13 minute read
COPD – 2020 a paradigm shift
AUTHORS: Dr Ciara Ottewill, SpR in Respiratory Medicine; and Prof Stephen J Lane, Consultant Respiratory Physician, Department of Respiratory Medicine, Tallaght University Hospital
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality worldwide. Though classically it is associated with smoking, it is estimated that up to 20-45 per cent of those with COPD worldwide are non-smokers. The majority of studies to date focus on latestage disease, however, it is becoming increasingly clear that a focus on primary prevention of the disease is needed. Early detection of those with pre-COPD and early COPD should become priorities going forward.
Background
COPD is the third common cause of death worldwide,1 and is estimated to affect up to 12 per cent of the world population. It is the cause of over three million deaths annually, far surpassing the current annual death rate from Covid-19 (which at time of publication was estimated by the World Health Organisation (WHO) to have surpassed one million in its first 10 months). It is among the most common causes of death in Ireland, surpassed only by cardiovascular disease and cancers. The OECD estimates that COPD attributes to approximately 40 per cent of respiratory mortality in Ireland.
The prevalence of COPD appears to be increasing globally, in particular in countries including China and India. This increase appears to be primarily in non-smokers. It is estimated that approximately 25 per cent of the Irish population diagnosed with COPD are non-smokers. Prevalence of non-smokers with the diseases varies globally, with 45 per cent of patients in South Africa having never smoked. The reasons for non-smoking related COPD are becoming clearer and can be related to intrauterine factors, early life factors, exposure to inefficient combustion of biomass fuels in poorly ventilated houses, and second hand smoke for example. Prevalence of COPD in smokers, in contrast, appears to be relatively static, with an estimated 40-50 per cent of smokers developing the disease. Genetic factors, including α-1-antitrypsin deficiency, are also known to contribute to the disease.
COPD trends in Ireland in 2020
Ireland has one of the highest rates of hospitalisation for COPD in the OECD, at approximately 400 admissions per 100,000 population, which is among the highest rates of hospital admissions for COPD globally. Though over the past 15 years there has been a notable international
decrease in mortality secondary to cancer and cardiovascular disease, including myocardial infarction, mortality from COPD remains high. COPD accounts for the primary diagnosis in over 1,500 deaths annually in Ireland, which is second only to lung cancer in causes of death from respiratory disease, as is outlined in the 2018 Respiratory Health of the Nation document from the Irish Thoracic Society. COPD is more prevalent in lower socioeconomic groups within Ireland.
Pathophysiology
Exhalatory airflow obstruction (afterload) and hyperinflation (preload) There are two key pathological changes in COPD, ie, chronic bronchitis and emphysema. Chronic bronchitis and emphysema results in either anatomical or functional airflow obstruction. This obstruction occurs throughout the entire length of the non cartilaginous, smooth muscle containing tracheobronchial tree. The obstruction occurs predominantly during exhalation as the airways are naturally more narrowed then than during inhalation. Thus there is increased ‘afterload’ to the process of exhalation making it more difficult and lengthy for patients to exhale at rest and during exercise. Emphysema, in addition to exhalatory obstruction, results in increased lung compliance because of parenchymal destruction. Therefore in the ‘tug of war’ between the chest wall and lung parenchyma maintained by the pleura, the outward forces of the chest wall will overcome the inward forces of the damaged elastic lung resulting in an increase of volume trapped in the lung at the end of quiet exhalation or the FRC (functional residual capacity). This trapped volume results in significant ‘preload’ to the patients with predominant emphysema subtype. Ultimately the lung becomes hyperinflated and ‘barrel-like’ as the chest wall creeps up over the trachea. COPD patients find themselves ‘breath stacking’ because of this air trapping, which contributes substantially to breathlessness at rest and on exertion. Powerful treatments for COPD, including LAMAs (long-acting muscarinic antagonists) and LABAs (long-acting beta agonists), work by bronchodilating (as measured by an improvement in FEV1) the increased afterload of exhalatory airflow obstruction. In addition, along with lung volume reduction surgery (LVRS) and endobronchial valve therapy (EBV), they act by deflating the preload caused by the hyperinflated FRC (as measured by increasing the VC (vital capacity), which can often be significant).
