




We welcome contributions from Hepatitis SA members and the general public. SA Health has contributed funds towards this program.







We welcome contributions from Hepatitis SA members and the general public. SA Health has contributed funds towards this program.
When we at Hepatitis SA talk with members of the community about hepatitis C treatment, we usually point out that the current available cure works for more than 95% of people, and has little or no side-effects. This cure, consisting of a type of medication known as as direct-acting antivirals (DAAs), is available on the Pharmaceutical Benefits Scheme (PBS) in Australia.
A new, large-scale study by Gilead Sciences (one of the pharmaceutical companies that makes the medication, so they have a vested interest in its success) has demonstrated that the cure rate with sofosbuvir and velpatasvir (usually taken together over a 12-week period) is actually almost 99%.
The study, of more than 7000 patients in Australia, Canada, Europe, and the US, all treated with sofosbuvir/velpatasvir for hepatitis C virus infection reported a sustained virologic response (SVR) rate of 98.9%. SVR is when the virus is no longer detectable 12 to 24 weeks after treatment finishes.
“These results emphasize the broader applicability of these treatments worldwide.”
SVR rates were consistently high for both male and female patients, including those with genotype 3 (traditionally one of the harder genotypes of hepatitis C to cure), as well as those with cirrhosis.
“Our data show that even in the presence of more challenging factors like genotype 3 and cirrhosis, SVR rates remained above 97%, which is a strong indicator of the effectiveness of these therapies,” Dr Osinusi explained.

Dr Anu Osinusi
Dr Anu Osinusi (Vice President, Virology. Head, Hepatitis, Respiratory and Emerging Viruses at Gilead Sciences) said the results were really exciting. “The study demonstrates that the high effectiveness of sofosbuvir and velpatasvir in real-world populations aligns with previous findings, reinforcing the global applicability of current HCV treatment guidelines.”
“We’ve seen that these pan-genotypic direct-acting antiviral agents for hepatitis C have consistently shown high SVR rates across diverse populations,” Osinusi said.
While SVR rates remained high across both sexes and age groups, the study also examined what is known as the time-totreatment initiation (TTI), which is the interval between diagnosis and the beginning of therapy.
“The key message here is that minimizing the time to treatment initiation will result in better outcomes for all patients, reducing the risk of severe liver complications and increasing treatment rates across the board.
“The urgency to treat and minimise delays is crucial. Over time, it will result in fewer people having poor liver outcomes and will improve treatment rates for everyone infected with hepatitis C,” explained Dr Osinusi.
With cure rates at near-99% and better results from getting treated early, there has never been a better time get treated for hepatitis C.
READ MORE: bit.ly/SVR989
Held on 28 July, World Hepatitis Day is an internationally observed annual awareness day for viral hepatitis. It is one of nine global public health days officially mandated by the World Health Organization (WHO).
This year, the aim for World Hepatitis Day in Australia is to mobilise efforts to eliminate viral hepatitis by the WHO’s 2030 target. The month around World Hepatitis Day is busy with activities.
In South Australia, Hepatitis SA, in partnership with Relationships Australia, is running two free liver fibroscan clinics at suburban shopping centres. In addition, there are three free online information sessions (“Awareness starts with the ABCs”), open to anyone who is interested to learn about viral hepatitis, how to stay safe and how to support those living with chronic hepatitis B and C.
Clockwise: Parliament House glows green in 2023; Educator Yingbin in Point Pearce; and T-shirt painting at the Migrant Resource Centre

On 28 July, the Adelaide Oval, Footbridge and SA Parliament House will be lit green, as will the Elemental sculpture in Victor Harbor and Hepatitis SA’s premises in Hackney.
We are also leaning into the glowing green campaign by going green. Several groups, including nurses, community members and drug and alcohol service providers have taken up our invitation of decorating green T-shirts to wear in the week around 28 July. Kicking off was the Migrant Resource Centre in Elizabeth which had a fun-filled painting session. The T-shirt painting also capped off an education session at the Aboriginal Health Southern Yorke and Northern Local Health Network in Point Pearce the following week.
Read more about World Hepatitis Day on the Community News site (hepsa.asn.au/ communitynews) and the World Hepatitis Day site (worldhepatitisday.org.au).



