
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 04 | Apr 2025 www.irjet.net p-ISSN: 2395-0072


International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 04 | Apr 2025 www.irjet.net p-ISSN: 2395-0072
Dr. S. Kanmani1 , Mr. C. Rishikesh2
1Head of Department, Department of Civil Engineering, Anna University, Chennai, Tamil Nadu, India
2Post Graduate Student, Centre for Environmental Studies, Department of Civil Engineering, Anna University, Chennai, Tamil Nadu, India
Abstract - Electrochemical technologies, which use electrons to remove contaminants through redox reactions, can provide an elegant contribution to environmental control. The primary goal of electrochemical oxidation has been to produce carbon dioxide from oxidizable molecules. Leachate is a complicated effluent that comes from landfills and has the potential to have a significant influence on the environment. In order to comply with inland disposal regulations, this study attempts to treat the landfill leachate. In an electrochemical method, the removal of contaminants was investigated using various anode materials. In a batch electrolytic parallel plate reactor, leachate from two different packages were treated by electrochemical oxidation. The electrochemical process was carried out individually, using graphite as cathode and anode electrodes. The effects of the operating factors such as current density, Dilution factor, reaction time, chloride ion concentration that influence the removal of pollutant from Leachate electrochemically were studied.
Key Words: contaminants;electrochemicaloxidation;electrodes;Landfillleachate;redox
1.INTRODUCTION
Aiwabeletal..,(2011)saidthattheLandfillleachateisanimportantpollutionsourceoriginatedinmunicipallandfillsites. Landfillleachateisdefinedasthoseaqueousstreamsgeneratedasaconsequenceofrainwaterpercolationthroughwastes, biochemicalprocessesinthewaste’scellsandtheinherentwatercontentofthewastesthemselves.Leachatesmaycontainlarge amountsoforganicmatter,ofwhichhumictypeconstituentsareanimportantgroup,aswellasammonia-nitrogen,heavy metalsandchlorinatedorganicandinorganicsalts.RajanGandhimathietal.,(2013)discussedthatthedischargeoflandfill leachatecanleadtoseriousenvironmental problemsasthey maypercolate throughsoils andsub soils,causingextensive pollution of ground and surface waters if they are not properly treated and safely disposed. Leachate becomes ahead of wastewatersasbeingthemostdifficulttotreatasitisawastewaterwithacomplexandwidelyvariablecontentgenerated withinalandfill.Therefore,manypre-treatmentandcombinedtreatmentmethodshavebeenproventotreatleachate.Yunnen chenetal..,(2012)discussedthatthetypeofleachatedependsonfactorssuchasageandtypeoflandfill,pH,andBOD5/COD ratio.
Huankailietal.,(2023)investigatedthatduetoitseffectivenessandeaseinoperation,electrochemicaloxidationprocesshas recentlyreceivedsignificantattentionforwastewatertreatment.Theprocesshasshownitsefficacyforthedestructionof refractorypollutantssuchascyanideandEDTA,andalsoforcolourremoval.Ingeneral,pollutantscanbedestroyedelectrochemicallybydirectanodicoxidationorbyindirectoxidationManyresearchershaveinvestigatedtheelectrochemicaloxidation ofdifferentwastewaterswithvarioustypesofcompounds.Theseincludetextilewastewater,p-chlorophenolandpnitrophenol phenolicwastewaterandTanyolac,oliveoilwastewatertannerywastewateretal.,andpaintwastewater.
Xiannisongetal..,(2023)discussedthatthetreatmentofCLBEbyEOwithTi/RuO2-IrO2astheanodecanachieveconcomitantly organiccarbonremoval(COD)andnitrogenremoval(NH3-N).Thestatisticalanalysisrevealedthatcurrentdensity,A/V,andClconcentration were significantly and positively correlated with the removal of COD, NH3-N and TN, while d showed a nonmonotoniceffectonthem.TheinitialpHshowedanon-monotoniceffectontheremovalofCODthatwasfirstweakenedand thenpromoted,andwassignificantlyandpositivelycorrelatedwiththeremovalofNH3-NandTN.17Panizzaetal.,(2023) discussedthatinallanodesexceptgraphite,ammoniumremovaldominatedoverorganicoxidation.ATi/PbO2anodeisfoundas abetteranodethanTi/Ru-SnO2thoughBDDshowedbestperformanceamongthethreeanodesforbothCODandammonium removalaswellasintermsofcurrentefficiencyandoperatingcost.
