporosis. Similarly, alcohol consumption – more than one glass per day for women and two for men – can reduce bone mass and strength due to an imbalance in bone remodelling, and should be avoided.1,5
When is pharmacological intervention needed?
The American Association of Clinical Endocrinologists/American College of Endocrinology guidelines recommend that pharmacological treatment should be initiated for patients with:6
_ Osteopenia or low bone mass and a history of fragility fracture at the hip or spine
_ A T-score of −2.5 or less in the lumbar spine, femoral neck, total hip, or 33% radius despite the absence of a fracture
_ A T-score between −1.0 and −2.5 if the FRAX 10-year probability for a major osteoporotic fracture is greater than 20% or for a hip fracture is >3%.
How effective is pharmacotherapy?
An independent evidence review team performed a systematic review and network meta-analysis of osteoporosis treatments that analysed 34 randomised controlled trials and 36 observational studies. They found that in post-menopausal women with osteoporosis, bisphosphonates and denosumab have been shown to significantly reduce the risk of hip, vertebral, and other clinical fractures.7
Teriparatide reduces clinical and radiographic vertebral fractures, while selective estrogen receptor modulators are effective in reducing radiographic vertebral fractures but not clinical fractures.7
Sequential therapy, particularly using romosozumab followed by alendronate, has
demonstrated greater efficacy in reducing fractures than alendronate alone for women at very high risk of fractures. However, romosozumab carries a cardiovascular (CV) risk, making it unsuitable for those with a history of myocardial infarction or stroke.7 There is limited evidence on the impact of longer-term treatments, especially beyond three to four years. Bisphosphonates may offer additional benefits when extended beyond six years, although the risks of adverse effects such as osteonecrosis of the jaw and atypical femoral fractures increase with prolonged use. The optimal duration of treatment for other therapies, such as teriparatide, and denosumab, remains unclear.7
recommendations
An international multi-disciplinary working group of the European Society for Clinical and Economic Aspects of Osteoporosis,
Osteoarthritis, and Musculoskeletal Diseases recently published their recommendations for treating men living with osteoporosis.8
Extensive research supports the use of oral bisphosphonates, such as alendronate and risedronate, to improve BMD in men living with osteoporosis. A meta-analysis demonstrated significant increases in BMD at various sites, including the lumbar spine and femoral neck. 8
Zoledronate, administered intravenously, also significantly improved BMD and reduced vertebral fractures. Denosumab, administered as subcutaneous injections every six months, showed benefits in BMD accrual over two years in men living with osteoporosis. 8
Teriparatide has demonstrated significant improvements in BMD. Romosozumab also showed improvements in BMD, though its potential CV risks must be considered. 8
Testosterone replacement may benefit bone health in hypogonadal men, with increases in lumbar spine BMD observed in trials. However, robust evidence for fracture prevention is limited. While testosterone replacement might be considered for men with symptomatic deficiency, it should be combined with anti-osteoporosis medications for maximum fracture risk reduction. 8
Preventing bone loss requires a holistic approach, with lifestyle changes playing a crucial role. Regular weight-bearing and resistance exercises enhance bone strength and reduce fracture risk. Aerobic activities like Tai Chi further support bone health by improving balance and reducing fall-related injuries. Nutrition also plays a key role, with calcium- and vitamin D-rich diets essential for bone metabolism. Avoiding smoking and limiting alcohol consumption can also help maintain bone density. For those diagnosed with osteoporosis, pharmacotherapy is recommended, with treatments like bisphosphonates shown to reduce fracture risk. Combining a bone-friendly lifestyle with pharmacotherapy is crucial for optimal bone health.
References are available on request. SF

SOUTH AFRICA’S

BRAND*1
Effective reduction in blood pressure with significant reduction in CV mortality, myocardial infarction, stroke and LVH in at-risk hypertensive patients2,3
Significant renal-protective benefits in type 2 diabetic patients with nephropathy4
Affordable, making its antihypertensive, cardiovascular and renoprotective benefits accessible to more South Africans3,4,5
Reimbursed by medical aids on all plans#6
Plus is South Africa’s
Email: medinfo@accordhealth.co.za. Tel. No.: 011 234 5701/2. LOP/001/AUG24/AD. Abbreviations: CV: cardiovascular; LVH: left ventricular hypertrophy
References: 1. IMS MAT, June 2024. 2. Williams B, Mancia G, Spiering W, et al. ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021-3104. doi: 10.1093/eurheartj/ehy339. 3. Lindholm LH, Ibsen H, Dahlöf B, et al. LIFE Study Group. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002 Mar 23;359(9311):1004-10. doi: 10.1016/S0140-6736(02)08090-X. 4. Brenner BM, Cooper ME, de Zeeuw D, et al. RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861-9. doi: 10.1056/NEJMoa011161. 5. Database of Medicine Prices [30 August 2024]. Available from URL: https://www.health.gov.za/nhi-pee/. 6. Accord Data on File.

