HOSPITAL PROFESSIONAL NEWS IRELAND
Ireland’s Dedicated Hospital Professional Publication
Legal Category: Product subject to prescription which may be renewed (B).
Marketing Authorisation Number: EU/1/12/795/002; EU/1/12/795/007.
Marketing Authorisation Holder: AstraZeneca AB, SE-151 85 Södertälje, Sweden. Further product information available on request from: AstraZeneca Pharmaceuticals (Ireland) DAC, College Business and Technology Park, Blanchardstown Road North, Dublin 15. Tel: +353 1 609 71 00.
FORXIGA is a trademark of the AstraZeneca group of companies.
Veeva ID: IE-4697 Date of Prep: February 2023
IN THIS ISSUE:
NEWS: Ireland wins Medical World Cup Page 5
HOSPITAL
PHARMACY: Antimicrobial Resistance Position Paper Page 8
FEATURE: Vitamin D Intake and Status in Ireland
Page 14
MEDICINES: Opioid Prescribing Updates
Page 24
ONCOLOGY FOCUS: National Cancer Mission Hub Page 26
ONCOLOGY FOCUS: Prostate Cancer Page 36
STUDY: HIV Viral Suppression Page 70
HPN September 2023 Issue 112 HOSPITALPROFESSIONALNEWS.IE This Publication is for Healthcare Professionals Only
This is a promotional advertisement from LEO Pharma for IE healthcare professionals.

For the treatment of moderate to severe atopic dermatitis in adult and adolescent patients 12 years and older who are candidates for systemic therapy1

TIME TO PRESS PLAY
Prescribing Information for Adtralza® (tralokinumab) 150 mg solution for injection in pre-filled syringe
Please refer to the full Summary of Product Characteristics (SmPC) (www.medicines.ie) before prescribing.
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. Indications: Treatment of moderate-tosevere atopic dermatitis in adult and adolescent patients 12 years and older who are candidates for systemic therapy. Active ingredients: Each pre-filled syringe contains 150 mg of tralokinumab in 1 mL solution (150 mg/mL). Dosage and administration: Posology: The recommended dose of tralokinumab for adult and adolescent patients 12 years and older is an initial dose of 600 mg (four 150 mg injections) followed by 300 mg (two 150 mg injections) administered every other week as subcutaneous injection. Every fourth week dosing may be considered for patients who achieve clear or almost clear skin after 16 weeks of treatment. Consideration should be given to discontinuing treatment in patients who have shown no response after 16 weeks of treatment. Some patients with initial partial response may subsequently improve further with continued treatment every other week beyond 16 weeks. Tralokinumab can be used with or without topical corticosteroids. The use of topical corticosteroids, when appropriate, may provide an additional effect to the overall efficacy of tralokinumab. Topical calcineurin inhibitors may be used, but should be reserved for problem areas only, such as the face, neck, intertriginous and genital areas. If a dose is missed, the dose should be administered as soon as possible and then dosing should be resumed at the regular scheduled time. No dose adjustment is recommended for elderly patients, patients with renal impairment or patients with hepatic impairment. For patients with high body weight (>100 kg), who achieve clear or almost clear skin after 16 weeks of treatment, reducing the dosage to every fourth week might not be appropriate. The safety and efficacy of tralokinumab in children below the age of 12 years have not yet been established. Method of administration: Subcutaneous use. The pre-filled syringe should not be shaken. After removing the pre-filled syringes from the refrigerator, they should be allowed to reach room temperature by waiting for 30 minutes before injecting. Tralokinumab is administered by subcutaneous injection into the thigh or abdomen, except the 5 cm around the navel. If somebody else administers the injection, the upper arm can also be used. For the initial 600 mg dose, four 150 mg tralokinumab injections should be administered consecutively in different injection
IL, interleukin.

sites within the same body area. It is recommended to rotate the injection site with each dose. Tralokinumab should not be injected into skin that is tender, damaged or has bruises or scars. A patient may self-inject tralokinumab or the patient’s caregiver may administer tralokinumab if their healthcare professional determines that this is appropriate. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Precautions and warnings: If a systemic hypersensitivity reaction (immediate or delayed) occurs, administration of tralokinumab should be discontinued and appropriate therapy initiated. Patients treated with tralokinumab who develop conjunctivitis that does not resolve following standard treatment should undergo ophthalmological examination. Patients with pre-existing helminth infections should be treated before initiating treatment with tralokinumab. If patients become infected while receiving tralokinumab and do not respond to antihelminth treatment, treatment with tralokinumab should be discontinued until infection resolves. Live and live attenuated vaccines should not be given concurrently with tralokinumab. Fertility, pregnancy and lactation: There is limited data from the use of tralokinumab in pregnant women. Animal studies do not indicate direct or indirect harmful effects with respect to reproductive toxicity. As a precautionary measure, it is preferable to avoid the use of tralokinumab during pregnancy. It is unknown whether tralokinumab is excreted in human milk or absorbed systemically after ingestion. Animal studies did not show any effects on male and female reproductive organs and on sperm count, motility and morphology.
Side effects: Very common (≥1/10): Upper respiratory tract infections. Common (≥1/100 to <1/10): conjunctivitis, conjunctivitis allergic, eosinophilia, injection site reaction. Uncommon (≥1/1,000 to <1/100): keratitis. Precautions for storage: Store in a refrigerator (2°C-8°C). Do not freeze. Store in the original package in order to protect from light. Legal category: POM. Marketing authorisation number and holder: EU/1/21/1554/002. LEO Pharma A/S, Ballerup, Denmark. Last revised: October 2022
Reference number: REF-22407

Reporting of Suspected Adverse Reactions
Adverse events should be reported.
Reporting forms and information can be obtained from: HPRA Pharmacovigilance, Website: www.hpra.ie
Adverse events should also be reported to Drug Safety at LEO Pharma by calling +353 1 4908924 or e-mail medical-info.ie@leo-pharma.com
References: 1. Adtralza® SPC. 2. Duggan S. Drugs 2021;81(14):1657–1663. 3. Bieber T. Allergy 2020;75:54–62.
Date of preparation: December 2022
more at www.adtralza.ie Adtralza®
Learn
biologic
The first
licensed
that inhibits IL-13 alone, 1,2 a
key driver of atopic dermatitis signs and symptoms.3
IE/MAT-62263 Further information can be found in the Summary of Product Characteristics or from: LEO Pharma, Cashel Road, Dublin 12, Ireland. E-mail: medical-info.ie@leo-pharma.com
® Registered trademark
Not an actual patient. For illustrative purposes only. Individual results may vary.
Medical Drone delivery service starts in Ireland P4

Electronic Health Record System gains Investment P6
New discovery in Epilepsy Treatment P7
New Options for Prostate Surgery P12
Moderate-to-Severe Atopic Dermatitis P18
HIV Viral Suppression P70
Smart Devices in Diabetes Management P72
REGULARS
Oncology Focus: Cervical Cancer P41


Oncology Focus: Bladder Cancer P47
Oncology Focus: Non-Small Cell Lung Cancer P54
Feature: Osteoporosis P74
Editor
In one of our lead news stories, Bon Secours Health System (BSHS) has launched a groundbreaking clinical transformation project with healthtech firm MEDITECH which is to connect its entire hospital network using one electronic healthcare record (EHR).
The new 'BonsConnect' initiative, which will be the largest private hospital EHR system in Ireland, will improve patient safety, enhance clinical care, increase operational efficiency, and revolutionise the healthcare experience for the 300,000 patients Bon Secours treats annually.

Turn to page 6 to read the full story.
Meanwhile on page 8, we detail a recent Position Paper published by the European Association of Hospital Pharmacists on Infectious Diseases and Antimicrobial Resistance. Combating infectious diseases requires the implementation of a comprehensive intervention package comprised of measures including but not limited to prudent anti-infective use, vaccination and stewardship.
The Position Paper calls on national governments and health system managers to utilise the specialised background and knowledge of hospital pharmacists in multi-professional antimicrobial stewardship teams or other forms of antimicrobial governance in the hospital and in the community. It also underlines that hospital pharmacists are an integral part of the transfer of care to ensure that care could continue when patients leave the hospital.
Opioid analgesics are highly effective in the management of acute severe pain. Dr Cormac Mullins, Consultant in Anaesthesia and Pain Medicine at Cork University Hospital and South Infirmary Victoria University Hospital gives readers an overview of Opioid Prescribing from the recent updated CDC Prescribing Guidelines in this issue.
Hospital Professional News is a publication for Hospital Professionals and Professional educational bodies only. All rights reserved by Hospital Professional News. All material published in Hospital Professional News is copyright and no part of this magazine may be reproduced, stored in a retrieval system or transmitted in any form without written permission. IPN Communications Ltd have taken every care in compiling the magazine to ensure that it is correct at the time of going to press, however the publishers assume no responsibility for any effects from omissions or errors.
PUBLISHER
IPN Communications Ireland Ltd

Clifton House, Lower Fitzwilliam Street, Dublin 2 (01) 669 0562
GROUP DIRECTOR
Natalie Maginnis n-maginnis@btconnect.com
EDITOR
Kelly Jo Eastwood
EDITORIAL
editorial@hospitalprofessionalnews.ie
ACCOUNTS
Fiona Bothwell cs.ipn@btconnect.com
SALES EXECUTIVE
Avril Boyd avril@hospitalprofessionalnews.ie
SALES & TRAINING MANAGER
Swan Mude s.mude@hospitalprofessionalnews.ie
CONTRIBUTORS
Helena Scully | Eamon Laird
Kevin McCarroll | Martin Healy
Paul Sweeney | Padraig Daly
Dr Jason McGrath | Dr Gordon Daly

Mr Luke Cox | Dr Damir Vareslija
Amy Nolan | Rebecca Gorman
Dr Roisin McAvera | Zekiye Altan
Dylan Harvey | Rory Johnson
Professor Ronan Cahill | Martina Phelan
Ciara O’Connor | Mr Faraz Khan
Therea Lowry-Lehnen | Dr Cormac Mullins
James Bernard Walsh
Professor Donal Brennan
Professor William Gallagher
Professor David Galvin
Professor Leonie Young
Professor Fergal Malone
DESIGN DIRECTOR
Ian Stoddart Design
‘Despite a lack of evidence for their benefit in chronic pain, their use has increased substantially in many developed countries, including in Ireland. Increased prescribing rates can lead to opioid-use disorder and opioid diversion, with many patients reporting not disposing of finished opioid prescriptions’ he notes. You can read the full article starting on page 24.
The Special Focus for September is on Oncology, with some excellent clinically contributed articles covering a wide area of cancers. These include Professor David Galvin, Paul Sweeney and Padraig Daly discussing ‘Life Limiting Prostate Cancer,’ Professor Ronan Cahill and Mr Faraz Khan, University College Dublin and the Mater Misericordiae University Hospital who write on ‘Handheld Robotic Systems for Minimally Invasive Surgery’ and Dr Roisin McAvera who authors an article on page 52 about ‘Extramedullary Multiple Myeloma.’
I hope you enjoy the issue.

3 HOSPITALPROFESSIONALNEWS.IE | HPN • SEPTEMBER 2023 September Issue Issue 112 4
Contents Foreword
Clinical R&D: P80 12 7 72 HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication HOSPITALPROFESSIONALNEWS.IE @HospitalProNews HospitalProfessionalNews
Medical Drone Delivery Service in Ireland
Speed and timeliness are crucial when delivering vital medical and pharmaceutical items to patients and hospitals. Wing and Apian, a United Kingdom-based company connecting healthcare providers with drone operators and services, have partnered to begin medical drone delivery services in Ireland and jointly explore opportunities in the UK. We expect to begin working with hospitals and other care providers in Dublin later this year.
Over the coming months, Wing and Apian will work with healthcare and pharmacy partners to create a rapid medical delivery network in South Dublin. This efficient network, leveraging Wing’s aircraft and automation, will serve to improve the patient experience while reducing traffic congestion and emissions in the community. We look forward to engaging with the local community and stakeholders as we develop the operations and begin flights.
Wing’s highly automated delivery drones are well-suited for a range of useful healthcare applications; our technology is designed to deliver pharmacy items, laboratory samples, and medical devices and supplies very quickly in urban and suburban environments.

Together, Wing and Apian believe that healthcare should benefit from on-demand delivery much like consumers do in their personal lives. Medical drone delivery can provide a faster, more reliable, lower-cost solution than ground-based alternatives. We aim to address speed, inefficiencies, and also environmental challenges by reducing vehicles on the road. Wing’s operations require very little infrastructure and can be set
up in a range of spaces, making them suitable for a wide variety of healthcare facilities.
Regulatory progress in the European Union and the UK continues to open doors toward safe and beneficial drone services at scale. This operation will be Wing’s second in Ireland, following the public demonstration services in Lusk, where thoughtful input and the collaborative approach from the community have provided
Bon Secours open New Wards
invaluable feedback on the future of drone delivery. At Wing, we also look forward to future opportunities in the UK after years of collaboration with regulators and contributions to numerous policy forums.
Drone delivery in healthcare has a tremendous opportunity for scale, both in operational service and in benefits delivered to patients and providers. We’re pleased to invest in these outcomes alongside Apian.
patient centric, technologically advanced care to patients from North Dublin and further afield.
The new wards represent a ¤14 million investment and are part of Bon Secours Health System's ambitious ¤300 million national investment, aligned with their 2025 Strategic Plan.
Bill Maher, BSHS Group Chief Executive, highlighted that this expansion is part of a larger plan to upgrade our services continuously. Bon Secours Health System has consistently strived to combine the latest medical technologies with compassionate and personalized care, ensuring our patients receive the exceptional care they deserve. We remain dedicated to meeting the evolving needs of our patients and communities sustainably.
Sharon Morrow, Chief Executive of Bon Secours Hospital Dublin, underlined the significance of this milestone, as it enables us to deliver technologically advanced surgical and oncology services to patients in the region. Our commitment to advanced medicine and exceptional care remains unwavering as we prioritize patient well-being within a world-class and modern environment.

4 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Bon Secours Hospital recently welcomed Minister Paschal Donohoe to officially open the brand-new surgical and oncology day wards at the Bon
Secours Hospital Dublin. These new state-of-the-art facilities mark a significant milestone for healthcare in Dublin and increases the hospital’s capacity to provide
Minister Paschal Donohoe, Sharon Morrow and Bill Maher, Bon Secours Hospital Dublin
Lessons not being Learned
The Irish Hospital Consultants Association (IHCA) has commented on the publication of the independent report by the Mental Health Commission into the Child and Adolescent Mental Health Service (CAMHS).
The Association said, “The report published today by the Mental Health Commission, the scale and severity of the findings and recommendations must be taken as cause for serious action.
“Unfortunately, many of the findings are not surprising to those who are working within Ireland’s
mental health services on a day-to-day basis. We are trying to provide care in very complex and constrained conditions for young people who need care for urgent severe mental health crises – being able to ensure their timely assessment, care and follow-on monitoring is critical but incredibly challenging in the current environment.
“Time and time again, Consultants and others in the service have raised the serious concerns about staffing and capacity shortages and highlighted the impact this has
Hospital Pharmacy Congress
The next European Association of Hospital Pharmacists Congress will take place in Bordeaux, France, between 20 and 22 March 2024 and will focus on Sustainable healthcare – Opportunities & strategies.
The abstract submission is now open until 1 October 2023. All hospital pharmacists, other healthcare professionals, and scientists are strongly encouraged to submit their work for consideration by the EAHP Scientific Committee. If accepted, this could lead to the display at the annual EAHP Congress. The registrations for the Congress are also open and you can benefit from an Early Bird Rate.

on young people’s mental health and their ongoing care needs. Lessons are not being learned.
“Meanwhile, the growing deficits are stark. Currently CAMHS funding is approximately 0.63% of the overall Health Budget, at just ¤125.18m.
“This report confirms that the vast majority of CAMHS teams are significantly below the recommended staffing levels, some below 50% of recommended levels. Some of these services are missing a third of the required Psychiatry Consultants, as these
Ireland wins World Medical Cup
A team of medical doctors from Ireland have won the World Medical Football Championships.
The 11-strong Ireland team beat Britain 1-0 in Vienna to claim the crown after playing a gruelling six games in seven days.

They came out on top of 24 teams from around the world, with the winning goal scored by Dr Fergal Moran, nephew of Ireland football legend Kevin Moran.
Ireland’s team are a group of doctors of various specialities and grades who come together to compete under the Irish flag at the annual World Medical Football Championships. Their first venture into this long-running tournament (it began in Barcelona in 2004) came in Long Beach, USA in 2014. Since then they have competed in Barcelona, Leogang (Austria), Prague and Cancun.
The team also manage the longstanding annual Hospitals Cup competition, where teams from hospitals all over the country compete for the title. This tournament began as far back as 1948 and has been running consistently since. This year, teams from the Mater, St Vincent's,
the Federated Hospitals (St James & Tallaght), Cork, Waterford and Galway will compete for the title. The team says, “We seek to promote the value of team sport
permanent posts remain vacant or only filled on a temporary, agency, or locum basis.
“There are many strands that have to come together, involving all pillars of the health system –GPs, Consultants, Allied Health Professionals - to ensure these highly vulnerable patients are cared for as required.
“Our hope is that this report drives such a collaborative, whole-ofservice approach to ensuring an end-to-end care pathway under the oversight of dedicated clinical leadership for CAMHS.”
for physical & mental health to physicians, patients and the population at large, leading by example.”
The team of medical doctors from Ireland who won the World Medical Cup
5 HOSPITALPROFESSIONALNEWS.IE | HPN • SEPTEMBER 2023
News
¤25m investment in Electronic Health Record System
“BonsConnect is the next step in our digital journey and will change how care is delivered at our five hospitals, including our new hospital in Limerick, due to open in 2025”
Bon Secours Health System (BSHS) has launched a groundbreaking ¤25 million clinical transformation project with healthtech firm MEDITECH which is to connect its entire hospital network using one electronic healthcare record (EHR).
The new 'BonsConnect' initiative, which will be the largest private hospital EHR system in Ireland, will improve patient safety, enhance clinical care, increase operational efficiency, and revolutionise the healthcare experience for the
300,000 patients Bon Secours treats annually.
The new EHR project is part of a significant investment in state-ofthe-art equipment and technology within a wider ¤300 million national commitment by Bon Secours Health System as part of its 2025 Strategic Plan.
The BonsConnect project will create 30 new jobs in BSHS now, with a further 30 positions to be created in September. It will cover the full Bon Secours hospital

network in Cork, Galway, Limerick, Tralee, and Dublin, and is to be completed by the summer of 2025. To facilitate the execution of this project, Bon Secours Health Systems is partnering with Nordic, a global consultancy that exclusively supports healthcare systems to deliver digitally-enabled transformation. Leveraging their clinical expertise, extensive technical knowledge and strategic advice, they will play a crucial role in ensuring the project's successful implementation.
Steps to Eliminate Hepatitis C
This World Hepatitis Day, the HSE urged people at risk of Hepatitis C to order a free Hepatitis C test. Thousands of people at risk are now able to order a test to their home, as the HSE steps up its bid to eliminate the deadly disease.
The HSE National Hepatitis C Treatment Programme estimates that up to 3,000 people in Ireland may currently have the bloodborne virus, which infects the liver and if left untreated can cause serious and potentially life-threatening damage, leading to cirrhosis, possible liver failure and cancer – as well as a risk of spreading the disease to others.
Over 4,000 at-home Hepatitis C tests have been ordered and delivered since the HSE home test service went live in early April this year. The discreet, at-home tests are free to order online from www. hse.ie/hepc as part of the HSE’s Hepatitis C Treatment Programme, which has already treated over 7,000 people, 95 percent of whom are now cured.
The test involves a finger prick test, with a tiny blood sample dropped into a test tube, which is posted in a pre-paid envelope to a lab for analysis. Those who require follow up treatment will then be contacted and referred to a participating clinic or hospital.
Treatment for Hepatitis C is free, tablets are effective and welltolerated, with over 95% of people cured in as little as 8 to 12 weeks. The new home tests could help people unknowingly living with Hepatitis C to get a life-saving diagnosis and treatment sooner. The new self-test aim to reach people who may not be engaged with other services such as drug and alcohol support, as well as people who may have potentially been exposed to virus in the past, through previous injecting drug use, or they could have come into contact with infected blood through medical procedures, blood transfusions and blood
Bon Secours Health System
Group Chief Executive, Bill Maher, said, "Bon Secours Health System continues to be unrivalled in the quality of our service, combining the latest medical technologies and approaches with compassionate and personalised medical care. BonsConnect is the next step in our digital journey and will change how care is delivered at our five hospitals, including our new hospital in Limerick, due to open in 2025. This will lead to improved clinical decision-making, more efficient and accurate clinical documentation, and an increased level of access to the right information by the right person at the right time."
Helen Waters, Executive Vice President and Chief Operating Officer, MEDITECH, added, “We are excited that Bon Secours Health System has selected MEDITECH Expanse as the EHR to lead its digital transformation journey, and we look forward to supporting them in their mission to improve the health and wellbeing of their communities. Our new partnership provides an extraordinary opportunity to work together and use the latest technology to drive patient-centred care, improve patient access, and enhance the clinician experience while ensuring equitable access to quality care across the communities they serve.”
products, or equipment used in cosmetic services.
Professor Aiden McCormick, HSE Clinical Lead for the Hepatitis C Programme, said: “One of our biggest challenges is that as patient numbers get smaller, remaining Hepatitis C cases are harder to find and treat. Therefore, it’s vital that we offer a free, easy to access home test – especially for those who have been exposed to the virus but are reluctant to come forward. This latest tool is critical to ensuring more people can receive the treatment they need, or peace of mind, at the earliest opportunity. The results of these tests will help contribute to understanding the prevalence of Hepatitis C.”
6 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Molecule that could help Epilepsy Seizures
In a discovery that could lead to new treatments for epilepsy, a study led by RCSI University of Medicine and Health Sciences and FutureNeuro has identified a previously unknown ‘master controller’ of electrical signals in brain cells.
The study showed that the molecule could be a useful target for future medicines to help control seizures in epilepsy, which is one of the most common chronic brain diseases, affecting around 65 million people worldwide. Around one in three people with epilepsy have uncontrolled seizures despite being treated with currently available anti-seizure medication. The study, which was published in the journal Proceedings of the National Academy of Sciences of the United States of America (PNAS), took a broader look at what naturally controls how our brain cells communicate.
All of our brains need electrical signals to function. These signals begin with the entry of charged sodium ions into neurons through dedicated channels, which act like gates or doorways on the surface of the cells. In people with epilepsy these channels become overactive, leading to higher-frequency signals that generate seizures.
Calming effect
The ‘master controller’ molecule discovered by the team at RCSI and FutureNeuro, the SFI Research Centre for Chronic and Rare Neurological Disease, is called microRNA-335, a type of ribonucleic acid (RNA) that occurs naturally in our brain cells. By sticking to the messages that instruct how to assemble the channels, microRNA-335 lowers the amounts of sodium channels in brain cells.
In a series of experiments on human-derived cells growing in the lab, the study found that reducing the amount of microRNA-335 in neurons increased the amount of sodium ions that moved into the cells and caused the brain cells to become more excitable. This finding underscores the calming actions of microRNA-335.
The researchers also carried out early, pre-clinical tests in the lab using gene therapy that could deliver microRNA-335 specifically to neurons, and saw that it resulted in an encouraging reduction in seizures.
Finding this master-controller of sodium channels opens up opportunities to develop new treatments for controlling
Dr Mona Heiland, Postdoctoral Researcher, SFI FutureNeuro Centre and RCSI Department of Physiology and Medical Physics

seizures in epilepsy, according to researcher Dr Mona Heiland from FutureNeuro and RCSI Department of Physiology and Medical Physics.
“Overall, we think that microRNA-335 is acting as a regulator of brain excitability, and could be a potential new target for the treatment of drug-resistant epilepsies,” she said.
Seizure control
Professor David Henshall, Director of FutureNeuro and Professor of Molecular Physiology and Neuroscience at RCSI commented: “The discovery of this new potential treatment target opens up the possibility of long-lasting and improved seizure control for patients in the future.”
The highly collaborative international project involved
many partners, including the Interdisciplinary Nanoscience Centre at Aarhus University and Omiics, both in Denmark; the Epilepsy Center Frankfurt Rhine-Main at University Hospital Frankfurt in Germany; and the Department of Neuroscience, Physiology and Pharmacology at University College London. The research was funded by Science Foundation Ireland, Framework Programme 7 and the Welcome Trust.
Raising the Bar for Interventional Oncology
St. Vincent’s University Hospital (SVUH), a world-leading academic teaching hospital, is proud to announce its enrolment as an IASIOS Enrolled Centre, marking a significant milestone in its journey towards achieving IASIOS Accreditation in Interventional Oncology (IO).
IASIOS, the International Accreditation for Interventional Oncology Services, ensures the highest standards of quality and patient care in IO through its globally recognised accreditation programme. As a member of IASIOS, SVUH is committed to providing the highest quality care to its patients, aligning with internationally accepted standards.
IASIOS is based on the CIRSE Standards of Quality Assurance in Interventional Oncology, a document endorsed by the European Cancer Organisation (ECO) and 40 international societies. This enrolment demonstrates SVUH’s dedication

to delivering exceptional interventional oncology services.
Professor David Brophy, Consultant Diagnostic & Interventional Radiologist at St. Vincent’s University Hospital, said, “We are proud to join the IASIOS community as an Enrolled Centre. This is a significant milestone in our journey towards achieving IASIOS Accreditation. It reflects our unwavering dedication to providing the best possible care to our patients in the field of interventional oncology.”
SVUH extends its gratitude to Prof. David Brophy and the dedicated members of the Interventional Radiology Multidisciplinary Team for their invaluable contributions throughout the enrolment process. Their expertise and
commitment have played a pivotal role in this achievement.
As an IASIOS Enrolled Centre, SVUH becomes part of a global community of IO centres working collaboratively to develop and promote IO practice, while raising
awareness about its benefits among patients and medical providers. IASIOS community members have exclusive opportunities to learn from one another, network, and participate in various social events, seminars, and workshops.
7 HOSPITALPROFESSIONALNEWS.IE | HPN • SEPTEMBER 2023
News
Professor David Brophy with members of the Interventional Radiology Multidisciplinary Team
Hospital Pharmacy
Infectious Diseases and Antimicrobial Resistance Hospital Pharmacists Association adopts
new Position Paper
prevent the further spread of antimicrobial resistance, the European Association of Hospital Pharmacists (EAHP) urges an interprofessional approach in the healthcare setting and during the transition of care. Hospital pharmacists in Europe are ready to champion infection prevention and contribute and promote the prudent use of antimicrobials through the enforcement of antimicrobial stewardship. To improve patient outcomes proactive steps need to be taken.
Stewardship
This year's General Assembly of the European Association of Hospital Pharmacists (EAHP) adopted a new Position Paper on Infectious Diseases and Antimicrobial Resistance. Combating infectious diseases requires the implementation of a comprehensive intervention package comprised of measures including but not limited to prudent anti-infective use, vaccination and stewardship. In order to maintain the efficacy of antimicrobial drugs and to prevent the further spread of antimicrobial resistance, it is essential to have an interprofessional approach in the healthcare setting and during the transition of care.
The Position Paper calls on national governments and health system managers to utilise the specialised background and knowledge of hospital pharmacists in multi-professional antimicrobial stewardship teams or other forms of antimicrobial governance in the hospital and in the community. It also underlines that hospital pharmacists are an integral part of the transfer of care to ensure that care could continue when patients leave the hospital.
Investing in prevention strategies, infection control and immunisation should be considered and further consolidating the role of hospital pharmacists in European vaccination strategies is paramount. In this sense, the universal application of prevention and control measures published by the European Centre for
Disease Prevention and Control
and the World Health Organisation among healthcare professionals and the public it is recommended in the fight against infectious diseases. Considering the One Health Approach, adequate regulatory oversight and proper implementation of measures in the veterinary sector and the environment on global, European, national and local levels need to be addressed.
Governments need to make arrangements so that essential antibiotics in dosage forms and strengths appropriate for both adults and children will
be maintained on the market with contingency stock level arrangements and alternative production by hospital pharmacists enabled where necessary.
Below is a brief overview of the paper.
Combatting infectious diseases requires the implementation of a comprehensive intervention package comprised of measures including but not limited to prudent anti-infective use, vaccination and stewardship
To maintain the efficacy of antimicrobial drugs and to
The emergence of antibiotic resistance is widely recognised as a major public health problem. According to the European Commission and the European Centre for Disease Prevention and Control (ECDC), the number of patients in the EU that die each year as a result of infections caused by resistant bacteria increased from 25.000 in 2017 to 35.000 in 2020.
In the last decades, there has been dramatic growth in the ability of a microorganism to stop an antimicrobial from working against it. As a consequence, only a limited number of antibiotics are available for the treatment of infections caused by resistant bacteria. Other consequences for patients are that infections
EAHP calls on national governments and health system managers to utilise the specialised background and knowledge of the hospital pharmacist in multi-professional antimicrobial stewardship teams or other forms of antimicrobial governance in the hospital and in the community.
EAHP requires that hospital pharmacists are an integral part in the transfer of care to ensure that the patient care started in hospitals can be continued in the community.
EAHP asks for the inclusion of concrete measures, like the outcome measures provided by the Transatlantic Taskforce on Antimicrobial Resistance, in national action plans that increase the uptake of stewardship teams.
EAHP calls for further consolidating the role of hospital pharmacists in European vaccination strategies.
EAHP recommends the universal application of prevention and control measures by ECDC and WHO among healthcare professionals and the public in the fight against infectious diseases.
EAHP advocates for adequate regulatory oversight and proper implementation of measures in the veterinary sector and the environment on global, European, national and local levels.
EAHP demands increased investment to support the development of innovative proposals and the encouragement of research projects in new fields of infectious disease control such as immunotherapy and sustainability.
EAHP urges governments to make arrangements so that essential antibiotics in dosage forms and strengths appropriate for both adults and children will be maintained on the market with contingency stock level arrangements and alternative production by hospital pharmacists enabled where necessary.
8 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
persist, which results in longer hospital stays, higher healthcare costs, and an increased risk of the infection spreading to others. Thus, significant interprofessional actions are needed to ensure standard treatments and prevention of serious diseases with effective and safe medicines that are quality-assured, used appropriately, and accessible to all who need them.
The escalating threat of antimicrobial resistance is a global public health concern and now seriously jeopardises the effectiveness of standard treatments, rendering some ineffective for their approved indications. Antimicrobial stewardship (AMS) teams are an indispensable tool that promote the rational use of antimicrobial agents, selection of optimal drugs, dosing, duration of therapy, route of administration and the use of antibiotic susceptibility testing to reduce the use of broad-spectrum antibiotics. In Sweden, AMS has formed an integral part of the strategic programme against antibiotic resistance for the past 20 years. The first European adoption of the IDSA (Infectious Disease Society of America)
Practice Guidelines based on a new evaluation of the literature including European publications was performed in 2013 and published by an interdisciplinary working group from Austria, Germany and Switzerland.
The primary goal of AMS is to optimise clinical outcomes while minimising unintended consequences of antimicrobial use. Consequently, EAHP calls on national governments and health system managers to utilise the specialised background and knowledge of the hospital pharmacist in multi-professional antimicrobial stewardship teams or other forms of antimicrobial governance in the hospital and in the community.
Seamless transitions between the interfaces of different health settings need to be considered during the implementation of multi-professional antimicrobial stewardship teams or other forms of antimicrobial governance in the hospital and in the community. EAHP requires that hospital pharmacists are an integral part in the transfer of care to ensure that the patient care started in hospitals can be continued in the community.
Need for further implementation of antimicrobial stewardship programmes
AMS is still a long way from being routine in European hospitals. This is despite the scientific results of efficient reduction of antibiotic overuse, positive contributions to resistance development and even cost savings through AMS.
ECDC also supports the strengthening of the fight against AMR i.e. through an AMS toolkit with a special section including hospital pharmacists’ involvement. The EU Commission strongly supports AMS as an important tool in their publications, e.g. EU Guidelines for the prudent use of antimicrobials in human health. Besides the positive effects on patient treatment and sustainability of antibiotic therapy, there is also a cost-benefit described in the literature. EAHP asks for the inclusion of concrete measures, like the outcome measures provided by the Transatlantic Taskforce on Antimicrobial Resistance, in national action plans that increase the uptake of stewardship teams.
One Health Approach
Public health interventions, preventive measures and changes in human behaviour have proven to be effective tools against infectious diseases over many decades. The COVID-19 pandemic has put the spotlight on vaccination strategies, vaccine development and other preventative measures. To curb the spread of infectious diseases, the efforts of prophylaxis like vaccination and hygiene are important and need to be further enhanced. Prevention of infectious diseases should be strengthened by integrating vaccination planning developed for the fight against them and providing all healthcare professionals with access to vaccine records.
Hospital pharmacists – as part of the vaccination team across healthcare sectors – are raising awareness about vaccine safety, supporting vaccine administration processes and sharing clear information with citizens that have questions. They play a significant role in providing information to their fellow healthcare professional colleagues on vaccines, their development, differences and administration patterns and by that influence the perception of vaccines and their importance for combating diseases.
Hospital pharmacists, because of their specialised training are able to guarantee the safe handling of vaccines and are in a position to support the traceability and vigilance of vaccines. In the context of national vaccination awareness programmes the involvement of the expertise of hospital pharmacists as trusted sources of information is important for adequately informing the public. They are also essential societal pillars that lead by example by being vaccinated themselves and that provide objective and trusted information to improve public health. EAHP calls for further consolidating the role of hospital pharmacists in European vaccination strategies. Similarly to vaccination, existing prevention toolkits and hand hygiene as the single most important prophylactic measure need to be boosted. The prevention toolkit for healthcare professionals in hospitals and other healthcare settings of ECDC, to which EAHP contributed from the perspective of the hospital pharmacist, is for example a measure that should be promoted. EAHP recommends the universal application of prevention and control measures published by ECDC and WHO among healthcare professionals and the public in the fight against infectious diseases.
Incentives
Increasing resistance, particularly of carbapenem resistance and vancomycin resistance in Europe, means that specific funding actions are necessary for the benefit of the patient. Despite the commitments of the European Commission and others in relation to the support of research and developments further incentives are needed.
It is crucial that the revision of the EU’s general pharmaceutical legislation addresses the increase of European production sites to lower dependency on international markets and the development of new antibiotics. It is however questionable if transferable exclusivity vouchers are the right incentive to increase development, especially since any option of such upfront nature has not been tested before. Unpredictable results, including costs and also the lack of tailored conditions ensuring access to the new antibiotics, could be the consequence.
At the same time, a prolonged exclusivity period for the medicines
to which the voucher is applied puts respective patients at a potential risk of diminished competition in the market both in terms of supply chain fragility (resulting in secondary shortages) and also higher costs. In addition, quality metrics to discourage the development of unnecessary or ineffective antibiotics would need to be put in place. EAHP demands increased investment to support the development of innovative proposals and the encouragement of research projects in new fields of infectious disease control such as immunotherapy and sustainability.
Keeping old and established but still essential antibiotics on the market
In addition to the development of new antibiotics, universal access to old and established antibiotics that are being utilised in new ways needs to be maintained. The growing and more frequent shortages of antimicrobials are an additional contributing factor to AMR affecting the effectiveness of antimicrobials worldwide.30 The shortage of amoxicillin and amoxicillin/clavulanic acid has severely impacted treatment options for patients across Europe. Medicines shortages of antimicrobial drugs compromise their prudent use and must be avoided. Surveys conducted by EAHP in 2019 and 2020 showed that antibiotic shortages ranked among the top three medicinal products in shortage. Measures need to be taken by countries, including national governments, pharmaceutical industry, healthcare professionals, patients as well as other stakeholders at the pan-European level, to lower the risk of antibiotic shortages, especially since substitution supported by the AMS team is not always possible. Sweden and the UK are already piloting subscription-based models that provide a sufficient antimicrobial product supply guarantee. Studies that gather further information on this, including those securing access to essential legacy/establish antibiotics, should be supported. EAHP urges governments to make arrangements so that essential antibiotics in dosage forms and strengths appropriate for both adults and children will be maintained on the market with contingency stock level arrangements and alternative production by hospital pharmacists enabled where necessary.
9 HOSPITALPROFESSIONALNEWS.IE | HPN • SEPTEMBER 2023
News
Hospital Capacity Deficits hit Young People
The Irish Hospital Consultants Association (IHCA) has expressed its continuing concern at the excessive number of children waiting for an appointment to be treated or assessed in public hospitals.
The warning from Consultants comes as the latest National Treatment Purchase Fund (NTPF) figures released reveal that 895,700 people were on some form of hospital waiting list at the end of July, including almost 100,800 children and young people. While a recent report highlighted the extent of extreme capacity shortages in Ireland’s Child and Adolescent Mental Health Services (CAMHS), the IHCA’s analysis shows that similar capacity deficits are resulting in lengthy waiting lists across a
number of paediatric specialties, including Ear, Nose & Throat (ENT), Dermatology, Orthopaedics and Cardiology – with some being forced to wait months or years for assessment or treatment.
The IHCA said that difficulty in filling permanent Consultant posts and growing hospital and mental health capacity deficits against increases in demand are the root causes of the unacceptably long child waiting lists. One in five (20,600) children are waiting longer than a year for treatment or assessment by a hospital Consultant. Latest HSE data reveals the number of unfilled permanent Consultant posts has risen to a record 933.2 This is the highest Consultant vacancy rate ever.
Hospital diagnostics are not included in NTPF data which
accounts for an additional 8,916 children awaiting CTs, MRIs or ultrasounds at the three Dublin paediatric hospitals alone, bringing the total number awaiting care to almost 110,000 – or 1 in 12 children in the country.3
In addition, a near record 4,421 children4 were on separate CAMHS waiting lists at the end of May 2023 – 128 (3%) additional children added so far this year.5 The CAMHS waiting list has increased by almost a quarter (+865 or 24%) since the start of 2022 and has almost doubled (+2,094 or +90%) since the start of 2020.6
Commenting on today’s waiting lists, IHCA President Professor Robert Landers, said, “The monthly NTPF figures have recorded over 100,000 children on waiting lists for hospital care for
Irish National Orthopaedic Register
Mr John Kelly, Orthopaedic Consultant Clinical Lead
the fifth consecutive month, with one in five of these children waiting longer than a year to be treated or assessed in public hospitals. This is resulting in thousands of children not getting the care they need in a timely way, and the real possibility that they will suffer serious and lasting health and developmental issues that could have been reversed or mitigated against if only they were seen in time.
“As Consultants, we need and want sustainable solutions to help alleviate this distress and provide the care these children so desperately need. However, we have unresolved hospital capacity deficits and Consultant vacancies that is not being addressed urgently enough. These twin deficits must be addressed by the Government in October’s Budget.”
The National Office of Clinical Audit (NOCA) is pleased to announce that Sligo University Hospital (SUH), which is part of the Saolta Hospital Group, has become the 18th hospital in Ireland to roll out the Irish National Orthopaedic Register (INOR). The aim of this initiative is to monitor and improve the quality of care for individuals receiving joint replacement surgery in Ireland.
INOR is a secure, web-based, real-time system which provides a national electronic register of patients receiving joint replacement surgery in Ireland. Elective orthopaedic hip and knee replacement records will now be available nationally in a central register for the first time. The register will collate information from Sligo and will support early detection of implant performance
and improve the efficiency of the recall and review process.
Mr John Kelly, Clinical Lead for the INOR Project in Sligo University Hospital said: “The INOR is a great development for SUH. Sligo is now at the forefront of Arthroplasty use in Ireland and this leverages emerging technology and infrastructure to enable better surgical outcomes for our patients.”
Following patient consent, the INOR will collect information electronically at pre-operative, surgical and post-operative assessment stages, from patients who are undergoing joint replacement surgery. This will in turn support early detection of implant performance and improve patient experience with their implant over a longer time.
Mr James Cashman, National Clinical Lead for INOR welcomed the introduction of INOR to Sligo
University Hospital. He highlighted the “important contribution INOR makes to audit and good clinical governance, as well as allowing us to expedite notifying patients in the unlikely event of an implant recall occurring.”
Over the past three months, much work was undertaken by the local SUH implementation team for INOR in conjunction with the INOR national team to ensure implementation readiness across all relevant areas of Sligo University Hospital.

Gráinne McCann, General Manager of Sligo University Hospital welcomed the implementation, saying “We are pleased to formally become part of this national register so that we can continuously monitor and improve services we provide to our patient, ensuring quality standards of care within our services.”
INOR went live in May 2016 and is managed by NOCA, in conjunction with the HSE Office of the Chief Information Officer and clinically supported by Irish Institute of Trauma and Orthopaedic Surgeon (IITOS). Roll-out of INOR to the remaining public elective orthopaedic surgery sites is underway and will be completed by early 2023. Implementations of the private hospital are also under way.
10 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
From left: Micheál Bailey, Irish National Orthopaedic Register; Farnan Golden, IT; Aine Duffy, Arthroplasty Nurse Specialist; Caroline Quinn, CNM3 Perioperative Directorate; Ruth McManus, CNM 2, PAC; Michelle Gilroy, CNM2, TQIP; and
Viatris
Empowering people
worldwide to live healthier at every stage of life

With 1,600 people working across five sites in Ireland, Viatris provides access to medicines, develops innovative solutions and improves healthcare for patients.
Newenham Court, Malahide Road, Dublin 17, Dublin, Ireland.
Viatris.ie
Job Code: CC-2023-001
Date of Preparation: July 2023
www.viatris.ie
New Option for Prostate Surgery
A team led by Consultant Urologist Prof Rustom Manecksha recently carried out a new type of minimally invasive procedure treating BPH in the Reeves Day Surgery Centre at TUH

Our hospital and in particular the urology service has a long tradition of innovation, the introduction of this new therapy is a welcome addition to the number of ways we can treat an enlarged prostate, many of which are minimally invasive. This means our patients spend less time in hospital and have a shorter recovery time so they can get back to living their lives.”
prostate tissue. The treated tissue is then gradually reabsorbed by the body, reducing the size of the prostate and easing the symptoms for the patient. It is typically performed as a day procedure, using sedation or a short general anaesthetic.
One in four men over the age of 40 in Ireland will suffer from an enlarged prostate gland. In medical terms this is referred to as Benign Prostatic Hyperplasia (BPH) and in simpler terms an enlarged prostate*.
Earlier last month, a team led by Consultant Urologist Professor Rustom Manecksha carried out
Safety Notice
a new type of minimally invasive procedure treating BPH in the Reeves Day Surgery Centre at Tallaght University Hospital.
Professor Rustom Manecksha, Consultant Urologist who carried out the procedures commented “This week we have provided an alternative treatment for what is a common medical problem.
The innovative treatment (Rezūm) uses water vapour therapy to target and shrink the excess prostate tissue. When the steam contacts the prostate tissue the stored energy is release into the tissue. In time the body absorbs the treated tissue, reducing the size of the prostate. This relieves the urinary obstruction and improves urinary flow.
During the procedure, a specialised device is directed to deliver small bursts of heated water vapor directly into the
It is a new and alternative treatment option, which adds to the options already offered, including UroLift, which the Urology team in TUH introduced in 2019. Plasma vaporisation and bipolar prostate resection, Rezūm is a new and effective alternative to traditional surgical treatments for BPH, providing patients with symptom relief with minimal side effects and a quicker recovery time.
*When the prostate becomes enlarged it places pressure on the bladder and urethra (the tube that urine passes through). This can cause dribbling at the end passing urine, often feeling like the bladder has not fully emptied, needing to go to the toilet often even during the night leaving you feeling tired. If it is ignored and not treated it can lead to bigger problems with the kidneys and the bladder.
The marketing authorization holder of Simponi, Janssen Biologics B.V., and the local representative, Merck Sharp & Dohme Ireland (Human Health) Limited, in agreement with the European Medicines Agency and the Health Products Regulatory Authority, would like to inform you of the following: Summary
• Accidental needle stick injuries, bent or hooked needles, and device actuation failure have been reported for the Simponi SmartJect pre-filled pen.
• Instructions for use have therefore been revised as follows:
o Do not put the cap of the pre-filled pen back if removed, to avoid bending the needle.
o Only inject in the thigh or abdomen.
o Use a two-hand approach to administer the injection (one hand to hold the pre-filled pen and the other hand to press the blue button to start the injection).
o Do not pinch the skin, when positioning the pre-filled pen and when administering the injection.
• The device must be pushed against the skin until the green safety sleeve slides completely into the transparent cover BEFORE the blue button is pressed. Only the wider portion of the green safety sleeve remains outside of the transparent cover.
• All patients/caregivers, including those previously trained on the SmartJect pre-filled pen, should be instructed on the proper use of the device in accordance with the revised instructions for use.
Royal College of Physicians of Ireland Annual Symposium - St Luke’s open for bookings
16 – 20 October 2023 | Hybrid event
The Royal College of Physicians of Ireland Annual Symposium - St Luke’s is open for bookings. The symposium will take place online and in person from 16 to 20 October 2023 touching on the themes of clinical leadership, resilience, and climate and health. Throughout the symposium, these themes will be explored through a series of exciting events including a public art exhibition, our annual public meeting, the symposium day, and the ever-popular Heritage event. The Annual Stated Meeting will take place on 18 October 2023.

12 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Upskilling with Online CPD


RCSI University of Medicine and Health Sciences has created a new suite of online CPD short courses for healthcare practitioners looking to upskill and advance their careers.
The short continuous professional development (CPD) courses are provided via RCSI’s digital learning platform, RCSI Online, and are available to those working in healthcare and allied healthcare roles anywhere in the world. Running over five-week terms, the courses are delivered by experts
and time-independent. We are developing a growing portfolio of topics relevant to the current and anticipated needs of healthcare professions, with content delivered by subject-matter experts with industry and sectoral experience and expertise.”

To learn more about the short courses available on RCSI Online, visit https://www.rcsi.com/online/ courses/short-courses
Future of Future of pharmacIE pharmacIE




13 HOSPITALPROFESSIONALNEWS.IE | HPN • SEPTEMBER 2023
News
Connect with future pharmacists at APPEL's exclusive careers event. Book your ticket now! TICKETS Friday, 20th October 1pm - 4pm RCSI, 123 St Stephen
Vitamin D Intake
Vitamin D intake and status in Ireland: a narrative review part 1

