Understanding Your Test Results
The IMF InfoLine team is here to support you and your loved ones with the most up-to-date information about myeloma
Call the IMF InfoLine at 1.800.452.CURE (toll-free in the U.S. & Canada) or 1.818.487.7455 (worldwide), or email InfoLine@myeloma.org with your questions, or if you wish to discuss the contents of this booklet.
Want answers and don’t want to wait?
Get the answers you need anytime from Myelo® , your 24/7 generative AI assistant that is designed to support you on your journey with myeloma. Ask Myelo your questions at myeloma.org Join the Myeloma Knowledge Platform Visit myprofile.myeloma.org and create your personal online IMF account to receive the most helpful content recommendations tailored to support your unique myeloma journey.
You are not alone
The International Myeloma Foundation (IMF) is here to help you. The IMF is committed to providing information and support for patients with multiple myeloma (which we refer to simply as “myeloma”) and their care partners, friends, and family members.
We achieve this through a broad range of resources available on our website myeloma.org, and through numerous programs and services such as seminars, webinars, workshops, and the IMF InfoLine, which consistently provides the most up-to-date and accurate information about myeloma in a caring and compassionate manner. Contact the IMF InfoLine at 1.818.487.7455 or InfoLine@myeloma.org.
What you will learn from this booklet
Myeloma is a cancer that is not known to most patients at the time of diagnosis. If you have myeloma, it is important and helpful for you to learn about your disease, its treatment options, and supportive care measures in order to play an active role in your own medical care and to make good decisions about your care in partnership with your doctor.
If you are a patient with myeloma, we suggest that you read the IMF’s publication, Patient Handbook for Multiple Myeloma, which will help you to better understand this disease. In addition, this booklet will direct you to resources that may be relevant in your particular case. All IMF publications are free-of-charge and can be read, downloaded, or requested in printed format at publications.myeloma.org.
The IMF’s Understanding-series publications address specific drugs, drug classes, and combination therapies used to treat myeloma. These booklets also discuss supportive care measures that may help manage the symptoms and side effects of myeloma and its treatments.
Words in bold+blue type are explained in the IMF’s companion publication, Understanding Myeloma Vocabulary, a comprehensive glossary that also can be helpful in discussions with your doctor. Myeloma is complicated, but the language that describes it doesn’t have to be hard to understand.
If you are reading this booklet in electronic format, the light blue links will take you to the corresponding resources.
This booklet discusses the tests that are used to diagnose, monitor, and assess myeloma status throughout the disease course.
Important note about your test results
Do NOT compare your test results to those of other patients as each case of myeloma is unique.
Test results best reflect a myeloma patient’s status when followed and reviewed over time. No single test is adequate to tell the whole story of a patient’s current myeloma status. A trend or pattern reveals more than a single test result or study. Each test can be thought of as a piece of a puzzle; only when the pieces are assembled correctly can a patient and an experienced hematologist or oncologist make the proper inferences and decisions.
Laboratory test results are usually reported as a quantity in relation to a “reference range.” Each institution or laboratory has its own “normal” range, which may be different from another institution or laboratory, and different from the sample reference ranges published in this booklet.
Some tests are not fully automated, and human subjectivity can have an impact on the results. In addition, factors that have nothing to do with your myeloma can affect your test results.
It is important for you to request and collect a copy of your own comprehensive medical records, including all your laboratory tests, imaging studies, genetic testing, and biopsy reports. If you have access to your medical records in digital format, copy and save the files in a password-protected healthcare folder you set up for this purpose on your personal device or cloud storage of your choice. If electronic records are not available, request paper records, then create digital copies by scanning or photographing them.
Precursors to myeloma Monoclonal gammopathy of undetermined significance
All patients who develop myeloma have previously had a benign condition called monoclonal gammopathy of undetermined significance (MGUS, pronounced “EM-gus”). Generally, MGUS is asymptomatic. It is usually discovered through laboratory testing that is either routine or performed for other phenomena.
While all patients with active myeloma once had MGUS, only 20% of patients with MGUS will eventually progress to active myeloma. The risk of progression from MGUS to active myeloma is 1% per year. Patients with MGUS have relatively low levels of monoclonal protein (myeloma protein, M-protein) in the serum and/or urine, and their bone marrow plasma cell levels are less than 10%.
Table 1. MGUS, SMM, and active myeloma
Presence of myeloma defining events no no yes
Likelihood of progression ~1% per year ~10% per year not applicable
Treatment or observation observation only observation; treatment* treatment
*Treatment in the setting of a clinical trial or for high-risk patients likely to progress to active myeloma within 2 years.
1. Kyle et al. N Engl J Med. 2007;356:2582-2590.
2. International Myeloma Working Group. Br J Haematol. 2003;121:749-57.
3. Jagannath et al. Clin Lymphoma Myeloma Leuk. 2010;10:28-43.
4. Kyle et al. Curr Hematol Malig Rep. 2010;5:62-69.
5. Mateos et al. Blood 2009;114: abstract 614.
6. Durie and Salmon. Cancer 1975;36:842-854.
7. Durie et al. Leukemia 2006;20:1467-1473.
8. Rajkumar et al. Lancet Oncol. 2014;15:e538-e548.
Patients with MGUS must be monitored carefully by an experienced hematologist. The laboratory tests used to diagnose and monitor MGUS are discussed in detail in the IMF’s publication Understanding MGUS and Smoldering Multiple Myeloma.
Smoldering multiple myeloma
Smoldering multiple myeloma (SMM, pronounced “ess-em-EM”) is an asymptomatic precursor state of active myeloma. Patients with SMM have between 10% and 60% myeloma cells present in the bone marrow.
For patients with standard-risk SMM, the risk of progression to active myeloma is 10% per year for the first 5 years, then 3% per year for the next 5 years, then 1%–2% per year for the next 10 years. Patients with SMM must be monitored carefully at regular intervals by an experienced hematologistoncologist, preferably by a myeloma specialist.
The diagnostic criteria for SMM, an individual patient’s risk of progressing to active myeloma, and recent clinical trial experience with observation vs. treatment of high-risk SMM are discussed in detail in the IMF’s publication Understanding MGUS and Smoldering Multiple Myeloma.
