RESPIRATORY MEDICINE
RESPIRATORY MEDICINE
LATEST APPROACHES TO
ALLERGIC RHINITIS COPD
GOLD 2022 updates
Pulmonary rehab for
LONG COVID
LATEST APPROACHES TO
ALLERGIC RHINITIS COPD
GOLD 2022 updates
Pulmonary rehab for
LONG COVID
ASTHMA IN FOCUS
ASTHMA IN FOCUS



VOL 8 ● ISSUE 5 ● 2022
VOL 8 ● ISSUE 5 ● 2022
For patients not adequately controlled on dual therapy with moderate to severe COPD
UNLEASH THE PROTECTION
OF TRIXEO1,2
Significant protection against exacerbations*
TRIXEO Aerosphere is indicated as a maintenance treatment in adult patients with moderate to severe chronic obstructive pulmonary disease (COPD) who are not adequately treated by a combination of an inhaled corticosteroid and a long-acting beta2-agonist or combination of a long-acting beta2-agonist and a long-acting muscarinic antagonist.1

*Significant reductions in the rate of moderate or severe exacerbations vs LAMA/LABA (24%, n=2137 vs n=2120, annual rates 1.08 vs 1.42, 95% CI 0.69–0.83; p<0.001) and ICS/LABA (13%, n=2137 vs n=2131, annual rates 1.08 vs 1.24, 95% CI 0.79–0.95; p=0.003).1,2 COPD, chronic obstructive pulmonary disease; ICS, inhaled corticosteroid; LABA, long-acting beta2-agonist; LAMA, long-acting muscarinic antagonist. In the clinical trial programme for TRIXEO, LAMA/LABA refers to glycopyrronium/formoterol fumarate and ICS/LABA refers to budesonide/formoterol fumarate. ©AstraZeneca 2022. All rights reserved.
ABRIDGED PRESCRIBING INFORMATION
TRIXEO AEROSPHERE® 5 micrograms/7.2 micrograms/160 micrograms pressurised inhalation, suspension (formoterol fumarate dihydrate/glycopyrronium/budesonide)
Consult Summary of Product Characteristics (SmPC) before prescribing. Indication: Trixeo Aerosphere is indicated as a maintenance treatment in adult patients with moderate to severe chronic obstructive pulmonary disease (COPD) who are not adequately treated by a combination of an inhaled corticosteroid and a long acting beta2 agonist or combination of a long-acting beta2 agonist and a long acting muscarinic antagonist.
Presentation: Pressurised inhalation, suspension. Each single actuation (delivered dose, ex-actuator) contains 5 micrograms of formoterol fumarate dihydrate, glycopyrronium bromide 9 micrograms, equivalent to 7.2 micrograms of glycopyrronium and budesonide 160 micrograms.
Dosage and Administration: The recommended and maximum dose is two inhalations twice daily (two inhalations morning and evening). If a dose is missed, take as soon as possible and take the next dose at the usual time. A double dose should not be taken to make up for a forgotten dose. Special populations: Elderly:
No dose adjustments required in elderly patients. Renal impairment: Use at recommended dose in patients with mild to moderate renal impairment. Can also be used at the recommended dose in patients with severe renal impairment or end-stage renal disease requiring dialysis, only if expected benefit outweighs the potential risk. Hepatic impairment: Use at recommended dose in patients with mild to moderate hepatic impairment. Can also be used at the recommended dose in patients with severe hepatic impairment, only if expected benefit outweighs the potential risk. Paediatric Population: No relevant use in children and adolescents (<18 years of age). Method of administration: For inhalation use. To ensure proper administration of the medicinal product, the patient should be shown how to use the inhaler correctly by a physician or other healthcare professional, who should also regularly check the adequacy of the patient’s inhalation technique. Patients who find it difficult to coordinate actuation with inhalation may use Trixeo Aerosphere with a spacer to ensure proper administration of the medicinal product.. Contraindications: Hypersensitivity to the active substances or to any of the excipients.
Warnings and Precautions: Not for acute use: Not indicated for treatment of acute episodes of bronchospasm, i.e. as a rescue therapy. Paradoxical bronchospasm: Administration of formoterol/glycopyrronium/budesonide may produce paradoxical bronchospasm with an immediate wheezing and shortness of breath after dosing and may be life threatening. Treatment should be discontinued immediately if paradoxical bronchospasm occurs. Assess patient and institute alternative therapy if necessary. Deterioration of disease: Recommended that treatment should not be stopped abruptly. If patients find the treatment ineffective, continue treatment but seek medical attention. Increasing use of reliever bronchodilators indicates worsening of the underlying condition and warrants reassessment of the therapy. Sudden and progressive deterioration in the symptoms of COPD is potentially life threatening, patient should undergo urgent medical assessment. Cardiovascular effects: Cardiovascular effects, such as cardiac arrhythmias, e.g. atrial fibrillation and tachycardia, may be seen after the administration of muscarinic receptor antagonists and sympathomimetics, including glycopyrronium and formoterol. Use with caution in patients with clinically significant uncontrolled and severe cardiovascular disease such as unstable ischemic heart disease, acute myocardial infarction, cardiomyopathy, cardiac arrhythmias and severe heart failure. Caution should also be exercised when treating patients with known or suspected prolongation of the QTc interval (QTc > 450 milliseconds for males or > 470 milliseconds for females), either congenital or induced by medicinal products. Systemic corticosteroid effects: May occur with
any inhaled corticosteroid, particularly at high doses prescribed for long periods. These effects are much less likely to occur with inhalation treatment than with oral corticosteroids. Systemic effects include Cushing’s syndrome, Cushingoid features, adrenal suppression, decrease in bone mineral density, cataract and glaucoma. Potential effects on bone density should be considered particularly in patients on high doses for prolonged periods that have co existing risk factors for osteoporosis. Visual disturbances: May be reported with systemic and topical corticosteroid use. If patient presents symptoms such as blurred vision or other visual disturbances, consider ophthalmologist referral for evaluation of possible causes which may include cataract, glaucoma or rare diseases such as central serous chorioretinopathy (CSCR). Transfer from oral therapy: Care is needed in patients transferring from oral steroids, since they may remain at risk of impaired adrenal function for a considerable time. Patients who have required high dose corticosteroid therapy or prolonged treatment at the highest recommended dose of inhaled corticosteroids, may also be at risk. These patients may exhibit signs and symptoms of adrenal insufficiency when exposed to severe stress. Additional systemic corticosteroid cover should be considered during periods of stress or elective surgery. Pneumonia in patients with COPD: An increase in the incidence of pneumonia, including pneumonia requiring hospitalisation, has been observed in patients with COPD receiving inhaled corticosteroids. Remain vigilant for the possible development of pneumonia in patients with COPD as the clinical features of such infections overlap with the symptoms of COPD exacerbations. Risk factors for pneumonia include current smoking, older age, low body mass index (BMI) and severe COPD. Hypokalaemia: Potentially serious hypokalaemia may result from ß2-agonist therapy. This has potential to produce adverse cardiovascular effects. Caution is advised in severe COPD as this effect may be potentiated by hypoxia. Hypokalaemia may also be potentiated by concomitant treatment with other medicinal products which can induce hypokalaemia, such as xanthine derivatives, steroids and diuretics.
Hyperglycaemia: Inhalation of high doses of ß2-adrenergic agonists may produce increases in plasma glucose. Blood glucose should be monitored during treatment following established guidelines in patients with diabetes. Co-existing conditions: Use with caution in patients with thyrotoxicosis. Anticholinergic activity: Due to anticholinergic activity, use with caution in patients with symptomatic prostatic hyperplasia, urinary retention or with narrow-angle glaucoma. Patients should be informed about the signs and symptoms of acute narrow-angle glaucoma and should be informed to stop using this medicinal product and to contact their doctor immediately should any of these signs or symptoms develop. Co-administration of this medicinal product with other anticholinergic containing medicinal products is not recommended. Renal impairment: Patients with severe renal impairment (creatinine clearance of <30 mL/min), including those with end-stage renal disease requiring dialysis, should only be treated with this medicinal product if the expected benefit outweighs the potential risk. Hepatic impairment: In patients with severe hepatic impairment, use only if the expected benefit outweighs the potential risk. These patients should be monitored for potential adverse reactions..
Drug Interactions: Co-treatment with strong CYP3A inhibitors, e.g. itraconazole, ketoconazole, HIV protease inhibitors and cobicistat-containing products are expected to increase the risk of systemic side effects. Should be avoided unless the benefit outweighs the increased risk, in which case patients should be monitored for systemic corticosteroid adverse reactions. This is of limited clinical importance for short-term (1-2 weeks) treatment. Since glycopyrronium is eliminated mainly by the renal route, drug interaction could potentially occur with medicinal products affecting renal excretion mechanisms. Other antimuscarinics and sympathomimetics: Co-administration with other anticholinergic and/or long-acting ß2-adrenergic agonist containing medicinal products is not recommended as it may potentiate known inhaled muscarinic antagonist or ß2-adrenergic agonist adverse reactions. Concomitant use of other beta-adrenergic medicinal products can have potentially additive effects. Caution required when prescribed concomitantly with formoterol.
Medicinal product-induced hypokalaemia: Possible initial hypokalaemia may be
potentiated by xanthine derivatives, steroids and non potassium sparing diuretics. Hypokalaemia may increase the disposition towards arrhythmias in patients who are treated with digitalis glycosides. β-adrenergic blockers: ß-adrenergic blockers (including eye drops) can weaken or inhibit the effect of formoterol. Concurrent use of ß-adrenergic blockers should be avoided unless the expected benefit outweighs the potential risk. If required, cardio-selective ß-adrenergic blockers are preferred. Other pharmacodynamic interactions: Concomitant treatment with quinidine, disopyramide, procainamide, antihistamines, monoamine oxidase inhibitors, tricyclic antidepressants and phenothiazines can prolong QT interval and increase the risk of ventricular arrhythmias. L-dopa, L-thyroxine, oxytocin and alcohol can impair cardiac tolerance towards beta2-sympathomimetics. Concomitant treatment with monoamine oxidase inhibitors, including medicinal products with similar properties such as furazolidone and procarbazine, may precipitate hypertensive reactions. Elevated risk of arrhythmias in patients receiving concomitant anaesthesia with halogenated hydrocarbons.
Pregnancy and Lactation: Administration to pregnant women/women who are breast-feeding should only be considered if the expected benefit to the mother justifies the potential risk to the foetus/child.
Ability to Drive and Use Machines: Dizziness is an uncommon side effect which should be taken into account.
Undesirable Events: Consult SmPC for a full list of side effects. Common (≥ 1/100 to < 1/10): Oral candidiasis, pneumonia, hyperglycaemia, anxiety, insomnia, headache, palpitations, dysphonia, cough, nausea, muscle spasms, urinary tract infection. Uncommon (≥ 1/1,000 to < 1/100): Hypersensitivity, depression, agitation, restlessness, nervousness, dizziness, tremor, angina pectoris, tachycardia, cardiac arrhythmias (atrial fibrillation, supraventricular tachycardia and extrasystoles), throat irritation, bronchospasm, dry mouth, bruising, urinary retention, chest pain. Very Rare (< 1/10,000): Signs or symptoms of systemic glucocorticosteroid effects, e.g. hypofunction of the adrenal gland, abnormal behaviour. Not known: Angioedema, vision blurred, cataract, glaucoma.
Legal Category: Product subject to prescription which may be renewed (B)
Marketing Authorisation Number: EU/1/20/1498/002 120 actuations
Marketing Authorisation Holder: AstraZeneca AB, SE-151 85, Södertälje, Sweden. Further product information available on request from: AstraZeneca Pharmaceuticals (Ireland) DAC, Block B, Liffey Valley Office Campus, Dublin 22. Tel: +353 1 609 7100.
TRIXEO and AEROSPHERE are trademarks of the AstraZeneca group of companies.

Date of API preparation: 10/2021
Veeva ID: IE-3166
Adverse events should be reported directly to; HPRA Pharmacovigilance, Website: www.hpra.ie Adverse events should also be reported to AstraZeneca Patient Safety on Freephone 1800 800 899
1. TRIXEO AEROSPHERE 5 micrograms/7.2 micrograms/160 micrograms pressurised inhalation, suspension. Summary of Product Characteristics. Available at www.medicines.ie
2. Rabe KF et al. Triple inhaled therapy at two glucocorticoid doses in modeate-tovery-severe COPD. N Engl J Med. 2020;383:35–48. doi: 10.1056/NEJMoa1916046
COPD. N Engl J Med. 2020;383:35–48. doi: 10.1056/NEJMoa1916046
Veeva ID: IE-3706 Date of Preparation: March 2022
NEW NEW
Bringing the focus back to day-to-day respiratory care
not afford to pay for the cost of getting a new prescription. A further 16 per cent of parents/ guardians reported having had to forgo other essential items in order to purchase their children’s asthma medications.
Welcome to the latest edition of Update Respiratory Medicine.
Asthma awareness week took place earlier this month and saw the publication of a new survey by the Asthma Society of Ireland, which focused on the experience of children with asthma – revealing some concerning findings.
One-in-10 children in Ireland have asthma and one-in-five will develop it at some point in their childhood. There are a number of factors that are key to making Ireland an ‘asthma-safe country’ for children, according to the Society, which include timely access to appropriate healthcare, affordable asthma medication, and support for parents and children to learn more about the illness and to build self-management skills.
However, the Society’s survey of Irish parents or guardians about their children’s asthma found that 73 per cent of surveyed parents have experienced anxiety around managing their child’s asthma and 28 per cent indicated they experienced this anxiety ‘always or often’.
The survey also found that 20 per cent of children with asthma worry always or often about having an attack, and the same number worry always or often about having to take their medication in public.
Worryingly, just over a quarter (26 per cent) of parents struggle with the cost of their child’s asthma medications – 5 per cent said they have forgone buying their child’s asthma medications as they could not afford the medication or device, while 5 per cent had forgone buying their child’s medication because they could
Rationing or forgoing asthma medication can lead to a serious exacerbation of symptoms, which can escalate to a dangerous asthma attack. The Asthma Society has been calling for the inclusion of asthma medications on the HSE’s Long-Term Illness Scheme since the organisation’s inception in 1973, but successive governments have refused to update the scheme.
The Society’s survey also highlighted the impact of the coronavirus pandemic on asthma in children – 19 per cent of respondents reported avoiding or delaying going to hospital for their child’s asthma due to fears of them contracting Covid-19, while almost 59 per cent of respondents reported being unsure whether their child’s symptoms were caused by asthma or Covid-19. As a result, almost 16 per cent reported that this uncertainty resulted in a delay in appropriate treatment for the child.
Commenting on the findings, Sarah O’Connor, CEO of the Asthma Society of Ireland, said: “When we looked at the survey results, they really do speak volumes about Ireland’s status as an ‘asthma-safe’ country for children. In a year when the paediatric Model of Care for asthma is being developed by the HSE, we believe it is imperative to note that Ireland is not currently an ‘asthma-safe country’ for children.”
To coincide with asthma awareness week, this edition of Update has a special focus on asthma, with articles on asthma in women, asthma in children, and an overview of the HSE’s new end-to-end Model of Care for Adult Asthma.
There is also an update from the Irish Lung Fibrosis Association, an overview of an

innovative singing exercise programme for pulmonary fibrosis, as well as expert clinical articles on the latest 2022 COPD GOLD guidelines and the latest approaches to managing allergic rhinitis, as well as an update on the roll-out of the HSE’s pulmonary rehabilitation teams for COPD, and an interview with one of the clinicians behind important new Covid-19related Irish research. Speaking of Covid-19, this edition of Update features an expert overview of the role of pulmonary rehabilitation in patients suffering from long Covid, which is an issue that will unfortunately be with us for the foreseeable future and clearly needs more resources to support these patients and their clinicians.
In addition, there is a brief look at continuing international and national efforts to tackle TB, which remains one of the world’s biggest infectious causes of mortality and morbidity and is on the rise again following the impact of the pandemic.
Also returning to our shores this past winter after a brief disappearance was influenza, with this edition containing a summary of the latest data on cases to-date and vaccination uptake in older adults.
Finally, there is a meeting report on Cystic Fibrosis Ireland’s 2022 Annual Conference, which heard about the very positive impacts the latest treatments for this disease are having.
So all-in-all, this is a packed issue that should hopefully prove interesting and useful to all our readers. Thank you to all our expert contributors for taking the time to share their knowledge and advice for the betterment of patient care.
We always welcome new contributors and suggestions for future content, as well as any feedback on our content to-date. Please contact me at priscilla@mindo.ie if you wish to give any feedback or contribute an article. ■
1 Respiratory Medicine | Volume 8 | Issue 5 | 2022
A message from Priscilla Lynch, Editor
28 Pulmonary
32 SingStrong: Singing for pulmonary fibrosis
Editor Priscilla Lynch priscilla@mindo.ie
Sub-editor Emer Keogh emer@greenx.ie
Creative Director Laura Kenny laura@greenx.ie
34 Irish Lung Fibrosis Association update
36 Cystic Fibrosis Ireland 2022 Annual Conference report 39 Influenza in Ireland –the
Battling TB –
Advertisements Graham Cooke graham@greenx.ie Administration Daiva Maciunaite daiva@greenx.ie
Update is published by GreenCross Publishing Ltd, Top Floor, 111 Rathmines Road Lower, Dublin 6 Tel +353 (0)1 441 0024 greencrosspublishing.ie
© Copyright GreenCross Publishing Ltd 2022
The contents of Update are protected by copyright. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form by any means – electronic, mechanical or photocopy recording or otherwise – whole or in part, in any form whatsoever for advertising or promotional purposes without the prior written permission of the editor or publisher.
Disclaimer
The views expressed in Update are not necessarily those of the publishers, editor or editorial advisory board. While the publishers, editor and editorial advisory board have taken every care with regard to accuracy of editorial and advertisement contributions, they cannot be held responsible for any errors or omissions contained.
GreenCross Publishing is owned by Graham Cooke graham@greenx.ie
Contents 04 Covid-19 and ARDS: Irish research breakthrough 06 Latest approaches to allergic rhinitis 13 New HSE end-to-end Model of Care for Adult Asthma 14 Asthma in women 16 Asthma in children 19 COPD GOLD 2022 updates 26 HSE COPD pulmonary rehab update
rehab
for long Covid
of
updates from home and abroad
return
flu 40











A new horizon for Covid-19 treatment – Irish research
AUTHOR: Pat Kelly
As the evolving Covid-19 situation continues to move the ground under clinicians and researchers alike, a recent collaboration has provided hope for patients who have become critically ill with the virus, as well as their physicians. Working in partnership, the researchers from the RCSI and Beaumont Hospital in Dublin investigated the efficacy of an anti-inflammatory protein, alpha-1 antitrypsin (AAT), to treat Covid-19 patients who have progressed to acute respiratory distress syndrome (ARDS).
The study is notable for representing a number of ‘firsts’ – it was the first randomised, controlled trial of AAT for ARDS; the first randomised, controlled trial of AAT in the ICU; and it was the first trial of its kind of a Covid-19 therapeutic in Ireland. In designing the trial, the authors were acutely aware that treatment options for Covid-19 patients with ARDS are particularly limited.
AAT is a naturally occurring human protein that is produced by the liver and released into the bloodstream. AAT normally acts to protect the lungs from the destructive effects of common illnesses. In this trial, the researchers used AAT that had been purified from the blood of healthy donors in an effort to reduce inflammation in severely ill patients with ARDS associated with Covid-19. Overall, the results showed that treatment with AAT led to decreased inflammation after just one week and was safe and well tolerated. In addition, the treatment did not interfere with the patients’ own protective response to Covid-19.
Potential
Prof Gerry McElvaney of the RCSI Department of Medicine and Beaumont

