

THANK YOU TO OUR SPONSORS!



A Tale of THREE Meetings!
1. ASCO – American Society of Clinical Oncology (Chicago, IL)
45,000 attendees in all areas of Oncology
2. International Myeloma Working Group (IMWG) Summit (Milan, Italy)
Nearly 200 attendees including IMWG members
3. European Hematology Association (EHA) Annual Congress – Milan, Italy)
18,000 attendees in all areas of Hematology



1. SCREENING FOR MYELOMA
Timing of MM initiation

Age of first chromosomal gain to age of sample collection was median 30 years.






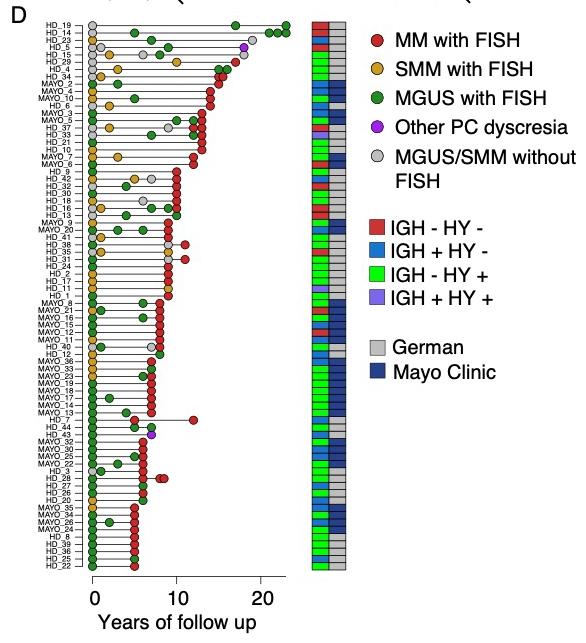
Heidelberg
Weinhold Raab Poos Mayo Clinic
Baughn Kumar Rajkumar
Maura F et al. Nat Gen 2025 in press; Rustad et al. Nat Comm 2020;
Oben et al. Nat Comm 2021; Maura et al CCR 2021; Samur et al. Blood 2024;
Iceland Screens, Treats, or Prevents Multiple Myeloma
Control arm
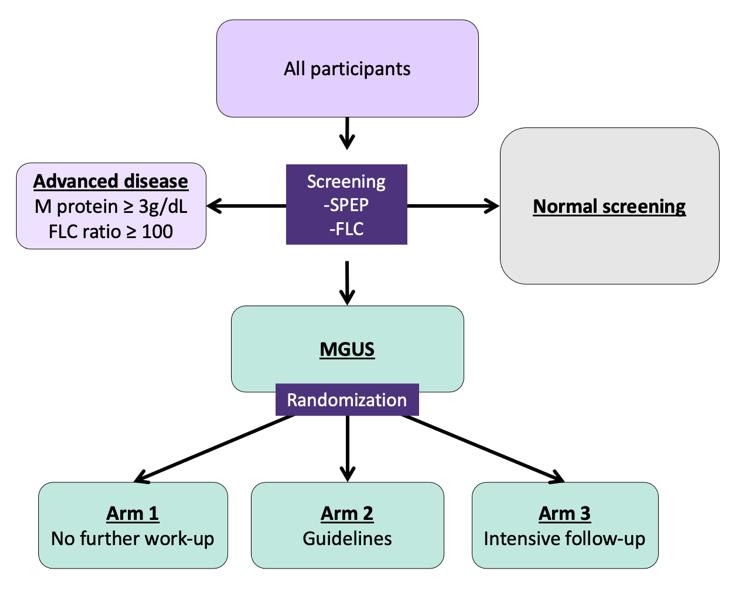
Inclusion criteria:
-Born ≤1975
-Icelandic resident

Exclusion criteria:
-Previous lymphoproliferative disease
Only arm 2 and 3:
-Previously known MGUS (excluded for this analysis)
Psychiatric health: Assessed at registration, after MGUS notification, and annually
Intervention arm
Rögnvaldsson S et al. (2021) Blood Cancer Journal; 11, 94

Dr. Joe’s Take on Screening
This is a MASSIVE and critical study to educate us about the feasibility and usefulness of screening It appears that the “events” that lead to MM start in the YOUNG!
All screening programs are focused by age, gender, family history, etc and we should expect the same in MM – especially as it is twice as common in patients of African descent
The ISTOPMM trial will likely answer the key question of whether or not screening saves lives in the next 2 years...
What does the panel think of Screening?

2. SMOLDERING MYELOMA
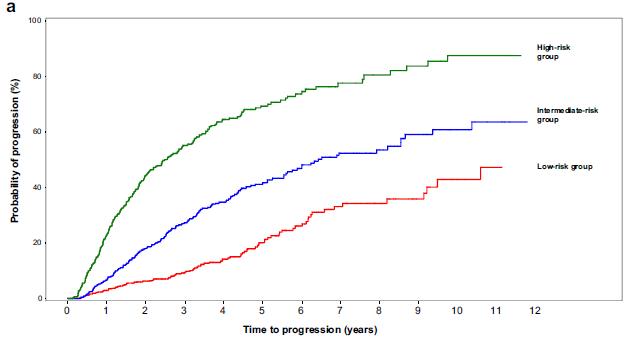
The 20-2-20 model
Risk factors
•
• Single center
• n = 421 patients, with SMM diagnosis in 2003-2015
• Excluded those with SLiM (retrospectively)
• Advanced imaging in 124 (29.4%)
• Multicenter
• n = 1363 patients, with SMM diagnosis in 2004-2018
• Excluded those with SLiM
• Advanced imaging in unknown number
Mateos
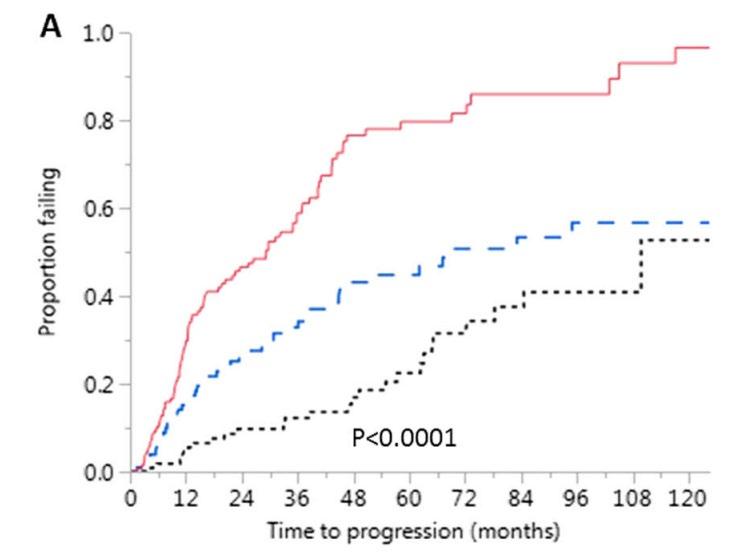
AQUILA: Progression to MM by IMWG SLiM-CRAB Criteria (IRC Assessment)
treatment
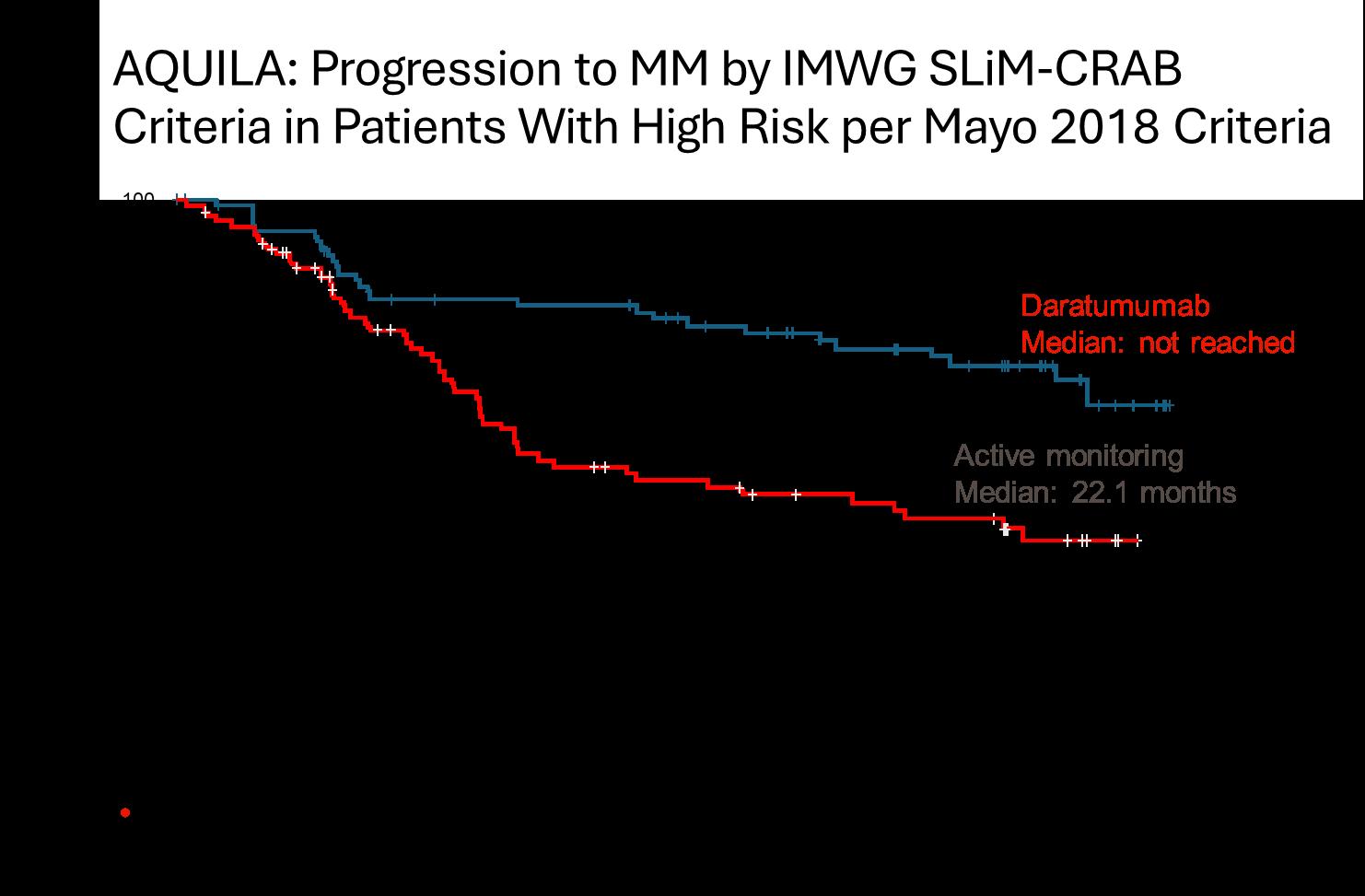
• DARA significantly reduced the risk of progression to MM or death by 51% versus active monitoring for all subsets and risk categories, more pronounced in high risk SMM
• The benefit continued beyond 36 months
• May 20, 2025: FDA ODAC positive vote
SMM: where are we today?
• We now have four options for HR SMM
• Early intervention with daratumumab (when approved)
• Close follow up with labs (Q 3-4 months) and modern imaging techniques (Q year)
• Enrollment in trials with combinations of current agents active in MM (PI,IMiDs,anti-CD38)
• Enrollment in trials with curable intent (HDT, CARs, Bispecifics)
• Decisions may be based on patient’s wish, fitness/frailty, comorbidities, life expectancy
• No need for trials in low risk SMM
• For intermediate risk SMM: we need trials with innovative approaches to intercept evolution to myeloma
• Research to identify risk factors at baseline that may move patients to an early myeloma category
• Research to develop dynamic models which are more informative for the daily management of patients (evolving patterns)
Dr. Joe’s Take on Smoldering Myeloma
• The AQUILA trial study was critical to really prove we can delay the progression to active myeloma and even improve survival with 3 years of daratumumab
• It underscores the important of a DISCUSSION with the health care team as many options can be offered to patients with high risk smoldering MM
• There will me MANY more trials coming in this area, with even more intense therapies like combinations and even CAR T Cell therapy...
What does the panel think of treating Smoldering?
(We have the PIs of the key Lenalidomide studies here!)