Please note that not all cases of active myeloma are preceded by SMM. An individual with MGUS can progress directly to active myeloma without first progressing to SMM.
Active myeloma
Myeloma cells are malignant (cancerous) plasma cells that do not make functioning antibodies, but instead produce an abnormal M-protein that can lead to organ and tissue damage. Myeloma most often grows in the
marrow within the bones of the spine, skull, pelvis, rib cage, shoulders, and hips. The presence of myeloma cells within the bone marrow can also lead to other medical problems.
Myeloma is a highly treatable disease. Many effective therapies are approved for use in myeloma by the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and by other regulatory agencies. Myeloma research is currently taking place around the world, adding more promising therapies to the growing list of treatment options.
Many myeloma patients lead full and productive lives for years, even decades, after diagnosis. Survival and quality of life of myeloma patients are improving steadily. Learning about myeloma and understanding how it is diagnosed, assessed, treated, and monitored can help patients and their loved ones reduce their anxiety and gain a sense of control.
Your test results
Testing is the most important tool that your doctor uses in order to:
¡ Diagnose MGUS, SMM, or active myeloma.
¡ Assess the stage of your myeloma.
¡ Assess your genetic risk factors.
¡ Assess the risk of disease progression.
¡ Assess your response to treatment.
¡ Monitor you during periods of remission.
¡ Determine if treatment needs to be started.
¡ Monitor for disease-related and treatment-related side effects.
Many tests may be required. Some tests may be used to assess other diseases that you may have had before or after the diagnosis of myeloma. Make sure you know which tests are for myeloma and which are for other medical issues. Tests for myeloma fall into three major categories:
1. Blood and urine tests.
2. Bone marrow tests.
3. Imaging studies.
It is always best to discuss your test results with the doctor treating your myeloma. The key points to discuss with your doctor are:
¡ Is your lab test result outside the normal range or not?
¡ Does your test result show a change from prior testing?
¡ If your test shows an unexpected change, ask your doctor to repeat the test.
1. Sample test results
H (for “high”) indicates that your test result is higher than the upper end of the reference range.
L (for “low”) indicates that your result is lower than the reference range.
Blood and urine tests
Units of measurement
¡ Lab results can vary depending on whether they measure grams or milligrams, liters or deciliters, etc. Note to patients in the United States: There is no time like the present to brush up on the metric system!
¡ Make sure you know the units that are used to measure your blood and urine samples. For example, if your sample is measured in grams per deciliter (g/dL), the resulting number will be one tenth of the result written in grams per liter (g/L). Another way to express this is: 0.1 g/dL = 1 g/L or 1 g/dL = 10 g/L. For example, one lab may show that you have 0.3 g/dL of M-protein but another lab may express the result as 3.0 g/L of M-protein. While the results are expressed in different units, the quantity is the same because 3.0 g/dL = 30 g/L.
Figure
Other variables
Your lab results can be affected by many factors:
¡ Other medications and supplements that you may be taking.
¡ The amount and type of fluids you have consumed.
¡ Whether or not you have eaten prior to the test.
Consult with your doctor to make sure there are no special instructions about medications, supplements, food, or drink prior to appointments when your blood and/or urine are collected.
Interpreting and comparing test results
Patient-to-patient differences
DO NOT COMPARE YOUR LAB RESULTS TO THOSE OF OTHER PATIENTS. Each patient’s myeloma is unique. For example, one patient’s test result might confirm stable remission, while another patient with the same test result might have active disease.
Changes over time
Myeloma is a cancer that evolves biologically, so a test that is a reliable marker for you when you are diagnosed may not be a reliable test for you later on. Conversely, a test that is not highly useful for you when you are diagnosed may be your most valuable assessment tool later in the disease course.
Importance of patient education
It is important for patients to educate themselves about how their myeloma is diagnosed, treated, and monitored, as well as the available supportive care. You can gain this understanding through discussions with your doctors and nurses.
Both the IMF International Myeloma Working Group (IMWG) and the National Comprehensive Cancer Network (NCCN) provide guidelines for appropriate tests to be done throughout the myeloma disease course, from initial diagnostic workup, to monitoring response to therapy, to monitoring for treatment side effects, to monitoring for relapse. At diagnosis, these tests should always be accompanied by an appointment during which your doctor takes your complete medical history, speaks with you about your health, and performs a physical exam – the “hands-on” part of diagnosing and assessing a patient.
Complete blood count (CBC)
The CBC is one of the main tests needed for diagnosing and monitoring myeloma patients. Many cases of MGUS, SMM, and myeloma are identified as the result of blood tests routinely ordered as part of an annual medical exam. The CBC is a blood test that quantifies all the cells that make up the solid parts of blood.
All of your blood cells – red blood cells (RBC), white blood cells (WBC), and blood-clotting cells called platelets – are made in the bone marrow, which is where myeloma grows. Both myeloma itself as well as many treatments for myeloma affect the ability of new blood cells to grow in the bone marrow. Your CBC will be monitored carefully throughout your treatment course to make sure that your blood cell counts are not decreasing to dangerous levels. Sometimes, patients must have a CBC every week to make sure that a particular treatment is not taking a toll on one or more of the blood cell types. CBC tests measure the following components:
Red blood cells (RBC)
REFERENCE RANGE (1012 = 1 trillion) for males 4.32–5.72 x 1012/L for females 3.90–5.03 x 1012/L Red blood cells are also called erythrocytes. These cells in the blood contain hemoglobin, deliver oxygen to all parts of the body, and take away carbon dioxide. Red blood cells are usually the first to decrease in number in response to active myeloma.
Hemoglobin (hgb)
REFERENCE RANGE for males 13.5–17.5 g/dL (135–175 g/L) for females 12.0–15.5 g/dL (120–155 g/L)
Hemoglobin, the most important part of the red blood cell, is a protein that transports oxygen to the organs and tissues of the body. A low hemoglobin count can be a sign of anemia, one of the CRAB criteria that are characteristic of active myeloma: elevated Calcium, Renal (kidney) dysfunction, Anemia, and Bone disease. The doctor will keep a close watch on your hemoglobin throughout your myeloma disease course, as it can be an early indicator of myeloma activity in the bone marrow.
Hematocrit (hct)
REFERENCE RANGE
Hematocrit is the volume percentage of red blood cells in the blood. Usually the percentage of RBC is about 45% for men and 40% for women.