Hospital, Dublin, is a senior author of the new research, which has been published in Med. He spoke with Update about the potential for this treatment approach to save lives and add a new option to the treatment armament of physicians dealing with these extremely vulnerable patients.
“From the very beginning of Covid, we had been working on trying to understand the inflammation in the lungs and the rest of the body that occurred because of Covid infection,” Prof McElvaney, a Consultant Respiratory Physician, explained. “We got a good handle on that. We learned early on that there are certain pro-inflammatory proteins in the blood that were found in Covid infections. We worked on ways of understanding those, because rather than just taking a ‘blanket’ approach and inhibiting those proteins, we wanted to find out what they caused and whether there was a physiological way to inhibit them,” he told Update
“We hit on the idea that although these individuals with severe Covid infection – we are talking about people who are on ventilators, for example –although they had a good, robust antiinflammatory response, in some cases it wasn’t effective enough to save them. We were working with colleagues in the US initially, and we found that there was a lot more inflammation than we had thought,” he said.
One of the human body’s main antiinflammatory agents is the AAT protein, and this prompted Prof McElvaney and his colleagues to measure the levels of AAT in blood. They found that in people with severe Covid-19 infection, whilst the levels of it were high, they were not sufficiently high enough to protect these patients’ lungs. “This gave us the impetus to design a study whereby we gave extra alpha-1 to people with Covid infection who are on ventilators. The idea was – because we weren’t 100 per cent sure it would work –that we wanted to compare it to placebo, so some people got placebo, and some got alpha-1,” he explained.
In the study, alpha-1 was given in two dosages: As a single once-off intravenous dose, followed by placebo once a week; and in the other group, once a week for four weeks. “We wanted to see whether giving this intravenous alpha-1 would decrease the inflammatory burden in people with Covid-19,” said Prof McElvaney. “Although it was a small study, we also wanted to see if it would have any physiological effect. It was a double-blinded, placebo-controlled trial with a relatively small number of patients, but we powered it; we knew how many people we would have to evaluate to see whether we would get an effect.”
4 Volume 8 | Issue 5 | 2022 | Respiratory Medicine
Prof Gerry McElvaney
Bonus findings
“We found that the key parameter that we were looking at was the effect of alpha-1 on a specific pro-inflammatory protein called interleukin-6 [IL-6] at day seven,” he continued. “We showed that intravenous alpha-1 decreased IL-6 at day seven, whereas with people given placebo, the IL-6 continued to rise at day seven.” There were unexpected bonus findings associated with the study, Prof McElvaney pointed out. “We also found that alpha-1 had other anti-inflammatory effects too – it actually decreased other proinflammatory cytokines.
“There were two other interesting things arising from that,” he said. “When steroids are administered, they seem to have great effect in inhibiting everything, but alpha-1 seemed to be a bit more selective. It inhibited pro-inflammatory cytokines, but did not inhibit anti-inflammatory cytokines, so this was a quite interesting effect. It gave some indication, but which was not statistically significant, in having an effect on time to extubate.” Prof McElvaney also noted that the alpha-1 used in the study was plasma-purified alpha-1, a protein that has already been used for many years throughout Europe and the US to treat patients with alpha-1 deficiency. “Unfortunately, in Ireland, our health service doesn’t reimburse for that, so we only have a small number of people in Ireland receiving plasma-purified alpha-1 antitrypsin for alpha-1 deficiency; a very small number compared to other countries. But we had a good idea about how it works because of that.”
In addition to its promising potential for Covid-19 treatment, Prof McElvaney’s research opens the door for clinical possibilities in a wider range of conditions. “What we really know is, it’s good in treating inflammation,” he said. “For example, in people with ARDS, even if it is not caused by Covid, theoretically, alpha-1 could work there too.
“There is good US data. Alpha-1 is effective in people with alpha-1 deficiency; it
may also be very useful in people with bronchiectasis or even cystic fibrosis bronchiectasis, where there is a lot of inflammation in the lungs, and we have actually been looking at that. There are a few other conditions [it could be applied to] – there is a very interesting skin condition associated with alpha-1 deficiency called panniculitis. We know that intravenous alpha-1 melts that away; it just cures it straight away, so it could also be used for certain skin conditions. Also, there is a series of conditions called vasculitides, and we know that alpha-1 may have a role in treating some of these vascular diseases as well. So the big ones [conditions] as I see it are in alpha-1 deficiency; ordinary COPD; ARDS, be it Covid- or non-Covid related; bronchiectasis, either cystic fibrosis or non-CF bronchiectasis; and panniculitis or vasculitis.”
into getting reimbursement for those in this country, which to me is bizarre.”
Variants
In the context of Covid-19, is this treatment breakthrough effective across all variants? “The people we looked at specifically were the very sick people,” Prof McElvaney said. “We looked at people on ventilators, for example, but it could be even more effective in people who are not yet on ventilators. We have no data on this, but it may actually even prevent the need for ventilation in some people, if it is given early enough. At that stage, the ‘horse has bolted’ so to speak, so it doesn’t really matter what variant they have. At that point, you are in an extreme situation because your lungs are so inflamed so at that point, the variant is irrelevant.
“Interestingly,” he continued, “alpha-1 may have another effect on Covid – it may also affect the ability of the virus to get into cells. One of the ways by which the SARS-CoV-2 virus gets into cells is that it is modified by a protease inside the cell, and alpha-1 inhibits that protease. So in addition to its anti-inflammatory effect, it may also have an antiviral effect. It is the second-most common protein in the body and it changes according to certain inflammatory processes.
Taken on the whole, the results of this translational research should open the door to reimbursement for alpha-1 as a therapy, Prof McElvaney suggested. “We are falling well behind the rest of Europe in this regard; most European countries pay for this [therapy]. In fact, we are conducting a big study at the moment with alpha-1 groups in Switzerland and Austria and the difference between us and them is that perhaps 50-to-60 per cent of their patients are on intravenous augmentation therapy, and we have less than 5 per cent of our people on it.” This highlights the acute problem in Ireland of gaining reimbursement for rare diseases: “And if you put this in context, even with the superdrugs we have for cystic fibrosis, there was an inordinate amount of effort that went
“We know that in Covid, the patients’ response to severe infection is not only to increase the amount of alpha-1 they have, but also to add certain sugars to it to make it more effective in being anti-inflammatory – there’s just not enough of it.”
Reference
McElvaney OJ, McEvoy NL, Boland F, McElvaney OF, Hogan G, Donnelly K, et al. A randomised, double-blind, placebo-controlled trial of intravenous alpha-1 antitrypsin for ARDS secondary to Covid-19. Med (N Y). 2022 Apr 8;3(4):233-248.e6. doi: 10.1016/j. medj.2022.03.001
5 Respiratory Medicine | Volume 8 | Issue 5 | 2022
We learned early on that there are certain proinflammatory proteins in the blood that were found in Covid infections
Allergic rhinitis in focus
AUTHOR: Dr Iseult Sheehan, Clinical Director, Allergy Ireland (www.allergy-ireland.ie)
CASE REPORT
A 15-year-old boy is brought to our clinic by his mother in July, referred by his GP. He has had significant allergic rhinitis symptoms since early childhood. The symptoms are mainly present from May to August.
His main symptoms include rhinorrhoea, sneezing, nasal congestion associated with pruritis to his nose, palate, and occasionally his arms and legs. He also experiences features of allergic conjunctivitis with itchy, watery eyes, and swelling intermittently.
He has recently been experiencing asthma and eczema flare-ups on high pollen count days.
His symptoms are preventing him from sleeping. In addition, he has just completed his Junior Certificate exams. His ability to concentrate and study was hindered and his exam performance was reduced compared to his mock exams.
His parents are very concerned about his Leaving Certificate exams in three years. He is a motivated student with ambitions to attend university. He has
Allergic rhinitis (AR) is a common condition with a global impact. In Ireland, at least onein-five people suffer with AR.1 The economic impact is striking. The European Union recently estimated that the indirect cost of undertreated AR on work productivity may cost between €30-50 billion per year.2
The symptoms of AR are often considered to be trivial and as such AR is underdiagnosed and undertreated. However, the burden of this disease is significant with a reduced qualityof-life for these individuals. It has been shown to affect cognitive and psychomotor function and patients describe the impact on sleep as considerably debilitating.
been taking multiple antihistamines daily, which he thinks might be contributing to his fatigue. He is also using an intranasal corticosteroid.
Examination
The boy appeared tired with dark rings under his eyes. He was visibly mouth breathing.
Flexible nasoendoscopy confirmed rhinitis with significantly oedematous turbinates bilaterally. There was increased mucus and visible mucosal pallor. There were no polyps and no septal deviation. Chest and eye examination was normal.
Skin prick testing was performed, which confirmed a strong sensitisation to grass pollen.
Management
Allergen avoidance measures were discussed and daily saline irrigation of the nasal cavity advised. Given the severity of the symptoms, a short course of topical intranasal corticosteroid drops (Betamethasone) was used followed by the commencement of a combination
intranasal corticosteroid and antihistamine spray. Topical mast-cell stabiliser (sodium cromoglycate) eye drops were advised. His symptoms improved and at a review in August he was almost symptom free.
Follow-up
The following year he was commenced on a similar plan from early April. However, on review in early June his symptoms were persistent. A trial of a leukotriene receptor antagonist was commenced, which significantly improved symptom control.
Following the pollen season, he was commenced on sub-lingual grass pollen immunotherapy and tolerated it well. He continued to take immunotherapy daily and the April prior to his exams he recommenced the medication plan as before. His exams went well and he remained asymptomatic throughout. He will continue to take immunotherapy to complete a threeyear treatment plan. As a result, it is expected that he will require less medications to control his allergic rhinitis in the future.
While struggling with AR symptoms, the ability to participate in social and sporting activities is reduced and missed days at work are a feature.
In addition, AR has a worrying impact on a child’s education. Missed or unproductive days at school are common. This becomes particularly apparent during hay fever
season, which coincides with exam time. A UK study of teenagers found that there was a reduction in exam performance for those with seasonal AR compared with other times of the year.3 This is most relevant for Leaving Certificate students and those in university.
Epidemiology
It is estimated that AR affects at least
6 Volume 8 | Issue 5 | 2022 | Respiratory Medicine
The symptoms of AR are often considered to be trivial and as such AR is underdiagnosed and undertreated
400 million people worldwide and the prevalence within Europe is between 17and-29 per cent.1 The UK has a prevalence of 26 per cent1 and Ireland is likely to be similar to this.
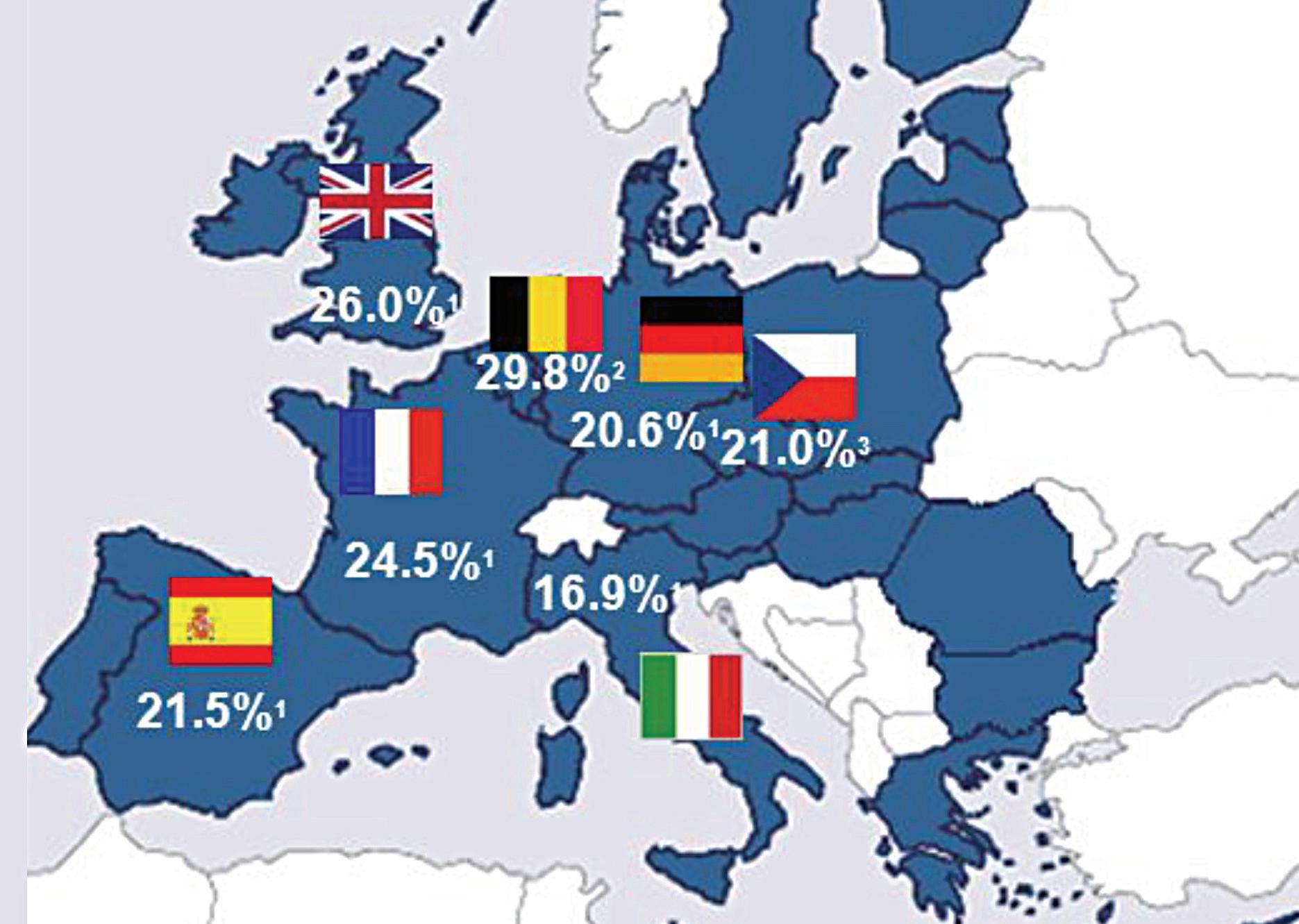
AR will often begin early in life, but prevalence increases with age. The International Study of Asthma and Allergies in Childhood (ISAAC, 2006) phase 3 study demonstrated this, showing a 5 per cent prevalence in those aged three years, an 8.5 per cent prevalence in those aged six-to-seven years, and a 14.6 per cent prevalence in those aged 13-to-14 years.4
What is most concerning is that the prevalence of AR is increasing globally; as was corroborated by this ISAAC study, which found an increase in prevalence of AR from 13-to-19 per cent over an eight-year period in a cohort of 13-to14 year olds.4 A smaller study in Cork demonstrated an increase in prevalence from 7.6 per cent to 10.6 per cent over a five-year period in a cohort of six-to-nine year olds. 5
Nature versus nurture
The cause for this rising prevalence is unclear, although risk factors may include
overuse of antibiotics, exposure to air pollution, maternal/passive smoking, and climatic factors among other theories.6
Certainly, environmental exposures are key to understanding the rising prevalence of allergies. The ‘hygiene hypothesis’ was proposed as an explanation whereby the
more sterile Western lifestyle was reducing infections and resulting in less type 1 immune responses. More recently, there is a better insight into the development of allergen tolerance with the microbiome during early life being an essential component. Antibiotic use will disrupt this, among other environmental factors.

7 Respiratory Medicine | Volume 8 | Issue 5 | 2022
FIGURE 2: Allergic sensitisation. Adapted from Bousquet et al. Nature Reviews Disease Primers, 202010
FIGURE 1: Prevalence of AR in Europe. Adapted from Bauchau et al. Eur Respir J 20041 (Figure from Stallergenes)
Exposure to irritants such as cigarette smoke and air pollution, particularly diesel exhaust fumes, has been shown to contribute to and exacerbate AR.
In addition, global warming is seen to be playing a role in Ireland, with milder weather resulting in prolongation of pollen and spore seasons. This is confounded by the introduction of new pollens such as ragweed, which would usually be a common allergen in North America and continental Europe.
Nevertheless, AR appears to be the consequence of environmental exposures in those with a genetic vulnerability. Indeed, genetic predisposition or atopy accounts for at least 50 per cent of AR cases,7 and genetic
studies have demonstrated that multiple susceptible loci can contribute to AR alone.8
Multimorbid AR
Multimorbid AR is whereby AR and asthma or atopic dermatitis co-exist. Interestingly, a differing variety of genetically susceptible loci are attributable to multimorbid AR, for example, IL-5 and IL-33 for those with AR and asthma. 8
AR is a risk factor for asthma. In fact 90 per cent of asthmatics have AR and 30-to40 per cent of those with AR have asthma.9
A ‘united airways’ disease approach to management is the more favoured approach in recent years. Moreover, the treatment of nasal inflammation in asthmatics has been shown to improve
outcomes. This highlights the importance of assessing for both asthma and rhinitis in these patients.
AR can also be associated with comorbid dermatological conditions, such as atopic dermatitis and urticaria, upon exposure to an allergen. Interestingly, the treatment of AR can very often result in improvements in these dermatological conditions.
Presentation
AR is an IgE-mediated inflammatory reaction following exposure to an allergen. This results in inflammation of the nasal lining and/or conjunctiva. The symptoms characteristically include rhinorrhoea, nasal obstruction, sneezing, and nasal itching.

Additionally, symptoms will often include an itchy palate and irritated, watery itchy eyes with associated periocular oedema or dark rings under the eyes (allergic shiners).
Patients can experience fatigue, snoring, mouth breathing due to nasal obstruction, and a feeling of heaviness in the head or a ‘fuzzy’ head. If the sinuses are affected the patient may experience sinus pressure and headaches and a post-nasal drip.

8 Volume 8 | Issue 5 | 2022 | Respiratory Medicine
IMAGE 1: Allergic shiners
IMAGE 2: Allergic salute
FIGURE 3: AR classification. Adapted from Aria Guideline 20199
120mg and 180mg Film-coated tablets
A NEW generation antihistamine offering non-drowsy, long-lasting relief from allergy symptoms


























Abbreviated Prescribing Information



Telfast 120 and 180 mg film-coated tablets
Each tablet contains 120 or 180 mg fexofenadine hydrochloride.
Presentation: Telfast 120 mg: Peach, capsule-shaped, film-coated tablet with 012 on one side and a scripted e on the other side. Telfast 180 mg: Peach, capsule-shaped, film-coated tablet with 018 on one side and a scripted e on the other side. Indications for Telfast 120 mg: Telfast 120 mg is indicated in adults and children 12 years and older for the relief of symptoms associated with seasonal allergic rhinitis. Indications for Telfast 180 mg: Telfast 180 mg is indicated in adults and children 12 years and older for the relief of symptoms associated with chronic idiopathic urticaria. Dosage: Adults and children aged 12 years and over: One tablet once daily before a meal. Not recommended for children under 12 years. Studies in special risk groups (elderly, renally or hepatically impaired patients) indicate that it is not necessary to adjust the dose of fexofenadine hydrochloride in these patients.
Method of administration: Oral. Contraindications: Hypersensitivity to the active substance or any of the excipients. Warnings and precautions: There is limited data in the elderly and renally or hepatically impaired patients. Fexofenadine hydrochloride should be administered with care in these special groups. Patients with a history of or ongoing cardiovascular disease should be warned that, antihistamines as a medicine class, have been associated with the adverse reactions tachycardia and palpitations. Interactions: Fexofenadine does not undergo hepatic biotransformation and therefore will not interact with other medicinal products through hepatic mechanisms. Coadministration of fexofenadine hydrochloride with erythromycin or ketoconazole has been found to result in a 2–3 times increase in the level of fexofenadine in plasma. The changes were not accompanied by any effects on the QT interval and were not associated with any increase in adverse reactions compared to the medicinal products given singly. Animal studies
have shown that the increase in plasma levels of fexofenadine observed after coadministration of erythromycin or ketoconazole, appears to be due to an increase in gastrointestinal absorption and either a decrease in biliary excretion or gastrointestinal secretion, respectively. No interaction between fexofenadine and omeprazole was observed. However, the administration of an antacid containing aluminium and magnesium hydroxide gels 15 minutes prior to fexofenadine hydrochloride caused a reduction in bioavailability, most likely due to binding in the gastrointestinal tract. It is advisable to leave 2 hours between administration of fexofenadine hydrochloride and aluminium and magnesium hydroxide containing antacids. Fertility, pregnancy and lactation: Fexofenadine hydrochloride should not be used during pregnancy unless clearly necessary. Fexofenadine hydrochloride is not recommended for mothers breast-feeding their babies. No human data on the effect of fexofenadine hydrochloride on fertility are available. In mice, there was no effect on fertility with fexofenadine hydrochloride treatment. Driving and operation of machinery: On the basis of the pharmacodynamic profile and reported adverse reactions it is unlikely that fexofenadine hydrochloride tablets will produce an effect on the ability to drive or use machines. In objective tests, Telfast has been shown to have no significant effects on central nervous system function. This means that patients may drive or perform tasks that require concentration. However, it is advisable to check the individual response before driving or performing complicated tasks. Undesirable effects: Headache, drowsiness, dizziness, nausea. Refer to Summary of Product Characteristics for other undesirable effects. Pack size: 30 tablets. Marketing authorisation holder: Opella Healthcare, 82 Avenue Raspail 94250, Gentilly, France SAS T/A Sanofi. Marketing authorisation number: PA23180/003/002-003. Medicinal product subject to medical prescription. A copy of the SPC is available on request or visit www.clonmelhealthcare.ie. Last revision date: March 2022.



Distributed in Ireland by Clonmel Healthcare Ltd. 2022/ADV/TEL/067H
Compression of the olfactory nerve due to oedema within the nasal cavity can result in an altered sense of smell and/or taste.
Pathophysiology
There are two phases which are paramount to the development of an allergy. Phase one occurs when an atopic individual is first exposed to the allergen. The allergen is taken up by antigen-presenting cells, particularly dendritic cells (DC), and is processed into peptide fragments. The DC will move through the lymphatics towards the lymph node where it will present this peptide fragment to a naïve T-cell.
The naïve T-cell becomes activated to express cytokines, particularly IL-4, which drives the differentiation of these cells to Th2 helper cells. An environment rich in cytokines IL-4 and IL-13 is created and is responsible for inducing IgE production from B-cells. Additionally, IL-5 is responsible for eosinophil recruitment and activation. The cytokine profile is vital as it determines a Th2 immune response.
In the meantime, T-cell dependent activation of B-cells stimulates further cytokine production, particularly IL-4, and promotes irreversible immunoglobulin class switching to allergen-specific IgE antibodies.
Allergen-specific IgE will attach to mast cells and basophils. This is referred to as primary sensitisation. In addition, memory B-cells are generated and a small number of memory T-cells remain.
Phase two occurs on subsequent exposure to this allergen. The allergen binds to the sensitised mast cells, triggering degranulation of the mast cell; releasing pre-stored and newly synthesised inflammatory mediators such as histamine, leukotrienes, and prostaglandins. These contribute to vascular permeability, eosinophil infiltration, and increased mucus production.
Furthermore, with repetitive allergen exposure, nasal priming occurs. This appears to cause an accumulation of

effector cells in the nasal mucosa and results in a hyper-responsiveness to the allergen and prolongation of symptoms. In addition, there appears to be a neural component to this hyper-responsiveness. Changes to the sensory nerves of the nose have been demonstrated in those with AR. In addition, innate immune responses can be initiated in the nasal epithelium by allergens directly compromising the epithelium and resulting in the release of alarmins such as IL-33, further activating the inflammatory response.
Classification of AR
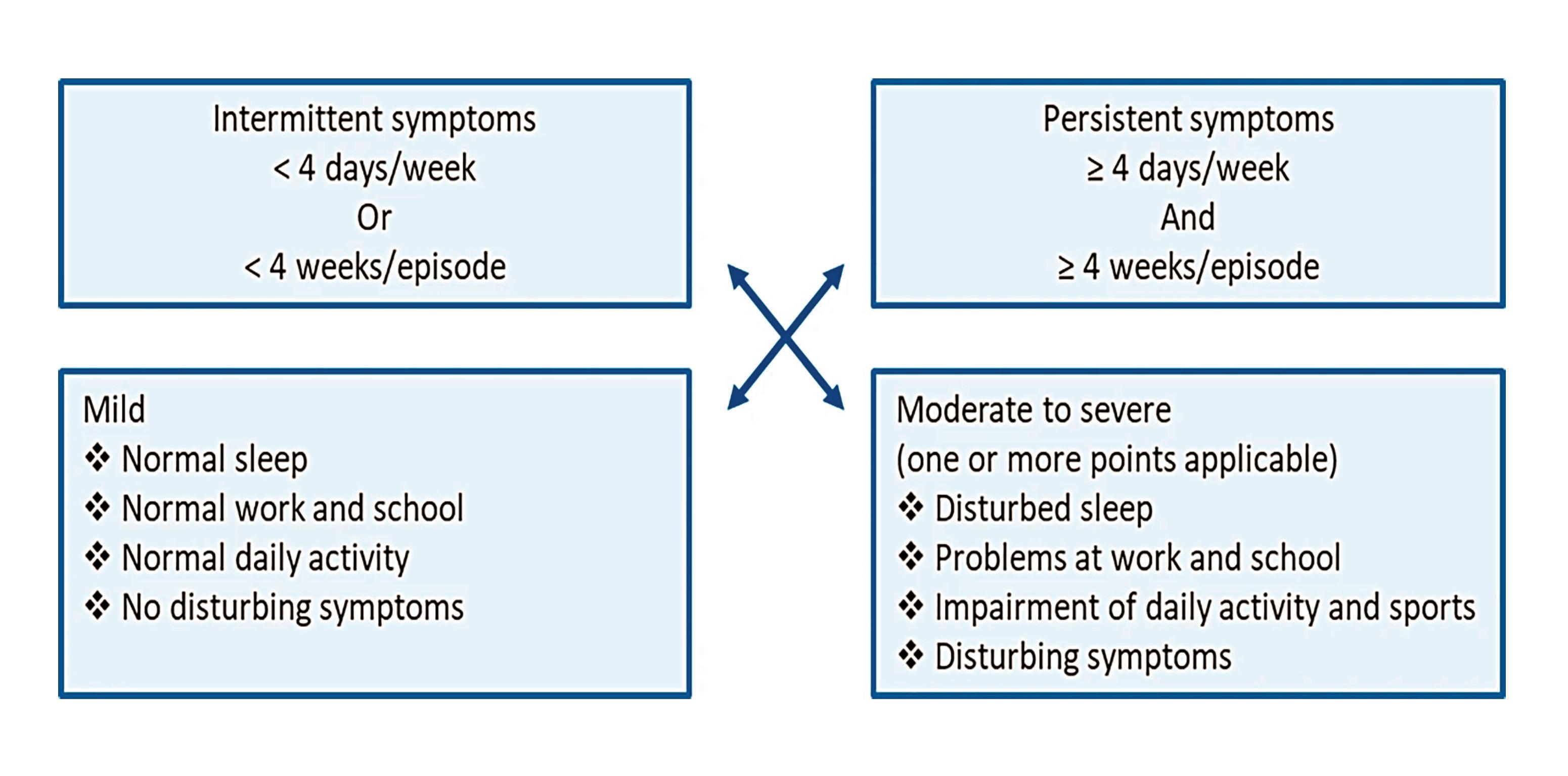
AR can be divided into seasonal and perennial based on allergen triggers. Seasonal rhinitis includes sensitisation to grass, tree, or weed pollen, and fungal spores. Whereas perennial rhinitis is commonly triggered by house dust mite or animal dander. This classification system is effective at giving a likely diagnosis of the trigger, which assists with recommending appropriate avoidance measures.
However, a new classification system focusing on the functional ability of the patient, including the frequency and severity of symptoms, has become a much more effective tool for making treatment decisions. This guideline was developed by Allergic Rhinitis and the Impact on Asthma (ARIA) in collaboration with the World Health Organisation (WHO).9
Diagnosis of AR
The diagnosis of AR is generally based on clinical symptoms. However, skin-prick allergy testing or specific IgE blood testing can be used to confirm the allergen trigger.
In addition, it is vital to examine the nose whereby you will often see bulky oedematous turbinates with visible increased mucus production (Images 3 and 4). Pallor of the mucosal lining is often present, particularly in longstanding cases. Occasionally, the mucosa will lose its smooth appearance and instead will have ridges and pitting from chronic allergic challenge. Pre-polypoid tissue can occasionally be present.

Non-pharmacological management
Allergen avoidance should be discussed. Nevertheless, avoidance alone is generally not sufficient to manage symptoms. In cases where the allergen trigger is animal dander, avoidance is effective if the animal is removed from the home.
Smoking cessation should be advised always. Smoking can be associated with chronic nasal symptoms and may even be associated with the development of polyposis. Passive smoking or ‘vaping’ appear to carry similar risk.
Saline irrigation is an effective way to directly cleanse the nasal cavity with the resultant reduction of mucus, inflammatory mediators, and bacterial burden. It has also been shown to improve mucociliary function.
Pharmacological management
In patients with mild intermittent symptoms an antihistamine is
10 Volume 8 | Issue 5 | 2022 | Respiratory Medicine
IMAGE 4: Bulky oedematous inferior turbinate with mucosal pallor
IMAGE 3: Normal inferior turbinate
symptom control”1
effective allergic symptom control”1 moderate / severe ic


For moderate / severe allergic rhinitis
Dymista is indicated for the of moderate to severe perennial allergic rhinitis with either intranasal glucocorticoid is not considered sufficient.
tions: Dymista is indicated for the relief of symptoms of moderate to severe seasonal and perennial allergic rhinitis if monotherapy with either intranasal antihistamine or glucocorticoid is not considered sufficient.2
INFORMATION:
Dymista (azelastine hydrochloride / fluticasone propionate) 137 micrograms / 50 micrograms per actuation, Nasal Spray, Suspension. Please refer to Summary of Product Characteristics (SmPC) before prescribing. Indications, Dosage and Posology: Relief of symptoms of moderate to severe seasonal and perennial allergic rhinitis if monotherapy with either intranasal antihistamine or glucocorticoid is not considered sufficient. Posology, For full therapeutic benefit regular usage is essential. Contact with the eyes should be avoided. Adults and adolescents (12 years and older), One actuation in each nostril twice daily (morning and evening). Children below 12 years, Dymista Nasal Spray is not recommended for use in children below 12 years of age as safety and efficacy has not been established in this age group. Elderly, No dose adjustment is required in this population. Renal and hepatic impairment There are no data in patients with renal and hepatic impairment.
Duration of treatment: Dymista Nasal Spray is suitable for long-term use. The duration of treatment should correspond to the period of allergenic exposure. Method of administration: Dymista Nasal Spray is for nasal use only.


