



“The best way to predict the future is to invent it.”







“The best way to predict the future is to invent it.”


Innovation is the key to progress!
Thepathfromscientificdiscoverythroughlife-savingmedicationdevelopment
requiresfastandpreciseallianceworkalongwithinnovativemodern approaches.Organizationswhichefficientlymanagethiscomplex developmentalpathwayplayavitalroleinmakingnoveltreatmentsavailableto patientswhoneedthem.
Altasciencesservesasamodelorganizationwhichdeliversunifiedsolutionsfromstart tofinishacrossallstagesofearly-phasedrugdevelopment.Theorganizationapplies theircombinedcapabilitiesforpreclinicalandclinicalsolutionstogetherwithdrug formulationmanufacturingandanalyticalservicesalongsidebioanalysistospeedup developmentschedules.ThroughitsholisticmodelAltasciencessimultaneously optimizesoperationswhilenurturinginnovativepracticeswhichresultsinsafeand rapidmarketentryoftherapies.
TheinnovativeProactiveDrugDevelopmentapproachdemonstratesAltasciences’ dedicationtofindingnewsolutions.Throughearlyidentificationandhandlingof anticipatedchallengesthecompanyreducestheriskfactorsandacceleratesR&D progressfrompre-clinicalphasestoclinicalassertionstages.Thisstrategicapproach bothspeedsupdevelopmentprocessesanddeliverspreciseexecutionofeachphaseto providequickertherapiestopatientsinneed.
InInsightsCare’srecentissueEliteEdition:InnovativeCROPioneeringExcellence inEuropehighlightsthetransformativeworkofAltascienceswhilerecognizingtheir dedicationtoleadingearly-phasedrugdevelopment.InsightsCaretakespridein showcasingorganizationslikeAltascienceswhichexemplifyinnovativeleadership sincetheirgroundbreakingpracticesadvancehealthcareprogresswhileimproving patientoutcomes.
Hope you have an informative read ahead!




Visualiser
David King
Business Development Manager
Emily Jones
Technical Head
Jacob Smile
Digital Marke�ng Manager
Alina Sege
Editor-in-Chief Chidiebere Moses Ogbodo
Managing Editor Pearl Shaw
Art
Samuel Mar�nez
Marke�ng Manager
Bill Thompson
Execu�ve Editor
Natalie May
Co-designer Paul Belin
Business Development Execu�ves
Anna Smith, Jack Miller
Assistant Technical Head
Joseph Taylor
Assistant Digital Marke�ng Manager
Daniel Jones
Research Analyst
Eric Smith
Circula�on Manager
James Carter
Art & Picture Editor
Grace Brown
Sales Execu�ves
Mike, Carl, Kris
Technical Consultants David, Robert
SME-SMO Execu�ve Gemson
Email:

Email: info@insightscare.com For Subscrip�on: www.insightscare.com

We

ChrisPerkin CEO Altasciences


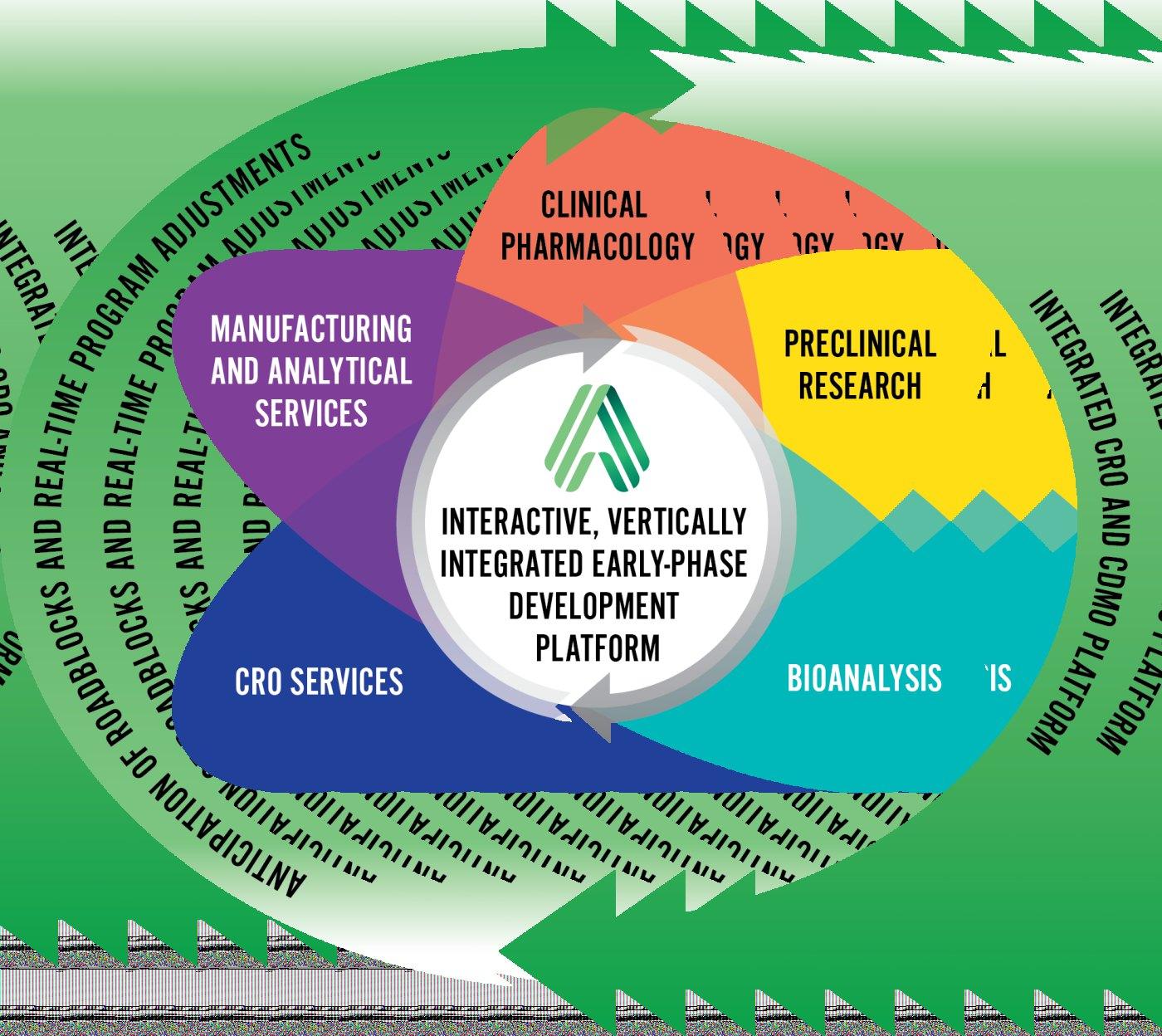
Revolutionizing Early-Phase Drug Development
Innovation can mean doing something differently—finding a new way. And that’s what we’ve done at Altasciences.
Intherelentlesspursuitofadvancinghealthcare,the
roleofcontractresearchorganisations(CROs)has becomepivotal.Leadingthewayinthisdomainis Altasciences—aCRO/CDMOdistinguishedbyitsunique approachandvisionaryoutlook.
ChrisPerkin,theCEOofAltasciencessince2010, embarkedonamissiontounitebrilliantscientificmindsto transformtheoutsourcingparadigmfordrugdevelopment. Altasciencesdoesn’tmerelyprovideresearchservices;it craftscomprehensivesolutionsthattraversetheentire spectrumofearlydrugdevelopment—frompreclinical researchtoearly-phaseclinicaltrialsandbeyond.
Altasciences’significantfootprintspansninefacilities acrossCanadaandtheU.S.,complementedbyastrategic presenceintheUK.Whatsetsthemapartistheir specialisationinseamless,end-to-endearly-phasedrug development.Thearrayofservicesoffered—preclinicaland clinicalsolutions,drugformulation,manufacturing, analyticalservices,bioanalysis,andthefullscopeof supportingCROservices—positionsAltasciencesasanallin-onesolutionforsponsors.
Our recent interview with Chris provides insight into the pioneering role of Altasciences in evolving the CRO industry!
Canyougiveusabriefoverviewofyourcareerinthe drugdevelopmentindustry?
