Biportal Endoscopic
Spine Surgery: Overview and Applications
Biomechanics of Zero-Profile
Anterior
Cervical Discectomy and Fusion
Precision in Practice: Alignment in Non-deformity
Spine Surgery
Latest Report on Coflex and Interspinous Devices
Osteoporosis and Spine Surgery
Postoperative Dysphagia
After Anterior
Cervical Spine Surgery
Telehealth in Spine Surgery
Vertebral COLUMNS
International Society for the Advancement of Spine Surgery
SPRING 2024
Academic
PLUS INSIDE
Mentorship and Productivity
ENDOSCOPY Biportal Endoscopic Spine Surgery: Overview and Applications
BIOMECHANICS
Biomechanics of Zero-Profile Anterior Cervical
Discectomy and Fusion
CLINICAL PRACTICE
Precision in Practice: Alignment in Non-deformity
Spine Surgery
SURGICAL DEVICES
Latest Report on Coflex and Interspinous Devices
BONE HEALTH
Osteoporosis and Spine Surgery
COMPLICATIONS
Postoperative Dysphagia After Anterior Cervical Spine Surgery
HEALTHCARE DELIVERY
Telehealth in Spine Surgery
Spring 2024
Editor in Chief
Kern Singh, MD
Editorial Board
Peter Derman, MD, MBA
Brandon Hirsch, MD
Sravisht Iyer, MD
Yu-Po Lee, MD
Sheeraz Qureshi, MD, MBA
Managing Editor
Audrey Lusher Designer CavedwellerStudio.com
Vertebral Columns is published quarterly by the International Society for the Advancement of Spine Surgery. ©2024 ISASS. All rights reserved. Opinions of authors and editors do not necessarily reflect positions taken by the Society. This publication is available digitally at www.isass.org/news/vertebralcolumns-Spring-2024
3 8 12 15 21 24 29 33 isass.org Spring 2024 Vertebral Columns EDITORIAL Academic Mentorship and Productivity
Become a member today https://www.isass.org/about/membership/
From the
Academic Mentorship and Productivity
Mentorship is pivotal in facilitating the education and professional development of both physicians and scientists, especially within the sphere of academic medicine. Young spine surgeons embarking on careers in academia are presented with an environment conducive to nurturing future surgeons, contributing substantially to spine literature, and delivering exemplary clinical care.1–3 The impact of mentorship on academic clinicians is multifaceted and profound. First, research indicates that mentored surgeons are more likely to secure NIH-funded research grants and demonstrate heightened academic productivity. 4,5 Second, mentored clinicians exhibit increased publication rates, accelerated academic promotion, and enhanced self-efficacy in accomplishing academic goals. 5,6 Additionally, studies have shown that mentored clinicians report greater career satisfaction compared to their non-mentored counterparts, as evidenced by surveys conducted across various medical schools.7,8, 9–11 These findings underscore the invaluable role of mentorship in shaping the careers and professional fulfillment of individuals in academic medicine.
As the field of academic spine surgery grows, so does the demand for exemplary mentors. 9 Regrettably, a persistent lack
of motivated and seasoned mentors has been cited as a barrier to successful research completion that keeps young physicians from selecting a path in academic medicine.12–14

Andrea M. Roca, MS

Fatima N. Anwar, BA

While there is currently no published data regarding the size or productivity of the academic spine surgeon workforce, there are indications suggesting that a comparable trend may be taking place within our field. 9 Given that early and ongoing mentorship strongly influences future commitment to research endeavors, it is crucial for practicing spine surgeons to comprehend the pivotal role of mentoring in creating surgeons who also pursue research. Mentorship is frequently overlooked as a primary strategic focus, yet it is essential for fostering diversity of leaders to carry out the organization’s mission.11 In the highly competitive field of spine surgery, institutions that foster a culture of mentorship and prioritize the vertical exchange of knowledge across career stages can potentially help reduce the slope of the learning process for young spine surgeons. Given the current situation
Alexandra C. Loya, BS

Srinath S. Medakkar, BS

Kern Singh, MD
3 isass.org Spring 2024 Vertebral Columns
EDITORIAL
Department of Orthopaedic Surgery at Rush University Medical Center in Chicago, Illinois.

where fewer academic spine surgeons are expected to generate a greater amount of evidence for informed decision-making in spine surgery, the imperative for recruiting and retaining academic spine surgeons has reached an all-time high. 9
Early Support and Disparities
In the early stages of an emerging academic spine surgeon’s journey, mentors play a crucial role in helping them integrate into the academic spine community and gain visibility among influential figures. However, mentoring goes beyond simply observing and imitating a mentor’s behaviors; it involves guiding the mentee in their personal and professional development. As described by Straus and Sackett, 3 mentoring is a process in which an experienced and empathetic mentor guides the mentee in developing and reassessing their ideas and learning. 3 This includes tasks like recommending participation in meetings,
research projects, and committees to introduce the mentee to key individuals in the spine community. Additionally, mentors can help mentees access grant funding and connect with experts in areas such as data management and statistical analysis. Acting as sponsors, mentors endorse mentees by highlighting their valuable skills and knowledge to others. In this role, mentors serve as knowledgeable guides, familiarizing mentees with the institutional landscape and advising them to avoid common mistakes due to lack of experience. Research by Stamm and Buddeberg-Fischer15 found that having a mentor and receiving career support correlated positively with both objective and subjective career success. Good mentors help mentees identify potential pitfalls, avoid unproductive collaborations, steer clear of time-consuming tasks that offer little career advancement, and shield them from unsupportive colleagues. 9,11
4 isass.org Spring 2024 Vertebral Columns EDITORIAL
Dr. Kern Singh after leading a Grand Rounds session for residents of Rothman Orthopaedics at Jefferson Health in March 2024.
Gender and racial disparities are apparent within orthopedic surgery, with women being notably underrepresented in terms of quantity, rank, and academic output.16 The proportion of Black (2%) and Hispanic (2%) spine division chiefs mirrors their respective percentages within orthopedic surgery.17,18 The limited presence (1%, n = 1) of women as spine surgery division chiefs underscores the necessity of examining factors that may discourage female orthopedic residents from pursuing a spine surgery fellowship.17 Cannada et al highlighted that spine fellowships attract the lowest rate of female applicants at 3% (compared to 25% for pediatric orthopedic fellowships).19 Continuing mentorship and pipeline programs are important to get more women, Black, and Hispanic surgeons into orthopedic surgery. 20 Spine societies could also think about mentorship programs to get more female orthopedic residents interested in spine surgery. An idea used in other fields to boost diversity is to hire diverse faculty members for leadership roles, like Division Chief, which helps bring in and keep more diverse faculty and residents in the department.17
Productivity
Khan et al conducted an assessment involving 1225 academic neurosurgeons across 99 U.S. departments and observed differing mean h-index values among neurosurgical subspecialties. 21 General, spine, and pediatric neurosurgeons exhibited the lowest mean h-indexes ranging from 10 to 14. Similarly, within the orthopedic
literature, various subgroups have revealed mean h-indexes of around 15 for American Orthopedic Society for Sports Medicine fellowship faculty, 12.8 ± 13.8 for adult total joint reconstruction surgeons, and 10.2 ± 9.9 for full-time academic hand surgeons associated with fellowship programs. 22–24
A study by Post et al found neurosurgical spine surgery displayed a higher mean h-index than orthopedic spine surgery. However, the distinctions in academic productivity and practice duration are primarily observed during the early career stages and suggest that those who achieve a higher mean h-index may simply be a result of having a longer residency duration and career, which allowed for more time to conduct and publish research. 25,26 Additionally, only neurosurgeons holding the rank of assistant or associate professor exhibited a significantly higher mean h-index compared to their orthopedic counterparts at the same rank. 25
Characteristics of Successful Mentors
Effective mentors prioritize their mentoring responsibilities, consistently investing effort to nurture the relationship and convey to their mentees that their success is valued and worth pursuing. Mentors help mentees in creating both short- and long-term personal and career goals that are ambitious yet attainable to ensure continued growth and development. By identifying mentees’ skills, strengths, and areas for improvement, mentors assist them in striving toward these goals. 9,27
5 isass.org Spring 2024 Vertebral Columns EDITORIAL
Accomplished mentors have progressed sufficiently in their own careers to possess the professional attributes necessary for effectively navigating the mentor–mentee relationship. With years of experience in their field, successful research mentors have attained a level of academic achievement that enables them to actively support their mentees’ careers without concerns regarding competition over matters such as authorship, research funding, or recognition. 27 Additionally, seasoned mentors have cultivated a broad network of academic connections, exposing their mentees to potential collaborative opportunities with future partners. 27 Moreover, as esteemed members of the academic community, mentors’ advocacy for their mentees is likely to be regarded by others as a genuine and reliable evaluation of the junior colleague’s abilities and qualifications, aimed at facilitating positive career advancements for the mentee. 9
Characteristics of Successful Mentees
The most effective mentees demonstrate reliability and accountability, consistently delivering completed projects ahead of agreed-upon deadlines.14 They value their mentor’s time and other commitments, ensuring adequate turnaround time for tasks such as proofreading manuscripts and drafting reference letters. Moreover, while it’s not obligatory for mentees to always heed their mentor’s advice or pursue every presented opportunity, they should recognize the effort invested by the mentor
in creating such opportunities and remain receptive to mentor feedback. 28 With the assurance that their mentor has their best interests at heart and is committed to their personal and professional growth, mentees can perceive constructive criticism as just that, thus avoiding hurt feelings and bruised egos. 9,13
Conclusion
Mentorship is incredibly important for guiding the careers of young spine surgeons by offering them a great chance to learn, conduct research, and excel clinically in a supportive environment. The benefits of mentorship are many, including getting research grants faster and advancing in one’s career more quickly. However, there’s a shortage of mentors, which makes it tough for new spine surgeons to succeed. To fix this, we need to keep supporting mentorship programs to encourage more women, Black, and Hispanic surgeons to join orthopedic surgery and spine specialties. Also, having diverse leaders can help attract and keep a diverse team. As the field of academic spine surgery grows, having great mentors becomes even more important. We need to figure out what might stop young doctors from choosing careers in academic medicine and work to fix it. By providing strong mentorship and supporting the next generation of spine surgeons, we can ensure our field keeps moving forward and succeeding. l
6 isass.org Spring 2024 Vertebral Columns
EDITORIAL
EDITORIAL
References
1. Chopra V, Arora VM, Saint S. Will you be my mentor? Four archetypes to help mentees succeed in academic medicine. JAMA Intern Med. 2018;178:175–176.
2. Sambunjak D, Straus SE, Marusic A. A systematic review of qualitative research on the meaning and characteristics of mentoring in academic medicine. J Gen Intern Med. 2010;25:72–78.
3. Straus SE, Sackett DL. Clinician-trialist rounds: 7. Mentoring: why every clinician-trialist needs to get mentored. Clin Trials. 2011;8:765–767.
4. Curtis P, Dickinson P, Steiner J, Lanphear B, Vu K. Building capacity for research in family medicine: is the blueprint faulty? Fam Med. 2003;35:124–130.
5. Orandi BJ, Blackburn S, Henke PK. Surgical mentors’ and mentees’ productivity from 1993 to 2006. Am J Surg. 2011;201:260–265.
6. Wise MR, Shapiro H, Bodley J, et al. Factors affecting academic promotion in obstetrics and gynaecology in Canada. J Obstet Gynaecol Can. 2004;26:127–136.
7. Benson CA, Morahan PS, Sachdeva AK, Richman RC. Effective faculty preceptoring and mentoring during reorganization of an academic medical center. Med Teach. 2002;24:550–557.
8. Palepu A, Friedman RH, Barnett RC,et al. Junior faculty members’ mentoring relationships and their professional development in U.S. medical schools. Acad Med. 1998;73:318–323.
9. Goldstein C. Success in academic spine surgery: the role of mentoring. Evid Based Spine Care J. 2023;4:90–95.
10. Steelman K, Fleifel D, Waheed M, Vaidya R. Mentorship in a surgical residency: a comprehensive review of the literature. Cureus. 2023;15:e43422.
11. Choi AMK, Moon JE, Steinecke A, Prescott JE. Developing a culture of mentorship to strengthen academic medical centers. Acad Med. 2019;94:630–633.
12. Brand RA, Hannafin JA. The environment of the successful clinician-scientist. Clin Orthop Relat Res. 2006;449:67–71.
13. Cohen JG, Sherman AE, Kiet TK, et al. Characteristics of success in mentoring and research productivity—a case-control study of academic centers. Gynecol Oncol. 2012;125:8–13.
14. Entezami P, Franzblau LE, Chung KC. Mentorship in surgical training: a systematic review. Hand. 2012:7;30–36.
15. Stamm M, Buddeberg-Fischer B. The impact of mentoring during postgraduate training on doctors’ career success. Med Educ . 2011;45:488–496.
16. Poon S, et al. Current trends in sex, race, and ethnic diversity in orthopaedic surgery residency. J Am Acad Orthop Surg. 2019;27:e725–e733.
17. Mesfin A, Huber A, Denasty A, Maqsoodi N. What are the academic and demographic characteristics of orthopaedic spine surgery division chiefs? Spine J. 2022;11:100147.
18. Donnally CJ 3rd, Schiller NC, Butler AJ, et al. Trends in leadership at spine surgery fellowships. Spine . 2020;45:E594–E599.
19. Cannada LK. Women in orthopaedic fellowships: what is their match rate, and what specialties do they choose? Clin Orthop Relat Res . 2016;474:1957–1961.
20. Adelani MA, Harrington MA, Montgomery CO. The distribution of underrepresented minorities in U.S. orthopaedic surgery residency programs. J Bone Joint Surg Am. 2019;101:e96.
21. Khan NR, Thompson CJ, Taylor DR, et al. An analysis of publication productivity for 1225 academic neurosurgeons and 99 departments in the United States. J Neurosurg. 2014;120:746–755.
22. Cvetanovich GL, Saltzman BM, Chalmers PN, et al. Research productivity of sports medicine fellowship faculty. Orthop J Sports Med. 2016;4:2325967116679393.
23. Lopez J, Susarla SM, Swanson EW, Calotta N, Lifchez SD. The association of the H-Index and academic rank among full-time academic hand surgeons affiliated with fellowship programs. J Hand Surg Am. 2015;40:1434–1441.
24. Khan AZ, Kelley BV, Patel AD, McAllister DR, Leong NL. Academic productivity among fellowship associated adult total joint reconstruction surgeons. Arthroplast Today. 2017;3;298–302.
25. Post AF, et al. Academic Productivity of Spine Surgeons at United States Neurological Surgery and Orthopedic Surgery Training Programs. World Neurosurg. 2109;121:e511–e518.
26. Dijanic CN, et al. Evaluation of the National Institutes of Health–supported relative citation ratio among American orthopedic spine surgery faculty: a new bibliometric measure of scientific influence. Spine J. 2022;11:100143.
27. Jochemsen-van der Leeuw HGAR, van Dijk N, van Etten-Jamaludin FS, Wieringa-de Waard M. The attributes of the clinical trainer as a role model: a systematic review. Acad Med. 2013;88: 26–34.
28. Straus SE, Johnson MO, Marquez C, Feldman MD. Characteristics of successful and failed mentoring relationships: a qualitative study across two academic health centers. Acad Med. 2013;88:82–89.
7 isass.org Spring 2024 Vertebral Columns
Biportal Endoscopic Spine Surgery
Overview and Applications