For full therapeutic benefit regular usage is essential. Contact children below 12 years of age as safety and efficacy has not only.
Spray must be primed by pressing down and releasing the seconds by tilting it upwards and downwards and the protective protective cap to be replaced. Presentation: Nasal Spray, drug interactions in patients receiving fluticasone propionate to the patient outweighs the risk of systemic corticosteroid corticosteroids and may vary in individual patients and between rarely, a range of psychological or behavioural effects including propionate in patients with severe liver disease is likely to significant adrenal suppression. If there is evidence for higher the lowest dose at which effective control of the symptoms considered whenever other forms of corticosteroid treatment receiving prolonged treatment with nasal corticosteroids is Visual disturbance may be reported with systemic and topical cataract, glaucoma or rare diseases such as central serous and/or cataracts. If there is any reason to believe that adrenal operation or injury to the nose or mouth, the possible benefits contraindication to treatment with Dymista Nasal Spray. Dymista contains plasma concentrations of fluticasone propionate are achieved propionate are unlikely. A drug interaction study in healthy subjects postmarketing use, there have been reports of clinically significant drug expected to increase the risk of systemic side-effects. The other inhibitors of cytochrome P450 3A4 produce negligible cytochrome P450 3A4 inhibitors (e.g. ketoconazole), as there is doses have been performed. However, they bear no relevance central nervous medications because sedative effect may are no or limited amount of data from the use of azelastine SmPC) Lactation: It is unknown whether nasally administered newborns/infant. Undesirable effects: Very common (≥1/10): adverse reactions: Reporting suspected adverse reactions via HPRA Pharmacovigilance, Website: www.hpra.ie Adverse renewed (B)
Instruction for use Preparing the spray: The bottle should be shaken gently before use for about 5 seconds by tilting it upwards and downwards and the protective cap be removed afterwards. Prior to first use Dymista Nasal Spray must be primed by pressing down and releasing the pump 6 times. If Dymista Nasal Spray has not been used for more than 7 days it must be reprimed once by pressing down and releasing the pump. Using the spray: The bottle should be shaken gently before use for about 5 seconds by tilting it upwards and downwards and the protective cap be removed afterwards. After blowing the nose the suspension is to be sprayed once into each nostril keeping the head tilted downward (see figure in section 4.2 of the SmPC). After use the spray tip is to be wiped and the protective cap to be replaced. Presentation: Nasal Spray, suspension.
Contraindications: Hypersensitivity to the active substances or to any of the excipients listed in section 6.1 of the SmPC. Warnings and precautions: During post-marketing use, there have been reports of clinically significant drug interactions in patients receiving fluticasone propionate and ritonavir, resulting in systemic corticosteroid effects including Cushing’s syndrome and adrenal suppression. Therefore, concomitant use of fluticasone propionate and ritonavir should be avoided, unless the potential benefit to the patient outweighs the risk of systemic corticosteroid side-effects (see section 4.5 of the SmPC). Systemic effects of nasal corticosteroids may occur, particularly when prescribed at high doses for prolonged periods. These effects are much less likely to occur than with oral corticosteroids and may vary in individual patients and between different corticosteroid preparations. Potential systemic effects may include Cushing’s syndrome, Cushingoid features, adrenal suppression, growth retardation in children and adolescents, cataract, glaucoma and more rarely, a range of psychological or behavioural effects including psychomotor hyperactivity, sleep disorders, anxiety, depression or aggression (particularly in children). Dymista Nasal Spray undergoes extensive first-pass metabolism, therefore the systemic exposure of intranasal fluticasone propionate in patients with severe liver disease is likely to be increased. This may result in a higher frequency of systemic adverse events. Caution is advised when treating these patients. Treatment with higher than recommended doses of nasal corticosteroids may result in clinically significant adrenal suppression. If there is evidence for higher than recommended doses being used, then additional systemic corticosteroid cover should be considered during periods of stress or elective surgery. In general, the dose of intranasal fluticasone formulations should be reduced to the lowest dose at which effective control of the symptoms of rhinitis is maintained. Higher doses than the recommended one (see section 4.2 of the SmPC) have not been tested for Dymista. As with all intranasal corticosteroids, the total systemic burden of corticosteroids should be considered whenever other forms of corticosteroid treatment are prescribed concurrently. Growth retardation has been reported in children receiving nasal corticosteroids at licensed doses. Since Dymista is also given to adolescents, it is recommended that the growth of adolescents receiving prolonged treatment with nasal corticosteroids is regularly monitored, too. If growth is slowed, therapy should be reviewed with the aim of reducing the dose of nasal corticosteroid if possible, to the lowest dose at which effective control of symptoms is maintained. Visual disturbance may be reported with systemic and topical corticosteroid use. If a patient presents with symptoms such as blurred vision or other visual disturbances, the patient should be considered for referral to an ophthalmologist for evaluation of possible causes which may include cataract, glaucoma or rare diseases such as central serous chorioretinopathy (CSCR) which have been reported after use of systemic and topical corticosteroids. Close monitoring is warranted in patients with a change in vision or with a history of increased ocular pressure, glaucoma and/or cataracts. If there is any reason to believe that adrenal function is impaired, care must be taken when transferring patients from systemic steroid treatment to Dymista Nasal Spray. In patients who have tuberculosis, any type of untreated infection, or have had a recent surgical operation or injury to the nose or mouth, the possible benefits of the treatment with Dymista Nasal Spray should be weighed against possible risk. Infections of the nasal airways should be treated with antibacterial or antimycotical therapy, but do not constitute a specific contraindication to treatment with Dymista Nasal Spray. Dymista contains benzalkonium chloride. Long term use may cause oedema of the nasal mucosa. Interactions with other medicinal products and other forms of interactions: Fluticasone propionate Under normal circumstances, low plasma concentrations of fluticasone propionate are achieved after intranasal dosing, due to extensive first pass metabolism and high systemic clearance mediated by cytochrome P450 3A4 in the gut and liver. Hence, clinically significant drug interactions mediated by fluticasone propionate are unlikely. A drug interaction study in healthy subjects has shown that ritonavir (a highly potent cytochrome P450 3A4 inhibitor) can greatly increase fluticasone propionate plasma concentrations, resulting in markedly reduced serum cortisol concentrations. During postmarketing use, there have been reports of clinically significant drug interactions in patients receiving intranasal or inhaled fluticasone propionate and ritonavir, resulting in systemic corticosteroid effects. Co-treatment with other CYP 3A4 inhibitors, including cobicistat-containing products is also expected to increase the risk of systemic side-effects. The combination should be avoided unless the benefit outweighs the increased risk of systemic corticosteroid side-effects, in which case patients should be monitored for systemic corticosteroid side-effects. Studies have shown that other inhibitors of cytochrome P450 3A4 produce negligible (erythromycin) and minor (ketoconazole) increases in systemic exposure to fluticasone propionate without notable reductions in serum cortisol concentrations. Nevertheless, care is advised when co-administering potent cytochrome P450 3A4 inhibitors (e.g. ketoconazole), as there is potential for increased systemic exposure to fluticasone propionate. Azelastine hydrochloride No specific interaction studies with azelastine hydrochloride nasal spray have been performed. Interaction studies at high oral doses have been performed. However, they bear no relevance to azelastine nasal spray as given recommended nasal doses result in much lower systemic exposure. Nevertheless, care should be taken when administering azelastine hydrochloride in patients taking concurrent sedative or central nervous medications because sedative effect may be enhanced. Alcohol may also enhance this effect (see section 4.7 of the SmPC). Fertility, pregnancy and lactation: Fertility: There are only limited data with regard to fertility (see section 5.3 of the SmPC). Pregnancy: There are no or limited amount of data from the use of azelastine hydrochloride and fluticasone propionate in pregnant women. Therefore, Dymista Nasal Spray should be used during pregnancy only if the potential benefit justifies the potential risk to the foetus (see section 5.3 of the SmPC) Lactation: It is unknown whether nasally administered azelastine hydrochloride/metabolites or fluticasone propionate/metabolites are excreted in human breast milk. Dymista Nasal Spray should be used during lactation only if the potential benefit justifies the potential risk to the newborns/infant. Undesirable effects: Very common (≥1/10): Epistaxis Common (>1/100, <1/10): Headache, dysgeusia (unpleasant taste), unpleasant smell For details of uncommon, rare and very rarely reported adverse events and those of unknown frequency, see SmPC. Reporting of adverse reactions: Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance, Website: www.hpra.ie Adverse reactions/events should also be reported to the marketing authorisation holder at the email address: pv.ireland@viatris.com or phone 0044(0)8001218267. Legal Category: Product subject to prescription which may be renewed (B)
Summary of Product Characteristics.
ine Hydrochloride/Fluticasone Propionate
“Provides effective allergic rhinitis
Job code: DYM-2022-0025 Date of Preparation : February 2022 Viatris.com For more information, please refer to Summary of Product Characteristics References: 1. Meltzer E et al. Clinically relevant effect of a new intranasal therapy (MP29-02) in allergic rhinitis assessed by responder analysis. Int Arch Allergy Immunol. 2013;161(4):369–77. 2. Dymista Nasal Spray Summary of Product Characteristics. ABBREVIATED PRESCRIBING
Marketing Authorisation Number: PA2010/059/001 Marketing Authorisation Holder: Mylan IRE Healthcare Limited, Unit 35/36, Grange Parade, Baldoyle Industrial Estate, Dublin 13, Ireland Full Prescribing Information available on request from: Viatris, Dublin 17. Phone 01 8322250. Date of revision of Abbreviated Prescribing Information: 10 Nov 2021 Reference Number: IE-AbPI-Dymista-v003
often effective. Second-generation antihistamines are recommended as they carry less cholinergic and sedating side-effects. Oral or nasal decongestants can be used as a rescue medication, but for no longer than five days to avoid rebound symptoms.
The ARIA guideline recommends intranasal corticosteroids as the firstline treatment for moderate-to-severe intermittent or persistent AR.9 A low bioavailability is recommended and so newer generation intranasal corticosteroids are preferred.
If the nasal cavity is very obstructed, a nasal spray may not be effective until the oedema has been reduced using intranasal corticosteroid drops. Should this not be effective, a combination intranasal treatment is now available combining corticosteroid and antihistamine.
Eye symptoms can be managed conservatively with cold compresses and tear supplements. However, if these
symptoms persist, it is advisable to consider oral and topical antihistamines, topical mast cell stabilisers (sodium cromoglicate), or decongestants. Topical corticosteroids should ideally be prescribed under the care of an ophthalmologist.
If there is evidence of lower airway irritability or asthma, a leukotriene receptor antagonist can be trialled. In severe cases, short courses of oral corticosteroids are occasionally required.
Newer treatment options:
Immunotherapy
Immunotherapy has been shown to significantly reduce symptoms and medication requirements and is recommended by the ARIA guideline. Additionally, the Global Initiative for Asthma (GINA) 2020 guideline recommends that immunotherapy be considered for asthmatics sensitised to dust mite.11 Immunotherapy involves exposing a patient to minute quantities of the allergen trigger, allowing the
immune system to build up a tolerance. It is essentially like a vaccination. It can be given as a subcutaneous injection or as a sublingual tablet. Sublingual therapy is used predominantly in Ireland and is currently available for grass pollen, dust mite, and tree pollen. Compliance is crucial and regular follow-up advised. It is usually a three-year process whereby the patient takes it daily. It is highly effective and well-tolerated.
Newer treatment options: Endonasal phototherapy
Phototherapy is well-established for skin conditions and is now being used within the nasal cavity to manage AR. It uses UVA (25 per cent), UVB (<5 per cent) and visible light (70 per cent) to induce a local immunosuppressive effect by inhibiting allergen-induced histamine release from mast cells and inducing apoptosis of T-lymphocytes and eosinophils. It essentially desensitises the nasal cavity, thus reducing symptoms. It is particularly useful when pharmacological treatment is insufficient or contraindicated.
References
1. Bauchau V, Durham SR. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Resp J 2004; 24(5): 758
2. Zuberbier T, Lötvall J, Simoens S, Subramanian SV, Church MK. Economic burden of inadequate management of allergic diseases in the European Union: A GA(2) LEN review. Allergy 2014; 69(10): 1275-9
3. Walker S, Khan-Wasti S, Fletcher M, Cullinan P, Harris J, Sheikh A. Seasonal allergic rhinitis is associated with a detrimental effect on examination performance in United Kingdom teenagers: Case-control study. J Allergy Clin Immunol 2007; 120(2): 381-7
4. Asher MI, Montefort S, Björkstén B, et al. Worldwide time trends in the
prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 2006; 368(9537): 733-43
5. Duggan EM, Sturley J, Fitzgerald AP, Perry IJ, Hourihane JO. The 2002-2007 trends of prevalence of asthma, allergic rhinitis, and eczema in Irish schoolchildren. Pediatr Allergy Immunol 2012; 23(5): 464-71
6. Asher MI, Stewart AW, Mallol J, et al. Which population level environmental factors are associated with asthma, rhinoconjunctivitis, and eczema? Review of the ecological analyses of ISAAC Phase One. Respir Res 2010; 11(1): 8
7. Zacharasiewicz A, Douwes J, Pearce N. What proportion of rhinitis symptoms is attributable to atopy? J Clin Epidemiol 2003; 56(4): 385-90
8. Lemonnier N, Melén E, Jiang Y, et al. A novel whole blood gene expression signature for asthma, dermatitis, and rhinitis multimorbidity in children and adolescents. Allergy 2020; 75(12): 3248-60
9. Bousquet J, Schünemann HJ, Togias A, et al. Next-generation allergic rhinitis and its impact on asthma (ARIA) guidelines for allergic rhinitis based on grading of recommendations assessment, development and evaluation (GRADE) and real-world evidence. J Allergy Clin Immunol 2020; 145(1): 70-80.e3
10. Bousquet J, Anto JM, Bachert C, et al. Allergic rhinitis. Nat Rev Dis Primers 2020; 6(1): 95
11. Global Initiative for Asthma. Global strategy for asthma management and prevention. Updated 2020. Available at: https://ginasthma. org/. [Last accessed on 3rd January 2021]
12 Volume 8 | Issue 5 | 2022 | Respiratory Medicine
The End to End Model of Care for Asthma
AUTHOR: Ms Ruth Morrow, Registered Advanced Nurse Practitioner (Primary Care); Respiratory Nurse Specialist (WhatsApp Messaging Service Asthma Society of Ireland); and Nurse Educator and Consultant
Asthma is the most common chronic respiratory disease in Ireland, with approximately one-in-10 of the population having asthma. Asthma control remains suboptimal in a large proportion of patients, which places significant health, social, and economic burden on the community and on healthcare. The reasons why asthma control remains poor is multi-factorial, but fragmented and unstructured care is believed to be an important contributory factor. The cost of asthma care in Ireland is over €500 million per annum, most of which is in secondary care.
The HSE’s new End to End Model of Care (MOC) for Asthma has been developed in consultation with a wide range of stakeholders including nurses, consultants, GPs, physiotherapists, patients, and patient support organisations. It covers the full spectrum of care provided in both hospital and in the community with a focus on developing partnerships between acute hospital services, general practice and community services, with the patient and his/her family being central to the model. The End to End MOC for adult asthma has been developed in tandem with the HSE strategy for chronic disease management. It outlines the structures that we should adhere to and adopt in the care of patients with, or at risk of, asthma. This MOC is guided by national and international best practice.
The document is not meant to be a guideline document outlining interventions to be used in varied clinical circumstances that present when managing patients with asthma. In this regard the National Clinical Care Programme (NCP) Respiratory endorses the guidelines produced and updated regularly by the Irish Thoracic Society (ITS), the ICGP, and Global Initiative for Asthma (GINA). However, the MOC document details how patients should be able to access care at various stages of their asthma and also outlines the roles and
responsibilities of the healthcare professionals (HCPs) providing this healthcare. It is envisaged that the implementation of this MOC will result in a reduction in the variation of care delivered to patients with asthma in Ireland, and additionally result in an improvement in their asthma control, clinical outcomes and quality-of-life.
The MOC seeks, through the implementation of its guidelines, to improve the standard of care provided to adult asthma patients in all healthcare settings, with a particular focus on primary care where the majority of asthma is managed. This MOC will place a particular focus on the ‘at-risk’ patients who are vulnerable to developing asthma and those at risk of experiencing an acute asthma event. This includes those in lower socio-economic groups, smokers, patients with multiple co-morbidities, and those with psychological problems.
The implementation of the MOC aims to ensure that optimum care is delivered using the principles of Sláintecare; so people with asthma receive the right care at the right time in the right place.
The spectrum of services, ranging from primary prevention to tertiary care, includes:
Primary prevention and health promotion.
Risk factor identification and management.
Early detection of asthma and its diagnosis.
Secondary prevention.
Primary care management of asthma.
Shared primary and secondary care management of asthma.
Secondary care management of chronic asthma.
Tertiary care.
In addition to guiding the delivery of the aforementioned objectives, this End to End MOC for adult asthma reflects the key reform themes identified by the HSE to improve the health of the population and to
reshape where and how healthcare services are provided in Ireland. These themes include improving population health, delivering care closer to home, developing specialist hospital care networks, and improving quality, safety, and value.
MOC scope
The scope of this MOC is to define the services required to support the general population of adults in the management of their asthma. It includes health services operated and funded by the HSE and includes community-based services as well as access to hospital-based secondary and tertiary care services if required. It acknowledges that specific health and social care settings, high-risk and vulnerable groups will require additional interventions and support. Working with other relevant national clinical programmes (paediatric and neonatal) and services, this MOC will inform the future development of shared pathways, policies, strategies and services to improve health outcomes in these settings.
Supporting documents include clinical guidelines published by the ICGP, ie, Asthma – Diagnosis, Assessment, and Management in General Practice Quick Reference Guide. The National Clinical Guideline for the Management of an Acute Asthma Attack in Adults (NCEC) is also referred to in this document. International clinical guidelines, such as those from GINA (2021), underpin the diagnosis and management of asthma.
Future development includes the NCP Respiratory collaborating with NCP Paediatrics and Neonatal to form a paediatric working group to develop Part 2: Paediatric Asthma.
Download the End to End MOC at: www.hse.ie/eng/about/who/cspd/ncps/asthma/ resources/end-to-end-model-of-care-for-asthma.pdf for more information.
13 Respiratory Medicine | Volume 8 | Issue 5 | 2022
Women and asthma
AUTHOR: Ms Ruth Morrow, Registered Advanced Nurse Practitioner (Primary Care), Respiratory Nurse Specialist (WhatsApp Messaging Service Asthma Society of Ireland), and Nurse Educator and Consultant
During childhood, boys have nearly twice the risk of developing asthma over girls. This changes once children reach the age of 12/13 years. Sex hormones, genetics, social and environmental factors, and responses to asthma treatments are important factors in the sex differences observed in asthma incidence, prevalence, and severity.
In childhood, obesity, regardless of physical fitness, is associated with higher asthma prevalence and morbidity in girls, but not in boys. In girls older than 11 years and women, asthma is five-to-seven times more common in obese people compared to those of normal weight (Koper et al, 2017).
Asthma prevalence is higher in women who have multiple pregnancies, women whose periods started earlier in life, and women with hormonal disturbances such as polycystic ovarian syndrome (PCOS) (Morales-Estrella et al, 2018). Women who are diagnosed with endometriosis also have an increased risk of asthma. A study by Morales-Estrella et al (2018) showed that 23.8 per cent of women who had endometriosis developed asthma, compared with 13.2 per cent of women who were taking oral contraceptives (OCS).
Testosterone, which increases in boys from the age of 12/13 years, has an antiinflammatory effect in the airways and is thought to be one of the reasons why asthma is less prevalent in boys at this age. Female hormones increase at this age in girls, which is thought to increase the risk of developing asthma and increase symptoms in those who are already diagnosed with asthma.
As adults, women have an increased prevalence and severity of asthma. For women, fluctuations in sex hormone levels during puberty, the menstrual cycle,
pregnancy, and menopause are associated with asthma (Nowrin et al, 2021). Later in life, asthma incidence and severity are higher in women than in men, and highest in women between the fourth and sixth decade of life. During adulthood there is a shift to a female predominance, which affects mainly non-atopic asthma. In the elderly, the gender-related differences decrease. As testosterone levels decrease in older men, the incidence of asthma can also increase in this age group (Koper, 2017).
In addition, pathophysiological abnormalities can be seen, which includes blood eosinophilia which seems to be more prominent in girls with asthma, but in adipose tissue. Girls with asthma tend to have a higher prevalence of noneosinophilic asthma (60 per cent) compared to corresponding boys (30.8 per cent).
Is asthma worse for women?
Severe asthma primarily affects boys before and at school entry age, as well as women around the time of menopause. Women also develop ‘corticosteroid-resistant’ or difficult-to-treat asthma more often than men (Moore et al, 2007).
Studies show that compared to men, women can have worse symptoms more often:
Women are more at risk of acute asthma flare-ups and are admitted to hospital more often with their asthma.
Women who develop asthma for the first time later in life, after menopause, are more likely to have asthma that is difficult to control, and to need specialist care and treatments to help deal with their symptoms.
Lung function starts to decline after about the age of 35 years in both males and females. For women it declines more quickly after the menopause.
Statistics show that women with asthma aged over 65 years are more at risk of lifethreatening asthma attacks.
Women who develop asthma at perimenopause tend to be less atopic, less corticosteroid-responsive, and obese, with steroid refractory asthma (Moore et al, 2007, Wu et al, 2014). These women frequently require high doses of inhaled corticosteroids (ICS) to manage their asthma. Their asthma tends to be difficult to manage and have a higher rate of healthcare utilisation and poorer health outcomes.
Hormones and asthma
Women are more likely to notice worse symptoms around times of hormonal change like puberty, menstruation, pregnancy, and perimenopause. Not all women are affected.
One-third of women report worse asthma symptoms before or during a period.
Some women, particularly those with severe asthma, have worse symptoms during pregnancy. Although many women notice an improvement or no change at all when they’re pregnant.
Asthma symptoms can get worse during peri-menopause.
Women who have never had asthma can develop asthma at peri-menopause.
Hormones can be an asthma trigger in their own right, but they can also make the woman more sensitive to other triggers, such as hay fever or colds and flu. It is not yet clear why this is the case. It could be because it increases inflammation in the body and causes inflammation in the airways.
Do women have different asthma triggers?
Women can also have all the same triggers as men, but some of these triggers may be worse for women or affect them more often. For example:
Food allergies are more common in women than men, with female hormones making them worse.
Cigarette smoke can affect women more
14 Volume 8 | Issue 5 | 2022 | Respiratory Medicine
than men. Women and girls may be more sensitive to cigarette smoke and girls with asthma who start to smoke may take longer and need more help to quit.
Stress, anxiety, and depression are more common in women, particularly older women who tend to be carers more often.
Indoor triggers such as cleaning products, cooking fumes and house dust mites, may affect women more as statistics show they’re more likely to be doing the cleaning at home.
How can women lower their asthma risk?
Having an annual asthma review, including assessment of symptoms, checking adherence and inhaler technique, and a review of their asthma ‘Action Plan’ can benefit women. At other times women should be advised that as they approach the peri-menopause, symptoms and asthma control may worsen and they should be advised to have an asthma review with adjustment of treatment if required.
Risk can also be lowered by:
Taking the controller medicine every day as prescribed so that they are less likely to react to any asthma triggers, including hormones.
Keeping a symptom diary to help find out if hormones are triggering asthma symptoms around their menstrual period.
Keeping an eye on weight. Being obese increases the risk of asthma symptoms worsening as women get older. It also increases the risk of women getting asthma for the first time around menopause.
Discussing the woman’s risk of osteoporosis. Being on higher doses of inhaled steroids or needing regular or longterm courses of steroid tablets increases the risk of osteoporosis. Women are four times more at risk than men of developing osteoporosis. In women who have asthma, the chances of developing osteoporosis are slightly higher than average.
Being aware of how other conditions could make asthma worse – for example, acid reflux, which is more common in women.
Pain relief, contraceptives, HRT and asthma
Around 20 per cent of women with asthma
experience worsening of their asthma premenstrually. These women tend to be older and have more severe asthma, a higher BMI, and have had asthma for a longer time (GINA, 2021). They also tend to have more menstrual abnormalities such as dysmenorrhoea, shorter menstrual cycles, and longer menstrual bleeding. Paracetamol is usually safe, but non-steroidal antiinflammatory tablets (NSAIDs), such as ibuprofen (eg, Nurofen), mefenamic acid (eg, Ponstan), and aspirin, may worsen asthma symptoms or trigger an asthma flare-up in some women. OCS and leukotriene receptor antagonists may be helpful for these women.
OCS (either the combined pill or the progestogen-only pill) are safe to take. Taking them at the same time as usual asthma medication will not affect the efficacy of either medication.
The morning-after pill, ellaOne, is not recommended for women with severe asthma. Some OCS are not recommended for women taking theophylline, as plasma concentrations of theophylline are increased.
Data from 3,257 pre-menopausal Scottish women showed that hormonal contraceptives reduced asthma incidence and decreased asthma-related healthcare utilisation, driven by a significant decrease in lean women, as well as decreased wheezing in asthma patients (Nwaru BI, Sheikh A, 2015). In a study by Morales-Estrella et al (2018), the prevalence of asthma was higher in women taking OCS than those who weren’t (14.3 per cent vs 8.8 per cent).
HRT also has asthma benefits and asthma risks:
Some research shows that HRT may increase the risk of women getting asthma for the first time.
Some studies show that HRT improves symptoms in women who already have asthma.
Generally, symptoms improve after the menopause, but this is not the case for women taking HRT.
Asthma and pregnancy
Asthma control often changes during
pregnancy – in approximately onethird of women their asthma symptoms worsen, one-third may improve, and the remaining third remain unchanged.
Exacerbations are common in pregnancy, particularly in the third trimester. Uncontrolled asthma and exacerbations may be due to mechanical or hormonal changes or due to the stopping or reduction of medications due to concerns by the mother or healthcare provider.
Pregnant women appear to be more susceptible to viral respiratory infections, including influenza.
Poor asthma control and exacerbations are associated with worse outcomes for the baby (low birth weight, pre-term weight, increased perinatal mortality) and the mother (pre-eclampsia). If asthma is well controlled during pregnancy, there is little or no increased risk of adverse maternal or foetal complications (GINA, 2021).
The advantages of actively treating asthma in pregnancy outweighs any potential risks from regular controller and reliever medications. Using medications to achieve good asthma control and prevent exacerbations is justified, even if their safety in pregnancy has not been proven. The use of ICS, montelukast, or theophylline is not associated with an increase of foetal abnormalities. There is plenty of evidence which shows that ICS reduce the risk of exacerbations during pregnancy, and stopping ICS during pregnancy is a significant risk factor for exacerbations.
During labour and delivery, women should be advised to continue their usual controller medications and use their reliever if needed (GINA, 2021). Acute exacerbations are not common during labour, but bronchoconstriction may be induced by hyperventilation and should be managed using short-acting bronchodilators.
References on request
15 Respiratory Medicine | Volume 8 | Issue 5 | 2022
Asthma in children
AUTHOR: Ruth Morrow, Registered Advanced Nurse Practitioner (Primary Care), Respiratory Nurse Specialist (WhatsApp Messaging Service, Asthma Society of Ireland), and Nurse Educator and Consultant
Asthma is a heterogeneous disease, usually characterised by chronic airway inflammation, which is defined by the history of respiratory symptoms such as wheeze, shortness of breath, chest tightness, and cough that vary over time and in intensity, together with variable expiratory airflow limitation (GINA, 2021).
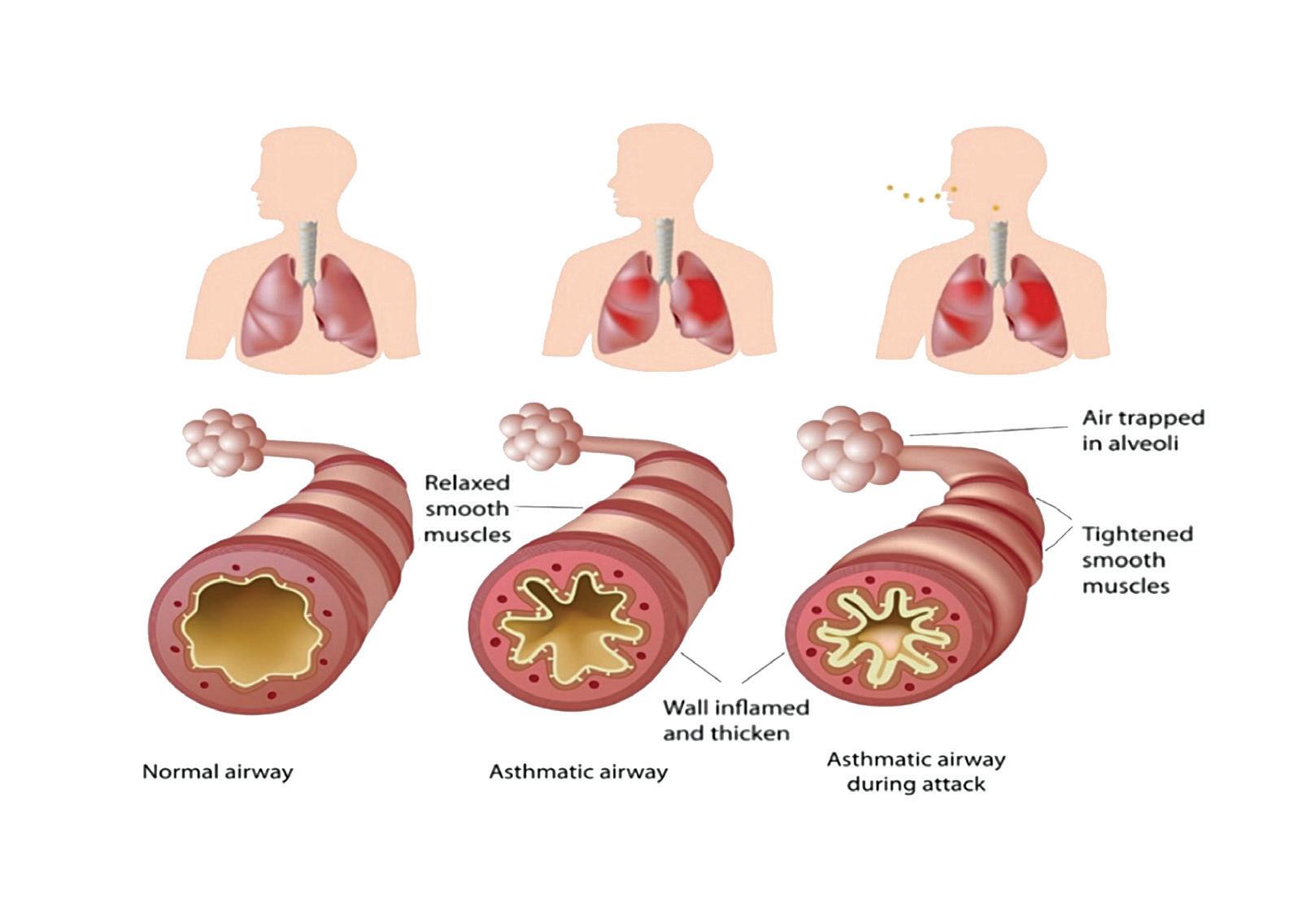
Asthma is one of the most common chronic diseases worldwide affecting an estimated 300 million. Prevalence is increasing in many countries, especially in children. Asthma is a major cause of school and work absence (Manning et al, 2005). The Asthma Society of Ireland estimates that that one-in-five children experience asthma at some stage in their life. Poorly-controlled asthma is expensive in terms of hospitalisations, visits to out-of-hours services, days missed from school, and the negative impact on quality-of-life for children and their families. The Asthma Insights and Reality in Europe study revealed that a child with asthma will lose 10 days from school per year (Manning et al, 2005, Manning et al, 2007).
Pathophysiology
Asthma is characterised by episodes of obstruction and an increase in bronchial reactivity as a result of exposure to trigger factors. It is a chronic inflammatory process with structural changes in the airways, which may become permanent if left untreated. Damage to the endothelial layer occurs, which allows infiltration of the mucosa by inflammatory mediators such as histamine. Smooth muscle hypertrophy and hypertrophy of the mucus glands ensues, with production of thick secretions causing mucous plugging. The number of blood vessels are also increased (Figure 1). As a result, the bronchoalveolar walls become thickened and the lumen size is reduced, resulting in wheezing, shortness of breath, cough and chest tightness. These changes are apparent even in mild disease, which provides health professionals with the rationale for prompt and continued use of inhaled corticosteroids (ICS).
Assessment and diagnosis of asthma
In children, asthma can be difficult to diagnose due to various differential diagnoses and the inability to perform peak flow readings in children under five years of age. A comprehensive and accurate history taking is essential and should include family history, medical and surgical history of the child, birth history, medications, and trigger factors. Children born prematurely are at higher risk of developing asthma. Symptom history includes duration, type, onset, and pattern of symptoms. Symptom history can be difficult to obtain, with some parents being vague about symptoms and the use of a symptom diary is useful in these situations.
The Global Initiative for the diagnosis and Management of Asthma (GINA) provides professionals with a guide for symptom patterns in children, which is useful in determining the probability of an asthma diagnosis (Figure 2). In children aged over five years, it is useful to carry out a peak flow diary for two weeks with the child recording their peak flow twice daily. Reversibility testing to salbutamol can also be carried out for these
children, with an increase in peak expiratory flow rate (PEFR) of 12 per cent indicating a positive diagnosis for asthma.
There is an increased probability that symptoms are due to asthma if:
There is more than one type of symptom (wheeze, shortness of breath, cough, chest tightness).
Symptoms are often worse at night or in the early morning.
Symptoms vary over time and in intensity.
Symptoms are triggered by viral infections, exercise, allergen exposure, changes in weather, laughter, and irritants such as car exhaust fumes, smoke or strong smells.
There is a decreased probability that symptoms are due to asthma if:
The cough is isolated with no other respiratory symptoms.
There is chronic production of sputum.
There is shortness of breath associated with dizziness, light-headedness or peripheral tingling.
Symptoms are exercise-induced dyspnoea with noisy inspiration (stridor).
16 Volume 8 | Issue 5 | 2022 | Respiratory Medicine
Physical assessment includes height and weight measurement. Chest examination to include chest expansion, percussion, and auscultation. Spirometry may also be performed in children over five years. Chest x-ray is not indicated for the diagnosis of asthma unless the child fails to respond to inhaled treatment. Allergy testing may be useful in helping to identify possible trigger factors. Forced exhaled nitrous oxide (FENO) testing can be helpful to confirm the diagnosis of asthma.
Assessment and identification of trigger factors can be challenging, and very often parents may not be aware of the potential trigger factors. Asking the parent to observe for potential trigger factors and use a symptom tracker over a period of time can be useful. Trigger factors can be inhaled (house dust mite, pollen, strong odours, smoke, animal dander), swallowed (foods, food additives and preservatives, and medications such as ibuprofen and beta-blockers), and non-allergic such as exercise, laughing or crying.