Published online by Cambridge University Press: 14 February 2023
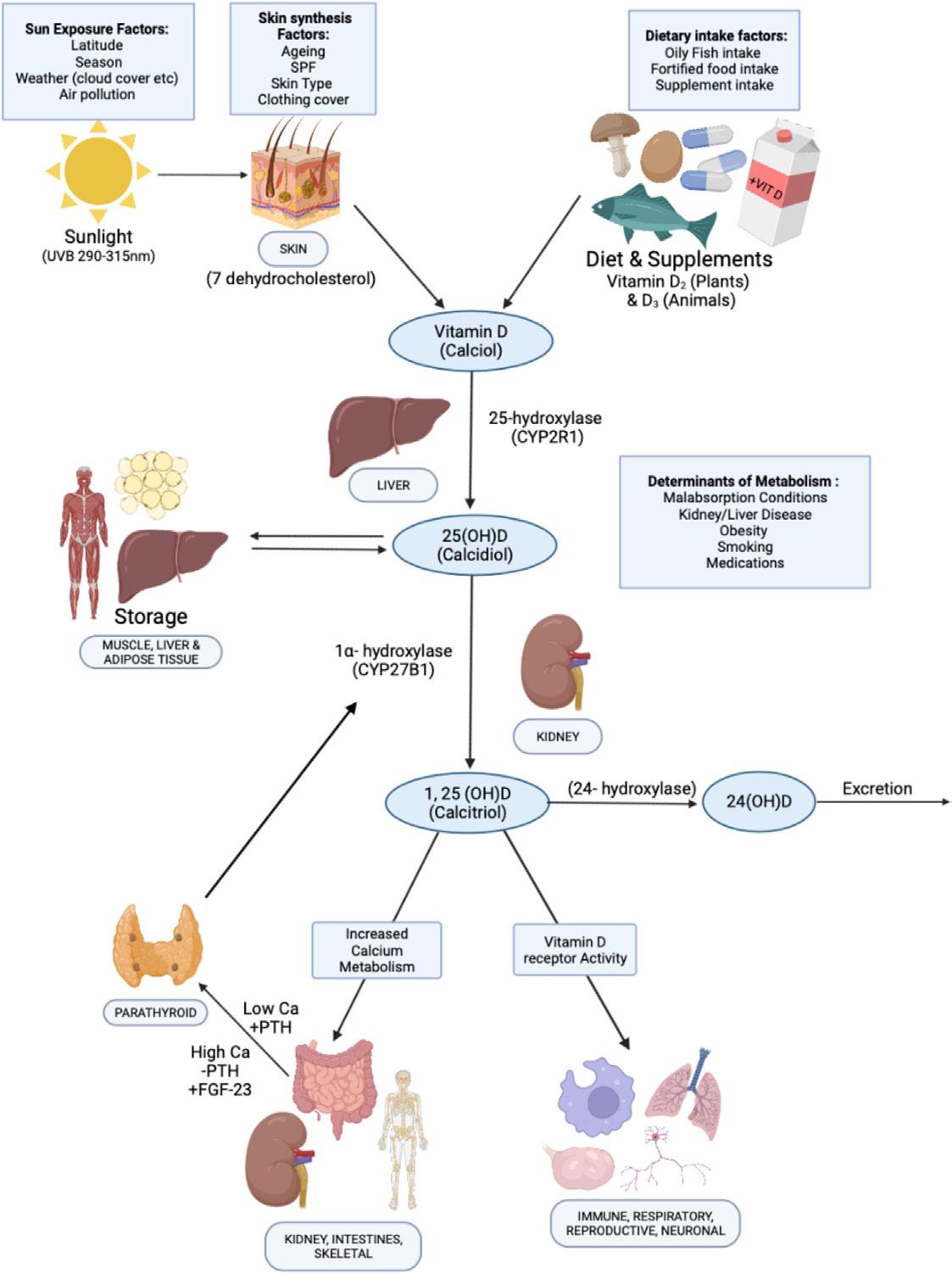
Vitamin D is crucial for musculoskeletal health, being required for the adequate absorption of calcium from the gastrointestinal tract. Vitamin D is a secosteroid synthesised via the action of UVB light on the skin, forming cholecalciferol (vitamin D3) (Figure 1).1 While this is the predominant physiological source of vitamin D, it can also be obtained from the diet in animal and plant foods (ergocalciferol or vitamin D2) and from fortified foods.2, 3 Its role in bone health is well established,4 with deficiency increasing the risk of rickets in childhood and osteomalacia in adults.1 Secondary hyperparathyroidism due to vitamin D deficiency can result in musculoskeletal pains and muscle weakness.5 Peak bone mass, which may determine up to 60 % of osteoporosis risk in later life can only be achieved with sufficient vitamin D and calcium intake.1 The role of vitamin D may also extend beyond bone health. For example, vitamin D receptors are found in numerous cells including immunological (T- and B-cells), osteoblasts, β cells and mononuclear cells, and in many organs such as the brain, heart, reproductive and the gut.6 Interaction of transcription factors [1,25-hydroxyvitamin D2 (1,25(OH)D2)] with the vitamin D receptors modulates gene expression, influencing numerous physiological functions including anti-cancer, immunological and
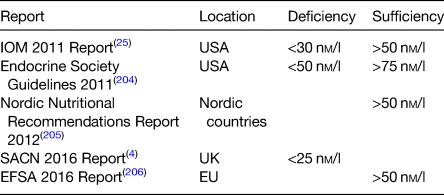
anti-inflammatory effects.7, 8 Thus, it may be involved in the pathogenesis of hypertension, stroke and CVD and may also play a role in immunity, autoimmune diseases, type I and II diabetes, multiple sclerosis, cancer, depression and dementia.6, 8, 12 While plausible physiological mechanisms exist for these potential extra-skeletal effects, evidence from robust randomised controlled trials is limited and causality has not been established.4, 6 However, maintaining adequate vitamin D status (>50 nM/l) has been associated with decrease in allcause mortality in a recent large prospective cohort study.13
Vitamin D status is determined by a number of intrinsic, environmental and lifestyle factors. Biosynthesis of vitamin D from UVB sunlight (290–315 nm) is dependent on the correct latitude, and for countries above 40°N such as Ireland (52–55°N) this is negligible between October and March.3, 14 Cloud cover, time of day, altitude and air pollution can also affect production and give rise to regional variations in status.15 Factors including age, skin type, sunscreen use and clothing cover also determines dermal synthesis.16 Finally, the absorption and bioavailability of vitamin D is affected by malabsorption conditions (e.g. Crohn's/coeliac disease), medication, smoking and obesity.3, 17
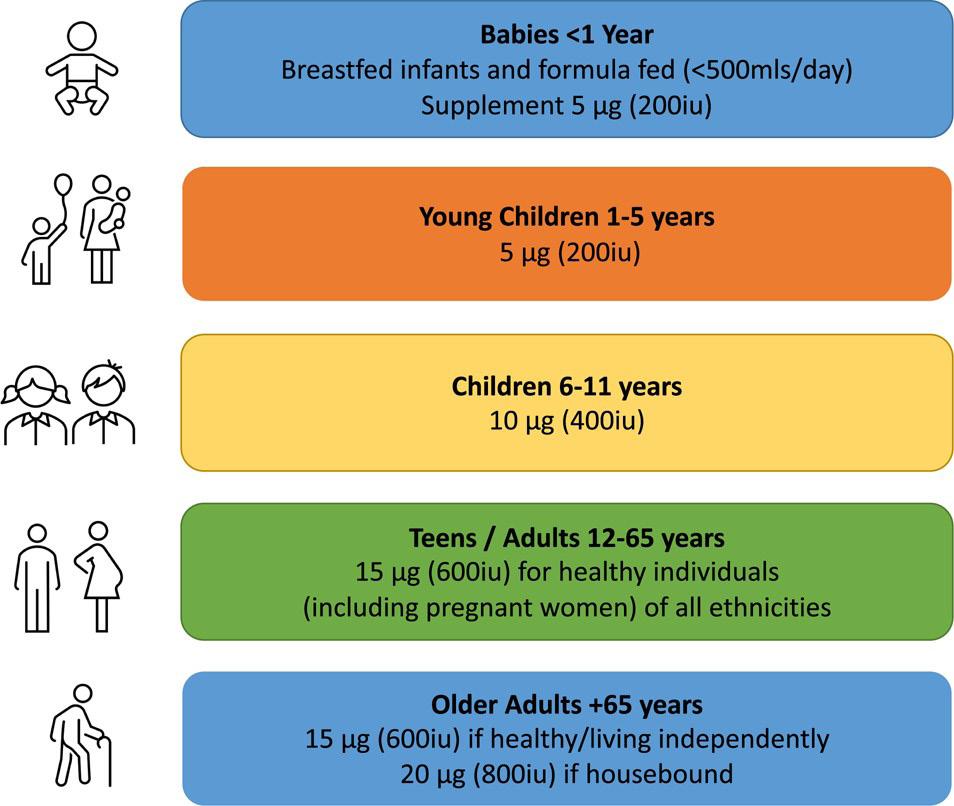
As a result of limited sun exposure, the Irish population is dependent on dietary sources of vitamin D, though intakes remain low, and most do not meet the RDA.18 The RDA as set by the Food Safety Authority of Ireland varies by age as shown in Figure 2.19–22 In addition, the proportion at risk of deficiency is rising due to demographic and
other changes.20 For example, the population is ageing, with the proportion over 65 years set to double by 205023 and there has also been an increase in those of ‘non-white’ ethnicity.24 Levels of overweight and obesity are also on the rise, increasing from 55 and 19 % in 2006 to 61 and 25 %, respectively, in 2016.23 While there are relatively few cases of rickets, the number reported has increased with twenty-three cases recently described in two Dublin hospitals.20 For these reasons, knowledge of both trends and current vitamin D status and intake is important in several subgroups of the population. There is no universal agreement on definitions of deficiency and sufficiency by professional bodies (Table 1). Vitamin D > 125 nM/l is suspected by the National Academy of Medicine as being harmful to health with potential negative effects on falls, depression and possibly other outcomes including cancer and all-cause mortality in some studies.25, 27 However, the National Academy of Medicine takes a precautionary approach that also factors in ethnic/genetic differences so as to maximise public health protection.27 Despite this, overt vitamin D toxicity causing hypercalcaemia is rare and usually occurs at levels above 375 nM/l.4, 28
Due to the limited half-life of the biologically active 1,25(OH)D (4–6 h) and its tight feedback control, vitamin D status is assessed by monitoring concentrations of
25(OH)D (half-life 3–4 weeks) which is under no feedback regulation.29 There are several types of vitamin D analytical techniques, with varying sensitivities and specifications.30 These include binding assays; RIA, chemiluminescence immunoassay, protein-binding assay, and bioanalytical assays such as HPLC and liquid chromatography tandem mass-spectrometry (LC-MS/ MS). Binding assays are relatively quick and inexpensive but are subject to interference from other vitamin D metabolites and may overestimate 25-hydroxyvitamin D [25(OH)D] concentration.29 HPLC and LC-MS/MS allows for the quantification of a large number of samples, but require more technical skill.30 LC-MS/ MS is now considered the gold standard of vitamin D assessment and allows for the measurement of both 25(OH)D2 and 25(OH)D3.29 The vitamin D standardisation programme and vitamin D external quality assessment scheme were developed to improve the accuracy and repeatability of vitamin D assessments.31, 32 Vitamin D intake is typically assessed with FFQ that include 24-h re-calls and 3–7-d food diaries. Weighed food diaries are most commonly utilised in Irish national nutrition surveys and calculate vitamin D intake using nutrient composition databases.33
14 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Helena Scully
To date, no study has comprehensively reviewed vitamin D research
Helena Scully, Kevin McCarroll, Martin Healy, James Bernard Walsh and Eamon Laird Mercers Institute for Research on Ageing, St James's Hospital, Dublin School of Medicine, Trinity College Dublin
Table 1. Serum 25-hydroxyvitamin D recommendations
15 HOSPITALPROFESSIONALNEWS.IE | HPN • SEPTEMBER 2023 News
Figure 1. Vitamin D metabolism. 25(OH) D, 25-hydroxyvitamin D; Ca, calcium; FGF-23, fibroblast growth factor 23; PTH, parathyroid hormone; SPF, sun protection factor
Vitamin D Intake
multivitamin containing vitamin D, 74 3 % were actually taking some form of supplementary vitamin D.61 Importantly, less supplement use in pregnancy has been associated with an increased risk of low vitamin D status in Irish infants.49 Supplementation in Irish pregnant women was also the strongest predictor of 25(OH)D > 30 nM/l with Caucasian females more likely to supplement than those of other ethnicities.37
Children/adolescents
To date, no study has comprehensively reviewed vitamin D research with regards to vitamin D status and intakes on the island of Ireland. We aim to summarise the peer-reviewed studies and official reports published since 2010 or earlier for specific subgroups where no other data was available. For the purpose of this review, we defined deficiency as <30 nM/l and excess as >125 nM/l unless otherwise stated.25
Vitamin D status and intake by population categories
Pregnancy and fertility
The largest Irish study (n 1768) in pregnant women found a prevalence of deficiency of 17 %(Reference Kiely, Zhang and Kinsella34), which was similar to other large studies where it ranged between 15 and 17 %.35, 36 In other studies, it varied between 13 and 65 % but sample sizes were small and were not likely to be representative.37, 40 In general, deficiency was less prevalent in early pregnancy (13–29 %)34, 36, 38, 41, 47 with rates increasing with gestation in most studies.41, 42, 45,47 A high proportion of mothers (25–65 %) were also found to be deficient at
delivery.39, 40 As expected, a seasonal variation in vitamin D deficiency in pregnancy was found,34, 38, 43, 45, 48, 50 with the lowest prevalence of 3–7 % detected in summer/autumn.34, 38
The prevalence of levels <50 nM/l in the largest studies were between 42 and 44 %(Reference Kiely, Zhang and Kinsella34–Reference Hemmingway, Kenny and Malvisi36), while in smaller studies variance was pronounced (44–91 %)(Reference O'Callaghan, Hennessy and Hull38,Reference Onwuneme, Martin and McCarthy40) . A seasonal effect was also evident, with prevalence generally higher in winter v. summer(Reference Kiely, Zhang and Kinsella34,Reference O'Callaghan, Hennessy and Hull38,Reference
O'Brien, O'Sullivan and Kilbane45,Reference Holmes, Barnes and Alexander48) . Prevalence of levels below <50 nM/l is similar to some pregnancy studies in Northern Europe where it affected approximately 50 % in the UK(Reference Gale, Robinson and Harvey51) and Belgium(Reference Vandevijvere, Amsalkhir and Van Oyen52). Only one Irish study (n 138) looked at men and women
undergoing fertility treatments and found that one out of five was deficient(Reference Neville, Martyn and Kilbane53).
Dietary and supplement intakes in pregnancy
Dietary intakes in pregnant women ranged between 1 9 and 10 7 μg/d,46, 54 with 80–99 % not meeting the recommendations.54, 56 By comparison, in the UK, 98–100 % were found not to meeting the advised intake of 10 μg/d.57
In Ireland the RDA in pregnancy is no different from the general adult population at 15 μg/d.22 However, evidence suggests that pregnant women require 20 μg/d to meet sufficiency (>50 nM/l)58 with 10–15 μg/d advised by a European consortium.59 In Finland, the 2003 food fortification of liquid milk resulted in a significant increase in vitamin D intakes in pregnant women,60 and as such should be considered as a public health measure to improve vitamin D status in Ireland.
Nearly 40 % of pregnant Irish women reported taking a specific vitamin D supplement in a Dublin study (n 175) in 2016, though the sample was from a confined area.61 However, when including a
In the largest study to date (n 5524), 15 % of children (5–19 years) were deficient,62 while the second largest (n 1226) found a deficiency rate of 23 %,63 though both were conducted in the Dublin area. In most studies deficiency prevalence varied between 5 and 23 %.62, 68 The most recent nationally representative study that measured vitamin D levels in teenagers (aged 13-18 years) found that 21.7% were deficient though sample size was small (n=246).69 The highest prevalence of deficiency (63–68 %) was reported in adolescents (aged 12 or 15 years) in Northern Ireland.70 However, vitamin D was assessed over 20 years ago, was not checked in the months of July or August and the study population was derived after stratified sampling so may not be more broadly representative. Furthermore, the results are discordant with other studies. As expected, the prevalence of deficiency was lower in the summer66, 71, 72 and higher in winter when it affected 18–30 % of teens,71, 72 and 26 % of children aged 1–17.63 In general, female children also had lower vitamin D status,63, 65, 70, 73, 74 but not in all studies.64
Notably, there was a ‘U-shaped’ relationship between deficiency prevalence and age, being lower in younger children (1–12 years) and greatest in adolescents and older children (>12 years). 62, 63, 65 For example, a recent large study found a greater prevalence of deficiency in over v. under 12s (24 v. 16 %).63 Lower rates of deficiency (2 %) have been found in toddlers (aged 2 years) and children under 5 (13 %),64, 66 with higher 25(OH)D also reported in those under v. over 4 years (61 0 v. 46 1 nM/l, P < 0 001).65 Similarly, in a recent large study, deficiency was lower
16 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Figure 2. Irish vitamin D supplement recommendations by age group
(5 %) in toddlers (1–4 years) but much higher (15 4 %) in older children (5–19 years).62 Better vitamin D status in younger children (<5 years) may relate to Ireland's infant supplementation policy.75 Conversely, lower rates of supplements and fortified food consumption has also been found in Irish teens compared to younger children.76 One study also reported that older teens (15–18 years) had lower mean 25(OH)D and were more likely to be deficient than younger teens (13–15 years).69 Greater screen time/sedentary behaviour and increased obesity rates in this age group may be important factors.77, 79 Socioeconomic status may also explain some of this variation as it has been associated with a higher prevalence of deficiency in Irish children.63 Overall, reports are broadly consistent with findings in northern European countries (47–69°N).80, 83 and in the UK 19 % of 11–18-year-olds were deficient, compared to just 2 % of those aged 4–10.84
Despite in general, less deficiency in younger children, this was not apparent for those aged <1 year. For example, deficiency in new-borns was high ranging from
34 to 63 % (based on cord blood samples),35, 39, 42, 49 while in preterm infants 64 % were deficient at delivery.40
Overall, approximately half of children aged 1–17 years had levels <50 nM/l,62, 63, 65, 68, 69 with a seasonal variation identified,63, 66, 73 similar to findings in the European Union (EU).75, 80, 85 Similar to deficiency, prevalence was lower in younger children (1–5 years), at 21–39 %.62, 64, 66 and higher in teens at 36–89 % as in UK studies.81 The prevalence of 25(OH)D < 50 nM/l in new-born cord blood was particularly high (between 80 and 92 %),35, 39, 42, 49 and similar (79–92 %) in preterm and term infants.35, 40, 42, 44, 49
Dietary and supplement intakes in children
The first nationally representative dietary survey in 2010/2011 in Irish children aged 12–59 months found that 70–84 % had intakes <5 μg/d (mean of 3 2 μg/d).86 In a small study (n 97) of 5-year-olds in 2019, intake remained low with just 6 2 % having consuming above 5 μg/d.64 Recent nationally representative surveys of older children (5–12 years) and teens (13–17 years) found the majority (94 %) had intakes <10 μg/d,
with little improvement between 2003/2004 and 2017/2018.78, 87, 88 In fact, comparing surveys, intakes had improved only a little, from 2 7 to 3 7 μg/d for teens and from 2 3 to 4 2 μg/d for children.87,89–91 Similarly, in a recent nationally representative study of teenagers (aged 13-18).69
In Irish infants, milk/formula comprised of 29 % of total intakes,86 similar to that in UK children aged 12–18 months.92 Milk/products were also the greatest source of vitamin D in children <4 years in Ireland followed by meat and its products as found in the UK.86, 92 However, in Irish children aged 5–12 years, fortified cereals were the largest contributor, followed by meat and then milk products87 as also identified in Belgium and the UK.81, 93 Meat and its products also account for the primary source of vitamin D in Irish children over 13,78,94 despite its relatively low content. This is also reflected in the UK, where it comprised of 35 % of dietary vitamin D intake.4
Children aged 1–5 years in Ireland are recommended to receive 5 μg/d, supplementing if necessary, with older children (aged 6-11
years) advised to consume 10 μg/d.20, 22 Overall, supplements are an important contributor to vitamin D status in children and adolescents in Ireland,65, 95 as found elsewhere in Europe.96, 98 The most recent national dietary survey (2017/2018) of Irish children aged 5–12 years indicated that just 10 % consume a vitamin D supplement87 which compares to 17 % in a representative sample (aged 1–4 years).95 Supplement use has also been found to decrease with age, with 21 % of 5–8, 16 % of 9–12 and 15 % of 13–17-year-olds consuming a vitamin D containing supplement.65, 66, 76, 95 Similar findings have been reported in the UK, with higher supplementation rates in younger children (14–16 %) compared to teens (5–6 %.57 Indeed, since the introduction of an infant supplementation policy in Ireland, initiation of a 5 μg/d supplement from birth increased to 92 %, with 30 % of parents compliant during the first year.99 In one study, supplement use was reported in 23 % of Irish children (aged 1–17) attending hospital though this could be due to underlying medical reasons.65
References available on request
17 HOSPITALPROFESSIONALNEWS.IE | HPN • SEPTEMBER 2023
Dermatology
Moderate-to-Severe Atopic Dermatitis
Written by Alan D. Irvine, Thishi Surendranathan, Liz Hennessy, Ivana Rajkovic, Annette
Dearbhla Coughlan, Sean Conlon, Richard Hudson
Alan Irvine
of care for paediatric AD in Europe. Furthermore, to our knowledge, there is no real-world evidence (RWE) study focusing on an Irish population of children and adolescents with moderateto-severe AD. The RWE study reported here is the first of its kind to systematically capture data on clinical characteristics, secondary care HCRU, and patient-reported outcomes (PROs) associated with treatment of moderate-to-severe AD, in children and adolescents in a single centre in Ireland.
Patients and Methods
Study design
Durkan,

primary care in Dublin, Ireland and secondary care nationally. Data collection was from October 2020 to June 2021.
It is estimated that in the Ireland 14.3% of children 13–14 years of age ever experience the inflammatory skin disorder atopic dermatitis (AD).1 AD has a profound impact on the quality of life (QoL) of patients and their relatives, affecting multiple aspects of psychological, social and physical functioning.2-7 Therapeutic guidelines recommend that firstline treatment aims to control flare with local anti-inflammatory agents, including topical corticosteroids (TCS).8 For more severe AD cases, ultraviolet light and systemic immunosuppressants are suggested.8
Recognition of immunological disease mechanisms in AD has led more recently to the identification of different therapeutic targets and development of novel therapies. Regulatory approval has been gained for several of these for moderate-to-severe AD, including biologic therapies targeting specific immune pathways.9 Some biologics are now licensed for use in adolescents and/or children. However, the treatment of childhood AD remains challenging since there can be inadequate response to available therapies, and the more advanced immunological therapies are limited to late line use and are costly. This means determining when to prescribe an advanced therapy and which therapy to use is not necessarily straightforward.10
AD presents a considerable socioeconomic burden. Healthcare resource utilisation (HCRU) increases with disease severity,11 and is significantly higher after systemic therapy initiation.12 While the Irish dermatology programme ensures patients are appropriately seen, assessed, and treated,13 limited data are available describing the HCRU associated with current standard
This was a retrospective, noninterventional, single-centre chart review of 31 patients with AD aged 6–17, conducted in the public healthcare service at Ireland's largest paediatric hospital, Children's Health Ireland, at Crumlin where around 90% of all Irish children treated with systemic therapies for AD are managed. Referrals are made from
Supplementary Figure 1 Study design schematic illustrating the retrospective observational period
Patients meeting the eligibility criteria were selected consecutively, starting with the most recently consulting patient, and working backwards until the patient target was reached (Supporting Information: Figure 1). Data were abstracted from medical records, starting from the first secondary care appointment post 1 January 2014 when patients were 6–17 years of age, up until the most recent date/ event available when patients were also under 18 years of age. A minimum of 12 months of data was abstracted for each patient from their first secondary care appointment after January 2014. Anonymised data were collected onto a standardised, purposedesigned electronic Case Report Form (eCRF) by hospital research staff.
Supplementary Figure 1 Study design schematic illustrating the retrospective observational period
Supplementary Figure 1 Study design schematic illustrating the retrospective observational period
BASELINE
Date of pa�ent ’s first secondary care appointment occurring post January 1st 2014a
BASELINE
Date of pa�ent ’s first secondary care appointment occurring post January 1st 2014a
Most recent AD consulta�onb
Most recent AD consulta�onb
Eligible pa�ents were iden�fied consecu�vely by the Site; Sites were asked to start with the most recently consul�ng pa�ent with AD, working backwards un�l a pa�ent mee�ng the study criteria was found, and un�l the total study sample was reached
Eligible pa�ents were iden�fied consecu�vely by the Site; Sites were asked to start with the most recently consul�ng pa�ent with AD, working backwards un�l a pa�ent mee�ng the study criteria was found, and un�l the total study sample was reached
Minimum of 12 months data were captured post first secondary care appointment Relevant details around diagnosis were also captured
Minimum of 12 months data were captured post first secondary care appointment Relevant details around diagnosis were also captured
a Patient must be aged 6–17 years at the Baseline time point. The Baseline date will vary for all patients;
b Patient must be younger than 18 years at the Baseline time point.
a Patient must be aged 6–17 years at the Baseline time point. The Baseline date will vary for all patients;
b Patient must be younger than 18 years at the Baseline time point.
18 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Dr
A real-world retrospective observational study exploring resource use for secondary care management of moderate-to-severe atopic dermatitis in children and adolescents at a single site in Ireland
THE ITCH & RASH OF MODERATE TO SEVERE ATOPIC DERMATITIS 1

RINVOQ demonstrated in an integrated analysis of the MEASURE UP 1 & 2 monotherapy studies at Week 16:
• EASI 75 skin clearance rates of 76% and 65% for patients treated with RINVOQ 30 mg QD and 15 mg QD, respectively, vs 15% with placebo (p<0.001 for both comparisons)2
• Itch reduction (mean percent change from baseline in Worst Pruritus NRS) of –70% and –58% for RINVOQ 30 mg QD and 15 mg QD, respectively, vs –24% with placebo (p<0.001 for both comparisons)2
RINVOQ, the first and only oral JAK inhibitor indicated for the treatment of both adults and adolescents ≥12 years* with moderate to severe Atopic Dermatitis in the EU**2-4
RINVOQ®▼ (upadacitinib) 15 mg prolonged-release tablets; 30mg prolonged-release tablets; 45 mg prolongedrelease tablets. Refer to Summary of Product Characteristics (SmPC) for full prescribing information. PRESENTATION: Each tablet contains upadacitinib hemihydrate, equivalent to 15mg of upadacitinib in the 15mg tablet, 30mg in the 30mg tablet and 45mg in the 45mg tablet. INDICATION: Treatment of moderate to severe active rheumatoid arthritis (RA) and active psoriatic arthritis (PsA) in adult patients who have responded inadequately to, or who are intolerant to one or more disease-modifying anti-rheumatic drugs (DMARDs). RINVOQ may be used as monotherapy or in combination with methotrexate. Treatment of active non-radiographic axial spondyloarthritis (nr-axSpA) in adult patients with objective signs of inflammation as indicated by elevated C-reactive protein (CRP) and/or magnetic resonance imaging (MRI), who have responded inadequately to nonsteroidal anti-inflammatory drugs (NSAIDs). Treatment of active ankylosing spondylitis (AS) in adult patients who have responded inadequately to conventional therapy. Treatment of moderate to severe atopic dermatitis (AD) in adults and adolescents 12 years and older who are candidates for systemic therapy. Treatment of adult patients with moderately to severely active ulcerative colitis (UC) or moderately to severely active Crohn’s disease (CD) who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent. DOSAGE AND ADMINISTRATION: Intended for use under guidance and supervision of a physician experienced in diagnosis and treatment of conditions for which upadacitinib is indicated. Dosage: RA, PsA, AS, and nr-axSpA: Recommended oral dose is 15mg once daily. Consideration should be given to discontinuing treatment in patients with AS and nr-axSpA who have shown no clinical response after 16 weeks of treatment. Some patients with initial partial response may subsequently improve with continued treatment beyond 16 weeks. AD: Adults: Recommended oral dose is 15 mg or 30 mg once daily based on individual patient presentation (see SmPC for details). A dose of 15 mg is recommended for patients at higher risk of venous thromboembolism (VTE), major adverse cardiovascular events (MACE) and malignancy. The lowest effective dose to maintain response should be used. For patients ≥ 65 years of age, the recommended dose is 15mg once daily. Adolescents from 12 to 17 years weighing at least 30 kg: Recommended oral dose is 15mg once daily. Consideration should be given to discontinuing upadacitinib treatment in any patient who shows no evidence of therapeutic benefit after 12 weeks of treatment. UC: Induction; Recommended oral dose is 45mg once daily for 8 weeks. For patients who do not achieve therapeutic benefit by week 8, 45mg once daily may be continued for an additional 8 weeks. Discontinue treatment in patients with no evidence of therapeutic benefit by week 16. Maintenance; Recommended oral dose is 15mg or 30mg once daily based on individual patient presentation (see SmPC for details). A dose of 15 mg is recommended for patients at higher risk of VTE, MACE and malignancy. The lowest effective dose to maintain response should be used. Patients ≥ 65 years of age: recommended dose is 15 mg once daily. In patients who have responded to treatment with upadacitinib, corticosteroids may be reduced and/or discontinued in accordance with standard of care. For patients with UC receiving strong inhibitors of cytochrome P450 (CYP) 3A4 the recommended induction dose is 30 mg once daily and maintenance dose is 15 mg once daily. CD: Induction; Recommended oral dose is 45mg once daily for 12 weeks. For patients who have not achieved adequate therapeutic benefit after the initial 12-week induction, 30mg once daily may be considered. For these patients, upadacitinib should be discontinued if there is no evidence of therapeutic benefit after 24 weeks of treatment. Maintenance; Recommended oral dose is 15mg or 30mg once daily based on individual patient presentation (see SmPC for details). A dose of 15mg once daily is recommended for patients at higher risk of VTE, MACE and malignancy. The lowest effective dose to maintain response should be used. Patients ≥ 65 years of age: recommended dose is 15 mg once daily. In patients who have responded to treatment with upadacitinib, corticosteroids may be reduced and/or discontinued in accordance with standard of care. For patients with CD receiving strong inhibitors of cytochrome P450 (CYP) 3A4 the recommended induction dose is 30 mg once daily and maintenance dose is 15 mg once daily. (see SmPC for more details). Special Populations: Elderly: Upadacitinib should only be used in patients ≥65 years if no suitable treatment alternatives are available. For AD patients ≥ 65 years of age, a dose higher than 15mg once daily is not recommended. For RA, PsA, AS and nr-axSpA there are limited data for patients 75 years of age and older. For UC and CD maintenance therapy, a dose higher than 15 mg once daily is not recommended in patients ≥ 65 years of age. For UC and CD patients ≥ 75 years of age the safety and efficacy of upadacitinib has not been established.
Renal: No dose adjustment required in mild-moderate renal impairment. Patients with severe renal impairment should use upadacitinib with caution (See SmPC for dose adjustments). Upadacitinib has not been studied in subjects with end stage renal disease and is therefore not recommended for use in these patients. Hepatic impairment: No dose adjustment is required in patients with mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment. Should not be used in patients with severe (Child-Pugh C) hepatic impairment. Paediatric Population: Safety and efficacy in RA, AS, PsA, nr-axSpA, UC and CD in children and adolescents aged 0 to 18 years and in AD in children under 12 years of age has not been established. No clinical exposure data are available in AD adolescents < 40 kg. CONTRAINDICATIONS: Hypersensitivity to active substance or excipients. Active tuberculosis (TB) or active serious infections. Severe hepatic impairment. Pregnancy. SPECIAL WARNINGS AND PRECAUTIONS: See SmPC for full details.
Upadacitinib should only be used if no suitable treatment alternatives are available in patients: 65 years of age and older; with history of atherosclerotic cardiovascular disease or other cardiovascular risk factors (such as current or past long-time smokers) or with malignancy risk factors (e.g. current malignancy or history of malignancy).
Immunosuppression: Combination use, with immunosuppressants e.g. azathioprine, 6-mercaptopurine, ciclosporin, tacrolimus, and biologic DMARDs or other JAK inhibitors has not been evaluated in clinical studies and is not recommended as the risk of additive immunosuppression cannot be excluded. Serious infections: Serious and fatal infections have been reported. Caution when treating patients ≥65 years. Frequently reported – pneumonia and cellulitis. Bacterial meningitis and sepsis have been reported. Opportunistic infections reported – TB, multidermatomal herpes zoster, oral/oesophageal candidiasis, and cryptococcosis. Do not initiate treatment in patients with active, serious infection, including localised infections. Consider the risk/benefit of treatment in patients with: chronic or recurrent infection, history of serious or opportunistic infection, those exposed to TB, those who have resided or travelled in
areas of endemic TB or endemic mycoses,those with underlying conditions that may pre-dispose patients to infection, those with diabetes. Closely monitor patients for signs and symptoms of infection during and after treatment. Interrupt treatment if the patient develops a serious/opportunistic infection. If infection is controlled, resume treatment following risk/benefit consideration. A higher rate of serious infections was observed with upadacitinib 30mg compared to upadacitinib 15mg. Tuberculosis: Pre-screen patients for active or latent TB. RINVOQ should not be given to patients with active TB. Consider anti-TB therapy in patients with previously untreated latent TB or in patients with risk factors for TB infection. Consult with a physician with experience in TB therapy to assess whether initiating anti-TB therapy is appropriate for an individual patient. Monitor patients for the development of signs and symptoms of TB, including patients who tested negative for latent TB infection prior to initiating therapy. Viral reactivation: Cases of herpes virus reactivation have been reported e.g. herpes zoster. If herpes zoster is reactivated, consider interruption of upadacitinib therapy until the episode resolves. Screen for viral hepatitis and monitor regularly for reactivation before starting and during therapy with upadacitinib. Patients, positive for hepatitis C antibody/hepatitis C virus RNA and hepatitis B surface antigen/hepatitis B virus DNA were excluded from clinical studies. If hepatitis B virus DNA is detected while receiving RINVOQ, a liver specialist should be consulted. Vaccination: Use of live, attenuated vaccines during or immediately prior to therapy is not recommended. It is recommended that patients be brought up to date with all immunisations, including prophylactic zoster vaccinations prior to starting therapy, in agreement with current immunisation guidelines. Malignancy: Lymphoma, NMSCs and other malignancies have been reported in patients receiving JAK inhibitors, including upadacitinib. There is a higher rate of malignancies and NMSCs with upadacitinib 30mg compared to 15mg. Periodic skin examination is recommended for all patients, particularly those with risk factors for skin cancer. See SmPC for full details. Haematological abnormalities: Do not start therapy if Absolute Neutrophil Count <1 x 109 cells/L, Absolute Lymphocyte Count <0.5 x 109 cells/L and Hb <8 g/dL. Monitor patients and temporarily stop therapy if abnormal haematological parameters as specified are detected during routine patient management. Gastrointestinal perforations: Events of diverticulitis and gastrointestinal perforations have been reported. Use with caution in patients who may be at risk for gastrointestinal perforation (e.g., patients with diverticular disease, a history of diverticulitis, or who are taking non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, or opioids). Patients with active Crohn’s disease are at increased risk for developing intestinal perforation. Patients presenting with new onset abdominal signs and symptoms should be evaluated promptly for early identification of diverticulitis or gastrointestinal perforation. Major adverse cardiovascular events: Events of MACE were observed in clinical studies of upadacitinib (See SmPC for full details). Lipids: Monitor total cholesterol, low-density lipoprotein (LDL) and high-density lipoprotein (HDL) at 12 weeks and thereafter according to international guidelines for hyperlipidaemia. Elevated LDL in clinical trials decreased to pretreatment levels in response to statin therapy, although evidence is limited. The effect of these lipid parameter elevations on cardiovascular morbidity and mortality has not been determined. Hepatic transaminase elevations: Monitor liver enzymes at baseline and thereafter according to routine patient management. Prompt investigation of the cause of liver enzyme elevation is recommended to identify potential cases of drug-induced liver injury. In such cases therapy should be interrupted until this diagnosis is excluded. Venous thromboembolism: Deep vein thrombosis (DVT) and pulmonary embolism (PE) were observed in clinical trials for upadacitinib. Use therapy with caution in patients with known VTE risk factors other than cardiovascular or malignancy risk factors. Risk factors include previous VTE, patients undergoing major surgery, immobilisation, use of combined hormonal contraceptives or hormone replacement therapy, and inherited coagulation disorder. Patients should be re-evaluated periodically during upadacitinib treatment to assess for changes in VTE risk and treatment should be discontinued in patients with suspected VTE. Hypersensitivity: Anaphylaxis and angioedema have been reported in patients receiving upadacitinib. Discontinue treatment and institute appropriate therapy if significant hypersensitivity occurs. INTERACTIONS: Upadacitinib is metabolised mainly by CYP3A4 and plasma exposures may be affected by CYP3A4 inhibitors or inducers. Upadacitinib 15 mg once daily should be used with caution in patients receiving chronic treatment with strong CYP3A4 inhibitors. Upadacitinib 30 mg once daily dose is not recommended for patients with atopic dermatitis receiving chronic treatment with strong CYP3A4 inhibitors. For patients with ulcerative colitis or Crohn’s disease using strong CYP3A4 inhibitors, the recommended induction dose is 30 mg once daily and the recommended maintenance dose is 15 mg once daily (see Dosage and Administration). Avoid food and drink containing grapefruit during treatment with upadacitinib. See SmPC for full details. FERTILITY, PREGNANCY AND LACTATION: Upadacitinib is contraindicated during pregnancy. Women of childbearing potential should be advised to use effective contraception during treatment and for 4 weeks following the final dose of upadacitinib. Female paediatric patients and/or their parents/caregivers should be informed about the need to contact the treating physician once the patient experiences menarche while taking upadacitinib. Upadacitinib should not be used during breast-feeding. The effect of upadacitinib on human fertility has not been evaluated.
ADVERSE REACTIONS: Refer to SmPC for full details on adverse reactions. Very common adverse reactions (≥1/10): upper respiratory tract infections, acne. Common adverse reactions (≥1/100 to <1/10): bronchitis, herpes zoster, herpes simplex, folliculitis, influenza, urinary tract infection, pneumonia, non-melanoma skin cancer, anaemia, neutropaenia, lymphopaenia, urticaria, hypercholesterolaemia, hyperlipidaemia, cough, abdominal pain, nausea, rash, fatigue, pyrexia, increased blood CPK/ALT/AST, increased weight and headache.
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance; website: www.hpra.ie.
LEGAL CLASSIFICATION: POM (S1A). MARKETING AUTHORISATION NUMBER/ PRESENTATION: EU/1/19/1404/001: RINVOQ 15 mg prolonged-release tablets in cartons of 28 tablets; EU/1/19/1404/006: RINVOQ 30 mg prolonged-release tablets in cartons of 28 tablets; EU/1/19/1404/010: RINVOQ 45 mg prolonged-release tablets in cartons of 28 tablets.
MARKETING AUTHORISATION HOLDER: AbbVie Deutschland GmbH & Co. KG, Knollstrasse, 67061 Ludwigshafen, Germany. Further information is available from AbbVie Limited, 14 Riverwalk, Citywest Business Campus, Dublin 24, Ireland. DATE OF REVISION: April 2023. PI-1404-010

Abbreviations: EASI – eczema activity and severity index; NRS – numerical rating scale; QD – once daily;* The recommended dose of RINVOQ is 15mg once daily for adolescents weighing at least 30kg. The safety and efficacy of RINVOQ in children with atopic dermatitis below the age of 12 years have not been established. No data are available. No clinical exposure data are available in adolescents <40kg. The posology in adolescent patients 30kg to <40kg was determined using population pharmacokinetic modelling and simulation.2 ** European Union

References: 1. Langan S. et al. Atopic dermatitis. The Lancet. 2020;396(10247):345-360. DOI: https://doi.org/10.1016/S0140-6736(20)31286-1. 2. RINVOQ Summary of Product Characteristics, available on www.medicines.ie3. Baricitinib Summary of Product Characteristics, available on www.medicines.ie 4. Abrocitinib Summary of Product Characteristics, available on www.medicines.ie

©2023 AbbVie Inc. All rights reserved. IE-RNQD-230022 | May 2023
HELP YOUR PATIENTS
Dermatology
BOX 1. PATIENT ELIGIBILITY CRITERIA, STUDY MEASURES, AND ANALYSES Patient eligibility criteria
Patients included in the study were children aged 6–11 years and adolescents aged 12–17 years with a diagnosis of moderate-to-severe atopic dermatitis (AD). The two cohorts were defined by age at first consultation. The diagnosis of moderate-to-severe AD was defined as patients who at some point since referral to secondary care and while aged 6 years or older, had experienced inadequate disease control with systemic therapy(ies), or required but were contraindicated to systemic therapy(ies), as confirmed by their clinician. Patients also had to be aged 17 years or younger at the initial referral appointment to secondary care, and still be under 18 years of age at the most recent consultation, thus ensuring collection of at least 1 years' worth of data. Patients had to have been seen in a secondary care clinic on 1 January 2014 or later to avoid capturing more historic data, and have at least 12 months of data in their medical records post 1 January 2014, while aged 6–17 years. Patients were excluded if they had been treated with biologic therapy for their AD, and/or had been involved with a clinical trial for an investigational drug for AD during the observation period.
Study measures
Data were collected on patient demographics and clinical characteristics; patient healthcare resource utilisation, including consultation history, hospitalisations, and treatment history; treatment-related outcomes; and, where available in the existing medical records. Patient-reported outcomes (PRO) data included the dermatology life quality index-children's version,1 the patient orientated eczema measure,2 and the EuroQol-5 dimension questionnaire.3 PRO scores were reported as ‘latest score’ where patients had only one available score, and as ‘earliest score’ and ‘latest score’ where patients had >1 score.
Analyses
All analyses were descriptive. Means and standard deviations, and medians and ranges (minimum and maximum values) were calculated for continuous variables; frequency and percentages were calculated for categorical variables. Analyses were based on observed data only. Missing data were not imputed and, for some questions, ‘don't know’ or ‘unknown’ were valid responses. The number of patients included for any analysis could therefore vary. Where data are missing, the number of patients is reported since this differs from the total cohort population. Both child and adolescent cohorts are included in the total patient sample. Due to the low numbers of adolescents (n = 9) recruited, data for the total patient sample and the child cohort only are reported for the majority of analyses.
Descriptive statistics were generated using the software package STATA® Version 16 or later (StataCorp, 2015. Stata statistical software: Release 16 [StataCorp LP]).
Patient inclusion/exclusion criteria
Patients were children aged 6–11 years and adolescents aged 12–17 years with a diagnosis of moderateto-severe AD (Box 1 above).
Results
Patient demographics
Thirty-one patients were included, made up of 22 children and 9 adolescents.
For the total 31 patient sample, mean (SD) duration, that is, time between first and most recent consultations during the observation period was 4.4 (1.9) years. Males predominated (total sample 61.3%; children, 59.1%) and, based on body mass index, 84.6% of all patients had a healthy body weight.
Patients had a mean (SD) of 2.7 (1.5) comorbid conditions, the majority of which were atopic in nature. Children most frequently had food allergies (68.2% of patients), allergic rhinitis (59.1%), and asthma (59.1%); adolescents mostly had asthma (55.6%) and
food allergies (44.4%). Infectious episodes were reported for over one-third of the total population (n = 11, 35.5%).
Patient clinical characteristics and clinical investigations
The majority of patients (n = 25, 80.6%) had a clinical phenotype of mixed AD (defined as concomitant respiratory allergies). This was higher (n = 20, 90%) in the child cohort. Three patients (9.7%; all adolescents) had ‘pure’ AD without atopic comorbidities (defined as no associated respiratory conditions). Affected body surface area (BSA) was reported at earliest consultation during the observation period for only three patients (BSA range 50%–90%).
Considering BSA and other relevant factors, AD was severe in 74.2% (n = 23) of patients at first referral. Mean (SD) patientreported flare episodes/patient/ year of observation was 2.0 (1.1).
A mean (SD) of 18.5 (12.2) tests/ scans/procedures per patient/ year of observation were reported; those most frequently performed were full blood count (n = 31,
100%) and renal function tests (n = 30, 96.8%). Half of children were tested for food allergies (n = 11, 50.0%).
Treatment history
A total of 412 prescriptions were recorded, of which 298 (70.1%) were for patients in the children's cohort. Overall, patients had each tried a mean (SD) of 13.4 (4.8) different prescribed treatments over the observation period and there was a mean of 3.7 (2.2) treatments/patient/year of observation. The most frequently prescribed treatment class was TCS (n = 200 prescriptions, 48.5%).
From start to end of treatment, patients had been using TCS for a mean (SD) of 38.6 (41.1) months, with treatment ongoing for 72 (36.0%) courses of these treatments. The most common stated reason for discontinuation of TCS treatment was course completed (n = 63, 49.2%).
Fewer than 5% of prescriptions each were for nonsteroidal topical treatments (all for tacrolimus),
which were used for the longest duration, amounting to a mean of 54.4 (41.4) months, and systemic corticosteroids (SCS; all oral or injections of prednisolone) that were used for a mean (SD) of 2.0 (3.1) months. As with TCS, nonsteroidal topical treatments were discontinued for reasons of ‘condition improved’ and ‘course completed’ (each n = 4/17, 30.8%), and all SCS were discontinued as the course of treatment was completed (n = 9, 100%).
Of the total 44 systemic immunosuppressant prescriptions, the most frequently prescribed were methotrexate (n = 30, 68.2%), ciclosporin (n = 7, 15.9%), and azathioprine (n = 4, 9.1%). Collectively, these were used for a mean (SD) of 27.6 (21.1) months. Treatment was ongoing for over half of these prescriptions (n = 25, 56.8%), with discontinuations due mainly to primary (n = 3/44, 15.8%) and secondary (n = 6/44, 31.6%) failure.
Only 3 (0.7%) prescriptions were for phototherapy, with a mean (SD) duration of 1.2 (0.6) months. ‘Other’ treatments, which included wet wraps, bleach baths, antihistamines, analgesics, and anti-infectives, were also frequently prescribed (n = 139 prescriptions, 33.7%), two-thirds of which were discontinued as the course was completed (n = 55, 66.2%).
Healthcare resource utilisation Consultations
Time from first to most recent consultation/patient (the observation period) was 4.4 (1.9) years. During this time, there was a mean (SD) of 15.3 (6.3) consultations/patient (virtual consultations, mean [SD] 1.2 [0.4]), or an annualised rate of mean (SD) 3.2 (1.3) consultations/patient/year of observation, suggesting that on average patients were attending the secondary care clinic every 4 months. The most frequently selected reasons for a consultation were for a routine appointment (total sample 46.9%; child cohort 50.1%) and follow up (total sample 43.8%; child cohort 40.6%), mainly for a clinical/treatment review. Flare was the reason cited for ≤10% of consultation visits (total sample 5.9%; child cohort 6.7%).
Over 70% of all consultations were with consultant dermatologists, and over 80% were with dermatology trainees; around 40% were with nurse specialists. Time allocated for consultant
20 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
dermatologist/paediatrician/ registrar consultations for a clinical/treatment review or followup were 15 min each.
Hospitalisations
Across all 31 patients, there was a mean (SD) of 0.4 (0.7) AD-related hospitalisations/patient during the observation period, and 0.03 (0.04) AD-related hospitalisations/ patient/year of observation. While the observed annualised rates were low, a quarter of patients (8/31, 25.8%) were hospitalised at least once during the observation period. For these patients, there was a mean (SD) of 1.5 (0.5) AD-related hospitalisations/patient, and 0.07 (0.04) AD related hospitalisations/ patient/year of observation.
The majority (75.0%) of patients hospitalised were children.
The eight patients hospitalised were admitted on a total of 12 occasions; six of those patients were children (i.e., 27.3% of the 22 children in the cohort) and they were hospitalised on 9 occasions. The total hospital stay during the observation period was 6.6 nights/ hospitalised patient (child cohort: 6.3), and 4.4 nights/hospitalisation (child cohort: 4.2). Over 90% of hospitalisations (child cohort: 88.9%) were due to flare, and over 80% (child cohort: 77.8%) for treatment administration.
Although none of the 12 hospitalisations required an ambulance call out, 7 (58.3%; child cohort n = 5, 55.6%) were classed
as an emergency visit, while 5 (41.7%; child cohort: n = 4, 44.4%) were elective.
Patient reported outcomes
The Dermatology Life Quality Index-Children (cDLQI) (score range 0–30) was the only PRO with available data. Data for the cDLQI were available for 29 (93.5%) patients, with a mean (SD) of 5.3 (3.4) scores captured/patient.
The earliest mean (SD) cDLQI score for the overall population was 9.7 (6.2), which reduced at the latest cDLQI score to 6.6 (5.5). The cDLQI score indicated that, the change in HRQoL from earliest to latest cDLQI score improved for 72.0% (n = 18) of patients and deteriorated in 20.0% (n = 5). The minimally clinically important difference (MCID) for cDLQI has been estimated as a 6-point change, indicating that the observed reductions may not be clinically meaningful.14
Discussion
There are no data available on the impact of AD on the health service and treatment of young people in Ireland. For this reason, we undertook this study to assess the treatment journey and effects of treatment in children and adolescents with moderate-tosevere AD at Children's Health Ireland, Crumlin, the major AD centre and largest paediatric hospital in Ireland. This RWE study, covering an observation period of mean 4.4 years/patient,
is the first study to highlight the substantial HCRU burden due to tests, procedures, prescriptions, therapy lines, consultations, and hospitalisations for AD in Ireland. Our results reflect a similar previous UK study in children and adolescents.15
Significant exacerbation of AD (flares) causes considerable distress to patients and we found that the moderate-to-severe AD patient cohort studied here experienced two flare episodes/ patient/year of observation. We recognise that this is considerably lower than the nine flares/ year reported in another realworld interview-based study of >900 children and adolescents with moderate-to-severe disease.16 However, reasons for the discrepancy may include differences in data source (i.e., medical records vs. interview), over-diagnosis through selfreporting, treatment compliance issues, or discrepancies between physician medical coding and patient perception.
The average number of tests, scans and procedures were high for this patient group, with each person having a mean of 18.5 per year of observation. All patients had full blood counts and all but one had liver/renal function tests. Immunoglobulin E (IgE) level was evaluated in many patients, yet, while a typical feature, IgE elevation is not present in all AD cases.17, 18 These tests alone place a substantial burden on patients and healthcare providers.
Our data suggest that clinicians generally followed treatment guidelines,8, 18, 19 initially prescribing TCS to control inflammation and systemic immunosuppressants for the most severe cases. Each patient studied used over three pharmacotherapies a year. TCS prescriptions predominated, with any discontinuation primarily because the patients' condition improved or the treatment course had been completed. In contrast, systemic immunosuppressant prescriptions were relatively rare and mostly ongoing, with discontinuations mainly due to treatment failure. Similarly in the US, evaluation of current treatment among >600,000 paediatric AD patients within a claims database found that about 80% of patients were prescribed ≥1 AD medication, and 87% were prescribed a TCS; SCS were prescribed in 14.7% of all patients and immunosuppressant use was rare (0.2%).20
Our data also indicate that current treatment options are not always effective in managing moderateto-severe AD symptoms, leaving patients with inadequately controlled disease. Generally, other real-world studies have reported poor disease control across all AD severity levels,21-23 and suggested a lack of effective long-term treatment options.24
We found that children and adolescents with moderate-tosevere AD attended a consultation every 4 months, mostly for routine and follow-up appointments. Notably, while overall rates of hospitalisation were low per patient year of observation, overall, the data suggest a onein-four chance of hospitalisation in 6–17-year-olds with AD over a ~4-year period. Additionally, those hospitalised spent approximately six nights in hospital, with each stay lasting longer than four nights, and over half were classified as an emergency. To set this in a wider international context, other studies have found that children with AD use more health services, are prescribed more therapies, are more likely to visit emergency departments, and be hospitalised than their counterparts without AD.25-28 For example, a US study reported that, among 1207 children with AD, there were 1641 hospitalisations with ≥1 overnight stay, amounting to 239,832 annual hospitalisation days.28 Additionally, those with AD had significantly higher odds of having 1–2 and ≥3 overnight hospitalisations in the past year
21 HOSPITALPROFESSIONALNEWS.IE | HPN • SEPTEMBER 2023
Dermatology
compared with children without AD (adjusted odds ratio, 1.17 and 1.64, respectively).28 However, hospitalisations in other scenarios are not comparable for the total number of cases studied in other papers.
The paediatric AD cDLQI scores observed in our study suggest that, despite some improvement over the observation period, AD had a significant negative impact on the QoL of more than one-third of children (not assessed statistically and small sample size). This is in line with other studies in children and adolescents reporting similar point estimates for cDLQI scores,
NEWS
Children's Health Ireland receives referrals from all primary care in Dublin and from secondary care nationally with around 90% of all Irish children treated with systemic therapies for AD managed within the service.
Conclusions
indicating the high negative impact of AD, particularly for those with severe disease.3, 4, 22, 29
This study is not without limitations. This data set represents the first study describing the treatment pathway and HCRU for children and adolescents with AD in the Irish healthcare setting and was carried out in a major influential centre that is the largest children's hospital in Ireland. While unlikely, it may differ from other sites which could have slightly different AD management practices. The findings were limited by reliance on data available from patient medical records only and patient enrolment
depended on the physicians' judgement of disease severity. Although reflective of routine care, this did result in some missing data, and in particular, PRO scores were not complete. Sourcing data from hospital records alone also means their further characterisation is warranted. Additionally, the study design imparted a recruitment/selection bias, since patients at the lowest severity level of AD are evaluated less frequently, and more patients with severe AD than moderate AD were selected. We also acknowledge that the total sample size is relatively small compared to other international studies.
Medical Council launch Questionnaire
This is the first study examining paediatric AD HCRU in Ireland. We found that moderate-to-severe paediatric AD is associated with complex treatment patterns and high healthcare burden in Irish outpatient secondary care. Annualised per person, hospitalisation rates are low but, for the 25% of patients who were hospitalised during the study, stay was protracted and, in some cases, repeated. Observed overall improvement in QoL was small and below the MCID for cDLQI. These data were collected from records from the main treating centre in Ireland. This may represent a limitation as primary care provision has been excluded from the analysis. We recognise that further work is needed to fully characterise the national paediatric AD burden to the Irish healthcare system but these findings highlight the significant burden that currently exists and were collected within a service which treats around 90% of all Irish children with systemic therapies for AD… As biologic therapies become increasingly available for the 6–17-year-old age group over the next few years, the data reported here may provide further justification for their uptake and use in paediatric patients with severe disease.
References available on request
The Medical Council has announced that it is developing its five-year strategic plan, to cover the period 2024 – 2028. To inform its development, a public consultation has been launched for a three-week period from 15th August until 5th September 2023.
The Medical Council is inviting members of the public, including patients, the medical profession, media, and other stakeholders, to give their views by participating in the survey.
This public consultation is available in an online survey questionnaire format. It will enable the Medical Council to obtain a broad range of feedback from individuals and organisations and inform its draft strategic plan.
The Medical Council has been following its current Statement of Strategy since 2019. This new strategic plan is the fourth strategic plan since the introduction of the 2007 Medical Practitioners Act and will be a progression of the previous 2019 – 2023 strategy.
The public consultation survey questionnaire is available at www.medicalcouncil.ie
It closes on Tuesday 5th September 2023.
22 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
YOUR ALL-IN-ONE
With all the benefits you expect from an adalimumab biosimilar, and more

Patient friendly:
Citrate-free and concentrated formula with a 50% lower injectable volume* to reduce pain following injection1,2
Experienced: European production supported by over 20 years of biosimilars experience**
Flexible: Two subcutaneous administration options
The new adalimumab biosimilar from CLONMEL HEALTHCARE1
THE MEDICINES MANAGEMENT PROGRAMME RECOMMENDS
HUKYNDRA AS A BEST VALUE BIOLOGICAL MEDICINE FOR ADALIMUMAB3
ABBREVIATED PRESCRIBING INFORMATION. Hukyndra 40 mg solution for injection in pre-filled syringe. One 0.4 ml single dose pre-filled syringe contains 40 mg adalimumab. Hukyndra 40 mg solution for injection in pre-filled pen. One 0.4 ml single dose pre-filled pen contains 40 mg adalimumab. Indications: 1) Rheumatoid arthritis 2) Juvenile idiopathic arthritis 3) Axial spondyloarthritis 4) Psoriatic arthritis 5) Psoriasis 6) Paediatric plaque psoriasis 7) Hidradenitis suppurativa (HS) 8) Crohn’s disease 9) Paediatric Crohn’s disease 10) Ulcerative colitis 11) Paediatric ulcerative colitis 12) Uveitis 13) Paediatric uveitis. Dosage: Refer to Summary of Product Characteristics. Method of administration: Hukyndra is administered by subcutaneous injection. Full instructions for use are provided in the package leaflet. Hukyndra is only available as 40 mg pre-filled syringe and 40 mg pre-filled pen. Thus, it is not possible to administer Hukyndra to patients that require less than a full 40 mg dose. If an alternate dose is required, other adalimumab products offering such an option should be used. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Active tuberculosis or other severe infections such as sepsis, and opportunistic infections. Moderate to severe heart failure (NYHA class III/IV). Warnings and precautions: Infections (Serious infections, Tuberculosis, Other opportunistic infections), Hepatitis B reactivation, Neurological events, Allergic reactions, Immunosuppression, Malignancies and lymphoproliferative disorders, Haematologic reactions, Vaccinations, Congestive heart failure, Autoimmune processes, Concurrent administration of biologic DMARDS or TNF-antagonists, Surgery, Small bowel obstruction, Elderly and Paediatric population. Interactions: Refer to Summary of Product Characteristics. Fertility, pregnancy and lactation: Women of childbearing potential should consider the use of adequate contraception to prevent pregnancy and continue its use for at least five months after the last Hukyndra treatment. Hukyndra should only be used during pregnancy if clearly needed. Hukyndra can be used during breast-feeding. Driving and operation of machinery: Hukyndra may have a minor influence on the ability to drive and use machines. Vertigo and visual impairment may occur following administration of Hukyndra. Undesirable effects: Very common: Respiratory tract infections (including lower and upper respiratory tract infection, pneumonia, sinusitis, pharyngitis, nasopharyngitis and pneumonia herpes viral), Leukopenia (including neutropenia and agranulocytosis), Anaemia, Lipids increased, Headache, Abdominal pain, Nausea and vomiting, Elevated liver enzymes, Rash (including exfoliative rash), Musculoskeletal pain, Injection site reaction (including injection site erythema). A copy of the SmPC is available upon request or go to www.clonmelhealthcare.ie Pack size: Hukyndra 40 mg solution for injection in pre-filled syringe - 2 pre-filled syringes + 2 alcohol pads, Hukyndra 40 mg solution for injection in pre-filled pen - 2 pre-filled pens + 2 alcohol pads. Marketing authorisation holder: STADA Arzneimittel AG, Stadastrasse 2-18, 61118 Bad Vilbel, Germany. Marketing authorisation numbers: EU/1/21/1589/002, EU/1/21/1589/005. Distributed by Clonmel Healthcare Ltd. Medicinal product subject to medical prescription. Date last revised: December 2021. 2023/ADV/HUK/130H. Date of Preparation: May 2023.

*When compared with Humira®. For example, 0.4 mL instead of 0.8 mL for a Hukyndra® 40 mg dose. **STADA founded Bioceuticals Arzneimittel AG in 2000.
1. Hukyndra® SmPC (Jan. 2022).
2. Nash P et al. Rheumatol Ther 2016;3:257–270. 3. Best-value biological medicines https://www.hse.ie/eng/about/who/cspd/ncps/medicines-management/best-value-medicines/best-valuebiological-medicines/
http://biosimilars.stada
40 mg 40 mg
Opioids
Opioid Prescribing Updates
Opioid Prescribing Updates from the Updated CDC Opioid Prescribing Guidelines
Written by Dr Cormac F. Mullins, MB, BAO, BCh, FCAI, FFPMCAI, DPMCAI, MSc Consultant in Anaesthesia and Pain Medicine, Cork University Hospital and South Infirmary Victoria University Hospital

drcormacmullins@gmail.com
A 45-year-old gentleman was admitted to hospital with a fractured tibial plateau following a road traffic collision. He was admitted under the orthopaedic team and placed on the emergency surgical theatre list. Due to a high volume of trauma patients admitted to the hospital, there were significant waiting times for access to theatre. The orthopaedic intern on the ward prescribed paracetamol and modified-release oxycodone 20mg twice daily, which was uptitrated to 30mg twice daily after three days. Later that week, he underwent operative fixation of the fracture. The anaesthetic team avoided regional anaesthesia due to the risk of compartment syndrome. His pain did not decrease in the expected manner postoperatively and this affected his engagement with physiotherapy. His modified-release oxycodone was uptitrated to 40mg twice daily. Due to substantial bed pressure that existed in the hospital, he was discharged on the third postoperative day with a script for analgesia and a follow-up appointment in the orthopaedic clinic in 6 weeks’ time. The discharging intern made sure he had sufficient analgesia to cover him for this period. When he presented to a busy orthopaedic clinic 6 weeks’ later, he remained on modified-release
oxycodone. He requested that his script be refilled and this request was granted.
Opioid analgesics are highly effective in the management of acute severe pain. However, their use in a chronic setting is associated with an increased risk of opioid misuse, overdose and tolerance among other risks. A systematic review conducted by the Agency for Healthcare Research and Quality concluded that insufficient evidence existed for the long-term benefits of opioids in chronic pain.1 As higher doses are often required in order to maintain adequate analgesia, dose-related risks such opioidinduced respiratory depression, opioid-induced bowel dysfunction and dependence become more common. In addition, their use in the elderly comes with an increased risk of cognitive impairment and falls. Despite a lack of evidence for their benefit in chronic pain, their use has increased substantially in many developed countries, including in Ireland.2 Increased prescribing rates can lead to opioid-use disorder and opioid diversion, with many patients reporting not disposing of finished opioid prescriptions.
The 2016 Centers for Disease Control and Prevention (CDC) guideline for Prescribing Opioids for Chronic Pain was released
to provide clinicians with a tool to assess the benefits and risks of opioid prescribing in chronic pain.3 Its introduction in the USA co-incided with rapid reductions in opioid dispensing rates per capita, with reductions in overall and high-risk prescribing, and increases in prescribing rates of non-opioids.4 Subsequent policies and legislation that arose out of this guideline, along with inflexible application of its recommendations, led to reports of overzealous tapering regimens, undertreated pain, acute withdrawals and accompanying psychological distress and unnecessary patient harm.5 These reports led to the development of a revised CDC opioid guideline in 2022 with a greater emphasis on patientcentred care, individualised care plans and flexibility.6 In addition to this, more recent evidence was included to support its prescribing recommendations in acute pain (<1 month duration) and subacute pain (1-3 months’ duration).
The new CDC guideline includes evidence from recent systematic reviews that non opioid medications are at least as effective as opioids for improving pain, function, or both, in many types of acute pain conditions, such as low back pain, neck pain, musculoskeletal conditions, minor surgery, dental procedures, headache or kidney stones. The guideline recommends that nonopioids are maximised where appropriate and where no contra-indication exists (e.g., oral or topical nonsteroidal anti-inflammatory medications). Nonopioids can include nonpharmacological interventions, such as exercise and psychological therapies, nonopioid medications, including serotonin and norepinephrine reuptake inhibitor (SNRI) antidepressants, pregabalin and gabapentin, nonsteroidal antiinflammatory drugs (NSAIDs), and paracetamol. Nonopioid therapies are preferred in subacute pain and chronic pain. In moderate-tosevere pain where nonopioids are insufficiently effective or contraindicated, opioids can provide
a useful role. Like the previous guideline, the 2022 guideline recommends that opioids be prescribed at the lowest effective dose for no longer than the expected duration of severe pain. Unlike in the case above, patients should not be provided with extended scripts for opioid medication, especially for acute or subacute pain. Tapering should be instituted where opioids are used continuously for greater than a few days.
Both 2016 and 2022 CDC guidelines advise that when commencing opioids for subacute or chronic pain, an assessment should take place of the expected benefits, risks and goals of treatment. The goals of treatment can be considered in terms of reductions pain intensity and improvements in activities of daily life. If the agreed thresholds for treatment goals are not reached after a reasonable trial, or if the side effects exceed the benefits, then the trial should be considered unsuccessful and the opioid should be weaned and stopped. Unlike in the case above, when commencing opioids for acute, subacute, or chronic pain, immediate-release opioids should be prescribed instead of modified-release or long-acting preparations.6 Other speciality bodies have release statements and publications warning of the increased risks associated with long-acting opioids, including opioid-use disorder, prolonged hospital admission, respiratory depression, and death.7,8
Extended-release preparations were introduced into surgical practice in the mistaken belief that they provided superior pain relief and reduced nursing workload. Marketing campaigns promoted the idea of steady state plasma concentrations and a simple dosing schedule. However, the trajectory of acute pain and postoperative pain is typically one of intermittent exacerbations of pain, especially associated with mobilisation, physiotherapy and exercise, which requires additional analgesia on an as required basis to facilitate such activity. Therefore, many patients require
24 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
additional doses of immediate release opioids on top of their extended-release opioid, which can contribute to additional side effects and worse pain control.9 The FDA has advised that some extended-release preparations should only be considered where patients have received a required dosage of an immediate-release preparation for at least one week.6
The new 2022 guideline specifically recommends that in cases of subacute opioid therapy (1 to 3 months), opioids should not be re-prescribed without carefully reassessing the goals, benefits and risks again, in order to prevent progression to unintentional long-term opioid therapy. This is illustrated in the case above. Frequently, opioids may be commenced in hospital and renewing the prescription may assumed by a different clinician, who may be unaware that the opioid was only commenced recently, and therefore, its continued
use should be re-assessed. Furthermore, many patients do not get a benefit in pain reduction or improvement in function from increasing opioid doses to greater than 50mg Morphine Milligram Equivalents (MME) per day and are likely to lead to diminishing returns, but with a substantially increased risk of side effects.
Where patients are already receiving opioids, the new guideline outlines how clinicians can work together with patients to determine whether opioids should be discontinued and how this can be done. Again, the benefits and risks of continuing opioid therapy should be weighed against the benefits and risks of stopping opioids. The guideline emphasises the importance of patient-centred care and shared decision-making especially as there may be reluctance or fear on the part of the patient to reduce their opioids. General principles are emphasised, rather than hard cut-offs, to avoid the
application of inflexible standards that may be misapplied in certain circumstances. Tapering should be done slowly, especially where opioids have been used for a longer timeframe (e.g., ≥1 year), and reductions of approximately 10% per month should be better tolerated by patients than more rapid tapers. Patient motivation is optimal for successful tapers. Stopping opioid therapy after prolonged use can be very challenging and it is important that patients have effective support and do not feel abandoned. Clinicians should offer follow-up to patients at frequent intervals (ideally, at least monthly), and multidisciplinary input other team members including pharmacists and nursing is highly valuable. Tapers may have to be paused and recommenced if withdrawal symptoms appear or when the patient feels ready. Ideally, opioid tapering should be performed within the context of a multimodal and multidisciplinary
approach to the patient’s pain management that is individualised and incorporates evidencebased treatment strategies and psychological support with the goal of improving patients’ function and quality of life, while minimising the risks of opioid therapy.