Nidheshetal.,(2023)saidthatLeachatetreatmentusingelectrochemicallyactivatedpersulfatebyexternallyaddedferrousions intotheleachatesystemhavebeencarriedouteffectively.ThetreatmentprocessesincludeanodicoxidationofPt/Ti,sulphate

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 04 | Apr 2025 www.irjet.net p-ISSN: 2395-0072
radicalgeneratedfromthecathodicreductionofpersulfateanditsactivationviaexternallyaddedFe2+ionsinwatermedium Hydroxylradicalsandactivechlorinestimulatedinthesystemduetoanodicoxidationandindirectelectro-chemicaloxidation processes accordingly enhance COD reduction in leachate. %ICE relies on persulfate concentration and escalates with augmentationofCODremovalrate.Huiansuetal.,(2023)saidthatthepollutantssuchasCOD,NH4+-N,S2−,turbidity,and heavymetalscouldbesignificantlyremovedbytheelectricalflocculationandelectrochemicaloxidationintheBEF-Osystem. Additionally,EBRwasusedtodeeplytreatBEF-Osystemeffluentandachievedasatisfactorytreatmenteffectonthemature landfillleachate.Shenetal.,(2006);Irdemezetal.,(2006);Lietal.,(1995)Isaetal..,(2008)investigatedthattheElectrochemical degradationofstabilizedlandfillleachatewasinvestigatedbyemployingaflowelectrochemicalreactorandusingTi02anode andTicathode.
ManytypesofelectrodeshavebeeninvestigatedforelectrochemicaltreatmentsuchasTi02,Ti,Fe,Pb02/Ti,Sn02/Ti,graphite andaluminium.AbdulhussianAabbasetal..,(2016)saidthattheelectrodegradationwasanalternativemeanstobreakdown recalcitrantorganiccompoundsinlandfillleachate.Duetohighenergyconsumption,however,thistechnologyismore18 expensivethanothertreatmentmethods.Asaresult,thistreatmenttechniquehasbeeninvestigatedlessextensivelyforthe treatmentofstabilizedleachate.
Chiangetal.(1995)investigatedthatcomparedtheefficacyoffouranodematerialsintermsofCODandammoniumnitrogen removalofalowBOD/CODratio(0.2)landfillleachateandtheefficacyorderhasbeenobtainedasSn-Pd-Ruoxide-coated titanium(SPR)>Ru-Tioxide-coatedtitanium(DSA)>Ti/PbO2>graphiteduetohighelectrocatalyticactivityofSPRanode.They haveconfirmedtheproductionofchlorine/hypochloriteasmainresponsiblespeciesfororganicoxidationandammonium nitrogenremovalusingalltheanodesbyconductingexperimentsusingsalinewater.Fernandesetal.(2016)investigatedthat duringtheinitialperiodofelectrolysis,organicoxidationreactiondominatedoverammoniumremovalbutlater,after50%COD removal,ammoniumremovalrategetacceleratedthroughhypochlorite(ClO)formation.Fernandesetal.(2016)alsocompared theperformancesofTi/Pt/PbO2,Ti/Pt/SnO2-Sb2O4andBDDanodeswhereTi/Pt/PbO2anodewasfoundasmostsuitable anodematerialyieldinglowerenergyconsumption,highernitrogenremoval,andlowernitrateformationthanBDDanode.The indirectoxidationthroughchlorine/hypochloritewasthedominantmechanism.
Therearethreemaindirectionsinthedevelopmentofelectrochemicaltreatmentprocessappliedtowastewaterscontaining variousimpurities.Theyareasfollows:
1) Removalofdissolvedimpurities(mostlyorganicmatter)fromwasteeffluentintheformofnon-toxic(orlesstoxic) andinsomecases,waterinsolubleproductsasaresultofanodicoxidationandcathodicreduction.
2) Removalofdissolvedimpurities(mainlyinorganicmatter)fromwastewatersbyelectrodialysis,simultaneously utilizingrecoveredproducts.
