

LIFE SCIENCES

ECBuild, LLC is a client-focused Construction Management firm specializing in Life Science, Laboratory and Regulated Pharmaceutical Manufacturing facilities.
We understand that each project has its own set of challenges, and we tailor our services to ensure successful outcomes. This is realized through the depth of knowledge in our teams as well as the variety of delivery methods we offer, including CM@ Risk, Lump Sum, ECPM, and Design-Build.
Our market profile within the Pharmaceutical and Life Sciences industry includes:
– cGMP Manufacturing Facilities
– Medical Device
– Aseptic Filling
– Oral Solid Dosage
– Cell & Gene Therapy Commercial Production Facilities
– Blood Processing Facilities
– Research Laboratories
– Process Development Laboratories
– QA/QC Laboratories
– Pilot Plant Facilities
– Chemical Plants

Services:
Pre-Construction
Procurement
Construction Phase
Construction Management
Project Management
Office Locations:
Atlanta, GA
Baltimore, MD
Ft. Worth, TX
Philadelphia, PA
Raleigh, NC
Wayne, PA
Services:
Architecture
Engineering
Interior Design Planning
Office Locations:
Atlanta, GA
Baltimore, MD
Charlotte, NC
Ft. Worth, TX
Irvine, CA
New York, NY
Philadelphia, PA
Raleigh, NC
San Diego, CA Wayne, PA
One Team. One Common Goal.
History
After working together successfully for over two decades and completing over 100 projects, the founding members of ECBuild decided to join forces in 2019 to start our own Construction Management company - ECBuild. Our goal is to provide superior construction management services to better serve our clients. Our hands-on approach to project management is based on proactive communication and mutual respect.
Our partner, EwingCole, is a nationally recognized, award-winning architecture, engineering, interior design and planning firm, offering services in a range of market sectors including Science & Technology, Healthcare, Government, Academic and Sports & Entertainment.
Success
For over two decades, the principal members of ECBuild have worked with EwingCole - as well as other design firmsearning a reputation for efficiently and effectively delivering projects on schedule and within budget.
Given the support and trust of our combined long-term clients, we have been able to strategically accelerate our project capacity and win new work. ECBuild provides full pre-construction and construction services with offices in Georgia, Maryland, North Carolina, Pennsylvania and Texas.
Together we have established a company that provides an integrated approach based on industry knowledge, design and construction know-how, and a commitment to unparalleled client service.

WHAT WE DO
Our clients are leaders in their respective industries. We partner with them to bring the latest construction management expertise to each project, to deliver buildings, spaces, and places that advance their mission.
Our diverse group of professionals take this responsibility seriously. As their trusted advisors, we are committed to helping our clients achieve their business and facility goals.

Construction Services
ECBuild provides a wide range of services at each stage of the construction process. Careful attention to pre-construction and procurement sets the framework for efficient implementation and delivery.
Pre-Construction Phase
– Critical Path Method Scheduling
– Cost Estimating
– Document Review
– Constructability Review
– Bid Package Strategy
– Establish Subcontractor Interest
– Long Lead Procurement Strategy
– Value Engineering Analysis
– Site Logistics Planning
– Quality Control Planning & Implementation
– Labor Availability Planning
– Submittal Schedule & Processing
– Coordination Review
– Materials & Equipment Procurement Schedule
– Scope Refinement
– Process Definition
Procurement Procedures
– Advertise locally to ensure maximum participation
– Pre-qualify subcontractors based on safety history & financial stability
– Solicit a minimum of three bids per bid package
– Conduct technical analysis of bids
– Resolve bid clarifications to comply with bid package documents
– Confirm insurance and bonding requirements can be met
– Resolve terms and conditions ensuring prime contract flow downs
– Package a conforming subcontract with relevant contract documents
– Submit a recommendation to award with supporting documentation
– Issue Notice to Proceed/Award based on Client’s approval


Construction Services
ECBuild’s team provides on-site services to manage the construction process through completion and close-out.
– Planning & Site Logistics
– Subcontractor Coordination & Management
– Develop & Track Monthly Reports
- Executive Summary
- Safety Recap
- Key Performance Indicators
- Schedule Update
- Project Billing
- Submittal and RFI Updates
– Provide Change Order Controls
– Establish Quality Management Plans
– Implement Job Safety Objectives
– Monitor & Enforce Jobsite Security Protocols
– Project Close-Out Services
– Commissioning/Validation Support



