Indications for the use of Carvykti
The FDA approval of Carvykti in February 2022 was based on data from the phase Ib/II CARTITUDE-1 clinical trial. Early, deep, and durable responses were observed in heavily pretreated patients after a single infusion of Carvykti. Of the 61 patients who were evaluable for minimal residual disease (MRD), 92% were MRD-negative at 10 -5. Updated results from this clinical trial demonstrated that responses deepened over time.
In April 2024, the FDA approved an expanded indication for the use of Carvykti® for the treatment of patients with RRMM who have received at least 1 prior line of therapy, including an immunomodulatory agent , a proteasome inhibitor, and an anti-CD38 monoclonal antibody. Carvykti was previously FDA-approved to be used after at least 4 prior lines of therapy.
The expanded FDA approval is based on data from the CARTITUDE-4 phase III clinical trial. In the Carvykti arm of the study, progression-free survival (PFS) was not reached at 24 months vs. PFS of 12 months with Pomalyst® (pomalidomide) + Velcade + dexamethasone [PVd] or Darzalex + Pomalyst + dexamethasone [DPd] in patients with relapsed and Revlimid-refractory myeloma who received 1 to 3 prior lines of therapy.
Carvykti is the first B-cell maturation antigen (BCMA) -targeted therapy approved for the treatment of patients with myeloma as early as first relapse. The new approval makes this important chimeric antigen receptor (CAR) T-cell therapy available to patients earlier in their treatment journey.
Carvykti continues to be studied in earlier-line treatment settings, including in newly diagnosed myeloma.
Figure 1. Blood cells that play a role in the immune system
Treatment with Carvykti
Carvykti is an immunotherapy that enhances the immune system, our body’s natural defense designed to destroy infected and malignant (cancerous) cells, and to remove cellular debris. The immune system is very complex. It includes white blood cells (WBC) and organs and tissues of the lymphatic system. Myeloma interferes with the normal function of the immune system.
Carvykti is a BCMA-directed genetically modified CAR T-cell therapy. Carvykti works by recognizing and binding to BCMA, a protein that is found almost universally on myeloma cells. This leads to the destruction of BCMA-expressing cells, thereby eliminating the myeloma cells. (BCMA is found on healthy cells as well.)
T cells are a type of WBC that originate in the bone marrow but mature in the thymus, a gland beneath the breastbone (sternum). T cells can be distinguished from other lymphocytes by the presence of a T-cell receptor (TCR) on the cell surface. T cells can recognize specific antigens and bind to them in order to surround and disable pathogens (infectious agents that cause disease).
T cells are one of the most important parts of our immune system; they recognize myeloma cells as foreign and cancerous. However, as myeloma grows, the number of T cells is reduced and their function blocked, thus allowing the myeloma to continue to grow. What is needed are T cells, which are capable of both recognizing myeloma cells as foreign and destroying them.
Each dose of Carvykti is customized using the patient’s own T cells that are administered as an intravenous (IV) infusion.
How Carvykti is given
Carvykti is currently administered only at certified treatment centers. Carvykti is delivered as a one-time intravenous infusion, manufactured for each individual patient using the patient’s own T cells. The treatment process generally takes 2 to 3 months and is comprised of the following steps:
1. Leukapheresis
Much like collecting stem cells for an autologous transplant, the patient’s own T cells are collected by a procedure called apheresis or leukapheresis. Whole blood is drawn from the patient, then passed through a machine that separates the blood into its individual components. The patient’s white blood cells are collected, and the remaining blood components are then immediately re-infused back into the bloodstream of the patient. This process may take 3 to 6 hours. If more T cells need to be collected, the procedure can be repeated.
