

COLOPHON
Production and Publication
Research & Training, Dept. of Radiology & Nuclear Medicine, Erasmus MC
Editor
Roos Murtagh
Assistant Editor
Shemara Mendes
Ting Ting Hu
Design & Photography
Vincent Blinde
Steven Ensering
Frank van der Panne
Maartje de Sonnaville
Printing
Ipskamp Printing Enschede
Visiting address
Department of Radiology & Nuclear Medicine
Erasmus MC
Dr. Molewaterplein 40 3015 GD Rotterdam
The Netherlands
Telephone: + 31 10 703 2277
Fax: + 31 10 703 4033 research.radiology@erasmusmc.nl
Post address
Department of Radiology & Nuclear Medicine
Erasmus MC
P.O. Box 2040
3000 CA Rotterdam
The Netherlands
Website http://www.erasmusmc.nl/radiologie
2021 Scientific Report department
of radiology & nuclear medicine
Aad van der Lugt, MD, PhD
Marion Smits, MD, PhD
Bench-to-Bedside
Esther AH Warnert, ir, PhD Theranostics of CNS and H&N tumours
Sophie Veldhuijzen van Zanten, MD, PhD
Ricardo Budde, MD, PhD & Alexander Hirsch, MD, PhD
Ivo G Schoots, MD, PhD
Edwin HG Oei, MD, PhD Improving
Adriaan Moelker, MD, PhD
MD, PhD
Myriam Hunink, MD,
Tonya White, MD,


2021 has been a special year for our department. This year has been a transition period in which the former chair prof.dr. Gabriel Krestin gradually handed over the lead of the department to me. During the final week of December multiple small farewell activities were organized for him. The activities were small because of the COVIDrestrictions and multiple because many colleagues liked to say goodbye, express their gratitude and recollect past highlights.
For me, it is a great honor to follow Gabriel Krestin as Head of Department. In the past months I have frequently heard the phrase: big shoes to fill. Gabriel has indeed achieved tremendous successes over the last two decades. The research in the department has achieved a world-standing over the years and also for 2021 several important milestones were reached.
We again managed to publish a record number of scientific papers and the citation score remained high. Twelve PhD defenses took place, most of them virtually. Two of the defenses were very special; Sebastian van der Voort defended his thesis with distinction and Ronald Booij was the first radiographer in the Netherlands to defend a PhD thesis.
Also several important grants were acquired, among them the prestigious ERC starting grant received by Julie Nonnekens for investigating the radiobiology of targeted radionuclide therapy.
The most important event was the assessment of the research performance with the Standard Evaluation Protocol (SEP) by independent international experts. In the final report we reached a score of excellence in all three evaluated domains: research quality, social relevance of the research, and viability of the research group. This report also provided useful advice how to further improve the quality of our scientific output.
It would require a huge effort to remain at the current scientific level in the coming years. However, all achievements mentioned in our scientific report would not have been possible without the tremendous efforts of very talented researchers. These talented and motivated people will continue to strive for the best results. Some of them have
PREFACE
recently been promoted. Ricardo Budde was appointed professor in Cardiovascular Imaging. Yann Seimbille, Laura Mezzanotte, Daniel Bos and Hieab Adams advanced to an associate professorship, and Gennady Roshchupkin and Sophie Veldhuijzen van Zanten to an assistant professorship.
These promotions are a reflection of their knowledge, skills and passion invested in scientific research and supervising new talents. With such individuals, we need not be afraid for the future.
In 2021, we further strengthened the collaborations with the Technical University of Delft. The Convergence initiative of the Erasmus MC, TU Delft and the Erasmus University will create new perspectives and opportunities for the role of technology in the medical domain. Our department is well suited and positioned to contribute to this program. We have already established five joint appointments with TU Delft and acquired several joint grants.
I would like to thank all of our researchers, PhD students, Post-docs and our supporting team for their continuous hard work. Today, research is teamwork and our team has strong and very dedicated players. I would also like to thank our external collaborators: our research partners from so many departments within Erasmus MC, our colleagues in radiology and other specialties from other universities worldwide, and particularly our partners in industry. I hope that the collaborations will continue also in the coming years.
Enjoy reading this annual report and expect more news from us in the coming years.
Aad van der Lugt, Professor and Chairman, May 2022
HIGHLIGHTS 2021
Honors & Awards

Stefan Roobol received a Young Investigator Award from the European Society of Radiation Research (ERRS) at the ERRS annual congress for his abstract ‘Live single cell tracking and deep learning-based analysis of DNA damage induction and repair following beta particle radionuclide therapy’.
Maryana Handula obtained the “Best Poster Award” for her presentation at the European Molecular Imaging Meeting (EMIM 2021).
Marion Smits was named ‘Most influential radiology researcher’ by AuntMinnie Europe.
Sebastian van der Voort won the YOUNG Medical Delta award and a Convergence Health and Technology Open Research Award.

Ivo Wagensveld received the EAU Prostate Cancer Abstract Award 2021 for the best abstract published on clinical and experimental studies in prostate cancer “A prospective multicenter comparison study of a risk-adapted ultrasound-directed and MRI-directed diagnostic pathway in prostate cancer suspected biopsy naïve men” at the annual congress of the European Association of Urology, virtual 2021.
Stephan Breda won the best oral presentation award during the ‘Star Paper Session’ of the annual scientific meeting of the Dutch Society for Sports Medicine (VSG) for his presentation entitled “Effectiveness of Progressive Tendon-Loading Exercise Therapy in Patients with Patellar Tendinopathy: A Randomised Clinical Trial.
Dianne van Dam-Nolen was award the best abstract prize at the European Congress of Radiology, for her abstract entitled: “A Prospective Multicenter Study To Improve Diagnosis Of High-Risk Carotid Plaques”.
Kimberlin van Wijnen received a Convergence Heath and Technology Open research Award.
Personal Grants
Vikram Venkatraghavan was awarded an Out of the Box grant from the DCVA Hearth-Brain Connection Crossroads consortium.

Julie Nonnekens received an ERC starting grant 2021 for the project “RADIOBIO: Deciphering the radiobiology of targeted radionuclide therapy: from subcellular to intra-tumoural analyses”.
Tessa Brabander received the KWF Young Investigator Grant in 2021.
Sophie Veldhuijzen van Zanten won the Young Scientific Talent Award of the Erasmus MC Foundation-Daniel den Hoed Fund.
Rianne van der Heijden was awarded a Bracco Fellowship for Translational Research in Advanced MRI which will allow her to spend two years as a Visiting Assisting Professor at the University of Wisconsin, Madison, USA as of April 2022.
Julia Neitzel was awarded a Marie Curie Global Fellowship for a collaboration between Harvard T.H. Chan School of Public Health and Erasmus MC on understanding resistance and resilience factors in the asymptomatic stage of dementia.
Gennady Roshchupkin, Frank Wolters and Jeremy Labreque all received a prestigious VENI grant which will allow them to continue their work for the Radiology & Nuclear Medicine and Epidemiology departments.
Appointments
Marleen de Bruijne was elected Fellow of the MICCAI Society.
In 2021, Esther Bron started as chair of the special interest group on Reproducibility and Open Science of the Deep Dementia Phenotyping (DEMON Dementia) Network.
Frederik Verburg was appointed Professor of Translational Nuclear Medicine.
Yann Seimbille was appointed a member of the editorial board of the European Journal of Nuclear Medicine and Molecular Imaging Radiopharmacy and Chemistry.
Esther Warnert was appointed a committee member of the Equality, Diversity and Inclusivity Task Force of the International Society of Magnetic Resonance in Medicine and VENA (the women in Academia network at the Erasmus MC).
Edwin Oei was appointed President of the Musculoskeletal MR Study Group of the International Society for Magnetic Resonance in Medicine (ISMRM).
Daniel Bos was appointed as Guest-Professor at the department of Cardiovascular Sciences at KU Leuven
Conferences, Courses, Special Lectures
Edwin Oei will co-hosted the International Workshop on Osteoarthritis Imaging (IWOAI) in Rotterdam from 30 June to 2 July 2021 which, after a period of lockdown, was attended by 25 in-person and 100 online attendees.
Edwin Oei delivered a prestigious invited lecture on the “Year in Review 2021: Imaging” during the virtual Osteoarthritis Research Society International (OARSI) World Congress on Osteoarthritis on 1 May 2021.
Marleen de Bruijne was the Program Chair of the 24th International Conference on Medical Image Computing and Computer Assisted Intervention.
Satellite Event
Kimberlin Wijnene, Meike Vernooij and Marleen de Bruijne helped to organise the “Where is VALDO: The Vascular Lesions Detection Challenge 2021” at the International Conference on Medical Image Computing and ComputerAssisted Intervention (MICCAI).
Shuai Chen won the PVS Segmentation Task (1st place) and all tasks CSVD quantification (2nd place) of the Where is VALDO Challenge as the BigrBrain team, MICCAI 2021.

Myriam Hunink organized and moderated an In Focus Programme for the European Congress of Radiology, Online March 2021, entitled ‘’Healthcare Professionals in Focus’’ about well-being and resilience, consisting of 4 sessions and 12 workshops.
Contributions to Guidelines
Weller M, Van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, Bendszus M, Balana C, Chinot O, Dirven L, French P, Hegi ME, Jakola AS, Platten M, Roth P, Rudà R, Short S, Smits M, Taphoorn MJB, Von Deimling A, Westhphal M, Soffietti R, Reifenberger G, Wick W, for the EANO Task Force on Diffuse Gliomas. European Association of Neuro-Oncology (EANO) guideline on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol 2021;18:170-186.
Le Rhun E, Guckenberger M, Smits M, Dummer R, Bachelot T, Sahm F, Galldiks N, De Azambuja A, Berghoff AS, Metellus P, Peters S, Hong Y-K, Winkler F, Schadendrof D, Van den Bent M, Seoane J, Stahel R, Minniti G, Wesseling P, Weller M, Preusser M. EANO-ESMO clinical practice guidelines for diagnosis and follow-up of patients with brain metastasis from solid tumours. Ann Onco 2021;32:1332-1347.
Van Straten M. Co-author of Dutch guideline ‘Imaging with ionizing radiation: guidance on risks, communication, and shielding’. Federation of Medical Specialists, Version 1, December, 2021.
Edwin Oei participated in the revision of the guideline Osteoporosis and Fracture Prevention (“herziening van de richtlijn Osteoporose en fractuurpreventie”) (Federatie Medisch Specialisten/Kennisinstituut) which will be published in 2022.
Visser (guidelines): Leidraad Onverwachte Bevindingen, NVvR, 17 juni 2021.
Ivo Schoots published the landmark paper of the “PI-RADS Committee Position on MRI Without Contrast Medium in Biopsy-Naive Men With Suspected Prostate Cancer. AJR 2021;216:3-19, as the leading author.
Visser JJ, Vonken EPA, Vries de M, Kors JA; Kritieke en onverwachte bevindingen in de radiologie: herziening van de leidraad; Imago maart en Memorad najaar 2021.
Petranović Ovčariček P, Giovanella L, Carrió Gasset I, Hindié E, Huellner MW, Luster M, Piccardo A, Weber T, Talbot JN, Verburg FA. The EANM practice guidelines for parathyroid imaging. Eur J Nucl Med Mol Imaging. 2021 Aug;48(9):28012822.
Booij R. Book Chapter “CT bij kinderen” in “Computer Tomografie” (in Dutch). ISBN: 978-90-368-2650-1.
Societal Impact
Jan- Jaap Visser gave an interview to BNR Beter regarding algorithms for lipotumours.
Dianne van Dam-Nolen was a panel member during a webinar with the Dutch Care Authority regarding Outcome-oriented care.
Esther Warnert contributed to the GliMR information video for patients and caregivers on data privacy in sharing medical data.

Karin van Garderen contributed to a promotional video regarding PhD for research software engineers.

Marion Smits was interviewed for an episode of "Het Hart van Rotterdam" regarding AI for brain tumour diagnosis.
Marion Smits attended a patient meeting of the brain tumour section of patient organisation ‘Hersenletsel’ (‘Brain injury’) on MRI of brain tumours: challenges and opportunities.
On The Lancet Healthy Longevity podcast: "In Conversation With ..... Jendé Zijlmans and Annemarie Luik": Dr Jendé Zijlmans and Dr Annemarie Luik discuss the interaction of cognitive reserve and brain reserve with frailty and the association with mortality risk. Based on fidings from an observational cohort study by authors: JL Zijlmans, S Lamballais, L Lahousse, MW Vernooij, KM Ikram, MA Ikram, AI Luik.

Julie Nonnekens was interviewed by the AD newspaper about her research on radioactive tracers for cancer treatment.

Medscape Medical News published an article in September; "Aspirin and Heparin Increase Bleeding Risk During EVT", following research conducted by Wouter van der Steen, Aad van der Lugt, Diederik Dippel and Bob Roozenbeek


Health-RI, a national initiative towards a data infrastructure for re-use of health data for research and innovation received a grant of 69 MEuro of the innovation fund. Wiro Niessen is board member of Health-RI and was co-PI of the grant proposal.
Extensive External Fundings
During 2021, the Department of Radiology & Nuclear Medicine received a number of external grants (please refer to the ‘Grants’ chapter for more information).
European Research Council Research and Innovation Action
KWF – Dutch Cancer Society
Clinical Trial Support
Daniel den Hoed Fonds Research & Innovation Action
GE Healthcare –Pain project Clinical Trial Support
Rankings
According to the Center for Science and Technology Studies (CWTS, Leiden/NL; period analyzed = 2012-2019), Erasmus MC Radiology & Nuclear Medicine has maintained a high citation record. The analysis reflects the broad spectrum of topics in which we do research and shows that we have again increased our publication volume and international impact. agency
Projects that received the most substantial funding are listed in the chart below.
Deciphering the radiobiology of targeted radionuclide therapy: from subcellular to intra-tumoural analyses
Salvage therapy with 225Ac-DOTATATE for patients with metastatic neuroendocrine tumors
Intra-arterial [225Ac]Ac-PSMA for progressive/ recurrent malignant glioma
Pinpointing the source of chronic pain and therapy response with whole-body 18F FDGPET/MRI
Julie Nonnekens€1.500.000
Tessa Brabander€542.000
Sophie Veldhuijzen van Zanten €478.000
Edwin Oei €347.000


CWTS Citation Analysis 2012-2020 The Center for Science and Technology Studies (CWTS, Leiden/NL) analyzed the citation behavior of scientific articles from the Departments of Radiology and of Nuclear Medicine published between 2012 and 2019. Citations were tracked throughout 2021. The analysis is performed in overlapping years to average out annual fluctuations. Furthermore, self-citations (author in common between citing and cited article), editorials, and abstracts are excluded from the analysis. More information at www.cwts.nl.
New facilities
Photon-counting CT
In April of 2021 the department of Radiology & Nuclear Medicine of Erasmus MC was the first hospital in The Netherlands, and second in the world, with the installation of the first clinical photon-counting CT (PCCT) scanner designed by Siemens Healthineers. The installation was part of a so-called ‘customer user test’ to provide Siemens with information on the stability, reliability, image quality and user experience on the system.
PCCT provides highly detailed, accurate and reproducible imaging of several diseases, as the detector counts the number of incoming photons and measures the photon’s energy, allowing to obtain and reflect spectral information. The technique further allows for imaging with superior spatial resolution, enabling to accurately distinguish structures down to 0.2 mm in size.
Therefore, PCCT enables the extraction of quantitative imaging biomarkers from biological tissues, e.g. cortical and trabecular bone structure. As the system is provided with dual-source technology, the increased spatial resolution and ability to obtain spectral information can be combined with very high temporal resolution. This allows to image fast moving objects like the heart or in non-cooperative (pediatric) patients. With this project Erasmus MC gained valuable experience of the PCCT technology in order to investigate new possibilities of imaging disease and how this may impact and improve patient care.

Icono
In 2018, when we moved to our new building, Siemens installed a Artis Q Biplane angiography system in the intervention complex in our department. It is used for neuro interventions and other interventions.
In 2020 Siemens offered to replace the Artis Q Biplane with their new biplane angiography system, the Artis Icono. The Icono is a very good solution for a wide range of interventional therapies. It has a completely new imaging chain, called OPTIQ. For our interventional radiologists the Icono offers better image quality and articact reduction, particularly for stroke treatment.
Although the Artis Q system was relatively new, we decided to replace it. The Icono was installed in the last two months of 2021. The installation was challenging, because some of the system’s cables run beneath our floor. This means they run above the ceiling of three ICU-boxes. In this period the ICU had a lot of COVID-patients, so timing was critical. Thanks to the help of our ICU colleagues, we managed to finish the installation on time. The Artis Q biplane was moved to the Sophia Children’s hospital, where it replaced the Pediatric Cardiology’s (very) old system.

CONVERGENCE
Erasmus MC, TU Delft, and EUR aim to become a global leader in Health & Technology Convergence
Novel scientific knowledge and the accelerating possibilities of technology hold great promises to address grand societal challenges. This is especially needed in the health domain.
Together, TU Delft, Erasmus MC and Erasmus University Rotterdam have what it takes to become a global leader in Health & Technology Convergence, shaping the future of health and healthcare in a transformative way. At the three institutions, over 300 principal investigators already cover the required range of disciplines. Moreover, Rotterdam and Delft offer an excellent ecosystem for health-tech research, innovation and economic activity. By truly converging our complementary expertise, we will be able to create a vibrant hub of over 30,000 researchers, students, clinicians and entrepreneurs, working together to improve health and societal participation for all.
The convergence themes aim to respond to urgent challenges, like staffing and healthcare expenses, and where many healthcare need currently remain unmet, the flagships aim to transform our healthcare system to become more proactive, personalized, precise, participatory and labor friendly. At the same time, the convergence approach of integrating knowledge, expertise and methodologies from different domains will foster groundbreaking new scientific discoveries, pushing the state of the art.
The convergence initiatives should cover research and innovation, education, talent development and shared facilities including state-of-the-art facilities in a real-world clinical context to stimulate interaction cutting-edge health-tech innovation. To maximize economic and societal impact, convergence initiatives will be designed for valorization by for example including the right people, involving stakeholders, designing business cases and roadmaps, and defining implementation vehicles.
Department of Radiology & Nuclear Medicine and the Convergence
As a large department with a lot of expertise and knowledge, we work with many technological devices, smart software programs and large image datasets. Image acquisition and interpretation requires in depth knowledge on medical physics. Image analysis requires engineering and programming skills. Today artificial intelligence (AI) could contribute tremendously to image acquisition, analysis and interpretation. An imaging department could play an important exemplary role in the healthtech convergence.

At the start in 2019, the Convergence initiative was built on the basis of four large research flagship programs. The Flagship programs defined scientific ambitions, together with a five-year research strategy in which a range of research questions are addressed. The envisaged results of the program should have the potential for a significant contribution to healthcare or scientific impact which can have long-term societal impact. The four defined flagships were:
1. Syn – Cells for health(care) – Nanobiology and molecular medicine: Utilize fundamental knowledge of molecular/cell biology to develop novel diagnostic and therapeutic approaches based on the engineering principles of cells, tissues and organs.
2. My Digital Twin – Health and data science: To collect data and health care data throughout life, and utilizing advanced disease risk models powered by artificial intelligence to promote individual health, reproduction, early disease detection, and more personalized treatment.

