80 minute read
Pati ent advocacy – improving the lives of people with lung fi brosis
Patient advocacy – improving the lives of people with lung fibrosis
AUTHOR: Ms Nicola Cassidy, on behalf of the Irish Lung Fibrosis Association
The Irish Lung Fibrosis Association (ILFA) is a patient organisation founded to support individuals with lung fibrosis and their families. Lung fibrosis is an underlying pathology in a number of interstitial lung diseases (ILD), the most prevalent of which is idiopathic pulmonary fibrosis (IPF). ILD is thought to affect around 1,000 patients in Ireland – only slightly less than those diagnosed with cystic fibrosis. It is characterised by increasing breathlessness with progressive impacts on patients’ abilities to engage in normal activities of daily living and deterioration in qualityof-life. With a median survival time from diagnosis of 4.5 years, the prognosis for patients with IPF is worse than many cancers. And yet despite the prevalence and the seriousness of ILD, many healthcare professionals, let alone lay people, remain unaware of this condition. Poor recognition is a considerable factor in the late diagnosis of ILD, which in turn affects patients’ ability to access timely pharmaceutical treatments, which can slow the progression of the disease and holistic treatments to maximise quality-of-life.
A critical part of ILFA’s work is patient advocacy; as an organisation we can have a greater voice than individual patients alone. There are many inadequacies in the diagnosis and management of ILDs and through engagement with multiple stakeholders, including politicians, healthcare professionals, the health service, industry representatives and more, we hope to address these unmet needs to improve the lives of those affected by ILDs.
We have recently had success in ensuring that ILD is recognised as a serious lung condition for the categorisation of people who are highly medically vulnerable to Covid-19. Lack of inclusion of ILD in the initial Health Protection Surveillance Centre (HPSC) and HSE guidelines on cocooning reflects the poor awareness of this condition. Lobbying on behalf of patients, families, and healthcare professionals resulted in revision of the HPSC guidance. Research has shown that patients with ILD are at significant risk of serious illness due to Covid-19 and it is essential for protection of patients that guidelines on cocooning reflect this.
Clinical care programme
A current focus of our ongoing work is to advocate for a HSE clinical care programme for lung fibrosis, as currently exists for COPD, asthma and cystic fibrosis and other conditions. Clinical care programmes provide benefits for patients and healthcare providers in clearly setting out a model for the provision of highquality care. National programmes ensure equity and timely access to appropriate care regardless of the patient’s location. Without such a unified approach, there are currently large discrepancies in the healthcare experiences of patients with ILD. Our research indicates that this is a priority area for ILFA’s advocacy work for patients, families, and healthcare providers working in this specialty, including the Irish Thoracic Society.
Transplantation
We are also engaged in work to support the enactment of legislation which would give more patients the opportunity to access lung transplantation, an effective ILD treatment for those eligible. Enactment of the Human Tissue Bill would introduce a system whereby everyone will be considered a potential organ donor unless they have registered their objection. Opt-out legislation has the potential to increase the number of organ donors and life-saving transplant operations that can take place. Additional funding to support staffing, infrastructure and resources for the national transplant centres is crucial as well as an ongoing national awareness campaign.
IPF registry
Another advocacy priority is lobbying for adequate national funding to support the National IPF Registry. The objective of the IPF Registry is to characterise the true incidence of IPF in Ireland, in order to plan for future healthcare needs. In addition, ILFA recently contributed to the public consultation on the Draft Recommendations on the Implementation of a National Electronic Patient Summary.
Long Covid
Lung fibrosis has been identified as one of the long term consequences of Covid-19 infection, so called ‘long Covid’. Research to characterise the relationship between Covid-19 and lung fibrosis is in its infancy, but available evidence suggests that there may be significant population morbidity arising from the persistent respiratory complications of Covid-19. ILFA may well be advocating for a rapidly growing patient population in light of this, giving our current work even greater urgency.
We would like to take this opportunity to thank all of those who support ILFA, most of whom are volunteers, and many who balance these commitments with busy jobs, for their work and dedication to improving the lives of people affected by lung fibrosis. We also wish to acknowledge the great work being done by respiratory healthcare professionals in supporting lung fibrosis patients in these challenging times. n
For more information please visit www.ilfa.ie
References available on request
Quadrivalent live attenuated infl uenza (LAIV) nasal vaccine for children
AUTHOR: Dr Tom Barrett, MB Dch MRCOG, Senior Medical O cer, HSE National Immunisation O ce
This year the seasonal infl uenza vaccination programme has been extended to all children aged two-to-12 years. The aim of the programme is to prevent infl uenza and its complications in children and to reduce the spread of infl uenza to others in the community. The infl uenza vaccine o ered to children is quadrivalent live attenuated infl uenza vaccine (LAIV), which is given intranasally, and is available free of charge from GPs and community pharmacists.
Infl uenza disease
Infl uenza is a very common acute viral respiratory illness which a ects all age groups. The virus is seen all year round, but peaks every winter. The degree of infl uenza infection is unpredictable; however, each year in Ireland infl uenza is responsible for between 200 and 500 deaths and as many as a 1,000 during a particularly severe season (2008/2009).
The 2020/2021 infl uenza season presents a signifi cant challenge to the delivery of healthcare services, both in primary care and in hospitals, especially as there is now a resurgence of cases of Covid-19. At an individual level, the consequences of dual infection with infl uenza and Covid-19 are unclear, but worse outcomes are likely, particularly for those at-risk.
To prevent cases of infl uenza in children and reduce the spread of infl uenza to others, the National Immunisation Advisory Committee (NIAC) has recommended infl uenza vaccine for all children (aged twoto-17 years inclusive).1
For the 2020/2021 infl uenza season, the Department of Health, on NIAC advice, has extended the infl uenza vaccination programme to children, providing funding



to the HSE to o er quadrivalent LAIV vaccine free to all children aged two-to-12 years inclusive.2
The aim of the extension of the infl uenza programme to children is to reduce: ▸ morbidity and mortality from infl uenza in children; ▸ the number of people with infl uenza; ▸ the number of hospital admissions; ▸ transmission of infl uenza to the elderly and persons in at-risk groups; ▸ transmission to healthcare workers in families with children; ▸ absenteeism of children from school and their parents from work.
Children outside of the two-to-12 age group (aged six months to <two years and aged 13 years and older) who have an underlying chronic medical condition should receive quadrivalent inactivated infl uenza vaccine (QIV) as in previous years.
Infl uenza in children
Children are among the most susceptible to infl uenza infection. It is estimated that

20-30 per cent of children develop infl uenza during each infl uenza season compared to fi ve-to-10 per cent of adults.3 Children, because they have limited pre-existing immunity, are primary vectors of infl uenza transmission in the community and shed the virus at higher viral titres. Children transmit the infl uenza virus for a longer period than adults; they can transmit the infl uenza virus for 10 or more days, compared to six days in adults, therefore increasing spread of the disease.
Approximately 10 per cent of children under 15 years attend their GP with infl uenza in an average infl uenza season. Infl uenza is an important cause of pneumonia, bronchitis, otitis media, croup and bronchiolitis in children. Incidence rates are highest in the younger age groups leading to high rates of excess outpatient visits, hospital admissions and antibiotic prescriptions.
In Ireland during the 2018/2019 infl uenza season, 1,245 children were hospitalised with infl uenza. Children aged less than fi ve years had the second highest
hospitalisation rates for influenza after those aged 65 years and older.
Between the 2009/2010 and 2018/2019 influenza seasons: ▸ 4,750 children aged 0-to-14 have required hospitalisation as a result of influenza, ▸ 183 requiring critical care, and ▸ 41 children died.4
Influenza vaccination for children in other countries
Nine European countries, the US, Canada and Australia currently recommend influenza vaccine for children. The UK gives LAIV to children. Finland, the US and Canada give LAIV or QIV to children.5, 6, 7
In the US, LAIV has been recommended since 2004. LAIV has been authorised for use in Canada since 2011.
In 2013, the UK introduced trivalent LAIV for two- and three-year-olds with pilot programmes for primary school children. Quadrivalent LAIV was introduced during the 2014/2015 influenza season. The programme has extended year on year to include all children from two up to 11 years.
In Finland, annual inactivated influenza vaccine was recommended for children aged six-to-35 months in 2007. LAIV was introduced in 2015 to enhance vaccine uptake. Since then, all two- and threeyear-old children have been eligible for vaccination with either LAIV or inactivated influenza vaccine. The programme has recently been extended to include all children to six years of age.
In Ireland, for the 2020/2021 influenza season, vaccination of children will help minimise the burden of influenza, by preventing influenza in children and the transmission of influenza from children to those in at-risk groups. This should reduce morbidity from influenza as well as influenza-related hospital admissions in all the at-risk groups.
This is especially important this coming influenza season, to minimise the impact on our health services from dual outbreaks of influenza and Covid-19.
LAIV for 2020/2021 influenza season:
The vaccine used is quadrivalent LAIV Fluenz Tetra, manufactured by Astra Zeneca, which will be offered to all children aged two years to 12 years. It is administered intranasally.
The vaccine contains the following four vaccine virus strains as recommended by the World Health Organisation (WHO):
1. an A/Guangdong-Maonan/SWL1536/2019 (H1N1)pdm09-like virus; 2. an A/Hong Kong/2671/2019 (H3N2)-like virus; 3. a B/Washington/02/2019 (B/Victoria lineage)-like virus; 4. a B/Phuket/3073/2013 (B/Yamagata lineage)-like virus.
LAIV may contain residues of egg proteins (ovalbumin), maximum amount of less than 0.024 micrograms and gentamicin. LAIV does not contain thiomersal or latex.
LAIV effectiveness
Since LAIV contains live attenuated viruses, it mimics natural infection with wild-type viruses, with the development of both mucosal and systemic immunity. Local mucosal antibodies protect the upper respiratory tract and may be more important for protection than serum antibodies, inducing more durable immune memory and so providing better long-term protection to children than inactivated influenza vaccines such as QIV.
In some studies, LAIV has been shown to be more effective in children compared with inactivated influenza vaccines.
In addition, LAIV may offer some protection against strains not contained in the vaccine, as well as virus strains that have undergone antigenic drift.
A recent meta-analysis of LAIV suggested an efficacy against confirmed disease of 83 per cent (95 per cent CI 69-91 per cent).8
The UK pilot primary school programme introducing LAIV was evaluated in 2014/2015 and showed: ▸ 94 per cent reduction in primary school age children GP influenza-like consultations; ▸ 74 per cent reduction in primary school age emergency department attendances with respiratory complaints; ▸ 93 per cent reduction in primary school age confirmed influenza hospitalisations; ▸ 59 per cent reduction in adults GP influenza-like illness consultations.9
LAIV dose and administration
Each LAIV vaccine comes as a prefilled nasal applicator and each applicator contains 0.2ml nasal suspension. The vaccine is administered by the nasal route. One dose of LAIV is 0.2ml administered in divided doses into each nostril, ie, 0.1ml in each nostril.
If the child’s nose drips after vaccination, the vaccine dose does not need to be repeated. The vaccine is immediately absorbed after administration. Parents and guardians should be reassured the vaccine is still effective if this occurs. For the same reason the vaccine does not need to be repeated if the child sneezes or blows their nose after vaccination.
LAIV can be given together with or at any time before or after the administration of any other live attenuated (eg, MMR) or inactivated vaccines.
GROUP
Medically at risk AGE
two-to-eight years
PREVIOUS VACCINATION
Have never had any influenza vaccine
Have had any influenza vaccine before
DOSE
Two doses four weeks apart
One dose
Gelatin in LAIV
inhibitors (eg, ipilimumab plus nivolumab) because of a potential association with immune related ▸ Those with a cranial CSF leak. LAIV, like some other vaccines, contains gelatin derived from pork, which is highly purified and hydrolysed and acts as a stabiliser. Gelatin in vaccines may cause concern to some members of the Muslim community.
