May
Natalie
May
Innovation can inspire, but real change is unforgettable. Taiwan’s
healthcare companies are showing how smart ideas can meet human needs.Everynewsolutionbeginswithcuriosityandawishtomakelife better Fromlife-savingtreatmentstodigitaltoolsthatbringcaretopatients, thesecompaniesaretransforminghealthcare.
Technology grabs attention, but the real heart of the story is the people behindit.Scientists,engineers,andinnovatorsareaskingbigquestionsand buildingsolutionsthatreallyhelp.Theyaren’tafterawards;theyjustwant tomakelifebetter.Everynewtreatmentortoolbringshope,comfort,and bettercaretothepeoplewhoneeditmost.
In our recent edition, Taiwan’s Trailblazing Healthcare Companies: Shaping2025andBeyond,featuredin Insights Care,wecelebratethese visionariesandthestoriesbehindtheirsuccess.Youwillfindcompaniesthat are leading the way, learn about the ideas behind their work, and feel the energyofanindustrythatcaresasmuchasitinnovates.Theirstoriesshow thathealthcareisnotjustaboutscience.Itisaboutpeople,compassion,and thecouragetocreateabetterfuture.
Asyouexplorethesepages,letyourselfbeinspired.Thefutureofhealthcare isbeingwrittentoday,andTaiwan’scompaniesareprovingthatinnovation withheartcantrulychangetheworld.
HappyReading!
20
Telehealth and Beyond Taiwan’s Response to Modern Healthcare Demands The Front Page Exclusive The Story Within Industry Insights
06 ScinoPharm Taiwan, Ltd. Supporting Global Healthcare with Integrated Drug Solutions
16 Balancing Act How Taiwan’s Healthcare System Manages Accessibility and Affordability
26 T-E Pharma Holding Transforming Treatment with Modular and Targeted Therapeutics





Brief Featuring Person Organiza�on
Amaran Biotech amaranbiotech.com
Bioguard Corpora�on bioguardlabs.com
Tessie Che Chairperson and General Manager
Edward Lai President
Crystalvue crystalvue.com.tw
ScinoPharm Taiwan, Ltd. scinopharm.com
T-E Meds and Immunwork, Inc. temeds.com immunwork.com
CP Chuang COO
Li-An(Susan) Lu CEO
Dr. Tse-Wen Chang Founder
Amaran Biotech is a CDMO specializing in biopharmaceu�cals, offering process development, cGMP manufacturing, and expert asep�c fill-finish services.
Bioguard Corpora�on provides veterinary diagnos�cs, offering rapid tests, PCR systems, and lab services, cer�fied ISO/IEC 17025, serving Taiwan and China.
Crystalvue specializes in ophthalmic medical devices, offering fundus cameras and OCT systems, with global ODM/OEM services and high-quality cer�fica�ons.
ScinoPharm Taiwan specializes in high-quality APIs, focusing on oncology and CNS drugs, offering end-to-end R&D and commercial-scale produc�on.
T-E Meds specializes in developing an�body-drug conjugates (ADCs) and an�body-radionuclide conjugates (ARCs) using its proprietary mul�-arm linker technology. Its sister company, Immunwork, focuses on ultra-long-ac�ng pep�de drugs for endocrine diseases.


