
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 11 Issue: 03 | Mar 2024 www.irjet.net p-ISSN: 2395-0072


International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 11 Issue: 03 | Mar 2024 www.irjet.net p-ISSN: 2395-0072
Purushottam Kumar*, B.P. Singh
Department of Physics, Dr. Bhimrao Ambedkar University, Agra 282002 (U. P.) India
Abstract - Batteries are essentially a form of storage that consists of two electrodes submerged in an electrolyte. This electrolyte acts as a conduit for the ion exchange that generates electricity. There has been extensive research done on various battery technologies and applications, and batteries of all shapes and sizes are thought to be one of the bestwaystostoreenergy.However, theenvironmentaleffects ofwidespreadbatteryuseremainasignificantissuethatneeds further investigation. Li-ion batteries are the preferred technology for hybrid and fully electric vehicles, power tools, and portable gadgets due to their unmatched combination of high energy and power density. Li-ion batteries will dramatically reduce greenhouse gas emissions if electric vehicles (EVs) replace the majority of gasoline-powered transportation. The main Li-ion battery components are presented and contrasted in this study, along with the accompanyingbatterymanagementsystemsandmethodsfor enhancing battery efficiency, capacity, and lifespan. Battery performance is shown to be critically dependent on material andthermalproperties.ThephysicalimplementationofLi-ion batteries, the positive and negative electrode materials, and the electrolytes are discussed.
Key Words: Li-ion batteries, different types of batteries, greenhouse gases, battery management systems, battery efficiency, electrode materials.
1.Introduction:
Anodeandcathodeelectrodesinanelectrolyteact asthebasicbuildingblocksofbatteries.Theionexchange thatproduceselectricityusesthiselectrolyteasachannel. Eachbatteryhasuniqueadvantagesanddisadvantages,but becausetorecentadvancementsinLi-iontechnology,these batteriesarenowthemarketleaderforusageinthemajority of handheld and portable electronics as well as electric vehicles[1].Thekeyreasonsforthisaretheirhighefficiency, lengthycyclelife,andspecificenergy(Wh/kg).Theydohave disadvantages, such costing a lot of money and requiring complexsafetyandmonitoringsystems.
Because of its unmatched mix of high energy and power density, lithium-ion batteries are the preferred technology for portable gadgets, power equipment, and hybrid and completely electric cars. If most gasolinepoweredvehiclesarereplacedbyelectricvehicles(EVs),Liion batteries will significantly reduce greenhouse gas emissions.Thehighenergyefficiencyoflithium-ionbatteries may also allow for their use in a range of electric grid applications, such as improving the quality of energy
obtained from renewable sources like wind, solar, geothermal, and other energy sources, thereby encouraging their wider adoption and the growth of an energysustainable economy. There has recently been a lot of researchinthisfieldasaresultoftheincreasedinterestin Li-ion batteries from both commercial and government fundingorganizations[2].
Lithium-ionbatteries(LIBs)havedrawnthemost attention and importance among the created batteries in recent years. LIBs have a long service life, high coulombic efficiency, high discharge power, and high energy density when compared to other batteries [3,4]. Due to these qualities,LIBshavemadegreatstridesinavarietyoffields, including stationary applications, portable and flexible electronics,andelectricvehicles.Theneedtooftenupdate the community is vital because the subject of LIBs is developingquicklyanddrawingmoreresearchers[5].
In Li-ion batteries, which may be thought of as energystoragedevicesthatdependoninsertionprocesses frombothelectrodes,lithiumionsactasthechargecarrier. Given this broad definition, there are different cell chemistriesthatmakeuptheLi-ionbatteryfamily[6].Most Li-ionbatteriesincludenegativeelectrodesmadeofcarbon orlithiumtitanate(Li4Ti5O12),whileresearchisalsobeing doneonnovelmaterialssuchlithiummetalandlithium(Si) alloys.Topromoteiontransport,differentlithiumsalts,such asLiPF6,aretypicallycoupledwithanorganicsolvent,such as diethyl carbonate, depending on the electrode material chosen. A dividing membrane allows lithium ions to pass between the electrodes, preventing an internal reaction [7,8].
Lithium-ion Using Li-ion batteries in highperformance electric vehicles is a desirable option. Li-ion offers a very high specific energy and a lot of chargedischarge cycles as compared to other rechargeable batteries.Additionally,thepriceisfair[9].Li-ionbatteries are therefore favored over alternative options like nickelmetal-hydride and silver-zinc batteries. However, Li-ion batteriesarenowonlyofferedintinysizesonthemarket.In order to attain the appropriate battery sizes, several cells must be joined in series and parallel topologies. Making highlyefficient,veryreliablebatterypacksforuseinelectric cars is a challenge because of these and safety concerns [10,11].

