HARVEST GUIDE





Maturity Monitoring
The ETS Grape Maturity Monitoring Panels provide sets of analyses requested to monitor fruit maturity.
ETS Juice Panel
Get the complete picture for informed winemaking with the harvest Juice Panel and insights from the 2022-2024 Harvests.
Scorpions:
Find out what microbes are coming in on your grapes using Scorpion diagnostics.
Non-Saccharomyces Yeasts Diagnostics
pages 01-02
pages 03-28
pages 29-30
The PCR-based diagnostics can be used to determine implant success with commercial strains of non-Saccharomyces yeasts. pages 31-32
Yeast Viability by Flow Cytometry
The concentrations of total and viable yeast are important indicators of fermentation health.
Measuring Proteins in Juice
ETS has developed Enzyme-Linked Immunosorbent Assays (ELISA) to monitor proteins associated with heat instability.
Volatile Acidity
Recognize the microbes and conditions that lead to VA formation to develop an effective monitoring and prevention program.
Potential Alcohol
Get a closer look at using glucose+fructose analysis to estimate potential alcohol.
Sugar Analysis
Sugar can mean a number of things. See what’s behind your “Residual Sugar” numbers.
Phenolics Program
ETS offers a full suite of advanced HPLC-based analytical tools to evaluate phenolic compounds in grapes and wines.
Phenolic Maturity
The ETS Red Grape Phenolic Panel
Case Study: Harsh Tannins
pages 33-33
pages 35-36
pages 39-40
pages 41-42
pages 43-44
pages 45-49
The ETS Grape Phenolic Panel in action page 50
Aromas
Detect and prevent common (and uncommon) sensory flaws.
The Impact of Wildfires
ETS offers an extended panel for volatile smoke markers and a glycosylated markers panel this harvest.
Wildfire Impact FAQ
Your most-asked questions, answered.
Comparative Tool for Grapes
Instantly compare your smoke marker levels in grapes to the ETS Baseline Database through your ETS Client Portal.
Harvest Toolkit
This short guide will give you the highlights of our most-requested Harvest testing.
Harvest Satellite Analysis
Use this quick reference to see which Juice Analyses are offered at your local lab.
Sampling and Shipping
Make sure you get the most out of your results using these sampling guidelines.
pages 51-52
pages 53-58
pages 59-62
pages 63-64
pages 66-68
pages 71-72
pages 73-74
Our Locations p. 76- St. Helena p. 77- Healdsburg p. 78- Paso Robles p. 79- Newberg p. 80- Walla Walla

MONITORING PANEL
The ETS Grape Maturity Monitoring Panel provides a set of analyses requested to monitor fruit maturity.
This panel includes the traditional measurements of juice solids (Brix) and acidity (Titratable Acidity and pH), in addition to a more accurate determination of fermentable sugars (glucose + fructose). Malic acid analysis is included, as the degradation of this organic acid is a well-known marker of ripening.
The panel provides berry size parameters (volume and weight), as well as a measurement of sugar per berry. Sugar per berry is a calculation based on the average berry volume as measured by the Dyostem system. It also provides an assessment of berry volume variability expressed as a Coefficient of Variation (%) and a histogram of the berry volume distribution.
Monitoring Sugar per Berry allows growers to determine the duration and rate of sugar loading. Post veraison, vines synthesize and actively transport sugar into berries. At the end of this phase, Brix usually keeps increasing due to berry dehydration. Monitoring Brix cannot determine when the sugar loading phase stops. When sugar loading stops, the actual quantity of sugar accumulated in each berry remains unchanged (see Fig 1).
The duration of the sugar loading phase, the time at which this phase ends, as well as the Brix achieved at that time, are all important indicators of vine growth conditions. Excessive or insufficient vigor, water availability, and resistance to heat stress all have an impact. Vines under moderate hydric stress typically reach between 21 and 23 Brix at the end of sugar loading. Vines with excessive vigor typically see their sugar loading stop before these levels are reached, as vegetative growth may compete with sugar synthesis and accumulation.
These vines often tend to “shut down” more easily in response to heat waves, which can prompt a sudden end of sugar accumulation in berries. Vines under excessive water stress, subject to nutrient deficiencies or diseased, also have difficulty reaching typical Brix levels. At the end of sugar loading, a variety of maturity events are triggered that influence the development of grape aroma compounds and phenolics. This is often used as an indication of when to start grape phenolics measurements (see Vineyard Decisions – Grape Phenolic Panel p. 44)

ETS offers Vivelys Dyostem ® analysis to help characterize vineyards, closely monitor grape ripening, and make more informed harvest decisions.


Winemakers rely on juice chemistry analysis for a complete picture of must composition at harvest that goes beyond traditional TA, pH, and °Brix. This is critical as juice chemistry is often different from vintage to vintage. Combining modern tools gives vital insights to make informed vineyard management decisions, choose harvest dates, predict/adjust wine composition and facilitate fermentations.


A thorough analytical picture gives winemakers the ability to better predict wine composition, and plan appropriate winemaking strategies in response to changing must compositions. Whether you analyze the free-run juice, monitor midfermentation chemistry, or do both, it is important to understand the analytical results in the context of fermentation process stages.
Analyzing juice pre-fermentation provides data that enables winemakers to identify anything unusual about the current vintage in comparison with previous vintages. Such insights can help inform winemaking decisions including acid adjustments and fermentation strategy.
Clients sometimes ask why they see a difference in the concentration of acids, or potential alcohol vs. final alcohol, when comparing their juice samples to postfermentation samples.
It is not unusual to observe differences between the levels of acids, potassium and sugar/potential alcohol in different samples from the same vineyard. Variations can also occur, depending upon sampling strategies and how representative the samples are of the vineyard.
How juice samples are prepared from grape samples for analysis matters. On page 73, we present typical sample preparation procedures.
In white winemaking, the differences in composition between free run juices and the different press fractions are well known.
In red winemaking, free-run juices obtained after filling tanks may not reflect the actual
content of the tank, since components such as acids and potassium can initially be sequestered at high levels in grape tissue next to the skin. As the grape tissue breaks down during cold soak, fermentation, and maceration, the resulting extraction of acids and potassium from the tissue into the juice can contribute to the observed differences.
Likewise, the sugar in raisins or shriveled grapes may take a long time to release during red winemaking, causing an underestimate of fermentable sugar and therefore potential alcohol. Mid-point analysis on the fermenting wine (analyzing glucose+fructose and ethanol) may give a more accurate picture of fermentable sugar and potential alcohol.
All of these factors can contribute to the differences observed between juice samples and the final wine composition.
Because the must components are in a state of flux from cold soak to post malolactic fermentation, many winemakers prefer to make incremental adjustments rather than rely on one initial adjustment. Winemakers who are targeting a certain TA and pH or ethanol level, for instance, often check their wine chemistry again at the fermentation midpoint. These mid-point numbers are used to make ongoing and final fermentation adjustments.


It’s important to note that in ripe fruit, glucose + fructose numbers often appear higher than the corresponding °Brix results. This is because °Brix is measured as a percentage by weight, meaning brix values are greatly influenced by the density of juice. Glucose + Fructose is measured as weight by volume and is independent of juice density. A must with 23.3 °Brix will not have 23.3% by volume fermentable sugar.

°Brix is not a true measure of fermentable sugar. Two juices with identical °Brix may have very different final alcohol concentrations due to varying amounts of fermentable sugars
Sugar concentration increases rapidly in grapes as they mature. This increase is usually due to sugar movement from the leaves to the fruit. During the final stages of berry development, dehydration may also contribute significantly to the final sugar concentration. Rates of sugar accumulation do vary from vintage to vintage.
°Brix is a measure of soluble solids in juice and must. The soluble solids in grape juice are primarily sugars. Other soluble solids, including organic acids can have a significant impact on brix, especially with unripe grapes. °Brix is used as an estimate of sugar concentration and often as a predictor of potential alcohol, but is not a true measure of fermentable sugar. Two juices with identical °Brix may have different final
alcohol concentrations due to varying amounts of fermentable sugars. Observations over previous vintages indicate that the amount of glucose + fructose per degree brix varies slightly from vintage to vintage. This can have a significant impact on potential ethanol predictions based solely on Brix. That said, many people use Brix to monitor maturity or sugar accumulation.
The sum of glucose + fructose measures the two main sugars present in juice that can be fermented by yeast. This analysis provides a sound basis for estimates of potential ethanol in the wine. Fermentable sugar analysis is an important supplement to °Brix testing when final ethanol predictions are critical.
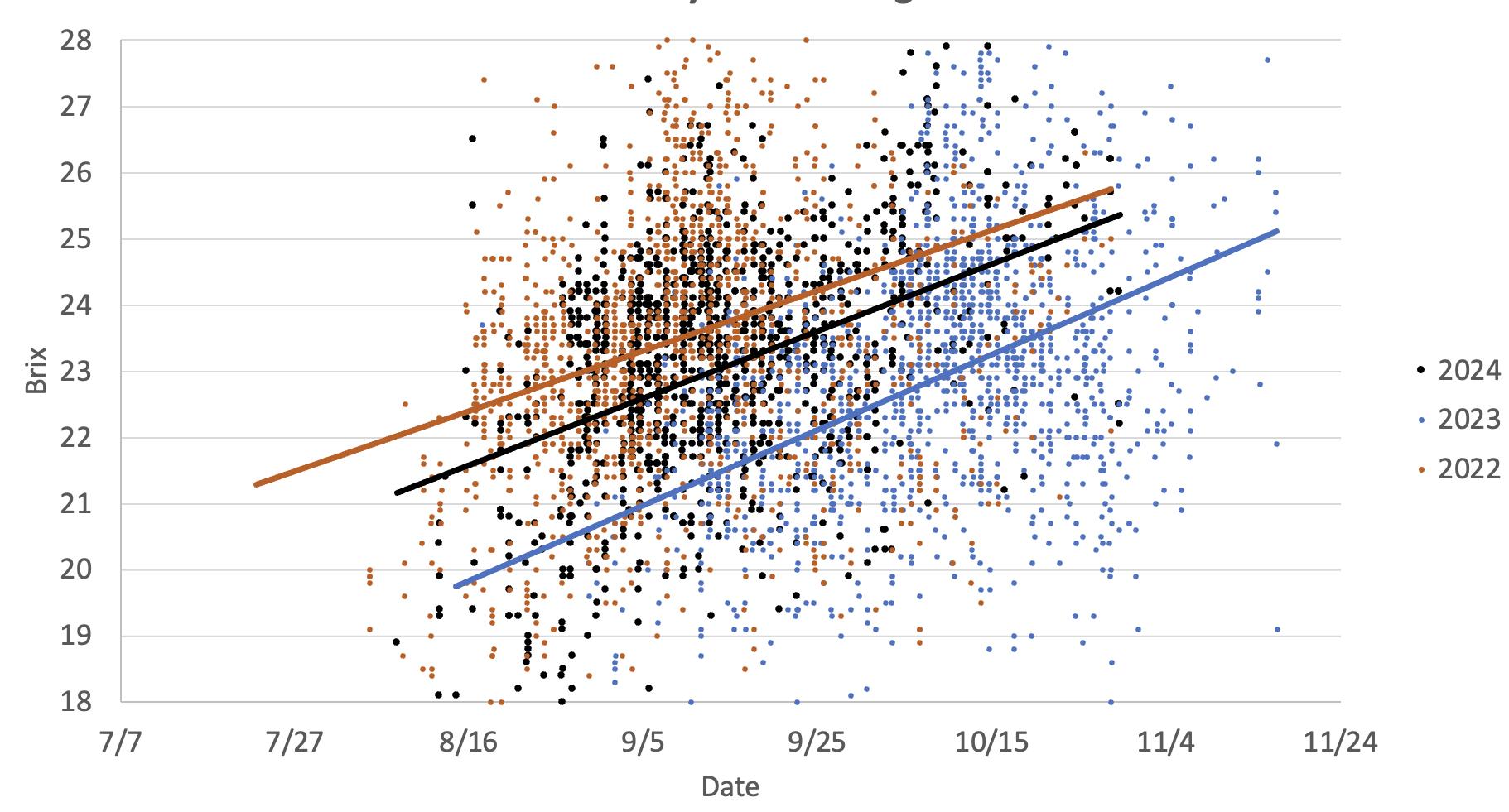
This graph illustrates the different °Brix accumulation rates in two warm vintages (2024 and 2022) versus a cooler vintage (2023). It is evident the °Brix reaches higher values earlier in the season during warm vintages as compared to the cooler vintage.
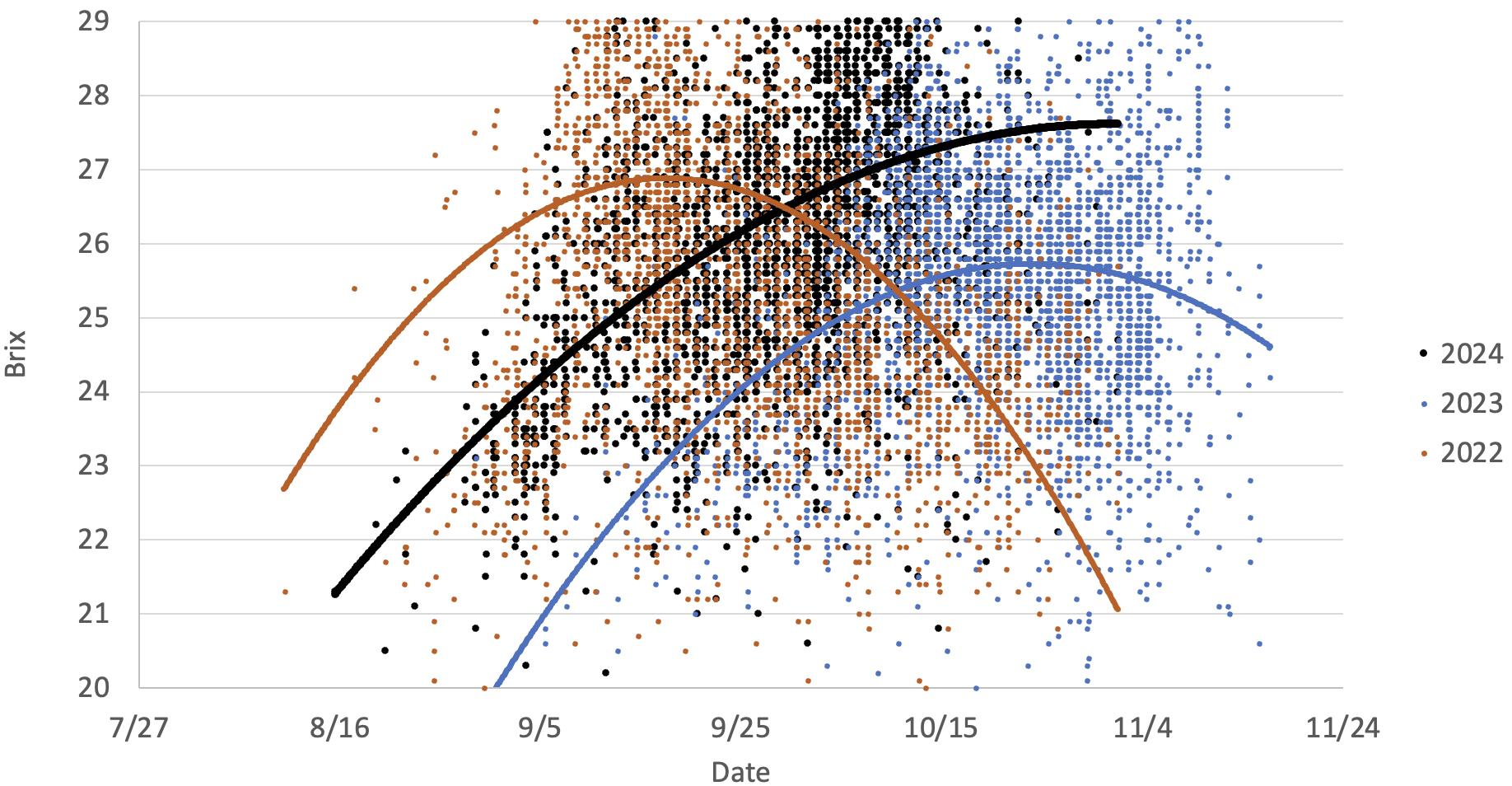
This graph illustrates the different °Brix accumulation rates in two warm vintages (2024 and 2022) versus a cooler vintage (2023). It is evident the °Brix reaches higher values earlier in the season during warm vintages as compared to the cooler vintage. Note the delayed harvest in 2023 as compared to 2022 and 2024. The 2022 data is presented as an exponential curve to highlight the impact of vine damage caused by the mid-September heat waves vines resulting in cessation of sugar production.

luggish and stuck fermentations, coupled with sulfide formation, have become increasingly common and are often associated with deficiencies of yeast assimilable nitrogen in the must. However, excessive concentrations of certain nitrogen compounds have been associated with microbial spoilage and other fermentation problems.
Knowledge of nitrogen status is critical for effective fermentation management. Nitrogen compounds are essential macronutrients for yeast, and are required for cell growth, multiplication, and yeast activity.
As with other juice chemistry components, YAN (Yeast Assimilate Nitrogen) values can fluctuate vintage to vintage
due to changes in ammonia and/or NOPA (Alpha Amino Nitrogen). Monitoring during harvest enables comparison with past vintages. There can be large differences in the amount of YAN in different varietals and in different geographical regions.
Ammonia and NOPA can change independently of each other resulting in different ratios of these two YAN components.
Yeast assimilable nitrogen includes both NOPA and ammonia. Analysis of only alpha amino nitrogen or only ammonia nitrogen does not provide an accurate indication of total nitrogen status for a given must.
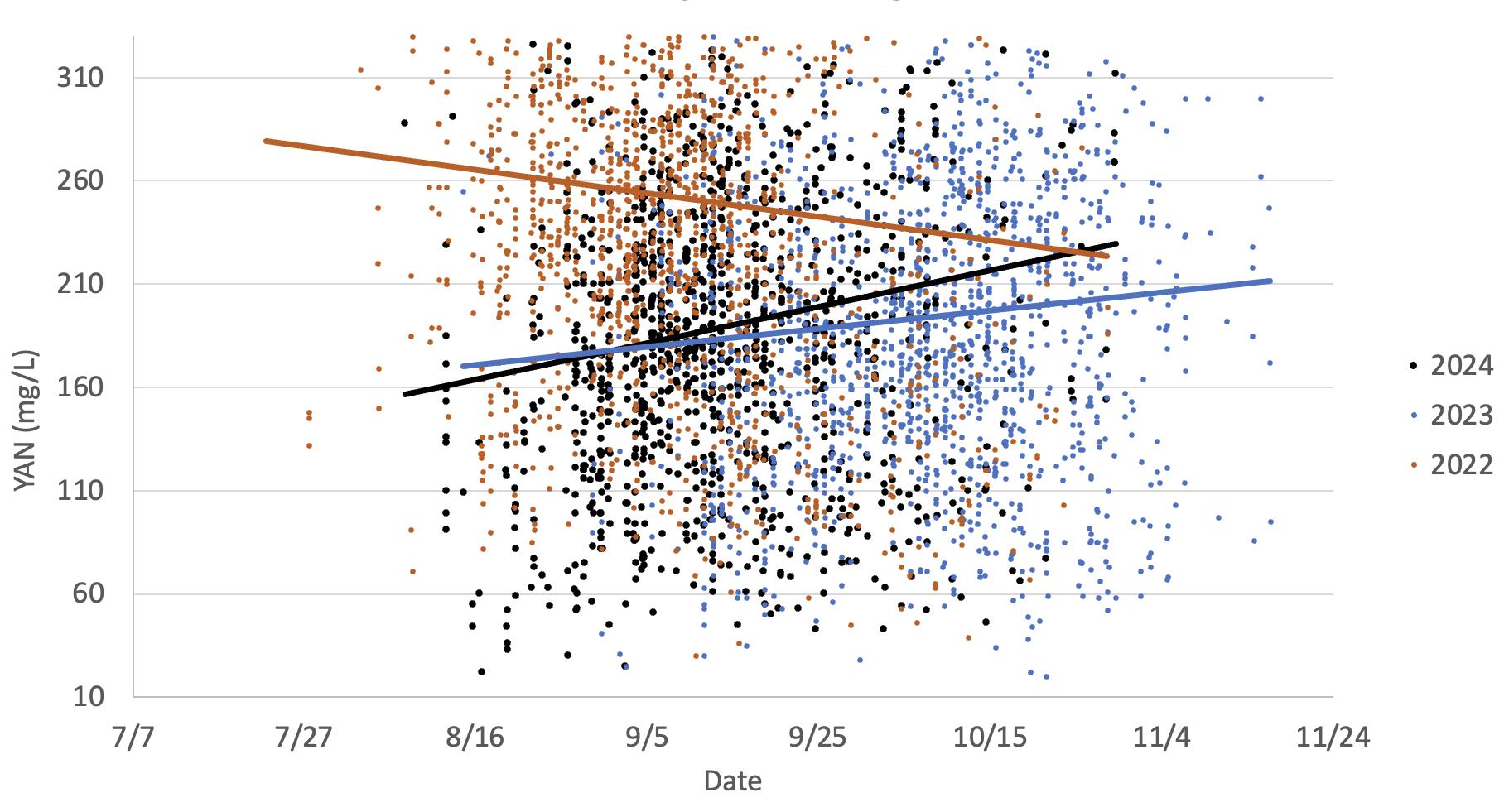
This graph illustrates the differences in Chardonnay grape YAN levels in two warm vintages (2024 and 2022) versus a cooler vintage (2023). Loss of berry water can result in concentration of grape metabolites, including YAN. Many factors, including ground water, previous vintage harvest dates, and vineyard practice can impact the amount of YAN in grapes.

This graph illustrates the differences observed in Cabernet Sauvignon YAN accumulation rates in two warm vintages (2024 and 2022) versus a cooler vintage (2023). Loss of berry water in hotter years can result in concentration of grape metabolites, including YAN. This would be more noticeable in fruit harvested late in the season. Many factors, including ground water, previous vintage harvest dates, and vineyard practice can impact the amount of YAN in grapes.

