2025 IMWG Summit Sponsors




SESSION 5: WORKING COMMITTEE REPORTS

SMOLDERING MULTIPLE MYELOMA COMMITTEE

BONE DISEASE COMMITTEE

Bone Disease Sub-Committee of the International Myeloma Working Group
IMWG Summit 2025
Updates from Projects discussed during IMWG Summit 2024
Project 1. Retrospective Study on the Safety of Denosumab in patients with CrCl<30 ml/min
• No data on denosumab in this setting of Myeloma patients
• Collect the data focusing on safety, but also in efficacy
• Group decides to go on for an IMWG paper and abstract for IMS/ASH 2024 based on the patients’ data we collect within the next year
Evaluation of the safety
and efficacy
of denosumab
in patients with multiple myeloma and severe renal impairment
Data sent from 6 IMWG centers
1. Department of Clinical Therapeutics, Alexandra General Hospital, National and Kapodistrian University of Athens, Athens, Greece (E. Terpos, I. Ntanasis-Stathopoulos)
2. Memorial Sloan Kettering Cancer Center, Weill Cornell Medical College, New York, NY, USA (S. Usmani, Carlyn Rose Tan)
3. University College London, London, UK (C. Kyriakou)
4. Department of Haematology, Odense University Hospital, Odense, Denmark (N. Abildgaard)
5. Universitaire Ziekenhuizen Leuven, Leuven, Belgium (M. Delforge)
6. Hospital Italiano de Buenos Aires, Buenos Aires, Argentina (D. Fantl)


IMWG Bone Subcommittee designed a retrospective study to evaluate denosumab efficacy and safety in MM patients with severe RI (eGFR based on CKD-EPI <30 ml/min/1.73m2).
A multi-institutional chart review was performed and data from patients diagnosed with symptomatic MM and RI, under active antimyeloma treatment and concurrent denosumab, were analyzed.
BONE SUBCOMMITTEE STUDY
E. TERPOS1, I. NTANASIS-STATHOPOULOS1, C. KYRIAKOU2, C. TAN3, N. ABILDGAARD4, M. DELFORGE5, D. FANTL6, C.
SEEHAUS6, E. CAMEIRO7, C. JOAO8,9, S. USMANI3
1. Department of Clinical Therapeutics, Alexandra General Hospital, National and Kapodistrian University of Athens, Athens, Greece.
2. Department of Haematology, University College London Hospital, London, UK
3. Memorial Sloan Kettering Cancer Center, Weill Cornell Medical College, New York, NY, USA.
4. Department of Haematology, Odense University Hospital, Odense, Denmark
5. Universitaire Ziekenhuizen Leuven, Leuven, Belgium ;
6. Hospital Italiano de Buenos Aires, Buenos Aires, Argentina
7. Myeloma Lymphoma Research Group, Champalimaud Experimental Clinical Research Programme, Champalimaud Foundation, 1400-038 Lisbon, Portugal
8. NOVA Medical School, Universidade Nova de Lisboa, Lisboa, Portugal; Hemato-Oncology Unit, Champalimaud Foundation, Portugal

Best response to MM treatment for the whole cohort was as follows:
• 8 (8.2%) patients achieved ≥CR,
• 35 (35.7%) VGPR and
• 24 (24.5%) PR.
• Median time to response was 42 (28-90) days. Regarding best renal response:
• 9 (9.2%) patients achieved CRrenal, • 12 (12.2%) PRrenal and 31 (31.6%) MRrenal.
• Median time to renal response was 30 (20-42) days

EVALUATION OF THE SAFETY AND EFFICACY OF DENOSUMAB IN PATIENTS WITH MULTIPLE MYELOMA AND SEVERE RENAL IMPAIRMENT; RESULTS FROM AN IMWG BONE SUBCOMMITTEE STUDY
E. TERPOS1, I. NTANASIS-STATHOPOULOS1, C. KYRIAKOU2, C. TAN3, N. ABILDGAARD4, M. DELFORGE5, D. FANTL6, C.
SEEHAUS6, E. CAMEIRO7, C. JOAO8,9, S. USMANI3
1. Department of Clinical Therapeutics, Alexandra General Hospital, National and Kapodistrian University of Athens, Athens, Greece.
2. Department of Haematology, University College London Hospital, London, UK
3. Memorial Sloan Kettering Cancer Center, Weill Cornell Medical College, New York, NY, USA.
4. Department of Haematology, Odense University Hospital, Odense, Denmark
5. Universitaire Ziekenhuizen Leuven, Leuven, Belgium ;
6. Hospital Italiano de Buenos Aires, Buenos Aires, Argentina
7. Myeloma Lymphoma Research Group, Champalimaud Experimental Clinical Research Programme, Champalimaud Foundation, 1400-038 Lisbon, Portugal
8. NOVA Medical School, Universidade Nova de Lisboa, Lisboa, Portugal; Hemato-Oncology Unit, Champalimaud Foundation, Portugal
• Fifty-one (52.0%) patients in our cohort developed hypocalcemia:
• 18 grade 1 (35.3%),
• 13 grade 2 (25.5%),
• 17 grade 3 (33.3%) and
• 3 grade 4 (5.9%) almost four times higher than the reported incidence for patients with normal renal function or mild/moderate RI.
• Lower baseline calcium levels and higher denosumab dose (120 vs. 60 mg) were associated with hypocalcemia.
• There were 3 (3.1%) cases of osteonecrosis of the jaw and no case of new skeletal-related events (SREs).