Design-Build Capabilities
Our unique relationship with EwingCole offers our clients the opportunity to realize their project using Design-Build methodology. We offer unparalleled access to information and streamlined coordination between design and construction. Our design and construction teams have worked together completing many successful projects. ECBuild Construction Management Services are also offered in collaboration with other design firms.
Design & Planning
– Site Planning
– Master Planning
– Feasibility Studies
– cGMP Planning
– Laboratory Planning
– Strategic Facilities Planning
– Architectural Design
– Interior Design
– Process
– Instrumentation & Controls
– Automation
– Visualization & Animation Engineering
– Mechanical/HVAC
– Building Automation Systems
– Electrical
– Lighting
– Plumbing
– Fire Protection/Life Safety
– IT/Telecommunications
– Structural
WHY WE DO IT
Our vision is to produce outstanding projects for clients in the Life Sciences industries, many of whom are at the forefront of developing products and therapies that save lives.
Being a part of that process, to improve the quality of life through bioscience, underlies our commitment to building high quality facilities that meet the rigorous standards for regulations and design. ECBuild has specialized experience in virtually all project types within the Life Sciences industry.
Cell & Gene Therapy
Cell & Gene Therapy (C>) facilities are highly regulated to allow the manufacture of minimally manipulated, complex biotechnology products. These facilities focus on clinical research studies, technology development, and full-scale production. Product manufacturing suites designed for cGMP and GTP (good tissue practices) need to be adaptable to most biologic and cellular processes.

Construction issues include:
– ISO 7 Classification standards for production suites must be met, requiring clean room panel construction.
– Emergency power is typical for all critical manufacturing processes.
– Temporary/Permanent access in suites to set process equipment.
– Utilities organized for easy maintenance.
– Accommodation of LN2 and CO2 bulk tanks and delivery access.



Medical Device
The Medical Device Industry manufactures a wide range of products that diagnose, monitor, and treat diseases and health conditions that affect humans.
Construction issues include:
– ISO Classification ranges based on products. ISO 7 to ISO 9 are typical industry standards.
– Finishes can sometimes be down graded resulting in cost savings opportunities.
– Equipment for manufacturing process is often procured in the EU which opens a series of challenges including customs, approval by local authority having jurisdiction, permitting strategy, UL vs. CE certification, metric connections, and establishing handshakes to avoid scope creep.
– A detailed logistics and rigging plan helps to shorten the installation timeline.
– In manufacturing plants where process systems are complex, lead times on equipment are critical path for most projects.

Aseptic Fill/Finish
Aseptic Fill/Finish is one of the most critical processes for the biopharmaceutical manufacturing of parenteral and other sterile drugs. The process relies on the sterile filtration of the formulated liquid drug product which is then aseptically filled and sealed in pre-sterilized containers (vials, bottles, syringes) within a strict particle controlled filling environment. Subsequent primary and secondary packaging operations ensure the product integrity.

Construction issues include:
– ISO Classification ranges based on ISO 7 to ISO 5 Classification standards are required in production suites, which requires clean room panels construction.
– Depending on process batch sizes, Compounding Reactors can vary from 500 liters to 20,000 gallons. Installation and protection of the larger vessels during construction requires a detailed logistics plan.
– Process piping installation is critical for the performance of this type of facility. A detailed QA/QC plan helps to ensure compliance.
– Passivation approach and implementation timing requires careful coordination between the construction team and the Client, especially with tie-ins to existing systems.
– In applications where CIP and SIP is a process requirement, lead time for block body valves is typically critical path.

Chemical Plants
ECBuild has extensive experience in the implementation of safe handling, receiving, storage and distribution system for solvents and other hazardous materials. Our team is familiar with OSHA Process Safety Management (PSM) requirements including specific documentation to be included as part of technical operating procedures.

Construction issues include:
– Electrical Classification for equipment / instrumentation could require long lead time.
– Fabrication and delivery schedules need to be factored into overall construction schedules.
– A clear delineation of classified boundaries on a project can often yield cost savings.
– Fire protection systems and construction of containment areas require attention to detail, such as costly fireproofing materials for tank legs and specialized coatings.
– Infrastructure requirements are typically complex, requiring technical expertise in the field. Process Waste, Air Emissions and Distillation systems, for example, need close coordination with specialty vendors and often resulting in specialized erection logistics plans. Due to the fabrication timeline of these systems, early procurement packages are often required.