nine-to-12 years Not relevant One dose The National Immunisation Office (NIO) has received a statement from
Healthy two-to-12 years Not relevant One dose the Chair of the Irish Council of Imams TABLE 1: Doses of LAIV required for children aged two-to-12 years stating that vaccines containing gelatin are NIAC has recommended healthy children The following are NOT further details. require one dose of LAIV. However, children contraindications in a medically at-risk group aged two-to- ▸ Asymptomatic HIV infection. LAIV side effects eight years inclusive are recommended two ▸ Children receiving: Very common or common doses of LAIV if they have never had any ● topical or inhaled corticosteroids. (More than one-in-10 to one-in-100): influenza vaccine before (Table 1). The two ● low dose systemic corticosteroids. Nasal congestion/rhinorrhoea, decreased doses should be given four weeks apart. ● replacement therapy corticosteroids appetite, malaise, fever, headache, and (eg, adrenal insufficiency). myalgia. In post-marketing surveillance, Contraindications to LAIV overall rates of fever were similar to the rates ▸ Anaphylaxis following a previous dose of Precautions following other childhood vaccines and were influenza vaccine or any of its constituents ▸ Defer until recovered from an acute generally mild and of short duration. (other than ovalbumin – see precautions) severe febrile illness. ▸ Asthma ▸ As LAIV has an ovalbumin content <0.1 Very rare (less than one-in-10,000): ● Acute exacerbation of symptoms micrograms per dose, it can be given to Immediate allergic reactions. increased wheezing and/or additional children with confirmed egg anaphylaxis Very rare cases of Guillain-Barré syndrome bronchodilator treatment in the last or egg allergy in a primary care setting (GBS) have been observed in the post72 hours. except children who have required ICU marketing setting following influenza ● Seek specialist advice if on regular admission to hospital for a previous severe vaccination. The risk of GBS following oral steroids or previous ICU admission anaphylaxis to egg who should be given influenza infection is significantly greater ▸ Significant immunosuppression due to LAIV in hospital. than that following influenza vaccination. disease or treatment. ▸ Aspirin/salicylates should not be ▸ Children who live with severely used for four weeks after vaccination Can LAIV vaccine cause immunosuppressed persons (ie, post unless medically indicated, as Reye’s virus shedding? haematopoietic stem-cell transplant syndrome has been reported following The attenuated vaccine viruses in LAIV are (HSCT)). the use of salicylates during wild-type cold adapted. They can replicate at the lower ▸ Concomitant use of aspirin/salicylates. influenza infection. temperatures found in the nose, but cannot ▸ Influenza antiviral medication within the ▸ Avoid influenza antiviral medication for replicate efficiently at body temperature previous 48 hours. two weeks post vaccination. elsewhere in the body. ▸ Those with severe neutropaenia (absolute ▸ Children aged two-to-12 years for whom neutrophil count <0.5 × 109/L) to avoid an LAIV is contraindicated should be Vaccinated children can shed the acute vaccine related febrile episode. offered QIV (provided there are no attenuated virus for a few days after ▸ Those on combination checkpoint contraindications to QIV). vaccination, but the virus that is shed adverse reactions. Vaccinated children can shed the attenuated virus ▸ Those post cochlear implant until the for a few days after vaccination, but the virus that risk of CSF leak has resolved – consult with the relevant specialist. is shed cannot cause infection permitted. See www.immunisation.ie for
HSE SEASONAL INFLUENZA VACCINATION PROGRAMME 2020/21 QUADRIVALENT LIVE ATTENUATED INFLUENZA VACCINE (LAIV) AND QUADRIVALENT INACTIVATED INFLUENZA VACCINE (QIV)
Name
Manufacturer Who
What
Contraindications
Precautions
Adverse reactions LAIV QIV
Fluenz Tetra (egg-based) Quadrivalent Influenza vaccine (split virion, inactivated) (egg-based)
Astra Zeneca
All aged two-to-12 years (at the time of vaccination)
1 dose (healthy children) 2 doses if: ▶ in a risk group ▶ and aged two-to-eight years ▶ and never had influenza vaccine before
Intranasal Sanofi Pasteur
In a risk group aged six months to <two years, and 13-to-64 years All aged 65 and older Two-to-12 years if LAIV is contraindicated 1 dose 2 doses if: ▶ never had influenza vaccine before ▶ and aged two-to-eight years ▶ or post HSCT or solid organ transplant
Intramuscular
Anaphylaxis following a previous dose of influenza vaccine or any of its constituents (except ovalbumin) Severe neutropaenia (absolute neutrophil count less than 0.5 x 109/l) On combination checkpoint inhibitors (eg, ipilimumab plus nivolumab)
Asthma - acute exacerbation of symptoms, increased wheezing and/or additional bronchodilator treatment in the last 72 hours Seek specialist advice if on regular oral steroids or previous ICU admission Concomitant use of aspirin/salicylates Influenza antiviral medication in the previous 48 hours Pregnancy Significant immunosuppression due to disease or treatment Those who live with severely immunosuppressed persons (eg, post HSCT)
Acute severe febrile illness, defer until recovery
Children who required ICU admission for a previous severe egg anaphylaxis should be given LAIV in hospital No aspirin/salicylates for four weeks after vaccine due to risk of Reye’s syndrome Avoid antiviral medication for two weeks after vaccine Seek specialist assessment for those who required ICU admission for a previous severe egg anaphylaxis Separate QIV from PCV vaccine by at least one week for children aged 12-to-23 months
Very common or common: Nasal congestion/ rhinorrhoea, decreased appetite, malaise, fever, headache, and myalgia. (Fever rates similar to those after other childhood vaccines; generally mild and of short duration) Very common: Injection site pain and swelling Fever, fatigue, myalgia, and irritability in young children Common: Drowsiness, sweating, and arthralgia
Very rare: Immediate allergic reactions Guillain-Barré syndrome (risk of GBS following infection is much greater than that post vaccination)
cannot cause infection. Peak incidence of shedding occurred two-to-three days postvaccination in Fluenz clinical studies.10
Each chapter of the the NIAC immunisation guidelines advise: “In some circumstances, advice in these guidelines may differ from that in the Summary of Product Characteristics (SmPC). When this occurs, the recommendations in these guidelines, which are based on current expert advice from NIAC, should be followed.”
NIAC advice, based on the current expert guidance, supersedes the advice in the licensed SmPC.
NIAC guidelines advise that: “Children who live with severely immunosuppressed persons requiring isolation (eg, post HSCT) should not receive the Quadrivalent Live Attenuated Influenza (LAIV) nasal vaccine.” This is a precautionary measure.
Therefore NIAC advice is that LAIV vaccine can be given to any child for whom the LAIV vaccine is not contraindicated, who
is living with any other person unless the adult is in isolation, eg, following a HSCT.
Children who are vaccinated with LAIV can ‘shed’ very small amounts of the weakened virus that is in the vaccine for a few days after vaccination. But the weakened viruses do not cause flu infection in others, or in the person vaccinated.
NIAC makes no recommendation that children avoid other vulnerable people including a parent who is on chemotherapy or immunotherapy.
NIAC advises: “Millions of doses of LAIV have been administered in the US for over 10 years and serious illness amongst immunocompromised contacts inadvertently exposed to vaccine virus has never been observed.”
Increasing uptake of influenza vaccine
Research, both in Ireland and elsewhere, has consistently shown that doctors and other healthcare professionals are the most trusted sources of information on vaccination. A recommendation by a trusted healthcare professional has been shown to increase vaccine uptake.
Reminders to patients about vaccination have also been shown to increase vaccine uptake, be that by text, phone, email or letter.
This influenza season, your recommendation to get the influenza vaccine to parents of children and other at-risk groups will be even more important, in order to maximise uptake.
Summary
The introduction of the LAIV vaccine for children will help minimise the burden of influenza by protecting children from influenza and preventing transmission of influenza from children to those in at-risk groups.
The NIO has developed an e-learning module for vaccinators available at www.HSEland.ie. The www.immunisation.ie website is updated with details on the LAIV vaccine.
The NIO has produced information materials on LAIV for parents and for vaccinators and copies have been distributed to vaccinators with additional copies available to order.
Influenza remains a major public health issue and never more so than during the Covid-19 pandemic. Influenza vaccination is the best intervention available. Extending
the influenza vaccination programme to children aged two-to-12 years aims to protect children and reduce transmission to others, thereby reducing the burden on our health services at this crucial time. n
The following resources on LAIV are available from the NIO:
▸ Frequently asked questions for healthcare professionals: https://www. hse.ie/eng/health/immunisation/pubinfo/fluvaccination/faqchild.pdf ▸ Algorithms on flu vaccination for children for healthcare professionals: https://www.hse.ie/eng/health/immunisation/ pubinfo/flu-vaccination/laivalgorithm.pdf ▸ Information materials for parents in different languages and easy-read guides: https://www.hse.ie/eng/health/immunisation/ pubinfo/flu-vaccination/information/ ▸ Commonly asked questions and answers on the safety of LAIV for parents: https:// www.hse.ie/eng/health/immunisation/pubinfo/ flu-vaccination/flu-vaccine-for-children/ fluvaccineqas.html
References
1. Royal College of Physicians of Ireland. Immunisation Guidelines for Ireland. Influenza Chapter https://www. hse.ie/eng/health/immunisation/hcpinfo/ guidelines/chapter11.pdf 2. Seasonal Influenza Programme 2020/2021 https://www.hse.ie/eng/health/ immunisation/hcpinfo/fluinfo/ 3. Burden of paediatric influenza in Western Europe: a systematic review Antonova E A, Rycroft C E et al. BMC Public Health 12, Article number: 968 (2012) 4. Health Protection Surveillance Centre (HPSC) Annual Epidemiological Report, Influenza and Other Seasonal Respiratory Viruses in Ireland, 2018/2019 (December 2019), https://www.hpsc.ie/a-z/ respiratory/influenza/seasonalinfluenza/ surveillance/influenzasurveillancereports/ previousinfluenzaseasons surveillancereports/20182019season/ Influenza per cent202018-2019 per cent20Season_Summary.pdf 5. The NHS national flu immunisation programme 2020/21. https:// www.england.nhs.uk/wp-content/ uploads/2020/05/national-fluimmunisation-programme-2020-2021.pdf 6. Finnish Institute for health and welfare. Vaccination programme for children and adolescents [online]. Available 7.
8.
9.
10. from: https://thl.fi/en/web/vaccination/ national-vaccination-programme/ vaccinationprogramme-for-children-andadolescents Canadian Immunisation Guide. Chapter on Influenza and Statement on Seasonal Influenza Vaccine for 2020–2021 https:// www.canada.ca/en/public-health/services/ publications/vaccines-immunization/ canadian-immunisation-guide-statementseasonal-influenza-vaccine-2020-2021. html#I1 Live-Attenuated Influenza Vaccine Effectiveness in Children from 2009 to 2015–2016: A Systematic Review and Meta-Analysis. Caspard H, Mallory R M, Yu J, and Ambrose C S. Open Forum Infect Dis. 2017 Summer; 4(3): ofx111 Uptake and impact of vaccinating school age children against influenza during a season with circulation of drifted influenza A and B strains, England, 2014/15 Pebody RG, Green HK, Andrews N, Zhao H, Boddington N, et al. Surveillance and outbreak report https://www. eurosurveillance.org/images/dynamic/EE/ V20N39/art21256.pdf Quadrivalent Live Attenuated Influenza (LAIV), nasal, Fluenz Tetra (AstraZeneca Pharmaceuticals (Ireland) DAC) SmPC http://www.hpra.ie/docs/defaultsource/vaccines-smpcs/fluenz-tetra_ smpc_07_2020.pdf?sfvrsn=2
Covid 19 - when to consider lung transplantation?
AUTHOR: Dr Niamh Boyle, Respiratory Registrar, and Dr Michelle Murray, Consultant Lung Transplant Physician, Mater Misericordiae University Hospital, Dublin
The true impact of the Covid-19 pandemic on our healthcare system remains unknown. However, it is already clear that the field of lung transplantation medicine is faced with new challenges to overcome. Questions are being raised about the potential role of lung transplantation post SARS-CoV-2 lung infection. However, as the disease course is yet to be fully elucidated, the indications and considerations for lung transplantation are uncertain. It is well-known that SARSCoV-2 can cause acute respiratory failure and acute respiratory distress syndrome (ARDS), however, whether it can cause worsening of a pre-existing respiratory disorder or lead to pulmonary fibrosis has not been fully established. Many respiratory viruses are known to cause ARDS and while we are familiar with lung recovery rates and survival in influenza viruses, ARDS secondary to SARS-CoV-2 infection poses a remarkable challenge.
SARS-CoV-2 infection
Uncertainty exists as to whether transplantation should be considered for either acute Covid-19 disease or for the potential sequelae. The manifestation of Covid-19 in the acute setting varies from a mild disease course to ARDS requiring intensive care admission. Approximately 10 per cent of patients will develop ARDS. In this group, mortality rates of 60 per cent have been reported. The long-term manifestations will become apparent in time. However, extrapolating from the acute findings and the experience with ARDS, post SARS-CoV-2 fibrosis is a potential complication, particularly in those with an underlying lung disease or those who have survived an ICU admission.
Acute setting
Lung transplantation is seldom an option for acutely critically ill patients and in this setting the long-term prognosis is likely to be poor. Cases of lung transplantation as a therapy for patients with acute SARS-CoV-2 respiratory failure have been reported in China, Europe and the US. While initial reports have been acceptable, it is likely that more transplantations have occurred, but have not been reported due to poor outcomes and related publication bias. Nevertheless, these initial cases highlight the challenges associated with transplantation in the acute setting.
Firstly, many of the patients who progress to respiratory failure requiring ICU admission secondary to SARS-CoV-2 have multiple co-morbidities including elevated body mass index (BMI), coronary artery disease and diabetes mellitus. These conditions can increase their risk of posttransplant complications and preclude them from transplantation irrespective of SARS-CoV-2 infection. These patients with advanced respiratory failure requiring ventilatory or extra-corporeal membrane oxygenation (ECMO) support are at a particular risk for complications such as muscle wasting, malnutrition, renal disease, and multi-organ damage, which renders them an unsuitable transplant candidate. Older frail patients also have a poor prognosis from lung transplantation in this setting.
Secondly, transplantation should only be considered in end-stage lung disease. At present, the recovery time for Covid19-related ARDS is unknown. Emerging reports suggest it is similar to ARDS due to other causes. It is well known that nonfunctioning lungs can recover after weeks to months of ECMO support following infection with influenza or a bacterial pneumonia. Thus, adequate time should
Age Physical Rehab
Standard Transplant Considerations
Other Organ Dysfunction
FIGURE 1: Transplant Covid-19 testing protocol
Social Support
Acute illness Severe, refractory ARDS
BMI Timing Reversibility Specific COVID-19 Considerations
Transplant Consent Sars-Co-V PCR
Center Experience
be given to allow for lung recovery prior to deeming the SARS-CoV-2 related lung disease irreversible. The International Society of Heart and Lung Transplantation (ISHLT) suggests lung transplantation could be considered after at least 28 days from the onset of lung injury. While transplant may initially appear as a panacea, the longterm survival rates of transplantation are approximately 60 per cent at five years, thus if native organ function can recover, this is often preferable.
In addition, the rapid spread and high infectivity rate of SARS-CoV-2 represents a complex challenge which has important implications across the entire field of transplantation. Transmission of infection to the clinical team is a distinct possibility, especially in the setting of lung transplantation, in which organ retrieval and transplant surgery are considered aerosol-generating procedures. It requires a significant change in practice for teams involved in organ procurement and recipient hospitals carrying out the transplant surgery. At all stages in the transplant pathway, safety of staff should be paramount. The ISHLT consensus recommends waiting at least 14 days after the confirmation of SARS-CoV-2 and two negative PCR tests 48 hours apart, prior to transplantation. A significantly higher mortality in PCR-positive patients, even in the absence of symptoms, has also been reported. The Department of Cardiothoracic Surgery and Transplant Medicine in the Mater has continued to perform heart and lung transplantation safely during the current pandemic by devising protocols for patients awaiting transplantation. See Figure 1.
Finally, of the reported cases of lung transplantation in the literature to date, all of the patients were bridged with ECMO. Many transplant centres have experience with ECMO-bridging, which is considered high-risk transplantation, and as such outcomes are intimately related to centre volume. Many centres have shown that outcomes are worse for patients who are elderly or non-ambulatory whilst on ECMO. Overall in the acute setting, in
Active transplant recipient at home/called in for transplant
Negative COVID-19 swab Positive COVID-19 swab
Proceed with Transplant
FIGURE 2: Transplant pathway
Temporarily suspend from the active transplant list
Recovered and symptom free for 28 days
Positive Covid-19 swab Two negative nose/throat swabs 24 hours apart
Remain suspended from the active transplant list
Weekly Gene Xpert and Flow Flex PCR testing
Two negative NP 24-48 hours apart Relist for transplant after MDT discussion
Covid testing on all potential recipients If negative; proceed with transplant If positive; defer transplant Relist for Transplant
patients with ARDS secondary to SARSCoV-2, consideration for potential lung transplantation should only be considered in those who are likely to have a good postoperative outcome. As such, both standard transplantation considerations and SARSCoV-2 specific considerations need to be assessed. See Figure 2.
