Welcome!
Thank you for joining us today for the June 24th, 2023, International Myeloma Foundation’s Regional Community Workshop –Atlanta.


Thank you for joining us today for the June 24th, 2023, International Myeloma Foundation’s Regional Community Workshop –Atlanta.












June 24, 2023, Agenda

9:00 – 9:15 AM Welcome and Announcements
Kelly Cox, Director of Support Groups, Senior Director Regional Community Workshops

9:15 – 9:45 AM Myeloma 101

Craig Hofmeister, MD, MPH – Winship Cancer Institute of Emory University
9:45 – 9:55 AM Q&A
9:55 – 10:40 AM Life is a Canvas, You are the Artist
Charise Gleason, MSN, NP-C, AOCNP – Winship Cancer Institute of Emory University; IMF Nurse Leadership Board
10:40 – 10:50 AM Q&A 10:50 – 11:00 AM COFFEE BREAK 11:00 – 11:45 AM Frontline Therapy
Ajay Nooka,MD, MPH – Winship Cancer Institute of Emory University
11:45 – 11:55 AM Q&A
11:55 AM – 12:40 PM LUNCH BREAK
June 24, 2023, Agenda
12:40 – 1:00 PM Local Patient & Care Partner Panel
Bob Cain, Patient & Molly Lay, Care Partner; Ted Price, Patient & MattiePrice, Care Partner
1:00 – 1:10 PM Q&A
1:10 – 1:30 PM Maintenance Therapy
Ajay Nooka,MD, MPH – Winship Cancer Institute of Emory University
1:30 – 1:40 PM Q&A
1:40 – 2:25 PM RelapsedTherapies & Clinical Trials

Craig Hofmeister, MD, MPH – Winship Cancer Institute of Emory University
2:25 – 2:35 PM Q&A
2:35 – 2:45 PM Closing Remarks

2:45 – 3:00 PM Coffee / Network


. The IMF provides FREE resources to help both patients and families.
Established in 1990, the IMF’s InfoLine assists over 4600 callers annually and answers questions across a wide variety of topics including:
Frequenttopics:
✓ Newly Diagnosed MM/Myeloma101

✓ TreatmentOptions
✓ Transplant
✓ Maintenance
✓ Side Effects
✓ Relapsed/RefractoryMM
✓ ClinicalTrials



✓ Resources for Drug Access/FinancialSupport/Local Support
✓ Referralsto MyelomaSpecialistsWithin/Outsidethe US
✓ Health Issues Related to Myeloma
✓ CaregiverSupport
Paul Hewitt, Missy Klepetar,and Deb Verla






A core missionof the IMF is to providethoroughand cutting-edgeeducationto the myeloma community.




























We don’t cure the vast majority of patients with myeloma because we don’t kill the myeloma initiating cells.
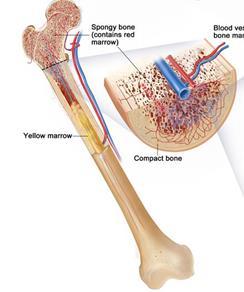
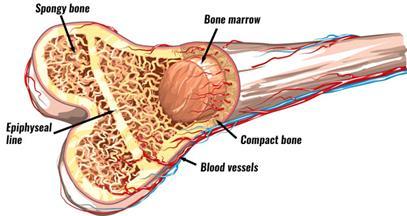
Lymphoid progenitor
Myeloid progenitor
Left: courtesy of National Cancer Institute

Middle: courtesy of My Life Stages
Right: courtesy of UF Health
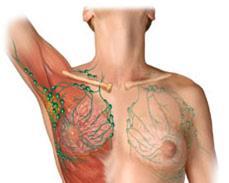
Lymphocyte
Plasma cells Lymphoma
Multiple Myeloma
Clogs the kidneys
causing kidney
failure
Monoclonal protein




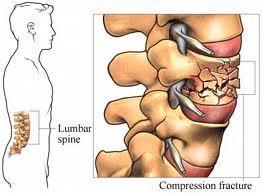
Fractures
Hypercalcemia
Infections
Anemia
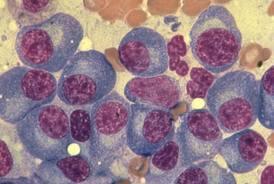
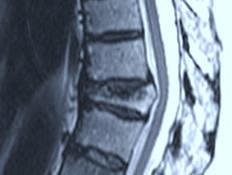
Obtain a bone biopsy at this vertebra showing the cells shown on the right panel Patient presents with worsening back pain
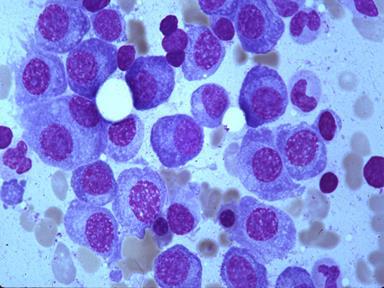
Pathologist reviews slides and documents clonal plasma cells
The most commonsigns are fractures, anemia,and kidney failure
CRAB CRITERIA
Calcium → High calcium
Renal → Kidney failure
Anemia → Fewer red blood cells
Bone → Holes in bones causing spontaneous fractures
MGUS
Monoclonal

Gammopathy
Undetermined
Significance
Smoldering Myeloma

Likelihood of multiple myeloma
S-LI-M CRAB criteria
95% in 2 years
Sixty percent plasma cells on bone marrow biopsy
70% in 2 years LIght chain ratio ≥ 100
75% in 2 years
MRI abnormalities (≥ 5 mm) in 2+ sites
These patients have not yet proven they have cancer, so avoid or embrace treatment at your own risk
100%
CRAB
Calcium elevation
Renal damage
Anemia
Bone holes
MGUS
▪ Small monoclonalor abnormal serumfree light chain ratio
▪ Clonalplasma cells in marrow < 10%
▪ No SLiM-CRAB criteria
Smoldering Myeloma
▪ M protein≥ 3 g/dL(serum) or ≥ 500 mg/24 hrs (urine)
▪ Clonal plasma cells in marrow 10-60%
▪ No SLiM-CRAB criteria
Multiple Myeloma

▪ Clonal marrow plasma cells
≥ 10% or ≥ 1 biopsy-proven plasmacytoma

▪ 1+ SLiM-CRAB criteria
AL Amyloidosis
▪ Amyloid-related systemic syndrome (renal, liver, heart, GI, PNS)
▪ Congo red in any tissue established to be light-chain related
▪ Circulating monoclonal protein or clonal marrow plasma cells

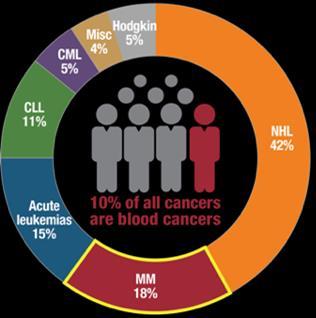
1 in 125 over a lifetime
1.8% of all new cancer cases in the U.S.
32,110 new cases in the U.S. in 2019
https://www.cancer.org/cancer/multiple-myeloma/about/key-statistics.html
More than one first degree relative of a myeloma patient
Gaucher’s disease 02
● Twofirst degreerelatives>100x therisk
● One first degreerelativeis4x aslikely to developmyeloma
Increasedrisk of myelomaor lymphoma 30-fold




Averageageofmyelomapatientis 70 y.o.
Black Americans 04
Black patientsare twiceaslikely to developmyelomaaswhiteones, who aretwice aslikely as pacific islanders
“You have high risk monoclonal gammopathy of undetermined significance (MGUS), so please come back in 3 months for a blood test.”
Risk means the odds of developing multiple myeloma.
Because of your high risk disease, we will always need to treat you with 3- or even 4drug cocktails.
High risk means less likely to respond to drugs and for a shorter time is more treatment resistant. Low risk is easier to treat and patients on average live longer.
“Your only lytic lesion is in your skull, so your myeloma bone disease is low risk.”
If you only have a few bones known to be affected by myeloma, and they are not weight-bearing, you have a lower risk for fracture.
The meaning of “risk” depends on the context.
LOW RISK (easier to control)
8-12 year average overall survival
High Risk (difficult to control)
2-5 year average overall survival


When a patient with lung, colon, breast, or prostate cancer hears Then that means
“You have early stage cancer.”
You likely have stage 1 or 2 cancer, it is isolated to a small part of the affected organ, and there is a better than 50% chance of cure.
“Your cancer is metastatic.”
Your cancer is spread throughout your body, and curative surgery is not an option. We found this cancer late in its course after it spread. Your survival is shorter than if we had found this cancer significantly earlier.
In most cancers, stage is a synonym for control.
When I say ‘stage’ to a myeloma patient What does that mean “We expect you to have easier to treat myeloma” You have stage 1 myeloma
“Just like 60% of my myeloma patients, I don’t know whether you have easier or harder to treat myeloma.”
You have stage 2 myeloma
“We expect you to have harder to treat myeloma.” You have stage 3 myeloma.
In myeloma, stage is a synonym for risk.
How are ‘stage’and ‘risk’different than ‘control’?
Kidney output, bone marrow health, and bone disease as good as it’s going to get
Kidneys may worsen and hemoglobin may drop. New holes in the bones are unlikely to develop. Calcium is may slowly rise
Expect new fractures, anemia, and kidney failure
Antibody = Immunoglobulin. These are proteins produced by normal plasma cells to fight infection and by myeloma cells. They are used by doctors to assess how many myeloma cells are in the patient.
A complete antibody

Heavy chain plus light chain
LIGHT CHAINS
kappa and lambda
HEAVY CHAINS IgA, IgG, IgM, IgD










Total Antibody #



Monoclonal immunoglobulin amt (M-spike or M-protein or paraprotein)
Monoclonal: all the same antibody













M-protein is the amount of antibodies made by your myeloma cells




Test Names
What is the Test Measuring IgG Measures level of Immunoglobulin G.
Serum Protein Electrophoresis (SPEP)
Monoclonalimmunoglobulin (M-spike,M-protein)
Test measures the monoclonal protein, made by the myeloma cells, and this is a subset of the total IgG.
Serum Free Light Chain Measures involved light chain, kappa or lambda, in the blood
IgA (Immunoglobulin A)
What is the Test Measuring
Measures level of Immunoglobulin A
Measuresinvolved light chain, kappa or lambda, in the blood
In most patients with an IgA myeloma, I don’t depend on the SPEP because it is often inaccurate - this is not the case with IgG myeloma.
Serum Free Light Chain
What is the Test Measuring
Measures involved kappa light chainsin the blood
Serum Free Light Chain
What is the Test Measuring
Measures involved lambda light chainsin the blood
Patient is asymptomatic Symptoms
IgG or IgA or Kappa or lambda light chains or SPEP (aka Mspike)
In these patients, a clinical trial is often ideal whenever they are eligible





Charise Gleason, MSN, NP-C,


IMF Nurse Leadership Board, Winship
Cancer Institute of Emory University

Charise Gleason, MSN, NP-C, AOCNP
Winship Cancer Institute of Emory University
COLOR WHEEL OF TREATMENT

Myeloma treatment
Infection and Side Effect Management
Know your care team, Telehealth & Meeting Prep, & Shared Decision Making

• Rapid and effective disease control
• Durable disease control

• Minimize side effects
• Allow for good quality of life
• Improved overall survival


• Prevent disease- and treatmentrelated side effects
• Optimize symptom management

• Allow for good quality of life
DISCUSS GOALS AND PRIORITIES WITH YOUR HEALTHCARE TEAM
Transplant
Eligible Patients
Transplant
Ineligible patients
Initial Therapy
Transplant
Consolidation
Maintenance
Treatment of Relapsed disease
Everyone
Consolidation/ Maintenance/ Continued therapy
Supportive Care

• There are no black and white answers to deciding to undergo a transplant
• Undergoing transplant is a commitment for both you and your care partner
• Understanding the process will help bring to focus elements needed to decide if/when to undergo transplant

Clinical Experience Data from Research
Patient Preference
Adapted from Philippe Moreau, ASH 2015



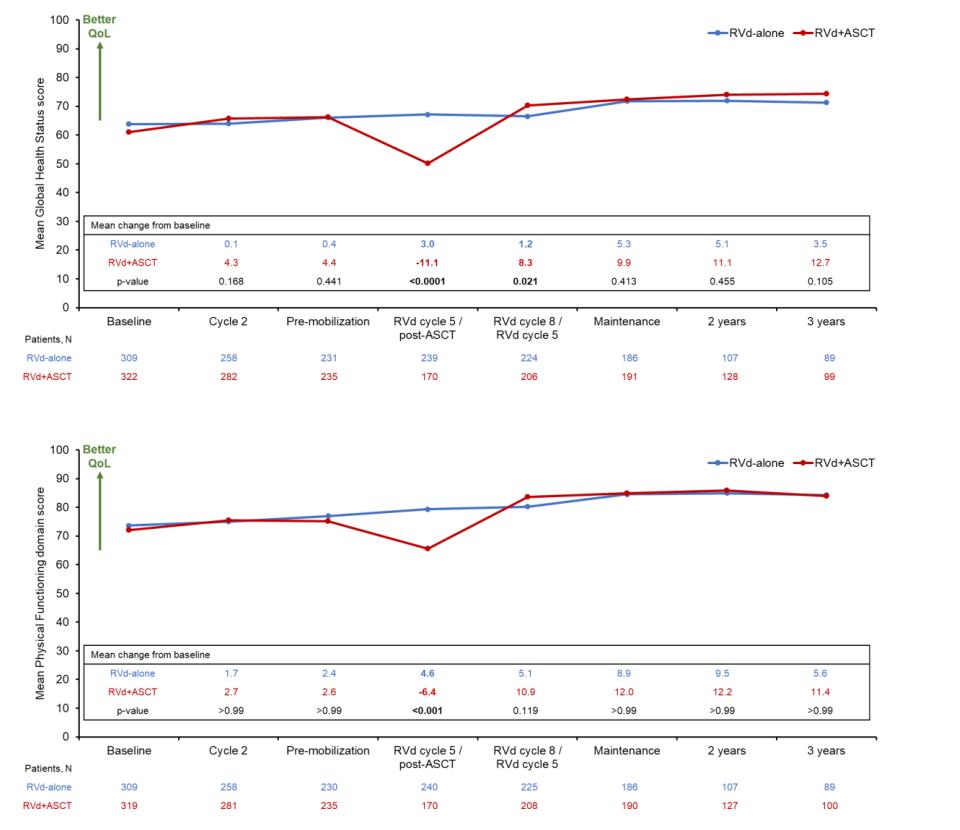
A meta-analysis identified the most common patient-reported symptoms and impact on QOL, and were present at all stages of the disease. Symptoms resulted from both myeloma disease and treatment, including transplant, and were in these categories:
Physical
• Fatigue
• Constipation
• Pain
• Neuropathy