Ihavebeeninthedrugdevelopmentindustryforalmost50 years.Istartedasapreclinicalstudydirectorin1975in England,andstayedinthepreclinicalspaceuntil2010, withstopsalongthewayinthepharmaceuticalindustryand amovetoCanada.IwentfromStudyDirectortoDirector ofToxicology,toGeneralManager,toSeniorCorporate
VicePresidentofalargeinternationalCRO.By2004,I managedthatsameCRO’spreclinicalsites:fourintheU.S., twoinCanada,andoneinChina.Since2010,I’vebeen CEOofwhatisnowAltasciences.
WhatinspiredyoutojoinAltasciences?
BeforejoiningAltasciences,Ioftenquestionedwhyallthe piecesneededfordrugdevelopment,particularlyearlydrug development,hadtobeseparate—contractingdifferent CROsfordifferentphasesandservices.Itnevermadesense tome.TheideaofasingleCROthatcouldmoveadrug throughalltheearlystagesofdevelopmenthadbeentalked aboutforyears,butnothinghadreallybeendonetomakeit happen.Iwasinspiredtotransformthisoutsourcing paradigm,andthat’sexactlywhatwe’veaccomplishedat Altasciences.
WhatarethecorevaluesuponwhichAltasciencesis built,andwhatisthemissionoftheorganisation?
Ourmissionisstraightforward:Wehelpsponsorsreach criticaldecision-makingmilestonesfasterbyimprovingthe speedandeaseoftheoveralldrugdevelopmentprocess.In fact,unlikemostintheindustry,weviewtheprocessasa continuumwithintersectingparts,insteadofaseriesof steps.
Intermsofvalues,webelieveincommunication, transparency,dependability,honesty,andgoodscience.We treatourclientslikecolleaguesandtheirprojectsasour own.Becauseweoftencollaboratewithsponsorsonmore thanonephaseofaprogramme,wereallygettoknowthem andeverydetailoftheirprojects,andarebestpositionedto supportthemthroughtheirjourney Thisiswhatwemean whenwesaywe“moveinunisontodeliverabigimpact withapersonaltouch.”