In Australia, there are two diseases which, together, kill nearly 1000 people every year. They affect close to 300,000 people and are also the leading causes of rising liver cancer deaths. Here’s the puzzle: we have the tools to stop this.
One disease – hepatitis C – is curable, and there is a vaccine for the other – hepatitis B. Yet hepatitis B cases, and related deaths, are still increasing and needless hepatitis C deaths continue.
Experts will tell you the reasons for this are complex; nonetheless, there are simple steps which can be taken now that will reduce the grief caused by these two chronic diseases. One in three people living with chronic hepatitis B have not been diagnosed, and one in five of those with chronic hepatitis C are not diagnosed. Going to your GP to ask for tests and scans can be challenging for anyone. Add to that community-based testing.
World Hepatitis Day on 28 July is a time to highlight the need for action. To address the alarming increase in hepatitis B cases and related deaths, Hepatitis Australia is calling for:
• expansion of hepatitis B testing by offering it to all people over the age of 25 (people born before routine vaccination); and for whom there’s no record of hepatitis B status.
• strengthening monitoring of hepatitis B vaccination given after birth, to ensure all babies in Australia receive the required doses on time.
• establishment of a hepatitis B care facilitation and monitoring program to reach the 75% of people living with hepatitis B who are not in regular recommended care, in order to connect them to ongoing care and support.


Australia’s next National Hepatitis B Strategy and National Hepatitis C Strategy outline Australia’s commitment to eliminating these viruses as public health threats by 2030.
The national strategies specify national targets for elimination:
Reduce new hepatitis B infections
Less than 1 in 1,000 children under 5
Reduce new hepatitis C infections by 90% Fewer than 5 new cases per 100,000
Reduce deaths from hepatitis B by 30%

At current rates of progress, Australia will not achieve any of its viral hepatitis elimination targets. With 2030 fast approaching, the TIME FOR ACTION is now! Australia can lead the world in hepatitis elimination.
Nearly 220,000 people in Australia are living with chronic hepatitis B.1 In 2023, 460 people died as a result of hepatitis B.2
This resource has been reviewed by leading research institutes, the WHO Collaborating Centre for Viral Hepatitis at the Doherty Institute and The Kirby Institute.
Deaths from hepatitis B are avoidable
There is a vaccine and effective treatments to manage hepatitis B.

Nearly 1 in 20 babies born to mothers with hepatitis B aren’t receiving the birthdose vaccine in time.

One in three people living Three quarters of all people
Almost half of all people with chronic hepatitis B have received care at any time the last 10 years.

preferred language.
The people living with chronic hepatitis B who are not receiving guidelines-based care risk developing severe liver disease and liver cancer in the future.
70,000 530 63%
63% of people in Australia have received hepatitis C cures since 2016.10
However, there are still nearly 70,000 people living with hepatitis C in Australia11
Australia must TAKE ACTION to eliminate hepatitis B by:
In 2023, an estimated 530 people died as a result of hepatitis C.12
Deaths from hepatitis C are avoidable
Strengthening monitoring of the hepatitis B ‘birthdose’ to ensure all babies in Australia receive early
Nearly one in five people living with chronic hepatitis C have never been diagnosed. Additionally, one in ten people living with hepatitis C have only been partially diagnosed because they have not received confirmatory testing to know if their hepatitis C is chronic.13
Rolling out a nationwide universal offer of hepatitis B testing for people over 25 who were born before routine vaccination and whose hepatitis B status has not been documented.
Establishing a National Hepatitis B Monitoring and Care Facilitation Program to connect the three-quarters of people living with hepatitis B currently not receiving guideline-based care to ongoing care and support.
Many people who were diagnosed in the past are disengaged from care and may have been lost to follow up14. Many will need to be found again.
Prisons are now the primary sites of transmission nationally and help sustain the hepatitis C epidemic in Australia. In December 2024 the prison population nationally was 44,000.15 Almost half report injecting drug use and 8% are estimated to be living with hepatitis C. Nationally in 2023 people in prisons accounted for 42% of all people treated for hepatitis C. People in prison are being needlessly infected (and reinfected following cure) because the current responses are not sufficient to prevent transmission.16