3. Deep imaging – Biomedical imaging: To advance detector technology, image reconstruction, imaging tracers, and image analysis to increase the diagnostic and prognostic information that can be derived from imaging data, and to improve image guided interventions.
4. Augmenting humans – Smart instruments and interventions: To augment the clinician with artificial intelligence and smart devices to improve the quality of cure and care, and to augment the patient with technology to promote and manage their health and social participation.
To practically kick off the convergence initiative, 36 postdoc positions were created. Each flagship program defined 4 subthemes as a topic for the research performed by two postdocs. Thereby, 4 postdoc positions were created to
tackle ethical issues related to technology in healthcare.
The department of Radiology & Nuclear Medicine participates in six of the subthemes.
Flagship: Augmenting humans - Smart instruments and interventions
Combining the Smart Knife with Augmented Reality
• Objective 1: to develop and assess a navigation-less AR approach for liver surgery
• Objective 2: to develop an electrosurgical knife providing real-time tumor margin assessment Postdoc
Pierre Ambrosini
Pouva Jelvehgaran
PI’s Erasmus MC
Tessa van Ginhoven
Cees Verhoef
Theo van Walsum
Precision diagnostics of cartilage load in Knee Osteo Arthritis
PI’s TU Delft
Ricardo Guerra Marroquim
Jenny Dankelman
Benno Hendriks
• Objective 1: to build and technically validate a laboratory employing biplanar fluoroscopy in a gait lab
• Objective 2: Clinical validation in a series of patients with KOA
Postdoc
Mariska Wesseling
Erin Macri
PI’s Erasmus MC
Sita Bierma-Zeinstra
Edwin Oei
Jos Runhaar
PI’s TU Delft
Jaap Harlaar
Amir Zadpoor
Ajay Seth
Optically guided endovascular thrombectomy in patients with large-vessel ischemic stroke
• Objective 1: Development of NIR-DCS for functional monitoring of brain in patients
• Objective 2: Relate CT/OCT image properties to mechanical properties of trombus, to define retriever properties
Postdoc
Rachel Cahalane
Esteban Venialgo
Quantitative Susceptibility MRI
PI’s Erasmus MC
Aad van der Lugt
Frank Gijsen
PI’s TU Delft
Nandini Bhattacharya
Aimée Sakes
Flagship: Deep imaging – Biomedical imaging
• Objective 1: obtaining vasculature signatures from susceptibility differences with novel, robust and accelerated MRI
Postdoc
Samy Abo Seada
PI’s Erasmus MC
Marion Smits
Marleen de Bruijne
Juan Hernandez-Tamames
Alexander Hirsch
Arnaud Vincent
Michelle Michels
Deep Imaging-Genetics for Osteoarthritis
PI’s TU Delft
Frans Vos
Sebastian Weingärtner
• Objective 1: to improve early diagnosis of OA and prediction of OA progression by combining imaging and genetics data using deep learning
• Objective 2: to enhance the understanding of underlying disease processes, using deep causal modelling of imaging and genetics data
Postdoc
Stephan Bongers
Jukka Hirvasniemi
PI’s Erasmus MC
Stefan Klein
Gennady Roshchupkin
Wiro Niessen
Edwin Oei
Joyce van Meurs
Jos Runhaar
Dieuwke Schiphof
Broad Spectrum, High Precision Theranostics Cancer Therapy
PI’s TU Delft
Marco Loog
Jesse Krijthe
Frans Vos
Marcel Reinders
Sita Bierma-Zeinstra
Samantha Copeland
• Objective 1: to develop FAP tracers labeled with (i) theranostic radionuclides for image guided precision destruction of cancer cells (ii) fluorescent dyes for accurate tumor resection
Postdoc
Mark Hoorens
Arif Muhammad
PI’s Erasmus MC
Yann Seimbille
Marion de Jong
PI’s TU Delft
Marlies Goorden
Freek Beekman
A second initiative to boost the convergence was announce mid 2021 with the Open Mind calls. These Open Mind calls invited young researchers to come up with novel, innovative ideas on research, education, facilities, (data) infra-
structure or fast track innovation. Regarding these Open Mind Call projects, the department of Radiology & Nuclear Medicine is involved in the four following projects:
Open Mind Call projects
O2-Sense, converging on wearable oxygen monitoring for brain tumor patients
The delivery of oxygen to brain tissue is of utmost importance for healthy brain functioning. Impaired oxygen delivery to brain tissue causes hypoxia, which has severe consequences. In brain tumors hypoxia promotes aggressive tumor growth and increases resistance to therapy. To accurately measure cerebral oxygenation across the tumor, currently either PET or MRI needs to be used. These technologies take a ‘snapshot’ measurement of oxygenation status for which patients need to travel to a hospital for long scans in bulky equipment, operated by professional staff after which image analysis is done by medical specialists. Additionally, in follow-up patients undergo this process repeatedly for accurate monitoring, giving a lot of stress and burden to the patient.
PI Researchers Erasmus MCResearchers TU DelftResearchers EUR
Esther WarnertSamy Abo Seada
Alina Rwei
Sebastian Weingartner
Scanning Confocal Nuclear Microscope for improved Radiopharmaceutical Imaging
Marleen de Mul
Radioactively labeled drugs (radiopharmaceuticals) can be used to diagnose and treat various diseases such as cancer. In order to visualize, understand and optimize how these radiopharmaceuticals target (cancer) cells, high resolution nuclear scanning is essential. In this project, we will experimentally realize world’s first Scanning Confocal Nuclear Microscope (SCNM) and perform validation tests in mice. The SCNM that we will build, will be mounted onto a nuclear scanner (VECTor) previously developed at TU Delft and available at Erasmus MC, and will allow high resolution 3D imaging of radiopharmaceuticals. Conventional nuclear imaging reaches a resolution of 120µm (in the VECTor system), while our simulations indicate that the newly designed SCNM has 3 to 4 times better resolution. This highly increased image resolution of the SCNM will allow a more detailed analysis of the localization of the radiopharmaceuticals, enabling better analysis of (novel) diagnostic and therapeutic agents. After this project is finished, the SCNM has the potential to be commercialized by our partner MILabs (spin-off from TU Delft), so users from all over the world could benefit from this improved imaging technology.
PI Researchers Erasmus MCResearchers TU Delft
Julie Nonnekens
Advancing cancer treatment with CERN technology
Marlies Goorden
Freek Beekman
Alpha radionuclide therapy is very promising treatment which can considerably prolong life expectancy of terminal patients and even sometimes leading to complete cure. However, this therapy is still facing many challenges, such as loss of the alpha radionuclides (upon decay) and subsequent damage to healthy tissue. In order to improve this therapy, the distribution of the alpha radionuclides and the biological damage they can induce should be studied. In this proposal, the TU Delft and Erasmus MC join forces by developing a novel detection technique to assess this distribution and link it to biological experiments, helping to better design this therapy for the benefit of the patient.
PI Researchers Erasmus MCResearchers TU Delft
Sofia Koustoulidou Yann Seimbille
Maryana Handula
Julie Nonnekens
Neurodegeneration beyond DTI
Antonia Denkova
Jeroen Plomp
Brain white matter is composed of many different tissue types. Current brain MR imaging techniques for studying microstructure mostly focus on a single technique: diffusion-weighted MRI (DWI). In this project we devise a new biomarker for macromolecule tissue volume (MTV) using Proton Density-weighted imaging and AI techniques. MTV is recently shown to be an effective measure of myelin content that can provide additional insight into brain microstructure. However, measurement of MTV currently relies on quantitative MRI (qMRI) and a large dataset of combined qMRI and DWI does not currently exist. Therefore, by combining knowledge and data from TU Delft and Erasmus MC, we propose to devise a new “bridging” biomarker that is both indicative of MTV and derivable from existing data to investigate the true added value of MTV for studying neurodegeneration. This novel MTV biomarker could open up new studies into the brain microstructure and thus enrich the derivable information from running studies. It can possibly lead to a valid biomarker for diagnosis, prognosis, and following of therapy of neurodegenerative disease.
PI Researchers Erasmus MCResearchers TU Delft
Bo Li
Esther Bron
Wiro Niessen
Meike Vernooij
Dirk Poot
Frans Vos
Martijn Nagtegaal
Recently, some new development in the portfolio of the Convergence have been implemented. Now the organizational structure consists of three layers: strategic, coordination, and execution. Under these layers, societal themes and programs are established:
Societal themes
Resilient Delta
Tackling today’s global societal challenges requires resilience, especially in the delta regions, which are home to more than two-thirds of the world’s largest cities and are at risk from rising sea levels owing to their geographical location. Within Resilient Delta we work in an interdisciplinary way in the academic field, collaborating with societal partners to design resilience solutions in the real-world dynamics of our living lab, the Rotterdam delta.
Health & Technology
TU Delft, Erasmus University Rotterdam and Erasmus MC are joining forces and integrating knowledge, expertise and methodology. Through convergence, we will form novel frameworks that foster scientific discovery and technological innovation in the field of health and healthcare.
AI, Data & Digitalisation
The digital transformation is irrevocable, moving at lightning speed and has significantly transformed over the past decade. Artificial Intelligence (AI) plays a leading role in digitization. The application possibilities of AI are endless. The socio-economic impact is expected to be huge and relate to many of the challenges we face. Within the theme AI, Data & Digitization, we look together how we can contribute 'with' and 'in' AI to these challenges in society and healthcare, and how scientifically a lasting leading role can be assumed in this area worldwide. If we do this together and from different disciplines, we can take big steps. That is why universities and university medical centers in South Holland are joining forces in the knowledge cluster for 'AI, Data & Digitization'.
Programs
Pandemic & Disaster Preparedness Center
Together, we will reduce risks and build resilience through effective disaster prevention, preparedness, and recovery management.
Healthy Start
We believe every child and young adult should have the opportunity to reach their full developmental potential. Within Healthy Start we explore the early-life origins of disparities in health and wellbeing from a transdisciplinary perspective. In this way we can identify early-life opportunities and co-create innovative preventive strategies with our partners, leading to better health, wellbeing and participation for future generations.
The convergence cooperation is foreseen to grow to an annual budget of about 63 million euro. Regarding a campus and shared facilities, Convergence proposals are written to map important and promising collaborations enabling the Convergence to target major breakthroughs in health and science. End 2021 a new call has been announced to form flagships in which groups from Erasmus MC, Technical University Delft and Erasmus University collaborate. After review flagships will be selected that will receive substantial funding for 5 years.
Flagship proposals
Flagship proposals Pis Collaborators
Deep medical imaging of Structure, Physiology and Function
THERANOSTICS
I-GUIDE: Image guided minimally invasive interventions
Radiation Therapy Centre 2030: personalized Self-Steering Radiotherapy
Smart OR 2030
Healthy joints
Marion Smits
Martin Verweij (TUD)
Rik Wehrens (EUR)
Yann Seimbille
Antonia Denkova (TUD)
Lucas Goossens (EUR)
Aad van der Lugt
Ken Redekop (EUR)
Jenny Dankelman (TUD)
Remi Nout
Hedwig Blommestein (EUR)
Dennis Schaart (TUD)
Eppo Wolvius
John van den Dobbelsteen (TUD)
Welmoed van Deen (EUR)
Sita Bierma-Zeinstra
Jaap Harlaar (TUD)
Inge Merkelbach (EUR)
Dirk Poot, Juan Hernandez Tamames, Esther Warnert, Marleen de Bruijne, Esther Bron, Alexander Hirsch, Edwin Oei, Wiro Niessen
Laura Mezzanotte, Simone Dalm, Julie Nonnekens, Mark Konijnenberg, Frederik Verburg
Theo van Walsum, Adriaan Moelker
Aad van der Lugt, Marcel van Straten, Marleen de Bruijne, Martijn Starmans
Stefan Klein, Wiro Niessen, Gennady Roshchupkin, Theo van Walsum
Edwin Oei, Stefan Klein, Jukka Hirvasniemi, Rianne van der Heijden
Under review
Under review
Under review
Under review
Under review
Under review
Education
Erasmus MC, the Delft University of Technology (TUD) and the LUMC offer unique interdisciplinary joint-degree programs such as Clinical Technology (BSc) and Technical Medicine (MSc). The programs are multidisciplinary and link science and technology with clinical practice and its professional medical procedures. It combines a thorough understanding of the functioning of the human body and the influence of disease processes with an
Education typeCourse
equally comprehensive understanding of medical technology and professional medical procedures. Nanobiology (Bsc and MSc) is a joint degree between EMC and TUD. In this program, methods and theories from physics, and molecular biology are integrated, using tools from math and computer modelling to further understanding of the molecular basis of life.
ECTSCoordinator Additional lecturers
MSc Technical Medicine Coordinator Erasmus MC Jifke Veenland
Track coordinator Imaging & Interventions Jifke Veenland
Advanced Image Acquisition5 Wiro NiessenMarcel van Straten
Molecular Imaging 5 Laura GravenMark Konijnenberg, Yann Seimbille, Anita Harteveld
Advanced Image Processing5 Jifke VeenlandMartijn Starmans, Karin van Garderen, Jose Castillo Tovar
Python
2.5 Theo van WalsumHakim Achterberg, Marcel Koek
Machine Learning 2.5 Jifke VeenlandMartijn Starmans, Karin van Garderen, Hakim Achterberg
Supervision honours student
Supervision internships
Supervision graduation projects
Marcel Segbers, Jifke Veenland
Edwin Oei, Jifke Veenland, David Hanff, Adriaan Moelker, Frederik Verburg, Matthijs van der Sluijs, Gennady Roshchupkin, Dirk Poot, Juan Hernandez, Marcel Segbers, Theo van Walsum, Anita Harteveld, Stefan Klein, Ruisheng Su, Luisa Sanchez Brea, Danilo Andrade de Jesus
Marion Smits, Esther Bron, Esther Warnert, Jifke Veenland, Edwin Oei, Theo van Walsum, Ruisheng Su, Mohamed Benmahdjoub, Matthijs van der Sluijs
BSc Clinical Technology Imaging 6.5 Jifke VeenlandMarcel van Straten
Minor Medicine for TUD students
Image Processing 3 Jifke VeenlandMartijn Starmans
1.5 Marcel van StratenJifke Veenland
BSc Nanobiology Lab Course 1 and 2 Laura Mezzanotte
RESEARCH FOCUS AREAS
The department of Radiology & Nuclear Medicine is committed to perform high-quality and high-impact research in all areas of the biomedical imaging discipline from technology development and fundamental discoveries, to translational, clinical, and population levels. The individual research lines (30) are organized within four main research focus areas. A research line is defined as a distinct research topic within a main focus area with its own strategic plan, coordinated by a Principal Investigator (PIb) in a tenured position at the level of

assistant professor or above, substantial external funding, and a group of at least two PhD students. Research content and strategy are discussed in the Research Committee formed by all PIs of the department (meeting once every two months). Daily business is the responsibility of the Research Managing Board consisting of representatives (coordinators) of the four focus areas, the head of the department, and the head of research & education. Focus area coordinators are responsible for communication and coordination within the focus area.
Focus area 1: BIOMEDICAL IMAGE ACQUISITION & ANALYSIS
Advances in medical imaging have drastically increased the ability to (non-invasively) study both anatomy and function. In addition, imaging data are increasingly complemented by other types of data, including -omics, lifestyle and environmental data. With these advances, the sheer size, complexity, and heterogeneity of biomedical (imaging) data have increased enormously, and the challenges to optimally use this information for biomedical research and clinical practice have grown accordingly. At the same time, methods for the automated analysis of these data have also increased tremendously. This especially applies to the analysis of biomedical (imaging) data with artificial intelligence techniques, which will have an enormous
impact on disease prevention, cure and care. This research group is at the forefront of these developments. Its focus is to develop advanced image processing and machine learning techniques to optimize both the acquisition and analysis of biomedical imaging data with the aim to develop novel diagnostic, prognostic, therapy planning and therapy monitoring tools, and to develop techniques to support image-guided interventions and surgery. In addition, the group develops methods for the integrated analysis of imaging, -omics and clinical outcome data to improve the understanding of disease aetiology and improve risk prediction. The group is also involved in establishing the health data infrastructure to support this research. In this way, the group’s research contributes to and facilitates the implementation of ‘integrated diagnostics’ in clinical practice.

Focus area 2: MOLECULAR IMAGING & THERAPY
Research in the Molecular Imaging and Therapy focus area ranges from fundamental, to preclinical and clinical projects. The aim is to study molecular and cellular events in a non-invasive manner and to develop new (radionuclide) treatment modalities for cancer. This is accomplished by combining forces in radiopharmaceutical chemistry, genetic engineering of reporter genes and radiobiology to create new tools which are essential to understand and optimize treatment- and imaging

modalities. Follow-up preclinical research in optical and multi-modal imaging, and radionuclide therapy will pave way for translation into clinical validation and implementation of novel approaches for radionuclide imaging and therapy. In specific, the research focuses on the development of contrast agents, reporter genes, radiopharmaceuticals and multimodality agents for MRI, optical, optoacoustic and/or radionuclide imaging and therapy, as well as their functioning within the cell and/or whole organism, their preclinical validation, and their clinical translation, to ultimately improve the cure rate and quality of life of patients.
Focus area 3: CLINICAL IMAGING
The Clinical Imaging focus area investigates the clinical value of (new) imaging technologies and imaging biomarkers, following a structured order of investigations. The aim is to validate and implement new technologies in diagnostic imaging and image-guided therapies. Image acquisition of new technology is optimized in phantom studies and in both volunteer and patient studies. The robustness of imaging biomarker extraction is assessed with a focus on accuracy, repeatability and reproducibility. The diagnostic accuracy of new imaging technology is investigated by comparing this imaging data to reference standards such as histopathology, other biomarkers or other imaging modalities. The possible automation of imaging biomarker extraction is investigated in collaboration with the Biomedical Image Acquisition & Analysis research line. The researchers perform clinical studies in which imaging biomarkers are related to other -omics and assess clinical relevance by evaluating diagnostic confi-

dence regarding clinical decision-making and impact on treatment planning. They evaluate the prediction of outcome or treatment response based on imaging biomarkers for precision medicine with a focus on prediction rules, including quantitative imaging biomarkers, radiomics features and deep-learning algorithms. Accurate response assessment is of the utmost importance in the context of newly-developed treatments (endovascular treatment, cancer treatments). Multi-centre (clinical) trials are used to assess and evaluate imaging biomarkers of disease activity and response to treatment. These include conventional and advanced physiological imaging markers such as perfusion and diffusion weighted imaging techniques. The topics currently of primary interest are neuroimaging (vascular disease, tumours, neurodegeneration), cardiac imaging (coronary artery disease, endocarditis, cardiomyopathies), musculoskeletal imaging (sports injuries, osteoarthritis), abdominal imaging (liver tumours, prostate cancer), paediatric lung imaging and advanced image-guided interventions.
Focus area 4: IMAGING IN HEALTH SCIENCES
The Imaging in Health Sciences focus area encompasses four population-based research lines (Population Imaging, Paediatric Population Neuroimaging, Imaging of Arteriosclerosis, and Precision Epidemiology) and one methodology-focused research line (the Assessment of Radiological Technology (ART) group). Central to this focus area is the integration of epidemiological methods and imaging techniques
across the spectrum of healthy individuals to diseased populations, spanning from fetal life to old age. The ultimate aims are: to better understand typical and atypical development (i.e., in childhood); to identify health-related factors that can improve public health and inform healthcare policy; to unravel the aetiology of illnesses; and to improve disease prediction and decision-making in clinical practice. The emphasis on epidemiological methods is reflected in the joint appointment of four PIs from this research focus area in the Department of Epidemiology.
RESEARCH STAFF
Full Professors
Marleen de Bruijne, PhD
Ricardo PJ Budde, MD, PhD
Pim J de Feyter, MD, PhD
Willem A Helbing, MD, PhD
MG Myriam Hunink, MD, PhD
Gabriel P Krestin, MD, PhD, FACR, FRCR
Clemens WGM Löwik, PhD
Aad van der Lugt, MD, PhD
Wiro J Niessen, PhD
Marion Smits, MD, PhD
Juan A Hernández Tamames, PhD
Harm AWM Tiddens, MD, PhD
Meike W Vernooij, MD, PhD
Frederik A. Verburg MD, PHD
Associate Professors
Hieab HH Adams, MSc, PhD
Esther E Bron, MSc, PhD
Daniel Bos, MD, PhD
Filippo Cademartiri, MD, PhD
Alexander Hirsch, MD, PhD
Ivo Schoots, MD, PhD
Stefan Klein, PhD
Koen Nieman, MD, PhD
Laura Mezzanotte, PhD
Edwin HG Oei, MD, PhD
Yann Seimbille, PhD
Theo van Walsum, PhD
Tonya JH White, MD, PhD
Frans Vos, PhD
Assistant Professors
Tessa Brabander, MD, PhD
Pierluigi Ciet, MD, PhD
Simone U Dalm, MSc, PhD
Gyula Kotek, MD, PhD
Adriaan Moelker, MD, PhD
Jacob J Visser, MSc, MD, PhD

Julie Nonnekens, PhD
Dirk HJ Poot, PhD
Bob Roozenbeek, MD, PhD
Marcel van Straten, PhD
Jifke F Veenland, PhD
Sophie EM Veldhuijzen van Zanten, MD, PhD
Henri A Vrooman, PhD
Esther AH Warnert, PhD
Post-Docs & Junior Researchers
Samy Abo Seada, PhD
Hakim C Achterberg, MSc, PhD
Pierre Ambrosini, PhD
Danielle ME van Assema, MD, PhD
RH (Erik) de Blois, PhD
Daan Caudri, MD, PhD
Roy S Dwarkasing, MD, PhD
Tavia Evans, MSc, PhD
Rianne van der Heijden, MD, PhD
Jukka Hirvasniemi, PhD
Mark Hoorens, MSc, PhD
Danilo Andrade de Jesus, PhD
Hoel Kervadec, PhD
Mark W Konijnenberg, PhD
Sofia Koustoulidou, MSc, PhD
Jeremy Labrecque, MSc, PhD
Bo Li, MSc, PhD
Hanyue Ma, MSc, PhD
Julia Neitzel, PhD
Inge-Marie Obdeijn, MD, PhD
Kranthi Panth, PhD
María Rodriguez-Ayllon, PhD
Gennady Roshchupkin, MSc, PhD
Luisa Sánchez Brea, PhD
Rebecca ME Steketee, MSc, PhD
Maarten GJ Thomeer, MD, PhD
Erik Vegt, MD, PhD
Natalia Vilor-Tejedor, PhD
Ivo Wagensveld, MD, PhD
Frank J Wolters, MD, PhD
PhD Students
Abdullah Thabit, MSc
Adriaan Coenen, MD
Ahmad Alafandi, MD
Aikaterini Tziotziou, MSc
Alexandra Cristóbal Huerta, MSc
Ali R Wahadat, MD
Angelina Pieters, MD
Anouk C de Jong, MD
Antonio Garcia-Uceda Juarez, MSc
Arno van Hilten, MSc
Bas A de Vries, MSc, PhD 21’
Bernadette BLJ Elders, MD
Chaoping Zhang, MSc
Chintan Chawda
Crispijn van den Brand, MD
Daniël F Osses, MSc, MD, PhD 21’
Danny Feijtel, MSc
Desirée K de Vreede, MSc, MD
Dorottya Papp, MSc
Douwe J Spaanderman, MsC
Dianne van Dam-Nolen, MSc, MD
Duygu Harmankaya, MD
Dylan Chapeau, MSc
Eline AM Ruigrok, MSc
Eline J Vinke, MSc
Eline Hooijman, MSc
Eline Krijkamp, MSc
Emanoel R Sabidussi, MSc
Érika Murce Silva, MSc
Fatemehsadat Arzanforoosh, MSc
Fatih Incekara, MD, PhD 21’
Fay Nous, MD
Federico Mollica, MD
Fjorda Koromani, MSc, MD
Frank-Jan H Drost, MSc, MD
Gerda Bortsova, MSc
Giulia Colzani, BSc
Giorgia Zambito, MSc, PhD 21’
Guilia Tamborino, MSc
Ilanah Pruis, MSc
Ilva Klomp, MSc
Isabelle van der Velpen, MD
Jan A van der Voet, MSc, MD
Janine van der Toorn, MSc
Jason Beaufrez, MSc
Jendé Zijlmans, MD, MSc
Jennifer Meerburg, MD, PhD 21’
Jiahang Su, MSc
Jie Deng, MD
Joost Verschueren, MD
Jose M Castillo Tovar
Joyce van Arendonk, MSc
Karin van Garderen, MSc
Kemal Sumser, MSc
Kim van Wijnen, MSc
Krishnapriya Venugopal, MSc
Kristine Dilba, MD
Laura Núñez González, MSc
Laurens Topff, MD
Lennard Wolff, MD
Lisa Caulley, MPH, Md
Lorain Geenen, MSc
Luke G Terlouw, MD
Marc CM Stroet, MSc
Marguerite Faure, MD
Marijn Mostert, MSc
Marleen M van den Heuvel, MD
Marjolein Dremmen, MD
Marjolein Verhoeven, MSc
Mathijs Rosbergen, MSc
Martijn Starmans, MSc
Matthijs P van der Sluijs, MD
Maryana Handula, MSc
Mohamed Benmahdjoub, MSc
Nadinda van der Ende, MD
Natasa Gaspar, MSc, PhD 21’
Neslisah Seyrek, MD
Nienke D Sijtsema, MSc
Nikki van der Velde, MD
Nikki Boodt, MSc, MD
Noémie Minczeles, MD
Noor Samuels, MD
Núria Jansen, MSc
Pinar Yilmaz, MD
Priciana Paraiso, PharMD
Qianting Lv, MD
Riwaj Byanju, MSc
Rob A van de Graaf, MD, PhD 21’
Roisin MC Morrow, MSc
Ronald Booij, MSc, PhD 21’
Ruisheng Su, MSc
Sander Lamballais, MSc
Sanne den Hartog, MD

Sebastian van der Voort, MSc, PhD 21’
Simran P Sharma, MD
Shuai Chen, MSc
Sophie Derks, MD, MSc
Stefan J Roobol, MSc, PhD 21’
Stijntje Dijk, MSc, MD
Sui Wai Ling, MD
Stephan J Breda, MD
Subhradeep Kayal, MSc
Sven PR Luijten, MD
Taihra Zadi, MSc
Theresa V Feddersen, MSc
Thom Reuvers, MSc
Thomas Phil, BSc
Tiny Cox, BSc
Tijmen A van Zadelhoff, MD
Tong Wu, MD
Tyrillshall Damiana, MSc
Vikram Venkatraghavan, MSc, PhD ‘21
Wiebe G Knol, MD
Wietske Bastiaansen, MSc
Wouter Teunissen, MSc
Wouter van der Steen, MD
Wytse van den Bosch, MD
Yifan Wang, MD
YuanYuan Sun, MSc
Yulun Wu, MSc
Yuxin Chen, MD
Zarha Sedghi Gamechi – PhD 21’
Visiting Scientists
A. Lavrova – ESR Bracco Fellow, St. Petersburg, Russia
Alan Chan – Visiting Senior Scientist
Aleksei Tiulpin – University of Oulu/FI
Annemieke van Beek, MSc (personnel van Adams)
Brian Berghout – PhD student Epidemiology
C. Tseng – PhD student TU Delft
Cevdet Acarsoy – PhD student Epidemiology
D. van Dorth – PhD student LUMC
Enzo Kerkhof – TU Delft, Leiden University
Fenna ten Haaf – EUR MSc thesis student
Ivan Dudurych – University Hospital, Groningen
Jesus Melgarejo – PhD student KU Leuven
Jet Peek – TU Delft, Leiden University
Joost Wooning – TU Delft
M. Rosbergen – MSc student TU Delft
Mathias Polfliet, MSc – Free University Brussels/BE
Mika W Vogel, PhD – ASL Scientist & Team Leader
ASL Scientists Europe, GE Healthcare
Mikolaj Pawlak, MD, PhD – University of Poznan/PL
Myrthe van den Berg – TU Eindhoven
Rita Marques – University of Coimbra
Samantha de Graaf – TU Delft
Silas Orting, MSc – University of Copenhagen/DK
Tessa Kos – TU Delft, Leiden University
Tim van den Beukel – PhD student UMC Utrecht
Vania Silva – University of Coimbra
Vincent Hellebrekers – TU Delft
Vincent van Ginneken – Visiting Senior Scientist
Yannick Kaiser – PhD student Amsterdam – UMC
Z.S. Erdal – Ankara, Turkey

Unit Research & Training
Adriaan Versteeg – IT Architect/Scientific Programmer
Andrea Gutierrez – IT Architect/Scientific Programmer
Alexander Harms – IT Architect/Scientific Programmer
Daan van der Velden – Post Processing
Dennis Kuijper – Coordinator R&I Nuclear Medicine
Ezgi Çetin – Medical Student
Hakim C Achterberg, MSc – IT Architect/Scientific Programmer
Henri Vrooman – IT Architect/Scientific Programmer
Ivan Bocharov – IT Architect/Scientific Programmer
Jan de Swart – Imaging Specialist
Jean-Baptiste J.C. Aarssen – Coordinator R&I MNAA
Joël de Groen – CT Technician
Laurens Groenendijk – Data Manager
Leontien Heiligers – Trial Office Coordinator
Lisette de Kreij-de Bruin – Research Technician
Mahlet Birhanu – IT Architect/Scientific Programmer
Marcel Koek, MSc – IT Architect/Scientific Programmer
Marcel L Dijkshoorn – Research Technologist CT
Mariëtte PC Kemner van de Corput, PhD –Head LungAnalysis
Maryana Handula – Research Technician
Michelle de Bloeme-Hus – Coordinator R&I Interventional
Milja de Bruine – Imaging Trial Office
Rachida Hadouch – Radiology Assistant MRI Ommoord
Renée AL Leenaars – Imaging Trial Office/Research Assistant
Ronald Booij – Coordinator R&I CT
Priscilla van Andel – Secretary R&T
Sylvia Bruininks – Coordinator R&I MRI
Sophie Nottle – Imaging Trial Office
Yvonne JGM Martens-Griep – Project Monitor
Additional Scientific Support Staff
Chantal van Santen – Paauw – ICT Tech
David W de Vries – Manager ICT & Engineering
Jeffrey Langerak – ICT Tech
Jeffrey Slangen – ICT Tech
Mart CM Rentmeester, PhD – ICT Tech
Natasja M Gouweleeuw – Advisor Finance
LCJ (Bert) van Heerebeek – ICT Tech
Robert Helder – Biomedical Engineer
Renald Slag – Biomedical Engineer
Yuri Versteeg – ICT Tech
Paul A Visser – Biomedical Engineer
Piotr A Wielopolski, PhD – MR Physicist
Rob Zandstra – Biomedical Engineer

RESEARCH SUPPORT
The department Radiology & Nuclear Medicine contains two large sections, Patient Care and Research & Education. Monique de Waard is director of Research & Education and is responsible for managerial, financial, and strategic issues. She provides management reports for several output overviews and plays an important role in project management. Priscilla van Andel works as her secretary and has a huge role in supporting Monique, but she also supports researchers with organizational issues. Joyce Pijnappel and Kirsten Raaijmakers both are staff advisors. Wouter Roobol, Aart Hemker, Lyda Kramp and Lydia Wielemaker and Lonneke Vos, staff from the management office of the Theme Diagnostics & Advice, support us with regard to project administration, financial administration and human resource management. The staff office together with the unit Research production provides individual researchers with top-quality support for organizational, management, legal, ethical, financial, administrative, or other research issues. This way our researchers can focus fully on their research projects.
The Research Committee forms the center of all research activities of the department and meets once every two months. Members of the committee are full professor, associate professors and assistant professors and are leading a research group as Principle Investigator. In 2021 30 research groups were organized within four main research focus areas (Figure 1).
A research group is defined as a distinct research topic within a main focus area with its own strategic plan, coordinated by a Principal Investigator in a tenured position at the level of assistant professor or above, with substantial external funding and a group of at least two PhD students. The research committee discusses new research opportunities and strategies, and monitors the quality of research within the department. To encourage collaboration within the department, a member of the committee presents his/ her long and short-term research plans during Research Committee meetings. The committee exists of several subcommittees and working groups like: research strategy, data management, scientific integrity and communication. The committee gets advice from several working groups, who, for example, prepare policy documents, communication items or analyze output factors.

