How to repair a damaged heart?

A blockage in one of the arteries that brings blood to the heart leads to an ischemia, restricting the flow of essential nutrients and damaging cardiac muscle, with lasting effects on heart function. Researchers in the BRAV3 project are working to develop a personalised, biological device to support a damaged heart, as Dr Manuel Mazo Vega explains.
The effective function of cardiac muscle depends on a continuous supply of nutrients, in particular oxygen, and an occlusion or blockage in the arteries that bring blood to the heart can rapidly have serious consequences. During a myocardial infarction an arterial blockage causes an ischemia, which restricts the flow of blood and essential nutrients to the heart. “When an artery is occluded cardiac muscle starts dying within minutes,” explains Dr Manuel Mazo Vega, coordinator of the Regenerative Medicine Research Group at the University of Navarra in Spain. While most patients in developed countries survive a myocardial infarction, it does affect their long-term heart function. “When cardiac muscle dies the organism starts a repair process very similar to what happens when you cut your skin. It creates some blood clots, then fibroblasts – which are like stromal cells –that synthesise the extracellular matrix. So they create a scar, which prevents the heart from bursting,” continues Dr Mazo Vega. “However, this scar is there permanently, and the heart has less muscle to contract, so function declines. This leads to a series of problems that become chronic.”
BRAV3 project
This issue is central to the work of the BRAV3 project, an EU-funded initiative bringing together partners across Europe, coordinated by Prof. Felipe Prósper from the University of Navarra. The aim in the project is to essentially develop a new method of repairing a heart damaged by ischemic cardiomyopathy and restoring its function, building on earlier research into induced pluripotent stem cells (iPSCs). “iPSC technology was developed in 2006. With this technology we can take any cell – usually a blood cell or a skin cell – and turn it into a stem cell that will later be transformed into a cardiac cell,” says Dr Mazo Vega. This opens up the possibility of producing cardiac cells, which can then potentially be injected back into a damaged heart, yet Dr Mazo Vega says this approach is not very effective.
“Around 90-95 percent of the cells injected