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 11 Issue: 03 | Mar 2024 www.irjet.net p-ISSN: 2395-0072
In recent years, Li-ion batteries, one of the most technologically advanced rechargeable batteries, have receivedalotofattention.Theyareonlyfoundincellphones andlaptopcomputers,althoughtheynowholdamonopoly on mobile power sources for portable electronics [12]. Around20yearsago,roughlyaroundthesametimewhen Li-ionbatterieswerefirstmadeavailableforpurchase,the personal digital electronic revolution began, and Li-ion batteriesarenowregardedasitsdrivingforce.Asonemay havealreadyobservedfromhisorherdailylife,betterLi-ion batteries are constantly needed to support the growing functionalityofmobileelectronics.Forinstance,charginga cell phone with more features less frequently than one's presentphonewillenhanceone'squalityoflife.
Electric and hybrid vehicles, which demand nextgenerationLi-ionbatterieswithnotonlyhighpower,high capacity, high charging rate, and long life, but also significantlyenhancedsafetyperformanceandlowcost,are another significant expanding market for Li-ion batteries. Obama's administration in the United States has an extremely ambitious target of one million plug-in hybrid automobiles on the road by 2015. Similar strategies are beingusedgloballytopromotehybridandelectriccars.To underscorethewidespreadinterestinLi-ionbatteries,the Foreign Policy magazine even wrote an article headlined "Thegreatbatteryrace"[14].
Particularlyasdemandfromelectricvehiclesrises, Li-ion battery demand is rising quickly. To meet the demands from consumer use and electric vehicles, it is anticipated that roughly 100 GW hours of Li-ion batteries will be needed [15]. By 2018, the latter will account for around 50% of Li-ion battery sales. Additionally, Li-ion batteries will be used to smooth the gap between energy supplyanddemandinordertobuffertheintermittentand erraticgreenenergysupplyfromrenewableresourceslike solar and wind. For instance, additional solar power producedduringthedaycanbestoredinLi-ionbatteriesto provide power at night when there is no sun light. Largescale Li-ion battery production for grid applications will necessitate low-cost next-generation battery production [16].
Fig. 1. Intwodecades,Li-ionbatterydemandwill increase.Withpermission[17],reproduction.
Over the past 10 years, the number of scientific papers has increased tenfold as a result of the call for sustainable bio-based electricity storage systems [17–18]. Numerousstudieshaveaddressedtheuseofcarbonanodes made from biomass in batteries to date. The primary propertiesofbio-basedcarbonanodesforbatteriescanbe distilleddowntoafewkeybenefits,suchas:
Excellentphysical-mechanicalstabilityisprovided bythewideinterlayergapsduringtheionintercalation/deintercalationprocess[19].
Many,variedsurfacefunctionsthatenhancecharge transfer.
High specific surface area (SSA), well-developed pore architectures with various nanoscale sizes, superiorthermalstability,andquickmasstransfers [20]areallpositivecharacteristics.
Toimprovetheelectrochemicalperformance(such as lifetime, capacity, and safety), the surface and structure of bio-based carbons can be easily adjusted or altered in terms of their chemical structure and surface functions, such as via heteroatomdoping(nitrogen,oxygen,sulphur,etc.).
The development of multiple carbon anodes employingvariousbiomasscarbonsandcomposites is made easier by the vast availability and accessibilityofthesematerials[21].
Lithium-ion batteries (LIBs) are the battery technologythathasbeenaroundthelongestandiscurrently the most used in our society. It has helped make portable electronicsdevicespossibleandisrevolutionizingtheauto sector.Intenseresearchisbeingdoneonhighenergydensity batteries all around the world, and LIBs, which have emerged as one of the most promising energy storages

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 11 Issue: 03 | Mar 2024 www.irjet.net p-ISSN: 2395-0072
despiterisingcostsanduncertaintysurroundingthesource ofcomponentmaterials,aregrowinginpopularity.
Li and Gr are becoming increasingly important strategicresources,nonetheless,asaresultoftheincreasing demand and uneven geographic distribution [22–24].
Lithium-ionbatteries(LIB)useLi4Ti5O12(LTO)orGrasthe anode and lithium-ion cathode materials such as lithium cobalt oxide (LCO), lithium iron phosphate (LFP), lithium manganese oxide (LMO), lithium nickel-manganese-cobalt (NMC),andlithiumnickel-cobalt-aluminumoxide(NCA).The majority of LIBs in the world nearly 95% are made in AsiannationsincludingChina,Taiwan,SouthKorea,Japan, andSouthKorea.However,duetoitslimitedenergycapacity andunreliableoperation,Grhasbeenwidelyemployedasan anode material for LIBs and was unable to meet the requirements.
Incontrast,anumberofanodematerialshavebeen extensivelyresearchedandattemptedtoreplaceGr.These materialsincludesilicon,tin,simplebinarytransitionmetal oxides, even Li metal for solid-state batteries, and particularlybiomass-derivedcarbonmaterialswithspecial morphologiesandstructuresbecausetheytypicallyexhibit highspecificcapacity[25,26].