• Impaired Physical Functioning
• Sexual Dysfunction

Psychological
• Depression
• Anxiety
• Sleep Disturbance

• Decreased Cognitive Function
• Decreased Role & Social Function

Financial
• Financial burden (80%)
• Financial toxicity (43%)

All can affect quality of life and relationships
Fatigue is the most common reported symptom (98.8%)
Sources include anemia, pain, reduced activity, insomnia, treatment toxicity, bone marrow suppression

• Anxiety reported in >35%


• Depression nearly 25% Financial concerns, disease progression, end-of-life, and change in social and sexual function were highlighted sources
Often, people do not share these symptoms with their provider. Talk to your provider about symptoms that are not well controlled or thoughts of self harm. Help is available.
&
Steroid Synergy
Steroids are a backbone and work in combination to enhance myeloma therapy
• Consistent schedule (AM vs. PM)
• Take with food
• Stomach discomfort: Over-the-counter or prescription medications
• Medications to prevent shingles, thrush, or other infections
Steroid Side Effects
• Irritability, mood swings, depression
• Difficulty sleeping (insomnia), fatigue

• Increased risk of infections, heart disease
• Muscle weakness, cramping

• Increase in blood pressure, water retention
• Blurred vision, cataracts
• Flushing/sweating
• Stomach bloating, hiccups, heartburn, ulcers, or gas
• Weight gain, hair thinning/loss, skin rashes
• Increase in blood sugar levels, diabetes

Rajkumar SV, Jacobus S, Callander NS, Fonseca R, Vesole DH, Williams ME, Abonour R, Siegel DS, Katz M, Greipp PR, Eastern Cooperative Oncology Group (2010) Lenalidomide plus highdose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol 11(1):29–37.
Shortness of Breath
CRS is a common but usually mild side effect

Headache
Encephalopathy
Confusion
Seizures
Altered wakefulness
NEUROTOXICITY

Neurotoxicity is a rare but serious side effect
Tremors
Hallucinations
Facial nerve palsy
Ataxia Apraxia
7-10 fold increased risk of bacterial and viral infections for people with myeloma
• Report fever of more than 100.4°F, shaking chills even without fever, dizziness, shortness of breath, low blood pressure to HCP as directed.

• Compromised immune function comes from multiple myeloma and from treatment.
• Infection is serious for myeloma patients!


Infection Prevention Tips
Good personal hygiene (skin, oral)
Environmental control (wash hands, avoid crowds and sick people, etc)
COVID: The Best Way to Prevent Illness Is to Avoid Being Exposed to the Virus
Spread mainly through respiratory droplets that are produced by cough, sneezing and talking. More droplets with louder talking, yelling, singing

Growth factor (Neupogen [filgrastim])

Immunizations (NO live vaccines)
Medications (antibacterial, antiviral)
As recommended by your health care team
• Get COVID Vaccine + Booster: Excellent protection against severe disease, but vaccine effectiveness may be lower in people with compromised immune systems
• Wear a High-quality Mask: Respiratory droplets can spread disease; a high-quality mask can prevent exposure to airborne viral particles
• Avoid Crowds & Sick People
• Physical Distance & Outdoors: Close contact and indoor locations increases risk of spread
• Wash Your Hands: Less common to get from a hard surface
Diarrhea may be caused by medications and supplements
• Laxatives, antacids with magnesium
• Antibiotics, antidepressants, others
• Milk thistle, aloe, cayenne, saw palmetto, ginseng
• Sugar substitutes in sugar free gum
Avoid caffeinated, carbonated, or heavily sugared beverages
Take anti-diarrheal medication
• Imodium®, Lomotil®, or Colestid if recommended
• Fiber binding agents – Metamucil®, Citrucel®, Benefiber®
• Welchol® if recommended

Constipation may be caused by
• Opioid pain relievers, antidepressants, heart or blood pressure medications, others
• Supplements: Calcium, Iron, vitamin D (rarely), vitamin B-12 deficiency
Increase fiber
• Fruits, vegetables, high fiber whole grain foods
• Fiber binding agents – Metamucil®, Citrucel®, Benefiber®
Fluid intake can help with both diarrhea and constipation, and good for kidneys. Discuss GI issues with health care providers to identify causes and make adjustments to medications and supplements.

Pain can significantly compromise quality of life

Sources of pain include bone disease, neuropathy and medical procedures
Management
• Prevent pain when possible
• Bone strengtheners to decrease fracture risk; anti viral to prevent shingles; sedation before procedures
• Interventions depends on source of pain
• Monitor serum calcium levels
• Imaging may be needed depending on type and location of pain (eg, MRI, PET-CT)
• May include medications (eg bone modifying agents), activity, surgical intervention, radiation therapy, etc

• Complementary therapies (Mind-body, medication, yoga, supplements, acupuncture, etc)
Tell your health care provider about any new bone pain or chronic pain that is not adequately controlled
Peripheral neuropathy: damage to nerves in extremities (hands, feet, or limbs)
• Numbness
• Tingling
• Prickling sensations
• Sensitivity to touch
• Burning and/or cold sensation
• Muscle weakness
Prevention / management:
• Bortezomib once-weekly or subcutaneous administration

• Massage area with cocoa butter regularly
• Supplements:
• B-complex vitamins (B1, B6, B12)
• Folic acid, and/or amino acids but do not take on day of Velcade® (bortezomib) infusion
• Safe environment: rugs, furnishings, shoes

If PN worsens, your HCP may:
• Change your treatment
• Prescribe oral or topical pain medication
• Suggest physical therapy
Report symptoms of peripheral neuropathy early to your health care provider; nerve damage from PN can be permanent if unaddressed
Financial burden comes from
• Medical costs

• Premiums
• Co-payments
• Travel expenses
• Medical supplies

• Prescription costs
• Loss of income
• Time off work or loss of employment
• Caregiver time off work
Contact the Social Services department at your hospital or clinic to talk to a social worker for assistance.
Funding and assistance may be available
• Federal programs
• Pharmaceutical support
• Non-profit organizations
• Websites:
• Medicare.gov
• SSA.gov
• LLS.org
• Rxassist.org
• NeedyMeds.com
• HealthWellFoundation.org
• Company-specific website
Manage stress
• Rest, relaxation, sleep hygiene
• Mental health / social engagement
• Complementary therapy
Maintain a healthy weight
• Nutrition
• Activity / exercise
Preventative health care
• Health screenings, vaccinations
• Prevent falls, injury, infection
• Stop smoking
• Dental care
Maintain renal health
• Myeloma management
• Hydration
• Avoid renally-toxic medications
– Dose adjust to renal function

• Diabetes management
Protect your bones
• Nutrition, Calcium + D supplement
• Weight-bearing activity / walking
• Bone strengthening agents
Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Dimopoulous M, et al. Leukemia. 2009;23(9):1545-56. Brigle K, et al. CJON. 2017;21(5)suppl:60-76. Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Faiman B, et al. CJON. 2011;15suppl:66-76. Miceli TS, et al. CJON. 2011;15(4)suppl:9-23.
“An ounce of prevention is worth a pound of cure.” Benjamin Franklin
Care partner support is essential for the entire transplant and CAR T processes particularly
• Sedated procedures; Education sessions
• Assistance with daily activities, managing medications and alerting the medical team of changes
• Continued support and assistance is often needed in the early days after returning home. Less assistance will be needed as time goes on.


Care partner can be one person or a rotation of many people.


You are central to the care team








Be empowered
• Ask questions, learn more
• Participate in decisions
Communicate with your team
• Understand the roles of each team member and who to contact for your needs

• Participate in support network








Come prepared:
• Bring a list of current medications, prescribed and over the counter
• Write down your questions and concerns. Prioritize them including financial issues

• Have there been any medical or life changes since your last visit?
• Current symptoms - how have they changed (improved, worsened, stable)? Keep a symptom diary. Bring it along
• Communicate effectively: your health care team can’t help if they don’t know
• Know the “next steps”, future appointments, medication changes, refills, etc
Check with your healthcare team –Is telemedicine an option?
Similar planning for “in-person” appointment PLUS:
• What is the process and what technology is needed?

• Plan your labs: are they needed in advance? Do you need an order?
• Plan your location: quiet, well-lit location with strong wi-fi is best
• Plan yourself: consider if you may need to show a body part and wear accessible clothing
• Collect recent vital signs (blood pressure, temp, heart rate) self-serve blood pressure cuff is available at many pharmacies and for purchase
Be empowered to be part of the treatment decision-making

• Ask for time to consider options (if needed/appropriate)
• Understand options; consider priorities
• Use reliable sources of information
• Use caution considering stories of personal experiences
• Consider your goals/values/preferences
• Express your goals/values/preferences; create a dialog

• My top priority is [goal/value]; additional [preferences] are also important.
• I think [treatment] may be a good choice given my priorities… What do you think?
• Arrive at a treatment decision together


























❖ The Atlanta Area Multiple Myeloma Support Group



❖ Meets virtually on the First Saturday of each month at 11AM Eastern
❖The Southside
Atlanta Multiple Myeloma Support Group
❖Meets virtually on the Fourth Saturday of each month at 10AM Eastern

A local and national movement to improve the short- and long-term outcomes of African Americans with myeloma


Publications
Mentorship of Medical Students
https://m-poweratlanta.myeloma.org/






















Facebook Live
Free Courses for Drs and Nurses
Collaborate with local people
November 2021, M-Power Community Workshop

Social Media
Community Workshops
Educational Postcards
Community Events
Myeloma Button at Grady























 Ajay Nooka, MD, MPH
Ajay Nooka, MD, MPH


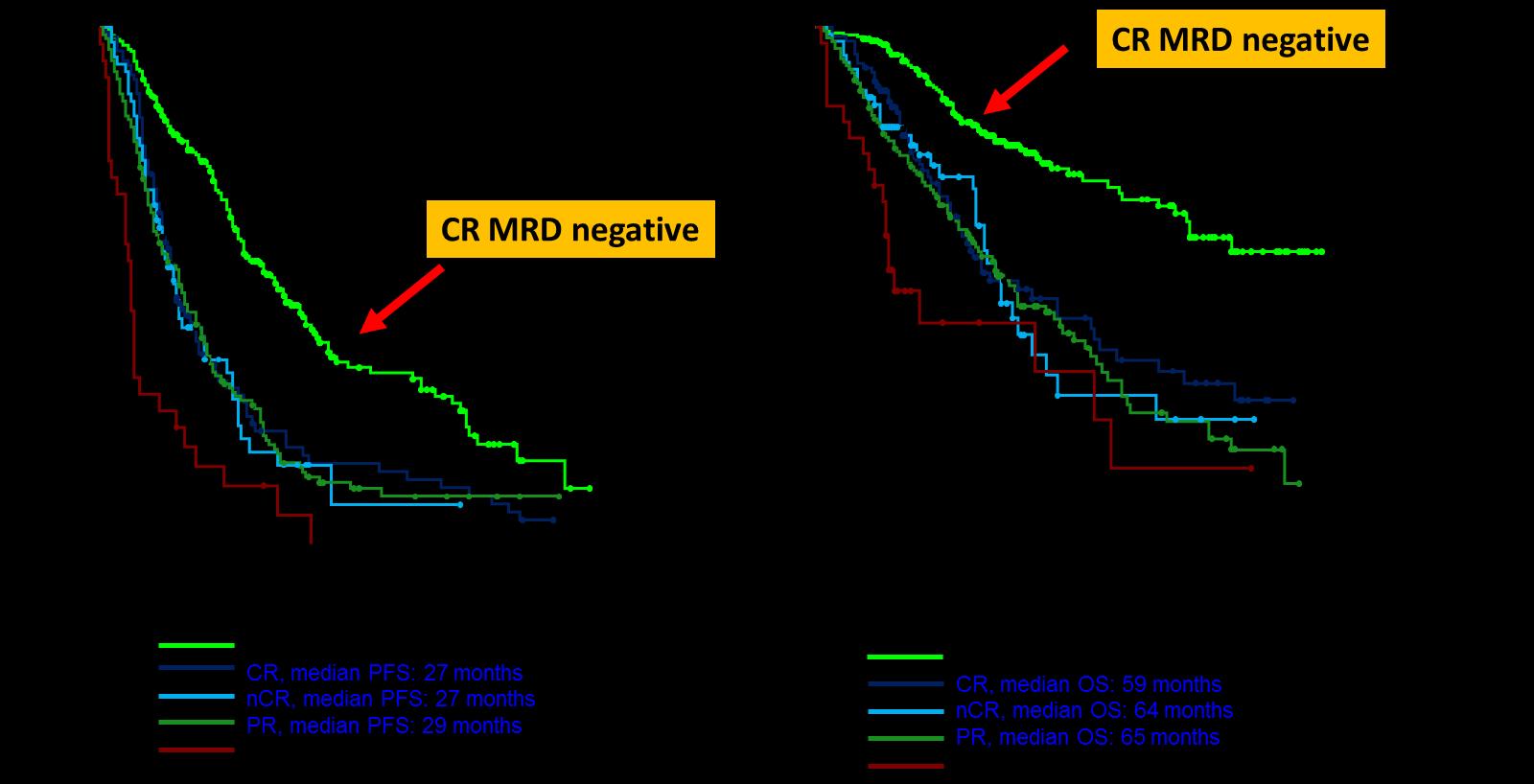
• Review the importance of DEPTH of response in early treatment of myeloma and the increasing use of MRD testing
• Discuss emerging approaches in transplant eligible patients, including quadruplet therapy and stem cell transplantation
• Outline the approach to a patient not going to transplant and how to optimize continuous therapy

