
2025 IMF PATIENT AND FAMILY SEMINAR
PHILADELPHIA, PA

MAY 2 & 3, 2025
Thank you to our sponsors!















MAY 2 & 3, 2025
Thank you to our sponsors!












Please return your program evaluations on your way out – whether you stay for the full program, or only one session, your feedback is invaluable to our team. Thank you for taking the time completing this.




Welcome & Announcements
Robin Tuohy, Vice President, Patient Support International Myeloma Foundation
IMF Update
Diane Moran, RN, MA, EdM CEO, Senior VP Strategic Planning, International Myeloma Foundation
Understanding Clinical Trials
Joseph Mikhael, MD, MEd, FRCPC, FACP, FASCO, Chief Medical Officer, International Myeloma Foundation
Fireside Chat: What is the Future of Myeloma? With Q&A
Joseph Mikhael, MD, MEd, FRCPC, FACP, FASCO, Chief Medical Officer, International Myeloma Foundation
David Vesole, MD, PhD
John Theurer Cancer Center, Hackensack University Medical Center
MedStar Georgetown University Hospital
BREAK
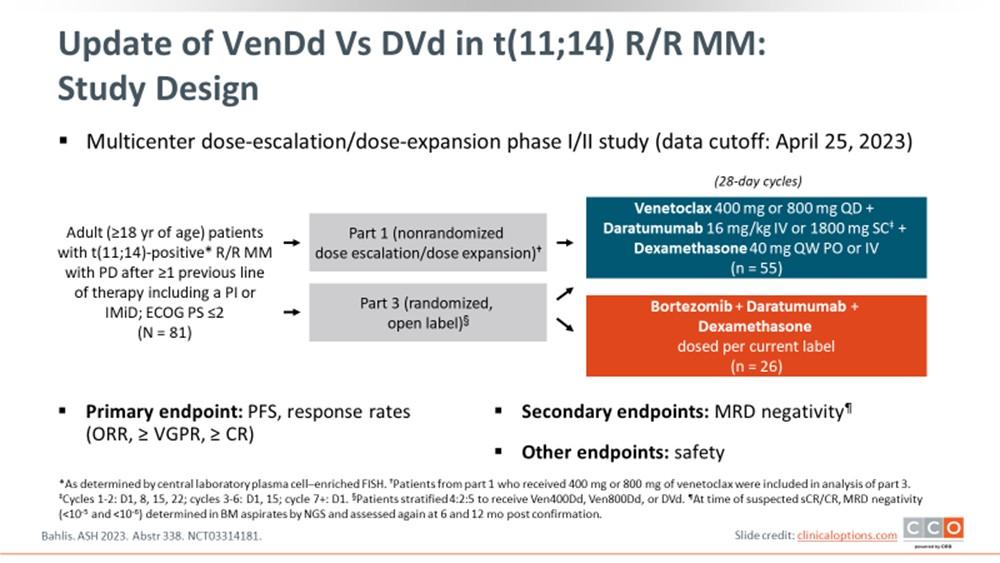
Breakout Sessions #1: Treating Myeloma
Breakout A: Newly Diagnosed: Frontline Therapy
Dan Vogl, MD, MSCE
Abramson Cancer Center, University of Pennsylvania, PA
Breakout B: Managing Relapsed Myeloma
Presentation Slides: Are available by scanning the QR code, Instructions are on the QR code handout on each table.
Restrooms: Restrooms are located outside the ballroom to your right, past the stairs and down the hallway.
Badge Holders: Please return your badge holders and we can recycle them.

We greatly appreciate your time and feedback!











Special interest groups are designed as a supplemental support for specific populations of patients, in addition to their local Support Groups
Las Voces de Mieloma-founded in 2022
Designed for Spanish speaking patients only
Living Solo & Strong with Myelomafounded in 2022
Designed for patients without a care partner


High Risk Multiple Myeloma-founded in 2023
Designed to address the needs of the high-risk MM population
Care Partners Onlyfounded in 2024
Designed to address the needs and concerns of care partners
Veterans SIG-founded in 2025
For those who served our country
Smolder Bolder-founded in 2023
Created for people living with Smoldering Multiple Myeloma
MM Families-founded in 2021
For patients/care partners with young children

Philadelphia Multiple Myeloma Networking Group
Meets virtually on the 2nd Saturday of each month at 1:30 PM Eastern
Hackensack Multiple Myeloma Support Group
Meets virtually on the 3rd Thursday of each month at 10:30 AM Eastern

Central New Jersey Multiple Myeloma Support Group
Meets virtually on the 1st Wednesday of each month at 6:30 PM Eastern
Delaware Multiple Myeloma Support Group
Meets sometimes virtually on the 3rd Wednesday of each month at 6:00 PM & sometimes in-person on the 3rd Saturday of each month at 11:00 AM Eastern
Assistance with understanding lab results, terminology and disease state
Preparing for medical visits
Resource Information:
• Financial & Emotional Support
• Expert Myeloma Referrals
“Thank you so much for the informative conversation and all the time you spent listening and helping me decipher the MM lingo. What an amazing service!”


“Thank you for your response and excellent question suggestions for my hematology team.”






Tip Cards
Myeloma Minute Weekly Updates


Myeloma Today Quarterly News
























local myeloma experts


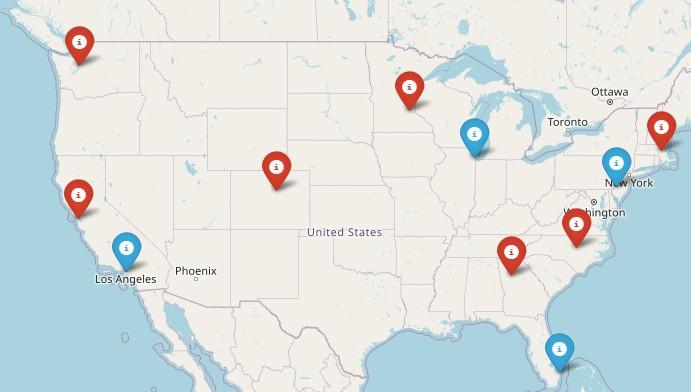

• Boca Raton, FL – March 14 – 15
• Philadelphia, PA – May 2 – 3
• Los Angeles, CA – August 15 – 16
• Chicago, IL – October 3 – 4

• Virtual - March 4
• San Francisco, CA - March 29
• Atlanta, GA - April 5
• Edina, MN - April 26
• Denver, CO - June 21
• Virtual – July 29
• Seattle, WA - August 9
• Waltham, MA - September 27
• Raleigh-Durham, NC - November 15
• Virtual – November 18






Diane Moran, RN, MA, EdM Chief Executive Officer
Sr VP
Strategic Planning
with the IMF since April 2006

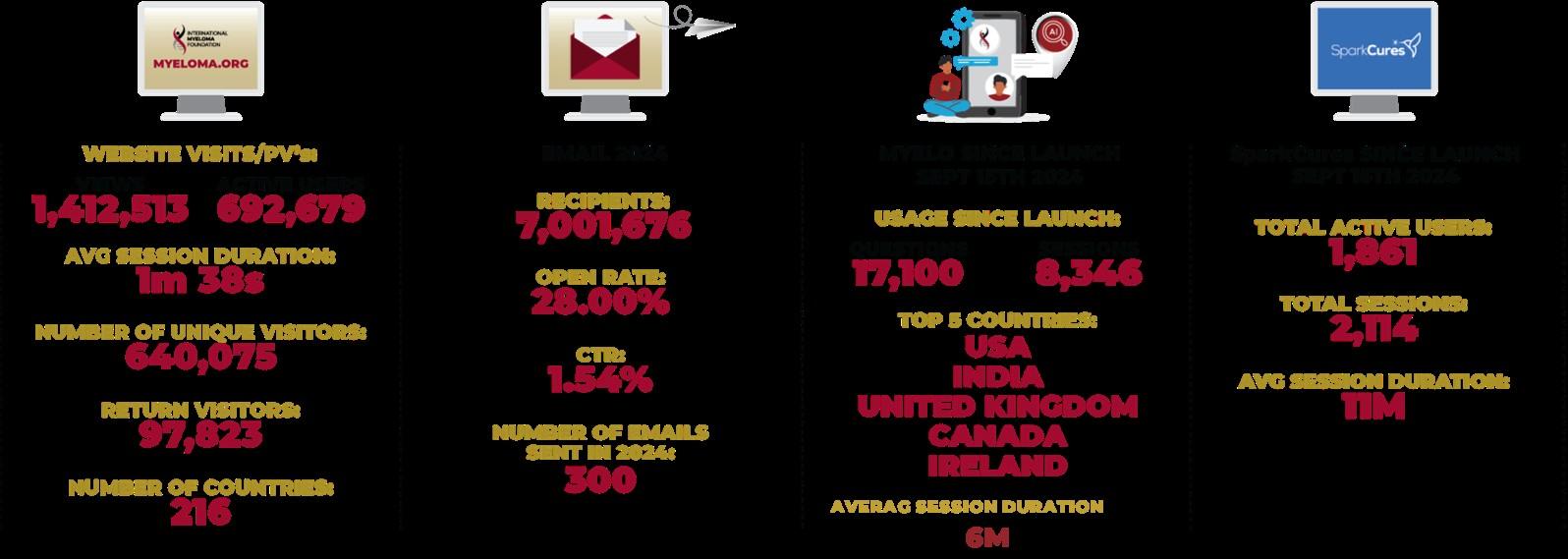
900,000 + views

• Support Groups
• InfoLine
• PFS
• RCW

3600 Nurses serving 23,000 patients with MM
• Nurse Leadership Board
• ONS Symposium
• Myeloma University






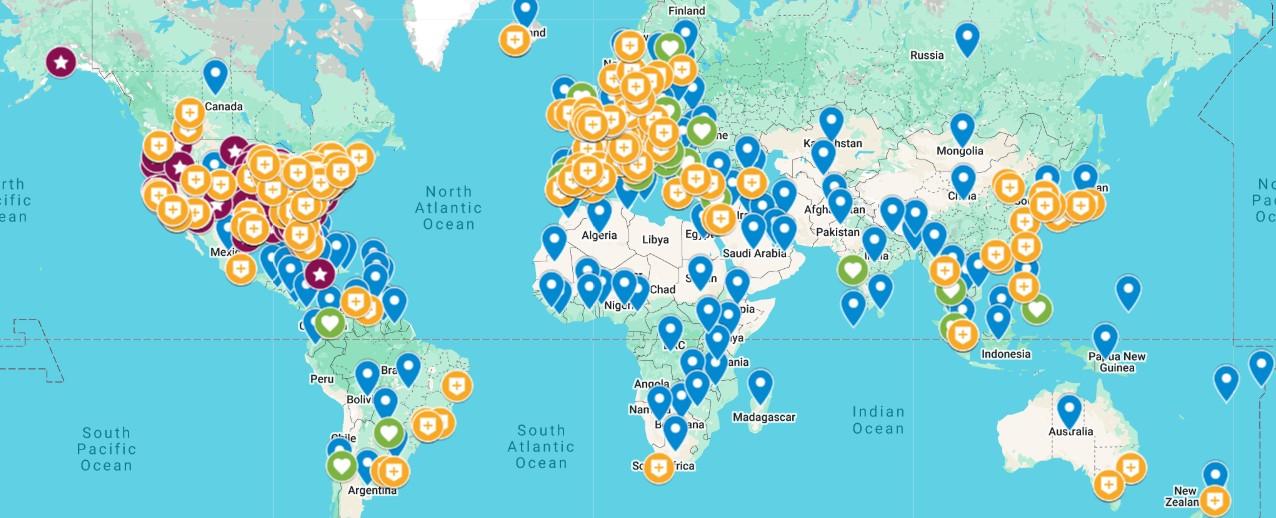
InfoLine Support groups GMAN Members IMWG Members

A world where every myeloma patient can live life to the unburdened by the disease.
Joseph Mikhael, MD, MEd, FRCPC, FACP, FASCO, Chief Medical Officer ,International Myeloma Foundation
David Vesole, MD, PhD
John Theurer Cancer Center, Hackensack University Medical Center MedStar Georgetown University Hospital

Joseph Mikhael MD, MEd, FRCPC, FACP, FASCO Chief Medical Officer, International Myeloma Foundation
Professor, Translational Genomics Research Institute, City of Hope Cancer Center


Provide The Rationale For Clinical Trials
Outline The Phases Of Clinical Trials
Discuss The Risks And Benefits Of Clinical Trials




The drive of research has brought us to where we are
No one is expected to be a “guinea pig” with no potential benefit to them
Research is under very tight supervision and standards
Open, clear communication between the physician and the patient is fundamental Driving research forward!




MYTH: If I participate in a clinical trial, I might get a placebo, not active treatment
MYTH: If I participate in a clinical trial, I can’t change my mind
• Phase 1 and 2, everyone gets active treatment
• Phase 3 standard of care vs new regimen: often standard regimen with/without additional agent in MM trials
• Patients can withdraw their consent for clinical trial participation at any time

MYTH: Clinical trials are dangerous because they have new medicines and practices
• Some risk is involved with every treatment, but medicines are used in clinical trials with people only after they have gone through testing to indicate that the drug is likely to be safe and effective for human use



MYTH: Clinical trials are expensive and not covered by insurance
• Research costs are typically covered by the sponsoring company
• Standard patient care costs are typically covered by insurance
• Check with clinical trial team/insurers; costs such as transportation, hotel, etc may not be reimbursed and are paid by patient
PhRMA website. Accessed March 25, 2024. https://phrma.org/-/media/Project/PhRMA/PhRMA-Org/PhRMA-Org/PDF/A-C/CLINICAL-TRIALS-MYTH-FACT-PRINT.pdf?hsCtaTracking=f6689b95-1626-40d9-8c87-c6b 8d31600a4%7C35221aa8-d487-4db3-9416-b9c3c35e3bac
.



Every patient is unique and must be viewed that way
Benefits of trials are numerous and include:
Early access to “new” therapy
Delay use of standard therapy
Contribution to myeloma world – present and future
Financial access to certain agents
Must be balanced with potential risks
“Toxicity” of side effects
Possibility of lack of efficacy


Identify a target for therapy in the laboratory
Confirm the anticancer activity in laboratory and animal studies
Clinical trials (human studies) to determine safety, dosing and effectiveness
The whole process costs millions of dollars and years of effort!