The central tenets of the updated CDC guidelines for opioid prescribing emphasise the importance of ensuring adequate access to effective pain management with an emphasis on improving function and quality of life, while minimising the risks associated with prescription opioid therapy. Communication between healthcare professionals and patients around the risks and benefits of opioids are central to ensuring patient-centred approaches to pain management and addressing the unique needs of every patient.
References available on request
25 HOSPITALPROFESSIONALNEWS.IE | HPN • SEPTEMBER 2023
Oncology Focus: Cancer Research

Establishing a National Cancer Mission Hub
The NCCP Collaborates with the All-Island Cancer Research Institute (AICRI) to establish a National Cancer Mission Hub in Ireland
Establishing a Na�onal Cancer Mission Hub
Establishing a Na�onal Cancer Mission Hub
heading: The NCCP Collaborates with the All Island Cancer Research Ins�tute (AICR
Establishing a Na�onal Cancer Mission Hub
heading: The NCCP Collaborates with the All Island Cancer Research Ins�tute (AICR


Na�onal Cancer Mission Hub in Ireland
Na�onal Cancer Mission Hub in Ireland

Donal Brennan (NCCP) William Gallagher
Establishing of Cancer Mission
Hubs: Networks and Synergies (ECHoS) is a new three-year European consortium supported by the EU Mission on Cancer and brings together from 58 organisations from governmental, healthcare, research, academic and non-profit sectors distributed across 28 countries (¤6 million funding 2023-2026).
heading: The NCCP Collaborates with the Cancer Research Ins�tute (AICR to establish Na�onal Cancer Mission Hub in Ireland


Donal Brennan (NCCP) William Gallagher
moving policy-making processes towards people-centric healthcare and research systems in ways that cannot be achieved through individual efforts and fragmented ini�a�ves.
The NCMHs will encourage participation from stakeholders and citizens in cancer-related policy dialogues at EU, national, regional and local levels It will help to advance better integration of cancer research and policy. Further information on the hubs is available on the ECHoS website (https://cancermissionhubs.eu/)
National Cancer Mission Hubs (NCMHs) will be created in 28 countries (25 Member States, 3 Associated Countries). Ireland is represented within the ECHoS project by the HSE National Cancer Control Programme (NCCP: https://www.hse.ie/eng/ services/list/5/cancer/) as the primary national lead and the AllIsland Cancer Research Institute (AICRI: https://www.aicri.org/) as co-participant. Both the NCCP and AICRI will collaborate closely with the consortium coordinators and leading experts across Europe, to establish a National Cancer Mission Hub (NCMH) in Ireland. In terms of project leadership, Prof. Donal Brennan is the National Clinical Lead for Cancer Research and chairs the NCCP Cancer Research Group. Prof. William Gallagher is a cancer biologist based in University College Dublin and co-lead of AICRI. Both the NCCP and AICRI will collaborate closely to establish a NCMH in Ireland. This combined effort
will coordinate research and innovation and healthcare actions on cancer, moving policy-making processes towards people-centric healthcare and research systems in ways that cannot be achieved through individual efforts and fragmented initiatives.
The NCMHs will encourage participation from stakeholders and citizens in cancer-related policy dialogues at EU, national, regional and local levels. It will help to advance better integration of cancer research and policy. Further information on the hubs is available on the ECHoS website (https://cancermissionhubs.eu/)
Representatives of AICRI and the NCCP attended the launch of the ECHoS project in Lisbon in early May 2023. The kick-off meeting in Lisbon allowed the project partners to meet, to learn more about the European Strategy on Cancer and hear about other EU-funded projects in cancer research and service delivery. The launch meeting provided a better understanding of EC expectations for ECHoS and the National Cancer Mission Hubs. Partners also learned more about the ECHoS project’s six work packages.
Bridging cancer care, cancer policy and research
Bridging cancer care, cancer policy and research

This establishment of a NCMH in Ireland aligns with Ireland’s National Cancer Strategy 2017-26, which has a particular focus on involving patients in their own cancer care and further developing the role of research to improve cancer services, as well as aligning with the principles of Sláintecare and the involvement of citizens in how Ireland shapes its health services. It is a key step towards strengthening the voice of national stakeholders in cancer policies in Europe. It will foster national and international collaboration, promote inclusivity, and drive innovation in cancer care. It will also help to accelerate cancer
Establishing of Cancer Mission Hubs: Networks and Synergies (ECHoS) is a new three-year European consortium supported by the EU Mission on Cancer and brings together from from governmental, healthcare, research, academic and non-profit sectors across 28 countries (€6 million funding 2023-2026)
Establishing of Cancer Mission Hubs: Networks and Synergies (ECHoS) is a new three European consortium supported by the EU Mission on Cancer and brings together from from governmental, healthcare, research, academic and non-profit sectors across 28 countries (€6 million funding 2023-2026)
Establishing of Cancer Mission Hubs: Networks and Synergies (ECHoS) European consortium supported by the EU Mission on Cancer and ations from governmental, healthcare, research, academic across 28 countries (€6 million funding 2023-2026)
Representatives of AICRI and the NCCP attended the launch of the ECHoS project in Lisbon in early May 2023. The kick-off meeting in Lisbon allowed the project partners to meet, to learn more about the European Strategy on Cancer and hear about other EU-funded projects in cancer research and service delivery. The launch meeting provided a better understanding of EC expectations for ECHoS and the National Cancer Mission Hubs. Partners also learned more about the ECHoS project’s six work packages
Na�onal Cancer Mission Hubs (NCMHs) will be created in 28 countries (25 Member States, Ireland is represented within the ECHoS project by the HSE htps://www.hse.ie/eng/services/list/5/cancer/) as the primary na�onal lead and the All-Island Cancer Research Ins�tute (AICRI: par�cipant. Both the NCCP and AICRI will collaborate closely with the consor�um coordinators and leading experts across Europe, to establish a Na�onal Cancer Mission Hub (NCMH) in Ireland. In terms of project leadership, Prof. Donal Brennan a�onal Clinical Lead for Cancer Research and chairs the NCCP Cancer Research Group. Prof. William Gallagher is a cancer biologist based in University College Dublin and co-lead Both the NCCP and AICRI will collaborate closely to establish a NCMH in Ireland. This research and innova�on and healthcare ac�ons on cancer,
Na�onal Cancer Mission Hubs (NCMHs) will be created in 28 countries (25 Member States, Ireland is represented within the ECHoS project by the HSE Na�onal Cancer Control Programme (NCCP: htps://www.hse.ie/eng/services/list/5/cancer/) as primary na�onal lead and the All-Island Cancer Research Ins�tute (AICRI: par�cipant. Both the NCCP and AICRI will collaborate closely with the consor�um coordinators and leading experts across Europe, to establish a Na�onal Cancer Mission Hub (NCMH) in Ireland. In terms of project leadership, Prof. Donal Brennan a�onal Clinical Lead for Cancer Research and chairs the NCCP Cancer Research Group. Prof. William Gallagher is a cancer biologist based in University College Dublin and co-lead Both the NCCP and AICRI will collaborate closely to establish a NCMH in Ireland. combined effort will coordinate research and innova�on and healthcare ac�ons on cancer,
Na�onal Cancer Mission Hubs (NCMHs) will be created in 28 countries Associated Countries) Ireland is represented within the ECHoS project Cancer Control Programme (NCCP: htps://www.hse.ie/eng/services/list/5/cancer/)


primary na�onal lead and the All-Island Cancer Research htps://www.aicri.org/) as co-par�cipant. Both the NCCP and AICRI with the consor�um coordinators and leading experts across Europe, Cancer Mission Hub (NCMH) in Ireland. In terms of project leadership, a�onal Clinical Lead for Cancer Research and chairs the NCCP Prof. William Gallagher is a cancer biologist based in University College AICRI. Both the NCCP and AICRI will collaborate closely to establish combined effort will coordinate research and innova�on and healthcare
research in Ireland. The NCMH is an opportunity to tailor our cancer landscape to the precise needs of our patients and society and it is expected to develop some guidelines and tools that can help other organisations to implement relevant, impact-driven, research and policy dialogues in meaningful areas of health and beyond.
Time to Act
This establishment of a NCMH in Ireland aligns with Ireland’s Na�onal Cancer Strategy 201726, which has a par�cular focus on involving pa�ents in their own cancer care and further developing the role of research to improve cancer services, as well as aligning with the principles of Sláintecare and the involvement of ci�zens in how Ireland shapes its health services. It is a key step towards strengthening the voice of na�onal stakeholders in cancer policies in Europe. It will foster na�onal and interna�onal collabora�on, promote inclusivity, and drive innova�on in cancer care. It will also help to accelerate cancer research in Ireland
The NCMH is an opportunity to tailor our cancer landscape to the precise needs of our pa�ents and society and it is expected to develop some guidelines and tools that can help other organisa�ons to implement relevant, impact-driven, research and policy dialogues in meaningful areas of health and beyond
Cancer represents a global health challenge and the fight against cancer can no longer be an isolated endeavour. Currently, research, innovation and healthcare initiatives are largely siloed within specific communities and the engagement of citizens
26 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Professor Donal Brennan
Written by Professor Donal Brennan (NCCP), Professor William Gallagher (AICRI), Dr Rosemarie Gannon (AICRI) and Dr Ozlem McDonnell (NCCP)
Professor William Gallagher
1
Written by Professor Donal Brennan (NCCP) and Professor (AICRI)
1
1
Dr Rosemarie Gannon (AICRI) Dr Ozlem McDonnell (NCCP)
Available in 3 flavours
Consider Fresubin®




3.2 kcal Drink as a shot
Vanilla-Caramel
Hazelnut
Mango


(Actual dosage is at the recommendation of the healthcare professional)
VITAMIN HIGH ENERGY 400 kcal
PROTEIN 20g D10µg/125ml
per 125ml bottle
When you’re looking for a low volume, energy dense ONS*, why not choose the one with the most protein per 125ml bottle?**
*Oral nutritional supplements

**mims.ie October 2022, Republic of Ireland

T:
F: +353 (0)1 849 6949 www.fresenius-kabi.ie


Date of Preparation: October 2022
Ref: IE-Fre3.2-2200006
Fresenius Kabi Ireland Unit 3B Fingal Bay, Balbriggan Co. Dublin, Ireland.
+353 (0)1 841 3030
existent. Supported by the European Bea�ng Cancer Plan and the EU Mission on Cancer, cancer occupies a central place within the European poli�cal agenda. Europe’s poli�cal leaders are now championing a pan-European coopera�on towards a Health in All Policies (HiAP) approach, and a data-informed, ci�zen-focused, research-driven agenda. The �me to act is now
Oncology Focus: Cancer Research
this event. There will be five main sessions as follows:
Session 1: Ireland’s Participation in EU Cancer Mission Projects
Session 2: How Can We All Benefit from Europe’s Beating Cancer Plan?
Session 3: The Lived Experience at the Heart of National and European Cancer Initiatives
Session 4: Co-ordination of Cancer Research at National and European Levels
Session 5: Engagement with Ireland’s National Cancer Mission Hub going forward
is either poor or non-existent. Supported by the European Beating Cancer Plan and the EU Mission on Cancer, cancer occupies a central place within the European political agenda. Europe’s political leaders are now championing a pan-European cooperation towards a Health in All Policies (HiAP) approach, and
a data-informed, citizen-focused, research-driven agenda. The time to act is now.
AICRI, in collaboration with the NCCP, is organising an EU Cancer Mission Day – Ireland in Dublin on 26th September 2023. This event will provide a platform for academic researchers, clinician scientists and other healthcare

professionals, charity partners and policymakers, as well as those with a lived experience of cancer, to come together and share their insights, knowledge and experiences to explore innovative approaches to address cancer challenges. Minister for Health, Stephen Donnelly, TD, will provide a keynote address at
AICRI, in collabora�on with the NCCP, is organising an EU Cancer Mission Day – Ireland in Dublin on 26th September 2023. This event will provide a pla�orm for academic researchers, clinician scien�sts and other healthcare professionals, charity partners and policymakers, as well as those with a lived experience of cancer, to come together and share their insights, knowledge and experiences to explore innova�ve approaches to address cancer challenges. Minister for Health, Stephen Donnelly, TD, will provide a keynote address at this event. There will be five main sessions as follows:
Attendance in-person at this event is now sold out, however, if you are most welcome to join the event online by registering on Eventbrite (https://www.eventbrite.ie/e/eucancer-mission-day-ireland-onlinetickets-682109025457) and to view the agenda.
Contact: Dr Ozlem McDonnell, NCCP ECHoS Project Coordinator (ozlem.mcdonnell@cancercontrol.ie)
Dr Rosemarie Gannon, AICRI Project Manager (rosemarie.gannon@ucd.ie)
Session 1: Ireland’s Par�cipa�on in EU Cancer Mission Projects
Oncology Focus News
Session 2: How Can We All Benefit from Europe’s Bea�ng Cancer Plan?
Session 3: The Lived Experience at the Heart of Na�onal and European Cancer Ini�a�ves
Session 4: Co-ordina�on of Cancer Research at Na�onal and European Levels
Launch of Hereditary Cancer Model of Care
Session 5: Engagement with Ireland’s Na�onal Cancer Mission Hub going forward
Atendance in person at this event is now sold out, however, if you are most welcome to join the event online by registering o htps://www.eventbrite.ie/e/eu-cancermission nd to view the agenda.
The National Cancer Control Programme recently launched a national Hereditary Cancer Model of Care at our offices on Parnell Street, which was the culmination of many months of work by a broad multidisciplinary Advisory Group.

This Hereditary Cancer Model of Care provides the blueprint for the necessary development of services for the identification and management of people with an inherited cancer predisposition (e.g.
Lynch syndrome or BRCA gene cancer risk), in order to harness the power of genetics to reduce cancer risk, and improve patient outcomes and quality of life. It complements the National Strategy for Accelerating Genetic and Genomic Medicine in Ireland (launched in Dec 2022), which addresses a number of the key enablers such as building the genetics and genomics workforce and strengthening our infrastructure.
Speakers at the launch gave an overview of the Model of Care and of initiatives underway within hereditary cancer services. Mr Pat Fahey and Ms Jean O’Neill provided valuable insight to the patient experience of living with an inherited predisposition to cancer. The model of care will ensure/ support the development of:
• Development of national guidance to underpin standardised best practice, including mainstreaming of cancer genetic testing
• Equitable access to hereditary cancer services for all
• Clear governance and clarity regarding the roles of national genetics and genomic structures, specialist cancer genetics services and regional cancer predisposition services, within an overarching model of care
• Commitment to the Sláintecare principles of right person, right place, right time – through the integration of genetic
assessment and testing into regional services, with access to specialist genetic expertise as required
• Maximal use of technological solutions

• Timely access to genetics assessment and testing, including specialist cancer genetics expertise as required
• Timely access to clinical management of cancer predisposition, including risk reduction options
• Comprehensive and coordinated approaches to ongoing management
• Quality assurance of service
• Access to high quality patient information, psychological support and peer support for patients
• Education and training of health care professionals, including accredited training for those delivering mainstreamed pathways
28 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Speakers at the launch (L to R) – Rory Kennelly, Noelle Cullinan, David Gallagher, Bernie Carter, Jean O’Neill, Yvonne Hanhauser, Marie Duff, Pat Fahey, Risteárd Ó Laoide, Triona McCarthy

Our annual listing celebrating innovation and excellence in healthcare is coming soon. PROFESSIONAL 100 PROFESSIONAL 100 To request an entry or nomination form please contact: sarah@hospitalprofessionalnews.ie 2 0 2 3 NO M I N A T I O N S NO W O P E N HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication To request an entry or nomination form please contact: kelly-jo@ipn.ie or danielle@hospitalprofessionalnews.ie PROFESSIONAL 100
Oncology Focus: Melanoma

Melanoma-related costs by disease stage and phase of management in Ireland
1Clinical Costing Solutions, Belfast 2Tallaght University Hospital, Tallaght 3HSE National Cancer Control Programme (NCCP) 4St. James and Tallaght University Hospitals, Department of Medicine, Trinity College Dublin 5Sligo General Hospital, Sligo
Melanoma is the fifth most commonly diagnosed cancer in both men and women in Europe1 and has one of the fastest growing incidence rates globally.2 If 2020 rates continue, the global burden from melanoma is estimated to increase by ~50% by 2040 presenting an important challenge to cancer control and public health globally.2
In Ireland, over 1000 people are diagnosed with melanoma annually, a number which is predicted to triple between 2015 and 2045.3 Harmful exposure to UV radiation (mostly solar radiation) is the most avoidable cause of melanoma risk and mortality, with many economic evaluations reporting favourable benefits from preventative intervention.4 Although most cases are caught early, 10% of melanomas in Ireland are still diagnosed at Stage III or IV where 5-year survival rates for distant disease are much lower than for local disease (94.60 versus 32.68%).5


Recent advances in treatment have transformed the care of melanoma patients, delivering meaningful improvements in survival; however, the extremely high cost of such
treatments presents a challenge for funders.6 The impact of early detection on extended survival and subsequent treatment costs has increased the interest in estimating the financial costs associated with melanoma and the associated cost-effectiveness of melanoma screening strategies.4 7 8
Numerous studies have explored the cost burden of melanoma;4,9–36 however, many of these findings are now obsolete as they were undertaken before the introduction of high-cost systemic drugs used for adjuvant and advanced melanoma treatment.
The aim of this study was to provide robust estimates of the stage-specific costs of melanoma over a 5-year time frame from the perspective of the Irish healthcare system to inform budgetary planning and underpin future economic evaluation of primary prevention to enable efficient allocation of public funds.
Methods
A model of cumulative cost depending on the initial stage at diagnosis (Stage 0/1A, Stage IB, Stages IIA–IIC, Stages IIIA–IIIC and Stage IV) was constructed, following the newly diagnosed
patient for 5 years, when lymph node, in-transit or distant metastases are most likely to occur resulting in diagnostic procedures, surveillance and additional treatment. Tromme et al. 9 developed a model to estimate the stage-specific economic burden of melanoma in Belgium (Fig. 1), which has subsequently been used to estimate costs in Brazil.33 We adopted a similar approach, modifying the model to reflect local data availability. In brief, during each annual cycle, and for each stage of disease, patients have a probability of transitioning to another stage. The model incorporated ‘timedependence’ such that the risk of progression, recurrence or death was dependent on duration in each stage. Our approach differed from Tromme et al 9 as we had access to disaggregated data for Stage IIIB/C (which was unavailable from the Belgian registry) hence intransit metastases were included as Stage IIIB; death was not disaggregated into ‘deaths from melanoma’ and ‘other causes’. In the interests of parsimony, we did not explicitly account for costs associated with false positives in the diagnostic phase of treatment or for multiple lesions. Transition probabilities were assumed the same for both genders and all ages and strictly local recurrences were not included because of their rarity.
A melanoma care pathway was developed for each stage of disease based on populationlevel data supplied by the National Cancer Registry Ireland (NCRI), where completeness of registration is estimated to be in excess of 96% of all diagnosed cancers.37 Once developed, the care pathway was reviewed by a multidisciplinary team comprising dermatologists (3), a medical oncologist, a health economist, public health medicine consultants
(2), oncology pharmacists (2) and representatives from the National Cancer Control Programme (NCCP) (4) who were convened to oversee the construction, population and validation of the model. Additional expertise was supplied by the NCRI, the Healthcare Pricing Office (HPO) and the Primary Care Reimbursement Service (PCRS) of the Health Service Executive (HSE) in Ireland.
All patients entering the melanoma care pathway were referred by their GP (via the e-referral system) to the skin cancer clinic. Here they were diagnosed, staged (including imaging, dependent on initial preliminary or succeeding clinicpathological staging), discussed at a melanoma multidisciplinary team meeting (MDTM) and given a definitive diagnosis based on a surgical excision of the melanoma.
Patients with Stage 0 or IA disease underwent no further treatment. A proportion of Stage IB and IIA patients underwent sentinel lymph node biopsy (SLNB) and (where appropriate) were discussed at an Oncology MDTM (on at least one occasion). Patients with a positive SLNB underwent follow-up completion lymphadenectomy. Patients with Stage IV disease underwent surgery or stereotactic radiation for removal or treatment of oligometastatic melanoma. A proportion of patients with Stage IV BRAF mutant melanoma were treated with combination BRAF_ MEK inhibitors (it should be noted that the reporting period partly precedes national reimbursement approval of adjuvant Dabrafenib and Trametinib for public patients with Stage III melanoma). Reimbursement approval of these drugs will have financial implications arising from increased prescribing.
30 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Patricia Heckmann
Written by Grainne E Crealey1, Caitriona Hackett2, Katharine Harkin3, Patricia Heckmann3, Fergal Kelleher4, Áine Lyng3, Triona McCarthy3, Maria McEnery3, Clare Meaney3, Darren Roche5, Anne-Marie Tobin2
Dr Anne-Marie Tobin
A schedule of follow-up visits specific to each disease stage was detailed in the care pathway. Patients with Stage 0/1A disease had two appointments over the following 12 months. The frequency of clinic visits increased with stage of disease (e.g. patients in Stage IIIB/C had four appointments per year in Years 1–3 and twice annually in Years 4–5). In addition, a schedule of investigations accompanied such visits (biannual CT PET + MRI, Years 1–3; annually thereafter until Year 5). Patients suitable for systemic therapy followed a separate schedule of clinic visits, blood tests and imaging.
Data used to populate the model
Data on the distribution of patients across disease stages and resource use estimates (by stage) were supplied by the NCRI for all patients with an ICD-10 principal code for malignant melanoma of the skin (C43). At the time of this study, the most recent available data were for 2016 (n = 1140 patients). The probability of a patient receiving a treatment, alone or in combination, was tabulated by stage and site. Additional data (based on ICD-10 principal code) were obtained from the Hospital Inpatient Enquiry team. Supplementary data relating to diagnostic work-up and adherence to surveillance protocols were obtained by undertaking a retrospective chart review of
melanoma patients from a large teaching hospital (n = 70, followup from 12 to 40 months).
In Ireland, data on medication use are captured by the PCRS database; however, it was not possible to isolate resource use by ICD-10 principal code. Data on treatment regimens (by stage) are collected by NCRI; however, this does not extend to the dose and duration of radiotherapy, chemotherapy, immunotherapy and targeted drugs. Hence conservative estimates based on NCCP approved treatment regimens for melanoma and validated by expert opinion within the group (Oncology Consultant and Senior NCCP pharmacists) were employed.
Following treatment, individuals have a stage-specific risk of remaining in remission, having a recurrence, distant metastases or death. In the absence of data on disease free progression for this patient population, data on the probability of dying in a 12-month period (using intervalspecific observed survival by stage for Years 1–5) were supplied by the NCRI. The probability of recurrence was based on recurrence-free survival rates presented in Leiter et al 38 and further assumed that 77% of initial recurrences were locoregional with the remainder classified as distant metastases.
Valuation of resources
Direct healthcare costs were collected from the perspective of the publicly funded Irish health and social care system (HSE) for 2020 (¤). Costs associated with diagnosis, in-patient and out-patient procedures, systemic therapy, relapse and supportive care were included. All costs were adjusted to 2020 (¤) using the Consumer Price Index for Health (Central Statistics Office www.cso.ie) and discounted at a rate of 4% per annum as per best practice guidelines for performing Health Technology Assessment in Ireland.39
Nationally produced unit costs for individual procedures are not produced in Ireland, hence procedures captured by NCRI were mapped across to Diagnosis Related Groups codes (DRGs) compiled by the Health Pricing Office (HPO) using the ICD-10 Principal code for Melanoma (C43). This work package involved assessing the frequency with which procedures were performed in conjunction with expert opinion from HPO senior analysts and clinical expert opinion to construct frequency weighted mean costs (reflecting level of complexity and whether it was performed as an inpatient or day case procedure). Analysts in HPO were consulted to ensure the ‘DRG cost bundle’ avoided double counting (specifically with respect to blood tests and imaging).
The cost of immunotherapy regimens approved for use by the HSE for treatment of melanoma was provided by the NCCP https:// www.hse.ie/eng/services/list/5/ cancer/profinfo/chemoprotocols/ melanoma/. Costs were based on list price including VAT (23%) plus an administration cost (DRG code R63Z from the Admitted Patient Price List 2020 for chemotherapy day case). The monitoring and management of adverse events (AEs) (which is not included in the administration cost DRG) were calculated separately based on reported AE frequency from clinical trials and using a list of costs from the ABF Admitted Patient Price List 2020. In the base-case analysis, costs associated with 12 months of therapy were applied to all patients residing in that state. The cost of supportive care was derived from estimates of the cost of palliative care in the Irish healthcare system utilizing only that portion of cost attributable to specialist palliative and hospital care costs (¤11,685).40

Sensitivity analysis
One-way and probabilistic sensitivity analyses (PSA) were undertaken to explore the impact of parameter uncertainty on the total cost estimates. Parameters associated with most uncertainty were subjected to one-way sensitivity analysis. PSA were conducted by running the model with a hypothetical cohort of 10,000 patients calculating cost estimates for each simulation. In each simulation, the value for each parameter was varied across a probability distribution reflective of the properties of the parameter under consideration and data informing them. Probabilities were modelled using beta distributions and parameterized with the sample size from the relevant published literature or data from NCRI. Costs were specified as gamma distributions as they are constrained to be positive, continuous and highly skewed.
All analyses were undertaken in TreeAge Pro 2021 (TreeAge Software, Inc., Williamstown, MA) and reported in line with The Consolidated Health Economic Evaluation Reporting Standards 2022 statement.41
Results
Patients with the best prognosis (Stage IA) were the least costly to treat and manage compared with
31 HOSPITALPROFESSIONALNEWS.IE | HPN • SEPTEMBER 2023
those with the worst prognosis (Stage IV). The cost of managing a case of melanoma diagnosed at Stage IV was over 25 times more expensive than managing a case diagnosed at Stage IA (Stage IV ¤122,985 versus Stage IA ¤4269).
Sensitivity analysis
The 95% confidence interval (CI) for the average (stage-weighted) cost per person for managing a case of melanoma over a 5-year period (base case ¤20,268) was estimated from the PSA and ranged from ¤16,126 to ¤25,071. To reflect changes in practice since data were obtained from NCRI (for 2016), we assumed that 70% of patients in Stage IIIA and 100% of patients in Stages IIIB and IIIC received immunotherapy or targeted drugs for a maximum of 12 months, and 100% of Stage IV patients for a maximum of 24 months. This assumption was based on expert opinion within the group. Under these assumptions the stageweighted average cost per person almost doubled from ¤20,268 (CI: ¤16,126–¤25,071) to ¤37,715 (CI: ¤32,619–¤43,019). In the basecase analysis, we assumed that all patients consumed 100% of prescribed medication. When we assumed that 88% of doses were consumed (as per Pike et al 29) the stage-weighted average cost per person fell to ¤18,561 (¤14,915–¤22,793). A flat-rate of 88% was used; however, it is noteworthy
Oncology Focus: Melanoma
that Pike et al 29 also adjusted the quantity of doses consumed downward on a monthly basis by drug type.
In the base-case analysis, we assumed that 77% of recurrences were locoregional, and the rest were distant. When we assumed a 20% reduction in locoregional progression, the stage-weighted average cost per person rose to ¤21,079 (¤16,681–¤26,180) and similarly fell to ¤19,461 (¤15,569— ¤23,988) when we assumed a 20% increase.
Interval-specific recurrence rates for each stage were employed in the model.38 When these recurrence rates were varied by 20% around the base case estimate, the stage-weighted average cost per patient changed from ¤19,217 (CI: ¤15,388–¤23,680) to ¤21,476 (¤13,365–¤26,658).
Discussion
Main findings of this study
Healthcare costs for those diagnosed with Stage IV disease (over a 5-year time horizon) were over 25 times higher than for those diagnosed at Stage IA. Given prognosis is also superior, it underscores the importance of early detection and intervention. The cost of managing melanoma was particularly sensitive to those factors that contribute to the cost of immunotherapeutic and targeted drugs (such as dose, duration and choice of agent).
Oncology Focus News
Despite the use of sensitivity analysis to explore potential sources of uncertainty, it is clear that the collection of ‘real-world’ data relating to ‘key’ cost drivers is crucial if we are to understand the distribution of costs and accurately estimate the costeffectiveness of early intervention and treatment strategies.
What is already known on this topic
Our study echoes findings in the literature regarding the relationship between disease stage and cost.10 20–22 27 32 33 However, the difference in costs between stages has changed dramatically with the introduction of immunotherapeutic and targeted drugs rendering findings from earlier papers less relevant.
Our findings support those found in other recent studies, which have differentiated cost by stage of disease. One-year costs within the Italian health service ranged from ¤1,837 to ¤66,950 producing a cost ratio of 31.27 (between Stages I and IV).32 In the Spanish health care system, costs estimated over a 10-year time frame ranged from ¤1689 (Stage I) to ¤88,268 (Stage IV) reporting a doubling in costs where there was lymph node involvement, and treatment costs that were 52-fold higher for those with the least as opposed to most favourable prognosis.31 Similarly, spending on advanced melanoma in Brazil
(estimated over a 3-year time frame) was reported as being up to 34-fold higher in the public health system compared with lower-stage disease.33 In the Netherlands, the real-world healthcare costs of melanoma were explored, and since the introduction of immunotherapeutic and targeted drugs, systemic therapies now account for 84% of costs, with costs differing substantially between patients who receive systemic therapy (¤105,078) and those who do not (¤7988).6
What this study adds
This is the first study to estimate the healthcare costs for melanoma patients by disease stage in Ireland. A strength of this study is that real-world data were used to populate the model that contrasts with other studies that have estimated resource utilization using national guidelines, ‘best practice’ or expert opinion/elicitation. Our study will enable robust estimation of the cost-effectiveness of prevention strategies in the Irish healthcare setting, and provide a blue-print for estimating the stagespecific costs of other cancers in Ireland. It has also clarified where best to focus resources with respect to data collection and linkage efforts moving forward.
References available on request https://academic.oup.com/jpubhealth/ advance-article/doi/10.1093/pubmed/ fdac154/7160043
The Economic Cost of Melanoma Skin Cancer in Ireland
New research conducted by the HSE National Cancer Control Programme (NCCP) highlights the difference between the economic cost of diagnosing and treating a person with the earliest stage of melanoma skin cancer (stage IA) compared to those with late stage melanoma skin cancer (stage IV).
Early diagnosis of skin cancer is easier to treat and the patient is more likely to recover well, resulting in reduced economic cost for the health service and the patient.
The study1 found the cost of diagnosing and treating a person
with late stage melanoma was more than 25 times higher than for a person with early stage disease (stage IV ¤122,985 versus stage IA ¤4,269). The study did not take into account the additional wider economic and personal costs of melanoma skin cancer to patients, their families and society.
In Ireland, over 1,000 people are diagnosed with melanoma skin cancer each year, a number which is predicted to double between 2015 and 2045. Melanoma skin cancer is the most dangerous form of skin cancer, as it can spread to other organs in the body and can be fatal.
Skin cancer is one of the most preventable cancers.

Minister for Public Health, Wellbeing and the National Drugs Strategy Hildegarde Naughton TD said, “This research reinforces the importance of prevention and early detection. We cannot treat our way out of rising skin cancer rates, so by working to reduce the number of cases every year we can also reduce the large cost burden that this disease places on our health services.
“We know that skin cancer is largely preventable, so I would urge people to be vigilant in reducing their risk by being
SunSmart and checking skin regularly for any changes.”
Dr Triona McCarthy, Consultant in Public Health Medicine, HSE NCCP added, “Investment in skin cancer prevention and early detection programmes show strong potential for health and economic benefits. The National Skin Cancer Prevention Plan 2023 – 2026 is focused on reducing risk across the population, by raising awareness of the actions we can all take to reduce our risk of skin cancer such as following the SunSmart 5 S’s and avoiding use of sunbeds, and it sets out how we can support people to adopt these behaviours.”
32 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Oncology Focus: Absract

P53 Incidence of merkel cell carcinoma in the Republic of Ireland (1994–2019)
Written by Claire Doyle, Marta Costa Blasco, Orla McFeeley, Paula Beatty, Sarah O’Mahoney, Lisa Murphy, Madonna Andrawis and Anne Marie Tobin Tallaght University Hospital, Dublin
Abstract
Merkel cell carcinoma (MCC) is a rare, aggressive, neuroendocrine cutaneous malignancy most commonly observed in those aged over 65 years. MCC is twice as common in males as in females and is 25 times more common in
males that was not observed in females. This study adds to the body of epidemiological data on MCC.
those with fair skin than in those with darker skin types. Solid organ transplant recipients have a 24fold increased risk of developing MCC. There has been a 95% increase in the incidence of MCC in the USA from 2000 to 2013, with further increases predicted. The

aim of this study was to investigate the trends of incidence of MCC in the Republic of Ireland. Agestandardized rates were calculated using the 2013 European standard population. There were 447 histologically confirmed MCCs diagnosed between 1994 and
2019. Forty-nine per cent of cases (n = 220) were in males. Median age was 80 years (range 15–19 to 95+). The median age for males was 78 years and the median age for females was 82 years. On average, there were seven cases of MCC diagnosed annually between 1994 and 1998 vs. 27 cases of MCC annually diagnosed between 2015 and 2019—almost a quadrupling of case numbers. The age-adjusted incidence rate of MCC for both male and females is detailed in Table 1. Previous literature has indicated that MCC is twice as common in males than in females. Our cohort differed in that there was a slight female predominance in patients diagnosed with MCC. This is in keeping with published literature from Northern European groups, which have also demonstrated a female predominance in MCC. While the absolute number of MCC diagnosed in Ireland has increased in both males and females, the age-adjusted incidence rate is increasing in males, but there has not been a significant change in females. Other Northern European groups have also demonstrated an increase in the incidence of MCC in males that was not observed in females. This study adds to the body of epidemiological data on MCC.
Age-adjusted incidence rates of Merkel cell carcinoma (MCC)
33 HOSPITALPROFESSIONALNEWS.IE | HPN • SEPTEMBER 2023
Dr Anne-Marie Tobin
Table
1
Table
Age-adjusted incidence rates of
carcinoma (MCC) Cases of MCC Age-standardized rate (per 100 000 person years) SE 95% confidence interval Males 1994–1999 23 0.52 0.13 0.27–0.77 2000–2004 21 0.37 0.08 0.21–0.53 2005–2009 35 0.79 0.15 0.50–1.08 2010–2014 66 1.19 0.16 0.88–1.50 2015–2019 75 1.05 0.12 0.81–1.29 Females 1994–1999 26 0.36 0.07 0.22–0.50 2000–2004 44 0.67 0.10 0.47–0.87 2005–2009 54 0.79 0.11 0.57–1.01 2010–2014 45 0.57 0.09 0.39–0.75 2015–2019 58 0.65 0.09 0.47–0.83
1
Merkel cell
New prostate cancer biomarkers provide hope to millions of men
advanced and aggressive the cancer is and whether immediate treatment is needed.”
Trinity researchers - as part of an international team of scientists - have identified three new biomarkers for prostate cancer to help identify and differentiate potentially aggressive cases of the disease.
An international team of scientists led in Trinity College by Professor John O’Leary, Consultant Histopathology, School of Medicine, and Professor Doug Brooks at the University of South Australia (UniSA) has made a landmark breakthrough which will assist pathologists when visualising prostate cancer in patient tissue samples.

The research has been published in the international journals Pathology and Cancers.
The team has identified three new biomarkers which will allow pathologists to determine which patients require immediate, radical
treatment compared to those who need close monitoring.
With more than one million men diagnosed with prostate cancer worldwide each year, the research breakthrough is significant. Prostate cancer incidence in Ireland is currently the highest in Europe (GLOBOCAN, 2020). The number of deaths from prostate cancer was 554 per year, during the period 2016-2018. This represents 20.2 deaths per 100,000 of the population, attributing to 12% of male cancer deaths (NCRI, 2021).
The UniSA-based team has collaborated with the Australian company Envision Sciences on the technology to improve patient management and treatment outcomes.
Speaking on the discovery, Professor John O’Leary said,
“This discovery made with colleagues from the University of South Australia, University of Adelaide, Royal Melbourne Institute of Technology, and the Prostate Cancer Research Consortium (PCRC), the biobank for which is maintained and curated by colleagues at UCD, marks an important breakthrough in prostate cancer diagnostics and prognostics and offers for the first time a precision medicine approach in prostate cancer diagnostics allowing pathologists to a). define the entire extent of the prostate cancer in a specimen; b). establish whether it is indolent or aggressive disease and define the risk of disease recurrence and metastasis and c). define the metabolic profile of the tumour to decide whether it is using sugar or lipid metabolism, which also defines outcome of the disease.”
Professor Doug Brooks (UniSA), also said, “It is anticipated this will lead to long-term improvements in the way prostate cancer is diagnosed and graded.
“The biomarkers are remarkably sensitive and specific in accurately visualising the progress of the cancer and confirming its grade. This discovery has led to the commercial development of a test designed to determine how
Envision Sciences, which funded the development and translation of the technology at UniSA, has signed a commercialisation agreement with the largest tissue diagnostic pathology company in the US, Quest Diagnostics, to take the technology into clinical practice.
Pending a successful outcome in the US, it is expected that clinical trials using the innovative technology will be undertaken in Australia.
Professor O’Leary concluded, “This discovery and spin-out is transformative and disruptive in terms of prostate cancer diagnostics. Its adoption by Quest Diagnostics as a laboratory developed test (LDT) now ensures that the markers will be used in routine practice in the US. Our ambition is to see these markers used in Europe and Austral-Asia as well.”
The Trinity College Dublin team (including affiliated hospital sites) led by Professor John O’Leary includes Dr Prerna Tewari, Dr Sharon O’Toole, Dr Cara Martin, Dr Victoria Malone, Dr Laura Edgerton, Dr Mark Bates, Dr Mark Ward, Dr Sheena Heffernan, Dr Sarah Ni Mhaolcatha, Dr Roisin O’Connor, Dr Helen Keegan, Dr Marguerite Carter, Dr Katie Ryan, Dr Sophie Prendergast, Mr Nathan Brady, Mr Andreas Clarke, Dr Bashir Mohammed, Dr Filip Sokol.
Providing Faster Diagnostics and Cancer Care

A new collaboration has transformed healthcare in Northern Ireland by providing a more precise and faster diagnosis service, as well as better treatment options for people with cancer.
The Precision Medicine Centre (PMC) at Queen’s has developed a partnership with the Regional Molecular Diagnostic Service (RMDS) at the Belfast Health and Social Care Trust (BHSCT), to implement genomic technology for the diagnosis of cancer patients.
The new Service integrates an innovative genomic test developed by the Precision Medicine Centre
at Queen’s into the diagnostic workup of cancer patients in Northern Ireland to ensure they can access a much wider range, and the latest and most effective cancer treatments available in the rest of the UK and EU.
The Service uses comprehensive genomic profiling for diagnostic purposes that looks for mutations and other alterations in DNA of cancer biopsies. Finding these DNA alterations allows patients to receive targeted treatments that can improve their survival and quality of life, compared to conventional cancer treatments.
In addition, it is more costefficient to provide than the standard testing system previously used in Northern Ireland and allows patients to be enrolled in new clinical trials exploring novel therapies for cancer treatments, which can help improve patient outcomes.
David Gonzalez de Castro, Professor of Genomic Medicine at Queen’s and Consultant Clinical Scientist at the BHSCT, explains: “Genomic medicine offers very real and exciting prospects for healthcare in Northern Ireland in terms of early detection, faster diagnosis, and personalised
and better treatment for cancer patients.
“This collaboration ensures our patients are offered a first-class homegrown genomic diagnostic service and is a fine example of how the Precision Medicine Centre at Queen’s is working with our partners to deliver for our patients and supporting the NHS at a challenging time. This represents the first example of an innovative collaboration model that will extend to other diagnostic modalities, making the most of this strategic partnership for the benefit of our patients.”
34 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE Oncology
News
Focus:
Professor Doug Brooks at the University of South Australia (UniSA)
Oncology Focus: Ovarian Cancer
Harnessing Proteins to Stop Ovarian Cancer
A major new study, published in Nature, has discovered the antitumour activities of the immune protein interferon epsilon, which in pre-clinical models blocks the metastasis of ovarian cancer cells by instructing immune cells to kill the cancer cells.
The search for preventions and cures for the most prevalent and deadly form of ovarian cancer has now turned inward with the discovery of the anti-tumour activities of an immune protein called ‘interferon epsilon’. This research is published today in the journal Nature and was co-led by Trinity College Dublin researcher Dr Nollaig Bourke.
Interferons (IFN) are a type of immune signalling protein, or cytokine, which can kill tumour cells and activate anti-tumour immune responses.
Dr Nollaig Bourke, School of Medicine - who worked with colleagues in the Hudson Institute of Medical Researchin Melbourne, Australia, before returning to establish her own research immunology group at Trinity –explained, “Early in this study we found that women with high grade serous ovarian cancer no longer had the normal expression of an immune protein called interferon epsilon in their female reproductive tract. This was really interesting to us as we knew that interferon epsilon was part of a family of proteins known for their anti-tumour activities and we wondered what would happen if we could try restore this lost expression – could
giving interferon epsilon back help block the growth of ovarian cancer cells and therefore prevent the growth of primary and secondary tumours?”
A key motivation for the team is the poor survival rate for women with ovarian cancer, particularly high-grade serous ovarian carcinoma (HGSOC). Patients are often not diagnosed until later stages of the diseases when cancer cells have metastasised from the initial tumour to other sites in the body, making the disease really difficult to treat. About 400 women in Ireland are diagnosed with ovarian cancer each year.
A major advance in cancer treatment in recent years has been cancer immunotherapies, where the immune system is harnessed to kill cancer cells. The researchers received pilot funding from the US Defence Department’s Ovarian Cancer Research Programme to assess interferon epsilon as a novel immunotherapy in ovarian cancer.


Dr Bourke continued, “What was really striking about our study was that when we gave interferon epsilon in our pre-clinical models, it was able to directly kill tumour cells but more importantly, it was really good at instructing immune cells in the body to target and kill cancer cells involved in metastasis, thus blocking the development of secondary tumours.”
This study was made possible by the team of expert immunology scientists and oncology clinicians
who collaborated to make these discoveries, including former Trinity alumni Dr Nicole Campbell and Dr Niamh Mangan. Dr Campbell, who joined the research team after completing a PhD in Trinity, is the publication’s joint first author and has been working for several years on understanding how these treatments which target the body’s immune system can be optimised to improve its ability to fight the tumour. Dr Campbell said, “Cancer immunotherapies have been very successful in the treatment of other types of cancer, but they have had limited success in ovarian cancer – we’re looking to change that.
“We know that in High Grade Serous Ovarian Cancers (the commonest form of ovarian cancer) tumour cells recruit and activate ‘immunosuppressive’ cells which prevent anti-tumour immune cells from killing tumour cells, so we’re aiming to develop
new therapeutics which can reverse that process and improve survival rates.”

Professor Paul Hertzog, Centre For Innate Immunity & Infectious Diseases, Hudson Institute of Medical Research, who co-led the study with Dr Bourke, added, “We now know that Interferon epsilon is naturally made in the (epithelial) cells lining organs such as the female reproductive tract where we knew it acts as a natural booster of immunity to infections. Our recent discovery is that it also acts as a tumour suppressant, and that it is lost during the process of ovarian tumour formation.
We know from pre-clinical models that administering it will dramatically inhibit ovarian cancer growth, particularly in cases where the cancer has metastasised into the peritoneal cavity.”
What’s Next?
The next step is to understand which immune cells are important to the anti-tumour activity of IFN epsilon, and to test whether it is effective on human cells.
Combining studies in mice and using the by-products of human ovarian cancer treatment, the team now aim to better understand how IFN epsilon works to protect against ovarian cancer and move a big step closer to ‘first-inhuman’ clinical trials. The goal is to develop IFN epsilon as a new immunotherapy for ovarian cancer.
Further Reading
To read the paper ‘Interferon-ε is a novel tumour suppressor and restricts ovarian cancer’ published in Nature follow this link: https:// www.nature.com/articles/s41586023-06421-w
35 HOSPITALPROFESSIONALNEWS.IE | HPN • SEPTEMBER 2023
Professor Paul Hertzog, Centre For Innate Immunity & Infectious Diseases, Hudson Institute of Medical Research
Oncology Focus: Prostate Cancer
Life Limi�ng Prostate Cancer
Life Limiting Prostate Cancer
Life Limi�ng Prostate Cancer
Sub-heading: The Dilemma of Iden�fying Cancer:
The Dilemma of Identifying and Treating Life limiting Prostate Cancer: Prostate Check may be the future of Men’s cancer screening
Prostate Check may be the future of Men’s
Sub-heading: The Dilemma of Iden�fying and Trea�ng Life limi�ng Prostate Cancer:
Written
by David Galvin MD FRCS(Urol), Associate Professor,
UCD Consultant

Prostate Check may be the future of Men’s cancer screening
Mater and St Vincent’s hospitals Dublin; Paul Sweeney, Consultant Urologist, Mercy hospital, Cork and Padraig Daly, Consultant Urologist, University hospital Waterford
 Urologist,
Urologist,
On Behalf of the Irish Society of Urology (ISU)
Background
In February 2021, the European Commission (EC) announced the EU Cancer plan, to tackle Cancer across Europe, from prevention, to screening and diagnosis, to treatment and palliation. One of the more sensitive goals was the expansion of screening from Breast, Cervical and Colorectal cancers, to include Lung, Stomach and Prostate. This was not greeted across Europe with enthusiasm in all countries, as it would require significant resources and place a major strain on a number of healthcare systems across the EU. In order to inform a European early detection / screening program, further information and research is required. Previous screening programs in prostate cancer have been established around the world without patient engagement, and led to poor outcomes, over-treatment and screening was therefore generally not recommended. The European Association of Urology has led a successfully application (known as PRAISE-U) involving 25 countries across Europe who over a total
Writen by David Galvin MD FRCS(Urol), Associate Consultant Urologist, Mater and St Vincent’s hospitals Dublin; Paul Sweeney, Consultant Urologist, Mercy Consultant Urologist, University hospital Waterford

Writen by David Galvin MD FRCS(Urol), Associate Professor, UCD Consultant Urologist, Mater and St Vincent’s hospitals
On Behalf of the Irish Society of Urology (ISU)
Dublin; Paul Sweeney, Consultant Urologist, Mercy hospital, Cork and Padraig Daly, Consultant Urologist, University hospital Waterford
On Behalf of the Irish Society of Urolo Background

Background
will all be analysed centrally by the EAU Investigators. In essence we will have Irish data, on Irish men assessing a novel home PSA testing system as a potential screening method in our commonest male cancer. We will learn from the experience of other European countries, and they from ours. Other work packages are examining the current status of screening across Europe, the needs of countries and the design and implementation of a screening protocol through knowledge sharing.
of 6 work packages in 3 years will provide the much needed information to inform a successful screening program. Work package 4, involves the establishment of national pilot programs in 5 countries, and Ireland, through the Irish Society of Urology (ISU) has been invited to establish a research screening program.
Irish Pilot: Prostate Check
2026. The Irish proposal brings together two partners, firstly an academic hub in University College Dublin (UCD), and secondly the Health Service Executive (HSE).
Irish Pilot: Prostate Check
The ISU, the National Screening Service (NSS) and the National Cancer Control Program (NCCP) have developed an innovative prostate cancer screening pilot program, known as Prostate Check that is being funded by the EC through PRAISE-U . The program will involve doctors (both GPs and Urologists), Irish men (aged 50-70 years) and their families. Feedback from all involved will inform the design of a future PSA based risk-stratified national screening system. The Irish data collected will be fed to the PRAISE-U Investigators (based in Erasmus University in Rotterdam) who will put together a European wide report to the European Commission by April
In February 2021, the European Commission (EC) Cancer across Europe, from preven�on, to screening pallia�on. One of the more sensi�ve goals was the Cervical and Colorectal cancers, to include Lung, across Europe with enthusiasm in all countries, as place a major strain on a number of healthcare systems European early detec�on / screening program, further Previous screening programs in prostate cancer have without pa�ent engagement, and led to poor outcomes therefore generally not recommended The European successfully applica�on (known as PRAISE-U) involving a total of 6 work packages in 3 years will provide successful screening program. Work package 4, involves programs in 5 countries, and Ireland, through the invited to establish a research screening program.
The EAU Screening Algorithm
So what’s new?
Don’t GPs already have guidelines on PSA testing from the NCCP?

Irish Pilot: Prostate Check
In February 2021, the European Commission (EC) announced the EU Cancer plan, to tackle Cancer across Europe from preven�on, to screening and diagnosis, to treatment and pallia�on. One of the mo was the expansion of screening from Breast, Cervical and Colorectal cancers, to include Lung, Stomach and Prostate. This was not greeted across Europe with in all countries, as it would require significant resources and place a major strain on a number of healthcare systems across the EU. In order to inform a European early detec�on / screening program, further informa�on and research is required. Previous screening programs in prostate cancer have been established around the world without pa�ent engagement, and led to poor outcomes, over-treatment and screening was therefore generally not recommended The European Associa�on of Urology has led a successfully applica�on (known as PRAISE-U) involving 25 countries across Europe who over a total of 6 work packages in 3 years will provide the much needed informa�on to inform a successful screening program. Work package 4, involves the establishment of na�onal pilot programs in 5 countries, and Ireland, through the Irish Society of Urology (ISU) has been invited to establish a research screening program.
Men aged 50-70 years will be randomly identified from the Screening service, and will be invited to the pilot, giving their consent if they wish to be involved and for their data to be collected (via the website prostatecheck. ie). Partnering with a test provider, a home based PSA blood testing kit will be sent to the patient, who will perform a finger prick blood sample and return the sample for PSA testing in the mail to the laboratory. This means that GP practices will no longer be burdened with blood testing and referrals. All the data will be stored securely in UCD. All of the pilot programs will follow the EAU algorithm (Image 1). Men with high PSA readings will be seen automatically in the successful Rapid Access Prostate Clinics (RAPC) for MRI imaging, possible biopsy and treatment if needed. This data, and feedback from doctors, patients and families
The ISU, the Na�onal Screening Service (NSS) and (NCCP) have developed an innova�ve prostate cancer Prostate Check that is being funded by the EC through doctors (both GPs and Urologists), Irish men (aged from all involved will inform the design of a future screening system. The Irish data collected will be
The ISU, the Na�onal Screening Service (NSS) and the Na�onal Cancer Control Program (NCCP) have developed an innova�ve prostate cancer screening pilot program, known as Prostate Check that is being funded by the EC through PRAISE-U . The program will involve doctors (both GPs and Urologists), Irish men (aged 50-70 years) and their families. Feedback from all involved will inform the design of a future PSA based risk-stra�fied na�onal screening system. The Irish data collected will be fed to the PRAISE-U Inves�gators (based in

Men who visit GPs get blood tests. In other words, diabetics, those on statins and the chronically morbid, alongside those on medical cards or who can afford GPs, get diagnosed most often in Ireland. This is ad-hoc and opportunistic screening is very different from targeting the population of men most at risk at dying from prostate cancer, in fact these are probably the co-morbid population that would not be the target of a screening program.
Data shows that at the age of 45-50, men with the highest PSA levels are most at risk from developing serious prostate cancer (ref 1). In fact, 90% of men who subsequently die from prostate cancer, the vast majority are in highest quartile (top 25%) of PSA levels aged 45-50, suggesting that early diagnosis and intervention in this group, will have a considerable impact on mortality (ref 2). At the other end of the spectrum, we know that men whose PSA is <1ng/ml at the age of 50, and <2ng/ml at the age of 60, have very low risk of prostate cancer
36 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
David Galvin
Paul Sweeney
Padraig Day
death, and therefore PSA testing can be deferred in this low risk population as per the EAU algorithm (ref 3).