The Front Page Exclusive


The pharmaceutical industry is shifting quickly. Scientific progress is fast, rules are more complex, and there’s an urgent need for new medicines Companies face challenges beyond just research. Success nowmeansbeingflexibleinstrategy,stronginoperations,and uncompromising in quality to deliver medicines that truly makeadifference.Thosewhocansimplifythejourneyfrom discoverytodeliverywhilekeepingsupplychainssteadyare theonesshapingthefutureofhealthcare.
Since 1997, has earned a solid ScinoPharm Taiwan, Ltd. reputation as a trusted global partner It provides complete pharmaceutical services, from creating tough active ingredients to producing complex injectable medicines Beyond technical execution, ScinoPharm adds real strategic value by accelerating development, limiting handoffs, and ensuring reliable supply chains for complex therapies. Leading this charge is Li-An (Susan) Lu, the company’s President and CEO, whose strategic insight and purposeful leadershiparedrivingbetterhealthoutcomesworldwide.
Guided by Susan Lu’s vision, ScinoPharm has transformed from a pure API manufacturer into a comprehensive pharmaceuticalpowerhouse.Rightnow,thecompanyworks closely with innovators, specialty pharma, and generic drug makers to deliver important medicines to patients. Looking ahead to 2025 and beyond, it’s committed to keeping high scientificstandards,followingstrictregulations,andrunning smoothly—allmotivatedbyastrongdedicationtothepeople itserves.
Let’s see how ScinoPharm Taiwan combines cutting-edge science and strategic vision to accelerate drug development andimprovepatientoutcomesworldwide!
While her path to pharmaceutical leadership might seem unconventional,SusanLu’scareerhasbeendefinedbyaclear focus on strategic growth and operational excellence. “Although my background does not originate from the pharmaceuticalorhealthcaresectors,myprofessionalcareer has consistently centered on corporate strategy, organizational leadership, and operational excellence—with afocusondrivingbusinessgrowth,streamliningoperations, andcreatinglong-termvalue,”sheexplains.
Whatinspiredhertostepintothiscomplexindustrywasthe opportunitytoleveragethoseskillsinasectorwithprofound societal impact “This is an industry where advanced scientific knowledge, innovation, and deep intellectual rigor
converge in service of a vital mission: improving patient outcomes.Thatpurposeresonatesstronglywithme.”
Taking on the role of President and CEO was a deliberate decision driven by purpose as much as ambition. “I believe that strong leadership and sound strategy not only drive commercial success but also empower an organization to deliverlasting,positiveimpacttosociety.”Throughthislens, Susan applies her expertise to help translate scientific innovationintoaccessible,meaningfulsolutionsforpatients worldwide.

Sinceitsinceptionnearlythreedecadesago,ScinoPharmhas continuously expanded and refined its capabilities, steadily progressing from a pure-playAPI manufacturer into a fully integratedCDMO.“Apivotalmilestonewasreached12years ago when we strategically expanded into finished dosage formulations, transforming into a fully integrated pharmaceutical organization ” This strategic evolution broadened the company’s technical expertise and strengthened its competitive position along the pharmaceuticalvaluechain.
Throughout its growth, ScinoPharm has maintained an unyielding commitment to quality, regulatory compliance, and operational excellence. Its facilities undergo rigorous audits from global health authorities and industry partners alike, achieving the remarkable feat of zero-deficiency outcomes multiple times. Such consistency is no small feat, especiallygiventhevolatilityanddisruptionsinglobalsupply chains over recent years. Despite these challenges, “Even under pressure, we maintained uninterrupted operations and continued to fulfill the rigorous expectations of both regulatorsandcustomers.”
This resilience stems from a foundational sense of responsibility—notonlytocustomersandregulatorsbutmost importantly, to the patients who rely on ScinoPharm’s products. “We remain committed to ensuring the stable supply of high-quality medicines, upholding the highest standardsofintegrity,quality,andoperationaldisciplineinall aspectsofourwork.”Thisethosunderpinseverydecisionand guidesthecompany’songoingpursuitofexcellence.
DefiningDifferentiationinaCrowdedMarketplace
In a global pharmaceutical ecosystem teeming with capable manufacturers and CDMO providers, differentiation hinges onmorethanjustmeetingstandards—itdemandsarelentless
“Although my background does not originate from the pharmaceutical or healthcare sectors, my professional career has consistently centered on corporate strategy, organizational leadership, and operational excellence—with a focus on driving business growth, streamlining operations, and creating long-term value.”





drive to exceed them.As Susan Lu states, “At ScinoPharm, meeting baseline qualifications has never been a differentiator; it is a prerequisite.” The company’s competitive edge lies in its commitment to elevate these standardsconsistentlyovertime.
Technological innovation is central to this pursuit Continuous investment in scientific capabilities, process optimization, and manufacturing technologies keeps ScinoPharmattheforefrontofindustryadvancements.“Our teams proactively integrate new methodologies, improve platformefficiency,anddriveinnovationacrossallaspectsof our operations.”This proactive mindset ensures compliance is coupled with forward-thinking excellence, enabling the company to navigate complex, high-barrier pharmaceutical manufacturingwithconfidence.
Beyond technology, trust and long-term partnerships are pillars of ScinoPharm’s value proposition. Many client

relationships span years or even decades, grounded in a shared commitment to quality, transparency, and consistent delivery.“OurpartnersrecognizethatScinoPharmoffersboth the technical depth and the operational stability needed to support complex programs from development through commercial supply.” This blend of scientific excellence and reliability empowers clients to innovate fearlessly, knowing theirsupplychainpartnerisequallysteadfast.
In recent years, ScinoPharm has achieved several key milestones that showcase its operational excellence and regulatory rigor “Post-COVID-19, as travel and movement returned to normal, our API and sterile injectable facilities havesuccessfullyundergonemultipleU.S.FDAinspections. This continues our pre-pandemic track record of receiving zero 483s and serves as a compelling testament to the robustness and consistency of our quality systems.” Susan