1.2 Batteries of Various type:
There are two basic types of batteries:
1. Primarybatteries
2. Secondarybatteries
1. Primary batteries: Primarybatteriesare“singleuse”andcannotberecharged[27].
Example:
AlkalineBatteries.
Lead-acidBatteries.
Ni-MHBattery.
Ni-CdBattery.
Li-Pobattery.
Alkaline battery:
Example: Dcells,Ccells,AACells,AAACells,AAAA cells,NCells,9Voltcells,ButtonCells.
Lead-acid Batteries:
Example: Flooded Acid, Gelled Acid, Advanced AGM (AbsorbedGlassMat).
Ni-MH Battery: NiMHhydridebatteriesareusedin. Example Batteries for hybrid cars, electric razors and toothbrushes,cameras,camcorders,phones,pagers,anda widerangeofotherdevicesarejustafewexamples.
Ni-Cd Battery:
Example: Specialty Emergency lighting, cordless and wireless phones, and other devices all employ Ni-Cd
batteries. There are two different types of NiCd batteries: sealed and vented. Both have relatively low internal resistanceandmaydeliversignificantsurgecurrents[28].
Li-Po battery:
Example: LiPocellsprovidemanufacturerswithcompelling advantages.Theycaneasilyproducebatteriesofalmostany desired shape. For example, the space and weight requirementsofmobiledevicesandnotebookcomputerscan be met. They also have a low self-discharge rate, which is about5%permonth.
2. Secondary batteries: Asecondarybatteryisaportable voltaiccellthatisrechargeable[29].
Examples:
Nickel-cadmium(NiCd)
Leadacid,andlithium-ionbatteries.
Nickel-cadmium (NiCd): Thenickel-cadmiumbattery (commonly abbreviated NiCd or NiCad) is a type ofrechargeable battery using nickel oxide hydroxide andmetalliccadmiumaselectrodes.Theabbreviation NiCadisaregisteredtrademarkofSAFTCorporation, althoughthisbrandnameiscommonlyusedtodescribe allnickel-cadmiumbatteries.
Lead acid, and lithium-ion batteries: Lead acid batteriesarecurrentlyproducedinthreedifferentforms, andeachtypecanbedesignedandconstructedforeither starting or deep cycle uses. These varieties include AdvancedAGM(AbsorbedGlassMat),FloodedAcid,and GelledAcid.
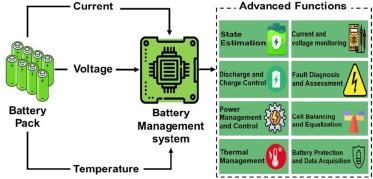
Battery Management System:
Inordertopreventanydetrimentaleffectsonthe power-intakeprofile,abattery-managementsystem(BMS) recognizes unexpected circumstances and confirms the appropriate strategy for controlling the battery's temperaturebehavior.BMSisessentialtothereliableand effective operation of batteries. It makes use of state estimation,avastareaofstudy.
Theconsequencesofoperationsatvariousstates, powerrequirements,temperatures,andhealthstatesshould be minimized via BMS design. It often uses model base estimation,which,inordertofunctioneffectively,needsa precisebatterymodelandastrongestimatingapproach.A well-designed BMS is necessary to support the safety, dependability, and overall performance of lithium-ion batterysystemssinceLIBschargefasterthanconventional batterytechnologies[30].
AI strategies in battery management systems:
Awell-designedBMSiscrucialtosupportthesafety, dependability, and overall performance of lithium-ion batterysystemssinceLIBschargefasterthanconventional

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 11 Issue: 03 | Mar 2024 www.irjet.net p-ISSN: 2395-0072
batterytechnologies.DuetothefactthataLi-ionbatteryisa highlytime-variant,non-linear,andcomplexelectrochemical system,itisdifficulttoestimatetheSOCofaLi-ionbattery accurately.Thedirectmeasurementapproach,bookkeeping estimation method, model-based method, and computer intelligencemethodarethefourmajorcategoriesintowhich the SOC estimating methods have been divided. Their benefits, shortcomings, and estimation mistakes from previousstudiesarediscussedcritically.Toenhanceonline estimation,certainsuggestionsaremadedependingonthe advancementoftechnology[31].
The BMS is in charge of cell balance, thermal management,safety,andtheSOC,SOH,SOP,andremaining usablelife(RUL)ofthebatterypack.
A battery is controlled by a software and hardware system known as a battery management system (BMS) in order to work properly [32]. A BMS is composed of numerousfunctionalcomponents,includingastatemachine, temperaturemonitors,areal-timeclock,acut-offfieldeffect transistor,acellvoltagemonitor,andacellvoltagebalance [33]. The market is flooded with many types of BMSintegrated chips. For various systems, the functional componentsarearrangeddifferently;theycouldbeanything from a straightforward analogue front end with a microcontroller capable of balancing and monitoring to a stand-alonefullyautomated.
