

2025 IMF REGIONAL COMMUNITY WORKSHOP
DENVER, CO

JUNE 21, 2025

Thank you to our speakers & our sponsors!









Welcome & Introductions
Robin Tuohy
Vice President, Patient Support
International Myeloma Foundation
Understanding Myeloma Basics
Daniel Sherbenou, PhD, MD
University of Colorado Hospital, Aurora, CO
IMF REGIONAL COMMUNITY WORKSHOP
DENVER MORNING AGENDA

(Video) Closing the Gap: Health Disparities in Myeloma
Joseph Mikhael, MD, MEd, FRCPC, FACP, FASCO,
Chief Medical Officer, International Myeloma Foundation
Advancing Treatment Options Through Clinical Trials
Peter Forsberg, MD
Colorado Blood Cancer Institute, Denver, CO
Q&A with Panel
Coffee Break
Breakout: Frontline or Relapsed Treatment Approaches
-NDMM: Getting Started with Myeloma Management
Daniel Sherbenou, PhD, MD
-RRMM: Continuing the Myeloma Treatment Journey
Peter Forsberg, MD
Housekeeping Items
Presentation Slides: Are available by scanning the QR code, Instructions are on the QR code handout on each table.
Program Evaluations: evaluations at the end of today.
Restrooms: Bathrooms are located on this level, outside the meeting room you will turn right, then left and go past the elevators. Turn Right and the bathrooms will be at the end of the hall.
Parking: If you have parked at the hotel today, please check in with an IMF team member at the registration desk. There is no charge for parking, but we would record the number of our attendees who have parked at the hotel.

Badge Holders: Please return your badge holders and we can recycle them.
We greatly appreciate your time and feedback!


IMF: Who we Are
Founded 35 years ago, the International Myeloma Foundation (IMF) is the global leader in multiple myeloma with the mission of improving the quality of life of myeloma patients while working toward prevention and a cure.
We envision a world where every myeloma patient can live life to the fullest, unburdened by the disease.


Our Four Pillars

Strateg
y


• The IMWG comprised of hundreds of the best and brightest doctors and researchers globally set the guidelines for treating myeloma around the world.
• IMF advocates for patients globally helping to provide access for underserved communities. This includes our M-Power Project meeting the underserved where they are.
Raise the Bar
• IMF holds research focused summits & informational patient meetings, including patient & family seminars, regional community workshops to name a few, reaching thousands of patients, care partners and HCPs globally.
Broaden our Reach
• IMF research encompasses a multitude of Studies and Cure trials across the world.
• We run GMAN a global myeloma action network brining myeloma leaders across the world together to align and support our efforts globally.
• SUPPORT Studies show that social support can greatly improve the quality of life of people with cancer. The IMF offers more than 150 myeloma support groups across North America, including groups for Spanish-speakers, people with smoldering myeloma, care partners, veterans, high-risk patients, patients with young families, and those without care partners.


• One such trial, iStoppMM is THE Largest clinical trial of its kind with almost half of the Iceland population sampled and over 70k screened for MGUS.
• Two of our social media focused tentpole marketing campaigns MAM and BCAM reach over 70 million on social across over 60 countries annually.
Examine the why of all our actions to ensure they are purpose-driven, meaningful, and effective.
Address unmet patient needs by expanding our reach to diverse & underserved populations in everything we do.

Innovate Every Step of The Way
• We advocate for patients on capitol hill, helping to facilitate FDA drug approvals and fight for reduced drug prices.
• Immune Therapy Registry and Tissue Bank collecting samples of patients to further understand high risk and relapsed patients.
• We produce hundreds of publications outlining patient care, signs & symptoms, treatment options & ways to cope and live well with Myeloma.
The IMF InfoLine answers myeloma-related questions. Myelo, the IMF’s generative AI assistant, is available 24/7 to help you find the right resources.

Provide those who need it most with what they need the most, throughout their myeloma journeys.
The IMF Support Group Team is Here
For You!
Shared Experiences Help to Better Understand the Myeloma Journey
• Support Groups empower patients & care partners with information, insight & hope
• The IMF provides educational support to a network of over 150 myeloma specific groups
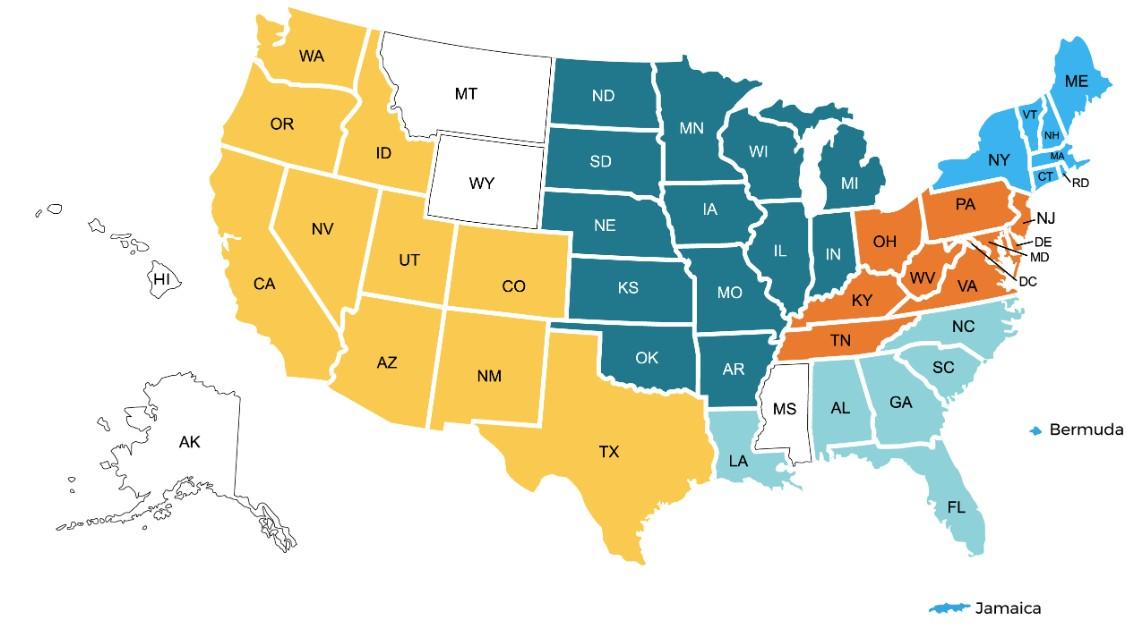
150+ US Support Groups
Over 200 Support Group Visits/year







Special Interest Groups
Special interest groups are designed as a supplemental support for specific populations of patients, in addition to their local Support Groups
Las Voces de Mieloma-founded in 2022
Designed for Spanish speaking patients only
Living Solo & Strong with Myeloma founded in 2022
Designed for patients without a care partner

High Risk Myelomafounded in 2023
Designed to address the needs of the high-risk MM population
Care Partners Onlyfounded in 2024
Designed to address the needs and concerns of care partners
Veterans Only founded in 2025
For those who served our country
Smolder Bolderfounded in 2023
Created for people living with Smoldering Myeloma
MM Familiesfounded in 2021
For patients/care partners with young children

Multiple Myeloma Support Group
Meets in a hybrid fashion on the 2nd Saturday of each month at 9:00AM Mountain Time

Denver
Care Partners Support Group
Meets virtually on the 4th Wednesday of each month at 7:00PM Mountain

IMF InfoLine
Connecting Patients to Resources…
Shortening “Time to Hope” for Over 1,000 First-Time Callers Each Year
Assistance with understanding lab results, terminology and disease state
Preparing for medical visits
Resource Information:
• Financial & Emotional Support
• Expert Myeloma Referrals
“Thank you so much for the informative conversation and all the time you spent listening and helping me decipher the MM lingo. What an amazing service!”



“Thank you for your response and excellent question suggestions for my hematology team.”









Written Education
Understanding Booklets
Tip Cards
Myeloma Minute Weekly Updates
Myeloma Today Quarterly News







































Live Patient Education
4 PATIENT & FAMILY
including world-renowned experts 10 MYELOMA COMMUNITY WORKSHOPS including local myeloma experts

Locations


2025 Live Patient Education
Patient & Family Seminars
• Boca Raton, FL – March 14 – 15
• Philadelphia, PA – May 2 – 3
• Los Angeles, CA – August 15 – 16
• Chicago, IL – October 3 – 4

Myeloma Community Workshops
• Virtual - March 4
• San Mateo, CA - March 29
• Atlanta, GA - April 5
• Edina, MN - April 26
• Denver, CO - June 21
• Virtual – July 29
• Seattle, WA - August 9
• Waltham, MA - September 27
• Raleigh-Durham, NC - November 15
• Virtual – November 18
Scan for Upcoming Events!






Understanding Myeloma Basics
Daniel Sherbenou, PhD, MD
University of Colorado Hospital, Aurora, CO
Understanding Myeloma Basics
Daniel Sherbenou, MD, PhD
6/21/2025

Plasma Cell Program
MM Learning Objectives
1. Basic Biology and Epidemiology
2. Plasma Cell Targeted Therapies
3. Response Criteria and MRD
4. First Line Treatment
5. Treatment of Relapsed Disease
MM Learning Objectives
1. Basic Biology and Epidemiology
2. Plasma Cell Targeted Therapies
3. Response Criteria and MRD
4. First Line Treatment
5. Treatment of Relapsed Disease

Multiple Myeloma: Historical Perspective

1st patient diagnosed with bone pain and and "animal matter" in urine Termed Multiple Myeloma



Bone Marrow involvement recognized
Peak on serum protein electrophoresis noted



1850 1873 1889 1898 1939




High-dose therapy with autologous stem cell support/ thalidomide
Kyle RA et al. Mayo Clin Proc. 2003;78:21
Barlogie B et al. N Engl J Med. 1984;310:1353; Berenson JR et al. N Engl J Med. 1996;334:488; Alexanian R et al. Ann Intern Med. 1986;105:8; Bergsagel D. Cancer Chemother Rep. 1962;21:87; Salmon SE et al. Cancer Chemother Rep. 1967;51:179; Rousselot P et al. Cancer Res. 1999;59:1041;McElwainTJ, Powles RL. Lancet. 1983;2:822
Combinations
Multiple Myeloma: the 21st Century


Daratumumab Approved as Monotherapy





Acute Myeloid Leukemia
Multiple
Myeloma Originates from the Plasma Cell
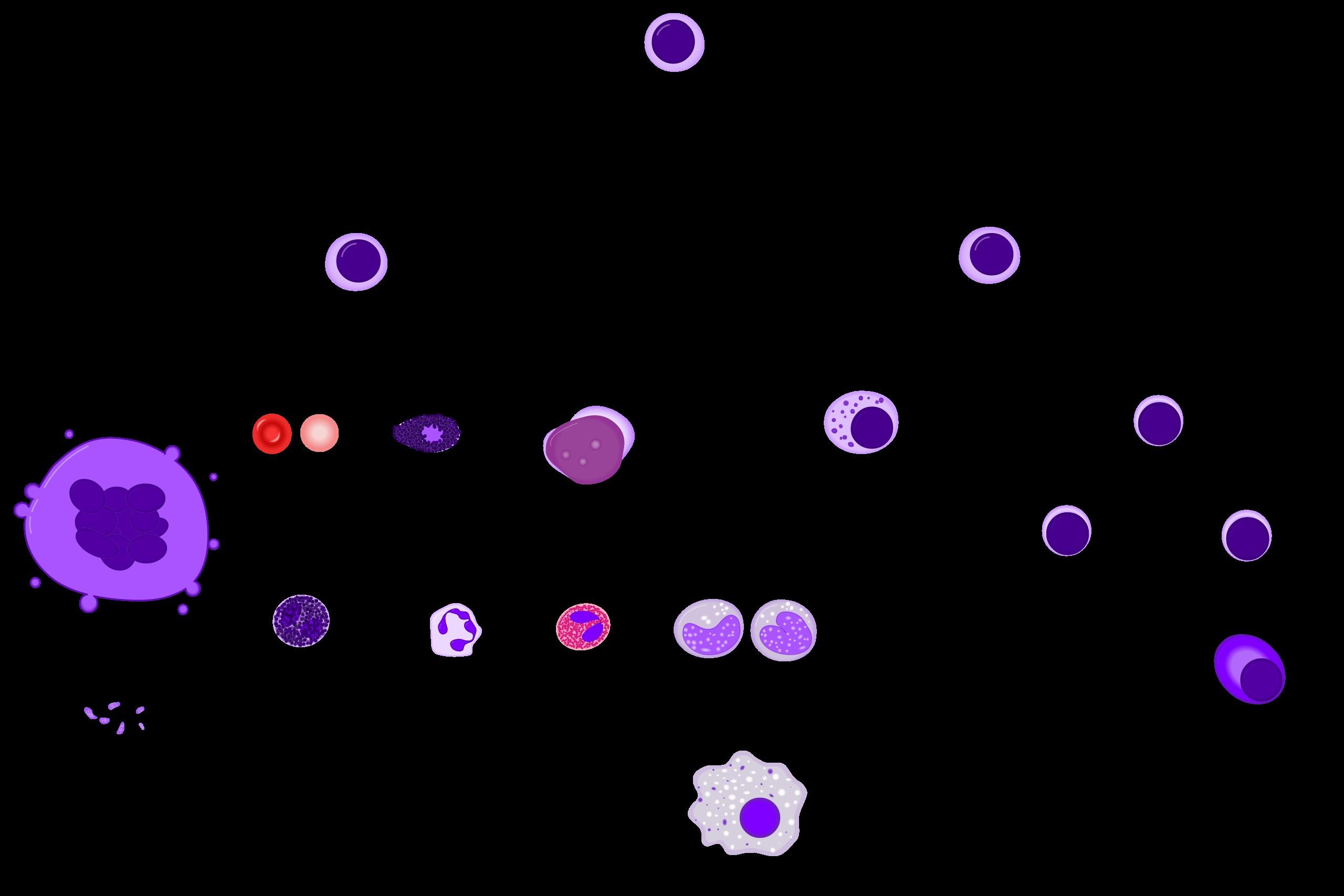
Chronic Myeloid Leukemia (HSC)
Acute Lymphoid Leukemia
Lymphoma
Multiple Myeloma
Multiple Myeloma: The Treatment Landscape is Transforming
IMiDs, PIs, mAbs
CAR-T, bsAbs
IMiDs – Immunomodulatory Drugs
PIs – Proteasome Inhibitors
mAbs – monoclonal antibodies
CAR-T – chimeric antigen receptor T cells bsAbs – bispecific antibodies
Patients now average greater than 10 years living with the disease with optimal treatment
Myeloma eventually develops drug resistance to the key drugs—Immunomodulatory Drugs,
Chimeric Antigen T-cells (CAR-T) and Bispecific Antibodies (bsAbs) have emerged with
Comparison of Blood Cancers by Survival, Age
of Onset and Incidence

*Note: this slide is outdated

Acquired Genetic Mutations Generally Favor
Cell Doubling, Genomic Instability and Survival
Translocation
‘Donors’
•14q32: Heavy chain
•2p12: Kappa
•22q11: Lambda
Translocation ‘Acceptors’
•11q13: most common (cyclin D1, 30%)
•4p16 (FGFR3, MMSET, 25%)
•8q24 (MYC, 5%)
•16q23 (MAF, 1%)
•6p25 (IRF4, rare)
Point Mutations
• p53
• K-Ras
• N-Ras
• B-RAF
• IDH1 and IDH2
Gains and deletions
• Hyperdiploid
• 13 deletion – 10-50%
• Deletion 17p (p53)
• Gain of chromosome 1q (30%)
• Loss of chromosome 1p
Chromosome
Grains/Losses
Translocations
(Hybrid Chromosomes)

Blood Cancer and BMT Program
SCHOOL
higher risk
*also elevated B2MG with normal kidney function
Normal Chromosomes
Point Mutations
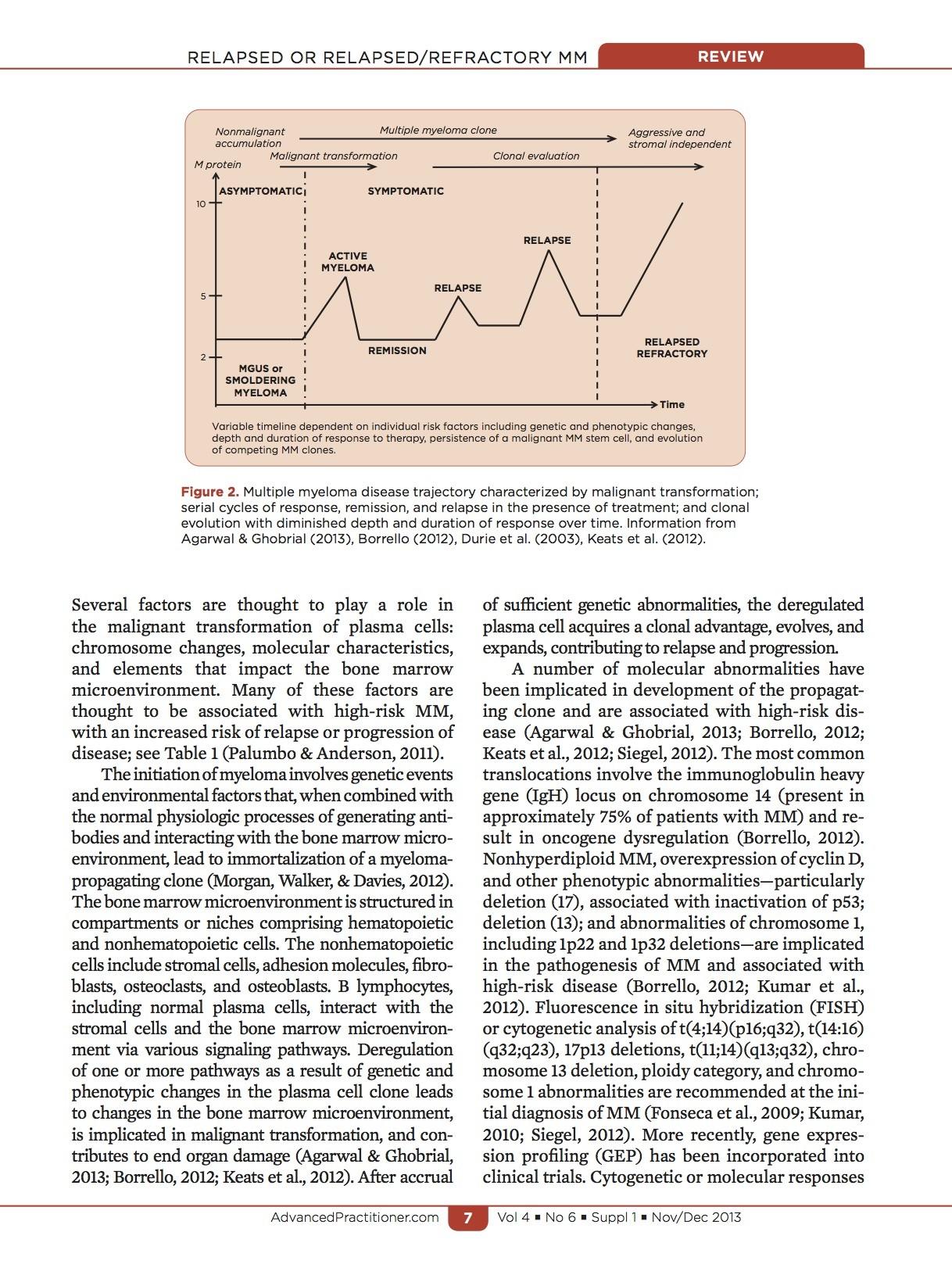
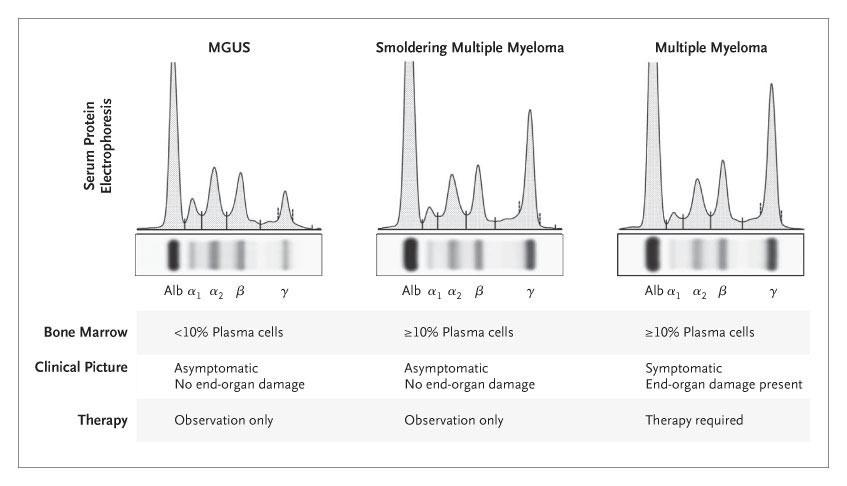
Disease Spectrum MGUS SMM

Monoclonal Gammopathy of Undetermined Significance (MGUS)
•1% per year risk of progression
Smoldering Multiple Myeloma (SMM)
•10% per year risk of progression for first 5 years
•3% per year for next 5 years
•1% per year thereafter
Multiple Myeloma (MM)
Secretion of Monoclonal Antibody (M-protein)
Serum Free Light Chains
Kyle R et al. NEJM 2007;356:2582-

Edition, 2004
Kyle RA and Rajkumar SV. Cecil Textbook of Medicine, 22nd
MM Learning Objectives
1. Basic Biology and Epidemiology
2. Plasma Cell Targeted Therapies
3. Response Criteria and MRD
4. First Line Treatment
5. Treatment of Relapsed Disease
Myeloma Toolbox: The Current Treatment Options
Proteasome Inhibitors
Bortezomib (Velcade)
Carfilzomib (Kyprolis)
Ixazomib (Ninlaro)
Alkylator Chemo
Cyclophosphamide
Melphalan
Bendamustine
Immunomodulatory
Drugs (IMiDs)
Thalidomide (Thal)
Lenalidomide (Revlimid)
Pomalidomide (Pomalyst)

Monoclonal Antibodies
Daratumumab (Darzalex)
Isatuximab (Sarclisa)
Elotuzumab (Eloquist)
Idecabtagene vicleucel (Abecma)
Ciltacabtagene autoleucel (Caryvkti)
Bispecific Antibodies
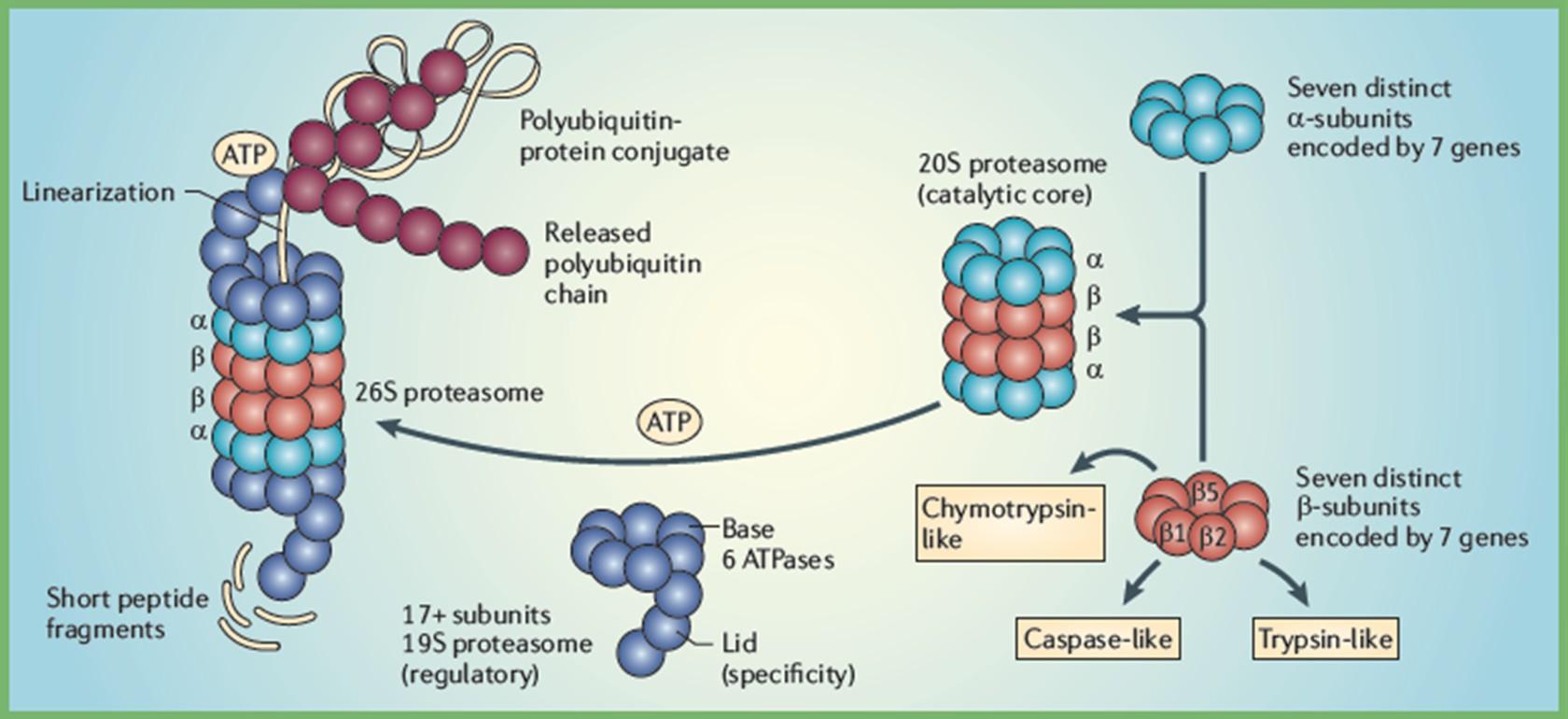
Proteasome Inhibitor Mechanism of Action
Proteasome Inhibitors (PIs)