This article was independently sourced by

In 2019, 1.3 billion people were living with hypertension, defined by systolic blood pressure (SBP) ≥140 mmHg and diastolic blood pressure (DBP) ≥90 mmHg. SBP is often referred to as a ‘silent killer’ and causes >10 million deaths annually, making it the leading global health risk.1
Globally, about 33% of adults between 30- and 79-years have hypertension, but only 54% are diagnosed, 42% receive treatment, and just 21% have it under control. In Africa, hypertension affects 36% of adults, with 43% diagnosed, 27% receiving treatment, and only 12% having it under control. If countries can achieve a 50% hypertension control rate by 2050, the World Health Organization (WHO) estimates that 76 million cardiovascular
(CV) deaths and 450 million disabilityadjusted life years could be prevented.1
Three common triggers for hypertension are high sodium intake, alcohol consumption, and tobacco use. In Africa, sodium intake averages 2687mg/day, exceeding the recommended <2000mg/ day. Alcohol, while temporarily lowering BP, often leads to a rebound increase and chronic hypertension, making its reduction
crucial for mitigating hypertension-related health issues. Tobacco use was responsible for 8.7 million deaths globally in 2019, with acute effects on BP due to sympathetic nervous system stimulation, although the long-term impact on chronic hypertension is less clear.1
Effective interventions
Pharmacological treatment for hypertension has been around since the 1950s, contributing to ~20% of the decline in
CV mortality in high-income countries over the past four decades.1
The WHO sets treatment goals of BP <140mmHg/90mmHg for patients without comorbidities, while for those with CV disease (CVD) or high CV risk, including diabetes or chronic kidney disease (CKD), the target is SBP <130mmHg.1
The 2024 European Society of Hypertension (ESH) guidelines support these targets, recommending a target SBP of <130mmHg and DBP <80mmHg for most patients up to 79-years. Lifestyle interventions have also proven effective in reducing blood pressure, including adopting a healthy diet, regular physical activity, weight reduction, sodium restriction to <5g/ day, increasing potassium intake, limiting alcohol intake, quitting smoking, and managing stress. 2
The WHO recommends starting antihypertensive medication for individuals who have an SBP of ≥140mmHg, or a DBP of ≥90mmHg. For those with existing CVD and an SBP between 130mmHg139mmHg, treatment is also recommended. Additionally, individuals without CVD but with high CV risk factors such as diabetes or CKD, and an SBP in the 130mmHg139mmHg range, should also begin treatment.1
When starting anti-hypertensive therapy, the WHO suggests conducting laboratory tests to screen for comorbidities and secondary hypertension, provided these tests do not delay the initiation of treatment. CVD risk assessment should be carried out if feasible but should not hinder treatment initiation.1
For first-line pharmacological treatment, the WHO recommends using drugs from one of three classes:1
1 Thiazide and thiazide-like diuretics.
2 Angiotensin receptor blockers (ARBs) or angiotensin-converting enzyme inhibitors (ACEis).
3 Long-acting dihydropyridine calcium channel blockers (CCBs).
Anti-hypertensives: Who and what?
According to the ESH guidelines, the pharmacological treatment of hypertension involves a stepwise approach, starting with combination therapy for most patients. The initial combination typically includes an ACEi or ARB with a CCB or a thiazide/ thiazide-like diuretic. If well tolerated, the dosage can be increased to achieve up to 60% control of BP. 2
For patients requiring further
control, a triple combination therapy is recommended, adding another agent to the initial combination, potentially achieving up to 90% control. 2
Monotherapy should be reserved for selected patients, such as those with low-risk hypertension, high-normal BP with very high CV risk, or frail and elderly patients. Beta-blockers (BBs) can be used as monotherapy or at any step of combination therapy. 2
In cases of true resistant hypertension, where BP remains uncontrolled despite the use of three anti-hypertensive agents, additional therapies such as spironolactone or other mineralocorticoid receptor antagonists can be considered. Renal denervation may be an option for patients with an estimated glomerular filtration rate >40ml/min/1.73 m². 2
International guidelines recommend ARBs or ACEis as first-line therapies for patients with various comorbidities
Peresuodei et al conducted a systematic review comparing the safety and efficacy of ARBs and ACEis in the management of hypertension. The review included 10 studies and data of >1.6 million patients. 3
ARBs work by competitively binding to angiotensin-2 receptors, preventing its activation, while ACEis block the conversion of angiotensin (AT)-1 into the active form, AT-2. 3
The team found that ARBs and ACEis are equally safe and effective in managing hypertension. However, in terms of tolerability, the review noted that ARBs were better tolerated than ACEis. 3
Patients on ACEis were more likely to experience dry cough and angioedema compared to those on ARBs. Overall, ARBs had a lower rate of adverse effects (AEs). 3
According to Dézsi, the introduction of ARBs represents a significant milestone in the management of hypertension,
expanding personalised treatment options, particularly for patients intolerant to ACEis. 4 International guidelines recommend ARBs or ACEis as first-line therapies for patients with various comorbidities, including microalbuminuria, renal dysfunction, CKD, metabolic syndrome, diabetes, atherosclerosis, stable angina, a history of myocardial infarction (MI), atrial fibrillation (AFib), and heart failure (HF). 4
A large-scale observational study involving >3 million patients commencing anti-hypertensive treatment with either an ARB or ACEi found no significant difference in effectiveness between the two classes in preventing acute MI, HF, stroke, or composite CV events. However, ARBs demonstrated a better safety profile, with lower risks of acute pancreatitis, angioedema, cough, and gastrointestinal bleeding. 5
Currently, guidelines do not specify which ARB should be used for patients with various comorbidities, although several studies have indicated that certain ARBs can provide additional beneficial effects. 4 Among ARBs, telmisartan is unique, as it is the only ARB approved for reducing CV morbidity in patients with atherothrombotic CV disease. This approval is based on findings from the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial, which demonstrated that telmisartan offers similar reductions in composite endpoints of CV death, MI, stroke, or HF hospitalisations compared to ramipril. Additionally, the Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease reported that telmisartan was linked to reduced CV hospitalisations, lower left ventricular hypertrophy, and decreased incidence of both macrovascular and microvascular events, including microalbuminuria. A combined analysis with data from the Prevention Regimen for Effectively Avoiding Second Strokes study also revealed telmisartan’s significant benefits in reducing CV death, MI, and stroke. Furthermore, telmisartan has been shown to significantly reduce the recurrence of AFib compared to carvedilol, amlodipine, and ramipril. 4
A meta-analysis of eight trials found that telmisartan outperformed other ARBs in reducing fasting plasma glucose and increasing adiponectin levels. At an 80mg dose, telmisartan lowered fasting plasma insulin levels and improved insulin resistance, measured by the homeostasis model assessment, and was associated with a 16% reduction in the risk of newonset diabetes compared to placebo. 4
The Angiotensin II Receptor Blockade in Obese Patients with Hypertension and Insulin Resistance study demonstrated that telmisartan significantly improved the hyperinsulin response to glucose loading in obese patients with hypertension and insulin resistance, along with improvements in vascular inflammation, reductions in visceral fat, and increases in serum adiponectin. 4
Telmisartan also shows promise in improving proteinuria and preventing progression to end-stage renal disease (ESRD). It significantly reduced urinary albumin/protein excretion and the urinary albumin/protein to creatinine ratio by 20% and 14%, respectively, compared to other ARBs and ACEis, resulting in an overall reduction of 40%. 4
Furthermore, telmisartan appears to protect against hypertension-related cognitive decline through angiotensin 1 receptor blockade and partial activation of PPAR- γ in the hippocampus, significantly restoring cognitive functions impaired by chronic stress and reducing forgetfulness. 4
Effective antihypertensive treatment must sustain BP control, particularly in the last six hours of the dosing period or following a missed dose. Early morning BP surges and 24-hour mean BP are associated with CV events and target-organ damage. 6
A key advantage of telmisartan is its long half-life, providing 24-hour protection. Lacourcière et al compared telmisartan’s long half-life with the shorter half-life valsartan during the last six hours of treatment and after a missed dose. 6,8
In their study, participants received once-daily telmisartan (40mg-80mg) or valsartan (80mg-160mg) for eight weeks. Results showed that during the last six hours of treatment, telmisartan reduced DBP by 7.6mmHg compared to 5.8mmHg with valsartan, with SBP reductions of 11.1mmHg for telmisartan versus 9.1mmHg for valsartan. 6
After a missed dose, telmisartan reduced 24-hour mean DBP by 7.2mmHg versus 5.5mmHg with valsartan, and SBP reductions were 10.7mmHg with telmisartan compared to 8.7mmHg with valsartan. The authors concluded that telmisartan consistently provided greater BP reductions, especially in the last hours of the dosing interval, making it particularly beneficial for patients with poor adherence to treatment. 6
In the Study of Micardis (telmisartan) in Overweight/Obese Patients with Type 2 Diabetes and Hypertension, 840 patients were randomised to receive telmisartan 80mg or valsartan 160mg daily for four weeks, followed by HCTZ 12.5mg for six weeks.7
Results showed that telmisartan/HCTZ significantly reduced mean BP in the last six hours of the dosing interval compared to valsartan/HCTZ, with greater reductions in SBP and DBP.7
Overall, telmisartan combined with HCTZ provided more significant BP reductions, particularly during early morning hours, making it a more effective option for managing high-risk patients with hypertension and type 2 diabetes.7
The fixed-dose combination of telmisartan and hydrochlorothiazide (HCTZ) is used for adults whose BP is inadequately controlled with telmisartan alone. Studies demonstrate that adding HCTZ to telmisartan significantly improves BP control, with reductions in ambulatory SBP and diastolic DBP of 21.5/14.6mmHg over 24-hours, 21.8/14.9mmHg during the day, and 20.4/13.7mmHg at night. 8,9,10,11
Concerns have been raised regarding the metabolic side effects of thiazide diuretics, particularly their impact on glucose tolerance. Combining ARBs with HCTZ can mitigate some of the metabolic effects associated with thiazide diuretics. For instance, ARBs reduce the risk of hypokalaemia, and their use may also
counterbalance the tendency of thiazides to cause hyperglycaemia and new-onset diabetes. Studies have shown that ARBs are linked to fewer cases of new-onset diabetes compared to certain other agents.12
Losartan has demonstrated benefits in reducing the relative risk of the composite endpoint of death, MI, or stroke by 13% in the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) study. Greater benefits were observed in diabetic patients, where mortality was reduced by 39%. 4
The Evaluation of Losartan in the Elderly (ELITE) I and II trials showed that, in elderly patients living with HF, treatment with losartan provided similar outcomes to captopril regarding allcause mortality, sudden death, and resuscitated arrests. In the ELITE I study, losartan also showed a lower mortality rate, primarily due to a greater reduction in sudden cardiac death. Additionally, using a 150mg dose of losartan provided further benefits compared to the 50mg dose, reducing the rate of death or HF hospitalisation and improving left ventricular ejection fraction. 4
Post-hoc analyses indicate that losartan, valsartan, and candesartan are associated with a 20%-35% reduction in the incidence of new-onset AFib. Losartan also effectively reduced the maximum and total duration of paroxysmal AFib in patients with sick sinus syndrome, without causing significant haemodynamic changes. 4
Studies have shown that losartan reduces the risk of ESRD by 28% and lowers urinary protein excretion by 35% in patients with diabetic nephropathy, compared to placebo. 4
Treatment with losartan has also been shown to enhance erectile function, sexual satisfaction, and frequency of sexual activity in hypertensive patients. In diabetic patients, losartan alone or in combination with tadalafil significantly improved erectile dysfunction (ED), with the greatest benefit observed in those with mild to moderate ED. 4
Additionally, losartan uniquely lowers serum uric acid (SUA) levels, which are associated with an increased risk of gout. Compared with a low dose (50mg), highdose losartan (150mg) reduced SUA by -0.27mg/dl. High-dose losartan reduced the incidence of hyperuricaemia.13
A real-world study assessed the effectiveness of combining losartan 50mg with HCTZ 12.5mg in managing isolated systolic hypertension among patients with various comorbidities. The analysis included 15 846 patients diagnosed with SBP >140mmHg and DBP <90mmHg, excluding those with diabetes or CKD. The most prevalent CV risk factor was hypercholesterolaemia (48.1%), followed by obesity (16.3%). Other conditions included cerebrovascular diseases (9.6%), ischaemic heart disease (7.9%), and left ventricular hypertrophy (4.6%). The proportion of patients with complications varied by age: 62% for ≤64 years, 69% for 65-74 years, and 67% for ≥75 years. Mean BP improved from 156/78mmHg initially to 140/72 mmHg at one month and 134/72mmHg at six months.14
Laboratory values for total cholesterol, uric acid, haemoglobin A1c, and serum potassium remained stable. Adverse effects such as orthostatic hypotension and significant BP drops were rare. This combination therapy proved effective and safe in achieving the recommended BP target of <140mmHg.14
Amlodipine is a widely used CCB that effectively lowers blood pressure by inhibiting calcium influx into vascular smooth muscle cells, leading to vasodilation. It is particularly beneficial for patients with isolated systolic hypertension, a common condition in the elderly. Amlodipine has a favorable side effect profile and is well-tolerated by most patients. Additionally, it has been shown to reduce the risk of CV events, including stroke and MI.15
According to the 2024 European Society of Cardiology (ESC), combination therapy including a CCB combined with either a thiazide diuretic or a renin-angiotensin system blockers (ACEis or ARBs) should be considered in patients of African descent
who require BP-lowering treatment.16
The ESC recommends BBs in specific situations, such as managing angina, HF, or post-MI, and for controlling heart rate, where they play a crucial role in therapy. In these cases, second-generation (cardio-selective) and third-generation (vasodilating) BBs are recommended. However, BBs are generally less effective than ACEis, ARBs, CCBs, or diuretics in preventing stroke and have a higher discontinuation rate due to side effects. Additionally, the use of BBs and diuretics, particularly in combination, is linked to an increased risk of new-onset diabetes in susceptible patients.1
In conclusion, patient selection is crucial in hypertension management, and telmisartan offers significant advantages, particularly for those with comorbidities such as atherosclerosis, diabetes, and CKD. Its long half-life ensures sustained BP control, particularly in the last hours of the dosing interval, making it beneficial for patients with adherence challenges.
Telmisartan also provides additional CV protection, reducing the risk of MI, stroke, and HF hospitalisations, while improving insulin resistance and reducing proteinuria.
Patients with hypertension who struggle with early morning BP surges or those who require better 24-hour BP control may particularly benefit from telmisartan. Furthermore, its fixed-dose combination with HCTZ enhances blood pressure control while minimizing metabolic side effects at lower doses. Overall, telmisartan is an excellent option for high-risk patients requiring both BP management and CV protection.
Losartan is beneficial for patients with diabetes and CVD, while CCBs are effective in reducing BP by relaxing blood vessels, and BBs are often used for patients with HF or arrhythmias.
1. World Health Organization. Global report on hypertension: the race against a silent killer. 2023 [Internet]. Available at https://www.who.int/ publications/i/item/9789240081062
2. European Society of Hypertension. 2024 European Society of Hypertension clinical practice guidelines for the management of arterial hypertension. European Journal of Internal Medicine, 2024.
3. Peresuodei TS, et al A Comparative Study of the Safety and Efficacy Between Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers on the Management of Hypertension: A Systematic Review. Cureus, 2024.
4. Dézsi CA. The Different Therapeutic Choices with
ARBs. Which One to Give? When? Why? Am J Cardiovasc Drugs, 2016.
5. Chen RJ, et al Comparative First-Line Effectiveness and Safety of ACE (Angiotensin-Converting Enzyme) Inhibitors and Angiotensin Receptor Blockers. A Multinational Cohort Study. Hypertension, 2021.
6. Lacourcière Y, Krzesinskib J-M, Whitec WB, et al Sustained antihypertensive activity of telmisartan compared with valsartan. Blood Pressure Monitoring, 2004.
7. Sharma AM, Davidson J, Koval S, Lacourcière Y. Telmisartan/hydrochlorothiazide versus valsartan/ hydrochlorothiazide in obese hypertensive patients with type 2 diabetes: the SMOOTH study. Cardiovascular Diabetology, 2007.
8. Gosse P. A review of telmisartan in the treatment of hypertension: blood pressure control in the early morning hours. Vasc Health Risk Manag, 2006.
9. Professional Information. Telmisartan/ Hydrochlorothiazide 40 mg/12.5 mg tablets. 2023 [Internet]. Available at: www.hpra.ie/img/ uploaded/swedocuments/Licence_PA2315-107001_12042023131159.pdf
10. Fogari R, et al Effectiveness of hydrochlorothiazide in combination with telmisartan and olmesartan in adults with moderate hypertension not controlled with monotherapy: a prospective, randomized, open-label, blinded end point (PROBE), parallel-arm study. Curr Ther Res Clin Exp, 2008.
11. Kjeldsen SE, et al. Telmisartan and hydrochlorothiazide combination therapy for the treatment of hypertension. Curr Med Res Opin, 2010.
12. Weir MR, Bakris GL. Combination therapy with Renin-Angiotensin-aldosterone receptor blockers for hypertension: how far have we come? J Clin Hypertens (Greenwich), 2008.
13. Ferreira JP, Zannad F, Kiernan MS, Konstam MA. High- versus low-dose losartan and uric acid: An analysis from HEAAL. Journal of Cardiology, 2023.
14. Suzuki H, et al Antihypertensive effectiveness of combination therapy with losartan/ hydrochlorothiazide for ‘real world’ management of isolated systolic hypertension. Therapeutic Advances in Cardiovascular Disease, 2015.
15. Bulsara KG, et al. Amlodipine. [Updated 2024 Apr 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK519508/
16. McEvoy JW, et al. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension. European Heart Journal, 2024. SF
Scan for the quiz

