Regulatory Register
Winter 2023

NMPF Files FOIA with FDA on Plant-Based Beverages
For decades, NMPF has been baffled by why the Food and Drug Administration (FDA) has not enforced its standards of identity, especially given that it results in human health harm. To better understand what FDA has received and has been working on, NMPF sent a Freedom of Information Act (FOIA) request on Feb. 3 for all information related to plant-based labeling, the use of the term “animal-free,” the negative human health consequences due to mislabeling of plantbased products and much more.
The FOIA is especially important to hold FDA accountable, as a new frontier in mislabeling that’s arising with the development of socalled “lab-based” dairy alternatives, NMPF wrote in the FOIA.
“As synthetic foods begin to appear in the marketplace, NMPF anticipates that FDA
Labeling and Standards
will again turn a blind eye to misleading and unlawful labeling practices by synthetic food manufacturers of purported ‘dairy’ products, to the detriment of consumer understanding,” NMPF wrote. “Dairy foods are extremely complex, offering essential nutrients, numerous high-quality proteins, micronutrients and hundreds of fatty acids, all of which interact with each other and deliver one of the most nutritious foods in the marketplace.”
NMPF anticipates that FDA will again turn a blind eye to misleading and unlawful labeling practices by synthetic food manufacturers of purported ‘dairy’ products, to the detriment of consumer understanding

*
FDA Amends Yogurt Standard of Identity
FDA issued a response to requests from Chobani and the International Dairy Foods Association (IDFA) for public hearings on six issues relating to the yogurt standard of identity on Dec. 14.
The yogurt standard of identity was published on Jun. 21, 2021. Certain provisions of the yogurt standard were stayed while FDA reviewed the requests. FDA has now denied the requests for hearings, modified the yogurt standard in response to the objections and lifted the stay. The rule became effective on Jan. 17, with a compliance date of Jan. 1, 2024.
The six provisions to which IDFA (5) and Chobani (1) objected to and requested public hearings on, and their outcomes:
1. FDA denied the request for a hearing on minimum titratable acidity of not less than 0.7% or a pH of 4.6 or lower.
2. FDA denied the request for a hearing on the requirement that cream can only be added before culturing.
3. FDA denied the request for a hearing on the requirement that if vitamin D is added, it must be not less than 25% of the daily value (DV) and agreed to modify the standard to set the minimum level at 10% of the DV as IDFA requested.
4. FDA denied the request for a hearing to eliminate the 3.25% minimum milkfat requirement. But FDA modified the general definition and standard of identity under 21 CFR 130.10 to permit the use of fat-containing flavors (such as coconut flakes, chocolate, etc.) in lower fat yogurt.
5. FDA denied the request for a hearing on the exclusion of the use on safe and suitable non-nutritive sweeteners and modified the standard to permit the use nonnutritive sweeteners as optional ingredients as IDFA had requested.
6. FDA denied Chobani’s request for a hearing on the exclusion of ultrafiltered milk as a basic dairy ingredient.
NMPF notes that the new yogurt standard of identity strongly defends the importance of standards of identity, stating “any food that purports to be or is represented as yogurt, must conform to the definition standard of identity for yogurt.” That’s an important principle in holding plant-based products accountable for using dairy names in violation of standards of identity.
The new yogurt standard of identity strongly defends the importance of standards of identity, stating “any food that purports to be or is represented as yogurt, must conform to the definition standard of identity for yogurt.”
USDA Proposes Two Nutrition Rule Updates Affecting Dairy
The United States Department of Agriculture (USDA) has recently released two new rules updating both the Supplemental Nutrition Program for Women, Infants and Children (WIC) and the school meal standards.
On Nov. 17, USDA released a proposed rule updating the current WIC food program, which included proposed cuts to the monthly milk allotments across all food packages. Some positive proposed changes included flexibilities for yogurt and cheese substitution; yogurt container size flexibility; and the authorization of lactose-free milk, which would be positive for providing dairy’s benefits to lactose-intolerant populations that currently have imperfect access to dairy. Still, decreasing the overall amount of dairy offered in the WIC packages decreases access to the vital nutrients dairy has to offer and has the potential to lead to overall lower WIC program participation.
USDA also released a proposed rule on Feb 3. updating the school meal standards which would go into effect, once finalized, in the 2024-2025 school year. The rule offers two alternative options for school milk which USDA is offering for comments:

• Option 1: Only allows unflavored milk to be served to grades K-8; fat-free and low-
Nutrition and Food Safety
fat flavored and unflavored milk can be served to kids in grades 9-12; and
• Option 2: Fat-free and low-fat flavored and unflavored milk are available to all grades, following the added sugar limit proposed in the rule.
The rule also proposes adding weekly added sugar limits that would align with the Dietary Guidelines for Americans guidance of no more than 10% of calories per week from added sugars. Proposed added sugar productspecific limits would include a flavored-milk limit of 10 grams of added sugars per 8 fluid ounces and a yogurt limit of no more than 12 grams of added sugars per 6 ounces.
NMPF will submit comments on both rules.
Decreasing the overall amount of dairy offered in the WIC packages decreases access to the vital nutrients dairy has to offer and has the potential to lead to overall lower WIC program participation.
HHS, USDA Announce Dietary Guidelines Advisory Committee Members,


First Public Meeting
The Departments of Health and Human Services (HHS) and USDA announced on Jan. 19 the members of the 2025 Dietary Guidelines Advisory Committee. The committee, which includes 20 nutrition and public health experts, is tasked with writing the scientific report that informs development of the 2025-2030 Dietary Guidelines for Americans (DGAs), which informs federal nutrition program offerings.
In addition to announcing the Advisory Committee, the departments also opened a
public comment period that will run through the duration of the committee’s work. The first public meeting of the Advisory Committee is taking place on Feb. 9-10, and anyone can register and attend virtually.
The guidelines, updated every five years, provide advice on what to eat and drink to meet nutrient needs. They also determine what is served in USDA’s school meal programs. The last edition, released in 2020, continued to recommend three servings of dairy a day, recognizing dairy as a source of essential nutrients including three nutrients of public health concern. NMPF is active in the dietary guidelines process, providing both oral and written comments to USDA and HHS.
Sustainability and the Environment
NMPF Urges USDA to Elevate Dairy in Conservation Programs

NMPF submitted comments Dec. 21 to USDA’s Natural Resources Conservation Service urging it to prioritize critical opportunities for dairy as it implements new climate-smart conservation funding in the Inflation Reduction Act enacted in August.
In its letter, NMPF urged USDA to develop new initiatives focused on manure and feed management, both of which will help dairy farmers advance their sustainability leadership as the sector works to fulfill its voluntary, producer-led goal of becoming greenhouse gas neutral or better by 2050. NMPF supported the Inflation Reduction Act’s $20 billion in landmark new funding for farm bill conservation programs.
First, NMPF recommended a multi-pronged new manure management initiative within the Environmental Quality Incentives Program (EQIP). This effort would focus on reducing methane emissions associated with manure handling and storage by concentrating on investments in waste separation and handling as well as methane digesters, which can capture as much as 80 percent of the methane resulting from a waste stream. NRCS has existing practice standards for waste separation facilities (CPS 632), waste treatment (CPS 629), and digesters (CPS 366) that could be used today for this new initiative. This approach would appeal to those operations that have the capacity and manure volumes to make the operation of a methane digester successful. NMPF also urged NRCS to include CPS 629 in its NRCS Climate Smart
Mitigation Practice list, so as not to limit the options available for dairy producers under IRA funding.
NMPF also urged NRCS to develop a feed management conservation initiative, offering support in both the EQIP and the Conservation Stewardship Program. Dairy producers are interested in new approaches to feed management that can reduce enteric methane emissions and subsequently reduce GHG emissions from dairy production. Enteric methane emissions, including gas released from cow eructation, account for approximately one-third of a dairy farm’s GHG footprint.
NMPF urged USDA to develop new initiatives focused on manure and feed management, both of which will help dairy farmers advance their sustainability leadership as the sector works to fulfill its voluntary, producer-led goal of becoming greenhouse gas neutral or better by 2050.
NMPF Urges Sped-Up FDA Approval of Climate-Friendly Feed Additives
NMPF called on FDA to use existing legal authority to modernize its regulations allowing for faster approval of animal-feed additives that reduce greenhouse gas emissions, submitting comments on Nov. 16 to the agency that highlighted the need for urgent action to enhance dairy’s role as a climate solution.
“Innovative and voluntary solutions are needed to reduce greenhouse gas (GHG) emissions, including methane,” said Dr. Jamie Jonker, NMPF’s chief science officer, in the comments . “Enteric emissions directly from cows currently account for roughly one third of all GHG emissions from dairy farms and present an important area of opportunity
for methane reductions. Feed composition changes can directly or indirectly reduce enteric emissions resulting from livestock.”
While animal-feed additives are a promising path toward a net-zero future for dairy as outlined in industry goals, the pace of their approval lags that of competitors such as the European Union due to current FDA processes. By streamlining bureaucracy and allowing feed-additives to be treated as foods rather than as drugs, the United States can maintain and advance its global leadership in sustainability, Jonker wrote.
Through the U.S. Dairy Net Zero Initiative, a collaboration across dairy organizations, dairyfarm research is advancing new technology and new market development opportunities to make sustainability practices more accessible and affordable to farms of all sizes, including enteric methane reduction.