Innovations in surgical technique continue to reduce the morbidity of spine surgery and improve patient outcomes. Spinal endoscopy represents a new advancement in minimally invasive spine surgery, providing patents with an alternative to existing approaches. Interest in endoscopic techniques has increased significantly over the past 5 years. A current PubMed search of “spine biportal endoscopy” reveals 173 publications spanning the years 2021–2024, compared with 67 articles published from 2016–2020.
Spinal endoscopy expands upon the well-established advantages of traditional minimally invasive spine surgery. 1 The smaller diameter of endoscopic portals leads to even less trauma to the surrounding muscles and soft tissues, reducing postoperative pain and speeding recovery. Modern endoscopes are equipped with high resolution cameras capable of providing extremely high magnification. This technology produces superior visualization of the neural elements, augmenting the precision and safety of the procedure. Furthermore, the enhanced visualization and small working channels utilized in endoscopy allow for effective treatment of foraminal pathology, which has historically
been difficult to treat without a combined arthrodesis. Spinal endoscopy may also be performed “awake,” mitigating the risks of general anesthesia and allowing for real time patient feedback related to neural manipulation. These potential advantages position spinal endoscopy as a promising alternative to traditional tubular approaches in spine surgery.
Endoscopic Techniques
Several techniques exist within spinal endoscopy, each offering its own set of advantages. Endoscopic approaches are performed via either an interlaminar or a transforaminal technique. Either one or multiple working portals may be used. Uniportal approaches involve insertion of an endoscope with a central working channel through a single incision. This approach is favored for its simplicity and reduced trauma to the surrounding tissues, leading to less postoperative pain and faster recovery times. Biportal endoscopy involves the use of two separate portals: one for insertion of the endoscope and another for introducing surgical instruments. This technique allows for manipulation of surgical instruments independent of the endoscopic camera and may be more ergonomically familiar to surgeons new to endoscopy. The choice between uniportal and biportal techniques
8 isass.org Spring 2024 Vertebral Columns
From The CORE Institute in Phoenix, Arizona. ENDOSCOPY
Brandon P. Hirsch, MD
is influenced by surgeon preference and also by the type of pathology being treated.
Uniportal Endoscopy
Uniportal endoscopy via a transforaminal approach was first introduced by Kambin in the early 1990s for the treatment of disc herniations. 2 Since that time, advancements in technology for bony resection have also allowed for the treatment of stenosis. Techniques for interbody fusion via a uniportal technique have also been published. 3 While many expert endoscopic surgeons routinely utilize uniportal approaches, this technique has some inherent limitations. Because instruments are placed through a working portal integrated into the endoscope, the surgeon may only access pathology directly in line with the camera. Retraction of the neural elements must be performed using the endoscopic cannula; thus, any attempts to adjust the line of sight of the camera will also require applying force to the nerve root. The size of endoscopic burrs and drills is also limited by the diameter of the central working cannula, which can reduce the efficiency of bony resection.
Biportal Endoscopy
Biportal endoscopy, first described by De Antoni et al, 4 addresses many of these limitations by decoupling placement of surgical instruments from the endoscope. As a result, instruments can generally be introduced via the working portal without compromising visualization. Nerve root retraction for discectomy can also be accomplished with traditional instruments
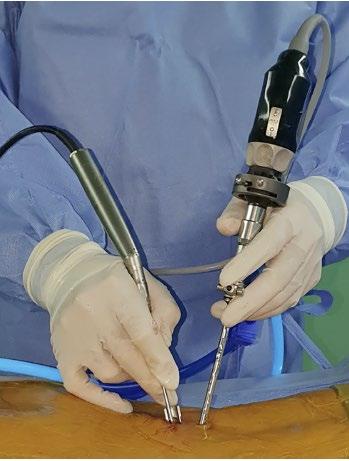
rather than requiring the endoscope to be used. This allows for improved visualization and, in theory, safer manipulation of the neural elements. The working portal diameter is not limited by the size of the endoscope, so larger burs and endoscopic shavers can be used to speed bony resection during laminectomy or facetectomy. In addition, the triangulation afforded by the use of two portals is familiar to orthopedic
9 isass.org Spring 2024 Vertebral Columns
ENDOSCOPY
Figure 1. Biportal endoscopic spine surgery utilizes two working channels and continuous fluid irrigation to improve surgical field visualization. Reprinted from Kang et al. Biportal endoscopic technique for transforaminal lumbar interbody fusion: review of current research. Int J Spine Surg. 2021;15:S84-S92.

surgeons with training in arthroscopy and may help reduce the learning curve. 5 An additional advantage of biportal endoscopy includes the use of existing, nonspecialized endoscopic equipment and instruments. A traditional 0- or 30-degree arthroscope is typically used along with existing radiofrequency (RF) probes, arthroscopic shavers, and burs, which are traditionally used for arthroscopy. In addition, standard curettes, probes, nerve root retractors, as well as Kerrison and pituitary rongeurs may be used without modification. This ability to use standard, existing instrumentation reduces the per case cost for biportal endoscopy, eliminating a significant barrier to adoption.
Biportal endoscopy can be used for a wide range of pathology including disc herniations, spinal stenosis, and metastatic disease and can also be used for posteriorly based interbody fusions. 6 The biportal technique is performed with the patient in the prone position, using a radiolucent table to facilitate fluoroscopic guidance. Two portals are planned, typically using spinal needles to ensure appropriate trajectories. Two subcentimeter incisions centered on the medial aspect of the pedicles above and below the level of interest are made. The cranial incision is typically used as the viewing portal for the endoscope and the caudal portal is used for instruments. Dilators are then introduced and are used to palpate the spinolaminar junction. The dilators are then used to create a potential space beneath the paraspinal musculature overlying the interlaminar window. The endoscope is then introduced along with either the RF probe or soft tissue shaver. Irrigation is utilized via the endoscope. Soft tissue overlying the lamina is then resected until visualization of the posterior elements is achieved. The remainder of the procedure is then carried out in a fashion very similar to traditional decompression. If an interbody fusion is planned, standard osteotomes and/or burrs are used to resect the facet joint complex and carry out discectomy. Endplate preparation and graft placement are then performed in a traditional manner under direct visualization. Percutaneous pedicle screw placement is then carried out under image guidance in the typical fashion. Some experts recom -
10 isass.org Spring 2024 Vertebral Columns
Figure 2. Intraoperative image of the L4-5 lateral recess and traversing nerve root with cottonoid in place.
ENDOSCOPY
ENDOSCOPY
mend drain placement to facilitate egress of hematoma and/or irrigation on the day of surgery.
Conclusion
The learning curve in biportal endoscopy is important to understand for those considering adopting the technique. Surgeons must become adept at coordinating the use of two separate portals for visualization and instrumentation, as well as developing the necessary hand-eye coordination and depth perception to navigate within confined spaces. Additionally, mastering the nuances of tissue manipulation and instrument handling, as well as understanding anatomical landmarks through the endoscope’s lens, is essential. As surgeons gain experience, they gradually become more efficient, improving surgical outcomes and reducing operating times. Achieving proficiency in biportal
Biportal endoscopy can be used for a wide range of pathology, including disc herniations, spinal stenosis, and metastatic disease, and can also be used for posteriorly based interbody fusions.
References
1. Lokhande P V. Full endoscopic spine surgery. J Orthop. 2023;40:74–82.
2. Kambin P. Arthroscopic microdiskectomy. Mt Sinai J Med. 1991;58:159–164.
3. Wagner R, Haefner M. Uniportal endoscopic lumbar interbody fusion. Neurospine. 2020;17:S120.
4. De Antoni DJ, Claro ML, Poehling GG, Hughes SS. Translaminar lumbar epidural endoscopy: anatomy, technique, and indications. Arthroscopy. 1996;12:330–334.
5. Jitpakdee K, Liu Y, Heo DH, Kotheeranurak V, Suvithayasiri S, Kim JS. Minimally invasive endoscopy in spine surgery: where are we now? Eur Spine J. 2023;32:2755–2768.
endoscopy typically involves a steep initial learning curve, with skills and proficiency continuing to evolve with ongoing practice and mentorship.7,8 Continuous education, training workshops, and proctoring opportunities will help surgeons overcome the challenges of the learning curve and achieve proficiency in biportal endoscopic techniques. l
6. Sakhrekar R, Ha JS, Han H-D, Kim DH, Kim CW, Kulkarni S. The past, present, and future of unilateral biportal endoscopy with a technical note on novel endoscopic visualization pedicle screw insertion technique and UBE-transforaminal lumbar interbody fusion technique with literature review. J Orthop Case Rep. 2023;13:165.
7. Park SM, Kim HJ, Kim GU, et al. Learning curve for lumbar decompressive laminectomy in biportal endoscopic spinal surgery using the cumulative summation test for learning curve. World Neurosurg. 2019;122:e1007–e1013.
8. Wang W, Liu Z, Wu S, et al. [Research of learning curves for unilateral biportal endoscopy technique and associated postoperative adverse events]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2022;36:1221–1228.
11 isass.org Spring 2024 Vertebral Columns
Biomechanics of Zero-Profile Anterior Cervical Discectomy and Fusion



