
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 11 Issue: 04 | Apr 2024 www.irjet.net p-ISSN: 2395-0072


International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 11 Issue: 04 | Apr 2024 www.irjet.net p-ISSN: 2395-0072
Abhinaya K1 , Akhila K2, Bharath VJ3, Rohith TP4 , Rohini P5
1,2,3,4 , Student, Department of Civil engineering, Jawaharlal College of Engineering and Technology, Ottapalam, Kerala, India
5Assistantprofessor, Department of Civil Engineering, Jawaharlal College of Engineering and Technology, Ottapalam, Kerala, India ***
Abstract Geopolymer mortar is a type of mortar that is made usinggeopolymerbindersinsteadoftraditionalcementbasedbinders. Geopolymer binders are typically derivedfrom industrial by-products or natural materials and have lower environmental impact compared to cement. Geopolymer mortars offer high strength, durability, and chemical resistance properties, making them suitable for a variety of construction applications. Geopolymer Concrete has gained attention to the development of pavement repairs, Airport highwaysandothercivilengineering structures.Inthispaper a comprehensive study was carried out for compressive strength of Geopolymer mortar using Fly Ash. The compressive strength of Geopolymer mortar prepared by replacing 100% cement with Fly Ash was checked. Alkali activators suchas sodiumhydroxideandsodiumsilicatewere usedas binders.Compressive strength werecheckedat7days and 14 days by performing compression test and it was observed that from the result of optimum molarity dosage of M ,the compressive strength of Geopolymer
Key Words: FlyAsh,sodiumHydroxide,SodiumSilicate,M Sand,Geopolymerization,Geopolymermortar.
Inordertoreduceenvironmentaldeteriorationandaddress climate change concerns, the global construction industry hasbeenunderincreasingpressuretoembracesustainable techniques and materials in recent years. The creation of substitutebindersforPortlandcement,whichisnotorious for its high carbon footprint and energy-intensive manufacturing process, is one area of great interest and innovationinthefieldofsustainablebuildingmaterials.A viable option that provides a sustainable substitute for conventional cement-based materials is geopolymer technology.
ThemanufactureofPortlandcement,avitalcomponentof traditional mortars and concrete, accounts for a sizeable amountoftheworld'scarbondioxide(CO2)emissions.Hightemperaturekilnfiringisastepintheprocessthatreleases CO2asabyproductandconsumesa significantamount of energy.Inaddition,habitatdevastationand environmental degradationarecausedbytheminingofrawmineralslike limestone. There is a pressing need to lessen the environmentalimpactofcementproductionasurbanization
and infrastructure development drive up demand for buildingmaterials
Thebuildingsectoralsofacesagreatdealofdifficultydueto thelossofnaturalresources,includingthesupplyofhighquality aggregates for the manufacturing of concrete. Conventional river sand mining has resulted in habitat destruction, bank erosion, and environmental damage. In addition, the lack of appropriate aggregates has led to increased expenses and logistical difficulties for building projects.
Examining sustainable aggregates and alternative binder systemsthatprovideperformanceonparwithorbetterthan traditional materials while reducing their environmental impact is imperative in light of these difficulties. By using locally accessible minerals and industrial by-products, geopolymer technology offers a creative solution to these problems by producing high-performance mortars and concretewithalowercarbonfootprintandresourceusage.
With potential advantages in terms of performance, economy, and the environment over traditional Portland cement-based mortar, geopolymer mortar has become a viable substitute. Through a process known as geopolymerization, inorganic polymers known as geopolymers are created from aluminosilicate precursors, such as fly ash, slag, or other industrial by-products. Geopolymerization can be accomplished at ambient temperature or mild heat, resulting in reduced energy consumption and CO2 emissions than Portland cement, which requires high-temperaturecalcination and releases considerablevolumesofCO2
2. THE METHODOLOGY FOLLOWED IN THIS PROJECT
Collection of materials like Fly ash, M sand, sodium silicateandsodiumhydroxide.
Selectingdimensionofcube.
Preparationofmixproportions.
Castingcubesforcompressiontest.
Testonspecimen
Arrivingatconclusion