“Our ultimate goal is to support patients through more effective, accessible, and high-quality pharmaceutical solutions, while serving as a trusted, long-term partner for our clients navigating the complexities of an increasingly dynamic global healthcare landscape.”

explainsthattheseinspectionscoveredawiderangeofsterile injectable formats—vials, liquids, cartridges, and prefilled syringes—highlighting the company’s expertise in handling complex,high-potencysterilemanufacturing.
Onthecommercialfront,ScinoPharmremainsavitalpartner insupplyingoncologyinjectablestotheU.S.marketandhas also supported successful launches for treatments targeting epilepsy, Alzheimer’s disease, and other critical conditions through its API supply These achievements reflect the company’s steadfast commitment to both innovation and quality.
Progress in its own product pipeline further underscores ScinoPharm’s growth. “One vial-format injectable and one liquid injectable product have received U.S. FDAapproval, while ANDA submissions for our cartridge and prefilled syringeproductsarecurrentlyunderreview.”Susannotesthat managingthesecomplexformats—ofteninvolving


“Post-COVID-19, as travel and movement returned to normal, our API and sterile injectable facilities have successfully undergone multiple U.S. FDA inspections. This continues our pre-pandemic track record of receiving zero 483s and serves as a compelling testament to the robustness and consistency of our quality systems.”


high-potency compounds and cold-chain requirements demonstrates their advanced technical capabilities and regulatory strength. These breakthroughs signifythecompany’sevolutionfromapureAPIsupplierto an integrated drug solutions provider with both proprietary productsandCDMOofferings.
Envisioning the future, ScinoPharm focuses on advancing verticalintegrationandexpandingintherapeuticareaswhere itcancreatethemostimpact.“Wearebuildingonourproven legacy in active pharmaceutical ingredients (APIs) while further expanding our capabilities in complex injectable formulations and combination products involving medical devices.” This comprehensive strategy simplifies supply chainsandbetteraddressespatientneeds.
The company targets key fields such as oncology, hematology,metabolicdisorders,andcentralnervoussystem diseases areas with significant unmet needs and rapid innovation. By aligning strengths in process development, complex manufacturing, and regulatory compliance, ScinoPharm aims to turn scientific excellence into real therapeuticprogress.
“Our ultimate goal is to support patients through more effective, accessible, and high-quality pharmaceutical solutions,whileservingasatrusted,long-termpartnerforour clients navigating the complexities of an increasingly dynamic global healthcare landscape.”This vision reflects a dual commitment to patient outcomes and sustainable partnerships.
ScinoPharm integrates environmental, social, and governance(ESG)principlesdeeplyintoitsoperations,with theCEOpersonallyoverseeingthiscommitment.
On the environmental side, the company focuses on waste reduction,energyefficiency,waterrecycling,andlow-carbon processes. Since 2023, it has voluntarily assessed productlevel carbon footprints following the Greenhouse Gas Protocolforgreatertransparency
Social responsibility includes fostering a diverse and inclusive workplace, enhancing health and safety, providing mental health support, and investing in employee development.Italsopromotesresponsiblesupplychainsand communityengagement.
In governance, “we have enhanced the oversight role of our Board and embedded sustainability into our governance framework through a cross-functional ESG structure encompassing risk management, regulatory compliance, cybersecurity,anddataprotection.”SusanLuemphasizesthat these efforts not only support steady growth but also build credibilityandtrustintheglobalpharmaceuticalecosystem.
Supply chain stability is fundamental to ScinoPharm’s operational excellence To navigate ongoing global disruptions, the company employs a comprehensive risk management strategy combining real-time supply chain monitoring with strategic diversification across suppliers, manufacturing partners, and geographic locations. Maintainingadequateinventoryreservesfurtherstrengthens itsabilitytoabsorbunforeseenchallenges.
Additionally, proactive scheduling of select product manufacturingallowsScinoPharmtoanticipateandmitigate supplyvolatilityeffectively
On the regulatory front, a dedicated team continuously monitors evolving global requirements, ensuring timely compliance and alignment with market standards. This integrated approach to managing both operational and regulatoryrisksempowersScinoPharmtomaintainseamless supply continuity for clients while safeguarding its own growthambitions.
ExcellenceandinnovationarecornerstonesofScinoPharm’s organizationalculture.Thecompanyfostersanenvironment ofself-awarenessandcontinuousimprovement,encouraging teams to identify gaps, challenge limits, and expand capabilities.
“We believe that true excellence demands purposeful innovation.”This ethos is supported by structured programs such as cross-functional projects, technology scouting, and open forums designed to generate actionable ideas that enhanceprocessesandunlocknewvalue.
Collaboration and shared learning are fundamental. The companyactivelypartnersexternallytoaccelerateknowledge transfer and integrates external expertise internally to maintainagilityandcompetitiveedge.