Figure 1: The individual research lines (30) are organised within four main research focus areas.
Our PhD students have a hierarchal appointment within the section Research & Training. Their operational appointment is within the research group they work in. PhD student review meetings are organized regularly with a sub-committee of the Research Committee. The students are asked to present their research, education and thesis planning. The subcommittee advices, asks questions related to research integrity and data management, and observes whether the student complies with the departmental and institutes procedures and policies. Once a year, the Research Committee invites all PhD students for the Graduate Student Dinner. This dinner aims to bring PhD students and members of Research Committee closer together. In 2021 unfortunately, due to the Covid-19 pandemic, the dinner was cancelled for the second year in a row.
The unit Research production consists of the following groups of employees with a role in research support:
Imaging Trial Office (ITO)
The ITO is part of the unit Research production. The office provides high quality scientific research support to all researchers from the department as well as researchers from other departments. The ITO prepares Institutional Review Board (IRB) protocols and function as the primary contact point for the IRB. They provide study volunteers, take oral questionnaires, liaise with the clinic to arrange logistics, and assemble, enter, and track data, and anonymize images and perform image analysis. They also advice on laws and regulations and perform quality controls to assure performance levels, monitor projects and they manage all aspects of service projects freeing our researchers and radiologists of this burden.
The data manager is specialized in data safety and privacy, and development of (clinical trial) databases, which extends the level and range of support offered. The clinical trial monitor oversees the conduct of clinical trials and ensures that these trials are conducted according to protocol, GCP, SOPs and regulatory requirements.
Research technicians
Research technicians at our department work within the pre-clinical research groups. They support and execute fundamental research and animal experiments and carry out histological, radiochemical, molecular and imaging techniques.
Research MBB
Research MMB are (specialized) radiographers and medical nuclear technicians executing data collecting at the different modalities. They guide the introduction of new technologies. They scan study participants for diagnostic- or therapeutic research projects, collect data for scientific projects and provide post processing of radiologic images. This involves, for example, volumetry of liver and lung measurements on CT images and a variety of other services for patient care as well as research projects.
Coordinators Research & Innovation
Each clinical unit has its own Coordinator Research & Innovation who is responsible for the organization of research support within their own units as well as the translation of research results into clinical practice. Together with colleagues like researchers, PhD students, ITO, but also research MBB, radiologists and clinical physicists they take care of development and optimization of research protocols and give advice on the use of the protocols. In 2021 the group of four coordinators in the units MRI, CT, Nuclear Medicine and Intervention has been expanded with a Coordinator Research & Innovation for the MNAA unit
ICT administrators
ICT support staff, part of the Unit Technical Support, maintains our Picture Archiving and communication System (PACS) 24/7. They are also responsible for other software, varying from general office programs to medical software to specific research applications, and maintain and troubleshoot the hundreds of laptops, desktops, workstations, servers, and other computer equipment used in our department. Large scale medical studies pose technical and administrative challenges.
IT developers and research infrastructure
Large scale medical studies pose technical and administrative challenges. The BIGR Infrastructure Group design and develop an IT infrastructure to solve these challenges and make medical imaging research reproducible, more robust and more consistent. We are applying our infrastructure and knowledge in local Erasmus MC projects (e.g. RSS, GenR, Research Suite), national projects (e.g. CVON, BBMRI, CONTRAST, Health-RI) and international projects (Euro-BioImaging, EuCanImage, EuCanShare).
We are also responsible for hosting the medical imaging archive XNAT in Erasmus MC and Health-RI. We deliver software and infrastructure that support researchers, as such we work together with a long list of researchers in and out of our department to create the best possible solutions.
We have created a reference IT infrastructure using a modular approach, so we can suit all projects and studies that need to deal with medical imaging data and data analysis. The modules can be rearranged and configured to fit the specific needs. In Figure 2 a schematic overview of the infrastructure is given.
Biomedical engineers
Our biomedical engineers, part of the Unit Technical Support, play an important role in the acquisition and installation of imaging equipment, both for clinical work and research. The technical support team tests and validates new equipment before it is used for patient care or research, assuring image quality and patient safety. Their work allows researchers to acquire validated and reliable data for their research projects.
ERGO and Generation R Study
At the ERGO center in Ommoord are MRI scans for the Rotterdam study performed. The medical student team of Generation R and ERGO support our research organization. For the Generation R study they make MRI scans of children and their parents. For the ERGO study they assist with the acquisition of MRI scans. After the MRI they are responsible for taking movement tests to screen for Parkinson, a walking test and a polyneuropathy screening including an EMG and a questionnaire.

Figure 2: Reference infrastructure for handling data and analysis in projects and studies involving medical imaging data.
Ranking
The final report of the 2013-2018 research evaluation, conducted by an external independent committee using the Standard Evaluation Protocol ( SEP ), was completed in 2021. The assessment committee reached an opinion about the research based on a self-evaluation of the research unit, additional documents and interviews that took place during the virtual site visit. The committee gave our department an excellent score in terms of research quality, social relevance and viability, the three main issues on which all departments of Erasmus MC were evaluated. The department received 5 recommendations for improvement for which follow-up actions were formulated. For each theme within Erasmus MC, the follow-up actions of all departments were published together with the final SEP report.

Notable achievements/efforts/milestones for 2021
• Involvement with the Research Suite has intensified and we are jointly developing infrastructure for automating the availability of de-identified and consent checked (linked) clinical imaging data in the Health Data Platform based on research questions. [HDP, Research Suite]
• Developing metadata models (e.g. DICOM-MIABIS) for DICOM data in catalogs together with EIBIR, this helps data become findable. [EuCanImage, euCanSHare, (local) Health-RI]
• Build the infrastructure for translating the LowGrade Glioma analysis pipeline of Karin van Garderen and Marion Smits to the clinic for research purposes [Ease]
• We were involved in setting up the Erasmus Imaging Office, an initiative from our department to handle imaging-related requests from internal and external partners.
• Pushing innovations and contributed to a national trust framework in Federated and Distributed Learning [NCDC, Health-RI: Personal Health Train]
• Build DICOM Data ingestion systems from different data sources e.g. CMRad, PACS, VNA and various other DICOM based archives [EuCanImage]
• Development of data models that allow us to link imaging (XNAT) and non-imaging data (EGA-CRG) [EuCanImage]
• We helped setup and maintain the Erasmus MC GPU Cluster [Research Suite]
RADIOPHARMACY
Radiopharmaceuticals have become an integral part of modern medicine and are expected to play an increasingly important role in an ever-expanding range of diseases. In addition to the registered diagnostic and therapeutic applications of radiopharmaceuticals, there are many ideas within nuclear medicine about new applications and/or new radiopharmaceuticals/tracers.
The Radiopharmacy Unit is formed last year and creates a fruitful bridge and collaboration between the departments of Pharmacy and the Radiology & Nuclear Medicine. The team consists of a hospital pharmacist, quality advisors, pharmacy assistants, Radiopharmaceutical Laboratory Technologists (RLT) and coordinating radiation experts. The core focus of all the activities performed by the team is the sterile production of radiopharmaceuticals and the clinical implementation of innovative and new radiopharmaceuticals in compliance with Good Manufacturing Practice (GMP).

Nuclear medicine is pre-eminently a multidisciplinary field. The Radiopharmacy Unit works closely with the clinical radiochemistry team. In addition, the team collaborates with, among others, clinical physicists, physicians and nuclear medicine technologists.
As a team we would like to encourage all physicians and scientists to apply the most effective diagnostic and therapeutic radiopharmaceuticals, regardless of the complexity of required synthesis. We strive to contribute to the adequate preparation of regular and innovative radiopharmaceuticals and to provide Erasmus MC with a unique
opportunity in the field of applications of radiopharmaceuticals by deploying unique and specialist chemical analyst knowledge with a strong focus on quality and reliability.
Last year, the team has ensured that the laboratories where the pharmaceuticals are produced and prepared have obtained a manufacturer’s license. This enables Erasmus MC to be the first center in the Netherlands to prepare Actinium based radiopharmaceuticals for clinical studies.

With the license, the future is bright regarding the development of new radiopharmaceuticals for clinical applications. Besides, the unit is working on innovative ideas regarding treatment of patients and scientific research projects. The table below provides an overview of the Radiopharmaceuticals we aim to develop for clinical studies and clinical use.
Picture: extraction of Actinium by a Nuclear Analyst in the glovebox

Clinical radiochemistry
The (clinical) radiochemistry group is responsible for the implementation of i.e. new radionuclides, radiopharmaceuticals, labelings and detection techniques, automatizations, quality controls and implementation of new methodologies. Here we develop and optimize the different techniques and make them suitable for GMP production. We also facilitate required quality controls, implement new lab facilities and write together with different specialisms required documents, for example the Investigational Medicinal Product Dossier (IMPD) which is needed to start a clinical trial with a novel investigational drug.

Picture: Liz Krijnen (RLT) working with a specific administration system designed for the radioactive iodine-131-MIBG. Therapy for Neuro-Endocrine Tumors (NETs) is based on this iodine-131-MIBG.
From left to right: Joyce Pijnappel (manager radiofarmacy), Emar Thomasa (RLT + coordinating radiation expert), Figen Kahyargil (pharmacist), Pieter Meppelink (RLT), Jean Baptiste Aarssen (RLT), Simone Morelis (RLT), Heleen Voorwinden (quality advisor), Linda de Jong (RLT + coordinating radiation expert), Elly de Wit (quality advisor), Vera van den Broek (farmacy assistant), Verna de Korte (former RLT), Erik de Blois (clinical radiochemist), Eline Hooijman (PhD clinical radiochemistry), Stijn Koolen (Hospital Pharmacist). Liz Krijnen, Vaios and Bonny are missing in this picture of the team.
Clinical Radiopharmaceuticals we aim to implement in (2021-2023)
Isotope
Actinium-225
Peptide
PSMA
Dotataat
Lutetium-177PSMA (started: 22-02-2022)
NeoBOMB1
Dotataat (inta-arterial)
Gallium-68
Thorium-227
Pentixafor (CXCR4)
PSMA
NeoBOMB`1
PSMA
Fluor-18 DPA
Fluciclatide
Ammonia(13NNH3) not applicable
IMAGING FACILITIES
Magnetic Resonance Imaging
Brand Equipment
GE Healthcare
7.0T Discovery MR901 (pre-clinical) 2010AMIE Facility
3.0T Discovery MR750W 2012Sophia
3.0T Discovery MR750 2011Central Hospital
3.0T Signa Premier 2009Central Hospital
1.5T Signa Explorer 2016Sophia
1.5T Discovery MR450W 2014Central Hospital
1.5T Discovery MR450W 2011Cancer Institute
1.5T Signa Artist 2018Central Hospital
1.5T Signa Artist 2018Central Hospital
1.5T Signa Explorer 2019Population Imaging Center
X-Ray Computed Tomography
Brand Equipment
Siemens Somatom Definition DRIVE 2016Sophia
Somatom Definition Edge Twinbeam 2016Central Hospital
Somatom Force 2014Central Hospital
Somatom Definition Edge 2012Central Hospital
Somatom Definition Edge 2018Central Hospital
Somatom Definition Edge Plus 2017Central Hospital
Somatom Definition Edge Plus 2017 Central Hospital
Somatom On.Site (clinical use test) 2020 Central Hospital
Naeotom Alpha Photon Counting CT 2021 Central Hospital
Single Photon Emission Computed Tomography (SPECT)-based Imaging
Brand
Siemens Symbia T16 SPECT-CT
Symbia S SPECT
Positron-Emission Tomography (PET)-based Imaging
Biograph mCT 40 PET-CT
mCT 128 PET-CT
Angiography, Interventional Radiology, and Fluoroscopic Imaging
Mammography
Brand Equipment
Year of acquisition Location Hologic 3Dimensions 2017Central Hospital 2018Central Hospital
Affirm Prone Biopsy system 2017Central Hospital
Ultrasonic Imaging
Brand Equipment
Year of acquisition Location
Siemens ABVS 2012Central Hospital Philips Epiq 5 2014Central Hospital 2014Central Hospital iU22 2012Central Hospital 2012Central Hospital 2012Sophia 2012Sophia Epiq 7 2019Central Hospital 2019Central Hospital 2019Central Hospital 2019Central Hospital 2019Central Hospital
Esaote My Lab Twice 2015Central Hospital 2010Central Hospital
Conventional X-Ray Imaging
Brand
Equipment
Year of acquisition Location
Siemens Mobilett MiraMax 2016Central Hospital 2016Central Hospital 2016Sophia
Ysio wi-D 2012Central Hospital 2009Central Hospital 2009Central Hospital
Ysio Max 2018Central Hospital 2018Central Hospital 2018Central Hospital
Cios Alpha 2017Central Hospital 2017Central Hospital 2017Central Hospital 2017Central Hospital
Carestream DRX Revolution 2012Central Hospital 2001Central Hospital
Philips C-arm Veradius 2011Sophia
C-arm Pulsera 2009Central Hospital
C-arm Unity 1998Sophia
Digital Diagnost C90 2020Sophia 2020Sophia
Oldelft BeneluxTriathlon Trauma DR 2014Central Hospital 2009Central Hospital 2009Central Hospital
Hologic Fluroscan Insight Mini C-arm 2008Central Hospital Insight FD Flex 2021Sophia
Demedis Dental Ortophos XG3DS 2005Central Hospital Ortophos 3 DS 2003Sophia
Oldelft BeneluxPlanmeca ProMax 2D S3 2020Sophia
EOS ImagingEOS 2020Sophia
DEXA systems
Brand Equipment
GE iDEXA Dual-Energy X-ray Absorptiometry System
Information & Communication Technology
Brand Equipment
Medis Medis Suite MR
Philips IntelliSpace Portal
ScintomicsLabeling software
GE HealthcareAW Server
Siemens SyngoVia
TEMA SinergieDispensing software
Hermes Application Server
Year of acquisition Location
2014 Central Hospital
Year
2016Central HospitalMRI
2015All CT, MRI
2014Central Hospital Robotica Robot Synthesizer
2012All MRI
2012All CT
2011Central HospitalPET
2011Central Hospital Dispensing robot and Dose calibrators
2011Central HospitalSPECT Gold3 PACS
2011Central HospitalSPECT
2011Cancer InstituteSPECT
Comecer IBC Holtlab Management Software2008Central HospitalDose calibrators
Merge CADSTREAM
Hologic Softcopy Workstation
2006Cancer InstituteMRI
2016Cancer InstituteMammography
Equipment
Laboratory Facilities
Brand Equipment
Number
Scintomics Robotics Module 1 12015Radiochemistry
Robotica Robot Synthesizer 12014Central Hospital
Eckert & ZieglerRobotics Module 2 12011Radiochemistry
TEMA SinergieDispensing Robot 12011Central Hospital
Actuator Dispensing Robot with Lift 12011Central Hospital
Wallac/PerkinElmerWizard 2" 2480 Automatic Gamma Counter12015Radiochemistry 12011Central Hospital 12011AMIE Facility
Wizard 3" 1480 Automatic Gamma Counter1<2010Central Isotope Lab
Comecer Dose Calibrator
102004-2019Central Hospital 22008Central Isotope Lab 12008Radiochemistry 12010AMIE Facility
Interflow Laminar Flow Cabinet 72008-2013Central Hospital
2<2010-2011Central Isotope Lab
ISOMED 2101 1 Probe Counter Perfusion Type 723 070 12014Central Hospital
Metorx Germanium Detector + Multi-channel Analyzer
12012Radiochemistry
12011Central Hospital
BrightSpec bSCAN Thin-Layer Radio-Chromotography scanner 12015Radiochemistry
12019Central Hospital
Waters Alliance e2695 HPLC with a 2998 PDA detector + Canberra radioactivity detector 12015Radiochemistry
Acquity Arc (U)HPLC with a 2998 PDA detector + Canberra radioactivity detector 12019Radiochemistry
Acquity H-Class Ultra-Performance Liquid Chromatography (UPLC) with a 2998 PDA detector + Bpad radioactivity detector
Alliance e2695 HPLC with a 2998 PDA detector + Canberra radioactivity detector
Thermo Fisher Scientific
12011Radiochemistry
12019Radiochemistry
Alliance e2695 HPLC with a 2998 PDA detector + Bram and Flow radioactivity detector 12019Central Hospital
Liquid Chromatograph/Mass Spectrometer (LC/MS) Quantum Ultra
12017Radiochemistry
Biotage Microwave Biotage Initiator 12019Central Hospital
Leica SP8 confocal microscope 12020Central Isotope Laboratory – faculty building


BIOMEDICAL IMAGE ACQUISITION & ANALYSIS
Prof. Juan Hernandez-Tamames received his MSc degree in Physics from Complutense University in Madrid (Spain) in 1992. He received his PhD degree (cum laude) in Biomedical Engineering from Polytechnic University also in Madrid in 1999 with a dissertation about Wavelet Transforms in fMRI. Between 1999 and 2002 he obtained several academic positions as Assistant Professor at Complutense University and at Rey Juan Carlos University in Madrid. In 2000 he was visiting professor at the Institute of Psychiatry in London (King’s College of London). In 2002 he was appointed as Associate Professor at Rey Juan Carlos

University. From 2004 to 2015 he was the Head of Medical Image Analysis and Biometry Lab at Rey Juan Carlos University. From 2007 to 2014 he was the head of the Electronics Department at Rey Juan Carlos University. From 2008 to 2014 he was the director of the MR Physics Group at the Queen Sofia Research Center for Alzheimer’s Disease in Madrid. From 2010 to 2015 he was faculty of the MIT program M+Vision for medical imaging training and mentoring. Since 2020 he has a double appointment in the department of Imaging Physics in TU Delft j.hernandeztamames@erasmusmc.nl
MAGNETIC RESONANCE PHYSICS IN MEDICINE
JUAN A HERN
Á
NDEZ TAMAMES, PHD
full professor

Context
Magnetic Resonance physics in medicine is continuously evolving and improving. This research line tries to keep the Radiology and Nuclear Medicine department update to the latest MR techniques to facilitate clinical research and the best patient care at Erasmus MC.
The primary role of the MR Physics group in the Radiology and Nuclear Medicine department is to implement and develop novel MR imaging techniques. To Improve reproducibility and sensitivity is necessary to take MR beyond morphology-based diagnosis. The underlying physical parameters and their connection to biological processes and pathologies offer the potential for making MRI a quantitative diagnostic tool. We explore new quantitative MR techniques to establish pathology specific cut-off values and to improve the performance of Radiomics and Deep Learning Methods with more accurate quantitative biomarkers.
Top Publications 2021
Kotek, G., Nunez-Gonzalez, L., Vogel, M. W., Krestin, G. P., Poot, D. H., & Hernandez-Tamames, J. A. (2021). From signal-based to comprehensive magnetic resonance imaging. Nature Scientific reports, 11(1), 1-13.
Pirkl, Carolin M., et al. "Accelerated 3D whole-brain T1, T2, and proton density mapping: feasibility for clinical glioma MR imaging." Neuroradiology 63.11 (2021): 1831-1851.
Nunez-Gonzalez, Laura, et al. "Accuracy and repeatability of QRAPMASTER and MRF-vFA." Magnetic Resonance Imaging 83 (2021): 196-207.
Warnert, Esther AH, et al. "Mapping tumour heterogeneity with pulsed 3D CEST MRI in non-enhancing glioma at 3 T." Magnetic Resonance Materials in Physics, Biology and Medicine (2021): 1-10.
MR Research Projects: Objectives & Achievements
The activities of the MR Physics group are driven by clinical research lines of the Radiology and Nuclear Medicine department such as musculoskeletal research (with Edwin Oei), Lung MRI (with Harm Tiddens and Pier Luigi Ciet) and neuro-oncology (with Marion Smits). Besides the clinical research lines, it is important to notice that several fruitful projects are carried out on technical developments.
A very successful cooperation is taking place with the Radiotherapy department: Hyperthermia Unit of the Radiation Oncology Department (Gerard van Rhoon, Maarten Paulides) and Radiotherapy Planning (Steven Petit, Gerda Verduijn, Mischa Hoogeman and Marta Capala). These collaborations have respectively been granted: two KWF projects for “Hyperthermia treatment and MR thermometry” and “HolistiC early respOnse assessMent for oroPharynxgeaL cancEr paTiEnts (COMPLETE project)” and EU project for “Only MR Radiotherapy Planning with Deep Learning (DL)”.
Artificial Intelligence (AI) is a revolution in Medicine but particularly in Radiology. The MR Physics group has initiated several initiatives to link MR Physics and Artificial Intelligence. Three research projects on this topic were granted: one MRACE project, one EU EIT Healthcare project and, finally, one project sponsored by GE Healthcare.
The MR Physics group is actively participating in the Erasmus MC – TU Delft convergence. We are leading, jointly with Marion Smits Alexander Hirsch and Marleen de Bruin and in collaboration with Sebastian Weingärtner (Assist. Pr. In TU Delft) an innovative project in Quantitative Susceptibility Mapping for cardiac and neuro applications.
Samy Abo Seada is the postdoc of the convergence initiative and the project leader of research project granted in 2021 by the Dutch Parkinson & Parkisonisms Foundation.
Next paragraphs contain a list of the project-based activities of the MR Physics group, complementary to the projects in aforementioned research lines.
MR Physiological Signature and Artificial Intelligence
AI is becoming a revolution in Medical Imaging and aid diagnosis. However oncologists, neurologists, radiologists, specialists in general, need to fill the gap between AI and physiology.
Additionally, the MR Physics group wants to contribute to answer an important question for our clinical researchers: to what extent quantitative MR can provide reliable physiological information from the microscopic level .
In collaboration with GE healthcare we conducted a research to simultaneously fill the gap and to give a meaningful answer connecting MR, physiology and AI.
In the IGENE project led by Marion Smits (Pag 236) and with the outstanding collaboration of Esther Warnert (who recently received a VENI grant) (Pag 248), we investigate how Multi-parametric and synthetic MR can help in distinguishing molecular profiles in brain tumors using advance MR and AI.
This project includes the most advanced MR sequences optimized for brain tumors: Chemical Exchange Saturation Transfer (CEST) for amide, amine and PH assessment in the tumor, Enhanced Arterial Spin Labeling (eASL), Hybrid Gradient Echo Spin Echo (HEPI) for micro vessels architecture assessment.
Figure 1 Shows the prediction of contrast enhancing tumor only using quantitative parametric maps.

Figure 1. 73% of sensitivity and specificity in predicting enhancing only based on cut-off parametric values.
MRACE Project: EMC-HARPS. AI Harmonization.
EMC-HARPS is the acronym of a MRace project, granted along 2018: “Erasmus MC Harmonized Acquisition in Resonators for Population Studies based on MR-Signature and Machine Learning”. Long-term population studies such as Rotterdam or Generation R studies are forever bound to a particular scanner and software platform in order to avoid variabilities. However it could become an important drawback because the intrinsic hardware and software obsolescence impeding software upgrading and, eventually, scanner renewal.
The objective of this project is to demonstrate how AI could help MR Physics to achieve an impossible goal so far: to produce “scanner-independent MR images” from any subject for avoiding inter-scanner-platforms variability.
We will train a convolutional neural network to learn differences between scanners and reproduce images from one scanner as obtained in a different one. In other words, “AI could help us to tune scanners to exactly produce the same outcome as harps in an orchestra”
It is also important to notice that NVIDIA company has granted this project as well. Bryan Lusse who develop a CNN to predict parametric maps from weighted images.