ISHLT and Cypel M et al have outlined important considerations, which take into account candidate, disease and transmissibility factors in addition to rehabilitation potential, centre experience and equity of access to an organ:
Candidates 1. Should be under 65 years of age; 2. Should have single organ disease; 3. Should not have any other co-morbidities precluding from transplant; 4. Should be able to give informed consent.
Disease 5. Appropriate time should be given for lung recovery; 6. Radiology should suggest irreversibility.
Rehabilitation 7. Candidate should be ambulatory with good rehabilitation potential.
Infection 8. Candidate should have negative PCR result or sample showing absence of viable virus.
Centre 9. Should have experience in ECMObridging for ARDS; 10. Should have access to broad donor pool with a low wait-list mortality rate.
Post-acute setting
Pulmonary fibrosis is a well-recognised complication of ARDS with a substantial proportion of patients experiencing residual pulmonary function impairment and radiographic evidence of pulmonary fibrosis. Emerging reports suggest that some patients who survive the acute insult from SARS-CoV-2, may go on to develop pulmonary fibrosis resulting in end stage lung disease. The spectrum of fibrotic disease observed with SARS-CoV-2 ranges from fibrosis associated with organising pneumonia to severe acute lung injury with progression to extensive fibrotic change. Two studies from China provide us with some early figures following discharge. Functionally, 47 per cent of patients had impaired gas transfer and 25 per cent had reduced total lung capacity after discharge which correlated with disease severity.
Overall in the acute setting, in patients with ARDS secondary to SARS-CoV-2, consideration for potential lung transplantation should only be considered in those who are likely to have a good post-operative outcome
Radiographically, 65 per cent of patients had complete resolution of radiographic findings four weeks post-discharge.
Until robust long-term data is available, we can only draw from prior experience with ARDS and other strains of the coronavirus; Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV, SARS) and Middle Eastern Respiratory Syndrome Coronavirus (MERS-CoV, MERS). Studies have reported up to 30 per cent of patients with SARS and MERS with interstitial abnormalities radiologically and physiologically. A study of 71 patients with SARS showed that interstitial abnormalities and functional decline recovered two years post infection and at 15 years, 4.6 per cent of patients had persistent abnormalities. In a study of 36 patients who had recovered from MERS, chest radiographs approximately one month after hospital discharge showed abnormalities in one-third of patients. These results may appear reassuring, however, long-term outcomes secondary to SARS-CoV-2 may not be comparable. The population with severe SARS-CoV-2 respiratory disease have more co-morbidities and an older age profile than those that were enrolled in these longitudinal studies.
The trajectory of pulmonary fibrosis is variable. Some patients have a rapidly progressive disease course. It is postulated that aberrant immune pathways promote pulmonary fibrosis, possibly as the end result of a cytokine storm, however, the factors that determine which patients develop fibroproliferative ARDS are not well understood. Interestingly, the risk factors for severe SARS-CoV-2 are similar to those of idiopathic pulmonary fibrosis; male gender and advanced age. We must identify patients at risk of pulmonary fibrosis as there is evidence that the magnitude of the fibroproliferative response is related to long-term healthrelated quality-of-life.
Conclusion
The Covid-19 pandemic is uncharted territory. Transplant programmes around the world are endeavouring to continue to assess typical candidates with reduced elective beds, to perform life-saving surgery with limited critical care capacity and to safely deliver post-transplant care. While striving to continue usual care, new clinical questions arise. What is the optimal management of SARS-CoV-2 in transplanted patients and is there a therapeutic role for transplant in severe SARS-CoV-2-related lung disease? With these unanswered questions, comes the need to establish systems of care and resources to deal with the expected increase in referrals to transplant centres and programmes, such as pulmonary rehab, to optimise potential candidates. Should lung transplant be added to the armamentarium of treatments for patients with SARS-CoV-2 related ARDS or pulmonary fibrosis? Only time will tell, but hopefully understanding the complexities related to this will be beneficial to referring physicians now. ■
References on request
Acute respiratory viral infections
AUTHOR: Dr Amy Rigby, Consultant in Respiratory and General Internal Medicine, Beacon Hospital, Dublin
Acute respiratory tract infections are the cause of significant morbidity and mortality worldwide and are rivalled only by diarrhoea as the leading cause of death in developing countries. An adult may experience two-to-four upper respiratory infections a year, and a child may suffer up to 10. Thus the burden of viral respiratory tract infections, both clinical and economic, is significant. Viral respiratory pathogens may cause a number of clinical syndromes. Except for a few notable cases (adenoviruses, coronavirus) and rare cases of extrapulmonary dissemination of other respiratory viruses, replication of respiratory viruses generally occurs only in the respiratory mucosa of humans. Acute respiratory tract infections usually start in the upper respiratory tract; spread to the lower airways occurs in two-to-four days either by continuous spread or by inhalation of aerosols.
Viral pharyngitis
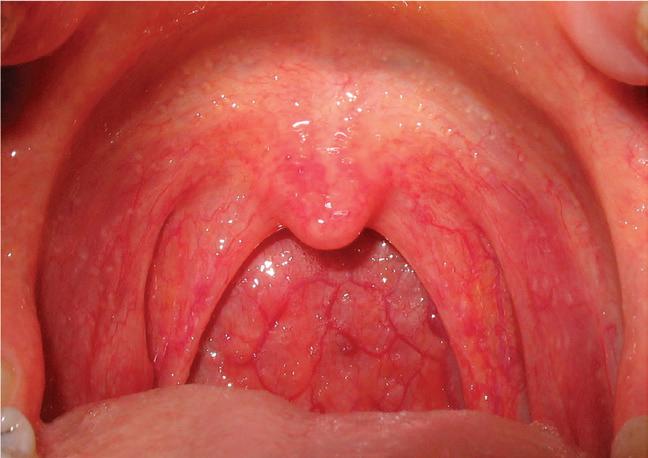
Pathogenicity of a particular virus depends on how a number of factors interact, in particular the number of infectious particles, how they reach the target tissue, the rate of multiplication, the effect of the virus on cell functions, and the host’s immune response. There is significant overlap in aetiology and symptoms of infections, so that even defining the exact syndrome (eg, common cold or influenzalike illness) can be difficult.
The sore throat that results from the common cold is caused by excretion of chemical inflammation mediators (prostaglandins and bradykinin) that stimulate pain nerve endings rather than a true pharyngitis, although cytomegalovirus (CMV) and Epstein–Barr virus (EBV) can cause true pharyngitis (infection and inflammation of the pharynx itself).
Viral pharyngitis
Mucosal infection of the lower respiratory tract leads to destruction of epithelial cells, submucosal hyperaemia, oedema of airways, and may cause a degree of haemorrhage; these will result in the clinical syndrome of viral pneumonia. For a pathogen to cause pneumonia, it must reach the alveolar space and have a large enough inoculum, or overwhelm the host’s already impaired immune system.
Rhinovirus and coronavirus generally do not cause significant damage to the airway cells; thus the common cold is associated with little coughing, whereas the influenza
Syndrome
Common cold
Croup
Influenzalike illness
Pneumonia
Causative viruses
▸ Rhinovirus/coronavirus
▸ Influenza, parainfluenza, enteroviruses, adenoviruses, human metapneumovirus (HMPV), RSV
▸ Parainfluenza viruses
▸ Influenza, RSV
▸ Influenza viruses
▸ Parainfluenza viruses, adenoviruses
▸ Influenza viruses/RSV/ adenoviruses
▸ Parainfluenza viruses, enteroviruses, rhinoviruses, HMPV, coronaviruses
TABLE 1: Acute respiratory tract infections virus may cause more significant damage to the respiratory epithelium (resulting in increased cough with influenza infection).
Diagnosis
Identifying the causative pathogen in viral respiratory tract infections can be problematic. Novel viruses continue to be identified, including bocavirus, WU/KI polyomaviruses, and of course the novel coronavirus, Covid-19.
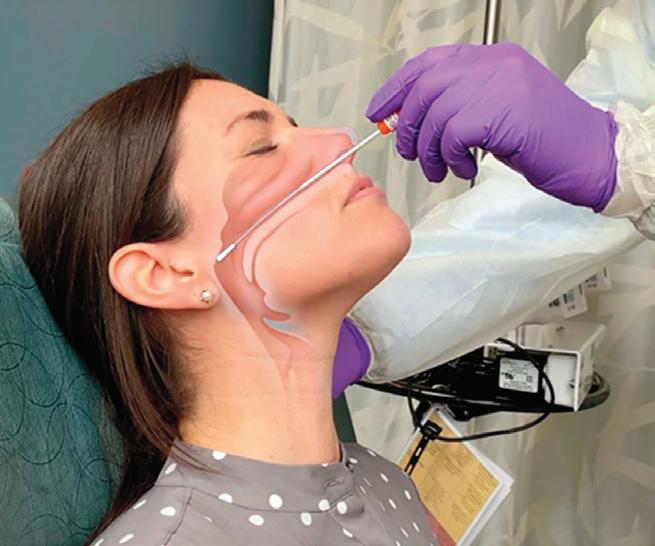
Rapid point of care tests are available, but only for a select few viruses (influenza and respiratory syncytial virus (RSV)), and these are antigen-based. They are less sensitive than laboratory tests.
PCR-based testing may be performed as a multiplex panel or individually (influenza, RSV, herpes simplex virus (HSV) and CMV). This is more sensitive than point of care tests.
For viruses that can be shed for prolonged periods (such as bocavirus, rhinoviruses, and some adenoviruses) or are latent and can reactivate (HSV and CMV), it is again more difficult to confirm a causal role. Thus demonstration of increasing viral load by quantitative PCR may be necessary should the pathogenic role of the virus need to be confirmed. Many viruses may be detected via ELISA (rapid antigen testing) but this may not indicate active disease, and these may coexist with bacterial pathogens.
Treatment
Treatment for viral respiratory infections is usually supportive. Occasionally specific antiviral therapy exists, such as oseltamivir/ zanamivir for influenza (given within the first 48 hours, longer if the illness is severe or the patient immunocompromised) and ribavirin for RSV in immunocompromised patients. Pleconaril was developed for the common cold, but its side effects outweighed the benefits.
Influenza treatments target neuraminidase, an enzyme of the viral capsid that is required for intercellular viral propagation. Oseltamivir and zanamivir are selective inhibitors of this enzyme and may contain infection during peak viral replication period (the first 24-72 hours), thus improving clinical symptoms and reducing morbidity and mortality.
Particularly within the immunocompromised population, ribavirin has been used also against metapneumovirus, and intravenous acyclovir may be considered for treatment of varicella zoster pneumonia in this population.
Antibiotics should not be used as prophylaxis against secondary bacterial infection, with the exception being given to those with chronic lung conditions.
Viral pneumonia
Viruses are the most commonly identified pathogens in severe communityacquired respiratory infections, with 25 per cent of community-acquired pneumonia being viral (this proportion is higher in children due to large proportion of RSV). It is most common in those at extremes of age and in the immunocompromised. It is also of particular concern during pregnancy due to the increased mortality rate even in otherwise healthy women. It is no longer a diagnosis of exclusion, as identification of the causative pathogen may often be obtained via PCR/ELISA testing. Cultures are gold standard but it may take weeks to get a result. The epidemiology of pneumonia is changing due to a number of factors, including introduction of the pneumococcal conjugate vaccine and declining rates of cigarette smoking (both associated with a reduction in the number of pneumococcal pneumonias), and an ageing population (age being a risk factor for viral pneumonia). Older people are 10 times more likely to develop viral pneumonia, which is associated with increased morbidity/mortality. Additionally, viral respiratory pathogens have an increased role in pathogenicity
Chest x-ray of Pneumocystis jirovecii pneumonia in an immunocompromised patient
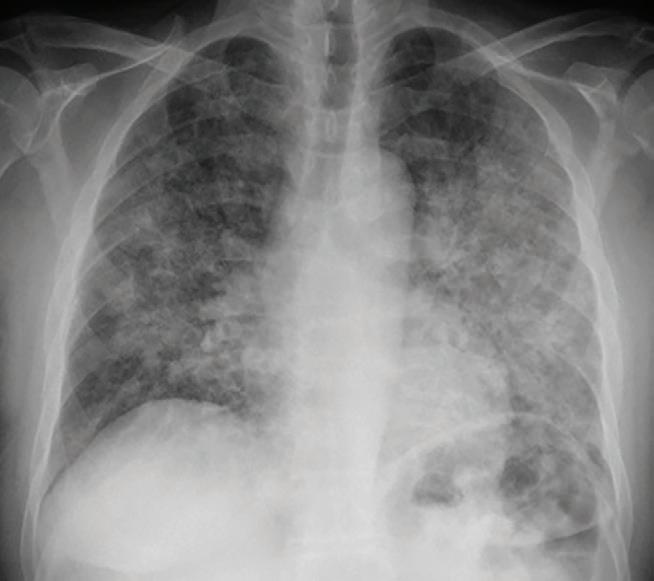
and as cofactors with bacterial pneumonia in the immunocompromised. It is vital to remember that viral pneumonia in the immunocompromised may present very differently, and that imaging may be much more dramatic than in the immunocompetent patient.
The US Centers for Disease Control and Prevention (CDC) Etiology of Pneumonia in the Community (EPIC) study found that 23 per cent of hospitalised patients were positive for viruses, and ICU studies identify viruses in 18-41 per cent of patients. A systematic review including over 10,000 patients identified viral infections in 25 per cent of those with community-acquired pneumonia; this number increased to 44 per cent in studies where >50 per cent had a lower respiratory sample obtained.
Viral pneumonia has multiple aetiologies. It may occur with severe measles/varicella or secondary to generalised infection with EBV or CMV. Influenza is the most common cause across the population. Rhinovirus is commonly identified in patients admitted with community-acquired pneumonia, but the significance of this remains unclear.