Arobusttalentdevelopmentframeworksupportsemployees at every career stage, offering tailored technical training, leadership programs, and mentorship opportunities aligned with strategic needs This comprehensive approach empowers employees to grow while advancing organizationalgoals.
Recognizing the complexity of modern drug development, ScinoPharmpositionsitselfasastrategiccollaboratorrather than merely a service provider Deep partnerships with biotech and pharmaceutical companies span the entire value chain—fromclinicaldevelopmentandregulatorystrategyto formulationandcommercialization.
By leveraging expertise in high-potency APIs and complex sterile injectable formulations, ScinoPharm adds technical depth and manufacturing reliability that complement partners’innovativestrengths.“Thesecollaborationsnotonly helpourpartnersadvancetheirprogramsmoreefficientlybut also enable us to broaden our own product portfolio and expandourparticipationinregionalandglobalmarkets.”
Eachpartnershiprepresentsaco-creationplatform,fostering sharedsuccessandreinforcingresilienceinarapidlyevolving healthcarelandscape.
Lookingbeyondthecompany’simmediatehorizon,SusanLu offers thoughtful advice for aspiring biotech entrepreneurs and young professionals. She emphasizes the importance of passion,purpose,andacomprehensiveunderstandingofthe pharmaceuticalecosystem.
“Whether you’re an entrepreneur or a young professional, I encourageyoutocontinuouslydevelopasolidunderstanding of the entire pharmaceutical value chain—from scientific ideation and R&D to regulatory pathways, manufacturing, and commercialization.” This broad perspective empowers individualstomakeinformed,strategicdecisionsthroughout theircareers.
Equally critical is maintaining humility, embracing lifelong learning, and fostering collaborative mindsets. “Whether within your own organization or across partnerships, shared effortsaccelerateinnovationandexpandyouroutlook.”
Finally,Susanwarmlyinvitesemerginginnovatorstoexplore strategic collaboration with ScinoPharm, leveraging the company’s extensive CMC capabilities and regulatory expertise to transform promising science into meaningful patientoutcomes.








www.insightscaremagazine.com
Taiwan’s health care system has been widely considered to be among the world’s best and most equitable. At its heart, it is an accessibility- and affordability-based system that grants citizens access to quality and timely care without placing economic strain. Its secret to success, however, is a precarious balancing act betweencoverageuniversalityandcostcontainment,onethat manynationsfindhardtoreplicate.
The pillar of Taiwan’s healthcare system is the single-payer NationalHealthInsurance(NHI)thatbeganin1995.Unlike most countries’ application of multi-payer systems, NHI consolidates financing and administration within a single national plan to allow for efficient provision of care. The scheme applies to every citizen and legal resident and is graduatedbyincome,hencemakingthesystemprogressive. This is a system that provides health care to every aspect of society, and not just low-income patients, the aged, and the chronicallyill.
Access to health care in Taiwan is unparalleled. The NHI coversabroadrangeofservices,frompreventivehealthand outpatient care to surgery and medication. The clinics and hospitalsaretidilydistributedgeographically,andruralareas arespecificallytargetedtopreventthelackofaccess.Patients
desire brief waits and to choose their carers, and therefore there is a competitive market driving high quality care. Technology has also been used inTaiwan to provide greater access.TheNationalHealthInsuranceAdministrationholdsa completeandcurrentelectronicrecordofpatientinformation and enables doctors to instantly view medical history and prevent duplicate procedures or testing. Affordability is the secondpillarofthesuccessofTaiwan’shealthcare.
Althoughthesystemofferscomprehensiveservices,itiscostsubsidizing The premiums are low compared to most developed Western countries, and out-of-pocket costs are minimal. Co-payments for hospitalization and doctor visits are limited to prevent excessive payments by patients. In addition, the NHI negotiates with medicine companies and medicalsuppliersdirectlytosecurefairpricesformedicines and procedures. This negotiating strength maintains health care costs in check and allows patients to gain worthwhile treatment The system-financing method also assists in maintaining the affordability of healthcare. NHI funds are derived primarily from contributions from payroll, supplemented by government and self-employed premium payments.Bythismeans,thecostissharedacrosssocietyso nosegmentisputunderpressure,andevenpoorpayerscanbe afforded treatment. The government further strictly controls