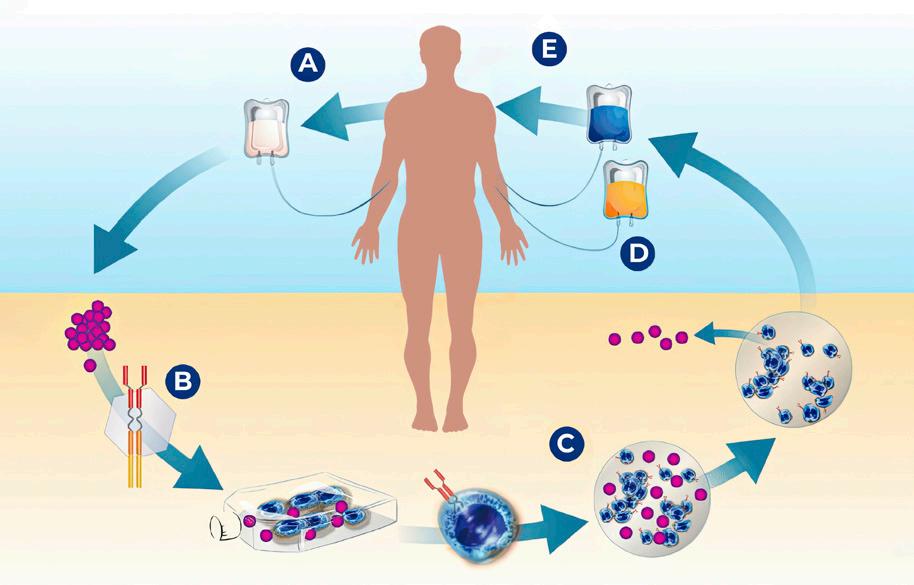
Figure 2. The mechanism of action of CAR T-cell therapy
A. The patient’s T cells are collected from the bloodstream; B. the T cells are modified to produce receptors on their surface that recognize the patient’s myeloma cells; C. the T cells are manufactured in large numbers; D. the engineered T cells are reinfused into the patient to seek out and kill the patient’s myeloma cells; E. reinfused T cells seek out and kill the patient’s myeloma cells.
2. Modification and manufacturing of T cells
The white blood cells collected during leukapheresis are sent to a manufacturing facility where your T cells are separated out. These T cells are genetically modified to make Carvykti, which will recognize and attack BCMA on the surface of your myeloma cells. This is done by inserting a gene into the T cells, which makes the cells express a CAR on the cell surface, then multiplying the T cells in the lab to manufacture millions of cells. Making your Carvykti CAR-T cells takes approximately 4 to 5 weeks from the time your cells are received at the manufacturing laboratory, but the time may vary.
3. Pre-infusion chemotherapy
While your Carvykti is being made, you may receive chemotherapy to treat your myeloma so that it doesn’t get worse. Then, for the 3 days prior to your infusion of Carvykti, your doctor will give you chemotherapy to help prepare your body to receive the infusion of the genetically modified T cells.
4. Infusion of Carvykti
After your treatment with pre-infusion chemotherapy is completed, and before you begin to receive Carvykti, you may be given medicines
to prevent or lessen a possible allergic reaction to Carvykti and to reduce fever. You will then receive your one-time infusion of Carvykti T cells through an intravenous catheter (tube). Due to the specialized nature of T-cell therapy, Carvykti must be administered at a certified treatment center. The infusion lasts approximately 30 to 60 minutes. After the infusion of Carvykti, the genetically modified T cells will begin to seek out your myeloma cells and attack them.
5. Monitoring
After your infusion of Carvykti, your doctor will monitor you for at least 10 days to observe that your treatment is working, and to check for any signs or symptoms of an adverse reaction that may occur. Blood tests will be used to track your progress. You must stay close to the center where you received your Carvykti treatment for at least 4 weeks.
Be sure to discuss with your doctor a plan for your long-term monitoring and follow-up.
Effects on ability to drive and operate machinery
For at least 8 weeks following your Carvykti infusion, you may be at risk for temporary memory and coordination problems, sleepiness, confusion, dizziness, and seizures. Don’t drive, operate heavy machinery, or engage in any activities that could be dangerous or hazardous if you are not mentally alert.
Warnings and precautions
Having Carvykti in your blood may cause some commercial tests for the human immunodeficiency virus (HIV) to give you a false-positive result, even when you may be HIV-negative.
Risk Evaluation and Mitigation Strategy (REMS)
The FDA requires a Risk Evaluation and Mitigation Strategy (REMS) program if a specific drug or treatment has serious safety concerns. REMS programs support the use of such drugs or treatments and help ensure that the potential benefits outweigh the risks. The FDA has determined that a REMS program is necessary with Carvykti. Therefore, Carvykti is available only through the CARVYKTI REMS program. Visit carvyktirems.com or call 1.844.672.0067 for more information.
Cytokine release syndrome (CRS)
Cytokines are proteins secreted by cells which can stimulate or inhibit growth/activity in other cells. In patients with myeloma, cytokines are produced in the bone marrow and circulate in the bloodstream. Cytokines are normally released in response to infection.
Cytokine release syndrome (CRS) is a potentially fatal, uncontrolled immune reaction in which cytokines become highly elevated and trigger an over-
whelming immune system response. A “cytokine storm” can seriously damage body tissues and organs.