WOTUS Disappoints Dairy
A final Waters of the United States (WOTUS) rule released Dec. 30 disappointed virtually very agriculture group studying this issue and drew criticism from NMPF for adding unnecessary confusion for farmers.
NMPF supported the Navigable Waters Protection Rule (NWPR) that had previously guided WOTUS policy and was disappointed to see the current EPA repeal it. However, the new rule wasn’t a complete retreat from that regulation, including some exemptions that will hopefully bring agriculture some clarity. Still, EPA is reintroducing considerable ambiguity in this version of the rule as it attempts to determine what is a ‘Water of the US’ as seen in the treatment of ditches, ephemeral streams and groundwater, all of which were largely categorically out under the NWPR. Two lawsuits have already been filed against the new rule, with many more to come.
GHG Protocol Survey Open Through March 14
NMPF, along with USDEC, Newtrient, IDFA and dairy companies, are participating in a coordinated effort through the Innovation Center for U.S. Dairy to develop messaging for U.S. dairy organizations to consider when crafting their response to the GHG Protocol’s Survey on Need for GHG Protocol Corporate Standards and Guidance Updates. The survey, due Mar. 14, is a welcome opportunity to provide feedback and suggestions to the standards that shape the sustainability data requests that customers make of their dairy suppliers.
GHG Protocol’s standards provide the globally recognized methodologies for measuring GHG emissions, including the Scope 3 emissions tracking that FARM Environmental Stewardship enables. Companies across all industries, including within the dairy supply chain, use these standards for their GHG reporting because they underpin prominent GHG reporting frameworks, such as the Science Based Targets initiative and the Task Force on Climate-Related Financial Disclosures.
Several of GHG Protocol’s standards and guidance documents have not been updated in more than a decade. As such, this is a rare opportunity to share U.S. dairy’s perspective and encourage updates to make GHG accounting and reporting more practical to implement.
EPA Releases Effluent Guidelines Program Plan 15; Announces CAFO Study
A new EPA plan to study and develop PFAS pollution limits and other measures is underway, NMPF was notified on Jan. 20, before the agency released its multifaceted Effluent Guidelines Program Plan 15.
The plan lays out how the agency will develop technology-based pollution limits and conduct studies on wastewater discharges from industrial and other sources. Specifically, the plan focuses on evaluating nutrient and per- and polyfluoroalkyl substances (PFAS) discharges.
The plan includes conducting a new study on concentrated animal feeding operations
(CAFOs), examining the extent to which CAFOs discharges from production and land application areas reach waterways that fall under the Waters of the United States (WOTUS) rule. The study will take years to complete; in addition to it EPA wants to gather information on new technologies and practices aimed to reduce discharges coming from the production and land application areas.
EPA will also look at the economic vitality of the regulated community as any future changes must be economically feasible. This information will then be used to determine if a revision to the effluent and pretreatment standards is warranted.
NMPF will work with EPA and monitor this issue closely.
FDA’s Center for Veterinary Medicine Testing Feed Samples for PFAS
NMPF staff met with the Food and Drug Administration Center for Veterinary Medicine (FDA CVM) after learning that FDA has started a new sampling project to explore levels of per- and polyfluoroalkyl substances (PFAS) in livestock feed samples The assignment will help the dairy industry understand if PFAS chemicals are getting into feed or not and if so at what levels. NMPF believes that no PFAS will be found, which will help push back on those who try to portray this as a dairy issue.
The project is set to run through the year, and FDA has assured NMPF it isn’t focused on dairy and is intended only to gain more knowledge around PFAS. FDA CVM plans to collect 60 different samples from randomly selected locations throughout the country. The samples will comprise 20 corn silage, 20 alfalfa hay and 20 corn grain samples. To date, 27 samples have been collected and 14 have been tested. All have come back nondetect for PFAS. These results thus far are unsurprising, given that all PFAS chemicals are synthetic and shouldn’t be present on a farm or in crops grown on a farm unless the farm is adjacent to a military base, landfill, industrial site, or land applied sewage sludge to cropland.