expenditure and alters premiums or reimbursement rates as requiredsoasnottorunonadeficit.
Against such accomplishments, however, Taiwan’s healthcaresystemremainschallengedtoprovideaccessibility asmuchasaffordability
Hanging over the horizon is the cost of an aging society Taiwan, like most of the developed nations, is confronting accelerated population transformation with an increasing numberofolderresidentswhohavetoseedoctorsmoreoften and at higher cost. Serving this population on a fiscally sustainable basis will require innovative policy steps like preventive care programs, disease management of chronic conditions,andshrewduseofmedicalresources.Thesecond is a balance between quality and cost. While system design promotesefficiency,providersareatriskofbeingencouraged to treat more in order to receive greater revenues and thus compromisethequalityofcare.Measurementofperformance andstandardizedprotocolareamongcontrolsthegovernment has established to manage such risks, but vigilance is a perpetualneed.
Taiwan’s experience offers valuable lessons for other countries seeking to achieve universal healthcare. By focusing on accessibility and affordability, the NHI demonstratesthatcompletecoveragecanbeachievedwithout going broke. The key strategies are single-payer to make administration simpler, progressive funding to be equitable, widespreadprovisionofservices,andcostprudencethrough negotiation and vigilance. While there are still challenges, Taiwan’s healthcare system keeps evolving with the perfect balance always directed towards human health. In brief, Taiwan’s health system is a delicate harmony between affordability and accessibility. With good planning and continuous improvement, it provides universal protection, high quality of care, and financial security to all. Naturally, demographicandeconomicpressuresthatwilltestthesystem overthenextfewyearscannotbewashedaway However,its guidingprinciplesandinnovativestrategiesofferaninspiring modelfornationsstrivingtodeliverequitableandsustainable healthcare.
-Pearl Shaw