• Overall,ourfindingssuggestthat denosumab is effective and safe for MM patients with severe RI, provided that proactive measures are taken to mitigate hypocalcemia.
• Possibly 60 mg, monthly, is sufficient for these patients to prevent both SREsandhypocalcemia.
• However, further prospective research with larger cohort and longer follow-up period will confirm these results and refine treatment guidelines.
Project 1. Retrospective Study on the Safety of Denosumab in patients with CrCl<30 ml/min - Plan
• Anyone who is interested in this study can send us data
• Aim to publish the paper by the end of this year (hope to have acceptance by the IMWG breakfast meeting at ASH 2025)
Project 2. Building a platform for collaborative imaging studies
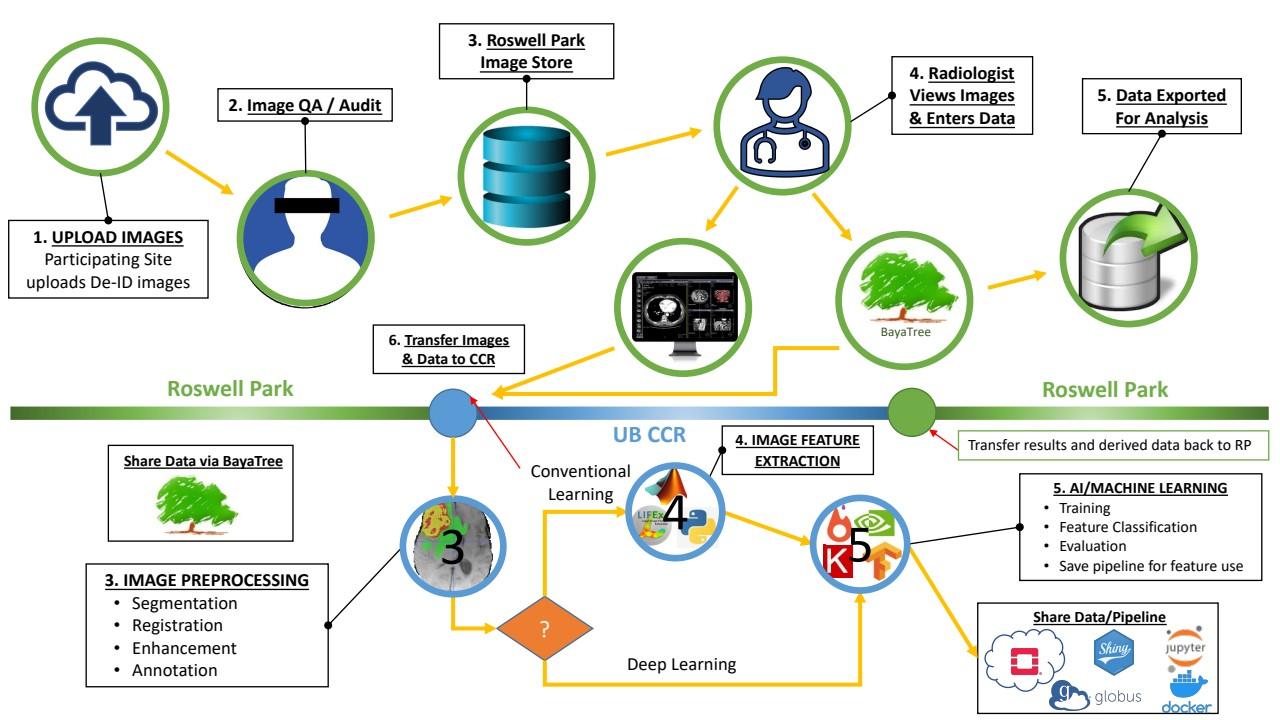
Project 2. Building a platform for collaborative imaging studies
Concept Developmen t of Online Platform
Obtain IRB approval/ data share agreement Beta-testing Platform Upload images and clinical data
Image evaluation by radiologists
Data analysis
Publication
Background
Utility of 18FDG-PET/CT for Risk Prediction in Relapsed/Refractory Multiple
Myeloma Patients Undergoing
CAR-T Cell Therapy: An Analysis of Baseline and Early Assessment Scans After One and Three Months
Tamariz-Amador, L.E; Alfonso-Piérola, A; Palacios-Berraquero, M.L; Huerga-Domínguez, S; Panizo-Inogés, M; Rifón, JJ; Villar, S; Marcos-Jubilar, E, Valencia-Espinoza; M; Prósper, F; Betech-Antar, V; San-Miguel, JF; GarcíaVelloso, MJ; Rodríguez-Otero, P.
2.A. Image Analysis in Patients on Immunotherapies
=> Background
The presence of FL, PMD, EMD, and bone marrow uptake was assessed at different time points



2.A. Image Analysis in Patients on Immunotherapies
=> Background
At baseline EMD was the only variable associated with inferior PFS and OS


2.A. Image Analysis in Patients on Immunotherapies
=> Background
A negative scan at month 1 had no significant impact on PFS, but did have on OS
A negative scan at 3 months was associated with both improved PFS and OS
The presence of EMD at 3 months was still associated with worse PFS and OS
Conversely to basal scans, persistent hypermetabolic PMD at month 3 was associated with inferior PFS and OS
2.A. Image Analysis in Patients on Immunotherapies
=> Participating Centers
University of Bologna, Italy
German Cancer Research Center, Heidelberg, Germany
Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA
University of Navarra, Spain
University of Nantes, France
Columbia University New York, NY, USA
University College, London, UK
University of Calgary, Canada
Shanghai Jiaotong University, China
Champalimaud Foundation, Lisbon, Portugal
Memorial Sloan Kettering Cancer Center, New York, NY, USA
University of San Francisco, CA, USA
University of Oslo, Norway
Icahn School of Medicine at Mount Sinai, NY, USA
MD Anderson CCC, Houston, TX, USA
University of Athens, Greece
University of Dresden, Germany
2.A. Image Analysis in Patients on Immunotherapies
OBJECTIVES
Primary:
• To collect imaging and serological data from patients on immunotherapy to assess patterns of response (remission versus progression versus suspected pseudoprogression) and frequency of these patterns
Secondary:
• To analyze the prognostic significance of the different response patterns for PFS and OS
• To define new criteria for imaging response in patients with multiple myeloma on immunotherapies
Exploratory:
• To apply computer-based image analysis to identify additional characteristics of focal lesions in patients with different response patterns
• To compare different imaging techniques (PET versus MRI (including DWI) versus CT)
PET/CT/DWI-MRI
response
CR (MRD)
2.B. Updated definitions of imaging response
Definition
Uptake ≤ liver pool (Deauville scale < 4) by PET or RAC-1 by DWI-MRI in ALL localizations BM/FL/PSD/EMD, irrespective of the reduction of soft-tissue plasmacytomas size.
Decrease of EITHER NUMBER OF FLs/PSD/EMD, WITH STABLE SUV (DS 4-5), OR ACTIVITY (SUV reduction at least 25%), WITH STABLE NUMBER of FLs/PMD/EMD, OR BOTH or RAC-2 at MRI, compared to baseline, irrespective of the reduction of soft-tissue plasmacytomas size.
SD
PD
No significant modification of BM/FL/EMD/PSD uptake or at MRI (RAC-3) compared with baseline, irrespective of soft-tissue plasmacytoma size
New lesion (FL/EMD/PSD) compared with baseline imaging, both in the functional part (PET DS > 4 or MRI RAC 4 or 5) or at CT if different technique applied caution recommended
• First evaluation after 3 months from the end of therapy (to avoid as much as possible background influence/tumor flare after CART/ bone regeneration). Once CR established, no other evaluation requested until suspect of progression, unless high risk context (i.e. EMD, post CART…)
• If persistent metabolic/MRI lesion or doubtful evaluation, repeat after 3-6 months, until CMR/RAC-1 or PD
New Projects
CTCs in patients with Solitary Bone Plasmacytoma
• CTCs have prognostic value in NDMM patients
• No data on CTCs kinetics before and after radiotherapy
• Better definition of the disease – possible exclusion from definition the entity of solitary plasmacytoma with minimal marrow involvement
• NGFC? or NGS?
Phase 2 Randomised Trial: Romosozumab in Newly Diagnosed Multiple Myeloma
Dr
Georgia McCaughan, Prof Peter Croucher & Prof Evangelos Terpos