Figure 2. Prediction of parametric maps from weighted images using U-AttenNet. Left column contains the ground truth parametric maps. The figure shows model prediction of T1, T2, and PDmaps for models with access to different combinations of the input data (left to right).
KWF-Project. Multi-coil magnetic resonance guided hyperthermia for precision treatment of advanced head and neck carcinoma.
In collaboration with Radiation Oncology Department, Hyperthermia Unit, in 2018 we were granted by KWF to develop a dual MR coil for Hyperthermia. In adjuvant mild hyperthermia (HT) of sensitive regions like the head and neck (H&N) real time 3D temperature (T) monitoring is critical for accurate application of thermal doses within the target. Theresa Feddersen is a PhD student involved in this project and Kemal Sumser has already defended his PhD thesis over this topic in 2021.


Figure 3. Picture of MRcollar applicator prototype and simulation of tumor treatmet with the hypercollar coil.
Theresa Feddersen is exploring novel MR sequences for improving patient comfort along the MR acquisitionand hopefully increasing the MR thermometry accuracy and precision.
EU EIT Health Project. Deep MR-only Radiotherapy
In collaboration with the Radiotherapy department and General Electric Healthcare we were granted by EU to develop a technology that eventually could avoid CT scanning for Radiotherapy Planning. We will use deep learning for a perfect delineation of the bone and target areas for radiotherapy of head and neck tumours and pelvic tumours. As an important part of this project, GE has developed a new multi-parametric silent zero TE sequence, capable of capturing signal from the bone with MR paving the way of being used for PET-MR.
Next figure shows head and neck obtained with the silent Zero TE sequence and pseudo-CT conversion.:


Next figure shows the Radiotherpy planning based on pseucdo CT compared to CT.

3D Signal Evolution - Transient Imaging. The “MR Signature”
In collaboration with GE Healthcare (Mika Vogel, PhD), the MR Physics group (Gyula Kotek Phd, Pag 50, Willem van Valenberg PhD Pag 52, Dirk Poot PhD Pag 66 and Juan A Hernández-Tamames) are developing the 3D version of “MR Signature”
The next figure corresponds to the 2D proof of concept published last year:

7. 2D
T1, T2, T1/T2 , B0 and B1 maps, simultaneously obtained with MR and Synthetic Images derived from the 2D multiparametric maps.
Figure 4. Head and Neck Multiparametric silent Zero TE acquisition.
Figure 5. Pseudo-CT 3D Rendering from ZTE MR.
Figure 6. Radiotherapy Planning based on Pseudo CT acquisition.
Figure
Proton Density,
Oropharynx
Tumors. Response assessment with Arterial Spin Labelling and Multi-parametric
Mapping.
Nienke Sijstema is the PhD student focused on this project. She is investigating quantitative biomarkers for response assessment in relevant regions of he neck. Next figure shws some of the results.

Figure 8. Example of the (a) T2w image, (b) T1 map aDelineations are shown of submandibular glands (left column), the tonsils (the two medial ROIs in the middle column) and parotid glands (right column). Note that delineations of the parotid glands are also visible in the middle column.
Erasmus MC – TU Delft Convergence Project: The Heart and Brain Connection. QSM and AI in Cardio and Neuro.
In 2019 we have initiated a hopeful promising and tight convergence with TU Delft. The MR Physics group is fully involved leading with Sebastian Weingärtner (Assist. Pr. In TU Delft), Frans Vos (Assoc. Pr. In TU Delft), Alexander Hirsch MD, PhD, (Pag 258), Marion Smits MD, PhD, (Pag 263) and Marleen de Bruijne, PhD, (Pag 88) one of the flagship convergence projects.
Samy Abo Seada, who is investigating the clinical applicability in several pathologies, in particular, in Parkinson and Parkinsonisms.

We have been successfully granted by the Dutch “Parkinson Veriniging” to develop an advanced MR protocol for early diagnoses of parkinsonisms.

Figure 10. Convergence project in collaboration with TU Delft.
Figure 11. QSM image generated using AI.
Lung MR Research
In collaboration with Pierluigi Ciet (Pag. 327), MD, PhD and Prof Harm Tiddens (Pag. 320) and the outstanding support of Piotr Wielopolski , our PhD student Dory Papp (Pag. 56) also collaborating with Marleen de Bruijne (Pag. 88) and her PhD student José Castillo-Tovar ( Pag. 85), we are applying AI in 4D-ZTE sequences to generate clinically relevant images from free breathing. It has important applications in pediatrics imaging of lung in free and irregular breathing.

Placenta Study
In collaboration with Dirk Poot (Pag. 66) and Stefan Klein (Pag. 118), we have initiated an interesting collaboration win AnneMarie Mulder, from the Gynecology department to assess placenta condition with MR. With Marteen Thomeer we have developed an advanced protocol with oxygen challenge to evaluate placenta function.



13. Anatomical and Functional MR images obtained on the Premier MR Scanner with the Air Coil. Internal cotyledons of the placenta where mother and fetus circulation and exchanging oxygen and nutrients can be depicted.
Pr. Juan A. Hernández-Tamames has participate as MRI expert in the NIH working group of Human Placenta Project 2021 (HPP2021).
Expectations & Directions
In MR, the ambition is to grow, attract further talented PhD students and post-doctoral fellows in order to widen and solidify the technology related expertise in the group. The group needs to play two main roles: a) provide service for clinical researchers b) contribute to MR technology through innovation in novel imaging techniques.
The group needs to increase the technical support staff to guarantee further developments and the maintenance of the current ones.
The growing number of partnerships with researchers and manufacturers of different fields requires competent MR Physicists who channel their knowledge to the respective medical or technical fields with a strong hinterland: a coherent group of MR Physicists. The knowledge in the field of MR Physics is quite broad ranging from handson electronics to theoretical physics through computer simulation and experimental skills. Sub-specialization within the group needs to be established.
Funding
Samy Abo, Anke van der Eerden, Agnita Bonn, Juan A. Hernandez-Tamames. Advanced MR Protocol for Parkinsonisms. Dutch Parkinson Vereniging. 2021-2023.
Hernández-Tamames Juan A, Tonya White, Meike Vernooi. MRace project. HARPS: Harmonization of Erasmus MC Resonators for Population Studies. 2019-2021.
Hernández-Tamames, Gyula Kotek. Signal Evolution Transient Imaging II. GE Healthcare WorkStatement. 2020-2022.
Paulides, Maarten (Radiation Oncology), Van Rhoon Gerard (Radiation Oncology) , Franckena Martina (Radiation Oncology), Hernández-Tamames Juan A. (Radiology): Netherlands Cancer Society Grant 2018-2021: “Multi-coil magnetic resonance guided hyperthermia for precision treatment of advanced head and neck carcinoma”
Florian Wiesinger (GE), Steven Petit, Hernández-Tamames Juan A, et al. EU EIT Health 2019-2022. “Deep MR-Only Radiotherapy”
Figure 12. Lung reconstruction using Deep Learning
Figure
Steven Petit, Hernández-Tamames Juan A, Aad van der Lugt et al. Elekta Research grant. 2018-2022. “Oropharynx Cancer”
Steven Petit, Hernández-Tamames Juan A, Aad van der Lugt, et al. “COMPLETE Project for Holistic Assessment of Oropharingheal Cancer”. 2019-2023. “Oropharynx Cancer"
Additional Personnel
Mika W Vogel, PhD ASL Scientist & Team Leader ASL Scientists Europe, GE Healthcare
Mika Vogel is a GE Scientist who operates primarily from the Erasmus MC Dept of Radiology. He supports the collaborative research that we perform with GEHC as well as advising on matters involving MR. For GE Healthcare, Mika manages the post-market advanced technology software modules. These modules are intended to go to different collaborator sites, and undergo design control and are subject to regulatory requirement for their distribution.

MRI PHYSICS
Assistant Professor GYULA KOTEK, PHD
MRI PHYSICIST

IGyula Kotek received his MSc (Physics) from the Eötvös Loránd University, his PhD (Physics) from University of Szeged. In the 90’s he followed this with post-graduate work at the Research Institute of Technical Physics, Budapest/HU, the New York Medical College, NY/USA, and the Max Planck Institute, Munich/DE. From 2003 he works in MR Imaging research and medical physics. He joined Erasmus MC in 2008, where he has been working since with an interruption 2014-2016, when he has spent two years as research coordinator for PET/MRI studies. His expertise is in MR Imaging Physics, pulse sequences, MRI coils, radiotherapy treatment planning, bio-physical modeling. g.kotek@erasmusmc.nl
n 2021 Gyula has been the project manager and senior scientific contributor of the SETI (Signal Evolution Transient Imaging) project: a technology development project in collaboration with General Electric Healthcare in the field of fast quantitative transient imaging. The goal of this current phase (SETI II.) is the acceleration of the previously proved concept as published in a Nature Scientific Report. He is driving the technology development in close collaboration with Dirk Poot (EMC) and Mika Vogel (GEHC).
His main responsibility in the MR Physics team is acquisition techniques: sequences and implementation on clinical scanners.
He is the lead-inventor of two patents in the MR Signature technique in the field of fast quantitative image acquisition.
Gyula has been supervising two PhD students Dorottya Papp (lung and musculoskeletal MRI) and Laura Nunez Gonzales (fast quantitative techniques: MR Fingerprinting, QTI, MR Signature) and post-doctoral Willem Valenberg dedicated to the SETI II. project.
His main interest is the development and implementation of MRI acquisition techniques and quantitative parametric mapping. His continued effort and ambition are to elevate the department’s role in the cooperation with GEHC as technology contributor in MR Imaging.
Gyula is participating in the department’s PET-MRI workgroup.

Example of 3D parametric maps acquired with the patented technique developed in collaboration with GEHC. In vivo maps: B0, B1+, PD, T1, T2. Phantom maps and comparison of derived parameters to reference values.
Forward
In the SETI research line, he will focus on bringing the original concept to clinical application. His ambition in 2022 is to establish a matrix organization with the goal to strengthen the PET-MRI technology related research at Erasmus MC. He continues his work on establishing the theoretical novel concepts in MR Imaging techniques within and beyond the framework of the cooperation with GE Healthcare. His continued effort is to support MSK and lung imaging with his PhD students.

QUANTITATIVE SUSCEPTIBILITY MRI: DEEP INSIGHTS IN CARDIO- AND NEURO-VASCLATURE
SAMY ABO SEADA, PHD
Post-doctoral researcher
Project Funding Convergence project Erasmus MC – TU Delft
Research period July 2020 – July 2022
Email s.aboseada@erasmusmc.nl
This project is a collaboration between the Erasmus MC Department of Radiology & Nuclear Medicine and the Department of Imaging physics at TU Delft.
Te project I am working on investigates clinical uses of a novel MR imaging technique known as Quantitative Susceptibility Mapping (QSM). QSM is sensitive to tissue susceptibility, and its signal is sourced from tissue iron, myelin and calcium concentrations. Tissue iron is of particular interest as it is related to several brain diseases. A good example is the difference of iron accumulation in the basal ganglia for different forms of parkinsonism, such Parkinson’s Disease, Multiple System Atrophy and Progressive Supranucleur Palsy. Another example, is the use of QSM to monitor non-enhancing multiple sclerosis lesions. In this case QSM is sensitive to the iron in the inflammatory microglia.
My first objective is to translate this novel technique into a clinical tool in collaboration with clinicians at the Erasmus MC. To do I hope to demonstrate its usefulness and applicability in a research project aiming to better diagnose atypical parkinsonism disease at an early stage, to give patients the correct diagnosis as early as possible. Other clinical applications I am interested in are cardiac QSM and using QSM in the placenta.
In July 2021 we received research funding from Parkinson NL to initiate an observational pilot study on patients with atypical parkinsonsism. We started the APqMRI study to investigate the benefits of quantitative MRI methods (QSM, atrophy and DTI) as well as neuro-melanine MRI for diagnosing atypical parkinsonisms. We collaborate with a Agnita Boon, movement disorder specialist at the department of neurology, and Anke van der Eerden, neuroradiologist at our department.
I also collaborate with Esther Warnert, PI at our department, and researchers from TU Delft, on measuring oxygen levels with a wearable device and correlating these measurements with novel MRI oxygen mapping techniques.
I have wider interests in MR physics and image processing techniques in different anatomies, such as cardiac QSM and using AI for inverse problems.

WILLEM VAN VALENBERG, PHD ACCELERATED QUANTITATIVE MRI
Project Funding GE Healthcare
Research period September 2020 – September 2022
Email w.vanvalenberg@erasmusmc.nl
This project is collaboration between the Departments of Radiology & Nuclear Medicine and Epidemiology.
Quantitative MRI methods measure the tissue properties that determine the contrast in MR images. These measured properties are more reproducible than conventional images, and can therefore improve the comparison of exams between scanners or over time. However, quantitative methods typically require a substantial amount of scan time which limits their clinical applicability.

This project aims to reduce scan time of quantitative MRI through a combination of different techniques. One is to decrease the number of measurements by increasing their readout time. The blurring of images due to the longer readout is reduced by modelling the off-resonance effects during image reconstruction (Figure 1). A further decrease of measurements is obtained by undersampling the data, at the cost of image artifacts. These artifacts can be reduced by combining information from different measurement coils (SENSE), and/or difference images (SC), see Figure 2. Combining both techniques greatly reduces scan time at little cost of the resulting parameter maps.

Figure 2. Comparison of T1 and T2 parameter maps from a reference (fully-sampled) acquisition and from an acquisition with an undersampling factor of 4, where the image reconstruction was done using parallel imaging (SENSE) and parallel imaging with subspace constraint (SENSE+SC). The addition of the subspace constraint strongly reduces the error in the parameter maps.
Figure 1. Image reconstructions from acquisition with long spiral readout (22 ms). The image blurring due to the long readout duration can be reduced by correcting for off-resonance effects in the reconstruction.

FAST IMAGING TECHNIQUES IN MR
ALEXANDRA CRISTÓBAL HUERTA, MSC
PhD Student
Advisors Juan Antonio Hernández Tamames & Dirk Poot
Project Funding
Research period June 2015– August 2019
Email a.cristobalhuerta@erasmusmc.nl
Preserving image quality while reducing scanning time is one of the major challenges in MR imaging. In this thesis, we are investigating several approaches to accelerate high-resolution structural imaging of the brain and knee, while image quality is preserved. The Fast Spin Echo sequence (FSE) was chosen as sequence to investigate. Firstly, I evaluated different reconstruction techniques, such as parallel imaging (PI), compressed sensing (CS) and half Fourier (HF) to obtain accelerated high-resolution images without artifacts. After, I implemented and investigated the 3D Gradient and Spin Echo sequence (GRASE) to further accelerate high-resolution imaging in brain and knee. I proposed and evaluated several k-space trajectories and k-space undersampled grids for integrating PI and CS in 3D-GRASE in brain and knee applications. These trajectories minimize the artifacts introduced by mixing spin-echoes and gradient-echoes in the k-space.
If an optimal trajectory is not used in the acquisition of 3D-GRASE images, artifacts can appear. In order to overcome this limitation and generate images more similar to 3D-FSE, I am investigating a deep learning approach where 3D-FSE images from the knee are used as ground truth. This network learns the corrections that should be made in a 3D-GRASE image to look like a 3D-FSE image, correcting artifacts and contrast. For this task, I evaluated the performance of a 3D U-Net architecture together with three different loss functions: DDSIM, L2 and perceptual. Results using a perceptual loss function show that global artifacts can be removed from the images, achieving a more similar 3D-FSE image from a non-optimal 3DGRASE acquisition.

Figure 1. PD-weighted knee images for a second subject in the sagittal, coronal and axial planes obtained for A) 3D-FSE, B) 3DGRASE and C) 3D U-Net with perceptual loss..

THERESA V FEDDERSEN, MSC IMPROVING MR THERMOMETRY FOR HYPERTHERMIA TREATMENTS
PhD Student
Advisors M.M. Paulides, G.C. van Rhoon, D.H.J. Poot & J.A. Hernández Tamames
Project Funding KWF
Research period November 2018 – October 2022
Email t.feddersen@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Radiotherapy
The current clinical standard for temperature monitoring during deep hyperthermia (HT) treatments is to use invasive or intraluminal catheters. These give limited (point specific) information from few probes. The possibility of producing noninvasive 3D temperature maps in real-time using an MRI is therefore very attractive. Temperature changes in tissues can be detected by different methods, the most used being the proton resonance frequency shift (PRFS), due to its linear increase with temperature and relative tissue independence (with the exception of fat). However, the changes that are being detected are very small and thus this method is especially susceptible to motion artefacts and magnetic field stability. To achieve the SNR and stability needed for accurate temperature maps during the substantial treatment time of 90 minutes, we need to find a way to deal with these difficulties.
One way to improve MR thermometry (MRT) is to increase the SNR by using a dedicated applicator coil that also acts as a receiver. The MRcollar (Figure 1) has recently been developed for the head & neck and can be used in an MRI without disturbing the magnetic field noticeably.

In order to develop a motion robust multi-coil multiecho MRT protocol, firstly the performance of different multi-echo gradient echo sequences was compared in phantom. an example is presented in Figure 2. We found the performance of the 3D sequence (IDEAL IQ) most promising.
A comparison of the same sequences in 10 healthy volunteers, also showed the 3D GRE sequence as the most stable over time. Regarding the post-processing pipeline for volunteers, we have implemented rigid image registration to correct for inter-scan motion and developed an automatic fat selection tool in order to improve the B0 drift correction.
Further, we have commenced investigations into the feasibility of silent MRT, acceleration using parallel imaging during MRT, and whether the found improvements using the 3D implementation translate to the pelvis applicator.
Due to the COVID-19 pandemic, the access to the hospital and hence also the ability to conduct experiments was limited. The time was used to write a review article: “Clinical Performance and Future Potential of Magnetic Resonance Thermometry in Hyperthermia”, published in Cancers.

Figure 1 Head & neck applicator for MR guided HT
Figure 2 Temperature change over time

FAST MULTI-PARAMETRIC ACQUISITION METHODS FOR QUANTITATIVE BRAIN MRI
LAURA NÚÑEZ GONZÁLEZ, MSC
PhD Student
Advisors Dirk Poot, Gyula Kotek & Juan Antonio Hernández Tamames
Project Funding
Research period April 2016 – May 2022
Email l.nunezgonzalez@erasmusmc.nl
Nowadays, the feasibility of rapidly obtaining at once quantitative PD, T1 and T2 maps of the brain has been proven and demonstrated with several techniques such as MR-Fingerprinting (MRF) or MAGiC. We focused on exploring beyond this by using existing techniques differently and creating a new sequence to obtain more information.
We used data acquired using MRF scheme to obtain multiple components per voxel instead of the usual singlecomponent estimation. By applying the Sparsity Promoting Iterative Joint Non-negative least squares (SPIJN) algorithm to the MRF data, we obtained fraction-tissue maps (figure 1) for white matter, gray matter, myelin and cerebral spinal fluid among others.
Also, we designed a new sequence named “Multi-Phase balanced, non-Steady State Free Precession” (MP-b-nSSFP), which consist of the repetition of a 4-pulses block from which it is possible to obtain not only the intrinsic parameters (T1, T2 and PD) but also extrinsic parameters such as B0 and B1 inhomogeneities (Figure 2).


2. Parametric maps derived with the MP-b-nSSFP sequence: Proton density (a), T1 (b), T2 (c) B1 scaling factor (d) and B0 inhomogeneities (e).
Figure 1. Central slice of the SPIJN-MRF maps for different tissue types of a volunteer scanned 8 different days.
Figure

APPLICATION OF ZTE IN LUNG IMAGING
DOROTTYA PAPP, MSC
PhD Student
Advisors Juan Antonio Hernandez Tamames & Gyula Kotek
Project Funding
Research period November 2017 – May 2022
Email d.papp@erasmusmc.nl
MRI has recently emerged as a potential clinical tool that can produce high resolution images of structural lung changes similar to Computed Tomography (CT) scans, thanks to the use of ultrashort TE readouts, but without using ionizing radiation. Thanks to these developments, pediatric patients with chronic lung disease, such as cystic fibrosis (CF), can undergo routine monitoring with CT like image. With the help of Piotr Wielopolski and Pierluigi Ciet we investigate three different MRI sequences (Ultrashort Echo Time - UTE, Zero Echo Time - ZTE3D vnav and ZTE 4D). We found so far that UTE and ZTE readouts provide similar image quality for lung MRI to assess structural changes. Conversely, detection of low density region is limited both in UTE and ZTE compared to CT, likely due to free-breathing conditions. Short TE breath-hold SPGR scan still provide the best CNR to detect air trapping with MRI. One of the advantages of ZTE sequences is the comfort it can give to pediatric patients due to the fact it
is ‘noiseless’. We are working on a reconstruction method which can give the same image quality with ZTE 4D freebreathing as the breath-hold SPGR sequence.
Although fully convolutional neural networks (FCNNs) have been widely used for MR imaging, they have not been extended for improving free-breathing lung imaging yet. With Jose M. Castillo T. our aim was to improve the image quality of ZTE4D using a FCNN trained on breath hold ZTE (ZTE-BH) images to improve the image quality of ZTE4D in free-breathing. It helps the clinical work with patients with whom it is impossible to obtain breath-hold imaging. Our model obtained a MSE of 0.08% on the validation set. When tested on unseen data the predicted images from our model had improved visual image quality and artifacts were reduced in free-breathing ZTE4D.
Figure 1. Flow diagram of the proposed framework. The first step was the modification of the original breath-hold images, followed by the training of the FCNN, then the denoising of the original ZTE4D images.


NIENKE SIJTSEMA, MSC RESPONSE ASSESSMENT OF HEAD AND NECK CANCER
PhD Student
Advisors Juan Antonio Hernandez Tamames, Steven Petit, Mischa Hoogeman & Dirk Poot
Project Funding Elekta AB, Stockholm, Sweden
Research period November 2017 – May 2021
Email n.sijtsema@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Radiotherapy.
One of the aims of my PhD project is to find and optimize MRI techniques that yield relevant biomarkers for treatment response prediction of head and neck cancer. These biomarkers will allow us to adjust the treatment at an early stage in the treatment process and on a patient by patient basis.
So far, we optimized and implemented Non-Gaussian Intravoxel Incoherent Motion (NG-IVIM) imaging, a form of DWI, for head and neck. From this we obtain the apparent diffusion coefficient (ADC) as well as parameters related to perfusion and tissue cellularity. This sequence is now used in the COMPLETE study, where we aim to relate the change in DWI parameters during radiotherapy to tumor response and overall survival. Additionally, we are looking into the difference between human papilloma virus (HPV) positive and negative oropharyngeal tumors in terms of NG-IVIM.
In the past year, we have also optimized and evaluated arterial spin labeling (ASL), a perfusion MRI technique, for head and neck. First, this will be done in healthy volunteers
to establish a range normative values for healthy tissues. In the future, ASL might be a useful tool for early prediction of tumor response and overall survival, as well as for early prediction of radiation toxicity in healthy tissues. A next step will be to compare the blood flow obtained from ASL to the perfusion parameters obtained from NG-IVIM.
In addition to this, we are also interested in retrospective assessment of toxicity (i.e. response of healthy tissue to radiation), and we have investigated risk factors for development of osteoradionecrosis in the mandible, as well as the interaction between the osteoradionecrosis location, the dose to that location and the location of teeth extractions.
Figure 1. Blood flow map obtained from ASL from a healthy volunteer, overlayed on the synthetic T2w image. ROIs are shown in blue (left; submandibular glands, middle; tonsils and parotid glands, right; parotid glands)
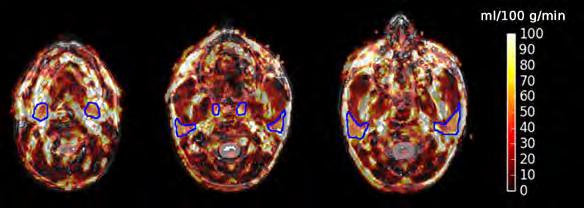

Advisors
KRISHNAPRIYA VENUGOPAL, MSC VASCULAR SIGNATURE MAPPING OF GLIOMA
PhD
Student
Juan Antonio Hernandez Tamames, Matthias van Osch, Dirk Poot & Esther Warnert
Project Funding NWO domain AES
Research period February 2020 – January 2024
Email k.venugopal@erasmusmc.nl
Dynamic Susceptibility Contrast (DSC) imaging commonly uses GRE-EPI to trace the first passage of a GBCA to measure relative cerebral blood volume (rCBV). Vessel architecture imaging (VAI) is an emerging DSC variant with combined GRE/SE-EPI where the sensitivity of SE to smaller vessel radii (R) is exploited. We propose a MR vascular fingerprinting (MRVF) approach to disentangle VAI biomarkers: rCBV, R and permeability (k) during bolus injection. In glioma patients we compared MRVF to a conventional vessel size imaging (VSI) technique.
Hybrid EPI (HEPI, a fast acquisition technique combining GRE and SE) DSC were acquired from patients with confirmed diagnosis of glioma. Dynamic images (TR/TEGRE/ TESE 1.5s/20ms/70ms, 15 slices, 3T, MR750, GE) were acquired during bolus injection of 7.5ml of GBCA (Gadovist, Bayer, GE) after a preload of equal size. We imported the HEPI sequence as played out on the scanner into a Bloch based DCE simulation tool that simulates CA extravasation, diffusion, and MR signal. A dictionary was created by simulating 5 vessels with varying k, R and rCBV. For each dictionary atom the simulation included 400s to cover prebolus and delay, followed by 100s of the main bolus and HEPI-acquisition. The dictionary was matched to the patient data (4 patients, confirmed molecular status after surgery) by allowing a separate scaling factor for the GRE and SE parts to compensate for baseline (T2) differences between dictionary and in-vivo signals. The parameters obtained were compared with those estimated using the VSI method.
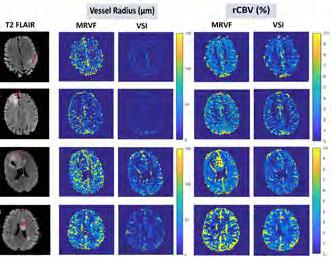
Figure 1. T2 FLAIR images of the 4 patient datasets of slices showing the glioma (marked by the red arrows) and the corresponding vessel radius and rCBV maps obtained using the MRVF and the VSI methods
Figure 1 shows for each patient a slice of the T2 FLAIR with the corresponding parametric maps obtained by the two methods. It is observed that both techniques can differentiate normal and tumor tissues, show similar patterns, although also quantitative differences. By dictionary matching of HEPI data a good match was found between simulated and in-vivo data. Further research will study whether permeability is essential for the dictionary for DSC with preload.
This project is a collaboration between the Departments of Radiology & Nuclear Medicine, Erasmus MC and Leiden UMC.

