2 minute read
The history of oscillatory reactions
Th e e B lousov-Zhabotisnky react i o n
The story of development and discovery of a new field of chemistry through rejection, failed publications and ultimately, scientific collaboration.
Advertisement
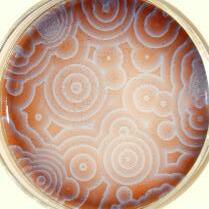
The Belousov- Zhabotisnky reaction is an oscillating reaction that was discovered by Soviet scientist Boris P. Belousov in 1951, when he was trying to investigate the reaction mechanisms of the citric acid cycle using Cerium ions, citric acid and bromate ions. He stumbled upon a reaction which seemed to show periodic colour changes, which continued for an hour before finally coming to a stop. This finding was fascinating as this seemed to directly violate the second law of thermodynamics. Compared to the 1950s, oscillatory and nonequilibrium reactions are a well known field of chemistry in the modern day; since the early 19th century, oscillating reactions have had applications in electrochemical cells which produce alternating current (G.T. Winfree), to measuring rates of chromium dissolution (Ostwald). Although oscillating reactions have been around for such a long time, our understanding of them was a lot different back in the days of G.T. Winfree. For one, it was a widely accepted belief that oscillating reactions had to be heterogeneous, meaning the reactants had to
be in different states. This was an accepted fact by the scientific community until Alfred J. Lotka proposed several kinetic models of an idealised homogenous oscillating reaction. This was received by William C. Bray, who was the first man to discover a genuine homogenous oscillatory reaction, the BrayLiebhaufsky reaction. The Bray-Liebhafsky reaction was described as a ‘chemical clock’ by Bray in 1921. It is the catalytic conversion of hydrogen peroxide to oxygen and water by iodate, the anion of iodic acid. When this reaction was first discovered, it was criticised by most chemists and the oscillations were said to occur due to heterogeneous impurities. The Belousov- Zhabotinsky reaction is considered to be the immediate descendant of the Bray- Liebhafsky reaction. It was also a homogenous oscillating reaction, and thus was wholly rejected by the scientific community for being in violation of the second law of thermodynamics. In fact, the paper that Belousov wrote was rejected by 2 scientific journals over a span of 7 years, making Belousov swear off publications for the rest of his career. He was told by the editors of the journals that they would only publish the reaction if he also presented a correction of the second law of thermodynamics in favour of his reaction, which he couldn’t achieve. Later, with the help of Western scientists, namely Field, Koros and Noyes, it was shown that the reaction was not actually violating any fundamental laws, as equilibrium was actually reached when oscillations stopped, and the Belousov-Zhabotinsky reaction went on to influence the creation of a new field of non-equilibrium dynamics. However, in the modern day and age, the Belousov- Zhabotinsky reaction is widely
used in areas such as self-oscillating gels and polymers which are commonly used in biomimetic actuators in order to gain a better understanding of autonomous biological processes, and self-actuating gel pumps that don’t require electrical components and are driven by chemo-mechanical energy from the reaction.