Figure 2. Blood composition
Red blood cells
White blood cells
Platelets
Plasma
Slaybaugh Studios
White blood cells (WBC)
REFERENCE RANGE (109 = 1 billion)
3.5–10.5 x 109/L
White blood cells make up the body’s immune system. They fight foreign substances that enter the body, including bacteria, viruses, and toxins. Low WBC counts can result from many types of treatment for myeloma, which can further diminish your ability to fight disease. Your WBC count will be followed carefully during your treatment for myeloma.
Neutrophils
REFERENCE RANGE
1.7–7.0 x 109/L
Neutrophils are a type of WBC that helps fight infections, particularly those caused by bacteria and fungi. A low neutrophil count is called neutropenia, a condition that results in susceptibility to infection. Your doctor will check your neutrophil count, sometimes expressed as ANC (Absolute Neutrophil Count, which measures both mature and immature neutrophils), to make sure that it is safe to give you a dose of a particular treatment.
Platelets
REFERENCE RANGE
150–450 x 109/L
Figure 3. Blood cells that play a role in the immune system
Platelets (thrombocytes) are blood cells that help the blood to clot and prevent bleeding. Although low hemoglobin is a more common blood-related symptom of myeloma than is a low platelet count (thrombocytopenia), some patients do have low platelets at diagnosis as a result of their myeloma. In addition, certain treatments for myeloma, in particular the proteasome inhibitors Velcade® (bortezomib), Kyprolis® (carfilzomib), and Ninlaro® (ixazomib) can also cause low platelets, resulting in severe bruising or bleeding. Your platelet count should be monitored throughout your disease course.
Hematopoietic stem cell Monocyte
B lymphocyte
Macrophage
Lymphocyte
T lymphocyte
NK cell
Plasma cell
Dendritic cell
Myeloma cell (malignant plasma cell)
Chemistry profile
The comprehensive metabolic panel (CMP) is a key test in the diagnosis and monitoring of myeloma. Along with the CBC, it is part of a routine physical exam. The individual CMP tests recommended for myeloma patients by both the IMWG and the NCCN are:
BUN (blood urea nitrogen)
REFERENCE RANGE
7–20 mg/dL
This test provides information about how well your liver and kidneys are functioning.
Serum creatinine
REFERENCE RANGE
0.6–1.3 mg/dL
This test is used to assess the “R” in the CRAB criteria – Renal (kidney) function. Creatinine is a waste product from the normal breakdown of muscle tissue. It is filtered through the kidneys and excreted in urine. Measurement of the serum creatinine level is a useful indicator of how well your kidneys are functioning. Kidney function can be seriously affected by light chain proteins, so it’s very important to assess kidney function at diagnosis and at regular intervals thereafter, particularly if you have light chain Bence-Jones protein in your urine. Kidney function can also be affected by high levels of calcium in the blood, which can result from myeloma-induced bone breakdown.
Creatinine clearance
REFERENCE RANGE (mL/min = milliliters per minute) for males 97–137 mL/min for females 88–128 mL/min
Creatinine clearance is the amount of blood per minute that the kidneys can make creatinine-free. The measurement of creatinine clearance helps provide information about kidney function. It requires both 24-hour urine collection and a blood sample. Creatinine clearance (and therefore kidney function) declines naturally with age, explaining the wide range of normal values. People over 60 – the vast majority of myeloma patients – may have an apparently normal serum creatinine level but have a low rate of creatinine clearance. The 24-hour urine sample provides a more accurate assessment of decline in kidney function than does the serum creatinine test.
Creatinine clearance of less than 40 mL per minute is considered a sign of early active myeloma in a patient who otherwise has no CRAB features. These patients should be treated rather than merely observed for disease progression.
Estimated glomerular filtration rate (eGFR)
REFERENCE RANGE
90–120 mL/min/1.73 m2
The eGFR is used in conjunction with the measurement of creatinine in the serum to screen for and detect kidney damage. It is estimated rather than actual because it is calculated from the serum rather than from a 24-hour urine sample. It is usually calculated automatically at the time the creatinine is measured. This test is not accurate for people who are older than 70, very overweight, very muscular, or pregnant.
Calcium
REFERENCE RANGE
9–10.5 mg/dL
This test is used to assess the “C” in the CRAB criteria – elevated Calcium in the blood. Calcium is stored in the bones and is released as part of normal bone remodeling (the body constantly breaks down and rebuilds bone). Myeloma grows in the bone marrow, where it changes the environment inside the marrow and causes a cascade of cellular events that can result in increased bone breakdown. Increased bone breakdown results in both an increased level of calcium in the blood and an increased risk of fractures. A high blood calcium level can also damage the kidneys.
Total protein
REFERENCE RANGE
6–8 g/dL
Total protein measures the total amount of blood protein, including both albumin (the most plentiful protein in the blood) and globulin. If M-protein is present in the blood, it will increase the amount of blood globulin, causing the amount of total blood protein to rise. At diagnosis, an elevated total protein should prompt a doctor to order additional, more specific tests to see if the source of elevated globulin protein might be from myeloma. If you have been diagnosed and treated for active myeloma, your doctor will use more specific tests to assess the amount of M-protein in your blood and/or urine.
Tests to assess M-protein
Serum quantitative immunoglobulins (QIg)
REFERENCE RANGE for patients ≥ 18 years old
IgG 767–1,590 mg/dL
IgA 61–356 mg/dL
IgD ≤ 10 mg/dL
IgE ≤ 214 mg/dL
IgM 37–286 mg/dL
QIg testing measures the total amount of each primary immunoglobulin or antibody class (also called an “isotype”) in the serum. It measures both
polyclonal (normal) and monoclonal (myeloma-related) immunoglobulins, so if an increase in one of the antibody isotypes is found, further testing with electrophoresis is required to determine if the elevation is caused by the presence of monoclonal immunoglobulin protein. QIg is often the preferred way to assess IgA protein, because IgA may be difficult to quantify with electrophoresis. Note: The ranges for IgD and IgE are reported differently from those for IgG, IgA, and IgM.