Bortezomib
Carfilzomib
Ixazomib
Illustration by Gwen Tice
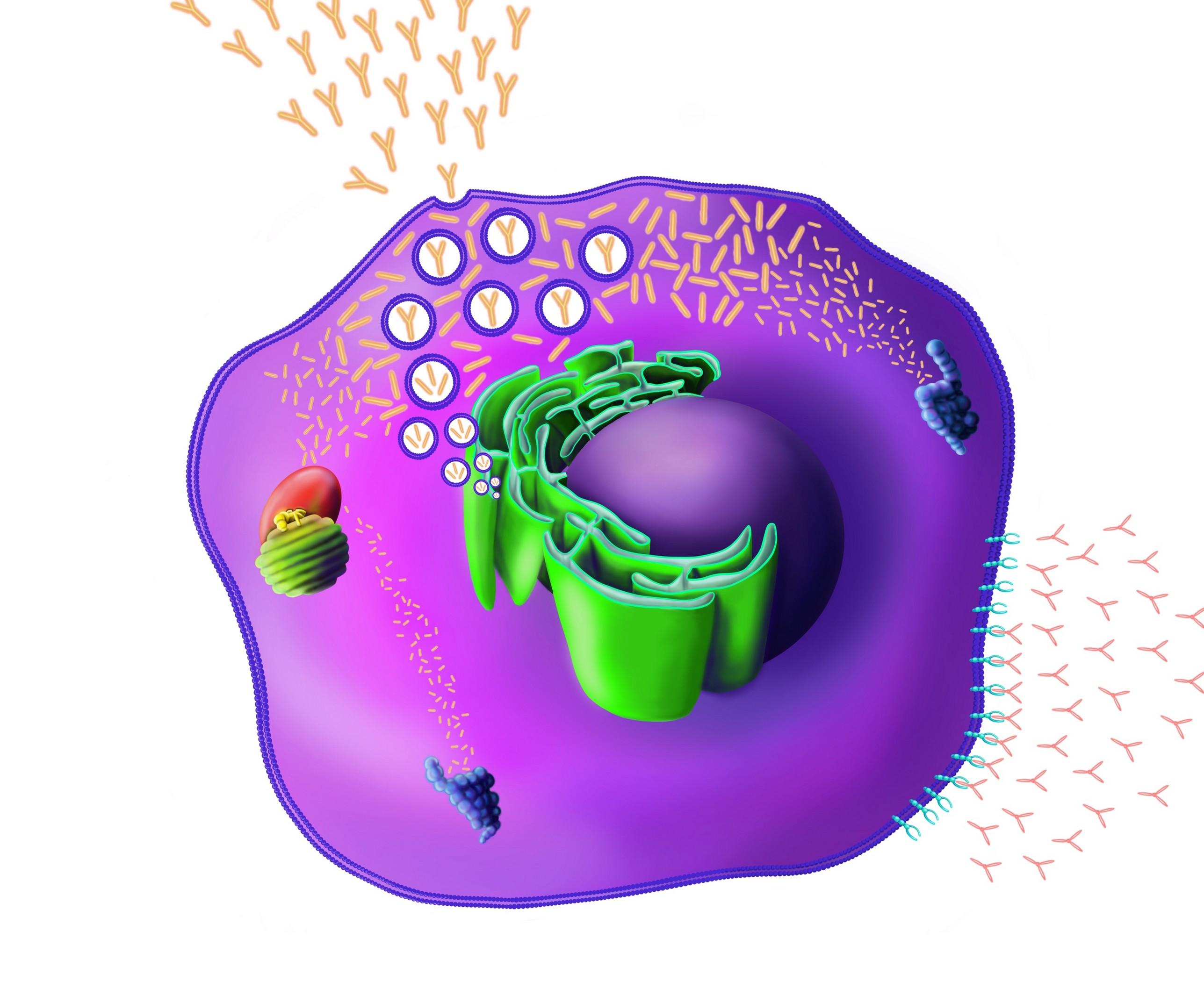
IMiD
monoclonal antibody
IMiD: Unique Mechanism of Action
E3 Ubiquitin Ligase Complex Proteosome
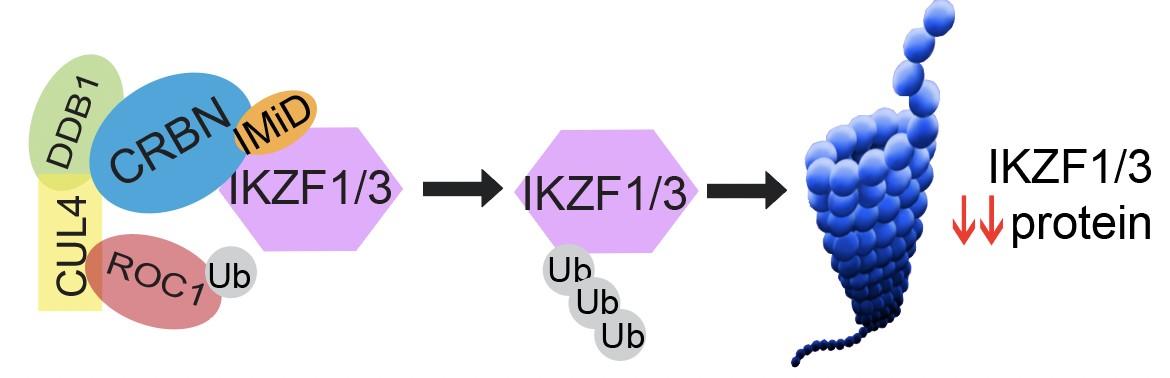

Ikaros 1 = IKZF1
Ikaros 3 = IKZF3
CRBN = Cereblon
Ub = ubiquitin
Unknown mechanism Survival, proliferation
Irf4 Myc
Immunotherapy: Diverse Mechanisms for Myeloma
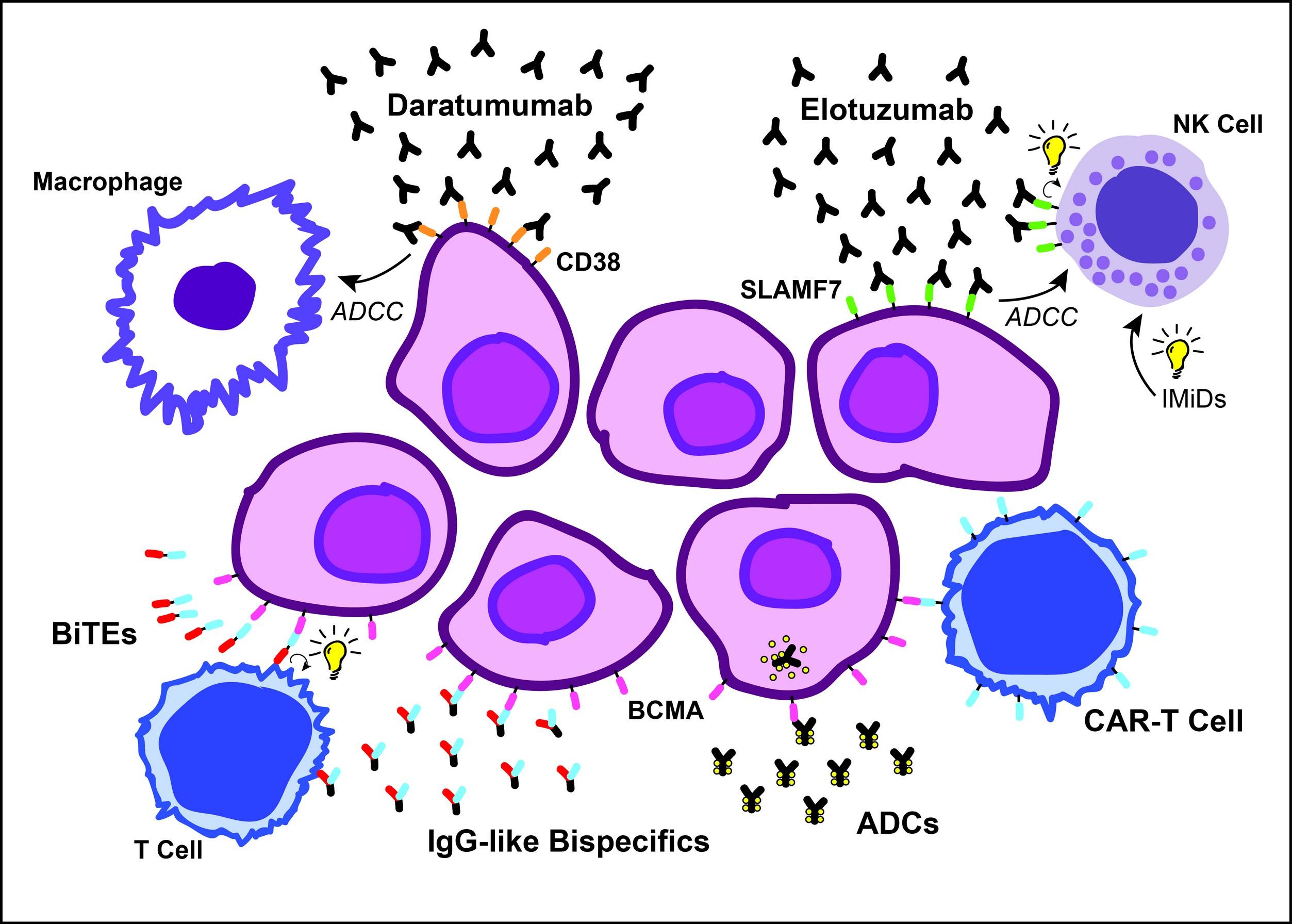
Sherbenou et al, Clin Lymphoma Myeloma Leuk, 2107
Multiple Myeloma Drug Side Effects
• Lenalidomide: decreased neutrophil count, diarrhea (bile acid malabsorption → bile acid sequestrant), clotting (preventative medication needed), rash, increased risk ~1% for secondary malignancies
• Thalidomide: neuropathy (plus above, should be avoided)
• Velcade: sensory neuropathy (less with subcutaneous route)
• Carfilzomib: cardiotoxicity (rare if given weekly)
• Daratumumab: infusion-related reactions (usually first injection only)
• Dexamethasone: insomnia, mood disturbance, elevated blood sugar, pneumocystis pneumonia (preventative antibiotic needed with high dose Dex)
• Melphalan: bone marrow suppression, nausea/vomiting, diarrhea
• CAR-T Cells, bispecific antibodies: cytokine release syndrome (CRS), ICANS (neurotoxicity syndrome), delayed neurotoxicities (with Carvykti)
MM Learning Objectives
1. Basic Biology and Epidemiology
2. Plasma Cell Targeted Therapies
3. Response Criteria and MRD
4. First Line Treatment
5. Treatment of Relapsed Disease
Myeloma Response to Treatment
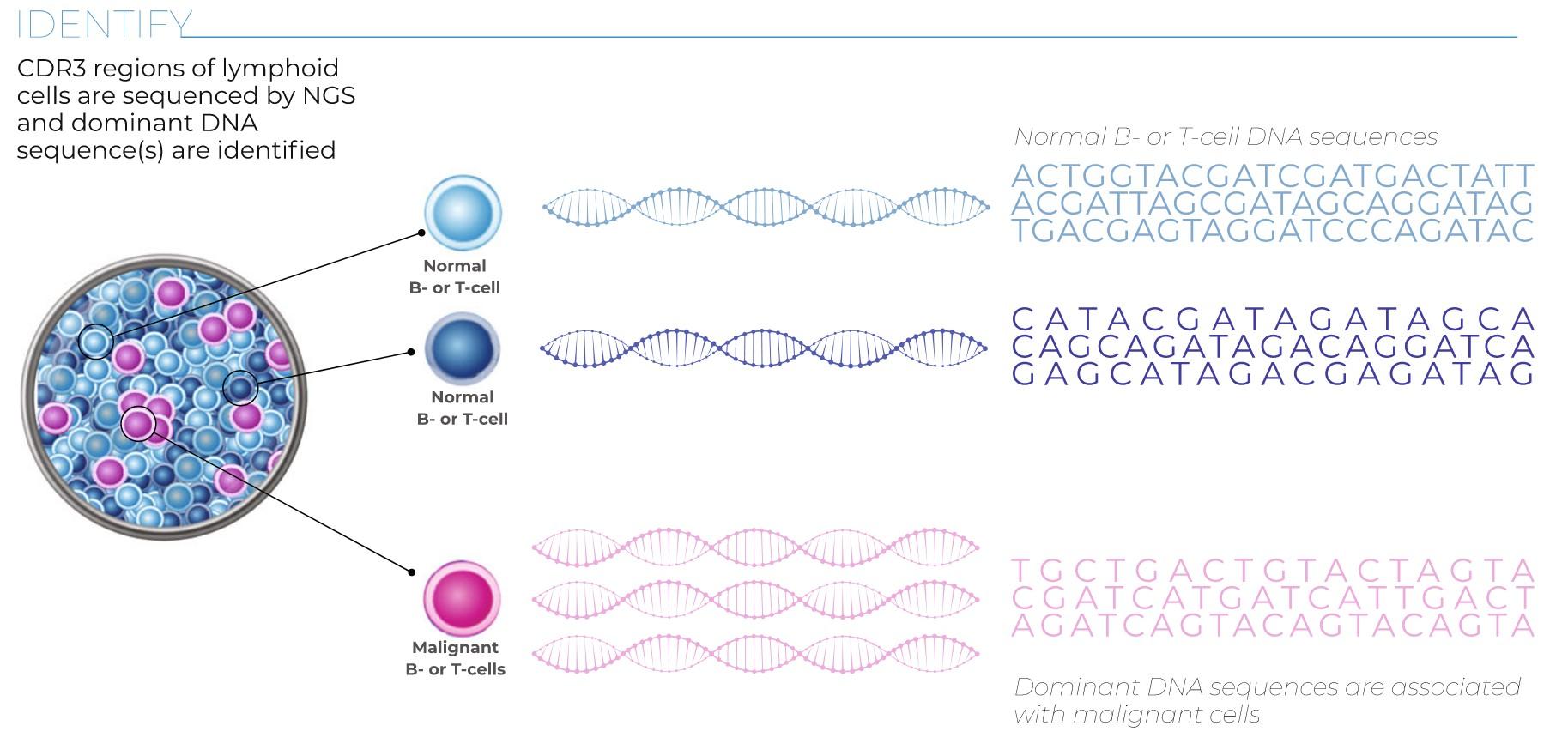
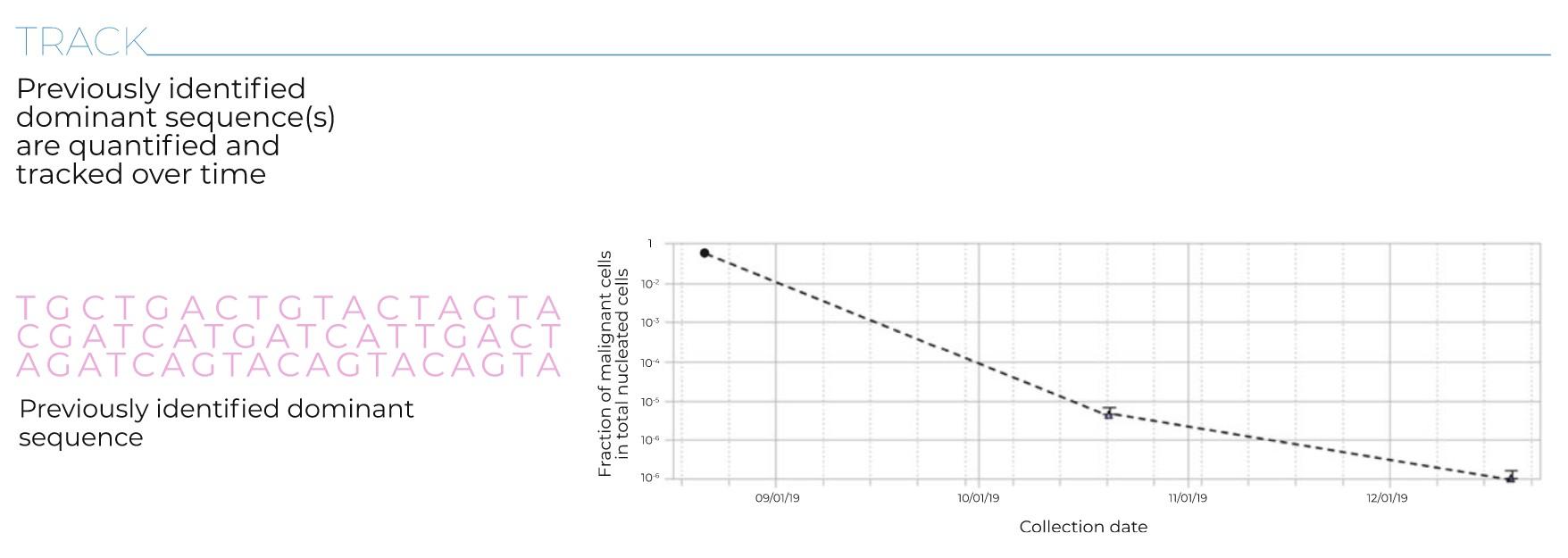
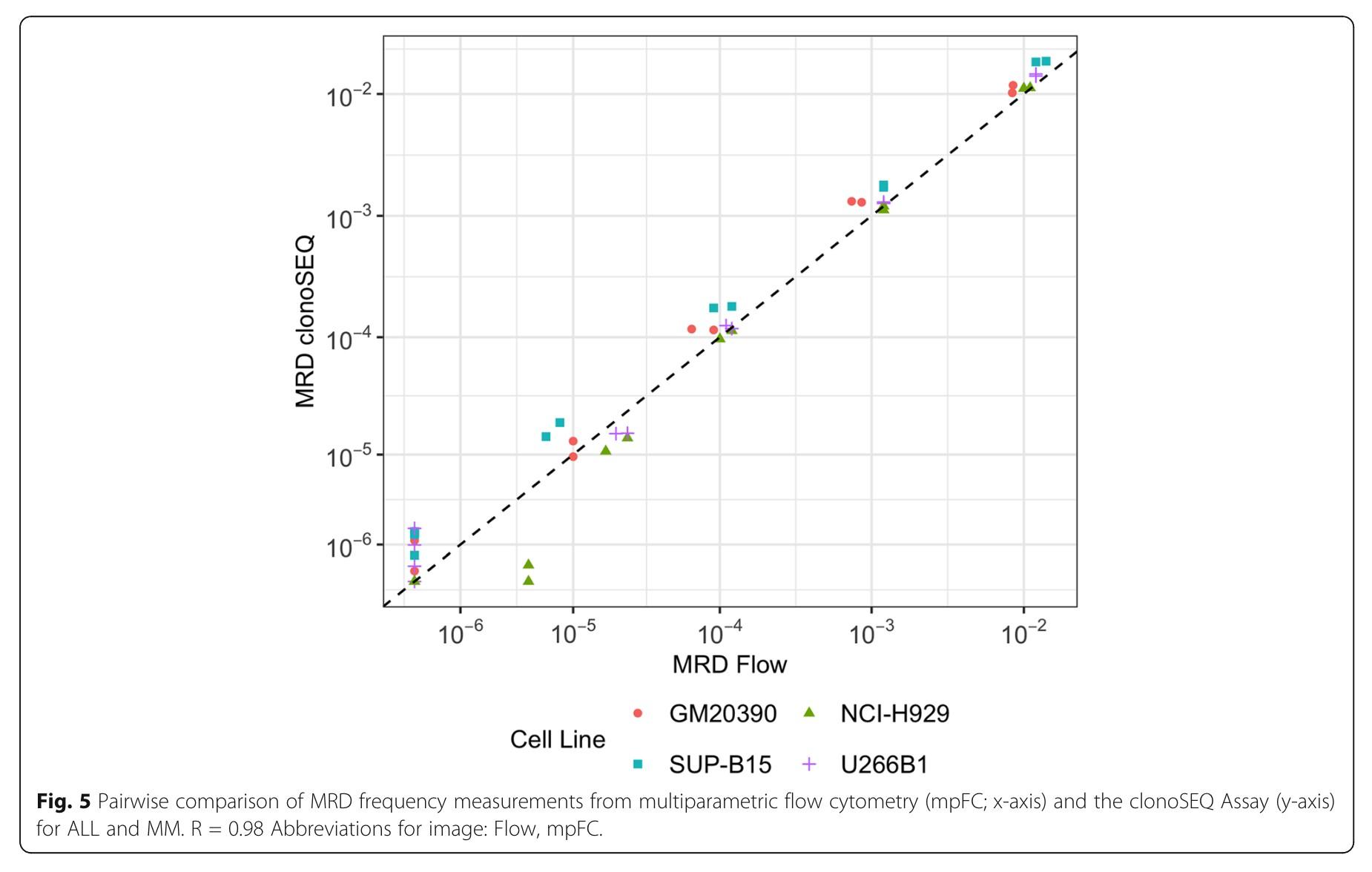
Pearson’s
MM Learning Objectives
1. Basic Biology and Epidemiology
2. Plasma Cell Targeted Therapies
3. Response Criteria and MRD
4. First Line Treatment
5. Treatment of Relapsed Disease
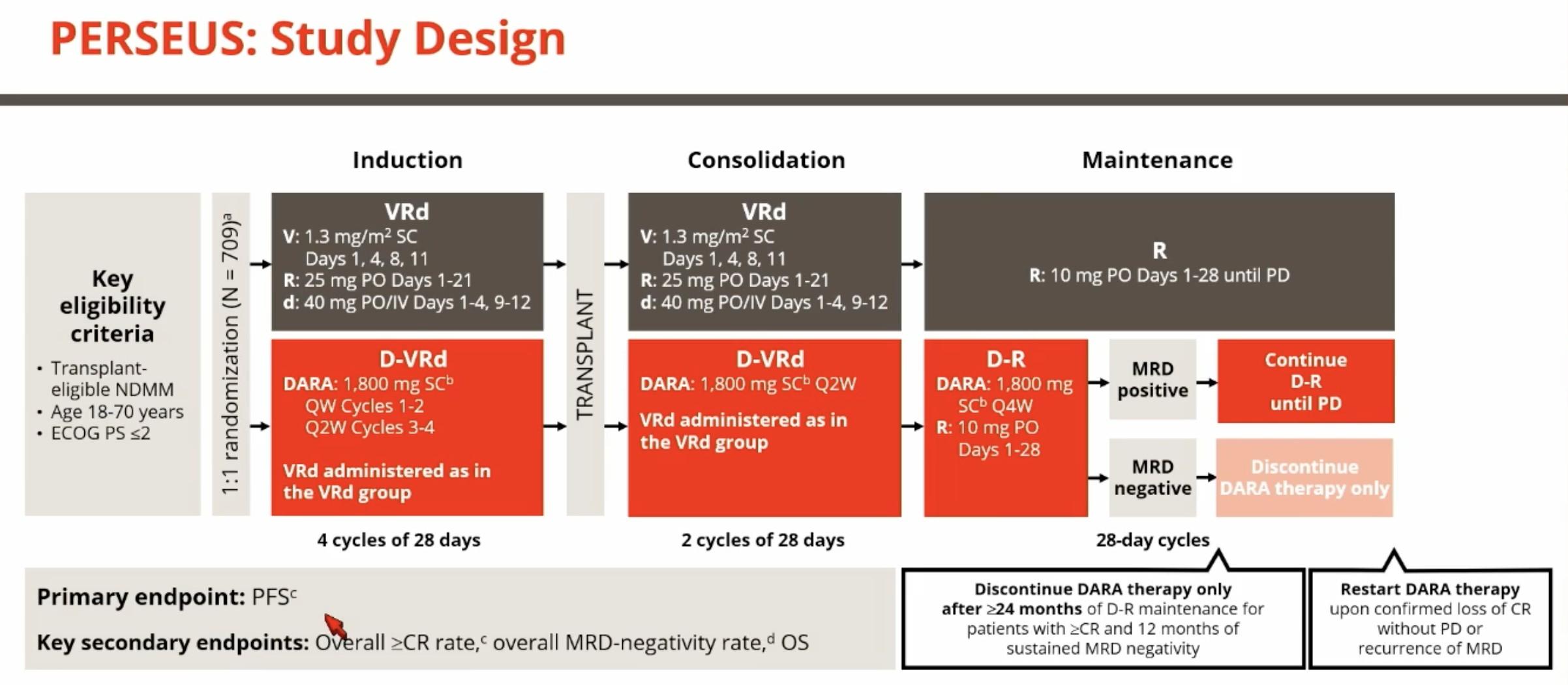
MM Learning Objectives
1. Basic Biology and Epidemiology
2. Plasma Cell Targeted Therapies
3. Response Criteria and MRD
4. First Line Treatment
5. Treatment of Relapsed Disease
Chimeric Antigen Receptor T cell (CAR T)
Therapies in MM
Abecma approved March 2021
• ORR: 73%
• Median PFS: 8.8 mo
Carvykti approved Feb 2022
• ORR: 98%
• median PFS= 27 mo, 2-yr PFS 60.5%
Munshi et al, NEJM. 2021
Martin et al, Blood. 2021
Usmani et al, ASCO Meeting. 2022
Yu B, Jiang T, & Liu D, J. Hematol. Oncol. 2020
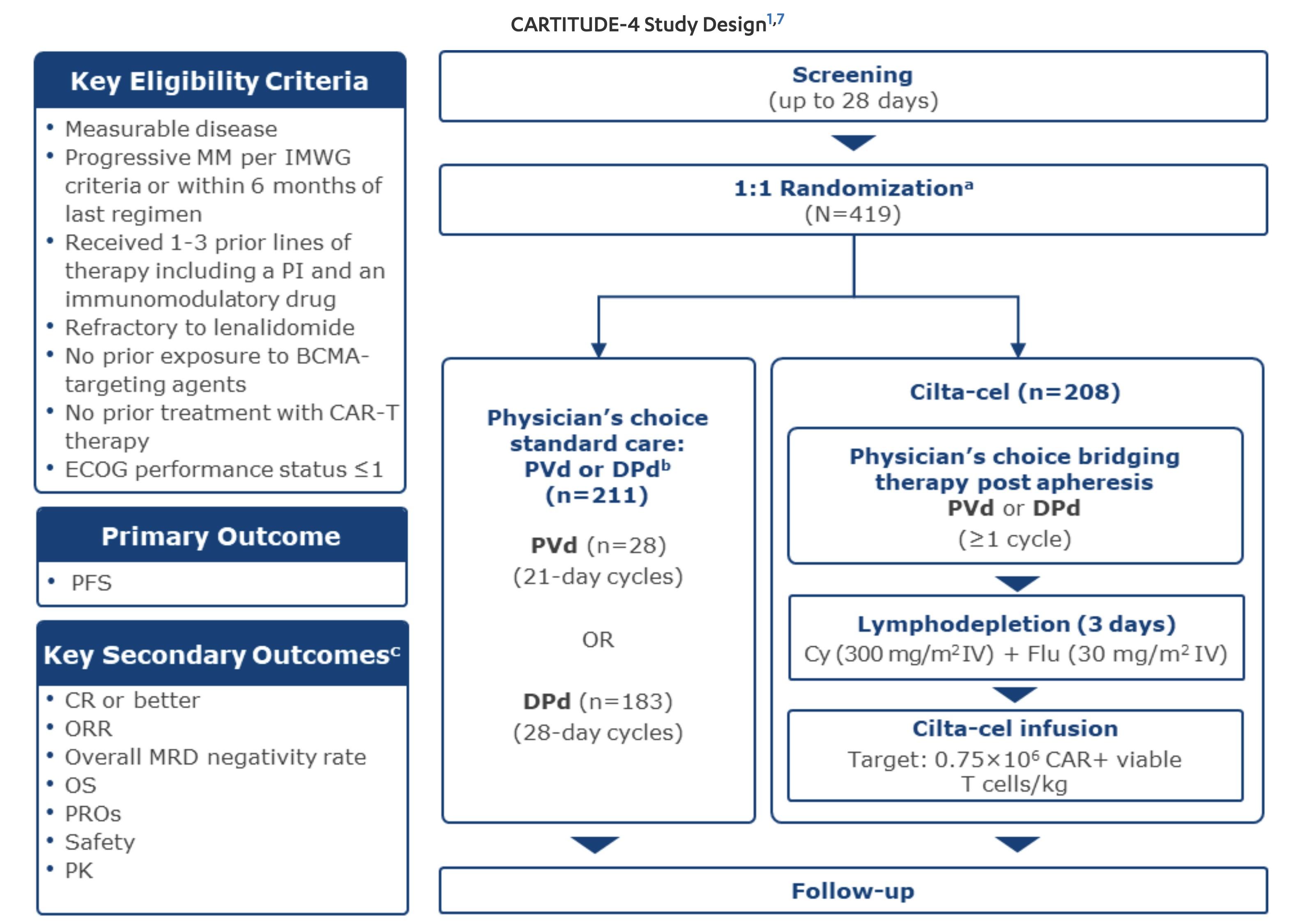
CARTITUDE-4: Cilta-Cel
Showed Superior Efficacy in Early Relapse
MM Compared to SOC Regimens
• During the first 8 weeks 22 patients died in cilta-cel arm vs 8 in SOC (before cilta-cel given)
• ORR 84.6% vs 67.3%
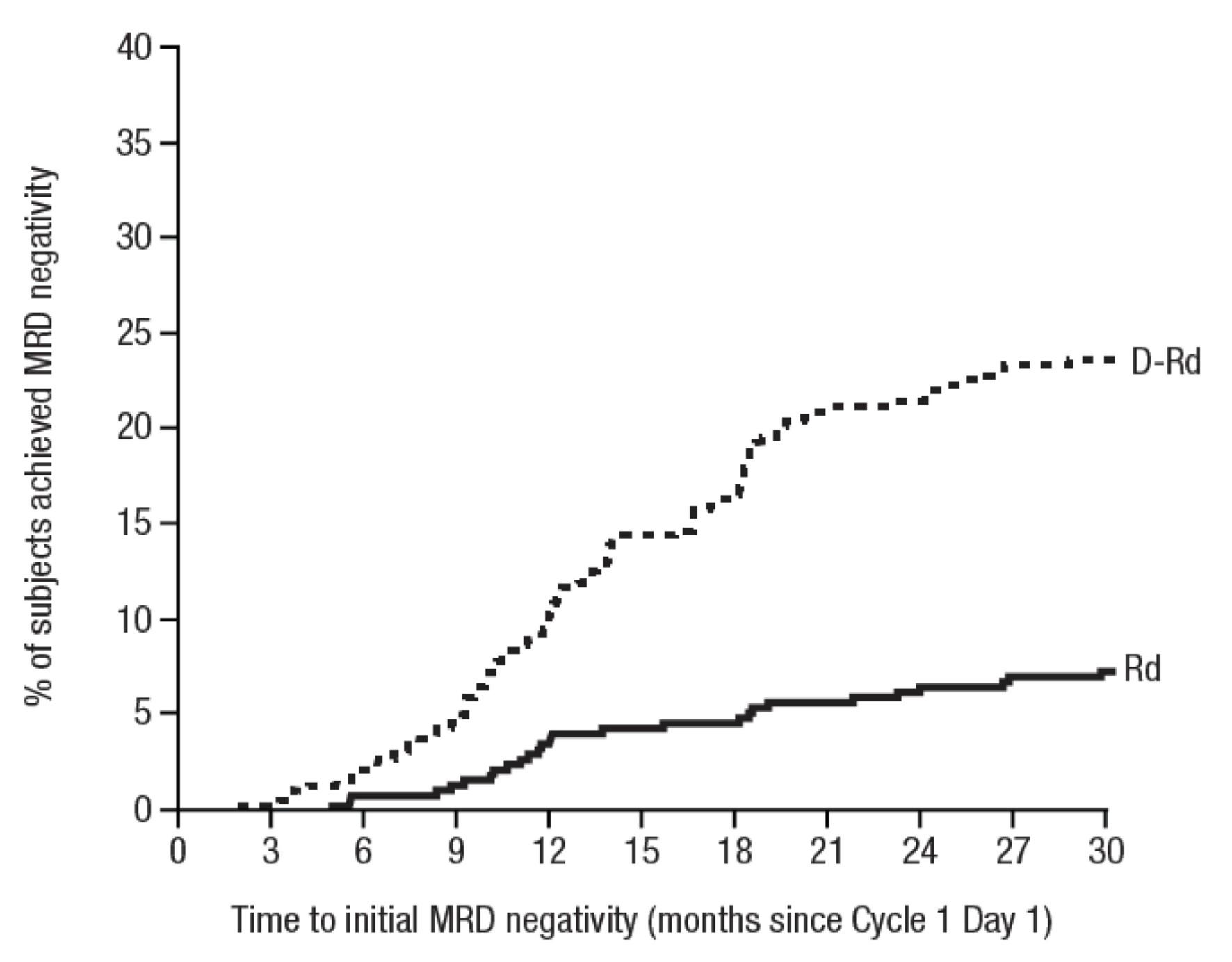
• Any time MRD 10-5 60.6% vs 15.6%
• PFS HR 0.26 for progression or death
• Updated Results* 30-month PFS 59.4% vs 25.7%
• Led to approval with one prior line April 2024