Most agents are tested in lab models
Various “myeloma cell lines”, also known as “in vitro”
Next step is animal model
We are more like mice than you think!!
Earliest study in Phase I is called “First in Human”
Often uses extremely low dose of drug to ensure safety


All patients receive the experimental therapy
Phase 1 trials find the optimal dose of a new drug or drug combination
Patients get higher doses as the study continues
Determine side effects of new drugs or combinations
Explore how the drug is metabolized by the body

Important for all stages of myeloma



Determine if a new drug or combination is effective against the cancer
May be added to a Phase 1 study once the ideal dose is found
Patients usually receive the experimental therapy
In some cases, the study may include two “arms” comparing either two different doses or a different treatment (another combination of drugs)


Highest form of clinical evidence. Typically, a large number of patients are required…usually required for full FDA approval
Patients receive either an experimental therapy (one or more drugs) or the current standard treatment
o The patient is randomly assigned to a treatment—a process called “randomization”
o Neither the physician or the patient can determine which treatment is given

May be placebo controlled, if no standard treatments are available
Very closely monitored for effectiveness and side effects

Preclinical
ANIMAL STUDIES: Examine safety and potential for efficacy
PHASE 1
PHASE 2
FIRST INTRODUCTION OF AN INVESTIGATIONAL DRUG INTO HUMANS
• Determine metabolism and PK/PD actions, MTD, and DLT
• Identify AEs
• Gain early evidence of efficacy, studied in many conditions; typically, 20 to 80 patients; everyone gets agent
• Determine short-term AEs and risks; closely monitored
• Includes up to 100 patients, typically
PHASE 3

PHASE 4
INFORMATION COMPARED TO STANDARD OF CARE
• Placebo may be involved if no standard of care exists; hundreds to several thousand patients
• Often multiple institutions; single or double blind; sometimes open label
APPROVED AGENTS IN NEW POPULATIONS OR NEW DOSE


• Patients will receive, at a minimum, the best standard treatment
• If the new treatment or intervention is proven to work, patients may be among the first to benefit
• Patients have a chance to help others and improve cancer care


• New treatments or interventions under study are not always better than, or even as good as, standard care
• Even if a new treatment has benefits, it may not work for every patient
• Health insurance and managed care providers do not always cover clinical trials



Patients may:
• Be unaware of clinical trials
• Lack access to trials

• Fear, distrust, or be suspicious of research
• Have practical or personal obstacles
• Face insurance or cost problems
• Be unwilling to go against their physicians’ wishes
• Not have physicians who offer them trials
• Have a disconnect with their healthcare team


There has been a lack of diverse representation in clinical trials in myeloma.
In the U.S., approximately 20% of all myeloma patients are of African descent, but only 5%–8% of patients in myeloma clinical trials are of African descent.
This is significant for the following reasons:
All patients of all races and ethnicities should be able to benefit from clinical trials.
Diverse patient representation in clinical trials is required to ensure that the outcomes are applicable to all patients.
Reasons for underrepresentation in clinical trials are complex and include:
Systemic racism, accessibility of clinical trials, sensitivity to diversity by medical professionals

Misconduct in medicine in the past, the lack of trust in the system, and more.


[P]eople from racial and ethnic minorities and other diverse groups are underrepresented in clinical research. This is a concern because people of different ages, races, and ethnicities may react differently to certain medical products.
– FDA

Leadership and commitment

Community engagement practices
Investigator hiring, training, and mentoring practices


Patient engagement practices

US Cancer Centers of Excellence: Strategies for Increased Inclusion of Racial and Ethnic Minorities in Clinical Trials

How does the study work? How often will I need to see my doctor or visit the cancer center?

Will I need to undergo additional tests?
What is currently known about the new drug or combination?


What benefits can I expect?
What side effects should I expect? Who should I notify if I have side effects?


Can I take my vitamins or other medications?

Can I get the treatment with my local doctor?



Will my insurance pay for my participation in the clinical trial?


Discuss with your physician if you are eligible for a clinical trial
Work with your physician to determine the best trial for you
Meet with the clinical research nurse or trials coordinator to discuss the trial
Carefully review the provided “Informed Consent” Describes the study and any potential safety concerns related to the experimental medication



Clinicaltrials.gov https://clinicaltrials.gov/



ncreasing-diversity-in-cancer-clinical-research






Joseph Mikhael, MD, MEd, FRCPC, FACP, FASCO, Chief Medical Officer ,International Myeloma Foundation
David Vesole, MD, PhD
John Theurer Cancer Center, Hackensack University Medical Center MedStar Georgetown University Hospital


WHEN YOU RETURN FROM BREAK PLEASE HEAD TO YOUR SELECTED BREAKOUT SESSION:
BREAKOUT A: NEWLY DIAGNOSED: FRONTLINE
THERAPY
Please move to Aria B
BREAKOUT B: MANAGING RELAPSED MYELOMA
Please remain in this room
Thank you to our sponsors!













David Vesole, MD, PhD
John Theurer Cancer Center, Hackensack University Medical Center
MedStar Georgetown University Hospital

David Vesole, MD
John Theurer Cancer Center, Hackensack University Medical Center
MedStar Georgetown University Hospital
2025 IMF Philadelphia Patient and Family Seminar
At the conclusion of this presentation, you should be better able to:
1) Know what is considered early and late relapsed/refractory multiple myeloma
2) Know the options available to treat patients with relapsed/refractory multiple myeloma.
3) Know what to expect on CAR-T cell therapy or with a bi-specific Tcell engager for relapsed/refractory myeloma.

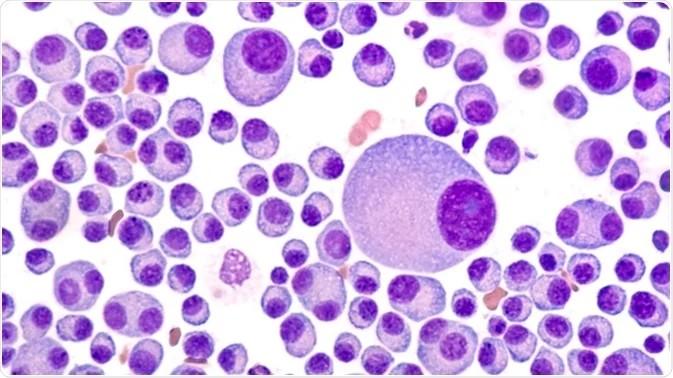
– Produce large quantities of abnormal antibodies:
– monoclonal or M proteins, – light chains (Bence-Jones)
– Crowd out and impair production of normal blood cells and antibodies

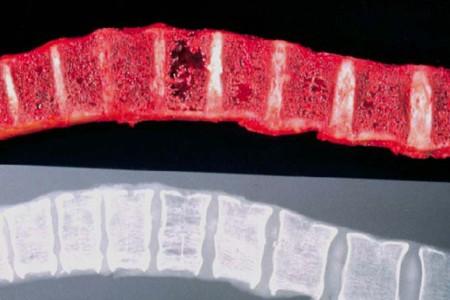
• Fractures
• Osteoporosis (or osteopenia)
• Plasmacytoma (soft tissue tumor) of the bone



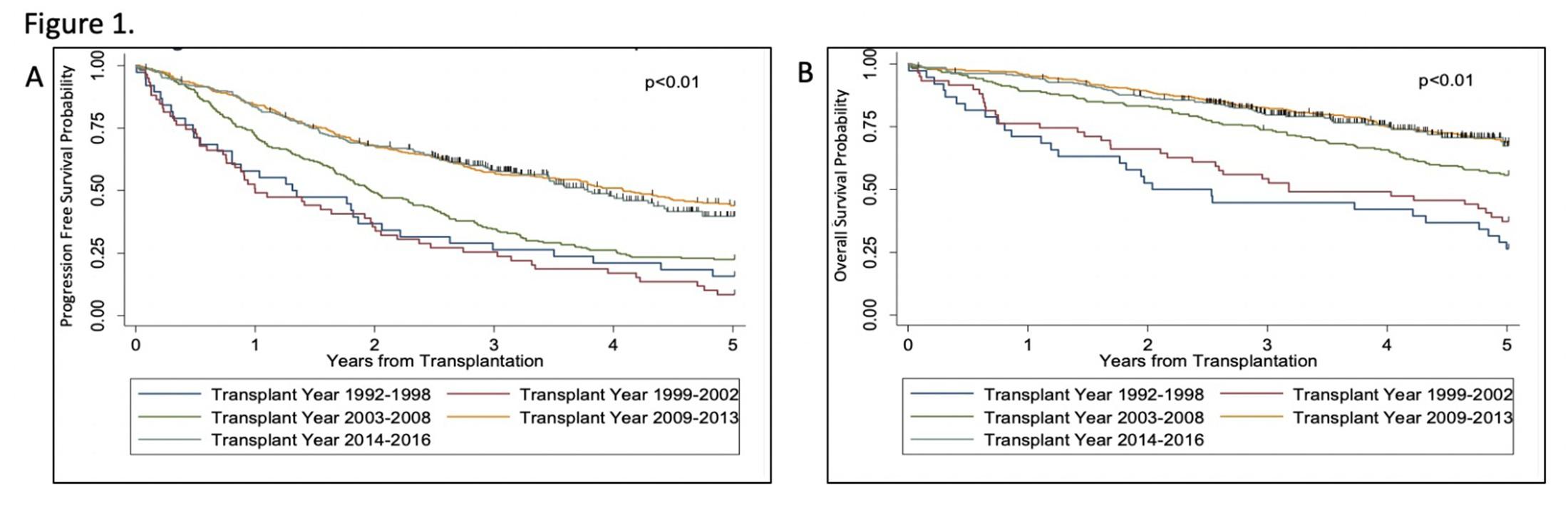
Improvement in Survival of Multiple Myeloma Patients: A Long-Term Institutional Experience, Blood, 2019,
Jordan Nunnelee, BA,Qiuhong Zhao, MS,Don M. Benson, Jr., MD PhD,Ashley E. Rosko, MD,Maria Chaudhry, MD,Naresh Bumma, MD,Abdullah Mohammad Khan, MBBS,MSc,Srinivas Devarakonda, MD,Yvonne A. Efebera, MD MPH,Nidhi Sharma, PhD,