Ammonia is the form of nitrogen nutrition most easily assimilated by yeast. Wineries routinely supplement nitrogen deficient musts with diammonium phosphate at the start of, or during fermentation to provide adequate nitrogen levels. Additional ammonia analysis and adjustments during fermentation may also be beneficial in minimizing the risk
of stuck fermentation and sulfide formation. Ammonia results are expressed as mg NH3 per liter. These values may be expressed as nitrogen equivalents by multiplying NH3 results by 0.82. Monitoring ammonia trends is important to understand changes in the levels of this rapidly available nitrogen compound.
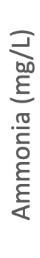
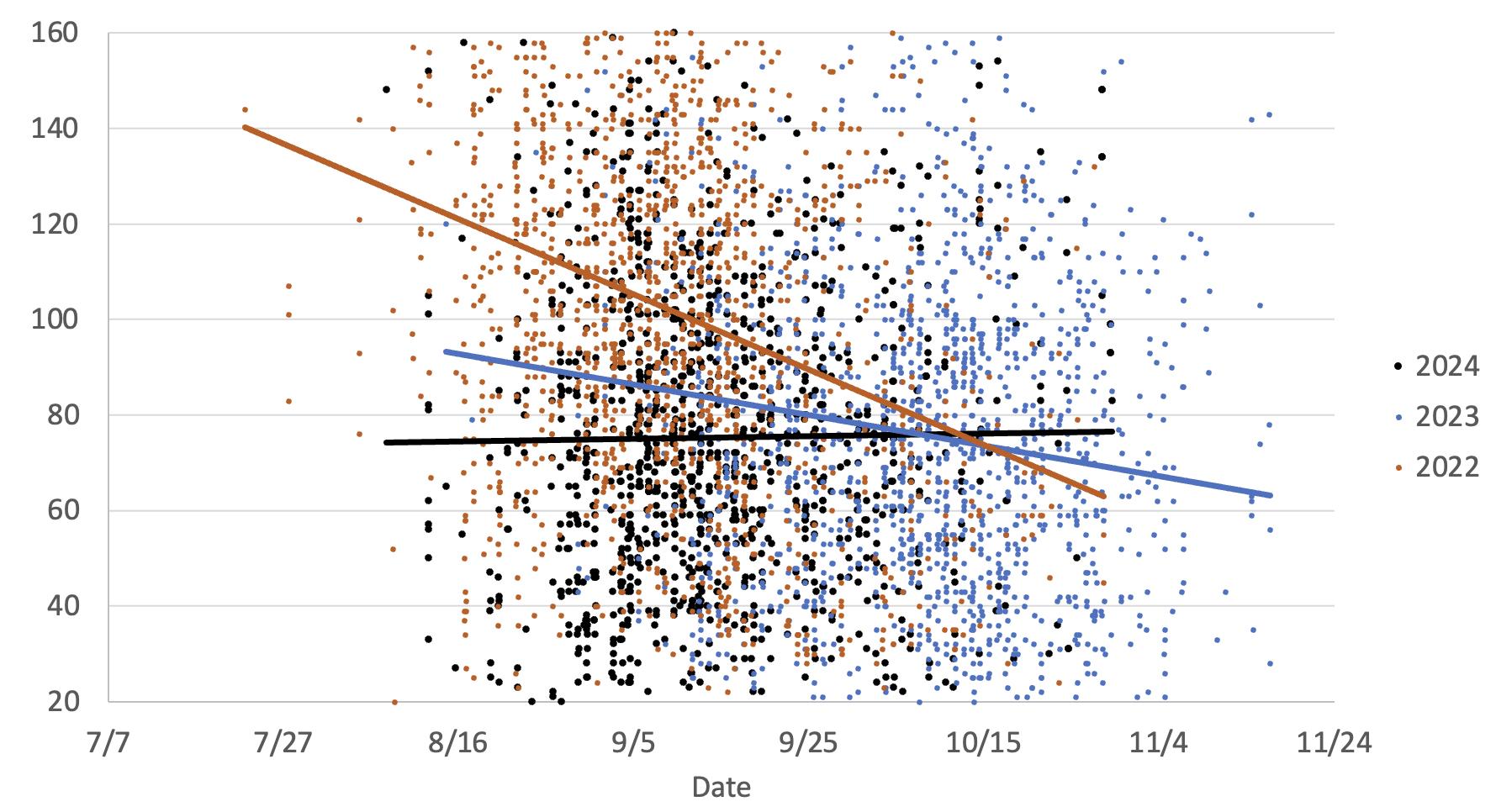
This graph illustrates the differences in Chardonnay grapes ammonia levels in two warm vintages (2024 and 2022) versus a cooler vintage (2023). There doesn’t appear to be any trends related to temperature. Ammonia levels generally decrease or stay flat during maturation. Slight increases could be due to berry water loss.
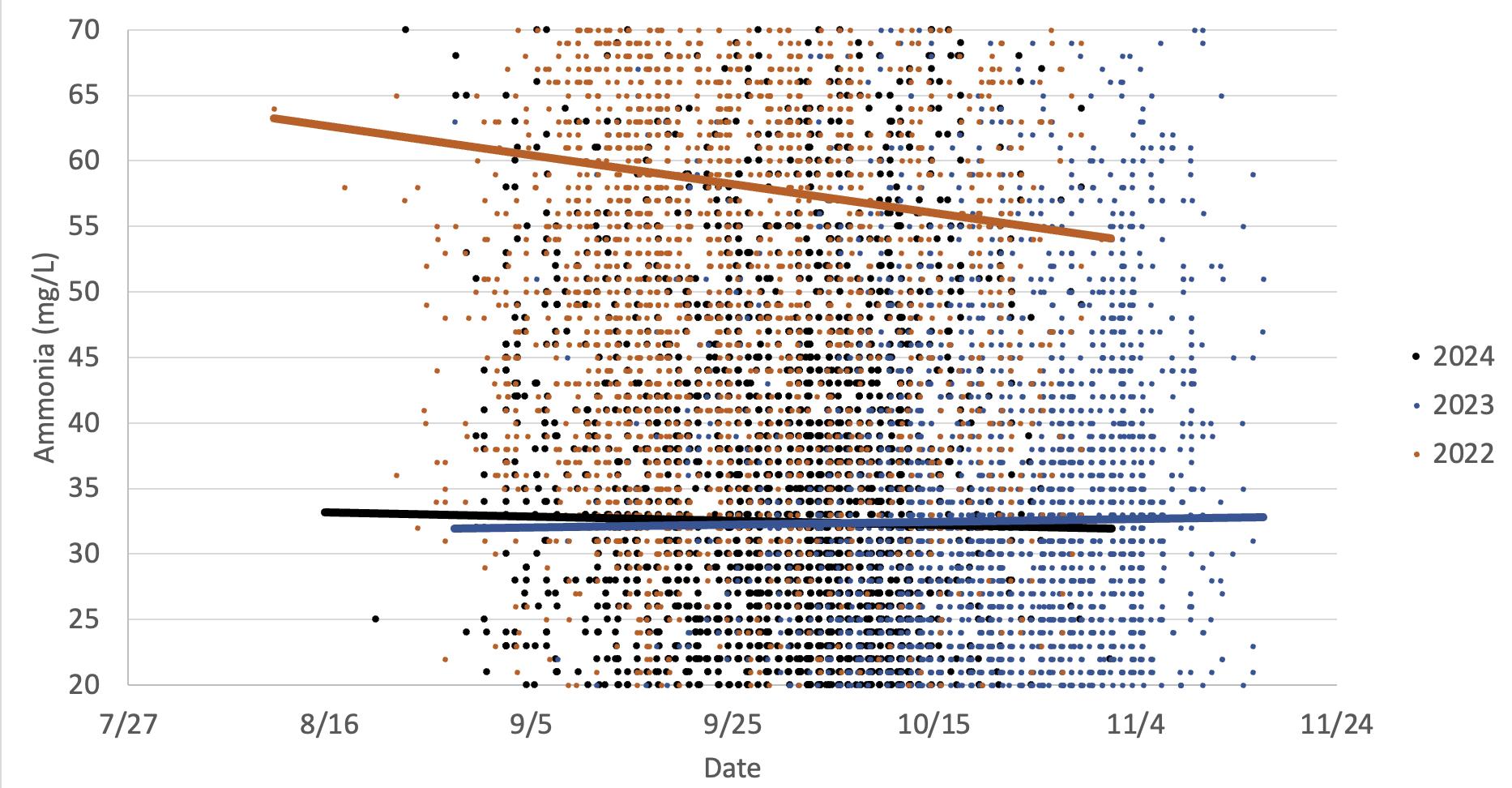
This graph illustrates the differences in Cabernet Sauvignon grapes ammonia levels in two warm vintages (2024 and 2022) versus a cooler vintage (2023). There doesn’t appear to be any trends related to temperature. Ammonia levels generally decrease or stay flat during maturation. Slight increases could be due to berry water loss.

Amino nitrogen, otherwise referred to as “Nitrogen by OPA”, or NOPA, is determined using a method specific for alpha amino groups. It is a measurement of primary amino acids usable by yeast. NOPA does not include proline, which is not
utilized by yeast, nor ammonia. NOPA results are expressed as mg nitrogen per liter. NOPA generally trends upward and is usually the largest component of the YAN.
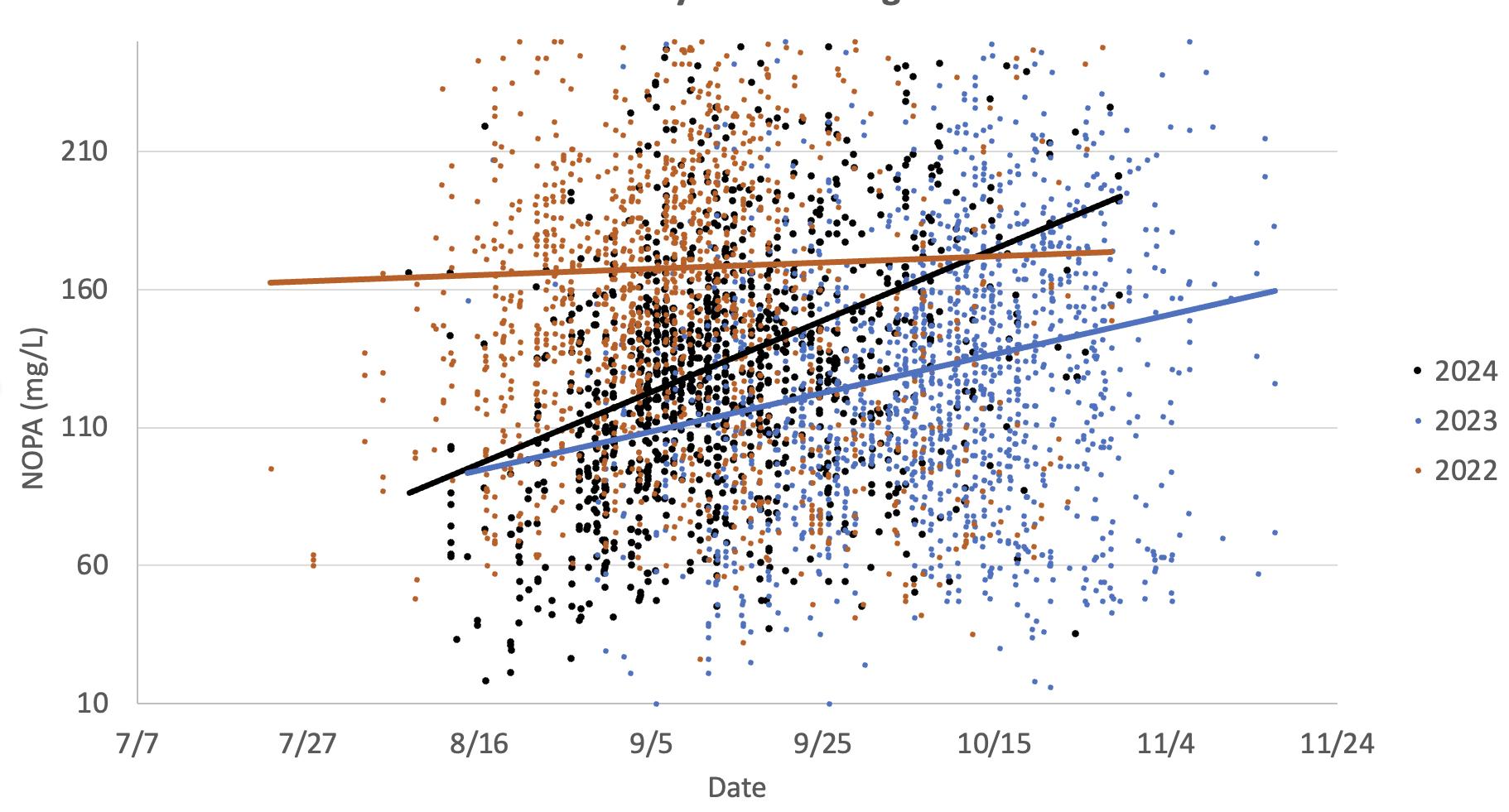
This graph illustrates the changes in Chardonnay grapes NOPA accumulation rates in two warm vintages (2024 and 2022) versus a cooler vintage (2023). The data indicates higher NOPA in warmer years. NOPA levels generally increase during maturation due to protein synthesis and accumulation. Increases towards the end of harvest could be due to berry water loss.
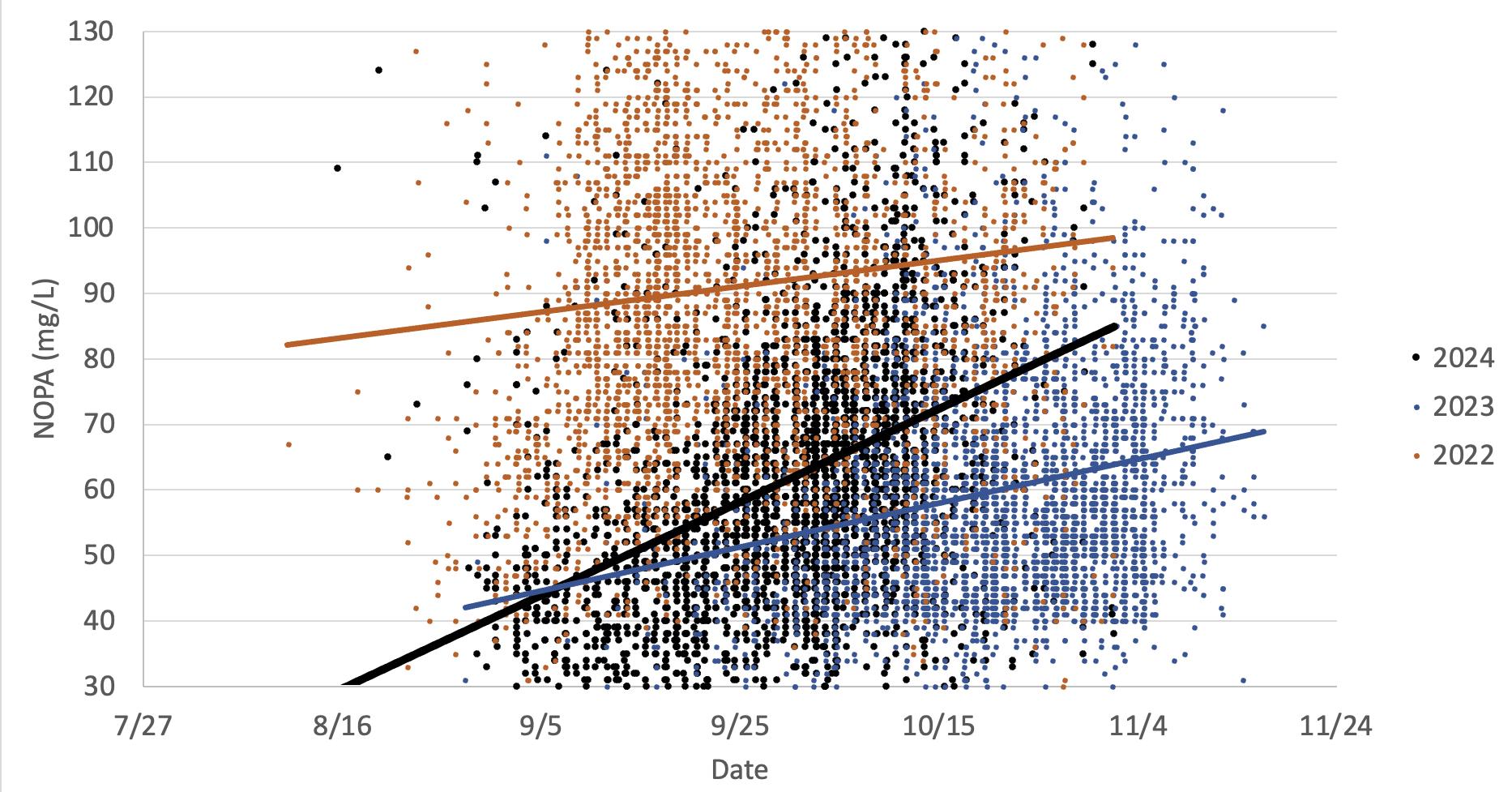
This graph illustrates the changes in Cabernet Sauvignon grapes NOPA accumulation rates in two warm vintages (2024 and 2022) versus a cooler vintage (2023). The data indicates higher NOPA in warmer years. NOPA levels generally increase during maturation due to protein synthesis and accumulation. Increases towards the end of harvest could be due to berry water loss.


The acid composition of must is a complex balance of free hydrogen ions, acids, acid salts, and cations. Concentrations of these various components and their interactions influence many winemaking parameters.
The principal objective of acid management is to achieve and maintain a pH favorable to optimum wine balance and stability.
Vintage variations in any of the components that impact acid balance can result in unexpected changes in the final pH and TA of a wine.
Monitoring the parameters that contribute to pH and TA is an important preview to how the vintage will affect overall acid balance in the wine.

Tartaric acid is one of the two major organic acids found in grapes. It accumulates in grape tissue early during development and declines during ripening due to berry growth and dilution. Tartaric acid is not usually metabolized in grapes. It is present in grapes, must, and wine as a free acid and weak acid-salt complex. Tartaric acid-salts may precipitate, primarily as potassium bitartrate and calcium tartrate.
Both the formation and solubility of salts are affected by a balance of components that are in flux throughout the early life of a wine. An increase in the ratio of the free tartaric acid to the tartaric acid salts will cause a decrease in pH. This will affect the flavor, balance, and stability of the final product.
Tartaric acid is commonly used to adjust the acid balance of juices and wines. Understanding tartrate interactions is critical in designing appropriate acidification strategies.
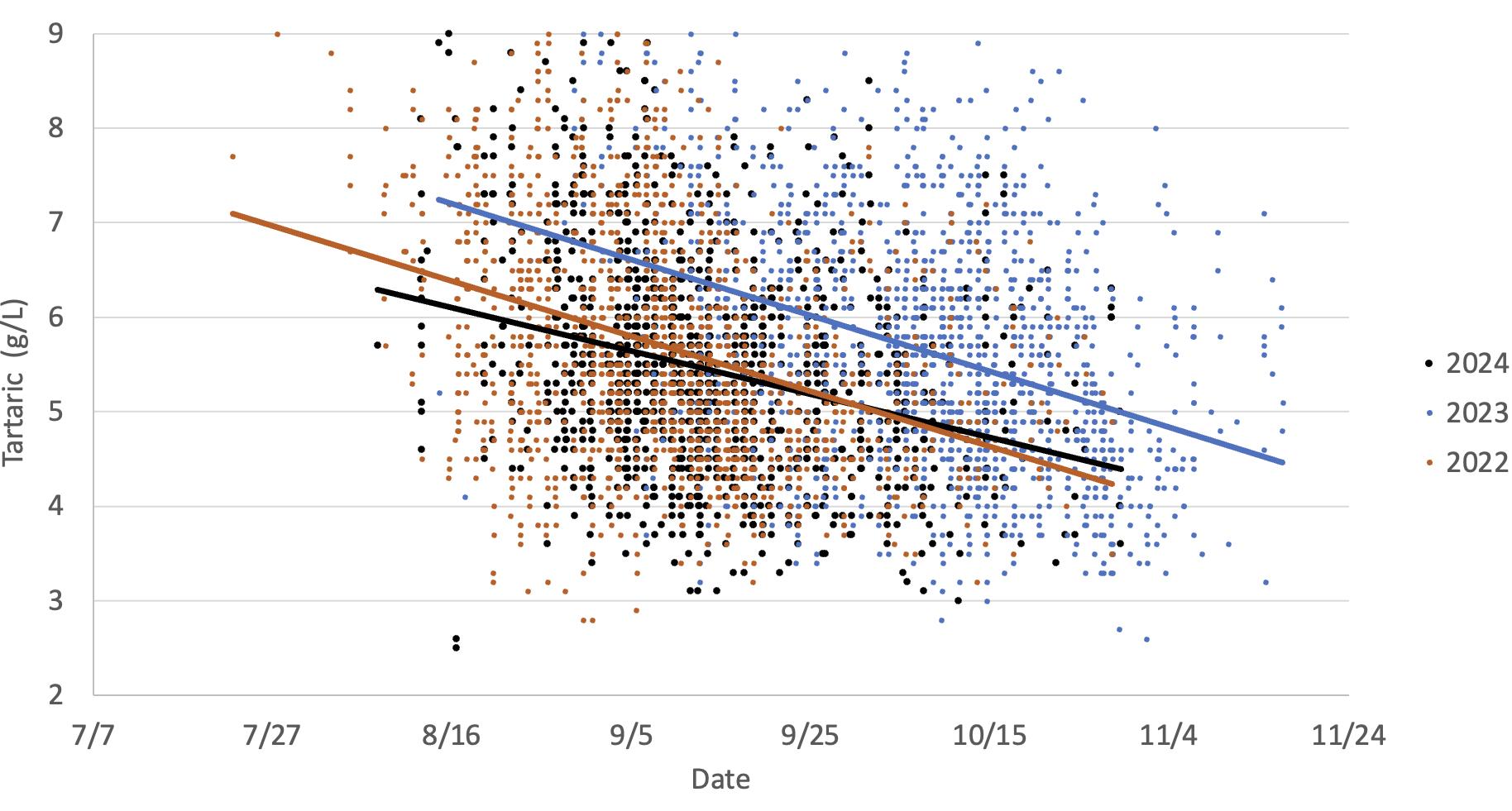
This graph illustrates the differences in Chardonnay grapes tartaric acid loss in two warm vintages (2024 and 2022) versus a cooler vintage (2023). The data indicates tartaric acid drops as the grapes mature, which occurs earlier in warmer years.
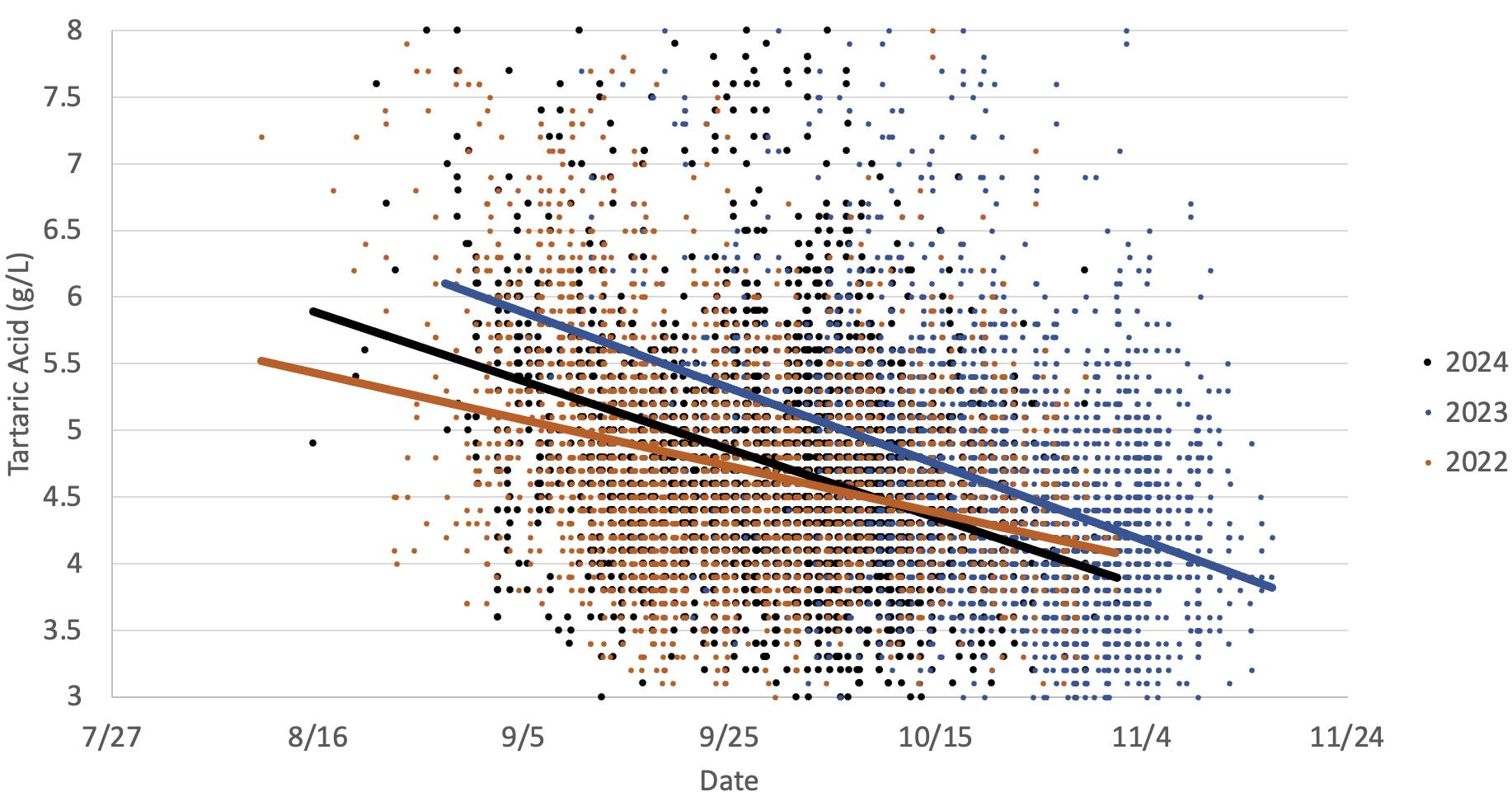
This graph illustrates the differences in Cabernet Sauvignon grapes tartaric acid loss in two warm vintages (2024 and 2022) versus a cooler vintage (2023). The data indicates tartaric acid drops as the grapes mature, which occurs earlier in warmer years.