Serum protein electrophoresis (SPEP)
REFERENCE RANGE
albumin 3.3–5.7 g/dL
alpha-1 0.1–0.4 g/dL
alpha-2 0.3–0.9 g/dL
beta-2 0.7–1.5 g/dL
gamma 0.5–1.4 g/dL
SPEP is one of the most important tests used to diagnose and assess the status of myeloma. It measures the amount of M-protein that is made by myeloma cells. The amount of M-protein production is linked to the number and/or activity of myeloma cells, except in the case of non-secretory myeloma.
Table 2. Isotypes of immunoglobulins
kappa lambda
IgG kappa IgG lambda
IgA kappa IgA lambda
IgM kappa IgM lambda
IgD kappa IgD lambda
IgE kappa IgE lambda
Figure 4. Structures of immunoglobulins
IgG, IgE, IgD
IgM
SPEP separates and quantifies proteins based on their electrical charge, size, and shape. As you learned above, the two types of protein in serum are albumin and globulin. Although there is only one type of albumin, there are sub-types of globulin, usually appearing on SPEP as alpha-1, alpha-2, beta-1, beta-2, and gamma globulins. Immunoglobulin proteins are gamma globulins, and usually, M-protein produced by myeloma cells will separate out in the gamma region of the graph. The exception to this rule is IgA protein, which may sometimes migrate to the beta or even the alpha-2 region. If this occurs, other tests can be more useful than SPEP.
IgA
Figure 5. SPEP test results
Abnormal result with myeloma cells producing the M-protein, creating an M-spike in the beta-2 region
Monoclonal immunoglobulin protein appears on the results graph as a narrow spike: Because all the cells in the M-protein are identical and have the same electrical charge, they gravitate to the same small gamma region and form a peak or spike on the graph. The M-spike is the telltale indicator of myeloma protein in the blood, a marker for the activity of myeloma cells. By calculating the area under the curve (AUC) of the spike and then subtracting the amount representing normal immunoglobulins, the pathologist can quantify the amount of M-protein.
Abnormal result with myeloma cells producing the M-protein, creating an M-spike in the gamma region
Serum albumin accounts for 55% of the total protein in the clear liquid part of the blood and is produced in the liver. When myeloma is active, it stimulates the production of certain cellular proteins (cytokines) that impede the ability of the liver to produce albumin, and the level of albumin in the serum drops. According to the revised International Staging System (R-ISS), albumin is one of two blood proteins used to predict the behavior of myeloma cells at diagnosis; the other is serum beta-2 microglobulin (β2-microglobulin, β2M, or β2M).
Urine protein electrophoresis (UPEP)
REFERENCE RANGE
globulins in the urine no significant amount total protein < 167 mg/24 hours urine albumin < 5 mg/dL
Approximately 30% of myeloma patients have light chain protein in their urine as well as heavy chain protein in the blood. Approximately 15%–20% of patients have myeloma cells that produce only light chains and no heavy
Normal SPEP result
chains. Although fragments of heavy chain proteins can be found in the urine, intact heavy chains are too large to fit through the capillaries that send blood to the kidneys for filtration. Light chains, however, are so small and light in molecular weight (hence the name “light” chains) that they can easily pass through the capillaries and enter the kidneys, and from there, to the bladder and into the urine.
Patients submitting a specimen for UPEP must collect urine for 24 hours. A 24-hour urine specimen provides a good indication of average amounts of different proteins in the urine. UPEP separates the proteins according to their size and electrical charge, and quantifies the amount of light chain protein.
Immunofixation electrophoresis (IFE)
The IFE laboratory test identifies the type of M-protein (heavy chain IgG, IgA, IgD, IgE, or IgM; kappa or lambda light chain). It does not measure the amount of M-protein, so there is no reference range. Urine IFE is performed on a 24-hour urine collection. The test result is either negative or positive for the presence of a specific type of M-protein. Serum or urine immunofixation that is negative for an M-protein is considered normal. SPEP and IFE provide different but complementary information. You need to be aware of both the amount (SPEP) and type (IFE) of immunoglobulin protein made by your myeloma cells.
The IMWG Uniform Response Criteria for Multiple Myeloma define complete response (CR) to therapy as:
¡ Negative immunofixation on the serum and urine, and
¡ disappearance of any soft tissue plasmacytomas, and
¡ ≤ 5% plasma cells in the bone marrow.
Serum free light chain (sFLC)
REFERENCE RANGE
mg/dL
mg/dL
The reference range cited above is used by the Mayo Clinic. Your laboratory may use a different reference range.
Immunoglobulins are made up of heavy chains and light chains, which are usually bound together as “intact immunoglobulins.” Plasma cells produce more light chains than heavy chains, and these unbound light chains circulate freely in the blood. These “free” light chains are present in both healthy individuals and in patients with myeloma. Healthy individuals have many intact antibody molecules circulating in their blood, but they have fewer free light chains.
Normal
High
Low
Table 3. Possible serum free light chain (sFLC) test results
Interpretation
No evidence of monoclonal light chains. Confirm with other lab tests.
Monoclonal kappa free light chains
Monoclonal lambda free light chains
Plasma cells of both light chain types are secreting antibodies (possible infection, autoimmunity, or kidney damage). Normal
General immune suppression (possibly due to chemotherapy or other reasons).
Possible low-level monoclonal kappa free light chains or immune suppression of lambda. Low
Possible low-level monoclonal lambda free light chains or immune suppression of kappa.
“Slightly” high “Slightly” high Normal or “slightly” high
Possible kidney damage (“slightly” = up to approximately 3.1).
Figure 6. Example of light chain levels and number of myeloma cells in two different myeloma patients
Patient 1:
More myeloma cells produce fewer light chains
Free light chains Cells
Patient 2: Few myeloma cells produce more light chains
Number of myeloma cells
Free light chain levels
The sFLC test can show if the myeloma cells are releasing kappa free light chains at a high level and lambda light chains at a normal or lower level. The sFLC test can also show if the myeloma cells are secreting lambda free light chains at a higher level and kappa free light chains at a normal or lower level.
Some patients’ myeloma cells secrete both heavy and light chains. Some secrete only heavy chains. Some secrete only light chains; this is called Bence-Jones myeloma. Some patients’ myeloma cells may secrete many more light chains at relapse than they did prior to treatment.