• Relapsed: Reappearance of the disease
• Refractory: Treatment that someone is currently taking no longer works.
• Progression: Increase in tumor markers from the blood or urine tests (M-spike or light chains), OR an end-organ symptom (bone lesion, high calcium).
• Line of therapy: Change in treatment that is not working or has unmanageable side effects
(Note: induction therapy + stem cell transplant + maintenance is ONE line of therapy)
Myeloma returns after 1 to 3 prior lines of therapy
Myeloma returns after 4 or more prior lines of therapy
• Disease progression (by serum or urine markers or by symptoms)
• While on or within 60 days of a
Proteasome inhibitor
• Bortezomib
• Carfilzomib
• Ixazomib
Immunomodulator
• Thalidomide
• Lenalidomide
• Pomalidomide
• Daratumumab
• Isatuximab
Proteasome Inhibitors
IMiDs Chemotherapy and Steroids
Bortezomib
Lenalidomide
Carfilzomib
Thalidomide
Ixazomib
Pomalidomide
KD-PACE/VDPPACE/VDR-PACE/ BEAM
Bi-Specific TCell Engagers Small Molecule Inhibitors
BCMA: Teclistimab
Elranatamab
Cyclophosphamide
Melphalanlow/high dose
Bendamustine*
Dexamethasone, prednisone, solumedrol
GPRC5D: Talquetamab
Selinexor (XPO-1 inhibitor)’
Venetoclax* (BCL2inhibitor)
Mono-Abs or AntibodyDrug Conjugates
Daratumumab (CD-38)
Isatuximab (CD38)
Elotuzumab (SLAM-F7)
Belantamab (BCMA-ADC)*
Triplet drug combinations
Early relapse
After 1 to 3 prior therapies
CAR T-cell therapy
(If rev-refractory)
Clinical trials
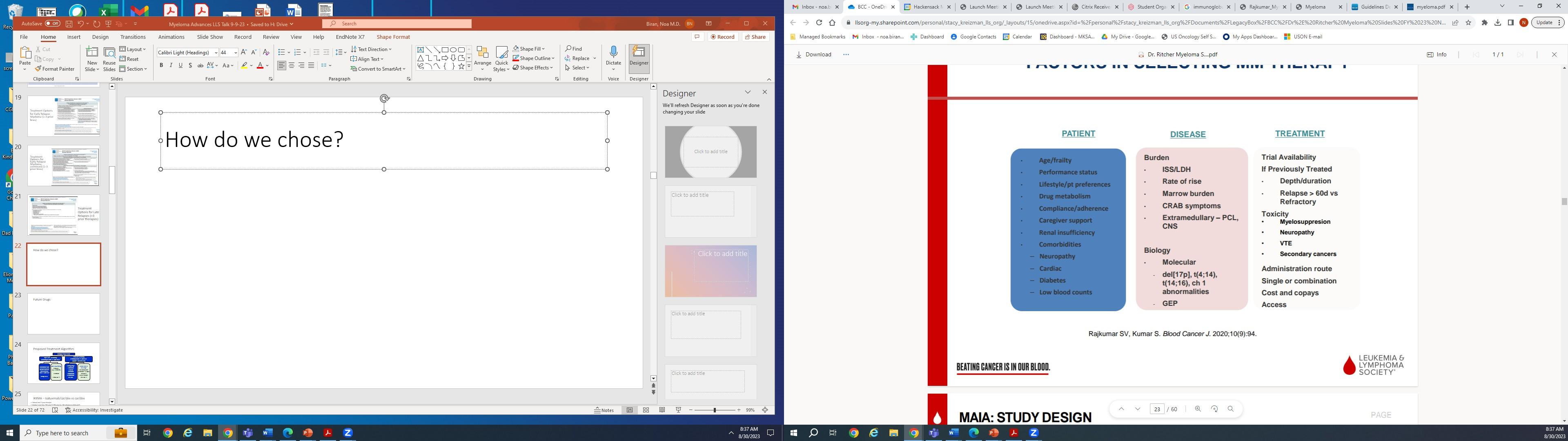
How do we decide?
• Rate of progression
• FISH and mutation status
• Presence or absence of symptoms
• Patient’s other conditions (comorbidities)
• Social situation – distance from cancer center, social support, financial situation, driving ability
• Prior treatment history – what worked before may work again
If you are refractory to… Your specialist might recommend…
Daratumumab or Isatuximab
Carfilzomib + Lenalidomide/Pomalidomide + Dexamethasone
Bortezomib
Isatuximab + Carfilzomib + Dexamethasone
Lenalidomide
Daratumumab+ Ixazomib or Pomalidomide+ Dexamethasone
Triplet/quad regimens
CAR T-cell therapy
Late relapse
After 4 or more prior therapies
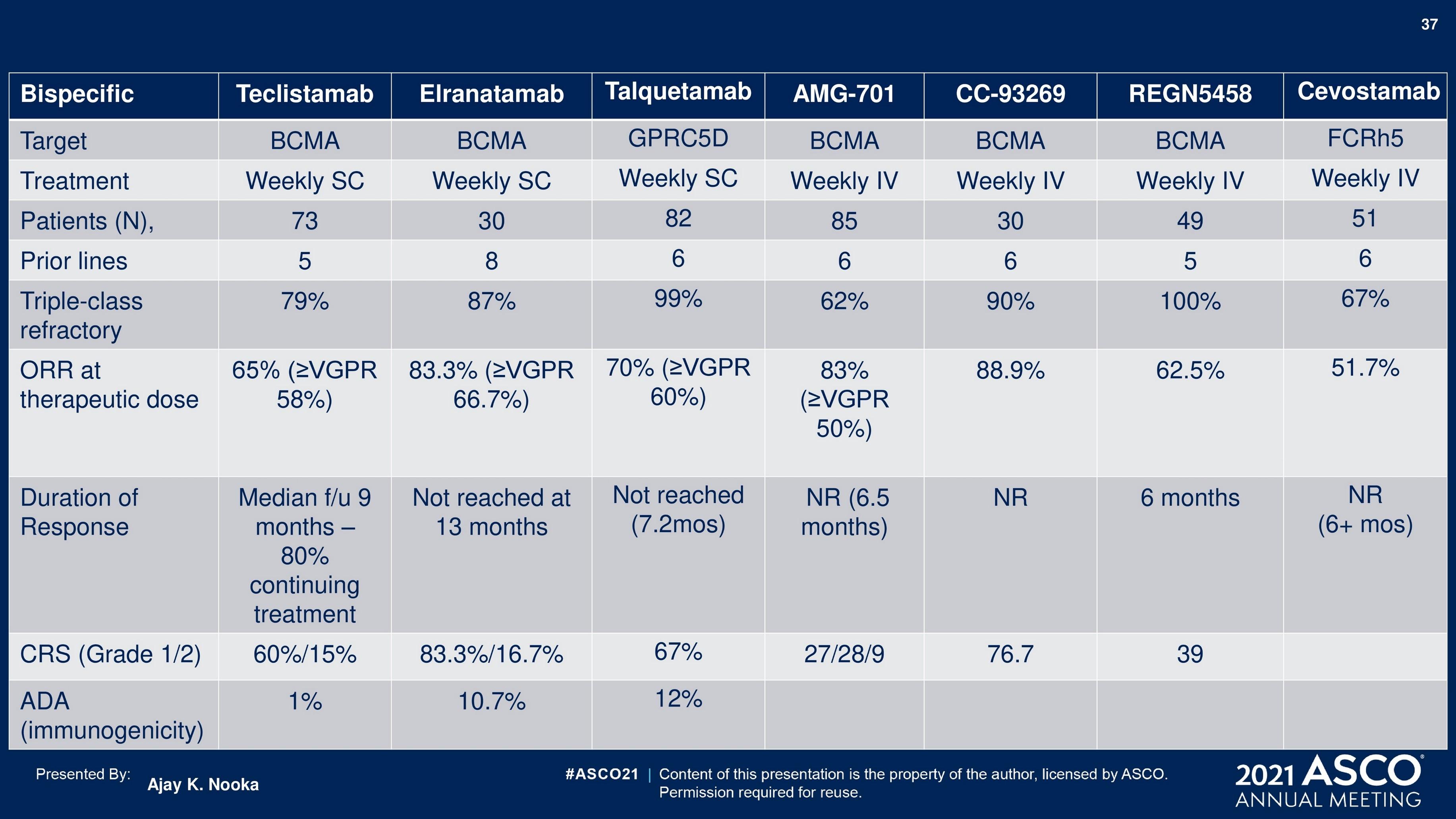
Bispecific Antibodies
How do we decide?
• Rate of progression
• FISH and mutation status
• Presence or absence of symptoms
• Patient’s other conditions (comorbidities)
• Social situation – distance from cancer center, social support, financial situation, driving ability
• Prior treatment history – what worked before may work again
Clinical trials
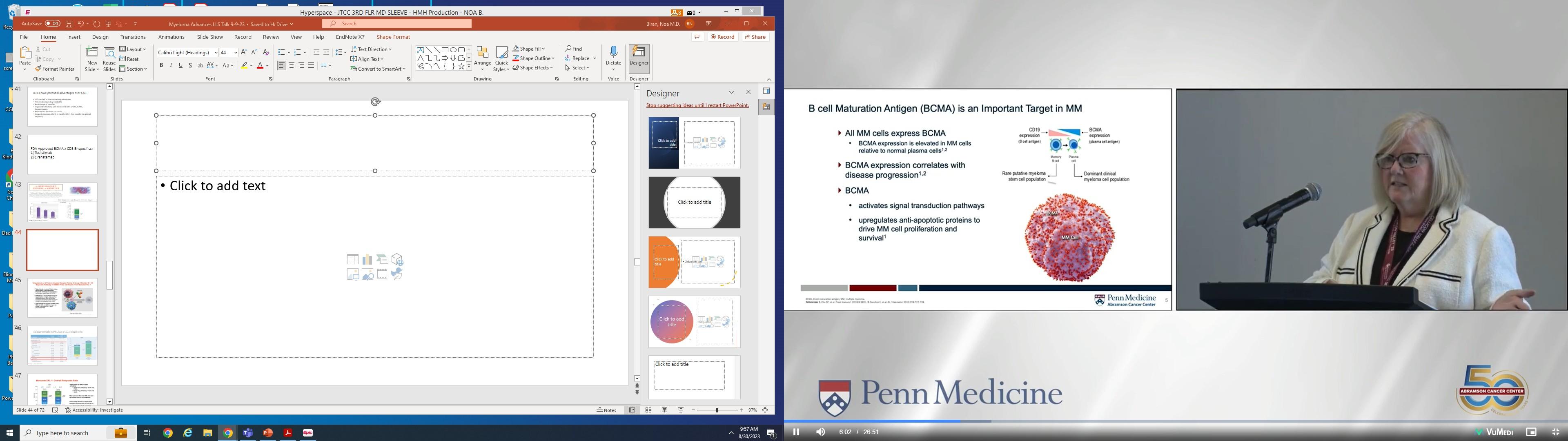
• Your body’s own T-cells are removed from the body and modified to have the ability to find and destroy multiple myeloma cells.
• The CAR-T cells, once modified, target a protein called BCMA on the myeloma cells.
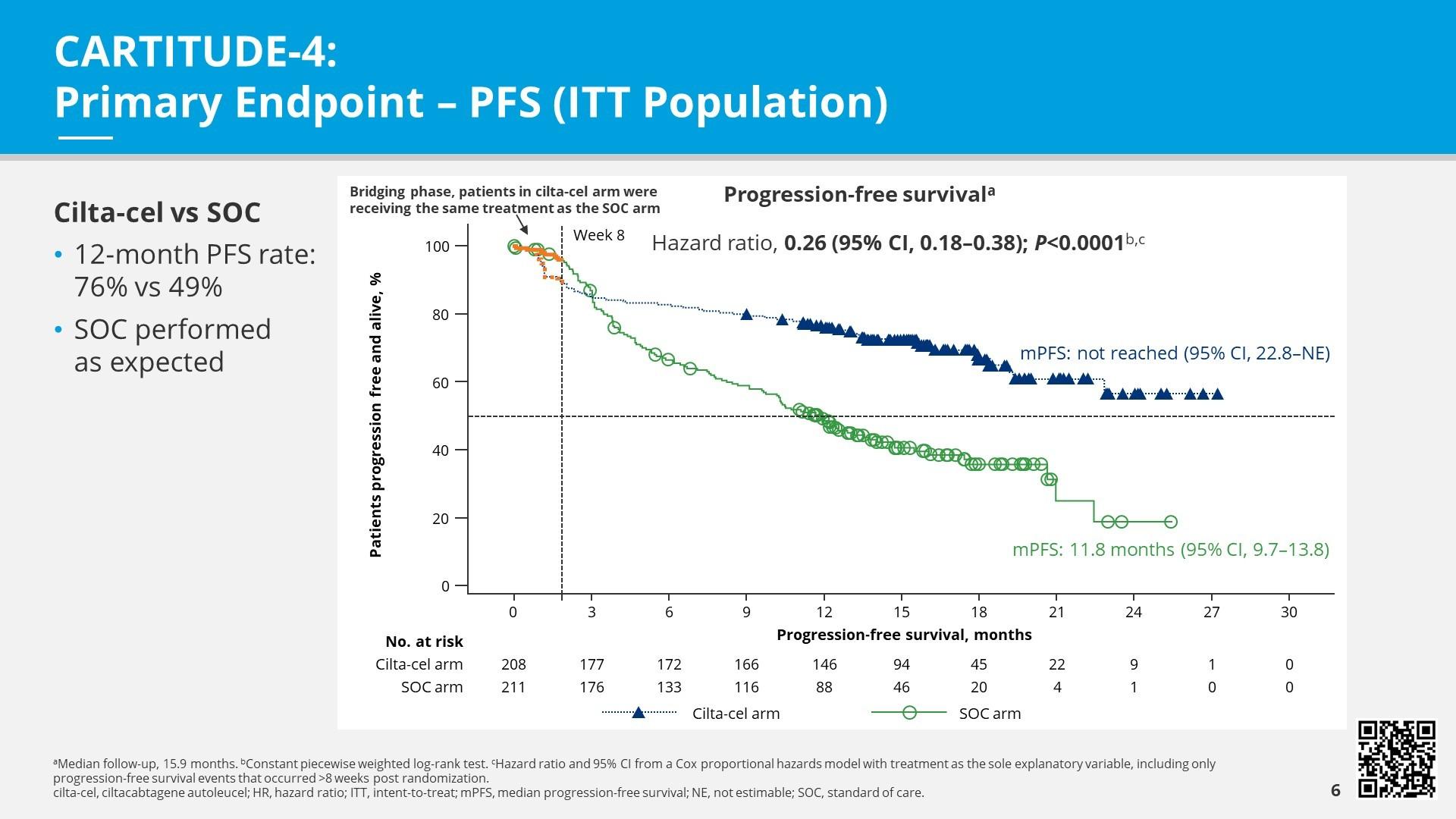
• There are 2 FDA approved CAR-T cell therapies: Ide-cel (Abecma) and Cilta-cel (Carvykti).
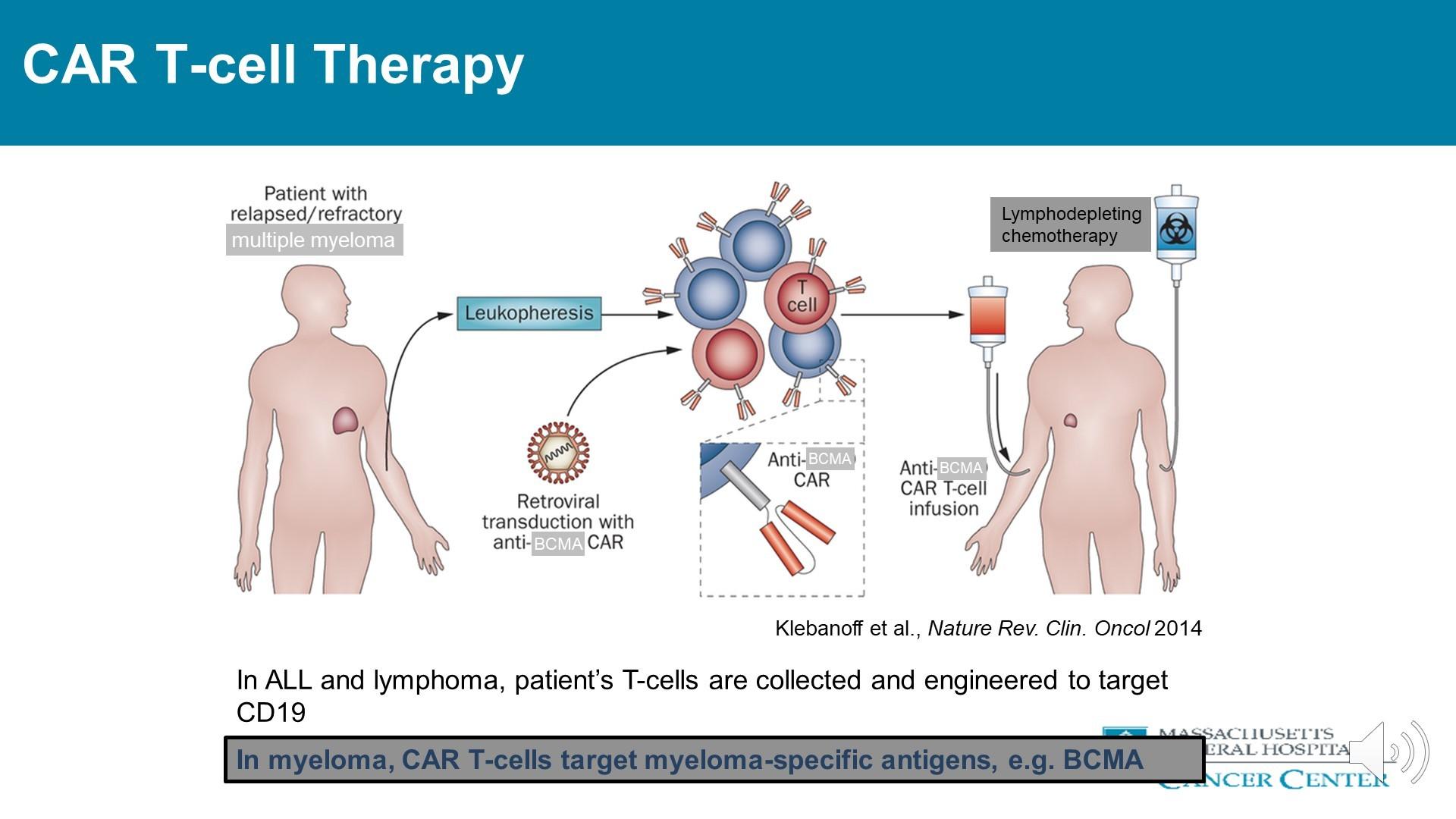
In myeloma-CAR-T cells target myeloma-specific antigens, i.e. BCMA
1) T-cell collection – One time only
2) CAR-T cell manufacturing – Entire process takes several weeks. Bridging therapy (to treat the myeloma) may be required.
3) Lymphodepletion chemotherapy – Treatment to make room for the CAR-T cells.
4) CAR-T infusion – Hospitalization times may vary.
5) Post-CAR-T cell monitoring – Caregiver is necessary for at least 4 weeks after treatment. Side effects and disease monitoring will occur.
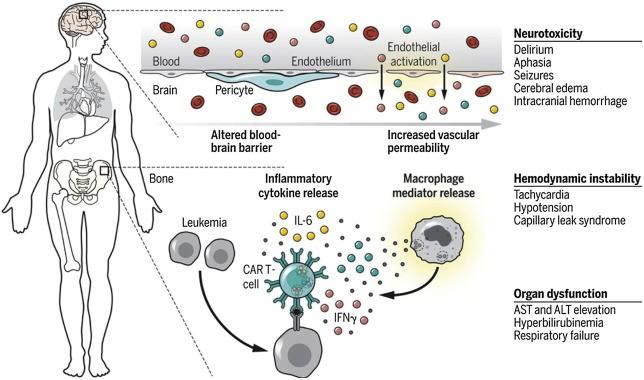
Side Effect Symptoms
Cytokine release syndrome (CRS)
• Fever
• Difficulty breathing
• Dizziness
• Nausea
• Headache
• Rapid heart beat
• Low Blood pressure
Neurotoxicity (ICANS)
• Headache
• Confusion
• Language disturbance
• Seizures
• Delerium
• Brain Swelling
Onset After CAR T-Cell Infusion
Duration Management
1–9 days
5–11 days
• Actemra (tocilizumab)
• Corticosteroids
• Supportive care
2–9 days
3–17 days
• Antiseizure medications
• Corticosteroids
Hygiene and Environment
Possible Transfusions
Preventative Antiviral Medication
Infection Prevention
Vaccinations
In some cases, preventative antibiotics and antifungals
Monthly IV
Immunoglobulin