Dr.Tse-WenChang Founder T-EMedsand Immunwork,Inc



-EPharmaHoldingunitestwo companies ImmunworkandT-EMeds—under oneparentorganizationfoundedbyDr.Tse-Wen Chang,abiotechpioneerwithdecadesofexperiencein antibodytherapiesandimmunology Immunworkfocuses onchronicandmetabolicdiseases,withastrongpipelineof long-actingpeptidedrugsthatsimplifytreatmentschedules andimprovepatients’qualityoflife.T-EMedsspecializes intargetedcancertreatments,developingadvanced antibody-drugconjugates(ADCs)andantibodyradionuclideconjugates(ARCs)designedtomaximize efficacywhileminimizingsideeffects.
Bothcompaniesoperateindependently,butshare knowledgeandresourceswithintheholding.This collaborativestructureacceleratesprogressfromresearchto clinicaltrialsandtacklescomplicatedmedicalchallenges moreefficiently.Italsoallowseachcompanytobuildonits strengthswhilecontributingtoabroaderportfolioof innovativetherapies.
SupportedbyTaiwan’sthrivingbiotechcosystem—characterized bystrongresearchinfrastructureandhighlyskilled talent—T-EPharmaHoldingiswell-positionedtorefine andbringtherapiesclosertopatients.By2025,thegroup aimstodelivertreatmentsthatarenotonlyinnovativebut alsopracticalandaccessible.Together,ImmunworkandT-E Medsarepoisedtomakeasignificantimpactonglobal healthcare,improvingoutcomesacrossawiderangeof diseases.
Let’s delve into the interview details below!
Couldyoushareyourpersonaljourneyinthehealthcare andbiotechfield,andhowitledtothefoundingofT-E PharmaHoldinganditstwosubsidiaries,T-EMedsand Immunwork?
Ihadbeenworkinginbiotechsincethelate1970s.Iwas partofthecoreteamdevelopingOKT3,whichdefinesthe antigenCD3,inearly1980atOrthoPharmaceutical,J&J, inRaritan,NewJersey.IcofoundedTanoxinHoustonin 1986andinventedtheanti-IgEtherapyin1987,whichled tothedevelopmentofXolair
Intheearlypartofthe2010s,severalimmunotherapiesfor cancer,includingantibody-drugconjugates,CAR-T,and immunecheckpointinhibitors,whichhadbeenexploredby immunologistsfordecades,allbegantoblossom.Ijoined thecheerstowitnessthehistoricalperiodofFDAapproval ofseveralnewdrugsinthesefields,butIrecognizedthat therewerestillchallengesahead.Manyofthenew therapiesthathadbeenapprovedorwereunder developmentcouldachieveresponseratesoflessthan50%, andsomeevenhadonly20or30%.
Onemajorreasonfortheseshortcomingswasthatthedrugs hadtoxicities,andpatientscouldnottoleratehigherand moreeffectivedoses.Irationalizedthatiflargerproportions ofthedrugwerebroughttothediseasesitesandsmaller proportionswereinotherpartsofthebody,thedrugscould achievehigherefficacyandcauselesstoxicity.Ithus
“By organizing each sub-platform under a dedicated company, T-E Pharma Holding ensures clear strategic focus for each technology line while maintaining an integrated innovation ecosystem. The synergy between Immunwork and T-E Meds enables efficient knowledge sharing, optimized resource allocation, and cohesive portfolio management, accelerating innovation and amplifying the collective impact of the holding company on global healthcare.”
conceptualizedthedevelopmentof“T-Epharmaceuticals,” whichcontainbothtargetingandeffectormoieties.Under thisconceptualdirection,Iinventedthe“multi-armlinker” technology Thesenewinventionspropelledmetofound Immunworkin2014.
HowdoT-EMedsandImmunworkcomplementeach other’smissionswithintheholdingstructure,andhow doesthissynergyaccelerateinnovation?
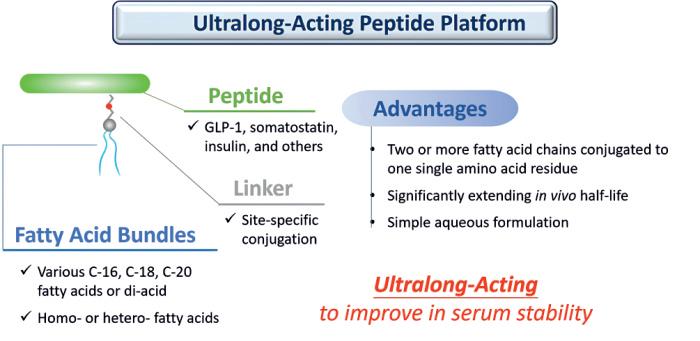
T-EPharmaHoldingownsanddrivesthedevelopmentof innovativetherapeuticsbasedonitsproprietaryT-E Pharmaceuticalsplatform,appliedthroughtwospecialized sub-platforms.ImmunworkfocusesontheFattyAcid Bundleplatformtocreateultra-long-actingpeptidedrugs, withleadcandidateslikeTE-8105andTE-8214currently advancinginclinicaltrials.Meanwhile,T-EMedsleadsthe DrugBundleplatform,dedicatedtodevelopingADCsand ARCsfortargetedcancertherapies.
Byorganizingeachsub-platformunderadedicated company,T-EPharmaHoldingensuresclearstrategicfocus foreachtechnologylinewhilemaintaininganintegrated innovationecosystem.ThesynergybetweenImmunwork andT-EMedsenablesefficientknowledgesharing, optimizedresourceallocation,andcohesiveportfolio management,acceleratinginnovationandamplifyingthe collectiveimpactoftheholdingcompanyonglobal healthcare.
Whatarethemostsignificantscientificortechnological breakthroughseachcompanyhasachievedinrecent years?
T-EPharmahaspioneeredamodulardrugdesignapproach throughitsproprietaryT-EPharmaceuticalsplatform, characterizedbytwocoreinnovations:
• T-EDrugConcept:ThecombinationofTargeting(T) andEffector(E)moietiesinapharmaceuticalmolecule toimproveefficacyandsafety.Example:TE-6168(by Immunwork),anovelfibrin-targetingthrombolyticfor ischemicstroke.
• Multi-ArmLinkerTechnology:Apatented,modular systemenablingflexible,efficientassemblyofcomplex drugmolecules—formingtwosub-platforms:
1. FattyAcidBundleplatform(Immunwork):Enables ultra-long-actingpeptidedrugs(e.g.,TE-8105andTE-