Partial response
50% reduction in M protein
Very good partial response
90% reduction in M protein
immunofixation positive only





Complete remission
No M-protein
immunofixation negative


Minimal Residual Dis
Minimal Residual Dis

Next Generation Molecular testing

MRD refers to the persistence of residual tumor cells after treatment and is responsible for relapse1

Current techniques can detect MRD with a sensitivity of 10-6 for MM cells2
MR, minimal response; neg, negative; pos, positive; R, relapse
1. Adapted from Hauwel M, Matthes T. Swiss Med Wkly 2014:144:w13907
2. Biran N, et al. Curr Hematol Malig Rep 2014;9:368–78
Newly Diagnosed MM and Risk Stratified
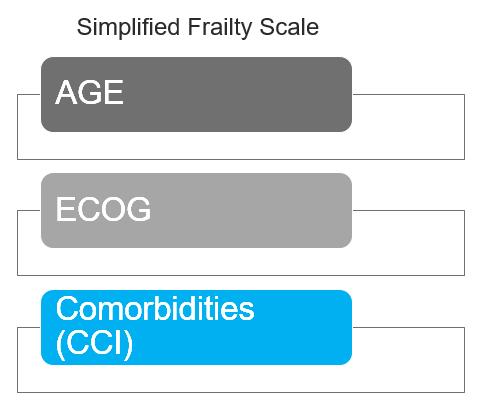
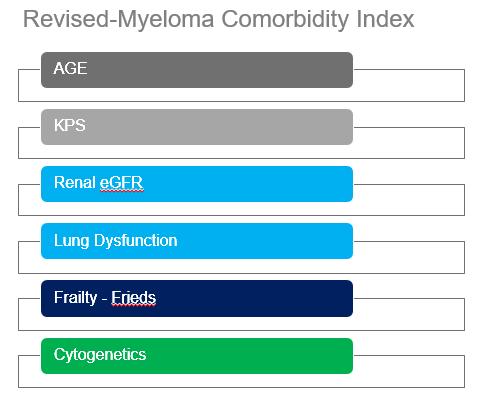
Factors to be considered for ASCT
Age, performance status (PS), comorbidities (R-MCI score, HCT-Cl) and organ function
ASCT Eligible
ASCT Ineligible
1. Most patients will be given a combination of drugs to control the disease quickly
2. We don’t “save the best for last” because early therapies have a long term effect on survival
3. We seek a DEEP and DURABLE response
4. We mix and match from the 3 major classes of drugs and add steroids:
Proteasome Inhibitors – most often botezomib (Velcade)
Immunomodulatory Drugs – lenalidomide (Revlimid)
Monoclonal Antibodies – daratumumab (Darzalex)
5. We decide early on whether or not someone will have a stem cell transplant
Induction
• Velcade/Revlimid/Dex:(VRD)
• Velcade/Thalomid/Dex:(VTD)
• Velcade/Cytoxan/Dex:(CyBorD)

• Darzalex/Revlimid/Dex:(DRD)
• Darzalex/Velcade/Melphalan/Dex
• Darzalex/Velcade/Thalidomide/Dex
• Kyprolis/Revimid/Dex(KRD)
• Darzalex/Velcade/Revlimid/Dex: Dara-RVD
• Ninlaro/Revimid/Dex(IRD)
• Clinicaltrials
Consolidation
Maintenance
• Stem Cell Transplant
• Continue Induction
• Clinicaltrial
• Revlimid
• Velcade
• Ninlaro
• Observation
• Thalidomide
• Revlimid/Dara
• Clinicaltrial


Rescue
Dara+Pomalyst+Dex
Kyprolis+Pomalyst+Dex
Cytoxan+Pomalyst+Dex
Ninlaro+Pomalyst+Dex
Elo+Pomalyst+Dex
Elo+Thaliomide+Dex
Dara+Kyprolis+Dex
Kyprolis+Revlimid+Dex
Elo+Revlimid+Dex
Dara+Revlimid+Dex
Dara+Velcade+Dex
Elo+Velcade+Dex
Cytoxan+Kyprolis+Thalidomide+dex
4 drug therapies of novel agents
Ninlaro+Cytoxan+Dex
Velcade+ Cytoxan+Dex
Velcade+ Pomalyst+Dex
Chemotherapy
Selinexor+Dex
Selinexor+Velcade
Selinexor+ Dara
Isatuximab(Sarclisa)+





Pomalyst+Dex
Darzalex Faspro (under skin)
Ide-cel CAR-T (FDA approved 3/26/2021)
Cilta-cel CAR-T (FDA approved 2/28/2022)
Teclistamab (FDA approved 10/25/2022)
maintenance 13 cycles (10-15 mg/d)

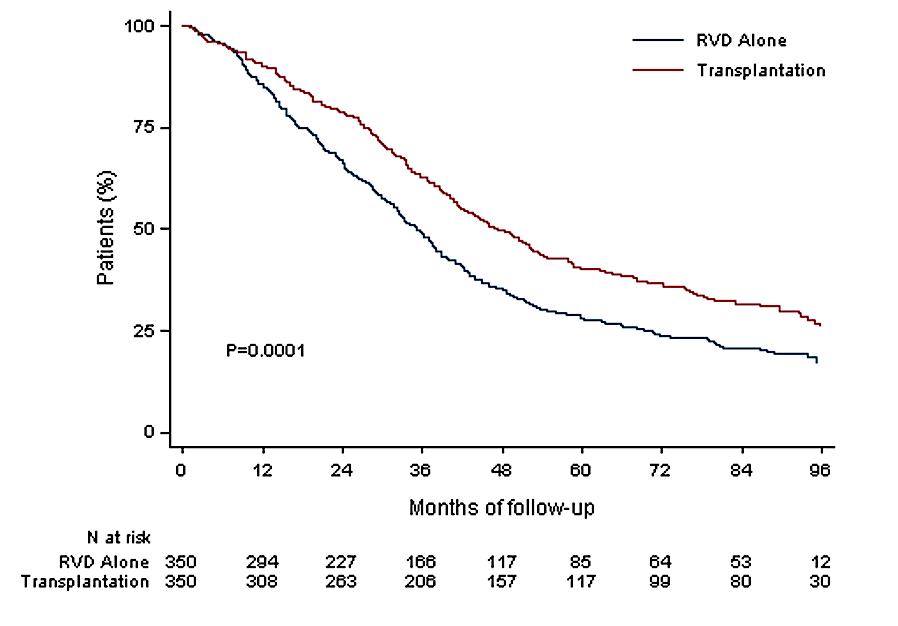
Median follow up 89.8 months
HR (95CI) 0.70 [0.59;0.83]
Median PFS 47.3 months (Transplantation, arm B)

Median PFS 35 months (RVD alone, arm A)
30% reduction in the risk of progression or death in patients receiving transplant
Median follow up 89.8 months
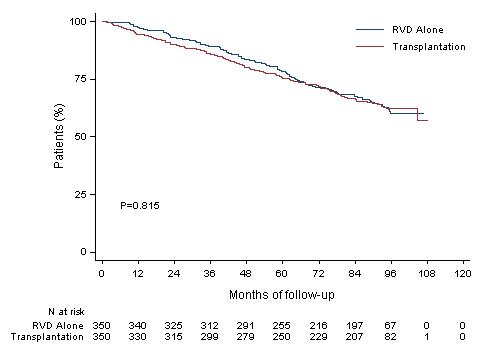
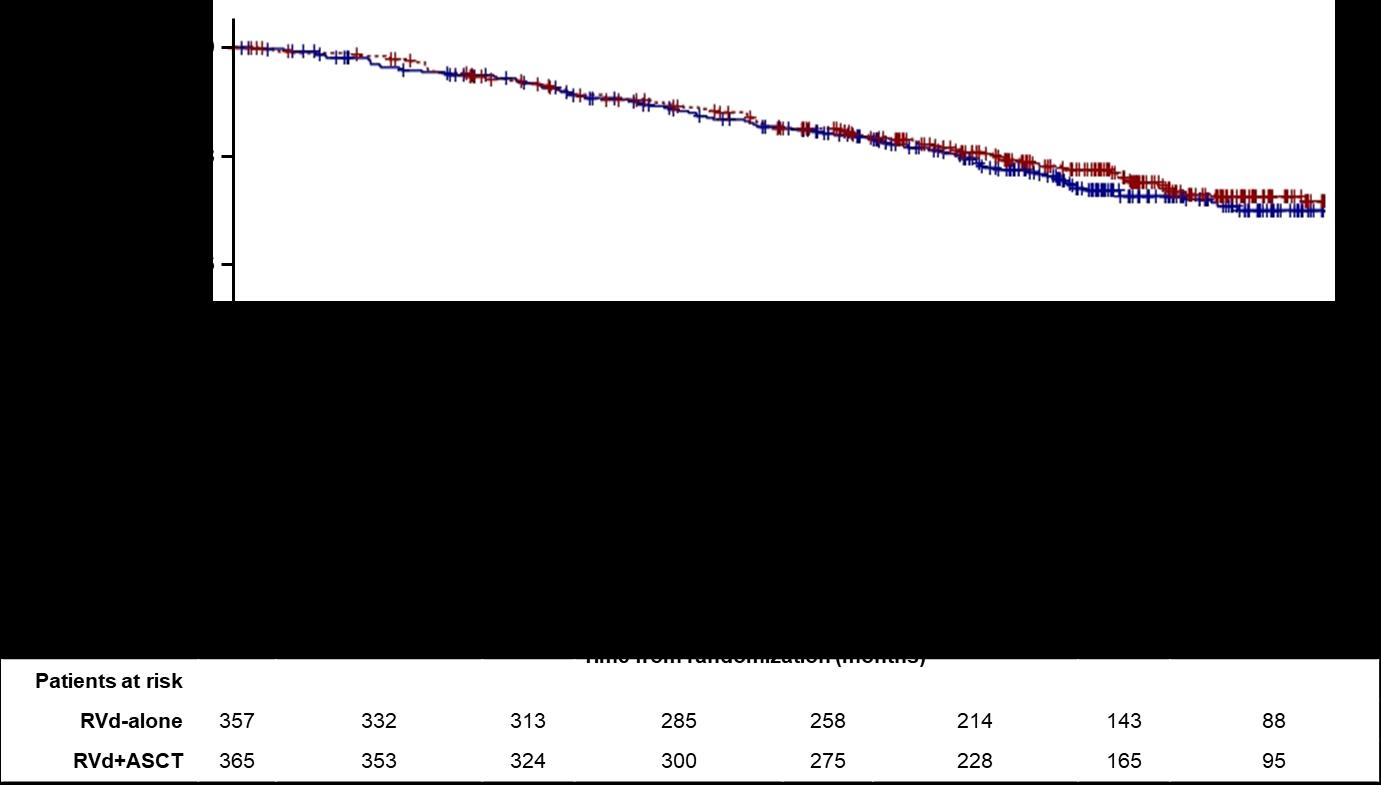
HR (95CI) 1.03 [0.8;1.32]
8y-OS 62.2% (Transplantation, arm B)