Although there are features suggestive of bacterial versus viral aetiology (adult age group, raid onset of illness, elevated white cell count and neutrophilia are suggestive of bacterial aetiology, whereas interstitial bilateral infiltrates rather than lobar alveolar infiltrates suggest a viral cause), there is such significant overlap that these features are not clinically useful.
As testing improves, the question becomes if/when/how to test. Does testing improve antimicrobial stewardship and allow early commencement of antiviral therapy? Or merely identify pathogens of uncertain clinical significance and increase costs? In multiple observational studies of influenza patients, viral testing was infrequently found to alter management. In one study 75 per cent of patients with PCR-confirmed influenza continued to receive antibiotics, and in another over 60 per cent of low-risk patients with a positive rapid viral test continued to receive antibiotics. The clinical significance of a positive respiratory viral test result remains unclear.
Rapid tests for biomarkers of bacterial versus viral infection are increasingly being looked to in an attempt to address these issues. These include: ▸ CRP; ▸ Procalcitonin; ▸ TNF-related apoptosis-inducing ligand (TRAIL); ▸ IP-10; ▸ Myxovirus resistance A (MxA).
CRP
CRP is an acute phase reactant. Its production is stimulated during infection or tissue inflammation, when interleukin-6 (IL-6), interleukin-1b (IL-1b), and tumour necrosis factor-alpha (TNF-a) stimulate hepatocytes to synthesise CRP.
CRP levels tend to be much higher in bacterial infections than in those of viral aetiology, and due to its short half-life serial measurements may also be used to ensure appropriate response to therapy. However, a moderately elevated CRP (1060mg/l) may also be seen in viral infections (particularly those caused by influenza virus and adenovirus), and as such there is significant overlap between viral and bacterial aetiologies. In systematic reviews it has been found to be neither sufficiently sensitive to rule out nor sufficiently specific to rule in a bacterial cause for an infiltrate on chest x-ray. CRP peaks between three-tofive days after a viral challenge; thus raised CRP values from viral infection during the first seven days may lead to mistaken prescription of antibiotics. However, CRP may also be more specific for predicting bacterial aetiology after the first week, when elevated levels may suggest secondary bacterial confection.
Procalcitonin
Procalcitonin is an acute phase reactant released by leukocytes. As viral infections are less likely to result in a leukocytosis, acute phase reactants are thought to be a marker of bacterial aetiology. Production of procalcitonin requires circulating tumour necrosis factor (TNF-alpha); in viral infections, macrophages produce interferon-alpha (INF) that can inhibit TNF-alpha and so suppress elevation of procalcitonin; thus viral infections tend not to increase procalcitonin.
However, a meta-analysis of 12 studies (over 2,000 patients) found that both sensitivity and specificity of procalcitonin as a determinant of viral versus bacterial aetiology were too low and too variable to be used for decision-making.
TRAIL and IP-10
TNF-related apoptosis-inducing ligand (TRAIL) and interferon-gamma-inducedprotein-10 (IP-10) have been shown to predict viral infection, particularly in combination. Both are induced in viral infection and suppressed in bacterial infection, thus elevated levels are suggestive of viral aetiology.
Myxovirus A (MxA)
Myxovirus resistance genes mx1 and mx2 belong to the class of IFN-stimulated genes that are induced by viruses. After viral infection, IFNs bind with their receptors on the surface of uninfected cells, which activates mx1 and mx2. These then produce antiviral proteins MxA and MxB. The MxA protein is selectively increased in patients with viral infections and may therefore be useful biomarkers for viral infection.
Combination tests
Newer combinations of viral-induced proteins that complement routinely used bacterial-induced proteins (such as CRP and procalcitonin) are being developed to increase capacity to accurately distinguish between viral and bacterial aetiologies. Studies of combinations of MxA and CRP have demonstrated the capacity to correctly identify 92 per cent of patients without infection, 80 per cent of patients with confirmed bacterial infection, and 70 per cent of patients with established viral infection. A combination of CRP + TRAIL + IP-10 has been shown in several studies to have a significantly greater efficacy in the differential diagnosis of bacterial and viral infections than not only individual biomarkers (CRP, procalcitonin, white cell count, IL-6), but also currently used combinations of these biomarkers.
Post-viral cough
Viral infections result in an immune response that in particular stimulates T-lymphocytes. Th2 response leads to wheeze and airway inflammation. Postviral cough is a very common complaint presenting to medical practitioners. It may last three-to-eight weeks, and is the result of the inflammatory response, resulting in bronchial hyper-responsiveness, mucus hypersecretion, and impaired mucociliary clearance. When the larynx is inflamed and hyperreactive, coughing may continue to occur in response to stimuli that would not generally cause coughing (such as cold air). A spectrum exists between viral-induced post-viral cough and exacerbations of asthma following viral infection. Stimulation of allergic pathways in susceptible individuals may continue even after the acute viral infection has resolved. Viral respiratory tract infections may cause wheeze in many asthmatics and are the underlying cause for more than half of acute exacerbations of asthma. Even following a simple rhinovirus infection, allergen-induced production of histamine and late-phase reactions secondary to eosinophil activation may persist for fourto-six weeks.
Pure post-viral cough remains a diagnosis of exclusion. Imaging will be normal. Treatment (if required), includes an antihistamine, bronchodilators, and inhaled corticosteroids. Occasionally oral corticosteroids may be trialled.
Summary
Multiple viruses exist that may cause a number of syndromes. Diagnosis is usually clinical, although there is an increasing push for a specific diagnosis in order to reduce the use of antibiotics. Significant cross over exists between viral and bacterial aetiologies, and current research is focused on ways to differentiate between the two. Unusual presentations may occur in the immunocompromised. Few treatment options are available other than supportive care. Finally, post-viral cough is common and may present similarly to asthma, although it should resolve completely (but may take several months). n
References on request
Positive developments within the field of cystic fibrosis
AUTHOR: Sarah Tecklenborg, Senior Research and Policy Coordinator, Cystic Fibrosis Ireland
At the recent European Cystic Fibrosis Society conference, held online on 24-25 September, Prof Ed McKone, Consultant Respiratory Physician, St Vincent’s University Hospital, Dublin, outlined the evolving natural history of cystic fibrosis (CF) in Ireland, the clinical characteristics of CF at the different life phases and the treatment challenges that arise therein.
There have been large improvements over the last 60 years in CF survival. Many of these improvements were being seen even before the advent of CFTR modulators – the cystic fibrosis transmembrane conductance regulator modulator therapies, which are designed to correct the malfunctioning protein made by the CFTR gene in people with CF. Much of the improvements in survival and life expectancy have been attributed to antipseudomonal antibiotics, improved nutrition, the expertise of the multidisciplinary care teams and our ability to detect CF early and begin treatment.
New research published in the European Journal of Human Genetics has shed light on some of the outcomes over 6.5 years of the inclusion of CF in the Newborn Screening Programme in Ireland. The overall incidence of CF was found to be onein-2,570 births, with an estimated carrier frequency of one-in-25.3. This is lower than the previously reported one-in-19 carrier frequency in Ireland.
The likely cause of this decrease in incidence in the Republic of Ireland is increased ethnic diversity arising from inward migration. The authors recommended that in the case of counselling for a couple of Irish origin, the carrier rate of one-in-19 should still be Prof Eileen Savage

used. Ireland continues to have one of the highest incidences of CF in the world.
Transition
Prof McKone noted the decline in physical markers of health experienced by people with CF in adolescence and early adulthood. The transition from paediatric to adult care and greater selfmanagement of their condition is a critical time for people with CF. This period is characterised by a decline in lung function and nutritional status, increases in the detection of pseudomonas and MRSA bacteria, and is associated with increasing rates of depression and anxiety.
In a joint Cystic Fibrosis Ireland (CFI) and Health Research Board-funded study, Vice Dean for Graduate Studies in the College of Medicine and Health in University College Cork (UCC) Prof Eileen Savage (PhD) and colleagues from UCC used a multivariate analysis to examine the association between socio-demographic variables (age, sex), physical (FEV1, BMI) and mental health variables and healthrelated quality-of-life (HRQOL) among a sample of adolescents and adults with CF aged >14 years in Ireland. The study found that mental health variables, specifically depression and anxiety, were more strongly associated with HRQOL than physical variables. The results indicate that as CF disease progresses and physical health declines, maintaining mental health is essential to ensure people with CF have the highest HRQOL possible.
Dr Jennifer Cronly (PhD), School of Nursing and Midwifery, UCC, and Prof Savage also investigated the role of agency in empowering young people to take control of their own disease management during adolescence. Their research highlighted the threats negative interactions with healthcare professionals may have for this patient group in the development of agency. The authors noted the need for respect and encouragement of young people as they go through the process of transition and to allow adolescents to have an active role in the decision-making process around their own care.
Covid-19
Mental health concerns are not only prevalent during adolescence, but throughout the life course for people with long-term health conditions. The coronavirus pandemic has had an unprecedented impact on those with underlying health conditions including people with CF. In the early period of the pandemic, people with CF were considered extremely medically vulnerable and were advised to cocoon for their own safety. This caused high levels of stress and concern.
A survey undertaken by CFI in late March 2020 identified high levels of
anxiety among people with CF and their carers, and observed major concerns about the unknown health implications of coronavirus and CF, fears of possible medication shortages or supply issues, and the mental health and practical implications of cocooning. In light of new data on the nature of Covid-19 disease and CF, people with CF are now considered high risk from Covid-19 if their CF is stable, and extremely medically vulnerable if their CF is unstable or they are post-transplant. While data has shown that coronavirus is not as severe in people with CF as originally feared, it is not a benign disease.
Data from the European Cystic Fibrosis Registries up to 18 September identified 138 cases of Covid-19 among people with CF. Where information was known, 20 per cent (n=27) of these infections were asymptomatic and 79 per cent (n=102) were symptomatic. Among symptomatic patients, 60 per cent required hospitalisation, 16 per cent of these were treated in ICU and sadly 25 per cent (n=4) of those in ICU died. The incidence of Covid-19 among people with CF in the EU was 0.2 per cent with a 2.9 per cent case fatality rate. Both of these are lower than the incidence and case fatality rate among the general population in the EU for the same time period (4.5 per cent and 0.5 per cent respectively). This may reflect the enhanced precautions taken by people with CF and their family members and carers to prevent infection.
These enhanced precautions, and the duration for which people with CF have had to endure them, may not come without consequences. Prof Patricia Fitzpatrick, Professor of Epidemiology and Biomedical Statistics in University College Dublin, is currently leading a research project to assess the impact the Covid-19 pandemic and cocooning have had on the daily lives of people with CF. The research will investigate how people with CF and their parents/carers have managed their daily lives throughout the pandemic; assess their access to hospital visits and routine CF care; examine precautions taken by them; and report on the impact on mental health and the stress management techniques used to cope.
Ivacaftor/tezacaftor/elexacaftor
While Covid-19 has cast a grey uncertainty across the future, for many people with CF the horizon is looking brighter than ever. On 21 August, it was announced that the European Commission had granted marketing authorisation for Kaftrio (ivacaftor/tezacaftor/elexacaftor) in a combination regimen with ivacaftor to
treat people with CF aged 12 and over with one F508del mutation and one minimal function mutation, or two F508del mutations in the CFTR gene. Thanks to the innovative ‘Portfolio Agreement’ signed in 2017 between the HSE and Vertex Pharmaceuticals, the treatment regime is now available to eligible patients in Ireland.
Ms Sally Ann Lynch and Ms Tara Clark, Department of Clinical Genetics, Children’s Health Ireland at Crumlin, suggest that up to 95 per cent of people with CF may be eligible for a modulator therapy in Ireland based on their genotypes. A recent European study using CF registry data (currently unpublished) goes further and found that up to 97.7 per cent of adults with CF in Ireland will be eligible for at least one of the modulator drugs – this would be the highest proportion of eligible adults throughout Europe.
RECOVER
Ivacaftor/tezacaftor/elexacaftor has been shown to be effective in multiple clinical trials, however, the real-world impact of this drug on the lives of people with CF may be even greater. The RECOVER project, led by Prof Paul McNally, Associate Professor of Paediatrics, RCSI, will investigate the real-world effect of the new CF medications on clinical outcomes in people with CF. The project is funded by the Cystic Fibrosis Foundation in the US (€2.85 million), the CF Trust (UK) (£112,000), and Cystic Fibrosis Ireland (€100,000). RECOVER is a multi-centre cohort study, which will take place in eight sites across Ireland and the UK over a three-year period.
It will collect information on a range of health data and clinical end-points such as lung clearance index, chest CT, gastrointestinal symptoms and inflammation, and medication adherence. ‘Real-world’ studies examine how drugs impact on a broad range of patients, many of whom would have been excluded from the initial clinical trials due to poor underlying health or the existence of comorbidities. The data can confirm trial findings and shed light on the overall impact of the drug on the day-today lives of people with CF and not just on a limited range of biomarkers or a specific cohort.
With more and more people with CF living for longer, clinicians are having to detect and treat current and emerging ageing comorbidities in this group, including those already known to be associated with older age and those possibly resulting from long-term use of new therapies. People with CF are also turning their minds to the need for better educational opportunities, long-term careers and pension planning. A normal life is becoming less of an extraordinary ambition for people with CF.
Cystic Fibrosis Ireland is committed to leaving no one behind in the quest for improved quality-of-life for all people with CF.
Non-tuberculous mycobacteria: An overview
AUTHORS: Dr David Quigley, Respiratory Registrar; and Prof Anne Marie McLaughlin, Respiratory Consultant, St James’s Hospital, Dublin
Non-tuberculous mycobacteria (NTM) are ubiquitous organisms found in the environment. NTM comprises over 160 subspecies. Data from 2013 identifies Mycobacterium avium complex (MAC) as the most frequently isolated form of NTM in Europe with a prevalence of 38 per cent of all NTM. A total 43 per cent of all NTM in Ireland represented MAC in subgroup analysis of this data, with only Sweden (67 per cent) and Germany (55 per cent) having higher levels in Europe. It predominantly affects northern European over southern European countries. Transmission is generally based on complex host-pathogen factors not yet fully understood. The (Mycobacterium) M.kansaii, M.xenopi, M.almonese, and M.abscessus subspecies are encountered less frequently in Europe but are common in other parts of the world. They have been identified in soil, shower heads and water reservoirs. There is also data for humanhuman transmission with M.abscessus in individuals with cystic fibrosis (CF). NTM have the potential to cause multisystem disease in both humans and animals.