NEW HARDWARE AVENUES FOR ENABLING MAGNETIC RESONANCE THERMOMETRY GUIDED RADIO FREQUENCY
HYPERTHERMIA TREATMENT IN THE HEAD & NECK
KEMAL SUMSER, MSC
PhD Awarded 2 June 2021
Advisors Gerard van Rhoon, Juan Antonio Hernandez-Tamames & Margarethus Marius Paulides
Project Funding
Short CV
Erasmus MC Fellowship: Temperature-based transient perfusion imaging for personalized cancer therapy
Kemal Sumser was born in Ankara, Turkey in 1991. He received his B.Sc. and M.Sc. degrees in electrical and electronics engineering from Middle East Technical University, Ankara, Turkey and Ph.D. from Erasmus University Medical Center, Rotterdam, The Netherlands. He is currently working as postdoctoral research at Erasmus MC, Department of Radiotherapy. His research activity mainly concerns hyperthermia treatments, electromagnetic and thermal modeling of biological tissues, and MRI guided treatments.
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Radiotherapy.
The beneficial effect of hyperthermia as adjuvant to radiotherapy in head and neck cancers are well established with clinical trials. In general, the benefits of hyperthermia are found to be depend on thermal dose. To improve the delivery of thermal dose, a great deal of work has been and still is conducted on device development, treatment planning, patient modeling, and treatment monitoring. Treatment monitoring is an important aspect to ensure correct assessment of treatment quality and dose delivery, as well as validation of modeling and planning. However, in clinic, target temperature increase information is seldom available and clinicians rely on pretreatment SAR calculations. MR thermometry during head and neck hyperthermia is a potential solution to improve 3D information of the applied temperature distribution, but it at present is not yet clinically available and the required temperature measurement accuracy demands technology beyond the

current available state of the art. This thesis starts with first presenting current state of the art MR compatible RF hyperthermia devices. From it, it follows that the field lacks standardization in validation and needs an MRI compatibility benchmark. Secondly it shows that MRT guidance during hyperthermia treatment is required due to the effect of the large vessel network in head and neck region on the temperature distribution. Thirdly, it describes the design and validation of a MR compatible head and neck hyperthermia applicator that can heat at equal quality as the non-MR compatible HYPERcollar3D. Fourthly, the apparent MRT errors due to the water bolus can be effectively and economically solved by doping the water bolus with contrast agents. Lastly, it shows that integration of multi-channel receiver coils in a phased array of RF-antennas is feasible and improves the SNR. These results validate our hypothesis that a suitable combination of hardware technologies enables precise monitoring of the temperature distribution which on its turn will provide the important information to exploit optimal SAR steering during MRT guided H&N HT.

https://repub.eur.nl/ pub/135610

Marcel van Straten (1974) studied Applied Physics at Delft University of Technology. His MSc project was on contrast agents in MRI. He investigated the relationship between magnetization relaxation times and concentration of contrast agents. His PhD project at the Academic Medical Center in Amsterdam focused on the application of image registration techniques in spiral CT. One of the applications provided a fully automatic technique to remove obscuring bone
structures and calcifications from CT angiography images. After that he was a postdoctoral researcher at the Institute of Medical Physics of the University of Erlangen-Nuremberg. He worked on the optimization and dosimetric aspects of CT. In 2008 he joined the Department of Radiology of Erasmus MC. In 2013 he has been granted certification as a medical physics expert. marcel.vanstraten@erasmusmc.nl
MARCEL VAN STRATEN, PHD assistant professor PHYSICS IN CT TECHNOLOGY

Context
In CT, image quality is influenced by many factors. It starts with the scanner’s hardware and acquisition protocol and ends with the reconstruction technique and post-processing techniques applied. In clinical practice, the radiologist would like to have the best possible image quality at the lowest radiation dose possible for a specific diagnostic task.
Besides the need for optimization of the acquisition and reconstruction technique, there is a need for a smooth introduction of these optimized techniques into clinical practice. Our research focuses on the standardization and optimization of image quality in x-ray computed tomography (CT) based on the laws of physics and driven by technological innovations.
Top Publications 2021
van der Werf NR, M van Gent, R Booij, D Bos, A van der Lugt, RPJ Budde, MJW Greuter, M van Straten. Dose Reduction in Coronary Artery Calcium Scoring Using Mono-Energetic Images from Reduced Tube Voltage Dual-Source Photon-Counting CT Data: A Dynamic Phantom Study. Diagnostics (Basel) 11(12):2192 (2021)
Booij R, M van Straten, A Wimmer, RPJ Budde. Influence of breathing state on the accuracy of automated patient positioning in thoracic CT using a 3D camera for body contour detection. Eur Radiol. [Epub ahead of print] (2021)
van der Werf NR, R Booij, B Schmidt, TG Flohr, T Leiner, JJ de Groen, D Bos, RPJ Budde, MJ Willemink, MJW Greuter. Evaluating a calcium-aware kernel for CT CAC scoring with varying surrounding materials and heart rates: a dynamic phantom study. Eur Radiol. 31(12):9211-9220 (2021).
Research Projects: Objectives & Achievements
Evaluation of technological innovations
New acquisition hardware or new reconstruction methods always claim to improve the image quality of CT scans or to reduce the dose without affecting the image quality. In order to assess the value of newly introduced acquisition and reconstruction techniques, it is of utmost importance to use objective evaluation methods to bring forth both benefits and limitations of these developments. Our research focuses on the development and application of such evaluation methods. In 2021, a dual-source photon-counting detector-based CT scanner (NAEOTOM Alpha, Siemens Healthineers) became available for routine clinical use. Our department was as one of the first sites worldwide able to evaluate this quantum leap in CT technology (see highlights).
Building a knowledge base
After a successful evaluation and optimization of the acquisition and reconstruction technique, there is the need for a smooth introduction into clinical practice. This can be a complex optimization process. Changing system properties, acquisition parameters, or reconstruction parameters, will influence both radiation dose and image quality. Optimization via a human interface might be time consuming and error prone. We use the knowledge generated in our research group to build a knowledge base on the performance of a CT scanner in various situations that we use for the automation of the CT operating procedure. Our goal is that the automated procedure makes the best use of the latest technological innovations for a given diagnostic question. In 2021, PhD student Ronald Booij successfully defended his thesis on the ‘knowledgeable’ CT scanner that focuses on the evaluation and implementation of technological solutions to automate and/or improve CT imaging (see his page for details).
Standardization of CT imaging of the lung s
CT has utility in Cystic Fibrosis (CF) research if it is sensitive enough to detect changes with therapy or disease progression. We work on the standardization and optimization of CT which is a prerequisite for unbiased automated analyses and reduced observer variability.
Smart*Light
Research project Smart*Light aims to develop a compact and bright X-ray source with tunable X-ray energy. To achieve this, a consortium of 12 partners in the Netherlands and Flanders, including Erasmus MC collaborates closely. The Smart*Light X-ray source can expectedly be applied in clinical diagnostics (besides materials science research and for the investigation of important artworks). The X-ray source design has been finalized. Production, however, suffered from serious delays due to limited availability of hardware and qualified personnel.
Expectations & Directions
In CT, objective quantification of the performance of new technology and algorithms will allow us to determine their impact on the image quality and thus on the diagnostic performance. The knowledge obtained by the studies described above will allow for building a knowledge base that can be used for the development of a ‘knowledgeable CT scanner’ and for the automation of the CT operating procedure.
Funding
Ronald Booij received an Academy Van Leersum grant of the Academy Medical Sciences Fund, Royal Netherlands Academy of Arts & Sciences for a visiting project regarding “Clinical application of photon counting CT: a quantum leap in medical imaging” at CMIV, Linköping University, Sweden.
Invited Lectures
Booij R. “De ‘intelligente’ CT-scanner: Optimalisatie van stralingsdosis en beeldkwaliteit”, Kalcio Healthcare, Leusden, The Netherlands, 14 October 2021
Booij R. “Difficult issues in coronary CT imaging”, European Society of Cardiovascular Radiology, online edition, 20 – 23 October 2021
Booij R. “The knowledgeable CT scanner: Protocol optimalisatie?!”, Parkstad CT Symposium, The Netherlands, May 2021. (online)


Virtual mono-energetic CT images of an anthropomorphic abdomen phantom with circular cavities containing water with various concentrations of (iodinated) contrast agents.
Highlights
In 2021, multiple (phantom) studies were performed on the new dual-source CT scanner fully equipped with photoncounting detectors instead of energy-integrating detectors. Results were presented at the 2021 annual meeting of the Radiological Society of North America (RSNA) and the first manuscripts have been accepted for publication. A study conducted and published by Van der Werf et al. was related to coronary artery calcium (CAC) quantification with photon-counting CT. In this study, the ability of data acquisition at several x-ray tube potentials, while reconstructing images at the same virtual monoenergetic energy was employed. The ability of using a patient-size specific tube potential resulted in a radiation dose reduction of up to 67% for medium and high density CAC, without changing quantification results.
Booij et al. presented at the annual meeting of the RSNA another study on the improved visualization of iodinated contrast-agents with the aid of virtual monoenergetic imag-
Niels van der Werf, Medical Physicist
Left: image from a photon-counting CT scanner with a superior contrast-to-noise ratio. Right: image from a conventional CT scanner. Energy level 40 keV and W/L 2000/500 HU for both images.
ing at low keV-levels when compared with state-of-the-art dual-energy CT based on conventional energy-integrating detectors (see figure).
Research on characterizing imaging performance with a standard water phantom was extended to photon-counting CT by Van Straten et al. and presented at the RSNA. Despite the relatively simple nature of the phantom, it allowed for a detailed analysis of the impact on image quality of improvements in both the detector hardware and the iterative reconstructing technique used on this scanner. At a given radiation dose, image noise properties and spatial resolution were better with photon-counting CT than with conventional CT. The planned system updates (early 2022) are expected to result in many more interesting research opportunities.
Additional Personnel
Marcel L. Dijkshoorn - Research Technologist CT
Niels van der Werf (1988) studied Applied Physics and Biomedical Engineering at the University of Groningen. After his medical physicist residency at the Albert Schweitzer hospital, he joined the department of Radiology & Nuclear Medicine of the Erasmus MC in 2019. Alongside this residency, he started as a PhD student at the Radiology department of the University Medical Center Utrecht focusing on the diagnostic performance of coronary artery calcium detection and quantification with computed tomography. The target end-date of this PhD is before the summer of 2022.


THE “KNOWLEDGEABLE” CT SCANNER: OPTIMIZATION BY TECHNOLOGICAL ADVANCEMENTS
RONALD BOOIJ, PHD
PhD Awarded 23 February 2021
Advisors Gabriel Krestin, Marcel van Straten & Ricardo Budde
Project Funding Erasmus MC Radiology
Short CV Ronald Booij was born on April 17, 1980 in Rozenburg, The Netherlands. He started the in-service education Radiographer at the Dijkzigt hospital (now Erasmus MC) in 1997. He obtained postgraduate degrees in CT (2004) and in “supervisor in health care” (2007). In 2009 he became the coordinator of Research & Innovation of the unit CT. From 2011, he became teacher of several postgraduate education in CT in The Netherlands and a regular contributor to (inter)national conferences. He was an EFRS expert committee member for medical imaging, representing CT. In April 2017 he obtained the degree of Master of Science (MSc) in medical imaging & radiation oncology at the Inholland university of applied sciences in Haarlem, The Netherlands. Subsequently, he started his PhD project at the Erasmus MC Rotterdam, entitled: the “knowledgeable” CT scanner under supervision of Professor Gabriel Krestin which resulted in this thesis.
Optimization of a computed tomography (CT) exam can be challenging, as there is a wide variety in patient characteristics, continuously emerging technologies, increased workload and the increase of the number of interrelated acquisition and reconstruction parameters to be adjusted. In addition, the variety of patients and referral questions necessitates different acquisition strategies. Therefore, artificial intelligence (AI) is integrated in most of the steps of the imaging chain to make procedures more accurate, standardized and supports the users. Even though AI is able to “learn” from analyzing more and more cases, it remains important to know and monitor their performance, as well as to determine their accuracy. This thesis focuses on evaluation and implementation of technological solutions to automate and / or improve distinct aspects of the CT imaging chain. We conclude that knowledgeable solutions like AI should be embraced, as they can be an important factor in assisting medical healthcare workers and may also lighten the workload. In addition, a high knowledge base among the users of intelligent machines is essential for the development and correct application of AI. The human side remains necessary for further optimization in clinical practice and for future developments. Therefore, optimization is achieved by deploying smart technologies and by merging of knowledge obtained by man and machine, creating opportunities for a “knowledgeable” CT scanner. Ultimately, this may lead to a symbiosis between man and machine.


https://repub.eur.nl/pub/133040

Dirk Poot is heading the quantitative MRI reconstruction research line. He is affiliated with the Biomedical Imaging Group Rotterdam (BIGR, http://www.bigr.nl) and the MR physics group. In 2005, Dirk received his MSc degree from the faculty of Applied Physics at the Delft University of Technology, Delft/NL. In 2010 he obtained his PhD degree at the Visionlab, University of Antwerp, Antwerp/BE, for his research on reconstruction and statistical processing of Magnetic Resonance Images. He is Work Package leader in the H2020 MSCA project B-Q MINDED. His current research interests include MR image acquisition, reconstruction, quantification, and motion compensation. d.poot@erasmusmc.nl
QUANTITATIVE MRI RECONSTRUCTION
DIRK
POOT,
PHD assistant professor

Context
Quantiative MRI is becoming increasingly relevant in the era of precision medicine. Current clinically used MRI protocols are still mostly limited to weighted images, such as T1-weighted or T2-weighted. This delivers images optimized for visual inspection by a radiologist that is looking for structural abnormalities. However, these images do not provide measurements of the actual magnetic resonance properties of the tissue; e.g. the T1 or T2 relaxation time, or the diffusion or perfusion rate. Also, there might be substantial variability in the images between scanners, or even from the same scanner at different moments in time. This lack of standardization hampers the detection of subtle diffuse, disease induced, changes in the tissues. The key objective of quantitative MRI is to complement the qualitative images with quantitative measurements of tissue properties.
Top Publications 2021
Zhang C, Klein S, Cristobal-Huerta A, HernandezTamames JA, Poot DHJ. APIR4EMC: Autocalibrated parallel imaging reconstruction for extended multicontrast imaging. Magn Reson Imaging. 2021
Sabidussi ER, Klein S, Caan MWA, Bazrafkan S, den Dekker AJ, Sijbers J, Niessen WJ, Poot DHJ, Recurrent inference machines as inverse problem solvers for MR relaxometry, Media 2021
Nunez-Gonzalez L, Kotek G, Gómez PA, Buonincontri G, Vogel M, Krestin GP, Poot DHJ, Hernandez-Tamames JA, Accuracy and repeatability of QRAPMASTER and MRF-vFA, Magn Reson Imaging. 2021
Research Projects: Objectives & Achievements
My research line focusses on quantitative MRI reconstruction, in close collaboration with the MR physics group of J.A. Hernandez-Tamames (page 42) as well as the image registration group of S. Klein (page 118). The aim of quantitative MRI is to objectively measure tissue properties such as for example the T1, T2(*) relaxation times or tissue perfusion. This is done by acquiring several images with specific differences in their acquisition settings. The intensity of the acquired images is fitted to a model that is derived from the MR physics of the acquisition method.
Acceleration of acquisition
Within the European Horizon2020 B-Q MINDED project, we are accelerating quantitative MR imaging to overcome the long acquisition time of usual quantitative MRI approaches. Traditional approaches sample the k-space of each contrast weighted image sufficiently to reconstruct images for each contrast separately. In the B-Q MINDED project we aim to severely under-sample the k-spaces, such that normal image reconstruction fails. By using the known relations among the different contrast weightings in the reconstruction process we increasingly are able to still obtain high-quality images and tissue property maps.
Motion compensation
Subject motion is a major cause of low quality or failed MRI exams. For quantitative MRI acquisitions this situation is even worse, as typically they are longer than the acquisition of traditional weighted images. Additionally, in the analysis, images are combined, increasing the sensitivity to motion. Hence a major focus of my research line is to compensate for subject motion. By exploiting the known relations among the images as well as by adjusting the acquisition to acquire some reference data to allow identification and subsequent compensation of unavoidable subject motion.
Acceleration of reconstruction
The advanced methods that we develop for high quality reconstruction from highly accelerated scans may have long computation times. Even though computers get faster every year, innovative methods to improve computation time are needed. With novel work on deep learning based image reconstruction we are accelerating the reconstruction process to achieve clinically acceptable reconstruction times for the advanced methods.

In-vivo T1 map, where the novel deep learning method Recurrent Inference Machine (RIM) outperforms the standard estimation procedure (MLE) as well as a reference deep learning method (ResNet).
Expectations & Directions
In the upcoming years, we aim to further develop the quantitative MR image acquisition and reconstruction methods. In parallel the novel developments will be applied in clinical research projects for further evaluation.
Funding
Jan Sijbers (Universiteit Antwerpen), Wiro Niessen, Dirk Poot, Stefan Klein, and consortium partners: EU Horizon2020 MSCA-ITN, 2018-2022: “B-Q MINDED –Breakthroughs in Quantitative Magnetic resonance ImagiNg for improved DEtection of brain Diseases”.
Marion Smits, Thijs van Osch, Juan A. Hernández-Tamames, Stefan Klein, Dirk H.J. Poot, Vascular signature mapping of Brain Tumor Genotypes, NWO-TTW
Highlights
Riwaj Byanju published his first paper
Emanoel Sabidussi published his first paper
Chaoping Zhang published his second paper
6 MSc/BSc students worked on a project in this theme

OPTIMAL PARAMETER ESTIMATION FROM
INTRA-SCAN
Advisors Dirk Poot & Stefan Klein
Project Funding B-QMINDED
Research period April 2018 – March 2022
Email r.byanju@erasmusmc.nl
The main obstacle in the clinical implementation of Q-MRI is the long scan time required. Q-MRI acquisitions require the acquisition of different contrasts, thus also have redundancy in the temporal dimension. Exploiting this can reduce acquisition time. My project aims to improve acquisition settings to reduce acquisition time and make Q-MRI clinically feasible.
Previously, we developed a theoretical metric that can be used to get time-efficient scan settings, which leverages redundancy in the temporal dimension to reduce scan time.
This year, our focus was on Myelin water fraction (MWF), an imaging biomarker of Myelin content. MWF has the potential to be used in the early detection and diagnosis of many brain disorders. Multiple Spin echo (MSE) is
RIWAJ BYANJU, MSC
MODULATED MR DATA/BQMINDED PhD Student
Within this H2020-MSCA-ITN project B-Qminded (bqminded.eu) we collaborate with 4 industrial and 5 academic partners in the Netherlands, Belgium, Germany, and United Kingdom.
considered the gold standard for acquiring MWF maps; however, long scan time has limited its clinical use. We developed advanced reconstruction techniques to produce MWF maps from highly undersampled MSE scans to reduce scan time. The reconstruction technique compliments the undersampled data by sharing information across the contrasts.
We extended our reconstruction technique to GRASE acquisition (developed in the MR physics group of Juan) for even faster scans of MWF maps. Using highly accelerated GRASE scans could potentially enable the acquisition of MWF maps in clinically acceptable acquisition times. in-vivo scans have shown promising results. Further verification with volunteer scans that will compare it with the reference MSE and state-of-the-art acceleration technique is planned.

T1 map, acceleration factor 32x enabled by using redundancy in the contrasts.

B-Q MINDED: ADVANCED DEEP LEARNING METHODS FOR QUANTITATIVE MRI
EMANOEL R SABIDUSSI, MSC
PhD Student
Advisors Dirk Poot, Stefan Klein & Wiro Niessen
Project Funding H2020 MSCA ITN – B-Q MINDED
Research period September 2018 – 2022
Email e.ribeirosabidussi@erasmusmc.nl
This project is hosted by the Biomedical Imaging Group Rotterdam. Within the H2020-MSCA-ITN project B-Q Minded (bqminded.eu) we collaborate with 4 industrial and 5 academic partners in the Netherlands, Belgium, Germany, and United Kingdom.
Current use of MRI is mostly limited to the evaluation of weighted images: qualitative contrast-based images that are optimized for visual analysis. However, the image contrast in conventional MRI is influenced by a number of external factors besides tissue specific properties, which limits the reliable characterization of their magnetic properties.
The goal of my research is to develop novel deep learning methods that allow the processing of weighted images into quantitative parametric maps. These quantitative maps measure the inherent magnetic resonance properties of the tissues and are, therefore, much more sensitive to changes in physiology than conventional MRI.Previously, we successfully applied the Recurrent Inference Machines (RIMs) to extract the T1 and T2 parameters from weighted images, with lower error than other methods.
This year, we focused on Diffusion MRI, a technique that allows us to infer the micro-structure of biological tissues by analyzing how water diffuses within and between these structures.
Experiments with simulated and real data show that our method is robust to changes in acquisition protocols, and, in many cases, have lower error than the current state-ofthe-art in Diffusion Tensor Imaging.

Diffusion Tensor Experiments with dtiRIM and MRTrix3 (IRLLS). 4 figures on top show results for simulation data. In each, the bottom rows are results from dtiRIM. We observe a much lower bias and standard deviation for both FA and MD with the dtiRIM. The bottom figure shows Direction Color Encoded FA map for both methods in an in-vivo datset.

ADVANCED RECONSTRUCTION METHODS FOR QUANTITATIVE MRI
CHAOPING ZHANG, MSC
PhD Student
IAdvisors Wiro Niessen, Stefan Klein & Dirk Poot
Project Funding China Scholarship Council (CSC)
Research period September 2015 – January 2020
Email c.zhang@erasmusmc.nl
maging time is an important issue in MRI, for patient comfort, scanning cost, and reduction of potential subject motion. In Chaoping Zhang’s study, he aims to develop motion compensation method and fast imaging techniques including APIR4GRASE, APIR4EMC, APIR-Net. In this year, he further validated the APIR4EMC technique.
APIR4EMC aims at a faster or higher image quality reconstruction for multi-contrast imaging. Multi-contrast images of the same region, like T1, T2, proton density, and FLAIR images, are routinely acquired in clinical MRI, and share the same anatomy. Thanks to the compensation of signal evolution along the echo train of different contrasts, APIR4EMC is able to exploit the signal correlation among contrasts to improve the image quality of the reconstruction, and thus facilitate acquisition with higher subsampling factors. Compared to the conventional single contrast reconstruction method GRAPPA, with the same accelerated acquisition, APIR4EMC is able to reduce artifacts and improve SNR of the reconstructed images for all contrasts, and thus enables increased acceleration capability for multi-contrast acquisitions.
In the in-vivo experiments, using the optimized k-space sampling pattern, APIR4EMC achieved good quality reconstruction with 1 mm3 isotropic 3D brain acquisition with contrasts of T1, T1-Fatsat, T2, PD, and FLAIR that were acquired in as little as 7.5 min with the acceleration factor of 9.

Figure 1. The reconstructed in-vivo brain images of all contrasts by GRAPPA and APIR4EMC with subsampling factors 6 and 9. For each of them, one axial slice is shown. Red arrows point to failed GRAPPA reconstructions with strong aliasing artifacts.
JOINT APPOINTMENT IN TU DELFT
Wiro Niessen is full professor in Biomedical Image Analysis at Erasmus MC and Delft University of Technology. He is Medical Delta Professor, director of the Medical Delta Imaging Institute and leading the Biomedical Imaging Group Rotterdam.
He is Associate Editor of Medical Image Analysis and the International Journal of Computer Assisted Radiology and Surgery. He is Chief Technology Officer of Health-RI, which aims to establish a national health data infrastructure.