Anterior cervical discectomy and fusion (ACDF) is used to treat a variety of cervical spine abnormalities, including traumatic injuries, neoplasms, infections, and degenerative disorders. Pioneers of ACDF in the 1950s used cylindrical or tricortical bone grafts without plating. However, in the 1970s, ventral cervical plating was introduced to prevent bone graft migration as well as to decrease the compression and shear forces to which the operative segment was exposed. 1 The principles of creating a sound ventrally based construct include distraction, central compression (tension band), and cantilever beam fixation. 1 While a rigid construct is preferred in trauma scenarios, some have questioned its necessity in elective degenerative scenarios. Moreover, anterior cervical plates are not benign, with reported complications including esophageal damage, neurovascular injuries, and dysphagia. 2,3
Zero-profile stand-alone cages were designed to address these complications while providing stability and facilitating fusion. Theoretically they allow for decreased soft tissue dissection and retraction, decreased mass effect on retropharyngeal structures, and decreased adjacent segment degeneration. 4 However, this newer technique may be more prone to cage subsidence, graft collapse, cervical dislocation, and cervical kyphosis. 5 With advancements in cervical instrumentation, it is important that spine surgeons appreciate the biomechanical characteristics of stand-alone technologies in comparison to plate and cage constructs.
While the placement of an intervertebral graft facilitates anterior fusion, removing the anterior and posterior longitudinal ligaments diminishes stability in flexion and extension. Cervical plates help restore this sagittal stability and increase rates of fusion. Shimamoto et al evaluated the biomechanical performance of stand-alone single-level cervical cages without integrated fixation in a cadaveric study and demonstrated that the flexion-extension and lateral bending range of motion at C4-C5 increases following discectomy and cage insertion without plate fixation, whereas axial rotation does
12 isass.org Spring 2024 Vertebral Columns
BIOMECHANICS
From Somers Orthopaedic Surgery & Sports Medicine Group in Kisco, New York (Dr Perfetti) and the Texas Back Institute in Plano, Texas (Drs Satin and Derman).
Dean C. Perfetti, MD, MPH
Alexander M. Satin, MD
Peter B. Derman, MD, MBA
not increase when compared to the intact spine. 6 Hart et al studied 2 different subaxial levels, C3-4 and C5-C6, and found that single threaded cylindrical stand-alone cages without integrated fixation did not provide mechanical resistance in lateral bending and axial rotation.7 Initial range of motion was significantly greater for cadavers implanted with a stand-alone cage compared to those with a structural graft and plate (9.1° ± 3.7° vs 5.8° ± 2.4°, p = 0.04).7 However, next generation low-profile or no-profile devices with integrated fixation have performed better than earlier standalone generations. 8
Biomechanical testing on human cadavers has shown that integrated low-profile plate and spacer constructs as well as integrated spacer constructs alone can have biomechanical profiles like that of non-integrated plate and spacer constructs. Scholz et al demonstrated that low-profile anchored spacers decreased range of motion compared to the intact motion segment and the difference in range of motion was not statistically significant when compared to a cage and plate design (both locking and dynamic plates) in a cadaveric model. 9
BIOMECHANICS
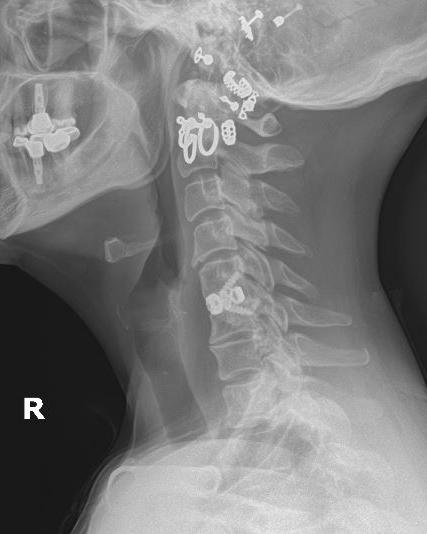
Similarly, Stein et al reported that 3-screw zero profile devices were not significantly different in range of motion reduction compared to traditional ACDF plate and cage constructs. 10 Moreover, Majid et al
Theoretically, zero-profile devices allow for decreased soft tissue dissection and retraction, decreased mass effect on retropharyngeal structures, and decreased adjacent segment degeneration. However, this newer technique may be more prone to cage subsidence, graft collapse, cervical dislocation, and cervical kyphosis.
13 isass.org Spring 2024 Vertebral Columns
evaluated 2-screw and 4-screw integrated plate spacer constructs and reported no significant differences in range of motion reductions in comparison to each other or to a separate ACDF spacer and plate construct. 11 Because the relatively acute cephalad-caudal screw angulation of integrated stand-alone devices can make screw placement difficult, anchored or bladed devices have been introduced to provide fixation with in-line deployment. A cadaveric study comparing the biomechanical properties of screws, anchors and blades in integrated devices found biomechanical equivalence in all loading modes (flexion-extension, lateral bending, and axial rotation).12
References
1. Ugokwe K. “Chapter 62: Anterior Subaxial Cervical Fixation Techniques.” In: Benzel E, ed. Benzel’s Spine Surgery: Techniques, Complication Avoidance and Management . 4th ed. 2016.
2. Wang Z, Jiang W, Li X, et al. The application of zero-profile anchored spacer in anterior cervical discectomy and fusion. Eur Spine J. 2015;24:148-154.
3. Chen Y, Chen H, Wu X, Wang X, Lin W, Yuan W. Comparative analysis of clinical outcomes between zero-profile implant and cages with plate fixation in treating multilevel cervical spondylotic myelopathy: a three-year follow-up. Clin Neurol Neurosurg. 2016;144:72-76.
4. Ahn SS, Paik HK, Chin DK, Kim SH, Kim DW, Ku MG. The fate of adjacent segments after anterior cervical discectomy and fusion: the influence of an anterior plate system. World Neurosurg. 2016;89:42-50.
5. Kwon WK, Kim PS, Ahn SY, et al. Analysis of associating factors with C2-7 sagittal vertical axis after two-level anterior cervical fusion: comparison between plate
Further adding to decision-making complexity are the material characteristics, including modulus of elasticity, shape, and microporosity, as well as the number of levels fused. Segmental stability decreases with the number of instrumented segments regardless of the implant used. However, cage plus plate constructs in 2- and 3-level instrumentation are biomechanically stiffer with a significantly lower range of motion in flexion and extension compared to multi-level anchored devices.13 Nonetheless, zero-profile integrated ACDF devices rival the biomechanical profiles of traditional ACDF plate and cage constructs for single-level degenerative disease. l
augmentation and stand-alone cages. Spine (Phila Pa 1976). 2017;42:318-325.
6. Shimamoto N, Cunningham BW, Dmitriev AE, Minami A, McAfee PC. Biomechanical evaluation of stand-alone interbody fusion cages in the cervical spine. Spine . 26(19):E432-E436.
7. Hart R, Gillard J, Prem S, Shea M, Kitchel S. Comparison of stiffness and failure load of two cervical spine fixation techniques in an in vitro human model. J Spinal Disord Techn. 2005;18:S115-S118.
8. Panchal R, Gandhi A, Ferry C, Farmer S, Hansmann J, Wanebo J. A biomechanical evaluation of a next-generation integrated and modular ACDF device possessing full-plate, half-plate, and no-profile fixation iterations. Global Spine J. 2019;9(8):826-833.
9. Scholz M, Reyes P, Schleicher P, et al. A new stand-alone cervical anterior interbody fusion device: biomechanical comparison with established anterior cervical fixation devices. Spine . 2009;34(2):156-160.
10. Stein MI, Nayak AN, Gaskins RB 3rd, Cabezas AF, Santoni BG, Castellvi AE. Biomechanics of an integrated interbody device versus ACDF anterior locking plate in a single-level cervical spine fusion construct. Spine J. 2014;14(1):128-136.
11. Majid K, Chinthakunta S, Muzumdar A, Khalil S. A comparative biomechanical study of a novel integrated plate spacer for stabilization of cervical spine: an in vitro human cadaveric model. Clin Biomech (Bristol, Avon). 2012;27(6):532-536.
12. Arnold PM, Cheng I, Harris JA, et al. Single-level in vitro kinematic comparison of novel inline cervical interbody devices with intervertebral screw, anchor, or blade. Global Spine J. 2019;9(7):697-707.
13. Scholz M, Schleicher P, Pabst S, Kandziora F. A zero-profile anchored spacer in multilevel cervical anterior interbody fusion: biomechanical comparison to established fixation techniques. Spine (Phila Pa 1976). 2015;40(7):E375-E380.
14 isass.org Spring 2024 Vertebral Columns
BIOMECHANICS
From the 1Hospital for Special Surgery in New York, New York; 2University Medical Center Hamburg-Eppendorf in Germany; and 3University of Tsukuba, Institute of Medicine, Department of Orthopaedic Surgery in Tsukuba, Japan.
Precision in Practice
Alignment in Non-deformity Spine Surgery
In the realm of spine surgery, the focus on sagittal alignment has traditionally been synonymous with deformity correction. However, within the landscape of degenerative and minimally invasive procedures, its importance is increasingly recognized.1-3 Sagittal alignment refers to the optimal curvature of the spine when viewed from the side, which is crucial for maintaining stability and balance and mitigating degenerative processes.
In non-deformity cases, such as lumbar spinal stenosis or degenerative disc disease, the impact of sagittal alignment on surgical outcomes is profound yet often underestimated. The relationship between sagittal balance and clinical symptoms is intricate; deviations from the norm can lead to functional impairments, pain, and compromised quality of life.1,3,4
Moreover, in the era of minimally invasive spine surgery (MIS), understanding sagittal alignment becomes even more critical. While MIS offers the advantage of reduced tissue trauma and quicker recovery, its success hinges upon meticulous preoperative planning and intraoperative precision, including alignment restoration.1,2,5
CLINICAL PRACTICE
What We Learned From Spinal Deformity Correction
The alignment of the spine profoundly impacts a person’s movement, posture, and overall functionality.1 The human spine underwent a highly complex biomechanical evolution, facilitating bipedalism through distinctive skeletal adaptations, ultimately forming the characteristic “S-shape” spinal curvature. This curvature’s stability is intricately governed by a specialized network of muscles, ligaments, and vertebrae interactions, ensuring efficient weight distribution, shock absorption, and support during upright locomotion.1 Any pathology that alters this equilibrium instigates sagittal malalignment and its compensatory mechanisms.1 Since thoracic pathologies are infrequent in non-deformity patients, alignment assessment in non-deformity patients predominantly centers around the cervical and lumbar regions.
Achieving sagittal alignment involves balancing the cranium, spine, and pelvis through key measurements: pelvic incidence (PI), pelvic tilt (PT), lumbar lordosis (LL), thoracic kyphosis (TK), cervical lordosis (CL) and C7 plumb line/sagittal vertical axis (SVA).6,7 Each person’s individual measurements vary