FIGURE 1: Fletcher-Peto Curve, illustrating the decline in FEV1% in those with COPD compared to the general population
Naming, staging, and classification
COPD, as we understand it today, was defined in terms of the clinical phenotypes around the time of World War II. The poor prognosis ‘blue bloater’, having a characteristic hypoxaemic respiratory failure, had airflow obstruction secondary to chronic bronchitis on post-mortem. The better prognosis breathless ‘pink puffer’ had airflow obstruction secondary emphysematous parenchymal destruction on post mortem. In the 1950s it became possible to measure this obstruction in vivo using spirometry, and spirometry currently remains a crucial tool in both the diagnosis of COPD and in the staging of disease. In 1997 the Global Obstructive Lung Disease (GOLD) initiative formally
Category
GOLD 1
GOLD 2
GOLD 3
GOLD 4 Severity
Mild
Moderate
Severe
Very severe
FEV1% predicted
>80%
50%<FEV1< 80%
30%<FEV1 <50%
<30%
FIGURE 2.A: GOLD Classification 1997 to 2011 named this disease COPD. Expiratory airflow obstruction was defined as a ratio of FEV1/FVC <0.7, ie, COPD patients, in contrast to patients without COPD, could not exhale greater than 70 per cent of their VC in the first second of a standardised forced exhaled manoeuvre post a shortacting bronchodilator. In the presence of airflow obstruction, the biomarker of annualised percentage FEV1 was then used to stage the disease from 1 through to 4 in terms of severity as in Figure 2A, based on the Fletcher-Peto curves (Figure 1, above) validated by the larger Framingham Study.2
Since 2011, with the realisation of the limitation of outcome determination based on annualised percentage FEV1 alone, GOLD modified their classification with more emphasis being placed on symptom or breathlessness scores, eg, the CAT score or MRC dyspnoea scale, in addition to exacerbation history with grading now becoming ABCD rather than stage 1-4 (Figure 2B). In 2017, spirometry was removed from staging and used for diagnosis only. Grading of prognosis now was now to be based on symptom scores and exacerbation history alone once airflow obstruction had been confirmed. This of course makes it much easier to use in primary care. A GP simply has to ask the patient how breathless they are and interrogate their exacerbation history, ie, a breathless patient on hills, with two exacerbations within the last 12 months, in the presence of airflow obstruction indicates grade D and interventions can be planned accordingly.2
C D
A B
>two exacerbations or >one leading to hospitalisation 0 or one exacerbation (not leading to hospitalisation) MmMRC 0-1 CAT <10 mMRC >2 CAT >10
FIGURE 2.B: GOLD Classification 2011 -2017. Symptom and Exacerbation Rate A-D
Natural history, pre-COPD, and early COPD
Approximately 100,000 COPD patients have been studied in randomised, placebo controlled interventional trials in recent years. The average age at enrolment is 62 years and the average per cent predicted FEV1 is 50 per cent. These studies are thus focussing on very late phase disease. The endpoints studied are changes in lung function, breathlessness, exacerbation rate and all-cause mortality. Invariably these studies show some improvements in the above endpoints, but what is striking is that there remains a huge unmet need in these endpoints despite interventions.
This has now focused intense interest on the detection of pre-COPD and early COPD. There is thus now a realisation that focussing on the sole trajectory of FEV1 decline from a supposed always normal 100 per cent baseline in one’s late 20s gives a very incomplete picture as to the natural
1. Trajectory 1 indicates normal gain in lung function and normal decline, which does not result in COPD 2. Trajectory 2 indicates failure of gain of lung function, but a normal decline and may or may not result in COPD
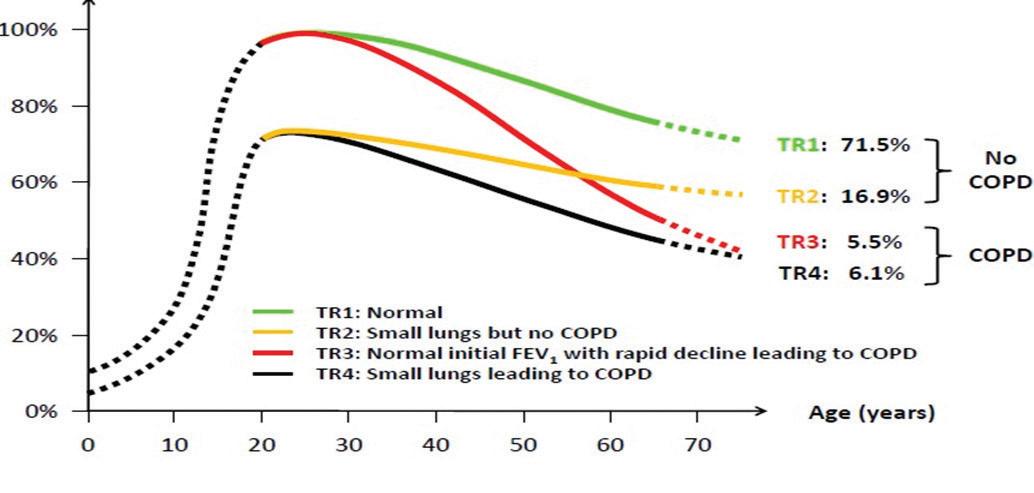
FIGURE 3: COPD FEV1 trajectories 3. Trajectory 3 is what we currently base all our insight on in COPD and will be very familiar to most of us, ie, normal gain and an accelerated decline 4. Trajectory 4 illustrates failure of gain and accelerated decline, which will result in COPD2
course of this disease.3 It is now accepted that there are at least four different trajectories of FEV1 over time and that the starting point should be at birth or as near to it as possible. Figure 3 (above) illustrates this.
