Use of Microfluidics Technology to Model Pathogenic Protein Seeding in Neurodegeneration in vitro
Dr Eve Corrie,1 Dr Rebecca Kelly,1 Dr Matthieu Trigano,1 and Dr Emma V. Jones1
1Medicines Discovery Catapult, Block 35, Alderley Park, Cheshire, SK10 4ZF, UK.
Introduction
Many neurodegenerative diseases are associated with the presence of misfolded, aggregating proteins within the brain, leading to cytotoxicity and cell death. The prion hypothesis states that these toxic species spread through the brain via anatomically connected regions, leading to widespread neurodegeneration.
Examples of prion-like proteins in neurodegeneration:
• α-synuclein – Parkinson’s disease, dementia with Lewy bodies, multiple system atrophy
• β-amyloid – Alzheimer’s disease
• TDP43 – Amyotrophic lateral sclerosis, frontotemporal dementia
• Tau – Alzheimer’s disease, corticobasal degeneration, frontotemporal dementia, chronic traumatic encephalopathy, progressive supranuclear palsy
Compartmentalised Microfluidics Devices for Neuronal Culture

eNuvio Omega4 device

• Two open chambers connected by 10µm microchannels –narrow enough to maintain separation of cell bodies but allow projection of neurites between chambers
Fluidic isolation maintained by asymmetric volume loading to ensure unidirectional flow of fluid
• Neuron → neuron
• Neuron → non-neuronal cell (e.g. glia, peripheral cells, myocytes)
Pathogenic misfolded protein
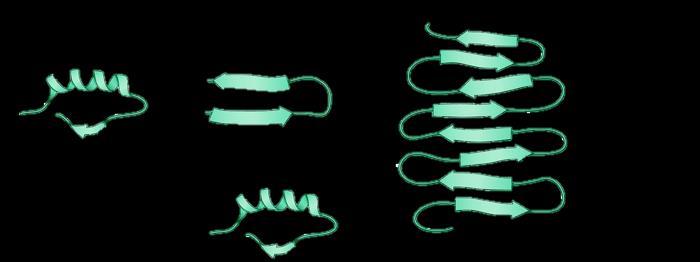
Native protein Native protein
Protein aggregate

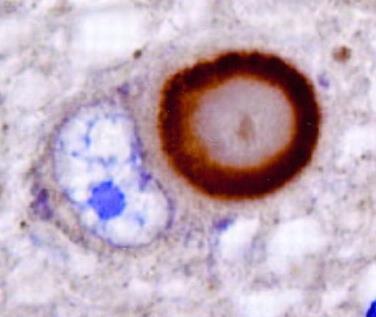
α-synuclein
Lewy Body1
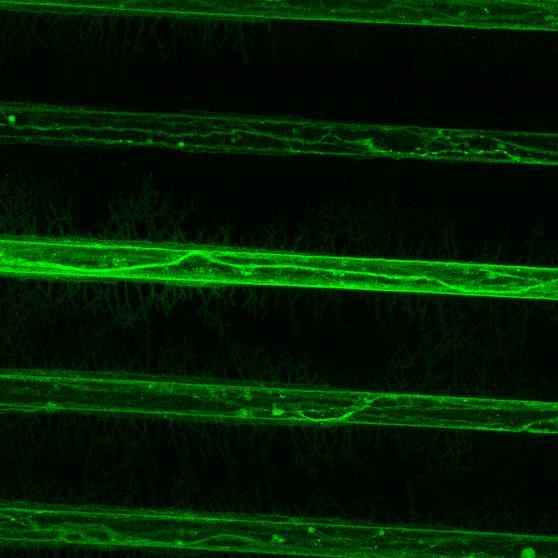
Projection of iPSC-derived glutamatergic cortical neurons through microchannels, imaged at 2 weeks in culture
• ioGlutamatergic neurons (bit.bio) were labelled with live neuron dye Neurofluor NeuO