The sFLC test is useful for patients with any type of myeloma, including low secreting or non-secretory myeloma, and at different stages of disease, during treatment and after treatment. Your sFLC test results are a meaningful way to monitor your myeloma when the tests are tracked over time. Do not compare your sFLC test results with other patients.
The sFLC test is also used in the diagnosis and monitoring of patients with MGUS, and in monitoring patients with SMM. For more information, read the IMF’s booklet Understanding MGUS and Smoldering Multiple Myeloma.
Mass spectrometry
Mass spectrometry (“mass spec”) is a simple blood test, which is a highly sensitive new method to detect and measure the precise amount of M-protein in the blood. Mass spec is helpful in the diagnosis and monitoring of MGUS, SMM, and active myeloma. During assessment of response to treatment, much deeper levels of response and much earlier indicators of response can be detected.
PROGNOSTIC FACTOR
ISS Stage I
CRITERIA
Serum β2-microglobulin < 3.5 mg/L, serum albumin ≥ 3.5 g/dL II Not ISS stage I or III
III
Serum β2-microglobulin ≥ 5.5 mg/L
CA by FISH High Risk Presence of one or more of the following: del(17p), t(4;14), t(14;16)
Standard Risk No high-risk CA
LDH Normal
High
Serum LDH < the upper limit of normal
Serum LDH > the upper limit of normal
A new model for risk stratification for multiple myeloma
R-ISS Stage I
ISS stage I and standard-risk CA by FISH and normal LDH
II Not R-ISS stage I or III
III
ISS stage III and either high-risk CA by FISH or high LDH
CA, chromosomal abnormalities; FISH, fluorescence in situ hybridization; ISS, International Staging System; LDH, lactate dehydrogenase; R-ISS, Revised International Staging System.
Table 4. Risk factors for myeloma and the R-ISS
While mass spec is a simple blood test, it is currently conducted at only a few reference laboratories, such as Mayo Clinic Medical Labs in Rochester, Minnesota. It is anticipated that mass spec will be approved by the FDA in the near future, which would increase access to this test.
In February 2021, Blood Cancer Journal published a report by the International Myeloma Working Group (IMWG) Mass Spectrometry Committee on the benefits of mass spec methods in diagnosing and monitoring patients with myeloma and related disorders. Mass spec can distinguish between very low levels of M-protein and blood levels of monoclonal antibodies used to treat myeloma. Making this distinction can confirm if the patient has achieved CR.
In December 2022, at the 64th Annual Meeting & Exposition of the American Society of Hematology (ASH), a research study was presented that compared the impact of mass spec in peripheral blood vs. next-generation flow (NGF) in bone marrow to assess minimal residual disease (MRD) in newly diagnosed myeloma patients receiving maintenance as part of the GEM2014MAIN clinical trial. The complementary testing in this study demonstrated similar ability to predict a shorter or a longer remission. Since mass spec is a test performed in the blood, it can be used as a screening method: if mass spec is negative, then this could indicate a need for bone marrow testing to check if residual disease can be found.
Other useful blood tests
Beta-2 microglobulin (β2-microglobulin, β2M, β2M)
REFERENCE RANGE
0.70–1.80 mg/L
The serum β2M level indicates the amount and activity of the underlying myeloma. It is one of two blood proteins (the other is serum albumin) included in the revised International Staging System (R-ISS) to help understand the potential for the aggressiveness of newly diagnosed myeloma. In addition to its function in staging myeloma during the initial work-up, β2M can be used to evaluate disease activity and to monitor response to treatment. A serum β2M of < 3.5 mg/L is considered stage I; between 3.5 mg/L and 5.5 mg/L is stage II; and ≥ 5.5 mg/L is stage III.
Lactate dehydrogenase (LDH)
REFERENCE RANGE
105–333 IU/L (international units per liter)
High LDH can be a sign of aggressive disease and is therefore included in the R-ISS to help determine prognosis. LDH is an enzyme found in almost all body tissues. It plays an important role in cellular respiration, the process by which glucose (a sugar) is converted into usable energy for cells. Although LDH is abundant in tissue cells, blood levels of the
enzyme are normally low. However, when tissues are damaged by injury or disease, they release more LDH into the bloodstream. LDH rises when myeloma is actively growing.
C-reactive protein (CRP)
REFERENCE RANGE
≤ 8.0 mg/L (in healthy individuals, < 8 mg/L)
CRP is an indicator of inflammation, and its measurement is used routinely in assessing heart disease and autoimmune diseases. CRP is produced by the liver and released into the bloodstream within a few hours after tissue injury, the start of an infection, or another cause of inflammation. Increased levels of CRP indicate active myeloma.
Glucose
REFERENCE RANGE
70–100 mg/dL (fasting blood glucose level)
The level of glucose, a major source of energy for most cells, should be established before you start treatment, and should be monitored carefully if you are taking dexamethasone or another glucocorticosteroid. These steroids, which are common components of myeloma treatment regimens, can cause the level of blood glucose (sugar) to rise. If not controlled, elevated blood sugar can result in diabetes.
Bone marrow tests
Myeloma cells grow in the bone marrow, and the only way to assess their properties is to remove a sample of these cells and to analyze them. Some of these tests can be done by a pathologist looking through a microscope and others require sophisticated computers with customized software programs. The aim is to assess how many myeloma cells there are, what they look like as compared to normal plasma cells, what their cellular genetics are, how rapidly they’re reproducing, and which type of antibodies they express.
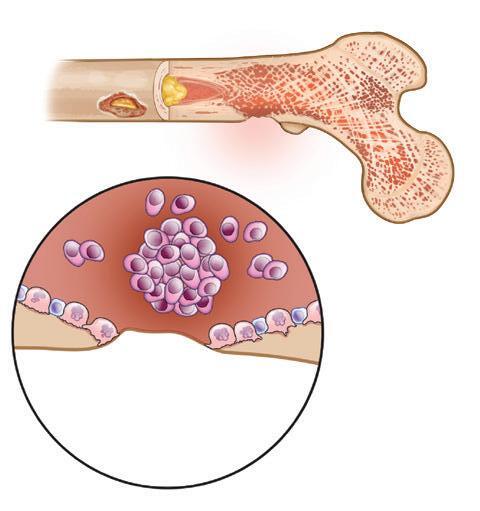
Figure 7. Healthy bone compared to bone damaged by myeloma
Bone marrow aspiration and bone marrow biopsy are routinely performed at diagnosis and are ordered at the doctor’s discretion - + Myeloma cells Lytic lesion
HEALTHY BONE
MULTIPLE MYELOMA
after treatment, annually during periods of remission, and at other points when the doctor deems it necessary to determine a patient’s status. Bone marrow biopsy is also a reliable way to monitor the status of patients with oligosecretory or non-secretory myeloma; also, SFLC can be used in a number of such cases.