PFAS encompasses a group of 5,000 synthetic chemicals, commonly used in non-stick products and firefighting foam. Concerns over their potential environmental and health impacts continued to gain awareness over the past decade. A handful of isolated incidents have directly impacted dairy farms, in some instances, preventing farmers from shipping milk. However, PFAS continues to be a watercentric issue, with hundreds or thousands of drinking water sources contaminated. Little to nothing is known about how PFAS is transferred through the food chain. NMPF will continue to work with FDA and monitor the findings of this feed study.

Animal Health
Annual FDA Drug Residue Report Indicates Progress
Less than eight-hundredths of one percent – only 1 out of every 12,400 milk tankers – tested positive for antibiotic residues last year, according to the 2022 National Milk Drug Residue Database annual report released on Dec. 15 by the U.S. Food and Drug Administration. The data illustrates the continued long-term national pattern of improvements in milk quality practices by the industry.
Of the approximately 3.3 million milk pickup tankers tested in the past year, only 269 (0.08%) yielded a positive result. The number of samples tested (tankers, packaged products, producer samples) and reported positive decreased from 451 in 2021 to 424 in 2022; only two pasteurized packaged dairy products (29,195) tested were found to have an antibiotic residue. The 38,000 pounds of product were destroyed and not sold to the public.
The full report is available here. Source: FDA Center for Veterinary Medicine
Stepped-Up Raw Milk Multi-Drug Residue Monitoring Begins
FDA has begun a three-year National Multi-Drug Residue Raw Milk Monitoring Project that will influence whether changes to the required testing for milk are needed. The new monitoring project will test for 19 additional drug residues.

FDA currently has a robust sampling program that requires testing for the beta-lactam family of drugs. This monitoring has been highly effective at protecting public health and providing credible data to demonstrate the significant decline in residue incidents over the past 30 years.
NMPF and its members have worked cooperatively with FDA and state regulatory agencies since 2019 to evaluate whether the current animal drug residue monitoring program for the U.S. milk supply should be expanded to include additional drugs. The information gathered during this monitoring project will guide the food safety risk assessment process to ensure drug residue testing requirements reflect the latest science, thus affirming the safety of U.S. milk.
The project will not affect current required monitoring or testing at the farm and processor levels. State regulatory officials will sample milk trucks at dairy processing plants. The samples will be double-blinded and, therefore, untraceable to an individual farm or processing plant prior to testing.
19
The project will test for drug residues:
Gentamicin
Neomycin
Florfenicol
Ivermectin
Thiabendazole
Doramectin
Eprinomectin
Enrofloxacin
Tylosin
Erythromycin
Flunixin
Naproxen
Phenylbutazone
Sulfadimethoxine
Sulfamethazine
Sulfaquinoxaline
Oxytetracycline
Tetracycline
Chlortetracycline
NMPF Joins with Other Livestock Groups in Commenting on the Regulation of Animal Biotechnology
NMPF joined a coalition of livestock and allied organizations on Feb. 3 to identify ambiguities, gaps, inefficiencies, and uncertainties in the Coordinated Framework for the Regulation of Biotechnology. Specifically, the coalition repeated the concern with the existing regulatory approach under FDA for animals containing intentional genomic alterations (IGA).
The FDA‘s one-size-fits-all regulatory approach for IGAs, which contorts a statutory authority designed for conventional animal drugs, is ambiguous, inefficient, and often imposes requirements that are not scientifically justifiable on academics and developers. It is certainly not fit for regulating low-risk genetic improvements in animals intended
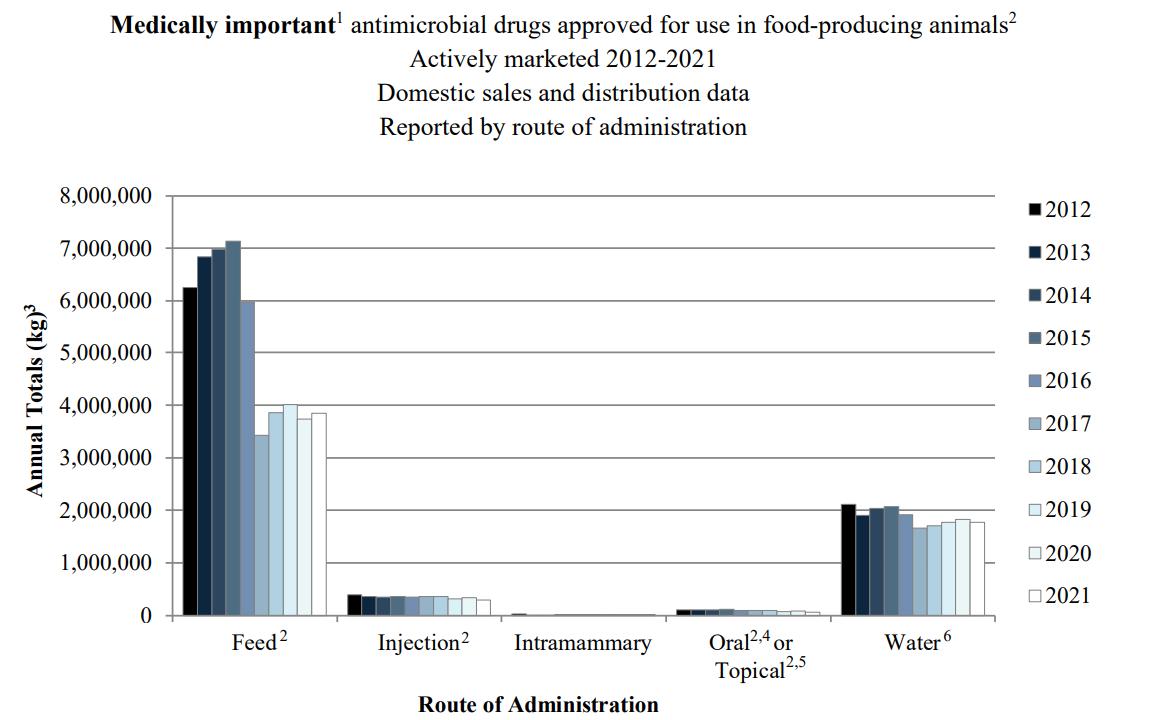
Sales of Antimicrobial Drugs Approved for Use in FoodProducing Animals Declines