15 isass.org Spring 2024 Vertebral Columns
Annika Heuer, MD1,2
Eric Kim, BS1
Myles Allen, MBChB1
Tomoyuki Asada, MD13
Sheeraz A. Qureshi, MD, MBA1
slightly, with normal ranges typically falling within the following ranges: PI = 55° ± 10°, PT <20°, LL = 60°, TK = 20°–50°, CL = 20°–40°, and SVA with a C7 plumb line distance = ± 4–5 cm.8-10 However, with advancing age, individuals tend to adopt a more forward-leaning posture and seem to tolerate slightly more positive sagittal alignment values. Factors contributing to sagittal imbalance include any condition that shifts the body’s center of gravity forward. To counterbalance forward bending, the cervical spine can adjust with an exaggerated lordotic curve to achieve a stance that appears more neutral.10,11 Furthermore, compensation can include increased pelvic tilt and knee flexion, which leads to a physically straining, fatiguing position to maintain. 8,12
This imbalance is not exclusively the result of classical deformity-related pathologies. Degenerative deterioration of spinal discs can lead to small segmental alterations accompanied by restricted movement; it amplifies mechanical stress on adjutant segments, subsequently leading to a cumulative effect that can cause persistent sagittal imbalance.8,12
Given the considerable variability in spinal alignment among individuals, meticulous preoperative planning becomes paramount to optimize clinical outcomes effectively. Furthermore, age-related degenerative changes significantly contribute to malalignment, emphasizing the necessity for adjusting realignment strategies based on a patients’ age and specific conditions. 8,9 To address these challenges, Lafage et al proposed age-adjusted alignment thresholds for adult spinal deformity correction aimed at enhancing postoperative results and overall patient
satisfaction. Their analysis could show that younger patients necessitate a more rigorous correction to align with the Oswestry Disability Index (ODI) and Short Form-36 Physical Component Score results.13
The presence of spinal misalignment is associated with symptoms such as back pain, radiculopathy, and postural instability. This underscores the imperative for attaining optimal alignment postoperatively to foster improvement in patients’ conditions.1,4,9 Further highlighting the significance of spinopelvic alignment, recent studies have demonstrated a correlation between restored alignment and improvements in patient-reported outcome measures, including pain reduction, enhanced function, and improved quality of life.1,2 Utilizing discharge disposition as a proxy for positive postoperative results, Macki et al found that individuals with improved sagittal balance after MIS lumbar interbody fusion for degenerative spine disease were more likely to be discharged to a rehabilitation facility rather than home. In their patient cohort, each 1-cm enhancement in postoperative sagittal balance increased the likelihood of a discharge to their home by 27%. 2
Precision in Practice: Alignment in Non-deformity Spine Surgery
Assessing spinal alignment in non-deformity patients serves as a valuable tool to understand a patient’s condition, determining appropriate treatment methods, assessing complication risks, and even predicting patient-reported postoperative outcomes.1,9
A substantial amount of research has shed light on the relationship between the
16 isass.org Spring 2024 Vertebral Columns
CLINICAL PRACTICE
alignment of the cervical spine and cervical degenerative diseases in patients who do not inherently have a spinal deformity. Although these local effects have been examined, few studies explored the value of global spinal alignment, from the thoracic region down to the pelvis, in patients requiring surgery for cervical degeneration so far. 9,11
The prevalence of clinical adjacent segment pathology requiring surgery after anterior cervical decompression and arthrodesis has been reported to be 9% at 5 years, 12% at 6 years and 19.2 % at 10 years. Several risk factors for the development of such clinical adjacent segment pathology have been reported, including cervical malalignment.14 Katsuura et al illustrated how postoperative cervical kyphotic changes at the fused segment contribute to degenerative changes in adjacent intervertebral levels following anterior cervical discectomy and fusion for degenerative disorders. The authors noted degenerative alterations in adjacent intervertebral levels in 77% of cases where kyphotic changes occurred at the fused segment during a mean follow-up period of 9.8 years.15 Similarly, Park et al, identified cervical malalignment following anterior cervical discectomy and fusion at C5-6 as a precursor to adjacent segmental disease, necessitating additional surgery. The most significant disparity among patients necessitating surgery for adjacent segment pathology was demonstrated to be the distance between the C2 to C7 sagittal plumb lines and the distance from the fusion mass to the C7 plumb line.14 Lee et al highlighted that laminoplasty procedures can promote kyphotic cervical changes and subsequently
lead to adjacent segmental disease. Their study revealed a significant association between the T1 slope and the development of a kyphotic deformity. The T1 slope predicted postoperative kyphotic changes in the cervical spine following laminoplasty with a cut-off point of 29°. The authors concluded that patients with a higher T1 slope required greater lordotic force, rendering them more susceptible to these changes postoperatively.16 In agreement with this, Kim et al reported that laminoplasty can worsen kyphotic alignment and should only be considered for patients with cervical lordosis or flexible/reducible kyphosis.17
Iyer et al showed that a decreased T1 slope minus CL and increased C2-C7 SVA were independent predictors of cervical deformity and high preoperative pain score measured by the neck disability index in patients with degenerative cervical pathologies.18
The significance of both regional and global spinal alignment is critical, especially when considering the impact of degenerative changes on overall posture and balance. To accurately assess global sagittal balance—including the regional cervical spine—it is often necessary to conduct full-length standing radiographic examinations, though these are not always considered essential for all patients with an isolated cervical pathology in daily clinical practice. However, there is a growing need for further research to understand the interactive effects of these factors better and to establish the value of comprehensive radiographic evaluation in the planning stages of spinal surgery. 9,19
In lumbar degenerative spine disease, similar findings were observed. Miyakoshi
17 isass.org Spring 2024 Vertebral Columns
CLINICAL PRACTICE
et al observed that patients suffering from both lumbar spinal stenosis and chronic low back pain tended to have a more pronounced lumbar kyphotic component than individuals with lumbar spinal stenosis alone. 20 Endo et al discovered that patients with lumbar disc herniation displayed an altered sagittal spinal alignment compared to healthy peers, characterized by an anterior shift in the C7 plumb line (SVA), reduced lordosis, and a more vertical sacrum position. They also found correlations between SVA and L1-S1, overall spinal posture, and clinical symptoms measured by the Japanese Orthopedic Association score. Postoperatively, abnormal posture improved within 6 months alongside subjective symptom enhancement on the Japanese Orthopedic Association scale. Their research suggests that in lumbar disc herniation patients, sagittal balance alterations might be secondary to factors such as segmental discopathy, disc height loss, postural changes to alleviate pain, or foraminal stenosis rather than intrinsic structural deformity. 21 Similarly, Bouknaitir et al found that sagittal balance and PROMs showed a postoperative improvement within 6 months in patients older than 60 years who underwent decompression alone for lumbar spinal stenosis. Furthermore, SVA correlated significantly with preoperative ODI and visual analog scale for leg pain scores. 22 Acknowledging and addressing the compensatory responses is crucial for managing spinal alignment and balance when treating degenerative spinal conditions.10
Although multiple studies could show that postoperatively mismatched spinopelvic pa-
rameters increase the incidence of adjacent segment degeneration and/or failure requiring reoperation following short-segment lumbar fusion, a high prevalence of postoperative iatrogenic sagittal plane deformity remains despite advances in segmental fixation and the power of newer interbody devices.1,23-26 In a retrospective review of postoperative lumbar radiographs from 578 patients undergoing 1- or 2-level lumbar fusion across 18 US-based institutions, findings indicated that 63% of patients maintained their alignment, with 9% experiencing alignment restoration. However, 21% of patients did not achieve correction, and 7% had worsened alignment following the procedure. 23
The current literature underlines the detrimental consequences of such results for the individual patient. Patient-reported outcomes rated by ODI and pain scores was influenced by the overall postoperative sagittal balance as defined by SVA after surgical treatment for degenerative spondylolisthesis. Furthermore, the study showed that SVA negatively correlated with Short Form-36 scores and positively correlated with ODI scores, suggesting that a more positive SVA leads to a decline in physical function and worsened spine-specific disability. 24 Yamasaki et al found that the risk of adjacent segment disease (ASD) was significantly higher, by a factor of 5.1, in patients with a preoperative PT exceeding 22.5° after undergoing transforaminal lumbar interbody fusion. They concluded that a primary surgical goal should be the prevention or restoration of sagittal balance after surgery, especially in patients with a large preoperative PT. 25
18 isass.org Spring 2024 Vertebral Columns
CLINICAL PRACTICE
CLINICAL PRACTICE
This conclusion was further validated by Tempel et al, who described ASD as a global disease process as opposed to a focal condition. They demonstrated a strong association between a high PI-LL mismatch and the development of symptomatic ASD requiring revision lumbar surgery after single-level transforaminal lumbar interbody fusion for degenerative spondylosis or low-grade spondylolisthesis. For each 1° increase in PI-LL mismatch (preoperative to postoperative), the odds increased by 1.3- and 1.4-fold, respectively, while a mismatch greater than 11° had a positive predictive value of 75%. 26
These studies highlight the importance of preoperative alignment and the pursuit of optimal postoperative correction in patients with degenerative spine pathology to optimize the clinical outcomes and postoperative complications.
Enhancing Precision
Innovative technology and techniques have revolutionized MIS within the past decade. Precision and accuracy are paramount to achieving optimal patient outcomes. 27,28 The integration of robotics and navigation systems into surgical workflows represents a significant advancement. Specifically, robotic assistance and navigation technology offer substantial benefits in optimizing sagittal alignment planning for spine surgery. Through precise preoperative imaging analysis and intraoperative guidance, robotics enable surgeons to meticulously plan and execute corrective maneuvers that were preoperatively tailored to each patient’s unique spinal anatomy. 28,29 Furthermore, navigation
systems provide real-time intraoperative feedback on spinal alignment parameters, allowing surgeons to make immediate adjustments during the procedure to achieve optimal sagittal balance. 30 By integrating these technologies, surgeons can enhance the accuracy of alignment restoration, minimize intraoperative complications, and improve postoperative outcomes, ultimately leading to better patient satisfaction and quality of life. 27 This trend is poised to persist, propelled by ongoing innovations like robotics, virtual reality, and augmented reality. These technological strides will further amplify the significance of 3-dimensional navigation in MIS, offering unprecedented precision and accuracy in treatment. 27,28
Advancing Surgical Outcomes and Quality of Life
Over the past decade, advancements in our comprehension of both regional and global spine alignment have transcended mere deformity correction. Emphasizing regional spinal alignment is crucial considering its profound influence on stability, balance, and degenerative processes. Physiological variations, age-related changes, and pathological malalignment directly impact not only radiographic outcomes but also patient-reported outcome measures. Enhancing our understanding can lead to improved surgical outcomes and a higher quality of life. Surgical interventions should consider both regional and global spinal alignment, necessitating meticulous preoperative planning aligned with patient-centered goals, especially in the era of MIS techniques. l
19 isass.org Spring 2024 Vertebral Columns
References
1. Diebo BG, Balmaceno-Criss M, Lafage R, et al. Sagittal alignment in the degenerative lumbar spine: surgical planning. J Bone Joint Surg Am. 2024;106(5)445-457.
2. Macki M, Fadel HA, Hamilton T, et al. The influence of sagittal spinopelvic alignment on patient discharge disposition following minimally invasive lumbar interbody fusion. J Spine Surg. 2021;7(1):8-18.
3. Champagne PO, Walsh C, Diabira J, et al. Sagittal balance correction following lumbar interbody fusion: a comparison of the three approaches. Asian Spine J. 2019;13(3):450-458.
4. Hiyama A, Katoh H, Sakai D, Sato M, Watanabe M. Effects of preoperative sagittal spinal imbalance on pain after lateral lumbar interbody fusion. Scientific Reports. 2022;12(1):3001.
5. Trenchfield D, Lee Y, Lambrechts M, et al. Correction of spinal sagittal alignment after posterior lumbar decompression: does severity of central canal stenosis matter? Asian Spine J. 2023;17(6):1089-1097.
6. Pumberger M, Schmidt H, Putzier M. Spinal deformity surgery: a critical review of alignment and balance. Asian Spine J. 2018;12(4):775-783.
7. Park C, Agarwal N, Mummaneni PV, Berven SH. Spinopelvic alignment: importance in spinal pathologies and realignment strategies. Neurosurg Clin N Am. Oct 2023;34(4):519-526.
8. Kim D, Davis, DD., Menger, RP. Spinal Sagittal Balance . Treasure Island, FL: StatPearls Publishing; 2024.
9. Patel PD, Arutyunyan G, Plusch K, Vaccaro A Jr, Vaccaro AR. A review of cervical spine alignment in the normal and degenerative spine. J Spine Surg. 2020;6(1):106-123.
10. Wang Y, Li XY, Zhu WG, Liu CX, Kong C, Lu SB. Compensatory classification in spine sagittal malalignment with lumbar degeneration. BMC Musculoskelet Disord. 2023;24(1):229.
11. Fujimori T, Le H, Schairer W, et al. The relationship between cervical degeneration and global spinal alignment in patients with adult spinal deformity. Clin Spine Surg. 2017;30(4):E423-e429.
12. Schwab F, Patel A, Ungar B, Farcy JP, Lafage V. Adult spinal deformity-postoperative standing imbalance: how much can you tolerate? An overview of key parameters in assessing alignment and planning corrective surgery. Spine (Phila Pa 1976). 2010;35(25):2224-2231.
13. Lafage R, Schwab F, Challier V, et al. Defining spino-pelvic alignment thresholds: should operative goals in adult spinal deformity surgery account for age? Spine . 2016;41(1):62-68.
14. Park MS, Kelly MP, Lee DH, Min WK, Rahman RK, Riew KD. Sagittal alignment as a predictor of clinical adjacent segment pathology requiring surgery after anterior cervical arthrodesis. Spine J. 2014;14(7):1228-34. doi:10.1016/j.spinee.2013.09.043
15. Katsuura A, Hukuda S, Saruhashi Y, Mori K. Kyphotic malalignment after anterior cervical fusion is one of the factors promoting the degenerative process in adjacent intervertebral levels. Eur Spine J. 2001;10(4):320-324.
16. Lee JS, Son DW, Lee SH, Kim DH, Lee SW, Song GS. The predictable factors of the postoperative kyphotic change of sagittal alignment of the cervical spine after the laminoplasty. J Korean Neurosurg Soc. 2017;60(5):577-583.
17. Kim SW, Jang SB, Lee HM, et al. Analysis of cervical spine alignment and its relationship with other spinopelvic parameters after laminoplasty in patients with degenerative cervical myelopathy. J Clin Med. 2020;9(3):713.
18. Iyer S, Nemani VM, Nguyen J, et al. Impact of cervical sagittal alignment parameters on neck disability. Spine . 2016;41(5):371-377.
19. Lee JK, Hyun SJ, Kim KJ. Optimizing surgical strategy for cervical spinal deformity: global alignment and surgical targets. Neurospine . 2023;20(4):1246-1255.
20. Miyakoshi N, Hongo M, Kasukawa Y, Ishikawa Y, Shimada Y. Prevalence, spinal alignment, and mobility of lumbar spinal stenosis with or without chronic low back pain: a community-dwelling study. Pain Res Treat . 2011;2011:340629.
21. Endo K, Suzuki H, Tanaka H, Kang Y, Yamamoto K. Sagittal spinal alignment in patients with lumbar disc herniation. Eur Spine J. 2010;19(3):435-438.
22. Bouknaitir JB, Carreon LY, Brorson S, Andersen M. Change in sagittal alignment after decompression alone in patients with lumbar spinal stenosis without significant deformity: a prospective cohort study. J Neurosurg Spine . 2022:1-7.
23. Leveque J-CA, Segebarth B, Schroerlucke SR, et al. A multicenter radiographic evaluation of the rates of preoperative and postoperative malalignment in degenerative spinal fusions. Spine . 2018;43(13):E782-E789.
24. Radovanovic I, Urquhart JC, Ganapathy V, et al. Influence of postoperative sagittal balance and spinopelvic parameters on the outcome of patients surgically treated for degenerative lumbar spondylolisthesis. J Neurosurg Spine . 2017;26(4):448-453.
25. Yamasaki K, Hoshino M, Omori K, et al. Risk factors of adjacent segment disease after transforaminal inter-body fusion for degenerative lumbar disease. Spine (Phila Pa 1976). 2017;42(2):E86-e92.
26. Tempel ZJ, Gandhoke GS, Bolinger BD, et al. The influence of pelvic incidence and lumbar lordosis mismatch on development of symptomatic adjacent level disease following single-level transforaminal lumbar interbody fusion. Neurosurgery. 2017;80(6):880-886.
27. Hussain I, Cosar M, Kirnaz S, et al. Evolving navigation, robotics, and augmented reality in minimally invasive spine surgery. Global Spine J. 2020;10(2 Suppl):22s-33s.
28. Vo CD, Jiang B, Azad TD, Crawford NR, Bydon A, Theodore N. Robotic spine surgery: current state in minimally invasive surgery. Global Spine J. 2020;10(2 Suppl):34s-40s.
29. Farber SH, Pacult MA, Godzik J, et al. Robotics in spine surgery: a technical overview and review of key concepts. Front Surgery. 2021;8:578674.
30. Huang M, Tetreault TA, Vaishnav A, York PJ, Staub BN. The current state of navigation in robotic spine surgery. Ann Transl Med. 2021;9(1):86.
20 isass.org Spring 2024 Vertebral Columns
CLINICAL PRACTICE
Latest Report on Coflex and Interspinous Devices
More than 600,000 patients are treated for lumbar spinal stenosis in the United States annually.1 A combination of disc degeneration resulting in disc bulging and osteophyte formation from facet arthropathy causes a circumferential narrowing of the spinal canal from bone stenosis. In addition, buckling of the ligamentum flavum further narrows the spinal canal. With an aging population, the number of patients with lumbar spinal stenosis will continue to increase every year. Lumbar stenosis can present with back pain, leg pain, and symptoms of neurogenic claudication. This can result in decreased function, impacting a patient’s ability to walk and their quality of life. Patients can be treated with physical therapy, medications such as nonsteroidal anti-inflammatory drugs, and nerve pain medications such as gabapentin and pregabalin. Epidural steroid injections can also be considered. When nonoperative treatments have failed, surgical options can be considered.
Lumbar decompression procedures have been proven to be an effective treatment for lumbar stenosis. Javid et al 2 performed a prospective study on 170 patients who underwent surgery for lumbar stenosis (86 patients), lumbar stenosis and herniated disc (61 patients), or lateral recess stenosis (23 patients). For patients with lumbar stenosis, the success rate was 88.1 % at 6 weeks and 86.7% at 6 months. For patients with lumbar stenosis and disc
herniations, the success rate was 80% at 6 weeks and 77.6% at 6 months. In another study, Silver et al3 performed a retrospective study on 258 consecutive decompressive lumbar laminectomies performed on 244 patients presenting with spinal stenosis. Overall, a high degree of success (93% pain relief, 95% return to normal activity) was achieved in the short term, which was supported by the longer-term follow-up data (64% pain relief, 56% activity return, 75% satisfaction).
 Yu-Po Lee, MD
Yu-Po Lee, MD
However, there are limitations and potentially adverse effects from lumbar decompression procedures. Katz et al4 performed a retrospective review on 88 consecutive patients who had had a laminectomy for degenerative lumbar stenosis. One year postoperatively, 6% of the patients had a second operation and another 6% still had severe pain. By the time of the last follow-up 3 years later, 17% of the original 88 patients had a repeat operation because of instability or stenosis. Also, an additional 30% still had severe pain. In another study, Bydon et al5 performed a retrospective review on 500 patients who underwent a 1-3 level lumbar laminectomy for degenerative lumbar diseases. Lumbar discectomies and fusions were excluded. The authors found that 72 patients (14.40%) required reoperations for progression of degenerative disease over a mean of 3.40
21 isass.org Spring 2024 Vertebral Columns
From UCI Health in Orange County, California. SURGICAL DEVICES
SURGICAL DEVICES