3) Removalofdissolvedandfinelydividedindispersedstate(includingemulsified)insolubleimpurities(bothorganic andinorganic)byelectrocoagulationmethodsandelectroflocculationprocess(DengandEnglehardt,2006).
2.1 ADVANTAGES OF ELECTROCHEMICAL PROCESS
Theadvantagesofelectrochemicaloxidationare:
A. Lesslandarearequiredandpollutionfree.
B. Doesnotleaveanyresidueandnoadditionalreagentsarerequired.
C. Continuousprocesswithrelativelyhighflowratescanbedesigned.
D. Nophasechangesarerequiredandhighcurrentefficienciescanbeattained.
2.2 LIMITATIONS OF ELECTROCHEMICAL PROCESS
a) ElectricityrequirementandhighpHmayaffecttheelectrodes.
b) Itisusuallynecessarytodoamultiplepassoftheelectrolytetoachieveahighremovalofitsioniccontents.Asingle passusuallyremoveslessthan50%(BhaskarRajuetal.,2009).

International Research Journal of
Volume: 12 Issue: 04 | Apr 2025 www.irjet.net
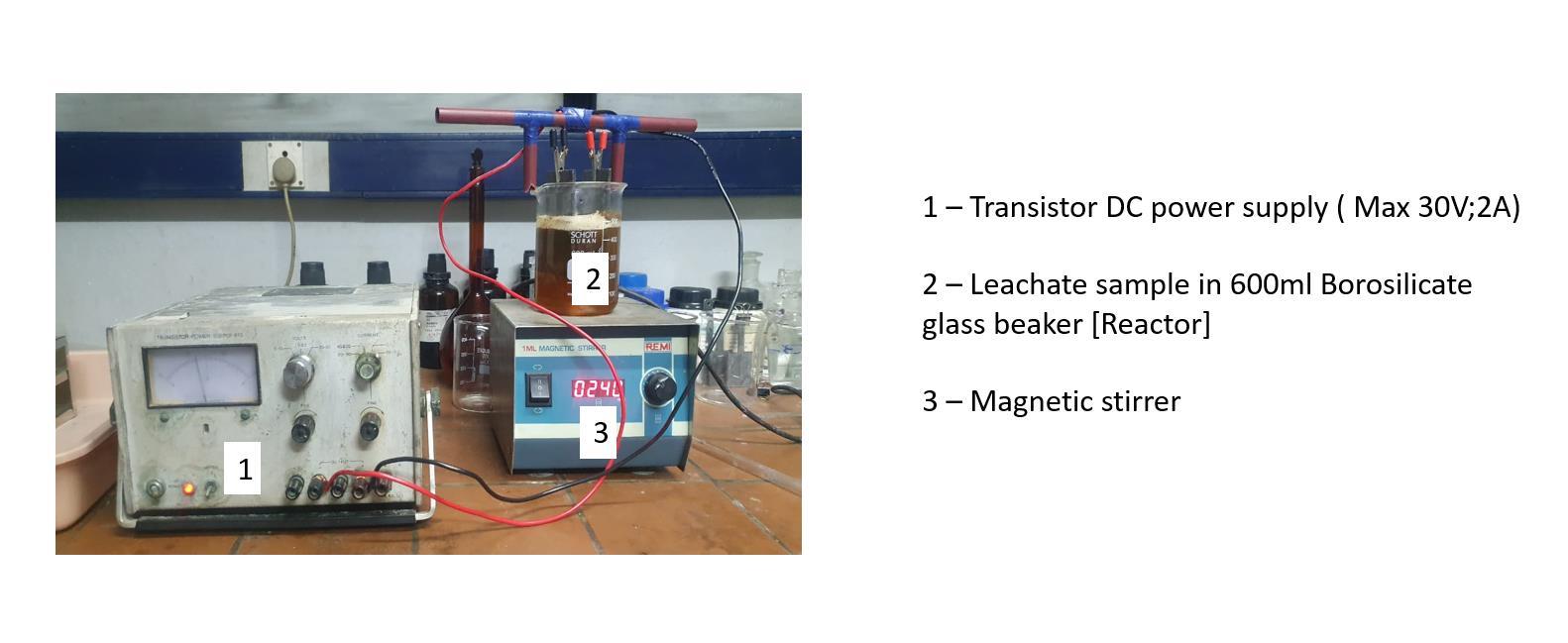
Theexperimentwasconductedbybatchprocess.Anundividedcellof500mlcapacity(GlassBeaker)wereusedthroughout thestudy.Theexperimentalapparatuswasconstructed.Theanodeandcathodewerepositionedverticallyandparalleltoeach otherwithaninnerelectrodegapof2cm.Theseelectrodesweredippedintheelectrolytesolutioninsubmergenceof7cm. WireswereusedformakingconnectionsbetweentheelectrodesandDCpowersystem.Thepositiveterminalisconnectedtothe anode and negative terminal to the cathode. The reactor was kept in a glass bowl containing water to maintain constant temperatureoftheelectrolytecell.Thesolutionwaskeptconstantlystirredwithamagneticstirrerinordertomaintainuniform concentrationoftheelectrolytesolutionasshowninFigure1

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 04 | Apr 2025 www.irjet.net
Note: Sample 1 is the leachate sample collected from Package 1 from Perungudi dumpsite.