High Purity Systems
The design of High Purity Water generation, storage and distribution systems for biopharmaceutical manufacturing begins with a solid understanding of the regulatory requirements for the various grades of high purity water as applied to cGMP processes including RO/DI, Purified Water (PW) and Water for Injection (WFI).
Construction issues include:
– These systems can be simple or extremely complex.
– Depending upon the chemistry of the incoming water, for example, additional upstream pre-processing steps (singularly or combined) could be required to achieve high purity.
– Lead times for equipment can also be challenging if the project is not properly planned.
– When replacing or adding capacity to an existing system, a logistics plan - including shut down activities and passivation approach - is prepared in advance of the installation and reviewed with the Client.
– Water systems infrastructure, from pre-treatment to generation, requires a full understanding of the system operation and its components to ensure that the procurement of the system is completed without gaps.


Laboratories
Laboratory facilities are complex, technically sophisticated, and mechanically intensive structures that are expensive to build and operate. Hundreds of decisions must be made before and during new construction or renovation that will determine how successfully the facility will function when completed as well as how successfully it can be maintained once put into service.
Construction issues include:
– Clear definition of laboratory equipment and associated utilities is a must to ensure project success.
– Lead times on highly specialized Fume Hoods or custom casework components need to be identified early to validate fabrication and project timelines.
– Equipment final installation requires a logistics plan to establish potential bottlenecks and determine “leave outs” prior to construction completion.
– Coordination of HVAC control devices, valves and electrical panels are ideally accessed from outside of the lab, to minimize disruption or contamination, once the facility is in operation.
– Use of BIM modeling is essential to ensure coordination of trades and avoid device installation conflicts.


HOW WE DO IT
We use our experience to benefit our clients. At the heart of it, our success is based on our excellent track record and collective experiences within the industry.
We bring together technical expertise, creativity, technology and best construction management practices to satisfy the facility needs of our clients. We bring a culture of safety first. We are accustomed to working in complex environments that require validated systems and critical utilities, including ISO classified spaces and continuous 24/7 manufacturing.
We develop project specific execution plans that enable construction while maintaining facility operations. Our methodology is supported by detailed programs for Safety, Quality Control, and Schedule/Cost Management.

Experience
ECBuild has significant, relevant experience designing and building facilities for EU and FDA-regulated pharmaceutical, biomed and medical device manufacturing companies that must comply with current Good Manufacturing Practices (cGMP) and Good Laboratory Practices (GLP). These facilities require special measures to maintain cleanliness, close coordination during shutdown activities and prompt Turn Over Packages (TOP) documentation turnover.
Our staff consists of a diverse team of construction experts that understand cGMP spaces, critical utilities and process systems. We deploy the latest 3D BIM technologies, fabrication methods, industry-leading safety best practices, and quality control procedures on every project. During construction, our team takes significant measures to ensure that compliance with cGMP protocols are implemented.
ECBuild is focused on delivering projects that fulfill the specific needs and objectives of our clients.
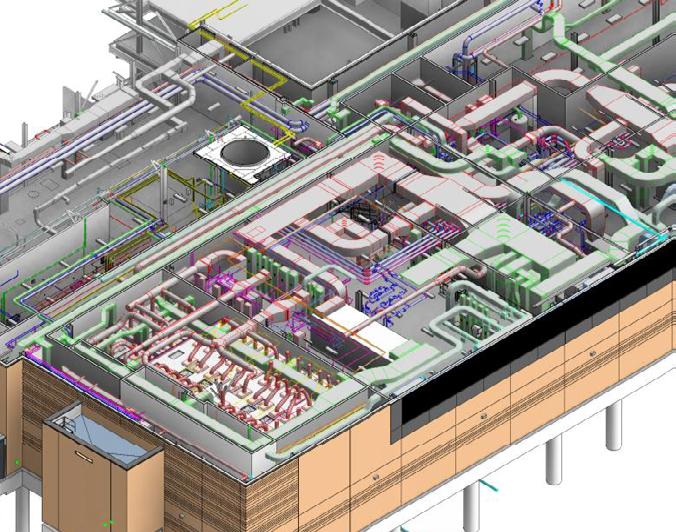