He is fellow of the Medical Image Analysis and Computer-Assisted Intervention Society.
Wiro Niessen is co-founder and scientific lead of Quantib BV, an Erasmus MC spin-off which develops AI methods to support image-based disease detection, diagnosis, and therapy planning. Wiro Niessen is member of the Netherlands Royal Academy of Arts & Sciences and winner of the Simon Stevin award, the largest prize for Applied Sciences in the Netherlands. w.niessen@erasmusmc.nl
QUANTITATIVE BIOMEDICAL IMAGING, IMAGING GENETICS & AI IN RADIOLOGY

Context
Advances in imaging devices have drastically increased our capabilities to (non-invasively) study both anatomy and function non-invasively. In addition, imaging data are increasingly complemented with other types of data, including genetic and metabolomics data. With these advances, the sheer size, complexity, and heterogeneity of biomedical (imaging) data has increased enormously, and the challenges to optimally use this information for biomedical research and clinical practice have increased accordingly.
At the same time, methods for the automated analysis of these data have increased tremendously. Especially the analysis of biomedical (imaging) data with artificial intelligence techniques has taken an enormous flight and by 2030 it it expected to have dramatically changed the landscape of the healthcare system.
Top Publications 2021
A. van Hilten, S. A. Kushner, M. Kayser, M.A. Ikram, H. H. Adams, C.C.W. Klaver, W. J. Niessen, G. V. Roshchupkin “GenNet framework: interpretable deep learning for predicting phenotypes from genetic data” Communications Biology, 4(1), 2021
T.J.M. Castillo, M.P.A. Starmans, M. Arif, W.J. Niessen, S. Klein, C. Bangma, I. Schoots, J. Veenland “A multicenter, multi vendor study to evaluate the generalizability of a radiomics model for classifying prostate cancer: high grade versus low grade. Diagnostics 11(2), 2021.
B. Li, W.J. Niessen, S. Klein, M. de Groot, M.A. Ikram, M. W. Vernooij, E. E. Bron “Longitudinal diffusion MRI analysis using Segis-Net: a single step deep-learning framework for simultaneous segmentation and registration.” NeuroImage 235, 2021.
This research group is at the forefront of these developments; its focus is to provide an infrastructure for, and to develop advanced image processing and machine learning techniques to analyse large biomedical imaging resources and clinical imaging data. The ultimate aim of this research is to develop novel diagnostic, prognostic, therapy planning and therapy monitoring tools. In addition, the group develops methods for linking imagederived features to other (e.g. genetic data) and methods for the integrated analyses of these complementary data to understand disease etiology and investigate the potential for disease staging and prognosis using such analyses.
The main activities in the group involve: (i) the development of an IT infrastructure to support large scale image analysis, and re-use of healthcare data for research and innovation (ii) the discovery and development of quantitative imaging biomarkers and their standardized extraction, (iii) the development and implementation of methods for the integrated analysis of imaging and genetic data (imaging genetics and radiogenomics), and (iv) the development and application of radiomics and deep learning techniques to improve tumor classification and therapy selection in oncology.
Research Projects: Objectives & Achievements
Image data science infrastructure
In this research theme we develop and maintain an image data infrastructure to support research and innovation for data-driven health. In 2021, a major milestone was reached by receiving a 69 MEuro national innovation grant by Health-RI (Wiro Niessen is board member of Health-RI) for establishing a national health innovation data infrastructure for re-use of date for research and innovation. The efforts in our group will link to the efforts of Health-RI.
The infrastructure we establish supports multicenter clinical and population imaging studies. Next to facilities to store and access imaging data, and link them to relevant other data (clinical data, genetic data), the infrastructure supports the standardized extraction of quantitative imaging biomarkers. This for example supports research to validate and implement quantitative image analysis techniques to improve the (early and differential) diagnosis and management of neuro-degenerative and neurovascular diseases. We have developed and implemented a library of image processing tools for the accurate and reproducible quantification of brain morphol-
ogy, function, and brain changes, both in development, healthy ageing, and disease. These techniques have been implemented in an IT framework facilitating standardize and reproducible extraction of these imaging biomarkers. By applying these techniques to large population and clinical cohorts, we support research in disease etiology, develop methods for early and differential diagnosis of neurodevelopmental and neurodegenerative disease, and assist in therapy planning and monitoring. This research line has many collaborative efforts with the other research lines in the Biomedical Imaging Group Rotterdam, there exists a strong collaboration with TU Delft as part of Erasmus MC TU Delft convergence, and with TU Delft and Leiden University Medical Center, through the Medical Delta Imaging Institute, with the ESFRI projects BBMRI and EuroBioImaging. The group contributes to valorization of research through public private partnerships, and has multiple projects with e.g. Quantib, Philips and GE.
In 2021, with our expertise and infrastructure, we supported both the Erasmus MC EraCore study, for observational studies on Covid-19 patients, and further developed a national portal for observational Covid-19 research. This portal is facilitated by harmonizing clinical data across Dutch University Medical Centers. Finally, we have participated in pilot projects to facilitate distributed learning across multiple institutes, both nationally and internationally. In the coming years research and infrastructural activities to facilitate distributed learning in medical imaging and imaging genetics will increase, and we will develop this in collaboration with Health-RI and the Dutch AI coalition.
Imaging Genetics, radiomics and radiogenomics
We have significanlty increased our research into deep learning techniques to directly link genetic data to relevant clinical outcomes. An open source software framework (Gennet) for this has been developed further. In collaboration with NVIDIA we have worked on a federated implementation of this framework, to support multicenter studies in this domain. We have continued our projects in the field of radiomics.
Expectations & Directions
In 2022 will increasingly develop methods for federated analysis, both in our imaging, as in our genetic data analysis project. Also, increasingly we will develop methods for the integrated analysis of multiple data types. In collaboration with Health-RI we will further develop an infrastructure to support the development and valida-
tion of AI methods in a multicenter setting. There will be increased interest in the development of trustworthy AI, in order to facilitate responsible use of novel methods in clinical practice. We will start two new AI labs (ICAI labs) with 10 PhD students as part of the NWO long term programme project. Also, we will start a number of new AI projects in cardiology and neurology. Finally, we will contribute significantly to the convergence initiative with TU Delft and EUR, and we will further contribute to the Erasmus MC and national Covid-19 data portals.
Funding
Wiro Niessen, Gerrit Meijer, Leone Flikweert, Ruben Kok, Health-RI (national) innovation fund grant for health data infrastructure.
Aad van der Lugt, Wiro Niessen, Stefan Klein, Daniel Bos and consortium partners: European Commission Horizon 2020 grant: “EuCanImage: A European Cancer Image Platform Linked to Biological and Health Data for NextGeneration Artificial Intelligence and Precision Medicine in Oncology.”
Niessen, Wiro, Daniel Bos , and consortium partners: NWO Big Data Grant, 2019-2023: “MyDigiTwin: Your Digital Twin to improve early detection and promote risk selfmanagement of cardiovascular disease.”
Wiro Niessen, Ivo Schoots, Jifke Veenland , C. Bangma: TKI MRI prostate project.
Wiro Niessen, Mark van Buchem, Frans Vos: Medical Delta 3.0 Dementia & Stroke 2018 – 2022
Theo van Walsum, Wiro Niessen, Stefan Klein and consortium partners 2018-2021: H2020 Project Merlin
Aad van der Lugt, Wiro Niessen and consortium partners: CVON project CONTRAST: 2017-2022.
Niessen, Wiro, Ivo Schoots, Jifke Veenland, Stefan Klein and consortium partners: STW Perspectief programme “STRATEGY” (radiomics; two STW projects)
Vernooij, Meike, Wiro Niessen, Stefan Klein and consortium partners: EU H2020 project EuroPond 2016-2021
Niessen, Wiro: Simon Stevin Meester award; 2015-2022
Wiro Niessen, Aad van der Lugt 2011 – 2021 BBMRI 2.0 project “Linking population imaging and biobanking”
Invited Lectures Wiro Niessen
Dec 2021: Netherlands Royal Science Academy: “Towards a National Institute for Data/Model driven Life Sciences”
November 2021: “Health-RI, Health-data infrastructure for research and innovation”, Digital Health Summit, Munich.
November 2021: “Medical Image Analysis with AI for Improved Disease Diagnosis and Prognosis”, Guus Schrijvers Academie, Ede-Wageningen.
October 2021: “How big data an AI facilitate precision Medicine”, G-STIC conference, Dubai (hybrid).
October 2021: Keynote Prinses Maxima Center conference “ Medical Image Analysis with AI for Improved Disease Diagnosis and Prognosis”
October 2021: How Big Data & AI Facilitate Precision Medicine, the Medical Imaging Perspective, Bataafsch Genootschap Steven Hoogendrijk Prijs symposium lecure, Rotterdam.
September 2021: “How Big Data & AI Facilitate Precision Medicine, the Medical Imaging Perspective”: AI Automata conference, Zurich (remote).
September 2021: “AI, machine learning and deep learning”, Conference RIVM: population research on cancer, online.
May 2021 “The role and impact of R&D synergies between academia and industry in Europe, SIIM conference, USA (online).
April 2021 “Towards the responsible introduction of AI in clinical practice; applications in neurology and oncology”, Invited lecture Vanderbilt University
March 2021 “AI for the interpretation of medical imaging and genetic data: opportunities and challenges to translate to clinical practice” Dutch-Israel AI symposium (online).
March 2021 “AI for prostate cancer diagnosis and prognosis on multiparametric MRI, American College of Radiology meeting (online).
January 2021, Keynote Lecture Kindersymposium “Biomedical imaging and genetic (BIG) data analysis with AI for precision medicine” AMC, Amsterdam.
• Health-RI, a national initiative towards a data infrastructure for re-use of health data for research and innovation received a grant of 69 MEuro of the innovation fund. Wiro Niessen is board member of Health-RI and was co-PI of the grant proposal
• A biologically interpretable neural network architecture for phenotype prediction was developed using knowledge such as gene annotations and meQTL data, to connect gene-expression and methylation data to their corresponding genes
• First step towards multicenter distributed development and deployment of AI tools was achieved in a collaboration with the Netherlands AI Coalition.
Additional Personnel Highlights
Marcel Koek, MSc
Marcel Koek, MSc, is leading the IT infrastructure group, with the main focus to support the standardized extraction of quantitative imaging biomarkers from multicenter imaging studies, and linking the derived measures to other data.

Hakim Achterberg, MSc
Hakim Achterberg, MSc, is IT architect and scientific programmer, contributing to the BIGR IT infrastructure. Also, he is has large expertise in image analysis workflows and neuro image analysis.

Adriaan Versteeg
Adriaan Versteeg is scientific programmer, contributing to the BIGR IT infrastructure. He has large expertise in XNAT, image analysis workflows and neuro image analysis.




Andrea Gutierrez
Andrea Gutierrez is scientific programmer, contributing to the BIGR IT infrastructure to support multicenter biomedical imaging research projects.
Mahlet Birhanu
Mahlet Birhanu is scientific programmer, contributing to the BIGR IT infrastructure. She has large expertise in medical image analysis.
Ivan Bocharov
Ivan Bocharov is scientific programmer, contributing to the BIGR IT infrastructure to support multicenter biomedical imaging research projects.
IMAGE ANALYSIS IN ONCOLOGY
JIFKE VEENLAND, PHD
Assistant Professor
JOINT APPOINTMENT IN MEDICAL INFORMATICS

Jifke Veenland obtained her MS degree in Medicine at Groningen University and her MS degree in Informatics at Leiden University. She received her PhD degree at the Erasmus University Rotterdam (Depts of Radiology and Medical Informatics) at 1999. She worked from 1997 as a researcher in automated image analysis at KPN Research before she joined in 2000 the image processing group of the Departments of Radiology and Medical Informatics. Focus in research is on tissue characterization and quantification of heterogeneity in MRI tumor images.
Next to that, she is coordinator of the MSc track Imaging & Interventions of Technical Medicine, EMC-coordinator of the MSc Technical Medicine, project leader of the ErasmusArts 2030 Technology group, coordinator of the modules Imaging and Image Processing for the BSc Clinical Technology. For the MSc Technical Medicine, she coordinates the modules Advanced Image Processing and Machine Learning. j.veenland@erasmusmc.nl
In cancer imaging, advances in MR hardware and software have resulted in the ability to visualize biochemical processes superimposed on anatomic images. For example, the oxygenation status, the acidity, the Brownian motion of water molecules and the blood perfusion can be imaged. Currently, there is a strong interest in determining the value of these functional characteristics as non-invasive biomarkers to evaluate treatment response and outcome. In order to sensitively and reproducibly measure, changes in, these functional parameters, robust and automated processing tools are needed. This research line aims to develop and evaluate image processing techniques for visualization, quantification and integrated analysis of anatomical and functional cancer imaging data. This work is performed in close collaboration with Radiology, Nuclear Medicine, Radiotherapy and Urology. Different research lines are defined 1) radiomics and 2) developing biomarkers
Radiomics for Prostate Cancer
Prostate cancer (PCa) is the most common malignancy and second leading cause of cancer-related death in men. High-grade PCa has the highest potential to metastasize and these patients are therefore faced in general with the poorest prognosis. Patients with low-grade PCa usually die of other causes than PCa.
Overdiagnosis of low-grade PCa and consequently overtreatment is a significant problem in current practice. Therefore accurate discrimination between high- and low-grade PCa is critical for risk stratification and clinical decision-making. Such discrimination should be based on prognostic biomarkers. Since PCa can display significant intra-tumor heterogeneity, the challenge is to characterize the tumor tissue phenotype and genotype in order to locate the high-grade tumor foci or dominant lesion(s). Multiparametric MRI (mpMRI) visualizes and quantifies cell density and tissue perfusion/permeability in a non-invasive manner. Using texture analysis methods, the local environment in the tumor can be quantified, yielding a wealth of Radiomics features, and aid in discriminating normal or benign tissue, low-grade and high-grade PCa. By establishing a 3D-correspondence between tumor histology and mpMRI, the selection of Radiomics features that quantify most accurately and robust the cancer tissue becomes feasible. Robust and accurate imaging biomarkers may help in further risk stratification and clinical decision-making and may lead to reduction in overdiagnosis and overtreatment. In this project we aim to develop a high risk PCa classifier based on mpMRI radiomic features.

Figure 1. The top row shows in overlay the ground truth (in red) as delineated by the radiologist and proven by targeted biopsy as significant PCa. The lower row shows the segmented significant PCa lesion (in pink) by the CNN model. All images show the same axial slice as 2D view of mpMRI images (a+e T2w images, b+f DWI b800, c+g ADC map) of the prostate with the reference ground truth (d) and the segmented PCa lesion by the model (h).
Multi-Center, Multi-Vendor study to evaluate the generalizability of a radiomics model for classifying prostate cancer
Radiomics applied in MRI has shown promising results in classifying prostate cancer lesions. However, many papers describe single-center studies without external validation. The issues of using radiomics models on unseen data have not yet been sufficiently addressed. The aim of this study is to evaluate the generalizability of radiomics models for prostate cancer classification and to compare the performance of these models to the performance of radiologists. Multiparametric MRI, photographs and histology of radical prostatectomy specimens, and pathology reports of 107 patients were obtained from three healthcare centers in the Netherlands. By spatially correlating the MRI with histology, 204 lesions were identified. For each lesion, radiomics features were extracted from the MRI data. Radiomics models for discriminating highgrade (Gleason score ≥ 7) versus low-grade lesions were automatically generated using open-source machine learning software. The performance was tested both in a single-center setting through cross-validation and in a multi-center setting using the two unseen datasets as external validation. For comparison with clinical practice, a multi-center classifier was tested and compared with the Prostate Imaging Reporting and Data System version 2 (PIRADS v2) scoring performed by two expert radiologists. The three single-center models obtained a mean AUC of 0.75, which decreased to 0.54 when the model was applied to the external data, the radiologists obtained a mean AUC of 0.46. In the multi-center setting, the radiomics model obtained a mean AUC of 0.75 while the radiologists obtained a mean AUC of 0.47 on the
same subset. While radiomics models have a decent performance when tested on data from the same center(s), they may show a significant drop in performance when applied to external data. On a multi-center dataset our radiomics model outperformed the radiologists, and thus, may represent a more accurate alternative for malignancy prediction.
Validation comparing deep learning and radiomics
Many methods to improve significant prostate cancer diagnosis (PCa) are being reported in literature. These methods are based either on deep learning or radiomics. However, there is lack of scientific evidence comparing these methods on the same external validation sets. The aim of this study was to compare the performance of a deep learning model with the performance of a radiomics model for significant PCa diagnosis on various cohorts. We collected multiparametric Magnetic Resonance images and pathology data from four patient cohorts (644 patients in total). One of the cohorts was used to develop a deep learning model and a radiomics model. Both models were tested on the three remaining cohorts. The comparison shows that whereas the performance is similar on the training cohort, the radiomics model outperformed the deep learning model in all the testing cohorts, making it a more accurate tool to detect clinical significant prostate cancer.
Matching MRI with histology: detecting cribriform growth
Recently is has been shown that prostate cancer with cribriform growth, detected in histology, is associated with adverse clinical outcomes. Magnetic resonance imaging (MRI) has considerable potential in non-invasive tumor characterization, as a multitude of sequences can be employed. In this project, we aim to identify the radiomic properties of cribriform lesions.
DEVELOPING NEW IMAGING BIOMARKERS
BASED ON QUANTITATIVE, PHYSICAL MODELS
FRANS VOS, PHD
Assistant Professor
JOINT APPOINTMENT AT TU DELFT

Frans Vos (1969) obtained his MSc both in Computer Science and Medical Informatics from the University of Amsterdam in 1993. He performed his graduation work at Yale University in the Biomedical Imaging Sciences Division, where he was supervised by Jim Duncan. In 1998 he got his PhD from the Vrije Universiteit of Amsterdam. Frans envisions that improvements in medical imaging are needed to enhance disease prevention, early diagnosis, and the effectiveness of therapy. All techniques developed under his supervision are characterized by clever modelling, incorporation of prior (clinical) knowledge and relying strongly on the physical principles of image formation. He has a strong background in the quantitative analysis of MR images and the extraction of imaging biomarkers. Frans is currently associate professor with both the Department of Imaging Physics at Delft University of Technology and the Department of Radiology at the Erasmus MC. He is (joint-) head of the section Medical Imaging at TU Delft and coordinator of the track medical physics of the master’s programme in biomedical engineering at TU Delft. His primary aim is to bridge the gap between more fundamental research into the physics of imaging techniques and their application in clinical practice. f.m.vos@tudelft.nl
2021 was the year during which important steps were made in several projects in which the Departments of Imaging Physics at TU Delft and Radiology at Erasmus MC collaborate. What is more, approximately 10 undergraduate students in biomedical engineering and applied physics from TU Delft graduated on master-thesis projects in the Department of Radiology. Furthermore, several new research projects were started in which undergraduate and PhD students, postdocs, technical and clinical researchers and clinicians from both institutions collaborate.
Developing biomarkers of radiationinduced toxicity in brain tumour patients based on advanced MRI
Photon radiation is commonly used in radiotherapy, although it can have a detrimental effect on the healthy tissue surrounding the treatment site. In particular, it has been shown to harm the microvasculature of healthy brain tissue, which is at the basis of damage to the white matter microstructure.
In 2020 the RIGEL study (Radiotherapy in IDH mutated glioma) started in which patients are imaged prior to and after radiotherapy. Specifically, Dynamic Contrast Enhanced (DCE), Dynamic Susceptibility Contrast (DSC) are applied

Figure 1. Illustration of AIF measurements in one representative patient in one particular region. This region was manually selected in the DCE images (a) and subsequently projected onto registered DSC images (b). Contrast concentration change measured in the DCE and DSC series in the voxels from the selected region (c) and the mean curve (d). Blue lines are measurements from DSC MRI (left y axis); red lines represent measurements from DCE MRI (right y axis).
for measurement of properties of the microvasculature. Ultimately, the project aims at creating new MR imaging and image analysis techniques for the development of biomarkers of radiation toxicity solving limitations of currently available methods.
In 2021, we performed combined DCE and DSC imaging in five patients suffering from low-grade glioma included in the RIGEL study. The estimation of tissue properties from these data is based on indicator dilution theory and is driven by the measurement of the Arterial Input Function (AIF). The AIF represents the time varying contrast agent concentration supplied to the tissue and is usually measured in a feeding artery. An important issue, however, is that measuring the AIF’s absolute contrast agent concentration is not possible due to uncertainties in the relation with the measured R2*-weighted signal. We used the presently included patients to compare (1) the AIF determined from DCE-MRI with the AIF from DSC MRI; (2) the estimated perfusion properties from DSC data based on a DCE-driven AIF with estimation of those properties using a DSC-based AIF.
We have found that the DCE-derived AIF significantly correlates with DSC-derived AIF, and yields more stable measurements than the DSC-derived AIF among different voxels in the same artery (less variation in peak height while sharing similar patterns).This might indicate that the quantitative DCE AIF could replace theDSC-driven AIF in order yield more stable estimation. In this project there is close collaboration between the Departments of Radiology (Vos, Smits) and Radiotherapy (Jaspers, Mendez-Romero) at EMC, the Department of Imaging Physics at TU Delft (Vos, Tseng), and the Gorter Center at LUMC(Van Osch).
Multi-component parameter mapping in quantitative MRI
Quantitative MRI (qMRI) methods estimate physical parameters that underlie the MR signal. These parameters can describe processes such as magnetization relaxation, water diffusion, blood perfusion, and oxygen consumption. Changes in such properties of tissues have been linked to various pathologies. Magnetic Resonance Fingerprinting (MRF) is a recently introduced paradigm to acquire multiple of such parameters within a short scan time. This project on multiparametric MRI mapping focuses on solving issues related partial volume effects. We target to achieve this by assuming that only a limited number of tissue components are present across a large imaging volume (e.g. the brain).
In 2021 a evaluation study was performed using previously acquired data to assess the repeatability of our method. These data concern 5 subjects that are scanned 8 times with the same fingerprinting protocol, making it possible to assess the repeatability. We have found that MRF provides accurate and precise tissue relaxation parameter estimations taking into account intrinsic partial volume effects. It facilitates obtaining tissue fraction maps of prevalent tissues including myelin water which can be relevant for evaluating diseases affecting the white matter.
Furthermore, we developed efficient algorithms to obtain multi-component MR fingerprinting (MC-MRF) estimations directly from highly undersampled data. We found that our new methods come with decreased sensitivity to undersampling artifacts and improved noise resilience compared to existing techniques. In this project clinical researchers from the Department of Radiology at EMC (Vos, Poot, Tamames) collaborate with technical researchers from the Deparment of Imaging Physics at TU Delft (Vos, Nagtegaal) and the Gorter Center at LUMC (Van Osch, De Bresser).
What lies ahead in 2022?
In the coming year we expect a new TU Delft-Erasmus MC convergence project on Quantitative Susceptibility MRI to accelerate. This project particularly aims at advancing two clinical research areas: 1) Quantitative functional imaging of brain activity for presurgical planning of brain tumor resection and 2) microvascular integrity in cardiomyopathies.
We expect that in 2022 a postdoctoral researcher will start to work on this topic in the Department of Imaging Physics at TU Delft. In this program the Department of Radiology (Tamames, Vos, Smits, de Bruijne) works intensely together with the Department of Imaging Physics at TU Delft (Vos, Weingartner). As such this is a clear example of how technical knowledge and clinical expertise can strengthen each other. What is more, similar collaborative projects are expected to be initiated in 2022, especially on Deep Learning.