HH is a measure of free hydrogen ions in solution (which corresponds to the chemical definition of acidity) and is used as a gauge of wine acidity.
Wine color, potassium bitartrate stability (cold stability), calcium stability, and molecular SO2 level are directly related to wine pH. pH is also critical in relationship to microbial stability, interactions of phenolic compounds, and color expression.
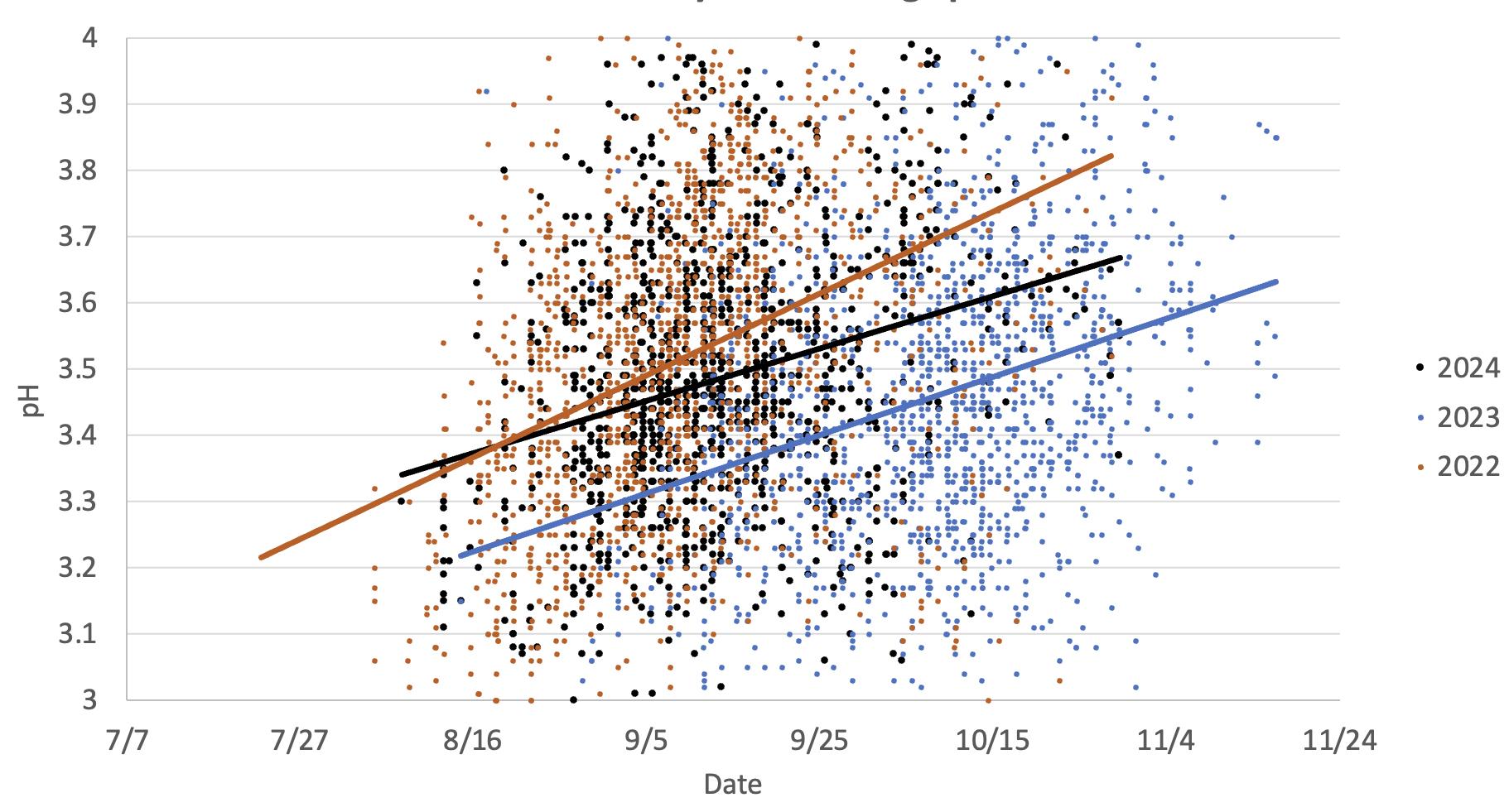
This graph illustrates the pH changes in Chardonnay grapes observed in two warm vintages (2024 and 2022) versus a cooler vintage (2023). The data indicates pH increases as the grapes mature, primarily due to increases in potassium and decreases in malic acid, which occur earlier in warmer years.

This graph illustrates the pH changes in Cabernet Sauvignon grapes observed in two warm vintages (2024 and 2022) versus a cooler vintage (2023). The data indicates pH increases as the grapes mature, primarily due to increases in potassium and decreases in malic acid, which occur earlier in warmer years.

Malic acid accumulates early in berry development and declines during ripening due to dilution and respiration. Viticultural practices and grape cluster environments may directly affect respiration rates of malic acid. Malic acid levels affect pH and titratable acidity.
Malic acid is converted to lactic acid during malolactic fermentation, resulting in the loss of an acid group. The effect of this acid reduction on pH depends upon the initial amount
of malic acid and buffer capacity of the wine. Malolactic fermentation in wines containing low levels of malic acid and high buffer capacity will have little impact on wine pH. Malolactic conversion in wines with high malic acid and low buffer capacity can result in a substantial pH increase. Malic acid tends to be higher in cooler vintages.
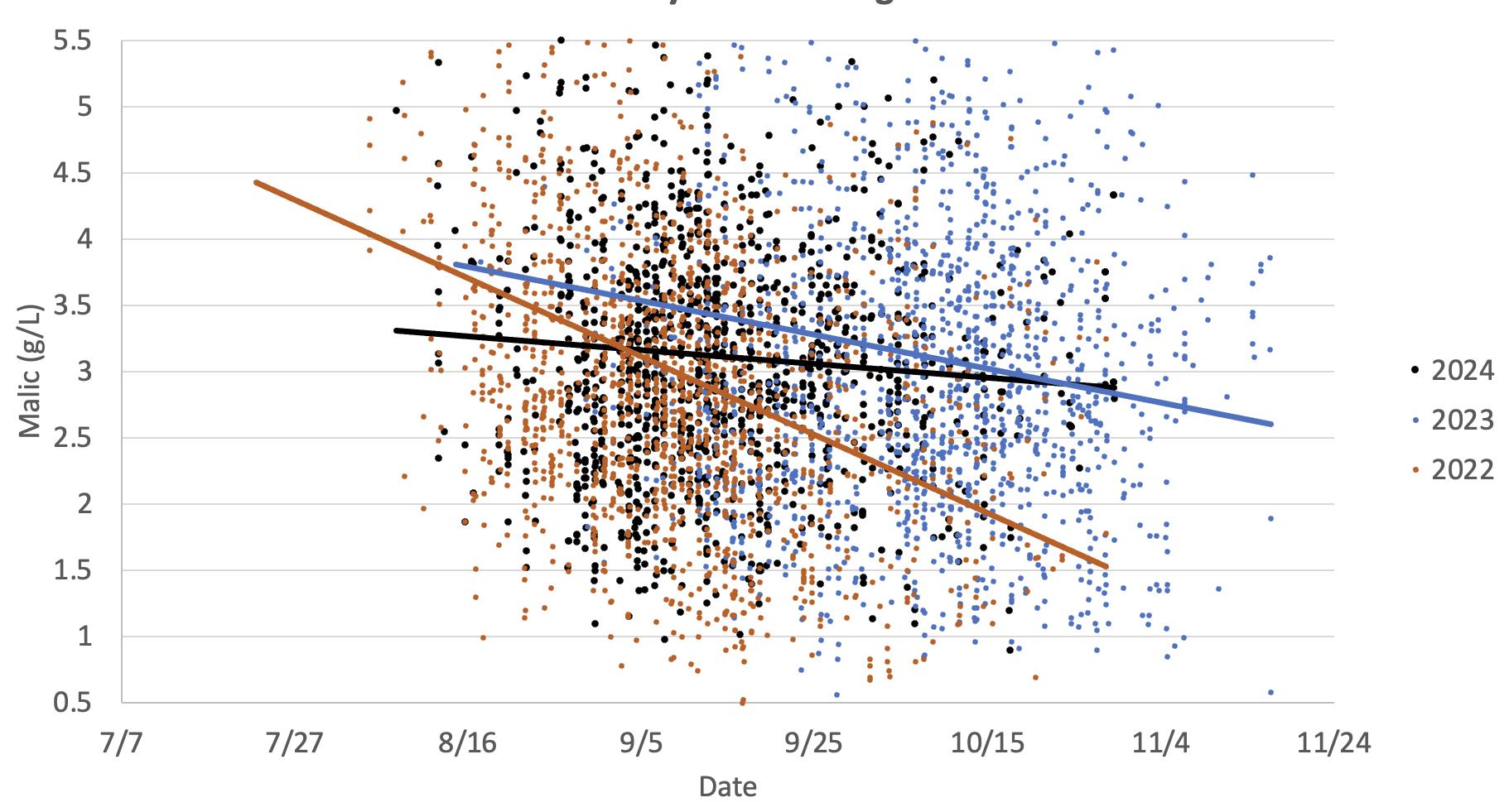
This graph illustrates the loss of malic acid in Chardonnay grapes observed in two warm vintages (2024 and 2022) versus a cooler vintage (2023). The data indicates malic acid is still being produced. This is primarily due to increased respiration, which can be accelerated with reduced differences in diurnal temperature shifts in warmer years.
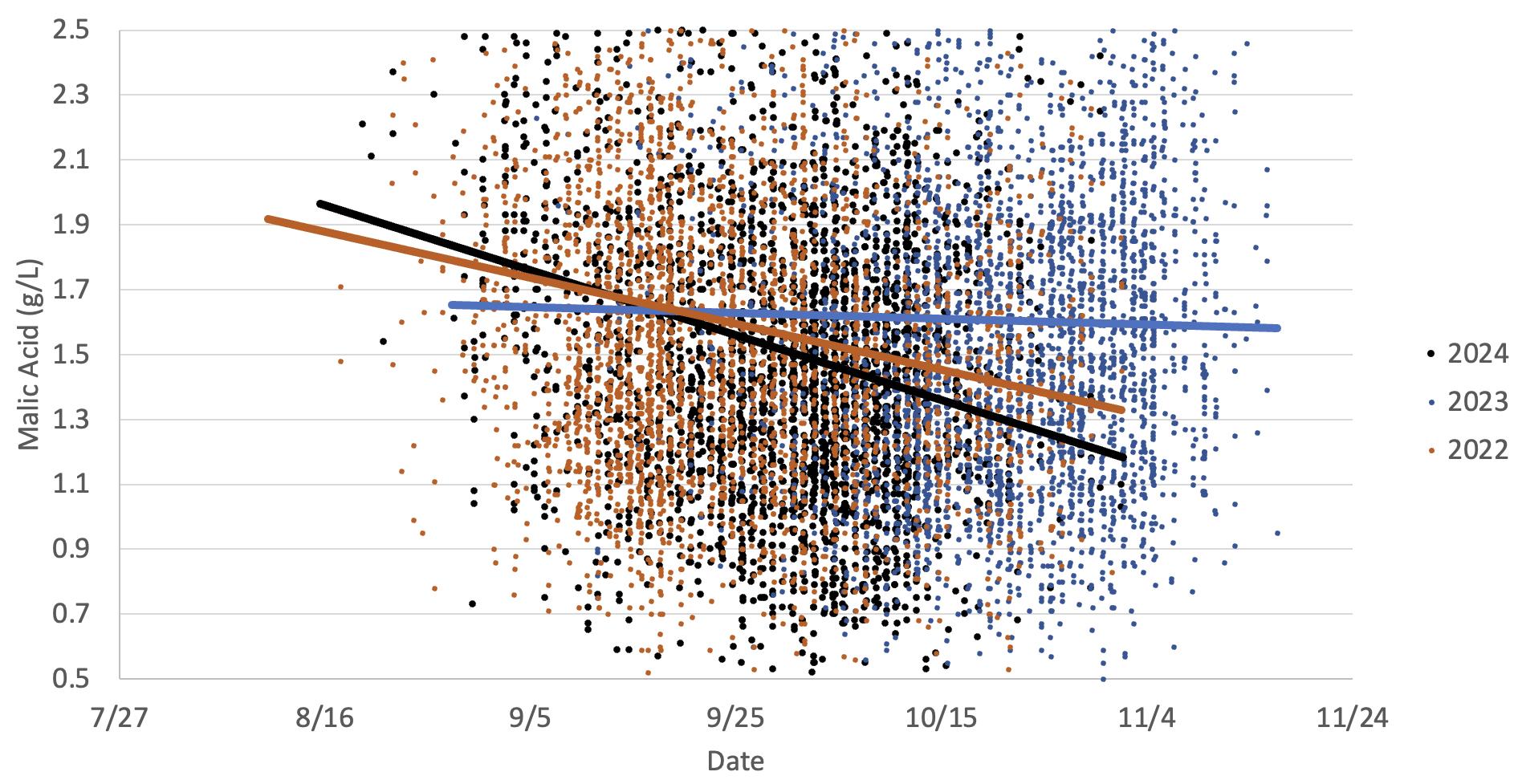
This graph illustrates the loss of malic acid in Cabernet Sauvignon grapes observed in two warm vintages (2024 and 2022) versus a cooler vintage (2023). The data indicates malic acid is still being produced. This is primarily due to increased respiration, which can be accelerated with reduced differences in diurnal temperature shifts in warmer years.

Titratable acidity (TA) measures total available hydrogen ions in solution. This measurement includes both the free hydrogen ions and the undissociated hydrogen ions from acids that can be neutralized by sodium hydroxide.
TA is the most widely used measurement of acidity in wine.
Although generally considered a simple parameter, titratable acidity is actually a reflection of complex interactions between the hydrogen ions, organic acids, organic acid-salts, and cations in solution. Often there is no direct correlation between TA and pH. Two musts with similar titratable acidity may have very different pH values.
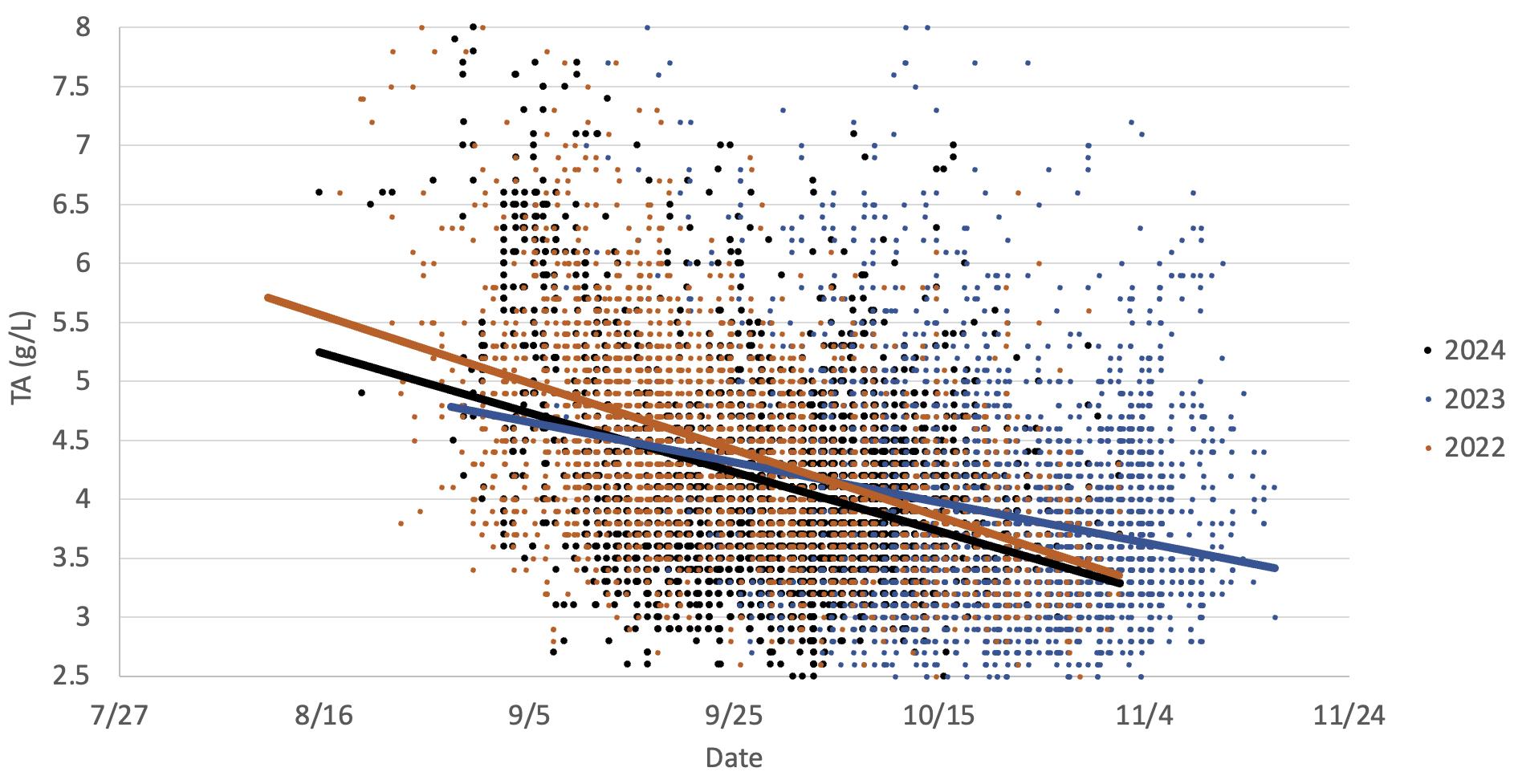
This graph illustrates the drop in titratable acidity observed in Chardonnay grapes in two warm vintages (2024 and 2022) versus a cooler vintage (2023). The data indicates titratable acidity drops as the grapes mature, which occurs earlier in warmer years. This is primarily due to increases in potassium and decreases in malic acid as the grapes mature.
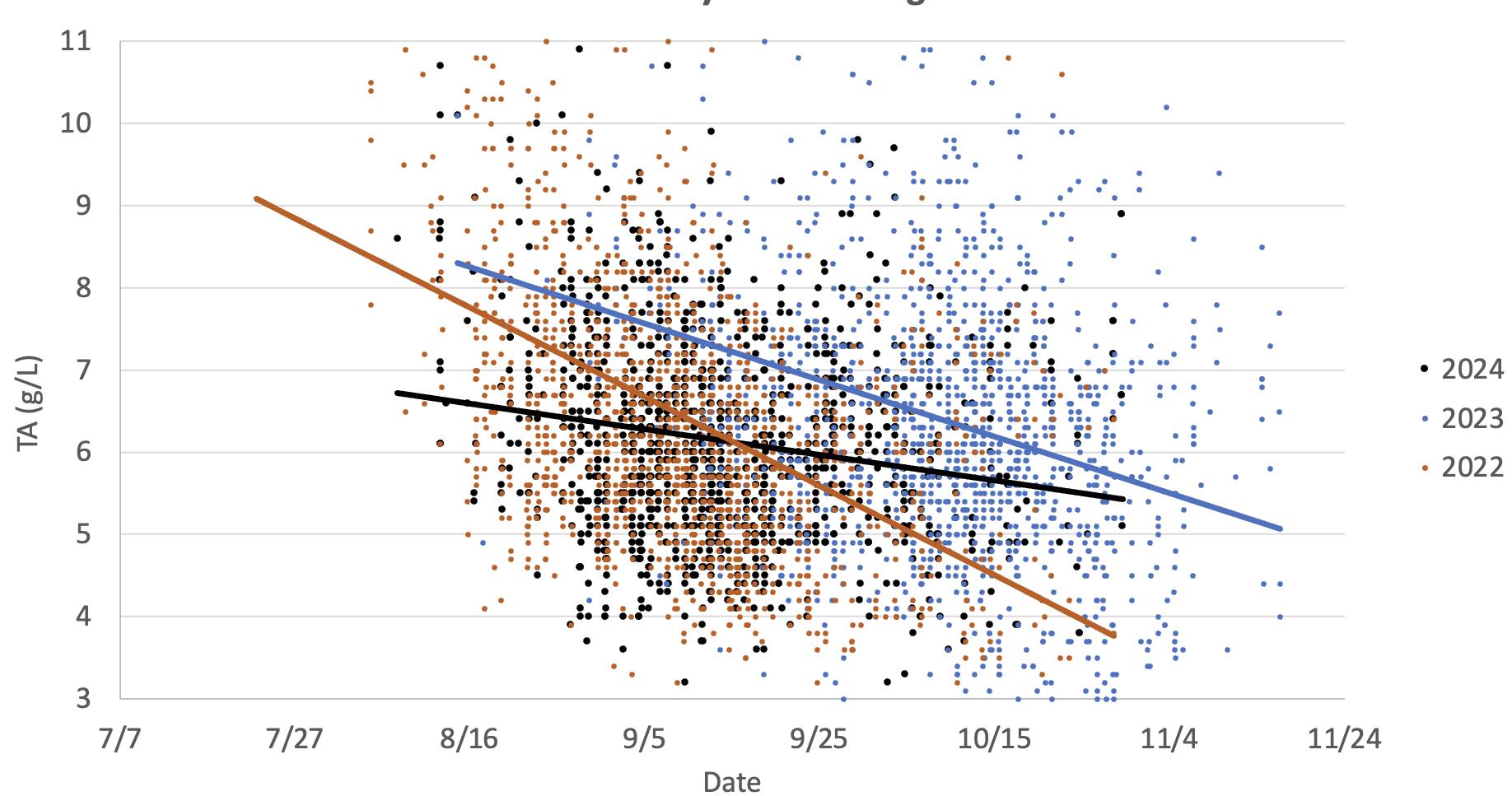
This graph illustrates the drop in titratable acidity observed in Cabernet Sauvignon grapes in two warm vintages (2024 and 2022) versus a cooler vintage (2023). The data indicates titratable acidity drops as the grapes mature, which occurs earlier in warmer years. This is primarily due to increases in potassium and decreases in malic acid as the grapes mature.