into the heart disappear within 24 hours,” he explains. “Over the last 20-25 years there’s been increasing interest in building tissues and organs in the lab. People are trying to get input from different fields, including stem cells, tissue engineering, and cardiology for example, to build something meaningful in terms of treating patients.”
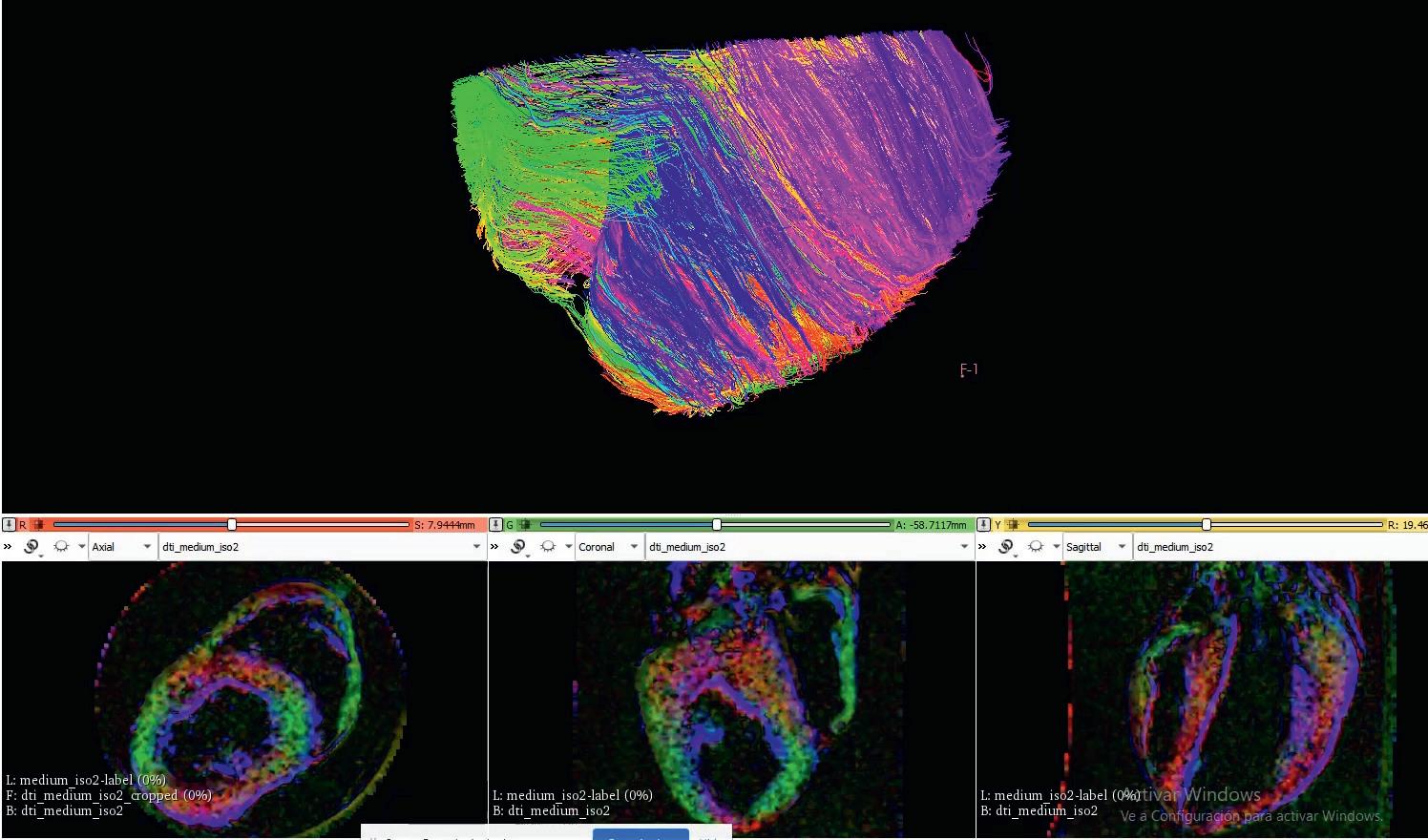
throughout its thickness. This is how the heart has evolved over millions of years in order to pump blood as efficiently as possible,” he outlines. Researchers are using magnetic resonance imaging (MRI) on pigs’ hearts to determine this structure to a high degree of precision, which can then provide a solid basis for designing and producing
Over the last 20-25 years there’s been increasing interest in building tissues and organs in the lab. People are trying to get input from different fields, including stem cells, tissue engineering, and cardiology, to build something meaningful in terms of treating patients.
The BRAV3 project is now working towards this wider goal, with Dr Mazo Vega and his colleagues bringing together these different strands of research to develop a device called BioVAD (Biological Ventricular Assisting Device), which will be tested on pigs. Evidence shows that for such a device to work effectively in the heart, it needs to closely resemble natural structures, so Dr Mazo Vega says the BioVAD has to mimic the structure of the cardiac tissue that it is going to replace on a 3-dimensional level.
“The orientation of the different layers of tissue in the heart changes slightly
the BioVAD. “We can then use 3-D printing, and other materials and technologies, to structure stem cells in such a way that they can produce this tissue in a way that mimics the structure we see from the MRI images,” says Dr Mazo Vega.
A lot of input is being provided here by computational models, which help researchers identify the key points that need to be addressed within the structure of the BioVAD, without the need for expensive tests. Cells in the device are arranged on a polymeric scaffold, and a lot of attention in the project is focused on ensuring the cells
are oriented in the ideal way, which Dr Mazo Vega says is crucial to the effectiveness of the BioVAD in helping to restore cardiac function. “We can develop cardiomyocytes in the lab, muscles which contract. But if you have one cell contracting in one direction, and others contracting in different directions, then the sum of all these forces is going to be very minor,” he points out. The orientation of cardiac layers varies across the thickness of the heart, which is what researchers in the project are seeking to replicate. “With our 3D printing technology we can build scaffolds with very different geometries. This geometry is then going to affect the functionality of the tissue that we build,” continues Dr Mazo Vega. “We can build very, very thin fibres that can help cells to align themselves in certain orientations.”
The project team have investigated a variety of different geometries, and the results have been put into computational models, which provide deeper insights into how cellular orientation affects function. So far this work has centered on relatively thin layers of the myocardium, with researchers planning to investigate thicker layers of tissue in future, which will add a further degree of complexity. “Vascularisation is an important consideration in this respect.