The Challenge for Patients:
Patients continue to experience skeletal related events
• 45% of patients on anti-resorptive therapy will have a skeletal related event
• Anti-resorptive treatments only stop further bone loss
• Treatments that build new bone are required
• Romosozumab, an antibody against sclerostin, is a new bone anabolic drug

Raje et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: an international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncology. Volume 19, Issue 3, March 2018, Pages 370-381
Sclerostin: A
Pivotal Regulator of Bone Formation
Mutations in sclerostin lead to heavier bones

Inhibiting sclerostin leads to increased bone mass

et al 2014
Romosozumab in Post-Menopausal Women
with Osteoporosis and Fracture: BMD and Turnover Markers

KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, Maddox J, Fan M, Meisner PD, Grauer A. Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. N Engl J Med. 2017 Oct 12;377(15):1417-1427. doi: 10.1056/NEJMoa1708322. Epub 2017 Sep 11. PMID: 28892457.
Romosozumab in Post-Menopausal Women with Osteoporosis
and Fracture: BMD and Turnover Markers

Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, Maddox J, Fan M, Meisner PD, Grauer A. Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. N Engl J Med. 2017 Oct 12;377(15):1417-1427. doi: 10.1056/NEJMoa1708322. Epub 2017 Sep 11. PMID: 28892457.
Anti-Sclerostin Antibody Treatment in Models of Myeloma:
Improved Efficacy in Combination with Zoledronate
In pre-clinical models of myeloma bone disease antisclerostin treatment:
• Increases bone formation
• Stops myeloma bone disease
• Increase resistance to fracture
• Improves efficacy when combined with zoledronic acid


Addressing the Challenge for Patients:
A Trial of the Anti-Sclerostin Antibody – Romosozumab – in Myeloma
• Pilot trial of romosozumab in Myeloma
• St Vincent’s Hospital Sydney/Garvan partnership
• 12 patients with skeletal related event who have received prior zoledronic acid
• Romosozumab 210mg subcutaneously for 12 months

• Primary endpoints – safety and bone turnover markers
• Secondary endpoints - bone mineral density at 6 and 12 months, skeletal related events, progression free survival
• Tertiary endpoints – Single cell analysis, CT analysis of bone lesions

Interim Analysis of Pilot Study
Characteristic
Preliminary analysis of first 8 patients: • BMD decreased at baseline • No adverse events
• Formation markers increased
• Resorption markers decreased
• BMD increased at 6/12 months
• No new skeletal events
Bone Mineral Density T Score (IQR)
Interim Analysis of Pilot Study
Preliminary analysis of first 8
patients:
• BMD decreased at baseline
• No adverse events
• Formation markers increased
• Resorption markers decreased
• BMD increased at 6/12 months
• No new skeletal events
Change in P1NP and β-CTX Levels
Interim Analysis of Pilot Study
Preliminary analysis of first 8
patients:
• BMD decreased at baseline
• No adverse events
• Formation markers increased
• Resorption markers decreased
• BMD increased at 6/12 months
• No new skeletal events
A Proposal for a Randomized Phase II Study of Romosozumab
• Patients with newly diagnosed multiple myeloma with bony disease
• Randomised to 12 months of:
• Zoledronic acid and romosozumab vs.
• Zoledronic acid
• Primary endpoint:
• % Change from baseline in bone mineral density at the lumbar spine at 12 months
• Secondary endpoints:
• P1NP and beta-CTX at cycle 1, 2, 3, 6 and 12 months (median % change)
• Skeletal related events
• Myeloma progression free survival
• Improvement in lytic disease on CT
• Quality of life: BPI-SF, EORTC QLQ-C30 and QLQ-MY20
Trial Design
Key eligibility Newly diagnosed MM
Bony disease Planned induction
Romosozumab 210mg every 28 days
Zoledronic acid 4mg every 28 days
Primary endpoint
• % Improvement in BMD at Lumbar spine at 12 months
Zoledronic acid 4mg every 28 days
Secondary endpoints
• Change in P1NP and betaCTX
• Skeletal related events
• Progression free survival
• Improvement in lytic disease on CT imaging
• Quality of life
Statistical Assumptions
• Sample size: 100 patients, 50 patients in each arm
• Assumption of an increase in lumbar spine BMD of 4.1% (Muchtar et al, McClung et al) with bisphosphonate therapy
• Numbers based on a 90% power to detect a >5% increase of lumbar spine BMD at 12 months with addition of romosozumab with a significance level of 0.05
• Assumed compliance rate 85%
Thank you