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 11 Issue: 04 | Apr 2024 www.irjet.net
3.1 Fly Ash
A fine, powdery substance known as fly ash is produced when thermal power plants burn pulverized coal. It is removed from coal-fired power stations' chimneys and collectedinthefluegases.Flyashcanbeusedtoconcreteas anadditionalcementitiousingredientbecauseitishighin iron, silica, and alumina. "The finely divided residue that resultsfromthecombustionofgroundorpowderedcoaland thatistransportedbyfuelgasesfromthecombustionzone to the particle removal system" is the definition of fly ash given by ACI 116 R. In thermal power plants, fly ash is a byproduct of burning pulverized coal. Table 1 lists the differentconstituentsandflyash'schemicalcomposition.
Table -1: Chemicalcompositionofflyash.
SI.
3.2
3.2.1
Water glass, or sodium silicate, is a crucial component in geopolymerconcrete.It functionsasanactivator,forming the geopolymer binder through reactions with aluminum silicate and other substances. Sodium silicate aids in the creationofastrongandlong-lastinggeopolymermatrixand supplies alkalinity. The Prowess Company provides the necessaryquantityofsodiumsilicateforcollection.Infigure 1,sodiumsilicateisdisplayed.

Causticsoda,orsodiumhydroxide,isan alkalineactivator thatisutilizedingeopolymermortarcompositions.Sodium hydroxide starts the chemical reaction required for geopolymerizationwhencombinedwithflyashandsodium silicate (water glass), two more activators.as depicted in Figure2,sodiumhydroxide

The product is obtained as pellets with a purity of 98% sodium hydroxide. To get the molarity, these pellets are combinedwithdistilledwaterinthe appropriateratio.To ensure that the alkali liquid undergoes no additional chemical reaction, distilled water is used. Once sodium hydroxidehasbeenaddedtothewater,thoroughlyagitate the mixture until all of the pellets have dissolved. As the pelletsdissolveandthewatertemperaturerises,takegood careofyourskin.Thewater'stemperaturehasreachedthe point when it boils. After that, the solution is allowed to thoroughlycool.Thesolutioncoolsdownoverthecourseof fourtofivehours.
A1Msodiumsilicatesolutionismade.Afterthat,themixture waslet tostandfor roughly a full dayin order tocreate a uniformmixture.Inthiscase,fourdistinctmolaritytypes 8M,10M,12M,and14M havebeenexamined.providesthe amountofsodiumhydroxidethatmustbemixedwithwater to produce 1000 grams of NaOH solution, while Table 1

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 11 Issue: 04 | Apr 2024 www.irjet.net p-ISSN: 2395-0072
displaysthedensityandmolecularweightofvariousmolar solutions.
3.3 M sand
Manufacturedsand,orMsand,isakindofsandmadefrom crushed hard granite rocks. It serves as a natural sand substituteinconstructionprojectsandisanalternativeto riversand.Typically,Msandparticlesrangeinsizefrom150 micronsto4.75mm.Sinceitisfinerthansandfromanatural river,itcanbeusedforavarietyofconstructionprojects.
4.1 Mould preparation
Moulds of size 70.6x70.6x70.6mm are prepared with multiwoodsheetswithsufficientthicknessasshowninthe figure3.

4.2 Preparation
Thebasicdifferencebetweenordinarycementmortarand geopolymermortaristhebinder.Thesilicaandaluminum oxidepresentinFlyashandreactswithalkaliliquidtoform a geopolymer paste which on heating goes through a geopolymerizationprocess.
1. In the study work, low calcium, class F dry fly ash are obtainedfromMalabarcementsisusedasbasematerialto makethegeopolymers.
2.Sodiumsilicate(Na2SiO3)mixedwithsodiumhydroxide (NaOH)asanalkalineactivatorhasbeenusedinthisstudy, NaOH in pellet form with 98% purity & Na2SiO3 is in powderedformconsistofNa2O=9.4%andSiO2=30.1%
3. TheratioofNa2SiO3&NaOHiskeptas1.
4. Oven dry curing temperature i.e., 60ºC for time in 48 hoursiskeptconstant.
5.Theratioofalkaliactivatortoflyashisconsidered0.8.
Theprocessofmakinggeopolymermortarinvolvestheuse ofregularPortland cement. BothregularPortlandcement mortar and geopolymer mortar are made using the same technique. The three primary ingredients used to make mortarareflyash,Msand,andalkaliactivatorsolution.The sandiscleanedinaccordancewithIS2116-1980bypassing itthrougha4.75mmsieve.Aflyashtosandratioof1:3is assumed.Afterthoroughlycombiningthesandandflyash withatrowelorbyhand,averyslowadditionofalkaliliquid is made. For two to three minutes, mix. The cementitious materialandalkaliliquidbinderarethoroughlycombined. Followingthemixingofthecombinationandcubeformation (asperIS2250(1981),themixtureisbakedfor48hoursat 60degreesCelsiustocureit.After48hoursintheoven,the compressivestrengthtestforeachmolarity'scubewill be conducted. Likewise, after 7, 14, and 28 days, the mortar cubeofeachmolaritywillundergoacompressivestrength test. For curing, geopolymer mortar blocks are stored outside.Showninfigure4and5.