The bone marrow is composed of both solid and liquid matter: The solid part is a sponge-like structure consisting of a fibrous network filled with liquid; the liquid portion contains bloodmaking (hematopoietic) stem cells, blood cells in various stages of maturation, and such raw materials required for cell production as iron, folate, and vitamin B12. A complete bone marrow exam requires both an aspirate of the liquid portion of the marrow and a core specimen of the solid portion, which includes a piece of the bone tissue with its enclosed marrow. To make the process less uncomfortable, bone marrow aspiration and biopsy should be performed by experienced technicians using anesthetics and improved needles.
Bone marrow aspiration and core biopsy
Plasma cell percentage NORMAL RANGE
1%–2% (Note: < 5% is CR, or complete response)
The pathologist will examine the bone marrow aspirate or core under the microscope and determine the percentage of plasma cells in the sample. Normal bone marrow has about 2% or fewer plasma cells. The presence of 60% or more plasma cells in the bone marrow is an independent myeloma-defining event.
Although myeloma cells aren’t distributed evenly throughout the bone marrow in the skeleton, the iliac crest is most often chosen as the biopsy site because it provides a representative sample.
Plasma cell morphology
The appearance of myeloma cells is distinct, with large nuclei that make the cells look like pimiento-stuffed olives. The appearance and number of these cells are recorded by the pathologist. Words such as
Site of biopsy
Figure 8. Bone marrow biopsy
“mature,” “immature,” or “atypical” are used to describe the plasma cells. Generally, “mature” cells suggest a better prognosis than “immature” or “atypical” plasma cells.
Specimen quality
Based on the quality and condition of the sample under the microscope, the doctor can ascertain if the sample is representative of what is going on in the patient’s bone marrow.
Immunohistochemistry and flow cytometry
Immunohistochemistry (IHC), also called immunophenotyping, is the process of detecting antigens in tissue samples by introducing antibodies that bind to them.
IHC is one of the tests used to determine stringent complete response (sCR) to therapy as defined by the IMWG Uniform Response Criteria. In addition to the criteria for CR, the IMWG criteria for sCR include a normal free light chain ratio and the absence of clonal plasma cells in the bone marrow by immunohistochemistry or immunofluorescence.
Immunophenotypic analysis of a myeloma patient’s bone marrow identifies myeloma protein markers, if they are present. A fluorophore, or fluorescent marker, is attached to each antibody, which glows when it finds the correct antigen on the surface of the myeloma cells. Several antibodies are usually used simultaneously; the fluorophores are given different colors (fluorochromes) for each antibody. The bone marrow sample cells and selected antibodies are sent through a flow cytometer, which is a laser-based instrument that reads the fluorophores and identifies and sorts the myeloma cells.
NGF cytometry, a highly accurate way to detect minimal residual disease after treatment, uses many antibodies and myeloma-specific computer software to rapidly perform eight-color immunophenotyping of myeloma cells. This test can detect 1 myeloma cell per approximately 1,000,000 bone marrow cells in a laboratory sample.
Cytogenetics
Human beings have two copies of each of their 23 chromosomes in every cell in their bodies. Standard cytogenetics is the assessment of the chromosomes in dividing cells after brief culture in the laboratory. Since the active growth rate of myeloma cells is usually fewer than 3%, this provides an incomplete assessment of any chromosomal changes present. Nonetheless, if abnormalities are noted, they are important, because they appear in the few cells that are actually growing.
Figure 9. Karyotype analysis of
This test may be performed on the bone marrow of newly diagnosed myeloma patients and then performed again after treatment to see if the therapy has eliminated all the cells with chromosomal abnormalities. Standard cytogenetics may also be performed at relapse to help determine if it is time to resume therapy, and if one therapy might be preferable to another.
In the laboratory, after the bone marrow specimen is allowed to grow in a special dish, the cells are stained and photographed to provide a karyotype that shows the number and arrangement of the chromosomes. The chromosomes can be karyotyped only if the cells are undergoing division. Karyotyping is particularly valuable for identifying higher-than-average-risk myeloma in patients with fewer than two copies of each chromosome (hypodiploidy) and in those whose 13th chromosome is partially deleted during cell division (called “del 13” or “13q-“).
FISH
Fluorescence in situ hybridization (FISH) is a test used to assess genetic risk based on chromosomal abnormalities. FISH is not a substitute for karyotyping but is complementary to it. FISH is the assessment of the chromosomes of all myeloma cells in a bone marrow sample. FISH allows detection of changes whether myeloma cells are growing or not.
FISH can detect numerical and structural chromosomal abnormalities. The cells are fixed in paraffin wax, then fluorescent probes that bind to certain sequences of the chromosome are attached, and each chromosome can be identified by a different color.
Chromosomes are made up of two chromatids paired in an X form, with the X shorter at the top and longer on the bottom. The “short arms” at the top half of the X are labeled “p” and the “long arms” at the bottom are labeled “q.” During normal cell division, the chromosomes divide in two, each single chromatid forming a duplicate of its genetic material in a new cell.
FISH is capable of detecting chromosomal translocations that can occur when gene sequences of one chromatid get shifted over to another chromatid during cell division, and the colors of the fluorescent probes from one chromosome appear in a different chromosome. Chromosomal deletions can be detected when a fluorophore color is absent.
FISH results have been incorporated into the revised International Staging System (R-ISS) for myeloma because they provide a powerful tool for predicting risk and survival in myeloma. The following cytogenetic abnormalities are considered to confer high risk:
¡ t(4;14) (translocation of gene segments from chromosome 4 to 14).
¡ 17p–, del 17p (deletion of the short arm of chromosome 17).
¡ t(14;16) (translocation of gene segments from chromosome 14 to 16).