By aligning our services, teams, and sites, we eliminate communication gaps and transfer delays between various development stages.


Our vision will not change—we will continue to be the one-stop solution for early-phase drug development.
WhatmakesAltasciencesthemostpowerfuland integratedearly-phasedrugdevelopmentsolutioninthe industry?
I’llgiveyouthreewords:ProactiveDrugDevelopment. Thesewordsencapsulateourapproachandourpromiseto ourpartners.Weuniteallearly-phaseCROandCDMO activitieswithinoneorganisation,Altasciences—removing theneedformultipleserviceproviders,mitigatingrisk,and safelycondensingtimelines.Withteamsandsystemsthat talktoeachother,andoperationsthatarecentrally coordinated,theclientneverhastorepeatthemselvesas theymovetheirnewdrugthroughthedevelopmentprocess, ensuringasmootherandfastertransitionintothenext stages.
Wecansavesponsorsupto40%intime.Inturn,clientscan meettheirmilestoneswithease,gettingbetterdrugstothe peoplewhoneedthem,faster
HowdoyouensurethatAltasciencesstaysatthe forefrontofinnovationintheCROindustry,andwhat roledoyouplayindrivingthisinnovation?
Tome,innovationisnotsynonymouswith“new.” Innovationcanmeandoingsomethingdifferently—finding adifferentway Andthat’swhatwe’vedoneatAltasciences. Howwehaveintegratedandlinkedallthepiecesofearlyphasedrugdevelopmenttogetherisnotrocketscience,but itisinnovativeinourindustry Welistenedtowhat sponsorsweresayingabouttheirdrugdevelopmentpain pointsandcreatedsolutionsthataddressedthemhead-on. Andwecontinuetolistenandadapt—thisishowwestayat theforefrontofinnovation.
Overthelastfiveyearsalone,wehavesignificantly increasedcapacityandourportfoliothroughboth
acquisitionsandorganicgrowthtomeetthegrowingneeds ofourclients—we’veaddedsixlocations:aCDMOfacility inPhiladelphia,athirdclinicalunitinLosAngeles,and threemorepreclinicalfacilitiesinScranton,Sacramento, andColumbia;aswellasanofficeintheUK.We’vealso expandedmanyofourfacilities,themostrecentbeingan expansionofthebioanalyticallaboratoryatourpreclinical facilityinColumbia,aswellasa35,000-square-foot additiontoourCDMOfacility.
Wedriveinnovationthroughbothourscience(thecomplex researchwedo)andknowledgesharing.Wehavepublished almost50issuesofourownscientificjournal(The Altascientist),aswellasaplethoraofeBooks,audiobooks, webinars,andpodcasts.Wealsohostscientifictalksand presentscientificpostersatindustryconferences,in additiontoorganisingourownsymposia.Allofwhichgive opportunitiestoexchangewithandlearnfromother industryleaders.
CanyousharesomeexamplesofAltasciences’ pioneeringexcellenceinearly-phasedrugdevelopment inEurope?
NovoHoldings’acquisitionofAltasciencesin2021helped usincreaseourpresenceinEuropeandshortlyafter,we establishedanofficeintheUK—astrategicmovethat demonstratesourcommitmenttoprovidingenhanced servicesandsupporttoourpartnersandclientsinEurope. OurprogrammemanagersinEuropeareabletouseand sharetheirknowledgeofregionalnuances,fosterstrong relationships,andprovidetailoredsolutionstomeetthe specificneedsofourEuropeanclients.
Wehaveproactivelyformulatedrobuststrategiestosupport Europeancountriesinnavigatingregulatoryshifts, particularlyintheconductoffirst-in-humantrials.
WithMHRAandEUregulatorytimescalescausinglengthy delays,ourabilitytopursueINDsintheU.S.,andCTAsin Canada,isahugebenefittoourEuropeanclients.Wehave hostedtwowell-attendedsymposiainthepasttwoyearsin theUKtoexchangewithpeersandleadadiscussionabout fundraising,thestateofdrugdevelopmentinEurope,anda wealthofresearchtopics.
WithAltasciences,locationisnotanissue.Weharnessa proprietarycommunicationplatformandcentralised schedulingsystemtodeliveraseamlessprovisionofour expertise,irrespectiveofgeographicalboundaries,ensuring efficiencyandaccessibilityforourpartners.
WhatsetsAltasciencesapartfromotherCROsin Europe,andwhatuniquevaluedoesitoffertoclients?
AtAltasciences,wehavetheabilitytotakeadrugfrom preclinicaltestingintoclinicaldevelopment,including proofofconcept.Wecanalsosupportclientswiththeir drugmanufacturing.Thisisuniqueinthecurrentmarket, anditallowsEuropeanclientstopartnerwithoneCROfor theirearly-phasedrugdevelopment.
Ourprogrammemanagementteamfollowstheclient’s moleculeacrosseachofthesephases,providinga centralisedpointofcontact.Additionally,withprogramme managersacrossNorthAmericaandintheUK,sponsors canbenefitfromadualprojectmanagementstructure.With teamsworkingacrossvarioustimezones,ourprogramme andprojectmanagerscaneasilyprovideatleast12hoursof availabilityeachday.
Ourscientificandbusinessadvisoryteamsincludethree advisorsintheUK.Theyhaveadeepunderstandingofthe Europeanmarketandarereadilyavailabletoclients.
Whataresomeofthebiggestchallengesyouhavefaced asCEOofAltasciences,andhowhaveyouovercome them?
IjoinedAltasciencesbecauseIsawanopportunityto addresssponsors’painpoints.Iknewthat,asCEO,Icould implementmyvisionofaone-stop,integratedearlydrug developmentsolutioncompany.Atthetime,thiswasa revolutionaryidea,sothebiggestchallengewasobtaining buy-infrominvestors,employees,andpotentialclients.The firststepwasbuildingtherightteamoflike-minded individuals.Then,wehadtocommunicateourvision clearlyandeffectively
Mostofthesechallengesweresurmountedbyestablishinga stronginternalfoundation.Thiswasachievedthrough feedbackfromfocusgroupswithclientsandprospective clients,aswellasconsistent,candid,andopen communicationwithallstaffmembers,receptivenessto constructivecriticismandnovelideas,implementationof newprocessesandproprietarysoftware,andmost importantlytheacknowledgmentandcelebrationofteam accomplishments.Theprincipleofintegrationemergedas thecornerstoneofthecompany,recognisingthatithadto beingrainedinourculturefromtheverybeginningand couldnotbeback-engineered.
Howdoyoubalancetheneedforqualityand compliancewiththedriveforinnovationandefficiency intheservicesthatAltasciencesprovides?
QualityatAltasciencesmeansensuringourservicesare reliable,effective,andsafe,aswellasinlinewithall regulatoryguidelines.Rigorousqualitycontrolmeasures areembeddedinallaspectsofouroperations.


We treat our clients like colleagues and their projects as our own.