International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 11 Issue: 04 | Apr 2024 www.irjet.net p-ISSN: 2395-0072
5.1 Density test
Resultsobtainedfromdensitytestsaregivenbelow
(i)Thedensityofmortarcubesof8M,10M,12M,14and16M after48hoursofovendryingisshownbelow.
Table -2: Densityafterovendrying
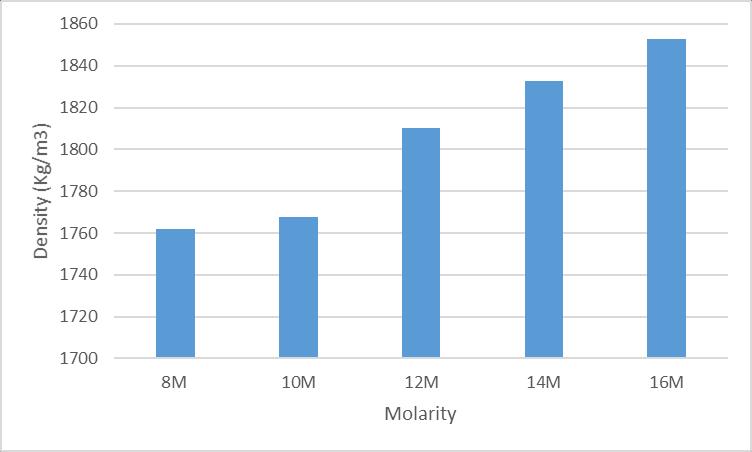
6:Densityafterovendrying
After measuring the density of mortar cubes of different molaritiesafter48hoursofovendrying,thehighestdensity was obtained in mortar cubes with 16M NaOH. And the lowerdensityisobtainedinmortarcubesof8M.
(ii)Thedensityofmortarcubesof8M,10M,12M,14and 16Mat7thand14thdayisshownintable3
Table -3: Densityafterambientcuring
After measuring the density of mortar cubes of different molaritiesthataresubjectedtoambientcuring,thehighest densitywasobtainedinmortarcubeswith16MNaOH.And thelowerdensityisobtainedinmortarcubeswith8MNaoH asshowninfig7
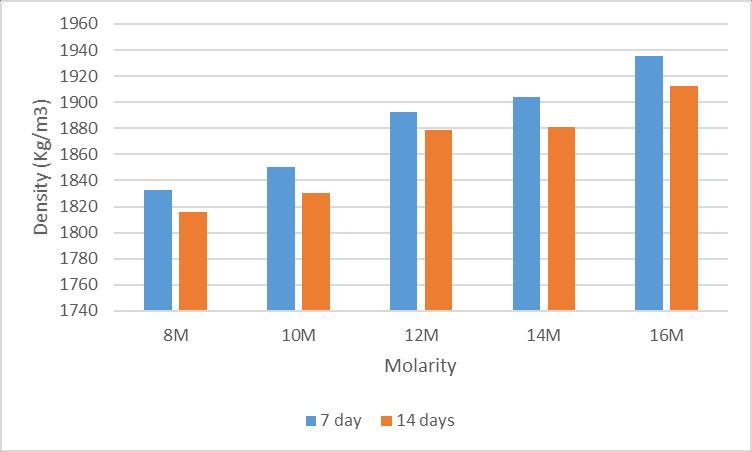
Fig7:Densityafterambientcuring
5.2 Compressive strength test
Resultsobtainedfromcompressivestrengthtestsaregiven below.
(i) compressivestrengthofovendriedmortarcubes.
Thecompressivestrengthofmortarcubesafter48hoursof ovencuringisshownintable4
Table – 4:Compressivestrengthafterovencuring
Here after 48hours of oven curing of mortar cubes at a temperature of 60 degrees Celsius, the highest value compressive strength is obtained from the geopolymer mortarmadefrom14MNaOHandleastvaluecompressive strengthisobtainedfrommortarmadefrom8MNaOH.The FollowingChartShowstheCompressivestrengthofMortar withDifferentMolaritiesasshowninfigure8