years. Of the 72 patients undergoing reoperations, 55.56% underwent decompression alone, while 44.44% underwent decompression and posterolateral fusions.
The issue with lumbar decompression procedures is that they are capable of destabilizing the spine because bone, ligament, and disc are being removed. Also, the muscles, which are the dynamic stabilizers of the spine, have been disrupted. So, there is the risk that the spine becomes unstable enough after a lumbar decompression that a fusion is necessary. Another reason why patients may fare poorly after a lumbar decompression is because the spine is not fused. While this preserves motion, this then allows discs to herniate once more causing bone spurs to regrow. This can lead to recurrent stenosis and pain. Therefore, there are many shortcomings of lumbar decom-
Figure. Anteroposterior (A) and lateral (B) image of a patient who had lumbar stenosis and degenerative scoliosis who was treated with L2-3, L3-4, and L4-5 laminotomies and interspinous spacers.
pression procedures despite overall having positive clinical outcomes.
To treat the instability caused by lumbar decompression procedures, surgeons can fuse the unstable segments. However, there are complications associated with fusion surgery. These complications include an increased rate of infection, increased risk of nerve injury, pseudarthrosis, and a potential increased risk of adjacent segment degeneration.6-8 To counteract the potential instability caused by lumbar decompression procedures while giving surgeons an alternative to fusion, interspinous spacers were developed. Once such device is the Coflex (Figure).
The Coflex is a U-shaped titanium alloy device that is compressible but restricts motion. This device can be inserted between the spinous processes to reduce motion and permit more
22 isass.org Spring 2024 Vertebral Columns
A B
physiological loading to the spine. There are studies showing favorable results with the Coflex device. Errico et al9 evaluated 127 patients who had the Coflex device implanted. At the mean follow-up of 6.3 years, 7% of patients were unsatisfied, 46% were satisfied, and 46% were very satisfied with their clinical outcome.
However, what do the latest studies on the Coflex device show? Zhong et al10 performed a retrospective review on 83 patients who were treated with the Coflex device versus a single level laminectomy. The authors noted that the Coflex patients had higher estimated blood loss (97.50 ± 77.76 vs 52.84 ± 50.63 mL, p = 0.004), longer operative time (141.91 ± 47.88 vs 106.81 ± 41.30 minutes, p = 0.001), and longer length of stay (2.0 ± 1.5 vs 1.1 ± 1.0 days, p = 0.001).
Total perioperative complications (21.7% vs 5.4%, p = 0.035) and instrumentation-related complications were also higher in the Coflex group (10.9% vs 0% laminectomy group, p = 0.039). Therefore, in patients who had a
References
1. Katz JN, Zimmerman ZE, Mass H, Makhni MC. Diagnosis and management of lumbar spinal stenosis: a review. JAMA . 2022;327(17):1688-1699.
2. Javid MJ, Hadar EJ. Long-term follow-up review of patients who underwent laminectomy for lumbar stenosis: a prospective study. J Neurosurg. 1998;89(1):1-7.
3. Silvers HR, Lewis PJ, Asch HL. Decompressive lumbar laminectomy for spinal stenosis. J Neurosurg. 1993;78(5):695-701.
4. Katz JN, Lipson SJ, Larson MG, McInnes JM, Fossel AH, Liang MH. The outcome of decompressive laminectomy for degenerative lumbar stenosis. J Bone Joint Surg Am. 1991;73(6):809-16.
single-level lumbar laminectomy, the Coflex device did not improve overall outcomes. In another study, Li et al11 reviewed 26 studies on the Coflex device and found that compared to lumbar fusion, the Coflex device had a shorter operation time, less intraoperative blood loss, and shorter hospital stays. The Coflex group also had a lower visual analog scale score, lower total complications rate, and higher range of motion ( p < 0.00001).
In conclusion, the current literature shows that there is promise with the Coflex device in treating degenerative lumbar stenosis. In simple stenosis cases, the device may not be warranted because the increased cost of the device does not improve overall outcomes. However, in cases where there are no obvious signs of instability but there are concerns of potential instability after decompression, the Coflex device may be a good alternative to fusion. Further studies are needed to better elucidate the role of the Coflex device. l
5. Bydon M, Macki M, Abt NB, et al. Clinical and surgical outcomes after lumbar laminectomy: an analysis of 500 patients. Surg Neurol Int . 2015;6(suppl 4):S190-S193.
6. Veronesi F, Sartori M, Griffoni C, et al. Complications in spinal fusion surgery: a systematic review of clinically used cages. J Clin Med. 2022;11(21):6279.
7. Goh GS, Tay YWA, Yue WM, Guo CM, Tan SB, Chen JL. What are the patient-reported outcomes, complications, and radiographic results of lumbar fusion for degenerative spondylolisthesis in patients younger than 50 years? Clin Orthop Relat Res . 2020;478(8):1880-1888.
8. Okuda S, Iwasaki M, Miyauchi A, Aono H, Morita M, Yamamoto T. Risk factors for adjacent segment de -
generation after PLIF. Spine (Phila Pa 1976). 2004;29(14):1535-1540.
9. Errico TJ, Kamerlink JR, Quirno M, Samani J, Chomiak RJ. Survivorship of coflex Interlaminar-Interspinous Implant. SAS J. 2009;3(2):59-67.
10. Zhong J, O’Connell B, Balouch E, et al. Patient outcomes after single-level coflex interspinous implants versus single-level laminectomy. Spine (Phila Pa 1976). 2021;46(13):893-900.
11. Li T, Yan J, Ren Q, Hu J, Wang F, Liu X. Efficacy and safety of lumbar dynamic stabilization device coflex for lumbar spinal stenosis: a systematic review and meta-analysis. World Neurosurg. 2023;170:7-20.
23 isass.org Spring 2024 Vertebral Columns
DEVICES
SURGICAL
BONE HEALTH
Osteoporosis and Spine Surgery