The differential diagnosis for asthma includes inhaled foreign body, cystic fibrosis, primary ciliary dyskinesia, bronchopulmonary dysplasia, and immune deficiency. Failure to respond to treatment warrants further investigation to outrule any of these conditions.
Management of stable asthma
The goals of asthma management are:
1. Symptom control: To achieve good control of symptoms and maintain normal activity levels.
2. Risk reduction: To minimise future risk of exacerbations, fixed airflow limitation, and medication side-effects.
Assessment of asthma control involves assessing symptoms over the previous four weeks and assessing risk factors for poor outcomes. Treatment issues should also be addressed at every visit and should include:
Checking inhaler technique and adherence.
Asking about side-effects.
Reviewing the child’s written asthma action plan.
Exploring the parent’s attitudes and goals for their asthma.
GINA (2021) provides a step-wise approach for the pharmacological management of asthma. In a recent change to the guidelines, it is outlined when ICS should be used at the same time as when salbutamol is used. Children who are using salbutamol more than twice a week should be commenced on ICS. If the child is not controlled on the appropriate step, inhaler technique and adherence to medication should be reviewed
before moving up to the next step. Once optimal control has been achieved for at least three months, then the medication can be titrated down.
A metered dose inhaler (MDI) with spacer is the first choice for the delivery of medication in stable asthma for children under five and up to the age of seven/eight years. After this, a dry powder device or breath-actuated device may be an option for the delivery of treatment.
Nebulisers should be reserved for the management of severe, acute exacerbations of asthma.
Side-effects of ICS medication include dysphonia and oral candidiasis and parents should be educated in the avoidance of these side-effects, ie, rinsing the mouth out and brushing their teeth. Where masks are used it is vital to ensure that the mask is the correct size and fits correctly to form a seal around the mouth and nose, so as to avoid deposition of the drug into the eyes. The face should be washed following administration of ICS.
Risk factors for poor outcomes
Children who experience uncontrolled asthma symptoms and had one or more exacerbation in the previous year.
The start of the child’s usual ‘flare-up’ season (especially if autumn).
Has major psychological or socio-economic problems for child or family.
Poor adherence with controller medication, or
Children with incorrect inhaler technique are at risk of an exacerbation in the coming months.
Asthma and Covid-19 in children with asthma
A study by Shi et al (2021) explored data from primary care, community prescribing, hospital admissions, and deaths. It provides a detailed insight into the risk of severe SARSCoV-2 in children aged five-to-17 years with asthma in Scotland. The findings suggest that compared with children without asthma, the risk of admission to hospital with Covid-19 is increased in children with a diagnosis of asthma, particularly when they have previously been admitted to hospital with asthma or have required two or more courses
17 Respiratory Medicine | Volume 8 | Issue 5 | 2022
FIGURE 2: Symptom pattern in children under five years of age (GINA, 2021)
SYMPTOMS MILD SEVERE
administrations of inhaled SABA, even if the child shows other clinical signs of improvement.
3. Unable to be managed at home:
Social environment that impairs delivery of acute treatment.
Parent/carer unable to manage child at home.
of oral corticosteroids in the 24 months before the study start date in March 2020.
Assessment and management of acute asthma
Accurate and timely assessment of acute asthma exacerbations should be carried out to ensure a successful outcome. Table 1 differentiates between a mild and severe acute exacerbation.
The management of acute asthma includes:
1. Oxygen therapy – 24 per cent delivered by face mask (usually 1L/min) to maintain oxygen saturation 94-to-98 per cent.
2. Inhaled short-acting bronchodilator –two-to-six puffs of salbutamol by spacer, or 2.5mg by nebuliser, every 20 minutes for first hour, then reassess severity. If symptoms persist or recur, give an additional two-to-three puffs per hour. Admit to hospital if >10 puffs required in three-to-four hours.
3. Oral corticosteroids – give initial dose of oral prednisolone (1-2mg/kg up to maximum of 20mg for children <two years; 30mg for two-to-five years).
4. Additional treatments can include – for moderate/severe exacerbations, give two puffs of ipratropium bromide 80mcg (or 250mcg by nebuliser) every 20 minutes for one hour only.
All children who experience an acute exacerbation of asthma should be reviewed six
hours after their event if they are not referred to secondary care. Criteria for immediate transfer to secondary care include:
1. Features of severe exacerbation at initial or subsequent assessment:
Child is unable to speak or drink.
Cyanosis.
Subcostal retraction.
Oxygen saturation <92 per cent when breathing room air.
Silent chest on auscultation.
2. Lack of response to initial bronchodilator treatment:
Lack of response to six puffs of inhaled short-acting beta agonist (SABA) (two separate puffs, repeated three times) over one-to-two hours.
Persisting tachypnoea despite three
References
1. Bardach NS, Harder VS, McCulloch CE, Thombley R, Shaw JS, Hart VC, Cabana MD. Follow-up after asthma emergency department visits and its relationship with subsequent asthma-related utilisation. Acad Pediatr 2022 Apr;22(3S):S125-S132. doi: 10.1016/j. acap.2021.10.015
2. Central Statistics Office. 2011, Quarterly National Household Survey
3. Global Initiative for Asthma, 2021, Pocket guide for the diagnosis, management, and prevention of asthma. Available at: https:// ginasthma.org/
A follow-up review should be organised after an asthma exacerbation to review the child/adolescent asthma plan and medication. This also provides an opportunity to discuss the potential trigger for this exacerbation and the prevention of future exacerbations. A study carried out by Bardach et al (2021) concluded that for children and adolescents with an asthmarelated emergency department visit, having a follow-up within 14 days is associated with lower rates of subsequent asthma-related emergency department revisits in the shortterm (within 60 days) and the long-term (within 365+ days) (Bardach et al, 2021).
Conclusion
This article has explored asthma in children, which can be difficult and challenging to diagnose and treat. Asthma is the most common chronic condition; affecting over 10 per cent of children. The burden of childhood asthma is significant in terms of healthcare costs and the impact on the child’s and family’s quality-of-life.
4. Manning P, Greally P, Shanahan E. Asthma control and management: A patient’s perspective. Ir Med J. 2005 NovDec;98(10):231-2, 234-5
5. Manning PJ, Goodman P, O’Sullivan A, Clancy L. Rising prevalence of asthma, but declining wheeze in teenagers (1995-2003): ISAAC protocol. Ir Med J. 2007 Nov-Dec;100(10):614-5
6. Shi T, Pan J, Katikireddi SV, McCowan C, Kerr S, Agrawal U, et al. Risk of Covid-19 hospital admission among children aged 5-17 years with asthma in Scotland: A national incident cohort study. Lancet Respir Med. 2022 Feb;10(2):191-198. doi: 10.1016/S2213-2600(21)00491-4
18 Volume 8 | Issue 5 | 2022 | Respiratory Medicine
Altered consciousness No Agitated, confused or drowsy Oximetry on presentation (SaO2) >95% <92% Speech Sentences Words Pulse rate <100 beats/min >200 beats/min (0-3 years) >180 beats/min (4-5 years) Central cyanosis Absent Likely to be present Wheeze intensity Absent Chest may be quiet TABLE 1: Assessment of acute exacerbation of asthma (GINA, 2021)
GOLD 2022 guidelines update
Chronic obstructive pulmonary disease (COPD) is a respiratory disease characterised by persistent respiratory symptoms and airflow limitation, which is usually progressive. This disease is both preventable and treatable. An estimated 380,000 people in Ireland have COPD, but only 110,000 have been diagnosed. COPD is a significant cause of mortality in Ireland and has one of the highest age-standardised death rates from COPD compared to other European countries. Ireland also has the highest COPD hospitalisation rates among countries in the OECD.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) has produced annual reports offering recommendations on the management of COPD since 2001. The 2022 GOLD guidelines offer important updates for pharmacological management, including new evidence around the use of blood eosinophils as a biological marker to guide treatment and predict patients at higher risk of decline in lung function. This has important implications for general practice, especially during the follow-up of COPD patients at chronic disease management (CDM) reviews.
The GOLD 2022 guidelines also include new definitions for COPD classification, such as early COPD, mild COPD, COPD in young people, and pre-COPD. More research is needed into these particular groups, but it highlights the importance of detecting COPD earlier so that interventions to prevent disease progression can be started.
Diagnosis and initial assessment
There have not been any significant changes in the chapter on diagnosis and initial assessment. A diagnosis of COPD should be considered in any patient presenting with a history of dyspnoea,
chronic cough or sputum production, and/ or a history of exposure to risk factors such as cigarette smoking or occupational exposures, eg, dust, fumes.
The presence of symptoms and spirometry showing a post-bronchodilator FEV1 / FVC less than 0.7 is necessary to make the diagnosis of COPD. It is important that the spirometry used to diagnose COPD is accredited and of high quality to ensure proper diagnosis.
The goals of COPD assessment are to determine the level of airflow limitation,
its impact on patients’ health, and the risk of future events (such as exacerbations, hospital admissions or death).
The GOLD report recommends the following must be considered in the assessment:
Severity of airflow limitation spirometry;
The nature and severity of the patients symptoms;
History of moderate and severe exacerbations, and risk of further exacerbations;
Presence of comorbidities.
First, patients are classified into GOLD groups 1-4 based on their post-bronchodilator FEV1 result, as shown in Table 1. However, there is only a weak correlation between FEV1 results, symptoms, and impairment of the patient’s health status, so it is necessary to also assess the patient’s symptoms formally.
The GOLD ABCD group stratification tool is used to guide initial treatment and is determined by assessment of the patient’s
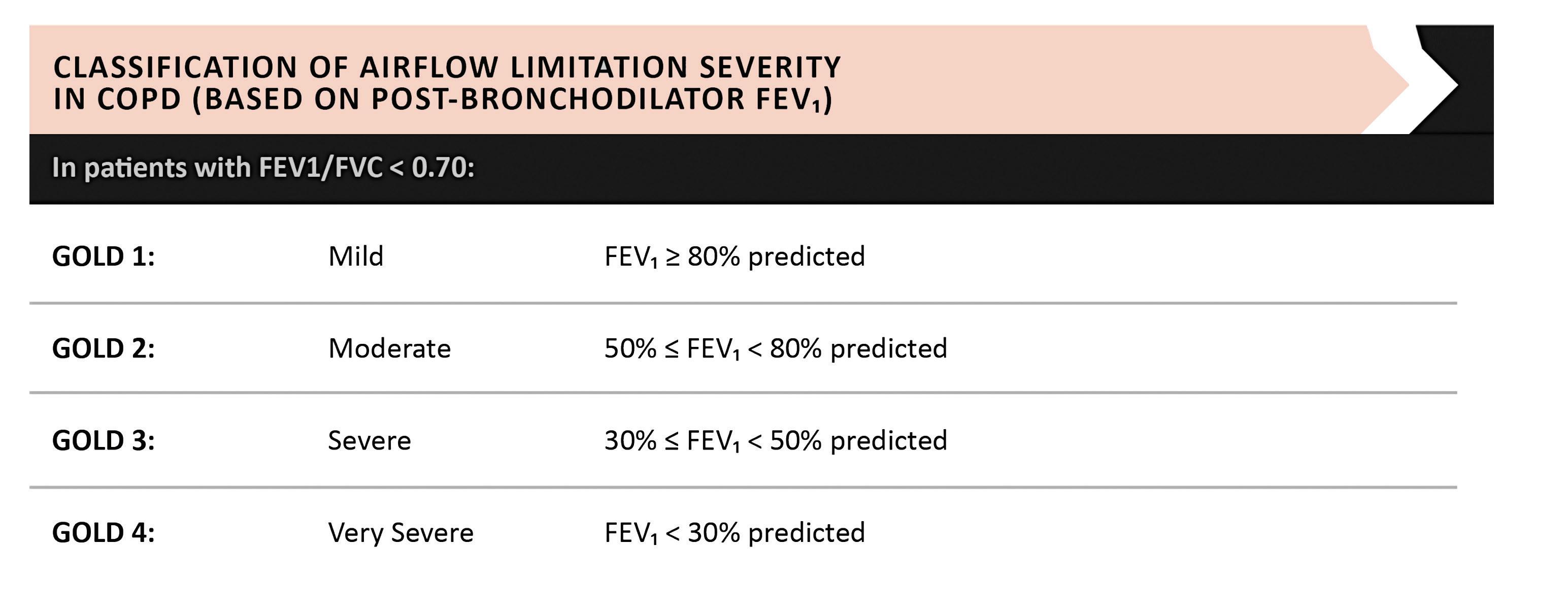
19 Respiratory Medicine | Volume 8 | Issue 5 | 2022
AUTHORS: Dr Dermot Nolan, HSE National Asthma Programme GP Lead; and Dr Donna Sinnott, GP Registrar, Tramore Medical Clinic, Co Waterford
GOLD guidelines on airflow limitation
It is important that the spirometry used to diagnose COPD is accredited and of high quality to ensure proper diagnosis
MODIFIED MRC DYSPNOEA SCALE


20 Volume 8 | Issue 5 | 2022 | Respiratory Medicine
FIGURE 1: GOLD ABCD assessment
For patients not adequately controlled on dual therapy with moderate to severe COPD
UNLEASH THE PROTECTION
OF TRIXEO1,2
Significant protection against exacerbations*
TRIXEO Aerosphere is indicated as a maintenance treatment in adult patients with moderate to severe chronic obstructive pulmonary disease (COPD) who are not adequately treated by a combination of an inhaled corticosteroid and a long-acting beta2-agonist or combination of a long-acting beta2-agonist and a long-acting muscarinic antagonist.1