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 11 Issue: 04 | Apr 2024 www.irjet.net p-ISSN: 2395-0072
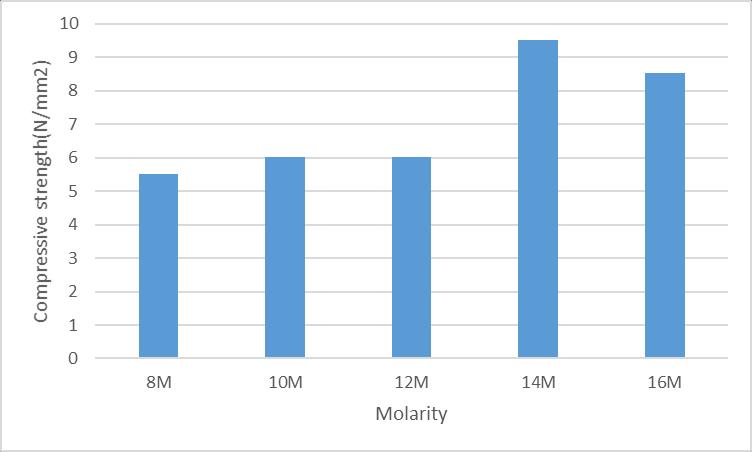
ovencuring
(i) compressive strength of mortar cubes after ambient curing
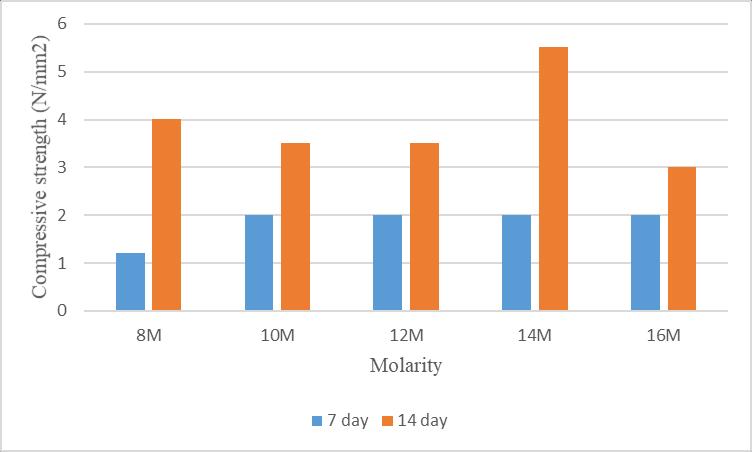
The compressive strength of mortar cubes after 7 and 14 daysofambientcuringareshownintable5.
Table – 5:Compressivestrengthafterambientcuring
5.3 Water absorption test
Results obtained from water absorption tests are given below;
Table – 5:waterabsorptiontestofovendriedsamples
MOLARITY WEIGHT BEFORE ABSORPTION OFWATER
Thefollowingchartshowsthecomparisonofcompressive strengthofdifferentmolaritiesofgeopolymermortarcubes after7and14daysofambientcuring.Fromthischartitis observedthat,thecompressivestrengthofmortarcubeis increasingbyincreasingthemolarityofNaOH.
Hereaftercomparingtheresults,themaximumcompressive strengthisobtainedfrommortarwith14MNaOHisshown infigure9
The following chart shows the percentage of water absorptionofmortarcubesmadeusingdifferentmolarities of sodium hydroxide. Here the percentage of water absorptionisreducingwithanincreaseinmolarityexceptin 16M.incubemadewith16MNaOH,thepercentageofwater absorptionincreasesslightlycomparedto14M.
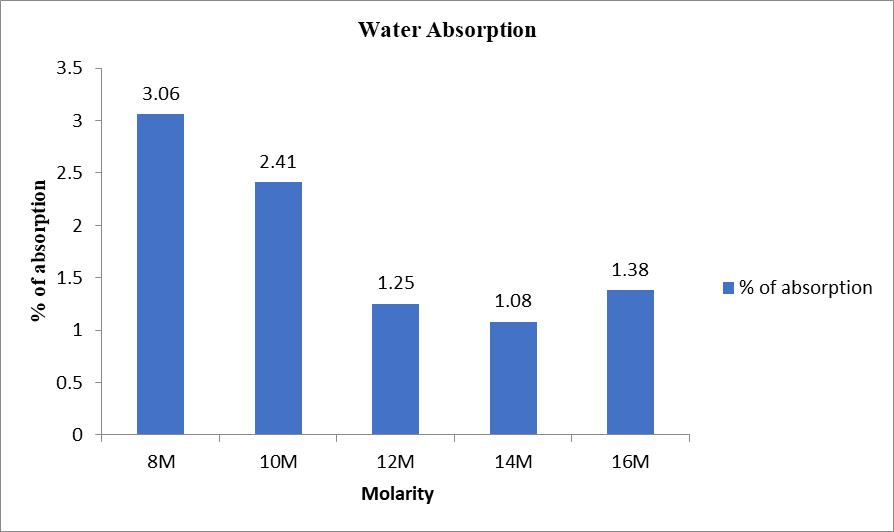
10:waterabsorptiontestofovendriedsamples