This article was independently sourced by Specialist Forum.
Warfarin, a vitamin K antagonist (VKA), has long been the standard treatment for atrial fibrillation (AFib), supported by strong evidence for preventing stroke and thrombotic events. However, its clinical use presents challenges such as slow onset, numerous drug and food interactions, and an increased risk of major bleeding, necessitating frequent monitoring.
The introduction of non-VKA oral anticoagulants (NOACs) has significantly improved AFib management, offering better safety profiles, fewer interactions, and enhanced patient adherence.
AFib is the most common sustained cardiac
This CPD activity was made possible by Sanofi. The content was independently sourced.

arrhythmia in adults globally, associated with significant morbidity, mortality, and a rising economic burden. The condition’s prevalence, currently estimated between 2% and 4% in adults, is expected to increase due to aging populations and improved diagnostics. Age, hypertension, diabetes, and heart failure
are major risk factors, contributing to its growing prevalence. Diagnosis requires ECG documentation showing irregular heart rhythms without distinct P-waves.
To access the article and quiz, go to https://www.medicalacademic.co.za/ courses/ SF






















This article was independently sourced by Specialist Forum.
It is estimated that ~10% of adults ≥75-years have atrial fibrillation (AFib). AFib results in uncoordinated contractions of the heart’s atria, which can lead to the formation of blood clots. These clots increase the risk of stroke five-fold and can also contribute to cognitive decline, renal impairment, and mesenteric ischaemia.1
Studies show that ~20% of strokes occur in patients with known AFib who were either inadequately anticoagulated or inappropriately treated with antiplatelet drugs.1
Stroke recovery can be complicated by venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE). The risk of VTE is highest within the first three months following a stroke, decreasing rapidly afterward. 2
Although symptomatic PE occurs in only about 1% of stroke survivors within the first two- to four-weeks, it remains a significant cause of preventable death, accounting for up to 30% of post-stroke fatalities. Strokerelated VTE has also been linked to increased disability at six months and lower survival rates at 30-days and one-year post-stroke.2 It is therefore extremely important to evaluate patients’ stroke risk, and those identified as high-risk should start treatment with non-vitamin K oral anticoagulants (NOACs) as the primary antithrombotic therapy, according to Giskes et al 1 NOACs are direct-acting medications that specifically target either thrombin (factor IIa) or activated factor X (Xa). Evidence indicates that NOACs are at least as effective as, if not superior to, warfarin in reducing the risk of
stroke, with similar or lower rates of bleeding compared to warfarin.1
NOACs reduce stroke risk by ~70% and mortality by ~30%. Intracranial haemorrhage (ICH) rates are significantly lower with NOACs compared to warfarin, and the incidence of major gastrointestinal (GI) bleeding is comparable between NOACs and warfarin.1
NOACs offer practical benefits in clinical settings, including fewer food and drug interactions and the absence of the need for frequent monitoring. They achieve full anti-coagulation within one to two hours of dosing due to their rapid onset.1
Are NOACs universally suitable for all patients?
In South Africa, the available NOACs include dabigatran, a direct inhibitor of factor IIa, and apixaban and rivaroxaban, which are direct inhibitors of factor Xa.1
All three NOACs are indicated for the prevention of VTE in patients undergoing hip or knee replacement surgery, the reduction of stroke and SE risk in patients living with AFib, and the treatment and prevention of recurrent DVT PE. 3,4,5
Dabigatran, apixaban, and rivaroxaban each serve these purposes, with slight
variations. Apixaban additionally reduces the risk of mortality in patients living with NVAFib with one or more risk factors. 3,4,5 Rivaroxaban and apixaban are currently among the most prescribed NOACs. However, their distinct pharmacokinetic and pharmacodynamic profiles can influence their safety and effectiveness differently. 6
Deciding to anticoagulate a patient involves assessing the need for anticoagulant therapy. The CHA2DS2-VASc score has expanded eligibility for anticoagulants to 90%-95% of patients, compared to the previous 66% with the CHADS2 score.9
Given the low bleeding rates of NOACs, most patients living with NVAFib should receive anticoagulant therapy. Exceptions are patients with active bleeding, recurrent bleeding from anticoagulants, or a CHA2DS2-VASc score of 0. For patients who had major bleeding with warfarin, alternatives like apixaban or rivaroxaban should be considered before eliminating anticoagulation.9
According to Bonde et al, clinical guidelines do not endorse a particular NOAC over

others for stroke prevention in AFib. As a result, there is considerable variability in the choice of NOACs, leading to similar patients potentially receiving different NOACs depending on the treatment facility.7
Patient profiles that can aid in tailoring anticoagulation therapy include:9,10 _ Apixaban is preferred due to its consistent efficacy in reducing bleeding outcomes, including intracranial bleeding. For younger patients with good kidney function and no bleeding history, apixaban offers significant protection from stroke.
_ Apixaban is favoured over rivaroxaban for patients with a high bleeding
risk due to its lower incidence of bleeding complications.
_ Apixaban is recommended as it is associated with a lower rate of GI bleeding compared to rivaroxaban.
_ Apixaban is suitable for patients with chronic kidney disease (CKD), including those with end-stage renal disease, as it does not require dose adjustment based on CKD stage.
_ Rivaroxaban, with its once-daily dosing, can be beneficial for improving patient adherence compared to medications requiring multiple daily doses. However, comparisons between once-daily and twice-daily dosing regimens reveal that
despite a higher percentage of prescribed doses being taken with once-daily dosing, twice-daily dosing ensures a greater degree of continuity in drug action.
For the prevention of VTE following elective hip or knee replacement surgery, the recommended dose of apixaban is 2.5mg taken orally twice daily. The first dose should be administered 12- to 24-hours post-surgery. For hip replacement surgery, the treatment duration is recommended to be 32- to 38- days, while for knee replacement surgery, it is 10- to 14-days. 4
According to Agnelli et al, the decision to extend treatment is challenging. In the Apixaban for the Extended Treatment of Venous Thromboembolism (AMPLIFY-EXT) the team set about evaluating the efficacy and safety of two doses of apixaban compared to placebo in patients with VTE who had completed six- to 12-months of initial anticoagulation therapy and were uncertain about continuing treatment.11
Agnelli et al evaluated the efficacy and safety of two apixaban doses (2.5mg and 5mg twice daily) compared to placebo. The study medications were administered for 12 months.11
The results showed that among 2486 randomised patients (with 2482 included in the intention-to-treat analysis), the rate of symptomatic recurrent VTE or death from VTE was significantly lower in both apixaban groups compared to the placebo group.11
Specifically, 8.8% of placebo recipients experienced these outcomes, while only 1.7% of those receiving either the 2.5mg or 5mg dose of apixaban did. The difference between apixaban and placebo was 7.2 percentage points for the 2.5mg dose and seven percentage points for the 5mg dose, with both comparisons reaching statistical significance.11
Regarding bleeding risks, major bleeding occurred in 0.5% of the placebo group, 0.2% in the 2.5mg apixaban group, and 0.1% in the 5mg apixaban group. Clinically relevant non-major bleeding rates were slightly higher in the apixaban groups compared to placebo. The rate of death from any cause was also lower in the apixaban groups compared to the placebo group.11
In conclusion, extended treatment with apixaban, whether at a treatment dose of 5mg or a prophylactic dose of 2.5mg, effectively reduced the risk of recurrent VTE without increasing the rate of major bleeding.11
For the prevention of stroke and SE in patients with NVAFib, the recommended
dose is 5mg taken orally twice daily. However, for patients who meet at least two of the following criteria: Age ≥80-years, body weight of ≤60kg, or serum creatinine levels of ≥1.5mg/dl or higher –the recommended dose is 2.5mg twice daily. 4
In the Apixaban Versus Aspirin in Patients with Atrial Fibrillation (AVERROES) trial, 5599 patients living with AFib and at least one additional stroke risk factor, who were unsuitable for VKA therapy, were enrolled at 522 sites across 36 countries.12
Patients were randomised to receive apixaban (n=2808) or aspirin (n=2791). After a mean follow-up of 13-months, the trial was stopped early due to the clear benefit of apixaban, which significantly (55%) reduced the risk of stroke or SE compared to aspirin with similar major bleeding risk.12
Following the double-blind phase, an open-label extension was initiated, allowing participants to continue receiving apixaban. Of the 5599 participants, 58.5% continued apixaban treatment during the open-label extension, with a median follow-up of three years.12
During this period, the annual rates of stroke or SE, haemorrhagic stroke, and major bleeding were 1.0%, 0.3%, and 1.2%, respectively. These event rates were consistent with those observed during the initial trial phase.12
An analysis of all patients who received apixaban from the start of AVERROES to the end of the extension showed similar results, supporting the long-term efficacy and safety of apixaban in patients living with AFib.12
In a retrospective cohort study by Ray et al, the team compared major ischaemic and haemorrhagic outcomes between apixaban and rivaroxaban in patients ≥65-years (n=581 451). Follow-up was four years.13
The primary outcome was a composite of major stroke/SE and haemorrhagic (ICH and other significant bleeding) events. Secondary outcomes included non-fatal extracranial bleeding and total mortality.13
Results showed that the adjusted primary outcome rate for rivaroxaban was 16.1 per 1000 person-years compared to 13.4 for apixaban, indicating a higher risk associated with rivaroxaban. Rivaroxaban also had an increased risk for both major ischaemic events (8.6 vs 7.6 per 1000 person-years) and haemorrhagic events (7.5 vs 5.9). Additionally, patients on rivaroxaban experienced higher rates of non-fatal extracranial bleeding (39.7 vs 18.5) and total mortality (44.2 vs 41).13
In conclusion, both apixaban and rivaroxaban offer significant benefits in managing patients living with AFib. Apixaban is generally preferred in patients at higher risk of bleeding, particularly those with CKD or a history of GI bleeding. For extended treatment, apixaban effectively reduces the risk of recurrent VTE without increasing major bleeding, providing a safe and efficient option for longterm anticoagulation therapy. Tailoring patient selection based on individual risk profiles remains essential.
References
1. Giskes K, Lowres N, Hespe C, Freedman B. Stroke Risk Mitigation Prescribing and Monitoring Anticoagulation in Atrial Fibrillation. Modern Medicine, 2024.
2. Tøndel BG, Morelli VM, Hansen JB, Braekkan SK . Risk factors and predictors for venous thromboembolism in people with ischemic stroke: A systematic review. J Thromb Haemost, 2022.
3. Professional Information. Pradaxa. 2022. [Internet]. https://pi-pil-repository. sahpra.org.za/wp-content/uploads/2022/08/pi-pradaxa-17aug2022.pdf
4. Professional Information. Apixaban. 2024 [Internet]. Available at: https:// pi-pil-repository.sahpra.org.za/wp-content/uploads/2024/04/Final_PIL_ Apixaban-Accord_Applicant-1.pdf
5. Professional Information. Xarelto. 2022 [Internet]. Available at: https://pi-pilrepository.sahpra.org.za/wp-content/uploads/2022/05/approved-xarelto-15and-20-pi-05.2022.pdf
6. Bonde AN, Martinussen T, Lee CJ-Y, et al. Rivaroxaban Versus Apixaban for Stroke Prevention in Atrial Fibrillation. An Instrumental Variable Analysis of a Nationwide Cohort. Circ Cardiovasc Qual Outcomes, 2020.
7. Professional Information. Ixarola. 2022 [Internet]. Available at: https://pi-pilrepository.sahpra.org.za/wp-content/uploads/2022/05/approved-ixarola-1520-pil-05.2022.pdf
8. Byon W, Garonzik S, Boyd RA, Frost CE. Apixaban: A Clinical Pharmacokinetic and Pharmacodynamic Review. Clinical Pharmacokinetics, 2019.
9. Schaefer JK, McBane RD, Wysokinski WE. How to choose appropriate direct oral anticoagulant for patients with nonvalvular atrial fibrillation. Ann Hematol, 2016.
10. Vrijens B, Heidbuchel H. Non-vitaman K antagonist oral anticoagulants: considerations on once- vs. twice-daily regimens and their potential impact on medication adherence. Europace, 2015.
11. Agnelli G, Buller HR, Cohen A, et al Apixaban for Extended Treatment of Venous Thromboembolism. NEJM, 2013.
12. Benz AP, Eikelboom JW, Yusuf S, et al. Long-Term Treatment with Apixaban in Patients with Atrial Fibrillation: Outcomes during the Open-Label Extension following AVERROES. Thromb Haemost, 2021.
13. Ray WA, Chung CP, Stein M, et al Association of Rivaroxaban vs Apixaban with Major Ischemic or Hemorrhagic Events in Patients with Atrial Fibrillation. JAMA, 2021. SF
Scan for the quiz