All men aged 50-70 years, particularly those with a first or second degree relative with prostate cancer, or BRCA carriers are most at risk. Those with PSA readings >3ng/ml will be referred in to the RAPC. There, men will be risk stratified using their family history, prostate size and the EAU risk calculator (known to reduce the need for biopsy). In this way, the valuable resources in hospital will be respected, and used efficiently. And reduce the burden on GP practices and the need for blood testing in the community. MRI imaging allows for the use of the standardised prostate

imaging score (PIRADS) which will determine the need for a targeted biopsy. All of this is designed to reduce the number of unnecessary biopsies, over-diagnosis and subsequent over-treatment, which many screening programs can fall foul of. If necessary men can avail of active surveillance, focal therapy and either minimally invasive surgery or radiotherapy. Our cancer centers are now supported by advanced nurse practitioners (ANP) in prostate cancer survivorship and self-supported follow up pathways funded by the NCCP and HSE.
Conclusion
Over the next 3 years, Ireland plans to complete this pilot study. Men will be invited to screening
from mid-2024 over a 12 month period, and just 5000 men will be recruited in the initial pilot. It is our hope that the home finger prick blood sample will appeal to men, and can piggyback on the already well established and successful bowel screening efforts. Certainly this smart and humble approach to male cancer screening will hopefully avoid the mistakes of the past, and perhaps will lead to a new paradigm, where men will engage in their own healthcare through cancer screening programs that can be scaled to include other diseases in the future.
References
1. Ulmert D, Cronin A, Vickers A, Lilja H. PSA at or before the age
of 50 as a predictor of advanced prostate cancer diagnosed up to 25 years later: A case control study. BMC Med. 2008 Feb 15;6:6
2. Vickers A, Ulmert D, Lilja H. Strategy for detection of Prostate Cancer based on relationship between PSA at age of 40-55 years and long term risk of metastases. BMJ 2013 Apr 15;346:f2023.
3. Sjoberg D, Vickers A, Ulmert D, Lilja H. Twenty-year Risk of Prostate Cancer Death by Midlife Prostate-specific Antigen and a Panel of Four Kallikrein Markers in a Large Populationbased Cohort of Healthy Men. BMJ. 2010 Sep 14;341:c4521.
Image
37 HOSPITALPROFESSIONALNEWS.IE | HPN • SEPTEMBER 2023
1. EAU Prostate Screening Algorithm
Oncology Focus: Metastasis
Breast cancer brain metastasis: insight into molecular mechanisms and therapeutic strategies
Department
Introduction: Breast Cancer

Brain Metastasis - A Growing Health Challenge
Breast cancer is the most commonly diagnosed cancer among women worldwide, with an increasing incidence in recent years. Although early detection and advances in treatment have significantly improved patient outcomes, metastatic breast cancer remains a major clinical challenge.1 Metastasis, the spread of cancer cells from the primary tumour to distant organs, is responsible for most breast cancer-related deaths. One of the most concerning sites of metastasis is the brain, as breast cancer brain metastases (BCBM) are associated with high morbidity and mortality. The rising incidence of BCBM can be attributed to several factors:2
Improved systemic treatment: Advances in targeted, hormonal, and immunotherapies have led to better primary tumour control and reduced distant metastases. Consequently, breast cancer patients live longer, increasing the likelihood of brain metastases developing over time.
Limited penetration of drugs into the brain: Many systemic treatments used for breast cancer cannot cross the blood-brain barrier (BBB) effectively. This
highly selective barrier protects the brain from potentially harmful substances. However, the limited penetration of these drugs into the brain can result in inadequate treatment of cancer cells that have already metastasized to the brain or are in the process of doing so. This may lead to the development and growth of BCBM even when the primary tumour and other metastases are well controlled.
Improved diagnostic techniques: The development and widespread use of advanced imaging techniques, such as magnetic resonance imaging (MRI), have made it possible to detect brain metastases at earlier stages and with greater accuracy. However, this has also increased the reported incidence of BCBM. Moreover, specific breast cancer subtypes are more likely to develop BCBM,1, 3 including:
HER2-positive breast cancer: This subtype, characterized by the overexpression of the human epidermal growth factor receptor 2 (HER2), has a higher propensity for brain metastasis. Targeted therapies against HER2, such as Trastuzumab and Pertuzumab, have improved the overall survival of patients with HER2-positive breast cancer. Still, they have limited penetration across the BBB, continuing the risk of BCBM.
Triple-negative breast cancer (TNBC): TNBC lacks the expression of estrogen receptor (ER), progesterone receptor (PR), and HER2, which makes it more aggressive and challenging to treat. This subtype is associated with a higher risk of developing BCBM, partly due to its aggressive biology and the lack of targeted therapies available.
Unravelling the Mechanism of Action: How PARP Inhibitors Target DNA Repair to Combat Cancer

PARP (poly ADP ribose polymerase) enzymes play a crucial role in the repair of singlestrand DNA breaks through the base excision repair pathway. Inhibition of PARP enzymes by PARP inhibitors leads to accumulating unrepaired singlestrand DNA breaks, which can be converted into double-strand breaks during DNA replication. Since cells with BRCA mutations have an impaired ability to repair double-strand breaks through homologous recombination (HR) repair, accumulating these breaks can lead to genomic instability and, ultimately, cell death.4 The mechanism of action of PARP inhibitors is summarized in Figure 1
Emerging evidence has revealed that PARP inhibitors can also be

effective in HR-deficient (HRD) cells independent of BRCA mutations.5 HRD can be caused by various factors, including mutations in other HR-related genes (such as RAD51 or PALB2), epigenetic changes, or alterations in gene expression. This has broadened the potential application of PARP inhibitors to a broader range of cancers with HRD beyond those with BRCA mutations.
PARP inhibitors have shown promise as a therapy for breast cancer patients, particularly those with germline BRCA1/2 gene mutations. However, their potential role in treating breast cancer brain metastasis remains an area of ongoing research. Interestingly, recent studies have suggested that specific PARP inhibitors, such as Pamipirab, can, to some extent, cross the BBB, making them potential candidates for treating brain metastasis.6 First, however, it is crucial to develop reliable biomarkers to identify patients with HRD who are most likely to benefit from PARP inhibitor therapy, irrespective of their BRCA status.
Expanding the Scope of PARP Inhibitor Therapy: Harnessing HRD Biomarkers Beyond BRCA Mutations
Numerous potential biomarkers and assays have been developed

38 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Dr. Jason McGrath
Dr. Jason McGrath (Postdoctoral researcher), Dr. Gordon Daly (Ph.D. researcher), Mr. Luke Cox (Research assistant), Dr. Damir Vareslija (Lecturer and Principal Investigator), and Prof. Leonie Young (Professor, Scientific Director Beaumont RCSI Cancer Centre).
of Surgery, Royal College of Surgeons in Ireland & Beaumont Hospital.
Dr. Gordon Daly
Dr. Damir Vareslija & Professor Leonie Young
Figure 1. Mechanism of PARP inhibitors in healthy and diseased breast cells. In functional BRCA cells (top), PARP inhibitors disrupt the repair of single-strand DNA breaks, thereby inducing DNA damage and destabilizing cell growth. In BRCA-mutated cells (bottom), PARP inhibitors prevent the repair of single-strand DNA breaks, accumulating double-strand breaks. With the BRCA repair pathway dysfunctional, these breaks cannot be repaired, leading to cell death. The schematic illustrates the versatile role of PARP inhibitors in both BRCA contexts. *PARP inhibitors are also effective in cells deficient in homologous recombination proteins (RAD51 or PALB2) independent of BRCA mutational status. This diagram was created using Biorender.com
or are under investigation to assess HRD in tumour samples:7, 8
Genomic Scar Assays: These assays measure specific genomic aberrations or "scars" characteristic of HRD tumours. The Myriad myChoice HRD and Foundation Medicine's FoundationFocus CDxBRCA tests are the most well-known genomic scar assays. Both of these tests assess three genomic parameters: loss of heterozygosity (LOH), telomeric allelic imbalance (TAI), and large-scale state transitions (LST). A combined HRD score is calculated based on these parameters, with higher scores indicating a higher likelihood of HRD.
Mutational Signatures: Specific patterns of somatic mutations, known as mutational signatures, can provide insights into a tumour's underlying DNA repair deficiencies. Whole-genome or whole-exome sequencing data can be used to identify these mutational signatures, which may help to determine whether
a tumour is HRD and potentially responsive to PARP inhibitors.
Gene Expression Profiling: Measuring the expression levels of specific genes involved in the HR pathway can help to identify tumours with HRD. Gene expression profiling can be performed using RNA sequencing or microarray analysis techniques. A distinct gene expression pattern associated with HRD may indicate that the tumour could be sensitive to PARP inhibitor therapy.
Functional Assays: These assays directly measure the activity of the HR pathway in tumour cells. For example, the RAD51 foci formation assay evaluates the recruitment of RAD51 to DNA damage sites, which indicates functional HR. A reduced capacity to form RAD51 foci may suggest HRD and potential sensitivity to PARP inhibitors.
While these biomarkers and assays show promise in identifying HRD tumours that may benefit from PARP inhibitor therapy, further research is needed to

validate their accuracy and clinical utility. Furthermore, as our understanding of HRD improves, a combination of multiple biomarkers will likely be used to select patients who are most likely to respond to PARP inhibitors, regardless of their BRCA status.
Adapting to Survive: The Complex Dynamics of Resistance to PARP Inhibitor Therapy
Unfortunately, both mutatedBRCA and non-mutated-BRCA tumours can develop resistance to PARP inhibitors through various mechanisms, summarized in Figure 2. Some of the common factors contributing to PARP inhibitor resistance include:9, 10
Reduced drug uptake or increased efflux: Resistance to PARP inhibitors may arise from alterations in drug transport mechanisms (i.e., ABCB1 network), such as reduced drug uptake into cancer cells or increased drug efflux, which decreases intracellular drug concentrations and ultimately reduces the drug's efficacy.
Alterations in PARP expression or activity: Changes in the expression or activity of PARP enzymes can contribute to resistance. For example, some tumours develop resistance by downregulating PARP1 expression, rendering the PARP inhibitor ineffective. In other cases, the cancer cells might express PARP mutants resistant to the inhibitor's effects.
Restoration of homologous recombination (HR): PARP inhibitors exploit synthetic lethality by targeting cancer cells with HR deficiencies. However, tumours can develop resistance by restoring HR function, which can occur through secondary mutations in the BRCA1/2 genes or upregulation of other genes involved in the HR pathway.
Activation of alternative DNA repair pathways: Tumour cells can overcome PARP inhibitorinduced DNA damage by compensating with alternative DNA repair pathways, such as non-homologous end joining (NHEJ), allowing them to repair and survive DNA damage.
Epigenetic changes: Alterations in the epigenetic regulation of DNA repair genes can also contribute to PARP inhibitor resistance. For example, changes in the methylation status of specific genes involved in the HR pathway might restore HR function,
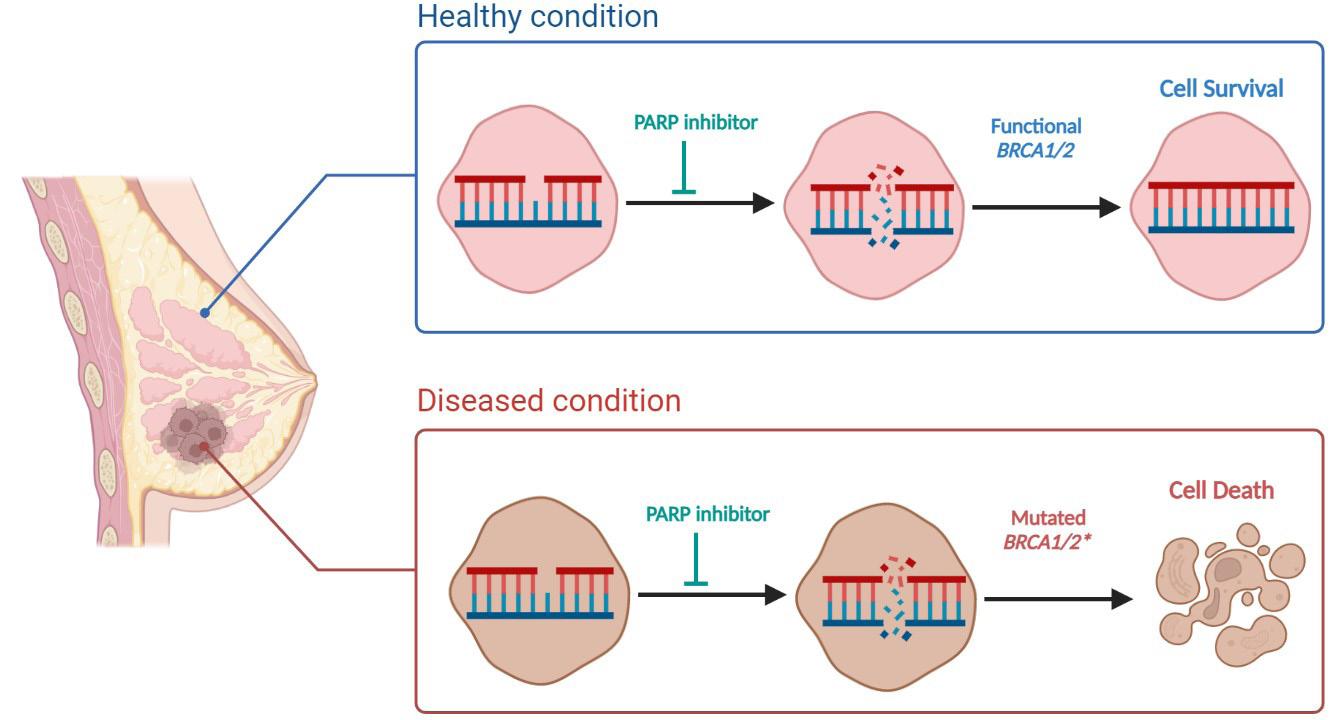
39 HOSPITALPROFESSIONALNEWS.IE | HPN • SEPTEMBER 2023
enabling the cancer cells to repair DNA damage more efficiently. Adaptation of cellular signalling pathways: Cancer cells can adapt to PARP inhibitors by activating alternative signalling pathways that promote cell survival, proliferation, and DNA repair. These adaptive changes can help the cells overcome the cytotoxic effects of PARP inhibitors and contribute to therapy resistance.
To overcome PARP inhibitor resistance, researchers are exploring combination therapies targeting multiple pathways, developing new PARP inhibitors with improved efficacy, and better identifying patients most likely to benefit from PARP inhibitor treatment, and just as significantly to help maintain a patient's quality of life by reducing drug concentrations.
Boosting the Battle: Approaches to Enhance PARP Inhibitor Sensitivity
Several ongoing clinical trials are exploring novel strategies
Oncology Focus: Metastasis
Figure 2. Common mechanisms responsible for PARP inhibitor resistance in cancer. Increased drug efflux: Cancer cells are known to upregulate drug transporters on the cell surface, which are more efficient at eliminating drugs, including PARP inhibitors (PARPi), thus increasing drug resistance. Mutated PARP1: Alterations in PARP1 expression or mutated PARP1 significantly prevent cell death by PARPi due to reduced drug activity. Restoring HR: Homologous recombination (HR) can be restored in tumours by a secondary mutation of BRCA or upregulating other HR proteins (Rad51 or PALB2) to compensate for the loss of BRCA, consequently resulting in treatment resistance. This diagram was created using Biorender.com
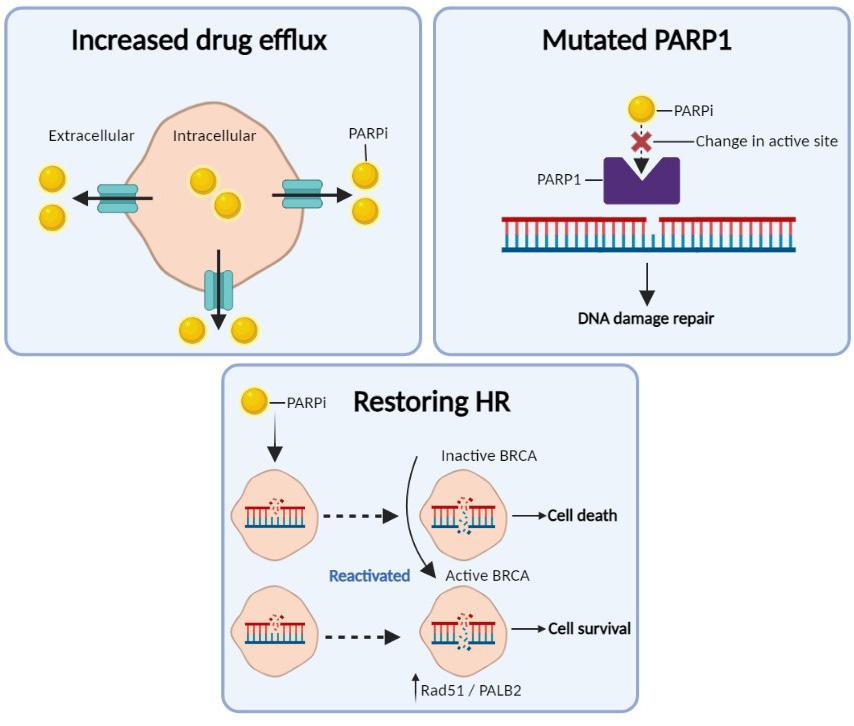
to overcome PARP inhibitor resistance in cancer treatment, including breast cancer. Some of these exciting trials include:
NCT02157792:11 This Phase I study evaluates the combination of Berzosertib, an ATR kinase inhibitor, with the PARP inhibitor Olaparib in patients with advanced solid tumours, including those who have progressed on prior PARP inhibitor therapy. The rationale behind this combination is that ATR inhibition can further compromise DNA repair pathways in cancer cells, potentially enhancing the cytotoxic effects of PARP inhibitors.
NCT03579316:12 This Phase II trial investigates the combination of Adavosertib, a WEE1 inhibitor, with Olaparib in patients with advanced solid tumours, including those resistant to prior PARP inhibitor treatment. WEE1 inhibition may cause replication stress and genomic instability, which could sensitize cancer cells to PARP inhibition.
NCT02861573:13 This Phase I/II trial evaluates the combination of the immune checkpoint inhibitor Pembrolizumab with Olaparib in patients with advanced solid tumours, including those who have received prior PARP inhibitor therapy. Combining PARP inhibitors with immunotherapies might help overcome resistance by stimulating anti-tumour immune responses.
NCT02734004:14 This Phase Ib/ II trial assesses the combination of the anti-PD-L1 antibody Durvalumab with Olaparib and chemotherapy in patients with advanced solid tumours, including those resistant to prior PARP inhibitors. This trial aims to determine whether adding immune checkpoint blockade and chemotherapy to PARP inhibitor therapy can improve outcomes in resistant patients.
Future implications:
The use of PARP inhibitors in BCBM holds significant promise for improving patient outcomes. By

exploiting DNA repair deficiencies in cancer cells, PARP inhibitors have shown imposing activity in treating BRCA-mutated and HRD breast cancer. However, resistance to PARP inhibitors remains a significant challenge. Ongoing clinical trials exploring novel strategies to overcome resistance, such as combining PARP inhibitors with immunotherapy or other targeted agents, offer hope for further improving the effectiveness of these drugs. Additionally, identifying reliable biomarkers to predict response to PARP inhibitors in HRD patients, regardless of BRCA status, could help expand the patient population that may benefit from this treatment. With continued research, PARP inhibitors may become an essential component of the treatment armamentarium for BCBM and improve outcomes for patients with this devastating condition.
References available upon request
40 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Oncology Focus: Cervical Cancer

Vaccination of children and teenagers to prevent cervical cancer
and gynaecology review
Written by Ciara O'Connor1 and Fergal Malone2
1RCSI medical student

2Professor and Chair of Obstetrics and Gynaecology, Royal College of Surgeons, and Rotunda Hospital, Dublin
HPV genomes found in cervical carcinomas
HPV viruses cloned
HPV defined as large, closely-related family: >100 types, 30-40 anogenital
Mechanisms of transformation elucidated (true ‘tumour virus’)
Epidemiological case-control studies completed
15-20 HPV types classified as oncogenic
is
FIGURE 1:
ME. Prevention of cervical cancer through vaccination of adolescents. Clin Pediatr (Phila) 2006; 45 (5): 393-8.
FIGURE 1: Discovery of the link between human papillomavirus and cervical cancer. Adapted from: Pichichero ME. Prevention of cervical cancer through vaccination of adolescents. Clin Pediatr (Phila) 2006; 45 (5): 393-8.
afford some protection against HPV transmission, although this protection is incomplete.5
Introduction
The aim of this review was to examine the evidence for and against vaccination of children and teenagers against HPV and the development of cervical cancer.12
Methods
The World Health Organisation commented recently that a vaccine to prevent oncogenic HPV infection or premalignant cervical lesions from progressing to cancer would offer a cost-effective long-term strategy to reduce the cervical cancer burden, particularly for developing countries where effective screening programmes are not available.12
The aim of this review was to examine the evidence for and against vaccination of children and teenagers against HPV and the development of cervical cancer.12
Methods
A MEDLINE and PubMed search (1980-2007) was performed using the search terms ‘HPV vaccination’, ‘HPV vaccination and children’, ‘Cervical cancer and HPV’, and ‘CIN’. An English language restriction was imposed and the search continued up to August 20, 2007. The references cited in review articles were examined for potentially eligible studies. The literature search yielded 160 papers, of which 39 were considered eligible for inclusion.
Results
HPV and cervical cancer
In disease control, prevention is superior, safer, and generally more cost-effective than treating illnesses after they develop. Vaccination, which is the administration of antigenic material to produce immunity to a disease, is one of the most important preventive measures currently at our disposal. The immune response to a vaccine may be cell-mediated or antibodymediated, or both. Human papillomaviruses (HPVs) comprise a group of about 100 DNA nonenveloped viruses. The burden of disease related to HPV is substantial. Variable proportions of penile,1 vaginal,2 urethral,3 and head and neck cancers have been found to contain carcinogenic HPV.4-6 Cervical cancer is the most important manifestation of genital HPV infection, and is one of the leading causes of cancer mortality in women worldwide. The global disease burden of cervical cancer is estimated at 470,000 new cases, and 230,000 deaths, every year. Almost 80% of cases occur in developing countries, where it is the most
common cancer among women.7 In Europe, 15,000 women die from cervical cancer each year, and it is the second most common form of cancer seen in women under 35 years of age. In Ireland, there are approximately 180 new cases annually, and about 80 deaths a year are caused by cervical cancer.8 Cervical cancer primarily affects women between 39 and 45 years of age, thereby representing an important opportunity cost in terms of potential years of life lost. The impact of the disease is accentuated further by the average age at death, often when women are still raising children. In the United States, it is estimated that over six million people are infected with genital HPV.9 Data from the National Health and Nutrition Examination Survey found a 26.8% prevalence of HPV among US girls and women, with increasing prevalence each year from ages 14 to 24 years, followed by a gradual decline in prevalence until 60.10 The risk factors for acquisition of HPV infection are shown in Table 1. Infection is extremely common in young women in their first decade of sexual activity.11 Condoms
Key landmarks in the discovery of the link between HPV and cervical cancer are illustrated in Figure 1. The first recognition of this relationship was made by zur Hausen in the 1970s.13 Using sensitive molecular polymerase chain reaction (PCR) techniques, Walboomers et al detected HPV DNA in 99.7% of a series of cervical cancers.14 More than 100 strains of HPV exist, but types 16 and 18 are the most prevalent oncogenic strains of the virus, with HPV 16
The World Health Organisation commented recently that a vaccine to prevent oncogenic HPV infection or premalignant cervical lesions from progressing to cancer would offer a cost-effective long-term strategy to reduce the cervical cancer burden, particularly for developing countries where effective screening programmes are not available.12

RCSIsmj obstetrics and gynaecology
A MEDLINE and PubMed search (1980-2007) was performed using the search terms ‘HPV vaccination’, ‘HPV vaccination and children’, ‘Cervical cancer and HPV’, and ‘CIN’. An English language restriction was imposed and the
TABLE 1: Risk factors for human
Women
Young age (peak age group, 20-24 years)
Increased number of sexual partners
First sexual intercourse at an early age
Male partner sexual behaviour
Smoking
Oral contraceptive use
Uncircumcised male partners
Men
Young age (peak age group, 25-29 years)
Increased number of sexual partners
Lack of circumcision
Taken from: Pichichero ME. Prevention of cervical cancer through vaccination of adolescents. Clin Pediatr (Phila) 2006; 45 (5): 393-8.
Introduction
FIGURE cancer. vaccination
In disease control, prevention is superior, safer, and generally more cost-effective than treating illnesses after they develop. Vaccination, The prevent
41 HOSPITALPROFESSIONALNEWS.IE | HPN • SEPTEMBER 2023
Discovery of the link between human papillomavirus and cervical cancer. Adapted from: Pichichero
papillomavirus infection.
29/01/2008 15:55 Page 42
Vaccination,
In
35
RCSILayout-AW
is
cancer.8 of of
Ciara O'Connor
Professor Fergal Malone
Oncology Focus: Cervical Cancer
TABLE 2: Comparison of human papillomavirus vaccines.
Gardasil (quadrivalent vaccine)
Manufacturer Sanofi Pasteur MSD
European license
Granted September 2006
Licensed for use in Females 9-26 years
What it is
Cervarix (bivalent vaccine)
GlaxoSmithKline
Not granted yet; anticipated in 2008
Females 10-55 years
L1 VLP vaccine in aluminium-containing vaccine in aluminium-containing suspension. Volume 0.5ml suspension. Volume 0.5ml
L1 VLP (vaccine-like particle)
Dosage 3-vaccine course
3-vaccine course
Administration Doses at 0, 2 and 6 months, Doses at 0, 2 and 6 months, given by deep IM injection to given by deep IM injection deltoid or anterolateral thigh to deltoid or anterolateral thigh
Targets
HPV 6/11/16/18
Efficacy 100% against precancerous lesions
Common adverse effects
HPV 16/18 (may also target 31/45)
100% against precancerous lesions associated with HPV 16- and associated with HPV 16 and 18 18-related cervical cancer
• Local injection site pain (up to 86%)
• Erythema (up to 41%)
• Swelling (up to 37%)
• Local injection site pain (up to 93%)
• Erythema (up to 36%)
• Swelling (up to 34%)
• Headache (most common systemic adverse event)• Headache (most common systemic adverse effect)
Adapted from: Kenny N. HPV vaccine update. World of Irish Nursing 2007; 15 (2).
changes.19-22 Most women will get oncogenic HPV at some stage in their lives, usually in their teens or twenties, and it will pass harmlessly. A very small proportion persists, and becomes integrated into the cell DNA. These pose a risk of cancer to the woman in question. About 500,000 precancerous lesions of Grade 2 and 3 (CIN 2 and 3) are diagnosed each year in the United States, and about 50-60% are attributable to HPV 16 and HPV 18.23
search continued up to August 20, 2007. The references cited in review articles were examined for potentially eligible studies. The literature search yielded 160 papers, of which 39 were considered eligible for inclusion.
Results
HPV and cervical cancer
HPV vaccines
intraepithelial lesions (LSIL) and Grade 1 cervical intraepithelial neoplasia (CIN) changes.19-22 Most women will get oncogenic HPV at some stage in their lives, usually in their teens or twenties, and it will pass harmlessly. A very small proportion persists, and becomes integrated into the cell DNA. These pose a risk of cancer to the woman in question. About 500,000 precancerous lesions of Grade 2 and 3 (CIN 2 and 3) are diagnosed each year in the United States, and about 50-60% are attributable to HPV 16 and HPV 18.23
against either incident or persistent HPV infection.26
profoundly reduce the risk of CIN in about 10 years.
HPV vaccines

Two vaccines have been developed to prevent HPV infection and details of these are shown in Table 2. Randomised controlled trials of HPV vaccines have demonstrated their immunogenicity and ability to prevent incident and persistent type-specific HPV infection and associated cytological abnormalities in the cervix.24
Intramuscular injection of either vaccine induces high titres of neutralising antibody, at more than 50 times the titres induced by natural infection.25 Phase II trial results for both vaccines have shown efficacy estimates of 100% against either incident or persistent HPV infection.26
In a seminal randomised, double blind, controlled trial to assess the efficacy, safety, and immunogenicity of a bivalent HPV 16/18 L1 virus-like particle vaccine (similar to Cervarix), Harper et al randomised 1,113 women aged between 15 and 25 years. These women received three doses of either the vaccine or placebo on a 0-, 1-, and 6-month schedule in North America and Brazil. Women were assessed for HPV infection for up to 27 months by cervical
Key landmarks in the discovery of the link between HPV and cervical cancer are illustrated in Figure 1. The first recognition of this relationship was made by zur Hausen in the 1970s.13 Using sensitive molecular polymerase chain reaction (PCR) techniques, Walboomers et al detected HPV DNA in 99.7% of a series of cervical cancers.14 More than 100 strains of HPV exist, but types 16 and 18 are the most prevalent oncogenic strains of the virus, with HPV 16 accounting for more than 60% of cervical cancers, and HPV 18 responsible for another 10%.15-18 The great majority of HPV infections, including infection by carcinogenic types, are transient, and resolve or become undetectable within a year or two, sometimes causing mild cytopathological changes, including atypical squamous cells (ASC), low-grade squamous
Two vaccines have been developed to prevent HPV infection and details of these are shown in Table 2. Randomised controlled trials of HPV vaccines have demonstrated their immunogenicity and ability to prevent incident and persistent type-specific HPV infection and associated cytological abnormalities in the cervix.24 Intramuscular injection of either vaccine induces high titres of neutralising antibody, at more than 50 times the titres induced by natural infection.25 Phase II trial results for both vaccines have shown efficacy estimates of 100%
In a seminal randomised, double blind, controlled trial to assess the efficacy, safety, and immunogenicity of a bivalent HPV 16/18 L1 virus-like particle vaccine (similar to Cervarix), Harper et al randomised 1,113 women aged between 15 and 25 years. These women received three doses of either the vaccine or placebo on a 0-, 1-, and 6-month schedule in North America and Brazil. Women were assessed for HPV infection for up to 27 months by cervical cytology and self-obtained cervico-vaginal samples, and for vaccine safety and immunogenicity. The vaccine efficacy against incident infection was 91.6% (95% CI, 64.5–98.0) and 100% against persistent infection (95% CI, 47.0–100), as well as also protecting against HPV-associated cytological abnormalities and lesions.27 In a follow-up study in 2006, Harper et al found that more than 98% of subjects who received all three doses remained seropositive for HPV 16/18.28 While it is true that vaccination may not have an effect on cervical cancer for 20 to 30 years, vaccination could
cytology and self-obtained cervico-vaginal samples, and for vaccine safety and immunogenicity. The vaccine efficacy against incident infection was 91.6% (95% CI, 64.5–98.0) and 100% against persistent infection (95% CI, 47.0–100), as well as also protecting against HPV-associated cytological abnormalities and lesions.27 In a follow-up study in 2006, Harper et al found that more than 98% of subjects who received all three doses remained seropositive for HPV 16/18.28 While it is true that vaccination may not have an effect on cervical cancer for 20 to 30 years, vaccination could profoundly reduce the risk of CIN in about 10 years.
Two major randomised controlled trials – the FUTURE (Females United to Unilaterally Reduce Endo/Ectocervical Disease) II trial29 and the PATRICIA (PApilloma TRIal against Cancer In young Adults) study30 – were included in a systematic review by Rambout et al 31 This systematic review found that HPV vaccination was highly effective in preventing vaccine type-specific HPV infection, and precancerous cervical disease, particularly among women aged 15 to 25 years who received all three vaccine doses, had no more than six lifetime sexual partners, and no prior abnormal results from cervical screening. The FUTURE II study found that in young women who had not previously been infected with HPV 16 or HPV 18, those in the vaccine group had a significantly lower occurrence of highgrade CIN related to HPV 16 or HPV 18 compared with the placebo group.29 Both trials demonstrated high efficacy against CIN 2 or worse related to HPV types 16 and 18, with a mean follow-up of three years (FUTURE II) and 15 months (PATRICIA), respectively.31
Two major randomised controlled trials – the FUTURE (Females United to Unilaterally Reduce Endo/Ectocervical Disease) II trial29 and the PATRICIA (PApilloma TRIal against Cancer In young Adults) study30 – were included in a systematic review by Rambout et al 31 This systematic review found that HPV vaccination was highly effective in preventing vaccine type-specific HPV infection, and precancerous cervical disease, particularly among women aged 15 to 25 years who received all three vaccine doses, had no more than six lifetime sexual partners, and no prior abnormal results from cervical screening. The FUTURE II study found that in young women who had not previously been infected with HPV 16 or HPV 18, those in the vaccine group had a significantly lower occurrence of high-grade CIN related to HPV 16 or HPV 18 compared with the placebo group.29 Both trials demonstrated high efficacy against CIN 2 or worse related to HPV types 16 and 18, with a mean follow-up of three years (FUTURE II) and 15 months (PATRICIA), respectively.31
42 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Volume 1: Number 1. 2008 | Page 43 RCSIsmj obstetrics and gynaecology
review
RCSIsmj obstetrics and gynaecology review
HPV VIRUS Over 100 subtypes, 40 infect the genital tract, half of these are oncogenic
18 months EXPOSURE Primary mode of transmission is by sexual intercourse, lifetime risk of infection 80%
vaccinated, more than 1,300 deaths from cervical cancer during their lifetimes would be averted,37 but as the United States does not have a national cervical screening programme, their cost-effectiveness calculations may be skewed.
INFECTION
Very common but mostly transient with spontaneous resolution within 18 months
18 months
PERSISTENCE
Less than 20% of women show signs of persistent infection
1-5 years
MALIGNANT TRANSFORMATION
Viral DNA integrates into the cells of the dividing cervix
PRECANCER Abnormal cells divide more rapidly lacking quality assurance of cell death
10 years
CANCER 99% of cervical cancers are positive for high-risk HPV
FIGURE 2: Natural history of human papillomavirus infection.
Adapted from: presentation by Dr Grainne Flannelly entitled ‘HPV Vaccination’, to the National Advisory Group, March 2007 Discussion
The estimated cost of the quadrivalent HPV vaccine is $360 in the USA, and ¤450 in Europe for a three-course series, making it among the most expensive of all vaccines.38 If consumers or insurers were to pay, poor and uninsured persons would be unable to afford the vaccine, further exacerbating health disparities. If the government is to pay, this will require funds from the central budget, perhaps reducing health spending for other programmes.

Cost-effectiveness studies of HPV vaccination have had variable results, depending on assumptions about effectiveness and safety.36 The major financial benefit of the vaccine to the Irish health service is likely to be the prevention of anogenital warts that the quadrivalent virus affords. Anogenital warts represent about 10% of sexually-transmitted HPV infections and 4,147 cases of anogenital warts were notified in Ireland in 2004. 8 A research group at Stanford University found that if all 12-year-old girls currently living in the United States were vaccinated, more than 1,300 deaths from cervical cancer during their lifetimes would be averted,37 but as the United States does not have a national cervical screening programme, their cost-effectiveness calculations may be skewed.
of Texas (by executive order) and Virginia made quadrivalent HPV vaccine mandatory for girls entering sixth grade.43 However, the Texas legislature recently voted to overturn the governor’s order, and the state of Virginia granted parents generous “opt-out” provisions.44 Nearly 20 additional states are considering similar legislation.45 Research in the US on the issue of mandatory vaccination concluded that making the HPV vaccine mandatory might paradoxically contribute to long-standing parental concerns about the safety of school-based vaccination, and increase parental and public apprehension about the vaccine.46
The estimated cost of the quadrivalent HPV vaccine is $360 in the USA, and € 450 in Europe for a three-course series, making it among the most expensive of all vaccines. 38 If consumers or insurers were to pay, poor and uninsured persons would be unable to afford the vaccine, further exacerbating health disparities. If the government is to pay, this will require funds from the central budget, perhaps reducing health spending for other programmes.
Australia already has a government-funded programme offering vaccination to girls and young women aged 12 to 26 years.47 The United Kingdom has just announced that universal HPV vaccination of girls aged 12 to 13 years will begin in September 2008, and the UK Heath Secretary Alan Johnson has confirmed that the cervical screening programme will continue after the introduction of the HPV vaccine. It is expected that they will use the quadrivalent vaccine Gardasil.48
Discussion
It is important to vaccinate patients before the age at which exposure to HPV is likely to occur, and from a public health perspective, routine vaccination before sexual debut or shortly thereafter is important. Recent Irish data found that 12% of women had their first experience of vaginal intercourse before the age of 17.8 years,32 and a poll of 15- to 17-yearolds found that one in four had had sex.33 The 2007 guidelines from the American Cancer Society recommend routine HPV vaccination for females aged 11 to 12 years but also state that females as young as nine years may receive the vaccine.34
Brabin et al used a questionnaire to randomly sample parents of pupils aged 11 to 12 years in seven different secondary schools in the city of Manchester.35 They found a HPV vaccine uptake rate of 80% was achievable if the vaccine was perceived as safe and effective. However, the authors also found that most parents lacked knowledge about HPV, and some were concerned about sexual health issues that would arise as part of a HPV vaccine programme, as well as the possibility that administering the vaccine might actually encourage promiscuity.35
It is important to vaccinate patients before the age at which exposure to HPV is likely to occur, and from a public health perspective, routine vaccination before sexual debut or shortly thereafter is important. Recent Irish data found that 12% of women had their first experience of vaginal intercourse before the age of 17.8 years,32 and a poll of 15- to 17-year-olds found that one in four had had sex.33 The 2007 guidelines from the American Cancer Society recommend routine HPV vaccination for females aged 11 to 12 years but also state that females as young as nine years may receive the vaccine. 34
In a population-based study to assess parental consent and potential HPV vaccine uptake, Brabin et al used a questionnaire to randomly sample parents of pupils aged 11 to 12 years in seven different secondary schools in the city of Manchester. 35 They found a HPV vaccine uptake rate of 80% was achievable if the vaccine was perceived as safe and effective. However, the authors also found that most parents lacked knowledge about HPV, and some were concerned about sexual health issues that would arise as part of a HPV vaccine programme, as well as the possibility that administering the vaccine might actually encourage promiscuity. 35
In a population-based study to assess parental consent and potential HPV vaccine uptake,
Cost-effectiveness studies of HPV vaccination have had variable results, depending on assumptions about effectiveness and safety.36 The major financial benefit of the vaccine to the Irish health service is likely to be the prevention of anogenital warts that the quadrivalent virus affords. Anogenital warts represent about 10% of sexually-transmitted HPV infections and 4,147 cases of anogenital warts were notified in Ireland in 2004.8 A research group at Stanford University found that if all 12-year-old girls currently living in the United States were
In the wake of concerns about measles-mumps-rubella (MMR) vaccines, parents have become more cautious about vaccine safety and some parents are less trusting of scientific evidence.39,40 There are scant data on the duration of HPV vaccineinduced immunity. Following naturally occurring HPV infection, many women do not develop detectable HPV antibodies and, in the case of HPV 16, the available serological assays detect type-specific antibodies in only 54-60% of infected women.20 Therefore, longer-term follow-up of vaccinated subjects cannot rely on routine measurement of HPV vaccine-induced antibody titres. Furthermore, because of the extended time involved in the progression from HPV infection to cervical cancer, it will be many years before a reduction in cancer incidence and mortality rates can be verified within a vaccinated population. The Canadian federal government recently announced a $300 million investment in a programme for vaccinating girls and women with the currently available Gardasil vaccine. Questions have been raised, however, as to whether a universal immunisation programme is, at this time, premature and could have unintended negative consequences for individuals and for society as a whole.41 A repeated comment is that there is no epidemic of cervical cancer in Canada to warrant the sense of urgency for a vaccination programme.
In the wake of concerns about measles-mumps-rubella (MMR) vaccines, parents have become more cautious about vaccine safety and some parents are less trusting of scientific evidence.39,40 There are scant data on the duration of HPV vaccine-induced immunity. Following naturally occurring HPV infection, many women do not develop detectable HPV antibodies and, in the case of HPV 16, the available serological assays detect type-specific antibodies in only 54-60% of infected women.20 Therefore, longer-term follow-up of vaccinated subjects cannot rely on routine measurement of HPV vaccine-induced antibody titres. Furthermore, because of the extended time involved in the progression from HPV infection to cervical cancer, it will be many years before a reduction in cancer incidence and mortality rates can be verified within a vaccinated population. The Canadian federal government recently announced a $300 million investment in a programme for vaccinating girls and women with the currently available Gardasil vaccine. Questions have been raised, however, as to whether a universal immunisation programme is, at this time, premature and could have unintended negative consequences for individuals and for society as a whole.41 A repeated comment is that there is no epidemic of cervical cancer in Canada to warrant the sense of urgency for a vaccination programme.
According to the 2006 Canadian cancer statistics,42 cervical cancer is the 11th most frequent cancer affecting women, and the 13th most common cause of cancerrelated deaths, accounting for approximately 400 deaths per year. Earlier this year the states
According to the 2006 Canadian cancer statistics,42 cervical cancer is the 11th most frequent cancer affecting women, and the 13th most common cause of cancer-related deaths, accounting for approximately 400 deaths per year. Earlier this year the states of Texas (by executive order) and Virginia made quadrivalent HPV vaccine mandatory for girls entering sixth grade.43 However, the Texas legislature recently voted to overturn the governor’s order, and the state of Virginia granted parents generous “opt-out” provisions.44 Nearly 20 additional states are considering similar
In Ireland, the Department of Health and Children has not yet introduced routine cervical cancer vaccination, or even a systematic screening programme. In January 2006, the Minister for Health and Children, Mary Harney, announced the rollout of the Irish Cervical Screening Programme (ICSP). In parallel with the ICSP, she announced the establishment of the Board of the National Cancer Screening Service and, in a press release, stated that: “issues to be examined included the effectiveness and cost-effectiveness of the vaccine, categories of women who should be vaccinated, immunity duration and booster requirements, and the implications for the national screening programme”.49 In a properly screened programme, the vaccination of young girls will prevent CIN and colposcopy, as well as prevent cervical cancer. Vaccination provides a new approach to preventing cervical cancer in Ireland and in other countries with a high incidence of the disease and zero or poorly developed screening programmes.47 It is conceivable that if a vaccination programme were introduced in the absence of a screening programme, the rate of cervical cancer could actually increase. This message is reiterated by Adams et al, who
43 HOSPITALPROFESSIONALNEWS.IE | HPN • SEPTEMBER 2023
Page 44 | Volume 1: Number 1. 2008
FIGURE 2: Natural history of human papillomavirus infection. Adapted from: presentation by Dr Grainne Flannelly entitled ‘HPV Vaccination’, to the National Advisory Group, March 2007.
Oncology Focus: Cervical Cancer

stress the need to implement or continue a cervical screening programme to complement vaccination, which will optimise prevention in those who receive the vaccine and prevent cervical cancer in older women where the value of vaccination is currently unclear.50 Vaccination will reduce the risk of cervical cancer by another 20%.37
Conclusion
HPV vaccination has high efficacy against the major HPV types that cause life-threatening disease. Vaccination appears to be safe, and so delaying vaccination may mean that many women will miss an opportunity for long-lasting protection against cervical cancer. On the other hand, a cautious approach may be warranted in the light of important unanswered questions about overall vaccine efficacy, duration of protection and possible adverse effects.50 The advent of HPV vaccines represents the most important breakthrough to date in cervical cancer prevention, and will have positive consequences for public health worldwide.
Acknowledgements
I would like to thank my family for their encouragement to write this paper, and Professors Malone and Prendeville for their helpful comments.
References
1. Rubin MA, Kleter B, Zhou M et al. Detection and typing of human papillomavirus DNA in penile carcinoma: evidence for multiple independent pathways of penile carcinogenesis. Am J Pathol 2001; 159 (4): 1211-8.
2. Daling JR, Madeleine MM, Schwartz SM et al. A population-based study of squamous cell vaginal cancer: HPV and cofactors. Gynecol Oncol 2002; 84 (2): 263-70.
3. Cupp MR, Malek RS, Goellner JR, Espy MJ, Smith TF. Detection of human papillomavirus DNA in primary squamous cell carcinoma of the male urethra. Urology 1996; 48 (4): 551-5.
4. Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med 2001; 345 (26): 1890900.
5. Herrero R, Castellsague X, Pawlita M et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 2003; 95 (23): 1772-83.
6. Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev 2005; 14 (2): 467-75.
7. Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer 2001; 94 (2): 153-6.
8. Kenny N. HPV vaccine update. World of Irish Nursing 2007; 15 (2).
9. Weinstock H, Berman S, Cates W Jr. Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health 2004; 36 (1): 6-10.
10. Dunne EF, Unger ER, Sternberg M et al. Prevalence of HPV infection among females in the United States. JAMA 2007; 297 (8): 813-9.
11. Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet 2007; 370 (9590): 890-907.
12. WHO. Human papillomavirus vaccines against cervical cancer. (Accessed August 17, 2007, at www. who.int/vaccines/en/olddocs/ humanpapill.shtml.)
13. McIntyre P. Finding the viral link: the story of Harald zur Hausen. Cancer World 2005; 7: 32–7.
14. Walboomers JM, Jacobs MV, Manos MM et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189 (1): 12-9.
15. Munoz N, Bosch FX, de Sanjose S et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348 (6): 518-27.
16. Bosch FX, Manos MM, Munoz N et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst 1995; 87 (11): 796-802.
17. Clifford GM, Smith JS, Plummer M, Munoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a metaanalysis. Br J Cancer 2003; 88 (1): 63-73.
18. Schiffman M, Castle PE. Human papillomavirus: epidemiology and public health. Arch Pathol Lab Med 2003; 127 (8): 930-4.
19. Cuschieri KS, Cubie HA, Whitley MW et al. Multiple high risk HPV infections are common in cervical neoplasia and young women in a cervical screening population. J Clin Pathol 2004; 57 (1): 68-72.
20. Ho GY, Studentsov YY, Bierman R, Burk RD. Natural history of human papillomavirus type 16 virus-like particle antibodies in young women. Cancer Epidemiol Biomarkers Prev 2004; 13 (1): 110-6.
21. Moscicki AB, Shiboski S, Hills NK et al. Regression of low-grade squamous intra-epithelial lesions in young women. Lancet 2004; 364 (9446): 1678-83.
22. Woodman CB, Collins S, Winter H et al. Natural history of cervical human papillomavirus infection in young women: a longitudinal cohort study. Lancet 2001; 357 (9271): 1831-6.
23. Clifford GM, Smith JS, Aguado T, Franceschi S. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. Br J Cancer 2003; 89 (1): 101-5.
24. Villa LL, Costa RL, Petta CA et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multi-centre phase II efficacy trial. Lancet Oncol 2005; 6 (5): 271-8.
25. Harro CD, Pang YY, Roden RB et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J Natl Cancer Inst 2001; 93 (4): 284-92.
26. Koutsky LA, Ault KA, Wheeler CM et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med 2002; 347 (21): 1645-51.
27. Harper DM, Franco EL, Wheeler C et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 2004; 364 (9447): 1757-65.
28. Harper DM, Franco EL, Wheeler CM et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367 (9518): 1247-55.
29. FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356 (19): 1915-27.
30. Paavonen J, Jenkins D, Bosch FX et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-likeparticle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007; 369 (9580): 2161-70.
31. Rambout L, Hopkins L, Hutton B, Fergusson D. Prophylactic vaccination against human papillomavirus infection and disease in women: a systematic review of randomised controlled trials. CMAJ 2007; 177 (5): 469-79.
32. Irish Study of Sexual Health and Relationships Report. Dublin; 2006, October 16.
33. One in four 15-17 year olds have had sex – poll. Irish Times, September 19, 2003.
34. Saslow D, Castle PE, Cox JT et al. American Cancer Society Guideline for human papillomavirus (HPV) vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin 2007; 57 (1): 7-28.
35. Brabin L, Roberts SA, Farzaneh F, Kitchener HC. Future acceptance of adolescent human papillomavirus vaccination: a survey of parental attitudes. Vaccine 2006; 24 (16): 3087-94.
36. Elbasha EH, Dasbach EJ, Insinga RP. Model for assessing human papillomavirus vaccination strategies. Emerg Infect Dis 2007; 13 (1): 28-41.
37. Sanders GD, Taira AV. Costeffectiveness of a potential vaccine for human papillomavirus. Emerg Infect Dis 2003; 9 (1): 37-48.
38. Gardner A. Drugmaker assists in pushing for mandate for HPV vaccination. Washington Post, February 11, 2007.
39. Fredrickson DD, Davis TC, Arnould CL et al. Childhood immunisation refusal: provider and parent perceptions. Fam Med 2004; 36 (6): 431-9.
40. Bardenheier B, Yusuf H, Schwartz B, Gust D, Barker L, Rodewald L. Are parental vaccine safety concerns associated with receipt of measles-mumps-rubella, diphtheria and tetanus toxoids with acellular pertussis, or hepatitis B vaccines by children? Arch Pediatr Adolesc Med 2004; 158 (6): 569-75.
41. Lippman A, Melnychuk R, Shimmin C, Boscoe M. Human papillomavirus, vaccines and women’s health: questions and cautions. CMAJ 2007; 177 (5): 484-7.
42. Canadian Cancer Statistics. Toronto; 2006.
43. Gostin LO, DeAngelis CD. Mandatory HPV vaccination: public health vs. private wealth. JAMA 2007; 297 (17): 1921-3.
44. Saul S, Pollack A. Furore on rush to require cervical cancer vaccine. New York Times, February 17, 2007.
45. HPV vaccine: Introduced Legislation. 2007. (Accessed August 14, 2007, at www.ncsi.org/programs/health/ HPVvaccine.html#hpvlegis.)
46. Colgrove J. State of Immunity: The Politics of Vaccination in Twentieth Century America. Berkeley: University of California Press, 2006.
47. Raffle AE. Challenges of implementing human papillomavirus (HPV) vaccination policy. BMJ 2007; 335 (7616): 375-7.
48. HPV vaccine recommended for NHS immunisation programme. 2007.(Accessed November 11, 2007, athttp://www.gnn. gov.uk/environment/fullDetail. asp?ReleaseID=325799&News AreaID=2&NavigatedFromDepartm ent=False.)
49. First Major Irish Study Of Sexual Health And Relationships Launched. 2006.(Accessed August 15, 2007, at www.dohc.ie/press/ release/2007/20070131c.html.)
50. Adams M, Jasani B, Fiander A. Human papillomavirus (HPV) prophylactic vaccination: challenges for public health and implications for screening. Vaccine 2007; 25 (16): 3007-13.
44 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Oncology Focus: Robotics
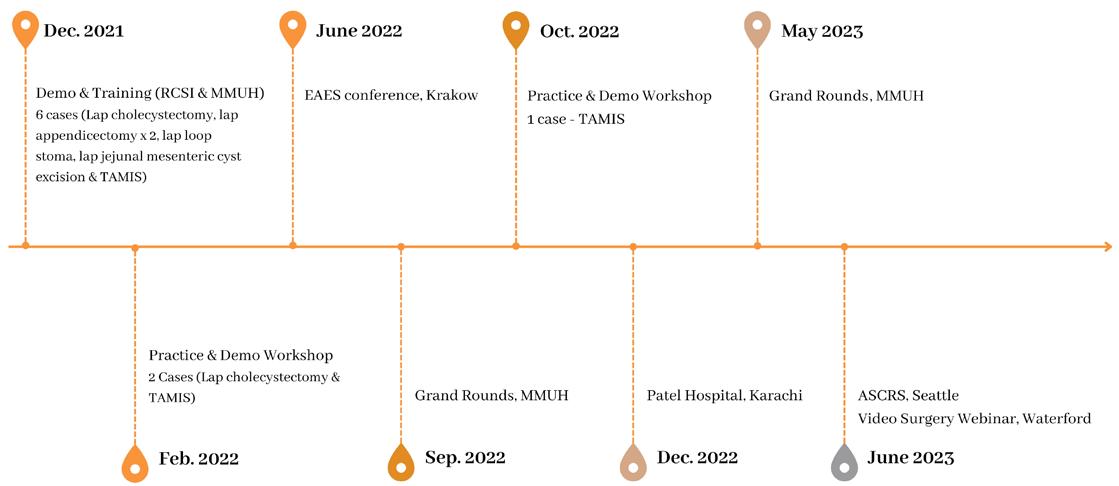

Handheld Robotic Systems for Minimally Invasive Surgery
 Written by Professor Ronan Cahill & Mr Faraz Khan, University College Dublin; Mater Misericordiae University Hospital
Written by Professor Ronan Cahill & Mr Faraz Khan, University College Dublin; Mater Misericordiae University Hospital

Minimally invasive surgery (MIS) for abdominopelvic disease has been available for patients now for over three decades in the form of laparoscopic or “keyhole” surgery. It has proven beneficial for patients in terms of postoperative recovery with less wound complications and less chance of peritoneal adhesions as well as shorter hospital stay and faster return to full activity rates. More recently increasingly sophisticated and expensive hardware platforms in the form of Robotic Assisted Surgery have been added to the MIS option to allow surgeons perform operations in a similar fashion to those now commonly done laparoscopically with added technological capability such as allowing the on-screen visualisation to be controlled directly by the operator, the provision of immersive and often 3-dimensional viewing and, perhaps especially, highly articulating instrument tips. An example of how articulating tips could be an advantage is suturing. Whereas the more standard laparoscopic needle holder devices simply open and close at its end where the needle is grasped with the instrument shaft itself being a straight, rigid “stick”, in robotic surgery, the end effector
of the instrument has “degrees of freedom” of movement allowing for various angulations and rotational movements relative to the shaft making it easier to perform tasks needing finer detail. It is hoped that these technological capabilities will overtime allow more complex operations be envisaged and/or that existing MIS operations can be done more easily by surgeons trying to learn operations.
In the last 5 years there have been additional automational advances in laparoscopic surgical instrumentation aimed at obviating the need for great investment in time and capital expenditure associated with current incumbent large-scale robotic platforms. This has enabled articulating end effectors for handheld instruments allowing for robotic-like dexterity and manoeuvrability within the standard MIS set up and workflow. While this capability can be achieved to some degree by clever mechanical engineering alone, miniaturised electromechanical motors are now available that can “robotise” instrument tip movement making for intuitively controlled fine movements. HandX™ by Human Xtensions is the first CE marked computerised smart software driven laparoscopic instrument that gives the same degree of control and articulation
as one would get in robotic surgery but that also fits immediately with standard laparoscopic workflows, set-ups and indeed pricing. This device is lightweight and compact and so can be operated handheld. Its provided with singleuse, detachable end effectors (so far, the range of instruments include Fenestrated grasper, Needle holder, Monopolar hook, Monopolar spatula, Monopolar scissors, Self-Righting needle holder) that make it suitable for a wide variety of surgical approaches
and surgical environments allowing it offer a new niche between standard laparoscopic surgery and the now “traditional” robotic platforms without the problem of over-functionality. Simpler operations so can be performed more simply then the robotic equivalent while more complex laparoscopic procedure can be augmented by focussed application of the necessary technology capability just for the specific part requiring it. The disposable component aspect
45 HOSPITALPROFESSIONALNEWS.IE | HPN • SEPTEMBER 2023
Professor Ronan Cahill
Mr Faraz Khan
Timeline
of the instrument cuts down on maintenance contracts and the learning curve is that of a single instrument that can be applied to a variety of operations rather than needing a wholly changed procedural workflow across a spectrum of surgeries and even specialties. The minimal set up time enables fast procedure turnover and the device itself is comfortable to use single handedly. Importantly, the device can be used with existing small, 5mm trocars and can be utilised via existing port positions. Our journey of adoption of this handheld smart instrumentation began in December 2021 with initial team training in the use of HandX in learning sessions in our in-hospital dedicated surgical skills and development lab at the Mater Misericordiae University Hospital and a further session in the national surgical skills centre RCSI building on our prior experience with purely mechanical handheld articulating devices (namely FlexDexTM). The technology was separately also introduced including practical sessions to senior surgical trainees at the RCSI as part of a New Technologies training day. Such training sessions comprised of hands on training on a laparoscopic box trainer for the surgeons using the device. Immediately after our intensive training, with hospital approval, a series of operative cases were carried out over three days including elective & emergency surgeries (appendicectomies, cholecystectomy, intestinal stoma formation and cyst excision) where
Oncology Focus: Robotics

the device was used alongside existing laparoscopic surgical instrumentation after additional dedicated theatre team training for the set-up of the device. Following this initial experience, two shorter subsequent three day periods of HandX™ access were enabled in February & October 2022 allowing additional cases to be done including a series of Transanal Minimally Invasive Surgery (TAMIS) for significant rectal polyps and early cancer. Overall the device was successful deployed in three different theatres in two different hospitals (including our elective and emergency theatres in MMUH and one in Mater Private Hospital) with it being obvious to all how easy it was to move between the various locations, and most importantly, transfer the necessary set-up skills between the different theatre staff supporting the surgeries. Each time we used the device for a period we ensured there was a recap session, practice and access to our surgical skills lab including in particular simulation of TAMIS procedures. Along with the essential review and practice run for the operators, there were also additional demo workshops for other consultant colleagues and surgical trainees.
The experience with HAND-X for TAMIS is worth detailing more as here it has particular benefits for this operative access that requires operating akin to laparoscopic surgery but within the anatomical confines of the rectum. This needs a specific access device (called Gel-Point Path, Applied Medical) which creates a sealed space for
trocar and instrument access by means of a gas insufflator that is used to create a pneumo-rectum (essentially a gas filled rectum).
A pair of surgeons perform such operations, one with two laparoscopic instruments and an assistant holding and directing the camera with both sharing the visual display on a monitor, most often for the removal of large benign or early-stage cancerous growths which may not be amenable for excision via colonoscopy. This procedure requires dedicated training and surgical subspeciality interest and expertise and not all colorectal surgeons carry it out routinely because of this. With standard laparoscopic instruments, some steps indeed maybe a little difficult at times, especially when it comes to suturing closed a defect after resection within the constraints of the relatively narrow space as compared to abdominal laparoscopic procedures where there is generally significantly more room. In some institutions, robotic platform TAMIS operations have been carried out as surgeons try to obviate their difficulties with performing the operation in the standard way and more recently indeed some entirely new platforms are being developed to better enable such robotic access (the current robotic systems need a lot of set-up especially for such operations).
In our experience, HandX™ device helped us perform TAMIS procedures. We used it alongside the standard TAMIS set-up and instrumentation for circumferential

lesion marking and haemostatic full thickness excision as well as defect suturing. All procedures were completed within a satisfactorily efficient time frame (operating times <one hour) and all lesions were fully excised. Early in-patient recovery was very good giving us the impression that HandX™ capably facilitates robot-like instrument movement for TAMIS without needing new workflows. With time dedicated to instrument understanding and training, HandX™ certainly increased dexterity and its small operating room footprint was another advantage along with its greater cost-effectiveness compared to other robotic platforms. Hence it indeed seems to have the potential to be “the right-sized & specified robot for TAMIS”.
As a new innovation, we have wanted to make sure that our experience was also shared with a broader surgical audience. This included: two Surgical Grand Round presentations at MMUH, Dublin, an international training day presentation at Patel Hospital in Karachi, Pakistan, as well as national and international surgical societies such as a plenary session presentation at Waterford Surgical Video Webinar, a podium presentation at the European Association of Endoscopic Surgery (EAES) in Krakow, Poland and an invited podium talk at the “New Technologies Symposium” at the American Society of Colon and Rectal Surgeons conference, Seattle, USA. Overall so, HandX™ in our experience has proven to be a viable, smart laparoscopic instrument that can allow the spectrum of basic to advanced operation deployment that has proven to be great interest across the specialties of general and colorectal surgery. With a short training requirement, one can quickly adapt this technology and add to their MIS armamentarium. It gives robotic like dexterity within a very portable device that can be used in multiple theatre settings and can be made accessible to surgical trainees from an early stage in their careers across the various surgical specialities that make use of MIS.
Publications
1) M F Khan, E Kearns, R Cahill “Initial experience with the HandX device: training and initial laparoscopic case series – a video vignette” Colorectal Disease Journal. Oct 2022
2) M F Khan, E Kearns, R Cahill “Clinical Experience of HandX for Transanal Minimally Invasive Surgery (TAMIS)” DCR-D-22-00919R2 (in press)
46 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
HandX™ by Human Xtensions
Oncology Focus: Bladder Cancer
Bladder Cancer: Management and Outlook
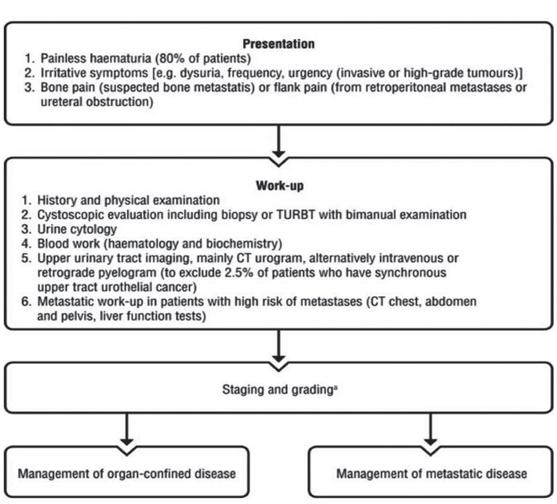
Bladder carcinoma (BC) is the most common neoplasm of the urinary system. It is the 10th most common cancer worldwide; the 6th most common cancer in men and the 17th most common cancer in women.
573,278 new cases of bladder cancer were diagnosed globally in 2020, and over 212,536 deaths due to BC were recorded.
We spoke with Theresa Lowry Lehnen, Clinical Nurse Practitioner and Associate Lecturer South East Technological University to find out more about the diagnosis, management and outlook for this condition.