3. FRONTLINE THERAPY
Subcutaneous Daratumumab (Dara) + Bortezomib/Lenalidomide/Dexamethasone With Dara + Lenalidomide Maintenance in Transplant-Eligible Patients
With Newly Diagnosed Multiple Myeloma: Analysis of Sustained Minimal Residual Disease Negativity in the
Phase 3 PERSEUS Trial
Philippe Moreau1, Pieter Sonneveld2, Hermann Einsele3, Hang Quach4, P Joy Ho5, Meral Beksac6, Cyrille Hulin7, Elisabetta Antonioli8, Xavier Leleu9, Silvia Mangiacavalli10, Aurore Perrot11, Michele Cavo12, Angelo Belotti13, Annemiek Broijl2, Huiling Pei14, Anna Sitthi-Amorn15, Robin L Carson15, Paula Rodríguez-Otero16, Meletios A Dimopoulos17, Mario Boccadoro18
1University Hospital Hôtel-Dieu, Nantes, France; 2Erasmus MC Cancer Institute, Rotterdam, Netherlands; 3Universitätsklinikum Würzburg, Medizinische Klinik und Poliklinik II, Würzburg, Germany; 4University of Melbourne, St. Vincent’s Hospital, Melbourne, VIC, Australia; 5Royal Prince Alfred Hospital, Sydney, Australia; 6Ankara University, Ankara, Turkey; 7Hôpital Haut Lévêque, University Hospital, Pessac, France; 8Careggi Hospital and University of Florence, Firenze, Italy; 9CHU Poitiers, Poitiers, France; 10Fondazione IRCCS Policlinico San Matteo, University of Pavia, Pavia, Italy; 11Centre Hospitalier Universitaire de Toulouse, Oncopole, Toulouse, France; 12IRCCS Azienda Ospedaliero-Universitaria di Bologna, Seràgnoli Institute of Hematology, Bologna University School of Medicine, Bologna, Italy; 13ASST Spedali Civili di Brescia, Brescia, Italy; 14Johnson & Johnson, Titusville, NJ, USA; 15Johnson & Johnson, Spring House, PA, USA; 16Cancer Center Clínica Universidad de Navarra, Cima, Pamplona, Navarra, Spain; 17National and Kapodistrian University of Athens, Athens, Greece; 18University of Turin, Turin, Italy

https://www.congresshub.com/Oncology/ AM2025/Daratumumab/Moreau
Copies of this presentation obtained through Quick Response (QR) Codes are for personal use only and may not be reproduced without permission from ASCO® or the author of this presentation.

Presented by P Moreau at the American Society of Clinical Oncology (ASCO) Annual Meeting; May 30–June 3, 2025; Chicago, IL, USA & Virtual
PERSEUS: Study Design
Maintenance
• Transplanteligible NDMM
• Age 18‒70 years
• ECOG PS ≤2
4 cycles of 28 days
28-day cycles
Continue DR until PD Stop Dara and Restart Dara continue R per criteria
• MRD-negativityc rate was defined as the proportion of patients achieving MRD negativity and ≥CR in the ITT population
– Patients who were not evaluable or had indeterminate results were considered MRD positive
– MRD was evaluated post consolidationd at the time of suspected CR/sCR; at 12, 18, 24, 30, and 36 months after cycle 1 day 1; and yearly thereaftere
aStratified by ISS stage and cytogenetic risk. bDara 1800 mg co-formulated with rHuPH20 (2000 U/mL; ENHANZE ® drug delivery technology, Halozyme, Inc., San Diego, CA, USA); VRd administered as in the VRd group. cMRD was assessed using the clonoSEQ assay (v.2.0; Adaptive Biotechnologies, Seattle, WA, USA) in patients with ≥VGPR post consolidation and at the time of suspected ≥CR. dIn patients with ≥VGPR. eIn patients who achieved CR/sCR and remained on study.
CR, complete response; Dara, daratumumab; DR, daratumumab and lenalidomide; DVRd, daratumumab, bortezomib, lenalidomide, and dexamethasone; ECOG PS, Eastern Cooperative Oncology Group performance status; ISS, International Staging System; ITT, intent-to-treat; MRD, minimal residual disease; NDMM, newly diagnosed multiple myeloma; PD, progressive disease; rHuPH20, recombinant human
PH20; R, lenalidomide; sCR, stringent

PERSEUS: Sustained
MRD-Negativity (10‒5) ≥CR Rates
by P Moreau at the American Society of Clinical Oncology (ASCO) Annual Meeting; May 30–June 3, 2025; Chicago, IL, USA & Virtual

Sustained MRD Negativity in the Phase 3
PERSEUS Trial: Conclusions
• Rates of relapse or progression within 18 months of treatment initiation (functionally high-risk disease) were lower than seen in previous daratumumab frontline trials1 and were halved with DVRd (3.1%; n=11) vs VRd (6.8%; n=24)
• DVRd + DR maintenance led to deeper and more durable responses, and was associated with improved PFS
– Higher rates of sustained MRD negativity (10‒5) ≥CR were achieved with DVRd vs VRd, with nearly two-
thirds of patients achieving sustained MRD negativity for ≥12 months and more than half achieving sustained MRD negativity for ≥24 months
– Sustained MRD negativity was associated with a PFS benefit, with >95% of patients with ≥12- or ≥24-month
sustained MRD negativity remaining progression free at 48 months
These results reinforce the consistent benefit of DVRd + DR maintenance and further support the PERSEUS regimen as SOC for TE NDMM
CR, complete response; DR, daratumumab and lenalidomide; DVRd, daratumumab, bortezomib, lenalidomide, and dexamethasone; NDMM, newly diagnosed multiple myeloma; PFS, progression-free survival; SOC, standard of care; TE, transplant-eligible; VRd, bortezomib, lenalidomide, and dexamethasone.
1 Gay F, et al. Hematology 2023;2023:433-42.