We can save sponsors up to 40% in time.
Weoperatewithinaframeworkofstrictregulationsand guidelineswithharmonisedSOPs,ensuringthatour servicescomplywithindustrystandards,legalmandates, andregulatoryrequirementsworldwide.Thisisnonnegotiable.
Ourpursuitofexcellencedoesn’tstopatadherenceto qualityandcompliance.Innovationandefficiencyare equallyintegraltowhoweare.Wecontinuouslyseek innovativesolutionsandworkflows,leveragingthelatest technologiesandmethodologiestoenhanceourservices, suchasdevelopingtheproprietarycommunicationplatform andthecentralisedschedulingsystemthatImentioned earlier Wealsocontinuallyoptimizeprocessesandrefine ouroperationstodeliverresultswithunmatchedefficiency
AtAltasciences,wetakeprideinpushingboundaries, exploringnewhorizons,andprovidingsolutionsthat anticipatetheevolvingneedsofourclientsandtheindustry, whilemaintainingthehigheststandardsofqualityand compliance.
HowdoesAltasciences’approachtoresearchand developmentdifferfromotherCROsinEurope?
ItcanbesummedupbythethreewordsIdefinedearlier: ProactiveDrugDevelopment.Ourabilitytosimplifyand streamlinebycentralisingdrugdevelopmentforsponsorsis whatmakesAltasciencesdifferent.Asponsorcanpartner withustoaccomplishalltheirearly-phasedrug development,eliminatingtheneedtocontractandmanage multiplevendorstogettheworkdone.Byaligningour services,teams,andsites,weeliminatecommunication gapsandtransferdelaysbetweenvariousdevelopment stages.Complementaryprogrammemanagementcentralises allscheduling,finance,andoverallcommunicationforour
clients—relievingtheadministrativeburdenontheirpart. Wearealsonotlimitedbyalinear,task-basedapproach. Oneexampleofthisishowwecanplanaclient’sclinical programmewhilepreclinicalsafetyassessmentisongoing sothattheycanstartfirst-in-humantrialsassoonasthey receiveregulatoryapproval.Withthistypeofparallel processing,wedelivervaluabledecision-makingdata, faster—withoutincreasingrisk.Inalltheseways, Altasciencesmakesthedrugdevelopmentprocesseasier andmoreefficientforthesponsor.
CanyouprovidesomeinsightsintoAltasciences’future plansanditsroleinshapingthefutureoftheCRO industry?
Ourvisionwillnotchange—wewillcontinuetobetheonestopsolutionforearly-phasedrugdevelopment.Weare committedtobuildingevenmorerelationshipswithclients forend-to-endprogrammesasthisiswheretheycan experiencethegreatestbenefitsofchoosingAltasciencesas theirpartner Toachievethat,we’lldoaswe’vealways done…nurturerelationships,buildstrategicpartnerships, andmeetclientswherevertheyareintheirjourney;we’ll continuetolistentoclients’needsandwants,andwilllook forwaystoprovideanevenmorecomprehensiveand strongersolution.Immediatefutureplansincludefurther expansionstoourpreclinicalsitesandbioanalytical laboratories.Additionally,wewillcontinuetogrowour presenceinEurope.
HowdoesAltasciencesintegratesustainabilitypractices intoitsoperationsforenvironmentallyresponsibledrug development?
Inourindustry,socialresponsibilitygoesbeyond compliance—it’safundamentalobligation.Wecontinueto striveforexcellencethroughdiversity,inclusion,anda
pledgetocreateapositivesocialchange.Ourgoalsinclude addressingcriticalissues,likeGHGandscience-based emissions,andupholdingtheUnitedNations’Global Compact’sTenPrinciples,whichcovershumanrights, labour,environment,andanti-corruption.Ourdedicationto ESGprinciplesformsthebedrockofourethos.
AllofourteamshavereceivedformaltrainingonESG principles.Wehavevariousprogrammesandpoliciesin placetosupportouremployees,andweworkhardtocreate apositiveworkenvironment.Weaimtosignificantly reduceouremissionsby2030.Furthermore,we’ve implementedethicalpoliciesinareassuchasanticorruption,dataprivacy,andinformationsecurity,andwe conductregularauditsofcontrolproceduresand informationsecurityriskassessments.
Whatadvicewouldyougivetobuddingentrepreneurs andenthusiastsaspiringtoventureintotheresearch space?
Don’tbeafraidtoquestionthestatusquo—toinnovate,to takechances,tochooseadifferentpath.Changeisslowin anindustrylikeours,andtheproven,establishedstandard isoftenpreferredoverout-of-the-boxthinking.WhenI startedonmyvisionforAltasciences,wereceivedalotof raisedeyebrowsbecauseithadneverbeendonebefore. Throughpassion,determination,andinnovation,wewere abletoprovethatthereisanotherwaytooutsource research;wedon’thavetosettleforthestatusquo. Perseveranceandagoodplanwillattractotherswhoare like-minded,andthatwillbecomeyourfoundation.