8y-OS 60.2% (RVD alone, arm A)
More than 60% of the patients in the two arms are alive after 8 years of follow-up
-Patients aged 18-65 yrs with symptomatic newly diagnosed MM following 1 cycle of RVD
-56 sites within the United States from 2010 to 2018
End Points of Study and Follow-up
• Primary end point: progression-freesurvival (time to next relapse)
• Secondary end points included:
• Response rates, overall survival, quality of life, and adverse events
• Follow-up on participantstatus: median of 6 years
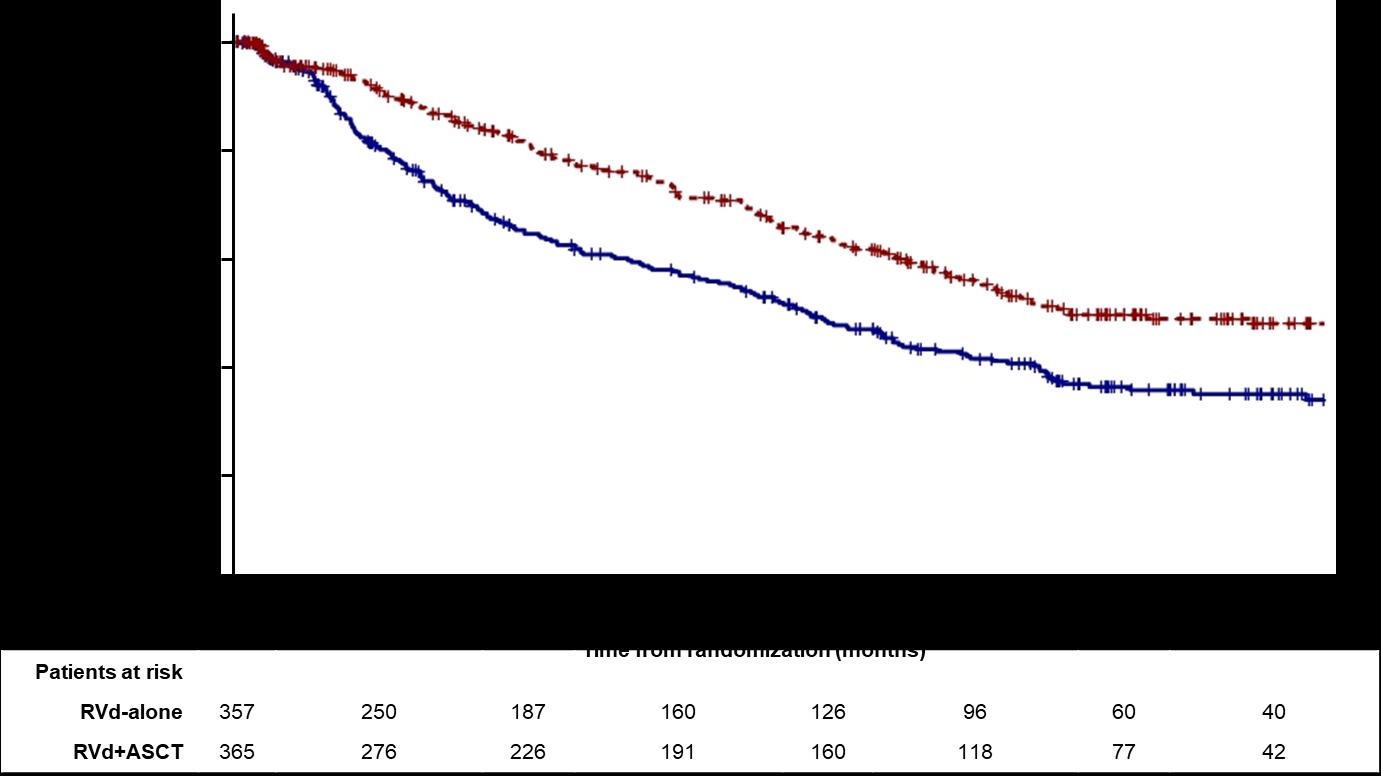
CI, confidence interval;HR,hazardratio; Data cut off: 12/12/21
Data cut off:12/12/21
*p-value adjusted using Bonferroni’s correction to control overall family-wise error rate for secondary outcomes
• ASCT remains very relevant and important in prolonging PFS in younger and eligible patients
• BUT it may not be mandatory in all eligible patients upfront
• As with other agents, we INDIVIDUALIZE the sequencingpatterns
• ASCT does carry genuine toxicity, short term and long term
• We may become callous to these toxicities
• Maintenance therapy remains an important part of myeloma therapy
Daratumumab (DARA) + Lenalidomide, Bortezomib, and Dexamethasone (RVd) in Patients With Transplant-eligibleNewly Diagnosed Multiple Myeloma (NDMM): Final Analysis of GRIFFIN
DouglasW. Sborov,1 JacobLaubach,2 JonathanL.Kaufman,3 BrandiReeves,4 CesarRodriguez,5
Ajai Chari,5 RebeccaSilbermann,6 LucianoJ.Costa,7 Larry D.AndersonJr.,8 Nitya Nathwani,9
Nina Shah,10 NareshBumma,11 SarahA.Holstein,12 Caitlin Costello,13 AndrzejJakubowiak,14
RobertZ. Orlowski,15 KennethH. Shain,16 AndrewJ.Cowan,17 HuilingPei,18 Annelore Cortoos,19
SharmilaPatel,19 ThomasS.Lin,19 PaulRichardson,2 SaadZ. Usmani,20 PeterM.Voorhees21
1HuntsmanCancerInstitute,Universityof Utah Schoolof Medicine, Salt LakeCity, UT, USA; 2Dana-FarberCancerInstitute,Harvard Medical School,Boston,MA, USA; 3WinshipCancerInstitute,EmoryUniversity, Atlanta, GA, USA; 4Universityof NorthCarolina –ChapelHill,ChapelHill,NC, USA; 5Icahn Schoolof Medicine at Mount Sinai, NewYork, NY, USA; 6Knight CancerInstitute, OregonHealth& ScienceUniversity, Portland,OR, USA; 7University of Alabamaat Birmingham,Birmingham,AL, USA;
8Departmentof InternalMedicine, DivisionofHematology/Oncology,UT SouthwesternMedicalCenter,Dallas,TX, USA; 9Judy andBernard BriskinCenterfor Multiple Myeloma Research,Cityof HopeComprehensiveCancerCenter,Duarte,CA, USA; 10Departmentof Medicine, Universityof CaliforniaSanFrancisco,SanFrancisco,CA, USA; 11Division of Hematology,The OhioState UniversityComprehensiveCancerCenter,Columbus,OH,USA; 12University of NebraskaMedicalCenter,Divisionof OncologyandHematologyDepartmentofInternalMedicine, Omaha,NE, USA; 13Moores CancerCenter, Universityof CaliforniaSanDiego,LaJolla, CA, USA; 14University of ChicagoMedicalCenter, Chicago,IL, USA; 15Departmentof Lymphoma/Myeloma,TheUniversityof Texas MD AndersonCancerCenter, Houston,TX, USA; 16Department of Malignant Hematology,H. LeeMoffitt CancerCenter, Tampa,FL, USA; 17Divisionof Medical Oncology, Universityof Washington,Seattle,WA, USA; 18JanssenResearch & Development,LLC,Titusville, NJ, USA; 19JanssenScientificAffairs, LLC, Horsham,PA, USA; 20Memorial SloanKetteringCancerCenter, NewYork, NY, USA; 21Levine CancerInstitute, Atrium Health,Charlotte,NC, USA
Scan the QR code.
https://www.congresshub.com/Oncology/IMS20 22/Daratumumab/Sborov
The QR code is intended to provide scientific information for individual reference, and the information should not be altered or reproduced in any way.

sCR, P= 0.0079a ≥CR, P= 0.0005a

• Rates of ≥CR improved over time and the deepest responses occurred at the end of study maintenance
• At all timepoints, response rates for D-RVd were consistently higher versus RVd
PR, partial response; SD/PD/NE, stable disease/progressive disease/not evaluable. a P value was calculated usingthe Cochran–Mantel–Haenszelchi-squre test. bResponse rates are from the primary analysis cutoff (median follow-up: 13.5 months), and the response-evaluable population included 196 patients (D-RVd, n = 99; RVd, n = 97). cResponse rates for the maintenance phase were evaluated at the time of final analysis (median follow-up: 49.6 months), and the response-evaluable population included 198 patients (D-RVd, n = 100; RVd, n = 98).
• Median follow-up: 49.6 months

• Median PFS was not reached in either group
• PFS was longer for D-RVd/DR versus RVd/R, with a clinically meaningful55% reduction in the risk of disease progression or death
• The separation of the PFS curves occurred beyond 1 year of maintenance
aHR and 95% CI from a Cox proportionalhazards model withtreatment as the sole explanatory variable and stratified with ISS staging (I, II, III) and baseline creatinine clearance (CrCl [30-50 mL/min or >50 mL/min]) at randomization. A hazard ratio <1 indicates an advantage for D-RVd. P-value is based on the log-ranktest stratified with ISS staging and baseline creatinine clearance at randomization.

Mathias Haenel2 , Igor W Blau2 , Dirk Hose1 , Anna Jauch1 , Baerbel Schurich1 , Kai Neben2 , An a Seckinger1 , Barbara eis2 , Christian Gerecke2 , Ingo G. H. Schmidt-Wolf2 , Katja Weise 2 , Christof Scheid2 , Hans Salwender2 ermany, 2GMMG, Germany, 3Division of Biostat stics, German Cancer Research Center He delberg, Germany
Induction phase (3 x 6-weekcycles)
zed (n = 504
s were excluded rom ITT due to rmed d agnosis of mu t p e mye oma requ ring systemic herapy ) cated
Pat en s no rece ving a oca
Maintenancephase (4-weekcycles)
In the PAd group 91 2% and n the VCD group 96 0% o he pa ients comp eted hree p anned nduct on cycles. Appl ed total bortezomib dose over al hree cycles was comparable n both, PAd and VCD arms. Response rates were simi ar n both induction reg mens (PAd vs. VCD) w th 34 3% vs. 37 0% of pat en s ach eving VGPR or be ter Non-inferiority o VCD compared to PAd was shown (one-sided p=0 0013). Similar resul s were obtained in he PP analysis. CR rates were 4 4% and 8 4% (PAd vs. VCD) and 21 1% and 22 3% (PAd vs. VCD) or near complete response (nCR) or bet er Part a response (PR) or better was reached n 72 1% vs. 78 1% o the pat en s (PAd vs. VCD) (f gure 3).
equa y d stributed for SS and Durie-Salmon d sease stage, enet c abnormal t es de et on (17p), ranslocat on t(4;14) and ni icant di ferences n pat ent age and d stribu ion o WHO
cs
Isa (IV) 10 mg/kg Cycle 1 Cycle 2–3 Bor (SC) 1.3 mg/m² Len (PO) 25 mg Dex (PO) 20 mg
F gure 3: Response rates after induction PAd or VCD induction therapy.
The proport on of pat ents w th any adverse event was comparab e in PAd vs. VCD (61 3% vs. 64 0%, p=0 58), but more serious adverse even s (SAEs) were observed during PAd nduct on (32 7% vs. 24 0%, p=0 04). VCD led o a sign f can ly higher proport on o eukocytopen a and neutropenia CTCAE grade 3 and 4 (PAd 11 3% vs. VCD 35 2% p=<0 001). The number of nfect ons (≥ CTCAE grade 2) and infect on-re a ed SAE was simi ar (PAd 24 6% vs. VCD 22 4% or AE, p=0 60 and PAd 12 9% vs. VCD 10 8% for SAE, p=0 49). Compared to he infect on rate (AE ≥ CTCAE grade 2) of 49% during PAD (dexamethasone 40 mg on days 1-4 9-12 17-20) in he HOVON65/GMMG-HD4trial a reduct on n MM5 during induction was observed Pre iminary data (412 pat en s) o numbers of col ected CD34+ stem cel s were comparab e (PAd median 9 8x106 vs. VCD med an 9 4x106 kg bodywe gh , p=0.15). n the PAd arm more deaths were observed compared to he VCD arm (5 vs 1).
Tab e 2 Tox c ty during nduction
Isa (IV) 10 mg/kg: Cycle 1 Cycle 2–3 Cycle 4+ Len (PO) 10 mg increased to 15 mg after 3 months
Dex (PO) 20 mg: first cycle
ASCT, autologous stemcell transplant;D, day;d/Dex, dexamethasone; HDT, high-dosetherapy;Isa, isatuximab; IV, intravenous; NDMM, newly diagnosedmultiple myeloma; PD, progressivedisease;PO, oral; R/Len, lenalidomide; SC, subcutaneous;Te, transplant eligible; V/Bor,bortezomib; RVd is off labeluse in some countries accordingtothe lenalidomide summary of productcharacteristics. 1. ClinicalTrials.gov: NCT03617731

a to evaluate PAd vs VCD induction prior to HDT followed by tenance – final analysis on induction therapy

Patients withMRD negativity at the end of induction therapy
Mathias Haenel2 , Igor W Blau2 , Dirk Hose1 , Anna Jauch1 , Baerbel Schurich1 , Kai Neben2 , An a Seckinger1 , Barbara eis2 , Christian Gerecke2 , Ingo G. H. Schmidt-Wolf2 , Katja Weise 2 , Christof Scheid2 , Hans Salwender2 ermany, 2GMMG, Germany, 3Division of Biostat stics, German Cancer Research Center He delberg, Germany
OR
andom zed (n = 504
VCD (n = 251)
n s were excluded rom ITT due to rmed d agnosis of mu t p e mye oma requ ring systemic herapy ) cated = 248) Ad and (n = 1
Rece ved a oca ed nterven on (n = 249) n = 234 n =
Pat en s no rece ving
a oca ed n erven on due to:
- non-comp ance (n = 1)
- w hdrawa of consen (n = 2
One pa ent excluded rom ITT (due o uncon rmed d agnosis of mu t p e mye oma requ ring systemic herapy) rece ved VCD therapy and was ncluded n safe y ana ysis
= 249
Excluded from PP ana ysis
- ncomp e e nduct on therapy (n = 5)
- missing response
assessmen (n = 3
- one pa en no TT not PP but Sa ety (see above)
In the PAd group 91 2% and n the VCD group 96 0% o he pa ients comp eted hree p anned nduct on cycles. Appl ed total bortezomib dose over al hree cycles was comparable n both, PAd and VCD arms. Response rates were simi ar n both induction reg mens (PAd vs. VCD) w th 34 3% vs. 37 0% of pat en s ach eving VGPR or be ter Non-inferiority o VCD compared to PAd was shown (one-sided p=0 0013). Similar resul s were obtained in he PP analysis. CR rates were 4 4% and 8 4% (PAd vs. VCD) and 21 1% and 22 3% (PAd vs. VCD) or near complete response (nCR) or bet er Part a response (PR) or better was reached n 72 1% vs. 78 1% o the pat en s (PAd vs. VCD) (f gure 3).
F gure 3: Response rates after induction PAd or VCD induction therapy.
Low number of not assessable/missing† MRD status: Isa-RVd (10.6%) and RVd (15.2%)
equa y d stributed for SS and Durie-Salmon d sease stage, enet c abnormal t es de et on (17p), ranslocat on t(4;14) and ni icant di ferences n pat ent age and d stribu ion o WHO
The proport on of pat ents w th any adverse event was comparab e in PAd vs. VCD (61 3% vs. 64 0%, p=0 58), but more serious adverse even s (SAEs) were observed during PAd nduct on (32 7% vs. 24 0%, p=0 04). VCD led o a sign f can ly higher proport on o eukocytopen a and neutropenia CTCAE grade 3 and 4 (PAd 11 3% vs. VCD 35 2% p=<0 001). The number of nfect ons (≥ CTCAE grade 2) and infect on-re a ed SAE was simi ar (PAd 24 6% vs. VCD 22 4% or AE, p=0 60 and PAd 12 9% vs. VCD 10 8% for SAE, p=0 49). Compared to he infect on rate (AE ≥ CTCAE grade 2) of 49% during PAD (dexamethasone 40 mg on days 1-4 9-12 17-20) in he HOVON65/GMMG-HD4trial a reduct on n MM5 during induction was observed Pre iminary data (412 pat en s) o numbers of col ected CD34+ stem cel s were comparab e (PAd median 9 8x106 vs. VCD med an 9 4x106 kg bodywe gh , p=0.15). n the PAd arm more deaths were observed compared to he VCD arm (5 vs 1).
Tab e 2 Tox c ty during nduction
Isa-RVd is the first regimen to demonstrate a rapid and statistically significant benefit from treatment by reaching a MRD negativity of 50.1% at the end of induction and to show superiority vs. RVd in a Phase 3 trial
*P value derivedfromstratifiedconditional logistic regressionanalysis
†Missing NGF-MRD values wereduetoeither patients’ loss to follow -upduringinductiontherapy or to missing bone marrow samples or technical failures in measurement countedas non-responders,i.e.NGF-MRD positive
CI, confidenceinterval;d,dexamethasone; Isa, isatuximab; ITT, intent-to-treat;MRD, minimal residualdisease;NGF, next-generationflow; OR, odds ratio; R, lenalidomide; V,bortezomib