The condition occurs four times more often in men than women. The age-standardised incidence rate per 100, 000 person-years is 9.5 for men and 2.4 for women worldwide, and in the EU, 20 for men and 4.6 for women.
Theresa told us, “Approximately 490 people in Ireland are diagnosed with bladder cancer annually. The incidence increases with age. Approximately 70 per cent of patients diagnosed with bladder cancer in Ireland are over 65 years of age, and up to 15% are diagnosed with metastatic disease at initial presentation.”
Urothelial carcinoma (UC) also known as transitional cell carcinoma (TCC) is the most common histologic type of bladder cancer, accounting for approximately 90% of cases. She adds, “The incidence of bladder cancer is twice as high in developing countries compared to developed countries. Most bladder cancers in developing countries are squamous cell carcinoma, and correlate with endemic schistosomiasis.”
There are three types of bladder cancer:
1. Transitional cell carcinoma (TCC) or urothelial carcinoma (UC) which begins in cells in the innermost tissue layer of the bladder. These cells can stretch when the bladder is full and shrink when it is emptied. Most bladder cancers begin in the transitional cells. Transitional cell carcinoma can be low-grade or high-grade:
• Low-grade transitional cell carcinoma often recurs after treatment, but rarely spreads into the muscle layer of the bladder or to other parts of the body.
• High-grade transitional cell carcinoma often recurs after treatment and often spreads into the muscle layer of the bladder, to other parts of the body, and to lymph nodes. Almost all deaths from bladder cancer are due to high-grade disease.
2. Squamous cell carcinoma begins in squamous cells, which are thin, flat cells lining the inside of the bladder. Cancer may form after long-term infection or irritation.
3. Adenocarcinoma: Cancer that begins in glandular cells that are found in the lining of the bladder. This is a very rare type of bladder cancer. Cancer in the lining of the bladder is called non-muscle-invasive bladder cancer (superficial). Cancer that has spread through the lining of the bladder, and which invades the muscle wall of the bladder or has spread to nearby organs and lymph nodes is called muscle invasive bladder cancer.
Risk Factors
There are multiple risk factors for bladder cancer, including increasing age, family history, genetic mutations, smoking, schistosomiasis infection, and occupational exposure to certain chemicals. The risk in smokers depends on smoking duration and intensity, and is 2 to 6 times higher than in non-smokers.

Theresa says, “Smoking is the most important risk factor for bladder cancer and thought to cause about half of all bladder cancers. Occupational exposure to paint, rubber, petroleum products, and dyes, and chemicals such as arylamine, aniline, phenacetin and arsenic are associated with bladder cancer, and exposure to the chemotherapy drug cyclophosphamide has been associated with an increased risk.
“Chronic urinary tract infections and infection with the parasite S. haematobium is also associated with an increased risk of bladder cancer, often squamous cell carcinomas. In developing
countries, schistosomiasis infection is an important cause of BC. Schistosoma haematobium ova embed in the bladder wall leading to irritation, chronic inflammation, squamous metaplasia, and dysplasia, with further progression leading to squamous cell carcinoma of the urinary bladder.
“Mutations in the p53 tumour suppressor gene, as well as abnormalities in chromosome 9, are common in invasive bladder cancer. Inherited mutations of two other genes, glutathione
S-transferase (GSTM1) and NAT2 (n-acetyltransferase), also increase risk for bladder cancer.”
Clinical Features
Bladder cancer typically presents with gross or microscopic haematuria. Less commonly, patients may complain of urinary frequency, nocturia, and dysuria; symptoms that are more common in patients with carcinoma in situ.
Other symptoms may include fatigue, weight loss, and a pelvic mass, according to Theresa, who adds that patients with upper
47 HOSPITALPROFESSIONALNEWS.IE | HPN • SEPTEMBER 2023
An interview with Theresa Lowry Lehnen, RGN, RNP, PhD, Clinical Nurse Specialist and Associate Lecturer with South East Technological University
Oncology Focus: Bladder Cancer
urinary tract urothelial carcinomas may present with pain resulting from obstruction by the tumour.
Diagnosis
“There are no screening tests for the early detection of BC,” she notes. “Despite the search for urinary biomarkers for the early and non-invasive detection of bladder cancer, no biomarkers are used at present in daily clinical practice. Diagnostic modalities used in diagnosing BC include Imaging (ultrasound, intravenous urography (IVU), computed tomography (CT), and magnetic resonance imaging (MRI)); cystoscopy, and biopsy.
“Cystoscopy is an effective but invasive tool to detect bladder cancer tumours. It is considered the gold standard and most useful diagnostic test. Direct visualisation of the bladder is necessary in all cases of haematuria, where there is a concern for bladder cancer, and no imaging modality is currently sensitive enough to replace cystoscopy.
“The main role of imaging in the assessment of haematuria is to evaluate the kidneys and ureters. Ultrasound or CT may be used, and guidelines differ in their recommendation regarding imagery. Ultrasound of the kidneys will identify most renal tumours; however, it can miss a small percentage of upper tract urothelial cancers which can be detected with CT scanning. Urine cytology remains an important diagnostic tool, and is a non-invasive diagnostic method where voided or instrumented urine is examined for exfoliated cancer cells. The overall sensitivity ranges from 28 to 100%, with a median of 44%. It has a high sensitivity for highgrade tumours, but low sensitivity for low-grade tumours, ranging only from 4% to 31%.
“Cytology is useful, particularly as an adjunct to cystoscopy, when a high-grade malignancy is present. A positive cytology indicates a urothelial tumour anywhere in the urinary tract, however, negative cytology does not exclude the presence of a tumour. In some cases, a bone scan or PET scan may be recommended, but are not normally required, especially with non-muscle invasive bladder cancer.”
Grading and Staging
Grading describes how quickly the cancer may grow and spread.
Stages of bladder cancer
• T4b: In distant metastases, the cancer has spread beyond the surrounding areas, to other parts of the body such as the liver, bones or lungs.
Nodes (N)
• N0: No cancer is found in any of the lymph nodes.

• N1: Cancer is found in one lymph node and is smaller than 2 cm.
• N2: Cancer is found in one lymph node and is bigger than 2 cm but less than 5 cm or the cancer has spread to more than one lymph node, but is smaller than 5 cm.
• N3: Cancer is found in at least one lymph node and is 5 cm in size.
Metastasis (M)
• M0: Cancer has not spread to other parts of the body.
• Low-grade bladder cancer: The cancer cells look only slightly abnormal, much like normal bladder cells. The cancer is usually slow-growing and less likely to spread than high grade bladder cancer.
• High-grade bladder cancer: The cancer cells look fairly or very abnormal and are more likely to grow quickly. Carcinoma in situ (CIS) is always classed as high grade.
Staging using TNM scores, describes how deeply the cancer has grown into the bladder and if it has spread to other parts of the body.TNM stage is the most important prognostic factor of urinary bladder carcinoma.
• Tumour (T): How deeply the tumour has grown into the bladder.
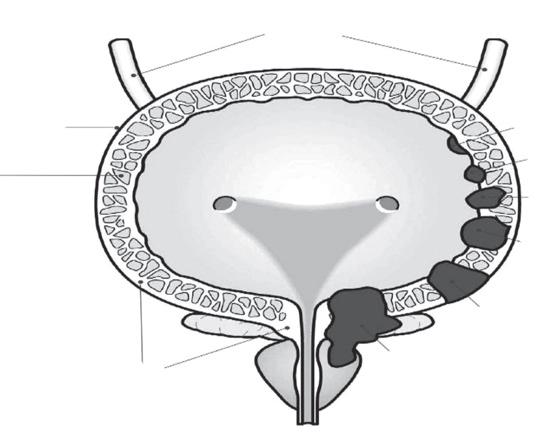
• Node (N): Is there cancer in the lymph nodes?
• Metastasis (M): Has the cancer has spread to other parts of the body?
The most common bladder cancer - non-muscle-invasive bladder cancer is staged as follows:
• Carcinoma in situ (CIS): This appears as flat, red areas in the bladder. This type of bladder cancer is more likely to come back after treatment, often as another non-invasive cancer in the bladder. CIS is always classed as high grade. It can grow more quickly and can become invasive, requiring
different treatment to other nonmuscleinvasive bladder cancers.
• Ta: The tumour is found as a papillary cancer growing only in the innermost lining of the bladder.
• T1: The tumour has started to grow into the connective tissue just below the bladder lining.
The rarer muscle-invasive bladder cancer is staged as follows:
• T2: The tumour has grown into the muscle layer in the bladder. It is divided into T2a and T2b.
• T2a: the cancer has grown into the superficial muscle
• T2b: the cancer has grown into the deeper muscle
• T3: The tumour has spread through the muscle layer to the outer fat layer around the bladder.
It is divided into T3a and T3b.
• T3a: microscopic invasion
• T3b: macroscopic invasion
Advanced / metastatic bladder cancer
• T4: The cancer has spread outside the bladder to other organs.
It is divided into T4a and T4b.
• T4a: With locally advanced disease, the cancer may have spread into the pelvic wall, the prostate in men, or the uterus or vagina in women.
• M1: Cancer has spread to other parts of the body. This is known as secondary, metastatic or advanced bladder cancer. M1 can be divided into M1a and M1b.
• M1a: Cancer has spread to the lymph nodes outside the pelvis.
• M1b: Cancer has spread to other parts of the body such as the bones, lungs and liver.
Treatment and Management
Bladder cancer is a prevalent disease with substantial morbidity and mortality which requires interprofessional medical and surgical management, she says. “Treatment is based on the pathological findings of the biopsy, with attention to histology, grade and depth of invasion.”
The most common types of treatments used for bladder cancer are surgery, chemotherapy, immunotherapy, targeted therapy and radiation therapy.
NMIBC- non-muscle-invasive bladder cancer
“NMIBC is the most common diagnosed bladder cancer and refers to bladder cancers confined to the mucosa (pTa) or lamina propria (Pt1), without invasion of the bladder wall muscle. CIS, a high-grade fat tumour confined to the mucosal layer, is also included in this group. pTa and pT1 appear as papillary type lesions and the number and size of tumours are important predictors for the risks of progression and reoccurrence. CIS tumours can appear as changes on the bladder mucosa and often
48 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Ureters Fat Muscle Connective tissue CIS Ta T1 T2 T3 T4
present with lower urinary tract symptoms rather than haematuria.
“Management of NMIBC includes transurethral resection of bladder tumour (TURBT), a diagnostic and therapeutic procedure that allows the collection of samples to determine the grade and stage of bladder cancer. The samples are sent for histological assessment. NMBIC has the potential to reoccur or progress to muscle invasive bladder cancer (MIBC).

“The European Organisation for Research and Treatment of Cancer (EORTC) developed a scoring model for predicting recurrence and progression. The scoring system is based on assigning points for- number of factors; tumour diameter; prior recurrence rate; category; concurrent CIS; and World Health Organisation (WHO) 1973 tumour grade.”
Theresa adds that surveillance after treatment such as trans urethral resection of bladder tumour (TURBT) uses different modalities including regular cystoscope evaluation, CT scan and cytology. Intravesical treatments including chemotherapy agents mitomycin, epirubicin and BCG are important in the reduction of recurrence and progression rates of NMBIC.
Management of MIBC- muscle invasive bladder cancer
“While most patients presenting with bladder cancer have NMIBC, up to 25 per cent have muscle
Bladder cancer stages
invasive bladder cancer (MIBC), where cancer cells are detected in the muscularis mucosa at the time of TURBT. Tumours that extend to the muscle layer are staged T2, to the perivesical fat T3, and those that invade other organs or the pelvic side wall, T4,” she says.
“Muscle invasive bladder cancer cannot be cured with endoscopic treatments alone and requires radical therapy. Even with radical treatments the five-year survival rate is approximately 50%.

Treatment options for MIBC include radical cystectomy, neoadjuvant chemotherapy (NAC), urinary reservoir reconstruction, trimodal therapy, radiotherapy, and endoscopic management.In radical cystectomy the prostate gland and seminal vesicles are also removed in men and the urethra is removed if a tumour is detected on urethral biopsy. In women the uterus, urethra and adjacent vaginal tissues are removed, however, the ovaries can usually be left in situ. Regional lymph nodes are also removed in both men and women.
“The addition of neoadjuvant chemotherapy (NAC) has been shown to account for a 5 per cent improvement in overall survival with radical cystectomy. NAC should be commenced as soon as possible and followed by surgery usually within six weeks. Ileal conduit is the most common method of urinary reservoir reconstruction in Ireland and the UK.
“The Studer neobladder is another technique, but is contraindicated
Cancer cells spread to other parts of the body
when a tumour has spread to the urethra. Trimodal therapy utilises TURBT, radiotherapy and chemotherapy. In carefully selected patients, results from trimodal therapy can match those of radical cystectomy. External beam radiotherapy should not be offered as a primary therapy and only be considered in patients unfit for cystectomy.
“Endoscopic resection and diathermy for patients with unresectable disease can improve the quality, but not quantity-oflife. Standard first-line treatment for metastatic urothelial cancer is gemcitabine/cisplatin (GC) or MVAC (methotrexate, vinblastine, adriamycin and cisplatin). Approximately 50% of patients are unfit for cisplatin-containing chemotherapy due to poor hearing, impaired renal function or comorbidity.”
Prognosis and complications
Bladder cancer is a prevalent disease with substantial morbidity and mortality that requires multidisciplinary medical and surgical management. NMIBC can be completely managed endoscopically with the occasional use of intravesical treatments. Strict surveillance is necessary to help prevent recurrence or progression.
Theresa adds, “MIBC is more progressive and treated with radical cystectomy/radical cystoprostatectomy and ileal conduit formation following NAC. Long-term follow-up is required to
monitor recurrence and functional deterioration. The prognosis of UC depends on multiple factors. “The TNM stage is the most important prognostic factor of urinary bladder carcinoma. The five-year overall survival for pT1 is 75%, pT2 50%, and pT3 20%.”
Up to 45% of patients will develop complications following radical cystectomy and ileal conduit formation. Main complications include vitamin B12 deficiency, metabolic acidosis, and deterioration of renal function, urinary tract infections, and anastomotic complications. The general 5-year survival rate for people with bladder cancer is 77%. However, survival rates depend on many factors, including the type and stage of bladder cancer that is diagnosed. Advanced and metastatic bladder cancer has a poor prognosis and median survival even with cisplatin-based chemotherapy is approximately 14 months.
Theresa concludes, “Ongoing clinical trials are increasingly showing an overall survival benefit with immunotherapy and targeted treatment for bladder cancer. Researchers are studying new combinations of chemotherapies and other medications to see if they are safe and more effective than other treatments. Clinical trials are underway to find a better way of reducing symptoms and side effects of current treatments to improve comfort and quality of life for patients.”
49 HOSPITALPROFESSIONALNEWS.IE | HPN • SEPTEMBER 2023
Superficial stage Muscle invasive stage Metastic stage
Tumor
Bladder lining Submucosa Muscle Fat
Oncology Focus: Childhood Cancer
Marking Childhood Cancer Awareness Month
 Written by Amy Nolan, Head of Children Adolescents and Young Adults and Rebecca Gorman, CAYA Advocacy Officer
Written by Amy Nolan, Head of Children Adolescents and Young Adults and Rebecca Gorman, CAYA Advocacy Officer

common, such as diabetes and epilepsy. The small numbers of children diagnosed each year makes it a challenge to detect in
primary care by a GP who may only see one case in their career; however, the individual risk for each child is quite high, and
GP’s need to be equipped with the educational resources and guidance to make detection and referral as streamlined as possible.


Some symptoms of childhood cancers
Most often, these signs and symptoms will not mean cancer
But, early detection saves lives So, talk to your GP if it's unusual, unexplained, and won’t go away
Balance, co-ordination, or speech problems
Loss of appetite or weight loss
An unexplained lump, swelling, or pain
Fits or seizures
Childhood Cancer in Ireland

Approximately 200 children are diagnosed with cancer each year in Ireland.1 Average survival rates continue to increase and are at 80%, however, it remains as the leading cause of death in children over the age of 1,2 and treatment leaves most survivors with long-term side effects. The Irish Cancer Society is one of many organisations marking childhood cancer awareness month this September, to support the families coping with this disease and work to improve treatments and support available to them in Ireland.
The incidence of cancer in children increases with age, to a moderate risk by 15 years (1 in 450) to a substantial risk by the age of 20 (1 in 320).3 This incidence is comparable to other childhood illnesses that are seen as more


Changes in eyes or vision: a squint, pain, or a white glow in photos
Swollen lymph nodes in the neck or groin

Feeling very tired most of the time
Regular night sweats or high temperatures
Feeling sick (nausea) or getting sick (vomiting)
Blood in pee or poo
Bruising, or bleeding under the skin that looks like a rash
Regular headaches and early-morning vomiting
www.cancer.ie/children
50 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Amy Nolan
Rebecca Gorman
Early Detection
The early detection and diagnosis of childhood cancer matters.
While childhood cancer is not preventable via lifestyle interventions, as is the case in many adult cancers, the early detection of cancer is the best way to help get the treatment required. Early detection, timely diagnosis and access to treatment and support are key in ensuring the least harsh treatment protocol can be used in each patient according to their needs. The later the cancer is diagnosed, the higher the chances that more intensive combinations of treatments will be required thereby increasing the burden of late effects in survivors. Indeed, research has shown that this is an issue in other European countries.4, 5 A protracted pathway to diagnosis reduces the health-related quality of life in adolescents and young adults, with increased mental health difficulties also experienced in this vulnerable group.6 As in the case in cancer care, the earlier it is diagnosed, the better.
Primary care is generally the first instance where childhood cancer can be detected. There is a challenge in diagnosis due to the diffuse clusters of symptoms that are often non-specific as well as the rarity of the disease. There is also no referral guidance in place in Ireland for GPs to equip them with the information needed to refer patients appropriately, and what symptoms require immediate or urgent referral for further testing. In the absence of this, helpful considerations include:
• Not to disregard the concerns or the “gut feeling” of parents or the practitioner
• 3 visits over 3 months with the same collection of cancer has a higher risk of it being cancer, and that
• Contacting a specialist in paediatric haematology/ oncology is a good step when the GP is concerned.7
Referral guidance for primary care is a key element in the early detection of childhood cancer and
New online Cancer Tool
A new online ‘European Cancer Pulse’ tool, created to compare cancer data across Europe, has highlighted that only 12 of the 27 EU Member States have an up-todate national cancer control plan.

The research has been published in the prestigious Lancet Oncology journal and was led by Professor Mark Lawler, Professor of Digital Health at Queen’s University Belfast, European Cancer Organisation (ECO) board member and Scientific Lead of the European Cancer Pulse project.
The new user-friendly tool features more than 120 data measurements – from 34 countries throughout the European Union, and beyond. It allows anyone to quickly and easily discover cancer care disparities between countries. For example, the inequality in relation to workforce issues: how Germany has 1,395 nurses per 100,000 inhabitants, whereas for the UK that number drops to 778.
Commenting on the results, Professor Lawler said, “How can you ever improve cancer care in a country that lacks an up-todate national cancer plan? The European Cancer Pulse shines a critical light on which countries are falling behind on cancer care – and specifically how. It empowers us to use data intelligence to identify the problems, but more importantly to do something about them.”
The new tool complements the existing European Commission’s Cancer Inequalities Registry, pulling together key additional facts and figures from many more European countries: How much is a country spending on cancer research? How many CT or PET scanners does it have? What percentage of citizens know about cancer risks and symptoms? How is the level of cancer care received influenced by a person’s income, gender, age, education, and other social factors?
campaigns to increase awareness works. The HeadSmart campaign in the UK to increase awareness of brain tumour symptoms in children, as well as amplifying existing referral guidance, decreased the diagnostic timeline from 14.4 weeks to 6.7 weeks, now one of the shortest timelines in the world.8
Excellent guidance exists as exemplars for the Irish referral pathway system: The UK have NICE Guidance with supported documentation from the Childhood Cancer and Leukaemia Group (CCLG).9 CCLG are also a partner in the “Childhood Cancer Smart” project,10 which aims to reduce the diagnostic timelines for childhood cancer by addressing delays and improving awareness of the symptoms of cancer in young people. Evidence-based measures to improve the early detection of childhood cancer includes referral pathways and guidance in primary care, training for healthcare professionals to detect the signs and symptoms of childhood cancer, and campaigns to increase
the awareness of symptoms among the public.
Childhood Cancer Awareness Month
This September, the Irish Cancer Society wants to highlight the solutions required to prioritise the early detection of childhood cancer. We continue to support primary care practitioners to empower them and ensure they have access to the referral guidance, educational resources and pathways required to get children diagnosed as soon as possible. Childhood cancer, while a rare disease, is life-threatening and leaves profound effects on the child and their family when diagnosed. This Irish Cancer Society provides many supports to these families, including through our advocacy efforts for this group and in the services we provide. For more info, see https://www. cancer.ie/cancer-information-andsupport/childrens-cancer
References available on request
Oncology Focus News
In Ireland, the Pulse specifically reveals that it takes on average over 20 months for new cancer medicines to be included in public reimbursement, making it among the longest in Western and Northern European countries, compared to four months in Germany for instance. Ireland also has challenges in relation to screening for colorectal cancer, with just 1 in 3 persons above 50 reporting having undertaken a test in the last three years, as compared to over 75% in Denmark. Meanwhile, Ireland is relatively well-equipped in terms of cancer workforce, being 37% and 45% over the European average for oncologists and nurse numbers, respectively.
Additional data is being added to the European Cancer Pulse regularly, helping to reflect the full scope of cancer research and care in Europe and to monitor improvements – or lack thereof.
This includes ongoing research on cancer workforce capacity limits and social inequities affecting marginalised populations in cancer. Emerging evidence shows that there is still a long way to go before equitable access to cancer prevention, research and care is achieved across Europe. Aside from existing inequities, persistent issues such as the health workforce crisis represent significant risks for deteriorating outcomes in the coming years. The influence of the COVID pandemic is also critical. Professor Lawler adds: “Our data intelligence indicates that solving these health and cancer disparities must be prioritised at a political level, both Europe wide and nationally, in order to improve the lives of those European citizens affected by cancer. The European Cancer Pulse represents a crucial asset that we must deploy effectively in our fight against cancer.”
51 HOSPITALPROFESSIONALNEWS.IE | HPN • SEPTEMBER 2023
Oncology Focus: Multiple Myeloma
Extramedullary Multiple Myeloma
Written by: Dr Roisin

Introduction
Multiple myeloma (MM) is the second most common blood cancer worldwide and is characterised by clonal proliferation of malignant plasma cells within the bone marrow. These MM plasma cells secrete a monoclonal antibody, often known as M-protein. This can lead to bone destruction, anaemia, myelosuppression and have implications on renal function and other systems.1 Although survival rates are constantly improving with the introduction of new therapies, MM is still incurable as patients will eventually relapse or stop responding to available therapies. Since MM cells usually reside in the bone marrow, most therapies work to target cell types within this microenvironment. However, in rare cases MM can present in a particularly aggressive form
whereby the cancer cells have become independent of the bone marrow and may infiltrate other organ systems (Figure 1). This is known as extramedullary MM (EMM) and occurs in only around 7% of patients at diagnosis (primary EMM), and up to 20% of patients at relapsed stage (secondary EMM).2 Even in the era of novel therapies, the incidence of EMM is increasing. Many hypothesise that EMM can arise from particularly aggressive MM clones originating in the bone marrow. Since most current therapies have been designed to target the bone marrow and its niche, it is possible that these therapies are driving malignant plasma cells to evolve and escape the bone marrow and present as EMM. With a particularly poor prognosis, it is important that we are aware of EMM, and improve our understanding of its presentation and pathogenesis. Here I give an overview of the disease, our current understanding of pathogenesis, and treatment approaches.
Definition
The definition of EMM can vary widely between groups but generally, it can be classified into two broad groups; bone-related/ osseous EMM and extraosseous
EMM. Bone-related EMM are soft tissue masses arising from bone and growing contiguously, whereas soft tissue masses tend to be in isolated extraosseous locations as a result of haematogenous spread.2, 3 Some groups restrict the definition to exclude bone-related EMM. EMM may occur at any anatomic site, but involvement of lymph nodes, skin, central nervous system (CNS) and effusions are more common. It is important to distinguish between osseous and extraosseous EMM as patients with extraosseous have a worse prognosis. Solitary plasmacytomas are explicitly excluded from EMM definition as these can occur in the absence of MM diagnosis.2 Additionally, plasma cell leukaemia (PCL) is an aggressive form of MM that appears when the presence of clonal plasma cells in peripheral blood is greater than 20%. However, it is also usually excluded from the definition of EMM since it is characterised as a unique entity with a defined clinco-pathological state.2
Detection and Incidence
In patients with a confirmed MM diagnosis, EMM is usually detected by identification of pathologic soft tissue masses using imaging techniques (computed tomography (CT)-scan, PET/ CT, magnetic resonance imaging (MRI), or ultrasound), biopsy or physical examination. Patients who present with EMM during disease progression tend to have lower
levels of serum M-protein, lower haemoglobin, and higher lactate dehydrogenase (LDH) than those with EMM at diagnosis.2

It is believed that the incidence of EMM is largely underestimated given the wide range of definitions used and methods used for detection. Moreover, many studies predate the use of routine sensitive imaging meaning EMM may have gone undetected. Reported incidence therefore varies across studies but overall, the incidence of EMM at relapse is much greater than at diagnosis, which is in concordance with its consideration to be a high-risk feature. Additionally, bone-related EMM is more common than extraosseous EMM. At diagnosis, extraosseous EMM is present in less than 5% of patients whilst bone-related EMM is reported in between 7 and 23% of patients.3 Alternatively at relapse, the incidence of extraosseous EMM ranges from 3 to 10%, and bonerelated from 6 to 23%.3
Pathogenesis and Genetic Hallmarks
There are very limited studies published addressing the underlying pathogenesis of EMM. We do know however that in MM, the bone marrow microenvironment has an extremely important role to play. Briefly, the bone marrow niche can activate signalling cascades that promote adhesion of MM
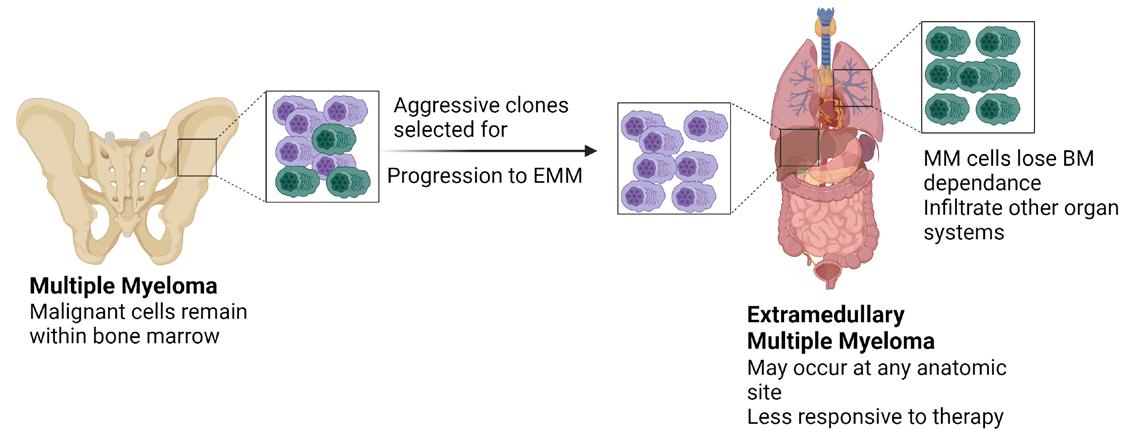
52 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
McAvera, Postdoctoral Researcher at Royal College of Surgeons in Ireland, Beaumont Hospital Dublin Campus
Figure 1 Extramedullary Multiple Myeloma
Classic MM is characterised by clonal proliferation of malignant plasma cells within the bone marrow. In rare cases, EMM may occur, usually at relapse. EMM cells can survive outside of the bone marrow and may infiltrate other organ systems. As a result it is particularly difficult to treat and has an inferior prognosis.
cells to the bone marrow, as well as tumorigenesis and even drug resistance. These signalling pathways are controlled by many factors including gene transcription and expression. A commonly employed pathway in MM is known as the RAS signalling pathway, and gene mutations in RAS pathway members are associated with EMM. Moreover, changes in the Wnt pathway signalling and interleukin-6 (IL-6) signalling have been shown in EMM, and are associated with migration and invasion of tumour cells.3, 4
For years now, cytogenetic abnormalities in MM are routinely tested for both at diagnosis and relapse. These are commonly translocations involving the IGH gene (chromosome 14) but also deletions such as del(17p) and chromosome amplifications such as gain(1q)/amp(1q), and these are usually detected using fluorescence in situ hybridisation (FISH). Several studies have investigated the incidence of these common markers in patients with EMM and generally, the markers associated with inferior prognosis are more frequent.5 This includes t(4;14), gain(1q), del(1p) and del13). On the other hand, a t(11;14) translocation is associated with standard-risk MM and is less likely to occur in patients with EMM. Most of these studies so far have only genomically characterised the bone marrow of EMM patients, but increasingly studies are using the tissue from extramedullary sites as well. This is crucial for the understanding of EMM disease biology, as clonal evolution plays a huge role and as such, spatial heterogeneity can occur i.e. patients present with differential cytogenetics/mutations/gene expression between anatomical sites. Many hypothesise that aggressive clones are selected for in the bone marrow, which are more likely to escape the bone marrow and infiltrate elsewhere.
Recent studies have also used gene expression based methods to understand EMM. Gene expression profiling (GEP) has become an alternative method to FISH for risk classification of MM. Although studies are limited, patients classified as high-risk by GEP are more likely to experience EMM.5 Additionally, more in-depth transcriptomic sequencing of EMM has revealed differentially expressed gene pathways that may contribute to pathogenesis. Single-cell sequencing approaches have also identified different cell subpopulations in extramedullary sites vs bone marrow, many of which are immune cell types.4
As mentioned previously, RAS pathway mutations are associated with EMM. DNAbased sequencing methods have also identified mutations in DNA repair pathways such as ATM and ATR, and important tumour suppressors such as TP53.5 I hope that further genomic studies will uncover mechanisms behind EMM progression, and possible new biomarkers to predict its occurrence.
Prognosis
EMM is described as a very aggressive subentity of MM as it is associated with high tumour burden. As a result, patients tend to have very exacerbated MM manifestations such as severe anaemia, thrombocytopenia, hypercalcaemia and high rates of renal impairment. Moreover, patients also present with high serum lactate dehydrogenase and serum β2 microglobulin level ≥ 5.5 g/dL.2 Generally, prognosis for EMM is very poor with a median overall survival of less than 1 year in patients who are refractory to standard treatments. If eligible, an autologous stem cell transplant (ASCT) is the most effective MM treatment, but even so, EMM prognosis is still only 3 years. Many other factors can also influence EMM prognosis, such as subtype and anatomical site. For example, in patients receiving an ASCT, progression free survival was longer in those with bone-related EMM compared to extraosseous (51.7 months vs 38.9 months).2 Additionally, patients presenting with secondary EMM have a worse prognosis than those with primary EMM. Moreover, EMM at certain sites such as blood, CNS, liver, lung and muscles has been associated with earlier mortality than in cases where these sites were not involved. More recently, studies have also shown that certain genetic abnormalities in EMM may influence patient outcome. For example, EMM patients with amp(1q) had inferior survival compared to those without amp(1q) and furthermore, the number of additional copies was also proportionately detrimental to outcome.6
Current and Future Treatment Strategies

Given the lack of consensus on EMM definition and its low incidence, most studies evaluating treatments for EMM have been of a retrospective nature. Additionally, most clinical trials tend to exclude patients with EMM making it even more difficult to assess novel agents in this setting. In general, all MM patients are widely grouped
as either transplant-eligible or transplant-ineligible at diagnosis based on factors such as age and co-morbidities.1 Standard of care induction therapy consists of a proteasome inhibitor (PI) such as bortezomib and an immunomodulatory agent (IMiD) such as lenalidomide. This is followed by stem cell harvest and an ASCT for transplant-eligible patients, whilst ineligible patients will receive longer induction therapy followed by maintenance therapy, commonly lenalidomide. Treatment approaches for MM patients with EMM remains the same as patients without EMM, and thus results remain unsatisfactory.2, 7 ASCT has indeed shown survival benefit for patients with both extraosseous and bone-related EMM. However, it still cannot fully overcome the poor prognosis of EMM. Likewise, regimens including bortezomib and/or IMiDs have improved survival of EMM overall, but when compared to those with only classic MM, prognosis is still inferior. The first generation IMiD thalidomide is associated with poor efficacy in EMM compared to second generation IMiDs lenalidomide and pomalidomide.7 Monoclonal antibodies have more recently been incorporated into MM regimens. One such example is CD38-targeting daratumumab, used mainly in the relapsed setting in combination with lenalidomide and dexamethasone (Dara-RD). This is described as one of the most beneficial regimens for relapsed MM. However, limited efficacy is observed in EMM, perhaps due to loss of CD38 expression.7 Other therapies currently approved for MM include elotuzumab (SLAMF7 monoclonal antibody), selinexor (nuclear export inhibitor) and venetoclax (BCL-2 inhibitor). Unfortunately, reports of these in EMM patients are currently lacking. Similarly, data is not yet available for the use of chimeric antigen receptor (CAR) T cell therapy or bispecific T-cell engagers (BiTEs) in EMM. Future trials should aim to incorporate EMM patients, and perhaps in the future regimens specific to EMM may become standard of care. MM with CNS (MM-CNS) involvement is extremely rare (<1%) and particularly difficult to treat due to novel agents’ lack of ability to cross the blood-brain barrier. However, several case reports show that the novel PI marizomib is much more effective at CNS penetration than previous PIs and as thus is beneficial for MM-CNS.7 Data is not yet available for this in EMM. With this in mind however, in the future we may move towards
specific targeted therapies for different anatomical sites where EMM may present.
Conclusion
EMM is an extremely high-risk feature in MM patients, which is associated with inferior response and survival. Due to its low prevalence and lack of documentation, our understanding of EMM is only beginning. It is clear that novel therapies have undoubtedly improved the prognosis for MM as a whole. However, for patients with EMM these improvements are not as pronounced and response remains unsatisfactory. EMM patients represent a particularly poor group to treat, and no specific regimen is currently available. We hope that as more clinical trials include patients with EMM, the most beneficial regimens will be uncovered, and perhaps down the line more targeted therapies for specific EMM subtypes. Additionally, more genomic studies are being performed on EMM and will contribute to our understanding of disease biology and molecular hallmarks/biomarkers. Perhaps, genomic characterisation in combination with clinical trials will answer questions regarding the role of clonal evolution in disease progression. Ultimately, risk-adapted therapy is the way forward for EMM.
References
1. Rajkumar SV. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022;97(8):1086-107.
2. Bhutani M, Foureau DM, Atrash S, Voorhees PM, Usmani SZ. Extramedullary multiple myeloma. Leukemia. 2020;34(1):1-20.
3. Bansal R, Rakshit S, Kumar S. Extramedullary disease in multiple myeloma. Blood Cancer J. 2021;11(9):161.
4. Ryu D, Kim SJ, Hong Y, Jo A, Kim N, Kim HJ, et al. Alterations in the Transcriptional Programs of Myeloma Cells and the Microenvironment during Extramedullary Progression Affect Proliferation and Immune Evasion. Clin Cancer Res. 2020;26(4):935-44.
5. McAvera R, Quinn J, Murphy P, Glavey S. Genetic Abnormalities in Extramedullary Multiple Myeloma. International Journal of Molecular Sciences. 2023;24(14).
6. Kriegova E, Fillerova R, Minarik J, Savara J, Manakova J, Petrackova A, et al. Whole-genome optical mapping of bone-marrow myeloma cells reveals association of extramedullary multiple myeloma with chromosome 1 abnormalities. Sci Rep. 2021;11(1):14671.
7. Li Y, Sun Z, Qu X. Advances in the treatment of extramedullary disease in multiple myeloma. Transl Oncol. 2022;22:101465.
53 HOSPITALPROFESSIONALNEWS.IE | HPN • SEPTEMBER 2023
Oncology Focus: Lung Cancer Immunotherapy through the Lens of Non-Small Cell Lung Cancer

 Written by Robyn Stanley1,†, Saoirse Flanagan1,†, David O’Reilly2, Ella Kearney1, Jarushka Naidoo2,3,4,*,‡ and Catríona M. Dowling
Written by Robyn Stanley1,†, Saoirse Flanagan1,†, David O’Reilly2, Ella Kearney1, Jarushka Naidoo2,3,4,*,‡ and Catríona M. Dowling
1School of Medicine, University of Limerick, V94 T9PX Limerick, Ireland
2Beaumont Hospital, D09 V2N0 Dublin, Ireland
3Department of Medicine, Royal College of Surgeons in Ireland, D02 YN77 Dublin, Ireland
4Sidney Kimmel Comprehensive Cancer Centre, Johns Hopkins University, Baltimore, MD 21218, USA
*Authors to whom correspondence should be addressed.
†These authors contributed equally to this work.
‡These authors also contributed equally to this work.
Immune Checkpoint Blockade Inhibitors (ICIs)
The development of immune checkpoint inhibitors (ICIs) has earned much interest in the field of immuno-oncology due to their significant success, in particular with improved survival in patients with difficult-to-treat cancers such as NSCLC.1
A key function of the immune system is to distinguish self from nonself. This is achieved by the detection and binding of a T cell receptor (TCR) to an antigen displayed by the major histocompatibility complex (MHC) on the surface of an antigen-presenting cell (APC).2 T cell activation is regulated
by several immune checkpoint pathways during the immune response, a process called peripheral tolerance [2]. At the centre of this process are the cytotoxic T-lymphocytesassociated antigen 4 (CTLA-4) and programmed death 1 (PD-1) immune checkpoint pathways. Cancer cells have the ability to avoid immune suppression by expressing checkpoint molecules such as PD-1, CTLA4 and programmed death ligand 1 (PD-L1).3
PD-1 is a member of the CD28 family of negative costimulatory receptors expressed on activated lymphocytes and monocytes. It modifies T cell activation by binding to its
ligands presented on APCs, i.e., PD-L1 and programmed death 2 (PD-L2). PD-1 plays an immunoregulatory role by reducing initial T cell activation, modifying T cell differentiation and effector functions, and supporting the development of immunological memory.2 PD-L1 is expressed on tumour cells, and when it binds to PD-1 on the T cell, T cellassociated kinases are inhibited, preventing the development of cytotoxic T cell response to tumours,4 thus stopping T cells from identifying and eradicating tumour cells (Figure 1 on page 56). Moreover, binding to PD-L1 can inhibit the proliferation of T lymphocytes and the production of cytokines such as IL-2 and IFN-Y, thus inhibiting the proliferation of B lymphocytes.4 This results in an overall weakening of the immune response. High expression of PD-L1 is observed in 24–60% of patients with non-small cell lung cancer (NSCLC),5 and this high expression of PD-L1 has been shown to result in a shorter survival and a poor prognosis for patients.4 The expression of PD-L1 is controlled by a number of factors such as transcriptional regulation,6,7,8 epigenetic regulation,9,10 post-translational modifications11,12,13 and metabolic reprogramming.14,15,16 Monoclonal antibodies which bind and block PD-1 receptors signals can reactivate the tumour-infiltering lymphocytes, allowing the recognition and elimination of malignant cells.17
CTLA-4 is a receptor on T cells that causes the inhibition of T cell priming, activation, and migration.18 CTLA-4 is highly
expressed on activated T cells where it competes with CD28 for binding to CD80 and CD86 expressed on APC (Figure 1).19 Consequently, the overexpression of CTLA-4 in the tumour micro-environment can act as a biomarker for prognosis and treatment of NSCLC.20 Targeting CTLA-4 can prevent the immunosuppressive CTLA-4 binding mechanisms by diminishing the co-stimulatory binding of CD28. This allows for the activation and proliferation of T cells at early stages prior to complementary ligand binding, leading to stimulatory signals which attack cancer cells.2
Currently six mAb targeting PD-1/ PD-L1 and one mAb targeting CTLA4 have been approved by the FDA for the treatment of NSCLC (Table 1 on page 58), which we will discuss in more depth in subsequent sections. There are also several other negative checkpoints emerging that suppress the immune system through ligand/receptor binding. These include T cell immunoglobulin, mucin-containing protein-3 (TIM-3), V-domain immunoglobulin suppressor of T cell activation (VISTA), ITIM domain T cell immunoreceptor (TIGIT), and Lymphocyte activation gene-3 (LAG-3).30 TIM-3 is expressed on activated CD4+ T cells (T helper cells) and negatively induces cytokines (Th1 and Th17). It also attaches to galectin-9, which leads to apoptosis of cytotoxic cells (CD4+ and CD8+).31 Galectin-9 is upregulated in cancer cells and suppresses an anti-tumour response through ligation with
54 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Jarushka Naidoo
Figure 1. Mechanisms of action of immune checkpoint inhibitors. T cells recognize tumour antigens at the MHC on antigen presenting cells (APC) by T cell receptors (TCR) displayed on T cells. The interaction between CD80/CD86 (also known as B7-1 or B7-2) on APC and CD28 mediates a T cell co-stimulation in conjugation with TCR signals emitted by the T cell. CTLA-4 on activated T cells interacts with either CD80 or CD86 ligands (with a higher affinity for CD28), thus stopping the T cell from sending inhibitory signal to a T cells. Monoclonal antibodies, anti-CTLA-4 (e.g., ipilimumab) block this inhibitory pathway, thereby restoring T cell activity and amplifying an immune response. Cytotoxic CD8+ specific T cells recognise tumour antigens at the MHC of the APC using TCR. PD-1 is expressed on activated T cells, and the binding of PD-L1 on APC to PD-1 results in an adaptive expression. The interaction between PD-1 and PD-L1 negatively regulates the anti-tumour T cell response and causes immunosuppression. Anti-PD-1 (e.g., pembrolizumab) and anti-PD-L1 mAbs (e.g., atezoliumab) block this inhibitory pathway, thereby restoring T cell activity and relieving the immunosuppression.
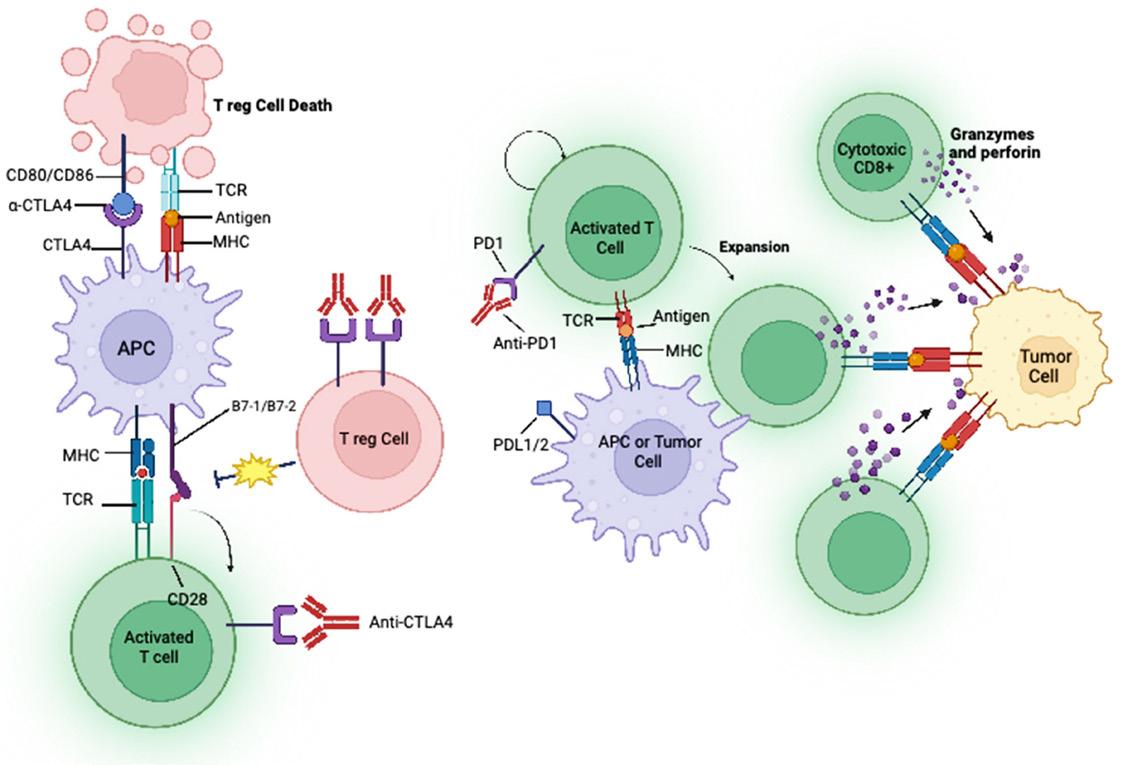
TIM-3.31 LAG-3 can also bind to galactin 3 in the TME, resulting in a reduction in anti-tumour response by inhibiting CD8+ T cells.32 TIGIT inhibits the immune system by competing with CD226 to interact with CD112 and CD155 which would normally activate T cells.33 VISTA is a member of the B7 family of checkpoints that are primarily expressed in hematopoietic cells and naïve CD4+ and Foxp3+ regulatory T cells. It can act as both a ligand and a receptor in negatively regulating immune responses [34]. VISTA has been shown to be more complicated than originally thought and its mechanism of action is not fully understood.
Some new studies have suggested a dual combination of ICIs may

prevent resistance and improve positive outcomes for patients; however, elevated side effects must be considered.30 Patients who do respond to ICIs show that tumour load, immunogenicity, and the extent of immunosuppression in the microenvironment are critical factors that determine the probability of a positive clinical response.35 Thus, ICI combination strategies could be the way forward. Dual regimes including anti-PD-1 in combination with less successful ICI such as anti-TIGIT and anti-LAG-3 are currently in clinical trials.35 For example in the CITYSCAPE trial, atezolizumab (anti-PD-L1) plus tiragolumab (anti-TIGIT) are being assessed for first-line treatment of NSCLC.36 Findings from this study have
demonstrated that titragolumab plus atezolizumab improved both the response rate and progression free survival compared with atezolizumab alone with a safe profile.36 Other combination strategies involving ICIs and other forms of immunotherapy include the use of adoptive cell transfer with ICIs. The most recent of which includes the use of nivolumab in combination with tumour infiltrating lymphocytes (TIL). A phase 1 clinical trial investigating the benefit of TILs administrated with nivolumab in patients with advanced NSCLC demonstrated effective T cell expansion in vivo and manageable toxicity to the patient,37 showing promise for the future.
CAR-T Cell Therapy
Another type of immunotherapy which has gained some attention in the lung cancer field is chimeric antigen receptor (CAR) T cells. CAR-T cells are T cells that have been genetically-modified to express the CAR protein, allowing T cells to recognize cancer cells, without relying on MHC,38 and trigger a downstream signalling cascade of T cell activation.39 CAR-T cell therapy is an approved therapy for haematologic malignancies such as B-cell leukaemia.40 Many studies have displayed successful anti-tumour activity in vitro41 and in vivo42 using CAR-T cells specific to NSCLC tumour-associated antigens such as B7-H3,41 GD242 and PTK7.43 These studies and others have led
55 HOSPITALPROFESSIONALNEWS.IE | HPN • SEPTEMBER 2023
Oncology Focus: Lung Cancer