Therefore, the focus of COPD has now shifted to failure of gain of FEV1 as much as accelerated decline. How else can we explain the huge prevalence of COPD in younger people in developing countries who are exposed to inefficient biomass combustion, high prevalence rates of TB, premature birth and excess chest infections inter alia?
Furthermore, this has now shifted our thinking to individuals with pre-COPD and early COPD. Pre-COPD are individuals with no symptoms as yet and normal lung function albeit at the lower limit of normal. What defines them is the trajectory of their lung function gain and decline. These subjects would have reduced gain in FEV1 at an early stage and an accelerated decline after adult peak as per trajectories 2 and 4 above. Early COPD are individuals with no or minimal symptoms whose percentage predicted FEV1 is lower than the expected range for any given age, ie, they are picked up on screening.1 As a result of this fundamental shift in our understanding of COPD towards earlier disease, annual measurement of FEV1 in the general population is now strongly advised in order that we may assess trajectories in individual patients.
Measurement of FEV1 should be carried out, like BP or blood sugar, at each GP visit in our view. If we do not do this we will continue to manage COPD in emergency departments (EDs) at the end stage of its natural history when there is little we can do about it. In the 1970s, ischaemic heart disease was managed in the ED when patients had heart attacks. Look how that has changed through earlier detection of risk factors, statins, and blood pressure management. We are now at the cusp of doing the same in COPD, but it has been a long time coming because of the nihilism surrounding COPD as a self-induced cigarette-induced disease and because of a fundamental failure of our understanding of this now ‘respectable’ disease.
In our view, with a huge effort into general population FEV1 screening and a shift of our interventional studies to earlier disease, we will at last make an impact on this devastating disease as depicted in the following figure. COPD is an international public health issue.
Management
Treatment recommendations for COPD are laid out in both the GOLD guidelines, and the ICGP COPD Quick Reference Guide. Current treatments in COPD aim to reduce symptoms, increase quality-of-life, and decrease exacerbation rates and mortality. Exacerbations of COPD, including viral exacerbations, have been shown to contribute significantly to COPD-related mortality.1 Slowing the rate of decline in FEV1 and decreasing exacerbation may certainly aid in improving overall survival. Through placing an emphasis on early disease detection, and slowing rate of decline in early stages of the disease may significantly reduce morbidity.
Lifestyle modifications
Present management options include non-pharmacological initiatives, including smoking cessation, placing an importance on vaccination to reduce exacerbation rates, and exercise, including pulmonary rehabilitation. Despite significant public health interventions, including plain packaging, increased taxes and indoor smoking bans, smoking remains the most common avoidable health risk in the EU.
Vaccination is a crucial intervention in COPD and viral infections including
influenza are key areas to target with regard to prevention of exacerbations.
Minimising exposure to inhaled environmental pollutants, and early aggressive treatments of childhood infections may also allow patients to reach optimal FEV1.
Pharmacological therapies
Pharmacological therapies are described in the GOLD Guidelines 2019, with a stepwise treatment approach, aiming to escalate or downgrade treatments as time progresses.