* Mateos et al. 2024 International Myeloma Society Annual Meeting; September 25-28, 2024; Rio de Janeiro, Brazil.
Abstract OA – 65.
CARTITUDE-4: Cilta-Cel
MM Compared to SOC Regimens
• NEJM Publication OS HR 0.78 at median 15.9-month follow-up
• Updated Results* OS HR, 0.55 (95% CI, 0.39-0.79) P = .000* at median 33.6-month follow-up
• Median OS for Cilta-cel still not reached
• 30-month OS 76.4%
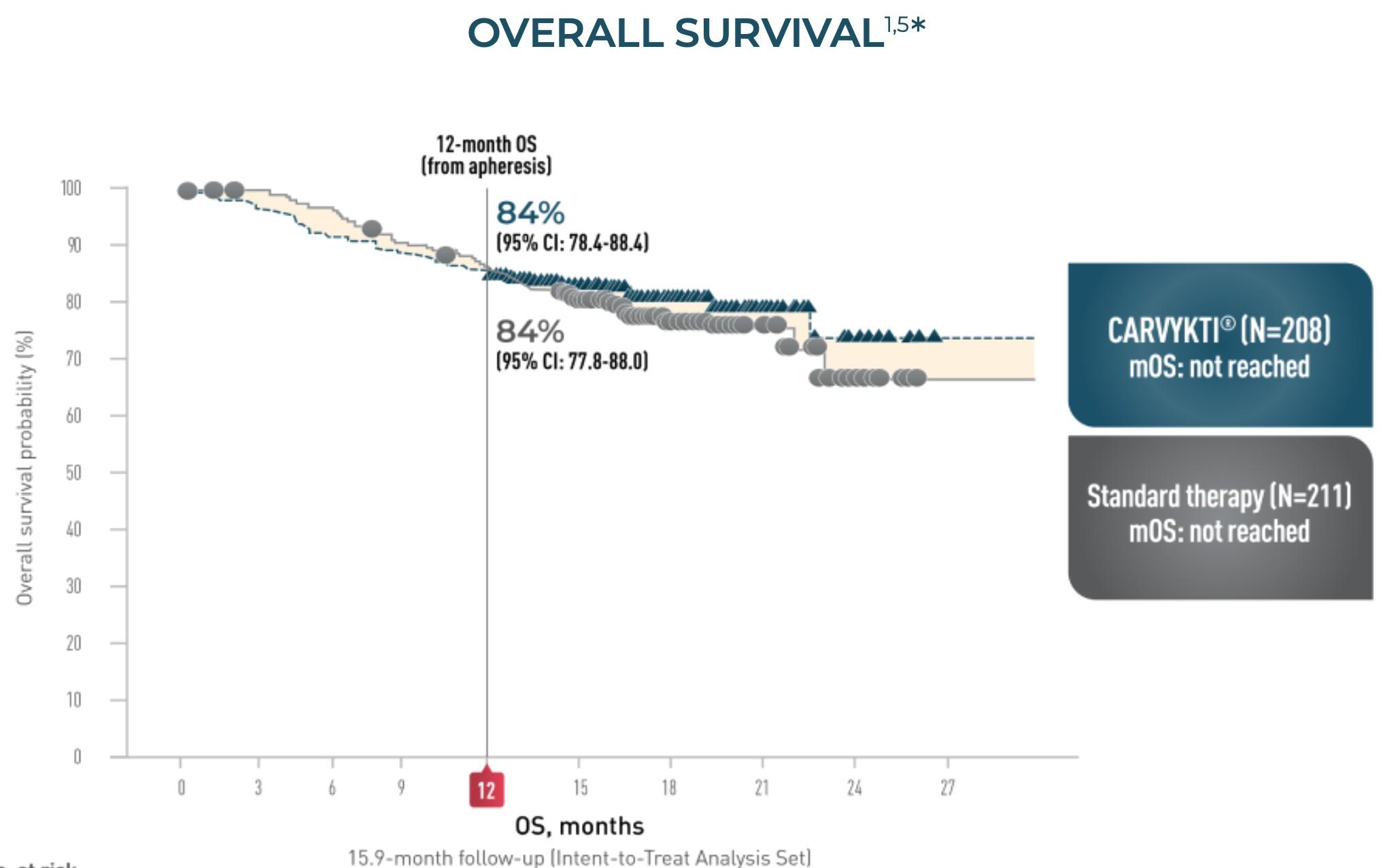
* Mateos et al. 2024 International Myeloma Society Annual Meeting; September 25-28, 2024; Rio de Janeiro, Brazil.
Abstract OA – 65.
Myeloma
Drug
Sensitivity
Testing (My-DST): Our Approach to Personalized Medicine and Biomarker Development
Patients are Treated with Multiple Lines of Therapy


MM Partner MD
• Jorge Monge
Lab Team
• Zach Walker
• Sarah Parzych
• Kady Dennis
• Lorraine Davis
• Alana Keller
Name
Leadership
• Dan Pollyea
• Craig Jordan
Clinical Team
• Nadine Martin
• Lindsey Rodriguez
• Hali Handorf
• Kelly Sidor
• Kelly O’Brien
• Jenni Tobin
• Kelsey Marciano
• Rebecca Rezac
Transplant Team
• Sarah Allen
• Megan Gager
daniel.sherbenou@cuanschutz.edu

Trial Team
• Derek Schatz
• Natalie Pfenning
• Rona Wang
• Alfonso Roque
• Meghan Straubel
• Keegan Carroll
• Phuong Ho
CAR-T Team
• Mia Etre
• Chloe Wood
• Ellen Velisek

Closing the Gap: Health Disparities in Myeloma (Video)
Joseph Mikhael, MD, MEd, FRCPC, FACP, FASCO Chief Medical Officer, International Myeloma Foundation

Advancing Treatment Options Through Clinical Trials
Forsberg, MD
Colorado Blood Cancer Institute, Denver, CO
Peter
Advancing Treatment Options Through Clinical Trials
IMF Denver Regional Community Workshop
Peter Forsberg MD Co-Director of Plasma Cell Disorders
Colorado
Blood Cancer Institute


Clinical Trials - Overview
Remember some of the important principles of clinical trials:
• The drive of research has brought us to where we are
• No one is expected to be a “guinea pig” with no potential benefit to them, there are strong checks/balances to prevent this
• Clinical research is maintained under very tight supervision and standards
• Open, clear communication between the physician and the patient is fundamental


Clinical Trials – Why Me??
• Every patient is unique and needs to be viewed that way
• Benefits of trials are numerous and include:
• Early access to “new” therapy
• Delay use of standard therapy
• Contribution to myeloma world – present and future
• Financial access to certain agents
• Must be balanced with potential risks
• “toxicity” of side effects
• Possible lack of efficacy
• Added time and effort


Overview of New Drug Development
Identify a target for therapy in the laboratory
Confirm the anticancer activity in laboratory and animal studies
Clinical trials (human studies) to determine safety, dosing and effectiveness
The whole process costs millions of dollars and years of effort!


Clinical Trials - Phases
Phase I Phase II Phase III
Tests safety Tests how well treatment works Compares new treatment to standard treatment


Phase 1 Clinical Trials
• All patients receive the experimental therapy
• Phase 1 trials find the optimal dose of a new drug or drug combination
• Patients get higher doses as the study continues
• Determine side effects of new drugs or combinations
• Explore how the drug is metabolized by the body
• Most often done in setting when few established options available


Phase 2 Clinical Trials
• Determine if a new drug or combination is effective against the cancer
• More information about side effects/safety
• May be added to a phase 1 study once the ideal dose is found
• Patients usually receive the experimental therapy
• In some cases, the study may include two (or more) “arms” comparing either different doses or combinations of the experiment therapy


Phase 3 Clinical Trials
• Highest form of clinical evidence. Typically a large number of patients are required…usually required for full FDA approval
• Patients receive either an experimental therapy (one or more drugs) or the current standard treatment
• The patient is randomly assigned to a treatment—a process called randomization
• Neither the physician or the patient can determine which treatment is given
• May be placebo controlled, if no standard treatments are available
• Very closely monitored for effectiveness and side effects


Clinical
trial study design or protocol
• Each cancer clinical trial has a written detailed study design called a protocol that includes:
• Why the clinical trial is needed
• Purpose of the clinical trial
• What drug or drug(s) are being tested, with a treatment and follow-up schedule
• Safety measures throughout the clinical trial program
• How outcomes will be measured
• Who is eligible for the clinical trial
• How the clinical trial will be organized, one site or multiple sites
• If the clinical trial is a multi-site trial, all participating physicians must follow the same protocol


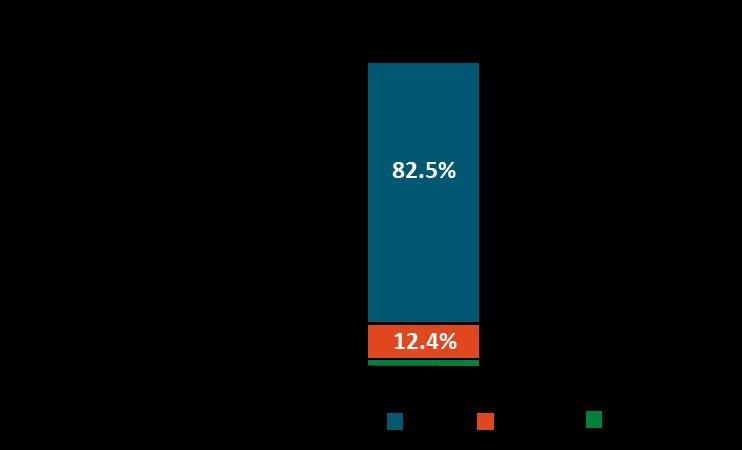
Diversity in Clinical Trials
• There has been a lack of diverse representation in clinical trials in myeloma.
• In the U.S., approximately 20% of all myeloma patients are of African descent, but only 5%–8% of patients in myeloma clinical trials are of African descent.
• This is significant for the following reasons:
• All patients of all races and ethnicities should be able to benefit from clinical trials.
• Diverse patient representation in clinical trials is required to ensure that the outcomes are applicable to all patients.
• Reasons for underrepresentation in clinical trials are complex and include accessibility of clinical trials, sensitivity to diversity by medical professionals, misconduct in medicine in the past, the lack of trust in the system, and more


Commonly Asked Questions
How does the study work? How often will I need to see my doctor or visit the cancer center?
Will I need to undergo additional tests?
What is currently known about the new drug or combination?
What benefits can I expect?
What side effects should I expect? Who should I notify if I have side effects?
Can I take my vitamins or other medications?
Can I get the treatment with my local doctor?
Will my insurance pay for my participation in the clinical trial?










Considering Entering a Clinical Trial?
• Discuss whether or not you are eligible for a clinical trial with your physician
• Work with your physician to determine the best trial for you
• Meet with the clinical research nurse or trials coordinator to discuss the trial
• Carefully review the provided “Informed Consent”
• Describes the study and any potential safety concerns related to the experimental medication




Ongoing Trials in Myeloma
SO many areas being studied right now including…
• Innovating with CAR T Cell therapy (use earlier in myeloma, new targets, faster manufacturing)
• Expanding on bispecific therapies (use in earlier lines, without hospitalization, as part of combinations, new targets) and even trispecifics
• New molecules – CelMods, antibody drug conjugates
Trials happening NOW may pave the path towards cure for many MM patients



Q&A WITH PANEL
Housekeeping Items
Presentation Slides: Are available by scanning the QR code, Instructions are on the QR code handout on each table. Program Evaluations: evaluations at the end of today.
Restrooms: Restrooms are located outside the ballroom to your right Badge Holders: Please return your badge holders and we can recycle them.

We greatly appreciate your time and feedback!


BREAK
WHEN YOU RETURN FROM BREAK
PLEASE HEAD TO YOUR SELECTED BREAKOUT SESSION:

BREAKOUT A: NDMM - GETTING STARTED WITH MYELOMA
MANAGEMENT
Dr. Daniel Sherbenou, PhD, MD
Please move to Vail Ballroom
BREAKOUT B: RRMM - CONTINUING THE MYELOMA TREATMENT JOURNEY
Dr. Peter Frosberg, MD
Please remain in this room

Thank you to our speakers & our sponsors!










Breakout A: NDMM - Getting Started with Myeloma Management
Dr. Daniel Sherbenou, PhD, MD

Newly Diagnosed: Getting Started with Myeloma Management
Dan Sherbenou, MD, PhD
6/18/2025

Upfront Quad Therapy


Image from Nisha Joseph’s ASH Presentation









Considerations During Upfront Treatment
• Anti-CD38 antibody should always be included in upfront treatment today
• Consider Levofloxacin (antibiotic) through induction for patients who are elderly or frail
• Consider “dose dense” Zometa upfront 3 monthly doses for patients with significant bone disease. Every 3 months is fine for all others. Dental clearance is needed prior to starting.
• Can consider delayed transplant in patients without high-risk disease who reach MRD-negative status pre-transplant
• Acyclovir prevention for everyone with multiple myeloma, can consider discontinuing one year after transplant, but I continue indefinitely

Breakout B
RRMM: Continuing the Myeloma Treatment Journey
Peter Forsberg, MD
Colorado Blood Cancer Institute, Denver, CO
Relapsed/Refractory Multiple Myeloma: Continuing the Myeloma Treatment Journey
IMF Denver Regional Community Workshop
Peter Forsberg MD Co-Director of Plasma Cell Disorders
Colorado Blood Cancer Institute


Disclosures
• Consultant – J&J, BMS, GSK, Sanofi, Kite, Pfizer
• Research Support – J&J, Pfizer, Abbvie, Kite, Astrazeneca, BMS, GSK


Lets start with some definitions
• Relapsed myeloma-
• Myeloma that becomes active after prior therapy
• Refractory myeloma-
• Myeloma that fails to respond to therapy or becomes active during therapy after initial control
• You can be refractory to certain therapies while still having multiple viable treatment options (even in the same drug class)
• You can receive a treatment but if it’s stopped before myeloma progresses we wouldn’t consider you refractory to it (we call this exposed)


Multiple Myeloma is Not One Disease!
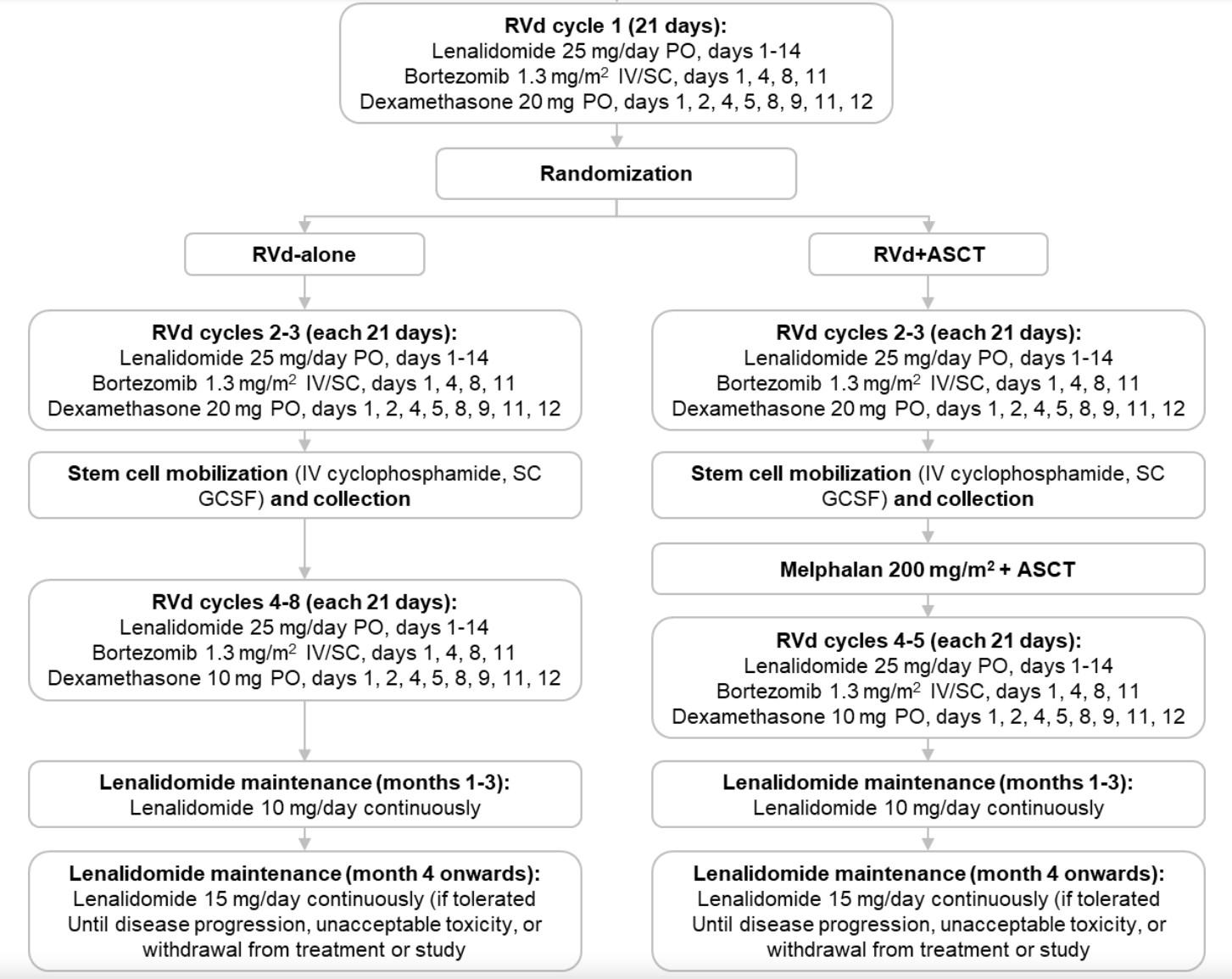
RVD+ASCT+Lenalidomide Maintenance
Relapse 2

Relapse 1
Relapse 3
Relapse 2
Relapse 1
Relapse 1
Time: Years!