Sample 2 is the leachate sample collected from Package 3 from Perungudi dumpsite.
Source: APHA(2005)andAPHA-AWWA-WPCF(1998)
Theresultsshowthatthevaluesareobtainedbycharacterisationofsample.Thesamplewascollectedfromperungudidumpsite package3.ThesamplewasfoundtobeslightlyalkalineinnatureatpHof7.8.ThesamplewasfoundtohavehigherCODand chloridesof1228.8mg/Land1149.65mg/Lduetopresenceofinorganicpollutantsandotherpersistentorganicpollutants.The samplehasshownhigherconductivityof5710µSduetopresenceofchlorides.Thesampleswillbetreatedwithelectrochemical oxidationandcomparedwiththestandardsofleachateaspermunicipalsolidwastemanagementrules2016.
Theresultsshowthatthevaluesareobtainedbycharacterizationofsample.Thesamplewascollectedfromperungudidumpsite package3.ThesamplewasfoundtobeslightlyalkalineinnatureatpHof7.93.ThesamplewasfoundtohavehigherCODand chloridesof4218.8mg/Land7867.7mg/Lduetopresenceofinorganicpollutantsandotherpersistentorganicpollutants.The sample has shown higher conductivity of 12810 µS due to presence of chlorides. The samples will be treated with electrochemicaloxidationandcomparedwiththestandardsofleachateaspermunicipalsolidwastemanagementrules2016.
Graphite is an electric conductor, consequently, useful in such applications as arc lamp electrodes. Commonly used inert electrodes:graphite(carbon).Itcanconductelectricityduetothevastelectrondelocalizationwithinthecarbonlayers( a phenomenoncalledaromaticity).Thesevalenceelectronsarefreetomove,soareabletoconductelectricity.Theelectrode materialthatwillbeemployedinthepresentstudywillbegraphitewiththedimensionof10X5cm.Theeffectiveareaofthe cathodewillbe50.0cm2.
AstabilizedDCpowersupply(0-50V)ispreferredasthesourceofelectriccurrentfortheexperiment.
Leachatesampleof500mleachwastakenforbatchanalysisfromtheperungudidumpsite.Thesamplesweretransportedto thelaboratoryandstoredinarefrigeratorat4oCpriortouseintheexperiments.Theelectrodeswereplacedverticallyand paralleltoeachotherintheelectrolyticreactorcontaining450mLofleachatesample.Thedistancebetweenthecathodeand anodewas2cm.Electrolyte,sodiumsulphateNaClwasaddedataconcentrationof(0.05mMto0.2mM)tothesamplesbefore eachexperiment.DCpowersupplyatcurrentdensityof20mA/cm2wasappliedinitiallytothereactor.Atdifferenttime intervalsthesamplewascollectedandstoredinairtightcontainerforfurtheranalysisinthelaboratory.Thesamplecollected wasanalysedforCODandcolour.Byvaryingthevoltagetothereactor,themaximumremovalefficiencywasobtained
5.1
Thestudywascarriedouttilltheoptimallevelofleachateattained.Withvaryingtimeinterval.Therangeofreactiontimewas [0-240]mins.

