Superalkali Aza-Cryptand Alkalide Ion Pairing Interactions Karishma Ramcharan, Daniel P. Miller*
Introduction The study of alkalides and their structure in solvent has been quite elusive due to the relative instability of superalkali-alkalides. Typically, alkalides decompose within a couple of hours/days due to their strong reducing potential. Therefore, a Density Functional Theory computational study was performed herein to investigate the structure of alkalides for Potassium and Sodium about the superalkali Potassium and Sodium Hexamethylated Aza-Cryptand [2.2.2].
Results
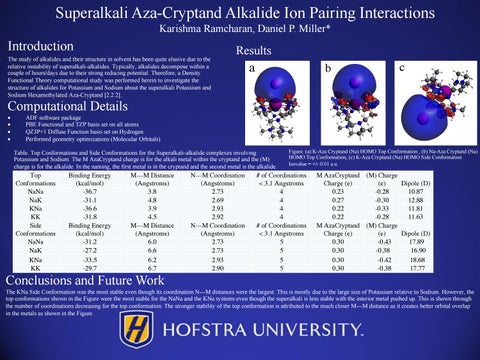
a
b
c
Computational Details • • • •
ADF software package PBE Functional and TZP basis set on all atoms QZ3P+1 Diffuse Function basis set on Hydrogen Performed geometry optimizations (Molecular Orbitals) Table. Top Conformations and Side Conformations for the Superalkali-alkalide complexes involving Potassium and Sodium. The M AzaCryptand charge is for the alkali metal within the cryptand and the (M) charge is for the alkalide. In the naming, the first metal is in the cryptand and the second metal is the alkalide.
Top Conformations NaNa NaK KNa KK Side Conformations NaNa NaK KNa KK
Binding Energy (kcal/mol) -36.7 -31.1 -36.6 -31.8 Binding Energy (kcal/mol) -31.2 -27.2 -33.5 -29.7
M---M Distance (Angstroms) 3.8 4.8 3.9 4.5 M---M Distance (Angstroms) 6.0 6.6 6.2 6.7
N---M Coordination (Angstroms) 2.73 2.69 2.93 2.92 N---M Coordination (Angstroms) 2.73 2.73 2.93 2.90
Figure. (a) K-Aza Cryptand (Na) HOMO Top Conformation , (b) Na-Aza Cryptand (Na) HOMO Top Conformation, (c) K-Aza Cryptand (Na) HOMO Side Conformation Isovalue = +/- 0.01 a.u.
# of Coordinations < 3.1 Angstroms 4 4 4 4 # of Coordinations < 3.1 Angstroms 5 5 5 5
M AzaCryptand (M) Charge Charge (e) (e) Dipole (D) 0.23 -0.28 10.87 0.27 -0.30 12.88 0.22 -0.33 11.81 0.22 -0.28 11.63 M AzaCryptand (M) Charge Charge (e) (e) Dipole (D) 0.30 -0.43 17.89 0.30 -0.38 16.90 0.30 -0.42 18.68 0.30 -0.38 17.77
Conclusions and Future Work The KNa Side Conformation was the most stable even though its coordination N---M distances were the largest. This is mostly due to the large size of Potassium relative to Sodium. However, the top conformations shown in the Figure were the most stable for the NaNa and the KNa systems even though the superalkali is less stable with the interior metal pushed up. This is shown through the number of coordinations decreasing for the top conformation. The stronger stability of the top conformation is attributed to the much closer M---M distance as it creates better orbital overlap in the metals as shown in the Figure.