We view the process as a continuum with intersecting parts, instead of a series of steps.

Thefutureof medicinerelieson research;it'sthroughrigorous studythatweunlockthe potentialfornewtreatments andtherapies.”

www.insightscare.com





Dataprivacydealswithprotectingpersonal
informationinsuchawaythatcollection,storage, processing,andtransmissionofthesamecomply withapplicablelaws.Cybersecuritygenerallyrelatestothe protectionofsystems,networks,andprogramsfrom cyberattacksorunauthorizedaccess,althoughthetwoterms areapplieddifferently Thecasesofdatabreach,among othertypesofcyberattacks,withinformationrelatedto internalandsensitivedataofpersonsorentitiesleadtohigh costsofdoingbusiness:financiallosses,reputational damage,lawsuits,anderosionofcustomerconfidence.
Theneedfordataprivacyandcybersecuritybecamean emphasiswiththearrivaloftheGeneralDataProtection RegulationintheEuropeanUnionandtheCalifornia ConsumerPrivacyActintheUnitedStates.These regulationsplaceonerousrequirementsonhow organizationsmusthandlepersonalinformation,withvery expensivefineswhennotfollowedproperly.Itbecomes importantthatCROsmakesuretheirorganizationsabideby theseandmanagethesecurityrisksassociatedwiththem effectively
Thesophisticationandcomplexityofmoderncyberthreats arehardforanorganizationtooutsmart.It’slikehackers constantlyfindnewwaysofexploitinganygiven weaknessesinthesystemandapplication.Thesetactics includephishing,ransomware,andsocialengineering.The ever-evolvingthreatlandscapewillkeepforcingtheCRO toreassessandrevisehiscybersecuritystrategytoavoid anypotentialbreach.

Moreover,thenewattackvectorsavailedbythenascent adoptionofIoT,cloud,andmobilearemoredifficultto manage.ItfallsupontheCROtoensuretheirorganization ispreparedtorepelsuchemergingthreatswithoutbeingan obstacletoinnovationortechnologyadoptions.
Asmentioned,regulatorycompliancerepresentsa significantissueforCROs.Afterall,lawsandregulations relatedtodataprivacyandcybersecurityareofteninflux, addingnewrequirementsyearly.CROsmustensuretheir organizationsaremeetingtheseregulations-whichmaybe differentfromoneregiontoanother-andseverepenalties comewithfailuretofulfillthem,thereforeunderminingan organization’sstandingandreputationinthefinancial spheres.
Itisprettycumbersomeandtime-consumingtostayabreast ofchangesinglobalregulatoryrequirementsand implementthesamewithinanorganization.Further,most organizationsoperateinmorethanonejurisdiction,making complianceverychallenging.TheChiefRiskOfficerhasto maintaincomprehensiveknowledgeregardinginternational dataprivacylawsandcoordinatewiththelegaland compliancedepartmentsregardingadherencetorelevant frameworks.
•