• Axons can be visualised within the channels and projecting well into the adjacent chamber
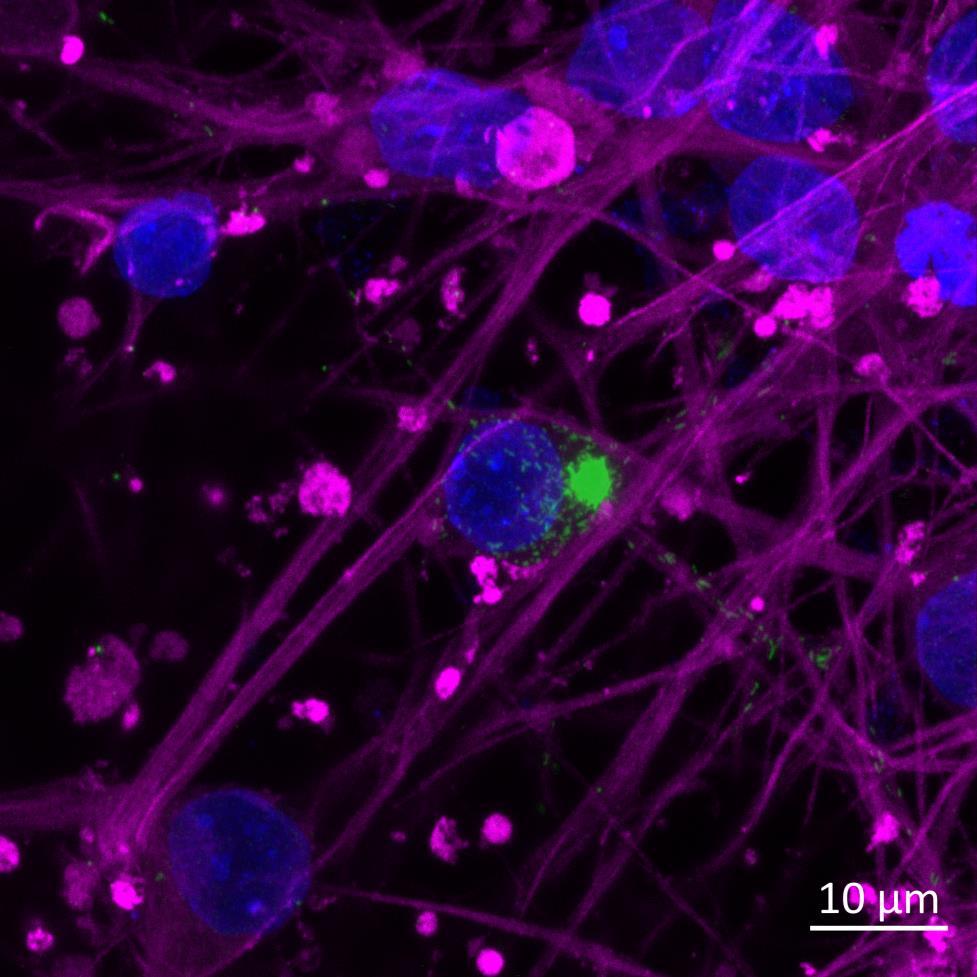
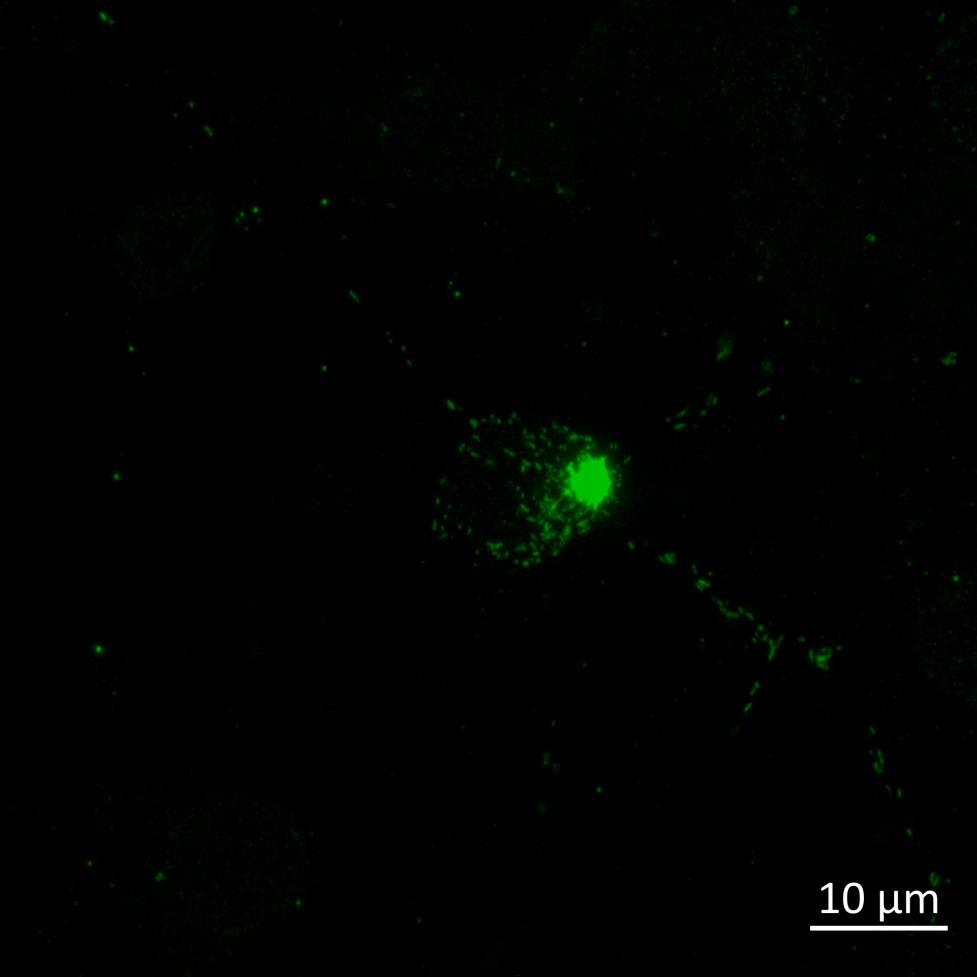
Inter-neuronal Seeding of Fluorescent α-synuclein Preformed Fibrils
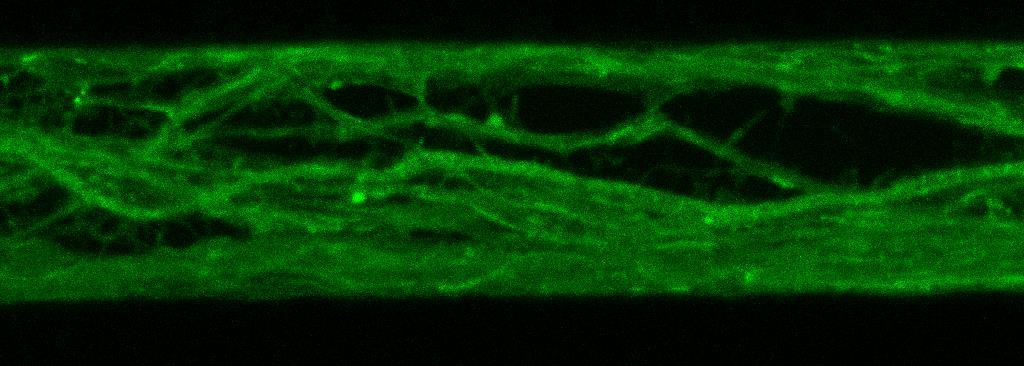


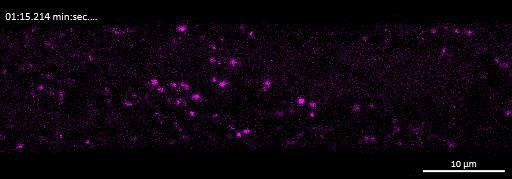

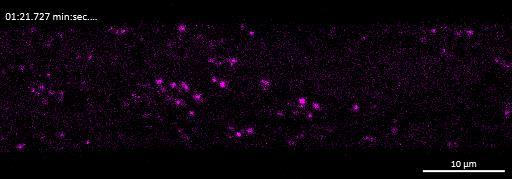
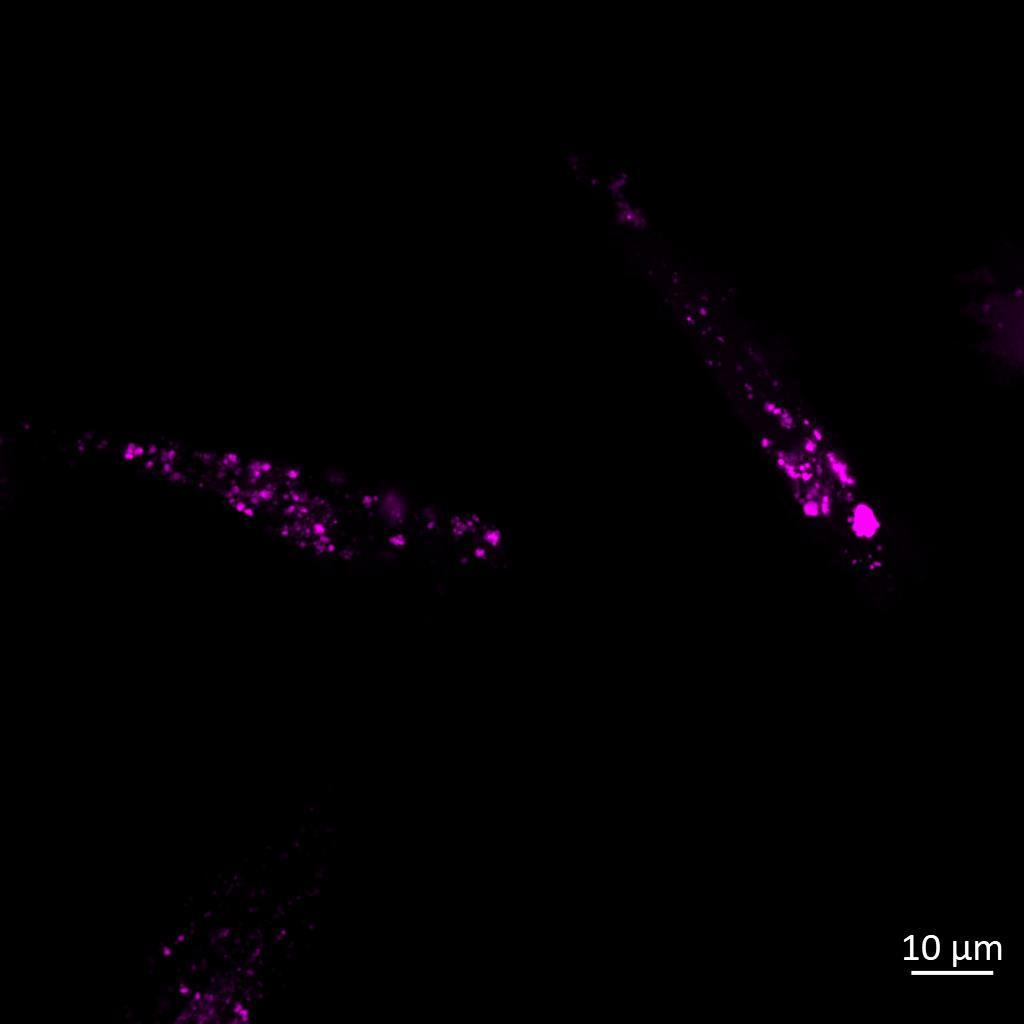
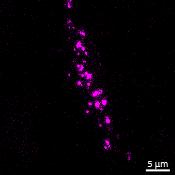
Live imaging showing internalisation and fast axonal transport of Alexa Fluor 594-labelled α-synuclein PFFs (a single puncta over time marked by arrow) applied to one “donor” chamber of microfluidics device