According to the latest figures provided by Hepatitis Australia* an estimated 220,000 people in Australia live with chronic hepatitis B, up 10% from 200,000 the previous year. Many of the communities with higher hepatitis B prevalence were born overseas and face barriers to accessing testing and care, including language and knowledge of the healthcare system.
The numbers reveal that one in three people with chronic hepatitis B have not been diagnosed, and three quarters have not received recommended care in the last 12 months. Furthermore half of all people living with chronic hepatitis B have not received care at any time in the last 10 years!
The story is even more dramatic with hepatitis C. There are cures. Full stop. Directacting antivirals, DAAs for short, have an almost 99% cure rate. You just take one or two tablets daily for eight or twelve weeks, depending on the particular medicine prescribed. Sadly, in Australia there are still 590 deaths each year as a result of hepatitis C, and 70,000 people still live with the risk of liver failure and liver cancer caused by this completely curable disease.
While 63% of people living with hepatitis C have been cured since 2016, one in five people with chronic hepatitis C have not been diagnosed. Additionally, one in ten people who tested positive for hepatitis C antibodies have not been tested to confirm if they have chronic hepatitis C. Many who had been diagnosed in the past, before cures were available, are disengaged from services and may be lost to health service follow up.
According to Hepatitis Australia, 84% of people with chronic hepatitis C either no longer inject drugs or contracted it in other ways. These people are usually not connected to services and as such are
unaware of hepatitis C testing and cure, and therefore at higher risk of serious liver disease.
It was also revealed that prisons are now the primary transmission sites where people are unnecessarily infected and reinfected due to inadequate prevention measures.
As part of its World Hepatitis Day call to action, Hepatitis Australia is pushing for:
• setting up of needle and syringe programs in Australian prisons.
• finding ways to engage with the 84% of people with chronic hepatitis C who are disengaged from services
• strengthening community-led engagement with people who inject drugs as a priority group in efforts to eliminate viral hepatitis.
If you don’t know your hepatitis B or C status, get tested! The tests are simple and free, and can be ordered by your GP. For hepatitis C, there are community-based testing clinics in metropolitan South Australia.
Hepatitis B and hepatitis C tests are NOT part of routine blood tests. If you need help talking to your GP about getting tested, give us a call on 1800 437 222. We can provide you with a referral letter and a testing request card to make the process easier for you.
To find out more about hepatitis C testing, where and when community-based testing is available and other ways you might easily get tested for hepatitis B and hepatitis C, visit our website (hepsa.asn.au) or email your questions to comms@hepsa.asn.au.
Testing saves lives, and the life saved could be yours.

