
14 minute read
Alexis Evans (Year 9
Ocean acidity and its impacts
Alexis Evans (Year 9) The Illawarra Grammar School, 10/12 Western Avenue, Wollongong, NSW, Australia, 2500
Advertisement
Introduction
The economies of many countries around the world are dependent upon the ocean, but it and all the organisms that reside in it are slowly being damaged by ocean acidification. Ocean acidification is “the reduction of pH of the ocean over an extended period of time, caused primarily by uptake of carbon dioxide (CO2) from the atmosphere” (NOAA n.d.). The pH scale is a measure of how acidic or basic a substance is, ranging from 14, meaning very basic, to 1, which is very acidic. A pH of 7 means that a substance is neutral, such as pure water. Seawater, with all of its additives, has a basic pH. Since the industrial age, carbon dioxide levels in the atmosphere have been rising due to the burning of fossil fuels for energy and land use change. The ocean absorbs approximately 30% of the carbon dioxide that is released into the atmosphere (NOAA n.d.). Though the effects of this carbon dioxide absorption are not outwardly noticeable, it can cause great damage beneath the surface (Smithsonian Ocean 2018).
When carbon dioxide is absorbed into water, a chemical reaction occurs which produces carbonic acid, which lowers the pH of the water to become more acidic. The ocean is very vast, and would require a great deal of carbon dioxide to change the pH of such a large expanse of water, but it is predicted that approximately 525 billion tons of carbon dioxide has been absorbed into the ocean since the industrial era, changing the ocean’s chemical make-up to become 30% more acidic (Smithsonian Ocean 2018). Because the change in pH has been so rapid, the fastest change in ocean chemistry in 50 million years, marine life that exists within the Earth’s oceans has not been given the time to adapt and protect itself from the changing environment. Some creatures’ shells dissolve in more acidic seawater, and corals are also more difficult to build and maintain in such a foreign environment (NOAA n.d.).
The chemistry behind the process of ocean acidification is quite simple; deceptively so for how much destruction it causes. When carbon dioxide enters the seawater and reacts with it to produce carbonic acid, the pH of the ocean is lowered. The creation of this acid binds carbonate ions, making less available for the corals, oysters, mussels and other sea creatures that need them to build shells and skeletons (Smithsonian Ocean 2018). Carbonic acid is a relatively weak acid, so can not actually change the entire ocean acidic, but it does make it lean dramatically towards being acidic. The ocean pH has dropped so far from 8.2 to 8.1 since the industrial revolution, when large amounts of carbon dioxide began to be emitted into the atmosphere, and is predicted to drop another 0.3 to 0.4 pH units by the end of the century (Smithsonian Ocean 2018).
This experiment aims to simulate the chemical reaction between water and carbon dioxide in ocean acidification and investigate the effect of the amount of sodium hydrogen carbonate as the base in a neutralisation reaction on the pH of water after carbon dioxide is produced and bubbled into the water to form carbonic acid. Because carbon dioxide is a gas and would be difficult to capture to use for the experiment, a neutralisation reaction will first be completed to the necessary carbon dioxide for the ocean acidification simulation reaction. A neutralisation reaction occurs between an acid and a base and makes the resulting solution one with a neutral pH of 7. However, for the resulting solution to be fully neutral, the acid and base must fully cancel each other out, which means they must be in equal amounts. The reactants that will be used in this investigation are acetic acid, commonly known as vinegar, and
sodium hydrogen carbonate. This reaction can be represented by the chemical equation:
CH3COOH + NaHCO3 → H2O + CO2 + NaC2H3O2 (vinegar + sodium hydrogen carbonate → water + carbon dioxide + sodium acetate).
The products of a neutralisation reaction are always water and a salt. In the case of the base in the reaction being a carbonate, such as sodium hydrogen carbonate, the reaction will also result in carbon dioxide. When this neutralisation reaction is completed, the carbon dioxide it produced will be transferred into a volume of water and the pH will be measured after the acidification reaction has taken place. Since the equipment needed to measure how much carbon dioxide is used in the secondary reaction is not available, the data collected will be of how much sodium hydrogen carbonate is added to the vinegar, since that amount will be proportional to the amount of carbon dioxide produced and transferred into the second reaction. The chemical equation for the main reaction of this investigation is: H2O + CO2 → H2CO3 (water + carbon dioxide → carbonic acid).