Dhakal B et al. ASCO 2023. Abstract LBA106. Einsele H et al. EHA 2023. Abstract S100.





Monoclonal antibody that can simultaneously bind to 2 different cell surface markers: one on the myeloma cell and one on a patient’s T-cells

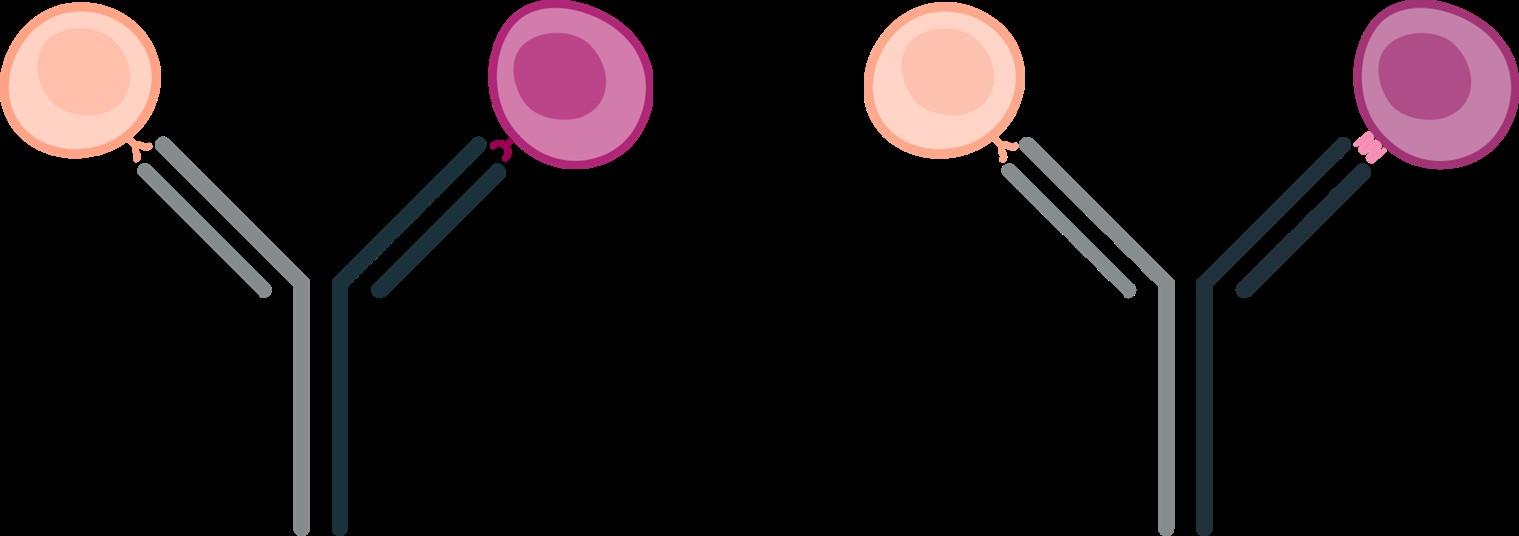

Readily available Precise dosing
Broad range of myeloma targets
Reduced rates of CRS and neurotoxicity Retreatment has been successful
• Administration is by an injection under the skin.
• To minimize side effects and to monitor closely, the first 2-3 doses are administered in the hospital.
• Requires ongoing administration until disease progression or unacceptable side effects.
• If CRS occurs – tocilizumab can be used.
• If neurologic toxicity occurs – dexamethasone can be used.
Lesokhin A et al, Nature Medicine, Aug 15 2023.