For the purpose of this review we will be concentrating on pulmonary disease as the most common presentation of NTM and one with which we are familiar in treating.
Host genetic and immune factors are thought to play a key role in susceptibility to NTM disease. Those with underlying lung disorders are at risk, particularly those with bronchiectasis, CF and in some cases severe emphysema. Other individual risks include low socioeconomic backgrounds, poor nutritional status and HIV, particularly in disseminated disease. It affects women significantly more than men overall. The Ontario study identified 74 per cent of cases to be women. MAC
has an even higher incidence in these patients with the overall phenotype previously referred to as ‘Lady Windermere syndrome’. These tend to be elderly, nonsmoking Caucasian females.
One study indicated patients with NTM have more low-frequency, proteinaffecting variants in immune, CFTR (cystic fibrosis transmembrane regulator), cilia, and connective tissue genes than their unaffected family members and control subjects. The authors suggest this may be related to the development of bronchiectasis initially and subsequent development of NTM due to further acquired immunodeficiency. Genetic variations in MST1R has been identified when associated with pectus excavatum and scoliosis. Heterozygous mutations in the CFTR gene have been associated with the disease relevant to an Irish population. A modest reduction in interferon-γ production and an increase in transforming growth factor (TGF)-β levels have been described.
In terms of true incidence of NTM, regional and global geographical factors are likely at play but universally rates seem to be increasing. In Europe, a study in the UK identified a rising incidence of NTM isolation driven almost exclusively by an increase in MAC disease with incidence rising from 5.6 per 100,000 to 7.6 per 100,000 over the period 2007-2012. Ringshausen et al reported a prevalence of 2.3 per 100,000 for NTM pulmonary
disease in 2009 in Germany rising to 3.3 per 100,000 in 2014. A concerning incidence of up to 14.5 per cent of NTM in CF patients has been reported in one study.
Improved diagnostic methods and increased physician awareness might have led to such increase in NTM infection. It has been suggested that its incidence is increasing due to ageing populations and immunosuppression. NTM disease has been associated with immunosuppressive medications such as azathioprine, cyclophosphamide, mycophenolate and cyclosporine, as well as with anti-TNFα agents. This raises questions for clinicians in screening for NTM before initiation of these therapies. Data from Denmark indicates patients with COPD on inhaled corticosteroids (ICS) have three-times the risk of developing NTM than those on non-steroid based therapy. Increased risk of NTM has also been seen in patients with asthma with long-term use of ICS. Those with obstructive lung disease and worsening infective symptoms should be considered for further investigation if there is any clinical/ radiological concern for NTM.
Classification of NTM
Runyon initially classified mycobacteria based on their growth patterns (rapid versus slow), whether they produced yellow pigment or not and if this was produced in the dark or only after light exposure. Class 1-3 where defined as slow growers with Class 4 rapid growers. Class 1 (photochromogens) produce a yellow pigment when exposed to light and most commonly include subtypes M.kansaii and M.simiae. Class 2 (scotochromogens) produce a yellow pigment in either light or dark with M.Gordonae the most identifiable subspecies here. Class 3 (nonchromogens) never produce pigment and includes MAC. Rapid growers include M.abscessus, which is especially pathogenic in CF patients.
Clinical presentation
Clinically, patients with NTM present with pulmonary symptoms such as productive cough, dyspnoea and occasionally haemoptysis which may give concern for TB. Constitutional symptoms include night sweats, weight loss, loss of appetite and general decline. Cavitary disease may be difficult to differentiate radiologically from TB in both conditions. Patient factors including birthplace, age, and presence of underlying lung disease have been shown to be helpful in differentiation. An interferon-gamma release assay is typically positive in TB and negative in NTM. Clinical symptoms can be minor including mild cough and mild fatigue. The NTM diagnosis may only become apparent after incidental radiological findings consistent with the disease with symptoms correlated retrospectively.
Diagnosis
As per the American Thoracic Society (ATS) guidelines, the diagnosis of NTM encompasses clinical (as described above), microbiological and radiological evidence. An isolated microbiological specimen does not necessarily indicate lung disease but can reflect colonisation or specimen contamination. The microbiologic criterion includes positive culture results from either: A minimum of two separate expectorated sputum samples, one bronchial wash/lavage (BAL) or a transbronchial/other lung biopsy with mycobacterial histopathologic features. Bronchoscopy with BAL is performed with patients off all antibiotic therapy when done for suspected NTM. If clinically suspected, NTM should be excluded before initiating long-term macrolide therapy in those with bronchiectasis to prevent future resistance.
Radiologically, the ATS criteria requires an observation of nodular or cavitary opacities on a chest x-ray or a CT scan that shows multifocal bronchiectasis with multiple small nodules. In more recent guidelines, the radiographic criterion has been interpreted to demand one-of-two different radiographic manifestations: Fibrocavitary or nodular bronchiectatic disease. Fibrocavitary disease demonstrates cavitary lesions predominantly in the upper lobes while nodular bronchiectatic disease presents as multifocal bronchiectasis, clusters of small nodules, and branching linear structures that commonly involve the right middle lobe and the lingular segment of the left upper lobe. The latter is more common in older, thin females. Mixed patterns are also seen. CT scanning is the modality of choice for assessing the anatomical evidence of disease in detail.
Treatment
In August of this year (2020) new ATS/ ERS/ESCMID/IDSA comprehensive guidelines were published. This group refer to the fact that although overall they recommend treatment in those with NTM disease, patient factors should be considered. This list is complex and nonexhaustive. Clinically, disease progression can vary amongst patients independent of perceived microbiological or radiological severity. Propensity for recurrence and reinfection despite appropriate treatment is high, especially in cases of nodular/ bronchiectatic disease, with rates of up to 50 per cent quoted in one study of 180 patients at four-year follow-up. There is evidence from Korea that up to half of patients with stable MAC disease that had not progressed clinically or radiologically at three years become culture negative during a six-year follow up period. On subgroup analysis, younger patients with higher BMI and AFBnegative smear disease were more likely to convert spontaneously. Conversely,
systemic symptoms including fever/fatigue, increased age, and low BMI were less likely and smear-positive patients were less likely to sputum convert.
The recent ATS/ERS/ESCMID/IDSA clinical guidelines for treatment of MAC encompass three regimens depending on clinical severity/radiological appearance (nodule versus cavitating) and previous treatment of disease. This has not changed significantly from previous ATS guidelines. For non-severe nodular/bronchiectatic disease it is recommended a macrolide, ethambutol and rifampicin are taken three times a week. For cavitary and recurring or clinically severe disease the above medications are given daily with the recommendation for an IV aminoglycoside for the first two-tothree months.
When considering a patient for treatment the disease severity, type of infectious species and patient preferences/ characteristics are evaluated in detail. Poor prognostic factors include cavitary disease, low BMI, low albumin, and/or elevated inflammatory markers at time of diagnosis. Cases in which severe clinical symptoms affecting overall health and functional status would be important to consider treatment. Those with baseline immunosuppression should also be considered for early intervention. Rapid radiological progression on follow up and decline in pulmonary function at follow up is also associated with poor prognosis and favours early treatment.
Duration of treatment and drug regimens vary depending on specific organism and susceptibility profile. In most cases treatment is continued until a patient is sputum negative for 12 months. In terms of specific microorganisms, more virulent and treatment-responsive forms of NTM such as M.kansaii favour a trial of treatment. In patients with M.kansasii pulmonary disease, we perform susceptibility-based treatment for rifampicin over empiric therapy as resistant disease has been associated with treatment failure. In patients with MAC and M.abscesses pulmonary disease a ‘watch and wait’ approach may sometimes be more appropriate. Susceptibility-based treatment for macrolides and amikacin over empiric therapy again is favoured in all organisms based on evidence of treatment failure in resistant disease. Close liaison with microbiology colleagues is important.
Full transparency regarding the risks and benefits of treatment is important in discussion with patients. NTM poses specific difficulties due to the variable treatment response clinically, duration of treatment, and unpredictable recurrence. Side effects of medications can be serious. Rifampicin is associated with hepatic dysfunction and amikacin has been associated with renal dysfunction. Regular monitoring of liver and renal function tests is crucial at two-to-three monthly intervals. Macrolides can cause cardiac arrythmias, so baseline ECG is important for QTC evaluation. Visual loss and ototoxicity are reported with ethambutol/ macrolides and aminoglycosides respectively. When concerns around adherence are present due to cognitive, social or functional impairment, a watch and wait approach is more appropriate. The risks of taking medications intermittently increases resistance profiles with no treatment preferable in these cases. Current ATS guidelines recommend treating until 12 months post-culture conversion. Sputum cultures are repeated at three-monthly intervals post treatment to ensure they remain negative, however, there is an argument that clinical and radiological improvement could be used rather as treatment endpoints.
Surgery has also been described in the literature as an adjunct treatment for NTM. ATS recommends it only for those with localised disease, limiting rate of progression, or refractory disease. One review collaborates positive data from several studies indicating it may still have a role, however, it is mostly reserved for those with resistant disease poorly responsive to medical therapy. Paradoxically, resistant disease is usually too widespread for surgical consideration by the time it is identified.
Inhaled amikacin can be used in treatment of NTM as per recent guidelines. Efficacy comes from five retrospective case series (138 patients) of treatment-refractory cases with no comparator arm. Sputum conversion ranged from 18-67 per cent and clinical response from 20-100 per cent depending on the series.
Novel inhaled therapies are currently being researched for NTM. Clofazimine inhalation suspension has showed promise in mouse models with concentrations in the lungs four times greater than equivalent oral dosing. Inhaled ciprofloxacin has showed some early promise in bronchiectasis treatment, but data for NTM at this stage is lacking.
Conclusion
NTM is increasing in incidence worldwide and is a common presentation in our centre. We believe treatment for NTM should be made after longitudinal assessment. Despite guidelines for radiological, clinical and microbiological characteristics that would support treatment, data in the literature can be conflicting due the variable nature of the disease. The right decision for a particular patient may prove difficult depending on their demographics. Basic medical ethical principles of beneficence, non-maleficence and informed consent are particularly relevant to this illness
Key learning points
NTM prevalence is increasing. Most common subtype in Ireland is
MAC infection. Clinical/microbiological/radiological and patient criteria determine severity and need for treatment. Novel inhaled therapies with lower side effect profiles and good efficacy may provide future options. ■
References on request
An overview of alpha-1 antitrypsin defi ciency in Ireland
AUTHORS: Dr Daniel Fraughen,1 Dr Tomás P Carroll,1,2, Geraldine Kelly,2 and Prof Gerry McElvaney,1 1Irish Centre for Genetic Lung Disease, RCSI Education and Research Centre, Beaumont Hospital, Dublin; 2Alpha-1 Foundation Ireland
The fi eld of alpha-1 antitrypsin defi ciency (AATD) has undergone exciting new changes in recent years with two clinical trials of novel oral medicinal compounds currently being undertaken in Beaumont Hospital, Dublin, to help those most severely a ected by the condition.
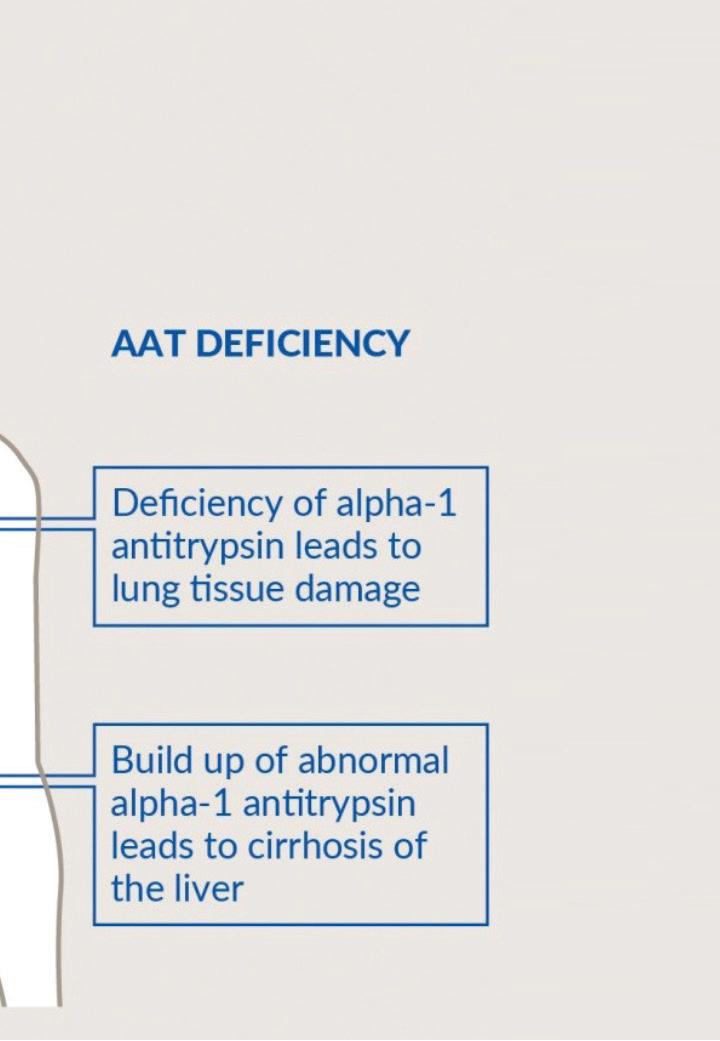
Alpha-1 antitrypsin (AAT) is an anti-protease, which is primarily produced in the liver and circulates in the blood (Figure 1). Its main site of action is in the lungs where it binds to neutrophil elastase (NE), inhibiting it in the process. If NE remains unbound due to AATD; it can result in premature emphysema. This manifests clinically as chronic obstructive pulmonary disease (COPD). The risk of COPD is greatly enhanced by the presence of environmental and cigarette smoke exposure.