*Significant reductions in the rate of moderate or severe exacerbations vs LAMA/LABA (24%, n=2137 vs n=2120, annual rates 1.08 vs 1.42, 95% CI 0.69–0.83; p<0.001) and ICS/LABA (13%, n=2137 vs n=2131, annual rates 1.08 vs 1.24, 95% CI 0.79–0.95; p=0.003).1,2 COPD, chronic obstructive pulmonary disease; ICS, inhaled corticosteroid; LABA, long-acting beta2-agonist; LAMA, long-acting muscarinic antagonist. In the clinical trial programme for TRIXEO, LAMA/LABA refers to glycopyrronium/formoterol fumarate and ICS/LABA refers to budesonide/formoterol fumarate. ©AstraZeneca 2022. All rights reserved.
ABRIDGED PRESCRIBING INFORMATION
TRIXEO AEROSPHERE® 5 micrograms/7.2 micrograms/160 micrograms pressurised inhalation, suspension (formoterol fumarate dihydrate/glycopyrronium/budesonide)
Consult Summary of Product Characteristics (SmPC) before prescribing Indication: Trixeo Aerosphere is indicated as a maintenance treatment in adult patients with moderate to severe chronic obstructive pulmonary disease (COPD) who are not adequately treated by a combination of an inhaled corticosteroid and a long acting beta2 agonist or combination of a long-acting beta2 agonist and a long acting muscarinic antagonist.
Presentation: Pressurised inhalation, suspension. Each single actuation (delivered dose, ex-actuator) contains 5 micrograms of formoterol fumarate dihydrate, glycopyrronium bromide 9 micrograms, equivalent to 7.2 micrograms of glycopyrronium and budesonide 160 micrograms.
Dosage and Administration: The recommended and maximum dose is two inhalations twice daily (two inhalations morning and evening). If a dose is missed, take as soon as possible and take the next dose at the usual time. A double dose should not be taken to make up for a forgotten dose. Special populations: Elderly:
No dose adjustments required in elderly patients. Renal impairment: Use at recommended dose in patients with mild to moderate renal impairment. Can also be used at the recommended dose in patients with severe renal impairment or end-stage renal disease requiring dialysis, only if expected benefit outweighs the potential risk. Hepatic impairment: Use at recommended dose in patients with mild to moderate hepatic impairment. Can also be used at the recommended dose in patients with severe hepatic impairment, only if expected benefit outweighs the potential risk. Paediatric Population: No relevant use in children and adolescents (<18 years of age). Method of administration: For inhalation use. To ensure proper administration of the medicinal product, the patient should be shown how to use the inhaler correctly by a physician or other healthcare professional, who should also regularly check the adequacy of the patient’s inhalation technique. Patients who find it difficult to coordinate actuation with inhalation may use Trixeo Aerosphere with a spacer to ensure proper administration of the medicinal product..
Contraindications: Hypersensitivity to the active substances or to any of the excipients.
Warnings and Precautions: Not for acute use: Not indicated for treatment of acute episodes of bronchospasm, i.e. as a rescue therapy. Paradoxical bronchospasm: Administration of formoterol/glycopyrronium/budesonide may produce paradoxical bronchospasm with an immediate wheezing and shortness of breath after dosing and may be life threatening. Treatment should be discontinued immediately if paradoxical bronchospasm occurs. Assess patient and institute alternative therapy if necessary. Deterioration of disease: Recommended that treatment should not be stopped abruptly. If patients find the treatment ineffective, continue treatment but seek medical attention. Increasing use of reliever bronchodilators indicates worsening of the underlying condition and warrants reassessment of the therapy. Sudden and progressive deterioration in the symptoms of COPD is potentially life threatening, patient should undergo urgent medical assessment. Cardiovascular effects: Cardiovascular effects, such as cardiac arrhythmias, e.g. atrial fibrillation and tachycardia, may be seen after the administration of muscarinic receptor antagonists and sympathomimetics, including glycopyrronium and formoterol. Use with caution in patients with clinically significant uncontrolled and severe cardiovascular disease such as unstable ischemic heart disease, acute myocardial infarction, cardiomyopathy, cardiac arrhythmias and severe heart failure. Caution should also be exercised when treating patients with known or suspected prolongation of the QTc interval (QTc > 450 milliseconds for males or > 470 milliseconds for females), either congenital or induced by medicinal products. Systemic corticosteroid effects: May occur with
any inhaled corticosteroid, particularly at high doses prescribed for long periods. These effects are much less likely to occur with inhalation treatment than with oral corticosteroids. Systemic effects include Cushing’s syndrome, Cushingoid features, adrenal suppression, decrease in bone mineral density, cataract and glaucoma.
Potential effects on bone density should be considered particularly in patients on high doses for prolonged periods that have co existing risk factors for osteoporosis. Visual disturbances: May be reported with systemic and topical corticosteroid use. If patient presents symptoms such as blurred vision or other visual disturbances, consider ophthalmologist referral for evaluation of possible causes which may include cataract, glaucoma or rare diseases such as central serous chorioretinopathy (CSCR). Transfer from oral therapy: Care is needed in patients transferring from oral steroids, since they may remain at risk of impaired adrenal function for a considerable time. Patients who have required high dose corticosteroid therapy or prolonged treatment at the highest recommended dose of inhaled corticosteroids, may also be at risk. These patients may exhibit signs and symptoms of adrenal insufficiency when exposed to severe stress. Additional systemic corticosteroid cover should be considered during periods of stress or elective surgery. Pneumonia in patients with COPD: An increase in the incidence of pneumonia, including pneumonia requiring hospitalisation, has been observed in patients with COPD receiving inhaled corticosteroids. Remain vigilant for the possible development of pneumonia in patients with COPD as the clinical features of such infections overlap with the symptoms of COPD exacerbations. Risk factors for pneumonia include current smoking, older age, low body mass index (BMI) and severe COPD. Hypokalaemia: Potentially serious hypokalaemia may result from ß2-agonist therapy. This has potential to produce adverse cardiovascular effects. Caution is advised in severe COPD as this effect may be potentiated by hypoxia. Hypokalaemia may also be potentiated by concomitant treatment with other medicinal products which can induce hypokalaemia, such as xanthine derivatives, steroids and diuretics.
Hyperglycaemia: Inhalation of high doses of ß2-adrenergic agonists may produce increases in plasma glucose. Blood glucose should be monitored during treatment following established guidelines in patients with diabetes. Co-existing conditions: Use with caution in patients with thyrotoxicosis. Anticholinergic activity: Due to anticholinergic activity, use with caution in patients with symptomatic prostatic hyperplasia, urinary retention or with narrow-angle glaucoma. Patients should be informed about the signs and symptoms of acute narrow-angle glaucoma and should be informed to stop using this medicinal product and to contact their doctor immediately should any of these signs or symptoms develop. Co-administration of this medicinal product with other anticholinergic containing medicinal products is not recommended. Renal impairment: Patients with severe renal impairment (creatinine clearance of <30 mL/min), including those with end-stage renal disease requiring dialysis, should only be treated with this medicinal product if the expected benefit outweighs the potential risk. Hepatic impairment: In patients with severe hepatic impairment, use only if the expected benefit outweighs the potential risk. These patients should be monitored for potential adverse reactions..
Drug Interactions: Co-treatment with strong CYP3A inhibitors, e.g. itraconazole, ketoconazole, HIV protease inhibitors and cobicistat-containing products are expected to increase the risk of systemic side effects. Should be avoided unless the benefit outweighs the increased risk, in which case patients should be monitored for systemic corticosteroid adverse reactions. This is of limited clinical importance for short-term (1-2 weeks) treatment. Since glycopyrronium is eliminated mainly by the renal route, drug interaction could potentially occur with medicinal products affecting renal excretion mechanisms. Other antimuscarinics and sympathomimetics: Co-administration with other anticholinergic and/or long-acting ß2-adrenergic agonist containing medicinal products is not recommended as it may potentiate known inhaled muscarinic antagonist or ß2-adrenergic agonist adverse reactions. Concomitant use of other beta-adrenergic medicinal products can have potentially additive effects. Caution required when prescribed concomitantly with formoterol.
Medicinal product-induced hypokalaemia: Possible initial hypokalaemia may be
potentiated by xanthine derivatives, steroids and non potassium sparing diuretics. Hypokalaemia may increase the disposition towards arrhythmias in patients who are treated with digitalis glycosides. β-adrenergic blockers: ß-adrenergic blockers (including eye drops) can weaken or inhibit the effect of formoterol. Concurrent use of ß-adrenergic blockers should be avoided unless the expected benefit outweighs the potential risk. If required, cardio-selective ß-adrenergic blockers are preferred. Other pharmacodynamic interactions: Concomitant treatment with quinidine, disopyramide, procainamide, antihistamines, monoamine oxidase inhibitors, tricyclic antidepressants and phenothiazines can prolong QT interval and increase the risk of ventricular arrhythmias. L-dopa, L-thyroxine, oxytocin and alcohol can impair cardiac tolerance towards beta2-sympathomimetics. Concomitant treatment with monoamine oxidase inhibitors, including medicinal products with similar properties such as furazolidone and procarbazine, may precipitate hypertensive reactions. Elevated risk of arrhythmias in patients receiving concomitant anaesthesia with halogenated hydrocarbons.
Pregnancy and Lactation: Administration to pregnant women/women who are breast-feeding should only be considered if the expected benefit to the mother justifies the potential risk to the foetus/child.
Ability to Drive and Use Machines: Dizziness is an uncommon side effect which should be taken into account.
Undesirable Events: Consult SmPC for a full list of side effects. Common (≥ 1/100 to < 1/10): Oral candidiasis, pneumonia, hyperglycaemia, anxiety, insomnia, headache, palpitations, dysphonia, cough, nausea, muscle spasms, urinary tract infection. Uncommon (≥ 1/1,000 to < 1/100): Hypersensitivity, depression, agitation, restlessness, nervousness, dizziness, tremor, angina pectoris, tachycardia, cardiac arrhythmias (atrial fibrillation, supraventricular tachycardia and extrasystoles), throat irritation, bronchospasm, dry mouth, bruising, urinary retention, chest pain. Very Rare (< 1/10,000): Signs or symptoms of systemic glucocorticosteroid effects, e.g. hypofunction of the adrenal gland, abnormal behaviour. Not known: Angioedema, vision blurred, cataract, glaucoma.
Legal Category: Product subject to prescription which may be renewed (B)
Marketing Authorisation Number: EU/1/20/1498/002 120 actuations
Marketing Authorisation Holder: AstraZeneca AB, SE-151 85, Södertälje, Sweden. Further product information available on request from: AstraZeneca Pharmaceuticals (Ireland) DAC, Block B, Liffey Valley Office Campus, Dublin 22. Tel: +353 1 609 7100.
TRIXEO and AEROSPHERE are trademarks of the AstraZeneca group of companies.

Date of API preparation: 10/2021
Veeva ID: IE-3166
Adverse events should be reported directly to; HPRA Pharmacovigilance, Website: www.hpra.ie Adverse events should also be reported to AstraZeneca Patient Safety on Freephone 1800 800 899
1. TRIXEO AEROSPHERE 5 micrograms/7.2 micrograms/160 micrograms pressurised inhalation, suspension. Summary of Product Characteristics. Available at www.medicines.ie
2. Rabe KF et al. Triple inhaled therapy at two glucocorticoid doses in modeate-tovery-severe COPD. N Engl J Med. 2020;383:35–48. doi: 10.1056/NEJMoa1916046
COPD. N Engl J Med. 2020;383:35–48. doi: 10.1056/NEJMoa1916046
Veeva ID: IE-3706 Date of Preparation: March 2022
NEW NEW
symptoms and exacerbation risk. The 2022 report emphasises that the assessment of a patient’s GOLD group is only recommended for determining initial therapy and not for the reassessment of patients at follow-up, which will be discussed below.
Assessment of symptoms can be carried out by using scales such as the modified Medical Research Council (mMRC) dyspnoea questionnaire or the COPD assessment tool (CAT). The mMRC is included as part of the CDM assessment of patients with COPD and measures breathlessness (Table 2). A patient is then awarded a grade between 0 and 4 based on severity of their breathlessness. While the mMRC can be a good measure of health status and predicts future mortality risk, it is well recognised that COPD causes symptoms beyond just dyspnoea. The CAT is a more comprehensive tool which looks at eight different domains such as cough, activity limitation, and energy levels, and asks a patient to rate their severity on a scale of 0-5.
Finally, the patient’s history of moderate or severe exacerbations, including hospitalisations, should be noted. The patient’s risk of exacerbations along with their level of symptoms is used to determine their GOLD group A-D as shown in Figure 1
Initiation of treatment
Recommendations on initial pharmacotherapy
in COPD are unchanged in the 2022 GOLD report (Figure 2). Patients in groups A, B, and C should generally be started on a single bronchodilator. There is no evidence to recommend one class of long-acting bronchodilator over another, so treatment choice should depend on the patient’s perception of symptom relief in groups A and B. For patients in group C, it is recommended that they are started on a long-acting muscarinic antagonist (LAMA) as studies have shown them to be superior to long-acting beta agonists (LABAs) for exacerbation prevention.
For GOLD group D patients (frequent exacerbations with more severe symptoms) there are three different options for initial treatment. The choice is influenced by symptom severity and blood eosinophil count. The first option is monotherapy with a LAMA. The second option of a LAMA/LABA combination is for patients with more severe symptoms (eg, CAT score ≥20). Combination therapy has been shown to be superior to single bronchodilator therapy for patient-reported outcomes in this group. The third option for group D patients is to start treatment with a LABA/inhaled corticosteroid (ICS). This option has the greatest likelihood of reducing exacerbations in patients with blood eosinophil counts >300 or patients with a history of asthma.

Blood eosinophil count and ICS use
The 2022 GOLD guidelines discuss new evidence
regarding the use of blood eosinophil count to help determine patients that will gain the most from the addition of an ICS. Multiple studies have shown the relationship between blood eosinophil counts and the effects of ICS with incrementally increasing benefit at higher eosinophil counts. With eosinophil counts <100, ICS-containing regimens have little or no effect, so patients falling beneath this threshold have a low likelihood of benefiting from ICS. An eosinophil count ≥300 can be used to identify patients with the greatest likelihood benefitting from ICS.
The thresholds of <100 and ≥300 cells should be seen as estimates rather than precise cut-off values that can predict different probabilities of treatment benefit. Other factors such as exacerbation risk, smoking status, and history of concomitant asthma or a history of mycobacterial infection, should also be considered when initiating ICS treatment. These findings are summarised in Figure 3, which can be used as a guide for both starting initial treatment and at follow-up.
The 2022 GOLD update also suggests that blood eosinophil count might serve as a prognostic biomarker for lung function decline. One study included showed a greater decline in FEV1 for patients who had mild/moderate COPD with higher eosinophil counts, while another showed that in young people without COPD a
22 Volume 8 | Issue 5 | 2022 | Respiratory Medicine
FIGURE 2: COPD treatment options per GOLD guidelines
Genuair®-has it ‘clicked’ yet?






The ONLY pre lled inhaler with visual and audible feedback for confirmed dose delivery1-4

Genuair - a simple to use inhaler for patients with COPD4




Abbreviated Prescribing Information
Eklira® Genuair® 322 micrograms inhalation powder. Please consult the Summary of Product Characteristics (SPC) for the full prescribing information. Presentation: Inhalation powder in a white inhaler with an integral dose indicator and a green dosage button. Each delivered dose contains 375 µg aclidinium bromide equivalent to 322 µg of aclidinium. Also, contains lactose. Use: Maintenance bronchodilator treatment to relieve symptoms in adult patients with chronic obstructive pulmonary disease (COPD). Dosage: For inhalation use. Recommended dose is one inhalation of 322 micrograms aclidinium twice daily. Patients should be instructed on how to administer the product correctly as the Genuair inhaler may work di erently from inhalers used previously. It is important to instruct the patients to read the Instructions for Use in the pack. No dose adjustments are required for elderly patients, or those with renal or hepatic impairment. No relevant use in children and adolescents.



Contraindications: Hypersensitivity to aclidinium bromide or to any of the excipients. Warnings




















and Precautions: Stop use if paradoxical bronchospasm occurs and consider other treatments. Do not use for the relief of acute episodes of bronchospasm. Use with caution in patients with myocardial infarction in the previous 6 months, unstable angina, newly diagnosed arrhythmia within the previous 3 months, or hospitalisation within the previous 12 months for heart failure functional classes III and IV. Dry mouth, observed with anticholinergic treatment, may be associated with dental caries in the long term. Use with caution in patients with symptomatic prostatic hyperplasia or bladder-neck obstruction or with narrow-angle glaucoma. Do not use in patients with rare hereditary problems of galactose intolerance, total lactose de ciency or glucose-galactose malabsorption. Interactions: Do not administer with other anticholinergic-containing medicinal products. No other interactions expected. Please consult the SPC for more details. Fertility, pregnancy and lactation: No data on use in pregnancy. Risk to newborns/infants cannot be excluded. Consider risk-bene t before using during lactation. Unlikely to a ect fertility at the recommended dose. Side-e ects: Common (1-10%): Sinusitis, nasopharyngitis, headache, cough, diarrhoea, nausea. Uncommon (0.1-1%): Dizziness, blurred vision, tachycardia, palpitations, dysphonia, dry mouth, stomatitis, rash, pruritus, urinary retention. Rare (0.01-0.1%): hypersensitivity. Not known: angioedema, anaphylactic reaction. Pack sizes: Carton containing 1 inhaler with 60 unit doses. Legal category: POM Marketing
Authorisation Number: EU/1/12/778/002 Marketing Authorisation holder: AstraZeneca AB, SE151 85 Södertälje, Sweden. Marketed by: A. Menarini Pharmaceuticals Ireland Ltd., Castlecourt, Monkstown Farm, Monkstown, Glenageary, Co. Dublin A96 T924. Further information is available on request to A. Menarini Pharmaceuticals Ireland Ltd. or may be found in the SPC. Last updated: February 2020
This medicinal product is subject to additional monitoring. This will allow quick identi cation of new safety information. Healthcare professionals are asked to report any suspected adverse reactions to: HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2, Tel: +353 1 6764971, Fax: +353 1 6762517, Website: www.hpra.ie, e-mail: medsafety@ hpra.ie. Adverse events should also be reported to A. Menarini Pharmaceuticals Ireland Ltd. Phone no: 01 284 6744.
Date of item: November 2020. IR-BRI-10-2020

Abbreviated Prescribing Information Brimica® Genuair® 340 micrograms/12 micrograms inhalation powder. Please consult the Summary of Product Characteristics (SPC) for the full prescribing information. Presentation: Inhalation powder in a white inhaler with an integral dose indicator and an orange dosage button. Each delivered dose contains 396 µg aclidinium bromide (equivalent to 340 µg of aclidinium) and 11.8 micrograms of formoterol fumarate dihydrate. Also, contains lactose. Use: Maintenance bronchodilator treatment to relieve symptoms in adult patients with chronic obstructive pulmonary disease (COPD). Dosage: For inhalation use. Recommended dose is one inhalation of 340 µg/12 µg twice daily. Patients should be instructed on how to administer the product correctly as the Genuair inhaler may work di erently from inhalers used previously. It is important to instruct the patients to read the Instructions for Use in the pack. No dose adjustments are required for elderly patients, or those with renal or hepatic impairment. No relevant use in children and adolescents. Contraindications: Hypersensitivity to the active substances or to any of the excipients. Warnings and Precautions: Do not use in asthma. Stop use if paradoxical bronchospasm occurs and consider other treatments. Do not use for the relief of acute episodes of bronchospasm. Use with caution in patients with myocardial infarction in the previous 6 months, unstable angina, newly diagnosed arrhythmia within the previous 3 months, or hospitalisation within the previous 12 months for heart failure functional classes III and IV. Discontinue if increases in pulse rate, blood pressure or changes in ECG occur. Use with caution in patients with a history of or known prolongation of the QTc interval or treated with products a ecting the QTc interval. Use with caution in patients with severe cardiovascular disorders, convulsive disorders, thyrotoxicosis and phaeochromocytoma. Hypokalaemia may occur, is usually transient and supplementation not needed. In patients with severe COPD, hypokalaemia may be potentiated by hypoxia and concomitant treatment. Use with caution in patients with symptomatic prostatic hyperplasia, urinary retention or with narrow-angle glaucoma. Dry mouth, observed with anticholinergic treatment, may be associated with dental caries in the long term. Do not use in patients with rare hereditary problems of galactose intolerance, the Lapp lactase de ciency or glucose-galactose malabsorption. Interactions: Do not administer with other anticholinergic and/or long-acting β2-adrenergic agonist containing medicinal products. Caution in use with methylxanthine derivatives, steroids, non-potassium-sparing diuretics, β-adrenergic blockers or medicinal products known to prolong the QTc interval. Please consult the SPC for more details. Fertility, pregnancy and lactation: No data on use in pregnancy. Consider risk-bene t before using during lactation. Unlikely to a ect fertility at the recommended dose. Sidee ects: Common (1-10%): Nasopharyngitis, urinary tract infection, sinusitis tooth abscess, insomnia, anxiety, headache, dizziness, tremor, cough, diarrhoea, nausea, dry mouth, myalgia, muscle spasms, peripheral oedema, increased blood creatine phosphokinase. Uncommon (0.1- 1%): Hypokalaemia, hyperglycaemia, agitation, dysgeusia, blurred vision, tachycardia, electrocardiogram QTc prolonged, palpitations, angina pectoris, dysphonia, throat irritation, stomatitis, rash, pruritus, urinary retention, increased blood pressure. Rare (0.01-0.1%): Hypersensitivity, bronchospasm, including paradoxical. Not known: anaphylactic reaction, angioedema. Pack sizes: Carton containing 1 inhaler with 60 unit doses. Legal category: POM Marketing Authorisation Number: EU/1/14/963/001 Marketing

Authorisation holder: AstraZeneca AB, SE-151 85 Södertälje, Sweden. Marketed by: A. Menarini Pharmaceuticals Ireland Ltd., Castlecourt, Monkstown Farm, Monkstown, Glenageary, Co. Dublin A96 T924. Further information is available on request to A. Menarini Pharmaceuticals Ireland Ltd. or may be found in the SPC. Last updated: October 2019


This medicinal product is subject to additional monitoring. This will allow quick identi cation of new safety information. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2; Tel: +353 1 6764971; Fax: +353 1 6762517. Website: www.hpra.ie; E-mail: medsafety@ hpra.ie. Adverse events should also be reported to A. Menarini Pharmaceuticals Ireland Ltd. Phone no: 01 284 6744.

References: 1 MIMS Ireland November 2020 2. Eklira® Genuair® Summary of Product Characteristics, last updated November 2019 3. Brimica® Genuair® Summary of Product Characteristics, last updated August 2019 4 Magnussen, H et al. COPD. 2019 Apr;16(2):196-205
LAMA + LABA LAMA
higher eosinophil count was associated with development of COPD.
Follow-up pharmacological treatment
Patients should be routinely reassessed to determine if their treatment is effectively managing their symptoms and reducing exacerbations. It is always important to assess the patient’s inhaler technique and adherence, as well as non-pharmacological approaches such as smoking cessation or pulmonary rehabilitation before making any adjustments to pharmacological treatment. In some cases it may be appropriate to simply switch inhaler device or molecules within the same class rather than escalating/ de-escalating treatment. Other causes of symptoms should also be considered and investigated as appropriate.
Follow-up treatment of COPD is not based on the patient’s ABCD group, but rather what their predominant symptoms are if not well controlled. GOLD proposes two different treatment algorithms depending on whether the patient’s predominant issue is dyspnoea or exacerbations (in the case where both dyspnoea and exacerbations are an issue, follow the exacerbations pathway) (Figure 4).
The first algorithm is for patients in whom breathlessness or exercise limitation is the predominant symptom. If the patient is on long-acting bronchodilator monotherapy, the use of two bronchodilators is recommended. If this doesn’t improve symptoms, the patient should be stepped down again to monotherapy. For patients who are already on a combination LABA/ICS, consider if the initial indication for ICS was appropriate. If ICS is appropriate, then a LAMA can be added in to start triple therapy. If, however, the initial indication for ICS was inappropriate then the patient should be switched to LABA/LAMA.
For patients in whom exacerbations are the predominant issue, the second algorithm should be followed. For patients on monotherapy with a LABA or LAMA consider escalating to combination therapy with either a LABA/LAMA or LABA/ICS. LABA/ICS may be preferred for patients with (i) a history of asthma, (ii) eosinophils ≥300 with one or more exacerbations a year, or (iii) eosinophils ≥100 with more than two moderate exacerbations/ one hospitalisation.
In patients who develop further exacerbations on LABA/LAMA treatment, blood eosinophil
counts should be performed to predict likelihood of beneficial response to the addition of ICS. Patients with eosinophils ≥100 should be escalated to triple therapy of LABA/LAMA/ICS. Patients with eosinophil counts <100 should be considered for the addition of roflumilast or a macrolide antibiotic. These medications should also be considered in patients still having exacerbations on triple therapy.

Newer studies have shown that regular use of some antibiotics (eg, azithromycin 500mg three times per week or erythromycin 250mg twice daily) can reduce the rate of exacerbations for one year in exacerbationprone patients. This benefit is less in active smokers, however. The safety profile of antibiotics such as azithromycin should also be considered before starting treatment as its use is associated with QTc prolongation and an increased incidence of bacterial resistance.
Roflumilast is a PDE4 inhibitor that reduces the number of exacerbations requiring systemic corticosteroids. It should be considered as an additional treatment in patients with an FEV<50 per cent predicted and chronic bronchitis, especially if they have had at least one exacerbation needing
24 Volume 8 | Issue 5 | 2022 | Respiratory Medicine
FIGURE 3:
hospital admission as it is reported the benefits are greater in patients with a history of exacerbations requiring admission.
Non-pharmacological treatment
It is crucial at all stages of COPD treatment to consider non-pharmacological management (such as smoking cessation and encouragement of regular exercise and a balanced diet). The recommendations for this remain largely unchanged in the latest GOLD guidelines.

Pulmonary rehabilitation is the most effective therapeutic strategy to improve shortness of breath, exercise tolerance, and
general health status of patients with COPD and is recommended for all patients with symptoms or at risk of exacerbations. New data included in the 2022 GOLD guidelines highlights the importance of rehabilitation shortly after a hospital admission for an exacerbation of COPD. There is a significantly lower mortality risk for those who start rehabilitation early versus patients who start rehabilitation late or not at all following hospital admission. There is also new guidance with regards to tele-rehabilitation, which shows that it is safe and has similar benefits to in-person programmes. Vaccinations remain an important part
of treatment of COPD. The 2022 GOLD guidelines include a new recommendation that all patients should receive Covid-19 vaccines in line with national guidance. It is also important to ensure patients are up-to-date with vaccinations such as influenza, pneumococcus, and Tdap (for adults not vaccinated in adolescence).
Reference
Global Initiative for Chronic Obstructive Lung Disease (2022).
2022 GOLD Reports. https://goldcopd. org/2022-gold-reports-2/.
25 Respiratory Medicine | Volume 8 | Issue 5 | 2022
FIGURE 4:
• DYSPNOEA • • EXACERBATIONS •
Update on roll-out of new pulmonary rehabilitation teams nationally
AUTHOR: Priscilla Lynch
Chronic obstructive pulmonary disease (COPD) is the most prevalent respiratory disease in Irish adults and is a major cause of morbidity and mortality. It is estimated that 380,000 people are living with COPD yet only 110,000 are diagnosed.
COPD has considerable impact on the quality-of-life of the patient, families, and carers. At least 1,500 patients die each year of this disease and over 15,000 patients are admitted to hospital with COPD in Ireland. Ireland has long had one of the highest COPD hospitalisation rates in the OCED.
The HSE National Clinical Programme for Respiratory launched the first NCEC Guideline No 27 Management of COPD with the Department of Health on 3 November 2021. The development of this national clinical guideline for COPD is a major step forward in that it will ensure that COPD patients across the country receive consistent and standardised care, based on the best available evidence.
The HSE and National Clinical Programme Respiratory End-to-End Model of Care for COPD continues to support people in community and hospital settings, with the establishment of specialist teams in the community, including respiratory integrated care teams. This will support the care of more patients in the community, and lessen the need for hospital-based care. Some of the services that will be made available in the community include evidence-based interventions such as pulmonary rehabilitation.
Pulmonary rehabilitation
Pulmonary rehabilitation involves supervised classes, twice-weekly for eight weeks and can be delivered in-person and online. It is strongly recommended in the management of patients with COPD by current national and international guidelines.
Pulmonary rehabilitation involves a very detailed assessment including:
Detailed respiratory history (PFTS, CXR, bloods).
Co-morbid conditions.
Considerations during exercise.
Inhaler review.
Oxygen requirement.
Smoking status.
Baseline respiratory symptoms and exacerbation history.
Subjective respiratory assessment: mMRC, CAT, Borg.
HADS questionnaire.
Health-related quality-of-life questionnaire.
Social history.
Physical ability.
Physiological baseline measurements.
Mobility distance.
Falls history.
Exercise testing.
Based on this assessment, exercise and selfmanagement interventions are then tailored to meet individual patient needs.
The HSE Respiratory National Clinical Programme is continuing the roll-out of 34 community-based dedicated pulmonary rehabilitation teams around the country, which will empower people living with COPD to improve their symptommanagement and decrease hospitalisations.
Angela Ryan, Programme Manager, HSE National Clinical Programme, Respiratory says: “As part of both the Integrated Care for Chronic Disease Management being rolled out in GP clinics and the National Respiratory Model of Care, patients with COPD and asthma are being actively monitored by their GP and practice nurse to improve management of their conditions. This programme facilitates care in the community, closer to home, and can facilitate referral to the local pulmonary rehabilitation team, based in the community.
“The pulmonary rehabilitation teams include dedicated teams of specialist physiotherapists and nurses. Pulmonary rehabilitation improves dyspnoea (breathlessness), health status and exercise tolerance in patients with stable COPD, and reduces hospitalisation in patients who have had a recent exacerbation. It leads to a reduction in symptoms of anxiety and depression. Education and self-management are core components of pulmonary rehabilitation. Selfmanagement education with a healthcare professional, improves health status and decreases hospitalisations and emergency department visits.
“Pulmonary rehabilitation is a comprehensive intervention based on thorough patient assessment followed by patient-tailored therapies that include, but are not limited to, exercise training, education, and self-management intervention aiming at behaviour change, designed to improve the physical and psychological condition of people with chronic respiratory disease and to promote the long-term adherence to healthenhancing behaviours,” explains Ms Ryan.
Dr Mark O’Kelly, GP, Rialto Medical, Dublin, said: “The experience in our practice has been that patients who have engaged in the community pulmonary rehab programmes have benefited greatly. They have a better understanding of their disease and are better equipped from a self-management perspective also. This means that they are having less flare ups of their disease and have improved symptom control also.”
COPD resources on request
COPD Support Ireland are available via www.copd.ie and the national COPD advice line can be contacted on 1800 83 21 46.
26 Volume 8 | Issue 5 | 2022 | Respiratory Medicine
Fictional patient, for illustrative purposes only
For COPD patients on treatment with ICS/LABA and at risk of exacerbation* 1
*A worsening of symptoms or a history of exacerbation treated with antibiotics or oral corticosteroids in the past 12 months
It’s the things you
do today
that
make a big difference to their tomorrows1-3
TRELEGY Ellipta provides your patients with statistically superior improvements in lung function and health-related quality of life, and reduction in annualised rate of moderate/ severe exacerbations** vs. budesonide/formoterol***1–3

**Moderate exacerbation is a worsening of symptoms or a history of exacerbation treated with antibiotics or oral corticosteroids. A severe exacerbation is a worsening in symptoms that required hospitalisation.
TRELEGY Ellipta (FF/UMEC/VI) 92/55/22 mcg OD is indicated for maintenance treatment in adult patients with moderate to severe COPD who are not adequately treated by a combination of an ICS and a LABA or a combination of a LAMA and a LABA1
▼This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.