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 04 | Apr 2025 www.irjet.net p-ISSN: 2395-0072
Theeffectofcurrentdensitywasvariedfrom[20-80]mA/cm2
5.3
TheeffectofadditionofelectrolyteNaClwasaddedtoenhanceremovalofpollutantfromlandfillleachate.Thedosagewas [0,500,1000mg/L].
5.4 DILUTION
Theeffectofdilutionwascarriedoutatvariousratiosrawleachate withnodilution[Noadditionofdistilledwater],1:1[ 250mlleachatesample+250mldistilledwater]dilution,2.3:1[350mlDistilledwater+150mlleachatesample]dilution.
6.1. Cod
ThebatchEOprocesswasconductedtochecktheremovalofCODfromtheleachate[Package3andpackage5].Thechemical oxidationdemandoftheeffluentsampleisdeterminedbyopenrefluxmethod,usingCODdigestionapparatus.Theabsorptionof thesampleisrecordedtomeasurecoloronSpectrophotometer,recordingthespectraover190nmto1000nmrange.
6.2. Final Optimization for Package 3 (Sample 2):
Theoptimizedconditionforreactiontime2hourshasremoved75.4%ofCOD.buttheCODvaluedoesnotmeetthestandards ofleachatementionedaspermunicipalsolidwastemanagementrules2016.ThefinalvalueofCODis561.72mg/l,whereas minimumCODvaluetobemaintainedasperMunicipalsolidwastemanagementrules2016is250mg/l.
Byincreasingthereactionfrom2hoursto4hours,TheCODremovalincreaseddrasticallyfrom75.4%to95%,thevalueis120.2 mg/l,whichisfavorabletomeettheminimumCODvalueasperMSWrules2016.
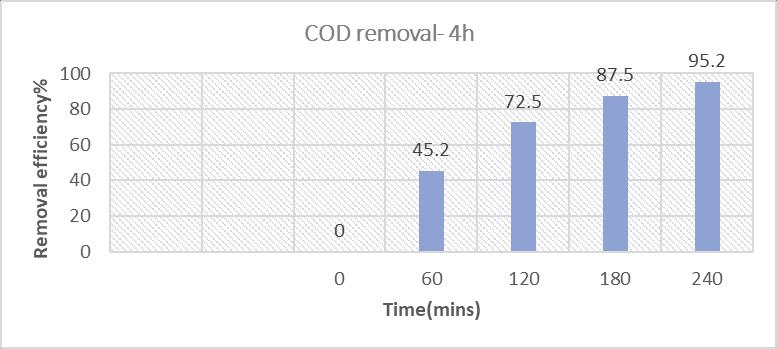
2: MaximumreductionofCOD
Colorremovalhasslightlyincreasedwhencomparedto2hoursofreactiontime.In2hoursofreactiontime,theabsorbance valueswerecalculated.Only19.2%ofcolorat526nm,7.1%ofcolorat436nm,5.8%ofcolorremovalat620nmhasbeen removedwhichisnotthatmuchsignificant.

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 04 | Apr 2025 www.irjet.net p-ISSN: 2395-0072
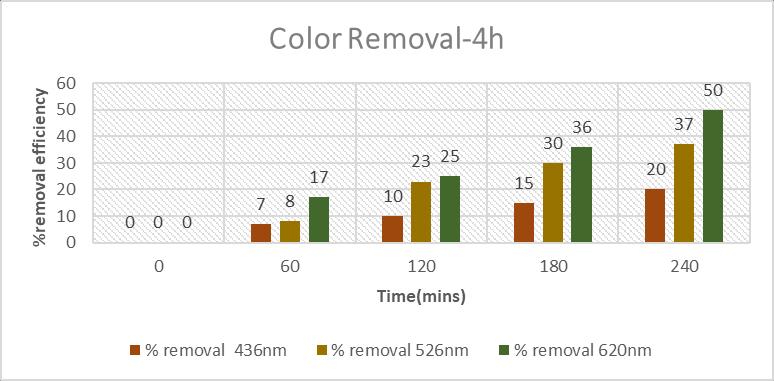
Inordertoincreasethecolorremoval,Reactiontimehasbeenincreasedfrom2hoursto4hoursinoptimizedcondition.From thereactionof4hours,Colourremovalhasbeenabsorbedindifferentabsorbancevalueswhichare20%colourremovalat 436nm,37%colourremovalat526nmand50%colourremoval in620nm.whichissignificant.Ifthereactiontimefurther increasedthecolourremovalalsoincreases.