The production of AAT protein is controlled by the SERPINA1 gene. Each person has two copies (alleles) of the SERPINA1 gene, which is located on the long arm of chromosome 14. The SERPINA1 alleles are co-dominant and are equally responsible for producing their own set of AAT protein. The protein produced by the normal SERPINA1 allele is called FIGURE 1: The role of AAT in normal and de cient individuals
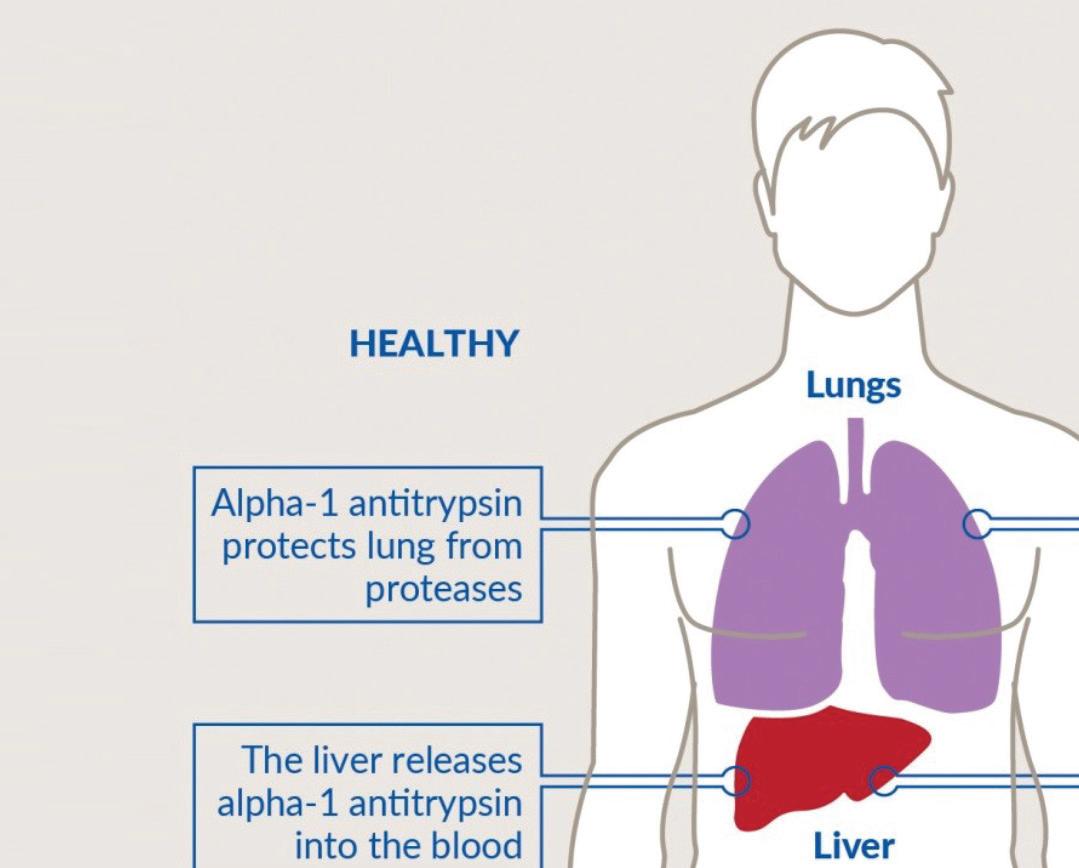
‘M’ AAT protein. If a person has two normal SERPINA1 alleles, then they will only produce M AAT protein – and their AAT phenotype will be MM.
Over 100 mutations in the SERPINA1 gene exist and cause the production of a variety of abnormal AAT variants with some quantitatively and/or qualitatively defective (Figure 1). Abnormal variants of
the AAT protein can be analysed by a process called isoelectric focusing or ‘phenotyping’ and this is how AATD can be diagnosed (Figure 2). Simply, AAT protein variants are separated on an agarose gel by electric current. Di erent variants of AAT protein travel through the agarose gel at di erent speeds and abnormal AAT protein variants are assigned di erent letters based on their migration speed. Common variants include
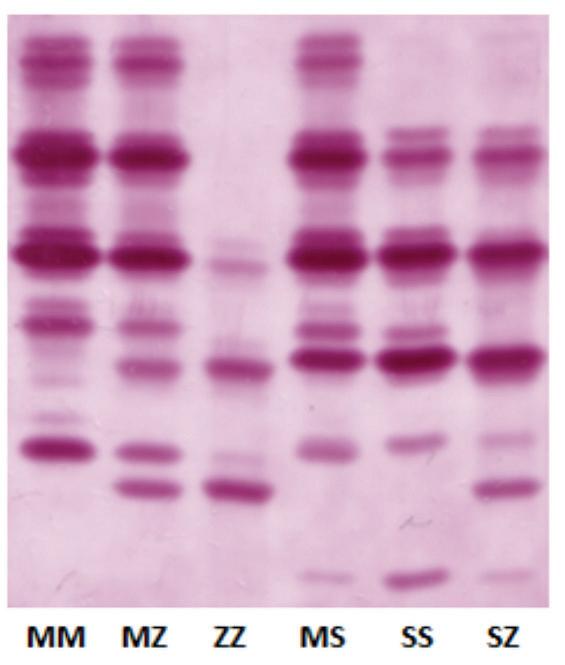
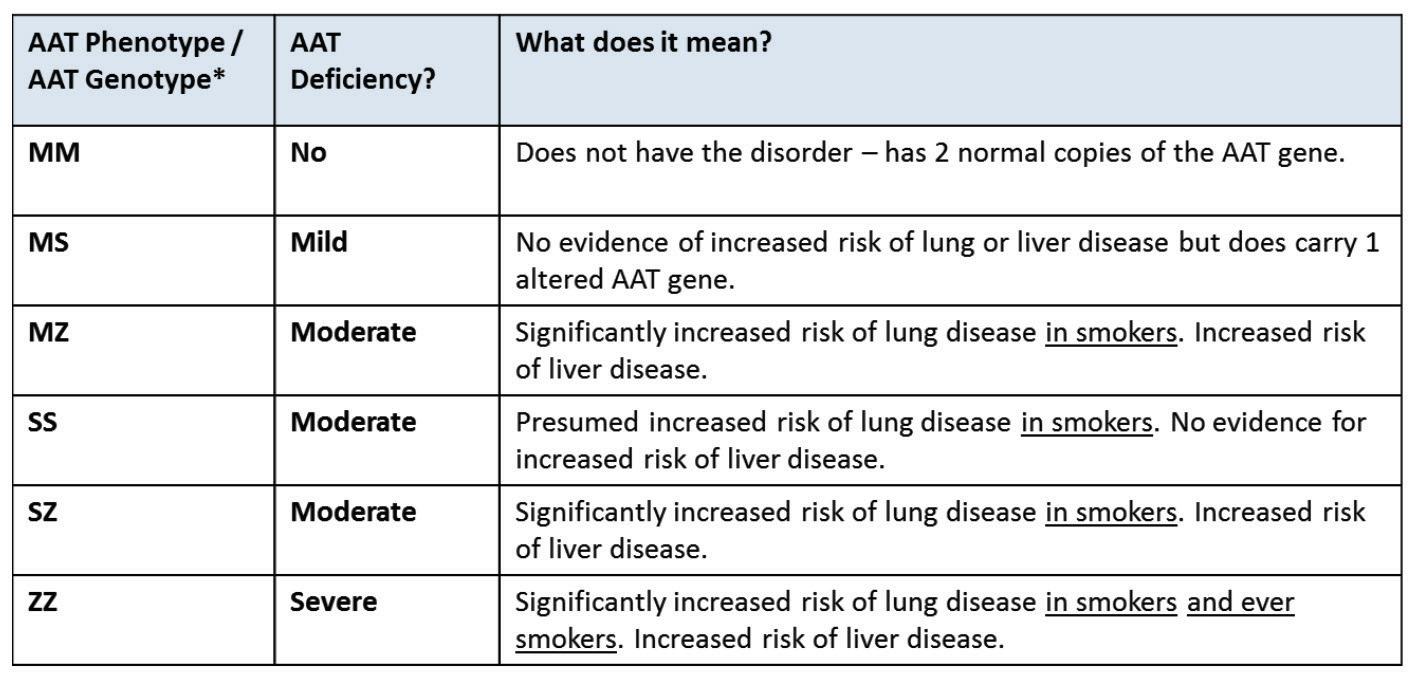
FIGURE 2: (LEFT) Typical isoelectric focusing gel used to diagnose AATD. The six most common phenotypes are shown with ZZ causing the most severe serum AAT de ciency (reduced by 80-85%) and posing the highest disease risk. (RIGHT) List of common AAT phenotypes and simple guide to risk (Note: phenotype reported when testing is on serum, genotype reported when testing is on DNA).
FIGURE 3: Typical AATD family tree. ZZ (severe AATD), MZ (moderate AATD), and MM (una ected) phenotypes are shown
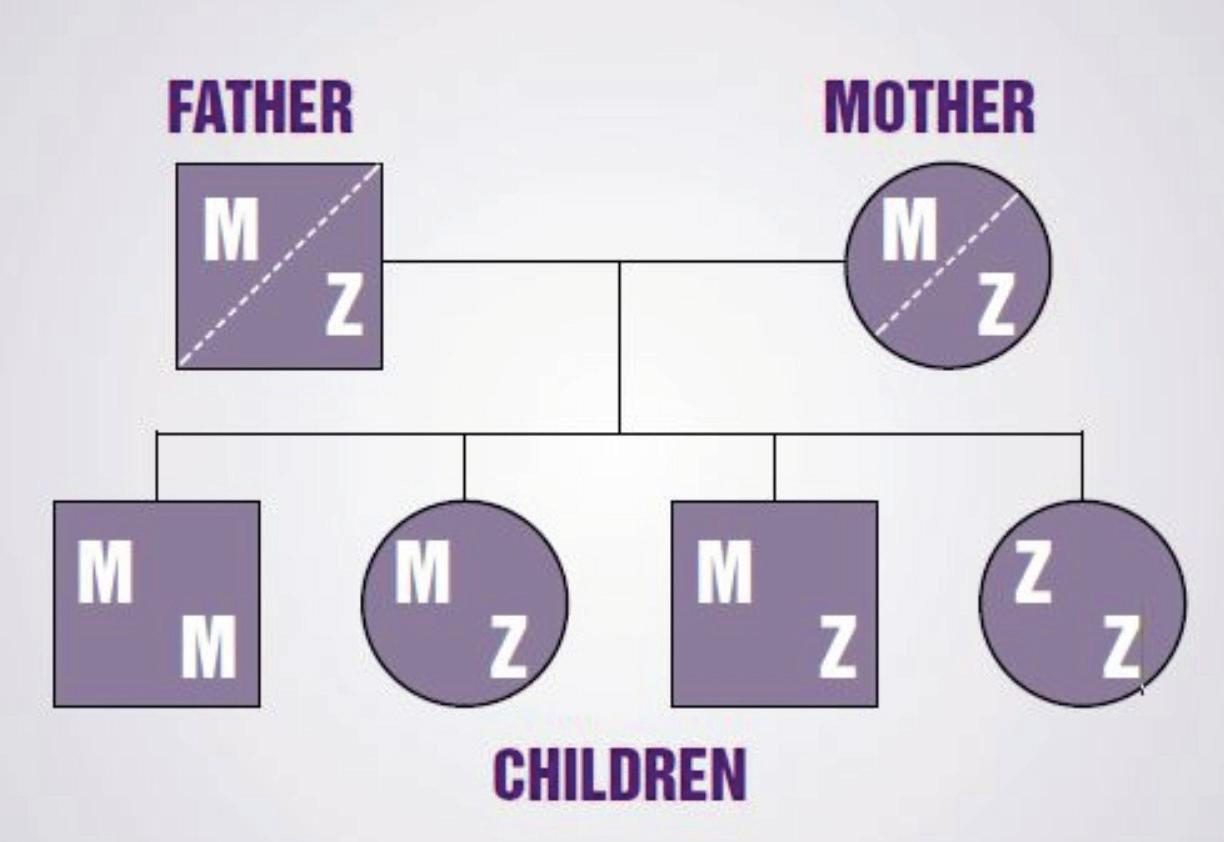
the ‘F (fast)’, ‘S (slow)’ and ‘Z’ AAT variants. As the SERPINA1 alleles are co-dominant; this can result in a spectrum of phenotypes of AAT protein such as MS (minimal risk), MI, MZ (moderate risk), IS, SS, SZ (moderate risk) and ZZ (severely a ected) phenotypes, for example, (Figure 3). It should be noted that extremely rare defi cient mutations exist where no detectable AAT protein is produced and these are called ‘null’ or ‘Q0’ mutations. In Ireland, for example, we have discovered two novel null mutations, named Q0dublin and Q0cork.
AATD is very common in Ireland. It is known from prevalence data that approximately one-in-25 carry a Z mutation and one-in-10 carry an S mutation on the island of Ireland (Carroll et al, 2011). Extrapolating from these frequencies; it is estimated that there are 3,000 patients with ZZ AATD in Ireland and a further 12,000 with SZ AATD. Other less severe phenotype combinations of AATD exist and while these have a lower disease risk; they can be clinically signifi cant in smokers where the risk of COPD is increased (eg, MZ) (Molloy et al, 2014). More information on this can be found at www.alpha1.ie.
Patients with AATD are classically diagnosed due to family screening, respiratory symptoms or mild hepatitis, but it is important to recognise that AATD can present to an outpatient clinic or a GP practice with a number of di erent and interesting clinical manifestations such as panniculitis (erythema nodosum), ANCAassociated vasculitis, cirrhosis, bronchiectasis, and di cult to control asthma. In patients with these conditions, it is worth considering and out-ruling a diagnosis of AATD.
To date in Ireland the national targeted detection programme has tested over 20,000 people for AATD. A total 380 patients with ZZ AATD have been identifi ed through this programme. This means there are approximately 2,600 people with severe AATD left to be identifi ed. These patients will miss out on opportunities for specialist care and behavioural change, but also to engage with clinical trials that are being conducted at the National Centre of Expertise for AATD at Beaumont Hospital.
The indications for screening for AATD follow World Health Organisation (WHO) and American Thoracic Society/ European Respiratory Society (ATS/ERS) recommendations and these include: All individuals with COPD/airfl ow obstruction (regardless of age/ethnicity/ smoking status). All individuals with unexplained liver disease, including neonates and the elderly. Parents, siblings and children of those with AATD. All individuals with asthma and airfl ow obstruction that is incompletely reversed by treatment. All individuals with unexplained bronchiectasis. All individuals with necrotising panniculitis. All individuals with ANCA-associated vasculitis (granulomatosis with polyangiitis).
