Pedagogy of Microfossil Identification: Learning Foraminifera Species Amanda Perrone and Dr. E. Christa Farmer Hofstra Geology, Environment, & Sustainability Abstract:
Identifying foraminifera fossils requires critical thinking skills and an understanding of the unique characteristics of each species.
a)
Results:
b)
Introduction:
At the pinnacle of our climate crisis, it is more imperative than ever that the Earth’s past climate is understood. Microfossils contain atomic information within their compounds that provide clues to what Earth was like in the past. Fossilized planktonic foraminifera are one type of microfossil that is commonly used as a paleoclimate proxy because of their widespread availability in the world’s oceans for millions of years. Differentiating the species is essential since there are hundreds of species with varying paleoclimate purposes (Katz et al.). For example, surface water foraminifera species can be used to approximate surface water temperature and the thermocline from their relative abundance (Katz et al.). Foraminifera samples used in this study are from sediment core site U1313 of the Integrated Ocean Drilling Program (IODP) (Expedition 306 Scientists, 2006), pictured in figure 1. Sediment samples were catalogued by meters composite depth, or MCD, of 3 cores combined (Expedition 306 Scientists, 2006). The species identification is pursued with the purpose of attained relative abundance for each species. In particular, the species which are selected for this study are Neogloboquadrina pachyderma, Neogloboquadrina incompta, and Turborotalita quinqueloba. The ratio of relative abundance of N. pachyderma to N. incompta represents temperature with a greater amount of N. pachyderma representing colder temperatures (Eynaud, 2011).
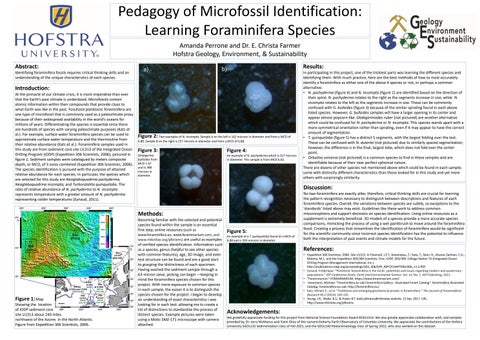
Figure 2: Two examples of N. incompta. Sample A on the left is 162 microns in diameter and from a MCD of 6.88. Sample B on the right is 257 microns in diameter and from a MCD of 6.88.
Figure 3: Globigerina bulloides from MCD 7.37 and is 398 microns in diameter.
Figure 4:
An example of N. pachyderma which is 317 microns in diameter. This sample is from MCD 6.02.
Discussion:
Methods:
Becoming familiar with the selected and potential species found within the sample is an essential first step; online resources (such as www.foraminifera.eu, www.foraminarium.com, and www.mikrotax.org/pforams) are useful as examples
Figure 1: Map
Showing the location of IODP sediment core site U1313 about 240 miles northwest of the Azores in the North Atlantic. Figure from Expedition 306 Scientists, 2006.
In participating in this project, one of the trickiest parts was learning the different species and identifying them. With much practice, here are the best methods of how to most accurately identify a foraminifera as either one of the above 4 species or not, or perhaps a common alternative: • N. pachyderma (figure 4) and N. incompta (figure 2) are identified based on the direction of their spiral. N. pachyderma rotates to the right as the segments increase in size, while N. incompta rotates to the left as the segments increase in size. These can be commonly confused with G. bulloides (figure 3) because of the similar spiraling found in each above listed species. However, G. bulloides samples will have a larger opening in its center and appear almost popcorn-like. Globigerinoides ruber (not pictured) are another alternative which could be confused for N. pachyderma or N. incompta. This species stands apart with a more symmetrical orientation rather than spiraling, even if it may appear to have the correct amount of segmentation. • T. quinqueloba (figure 5) has a distinct 5 segments, with the largest folding over the test. These can be confused with N. dutertei (not pictured) due to similarly spaced segmentation; however, the difference is in the final, largest lobe, which does not fold over the center point. • Orbulina universa (not pictured) is a common species to find in these samples and are identifiable because of their near perfect spherical nature. There are dozens of other species not mentioned above which could be found in each sample; some with distinctly different characteristics than those looked for in this study and yet more others with surprisingly similarity.
of verified species identification. Information such as a species, genus (helpful to see other species with common features), age, 3D image, and even test structure can be found and are a good start to grasping the distinctness of each specimen. Having washed the sediment sample through a 63-micron sieve, picking can begin —keeping in mind the foraminifera species chosen for this project. With more exposure to common species in each sample, the easier it is to distinguish the species chosen for the project. I began to develop an understanding of exact characteristics I was looking for in each test: allowing me to create a list of distinctions to standardize the process of distinct species. Example pictures were taken using a Motic SMZ-171 microscope with camera attached.
Figure 5:
An example of a T. quinqueloba found at a MCD of 6.88 and is 203 microns in diameter.
No two foraminifera are exactly alike; therefore, critical thinking skills are crucial for learning the pattern recognition necessary to distinguish between descriptions and features of each foraminifera species. Overall, the variations between species are subtle, so exceptions to the ‘standards’ listed above may exist. Guidelines like these work to address common misconceptions and support decisions on species identification. Using online resources as a supplement is extremely beneficial. 3D models of a species provide a more accurate species comparisons, mimicking the process of using a wet paintbrush to move around the foraminifera fossil. Creating a process that streamlines the identification of foraminifera would be significant for the scientific community since incorrect species identification has the potential to influence both the interpretation of past events and climate models for the future.
References: • Expedition 306 Scientists, 2006. Site U1313. In Channell, J.E.T., Kanamatsu, T., Sato, T., Stein, R., Alvarez Zarikian, C.A., Malone, M.J., and the Expedition 303/306 Scientists. Proc. IODP, 303/306: College Station TX (Integrated Ocean Drilling Program Management International, Inc.). http://publications.iodp.org/proceedings/303_306/EXP_REPT/CHAPTERS/306_11 2.PDF • Eynaud, Frédérique. "Planktonic foraminifera in the Arctic: potentials and issues regarding modern and quaternary populations." IOP Conference Series: Earth and Environmental Science. Vol. 14. No. 1. IOP Publishing, 2011. • “Foraminarium.” FORAMINARIUM, https://www.foraminarium.com/. • Hesemann, Michael. “Foraminifera.eu Lab Foraminifera Gallery - Illustrated Foram Catalog.” Foraminifera Illustrated Catalog, Foraminifera.eu Lab, http://foraminifera.eu/. • Katz, Miriam E., et al. "Traditional and emerging geochemical proxies in foraminifera." The Journal of Foraminiferal Research 40.2 (2010): 165-192. • Young, J.R., Wade, B.S., & Huber B.T. (eds) pforams@mikrotax website. 21 Apr. 2017. URL: http://www.mikrotax.org/pforams
Acknowledgements: We gratefully appreciate funding for this project from National Science Foundation Award #1911514. We also greatly appreciate collaboration with, and samples provided by, Dr. Jerry McManus and Yuxin Zhou of the Lamont-Doherty Earth Observatory of Columbia University. We appreciate the contributions of the Hofstra University GEOL135 Sedimentation class of Fall 2021, and the GEOL140 Paleoclimatology class of Spring 2022, who also worked on this dataset.