¡ 1q+ (gain of an additional long arm on chromosome 1).
While some chromosomal abnormalities signal aggressive myeloma, others have no negative prognostic impact. Nearly all myeloma patients demonstrate deletion of all or parts of chromosome 13 by FISH analysis, which is why deletion 13 is better determined by standard cytogenetics than by FISH.
The loss of the short arm of chromosome 17 by FISH analysis confers especially high risk because an important tumor suppressor gene, p53,
Figure 10. Fluorescence in situ hybridization (FISH) of a myeloma cell
Figure 11. Chromosomal abnormalities in high-risk myeloma
Deletion
Translocation
is located there. Tumor suppressor genes, also known as anti- oncogenes, control cell division and help prevent cancer cells from developing.
Therapy choices are often related to chromosomal status. For example, regimens that contain Velcade for induction and maintenance therapy are the preferred treatment for patients with t(4;14) myeloma. Pomalyst® (pomalidomide) is less effective than Velcade for t(4;14), but in studies thus far, Pomalyst is more effective than Velcade for overcoming the negative impact of del 17p.
Imaging studies
Bone disease is characteristic of myeloma, with 70%–80% of patients presenting with bone disease at diagnosis. For these patients, and for those who develop bone disease in the course of their myeloma, imaging studies are helpful to assess the amount and distribution of myeloma lesions within the bone marrow as well as the presence of a potential pathologic fracture. Repeated imaging is indicated if pain or nerve compression are likely induced by bone lesions, and to exclude extramedullary disease. Ask your doctor which imaging studies might be helpful for monitoring your myeloma. Hematologist-oncologists have a number of available options for imaging studies.
You may also find it helpful to read the IMF’s publication Understanding Treatment of Myeloma-Induced Vertebral Compression Fractures.
X-ray
For many years, X-rays of the whole body have been used to diagnose and assess myeloma-related bone damage. The use of more sensitive studies is now recommended due to the following limitations of X-rays:
¡ 30% or more of the trabecular bone (the spongy part of the bone containing fat and bone marrow, where myeloma cells grow) must be missing before an X-ray can reveal the damage. A study showed that bone loss in lumbar vertebrae can be seen on an X-ray only when 50%–75% of the trabecular bone has already been destroyed.
¡ X-ray is not a sensitive study for focal lesions in the bone marrow.
¡ The appearance of a lytic lesion on an X-ray does not change following therapy, even if there is no longer any active myeloma there.
¡ X-ray provides low visualization of the spine and pelvis.
¡ X-ray cannot accurately depict the cause of lesions in myeloma.
¡ Because wholebody X-ray (WBXR) requires 20 separate films, the study is time-consuming.
CT or CAT scan
Figure 12. X-ray of a hip fracture due to multiple myeloma
Computed tomography (CT), also called computed axial tomography (CAT), is a radiological study that uses X-ray technology to create a crosssectional, three-dimensional image of the inside of the body. It is a more precise study than X-ray and can provide clear, detailed images of bone.
¡ CT allows for the detection of small bone lesions that are not detectable by plain X-rays. In 20%–25% of patients with negative X-ray studies, whole-body CT will detect destructive bone lesions.
¡ CT can detect soft tissue masses that are not visible on X-ray.
¡ CT provides a more comprehensive assessment of fracture risk and the stability of collapsed vertebrae than X-ray.
To detect and document early bone disease, the IMWG recommends the use of whole-body low-dose CT (WBLDCT) as the preferred baseline imaging study for newly diagnosed myeloma patients. It has the following advantages:
¡ WBLDCT is faster and more convenient than WBXR.
¡ WBLDCT uses two to three times less radiation than conventional CT.
¡ WBLDCT does not require the use of contrast agents.
In 2017, the IMWG published a study, concluding that whole-body CT, either alone or as part of a PET-CT protocol, should be considered the current standard for the detection of osteolytic lesions in myeloma.
In 2018, the IMWG published its recommendations for acquisition, interpretation, and reporting of WBLDCT in patients with myeloma and other plasma cell disorders, thus establishing international protocol standards for this imaging study that can be followed by radiologists everywhere.
In 2019, the IMWG published guidelines on optimal use of imaging methods at different disease stages.
Despite its advantages, limitations of CT include:
¡ Like MRI, CT cannot be used for treatment monitoring because bone lesions in myeloma regress slowly or not at all, even in patients in complete remission.
¡ CT is not as sensitive as MRI in detecting lesions outside the bone marrow (extramedullary disease) or in the vertebrae and pelvis.
¡ CT is an expensive study.
¡ Even in low-dose format, CT uses an increased level of radiation as compared to X-ray or to MRI, which doesn’t use radiation at all.
MRI
Magnetic resonance imaging (MRI) is a non-invasive study that uses magnetic energy and radio waves, not radiation, to produce a detailed two- or three-dimensional image of structures inside the body. MRI scans map the location of water and fat in the body and produce detailed spatial images. MRI is a useful tool for diagnosing and monitoring myeloma because of its ability to image early focal lesions in the bone marrow. Because MRI creates images of soft tissue, it can show small clumps of myeloma in the bone marrow, plasmacytomas, and compression of the spinal cord by these masses.
The best setting for MRI is early in diagnosis. MRI is highly sensitive for the detection of focal lesions before bone destruction occurs. In a large comparative study of X-ray and MRI, 52% of patients had normal-appearing
whole-body X-ray but had focal lesions that were apparent on MRI. The IMWG guidelines on MRI state that because MRI is a more sensitive study than X-ray for focal lesions (before the appearance of lytic bone lesions), all SMM patients should undergo whole-body MRI (WBMRI) or spine and pelvic MRI if WBMRI is unavailable.
The limitations of MRI include the following:
¡ MRI is an expensive, timeconsuming procedure.
¡ Patients who have metal implants, and patients who are claustrophobic, cannot undergo MRI.
13. Example of an MRI study
¡ There is a lag time of about 9 months or longer before an MRI will look normal after an area of myeloma has been successfully treated. Because this leads to a high falsepositive rate, the IMWG guidelines state that the use of MRI “for the follow-up of patients, before or after different therapies, in the absence of clinical indications is not recommended.”