It is hypothesised that the outcome of this investigation will be that the carbon dioxide will make the water more acidic and lower the pH to below 7, in the acidic range. This is because when carbon dioxide meets water and reacts with it, it forms carbonic acid. Since water has a neutral pH neutral, the solution of the carbonic acid and water will then become more acidic with a lower pH. This means that the more sodium hydrogen carbonate is added to the vinegar in the neutralisation reaction, the more carbon dioxide will be produced and then transferred into the secondary reaction, and therefore more carbonic acid will be produced. Ultimately, the more sodium hydrogen carbonate is added to the neutralisation reaction, the lower and more acidic the pH will be from the water acidification reaction. If the impacts, causes and severity of ocean acidification can be understood, it can be properly addressed before it becomes an overly major issue, and all the economies in the world that rely upon fishing and the ocean to survive will prosper once again.
Methodology Independent variable:
The independent variable in this investigation is the amount of sodium hydrogen carbonate (commonly known as bicarb soda) added to the vinegar to react to form carbon dioxide.
Dependent variable:
The dependent variable in this investigation is the pH of the water and carbonic acid solution after the reaction between the sodium hydrogen carbonate and vinegar. The pH will be measured using a fully-charged pH probe inserted into the solution, and the results will be read in an exact number on the application that connects the probe to an electronic device.
Controlled variables:
There are several factors in this experiment that must be controlled. The amount of vinegar used in the chemical reaction to create carbon dioxide must be regulated across all tests to ensure that the amount of carbon dioxide can be proportional across all tests. If the amount of vinegar changes and the amount of sodium hydrogen carbonate changes, then it would be impossible to know how much carbon dioxide was entering the beaker of water. In that case, then the results of the experiment would be meaningless because the investigation would not be valid. This variable can easily be controlled, by simply deciding upon an amount to use for every test and measuring out that amount using an electric scale. The amount of vinegar that will be used is 80mL. The amount of water in the beaker must also be controlled throughout the investigation, because the pH would vary depending upon the concentration of the acid. The concentration of the acid would vary in
different amounts of water, affecting the validity of the results.
The amount of water in the beaker can be controlled in the same way as the amount of vinegar in the flask. For each repetition of the experiment, the same amount of water can be measured out into the beaker using the electric scale. 120mL of water will be used. The pH of the water the carbon dioxide will be bubbled into must also be controlled. It would be difficult to change the pH to make it exactly the same for each repetition without modifying the outcome of the experiment, but if the water is sourced from the same location it is most likely to have the same pH. The initial pH of the water will be measured using a pH probe, and if there is a larger difference than 0.5 between the pH, that water will not be used.
The water will be sourced from the same tap, so that should ensure the pH will be maintained across the investigation. To make sure that the measuring of the pH of the water is accurate every time, it must be ensured that when the sodium hydrogen carbonate reacts with the vinegar, the solution does not bubble up enough to reach the level of the side-arm in the flask and enter the beaker of water and carbon dioxide. If either of these reactants enter the water and carbon dioxide solution, they would affect the validity of the results, because the sodium hydrogen carbonate is a base and would increase the pH, while the vinegar is an acid and would decrease the pH. Any additive would therefore alter the pH that was given by the experiment, and render the results invalid. Finally, the method of measuring the pH in this investigation must be maintained so that the results can all be recorded in the same way. If universal indicator were used for one test, then a pH probe used for another, the results would not be comparable and would thus be meaningless. The method of pH measurement used in this investigation will be a pH probe.
Equipment list:
• 1 Side-arm flask • 1 Funnel • Vinegar • 100mL beaker • 150mL beaker • 200mL beaker • 1 Balloon • Sodium hydrogen carbonate • 1 pH probe • 1 Electric scale • 1 spoon • Water • Rubber tub
Method
1. The rubber tube was attached to the arm of the side-arm flask, and the flask was filled with 80mL of vinegar, which was measured into the 150mL beaker on the electric scale before it was poured into the flask. 2. For the first test, 3g of sodium hydrogen carbonate was measured into the 100mL beaker on the electric scale using the spoon. The sodium hydrogen carbonate was then poured, with the use of the funnel, into the balloon. 3. The opening of the balloon was stretched over the top opening of the side-arm flask so that the seal was airtight. It was done carefully to prevent any of the sodium hydrogen carbonate from being tipped into the flask prematurely. 4. The 200mL beaker was filled with 120mL of water from the tap, again using the electric scale. The pH probe was set up and inserted into the water. After the pH reading settled and had stopped changing to reach a definitive number, the pH probe was removed from the water. 5. The end of the rubber tube not attached to the side-arm flask was inserted into the water in the beaker and the opening was fully submerged.
