
8 minute read
Liam Harvey (Year 12
Future Steel
Liam Harvey (Year 12) The Illawarra Grammar School, 10/12 Western Avenue, Wollongong, NSW, Australia, 2500
Advertisement
Introduction
The Port Kembla Steelworks (PKSW) is an industrial facility located in the Illawarra region and is an asset to the steelmaking industry in Australia, as well as the economic prosperity of the Illawarra: The PKSW contributes approximately 9000 employment opportunities and $6.5 billion in output (‘The future of steelmaking’ 2021).
A crucial component of the steelmaking process is the blast furnace – PKSW has two available, and the No.6 Blast Furnace is undergoing a full replacement and repair known as a ‘reline’.
The No.6 Blast Furnace Reline Project presents an opportunity for the modification of current technologies and the addition and implementation of new, emerging technologies within the steelmaking process (Figure 1). Hence, this prompts an analysis of the current state of the blast furnace technology and an assessment of potential alternative technologies to meet the PKSW’s decarbonisation commitment of Net Zero greenhouse gas emissions by 2050 (‘The future of steelmaking’ 2021).
One such speculative technology that was addressed during the 2021 Blue Scope Town Hall was the electrolysis of iron ore – molten oxide electrolysis (MOE), which was identified as a possible route for the PKSW to pursue in its steel production and is the topic for this article.
Research Question + Sub questions The main, guiding research question is: Could molten oxide electrolysis (MOE) be a viable method of steel production in the future?
To answer this main question, a range of sub-questions first need to be addressed: - What is the typical method of steel production used? - What is MOE and how does it work? - What are the benefits of using MOE?

Background Research
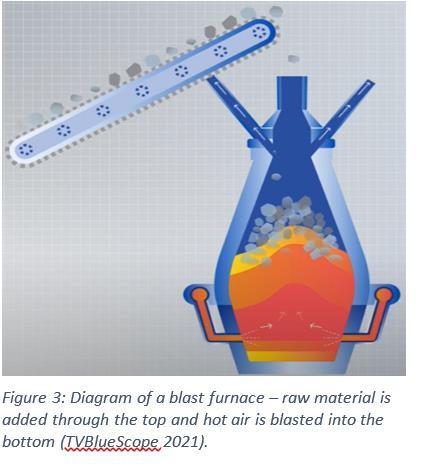
Firstly, the ‘bread and butter’ method of steel production, which is what PKSW uses. Two processes must occur before steel can be made: the creation of coke and the creation of sinter. Coke is created by obtaining coal from various suppliers and placing it into a group of ovens known as ‘batteries.’ The coal is baked in these ovens to burn off volatile components within the coal (Clean Air Council, 2016). The newly formed coke is immediately transferred along conveyor belts to the top of the blast furnace in preparation for the creation of iron. Sinter is created by creating a mixture of iron ore and flux (a blend of limestone, dolomite, and other components) and placing this mix onto a slow-rolling conveyor belt (Figure 2). This conveyor belt is constantly exposed to temperatures of 1000°C to harden this mix. At the end of the conveyor belt it is crushed, cooled, and sent to the top of the blast furnace (Illawarra Mercury, 2016) (Figure 3). Next, the coke and sinter are combined with more iron ore within the blast furnace where the following chemical reactions takes place to reduce the iron oxide into raw iron (Shelton Iron and Steel Co, n.d.): 3����2��3 +���� →����2 +2����3��4 ����3��4 +���� → ����2 +3������ ������+���� → ����2 +����,����:������+�� →����+���� Where the ����2 and ���� is produced by the reaction between the coke and the oxygen in the hot air injected into the blast furnace: ��+ ��2 →����2 ����2 +�� →2����
After this, the molten iron is sent to the Basic Oxygen Steelmaker (BOS) where it is combined with oxygen and scrap metal to produce steel, where leftover carbon from the blast furnace is removed: ��+ ��2 →����2 2��+ ��2 →2���� This achieves the correct blend of carbon within the steel, as the properties of the steel are dependent on the amount of carbon within the iron.
Next, the emerging steel production method known as molten oxide electrolysis (MOE) works by ripping apart iron oxides using an inert anode and an electric current, to produce molten iron without the release of greenhouse gases such as carbon dioxide typically produced in other methods (Boston Metal 2022): 2����2��3 +�� − → 4����+ 3��2