combination with nivolumab)
combination with platinum-based chemotherapy)
2022 (in combination with durvalumab and platinumbased chemotherapy)
to the development of clinical trials of CAR-T cell therapy in NSCLC. However, these trials are still in early phase and are yet to receive FDA approval. Current issues in using CAR-T cells to treat solid tumours include the harsh tumour microenvironment,44 resulting in T cell exhaustion and subsequent failure to activate their cytotoxic abilities. T cells are also unable to detect intracellular antigens,44 and most significantly, there has yet to be a surface antigen discovered that is as widely expressed as CD19 on B-cells seen in leukaemia.45
Oncolytic Virus Therapy
Oncolytic viruses are genetically modified to promote the targeting and destruction of specific cancer cells, while leaving selfcells untouched.46 The genetic modifications used in oncolytic virus therapy (OVT) remove the adenoviral genes that are used in normal cells for viral infection, but not in cancer cells.47 The primary mechanisms of oncolytic viruses include the lysis of tumour cells, followed by the promotion of anti-tumour activity,48 and changes in cytokine levels which generate a proinflammatory tumour microenvironment.49 Studies have also shown the ability of OVT to promote expression of PD-L1 on tumour cells, making them better targets for ICIs.50
OVT was first approved by the FDA as a treatment for unresectable melanoma patients in 2015,51 and there are ongoing trials examining the use of OVT in NSCLC patients (Table 2 on page 60). A phase I study demonstrated the successful
insertion of these oncolytic viruses into NSCLC cells while avoiding healthy tissue, and no adverse reactions were reported.49 The study assessed intravenous delivery (IV) of enadenotucirev (ColoAd1) in patients with resectable colorectal cancer, nonsmall cell lung cancer, urothelial cell cancer and renal cell cancer patients. Immunohistochemistry (IHC) analysis was utilised to investigate the success of IV delivery of the virus, and successful viral replication was observed, demonstrating effective delivery of the virus. Positive CD8+ T cell infiltration was also observed in patients’ tumour samples, indicating viral delivery can induce a successful immune response without causing any harm to the patient.49 While the use of OVT remains very much at its infancy in the NSCLC setting, it will be interesting to see how this develops in the future.
Immunotherapy for Advanced Stage NSCLC
In 2012, a phase I study was conducted to determine the safety and efficacy of nivolumab in patients with certain types of cancers. Surprisingly, this trial showed a response rate of 18% among patients with NSCLC and led to further clinical development and other anti-PD(L)1 agents in NSCLC.52 Checkmate-017 was a landmark phase III trial that examined the safety and efficiency of nivolumab versus docetaxel. The trial showed significantly improved overall survival (OS), overall response rate (ORR), and progression-free survival (PFS) with nivolumab over docetaxel in patients who had received one prior line of therapy.21 In March 2015, Checkmate-017 together with Checkmate-057, which had the same study design but was conducted in patients
with non-squamous NSCLC,53 led to the approval by the FDA of nivolumab as the first ICI for the treatment of patients with advanced NSCLC after platinumbased chemotherapy. The five year outcomes of pooled data from both these trials revealed that at five years, nivolumab continued to demonstrate a survival benefit compared with docetaxel, with an OS of 13.4% versus 2.6%, and a PFS of 8.0% versus 0%.54 This is a significant and life-changing advance for patients with NSCLC. The approval of nivolumab was quickly followed by the approval of subsequent ICIs (Table 1). In October 2016, results from the KEYNOTE-01023 and KEYNOTE-02422 trials led to the approval of the PD-1 inhibitor, pembrolizumab, for the treatment of patients with metastatic NSCLC. In KEYNOTE-024, pembrolizumab was compared with cytotoxic chemotherapy as a first-line treatment for patients with advanced NSCLC and a PD-L1 tumour percentage score of 50% or greater. Pembrolizumab was shown to have significantly longer PS and OS and fewer adverse effects compared with platinumbased chemotherapy and so became a new standard of care for the first-line treatment of patients with ‘PD-L1 high’ NSCLC.22 Following positive results from the POPLAR and OAK trials, another ICI inhibitor, atezolizumab, was approved for second-line treatment for patients with locally advanced or metastatic NSCLC.24,25 While anti-PD-(L)1 monotherapy had demonstrated benefit in a subset of patients with pre-treated NSCLC, it was also postulated that combination immunotherapy may be a useful strategy in NSCLC.
Ipilimumab and tremelimumab are CTLA-4 inhibitors previously
used in metastatic melanoma. In a phase II trial conducted more than 10 years ago, single agent ipilimumab combined with chemotherapy demonstrated modest benefit compared with ipilimumab monotherapy in NSCLC.55 However, the combination of ipilimumab and nivolumab had demonstrated deep and durable responses in patients with melanoma,56 and it was hoped this could be recapitulated in NSCLC. In early phase studies of this combination, it was clear that the doses used for melanoma resulted in unacceptable toxicity in patients with NSCLC. After modification of this dose to ipilimumab every 6 weeks, the Checkmate-227 explored this combination in a complex eight-arm clinical trial. Broadly, this study demonstrated a significant benefit in PFS and OS as well as durable responses in patients with both PD-L1 > 1% and PD-L1 < 1% NSCLCs, and is approved as a ‘chemotherapy-free’ option in the first-line treatment for patients with advanced PD-L1 > 1% NSCLC.27 In addition, a novel regimen from the Checkmate 9LA trial incorporating two doses of platinum-doublet chemotherapy in addition to ipilimumab and nivolumab also resulted in both PFS and OS benefits for this combination compared with chemotherapy alone, and is also an approved treatment option for first-line advanced NSCLC in all-comers for PD-L1 status.57 This regimen is thought to potentially provide the ‘neoantigen release’ of cytotoxic chemotherapy alongside the long-term durable outcomes seen with ipilimumab and nivolumab.
While the use of ICIs has undoubtably changed the landscape of patient care for NSCLC, there remains an urgent need to transform more patients
56 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Table 1. Immune checkpoint inhibitors approved for the treatment of NSCLC
Name Approval Type of MA Action Usage Reference Nivolumab March 2015 IgG4 PD-1 Stage III OR IV metastatic NSCLC. [21] Pembrolizumab October 2016 Humanized IgG4-K isotope antibody. PD-1 Stage IV metastatic NSCLC. [22,23] Atezolizumab October 2016 IgG1 PD-L1 Stage III or IV metastatic NSCLC. [24,25] Durvalumab February 2018 IgG1 k PD-L1 Stage III NSCLC [26] Ipilimumab May
IgG1 CTLA4 NSCLC [27] Cemiplimab November
(in
IgG4 PD-L1 Stage III OR IV metastatic NSCLC [28] Tremelimumab November
IgG2 PD-L1 Stage III OR IV metastatic NSCLC [29]
Table 1. Immune checkpoint inhibitors approved for the treatment of NSCLC.
2020 (in
2022
IMFINZI is reimbursed for the treatment of locally advanced, unresectable non-small cell lung cancer (NSCLC) in adults whose tumours express PD-L1 on ≥1% of tumour cells and whose disease has not progressed following platinum-based chemoradiation therapy.1
50 mg/mL concentrate for solution for infusion
In a post-hoc subgroup analysis of the PACIFIC study, WITH IMFINZI, the median overall survival (OS) at 5 years, was 63.1 months versus 29.6 months for the placebo arm (HR: 0. 61; 95% CI, 0.44-0.85)1*
*Patients were retrospectively tested for PD-L1 expression on tumour cell using the Ventana PD-L1 (SP263) IHC assay, where available. 63% of patients provided a tissue sample of sufficient quality and quantity to determine PD-L1 expression and 37% were unknown. This exploratory post-hoc 5-year OS analysis was conducted at ~5 years after last patient was randomised and was not powered to show statistical significance.
Primary analysis in the ITT population
Median OS†: Not reached vs 28.7 months with placebo (HR=0.68; 99.73% CI, 0.47-0.997); P=0.00252
Median PFS‡: 16.8 vs 5.6 months with placebo (HR=0.52; 95% CI, 0.42-0.65; P<0.0001)1
The primary 2-year OS analysis was conducted after 299 deaths for 42% maturity (61% of targeted events) with a median follow-up of 25.2 months. Reduction in the risk of death vs placebo was 32% (95% CI, 0.53-0.87).
Median OS was NR with IMFINZI (95% CI, 34.7-NR) vs 28.7 months with placebo (95% CI, 22.9-NR).1,2 ‡Measured based on RECIST v1.1 criteria by BICR. The primary PFS analysis was conducted after 371 events (81% of targeted 458 events) with a median follow-up of 14.5 months. Reduction in the risk of progression or death vs placebo was 48% (HR=0.52; 95% CI, 0.42-0.65). Median PFS was 16.8 months with IMFINZI (95% CI, 13.0-18.1) vs 5.6 months with placebo (95% CI, 4.6-7.8). 3
SAFETY INFORMATION
The most frequent any grade (>10%) adverse reactions were cough/productive cough (21.5%), diarrhoea (16.3%), rash (16.0%), pyrexia (13.8%), upper respiratory tract infections (13.5%), abdominal pain (12.7%), pruritus (10.8%), and hypothyroidism (10.1%)1
See SmPC for recommended treatment modifications for IMFINZI and management considerations.
ABBREVIATED PRESCRIBING INFORMATION
IMFINZI® (durvalumab) 50mg/ml Concentrate for Solution for Infusion
Consult Summary of Product Characteristics (SmPC) before prescribing.
Indication: Non-Small Cell Lung Cancer: • IMFINZI as monotherapy is indicated for the treatment of locally advanced, unresectable non-small cell lung cancer (NSCLC) in adults whose tumours express PD-L1 on ≥ 1% of tumour cells and whose disease has not progressed following platinum-based chemoradiation therapy. • IMFINZI in combination with tremelimumab and platinum-based chemotherapy is indicated for the first-line treatment of adults with metastatic NSCLC with no sensitising EGFR mutations or ALK positive mutations. Small Cell Lung Cancer: IMFINZI in combination with etoposide and either carboplatin or cisplatin is indicated for the first-line treatment of adults with extensive-stage small cell lung cancer (ES-SCLC). Biliary Tract Cancer: IMFINZI in combination with gemcitabine and cisplatin is indicated for the first-line treatment of adults with unresectable or metastatic biliary tract cancer (BTC). Hepatocellular Carcinoma: IMFINZI in combination with tremelimumab is indicated for the first line treatment of adults with advanced or unresectable hepatocellular carcinoma (HCC). Presentation: Each ml of concentrate for solution for infusion contains 50mg durvalumab. Dosage and administration: Treatment must be initiated and supervised by a physician experienced in the treatment of cancer. IMFINZI is administered as an intravenous infusion over 1 hour. Refer to ‘Method of administration’ in section 4.2 of SmPC. Patients with locally advanced NSCLC should be evaluated for treatment based on the tumour expression of PD-L1 confirmed by a validated test Monotherapy: For locally advanced NSCLC, the recommended IMFINZI dose is 10 mg/kg every 2 weeks or 1 500 mg every 4 weeks, until disease progression, unacceptable toxicity, or a maximum of 12 months. Combination therapy: For metastatic NSCLC, the recommended IMFINZI dose during platinum chemotherapy is 1 500 mg in combination with tremelimumab 75 mg and platinum-based chemotherapy every 3 weeks (21 days) for 4 cycles (12 weeks). For metastatic NSCLC, the recommended IMFINZI dose for post-platinum chemotherapy is 1 500 mg every 4 weeks as monotherapy and histology-based pemetrexed maintenance therapy every 4 weeks. A fifth dose of tremelimumab 75 mg should be given at week 16 alongside IMFINZI until disease progression or unacceptable toxicity. For ES-SCLC, the recommended IMFINZI dose in combination with chemotherapy is 1 500mg, every 3 weeks (21 days) for 4 cycles, followed by 1 500 mg every 4 weeks as monotherapy, until disease progression or unacceptable toxicity. For BTC, the recommended IMFINZI dose in combination with chemotherapy is 1 500 mg, every 3 weeks (21 days) up to 8 cycles, followed by 1 500 mg every 4 weeks as monotherapy until disease progression or unacceptable toxicity. For HCC, the recommended IMFINZI dose is 1 500 mg administered in combination with 300 mg tremelimumab as a single dose at Cycle 1/Day 1, followed by IMFINZI as monotherapy every 4 weeks until disease progression or unacceptable toxicity. Please consult Section 4.2 in the SmPC for more information. Dose escalation or reduction is not recommended. Treatment withholding or discontinuation may be required based on individual safety and tolerability. Suspected immune-mediated adverse reactions: Adequate evaluation should be performed to confirm aetiology or exclude alternate aetiologies. Based on the severity of the adverse reaction, IMFINZI and/or tremelimumab should be withheld and corticosteroids administered. Guidelines for the management of immune mediated adverse reactions are described in Table 2, Section 4.2 in the SmPC. Increasing dose of corticosteroids and/or using additional systemic immunosuppressants should be considered if there is worsening or no improvement. Upon improvement to ≤ Grade 1, corticosteroid taper should be initiated and continued over at least 1 month. After withhold, IMFINZI and/or tremelimumab can be resumed within 12 weeks if the adverse reactions improved to ≤ Grade 1 and the corticosteroid dose has been reduced to ≤ 10 mg prednisone or equivalent per day. IMFINZI and tremelimumab should be permanently discontinued for recurrent Grade 3 (severe) immune-mediated adverse reactions and for any Grade 4 (life-threatening) immune-mediated adverse reactions, except for endocrinopathies that are controlled with replacement hormones. Special populations: Elderly: No dose adjustment is required for elderly patients (≥ 65 years of age). Renal/Hepatic impairment: No dose adjustment is recommended in patients with mild or moderate renal or hepatic impairment. Data from patients with severe renal or hepatic impairment are too limited to draw conclusions on this population.
Contraindications: Hypersensitivity to the active substance or to any of the excipients. Warnings and precautions: Traceability: In order to improve the traceability of biological medicinal products, the tradename and the batch number of the administered product should be clearly recorded. Immune-mediated pneumonitis: Monitor for signs and symptoms of pneumonitis or radiation pneumonitis. Suspected pneumonitis should be confirmed with radiographic imaging and other infectious and disease-related aetiologies excluded. Immune-mediated hepatitis: Monitor alanine aminotransferase, aspartate aminotransferase, total bilirubin, and alkaline phosphatase levels prior to initiation of treatment and prior to each subsequent infusion. Additional monitoring is to be considered based on clinical evaluation. Immune-mediated colitis: Monitor for signs and symptoms of colitis/diarrhoea and intestinal perforation. Immune-mediated hypothyroidism, hyperthyroidism and thyroiditis: Monitor for abnormal thyroid function tests prior to and periodically during treatment and as indicated based on clinical evaluation. Immune-mediated adrenal insufficiency: Monitor for clinical signs and symptoms of adrenal insufficiency. Immune-mediated type 1 diabetes mellitus: Monitor for clinical signs and symptoms of type 1 diabetes mellitus including diabetic ketoacidosis. Immune-mediated hypophysitis/hypopituitarism: Monitor for clinical signs and symptoms of hypophysitis or hypopituitarism. Immune-mediated nephritis: Monitor for abnormal renal function tests prior to and periodically during treatment with IMFINZI. Immune-mediated rash: Events of Stevens-Johnson Syndrome or toxic
epidermal necrolysis have been reported in patients treated with PD-1 inhibitors. Monitor for signs and symptoms of rash or dermatitis (including pemphigoid). Immune-mediated myocarditis: Monitor for signs and symptoms of immune-mediated myocarditis. Immune-mediated pancreatitis: Monitor for signs and symptoms of immune-mediated pancreatitis. Other immune-mediated adverse reactions: Myasthenia gravis, myelitis transverse, myositis, polymyositis, meningitis, encephalitis, Guillain-Barre syndrome, immune thrombocytopenia, and cystitis noninfective have been observed. Monitor for signs and symptoms. All immune-mediated adverse events should be managed as recommended in Section 4.2 of the SmPC. Infusion-related reactions: Monitor for signs and symptoms of infusion related reactions. Consult section 4.2 of SmPC for management recommendations. Cholangitis and biliary tract infections: Closely monitor patients with BTC (especially those with biliary stents) for development of cholangitis or biliary tract infections before initiation of treatment and, regularly, thereafter. Metastatic NSCLC: Limited data are available in elderly patients (≥ 75 years). Careful consideration of the potential benefit/risk of this regimen on an individual basis is recommended. Patients excluded from clinical studies: Patients with: a baseline ECOG performance score ≥ 2; active or prior documented autoimmune disease within 2 years of initiation of the study; a history of immunodeficiency; a history of severe immune-mediated adverse reactions; medical conditions that required systemic immunosuppression, except physiological dose of systemic corticosteroids (≤ 10 mg/day prednisone or equivalent); uncontrolled intercurrent illnesses; active tuberculosis or hepatitis B or C or HIV infection or patients receiving live attenuated vaccine within 30 days before or after the start of IMFINZI. Durvalumab should be used with caution in these populations after careful consideration of the potential benefit/risk on an individual basis. The safety of concurrent prophylactic cranial irradiation (PCI) with IMFINZI in patients with ES-SCLC is unknown. Drug Interactions: The use of systemic corticosteroids or immunosuppressants before starting durvalumab, except physiological dose of systemic corticosteroids (≤ 10 mg/day prednisone or equivalent), is not recommended because of their potential interference with the pharmacodynamic activity and efficacy of durvalumab. However, systemic corticosteroids or other immunosuppressants can be used after starting durvalumab to treat immune-related adverse reactions. PK drug-drug interaction between durvalumab and chemotherapy was assessed and showed concomitant treatment did not impact the PK of etoposide, carboplatin or cisplatin. Additionally, based on population PK analysis, concomitant chemotherapy treatment did not meaningfully impact the PK of durvalumab. PK drug-drug interactions between durvalumab in combination with tremelimumab and platinum-based chemotherapy were assessed and showed no clinically meaningful PK interactions between tremelimumab, durvalumab or any of the chemotherapy drugs involved in the concomitant treatment. Pregnancy and Lactation: Durvalumab may cause foetal harm when administered to a pregnant woman and is not recommended during pregnancy and in women of childbearing potential not using effective contraception during treatment and for at least 3 months after the last dose. A decision must be made whether to discontinue breast-feeding or to discontinue or abstain from durvalumab therapy taking into account the benefit of breast-feeding for the child and the benefit of therapy for the woman. Undesirable events: Consult SmPC for full list of side-effects Adverse reactions included below are associated with IMFINZI as monotherapy, and IMFINZI in combination with chemotherapy, and IMFINZI in combination with tremelimumab and platinum-based chemotherapy, and IMFINZI in combination with tremelimumab. For more details refer to tables 3 and 4 in section 4.8 of SmPC. Very common (≥ 1/10; any grades): Upper respiratory tract infections, anaemia, leukopenia, neutropenia, thrombocytopenia, hypothyroidism, decreased appetite, cough/productive cough, diarrhoea, abdominal pain, constipation, nausea, vomiting, rash, alopecia, pruritus, arthralgia, pyrexia, fatigue, oedema peripheral, aspartate aminotransferase increased or alanine aminotransferase increased, pneumonia. Common (≥ 1/100 to < 1/10; any grades): Influenza, oral candidiasis, dental and oral soft tissue infections, febrile neutropenia, pancytopenia, adrenal insufficiency, hyperthyroidism, thyroiditis, hypopituitarism or hypophysitis, neuropathy peripheral, pneumonitis, dysphonia, stomatitis, amylase increased, lipase increased, colitis, pancreatitis, hepatitis, night sweats, dermatitis, myalgia, , blood creatinine increased, dysuria, infusion related reaction. Uncommon (≥ 1/1 000 to < 1/100; any grades): Interstitial lung disease, psoriasis, myositis, nephritis, immune thrombocytopenia, diabetes insipidus, type 1 diabetes mellitus, encephalitis, myasthenia gravis, meningitis, pemphigoid, polymyositis, cystitis noninfective. Rare (≥ 1/10 000 to < 1/1 000; any grades): Myocarditis. Not known: Noninfective encephalitis, Guillain-Barré syndrome, myelitis transverse, intestinal perforation, large intestine perforation. Legal Category: Product subject to prescription which may not be renewed (A) Marketing Authorisation
Number: EU/1/18/1322/001; EU/1/18/1322/002 Marketing Authorisation Holder: AstraZeneca AB, SE-151 85, Södertälje, Sweden. Further information available on request from: AstraZeneca Pharmaceuticals (Ireland) DAC, College Business and Technology Park, Blanchardstown Road North, Dublin 15 Tel: +353 1 6097100. IMFINZI is a registered trade mark of the AstraZeneca group of companies.
Date of API Preparation: 05/2023 Veeva ID: IE-4946

Adverse events should be reported directly to: HPRA Pharmacovigilance. Website: www.hpra.ie. Adverse events should also be reported to AstraZeneca Patient Safety on Freephone 1800 800 899 References: 1. Imfinzi-Ireland Summary of Product Characteristics. May 2023. 2. Antonia SJ, et al. N Engl J Med 2018;379:2342−2350. 3. Antonia SJ, et al. N Engl J Med 2017;377:1919-1929. IMFINZI is a trademark of the AstraZeneca group of companies. ©2023 AstraZeneca. All rights reserved. Veeva ID: IE-4726 | DOP: May 2023
from immunotherapy nonresponders to responders. Hence, current research strategies are focused on improving the response rate of ICIs, and these efforts are mainly centred around creating effective combination regimens with chemotherapy, radiotherapy, and other anti-cancer drugs. In 2018, the FDA approved the use of pembrolizumab in combination with chemotherapy for first-line treatment of metastatic nonsquamous NSCLC, independent of PD-L1 tumour expression status. This approval was based on the results of the KEYNOTE-189 trial which demonstrated a longer OS and PFS in patients receiving pembrolizumab in addition to standard chemotherapy of pemetrexed and a platinumbased drug compared with patients receiving chemotherapy alone.58 This combination regimen was also approved for first-line treatment in metastatic squamous NSCLC following the results of the KEYNOTE-407 trial, again independent of PD-LI tumour expression status.59 The positive results from the Impower150 trial led to the approval of atezolizumab with chemotherapy and bevacizumab for first-line treatment of metastatic nonsquamous NSCLC.60 Interestingly, it has been demonstrated that the combination of chemotherapy with bevacizumab induces proliferation of peripheral CD8 T cells, particularly memory and effector subsets,61 perhaps offering the rationale for
Oncology Focus: Lung Cancer

combining chemotherapy and bevacizumab with ICIs. This is also now an approved option for first-line treatment of advanced NSCLC independent of PD-L1 status, based on the phase III IMPower150 trial.60
Challenges Associated with the Use of Immunotherapy
Immune-Related Adverse Events (irAEs)
Despite the clinical benefit that arises from immunotherapy, more than 20% of patients experience immune-related adverse events (irAEs) from therapy, and the incidence may be >50% with combination approaches.118
Immune-related adverse events can be described as autoimmune conditions that can affect any organ system in the body after ICI administration.119 These toxicities have presented as a challenge for clinical practitioners and patients because rather than managing familiar side effects such as nausea, anaemia, and immunosuppression, they are now confronted with unfamiliar side effects, such as underactive pituitary glands and hepatitis.119
Immune-related adverse events vary in terms of their onset time, severity, and underlying biology.120 They can affect a broad range of organs and occur at any time during the patient’s treatment course. They most commonly occur in the first three months of treatment but have also been observed to occur long after ICI has been stopped.121 The time of irAEs occurrence gives an indication of the severity of the effects on the patient. For example, toxicity in the first year of therapy strongly correlated with long term toxicity beyond
1 year.120 Many ICI clinical trials have reported longer-term safety data, but information is still limited about the ongoing impact of the toxicities.121 Treatments for irAEs centre around glucocorticoids for acute irAEs (developed during ICI treatment) specifically, with good effects observed after several weeks. Although most irAEs resolve, some develop into a chronic state (develop after ICI treatment has terminated) and lifelong therapy such as hormonal supplementation or immunosuppression may be required.119
IrAEs are distinct from those that occur with traditional chemotherapy or other forms of anti-cancer therapy as they occur as a result of the immunologic mode of action of ICIs. There are limited data exploring the mechanisms that underpin the development of irAEs. However, T-cell,122,123,124 B-cell125 and macrophagerelated mechanisms123 have been identified. In a comprehensive clinical and translational study, the cytokine interleukins-6 (IL-6) was shown to be highly upregulated in the patient cohort after nivolumab treatment.124 Moreover, blocking IL-6 in mouse models could potentially mitigate autoimmunity and maintain, or even possibly boost, the tumour immunity.124 Recently, it was observed that the use of IL-6 blockade in a patient with ICI-induced irAEs led to successful mitigation of irAEs symptoms without compromising the ICI treatment.124 It has been demonstrated that changes in T cell populations occur early after ICI treatment,122 and these changes can also affect B cells and macrophages
directly or indirectly; however, the mechanism for this remains largely unknown.125 Early changes in B cells have also correlated with high rates of irAEs, indicating B cells may play an important role in driving irAEs.125 The link between T cells, B cells, and macrophages in relation to irAEs and the modes of actions they employ, is of major interest of the immunotherapy field at present.
It is critical that effective strategies are developed in the clinic to address the issue of irAEs associated with ICIs. Clinical experience of ICI toxicity develops local expertise in managing the diverse range of potential irAEs associated with ICIs. In recent years, there has been the publication of international guidelines for the management of irAEs to guide clinicians in the clinical management of these complex cases. These include the National Comprehensive Cancer Network (NCCN) and the European Society of Medical Oncology (ESMO) guidelines.126,127 These provide detailed decision assistance tools regarding risk stratification, early diagnosis, steroid administration and steroid sparing strategies for patients experiencing irAEs.
The management of irAEs ideally involves specialist teams involving a medical oncologist and an organ specialist (e.g., a respiratory physician in case of pneumonitis). In some institutions, this has been formalised into a dedicated irAE toxicity team. This form of multidisciplinary team has demonstrated feasibility and been shown to change patient management.128
58 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Table 2. Clinical trials investigating OVT in NSCLC.inhibitors approved for the treatment of NSCLC
ClinicalTrials.gov Identifier Status Phase Virus Cancer Type NCT03004183 Active, not recruiting Phase II ADV/HSV-tk NSCLC NCT05076760 Recruiting Phase I MEM-288 NSCLC NCT02879760 Completed Phase I/II Ad-MAGEA3 and MG1-MAGEA3 NSCLC NCT02053220 Completed Phase I Colo-Ad1 NSCLC NCT05602792 Recruiting Phase I/II T3011 NSCLC NCT00861627 Completed Phase II REOLYSIN® NSCLC NCT03767348 Recruiting Phase II RP1 NSCLC NCT04725331 Recruiting Phase I/II BT-001 NSCLC
Table 2. Clinical trials investigating OVT in NSCLC.
Critical areas of research include the appropriate risk stratification of patients and the development of biomarkers for early identification of irAEs. Prior to treatment initiation, risk stratification of those at high risk of irAEs is a critical step in ensuring patients are not exposed to an unacceptable level of risk. For example, it has been demonstrated that patients with a history of interstitial lung disease are at high-risk of developing ICI associated pneumonitis.129 In the case of cardiotoxicity, prospective data would suggest that baseline ECG/troponin can be helpful in identifying those patients most at risk of toxicity, and these patients may warrant close surveillance. It is likely that the future of ICI toxicity management will involve a refinement of our strategies to identify those patients most at risk through clinical studies and biomarker identification in tandem with improvements in immunosuppressive strategies.
Biomarkers
The reasons underlying why some patients with NSCLC achieve disease control from therapy, or develop toxicity, are incompletely understood. Hence, another major challenge facing the modern era of immunotherapy treatment lies in the development of efficient biomarkers to optimize patient selection. The expression of PD-L1 on tumour cells, quantified using IHC, is currently the most widely used and validated biomarker to guide the selection of patients to receive ICIs. PD-L1 expression has shown predictive value in many clinical trials in NSCLC, with correlations observed between clinical response and increased expression of PD-L1 on tumour cells.23,24,130,131 However, positive correlation of PD-L1 expression can only partially predict which patients will benefit from ICIs, and many trials have demonstrated responses irrespective of PDL1 expression status.5,21 This imperfection in the use PD-L1 as a biomarker of response could be attributed to many factors. For instance, differences exist in the specific types of assays that are utilized to assess PD-L1 expression in tumour tissues, and even within these assays it can prove difficult to score the PD-L1 expression consistently and accurately on tumour cells and immune cells.132 Moreover, intratumour heterogeneity (ITH) exists within the tumour of patients with NSCLC, and hence, the biopsy samples may not accurately reflect the expression of PD-L1 throughout the tumour.133,134 The
emerging use of liquid biopsies to assess the expression of PDL1 using cytology samples has shown great promise, and so may help overcome some of these challenges in the future.39
Tumour mutational burden (TMB) refers to the absolute number of non-synonymous mutations within a tumour, which leads to the generation of immunogenic neo-peptides displayed on the surface of tumour cells, and hence, is associated with a greater CD8+ T cell response following ICI treatment.135 In June 2020, the FDA approved the use of pembrolizumab for the treatment of unresectable and metastatic solid tumours with a high TMB. This approval was based on the results of the KEYNOTE-158 trial which demonstrated that a high TMB was associated with an increased objective response rate.136 However, similar to the use of PD-L1 expression, the predictive value of TMB is limited by the presence of ITH. A high ITH may result in the neoantigens only being present on a subset of cells and hence, the immune response may not be effective against the entire tumour.137 Moreover, sub-clonal neoantigens, which occur as a result of cytotoxicchemotherapy, give rise to high TMB and these sub-clonal neoantigens are associated with poor responders. This is in comparison to an enhanced response to ICI in patients with tumours enriched for clonal neoantigens.138 Hence, neoantigen ITH can also contribute to the limited predictive value of TMB, emphasizing the need for effective diagnostic techniques that examine the entire tumour.
Another biomarker of response which is currently gaining much attention in the field of NSCLC is the use of circulating tumour DNA (ctDNA). Cells release small double-stranded DNA fragments into the bloodstream during apoptosis and necrosis, termed circulating free DNA (cfDNA), and in cancer patients, small fractions of cfDNA can be shed from the tumour in the form of ctDNA.139 Several studies have demonstrated that the levels of ctDNA, as detected by a liquid biopsy, can predict response to ICI in patients with NSCLC.140,141,142 Moreover, ctDNA can be used for the detection of point mutations associated with sensitivity to ICI. For example, several studies have demonstrated that mutations in STK11 can predict the response to treatment,143 including ICIs144 in NSCLC. Other biomarkers
assessed by liquid biopsy which may offer potential in predicting the response to ICI in the future include peripheral blood cytokines,145 circulating non-coding RNA146 and the levels of various immune cell populations.147,148,149 Finally, biomarkers to predict irAEs are even less characterized. Some research suggests the composition of the gut microbiome in response to ICI can influence the development of irAEs;150,151 however, the mechanism of action remains largely unclear. Other potential irAEs biomarkers include baseline auto-antibodies,152 germline genetics,153 T cell and B cell populations,133 and shared T cell antigens.132 Given the adverse effects that ICI can have on patients, it is imperative that the field invests research in developing predictive biomarkers to optimise patient selection and deliver this therapy to patients most likely to benefit.
Future Outlook for the Use of Immunotherapy in NSCLC
ICIs have established efficacy in advanced and early-stage NSCLC, and it is unlikely that PD-1/PD-L1 monotherapy will improve on the already established benefit for patients. As mentioned previously in this review, recent interest lies in combining ICIs with novel combination strategies which could include radiation, novel ICIs or other novel systemic therapies. For example, neoadjuvant durvalumab was investigated with or without sub-ablative stereotactic radiotherapy (SBRT) in patients with resectable NSCLC.154 In this study patients received two cycles of neoadjuvant durvalumab +/− 3 fractions of SBRT (8 Gy * 3), followed by surgery. In the NEOCOAST study, the authors investigated neoadjuvant durvalumab +/ novel agents for resectable stage I–IIIA NSCLC.155 These novel agents included the anti-CD73 agent oleclumab, the anti-NKG2A monalizumab or the anti-STAT3 antisense oligonucleotide danavatirsen. Finally, the inhibition of novel checkpoints as a monotherapy or in combination with pre-existing ICIs is an emerging strategy in this field. LAG-3 suppresses T cell activation and cytokine secretion.156 Inhibitors of LAG-3 have already reached phase II and phase III clinical trials and relatlimab has been approved in combination with nivolumab for advanced melanoma.157 In NSCLC, the RELATIVITY study has commenced accrual and is
exploring the combination of relatlimab and nivolumab and chemotherapy in the advanced setting.158
Another critical area of research in this field is utilising ‘liquid biopsy’ assays to identify patients not responding to therapy at an early stage in their treatment paradigm so that therapy plans can be adapted thereafter. ‘Liquid Biopsy’ generally refers to the use of blood based (but can use other body fluids, e.g., breath) biomarkers to identify tumourbased signatures which could include cfDNA, ctDNA, circulating tumour cells (CTCs) and others.159
In an investigation of 67 patients with stage IV NSCLC, a ctDNA ‘molecular response’ in plasma 9 weeks post starting ICI was associated with a durable clinical benefit (defined as an ongoing response at 6 months post IO, 3.5% vs. 49.4%, p < 0.001). This study demonstrates the potential for ctDNA dynamics to identify responders to therapy.

In summary, it is likely that the future of ICIs in NSCLC will involve their combination with other systemic/local therapy but advances in therapeutics will be combined with novel diagnostics/ biomarkers (e.g., liquid biopsy) to improve patient selection for therapy.
Conclusions
The introduction of immunotherapy as a treatment option for patients with NSCLC has offered benefit and hope to selected patients. This is reflected in the survival benefit and improvements in the quality of life for these patients. However, the subset of patients who sustain a prolonged antitumour response remains relatively low. Continued development of effective immunotherapybased combination regimens and expansion into earlier stage NSCLC will hopefully increase the proportion of patients who respond to ICIs in the future. Moreover, a critical gap in the field is to develop predictive biomarkers to identify patients who will benefit most from ICI or develop toxicity. The identification of such biomarkers and their integration with clinical care and therapeutic decision-making would continue to ensure the impact of immunotherapy for NSCLC in the future.
References available on request
This article can be viewed in full via: Cancers 2023, 15(11), 2996; https://doi.org/10.3390/ cancers15112996
59 HOSPITALPROFESSIONALNEWS.IE | HPN • SEPTEMBER 2023
Oncology Focus: AML

Alterations of cohesin complex genes in acute myeloid leukemia: differential co-mutations, clinical presentation and impact on outcome
Acute myeloid leukemia (AML) is a genetically complex disease. The recently revised WHO classification acknowledges a variety of genetically defined alterations which constitute distinct disease entities.1 Correspondingly, our understanding of myeloid neoplasms moves away from somewhat arbitrary numerical counts of bone marrow blasts and toward an appreciation of genetic drivers of disease as is acknowledged in the revised International Consensus Criteria.2 On this basis, the recently revised European Leukemia Net (ELN) recommendations broaden the spectrum of clinically relevant genetic alterations with respect to individual patient risk warranting treatment that is adjusted to individual low-, intermediate-, and high-risk molecular alterations and cytogenetics.3 In these updated definitions, mutations of the cohesin subunit SA-2 (STAG2) are recognized as a defining alteration of AML with myelodysplasiarelated gene mutations (in absence of other defining alterations) irrespective of prior presence of myelodysplastic neoplasms.3 Further, mutated STAG2 is defined as a prognostic marker of high-risk (if not co-occurring with favorable risk AML subtypes) incentivizing intensive treatment and, potentially, allogeneic hematopoietic stem cell transplantation (HCT).3
STAG2, double-strand-break repair protein rad21 homologue (RAD21), and structural maintenance of chromosomes (SMC) proteins 1 A (SMC1A) and 3 (SMC3) form the four core units of the cohesin complex, a ring-like protein complex that encircles sister chromatids during replication and initiates metaphase-to-anaphasetransition upon sister chromatid release.4 Additionally, the cohesion complex plays a key role in regulation of both structure and function of chromatin where it is recruited to chromatin binding sites via CCCTF-binding-factor.5 Since the initial discovery of the cohesin complex in 1997(refs.6 7),
genetic alterations have been detected in a multitude of malignant neoplasms8 including bladder cancer,9 10 11 Ewing sarcoma,12 13 14 endometrial cancer,15 glioblastoma,16 and myeloid malignancies.
17 18 19 20 21 22 23 24
While initially inactivating mutations of the cohesin complex were thought to promote carcinogenesis via aberrant segregation of sister chromatids and subsequent aneuploidy, especially recent findings of altered cohesin subunits in commonly euploid myeloid malignancies (with the exception of myeloid leukemias associated with Down Syndrome18) hint at more complex mechanisms of pathogenesis.4 For instance, the finding that cohesin-CCCTF-binding-factor sites are frequently altered in cancer cells underlines the cohesins’ function in threedimensional chromosome organization as a key component of carcinogenesis in a variety of neoplasms.25,26,27 Further, inactivation of cohesin subunits may result in a complete collapse of topologicallyassociating-domain (TAD) structure.28 29 30 Additionally, mutated cohesin subunits appear to play a role in stemness and differentiation in hematopoietic stem cells (HSC). Inactivation of STAG2, RAD21, SMC1A, and SMC3 was found to promote stem cell self-renewal in human and mouse HSCs in vitro and subunit-specific knockout mice were found to bear changes in erythroid and myeloid differentiation mimicking myeloproliferative disorders similar to early human leukemogenesis.31 32 33 This results in a proliferation advantage hinting at a key function of the cohesin complex in regulating cellular differentiation.31 32 33
Taken together, these findings suggest a multi-facetted role of the cohesin complex and its individual subunits in human carcinogenesis. The impact of individually altered cohesin subunits on patient outcome in AML is unclear as
previous studies have suggested unfavorable,17 favorable19 as well as no prognostic impact.20 Therefore, we aimed to identify distinct co-mutational patters for mutations of STAG2 and other proteins of the cohesion complex that help to predict clinical outcomes in a large multicentric cohort of adult patients with AML.
Methods
Data set and definitions
We retrospectively analyzed a cohort of 1615 adult AML patients that were treated in previously reported multicenter trials (AML96(ref.34) [NCT00180115], AML2003(ref.35) [NCT00180102], AML60 + (ref.36) [NCT 00180167], and SORAML(ref.37) [NCT00893373]) or registered in the bio-registry of the German Study Alliance Leukemia (SAL [NCT03188874]) which encompasses 59 centers specialized in the treatment of hematologic neoplasms across Germany and the Czech Republic. Patients were eligible based on diagnosis of AML according to WHO criteria,1 age ≥ 18 years, curative treatment intent and available biomaterial at diagnosis. Prior to analysis, all patients gave their written informed consent according to the revised Declaration of Helsinki.38 All studies were approved by the Institutional Review Board of the Technical University Dresden (EK 98032010). Complete remission (CR) and survival times including event-free (EFS), relapse-free (RFS), and overall survival (OS) were defined according to ELN2022 criteria.3 Patients were retrospectively re-stratified into ELN2022 risk groups.3 Since patients from earlier clinical trials were only re-stratified according to ELN2022 criteria, study accrual was not influence based on ELN risk. A summary of individual study protocols is provided in table S1
AML was defined as de novo when no prior malignancy and no prior treatment with chemo- and/ or radiotherapy was reported.
AML was defined as secondary (sAML) when prior myeloid
neoplasms were reported, and therapy-associated (tAML) when prior exposure to chemo- and/or radiotherapy was reported.
Molecular analysis and cytogenetic analysis
Pre-treatment peripheral blood or bone marrow aspirates were screened for genetic alterations using next-generation sequencing (NGS) with the TruSight Myeloid Sequencing Panel (Illumina, San Diego, CA, USA) covering 54 genes (table S2) that are associated with myeloid neoplasms including full coding exons for SMC1A, RAD21, and STAG2 and relevant exons (10, 13, 19, 23, 25, and 28) for SMC3 according to the manufacturer’s recommendations as previously reported.39 40 DNA was extracted using the DNeasy blood and tissue kit (Qiagen, Hilden, Germany) and quantified with the NanoDrop spectrophotometer. Pooled samples were sequenced pairedend (150 bp PE) on a NextSeq NGS-instrument (Illumina).
Sequence data alignment of demultiplexed FastQ files, variant calling and filtering was performed with the Sequence Pilot software package (JSI medical systems GmbH, Ettenheim, Germany) with default settings and a 5% variant allele frequency (VAF) mutation calling cut-off. Human genome build HG19 was used as reference genome for mapping algorithms. Dichotomization of dominant and subclonal (or secondary) mutations was performed by comparing VAFs of detected mutations with VAFs of co-mutated driver variants. For resolution of putative subclonal mutations a minimum difference of 10% VAF was applied. For cytogenetic analysis, standard techniques for chromosome banding and fluorescence-in-situhybridization (FISH) were used.
Statistical analysis
Statistical analysis was performed using STATA BE 17.0 (Stata Corp, College Station, TX, USA). All tests were carried out as two-sided tests. Statistical significance was
60 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Figure 1 Graphic representation of the domain structure of STAG2 and positions of STAG2 mutations in 88 AML patients (A). Mutations of STAG2 are categorized by function (missense=blue, termination=red), clonal rank (dominant=red/subclonal=blue), number of mutations, and variant allele fraction, and associated co-mutations (B). For detailed information on co-mutations and results of individual significance tests, see Tab. S4. Survival analysis using the Kaplan-Meier method and log-rank test for event-free (C), relapse-free (D) and overall survival (E) differentiating between STAG2-mutated and STAG2-wildtype AML.
determined using a significance level α of 0.05. Fisher’s exact test was used to compare categorical variables. Normality was assessed using the Shapiro-Wilk test. If the assumption of normality was met, continuous variables between two groups were analyzed using the two-sided unpaired t-test. If the assumption of normality was violated, continuous variables between two groups were analyzed using the Wilcoxon rank sum test. With regard to outcome variables, patients were analyzed on a complete case basis. Univariate analysis was carried out using logistic regression to obtain odds ratios (OR). Time-to-event analysis was performed using Cox-proportional hazard models to obtain hazard ratios (HR) as well as the Kaplan-Meier-method and the log-rank-test. For survival times, OR and HR, 95%-confidenceintervals (95%-CI) are reported. Multivariable models were adjusted for ELN2022 categories and age. In the case of AML with mutated STAG2, additional adjustments were performed in multivariable analysis for frequently co-mutated genes with an established impact on patient
outcome according to ELN2022 definitions.3 Median follow-up time was calculated using the reverse Kaplan-Meier method.41
Results

Alterations of cohesin complex genes are recurrent events in AML with distinct clinical presentation
Alterations of any of genes of the cohesin complex (i.e. STAG2, RAD21, SMC1A and SMC3) were found in 184 of 1615 patients (11.4%). With the exception of one patient harboring both mutated STAG2 and SMC3, alterations of cohesin complex genes were found to be mutually exclusive. With respect to clinical presentation, patients harboring mutations in genes of the cohesin complex had significantly lower white blood cell count (WBC, median 12.1*109/l vs, 20.4*109/l, p = 0.001), peripheral blood blast count (PBB, median 25.5% vs. 41.0%, p = 0.020), and bone marrow blast count (BMB, median 54.0% vs. 63.5%, p < 0.001) at initial diagnosis. Mutations in the cohesin complex were
significantly associated with normal karyotypes (66.8% vs. 49.6%, p < 0.001), mutated TET2 (25.0% vs. 18.5%, p = 0.046), ASXL1 (17.9% vs. 7.1%, p < 0.001), and SRSF2 (16.8% vs. 5.0%, p < 0.001), while inframe mutations in CEBPA-bZIP (7.6% vs. 9.1%, p = 0.008) and mutated IKZF1 (0% vs. 3.2%, p = 0.007), TP53 (2.7% vs. 7.7%, p = 0.009), complex karyotypes (6.0% vs. 12.4%, p = 0.022) and inv16 or t(16;16) were rare (0.5% vs. 4.0%, p = 0.016) compared to cohesin wild-type AML. Median follow-up time for the entire cohort was 89.5 months (95%-CI: 85.5-95.4).
AML with mutated STAG2 shows a distinct co-mutational pattern and clinical presentation while patient outcome is not affected Mutations in STAG2 were the most frequent alterations of cohesin complex genes in the cohort (n = 88, 5.4%). The majority of STAG2 mutations were nonsense mutations (n = 82, 93.2%), while missense mutations
were rare (n = 6, 6.8%, Fig. 1A). Alterations of STAG2 were more often dominant (58.0%) rather than subclonal (42.0%). At initial presentation, AML patients harboring mutated STAG2 were significantly older (58 years vs. 55 years, p = 0.023) and had lower WBC (median 5.4 × 109/l vs. 20.7 × 109/l, p < 0.001), PBB (20.0% vs. 41.0%, p < 0.001), BMB (48.3% vs. 63.5%, p < 0.001) and LDH (median 342 U/l vs. 450.8 U/l, p = 0.001). Table S4 provides an overview of baseline characteristics of patients with STAG2-mutated AML. Alterations of STAG2 were frequently associated with normal karyotypes (75.0% vs. 50.4%, p < 0.001), mutated IDH2 (23.9% vs. 13.5%, p = 0.011), RUNX1 (18.2% vs. 8.8%, p = 0.007), BCOR (12.5% vs. 4.3%, p = 0.002), ASXL1 (31.8% vs. 6.9%, p < 0.001), SRSF2 (27.3% vs. 5.1%, p < 0.001), CUX1 (6.8% vs. 2.4%, p = 0.004), and ZRSR2 (4.5% vs. 1.4%, p = 0.044). Compared to STAG2-wild-type AML, in-frame mutations of CEBPA-bZIP (5.7% vs. 9.1%, p = 0.004) as well as mutated NPM1 (14.8% vs. 32.0%, p < 0.001) and FLT3-ITD (9.1% vs. 22.3%, p = 0.002) were significantly less common (Fig. 1B). With respect to patient outcome, we found no difference in CR rate between patients with mutated or wild-type STAG2 (63.6% vs. 66.8%, OR = 0.71, p = 0.138). EFS, RFS and OS did not differ. Clonality, i. e. whether mutated STAG2 was present in the dominant clone or detected at a subclonal level, did also not influence patient outcome. Mutations wof BCOR, RUNX1 and ASXL1 are established markers of adverse risk and mutated STAG2 has been added as an adverse marker in the recent ELN2022 recommendations.3 Since patients that harbored mutations of STAG2 showed a co-mutational pattern with significantly increased rates of comutated BCOR, RUNX1 and ASXL1, we evaluated their individual influence on outcome in multivariable models. With respect to achievement of CR, mutated STAG2 remained of no independent prognostic impact in a multivariable model adjusted for mutational status of BCOR, RUNX1, and ASXL1 while mutated RUNX1 and ASXL1 showed significantly decreased ORs to achieve CR (p < 0.001
61 HOSPITALPROFESSIONALNEWS.IE | HPN • SEPTEMBER 2023
Oncology Focus: AML

significantly increased rates of t(8;21) (20.0% vs. 3.5%, p = 0.002) while no other associations were found. Patients harboring SMC3 mutations showed significantly increased co-mutations of NPM1 (65.0% vs. 30.8%, p = 0.003) while no difference between mutated or wild-type SMC3 was found for other alterations. CR rate did not differ neither for patients with SMC1A mutations nor patients with SMC3 mutations when compared to wildtype patients. With regard to survival times, again no difference was found both for patients with SMC1A-mutated vs. SMC1Awildtype AML (Fig. 3C-E) as well as patients with SMC3 mutations when compared to their wildtype counterparts (Fig. 4C-E). Further analysis with respect to clonality (dominant vs. subclonal) of the specific mutations did not show any differences for CR rates, EFS, RFS, or OS both for AML with mutated SMC1A and SMC3
Graphic representation of the domain structure of RAD21 and positions of RAD21 mutations in 51 AML patients (A). Mutations of RAD21 are categorized by function (missense=blue, termination=red), clonal rank (dominant=red/ subclonal=blue), number of mutations, and variant allele fraction, and associated co-mutations (B). For detailed information on co-mutations and results of individual significance tests, see Tab. S10. Survival analysis using the Kaplan-Meier method and logrank test for event-free (C), relapse-free (D) and overall survival (E) differentiating between RAD21 -mutated and RAD21 -wildtype AML.
Discussion
for both) in this model. Regarding survival times, multivariable models adjusted for mutations of BCOR, RUNX1, and ASXL1 also showed no independent impact of mutated STAG2 on EFS, RFS, and OS. Contrastingly, mutated RUNX1 showed significantly increased HRs for EFS, RFS, OS, while mutated ASXL1 showed significantly increased HRs for EFS and OS, and mutated BCOR showed a significantly increased HR only for EFS in these multivariable models.
AML with mutated RAD21 shows a
distinct co-mutational pattern while RAD21 mutational status does not influence outcome
The second most common alteration was RAD21 which was detected in 51 patients (3.2%), again mostly being nonsense (n = 35, 68.6%) rather than missense mutations (n = 16, 31.4%, Fig. 2A). Further, alterations of RAD21 were mostly dominant (n = 36, 70.6%). With respect to baseline patient characteristics, patients harboring RAD21 mutations showed
significantly increased LDH (median 705.0 U/l vs. 440.2 U/l, p < 0.001) upon initial diagnosis. Patients with mutated RAD21 were most frequently categorized within the ELN2022 favorable risk group (51.0% vs. 35.2%, p = 0.037), while categorization in the ELN2022 adverse risk group was less prevalent (15.7% vs. 37.0%, p = 0.001). Concordantly, patients with mutated RAD21 commonly had normal karyotypes (70.6% vs. 50.8%, p = 0.009), while complex aberrant karyotypes were rare (2.0 vs. 12.0%, p = 0.009). Common co-mutations compared to patients with wildtype RAD21 were alterations in EZH2 (9.8% vs. 3.7%, p = 0.046), KRAS (13.7% vs. 5.0%, p = 0.016), CBL (7.8% vs. 1.8%, p = 0.017), and NPM1 (56.9% vs. 30.4%, p < 0.001, Fig. 2B). Mutated RAD21 was mutually exclusive with mutated SMC3, SMC1A and STAG2 as well as TP53 (p = 0.047). With respect to patient outcome, we found no differences in CR
rate, EFS, RFS and OS for patients with mutated vs. wildtype RAD21 in general. Clonality of mutated RAD21 (dominant clone vs. subclonal) did not affect CR rate, EFS, RFS or OS.
AML with either mutated SMC3 or SMC1A does not differ from SMC3- or SMC1A-wild-type AML regarding clinical presentation, co-mutations, and outcome
Twenty-five patients (1.5%) harbored alterations in SMC1A, while mutated SMC3 was found in 20 patients (1.2%). Alterations in both SMC1A and SMC3 were only detected as missense mutations (Fig. 3A, Fig. 4A) and the majority was found in the dominant clone (SMC1A: 60.0%, SMC3: 55.0%). There were neither differences in baseline clinical characteristics between patients with SMC1Amutated vs. SMC1A-wild-type AML nor between patients with SMC3-mutated vs. SMC3wild-type AML. With respect to co-mutations, patients harboring mutated SMC1A showed
In our retrospective multi-center cohort study in 1615 intensively treated AML patients, we were able to ascertain distinct patterns of changes in cohesin complex genes, identify their association with other recurrent genetic alterations and clinical presentation as well as confirm their mutual exclusivity which may serve as a target for therapeutic approaches. We confirm that mutations in the genes of the cohesin complex are recurrent genetic events in AML with a reported frequency between 5.913.0%(ref.15, 19, 20, 23, 24, 42) which is in line with our cohort where 11.4% of patients harbored an alteration of cohesin complex genes. In accordance with previous studies(ref.15 19 20 23 24 42), we found these mutations to be mutually exclusive with the exception of one patient bearing both mutated STAG2 and SMC3 The inactivation of more than one subunit of the cohesin complex may result in structural collapse of topologically associated domains which may explain the mutual exclusivity of these gene alterations.4 STAG2 may form the exception as it has a functional homologue in STAG1 that can potentially compensate for its malfunction in three-dimensional genome organization.29 In the early days of research into the role of the cohesin complex in
62 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
When choosing a treatment for adult patients with Ph+ CML resistant or intolerant to prior therapy 1

EXPAND YOUR EXPECTATIONS of long-term BOSULIF treatment









BOSULIF IS INDICATED FOR THE TREATMENT OF ADULT PATIENTS WITH:1



































































• newly-diagnosed chronic phase (CP) Philadelphia chromosome-positive chronic myelogenous leukaemia (Ph+ CML).


• CP, accelerated phase (AP), and blast phase (BP) Ph+ CML previously treated with one or more tyrosine kinase inhibitor(s) [TKI(s)] and for whom imatinib, nilotinib and dasatinib are not considered appropriate treatment options.

MARKETING AUTHORISATION HOLDER:








Rapid and deep molecular responses compared with imatinib
safety profile, comparable to imatinib, that shows low impact on cardiovascular health over




Once-daily dosing, taken with food
LEGAL CATEGORY S1A.
FURTHER INFORMATION IS AVAILABLE UPON REQUEST
Bosulif® (bosutinib)





















Prescribing Information: Please refer to the Summary of Product Characteristics (SmPC) before prescribing Bosulif 100 mg or 500 mg film-coated tablets.
Presentation: Film-coated tablet containing 100 mg or 500 mg bosutinib (as monohydrate).





Indications: Bosutinib is indicated for the treatment of adult patients with newly diagnosed chronic phase (CP) Philadelphia chromosome-positive chronic myelogenous leukaemia (Ph+ CML) or (CP), accelerated phase (AP), and blast phase (BP) Ph+ CML previously treated with one or more tyrosine kinase inhibitor(s) and for whom imatinib, nilotinib and dasatinib are not considered appropriate treatment options. Dosage: Therapy should be initiated by a physician experienced in the diagnosis and the treatment of patients with CML. Newly- diagnosed CP Ph+ CML: The recommended dose is 400 mg bosutinib daily with food. CP, AP or BP Ph+ CML with resistance or intolerance to prior therapy: The recommended dose of bosutinib is 500 mg taken orally once daily with food. In clinical trials, treatment with bosutinib continued until disease progression or intolerance to therapy. In the Phase 1/2 clinical study in patients with CML who were resistant or intolerant to prior therapy, dose escalations from 500 mg to 600 mg once daily with food were allowed in patients who failed to demonstrate complete haematological response (CHR) by Week 8 or complete cytogenetic response (CCyR) by Week 12 and did not have Grade 3 or higher adverse events possibly-related to the investigational product. In the Phase 3 clinical study in patients with newly diagnosed CP CML treated with bosutinib 400 mg, dose escalations by 100 mg increments to a maximum of 600 mg once daily with food were permitted if the patient failed to demonstrate breakpoint cluster region-Abelson (BCR ABL) transcripts ≤ 10% at Month 3, did not have a Grade 3 or 4 adverse reaction at the time of escalation, and all Grade 2 non-haematological toxicities were resolved to at least Grade 1. In the Phase 4 clinical study in patients with Ph+ CML previously treated with 1 or more TKI(s), dose escalations from 500 mg to 600 mg once daily with food were allowed in patients with unsatisfactory response or with signs of disease progression in the absence of any Grade 3 or 4 or persistent Grade 2 adverse events. For details of dose escalation and dose reduction guidelines for non-haematologic adverse reactions and for haematologic adverse reactions, refer to SmPC section 4.2. Patients with serum creatinine >1.5 x ULN were excluded from CML studies. Increasing exposure (area under curve [AUC]) in patients with moderate and severe renal impairment during studies was observed. For details of dosage in patients with moderate and severe renal impairment please refer to SmPC section 4.2. Caution should be exercised in patients with relevant cardiac disorders and in patients with recent or ongoing clinically significant gastrointestinal disorder (see section 4.4 of SmPC). No specific dose recommendation is necessary in the elderly (> 65 years). Since there is limited information in the elderly, caution should be exercised in these patients. The safety and efficacy of bosutinib in patients under 18 years of age has not been established. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Hepatic impairment. Special warnings and precautions for use:
Treatment with bosutinib is associated with elevations in serum transaminases (ALT, AST). Transaminase elevations generally occurred early in the course of treatment. Patients receiving bosutinib should have liver function tests prior to treatment initiation and monthly for the first 3 months of treatment, and as clinically indicated. Treatment with bosutinib is associated with diarrhoea and vomiting, therefore patients with recent or ongoing clinically significant gastrointestinal disorder should use this medicinal product with caution and only after a careful benefit-risk assessment. Patients with diarrhoea and vomiting should be managed using standard-of-care treatment, including an antidiarrhoeal or antiemetic medicinal product and/or fluid replacement. In addition, these events can also be managed by withholding bosutinib temporarily, dose reduction, and/or discontinuation of bosutinib (see SmPC sections 4.2 and 4.8). The antiemetic agent, domperidone, has the potential to increase QT interval prolongation and to induce “torsade de pointes”- arrhythmias; therefore, co-administration with domperidone should be avoided. It should only be used if other medicinal products are not efficacious. In these situations an individual benefit-risk assessment is mandatory and patients should be monitored for occurrence of QT prolongation.
Treatment with bosutinib is associated with myelosuppression, defined as anaemia, neutropenia, and thrombocytopenia. Complete blood counts should be performed weekly for the first month and then monthly thereafter, or as clinically indicated. Treatment with bosutinib may be associated with fluid retention including pericardial effusion, pleural effusion, pulmonary oedema and/or peripheral oedema. Patients should be monitored and managed using standard-of-care treatment. Elevation in serum lipase has been observed. Caution is recommended in patients with previous history of pancreatitis. Bosutinib
References
1. BOSULIF® Summary of Product Characteristics.
2. Gambacorti-Passerini C, Brümmendorf TH, Kim D-W, et al. Poster presentation 7009 at American Society of Clinical Oncology (ASCO) annual meeting; June 4-8, 2021.





















3. Cortes JE, et al. J Clin Oncol 2018;36:231–237.
4. Gambacorti-Passerini C, et al. Am J Hematol 2014;89:732–742.






may predispose patients to bacterial, fungal, viral or protozoan infections. Automated machine-read QTc prolongation without accompanying arrhythmia has been observed. Bosutinib should be administered with caution to patients who have a history of or predisposition for QTc prolongation, who have uncontrolled or significant cardiac disease including recent myocardial infarction, congestive heart failure, unstable angina or clinically significant bradycardia, or who are taking medicinal products that are known to prolong the QT interval. Monitoring for an effect on the QTc interval is advisable and a baseline ECG is recommended prior to initiating therapy with Bosutinib and as clinically indicated. Hypokalaemia or hypomagnesaemia must be corrected prior to bosutinib administration and should be monitored periodically during therapy.
Treatment with bosutinib may result in a clinically significant decline in renal function in CML patients. A decline over time in estimated glomerular filtration rate (eGFR) has been observed in patients treated with bosutinib in clinical studies. It is important that renal function is assessed prior to treatment initiation and closely monitored during therapy with bosutinib, with particular attention in those patients who have pre-existing renal compromise or in those patients exhibiting risk factors for renal dysfunction, including concomitant use of medicinal products with potential for nephrotoxicity, such as diuretics, angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers and nonsteroidal anti-inflammatory drugs (NSAIDs). According to population pharmacokinetic analyses, Asians had a lower clearance resulting in increased exposure. Therefore, these patients should be closely monitored for adverse reactions especially in case of dose escalation. Bosutinib can induce severe skin reactions such as Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. Bosutinib should be permanently discontinued in patients who experience a severe skin reaction during treatment. Due to the possible occurrence of tumour lysis syndrome (TLS), correction of clinically significant dehydration and treatment of high uric acid levels are recommended prior to initiation of bosutinib (see SmPC section 4.8). Reactivation of hepatitis B (HBV) in patients who are chronic carriers of this virus has occurred after these patients received BCR-ABL tyrosine kinase inhibitors. Some cases resulted in acute hepatic failure or fulminant hepatitis leading to liver transplantation or a fatal outcome. Patients should be tested for HBV infection before initiating treatment with Bosulif. Experts in liver disease and in the treatment of HBV should be consulted before treatment is initiated in patients with positive HBV serology (including those with active disease) and for patients who test positive for HBV infection during treatment. Carriers of HBV who require treatment with Bosulif should be closely monitored for signs and symptoms of active HBV infection throughout therapy and for several months following termination of therapy. Exposure to direct sunlight or UV radiation should be avoided or minimised due to the risk of photosensitivity associated with bosutinib treatment. Patients should be instructed to use measures such as protective clothing and sunscreen with high SPF. The concomitant use of bosutinib with strong or moderate CYP3A inhibitors/inducers should be avoided as an increase/decrease in bosutinib plasma concentration will occur. Grapefruit products, including grapefruit juice and other foods that are known to inhibit CYP3A should be avoided. Drug interactions: The concomitant use of bosutinib with strong CYP3A inhibitors (including, but not limited to itraconazole, ketoconazole, posaconazole, voriconazole, clarithromycin, telithromycin, nefazodone, mibefradil, indinavir, lopinavir/ritonavir, nelfinavir, ritonavir, saquinavir, boceprevir, telaprevir, grapefruit products including grapefruit juice) or moderate CYP3A inhibitors (including, but not limited to fluconazole, ciprofloxacin, erythromycin, diltiazem, verapamil, amprenavir, atazanavir, darunavir/ritonavir, fosamprenavir, aprepitant, crizotinib, imatinib) should be avoided, as an increase in bosutinib plasma concentration will occur. Refer to section 4.5 of the SmPC for further details. If a strong or moderate CYP3A inhibitor must be administered during bosutinib treatment, an interruption of bosutinib therapy or a dose reduction in bosutinib should be considered. The concomitant use of bosutinib with strong CYP3A inducers (including, but not limited to carbamazepine, phenytoin, rifampicin, St. John’s Wort), or moderate CYP3A inducers (including, but not limited to bosentan, efavirenz, etravirine, modafinil, nafcillin) should be avoided, as a decrease in bosutinib plasma concentration will occur. Caution should be exercised when administering bosutinib concomitantly with proton pump inhibitors (PPIs). Short-acting antacids should be considered as an alternative to PPIs and administration times of bosutinib and antacids should be separated (i.e. take bosutinib in the morning and antacids in the evening) whenever possible. Bosutinib should be used with caution in patients who have or may
Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Bruxelles, Belgium. MARKETING
develop prolongation of QT, including those patients taking anti-arrhythmic medicinal products or other medicinal products that may lead to QT prolongation. Refer to sections 4.4 and 4.5 of the SmPC for further details. Fertility, pregnancy and lactation: Not recommended in pregnancy or whilst breast feeding. Bosutinib has the potential to impair reproductive function and fertility. Men being treated with bosutinib are advised to seek advice on conservation of sperm prior to treatment because of the possibility of decreased fertility due to therapy with bosutinib. Driving and operating machinery: Bosutinib has no or negligible influence on the ability to drive and use machines. Undesirable effects: Very common adverse events are: respiratory tract infection (including lower respiratory tract infection, respiratory tract infection viral, upper respiratory tract infection, viral upper respiratory tract infection), nasopharyngitis, thrombocytopenia (including platelet count decreased), neutropenia (including neutrophil count decreased), anaemia (including haemoglobin decreased, red blood cell count decreased), decreased appetite, dizziness, headache, pleural effusion, dyspnoea, cough, diarrhoea, vomiting, nausea, abdominal pain (including abdominal discomfort, abdominal pain lower, abdominal pain upper, abdominal tenderness, gastrointestinal pain), rash (including rash macular, rash maculo-papular, rash papular, rash pruritic), pruritus, arthralgia, back pain, oedema (including eyelid oedema, face oedema, generalised oedema, localised oedema, oedema peripheral, periorbital oedema, periorbital swelling, peripheral swelling, swelling, swelling of eyelid), pyrexia, fatigue (including asthenia, malaise), lipase increased (including hyperlipasaemia), alanine aminotransferase increased (including alanine aminotransferase abnormal), aspartate aminotransferase increased, blood creatinine increased. Commonly reported adverse events are: pneumonia (including atypical pneumonia), pneumonia bacterial, pneumonia fungal, pneumonia necrotising, pneumonia streptococcal), influenza (including Influenza H1N1), bronchitis, leukopenia (including white blood cell count decreased), drug hypersensitivity, dehydration, hyperkalaemia (including blood potassium increased), hypophosphataemia (including blood phosphorus decreased), dysgeusia, tinnitus, pericardial effusion, hypertension (including blood pressure increased, blood pressure systolic increased, essential hypertension, hypertensive crisis), pulmonary hypertension (including pulmonary arterial hypertension, pulmonary arterial pressure increased), respiratory failure, gastrointestinal haemorrhage (including anal haemorrhage, gastric haemorrhage, intestinal haemorrhage, lower gastrointestinal haemorrhage, rectal haemorrhage, upper gastrointestinal haemorrhage), pancreatitis (including pancreatitis acute), gastritis, hepatotoxicity (including hepatitis, hepatitis toxic, liver disorder), hepatic function abnormal (including hepatic enzyme increased, liver function test abnormal, liver function test increased, transaminases increased), photosensitivity reaction (including polymorphic light eruption), urticaria, acne, myalgia, acute kidney injury, renal failure, renal impairment, chest pain (including chest discomfort), pain, electrocardiogram QT prolonged (including long QT syndrome), amylase increased (including hyperamylasaemia), blood creatine phosphokinase increased, gamma glutamyltransferase increased, blood bilirubin increased (including hyperbilirubinaemia, bilirubin conjugated increased, blood bilirubin unconjugated increased). Refer to section 4.8 of the SmPC for further information on side effects, including description of selected adverse reactions.
Legal Category: Product subject to prescription which may not be renewed (A): S1A. Marketing
Authorisation Numbers: EU/1/13/818/001 – 100 mg (28 tablets); EU/1/13/818/003 – 500 mg (28 tablets).
Marketing Authorisation Holder: Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Bruxelles, Belgium.
For further information on this medicine please contact: Pfizer Medical Information on 1800 633 363 or at medical.information@pfizer.com. For queries regarding product availability please contact: Pfizer Healthcare Ireland, Pfizer Building 9, Riverwalk, National Digital Park, Citywest Business Campus, Dublin 24 + 353 1 467 6500.
Last revised: 04/2022.


Ref: BO 16_0.