Despite having all the tools to combat liver cancer, a lack of prioritisation and action has resulted in an increase in mortality worldwide. These deaths are entirely preventable if people are given timely access to hepatitis testing, treatment and vaccination.
Liver cancer is the third most common cause of cancer deaths worldwide, with the majority of liver cancer cases caused by viral hepatitis. The tools to eliminate hepatitis exist, with modelling finding that meeting the WHO hepatitis elimination targets for testing and treatment by 2030 (now just 5 years away) is cost-effective for health systems. But around the world, incorporating funded hepatitis interventions into cancer control strategies has been slow, with the number of liver cancer cases caused by hepatitis expected to double by 2040.
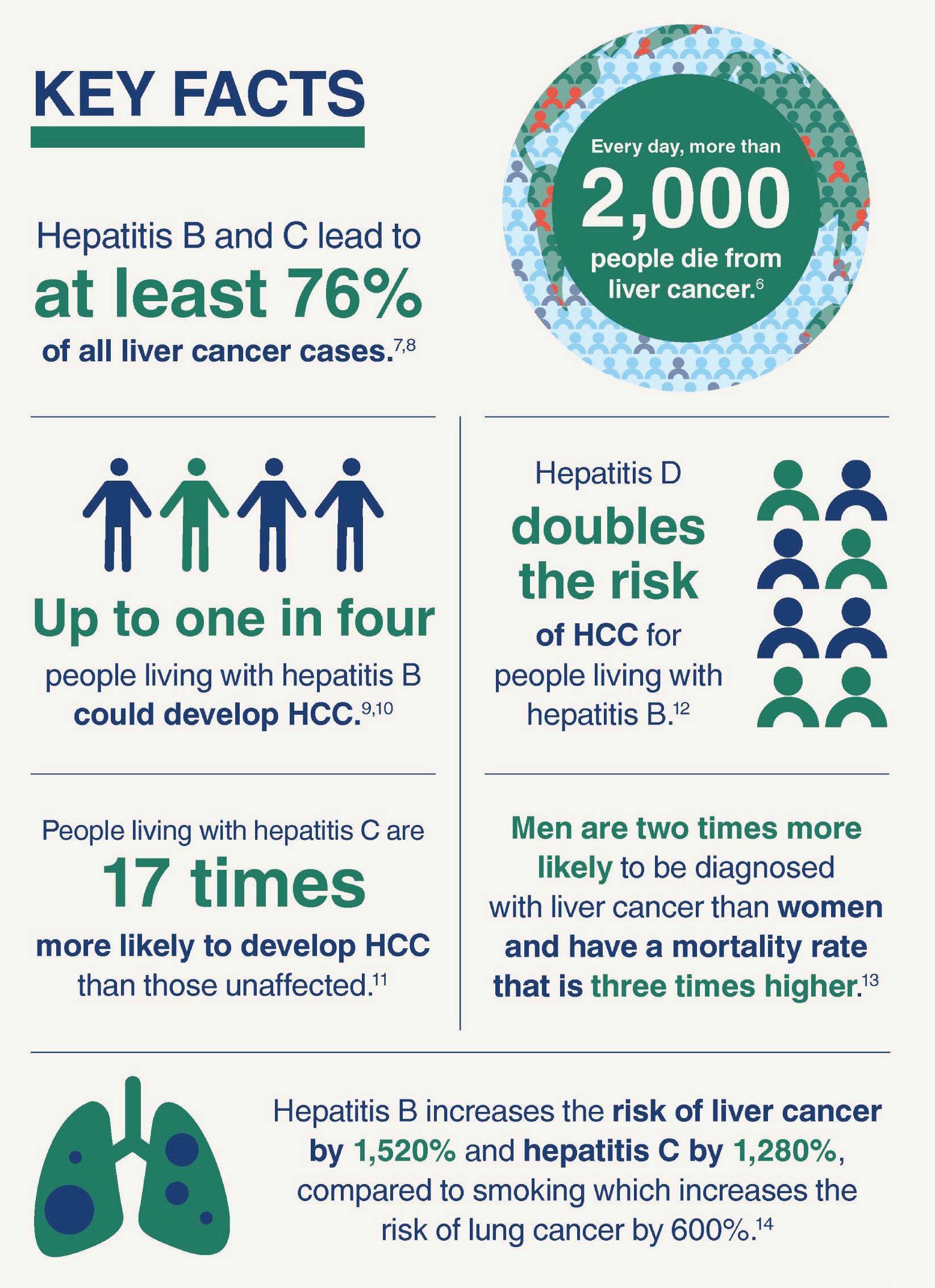
Research conducted by the World Hepatitis Alliance (WHA) has also found that 42% of people globally are not aware that viral hepatitis is the leading cause of liver cancer. This means that the urgency to make governments act is not always going to come from the people they represent.
To help address this, WHA has prepared a white paper, Beating cancer through hepatitis elimination – working together to reduce NCDs (non-communicable diseases), which sets out a number of calls to action for policymakers which if implemented could be transformative for people at risk of liver cancer.
The paper demonstrates that we need to integrate hepatitis testing, treatment and vaccination into funded national cancer programmes to decrease the incidence of liver cancer, increase early diagnosis, and reduce mortality. The paper calls for action in five main areas. •
Implement national awareness campaigns linking hepatitis and liver cancer to increase uptake of preventative services, including hepatitis testing, treatment and vaccination.
Expand timely access to universal hepatitis B vaccination at birth to prevent mother-tochild transmission.
Integrate costed hepatitis B vaccination for children and adults into all national immunisation schedules and cancer control plans and strategies, with indicators as part of monitoring and evaluation.
Testing for viral hepatitis
Integrate costed testing for viral hepatitis into national cancer and NCD prevention

and control strategies and programmes with indicators included as part of monitoring and evaluation.
Increase training for healthcare professionals on hepatitis and its connection to liver cancer to expand access to testing in clinical settings.
Enable primary health services, communitybased organisations and trained peers to offer community-based testing for hepatitis, in addition to testing provided by healthcare workers.
Antiviral treatment
Include hepatitis treatment within national cancer and NCD action plans, strategies and programmes as it prevents or slows the progression of chronic hepatitis to liver cancer.
Ensure equitable, affordable and continuous access to hepatitis treatment by expanding eligibility and offering treatment in accessible settings, such as primary healthcare and antenatal care.
Implement and expand HCC surveillance programmes that include all people living with or cured of hepatitis to increase early diagnosis and treatment for liver cancer.
Invest in research and development of accurate and more sensitive surveillance and diagnostic methods for HCC to improve patient outcomes.
Strengthen and link cancer and hepatitis data registries to improve monitoring and evaluation and inform health systems responses to liver cancer.
Improve understanding of barriers to accessing HCC surveillance programmes to increase uptake.
Empower civil society organisations and peers to support HCC monitoring programmes to improve uptake of liver cancer screening.
•
The paper emphasises just how costeffective hepatitis B vaccination really is in preventing hepatocellular carcinoma cases and reducing hepatitis B-related mortality. Globally, increases in hepatitis B vaccination programmes have resulted in decreased hepatitis B-related liver cancers. Modelling by the Center for Disease Analysis (CDA) Foundation in several countries with different income levels but similar prevalence of hepatitis B and C found that reaching the WHO hepatitis elimination targets for testing and treatment by 2030 was either highly cost-effective or cost-saving in all countries by 2045.
In late May, WHA hosted a webinar to launch the white paper, discussing the recommendations and talking about how, by integrating hepatitis and cancer control strategies, we can reduce the number of liver cancer cases globally. You can watch it at youtube.com/watch?v=uM7W9SEchq8
Policy makers must act now to integrate costed hepatitis services into fully funded national cancer control policies and programmes to reduce the burden of noncommunicable diseases. By integrating hepatitis and non-communicable disease programmes, policy makers can make health systems stronger and more cost-effective. With concerted effort, together we can prevent liver cancer by eliminating hepatitis.
READ MORE: worldhepatitisalliance.org/whitepaper