8214)withextendedhalf-lifeandimprovedsolubility.
2. DrugBundleplatform(T-EMeds):Enablessitespecific,dual-payloadADCswithhighDAR,e.g.,TE1146formultiplemyeloma.
Todate,T-EPharmaholdsover90globalpatentsand continuesadvancingfirst-andbest-in-classtherapeutics.
HowhasTaiwan’sbiotechecosystemandregulatory landscapesupportedyourgrowthandglobalambitions?
Taiwan’sbiotechecosystemhasprovidedstrongsupportfor ourgrowththroughgovernment-ledinitiatives,including innovation-friendlypoliciesthatencouragecutting-edge research.TheregulatorylandscapeinTaiwanoffersa supportiveandwell-structuredclinicaltrialenvironment, whichfacilitatesefficientexecutionoftrialsandhelpsus advanceournoveltherapies.


Moreover,Taiwan’srichtalentpoolandclose collaborationsbetweenacademiaandindustrystrengthen ourresearchanddevelopmentefforts.Lookingahead, Taiwan’sstrategiclocationandrobustsupplychain infrastructurewillfurtherenhanceourabilitytoexpandand operateglobally.Together,thesefactorscreateanideal environmentthatsupportsourproprietaryplatform technologiesanddrivesustowardachievingourglobal ambitions.
WhatpressingmedicalchallengesareT-EMedsand Immunworkaimingtosolve,andhowareyoursolutions differentfromthoseinthemarkettoday?
Immunworkfocusesonchallenging,chronicdiseaseslike obesity,type2diabetes,fattyliverdisease,neuroendocrine tumors,andacromegaly,whichrequirelong-termtreatment butlackeffectivetherapies.Ourproprietary“FattyAcid Bundle”platformenablesultra-long-actingformulations thatreduceinjectionfrequency,improvingpatient adherenceandqualityoflife.LeadcandidatesTE-8105and TE-8214areinclinicaltrials,showinggoodtolerabilityand promisingbenefits.
T-EMedsusesaproprietaryenzyme-free,site-specific conjugationtechnologywithits“Multi-ArmLinker”and “DrugBundle”platformstocreatehigh-DARADCs/ARCs withsuperioryield,purity,andflexibledesign.Wecan controlpayloadtype,number,andproperties,even
attachingtwodistinctpayloadstooneantibodyinasingle step.Ourleadcandidate,TE-1146,hasdemonstratedstrong preclinicalefficacyandismovingtowardfirstinhuman trialin2026,offeringbest-in-classpotentialfordifficult-totreatcancers.
HowdoesT-EPharmaHoldingensurethatinnovationis pairedwithaccessibility,affordability,andlong-term sustainability?
Weadheretoasetofverystrictcriteriatodesignand screenourproductcandidates,sotobehighlyconfidentthat ourcandidatesarecompetitiveamongtheproductsbeing developedfortherelateddiseaseindicationsbyvarious companies.Ourproductcandidatesshouldhavesubstantial differentiationfromotherproductswhicharealready approvedandmarketedorareunderdevelopment.Among thecriteriaweadoptarethatourproductcandidateshave convincingpharmacology,theirmanufacturingisfeasible, areadequatelyprotectedbyourpatentsorpatent applications.Theproductcandidatesaremoreefficacious, orsafer,orlesscostly Webelievethatseveralofour products,e.g.TE-8214andTE-8105,fitthesecriteria.
WheredoyouenvisionT-EMeds,Immunwork,andT-E PharmaHoldingasawholefiveyearsfromnow,and whatlegacydoyouhopetobuild?
Weaspiretocontinuetogrow,buildingvaluesby developingproductcandidatesthatfitintoourcriteria, advancingthesecandidatesthroughclinicaldevelopmentby ourselvesorbycollaboratingwithpartners.Infiveyears fromnow,wehopethatT-EPharmahasoneormore products,ascendingfromtheportfoliosofImmunwork,or T-EMeds,orboth,thatlookverypromisingtobeaworldclasspharmaceuticalproduct.WealsohopeT-EPharmais onthewaygrowingtobeaworld-classbiotechcompany
Toknowmoreabouttheirwork,youcanvisit: Immunworkwebsite T-EMedswebsite LinkedIn