PP-BOS-IRL-0182
Date of Prep: July 2023
AUTHORISATION NUMBER(S): Bosulif 100 mg lm-coated tablets EU/1/13/818/001 EU/1/13/818/002 EU/1/13/818/005 Bosulif 400 mg lm-coated tablets EU/1/13/818/006 EU/1/13/818/007 Bosulif 500 mg lm-coated tablets EU/1/13/818/003 EU/1/13/818/004
5. Cortes JE, et al. Presented at the American Society of Clinical Oncology (ASCO) Annual Meeting, 2018; Oral presentation 7002. 6. Gambacorti-Passerini C, et al. Haematologica 2018;103:1298–1307. 7. Valent P, et al. Blood 2015;125:901–906. * Low cardiac events occurrence defined as 5.2% for BOSULIF® vs 5.3% for imatinib in clinical trials3
1,2
Oncology Focus: AML

Graphic representation of the domain structure of SMC1A and positions of SMC1A mutations in 25 AML patients (A). Mutations of SMC1A are categorized by function (missense=blue, termination=red), clonal rank (dominant=red/ subclonal=blue), number of mutations, and variant allele fraction, and associated co-mutations (B). For detailed information on co-mutations and results of individual significance tests, see Tab. S13. Survival analysis using the Kaplan-Meier method and logrank test for event-free (C), relapse-free (D) and overall survival (E) differentiating between SMC1A-mutated and SMC1A-wildtype AML.
carcinogenesis, it has been hypothesized that its malfunction may lead to aberrant segregation of sister chromatids and consequently aneuploidy as a major driver in neoplastic transformation.8 However, especially studies investigating mutations of the cohesin complex in myeloid neoplasms have refuted this claim since these alterations are commonly found in AML with euploid karyotypes.20, 24, 42 Correspondingly, the rate of patients with normal karyotypes in our cohort was significantly increased while the rate of complex aberrant karyotypes was significantly decreased for patients with cohesin-mutated AML. These findings suggest alternate contributions of cohesin in carcinogenesis rather than mere aneuploidy and chromosomal instability. Alterations of cohesin subunit genes have both been described as early and late events in
leukemogenesis17 20 24 31 32 43 suggesting a passenger rather than a driver function. However, in our cohort, the majority of cohesion complex mutations were detected in dominant clonal constellations, pointing at a potential role as an early event during AML initiation. Likewise, cohesin plays an important role in regulating the stemness and pluripotency of stem cells.31,32,33 Thus, an interplay of alterations of cohesin genes with other genetic events in driver genes such as NPM1 likely promotes malignant transformation. Mutated NPM1 has been associated with alterations of cohesin complex genes.19, 20 An interaction of cohesin proteins with NPM1 could be mediated by CCCTC-binding factor— a transcription factor that regulates tumor suppressor loci—which has been shown to bind and interact with both,44 45 potentially contributing to their
role in stem cell self-renewal.46 47 In our cohort, we found mutated SMC3 and RAD21 to be associated with mutated NPM 1 while NPM1 mutations were less frequently associated with mutated STAG2. In comparison to their wildtype counterparts, patients with mutated STAG2 more frequently also had mutated IDH2, TET2, BCOR, ASXL1, SRSF2, and ZRSR2. Further, patients with alterations of RAD21 showed increased rates of co-occurring mutations in EZH2, KRAS, and CBL besides NPM1. An association of cohesin mutations wit mutated TET2, ASXL1, BCOR, and EZH2 has previously been reported,24 42 however, it is important to note that different subunits of the cohesin complex show different co-mutational patterns.
The prognostic impact of cohesin mutations in AML has been unclear as studies are not only limited but also report
conflicting results. Tsai et al.19 report increased OS and disease-free survival for patients with cohesin mutations, which was confirmed by multivariable analysis in a cohort of 391 patients with de novo AML. In contrast, Thol et al.20 found no impact of cohesin mutations on CR rate, RFS, and OS in a cohort of 389 intensively treated AML patients. In MDS, Thota et al.24 reported decreased OS for patients with cohesin mutations, especially in STAG2-mutated MDS for patients who survived beyond 12 months. Commonly, previous studies were limited in sample size, often ranging between 300 and 600 patients. In our comparatively large cohort of 1615 intensively treated AML patients, we did not find a significant impact of any gene alterations of cohesin subunits on CR rate, EFS, RFS, or OS. The recently revised ELN2022 recommendations3 introduce mutated STAG2 as a prognostic marker of adverse risk (if no markers of favorable risk are co-occuring). Multivariable models adjusted for mutation status of BCOR, ASXL1, and RUNX1, which were more prevalent in STAG2-mutated AML patients, demonstrated no independent impact of mutated STAG2 on patient outcome while these comutations had varying individual prognostic impact. Several reports agree that STAG2 mutations are associated with sAML, and thus, as a part of corresponding compound attributes they are associated with the overall adverse impact of sAML on outcome.17 43 However, these mutations contribute only a minor part of this compound attributes. According to our observations such an adverse effect on outcome cannot be verified for the presence of STAG2 mutations per se. While the cohesin complex undoubtably plays a role in leukemogenesis, given the ambiguity of existing reports on cohesin’s (and STAG2’s) role in AML prognostication19, 20, 24 caution may be warranted with respect to determining patient risk and ultimately treatment allocation. Nevertheless, it should be acknowledged that our study is limited by the fact that results are only available for intensively treated patients. The extent to which the reported results are also transferable to patients which receive less intensive regimens or targeted therapy remains to be evaluated.
64 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Graphic representation of the domain structure of SMC3 and positions of SMC3 mutations in 20 AML patients (A). Mutations of SMC3 are categorized by function (missense=blue, termination=red), clonal rank (dominant=red/ subclonal=blue), number of mutations, and variant allele fraction, and associated co-mutations (B). For detailed information on co-mutations and results of individual significance tests, see Tab. S14. Survival analysis using the Kaplan-Meier method and logrank test for event-free (C), relapse-free (D) and overall survival (E) differentiating between SMC3-mutated and SMC3-wildtype AML.
While the prognostic impact of cohesin alterations in AML remains elusive, their co-mutational pattern with respect to mutual exclusivity may make them a viable option for targeted therapy. Mutually exclusive gene alterations may be utilized therapeutically via synthetic lethality.48 If the alteration of one mutated gene provides a cancerous cell with a survival advantage as long as a second gene remains unaltered, the alteration or inhibition of the second gene or its gene product may confer apoptosis specifically in cells carrying the initial alteration.49, 50 Synthetic lethality via inhibition of mediators of replication fork stability such as poly ADP-ribose polymerase (PARP) has been demonstrated in BRCA-mutated breast, ovarian, pancreatic, and prostate cancer.51 The functional homologues STAG1 and 2 offer the possibility for a synthetically lethal therapeutic strategy via PARP inhibition. In glioblastoma cells, Bailey et al.52 have demonstrated
that mutated STAG2 significantly increases the sensitivity to PARP inhibition. Further, Black et al.53 found STAG2-deficient leukemic cells to bear a significantly higher susceptibility to treatment with talazoparib. Currently, a phase 1 study is ongoing investigating the safety and efficacy of talazoparib for cohesin-mutated AML and MDS with excessive blasts (NCT03974217).54
In summary, we report distinct co-mutational and clinical patterns for mutated STAG2 and RAD21 in a large sample of AML patients while mutated SMC3 and SMC1A lacked such patterns. However, no cohesin subunit—including mutated STAG2 that was recently added to the ELN2022 criteria as a marker of adverse risk—showed any impact on patient outcome regarding the achievement of CR, EFS, RFS, or OS. While we did not find a prognostic impact of cohesin alterations in AML, their mutual exclusivity may make them a potential target for therapeutic approaches based on synthetic lethality.
References available on request
Author information
Author notesThese authors contributed equally: Jan-Niklas Eckardt, Sebastian Stasik, Jan Moritz Middeke, Christian Thiede.
Authors and Affiliations
1. Department of Internal Medicine I, University Hospital Carl Gustav Carus, Dresden, GermanyJan-Niklas Eckardt, Sebastian Stasik, Christoph Röllig, Frank Kroschinsky, Johannes Schetelig, Martin Bornhäuser, Jan Moritz Middeke & Christian Thiede
2. Division of Health Care Sciences, Dresden International University, Dresden, Germany - Jan-Niklas Eckardt, Tiago Cerqueira & Timo Siepmann
3. German Cancer Research Center (DKFZ) and Medical Clinic V, University Hospital Heidelberg, Heidelberg, Germany - Tim Sauer & Carsten Müller-Tidow
4. Department of Internal Medicine II, Jena University Hospital, Jena, Germany - Sebastian Scholl & Andreas Hochhaus
5. Department of Hematology, Oncology, Hemostaseology, and Cell Therapy, University Hospital RWTH Aachen, Aachen, Germany - Martina Crysandt & Tim H. Brümmendorf
6. Medical Clinic III, St. MarienHospital Siegen, Siegen, Germany - Ralph Naumann
7. Medical Clinic II, University Hospital Frankfurt, Frankfurt (Main), Germany - Björn Steffen & Hubert Serve
8. Medical Clinic and Policlinic II, University Hospital Würzburg, Würzburg, Germany - Volker Kunzmann & Hermann Einsele
9. Department of Hematology, Oncology and Palliative Care, Rems-Murr-Hospital Winnenden, Winnenden, GermanyMarkus Schaich
10. Department of Hematology, Oncology and Immunology, Philipps-University-Marburg, Marburg, Germany - Andreas Burchert & Andreas Neubauer
11. Department of Internal Medicine V, Paracelsus Medizinische Privatuniversität and University Hospital Nurnberg, Nurnberg, Germany - Kerstin Schäfer-Eckart
12. Department of Medicine A, University Hospital Münster, Münster, Germany - Christoph Schliemann & Wolfgang E. Berdel
13. Medical Clinic V, University Hospital Erlangen, Erlangen, GermanyStefan W. Krause
14. Medical Clinic III, Chemnitz Hospital AG, Chemnitz, GermanyRegina Herbst & Mathias Hänel
15. Department of Hematology, University Hospital Essen, Essen, Germany - Maher Hanoun
16. Medical Clinic II, St. Bernward Hospital, Hildesheim, GermanyUlrich Kaiser
17. Department of Hematology, Oncology and Palliative Care, Robert-Bosch-Hospital, Stuttgart, Germany - Martin Kaufmann
18. Department of Internal Medicine, Hematology and Oncology, Masaryk University Hospital, Brno, Czech Republic - Zdenek Rácil & Jiri Mayer
19. Medical Clinic I Hematology and Celltherapy, University Hospital Leipzig, Leipzig, GermanyUwe Platzbecker
20. Department of Internal Medicine, University Hospital Kiel, Kiel, Germany - Claudia D. Baldus
21. DKMS Clinical Trials Unit, Dresden, Germany - Johannes Schetelig

22. Department of Neurology, University Hospital Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany - Timo Siepmann
23. German Consortium for Translational Cancer Research DKTK, Heidelberg, Germany - Martin Bornhäuser
24. National Center for Tumor Disease (NCT), Dresden, GermanyMartin Bornhäuser
65 HOSPITALPROFESSIONALNEWS.IE | HPN • SEPTEMBER 2023
Oncology Focus: Treatment

Empowering Lung Cancer Care: RNA Therapeutics Expand Treatment Frontiers

 Written by Zekiye Altan1, Dylan Harvey1, Rory Johnson1,2,*
Written by Zekiye Altan1, Dylan Harvey1, Rory Johnson1,2,*
1School of Biology and Environmental Science, University College Dublin, Dublin D04
V1W8, Ireland
2Conway Institute for Biomolecular and Biomedical Research, University College Dublin, Dublin D04
V1W8, Ireland
*Correspondence to: rory.johnson@ucd.ie

The primary challenges in treating lung cancer revolve around therapy resistance, the potential for toxic side effects, and the lack of available targeted therapies (Figure 1). In this context, the need to find more effective solutions against the challenges of lung cancer treatment has become inevitable. The limitations in treatment options, especially for advancedstage lung cancer patients, have necessitated the development of novel and effective approaches. This article will discuss a promising new type of therapy targeting a fundamental yet often overlooked molecule - RNA- and its potential to overcome these challenges in lung cancer treatment.
Challenges in Lung Cancer Treatment
Lung cancer stands as the leading cause of cancer-related deaths, both in Ireland and worldwide, making it a prominent contributor to the global burden of cancer mortality. Particularly, factors such as tobacco usage, exposures to substances like asbestos and radon, are among the key risk factors in the development of lung cancer. While therapy resistance remains a primary concern, conventional approaches such as surgical procedures, radiotherapy, and chemotherapy can result in notable success for select patients. However, a frequent outcome is the emergence of severe side effects, which can significantly diminish the quality of life for many individuals. Additionally, the effectiveness of these treatment methods remains limited, particularly for those with advanced-stage lung cancer, leading to reduced survival rates.
66 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Zekiye Altan
Dylan Harvey
Rory Johnson
Figure 1. Current treatment approaches and future therapeutic perspectives for lung cancer therapy
RNA Therapeutics: A Promising Frontier in Lung Cancer Treatment
In recent years, an exciting new therapeutic modality has emerged in the form of ‘RNA therapeutics’ (RNATX). RNA, typically associated with messenger RNA (mRNA), are often thought to be transient molecules derived from proteincoding genes which are translated into proteins in our cells. However, another class of RNA molecules called long noncoding RNA (lncRNA) which do not encode for proteins and far outnumber such protein-coding genes, exist and function as mature RNA molecules throughout our cells. RNA therapeutics is based upon a core feature of nucleic acids – sequence complementarity, allowing for precise targeting of gene targets. RNATX encompasses strategies to target cellular nucleic acids using, for example, antisense oligonucleotides (ASOs), which are artificially created therapeutic RNA molecules, introduced into cells to specifically target cancer-associated RNA, including both mRNA (protein-coding) and lncRNA (non-protein-coding). The general goal of RNATX is either to deplete cancer-promoting genes, or provide tumor-suppressing RNA molecules for therapy. In all cases, artificially created therapeutic RNA are delivered to the patient through a variety of means such as systemic delivery as required for lung cancer.
RNATX induces and orchestrates a variety of cellular responses. These responses range from guiding protein synthesis and disrupting conventional protein production to serving as targeted agents, all contingent upon the specific RNA sequence utilized. The spotlight on RNATX has intensified, particularly due to its remarkable success in swiftly generating COVID-19 vaccines. However, it's worth noting that the potential of RNATX extends far beyond vaccines. For instance, in the realm of cancer treatment, RNATX presents a remarkable case as seen through the application of ASOs in targeting the well-explored oncogenic JAK-STAT pathway. Researchers undertook this approach in the context of patients diagnosed with diffuse large B-cell lymphoma, resulting in impressive efficacy and safety outcomes.
RNATX presents a host of advantages over conventional treatments, including costeffectiveness, minimal side effects, and the precision targeting of tumor cells. This, in turn, carries the potential to usher in a paradigm shift in lung cancer

treatment, which urgently requires novel therapeutic approaches.
The allure of RNATX in lung cancer treatment lies in its potential to surmount drug resistance and unintended off-target interactions, both key issues in lung cancer treatment. In contrast to traditional chemotherapy or radiation which often influences both healthy and cancerous cells, RNATX, can be engineered to zero in on highly expressed lncRNA genes, primarily found in cancer cells. LncRNA genes although vast in number (over 50,000) still remain poorly understood. They are positioned as ideal targets for RNATX as they exhibit higher levels of cell and cancer specificity, compared to mRNA or protein-coding genes. Both the level of gene targeting precision and tumor-specificity holds the promise of minimizing collateral damage to healthy tissues, thus elevating the overall quality of life for patients.
Moreover, the adaptable nature of RNATX provides a versatile platform, capable of being tailored to various types of lung cancer, accommodating even specific genetic mutations within tumors. This adaptability is especially pivotal in tackling the heterogeneous nature of lung cancer, where tumors exhibit diverse genetic profiles and distinct responses to treatments. Hence, RNATX may be fashioned into a truly ‘personalized’ therapy for every individual tumor.
Nevertheless, every type of therapy depends on identifying targets, or vulnerabilities in the diseased cell. Despite the significant promise held by RNATX, identifying optimal molecular
targets for these therapies remains a challenge due to our incomplete knowledge of the disease. The selection of appropriate target genes or proteins is pivotal to ensure treatment effectiveness while mitigating the risk of undesired outcomes.
To navigate these challenges, selecting optimal ASO therapy targets demands critical criteria. These targets should play vital roles in lung cancer tumorigenesis, remain exclusive to cancer cells, be susceptible to ASO intervention, and boast robust validation through extensive studies. Furthermore, these targets should exhibit minimal toxicity and demonstrate the potential to synergize effectively with other treatment modalities. This process necessitates comprehensive research to unravel the molecular pathways driving lung cancer progression, identifying critical genes contributing to tumor growth, metastasis, and treatment resistance.
This is where the application of advanced technologies such as high-throughput sequencing, bioinformatics, and computational modeling comes into play. By identifying genes frequently mutated in a significant portion of lung cancer patients, such as EGFR, ALK, KRAS, and PD-L1, or pathways unique to lung cancer cells, scientists can meticulously devise RNA-based interventions that disrupt these processes with precision. This could potentially trigger tumor regression or even eradication.
In summary, RNATX offer a targeted and adaptable approach
that holds immense promise for revolutionizing lung cancer treatment by minimizing side effects and optimizing patient outcomes. Yet, unlocking this potential hinges on a profound understanding of lung cancer biology and the meticulous identification of optimal therapeutic targets through cutting-edge technological methods. This could mark the dawning of a transformative era in effective and personalized lung cancer treatments.
LncRNAs in Cancer: Unraveling Roles and Therapeutic Avenues
In the unfolding narrative, long non-coding RNAs (lncRNAs) emerge as promising RNATX targets, representing a vast class of RNA molecules that primarily do not encode proteins. This challenges the conventional notion that RNAs predominantly act as intermediaries for protein synthesis, as stipulated by the central dogma. Unlike the role of messenger RNA (mRNA) in relaying genetic instructions from DNA to proteins, lncRNAs instead exist as functional, mature RNA molecules. LncRNAs bring to light the intricacies and diversity inherent in the genetic landscape. Despite their limited or absent protein-coding capacity, lncRNAs wield a multifaceted influence across various cellular processes. Through modulation of gene expression, interaction with other RNA molecules, and orchestration of intricate regulatory networks,
67 HOSPITALPROFESSIONALNEWS.IE | HPN • SEPTEMBER 2023
Figure 2. Regulatory mechanisms of lncRNAs in the cell
lncRNAs unveil a comprehensive layer of genetic control extends beyond than that of proteincoding genes.
LncRNAs have been shown to possess a wide spectrum of regulatory functions, from chromosome dosage compensation to modulation of chromatin states, cell differentiation, and basic cellular functions (Figure 2). However, the specific functions and mechanisms of many of these lncRNAs remain unclear. At this juncture, further research is needed to better comprehend the role of lncRNAs in cancer.
In the realm of cancer biology, the allure of lncRNAs lies not only in their potential to expand our understanding of basic disease processes, but also in their potential as groundbreaking therapeutic targets. Herein lies the question of paramount importance: why are lncRNAs more attractive therapeutic prospects than widelystudied proteins? The answer lies in their unique attributes. Generally, lncRNAs are more cell-type and cancer-specific than protein genes. They also outnumber protein genes over 2.5 times and have more specific downstream genetic regulatory networks. These attributes place lncRNAs as ideal candidates for precision RNATX due to the
Oncology Focus: Treatment

intrinsic specificity of both their expression and function, as compared to proteins. The ability to orchestrate multiple processes simultaneously grants them an unprecedented level of influence over cellular behavior. Moreover, lncRNAs can serve as central regulators, modulating entire cascades of gene expression, thereby holding the power to delicately balance aberrant cellular states – a feat that proteins alone often do not to accomplish.
LncRNAs play a key role in finely modulating gene expression, determining the phenotype of cancer cells, and influencing regulation of cellular processes. Aberrant lncRNA expression in cancer cells has been shown to impact fundamental cancer mechanisms such as cellular proliferation, apoptosis, invasion, and metastasis. Furthermore, lncRNAs can intricately regulate signaling pathways and precisely cell cycle regulation, thereby influencing the tumor microenvironment. These critical findings underscore the potential of lncRNA differential expression in cancer as viable therapeutic targets.
The development of lncRNAbased therapeutics holds a crucial role in lung cancer treatment. These approaches offer a tailored strategy that aims to disrupt
cancer cell functions, halt growth and spread, while also targeting treatment-resistant cells. The specific high expression of oncogenic lncRNAs in cancer cells indicates their potential use as therapeutic targets, with the potential to minimize side effects by sparing non-tumor cells.
While previous studies have identified a range of differentially expressed lncRNAs in lung cancer, our knowledge of their specific functions in lung cancer remains limited and requires further investigation.
Targeting lncRNAs with ASOs
Traditionally, targeting RNA molecules with small molecule inhibitors has been challenging due to a limited understanding of the dynamic tertiary structures of RNA molecules. However, ASOs offer a promising avenue for lncRNA targeting. ASOs are synthetic RNA molecules designed solely based on sequence information, capable of specifically acting on genes or transcripts using principles of sequence complementarity.
Mechanisms of ASOs
ASOs can modulate target RNA levels through various mechanisms. Some mechanisms involve directing the activity of RNase H for RNA degradation
of the target genes, while others inhibit translation (with mRNA) or biogenesis of the target RNA. Additionally, ASOs can impact RNA splicing, polyadenylation, and cellular localization (Figure 2). The specific mechanism depends on ASO design and therapeutic objectives.
In conclusion, utilizing ASOs to target lncRNAs presents a promising strategy in cancer treatment. The distinct attributes of lncRNAs and the flexibility of ASOs position them as compelling candidates for therapeutic interventions. Preclinical and clinical investigations provide evidence of the potential of lncRNA-targeted therapies. Ongoing research holds the potential to develop effective treatments for cancer and other diseases, harnessing the capabilities of lncRNAs and ASOs.
ASO-Based Approaches and Their Clinical Implications
Despite the formidable challenges encountered in developing lncRNA-based therapies, our research underscores substantial advancements in this field. Leveraging ASOs and CRISPR-based methods, we
68 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Figure 3. Targeting of LncRNAs with ASOs Therapy to Cancer Treatment
Advantages of ASO-Based Therapy
Specificity: ASOs can be tailored for high specificity towards the target RNA, minimizing off-target effects.
Potency: ASOs can exhibit high potency, effectively reducing target RNA levels.
Allele-Specific Suppression: ASOs can discern between transcripts differing by a single nucleotide, enabling allelespecific suppression of mutant genes.
Versatility: ASOs can influence both rare and highly abundant transcripts, including specific splice variants and noncoding RNAs.
Summary of ASO-based Cancer Treatment
Overview of ASO Therapy
Clinical Trials and Preclinical Studies
ASOs have shown promising results in clinical trials for various diseases.

Kynamro® is an ASO drug targeting ApoB, FDA-approved for treating homozygous familial hypercholesterolemia.
Currently, more than 30 ASO drugs, including those targeting lncRNAs, are in preclinical or clinical testing.
ASOs in Cancer Treatment
Our preclinical studies have demonstrated the potential of ASO-based therapies to effectively reduce specific lncRNA levels.
Our findings reveal that ASO-mediated knockdown of lncRNAs can regulate key cancer-related phenotypes in NSCLC cells and 3D lung cancer models.
Future Directions and Challenges
A deeper understanding of lncRNA functionality is essential.
Design and optimization of ASOs are critical for effective targeting. Advances in ASO chemistry and delivery systems are pivotal.
Novel delivery strategies, such as nanoparticle-based systems, can enhance ASO-based therapeutic efficiency.
harness targeted precision to modulate lncRNA expression, potentially mitigating side effects. Nonetheless, it's imperative to acknowledge inherent obstacles, encompassing effective ASO delivery to target tissues, the risk of unintended interactions leading to off-target effects, and the potential for immune responses following therapy. Concurrently, the evolution of nanoparticlebased delivery systems and other innovative technologies holds promise in surmounting these challenges, thereby amplifying the efficacy, specificity, and overall safety of lncRNA-based therapeutics. Navigating these intricacies demands a fusion of cutting-edge science and unwavering dedication, propelling transformative strides in patient care and treatment paradigms.
ASO therapy extends its potential across a spectrum unmet medical needs. Notably, it shows promise in tackling neurodegenerative disorders like spinal muscular atrophy and ALS, genetic anomalies such as Duchenne muscular dystrophy and cystic fibrosis, and even cancer, where ASOs could perturb tumor growth by precisely targeting oncogenes. In the realm of cancer, numerous lncRNAs have surfaced as viable therapeutic targets across diverse cancer types, with encouraging preclinical findings. For
instance, MALAT1 and HOTAIR, implicated in lung and breast cancer progression respectively, exemplify potential targets for tailored treatment approaches. Clinical trials validating the reliability and effectiveness of lncRNA-based interventions are imperative to substantiate their worth in cancer therapy.
ASOs, as versatile therapeutic tools, can be administered through various routes based on the target tissue. Intravenous (IV) injection is a common method for achieving systemic delivery, reaching multiple organs and tissues, including the lung. Additionally, subcutaneous injection offers a means for localized effects or the precise targeting of specific tissues. This multifaceted administration approach aligns ASO therapies with the diverse requirements of patients and diseases, enhancing their clinical applicability.
In summation, the promise of ASO therapy in addressing an array of maladies via pinpointed genetic and molecular modulation is undeniable. While challenges persist, ongoing research and clinical endeavors propel the field forward, highlighting the potential for ASOs to emerge as invaluable instruments within the medical armamentarium.
Conclusion
As the GOLD Lab team, our transition from the University of Bern to University College Dublin (UCD) has ushered in a new era of exploration, one focused on the untapped potential of lncRNAs as therapeutic targets in cancer, with focus on lung cancer. Through a blend of computational approaches and hands-on experimentation, our goal has been to not only innovate within lncRNA therapeutics but also empower fellow researchers with our insights.
Our journey exploring the genomic dark matter of cancer, particularly non-small cell lung cancer (NSCLC) and KRAS-mutant NSCLC, has been marked by synergy between bioinformatics expertise and cutting-edge CRISPR screens. Our screens encapsulate crucial cancer hallmarks, offering a novel perspective for understanding the complexities of lung cancer. Our unique pipeline, designed from the ground up, identified 80 oncogenic lncRNAs that emerged as overexpressed specifically in NSCLC.
This select group of lncRNAs has not remained a mere discovery but has become the bedrock of our mission—a mission driven by the pursuit of understanding. As we delve into their functional roles, we're unraveling the intricate
choreography that these lncRNAs perform within the complex pathways of tumorigenesis and cancer progression. And as we look toward translation to the clinic, combining cutting-edge technologies with the application of ASOs, we envision breakthroughs in developing targeted therapies at the frontier of RNATX. Our journey, while ambitious, has not been without its challenges. Through the crucible of in vivo and in vitro experiments employing highprecision ASOs, we've validated our curated list of target lncRNAs we had identified. The fusion of data-driven discovery and clinical significance underscores the importance of our research not just as a theoretical exercise, but as a stepping stone toward efficient cancer treatment. We believe in the transformative power of our work, requiring validation through rigorous experimentation, to unlock promising therapeutic avenues in the fight against lung cancer. Our group aims to provide promising ASOs to progress onto clinical trials and further investigations, bridging the gap between our discoveries and tangible patient outcomes. With every confirmed hypothesis and validated ASOs, our efforts progress towards therapies which we hope leads to extended lives, improved quality of life, and elevated survival rates for lung cancer patients.
69 HOSPITALPROFESSIONALNEWS.IE | HPN • SEPTEMBER 2023
HIV Updates
The Role of HIV Viral Suppression
in May 2023 to assess community experiences of the 2022-2023 mpox outbreak in Europe and the Americas.
that work for HIV will also be beneficial for addressing future pandemics."
The World Health Organization (WHO) is releasing new scientific and normative guidance on HIV at the 12th International IAS (the International AIDS Society) Conference on HIV Science.

New WHO guidance and an accompanying Lancet systematic review released recently describe the role of HIV viral suppression and undetectable levels of virus in both improving individual health and halting onward HIV transmission. The guidance describes key HIV viral load thresholds and the approaches to measure levels of virus against these thresholds; for example, people living with HIV who achieve an undetectable level of virus by consistent use of antiretroviral therapy, do not transmit HIV to their sexual partner(s) and are at low risk of transmitting HIV vertically to their children. The evidence also indicates that there is negligible, or almost zero, risk of transmitting HIV when a person has a HIV viral load measurement of less than or equal to 1000 copies per mL, also commonly referred to as having a suppressed viral load.
Antiretroviral therapy continues to transform the lives of people living with HIV. People living with HIV who are diagnosed and treated early, and take their medication as prescribed, can expect to have the same health and life expectancy as their HIV-negative counterparts.
“For more than 20 years, countries all over the world have relied on WHO’s evidence-based guidelines to prevent, test for and treat HIV infection,” said Dr Tedros Adhanom Ghebreyesus, WHO Director-General. “The new guidelines we are publishing will help countries to use powerful tools have the potential to transform the lives of millions of people living with or at risk of HIV.”
At the end of 2022, 29.8 million of the 39 million people living
with HIV were taking antiretroviral treatment (which means 76% of all people living with HIV) with almost three-quarters of them (71%) living with suppressed HIV. This means that for those virally suppressed their health is well protected and they are not at risk of transmitting HIV to other people. While this is a very positive progress for adults living with HIV, viral load suppression in children living with HIV is only 46% - a reality that needs urgent attention.
Here is an overview of other key scientific and normative updates being released by WHO at IAS 2023 conference:
HIV and Mpox
An analysis of global surveillance data reported to WHO during the multi-country outbreak of mpox, identified that among more than 82 000 mpox cases, around 32 000 cases had information on HIV status. Among those, 52% were living with HIV, most being men who have sex with men (MSM); and more than 80% reported sex as the most probable route of getting infected with mpox.
Among 16 000 people diagnosed with mpox and living with HIV, around one quarter (25%) had advanced HIV disease or immunosuppression – leading to an increased risk of hospitalization and death. People living with HIV who were taking HIV treatment and with good immunity had similar hospitalization and death outcomes as those who were HIV negative.
In the light of these findings, WHO recommends countries integrate mpox detection, prevention, and care with existing and innovative HIV and sexually transmitted infection prevention and control programmes.
To understand how to better prepare for and respond to future increases in mpox transmission, WHO led a rapid electronic survey
More than 24,000 people participated in the survey which focused on men who have sex with men, and trans and genderdiverse people, with 16,875 eligible individuals completing the survey. Almost 51% changed their sexual behaviour (such as reducing the number of sexual partners), and 35% had maintained these changes one year later. Findings from this survey provide valuable insights into the experiences and needs of affected communities and emphasize the importance of increasing access to mpox vaccination and diagnostics globally.
HIV and COVID-19
An updated analysis from WHO global clinical platform for COVID-19 up through May 2023 revealed a persistent high risk of death in people living with HIV hospitalized for COVID-19 across pre-Delta, Delta and Omicron variant waves, with an overall in-hospital mortality rate of 20%24%. For people without HIV, the risk of death fell during the Omicron variant wave by 53%— 55% compared to pre-Delta and Delta variant waves; but for people living with HIV, the percentage decline in mortality during the Omicron wave period compared to the other waves was modest (16%-19%). This difference resulted in a 142 times increased risk of death among people living with HIV when compared with people without HIV during the Omicron wave period.
Risk factors for in-hospital death that were common across all variant waves of the pandemic were low CD4 count (less than 200 cells per m3), and severe or critical COVID-19 illness at hospital admission.
“Uncontrolled HIV remains a risk factor for poor outcomes and death in the mpox outbreak and COVID-19 pandemic”, said Dr Meg Doherty, Director of WHO’s Global HIV, Hepatitis and Sexually Transmitted Infections Programmes. "We must ensure the integration of HIV considerations in pandemic preparedness and response. Protecting people living with HIV from future pandemics is vital and reinforces the need to ensure access to HIV testing and treatment and preventive vaccines for mpox and COVID-19 to save lives; community-led responses
Optimizing HIV testing services through expanded testing options and simplified service delivery
With new recommendations on HIV testing, WHO is calling on countries to expand use of HIV self-testing and promote testing through sexual and social networks to increase testing coverage and strengthen uptake of HIV prevention and treatment services in high-burden settings and in regions with the greatest gaps in testing coverage. The recommendation comes at a pivotal time, where self-care and self-testing are increasingly being recognized as ways to increase access, efficiency, effectiveness and acceptability of health care across many different disease areas, including HIV.
Primary health care and HIV
A new policy framework on primary health care (PHC) and HIV will help decision-makers optimize work and collaboration underway to advance primary health care and disease-specific responses, including HIV. In the second year of implementation, the Global Health Sector Strategies on HIV, viral hepatitis and sexually transmitted infections for 2022-2030 actively advocate for synergies within the framework of universal health coverage and primary health care. “Ending AIDS is impossible without optimizing opportunities across and within health systems, including with communities and in the context of primary health care”, said Dr Jérôme Salomon, WHO Assistant Director-General, Universal Health Coverage, Communicable and Noncommunicable Diseases.
This latest research and guidance are being presented at a time when progress towards ending the global AIDS epidemic has lagged, after the COVID-19 pandemic; but the response is rapidly catching up, with some countries now charting a path to end AIDS, including Australia, Botswana, Eswatini, Rwanda, United Republic of Tanzania, and Zimbabwe and 16 other countries that are close to reaching the 9595-95 global targets, which aim for 95% of people living with HIV knowing their status, 95% of those diagnosed receiving ART and 95% of those on treatment having suppressed viral loads.
70 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
“The new guidelines we are publishing will help countries to use powerful tools have the potential to transform the lives of millions of people living with or at risk of HIV.”

Real World Data Tackling Diabetes
 Professor Edward Gregg, Head
Professor Edward Gregg, Head
medical research. These studies assign people at random into different groups for interventions, and then compare a new approach with the standard that is already in place. Over decades, this has led to changes in how to manage diabetes in the clinic, and it has improved quality of life for those living with diabetes.
Smart devices make it easy to track how many steps you take, the meals you eat and even your resting heart rate. But how can we use real-world data to help tackle the global challenge of diabetes?
A recently published review from Professor Edward Gregg, Head of RCSI’s School of Population Health, points to the benefits and
possible pitfalls of bringing data from the real world to bear on the future of managing diabetes.
One-tenth of the world’s adults now have diabetes, whereby the body cannot regulate blood sugar well. Over time this lack of control increases the risk of sight loss, kidney failure, limb amputation and heart disease. In Ireland alone, the number of people living with Type 2 diabetes has almost doubled in the past 15 years, accounting for more than ¤550 million per year in direct healthcare costs.
Traditionally, research on how to help prevent and manage diabetes has relied heavily on randomised clinical trials, a gold standard in
New data sources
However, large, randomised clinical trials are expensive, time-consuming and don’t always tell us what works best in practice and the real world. So, the prospect of using realworld data from patients, such as healthcare records, insurance claims and measurements as well as non-health data sources for information about behaviour or environmental conditions, is tantalising. These data sources can offer a cheaper, faster way to gather information, and often mean that a more diverse group of people and settings can be included in the study.
New Research on Iron Deficiency
New research has emerged highlighting that females are impacted by anaemia to a greater degree than males. The study analysed data from 1990–2021, and spotlighted that in 2021 alone, anaemia affected nearly 2 billion people. Leader of this research, Nick Kassebaum, University of Washington, Seattle, USA, described the motivation for their investigation: “There are still wide disparities when you narrow the focus on geography, gender, and age.”

Findings brought to attention that worldwide in 2021, 31% of
females had anaemia, compared with 18% of males. This gender difference was more pronounced during reproductive years (15–49), where anaemia prevalence was 34% in females and 11% in males. The leading cause of anaemia in 2021 was dietary iron deficiency, constituting 66% of total cases, affecting 825 million females, and 444 million males. Results also spotlighted gynaecological disorders and maternal haemorrhage as important contributors to anaemic burden in females of reproductive age. The main cause
in children under 5 years was also dietary iron deficiency, but haemoglobinopathies, infectious diseases, HIV/AIDS, and malaria were also important contributors in locations where anaemia is most prevalent.
Sub-Saharan Africa and South Asia are currently facing the greatest burden from anaemia; in 2021 the highest prevalence was observed in Western sub-Saharan Africa (47%), South Asia (36%), and Central sub-Saharan Africa (36%). Regions with the lowest anaemia rates were Australasia (6%), Western Europe (6%), and
Even so, the use of real-world data in research requires adapting our current standards for study design and analysis to account for the limitations of these new and non-traditional data sources. For example, researchers have less control over how real-world data is gathered, so the potential impact of bias and missing data needs to be considered in analyses to ensure reliable findings.
Where real-world information stands to make a difference too is in tracking the effectiveness of new evidence-based initiatives in diabetes care when they are introduced: real-world data and evidence will provide insights into how research discoveries make a difference to large populations.
At the School of Population Health, RCSI researchers are bringing their expertise together to strengthen approaches using new data sources. Doing this the right way will continue to unlock the benefits of the data all around us.
North America (7%). Mali, Zambia, and Togo each had greater than 50% anaemic burden, whilst Iceland, Norway, and Monaco all had lower than 5%.
Highlighting the implications of this research for clinicians, speaking about the underlying causes of anaemia they modelled, Kassebaum announced: “It’s very important for clinicians to treat these causes in parallel to the anaemia itself. We hope they use these data to design more comprehensive intervention and treatment plans.”
72 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
News
of RCSI’s School of Population Health
TIME ISN’T ON YOUR SIDE





WE’RE HERE TO MAKE IT WORK FOR YOU
Pharmacosmos knows waiting lists are growing, and this can be overwhelming.1–3


We’re here to support you in delivering the iron your patients need (up to 20 mg/kg) in fewer hospital visits than other fast, high-dose IV irons, giving you more time.4–9
Reach out to Pharmacosmos and see how Monover (ferric derisomaltose) can help you achieve more















Prescribing information

Monover ® (ferric derisomaltose) prescribing information










































Note: Before prescribing please read full Summary of Product Characteristics. Pharmaceutical form: Ferric derisomaltose is a dark brown, non-transparent solution for injection/infusion. Presentations: Iron in the form of ferric derisomaltose; 100 mg/ml available in vials of 100 mg/ml, 500 mg/5 ml and 1,000 mg/10 ml. Indications: Monover ® is indicated in patients ≥18 years for treatment of iron deficiency when oral iron preparations are ineffective or cannot be used or when there is a need to deliver iron rapidly. The diagnosis must be based on laboratory tests. Administration: Each IV iron administration is associated with a risk of a hypersensitivity reaction. Thus, to minimise risk, the number of single IV iron administrations should be kept to a minimum. The iron need can be determined using either the Simplified Table, or the Ganzoni formula, or a fixed dose of 1,000 mg can be given to patients ≥50 kg body weight followed by reevaluation for further iron need, please consult full Summary of Product Characteristics. Monover ® may be administered as an IV bolus injection of up to 500 mg at an administration rate of up to 250 mg iron/minute up to three times a week, during a haemodialysis session directly into the venous limb of the dialyser under the same procedures as outlined for IV bolus injection, or as an up to 20 mg iron per kg body weight infusion. If the iron need exceeds 20 mg iron per kg body weight, the dose must be split into two administrations with an interval of at least one week. It is recommended whenever possible to give 20 mg iron/kg body weight in the first administration. Dependent on clinical judgement the second administration could await follow-up laboratory tests. Doses up to 1,000 mg must be administered over >15 minutes; doses above 1,000 mg must be administered over ≥30 minutes. In case of infusion, Monover ® should be infused undiluted or diluted in 0.9% sodium chloride. For stability, Monover ® should not be diluted to concentrations less than 1 mg iron/ml and never diluted in more than 500 ml.
Contraindications: Non-iron deficiency anaemia, iron overload or disturbances in utilisation of iron, hypersensitivity to any of the ingredients, decompensated liver disease, or known serious hypersensitivity to other parenteral iron products.
Warnings/Precautions: Parenterally administered iron preparations can cause hypersensitivity reactions including potentially fatal anaphylactic/anaphylactoid reactions. The risk is enhanced for patients with known allergies, a history of severe asthma, eczema or other atopic allergy, and in patients with immune or inflammatory conditions. Monover ® should only be administered in the presence of staff trained to manage anaphylactic reactions where full resuscitation facilities are available (including 1:1000 adrenaline solution). Each patient should be observed for at least 30 minutes following administration. If hypersensitivity reactions or signs of intolerance occur during administration, the treatment must be stopped immediately. In patients with compensated liver dysfunction, parenteral iron should only be administered after careful benefit/risk assessment. Careful monitoring of iron status is recommended to avoid iron overload. Parenteral iron should be used with caution in case of acute or chronic infection. Monover ® should not be used in patients with ongoing bacteraemia. Hypotensive episodes may occur if intravenous injection is administered too rapidly. Caution should be exercised to avoid paravenous leakage when administering Monover ® Pregnancy: Monover ® use during pregnancy requires a careful risk/benefit evaluation. The treatment should be confined to second and third trimester only. In rare cases, foetal bradycardia has been observed in pregnant women with hypersensitivity reactions. The unborn baby should be carefully monitored during intravenous administration of parenteral irons in pregnant women. Undesirable effects: No very common (≥10 %) undesirable effects listed. Common undesirable effects (1 % to 10 %): nausea; rash; injection site reactions. For information on other undesirable effects, please consult full Summary of Product Characteristics. Legal Category: POM. Package Quantities: 5 vials of 1 ml; 5 vials of 5 ml; 2 vials of 10 ml. Marketing Authorisation Number/Holder: PA 0982/002/002, Pharmacosmos A/S, Roervangsvej 30, DK-4300 Holbaek, Denmark. Date of preparation: July 2022. Further information is available on request to Pharmacosmos UK.
Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie. Adverse events should also be reported to Pharmacosmos UK Ltd. E: pvuk@pharmacosmos.co.uk T: +44 1844 269 007
References: 1. The Irish Times. Public hospital waiting lists grow again in February. Available at: https://www.irishtimes.com/news/health/public-hospital-waiting-lists-growagain-infebruary-1.4824745. Accessed August 2023. 2. The Irish Times. Record 908,000 patients on public waiting lists. Available at: https://www.irishtimes.com/news/ health/%20record-908-000-patients-on-public-hospital-waiting-lists-1.4652295 Accessed August 2023. 3. HSE. 2022 Waiting List Action Plan. Available at: https://www. gov.ie/en/publication/323b5-the-2022-waiting-list-action-plan/. Accessed August 2023. 4. Kearns J, Jacob SG. Frontline Gastroenterol. 2020;12(4):265–271.
5. Minogue A, et al. Presented at BBTS Glasgow 2017. Poster P080. 6. Monover ® SPC. 2022. Available at: https://www.medicines.ie/medicines/monover-100mg-ml-solution-for-injectioninfusion-32932/spc. Accessed August 2023. 7. Gozzard D. Drug Des Devel Ther. 2011;5:51–60. 8. Bhandari S, et al. Nephrol Dial Transplant. 2021;36(1):111–120. 9. Pollock RF, Muduma G. Clinicoecon Outcomes Res. 2017;9:475–483.
IE-MONV-0723-00002. Date of preparation: August 2023.








Knowledge gap in a cross section of Irish general practitioners prescribing denosumab for osteoporosis
Denosumab is commonly used by general practitioners (GPs) in Ireland in the treatment of osteoporosis.1 It is a monoclonal antibody administered twice yearly as a subcutaneous injection and is often used in patients with contraindications to bisphosphonates including gastro-oesophageal reflux disease and renal impairment (eGFR < 30 ml/min).1 However, studies suggest that a significant proportion of Irish GPs use denosumab as a first line therapy which is generally recommended for bisphosphonates.2 Importantly, unlike bisphoshonates where drug holidays are appropriate for some patients, they are not compatible with denosumab with the anti-resorptive effects wearing off after 6 months, resulting in rebound bone loss and risk of vertebral fractures.1 In fact, all treatment gains in bone density can be lost within 12–24 months
of stopping. For this reason, if denosumab is initiated in primary care, consultation with secondary care colleagues may be advisable given the need to have a longterm personalised osteoporosis management plan in place to enable denosumab to be stopped in a managed way, as necessary.3
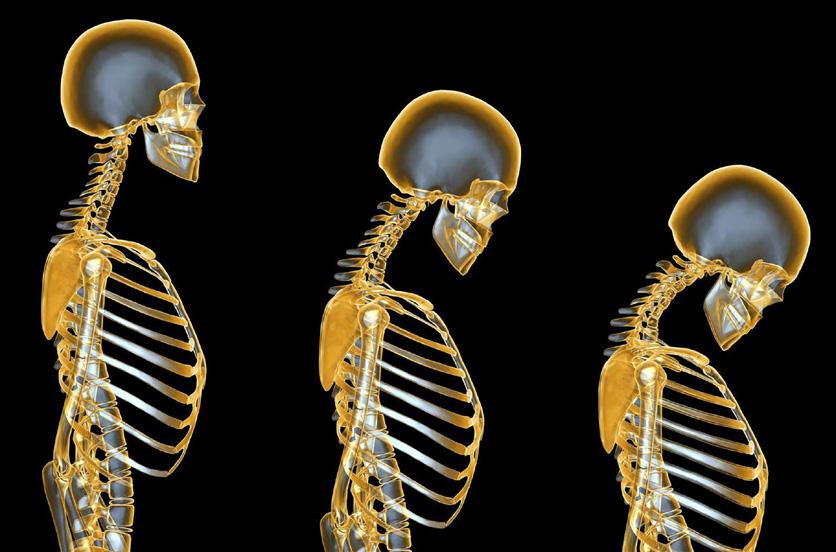
Guidelines have recently advised treatment with zoledronic acid if denosumab is stopped.3, 4 Oral bisphosphonates could also be cautiously considered in some patients with a short duration of therapy (< 2.5 years) providing there is monitoring with bone turnover markers (BTM).4 However, access to these options is difficult for GPs and even with follow-up therapy, bone loss can occur in a significant proportion of patients.1,5 For this reason, the decision to prescribe denosumab needs to be carefully considered given that there is no data on safety and
efficacy beyond 10 years.1 Indeed, in patients at high risk of fracture, remaining on therapy indefinitely may be appropriate.6
Finally, in routine clinical practice, GPs should not prescribe denosumab if there is hypocalcaemia and/or a vitamin D level below 50 nmol/l and also need to consider checking calcium levels post administration in high risk patients.7 Clinical monitoring of serum calcium levels prior to every dose is recommended but is not mandatory and clinical judgement should be applied on an individual patient basis.
We aimed to ascertain GP knowledge and practice with regard to denosumab including (1) use in the last year, (2) use as a first line therapy and reasons for same, (3) duration of treatment, (4) monitoring of blood biochemistry and knowledge of
recommendations for vitamin D status and calcium intake on treatment, (5) clinical staff administering the injection, (6) methods of recall for repeat injections and reliance on pharmacists for reminders, (7) knowledge of acceptable delay in receiving injections and perceived delays during COVID-19, (8) clinical practice if denosumab is stopped (after 2.5 years), as well as awareness of guidelines and if concerns about same and (9) perceived reasons for stopping denosumab.
Methods
GP principals/trainers and GP trainees registered with the Irish College of General Practitioners were contacted by email and invited to complete an online anonymous questionnaire in January 2022 using SurveyMonkey. This comprised 25 questions detailing information on GP demographics/practice characteristics and on knowledge and clinical practice regarding denosumab therapy. Differences in categorical responses to questions by GPs and practice type were explored with χ2�2 test and significance accepted when P < 0.05. Ethical approval was granted by the Irish College of General Practitioners (ICGP) ethics committee (Ref: ICGP_REC2021-T26)
Results
The survey was sent to 867 GPs and 17% (146) responded. Half (50%) comprised GP principals or trainers, the remainder GP trainees and two-thirds (67%) were female. The majority (74%) of practices were urban and 10% had one GP. Over a third (37%) were in a practice for more than 15 years, 12% for 5–14 years and 51% less than 5 years.
74 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Osteoporosis
Written by Eimear O’ Reilly1, Donal Fitzpatrick2,3, Rosaleen Lannon2,3 & Kevin McCarroll2,3
1Royal College of Surgeons, Dublin
2Bone Health Unit, St James’s Hospital, Dublin
3Mercer’s Institute for Research on Ageing, Dublin
BOTOX® is indicated for symptom relief in adults fulfilling criteria for chronic migraine (headaches on ≥15 days per month of which at least 8 days with migraine) in patients who have responded inadequately or are intolerant of prophylactic migraine medications1
Legal category POM (S1A). Marketing Authorisation Numbers: PA 1824/017/1-3. Further information is available on request from AbbVie Limited, 14 Riverwalk, Citywest Business Campus, Dublin 24, D24 XN32 or at www.medicines.ie
References:
1. BOTOX® Summary of Product Characteristics available on www.medicines.ie
IE-BCM-220002
March
Date of preparation:
2023
Osteoporosis
The vast majority (90%) had prescribed denosumab in the last year. Close to half (43%) had used it as a first line therapy with cited factors being gastrointestinal upset (49%), poor compliance with oral therapy (42%), convenience (32%), severe osteoporosis (14%) and renal impairment (7%). The most common time period envisaged for therapy duration was 3–5 years (50%) with 17% citing 1–3 years, 16% for 5–10 years, 2% more than 10 years and 15% lifelong use.
Over one in five (21%) responded that they did not check serum calcium prior to therapy and this was higher for GP registrars compared to trainers (26 vs 16%, P = 0.01). Approximately one in six (17%) cited checking serum calcium routinely after denosumab injections and 72% in patients at risk of hypocalcaemia. The minority (21%) did not assess total daily calcium intake and of 86 GPs who commented, 54% cited a recommended daily allowance in osteoporosis (≥ 1000 mg). Most (71%) considered that patients should have a vitamin D level of ≥ 50 nmol/l prior to starting denosumab, with 9% citing 30–50 nmol/l, 15% > 75 nmol/l and 20% none of these.
In most cases (63%), denosumab was administered by practice nurses only. Over one-third (40%) gave a reminder card for the next denosumab injection which was more likely in GP practices of ≥ 4 compared to 1–3 GPs (32 vs 27%, P = 0.01). Overall, 27% of GPs had alerts to remind patients of the need for repeat prescription. Systems included SMS text (13), phone call (5), email (3) and nurse call (3). Nearly half (49%) noted that they relied on pharmacists ‘some or most of the time’ to give reminders for a repeat prescription.
Over one-third (37%) were not aware that denosumab should not be delayed by > 7 months from the last injection with this being more likely in GP registrars compared to GP trainers (45 vs 29%, P = 0.04). The majority (71%) felt there was a delay in denosumab administration some or most of the time during COVID-19. There was no difference in the response by GP status (registrar vs trainer) as what to do if stopping denosumab after 2.5 years: 19% were unclear and might refer for specialist opinion, 41% cited a drug holiday and monitoring with DXA and about a third (38%) noted that an oral bisphosphonate or SERM should be started. Overall, 21% had no concerns about denosumab
being stopped but this varied significantly from 11% in trainers to 31% in registrars, P = 0.002. About one in five (18%) were aware of any recent guidelines on what do if stopping denosumab.
The main reason cited why GPs felt patients stopped denosumab was due to concern about being on treatment too long (43%), noncompliance (32%), adverse effects (29%) and perceived lack of benefit (14%). Individual comments included that ‘dementia patients family may forget to bring their relative to the practice’ and that ‘some patients become housebound and current resources do not support house calls for administration’. One GP commented that they do not use denosumab anymore as ‘it is too risky’.
Discussion
To our knowledge, this is the only study in Ireland that has explored both GP knowledge and clinical practice with regard to denosumab in the treatment of osteoporosis. We identified a knowledge gap in a number of areas, especially with regard to stopping treatment and follow-up therapy.
While the majority of GPs had used denosumab in the preceding year, close to half had prescribed it as first a first line treatment. In a recent study of 1146 Irish patients prescribed denosumab by GPs between 2012 and 2017, over half had no prior bone therapy suggesting a rate of first line use not explained by contraindications to other therapies.2 We found that in a third of the cases, ‘convenience’ was cited as an indication for first line use; however, no other Irish studies have explored the reasons for denosumab prescribing. Other studies elsewhere have shown that patients have a preference for a six monthly injection compared to weekly tablets, with convenience being identified as an important factor.8 This is likely to impact on GPs who take patient preferences into consideration when prescribing osteoporosis medications.9
About half of GPs envisaged therapy duration for 3–5 years with practice nurses administering the injection in most cases. However, it is unclear how this compares to practice elsewhere or whether the availability of nurses to administer injections could influence GPs prescribing of denosumab. A minority did not
check serum calcium prior to the injection though while assessing serum calcium is recommended before drug administration it is not mandatory. Indeed, during COVID-19, some guidelines waivered the advice to check serum calcium in all patients if normal in the previous year due to difficulties with accessing bloods and advised clinical judgement on an individual basis.10 On the other hand, about one in six checked calcium routinely post injection which is not necessary. Indeed, at the end of denosumab therapy (i.e. at about 6 months or more after the last injection), there may also be a mild hypercalcaemia associated with rebound phenomenon11 that could inadvertently lead to delay in the next injection. However, importantly, the majority of GPs did check serum calcium post injection in patients at risk of hypocalcaemia. Just over half of GPs cited a daily calcium intake of 1000 mg or more for patients with osteoporosis. By comparison, in a survey of GP knowledge of osteoporosis in the Czech Republic in 2017, 41% were reported to correctly state the recommended calcium intake.12
Most GPs had no alert systems to remind patients of their next dose and about half cited relying on pharmacists. While persistence with denosumab in Irish patients has been found to be 57% at 2 years, it has been reported to be higher in those with a medical card.2 Medical card holders in Ireland are entitled to medications at no cost, with reimbursement of their dispensing pharmacist by the Irish Department of Health. Pharmacy oversight of these prescriptions might contribute to this better persistence though avoidance of an ‘out of pocket’ expense is also likely to be an important factor.
A third were not aware of the need for timely denosumab administration (no longer than 7 months after the last injection) suggesting a lack of knowledge among some GPs of current guidelines. This was also more likely in GP registrars who might be less aware of recommendations. Perhaps not surprisingly, the majority of GPs felt there was a delay in denosumab injections during COVID-19 as has been reported elsewhere.13 Interestingly, the paradigm of drug holiday was considered by 41% if stopping denosumab despite the vast majority having concerns if there was therapy cessation.
However, a significant proportion were unclear as to what to do if stopping and might refer for specialist opinion. By comparison, in a recently published Australian study, GPs expressed uncertainty about when to stop denosumab, what to do when stopping, the risk of stopping without an alternative being prescribed, or what should be prescribed if a patient had previously had problems with bisphosphonates.9
Consistent with the above, we found that the majority of GPs had no knowledge of recent guidelines on what do if denosumab is stopped with just over one-third citing the use of an antiresorptive therapy after cessation. A previous study in Ireland found that 6% of patients who stopped denosumab were started on alternative treatments by their GP2 while in Australia, this was reported to be less than 20%.9 However, both studies reported on GP practices at a time when knowledge of the phenomenon of rebound bone loss on denosumab cessation was only emerging.1 GPs felt that the commonest reason for patients wanting to stop denosumab was concern about being on treatment too long which is a similar to what as been Identified for other osteoporosis drugs.14
We acknowledge that only 17% of GPs contacted replied to our survey which could bias the findings. However, the response rate to Irish GP surveys has been identified to be similarly low in other studies with the same methodology.15,16,17,18 The quality or representativeness of a survey also does not necessarily correlate with its size, and a lower response rate does not necessarily make a survey less accurate.19 Furthermore, previous research suggests that GPs with less interest in a topic may be less likely to engage in surveys.20,21 Therefore, this survey could potentially underestimate the knowledge gap identified.
In conclusion, we identified a knowledge gap with regard to denosumab prescribing among a sample of Irish GPs, particularly with regard to cessation of therapy and follow-up treatments. Our findings suggest that there is a need for education to increase awareness around denosumab use. It also highlights the need for reminder or recall systems in GP practices so as to avoid rebound fractures.9
References available on request
76 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Lead your patients to stronger bones with Prolia®1-4

