
7 minute read
DiSect
PDA development: Pancreatic cancer typically progress through several stages, with increased stromal infiltrate developing alongside. Credit Joanna Kelly.
Behind the building blocks of pancreatic cancer
A large proportion of a pancreatic tumour is comprised of stromal cells, which effectively protect the tumour and help it to grow. We spoke to Dr Claus Jorgensen about the work of the DiSect project in investigating communication between the tumour cells and the surrounding microenvironment, and its relevance to the wider goal of improving cancer treatment.
The nature of pancreatic cancer means it is difficult to diagnose the disease in its early stages, as the symptoms are not always easy to distinguish from other conditions. This means that most patients are diagnosed when the disease has advanced to a point where tumour cells have invaded the surrounding vessels or metastasised. “About 80 percent of pancreatic cancer patients are diagnosed at that stage. Only about 20 percent are diagnosed when the cancer looks like it’s been contained within the pancreas, where it hasn’t invaded structures and it hasn’t metastasized,” explains Dr Claus Jorgensen, a researcher at the Cancer Research UK Manchester Institute, the University of Manchester. Those patients who are diagnosed at a relatively early stage are often treated with surgery, and have a better long-term prognosis than patients with more advanced disease, who may need to be treated solely with chemotherapy. “Patients that get surgery have much better 5-year survival rates,” stresses Dr Jorgensen.
DiSect project
A deeper understanding of the structure and composition of a pancreatic tumour is central to improving this picture, a topic at the core of Dr Jorgensen’s work in the DiSect project. A pancreatic tumour is surrounded by a thick protective coating called the stroma, which accounts for a significant proportion of its overall composition. “When it’s fully developed as an adenocarcinoma, pancreatic cancer is a very stroma-rich tumour. It’s estimated that around 85 percent of a solid tumour is actually not tumour cells,” outlines Dr Jorgensen. In a healthy individual stromal cells play a supporting role to other cells, but Dr Jorgensen says that in patients with pancreatic cancer they are essentially hijacked. “Tumour cells start sending out signals that make the stromal cells behave in a manner that supports the tumour,” he says. “They hack normal developmental programmes, and try and utilise them to support tumour growth.”

Credit Christopher Below
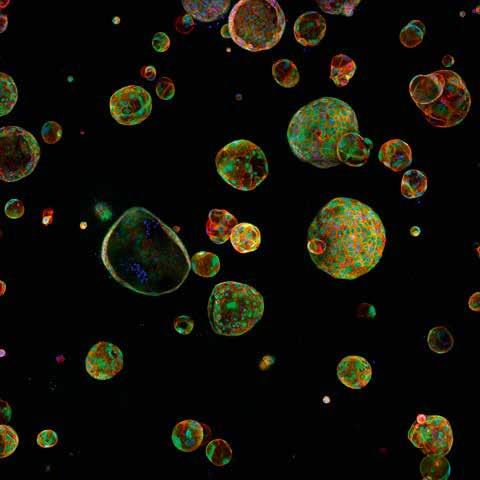
This means in pancreatic cancer that there is an expansion of stromal fibroblasts, which create an extra-cellular matrix resembling scar tissue, to support the tumour. There is also a regenerative response, where new cells come into the pancreas. “A lot of the population of new cells which you see in pancreatic cancer are part of the first response, where these cells come in and act in an immune suppressive way,” explains Dr Jorgensen. The stromal responses develop as the tumour evolves, although it’s not clear if the stroma develops in parallel with the tumour. “Are there any differences between the earlier and later stages of the stroma? In the DiSect project, we aim to better understand some of these mechanisms,” continues Dr Jorgensen. “We have a pretty good idea of the individual mutations acquired by the tumour cells. For example, the starting mutation that you see in pretty much all early lesions involves activating the oncogene called KRAS.”
Researchers have also identified a number of other important factors in tumour development, such as the loss of a tumour suppressor called p53, which plays an important role in preventing cancer formation. This information is being used in the project to replicate various stages of tumour development, from which Dr Jorgensen and his colleagues hope to gain deeper insights into how tumour cells engage with the stroma. “Does a tumour cell that has mutated KRAS and has lost its p53 function interact differently with the tumour microenvironment than a tumour cell at an earlier stage? How will the nature of these interactions shape the composition and function of the tumour microenvironment?” he outlines. Researchers are also investigating PanINs (Pancreatic Intraepithelial Neoplasia), a potentially malignant precursor to pancreatic cancer, but which often doesn’t develop into a tumour, which raises important questions. “Is that because the stroma, at that stage, restricts rather than promotes growth?” asks Dr Jorgensen.