Domestic sales and distribution of medically important antimicrobial drugs approved for use in food-producing animals decreased by less than one percent between 2020 and 2021, according to the U.S. Food and Drug Administration’s Center for Veterinary Medicine’s 2021 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals released Dec. 12. . Since a significant decrease in sales volume in 2017 caused by enhancements in the Veterinary Feed Directive, annual sales of medically important antimicrobials have remained at reduced levels. Compared to the 2015 peak, 2021 sales decreased 38 percent.
FDA reports sales by species, combining
for agricultural use, including gene edited varieties. To date, this regulatory approach has stifled the ability of academics and developers to bring important food and agricultural innovations to market that could benefit producers, consumers, and the environment.
Congress recently directed USDA to advance its own regulations pertaining to the movement of animals modified or developed by genetic engineering in consultation with FDA. Consistent with the direction from Congress and the objectives stated in the National Biotech and Biomanufacturing Initiative, the coalition urged USDA to expeditiously continue previous efforts to establish the department’s own regulations for the governance of animals developed with the use of genetic engineering to provide a clearer path to market for these important innovations and the benefits they confer.
beef, dairy, and bison antimicrobial sales and distribution so precise estimates for antimicrobial sales for dairy cattle are difficult to determine. However, the largest use of antimicrobials in dairy cattle is for mastitis during lactation and at dry off via intramammary infusion. In 2021, drugs sold for intramammary infusion purposes was less than 1 percent of total antimicrobial sales. Compared to 2012 (first year of the FDA report), intramammary antimicrobial sales have declined by 34 percent.
Jonker Appointed to Global AMR Leaders Group
Dr. Jamie Jonker has been appointed to the Global Leaders Group (GLG) on Antimicrobial Resistance which was established in November 2020 following the recommendation of the Interagency Coordination Group on Antimicrobial Resistance to strengthen global political momentum and leadership on AMR. The GLG collaborates globally with governments, agencies, civil society and the private sector through a One Health approach to advise on and advocate for prioritized actions for the
mitigation of drug resistant infections through responsible and sustainable access to and use of antimicrobials.
The GLG is administered by provided by the Quadripartite Joint Secretariat on Antimicrobial Resistance, a joint effort by the Food and Agriculture Organization of the United Nations (FAO), the United Nations Environment Program (UNEP), The World Health Organization (WHO), and the World Organization for Animal Health (WOAH). The GLG is currently chaired by the Honorable Mia Amor Mottley, Q.C., M.P., Prime Minister of Barbados.
 Source: Brenda Lashley, WHO/PAHO
Source: Brenda Lashley, WHO/PAHO
National Milk Producers Federation 2107 Wilson Blvd., Suite 600 Arlington, VA 22201 info@nmpf.org Phone: 703-243-6111


















 Source: Brenda Lashley, WHO/PAHO
Source: Brenda Lashley, WHO/PAHO