Theoptimizedconditionforthereactiontime2hourshasremoved87%ofCODwherethevalueofCODis162.7mg/lwhichis metthestandardsofmunicipalsolidwastemanagementrules2016.Whereasthecolourremovalisnotmuchefficientwhen comparedtoCOD.Toenhancethecolourremovalthereactiontimehasincreasedfrom2hoursto4hours.ThefinalCODvalueis 90.4mg/l.

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 04 | Apr 2025 www.irjet.net p-ISSN: 2395-0072
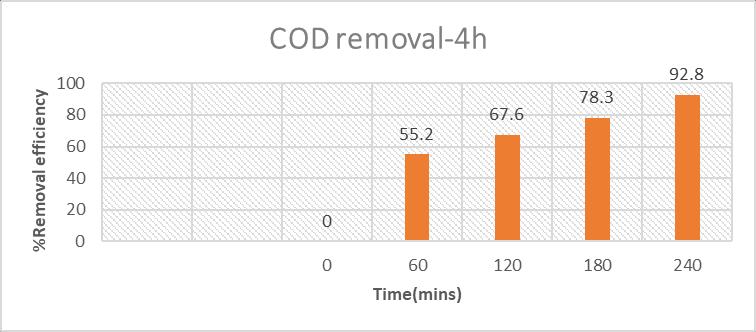
Colourremovalhasslightlyincreasedwhencomparedto2hoursofreactiontime.In2hoursofreactiontime,theabsorbance valueswerecalculated.Only23.3%ofcolourat526nm,17%ofcolourat436nm,50.3%ofcolourremovalat620nmhasbeen removedwhichisnotthatmuchsignificant.Inordertoincreasethecolourremoval,Reactiontimehasbeenincreasedfrom2 hoursto4hoursinoptimizedcondition.
Fromthereactionof4hours,Colourremovalhasbeenabsorbedindifferentabsorbancevalueswhichare43.5%colourremoval at436nm,62%colourremovalat526nmand63%colourremovalin620nm.whichissignificant.Ifthereactiontimeisfurther increased,thecolourremovalalsoincreased.

Increaseinreactiontime,thechlorideconcentrationinleachategetsconvertedtohypochlorite/chloriteafterprolongedperiod oftime,thishypochlorite/chloriteionisconvertedtohypochlorate/chlorateprecipitatewhichagglomerateswithdissolvedand suspendedsolidsinthesolution,therebycolourremovaloccurs.

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 04 | Apr 2025 www.irjet.net p-ISSN: 2395-0072


Thehighestremovalefficiencyofcolour63.4%at620nm,62.2%at526nmand40%at436nmforoptimumconditionsof variablesviz,pH=3.4andcontacttimeat240minsat60mA/cm2havebeenrecordedforleachatesamplefrompackage1. The highestremovalefficiencyofcolour20%at436nm,37%at526nmand50%at620nmforoptimumconditionsofvariablesviz, pH=3.4andcontacttimeat240minsat60mA/cm2havebeenrecordedforleachatesamplefrompackage3.
TheeffectivepercentageremovalofCODwas75.4%forreactiontimeof2hoursand95.2%forreactiontimeof4hoursfor leachatesamplePackage3.TheeffectivepercentageremovalofCODwas87.2%forreactiontimeof2hoursand92.5%for reactiontimeof4hoursforleachatesamplePackage1.
IncreaseinreactiontimeincreasestheCODandcolourremoval efficiencyandgraphiteelectrodeshelpsinreducingthe organiccontaminantsofleachateandalsootherpollutants.Theelectrodeischeap,economicallyavailableandeasytohandle.