BONE DISEASE COMMITTEE

IMMUNE THERAPY COMMITTEE
https:// myelomafoundation.share point.com/:p:/s/
IMFMeetingProgramResou rceHub/
EbXSrnEqNrNBkwdWyGW
IBxEBsx2hvN8kZTiWq2G
WlnONow?e=F2UiXt
IMWG Working Committee:
IMWG Meeting, Milan June 10, 2025
Hira Mian, MD
Yi Lin, MD PhD
Tom Martin, MD
IMWG Immune Therapy Committee
• Quarterly Committee Meetings
• March 21, 2025 (virtual)
• June 10, 2025 (IMWG Summit)
• September at IMS
• November 7, 2025 (virtual)
• IMF Database meetings
• Virtual Tissue bank meetings
IMF Database update
May 22, 2025
New Sites
Sites
Lead PI
Mount Sinai C.Rodriguez
University of Chicago
B. Derman
Emory J, Kaufman N.Joseph
University of Edmonton: university of Alberta I.Sandhu M. Chu
U Alabama S.Bal
Princess Margaret K.Stewart
U Pamplona P. Otero
Huntsman, Utah D. Sbovov
Univ of Kansas L.Shune
France P. Moreau
France M. Mohty
Germany H. Einsele
University of Ottawa A. McCurdy
Samsung Medical Center, Sungkyunkwan University School of Medicine
Utrecht, Netherlands
Dr. Kim
Rimke Oostvogels, Monique Minnema
DFCI-Harvard O. Nadeem
Brazil V. hungria
China Dr. Du
Australia A. Spencer
U of Calgary N. Bahlis
Manuscript Updates
Manuscripts Published
1. Mian, H., Martin, T.G., Pond, G.R. et al. Outcomes of frailty subgroups of older adults (age ≥ 70) treated with teclistamab: an International Myeloma Foundation immunotherapy database real-world analysis. Leukemia 39, 1252–1255 (2025). https://doi.org/10.1038/s41375-025-02552-3
2. Tan, C.R., Asoori, S., Huang, CY. et al. Real-world evaluation of teclistamab for the treatment of relapsed/refractory multiple myeloma (RRMM): an International Myeloma Working Group Study. Blood Cancer J. 15, 53 (2025). https://doi.org/10.1038/s41408-025-01259-z
Ongoing projects
Data being compiled
1. Hira Mian/Carlyn Tan: Early mortality and causes with immunotherapy
• Data being collected, plan for manuscript
2. Rakesh Popat - Real World Elranatamab Outcomes
• ASH submission planned
• Hoping to combine with IFM dataset
• Data cut off July 1st
Ongoing projects
Manuscripts currently being written
1. Carlyn Tan: Long-term Follow-up of Real-World Teclistamab for the Treatment of Relapsed/Refractory Multiple Myeloma (N=210)
• Will try to combine Data from Germany and Czech Republic +/- IFM
2. Murali Janakiram: Real world evidence with Talquetamab in a heavily pretreated population with MM (N= 118)
• 118 patients @ ASH = additional patients being added– excelspreadsheet being finalized end of May
3. Anupuma Kumar: Delayed neurotoxicity after CAR-T in multiple myeloma: (N=78)
New Proposals
1. Murali Janakiram/Darren Pan: Sequencing of ≥ 2 Immunotherapy
• Car-bispecific; Bispecific=>CAR; BCMA to GPRC5D; GPRC5D=>BCMA
2. Susan Bal/Hira Mian: Prophylactic Dexamethasone in CART
• Retrospective study: Can we look at database to see what is currently being done
3. Outcomes with Bela based combination therapy
IMWG Immune Therapy Committee Guidelines
Written
guidelines completed/planned
• Manuscript completed
• Costa, L.J., Banerjee, R., Mian, H. et al. International myeloma working group immunotherapy committee recommendation on sequencing immunotherapy for treatment of multiple myeloma. Leukemia 39, 543–554 (2025).
https://doi.org/10.1038/s41375-024-02482-6
• Manuscript planned
• Nooka, Terpos: Sequencing paper with belamaf incorporated and potentially strategies to think of with regards to dosing of drug, management of toxicity
• Rodriguez Otero, Chari, Usmani: T-cell engagers dosing.
IMWG Online Living Guidelines
• T-cell engagers outpatient management best practices
• Led by RN, APP, Pharmacist (Emory, UCSF, Mayo, UCL, others)
• CAR-T, T-cell Engagers updates
• IEC-late neurotoxicities: cranial nerve palsies, Guillaine Barre Syndrome, Parkinsonism, cerebellar toxicities
• IEC-enterocolitis
• Pre-emptive and alternative CRS management for T-cell engagers
Online Guideline: Prophylactic CRS management with T cell engagers
Preemptive Tocilizumab
Consider in patients with severe comorbidities, outpatient dosing per physician discretion, high tumor burden, or other conditions.
Single dose of IV Tocilizumab 8mg/kg 3h-4h hours prior to the first step-up dose of any bispecific antibody.
Other conventional premedication with dexamethasone, paracethamol and antihistamines remained unchanged.
Preemptive Dexamethasone
Consider in centers with limited access to tocilizumab.
Dexamethasone can be use to prevent CRS as a post-treatment drug. Dose range between 1020mg per physician discretion
24 and 48 hours after first step up dose
Online Guideline: Alternative T-cell engager CRS management
Dexamethasone treatment in low-grade CRS
Dexamethasone can be use for the management of grade 1 CRS, dose ranging from 10-20mg.
Consider in centers with limited access to tocilizumab or outpatient capability to manage CRS.
While rate of CRS with subsequent dose of T-cell engager step up dose between first treatment of CRS with tocilizumab or dexamethasone has not been formally studied in randomized trial, observation suggest that subsequent rate of CRS may be higher with dexamethasone. However subsequent episodes of CRS has also been managed with dexamethasone alone without the need for tocilizumab.
In the outpatient setting, consider a complete evaluation of the patient at the ER/hematology/oncology service for patients whose CRS do not respond to dexamethasone
Neutropenic fever and infection surveillance and management should be performed concurrently per institutional guideline
IMF Immune Therapy Data
Baseline Myeloma History
Subtypes of neurotoxicity (n=52)
• Median 4 prior lines of therapy (range 1-16)
• 67% progressed on last line
• 6% extramedullary disease
Baseline Neurological History
• No history of CNS myeloma or prior IT chemotherapy
• 1 (2%) had prior CNS radiation
• 10 (19%) had pre-existing neurological conditions
IEC-Parkinsonism Treatment –lessons learned
• Demographic trends – patients who developed Parkinsonism were predominantly male and White
• High peak inflammatory markers (ALC, ferritin, CRP) observed in Parkinsonism patients
• Cognitive and personality change may predate movement disorder
• Steroids were insufficient for full recovery
• Dopamine agonists had limited efficacy
• Early intervention with single dose of cyclophosphamide (2g/m2 IV) in the right patients may be key to recovery
• No evidence thus far of early relapse in those who received cyclophosphamide
Proposed Online Guideline Updates: IEC-Neurotoxicity (IEC-NT)
IEC-Parkinsonism (IEC-PKS)
Be vigilant of new-onset symptoms which can occur most commonly approximately 1-3 months post CAR-T infusion
Symptoms can be isolated movement, cognitive, and personality changes: stooped posture, bradykinesia, rigidity, paucity of speech, confusion, slowed responses, flat affect, micrographia
Do not need full presentation of all neurologic domains to prompt evaluation and consideration for management
Local clinic should contact CAR-T treatment centers to discuss coordination of evaluation and management
Recommend neurology consultation for comprehensive movement disorder evaluation, including MRI brain, lumbar puncture to rule out infectious etiology, and handwriting assessments for micrographia
Treatment experiences are limited to retrospective reports and case series
Symptoms resolution with IVIG, steroid, with and without dopamine agonist are limited and can be tried with initial presentation
Morbidity and mortality is high with severe cases, therefore alternative treatment should be considered when no improvement is seen in 1-2 weeks
Intrathecal chemotherapy and systemic high dose cyclophosphamide (1–2 g/m²) use have been reported with some patients having symptoms improvement and or resolution. High rate of infections have been seen with systemic cyclophosphamide, and antimicrobial prophylaxis and vigilant infection surveillance should be done.
Mitigating strategies
Myeloma disease burden reduction, when possible, prior to CAR-T infusion have been found to be associated with decreased incidence
Proposed Online Guideline Updates: IEC-Neurotoxicity (IEC-NT)
IEC-Guillaine Barre Syndrome
Consider this in the differential in patients with sudden onset of severe back pain after CAR-T infusion. Early signs may be confounded by patient’s history of bone disease from myeloma.
Prompt evaluation with neurologist consultation is warranted
Treatment experiences are limited with plasmapheresis, IVIG and steroid.
IEC-Cranial Nerve Palsy (IEC-CP)
Consider IEC-CP in patients presenting with new-onset cranial neuropathies approximately 35 weeks post CAR-T
most commonly facial nerve (cranial nerve VII) palsy, although cranial nerve III, V have also been reported
Can be unilateral or bilateral. More than 1 cranial nerve could be involved
Recommend neurology consultation, MRI brain, and lumbar puncture to rule out infectious etiology
Treatment experience from real world data, IMF Immunotherapy Data Registry and U.S. Myeloma Immunotherapy Consortium, suggest good response with steroid and IVIG
Median time to partial resolution was approximately 11 days, with full resolution typically achieved by 2 months.
Proposed Online Guideline update: Other Non-ICANS
Neurotoxicities
Bulbar and Cerebellar Toxicity associated with GPRC5d-targeted agents
Diagnosis
Consider in patients with unexplained ataxia, gait instability, dysmetria, bulbar aphasia or other bulbar or cerebellar symptoms occurred after the start of GPRC5D-targeting T-cell engager or CAR-T therapy.
Time to onset around 1st month but can be identified at a later time depending on the severity of the symptoms.
Recommend neurology consultation for comprehensive evaluation, including contrasted enhanced brain MRI, lumbar puncture to rule out infectious etiology, EEG. Other exams as deemed necessary.
Management
Pathogenesis is poorly understood
Recommended management includes:
Permanent interruption of the GPRC5d-targeting T-cell engager if grade 2 or higher
Consider early implementation of corticosteroids or IVIG, although no formal treatment has shown efficacy for symptom resolution
IEC-associated enterocolitis
Mayo Clinic Experience with Longer Follow-up
US MM Immunotherapy Consortium
• 14 cases across 10 U.S. Centers (2-4%)
• 13 cilta-cel; 1 ide-cel
• Likely not BCMA specific, 3 case reports with CD19 CAR-T
• Median time to onset 79 (22-210) days
• Median duration 76 (17-113) days
• Response to steroid (4/10); infliximab (3/6); vedolizumab (1/3)
• 5 deaths due to colitis or infection complications
• Important to escalate treatment early and monitor for C. Diff and CMV
Fortuna G et al. BCJ 2024.