joey@takenoteevents.co.za


The 2024 European Society of Cardiology guidelines recommend non-vitamin K antagonists (NOACs) over VKAs (eg warfarin) for preventing ischaemic stroke and thromboembolism in patients living with atrial fibrillation (AFib) – except for those with mechanical heart valves or moderate-to-severe mitral stenosis.1
For decades, warfarin was used as the primary anticoagulant. Warfarin achieves its anticoagulant effect by inhibiting clotting factors II, VII, IX, and X. However, it is prone to numerous drug and food interactions, requiring regular blood tests to maintain the INR within the therapeutic range. This demands significant patient time, medical resources, and careful dose adjustments by healthcare providers, which can be challenging. 2,3
In contrast, NOACs target a single clotting factor, such as factor Xa or factor IIa (thrombin), offering a more predictable and stable anticoagulant effect with fewer drug and food interactions and no need for routine monitoring. 3
In South Africa, three NOACs are available: Dabigatran, a direct inhibitor of factor IIa, as well as apixaban and rivaroxaban, which are direct inhibitors of factor Xa. All three NOACs are indicated for the reduction of stroke and systemic embolism (SE) in patients living with AFib. 4,5,6
Dabigatran was the first NOAC introduced (2010). In May this year, Pharma Dynamics introduced dabigatran etexilate, the first generic alternative to dabigatran. The new generic formulation is available in three strengths: 75mg, 110mg, and 150mg. 2,7
NOACs superior to warfarin
The superiority of NOACs as a class compared to warfarin has been shown in
numerous studies. NOACs have significantly improved the safety profile and treatment adherence of patients living with AFib. 2
A network-analysis by Chan et al (2024) showed that among the NOACs, only dabigatran had a lower risk of all-cause mortality than warfarin. Dabigatran was also associated with lower risks of major bleeding and intracranial haemorrhage (ICH). 8
Costs associated with stroke treatment and care
Stroke treatment and management impose a significant economic burden on healthcare resources in terms of direct medical expenses and indirect costs. In the

United States, expenditures on stroke treatment and care amount to ~R646bn annually and in Europe ~R1.312tn.9
In South Africa, the estimated total direct stroke treatment and care costs over a period of five-years were R7.3tn, with R2.6bn from inpatient care. The economic stroke burden was found to be higher in patients living with hypertension, cardiovascular diseases, and diabetes.10
What is the most costeffective anticoagulant?
Although NOACs are more expensive than warfarin, a cost-utility analysis comparing warfarin (target INR 2-3), apixaban (5mg twice daily), dabigatran (150mg twice daily), rivaroxaban (20mg once daily), and no treatment, found that all NOACs showed positive incremental net monetary benefits compared to warfarin, indicating their cost-effectiveness.9,11
per QALY in the United States) are considered good value for the health benefits they provide.12
In the South African context, a 2013 study by Bergh et al found that dabigatran was cost-effective, with an ICER of ~R93 290 per QALY gained, compared to warfarin. Avoiding INR testing with dabigatran reduces the ICER by up to 15.7%.13
Generic medicines contain the same active substances, strength, dosage form, and route of administration as their branded counterparts
According to a costing report compiled by the British National Institute of Health and Care Excellence, dabigatran offers a greater reduction in stroke risk, which significantly impacts expected costs and qualityadjusted life years (QALYs).11
Similarly, Wu et al found that based on the incremental costeffectiveness ratio (ICER) of ~R743 000 per QALY, NOACs are cost-effective. In many healthcare systems, treatments with an ICER below certain thresholds (eg typically ~R878 000 to ~R1.7m
Date: 2nd November




How effective is dabigatran etexilate and how does the cost compare to the originator?
Generic medicines are defined as those containing the same active substances, with identical strength, dosage form, and route of administration as their branded counterparts.14
They meet comparable standards for therapeutic equivalence. Unlike originator firms, generic manufacturers do not bear the research and development costs, allowing them to offer medicines at significantly lower prices – typically between 20% and 90% less.14
Generic medicines have become a crucial competitive factor in the pharmaceutical market, capturing significant market share once the patents of originator medicines expire. In South Africa, policies supporting generics, along with increased registration and improved patient acceptance, have resulted in a steady rise in the use of generic medicines.14
Dabigatran etexilate is a small molecule pro-drug with no direct pharmacological activity. Once administered orally, it is rapidly absorbed and converted to dabigatran by esterases in the plasma and liver. Dabigatran acts as a competitive, reversible direct thrombin inhibitor, the active component in plasma.15
Thrombin, a serine protease, converts fibrinogen into fibrin during coagulation, and inhibiting it prevents thrombus formation. Dabigatran also inhibits free thrombin, fibrin-bound thrombin, and thrombin-induced platelet aggregation.15
According to Sorenson et al, patients treated lifelong with dabigatran etexilate experienced fewer ICH (0.49 for dabigatran etexilate vs 1.13 for warfarin) and fewer ischaemic strokes (4.40 for dabigatran etexilate vs 4.66) per 100 patient-years.16






































The ICER of dabigatran etexilate was ~R183 000 per QALY compared to warfarin. This study highlights the cost-effectiveness of dabigatran etexilate as an alternative for stroke and SE prevention.16
While warfarin has long been the standard anticoagulant for stroke prevention in AFib, dabigatran offers clear advantages in both clinical outcomes and cost-effectiveness. Dabigatran provides a significant reduction in stroke risk and ICH compared to warfarin, with fewer drug and food interactions, and eliminates the need for routine INR monitoring, thus reducing overall healthcare costs.
Although dabigatran has a higher upfront cost, its longterm cost-effectiveness is well-documented, particularly when considering the avoidance of stroke-related costs.

Furthermore, the introduction of generic dabigatran etexilate in South Africa offers even greater cost savings, as generic versions are typically 20% to 90% cheaper than the originator, making it a more affordable and accessible option for both patients and healthcare systems. This reinforces the value of dabigatran, especially in its generic form, as a cost-effective alternative to warfarin for stroke prevention in atrial fibrillation.
References are available on request. SF