Graphiteelectrodeissensitiveforchlorideconcentrationandmaximumoperatinghoursof7hours.Electrochemicaloxidation providesseveraladvantagesforthepreventionandremedyofpollutionproblems.Theinherentadvantageisitsenvironmental compatibility as it uses a clean reagent, the electron , and there is little or no need for addition of chemicals. Likewise electrochemicaloxidationisgenerallycharacterizedbysimpleequipment,easyoperationandbriefretentiontime
[1] D.KornackandP.Rakic,“CellProliferationwithoutNeurogenesisinAdultPrimateNeocortex,”Science,vol.294,Dec.2001, pp.2127-2130,doi:10.1126/science.1065467.
[2] M.Young,TheTechnicalWriter’sHandbook.MillValley,CA:UniversityScience,1989.
[3] R.Nicole,“Titleofpaperwithonlyfirstwordcapitalized,”J.NameStand.Abbrev.,inpress.
[4] K.Elissa,“Titleofpaperifknown,”unpublished.
[5] Amokrane,A.,Comel,C.andVeron,J.(1997)‘Landfillleachatepre-treatmentbycoagulationflocculation’,WaterResearch, Vol.31,pp.2775–2782.
[6] APHA (2005) Standard Methods for the Examination of Water and Waste Water, 21st ed., American Public Health Association,WashingtonDC.

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 04 | Apr 2025 www.irjet.net p-ISSN: 2395-0072
[7] APHA-AWWA-WPCF(1998)StandardMethodsfortheExaminationofWaterandWasteWater,20thed.,AmericanPublic HealthAssociation,WashingtonDC.
[8] Aziz,H.A.,Alias,S.,Adlan,M.N.,Asaari,F.A.H.andZahari,M.S.M.(2007)‘Colorremovalfromlandfillleachatebycoagulation andflocculationprocess’,BioresourceTechnology,Vol.98,pp.218–220.
[9] BhaskarRaju,G.,ThalamadaiKaruppiah,M.,Latha,S.S.,Priya,D.L.,Parvathy,S.andPrabhakar,S.(2009)‘Electrochemical pretreatmentoftextileeffluentsandeffectofelectrodematerialsontheremovaloforganics’,ScienceDirect,Desalination, Vol.249,pp.167–174.
[10] Deng, Y. and Englehardt, J.D. (2006) Electrochemical Oxidation for Landfill Leachate Treatment, Department of Civil, ArchitecturalandEnvironmentalEngineering,McArthurBuilding,UniversityofMiami,USA.
[11] Enzminger,J.D.,Robertson,D.,Ahlert,R.C.andKosson,D.S.(1987)‘Treatmentoflandfillleachate’,JournalofHazardous Materials,Vol.14,pp.173–182.
[12] Mishra,R.,Gedam.N.,Waghmare.S.,Masid,S.andNeti,N.R.(2009)‘Landfillleachatetreatmentbythecombinationof physical-chemicalandelectrochemicalmethods’,JournalofEnvironScience&Engine,Vol.51,No.4,pp.315–320.
[13] Nisha Priya, M., Esakku, S. and Palanivelu, K. (2005) ‘Electrochemical treatment of landfill leachate’, Indian Chemical Engineer,CentreforEnvironmentalStudies,AnnaUniversity,pp.272–276.
[14] Rajkumar,D.,Palanivelu,K.andBalasubramanian,N.(2004)‘Combinedelectrochemicaldegradationandactivatedcarbon adsorptiontreatmentsforwastewatercontainingmixedphenoliccompounds,JournalofEnvironEngScience,Vol.4,pp.1–9.
[15] Tatsi,A.A.,Zouboulis,A.I.,Matis,K.A.andSamaras,P.(2003)‘Coagulation-flocculationpretreatmentofsanitarylandfill leachate’,Chemosphere,Vol.53,pp.737–744
[16] Vadivu,R.(2007)ManagementofReverseOsmosisRejectfromTextileIndustry,METhesis,CentreforEnvironmental Studies,AnnaUniversity,Chennai,India.
[17] Vlyssides,A.G.,Israilides,C.J.,Loizidou,M.,Karvouni,G.andMourafetiV.(1998)‘Electrochemicaloxidationoftextiledyeand finishingwastewaterusingPt/Tielectrode’,JournalofEnvironmentScienceandHealth,Vol.A33,No.5,pp.847–862.