This set up can be seen the diagram below. 6. While the rubber tubing was held in place in the beaker, the balloon attached to the top of the side-arm flask was inverted and the sodium hydrogen carbonate it contained was emptied into the vinegar within the flask. As the chemical reaction occurred between the sodium hydrogen carbonate and vinegar, the rubber tubing was held in place to ensure all carbon dioxide created from the primary reaction was captured to create the secondary chemical reaction with the water. 7. After the primary chemical reaction between the sodium hydrogen carbonate and vinegar had subdued and no more bubbles of carbon dioxide gas was being released into the water, the rubber tube was removed from the water and carbonic acid solution and the pH probe was once again inserted. The pH was measured. 8. The flask, rubber tubing, pH probe and beakers were rinsed out with water, and then the same process was repeated twice more with the amounts of sodium hydrogen carbonate of 5g and 7g. Diagram: Set up of the equipment in preparation for the experiment
Results
Water pH
Amount of sodium hydrogen carbonate (g) Initial Post-reactive
3 6.87 5.89 5 6.82 5.25 7 6.93 7.16
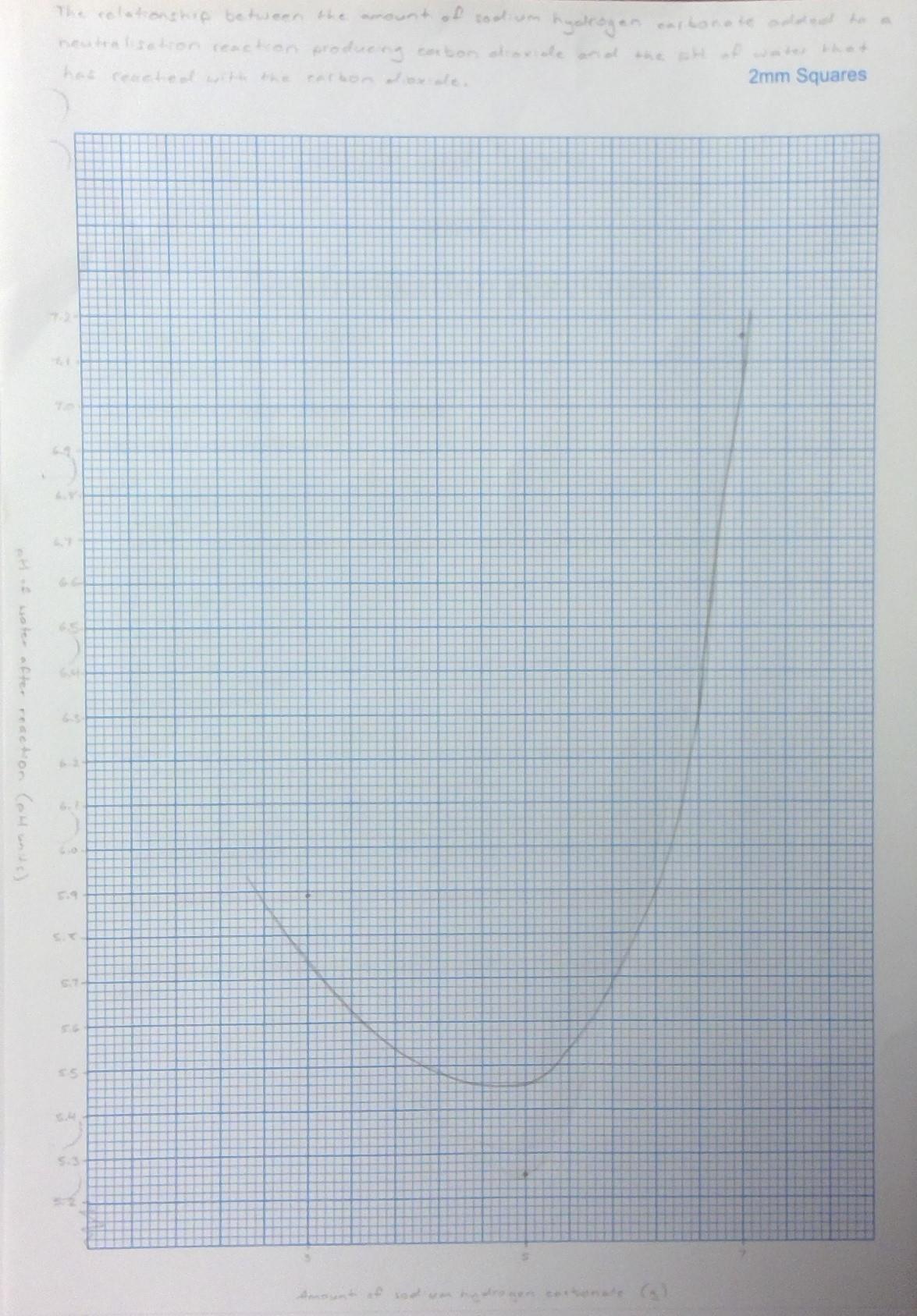
The graph is non-linear and does not show a relationship between the two variables. At first, when the independent variable increased, the dependent variable decreased, showing a negative relationship. Then, however, the dependent variable increased when the independent variable increased.