otassium is the primary cation present in grape tissue.
Potassium concentration in the berry is a function of root uptake and translocation. Both are strongly affected by viticultural factors including choice of rootstock, potassium fertilization, and canopy management.
Potassium moves into cells in exchange for hydrogen ions from organic acids. Potassium concentration is highest
near the grape skin. Crushing, skin contact, and pressing all influence potassium levels. Potassium has a strong influence upon pH, acid salt formation, tartrate precipitation, and buffer capacity. Decreases in juice potassium are associated with decreased juice pH. In the 2023 vintage, potassium levels were low which is often observed in cooler vintages.
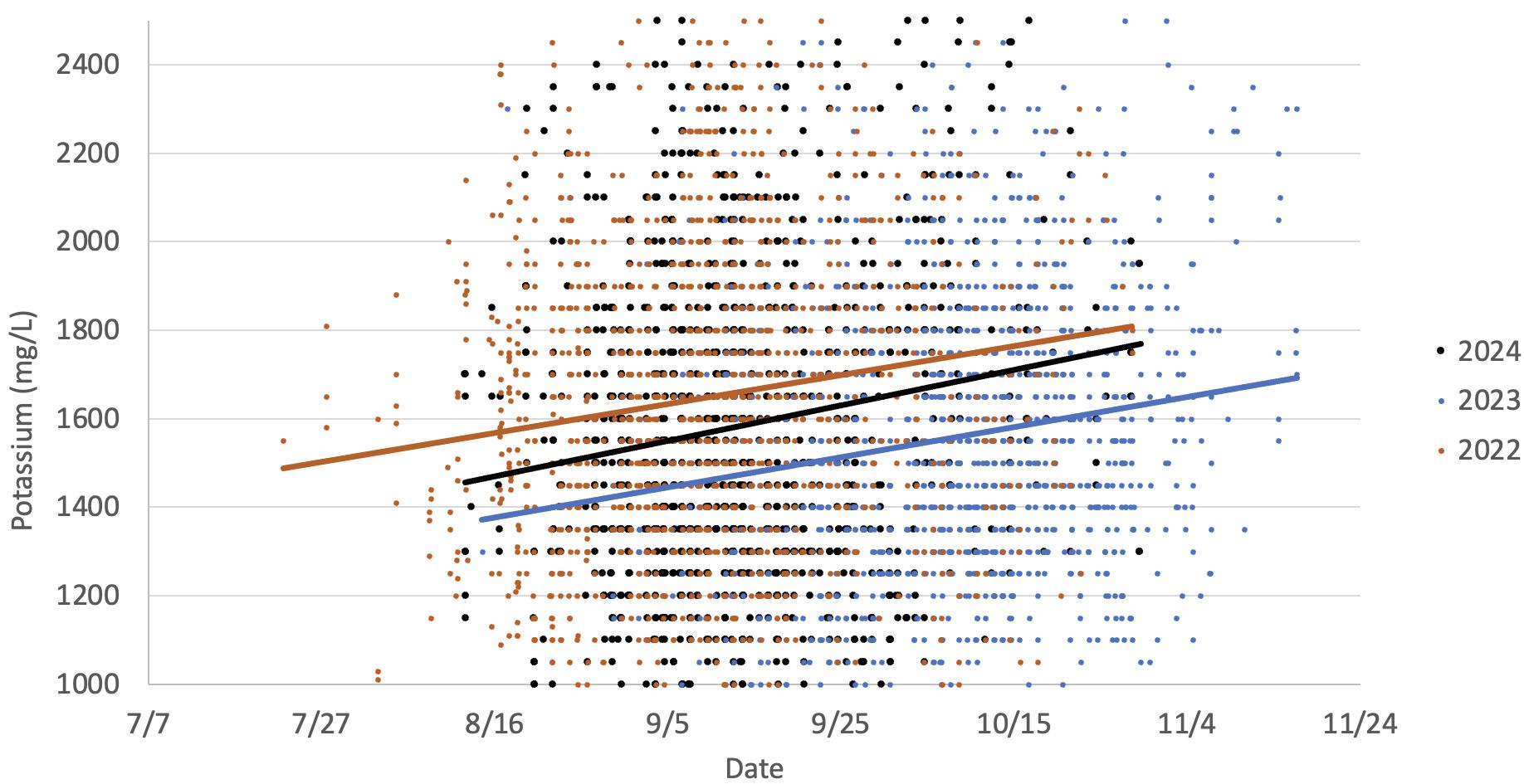
This graph illustrates the increase in potassium observed in Chardonnay grapes in two warm vintages (2024 and 2022) versus a cooler vintage (2023). The data indicates potassium increases as the grapes mature, which occurs earlier in warmer years. This is primarily due to increases in potassium transport from the leaves to the grapes as they mature.
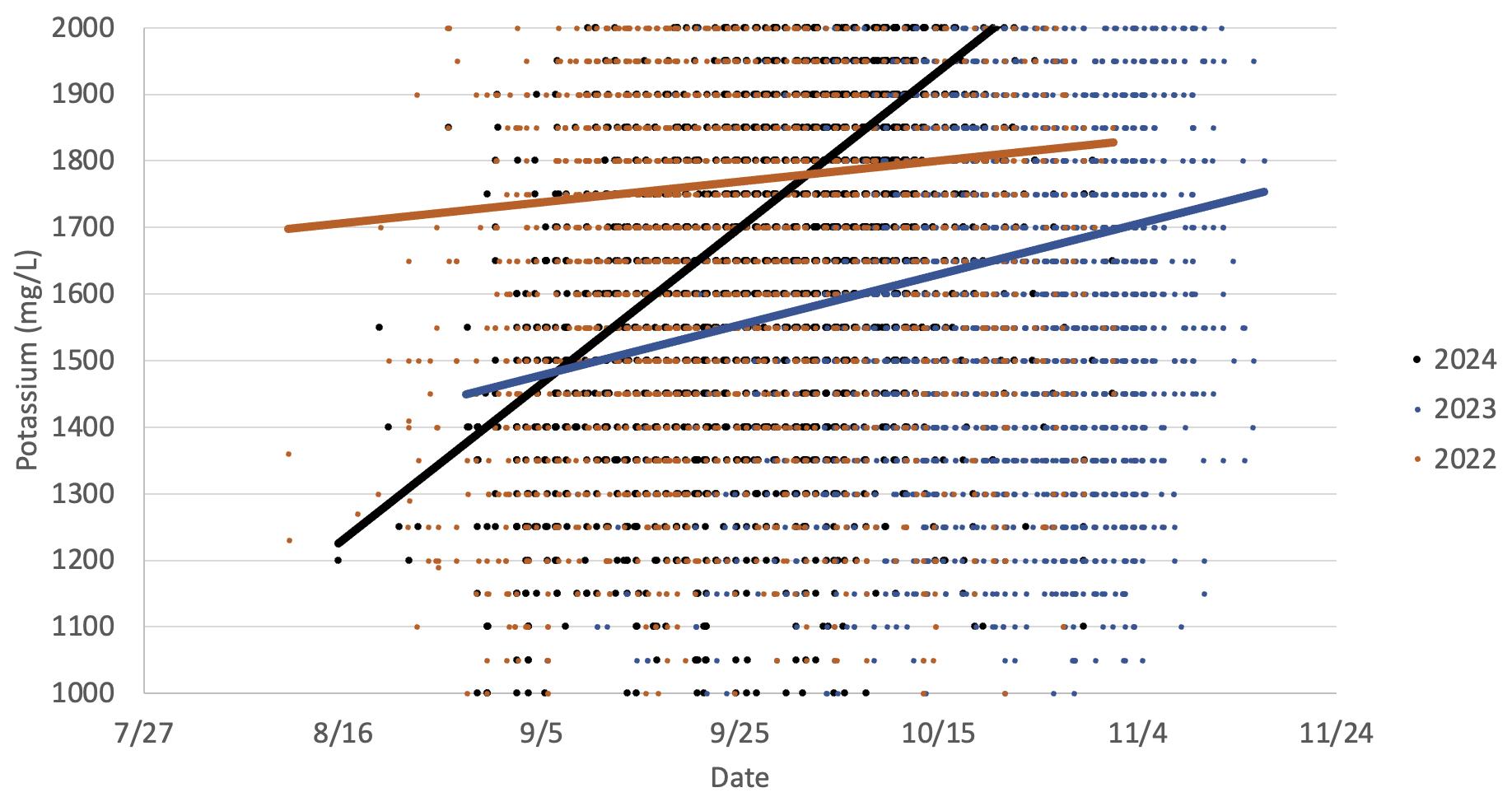
This graph illustrates the increase in potassium observed in Cabernet Sauvignon grapes in two warm vintages (2024 and 2022) versus a cooler vintage (2023). The data indicates potassium increases as the grapes mature, which occurs earlier in warmer years. This is primarily due to increases in potassium transport from the leaves to the grapes as they mature.
Conclusions: Juice chemistry is the foundation for the resulting wine. Producing wines with specific targets for ethanol and acid balance, while avoiding fermentation-related problems, requires a thorough understanding of the juice chemistry. Individual components of the juice can and do change from vintage to vintage. Winemakers aware of these changes will be able to adjust their winemaking process, where necessary, to achieve their targeted wine styles.

Incoming fruit serves as the most important vector for introduction of indigenous microbes into the winery. These microbes are often associated with sluggish fermentations and VA problems.
WHAT WE'RE LOOKING FOR...
ACETIC ACID BACTERIA
Acetic acid bacteria are commonly associated with grapes and the winery environment. The three groups of commonly detected acetic acid bacteria are Gluconobacter, Gluconacetobacter and Acetobacter. Both Gluconacetobacter and Acetobacter can generate acetic acid from ethanol in the presence of oxygen. The presence of these organisms can cause elevated volatile acidity in wines exposed to air.
Hanseniaspora (Kloeckera) is a wild apiculate yeast that is often present at high levels on incoming fruit. Hanseniaspora can initiate fermentation in the must and produce high levels of volatile acids, including acetic acid and ethyl acetate. It has been associated with acid rot in grapes infected by Botrytis cinerea. Population levels usually decline as alcohol concentration increases.
Pichia is a wild yeast that is often present at high levels on incoming fruit. Pichia can initiate fermentation, resulting in production of high levels of volatile acids, including acetic acid and ethyl acetate. These yeast have been associated with films formed in barrels and tanks during storage.
Identifying and quantifying yeast and bacteria that can cause spoilage during the winemaking process is the first step in preventing these spoilage problems.
Using Scorpions to see the full picture of spoilage microbes in the juice gives the winemaker better situational awareness. Potential problems can be identified during the cold soak process, in stuck or sluggish fermentations, or later during wine aging.
Volatile acidity, measured as acetic acid, can be formed throughout the winemaking process. Both acetic acid bacteria and strains of wild yeast – particularly Hanseniaspora and Pichia – are commonly linked to volatile acidity production prior to and in the early stages of fermentation.
Elevated VA levels often occur during the cold soak process, or between cold soak and fermentation during red wine production. The VA-producing spoilage microorganisms grow quickly during this time, producing increasing levels of acetic acid until fermentation conditions inhibit their growth.
In addition to causing sensory impacts, large populations of wild yeast can deplete the YAN in the must, resulting in a YAN deficiency for the Saccharomyces cerevisiae driving the fermentation. Winemakers who detect high levels of Hanseniaspora or Pichia in a must usually recheck YAN before yeast inoculation, and supplement YAN if necessary.
Likewise, if the Scorpions assay detects heterofermentative lactic acid bacteria, such as Lactobacillus brevis, L. kunkeei, L. hilgardii, L. fermentum, and Oenococcus
Production of high levels of volatile acidity prior to fermentation can also cause problems later in the production process, including possible impacts on the fermentation performance and wine sensory attributes.
Large numbers of Acetic Acid bacteria on incoming fruit can carry through the fermentation and cause problems with VA production when exposed to air during barrel aging.
oeni, in a juice, winemakers usually increase their monitoring of malic acid and microbe levels if the fermentation becomes sluggish or stuck. Early identification of the presence of these bacteria and recognizing the risk they pose to difficult fermentations is key to preventing VA formation in stuck fermentations.
MANY WINERIES AROUND THE WORLD USE GRAPE CONCENTRATE IN THE PRODUCTION OF THEIR WINES. ALTHOUGH GRAPE CONCENTRATE IS NOT THE ONLY SOURCE OF ZYGOSACCHAROMYCES YEAST, IT IS CERTAINLY A COMMON SOURCE FOR ZYGOSACCHAROMYCES TO BE INTRODUCED INTO THE WINERY.
The primary species of Zygosaccharomyces typically associated with grape concentrate are Zygosaccharomyces bailii and Zygosaccharomyces bisporus
Recently, a different species, Zygosaccharomyces rouxii , was isolated from a fermenting concentrate sample provided by a client. In response to this finding, we modified the design of the Zygosaccharomyces Scorpions primer/probe combination to detect more species. The new Zygosaccharomyces Scorpions diagnostic detects an additional 7 species of Zygosaccharomyces for a total of 9 species.
Zygosaccharomyces bailii
Zygosaccharomyces bisporus
Zygosaccharomyces rouxii
Zygosaccharomyces lentus
Zygosaccharomyces mellis
Zygosaccharomyces kombuchaensis
Zygosaccharomyces parabailii
Zygosaccharomyces pseudobailii
Zygosaccharomyces pseudorouxii


Several providers of commercial yeast to the wine industry are offering strains of non-Saccharomyces yeast for use in the winemaking process. These yeasts can be used as bioprotective agents to reduce the impact of indigenous nonSaccharomyces strains as well as a way to reduce SO2 use prior to fermentation. In addition, these yeast strains may provide improvements via increased acidity, aroma, complexity, structure, and mouthfeel.
In response to the increased use of these yeast strains by our clients, ETS has developed a series of PCR-based diagnostics to detect the presence of the individual strains; Metschnikowia pulcherrima, Lachancea thermotolerans, Torulapsora delbrueckii, and Pichia kluyveri
The PCR-based diagnostics can be used to determine implant success with the individual strains. They can also be used with the ETS Juice Yeast Scorpion Panel to look at the efficacy of these strains at reducing levels of Hanseniaspora uvarum and Pichia membranifaciens, commonly observed in must samples. The diagnostics can be requested for individual yeast species, or as a complete panel.
NON-SACCHAROMYCES YEAST PANEL
• Metschnikowia pulcherrima
• Lachancea thermotolerans
• Torulapsora delbrueckii
• Pichia kluyveri
The concentrations of total and viable yeast are important indicators of fermentation health. Flow cytometry provides a rapid and accurate means to monitor the concentration and viability of yeast throughout the fermentation process. ETS’ new automated analysis uses Flow Cytometry and the widely accepted dye exclusion method to determine cellular viability. Live yeast cells have selective cell membranes that exclude dye compounds. When a yeast cell
dies the membrane becomes permeable allowing dye to enter. Dead, or non-viable, cell DNA becomes stained and fluoresces. Individual cells are counted and photographed while passing through the flow cell. This automated method couples viability staining methods with advanced optics and flow cytometry to report total yeast count, % budding and % viability.

ETS has received an increasing number of requests to develop a method for determining the efficacy of bentonite fining in juice. Traditional Heat Stability tests can be difficult to perform on juice. ETS has developed methods to directly quantify two proteins produced by Vitis vinifera in response to biotic and abiotic stress. These proteins, chitinase and thaumatin-like protein (TLP), are very resilient and persist through the fermentation process. Both proteins
have been implicated in haze formation although chitinase may play a larger role in heat stability issues. ETS has developed Enzyme-Linked Immunosorbent Assays (ELISA) to detect the individual proteins. Extensive testing indicates a linear response between addition of bentonite and removal of both chitinase and TLP. The ELISA based analyses are applicable to monitoring bentonite fining of juice.
Juice Protein Panel
• Chitinase
• Thaumatin-Like Protein

Chitinase and NTU Response to Bentonite Addition in Juice
Response of Chitinase and TLP to Bentonite Addition in Juice

The two components commonly associated with “VA taints”, Acetic acid and ethyl acetate, can be formed by both yeast and bacteria - and in the case of bacteria, can be formed with or without oxygen present.
is strictly speaking a measure of the volatile acids in wine, although in the real world the contribution of volatile acids other than acetic acid is negligible. VA is a normal component of wines at moderate levels (normal concentrations range from 0.3-0.9 g/L of acetic acid), but very quickly becomes undesirable as levels rise. The sensory threshold is around 0.9-1.0 g/L, depending on the style of wine, and the U.S. government sets legal limits of 1.2 g/L in white wine and 1.4 g/L in red wine.

Acetic Acid can be produced prior to fermentation by Acetic Acid Bacteria and wild yeast in compromised fruit.
It’s unusual to see alcohol present in juice before fermentation, but if clusters experience fungal rot or other types of damage (such as bird or insect damage), wild yeast in the vineyard can begin fermenting the juice that is leaked out. Acetic Acid bacteria can then convert the alcohol to acetic acid, causing “sour rot” and leading to high VA levels before fermentation has even begun.
Ethyl Acetate is often produced in the early stages of fermentation, and can be a particular problem in native fermentations with a slow start. Native yeast, especially Hanseniaspora, are the main Ethyl Acetate producers at this stage. Note that the Hanseniaspora can consume most or all of the YAN in the must very early in the fermentation, although they will only produce alcohol up to around 6%. High levels of Hanseniaspora and low YAN concentrations can contribute to stuck fermentations.
From the chemistry standpoint, ethyl acetate, not being an acid, is not a component of VA. However, as an ester formed by ethanol and acetic acid, it is often linked to increased production of VA. From a sensory point of view, ethyl acetate is often classified as a “VA taint”, and its “nail polish remover” odor is often a telltale sign of high VA. Similar to acetic acid, there is a fine line between complexity and spoilage: small amounts of ethyl acetate can contribute “fruitiness/sweetness” or other positive characteristics to a wine at low levels. Normal concentrations are usually less than 100 mg/L, while the sensory threshold is generally 130-150 mg/L, depending on the wine style.
Acetic Acid production in primary fermentation is generally caused by yeast, including Saccharomyces and other species, but can also be formed by bacteria.
The native yeasts Hanseniaspora and Pichia can drive fermentations up until around 6-7% alcohol, at which point they become stressed by the alcohol. Saccharomyces is more competitive as it is tolerant to and produces higher alcohol levels. In certain situations, the native yeasts respond to the changing fermentation conditions by producing elevated levels of acetic acid.
Bacteria, usually Lactobacillus, can also generate acetic acid from sugar and can often produce high levels of VA in stuck and sluggish fermentations. Oenococcus oeni, the bacteria used for inoculating most malolactic fermentations can also produce acetic acid from fructose. This frequently occurs at the end of malolactic fermentation if there is still fermentable sugar remaining in the wine.
Ethyl Acetate production during fermentation is significantly impacted by the yeast strain and fermentation temperature. Although Saccharomyces cerevisiae will produce ethyl acetate, research has indicated that some of the Saccharomyces bayanus strains are more likely to form ethyl acetate in cold fermentations.
The amount of fermentable sugar (glucose and fructose) in juice and the average conversion rate of sugar into alcohol can be used to predict the potential alcohol level in wines.

Our clients have reported that glucose + fructose values improve the quality of their predictions, but it is important to remember that yeast populations and fermentation conditions vary, and any prediction of potential alcohol is only an approximation. Alcohol conversion ratios can be variable, so it is possible your actual alcohol may be lower or higher than the estimate.
Many of our clients have found that the conversion rates observed for their own yeasts and fermentation conditions remain relatively constant, and they use their internally observed conversion rates to calculate potential alcohol content based on their glucose + fructose values.
With white wines, predictions are usually fairly accurate. With red wine, however, getting a truly representative juice

sample can be a challenge and can affect potential alcohol predictions. A juice sample taken soon after a tank is filled may not take into account un-popped berries, unripe berries (less sugar and more acids), and raisins (sometimes an overlooked source of large amounts of sugar, acid, and potassium).
We suggest sampling after an initial 10°Brix drop, and analyzing the fermenting sample for glucose + fructose and alcohol simultaneously for a more accurate potential alcohol estimate. Proper sample preparation is key to accurate results. At ETS, juice samples are centrifuged before analysis, and then mixed by inversion to avoid stratification, ensuring the most accurate results. Particulates have a minimal impact on refractometry, but can have a large impact on densitometry results.

Predicting potential alcohol levels in finished wines sounds simple, but there is more than one way to measure “sugar”, and formulas to convert this sugar into potential alcohol often miss the mark.
The “old school” method was to multiply °Brix by 0.6. One degree Brix is defined as 1 gram of sucrose in 100 grams of aqueous solution. However, grape juice does not naturally contain sucrose, but rather glucose, fructose, and a variety of organic acids and other dissolved solids. So when used for grape juice, °Brix is actually just an approximation of dissolved sugar, not an accurate representation of the fermentable sugars, and using ºBrix for estimating potential alcohol adds an additional layer of uncertainty to alcohol predictions. Differences between ºBrix and actual fermentable sugar content are even more pronounced in high ºBrix fruit and in fruit affected by fungal growth.
How ºBrix is measured also has an influence. Differences exist between ºBrix by refractometry, densitometry
(using either hydrometers or digital instruments), and other secondary measurements. The differences among the various measurement techniques are quite unpredictable depending on sample composition.
A modern calculation that has proven to be more accurate uses glucose+fructose analysis, which provides a more accurate measurement of fermentable sugar levels compared to using ºBrix.
Note that in ripe fruit, glucose + fructose numbers often appear higher than the corresponding ºBrix results, because ºBrix is measured as a percentage by weight, meaning ºBrix values are greatly influenced by the density of the juice, while glucose + fructose is measured as weight by volume and is independent of juice density.
An official conversion rate formula used in Europe is: Potential Alcohol (% vol) = glucose + fructose (g/L) / 16.83. In practice, rounding the 16.83 conversion factor to 17 is common.
In the wine industry, a term like “sugar” can mean different things. Clients often request testing for “RS”, but this term can be very ambiguous.

°Brix is a measurement of the apparent concentration of sugar. It is commonly used for grape juice and must and is expressed as a percentage by weight (% w/w). One degree Brix is defined as 1 gram of sucrose in 100 grams of aqueous solution. When the solution contains dissolved solids other than pure sucrose, as is the case for grape juice and must, the °Brix is only an approximation of dissolved sugar.
In grape juice, Glucose + Fructose analysis measures the combined concentrations of the two main sugars present that can be consumed by yeast, also known as "fermentable sugars." Compared to °Brix, Glucose + Fructose can provide a better estimate of potential alcohol concentration after fermentation. In wine, “residual sugar” usually refers to the sum of Glucose + Fructose, an indication the amount of fermentable sugars remaining post fermentation, which is also an indication of ‘dryness’.
Sucrose is not captured by this test. If it has been used in the winemaking process (such as for chaptalization of must, secondary fermentation of sparkling wine or added as a sweetener) measurement of Glucose + Fructose alone is usually not adequate – instead see Glucose + Fructose (Inverted)
Historically, “Residual Sugar” was measured by the Reducing Sugar method. This test derives its name from the ability of most sugars in juice or wine to ‘reduce’ other compounds. The most common reducing sugars are glucose and fructose. However, the method does not distinguish between fermentable and non-fermentable sugars, or other ‘reducing’ compounds and these other compounds may contribute to reported results.
Because of these limitations, the Reducing Sugar method is no longer the preferred choice to monitor completion of primary fermentation.

The Glucose and Fructose Panel provides the individual levels of glucose and fructose, in addition to their combined concentration. This test is often requested to investigate or remedy stuck or sluggish fermentations.
Inverted Glucose + Fructose provides the sum of the concentrations of glucose and fructose after “inversion” of the sample. Inversion is a process by which sucrose is broken apart into glucose and fructose, so that it can be measured and included in the reported results. Hence, this test is useful when “Residual Sugar” is required after chaptalization of must, secondary fermentation of sparklings or whenever sucrose has been used as a sweetener in wine, other alcohol beverages or spirits.

ETS offers a full suite of advanced HPLC-based analytical tools to evaluate phenolic compounds in grapes, juice, fermenting must and wine. The range of phenolic analyses allows flexible use and implementation to suit individual needs.
GRAPE PHENOLIC PANEL
Phenolic compounds in red wine grapes are directly linked to eventual wine flavor, color and aging characteristics.
The grape phenolic panel can characterize site to site variation as well as within site differences. It works well as a prediction tool for describing vintage effects on potential phenolics and is a great tool for vineyard research projects. It is very sensitive to changes in phenolic compounds occurring during grape maturation. The changes in grape tannin are particularly important for red wine picking decisions. The grape phenolic panel can track changes in seed ripening, skin tannin extractability and tannin modification. Dilution and concentration effects on tannin and other phenolic components can be monitored with the Grape Phenolic Panel particularly when used in conjunction with Grape Water Content.
GRAPE PHENOLIC PANEL
Successful winemaking strategies require accurate information on grape composition. Winemakers use this panel during fermentation to reach target levels of tannin for specific wine styles, monitor seed extraction, adjust tannin modification through oxygenation and decisions on extended maceration and pressing.
RAPID PHENOLIC PANEL FOR WINE
Phenolic compounds are extracted from grapes during fermentation and maceration. Monitoring the phenolic composition of the must during fermentation can greatly enhance a winemaker’s control of the process.
Juice bleeds, fermentation temperatures, pump-over or punch down regimes, the use of rack-and-return, oxygen or air additions and press timings can all be fine-tuned with feedback on changes in phenolic composition. With this information, winemakers can adapt winemaking practices to fit the vintage and successfully create wines of a target style.
RAPID PHENOLIC PANEL
Phenolic composition is one of the main components of red wine style. The amount of tannin and its composition is the foundation of a wine’s structure. There are no “correct” values for these parameters. A winery must define their own style as a brand and for individual products within that brand.
Integrating phenolic information into stylistic choices requires an understanding of the impact of tannin on the sensory profile of wines. For a winery new to this information a good strategy is to analyze a selection of recent products. Recent production lots, finished wines and competitor’s products are good choices. Tasting products with analytical information allows winery staff to build the connections between taste and analytical information. Taste is the final arbiter of style but a clear understanding of the relationship of taste and analysis is needed to turn analytical information into action.
RAPID PHENOLIC PANEL
After the completion of fermentation/maceration, a wine lot typically represents a specific vineyard and fermentation tank. This is an excellent point for collecting quality control data. A comprehensive review of production lots is a powerful tool for monitoring block to block variation and the effects of winemaking practices. Analysis of finished production lots early in the vintage is very useful for changing fermentation practices and targets later in the vintage.
RAPID PHENOLIC PANEL FOR WINE OR RED WINE PHENOLIC PROFILE
Winemakers interested in consistent tannin and color levels benefit by comparing the phenolic profiles of bulk wines prior to blending. Potential blends can be compared to target phenolic levels and benchmarks prior to final blend.
RED WINE PHENOLIC PROFILE
Many wineries establish QC benchmarks for phenolic content immediately after bottling. This is especially useful for determining product consistency and for monitoring wine development during aging.
RED WINE PHENOLIC PROFILE
A historical review of products from within a winery and evaluation of similar products from other producers is an excellent way to establish phenolic benchmarks. This is often the first step in building an integrated program of phenolic analyses. A careful review of finished wines combined with sensory evaluation and market feedback can identify program strengths and weaknesses. The identification of desirable levels for key phenolic components creates targets that can be incorporated into process control points in the vineyard and winery.