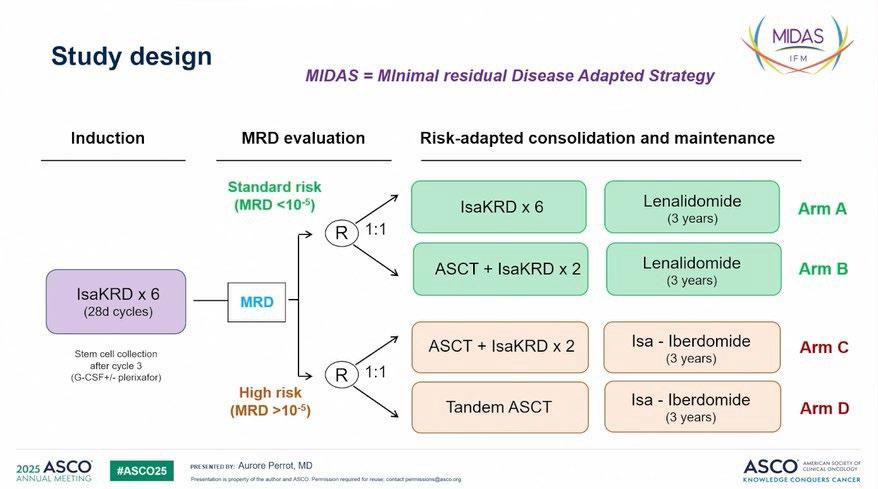
GMMG-CONCEPT Trial design
Stem cell mobilization after cycle 3
Isa: 10 mg/kg D1,8,15,22 in C1; D1,15 in C2+; K: 20 mg/m² D1,2 of C1; 36 mg/m² D8,9,15,16 of C1 and D1,2,8,9,15,16 in C2+; from 2021 onwards: 56 mg/m² on D1,8,15 and 70 mg/m² on D1,15 in maintenance; R: 25 mg D1-21 all Cycles; d: 40 mg D1,8,15,22 all Cycles (20 mg age >75).
Arm A: app. 15-18 months
Arm B: app. 12 months after inclusion
HRMM criteria: ISS stage II or III PLUS ≥1 of: del(17p), t(4;14), t(14;16) and/or ≥3 copies 1q21 (amp1q21)
Primary objective: MRD negativity after consolidation (NGF, 10-5)
Secondary objective: PFS; Selected tertiary objectives: ORR, OS
ASCT, autologous stem-cell transplant; d, dexamethasone; HDT, high-dose therapy; HRMM, high-risk multiple myeloma; Isa, isatuximab; ISS, International Staging System; K, carfilzomib; MRD, minimal residual disease; ND, newly-diagnosed; NGF, next-generation flow; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; R, lenalidomide; TE, transplant-eligible; TNE, transplant-ineligible. Following a protocol amendment in 2021, carfilzomib application was switched to once weekly 56 mg/m 2 .
Progression-free and Overall Survival
• Median follow-up of 43 months (0-90.2 months)
1st cohort: 69 months (0-90.2 months)
2nd cohort: 33 months (5.5-43.3 months)
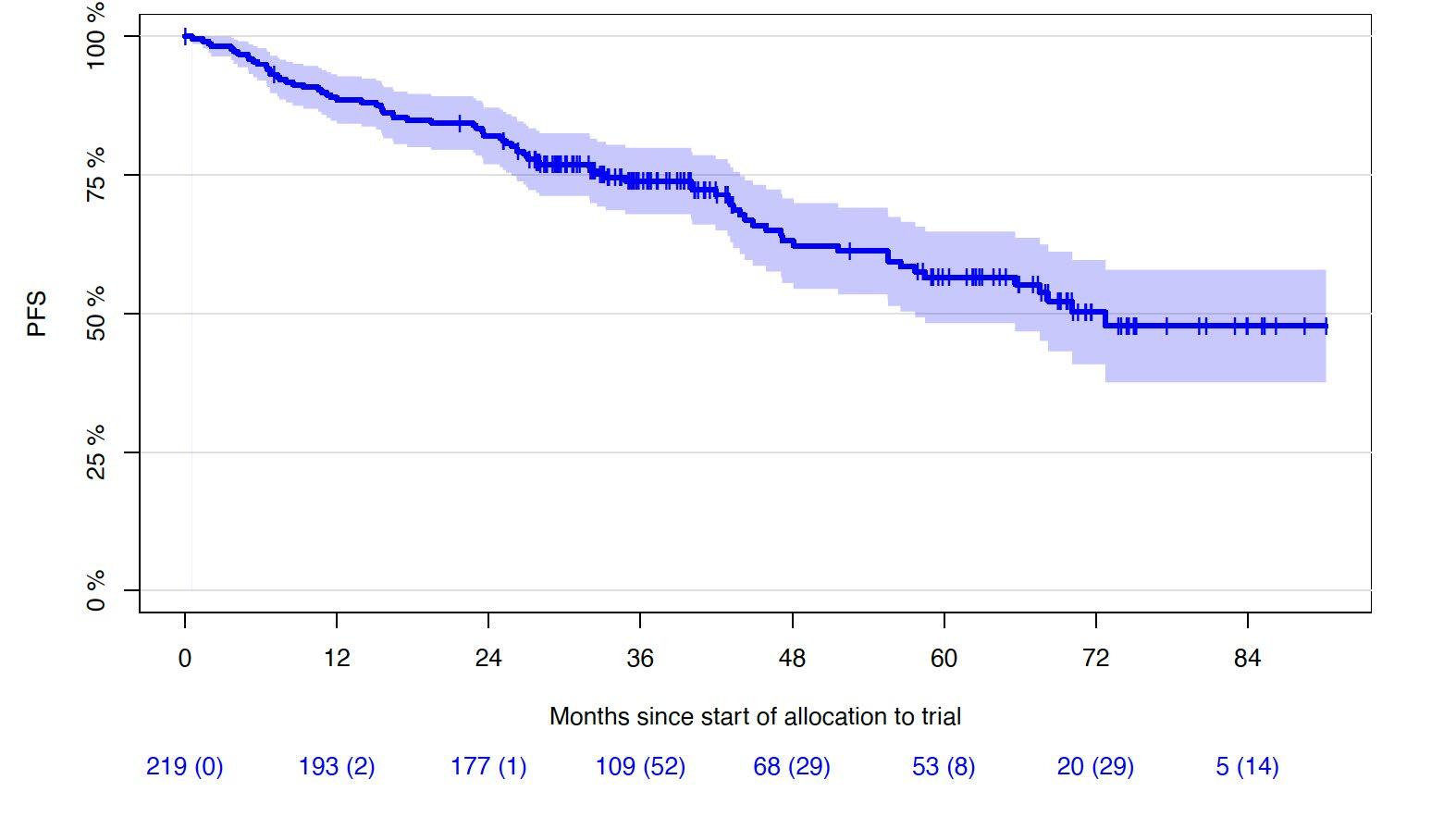
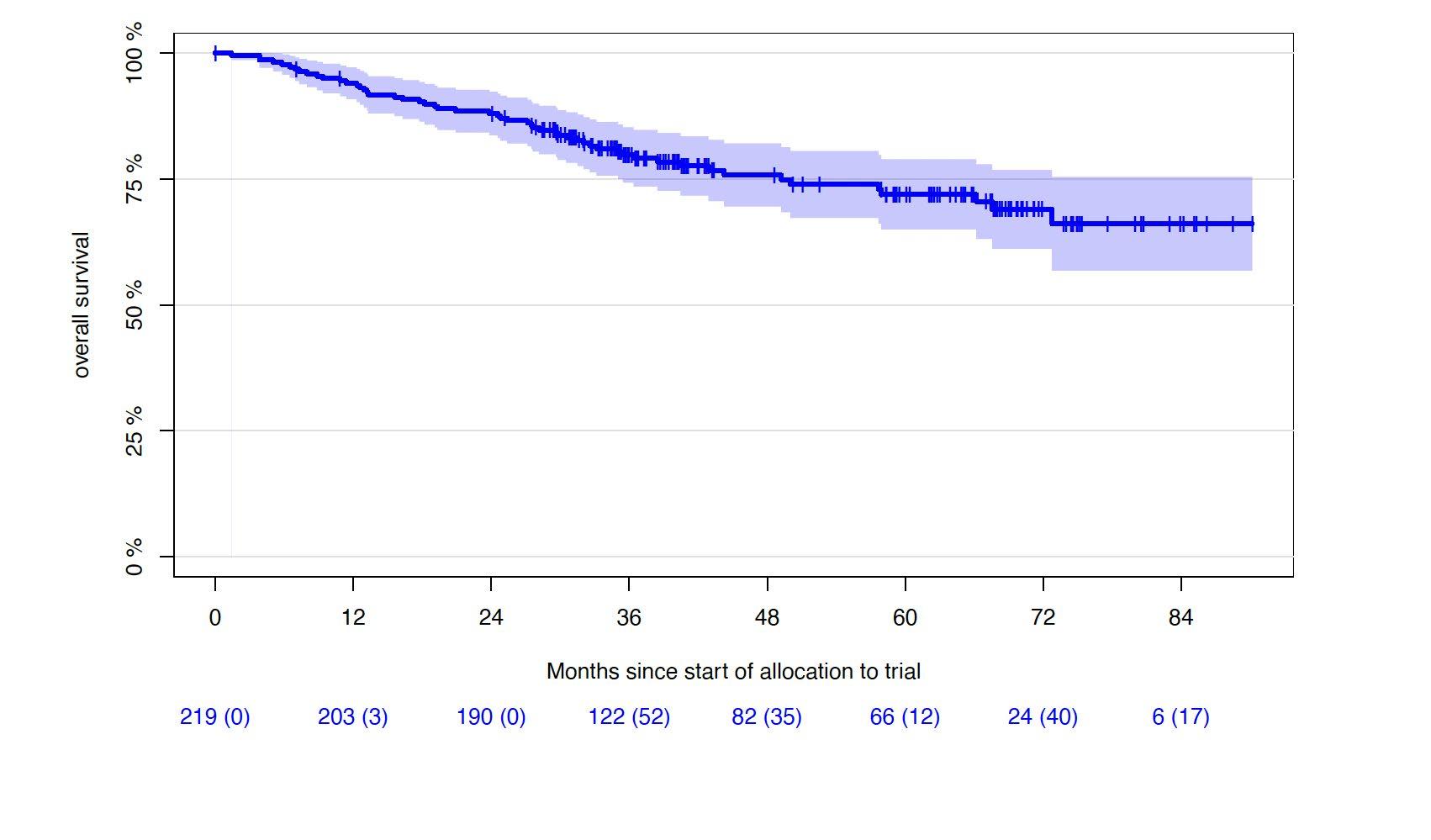
5Y-OS-rate: 72% (65.0-78.9%)
6Y-OS-rate: 69% (61.2-76.8%)
Lisa B. Leypoldt, MD
Results
Sustaining MRD-negativity was associated with increased PFS
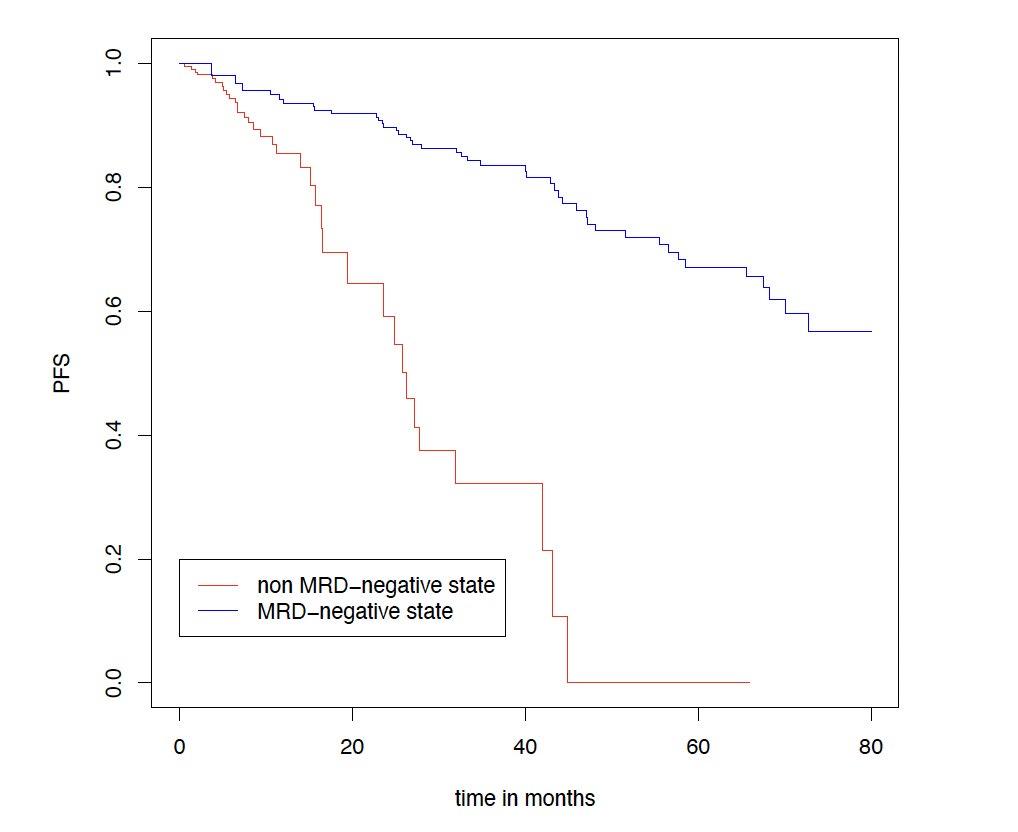
CI, confidence interval; HR: hazard ratio; MRD, minimal residual disease. Clinical data cut-off: April 28, 2025.
Multivariable time-dependent Coxregression analysis showed a prognostic PFS benefit for remaining in MRD negativity versus non–MRD negativity with a hazard ratio of 0.15 (95% CI, 0.07-0.29, p<0.0001)
Results for TE patients. Figure shows a Simon-Makuch plot illustrating the estimated survival while staying in the state of MRD-negativity compared to the estimated survival while staying in the state of MRD-positivity. Patients may switch between the two states under therapy
PRESENTED BY:
Lisa B. Leypoldt, MD
Other Studies
Belantamab mafodotin plus lenalidomide/dexamethasone in newly diagnosed intermediate-fit & frail multiple myeloma patients: Long-term efficacy and safety from the phase 1/2
BELARD clinical trial
Elranatamab in combination with daratumumab and lenalidomide (EDR) in patients with newly diagnosed multiple myeloma (NDMM) not eligible for transplant: Initial results from MagnetisMM-6 part 1