Discussion
The were no visible trends or relationships present in the results, and the aim of the investigation was not achieved. The aim was to investigate the effect of the amount of sodium hydrogen carbonate as the base in a neutralisation reaction on the pH of water after carbon dioxide is produced and bubbled into the water to form carbonic acid. The test using 7g of sodium hydrogen carbonate has an apparently outlying result, but this cannot be confirmed because the results were not reliable as they were not repeated at all. The hypothesis for this experiment was not supported by the gathered results, which was that as the amount of sodium hydrogen carbonate increased, the pH of the water would decrease to become more acidic. This was refuted because, while the first two results supported this, the last result did not.
The results of the experiment were not accurate, valid or reliable. There were several issues with how the investigation was carried out, the most important one being that there were no repetitions of the tests of each amount of sodium hydrogen carbonate. If each amount had been trialled at least three times, any outliers in the data could have been easily identified. The average could also have been found to have a more accurate representation of what the results were like. In addition, the accuracy was very low mostly in the third amount tested. Because so much vinegar was used in the neutralisation experiment, when 7g of sodium hydrogen carbonate was added, the chemical reaction bubbled up the neck of the flask and spilled out the rubber tubing into the beaker of water and carbon dioxide. This was a failure to control all the necessary variables, and greatly affected the validity of the result. Since the vinegar was still mostly at the bottom of the flask, the majority of the solution that entered the water beaker was sodium hydrogen carbonate, the base from the neutralisation reaction. Because it had a higher pH, it changed the results to be higher than they would have been had there been no sodium hydrogen carbonate in the beaker. Since bases have a higher pH, there was so much sodium hydrogen carbonate that it overpowered the amount of carbon dioxide to change the pH of the water to become higher and more acidic rather than lower and more basic.
The results, had the experiment been completed correctly, would have supported the hypothesis and shown that as the independent variable increased, the dependent variable decreased as the water and carbon dioxide solution became more acidic the more carbon dioxide was added. This is because whenever carbon dioxide molecules meet water molecules, a chemical reaction occur, which always produces carbonic acid, without exception. Since water is neutral with a pH of 7, the carbonic acid would lower the pH and turn the solution acidic. If this is not the case, then another factor would be interfering with the results such as in this investigation.
To improve the method, each change of the independent variable should be tested at least three times to improve the accuracy and reliability of the results and to remove any risk of unidentified outliers skewing the results away from what they should have been. Also, if less vinegar was poured into the flask for the neutralisation reaction, there would be much less risk of the solution bubbling up to interfere with the second reaction. This would make the results more valid because the base used in the neutralisation reaction would not be changing and influencing the measured pH of the water and carbon dioxide solution.
Conclusion
The aim of this investigation was to determine the effect of the amount of sodium hydrogen carbonate as the base in a neutralisation reaction on the pH of water after carbon dioxide is produced and bubbled into the water to form carbonic acid, and it was hypothesised that as the amount of sodium hydrogen carbonate was increased, the pH level of the water would decrease. The results showed no clear relationships between the two factors, therefore refuting the hypothesis, because in the first two tests, the pH did decrease, but in the final test it increased as the amount of sodium hydrogen carbonate did also. However, only one repetition was done of each change of the independent variable, so the experiment could not be considered reliable. Also, in the third test an amount of the products and reactants from the neutralisation reaction unintentionally entered the beaker of water and carbon dioxide, thereby making the results invalid and inaccurate. Even so, the findings of this investigation can contribute to helping understand the impact and issue of ocean acidification, and can provide a basis for a solution to be found to the threat it poses to the world’s oceans.
References
National Oceanic and Atmospheric Administration (NOAA) n.d., What is Ocean Acidification?, viewed 27 October 2022, <https://oceanservice.noaa.gov/facts/a cidification.html>. Smithsonian Ocean 2018, Ocean Acidification, viewed 27 October 2022, <https://ocean.si.edu/oceanlife/invertebrates/ocean-acidification>.