• No patients had a durable response to systemic corticosteroids.
• Three patients (Case 1, 2 and 3) received infliximab of which one (Case 2) achieved a durable response after 16 months.
• Two patients (Case 4 and 6) received Vedolizumab of which both achieved a durable response after 5 and 6 months.
• Two patients (Case 1 and 8) received high-dose Cyclophosphamide; both are yet to respond after 2 months.
• One patient (Case 3, with CD4+ LPD changes on duodenal biopsy) had highly refractory disease failing to respond to multiple lines.
• Myeloma response rate, 1-yr PFS and OS rate not different.
T-cell lymphoproliferative disorder with IECenterocolitis
Case Report after Cilta-Cel


mSMART Management of IEC-
Enterocolitis
• Typical onset 1-6 months after CAR-T infusion
• Can be severe and require hospitalization for IVF, electrolyte replacement and TPN
• Communicate with CAR-T treatment center to coordinate evaluation and management. Multi-disciplinary care with GI and ID.
• Recommended evaluations
• Stool: c. diff PCR, GI pathogen panel, calprotectin, (outpatient, less severe presentations: bile acids, pancreatic elastase)
• Blood: CMV PCR, TCR clonal rearrangement
• Imaging: CT abdomen and pelvis with contrast
• EGD and colonoscopy with biopsies
• Check on pathology for presence of T cells. Check TCR clonal rearrangement on biopsy specimen
mSMART Management of Severe IECEnterocolitis

Proposed Online Living Guideline Update: IEC-Enterocolitis
IEC-Enterocolitis
Diagnosis
Consider in patients with unexplained, non-bloody diarrhea with negative infectious work-up occurring 1-3 months post-CAR-T. This may also be seen post T-cell engagers.
Evaluation to include GI and ID specialists:
Perform endoscopic evaluation with biopsies that are specifically reviewed by a hematopathologist, as inflammation often resembles patterns seen in GVHD following allogeneic transplantation
Infections are common preceding or concurrent, should be evaluated and treated if identified
Perform T-cell receptor clonal rearrangement on GI biopsy as part of evaluation to identify T-cell lymphoproliferative disorder
Collaborate with product manufacturers to test for CAR-T presence on enteral biopsies
Management
Treatment experience are limited to retrospective reports & case series. Morbidity and mortality is high with severe cases. Consider TPN support early in severe cases.
IVIG and steroid, +/- budesonide can be trialed first. If lack of improvement in 1-2 weeks, consider stopping systemic steroid, and try infliximab or vedolizumab.
Consider T-cell modulating or lymphotoxic drug such as high dose cyclophosphamide in refractory cases or in cases of T-cell lymphoproliferative disorder
Remain vigilant of antimicrobial prophylaxis and infection surveillance during treatment
et
Virtual Tissue Bank Projects Resistance to immunotherapy
Resistance to IT Agents
Francesco Maura and Holy Lee
- Discussion
Post-BCMA therapy

Multiple Hot spots on BCMA


F, et al ASH 2024; Lee H, et al Nature Medicine 2023;29:2295-2306

Key point 7. Effector-to-target ratio (tumor burden) and baseline soluble BCMA levels
predict upfront anti-BCMA TCE response

Key questions guiding clinical and translational strategies in TCE resistance
• What is the optimal clinical method to detect emerging antigen escape clones in real time before clinical relapse - ctDNA, soluble antigen levels, WGS, targeted panels, flow/ IHC antigen expression
• How should longitudinal tissue collection protocols be designed to capture clonal evolution and emerging resistance - what should trigger sampling?
• Can baseline tumor burden/ heterogeneity/ T cell profile predict the likelihood or timing of relapse?
• Can rationally designed multi-specific TCEs, combinational strategies, or fixed duration therapies prevent functional antigen-loss?
Next steps – Virtual tissue bank
• Share sequencing and immune phenotyping data, linked to the IMF
Immune Therapy Data Registry
• Timepoints and archiving samples to enable near future sequencing studies - BM
• Pre-CART or TCE treatment, at time of PD/next treatment
• At fixed interval such as after 4 cycles of TCE
• When stopping TCE or going to maintenance
• Type of sequencing studies
• WGS at pre-treatment and PD/next treatment
• Targeted panels during surveillance
• Need for funding. Use testing that is CLIA to enable future use for clinical decision making
• Some lab may have cfDNA capability
• BM/PB immune panel
Prospective/Retrospective Database project –
IEC late neurotoxicities mitigating strategies
ALC peak as biomarkers to predict risk for delayed neurotoxicity (Mayo Clinic analysis)
*<0.05;
• Mayo Clinic 3 sites data (cases included in IMF Database Registry)
• Controls n=208
• IEC-NP (nerve palsies) n=15
• IEC-PKS (Parkinsonism) n=9
• Other risk factors by MVA
• Older age, ICANS, >1 dose tocilizumab, increased month 1 ferritin

10 mg PO twice daily for 3 days

ROC analysis identified ALCpeak ≥3.0 x 10^9/L as a viable threshold value (AUC=0.838)
ALCpeak ≥3.0 x 10^9/L has 21% AR for IEC-NP vs 1% if ALCpeak
<3.0x10^9/L
ALCpeak ≥3.0 x 10^9/L has 12% AR for IEC-PKS vs 1% if ALCpeak
<3.0x10^9/L
Thank you! Questions and Comments