First primary endpoint, end of induction MRD negativity by NGF (10-5), was met in ITT analysis
1.83 (95% CI 1.34–2.51)
Dara-KRd
• Daratumumab 16 mg/m2 days 1,8,15,22(days 1,15 C 3-6; day 1 C >6)
• Carfilzomib (20) 56 mg/m2 Days 1,8,15
• Lenalidomide 25 mg Days 1-21
• Dexamethasone40mg PO Days 1,8,15,22
MRD assessmentby NGS




”MRD-SURE” -Treatment-free observationand MRD surveillance*
• We are transitioning to quadruplets in frontline eligible patients
• BUT the optimal lengthof a quadrupletis still to be determined!
• Transplant still has a role in MM even with long term use of novel agents
• Consolidation therapy may deepen responses and should be considered in patients who have not achieved VGPR
• MRD guided discontinuation may be possible in lower risk groups but not high risk patients
Recommendation
Patients should be referred to a transplant center to determine transplant eligibility
Evidence Rating
Type: Evidence based
Evidence quality: Intermediate, benefit outweighs harm
Strength of recommendation: Moderate
Chronologic age and renal function should not be the sole criteria used to determine eligibility for SCT.
Type: Evidence based
Evidence quality: Intermediate, benefit outweighs harm
Strength of recommendation: Moderate


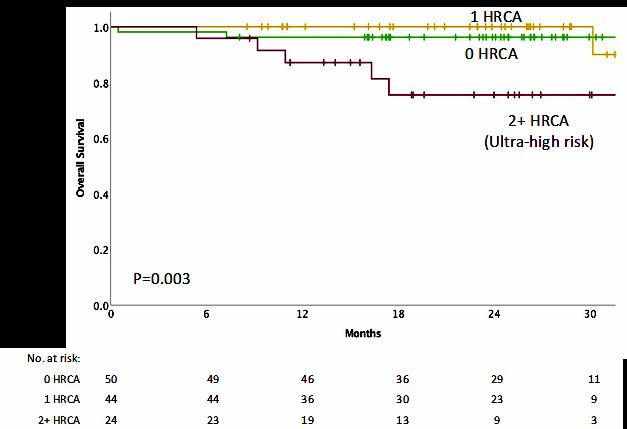
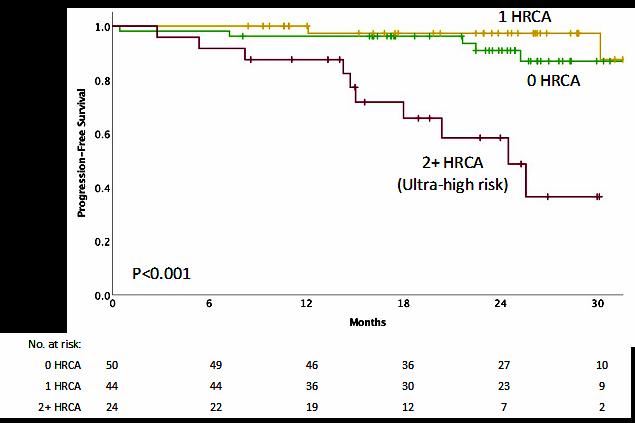
IMWG score of 1 = Intermediate-Fit
3-year OS was 76% (HR=1.61; 95% CI 1.02-2.56; p=.042)
Toxicities 16.7% (HR 1.23, 95% CI 0.89-1.71; p=.217) and
Discontinuation 20.8% (HR=1.41; 95% CI 1.00-2.01; p=.052).
‒ Patients were enrolled in MAIA from March 2015 through January2017
D: 16 mg/kg IV
Key eligibility criteria
• TIE NDMM
• ECOG PS score 0-2
• CrCl
≥30 mL/min
TIE,
aOn
QW Cycles 1-2, Q2W Cycles 3-6, then Q4W thereafter until PD
R: 25 mg PO Days 1-21 until PD
da: 40 mgb PO or IV Days 1, 8, 15, 22 until PD
Primary endpoint
• PFS
Rd
R: 25 mg PO Days 1-21 until PD
d: 40 mg PO Days 1, 8, 15, 22 until PD
D-Rd Cycles: 28 days
End-oftreatment visit (30days afterlast dose)
Longterm follow-up
Key secondary endpoints
• OS
• PFS2
• ORR
• CR/sCR rate
• MRD (NGS; 10–5) 1:1 randomisation
MAIA is a multicentre,randomised, open-label, active-controlled, phase3 studyof D-Rd versusRd alonein patientswith NDMM who are transplantineligible

transplant-ineligible; ECOG PS,EasternCooperative Oncology Group performance status; CrCl,creatinine clearance;IV, intravenous; QW, once weekly; Q2W, once every 2weeks; Q4W, once every 4 weeks; PD, progressive disease; PO, oral; ORR, overall response rate; CR,complete response;sCR, stringentcomplete response; MRD, minimal residual disease; NGS, next-generation sequencing; BMI, body mass index.
days when DARA is administered,dexamethasone willbe administeredto patients inthe D-Rd armandwill serve as the treatmentdose of steroid for that day, as well as therequired pre-infusion medication. bFor patients >75 years of age orwith BMI <18.5 kg/m2,dexamethasone was administered at a dose of 20mg QW.
Demographics and baseline characteristics were well balanced between arms

• D-Rd induced deeper responses with significantly higher rates of ≥CR and ≥VGPR, compared with Rd
• With >28 months of additional follow-up,responses deepened with continued DARA therapy

0.53; 95% CI, 0.43-0.66;

• D-Rd continued to demonstrate a significant PFS benefit, with median PFS not reached with D-Rd
• These data provide a new PFS benchmark in patients with NDMM who are transplant ineligible
HR, 0.68; 95% CI, 0.53-0.86;
D-Rd demonstrated a significant benefit in OS, with a 32% reduction in the risk of death, in patients with NDMM who are transplant ineligible

• Although ASCT remains the standard of care, use is likely to decline in patients who are 65-75 or with significant comorbidities
• Continuous therapy has resulted in better outcomes

• The balance of toxicity and efficacy is particularly important in this population
• ESPECIALLY with dexamethasone
• Most common approach is to select 2 agents from the 3 Novel Classes (PIs, IMiDs and MoAbs)
• Most will use DRD in standard risk patients
• Some may favor VRD in certain high risk patients
• DRD is more easily delivered and feasible
• D-VRD may well be a future standard of care even in these patients
Idecabtagene
Ciltacabtagene






11:55 AM – 12:40 PM LUNCH BREAK
12:40 – 1:00 PM Local Patient & Care Partner Panel
Bob Cain, Patient & Molly Lay, Care Partner; Ted Price, Patient & MattiePrice, Care Partner
1:00 – 1:10 PM Q&A
1:10 – 1:30 PM Maintenance Therapy
Ajay Nooka,MD, MPH – Winship Cancer Institute of Emory University 1:30 –
PM Q&A 1:40 – 2:25 PM RelapsedTherapies & Clinical Trials

Craig Hofmeister, MD, MPH – Winship Cancer Institute of Emory University 2:25 –
– 2:45 PM Closing Remarks 2:45 – 3:00 PM Coffee / Network




















Bob Fly Fishing on Flint River
Catch and Release

Molly Showing Nordic Walking
At Parkinson’s Move Day




• Discuss the principle of consolidation therapy and its application in myeloma

• Outline the major options for maintenance therapy
• Introduce the newer trend for the use of dual maintenance
• Provide an algorithm for maintenance based on risk status
• Induction: Intense and short term therapy with goal to achieve rapid remission

• Consolidation: Intense and shorter term therapy with goal of deep remission
• Maintenance: Less intense longer term therapy with goal of better PFS and OS
What does the Ideal Maintenance therapy look like?
• Deepen remission
• Prolong remission
• Easy to administer
• Minimal toxicity
CALGB 100104
(accrual8/2005 – 11/2009)
INDUCTION
ASCT
1:1 RANDOMIZATION
“NO EVIDENCE OF PD”
Primary Endpoint: PFS
LEN MNTCa (n = 231) PLACEBO (n = 229)
INTERIM AN Dec 2009
IFM 2005-02
(accrual6/2006 – 8/2008)
INDUCTION
ASCT
1:1 RANDOMIZATION
“NO EVIDENCE OF PD”
Primary Endpoint: PFS
LEN: 2 COURSES
GIMEMA (RV-MM-PI-209)
(accrual11/2007–7/2009)
2 × 2 DESIGN
LEN + DEX × 4 INDUCTION
Primary Endpoint: PFS
ASCT
MPR: 6 COURSES
LEN MNTCa (n = 307) PLACEBO (n = 307)
INTERIM ANALYSIS AND UNBLINDING
CROSSOVER BEFORE PD ALLOWED CONTINUED TREATMENT
LEN MNTCb (n = 67) NO TREATMENT (n = 68)
Jan 2010
CONTINUED
TREATMENT NO CROSSOVER BEFORE PD ALLOWED
ALL TREATMENT DISCONTINUED
Jan 2011
LEN MNTCb NO TREATMENT
PRIMARY ANALYSIS
CONTINUED TREATMENT CONTINUED TREATMENT
Targetpopulation of patientswithNDMM whoreceivedLENmaintenanceor placebo/no maintenanceafterASCT
a Starting dose of 10 mg/day on days 1-28/28wasincreasedto 15 mg/day if tolerated and continued until PD.
b Patients received 10 mg/day on days 1-21/28until PD.
ASCT, autologous stem cell transplant; LEN, lenalidomide; NDMM, newly diagnosed multiple myeloma; MNTC, maintenance; MPR, melphalan, prednisone, and Len; PD, progressive disease.
Attal et al ASCO 2016;McCarthyet al EHA 2016


