





Numbers of Salmonella Enteritidis (SE) infections in the Netherlands have increased since 2023 in both laying poultry and humans. New infections are also continuing to occur at elevated levels in 2025. The poultry industry is making various efforts to reduce the number of infections. There is continuous attention for hygiene measures and biosecurity both on and between farms.
Additional genetic testing of the salmonella strains found is being conducted at Royal GD on behalf of AVINED. Comprehensive, standardised questionnaires will provide additional information in the search for common sources where flocks are infected by the same SE strain. In addition, the list can be used for discussing risk factors and preventive measures.
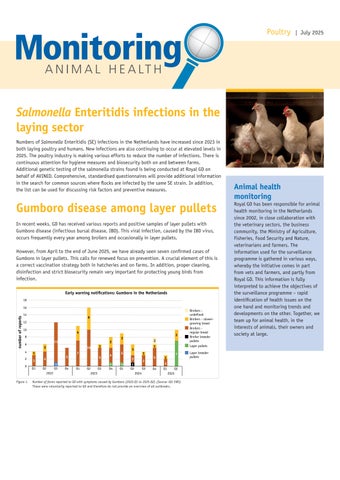
In recent weeks, GD has received various reports and positive samples of layer pullets with Gumboro disease (infectious bursal disease, IBD). This viral infection, caused by the IBD virus, occurs frequently every year among broilers and occasionally in layer pullets.
However, from April to the end of June 2025, we have already seen seven confirmed cases of Gumboro in layer pullets. This calls for renewed focus on prevention. A crucial element of this is a correct vaccination strategy both in hatcheries and on farms. In addition, proper cleaning, disinfection and strict biosecurity remain very important for protecting young birds from infection.