Two different experimental hepatitis B virus (HBV) treatments have shown very different results in their phase 2 trials in recent weeks. One, while initially promising, has failed to show high levels of response, while another has demonstrated very promising preliminary results.
New medications go through three phases of clinical trials on humans.
Phase 1: These are tests on whether a drug has harmful side-effects, and how much dosage is safe, and are usually conducted on up to 100 healthy people. They don’t test whether the medication actually works as a therapy.
Phase 2: These are tests on whether a drug actually works, usually conducted on 100-300 people with the disease to be treated. They often also test which dosage is the most effective.
Phase 3: These are tests, usually conducted in multiple locations with hundreds or thousands of people living with the disease to be treated. This allows the effectiveness, safety and dosage of the drug to be refined, and provides the information to doctors and consumers on how the drug is to be used safely.
The bad news is that Vir Biotechnology’s ‘functional cure’ combination therapy for hepatitis B has failed to meet the company’s hoped-for targets in its phase 2 trial. After 24 weeks of therapy using the drugs tobevibart and elebsiran, the researchers found that only 17% of patients with HBV achieved undetectable surface antigens (a protein found on the surface of the hepatitis B virus, used to detect its presence in the body), and even when pegylated interferon was added, only 21% of patients responded.
Taking every factor into account, the researchers determined only 11% and 15% of patients, respectively, achieved a functional cure for hepatitis B. This is not negligible, but not effective enough to make a dramatic change to hepatitis B treatment.
One bright spot in the research was that the treatment combination did eliminate hepatitis D in all six patients who were living with both the hep B and D viruses (hepatitis D is a virus that can only infect those already living with hepatitis B).
More promising, however, is the drug AHB137, developed by the pharmaceutical firm AusperBio. AHB-137 is intended to offer a functional cure by lowering the production of the proteins that HBV needs to replicate and infect cells. It belongs to a class of therapies called antisense oligonucleotides, which promote the destruction of specific target types of messenger RNA, which are proteins used, in this case, by the hepatitis B virus to sustain and grow itself.
In a recently completed phase 2 study in China, rapid reduction in hep B surface antigen was found in over 80% of participants. Even with those participants who had very high viral loads, almost half experienced this dramatic improvement. The effect was seen in patients who did a 12week and also patients who did a 16-week course of treatment, with very little difference between the two groups.
There were also very few reported sideeffects from the medication, boosting hopes that this could indeed be a new approach to hepatitis B treatment. “[These tests] demonstrate AHB-137’s potential as a backbone in achieving a functional cure for chronic hepatitis B,” said Dr Guofeng Cheng, AusperBio’s CEO.
Further AHB-137 phase 2 trials are now continuing to refine the treatment.
READ MORE:
bit.ly/vir_trial
bit.ly/ausperbio_trial

A study recently published by a gastroenterology research team in India, has identified a new ”phobia’ in people who have been cured of hepatitis C—the fear of reinfection. The study, published in the International Journal of Advanced Research, was headed by Dr Parveen Malhotra, and was run at the Department of Medical Gastroenterology, in the Post Graduate Institute of Medical Sciences (PGIMS), Rohtak.
Using direct-acting antivirals, more than 95% of people with hepatitis C can now be cured quickly and simply. Many people, however, have been living with the virus for years or even decades, and quite apart from any physical effects, this can create quite a heavy psychological and emotional burden.
Because of this, it makes sense that, once someone has been cured, it might not be so easy to actually feel free of the dangers of living with hepatitis C.
Dr Malhotra’s 5-year study looked at more than 5,400 cured patients. It found that, after successful treatment, many of the former patients will misread common symptoms or misunderstand antibody test results, and—


understandably, if mistakenly—believe that their hepatitis C infection has come back.
For example, 1,100 of the cured patients later returned to be tested again after receiving positive anti-HCV antibody test results,

usually from routine pre-surgical blood screening or blood donations. Nobody had explained properly to them that this result merely shows that a person has lived with hepatitis C in the past, but is not an accurate marker for currently active virus in the body, and so is exactly what you would expect in someone cured of a prior HCV infection.
Another 1,500 patients returned for tests after experiencing physical symptoms they associated with their past hepatitis C: symptoms like pain, stomach problems, itchy skin or sleep disruption. Understandably, this made them fear their hepatitis C had reappeared.
Living in fear:
by Russell Ferrer on Unsplash