Dr. Joe’s Take on Frontline
1. Quadruplets are the standard of care, and in transplant eligible patients, dual maintenance of daratumumab-lenalidomide provides the best sustained MRD
2. MRD will guide more therapy with time – in the interim, there is no more need of tandem transplant and the role of single transplant may be questioned if this correlates with PFS outcomes
3. High risk MM remains challenging to treat, but it requires an intense approach with sustained MRD as the goal
4. There is greater use of Carfilzomib as the PI in upfront quadruplets
5. Future options include Belantamab or Bispecific antibodies
What does the panel think?

4. EARLY RELAPSE


Isatuximab (Isa) subcutaneous (SC) via an on-body delivery system (OBDS) vs Isa intravenous (IV), plus pomalidomide and dexamethasone (Pd) in relapsed/refractory multiple myeloma (RRMM): Results of the randomized, non-inferiority, Phase 3 IRAKLIA study
Sikander Ailawadhi1, Ivan Špička2, Jin Lu3, Albert Oriol4, Silvia Ling5, Fredrik Schjesvold6, Alejandro Berkovits7, Marek Hus8, Meletios-Athanasios Dimopoulos9, Péter Rajnics10, Vania Hungria11, Maria del Rosario Custidiano12, Gurdeep Parmar13, Xavier Leleu14, Tondre T Buck15, Jiří Minařík16 , Rick Zhang17, Dorothée Sémiond17, Maya Stefanova-Urena17, Philippe Moreau18
1Division of Hematology/Oncology, Mayo Clinic, Jacksonville, FL; 2Departments of Medicine and Hematology, First Faculty of Medicine, Charles University and General Hospital, Prague, Czech Republic; 3Peking University People’s Hospital, Peking University Institute of Hematology, National Clinical Research Center for Hematologic Disease, Beijing, China; 4Institut Català d'Oncologia, Hospital Germans Trias i Pujol, Barcelona, Spain; 5Sydney Adventist Hospital, Wahroonga, NSW, Australia; 6Oslo Myeloma Center, Department of Hematology, Oslo University Hospital, Oslo, Norway; 7Inmunocel, Las Condes, Santiago, Chile; 8Samodzielny Publiczny Szpital Kliniczny, Lublin, Poland; 9Department of Clinical Therapeutics, National and Kapodistrian University of Athens, Athens, Greece; 10Mór Kaposi Teaching Hospital, Department of Hematology, Kaposvár and Faculty of Health Sciences, Institute of Diagnostics, University of Pécs, Pécs, Hungary; 11Department of Hematology and Oncology, Clínica São Germano, São Paulo, Brazil; 12Servicio de Hematología y Trasplante Hematopoyético Instituto Alexander Fleming Buenos Aires, Argentina; 13Illawarra Cancer Care Centre, Wollongong, NSW, Australia; 14Service d'Hématologie et Thérapie Cellulaire, CHU and CIC Inserm 1402, Poitiers Cedex, France; 15Spartanburg Medical Center, Center for Hematology/Oncology, Spartanburg, SC; 16Department of Hemato-Oncology, Faculty of Medicine and Dentistry, Palacký University and University Hospital Olomouc, Olomouc, Czech Republic; 17Sanofi, Cambridge, MA; 18Hematology Department, CHU Nantes, Nantes, France
On-body delivery injector (OBI)
• A sterile, single-use, userfilled* wearable injector, nested in a filling base1-4
• No batteries or electronics3,5
Pressing the center button initiates isatuximab SC delivery and signals completion1,3,6,7
Fill
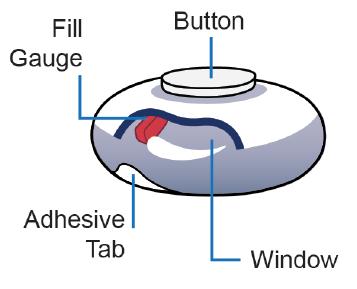
Window
Adhesive backing secures the device and assists with removal post-injection1,3,4,6