Slide stolen from Joe Mikhael
How about a few more definitions
• We categorize certain groups of drugs that act in similar ways to target MM as “classes”
• These include IMiDs (eg Revlimid, Pomalyst), proteasome inhibitors (eg Velcade, Kyprolis), monoclonal antibodies (eg Darzylex, Sarclisa)
• If you have received at least one IMiD, one PI and one antibody you’d be considered ”triple class exposed”
• If your myeloma has progressed while receiving at least one IMiD, one PI and one antibody you’d be considered “triple class refractory”
• A “line of therapy” is a treatment/combination or planned sequence of treatments to target myeloma


Early lines treatment are important!
1st Relapse 2nd 3rd 4th 5th and beyond
Fewer patient are eligible for therapy in each subsequent line of therapy (LOT)

adapted from: Yong, K et al. Br J Haematol 2016;175(2):252-264

Figure
MM Therapy, the Modern Era
Early 2000-2013: First wave of targeted therapy

Expansion of Monoclonal Antibodies

Myeloma Therapies in 2010
IMiDs
Proteasome inhibitors
Conventional chemotherap y
Other stuff


Lenalidomide (Revlimid)
Thalidomide
Bortezomib (Velcade)
Melphalan
Cytoxan
Bendamustine
Doxil
Clarithromycin (Biaxin)
Myeloma Therapies in 2015
IMiDs
Proteasome inhibitors
Monoclonal antibodies
Conventional chemotherap
Other stuff
Lenalidomide (Revlimid)
Bortezomib (Velcade)
Daratumumab (Darzylex)
Clarithromycin (Biaxin)
Pomalidomide (Pomalyst)
Ixazomib (Ninlaro)
Thalidomide

Carfilzomib (Kyprolis)
Elotuzumab (Empliciti)
Panobinostat (Farydak)

Cytoxan
Bendamustine
Doxil
Myeloma Therapies in 2025
IMiDs/Cereblon
Modifiers
Proteasome inhibitors
Monoclonal antibodies
CAR-Ts and Bispecifics
Conventional chemotherapy
Lenalidomide (Revlimid)
Bortezomib (Velcade)
Daratumumab (Darzylex)
Pomalidomide (Pomalyst)
Ixazomib (Ninlaro)
Elotuzumab (Empliciti)
Thalidomide
Carfilzomib (Kyprolis)
Isatuximab (Sarclisa)
Ida-Cel (Abecma)
Cilta-Cel (Karvykti)
Teclistamab (Tecvayli)
Elranatamab (Elrexfio)
Talquetamab (Talvey)

Other stuff
Melphalan
Belantamab Mafadotin (Blenrep)
Melflufan
Selinexor (Xpovio)
Cytoxan
Bendamustine
Doxil
Venetoclax (t11;14)
Panobinostat (Farydak)

Core MM Medicines- IMiDs
• Lenalidomide/Pomalidomide/Thalidomide
• Oral, targeted therapy
• Pomalidomide can be effective after lenalidomide (was developed this way), limited data on lenalidomide after pomalidomide
• Most patients receive lenalidomide in first line of MM therapy, by second line most patients have taken for extended period of time

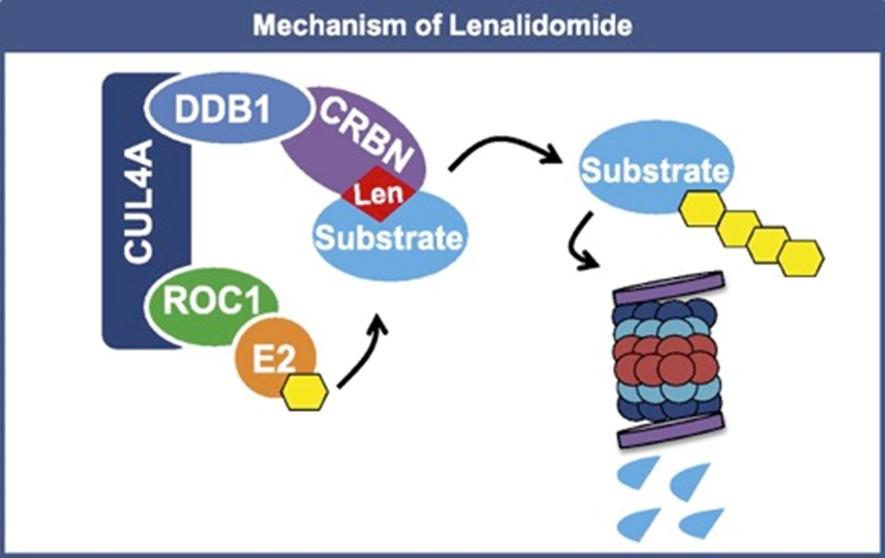

Core MM Medicines- Proteasome
Inhibitors
• Bortezomib (velcade), Carfilzomib (kyprolis)
• IV (bortezomib), subcutaneous (carfilzomib)
• Most often bortezomib used in newly diagnosed MM, carfilzomib after relapse
• Different side effects- neuropathy with velcade, cardiovascular with carfilz
• Carfilzomib likely more potent than velcade


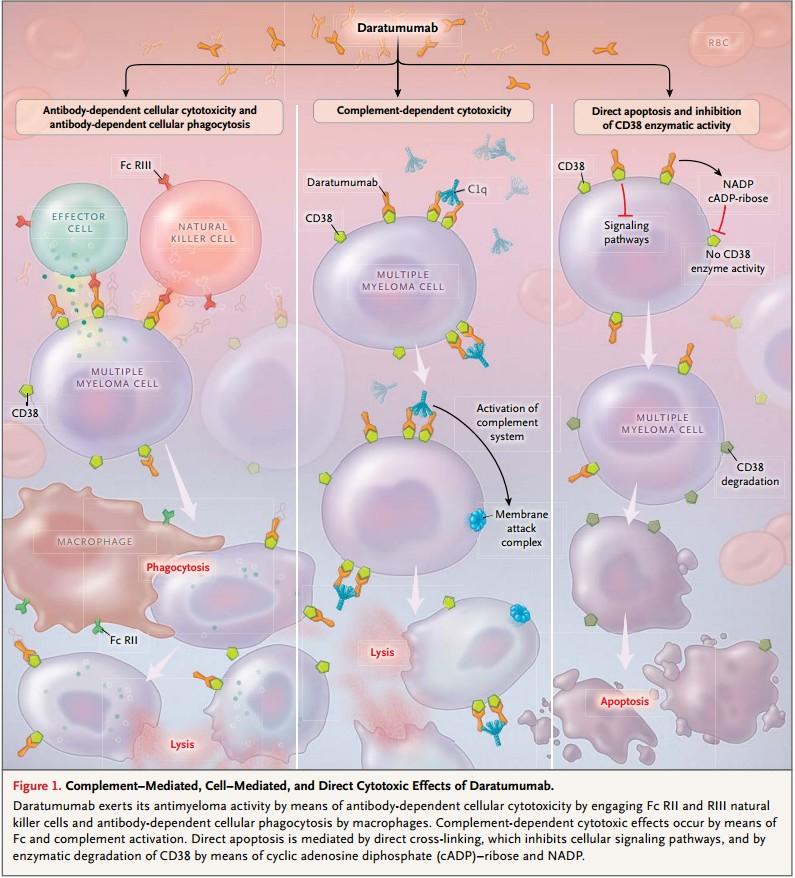
Core MM Medicines- Monoclonal antibodies
• CD38 targeting: Daratumumab (darzylex), Isatuximab (sarclisa)
• Would term these “Naked” antibodies
• Different than anti-body drug conjugate, bispecifics, etc
• Now used very consistently for newly diagnosed MM
• Good partners, limited side effects/quality of life impact
• Dara and Isa have very similar activity, if resistance to one develops likely resistant to other
• May redevelop some sensitivity with time off


Notable Phase 3 Combination Trials in RRMM


Some General Historical Principals of
Therapy in 2nd/3rd line setting
• We can combine any IMiDs, PI, CD38 monoclonal antibody and a few other things (low dose chemo, selinexor) with dex into a reasonable 3 drug combination
• Until a few years ago RRMM therapy largely meant 3 drug combos indefinitely
• Certain combos (Dara/Isa+Carfilzomib+Dex) may have longer average MM control but with more associated treatment visits over time
• ↑↑ efficacy ↓↓ convenience
• Other combos (Dara+Pomalidomide+Dex) have fewer treatments over time but shorter average MM control
• ↑↑ efficacy ↓↓ convenience
• What combos make sense depend on prior treatment, other medical issues, patient preference, etc


Where do we stand in RRMM in 2025?
• Primary question is no longer: Which combination therapy do we prefer?
• Now the primary question is: Should we pursue CAR-T in 2nd (or 3rd) line or not
• With each line of therapy scale tips more firmly towards T cell based immunotherapy


The Immunotherapy Revolution in MM is
Underway
Antibody–Drug Conjugates
Cytotoxic Payload

T-Cells
Antibodies or T-Cell Engagers
Cytotoxic Cytokines
Bispecific T-cell Engager
Antibody

Current CAR-Ts in MM
• T-cells engineered to target something on the surface of cancer cells
• Abecma: First CAR-T approved for use in relapsed MM in 2021
• Carvykti: Second approval in 2022
• Both initially approved for use in patients with 4 prior lines of therapy
• Mar 2024: Abecma approved in patients with patients with 2 prior lines of therapy
• April 2024: Carvykti approved in patients with 1 prior line of therapy

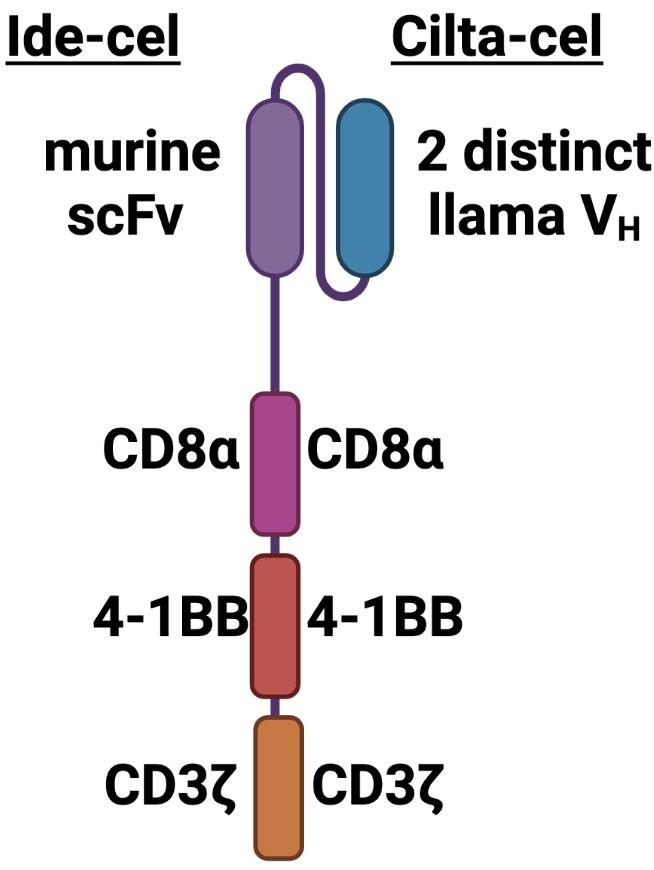
BCMA-specific extracellular scFv-targeting domain with 4-1BB co-stim and CD3ζ signaling domains
Contains 2 BCMA-targeting single-chain antibody designed to confer avidity

How does CAR-T work?
Leukapheresis Manufacturing Infusion
Collect patient’s white blood cells
Isolate and activate T-cells Engineer T-cells with CAR gene
Tumor cell

Targeting element (eg, CD19, BCMA)
Expand CAR T-cells Infuse same patient with CAR T-cells
Spacer
Transmembrane domain



Viral vector with CAR DNA CARengineered T-cell
Costimulatory domain (eg, CD28 or 4-1BB) CD3 (essential �� signaling domain)
Median manufacturing time: 17-28 days

















Patients undergo lymphodepleting (and possibly salvage/bridging) therapy

Majors. EHA 2018. Abstr PS1156. Lim. Cell. 2017;168:724. Sadelain. Nat Rev Cancer. 2003;3:35. Brentjens. Nat Med. 2003;9:279. Park. ASH 2015. Abstr 682. Axicabtagene ciloleucel PI. Tisagenlecleucel PI.


CAR T-Cell Therapy Patient Journey







CAR T: Expected Toxicities

Cytokine release syndrome (CRS) Neurotoxicity (ICANS)

Cytopenias

Infections

CRS ICANS
Onset 1 9 days after CAR T-cell infusion 2 9 days after CAR T-cell infusion
Duration 5 11 days 3 17 days
Symptoms
• Fever
• Difficulty breathing
• Dizziness
• Nausea
• Headache
• Rapid heartbeat
• Low blood pressure
• Headache
• Confusion
• Language disturbance
• Seizures
• Delirium
• Cerebral edema
Management
• Actemra (tocilizumab)
• Corticosteroids
• Supportive care
• Antiseizure medications
• Corticosteroids
*Based on the ASTCT consensus; †Based on vasopressor; ‡For adults and children >12 years; §For children ≤12 years; Only when concurrent with CRS
Xiao X et al. J Exp Clin Cancer Res. 2021;40(1):367. Lee DW et al. Biol Blood Marrow
Transplant. 2019;25:625; Shah N et al. J Immunother Cancer. 2020;8:e000734.

Late Line Carvykti: CARTITUDE-1
Trial

Most exciting data of 2023
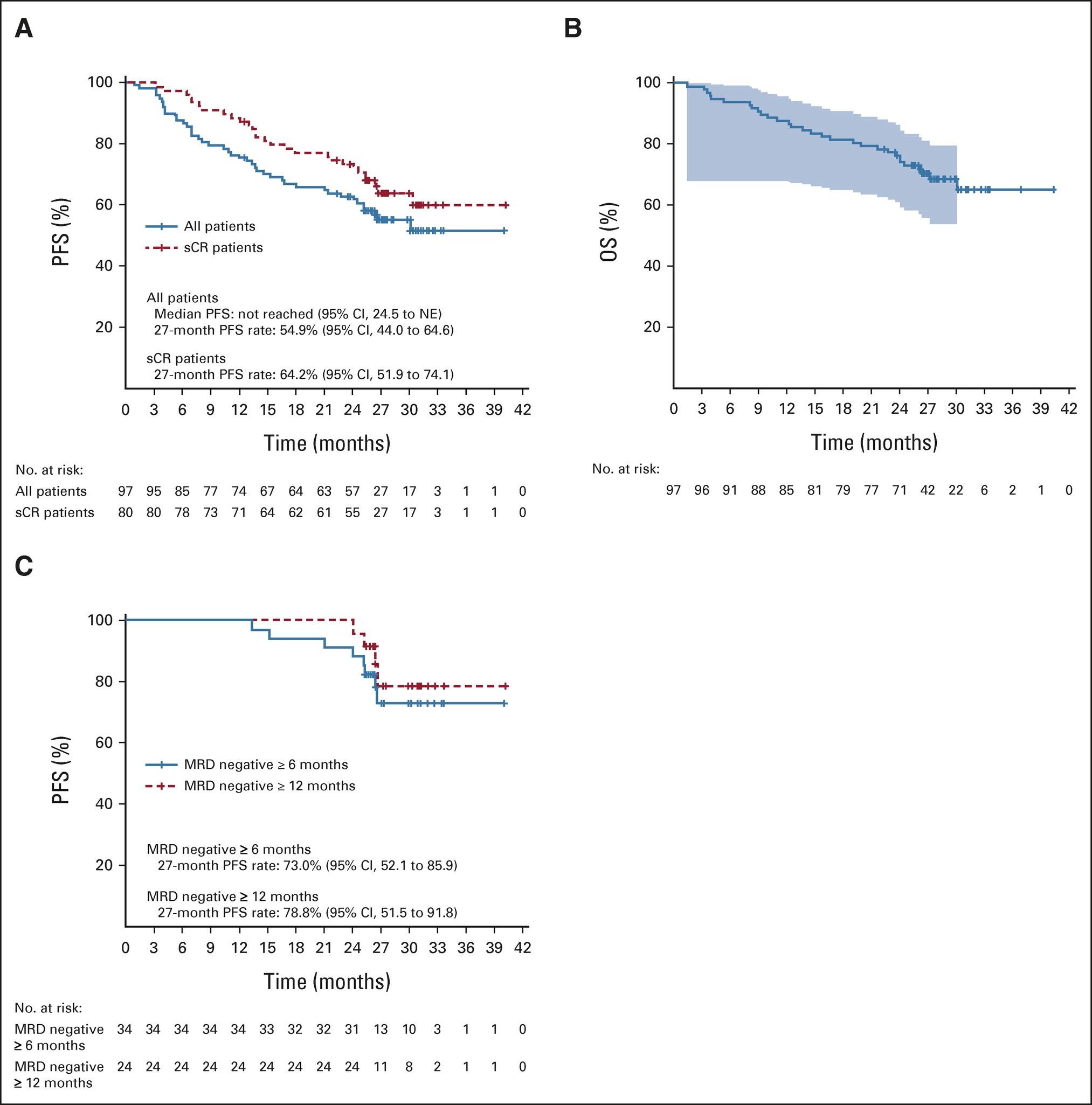
CARTITUDE-1 Long Term Follow-up
• The most exciting data of 2025
• Of 97 patients treated 32 with ongoing 5+ year remissions
• Average 6 prior therapies
• Median overall survival 60 months


Early Line Carvykti: CARTITUDE-4
Trial
• Carvykti vs standard of care in MM with 1-3 prior lines of therapy
• Lenaldiodmide refractory
• 15% triple refractory
• Comparison options: Pom/Vel/dex, Dara/Pom/dex

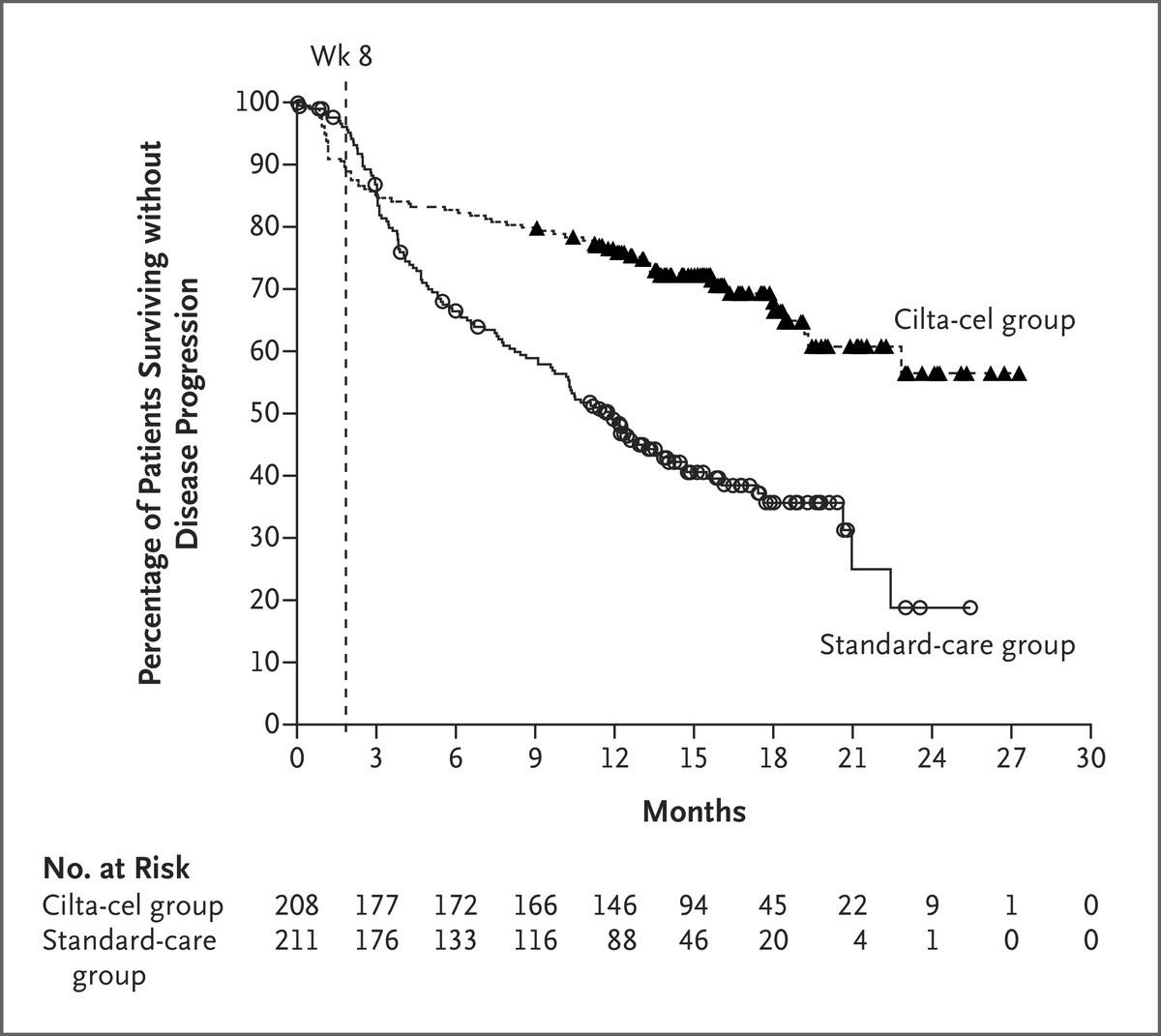

CARTITUDE-4 Safety
- Similar rates of infection, grade 3+ infection - 66% IVIg use in CAR arm - One primary concern with Carvykti is atypical neurological toxicity - Almost 5% on CARTITUDE-1 with late-onset movement or neurocognitive issues
- <1% in CARTITUDE-4 - 9% cranial nerve palsy
- Similar rate of secondary malignancy in both arms- So far

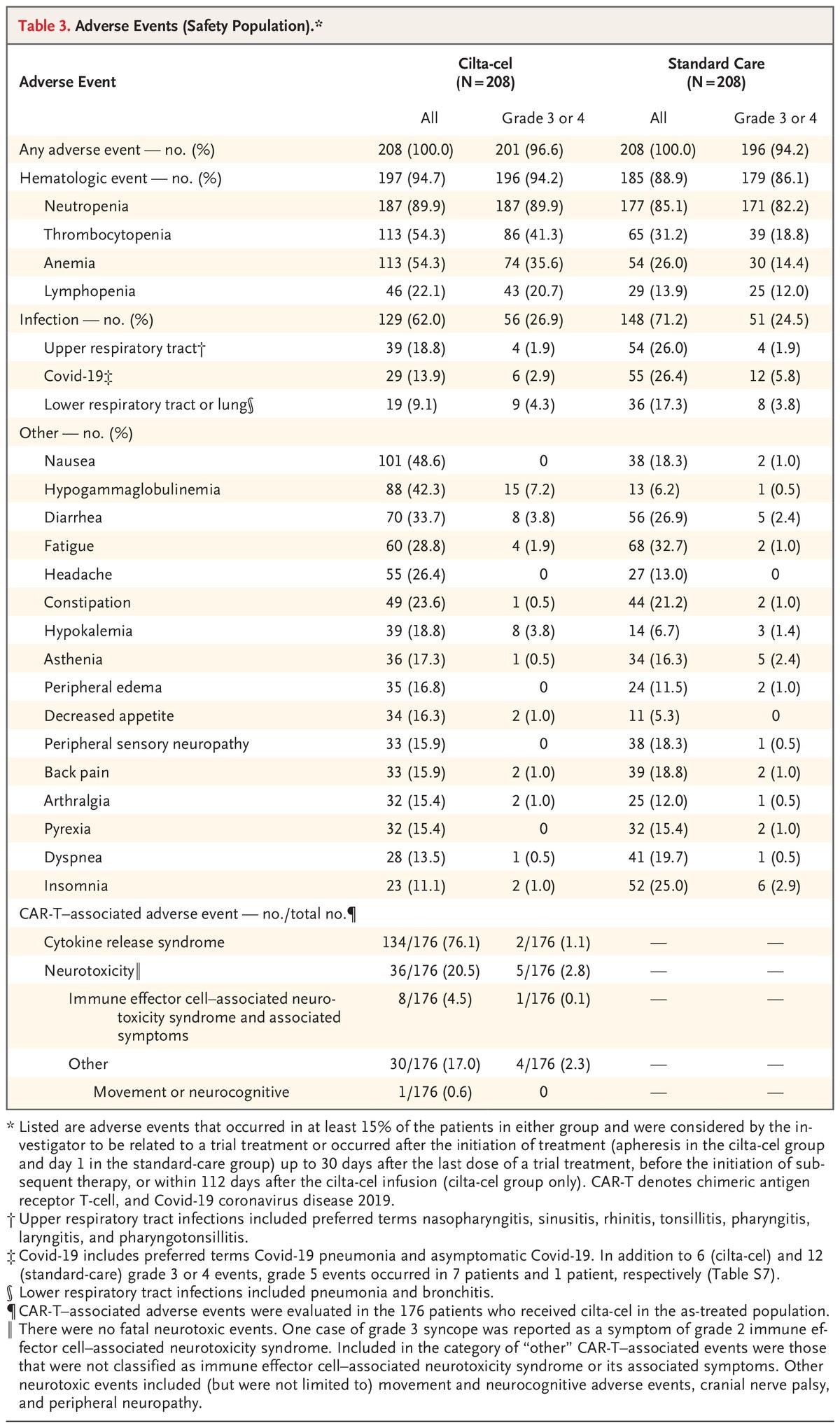




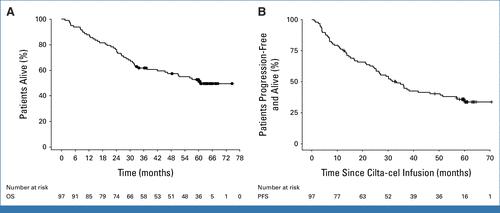
Overall Survival With Ciltacabtagene Autoleucel Versus Standard of Care in Lenalidomide-Refractory Multiple Myeloma:
Phase 3 CARTITUDE-4
Study Update
https://www.congresshub.com/Oncology/ IMS2024/Cilta-cel/Mateos-Cilta
1University Hospital of Salamanca/IBSAL/CIC/CIBERONC, Salamanca, Spain; 2Cancer Center Clinica Universidad Navarra, CIMA, IDISNA, Pamplona, Spain; 3Medical College of Wisconsin, Milwaukee, WI, USA; 4Centre Hospitalier Universitaire de Nantes, Nantes, France; 5CHU Poitiers, Poitiers, France; 6Amsterdam University Medical Center, Vrije Universiteit Amsterdam, Amsterdam, Netherlands; 7Stanford University School of Medicine, Stanford, CA, USA; 8Institut Català d’Oncologia and Institut Josep Carreras, Hospital Germans Trias i Pujol, Badalona, Barcelona, Spain; 9Tel Aviv Sourasky (Ichilov) Medical Center, Faculty of Medical & Health Sciences, Tel Aviv University, Tel Aviv, Israel; 10Peter MacCallum Cancer Centre, Melbourne and Royal Melbourne Hospital, Melbourne, Australia; 11Translation Laboratory, Centre of Excellence in Cellular Immunotherapy, Peter MacCallum Cancer Centre, Melbourne, Australia; 12Sir Peter MacCallum Department of Oncology, University of Melbourne, Parkville, Australia; 13Universitätsklinikum Würzburg, Medizinische Klinik und Poliklinik II, Würzburg, Germany; 14Fondazione IRCCS Istituto Nazionale dei Tumori Milano, University of Milano, Italy; 15Janssen Research & Development, Shanghai, China; 16Janssen Research & Development, Apex, NC, USA; 17Janssen Research & Development, Spring House, PA, USA; 18Cilag GmbH International, Zug, Switzerland; 19Janssen, Buenos Aires, Argentina; 20Janssen Research & Development, Beerse, Belgium; 21Janssen Research & Development, Neuss, Germany; 22Janssen Research & Development, Raritan, NJ, USA; 23Legend Biotech USA Inc., Somerset, NJ, USA; 24Royal Prince Alfred Hospital and University of Sydney, Sydney, Australia; 25University College London Hospitals, NHS Foundation Trust, London, UK