Since the early 2000s, ETS has offered grape testing for phenolic maturity through its Red Grape Phenolic Panel. This analysis is based on the partial extraction of berry samples, designed to simulate the extraction of phenolic compounds under winemaking conditions. The resulting extracts are analyzed using High Performance Liquid Chromatography (HPLC).
A key strength of our HPLC method is its ability to measure, in a single run, various classes of complex polyphenols - such as anthocyanins, tannins, and anthocyanin-tannin complexes (also known as polymeric anthocyanins) - as well as monomeric compounds like catechin, which serves as a marker for seed-derived phenolics. This unique capability provides valuable insights for both grape growers and winemakers.
Common applications of the Red Grape Phenolic Panel include:
• Monitoring phenolic maturity to determine optimal harvest timing
• Assessing the phenolic potential of grapes to evaluate their suitability for a desired wine style and price point
• Understanding and improving underperforming vineyards
• Supporting informed grape purchasing decisions
• Evaluating vintage differences
• Guiding winemaking decisions, such as whether to perform a saignée, based on analytical evidence
The ETS Red Grape Phenolic Panel assesses the three distinct components of Phenolic Maturity: anthocyanins, skin-derived tannins, and seed-derived tannins

Phenolic Maturity is often assessed by measuring a simple parameter, such as total anthocyanins, in grape homogenates. However, this approach has several limitations:
• The evolution of anthocyanins and tannins follows distinct pathways, meaning that one class of compounds cannot serve as a substitute for the other.
• It is essential to understand the origin of tannins: are they seed-derived or skin-derived? Seed-derived tannins are typically bitter and astringent, while skinderived tannins, which are considered “softer,” are key to mouthfeel, volume, and length.
• The extractability of skin and seed tannins changes in opposite directions as the grapes ripen.
• Phenolic Maturity should consider not just the overall concentration of polyphenols (anthocyanins and tannins), but also their extractability.
Pigments”, “Bound Anthocyanins”, “Pigmented Tannins”: Markers of
These challenges were clearly recognized by Bordeaux researchers in the early 1990s, leading to the development of the “Méthode Glories” assay. This method promised to finetune harvest dates and optimize polyphenol extraction based on the grape’s potential. Unfortunately, due to its time-consuming nature, labor-intensive process, and poor reproducibility, it fell short of becoming a practical tool for growers and winemakers. In contrast, the partial extraction strategy followed by HPLC analysis, developed by phenolics specialist Dr. Steve Price at ETS, has proven to be much more reliable and effective. This method has gained widespread adoption among our clients, both growers and winemakers, becoming more popular with each vintage. It allows for the assessment of the three distinct components of Phenolic Maturity: anthocyanins, skin-derived tannins, and seed-derived tannins. Additionally, it measures anthocyanin-tannin complexes (polymeric anthocyanins), which were once thought to form mainly during winemaking, but are actually produced in significant quantities in grape skins during the late stages of ripening. As an added benefit, the method also determines quercetin glycoside, a flavonoid produced by grapes for UV protection, which serves as a useful marker of
exposure.
Figure 1: Parameters reported in the ETS Red Grape Phenolic Panel, and typical ranges (mg/L) in Cabernet Sauvignon grapes “partial extracts” analyzed at ETS Laboratories. Note the wide ranges observed for all parameters.
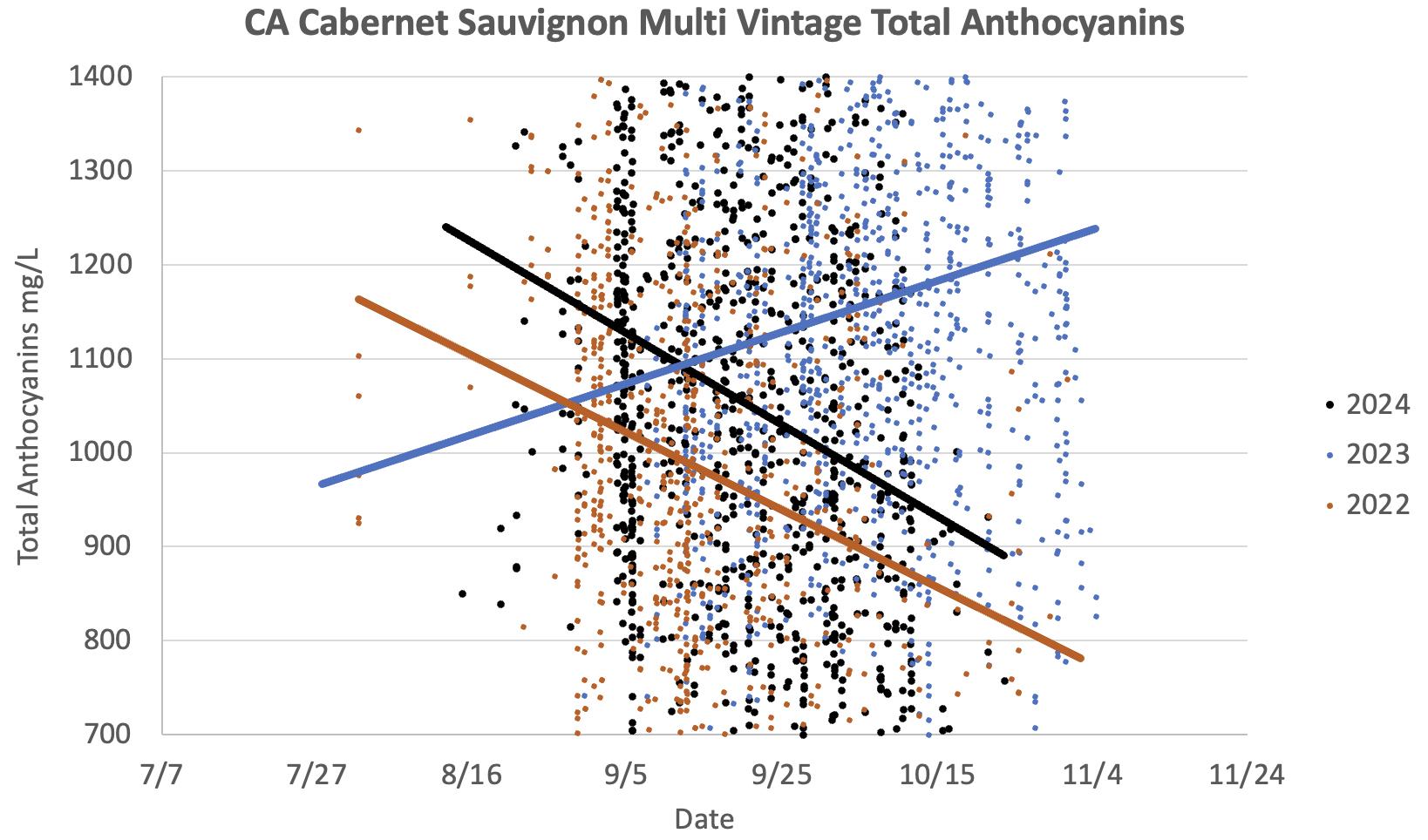
From the perspective of phenolic maturity, the vintages of 2022, 2023, and 2024 in California have been notably different, reflecting the vastly different climatic conditions over these three consecutive years. This year-to-year variation is clearly illustrated by the data we have collected, as shown in Figures 2 through 7, which highlight ripening trends in Cabernet Sauvignon grapes. In 2022, California experienced a warmer-than-average growing season, including a record-breaking 10-day heatwave in early September. This extreme heat had predictable effects on phenolic maturity:
• Total anthocyanin levels were low overall, indicating significant early degradation of these compounds due to the heat.
• Tannin levels started relatively low, suggesting that seeds were ripe early in the season. However, these levels increased as expected, due to the greater extractability of skin tannins, which is driven by skin senescence.
• Catechin levels were particularly low throughout the harvest, a clear indicator of a warm year, as seed hardening is driven by heat.
• As skins degrade, tannins combine with anthocyanins to form Polymeric Anthocyanins. Levels were initially high and increased rapidly during harvest, reflecting early and continued breakdown of skin cell structures.
• The Catechin/Tannin ratios were already quite low early in the season, indicating a smaller contribution of seedderived tannins than usual. In contrast, the Polymeric Anthocyanins/Tannin ratios started high and continued to increase sharply with hang time, confirming a larger contribution from skin-derived tannins.
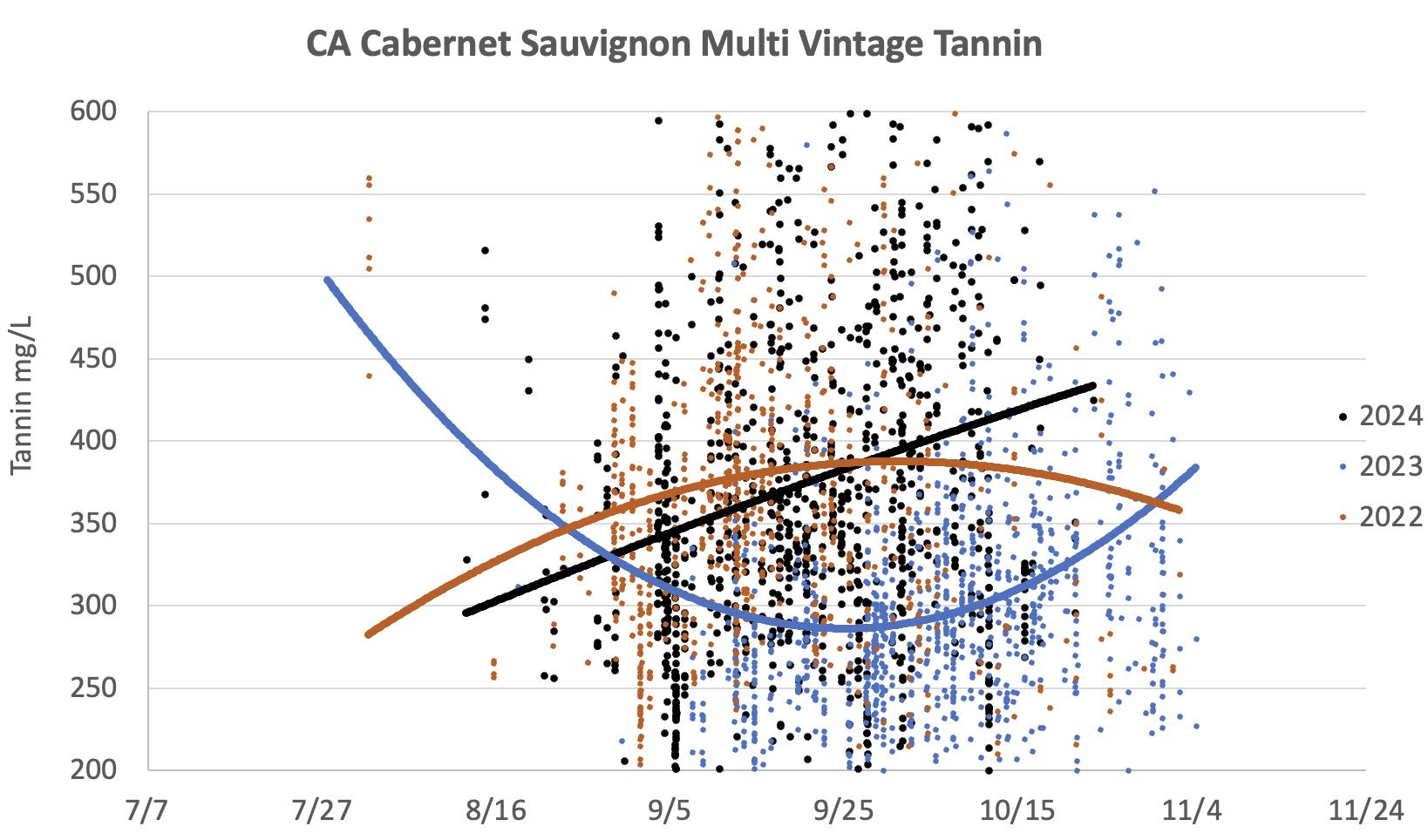

3: Tannins in Cabernet Sauvignon grape extracts during the 2022, 2023 and 2024 vintages. Note the low levels observed overall in 2023. That year, a “U-shaped” curve, due to the decline in seed tannins followed by the increase in skin tannins later in the season, was also observed.
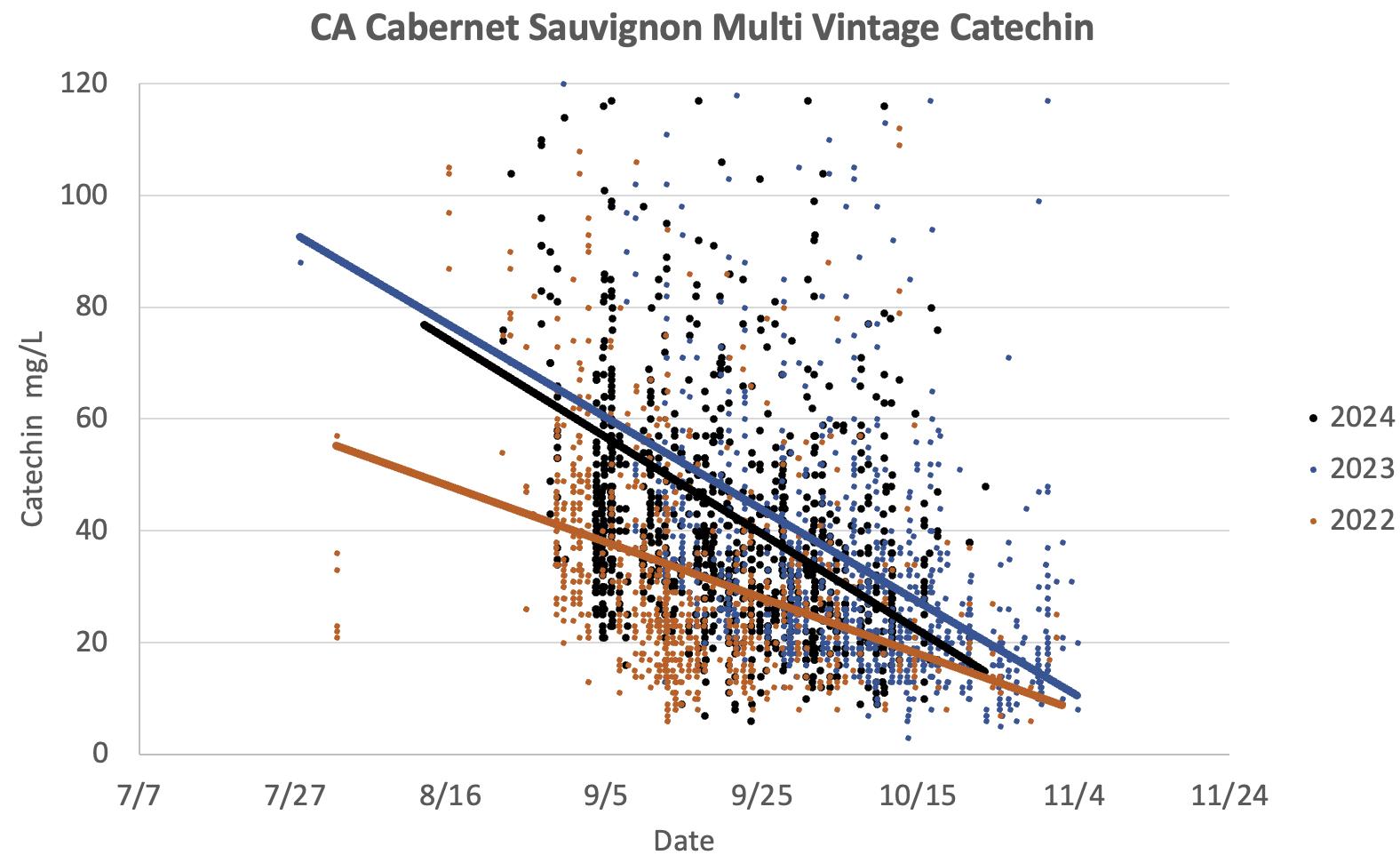
Figure 4: Catechin in Cabernet Sauvignon grape extracts during the 2022, 2023 and 2024 vintages. As seeds ripen, we always observe a decline during harvest season. Catechin levels were particularly low during the warmer 2022 vintage, indicating riper seeds.
In 2023, California experienced cooler-than-usual conditions, which led to contrasting effects on phenolic maturity compared to 2022. Clients began submitting samples later in the season:
• Total anthocyanin levels were high overall, due to reduced degradation. In a typical year in warm climates, harvest begins after the anthocyanin concentration reaches its maximum (referred to as the “anthocyanin peak”), after which levels generally decrease. However, 2023 was a notable exception, with anthocyanin levels continuing to rise throughout the harvest.
• Tannin levels were low overall. The trend observed in our data displayed a “U-shaped” curve, illustrating an early decline in seed tannins followed by an increase in the extractability of skin tannins.
• Catechin levels started high but gradually declined, indicating a delayed yet satisfactory seed hardening process.
• Polymeric anthocyanins levels were very low and increased only slowly during the harvest, indicating that the skins were resisting senescence.
• The Catechin/Tannin ratio started high but reached moderate levels as the season progressed. In contrast, the Polymeric Anthocyanins/Tannin ratio showed little change, reflecting a relatively stable contribution of skin tannins.
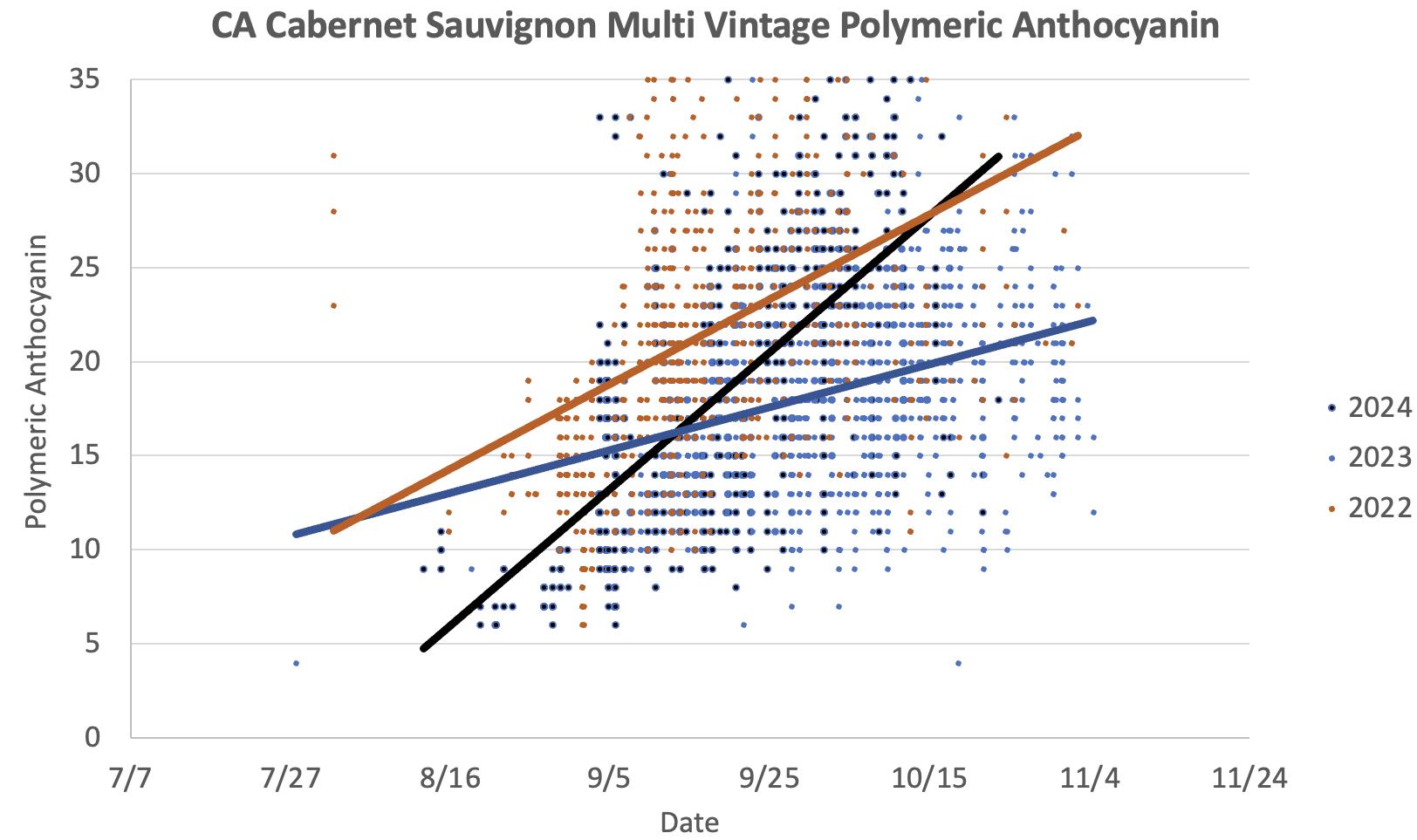
Figure 5: Polymeric Anthocyanins in Cabernet Sauvignon grape extracts during the 2022, 2023 and 2024 vintages. As skins degrade, tannins combine with anthocyanins, forming increasing levels of polymeric anthocyanins. During the cooler 2023 harvest, levels were low overall, and increased only very slowly.
In 2024, the growing season commenced with below-average temperatures, followed by a pronounced heat event in June and a subsequent heat wave in early October that brought a sudden conclusion to the harvest. Despite considerable heterogeneity among the samples analyzed, we noticed phenolic maturity parameters advancing rapidly over the course of the season:
• Total anthocyanin levels were fairly typical on average but exhibited a wide range of values.
• Tannin levels were low in early September, suggesting that the seeds were already ripe, while the skins were not ready yet to release their tannins. However, tannin extractability increased steadily throughout the month.
• Catechin levels started at moderate values, indicating relatively early seed maturity, and continued to decrease steadily.
• Polymeric anthocyanins began at lower levels but increased sharply in September, confirming rapid skin degradation and enhanced tannin extractability from the skins.
• Both the Catechin/ Tannin and Polymeric Anthocyanins/Tannin ratios moved in the right direction throughout September, indicating favorable maturation trends.
In conclusion, the 2022, 2023, and 2024 vintages will serve as key benchmarks for anyone looking to assess phenolic maturity. Together, they offer a comprehensive dataset that will be invaluable for comparing and evaluating future vintages.
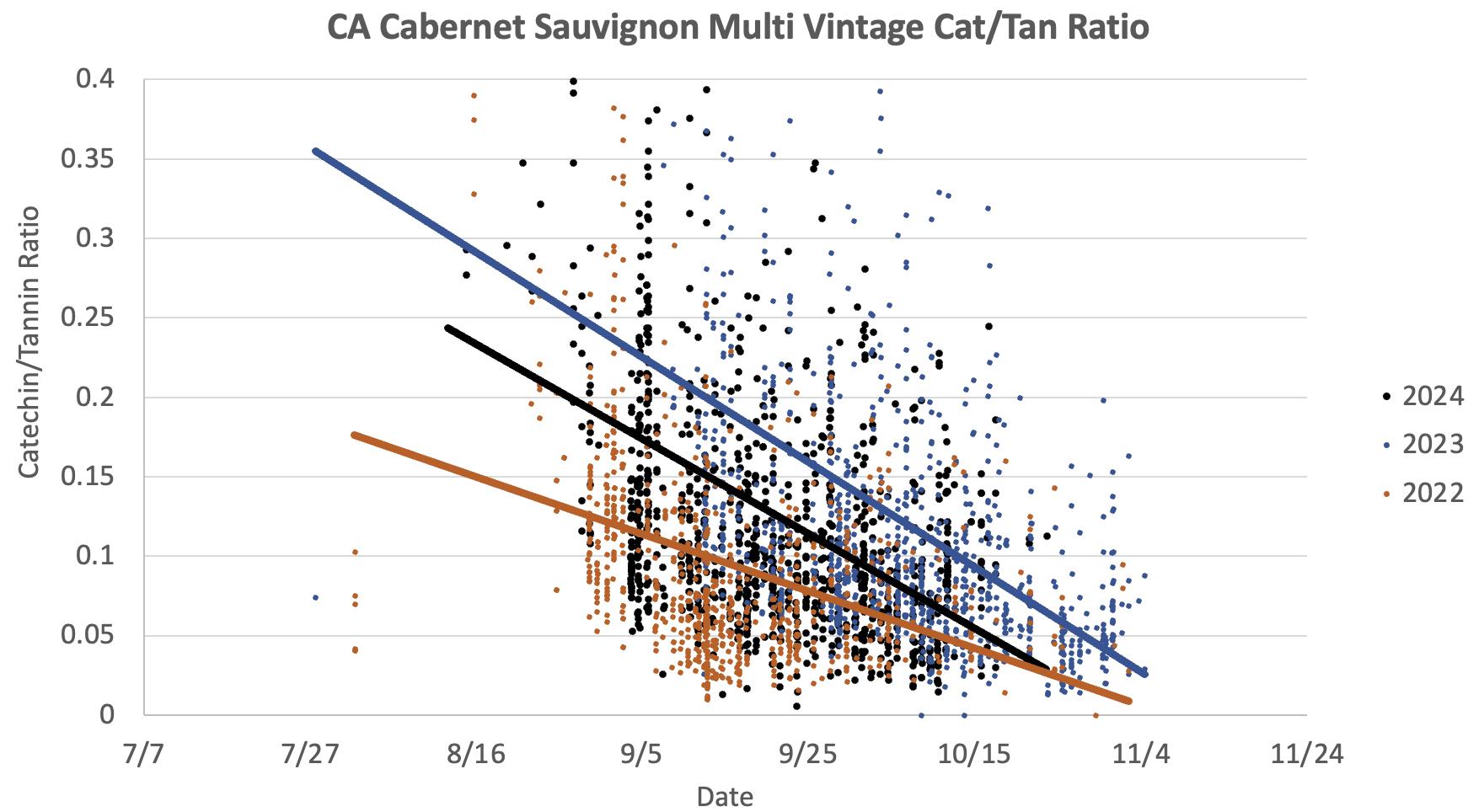
6: Catechin/Tannin ratios in Cabernet Sauvignon grape extracts during the 2022, 2023 and 2024 vintages. High Catechin/Tannin ratios indicate a higher contribution of seed-derived tannins to total tannins. Ratios usually decline very sharply during harvest. They were already low early in the season during the warmer 2022 vintage.
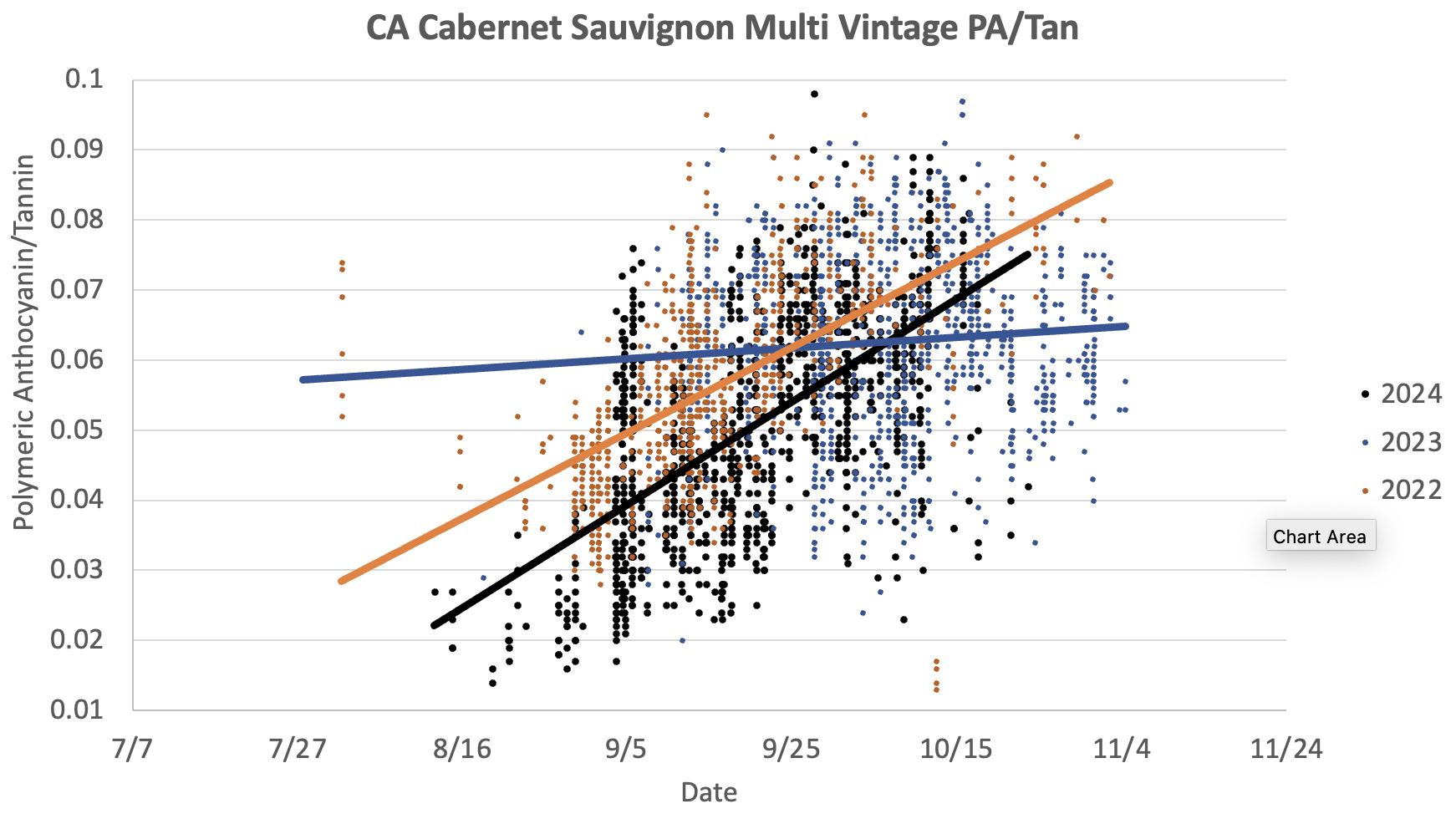
7: Polymeric Anthocyanins/Tannin ratios in Cabernet Sauvignon grape extracts during the 2022, 2023 and 2024 vintages. Higher ratios indicate skin degradation, and typically increase with hang time. The increase was barely noticeable during the cooler 2023 vintage.
BACKGROUND:
Wines from a young Syrah vineyard were extremely tannic and harsh.
ANALYSIS:
Client submitted grape samples for the ETS Grape Phenolic Panel
RESULTS:
CATECHIN TANNIN TOTAL ANTHOCYANINS QUERCETIN GLYCOSIDES POLYMERIC ANTHOCYANINS
TANNIN AND QUERCETIN GLYCOSIDES WERE BOTH VERY HIGH IN THE GRAPE SAMPLE. CATECHIN WAS QUITE LOW, PARTICULARLY FOR THE HIGH LEVEL OF TANNIN WITH A CATECHIN:TANNIN RATIO OF 0.008.
DISCUSSION AND ACTION:
Syrah grapes can have very high levels of tannin in the skins. Seeds, however, tend to ripen early so it is rare to see high catechin in Syrah grapes or seed tannin problems in wine. In this case, the problem seemed to be related to high skin tannin from excessive sun exposure. Skin tannin and quercetin glycosides increase with direct sun exposure. Sun exposed grapes synthesize quercetin glycosides in the skins to help block ultra violet light and simultaneously synthesize tannin to mitigate UV damage. The canopies were small and most grape clusters were in direct sunlight.
The client installed a shade cloth trial the following vintage and was able to reduce tannin and quercetin glycosides in the shaded treatments. Looking at vineyard variation, they found higher tannin in grapes from weaker sections of the vineyard and found a strong relationship between trunk diameter and grape skin tannin. Vines with smaller trunks had more tannin in the grape skins. Using trunk diameter as a guide, the client increased vineyard inputs in weaker areas to improve growth.
Shade cloth was installed in all the Syrah blocks resulting in reduced tannin levels in both grape and wine samples. Wines had improved textural qualities. As the vineyard matured, vine size and natural shading increased and tannin concentrations declined to non-problem levels.