• iPSC-derived glutamatergic cortical neurons were plated in both chambers of a microfluidics device
• Fluorescently labelled α-synuclein preformed fibrils (PFFs) were added to a fluidically isolated “donor” chamber of the microfluidics device
• Neurons were labelled with Neurofluor NeuO and imaged live over time following PFF addition
• Uptake of PFFs could be detected within neurons after direct application to the donor chamber
• Fast axonal transport of PFF positive puncta could be visualised within the microchannels of the microfluidics device at 24 hours after PFF addition
• Progressive accumulation of PFFs was then detected in neurons in the connecting “acceptor” compartment over time
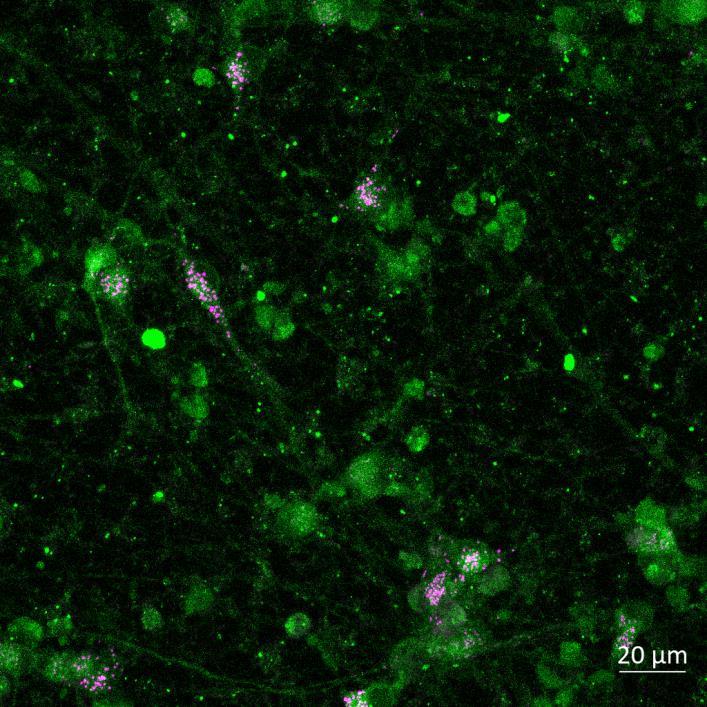
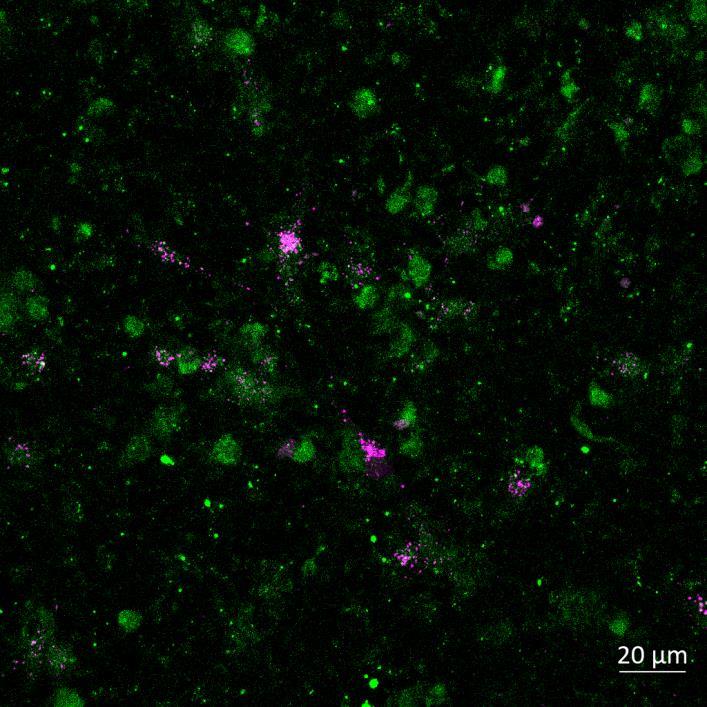
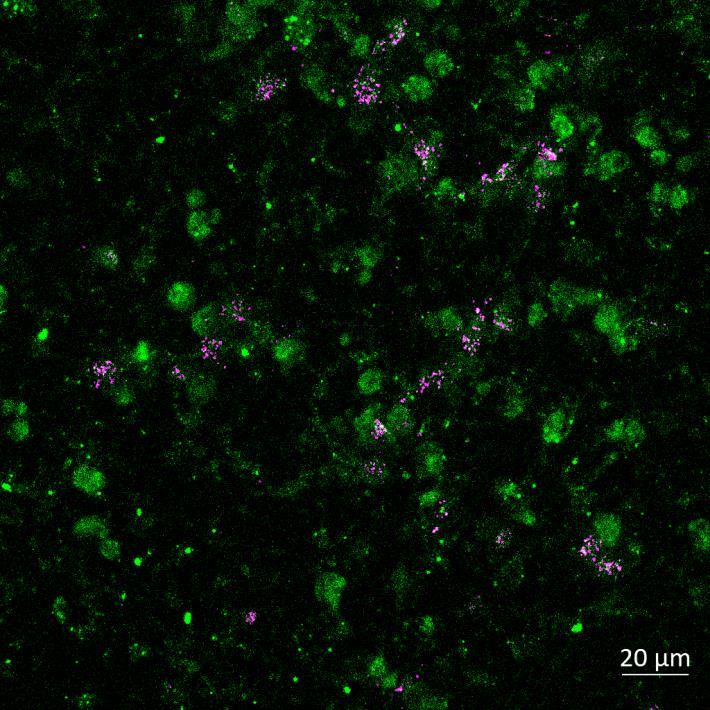

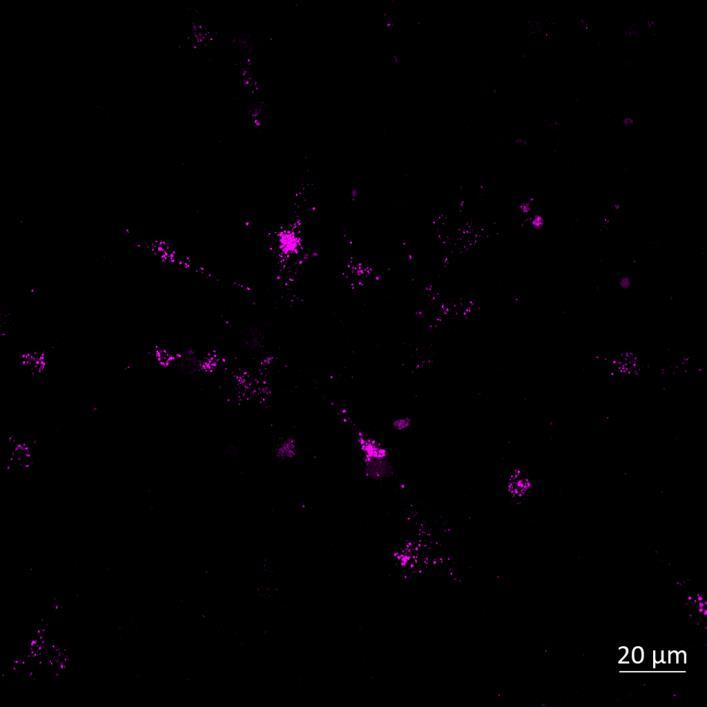

Phosphorylation and Aggregation of Native α-synuclein Induced by Exposure to Seeded Preformed Fibrils
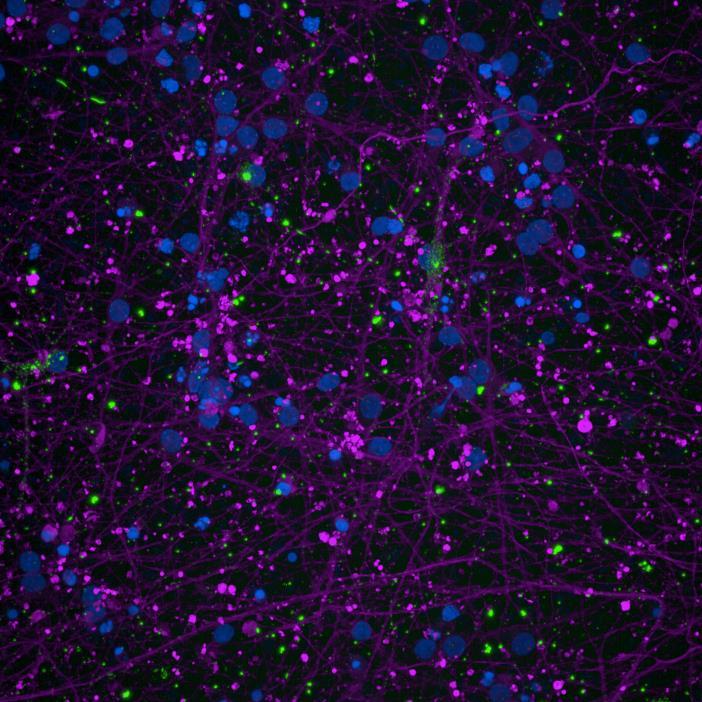
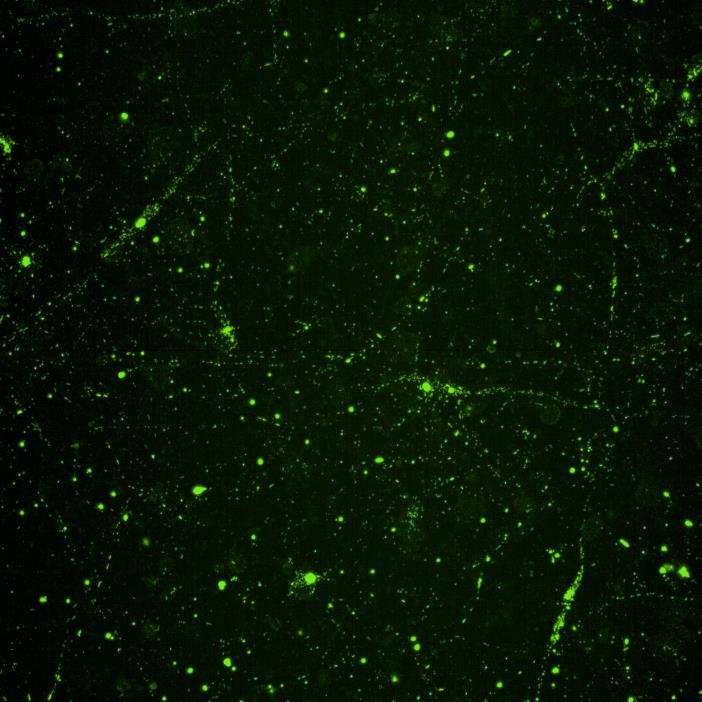
• Microfluidics devices were fixed at 2 or 3 weeks after PFF addition to “donor” chamber
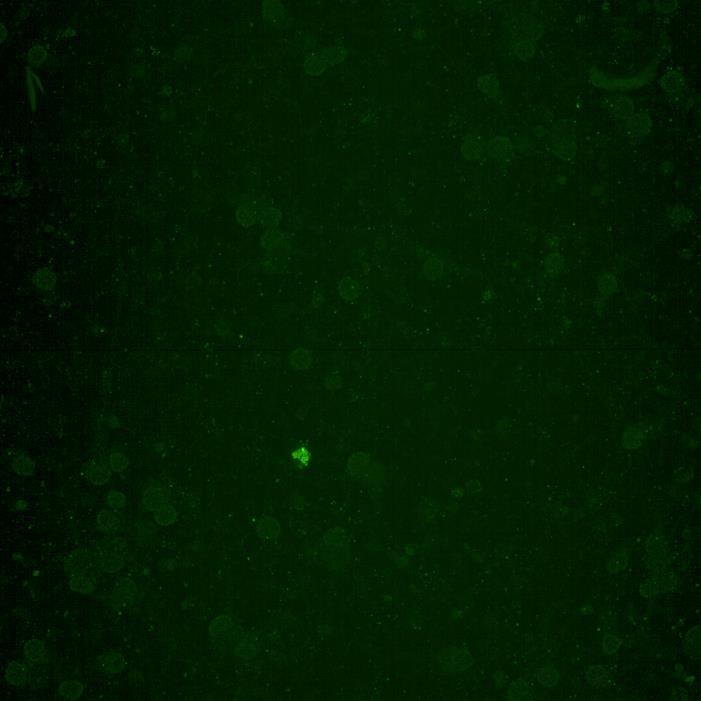