When we have a thicker layer of the myocardium, we will want to connect with the endogenous blood supply,” says Dr Mazo Vega. There are a variety of different types of cells in the heart, so the project team are working to identify the right combinations and proportions, alongside pursuing several other avenues of research. “In the project we have been investigating what the cardiac structure is like on a very precise level, then we want to translate this structure into 3-D printed scaffolds,” outlines Dr Mazo Vega. “What types of cells do we need to put on the scaffolds to develop the most therapeutically efficient tissue?”
Long-lasting support
This work is currently in progress, with researchers aiming to develop a device that can provide long-lasting support for people who have suffered cardiac damage, potentially enhancing quality of life and relieving the heavy burden that heart disease places on healthcare organisations. The initial aim is to develop a device that works effectively in pigs, bypassing testing on rodents, which Dr Mazo Vega says is not always a reliable guide to its effectiveness in larger animals. “We’ve seen that when new devices are tried on mice and rats they often work very well. But when this


is translated into a larger animal like a pig – usually as an intermediate step between the lab and the hospital – there is still an effect, but it is diminished,” he explains. If the device is shown to work effectively in pigs this will provide a stronger basis for its eventual translation to human patients, and a series of pilot tests have already been conducted, which will provide important insights into some practical issues. “We wanted to assess whether we can bring the cells from one country to another. Can we produce large scaffolds with tissues, and then put them into pigs?” outlines Dr Mazo Vega.
The size of the scaffold has evolved significantly over the course of the project, and an advanced design will soon be tested on a relatively small number of pigs, with researchers looking to assess whether it produces any functional effect. This study involves transplanting human cells, so it was necessary first to suppress the pigs’ immune systems – to prevent them from essentially eating up the cells – which proved challenging. “We expected immune-suppression regimes that we could use on pigs would already be available, but this wasn’t the case. Fortunately some people in the BRAV3 consortium were addressing this topic outside the scope of the project. We were able to tap into their knowledge, and now we’re moving forward with the tests,” says Dr Mazo Vega. While it’s not currently realistic to restore cardiac function to normal levels, Dr Mazo Vega hopes the device will have a positive impact, and
that the study will also help guide ongoing development. “The information gained from the study will help us iterate the scaffold design, to create something that is even more beneficial,” he continues. This device can be produced in different sizes to match the physiology of an individual, providing a more personalised means of treating ischemic heart disease.

The orientation of the different layers of tissue in the heart changes slightly throughout its thickness. This is how the heart has evolved over millions of years in order to pump blood as efficiently as possible.
The long-term vision is that a patient who has suffered a cardiac infarction would be assessed in hospital, and a BioVAD would then be produced, tailored to their specific circumstances and needs. “From the initial assessment we would find out the size of the ischemia, its position, and its geometry. That information would then be fed through the new computational models that have been developed in the project, and we would then print the BioVAD, based on knowledge of the infarction,” outlines Dr Mazo Vega. The focus in BRAV3 is ischemic heart disease, yet Dr Mazo Vega believes the project’s research also holds wider relevance to other areas of regenerative medicine. “Our workflow involves seeing a patient, getting an image of the damaged organ, and then producing a living tissue that is created for that specific problem. This kind of workflow could also be applied in other areas,” he says. While it is likely that different stem cells and printing technologies would be required, a similar workflow to that used in BRAV3 could be applied to treat other conditions, such as arthritis or large bone defects. “An individual patient would have a scan, then a cell-based construct could