Osteoporosis is an age-related, progressively debilitating disease characterized by diminished bone mass and disruption of the microarchitecture of trabecular and cortical bone. Approximately 54 million Americans have low bone mass or osteoporosis, and with an increased life expectancy and an expanding elderly population, the public health burden of osteoporosis continues to increase.1 Osteoporosis and low bone mass are especially relevant to spine surgery because approximately 20% of all patients undergoing spine surgery and up to 50% of women older than 50 years have osteoporosis. 2 This poses a unique challenge for spine surgeons because osteoporosis and low bone mass are associated with pseudoarthrosis, instrumentation failure, fracture, progressive kyphosis, and adjacent-level disc degeneration. 3,4 Given the propensity for low bone mineral density and the increasing age of the population undergoing spine surgery, orthopedic surgeons must consider optimizing bone quality to mitigate adverse surgical outcomes. This entails a comprehensive preoperative evaluation, including a thorough review of medical history to elucidate risk factors for osteoporosis, implementing nonsurgical modalities for treatment, and tailoring surgical treatment with patient-specific
instrumentation, cement augmentation, and perioperative anabolic and antiresorptive agent usage that may be better suited for patients with osteoporosis.
Prevention and Screening
Prevention is the most important principle in the management of osteoporosis, so orthopedic surgeons must recognize the numerous risk factors for primary osteoporosis. In addition to increasing age, tobacco smoking, a sedentary lifestyle, and alcoholism are known risk factors for primary osteoporosis. 5 It is also important to counsel patients on increasing peak bone mass through adequate caloric intake, appropriate dietary calcium, and vitamin D intake. 6 With rates ranging from 40% to 90%, diminished serum vitamin D levels are exceedingly common in adults and strongly associated with increased fracture risk and bone loss.7,8 In patients undergoing spine surgery, one study demonstrated that 47% of patients undergoing spinal deformity surgery and 64% of those undergoing cervical spine surgery had inadequate vitamin D levels (<30 ng/mL). 9 In addition to hypovitaminosis D, other causes of secondary osteoporosis orthopedic surgeons should be aware of include malabsorptive diseases, rheumatism, hypogonadism, and metabolic disorders.
The World Health Organization guidelines recommend that all postmenopausal
24 isass.org Spring 2024 Vertebral Columns
From the Department of Orthopaedic Surgery at Rush University Medical Center in Chicago, Illinois.
Luis M. Salazar, MD
Gregory Lopez, MD
women, men aged 50 years or older, and all individuals with known metabolic bone disease or a high number of osteoporosis risk factors undergo bone mineral density (BMD) screening.6 The gold standard screening tool for osteoporosis is measuring BMD using dual-energy x-ray absorptiometry (DEXA). In settings with limited access to DEXA scanners, ultrasonography is a cost-efficient alternative to assess bone quality. 10 Notably, recent evidence has demonstrated that computed tomography (CT) may serve as an adjunct screening and diagnostic tool for osteoporosis as measurement of Hounsfield units on CT images of the lumbar spine has been correlated with BMD.11 This is an important consideration as lumbar CT is routinely obtained in the majority of these patients for preoperative surgical planning. In addition to advanced imaging, patients undergoing elective spine surgery should also have a comprehensive metabolic laboratory evaluation, including serum osteocalcin, alkaline phosphatase, and markers for bone turnover, such as collagen cross-link degradation products in urine. 6 Other helpful laboratory tests that provide insight into secondary osteoporosis include serum calcium, phosphate, parathyroid hormone levels, and 25-OH vitamin D, the metabolically active circulating form of vitamin D.12
Medical Optimization
Currently, screening and management of osteoporosis are not common practices preceding spine surgery. One survey of 133 spine surgeons demonstrated that only
44% of them checked DEXA results before instrumented fusion, and only 12% checked metabolic laboratory results.13 This same survey revealed a large gap between the number of patients who sustain fragility fractures and those who receive appropriate treatment for osteoporosis. This is explained by the high level of discomfort with the medical management of osteoporosis among spine surgeons. Therefore, it is reasonable to promptly refer patients to primary care providers or endocrine specialists for partial or complete management of osteoporosis before any planned surgical procedure. Treatment of osteoporosis should be initiated in patients with osteopenia (T-score of -1.0 to -2.5) and either a 10-year risk of hip fracture >3% or a 10-year risk of any osteoporotic fracture >20%.14 These risks are predominantly determined by the Fracture Risk Assessment tool developed by the World Health Organization. In the United States, pharmacotherapy for osteoporosis includes oral vitamin D with or without calcium supplementation, bisphosphonates, recombinant human parathyroid hormone (rhPTH), human monoclonal antibodies, calcitonin, and hormone replacement therapy.
Vitamin D deficiency is an independent predictor of prolonged time to spinal fusion and pseudoarthrosis.15 Furthermore, dietary supplementation of vitamin D has been shown to improve fusion rates in rat models as well as patients undergoing transforaminal interbody fusion.16,17 Therefore, vitamin D supplementation should be considered prior to spine surgery. Similarly,
25 isass.org Spring 2024 Vertebral Columns
BONE HEALTH
rhPTH has demonstrated improved fusion rates and reduced rates of pedicle screw loosening.18,19 After a posterior interbody or transforaminal lumbar fusion, a randomized trial showed that weekly rhPTH treatment for 6 months resulted in improved time to fusion. 20 Notably, rhPTH seems synergistic when used with denosumab, a human monoclonal antibody. These two pharmacotherapies have been demonstrated to be more effective in improving BMD and accelerating spinal fusion compared with rhPTH alone. 21 Although these findings are promising, further investigation on denosumab and rhPTH is warranted prior to definitive recommendations.
On the other hand, the use of bisphosphonates in spine surgery remains controversial due to an early animal model study demonstrating a delay in fusion. 22 However, one meta-analysis of 8 randomized trials found no delay in bone healing following bisphosphonate treatment, 23 and another more recent systematic review showed no conclusive evidence of improved fusion after lumbar surgery. 24 Yet, there is a known increased risk of atypical femoral fractures and osteonecrosis of the jaw with long-term bisphosphonate use, and given the inconclusive effect on fusion and the unknown effects of long-term bisphosphonate use on the vertebral column, these drugs should be used with caution. 25
Calcitonin and selective estrogen receptor modulators (SERMs) are less studied in spine surgery and require patient-specific risk-benefit analysis. Calcitonin has been proven to be effective in reducing pain
caused by spinal fractures and in decreasing the risk of vertebral fracture incidence. 26,27 However, there is no conclusive evidence to support the notion that it enhances spinal fusion, and the U.S. Food and Drug Administration has associated the use of calcitonin with an increased risk of malignancy. 28 Similarly, there is a paucity of data reporting on the effects of SERMs on spine surgery outcomes; however, a study found that estrogen replacement therapy is a risk factor for deep venous thrombosis or pulmonary embolism after spine surgery. 29 Therefore, a patient-specific risk-benefit analysis is required prior to administrating calcitonin and SERMs in patients undergoing spine surgery.
Medical management of osteoporosis maximizes bone mineral density before surgery, and administering these drugs to patients undergoing spine surgery in the perioperative period may improve postoperative outcomes. Although the optimal time to treat osteoporosis before and after spine surgery has not yet been defined, orthopedic surgeons may advise patients who qualify for pharmacotherapy to begin their treatment and to consider postponing any surgical procedures for a minimum of 3 months. This is because 3 months after commencing treatment, bone turnover markers normalize and structural changes occur, leading to reduced fracture rates. 30
Surgical Optimization
BMD has significant implications on preoperative and intraoperative decision-making in spine surgery. Low BMD directly affects
26 isass.org Spring 2024 Vertebral Columns
BONE HEALTH
numerous biomechanical aspects of spine surgery, including fatigue failure, pullout strength, and insertional torque. 31 Namely, screw pull-out or cutout is the most common reason for instrumentation-related failure in the osteoporotic spine, so optimizing the bone-screw interface is paramount for achieving fixation. 32 The trajectory of pedicle screw insertion has been extensively studied as a method to improve fixation strength. In the thoracic spine, alignment paralleling the vertebral end plate maximizes insertional torque and pullout strength compared with the anatomical trajectory of pedicle screw placement. 31 Recent developments in pedicle screw design have also improved fixation in osteoporotic bone. Expandable pedicle screws may improve pullout strength as they allow for enhanced anchoring in bone without compromising integrity and have been shown to improve surgical outcomes in patients with osteoporosis. 33 One study compared patients with osteoporosis treated with expandable screws versus normal screws and found a fusion rate of 92.5% with expandable screws and 80.5% with normal screws. 34
Additionally, augmentation of pedicle screws with polymethyl methacrylate (PMMA) has also been extensively studied as a method to improve fixation strength and outcomes. PMMA augmentation increases pullout strength by 149%, decreases the loss of deformity correction, and is associated with increased fusion rates. 35,36 Moreover, vertebral augmentation with PMMA in patients with osteoporotic compression
fractures is an area of ongoing investigation. Early studies demonstrated equivocal outcomes between patients treated with percutaneous vertebroplasty compared with sham procedures. 37,38 However, more recent studies and meta-analyses have revealed favorable improvements in pain scores in patients who are treated with percutaneous cement augmentation compared with nonoperative management. 39,40 More specifically, patients who have acute fractures with persistent and severe pain <6 weeks from the fracture may experience significant pain relief with vertebral cement augmentation.41
Conclusion
As the elderly population continues to grow, osteoporosis is becoming increasingly common and can pose significant healthcare burdens. Therefore, spine surgeons should consider screening individuals at risk to mitigate adverse postoperative outcomes. Preoperative multidisciplinary medical management helps patients maximize bone mineral density and may improve fusion rates with select pharmacotherapy. Furthermore, identifying patients with osteoporosis allows for adequate preoperative planning and aids in intraoperative decision-making to improve fixation and enhance construct stability. Despite the challenges inherent in this patient population, with proper patient selection, preoperative optimization, and patient-specific surgical techniques, spine surgery can provide pain relief, deformity correction, and improved function. l
27 isass.org Spring 2024 Vertebral Columns
BONE HEALTH
BONE HEALTH
References
1. Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520-2526.
2. Wagner SC, Formby PM, Helgeson MD, Kang DG. Diagnosing the undiagnosed: osteoporosis in patients undergoing lumbar fusion. Spine (Phila Pa 1976). 2016;41(21):E1279-E1283.
3. DeWald CJ, Stanley T. Instrumentation-related complications of multilevel fusions for adult spinal deformity patients over age 65: surgical considerations and treatment options in patients with poor bone quality. Spine (Phila Pa 1976). 2006;31(19 Suppl):S144-151.
4. Johnsson KE, Willner S, Johnsson K. Postoperative instability after decompression for lumbar spinal stenosis. Spine (Phila Pa 1976). 1986;11(2):107-110.
5. Forstein DA, Bernardini C, Cole RE, Harris ST, Singer A. Before the breaking point: reducing the risk of osteoporotic fracture. J Am Osteopath Assoc. 2013;113(2 Suppl 1):S5-24; quiz S25.
6. Lane JM, Nydick M. Osteoporosis: current modes of prevention and treatment. J Am Acad Orthop Surg. 1999;7(1):19-31.
7. Cherniack EP, Levis S, Troen BR. Hypovitaminosis D: a widespread epidemic. Geriatrics. 2008;63(4):24-30.
8. Kocjan T, Tan TMM, Conway GS, Prelevic G. Vitamin D status in patients with osteopenia or osteoporosis--an audit of an endocrine clinic. Int J Vitam Nutr Res. 2006;76(5):307-313.
9. Stoker GE, Buchowski JM, Bridwell KH, Lenke LG, Riew KD, Zebala LP. Preoperative vitamin D status of adults undergoing surgical spinal fusion. Spine (Phila Pa 1976). 2013;38(6):507-515.
10. Komar C, Ahmed M, Chen A, et al. Advancing Methods of Assessing Bone Quality to Expand Screening for Osteoporosis. J Am Osteopath Assoc. 2019;119(3):147-154.
11. Schreiber JJ, Anderson PA, Rosas HG, Buchholz AL, Au AG. Hounsfield units for assessing bone mineral density and strength: a tool for osteoporosis management. J Bone Joint Surg Am. 2011;93(11):1057-1063.
12. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment,
and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-1930.
13. Dipaola CP, Bible JE, Biswas D, Dipaola M, Grauer JN, Rechtine GR. Survey of spine surgeons on attitudes regarding osteoporosis and osteomalacia screening and treatment for fractures, fusion surgery, and pseudoarthrosis. Spine J. 2009;9(7):537-544.
14. Unnanuntana A, Gladnick BP, Donnelly E, Lane JM. The assessment of fracture risk. J Bone Joint Surg Am. 2010;92(3):743-753.
15. Ravindra VM, Godzik J, Dailey AT, et al. Vitamin D levels and 1-year fusion outcomes in elective spine surgery: a prospective observational study. Spine (Phila Pa 1976). 2015;40(19):1536-1541.
16. Cho JH, Cho DC, Yu SH, Jeon YH, Sung JK, Kim KT. Effect of dietary calcium on spinal bone fusion in an ovariectomized rat model. J Korean Neurosurg Soc. 2012;52(4):281-287.
17. Xu Y, Zhou M, Liu H, et al. [Effect of 1,25-dihydroxyvitamin D3 on posterior transforaminal lumbar interbody fusion in patients with osteoporosis and lumbar disc degenerative disease]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2014;28(8):969-972.
18. Ohtori S, Inoue G, Orita S, et al. Teriparatide accelerates lumbar posterolateral fusion in women with postmenopausal osteoporosis: prospective study. Spine (Phila Pa 1976). 2012;37(23):E1464-1468.
19. Ohtori S, Inoue G, Orita S, et al. Comparison of teriparatide and bisphosphonate treatment to reduce pedicle screw loosening after lumbar spinal fusion surgery in postmenopausal women with osteoporosis from a bone quality perspective. Spine (Phila Pa 1976). 2013;38(8):E487-492.
20. Ebata S, Takahashi J, Hasegawa T, et al. Role of weekly teriparatide administration in osseous union enhancement within six months after posterior or transforaminal lumbar interbody fusion for osteoporosis-associated lumbar degenerative disorders: a multicenter, prospective randomized study. J Bone Joint Surg Am. 2017;99(5):365-372.
21. Ide M, Yamada K, Kaneko K, et al. Combined teriparatide and denosumab therapy accelerates spinal fusion following posterior lumbar interbody fusion. Orthop Traumatol Surg Res. 2018;104(7):1043-1048.
22. Hirsch BP, Unnanuntana A, Cunningham ME, Lane JM. The effect of therapies for osteoporosis on spine fusion: a systematic review. Spine J. 2013;13(2):190-199.
23. Xue D, Li F, Chen G, Yan S, Pan Z. Do bisphosphonates affect bone healing? A meta-analysis of randomized controlled trials. J Orthop Surg Res. 2014;9:45.
24. Stone MA, Jakoi AM, Iorio JA, et al. Bisphosphonate’s and intermittent Parathyroid Hormone’s effect on human spinal fusion: a systematic review of the literature. Asian Spine J. 2017;11(3):484-493.
25. Khosla S, Bilezikian JP, Dempster DW, et al. Benefits and risks of bisphosphonate therapy for osteoporosis. J Clin Endocrinol Metab. 2012;97(7):2272-2282.
26. Knopp JA, Diner BM, Blitz M, Lyritis GP, Rowe BH. Calcitonin for treating acute pain of osteoporotic vertebral compression fractures: a systematic review of randomized, controlled trials. Osteoporos Int. 2005;16(10):1281-1290.
27. Chesnut CH, Silverman S, Andriano K, et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study Group. Am J Med. 2000;109(4):267-276.
28. Komm BS, Morgenstern D, A Yamamoto L, Jenkins SN. The safety and tolerability profile of therapies for the prevention and treatment of osteoporosis in postmenopausal women. Expert Rev Clin Pharmacol. 2015;8(6):769-784.
29. Schulte LM, O’Brien JR, Bean MC, Pierce TP, Yu WD, Meals C. Deep vein thrombosis and pulmonary embolism after spine surgery: incidence and patient risk factors. Am J Orthop (Belle Mead NJ). 2013;42(6):267-270.
30. Anderson PA, Jeray KJ, Lane JM, Binkley NC. Bone health optimization: beyond own the bone: AOA critical issues. J Bone Joint Surg Am. 2019;101(15):1413-1419.
31. Lehman RA, Polly DW, Kuklo TR, Cunningham B, Kirk KL, Belmont PJ. Straight-forward versus anatomic trajectory technique of thoracic pedicle screw fixation: a biomechanical analysis. Spine (Phila Pa 1976). 2003;28(18):2058-2065.
32. Dodwad SNM, Khan SN. Surgical stabilization of the spine in the osteoporotic patient. Orthop Clin North Am. 2013;44(2):243-249.
33. Shea TM, Laun J, Gonzalez-Blohm SA, et al. Designs and techniques that improve the pullout strength of pedicle screws in osteoporotic vertebrae: current status. Biomed Res Int . 2014;2014:748393. doi:10.1155/2014/748393
34. Wu Z xiang, Gong F tai, Liu L, et al. A comparative study on screw loosening in osteoporotic lumbar spine fusion between expandable and conventional pedicle screws. Arch Orthop Trauma Surg. 2012;132(4):471-476.
35. Sawakami K, Yamazaki A, Ishikawa S, Ito T, Watanabe K, Endo N. Polymethylmethacrylate augmentation of pedicle screws increases the initial fixation in osteoporotic spine patients. J Spinal Disord Tech. 2012;25(2):E28-35.
36. Pfeifer BA, Krag MH, Johnson C. Repair of failed transpedicle screw fixation. A biomechanical study comparing polymethylmethacrylate, milled bone, and matchstick bone reconstruction. Spine (Phila Pa 1976). 1994;19(3):350-353.
37. Buchbinder R, Osborne RH, Ebeling PR, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009;361(6):557-568.
38. Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009;361(6):569-579.
39. Boonen S, Van Meirhaeghe J, Bastian L, et al. Balloon kyphoplasty for the treatment of acute vertebral compression fractures: 2-year results from a randomized trial. J Bone Miner Res. 2011;26(7):1627-1637.
40. Halvachizadeh S, Stalder AL, Bellut D, et al. Systematic review and meta-analysis of 3 treatment arms for vertebral compression fractures: a comparison of improvement in pain, adjacent-level fractures, and quality of life between vertebroplasty, kyphoplasty, and nonoperative management. JBJS Rev. 2021;9(10).
41. Lou S, Shi X, Zhang X, Lyu H, Li Z, Wang Y. Percutaneous vertebroplasty versus non-operative treatment for osteoporotic vertebral compression fractures: a meta-analysis of randomized controlled trials. Osteoporos Int. 2019;30(12):2369-2380.
28 isass.org Spring 2024 Vertebral Columns
Postoperative Dysphagia After Anterior Cervical Spine Surgery
The anterior approach to the cervical spine is safe and effective for various cervical pathologies, including degenerative, traumatic, congenital, and oncologic conditions.1,2 Postoperative dysphagia is one of the most common complications following anterior cervical spine surgery (ACSS). 2 Dysphagia involves dysfunction in the structures or neural control of any of the 3 normal phases of swallowing (oral preparatory, pharyngeal, and esophageal), which feature both voluntary and reflexive processes.3 Normal swallowing requires the careful coordination of over 25 pairs of muscles from the oral cavity, pharynx, larynx, and esophagus to ensure proper emptying of the nasopharynx and oropharynx with subsequent closing of the nasopharynx and larynx to facilitate the safe transit of foods and liquids and prevent aspiration. 3 The causes of postoperative dysphagia are multifactorial, often involving pharyngeal weakness secondary to soft tissue dissection and retraction, prevertebral soft tissue swelling, hematoma formation, direct nerve injury, direct esophageal injury, and inflammation secondary to hardware irritation and esophageal retraction.4
Dysphagia following ACSS is often transient, most commonly beginning
in the immediate postoperative period with an incidence ranging from 1% to 79% in the current literature.5 This wide range is likely related to differences in surgical techniques, surgical types, implants used, timelines for postoperative evaluation, and variations in definitions and assessment of dysphagia. 5 While often transient, postoperative dysphagia is associated with poorer outcomes in terms of quality-of-life assessments, hospital length of stay, in-hospital morbidity, direct costs, and readmission rates. 5
Clinical Signs/Symptoms and Assessment
Patients with postoperative dysphagia may present with reflexive coughing during or after swallowing, regurgitation, increased effort to swallow, and throat/chest congestion after eating. Impaired swallowing biomechanics feature thickening of the pharyngeal wall, dysfunctional pharyngeal constriction and peristalsis, increased transit time, reduced hyoid displacement, and decreased epiglottic inversion. 6 Significant postoperative dysphagia calls for immediate evaluation involving plain cervical radiographs to exclude structural causes (i.e., graft