Royal GD has been responsible for animal health monitoring in the Netherlands since 2002, in close collaboration with the veterinary sectors, the business community, the Ministry of Agriculture, Fisheries, Food Security and Nature, veterinarians and farmers. The information used for the surveillance programme is gathered in various ways, whereby the initiative comes in part from vets and farmers, and partly from Royal GD. This information is fully interpreted to achieve the objectives of the surveillance programme – rapid identification of health issues on the one hand and monitoring trends and developments on the other. Together, we team up for animal health, in the interests of animals, their owners and society at large.
During the first quarter of 2025, the diagnosis of tendon sheath inflammation caused by reovirus was made for 43 submissions. Six submissions were from regular broiler chickens and the remaining 37 were broilers of slower-growing breeds (see Figure 2). Despite reovirus necropsies being subsidized once again from this quarter onwards, this is similar to the fourth quarter of 2024, when reovirus necropsies were temporarily conducted outside the monitoring budget.
Other poultry
Broilers (slow-growing)
Broilers (regular)
No prominent clusters were observed in the genotyping. The most commonly found genotypes were groups 4 (including genotype 4.7) and 2 (see Figure 3).
At the start of the second quarter, it was relatively quiet. In April, four necropsies led to diagnoses of reoviral tendon sheath inflammation and reovirus was detected in the tendon sheaths of five submissions without presenting histological abnormalities consistent with reovirus infection. These latter submissions were largely about bacterial joint problems or developmental disorders.
From May onwards, the number of submissions rose again, with 31 diagnosed with reoviral tendon sheath inflammation in the first three weeks. With one exception, they were all broilers of slower-growing breeds. In particular, the reoviruses are all in genotypes 1 and 4; clustering can be seen in both gene groups, indicating that there may be common sources.
Vaccine strain Reference strain 2025-Q1 2025 up to week 3 of May
Reovirus isolates combined with tendon sheath inflammation during the period:
Slices: number of reovirus isolates
isolates*
isolates*
= genotype
Explanation of figure:
isolates* (each slice = 1 isolate)
Larger circles with several slices: multiple isolates that could not be distinguished from each other using the DNA fragment analysed. The distances between the various circles (measured along the connecting lines) show the degree of similarity: the shorter the distance, the greater the similarity.
More fowlpox outbreaks
The last quarter of 2024 saw an increase in the number of outbreaks of fowlpox among Dutch commercial poultry. The fowlpox virus strains from those outbreaks that were confirmed by GD (see Figure 4) were isolated and studied genetically using whole genome sequencing (WGS). What stands out is that the genetic material of another virus (reticuloendotheliosis virus, REV) appears to have become integrated into current European fowlpox virus strains.
Reticuloendotheliosis virus (REV) embedded in fowlpox virus REV is known for its ability to incorporate its own genetic material into that of the host during replication. That could be a cell from a chicken, but recent genetic research has now shown that this process also happened in fowlpox viruses. In a sense, the fowlpox virus has thus itself become infected with REV. This phenomenon is not limited to the Netherlands: other European countries have also reported fowlpox viruses in which REV genetic material is present. There is a possibility of the presence of REV affecting the virulence and other characteristics of fowlpox viruses in chickens. In addition, it cannot be ruled out that the REV could be released again from the fowlpox virus and that chickens may therefore also excrete REV after a fowlpox infection. That is a worrying possibility, as many European countries including the Netherlands have long been free of REV.
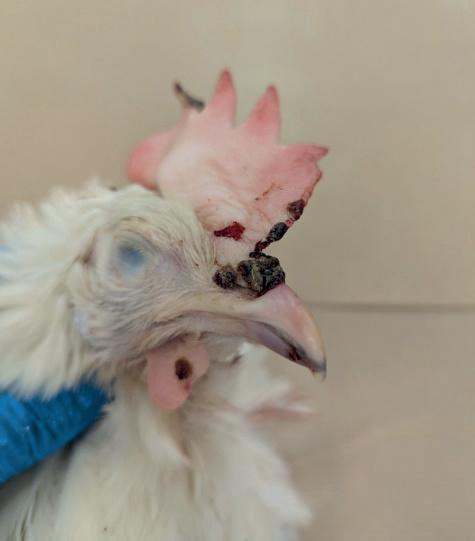
Given that outbreaks of the current fowlpox virus strains (containing embedded REV genes) lead to positive serology for REV, it is important to get a clear picture of the frequency of fowlpox outbreaks. A severe outbreak of fowlpox is obviously very noticeable (see Photo 1). There are worries that a proportion of fowlpox infections may be considerably milder and can be missed. Together with AVINED, GD is setting up a follow-up project to obtain a picture of the serological prevalence of fowlpox viruses among poultry. This project aims to obtain a detailed overview of the number of flocks that are serologically positive for fowlpox and how this relates to REV serology.
Disease/disorder/ health characteristic
Execution decree (EU) 2018/1882 of the Animal Health Law (AHL) (EU) 2016/429 (Category A disease)
Avian influenza (AI) in the Netherlands (H5/H7)
(Source: GD, WBVR, national government)
ND in the Netherlands
(Source: GD, WOAH)
Highly pathogenic AI (H5/H7)*: (first detection in flock)
* In commercial poultry and in backyard situations with >50 birds.
Serological monitoring by GD: (first detection in flock) (antibodies for H5/H7)
Commercial poultry: 0
(>50 birds)
Execution decree (EU) 2018/1882 of the Animal Health Law (AHL) (EU) 2016/429 (Categories B through E)
Avian influenza (AI) in the Netherlands (H5/H7)
(Source: GD, WBVR, national government)
Low pathogenic AI (H5/H7): (first detection in flock)
Campylobacteriosis No data available
Avian mycoplasmosis (Source: GD)
Mycoplasma gallisepticumA Serological monitoring by GD: Reproduction sector: Layer pullets: Layers: - not vaccinated and infected: - vaccinated and infected: Turkeys:
Cases in EWSC based on positive serology and/or voluntary PCR testing: Reproduction sector: Layers: Turkeys: Backyard poultry:
M. meleagridis (Source: GD)
Salmonellosis (non-zoonotic salmonella) (Source: GD)
Salmonella arizonae
Salmonella Gallinarum (SG) Commercial poultry: Backyard poultry: Number of farms/cases
Salmonella Pullorum (SP)
West Nile fever Not monitored
Article 2.1 Designation of animal diseases in the ‘Rules for Animal Health’ of the Dutch Animal Act Avian chlamydiosis (Source: GD)
Detected by GD:
Commercial poultry: Backyard poultry:
Disease/disorder/ health characteristic
(numbers at the farm level)
Article 2.2. Designation of zoonoses in the ‘Rules for Animal Health’ of the Dutch Animals Act Salmonellosis (zoonotic salmonella) (at the flock level) (Source: GD)
Salmonella Enteritidis Reproduction:
Salmonella Typhimurium Reproduction:
pullets:
Other types of Salmonella (S. Hadar, S. Infantis, S. Java, S. Virchow)
Reproduction:
Other WOAH-list poultry diseases in the Netherlands subject to compulsory notification Duck viral hepatitis (Source: GD)
Gumboro (IBD)
(Source: GD, EWS)
Infectious bronchitis (IB)
(Source: GD)
Infectious laryngotracheïtis (ILT)
(Source: GD, EWS)
Mycoplasma synoviaeB
(Source: GD)
Turkey
rhinotracheïtis (TRT) (Source: GD)
Detected by GD:
Reported in EWSC: Broiler breeder pullets: Broilers:
pullets:
poultry:
Types most commonly detected by GD:
Broilers: Layers:
Reported in EWSC: Layer pullets: Layers:
Broiler breeders:
Serological monitoring and/or dPCR GD:
Broiler pullets:
Broiler breeding:
Broiler breeder pullets:
Broiler breeders:
Layer pullets:
Layer rearing:
Layer breeder pullets
Layer breeders:
Layer pullets:
Layers:
Turkeys:
Detected by GD:
Broiler reproduction sector (incl. pullets):
Layer reproduction sector (incl. pullets):
Broilers:
Layer pullets:
Layers:
Meat turkeys:
Backyard poultry:
Royal GD P.O. Box 9, 7400 AA Deventer
Disease/disorder/ health characteristic
Other poultry diseases
Avibacterium paragallinarum
(Source: GD, EWS)
Histomonosis (Source: GD)
Pasteurella multocida (Source: GD)
Erysipelas (Erysipelothrix rhusiopathiae)
(Source: GD)
T. +31 (0)88 20 25 575
info@gdanimalhealth.com www.gdanimalhealth.com
Reported in EWSC: (see §5.6.1)
Layers: Small commercial farms (<250)
Backyard poultry:
Detected by GD: (see §5.5.1)
Reproduction (meat sector):
Reproduction (layer sector):
Layer pullets:
Layers: Meat turkeys:
Backyard poultry:
Detected upon necropsy: Layers:


Number of EWS reports

Continuation of
of farms
Detected by GD: Layers: Number of
Ç Moderate to marked increase
Ç Slight increase
- No change
È Slight decrease
È Moderate to marked decrease
A Based on serological monitoring
B Based on serological monitoring and/or differentiating Ms-PCR
C Early Warning System