Figure 2. Maps representing ground truth volume fractions (i.e. amount of tissue) in voxels across different tissue type (top), characterized by their particular T1 and T2 constants. Subsequent rows represent reconstruction erros with a state-of-art reference method (LR inversion), and two methods developed by us (MCADMM, k-SPIJN). Colors represent relative error. Observe the decrease root mean square error (RMSE).
TISSUE
LABELING AND TRACTOGRAPHY IN THE FIELD OF CRANIOSYNOSTOSIS
HENRI VROOMAN, PHD
Assistant Professor

Henri A. Vrooman was born in Rotterdam on April 2, 1959. He received the M.Sc. degree in physics (1986) and the Ph.D. degree in physics (1991) from Delft University of Technology (TU-Delft), the Netherlands. From 1986 to 1990, he was a Research Scientist at the Department of Image Processing and Pattern Recognition of the TUDelft. During that period he carried out research in the field of quantitative speckle interferometry. From 1990 until 2000 he was Assistant Professor at the Laboratory for Clinical and Experimental Image Processing of Leiden University Medical Center (LUMC), where he was involved in a number of medical image processing projects in cooperation with the Department of Radiology and other clinical departments of the LUMC. Since 2000 Henri is Assistant Professor at the Erasmus MC - University Medical Center Rotterdam, the Netherlands, where he started the Biomedical Imaging Group Rotterdam (BIGR). His research interests include digital image processing, pattern recognition, biomedical image processing techniques and diagnostic radiology. Recent focus is of his work is on neuroimaging and on the development of infrastructures for the processing of large data sets, especially important for Population Imaging.
h.vrooman@erasmusmc.nl
The year 2021 was a year with a lot of progression of our craniosynostosis projects. Firstly, we were able to develop a processing pipeline for the segmentation of neuro-tracts in controls and trigonocephaly patients. FSL preprocessing tools and autoPtx (open source tool) were used for this purpose. Because of inverse contrast between white and gray matter in young brains, and because of present pathology (deformed brain and skull), it took special efforts to produce the optimal processing steps. Secondly, two students from the Technical University Delft successfully finished their master thesis on the improvement of tissue segmentation tools for brain MRI images of young children and craniosynostosis patients. Atlas registration methods and tissue labeling algorithms were developed, on our CPU-cluster as well as with Deep Learning on our GPUs.
Diffusion Tensor Imaging of the frontal lobe
Children with trigonocephaly are at risk for neurodevelopmental disorders. It is, however, unclear whether neurodevelopmental disorders are caused by the outcome of an inborn brain anomaly or by restriction of brain development due to the fused metopic suture. Insight into the
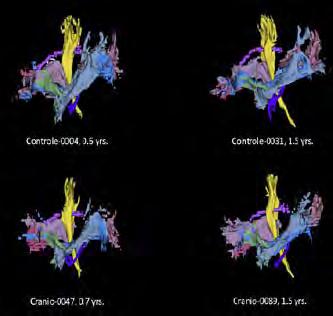
1. Tract Visualization. Segmentation of neuro-tracts in infant brains (controls and craniosynostose patients), computed by DTI-preprocessing and the open source autoPtx tool.
Figure
white matter microarchitecture of the unoperated brain of trigonocephaly patients can shed light on the pathogenesis of the related problems. The aim of this study was therefore, to investigate white matter properties of the frontal lobes in young, unoperated patients with metopic synostosis as compared to healthy controls using Diffusion Tension Imaging (DTI). Datasets of 46 patients with trigonocephaly with a median age of 0.49 years, for whom a surgical correction was considered, were compared with 22 controls.
White matter metrics derived from diffusion imaging of the following tracts in the frontal lobe were calculated using the FMRIB Software Library (FSL): the anterior thalamic radiation (ATR), cingulate gyrus part of the cingulum (CGC), uncinate fasciculus (UNC), the forceps minor (FMI) and the inferior fronto-occipital fasciculus (IFO). The Forceps major (FMA) was used as a control tract. The mean value of tract-specific fractional anisotropy (FA) and mean diffusivity (MD) were estimated for each subject and compared to healthy controls. By linear regression FA values per tract were assessed with trigonocephaly, sex, age and in a separate analysis volume as independent variables. A similar analysis was undertaken for the MD values.
Image registration for atlas-based analysis of brain regions
A brain atlas consists of one or multiple reference scans (e.g. T1w/T2w), each containing a set of labels belonging to the different brain regions. The atlas represents a brain with certain characteristics, such as a certain age. The atlas scan and corresponding labels can be registered to a patient image to obtain the labels for the individual patient.
Many adult brain atlases are available for atlas-based image registration; however, the availability of infant brain atlases is limited due to difficulties with manual segmentation. This is mostly caused by low tissue contrast, high image noise and movement artefacts. An atlas containing both T1w, T2w scans and a detailed parcellation map at different time points between the ages of 0 and 2 years is preferable. However, such an atlas is still missing. Also, no atlases are yet available that take any pathological cases into consideration. Therefore, atlases of healthy infants and healthy adults were used by our students.
We compared conventional and deep learning registration methods in a quantitative evaluation using synthetic data (i.e. deformed atlases) and in a qualitative experiment using registration of atlas scans to craniosynostosis scans. In addition to comparing registration methods, we evaluated the influence of using both T1-weighted and

T2-weighted scans and using an infant or adult atlas. Our qualitative results showed that head shape was registered well by both the conventional and the deep learning registration method, while the deep learning method performed better regarding registration of the ventricles. Quantitatively, our results showed that white matter structures were registered well. Regarding registration of the cortical brain regions, both methods resulted in a sub-optimal accuracy. We obtained the best results using the deep learning approach, probably as prior spatial information is incorporated in the training process.
Provision of image analysis and data management as a service
Finally, we had some nice professionalization of our Imaging Office in the last year. The Imaging Office is embedded in our department, and offers support for all medical imaging-related questions by clinicians, scientific researchers and companies.
The Imaging Office supports medical projects from planning to execution phases, and targets parties that would need access to medical imaging data and analysis tools. So, this includes service and support related to acquisition, storage and advanced analysis of radiological imaging data.
Figure 2. M-CRIB infant brain atlas with 100 labels. T1-weighted images (1), T2-weighted-images (2), and atlas images (3).

GENNADY ROSHCHUPKIN, PHD IMAGING GENETICS
Post-Doc
Project Funding Simon Stevin Meester; ERC Starting Grant
Research period March 2021 – March 2022
Email g.roshchupkin@erasmusmc.nl
This project is collaboration between the Departments of Radiology & Nuclear Medicine and Epidemiology.
Gennady is leading “Computational Population Biology” group in departments of Epidemiology, Radiology and Nuclear Medicine. His research focused on developing and application of methods for the integrative analysis of large-scale biological, epidemiological and clinical data. The group uses the latest approaches in machine learning, genomics, medical imaging and statistics to sort through increasingly rich and massive amount of data. The goals are to improve the understanding of how various omics affect the complex traits and to make use of such insights to improve the diagnosis, prevention and treatment of diseases whenever possible.
Since 2019 Gennady is chairing Machine Learning working group in The Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium.
Since 2021 Gennady is also leading Bioinformatics Working group in Genomics of MusculoSkeletal traits Translational Network. In 2021 Gennady, among others highly promising young scientists, got VENI grant from the ZonMw to further elaborate their own ideas during a period of three years. This grant will allow Gennady to work on his ideas regarding: Explainable Artificial Intelligence to unravel genetic architecture of complex traits.
While we have learned that most diseases have a genetic component, we are still far away from understanding the underlying processes. Using Artificial Intelligence, Gennady will investigate the complex relationship between DNA mutations and human health. This will be the basis for development of novel diagnostic, prognostic and therapeutic tools.


DEDICATED RADIOMICS FEATURES FOR GRADING PROSTATE CANCER
JOSE CASTILLO TOVAR
PhD Student
Advisors Wiro Niessen, Jifke Veenland & Ivo Schoots
Project Funding Netherlands Organisation for Scientific Research – TTW (project number 14932)
Research period May 1 2020 – Jan 2022
Email j.castillotovar@erasmusmc.nl
Prostate cancer (PCa) is the most common malignancy and second leading cause of cancer-related death in men. Whereas low-grade PCa are mostly indolent or slow growing tumors, high-grade PCa have the highest potential to metastasize and these patients are therefore faced with the poorest prognosis. Therefore, accurate discrimination between high- and low-grade PCa is critical for risk stratification and clinical decision-making.
In clinical practice, tumor grading is performed on prostate biopsy specimen, obtained by an invasive procedure of blinded systematically taken ultrasound guided biopsies. This procedure is associated with significant overdiagnosis and comorbidity.
Computed-aided diagnosis systems to improve significant prostate cancer diagnosis (PCa) are being reported in literature. These methods are based either on deep learning or radiomics. However, there is lack of scientific evidence comparing these methods on the same external validation sets.
During the last period we compare the performance of a deep learning model with the performance of a radiomics model for significant PCa diagnosis on various cohorts. We collected multiparametric Magnetic Resonance images and pathology data from four cohorts. One of the cohorts was used to develop a deep learning model and a radiomics model. Both models were tested on the three remaining cohorts. The comparison shows the radiomics model outperformed the deep learning model in all the testing cohorts, making it a more accurate tool to detect clinical significant prostate cancer.

Figure 1. ROC curve showing the performance of the Deep neural network (NN) and radiomics model on the external data sets.

COMPUTATIONAL AND IMAGING GENETICS
ARNO VAN HILTEN, MSC
PhD Student
Advisors Wiro Niessen & Gennady Roshchupkin
Project Funding Simon Stevin
Meester
Research period June 2018 – June 2022
Email a.vanhilten@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Epidemiology.
Predicting individual genetic risk is required for a future with precision and personalized medicine. Currently genetic risk predictions are mostly done by linear methods. Neural networks, state-of-the art predictors in many domains, are rarely used in population genetics.
During my PhD I developed GenNet, a framework for predicting traits and complex diseases using neural networks. In this framework, interpretable and memory-efficient neural network architectures are constructed by embedding biological knowledge from public databases, resulting in neural networks that contain only biological plausible connections (see figure). Additionally, the networks provide more information since they are interpretable. We can gain additional knowledge, for example how important a certain gene or pathway is, by inspecting the weights of the network. Similar to synapses in the brain, important connections are stronger and have higher weights.
This year we expanded the framework to use the whole genome. Additionally, we applied its principles to multi-omics data and finally we showed, using NVIDIA FLARE for distributed learning, that we can train a neural network with data from multiple sites without sharing individual level data. This allows us to train with more data, which will lead to better genetic risk prediction models, without having to worry about data security and privacy.

Figure 1. Schematic of distributed learning (Source NVIDIA). First a copy of the global model is sent to each institute. Each institute trains its neural network and sends model parameters back to the central site. The central site aggregates and updates its central model and sends the new model back for a new round until the global model does not improve further.
JOINT APPOINTMENT IN UNIVERSITY OF COPENHAGEN
Marleen de Bruijne is Professor of AI in Medical Image Analysis at Erasmus MC and University of Copenhagen. She received an MSc degree in physics (1997) and a PhD degree in medical imaging (2003), both from Utrecht University. Before joining the University of Copenhagen (2007) and Erasmus MC (2008) she was assistant professor and later associate professor at the IT University of Copenhagen. Marleen has (co-)authored 230 peerreviewed full papers in international conferences and journals , holds 7 patents, is the recipient of the NWO-VENI, NWO-VIDI, NWO-VICI, and DFF-YDUN awards, and is elected fellow of the MICCAI Society. She is program chair of MIDL 2020, MIDL

2021 and MICCAI 2021 and general co-chair of IPMI 2023. She is chair of the EMBS TC on Biomedical Imaging and Image Processing, and member of the IPMI and MICCAI boards, ISBI steering committee, and editorial boards of IEEE Transactions on Medical Imaging, Medical Image Analysis, Frontiers in ICT - Computer Image Analysis, and MELBA. Her research is in machine learning for quantitative analysis of medical images and computer aided diagnosis, with applications in pulmonary-, neuro-, and cardiovascular imaging. Next to the researchers introduced in the following pages,Marleen co-supervises Laurike Harlaar and Qianting Lv (Erasmus MC).
marleen.debruijne@erasmusmc.nl
AI IN MEDICAL IMAGE ANALYSIS
MARLEEN DE BRUIJNE, PHD
full professor

Context
The “AI in Medical Image Analysis” research line develops novel techniques for quantitative analysis of medical images, with a focus on machine learning – and especially deep learning –techniques and on large-scale image-based studies. An important theme is the development of machine learning techniques to predict disease directly based on imaging data. Using prediction models derived from a database of images for which the diagnosis has already been established or for which the future course of the disease is known from clinical followup, such techniques are more widely applicable and often more sensitive and robust than conventional image analysis methods. Another important theme is the development of robust and fair image analysis models based on clinically realistic situations. Machine learning techniques often work well on large, well-curated, fully annotated datasets, but how do we learn reliable models if datasets are small or heterogenous and have few, weak, or noisy annotations?
Currently our main application areas are in neuro-, vascular-, and pulmonary image analysis.
Top Publications 2021
Bortsova, G .; González-Gonzalo, C.; Wetstein, S. C.; Dubost, F.; Katramados, I.; Hogeweg, L.; Liefers, B.; van Ginneken, B.; Pluim, J. P. W.; Veta, M.; Sánchez, C. I. & de Bruijne, M . (2021), 'Adversarial Attack Vulnerability of Medical Image Analysis Systems: Unexplored Factors', Medical Image Analysis , 102141.
Gamechi, Z. S.; Arias-Lorza, A. M.; Saghir, Z.; Bos, D. & de Bruijne, M. (2021), 'Assessment of fully automatic segmentation of pulmonary artery and aorta on non-contrast CT with optimal surface graph cuts.', Medical Physics
S. Chen, Z. Sedghi Gamechi, F. Dubost, G. van Tulder, and M. de Bruijne, “An end-to-end approach to segmentation in medical images with CNN and Posterior-CRF,”Medical Image Analysis , p. 102311, 2021.
Research
Projects:
Objectives & Achievements
We develop novel methods for quantitative analysis of medical images. The focus is on fully automatic analyses, which makes our techniques ideally suited for large-scale imaging studies. We aim to develop techniques that are generic and that will often have multiple applications. For instance, the segmentation approach that we originally developed to segment the airway tree from CT images is now successfully applied to segment the lumen and outer wall of carotid arteries in MR and in ultrasound images and the aorta and pulmonary artery from CT; the appearance models we developed to analyze lung texture are also used in brain structure segmentation and to derive imaging biomarkers of dementia; and the conditional shape models we developed to predict the normal shapes of vertebrae in a patient-specific manner, to be used as a measure for vertebral deformity, were later used to predict the patient-specific heart motion in computer aided coronary interventions.
The different research lines are described briefly below and all group members describe their work in more detail in the next pages.
Pulmonary image analysis
Accurate and reproducible quantification of abnormalities in lung images is crucial to improve our understanding of development and progression of lung diseases, to assess the effect of treatment, and to determine prognosis in individual patients. Pulmonary image analysis at BIGR focuses on measuring structural lung damage in patients with cystic fibrosis (CF) both in very early and in advanced stages ― and on quantifying chronic obstructive pulmonary disease (COPD) from CT images. In close collaboration with researchers at University of Copenhagen and with the Erasmus Lung Imaging Group (ELIG) we have developed techniques to segment and measure the

Fig 1. Dynamic analysis of lung shape and volume provides image markers of diaphragm weakness in patients with neuromuscular disease. (Work by Gijs van Tulder and Laurike Harlaar)
dimensions of lungs, airways, and vessels, texture based methods to quantify parenchymal abnormalities, model-based image registration techniques to monitor localized changes, and motion analysis in dynamic MR and CT sequences of the breathing lungs to study respiratory insufficiency in patients with Pompe disease.
Vascular image analysis
Within this research line, we aim to develop imaging biomarkers of atherosclerosis from different imaging modalities. We work with in- and ex-vivo MRI, CT, ultrasound and histology images, and developed automated methods to segment the carotid arteries as well as different compo-
Fig 2. Fully automated approach to reliably segment the aorta and pulmonary artery from non-contrast CT. (Work by Zahra Sedghi Gamechi)

nents of atherosclerotic plaque including calcification, lipid, fibrous tissue, and intra-plaque hemorrhage. This provides measures of plaque volume and plaque composition, which are known predictors of whether a plaque is likely to rupture or not. We also investigate the value of more advanced imaging features as potential biomarkers; for instance, we found that ultrasound image texture characteristics correlate with future ischemic vascular events. In CT, we also assess the intracranial arteries and quantify intracranial calcifications (see Figure). Ultimately, this work should improve our ability to identify patients who have a high risk of suffering a stroke and who need surgical treatment.
Combining our work on pulmonary and cardiovascular image analysis, we have also developed techniques to quantify dimensions of the aorta and pulmonary artery, which are important predictors of exacerbations in COPD.
Neuro image analysis
Within neuro image analysis we develop techniques to quantify different aspects of neurodegenerative diseases, to facilitate clinical and epidemiological research in this area. We are working on techniques to automatically and robustly quantify markers of cerebral small vessel disease, including microbleeds, enlarged perivascular spaces, lacunes of presumed vascular origin, and white matter hyperintensities. We previously developed techniques to segment MR images of the brain intro gray matter, white matter and cerebrospinal fluid as well as techniques to perform brain structure segmentation as a starting point for volume and shape analysis. We have for instance shown that hippocampal shape derived from MRI scans is predictive for dementia years before clinical symptoms arise and that this provides additional predictive value over hippocampal volume.
Image analysis across imaging protocols
Software to analyze medical images often stops working properly when one switches to a new scanner type or changes the imaging protocol. This limits the applicability of such software and makes it difficult to compare data from different sites. In several projects we therefore investigate how learned models can be adapted to new types of image data and how image representations can be derived that are invariant to certain changes in scan protocols, such that multi-center or multi-scanner imaging data can be analyzed more robustly.
Learning from weak labels
Most machine learning approaches to quantitative image analysis need a large number of carefully, manually annotated images for model training. This requires that a) experts are not only able to assess the images visually, but also to indicate boundaries reliably, which may be problematic for example in case of diffuse abnormalities; and b) resources are available to perform annotation for the sole purpose of developing image analysis systems. Much more training data would be readily available if weaker labels that indicate for instance the presence, but not the location, of an abnormality could be exploited as well. In several projects we investigate how to learn from “weak” labels such as an estimate of the relative lung volume that is affected by disease as visible in CT, the number of observed lesions in a single slice in a brain MRI scan, or patient outcome measures.
Expectations & Directions
In the past few years we have turned to deep learning approaches in all our application areas. With these highly flexible models, attention to proper interpretation of which image features drive model predictions, understanding of the model’s failure modes, estimating model bias, and correction for possible confounding factors has become even more important than with more conventional learning techniques. Furthermore, in several of our studies longitudinal imaging data, where the same patient was scanned at multiple points in time, is becoming available. Although such data can be analyzed in the same way as is done for cross-sectional studies, by quantifying aspects in each individual image and then comparing the results of different time points, joint analysis of data of all time points may be more suitable to detect subtle changes. We are starting to develop dedicated end-to-end learning approaches to analyzing longitudinal data in several of our projects.
Funding
Bruijne, Marleen de : NWO ASPASIA 2020
Tamames , Weingartner, de Bruijne and consortium partners: ERASMUS MC - TU DELFT Convergence Program 2019-2022, Quantitative Susceptibility MRI: Deep insights in cardio- and neuro-vasculature
Bruijne, Marleen de : NWO VICI 2019—2024, “Learning imaging biomarkers: Machine learning techniques for data-driven disease prediction”
Wendelboe Nielsen, Olav (University of Copenhagen), Marleen de Bruijne , and consortium partners: RegionH 2019–2022, “Breath-CT : Diagnosing Patients Admitted with Breathlessness - Development and Validation of Machine Learning Algorithms based on Images from Computed Tomography”
Tiddens, Harm , Eva van Rikxoort, and Marleen de Bruijne : Netherlands CF foundation 2019–2022, “Computer assisted diagnosis for monitoring CF airway Disease”
Oudkerk, Vliegenthart, de Bruijne , and consortium partners: ZonMW Innovative Medical Devices Initiative - Technology for Sustainable Healthcare 2018–2023, “B3CARE”
Chen, Shuai : China Scholarship Council (CSC) PhD scholarship 2017–2021, “Machine learning in medical image analysis”.
Ginneken, Bram van, Marleen de Bruijne , and consortium partners: NWO-STW Perspectief Programme grant 2016–2023, DLMedIA: Deep Learning for Medical Image Analysis
Van Doorn, Pieter, Ans van der Ploeg, Harm Tiddens, Marleen de Bruijne: Prinses Beatrix Spierfonds 2015—2020, “MR imaging of respiratory muscle dysfunction in Pompe disease”
Elborn, Stuart (QUB), Harm Tiddens, Marleen de Bruijne, and consortium partners: Innovative Medicines Initiative (IMI) Grant 2015-2020: “iABC – Inhaled antibiotics in bronchiectasis and cystic fibrosis”
Invited Lectures
AI in clinical imaging, MRI Together: A global workshop on Open Science and Reproducible MR Research, December 2021
EPSRC Centre for Doctoral Training in Intelligent, Integrated Imaging In Healthcare (i4health), UCL, UK, Keynote, October 2021
Artificial Intelligence for Signal and Image Processing Workshop, University Paris-Saclay, September 2021
German workshop on Image Processing for Medicine 2021, Regensburg, Germany, Keynote, March 2021
European Conference of Radiology (ECR) - Artificial intelligence in radiology: the basics you need to know, March 2021
Highlights
Zahra Sedghi Gamechi successfully defended her PhD thesis “AUTOMATIC QUANTIFICATION OF THE AORTA AND PULMONARY ARTERY IN CHEST CT --- METHODS AND VALIDATION IN LUNG SCREENING “ in September 2021.
Marleen de Bruijne was the Program Chair of the 24th International Conference on Medical Image Computing and Computer Assisted Intervention.
Marleen de Bruijne was elected Fellow of the MICCAI Society.
Kim van Wijnen , Carole Sudre, Florian Dubost , Marius de Groot, and Marleen de Bruijne organized the successful Where is VALDO – Vascular Lesions Detection Challenge. 15 teams participated in this competition to compare their algorithms for microbleed, lacunes, and PVS detection.
Additional Personnel
Silas Orting, PhD, affiliated Postdoc
Emphysema is a pathology in chronic obstructive pulmonary disease (COPD), a leading cause of death worldwide. The extent and appearance of emphysema can be assessed in CT scans of the lungs. This project investigates machine learning approaches to estimate emphysema presence and extent. One of the main issues when applying supervised machine learning in medical image analysis is obtaining labels. Not only can the labeling procedure require medical expertise and be highly time-consuming and costly, it can also be very difficult, even for experts, to provide accurate labels. This thesis investigates three approaches to reducing the need for labels when training machine learning methods to assess emphysema: weakly supervised learning, crowdsourcing and learning from visual similarity. Silas was a PhD student at University of Copenhagen under supervision of Marleen de Bruijne and Jens Petersen. He successfully defended his thesis in April 2019 and is since (part-time) a postdoc under Marleen’s supervision.

This PhD is part of the B3CARE project with a focus on bronchial wall measurements on low-dose Thorax CT. The bronchial tree has a vast number of branches sequentially reducing in size. Research shows the small airways are the first to undergo remodeling in a diseased state, however manually quantifying these changes is incredibly time consuming and unreliable. Thus this project will collaborate with the BIGR to adapt an AI airway segmentation tool, and apply automated measurements to obtain bronchial biomarkers from ImaLife scans. These biomarkers will be matched to participant parameters to provide reference values, which may then be used to screen the general population for early signs of pulmonary disease.

Lacunes of presumed vascular origin are small cavities filled with fluid and are mainly located in the deeper parts of the brain. As one of the neuroimaging biomarkers for cerebral small vessel disease they are important to detect. However, as lacunes are small and can look very similar to other structures and lesions in the brain, manually detecting them can be a time consuming and difficult task. I developed an automated deep learning method to segment these lacunes. The most important challenge was the extreme imbalance between background and foreground since lacunes are very small and often occur with only a few. The method was developed using 3D brain MRI scans of the Rotterdam Scan Study. Project supervised by Kim van Wijnen and Marleen de Bruijne. Eline graduated in May 2021.
Shengnan Liu, associated researcher
I am a researcher specializing in intravascular image computing at Cardiology Department, EMC. My research concentrates on applying techniques of optical modeling, image analysis, classical machine learning, and deep learning to leverage large-scale clinical studies on coronary artery disease. Currently, I am working on structure segmentation in intracoronary optical coherence tomography images using a deep neural network. I also developed an automatic method for quantifying coronary calcium in intravascular ultrasound images using Support Vector Machine, which is further applied to analyze the development of reverse events after percutaneous coronary intervention.


Ivan Dudurych, joint PhD student with UMCG (prof R. Vliegenthart)
Eline Ooms, MSc thesis student

SUPERVISING NEURAL NETWORKS WITH HIGH-LEVEL DESCRIPTIONS
HOEL KERVADEC, PHD
Post-Doc
Project Funding NWO Vici: “Learning image biomarkers: machine learning techniques for data-driven disease prediction”
Research period September 2021 – September 2024
Email h.kervadec@erasmusmc.nl
Image semantic segmentation over the past few years has made tremendous progresses due in big parts to the development of deep learning-based methods that are fed with large, well-annotated and curated datasets of examples. Those annotations typically take the form of a pixel-wise mask, indicating exactly the boundary of the object to segment (be it an organ, blood vessel, tumor, …). As such, the most popular method to train neural networks simply learn the correct assignment for each pixel individually, without focusing at-all on the “big-picture”: the object shape, location and consistency. As such, Image segmentation is treated as a series of independent pixelwise classification decisions.

Preliminary works have demonstrated the feasibility of using more global shape-descriptors to supervise (or train) segmentation neural networks, without resorting to pixel-wise labels. Such way of supervising is not only intuitively closer to the way a human would describe the problem (as opposed to the very machine oriented pixel-wise label), but it also has the inherent potential to generalize across scans and across patients: the rough location of an organ is well known, and this information may be useful when training a neural network, without requiring per-scan annotations. A high-level description of the problem can also be a way to embed other prior anatomical knowledge at training time, in an intuitive and interpretable fashion.
A major part of this problem is therefore to develop more complex and descriptive descriptors that can be used to train neural networks and evaluate their performances. One goal is to have descriptions robust across time and
Figure 1. 3D representation of the heart (left-,right-ventricles, myocardium), with basic shape descriptors. Their range of value can enable us to express the limits of its motion.
space, to be able to describe once and supervise many scans without additional work.
Moreover, and interestingly, the less generalizable descriptions would be for abnormal and out-of-distribution cases: this can potentially be used as new and useful biomarkers for disease predictions.
Therefore, improved methods to measure and characterize objects could also prove to be valuable biomarkers for disease prediction , which could be then used in a (semi-)automatic setting . Compared to a fully image-based prediction model, the shape description would help with interpretability and explainability of the final prediction, ultimately increasing trust into the model.