However, of these 2,600 people who returned for tests, only 16 of them (or 0.6%) were found to have been reinfected.
“This confusion [about what antibody tests mean] causes unnecessary fear and anxiety,”
Dr Malhotra told The Tribune, India, after his results were published. He proposes the name ‘Malhotra Syndrome’ for this inaccurate fear of reinfection.
Dr Vani Malhotra, second author on the paper and Professor of Obstetrics and Gynaecology at PGIMS, explained further to The Tribune that “Most patients [affected by this fear] belong to lower-income groups and often connect every future health complaint to Hepatitis C, despite full recovery from the disease. They live in constant fear that the disease has returned.”
It remains to be seen if the results of this research can be replicated in other countries and communities, with similar rates of ‘Malhotra Syndrome’ and whether different levels of health education and socioeconomic status affect how much people live with a long-term fear of the return of hepatitis C.
The PGIMS offers free testing and treatment to people from the Indian state of Haryana, as well as those from surrounding states in northern India.
As of the end of 2024, the hospital has treated over 25,000 hepatitis C and 12,000 hepatitis B patients for free, and more than 38,000 endoscopies and 41,000 fibroscan tests have also been done free of cost to patients.
READ MORE: bit.ly/malhotra25

Supporting someone close to you who is struggling with drug or alcohol issues can be lonely and stressful. Most people are not equipped to deal with the challenge, and don’t seek help for fear of stigma and discrimination.
Tony Trimingham lived through that. After losing his son to a heroin overdose in 1997, and finding very little support available for himself and his family, he founded not-forprofit organisation Family Drug Support.
Family Drug Support (FDS) provides non-religious, non-judgmental, non-directive information and support to families, carers and friends of drug users across Australia. It offers services ranging from a 24/7 helpline and regular support groups to more intensive learning experiences including workshops and weekend courses. It also organises oneoff events to mark significant days.
FDS currently has regular activities in the ACT, New South Wales, Queensland, Victoria and South Australia. It runs 13 support groups in South Australia covering outer metropolitan areas and regional centres in Port
Augusta and Murray Bridge. Family Support Worker, Arneen Walden, who comes from a small South Australian mining town, says they aim to engage more hard-to-reach groups, including rural, Aboriginal and migrant communities.
Before joining FDS, Arneen had spent seven months working with Community Access and Services SA (CAaSSA), which provides alcohol and other drug services to diverse communities including people from South East Asia, Middle East and Africa. She hopes to use that experience to help families from those communities.
Family Support Worker, Jan Maguire, said one of the most important things FDS counsellors try to impart to people supporting a drug user is effective communication. “Often family members keep trying to ‘fix the problem’ … trying to get the person using drugs or alcohol to stop,” she said. “Instead, we encourage them to support the user to be safe, to reduce risks and harm.
“Our services are offered without judgement. We support those who want to save their re-
lationships by helping them to stay safe, stay alive and stay together. But we also support those who can’t cope anymore and need to step away.”
FDS support groups meet fortnightly, giving participants a safe space to share experiences and not feel alone. It is open to all; no membership is required. For families and carers who are open to learning more, FDS offers a four-day evidence-based program called Stepping Stones. The focus of this course is on taking care of yourself and effective communication.
Other activities include Stepping Forward a series of information sessions built around the topics: Families Stages of Change, Effective Communication, Alcohol and Other Drugs Information, Drug and Alcohol Treatment Options, Setting Workable Boundaries, Dealing with Conflict and Families and Ice. Stepping Forward may serve as a lead-in