29 isass.org Spring 2024 Vertebral Columns
COMPLICATIONS
From the Hospital for Special Surgery in New York, New York.
Eric Mai, BS
Joshua Zhang, BS
Cole Kwas, BS
Tomoyuki Asada, MD
Sravisht Iyer, MD
dislodgement, retropharyngeal abscess, and postoperative edema or hematoma).7 The workup may also include patient history, physical examination, laryngoscopy, and video radiographic swallow evaluation with the goals of characterizing the dysphagia (i.e., true dysphagia vs globus sensation or odynophagia, functional/motor dysphagia due to a structural cause, underlying systemic causes, mechanics of impairment, and aspiration risk).7 Patient questionnaires supplement these evaluation methods by providing a noninvasive means to capture the subjective nature of patients’ symptoms. While numerous instruments have been developed to assess dysphagia among different populations, there currently is no universally accepted single instrument. 6 Common instruments (validated and unvalidated) used in practice and cited in literature include the Bazaz Dysphagia Score, World Health Organization Dysphagia Grade, MD Anderson Dysphagia Inventory, Swallowing Quality of Life questionnaire, Eating Assessment Tool (EAT-10), Dysphagia Disability Index, and Dysphagia Short Questionnaire.1,2,8,9
Among these instruments, the Bazaz Dysphagia Score is currently the most widely used and cited instrument and involves patient-reported frequencies of difficulty swallowing liquids and solids to produce a severity of dysphagia grade. 6 Although its simple design has led to widespread use, it has not been formally validated. Reported incidence of dysphagia following ACSS based on literature using the Bazaz Dysphagia Score range from 20%–83% within 2 weeks postoperatively, 54.0%–59.6% at 1 month,
2.4%–18.6% at 6 months, and 1.1%–15.2% at 1 year.10 The EAT-10 questionnaire is also commonly used in clinical practice and involves 10 items assessing patients’ self-perceptions of dysphagia, with a score of ≥3 considered clinically meaningful dysphagia. 9 The EAT10 questionnaire can be completed in less than 2 minutes and has been shown to have excellent internal consistency, reproducibility, and criterion-based validity.9 In a prospective study, Ohba et al reported 34% of patients experiencing clinically significant dysphagia within 2 weeks postoperatively following ACSS, and 25.5% of patients with persistent dysphagia at 1-year follow-up.11
Risk Factors
A multitude of studies have attempted to define risk factors for the development of dysphagia following ACSS. While some risk factors are widely agreed upon, others remain controversial due to multiple studies demonstrating contradictory findings. Intraoperative considerations such as operative time, levels included in a surgery, instrumentation, and use of rh-BMP2 have all been associated with an increase in postoperative dysphagia incidence.12 Vaishnav et al found that operative time >61.5 minutes produced worse dysphagia scores ( p < 0.0001) at 6 weeks compared with baseline.12 Other studies have had similar findings and have reported a correlation between the numbers of levels operated on with increased dysphagia in later postoperative periods.10,13 Singh et al found that dysphagia occurs twice as often ( p < 0.001) in anterior cervical fusion of 3 or more levels than the same operation at 1-2
30 isass.org Spring 2024 Vertebral Columns
COMPLICATIONS
levels.14 There is also evidence that the specific levels being operated on can influence dysphagia incidence—operative levels at C3 or C4 have shown significantly higher risk of dysphagia than levels C5 to C7, possibly due to the typically thinner prevertebral tissue at the upper cervical levels. 2,15 Use of anterior cervical plating is widely believed to increase dysphagia rates. 16 Similarly, rhBMP-2 usage has been widely suspected to increase risk of dysphagia, but the literature has yielded conflicting findings.17,18 A number of patient-specific factors have also shown positive correlations with the development of postoperative dysphagia after cervical spine surgery. Comorbidities such as diabetes mellitus, obstructive sleep apnea, endocrine disorders, and high BMI all have exhibited such a pattern.13 Frailty is a measure of physiologic reserve that reflects patients’ comorbidities and has been shown to strongly predict postoperative outcomes in the spine literature. In a recent retrospective study, Asada et al found that frailty as measured by the modified Frailty Index-11 (mFI-11), was identified as a significant risk factor for postoperative dysphagia following ACSS.19
Prevention and Management
Intraoperative steroid administration has been a well-investigated method of minimizing dysphagia risk after cervical spine surgery. Most data indicate that local and intravenous steroid administration are both associated with a lower incidence of dysphagia at early postoperative follow-up after CDR and anterior cervical discectomy and fusion (ACDF), although evidence from
randomized controlled trials is limited.10,20
The efficacy of tracheal/esophageal traction exercises has also been studied as a strategy to prevent dysphagia. In a prospective randomized controlled trial, Chen et al found that patients undergoing anterior cervical fusion who performed tracheal/esophageal traction exercises for 3 days preoperatively had lower Bazaz dysphagia scores at early postoperative follow-up compared with controls. 20
Slimmer plate design has also been investigated as a means to reduce the rate of dysphagia, with Lee et al suggesting that a smaller and smoother profile plate, such as the Zephir (Medtronic Danek), is more effective in reducing the risk of dysphagia as compared to a larger plate, such as the Atlantis (Medtronic Danek). 8 Currently, the literature supports similar rates of postoperative dysphagia between ACDF and CDR in the immediate postoperative period, while ACDF has been shown to demonstrate higher rates of chronic dysphagia. 21,22
The primary goals of treatment for dysphagia are typically focused on maximizing food intake while minimizing aspiration and related adverse effects. Patients can learn compensatory and rehabilitative techniques for the safe transit of boluses through the pharynx while avoiding the adjacent airway, such as the modification of diet, enhancing sensory input prior to or during swallowing, protecting the airway with postural adjustments, or facial muscle exercises.6 Further medical or surgical intervention is often necessary if these strategies are ineffective, including placement of a feeding tube or true vocal cord injection.
31 isass.org Spring 2024 Vertebral Columns
COMPLICATIONS
COMPLICATIONS
Conclusion
Postoperative dysphagia is a prevalent, well-established, and multifactorial complication following ACSS that can contribute to significant morbidity and reduction in quality of life for patients. Understanding the risk factors and strategies for mitigating postoperative dysphagia is critical for in
References
1. Skeppholm M, Ingebro C, Engström T, Olerud C. The Dysphagia Short Questionnaire: an instrument for evaluation of dysphagia: a validation study with 12 months’ follow-up after anterior cervical spine surgery. Spine (Phila Pa 1976). 2012;37(11):996-1002.
2. Kalb S, Reis MT, Cowperthwaite MC, et al. Dysphagia after anterior cervical spine surgery: incidence and risk factors. World Neurosurg 2012;77(1):183-187.
3. Mendoza-Lattes S, Clifford K, Bartelt R, Stewart J, Clark CR, Boezaart AP. Dysphagia following anterior cervical arthrodesis is associated with continuous, strong retraction of the esophagus. J Bone Joint Surg Am. 2008;90(2):256-263.
4. Ian Dhar S, Wegner AM, Rodnoi P, et al. Fluoroscopic swallowing abnormalities in dysphagic patients following anterior cervical spine surgery. Ann Otol Rhinol Laryngol 2020;129(11):1101-1109.
5. Tsalimas G, Evangelopoulos DS, Benetos IS, Pneumaticos S. Dysphagia as a postoperative complication of anterior cervical discectomy and fusion. Cureus 2022;14(7):e26888.
6. Anderson KK, Arnold PM. Oropharyngeal dysphagia after anterior cervical spine
surgery: a review. Global Spine J. 2013;3(4):273-286.
7. Lee SH, Kim KT, Suk KS, Park KJ, Oh KI. Effect of retropharyngeal steroid on prevertebral soft tissue swelling following anterior cervical discectomy and fusion: a prospective, randomized study. Spine (Phila Pa 1976). 2011;36(26):2286-2292.
8. Lee MJ, Bazaz R, Furey CG, Yoo J. Influence of anterior cervical plate design on dysphagia: a 2-year prospective longitudinal follow-up study. J Spinal Disord Tech 2005;18(5):406-409.
9. Belafsky PC, Mouadeb DA, Rees CJ, et al. Validity and reliability of the Eating Assessment Tool (EAT-10). Ann Otol Rhinol Laryngol 2008;117(12):919-924.
10. Alentado VJ, Bisson EF, Potts EA. Dysphagia after cervical spine surgery: a review of risk factors and preventative measures. J Neurosurg Spine. 2022;38(3):382-388.
11. Ohba T, Hatsushika K, Ebata S, et al. Risk factors and assessment using an endoscopic scoring system for early and persistent dysphagia after anterior cervical decompression and fusion surgery. Clin Spine Surg. 2020;33(4):E168-E173.
12. Vaishnav AS, Saville P, McAnany S, et al. Is the likelihood of
counseling patients and providing effective management postoperatively. Future studies featuring larger prospective randomized cohorts may help clarify the variations in reported incidence, mechanisms, and risk factors of postoperative dysphagia, while further informing preventive and management strategies. l
dysphagia different in patients undergoing one-level versus two-level anterior cervical discectomy and fusion? Spine J. 2020;20(5):737-744.
13. Yew AY, Nguyen MT, Hsu WK, Patel AA. Quantitative risk factor analysis of postoperative dysphagia after anterior cervical discectomy and fusion (ACDF) using the Eating Assessment Tool-10 (EAT-10). Spine (Phila Pa 1976). 2019;44(2):E82-E88.
14. Singh K, Marquez-Lara A, Nandyala SV, Patel AA, Fineberg SJ. Incidence and risk factors for dysphagia after anterior cervical fusion. Spine (Phila Pa 1976). 2013;38(21):1820-1825.
15. Kang SH, Kim DK, Seo KM, Kim KT, Kim YB. Multi-level spinal fusion and postoperative prevertebral thickness increase the risk of dysphagia after anterior cervical spine surgery. J Clin Neurosci 2011;18(10):1369-1373.
16. Liu FY, Yang DL, Huang WZ, et al. Risk factors for dysphagia after anterior cervical spine surgery: a meta-analysis. Medicine (Baltimore). 2017;96(10):e6267.
17. Bellamy JT, Dilbone E, Schell A, et al. Prospective comparison of dysphagia following anterior cervical discectomy and fusion (ACDF) with and without rhBMP-2. Spine
J. 2022;22(2):256-264.
18. Lu DC, Tumialán LM, Chou D. Multilevel anterior cervical discectomy and fusion with and without rhBMP-2: a comparison of dysphagia rates and outcomes in 150 patients. J Neurosurg Spine. 2013;18(1):43-49.
19. Asada T, Singh S, Maayan O, et al. Impact of frailty and cervical radiographic parameters on postoperative dysphagia following anterior cervical spine surgery. Spine (Phila Pa 1976). 2024;49(2):81-89.
20. Chen Z, Wei X, Li F, et al. Tracheal traction exercise reduces the occurrence of postoperative dysphagia after anterior cervical spine surgery. Spine (Phila Pa 1976). 2012;37(15):1292-1296.
21. Smucker JD, Bassuener SR, Sasso RC, Riew KD. Comparison of long-term differences in dysphagia: cervical arthroplasty and anterior cervical fusion. Clin Spine Surg. 2017;30(8):E1160-E1164.
22. Skeppholm M, Olerud C. Comparison of dysphagia between cervical artificial disc replacement and fusion: data from a randomized controlled study with two years of follow-up. Spine (Phila Pa 1976). 2013;38(24):E1507-E1510.
32 isass.org Spring 2024 Vertebral Columns
HEALTHCARE DELIVERY
Telehealth in Spine Surgery
With the onset of the COVID-19 pandemic and the subsequent shutdown, innovative methods of providing patient care had to be developed. One of these innovations involved the use of telehealth patient visits to replace the traditional in-person visit. While the utilization of these visits was born out of necessity, the response to its adoption by patients has been favorable, with studies demonstrating an 87.7% satisfaction rate and 45.5% preference rate for telemedicine over an in-person visit.1 Despite such promising preliminary results, questions persist regarding the feasibility of initial evaluation of complex spine patients, functional evaluation of postoperative cases, and reimbursement methods by public and private payers.
Current Telehealth Utilization
Recent international cross-sectional studies have demonstrated contemporary telehealth utilization to be increasing over time despite the relaxation of pandemic lockdowns. In one study,2 more than 60% of providers performed at least 1 telehealth visit, with overall usage increasing nearly 4-fold. Additionally, nearly every surgeon reported a strong preference for at least 1 in-person visit prior to surgery. In particular, the telehealth model seems to be especially effective to for younger patients and patients who live far away from the treating provider.1 The cost benefit to the patient is also an important consideration, as studies have shown that telehealth visits save families an average of $223 per visit in transportation and work savings.3 Additionally,
improved patient compliance with appointments has the potential to save the healthcare system billions of dollars over time in lost productivity.4 Finally, the ability to evaluate patients across cities, states, and countries offers an increased referral network and capture area that could continue to be beneficial for spine surgeons over the coming years.
Limitations in Telehealth
 Vincent P. Federico, MD
Vincent P. Federico, MD