Firstly, iron oxide in a solid form is fed into the cell where the reaction will take place (Figure 4). Next, the iron oxide collects at the bottom of the cell, where it can be alloyed with other metals that change the properties of the steel. In this stage, carbon can also be added to allow the immediate creation of steel straight out of the MOE cell. Electricity is passed through the cell from the inert anode at the top of the cell, through the electrolyte bath which contains a range of elemental oxides and conducts the electricity to the cathode. As all of the other oxides contained within the electrolyte bath have a greater stability than iron oxide, it is the first to be separated when the electricity is sent through the cell (Soltoff, B 2022). This produces the molten iron which can be tapped through the bottom of the cell, and the only by-product throughout this process is oxygen gas – a much greener alternative to carbon dioxide.
Figure 1: Diagram of the cell used in MOE (Boston As long as the electricity used to melt the iron oxide
Metal 2022). is produced in a carbon neutral/negative manner, then the production of steel using the MOE cell will not contribute to global warming and the climate change crisis.
Hypothesis
Based on the preliminary research conducted, it is hypothesised that MOE could be a commercial method of producing steel in the future, but it may require more research to be a successful carbon-free alternative to tradition commercial steelmaking methods.
Further Research
Commercial utilisation of MOE compared to traditional steelmaking methods could potentially bring forth a range of benefits that see the technology being more advantageous to producers. As MOE can utilise any grade of iron ore due to its simplistic process, it is not restricted in the range of iron ore that it can use to produce high-grade molten iron. During the 2021 Blue Scope Town Hall, Head of Future Technologies Chris Page highlights the lack of range in reagents that Hydrogen DRI-EAF has: “there’s only a few types of ores that are suitable to go through the Hydrogen DRI and EAF process”. He also highlights that the majority of iron ore that the PKSW has access to does not fit the suitability category, hence limiting that technology. This highlights the flexibility and broad range of ores that MOE technology can accommodate, leading to a consistent production of iron/steel regardless of the availability of specific reagents, and a possible benefit to the adoption of this technology in the future by the PKSW. The reaction conditions used within MOE technology are like that of other steel production methods, yet the use of MOE requires less stages than Blast Furnace/BOF and EAF technologies. Furthermore, the use of MOE requires a liquid oxide electrolyte, which significantly varies compared to the reaction conditions of other technologies, which for Blast Furnace/BOF, requires injection of oxygen, as well as the presence of coke.

Furthermore, as there are less stages in MOE steelmaking compared to other methods, there is less exposure of the reagents to possible contaminants, automatically increasing the purity of any products. In addition, the purity of iron and steel produced in this method is guaranteed, as when the iron is melted, any impurities remain behind in the electrolytic fluid. Currently, the only functional MOE cell will produce less than 100 tons of steel per year, which is extremely small, and highlights the difficulties in the mass-manufacturing of such a new and emerging technology.
MOE is extremely limited in its industrial uses, as it has been essentially designed for a single purpose –to create iron and steel from iron oxides. However, NASA has conducted research into this technology for its viability in producing oxygen for life support on the moon, from lunar regolith – albeit not applicable to steelmaking (Science.gov, n.d.).
According to Boston Metals - the pioneers of MOE technology in steelmaking, the use of MOE is 25% more efficient than coal-based steelmaking. The use of MOE technology is up to 60% more energy efficient compared to the use of green hydrogen technology (Soltoff, B 2022). However, there would be difficulty in implementing MOE technology in current Blast Furnace/BOF steelmaking as they operate off of completely different systems, in comparison to green hydrogen, which already has the capital to be implemented as it is compatible with most DRI (direct reduced iron) systems (Soltoff, B 2022).
Furthermore, the MOE technology uses an extreme amount of power – 4 megawatt-hours of electricity to produce 1 ton of steel: the equivalent of powering an Australian home for 222 days (Adam Rusin Electrical 2017).
Conclusion
Based on the evidence compiled in this article, it can be ascertained that molten oxide electrolysis (MOE) is a very viable method of steel production in regard to its lack of carbon emissions, efficiency, and purity. However, production of MOE cells on a commercial scale needs to be achieved for this viability to become a reality, and the power consumption of each cell needs to be addressed to ensure this technology’s sustainability for future steel production, potentially as an alternative steel making process for Port Kembla Steelworks.
References
Adam Rusin Electrical 2017, Average kwh usage per day Australia, viewed 14 June 2022, http://www.arelectrical.com.au/tag/average-kwh-usage-per-dayaustralia/#:~:text=An%20average%20Australian%20house%20consumes,or%206570%20KW %20per%20hour. Bluescope 2022, About Us, viewed 13 June 2022, <https://www.bluescopeillawarra.com.au/about-us/>. Boston Metal 2022, Transforming Metal Production, viewed 13 June 2022, <https://www.bostonmetal.com/transforming-metal-production/>. Clean Air Council 2016, Coke and How it's Made, viewed 13 June 2022, <pacokeovens.org/what-is-coke/>.
Illawarra Mercury 2016, Steel Story Part 3, viewed 13 June 2022, <https://www.illawarramercury.com.au/steel-story-3/>. Science.gov n.d., Records for molten oxide electrolysis, viewed 14 June 2022, <science.gov/topicpages/m/molten+oxide+electrolysis.html>.
Shelton Iron and Steel Co n.d., How it works: The blast furnace, viewed 13 June 2022, <thepotteries.org/shelton/blast_furnace.htm>. Soltoff, B 2022, Green steel without hydrogen - can it work?, viewed 13 June 2022, <https://www.canarymedia.com/articles/clean-industry/green-steel-without-green-hydrogen-canit-work>. ‘The future of steelmaking’ 2021, Bluescope Steel, Port Kembla. TVBlueScope 2021, The Blast Furnace Process at Port Kembla, online video, 2 October, viewed 13 June 2022, <https://www.youtube.com/watch?v=qD2LPYZjdw4>. TVBlueScope 2018, The Story of Steel - Port Kembla Steelworks, online video, 31 August, viewed 13 June 2022, <https://www.youtube.com/watch?v=2hguRUF0P4