to doing the more intensive and therapeutic Stepping Stones course.
FDS also runs a half-day professional development workshop for people working with families dealing with a member who uses drugs or alcohol. This workshop aims to equip community sector workers with background knowledge and skills to help families to build resilience and improve the outcome for both the person who uses drugs and the family.
For those who have lost a family member to drugs or alcohol, FDS has started a monthly bereavement group. This will fill the gap for people who used to be in support groups but are left with nowhere to turn when death took away the person they were supporting.
Family Drug Support is funded by most state and territory health departments and staffed by trained workers and an army of volunteers. In South Australia alone, the organisation is supported by 35 volunteers. While services are open to all, they encourage people to join as members to get the quarterly magazine and discounts on resources.
Their national helpline is available 24 hours a day, seven days a week, staffed by welltrained volunteers who are fully debriefed after each shift. An annual retreat for volunteers provides opportunities to connect, refresh and update.
“People contact our support line when they need to dump or offload, need someone to listen to them, need information or when they are going to make a decision,” said Jan. “It’s often at a critical point, and we help them to find their way through it, without judgement.”
Head to the Family Drug Support website at fds.org.au for more details on events and locations. To get support, call 1300 368 186

This World Hepatitis Day we are focussing on testing. Early detection of viral hepatitis is vital to prevent avoidable deaths, like those caused by liver cancer.
Hepatitis B increases liver cancer risk by 1,520%, and hepatitis C increases the risk by 1,280% - compared to smoking which increases the risk of lung cancer by 600%.* Yet many people are unaware of the link between viral hepatitis and cancer, or of the other health conditions, such as diabetes and heart disease which are also associated with it.
This issue we focus on these related conditions, highlighting the need for people living with viral hepatitis to be aware of their condition and receive the care that they need.
* Beating cancer through hepatitis elimination: working together to reduce NCDs. World Hepatitis Alliance, Geneva, 2025.
Beating cancer through hepatitis elimination: working together to reduce NCDs World Hepatitis Alliance, Geneva, 2025. 13p.
Liver cancer is the third most common cause of cancer deaths worldwide, with the majority of liver cancer cases caused by viral hepatitis. Despite this, action to incorporate funded hepatitis interventions into cancer control strategies has been slow, with the number of liver cancer cases caused by hepatitis expected to double by 2040.
bit.ly/4lm6uGS

Liver disease is a significant risk factor for cardiovascular outcomes
European Association for the Study of the Liver (EASL), Geneva, 2023. 12p.
Liver disease activity (cT1) measured using LiverMultiScan was independently associated with a higher risk of cardiovascular disease and allcause mortality, independent of pre-existing metabolic syndrome, liver fibrosis or fat. bit.ly/livercardio

Roadmap to liver cancer control in Australia
Cancer Council Australia, Sydney, 2023. 19p.
Developed to reduce the disease burden, improve outcomes and improve survival rates for all Australians affected by liver cancer.
bit.ly/4nHsVYP

Risk factors (for liver cancer)
Hepatitis B Foundation, Doylestown PA, 2025. Webpage. Provides information about the major risk factors for liver cancer - hepatitis B and hepatitis C. Outlines additional risk factors including cirrhosis, excessive alcohol use and smoking, as well as obesity and diabetes.
bit.ly/rf_lc

Diabetes and hepatitis: what’s the link?
Hepatitis SA, Adelaide, 2024. Information about why people with hepatitis C are three times more likely to develop diabetes (including the functions of the pancreas and liver).
bit.ly/diabeteshep

Can a parent’s HBV affect their newborn’s heart?
Hepatitis SA, Adelaide, 2025.
A pair of Chinese studies have shown evidence that children of parents living with hepatitis B are more likely to be born with a heart defect.
bit.ly/hbvbabyheart



Hepatitis SA provides free information and education on viral hepatitis, and support to people living with viral hepatitis.
Postal Address:
Kaurna Country PO Box 782
Kent Town 5071
(08) 8362 8443 1800 437 222
www.hepsa.asn.au
Community News: hepsa.asn.au/ communitynews
Library: hepsa.asn.au/library


@HepatitisSA
@hepatitissa.asn.au.bsky.social
Resources: issuu.com/hepccsa
Email: admin@hepatitissa.asn.au
Free hepatitis A, B and C information, confidential and non-judgemental support, referrals and printed resources.
We can help. Talk to us. Call or web chat 9am–5pm, Mon–Fri
HEPATITIS SA BOARD
Chair
Arieta Papadelos
Vice Chair
Bill Gaston
Secretary
Sharon Eves
Treasurer
Michael Larkin
Ordinary Members
Lindy Brinkworth
Bernie McGinnes
Janice Scott
Memoona Rafique
Lucy Ralton
Tamara Shipley
Kerry Paterson (CEO)







Hepatitis SA has a wide range of hepatitis B and hepatitis C publications which are distributed free of charge to anyone in South Australia.
To browse our collection and place your orders, go to hepsa.asn.au/orders or scan the QR code below:

Viral Hepatitis Nurses are nurse consultants who work with patients in the community, general practice or hospital setting. They provide a link between public hospital specialist services and general practice, and give specialised support to general practitioners (GPs) to assist in the management of patients with hepatitis B or hepatitis C. With advanced knowledge and skills in testing, management, and treatment of viral hepatitis, they assist with the management of patients on antiviral medications and work in shared care arrangements with GPs who are experienced in prescribing medications for hepatitis C or accredited to prescribe section 100 medications for hepatitis B. They can be contacted directly by patients or their GPs:
CENTRAL ADELAIDE LOCAL HEALTH NETWORK
Queen Elizabeth Hospital
Phone: 0423 782 415, 0466 851 759 or 0401 717 953
Royal Adelaide Hospital
Phone: 0401 125 361 or (08) 7074 2194
Specialist Treatment Clinics
NORTHERN ADELAIDE LOCAL HEALTH NETWORK
Phone: 0401 717 971 or 0413 285 476
SOUTHERN ADELAIDE LOCAL HEALTH NETWORK
Phone: 0466 777 876 or 0466 777 873
Office: (08) 8204 6324
Subsidised treatment for hepatitis B and C are provided by specialists at the major hospitals. You will need a referral from your GP. However, you can call the hospitals and speak to the nurses to get information about treatment and what you need for your referral.
• Flinders Medical Centre Gastroenterology & Hepatology Unit: call 8204 6324
• Queen Elizabeth Hospital: call 8222 6000 and ask to speak a viral hepatitis nurse
• Royal Adelaide Hospital Viral Hepatitis Unit: call Anton on 0401 125 361
• Lyell McEwin Hospital: call Bin on 0401 717 971
Visit hepsa.asn.au: no need to log in, lots of info & updates
Visit hepsa.asn.au - no need to log in, lots of info & pdates
Follow Community News online: hepsa.asn.au/communitynews
Follo the HepSAY blog - hepsa.asn.a /blog
Order print resources at hepsa.asn.au/orders
SMS/WeChat us on 0403 648 348
Order print resources - hepsa.asn.a /orders/ Follo s on T i er @hep_sa or Facebook @Hepa sSA
Follow us on social: Bluesky @hepatitissa.asn.au.bsky.social or Facebook @HepatitisSA

Full range of syringes and needles. Water and filters also available in limited quantities for free.

Viral Hepatitis and Blood Safety Workforce Training!


Do you know where to refer clients living with viral hepatitis?
Do you know where to refer clients living with viral hepatitis?
Do you know if there is a cure?
Do you know if there is a cure?
Do you worry about blood exposure and needle-stick injury?
Do you worry about blood exposure and needle-stick injury?
Sessions cover a range of topics, including:
Sessions cover a range of topics, including:
Do you know where to refer clients living with viral hepatitis? Do you know if there is a cure? Do you worry about blood exposure and needle-stick injury?
Do you know where to refer clients living with viral hepatitis? Do you know if there is a cure? Do you worry about blood exposure and needle-stick injury?
• Basics about hepatitis A, B and C including the liver
• Basics about hepatitis A, B and C including the liver
Sessions cover a range of topics, including:
Sessions cover a range of topics, including:
• Transmission risks/myths
• Transmission risks/myths
• Basics about hepatitis A, B and
• Basics about hepatitis A, B and C
• Best practice around blood and blood exposure
• Best practice around blood and blood exposure
• Transmission risks/myths
• Transmission risks/myths
• Discrimination
• Discrimination
• Best practice around blood and blood exposure
• Best practice around blood and blood exposure
• Discrimination
• Discrimination
• Lived experience from the perspective of a Positive Speaker
• Lived experience from the perspective of a Positive Speaker
• Any other topics relevant to your workplace!
• Lived experience from the perspective of a Positive Speaker
• Any other topics relevant to your workplace!
• Lived experience from the perspective of a Positive Speaker
Cost: Free
Time: 1-2 hours
Cost: Free Time: 1-2 hours
Cost: Free Time: 1-2 hours
• Any other topics relevant to your workplace!
• Any other topics relevant to your workplace!
Cost: Free Time: 1-2 hours
Where: In-service: we will come to you or via online delivery
Where: In-service: we will come to you or via online delivery
Where: In-service: we will come to you!
Where: In-service: we will come to you!
Contact the Education Team on 8362 8443 or email education@hepsa.asn.au
Contact the Education Team on 8362 8443 or email education@hepsa.asn.au