While the increased utilization of telehealth is promising for its continued mass adoption, there are several limitations that must be addressed. Most notably, and particularly for evaluation of spine patients, the physical examination is an essential component to a comprehensive evaluation of a patient and their associated pathology. Motor, sensory, reflex, and central and/or peripheral nerve compression testing is all inherently easier and more reliable with an in-person examination. While several previously published studies have demonstrated best practices for a virtual physical examination, the limitations of the online platform persist.5,6 The potential long-term morbidity of missing an important piece of information, such as motor weakness, myelopathic symptoms, or other neurologic compromise, as a result of using telehealth cannot be overstated, and providers should remain cognizant of this as utilization continues to increase.
33 isass.org Spring 2024 Vertebral Columns
From the Department of Orthopaedic Surgery at Rush University Medical Center in Chicago, Illinois.
Arash Sayari, MD
HEALTHCARE DELIVERY
Additionally, the capital costs associated with purchasing the telehealth software, training yourself and your staff to use the software, and instructing complex spine patients on best methods to use the software, as well as performing physical examination maneuvers virtually, has the potential for reducing practice margins.7 The costs associated with increased time spent per visit can easily compound during a clinic, which can significantly reduce productivity over the course of time.
Telehealth Policy and Coverage
While the ability to provide accessible care for more patients in more locations can be a significant enhancement to the healthcare system, there are several complexities that require discussion. As telehealth is regulated on a state-by-state basis, there are limitations in what care surgeons can provide. Depending on the state, surgeons may be required to have a full state license to provide care or a telehealth-specific license for that state.8 These differing practices and qualifications can be burdensome for practices to keep track of but should remain at the forefront of any telehealth practice to avoid any compliance issues.
References
1. Satin AM, Shenoy K, Sheha ED, et al. Spine patient satisfaction with telemedicine during the COVID-19 pandemic: a cross-sectional study. Global Spine J. 2022;12(5):812-819.
2. Hayward K, Han SH, Simko A, James HE, Aldana PR. Socioeconomic patient benefits of a pediatric neurosurgery telemedicine clinic. J Neurosurg Pediatr. 2019;25(2):204-208.
3. Hare N, Bansal P, Bajowala SS, et al. Work Group Report: COVID-19: unmasking telemedicine. J Allergy Clin Immunol Pract 2020;8(8):2461-2473.e3.
4. Riew GJ, Lovecchio F, Samartzis D, et al. Telemedicine in spine surgery: global perspectives and practices. Global Spine J. 2023;13(5):1200-1211.
In addition to the medical licensing required to perform telehealth visits, coverage by both public and private insurances has required several renewals since its initial adoption during the pandemic. Most recently, the United States Congress extended coverage of Medicare telehealth services until December 31, 2024. This coverage included the evaluation and management codes most utilized by spine surgeons. Additionally, 98% of Medicare Advantage plans offer telehealth coverage. While individual private payers may have slight differences in their coverage and reimbursement, the ruling set by the Centers for Medicare and Medicaid Services offers a promising precedence for patients to have continued access to telehealth services.
Conclusion
Telehealth utilization in spine surgery has been met with enthusiasm and increasing adoption since the pandemic. While limitations remain, the opportunity to provide accessible care for patients who would otherwise be restricted is an essential component. Further long-term clarification on telehealth coverage and reimbursement is required to ensure there are no lapses in patient care for this increasingly adopted technology. l
5. Iyer S, Shafi K, Lovecchio F, et al. The spine telehealth physical examination: strategies for success. HSS J. 2021;17(1):14-17.
6. Sardar ZM, Coury JR, Luzzi AJ, Weidenbaum M, Riew KD. The telehealth spine physical examination: a practical approach learned during the COVID-19 pandemic. World Neurosurg. 2021;154:e61-e71.
7. Donnally CJ 3rd, Vaccaro AR, Schroeder GD, Divi SN. Is evaluation with telemedicine sufficient before spine surgery? Clin Spine Surg 2021;34(10):359-362.
8. Perez-Roman RJ, Trenchfield DR, Perez-Roman NI, Wang MY. The legal and socioeconomic considerations in spine telemedicine. Neurosurgery. 2022;90(4):365-371.
34 isass.org Spring 2024 Vertebral Columns



















 Yu-Po Lee, MD
Yu-Po Lee, MD









 Vincent P. Federico, MD
Vincent P. Federico, MD