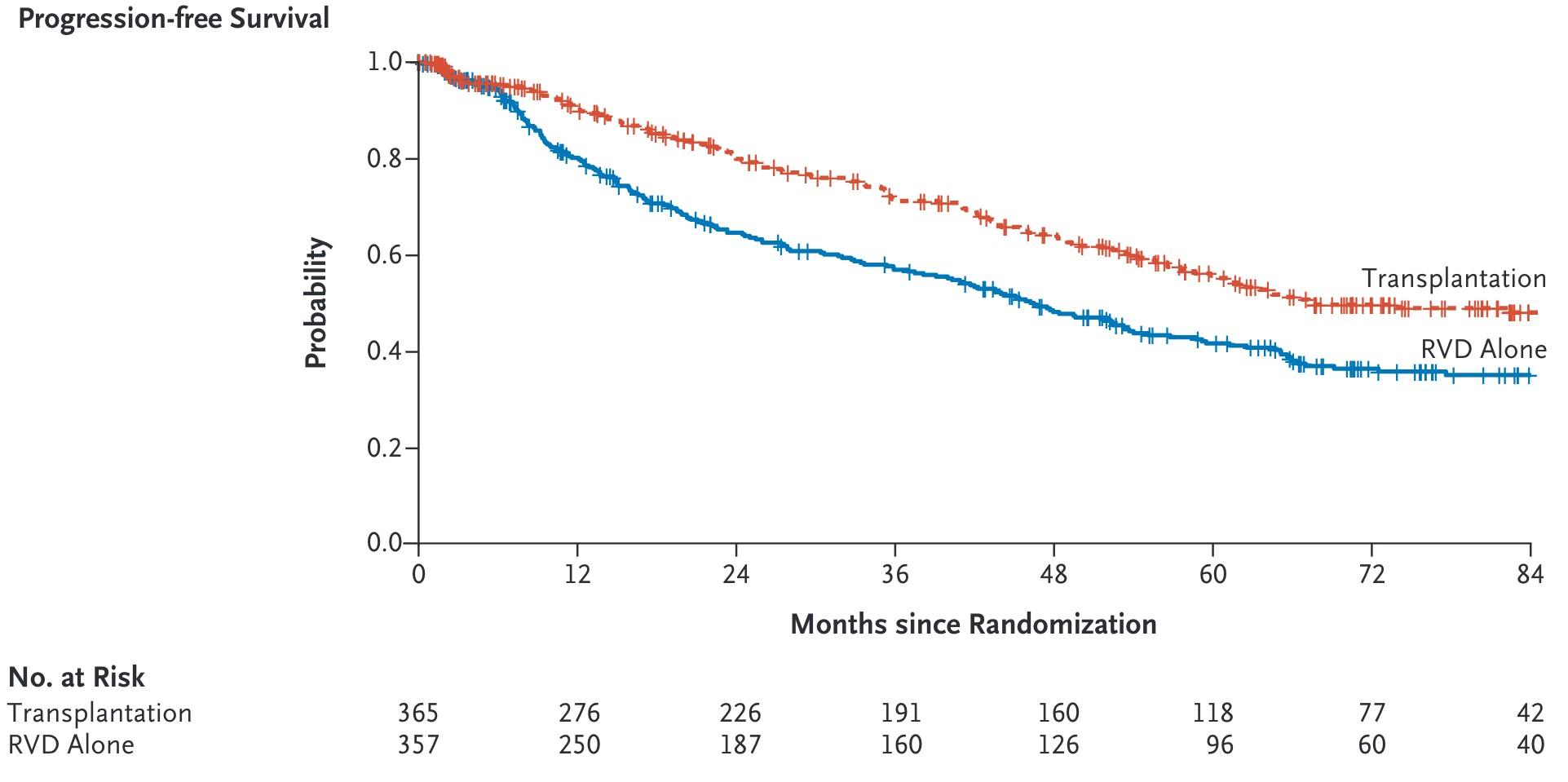
Long-Term CARTITUDE-4 Update (34 Months):
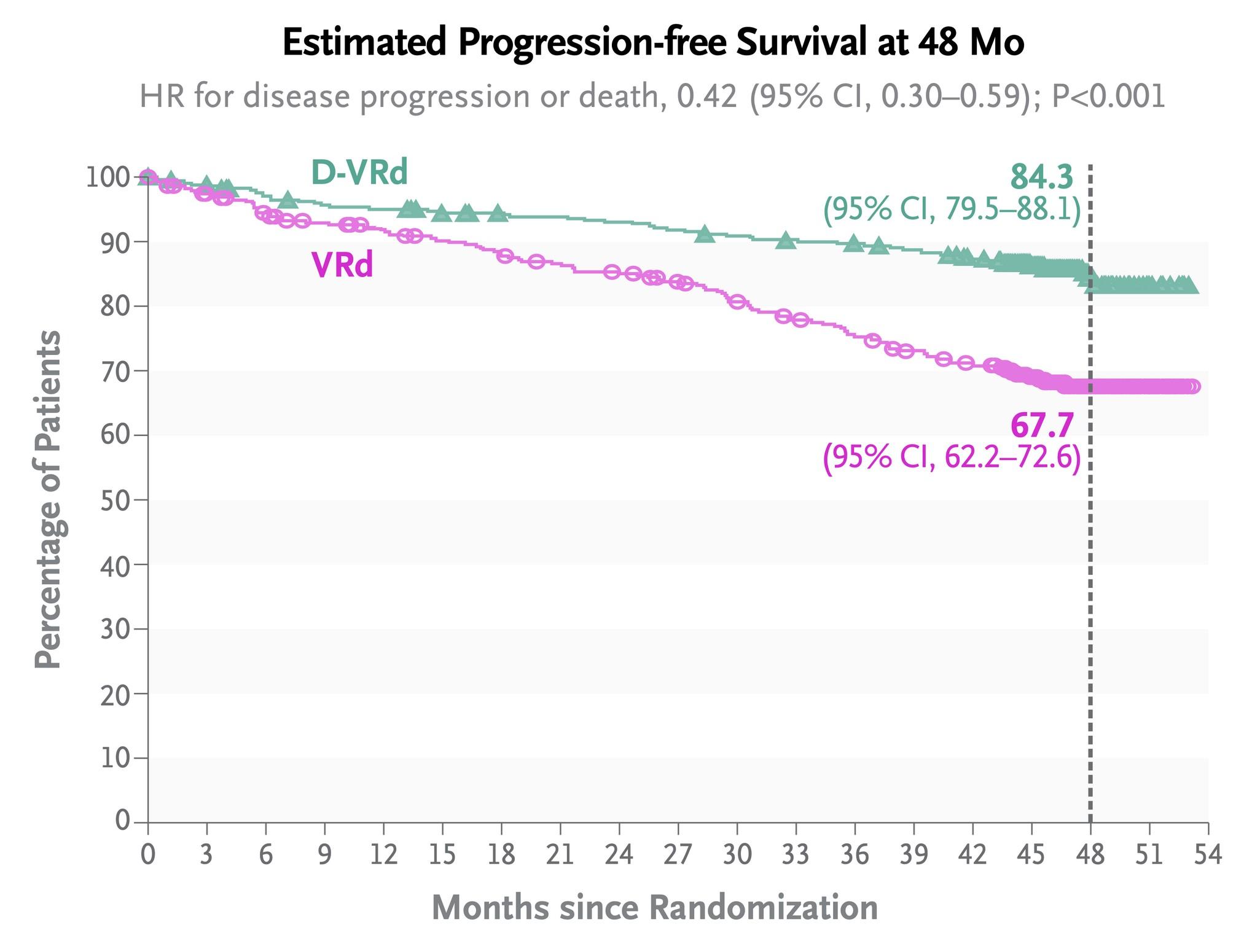
Cilta-cel Maintained Significant Improvement in Progression-Free Survival





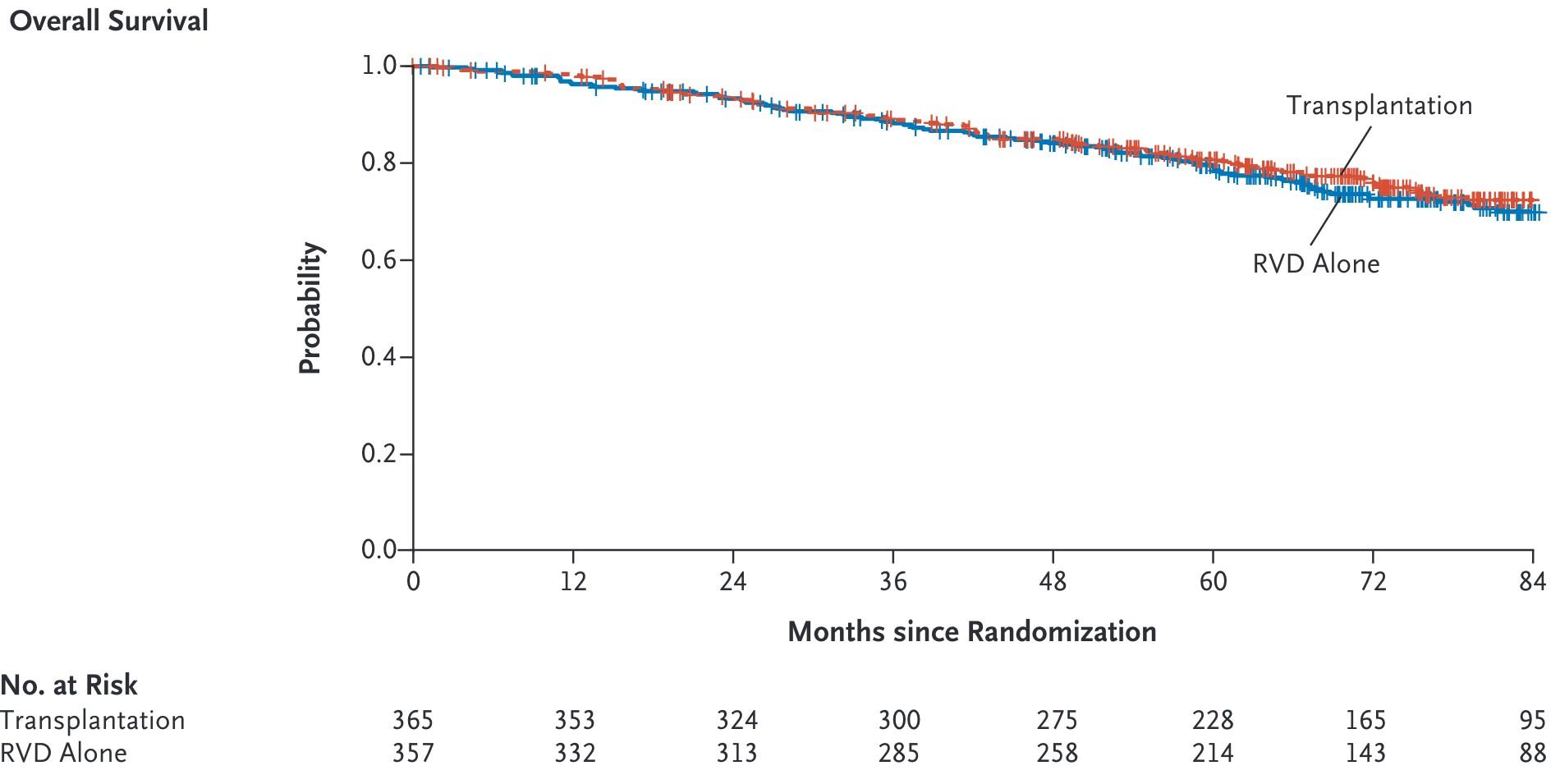
Long-Term CARTITUDE-4 Update (34 Months):
Cilta-cel Significantly Improved Overall Survival
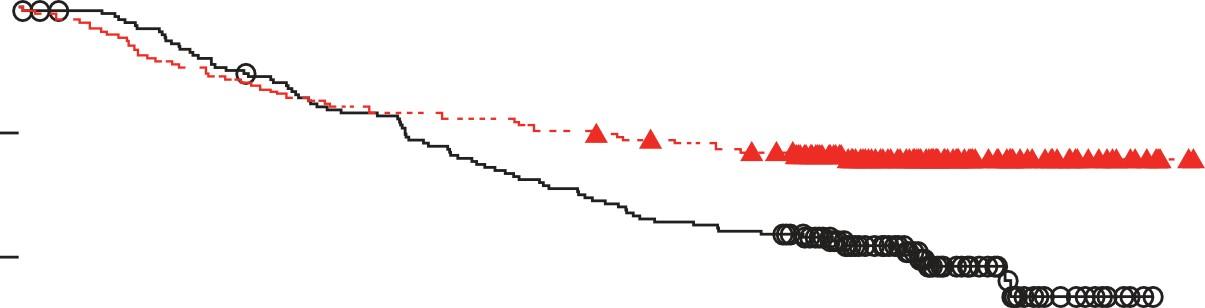






Long-Term CARTITUDE-4 Update (34 Months):
Consistent Overall Survival Benefit for Cilta-cel Across Prespecified
Subgroups
Cytogenetic risk at study entry
High riskd
Any of 4 markers abnormal
At least 2 of 4 markers abnormal
Excl. gain/amp(1q)
Standard risk
Refractory to PI + IMiD
Anti-CD38 + IMiD
PI + anti-CD38 + IMiD
Last line of prior therapy
Prior exposure to
Daratumumab
Bortezomib
Bortezomib and daratumumab
Daratumumab naive




If
we have positive phase
3 trials why not CAR-T for most in 2nd line?
• CAR obviously comes with baggage
• Cumbersome/complex
• Risk for neurotoxicity and secondary malignancy
• Important consideration but current CAR options may not fit needs for a significant portion of 2nd line patients
• So who should we prioritize for 2nd line CAR?


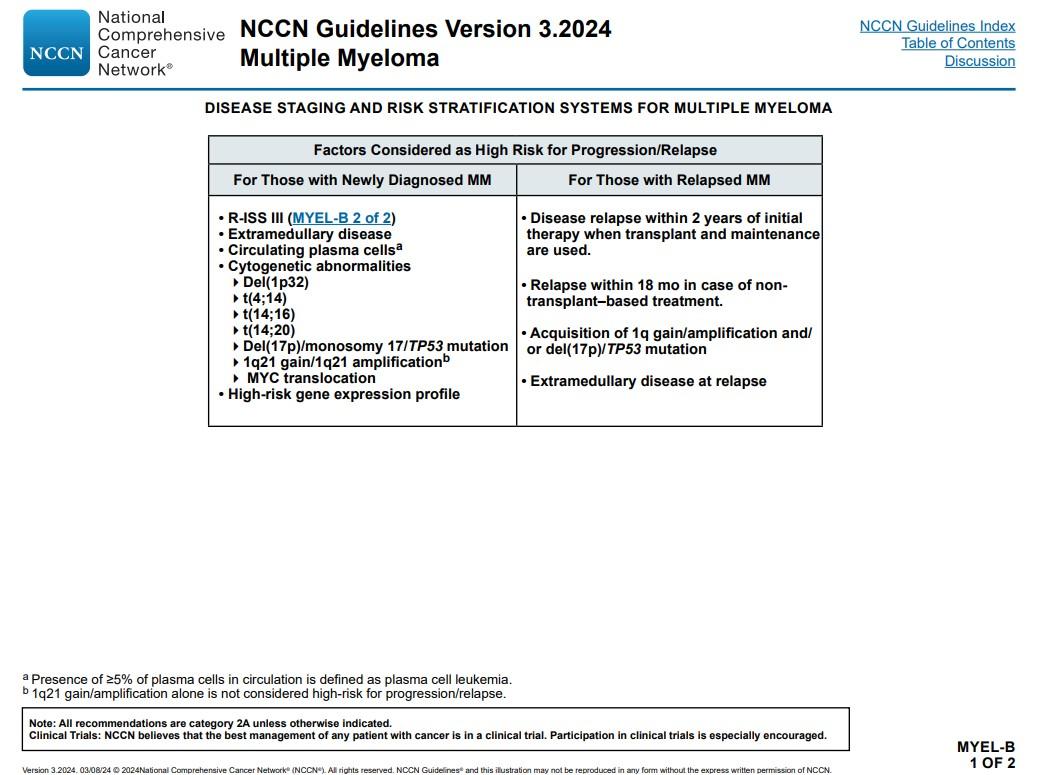
Functional high-risk MM was defined as progressive disease ≤18 months after receiving autologous stem cell transplant (ASCT) or the start of initial frontline therapy in patients with no ASCT
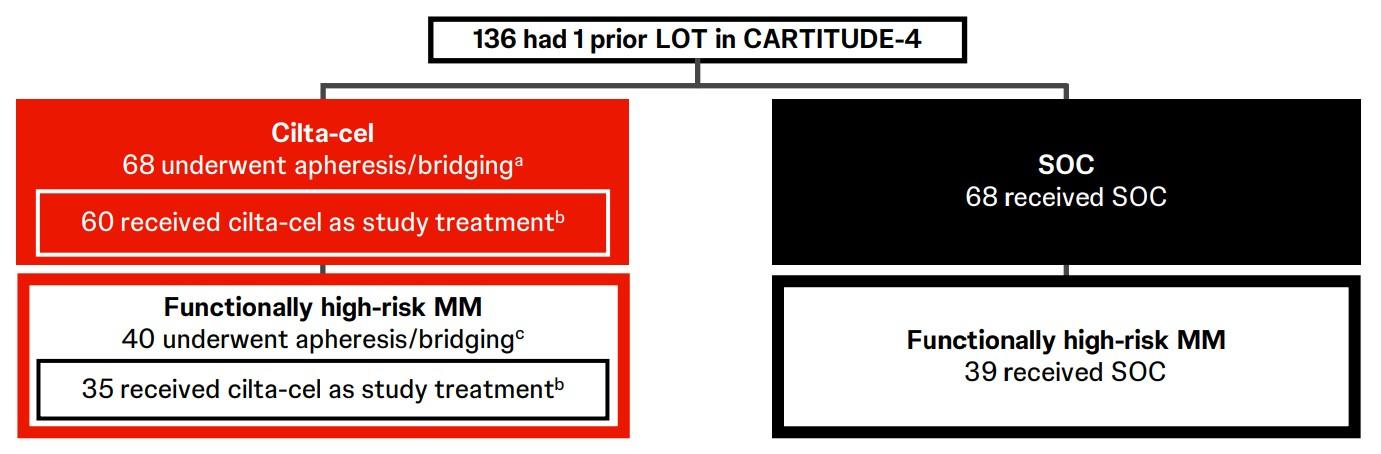
CARTITUDE-4
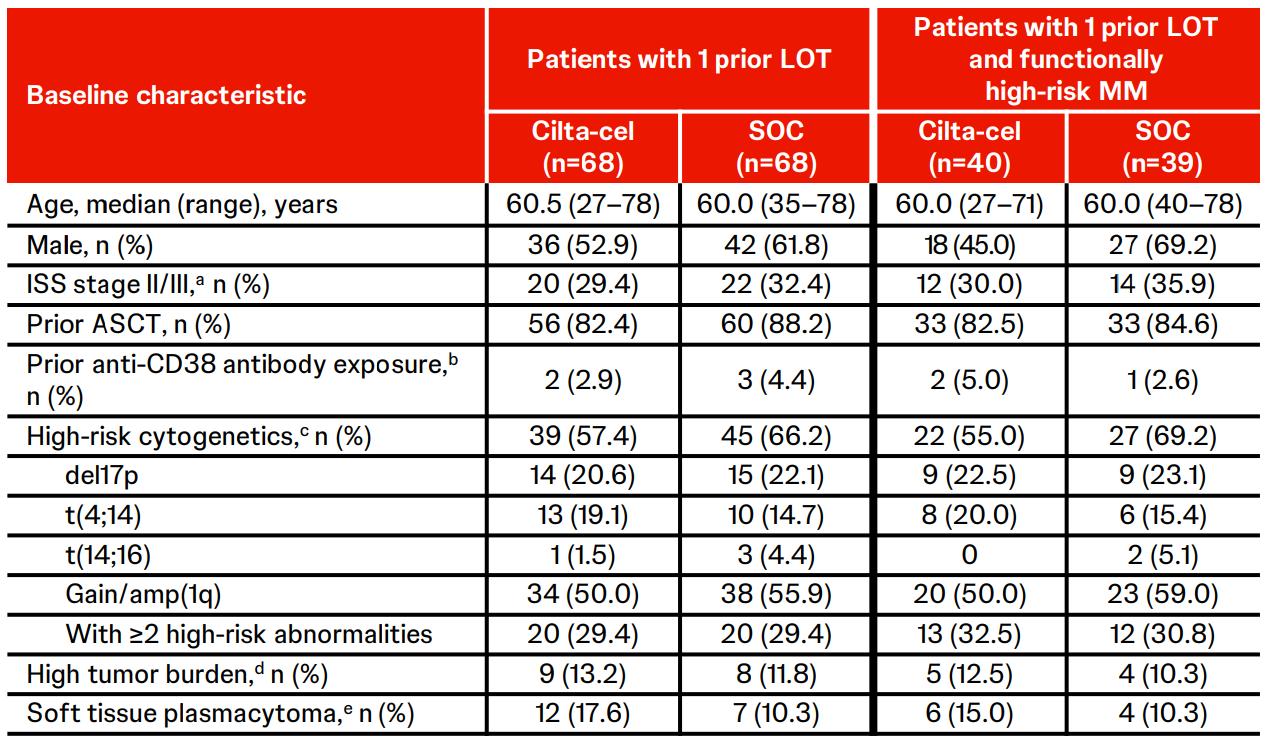

CARTITUDE-4 Subgroup
Analysis & Functional
High-Risk: PFS, Response & MRD Negativity
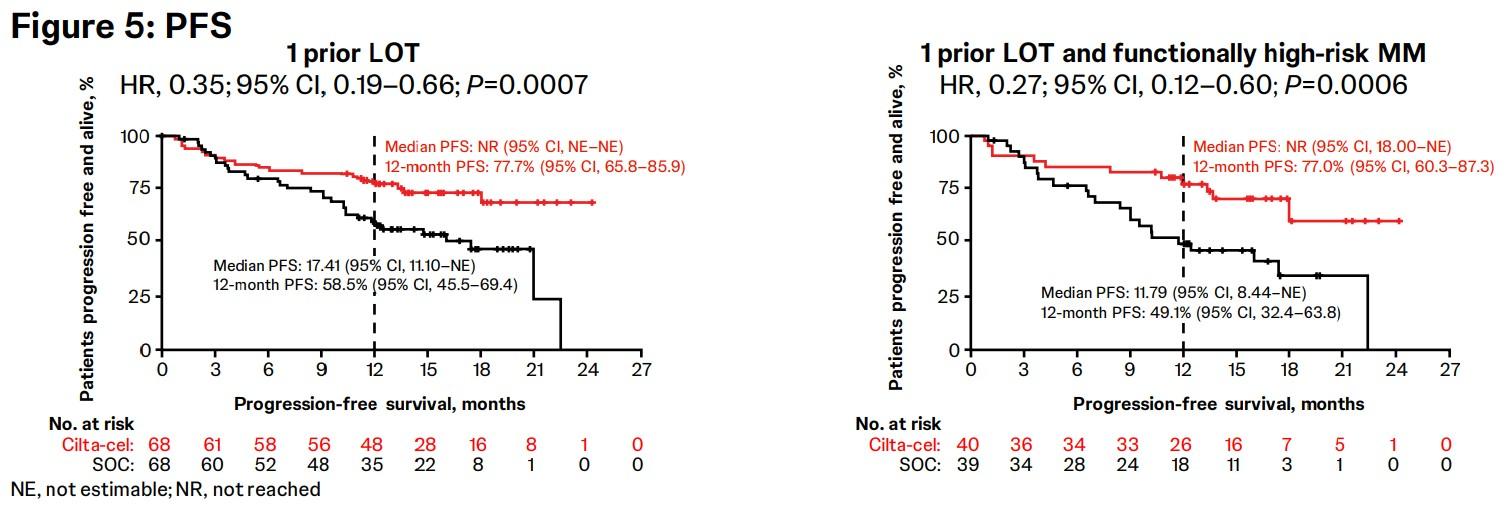
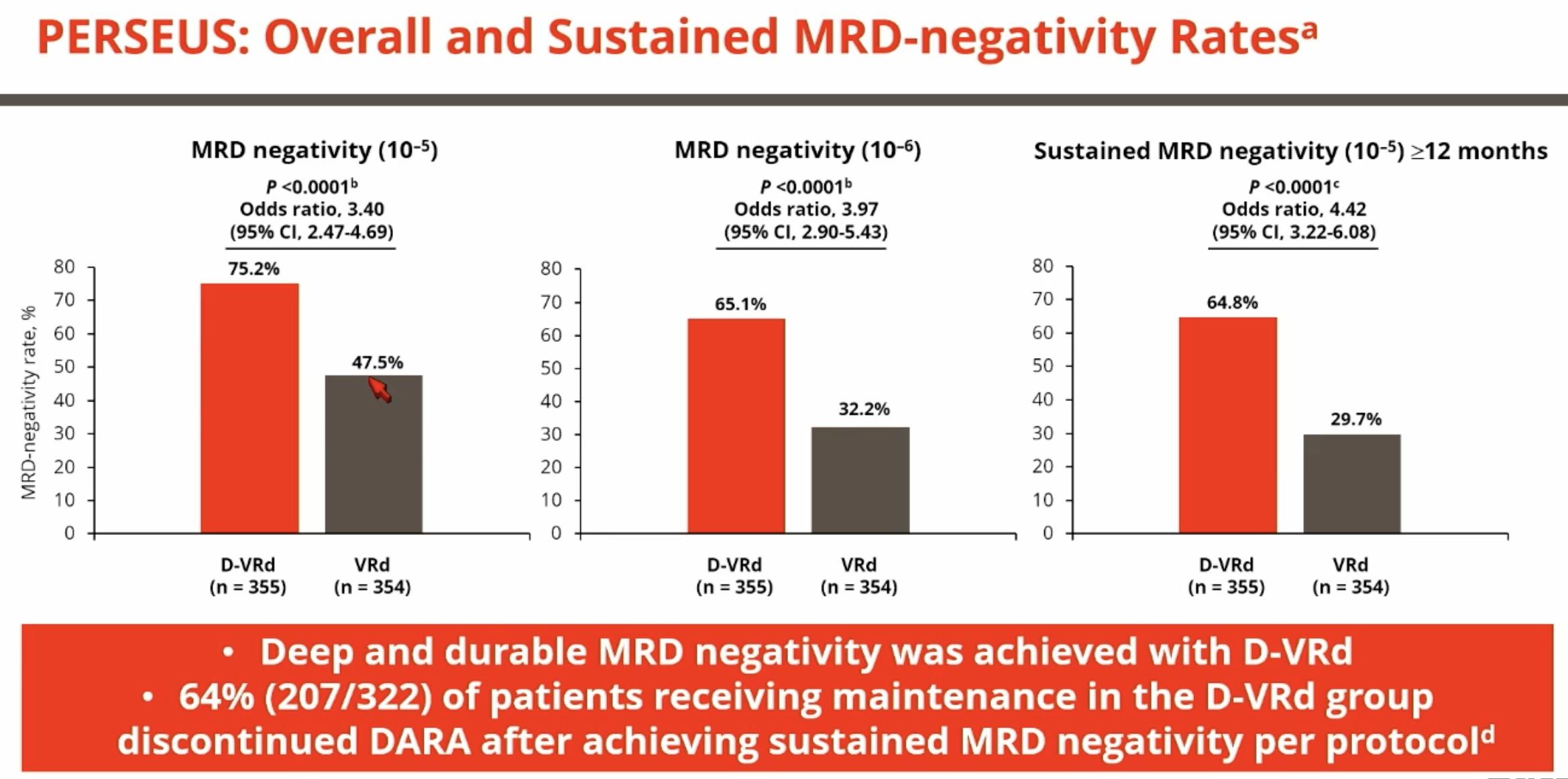
Overall response rate was 90% in cilta-cel patients with 1 prior LOT (n=61) and those with 1 prior LOT and functionally high-risk MM (n=35) demonstrated an ORR of 88%.
Patients with 1 prior LOT had ≥CR rate of 71% and those with 1 prior LOT and functional high-risk achieved a ≥CR rate of 68%.
Cilta-cel patients with 1 prior LOT showed an MRD-negativity (10-5) rate of 63% and those with 1 prior LOT and functional high-risk demonstrated an MRD-negativity rate of 65%
On the Horizon for CAR-T in MM
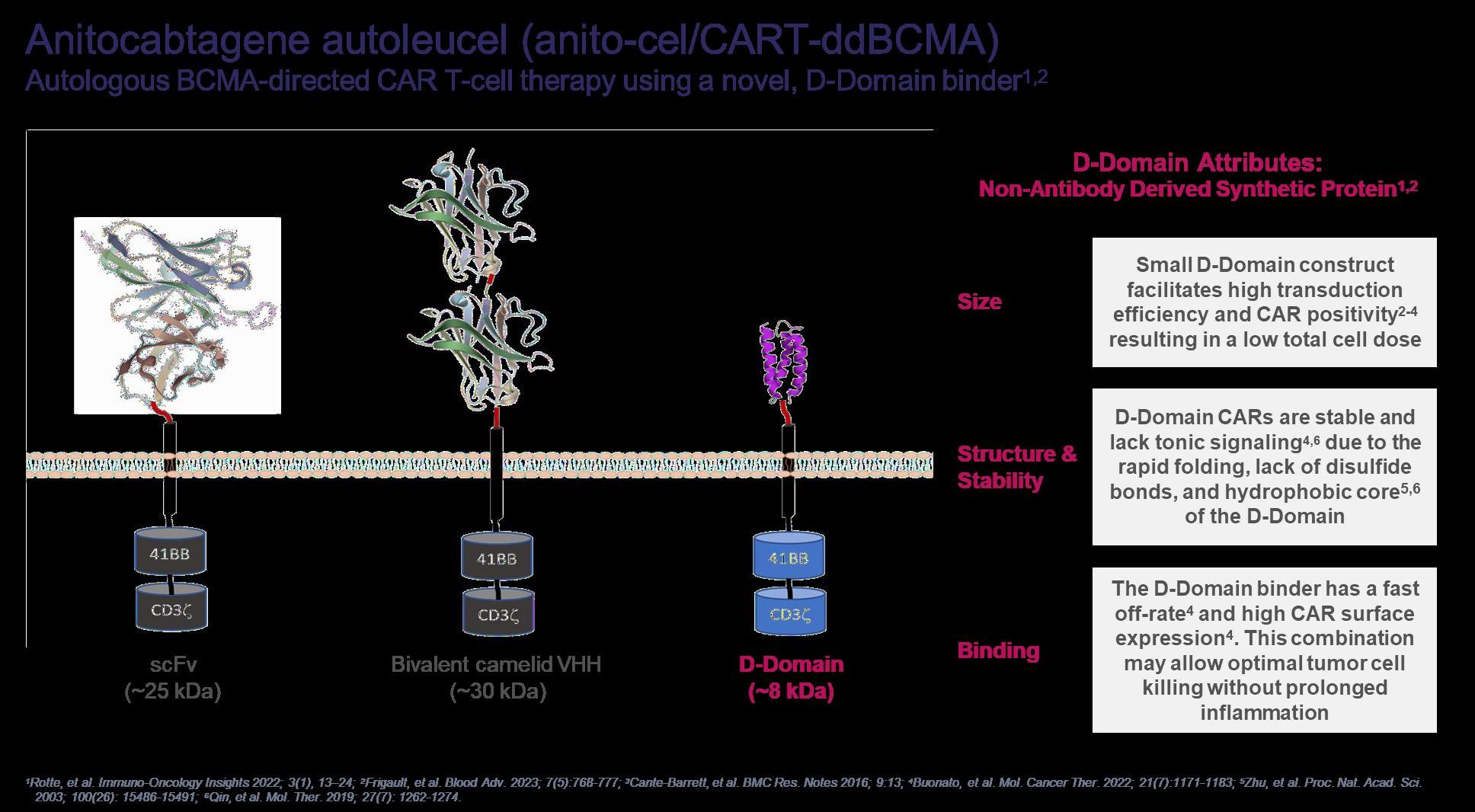
iMMagine-1: Phase 2 Study Design
Screening
Lymphodepleting chemotherapy
Cyclophosphamide 300 mg/m2
Key Eligibility Criteria