LAMA are a well-established treatment in COPD. Many studies have shown that LAMA, including tiotropium, improve FEV1, improve patient-reported qualityof-life, as well as decreasing rates of exacerbation and decreasing mortality. Despite this, more than 50 per cent of patients treated with LAMA monotherapy still experience breathlessness, regardless of disease severity. Meta-analysis has shown that LABA/LAMA in combination is superior to LAMA monotherapy in treating breathlessness. When compared to treatment with a combination of LABA/ ICS (inhaled corticosteroids), LABA/ LAMA therapy has also shown dual bronchodilator therapy is much more effective in reducing exacerbations.1
Addition of ICS to LABA/LAMA therapy has been shown to reduce patient symptoms and reduces moderate or severe exacerbations requiring hospitalisation, in those with an FEV1 <50 per cent, despite a small risk in community-acquired pneumonia. These findings are more prevalent in those patients with blood eosinophilia.
As such, the GOLD Guidelines 2019 now recommend peripheral blood eosinophils as a biomarker for the addition of ICS, with little effect in those with eosinophils 100cells/μl.2 Those with eosinophil levels of 100cells/μl typically respond well to the addition of ICS to their inhaled therapeutic regimen. ICS use in COPD has been shown to reduce annual exacerbation rates, improve quality-of-life, improve FEV1 and lead to a reduction in all-cause mortality across several studies.
Non-pharmacological therapies
Lung volume reduction surgery This invasive therapy has been shown to convey a survival benefit in those with severe upper lobe emphysema, with low exercise tolerance.
Endobronchial valve therapy This uses one-way stents, which allow air to escape during exhalation, while allowing no influx of air with inhalation, thereby allowing expansion of normal lung, with deflation of hyper-expanded bullous lung. These significantly increase FEV1, FVC and six-minute walk tests in patients with severe emphysema, with a resulting improvement in quality-of-life questionnaires.
Pulmonary rehabilitation This is a well-established treatment, which has been shown to reduce dyspnoea, improve exercise tolerance and patient-reported quality-of-life in patients with COPD.5
Summary
Despite a global decrease in smoking trends, COPD remains among the most common causes of death both in Ireland and internationally. It is a public health emergency, and going forward a focus must be placed on early detection of disease, to allow early interventions to slow decline in FEV1, and to reduce exacerbation rates. Efforts to do this must include serial FEV1 monitoring to diagnose pre-COPD, or early COPD, as well as to assess rate of decline. A focus must also be placed on identifying non-smokers with COPD, as up to onein-four patients in Ireland with COPD will have never smoked. It is crucial that an emphasis on primary prevention of disease rather than treatment of late stage disease is enforced going forward.
Therapies currently include a combination of lifestyle modification, and inhaled therapies, which are added in a step-wise fashion, with inhaled steroids addition guided by patient eosinophil count, as well as treatments including pulmonary rehabilitation, with the ultimate goal of minimising patient symptom burden, decreasing exacerbation rates, and thus slowing decline in FEV1. ■
References
1. Celli BR, Wedzicha JA. Update on Clinical Aspects of Chronic Obstructive Pulmonary Disease. Drazen JM, editor. N Engl J Med. 2019 Sep 26;381(13):1257–66. 2. GOLD-2019-v1.7-FINAL-14Nov2018WMS.pdf [Internet]. [cited 2020 Oct 20]. Available from: https://goldcopd.org/ wp-content/uploads/2018/11/GOLD2019-v1.7-FINAL-14Nov2018-WMS.pdf 3. Lange P, Celli B, Agustí A, Boje Jensen G, Divo M, Faner R, et al. Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. N Engl J Med. 2015 Jul 9;373(2):111–22 4. OECD, European Union. Health at a Glance: Europe 2018: State of Health in the EU Cycle [Internet]. OECD; 2018 [cited 2020 Oct 10]. (Health at a Glance: Europe). Available from: https://www.oecd-ilibrary.org/socialissues-migration-health/health-at-aglance-europe-2018_health_glance_ eur-2018-en 5. ICGP. Quick Reference Guide: Chronic Obstructive Pulmonary Disease. December 2019. [cited 2020 Oct 19]. Available from: https://www. icgp.ie/index.cfm?spPath=in_the_ practice/quick_reference_guides/ quick_reference_guides_ qrg_&resetcache=1&cachelist =page_in_the_practice_quick_ reference_guides_quick_reference_ guides_qrg_