A synopsis of these guidelines can be found on the Alpha-1 Foundation Ireland website at www.alpha1.ie.
Previously conducted research in the RCSI
A substantial amount of research has been conducted in the RCSI under Prof Gerry McElvaney in the past 20 years trying to understand the AAT protein and how its defi ciency a ects our health.
Previous family-based research has investigated to what degree AATD a ects those who are not yet symptomatic. A person who is known to have AATD invites their family members to be screened and to undergo spirometry in order to assess how AATD a ects those who have not yet presented themselves to a doctor for respiratory reasons.
These family-based studies were carried out in the past with the valuable assistance of a
large community of participants with MZ and SZ AATD. The SZ family study has led to a number of very important fi ndings about AATD. Through research carried out with the help of 44 Irish families containing at least one member with SZ AATD, it was found that SZ never-smokers demonstrated no increased risk of COPD when compared to their neversmoking MM and MS relatives (Figure 4, left). This has led to a challenge of the historic assumption that SZ AATD was a ‘severe’ phenotype and serious enough to require intravenous AAT replacement therapy.
A history of smoking combines with the SZ genotype to signifi cantly increase the severity of COPD when compared to ever-smoking MM and MS relatives (Figure 4, right). This means that smoking cigarettes (or long periods of exposure to workplace chemicals, dusts and fumes) is required before signifi cant lung disease will develop.
Furthermore, the SZ family study also found that a history of smoking alone is not associated with a greater rate of lung function decline, suggesting that stopping smoking prevents lung disease from getting worse. In real life terms, the knowledge is reassuring for
people with SZ AATD as it means their risk of lung disease is not the same as a more severe form of the condition.
People with the MS phenotype of AATD were no di erent to people with two normal copies of AAT (MM). This is reassuring for the large numbers of people who are MS (one-in-10 in Ireland are MS) as this means there is no evidence for any increased risk of lung disease due to this type. Many of these observations have been confi rmed in other large studies outside of Ireland.
The RCSI is currently leading further research projects in Ireland in trying to clarify the exact risk AATD poses to our population’s lung health, given the myriad ways AATD can a ect the lungs.
The future of AATD research
When a patient is referred to our National Centre of Expertise for AATD, they are given expert advice about their condition and given the opportunity to take part in our AATD registry.
Involvement in the AATD registry is of particular benefi t to patients who attend our
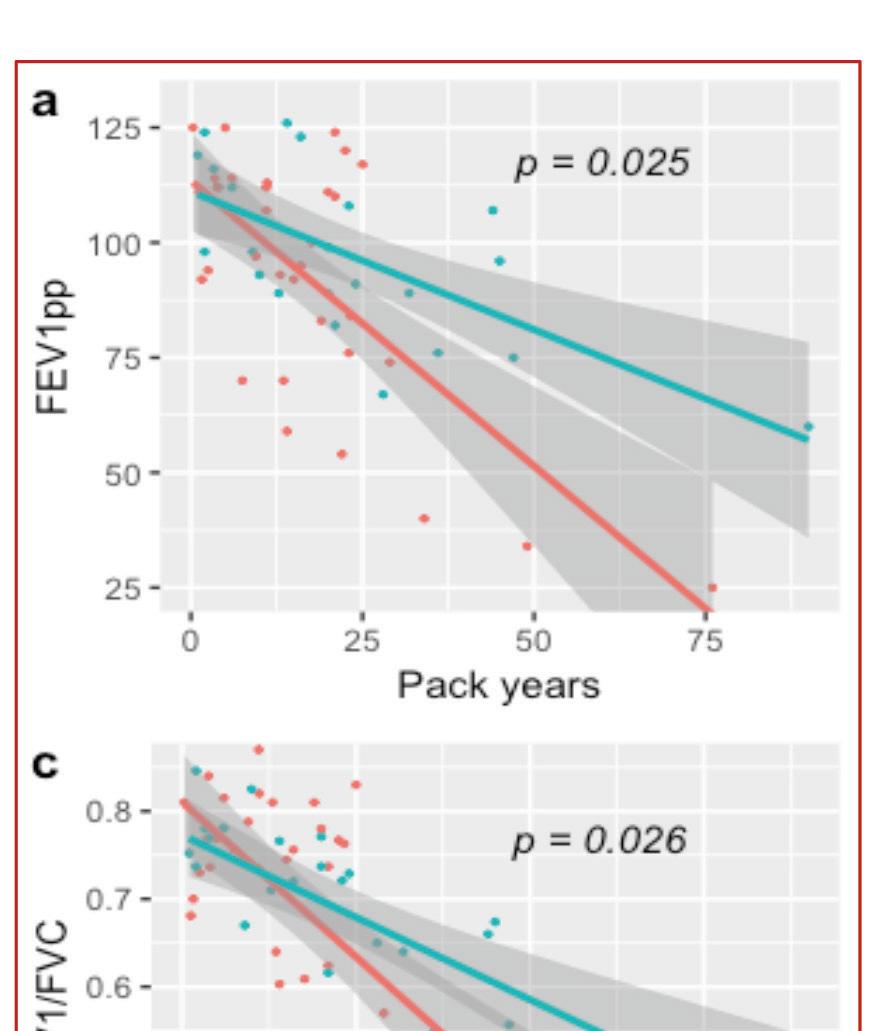
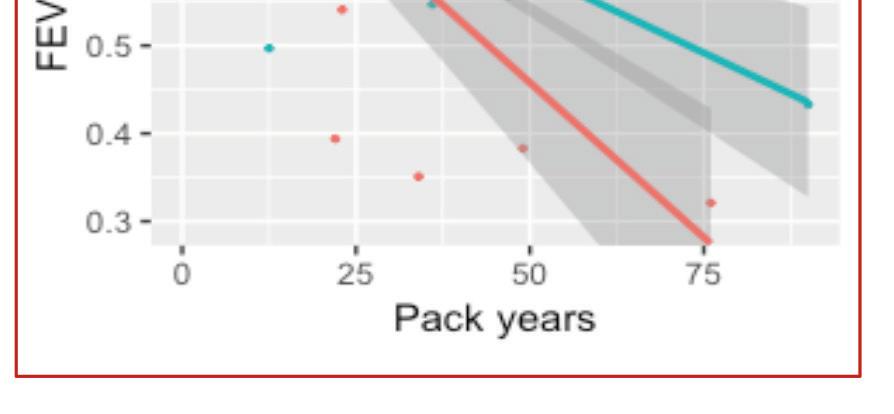
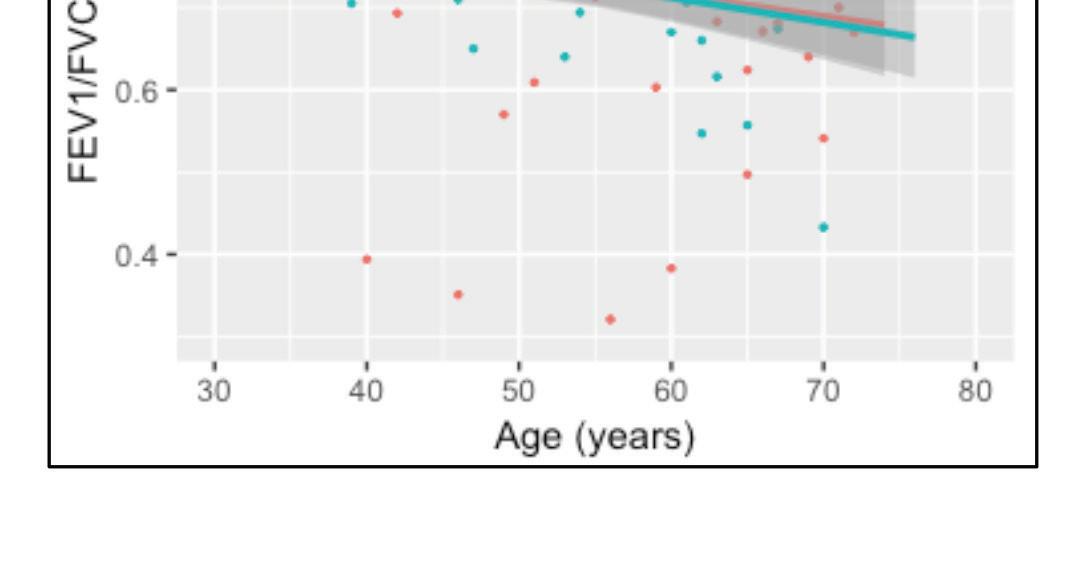
FIGURE 4: (LEFT) Lung function decline (both FEV1% predicted (pp) and FEV1/FVC) in never-smoking SZ AATD (red) compared to neversmoking normal risk MM and MS siblings (green) (RIGHT) Lung function decline in ever-smoking SZ AATD (red) compared to ever-smoking MM/MS siblings (green) AATD clinic as they can be easily identifi ed for inclusion in clinical trials that are currently being undertaken at RCSI Beaumont Hospital and worldwide.
One phase 2 clinical trial has already been completed in recent weeks in association with Vertex (VX19-814). This trial sought to increase circulating levels of AAT protein with an oral compound so it could act to prevent the damaging e ects of NE in the lung.
Another phase 2 clinical trial (VX19-864) of an oral compound will be conducted in Ireland in the coming months and will be recruiting patients with ZZ AATD in the very near future.
It should be noted that we accept referrals from all medical professionals in Ireland and are happy to see patients with any abnormal phenotype to help with genetic counselling around their condition and to explain the rationale behind various lifestyle modifi cations. We have a particular interest in patients with a ZZ AATD phenotype as they may be eligible for inclusion in current and all future trials.
It is hoped that at some point in the near future, a family-based study will be carried out involving patients with ZZ AATD and their siblings in order to establish the degree to which lung disease is present in asymptomatic ZZ AATD individuals. This will be a follow-on study from the MZ and SZ family studies. This new research will help us clarify to what extent people with ZZ AATD have disease before they present themselves to a healthcare professional with symptoms of lung disease. This will allow us to intervene very early in their disease trajectory and likely stave o some of the major complications of severe AATD.
Of the 380 patients found to have ZZ AATD, we have currently recruited approximately 220 to our registry. Given the ongoing trials and upcoming family-based studies, we would encourage referral to our outpatient clinic so these patients can be identifi ed more easily for inclusion into clinical trials. Further information can be found on www.alpha1.ie or by emailing us at alpha1@rcsi.ie.
Idiopathic pulmonary fibrosis and inhaled drug development
AUTHOR: Dr Cian O’Leary, PhD MPSI, RCSI School of Pharmacy and Biomolecular Sciences
Idiopathic pulmonary fibrosis (IPF) persists today as a devastating lung disease with a five-year survival of approximately 20 per cent, according to the European Respiratory Society. Although a rare disease in which the exact cause is unknown, its European incidence is near that of brain, stomach, and testicular cancers. For the approximate 200,000 people with IPF across Europe and North America, along with the additional 60,000 newly diagnosed cases annually, there is no current cure, with only two focused antifibrotic therapies available to slow the rate of fibrosis in the alveolar sacs and deterioration of pulmonary function: pirfenidone and nintedanib. These medications have certainly had a positive impact on the treatment of IPF and recent evidence has emerged that both confer a survival benefit, but they are not without their adverse drug reactions. In particular, gastrointestinal side effects such as nausea and diarrhoea can be significantly debilitating and lead to drug discontinuation in one in five patients. Patients can typically only tolerate one of these medications, with limited scope for combination therapy. Photosensitivity induced by pirfenidone can limit its use for those who are regularly outdoors, while both drugs are subject to drug-drug interactions that can complicate their use in patients with co-morbidities. Ultimately, additional therapies are needed for more effective clinical management of IPF, particularly when the condition is compared to other chronic respiratory diseases that have a vaster, versatile, armamentarium of therapies to select from.
New developments
In answer to this call, many exciting drug candidates in development are advancing through clinical trials and show great promise as new drug classes for IPF management, including the autotaxin inhibitor GLPG1690, the anti-CTGF monoclonal antibody pamrevlumab, and the ROCK inhibitor KD025. However, the development of inhaled therapies for IPF remains relatively unpursued. As observed in the management of conditions like asthma and COPD, inhaled therapy is effective through direct delivery of a lower drug dose to the disease site in the lungs; it also reduces systemic side effects and drug-drug interactions, facilitating combination therapy as part of an improved drug regimen. For IPF, an inhaled medicine could complement the use of oral pirfenidone and/or nintedanib, as well as other therapeutics that emerge from the clinical trial pipeline, to maximise improved patient outcomes. While lesser in number than oral or injectable therapies, several inhaled drug candidates are in the midst of clinical trials, with one of the most advanced in phase 2 trials being GB0139 by the biopharma spinout Galecto Biotech. Indeed, such is inhaled therapies’ potential to improve upon other routes of drug administration that the company Avalyn Pharma is pursuing the launch of inhaled pirfenidone, believing the same drug, delivered more efficiently, can provide better outcomes.
With all this information in mind, the logical question is why are other pharmaceutical companies not considering inhaled drug development? There are likely several factors in play here, but two prominent reasons are regulatory and clinical in nature. From a regulatory perspective, inhaled medicinal products require more extensive specification and characterisation than oral medicines, with consideration for accurate and reproducible specification for the inhaled device’s successful delivery of dose as a major hurdle. Of more concern to healthcare professionals, however, is whether it is feasible for sufferers of IPF with reduced pulmonary function to be able to inhale medicines adequately. The TOPICAL study, published last year, conducted a small trial to answer this question and show that alveolar deposition of drugs from nebulisers and pressurised metred dose inhalers (pMDI) is possible, encouraging further research into inhaled anti-fibrotic development.
Irish research
Within the School of Pharmacy and Biomolecular Sciences at the RCSI, my research group is actively exploring the development of such inhaled anti-fibrotic medicines. Our investigations are two-fold: First, to identify molecules with potential anti-fibrotic activity and to explore the therapeutic potential of these molecules when administered both as a standalone therapy and in combination with the current standard of care (pirfenidone or nintedanib); and second, to translate these molecules into an inhalable dosage form that could feasibly be used in the healthcare setting for the benefit of people with IPF. A key element of our current research programme is that we are repurposing off-patent drugs that have previously been used as oral medications for entirely different conditions, which have the advantage of decades of safety data in patients. Our research, kindly funded by the Irish Lung Fibrosis Association (ILFA), has begun to show that several of these drugs exhibit anti-fibrotic activity in cells isolated from IPF patients, and that this activity is potentially unique to the drug’s original mechanism of action. Now, by reformulating these drug molecules into inhalables so that lung cells can be directly exposed to them, we are unearthing new possibilities in IPF for safe medicines.