***Co-primary endpoints were change from baseline in trough FEV1 and SGRQ at week 24 (n=1810). A subset of patients (n=430) remained on blinded study treatment for 52 weeks. Trelegy showed an improvement in trough FEV1 of 171mL versus budesonide/formoterol (p < 0.001, 95% CI 148,194) at week 24. Trelegy showed an improvement in health-related quality of life (SGRQ) of 2.2 units (p <0.001, 95% CI 3.5, 1.0) at week 24. At week 52 in a subset of patients Trelegy showed a 44% reduction in annualised rate of moderate/severe exacerbations versus budesonide/formoterol (95% CI 15,63, p=0.006, Absolute difference 0.16).
TRELEGY Ellipta is generally well tolerated. Common adverse reactions include: pneumonia, upper respiratory tract infection, bronchitis, pharyngitis, rhinitis, sinusitis, influenza, nasopharyngitis, candidiasis of mouth and throat, urinary tract infection, headache, cough, oropharyngeal pain, constipation, arthralgia, back pain1 FF, fluticasone furoate; ICS, inhaled corticosteroid; LABA, long-acting ß2-agonist; LAMA, long-acting muscarinic antagonist; OD, once-daily; UMEC, umeclidinium, VI, vilanterol
References: 1. TRELEGY Ellipta SmPC 2019. 2. Lipson DA et al. Am J Respir Crit Care Med 2017; 196:438–446. 3. Lipson DA et al.N Engl J Med 2018; 378:1671–1680.
Trelegy▼ Ellipta (fluticasone furoate/umeclidinium/vilanterol [as trifenatate]) Prescribing information. Please consult the full Summary of Product Characteristics (SmPC) before prescribing Trelegy Ellipta (fluticasone furoate/umeclidinium/vilanterol [as trifenatate]) inhalation powder. Each single inhalation of fluticasone furoate (FF) 100 micrograms (mcg), umeclidinium bromide (UMEC) 62.5 micrograms and vilanterol as trifenatate (VI) 25 mcg provides a delivered dose of 92 mcg FF, 55 mcg UMEC and 22 mcg VI. Indications: Maintenance treatment in adult patients with moderate to severe COPD who are not adequately treated by a combination of an inhaled corticosteroid (ICS) and a long-acting ß2-agonist (LABA) or a combination of a LABA and a long acting muscarinic antagonist. Dosage and administration: One inhalation once daily at the same time each day. Contraindications: Hypersensitivity to the active substances or to any of the excipients (lactose monohydrate & magnesium stearate). Precautions: Paradoxical bronchospasm, unstable or life-threatening cardiovascular disease or heart rhythm abnormalities, convulsive disorders or thyrotoxicosis, pulmonary tuberculosis or patients with chronic or untreated infections, narrow-angle glaucoma, urinary retention, hypokalaemia, patients predisposed to low levels of serum potassium, diabetes mellitus. In patients with moderate to severe hepatic impairment patients should be monitored for systemic corticosteroid-related adverse reactions. Eye symptoms such as blurred vision may be due to underlying serious conditions such as cataract, glaucoma or central serous chorioretinopathy (CSCR); consider referral to ophthalmologist. Increased incidence of pneumonia has been observed in patients with COPD receiving inhaled corticosteroids. Risk factors for pneumonia include: current smokers, old age, patients with a history of prior pneumonia, patients with a low body mass index and severe COPD. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take Trelegy. Acute symptoms: Not for acute symptoms, use short-acting inhaled bronchodilator. Warn patients to seek medical advice if short-acting inhaled bronchodilator use increases. Therapy should not be abruptly stopped without physician supervision due to risk of symptom recurrence. Systemic effects: Systemic effects of ICSs may occur, particularly at high doses for long periods, but much less likely than with oral corticosteroids. Interactions with other medicinal products: Caution should be exercised with concurrent use of ß-blockers. Caution is advised when co-administering with strong CYP3A4 inhibitors (e.g. ketoconazole, ritonavir, cobicistat-containing products), hypokalaemic treatments or non-potassium-sparing diuretics. Co-administration with other long-acting muscarinic antagonists or long acting ß2-adrenergic agonists is not
Find
Start your patients on TRELEGY Ellipta today, expect more from tomorrow 1,2 Today.

TRELEGY. 2-3
recommended. Pregnancy and breast-feeding: Experience limited. Balance risks against benefits. Side effects: Common (≥1/100 to <1/10): pneumonia, upper respiratory tract infection, bronchitis, pharyngitis, rhinitis, sinusitis, influenza, nasopharyngitis, candidiasis of mouth and throat, urinary tract infection, headache, cough, oropharyngeal pain, arthralgia, back pain. Uncommon (≥1/1,000 to <1/100): viral respiratory tract infection, supraventricular tachyarrhythmia, tachycardia, atrial fibrillation, dysphonia, dry mouth, fractures. Rare (≥1/10,000 to <1/1,000): Hypersensitivity reactions, including anaphylaxis, angioedema, urticaria, and rash. Not known (cannot be estimated from the available data): vision blurred. Marketing Authorisation (MA) Holder: GlaxoSmithKline Trading Services Limited, 12 Riverwalk, Citywest Business Campus, Dublin 24, Ireland. MA No. [EU/1/17/1236/002]. Legal category: POM B. Last date of revision: September 2020. Code: PI-6725. Further information available on request from GlaxoSmithKline, 12 Riverwalk, Citywest Business Campus, Dublin 24. Tel: 01-4955000.
Adverse events should be reported to the Health Products Regulatory Authority (HPRA) using an Adverse Reaction Report Form obtained either from the HPRA or electronically via the website at www.hpra.ie. Adverse reactions can also be reported to the HPRA by calling: (01) 6764971. Adverse events should also be reported to GlaxoSmithKline on 1800 244 255.
Tomorrow.
out more here: www.trelegy.ie
request a visit from a GSK representative ©2020 GSK Group of Companies or its licensor Trademarks are owned by or licensed to the GSK Group of Companies
was developed in
or
TRELEGY Ellipta
collaboration with
PM-IE-FVU-ADVT-200014 | October 2020
The role of pulmonary rehabilitation following Covid-19 infection
AUTHOR: Dr Sarah O’Beirne, Consultant Respiratory Physician, Department of Respiratory Medicine, St Michael’s Hospital, Dun Laoghaire, and St Vincent’s University Hospital, Dublin
Coronavirus disease 2019 (Covid-19), caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, is a highly transmissible viral illness responsible for an ongoing global pandemic. Initial variants of the virus including the Alpha, Beta, and Delta variants have been associated with a high morbidity and mortality predominantly related
CASE REPORT 1
A 73-year-old male ex-smoker (30 pack years) with a history of Global Initiative for Chronic Obstructive Lung Disease (GOLD) II chronic obstructive pulmonary disease (COPD) with additional comorbidities, including hypertension and diabetes, developed Covid-19 infection during the first wave of the pandemic in March 2020. He was hospitalised and developed respiratory failure, which was managed with highflow oxygen therapy and did not require mechanical ventilation. On discharge from hospital he remained dyspnoeic and required 2L of oxygen (O2) for exertion. On assessment three months after his acute illness, he reported ongoing exertional breathlessness, anxiety, and fatigue. As a result he was referred for additional
evaluation and outpatient pulmonary rehabilitation. Chest x-ray demonstrated persistent, but improving pulmonary infiltrates and pulmonary function testing revealed moderate obstruction on spirometric testing consistent with his known GOLD II COPD, however, the diffusing capacity of the lung for carbon monoxide (DLCO) was reduced at 45 per cent of predicted; 10 per cent lower than prior testing. He walked a distance of 210m on a pre-rehabilitation six-minute walk test (6MWT) performed on 2L of O2 Respiratory questionnaires scores, including the COPD assessment tool (CAT) and the St George’s Respiratory Questionnaire (SGRQ), the Hospital Anxiety and Depression Scale (HADS), and the Post-Covid Functional Status (PCFS) scale, demonstrated significant symptom burden pre-rehabilitation.
to respiratory disease.1 After the acute illness, 10-to-30 per cent of individuals suffer from persistent symptoms such as shortness of breath, chest discomfort, fatigue, and neurocognitive difficulties. Ongoing symptoms may occur following severe disease requiring hospitalisation and in those with a milder initial illness without hospitalisation. The post-acute sequalae of Covid-19 infection, also termed ‘long Covid’ or ‘post-Covid-19 condition’ when they persist beyond
three months, can significantly affect quality-of-life (QOL) and productivity for many people and are a major concern both for individuals and healthcare planning. While there are no evidencebased treatment options as yet, there is a potential role for structured rehabilitation to improve symptoms and QOL in the condition. Case Report 1 and Case Report 2 are fictionalised cases representative of the typical patients frequently encountered in the post-Covid pulmonary rehabilitation programme (PRP) in our institution.
Persistent symptoms following Covid-19 infection
As of early May 2022, over 1.5 million Covid-19 cases had been reported in Ireland with more than 37,000 hospitalisations and over 7,000 deaths. However, the vast majority of infections are mild or moderate in nature and do not require hospitalisation. Common symptoms of acute Covid-19 infection (≤four weeks from infection onset) include fever, cough, shortness of breath, chest discomfort, and fatigue. For some individuals, the effects of Covid-19 can last
28 Volume 8 | Issue 5 | 2022 | Respiratory Medicine
The UK’s National Institute for Health and Care Excellence (NICE) define long Covid as signs and symptoms that develop during or after an infection consistent with Covid-19, continue for more than 12 weeks, and are not explained by an alternative diagnosis
CASE REPORT 2
A 42-year-old female who never smoked, with no significant past medical history. She was very active with an excellent preCovid exercise tolerance. She developed Covid-19 in mid-2020, reporting an initial mild infection with symptoms including fever, cough, shortness of breath, and loss of taste and smell, which did not require medical assessment or hospitalisation. After the acute phase she experienced persistent exertional dyspnoea and inability to return to her prior activity levels, severe fatigue, and poor concentration. She was unable to return to work as a nurse due to the severity of her symptoms. Extensive evaluation approximately three months after the acute illness, including chest x-ray, CT chest, full pulmonary function testing, and cardiac testing with echocardiogram and cardiac MRI, was normal. Based on her persistent, debilitating symptoms she was referred for outpatient pulmonary rehabilitation. Pre-rehabilitation
6MWT distance demonstrated a walk distance of 320m.
Respiratory questionnaires (CAT, SGRQ) and the HADS and PCFS were again consistent with the presence of significant symptoms and impairment in QOL.
well beyond the immediate illness, even in cases where this was mild. Approximately 10-to-30 per cent of patients who have tested positive for SARS-CoV-2 virus remain unwell beyond three weeks, and a smaller proportion – about 10 per cent –experience symptoms for months, which may be relapsing-remitting in nature.
The UK’s National Institute for Health and Care Excellence (NICE) define long Covid as signs and symptoms that develop during or after an infection
consistent with Covid-19, continue for more than 12 weeks, and are not explained by an alternative diagnosis. 2 Similarly, the World Health Organisation (WHO) consensus definition of the post-Covid condition is as occurring “in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually three months from the onset, with symptoms that last for at least two months and cannot be explained by an alternative diagnosis”. 3
organs such as the lungs, heart, and brain. Research into the pathogenesis of long Covid is urgently needed to better understand the symptoms and natural history of the condition, assist in prognostication, and to aid in development of effective therapies.
Long Covid sufferers can be divided into two main categories. The first group is those who developed a moderatesevere Covid-19 infection with direct organ injury related to the virus and/ or deconditioning related to the acute illness leading to persistent symptoms. These individuals frequently have evidence of persistent radiological and/ or physiological abnormalities and may be older with other co-morbidities. Based on initial illness severity, it could be anticipated that this group may have ongoing pulmonary abnormalities and require pulmonary rehabilitation.
Over 205 symptoms in 10 organ systems have been described in long Covid, and symptoms may fluctuate over time. The most prevalent symptoms reported are fatigue, shortness of breath, chest pain, post-exertional malaise, smell and taste disturbances, headache, poor concentration and ‘brain fog’, and sleep disturbance. Many individuals with long Covid are either unable to return to work or require an adjusted or reduced work schedule, with significant financial and workforce implications.
The underlying pathophysiology of long Covid remains unknown. A variety of mechanisms have been postulated including organ damage during the acute infection, a persistent hyperinflammatory state, inadequate antibody response, and ongoing viral activity. There may also be contributions from co-morbidities and deconditioning related to hospitalisation when present.
The SARS-CoV-2 entry receptor ACE2 is widely expressed, potentially explaining persistent symptoms related to multiple
The second group comprises those with an initial mild or even asymptomatic illness managed in the community whose symptoms persist from the acute illness or appear after recovery. They may present with prolonged multisystem involvement and significant disability and the functional disability may appear out of proportion to the degree of imaging and/or lung function impairment.
In both cases structured rehabilitation programmes and self-management strategies can play an important role in recovery from ongoing symptoms and functional impairment.
Pulmonary rehabilitation for Covid-19
As discussed, many patients, even those with initially mild symptoms, will have persistent physical, cognitive, and psychological disability, which impacts upon their QOL and economic productivity following Covid-19 infection. Given the heterogeneity of the persistent symptoms, there is a need for rehabilitation approaches that
29 Respiratory Medicine | Volume 8 | Issue 5 | 2022
Over 205 symptoms in 10 organ systems have been described in long Covid, and symptoms may fluctuate over time
address these physical and psychological issues in a holistic way. NICE defines rehabilitation as “a set of interventions designed to optimise functioning, health and wellbeing, and reduce disability in people with health conditions in interaction with their environment. In the context of ongoing Covid-19 symptoms, this may include providing information, education, supported selfmanagement, peer support, symptom management strategies, and physical rehabilitation.” 2
Comprehensive rehabilitation postCovid-19 has been recommended by the WHO and interim guidance has been released by the European Respiratory Society (ERS) regarding the role of pulmonary rehabilitation in the condition. 4 Initial recommendations were largely extrapolated from data related to the longer-term consequences and rehabilitation needs following the prior SARS and H1N1 outbreaks, and also studies in survivors of acute respiratory distress syndrome (ARDS), supported by the long record of success and safety of pulmonary rehabilitation in chronic lung disease. Now direct evidence supporting the benefits of rehabilitation post-Covid-19 infection is beginning to accumulate.
Pulmonary rehabilitation is a comprehensive intervention of patienttailored therapies focused on treatable traits including functional, behavioural and emotional traits. It utilises a multidisciplinary approach to guide rehabilitation, including physical, psychological, and psychiatric aspects of management. These programmes have been adapted and tailored for patients recovering from Covid-19 who are often younger and more likely to be in fulltime employment versus those living with chronic lung disease attending traditional pulmonary rehabilitation programmes. In addition to careful self-pacing of exercise to avoid postexertional malaise or exercise-induced
symptom flares, many post-Covid-19 rehabilitation programmes focus on teaching self-management skills to assist with fatigue management and neurocognitive symptoms. Psychological support and services to assist with a phased return to work are important components for many patients suffering from protracted symptoms.
Case reports outcomes
On completion of the PRP, the Case 1 patient reported reduced fatigue and overall improved QOL. This was accompanied by a significant improvement in 6MWT test distance to 330m and he no longer required ambulatory oxygen on re-assessment. Additionally, he experienced an improvement in CAT, SGRQ and HADS scores. At six-month follow-up the pulmonary infiltrates on chest x-ray had resolved and PFTs had returned to preCovid-19 values.
Similarly, Case 2 demonstrated improvement in 6MWT increasing to 480m following completion of the PRP. She continued to suffer from troublesome fatigue; however, with support and improved self-management skills she was able to manage ongoing fatigue symptoms better and instituted a phased return to work at 50 per cent
of her prior hours with a plan to gradually increase frequency and length of shifts as tolerated.
Summary
In Ireland, over 1.5 million people have contracted Covid-19 to-date. While the acute illness can be severe, leading to respiratory failure and in some cases death, particularly prior to the widespread availability of effective vaccines, the majority of individuals will experience only mild disease. However, 10-to-30 per cent report persistent symptoms that impact on QOL and productivity, including many individuals who had a mild initial illness. There is an increasing recognition of the large disease burden occurring following acute Covid-19 infection. Management requires a whole-patient perspective addressing the multi-system condition in a holistic, with growing evidence supporting a role for comprehensive rehabilitation in the condition. It is not yet known whether more recent variants in circulation including the Omicron variant, which appears to be associated with less severe illness, will also lead to protracted symptoms and impaired QOL. Regardless, it is likely that there will be a continued need for health systems across the world to respond to the aftermath of Covid-19 at least in the medium-term.
References
1. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708-1720
2. National Institute for Health and Care Excellence. Covid-19 rapid guideline: Managing the long-term effects of Covid-19. 2021. www.nice.org.uk/guidance/ng188
3. Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV, WHO Clinical Case Definition Working Group on Post-
Covid-19 Condition. A clinical case definition of post-Covid-19 condition by a Delphi consensus. Lancet Infect Dis 2022; 22: e102-e107. doi: 10.1016/S14733099(21)00703-9
4. Spruit MA, Holland AE, Singh SJ, Tonia T, Wilson KC, Troosters T. Covid-19: Interim guidance on rehabilitation in the hospital and post-hospital phase from a European Respiratory Society and American Thoracic Societycoordinated international taskforce. Eur Respir J. 2020 Aug 13;56(6):2002197. doi: 10.1183/13993003.02197-2020
30 Volume 8 | Issue 5 | 2022 | Respiratory Medicine

SingStrong – singing for better lung health in pulmonary fibrosis
Chronic respiratory disease in Ireland is a leading cause of morbidity and mortality and places a significant burden on healthcare resources. Living with a chronic condition such as chronic obstructive pulmonary disease (COPD), pulmonary fibrosis, and asthma can have a devastating impact on the patient and their families, impacting the holistic wellness of the person – physically, emotionally, socially, and economically.
Best practice advocates empowering people to self-manage their health and wellness over the long-term, with support. Group settings offer additional social connections, strengthen personal agency, and are particularly useful for psychosocial support. Because of the relatively larger proportion of patients with COPD, many interventions, including singing, have tended to focus on these populations (Lewis et al, 2016). However, given the severe trajectory of disease progression in pulmonary fibrosis in addition to the limited treatment options available, there is an urgent need to deliver novel strategies and nonpharmacological approaches to support this patient population.
SingStrong
The ‘SingStrong’ project is a clinicallybased singing and breathing retraining programme designed to address a host of bio-psychosocial problems through community-based group classes. This approach was developed by Dr Róisín Cahalan, Senior Physiotherapy Lecturer and researcher at the University of Limerick, and Limerick vocal coach and choir leader Ciara Meade.
The SingStrong programme, supported
by the Irish Research Council, was first delivered to community COPD support groups in Limerick, Clare, and Tipperary in 2019. An evaluation of the eightweek pilot trial was conducted with the assistance of HSE West clinicians. The 75 participants who attended at least half of the classes were included in the analysis, which showed significant improvements in physical endurance as well as nonsignificant improvements in spirometry
Pulmonary fibrosis patients
We explored the acceptability of the SingStrong programme for patients with pulmonary fibrosis in 2021, partnering with the Irish Lung Fibrosis Association (ILFA). The results of this feasibility trial have recently been published in Physiotherapy Research and Practice journal (Cahalan et al, 2022).
The weekly online programme conducted over 12 weeks was comprised of 45-minute classes of mindfulness, breathing retraining, vocal exercises and singing conducted by a trained vocal coach. People with pulmonary fibrosis were invited to participate and sessions were recorded for non-attenders. Participation in the research element of the programme was not required to attend the weekly classes.
and quality-of-life measures (Cahalan et al, 2021). A further study in a cohort of patients with ‘long Covid’ who undertook a modified online SingStrong programme reported improvements in physical symptoms related to breathlessness, breathing control, voice quality, and mental acuity, as well as improved qualityof-life and enhanced locus of control (Cahalan et al, 2022).
The SingStrong programme is flexible, appropriate for remote delivery, safe, and popular with patients. Members can dip in and out of the live online sessions and attend recorded resources at their convenience.
Results showed statistically significant improvements in self-reported qualityof-life and in symptoms measured by the IPF-PROM, a bespoke outcome measure for people with pulmonary fibrosis (Russell 2018). Class attendance also increased from 14 to 25 participants between weeks one and 12. Qualitatively, strong satisfaction with the way that classes were conducted and improved efficacy in self-management of lung health, in particular breathlessness, were reported.
Positive findings
The findings of this study echo and endorse previous findings that people with pulmonary fibrosis, as well as other chronic respiratory conditions, who undertake programmes such as SingStrong feel better and more empowered, even in the absence of objective physical improvements such as spirometry.
32 Volume 8 | Issue 5 | 2022 | Respiratory Medicine
AUTHORS: Dr Roisin Cahalan, Senior physiotherapy lecturer and researcher at the University of Limerick; and Dr Anne Marie Russell, Senior Lecturer, University of Exeter, UK, and Senior Clinical Fellow, Royal Devon University Healthcare NHS Foundation Trust
The ‘SingStrong’ project is a clinically-based singing and breathing retraining programme designed to address a host of biopsychosocial problems through community-based group classes
There are multiple physiological underpinnings for the efficacy of singing as an intervention in lung disease. These include utilisation of the cardiorespiratory system during persistent singing training, resulting in enhanced respiratory muscles and an optimised breathing mode. In addition, singing can also cause changes in neurotransmitters and hormones, including the upregulation of oxytocin, immunoglobulin A, and endorphins, which improves immune function and increases feelings of happiness. Many patients
Pulmonary fibrosis is a challenging condition with limited treatment options. The joy of the SingStrong programme and other similar offerings is that it is an exercise programme underpinned by clinical rationale and delivered with specific health aims in mind. But, because it is great fun, it does not feel like exercise in the conventional way. Therefore, compliance is excellent in comparison to traditional rehabilitation programmes, as participants are exercising without realising it.
for people suffering from pulmonary fibrosis and their families.
ILFA has adjusted to delivering supportive interventions for its members during the Covid-19 pandemic to support the needs of patients, their families and carers. Programmes informed by SingStrong and other novel approaches will continue to be delivered electronically and we hope in the future through hybrid approaches to assist patients, carers and healthcare professionals in non-pharmacological management of pulmonary fibrosis. ILFA will continue its advocacy role and collaborations with research partners to ensure that an eclectic range of exercise and wellbeing programmes are accessible and remain integral to living with pulmonary fibrosis to maximise quality-of-life.
Acknowledgments
with chronic disease display inefficient apical breathing patterns, using the smaller muscles of the neck and shoulder in preference to the primary respiratory muscles of the diaphragm and intercostal muscles. This can lead to disordered breathing, inefficient oxygenation of skeletal muscles, and over time reduced exercise endurance, anxiety, and a curtailed ability to participate in life.
Furthermore, in recognition of the strong adverse psychosocial impact of pulmonary fibrosis on the holistic wellness of the person, the SingStrong programme additionally facilitates optional breakout rooms for people to chat to others facing similar challenges. This has been a lifeline for many people living alone and cocooning due to the current pandemic. The online format has also allowed the delivery of seminars from relevant experts (physiotherapy, GP, dietician, psychologist), as requested by the members. All classes and talks were provided free-of-charge to participants and recorded to be made available to people who cannot attend live.
Interventions like SingStrong are only ever intended as adjuncts to mainstream clinical offerings, but may be important given the limited options for people with pulmonary fibrosis. Although these group-based singing classes may not lengthen life, they certainly appear to enhance life; a considerable gift
Thanks to ILFA for their support and funding the SingStrong programme for patients with pulmonary fibrosis. We would like to acknowledge the participants for their high level of engagement with the programme and contributions to the research associated with the SingStrong programme for patients with pulmonary fibrosis (feasibility study).
References
1. Lewis A, Cave P, Stern M, Welch L, Taylor K, Russell J, et al. Singing for lung health – A systematic review of the literature and consensus statement. NPJ Prim Care Respir Med. 2016 Dec 1;26:16080. doi: 10.1038/npjpcrm.2016.80
2. Cahalan R, Green J, Meade C, Griffin A. SingStrong: Singing for better lung health in COPD – A pilot study. Physiother Theory Pract. 2021 Mar 31:1–9. doi:10.1080/095939 85.2021.1907825
3. Cahalan RM, Meade C, Mockler S. SingStrong – A singing and breathing retraining intervention for respiratory and
other common symptoms of long Covid: A pilot study. Can J Respir Ther. 2022 Mar 9;58:20-27. doi: 10.29390/cjrt-2021-074
4. Cahalan R (b), Russell AM, Meade C, Hayes G. SingStrong – Singing for better lung health in pulmonary fibrosis: A feasibility study. Physiother Pract Res. 2022 April 19:1–9. doi: 10.3233/PPR-210622
5. Russell AM. Development and testing of an idiopathic pulmonary fibrosis (IPF) patient reported outcome measure (PROM). Doctoral thesis. 2018 National Heart and Lung Institute, Imperial College London. doi: 10.25560/86249
33 Respiratory Medicine | Volume 8 | Issue 5 | 2022
Interventions like SingStrong are only ever intended as adjuncts to mainstream clinical offerings, but may be important given the limited options for people with pulmonary fibrosis
Update from the Irish Lung Fibrosis Association
AUTHOR: Nicola Cassidy, Director of the Irish Lung Fibrosis Association
The Irish Lung Fibrosis Association (ILFA) provides research, education, and support for idiopathic pulmonary fibrosis (IPF). IPF is a progressive, debilitating lung condition that mostly affects older adults and leads to irreversible architectural lung damage, impairment of gas exchange, and respiratory failure.