Key attributes of isatuximab SC administration with OBI**
Flat dose†: No adjustments for body weight9,10
Hands-free administration‡,6,11-15
Hidden, ~30G needle: User does not see needle before, during, or after administration3,6,11,12,15,16
Sharps injury prevention feature7
No hyaluronidase1,3
10 mL injection volume9,10
Potential at-home administration¶,2,11,16,17 Adhesive
The window lets users track fill gauge progress during administration3,8
Individualized flow rate based on subcutaneous interstitial pressure13
*During clinical studies OBI is filled using a disposable luer lock syringe. Future iterations of the filling mechanism are under development; **The OBI is an investigational device; isatuximab SC formulation is not approved by any regulatory authority. Sanofi represent that the device is safe or effective for the purposes for which it is being investigated. †Flat dose investigated in TCD15484 study. ‡No user intervention required from when injection is started until completion (device ready for removal). ¶At-home administration of isatuximab SC by OBI will be tested in EFC15951.1. OBI, on-body injector; SC, subcutaneous. 1. Enable Injections. Press release. Available at: https://enableinjections.com/a-new-frontier-self-administration-of-lyophilized-large-volume-subcutaneous-biologics/ [accessed May 2025]; 2. Carey M. AJMC 2024;30:SP352; 3. Data on file; Technical Dossier Investigational OBDS EFC1595; 4. Quach H, et al. IMS 2023; Poster 306; 5. OncodrugDelivery. Wearable injectors. Available at: https://www.ondrugdelivery.com/wp-content/uploads/2019/09/Wearable-Injectors-ONdrugDelivery-Issue-100-September-2019-MR.pdf [accessed May 2025]; 6. Data on file; SAN-015369 Rev 02 CD38sc Transfer Needle IFU; 7. Data on file. Tested in according with U.S. FDA Guidance, Medical Devices with Sharps Injury Prevention Features, August 9, 2005; 8. Taylor NP. FiercePharma Press release. Available at: https://www.fiercepharma.com/drug-delivery/vcs-infuse-215m-into-sanofi-backed-wearablesubcutaneous-drug-delivery-player-enable [accessed May 2025]; 9. Quach H, et al. Haematologica 2024;109:4078–82; 10. Quach et al. ASCO 2022; P8025; 11. Desai M, et al. Drug Deliv 2025;32:2484278; 12. Sánchez Avello N, et al. IMS 2024; Poster P-418; 13. Press release. PR Newswire. Available at: https://www.prnewswire.com/news-releases/enable-injections-enters-into-strategic-partnership-with-sanofi-300859890.html [accessed May 2025]; 14. Sarclisa Press release 2025. Available at: https://www.sanofi.com/en/media-room/press-releases/2025/2025-01-09-06-00-00-3006798 [accessed May 2025]; 15. Rahman O. et al. ONdrugDelivery 2024;164:28–31; 16. Desai M, et al. Curr Med Res Opin 2024;17:1–12; 17. Enable injections. Meet enFuse. Available at: https://enableinjections.com/technology [accessed May 2025].
Image sources: Data on file; SAN-015369 Rev 02 CD38sc Transfer Needle IFU; Quach H, et al. IMS 2023; Poster 306; and Desai M, et al. Drug Deliv 2025;32:2484278.NCT05704049.
Gauge Button
Key takeaways

1
The results of IRAKLIA demonstrate that Isa SC on-body injector (OBI) offered the same level of efficacy and pharmacokinetics as Isa IV, meeting non-inferiority.

2 Patients receiving Isa SC OBI hands-free expressed higher satisfaction with their injection method than Isa IV patients.

3 Isa SC OBI had a similar safety profile to Isa IV and no unexpected safety signals.
Isa, isatuximab; IV, intravenous; MM, multiple myeloma; OBI, on-body injector; SC, subcutaneous.
a
DREAMM-7: study design
Recruitment period
≈13 months from FPI (May 7, 2020) to LPI (June 28, 2021)
Eligibility criteria
• Adults with MM
• ≥1 prior line of MM therapy and documented PD during or after most recent therapy
• No prior treatment with anti-BCMA
• Not refractory to or intolerant of daratumumab or bortezomib
Stratification:
• Prior lines of treatment (1 vs 2 or 3 vs ≥4)
• R-ISS stage (I vs II/III)
• Prior bortezomib (yes vs no)
Treatment period
Until end of study, withdrawal of consent, PD, death, or unacceptable toxicity
Cycles 1-8
Belamaf
2.5 mg/kg IV q3w +
bortezomib 1.3 mg/m2 SC on days 1, 4, 8, and 11 of cycles 1-8 (21-day cycles)
+
dexamethasone 20 mg on the day of and day after bortezomiba in cycles 1-8
Daratumumab
16 mg/kg IV qw in cycles 1-3; and q3w in cycles 4-8
+ Primary endpoint: PFS (IRC assessed)
bortezomib 1.3 mg/m2 SC on days 1, 4, 8, and 11 of cycles 1-8 (21-day cycles)
+ dexamethasone 20 mg on the day of and day after bortezomiba in cycles 1-8
Cycle 9+
Belamaf monotherapy
2.5 mg/kg IV q3w
Follow-up for PFS q3w
(for patients who discontinue due to reasons other than PD)
Disease assessments q3w
Follow-up for OS q12w
(for patients who discontinue due to PD or other reasons)
Daratumumab monotherapy
16 mg/kg IV q4w in cycle 9+
Median follow -up (ITT) 28.2 months (range, 0.1-40.0 months)
Disease assessment visits: q3w from cycle 1 day 1 until PD
Key secondary endpoints: OS, DOR, and MRD
Additional secondary endpoints: CRR, ORR, CBR, TTR, TTP, PFS2, AEs, ocular findings, and QOL
María Victoria Mateos, MD, PhD
DREAMM-7: ITT population
BVd led to a significant increase in PFS vs DVd
BVd demonstrated a statistically significant and clinically meaningful PFS benefit, with a median PFS that was 23 months longer than that with DVd (36.6 vs 13.4 months)
follow-up: 28.2 months (range, 0.1-40.0 months)
María Victoria Mateos, MD, PhD
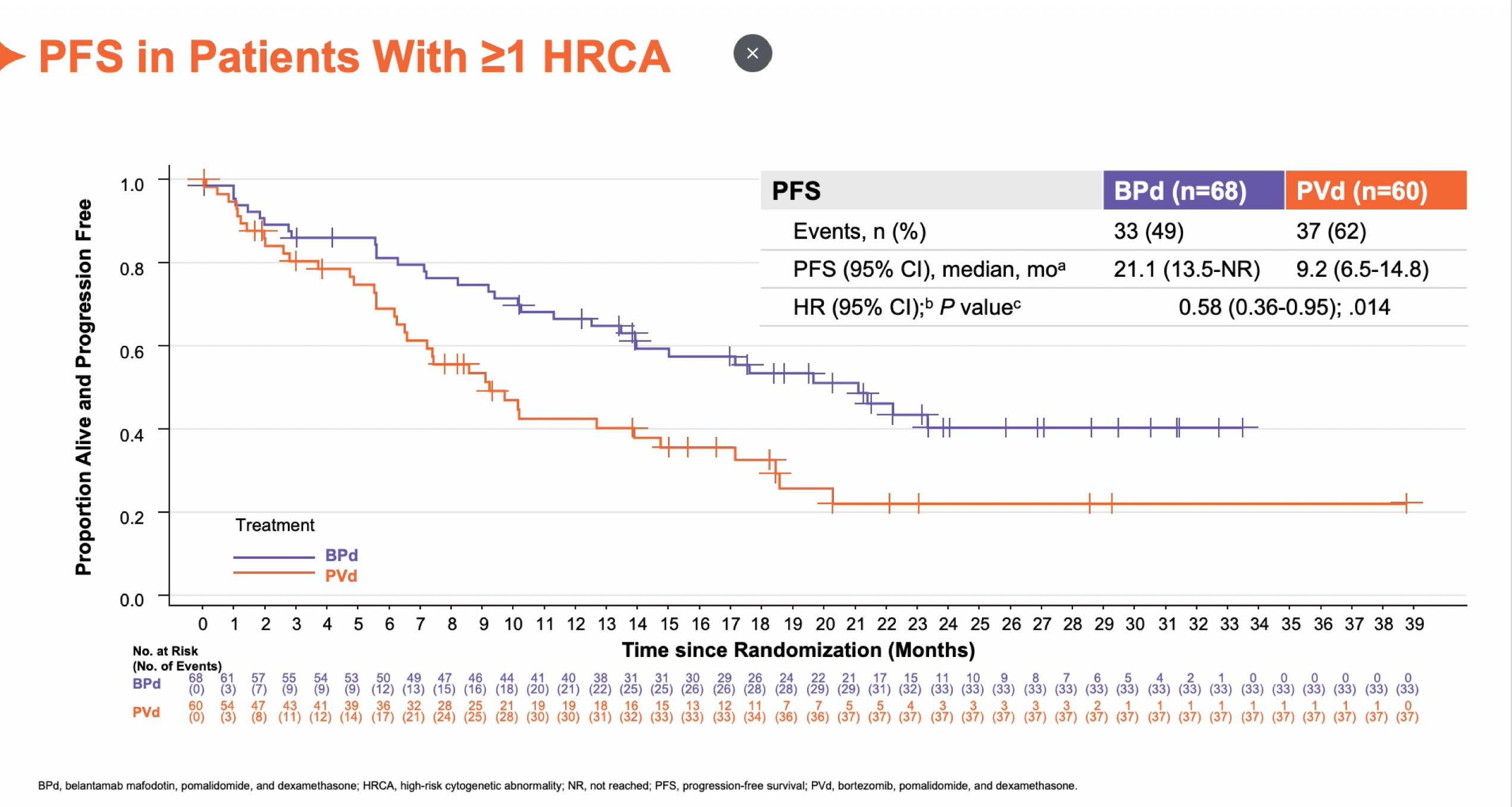