There are many different actuators, controllers, and sensors that the BMS in EVs may use. Batteries must be protected, operated within reasonable limits of voltage, current, and temperature, and have their characteristics correctly monitored by BMSs [34]. Modular architectures, centralisedsystems,anddistributedsystemshaveallbeen employed in hardware architecture. For maintaining and monitoring a battery's status, Richter and Meissner presented a layer structure [35]. According to Gold, the various functionalities of BMSs can be used to categorise them.Theseideascouldbeappliedtodevelopasubstantial frameworkwithfundamentalfunctionalit
Dataisgatheredatthemonitoringlayerbyseveralsensors housed within the battery pack. The vehicle control processor and each component of the battery pack are connected to the BMS. BMSs have a long history of using safety[36].
Cell balancing is done to improve battery performance withoutoverchargingandover-discharging;itaimstogroup cellswithcomparable SOClevels.Basedon the amount of charge each cell can hold, the controller will use a sophisticatedprogrammetogovernthechargingprocess.A preciseSOCcalculationofeachcellisthusacrucialpartof improving balance. Smart data evaluation is necessary to send battery fault warnings and detect out-of-tolerance conditions.
Historical data will be preserved and used to display the circumstancespriortoanalarmgoingoff[37].Usersmust haveaccesstothecrucial BMSdata through theinterface. Theremainingrangeoughttobeshownonthedashboard basedonthebattery'sSOC.Throughalarmsandreplacement suggestions,theconsumersmustalsobeinformedaboutthe predictionandestimationoftheaforementionedbattery.
The functionality of the BMS can be divided into the followingcategories.
• Defence: againsthighorlowtemperatures,overcharging, overdischarging,overcurrent,andshortcircuits.
• High-voltage ControlandSensing:monitortemperature, voltage,current,thermalcontrol,contactor,pre-charge,and groundfaultdetection.
• Diagnostics: estimations of state-of-life (SOL), state-ofhealth(SOH),andabusedetection.
Performance management, including computation of the powerlimit,cellbalancing/equalization,andSOCestimation.
•Interface:rangeestimation,reporting,datarecording,and communications.
3. Efficiency, Capacity, And Lifespan Materialand Thermal Characteristics:
Efficiency: Comparedtotheaverageleadacidbattery's85% efficiency, lithium batteries charge with a nearly 100% efficiency. This is particularly crucial when using solar power to charge because you want to get the most out of eachampbeforethesunsetsorisobscuredbyclouds[39]. Theyalsohaveanexcellenthigh-temperatureperformance, a high power-to-weight ratio, great energy efficiency, and minimalself-discharge.Themajorityoflithium-ionbattery partscanberecycled;however,thisisstillacostlyprocess fortheindustry[40,41].

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 11 Issue: 03 | Mar 2024 www.irjet.net p-ISSN: 2395-0072
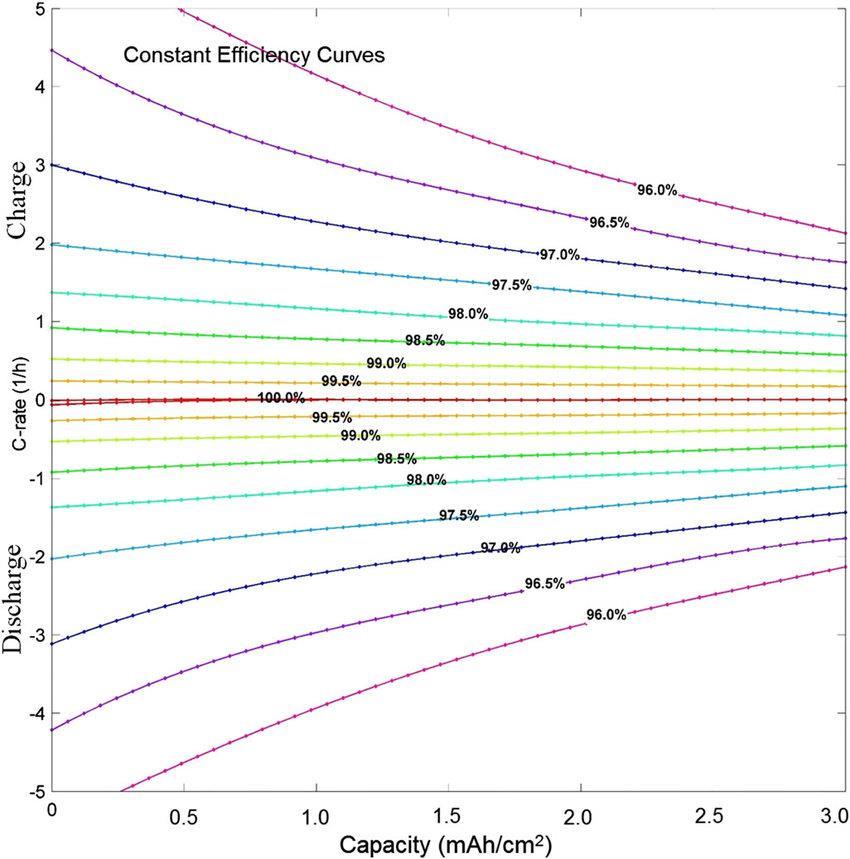
Fig. 4. Energyefficiencymapofatypicallithium-ionbattery family with a graphite anode and lithium iron phosphate (LFP)cathodethatoperatesatatemperatureof25°Candis chargedanddischargedwithinastate-of-chargeintervalof unity (SOC = 1). The color curves are constant energy efficiencycurves.