Fludarabine 30 mg/m2 Day -5, -4, -3
Anitocabtagene autoleucel manufacturing

Bridging therapy if necessary
Anitocabtagene autoleucel infusion Day 0

Response and safety assessments (up to 24 months)

Long term safety follow-up
Prior IMiD, PI, and CD38-targeted therapy
Received ≥3 prior lines of therapy
Refractory to the last line of therapy
ECOG PS of 0 or 1
Freeman et al, American Society of Hematology 2024, Abstract 1031
Evidence of measurable disease
Target Dose of 115 x 106 CAR+ T cells
Primary Endpoint:
ORR, per 2016 IMWG criteria
Key Secondary Endpoints: sCR/CR rate, per 2016 IMWG criteria
ORR in patients limited to 3 prior LoT, per 2016 IMWG criteria
Primary and key secondary endpoints to be assessed per Independent Review Committee (IRC); Investigator assessment of response per IMWG also permitted per protocol. CR, complete response; ECOG PS, Eastern Cooperative Oncology Group Performance Status; IMiD, immunomodulatory drug; IMWG, International Myeloma Working Group; LoT, line of therapy; ORR, overall response rate; PI, proteosome inhibitor; sCR,
Leukapheresis stringent complete response.
iMMagine-1: Overall Response Rate and MRD Negativity
Efficacy Evaluable Patients (N=86)
ORR=97%
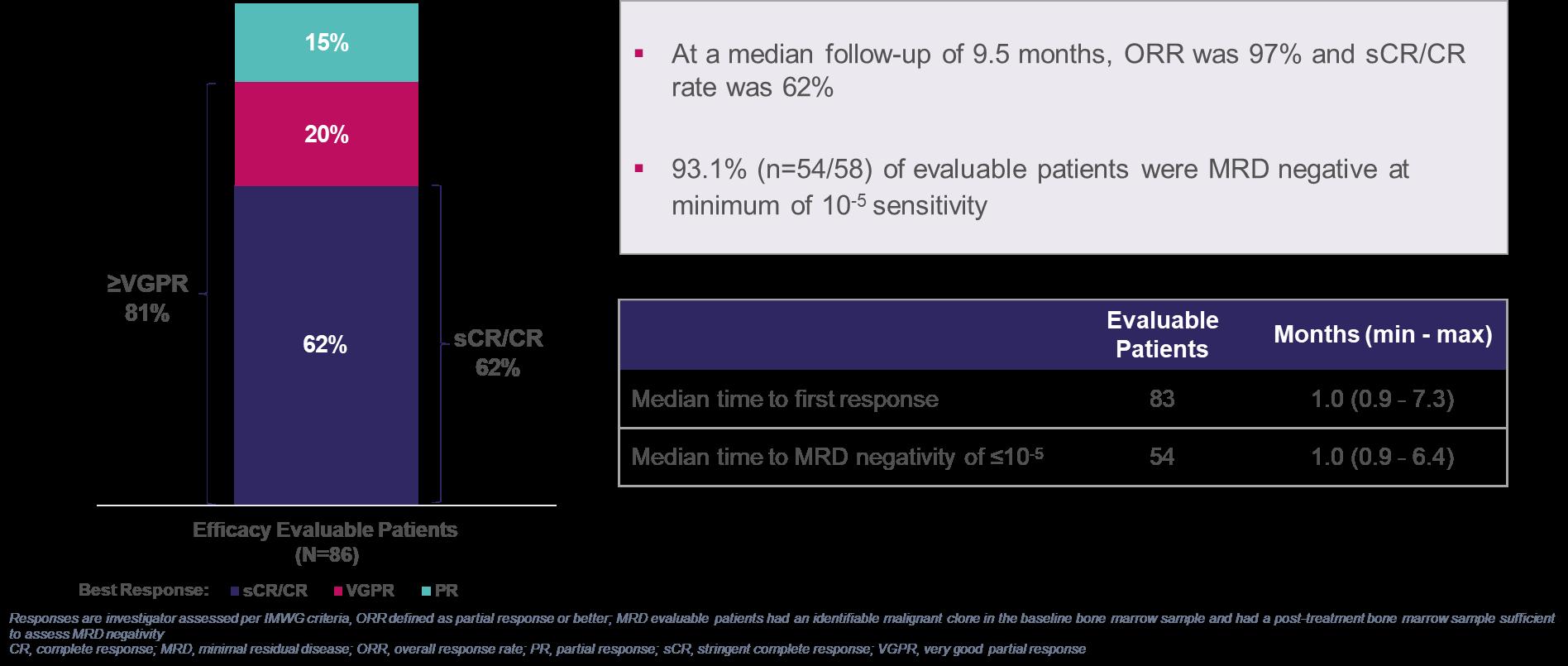
iMMagine-1: PFS and OS Rates
Estimated by Kaplan-Meier
Efficacy Evaluable Patients (N=86)
follow-up of 9.5 months (range 2 to 23 months)
iMMagine-1: Cytokine Release Syndrome
83% (81/98) of patients had CRS of any Grade; the median onset was 4 days
86% (84/98) of patients had CRS Grade 1 or less, including 17% (17/98) with no CRS
% of patients with either no CRS or CRS that resolved by:
≤7 days of anito-cel infusion: 63% (62/98)
≤10 days of anito-cel infusion: 92% (90/98)
≤14 days of anito-cel infusion: 98% (96/98)
Cytokine Release Syndrome (CRS) Per ASTCT criteria
Evaluable Patients N=98 Median onset (min-max) 4 days (1-17 days) Median duration (min-max) 3 days (1-9 days)
Supportive Measures
(71/98)
ventilation
CRS management per protocol was in line with standard medical practice with no prophylactic administration of tocilizumab or dexamethasone
For CRS onset in the first 48 hours, tocilizumab and dexamethasone were protocol recommended
For CRS onset after the first 48 hours, if tocilizumab was administered at investigator discretion, dexamethasone was also recommended
Grade 5 CRS occurred in a 76-year-old patient who had rapidly progressive disease between screening and baseline and did not receive bridging therapy
iMMagine-1: Immune-effector Cell-associated Neurotoxicity Syndrome (ICANS)
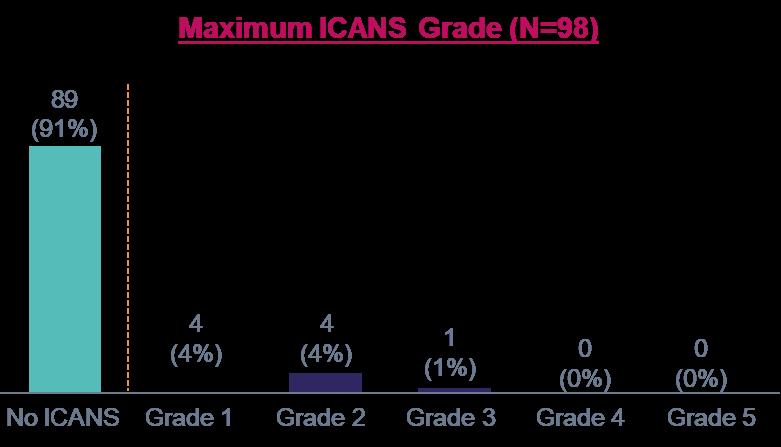
9% (9/98) of patients had ICANS of any grade; all cases resolved
No delayed or non-ICANS neurotoxicities were observed, including no incidence of Parkinsonism, no cranial nerve palsies, and no Guillain-Barré syndrome (n=98)
Similarly, no delayed or non-ICANS neurotoxicities have been observed in the Phase 1 study1 (n=38, median follow-up of 38.1 months with minimum follow-up of 25 months)
ICANS Per ASTCT criteria
Supportive Measures
a With the exception of n=1 Grade 1 ICANS (confusion) on day 34 post infusion that rapidly resolved
b With the exception of n=1 max Grade 2 ICANS with 29-day duration to resolution
1. Bishop, et al. Blood 2024; ASH Annual Meeting, Poster #4825.
Tocilizumab

• Now open and enrolling at CBCI


A little further on the Horizon for CAR-T in MM: GPRC5D Targeting CARs
• Arlocabtagene Autoleucel a GPRC5D-Targeted
CAR T-Cell Therapy: Updated Phase I Study Results at ASH
• 86 patients, 26 at RP2D (150 x 10^6 CAR cells)
• Median 5 prior lines of tx, ~50% prior BCMA tx
• ORR 91%, CR 48%
• Med PFS 18M, no diff if prior anti-BCMA
• Oral/skin/nail tox common but less severe than talquetamab
• Some atypical neurotox, diff than Carvykti (cerebellar)
• Also now open and enrolling at CBCI
CARs are cool but could bispecifics be even more impactful?!?
• Teclistamab (Tecvayli): Approved Dec 2022
• Elranatamab (Elrexfio) and Talquetamab (Talvey) approved Aug 2023
• Teclistamab and Elranatamab target BCMA
• Talquetamab targets GPRC5D
• All currently received accelerated approval after 4 prior lines of therapy including a PI/IMiD/CD38 MoAb
• Linvoseltamab (Regeneron BCMA target) FDA review set for July 10, 2025

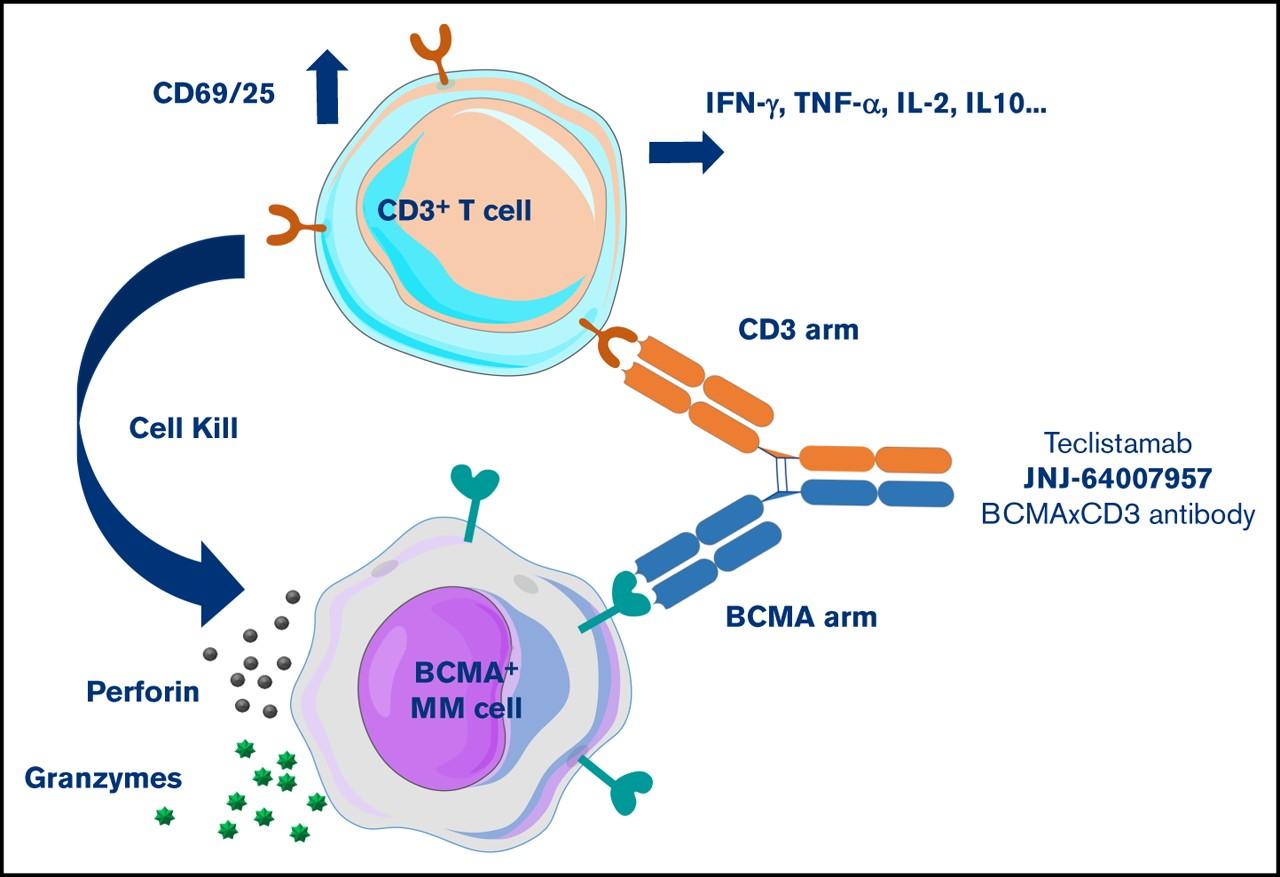

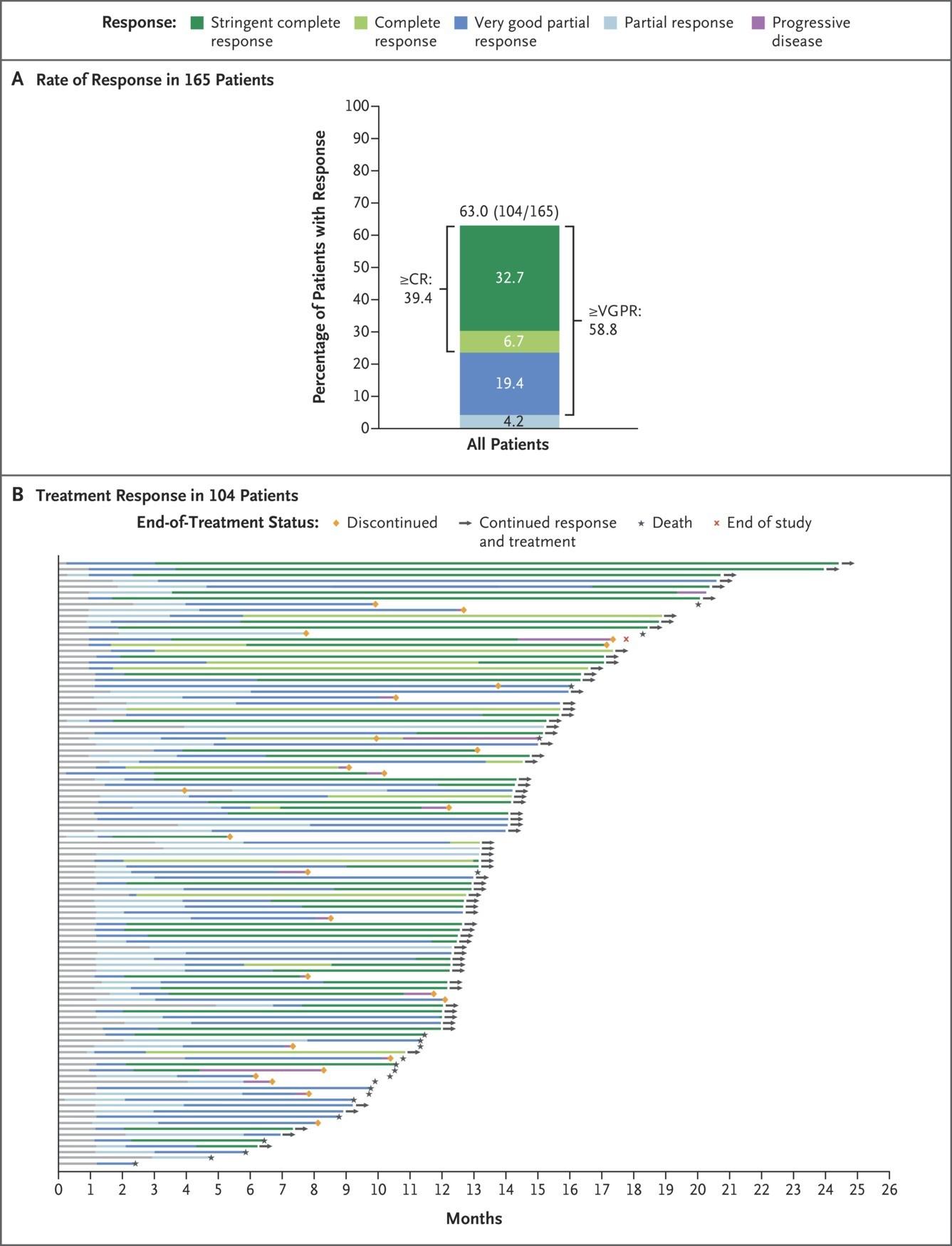
Teclistamab: MajesTEC-1 Trial
• 165 patients, median 5 prior lines of therapy
• 77% triple class refractory, 70% penta exposed
• Dosing: 2 step up doses then weekly initially
• CRS: All Gr 72%, <1% Gr 3/4
• 3% ICANS
• Infection: All Gr 76%, Gr 3/4 45%



Elranatamab- MagnetisMM-3 Trial
• 101 patient, median 5 prior lines
• 97% triple class refractory, 70% penta exposed
• Dosing: 2 step up doses 12mg->32mg then 76mg full dose then weekly, biweekly after
24 weeks monitoring for 48hrs between step-up dosing as inpatient
• CRS: 56%, no grade 3 or higher
• Infection 70%, grade 3+ 40%
• ICANS 3.4%


1. Lesokhin et al, Nature Medicine 2023 29; 2259-2267 2. Raje. ASH 2022. Abstr 158



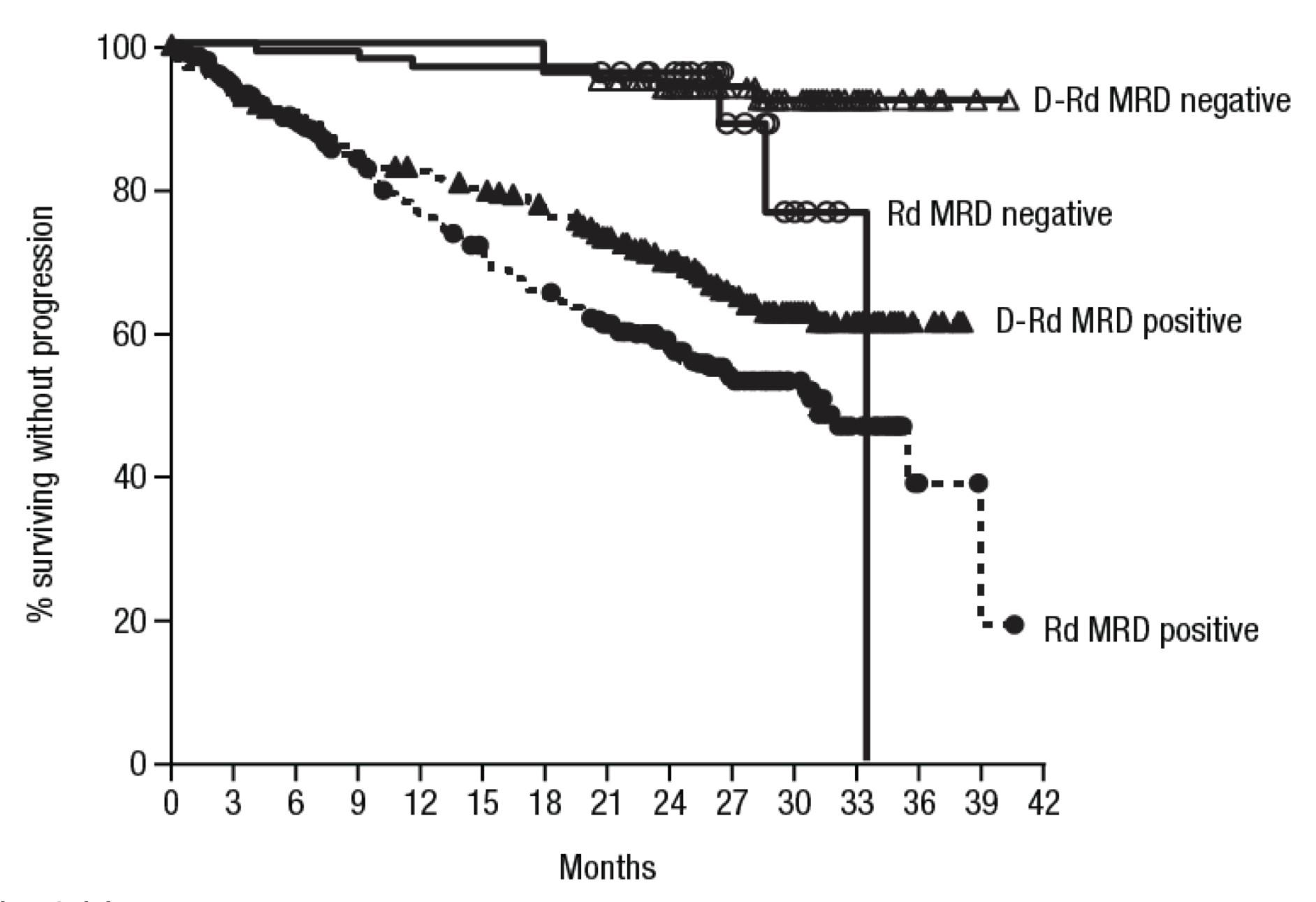
PFS and OS




• Median PFS was 17.2 (95% CI 9.8–NE) months (Figure 1) • Median OS was 24.6 (95% CI 13.4–NE) months (Figure 2)

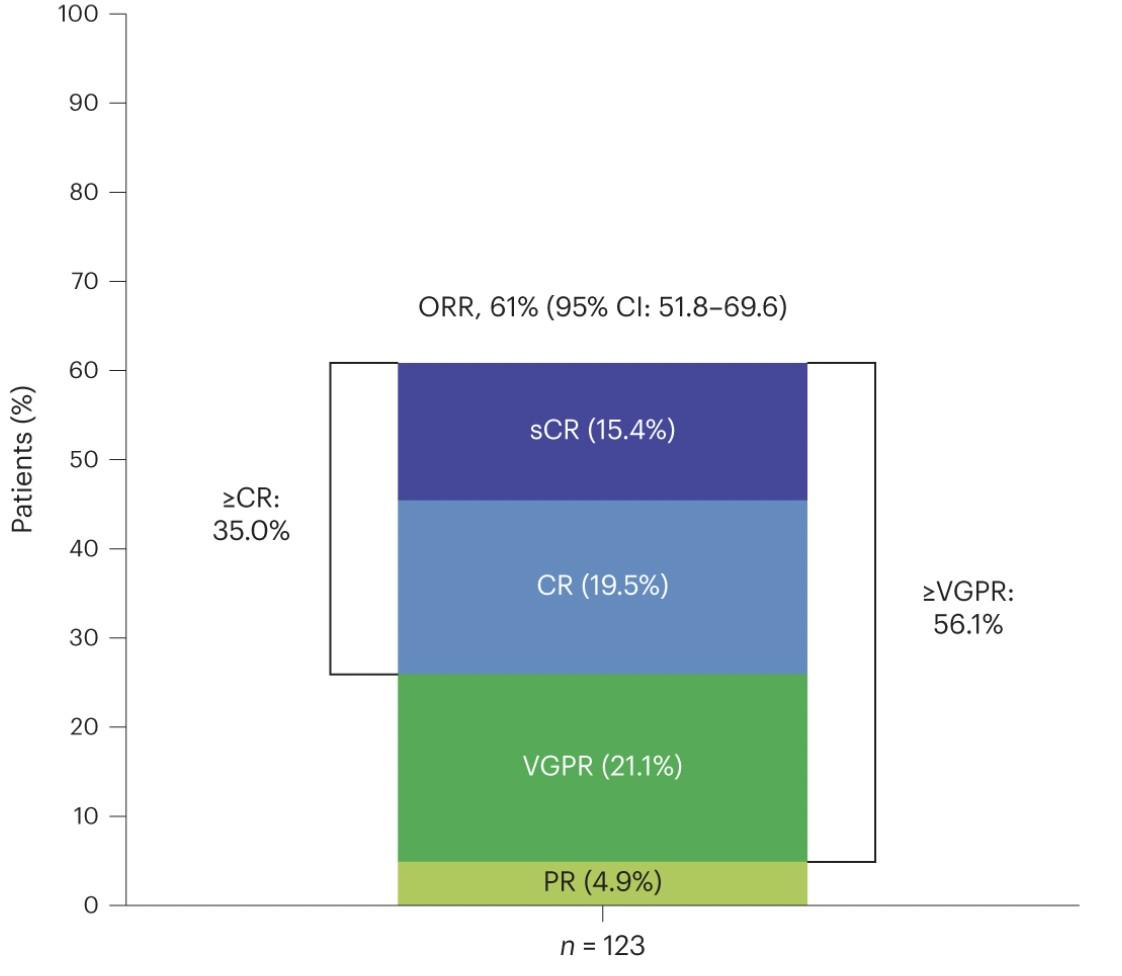
Prince HM et al.