¡ Treatment for myeloma will interfere with MRI results. If possible, myeloma patients should not start treatment before a scheduled MRI.
¡ The MRI scanning technique that is best for myeloma (with diffusionweighted imaging) has not been standardized and is not widely available.
PET or PET-CT scan
Positron emission tomography (PET) scanning is a “real-time” study that shows where, and to what extent, cancer cells are actively dividing in the body.
Before a PET scan, a patient is injected with a sugar-fluorine compound (FDG, or fluorodeoxyglucose) that is taken up by the body’s actively multiplying cells as fuel for cell division. When the body is scanned, the areas with the highest concentration of sugar-fluorine uptake glow from positrons emitted by the fluorine, revealing “hot spots” where rapid metabolism can indicate areas of active cancer cell division. This scan covers the whole body and is very sensitive in detecting potential tumor activity. It is measured in units of Standardized Uptake Value (SUV).
Figure
PET-CT is a highly accurate and valuable imaging technique used in diagnosis, therapy assessment, and prognosis of myeloma. It combines PET scan with CT in areas where there is high uptake of FDG. It provides information both about past damage and current myeloma activity, thus enabling the doctor to study changes over time. Because of its sensitivity and its ability to detect disease in areas outside the bone marrow, PET-CT has been included along with specialized testing of the bone marrow biopsy specimen to establish MRD-negative status following treatment.
The advantages of PET imaging include its ability to:
¡ Assess metabolic response to therapy. PET-CT is the preferred imaging study in this setting.
¡ Assess the status of patients with non-secretory myeloma, whose disease cannot be monitored with standard blood and urine tests.
¡ Detect lytic bone lesions at diagnosis as a baseline test before therapy.
¡ Predict progression-free survival (PFS) and overall survival (OS). Three or more PET-positive lesions are an independent predictor of poorer PFS and OS.
Disadvantages of PET include the following:
¡ It is time-consuming and expensive.
¡ Because areas of infection and inflammation can also take up FDG, PET scans can produce false-positive readings for cancer.
¡ There is some concern that skull lesions could be missed because of the normally high FDG uptake in the brain.
¡ As with MRI, therapy can interfere with PET results. Patients should not start therapy before a scheduled PET scan. Dexamethasone in particular is problematic.
Dexamethasone interferes with PET results by slowing down the entry of glucose into tumor cells. PET studies used to determine the effect of treatment should not be performed until after the patient has been off dexamethasone for 2–3 weeks, and before the patient starts the next cycle of dexamethasone.
Figure 14. F-fluorodeoxyglucose (FDG) PET-CT showing diffuse (D) and focal (F) myeloma lesions
In the U.S., the Centers for Medicare and Medicaid Services (CMS) currently covers the cost of one FDG PET scan and allows private health insurers who function as local Medicare contractors to decide whether or not to cover further PET scans. Doctors who wish to support the need for additional PET scans can do so based on the following justifications:
1. Myeloma recurrence.
2. Myeloma that is non-secretory.
3. Concern about infection or a second primary malignancy (SPM).
Bone densitometry
Bone density testing is useful for monitoring the effects of bisphosphonate therapy on the bones of patients who have diffuse thinning (osteopenia or osteoporosis) of the outer bone cortex. It is not a useful test in assessing myeloma bone disease.
Another bone-related test that is not useful in myeloma is the nuclear bone scan. Myeloma causes bone loss resulting in lytic lesions, which do not show up on a nuclear bone scan.
In closing
This booklet is not meant to replace the advice of your doctors and nurses who are best able to answer questions about your specific healthcare management plan. The IMF intends only to provide you with information that will guide you in discussions with your healthcare team.
To help ensure a good quality of life through effective treatment of your myeloma, you must play an active role in your own medical care. We encourage you to visit myeloma.org for more information and to join the Myeloma Knowledge Platform at myprofile.myeloma.org.
To receive the most up-to-date information about myeloma in a caring and compassionate manner, call the IMF InfoLine at 1.818.487.7455, email InfoLine@myeloma.org, or schedule a time to talk with an IMF InfoLine Coordinator at mmsm.link/infoline.
To get answers to your questions without having to wait, ask Myelo® anytime 24/7 at myeloma.org. This generative AI assistant is designed to help you find the right resources.
Use the hyperlinks and web addresses included in this publication for quick access to resources from the IMF. Sign up at subscribe.myeloma.org for our quarterly journal Myeloma Today and weekly e-newsletter Myeloma Minute, as well as alerts about IMF news, events, and actions.
The International Myeloma Foundation (IMF) is the global leader in myeloma. Our mission is to improve the quality of life of myeloma patients while working toward prevention and a cure. Since 1990, the IMF has been serving the myeloma community through the following four pillars:
RESEARCH At the IMF, finding a cure for myeloma is our top priority. The IMF Scientific Advisory Board (SAB) of leading myeloma experts identifies key opportunities to drive research forward. The IMF Black Swan Research Initiative® (BSRI®) is pushing the boundaries with early screening for a precursor condition of myeloma as well as cure-focused myeloma clinical trials. The IMF International Myeloma Working Group (IMWG) provides trusted guidelines for diagnosing, treating, and managing myeloma. We also fund innovative research through the IMF Brian D. Novis Research Grants.
EDUCATION Myeloma is a complex and unique journey for each patient. The IMF offers hundreds of videos and free publications in multiple languages to inform and empower patients and care partners to navigate their myeloma journey. All IMF seminars, webinars, and workshops are free-of-charge and designed to directly connect the patient community with expert myeloma clinicians. The IMF Nurse Leadership Board (NLB) provides recommendations for the management of myeloma. The IMF M-Power Project works to break down barriers and ensure health equity in underserved populations.
SUPPORT Studies show that social support can greatly improve the quality of life of people with cancer. The IMF offers more than 160 myeloma support groups across North America, including specialized groups for Spanish-speakers, people with smoldering myeloma, care partners of patients with myeloma, and patients who do not have care partners. The IMF InfoLine answers myeloma-related questions. Myelo®, the IMF’s generative AI assistant, is available 24/7 to help you find the right resources.
ADVOCACY In the U.S., the IMF Advocacy team represents your interests at the federal and state levels. Internationally, the IMF Global Myeloma Action Network (GMAN) works to improve patient access to treatments.