• Immunocytochemistry for β-tubulin and phosphorylated serine 129 α-synuclein was carried out
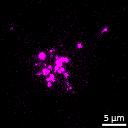
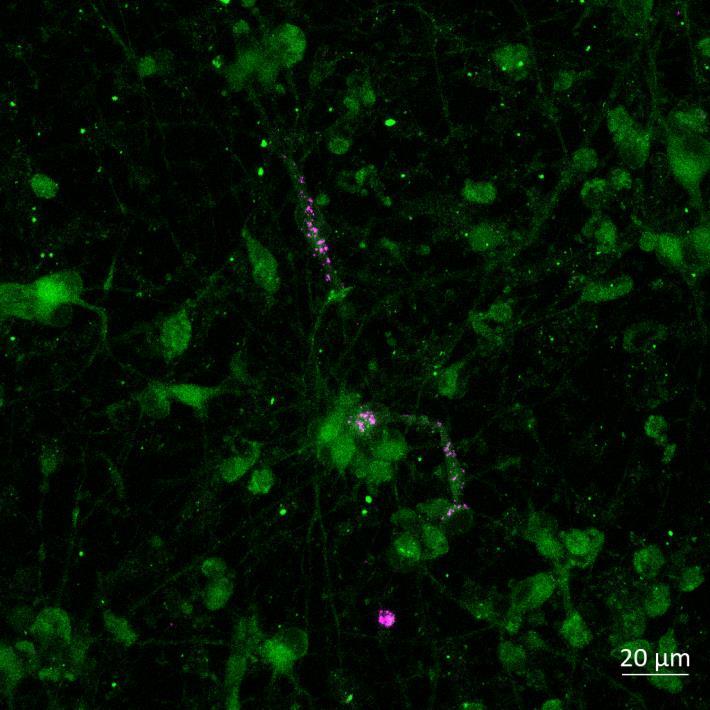
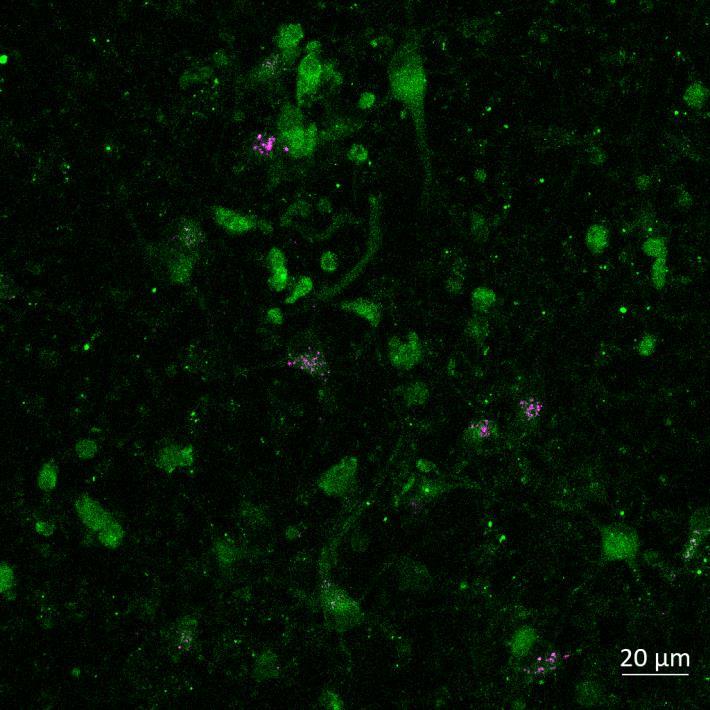
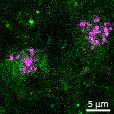
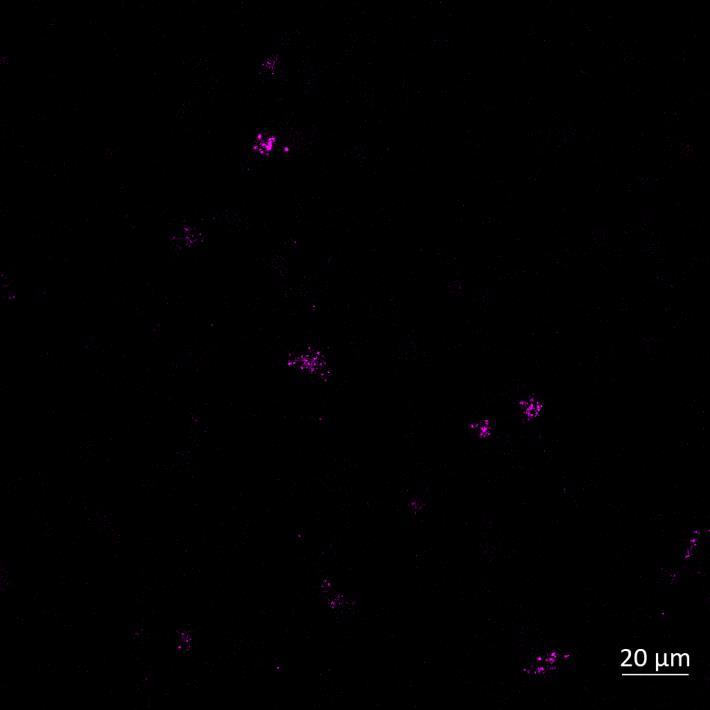
Lewy body-like perinuclear accumulation of phosphorylated serine 129 α-synuclein (arrows) in response to directly applied and seeded PFFs
• At 2 weeks, phosphorylated αsynuclein was detected in the “donor” chamber neurons only At 3 weeks, phosphorylated αsynuclein was additionally detected in neurons of the “acceptor” chamber