Since the discovery of eucalyptol (1,8-cineole) in red wines by ETS Laboratories in 2003, we’ve detected and measured eucalyptol in a large variety of wines exhibiting “eucalyptuslike” aromas. Flavors perceived during tasting were usually strongly related to concentrations of eucalyptol. Regardless of the grape variety, trace levels close to 1 ppb are associated with “fresh”, slightly “minty” notes. In the low ppb range, “minty” or “fresh bayleaf” aromas become stronger, and more easily identifiable as “eucalyptus” as concentrations increase. Wines with strong “eucalyptus” odors may contain more than 20ppb of eucalyptol.
Eucalyptol’s sensory impact in wine is considered more or less desirable depending on the grape variety. Wines from southern Rhône and Mediterranean varieties seem better able to accommodate the characteristic well. Moderate levels can be appreciated in wines from Bordeaux varieties. Whereas with Pinot Noir, even trace levels can detract from varietal expression.
Eucalyptol- Eucalyptus traits are considered typical in some “cult” wines; on the other hand, in excess this character can be overwhelming. Even a slight “eucalyptus” note can interfere with delicate varietal aromas, and can have a detrimental influence on certain grape varieties.
Application:
As eucalyptol may be mostly contributed by eucalyptus-derived MOG (leaves, bark debris…) hiding in grapes, analyzing grape samples before harvest is mostly pointless, and routine testing is not offered by ETS Laboratories. Wine analysis assists winemaking teams in objectively documenting their sensory impressions and managing a strong flavor component. Unlike IBMP and smoke-derived compounds (see p. 38), eucalyptol is relatively slow to extract during red winemaking, making shorter macerations a valid strategy to minimize impact. Winemakers who wish to minimize or maintain consistent levels of “eucalyptus” character will also benefit by determining eucalyptol concentrations in distinct wine lots prior to blending.


Isobutylmethoxypyrazine (IBMP) is the most important methoxypyrazine, a group of molecules responsible for very distinctive vegetal aromas in Sauvignon Blanc and a variety of red wines, mainly from the Cabernet family. In Sauvignon Blanc, these compounds add an often desired “grassy” character. In red wines however, the “green bell pepper” flavor is largely unpopular. Excessive IBMP levels lead to disappointing ratings and mixed success in the marketplace.
The “green bell pepper” flavor in wine depends primarily on IBMP levels in harvested grapes. Once grapes have been picked, IBMP levels are not easily altered by standard winemaking processes.

Application:
The intensity of “green bell pepper/grassy” characters in wines can be predicted by measuring IBMP in grapes right before harvest. Grape screening of IBMP helps identify “problem” vineyards or blocks.
Since IBMP decreases during grape maturation, monitoring IBMP levels throughout ripening is a unique tool for assessing “aromatic maturity”. It allows targeting harvest dates based on desired aroma characteristics.
Monitoring IBMP from the early stages of the ripening process can greatly improve fruit quality from underperforming vineyards. Levels in grapes are well known to be linked to vine vigor, canopy and water availability, with severe heat occasionally causing IBMP’s natural degradation to stop. Once the kinetics of IBMP accumulation and degradation in specific sites are understood, viticultural practices can be modified accordingly. Once grapes have been picked, IBMP levels are not easily altered by standard winemaking processes.
The IBMP potential of grapes can be grossly underestimated from juice samples, making whole berries the preferred sample in most cases. However, analyzing juice samples may be relevant in white winemaking.
Glutathione is not an aroma compound itself, but is a powerful antioxidant that protects white wines and rosés from oxidation and loss of aroma or flavor.
A low level of glutathione in grapes leads to lower levels in the juice, and early losses of aroma compounds.
Glutathione levels fluctuate during production, as the compound can be absorbed by yeast and then released after fermentation.
If final glutathione levels are low in young wines, the wines will experience faster loss of fresh varietal and fruity aromas, and poor aging potential.
Monitoring glutathione levels can be beneficial throughout the winemaking process to maximize white wine aroma and flavor, as well as prevent premature aging.
The glutathione content in grapes indicates their antioxidant potential, and can be influenced by a number of factors including soil nitrogen, vineyard practices, and grape maturity levels.
Analyzing changes in glutathione levels during production helps to pinpoint where in the process glutathione is being lost – often from contact with air or exposure to copper residues.
A testing program can also identify winemaking processes that boost glutathione release after fermentation, and increase levels in wines.
Glutathione, a natural grape antioxidant, can protect the aroma and flavor of white and rosé wines and prevents premature aging.
Note: Glutathione is extremely sensitive to contact with air. To help prevent oxidation of juice and wine glutathione samples, ETS provides complimentary sample tubes containing potassium metabisulfite for glutathione sampling.

Wildfire smoke impact on wine was identified as a serious problem after the 2003 wildfires in Australia and British Columbia. The California wine industry was affected due to wildfires in the summer of 2008, and smoke impact has been a concern for growers and wineries ever since. It has been reported that wildfire impact in grapes and wines is caused by a wide range of volatile phenols found in wildfire smoke. These compounds are absorbed and accumulate in berries. They eventually end up in wine where they can cause unwanted flavors. These off-flavors, described as “smoky”, “bacon”, “campfire”, and “ashtray”, are usually long lasting and linger on the palate even after the wine is swallowed.

During the 2008 California wildfires, ETS developed an analytical tool to screen grapes for the risk of smoke impact. This analysis measured trace levels of free guaiacol and 4-methylguaiacol in whole berries. In 2021, two additional panels, with a larger number of volatile (free) smoke markers and one with glycosylated (bound) markers were launched. These additional indicators in berries add insight that can help winemakers assessing their smoke exposure, and choose an appropriate course of action to mitigate the effects in their wines
Vine exposure to wildfire smoke can vary widely within a small geographic area, depending on proximity to the fires and wind conditions. Obtaining representative samples can be challenging. Mixing grape varieties in composite samples should be avoided, as grape cultivars often react differently to a similar exposure to smoke. Syrah grapes contain naturally occurring guaiacol and should never be mixed with grapes from other varieties prior to testing for wildfire smoke related compounds.
Smoke marker analysis on berry samples is usually required by crop insurance providers, although micro-ferment samples are becoming more widely accepted. Submit 200 to 300 loose berries, keeping them cold and undamaged as much as possible (do not crush them). When shipping samples, use hard plastic containers with icepacks in an insulated package. Avoid submitting cluster samples when possible, to avoid additional fees, and delays in receiving results. It is advisable to keep backup samples in a freezer.
Grape Samples Micro-ferments
It is possible to measure smoke exposure markers in juice samples, but the reported values may not represent the actual amounts of smoke compounds in the grape. We strongly recommend against submitting juice samples for pre-harvest screening. The majority of smoke compounds are located in the skins. Crushing the grapes does not release all of the skin’s smoke related compounds into the juice. For this reason, submitting whole berries for testing is preferred. We do not accept fermenting samples, which may constitute a safety hazard.
Wines from small-scale fermentations (“micro-ferments” or “bucket ferments”) may be tested for volatile smoke markers (basic or extended panels) and for glycosylated markers to complement pre-harvest grape testing. The pros and cons of both tests are outlined in Figure 1.
Sample Preparation Time (before sending to the laboratory)
Immediate >1 Week
Sensory Evaluation Not very useful
Useful, but difficult (need for multiple trained tasters including sensitive individuals)
Analysis Turnaround Time 1-3 days 1-3 days
Prediction of Smoke exposure in Production Wines
Indirect (variable “multipliers” between grape and wine results)
Reds: more direct (but delayed)
Whites: uncertain (ferment with skins for “worst case scenario”?)
Figure 1: Grape Samples vs. Small Scale Ferments (Microferments) as pre-harvest testing options for wildfire smoke impact
Analyzing production wines immediately after completion of primary fermentation allows a first assessment of wildfire impact. It is preferable to sample and analyze wines that have not come in contact with oak or oak-derived products which can contribute the same volatile compounds used as wildfire smoke markers.
It is still possible to get useful information for barreled wines from volatile smoke markers. Take samples from the most “neutral” barrels available and choose the extended panel of volatile (free) smoke markers rather than the basic volatile marker panel (guaiacols only). There is no issue analyzing oaked wines for glycosylated markers, since oak or oak products do not contain these compounds. Analyzing for glycosylated markers in wine is always relevant regardless of contact with oak.

ETS has been offering the Volatile Marker Basic Panel, consisting of guaiacol and 4-methylguaiacol, since 2008. These are the primary volatile markers used to indicate exposure of grapes to wildfire smoke. The Basic Panel has an excellent track record for assessing wildfire smoke exposure with pre-harvest grape samples, small-scale fermentation samples (micro-ferments), and production wines before contact with oak.
Notably, its utility was confirmed by the 2018 Mendocino Complex Fire Lake County Winegrape Commission Study. Participants in this study were the University of California Cooperative Extension (UCCE), the Australian Wine Research Institute (AWRI), AWRI Commercial Services, and ETS Laboratories. (Please see “From Blaze to Bottle: Smoke Gets in Your Wine”by Glenn McGourty in the January 2020 Issue of Wine Business Monthly).
ETS also offers an extended panel of volatile smoke markers. In addition to guaiacol and 4-methylguaiacol, this panel measures the secondary markers of smoke impact o-cresol, m-cresol, p-cresol, phenol, syringol and 4-methylsyringol.
This extended panel is available for grapes, small-scale fermentation samples (micro-ferments), and wines. Additional markers allow more complete assessments of wildfire impact compared to the basic panel, The Extended Panel provides useful information for moderately oaked wines, particularly those from “neutral” barrels, which often contribute low amounts of guaiacols making interpretation of results very difficult.
ETS offers a panel of glycosylated (bound) smoke markers using a state-of-the-art combination of solid phase extraction, liquid chromatography, and triple quadrupole mass spectrometry (SPE/HPLC/MS/MS - QQQ).
Over the past decades, ETS has worked behind the scenes with the Wine Institute Technical Committee, the AWRI, and several major wineries to reach consensus agreement on a common list of glycosylated markers. This group joined forces to have pure reference compounds (and their isotopic analogues) synthesized for each of the markers in that list. These analytical standards allow extremely reproducible quantitative results which are comparable between laboratories. ETS offers analysis for these compounds in grapes, micro-ferments, and wines.
Glycosylated smoke-derived compounds are not contributed by toasted oak, making them particularly useful to assess wildfire impact in oaked wines. The Smoke Glycosylated Markers Panel includes the primary glycosylated form for each volatile marker included in our Expanded Panel (Figure 2). They are not directly odor-active, but may reportedly contribute lingering aftertastes that are often experienced with impacted wines. One theory is that they may hydrolyze in wine, slowly releasing volatile “free” forms, causing smoke flavors to become more noticeable with time.

(“Free”) Markers
4-Methylguaiacol
ortho-, meta- and paraCresol
Phenol
(“Bound") Forms
Rutinoside
4-Methylguaiacol Rutinoside
Cresol Rutinoside
Phenol Rutinoside
Syringol Syringol Gentiobioside
4-Methylsyringol 4-Methylsyringol Gentiobioside
Figure 2: Compounds included in the ETS Smoke Expanded Volatile Markers (left column) and their main glycosylated (sugar-bound) forms included in the Glycosylated Markers panel (right column).