In the second instalment of our three-part series from Boehringer Ingelheim’s House of Stroke symposium, we explore key presentations on the comparative efficacy of thrombectomy versus thrombolysis and delve into the complexities surrounding atypical stroke cases.
Thrombectomy or trombolysis?
Dr Pradeep Rowji, neurologist based at Milpark Hospital in Johannesburg
When faced with the critical decision between thrombectomy and thrombolysis in the management of ischaemic stroke, it is essential to understand the personalised nature of these treatments. Each option offers distinct advantages, depending on the patient’s unique anatomy, the characteristics of the clot, and the location of the occlusion. One of the primary reasons to choose thrombectomy over thrombolysis lies in the ability to provide a highly personalised treatment. As experienced neuroradiologists emphasise, the approach can be tailored specifically to the patient’s vascular anatomy, particularly in older patients who often present with abnormally deformed vasculature. This personalisation begins with the choice of access, whether right or left radial, depending on the patient’s vessel architecture.
Thrombectomy allows for precision in navigating the sometimes-murky territory of the brain’s vasculature. The goal is to precisely reach the occluded vessel and remove the clot causing the stroke. Early investigations guide the intervention, ensuring that the procedure is both efficient and effective. When the clot is successfully removed and blood flow is restored, the result is often described as
perfection in stroke treatment.
Thrombectomy is particularly advantageous when dealing with large vessel occlusions, such as those involving the carotid arteries or the anterior circulation vessels. These occlusions can lead to anterior ischaemic strokes, where a significant portion of the brain’s blood supply is compromised. In these cases, the decision between thrombectomy and thrombolysis becomes crucial.
Thrombectomy is often the preferred choice when faced with complex occlusions, such as those involving the internal carotid artery (ICA) and the middle cerebral artery (MCA). These occlusions can take on a T shape (involving the ICA, MCA, and anterior cerebral artery) or an L shape (involving the ICA and MCA).
The challenge with thrombolysis alone in these cases is that long segment thrombi, often measuring several centimetres, are resistant to lytic agents. While thrombolysis may work in rare cases with specific types of clots, it is generally less effective in these scenarios.
Thrombectomy is considered a gamechanger in stroke treatment, especially when compared to thrombolysis. The success of mechanical thrombectomy is reflected in the number needed to treat (NNT), a key metric in medical treatment.
For thrombolysis, the NNT has historically
been around eight, meaning that eight patients need to be treated for one to benefit. However, with advances in thrombectomy techniques and technology, the NNT has been reduced to as low as 2.8 in some cases. This means that nearly one in every three patients treated with thrombectomy will have a positive outcome, a figure unmatched by other interventions, including cardiac or trauma surgery.
The precision and effectiveness of thrombectomy in removing clots and restoring blood flow lead to better tissue perfusion and significantly improved outcomes for patients. This approach reduces the risk of partial vessel opening, a common issue with thrombolysis, which can lead to higher mortality rates at three months post-treatment.
The success of thrombectomy also hinges on the timely recognition and treatment of stroke. Time is brain, and the faster a patient receives treatment, the better the outcome. When a patient arrives at the hospital, the first step is to assess their NIHSS score, which helps determine the severity of the stroke and the urgency of intervention. Patients with a score above 15 typically require immediate thrombectomy, while those with lower scores may be monitored or treated differently.
Advanced imaging, including NCCT scans or more sophisticated techniques, plays a
crucial role in guiding the decision-making process. In settings with limited resources, even basic imaging can be sufficient to identify candidates for thrombectomy.
Not your typical stroke – challenging cases and whether to thrombolyse
Dr Naeem Brey, neurologist with special interests in clinical neurology, electrophysiology and neuro-immunology
Strokes represent a significant global health issue, with ~22 million cases occurring annually. Developing countries, such as South Africa and India, are particularly affected, and the increasing incidence of strokes among younger populations has necessitated a re-evaluation of how these cases are defined and managed. Traditionally, stroke care has focused on older populations, generally those ≥80-years. However, younger patients, often defined as those <55-years, are a growing concern.
In this context, Dr Brey presented a challenging case involving a 25-year-old woman who suffered a severe stroke 35 hours after giving birth. Safety in pregnancy and lactation has not been established with the alteplase. The patient had high blood pressure (BP) and exhibited severe stroke symptoms, including hemiparesis and dysarthria.
Initial imaging revealed extensive damage, and the case posed a significant dilemma regarding the use of thrombolysis – a treatment that can be risky due to the potential for bleeding, especially in postpartum women.
Despite the high risks associated with thrombolysis, including the potential for postpartum haemorrhage, Dr Brey and his team decided to proceed with the treatment. This decision was informed by a thorough review of existing literature and consultations with other stroke specialists.
The outcome was positive: Although the patient experienced mild bleeding, it resolved without further complications. Remarkably, her condition improved rapidly, and within five days, she was able to walk with minimal assistance and care for her newborn. By three months, she had made a complete recovery.
Current guidelines do suggest that thrombolysis may be appropriate within a four-and-a-half-hour window if the benefits outweigh the risks. Documented cases, such as the one presented by Dr Brey, generally show positive outcomes without a significant increase in bleeding risks.
Patient selection is crucial in stroke management. Factors such as age,
comorbidities, BP, National Institutes of Health (NIH) Stroke Scale score, and imaging results should guide the decisionmaking process.
The advent of endovascular mechanical thrombectomy has also revolutionised stroke care, offering another treatment option that should not be withheld from pregnant patients. Studies have shown no significant difference in outcomes between pregnant and non-pregnant women, even though strokes during pregnancy tend to be more severe.
Dr Brey emphasised the importance of considering differential diagnoses in stroke-like presentations during pregnancy. Conditions such as Haemolysis, Elevated Liver Enzymes, Low Platelet Count, Posterior Reversible Encephalopathy, and Reversible Cerebral Vasoconstriction Syndromes can mimic stroke symptoms and must be ruled out.
These conditions can significantly influence treatment decisions and outcomes, particularly when considering thrombolysis in challenging situations.
The 2022 European Stroke Organization (ESO) guidelines are pivotal in navigating these complex decisions, even in scenarios lacking robust data. The ESO guidelines advocate for intravenous thrombolysis (IVT) and thrombectomy in eligible patients, even during atypical scenarios such as menstruation, pregnancy, and the postpartum period. They recommend considering these treatments even in menopausal patients, although the window for postpartum thrombolysis is generally limited to ten days.
In addition to the case of the young postpartum woman, Dr Brey also discussed a 94-year-old female patient with multiple comorbidities who presented with a high initial stroke score. Alteplase is not indicated for the therapy of acute stroke in children and adolescents <18-years or adults >80- years.
Despite significant improvement in her symptoms, her family chose not to proceed with thrombolysis due to the risks involved. The patient’s condition initially improved but later deteriorated due to sepsis, ultimately leading to her death.
This case underscores the complexities of decision-making in elderly patients, particularly those with multiple comorbidities. While initial improvements might suggest a favourable outcome, the risk of complications such as infection can drastically alter the prognosis.
The Third International Stroke Trial (2012), which expanded eligibility for thrombolysis to older patients, found that while mortality
at seven days was higher for those treated with alteplase, long-term outcomes at six months were similar between treated and untreated groups.
More recent trials have confirmed that thrombolysis in patients >80-years offers significant benefits in terms of independence, with no increase in longterm mortality. The ESO guidelines support the use of IVT for patients >80-years, backed by strong evidence. Alteplase is not indicated for the therapy of acute stroke in children and adolescents <18-years or adults >80-years.
Thrombectomy also plays a crucial role in stroke management for older patients. American guidelines suggest that while thrombectomy can be beneficial in the elderly, selection criteria should be individualised. Data from the CT perfusion to Predict Response to Recanalization in Ischemic Stroke Project trial (2017) indicates that bleeding rates plateau >60-years, suggesting that age alone should not be a contraindication for thrombectomy.
1
Thrombectomy is a highly effective treatment for ischaemic stroke, particularly in cases involving large vessel occlusions. By physically removing the clot, thrombectomy rapidly restores blood flow to the brain, significantly improving outcomes. This procedure is especially beneficial when thrombolysis alone may be insufficient, offering a greater chance of recovery with fewer longterm disabilities. Thrombectomy’s precision in targeting and removing the clot ensures that more patients experience positive outcomes, making it a critical intervention in modern stroke care.
2 Stroke management must evolve to address the growing incidence of strokes among younger populations and the complexities of treating pregnant and elderly patients.
Dr Brey’s case of a 25-year-old postpartum woman illustrates that thrombolysis, despite its risks, can lead to remarkable recovery when carefully considered. The 2022 ESO guidelines support thrombolysis and thrombectomy in diverse patient groups, including those with pregnancy-related strokes and older adults, highlighting the importance of tailored treatment strategies and thorough patient selection. SF
This
Dry eye disease (DED) is an inflammatory condition with similarities to autoimmune diseases. Environmental factors, infections, and genetic predispositions can trigger stress on the ocular surface, leading to an inflammatory response.1

DED can be classified into two main types: Aqueous-deficient, characterised by reduced tear production, and evaporative, marked by increased tear film.1,2
As the disease progresses, the two types often overlap –referred to as mixed mechanism disease or hybrid DED. Evaporative DED is more common than aqueous-deficient DED. This mixed aetiology affects between 30% and 70% of patients. 2,3,4
Meibomian gland dysfunction (MGD) is widely recognised as a leading cause of DED. Symptoms of DED include redness, burning, stinging, a sensation of a foreign body, itching, and sensitivity to light. Some patients also present with neuropathic pain. DED can significantly impair functional vision, particularly during activities like reading, using a computer, or driving.1,2,3
Tear hyperosmolarity and instability are the primary factors driving DED. Tear hyperosmolarity, where the tear fluid has a higher concentration of solutes compared to the surrounding epithelial cells, leads to reduced cell volume and increased solute concentrations. 4
In aqueous-deficient DED, hyperosmolarity is caused by a decrease in lacrimal gland secretion. For instance, reduced tear production due to age-related lacrimal gland dysfunction is a common example of this condition. In contrast, in evaporative DED, hyperosmolarity results from excessive evaporation of the tear film. 4
As DED worsens, it may involve a gradual loss of corneal sensitivity, leading to reduced compensatory responses, which further exacerbate the condition. In advancing aqueous-deficient DED, the tear film’s lipid layer spreads less effectively, a process worsened by diminished reflex tearing.4 Research suggests that this reduced lipid layer spreading may increase evaporation, transforming aqueous-deficient DED into mixed DED. Similarly, in evaporative DED, corneal damage and reduced sensory drive to the lacrimal gland can lead to increased tear hyperosmolarity, evolving the condition into mixed DED. 4
Treating










































































































































































