Development of CNS model for drug discovery using 3D printed human
iPSC-derived cells
Matthieu Trigano, Anna-Lena Zepernick, Eve Corrie, Emma V. Jones Medicines Discovery Catapult, Block 35, Alderley Park, Cheshire, SK10 4ZF, UK
Introduction
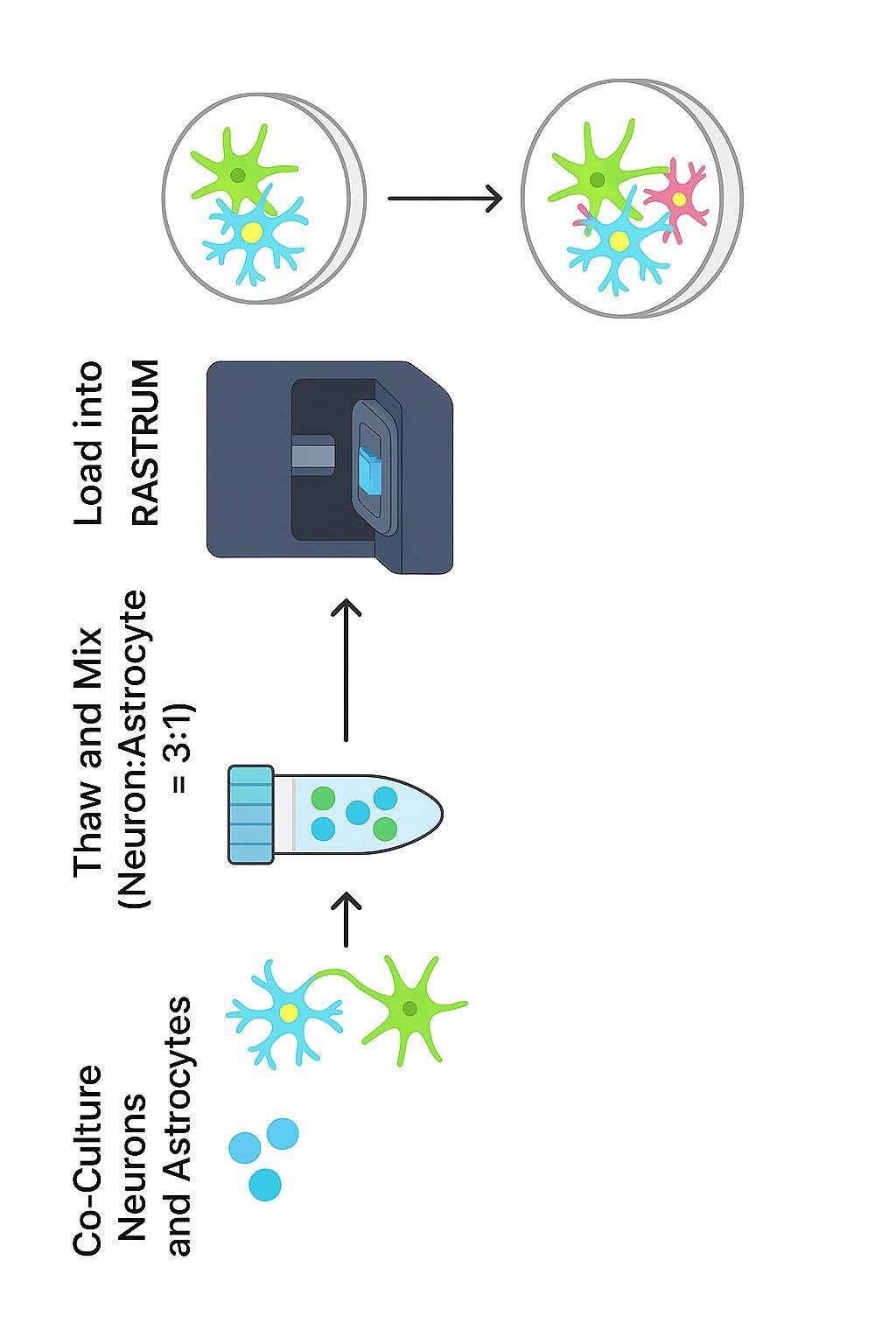
In the in vitro neuroscience team at MDC, our goal is to produce physiologically relevant central nervous system (CNS) cell models that will improve predictivity of assays