• Cytokine release syndrome
• Neurotoxicity
• Low blood counts
• Infections
Number of Infusions Single Continuous administration until progression
Hospitalization Required? Yes Yes in most institutions
Dependent on T-cell health? Yes, can have manufacturing failures Yes, may not be as effective with T-cell exhaustion
Bridging therapy needed? Yes, most of the time No
Caregiver needed? Yes No
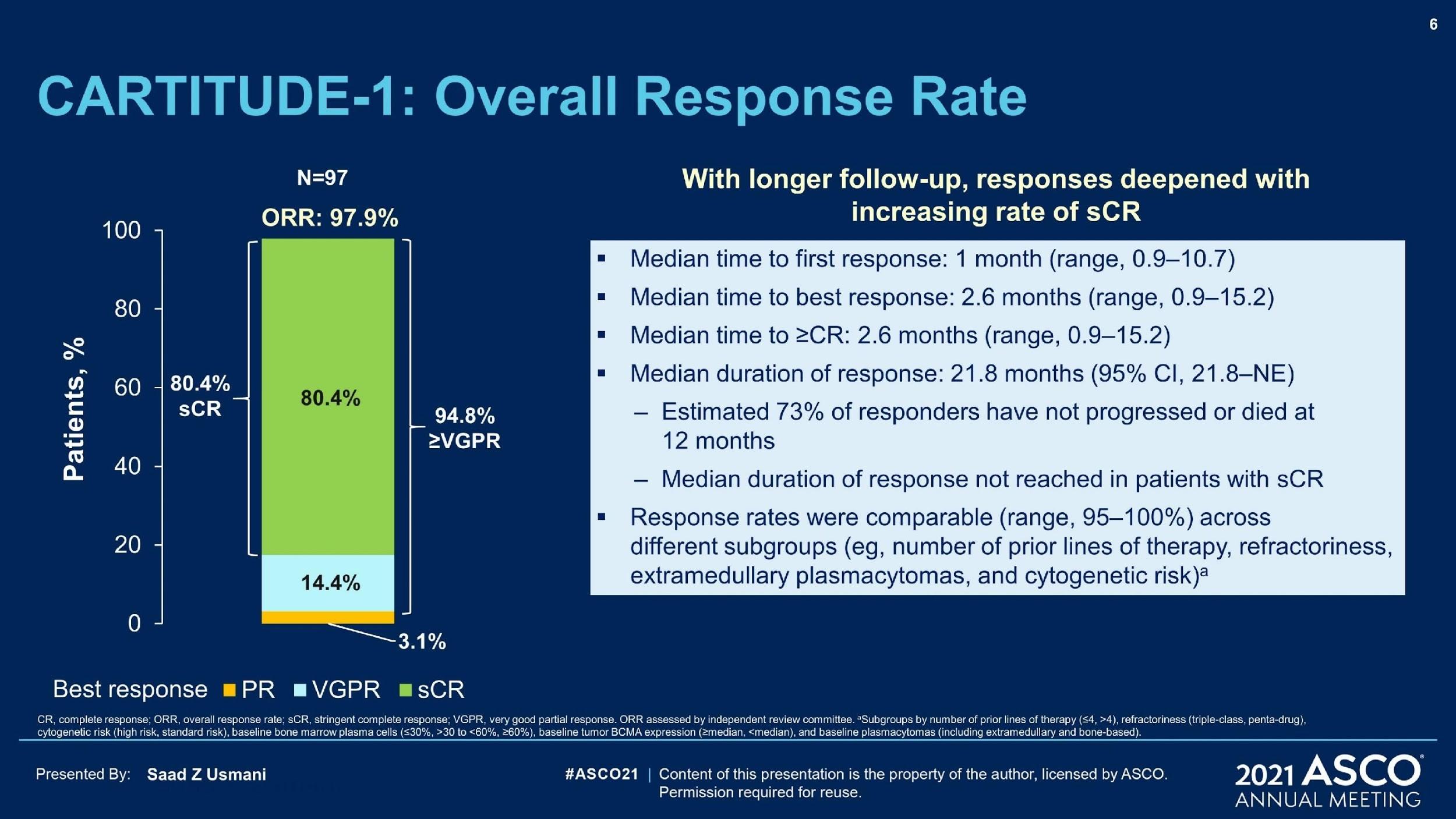
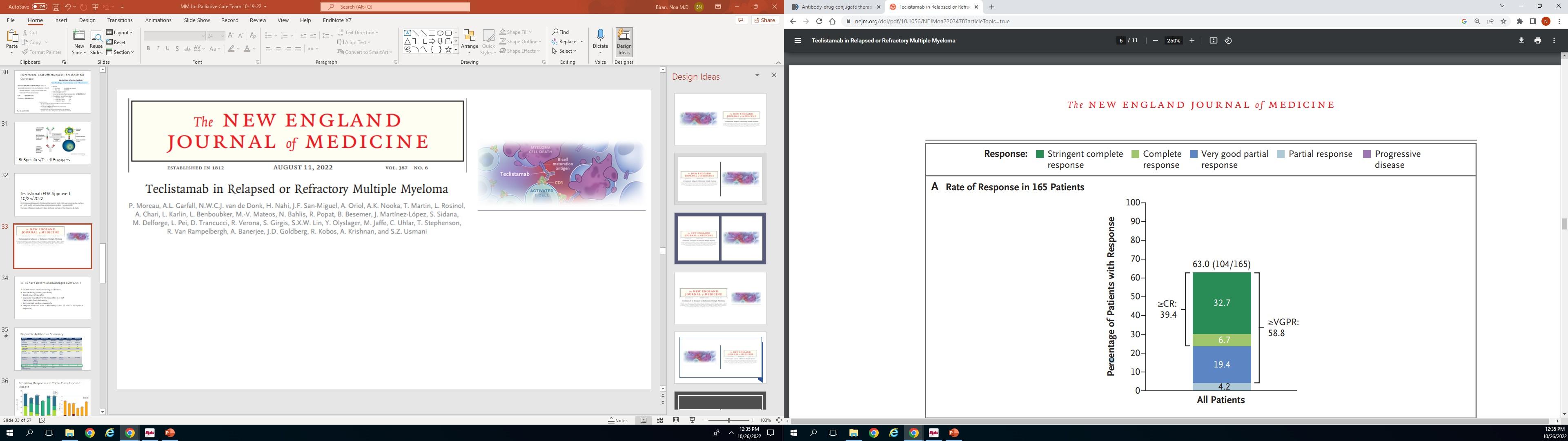
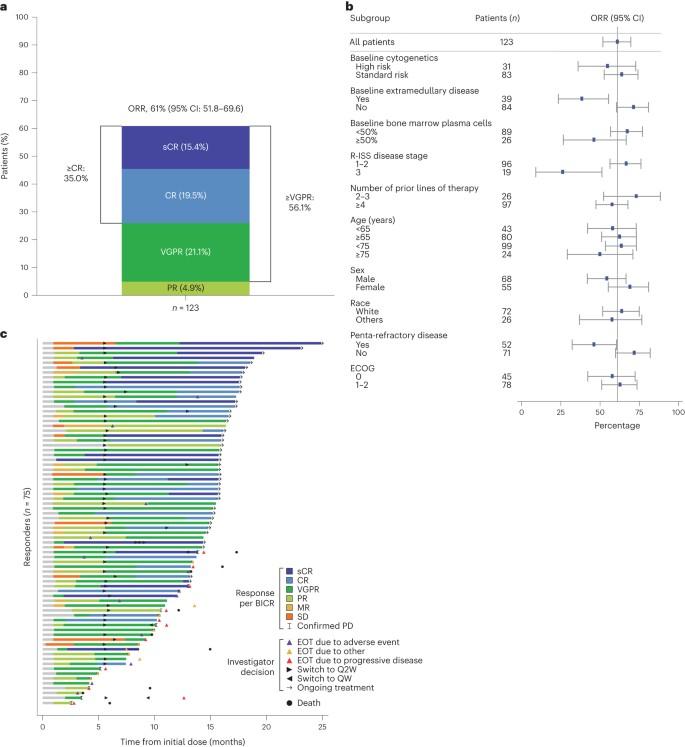
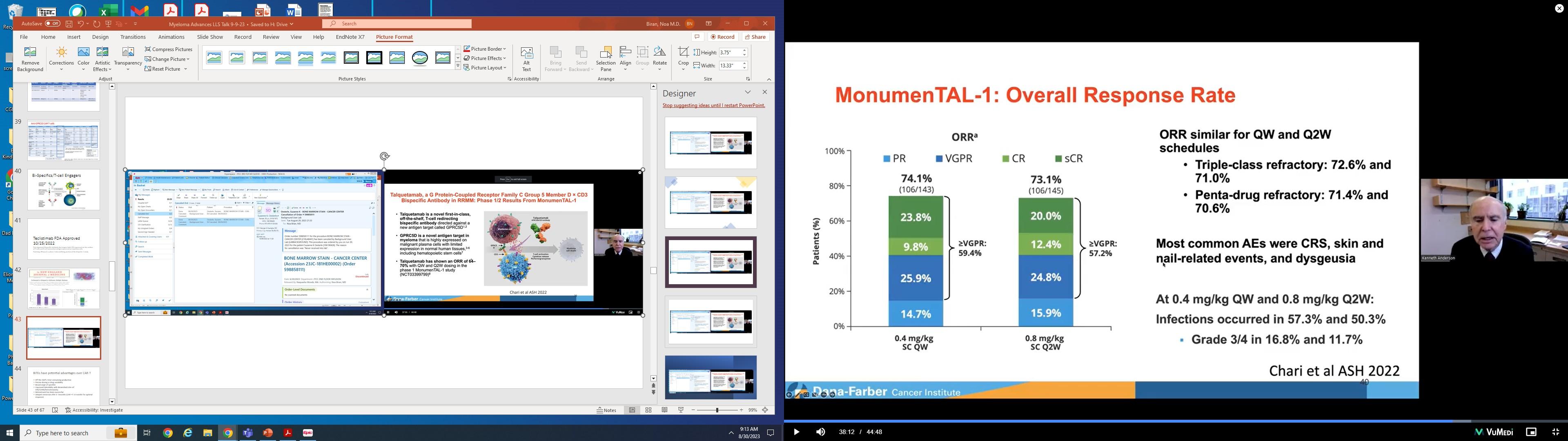
• LINKER-MM1 Study: Update shows 46% Complete Remission rate with the BCMA x CD3 bispecific antibody linvoseltamab.
• BMS986393 Study: CAR-T cell with a novel target against GPRC5D shows promising efficacy. Off-target effects include taste changes (18%), skin changes (20%), CRS (85%) and ICANS (10% and dose related).
• Arcellix CART-ddBCMA study: Specifically engineered to reduce side effects and improve efficacy. Early studies show 75% CR rate.
Monoclonal Antibodies and ADCs
TAK-079 (high-affinity CD38)
TAK-169 (CD38 fused with shiga-like toxin payload)
STRO-001 (CD74 ADC) FOR46 (CD46 ADC)
Indatuximab/BT062 (CD138-DM4 toxin)
CELMoDs/small molecule inhibitors
BiSpecifics in Combinations
Cellular Therapies
Iberdomide (cereblon E3 ligase)
CCC-92480 (cereblon E3 ligase)
Venetoclax (bcl-2)
BCMA x CD3: AMG-701
CC-93269
REGN5458
Elranatamab
GPRC5D x CD3
Talquetamab
Orva-cel LCAR-B38M Lummicar-2
ALLO-715
Descartes-011 and -018
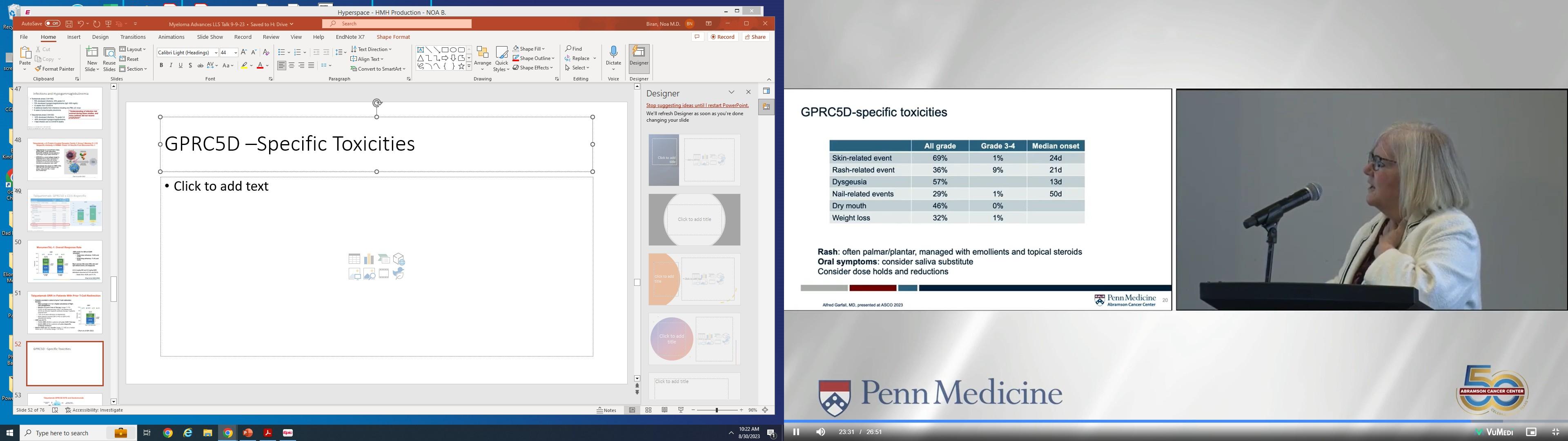
• Oral, potent novel CRBN E3 ligase modulator compound that co-opts Cereblon to enable enhanced degradation of target proteins including Ikaros and Aiolos
• Active in revlimid and pomalidomide-resistant myeloma cell lines and enhances cell mediated killing through immune stimulation.
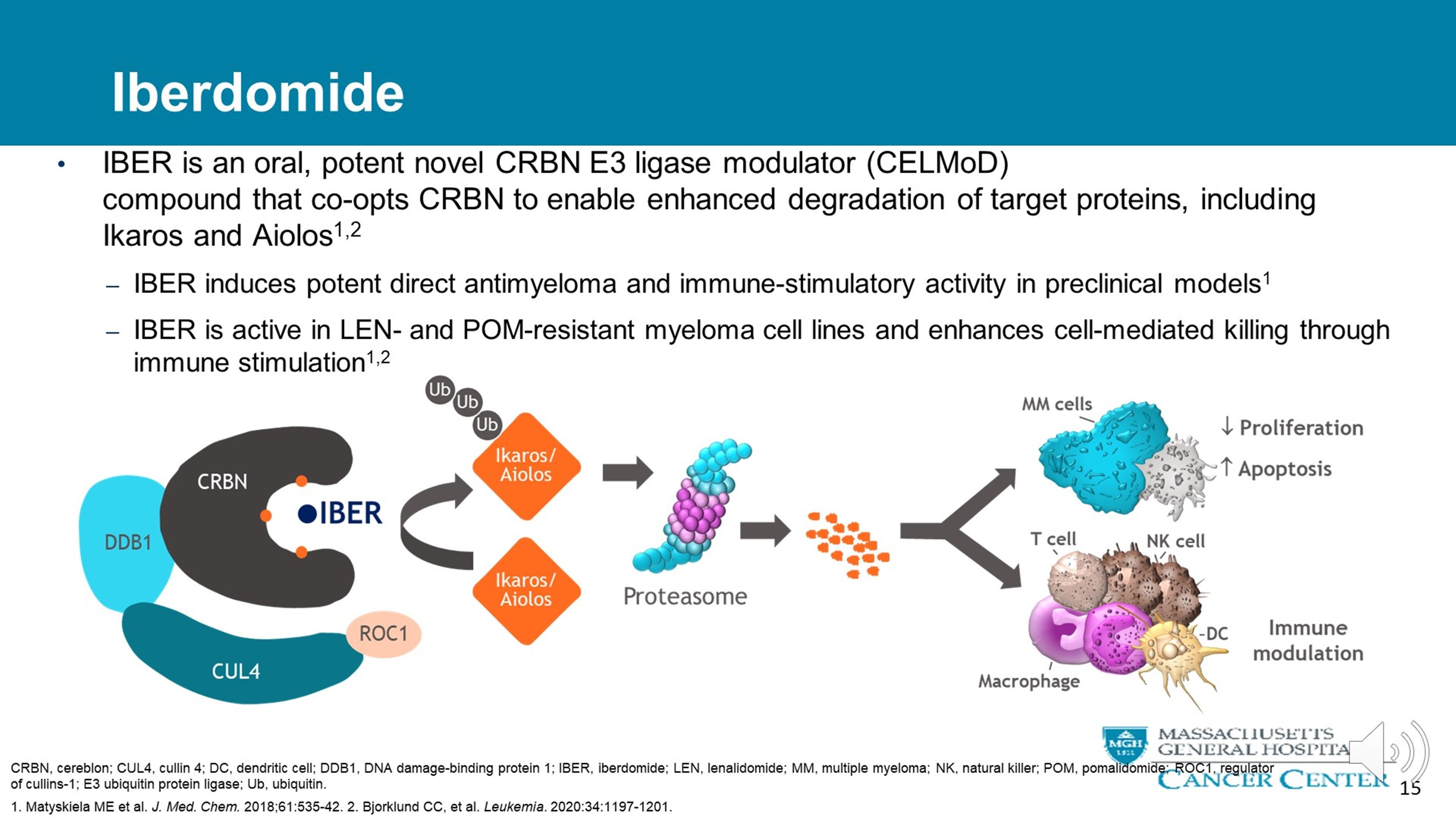
Matyskiela ME et al, J Med Chem 2018; 61:535-42. Bjorklund CC, et al,. Leukemia. 2020:34:1197-1201.
• Low blood counts
• Infections, in particular upper respiratory infections and pneumonia
• Nausea and decreased appetite
MA, et al. NEJM, June 1, 2024.
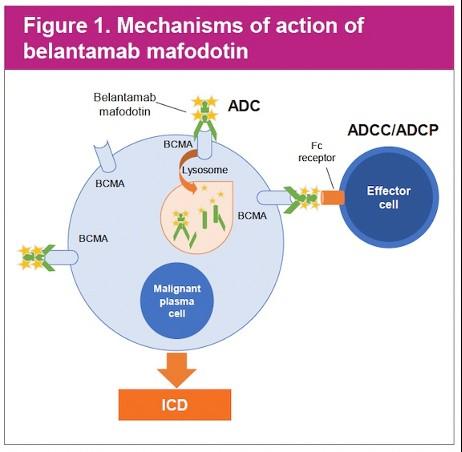
DREAMM-8: Belantamab + pomalidomide + dex versus Pomalidomide + Bortezomib + dex
• Improve symptoms
• Obtain a deep response
• MINIMIZE side effects, have a pro-active approach to reducing infections
• Use your best therapy early rather than reserve for later
• Consider Clinical Trials
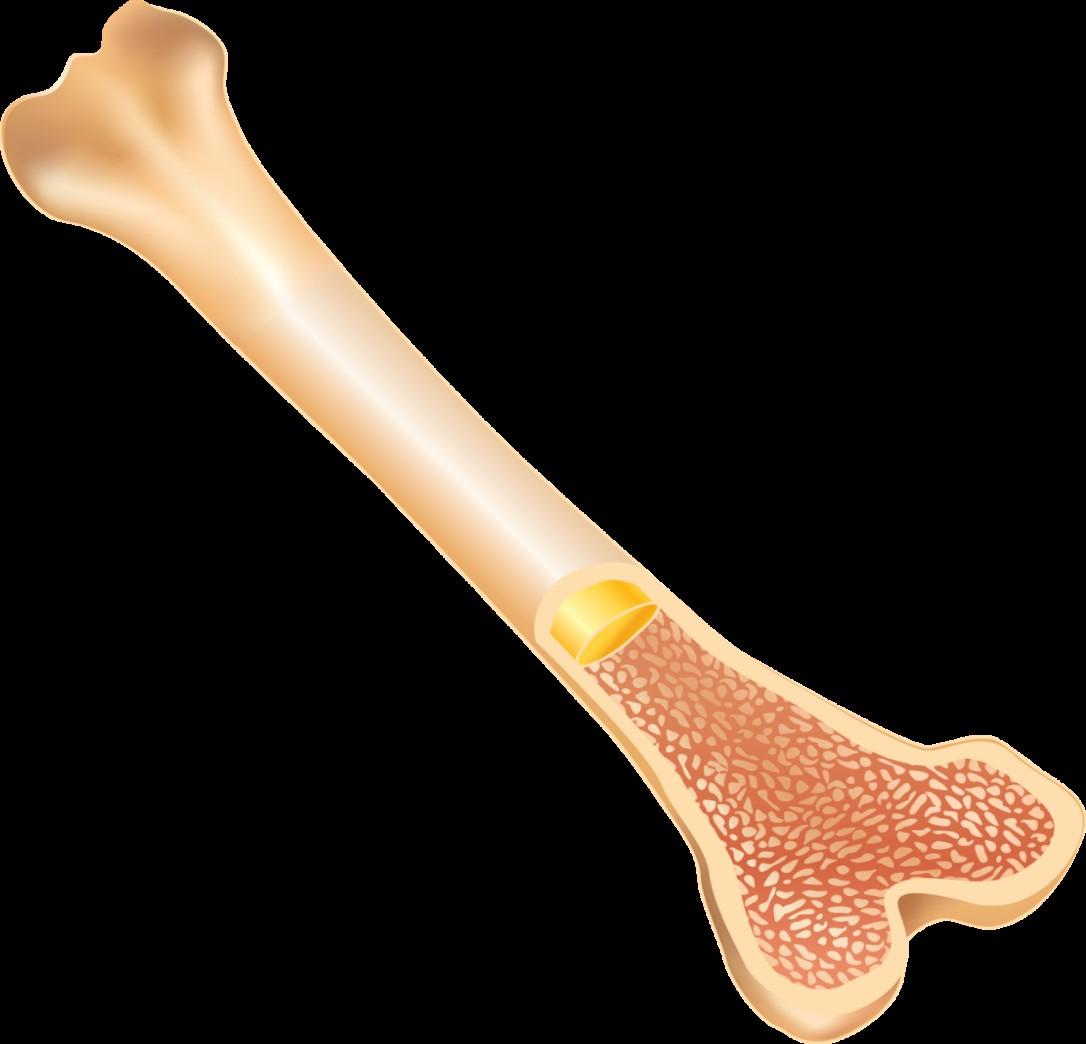