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 11 Issue: 04 | Apr 2024 www.irjet.net p-ISSN: 2395-0072
Resultsobtainedfromconsistencytestsareasshown, Weightofflyashtaken=400g
Table – 6:consistencyofflyash
%
Waterrequiredformakingstandardconsistencyofflyash is140mlfor400gm.
Standardconsistency(%)=(140/400)x100 =35%
Theconclusionthatcanbedrawnbasedontheresultsof researchontheeffectsNa2SiO3andNaOHonthe propertiesofgeopolymermortarareasfollows;
Geopolymermortaroffersasustainablealternative to traditional cement mortar, with benefits includingreducedenvironmentalimpactandgood performance.
Geopolymer technology reduces the carbon footprintassociatedwithcementproduction,asit reliesonindustrialwastematerials,contributingto sustainabilitygoals.
Maximumcompressive strengthafter 48hours of ovendryingisobtainedongeopolymermortar14M withavalueof9.42MPaandtheminimumvalueis obtainedongeopolymermortarwith8Mwhichis equal to 5.51 MPa. Based on this result, it can be concluded that the optimum mixture to get the maximumstrengthisusedNaOH14M.Basedonthe results, it can be concluded that the optimum molaritytogetmaximumcompressivestrengthis NaOH14M.
Theresultofcompressivestrengthofgeopolymer mortar is lower than cement mortar type S accordingtoASTMC1329-03.
Thegreaterthemolarityof NaOH,thegreaterthe densityofgeopolymermortarobtained
The percentage of water absorption reduces with increaseinmolarityofNaOHexcept16M.in16M
the percentage of water absorption increases slightly.
[1] Atabey,İsmailİsa,OkanKarahan,CahitBilim,andCengiz DuranAtiş,"Very high strength Na2SiO3 and NaOH activatedflyashbasedgeopolymermortar"(2020).
[2] D. R. Fitriani, “Pengaruh Modulus Alkali Dan Kadar Aktivator Terhadap Kuat Tekan Fly Ash-Based GeopolymerMortar,”p.58,2010.
[3] Kandoria, Vikrant & Kaur, Loveleen & Sharma, Dr. (2022).Development ofGeopolymer MortarUsingFly Ash. International Journal for Research in Applied Science and Engineering Technology. 10. 2375-2377. 10.22214/ijraset.2022.41139
[4] Tenepalli,J.S.andNeeraja,D,“PropertiesofclassFflyash basedgeopolymermortarproducedwithalkalinewater”, JournalofBuildingEngineering, 19 (2018).
[5] Thokchom, S., Ghosh, P., and Ghosh, S. (2011). “Durability of Fly Ash Geopolymer Mortars inNitric Acid–effect of Alkali (Na2O) Content.” Journal of Civil EngineeringandManagement,17(3),393–399