and translation to the One promising direction in the field is the use of advanced models which better mimic the interactions between cells extracellular matrix in vivo Increasing evidence suggests that models may more effectively reproduce the pathological disease processes In this study, we present a 3D bioprinted CNS generated from human induced pluripotent stem cells (iPSCs), embedded in a hydrogel scaffold using extrusion bioprinting technology This system allows for mono-, co-, and culture of different neural cell types using defined density matrix conditions
Optimizing and Characterizing 3D Co
Calcein labelling of neuron-astrocyte cocultures at multiple time points showed robust cell viability and increased culture complexity over time
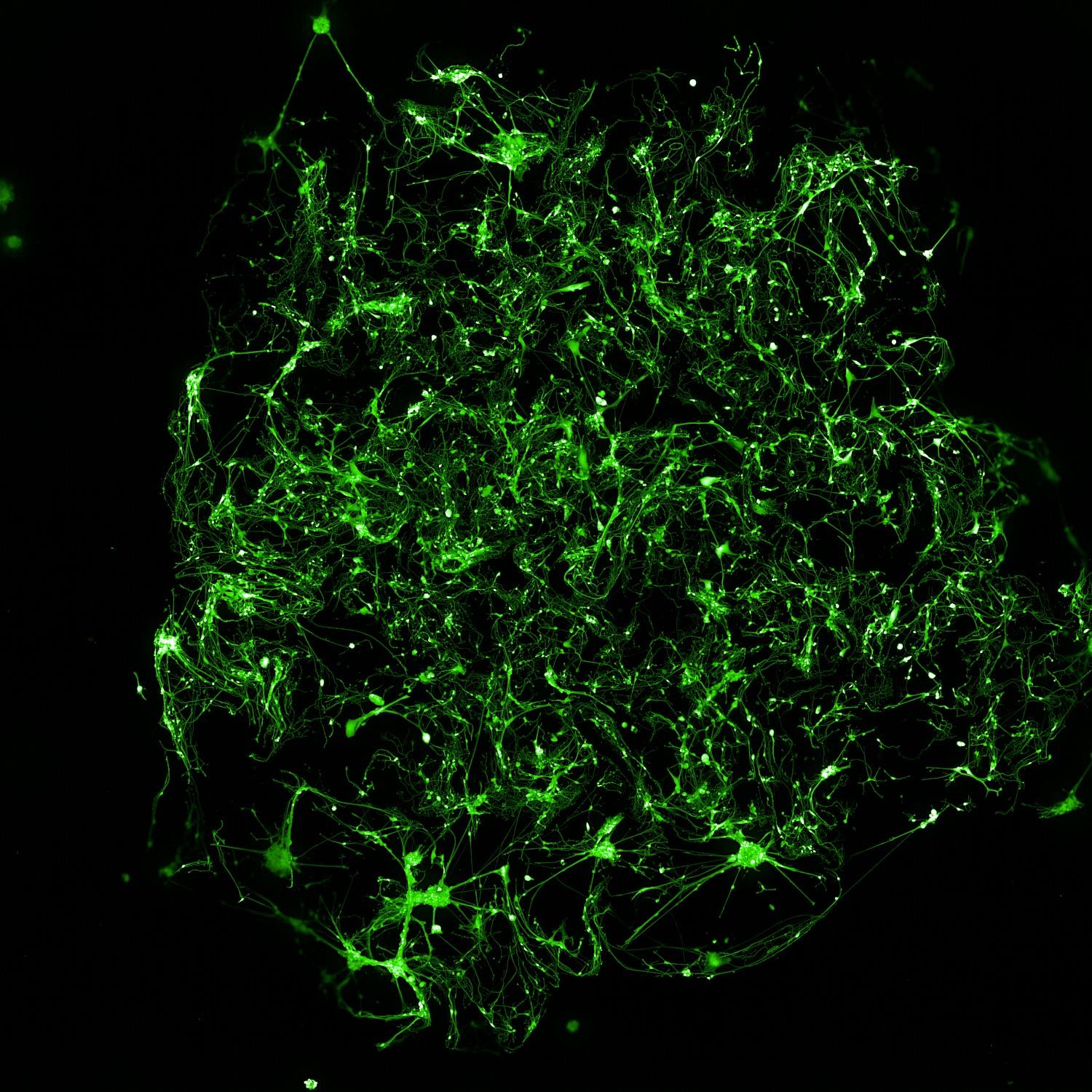



Methods








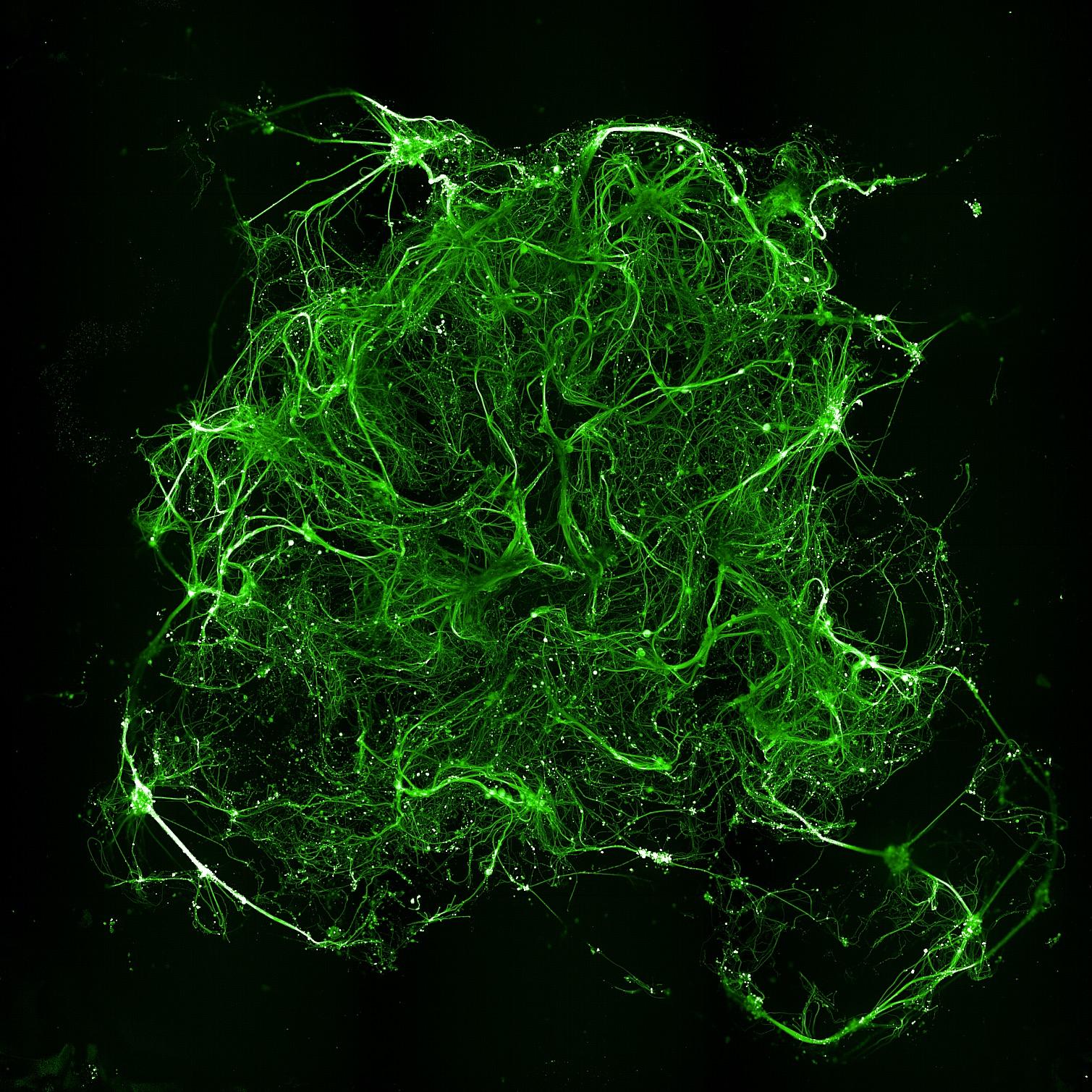
• Microglia actively migrated into the hydrogel matrix.