the time required for effective treatment. Each patient’s management plan will vary based on the underlying causes, disease severity, and external factors such as environmental conditions or medications. 4
After diagnosing DED and distinguishing between aqueous-deficient, evaporative, or mixed DED, the clinician’s first responsibility is to educate the patient about the condition, including treatment expectations and timelines. 4
For aqueous-deficient cases, treatment often begins with artificial tears, progressing to non-preservative solutions or higher-viscosity gels.
Artificial tears are a cornerstone in managing dry eye disease and play a role in treating corneal abrasions, promoting wound healing, managing pain and inflammation, addressing conjunctivitis and keratitis, rewetting and removing contact lenses, and aiding in foreign body removal. 4,5
Punctal occlusion may be considered for tear retention, especially in specific cases, though it remains debated in inflammatory conditions. 4
For evaporative cases, lid hygiene, warm compresses, and targeted therapies like antibiotics or anti-inflammatory treatments are also recommended. 4
Punctal occlusion can help retain tears and is especially useful for DED resulting from refractive surgery, contact lens wear, systemic diseases, or a rapid tear film break-up time. It is debated whether punctal plugs are suitable for patients with an inflammatory component, as they may prolong inflammation. 4
For those in adverse environments, moisture chamber goggles and humidifiers can reduce tear evaporation. In severe cases or when other treatments fail, autologous or allogeneic serums may be considered.
Oral secretagogues might also be an option for patients with aqueous-deficient or mixed DED, particularly if associated with Sjögren’s syndrome. 4
Artificial tears are available in both single-use and multi-dose formulations. Single-use units are preservativefree, while multi-dose packages typically contain preservatives to extend shelf life and prevent microbial growth. Newer formulations come in specially designed bottles that prevent micro-organism entry. 5,6
Preservatives like benzalkonium chloride (BAK) are associated with potential toxicity to the ocular surface, particularly with
long-term use. BAK can cause damage to corneal and conjunctival cells, delay corneal healing, and destabilise the tear film, leading to worsening symptoms. 6
Preservative-containing artificial tears are generally suitable for patients who require occasional use and do not have a history of ocular surface disease.
Preservative-free artificial tears are recommended for patients with severe DED who need frequent application throughout the day. 6
They are also preferred for individuals with known sensitivity to preservatives or existing ocular surface conditions, such as glaucoma or post-surgical recovery. 6
Preservative-free options significantly reduce the risk of preservative-induced ocular toxicity. Advances in multi-dose dispensers with unidirectional valves allow for preservative-free formulations while minimising the risk of contamination. 6
For patients with mixed DED, preservative-free propylene glycolhydroxypropyl guar (PG-HPG) nanoemulsion lubricant eye drops is a viable option, according to Silverstein et al. 7
PG-HPG features propylene glycol as the primary demulcent and employs a higher concentration of HPG gelling technology compared to previous formulations. This updated formulation includes a lipid excipient in nano-sized droplets that improves lipid coverage and provides a more translucent appearance.7
Upon application, the HPG/borate meshwork forms a protective, viscoelastic barrier on the ocular surface. As the pH normalises and sorbitol dilutes, this meshwork cross-links to maintain the barrier and slowly release lipids into the tear film.7
The inclusion of anionic phospholipid dimyristoyl phosphatidylglycerol enhances lipid stability by merging with existing lipids to address gaps caused by MGD. This nano-emulsion helps restore the tear film structure, prevent dry eye exacerbations, and support a healthier ocular surface.7
How effective are PG-HPG nanoemulsion lubricant eye drops?
In their phase IV study, Silverstein et al, evaluated the effectiveness of a single drop of PG-HPG nano-emulsion in relieving symptoms of DED. This formulation is now also available in preserved and multi-dose preservative-free options.7
Results showed significant symptom relief, with median score reductions of -1.0 at zero hours, -2 at four hours, and -2 at eight hours post-application. Both aqueous-deficient and evaporative
subtypes experienced a median symptom reduction of -2 at eight hours, while the mixed subtype had a reduction of -1.7
Median soothing sensation scores were 3 at zero hours, 4 at four hours, and 3.5 at eight hours. Tolerability was high across all DED subtypes, with most patients reporting low scores for burning, stinging, and foreign body sensations.7
The team concluded that PG-HPG nanoemulsion provides immediate and sustained symptom relief for up to eight hours in DED patients, with excellent tolerability.7
Rangarajan and Ketelson evaluated the effects of PG-HPG nano-emulsion on corneal epithelium models. Results demonstrated that PG-HPG nanoemulsion significantly improved moisture retention and hydration protection compared to a polyethylene glycol/ propylene glycol formulation and a vehicle control, with a hydration protection of 39.5% and surface hydration retention of 32.6% after desiccation. 8 Additionally, cell recovery from BAK damage was faster with PG-HPG nanoemulsion, and corneal permeability was reduced, indicating enhanced barrier function. The formulation also exhibited superior lubricity and elastic filament strength. PG-HPG nano-emulsion outperformed the other formulation and vehicle in all assessments. 8
Springs conducted a review of the safety and efficacy of PG-HPG nanoemulsion in treating DED. Results indicated that PG-HPG nano-emulsion exhibited viscoelastic properties optimised for ocular pH, mimicking the tear film elasticity and viscosity found in individuals without DED.9 Additionally, the formulation effectively reduced corneal and conjunctival staining, improved tear film stability, and maintained best-corrected visual acuity over time, with a low coefficient of friction in vitro.9
The PG-HPG nano-emulsion has shown significant efficacy in treating mixed DED. Studies have demonstrated its ability to provide immediate and sustained symptom relief for both aqueous-deficient and evaporative subtypes, with notable improvements in moisture retention, hydration protection, and barrier function. The formulation also reduced corneal and conjunctival staining, improved tear film stability, and maintained visual acuity. Overall, PG-HPG nano-emulsion stands out as an effective treatment option for mixed DED, offering enhanced protection and sustained relief with excellent tolerability. References are available on request. SF
Death toll from bacterial skin diseases:
In 2019, bacterial skin diseases were responsible for 72% of the 98 522 deaths due to skin and subcutaneous diseases worldwide.
Bacterial skin diseases:
Bacterial skin diseases represented 23% of global cases in 2019, with ~1.13 billion cases reported.
Global burden of skin diseases:
In 2019, fungal skin diseases accounted for 34% of new skin and subcutaneous disease cases globally, totalling ~1.65 billion cases.
Increased DALYs
In 2019, countries with a middle sociodemographic index recorded the highest number of DALYs for skin and subcutaneous diseases, highlighting significant health impacts in these regions.
Regional variation in skin conditions:
Fungal skin diseases and bacterial skin diseases were the leading contributors to new cases and deaths in most regions, including South Asia and Western sub-Saharan Africa.
Older adult concerns:
Decubitus ulcers, fungal skin diseases, and pruritus had higher age-standardised incidence rates and DALYs in older patients.
Fungal skin diseases by incidence:
Fungal skin diseases had the highest agestandardised incidence rate of 21 276.58 per 100 000 people.
Dermatitis incidence:
Dermatitis had an agestandardised incidence rate of 131.67 per 100 000 people, making it one of the most prevalent skin conditions globally.
Youth impact:
Scabies, acne vulgaris, and urticaria were most common in younger populations, with high disability-adjusted life years (DALYs) in the naught- to four-year age group.
Bacterial skin diseases death rate:
Bacterial skin diseases had the highest agestandardised death rate of 0.92 per 100 000 people.
Increase in acne:
Acne vulgaris saw an 18% increase in agestandardised incidence rate from 1990 to 2019, highlighting a significant increase in prevalence.
Decubitus ulcers growth:
New cases of decubitus ulcers increased by 106% from 1990 to 2019, underscoring a growing concern in skin and subcutaneous diseases.
This

Actinic keratoses (AK), also known as solar or senile keratoses, are benign intraepithelial neoplasms caused by long-term sun exposure. They typically appear as irregular, red, scaly papules or plaques on sun-exposed areas of the body such as the face, scalp, back of the arms, and dorsal aspect of the hands. AK lesions can disappear, persist or develop into squamous cell carcinoma (SCC, <1% per lesion per year).1,2
Studies show that the prevalence rates of AK are 18% in tropical, 2% in subtropical, and 18% in temperate regions. In individuals >60-years, the prevalence is ~15%, with men being more affected (24%) than women (14%).2
AK lesions are generally asymptomatic but can sometimes cause itching or stinging. The diagnosis of AK is primarily based on its clinical appearance, though non-invasive imaging techniques such as dermatoscopy, are useful diagnostic tools. Histomorphologically, AK have various variants, including atrophic, hypertrophic, acantholytic, pigmented, lichenoid, and Bowenoid types. 3
When multiple or recurrent AK lesions appear on sun-exposed skin, this is referred to as field cancerisation. Field cancerisation is often triggered by ultraviolet radiation damage to the p53 gene and can lead to the clonal expansion of cancer-primed cells, forming a contiguous patch of altered cells. 3
The primary goals of AK treatment are to eliminate as many clinical and subclinical
lesions as possible, achieve long-lasting remission, ensure a good cosmetic outcome, and prevent progression to invasive SCC. 3
The phrase ‘no pain, no gain’ is often linked to AK treatment, suggesting that effective therapies may come with some level of discomfort or side effects. Treatment plans should be tailored taking factors such as lesion characteristics, patient preferences, treatment availability, and cost into consideration. Urgent intervention is needed for rapidly growing, painful, or bleeding lesions to prevent complications.1
Treatment options for AK are categorised into lesion-directed and field-directed therapies. Lesion-directed treatments focus on individual lesions and include cryotherapy, curettage, and surgical excision, which are effective for targeting visible AK.1
Field-directed therapies treat larger areas of skin affected by sun damage and target both visible and subclinical lesions. Therapies include topical medications such as imiquimod and 5 - fluorouracil (5-FU), as
well as photodynamic therapy (PDT).1,3
The most widely used topical agents for the field-based treatment of multiple AK are imiquimod and 5-FU. In South Africa, imiquimod is indicated for the treatment of clinically typical, non-hyperkeratotic, non-hypertrophic AK on the face or scalp in adult patients. 5-FU is indicated for the treatment of AK. 4,5,6
Imiquimod is a topical immune-response modifier that acts as a toll-like receptor-7 agonist, stimulating the innate and adaptive immune systems. It activates dendritic cells, monocytes, macrophages, and Langerhans cells, promoting the release of cytokines and chemokines, while also inducing apoptosis in tumour cells. 3
Imiquimod cream is typically applied three times a week (eg Monday, Wednesday, and Friday) before bedtime and left on the skin for about eight hours. The initial treatment period lasts four weeks, after which a four-week break is recommended. During this break, a healthcare professional will assess the treated area to determine if the AK has cleared. If AK persists, treatment can be