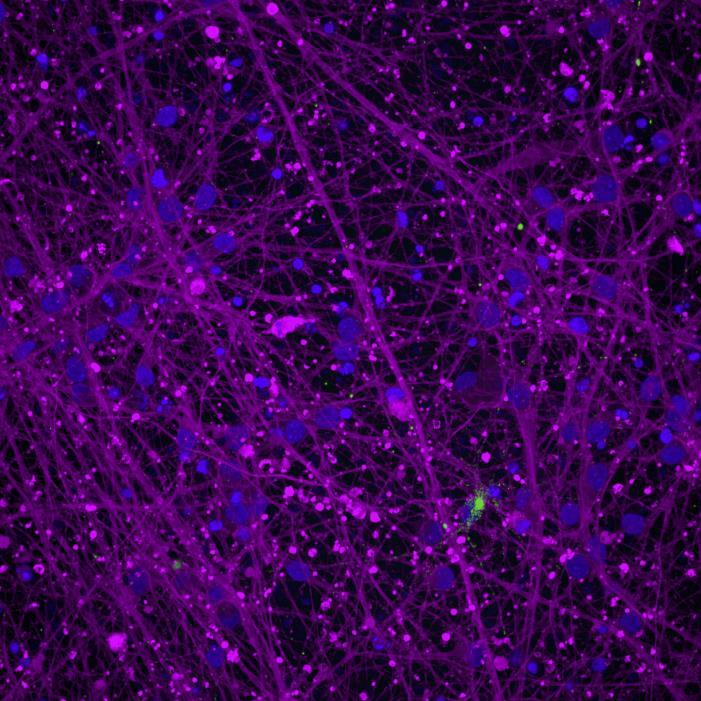
Neuron/Microglia Coculture in Microfluidics Devices
Compartmentalised cocultures of iPSC-derived glutamatergic cortical neurons and
•

•
•
•
• This technique could be widely applied to model the seeding of other aggregating neurodegenerative proteins “Donor”




Donor chamber – PFFs directly applied Acceptor chamber – seeded PFFs






Conclusions

• Uptake, axonal transport, seeding and subsequent phosphorylation and aggregation of α-synuclein can be modelled in microfluidics devices
• The effect of seeded α-synuclein to other cell types such as microglia could be assayed