•



Efficacy: ORR, MRD Negativity, and DOR

‒ sCR, 16.3%; CR, 21.1%; VGPR, 18.7%; PR
4.9%
‒-5 MRD negativity (10 ) rate was 90.3% in patients with ≥CR who were evaluable for MRD (n=31)
• Median DOR was NR (95% CI 29.4–NE) (Figure)
• Among responders per BICR who switched to Q4W dosing ≥6 months before the data cutoff (n=27), 25 (92.6%) maintained their response ≥6 months after the switch, including 22 (88.0%) who maintained ≥CR
• 1 (3.7%) patient had PD* and 1 (3.7%) patient permanently discontinued elranatamab 6 months after the switch to Q4W
*Per IMWG criteria in ≥1 assessment.
BICR = blinded-independent central review; CI = confidence interval; CR = complete response; DOR = duration of response; IMWG = International Myeloma Working Group; MRD = minimal residual disease; NE = not evaluable; NR = not reached; OR = objective response; ORR = objective response rate; PD = progressive disease; PR = partial response; Q4W = once every 4 weeks; sCR = stringent complete response; VGPR = very good partial response.
Median DOR, months (95% CI)
Patients with OR NR (29.4–NE)
Patients with ≥CR NR (NE–NE)
Patients with ≥VGPR NR (NE–NE)
rate for pts with ≥VGPR (95%
(51.4–76.0) 30-month rate for pts with
(Abstract 4738).
Prince HM et al.
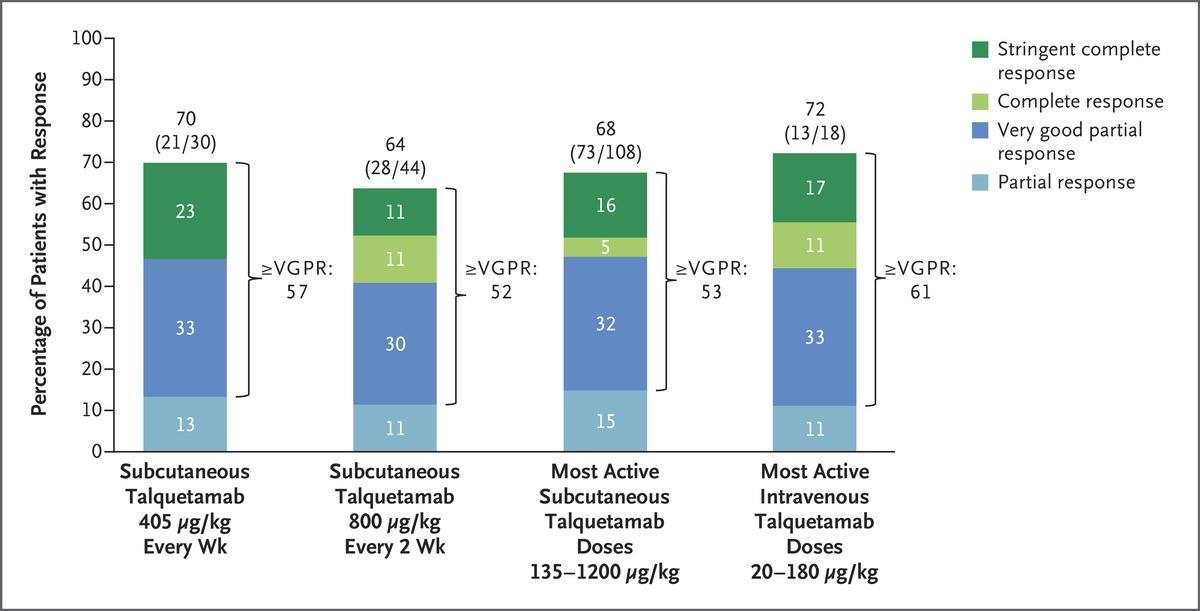
Talquetamab: MonumenTAL 1
• GPRC5D Targetting: Phase 1 dose escalation study with 232 patients
• 130 with subcutaneous dosing
• Dosing: 0.01mg/kg->0.06mg/kg->0.4mg/kg (weekly dosing) -> 0.8mg/kg (bi-weekly dosing)
48-72hrs with hospitalization between dosing
• Common adverse events included:
• CRS in 78% (only 1 grade 3/4)
• Skin-related events 69%
• Altered taste 59% (dry mouth very common)
• Nail related events 39%
• Infection: Around 65%, Grade 3+ 25%
Logistics of bispecifics
• CRS is very predictable- occurs during startup dosing, patients may need to do this at a high volume center
• Trials ongoing to try to prevent CRS before it develops
• Some centers hospitalize for initial dosing others don’t
• Dosing has evolved beyond initial development
• Less frequent dosing may help with infection risk, talquetamab toxicities
• Infection prevention key
• All patients need VZV and PJP prophylaxis
• IVIg for anyone with low antibody levels (essentially everyone)

OVERALL SURVIVAL & IVIG PROPHYLAXIS
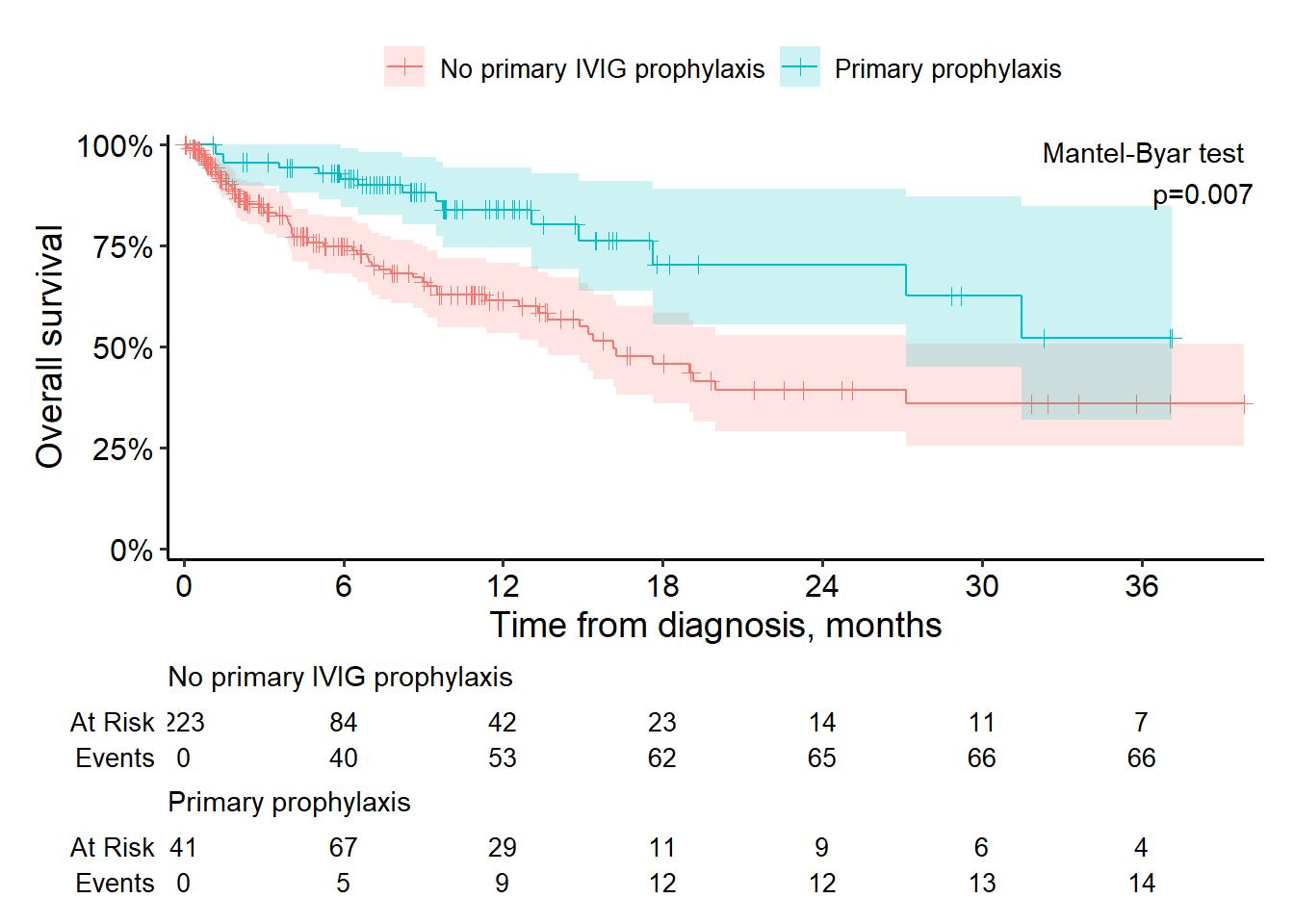


Emerging Bispecifics
• Linvoseltamab (LINKER-MM1 Trial1)
• D1 and 8 step-up, d15 full dosing, weekly week 3-14 then biweekly, every 4 weeks after 24 weeks if VGPR+
• ORR at RP2D 71%, estimated median DOR 29 months
• CRS 45%
• Etentamig (ABBV-383)
• Moving into phase 2s with q4 week dosing, single step up dose
• In dose optimization with 1 step up dose, q4wk subsequent dosing (n=23): CRS 30% Gr 2 4%, 2 patient received toci
• Cevostamab
• Novel target, FcRH5
• Step up dosing x 2 then q3 week
• Camma 23 (dosing after prior BCMA tx): Initial 11 patients after CAR-T ORR 73%

1. Bumma at al. JCO 2024
2. Mian et al, ASH 2024 Abstract 1985
3. Kumar et al. EHA Abstract 2024

JNJ-5322 BCMA×GPRC5D×CD3 Trispecific:
The Next Generation of Targeted Immunotherapies Reduces Side Effects and Enhances Efficacy
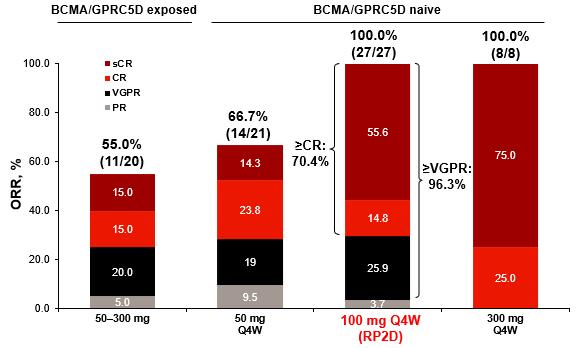




How do I choose between a CAR-T and a Bispecific?
CAR T-Cells
Personalized
Targeted immuno-cytotoxicity
Single infusion (“one and done”)
Potentially persistent
FACT-accredited center required (hospitalization likely required)
CRS and neurotoxicity; requires ICU and neurology services
Dependent on T-cell health (manufacturing failures)
Requires significant social support; caregiver required

Bispecific Antibody
Off the shelf
Targeted immuno-cytotoxicity
No lymphodepletion
Minimal steroids
Initial hospitalization required
CRS and neurotoxicity possible
Dependent on T-cell health (T-cell exhaustion)
Requires repeated administration

Return of Balantamab Mafadotin?
• Humanized, afucosylated IgG1 antiBCMA antibody
• Conjugated to a microtubule disrupting agent MMAF via a stable, protease resistant linker
• Accelerated approval 8/2020 following DREAMM-2 Trial, withdrawn 2/2023 after DREAMM-3 failed to reach primary endpoint
• 2 new positive trials in 2024
• DREAMM-8 Bela+Pd vs VPd
• DREAMM-7 Bela+Vd vs Dara-Vd
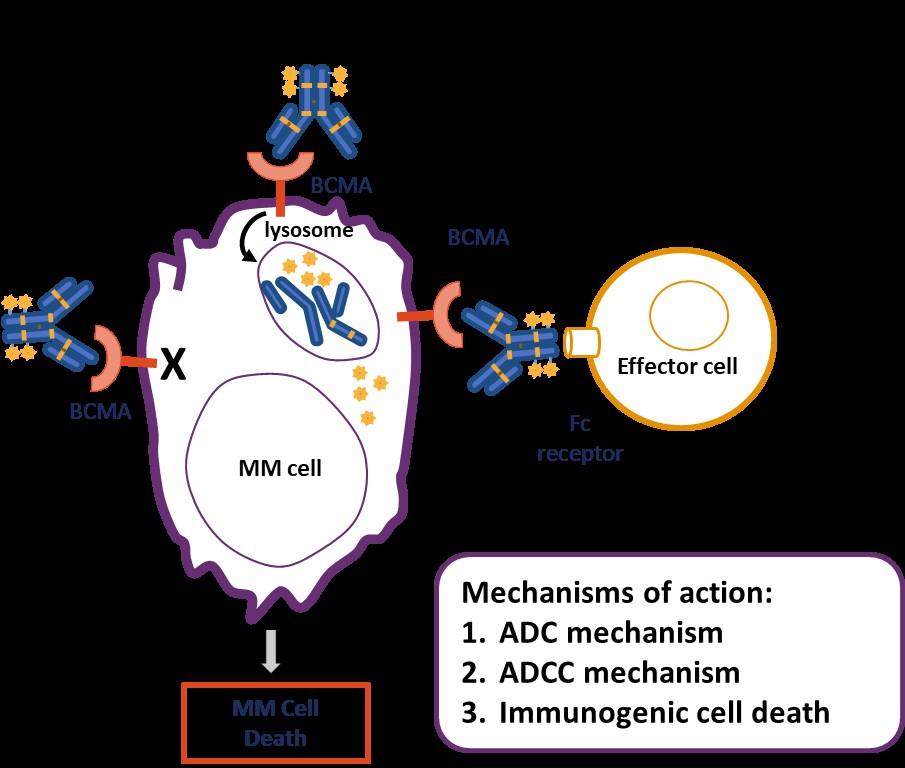
DREAMM-7: Belantamab Mafodotin
+ Vd vs Dara-Vd in R/R Multiple Myeloma
Multicenter, randomized, open-label phase III trial
Stratified by prior lines of tx (1 vs 2-3 vs ≥4), prior bortezomib (yes vs no), R-ISS (I vs II/III)
Adults with MM previously treated with ≥1 line of therapy; with PD on/after most recent therapy; no prior treatment with anti-BCMA–targeted therapy; not intolerant or refractory to bortezomib or daratumumab; ECOG PS 0-2 (N = 494)
Cycle 1-8
Belantamab mafodotin + Bortezomib +
Dexamethasone (BVd), 21-day cycles (n = 243)
Daratumumab + Bortezomib +
Dexamethasone (DVd), 21-day cycles (n = 251)
Belantamab mafodotin: 2.5 mg/kg IV Q3W cycle 1-8.
Cycle 9+
Belantamab mafodotin 2.5 mg/kg IV Q3W
Tx continued until PD, unacceptable toxicity, end of study, or consent withdrawal
Daratumumab 16 mg/kg IV Q4W
Bortezomib: 1.3 mg/m2 SC Days 1, 4, 8, 11 cycles 1-8 (21-day cycle).
Daratumumab: 16 mg/kg IV cycle 1-3 QW and 16 mg/kg IV Cycle 4-8 Q3W.
Dexamethasone: 20 mg on day of and day after bortezomib in cycle 1-8 in BVd and DVd regimens.
Follow-up Q3W for PFS and Q12W for OS if discontinued for reasons other than PD
Primary endpoint: PFS
Key secondary endpoints: OS, DoR, MRD negativity

DREAMM-7: Progression-Free Survival
PFS favored BVd vs DVd in subgroups, including patients with lenalidomide-refractory disease or those with high-risk cytogenetics
Early OS analysis, suggests meaningful OS benefit for BVd vs DVd (HR: 0.57)

DREAMM-7: Overall Survival
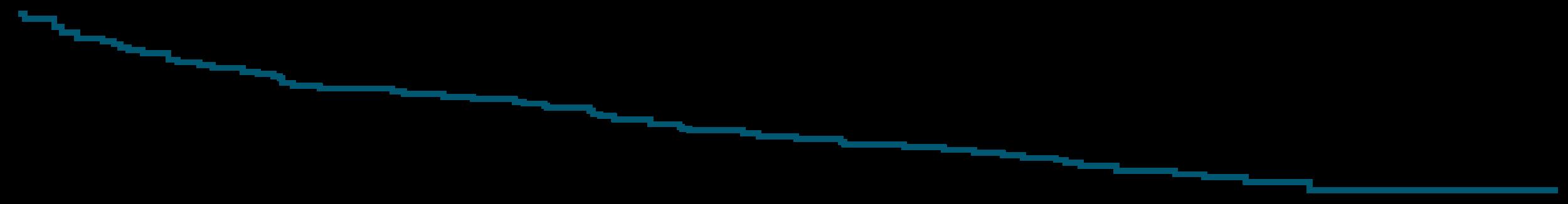
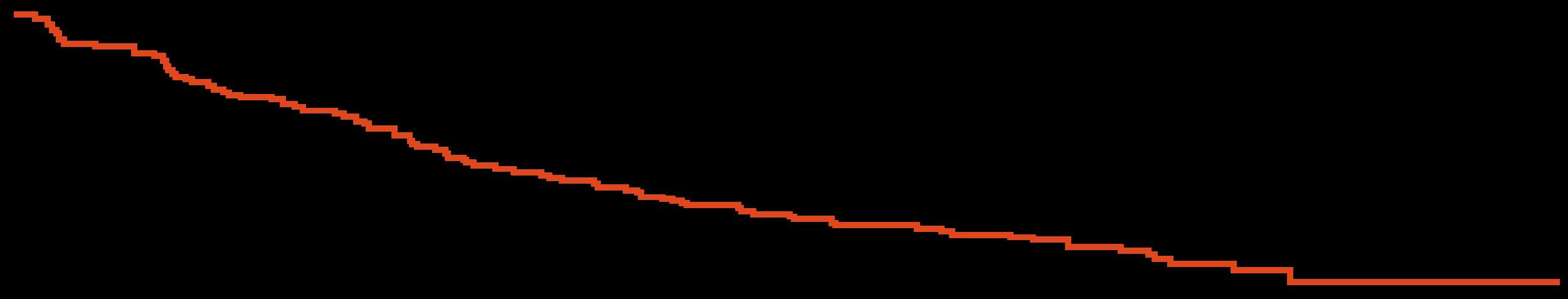
mOS was not reached
Modeling predicts a mOS of 84 mo with BVd vs 51 mo with DVd

DREAMM-7: Safety

Summary
• The myeloma landscape in 2025 is changing rapidly
• That impact is happening primarily in the RRMM setting
• This is likely to continue to shift over the next several years
• With most of these improvements we’re allowing MM patients to note just live longer but live better
• That’s really exciting!
•
It really, really is! It makes being an MM specialists one of the best jobs out there



LUNCH
Please remain in this room
Development Update: Partnering with the IMF
Sylvia Dsouza
Vice President, Development, International Myeloma Foundation
IMF REGIONAL COMMUNITY WORKSHOP
DENVER
AFTERNOON AGENDA

Seasons of Myeloma: Managing Side Effects and Living Well
Beth Faiman, PhD, MSN, APN-BC, AOCN, BMTCN, FAAN, FAPO
Nurse Leadership Board
Cleveland Clinic Taussig Cancer Institute, Cleveland, OH
Living the Myeloma Life: Local Patient & Care Partner
Pat Sullivan (Patient) and
Mark Cerda, PE (Care Partner)
Beyond Myeloma Therapy: Restoring Balance: Reiki and Holistic Approaches to Well-Being
Martha Lacy, MD
Mayo Clinic, Rochester, MN
Q&A with Panel
Closing Remarks
Robin Tuohy
Vice President, Support Groups, International Myeloma Foundation

Development Update: Partnering with the IMF
Sylvia Dsouza, Vice President,
Development
International Myeloma Foundation

Sylvia Dsouza Vice President Of Development
My Purpose At the IMF
• Support the mission and vision of the IMF
• Secure philanthropic support and resources for the IMF through diverse mechanisms
• Oversee a team of passionate and determined fundraising professionals who are committed to advancing the mission of the IMF
• Have the incredible honor of working with dedicated volunteers from the US and across the globe.
The International Myeloma Foundation
OUR VISION:
A world where every myeloma patient can live life to the fullest, unburdened by the disease.
OUR MISSION:
Improving the quality of life of myeloma patients while working toward prevention and a cure.

The Pillars Of The IMF

Research: The IMF is dedicated to finding a cure for multiple myeloma.

Education: The IMF provides education for patients, caregivers and health care providers.

Support: The IMF provides support for patients and caregivers through in-person and virtual support groups.
Advocacy: The IMF advocates for better access to care and more affordable options for myeloma patients.



Philanthropy: The Act of Giving To Improve WellBeing
The term originates from the Ancient Greek word "philanthrōpía," meaning "love of humanity"
•Volunteer
• Join your local support group/become a Support Group Leader (Nancy, SD)
• Join our grassroots patient Advocacy program (ex: Kathy from MN)
• Volunteer your time at local races organized by the IMF to engage the community (local co-chairs)
• Engage on social media to connect with others affected by myeloma and spread awareness and empower patients with knowledge and resources.
•Intellectual Capacity
• Offer your expertise as a speaker or panelist at events.
• Be a beta tester for various new tools and products and provide reviews & feedback
•Financial Support for Important Initiatives
• Make a philanthropic gift to support research, education, advocacy or patient support services and programs.

Philanthropy Fuels Our Mission
Philanthropy Comes In Many Forms
The term originates from the Ancient Greek word "philanthrōpía," meaning "love of humanity"

Peer-to-Peer (P2P) Fundraising
• Peer-to-Peer Fundraisers are created from YOUR ideas.
• Join or start an IMF P2P event. Engage family, friends, co-workers who support you. Let them show you that you are not alone.
Join the HOPE Society (Recurring Monthly & Annual Giving Program)
• Support IMF core programs, including educational events, publications, the toll-free InfoLine, and more.
• Start with a monthly contribution and when ready turn it into a yearly commitment.
• Help us cultivate the future in the quest to find a cure for myeloma by joining the International Myeloma Foundation's Hope Society.
Transformative Gifts (Major Giving and Principal Giving)
• Let your passion guide your gift.
• Gifts can also be designated, unrestricted, expendable and/or an endowment
Philanthropy Fuels Our Mission
IMF Events 2025: Join or Create an Event to Help Improve Lives, Find the Cure
Peer-to-Peer Fundraising:
• Laughs 4 Life
• 7th Annual Schleicher's Hikers 5K
• Mission for Matt (Dinner, and Dancing)
• Jack's Education and Experience Grant
• Czerkies Memorial Golf Outing
• Walkathon & BBQ To End Multiple Myeloma
• Row for a Cure
Miracles for Myeloma Virtual/In-Person 5K
Run/Walk Five events across the United States:
• Boca Raton, FL (03-15-2025)
• Los Angeles, CA (04-12-2025)
• Philadelphia, PA (05-03-2025)
• Clark, NJ (09-13-2025)
• Chicago, IL (10-04-2025)







What is your passion?









Giving Has Its Benefits
Monthly & Annual Giving – HOPE Society
• Join our flagship monthly and annual giving program, the HOPE Society.
• Get invited to Regional Salon Dinners in your area with IMF leadership and KOLs.
• Receive exclusive updates on research and trials fresh off the press.
• Play a pivotal role in supporting our four pillars.
• Support long-term initiatives that make a lasting difference.
Planned Giving
• Join the Brian D. Novis Legacy Society and make a planned gift!
• Gain immediate tax benefits
• Potentially increase your income during your lifetime.
• Continue to fund our core programs and four pillars.
• Make a bequest (a gift from your estate)
• Include a provision in your will or living trust.


• Designate us as a beneficiary of a life insurance policy, or retirement plan (IRA, 401(k), or 403(b).
• Include us in your will, a profound way to support the people and causes important to you.
Corporate and Foundation Gifts
• Your organization can contribute a corporate gift or foundation grant
• Provide seed funding that is necessary to accelerate the path to a cure.

Iceland Cycling Expedition (ICE):
IMF’s Signature Marquee Annual Endurance Event




ICE: Raising Awareness & Funding For Myeloma Research; Building Relationships


Three goals:
1. Raising awareness and visibility for multiple myeloma and a patient/care-partner journey (humanizing the disease)
2. Raising awareness and visibility for the International Myeloma Foundation
3. Raising critical funding to help accelerate research to find a cure for myeloma

Additional outcome that organically emerged – building a strong, interconnected myeloma community where all myeloma patients and their loved ones see hope, resilience and determination as part of the journey and feel empowered
#MyelomaICE Highlight: Film Festivals
ICE Film Documentary –– Why Film Festivals?
Submissions to film festivals reflects a unique and innovative approach to raising myeloma awareness and amplifying the IMF brand. Through creative storytelling, we introduce myeloma to diverse audiences, fostering emotional responses, educating members of the public, and inspiring curiosity.
Awards So Far:
• The World Film Festival in Cannes: Winner Best Documentary Short!
• Golden State Film Fest Audience Favorite Award!



• Phare Int'l Film Festival in Mauritius screening in February: Honorable Mention
• New York International Film Awards: Finalist
Screening Dates:
• April 12: WIFI Film Fest, Topeka, Kansas
• April 27: West Chester, PA, Film Fest
*Selections notifications extend until 10/20/25 by which point the short film will be publicly released.



Iceland Cycling Expedition 2025 Participants
• The 2025 Iceland Cycling Expedition will take place from Wednesday, August 27, 2025 through Tuesday, September 2, 2025.
• This signature marque event is a 6-day/7-night fundraising cycling challenge in the High Mountains of Iceland.
• 21 Participants (patients, care-partners, myeloma clinicians, sponsors)
• Applications for 2026 will open in October 2025. Follow us on Icelandcyclingexpedition.com and/or myeloma.org/ice2025
• To find out more about how to get involved with ICE contact Sylvia Dsouza at sdsouza@myeloma.org













Dr. Cristina Gasparetto Clinician & Researcher
Duke Cancer
Dr. Saad Usmani Clinician & Researcher
Memorial Sloan Kettering, NY
Huntsman Cancer Institute, UT
Dr. Jeffrey Zonder Clinician & Researcher Wayne State
Tatum Byhre Care Partner Minneapolis, MN
Troy Fischer Care Partner Bloomington, IL
Ashley Dieks Myeloma Survivor Garson, Ontario
Christian Hoff Myeloma Survivor Pittsboro, NC
Jered Haddad Myeloma Survivor Germantown, TN
Richard Pampe Myeloma Survivor Jackson, WY Benjamin Freund Myeloma
Katherine Podgorski Myeloma Survivor Toronto, Canada
Reach Out to the IMF Development Team
Learn more about our programs, projects, and initiatives at the IMF. We can help you find alignment with your own philanthropic passion and ways to deepen and strengthen your engagement with us.
Reach out to the IMF Development Team to start a conversation on how you can make a difference in the lives of the people impacted by myeloma.
Sylvia Dsouza- Vice President of Development
sdsouza@myeloma.org or (310)947-4126
Simona Grace- Director of Development, Major Gifts sgrace@myeloma.org or (818)487-7455 x 307
Alexandra Switzer- Assistant Director of Development Peer-toPeer Fundraising aswitzer@myeloma.org or (818)487-7455 x 315



Scan for Upcoming Events!