Capacity: Thespecificcapacitiesofpositiveelectrodesinthe range of 150-200 mAh g-1, which are constrained by the degreeoflithiumintercalationintotransitionmetaloxides, accountforthemajorityofthelithium-ionsystem'sbattery capacity.
Thermal Characteristics: The most significant factor affectingUPSbatteryageingandthepotentialcauseofearly batteryfailureishighambienttemperature[43]. Highertemperaturesresultina quickerchemicalreaction within the battery, which accelerates corrosion and increaseswaterloss[44].
Accordingtocalculations,atypicallithium-ionbatteryhasa positive electrode thermal conductivity of 5 W/(m/K), a separator thermal conductivity of 0.334 W/(m/K), and a negativeelectrodethermalconductivityof1.04W/(m/K)on average[45].
XRD(R-Hitachi,Tokyo,Japan)wasusedtoperformXraydiffraction.Transmissionelectronmicroscopy(TEM;J200C,Tokyo,Japan)andscanningelectronmicroscopy(SEM; J-6700, Tokyo, Japan) were used to analyse the materials' shape, size, and texture. The element distribution in the nanocomposites was investigated using Oxford-X energy dispersive X-ray spectroscopy (EDX) [46]. The thermogravimetricanalysis(TGA;STA409P-NETZSCH)was carriedoutinanairenvironmentwithanidealheatingrate (10°C/min),whileRamanspectroscopywasdoneusing a Renishaw-X Plus source. Utilizing [47], the chemical compositionandbondingofthesynthesizedmaterialswere verified.
The fabricated anode materials were characterized using electrochemical impedance studies (EIS; CHI660D, 0.01Hz-100 kHz, 5 mVs1) and Fourier-transform infrared spectroscopy(FTIR;IS50FT-IR).Thespecificsurfacearea was estimated using Brunauer Emmett Teller (BET; ASAP 2020M+CMICROMERITICS),andtheporesizedistribution was determined using the Barrett-Joyner-Halenda (BJH) method, which is based on N2 adsorption desorption isotherms[48].
Lithium-Ion Battery Features:
Size, fit, and performance of lithium-ion batteries can be tailored to the needs of the customer. The energy density of lithium-ion batteries is high (weight to volume ratio)[49].
Cellular voltage: The nominal voltage of lithium-ion batteriesis3.7volts per cell.Abattery pack canhaveany voltageinstepsof3.7voltsbyusingthecellsinseries.For instance,lithium-ionbatteriescanproduceabatteryof11.1 voltsusing3cells,14.8voltsusing4cells,and37voltsusing 10cells[50].
Lithium-Ion cells are connected in parallel to provide the necessary amp-hours (Ah). Depending on the needsoftheapplication,theAhsmightbeintherangeofa few amps to hundreds of amps. For instance, lithium-ion batteriescancreate7.8Ahbyconnectingthree2.6Ahcellsin parallelor26Ahbyconnectingten2.6Ahcellsinparallel. NumerouscellswithahighAhratingareavailablethatcan be utilized to give you the CAPACITY needed for your application[51].
Lithium-Ionhasanominalmaximumchargerateof 1C,whilelithium-polymerhasanominalmaximumcharge rateof2C.Therearecellswithupto10Cchargerates.You can create a Lithium-Ion battery pack that satisfies your needsbychoosingtherightcell.
Charge Technology: Only a charger made specifically for lithium-ion batteries with a normal Pack Protect circuit

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 11 Issue: 03 | Mar 2024 www.irjet.net p-ISSN: 2395-0072
should be used because those batteries have a complex chargingprofile.However,ifyouusetheSWEBMS(Battery Management System) with the built-in pulse charging, all you'llneedtochargethebatterypackisastraightforward DCpowersupplywithcontinuouspoweroryoumayconnect it directly to a solar panel using nothing but an isolation diode.Inotherwords,thesystemislessexpensivebecausea separateLi-Ionchargerisnotrequired.