CRS has occurred in some patients following treatment with Carvykti in clinical trials, including severe, life-threatening, or fatal reactions. Symptoms of CRS include fever, difficulty breathing, dizziness or lightheadedness, nausea, headache, tachycardia (fast heartbeat), low blood pressure, or fatigue. Severe or life-threatening CRS is treated with tocilizumab or tocilizumab together with steroids.
It is important to promptly alert your doctor if there is any change in your health or if you’re not feeling well while receiving treatment with Carvykti.
Neurologic toxicities
Immune effector cell-associated neurotoxicity syndrome (ICANS) has occurred in patients following treatment with Carvykti. ICANS often correlates with CRS but it can occur in the absence of CRS. After treatment with Carvykti, ICANS can occur before, during, or after CRS onset, or after CRS resolution. ICANS may be severe, life-threatening, or fatal.
Symptoms of neurologic side effects include but are not limited to the following: confusion, disorientation, loss of consciousness, seizures, tremor, slower movements, changes in personality, depression, tingling and numbness of hands and feet, leg and arm weakness, facial numbness, and difficulty speaking, reading, or writing. It is important to promptly alert your doctor if there is any change in your health or if you’re not feeling well.
HLH/MAS
Hemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS) are aggressive and life-threatening inflammatory conditions of excessive immune system activation. Fatal HLH/MAS reactions have occurred in patients following treatment with Carvykti. HLH/MAS can occur with CRS or with neurologic toxicities. It is important to promptly alert your doctor if there is any change in your health or if you’re not feeling well.
Cytopenias
Cytopenia is a condition in which there is a lower-than-normal number of one or more than one of your blood cell types. Prolonged or recurrent cytopenias have occurred following treatment with Carvykti, with bleeding and infection requiring support with growth factors or blood product transfusion. One or more recurrences of cytopenias are possible after partial or complete recovery of cytopenias. Neutropenia, a reduced level of a type of white blood cells called neutrophils, is associated with increased risk of infection. It is important to promptly alert your doctor if you develop fever, chills, or any other signs or symptoms of an infection.
Hypogammaglobulinemia
Hypogammaglobulinemia is a laboratory diagnosis made when the immune system is not producing enough immunoglobulin G (IgG) in the blood.
Hypersensitivity reactions
Hypersensitivity reactions are any undesirable reactions produced by the immune system. These reactions may be uncomfortable, damaging, or fatal. Patients receiving Carvykti must be monitored for hypersensitivity reactions during the infusion of Carvykti and for at least 2 hours after.
Secondary malignancies
A secondary malignancy is a new cancer that is unrelated to a pre-existing cancer diagnosis. Secondary cancers that are a consequence of treatment for the initial cancer may occur months or years after the initial treatment.
Possible common side effects of Carvykti
The most common nonlaboratory side effects that occurred in more than 20% of myeloma patients who received Carvykti are pyrexia (fever), CRS, hypogammaglobulinemia, hypotension, musculoskeletal pain, fatigue, pathogen-unspecified infections, cough, chills, diarrhea, nausea, encephalopathy, decreased appetite, upper respiratory tract infection, headache, tachycardia (fast heartbeat), dizziness, dyspnea, edema, viral infections, coagulopathy, constipation, and vomiting.
The most common laboratory side effects that occurred in 50% or more of myeloma patients who received Carvykti include thrombocytopenia, neutropenia, anemia, aminotransferase (enzyme) elevation, and hypoalbuminemia.
It is important to promptly alert your doctor if there is any change in your health or if you’re not feeling well. Be sure to inform your entire healthcare team that you have received Carvykti, as it may cause side effects that are severe or life-threatening. Immediately contact your doctor or get emergency help if you experience any of the following:
¡ Fever of 100.4°F/38°C or higher,
¡ Chills or shivering,
¡ Fast or irregular heartbeat,
¡ Difficulty breathing, shortness of breath,
¡ Very low blood pressure,
¡ Dizziness or lightheadedness,
¡ Effects on your nervous system, which may be subtle and can occur weeks after your infusion of Carvykti: feeling confused, disoriented, less alert, difficulty speaking, slurred speech, memory loss, difficulty
reading or writing or understanding words; loss of coordination affecting movement and balance, slower movements, changes in handwriting; personality changes including a reduced ability to express emotions, being less talkative, disinterest in activities, and reduced facial expression; tingling, numbness and pain in hands and/or feet, difficulty walking, leg and/or arm weakness, and difficulty breathing; facial numbness, difficulty moving muscles of face and eyes.