Seasons
of Myeloma: Managing Side Effects and Living Well
Beth Faiman, PhD, MSN, APN-BC, AOCN, BMTCN, FAAN, FAPO
Nurse Leadership Board
Cleveland Clinic Taussig Cancer Institute, Cleveland, OH
Seasons of Multiple Myeloma

Beth Faiman PhD, MSN, APN-BC, AOCN®, BMTCN®, FAAN, FAPO
Cleveland Clinic Taussig Cancer Institute & Nurse Leadership Board Member

Wintery Mix of Treatment Options Spring into Managing Side Effects
Summer of Success






Wintery Mix of Treatment Options

Diverse and Complex Treatment Combinations









Myeloma Treatment Common Combinations
Velcade® (bortezomib)
Lenalidomide
DVRd, VRd, Vd
DVRd, VRd, Rd
Kyprolis® (carfilzomib) KRd, Kd, DKd, Isa-Kd
Pomalyst® (pomalidomide) Pd, DPd, EPd, PCd, Isa-Pd
Darzalex® (daratumumab)
DVRd, DRd, DVd, DPd, DVMP, DKd
Ninlaro®(ixazomib) IRd
Empliciti® (elotuzumab) ERd, EPd
Xpovio® (Selinexor) XVd, XPd, XKd
Sarclisa® (Isatuximab) Isa-Kd, Isa-Pd
Blenrep® (Belantamab mafodotin) Bela-d
Abecma® (Idecabtagene Vicleucel) --
Carvykti™ (ciltacabtagene autoleucel)
Elrexfio™ (elranatamab)

Tecvayli® (teclistamab) --
Talvey™ (talquetamab) --
Venclexta® (venetoclax) Vd + ven
New agents or regimens in clinical trials are possible options
ASCT = autologous stem cell transplant; Bela = belantamab; C = cyclophosphamide; D = daratumumab; d = dexamethasone; E = elotuzumab; Isa = isatuximab; I = ixazomib; K = carfilzomib; M = melphalan; P = pomalidomide; R = lenalidomide; V = bortezomib; ven = venetoclax.


Stem Cell Transplant
ELIGIBILITY
Measuring Treatment Response
Determining Transplant Eligibility
Insurance Authorization Collecting Stem Cells



TRANSPLANT
High Dose Chemotherapy
Stem Cell Infusion
Supportive Care Engraftment
Duration: Approximately 2 weeks
Location: Transplant Center
Duration: Approximately 3-4 weeks
Location: Transplant Center

POST-TRANSPLANT
P H A S E 1 P H A S E 2 P H A S E 3

Restrengthening
Appetite recovery
“Day 100” assessment
Begin maintenance therapy
Duration: Approximately 10-12 weeks
Location: HOME





CAR T: Another Treatment Approach
Ask for a referral to CAR Tcell center as soon as it is possible as next treatment option (ie, before relapse)
Manufacturing takes
≈ 4 to 6 weeks
Bridging therapy may be needed
T-Cell Collection


No driving for 8 weeks
“One & Done” with continued monitoring
• Away from home
• Often some hospital stay
• Care Partner needed
• Side effect management
• CRS, ICANS
• Low blood counts
• Fatigue and fever
• Some patients need ongoing transfusion support


Bispecific Antibodies
• Different bispecific antibodies have differences in efficacy, side effects
– Available after 4 prior lines of therapy (or clinical trial)
– About 7 in 10 patients respond
– Off-the-shelf treatment; no waiting for engineering cells
– CRS and neurotoxicity
– Risk of infection
• BCMA target: greater potential for infection
– Tecvayli® (teclistamab)
– Elrexfio™ (elranatamab)
BISPECIFIC ANTIBODIES
• GPRC5D target: potential for skin and nail side effects, GI issues of taste change, anorexia and weight loss
– Talvey™ (talquetamab)







CAR T and Bispecific Antibodies: Unique Side Effects





CRS is a common but often mild & manageable side effect



CAR = chimeric antigen receptor; CRS = cytokine release syndrome. Oluwole OO, Davila ML. J Leukoc Biol. 2016;100:1265-1272. June CH, et al. Science. 2018;359:1361-1365. Brudno JN, Kochenderfer JN. Blood. 2016;127(26):3321-3330. Brudno JN, Kochenderfer JN. Blood Rev. 2019:34:45-55. Shimabukuro-Vornhagen, et al. J Immunother Cancer. 2018;6:56. Lee DW, et al. Biol Blood Marrow Transplant. 2019;25:625-638.




CAR T and Bispecific Antibodies: Unique Side Effects











Spring Into Managing Side Effects





The Early Bird Gets the Worm: Communicate Proactively with Your Healthcare Team
Your team may be able to help, but only if they know how you feel.
Unmanaged Myeloma can cause:
• Calcium elevation
• Renal dysfunction
• Low blood counts
• Infection Risk
• Blood clots
• Bone pain
• Neuropathy
• Fatigue

Side Effects of Treatment can
cause:
• GI symptoms
• Renal dysfunction
• Low blood counts
• Infection Risk



Tip: proactively discuss common side effects and what to do if they occur





How You Feel





• Blood clots
• Neuropathy
• Fatigue











Tip: Keep a Symptom Diary and bring it to appointments











Steroids enhance the effectiveness of other myeloma therapies
Your provider may adjust your dose. Do not stop or alter your dose of steroids without discussing it with your provider
Managing Steroid Side Effects
• Consistent schedule (AM vs. PM)
• Take with food
• Stomach discomfort: Over-the-counter or prescription medications
• Medications to prevent shingles, thrush, or other infections


Are Steroids Messing With Your Sunny Disposition?

Steroid Side Effects
• Irritability, mood swings, depression
• Difficulty sleeping (insomnia), fatigue
• Blurred vision, cataracts
• Flushing/sweating
• Increased risk of infections, heart disease
• Muscle weakness, cramping
• Increased blood pressure, water retention
• Stomach bloating, hiccups, heartburn, ulcers, or gas
• Weight gain, hair thinning/loss, skin rashes
• Increased blood sugar levels, diabetes


Infection Can Be Serious for People With Myeloma
Preventing infections is paramount.
Infection remains the leading cause of death in patients with multiple myeloma. Several factors account for this infection risk, including the overall state of immunosuppression from multiple myeloma, treatment, age, and comorbidities (e.g., renal failure and frailty).
IMWG Consensus guidelines and recommendations for infection prevention in multiple myeloma; Lancet Haematol.2022;9(2):143–161.
Infection Prevention Tips
Good personal hygiene (skin, oral)
Environmental control (avoid crowds and sick people; use a high-quality mask when close contact is unavoidable)



Report fever of more than 100.4°F, shaking chills even without fever, dizziness, shortness of breath, low blood pressure to HCP as directed.

As recommended by your healthcare team:
Immunizations:
Flu, COVID, RSV & and pneumococcal vaccinations; avoid live vaccines

Preventative and/or supportive medications (next slide)


Medications Can Reduce Infection Risk
Type of Infection Risk
Viral: Herpes Simplex (HSV/VZV); CMV

Medication Recommendation(s) for Healthcare Team Consideration
Acyclovir prophylaxis
Bacterial: blood, pneumonia, and urinary tract infection Consider prophylaxis with levofloxacin
PJP (P. jirovecii pneumonia)
Fungal infections
COVID-19 and Influenza
IgG < 400 mg/dL (general infection risk)
ANC < 1000 cells/μL (general infection risk)

Consider prophylaxis with trimethoprim-sulfamethoxazole
Consider prophylaxis with fluconazole
Antiviral therapy if exposed or positive for covid per institution recommendations
IVIg recommended
Consider GCSF 2 or 3 times/wk (or as frequently as needed) to maintain
ANC > 1000 cells/μL and maintain treatment dose intensity



GI Symptoms: Prevention & Management
Fluid intake can help with both diarrhea and constipation and helps kidney function
Constipation is more common in the induction phase
• Opioid pain relievers, antidepressants, heart or blood pressure medications (check with provider, pharmacist)
• Supplements: Calcium, Iron, vitamin D (rarely), vitamin B-12 deficiency Increase fiber
• Stay well hydrated
• Fruits, vegetables, high fiber whole grain foods
• Fiber binding agents – Metamucil® , Citrucel®, Benefiber®
Anorexia, the inability to eat, is common during transplant and resolves with time.
• Hydration is most important
• Small, frequent meals with a focus on protein intake
• You will work closely with a dietician to help monitor your calorie intake


Diarrhea is common during transplant and long-term maintenance therapy. Other medications and supplements
• Hydration is very important
• Electrolyte replacement is common
• Good skin care will help prevent irritation
• Stool exam may be needed to rule-out infection
• If no infection, anti-diarrheal medication may be prescribed



Discuss GI issues with healthcare providers to identify causes and make adjustments to medications and supplements


Management of Oral Side Effects
OTC dry mouth rinse, gel, spray are recommended. Advise patients to avoid hot beverages. Initiate anti-fungal therapy for oral thrush
Dexamethasone oral solutions “swish and spit” have been tried but with no proven benefit yet. Sour citrus or candies before meals are also recommended. Taste Changes


Dietary modifications with small bites, eating upright, and sips with food can help manage symptoms.
Some medications lead to weight gain, others to weight loss.
Dry mouth leads to taste changes which can lead to anorexia. Meet



Dry Mouth
Dysphagia
Catamero D, Purcell K, Ray C, et al. Presented at the 20th
Myeloma Society (IMS) Annual Meeting Nurse
September 27–30, 2023; Athens, Greece.

Skin and Nail Side Effects
Possible side effect to some treatments and supportive care medications
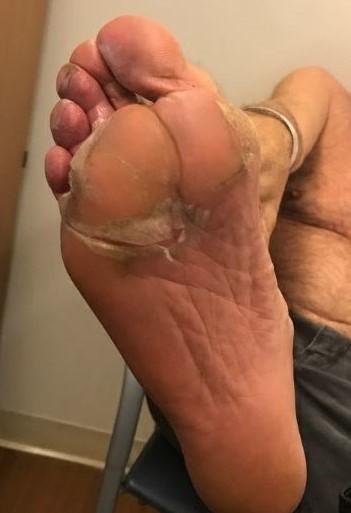
Skin Rash:
• Prevent dry skin; apply lotion
• Report changes to your care team
• Medication interruption or alternative, as needed
• Steroids:
– Topical for grades 1-2,
– Systemic and topical for Grade 3
• Antihistamines, as needed


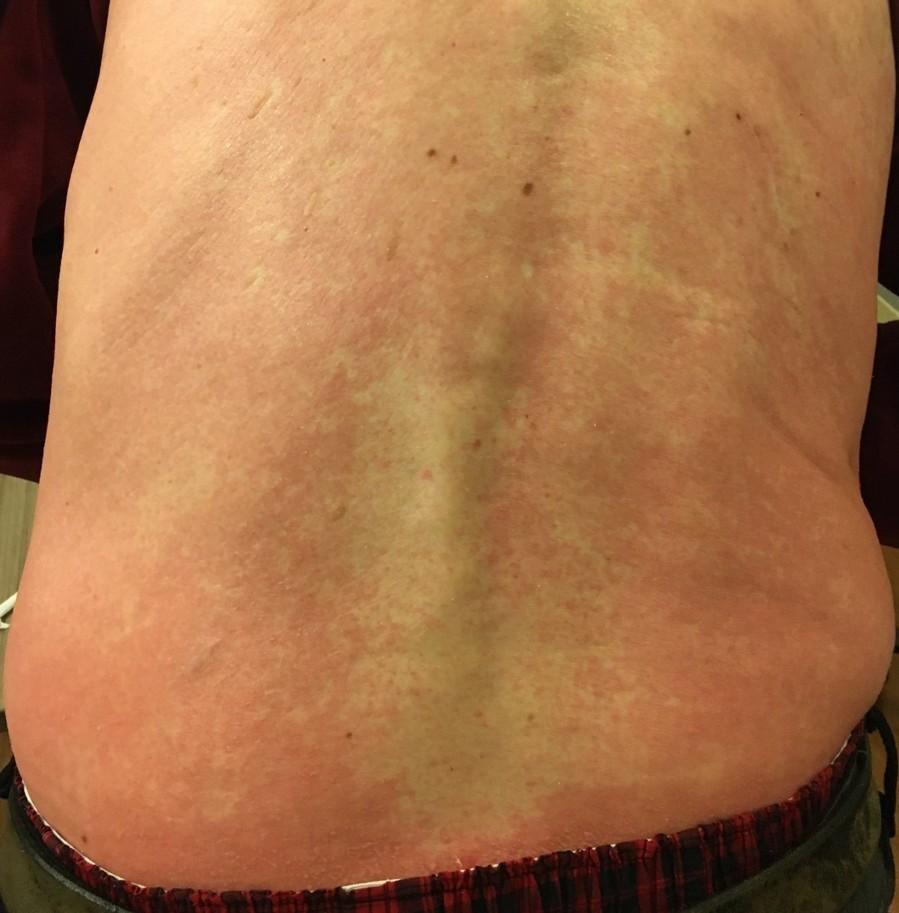


Nail Changes:
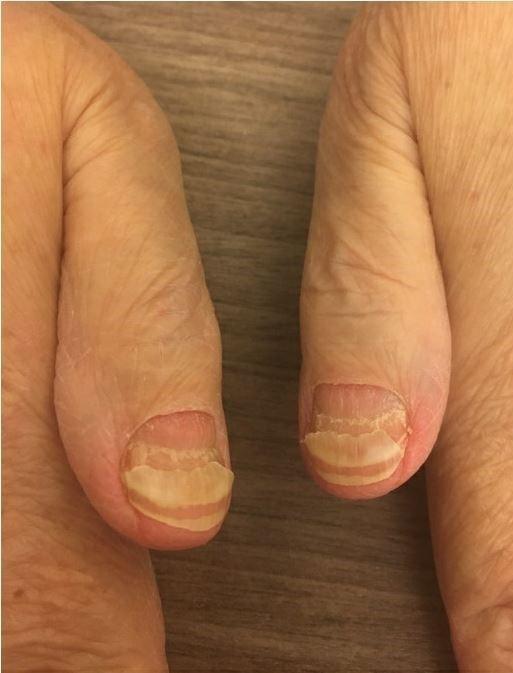
• Keep your nails short and clean. Watch for “catching and tearing”
• Apply a heavy moisturizer like Vaseline or salve. Wear cotton hand coverings to bed
• A nail hardener may help with thinning
• Tell the team if you have signs of a fungal infection, like thickened or discolored nails

Photos: Mount Sinai Hospital, NY, NY

Feel Like a Spring Chicken: Prevent and Manage Pain
Pain can significantly compromise quality of life
Sources of pain include bone disease, neuropathy and medical procedures
• Management
– Prevent pain when possible
• Bone strengtheners to decrease fracture risk
• Antiviral to prevent shingles
• Sedation before procedures
– Interventions depend on source of pain
• May include medications, activity, surgical intervention, radiation therapy, etc
• Complementary therapies (Mind-body, medication, yoga, supplements, acupuncture, etc)
• Scrambler therapy for neuropathy





Tell your healthcare provider about any new bone or chronic pain that is not adequately controlled


Peripheral Neuropathy Management
Peripheral neuropathy happens when there is damage to nerves in the extremities (hands, feet, limbs). Damage can be the result of myeloma, treatment or unrelated conditions (i.e. diabetes).
Symptoms:
• Numbness
• Tingling
• Prickling sensations
• Sensitivity to touch
• Burning and/or cold sensation
• Muscle weakness
Prevention / management:
• Bortezomib once-weekly and/or subcutaneous administration
• Massage area with cocoa butter regularly
• Neuroprotective Supplements:
– B-complex vitamins (B1, B6, B12)
– Green tea
• Safe environment: rugs, furnishings, shoes


If neuropathy worsens, your provider may:
• Adjust your treatment plan
• Prescribe oral or topical pain medication
• Suggest physical therapy
Report symptoms of peripheral neuropathy early to your healthcare provider; nerve damage from neuropathy can be permanent if unaddressed



Understanding Changes to Kidney Function
• Risk Factors
– Active multiple myeloma (light chains, high calcium)
– Other medical issues (ex: Diabetes, dehydration, infection)
– Medications (MM treatment, antibiotics, contrast dye)
– Poor Nutrition
• Prevention
– Stay hydrated – drink water
– Avoid certain medications when possible (eg, NSAIDs), dose adjust as needed
• Treatment
– Treatment for myeloma
– Hydration
– Dialysis




Many myeloma patients will experience kidney issues at some point; protecting your kidney function early and over time is important


Additional Supportive Care


Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Dimopoulous M, et al. Leukemia. 2009;23(9):1545-56. Brigle K, et al. CJON. 2017;21(5)suppl:60-76. Faiman B, et al. CJON. 2017;21(5)suppl:19-36.
Faiman B, et al. CJON. 2011;15suppl:66-76. Miceli TS, et al. CJON. 2011;15(4)suppl:9-23.


Summer of Success

Let the Sun Shine In
Fatigue
Fatigue is the most reported symptom.
Sources include anemia, pain, reduced activity, insomnia, treatment toxicity, bone marrow suppression 98.8%
Often, people do not share these symptoms with their providers. Talk to your provider about symptoms that are not well controlled or if you have thoughts of self-harm.


>35% of patients
of patients




Bee an Empowered Patient
Ask
questions
– What are my treatment options?
– What are the pros and cons of the different options?
– How will we know if treatment is working?
– What do the different labs mean?
– Who will be monitoring my labs?
– How can I access my test results (eg, patient portal)?
– What will we do if my treatment doesn’t work or quits working?
Participate in decisions
– Share your priorities and preferences
– Include care partner(s) in your discussion



Speak up if something seems different or unusual
– Normally 4 vials of blood but only drawing 3?
– Normally specialty pharmacy confirms delivery but haven’t heard from them this month?

Live in the sunshine, swim the sea, drink the wild air.
– When is my next appointment?
Communicate with your healthcare team
– Understand the roles of each team member
– Who to contact for your needs (eg, side effects, insurance issues, other)
Develop a support network
– Learn from others: IMF has many support groups or you can start one (IMF’s can help)


– Ralph Waldo Emerson

Care Partners Are Vital for Success


If you want to go fast, go alone, if you want to go far, go together
• Care partners may help with medical appointments, managing medication, daily living, physical assistance, emotional support, myeloma knowledge, healthy lifestyle, patient advocacy, financial decisions
• Care partners can be a spouse, close relative, a network of people (family, friends, neighbors, church members, etc)





African Proverb
• Caring for the Care Partner
– Recognize that caregiving is difficult/stressful
– Encourage care partners to maintain their health, interests, and friendships
– The IMF has information and resources to help care partners




Cultivate A Care Network



If you want to go fast, go alone, if you want to go far, go together
• Multiple studies demonstrate that strong social ties are associated with longevity, improved adherence to medical treatment and overall improved health outcomes
• Care partners can be a spouse, close relative, a network of people (family, friends, neighbors, church members, etc)
• Care partners may help with medical appointments, managing medication, daily living, physical assistance, emotional support, myeloma knowledge, healthy lifestyle, patient advocacy, financial decisions


• Caring for the Care Partner
– Recognize that caregiving is difficult/stressful
– Encourage care partners to maintain their health, interests, and friendships
– The IMF has information and resources to help care partners






Martino J, et al. Am J of Lifestyle Med. 2015;11(6):466-475. Yang YC, et al. Proc Natl Acad Sci U S A. 2016;113(3):578-583. Pinquart M and Duberstein PR. Crit Rev Oncol Hematol. 2010; 75(2):122–137.
African Proverb
IMF Care Giver Tip Cards

Enjoy Life’s Bounty

Harvest Good Health
Have a Primary Care Provider & Have Recommended Health Screenings
• Blood pressure
• Cholesterol
• Cardiovascular disease
• Diabetes
• Colonoscopy
• Women specific: mammography, pap smear
• Men specific: prostate
• Vision
• Hearing
• Dermatologic evaluation
• Dental checkups & cleaning

Develop & maintain healthy behaviors
• Good nutrition
• Regular activity
• Quit tobacco use
• Sufficient Sleep (next slide)
An ounce of prevention is worth a pound of cure. Benjamin
Franklin

Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Dimopoulous M, et al. Leukemia. 2009;23(9):1545-56. Brigle K, et al. CJON. 2017;21(5)suppl:60-76. Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Faiman B, et al. CJON. 2011;15suppl:66-76. Miceli TS, et al. CJON. 2011;15(4)suppl:9-23.






























Living the Myeloma Life: Local Patient & Care Partner
Pat Sullivan (Patient Advocate) &
Mark Cerda, PE (Care Partner)

Beyond Myeloma Therapy: Restoring Balance: Reiki and
Holistic Approaches to Well-Being
Martha Lacy, MD
Mayo Clinic, Rochester, MN

RESTORING BALANCE THROUGH SELF-CARE
Martha Lacy, MD
6/21/2025

WELCOME & OUR FOCUS TODAY

In my 33 years treating myeloma at Mayo Clinic, I was fortunate to see dramatic improvements in therapy and life span.
Yet, patients continue to suffer with stress, anxiety, and pain.
Today, we'll explore practical self-care tools for daily life.

THE MIND-BODY CONNECTION




Thoughts and emotions profoundly influence our physical health. By nurturing ourselves, we can positively impact our body's ability to cope and heal.

SIMPLE TOOLS THAT ANYONE CAN USE



Mindfulness Meditation

Connect with nature

Nourish your creativity

Reiki: access your vitality

MINDFULNESS: BEING PRESENT

Mindfulness is paying attention to the present moment without judgment.
It helps reduce worry about the past or future.
Mindfulness is a quality, meditation is a practice


MEDITATION: TRAINING YOUR MIND FOR CALM


Meditation trains your attention and awareness for a clear, calm mind.

No need for long sessions; even 2-5 minutes can make a difference.

Practical Tip: Explore guided meditations (many free apps/videos available) to help you get started.

Try this as a way to calm your nerves before an appointment or blood draw
TUNING INTO NATURE: THE HEALING OUTDOORS

Connecting with nature has profound healing benefits: Lowers stress hormones, improves mood, boosts immune function.

Practical Tip: Spend 10-15 minutes outdoors daily. Notice sounds, sights, and feelings
TAP INTO YOUR CREATIVITY

Engaging in creative activities like art, music, writing, or even gardening isn't just about making something beautiful – it's a powerful tool for well-being.

When we tap into our creativity, we unlock a host of benefits that can help us restore balance, manage stress, and reduce anxiety.

INTRODUCING REIKI: UNIVERSAL LIFE
FORCE ENERGY

Reiki (pronounced "Ray-key") is a Japanese technique for stress reduction and relaxation that promotes healing.

Based on the idea of "life force energy" (Ki) flowing through us.

When our Ki is balanced and flowing well, we feel healthy and vibrant.

HOW REIKI SUPPORTS WELL-BEING


A practitioner uses a gentle touch or places hands just above the body.This helps guide Universal Life Force Energy to areas where it's needed.
Benefits often include:
• Relaxation & Stress Relief
• Improved Mood
• Helps ease discomfort and boost energy.
• Better Sleep & Overall Calm
SURPRISE! YOU DON’T NEED TO BE IN THE SAME SPACE!

Reiki works at an energetic level; Practitioner and client don’t need to be physically together



We studied this in 30 myeloma patients at Mayo Clinic Originally conceived in 2019 to study in person reiki, by the time we were ready to launch in 2020, our methods had to be modified due to COVID-19 pandemic.

Patients randomly assigned to distance reiki weekly x 4, sham reiki weekly x 4, or no intervention

We found improved fatigue, pain interference and anxiety in the Reiki Group Cook, J et al Feasibility of Reiki in Multiple Myeloma: A randomized pilot on Health Related Quality of Life and Immune Function submitted to Integrative Medicine Reports
EXPERIENCING SELF-REIKI


You can learn Self-Reiki to bring comfort and calm to yourself anytime

Practical Exercise: Let's try a simple self-Reiki
session


BUILDING YOUR SELF-CARE TOOL KIT

Explore these tools and find what resonates with you.
Start small, be gentle and patient with yourself.
Notice your joy and empowerment grow as you learn to manage stress.
KEY TAKEAWAYS & EMPOWERMENT


You are empowered to actively participate in your well-being journey.

Small, consistent self-care actions create big positive impacts.


Embrace self-compassion. These practices complement your medical care; they don't replace it.



Apps & Websites: (e.g., Reiki.org, Calm, Headspace, Insight Timer for meditation).

Finding a Reiki Practitioner: Look for certified practitioners, ask for recommendations.

Book a reiki session with me (in person or distance): https://www.marthalacy.com


Q&A WITH PANEL

Closing Remarks
Robin Tuohy, Vice President, Patient Support International Myeloma
Foundation
Housekeeping Items
Presentation Slides: Are available by scanning the QR code, Instructions are on the QR code handout on each table.
Program Evaluations: evaluations at the end of today.

Parking: If you have parked at the hotel today, please check in with an IMF team member at the registration desk. There is no charge for parking, but we would record the number of our attendees who have parked at the hotel.
Badge Holders: Please return your badge holders and we can recycle them.
We greatly appreciate your time and feedback!



Thank you to our speakers & our sponsors!









OUR VISION:
A world where every myeloma patient can live life to the fullest, unburdened by the disease.
OUR MISSION:
Improving the quality of life of myeloma patients while working toward prevention and a cure.

IMF Core Values:
These are the core values we bring to accomplishing our mission each day.
Patient Centric
The patient experience is the focus of everything we do. Every interaction is an opportunity to establish a personal connection built on care and compassion which is the basis for continued support.
Respect All
As a team, we value honesty and transparency while creating a culture of mutual respect. We foster a myeloma community built on sincerity, authenticity, and kindness.
Excellence and Innovation
We value accountability, personal responsibility, and a steadfast commitment to excellence. We respect the legacy and reputation of our organization while seeking new solutions and advancements to improve outcomes, quality of life, and access to the best available resources for everyone impacted by myeloma.
Honor differences
We recognize each team member's skills and talents through collaboration and cooperation. Our programs aim to celebrate and support the diversity of our patients and their communities.