• Prevent bone disease from getting worse
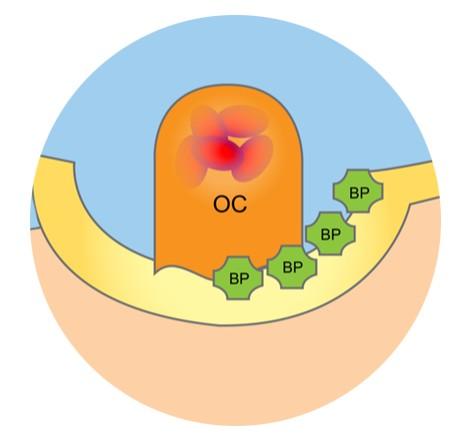


• Decreases pain and reduces skeletal related fractures



• IV infusion in doctor’s office every 3–4 weeks
• Kidney function can alter dosing or choice of drug
• Zometa (zoledronic acid): 15-minute infusion
• Aredia (pamidronate): 2-hour infusion
• Reduced kidney function
• Fracture of the femur
• Osteonecrosis of the jaw (ONJ)
• Low calcium levels (hypocalcemia)
859 patients
859 patients R
• Both equally effective in delaying a skeletal-related event
• Fewer side effects related to kidney toxicity with Xgeva
• Risk of ONJ is similar in both medications (1.2% higher risk with Xgeva)
Raje NS et al. J Clin Oncol. 2017;35: Abstract 8005. Terpos E et al. Haematologica. 2017;102: Abstract S782.
• Complete major dental work before beginning bisphosphonate therapy
• Practice good oral hygiene
• Schedule regular dental visits
• Inform the dentist re use of bisphosphonates/RANK-L inhibitor
• Keep oncologist informed of dental issues

• Relapsed or refractory myeloma occurs when myeloma recurs or is no longer responsive to treatment.
• Therapy choices will depend on the biology of the disease as well as patient factors and will be decided on by the physician, patient and caregivers.
• CAR-T and bispecific antibodies result in high response rates even in patients who have received several prior therapies.
• CAR-T can be used earlier in the lines of therapy whereas bispecific antibodies are approved for >4 prior lines.
• Side effects can be short or longterm and have to be managed in a multi-disciplinary approach.
• Clinical trials should also be considered in the relapsed setting.


Advocacy
What you need to know
Danielle Doheny, Director of Public Policy and Advocacy
Danielle Doheny IMF, Director of U.S. Policy & Advocacy


The Global Advocacy Team collaborates with multiple stakeholders to inform and influence decision-making on the critical healthcare issues that directly impact myeloma patients.

The U.S. Advocacy Team advocates for equitable access to timely diagnosis, innovative treatments and research on Capitol Hill and with key regulatory

The team advocates both alongside of and on behalf of the patient community that we serve.
Advocacy play a critical role to educate policymakers about the issues important to our community and motivate them to act.

The following policy principles are the foundation on which we prioritize our advocacy work.
1. Ensure Access to Care: We advocate for policies that ensure all myeloma patients have equitable, comprehensive, patientcentered care without insurance barriers that limit options or delay treatment initiation.
2. Eliminate Financial Barriers: We advocate for policies that allow myeloma patients access to treatments and supportive care interventions without facing financial hardships.
3. Advance Myeloma Research: We advocate for annual appropriations funding for myeloma research and the advancement of clinical trial eligibility and research protocols that ensure representation from diverse populations.



1. ENSURE ACCESS TO CARE
2. ELIMINATE FINANCIAL BARRIERS
3. ADVANCE MYELOMA RESEARCH
INSURANC E REFORM: DRUG ACCESS
Step Therapy Protocols Safe Step Act
INSURANC E REFORM: COINSURANCE
Oral Parity Cancer Drug Parity Act
INSURANC E REFORM: DRUG ACCESS PBM Reform PBM Reform Act
INSURANC
E REFORM: COPAYS Copay Accumulators HELP Copays Act
FEDERAL FUNDING
ANNUAL APPROPS
Annual Appropriations
NIH: National Cancer Institute, National Institute on Minority Health, ARPA-H
CDC: Comprehensive Cancer Control Initiative
DoD: Congressionally Directed Medical Research Program (CDMRP) for Myeloma.
MEDICARE REFORM:
PHYSICIAN ACCESS
Tele-Health/Medicine
Telehealth Modern. Act
MEDICARE REFORM: ANNUAL COST LIMITS
Inflation Reduction Act implementation Cap & Smoothing (MPPP), Drug Pricing, Drug Formularies
CLINICAL
TRIAL DIVERSITY
Primary care education, Focus on underserved, POC, rural settings and socioeconomically disadvantaged groups





No copays for vaccines under Part D
Insulin copays limited to $35/month


Expanded eligibility for the Extra Help Program
(Federal Low-Income Subsidy) to help pay premiums, deductibles, coinsurance & other costs
$3,250 annual cap (approx.) on out-of-pocket spending for prescription drugs under Part D (eliminating 5% coinsurance in catastrophic phase)

$2,000 annual cap on out-of-pocket spending for prescriptions under Part D
Option for a monthly payment program to “smooth out” total out-of-pocket spending throughout the year, with an overall monthly maximum
• Patients will need to enroll in the program (opt-in)
• The earlier in the year you join the program, the more you can benefit
• Your monthly bill may fluctuate somewhat
• No one will pay more than $2000 for the year

The Inflation Reduction Act (IRA) of 2022 is a federal law which aims to curb inflation by working to reduce the federal government budget deficit, lowering Medicare prescription drug prices and investing in domestic energy production.

1.
3.
• Inpatient hospital stays
• Care in a Skilled Nursing Facility
• Hospice Care
• *Does not cover regular MD visits or Rx drugs










Addressing healthcare barriers for multiple myeloma patients depends on winning over the hearts and minds of policymakers.
It is not enough to just identify an issue and have data-driven evidence/research to back it up. It is not even enough to work with coalition partners that agree with our point of view. We must convince policymakers to prioritize our issues, draft legislation and vote it into law.
Join Us! Join the Advocacy Team and share Your Story.






Patricia Mangan, RN, MSN, APRN-BC
Abramson Cancer Center, University of Pennsylvania
Patricia Mangan, RN, MSN, APRN-
BC University of Pennsylvania, Abramson Cancer Center Philadelphia, Pennsylvania

A presentation from the IMF’s Nurse Leadership Board


Focus on Managin g Sympto ms













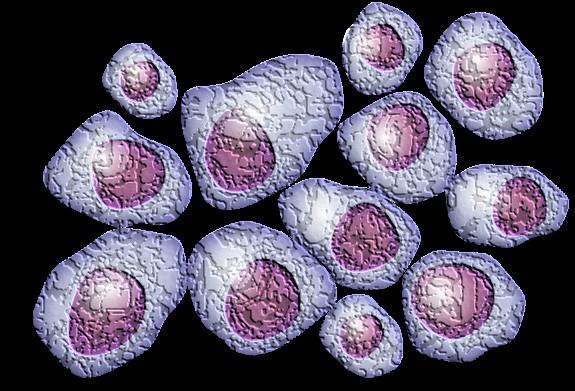
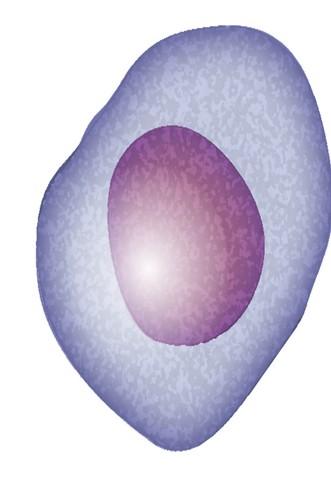
Plasma Cells come from white blood cells produced in the bone marrow and make many different antibodies to help fight infection (polyclonal).

In Multiple Myeloma, one plasma cell mutates, making many identical plasma cells (monoclonal).






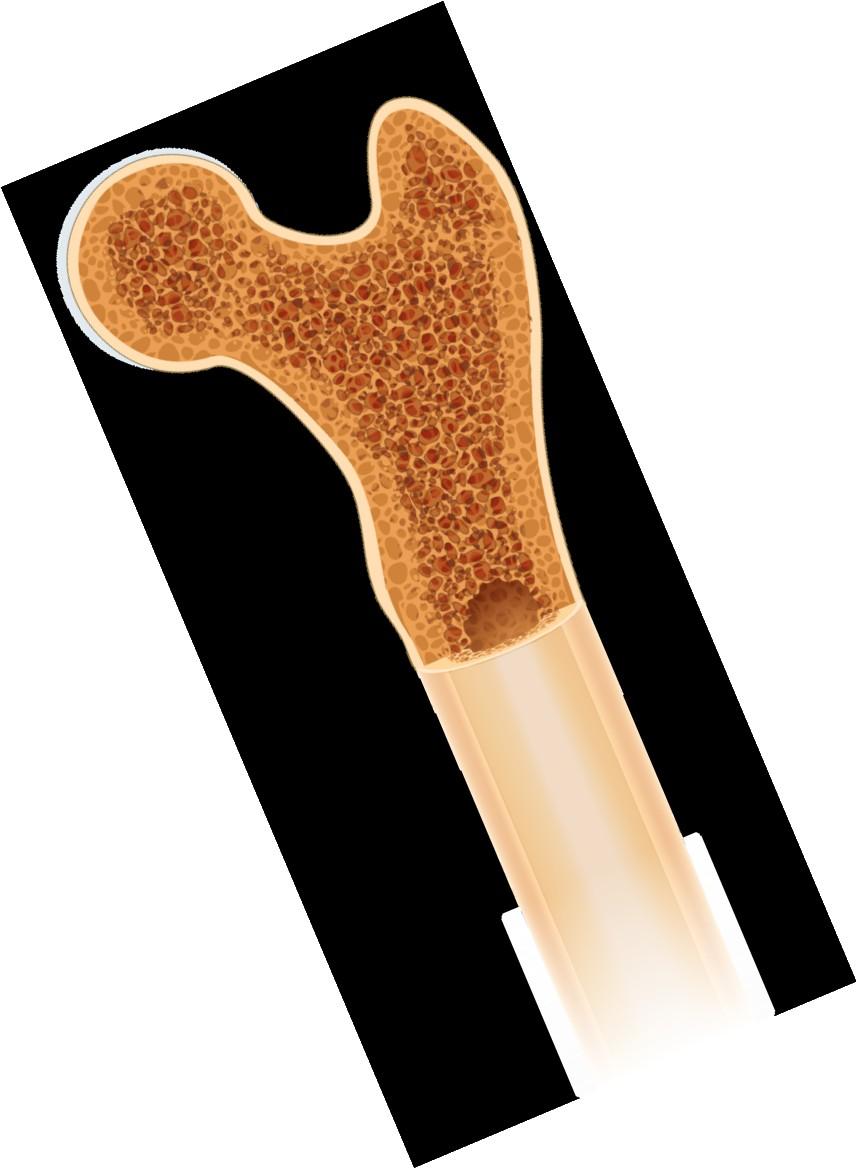
Bone marrow











Anxiety
Stress
Depression
Decreased red blood cells





Anemia & Fatigue


Decreased white blood cells

Myeloma protein in blood and urine
Changes in bone remodeling
Clonal myeloma plasma cells can cause many symptoms
– Crowd out normal bone marrow cells
– Produce myeloma protein
– Can cause kidney dysfunction
– Affect bone cells (balance of osteoclasts & osteoblasts)
Immune Dysfunction & Infection


Renal Dysfunction













Preventing infections is paramount.
Infection remains the leading cause of death in patients with multiple myeloma. Several factors account for this infection risk, including the overall state of immunosuppression from multiple myeloma, treatment, age, and comorbidities (e.g., renal failure and frailty).
IMWG Consensus guidelines and recommendations for infection prevention in multiple myeloma; Lancet Haematol.2022;9(2):143–161.
Good personal hygiene (skin, oral)
Environmental control (avoid crowds and sick people; use a high-quality mask when close contact is unavoidable)

Report fever of more than 100.4°F, shaking chills even without fever, dizziness, shortness of breath, low blood pressure to HCP as directed.
As recommended by your healthcare team:
Immunizations:
Flu, COVID, RSV & and pneumococcal vaccinations; avoid live vaccines
Preventative and/or supportive medications



Myeloma Treatment
Stay hydrated--drink water
Avoid certain medications
• IV contrast dyes
• NSAIDs like Advil (ibuprofen), Aleve (naproxen)
Be alert: symptoms of kidney dysfunction
• Fatigue and weakness
• Nausea and vomiting
• Foamy or dark urine
• Swelling in feet, ankles, or face
• Shortness of breath
• Persistent itching
• Loss of appetite
• Muscle cramps
• High blood pressure





Myeloma Treatment
Nutrition
Vitamin D
Calcium (if approved by doctor)
Weight-bearing activity (i.e., walking, standing, climbing stairs, stretching, dancing)
Bone-strengthening agents (prescribed by your healthcare team)

Report any new or worsening bone pain to your healthcare provider


Prevention
Sources of pain include bone disease, neuropathy and medical procedures.
Decrease fracture risk through myeloma treatment, bone strengthening agents, physical activity, preventative surgery
Prevent Nerve Damage: prevent shingles, manage diabetes, myeloma medication dosing and route of administration
Combine scheduled medical procedures, when possible (Ex. blood draw, biopsy), use sedation if available

Treatment
Interventions depend on source of pain, may include
Medications, Surgery, Radiation therapy, etc.
Physical therapy & continued activity, complementary therapies (Mind-body, meditation, yoga, supplements, acupuncture, etc.)
Scrambler therapy for neuropathy