It is vital that patients with pulmonary fibrosis engage in regular exercise to maintain their strength, muscle mass, physical and mental wellbeing, and independence. ILFA provides tailored exercise resources, including the 2,000 Steps Challenge walking pack, exercise DVD, yoga DVD, and TheraBands free-ofcharge to patients and respiratory healthcare professionals to promote exercise for pulmonary fibrosis patients
Meeting patients’ unmet exercise needs during the pandemic Pulmonary fibrosis was designated as an ‘extremely medically vulnerable’ condition for Covid-19 in 2020. Pulmonary fibrosis patients were advised to cocoon, follow public health guidelines, and take extra precautions to protect their health.
In April 2020, ILFA conducted a survey of our members (111 patients responded) to understand the impact of Covid-19. Almost half (47 per cent) said they were exercising less since Covid measures began, 42 per cent reported being worried, and 32 per cent stated they were anxious. The lack of exercise and negative feelings had the potential to impact patients’ health and wellbeing.
ILFA recognised that many patients were self-isolating, afraid to leave their homes, and fearful of undertaking exercise independently. To prevent deconditioning in this vulnerable cohort and keep patients engaged and committed to exercise, ILFA collaborated
with University Hospital Limerick to deliver a virtual exercise programme. Physiotherapistled weekly online exercise classes commenced in May 2020 using the Zoom platform and were advertised to ILFA members via the newsletter, website, and social media. Participants self-selected to register for the class and were provided with safety advice and information in advance. Classes are organised and supervised by an ILFA staff member.
The classes grew in popularity and in late 2020 an extra weekly class was scheduled due to demand. ILFA continues to deliver physiotherapist-led online classes twice a week and patients are referred by doctors, nurses, and physiotherapists working in hospital, community, and hospice settings.
Patient survey
In May 2021, participants taking part in classes were invited to complete an anonymous online survey to assess the impact of the virtual exercise classes.
A total of 53 participants with IPF responded to the survey. The majority (83 per cent) were aged over 60 years of age, 51 per cent were male, 36 per cent used oxygen all the time or most of the time, and 6 per cent were lung transplant recipients. Just 12 per cent of participants were diagnosed with IPF in the last year, 22 per cent between one-to-two years ago, 26 per cent between two-to-three years ago, and 24 per cent between three-to-five years ago; with 60 per cent of participants living outside Dublin.
At the time of the survey, just 11 per cent of participants were on a hospital waiting list for either face-to-face or virtual pulmonary rehabilitation. Only 34 per cent of respondents had previously attended a face-to-face pulmonary rehabilitation course and, of these, 39 per cent continued to exercise regularly when the course had finished. When attending
hospital courses, 89 per cent travelled by car and the average distance travelled was 8km for Dublin residents and 19km among those living outside Dublin (one person travelled over 50km to access classes).
A rating of ‘excellent’ was awarded for the classes by 73 per cent of respondents for help in overcoming anxiety about exercising online. The majority (80 per cent) stated that the instructions were excellent, and 77 per cent found it easy to join the class using Zoom.
Almost two-thirds (64 per cent) reported being more active because of the online exercise classes and 68 per cent reported being less fearful about doing exercise since starting the online classes. Overall, 83 per cent of patients who were previously not at all or not very active reported that they are now more active. Online exercise classes are safe and feasible for IPF patients and have provided substantial physical and emotional benefits. The survey highlights the lack of pulmonary rehabilitation availability to pulmonary fibrosis patients.
Acknowledgments
ILFA is grateful to Niamh Julian, Gordon Cagney, Petra Grehan, and Eimear Bell, respiratory physiotherapists, for their work and commitment to delivering the exercise classes.
References
1. Cassidy N, Sheahan D, Fox L, Brown L, Galvin L, Cassidy E, Sheridan M, O’Reilly KM. Perspectives of interstitial lung disease patients and carers during Covid-19. Irish Medical Journal, 114 (7):410
2. Abstracts from the Irish Thoracic Society Annual Scientific Meeting 2021. Ir J Med Sci 190, 159–211 (2021). doi: 10.1007/s11845021-02845-3
34 Volume 8 | Issue 5 | 2022 | Respiratory Medicine
umeclidinium/vilanterol


HAS POSITIVE HEAD-TO-HEAD DATA VS. ANOTHER ONCE-DAILY
In symptomatic patients with moderate COPD
*Anoro Ellipta compared to tiotropium/olodaterol showed statistical superiority on pre-specified secondary endpoint of trough FEV1 at 8 weeks in the Intent to Treat population. ITT population n=236 (180mL vs. 128mL in trough FEV1; Difference 52ml (p<0.001, 95% CI:28,77).
The primary endpoint of non-inferiority on trough FEV1 at Week 8 in the PP population was met. Non-inferiority was met for the primary endpoint at Week 8 in the PP population (n=227) (175mL Anoro Ellipta and 122mL tiotropium/olodaterol, 95% CI: 26, 80; p<0.001)1
Learn more by visiting: www.anoro.ie/headtohead
Anoro Ellipta is contraindicated for patients who are hypersensitive to the active substances or to any of the excipients. Anoro Ellipta is not indicated for the treatment of acute episodes of bronchospasm. Cardiovascular events, such as cardiac arrhythmias, may be seen after the administration of muscarinic receptor antagonists and sympathomimetic agents, including umeclidinium/vilanterol. Therefore, Anoro Ellipta should be used with caution in patients with severe cardiovasular disease. Due to antimuscarinic activity (i.e. LAMA class activity), umeclidinium/vilanterol should be used with caution in patients with urinary retention or with narrow-angle glaucoma.2
LAMA/LABA* 1
An 8-week, randomised, open-label, two-period crossover in symptomatic patients with moderate COPD (post bronchodilator FEV1 ≤70% and ≥ 50% of predicted value, mMRC≥2) and not receiving ICS at inclusion.1
COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; LABA, long-acting beta2-agonist; LAMA, long-acting muscarinic antagonist; mMRC, modified Medical Research Council scale; ITT, intent to treat; PP, per protocol.
Anoro Ellipta 55/22mcg is indicated as a maintenance bronchodilator treatment to relieve symptoms in adult patients with chronic obstructive pulmonary disease (COPD)2


▼This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information.
Anoro®▼ Ellipta® (umeclidinium bromide/vilanterol [as trifenatate]) Prescribing information (Please consult the full Summary of Product Characteristics (SmPC) before prescribing)
Anoro® Ellipta® 55/22mcg (umeclidinium bromide/vilanterol [as trifenatate]) inhalation powder. Each single inhalation of umeclidinium bromide (UMEC) 62.5 micrograms (mcg) and vilanterol (VI) 25mcg provides a delivered dose of UMEC 55mcg and VI 22mcg. Each delivered dose contains approx. 25 mg lactose. Indications: COPD: Maintenance bronchodilator treatment to relieve symptoms in adult patients with COPD. Dose and administration: Inhalation only. COPD: One inhalation once daily at the same time of the day. Contraindications: Hypersensitivity to the active substances or to any of the excipients (lactose monohydrate and magnesium stearate).
Precautions: Anoro Ellipta should not be used in patients with asthma. Treatment with Anoro Ellipta should be discontinued in the event of paradoxical bronchospasm and alternative therapy initiated if necessary. Cardiovascular effects may be seen after the administration of muscarinic receptor antagonists and sympathomimetics therefore Anoro Ellipta should be used with caution in patients with severe cardiovascular disease. Anoro Ellipta should be used with caution in patients with urinary retention, narrow angle glaucoma, convulsive disorders, thyrotoxicosis, hypokalaemia, hyperglycaemia and severe hepatic
impairment. No dose adjustment is required in renal or mild to moderate hepatic impairment. Patients with rare hereditary problems of galactose intolerance, the Lapp total lactase deficiency or glucose-galactose malabsorption should not use Anoro Ellipta. Acute symptoms: Anoro Ellipta is not indicated for acute episodes of bronchospasm. Warn patients to seek medical advice if short-acting inhaled bronchodilator use increases, a re-evaluation of the patient and of the COPD treatment regimen should be undertaken. Interactions with other medicinal products: Interaction studies have only been performed in adults. Avoid β-blockers. Caution is advised when co-administering with strong CYP3A4 inhibitors (e.g. ketoconazole, clarithromycin, itraconazole, ritonavir, telithromycin). Anoro Ellipta should not be used in conjunction with other long-acting β2-adrenergic agonists or medicinal products containing long-acting muscarinic antagonists. Caution is advised with concomitant use with methylxanthine derivatives, steroids or non-potassium-sparing diuretics as it may potentiate possible hypokalaemic effect of β2adrenergic agonists. Fertility, pregnancy, and breast-feeding: No available data. Balance risks against benefits. Side effects: Common: Urinary tract infection, sinusitis, nasopharyngitis,
References: 1. Feldman G.J et al. Adv Ther. 2017 Nov;34(11):2518-2533. 10.1007/s12325-017-0626-4.
2. Anoro Ellipta Summary of Product Characteristics. Available from: www.medicines.ie. Accessed April 2021.
ANORO ELLIPTA was developed in collaboration with ©2021 GSK group of companies. All rights reserved.
pharyngitis, upper respiratory tract infection, headache, cough, oropharyngeal pain, constipation and dry mouth. Uncommon: Hypersenstivity reactions including rash, tremor, dysgeusia, dysphonia, atrial fibrillation, supraventricular tachycardia, rhythm idioventricular, tachycardia, supraventricular extrasystoles and palpitations. Rare: Anaphylaxis, angioedema, urticaria, vision blurred, glaucoma, intraocular pressure increased, paradoxical bronchospasm, urinary retention, dysuria and bladder outlet obstruction. Frequency not known: Dizziness. Marketing Authorisation (MA) Holder: GlaxoSmithKline (Ireland) Limited, 12 Riverwalk, Citywest Business Campus, Dublin 24, Ireland. MA
Nr: 55/22mcg 1x30 doses [EU/1/14/898/002]. Legal category:
POM B. Last date of revision: January 2021. Job Ref: PI-3826. Further information available on request from GlaxoSmithKline, 12 Riverwalk, Citywest Business Campus, Dublin 24, Tel: 01-4955000.
Adverse events should be reported directly to the Health Products Regulatory Authority (HPRA) on their website: www.hpra.ie. Adverse events should also be reported to GlaxoSmithKline on 1800 244 255.
Anoro and Ellipta are registered trademarks of the GlaxoSmithKline group of companies



PM-IE-UCV-ADVT-210001
Date of Preparation: April 2021
Ambitions for continuing progress in cystic fibrosis care in Ireland
AUTHOR: Sarah Tecklenborg, Senior Research and Policy Coordinator, Cystic Fibrosis Ireland
The impact of new modulator therapies on the lives of people with cystic fibrosis (CF) and advances in care for people who are not eligible for modulator therapies were among the key issues addressed at Cystic Fibrosis Ireland’s (CFI) 2022 annual conference, which took place virtually on March 29-30.
There have been dramatic improvements in CF treatment in recent years, including the development of CF transmembrane conductance regulator (CFTR) modulator therapies and standardised multidisciplinary patient care. Roll-out of the new triple combination CFTR modulator compound, Kaftrio (Vertex pharmaceuticals), started in Ireland in September 2020.
RECOVER study
Prof Paul McNally, Consultant in Paediatric Respiratory Medicine and Director of Research and Innovation at Children’s Health Ireland at Crumlin, shared research into the impact of groundbreaking new therapies for people with CF.
Prof McNally leads the RECOVER study, an ongoing multicentre real-world study in eight centres in Ireland and the UK. Funded by CFI, the CF Foundation (US), and the CF Trust (UK), the study is examining in detail the impact of Kaftrio on the lives and health of people with CF. RECOVER is looking at the impact of Kaftrio as it is prescribed clinically. The project will gather both routine health data and less commonly used clinical endpoints such as the lung clearance index (LCI), chest CT, gastrointestinal symptoms, inflammation, and medication adherence, providing unique insights into the effects of the triple combination drug.
In the first 12 months of RECOVER, 116 adults and adolescents have been recruited across UK and Irish sites. In line with what was seen in clinical trials, the team have seen significant improvements in sweat chloride, lung function, and nutrition in people with CF taking Kaftrio.
In people with CF there is a problem in moving chloride across cell membranes, which lead to higher concentrations of salt in sweat. The study has shown that, for participants with two copies of the F508del mutation, more than 40 per cent had a sweat chloride level well within the ‘normal’ range while on Kaftrio. The significance of this genotype advantage for those with two copies of the F508del mutation remains unclear and ongoing work is needed to ascertain whether this leads to long-term improved outcomes for this subgroup.
The team have also identified significant improvements in outcomes not used in the original clinical trials, including the LCI (a sensitive lung function test) and exhaled nitric oxide (a marker for airway inflammation).
Three new sub-studies have been established as part of RECOVER: A psychology sub-study, in collaboration with Trinity College Dublin, examining experiences of young people (12-to-17 years) who have started taking Kaftrio; a study examining the impact of Kaftrio on nasal and sinus disease in children with CF aged six-to-11 years in collaboration with the RCSI and St James’s Hospital, Dublin; and, finally, a study with the University of Amsterdam using breath analysis to detect changes in airway metabolism and inflammation with Kaftrio treatment.
Prof McNally and his team hope to further their work to investigate the duration of beneficial effect from modulator therapies and whether disease can be prevented in children if they begin modulator therapy early enough.
Experiences of people living with CF
The voice of people living with CF was central to the conference. Not all people with CF have access to an effective therapy and among those who do, the experience can vary. For some, however, Kaftrio has been life changing.
“The last year of my life has been nothing short of a miracle and this is all down to the drug Kaftrio. Prior to December 2020, my life was getting more and more difficult to bear. I had constant chest infections, I couldn’t speak without violently clearing my throat, I had very low energy and regular hospital admissions. It really was becoming increasingly difficult for me to see myself living the life I wanted.
“In December 2020, with nervous excitement based on stories I had heard from the US, I started Kaftrio. Within a couple of hours it felt like a weight had been lifted off my chest. A week with little to no coughing turned into a month. Fourteen months later I can share with you that not only have I not needed one antibiotic for my chest or my sinuses, I have hardly coughed. I no longer have to plan my life around suspected hospital admissions. I no longer feel I need to sleep several times throughout the day and I no longer have to haggle with my medical team to prescribe me just one more oral antibiotic in a desperate attempt to avoid being locked in the CF isolation room in Christopher’s Ward. Thanks to Kaftrio, these are all things I no longer have
36 Volume 8 | Issue 5 | 2022 | Respiratory Medicine
to do. But let me tell you what I now can do.
“I can feel the air enter and exit my entire chest. I can laugh until my sides hurt without ending up in a coughing fit. I have healthier hair and brighter skin. I get up early to meditate and read instead of (doing) my rigorous CF treatments, and most of all I can plan for a bright future, knowing that my body will not let me down. Kaftrio has completely changed my life and I will never take that for granted.”
Orla Carrigan, person living with CF
CF treatment in the modulator era
As modulator therapies bring improvements in the health, symptom burden and experiences of people living with CF, questions are being asked by clinicians about what CF care now looks like in the era of modulator therapy.
Prof Barry Plant, Consultant Respiratory Physician and Director of the Adult CF Centre in Cork University Hospital, discussed inhaled antibiotic therapies and how they may be best used to protect lung health in the era of precision medicine.
CF is characterised by inflammation of the airway, chronic infections and progressive decline in lung function. Studies have shown that up to 25 per cent of CF exacerbations fail to recover to baseline pulmonary function levels after the exacerbation. Daily treatment regimens for CF include the use of inhaled antibiotics and mucolytics, which have led to significant improvements in respiratory disease and improved patient outcomes. Aerosolised antibiotics offer advantages over systemic therapy, as relatively high levels of the drug are delivered directly to the airways through a variety of delivery systems, including standard nebulisers or dry powder eFlow.
Patients on the new modulator therapies feel better, produce less sputum, and have less exacerbations. Prof Plant discussed his patients’ experiences on Kaftrio, noting that at three months post-
initiation of therapy only approximately 10 per cent of patients could provide a sputum sample. This poses problems with monitoring the microbiological status of patients as they cannot provide a sample to confirm whether they do, or do not, have an infection.
As patients experience less exacerbations and produce less sputum, some have been discontinuing their other timeconsuming burdensome therapies. A retrospective analysis of patient adherence to inhaled therapies before and after CFTR modulation with ivacaftor in the Cork CF clinic found there was a reduction in the frequency of use of inhaled therapies after starting the modulator.
In keeping with the increased liberation that Kaftrio has offered to people with CF, a more individualised approach to their inhaled antibiotics may help to optimise their adherence.
How to reduce the treatment burden associated with CF has been identified as a top research question for the CF community. Daily nebulised treatments, often taking up to two hours a day, are considered burdensome. There are groundbreaking trials underway to find out if people taking Kaftrio can safely start to reduce the number of treatments they have to manage as part of their daily healthcare routine. Two randomised studies, CF STORM (UK) and SIMPLIFY (US), aim to assess if stopping certain daily muco-active nebulisers is safe for people taking Kaftrio. The studies will have patients actively disengage from their daily nebulised mucoactive therapies (inhaled hypertonic saline and/or dornase alfa) and assess lung function over time.
If people with CF choose to give up their nebulised therapies without trials like STORM and SIMPLIFY, there would be no quantified evidence for clinicians to refer to and confidently advise their patients. In the short-term, Prof Plant recommends encouraging patients to continue to use
inhaled antibiotics, using personalised approaches to improve adherence where appropriate; “Modulator therapy works, but it probably works even better when used as an add-on to traditional CF therapies.”
Towards personalised medicine
Not everyone with CF is eligible for a modulator therapy like Kaftrio. Over 2,000 mutations in the CFTR protein, which causes CF, have been described. Many new drugs are mutation classspecific and are currently only being clinically tested in patients with specific mutations. These are often very common and well-described mutations. As a result, market authorisation and financial reimbursement of these drugs has been limited to specific subsets of people with CF. People with less common mutations, however, might also benefit from them.
Prof Kors van der Ent, Professor in Paediatric Pulmonology and coordinator of the European HIT-CF project, provided the meeting with an overview of the HIT-CF study, which aims to develop a path for access to therapies for individual patients who show response to therapy in a lab-based test. The HIT-CF project uses organoids, a tissue model derived from a person’s own cells obtained during a rectal biopsy. These organoids are used to test the efficacy of available therapeutics in a lab setting. Only patients with rare genotypes could participate in the study. Organoids from consenting participants will be stored in a biobank to use in future research and to test the efficacy of any future therapies, including genetic therapies. A followup clinical trial, CHOICES, is planned for later this year, which will assess the clinical impact of the therapy in people whose organoids responded highly. The trial aims to validate the organoid model of predicting clinical response to a therapy and this could pave the way for organoid-based personalised medicine in the future.
37 Respiratory Medicine | Volume 8 | Issue 5 | 2022


IN PRINT AND DIGITAL
For all that matters in medicine AVAILABLE
Influenza in Ireland – the return of flu
AUTHOR: Priscilla Lynch
Throughout the 2020/2021 influenza season (28 September 2020 – 23 May 2021) there was no evidence of influenza virus circulation in Ireland, according to the Health Protection Surveillance Centre (HPSC).
There were no confirmed influenza notifications reported on the Computerised Infectious Disease Reporting (CIDR) system during the 2020/2021 influenza season compared to 11,142 laboratoryconfirmed influenza notifications reported during the 2019/2020 season, and 7,939 during the 2018/2019 season. This absence occurred despite continued testing for influenza throughout the Covid-19 pandemic and an increase in sentinel GP influenza-like illness (ILI) testing by the National Virus Reference Laboratory (NVRL) during the 2020/2021 season.
This was the first time since influenza surveillance began in Ireland that no influenza circulated during the winter influenza season, and has been attributed to the impact of the Covid-19 pandemic and related restrictions on travel/movement/ mixing, and increased hygiene measures.
Minimal influenza activity was also observed globally during this time. The World Health Organisation (WHO) and the European Centre for Disease Prevention and Control (ECDC) reported influenza at much lower levels than normally expected for the 2020/2021 influenza season, with only sporadic detections of influenza A and B identified. Since the beginning of September 2021, however, there has been an increase in the number of influenza virus detections in Europe, albeit at lower levels than previous years.
Summary of influenza activity in Ireland 2021/2022
Since October 2021 (2021/2022 influenza season to week 17, 2022), a total of 1,947 laboratory-confirmed influenza cases in Ireland have been notified to the HPSC.
Currently influenza viruses are still circulating in Ireland, but are continuing to decrease.
A total of 48 confirmed influenza cases were notified during the week ending April 30 (week 17, 2022), compared to 135 cases in week 14 (ending April 10, 2022). The median age of the 65 notified cases in week 15 was 37 years. Of the 65 cases, 20 (30.8 per cent) were reported as hospital inpatients, with a median age of 33 years.
The overall influenza positivity rate reported from the NVRL was 6.8 per cent for week 16, and 7.7 per cent for week 15, compared to 13.4 per cent during week 13, 2022.
The sentinel GP ILI consultation rate decreased to 9.1/100,000 population during week 16, 2022, compared to 12.5/100,000 population during week 14.
For the 2021/2022 influenza season in Ireland (until end week 16, 2022), a total of 449 (23 per cent) cases out of a total 1,947 flu cases were reported as hospital inpatients, including 13 confirmed influenza cases admitted to critical care units.
To-date this season (end week 17, 2022), nine deaths in notified influenza cases have been reported.
A total of 21 influenza outbreaks have been notified – nine hospital outbreaks, two family outbreaks, seven nursing home outbreaks, one outbreak linked to a social gathering, and two in other healthcare settings.
Influenza A (H3) remains the predominant influenza virus circulating in Ireland during the 2021/2022 season to-date.
Vaccination uptake in older people
Every year the HSE recommends annual influenza vaccination to all individuals aged 65 years and older. Prior to the 2020/2021 season, flu vaccines and administration were
free only to all medical/GP visit cardholders in this age group.
In 2020, for the 2020/2021 season there was a change in policy in relation to access to free HSE flu vaccination services due to the Covid-19 pandemic, and anticipated pressures on the healthcare system. Under this policy change, all individuals aged 65 years and older were eligible for free flu vaccines and administration regardless of card status in order to minimise cost as a barrier to vaccination.
The average influenza vaccine uptake nationally in those aged 65 years and older attending GP clinics and pharmacies for vaccination was 70.5 per cent during the period September 2020 – August 2021. This represents a substantial increase from the 2019/2020 season.
The uptake is still below the recommended vaccine coverage for all EU Member States (75 per cent), but slightly above the highest uptake recorded during the 2008/09 season (70.1 per cent).
Variation in flu vaccination coverage was observed between age groups, with the highest uptake (79.5 per cent) in those aged 75 years and older and the lowest uptake in those aged 65-to-69 years (56.6 per cent). Variation in flu vaccination coverage was also observed between HSE areas, ranging from 62.8 per cent in HSE-NW to 75.1 per cent in HSE-SE.
References
1. Health Protection Surveillance Centre (HPSC). Influenza season 2020/2021 –Influenza vaccine uptake among those 65 years and older. EPI INSIGHT Vol 22 Issue 6. November 2021. Available at www.hpsc.ie
2. HPSC. Surveillance of influenza, respiratory syncytial virus and other respiratory viruses during the 2020/2021 influenza season. EPI INSIGHT Vol 22 Issue 6. November 2021. Available at www.hpsc.ie
39 Respiratory Medicine | Volume 8 | Issue 5 | 2022
Keeping up momentum in fighting TB
AUTHOR: Priscilla Lynch
In support of World TB Day, 24 March, the Forum of International Respiratory Societies (FIRS), of which the European Respiratory Society (ERS) is a founding member, called on the tuberculosis (TB) community to challenge itself to think differently, champion science, and embrace evidencebased innovation in order to end TB.
The World Health Organisation (WHO) recently noted that TB is only behind Covid-19 with regards to infectious diseaserelated mortality, with rates of TB infection increasing during the Covid-19 pandemic for the first time in over a decade.
With TB-related deaths also increasing for the first time in over a decade –1.5 million deaths in 2020 – TB must be treated as an emergency, said FIRS, calling on world leaders to keep the promises they made at the UN High-Level Meeting 2018 and invest to end TB.
Despite the limited funding (in particular when compared with Covid-19), there have been significant TB research triumphs in the past decade, such as:
Molecular tests that make diagnosis possible in less than two hours, rather than two-to-four weeks.
Treatment of multidrug-resistant TB has been shortened from two years of toxic, injectable agents to six months of an all-oral regimen.
Treatment of TB infection has been cut from nine months to as short as one-to-three months with safer and better tolerated regimens.
“These breakthroughs are testament to our commitment to science. In order to make the next great breakthrough in TB elimination we must nurture an environment where evidence-based innovation can flourish. It will be challenging to sustain such an environment
given the current hardships we are facing globally, including the millions of refugees fleeing the war in Ukraine,” said the ERS.
Ukraine has one of the highest instances of TB in the greater European area and onethird of those with TB are drug-resistant. The recent Russian invasion of Ukraine and subsequent influx of refugees into EU countries, including Ireland, is expected to impact on TB treatment in Ireland.
St James’s Hospital launches
Dr Noel Browne Medal in Tuberculosis for SpRs in respiratory medicine
The inaugural Dr Noel Browne Medal in Tuberculosis was presented on Wednesday, 4 May to Dr Ciara Ottewill and Dr Rachel Mulpeter by Ruth Browne, daughter of the late Minister for Health Noel Browne, in a ceremony at the RCPI in Dublin.
The prize was initiated by Prof Anne Marie McLaughlin, Consultant Respiratory Physician at St James’s Hospital, Dublin, to recognise excellence in SpRs in respiratory medicine across Ireland.
During the event, six finalists delivered presentations on their experiences in managing challenging cases of TB. The presentations delivered important learnings to assist in the treatment of TB at St James’s Hospital, as well as educating SpRs in respiratory medicine across Ireland in best practice of patient treatment and TB management.
Co-winner Dr Mulpeter presented on paradoxical reactions in TB, which can occur when medication is started in a patient who is already immunosuppressed due to coexisting conditions or medications.
Dr Ottewill in her presentation analysed the use of BPaL and BPaLM regimens in treatment of multidrug resistant TB
(MDRTB). The BPaLM regimen was approved as a shorter regimen in treating MDRTB this month.
Awarding the medal, Ms Browne said: “I am very proud to present the inaugural Dr Noel Browne Medal in Tuberculosis and to see the legacy of my father’s important work highlighted by St James’s Hospital. My father both lived with TB and lost his sister and mother to the disease. As Minister for Health from 1948-1951, he was devoted to improving TB care in Ireland with the ultimate goal of eradicating the disease.”
During the event, Prof McLaughlin delivered a lecture on the legacy of Dr Browne. “As Minister for Health, Noel Browne was a trailblazer in raising capital for improved health services in Ireland and implemented funding for new sanatoria and isolation facilities, which radically improved treatment of tuberculosis,” she explained.
St James’s, which is currently seeing an increase in TB cases, is a national leader in the management of TB, treating drug-resistant TB patients, patients who cannot tolerate particular medicines, and patients presenting with advanced disease, multiorgan disease, or significant disability.
The Irish Mycobacterial Reference Laboratory (IMRL) tests all samples taken from patients presenting at St James’s Hospital and assesses whether a particular isolate of TB is treatment-resistant.
St James’s three TB clinics are led by a multidisciplinary team including Prof McLaughlin, Prof Joseph Keane, Consultant Respiratory Physician, TB Clinical Nurse Specialist Lorraine Dolan, and a contact tracing clinic staffed by public health doctors and nurses who are also experienced in navigating cultural and language barriers when treating TB patients.
40 Volume 8 | Issue 5 | 2022 | Respiratory Medicine
Latest modules
Acute pain in primary care and community settings
1 CPD Hour
Author: Eamonn Brady MPSI, pharmacist, Whelehans Pharmacies, 38 Pearse St and Clonmore, Mullingar
Diagnosis and management of depression
2 CPD Hours
Authors: Dr Laura Ridgeway, Psychiatry Registrar, Saint John of God Hospital, Stillorgan, Co Dublin; and Dr Stephen McWilliams, Consultant Psychiatrist, Saint John of God Hospital, Stillorgan, Co Dublin, & Associate Clinical Professor, School of Medicine, UCD
Cholesterol and hypercholesterolaemia
2 CPD Hours
Authors: Dr Daniel Delaney, Registrar in Cardiology; and Prof David Burke, Consultant Cardiologist and Director of Cardiology, Cardiology Department, Beacon Hospital, Dublin
A B C doctorCPD.ie
www.medilearning.ie/doctorcpd Free CPD – now accessible on android, iPhone and tablet
Visit
Asthma*
Asthma*
KEEP ASTHMA TAMED
KEEP ASTHMA TAMED
KEEP ASTHMA TAMED