Overall, the future for inhaled medicines in IPF appears bright. Inhaled medicines can improve a patient’s situation by not just providing a new possibility of improving their condition, but also a means to improve quality of life by reducing adverse drug reactions. They are unlikely to be the definitive cure for IPF at present, but as part of a therapeutic regimen, they could provide the clinical management of IPF with a breath of fresh air.
For COPD patients on treatment with ICS/LABA and at risk of exacerbation*1
*A worsening of symptoms or a history of exacerbation treated with antibiotics or oral corticosteroids in the past 12 months

It’s the things you do today that make a big difference to their tomorrows1-3
Fictional patient, for illustrative purposes only
TRELEGY Ellipta provides your patients with statistically superior improvements in lung function and health-related quality of life, and reduction in annualised rate of moderate/ severe exacerbations** vs. budesonide/formoterol***1–3
Start your patients on TRELEGY Ellipta today, expect more from tomorrow1,2
**Moderate exacerbation is a worsening of symptoms or a history of exacerbation treated with antibiotics or oral corticosteroids. A severe exacerbation is a worsening in symptoms that required hospitalisation.
TRELEGY Ellipta (FF/UMEC/VI) 92/55/22 mcg OD is indicated for maintenance treatment in adult patients with moderate to severe COPD who are not adequately treated by a combination of an ICS and a LABA or a combination of a LAMA and a LABA1
Today. Tomorrow. TRELEGY.2-3
▼This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
***Co-primary endpoints were change from baseline in trough FEV1 and SGRQ at week 24 (n=1810). A subset of patients (n=430) remained on blinded study treatment for 52 weeks. Trelegy showed an improvement in trough FEV1 of 171mL versus budesonide/formoterol (p < 0.001, 95%
CI 148,194) at week 24. Trelegy showed an improvement in health-related quality of life (SGRQ) of 2.2 units (p <0.001, 95% CI 3.5, 1.0) at week 24. At week 52 in a subset of patients Trelegy showed a 44% reduction in annualised rate of moderate/severe exacerbations versus budesonide/ formoterol (95% CI 15,63, p=0.006, Absolute difference 0.16).
TRELEGY Ellipta is generally well tolerated. Common adverse reactions include: pneumonia, upper respiratory tract infection, bronchitis, pharyngitis, rhinitis, sinusitis, influenza, nasopharyngitis, candidiasis of mouth and throat, urinary tract infection, headache, cough, oropharyngeal pain, constipation, arthralgia, back pain1 FF, fluticasone furoate; ICS, inhaled corticosteroid; LABA, long-acting ß2-agonist; LAMA, long-acting muscarinic antagonist; OD, once-daily; UMEC, umeclidinium, VI, vilanterol References: 1. TRELEGY Ellipta SmPC 2019. 2. Lipson DA et al. Am J Respir Crit Care Med 2017; 196:438–446. 3. Lipson DA et al.N Engl J Med 2018; 378:1671–1680.
Trelegy▼ Ellipta (fluticasone furoate/umeclidinium/vilanterol [as trifenatate]) Prescribing information. Please consult the full Summary of Product Characteristics (SmPC) before prescribing. Trelegy Ellipta (fluticasone furoate/umeclidinium/vilanterol [as trifenatate]) inhalation powder. Each single inhalation of fluticasone furoate (FF) 100 micrograms (mcg), umeclidinium bromide (UMEC) 62.5 micrograms and vilanterol as trifenatate (VI) 25 mcg provides a delivered dose of 92 mcg FF, 55 mcg UMEC and 22 mcg VI. Indications: Maintenance treatment in adult patients with moderate to severe COPD who are not adequately treated by a combination of an inhaled corticosteroid (ICS) and a long-acting ß2-agonist (LABA) or a combination of a LABA and a long acting muscarinic antagonist. Dosage and administration: One inhalation once daily at the same time each day. Contraindications: Hypersensitivity to the active substances or to any of the excipients (lactose monohydrate & magnesium stearate). Precautions: Paradoxical bronchospasm, unstable or life-threatening cardiovascular disease or heart rhythm abnormalities, convulsive disorders or thyrotoxicosis, pulmonary tuberculosis or patients with chronic or untreated infections, narrow-angle glaucoma, urinary retention, hypokalaemia, patients predisposed to low levels of serum potassium, diabetes mellitus. In patients with moderate to severe hepatic impairment patients should be monitored for systemic corticosteroidrelated adverse reactions. Eye symptoms such as blurred vision may be due to underlying serious conditions such as cataract, glaucoma or central serous chorioretinopathy (CSCR); consider referral to ophthalmologist. Increased incidence of pneumonia has been observed in patients with COPD receiving inhaled corticosteroids. Risk factors for pneumonia include: current smokers, old age, patients with a history of prior pneumonia, patients with a low body mass index and severe COPD. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take Trelegy. Acute symptoms: Not for acute symptoms, use short-acting inhaled bronchodilator. Warn patients to seek medical advice if short-acting inhaled bronchodilator use increases. Therapy should not be abruptly stopped without physician supervision due to risk of symptom recurrence. Systemic effects: Systemic effects of ICSs may occur, particularly at high doses for long periods, but much less likely than with oral corticosteroids. Interactions with other medicinal products: Caution should be exercised with concurrent use of ß-blockers. Caution is advised when co-administering with strong CYP3A4 inhibitors (e.g. ketoconazole, ritonavir, cobicistat-containing products), hypokalaemic treatments or non-potassium-sparing diuretics. Co-administration with other long-acting muscarinic antagonists or long acting ß2-adrenergic agonists is not recommended. Pregnancy and breast-feeding: Experience limited. Balance risks against benefits. Side effects: Common (≥1/100 to <1/10): pneumonia, upper respiratory tract infection, bronchitis, pharyngitis, rhinitis, sinusitis, influenza, nasopharyngitis, candidiasis of mouth and throat, urinary tract infection, headache, cough, oropharyngeal pain, arthralgia, back pain. Uncommon
Find out more here: www.trelegy.ie or request a visit from a GSK representative or request a visit from a GSK representative
(≥1/1,000 to <1/100): viral respiratory tract infection, supraventricular tachyarrhythmia, tachycardia, atrial fibrillation, dysphonia, dry mouth, fractures. Rare (≥1/10,000 to <1/1,000): Hypersensitivity reactions, including anaphylaxis, angioedema, urticaria, and rash. Not known (cannot be estimated from the available data): vision blurred. Marketing Authorisation (MA) Holder: GlaxoSmithKline Trading Services Limited, 12 Riverwalk, Citywest Business Campus, Dublin 24, Ireland. MA No. [EU/1/17/1236/002]. Legal category: POM B. Last date of revision: September 2020. Code: PI-6725. Further information available on request from GlaxoSmithKline, 12 Riverwalk, Citywest Business Campus, Dublin 24. Tel: 01-4955000.
Adverse events should be reported to the Health Products Regulatory Authority (HPRA) using an Adverse Reaction Report Form obtained either from the HPRA or electronically via the website at www.hpra.ie. Adverse reactions can also be reported to the HPRA by calling: (01) 6764971. Adverse events should also be reported to GlaxoSmithKline on 1800 244 255.
TRELEGY Ellipta was developed in collaboration with
For COPD patients on treatment with ICS/LABA and at risk of exacerbation*1
*A worsening of symptoms or a history of exacerbation treated with antibiotics or oral corticosteroids in the past 12 months

It’s the things you do today that make a big difference to their tomorrows1-3
Fictional patient, for illustrative purposes only
TRELEGY Ellipta provides your patients with statistically superior improvements in lung function and health-related quality of life, and reduction in annualised rate of moderate/ severe exacerbations** vs. budesonide/formoterol***1–3
Start your patients on TRELEGY Ellipta today, expect more from tomorrow1,2
**Moderate exacerbation is a worsening of symptoms or a history of exacerbation treated with antibiotics or oral corticosteroids. A severe exacerbation is a worsening in symptoms that required hospitalisation.
TRELEGY Ellipta (FF/UMEC/VI) 92/55/22 mcg OD is indicated for maintenance treatment in adult patients with moderate to severe COPD who are not adequately treated by a combination of an ICS and a LABA or a combination of a LAMA and a LABA1
Today. Tomorrow. TRELEGY.2-3
▼This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
***Co-primary endpoints were change from baseline in trough FEV1 and SGRQ at week 24 (n=1810). A subset of patients (n=430) remained on blinded study treatment for 52 weeks. Trelegy showed an improvement in trough FEV1 of 171mL versus budesonide/formoterol (p < 0.001, 95%
CI 148,194) at week 24. Trelegy showed an improvement in health-related quality of life (SGRQ) of 2.2 units (p <0.001, 95% CI 3.5, 1.0) at week 24. At week 52 in a subset of patients Trelegy showed a 44% reduction in annualised rate of moderate/severe exacerbations versus budesonide/ formoterol (95% CI 15,63, p=0.006, Absolute difference 0.16).
TRELEGY Ellipta is generally well tolerated. Common adverse reactions include: pneumonia, upper respiratory tract infection, bronchitis, pharyngitis, rhinitis, sinusitis, influenza, nasopharyngitis, candidiasis of mouth and throat, urinary tract infection, headache, cough, oropharyngeal pain, constipation, arthralgia, back pain1 FF, fluticasone furoate; ICS, inhaled corticosteroid; LABA, long-acting ß2-agonist; LAMA, long-acting muscarinic antagonist; OD, once-daily; UMEC, umeclidinium, VI, vilanterol References: 1. TRELEGY Ellipta SmPC 2019. 2. Lipson DA et al. Am J Respir Crit Care Med 2017; 196:438–446. 3. Lipson DA et al.N Engl J Med 2018; 378:1671–1680.
Trelegy▼ Ellipta (fluticasone furoate/umeclidinium/vilanterol [as trifenatate]) Prescribing information. Please consult the full Summary of Product Characteristics (SmPC) before prescribing. Trelegy Ellipta (fluticasone furoate/umeclidinium/vilanterol [as trifenatate]) inhalation powder. Each single inhalation of fluticasone furoate (FF) 100 micrograms (mcg), umeclidinium bromide (UMEC) 62.5 micrograms and vilanterol as trifenatate (VI) 25 mcg provides a delivered dose of 92 mcg FF, 55 mcg UMEC and 22 mcg VI. Indications: Maintenance treatment in adult patients with moderate to severe COPD who are not adequately treated by a combination of an inhaled corticosteroid (ICS) and a long-acting ß2-agonist (LABA) or a combination of a LABA and a long acting muscarinic antagonist. Dosage and administration: One inhalation once daily at the same time each day. Contraindications: Hypersensitivity to the active substances or to any of the excipients (lactose monohydrate & magnesium stearate). Precautions: Paradoxical bronchospasm, unstable or life-threatening cardiovascular disease or heart rhythm abnormalities, convulsive disorders or thyrotoxicosis, pulmonary tuberculosis or patients with chronic or untreated infections, narrow-angle glaucoma, urinary retention, hypokalaemia, patients predisposed to low levels of serum potassium, diabetes mellitus. In patients with moderate to severe hepatic impairment patients should be monitored for systemic corticosteroidrelated adverse reactions. Eye symptoms such as blurred vision may be due to underlying serious conditions such as cataract, glaucoma or central serous chorioretinopathy (CSCR); consider referral to ophthalmologist. Increased incidence of pneumonia has been observed in patients with COPD receiving inhaled corticosteroids. Risk factors for pneumonia include: current smokers, old age, patients with a history of prior pneumonia, patients with a low body mass index and severe COPD. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take Trelegy. Acute symptoms: Not for acute symptoms, use short-acting inhaled bronchodilator. Warn patients to seek medical advice if short-acting inhaled bronchodilator use increases. Therapy should not be abruptly stopped without physician supervision due to risk of symptom recurrence. Systemic effects: Systemic effects of ICSs may occur, particularly at high doses for long periods, but much less likely than with oral corticosteroids. Interactions with other medicinal products: Caution should be exercised with concurrent use of ß-blockers. Caution is advised when co-administering with strong CYP3A4 inhibitors (e.g. ketoconazole, ritonavir, cobicistat-containing products), hypokalaemic treatments or non-potassium-sparing diuretics. Co-administration with other long-acting muscarinic antagonists or long acting ß2-adrenergic agonists is not recommended. Pregnancy and breast-feeding: Experience limited. Balance risks against benefits. Side effects: Common (≥1/100 to <1/10): pneumonia, upper respiratory tract infection, bronchitis, pharyngitis, rhinitis, sinusitis, influenza, nasopharyngitis, candidiasis of mouth and throat, urinary tract infection, headache, cough, oropharyngeal pain, arthralgia, back pain. Uncommon
Find out more here: www.trelegy.ie or request a visit from a GSK representative or request a visit from a GSK representative
(≥1/1,000 to <1/100): viral respiratory tract infection, supraventricular tachyarrhythmia, tachycardia, atrial fibrillation, dysphonia, dry mouth, fractures. Rare (≥1/10,000 to <1/1,000): Hypersensitivity reactions, including anaphylaxis, angioedema, urticaria, and rash. Not known (cannot be estimated from the available data): vision blurred. Marketing Authorisation (MA) Holder: GlaxoSmithKline Trading Services Limited, 12 Riverwalk, Citywest Business Campus, Dublin 24, Ireland. MA No. [EU/1/17/1236/002]. Legal category: POM B. Last date of revision: September 2020. Code: PI-6725. Further information available on request from GlaxoSmithKline, 12 Riverwalk, Citywest Business Campus, Dublin 24. Tel: 01-4955000.
Adverse events should be reported to the Health Products Regulatory Authority (HPRA) using an Adverse Reaction Report Form obtained either from the HPRA or electronically via the website at www.hpra.ie. Adverse reactions can also be reported to the HPRA by calling: (01) 6764971. Adverse events should also be reported to GlaxoSmithKline on 1800 244 255.
TRELEGY Ellipta was developed in collaboration with