Ciltacabtagene Autoleucel (Cilta-cel) vs Standard of Care (SOC) in Patients (pts) with Relapsed/Refractory Multiple Myeloma (MM): CARTITUDE-4 Survival Subgroup Analyses
Surbhi Sidana1, Joaquín Martínez-López2, Abdullah M Khan3, Albert Oriol4, Andrew Spencer5, Binod Dhakal6, Yaël C Cohen7, Cyrille Touzeau8,9,10, Dominik Dytfeld11, Hermann Einsele12, Jesús San-Miguel13, Salomon Manier14, Diana Chen15, Katherine Li16, Nina Benachour17, Carolina Lonardi18, Arnab Ghosh19, Nitin Patel20, Erika Florendo20, Simon J Harrison21,22,23
1Stanford University School of Medicine, Stanford, CA, USA; 2Hospital 12 de Octubre, Complutense University CNIO, MIC, Madrid, Spain; 3The Ohio State University Comprehensive Cancer Center, Columbus, OH, USA; 4Institut Català d’Oncologia and Institut Josep Carreras, Hospital Germans Trias Pujol, Badalona, Barcelona, Spain; 5Alfred Health-Monash University, Melbourne, VIC, Australia;6Medical College of Wisconsin, Milwaukee, WI, USA; 7Tel Aviv Sourasky (Ichilov) Medical Center, Faculty of Medical & Health Sciences, Tel Aviv University, Tel Aviv, Israel; 8Service d’hématologie, Centre Hospitalier Universitaire (CHU) Hotel Dieu, Nantes, France; 9Centre de Recherche en Cancérologieet Immunologie Intégrée Nantes Angers, INSERM, Centre National de la Recherche Scientifique, Université d’Angers, Université de Nantes, France;10Site de Recherche Intégrée sur le Cancer, Imaging and Longitudinal Investigations to Ameliorate Decision-making (ILIAD), French National Cancer Institute–French Ministry of Health–INSERM 12558, Nantes, France;11Poznan University of Medical Sciences, Poznań, Poland; 12Universitätsklinikum Würzburg, Medizinische Klinik und Poliklinik II, Würzburg, Germany; of Oncology, University of Melbourne, Parkville, Australia; 13Cancer Center Clinica Universidad de Navarra, CIMA, IDISNA, Pamplona, Spain;14University of Lille, CHU Lille, Lille, France;15Johnson & Johnson, Shanghai, China; 16Johnson & Johnson, Spring House, PA, USA; 17Johnson & Johnson, Beerse, Belgium; 18Johnson & Johnson, Buenos Aires, Argentina; 19Johnson & Johnson, Raritan, NJ, USA; 20Legend Biotech USA Inc., Somerset, NJ, USA; 21Peter MacCallum Cancer Centre, Melbourne, VIC, Australia, and Royal Melbourne Hospital, Melbourne, VIC, Australia; 22Translation Laboratory, Centre of Excellence in Cellular Immunotherapy, Peter MacCallum Cancer Centre, Melbourne, VIC, Australia; 23Sir Peter MacCallum Department of Oncology, University of Melbourne, Parkville, VIC, Australia Presented
https://www.congresshub.com/Oncology/ CONGRESS2024/Product/Author Last Name
Conclusions
• ITT analysis showed that cilta-cel improved PFS and OS vs SOC in all subgroups, including patients with EMD and 1 pLOT and beyond
• Compared with SOC, cilta-cel improved PFS and OS in patients with high-risk cytogenetics, suggesting it may overcome the poor prognosis associated with these high-risk features
• These data continue to support a positive benefit-risk ratio for cilta-cel in patients with lenalidomide-refractory MM as early as after first relapse
Cilta-cel improved PFS and OS vs SOC in all prespecified subgroups in CARTITUDE-4, including patients with standard and high-risk cytogenetics, extramedullary disease, and 1 pLOT and beyond
Other Studies
Linvoseltamab (LINVO) + carfilzomib (CFZ) in patients (pts) with relapsed/refractory multiple myeloma (RRMM): Initial results from the LINKER-MM2 trial

Dr. Joe’s Take on Early Relapse
1. The On Body Injector (OBI) for Isatuximab is a novel method for SQ delivery that is easy to administer and could even be used at home – it will make using Isatuximab dramatically easier
2. Belantamab will have a significant impact in early relapse due to its efficacy in triplet combinations with bortezomib and pomalidomide – its exact niche in sequencing therapies is yet to be determined, but will be attractive in more frail patients not eligible for CAR T
3. Linvoseltamab may be the next approved bispecific antibody – it does not appear to have a clear differentiating feature but combines well with other agents
What does the Panel think?


5. LATE RELAPSE
Long-Term (≥5 Year) Remission and Survival After Treatment With Ciltacabtagene Autoleucel in CARTITUDE-1
Patients With Relapsed/Refractory Multiple Myeloma
Peter M Voorhees1, Thomas G Martin2, Yi Lin3, Adam D Cohen4, Noopur Raje5, Myo Htut6, Abhinav Deol7, Mounzer Agha8, Jesus G Berdeja9, Binod Dhakal10, Andrzej J Jakubowiak11, Samir Parekh12, Hui Li13, Rocio Montes de Oca14, Huabin Sun15, Nikoletta Lendvai15, Deepu Madduri15, Mythili Koneru16, Nitin Patel16, Sundar Jagannath12
1Atrium Health/Levine Cancer Institute, Wake Forest University School of Medicine, Charlotte, NC, USA; 2University of California, San Francisco, CA, USA; 3Mayo Clinic, Rochester, MN, USA; 4Abramson Cancer Center, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA; 5Massachusetts General Hospital Cancer Center, Boston, MA, USA; 6City of Hope Comprehensive Cancer Center, Duarte, CA, USA; 7Karmanos Cancer Institute, Wayne State University, Detroit, MI, USA; 8UPMC Hillman Cancer Center, Pittsburgh, PA, USA; 9Tennessee Oncology, Nashville, TN, USA; 10Medical College of Wisconsin, Milwaukee, WI, USA; 11University of Chicago, Chicago, IL, USA; 12Icahn School of Medicine at Mount Sinai, New York, NY, USA; 13Johnson & Johnson, Shanghai, China; 14Johnson & Johnson, Spring House, PA, USA; 15Johnson & Johnson, Raritan, NJ, USA; 16Legend Biotech USA Inc., Somerset, NJ, USA
https://www.congresshub.com/Oncology/ AM2025/Cilta-cel/Voorhees
Copies of this presentation obtained through Quick Response (QR) Codes are for personal use only and may not be reproduced without permission from ASCO® or the author of this presentation.

Presented by PM Voorhees at the American Society of Clinical Oncology (ASCO) Annual Meeting; May 30–June 3, 2025; Chicago, IL, USA & Virtual
CARTITUDE-1 Long-Term Remission:
One-Third of Patients Were Progression-Free for ≥5 Years
Overall population (N=97); median follow -up: 61.3 months
Progression-free survival
Presented by PM Voorhees at the American Society of Clinical Oncology (ASCO) Annual Meeting; May 30–June 3, 2025; Chicago, IL, USA & Virtual
32 of 97 (33%) patients were treatment- and progression-free at ≥5 years

cilta-cel, ciltacabtagene autoleucel.
CARTITUDE-1 Long-Term
Remission: Conclusions
• One-third (33%, n=32) of the patients with a historical mPFS of <6 months remain progression-free for ≥5 years following a single cilta-cel infusion with no maintenance or subsequent therapy
– Of the progression-free patients, 12 from a single center with serial MRD assessments were all MRD- and imaging-negative at year 5 or longer, suggesting potential cure
• Long-term remission was not limited to standard-risk disease
– Patients with high-risk cytogenetics [ie, del17p, t(14;16), or t(4;14)] and those with extramedullary plasmacytomas were equally likely to be progression-free
• Patients in long-term remission had more immune-fit drug products and higher E:T ratio at peak expansion
• Median OS of 5 years sets a new benchmark in this population
Long-term follow -up from CARTITUDE-1, with one-third of patients remaining treatmentand progression-free for at least 5 years after a single infusion, shows the curative potential of cilta-cel in RRMM

First-in-Human Study of
(JNJ-5322), a Novel, Next-Generation Trispecific Antibody, in Patients With Relapsed/Refractory Multiple Myeloma:
Initial Phase 1 Results
Niels WCJ van de Donk1, Gala Vega2, Aurore Perrot3, Sébastien Anguille4, Albert Oriol5, Monique C Minnema6, Martin Kaiser7, Hans Lee8, Alfred L Garfall9, Jeffrey V Matous10, Larysa Sanchez11, Azra Borogovac12, Lionel Karlin13, Saad Z Usmani14, Joseph Weidman15, Sangmin Lee15, María-Victoria Mateos16, Paula Rodríguez-Otero17, Cyrille Touzeau18, Rakesh Popat19
1Amsterdam University Medical Center, Vrije Universiteit Amsterdam, Amsterdam, Netherlands; 2START Madrid-FJD, Hospital Fundación Jiménez Díaz, Madrid, Spain; 3Centre Hospitalier Universitaire de Toulouse, Oncopole, Toulouse, France; 4Vaccine and Infectious Disease Institute, University of Antwerp, Center for Cell Therapy and Regenerative Medicine, Antwerp University Hospital, Edegem, Belgium; 5Institut Català d'Oncologia and Institut Josep Carreras, Hospital Germans Trias i Pujol, Badalona, Barcelona, Spain; 6University Medical Center Utrecht, Utrecht, Netherlands; 7The Royal Marsden, NHS Trust, London, UK; 8The University of Texas MD Anderson Cancer Center, Houston, TX, USA; 9Abramson Cancer Center, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA; 10Colorado Blood Cancer Institute and Sarah Cannon Research Institute, Denver, CO, USA; 11Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA; 12City of Hope National Medical Center, Duarte, CA, USA; 13Centre Hospitalier Lyon Sud, Pierre-Bénite, France; 14Memorial Sloan Kettering Cancer Center, New York, NY, USA; 15Johnson & Johnson, Spring House, PA, USA; 16University Hospital of Salamanca/IBSAL/CIC/CIBERONC, Salamanca, Spain; 17Cancer Center Clínica Universidad de Navarra, CIMA, Pamplona, Spain; 18Service d’Hématologie, Centre Hospitalier Universitaire (CHU) Hotel Dieu, Nantes, France; Centre de Recherche en Cancérologie Et Immunologie Intégrée Nantes Angers, INSERM, Centre National de la Recherche Scientifique, Université d’Angers, Université de Nantes, Nantes, France; Site de Recherche Intégrée sur le Cancer, Imaging and Longitudinal Investigations to Ameliorate Decision-making (ILIAD), French National Cancer Institute–French Ministry of Health–INSERM 12558, Nantes, France; 19University College London Hospitals, NHS Foundation Trust, London, UK
Presented by NWCJ van de Donk at the American Society of Clinical Oncology (ASCO) Annual Meeting; May 30–June 3, 2025; Chicago, IL, USA & Virtual
https://www.congresshub.com/Oncology/ AM2025/Trispecific/Donk
Copies of this presentation obtained through Quick Response (QR) Codes are for personal use only and may not be reproduced without permission from ASCO® or the author of this presentation.