Experience
Our Science & Technology experience includes new and renovated facilities for manufacturing, quality control labs, vaccine development, regional blood processing centers, cell & gene therapy, research labs, clinical production facilities as well as offices, conferencing and workplace amenities.
Our unique relationship with EwingCole offers clients access to all aspects of facility planning and design for Science & Technology programs.
The ECBuild team is dedicated to delivering high quality, integrated construction solutions to help you achieve your vision.
Additional qualifications available upon request.
Project Management
ECBuild was created to support the construction management needs of our clients. To properly and efficiently manage the process, we must fully understand our client’s goals and objectives, milestones, deliverables, and end-user requirements. This enables our team to develop a detailed project plan. We establish key performance indicators based on the established project goals, so that our team can track the project progress.
We provide monthly status reports that measure the current status of the goals versus the set benchmarks.
We are committed to honest, forthright communications and value the trust our clients place in us as good stewards of their resources and investments.
Procore is our primary project management software platform for RFIs, Submittals, Daily Reports, Transmittals, Meeting Minutes, Contract Document Storage, Safety Inspections and Quality Control Inspections. We are well versed in Newforma, CMiC and Projectmates. 1 2
3
Cost & Schedule Management
These two components of the construction process - along with quality control - are intrinsically linked and critical to a project’s success. It is imperative to continuously evaluate and update the project schedule and costs to understand the scope of work completed and identify potential impacts as early as possible.
Our project managers and superintendents are trained in Primavera Scheduling Software and meet weekly
to monitor the project schedule and costs, in close coordination with subcontractors and suppliers. Inevitably, changes will occur during the process due to unforeseen conditions, user modifications, technology coordination or equipment upgrades, to name a few.
We understand the nuances of each phase of the construction life cycle, and the importance of verification, quality control and commissioning to ensure compliance and user satisfaction.
Identifying these issues early, and incorporating them into the schedule, allows the team to minimize and control any impacts. 4

WHAT WE VALUE
ECBuild brings people together into a cooperative effort to support the Life Science Industry.
We provide our clients with high quality facilities that enable research, development and manufacturing of products, cures and therapies to improve the quality of life. We value safety and collaboration on the construction site, which is based on respectful communication and the commitment to client success.

QA/QC Program
Quality Assurance and Quality Control processes are implemented to identify, correct, and document defects or deficiencies during the installation of the work. These tools are used for testing and inspection of completed work to ensure compliance with contract documents.
A full understanding of client requirements, as well as regulatory impacts, allows us to create a plan tailored to project needs.
Pre-Construction Meetings
We conduct Pre-Construction meetings with each trade prior to start of work to ensure full understanding of scope. Participants include core members of ECBuild and design team, subcontractor and QC personnel, and Owner’s representatives. Item include:
– Jobsite Safety Requirements
– Site Logistics and Protocols
– Subcontractor scope of work
– Contract drawings, details and specifications
– Shop drawings and submittals
– QA/QC Manual and Procedures
– Subcontractor and ECBuild QC checklists
Quality Control Manual
Developed at the outset of the project, the QC Manual identifies requirements of the contract documents, specifications, Owner cGMPs, SOP’s, URS and Technical Guidelines as well as procedures for executing a successful workplan, including:
– Submittal review
– Delivery inspection and documentation
– Subcontractor’s self-inspection and checklist sign-off
– ECBuild inspection and checklist sign-off
– Owner Inspection and checklist sign-off
Daily Observations and NonConformance
In addition to formal QA/QC checklists, daily observations are conducted by ECBuild Superintendents and QA/QC Personnel. Any issues flagged for nonconformance are tracked in Procore and shared with subcontractors in real time and at meetings. Items are tracked until corrections are completed and verified.
Total QA/QC Program
Our Project Quality Control Program must also work in concert with the Owner’s Quality Assurance Program. Our goal is to complete inspections, checklists and non-conformance items prior to Owner inspections or testing.
ECBUILD MISSION
To be a high valued construction partner delivering exceptional and consistent results to our clients and a sought-after workplace fostering professional growth opportunities for our employees.
ECBUILD CORE VALUES

Integrity.
It is how we act when nobody is watching.
Relationships.
We build long term and productive relationships founded on trust and mutual respect.

Collaboration.
We work as a team to foster a solution-oriented environment.

Invested.
We give our all and take pride in making a difference.

Driven.
We are inspired and committed to succeed.