be built to address the specific problem,” explains Dr Mazo Vega. The main focus is on ischemic cardiomyopathy however, and with the project set to conclude in late 2024, Dr Mazo Vega and the international BRAV3 research consortium are looking to build on what has been achieved so far. “We’ve now finalised a set of protocols to produce cells on large scales, as a cardiac infarction may affect up to 1 billion cells. Conventional cellular production methods cannot reach this kind of level, so we’ve set up new procedures, new bioreactors to produce cells in these kinds of quantities,” he outlines. “We’ve tuned the scaffold geometry to match what it needs to provide to the heart, and we are looking to stimulate these tissues to become stronger. Then we will go into pigs, for the final evaluation.”
Managing heart disease
The long-term aim is to develop a device to support healthy heart function in humans over the long-term, which could represent an attractive alternative to current methods of treating and managing ischemic heart disease. A patient living with the condition may need to take regular medication, and managing it can detract significantly from quality of life.
“A patient with ischemic heart disease may have to make regular visits to medical professionals. In the EU around 19 million people are living with the disease, so a lot of people are affected,” says Dr Mazo Vega. A reliable, effective means of supporting an injured heart could have a significant impact in this respect, both on individual patients and also healthcare organisations, which often devote large amounts of

resources to treating cardiovascular disease. Healthcare organisations do not have limitless resources however, and Dr Mazo Vega says cost-effectiveness has been a prominent consideration in the project.
“We incorporate highly detailed economic analysis in the project. We want to show that we are building something realistic in terms of cost,” he continues.
A key challenge here is to demonstrate that the BioVAD will reduce the demand for medical care and help enhance quality of life, bringing wider societal and economic benefits. A device that can help the heart function more effectively would help reduce the need for medication and regular trips to hospital, while also allowing hospitals to use their precious
resources and equipment for other urgent medical needs. “Large pieces of equipment are used in diagnosing and monitoring cardiac patients, like MRI machines and CT scans, and they are effectively bottlenecks in the healthcare system. These big pieces of equipment are also used for cancer patients, patients who have suffered trauma, and others,” continues Dr Mazo Vega. “We are part of a global research community, and we are contributing to progress in our field and helping to bring about change.”
 Reconstruction of cardiac fibre alignment in a porcine heart, showing how the orientation of cardiomyocytes varies. Different colours depict different orientations.
Project discussions during the last Consortium meeting at Leuven.
Discussions at 2023 BRAV3 Consortium meeting in Leuven, hosted by KUL.
Part of UNAV´s BRAV3 team: Dr. Mazo Vega, Dra Iglesias-García, Dra Flandes-Iparraguirre and Dra Montero-Calle.
Reconstruction of cardiac fibre alignment in a porcine heart, showing how the orientation of cardiomyocytes varies. Different colours depict different orientations.
Project discussions during the last Consortium meeting at Leuven.
Discussions at 2023 BRAV3 Consortium meeting in Leuven, hosted by KUL.
Part of UNAV´s BRAV3 team: Dr. Mazo Vega, Dra Iglesias-García, Dra Flandes-Iparraguirre and Dra Montero-Calle.
BRAV3
A cardiac regenerative medicine European initiative
Project Objectives
BRAV3 aims to provide a lifelong functional support to ischemic heart disease patients by combining computational analysis, 3D printing and regenerative medicine to develop a biological ventricular assist device (BioVAD). Its overarching goal is to bring the medical advances close to the bedside in the shortest time possible.
Project Funding
This project is funded by European Union´s Horizon 2020 research and innovation programme under grant agreement 874827.

Project Partners
BRAV3 brings together 14 different partners from six member states of the European Union. In line with the multidisciplinary spirit of the project, the consortium includesthree university hospitals, four universities, three technological research centres, three SMEs, an international company. Here you can find information about all of them: https://projectbrave.eu/partners/
Contact Details
Scientific Coordinator, Manuel M. Mazo Vega Investigador, Área de Terapia Celular Clínica Universidad de Navarra División de Tecnologías Avanzadas Cima Universidad de Navarra T: +34 948 194700
E: mmazoveg@unav.es
W: https://projectbrave.eu/
Professor Felipe Prósper MD, Ph.D Manu Mazo, Ph.D


Professor Felipe Prósper MD, Ph.D is Head of the Cellular Therapy Unit at the University of Navarra, where he also serves as co-director of the Haematology and Haemotherapy Unit. He is a member of the editorial board of 15 national and international scientific journals and has performed more than 30 clinical trials as a Principal Investigator.
Manu Mazo, Ph.D is group leader (PI) of the Cardiac Tissue Engineering research group at the Clínica and Cima Universidad de Navarra. His team conducts interdisciplinary research merging hiPSC biology, biofabrication and biomaterials for Regenerative Medicine and disease modelling.