Lithium-Ionbatterieshaveamaximumdischargerateof2C, whereas lithium-polymer batteries have a maximum dischargerateof3C(butsomelithium-polymercellshave dischargerateshigherthana30Crate)[53].
Discharge Temperature Range: Themaximumdischarge temperatureforlithium-ionandlithium-polymerisbetween -20Cand60C.Theenhancedlimitfordischargingdownto50°C has been chosen by SWE using empirical data and chemistry[54].
Storage: Recommended temperature range for storage:20°Cto60°C(storageattemperaturesbelow20°Creduces permanentcapacityloss).Recommendedvoltagerangefor shorttermstorageis3.0to4.2Vpercellinseries.storage periods:StoreLi-Ionbatteriesatabout75%capacity(3.85V to 4.0 V) and at low temperature to reduce permanent capacitylossoverlongstorageperiods[55].
5. Conclusions:
This work aimed to review batteries are fundamentallyastoragemediuminthebasicinformationthe introductionandtheworkingprincipaloftheli-ionbattery. In this study of the li-ion batteries and most advanced rechargeable batteries. They are currently the dominant mobile power sources for portable electronic devices, exclusively used in cell phones and laptop computers and sowfigsowinDemandforLi-ionbatteriesintwodecades Reproduced with permission. There are various type batteries, example and Battery Management System, AI strategiesinbatterymanagementsystems.Accordingtothe BMS's functionality may be categorized as followsProtection, High-voltage Control and Sensing, Diagnostics, Performance management, Interface, Capacity, Thermal Characteristics, Material Characterization. There are the Lithium-IonBatteryFeatures-VoltagePerCell,Capacity,Max Charge Rate, Charge Technology, Max Discharge Rate, DischargeTemperatureRange,Storage
Acknowledgements:
We wouldliketoexpressoursinceregratitudeto [Department of Physics, Institute of Basic Science, Dr Bhimrao Ambedkar University Agra.] for providing the necessaryresourcesandfacilitiestoconductthisresearch. Additionally,weextendourappreciationto[Prof.(Dr.)BP Singh (Dean Research)] for their invaluable guidance, support,andexpertisethroughoutthedurationofthisstudy.
Their mentorship has been instrumental in shaping the directionandoutcomesofthisresearchpaper.
References:
[1] B. Diouf and P. Ramchandra, "Potential of lithium-ion batteriesinrenewableenergy. “RenewableEnergyvol. 76, (2015),pp.375
[2] P Stephen, and R Socolow, "Stabilization wedges: solvingtheclimateproblemforthenext50yearswith currenttechnologies."sciencevol. 305.5686 (2004)pp. 968-972.
[3] Liu, Yuechen, et al. "A data-driven learning-based continuous-timeestimationandsimulationmethodfor energyefficiencyandcoulombicefficiencyoflithium-ion batteries."Energiesvol. 10.5 (2017)pp.597.
[4] Kane,N.Shashank,AshutoshMishra,andAnupK.Dutta. "Preface:Internationalconferenceonrecenttrendsin physics(ICRTP2016)."JPhysConfSer.Vol. 755, 2016, pp.1.
[5] M.Satalkar,N.Ghodke,andS.N.Kane."Influenceofhigh temperature sintering on the structural and magnetic Properties of Mn1− xZnxFe2O4." Journal of Physics: ConferenceSeries.Vol. 534,2014,pp.1.
[6] Han,Xuebing,etal."Acomparativestudyofcommercial lithium-ionbatterycyclelifeinelectricalvehicle:Aging mechanismidentification."Journalofpowersourcesvol. 251 (2014),pp.38-54.
[7] Han,Xuebing,etal."Acomparativestudyofcommercial lithium-ionbatterycyclelifeinelectricvehicle:Capacity loss estimation." Journal of Power Sources vol. 268, (2014),pp. 658-669.
[8] Faessler, Bernhard, "Stationary, second use battery energy storage systems and their applications: A researchreview."Energiesvol. 14.8,(2021),pp.2335.
[9] Xie, Yingchen, et al. "Inhomogeneous degradation inducedbylithiumplatinginalarge-formatlithium-ion battery."JournalofPowerSourcesvol. 542 (2022),pp. 231753.
[10] Ren,Dongsheng,etal."Acomparativeinvestigationof agingeffectsonthermalrunawaybehavioroflithiumion batteries." ETransportation vol. 2, (2019), pp. 100034.
[11] Coduri, Mauro, et al. "Rare earth doped ceria: The complexconnectionbetweenstructureandproperties." Frontiersinchemistryvol 6 (2018),pp.526.
[12] Comings, E. David, and K Blum, "Reward deficiency syndrome: genetic aspects of behavioral disorders." Progressinbrainresearchvol. 126,(2000)pp.325-341.