JNJ-5322 Trispecific: Novel Binding Domains
Targeting CD3, BCMA, and GPRC5D
Dual-Targeted Molecule to Bind Both BCMA and GPRC5D
Implications
• Enhanced myeloma cell targeting due to “double lock-down” effect of binding 2 myeloma antigens
• More comprehensive targeting of myeloma cells
• BCMA-/GPRC5D+, BCMA+/GPRC5D-, and dual BCMA+/GPRC5D+
• Prevention of antigen escape
Novel CD3, BCMA, and GPRC5D Binding Domains
• Potential to improve GPRC5Drelated safety profile
• Manageable CRS profile with only 1 step-up dose needed

JNJ-5322 Trispecific:
ORR in Patients Naive or Exposed to BCMA/GPRC5D Therapies

JNJ-5322 Trispecific: Treatment-Emergent Adverse Events
• At the RP2D, 1 DLT (neutropenia) and 1 grade 5 TEAE (pneumonia)
• Across all other doses, 4 DLTs (maculopapular rash, palmar-plantar erythrodysesthesia syndrome, pneumonia, and respiratory failure) and 4 grade 5 TEAEs (adenoviral encephalitis [drug related], embolic stroke, multiple organ dysfunction syndrome, and pulmonary hemorrhage)

TRIgnite-1 Study: Phase 1, First-in-Human, Dose-
Escalation Study of ISB 2001, a BCMA × CD38 × CD3
Targeting Trispecific Antibody in Patients with Relapsed/Refractory Multiple Myeloma (RRMM)
Eben I. Lichtman1, Amit Khot2, Bradley Augustson3, David Levitz4, Hanlon Sia5 ,
Nicole Wong Doo6, Michaela Liedtke7, Camille Martinet8, Vinu Menon9 ,
Andrew Garton9, Harjeet Sembhi 9, Maria Pihlgren9, Cyril Konto9, Beata
Holkova9, Lida Pacaud9 , Hang Quach10
UNC Lineberger Comprehensive Cancer Center, Chapel Hill, NC1; Peter MacCallum Cancer Centre, Melbourne, Australia2; Sir Charles Gairdner Hospital, Perth, Australia3; Montefiore Medical Park, Bronx, NY4; Pindara Private Hospital, Gold Coast, Australia, Gold Coast, Australia5; CONCORD HOSPITAL, Concord, Australia6; Stanford Cancer Center, Stanford, CA7; Medqualis, Montreal, QC8; IGI Inc, New York, NY9; University of Melbourne, St Vincent's Hospital, Melbourne, Australia10

for Relapsed/Refractory Multiple Myeloma
Key Attributes
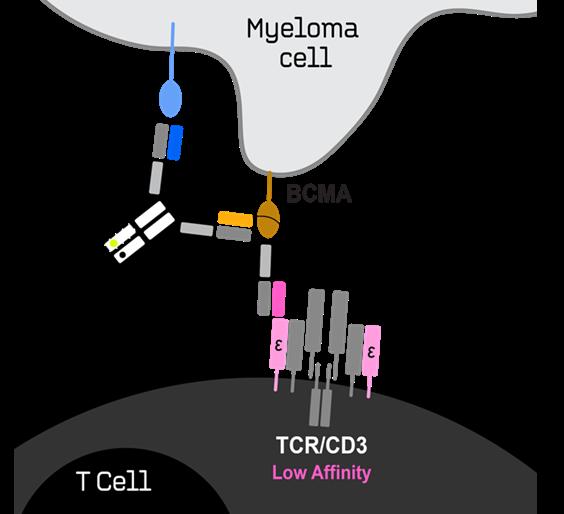
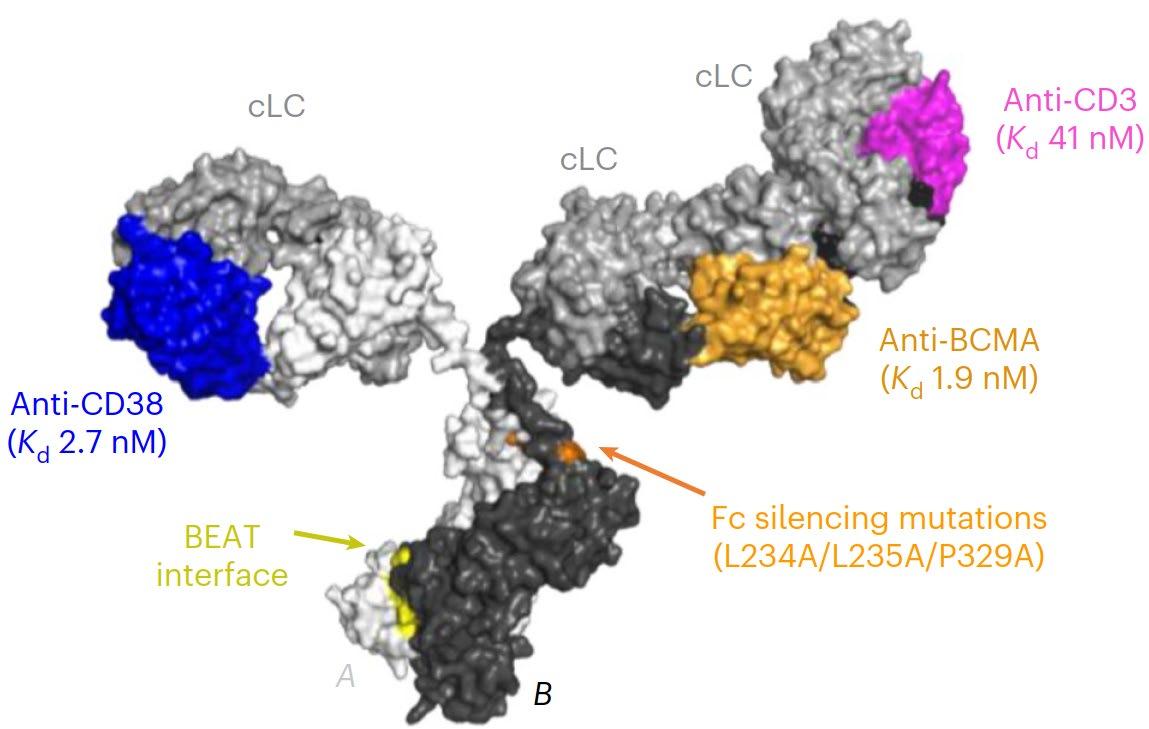
• Generated using IGI’s proprietary BEAT® protein platform
• Enhanced avidity-based binding to myeloma cells with both BCMA and CD38 Fab domains
• CD38 Fab domain targets nonoverlapping epitopes with Daratumumab
• Tuned BCMA>CD38>CD3 binding affinity and distal positioning of the CD38 vs CD3 binders drive potent tumor killing while minimizing CD38related off-tumor adverse events
TRIgnite-1 (ISB 2001): High
Response Rates in Difficult to Treat Patient Subgroups
Response in DL3 to DL9
Clinical Results from Dose-Escalation
of TRIgnite-1 Study with
ISB 2001, a Novel TREAT TM Trispecific Antibody
Dose escalation :
• Dose escalation completed with no DLTs observed up to 2700 µg/kg
• 35 heavily pretreated RRMM patients with ≥1-month follow-up included
• Favorable safety profile for a T-cell engager: Mild CRS, a single Grade 1 ICANS, well manageable neutropenias and infections enabling continuation of study treatment
• Deep and durable responses at active doses (≥ 50 µg/kg): ORR 79%, ≥CR 30%, ≥VGPR 64%, MRD negativity rate 75% with most responders still on treatment (median DOR not reached)
• Robust activity across key subgroups: Effective regardless of prior CAR-T, TCEs, BCMA therapies, CD38refractoriness, extramedullary disease, or high-risk cytogenetics
• Pharmacokinetics: Dose-proportional PK with a median half-life of 17 days supports less-frequent dosing.
Next Steps:
• Dose-expansion Part 2 is ongoing to establish RP2D and best dosing schedule for further development