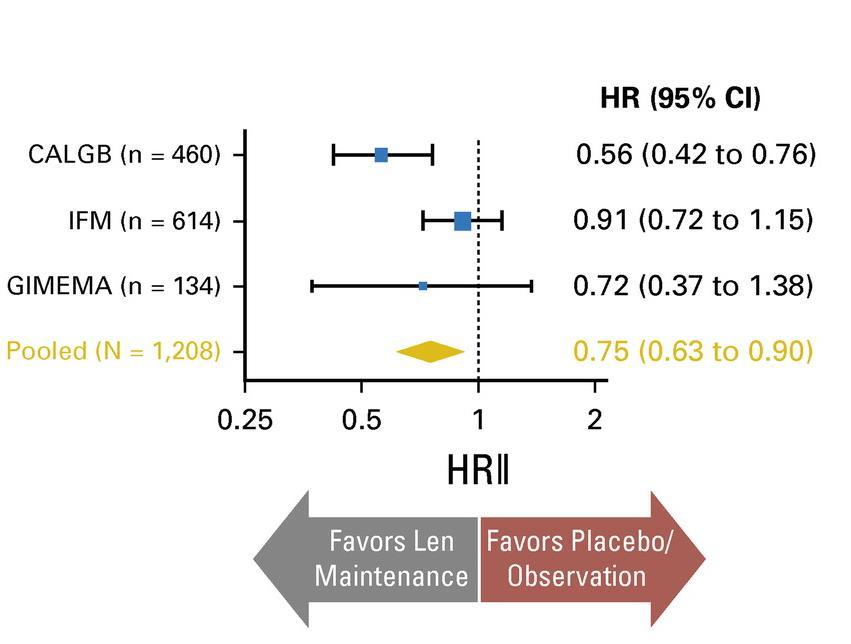
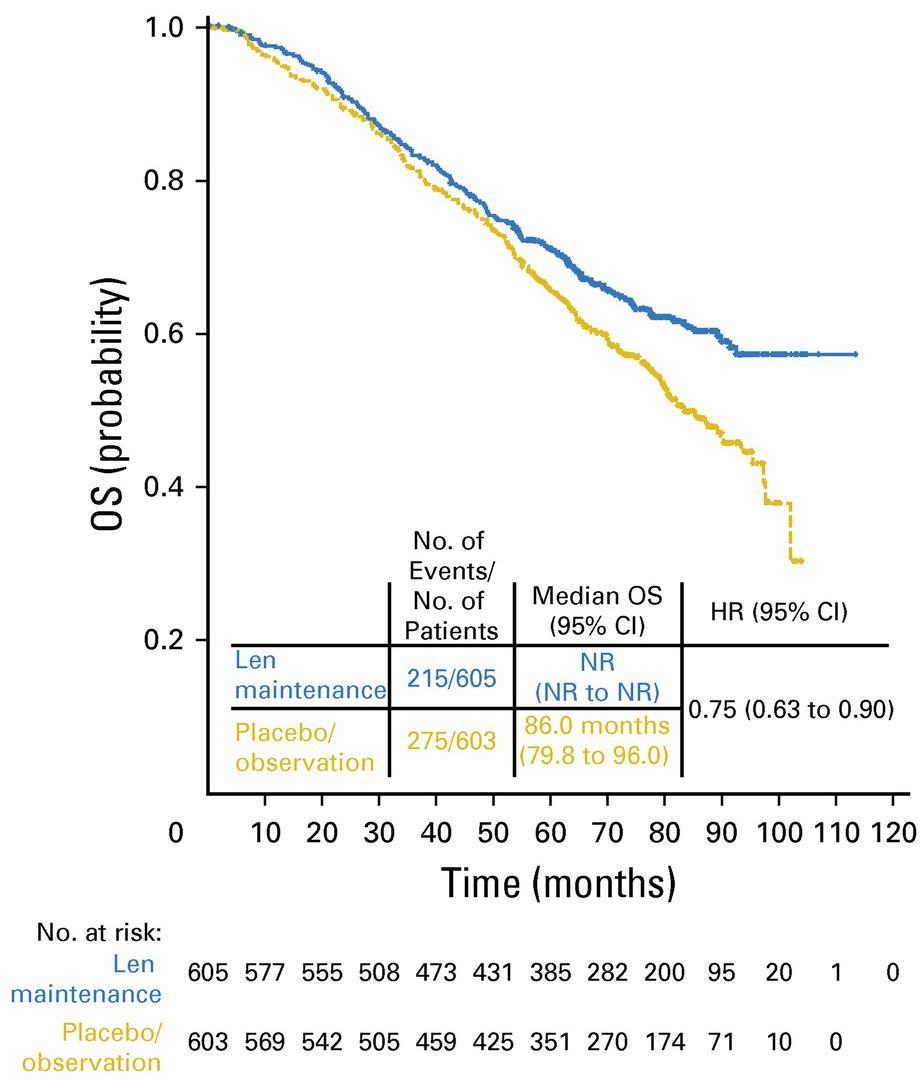 McCarthy et al. J Clin Oncol. 2017, 35:3279-3289.
McCarthy et al. J Clin Oncol. 2017, 35:3279-3289.

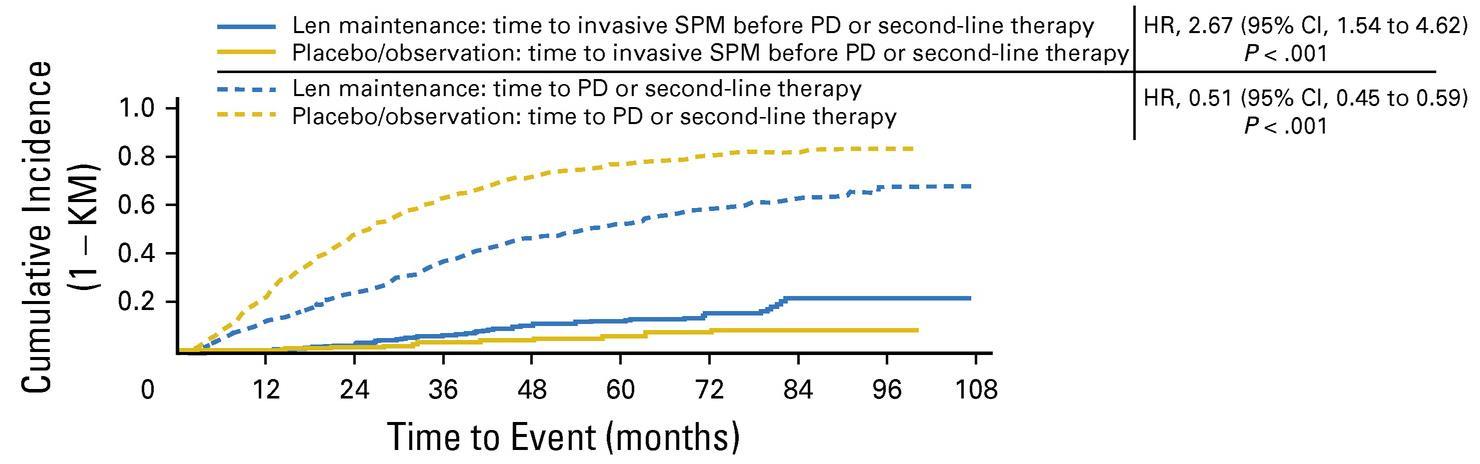
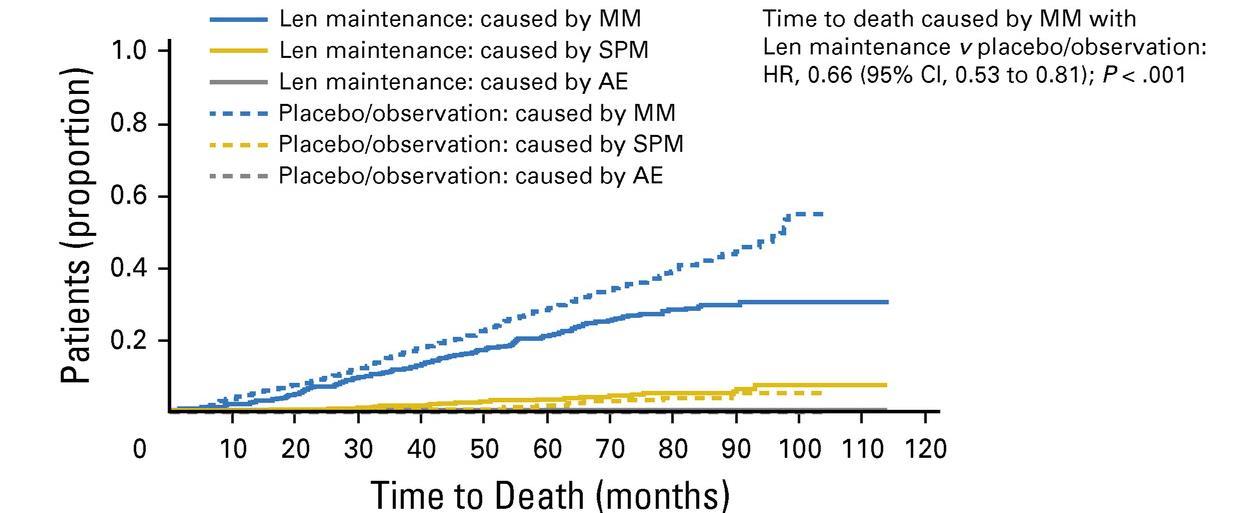
3 randomized trials: 1,209 patients:
• Median follow up 6.6 years
• PFS 52.8 months for lenalidomide vs 23.5 in placebo

• PFS2 also prolonged 73.3 months vs 56.7 (ie not creating more aggressive clone)
• Median overall survival: 86 months v. not reached: P = 0.001
• Benefit for ≤ PR as well as VGPR/CR patients
• 29% discontinuation rate with lenalidomide
• Second primary malignancy rate higher at 6.1% vs 2.8% in placebo after PD

Induction
NDMM
Maintenance
Lenalidomide
10 mg/day,days 1‒21/28
N=1551 (TE=828; TNE=723)
Medianfollow-up: 27 months (IQR 13‒43)
Exclusion criteria
R 1:1
Observation
• Failure to respond to lenalidomideas induction IMiD, or development of PD
• Previous or concurrent active malignancies
Treated on Myeloma XI inductionprotocols IQR, interquartile range; NDMM, newly diagnosed multiple myeloma; PD, progressive disease
Significant improvement in PFS from 18 to 36 months, HR=0.45


4x KCd
K: 36^ mg/m2 d 1-2,8-9,15-16
C: 300 mg/m2 d 1,8,15
d: 20 mg. d 1-2,8-9,15-16,22-23
4x KRd
K: 36^ mg/m2 d 1-2,8-9,15-16
R: 25 mg d 1-21
d: 20 mg. d 1-2,8-9,15-16,22-23
Intensification with high-dose melphalan followed by autologous stem-cell reinfusion MOBILIZATION
4x KRd
K: 36^ mg/m2 d 1-2,8-9,15-16
R: 25 mg d 1-21
d: 20 mg. d 1-2,8-9,15-16,22-23
4x KRd
K: 36 mg/m2 d 1-2,8-9,15-16
R: 25 mg d 1-21
d: 20 mg. d 1-2,8-9,15-16,22-23
4x KCd
K: 36 mg/m2 d 1-2,8-9,15-16
C: 300 mg/m2 d 1,8,15
d: 20 mg. d 1-2,8-9,15-16,22-23
4x KRd
K: 36 mg/m2 d 1-2,8-9,15-16
R: 25 mg d 1-21
d: 20 mg. d 1-2,8-9,15-16,22-23
R:10 mg days 1-21, until progressionor intolerance
K: 36 mg/m2 d 1, 2, 15, 16 up to2 years*
R:10 mg days 1-21, until progressionor intolerance
K: 36 mg/m2 d 1-2,8-9,15-16
R: 25 mg d 1-21
d: 20 mg. d 1-2,8-9,15-16,22-23
^20 mg/m2 on days 1-2, cycle 1 only. *Carfilzomib 70 mg/m2 days 1, 15 every 28 days up to 2 years for patients that have started the maintenance treatment from 6 months before the approval of Amendment 5.0 onwards.
NDMM, newlydiagnosedmultiplemyeloma,R1,firstrandomization(induction/consolidationtreatment);R2,second randomization (maintenancetreatment);ASCT, autologous stem-celltransplantation;K, carfilzomib;R, lenalidomide;C,cyclophosphamide;d,dexamethasone;KCd_ASCT, KCdinduction-ASCT-KCdconsolidation;KRd_ASCT,KRd induction-ASCT-KRdconsolidation;KRd12,12 cycles of KRd.
KRd_ASCT vs. KRd12 vs. KCd_ASCT KR vs. R
Medianfollow-upfromRandom1:51 months(IQR46‒55)
Medianfollow-upfromRandom2:37 months(IQR33‒42)
KRd_ASCT vs. KCd_ASCT: HR 0.54, 95% CI 0.38-0.78, p<0.001
KRd_ASCT vs. KRd12: HR 0.61, 95% CI 0.43-0.88, p=0.0084
KRd12vs. KCd_ASCT: HR 0.88, 95% CI 0.64-1.22, p=0.45
KR vs. R: HR 0.64, 95% CI 0.44-0.94, p=0.02294
3-year PFS reported in the figure. Random 1, first randomization (induction/consolidation treatment); ASCT, autologous stem-cell trasplantation; K, carfilzomib; R, lenalidomide; C, cyclophosphamide; d, dexamethasone; KCd_ASCT, KCd induction-ASCT-KCdconsolidation;KRd_ASCT, KRdinduction-ASCT-KRdconsolidation;KRd12,12cycles of KRd; Random 2,second randomization (maintenance treatment);p,p-value; HR, hazard ratio; CI, confidence interval.
• It appears that dual maintenance therapy prolongs PFS
• This occurs in both standard risk AND high risk patients
• It further opens the door to other dual maintenance strategies currently being used and explored:
• Lenalidomide + Bortezomib
• Lenalidomide + Ixazomib
• Lenalidomide + Daratumumab
• Others??

• Recall that GRIFFIN gave Dara for 2 years with lenalidomide in maintenance
• Responses continuedto deepen with Dara
• CASSIOPEIArandomized pts to no maintenance vs dara q 8 weeks
• Overall there was a benefit to having dara maintenance vs placebo
• However, if dara had been given at induction, that benefit did not seem to continue(ie If you had dara upfront, it didn’t add more to maintenance)
• We just don’t know what benefit it may add to lenalidomide

• Futurestudies with dara and Isatuximab will guideus
• Until then it is not generally used in this context
, Gareth Morgan
, Graham Jackson
On behalf of the Myeloma XI Trial Management Group and NCRI Haem-Onc Clinical Studies Group


Defining the optimum duration of lenalidomide maintenance after autologous stem cell transplant – data from the Myeloma XI trial.
N=1248
Median follow up: 44.7 months (IQR 32.4-62.7)
Median duration of lenalidomide therapy 28 cycles (range 1-96)
• Patients still on therapy 330/730 (45%)

• These data suggest an ongoing PFS benefit associatedwith continuinglenalidomide maintenancebeyond at least 4-5 years in the overall patientpopulation
• Even in patients with sustainedMRD negativity, thereis evidence of benefit from continuing lenalidomide maintenancefor at least 3 years in total
• Randomisedtrials to address the impact of stoppinglenalidomide maintenancein patients with sustainedMRD negativity could be considered, at no earlier than 3 years
• In patients who are MRD +ve these data support continuinglenalidomide untildisease progression
• No evidence of cumulative haematologicaltoxicity was identified
• These findings emphasisethe need for long term follow up of maintenancestudies to enable the exploration of suchquestions
• There is a planned powered OS updateof MyelomaXI in 2023

• Should post-transplant maintenance therapy be recommended for all patients?
• Yes
• Whichagent should be used?
• Lenalidomide remains the standard of care
• What is the optimal duration?
• Treatment until progression remains the standard of care
• What should patients with high-risk cytogenetics receive?
• Consider lenalidomide+ proteasome inhibitor; clinical trial
• Should MRD status dictate maintenance therapy?
• Not outside of a clinical trial
• What about Second Primary Malignancies?
• They are real, require a discussion and monitoring, but are outweighed by benefit