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 11 Issue: 03 | Mar 2024 www.irjet.net p-ISSN: 2395-0072
[13] E. Gray, MacA, et al, "Hydrogen storage for off-grid power supply." International Journal of Hydrogen Energyvol. 36.1,(2011),pp. 654-663.
[14] Xia, Y., et al, "The ABA-1 allergen of Ascaris lumbricoides:sequencepolymorphism,stageandtissuespecificexpression,lipidbindingfunction,andprotein biophysicalproperties."Parasitologyvol. 120.2, (2000), pp.211-224.
[15] Erdinc, Ozan, B. Vural, and M. Uzunoglu, "A dynamic lithium-ion battery model considering the effects of temperature and capacity fading." International ConferenceonCleanElectricalPower.IEEE,2009.
[16] Ghorbanzadeh,Milad,M Astaneh,andF Golzar,"Longterm degradation-based analysis for lithium-ion batteries in off-grid wind-battery renewable energy systems."Energyvol. 166,(2019),pp. 1194-1206.
[17] Alvira, Darío, D Antorán, and J. Joan, Manyà, "Plantderivedhardcarbonasanodeforsodium-ionbatteries: A comprehensive review to guide interdisciplinary research."ChemicalEngineeringJournalvol. 56, (2022), pp.137468.
[18] D. Reis, G Simões, et al, "Flexible supercapacitors of biomass-basedactivatedcarbon-polypyrroleoneggshell membranes." Journal of Environmental Chemical Engineeringvol. 9.5,(2021),pp. 106155.
[19] Reis, G S dos, et al, "A short review on the electrochemical performance of hierarchical and nitrogen-doped activated biocarbon-based electrodes forsupercapacitors."Nanomaterials vol. 11.2,(2021), pp. 424.
[20] Adeyemo, A. Ayotunde and T. Olumuyiwa, Amusan, "Modellingandmulti-objectiveoptimizationofhybrid energy storage solution for photovoltaic powered offgridnetzeroenergybuilding."JournalofEnergyStorage vol. 55,(2022),pp.105273.
[21] Guy, Marine, et al, "Process parameters optimization, characterization, and application of KOH-activated Norwaysprucebarkgraphiticbiocharsforefficientazo dyeadsorption."Moleculesvol. 27.2,(2022),pp.456.
[22] K.K.Constanti,etal,"Physicalchangeinpositive-plate material-an underrated contributor to premature capacityloss."Journalofpowersourcesvol.55.2(1995), pp.269-275.
[23] G. C. Zguris, "A review of physical properties of separators for valve-regulated lead/acid batteries." Journalofpowersources vol. 59.1-2,(1996),pp.131135.
[24] Hush,S.Noel,"Intervalence‐transferabsorption.Part2. Theoretical considerations and spectroscopic data." ProgressinInorganicChemistry(1967),pp. 391-444.
[25] A.Barroso-Bogeat, etal,"Temperaturedependenceof theelectricalconductivityofactivatedcarbonsprepared from vine shoots by physical and chemical activation methods."MicroporousandMesoporousMaterialsvol. 209,(2015),pp.90-98.
[26] Zhang,Yongzhi,etal,"Sodiumstorageinfluorine-rich mesoporous carbon fabricated by low-temperature carbonization of polyvinylidene fluoride with a silica template."RSCadvancesvol. 6.112, (2016),pp.110850110857.
[27] Chen,Kaixuan,etal,"Four-channelCWDMtransmitter chip based on thin-film lithium niobate platform." Journal of Semiconductors vol. 43.11, (2022), pp. 112301.
[28] Microcrystalline, Nanocrystalline, M. Masoud, and P. Heitjans. "Impedance Spectroscopy Study of Li lon DynamicsinSingleCrystal."
[29] Heitjans, Paul, et al, "NMR and impedance studies of nanocrystallineandamorphousionconductors:lithium niobate as a model system." Faraday discussions vol. 134,(2007),pp.67-82.
[30] Krishna,Gopal,etal,"Digitaltechnologyimplementation inbattery-managementsystemsforsustainableenergy storage: Review, challenges, and recommendations." Electronicsvol. 11.17,(2022),pp.2695.
[31] Ali, M. Umair, et al, "Towards a smarter battery managementsystemforelectricvehicleapplications:A critical review of lithium-ion battery state of charge estimation."Energiesvol. 12.3,(2019),pp.446.
[32] Lelie, Markus, et al, "Battery management system hardwareconcepts:Anoverview."AppliedSciencesvol. 8.4,(2018),pp.534.
[33] Uzair, Muhammad, G Abbas, and S Hosain, "Characteristics of battery management systems of electric vehicles with consideration of the active and passivecellbalancingprocess."WorldElectricVehicle Journalvol. 12.3,(2021),pp.120.