• Cells remained viable and embedded throughout culture.
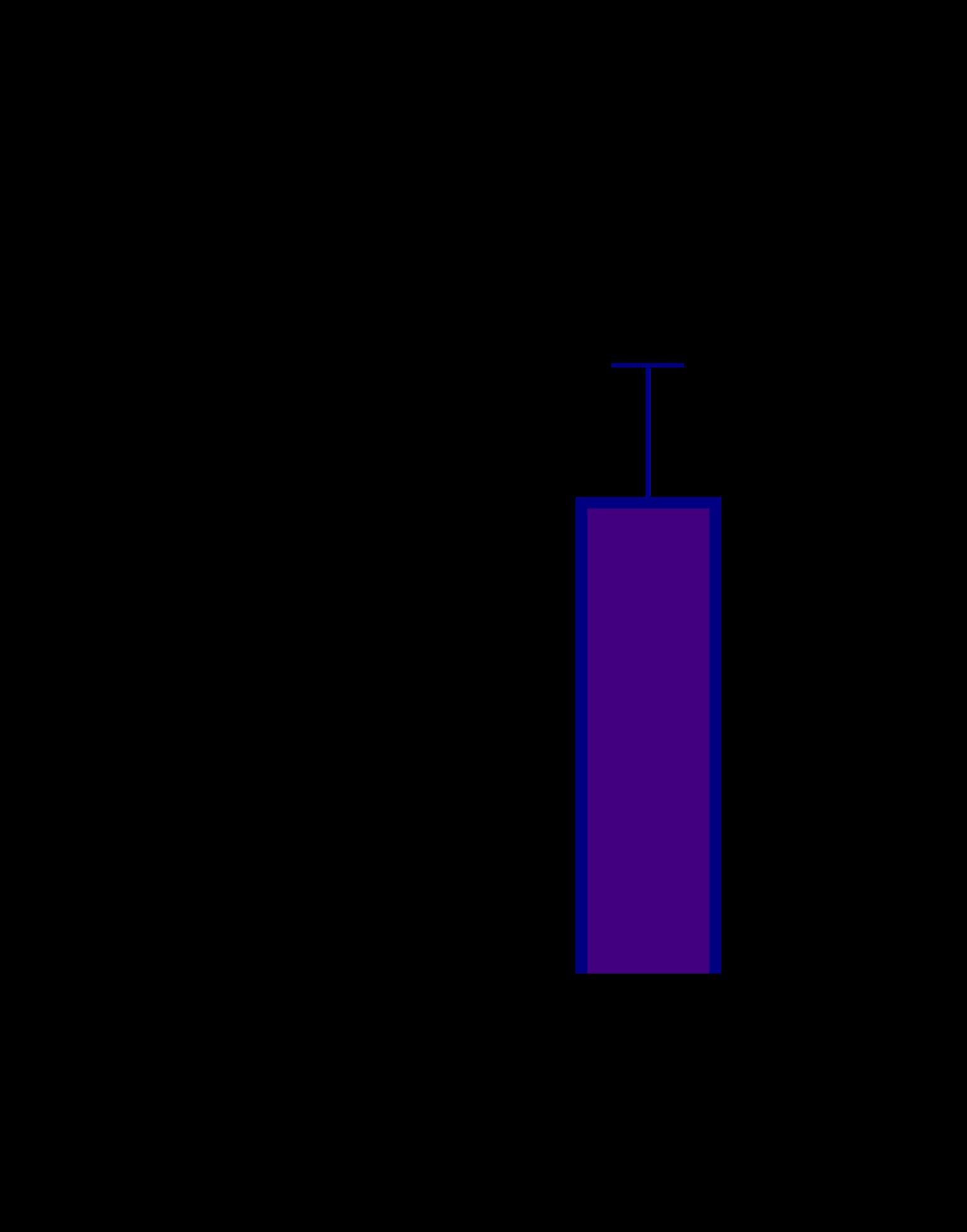
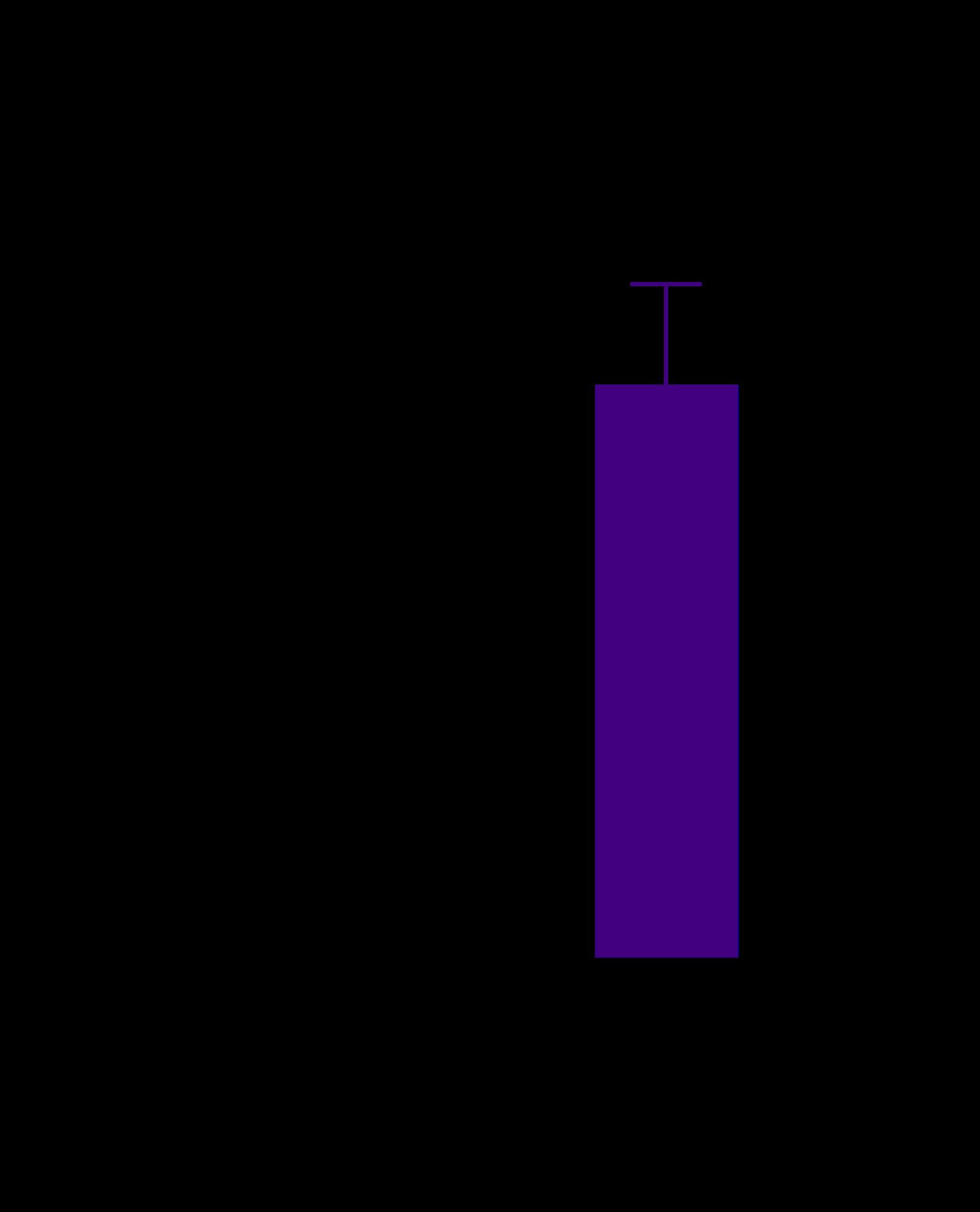
• We observed an increase level of IL6 and TNFα in 3D triculture



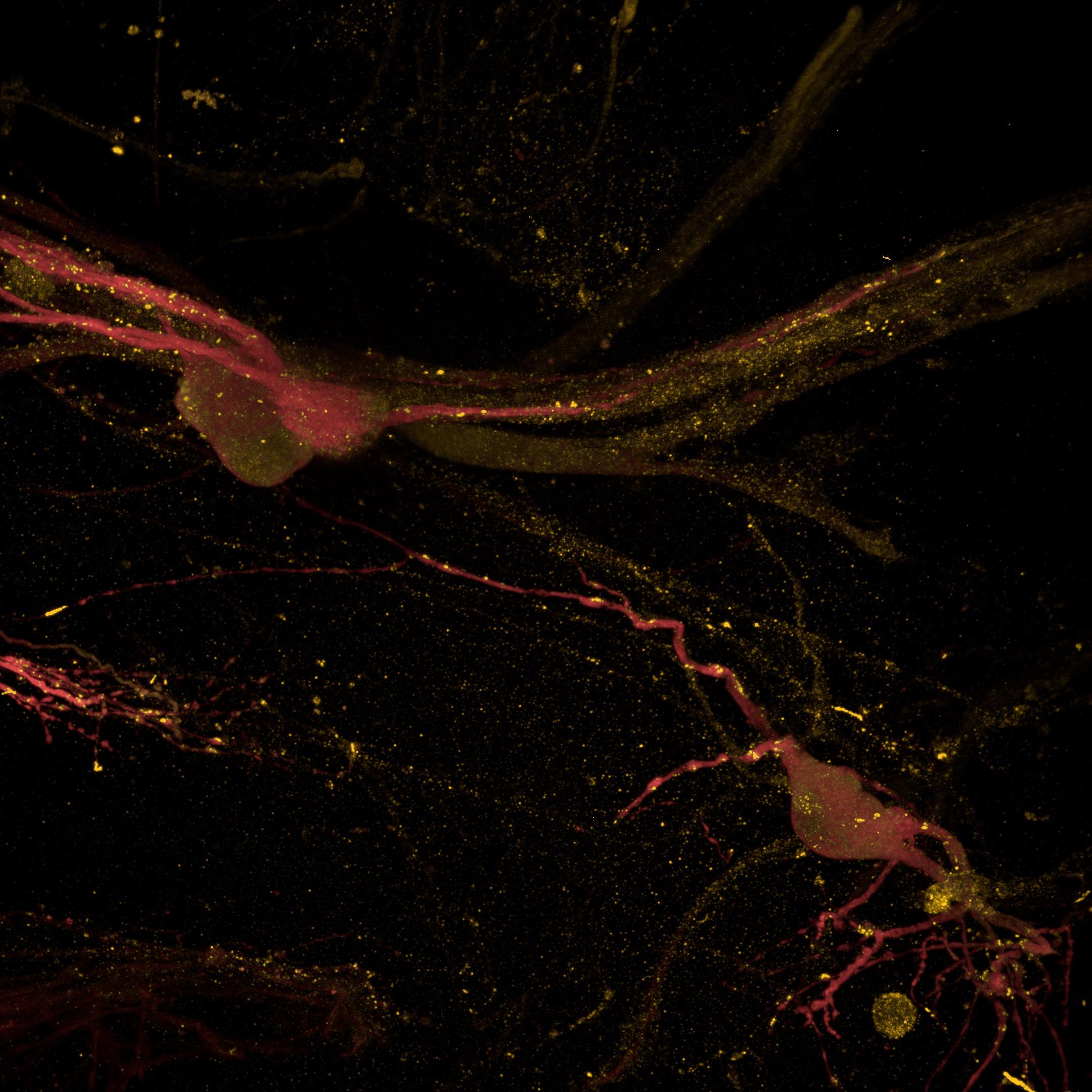