 Craig
Craig
 Hofmeister, MD, MPH
Hofmeister, MD, MPH


Dexamethasone
Solumedrol
Inhibitors
Velcade
Kyprolis
Ixazomib
7. Bispecific T-cell engagers
Teclistamab (Tecvayli)
Talquetamab
Revlimid
Pomalyst
Iberdomide
cc-92480
Darzalex Sarclisa
Selinexor (Xpovio)
6. Chimeric antigen

Receptor T-cells (CAR-T)
ABECMA (Ide-cel)
Carvykti (Cilta-cel)
Refractory to Lenalidomide
Not refractory to Dara (Darzalex)
Dara-refractory
Kyprolis Pom Dex (KPd) RajkumarSV. 2022

Second Relapse
• Is the patient t(11;14) and Venetoclaxnaïve? Do that.
• Next option is Selinexor-based, with a partner you can stomach
Third Relapse
• Is the patient t(11;14 and Venetoclaxnaïve? Do that.
• You are trying to figure out how to move to effective treatment:
Teclistamab(Tecvayli)
- Disease is poorly controlled
- They can handle weekly injections
ABECMA(Ide-Cel) or CARVYKTI (Cilta-Cel)
- Disease responsive

- Patient lives far away
Always be on the watch for a clinicaltrial – http://www.sparkcures.com
Rash This is not an allergy, it’s how the drug works. Can be managed by 1) Nonsedating anti-histamine (claritin); 2) Dose reduction; 3) Decrease dose frequency. Short-term steroid exposure (medrol dose pak) is OK.
Diarrhea Might be responsive to Imodium, but the diarrhea thought to be partly due to bile acid malabsorption. Second line is Welchol [1-6 tabs per day] or Colestid [2g twice daily]
Sedation Take at night in 95% of patients. Dose reduce for fatigue.
Chemo brain Dose reduce
Cramping Hydration
Blood clots Blood thinners.
There is no dose-response relationship for Revlimid > 10 mg or Pom > 2 mg, so don’t be afraid to dose reduce. There is almost no patient that can’t tolerate some useful dose of Revlimid.
Kidney failure category
How do we measure this accurately (look for eGFR or CrCl on your labs)
Prescribing insert recommendations
PRO TIP: Don’t even look at your creatinine. Follow your estimated glomerular filtration rate (eGFR) or your creatinine clearance (CrCl) to get a sense for your kidney function.
Shingles Be sure to take Valacyclovir (Valtrex) or Acyclovir (Zovirax) if you are taking these medications, whether you’ve had Shingles vaccines or not.
Diarrhea or constipation
Imodium for diarrhea, generic miralax or lactulose for constipation.
Tingling & numbness (peripheral nerves)
30% with Velcade, 5% with Ninlaro. Dose reduce! Most effective treatments are meds which decrease sensitivity (cymbalta > gabapentin & lyrica) and/or decrease transmission (opioids)
Low blood pressure (autonomic nerves)
30% with Velcade. Take a BP medication holiday as needed
High BP & heart failure
8-10% high BP with carfilzomib, 3% heart failure with carfilzomib.
Drug interactions Go ahead and drink green tea and take vitamin C if you really think extra vitamin C is helpful (it isn’t). The hype was overblown.
Darzalex and Sarclisa (CD38 antibodies)
● With dexamethasone 40+ mg per week, PJP prophylaxis is strongly recommended.
● If you have had some exposure to hepatitis B in the past, be sure to suppress it with entacavir or similar.
● Never, ever forget about Shingles prevention.
ABECMA & CARVYKTI (BCMA CARTs)
● Intravenous immuneglobulin (aka IVIG) for 6 months after infusion
Teclistamab (Tecvayli)
● Intravenous immuneglobulin (aka IVIG) for 6 months after infusion
● After the first cycle, don’t feel bad about skipping a dose as needed.
Xpovio (Selinexor)
● Start low – 40 mg weekly can be a therapeutic dose
● Olanzapine 5-10 mg at bedtime. Every night.
● Ondansetron 8 mg every 8 hrs as needed for nausea
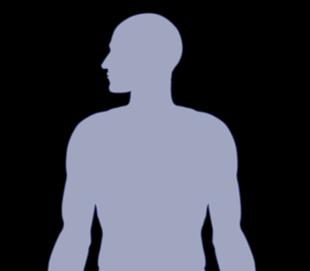
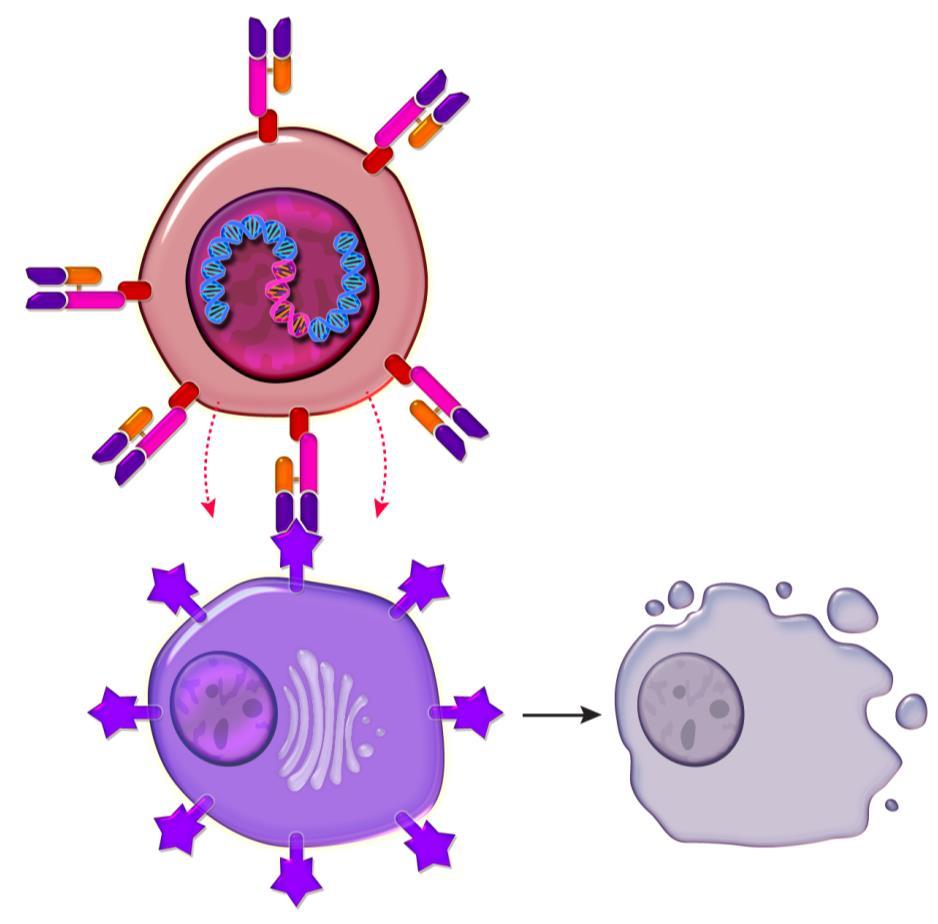
Virus containing instructions in its DNA to create a target on the cell surface to myeloma cells
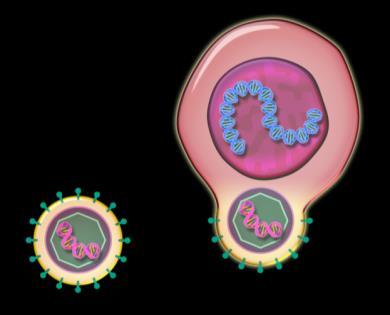
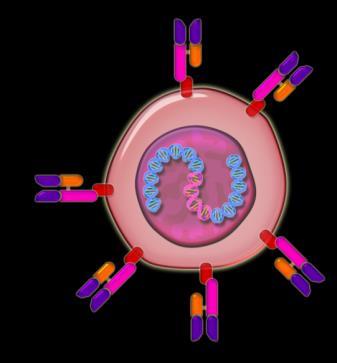

Chimeric antigen receptor enabled cell targeting the patient’s myeloma cell
T Cells (Immune cells) from the myeloma patient
Myeloma cell
Patient Journey: In the Trenches—
Clearance
LD Chemotherapy
▪ Clinical assessment
▪ Preinfusion disease staging
▪ Labs, education
▪ Notify manufacturer to deliver cells
▪ Washout guidance
▪ Schedule depends on product
▪ Largely outpatient
▪ Confirm cells on site before LD chemo
Admission If Inpatient
Beaupierre. J Adv Pract Oncol. 2019;10(suppl 3):29.
▪ Communication to inpatient
▪ TLC on admission request
▪ 7-10 day inpatientor outpatienttreatment

Infusion
▪ Usually <30 min
▪ Anticlimacticfor patient
▪ Vital signs
▪ Documentation
Beaupierre. J Adv Pract Oncol. 2019;10(suppl 3):29.
▪ Watch for side effects
▪ Evaluatefor myeloma response
▪ Referral back to primary oncologist depends on patientstatus, logistics (proximityof primary vs treating center), establishingeffective communication
▪ REMS requirement for patientto stay within 2 hr of treating center for 4 wk
§ Goalsof salvagetherapy:to stabilize disease
‒ Requires 1-2 week washout period before apheresis
Salvage therapy may be recommended during time between referral / consult and apheresis*
§ Goalsof bridgingtherapy:reducetumor burden, palliate symptoms,stabilizedisease and QoL, and to maintain functional reserveduringmanufacturing period
‒ Limit CRS/ICANSseverity by debulking
‒ Potential impact on CAR T-cell efficacy
§ Chooseleast toxic therapy, if possible, and allow hematologic recovery prior to LDC
For patients with difficult to control myeloma, bridging therapy may be recommended during time between apheresis and lymphodepleting chemotherapy
Jain. Biol Blood Marrow Transpl. 2019;25:2305. Kansagra. Bone Marrow Transplant. 2019;54:1868.
‒ Real-life time from pheresis to infusion is >30 days
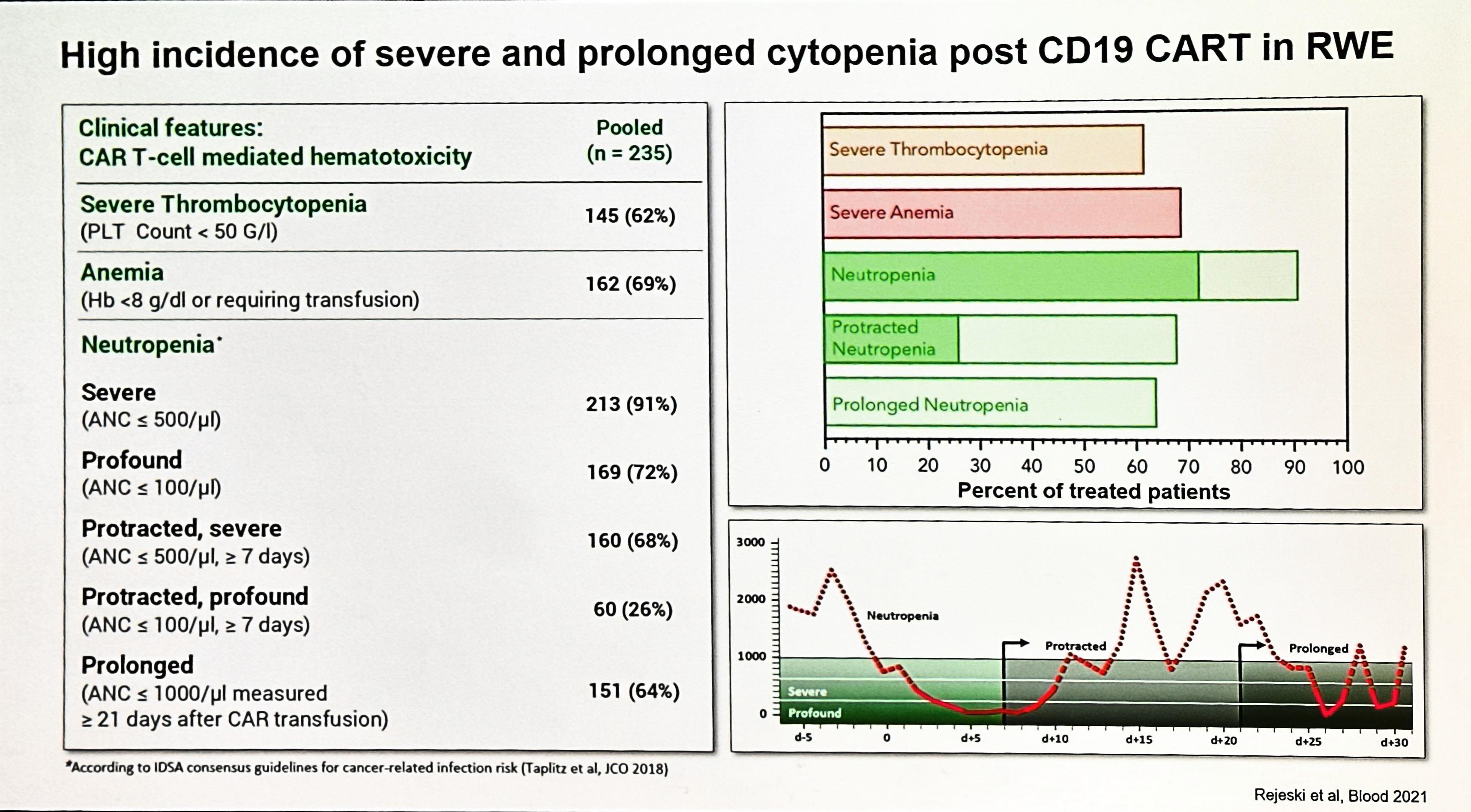
§ Most common toxicity of CAR T-cell therapy
§ Syndrome resulting from immune activation correlating with CAR T-cell expansion and elevations of inflammatory chemicals in the blood
‒ C-reactiveprotein and ferritin are cheap blood tests that rise with CRS
§ May present as noninfectious flulike syndrome
‒ Fever, malaise, myalgias
§ Can progress to life-threatening low blood pleasure, fluid filling up the lungs, and multiorgan failure
§ Onset usuallywithin first week after CAR T-cell therapy
§ Risk factors for severe CRS ≈ bad disease
‒ High disease burden
‒ Higher infused CAR T-cell dose
Cytokine release syndrome (CRS) Neurotoxicity (ICANS)