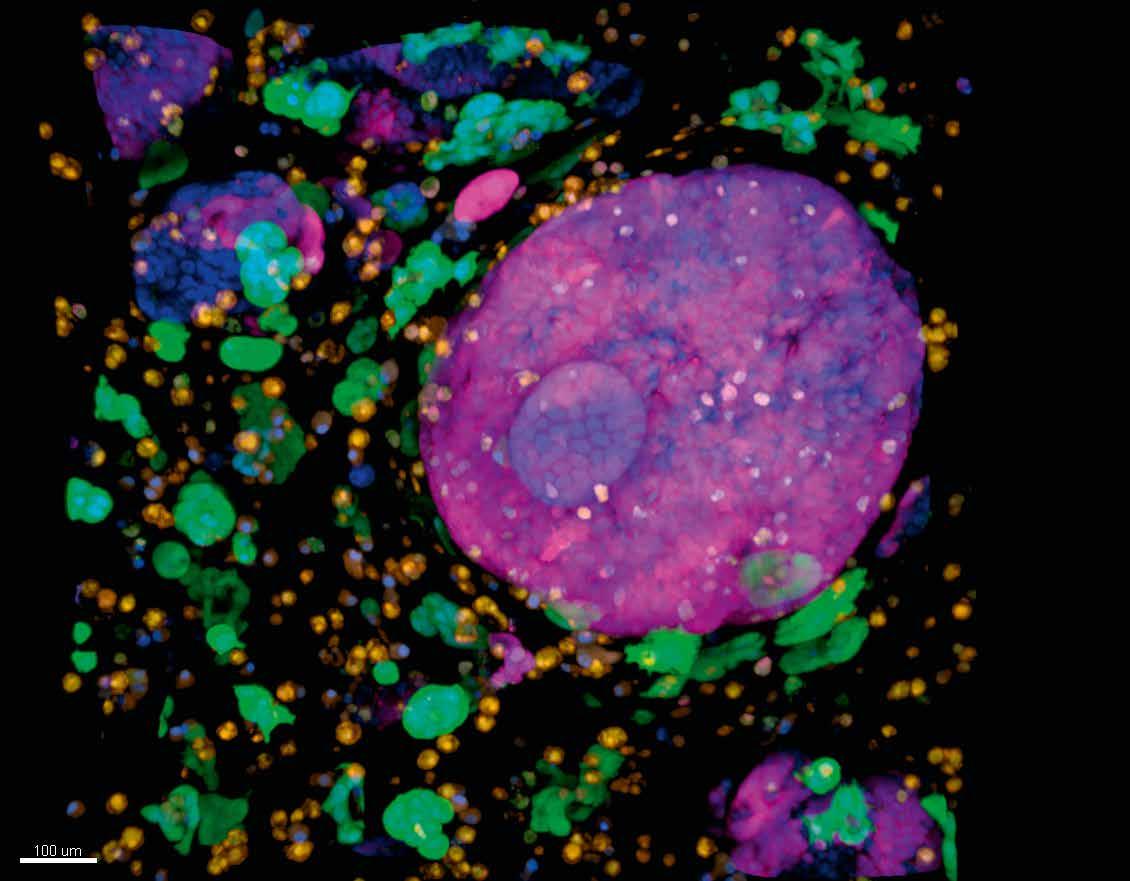
Background image courtesy of The National Cancer Institute.
Microenvironment
The aim here is to understand the nature of the relationship between tumour cells and the surrounding microenvironment. One part of this work involves investigating a heterogenous population of tumour cells and looking at how they interact with cells in the microenvironment. ”We isolate individual tumour cells from a tumour, then grow out clones. Then we study how those clones interact with the microenvironment,” explains Dr Jorgensen. Researchers have found that different tumour cells engage the microenvironment in different ways. “We’ve found that there are three general types of interaction with the microenvironment. That drives a different phenotype of the microenvironment, and that then engages the tumour cells differently,” continues Dr Jorgensen. “So essentially, you cannot assume that the microenvironment always engages tumour cells in a similar manner.”
Evidence suggests that the way the microenvironment engages tumour cells is tailored to the nature of those cells. This is an important observation in terms of the wider goal of moving towards more personalised treatment of pancreatic cancer. “Perhaps in future we need to think about profiling not just tumour cells, but also profiling the microenvironment, so that we have a more refined view of a tumour,” says Dr Jorgensen. In

another study within the project, Dr Jorgensen and his colleagues aim to take a very big-picture view of these tumours. “We’ve essentially mapped out all the individual cell populations that are in the tumour microenvironment, to really get a very detailed view of which cells are there,” he outlines. “We can study individual cells, but until we have an idea of what cells are in the microenvironment, their relative abundance and how they interact, it’s very difficult to develop better models.”
The wider objective in this research is to build a deeper understanding of pancreatic
cancer and contribute to the development of more effective therapies. Current treatment is based largely on chemotherapy, but there’s not been a lot of progress over recent years in terms of improving treatment; one factor behind this has been the relative lack of attention paid to the microenvironment. “One of the missing factors so far has been trying to modulate the microenvironment,” says Dr Jorgensen. Perturbing the cells in the microenvironment could make tumour cells more vulnerable to therapies, opening up new possibilities. “If we can understand the rules by which tumour cells depend on the microenvironment, then maybe we can change those rules and make them miss a growth signal. Will that then open up avenues for providing new therapies to target the tumour cells?” continues Dr Jorgensen.
DiSect The Tumour Stroma as a Driver of Clonal Selection
Project Objectives
Tumors are complex organs that, in addition to the transformed cancer cells, contain infiltrating host cells. It is widely accepted that infiltrating cells such as fibroblasts and immune cells influence the malignant behaviour of cancer cells. In this ERC funded project I am investigating how stromal cells support cancer cells and how specific mutations in the cancer cells modify these interactions.
Project Funding
This project is funded by the European Research Council. Total funding € 1 969 768.
Contact Details
Dr Claus Jorgensen Group leader Cancer Research UK Manchester Institute The University of Manchester Alderley Park Manchester SK10 4TG United Kingdom E: claus.jorgensen@cruk.manchester.ac.uk W: www.cruk.manchester.ac.uk
Dr Claus Jorgensen
Dr Claus Jorgensen obtained his PhD at the University of Southern Denmark in 2005. Following that, he moved to Toronto to train with Dr Tony Pawson, which is where he developed his fundamental interest in heterocellular signalling. He started his laboratory at The Institute of Cancer Research in 2010 and relocated to the CRUK Manchester Institute in 2014, where he is now a senior group leader.