IMMUNE THERAPY COMMITTEE
https:// myelomafoundation.share point.com/:p:/s/
IMFMeetingProgramResou rceHub/
EbXSrnEqNrNBkwdWyGW
IBxEBsx2hvN8kZTiWq2G
WlnONow?e=F2UiXt

COMBINED MASS SPECTROMETRY &
MRD COMMITTEE


Mass Spectrometry – Mayo Clinic Update
Curated by
Dr. David Murray, MD, PhD
Presented by Maria Alice Willrich, PhD
Associate Professor of Laboratory Medicine and Pathology
Co-director, Protein Immunology Laboratory
International Myeloma Working Group Summit 2025 | Milan, Italy June 10 &11




IgG, IgA , IgM, κ, λ Spectra
2D Spectra with Peaks highlighted Automated Analysis
Peak Analyzer Isotyper Interpreter Analyzer System

Current Detected M-Proteins

M-Protein, FLC and Ig Quant History



3D Spectra History
Lab Tech and Lab Director Review Manual regating if
TIMELINE OF MASS-FIX
Initial development of MALDI-TOF method
2014
2018 Clinical test go-live for serum IFE replacement
2016 Clinical Chemistry method publication
2024 Mass-fix quantitation SPEP replacement
2023 Clinical test go-live for urine IFE replacement
Performed >475,000 tests
1+ year course of MM treatment


EXAMPLE OF DYNAMIC RANGE IN AN IGG LAMBDA


M-protein slowly decreasing
IMPLICATIONS OF LOWER LOQ FOR M-PROTEIN

SPEP and IFE
• 65% of M-protein positive samples were below the LOQ of SPEP
• The range of IFE positive samples below the SPEP LOQ was 0.05 g/L to 40 g/dL by Mass-Fix
Mass-Fix
• 0.03% of M-protein samples had values below LOQ of Mass-Fix
MASS-FIX PROVIDES A LOT OF INFORMATION

ONGOING PROJECTS
DO LOW LEVEL M-PROTEINS PERSIST?

EXAMPLE OF LOW-LEVEL IGG KAPPA PERSISTENCE

4 years IgG kappa persisting at 0.04 g/L to 0.10 g/L

CAN MASS-FIX DETECT EARLY RELAPSE?



EARLY RELAPSE DETECTION IN AN OLIGO-SECRETORY IGG LAMBDA MM PATIENT




M-Protein 0.58 g/L
CAN HIGH RESOLUTION MASS-FIX ENHANCE MPROTEIN DETECTION

PLANNED WORKFLOW
MM diagnosed
MASS-FIX performed
• Isotype determined
• One sample used as baseline for accurate mass of M-protein determination
Treatment initiated
• MASS-FIX follow-up samples
• Identifies tmabs
MRD ASSAY CHARACTERISTICS AND PLAN FOR TESTING
Follow-up MASS-FIX
MALDI-TOF method
LOQ = 10 mg/dL
About 1/3 of MASS-FIX negatives are positive by hi-res MASS-FIX
Reflex to HiRes MASSFIX
High-resolution liquid chromatography MS on 7600 QTOF Qualitative only LOD = 0.3 mg/dL
BENEFITS
• Benefits of MASS-FIX are in the negatives
• Benefit of MRD testing in bone marrow are in the positives
• This approach is relatively simple
• Blood mass spectrometry, as an easier specimen type, can offer more information over time to understand the course of disease
• Go-live of MM MRD test is planned for Q1 2026 for Mayo Clinic patients
SOFTWARE TO COMBINE MASS-FIX AND HIGH-RESOLUTION MASS-FIX

High-Resolution Mass-Fix LOD ~ 0.003 g/L
ACKNOWLEDGMENTS















+ Protein Immunology Lab Technologists and Lab Assistants






Combined Mass Spectrometry and MRD Committee
Flow Cytometry - CIMA LAB Diagnostics
Hematology Department - Clinica and CIMA Universidad de Navarra
Spanish Myeloma Group (GEM)
EuroFlow Consortium
Additional thoughts
• Complementarity across different techniques: more studies are needed to identify optimal combination of methods, time points, treatment scenarios, making it cost-efficient and improving patients’ QoL.
• Mass spec is the future of M-component assessment: need studies comparing different methodologies for optimal use in different applications, and develop consensus/guidelines.
• MRD/PRD criteria to stop treatment and to define operational cure (as in the new response criteria): need to collect more data in ongoing and future studies.
• EMA questionnaire about MRD negative CR endpoint: EHA-EMA Joint Symposium session at 15.45 CEST, Friday 13.
• Further development of NGF MM-MRD: There are extensive requirements for analytical validation and clinical validation for an IVD cleared MRD test (as achieved for NGS). To support continued clinical validation of NGF, we propose a meta-analysis of existing high quality trial and real world data including MM MRD by NGF.
Possible algorithm to combine different methods and to monitor MRD/PRD

The assessment of MRD in BM should probably be the standard approach during the first year of treatment:
• Aligned with the MRD-negative CR end point
• Aligned with the time of best response achieved in different disease settings and treatment scenarios
• PRD using NGF/NGS will be often negative during the early and more intensive stages of treatment
• The use of MS may lead to some false-positive results owing to the long half-life of the M component
Monitoring PRD using NGF and MS

BloodFlow outperforms NGF and has independent prognostic value together with MRD assessment in BM


Complementarity between BloodFlow and MS

Double-negative MRD detection in PB and serum using BloodFlow and QIP-MS achieved a NPV of 84% (ie, MRD negativity in BM using NGF)
Improving PRD with dynamic monitoring using MS

Additional thoughts
• Complementarity across different techniques: more studies are needed to identify optimal combination of methods, time points, treatment scenarios, making it cost-efficient and improving patients’ QoL.
• Mass spec is the future of M-component assessment: need studies comparing different methodologies for optimal use in different applications, and develop consensus/guidelines.
• MRD/PRD criteria to stop treatment and to define operational cure (as in the new response criteria): need to collect more data in ongoing and future studies.
• EMA questionnaire about MRD negative CR endpoint: EHA-EMA Joint Symposium session at 15.45 CEST, Friday 13.
• Further development of NGF MM-MRD: There are extensive requirements for analytical validation and clinical validation for an IVD cleared MRD test (as achieved for NGS). To support continued clinical validation of NGF, we propose a meta-analysis of existing high quality trial and real world data including MM MRD by NGF.