[34] Balasingam, Balakumar, M Ahmed, and Krishna Pattipati."Batterymanagementsystems-Challengesand somesolutions."Energiesvol.13.11,(2020),pp.2825.
[35] Meissner,Eberhard,andG Richter,"Batterymonitoring and electrical energy management: Precondition for futurevehicleelectricpowersystems."Journalofpower sourcesvol. 116.1-2,(2003),pp.79-98.

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 11 Issue: 03 | Mar 2024 www.irjet.net p-ISSN: 2395-0072
[36] Merei,Ghada,C.Berger,andD.U.Sauer,"Optimization of an off-grid hybrid PV–Wind–Diesel system with differentbatterytechnologiesusinggeneticalgorithm." SolarEnergyvol. 97,(2013),pp.460-473.
[37] Xing, Yinjiao, et al, "Battery management systems in electricandhybridvehicles."Energiesvol. 4.11,(2011), pp.1840-1857.
[38] Troyk,R.Philip,andM A K Schwan,"Closed-loopclass E transcutaneous power and data link for micro implants."IEEETransactionsonBiomedicalEngineering vol. 39.6,(1992),pp.589-599.
[39] B. Jacobson and L. Nordberg., "Endoradiosondes for pressuretelemetering."IREtransactionsonbio-medical electronicsvol 8.3,(1961),pp.192-196.
[40] J. Nagumo et al, "Echo capsule for medical use (a batterylessendradiosonde)."IRETransactionsonBioMedicalElectronicsvol. 9.3,(1962),pp.195-199.
[41] Merei,Ghada,C.Berger,andD.U.Sauer,"Optimization of an off-grid hybrid PV–Wind–Diesel system with differentbatterytechnologiesusinggeneticalgorithm." SolarEnergyvol. 97,(2013),pp.460-473.
[42] Mackay,R.Stuart,"Biomedicaltelemetry:theformative years." IEEE Engineering in Medicine and Biology Magazinevol. 2.1,(1983),pp.11-17.
[43] Tong,S Jie,etal,"Off-gridphotovoltaicvehiclecharge usingsecondlifelithiumbatteries:Anexperimentaland numerical investigation." Applied energy vol. 104, (2013),pp.740-750.
[44] Marchetti,Dedaloetal,"QuickReportontheML=3.3on 1 January 2023 Guidonia (Rome, Italy) Earthquake: EvidenceofaSeismicAcceleration."RemoteSensingvol. 15.4,(2023),pp.942.
[45] Jung, Wongwan, etal,"Optimizationofhybridoff-grid systemconsistingofrenewablesandLi-ionbatteries." JournalofPowerSourcesvol. 451,(2020),pp.227754.
[46] Viole,Isabelle,etal,"Arenewablepowersystemforan off-grid sustainable telescope fueled by solar power, batteriesandgreenhydrogen."Energyvol. 282, (2023), pp.128570.
[47] Bai, Yao, et al, "Study on the COVID-19 epidemic in mainlandChinabetweenNovember2022andJanuary 2023, with prediction of its tendency." Journal of BiosafetyandBiosecurityvol. 5.1, (2023),pp.39-44.
[48] Penisa,N.Xavieryetal,"Projectingthepriceoflithiumion NMC battery packs using a multifactor learning curvemodel."Energiesvol. 13.20, (2020),pp.5276.
[49] Yi, Yikun, et al, "Progress and Prospect of Practical Lithium-Sulfur Batteries Based on Solid-Phase Conversion."Batteriesvol. 9.1,(2022),pp.27.
[50] Sun,Jiaxun,etal,"Riskassessmentanddrivingfactorsof trace metal (loid) s in soils of China." Environmental Pollutionvol. 309, (2022),pp.119772.
[51] Ma,Cong,P Fang,andTian-ShengMei,"Recentadvances in C–H functionalization using electrochemical transitionmetalcatalysis."AcsCatalysisvol. 8.8, (2018), pp.7179-7189.
[52] Ren,Yilun,etal,"Synergisticadsorption-electrocatalysis of2D/2DheterostructuretowardhighperformanceLi-S batteries." Chemical Engineering Journal vol. 439, (2022),pp.135535.
[53] Frey,Martin,etal,"Easilyaccessible,textilefiber-based sulfurizedpoly(acrylonitrile)asLi/Scathodematerial: correlating electrochemical performance with morphologyandstructure."ACSEnergyLettersvol. 2.3 (2017),pp. 595-604.
[54] Liu, Ying, et al, "Freestanding porous sulfurized polyacrylonitrile fiber as a cathode material for advanced lithium sulfur batteries." Applied Surface Sciencevol. 472,(2019),pp.135-142.
[55] Zhang, Yunyang, et al, "Se as eutectic accelerator in sulfurized polyacrylonitrile for high performance allsolid-state lithium-sulfur battery." Energy Storage Materialsvol. 21,(2019),pp.287-296.