6,7,8,9*
* Prolia® should not be stopped without considering alternative treatment in order to prevent rapid bone mineral density loss and a potential rebound in vertebral fracture risk.6
PROLIA® (denosumab) Brief Prescribing Information
Please refer to the Summary of Product Characteristics (SmPC) before prescribing Prolia. Pharmaceutical Form: Pre-filled syringe with automatic needle guard containing 60 mg of denosumab in 1 ml solution for injection for single use only. Contains sorbitol (E420). Indication: Treatment of osteoporosis in postmenopausal women at increased risk of fractures. Dosage and Administration: 60 mg Prolia administered as a subcutaneous injection once every 6 months. Patients must be supplemented with calcium and vitamin D. No dosage adjustment required in patients with renal impairment. Prolia should not be used in children aged < 18 years because of safety concerns of serious hypercalcaemia. Give Prolia patients the package leaflet and patient reminder card Re-evaluate the need for continued treatment periodically based on the benefits and potential risks of denosumab on an individual patient basis, particularly after 5 or more years of use. Contraindications: Hypocalcaemia or hypersensitivity to the active substance or to any of the product excipients. Special Warnings and Precautions: Traceability: Clearly record the name and batch number of administered product to improve traceability of biological products. Hypocalcaemia: Identify patients at risk for hypocalcaemia Hypocalcaemia must be corrected by adequate intake of calcium and vitamin D before initiation of therapy. Clinical monitoring of calcium levels is recommended before each dose and, in patients predisposed to hypocalcaemia, within 2 weeks after the initial dose. Measure calcium levels if suspected symptoms of hypocalcaemia occur. Renal Impairment: Patients with severe renal impairment (creatinine clearance < 30 ml/min) or receiving dialysis are at greater risk of developing hypocalcaemia. Skin infections: Patients receiving Prolia may develop skin infections (predominantly cellulitis) requiring hospitalisation and if symptoms develop then they should contact a health care professional immediately. Osteonecrosis of the jaw (ONJ): ONJ has been reported rarely with Prolia 60 mg every 6 months. Delay treatment in patients with unhealed open soft tissue lesions in the mouth. A dental examination with preventative dentistry and an individual benefit:risk assessment is recommended prior to treatment with Prolia in patients with concomitant risk factors. Refer to the SmPC for risk factors for ONJ. Patients should be encouraged to maintain good oral hygiene, receive routine dental check-ups and immediately report oral symptoms during treatment with Prolia. While on treatment, invasive dental procedures should be performed only after careful consideration and avoided in close proximity to Prolia administration. The management plan of patients who develop ONJ should be set up in close collaboration between the treating physician and a dentist or oral surgeon with expertise in ONJ. Osteonecrosis of the external auditory canal: Osteonecrosis of the external auditory canal has been reported with Prolia. Refer to the SmPC for risk factors. Atypical femoral fracture (AFF): AFF has been reported in patients receiving Prolia. Discontinuation of Prolia therapy in patients suspected to have AFF should be considered pending evaluation of the patient based on an individual benefit risk assessment. Long-term antiresorptive treatment: Long-term antiresorptive treatment may contribute to an increased risk for adverse outcomes such as ONJ and AFF due to significant suppression of bone remodelling.
Concomitant medication: Patients being treated with Prolia should not be treated concomitantly with other denosumab containing medicinal products. Warnings for Excipients: Patients with rare hereditary problems of fructose intolerance should not use Prolia. Interactions: Prolia did not affect the pharmacokinetics of midazolam, which is metabolized by cytochrome P450 3A4 (CYP3A4). There are no clinical data on the co-administration of denosumab
and hormone replacement therapy (HRT), however the potential for pharmacodynamic interactions would be considered low. Pharmacokinetics and pharmacodynamics of Prolia were not altered by previous alendronate therapy. Fertility, pregnancy and lactation: There are no adequate data on the use of Prolia in pregnant women and it is not recommended for use in these patients. It is unknown whether denosumab is excreted in human milk. A risk/ benefit decision should be made in patients who are breast feeding. Animal studies have indicated that the absence of RANKL during pregnancy may interfere with maturation of the mammary gland leading to impaired lactation post-partum. No data are available on the effect of Prolia on human fertility. Undesirable Effects: The following adverse reactions have been reported: Very common (≥ 1/10) pain in extremity, musculoskeletal pain (including severe cases). Common (≥ 1/100 to < 1/10) urinary tract infection, upper respiratory tract infection, sciatica, constipation, abdominal discomfort, rash, alopecia and eczema. Uncommon (≥ 1/1000 to < 1/100): Cellulitis, ear infection and lichenoid drug eruptions. Rare (≥ 1/10,000 to < 1/1,000): Osteonecrosis of the jaw, hypocalcaemia (including severe symptomatic hypocalcaemia and fatal cases), atypical femoral fractures, and hypersensitivity (including rash, urticaria, facial swelling, erythema and anaphylactic reactions). Very rare (< 1/10,000): Hypersensitivity vasculitis. Please consult the Summary of Product Characteristics for a full description of undesirable effects. Pharmaceutical Precautions: Prolia must not be mixed with other medicinal products. Store at 2°C to 8°C (in a refrigerator). Prolia may be exposed to room temperature (up to 25°C) for a maximum single period of up to 30 days in its original container. Once removed from the refrigerator Prolia must be used within this 30 day period. Do not freeze. Keep in outer carton to protect from light. Legal Category: POM. Presentation and Marketing Authorisation Number: Prolia 60 mg: Pack of 1 pre-filled syringe with automatic needle guard; EU/1/10/618/003. Price in Republic of Ireland is available on request. Marketing Authorisation Holder: Amgen Europe B.V., Minervum 7061, NL-4817 ZK Breda, The Netherlands. Further information is available from Amgen Ireland Limited, 21 Northwood Court, Santry, Dublin D09 TX31. Prolia is a registered trademark of Amgen Inc. Date of PI preparation: May 2022 (Ref: IE-PRO-0322-00006) Adverse reactions/events should be reported to the Health Products Regulatory Authority (HPRA) using the available methods via www.hpra.ie. Adverse reactions/events should also be reported to Amgen Limited on +44 (0)1223 436441 or Freephone 1800 535 160.
References:
1. Prolia® [denosumab], Summary of Product Characteristics.
2. Cummings SR et al, N. Eng. J Med 2009;361:756-765.
3. Holzer G et al, J Bone Miner Res 2009;24(3):468-74.
4. Poole K et al; J Bone Miner Res 012; 27 (suppl1):S44 abstract 1122.
5. Bone HG, et al; Lancet Diabetes Endocrinol. 2017;5:513-23;3-23.
6. Tsourdi E, et al. Bone. 2017;105:11–7.
7. Hernlund E, et al. Arch Osteoporos. 2013;8:136;
8. Kanis JA, et al. Osteoporos Int. 2019;30:3–44;
9. Brown JBMR 2013 Vol28 pp746-752.
Amgen Ireland 21 Northwood Court, Santry, Dublin 9, Ireland. © 2022 Amgen Inc. All rights reserved. IE-PRO-0522-00002. Date of preparation: June 2022 10 YEARS
5
DATA
Osteoporosis is a serious ongoing condition and ongoing treatment is required.
Pain
Waking up to Pain Chronic
Pain Ireland launches ‘Waking up to Pain’ campaign for Pain Awareness Month
Martina Phelan, Chair, Chronic Pain Ireland

Chronic secondary pain is chronic pain where the pain is a symptom of an underlying condition. Chronic secondary pain is organised into the following six categories:
Waking up to Pain
1. Chronic cancer-related pain is chronic pain that is due to cancer or its treatment, such as chemotherapy. This type of pain was represented for the first time in ICD-11.
Sub-heading; Chronic Pain Ireland launches ‘Waking up to Pain’ campaign for Pain Awareness Month
The World Health Assembly (WHA), a subsection of the World Health Organization (WHO), has declared September as Pain Awareness Month. Throughout the month of September, many organisations around the globe contribute and raise awareness of chronic pain. Chronic Pain Ireland is supporting the 2023 global campaign with the theme ‘Waking up to pain'.
The theme ‘Waking Up to Pain’ expresses the reality for up to 1 in 3 individuals in Ireland of waking up to pain each morning. Some people feel pain throughout the day, every day. Chronic Pain Ireland wants people in Ireland to wake up to the facts of chronic pain, which can affect anyone over their lifetime.
Waking up to the prevalence of pain: The University of Galway Prevalence, Impact and Cost of Chronic Pain (PRIME) study estimates up to 1 in 3 adults in Ireland experience chronic pain.
Waking up to the economic cost: The societal cost of chronic pain is significant in terms of healthcare costs and lost productivity. The Irish Pain Society estimates that chronic pain costs the Irish economy around ¤4.7 billion per year, more than 2.5% of GDP.
Waking up to the personal cost: According to The Irish Pain Society, 42% of people living with chronic pain think others doubt the existence of their pain even though 21% said their pain was so intense they wanted to die.
Waking up to the inadequacy of services: According to

What causes chronic pain? There are many causes of chronic pain such as an illness or injury where you recover, but pain persists. There may be an ongoing cause of pain, for example arthritis or cancer. Many people suffer chronic pain in the absence of any past injury or evidence of illness.
How is chronic pain treated?
The World Health Assembly (WHA), a subsection of the World Health Organization (WHO), has declared September as Pain Awareness Month. Throughout the month of September, many organisations around the globe contribute and raise awareness of chronic pain. Chronic Pain Ireland is supporting the 2023 global campaign with the theme ‘Waking up to pain'.
figures released by the Irish Pain Society, Ireland has just 27 dedicated pain consultants operating across the public system, meaning that 41% of patients are currently waiting more than 12 months for their first appointment with a chronic pain specialist, while 18% are waiting the same length of time for their first treatment.
2. Chronic postsurgical or posttraumatic pain is chronic pain that develops or increases in intensity after a tissue trauma (surgical or accidental) and persists beyond three months. It is also part of the ICD for the first time.
Chronic pain impacts on every aspect of a person’s life. A multidisciplinary approach to pain management is often required, providing interventions that help manage the pain. Public pain management programmes are available in some hospitals.
The theme ‘Waking Up to Pain’ expresses the reality for up to 1 in 3 individuals in Ireland of waking up to pain each morning. Some people feel pain throughout the day, every day. Chronic Pain Ireland wants people in Ireland to wake up to the facts of chronic pain, which can affect anyone over their lifetime.
● Waking up to the prevalence of pain: The University of Galway Prevalence, Impact and Cost of Chronic Pain (PRIME) study estimates up to 1 in 3 adults in Ireland experience chronic pain.
● Waking up to the economic cost: The societal cost of chronic pain is significant in terms of healthcare costs and lost productivity. The Irish Pain Society estimates that chronic pain costs the Irish economy around €4.7 billion per year, more than 2.5% of GDP.
How can Chronic Pain Ireland help?
● Waking up to the inadequacy of services: According to figures released by the Irish Pain Society, Ireland has just 27 dedicated pain consultants operating across the public system, meaning that 41% of patients are currently waiting more than 12 months for their first appointment with a chronic pain specialist, while 18% are waiting the same length of time for their first treatment.
Chronic pain, unlike acute pain, is pain that persists beyond the normal time of healing and this is generally accepted as pain that lasts longer than three months. The International Association for the Study of Pain (IASP) redefined pain in 2020 as “An unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage”. This expanded definition encompasses pain that arises in the absence of any evidence of tissue damage or disease.
Chronic pain, unlike acute pain, is pain that persists beyond the normal time of healing and this is generally accepted as pain that lasts longer than three months. The International Association for the Study of Pain (IASP) redefined pain in 2020 as “An unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage”. This expanded definition encompasses pain that arises in the absence of any evidence of tissue damage or disease.
3. Chronic secondary musculoskeletal pain is chronic pain in bones, joint and tendons arising from an underlying disease classified elsewhere. It can be due to persistent inflammation associated with structural changes or caused by altered biomechanical function due to diseases of the nervous system.
● Waking up to the personal cost: According to The Irish Pain Society, 42% of people living with chronic pain think others doubt the existence of their pain even though 21% said their pain was so intense they wanted to die.
Chronic Pain Ireland (CPI) is the national charity providing information, education and support services to people living with chronic pain, their families, friends, caregivers and other interested parties. We advocate on their behalf and work closely with all stakeholders.

Pain Awareness Month (PAM) Activities
Chronic secondary pain is chronic pain where the pain is a symptom of an underlying condition. Chronic secondary pain is organised into the following six categories:
4. Chronic secondary visceral pain is chronic pain secondary to an underlying condition originating from internal organs of the head or neck region or of the thoracic, abdominal or pelvic regions. It can be caused by persistent inflammation, vascular mechanisms or mechanical factors.
Chronic primary pain represents chronic pain as a disease in itself. Chronic primary pain is characterised by significant functional disability or emotional distress that is not better accounted for by another diagnosis. This may include chronic widespread pain, chronic primary musculoskeletal pain (previously termed "non specific"), as well as primary headaches and conditions such as chronic pelvic pain and irritable bowel syndrome. They are recognised as a group of chronic pain syndromes for the first time in the International Classification of Diseases 11th Revision – the global standard for diagnostic health information (ICD-11).
1. Chronic cancer-related pain is chronic pain that is due to cancer or its treatment, such as chemotherapy. This type of pain was represented for the first time in ICD-11.
Chronic primary pain represents chronic pain as a disease in itself. Chronic primary pain is characterised by significant functional disability or emotional distress that is not better accounted for by another diagnosis. This may include chronic widespread pain, chronic primary musculoskeletal pain (previously termed "non specific"), as well as primary headaches and conditions such as chronic pelvic pain and irritable bowel syndrome. They are recognised as a group of chronic pain syndromes for the first time in the International Classification of Diseases 11th Revision – the global standard for diagnostic health information (ICD-11).


5. Chronic neuropathic pain is chronic pain caused by a lesion or disease of the somatosensory nervous system. This category includes peripheral and central neuropathic pain. This type of pain is also newly represented in ICD-11.
6. Chronic secondary headache or orofacial pain contains the chronic forms of symptomatic headaches – those termed primary headaches in the International Classification of Headache Disorders 3rd edition (ICHD-3) are part of chronic primary pain – and follows closely the ICHD3 classification. Chronic secondary orofacial pain, such as chronic dental pain, supplements this section of ICD-11.
Chronic Pain Ireland are supporting our members during Pain Awareness Month by providing:
A 5-week self-management course based on the biopsychosocial model of pain.
Two 7-week Living Well with Chronic Pain programmes for members in collaboration with The Health Service Executive (HSE).
A 7-week Lifestyle Education Awareness Programme (LEAP) programme specifically for those living with fibromyalgia.
A facilitated chronic pain and mental health support group in partnership with Turn2Me.
Chronic Pain Ireland will host informative talks throughout September and have distributed information leaflets on chronic pain to pain teams and social prescribers across the country.
To learn more about our activities for Pain Awareness Month, visit www.chronicpain.ie.
References available on request
78 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE

HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication
Clinical R&D
XBIOTECH ANNOUNCES
FIRST PATIENT BEGINS NOVEL NATRUNIX THERAPY IN PHASE II RHEUMATOID ARTHRITIS (RA) CLINICAL TRIAL
XBiotech Inc. (NASDAQ: XBIT) has began treating the first patient in a phase II, double-blind, placebo-controlled, randomized clinical study to evaluate Natrunix as a new treatment for Arthritis. Natrunix blocks a key cause of inflammation involved in pain and joint destruction in rheumatoid arthritis (RA).
The primary endpoint of the Phase II is the American College of Rheumatology (ACR) 20% Response (ACR 20) rate at 12 weeks. Secondary and exploratory endpoints include ACR 50, ACR 70, numerical rating scale (NRS) for pain score, inflamed and painful joint counts, Health Assessment Questionnaire Disability Index (HAQ-DI), Routine Assessment of Patient Index Data 3 (RAPID-3), Clinical Disease Activity Index (CDAI) and safety. These endpoints reflect the expectation that Natrunix may provide needed improvement with respect to pain, tenderness, mobility of joints and quality of life. Approximately 210 subjects will be enrolled into three different arms in the study. All subjects will receive methotrexate (MTX) and be randomized to receive either one of two doses of Natrunix or placebo.
Natrunix is a monoclonal antibody indistinguishable from a naturally occurring antibody from a human donor. Natrunix binds and neutralizes the action of one of the most potent inflammation-causing substances known—interleukin-1. For decades, interleukin-1 was seen as the key target for drugs to treat RA. However, to date, drugs that block interleukin-1 have not lived up to expectations as therapies for RA. XBiotech believes it has solved this puzzle.
Interleukin-1 actually describes two separate and distinct molecules—IL-1a and IL-1b—that are produced at different times and places in the body. XBiotech is the first to develop candidate therapies—like Natrunix—that directly and specifically neutralize IL-1a. In recent years, world-class research has shown that in many cases IL-1a may be the crucial target for blocking disease-causing activities of the interleukin-1 inflammatory pathway. Natrunix thus holds promise as a new generation anti-inflammatory therapy for arthritis. As a naturally derived human antibody, it is also by design expected to be among
the safest and best tolerated medicines ever developed.
One in four adults, or over 50 million people in the United States are currently affected by Rheumatoid Arthritis, including 33% of those between the ages of 45-64 and 50% of person over 65 years of age. The number of persons affected by RA is expected to increase, with the CDC predicting that by the year 2040, 78.4 million adults, will suffer from RA in the United States. In addition, it is expected that 300,000 children will suffer from juvenile arthritis (Arthritis Foundation, 2023).
SPINAL INJURIES IRELAND (SII) CALLS ON BUSINESSES AND PUBLIC REPRESENTATIVES TO ‘BUDDY UP’
On average, three people sustain a Spinal Cord Injury (SCI) every week in Ireland with Spinal Injuries Ireland (SII) being the only charitable organisation dedicated to providing support for these 2,300+ people. This year, the charity aims to raise ¤100,000 by appealing to businesses and public representatives to get on the pathway to accessibility, and team up with service users of the charity by spending time in a wheelchair, as part of its largest national fundraising and awareness campaign for 2023 – ‘A Day in My Wheels’.
‘A Day in My Wheels,’ initially launched by SII in 2021, aims to highlight the need for a collaborative approach to create inclusive pathways to accessibility, while raising much-needed funds to support individuals living with SCI in Ireland.
The charity advocates on behalf of its members while also providing; one-to-one services from its community outreach team, and access to its trained peer mentor and comprehensive educational programmes. Another core element of SII’s work is counselling services for families and service users to help them cope with their new reality. This service delivery has changed post-Covid and in recent years, the charity has observed a significant increase in requests for these services.
In addition to all of these vital services, individuals and their families who are suddenly faced with SCI are faced with the cost of medical care, bowel and bladder care, pressure relieving equipment, and specialist requirements such as wheelchairs, hoists and adaptions to their car and home.
The aim of the campaign is not only to help the general public obtain a better understanding of living with this life-altering injury, but to offer employers and companies assistance to put the appropriate supports in place to help such individuals.
Fiona Bolger, CEO for Spinal Injuries Ireland (SII) explained that, “On average, three people sustain a Spinal Cord Injury (SCI) every week in Ireland. The impact can be devastating, and we want to be able to continue to provide support to our service users as they adjust to their life post-injury.
SII is inviting business leaders and public representatives (with up to four of their team members) to sign up now and join SII ambassadors and the Disability Federation of Ireland on Wednesday the 4th of October for approximately 2 hours at Cork County Hall for breakfast, talks and workshops. Following this, participants will have the opportunity to ‘buddy up’ with a service user and spend the afternoon using a wheelchair. The aim of the day is to provide a unique insight into the reality and accessibility issues those with SCI face every day, while raising much needed funds for SII.
Companies or individuals are invited to contribute ¤5,000 to participate in ‘A Day in My Wheels’ 2023, where funds will directly support the 2,300+ individuals in Ireland with a Spinal Cord Injury and their families. A similar event will also take place in Dublin on the 27th of September.
For more on Spinal Injuries Ireland and the services it provides, see spinalinjuries.ie
RANOLAZINE CLONMEL
Clonmel Healthcare is delighted to announce the launch of Ranolazine Clonmel 375mg, 500mg and 750mg prolonged-release tablets.
Full prescribing information is available on request or alternatively please go to www.clonmel-health. ie. Medicinal product subject to medical prescription.
Please contact Clonmel Healthcare on 01-6204000 if you require any additional information.
PA 126/356/1-3. PA Holder: Clonmel Healthcare Ltd., Clonmel, Co. Tipperary. Date prepared: July 2023. 2023/ADV/RAN/154H.
LETTERKENNY UNIVERSITY HOSPITAL TAKES PART IN MAGNET4EUROPE
Letterkenny University Hospital (LUH) is delighted be participating in a research project which aims to create a more positive work environment for nurses, with a focus on exemplary nursing practice. LUH is one of 67 hospitals across Europe participating in the Magnet4Europe project, it is an international programme designed to create a culture of excellence, and by doing so, improve the health and wellbeing of nurses in their workplace.
There is a large body of evidence which suggests that Magnet hospitals achieve higher employee retention rates, lower intention to leave, improved lower nurse burnout, higher levels of job satisfaction, better patient outcomes and improved patient safety. Magnet hospitals also report better clinical outcomes with shorter patient stay and reduced readmission rates.
80 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
The Magnet4Europe project spans across the EU, from Ireland to Sweden and the objective is to evaluate the impact of organisations redesign on nurses and clinician’s mental health and wellbeing in acute hospital settings.

In LUH a lot of work has been done to embed the Magnet model in the hospital. The Magnet model framework aims to ensure that the organisation’s policies, procedures, structures and supports promote staff wellbeing and empower staff to achieve excellence in patient care. It is important that staff are encouraged to access resources and are aware of how to do so. For example the hospital hosted a staff information day, which was an opportunity to showcase the wide range of staff supports already available and get feedback regarding what can be further done to support staff wellbeing.
Initiatives such as the launch of the LUH Rainbow badges, Africa Day and International Nurse Day celebrations ensure a culture of diversity and inclusion is visible within the organisation and that staff feel included, acknowledged and valued. Other plans involve optimising communication and being more innovative in how information is shared with staff, finding new ways to acknowledge and celebrate achievements, enhancing leadership capabilities within the nursing workforce and tailoring educational opportunities to the needs of staff.
The ‘Structural empowerment’ component of the Magnet model focuses on ensuring nurses are supported to be involved in
the decisions which affect their day-to-day work. This is done through having a clinical nursing voice on hospital committees and working groups and through the establishment of professional practice councils for different nursing grades.
The councils are nurse led and will be a voice of change within the LUH Nursing Service. They are forums for staff to convene to discuss nursing care issues and work together to find resolutions.
JANSSEN MARKS FIRST APPROVAL WORLDWIDE FOR AKEEGA® (NIRAPARIB AND ABIRATERONE ACETATE DUAL ACTION TABLET) WITH EC AUTHORISATION FOR THE TREATMENT OF PATIENTS WITH METASTATIC CASTRATION RESISTANT PROSTATE CANCER WITH BRCA1/2 MUTATIONS
The Janssen Pharmaceutical Companies of Johnson & Johnson have announced that the European Commission (EC) has granted marketing authorisation for AKEEGA® (niraparib and abiraterone acetate [AA]), in the form of a dual action tablet (DAT), given with prednisone or prednisolone, for the treatment of adults with metastatic castrationresistant prostate cancer (mCRPC) and BRCA1/2 mutations (germline and/or somatic) in whom chemotherapy is not clinically indicated.
Prostate cancer is one of the most common cancers in men in Ireland with about 3,940 men diagnosed each year. Despite treatment advances, mCRPC remains
an incurable, deadly disease. BRCA1/2 gene mutations have been identified in approximately 10-15 percent of mCRPC patients and are more likely to cause aggressive disease, poor outcomes, and a shorter survival time.
Professor Ray McDermott, Consultant Medical Oncologist, St Vincent’s University Hospital, Dublin said, “Metastatic castrationresistant prostate cancer remains a lethal disease, with high unmet needs in terms of treatment options, particularly for patients with BRCA1/2 gene mutations. The development of innovative therapies is vital to provide patients with new treatment options. The dual action tablet of niraparib with abiraterone acetate is a promising first line targeted treatment option for men with mCRPC and BRCA1/2 mutations. With BRCA testing now publicly available in Ireland, I hope this medicine will reach the right patients at the right time when available here.”
The EC authorisation, which also marks the first worldwide approval for AKEEGA®, is based on results of the randomised, double-blind, placebo controlled, Phase 3 MAGNITUDE study (NCT03748641). The trial assessed whether the addition of niraparib to AAP improved outcomes in those with untreated mCRPC, with or without alterations in homologous recombination repair (HRR) associated genes (which are involved in the repair of damaged DNA), including BRCA1/2. A total of 423 patients with HRR gene alterations were enrolled, 225 (53.2 percent) of whom had BRCA mutations, making it the largest cohort of BRCA1/2-positive patients with first line mCRPC in any clinical study to date.
Dr Thorsten Giesecke, General Manager, Commercial Business, Janssen Sciences Ireland UC, said: “Janssen’s legacy in innovation and the development of treatments for prostate cancer spans more than a decade. We remain committed to harnessing the power of science to bring solutions to patients at every stage of their cancer journey. The MAGNITUDE trial was prospectively designed as a precision medicine study to identify the specific population of patients who would most benefit from niraparib with AAP, and potentially increase the likelihood of treatment success. This approval marks an exciting step in the right direction, changing the outlook for patients with mCRPC for the better.”
The primary endpoint for MAGNITUDE was radiographic progression-free survival (rPFS), as analysed by blinded central review. Niraparib plus AAP significantly improved rPFS in all HRR-positive patients (Hazard Ratio [HR] 0.73; 95 percent Confidence Interval [CI], 0.56 to 0.96; p=0.022). This improvement was most pronounced in patients with BRCA1/2 gene mutations, where a statistically significant 47 percent risk reduction was observed for rPFS. With additional median follow-up at 24.8 months in the BRCA subgroup, rPFS by central review demonstrated a consistent and clinically meaningful treatment effect favouring niraparib plus AAP, with a median rPFS of 19.5 months compared with 10.9 months for placebo plus AAP. Additionally, there was a trend towards improved overall survival (OS) with niraparib plus AAP, strong improvement in time to symptomatic progression (TSP) and clinically meaningful improvement in time-to-initiation of cytotoxic chemotherapy (TCC). The observed safety profile of the combination of niraparib and AAP was consistent with the known safety profile of each agent.3 Of the patients with HRR gene alterations, 67 percent experienced Grade 3/4 adverse events (AEs) in the combination arm versus 46.4 percent in the control arm. The most common grade 3 AEs were anaemia (28.3 percent versus 7.6 percent) and hypertension (14.6 percent versus 12.3 percent) with niraparib and AAP versus placebo and AAP, respectively.15The combination of niraparib and AAP also maintained overall quality of life in comparison with placebo and AAP.
Niraparib is a highly selective poly adenosine diphosphate-ribose polymerase (PARP) inhibitor. Together with AA plus prednisone, the combination DAT regimen targets two oncogenic drivers in patients with mCRPC, namely alterations in the androgen receptor axis and in BRCA1/2. This is the first DAT formulation available in the European Union for patients with mCRPC with BRCA mutations.
Europe is the first region to approve AKEEGA® (niraparib and abiraterone acetate DAT), for the treatment of patients with BRCA-positive mCRPC, globally. In February 2023, Janssen submitted a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) seeking approval of this niraparib-based combination for the treatment of patients with BRCA-positive mCRPC.
81 HOSPITALPROFESSIONALNEWS.IE | HPN • SEPTEMBER 2023
From left: Elizabeth Neely, Assistant Director Of Nursing, Nurse Practice Development, LUH; Marie Callaghan, Assistant Director Of Nursing, Magnet Coordinator, LUH; Prof Jonathan Drennan, Irish lead, Magnet4Europe Project; Dr Anne Drake, Director of Nursing, LUH; Dr Noeleen Brady, Post-Doctoral Researcher, Magnet4Europe Project; and Sinead Fisher, Clinical Nurse Manager 3, Magnet, LUH
Clinical R&D
EVAXION PRESENTS PROMISING RESULTS ON EVX-B1, AN AI-DESIGNED VACCINE AGAINST STAPHYLOCOCCUS AUREUS
Evaxion Biotech A/S (NASDAQ: EVAX) (“Evaxion” or the “Company”), a clinical-stage biotechnology company specializing in the discovery and development of AI-powered immunotherapies, presents promising results of EVX-B1 at the Staphylococcal Diseases Gordon Research Conference in New Hampshire, USA.
“I am excited to present the impressive results of EVX-B1 at the Gordon Research Conference. The demonstration of full disease protection and complete bacterial clearance marks a significant advancement in the fight against this widespread and often antibiotic-resistant pathogen. With the many previous unsuccessful attempts to develop an effective S. aureus vaccine, EVX-B1 instills renewed hope in the battle against the bacteria,” said Steven Projan, PhD, Scientific Advisor to Evaxion and poster presenter.
EVX-B1 has been designed using Evaxion’s proprietary AI technology for vaccine target discovery. The AI technology has enabled the identification of novel S. aureus antigens, paving the way for the development of a vaccine with potential for broad protection against clinically relevant strains.
EVX-B1 induces strong immune responses, both cellular and humoral, that translate to a high level of protection in preclinical models for skin infection and sepsis. Importantly, EVX-B1 prevents both the development of disease and effectively eliminates the infection itself. In a 12-month immunogenicity study, EVX-B1 induced high and long-lasting antibody titers. A booster immunization substantially increased the immune response, protecting against S. aureus infection. The EVX-B1 vaccine candidate is ready for nonclinical development, CMC, and subsequent clinical testing.
“The remarkable performance of EVX-B1 in state-of-the-art animal models is a testament to our innovative approach and the power of computational target discovery. We are excited about the potential impact this vaccine could have in preventing S. aureus infections, which have long posed significant challenges to global health and where antibiotic resistance is developing fast,” said Birgitte Rønø, Chief Scientific Officer at Evaxion.
NEW RCSI MYHEALTH SERIES TO LOOK AT SCHOOL, STROKE AND SUPERBUGS
RCSI University of Medicine and Health Sciences has launched the 2023/24 programme for RCSI MyHealth, a video and podcast series that brings reliable and trustworthy healthcare information to the public.
The first three episodes in the new series will look at giving children the emotional tools to have a positive return to school, treatment and prevention of stroke, and antibiotic-resistant ‘superbugs’. As the series progresses in 2024, topics will include nutrition, heart health and dementia.
Running since 2017, the RCSI MyHealth lecture series aims to demystify common health concerns by drawing expertise and insight from RCSI researchers and international health experts at the cutting-edge of medical and healthcare developments.
The episodes are for anyone wanting to learn about common illnesses and health-related topics directly from leading healthcare experts and explore a wide range of areas in health and well-being. Previous episode topics have included menopause, vaping, cancer and positive ageing.
The upcoming episodes of the new 2023/24 RCSI MyHealth series are:
• 24 October – Stroke: Prevention and new developments in treatments and rehabilitation
• 14 November – Antibiotic resistant ‘superbugs’: Can they be stopped?
In the first episode airing on Tuesday, 29 August, researchers from the RCSI Centre for Positive Health Sciences (CPHS) will discuss how parents and caregivers can make the start of the school year a positive experience for children and teenagers. Chaired by Professor Ciaran O'Boyle, CPHS Director, it features Dr Jolanta Burke, Senior Lecturer in CPHS, Dr Trudy Meehan, Senior Clinical Psychologist specialising in child and adolescent mental health and lecturer in CPHS, and Dr Mary Collins, Chartered Psychologist and Senior Coach Practitioner, CPHS.
Speaking about the launch, Professor Hannah McGee, Deputy Vice Chancellor for Academic Affairs at RCSI, said: “This season of RCSI MyHealth builds on our commitment to enhancing human health through expert-driven
healthcare information. The topics covered in RCSI MyHealth are in direct response to public feedback and the most prevalent health issues affecting our population today. RCSI’s aim is to lead the world to better health and the RCSI MyHealth series is a core component of that, empowering the public to live longer, healthier and happier lives.”
RCSI University of Medicine and Health Sciences is ranked first in the world for its contribution to UN Sustainable Development Goal 3, Good Health and Well-being, in the Times Higher Education (THE) University Impact Rankings 2023.
ZENAS BIOPHARMA ANNOUNCES PUBLICATION OF PHASE 2 STUDY OF OBEXELIMAB, AN INVESTIGATIONAL TREATMENT FOR IGG4-RELATED DISEASE (IGG4-RD), IN THE LANCET RHEUMATOLOGY
Zenas BioPharma, a global biopharmaceutical company committed to becoming a leader in the development and commercialization of immunebased therapies, announces The Lancet Rheumatology has published findings from a Phase 2 study evaluating obexelimab for the treatment of patients with IgG4-Related Disease (IgG4-RD). Based on the results of this study, a Phase 3 study in patients with IgG4-RD is ongoing to further investigate the efficacy and safety of obexelimab administered as a subcutaneous injection.
IgG4-RD is a chronic, immunemediated fibro-inflammatory disease that can affect multiple organs including the major salivary glands, orbits, lacrimal glands, pancreas, biliary tree, lungs, kidneys, and retroperitoneum. Approximately 20,000 patients are diagnosed with IgG4-RD in the United States alone. Despite its increasing recognition, there remains a need for further research and effective therapeutic options for individuals living with this debilitating disease.
Across the world, the use of glucocorticoids is widely considered to be the standard of care for treating IgG4-RD. There are no approved treatment options for this condition. While commonly used, glucocorticoids and available B cell depleting therapies rarely lead to long-term, treatment-free remissions, and are associated with a high risk of toxicity in these patients. Such therapies also impair vaccine responses, including those for SARS-CoV-2 and influenza.
In a prospective, open-label, single arm, single-center pilot study to assess the efficacy and safety of obexelimab in the treatment of patients with IgG4RD (clinicaltrials.gov registration NCT02725476), obexelimab demonstrated strong improvement in the IgG4-RD Responder Index, a measure of disease activity, by inhibiting B cell function, without depleting B cells.
The published manuscript, titled “Obexelimab for the Treatment of Patients with IgG4-Related Disease: An Open-Label, SingleArm, Pilot Study to Evaluate Efficacy, Safety, and Mechanism of Action,” is available online and will appear in the August issue of The Lancet Rheumatology 2023;5(8) [E428-E429].
The following are the key findings in the paper:
• Obexelimab produced rapid, strong, and sustained clinical improvement, including complete remission (IgG4-RD Responder Index score of 0), in most patients with active IgG4RD.
• During obexelimab treatment, reductions in circulating B cells, including plasmablasts, were observed without evidence of cell death.
• Additionally, reduction of circulating B cells and rapid return to near normal levels after treatment discontinuation suggests that obexelimab may lead to B cell sequestration in lymphoid organs or the bone marrow.
• Obexelimab was well tolerated. The majority of treatmentrelated adverse events were grades 1 or 2, with the most common adverse events being gastrointestinal infusion-related events, most of which were mild.
“Our findings are a significant step forward in understanding the underlying mechanisms of IgG4-Related Disease; paving the way for more targeted treatment strategies,” said John Stone, MD, MPH, Professor of Medicine at Harvard Medical School, and the Edward A. Fox Chair in Medicine at Mass General Hospital. “Our team is honored to have our research recognized by The Lancet Rheumatology, and we are immensely grateful to the patients who participated in this groundbreaking study.”
ST VINCENT’S CELEBRATE MILESTONE
Just last month, St. Vincent’s University Hospital achieved a
82 SEPTEMBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
significant milestone in its Bariatric Surgery service as the hospital inaugurated the dedicated Bariatric Theatre and completed the 100th bariatric surgery for 2023. This accomplishment underscores the hospitals commitment to providing more timely and quality care to patients living with obesity and its complications.
"Today signifies great progress for the Bariatric Surgery service at SVUH & the National Weight Management Clinic in St Columcille’s Hospital as we inaugurate our dedicated Bariatric Theatre and successfully conclude our 100th bariatric surgery for the year 2023. We are also on the cusp of nearing the 1000-case milestone across the St. Vincent’s Hospital Group. A heartfelt thank you goes out to every individual who has played a role in this achievement. Your dedication, expertise, and tireless contributions have not only brought us to this juncture but have also set the stage for even greater developments in the future," said Professor Helen Heneghan, Consultant Bariatric & General Surgeon, SVUH & first female Prof of Surgery University College Dublin.
This milestone is a testament to the collaborative spirit of the exceptional multidisciplinary team who work across several sites - healthcare professionals, support staff, and administrators in SVUH, St Michael’s Hospital and St Columcille’s Hospital. Their unwavering commitment to the Bariatric Surgery Service has made achieving excellent outcomes possible.
REGIONAL HEALTH FORUM WEST ANNUAL GENERAL MEETING
At the Annual General Meeting of the Regional Health Forum West on Tuesday 27 June, the following members were elected to the
positions of Chair and Vice Chair of the Regional Health Forum West and Regional Health Forum Committee:
Regional Health Forum West
Chair - Cllr Donagh Killilea (FF), Galway County Council
Vice Chair - Cllr Ciaran Brogan (FF), Donegal County Council Regional Health Forum Committee

Chair - Cllr Declan McDonnell (NP), Galway City Council
Vice Chair - Cllr Michael Kilcoyne (NP), Mayo County Council
BOOTS IRELAND LAUNCHES
THIS YEAR’S BOOTS NIGHT WALK IN AID OF THE IRISH CANCER SOCIETY
Boots Ireland has partnered with Breast Cancer Survivor and Founder of the Good Glow Podcast Georgie Crawford to launch this year’s Boots Night Walk in aid of the Irish Cancer Society Night Nursing service which provides end-of-life care for people living with cancer in Ireland. The Irish Cancer Society Night Nursing service provides up to 10 nights of care and is free to people who avail of it. Boots Ireland has partnered with the Irish Cancer Society since 2012 and through the support of their team members, patients and customers have raised ¤2.8 million for the service so far, helping to provide over 8,000 nights of care.
This year, the Boots Night Walk is back, with Boots Ireland calling upon the public to sign up and walk 5km together this autumn in support of the Irish Cancer Society Night Nurses. The walk will take place on Friday the 8th September at Dublin’s Phoenix Park, or join us from afar to complete the walk a location of your choosing. Visit www.bootsnightwalk.com to make a donation or sign up* and start your own fundraising page to help raise money for this great cause.
NEW AMBASSADOR FOR IRISH HEART FOUNDATION
Irish rugby star Diarmuid Barron has revealed how the death of a young cousin from heart disease inspired him to learn life-saving cardiopulmonary resuscitation (CPR) skills.
Barron (25) who has been announced as an Irish Heart Foundation Ambassador, was speaking after being trained in CPR by secondary school students at Hazelwood College, Dromcollogher, Co Limerick, when he was presenting them with a CPR 4 Schools Special Recognition Award, as part of the Irish Heart Foundation’s CPR 4 Schools programme.

The programme, for post-primary schools across Ireland aims to train the next generation of lifesavers so they can respond swiftly and effectively in a cardiac emergency.
Barron, who recently helped Munster to win the United Rugby Championship in South Africa, said he knows from personal experience the devastating impact cardiovascular disease can have on lives.
83 HOSPITALPROFESSIONALNEWS.IE | HPN • SEPTEMBER 2023
Cllr Ciaran Brogan, Tony Canavan and Cllr Donagh Killilea
Munster rugby star Diarmuid Barron with teacher Michelle Herbert from Hazelwood College, Dromcollogher, Co Limerick.
Photo: SkyView Photography
TAKE IT ON WITH CIBINGO
CIBINQO is a convenient once-daily oral JAK1 inhibitor for moderate-to-severe atopic dermatitis (AD) that o ers1-4





















Significant skin clearance at week 12, with sustained control at week 481-3,5
Rapid itch relief, superior to dupilumab + TCS at week 2 for CIBINQO 200 mg + TCS, with significant results as early as day 41,6

A once-daily pill available in multiple doses that can be used with or without medicated topical therapies so you can tailor treatment to meet the individual needs of your patients1-3,7,8
CONSISTENT SAFETY PROFILE: Rigorously studied in >3500 patients across 7 clinical trials, including one ongoing LTE 9


PRESCRIBING INFORMATION CIBINQO® ▼ (ABROCITINIB)



Please refer to full Summary of Product Characteristics (SmPC) before prescribing Cibinqo®.


Presentation: Film-coated tablets containing 50 mg, 100 mg, or 200 mg abrocitinib. Each tablet contains 1.37 mg, 2.73 mg, and 5.46 mg of lactose monohydrate, respectively. Indications: For the treatment of moderate-to-severe atopic dermatitis in adults who are candidates for systemic therapy. Dosage: Treatment should be initiated and supervised by a healthcare professional experienced in the diagnosis and treatment of atopic dermatitis. Posology: The recommended starting dose is 100 mg or 200 mg once daily based on individual patient characteristics. A starting dose of 100 mg once daily is recommended for patients at higher risk of venous thromboembolism (VTE), major adverse cardiovascular event (MACE) and malignancy. If the patient does not respond adequately to 100 mg once daily, the dose can be increased to 200 mg once daily. A dose of 200 mg once daily may be appropriate for patients who are not at higher risk of VTE, MACE and malignancy with high disease burden or for patients with an inadequate response to 100 mg once daily. Upon disease control, dose should be decreased to 100 mg once daily. If disease control is not maintained after dose reduction, re-treatment with 200 mg once daily can be considered. The lowest e ective dose for maintenance should be considered. Discontinuation of treatment should be considered in patients who show no evidence of therapeutic benefit after 24 weeks. Cibinqo can be used with or without medicated topical therapies for atopic dermatitis. Treatment initiation: TTreatment should not be initiated in patients with a platelet count < 150 × 103/mm3, an absolute lymphocyte count (ALC) < 0.5 × 103/mm3, an absolute neutrophil count (ANC) < 1.2 × 103/mm3 or who have a haemoglobin value < 10 g/dL. Dose interruption: If a patient develops a serious infection, sepsis or opportunistic infection, dose interruption should be considered until the infection is controlled. Interruption of dosing may be needed for management of laboratory abnormalities. Missed doses: If a dose is missed, patients should be advised to take the dose as soon as possible unless it is less than 12 hours before the next dose, in which case the patient should not take the missed dose. Thereafter, dosing should be resumed at the regular scheduled time. Interactions: In patients receiving dual strong inhibitors of cytochrome CYP2C19 and moderate inhibitors of CYP2C9, or strong inhibitors of CYP2C19 alone (e.g., fluvoxamine, fluconazole, fluoxetine and ticlopidine), the recommended dose of Cibinqo should be reduced by half to 100 mg or 50 mg once daily. Treatment is not recommended concomitantly with moderate or strong inducers of CYP2C19/CYP2C9 enzymes (e.g., rifampicin, apalutamide, efavirenz, enzalutamide, phenytoin). In patients receiving acid reducing agents (e.g. antacids, proton pump inhibitors and H2 receptor antagonists), 200 mg once daily dose of abrocitinib should be considered (see section 4.5). Renal impairment: No dose adjustment is required in patients with mild renal impairment, i.e., estimated glomerular filtration rate (eGFR) of 60 to < 90 mL/min. In patients with moderate (eGFR 30 to < 60 mL/min) renal impairment, the recommended dose of Cibinqo should be reduced by half to 100 mg or 50 mg once daily. In patients with severe (eGFR < 30 mL/min) renal impairment, 50 mg once daily is the recommended starting dose. The maximum daily dose is 100 mg. Cibinqo has not been studied in patients with end-stage renal disease (ESRD) on renal replacement therapy. Hepatic impairment: No dose adjustment is required in patients with mild (Child Pugh A) or moderate (Child Pugh B) hepatic impairment. Cibinqo must not be used in patients with severe (Child Pugh C) hepatic impairment. Elderly: For patients 65 years of age and older, the recommended dose is 100 mg once daily. Paediatric population: The safety and e cacy of Cibinqo in children under 12 years of age have not yet been established. No data are available. Cibinqo has been studied in adolescents 12 to < 18 years of age. However, because of bone findings in juvenile rats (comparable to a 3 month old human), additional long-term data in growing adolescents is needed to conclude that the benefits outweigh the risks. Method of administration: This medicinal product is to be taken orally once daily with or without food at approximately the same time each day. In patients who experience nausea, taking Cibinqo with food may improve nausea. Tablets should be swallowed whole with water and should not be split, crushed, or chewed because these methods have not been studied in clinical trials. Contra-indications: Hypersensitivity to the active substance or to any of the excipients. Active serious systemic infections, including tuberculosis (TB), severe hepatic impairment, pregnancy, and breast-feeding.
Warnings and Precautions:
Abrocitinib should only be used if no suitable treatment alternatives are available in patients:
• 65 years of age and older;
• patients with history of atherosclerotic cardiovascular disease or other cardiovascular risk factors (such as current or past long-time smokers);
• patients with malignancy risk factors (e.g. current malignancy or history of malignancy)
Infections/serious infections: Serious infections have been reported in patients receiving Cibinqo. The most frequent serious infections in clinical studies were herpes simplex, herpes zoster and pneumonia. As there is a higher incidence of infections in the elderly and in the diabetic populations in general, caution should be used when treating the elderly and patients with diabetes. In patients 65 years of age and older abrocitinib should only be used if no suitable treatment alternatives are available. Treatment must not be initiated in patients with an active, serious systemic infection. Risks and benefits of treatment prior to initiating Cibinqo should be considered. Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with abrocitinib. A patient who develops a new infection during treatment should undergo prompt and complete diagnostic testing and appropriate antimicrobial therapy should be initiated. The patient should be closely monitored, and therapy should be temporarily interrupted if the patient is not responding to standard therapy. Tuberculosis: Tuberculosis was observed in clinical studies with abrocitinib. Patients should be screened for TB before starting treatment and yearly screening for patients in highly endemic areas for TB should be considered. Abrocitinib must not be given to patients with active TB. For patients with a new diagnosis of latent TB or prior untreated latent TB, preventive therapy for latent TB should be started prior to initiation of treatment. Viral reactivation: Viral reactivation, including herpes virus reactivation (e.g., herpes zoster, herpes simplex), was reported in clinical studies. The rate of herpes zoster infections was higher in patients who were treated with 200 mg, 65 years of age and older, with a medical history of herpes zoster, with a confirmed ALC < 1 × 103/mm3 prior to the event and patients with severe atopic dermatitis at baseline. If a patient develops herpes zoster, temporary interruption of treatment should be considered until the episode resolves. Screening for viral hepatitis should be performed in accordance with clinical guidelines before starting therapy and during therapy. Patients with evidence of active hepatitis B or hepatitis C (positive hepatitis C PCR) infection were excluded from clinical studies. Patients who were hepatitis B surface antigen negative, hepatitis B core antibody positive, and hepatitis B surface antibody positive had testing for hepatitis B virus (HBV) DNA. Patients who had HBV DNA above the lower limit of quantification (LLQ) were excluded. Patients who had HBV DNA negative or below LLQ could initiate treatment; such patients had HBV DNA monitored. If HBV DNA is detected, a liver specialist should be consulted. Vaccination: No data are available on the response to vaccination in patients receiving Cibinqo. Use of live, attenuated vaccines should be avoided during or immediately prior to treatment. Prior to initiating treatment with this medicinal product, it is recommended that patients be brought up to date with all immunisations, including prophylactic herpes zoster vaccinations, in agreement with current immunisation guidelines. Venous thromboembolism (VTE): Events of deep venous thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients receiving abrocitinib. In a large randomized active-controlled study of tofacitinib (another JAK inhibitor) in rheumatoid arthritis patients 50 years and older with at least one additional cardiovascular risk factor, a dose dependent higher rate of VTE including deep venous thrombosis (DVT) and pulmonary embolism (PE) was observed with tofacitinib compared to TNF inhibitors. A higher rate of VTE was observed with abrocitinib 200 mg compared to abrocitinib 100 mg. In patients with cardiovascular or malignancy risk factors abrocitinib should only be used if no suitable treatment alternatives are available. In patients with known VTE risk factors other than cardiovascular or malignancy risk factors, abrocitinib should be used with caution. VTE risk
factors other than cardiovascular or malignancy risk factors include previous VTE, patients undergoing major surgery, immobilisation, use of combined hormonal contraceptives or hormone replacement therapy, inherited coagulation disorder. Patients should be re-evaluated periodically during abrocitinib treatment to assess for changes in VTE risk. Promptly evaluate patients with signs and symptoms of VTE and discontinue abrocitinib in patients with suspected VTE, regardless of dose Major adverse cardiovascular events (MACE): Events of MACE have been observed in patients taking abrocitinib. In a large randomized active-controlled study of tofacitinib (another JAK inhibitor) in rheumatoid arthritis patients 50 years and older with at least one additional cardiovascular risk factor, a higher rate of major adverse cardiovascular events (MACE), defined as cardiovascular death, non-fatal myocardial infarction (MI) and non-fatal stroke, was observed with tofacitinib compared to TNF inhibitors. Therefore, in patients 65 years of age and older, patients who are current or past long-time smokers, and patients with history of atherosclerotic cardiovascular disease or other cardiovascular risk factors, abrocitinib should only be used if no suitable treatment alternatives are available. Malignancy (excluding non-melanoma skin cancer [NMSC]): Lymphoma and other malignancies have been reported in patients receiving JAK inhibitors, including abrocitinib. In a large randomized active controlled study of tofacitinib (another JAK inhibitor) in rheumatoid arthritis patients 50 years and older with at least one additional cardiovascular risk factor, a higher rate of malignancies, particularly lung cancer, lymphoma and non-melanoma skin cancer (NMSC) was observed with tofacitinib compared to TNF inhibitors. A higher rate of malignancies (excluding non-melanoma skin cancer, NMSC) was observed with abrocitinib 200 mg compared to abrocitinib 100 mg. In patients 65 years of age and older, patients who are current or past long-time smokers, or with other malignancy risk factors (e.g. current malignancy or history of malignancy), abrocitinib should only be used if no suitable treatment alternatives are available. Non-melanoma skin cancer: NMSCs have been reported in patients receiving abrocitinib. Periodic skin examination is recommended for all patients, particularly those who are at increased risk for skin cancer. Haematologic abnormalities: Confirmed ALC < 0.5 × 103/mm3 and platelet count < 50 × 103/mm3 were observed in less than 0.5% of patients in clinical studies. Treatment with abrocitinib should not be initiated in patients with a platelet count < 150 × 103/mm3, an ALC < 0.5 × 103/mm3, an ANC < 1.2 × 103/mm3 or who have a haemoglobin value < 10 g/dL. Complete blood count should be monitored 4 weeks after initiation of therapy and thereafter according to routine patient management. Lipids: Dose dependent increases in blood lipid parameters were reported in patients treated with abrocitinib compared to placebo. Lipid parameters should be assessed approximately 4 weeks following initiation of therapy and thereafter according to the patient’s risk for cardiovascular disease. The e ect of these lipid parameter elevations on cardiovascular morbidity and mortality has not been determined. Patients with abnormal lipid parameters should be further monitored and managed according to clinical guidelines, due to the known cardiovascular risks associated with hyperlipidaemia. Elderly: The safety profile observed in elderly patients was similar to that of the adult population with the following exceptions: a higher proportion of patients 65 years of age and older discontinued from clinical studies and were more likely to have serious adverse reactions compared to younger patients; patients 65 years and older were more likely to develop low platelet and ALC values; the incidence rate of herpes zoster in patients 65 years of age and older was higher than that of younger patients. There are limited data in patients above 75 years of age. Use in patients 65 years of age and older: Considering the increased risk of MACE, malignancies, serious infections, and all-cause mortality in patients 65 years of age and older, as observed in a large randomised study of tofacitinib (another JAK inhibitor), abrocitinib should only be used in these patients if no suitable treatment alternatives are available. Immunosuppressive conditions or medicinal products: Patients with immunodeficiency disorders or a first-degree relative with a hereditary immunodeficiency were excluded from clinical studies and no information on these patients is available. Combination with biologic immunomodulators, potent immunosuppressants such as ciclosporin or other Janus kinase (JAK) inhibitors has not been studied. Their concomitant use with abrocitinib is not recommended as a risk of additive immunosuppression cannot be excluded. Lactose: Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take this medicinal product. Drug Interactions: Potential for other medicines to a ect pharmacokinetics of abrocitinib: Abrocitinib is metabolised predominantly by CYP2C19 and CYP2C9 enzymes, and to a lesser extent by CYP3A4 and CYP2B6 enzymes, and its active metabolites are renally excreted and are substrates of the organic anion transporter 3 (OAT3). Therefore, exposures of abrocitinib and/or its active metabolites may be a ected by medicinal products that strongly inhibit or induce theses enzymes and transporter. Dose adjustments, as appropriate, may be required. Co-administration with products which increase gastric pH: When abrocitinib 200 mg was administered concomitantly with famotidine 40 mg, an H2-receptor antagonist, abrocitinib active moiety exposures decreased by approximately 35%. The e ect of elevating gastric pH with antacids, or proton pump inhibitors (omeprazole) on the pharmacokinetics of abrocitinib has not been studied and may be similar to that seen with famotidine. The higher 200 mg daily dose should be considered for patients treated concomitantly with products which increase gastric pH, as they may reduce the e cacy of abrocitinib. Potential for Cibinqo to a ect pharmacokinetics of other medicinal products: No clinically significant e ects of Cibinqo were observed in drug interaction studies with oral contraceptives. Caution should be exercised for concomitant use of abrocitinib with dabigatran. Caution should be exercised as the levels of P-gp substrates with a narrow therapeutic index, such as digoxin, may increase. Caution should be exercised when using abrocitinib concomitantly with narrow therapeutic index medicines that are primarily metabolised by CYP2C19 enzyme (e.g. S mephenytoin and clopidogrel). Dose adjustment may be required for other medicines primarily metabolised by CYP2C19 enzyme in accordance with their product information (e.g. citalopram, clobazam, escitalopram and selumetinib). Fertility, pregnancy, and lactation: Women of childbearing potential: Women of reproductive potential should be advised to use e ective contraception during treatment and for 1 month following the final dose of Cibinqo. Pregnancy planning and prevention for females of reproductive potential should be encouraged. Pregnancy: There are no or limited amount of data on the use of abrocitinib in pregnant women. Studies in animals have shown reproductive toxicity. Abrocitinib has been shown to cause embryo-foetal lethality in pregnant rats and rabbits, skeletal variations in the foetuses of pregnant rats and rabbits and to a ect parturition and peri/postnatal development in rats. Cibinqo is contraindicated during pregnancy. Breast-feeding: There are no data on the presence of abrocitinib in human milk, the e ects on the breast fed infant, or the e ects on milk production. Abrocitinib was secreted in milk of lactating rats. A risk to newborns/infants cannot be excluded and Cibinqo is contraindicated during breast feeding. Fertility: Based on the findings in rats, oral administration of Cibinqo may result in temporary reduced fertility in females of reproductive potential. The e ects on female rat fertility were reversible 1 month after cessation of abrocitinib oral administration. Driving and operating machinery: Cibinqo has no e ect on the ability to drive or use machines. Side E ects: The most commonly reported adverse reactions are nausea (15.1%), headache (7.9%), acne (4.8%), herpes simplex (4.2%), blood creatine phosphokinase increased (3.8%), vomiting (3.5%), dizziness (3.4%) and abdominal pain upper (2.2%). The most frequent serious adverse reactions are infections (0.3%) including herpes simplex, herpes zoster, and pneumonia. Refer to SmPC for further information on side e ects. Legal Category: S1A. Marketing Authorisation Numbers: EU/1/21/1593/002 - Cibinqo 50 mg (28 film-coated tablets), EU/1/21/1593/007 - Cibinqo 100 mg (28 film-coated tablets); EU/1/21/1593/012 - Cibinqo 200 mg (28 film-coated tablets). Marketing Authorisation Holder: Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Bruxelles, Belgium. For further information on this medicine please contact Pfizer Medical Information on 1800 633 363 or at medical.information@pfizer.com. For queries regarding product availability please contact: Pfizer Healthcare Ireland, Pfizer Building 9, Riverwalk, National Digital Park, Citywest Business Campus, Dublin 24 + 353 1 467 6500.
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 of the SmPC for how to report adverse reactions.
Last revised: 03/2023.
Ref: CQ 5_0 IE.
References: 1. Bieber T, Simpson EL, Silverberg JI, et al. N Engl J Med. 2021;384(12):1101-1112. 2. Simpson EL, Sinclair R, Forman S, et al. Lancet. 2020;396(10246):255-266. 3. Silverberg JI, Simpson EL, Thyssen JP, et al. JAMA Dermatol 2020;156(8):863-873. 4. Boeri M, Sutphin J, Hauber B, et al. J Dermatolog Treat. 2020 Nov 2:1-10. doi:10.1080/09546634.1832185. 5. Reich K, Silverberg JI, Papp K, et al. Presented at the Revolutionizing Atopic Dermatitis Virtual Conference; 13 June 2021. 6. Ständer S, Kwatra SG, Silverberg JI, et al. Am J Clin Dermatol. 2023;24(1):97-107. doi: 10.1007/s40257-022-00738-4. 7. Cibinqo Summary of Product Characteristics. 8. Blauvelt A, Silverberg JI, Lynde CW, et al. J Am Acad Dermatol. Published online 17 August 2021. doi: 10.1016/j.jaad.2021.05.075. 9. Simpson EL, Silverberg JI, Nosbaum A, et al. British Journal of Dermatology, 2023;188,supp2. doi: 10.1093/bjd/ljac140.025.
Although some clinical trials included adolescents, please note that CIBINQO is indicated for the treatment of moderate-to-severe atopic dermatitis in adults who are candidates for systemic therapy.
TCS includes low- to medium-potency topical corticosteroids, topical calcineurin inhibitors, or topical phosphodiesterase 4 inhibitors, per protocol guidance in JADE COMPARE.
Nonmedicated topicals were also required.1
AD=atopic dermatitis; JAK=Janus kinase; LTE=long-term extension; TCS=topical corticosteroid. © 2023 Pfizer Inc. All rights reserved. March 2023. PP-CIB-IRL-0103






Whether your patients’ moderate-to-severe AD is FIERCE or TAMER,












































































 Urologist,
Urologist,



















 Written by Amy Nolan, Head of Children Adolescents and Young Adults and Rebecca Gorman, CAYA Advocacy Officer
Written by Amy Nolan, Head of Children Adolescents and Young Adults and Rebecca Gorman, CAYA Advocacy Officer









































































































































































 Professor Edward Gregg, Head
Professor Edward Gregg, Head






























