Intoday’sinterconnectedworld,organizationsrelyona greatmanythird-partyvendorsandpartnersforeverything fromcloudstoragetodataanalytics.Aswitheverycoin, thereisaflipside:whilethesepartnershipsprovidegreat businessadvantages,theyalsoinvitecybersecurityanddata privacyrisks.TheCROhastotakestepswithaviewto assessingthesecuritymeasuresofthird-partyvendorsso thattheirpartnerswillfollowthesamesternstandardsthe organizationisfollowing.
Breachesthroughthird-partyvendorsarebecomingroutine, andeventsofthatkindnowhaveagreaterreachintothe integrityofbrandandprofits.Todevelopgoodpracticesby theCRO,periodicalauditingbyvendorsishighlyrequired andinvolvesbindingagreementssignedbythevendors outliningtheexpectationsaroundsecurity.Byandlarge,the exerciseisfoundcomplexandunwieldy.
• EmployeeTrainingandAwareness
Oneoftheweakestlinksinthechainwhenitcomesto cybersecurityistheemployeesthemselves.Nomatterhow advancedthetechnologicalsolutionorpoliciesare implemented,humanerrorstilltendstobetheleading factorindatabreaches,throughphishingattacks,poor passwordhabits,orotherformsofsensitiveinformation mishandlingthatplaceorganizationsatcyber-risk.
TheCROshouldpayextraattentiontocybersecurity awarenessanddataprivacytrainingforalllevelsof employees.Onlythencananysecurityculturedevelopment takeplaceinanorganization.Thishelpsanorganization reducerisksthroughregulartrainingandexercisesthat simulateattacks,clearlycommunicatepoliciesand procedurestotheemployeesforbetterawarenessto recognizethreats,andbestpracticesfordataprotection.
Mostcybersecurityanddataprivacymeasuresinvolvehuge investmentsintechnology,personnel,andtraining. However,inmanyorganizations,cybersecuritybudgetsare limited,andhencetheimplementationofcomprehensive securitymeasurescanbequitechallenging.TheCROmust balancetheneedforstrongcybersecuritywiththe organization’soverallfinancialconstraints.
Also,becausethespeedofchangeisrapidintermsof technologicalchanges,thesecuritysystemsalsohavetobe continuouslyupdated.Thus,aCROshouldhavefull involvementinthisdecision-makingwithexecutive leadership,resourceallocation,andcybersecurityconcerns remaininginfocus.
TheCROwillplayavitalroleintheworldofdataprivacy andcybersecuritychallenges.Theincreasingsophistication ofcyberthreats,coupledwithahardeningregulatory environment,requiresactiveriskmanagementpoliciesthat willbeinstrumentalinsensitivedataprotectionand businesscontinuity
Vigilance,investmentinappropriatetechnologies,and securityculturearethewaysinwhichCROswillleadtheir organizationsthroughthiscomplicatedlandscapeofdata privacyandcybersecurity.
-Natalie May
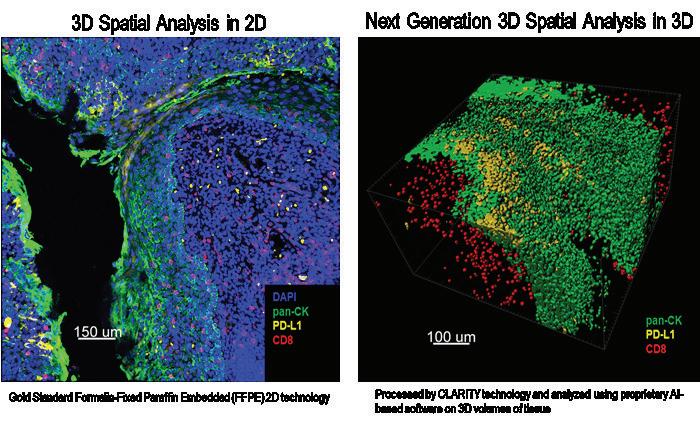

Intoday’sworldofclinicalresearch,thekeywordsare
efficiency,precision,andspeed.CROsneedtoconduct highlycriticalstudiesthathelpmedicaladvancements andregulatoryapprovals.Managementofclinicaltrials, coordinationbetweendifferentstakeholders,compliance withregulatoryrequirements,andvolumeofdatahave pushedcompaniestoacorner.Itisherethattheautomation ofworkflowhelpsinstreamliningprocesses,minimizing humanerrors,andimprovingproductivity.
Workautomationcanbedefinedasapplyingtechnologyto executerepetitiveandtime-consumingactivitiesofthe process.Inotherwords,theapplicationofsoftwareand otherdigitaltoolsforadministrativefunctionsandroutine datacollectionandotherrepetitiveactivitiesrelatedto investigationsinclinicalresearchstudiesfreesthe researchersandstafftodoothercriticalworksofdecisionmakingandanalysis.
Clinicalresearchdefinestheworkflowsinblockstructure coveringactivitiesrelatedtopatientrecruitment,regulatory approval,datacollection,monitoring,reporting,and trackingcompliance.Automationofworkflowwilloptimize theseprocessesthroughnotifications,allocation,and trackingtasksandapprovalswhilemanagingtheflowof dataautomatically.
BenefitofWorkflowAutomationinClinicalResearch
• IncreasedEfficiencyandProductivity
Themainbenefitofworkflowautomationisthatitenhances theefficiencyofoperations.Somefunctionsassociatedwith clinicalresearchincludeenrollmentofparticipants,data entry,sampletracking,andcasereportformmanagement. Automationfreestimeforresearchersandcoordinatorsto performsuchcomplexactivitiesasanalysis,patientcare, andclinicaldecision-making.