Phase 2 Study
of
Talquetamab
+
Teclistamab in
Patients With Relapsed/Refractory Multiple Myeloma and Extramedullary Disease: RedirecTT-1
Shaji Kumar1, María-Victoria Mateos2, Jing Christine Ye3, Shebli Atrash4, Hila Magen5, Hang Quach6, Michael P Chu7, Suzanne Trudel8, Joshua Richter9, Paula Rodríguez-Otero10, Hun Chuah11, Moshe Gatt12, Eva Medvedova13, Shahzad Raza14, Dok Hyun Yoon15, Tadao Ishida16, Jeffrey V Matous17, Laura Rosiñol18, Koichi Onodera19, Surabhi Bajpai20, Vikram Kurra20, Emma Scott21, Christoph Heuck21, Jenny Zhang21, Todd Henninger22, Lisa O’Rourke21, Payal Thakkar22, Mariacristina Festa23, Lin Huang21, Jiashen Lu24, Nicholas Au21, Maria Krevvata21, Ashwini Kumar21, Saad Z Usmani25, Yaël C Cohen26
1Mayo Clinic Rochester, Rochester, MN, USA; 2University Hospital of Salamanca/IBSAL/CIC/CIBERONC, Salamanca, Spain; 3MD Anderson Cancer Center, University of Texas, Houston, TX, USA; 4Levine Cancer Institute-Atrium Health, Charlotte, NC, USA; 5Chaim Sheba Medical Center, Ramat-Gan, Faculty of Medical and Health Sciences, Tel Aviv University, Tel Aviv, Israel; 6University of Melbourne, St Vincent’s Hospital, Melbourne, VIC, Australia; 7Alberta Health Services, Edmonton, AB, Canada; 8Princess Margaret Cancer Centre, Toronto, ON, Canada; 9Mount Sinai Medical Center, New York, NY, USA; 10Cancer Center Clínica Universidad de Navarra, Cima, Pamplona, Spain; 11Royal Perth Hospital, Perth, WA, Australia; 12Hadassah Medical Cener, Hebrew University of Jerusalem, Jerusalem, Israel; 13Knight Cancer Institute, Oregon Health and Science University, Portland, OR, USA; 14Taussig Cancer Institute, Cleveland Clinic, Cleveland, OH, USA; 15Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea; 16Japanese Red Cross Medical Center, Tokyo, Japan; 17Colorado Blood Cancer Institute and Sarah Cannon Research Institute, Denver, CO, USA; 18Hospital Clínic de Barcelona, IDIBAPS, Barcelona, Spain; 19Tohoku University Hospital, Sendai shi, Miyagi, Japan; 20Perceptive Imaging, Boston, MA, USA; 21Johnson & Johnson, Spring House, PA, USA; 22Johnson & Johnson, Raritan, NJ, USA; 23Johnson & Johnson, Leiden, Netherlands; 24Johnson & Johnson, Shanghai, China; 25Memorial Sloan Kettering Cancer Center, New York, NY, USA; 26Tel Aviv Sourasky (Ichilov) Medical Center, Faculty of Medical and Health Sciences, Tel Aviv University, Tel Aviv, Israel Presented by S
https://www.congresshub.com/EHA2025/Oncology/ Talquetamab/Kumar-Phase-2
The QR code is intended to provide scientific information for individual reference, and the information should not be altered or reproduced in any way

RedirecTT-1 Phase 2 Tal + Tec: Dual-Antigen Targeting in Patients With True EMD Led
to Higher ORR and ≥CR Rate
Tal + Tec combination in RedirecTT-1
Higher ORR and deeper responses in patients with EMD with Tal + Tec combination vs Tal or Tec monotherapy Tal or Tec monotherapy in patients with EMDc

Abstract S201
Phase 2 Registrational Study of Anitocabtagene Autoleucel for Relapsed and/or Refractory Multiple Myeloma (RRMM): Updated Results from iMMagine-1
Gurbakhash Kaur, MD; Ciara L. Freeman, MD, PhD; Binod Dhakal, MD; Richard T. Maziarz, MD; Natalie S. Callander, MD; Adam S. Sperling, MD, PhD; Carolina Schinke, MD; Andrzej J. Jakubowiak, MD, PhD; Noa Biran, MD; Douglas W. Sborov, MD, MS; Cindy Varga, MD; Larry D. Anderson, Jr., MD, PhD; Abhinav Deol, MD; Abraham S. Kanate, MD; Mehmet Hakan Kocoglu, MD; Melhem Solh, MD; Kamalika C. Banerjee, MS, MA; Krishna Rana, PharmD; Ana Kostic, MD; Enrique Granados, MD; Carolyn C. Jackson, MD, MPH; Christopher R. Heery, MD; Tim Welliver, MD, PhD; Krina Patel, MD, MSc; and Matthew J. Frigault, MD, MS
iMMagine-1: Overall Response Rate and MRD Negativity
Efficacy Evaluable Patients, N=117
iMMagine-1: PFS and OS Rates
Estimated by Kaplan-Meier
Median follow-up of 12.6 months (range: 5 to 29 months) PFS, progression-free survival; OS, overall survival
iMMagine-1: Immune-effector Cell-associated Neurotoxicity Syndrome (ICANS)

Maximum ICANS Grade (N=117)
Supportive Measures
8% (9/117) of patients had ICANS of any grade; all cases resolved
No delayed or non-ICANS neurotoxicities were observed, including no incidence of Parkinsonism, no cranial nerve palsies, and no GuillainBarré syndrome (n=117; median follow-up of 12.6 months, range: 5-29 months)
Similarly, no delayed or non-ICANS neurotoxicities have been observed in the Phase 1 study1 (n=38; median follow-up of 38.1 months, range: 25-56 months)
1. Bishop MR, et al. Blood (2024) 144 (Supplement 1): 4825 as presented in poster #4825 at ASH 2024. ASTCT, American Society for Transplantation and Cellular Therapy.
a With the exception of n=1 Grade 1 ICANS (confusion) on day 31 post infusion that rapidly resolved
b With the exception of n=1 max Grade 2 ICANS with 29-day duration to resolution
Dr. Joe’s Take on Late Relapse
1. The long term results of CARTITUDE-1 is VERY impressive with 33% of patients disease free at 5 years – it is triggering a discussion about CURE
2. The BCMA-GPRC5D trispecific has strong efficacy and significantly less toxicity than a combination of the drugs – it has the potential to supplant sequential use of bispecific antibodies
3. The BCMA-CD38 trispecific also has impressive efficacy and tolerability and may confer another option for relapsed MM
4. The combination of teclistamab and talquetamab is impressive in patients with EMD
5. Anitocel is a promising CAR T with a high response rate and minimal neurotoxicity
What does the panel think?


6. NEW DEFINITION OF HIGH RISK MM
IMS/IMWG consensus on high risk myeloma definition

in more than 20% of sorted plasma cells
2 among t(4;14) or t(14;16) or t(14;20)
Conclusion
• The IMS Panel recommends the use of this HRMM definition in all clinical trials going forward and in routine practice.
• The HR subset should represent around 20% of NDMM patients.
• Represents an important step toward risk-stratified therapeutic approaches in routine.
• We hope that this definition will promote the design and conduct of clinical trials FOCUSED on patients with HRMM.
• NGS-based definition, but available data with FISH (report the cut-offs positivity used).
• Risk features should be also evaluated at relapse* and prior to participation in a clinical trial.
(*the risk status at relapse supplants risk status at diagnosis, unless the patient was deemed high-risk at diagnosis).
• Do we need an ultra high risk definition ?
Dr. Joe’s Take on High Risk MM
This remains a very challenging area in MM as clearly outcomes are worse in high risk disease
There are many ways to define high risk, and this new definition narrows the group to about 20% of MM – but of course is not perfect
We need more options for high risk MM and hopefully this will help – especially those with “ultra” high risk disease....
What does the panel think?

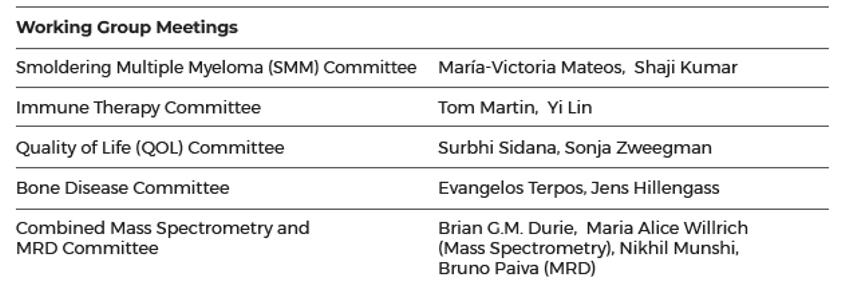
7. IMWG COMMITTEES
Final Takeaways
1. Frontline therapy has evolved to quadruplets in most patients, along with continued therapy with a CD38 Ab and lenalidomide
2. MRD will be more incorporated into our treatment strategy and may result in less use of transplant
3. CAR T cell therapy is emerging as the most effective therapy in relapsed MM – even in heavily relapsed patients outcomes are unprecedented with 33% disease free at 5 years
4. Other novel approaches with antibody-drug conjugates and bispecific antibodies will increase their presence in early lines of therapy in multiple combinations
All of this will provide for MANY more options for patients!


We Want to Hear From You
Feedback Survey
At the close of the meeting a feedback survey will pop up.
This will also be emailed to you shortly after the workshop.
Please take a moment to complete this survey.

THANK YOU TO OUR SPONSORS!



OUR VISION: A world where every myeloma patient can live life to the fullest, unburdened by the disease.
OUR MISSION:
Improving the quality of life of myeloma patients while working toward prevention and a cure.

IMF Core Values :
These are the core values we bring to accomplishing our mission each day.
Patient Centric
The patient experience is the focus of everything we do. Every interaction is an opportunity to establish a personal connection built on care and compassion which is the basis for continued support.
Respect All
As a team, we value honesty and transparency while creating a culture of mutual respect. We foster a myeloma community built on sincerity, authenticity, and kindness.
Excellence and Innovation
We value accountability, personal responsibility, and a steadfast commitment to excellence. We respect the legacy and reputation of our organization while seeking new solutions and advancements to improve outcomes, quality of life, and access to the best available resources for everyone impacted by myeloma.
Honor differences
We recognize each team member's skills and talents through collaboration and cooperation. Our programs aim to celebrate and support the diversity of our patients and their communities.