The Extended Volatile Panel (“free”) and Glycosylated Marker Panel (“bound”) currently offered by ETS and the AWRI represent the most technically sophisticated methods for determining if grapes or wine have been impacted by wildfire smoke. Although the Basic Volatile Panel provides adequate information for grape analysis, the strength of correlations observed between additional markers definitely increases the confidence of detecting smoke impact in grapes and wine.
What levels and patterns to expect in wines?
Here are our observations based on previous seasons smoke exposure data. When volatile markers are high, expect glycosylated markers to be high. This is illustrated in Figure 3, which shows levels observed in several Cabernet Sauvignon wines. These levels range from normal low baseline levels, observed without exposure to wildfire smoke, to extreme levels resulting from severe exposure. Based on multi-vintage observations, these patterns and ranges can be expected with most red wines. We generally observe lower levels in white and rosé wines due to reduced skin contact.
A notable exception is Syrah, now well known to contain naturally significant baseline levels of volatile guaiacol. However, baseline levels of the other volatile markers appear to be normal, making them useful to assess smoke impact.
Another oddity is Petit Verdot, which contains normal baseline levels of smoke markers. However, multi-year observations indicate it appears to be much more sensitive to smoke exposure, accumulating higher levels of smoke compounds than other varietals in adjacent rows. In Petit Verdot wines, the observed range is much larger than with any other variety, with measured volatile guaiacol exceeding 200 ug/L, and combined levels of glycosylated markers approaching 1,000 ug/L.
Patterns where volatile markers are relatively low, suggesting little or no smoke impact, co-occurring with relatively high levels of glycosylated markers occur less frequently. We have seen these patterns with California Sierra Foothills grapes in 2021 and wines from Rhone varieties. These patterns are particularly worrisome to winemakers since glycosylated (bound) smoke compounds, which are odorless, are reported to degrade with time, releasing volatile (free) odor-active compounds. Examples of such patterns are presented in Figure 4 (Three samples on the left).
On the other hand, we have observed a number of samples with the opposite pattern, meaning high volatile markers but relatively low levels of glycosylated markers. This was especially evident with 2020 Pinot Noir wines as seen in Figure 4 (Two samples on right).
It is not known if this opposite pattern is a recurrent occurrence that is specifically particular to Pinot Noir. Low levels of glycosylated markers may be caused by a weakened defense mechanism from the plant against smoke volatiles. This could possibly be related to the severe heat wave experienced all across the Western United States in midAugust 2020, which caused a reduction, or in some cases even “shut-down”, metabolism in Pinot Noir grapes.
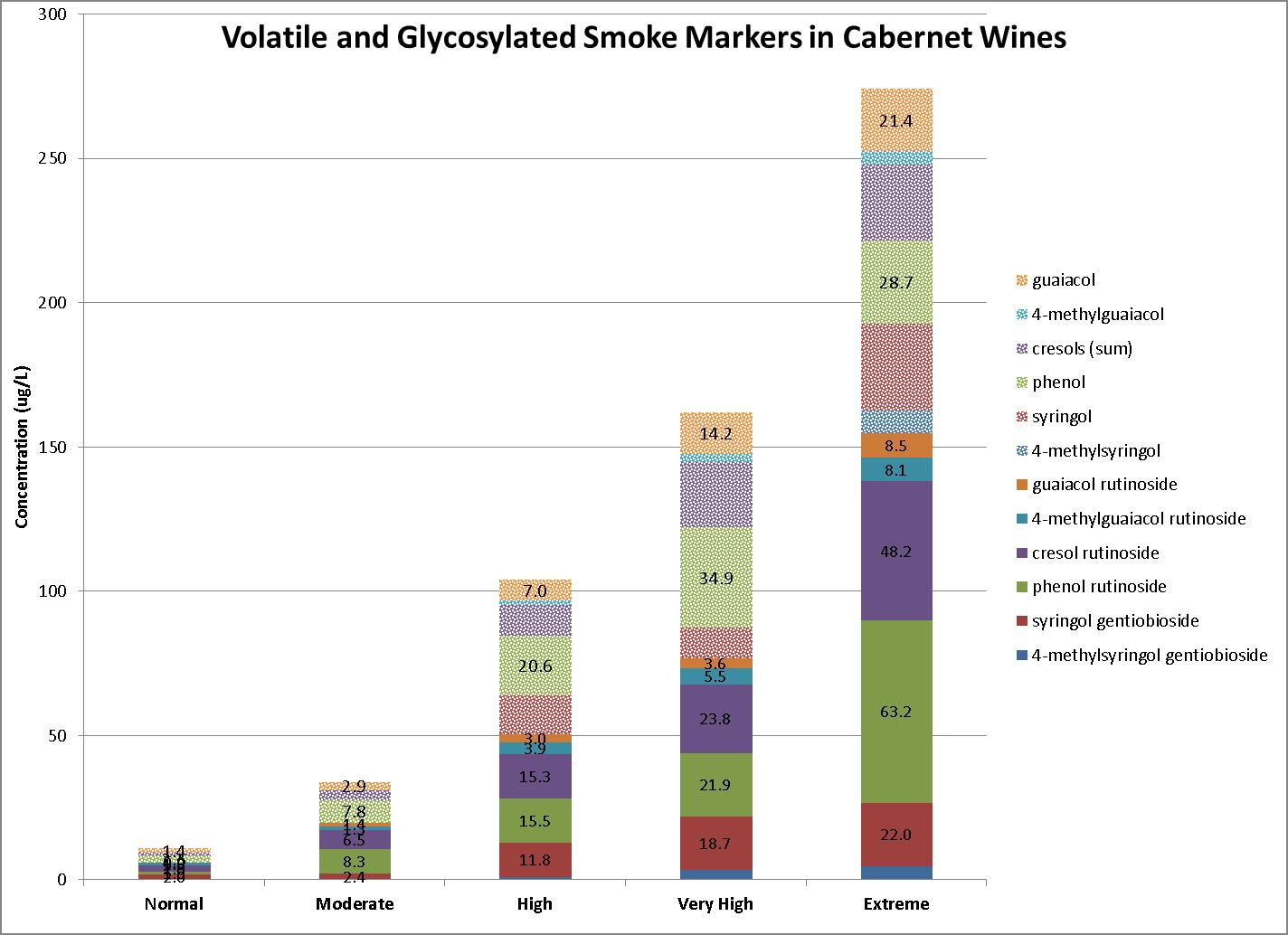
3: Range of volatile and glycosylated markers observed in 2020 Cabernet Sauvignon wines, from normal baseline to extreme levels resulting from severe exposure to wildfire smoke.
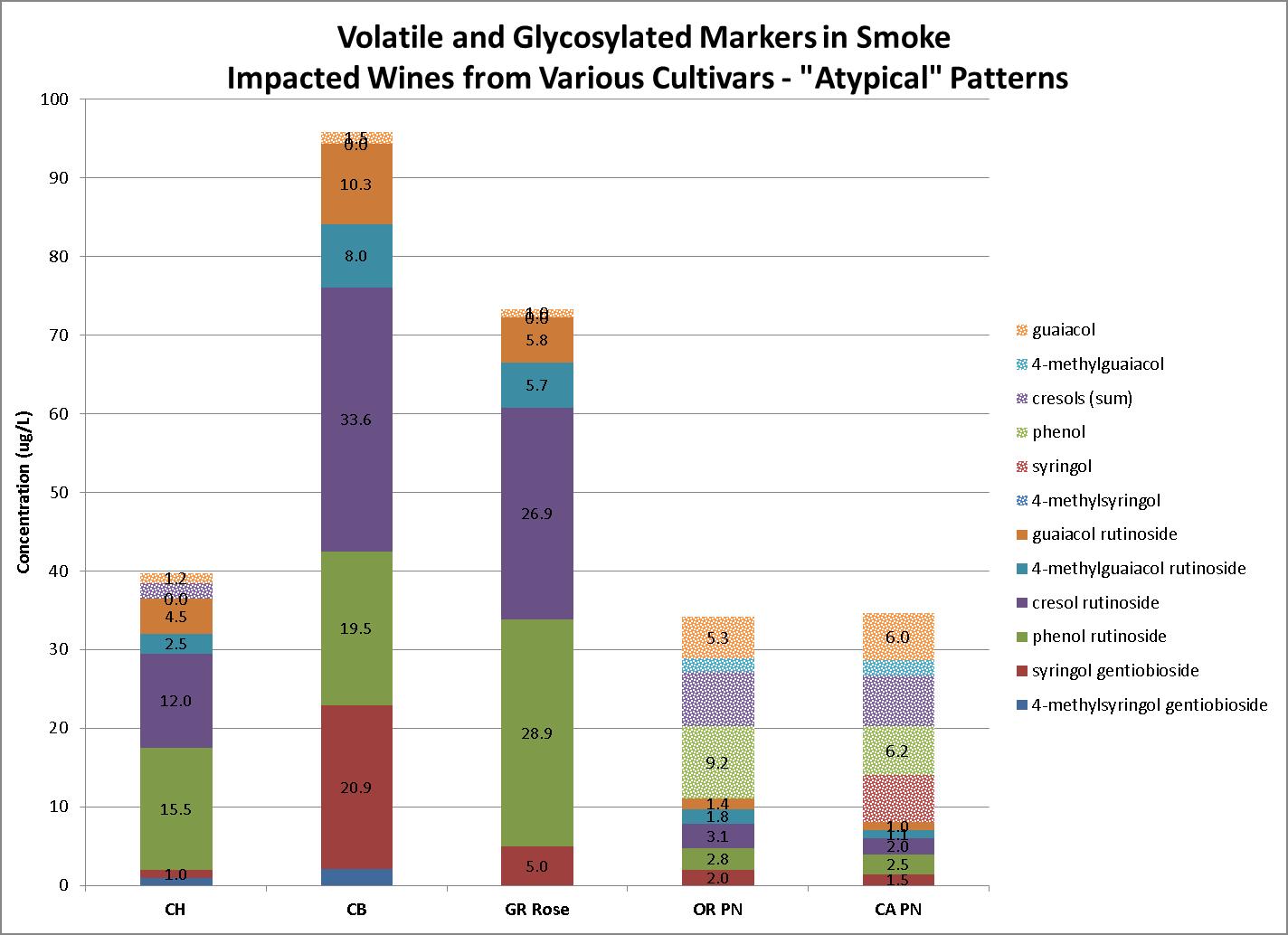
patterns between
very low levels of volatile markers with high glycosylated markers, while the two
opposite patterns.
I WANT TO TEST GRAPES FOR SMOKE, WHAT KIND OF SAMPLE SHOULD I BRING?
The preferred sample for red or white grapes is a representative 200-300 loose berry sample, with berries as intact as possible. Transport in small rigid “sandwich box” containers works well. This type of container is critical if you are shipping samples. Avoid submitting cluster samples. Additional cluster sample preparation time in the lab will delay results and there will be a processing fee. We do not recommend submitting juice samples
SHOULD I CRUSH BERRIES AND LET THEM SOAK IN THEIR JUICE BEFORE BRINGING THEM?
We do not recommend doing so. We must have consistent and uniform sample preparation for analyses to be comparable to baseline database concentrations. See pages 63-64.
WITH WHITE GRAPES, SHOULDN’T I BRING JUICE SAMPLES?
Since smoke compounds are primarily located in skins, we prefer whole berry samples for white grapes. Impact interpretation guidelines are similar for white and red grapes. With white grapes, smoke characters markers materializing in wine can be partially mitigated by minimizing skin contact. With red grapes, making a rosé by the direct pressing method can be a successful approach. In case of significant maceration with skins (e.g. following machine harvesting or intentional skin contact) the risk level may be equivalent with both red and white grapes.
ACAN YOU TEST FOR MORE THAN JUST “FREE” GUAIACOLS (GUAIACOL AND 4-METHYLGUAIACOL)?
Yes. We offer our extended volatile (free) markers panel for grapes and wines as well as our glycosylated (bound) glycosylated markers panel. See pages 57-58.
CAN I MIX BERRIES FROM DIFFERENT VARIETIES AND BRING A COMPOSITE SAMPLE?
This is not advisable, and is reportedly not acceptable for samples submitted for insurance purposes. Over the years we’ve seen drastic differences in pick-up of smoke compounds between grape varieties. For example, Petit Verdot is often much more impacted than other Bordeaux cultivars. We have also seen substantial differences in behavior between Chardonnay and Pinot Noir.
Syrah naturally contains variable amounts of guaiacol, the main smoke marker. This makes it impossible to assess smoke impact based on guaiacol only, whether with grapes or micro-ferments. Traditionally the only (but very imperfect) strategy available was to use other varieties grown next to Syrah blocks as “proxies”. Now availability of extended volatile and glycosylated markers panels can be utilized help directly assess Syrah samples.

Some laboratories offer testing after various acid and heat treatments, in an attempt to measure “Total” or “Bound” forms in their entirety. We have serious and continuing reservations about such tests, and do not offer them. Instead, for each of the volatile markers listed in our Expanded Panel, we can measure directly by HLPC/MS/MS its main glycosylated (sugar-bound) form (see figure 1). This is the strategy also adopted by the Australian Wine Research Institute (AWRI).
For grape samples the typical recommendation is about one to two weeks prior to harvest. Keep in mind that the impact of smoke is cumulative. “Negative” results early in the season may give a false sense of security, especially if more exposure to smoke happens. Of course, with microferments several days are required after grape sampling, in order to produce fermented samples that can be analyzed.
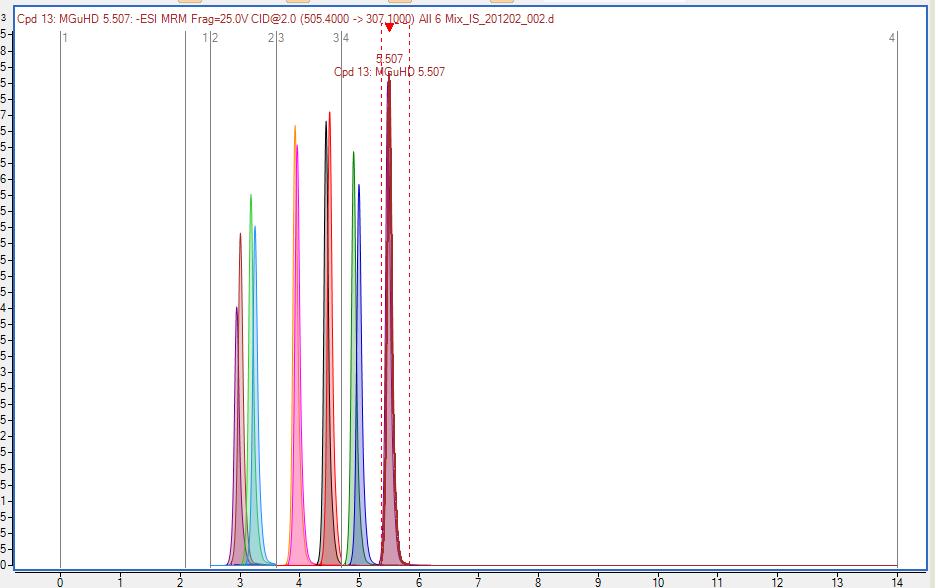
Figure 5: Glycosylated Smoke Markers and their isotopic analogues detected by LC/ MS/MS (ETS Laboratories). These standards make quantitative, accurate and reproducible results (comparable over a long period of time and between different laboratories) possible.
A step-by-step procedure for smallscale fermentations (micro-ferments) is available on the UC Davis Website:


I’D LIKE TO HAVE MICROFERMENTS TESTED. WILL ETS PREPARE THEM FOR ME?
We do not perform micro-ferments. For guidance, we recommend scanning the QR codes on the next page which will direct you to protocols from UC Davis, Washington State University, and the AWRI. At completion of fermentation, transfer the fermented wine into a bottle, let settle in fridge for a few hours, decant and submit sample in a 60 mL plastic tube. If a crop insurance claim is considered, check with your insurance provider if they accept micro-ferments.
AWRI protocol is found at:


DOES ETS RECOMMEND KEEPING PRE-HARVEST BACKUP SAMPLES?
Grape samples and micro-ferments serve two distinct purposes: helping with harvest decisions and serve as proof of smoke impact for insurance claims. Especially in the second case, it makes sense to keep backup samples, especially in the context of catastrophic wildfires.
Of course. Although each fire event is unique, our interpretation guidelines based on volatile (free) guaiacol derived from our experience since 2008 have stood the test of time, and have proven to be quite robust (Fig.6).
The presence of additional markers, with the extended volatiles marker panel, and the availability of a glycosylated markers panel, allow refining and confirming impact markers.
Comparison of grape results for extended volatile and glycosylated markers can be done easily online using our baseline database tool. See pages 63-64.
(OR MICRO-FERMENTS) TESTED “POSITIVE”. SHOULD I HARVEST?
Our interpretation guidelines are related to incremental risk scales, as there is no “magic number” below which no risk is present and above which wines are guaranteed to be impacted. Choosing to harvest or not will always be complex risk management decisions, loaded with painful consequences for both growers and wineries. Analytical results only help in making these difficult decisions.
Grapes and wines naturally contain low “baseline” levels of smoke markers, variable by grape variety and possibly by geographic origin. In most cases, we are confident that our current knowledge is adequate to help our clients with result interpretation.
The best and most valuable knowledge will always be obtained by creating your own database for grapes, microferments and/or wines from grapes not exposed to significant fire events.
For the most accurate turnaround time log in to your client portal and view analysis details for the relevant panels.
In case of catastrophic and widespread fire events, we will keep our TAT information for grape, micro-ferment and wine tests prominently displayed and updated regularly.
THE FOLLOWING FIGURES ARE BASED UPON THE ARTICLE “ FROM BLAZE TO BOTTLE: SMOKE GETS IN YOUR WINE” WINE BUSINESS MONTHLY JANUARY 2020
GUIDELINES FOR WHOLE BERRY TESTS (EXCLUDING SYRAH):
ANTICIPATED IMPACT IN WINE
GUIDELINES FOR MICROFERMENTS AND UNOAKED WINES (AGAIN EXCLUDING SYRAH):
Figure 6: Guidelines for volatile (free) guaiacol, the main marker of wildfire smoke impact, in grapes and micro-ferments and unoaked wines. Additional markers in the extended volatile panel as well as glycosylated markers allow refining and confirming assessments.
THESE GUIDELINES ARE FOR GUAIACOL, NOT SUM OF TWO COMPOUNDS. GUIDELINES ARE SIMPLY OBSERVATIONS BASED UPON PAST EVENTS, AND MAY OR MAY NOT APPLY TO CURRENT OR FUTURE EVENTS. ETS DOES NOT, AND WILL NOT, PROPOSE ACCEPTANCE OR REJECTION CRITERIA
Compare your smoke marker levels in grapes to the ETS Baseline Database through your ETS Client Portal.
Case #1:

All markers below reportable limits, or below baseline range = no evidence of exposure to smoke
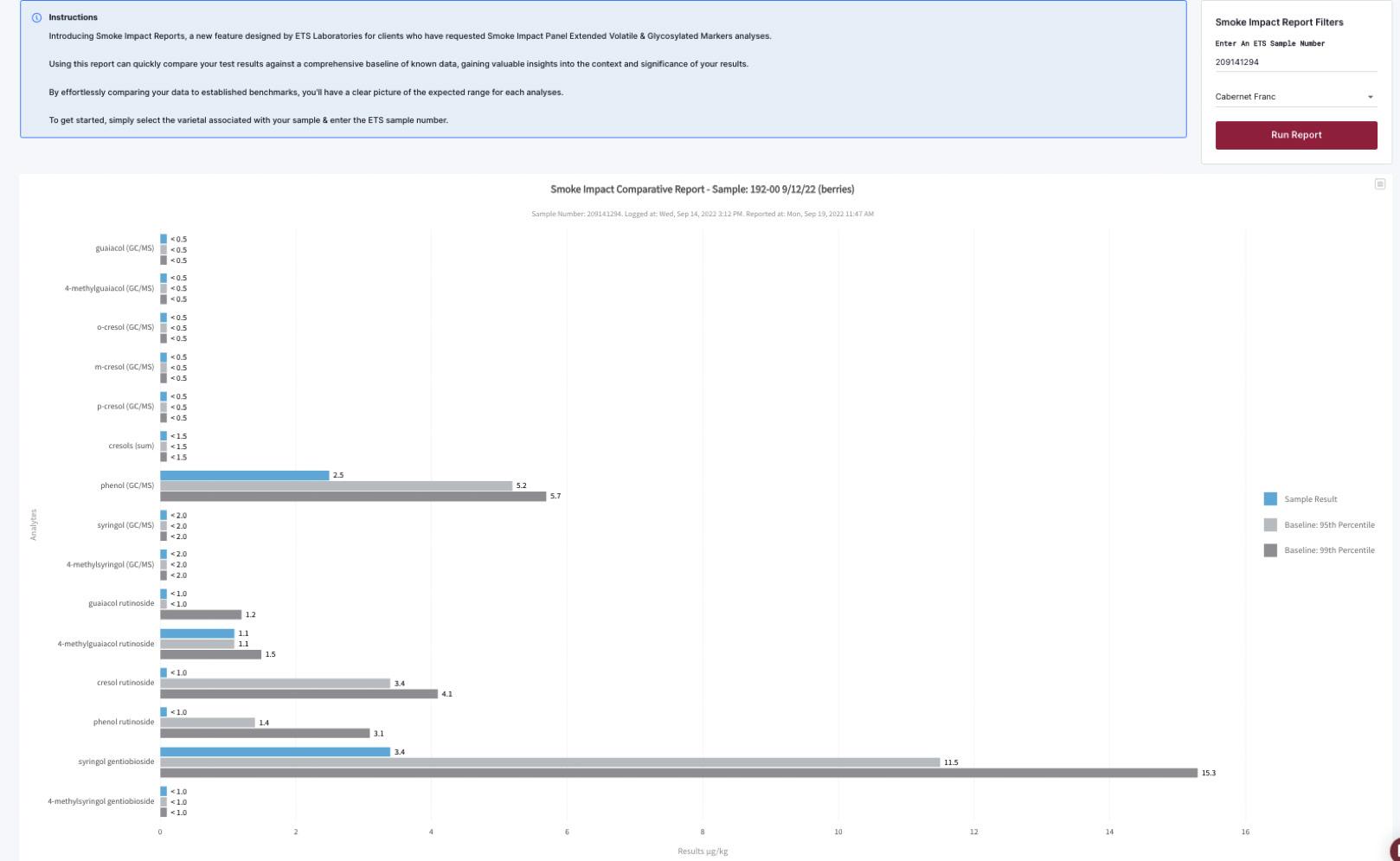
Blue: Your Sample Results
Grey: ETS Database Baseline Ranges

1. Log in to your ETS account
2. Click on the “Smoke Impact” icon on the left of your screen
3. Please read the Terms & Conditions carefully before continuing 4. A list of grape samples analyzed under your account will be displayed.
. Select one of your eligible samples
. Choose the varietal that matches your sample
. Click on the ‘Run Report’ Button

Case #2:
All markers (except syringols) above baseline range = clear evidence of exposure to smoke
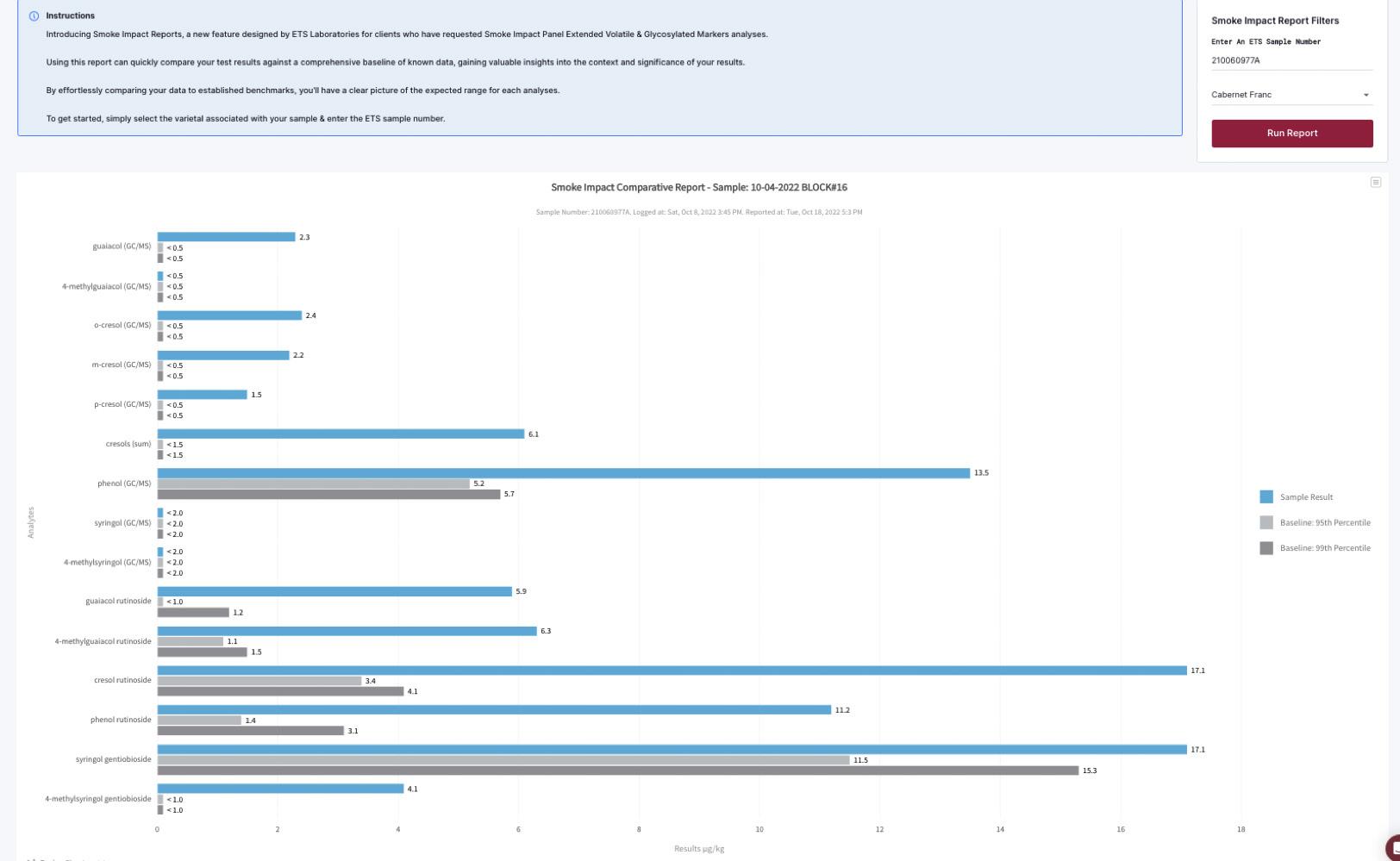
WARNING: Do NOT compare smoke markers results from any given laboratory to a baseline database from a different source.