You must not be given live vaccines for some time before and after Carvykti treatment. Talk to your doctor if you need to have any vaccinations.
Do not donate blood, organs, tissues, or cells for transplantation.
Support for Carvykti patients and care partners
The MyCARVYKTI™ Patient Support Program, sponsored by Janssen Biotech Inc. and Legend Biotech, is designed to help eligible patients and their care partners with support during treatment. Patients who meet financial and other eligibility requirements, and their care partners, may receive assistance with transportation, lodging, and out-of-pocket costs related to meals and other travel expenses associated with treatment at a certified treatment center. MyCARVYKTI™ Patient Support Specialists are available to help guide eligible patients through the enrollment process and assist with program benefits. Call 1.800.559.7875.
Carvykti in clinical trials
A clinical trial is a medical research study with people who volunteer to test scientific approaches to a new treatment or a new combination therapy. Each clinical trial is designed to find better ways to prevent, detect, diagnose, or treat a disease or a disorder and to answer scientific questions.
If you wish to explore participating in a clinical trial with Carvykti or any other myeloma therapy, be sure to discuss with your myeloma doctor all the potential risks and benefits that may apply to your particular case. For more information about what’s involved in study participation, read the IMF’s publication Understanding Clinical Trials in Myeloma.
The IMF has partnered with SparkCures to help myeloma patients discover and explore clinical trials across the U.S. that best match their needs. To identify a clinical trial personalized to your preferences, visit myeloma.org/sparkcures or contact the IMF InfoLine.
The clinicaltrials.gov online U.S. government database of clinical trials also provides information about research studies. However, the government does not review the safety and science of the studies submitted to this website.
In closing
This booklet is not meant to replace the advice of your doctors and nurses who are best able to answer questions about your specific healthcare management plan. The IMF intends only to provide you with information that will guide you in discussions with your healthcare team.
To help ensure a good quality of life through effective treatment of your myeloma, you must play an active role in your own medical care. We encourage you to visit myeloma.org for more information and to join the Myeloma Knowledge Platform at myprofile.myeloma.org.
To receive the most up-to-date information about myeloma in a caring and compassionate manner, call the IMF InfoLine at 1.818.487.7455, email InfoLine@myeloma.org, or schedule a time to talk with an IMF InfoLine Coordinator at mmsm.link/infoline.
To get answers to your questions without having to wait, ask Myelo® anytime 24/7 at myeloma.org. This generative AI assistant is designed to help you find the right resources.
Use the hyperlinks and web addresses included in this publication for quick access to resources from the IMF. Sign up at subscribe.myeloma.org for our quarterly journal Myeloma Today and weekly e-newsletter Myeloma Minute, as well as alerts about IMF news, events, and actions.
The International Myeloma Foundation (IMF) is the global leader in myeloma. Our mission is to improve the quality of life of myeloma patients while working toward prevention and a cure. Since 1990, the IMF has been serving the myeloma community through the following four pillars:
RESEARCH At the IMF, finding a cure for myeloma is our top priority. The IMF Scientific Advisory Board (SAB) of leading myeloma experts identifies key opportunities to drive research forward. The IMF Black Swan Research Initiative® (BSRI®) is pushing the boundaries with early screening for a precursor condition of myeloma as well as cure-focused myeloma clinical trials. The IMF International Myeloma Working Group (IMWG) provides trusted guidelines for diagnosing, treating, and managing myeloma. We also fund innovative research through the IMF Brian D. Novis Research Grants.
EDUCATION Myeloma is a complex and unique journey for each patient. The IMF offers hundreds of videos and free publications in multiple languages to inform and empower patients and care partners to navigate their myeloma journey. All IMF seminars, webinars, and workshops are free-of-charge and designed to directly connect the patient community with expert myeloma clinicians. The IMF Nurse Leadership Board (NLB) provides recommendations for the management of myeloma. The IMF M-Power Project works to break down barriers and ensure health equity in underserved populations.
SUPPORT Studies show that social support can greatly improve the quality of life of people with cancer. The IMF offers more than 160 myeloma support groups across North America, including specialized groups for Spanish-speakers, people with smoldering myeloma, care partners of patients with myeloma, and patients who do not have care partners. The IMF InfoLine answers myeloma-related questions. Myelo®, the IMF’s generative AI assistant, is available 24/7 to help you find the right resources.
ADVOCACY In the U.S., the IMF Advocacy team represents your interests at the federal and state levels. Internationally, the IMF Global Myeloma Action Network (GMAN) works to improve patient access to treatments.