The healthcare industry has evolved significantly in the last two years with technology innovations, demographics,andpatientexpectationchanges.One of the first countries to undergo change during the transition andmostresponsibleforhavingacost-effectiveandefficient healthsystemisTaiwan.Thecountryisquicklyconvergingto meet the current needs in the healthcare industry utilizing telehealth care, electronic medical records, and remote monitoring, among others, for convenience with quality services.
Telehealth, remote provision of health services by means of electronic communication, came into the healthcare of Taiwan. Driven at first by the COVID-19 pandemic, telemedicine allows patients to talk to doctors, get prescriptions, and follow up without hospitalization. It possessesseveraladvantages:itreducestheriskofspreadof infectiousdisease,enablesreliefofpressureonoverburdened hospitals, and provides access to care early in the course of diseasedevelopmenttothosewholiveinunderservedorrural communities. It provides continuity of care for chronically oriented diseases such as hypertension or diabetes through persistentvirtualfollow-upvisitsandlesslostworktime.
Taiwan’s National Health Insurance (NHI) scheme has been on the forefront of telehealth adoption. NHI does include telemedicine consultations, so it is accessible to the masses.
Theschemewouldmakeitappealingtopractitionersbecause itwouldreimburseforvirtualvisitsaswellasofficevisitsin suchamannerthatthepatientswouldnothavetopayextrafor it. In addition, there are government regulations for the qualityandsecurityofinternetconsultationsinordertoavoid misdiagnosis, data confidentiality, and privacy of information.
Taiwan’se-healthismorethantelemedicine.Taiwanspenta great deal building an island-wide system of electronic medical records (EMR). The patient’s history, lab results, images, and medication orders are entered into a secure, centralrepositoryandareaccessibletoauthorizedclinicians. Thesystemreducesduplicatetesting,enhancestheaccuracy ofdiagnosis,andstreamlinescollaborationamongspecialists. Thesystemalsoincreasespredictiveanalysisandpopulation health to allow the officials to monitor trends, redirect resourcesintheoptimalmanner,andreactinadvanceagainst public health crises Taiwan has even led in using telemonitoring and wearable technology Remote sensors monitoringvitalsigns,bloodglucose,orheartratewillbeable toprovideimmediatefeedbacktophysicians,andphysicians willhaveaquickerresponseifsomethinggoeswrong.
Toindividualresidentsorsinglepatientswholivealone,these technologies are an insurance blanket with the potential risk of continuous monitoring without the burden of repeated

hospitalstays.Theyhavealsobeenintegratedintotelehealth systems so that any alarm or anomaly is followed by an automatic virtual consultation, closing the care loop. PrioritizingpreventivemedicineisTaiwan’ssecondstrategy in its high-tech drive. It’s using electronic technology and telemedicine platforms for health promotion of healthy behavior, tracking immunization schedules, and health education Smartphone apps remind individuals to take medications, be tested for something, and follow doctors’recommended diet or exercise programs. As Taiwan transitionsfromreactivecaretoproactiveprevention,itaims to curb long-term healthcare spending while bringing in populationhealthgains.
With all these advances, however, come challenges. The largestchallengeisthedigitaldivide.Telemedicineandweb services are easily utilized by city dwellers, but elderly citizens or those dwelling in rural areas may lack the technologicalknowledgeorwebaccesstomakefulluseofit. Taiwan is attempting to fill the gap by making efforts at the societallevelsothatadequatetrainingandsupportarebeing madeavailablesothatnopartofsocietyremainsinthelurch. Ontopofthat,thereistheissueofbalancingvirtualandfaceto-face care for the health workers. Telehealth is definitely
suitable for consultation and follow-up, but there are some things one has to be on hand for, i.e., procedures, diagnosis, andemergencies.Itisaconstantjugglingacttodeterminethe levelofvalidvirtualandold-schoolcare.
Cybersecurity and information protection are also high priority With greater dependency upon digital media in healthcare,confidentialityofpatientinformationhasbecome thelargestissue.Taiwanhasimplementedstringentcontrols and complex encryption mechanisms to protect data, but vigilance and prompt response are required to stay ahead of constantly changing cyber attacks. Taiwan’s embracing of telehealth and use of technology in digital health is demonstrating its resolve to innovate against adversity Its incorporation with technology is making the nation stronger intheareasofaccess,effectiveness,andpatient-centeredness. Notonlyaretheseactionsmeetingneedsoftoday,butalsoare settingupthesystemforfuturecapacityinareasofpressureof agingpopulationandincreasedincidenceofchronicdisease.
Cumulatively, Taiwan’s transition away from an increase in healthcare demand illustrates the potential of telehealth and digitizationtoremakehealthcare.
-Pearl Shaw