extended for an additional four weeks, with a total maximum treatment duration of eight weeks. Clearance of AK should be evaluated four to eight weeks after each treatment cycle. 5
5-FU, a pyrimidine analogue and antimetabolite, works by inhibiting thymidylate synthase, an enzyme essential for DNA synthesis and RNA function. 5-FU can be applied once or twice daily for two- to four-weeks, up to a maximum of 12-weeks. 5-FU is effective for treating both single lesions and large areas of AK. 3
PDT is a minimally invasive treatment that targets malignant cells using photosensitisers like 5-aminolevulinic acid (5-AMA and 5-methyl aminolevulinate (5-MAL). These agents accumulate in abnormal cells and, when exposed to light, produce reactive oxygen species that cause cellular damage and necrosis. 3
Several studies have demonstrated the efficacy of imiquimod cream in treating AK. Imiquimod showed significant lesion reduction compared to a vehicle.7
A cycling treatment approach led to complete clearance in 82% of cases, while phase III trials reported clearance rates of 45.1% and 57.1% for imiquimod, with low discontinuation rates.7
Imiquimod, when combined with cryotherapy, further increased lesion clearance rates. In immunosuppressed patients, the cream was well tolerated without impacting graft function.7
The most common adverse effect of imiquimod cream is a localised skin reaction at the application site, including erythaema, itching, burning, and pain.7
In terms of long-term treatment, Del Rosso et al assessed multiple 16-week courses of imiquimod for treating extensive AK (mean treatment area of 625cm ²) Participants applied imiquimod twice weekly for 16 weeks, with additional courses available if needed. Most participants tolerated the treatment well, though 40.5% reported adverse events, and 31.4% experienced severe erythaema. 8
Overall, imiquimod reduced AK lesions by 80.2%, with a complete clearance rate of 36.4% and a partial clearance rate of 68.6%. Despite some skin reactions, multiple treatment courses effectively reduced AK in patients with large, affected areas. 8
AK is a chronic, unpredictable skin condition, characterised by the development of new lesions and recurring
episodes. Some lesions may regress spontaneously (rates range from 15% to 63% per year), however, despite initial clearance, long-term recurrence rates are high, ranging from 39% to 85%. Possible causes why AK may recur include nonadherence to treatment, misdiagnosis, or, though rare, malignant transformation to SCC.1,9,10,11
Given its superior longterm efficacy and cosmetic benefits, imiquimod should be considered a first-line treatment for AK
Because of its chronic nature, AK requires long-term treatment and management of lesions and the surrounding fields to prevent recurrence and potential progression to malignancy.1
Imiquimod cream has shown strong efficacy in the long-term treatment of AK, achieving high clearance rates in multiple studies. In a pilot study, 75% of AK lesions cleared with imiquimod compared to 12% in the control group.7
Other studies have demonstrated complete clearance rates of 45% to 57%, with lesion reductions of up to 86% following 16-week treatment courses. Long-term follow-up indicates sustained benefits, with many patients remaining clear one to two years after treatment and recurrence rates staying low.7
In combination with other treatments, such as cryotherapy, imiquimod further enhances clearance rates. When used postcryotherapy, lesion clearance increased significantly (87% vs 50% with cryotherapy alone). Additionally, lower concentrations (2.5% and 3.75%) of imiquimod also showed effective lesion clearance, with sustained results observed at one year. Overall, imiquimod has shown to be an effective long-term solution for AK management.7
Krawtchenko et al compared the clearance efficacy of cryosurgery, imiquimod, and 5-FU in treating AK. Initial clinical clearance was highest in the 5-FU group (96%), followed by imiquimod (85%) and cryosurgery (68%). Histological clearance rates were higher at 73% for imiquimod, followed by 67% for 5-FU, and 32% for cryosurgery.12
After 12-months, imiquimod showed the highest sustained clearance rate for individual lesions (73%) and total treatment field (73%), compared to 5-FU (54%) and cryosurgery (28%).12
Additionally, patients in the imiquimod group had the best cosmetic outcomes. Given its superior long-term efficacy and cosmetic benefits, imiquimod should be considered a first-line treatment for AK, concluded Krawtchenko et al.12
In their real-world study, Neugebauer et al compared the long-term efficacy of imiquimod and 5-FU in reducing the risk of recurrent AK. They found that although 5-FU was effective in the short-term, it is not more effective in the long-term (fiveyears) as with imiquimod. 4
According to Caperton and Berman , p atient preference should guide treatment, because clearance rates are significantly enhanced with good adherence to a treatment regimen.7
In their study, Caperton and Berman found that patients prefer imiquimod d ue to the ease, convenience, privacy, and autonomy associated with self-administration.7
Furthermore, a prospective, nonrandomised pilot study found that imiquimod achieved a median Treatment Satisfaction Questionnaire for Medication score of 72.7 for effectiveness, and a global satisfaction score of 69.4.13
Imiquimod scored a high satisfaction score regarding side effects, with a median of 93.8. Furthermore, 72% of imiquimodtreated patients expressed a preference for repeating the same treatment.13
AK are chronic and unpredictable, with long-term recurrence rates ranging from 39% to 85%. Despite initial clearance, AK lesions often reappear due to factors such as non-adherence to treatment or misdiagnosis.
Imiquimod cream has demonstrated strong efficacy in reducing AK recurrence, with complete clearance rates between 45% and 57% and lesion reductions of up to 86%.
Long-term studies show sustained benefits, with many patients remaining lesion-free for one to two years posttreatment. When combined with cryotherapy, imiquimod further enhances clearance rates and offers a reliable, longterm solution for managing recurrent AK. References are available on request. SF
This article was independently sourced by Specialist Forum.

Too many choices!
A practical approach to managing common urological problems
Benign prostatic hyperplasia

(BPH) is a common condition affecting older men, characterised by prostate gland enlargement, which results in urinary symptoms. Historically, BPH management focused on alpha-blockers and surgical interventions, such as transurethral resection of the prostate (TURP). However, advancements in medical treatments have diversified management strategies, leading to a more nuanced approach.
Currently, around 33% of men >50-years seek treatment for BPH symptoms, including reduced urinary flow, hesitancy, and increased nighttime urination. Initial evaluations involve comprehensive assessments and prostate-specific antigen (PSA) testing to rule out cancer. Alphablockers remain the mainstay of treatment, with newer selective agents like tamsulosin and silodosin offering improved efficacy and reduced side effects, particularly for older patients.
Selective alpha-blockers, while effective, can cause ejaculatory dysfunction due to smooth muscle relaxation, necessitating careful consideration in therapy selection. Combination therapy with 5-alpha reductase inhibitors, such as finasteride or dutasteride, may also be utilised but typically serves as an adjunct rather than first-line treatment. Surgical options remain significant, with TURP as the gold standard,
although less invasive techniques, like laser therapies, are becoming increasingly popular.
The evolution of BPH treatment highlights the importance of clear communication regarding potential side effects and patient expectations. Education on the impact of therapies on sexual function is crucial for maintaining patient satisfaction and optimising outcomes. Overall, while treatment options for BPH have expanded, the core principles of management continue to guide effective patient care.
To watch a replay of the webinar, click here or scan the QR code.
Back to the future: A review of PPIs


The introduction of omeprazole in 1989 marked a revolutionary advancement in the treatment of acid-related diseases, such as gastroesophageal reflux disease (GORD) and peptic ulcers.
As a proton pump inhibitor (PPI), omeprazole, along with other PPIs like esomeprazole and pantoprazole, effectively targets the proton pump in the stomach’s parietal cells, reducing gastric acid secretion. This mechanism provides significant symptom relief and facilitates
healing in conditions like GORD, peptic ulcers, and Zollinger-Ellison syndrome. PPIs bind irreversibly to the proton pump, leading to sustained acid suppression, even with their short half-life of one to two hours. After regular dosing, they achieve a steady-state inhibition of approximately 66% within two to three days, making them ideal for long-term management of acidrelated conditions. Individual PPIs vary in pharmacokinetics; for example, rabeprazole offers rapid onset, while lansoprazole has the highest bioavailability.
Despite their effectiveness, the long-term use of PPIs raises concerns about potential risks, including nutrient deficiencies and increased susceptibility to infections. Thus, while PPIs are crucial for managing certain conditions, physicians must evaluate the need for continued therapy on an individual basis, allowing for transitions to on-demand treatment where appropriate. Overall, PPIs have transformed acid-related disease management, offering flexible and effective treatment options tailored to patient needs.
To watch a replay of the webinar, click here or scan the QR code.

These webinars were sponsored by McLeods and are accredited for one (1) CPD point each. Once you have watched the replays, send an e-mail to john. woodford@newmedia.co.za and request to have your CPD point allocated to your profile on the HPCSA database. Include the webinar names and your HPCSA number in your e-mail.

Direct oral anticoagulants (DOACs) – also referred to as non-vitamin K antagonists – have become the primary treatment option for venous thrombosis, offering similar efficacy across the board but differing in safety profiles.
Among these, apixaban is notably preferred due to its strong safety record, particularly in high-risk populations and for long-term management of cancerassociated venous thrombosis (VTE).
Apixaban’s proven safety in managing conditions like pulmonary embolism and deep vein thrombosis (DVT) stems from its ability to significantly reduce major bleeding events, as seen in both clinical trials and real-world data.
A meta-analysis conducted in Amsterdam, comparing various DOACs, found no significant difference in recurrence rates. However, apixaban’s safety stood out, with a 40% reduction in major bleeding incidents, and data from the AMPLIFY trial showed a remarkable 69% reduction in major bleeding compared to other anticoagulants.
Real-world observational studies further emphasised apixaban’s safety, particularly in reducing risks associated with major bleeding and intracranial haemorrhage, a critical factor in avoiding fatal complications of VTE.
Furthermore, apixaban’s twice-daily dosing has proven to be safer for reducing major bleeding, even during the early phases of treatment for VTE. Across clinical trials and meta-analyses, apixaban consistently showed fewer bleeding events, which includes both major and clinically relevant non-major bleeding, when compared to its DOAC counterparts, such as rivaroxaban and dabigatran.
This favourable bleeding profile is significant, especially for patients who might otherwise discontinue anticoagulation therapy due to the inconvenience caused by frequent minor bleeding.
In terms of bleeding risks, apixaban offers key advantages, particularly in reducing intracranial bleeding by two-thirds. This
reduction is crucial because intracranial haemorrhages are often fatal.
Moreover, apixaban demonstrates a unique ability to lower the risk of gastrointestinal (GI) bleeding, which remains a concern with other anticoagulants, including rivaroxaban and warfarin. Real-world studies have consistently reaffirmed apixaban’s superior protection against GI bleeding, making it a safer option, particularly for patients at higher risk of this complication.
The benefits of apixaban extend beyond venous thrombosis to areas like atrial fibrillation and thromboprophylaxis for orthopaedic surgeries. In the ARISTOTLE trial, apixaban outperformed other treatments in stroke prevention and was associated with reduced major bleeding and overall mortality in patients with atrial fibrillation.
Similarly, for thromboprophylaxis following knee and hip replacement surgeries, apixaban lowered the occurrence of VTE without increasing bleeding risks –an advantage that distinguishes it from other DOACs in these settings.
When comparing anticoagulants like lowmolecular-weight heparin (LMWH), warfarin, and DOACs, particularly in terms of bleeding risks, studies such as the Einstein Extension study have provided valuable insights.
For instance, apixaban 2.5mg twice daily showed no significant difference in clinically relevant bleeding compared to other doses, reaffirming the safety of its dosing regimen.
Meanwhile, dabigatran exhibited a fivefold increase in clinically relevant bleeding, highlighting the importance of managing drug dosing to balance efficacy and safety. Apixaban’s lower trough levels and higher peaks contribute to its reduced bleeding risk, making it a more manageable option for clinicians.
The role of randomised controlled
trials (RCTs) versus real-world data is critical in assessing the performance of anticoagulants like apixaban. While RCTs are invaluable for establishing initial efficacy and safety, they often involve select populations and shorter follow-up periods.
In contrast, observational studies offer insights into how these drugs perform in broader, more diverse patient groups. For example, real-world data have been essential in understanding anticoagulant use in patients with chronic kidney disease (CKD) or obesity, populations often excluded from RCTs.
For patients with cancer-associated thrombosis, anticoagulation poses unique challenges due to higher recurrence rates and increased bleeding risks. DOACs, particularly apixaban, have demonstrated better safety profiles than LMWH in these patients.
In the Caravaggio study, which compared apixaban with LMWH, apixaban met the primary efficacy endpoint of non-inferiority while showing no significant difference in bleeding rates, reinforcing its status as a safer choice for patients at high risk, including those with GI or neurological cancers.
To watch a replay of the webinar, click here or scan the QR code.
The webinar is accredited for one (1) ethics point. Once you have watched the replay, send an e-mail to john.woodford@ newmedia.co.za and request to have your point allocated to your profile on the HPCSA database.
Include the webinar name and your HPCSA number in your e-mail. SF

for the PREVENTION of Stroke and Systemic Embolism in your patients with NON-VALVULAR ATRIAL FIBRILLATION2 &