COMBINED MASS SPECTROMETRY &
MRD COMMITTEE

QUALITY OF LIFE COMMITTEE
QoL Sub-Committee Meeting
June 10, 2025

Collaborative Effort!
Myeloma Experts and Researchers
1. Nadine Abdallah, Mayo Clinic, Rochester, MN
2. Rahul Banerjee, University of Washington, Seattle
3. Natalie Callandar, University of Wisconsin
4. Donna Catamero, Mount Sinai, NY
5. Rajshekhar Chakraborty, Columbia University
6. Gordon Cook, University of Leeds, UK
7. David Cucchi, Amsterdam UMC, Netherlands
8. Edvan Crusoe, Brazil
9. Michel Delforge, UZ Leuven, Belgium
10. Beth Faiman, Cleveland Clinic, OH
11. Doris Hansen, Moffitt Cancer Center
12. Jens Hillengass, Roswell Park Cancer Center
13. Murali Janakiram, City of Hope
14. Artur Jurczyszyn, Krakow, Poland
15. Charalampia Kyriakou, University College of London, UK
16. Hira Mian, McMaster University, Hamilton, Ontario
17. Roberto Mina, Italy
18. Simone Oerlemans, Netherlands
19. Charlotte Pawlyn, The Institute of Cancer Research, London, UK
20. Aurore Perrot, France
21. Borja Puertas Martinez, Spain <borjapuertas@usal.es>
22. Joshua Richter, Mount Sinai
23. Katja Weisel, Germany
24. Tanya Wildes, University of Nebraska
25. Christine Ye, MD Anderson Cancer Center
Statistical PRO Experts
Amylou Dueck, PhD Mayo Clinic, Arizona
Birgit Lissenberg, PhD, Netherlands
Regulatory Experts:
Vishal Bhatnagar, US FDA
Bindu Kanapuru, US FDA
Francesco Pignatti, EMA
Patient Advocates:
Eilidh Duncan, Myeloma Patient Europe
Poornima Parmesawaran, IMF Patient Board, US
PROPOSAL FOR GUIDELINES ON INVESTIGATING, ANALYSES AND REPORTING OF QOL IN MM CLINICAL TRIALS
1. INTRODUCTION - WHY TO MEASURE QOL?
SURBHI SIDANA AND SONJA ZWEEGMAN
2. QOL TOOLS IN CLINICAL MM TRIALS – THE PRO’S AND CON’S – WHICH TO USE?
RAHUL BANERJEE, CHARALAMPIA KYRIAKOU, MICHEL DELFORGE, DORIS HANSEN, ROBERTO MINA
3. STATISTICAL ANALYSES OF QOL, AND HOW TO DEFINE CLINICAL MEANINGFUL DIFFERENCES IN PRO DOMAINS
NADINE ABDALLAH, HIRA MIAN, DAVID CUCCHI, SIMONE OERLEMANS, BIRGIT LISSENBERG-WITTE, AMYLOU DUECK
4. PROS AS ENDPOINTS IN MYELOMA CLINICAL TRIALS – UNDER WHICH CONDITIONS
MURALI JANAKIRAM, NATALIE CALLANDER, BIRGIT LISSENBERG, VISHAL BHATNAGAR, BINDU KANAPURU, AMYLOU DUECK, FRANCESCO PIGNATTI
5. CONCLUSIONS AND GUIDELINES FOR INVESTIGATING, ANALYSES AND REPORTING OF QOL IN MM CLINICAL TRIALS
SURBHI SIDANA AND SONJA ZWEEGMAN
PROPOSAL FOR GUIDELINES ON INVESTIGATING, ANALYSES AND REPORTING OF QOL IN MM CLINICAL TRIALS
WHAT & WHEN
QOL TOOLS IN CLINICAL MM TRIALS – THE PRO’S AND CON’S – WHICH TO USE?
RAHUL BANERJEE, CHARALAMPIA KYRIAKOU, MICHEL DELFORGE, DORIS HANSEN, ROBERTO MINA
DESCRIPTION OF PRO AND QOL TOOLS IN CLINICAL MM TRIALS
Review of key trials in the last 15 years – leading to registration
2 SMM trials
12 NDMM trials
10 RRMM trial
Heterogeneous questionnaires – EORTC-QLQ C30 and MY20, EQ 5D 5L, FACT-G, FACT-MM and timepoints
Most used tool EORTC-QLQ C30 in combination with EORTC-MY20
However, in the US the NIH supports PROMISE-29
DIFFERENCES BETWEEN THE PROMIS-29 AND EORTC QLQ-30
Item/Domain
Pain Interference
Pain Intensity
Fatigue
Sleep Disturbance
Physical Function
Anxiety
Depression
Social Roles & Activities
Cognitive Function
Nausea / Vomiting
Dyspnea
Appetite Loss
Constipation / Diarrhea
Financial Impact
Global Health Status / QoL
(1 item)
(4 items)
(4 items)
(within scale)
(3 items)
(1 item only)
(indirect)
(indirect)
(less detailed)
WHAT
IS NOT CAPTURED?
• Specific side effects with novel immune therapies
• CRS, ICANS, late motor-neurotoxicity
• On-target, off-tumor symptoms
• GPRC5D – skin – nail – oral – GI
• BCMA – eye toxicity with ADC, severe and mild but chronic upper respiratory tract infections
WHAT & WHEN?
EORTC MY20 or specific PRO-CTCAE for myeloma-related symptoms? 3. Development of specific PRO-CTCAEs for what is not captured with certain therapies?
HOW?
STATISTICAL ANALYSES OF QOL, AND HOW TO DEFINE CLINICAL MEANINGFUL DIFFERENCES IN PRO DOMAINS
NADINE ABDALLAH, HIRA MIAN, DAVID CUCCHI, SIMONE OERLEMANS, BIRGIT
LISSENBERG-WITTE, AMYLOU DUECK
IT IS NOT ABOUT STATISTICS, BUT ABOUT CLINICAL MEANINGFUL DIFFERENCES
Minimal Important Difference (MID)
the smallest group-level difference in a score that patients or proxies perceive as meaningful, and that could prompt a change in clinical management
Responder Thresholds (RTs)/responder definitions (RDs)
to define individual-level changes considered meaningful
MORE THAN 1 METHOD TO DEFINE
CLINICALLY IMPORTANT DIFFERENCES
Method Description
Anchorbased Links PRO score changes to external criteria (e.g. patient-reported global ratingof change).
Distributionbased Uses statistical characteristics of the score distribution (e.g., 0.5SD, SEM) to estimate MID or RT.
Qualitative Derives thresholds through patient interviews or surveys identifyingwhat magnitude of change is personallymeaningful.
Triangulation Integrates all three methods to derive robust estimates of MIDs and RTs.
Strengths
Patient-centered; Directlylinks the score to a meaningful change in health perception
Easyto compute; no need for external anchor
Limitations
Requires valid and sensitive anchor; subject to recall bias or interpretation differences
Lacks patient input; Risk of over- or underestimatingclinical relevance as purely based on statistics; context-dependent
Deep insight into patients’ experiences; Can reveal differences across disease context or cultures
Maximizes validity; balances statistical, clinical, and patient-centered evidence
Resource-intensive; maylack generalizability
Requires integration of diverse methodologies and expertise
ANCHOR BASED
A patient rates their pain improvement as "a little better" on a 7-point global impression of change scale.
The average score change in those who said “a little better” becomes the MID
DISTRIBUTION BASED
0.5 SD: Half a standard deviation of the baseline scores or Standard Error of Measurement (SEM)
MORE THAN 1 METHOD TO DEFINE
CLINICALLY IMPORTANT DIFFERENCES
• And they all have been used – but not consistent
• All using different cutt offs
HOW?
MIDs or RTs?
Anchor or distribution-based or both?
And more in general – which statistical methods to use, how to report, how to handle missing data
Can we endorse consistent MIDs/RTs for EORTC QLQ-C30/MY20, PROMIS-29, and PRO-CTCAE?
10-point RT (within group) for EORTC scales – Coon et al., 2022
5-point MID (between groups) for EORTC scales
2-6 point RT (within group) for T-score PROMIS scales – Terwee et al., 2021
3-point MID (between groups) for T-score PROMIS scales – consensus meeting of PROMIS Leadership*