Managin g Sympto ms







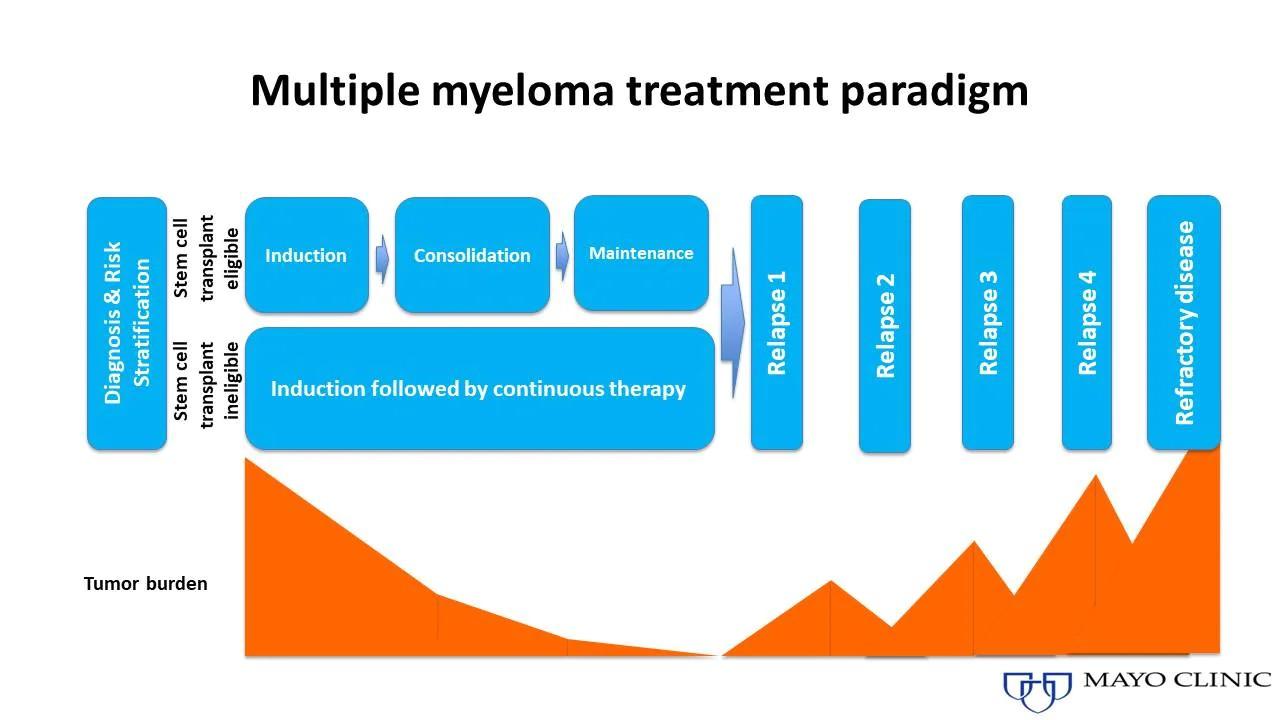
Induction

Consolidation
Initial treatments aimed at reducing the amount of myeloma cells
Intensification of treatment to deepen response. Either additional cycles of induction or autologous stem cell transplant (in eligible patients)

Prolonged lower-intensity treatment designed to sustain remission



Quadruplet therapy is preferred for nearly all patients with newly diagnosed myeloma

Anti-CD38 monoclonal antibody (mAb)
• Darzalex (daratumumab)
• Sarclisa (isatuximab)

Proteosome
Inhibitor (PI)
• Velcade (bortezomib)
• Kyprolis (carfilzomib)

Immunomodulator
y drug (IMiD)
• Revlimid (lenalidomide)
• Pomalyst (pomalidomide)

Steroid
• Decadron (dexamethasone)
• Prednisone
At infusion clinic: subcutaneous injection or infusion
Oral medication taken at home Supportive medication:
• Antiviral prophylaxis (i.e., acyclovir or valacyclovir) to prevent viral infections particularly shingles.
• Antibacterial agents (i.e., Bactrim, levofloxacin) to prevent bacterial infections.
• Aspirin or other anticoagulant therapy to reduce the risk of blood clots from IMiDs.
• Bone-strengthening agents (i.e., zoledronic acid, denosumab) to strengthen bones and protect against fractures.


Steroids enhance the effectiveness of other myeloma therapies

Your provider may decrease or discontinue the dose as myeloma responds to therapy.
Do not stop or alter your dose of steroids without discussing it with your provider
• Irritability, mood swings, depression
• Blurred vision, cataracts
• Increased risk of infections, heart disease
• Muscle weakness, cramping
• Increased blood pressure, water retention
• Difficulty sleeping (insomnia), fatigue
• Flushing/sweating
• Stomach bloating, hiccups, heartburn, ulcers, or gas
• Weight gain, hair thinning/loss, skin rashes
• Increased blood sugar levels, diabetes
Consistent schedule (AM vs. PM)
Take with food
Stomach discomfort: Over-thecounter or prescription medications
Medications to prevent shingles, thrush, or other infections
Rajkumar SV, et al. Lancet Oncol 11(1):29–37. King T, Faiman B. Clin J Oncol Nurs. 2017;21(2):240-249. Banerjee,R. et al. Blood 9.25.24


Peripheral neuropathy happens when there is damage to nerves in the extremities (hands, feet, limbs). Damage can be the result of myeloma, treatment or unrelated conditions (i.e., diabetes).
Symptoms: Numbness
Tingling
Prickling sensations
Sensitivity to touch
Burning and/or cold
sensation
Muscle weakness

Prevention / management:
Bortezomib once-weekly and/or subcutaneous administration
Massage area with cocoa butter regularly
Neuroprotective Supplements
• i.e., B-complex vitamins (B1, B6, B12)
Safe environment: rugs, furnishings, shoes
If neuropathy worsens, your provider may:
Adjust your treatment plan
Prescribe oral or topical pain medication
Suggest physical therapy
Nerve damage from neuropathy can be permanent.
Early reporting of symptoms may prevent worsening symptoms.
Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Tariman, et al. CJON.2008;12(3)suppl:29-36. Zhao T, et al. Molecules. 2022;27(12):3909.




Blood clots can cause swelling, pain, discoloration (DVT), shortness of breath, chest pain, sense of doom (PE). Blood clots
► HCPs may manage DVT/PE risk by
• Adjusting medications and schedules
• Prescribing blood-thinning medications according to assessed risk (DOAC, aspirin, warfarin, heparin)
• Balancing the risk of DVT and PE with that of bleeding with low platelets
► Additional strategies to reduce risk of clots:
• Anti-embolism stockings (elastic stockings)
• Exercise regimen
• Moving frequently when sitting long periods
• Travel precautions (foot/leg exercises, walking, aspirin if not already on blood thinner)
DVT=Deep Vein Thrombosis; PE=Pulmonary
Embolism
are serious and can be life threatening.



Family History
Obesity
Immobility
Smoking
Surgery
Rome, S, et al. Clin J Oncol Nurs. 2008;12(3)suppl:37-52. Faiman B. Clin J Oncol Nurs. 2016;20(4):E100-E105. De Stefano, et al. Hematologica, 2022



P H A S E 1

Measuring treatment response
Testing for Eligibility
Insurance authorization
Collecting stem cells

Duration: Approximately 2 weeks
Location: Transplant Center

P H
HD-Melphalan
Stem cell infusion
Supportive Care
• GI Management
• Transfusions
• Antibiotics
Hair Loss
Engraftment
Duration:
Approx. 3-4 weeks

Location:
Transplant Center


P H A S E 3

Restrengthening
Appetite recovery “Day 100” assessment
Begin maintenance therapy

Duration: Approximatel y 10-12 weeks
Location: HOME

stem cell transplant remains the standard of care for eligible patients


Fluid intake can help with both diarrhea and constipation and helps kidney function
Constipation is more common in the induction phase
Opioid pain relievers, antidepressants, heart or blood pressure medications (check with provider, pharmacist)
Supplements: Calcium, Iron, vitamin D (rarely), vitamin B-12 deficiency
Anorexia, the inability to eat, is common during transplant and resolves with time.
• Hydration is most important
• Small, frequent meals with a focus on protein intake
• You will work closely with a dietician to help monitor your calorie intake
Diarrhea is common during transplant and long-term maintenance therapy.
Other medications and supplements can cause GI issues.
Hydration is very important
Electrolyte replacement is common
Good skin care will help prevent irritation
Stool exam may be needed to rule-out infection
Increase fiber
Stay well hydrated
Fruits, vegetables, high fiber whole grain foods
Fiber binding agents – Metamucil® ,
Citrucel®, Benefiber®
If no infection, anti-diarrheal medication may be prescribed

Smith LC, et al. Clin J Oncol Nurs. 2008;12(3)suppl:37-52. Faiman B. Clin J Oncol Nurs. 2016;20(4):E100-E105.











Adapted from Durie B. Keats JJ, et al. Blood. 2012;120(5):1067-1076.


Myeloma Therapies Common Combinations
Belantamab mafodotinb Bela, BVd, BPd, BKRd
Bortezomib (SQ admin)
VRd, Vd, VCd
Carfilzomib KRd, Kd, Dara-Kd, Isa-Kd
Ciltacabtagene Autoleucel Cilta-Cel
Daratumumab Dara-Rd, Dara-Vd, Dara-Pd, Dara-VMp, Dara-Kd
Elotuzumab ERd, EPda
Idecabtagene Vicleucela Ide-Cel
Isatuximab Isa-Pda, Isa-Kd
Ixazomib IRd
Lenalidomide
VRd, Rd, KRd, Dara-Rd, ERd, IRd
Pomalidomidea Pda, Dara-Pd, EPda, PCdb
Selinexor Xd, XVd, XKdb, Dara-Xdb
New agents or regimens in clinical trials are always an option

Many treatment options are available.
More therapies are being studied
Clinical trials may be an option


a2 or more prior therapies. bOff-label; not currently FDA-approved. C = cyclophosphamide; d = dexamethasone; Dara = daratumumab; FDA = US Food and Drug Administration; E = elotuzumab; Isa = isatuximab; I = ixazomib; K = carfilzomib; M = melphalan; p = prednisone; P = pomalidomide; R = lenalidomide; SQ = subcutaneous; V = bortezomib; X = selinexor.
Rajkumar SV. 2024 Myeloma Algorithm. https://clinicaloptions.com/CE-CME/oncology/2024-mm-algorithm/18440-26989. Accessed 12.14.24. NCCN Guidelines®. Multiple Myeloma. V3.2024. Accessed March 15, 2024. Noonan K, et al. J Adv Pract Oncol. 2022;13(suppl 4):15-21. Steinbach M, et al. J Adv Pract Oncol. 2022;13(suppl 4):23-30. Moreau P, et al. Lancet Oncol. 2021;22(3):e105-e118. O’Donnell EK, et al. Br J Haematol. 2018;182(2):222-230. Mo CC, et al. EJHaem. 2023;4(3):792-810. Chang D. et al., Blood 2024, Abstract 2287.



BCMA target
• Abecma (Ide-Cel)
• Carvykti (Cilta-Cel)

1
Relapsed MM with 1-2 prior LOT Bridging therapy, if needed; Lymphodepleting therapy when CAR T cells are ready T Cell Infusion Close monitoring and Management of side effects
Relapsed MM after 4 prior LOT (or clinical trials)

TCE are innovative immunotherapies used in the treatment of relapsed multiple myeloma. These therapies work by redirecting the patient's own T-cells to recognize and attack myeloma cells.



3


2 a 2 b 4 5 HOME ! Apheresis to Collect T Cells T Cell Manufacturi ng
Bispecific antibodies
• About 7 in 10 patients respond
• Off-the-shelf treatment; no waiting for engineering cells
BCMA target: greater potential for infection
• Tecvayli® (teclistamab)
• Elrexfio™ (elranatamab)

Bispecific antibody


GPRC5D target: potential for skin and nail side effects, GI issues of taste change, anorexia and weight loss
• Talvey™ (talquetamab)
BCMA = B-cell maturation antigen; CAR = chimeric antigen receptor; GPRC5D = G protein–coupled receptor, class C, group 5, member D; MM = multiple myeloma; scFV = single chain fragment variable.

CAR T= Chimeric Antigen Receptor T Cell; LOT = Lines of
Hucks G, Rheingold SR. Blood Cancer J. 2019;doi:10.1038/s41408-018-0164-6.

Shah N, et al. Leukemia. 2020;34(4):985-1005. Yu B, et al. J Hematol Oncol. 2020;13:125.


CYTOKINE RELEASE
SYNDROME
• Fever
• Fatigue & Weakness
• Headache
• Nausea/Vomiting/Diarrhea
• Chills
• Low blood pressure
• Rapid heart rate

CRS is a common but often mild & managea ble side effect

Neurotoxicity is a rare but a serious side effect
• Difficulty breathing PREVENTION AND MANAGEMENT
• Disease management to reduce tumor burden
• Bispecific Step-Up Dosing (SUD)
• Tocilizumab
• Steroids
• Anti-Seizure medications
• Intravenous Immunoglobulin (IVIG)
• Close monitoring

ICANS AND NEUROTOXICITY
• Headache
• Difficulty concentrating
• Lethargy
• Agitation
• Hallucinations
• Tremors
• Aphasia (difficulty with speech, reading, writing, or understanding language)
• Confusion
• Memory loss
• Personality change
• Delayed Neurotoxicity can include Parkinsonism, Cranial Nerve Palsies and Peripheral Neuropathy/Guillan Barré syndrome (GBS)
CAR = chimeric antigen receptor. ICANS = Immune Effector Cell-Associated Neurotoxicity Syndrome Brudno JN, Kochenderfer JN. Blood. 2016;127(26):3321-3330. Lee DW, et al. Biol Blood Marrow Transplant. 2019;25:625-638. Kumar, et al. Blood (2024) 144 (Supplement 1): 4758.


Type of Infection Risk
Medication Recommendation(s) for Healthcare Team Consideration
Viral: Herpes Simplex (HSV/VZV); CMV Acyclovir prophylaxis
Bacterial: blood, pneumonia, and urinary tract infection
PJP (P. jirovecii pneumonia)
Fungal infections
COVID-19 and Influenza
Consider prophylaxis with levofloxacin
Consider prophylaxis with trimethoprimsulfamethoxazole
Consider prophylaxis with fluconazole
Antiviral therapy if exposed or positive for covid per institution recommendations
IgG < 400 mg/dL (general infection risk) IVIg recommended
ANC < 1000 cells/μL (general infection risk)

Consider GCSF 2 or 3 times/wk (or as frequently as needed) to maintain ANC > 1000 cells/μL and maintain treatment dose intensity


Anorexia (difficulty eating) Weight loss
• ASCT
• GPRC5D therapy
Steroids Weight gain, fluid retention
Excess hunger ASCT
GPRC5Ddirected therapy Opioids

Weight Loss
Weight Gain
Weight Management
• Monitor weight for significant loss or gain
• Adjust diet (reduce calories or add supplements )
• Work with a dietician
Smith LC, et al. Clin J Oncol Nurs. 2008;12(3)suppl:37-52. Faiman B. Clin J Oncol Nurs. 2016;20(4):E100-E105.