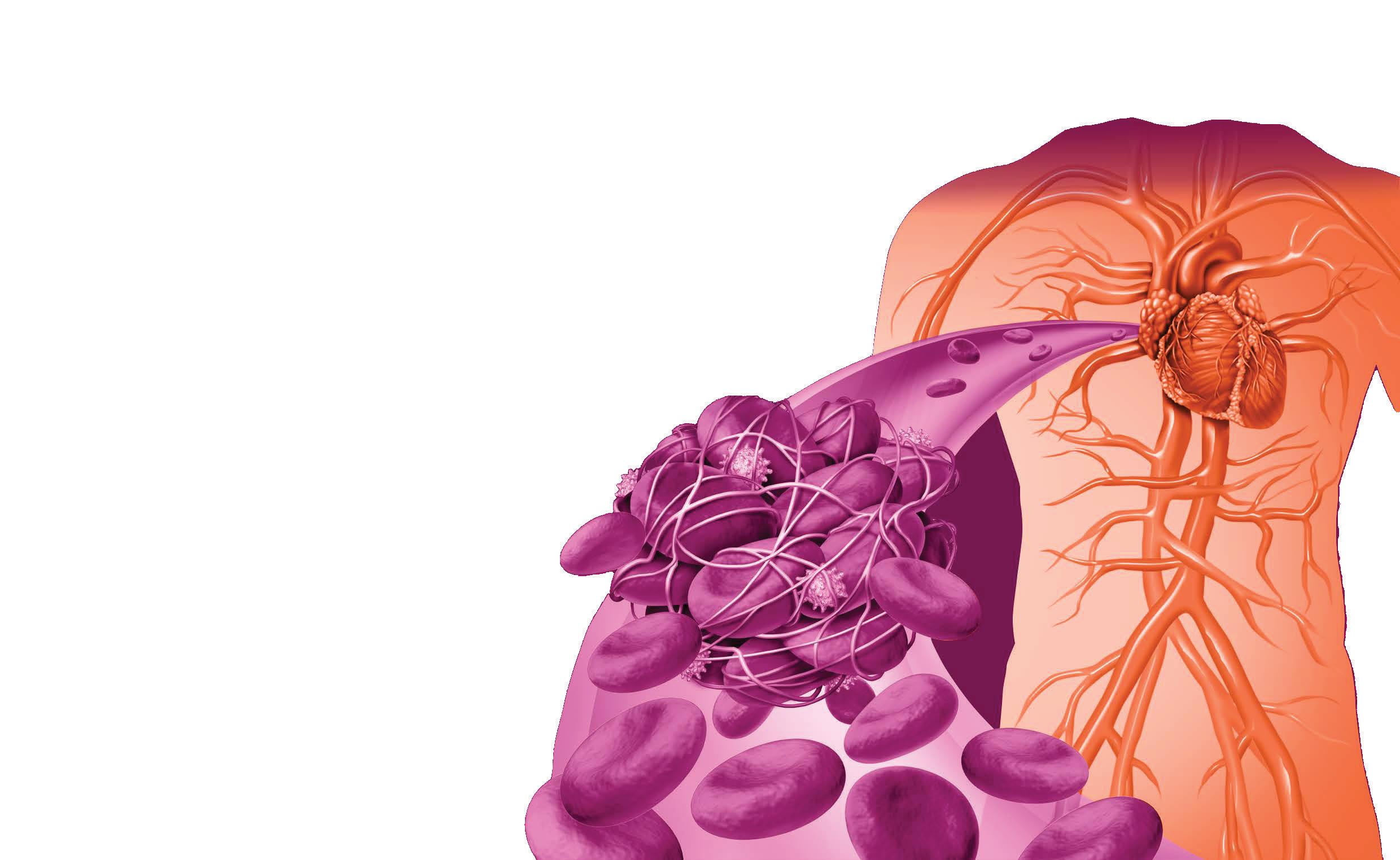
& for the TREATMENT of Deep Vein Thrombosis (DVT) and Pulmonary Embolism (PE) and PREVENTION of Recurrent DVT and PE.2 for the PREVENTION OF VENOUS THROMBOEMBOLIC EVENTS after elective knee and hip replacement surgery2 For ELIQUIS ® (apixaban) prescribing information, scan the QR code
References: 1. Data on file, IQVIA MIDAS® Q2’23 Sell-In/Sell-Out data 2. Pfizer Laboratories (Pty) Ltd. ELIQUIS® (apixaban) 2,5 mg and 5 mg Film-coated Tablets. Approved Package Insert – 11 July 2022. To access the ELIQUIS ® (apixaban) website, scan the QR code
S4 ELIQUIS® 2,5mg and 5mg Film-coated Tablets (Reg. No’s: 47/8.2/0463, 0464). Each film-coated tablet contains either 2,5 mg or 5 mg apixaban, respectively
To report an adverse event, please contact ZAF.AEReporting@pfizer.com . If you wish to contact Pfizer for any other purpose, please use contact details: +2711 320 6000 or 0860 734 937 (SA) . Monday-Friday 09h00-17h00.
Pfizer Laboratories (Pty) Ltd. Company Reg. No. 1954/000781/07. Building 2, 1st Floor, Maxwell Office Park, Magwa Crescent, Waterfall City, Midrand, Johannesburg, South Africa. Tel. No: 0 860 PFIZER (734937). Copyright © 2023. Pfizer Laboratories (Pty) Ltd. All rights rese rved P P - ELI - Z A F - 0493
This
Gastroesophageal reflux disease (GORD) is a prevalent condition affecting approximately 25% of the Western population, with a lower incidence in Asia. It is most commonly seen in individuals aged 60- to 70-years and is increasingly recognised in older adults.
GORD presents with a variety of symptoms, including heartburn, dysphagia, and nocturnal choking. Atypical symptoms such as chronic cough and laryngitis are also associated with the condition. Alarm symptoms, which necessitate urgent referral, include dysphagia, odynophagia, weight loss, and gastrointestinal bleeding. Diagnosis of GORD is multifaceted, involving empiric trials, endoscopy, and pH monitoring to evaluate acid exposure and symptom correlation. Manometry is also utilised to assess motility disorders related to GERD. The Montreal definition of GERD differentiates between non-erosive reflux disease (NERD) and erosive oesophagitis.
Treatment approach
The treatment of GORD aims to enhance lower oesophageal sphincter pressure, improve oesophageal acid clearance, facilitate gastric emptying, and protect the oesophageal lining. A threephase structure treatment approach is recommended and include:
1 Lifestyle modifications: Recommendations include elevating the head of the bed, reducing fat intake, quitting smoking, avoiding lying down after meals, losing weight, limiting alcohol, and wearing loose clothing. While these changes may not completely alleviate symptoms, they can significantly improve them.
2 Pharmacologic intervention :
Standard or high-dose antisecretory therapy is employed, with proton pump inhibitors (PPIs) proving more effective than H2 receptor antagonists in healing erosive oesophagitis and providing
symptom relief. Timing of medication administration is crucial, particularly for PPIs, which should be taken before meals for optimal effectiveness. Potential reasons for PPI therapy failure include adherence issues and weakly acidic reflux.
3 Surgical intervention : This is considered for patients unresponsive to pharmacologic treatment or those with severe complications. Laparoscopic fundoplication is presented as an effective option for refractory GORD, offering high patient satisfaction and long-term symptom relief, although it may lead to side effects like bloating and dysphagia. Alternative treatments, such as magnetic sphincter augmentation, show promise in improving quality of life and reducing PPI dependence.
Special considerations and unmet needs
In pregnancy, 66% of women experience heartburn, typically resolving post-delivery. Treatment during pregnancy should prioritise lifestyle changes, followed by antacids and H2 receptor blockers, or histamine-2 receptor antagonists, which are generally safe.
Unmet needs in GORD management include breakthrough symptoms and the necessity for improved medication adherence. The introduction of dexlansoprazole, a newer PPI, is highlighted for its superior acid control and longer duration of action, making it a promising option for GORD patients. Other treatment options include potassium-competitive acid blockers, pain modulators, and baclofen

for reducing transient lower oesophageal sphincter relaxation.
Endoscopic treatments are described as relatively new and may benefit select patients with well-documented GORD responsive to PPIs. The presentation includes case studies that illustrate the complexities of GORD management, emphasising the need for lifestyle changes, appropriate medication use, and potential surgical or endoscopic interventions for refractory cases.
A multifaceted approach is required to effectively manage GORD, integrating both medical and surgical options while addressing the challenges of longterm PPI therapy and the necessity for individualised patient care. By adopting a comprehensive treatment strategy, healthcare professionals can significantly improve the quality of life for patients suffering from GORD.
To watch a replay of the webinar, click here or scan the QR code.
This webinar was sponsored by Adcock and is accredited for one (1) CPD point. Once you have watched the replay, send an e-mail to john.woodford@newmedia.co.za and request to have your CPD point allocated to your profile on the HPCSA database. Include the webinar name and your HPCSA number in your e-mail.

THE ONLY PPI WITH A 2ND RELEASE FOR MAINTAINED RELIEF2,3

• the MOST POWERFUL inhibitory effect on the proton pump of ALL available PPIs.4
• TRUE once-daily dosing.5

References: 1. South African Medicine Price Registry. Database of Medicine Prices, 01 November 2023 [online]. [cited November 2023]; Available from URL: http://www.mpr.gov.za/.
2. Monthly Index of Medical Specialities. September 2023;63(No. 8):185-191. 3. Metz DC, Howden CW, Perez MC, et al. Clinical trial: dexlansoprazole MR, a proton pump inhibitor with dual delayed-release technology, effectively controls symptoms and prevents relapse in patients with healed erosive oesophagitis. Aliment Pharmacol Ther. 2009;29(7):742-54. doi: 10.1111/j.1365-2036.2009.03954.x. 4. Gąsiorowska A. The role of pH in symptomatic relief and effective treatment of gastroesophageal reflux disease. Prz Gastroenterol. 2017;12(4):244249. doi: 10.5114/pg.2017.72097. 5. Frye JW, Peura DA. Managing gastroesophageal reflux disease - comparative efficacy and outcomes of dexlansoprazole MR. Ther Clin Risk Manag 2015;11:1649-56. doi: 10.2147/TCRM.S66680. 6. Dexilant Professional Information. Takeda (Pty) Ltd, South Africa; August 2021. 7. Sharma P, Shaheen NJ, Perez MC, et al. Clinical trials: healing of erosive oesophagitis with dexlansoprazole MR, a proton pump inhibitor with a novel dual delayed-release formulation--results from two randomized controlled studies. Aliment Pharmacol Ther. 2009;29(7):731-41. doi: 10.1111/j.1365-2036.2009.03933.x. 8. Fass R, Chey WD, Zakko SF, et al. Clinical trial: the effects of the proton pump inhibitor dexlansoprazole MR on daytime and nighttime heartburn in patients with non-erosive reflux disease. Aliment Pharmacol Ther. 2009;29(12):1261-72. doi: 10.1111/j.1365-2036.2009.04013.x.
only dexlansoprazole available in South Africa. DDR: dual delayed-release; PPI: Proton pump inhibitor; QoL: quality of life. S4 DEXILANT 30 mg modified-release capsules, Reg. No.