Forinstance,automatedpatientrecruitmentsystemscan filtereligiblecandidateswitheaseaccordingtosetcriteria andsendnotificationsforfollow-upautomatically This reducestheworkloadinfindingparticipants,hence acceleratingthetimelineofclinicaltrials.
• ReducedErrorsandEnhancedAccuracy
Humanerrorisoneofthemajorcausesofdelayand discrepanciesinclinicaltrials.Dataentryerrors,missed deadlines,andincorrectreportingwillimpingeonthe integrityofaclinicalstudyandmayleadtononcompliance withregulatorybodies.
Automatingsuchworkflowprocessesreducesthe occurrenceofsucherrors.Forinstance,anautomateddata entrysystemlimitshumaninputting.Thedatafedintothe systemwillthusbeaptandconsistent.Alertscanalsobe automatedtonotifyresearchteamsaboutmissing information,discrepancies,ordeadlinesinmaintainingdata integrityandcompliance.
• ImprovedCollaborationandCommunication
Clinicalresearchrequiresinvolvementoftheresearcher,the regulator,theparticipantofthestudy,andthehealthservice provider Anymisunderstandingormisdirectedflowof informationcontributestoslowdownsandlogjamsinthe entiremultidisciplinaryprocess.
Automationofworkflowsfostersbettercommunicationand collaboration.Thismaybefacilitatedwithcloud-based updatesinreal-time,studydatasharing,andtrackingof tasks.Automatednotifications,reminders,andalertswill helpkeepallthepeopleinvolvedonthesamepagefor bettercoordinationandtimelyexecutionoftasks.
• BetterComplianceandReporting
Clinicaltrialsareasourceofgreatregulationbyagencies suchastheU.S.FoodandDrugAdministrationandthe EuropeanMedicinesAgency.Itiscriticalthatthese regulationsbefollowednotonlytoavoidpossiblelitigation butalsotokeeptheresearchbeyondlegalandethical review Automationofworkflowensuresthatregulatory requirementsateachstagearemet.
Automationofsystemstrackstheregulatory documentation,patientconsentforms,andotheractivities relatedtocompliance.Additionally,automatedreporting toolsgeneratereal-timecompliancereports,makingthe presentationofvaliddataeasiertobepresentedbyteamsto regulatoryagenciesandauditors,reducingthethreatof
non-complianceandguidingtheresearchprocess appropriately
• CostReduction
Clinicalresearchisoneofthemostexpensiveprocesses thatdemandshugeinvestmentsinbothmanpowerand materialresourcesandconsumesmuchtime.Itautomated theworkflowtoreducecostsbydecreasinghumanlabor andstreamliningoperations.Forexample,automateddata processingtoolsspeedupdataanalysisforearlier availabilitytoresearchteamswithoutusingexpensive manuallabor
Automationofrecruitment,follow-upofpatients,and managementofdocumentscouldreduceadministrative costssincemoreresourcesareavailableforapplicationin otherusefulareas,suchasenhancementofpatientcareor augmentationofresearchcapability.
• PatientRecruitmentandEnrollment
Anautomatedsystemforrecruitmentcanfindandcontact theeligibleparticipantswiththehelpofpredefinedcriteria. Infact,itwillprovidecontinuedmonitoringtobringon necessaryimmediateadjustmentsintherecruitment strategy.
• DataManagementandReporting
Thisincludesthefollowingactivities:dataprocessing,data cleaning,andreportingwiththehelpofworkflow automationsoftware.Automaticsystemsensureproper capturingofdataanditsprocessingaccuratelyinorderto givetimelyinsightswithminimalchancesforanerror.
• RegulatoryCompliance
Automatethetrackingofregulatorydocuments,approvals, andreportstostaycompliant.Workflowtoolscaneven triggerreminderstohelpmakesurecriticalregulatory deadlinesaremet.
• MonitoringClinicalTrials
Itmeansthatreal-timetrialprogressdatafromthe automatedmonitoringtoolscouldshowifmilestoneswere reached,whethertheadverseeventshavebeendocumented, andpatientsmonitoredaccordingtotheprotocol.
- Natalie May