Proactive asthma control that lasts1,2
Proactive asthma control that lasts1,2



RELVAR ELLIPTA (fluticasone furoate/vilanterol)
RELVAR ELLIPTA (fluticasone furoate/vilanterol)
Proactive asthma control that lasts1,2 RELVAR ELLIPTA (fluticasone furoate/vilanterol)
* Relvar Ellipta is indicated for the regular treatment of asthma in adults and adolescents aged 12 years and older where use of a combination medicinal product (long-acting β 2-agonist and inhaled corticosteroid) is appropriate: patients not adequately controlled with inhaled corticosteroids and ‘as needed’ inhaled short acting β 2-agonists or patients already adequately controlled on both inhaled corticosteroid and long-acting β 2 - agonist 2
* Relvar Ellipta is indicated for the regular treatment of asthma in adults and adolescents aged 12 years and older where use of a combination medicinal product (long-acting β 2-agonist and inhaled corticosteroid) is appropriate: patients not adequately controlled with inhaled corticosteroids and ‘as needed’ inhaled short acting β 2-agonists or patients already adequately controlled on both inhaled corticosteroid and long-acting β 2 - agonist 2
* Relvar Ellipta is indicated for the regular treatment of asthma in adults and adolescents aged 12 years and older where use of a combination medicinal product (long-acting β 2-agonist and inhaled corticosteroid) is appropriate: patients not adequately controlled with inhaled corticosteroids and ‘as needed’ inhaled short acting β 2-agonists or patients already adequately controlled on both inhaled corticosteroid and long-acting β 2 - agonist 2
For Healthcare Professionals only. Images used are for illustrative purposes only. Relvar is well tolerated. Most common adverse events are nasopharyngitis and headache2 PM-IE-FFV-ADVT-200005 December 2020
For Healthcare Professionals only. Images used are for illustrative purposes only. Relvar is well tolerated. Most common adverse events are nasopharyngitis and headache2 PM-IE-FFV-ADVT-200005 December 2020
For Healthcare Professionals only. Images used are for illustrative purposes only. Relvar is well tolerated. Most common adverse events are nasopharyngitis and headache2 PM-IE-FFV-ADVT-200005 December 2020
Relvar Ellipta was developed in collaboration with
Relvar Ellipta was developed in collaboration with
Relvar Ellipta was developed in collaboration with
References: 1. Bernstein DI et al. J Asthma 2015; 52: 1073-1083. 2. Relvar Ellipta SmPC, 2019, available on www.medicines.ie
Relvar® Ellipta® (fluticasone furoate/ vilanterol [as trifenatate]) Prescribing information (Please consult the full Summary of Product Characteristics (SmPC) before prescribing)
Relvar® Ellipta® (fluticasone furoate/ vilanterol [as trifenatate]) Prescribing information (Please consult the full Summary of Product Characteristics (SmPC) before prescribing)
Relvar® Ellipta® (fluticasone furoate/ vilanterol [as trifenatate]) Prescribing information
(Please consult the full Summary of Product Characteristics (SmPC) before prescribing)
Relvar® Ellipta® (fluticasone furoate/vilanterol [as trifenatate]) inhalation powder. Each single inhalation of fluticasone furoate (FF) 100 micrograms (mcg) and vilanterol (VI) 25mcg provides a delivered dose of 92mcg FF and 22mcg VI. Each single inhalation of FF 200mcg and VI 25mcg provides a delivered dose of 184mcg of FF and 22mcg of VI. Indications: Asthma: Regular treatment of asthma in patients ≥12 years and older where a long-acting β2-agonist and inhaled corticosteroid combination is appropriate and where patients are not adequately controlled on inhaled corticosteroids and ‘’as needed” short-acting inhaled β2-agonists, or where patients are already controlled on both inhaled corticosteroid and long-acting β2-agonist. COPD (Relvar 92/22mcg only): Symptomatic treatment of adults with COPD with a FEV1<70% predicted normal (post-bronchodilator) and an exacerbation history despite regular bronchodilator therapy). Dosage and administration: Inhalation only. Asthma: Patients with asthma should be given the strength of Relvar Ellipta containing the appropriate fluticasone furoate (FF) dosage for the severity of their disease. Prescribers should be aware that in patients with asthma, FF 100 mcg once daily is approximately equivalent to fluticasone propionate (FP) 250 mcg twice daily, while FF 200 mcg once daily is approximately equivalent to FP 500 mcg twice daily. Adults and adolescents ≥12 years: one inhalation once daily of: Relvar 92/22mcg for patients who require a low to mid dose of inhaled corticosteroid in combination with a long-acting β2-agonist. If patients are inadequately controlled then the dose can be increased to one inhalation once daily Relvar 184/22mcg. Relvar 184/22mcg can also be considered for patients who require a higher dose of inhaled corticosteroid in combination with a long-acting β2-agonist. Regularly review patients and reduce dose to lowest that maintains effective symptom control. COPD: one inhalation once daily of Relvar 92/22mcg. Contraindications: Hypersensitivity to the active substances or to any of the excipients (lactose
Relvar® Ellipta® (fluticasone furoate/vilanterol [as trifenatate]) inhalation powder. Each single inhalation of fluticasone furoate (FF) 100 micrograms (mcg) and vilanterol (VI) 25mcg provides a delivered dose of 92mcg FF and 22mcg VI. Each single inhalation of FF 200mcg and VI 25mcg provides a delivered dose of 184mcg of FF and 22mcg of VI. Indications: Asthma: Regular treatment of asthma in patients ≥12 years and older where a long-acting β2-agonist and inhaled corticosteroid combination is appropriate and where patients are not adequately controlled on inhaled corticosteroids and ‘’as needed” short-acting inhaled β2-agonists, or where patients are already controlled on both inhaled corticosteroid and long-acting β2-agonist. COPD (Relvar 92/22mcg only): Symptomatic treatment of adults with COPD with a FEV1<70% predicted normal (post-bronchodilator) and an exacerbation history despite regular bronchodilator therapy). Dosage and administration: Inhalation only. Asthma: Patients with asthma should be given the strength of Relvar Ellipta containing the appropriate fluticasone furoate (FF) dosage for the severity of their disease. Prescribers should be aware that in patients with asthma, FF 100 mcg once daily is approximately equivalent to fluticasone propionate (FP) 250 mcg twice daily, while FF 200 mcg once daily is approximately equivalent to FP 500 mcg twice daily. Adults and adolescents ≥12 years: one inhalation once daily of: Relvar 92/22mcg for patients who require a low to mid dose of inhaled corticosteroid in combination with a long-acting β2-agonist. If patients are inadequately controlled then the dose can be increased to one inhalation once daily Relvar 184/22mcg. Relvar 184/22mcg can also be considered for patients who require a higher dose of inhaled corticosteroid in combination with a long-acting β2-agonist. Regularly review patients and reduce dose to lowest that maintains effective symptom control. COPD: one inhalation once daily of Relvar 92/22mcg. Contraindications: Hypersensitivity to the active substances or to any of the excipients (lactose
Relvar® Ellipta® (fluticasone furoate/vilanterol [as trifenatate]) inhalation powder. Each single inhalation of fluticasone furoate (FF) 100 micrograms (mcg) and vilanterol 25mcg provides a delivered dose of 92mcg FF and 22mcg VI. Each single inhalation of FF 200mcg and VI 25mcg provides a delivered dose of 184mcg of FF and 22mcg of VI. Indications: Asthma: Regular treatment of asthma in patients ≥12 years and older where a long-acting β2-agonist and inhaled corticosteroid combination is appropriate and where patients are not adequately controlled on inhaled corticosteroids and needed” short-acting inhaled β2-agonists, or where patients are already controlled on both inhaled corticosteroid and long-acting β2-agonist. COPD (Relvar 92/22mcg only):

Symptomatic treatment of adults with COPD with a FEV1<70% predicted normal (post-bronchodilator) and an exacerbation history despite regular bronchodilator therapy). Dosage and administration Inhalation only. Asthma: Patients with asthma should be given the strength of Relvar Ellipta containing the appropriate fluticasone furoate (FF) dosage for the severity of their disease. Prescribers should be aware that in patients with asthma, FF 100 mcg once daily is approximately equivalent to fluticasone propionate (FP) 250 mcg twice daily, while FF 200 mcg once daily is approximately equivalent to FP 500 mcg twice daily. Adults and adolescents ≥12 years: one inhalation once daily of: Relvar 92/22mcg for patients who require a low to mid dose of inhaled corticosteroid in combination with a long-acting β2-agonist. If patients are inadequately controlled then the dose can be increased to one inhalation once daily Relvar 184/22mcg. Relvar 184/22mcg can also be considered for patients who require a higher dose of inhaled corticosteroid in combination with a long-acting β2-agonist. Regularly review patients and reduce dose to lowest that maintains effective symptom control. COPD: one inhalation once daily of Relvar 92/22mcg. Contraindications: Hypersensitivity to the active substances or to any of the excipients (lactose
monohydrate & magnesium stearate). Precautions: Pulmonary tuberculosis, severe cardiovascular disorders, heart rhythm abnormalities, thyrotoxicosis, uncorrected hypokalaemia or patients predisposed to low levels of serum potassium. chronic or untreated infections, diabetes mellitus. Paradoxical bronchospasm – substitute alternative therapy if necessary. In patients with hepatic with moderate to severe impairment 92/22mcg dose should be used. Acute symptoms: Not for acute symptoms, use short-acting inhaled bronchodilator. Warn patients to seek medical advice if short-acting inhaled bronchodilator use increases. Therapy should not be abruptly stopped without physician supervision due to risk of symptom recurrence. Asthma-related adverse events and exacerbations may occur during treatment. Patients should continue treatment but seek medical advice if asthma symptoms remain uncontrolled or worsen after initiation of Relvar. Systemic effects: Systemic effects of inhaled corticosteroids may occur, particularly at high doses for long periods, but much less likely than with oral corticosteroids. Possible Systemic effects include: Cushing’s syndrome, Cushingoid features, adrenal suppression, decrease in bone mineral density, growth retardation in children and adolescents, cataract, glaucoma. More rarely, a range of psychological or behavioural effects including psychomotor hyperactivity, sleep disorders, anxiety, depression or aggression (particularly in children). Increased incidence of pneumonia, including pneumonia requiring hospitalisation, has been observed in patients with COPD receiving inhaled corticosteroids. If a patient presents with visual disturbance they should be considered for referral to an ophthalmologist for evaluation of possible causes which may include cataract, glaucoma, or rare diseases such as central serous chorioretinopathy. Physicians should remain vigilant for the possible development of pneumonia in patients with COPD as the clinical features of such infections overlap with the symptoms of COPD exacerbations. Risk factors for pneumonia include: current smoking, older age, low body mass index and severe COPD. The incidence of pneumonia in patients with asthma was common at the higher dose of Relvar (184/22mcg). Patients with rare hereditary problems of galactose intolerance, the total lactase deficiency or glucose-galactose malabsorption
monohydrate & magnesium stearate). Precautions: Pulmonary tuberculosis, severe cardiovascular disorders, heart rhythm abnormalities, thyrotoxicosis, uncorrected hypokalaemia or patients predisposed to low levels of serum potassium. chronic or untreated infections, diabetes mellitus. Paradoxical bronchospasm – substitute alternative therapy if necessary. In patients with hepatic with moderate to severe impairment 92/22mcg dose should be used. Acute symptoms: Not for acute symptoms, use short-acting inhaled bronchodilator. Warn patients to seek medical advice if short-acting inhaled bronchodilator use increases. Therapy should not be abruptly stopped without physician supervision due to risk of symptom recurrence. Asthma-related adverse events and exacerbations may occur during treatment. Patients should continue treatment but seek medical advice if asthma symptoms remain uncontrolled or worsen after initiation of Relvar. Systemic effects: Systemic effects of inhaled corticosteroids may occur, particularly at high doses for long periods, but much less likely than with oral corticosteroids. Possible Systemic effects include: Cushing’s syndrome, Cushingoid features, adrenal suppression, decrease in bone mineral density, growth retardation in children and adolescents, cataract, glaucoma. More rarely, a range of psychological or behavioural effects including psychomotor hyperactivity, sleep disorders, anxiety, depression or aggression (particularly in children). Increased incidence of pneumonia, including pneumonia requiring hospitalisation, has been observed in patients with COPD receiving inhaled corticosteroids. If a patient presents with visual disturbance they should be considered for referral to an ophthalmologist for evaluation of possible causes which may include cataract, glaucoma, or rare diseases such as central serous chorioretinopathy. Physicians should remain vigilant for the possible development of pneumonia in patients with COPD as the clinical features of such infections overlap with the symptoms of COPD exacerbations. Risk factors for pneumonia include: current smoking, older age, low body mass index and severe COPD. The incidence of pneumonia in patients with asthma was common at the higher dose of Relvar (184/22mcg). Patients with rare hereditary problems of galactose intolerance, the total lactase deficiency or glucose-galactose malabsorption
monohydrate & magnesium stearate). Precautions: Pulmonary tuberculosis, severe cardiovascular disorders, heart rhythm abnormalities, thyrotoxicosis, uncorrected hypokalaemia or patients predisposed to low levels of serum potassium. chronic or untreated infections, diabetes mellitus. Paradoxical bronchospasm – substitute alternative therapy if necessary. In patients with hepatic with moderate to severe impairment 92/22mcg dose should be used. Acute symptoms: Not for acute symptoms, use short-acting inhaled bronchodilator. Warn patients to seek medical advice if short-acting inhaled bronchodilator use increases. Therapy should not be abruptly stopped without physician supervision due to risk of symptom recurrence. Asthma-related adverse events and exacerbations may occur during treatment. Patients should continue treatment but seek medical advice if asthma symptoms remain uncontrolled or worsen after initiation of Relvar. Systemic effects: Systemic effects of inhaled corticosteroids may occur, particularly at high doses for long periods, but much less likely than with oral corticosteroids. Possible Systemic effects include: Cushing’s syndrome, Cushingoid features, adrenal suppression, decrease in bone mineral density, growth retardation in children and adolescents, cataract, glaucoma. More rarely, a range of psychological or behavioural effects including psychomotor hyperactivity, sleep disorders, anxiety, depression or aggression (particularly in children). Increased incidence of pneumonia, including pneumonia requiring hospitalisation, has been observed in patients with COPD receiving inhaled corticosteroids. If a patient presents with visual disturbance they should be considered for referral to an ophthalmologist for evaluation of possible causes which may include cataract, glaucoma, or rare diseases such as central serous chorioretinopathy. Physicians should remain vigilant for the possible development of pneumonia in patients with COPD as the clinical features of such infections overlap with the symptoms of COPD exacerbations. Risk factors for pneumonia include: current smoking, older age, low body mass index and severe COPD. The incidence of pneumonia in patients with asthma was common at the higher dose of Relvar (184/22mcg). Patients with rare hereditary problems of galactose intolerance, the total lactase deficiency or glucose-galactose malabsorption
should not use Relvar. Interactions with other medicinal products: Interaction studies have only been performed in adults. Avoid β-blockers. Caution is advised when co-administering with strong CYP 3A4 inhibitors (e.g. ketoconazole, ritonavir, cobicistat-containing products). Concomitant administration of other sympathomimetic medicinal products may potentiate the adverse reactions of FF/VI. Relvar should not be used in conjunction with other longacting β2-adrenergic agonists or medicinal products containing long-acting β2-adrenergic agonists. Pregnancy and breast-feeding: Experience limited. Balance risks against benefits. Side effects: Very Common (≥1/10): Headache, nasopharyngitis. Common (≥1/100 to <1/10): Candidiasis of the mouth and throat, pneumonia, bronchitis, upper respiratory tract infection, influenza, oropharyngeal pain, sinusitis, pharyngitis, rhinitis, cough, dysphonia, abdominal pain, arthralgia, back pain, muscle spasms, fractures, pyrexia. Uncommon (≥1/1,000 to <1/100): Hyperglycaemia, vision blurred, extrasystoles. Rare (≥1/10,000 to <1/1,000): Hypersensitivity reactions including anaphylaxis, angioedema, rash and urticaria; palpitations, tachycardia, tremor, anxiety, paradoxical bronchospasm. Marketing authorisation (MA) Holder: GlaxoSmithKline (Ireland) Limited, 12 Riverwalk, Citywest Business Campus, Dublin 24, Ireland. MA Nrs: 92/22mcg 1x30 doses [EU/1/13/886/002]; 184/22mcg 1x30 doses [EU/1/13/886/005].
should not use Relvar. Interactions with other medicinal products: Interaction studies have only been performed in adults. Avoid β-blockers. Caution is advised when co-administering with strong CYP 3A4 inhibitors (e.g. ketoconazole, ritonavir, cobicistat-containing products). Concomitant administration of other sympathomimetic medicinal products may potentiate the adverse reactions of FF/VI. Relvar should not be used in conjunction with other longacting β2-adrenergic agonists or medicinal products containing long-acting β2-adrenergic agonists. Pregnancy and breast-feeding: Experience limited. Balance risks against benefits. Side effects: Very Common (≥1/10): Headache, nasopharyngitis. Common (≥1/100 to <1/10): Candidiasis of the mouth and throat, pneumonia, bronchitis, upper respiratory tract infection, influenza, oropharyngeal pain, sinusitis, pharyngitis, rhinitis, cough, dysphonia, abdominal pain, arthralgia, back pain, muscle spasms, fractures, pyrexia. Uncommon (≥1/1,000 to <1/100): Hyperglycaemia, vision blurred, extrasystoles. Rare (≥1/10,000 to <1/1,000): Hypersensitivity reactions including anaphylaxis, angioedema, rash and urticaria; palpitations, tachycardia, tremor, anxiety, paradoxical bronchospasm. Marketing authorisation (MA) Holder: GlaxoSmithKline (Ireland) Limited, 12 Riverwalk, Citywest Business Campus, Dublin 24, Ireland. MA Nrs: 92/22mcg 1x30 doses [EU/1/13/886/002]; 184/22mcg 1x30 doses [EU/1/13/886/005].
should not use Relvar. Interactions with other medicinal products: Interaction studies have only been performed in adults. Avoid β-blockers. Caution is advised when co-administering with strong CYP 3A4 inhibitors (e.g. ketoconazole, ritonavir, cobicistat-containing products). Concomitant administration of other sympathomimetic medicinal products may potentiate the adverse reactions of FF/VI. Relvar should not be used in conjunction with other longacting β2-adrenergic agonists or medicinal products containing long-acting β2-adrenergic agonists. Pregnancy and breast-feeding: Experience limited. Balance risks against benefits. Side effects: Very Common (≥1/10): Headache, nasopharyngitis. Common (≥1/100 to <1/10): Candidiasis of the mouth and throat, pneumonia, bronchitis, upper respiratory tract infection, influenza, oropharyngeal pain, sinusitis, pharyngitis, rhinitis, cough, dysphonia, abdominal pain, arthralgia, back pain, muscle spasms, fractures, pyrexia. Uncommon (≥1/1,000 to <1/100): Hyperglycaemia, vision blurred, extrasystoles. Rare (≥1/10,000 to <1/1,000): Hypersensitivity reactions including anaphylaxis, angioedema, rash and urticaria; palpitations, tachycardia, tremor, anxiety, paradoxical bronchospasm. Marketing authorisation (MA) Holder: GlaxoSmithKline (Ireland) Limited, 12 Riverwalk, Citywest Business Campus, Dublin 24, Ireland. MA Nrs: 92/22mcg 1x30 doses [EU/1/13/886/002]; 184/22mcg 1x30 doses [EU/1/13/886/005].
Legal category: POM B. Last date of revision: June 2019. Code: PI-2046. Further information available on request from GlaxoSmithKline, 12 Riverwalk, Citywest Business Campus, Dublin 24. Tel: 01-4955000. Adverse events should be reported directly to the Health Products Regulatory Authority (HPRA)
Legal category: POM B. Last date of revision: June 2019. Code: PI-2046. Further information available on request from GlaxoSmithKline, 12 Riverwalk, Citywest Business Campus, Dublin 24. Tel: 01-4955000.
Legal category: POM B. Last date of revision: June 2019. Code: PI-2046. Further information available on request from GlaxoSmithKline, 12 Riverwalk, Citywest Business Campus, Dublin 24. Tel: 01-4955000.
Adverse events should be reported directly to the Health Products Regulatory Authority (HPRA) on their website: www.hpra.ie. Adverse events should also be reported to GlaxoSmithKline on 1800 244 255.
www.hpra.ie. Adverse events should also be reported to GlaxoSmithKline on 1800 244 255.
Adverse events should be reported directly to the Health Products Regulatory Authority (HPRA) on their website: www.hpra.ie. Adverse events should also be reported to GlaxoSmithKline on 1800 244 255.
Asthma*
References: 1. Bernstein DI et al. J Asthma 2015; 52: 1073-1083. 2. Relvar Ellipta SmPC, 2019, available on www.medicines.ie
on their website:
2. Relvar Ellipta SmPC,
References: 1. Bernstein DI et al. J Asthma 2015; 52: 1073-1083.
2019, available on www.medicines.ie
















































































