• The 3D neuron-astrocyte model was amenable to immunocytochemistry labelling and high-content confocal imaging (Opera Phenix)
• βIII-tubulin (neurons) and GFAP (astrocytes) labelling revealed an intricate interaction between the two cell types
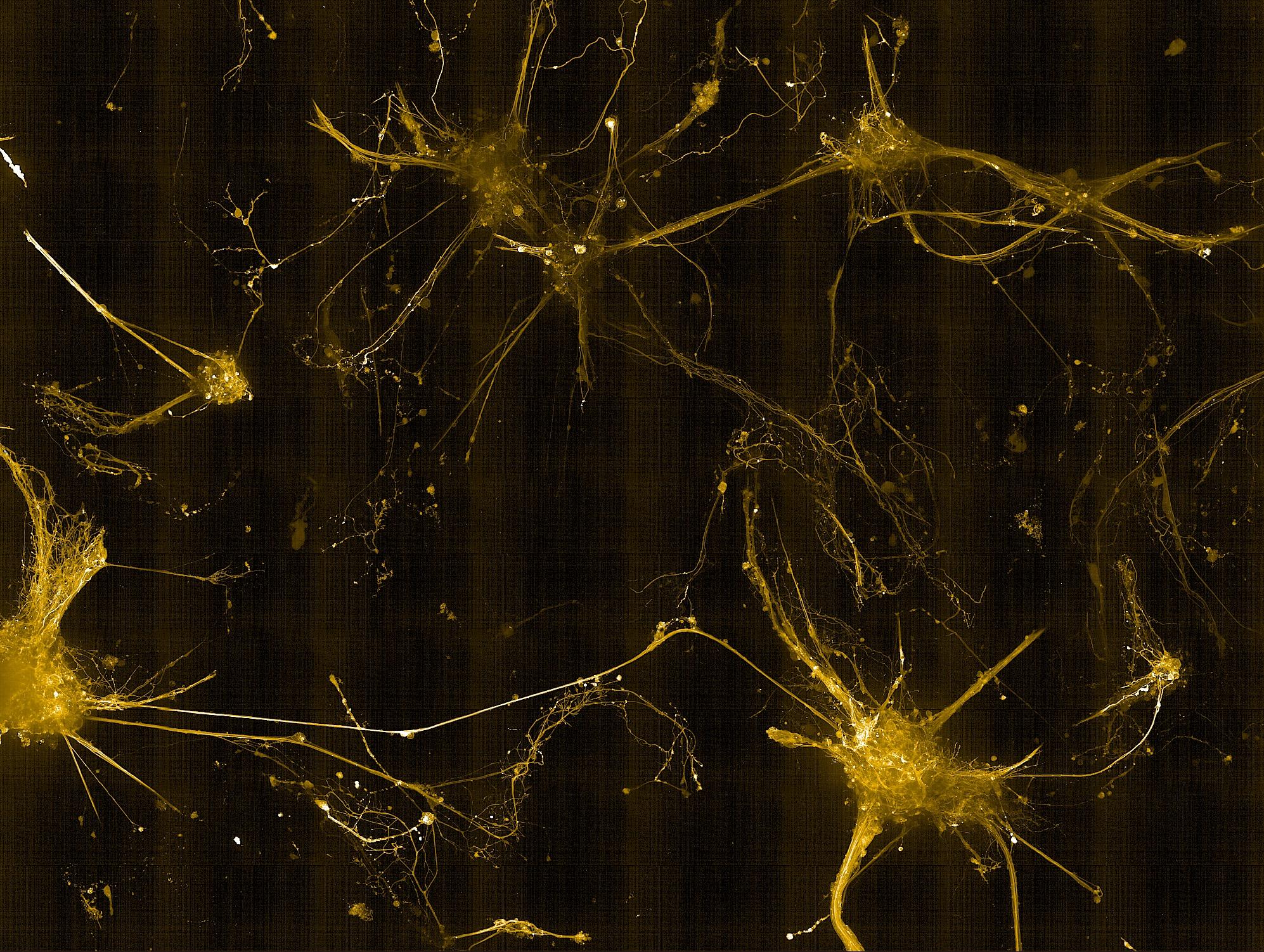
• Key pre- and post-synaptic proteins were successfully imaged, enabling the evaluation of neuronal maturity

Conclusion
• Established a 3D co- and tri-culture CNS model with neurons, astrocytes, and microglia amenable for drug discovery studies
• Detected mature synaptic protein expression in 3D cultures, indicating network formation
• Observed strong neuron–astrocyte interactions
• Plan to compare 2D vs 3D cultures to assess cellular and functional differences in model of CNS diseases