Xerostom
ia

OTC dry mouth rinse, gel, spray are recommended. Avoid hot beverages. Anti-fungal therapy for oral thrush.


Dysgeusi a

Dysphagi a



=Dry Mouth =Difficulty Swallowing =Taste Change
Dexamethasone oral solutions “swish and spit” may provide benefit. Sour citrus or candies before meals are also recommended.
Care

Attention to oral hygiene.
Regular dental cleaning and evaluation. Close monitoring for ONJ, oral cancer and dental caries
Dietary modifications with small bites, eating upright, and sips with food can help manage symptoms

Weight Monitorin g
Some medications lead to weight gain, others to weight loss. Meet with a Nutritionist
Consider diet changes, supplements


Work closely with your entire health care team to manage oral side effects.



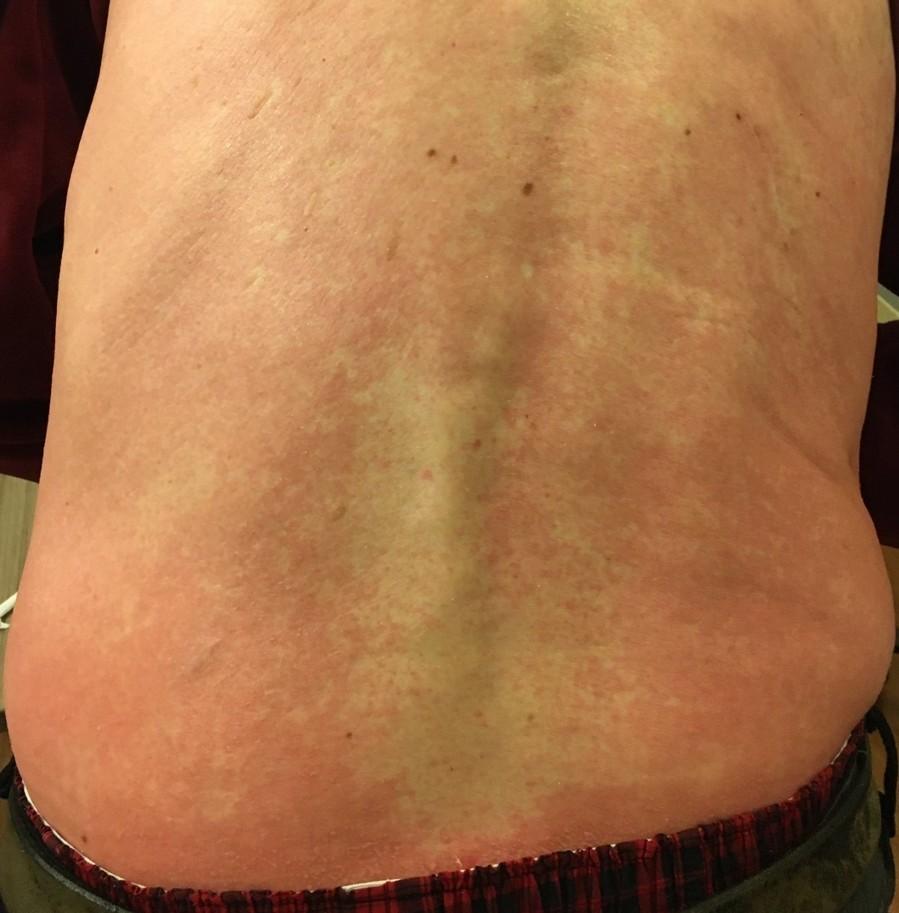
Skin Rash:
Prevent dry skin; apply lotion
Report changes to your care team
Medication interruption or alternative, as needed
Steroids:
• Topical for grades 1-2,
• Systemic and topical for Grade 3
Antihistamines, as needed

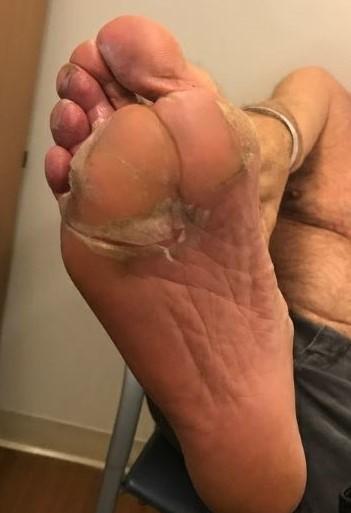
Nail Changes:
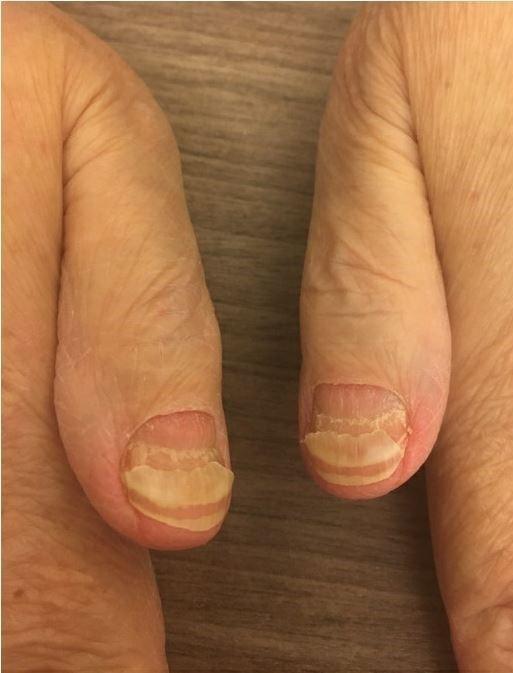

Keep your nails short and clean.
Watch for “catching and tearing”
Apply a heavy moisturizer like Vaseline or salve. Wear cotton hand coverings to bed
A nail hardener may help with thinning
Tell the team if you have signs of a fungal infection, like thickened or discolored nails












Fatigue is the most reported symptom. Sources include anemia, pain, reduced activity, insomnia, treatment toxicity, bone marrow suppression. Symptoms can improve with continued physical activity.
Symptoms are under-reported:
“I mentioned it before. Nothing can be done.” “I don’t want to be put on another medication.”

>35% of patients ≈25% of patients



• Mental health / social engagement
• Stress reduction; relaxation
• Sufficient Sleep
• Maintain a healthy weight; eat nutritiously
• Activity / exercise / prevent falls, injury
• Stop smoking Complementary or alternative therapy
• Have a PCP for general check ups, preventative care, health screenings, vaccinations
• Have specialists for dental care, eye exams/screening, skin cancer screening



Recommended Health
Screenings
• Blood pressure
• Cholesterol
• Cardiovascular disease
• Colonoscopy
• Dental checkups & cleaning
• Dermatologic evaluation
• Diabetes
• Hepatitis
• Hearing
• Vision
• Women specific: mammography, pap smear
Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Dimopoulous M, et al. Leukemia. 2009;23(9):1545-56.
• Men specific: prostate
Brigle K, et al. CJON. 2017;21(5)suppl:60-76. Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Faiman B, et al. CJON. 2011;15suppl:66-76. Miceli TS, et al. CJON. 2011;15(4)suppl:9-23.



Multiple studies demonstrate that strong social ties are associated with
• Increased longevity including people with cancer
• Improved adherence to medical treatment leading to improved health outcomes
• Lower risk of cardiovascular diseases
• Increased sense of purpose & life satisfaction
• Improved mood and happiness
• Reduced stress and anxiety
• Enhanced resilience
Care partners may help with medical appointments, managing medication, daily living, physical assistance, emotional support, myeloma knowledge, healthy lifestyle, patient advocacy, financial decisions
Care partners can be a spouse, close relative, a network of people (family, friends, neighbors, church members, etc)
• Recognize that caregiving is difficult/stressful

• Encourage care partners to maintain their health, interests, and friendships
• The IMF has information and resources to help care partners






































































“Thank You!”
From the NLB and the IMF




Joseph Mikhael, MD, MEd, FRCPC, FACP, FASCO, Chief Medical Officer, International Myeloma Foundation
•Health disparities are preventable differences in the burden of disease, injury, violence, or opportunities to achieve optimal health that are experienced by socially disadvantaged populations
- Centers for Disease Control (CDC)
•Health equity generally refers to individuals achieving their highest level of health through the elimination of disparities in health and health care






The core vision of this initiative is to improve the short- and long-term outcomes for African American patients with myeloma.
We want to empower patients and communities to change the course of myeloma…
Enhance access to optimal care by educating myeloma providers about the disparity and how to reduce it
Engage the community to increase awareness and provide support
Shorten the time to diagnosis by educating primary care providers to recognize the disease and order the right tests



April 2024
Multiple cities in Indiana
Annual Indiana Black Barbershop Health Initiative
• Health screenings for Black men on the 1st and 2nd Saturdays in April
• Shared materials on myeloma and M-Power for distribution in 18 barbershops in Evansville, Elkhart, and South Bend



June 20, 2024
New York, New York
50+ attended
76% African American
• 86% planned to share something they learned with their family, friend or healthcare provider
• 100% of attendees rated the program as excellent to very good
September 5, 2024
Charlotte, North Carolina

October 10, 2024
Richmond, Virginia
Primary Care Physician Dinner Meeting
• Presenting on multiple myeloma to the diverse community of healthcare professionals during the quarterly meeting of the Charlotte Medical Dental Pharmaceutical Society
40+ Attended
81% African American
• 88% plan to share something they learned with their family, friend, or healthcare provider
• 100% of attendees rated the program as excellent to very good

Juneteenth 2024: Abyssinian Church, Harlem, NYC

June21st , 2025

CelebratingJuneteenthinBrooklyn,NewYork!



















Our goal is to reduce DELAYS in diagnosis among African Americans by educating the primary care community with a focus on:
• Recognizing the signs and symptoms of myeloma
• Discriminating myeloma from other diagnoses such as diabetes
• Capturing an accurate diagnosis through proper use of testing
• Providing referral guidelines for Hematology and Oncology


• Grand Rounds

8,000
• Postcards mailed to 6,000+ PCPs in target cities
• Free PCP CME course “Don’t Miss Myeloma”
• Cobb Institute talk

• Talk at NMA Annual Meeting Dinner Meetings Articles and pending publications



Abstracts and Articles



• 12 1st – 3rd year medical students from all over the country met on August 5th in NYC at the NMA Annual Convention and Scientific Assembly
• Presented their posters, they worked on with a multiple myeloma experts immediately following the Jane Cooke Wright Symposium; which was dedicated to Dr. Edith Mitchell







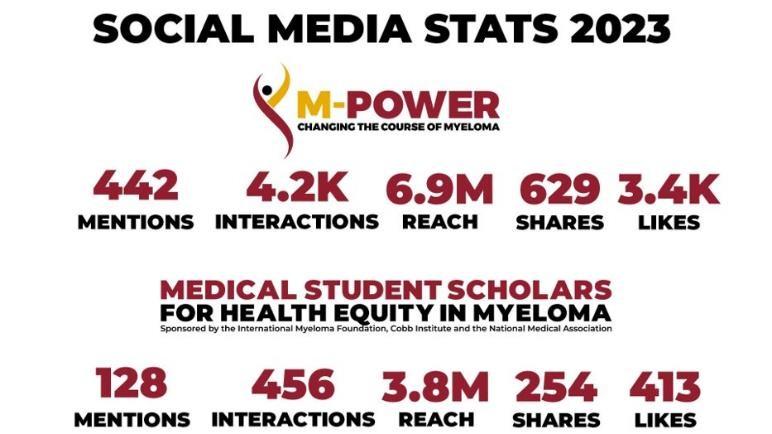
Over 400,000 visits to M-Power site!









M-Power Website:
•Web Stats: Over 40k Page views across main, city sites & myeloma.org
•Google PPC targeted web traffic
Email Stats:
•Total Sent: 18 emails
•Total Audience: 38k*
•Open Rate Avg: 31%*
*Note: We have continued to refine lists, contributing to a more engaged audience as evidenced in the Open Rates (The industry standard high-mark is 21%).
M-Minute Promotion Stats:
•Total Sent: 19 emails
•Total Audience: 323k
•Open Rate Avg: 38.91%
M-Power Related Video Stats:
• Total Views: Over 50k













Engage
• 2025 Juneteenth Workshop, NYC
• M-Power Community Workshop in Miami and Philly
• Expand online and social media strategy
• Primary care program in Charlotte
• Lab based education
• Electronic Medical Record Initiative
Enhance
• Diversity in Clinical Trial Academy as part of the Diversity in Clinical Trials initiative
• Nurse equity decision tool



•Be more conscious of the topics of health equity
•Evaluate the opportunities in your experience to reduce disparities
•Support the M-Power movement!




PLEASE HEAD TO YOUR SELECTED BREAKOUT SESSION:

BREAKOUT A: PATIENTS ONLY – LESSONS LEARNED
Please remain in this room
BREAKOUT B: CARE PARTNERS ONLY
Please move to Aria B

Michael Tuohy, 25-year Myeloma Patient, Support Group Leader

David Vesole, MD, PhD
John Theurer Cancer Center, Hackensack University Medical Center
MedStar Georgetown University Hospital
Dan Vogl, MD, MSCE
Abramson Cancer Center, University Of Pennsylvania, PA


Please return your program evaluations on your way out – whether you stay for the full program, or only one session, your feedback is invaluable to our team. Thank you for taking the time completing this.



Thank you to our sponsors!












OUR VISION:
A world where every myeloma patient can live life to the fullest, unburdened by the disease.
OUR MISSION:
Improving the quality of life of myeloma patients while working toward prevention and a cure.

These are the core values we bring to accomplishing our mission each day.
The patient experience is the focus of everything we do. Every interaction is an opportunity to establish a personal connection built on care and compassion which is the basis for continued support.
As a team, we value honesty and transparency while creating a culture of mutual respect. We foster a myeloma community built on sincerity, authenticity, and kindness.
We value accountability, personal responsibility, and a steadfast commitment to excellence. We respect the legacy and reputation of our organization while seeking new solutions and advancements to improve outcomes, quality of life, and access to the best available resources for everyone impacted by myeloma.
We recognize each team member's skills and talents through collaboration and cooperation. Our programs aim to celebrate and support the diversity of our patients and their communities.