Onset 1 day with Ide-Cel (Abecma) 7 days with Cilta-Cel (Carvykti)
Duration 5 11 days
Symptoms
Cytopenias Infections
• Fever
• Shortness of breath
• Low blood pressure
• Rapid heartbeat
Management Tocilizumab
2 9 days after CAR Tcell infusion
3 17 days
• Headache
• Confusion
• Language disturbance

• Seizures
Steroids
after CART infusion

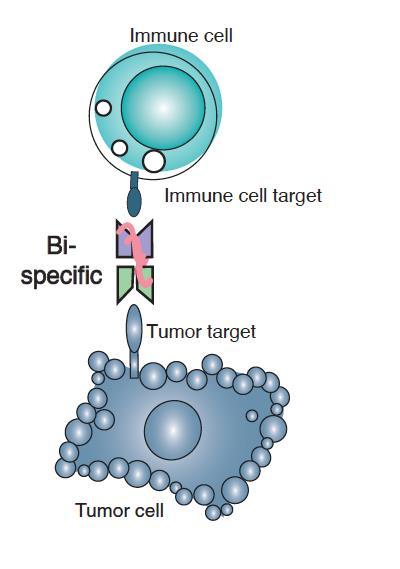
A bispecific antibody is a drug that has been designed to simultaneouslytarget two different proteins, often on two different cells.
Bispecific T-cell Engager (BiTe)
Bispecific Killer Engager (BiKE)
Trispecific Killer Engager (TriKE)

• BiTEs are fusion proteins consisting of two single-chain variable fragments (scFvs) of different antibodies

• One of the scFvs binds to T cells via the CD3 receptor, and the other to a tumor cell via a tumor specific molecule, leading the T-cell to secrete cellularpoisons that kill the tumor cell.
BiTE is a registered trademark of Amgen, so other companies avoid using the word and say bispecifics


Cytokine Release Syndrome (CRS)

Fever ± low blood pressure ± lungs filling up with fluid.
Treatment: Tocilizumab
Immune Effector
Cell-Associated Neurotoxicity Syndrome (ICANS)
Confusion → stupor → seizures
Treatment: Dexamethasone
Key Points – CAR T and Bispecifics
CAR T and bispecific antibodies are very active even in heavily pretreated patients.
Side effects of CAR T cells and bispecific antibodies include cytokine release syndrome, confusion, and low blood counts, all of which are treatable.
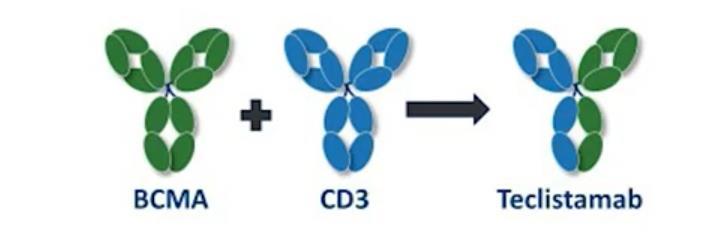
Abecma and Carvykti are only the first-generation CAR T cells and target the same protein. Different CAR Ts and different targets are on the way. Bispecific antibodies represent an “off-the-shelf” immunotherapy; Tecvayli was approved in October 2022.
Several additional bispecific antibodies are under clinical evaluation.

Wehave system issuesthat make clinicaltrial access inequitable.
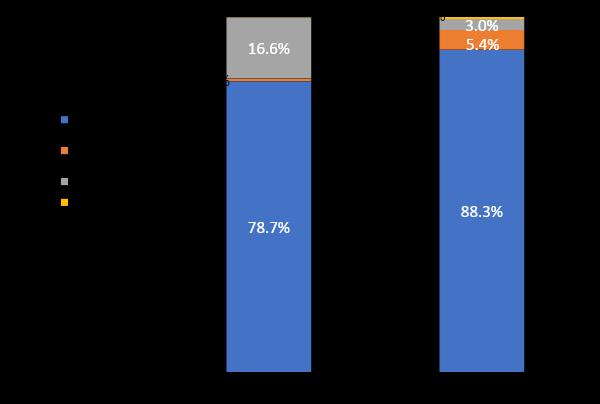
Source: FDA-AACR Workshop to Examine Underrepresentation of African Americans in Multiple Myeloma Clinical Trials. Washington D.C., Feb 2020
Ex-US (N = 17,104) US (N = 6,319)● New drug applications (2003-2017):low rate of AA patient enrollment in pivotal (4.5%) and international (1.8%) trials
● People of color with myeloma have had a smaller increase in population-level survival in the early 21st century than white patients
● Data from clinical trials show that mortality is similar for people of color, suggesting the population-level difference is due to utilization
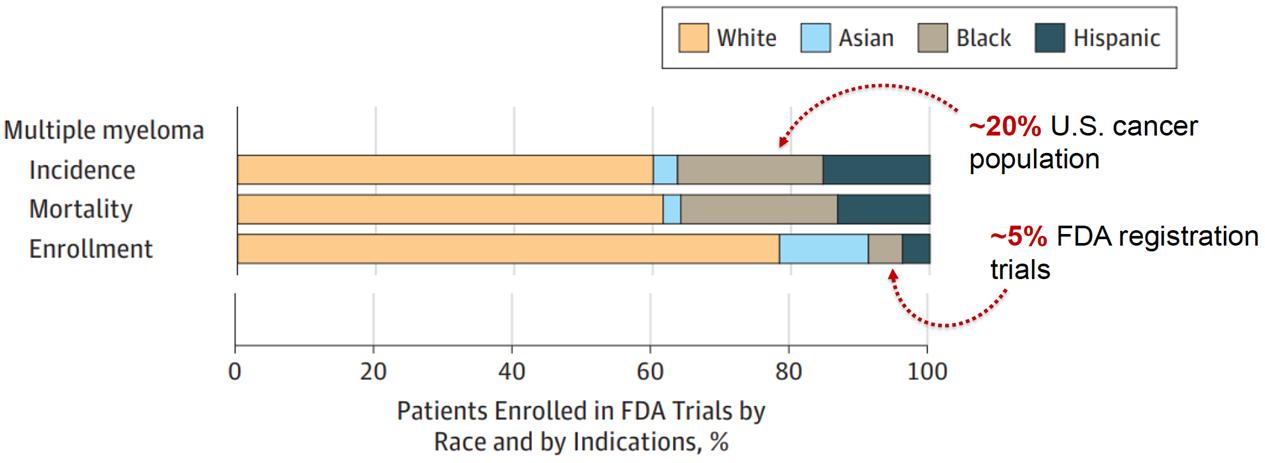

1. There is a longer time from symptoms to diagnosis among African Americans
2. African Americans are younger by about 5 years on average at diagnosis
3. MM and MGUS are more than 2x as common in African Americans
4. African Americans are less likely to receive the four T’s: Transplant, Triplets, Trials and CAR T
5. African Americans have biologic differenceswith more t(11;14) and less high-risk cytogenetics with deletion 17p
6. Survival outcomes in African Americans are HALF of what is seen in White Americans
7. African Americans can achieve equal or better outcomes when they receive therapy
It is a complex problem and requires a complex solution
Key themes of Success:
Awareness, Education, Advocacy and Empowerment in the lay community

Education, Cultural Competence, Access in the medical community
Policy, Expectations, Commitment in the regulatory and corporate community
This is impossible without genuine collaboration between ALL stakeholders
We believe the IMF is uniquely poised to address this issue and bring many of these key stakeholders together…

The core vision of this initiative is to improve the short- and longterm outcomes of African American patients with myeloma.
Engage the community to increase awareness and provide support
We want to empower patients and communities to change the course of myeloma…

Enhance access optimal care by educating myeloma providers about the disparity and how to reduce it
Shorten the time to diagnosis by educating primary care providers to recognize the disease and order the right tests
1931 : Decided to follow untreated men (study didn’t have funds for treatment) in order to show the need for syphilis treatment.
1932 : The men would be given periodic physical assessments and told they were being treated. Enrolled 399 African-Americans with syphilis to observe the natural history of syphilis.

1945 : Penicillin accepted as treatment of choice for syphilis.
1969 : CDC & AMA officiallysupport continuation of the study. OMG.
1972 : Washington Star broke the story.
1974 : Class action lawsuit settled - only $37K per patient.
1997 : Formal apology from President Clinton
2009 : The last widow receiving benefits from Tuskegee Health Benefit Program (THBP) dies
Each time they visited the doctors, the men thoughttheir condition was being treated and cured. Instead the “medicine” provided to them was fake. To ensure that the men would show up for a spinal tap, the doctors misled them with a letter fullof hype, “Last Chance for Special Free Treatment.”
https://en.wikipedia.org/wiki/Unethical_human_experimentation_in_the_United_States
National Research Act 1974
1. All persons, no matter how weakened, deserve protection
2. All research should maximize benefits, minimize harms
3. Research must not exploit those readily available or malleable Research on the fetus (1975)
No inducements will be offered to terminate a pregnancy. Individuals engaged in the research will have no part in any decisions as to the timing, method, or procedures used to terminate a pregnancy.
Research involving prisoners (1976)
From 1962 to 1966, for example, 33 pharmaceutical companies tested 153 experimental drugs at Holmesburg Prison (Philadelphia, PA). This stopped in 1976, and was modified in 1997 such that research on prisoners must present no more than minimalrisk
Research involving children (1977)
Defines children as vulnerable
Institutional review boards (1978)
Requires the establishment of committees to review each research project
• Healthy volunteers paid $2,060 to participatein first-in-humanstudy of a fatty acid amide hydrolaseinhibitor in hopes of treating anxiety and pain
• 90 people received lower doses without many side effects.
• The 6 participants thatbecame ill received repeat higher doses over the course of several days – the first was hospitalizedand later died, the trial was stopped the next day, but the other 5 all went to the hospitalover the next week.
● First in human phase I clinicalstudy was conductedfor a CD28superagonist antibody TGN1412 in six human volunteers.
● After the very first infusion,all 6 volunteers faced life-threateningconditions involving multiorgan failure for which theywere moved to the ICU.
● Moreover, when the last volunteer was to be infused, the first volunteer had already started showing adverse effects. Despitethis, the 6th volunteer was still infused in a private unit leased by the clinicalresearchorganization (CRO).
Set concrete targets for trial enrollment based on demographics, race, and disease biology. Expansion cohorts after primary accrual target(s) are met
Providegrant funding for studying subpopulations
SpecificPK/PD and efficacy/safetydata in people of color
Incentivize clinicaltrials in center where people of color are the majority
Increase representationof POC in facultyand clinicaltrial staff
Adjust hiring processesto limit implicitbias
Adjust accrual target(s) system-wide, protocol nonspecific
Wehave patient-level myths that persist that influence patients to make poor choices.
Each timeyou need treatment, see if a trial is appealing
Try something new if it fits, aka a clinical trial
In a diseasewith 100% risk of recurrence, would you be willing to try somethingdifferentif we knew that it wasn’t going to be worse (and might be better) than standard treatment?
This is the definitionof a clinical trial
Clinical trials are riskier than FDA-approved drugs
Scientific evidence shows that treatment on a clinicaltrial has as good a chance for success as standard treatment. The IRB and FDArequire this.
I
Current treatments = 100% risk of relapse.
Using new drugs or old drugs in a new way may improve results, inching you (and us) closer to a cure.
want the “gold standard” or “I don’t want to be a guinea pig”
Clinical trials should only be for people with no other options
All patients should consider trialsat all stages, e.g. newly diagnosed, maintenance, and for when they relapse.
Consult
If my doctor doesn’t mention clinical trials, it must not be right for me.
with a doctor that treats only myeloma, aka a myeloma specialist.
All oncologistsknow how to treat myeloma
A general oncologist could use a specialist’s help in dealing with this rare cancer.
The trial is more important than the patient.
Never. You can stop your participation on a clinical trial at ANY TIME and for ANY reason.
Any clinical trial is a good trial. Some clinical trialsare sponsored to expand indications of approved drugs to increase profit.
Some clinical trials require too many trips, too many biopsies –ask what is required and what is optional?

I don’t want to get the placebo. Practically no myeloma trials have a placebo arm, but there are still control and experimental ‘arms’.
1. Use the best available clinical trial search engine (www.sparkcures.com), but if you really want to know what trials are available, you have to physically show up.
2. The most common reason to not participate in a clinical trial is transportation.
a) How many extra trips? Is there money to decrease transportation and lodging costs?
b) How many extra procedures, like bone marrow biopsies?
3. What are the standard of care alternatives? The clinical trial may be the only good option, but it’s rarely the only option.
4. Is the principal investigator of the trial also your doctor? Let’s all be honest.
5. For therapeutic trials, don’t ask “What are the side effects?”, ask instead “What is the main side effect”? Most consents are >20 pages and time is short.
















Patient and Family Webinars: Los Angeles – August 18 & 19, 2023 – details to be announced,this event will havean international myeloma expert presenting!


Thank you for attending today's program!
June 24, 2023, International Myeloma Foundation’s Regional Community Workshop –Atlanta.