1-point RT (within group) for PRO-CTCAE – Lee et al., 2025
*https://www.healthmeasures.net/score-and-interpret/interpret-scores/promis/meaningful-change
TO FINALLY COME TO A(N) (END)POINT
PROS AS ENDPOINTS IN MYELOMA CLINICAL TRIALS
MURALI JANAKIRAM, NATALIE CALLANDER, BIRGIT LISSENBERG-WITTE, VISHAL BHATNAGAR, BINDU KANAPURU, FRANCESCO PIGNATTI, AMYLOU DUECK
1. When as a co-primary endpoints when as a secondary endpoint
2. Which requirements when included as an endpoint
3. How to check for bias in reporting
4. Endorse SISAQOL recommendations for statistical analysis of PROs
Proposed Guidelines
What to measure
1. Disease-related symptoms
2. Symptomatic adverse events (key AEs of interest based on trial)
3. Physical function
4. Role function
5. Overall side effect impact summary measure
6. Overall quality of life – dependent on many factors and may not solely represent treatment impact
1-5 are consistent with FDA Core Guidance
Key Recommended Instruments
Domain PRO Measures
1. Disease related symptoms: Pain, fatigue
2. Symptomatic AEs
3. Physical function
4. Role function (work, home, leisure)
5. Side effect summary score
EORTC QLQC30 or PROMIS-29
• PRO-CTCAE: Pick from item library
• EORTC QLQ-30 (nausea, vomiting, diarrhea, sleep)
• Specific symptom modules (vision, taste)
EORTC QLQC30 or PROMIS-29
EORTC QLQ-30 (No direct measure in PROMIS)
6. Overall QoL
EORTC library question 168 or GP-5 (FACIT library)
EORTC QLQC30
Note for PROMIS measures: not standalone question for overall QoL but available as summary measure
How often to measure: Active MM
Frequency:
On continuous Therapy
First 3-6 months during induction: Once a month
Month 6-24 months: Every 3 months 2 years: Every 6 months
At progression, and if feasible 1 additional timepoint soon after
Response adapted treatment stopping or escalation
More often: Monthly for at least 3 months after treatment change
QoL may improve after stopping and worsen with increased treatment burden
Special Considerations: BsAb and CAR-T
Frequency:
Bispecific Antibodies
In the step- up phase: consider more often in the first cycle (weekly)
CAR-T Cell Therapy
• At enrollment/apheresis (if significant interval in between, enrollment should be baseline)
• Prior to lymphodepletion
• Day 0, Day 14
• Month 1-6: Monthly
• Month 6-2 years: Every 3 months
• Beyond 2 years: Every 6 months
Extended bridging/induction approach: refer to continuous therapy .
Both enrollment/apheresis and pre-lymphodepletion are important timepoints as bridging represents a critical period with rapidly changing disease and symptom burden. Change should be described from apheresis to pre-LD and compared to both timepoints
Special Considerations: RCTs
• In a RCT with different types of treatment (Eg. CAR-T vs continuous, limited vs continuous duration BsAb), measurement frequency should align to the more intense arm.
• If two arms differ in unique toxicity, specific symptomatic AEs should be included to determine tolerability.
• Eg. In a hypothetical trial for DaraKRd vs DaraVRd, neuropathy and dyspnea/chest pain should be included as patient reported AEs
Special Considerations: Smoldering MM
• Important to measure more frequently and for longer
• Include PRO-CTCAEs and instruments to evaluate overall side effect bother
• Current measures for cancer population (EORTC) may not be appropriate
• Measures validated in general populations (PROMIS) may be more appropriate
• Consider development of additional measures
Regulatory Considerations
• In certain areas, regulatory requirements may necessitate the use of additional PROs
• Eg. Use of EQ5D in registrational trials for EMA
• Include as required by regional regulatory guidelines
Responder
Thresholds and Minimally Important Difference (MID)
Responder Threshold: Individual level change, MID: Group level change
Various methods to define: Anchor, distribution, qualitative
• For EORTC scale (100-point scale)
• Responder threshold: 10-point change
• MID: 5-point difference is clinically significant, as long as statistically significant
• For PROMIS T-score scales:
• Responder threshold: 5-point change is meaningful (literature reported 2-6 points)
• MID: 3-point difference to be clinically significant on T-score scale, as long as statistically signifcant
Working on consensus on subdomains
PRO-CTCAE: Descriptive analysis, but 1-point change is meaningful (like grade 2 vs
Special Considerations in Data Analysis
Report PRO change by key sub-groups of interest:
• Age group
• Baseline function/frailty
• Disease symptoms
• Consideration for Race/Ethnicity
PROs as Endpoints
• Limited ability to use PROs as a primary endpoint
• Key secondary or co-primary endpoint with traditional efficacy measures to define tolerability of treatment
• Pre-planned, with hypothesis and statistical analysis plan and plan for missing data (aligned with FDA guidance)
• Endpoints should include specific domains or symptoms, not overall QoL
• Examples: NDMM: Improvement in pain and fatigue can be an endpoint
Largely asymptomatic patients (smoldering MM, biochemical relapse, maintenance studies): Side effects/tolerability as endpoint
Pre-planned Robust Framework to Handle Missing Data
• Use Estimand framework to clearly specify components of analysis including handling intercurrent events (missing data, crossover)
• Prefer mixed modelling when estimating means at a fixed time point – plus sensitivity/supplemental data analysis varying assumptions around intercurrent events and missing data
• Other analysis possible depending on the endpoint selected (eg, responder definition)
• Efforts to include reporting of amount and reasons for missingness
• Awaiting updated SISAQOL guidelines and will align SISAQOL 2020 report. Coens et al.
Future Directions
Focus Planned Action

Comparison/correlation of scores b/w
EORTC QLQC30 and PROMIS-29
Validate in large datasets
Certain future studies in MM to include both and develop statistical approaches to compare
Surrogate endpoint analysis:
Optimal timepoints, degree of expected change and relationship between PRO change and efficacy measures
Consensus for responder thresholds/MID for subscales of EORTC and PROMIS-29
Evaluation in smoldering MM
Effort to evaluate individual patient level PRO data from large clinical trials (~ MRD effort)
As above
Review applicability of current measures and consider development of additional measures

QUALITY OF LIFE COMMITTEE

FUTURE PLANNING
Thank you to
2025 IMWG Summit Sponsors