The ETS Grape Maturity Monitoring Panels provide a set of analyses that growers and winemakers request to monitor fruit maturity, including Brix, Glucose + Fructose, TA, pH and malic acid, as well as berry size parameters and average sugar per berry. Monitoring sugar per berry allows growers to determine the duration and rate of active sugar loading, during which vines synthesize and actively transport sugar into berries. The time at which the sugar loading phase ends, and what level of Brix was achieved at that time, are both important indicators of wine growing conditions (excessive or insufficient vigor, water availability and resistance to heat stress).
For more details see pg. 1

Follow changes in grape phenolics during ripening, using the catechin and tannin "ripeness" index to monitor seed ripening, and quercetin analysis to monitor canopy effects on grape phenolics.
For more details see pg. 43

Wild yeast and bacteria from the vineyard may be introduced into the winery on the harvested fruit, causing spontaneous fermentation and/or spoilage. ScorpionsTM DNA analysis offers winemakers an early detection tool to identify these spoilage organisms.
Despite the best practice of modern winemaking methods, microbial contamination often occurs during wine production. Spoilage microbes are capable of survival and growth in the wine, potentially producing offflavors, off aromas, and turbidity. Microbiological contamination is often undetected until related problems in the wine become noticeable by sensory evaluation. Scorpions™ assays, based on specific genetic targets, detect the full range of wine and juice spoilage organisms. This genetic analysis method detects microbial populations directly in wine or juice. Results are routinely reported within two business days, giving winemakers the ability to address problems before wine defects occur.
Targeted genetic probes give the winemaker the ability to monitor only those specific spoilage organisms that have the potential to adversely impact wine quality, and to accurately measure populations down to extremely low levels.
For more details see pg. 29

Changes in grape water content influence finished wine composition and can be as important as standard sugar and acid measurements when making picking decisions. Grape water content is also very useful for understanding changes in TA, pH, ºBrix, and other harvest indicators.

Our most popular harvest panel offers a full range of grape and must analyses, combining more than 10 tests including fermentable sugar (to help estimate alcohol content) and YAN (yeast-assimilable nitrogen — to help predict sluggish or stuck fermentation and potential sulfide formation.)
For more details see pg. 2

Glutathione, a natural grape antioxidant, can protect the aroma and flavor of white and rosé wines and prevent premature aging. Glutathione levels fluctuate during production, as the compound can be absorbed by yeast and then released after fermentation.
For more details see pg. 52

The compounds in wildfire smoke are absorbed by vines and can cause unwanted flavors in wine. Analyzing for these compounds allows winemakers to screen grapes for the risk of smoke impact and work to mitigate its effects.
For more details see pg. 53

The compound responsible for a "green bell pepper" aroma in wine, IBMP, decreases quickly during maturation. But once grapes are picked, it is hard to control. Monitoring changes in grape IBMP directly influences final levels in wine, and is crucial in making picking decisions.
For more details see pg. 52

Eucalyptus character is a controversial sensory expression in red wines from California and countries with Mediterranean climates. Even a slight “eucalyptus” note can interfere with delicate varietal aromas, and can have a detrimental influence on certain grape varieties.
For more details see pg. 51

This comprehensive test panel checks grapes for Botrytis (using ScorpionsTM ) and laccase, detecting both the spoilage organism and its byproducts that can harm your wine.

Laccase is a polyphenol oxidase associated with rot caused by Botrytis. Elevated levels of laccase can result in oxidation of phenolic compounds that may cause color degradation or premature browning in red wines. In addition, laccase mediated oxidation can also affect the aroma profile of the wine.
Our automated method reports yeast viability and total cell count back to the client within hours, and the real-time microscopic flow image analysis examines 1,000 times the volume used in standard microscopic methods, vastly increasing the accuracy of your results.

By the end of maceration or fermentation, the tannin content of a wine is already fixed. Monitoring phenolics during this critical period allows winemakers to better control tannins by increasing or decreasing phenolic extraction.
For more details see pg. 45
ETS Laboratories offers DNA fingerprinting to distinguish between closely related strains of Saccharomyces cerevisiae. ETS MLVA technology allows winemakers to monitor yeast population in native fermentations and check the efficiency of inoculations with commercial strains.


Since we first opened our doors in St. Helena in 1978, we’ve grown alongside the wine industry, partnering with our clients as they’ve gone on to create many of the world’s finest wines. We’re continuing to invest not only in Napa Valley, but also in other rapidly growing wine regions by expanding our local and online services to support winemakers with advanced tools and technical assistance.
Here’s what to take advantage of in Harvest 2025:

Samples can be dropped off locally for any analysis ETS offers. These common harvest analyses will be run onsite for quicker turnaround.
ALL SATELLITES

PASO ROBLES, WALLA WALLA & NEWBERG
PASO ROBLES & NEWBERG
VISIT OUR WEBSITE FOR CURRENT PRICES, AND THE FULL LIST OF ONSITE WINE ANALYSES.
ANALYSIS
TECHNIQUE TURNAROUND
JUICE PANEL MINERVA SAME DAY
BRIX
GLUCOSE + FRUCTOSE
PH
TA (TITRATABLE ACIDITY)
TARTARIC ACID
L-MALIC ACID
POTASSIUM
NOPA
AMMONIA
VOLATILE ACIDITY
BUFFER CAPACITY MANUAL SAME DAY
ETHANOL MINERVA SAME DAY
FREE SO 2
FLOW INJECTION SAME DAY
TOTAL SO 2 FLOW INJECTION SAME DAY
TURBIDITY TURBIDIMETRY SAME DAY
RAPID PHENOLIC PANEL HPLC 1 DAY
GRAPE PHENOLIC PANEL HPLC 1 DAY
SCORPIONS BACTERIA
JUICE PANEL SCORPIONS™
SCORPIONS YEAST JUICE PANEL SCORPIONS™
SCORPIONS COMBINED JUICE PANEL SCORPIONS™ 2
It’s important to collect and handle harvest samples carefully to ensure accurate and representative results. We’ve collected our recommendations to help you get the most out of your harvest analyses.

Most harvest samples received at ETS come to the laboratory as juice.
Berries pressed for a juice sample should be selected from at least 20-40 different clusters, and can easily be pressed by hand in their collection bag – pour the juice into a standard ETS 60mL sample tube and label with your ETS client labels.
Samples should be kept cool to prevent fermentation.
Take 200-400 berries per block. Pick berries from random clusters on both sides of the row. Take berries from the top and bottom of both the front and back of each cluster.
*Samples submitted for berry analysis should contain only intact and undamaged fruit.
For grape phenolic testing, a representative sample is critical to obtain accurate results, especially in varietals with tight clusters. Samples for the Grape Phenolic Panel should include berries from at least 20-40 different clusters. Clusters can be collected either from harvest containers or directly from the vineyard.
To get a representative sample, all the berries must be stripped from the clusters and mixed before bagging a 300-400 berry sample for analysis (about 500g or 16 oz).
Samples should contain only intact and undamaged fruit to ensure accurate results.
We encourage clients to submit berry samples rather than whole clusters. If samples are submitted as clusters, ETS will prepare a berry sample for an additional fee.
Each bag of berries should be clearly labeled with the client name, sample ID, and analyses to be performed.
ETS provides free sample labels that are pre-printed and barcoded with your client ID – visit our website, or give us a call.
Don’t get caught empty handed – order complimentary tubes, pre-printed labels, and shipping pods to take the headache out of collecting and shipping samples.
1. Login to your ETS account and select the winery you're ordering supplies for.
2. Use the "Get Supplies" button on the dashboard to place a supply order.
Outside the range of our sample pickup service?
ETS offers free shipping kits, which include an insulated envelope and an ice pack, to help you easily send in samples no matter where you're located.

To prevent problems from fermentation, juice samples should be frozen or boiled for shipment, and clearly marked as either “FROZEN” or “BOILED” depending on the treatment used.
Boil samples with a loosely fitting cap to prevent evaporation and concentration. Do not over-boil.
Freeze the sample in a plastic ETS sample tube. Do not over-fill the tube – leave a small space for the sample to expand when frozen.
Never freeze samples in glass containers to prevent breakage and injury.
To ensure accurate results, it's important to avoid damaging DNA or killing yeast and bacteria:
° Keep samples cool with ice packs.
° Ship by overnight delivery using a parcel carrier like FedEx, UPS, or GLS.
Scorpions samples should not be frozen or boiled.

In addition to our St. Helena headquarters, we operate satellite laboratories across the West coast to bring advanced tools to winemakers' doorsteps and provide local support to other growing wine regions.
These quick guides provide a reference for the 2025 harvest, including weekend hours and convenient services to make it easier than ever to send in harvest samples, including dropbox locations and complimentary courier service.
As always, if you have any questions, just call your local lab and we'll be happy to help.
(707) 963-4806
899 Adams Street, Suite A St. Helena, CA 94574
SEE INSTRUCTIONS FOR SHIPPING JUICE SAMPLES – P. 41
ST. HELENA
Located at our laboratory, next to the main entrance.
Samples left overnight will be processed when we open the following business day.
HOURS
Every Harvest ETS offers extended and weekend hours. Our team is on-call to meet your needs.
Please check the ETS Website for our most up-to-date business hours.

To schedule on-call service, please call by 2pm on Friday: (707) 963-4806
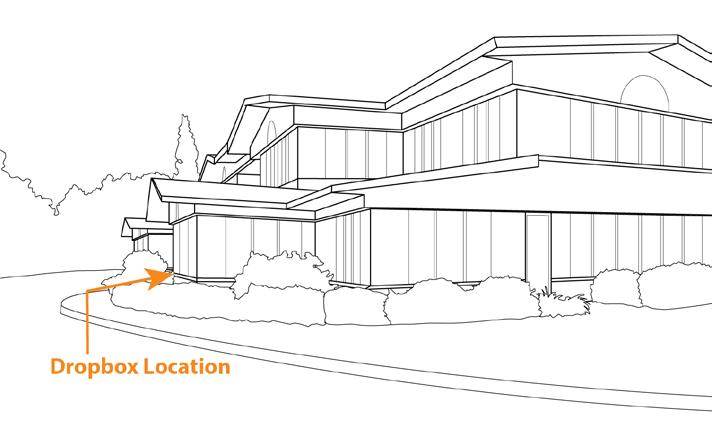
DROPBOX IS LOCKED FOR THE PIN CODE, CALL US, OR LOG IN TO YOUR ETS ACCOUNT AND VISIT “CONTACT”
Our complimentary courier service is available in Napa, Sonoma, and Mendocino counties every day ETS is open.
REQUEST A PICKUP
DEADLINE You can request a courier pickup by logging in to your ETS account, or by calling our lab.
Please request a pickup by 10 am
This allows us to ensure speedy turnaround on time-critical harvest analyses.
For the most up-to-date dropbox hours and pick up times please check our website: www.etslabs.com/contact.
LODI DROPBOX ESTATE CRUSH
SOUTH NAPA DROPBOX RUTHERFORD EQUIPMENT
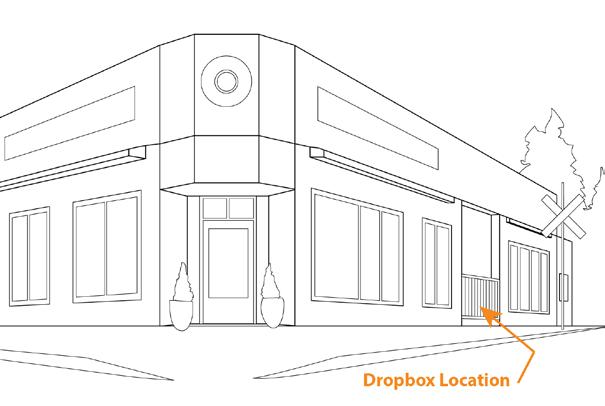
2 W. Lockeford Street Lodi, CA THE DROPBOX IS AVAILABLE WHEN ESTATE
759 Technology Way Napa, CA
PHONE (805) 434-9322
3320 Ramada Drive, Suite B Paso Robles, CA 93446
IF YOU NEED TO SHIP SAMPLES, PLEASE SEND THEM DIRECTLY TO OUR ST. HELENA LAB: 899 ADAMS STREET, SUITE A ST. HELENA, CA 94574
Every Harvest ETS offers extended and weekend hours. Our team is on-call to meet your needs.
Please check the ETS Website for our most up-to-date business hours.

To schedule on-call service, please call by 2pm on Friday: (707) 963-4806
Located at our laboratory, next to the front door.
Samples left overnight will be processed when we open the following business day.
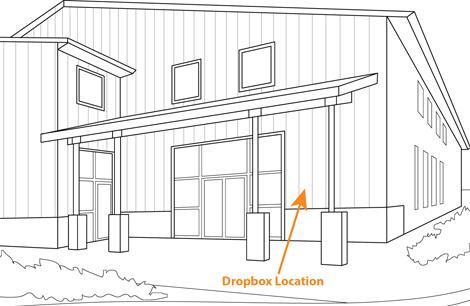
DROPBOX IS LOCKED. FOR THE PIN CODE, CALL US, OR LOG IN TO YOUR ETS ACCOUNT AND VISIT “CONTACT”
SANTA MARIA
2330 Westgate Road Suite 9 Santa Maria, CA, 93455
LOMPOC
333 North D Street Lompoc, CA, 93436
BUELLTON
90 Easy St, Buellton, CA 93427
FOR ALL CENTRAL COAST DROPBOXES, PLEASE DROP SAMPLES BY 11:00 AM FOR SAME-DAY DELIVERY MONDAY-FRIDAY
We're excited to offer complimentary courier service this September for our clients in the Central Coast.
REQUEST A PICKUP
You can request a courier pickup by logging in to your ETS account, or by calling our Paso Robles lab.
Please request a pickup by 10 am. This allows us to ensure speedy turnaround on time-critical harvest analyses. DEADLINE
SERVICE AREA
We're currently picking up samples in the Paso Robles and SLO areas. We will continue to expand as demand growsplease let us know if you'd like service.
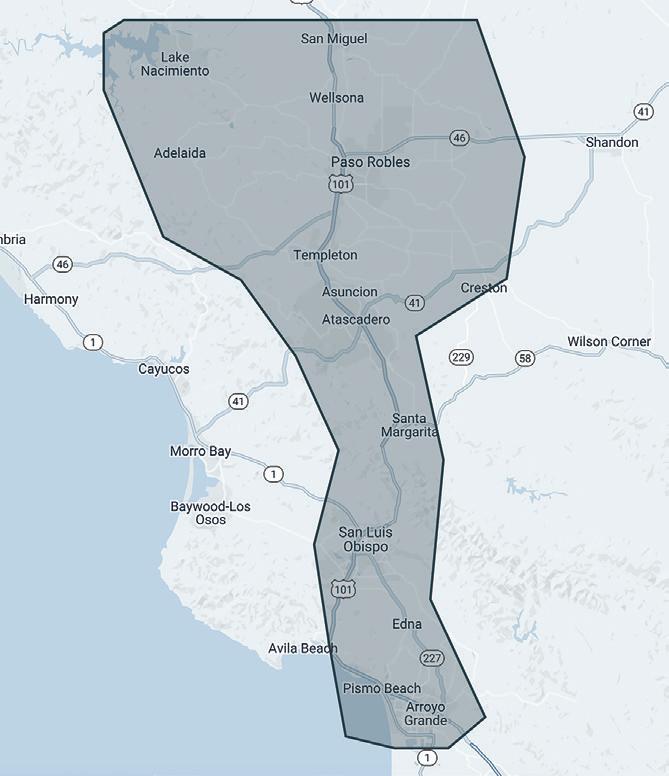
PHONE (707) 433-7051
190 Foss Creek Circle, Suite G Healdsburg, CA 95448
IF YOU NEED TO SHIP SAMPLES, PLEASE SEND THEM DIRECTLY TO OUR ST. HELENA LAB: 899 ADAMS STREET, SUITE A ST. HELENA, CA 94574
Every Harvest ETS offers extended and weekend hours. Our team is on-call to meet your needs.
Please check the ETS Website for our most up-to-date business hours.

To schedule on-call service, please call by 2pm on Friday: (707) 963-4806
Located at our laboratory, on the north side of the building.
Samples left overnight will be processed when we open the following business day.
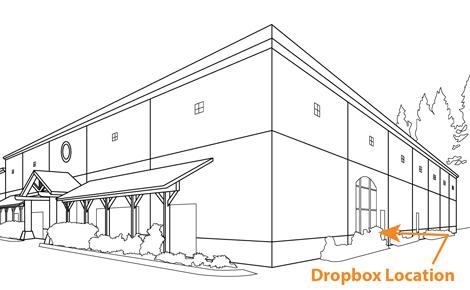
Our complimentary courier service is available in Napa, Sonoma, and Mendocino counties every day ETS is open.
REQUEST A PICKUP
You can request a courier pickup by logging in to your ETS account, or by calling our lab.
DEADLINE
Please request a pickup by 10 am
This allows us to ensure speedy turnaround on time-critical harvest analyses.
537-6245
214 W. Hancock Street Newberg, OR 97132
IF YOU NEED TO SHIP SAMPLES, PLEASE SEND THEM DIRECTLY TO OUR ST. HELENA LAB: 899 ADAMS STREET, SUITE A ST. HELENA, CA 94574
HOURS
Every Harvest ETS offers extended and weekend hours. Our team is on-call to meet your needs.
Please check the ETS Website for our most up-to-date business hours.

To schedule on-call service, please call by 2pm on Friday: (707) 963-4806
Located at our laboratory, next to the main entrance. Samples left overnight will be processed when we open the following business day. NEWBERG
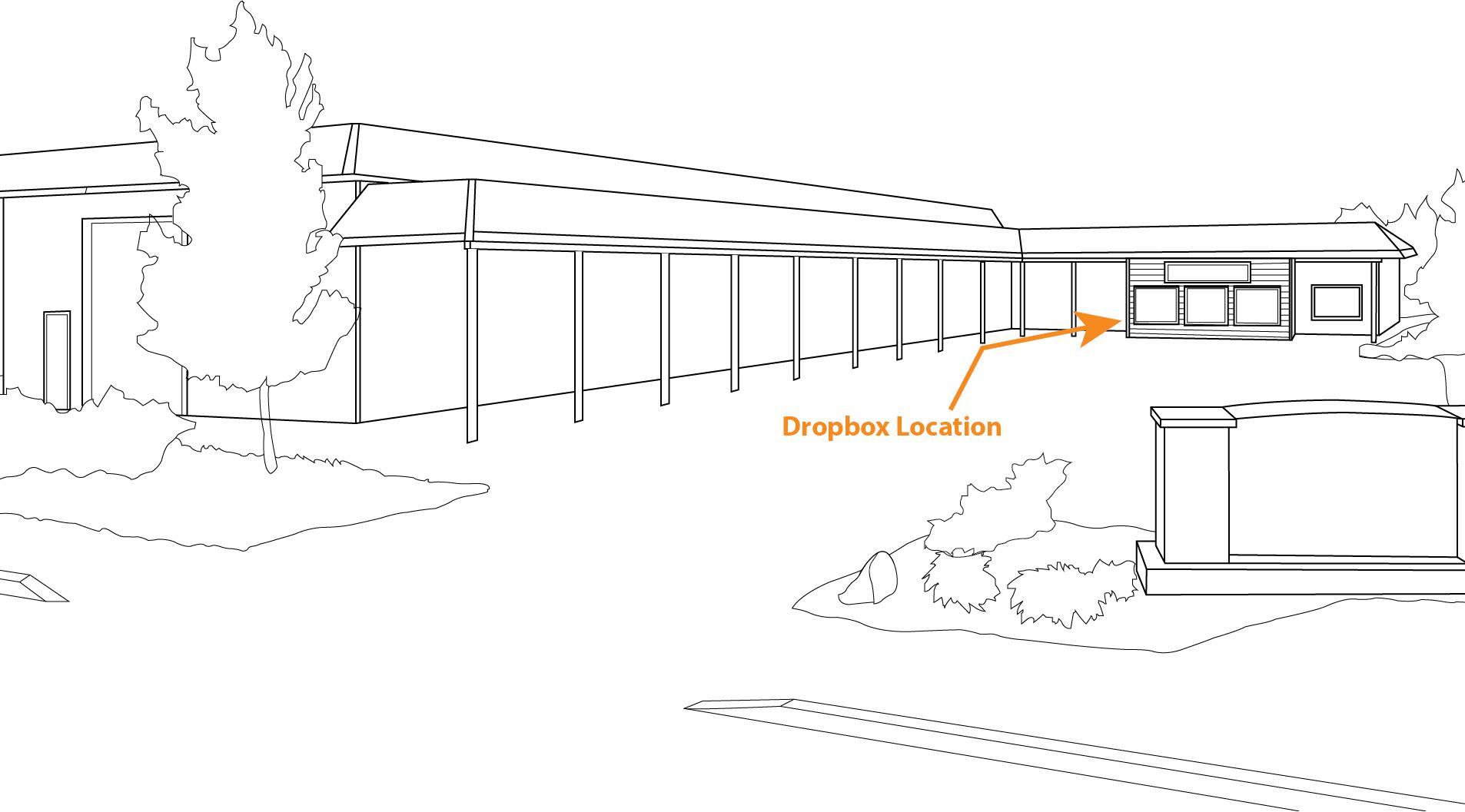
Our complimentary courier service is available in Salem, McMinnville, Newberg, and the surrounding areas every day ETS is scheduled to be open.
REQUEST A PICKUP
You can request a courier pickup by logging in to your ETS account, or by calling our lab.
DEADLINE
Please request a pickup by 10 am
This allows us to ensure speedy turnaround on timecritical harvest analyses.
(509) 524-5182
Every Harvest ETS offers extended and weekend hours. Our team is on-call to meet your needs.
Please check the ETS Website for our most up-to-date business hours.

To schedule on-call service, please call by 2pm on Friday: (707) 963-4806
DROPBOX LOCATIONS
PROSSER
WINEMAKERS LOFT
357 Port Ave Prosser, WA
PICKUP TIME: 10:45 AM
PLEASE DROP SAMPLES BY 10:30 AM FOR SAME-DAY DELIVERY MONDAY-FRIDAY
HOPTOWN PIZZA
2560 Donald Wapato Rd. Wapato, WA
PICKUP TIME: 10:00 AM
PLEASE DROP SAMPLES BY 9:45AM FOR SAME-DAY DELIVERY MONDAY-FRIDAY
3020 E. Isaacs Ave. Walla Walla, WA 99362
Located at our laboratory, next to the main entrance.
Samples left overnight will be processed when we open the following business day.
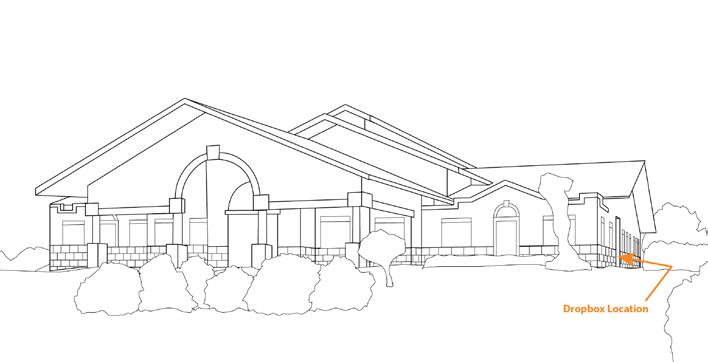
DROPBOX IS LOCKED. FOR THE PIN CODE, CALL US, OR LOG IN TO YOUR ETS ACCOUNT AND VISIT “CONTACT”
RED MOUNTAIN COOPER WINE CO. 35306 N Sunset Rd. Benton City, WA
PICKUP TIME: 11:30 AM
PLEASE DROP SAMPLES BY 11 AM FOR SAME-DAY DELIVERY MONDAY-FRIDAY
RICHLAND CENTRAL INDUSTRIAL SALES
PICKUP TIME: 12 PM 2235 Henderson Loop Richland, WA
PLEASE DROP SAMPLES BY 11:30 AM FOR SAME-DAY DELIVERY MONDAY-FRIDAY
Our complimentary courier service is available in Walla Walla and the surrounding areas every day ETS is scheduled to be open.
REQUEST A PICKUP
You can request a courier pickup by logging in to your ETS account, or by calling our lab.
DEADLINE
Please request a pickup by 10 am
This allows us to ensure speedy turnaround on time-critical harvest analyses.
14030 NE 145th St., Suite B Woodinville, WA
PICKUP TIME: 1 PM
PLEASE DROP SAMPLES BY 8 PM FOR OVERNIGHT SHIPMENT MONDAY-THURSDAY