Advisors
GERDA BORTSOVA, MSC AUTOMATIC DETECTION OF INTRACRANIAL CALCIFICATION S
PhD Student
Marleen de Bruijne & Wiro Niessen
Project Funding NWO TTW Perspective Program Deep Learning for Medical Image Analysis (DLMedIA): Weakly Labeled Deep Learning
Research period April 2017 – March 2022
Email g.bortsova@erasmusmc.nl
Intracranial arteriosclerosis is a major risk factor for stroke. An important indicator of intracranial arteriosclerosis is intracranial internal carotid artery calcification (ICAC). ICAC is thus a promising biomarker for the increased risk of cerebrovascular diseases for future clinical use. However, quantitative measurements of ICAC currently rely on time-consuming and error-prone manual annotations. This hampers research on the etiology and neurological consequences of ICAC and may hinder introducing ICAC-based biomarkers into clinical practice. In this project, we developed and validated a cheaper and faster alternative to manual assessment, a fully-automated deep-learning-based method for delineation of ICAC in non-contrast computed tomography. The method was trained and validated on Rotterdam Study dataset including 2,319 non-contrast head CT scans.
To evaluate the method, we compared manual and automatic assessment with respect to three aspects: 1) the agreement with the assessment by an independent observer, 2) the accuracy in delineating ICAC as judged by an expert via blinded visual comparison, 3) the association with stroke. The figure on the left shows a random sample of three regions provided to the expert for blinded visual assessment of how accurate manual and automatic segmentations are relative to each other.
Intraclass correlations between manual and automatic ICAC volume measures and between observers were 0.98 and 0.91, respectively. Of all manually delineated calcifications, 84% were detected by the automated method. Of all automatic detections, 12% were not delineated by the observers. This performance was comparable to an independent observer. Automatic delineations were more accurate than manual ones in the blinded visual comparisons. Analyzed further, 77% of ‘false positive’ and 28% of ‘false negative’ automatic delineations were

more accurate than their manual counterparts. Both automatic and manual measures were strongly associated with stoke.
In addition to the mentioned evaluations, we validated our method on an external dataset: 100 non-contrast head CT scans of trauma patients. The examples of scans with segmentations are shown on the figure on the left.
Intraclass correlation between manual and automatic ICAC volume measures was 0.94. Sensitivity and false positive rate were 73% and 81%, respectively (vs. 84% and 12% on Rotterdam Study dataset).
In conclusion, our method automates time-consuming manual ICAC assessment, while maintaining accuracy. The method can therefore be used to replace or augment manual assessment.
Figure 1. Manual and automated segmentations on scans from an external validation dataset Blue and red indicate manual and automatic segmentations, respectively.

IROBIN CAMARASA, MSC
Student
UNCERTAINTY QUANTIFICATION AND INTERPRETABILITY FOR MEDICAL IMAGING PhD
Advisors Marleen de Bruijne & Daniel Bos
Project Funding NWO-VICI Learning imaging biomarkers: Machine learning techniques for data-driven disease prediction
Research period October 2019 – October 2023
Email r.camarasa@erasmusmc.nl
n this project we develop new deep learning techniques to help assess the risk of stroke based on MR images of the carotid artery. Atherosclerotic plaque in the common and internal carotid artery is highly correlated with the risk of stroke. In clinical practice, atherosclerosis is assessed via the degree of stenosis; a measurement derived from different diameters of the lumen and wall of the carotid artery. Our first step was to develop an accurate segmentation of both the vessel lumen and wall. We use a U-net deep learning architecture to perform the segmentation. Subsequently, the diameter, the volume, and the shape can be used in stroke prediction models.
Deep learning techniques are facing two major obstacles to be used in a clinical context: the lack of interpretability of the models and the lack of uncertainty estimates of their prediction. This research aims to tackle both of those issues, providing more reliable and robust models.
We use data from both the care II study and the Plaque At RISK study (PARISK). PARISK study includes 200 patients with symptomatic carotid artery stenosis who are at high risk for recurrent stroke. There are 5 MR sequences per patient, acquired in different centers in the Netherlands. The care II study is a public dataset of 25 patients that had a recent ischemic stroke or transient ischemic.

In the past year, we worked on developing uncertainty-aware and interpretable deep learning algorithms. In terms of uncertainty assessment, we extended our validation and comparison of uncertainty methods based on the Monte-Carlo dropout Bayesian technique. We also worked on automated methods for direct artery diameter prediction. To make such models interpretable, we proposed a mathematically grounded technique to segment a carotid artery while training the model only on diameter annotations. In addition to estimating the maximum artery diameter, our algorithm provides the most likely lumen shape corresponding to this diameter. This type of more interpretable algorithms is essential to the deployment of artificial intelligence in clinical practice.

Figure 1. Overview of diameter extraction method
Figure 2. Boundary point extraction mechanism on a MR image of the carotid artery

Advisors
SEMI SUPERVISED LEARNING IN MEDICAL IMAG E ANALYSIS
SHUAI CHEN, MSC
PhD Student
Marleen de Bruijne & Gijs van Tulder
Project Funding China Scholarship Council (CSC)
Research period September 2017 – August 2021
Email S.chen.2@erasmusmc.nl
As a type of machine learning, Convolutional Neural Networks (CNN) have shown great potential in medical image analysis. CNN can extract rich 2D or 3D information from the original images and derive numerous image features at multiple scales automatically. With a well-designed training strategy, CNN has achieved state-of-the-art segmentation results in very short computation times in many applications.
However, a problem of CNN is that often, successful model training requires a large annotated dataset. Different from the situation in many applications in computer vision where the labels can be collected through crowdsourcing, the labels in medical imaging are very scarce and expensive to obtain because they require knowledge with expertise from radiologists. In practice, there usually exists a very limited amount of labeled data and a larger amount of unlabeled data for a specific segmentation task. Thus, methods that are able to learn from unlabeled data, such as semi-supervised learning and self-supervised learning, are desirable in many segmentation tasks.
We propose a new self-supervision task called source identification (SI), which is inspired by the classic blind source separation problem. Synthetic images are generated by fusing multiple source images and the network’s task is is to reconstruct the original images, given the fused images. A proper understanding of the image content is required to successfully solve this task, which makes it a useful task to pretrain a neural network with. During pretraining the network learns useful features for medical image understanding, before fine-tuning the model to perform good image segmentations. We test our method on segmentation of brain tumors as well as white matter hyperintensities from MRI The results show that the proposed SI task outperforms traditional self-supervision tasks including inpainting, pixel shuffling, intensity shift, and super-resolution.

Figure 1. Source-identification self-supervised learning framework

AUTOMATIC AIRWAY SEGMENTATION AND BRONCHIECTASIS QUANTIFICATION
ANTONIO GARCIA-UCEDA JUAREZ, MSC
PhD Student
Advisors Marleen de Bruijne & Harm Tiddens
Project Funding Innovative Medicines Initiative (IMI): Inhaled Antibiotics in Bronchiectasis and Cystic Fibrosis (iABC)
Research period November 2017 – November 2021
Email a.garciauceda@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Pediatrics
In this project we develop image processing methods to automatically segment (delineate) the bronchial tree from chest computed tomography (CT) scans, and to subsequently extract airway measures as imaging biomarkers. These biomarkers are useful for clinicians to quantify structural abnormalities in the airways due to lung diseases such as Chronic Obstructive Pulmonary Disease (COPD) and Cystic Fibrosis (CF). Automatic quantification of key lung features from CT scans can be more sensitive and reproducible than visual CT scoring methods, and require less effort from clinicians.
Segmenting manually the airways from chest CT scans is complex and tedious, requiring several hours per scan by trained annotators, and is prone to errors. Thus, an automated airway segmentation method is highly desirable.
However, this is challenging to build as the bronchial tree is a complex tree-like structure, with branches of various sizes and orientations. For this, we use state-of-the-art methods for biomedical image segmentation based on deep learning convolutional neural networks (CNNs), and in particular the U-Net architecture.
Having obtained airway segmentations, we automatically extract measures from the segmented branches to compute airway biomarkers. In particular, we study airway tapering, i.e. the reduction of airway diameter along a branch and after bifurcation; and the ratio of airway diameter to that of the adjacent artery. We apply these biomarkers to quantify bronchiectasis in patients with cystic fibrosis lung disease and with non-CF bronchiectasis.


Figure 1. Schematics of the proposed deep learning based airway segmentation method
Figure 2. Examples of airway branches that are correctly detected by the proposed method (U-Net) but missed by the previous method (LOP).

Advisors
SELF SUPERVISION AND DATA EFFICIENCY IN BIOMEDICAL IMAGE SEGMENTATION
SUBHRADEEP KAYAL, MSC
PhD Student
Marleen de Bruijne
Email s.kayal@erasmusmc.nl
Convolutional Neural Networks (CNNs), and variants thereof, have been particularly useful in medical image analysis. CNNs can be trained relatively easily to perform many kinds of image segmentation tasks, ranging from isolating abnormal tissue in a brain image to segmenting entire airway trees in a lung scan. However, learning tasks in the biomedical domain are often constrained by the lack of substantial labelled data, which is often difficult and time-consuming to obtain. In our project, we try to tackle this problem by proposing solutions on the lines of data augmentation and self-supervised learning.
Self-supervised learning points to methods in which neural networks are explicitly trained on large volumes of data, whose labels can be determined automatically and
inexpensively, to reduce the need for manually labeled data. Many ways of performing self-supervision exist, amongst which a popular way is the pre-train and finetune paradigm where: (1) a convolutional neural network is pre-trained on a proxy task for which labels can be generated easily, and (2) it is then finetuned on the main task using labeled data. Utilizing a suitable and complex proxy task, self-supervision teaches the network robust and transferable visual features. In our recent working paper, we propose a new self-supervision task, which is inspired by the classic blind source separation problem. Synthetic images are generated by fusing multiple source images and the network’s task is to reconstruct the original images, given the fused images, as show in Figure below.
Figure 1. The proposed source identification task. Three source images $s_1$, $s_2$, and $s_3$ are used for this illustration. Cross-patients SI (CSI) and within-patient SI (WSI) are two different strategies to extract source signals, which focus on learning features between different patients and within one individual patient respectively.


KIMBERLIN VAN WIJNEN, MSC DEEP TRANSFER LEARNING
PhD Student
Advisors Marleen de Bruijne & Meike Vernooij
Project Funding
NWO TTW Perspective Programme Deep Learning for Medical Image Analysis (DLMedIA): Deep Transfer Learning
Research period February 2019 – February 2023
Email k.vanwijnen@erasmusmc.nl
Dep learning techniques have shown great promise for automating the extraction of biomarkers from medical images. However, the application of these methods on new data is highly challenging. Differences e.g. in scanner protocol or patient characteristics, between the data the method was developed on and a new dataset, can greatly harm the accuracy and precision of the method on the new data. If the differences in data are large enough there is a considerable chance the method might not work at all. In this project we aim to develop domain adaptable deep learning techniques for neuroimaging biomarkers that are robust to these differences in data.
We have developed an automated deep learning method for the detection of enlarged perivascular spaces (PVS). The burden of enlarged PVS in the brain is an important emerging neuroimaging for cerebral small vessel disease (CSVD). Using a set of example scans with dot annotations of enlarged PVS, we were able to optimize a convolutional neural network to detect locations of enlarged PVS. We evaluated our method on a set of 1000 scans of the Rotterdam Scan Study and showed that the method could detect PVS comparably to a human rater in four clinically relevant regions. Our next focus will be on developing a method for segmenting enlarged PVS that is domain adaptable.
We organized a challenge in conjunction with the International Conference on Medical Image Computing and Computer Assisted Intervention (MICCAI) 2021 and presented the results as a satellite event at this conference. The VAscular Lesion DetectiOn challenge (or in short the “Where is VALDO” challenge) focused on the segmentation of enlarged PVS, microbleeds and lacunes. These important CSVD biomarkers are difficult to quantify with automated methods as these structures are extremely small and difficult to distinguish from mimics. We provided training data

Figure 1. Top left: enlarged PVS annotations shown in one hemisphere of the brain. Top right and bottom row: Crops of brain MRI scans with predictions by methods submitted to the “Where is VALDO” MICCAI challenge (in blue) and the raters annotations (in red), for the three tasks.
from three different centers with varying scan parameters. The participating teams developed methods and submitted them, which we evaluated on an independent, hidden test set. We received 19 submissions in total over all three tasks, with most focusing on microbleeds. The results were very promising, but there is still clearly more progress necessary to really solve these tasks, especially with respect to overcoming the extreme imbalance and performing consistently across datasets. Our aim was to encourage the development of reliable automated methods for quantifying these biomarkers. The challenge will remain open for new submissions and we look forward to seeing further advancement of these methods.

Advisors
AUTOMATIC QUANTIFICATION OF THE AORTA AND PULMONARY ARTERY IN CHEST CT
ZAHRA SEDGHI GAMECHI, PHD
PhD Awarded 21 September 2021
Marleen de Bruijne & Wiro Niessen
Project Funding Iranian Ministry of Science, Research, and Technology (MSRT)
Short CV Zahra Sedghi Gamechi was born in Urmia, Iran, on 5 May 1987. After finishing high school in Physics and Mathematics, Zahra studied Electrical Engineering (BSc) at Urmia University. Selected as an “Exceptional Talented Student”, she followed her Master’s studies in Communication Engineering, where she focused on pattern recognition, and medical image processing. During and after her Master’s studies, she worked as a lecturer and director of the Physics & Electrical Laboratory at Urmia University of Technology. In November 2014, Zahra started her PhD at the Biomedical Imaging Group Rotterdam at Erasmus MC, on the topic of model-based medical image analysis for vascular segmentation. From March 2019, Zahra began working as a data analytic/scientist at Almedne B.V.
Cardiovascular diseases and Chronic Obstructive Pulmonary Disease (COPD) are among the major leading causes of death globally. In the search for early identification of individuals at risk of cardiovascular disease in COPD, imaging-based assessments of the shape and size of the aorta and pulmonary artery have rapidly gained interest. Changes in these two large arteries may indicate cardiovascular diseases such as pulmonary hypertension and aortic aneurysm. Furthermore, the ratio of the diameter of the pulmonary artery to ascending aorta at the level of pulmonary artery bifurcation is shown to be associated with an increased risk of severe exacerbations and increased mortality in patients with COPD. Therefore, it is essential to accurately delineate and quantify the anatomy of these arteries. Since performing diameter measurements manually is laborintensive, automatic 3D segmentation and measurement techniques are desirable.
In my thesis, I aimed to develop and validate fully automatic segmentation and diameter measurement techniques to quantify the shape and size of the aorta and pulmonary arteries in CT scans. My thesis includes a method based on optimal surface graph cuts to segment the aorta and pulmonary arteries separately and extract landmarks for each vessel for automatic diameter and diameter ratio measurement. With this presented method I have investigated the thoracic artic diameters and the aortic growth rate in a large population. My thesis also presents a new deep-learning-based approach named


Posterior-CRF, for jointly segmenting the arteries. The methods presented in my thesis provide robust and reproducible results of sufficient accuracy and reliability for use in the clinical study.
https://repub.eur.nl/pub/135668
Theo van Walsum graduated in Informatics at the TU Delft in 1990. In 1995 he received his PhD on flow visualization at the Scientific Visualization Group of TU Delft. After one year at the LKEB (LUMC), where he started working on image processing, he became a Post-doc at the Image Sciences Institute (UMC Utrecht).
There he developed his interest in improving image guidance, and worked on several projects where rotational imaging was used for image guidance.
He got a position at the BIGR group at the Erasmus MC in February 2005 where

he is heading the “Image Guidance in Interventions and Therapy” theme group. This group focusses on improving image guidance by integrating pre-operative image information in the interventional situation for cardiovascular and abdominal applications. He is also involved in cardiovascular image processing; he co-organized three cardiovascular “Grand Challenges” and organizes the MICCAI workshops on medical imaging and stroke. He also is one of the PIs of the recently established Smart Surgery Lab. t.vanwalsum@erasmusmc.nl
IMAGE GUIDANCE IN INTERVENTIONS AND THERA PY
THEO VAN WALSUM, PHD associate professor

Context
In minimally invasive interventions, small incisions are used for diagnostic or interventional procedures, giving minimal trauma to the patient. However, direct eyesight on the anatomy of interest, such as in conventional surgical procedures, is lacking, and therefore imaging is used during these procedures. Commonly used imaging modalities in minimally invasive are fluoroscopy and ultrasound, which allow for instantaneous visualization of the interventional instruments in relation to the patient anatomy. Unfortunately, these modalities are limited in their visualization capabilities: ultrasound is often only available as 2D, is operator-dependent and hard to interpret, fluoroscopy only provides 2D projection images, does not have soft tissue contrast and uses ionizing radiation. These imaging modalities lack the wealth of information that is often available from diagnostic imaging. We intend to improve imaging in these minimally invasive interventions by integrating information available from preoperative imaging and planning. Additionally, we are working towards improved use of pre- and post-interventional imaging, e.g. by modeling patient anatomy on pre- interventional images, determining quantitative imaging biormarkers related to treatment and assessing therapy success on post-interventional images.
Top Publications 2021
Bakker, E., Dikland, F. A., van Bakel, R., De Jesus, D. A., Brea, L. S., Klein, S., ... & Paques, M. (2021). Adaptive optics ophthalmoscopy: a systematic review of vascular biomarkers. Survey of Ophthalmology.
Su, R., Sandra A. P. Cornelissen, M van der Sluijs, A C. G. M. van Es, W.H. van Zwam, D.W.J. Dippel, G. Lycklama, et al. “AutoTICI: Automatic Brain Tissue Reperfusion Scoring on 2D DSA Images of Acute Ischemic Stroke Patients.” IEEE Transactions on Medical Imaging 40, no. 9 (September 2021): 2380–91.
Benmahdjoub, M., W.J. Niessen, E.B. Wolvius, and T. van Walsum. “Virtual Extensions Improve PerceptionBased Instrument Alignment Using Optical Seethrough Devices.” IEEE Transactions on Visualization and Computer Graphics 27, no. 11 (November 2021): 4332–41.
Research Projects: Objectives & Achievements
The primary goal of this research line is to improve image guidance in interventions by introducing (preoperative diagnostic) 3D and 4D images and planning/models into the interventional suite / operating room.
Whereas navigation approaches have become state-of the art in brain surgery and orthopedics, application of this technology in e.g. cardiac and abdominal interventions has been hampered by continuous tissue motion and deformation. Our first focus is to develop robust techniques for integrating pre-operative information that can be used in case where tissue motion and deformation may occur. To this end, we are developing and evaluating methods to build pre-operative models for interventional planning, and for alignment of these pre-operative models with the interventional situation, by utilizing interventional images, position tracking information and motion/deformation models.
Conventional navigation approaches, where surgical instruments are shown in relation to pre-operative imaging on a 2D computer screen, result in continuous switching of focus (from screen to surgical field), and difficulties in hand-eye coordinates. Together with surgical departments, we are therefore working on mixed reality approaches that permit integration of 3D imagery in the surgical field of view. Main challenges are obtaining and mainlining accurate alignment of the images with the patient. These efforts take part in the newly established ‘Smart Surgery Lab’, were researchers from Erasmus MC and TU Delft collaborate.
In the field of cardiac interventions, we are strongly cooperating with the Department of Cardiology (Joost Daemen). We have been developing techniques to model

cardiac shape and motion, and to extract cardiac and coronary anatomy from CTA images, and based on this an approach for dynamic roadmapping.
In the field of interventional radiology, we are focusing on liver interventions, such as TACE, RFA, and TIPS. For TACE, a strategy similar to the cardiac interventions is employed: integrating high resolution 3D models of the vasculature in the X-ray imaging.
For RFA and TIPS, we are developing techniques that exploit the capabilities of modern real-time 3D ultrasound transducers to capture tissue motion during the intervention, which would allow more accurate integration of anatomical details from pre-operative 3D images. Additionally, we are working on approaches to track devices in interventional modalities: catheters in X-ray images and fluoroscopy, and needles in 2D and 3D ultrasound. These projects are performed in collaboration with Adriaan Moelker. In this research line, we have realized a prototype CT-based image guidance system for ablation of liver lesions. In the field of Mixed and Augmented Reality, we are collaborating with surgical disciplines (neurosurgeons, traumasurgeons, CMF surgeons, oncological surgeons). We have developed a AR-based navigation approach that can be utilized without adaptation of the AR device. Clinical prototypes of this approach are being evaluated in phantom studies, for e.g. craniosynostosis and spine surgery.
Expectations & Directions
The essence of our work is the modeling of the preoperative images, and the integration of the information obtained from pre-interventional imaging in the intervention.
After having developed tissue and motion modeling for cardiac interventions, we are now working towards integration of these models into the intervention. The two challenges to be addressed are: 1) initial alignment of the pre-operative information with the interventional scene, and 2) keeping this alignment up-to-date. Additionally, these technologies should be fast enough for deployment in an interventional setting. This will be our main focus of the coming years.
A similar strategy is followed for the abdominal interventions: the focus is shifting from pre-operative modeling to the robust and real-time integration of this information with the interventional imaging. The developments of in interventional imaging modalities (e.g. better X-ray detectors, having better contrast and dynamic range, and real-time 3D US transducers) are closely followed, and novel technologies, when appropriate will be integrated.
Figure 1. Augmented reality navigation: overprojection of structures on a skull phantom.


Figure 2. Automated processing of DSA images of stroke patients: left: automatic computation of autoTICI, right automated perforation detection.
Minimally invasive image guided interventions is a field that is still growing, in absolute numbers of interventions as well as in the variety of interventions that can be performed minimally invasively. Technology for improving image guidance will be relevant for novel interventions as well. We are actively exploring other applications and disciplines where similar approaches may be beneficial. Additionally, we are working on the assessment of the technology developed in clinical practice.
A new direction of research that was started together with CranioMaxilloFacial surgery is the use of mixed reality in complex surgeries. By integrating mixed reality with (neuro) navigation, we intend to provide even better visualization options compared to standard navigation. Additionally, such mixed reality approaches are expected to have a positive impact in training and education. To this end, a ‘Smart Surgery Lab’ was established, in which clinicians, the BIGR group and researcher from TU Delft will collaborate.
Funding
Xavier Levecq (Imagine Eyes), Theo van Walsum, Stefan Klein, Wiro Niessen and consortium partners: H2020 Ecsel program IA Grant 2018-2021: MERLIN: Multi-modal, multiscale retinal imaging.
Theo van Walsum, Wiro Niessen, Aad van der Lugt, Jorrit Glastra: Hartstichting PPS call, 2018-2021: Accurate: Automatic CTA image analysis to support treatment selection in acute stroke.
Theo van Walsum, Ad van Es, Danny Ruijters (Philips Healthcare): Health Holland TKI Call, 2019-2023: Q-Maestro: Quantitative Microvasculature AssEssment in projection angiography of ischemis STROke patients
Eppo Wolvius, Wiro Niessen, Theo van Walsum: Koers 23: TU-Delft – Erasmus MC Smart Surgery Lab.
Jenny Dankelman Kees Verhoef, … Theo van Walsum:TU Delft – Erasmus MC Convergence project: Smart Surgical Knife with AR: Combining Smart Knife with Augmented Reality.
Invited lectures
Theo van Walsum, Deep learning for image biomarker extraction, 5th DCVA Translational Cardiovascular Research Meeting 24 Juni 2021, Utrecht
Highlights
The Merlin project, an H2020 project in which the BIGR[eye] group participated, was finalized, and according to the reviewers, the project ‘performed beyond expectations”.
Four Clinical Technology BSc students, Fleur Lycklama à Nijeholt, Victoria Marting, Vivian van Asperen, and Josefien van den Berg did their final BSc project supervised by Matthijs vd Sluijs and Ruisheng Su on artery-vein separation in DSA images; the full paper based on this work was accepted at a conference. Two of the students will present the work at the conference in San Diego.
Additional Personnel
Konstantinos Ntatsis (Research Software Engineer)
Optical coherence tomography (OCT) is a medical device that, in the context of our current work, offers a three-dimensional image of the eye retina, which ophthalmologists can use to make clinical diagnoses for their patients. Depending on the patient’s ability to fixate on a target, their eye can make smaller or bigger involuntary movements, creating motion artifacts in the acquired volume and, hence, deteriorating its quality and hampering the work of the clinician. In the past year, we contributed in addressing this issue by developing a deep-learning registration technique that corrects motion in retinal OCT images in a fast and robust manner.

Samantha de Graaf, MSc thesis student (TU Delft)
An early and reliable outcome prediction in stroke patients is important for initiation of individual treatment. Based on the predicted outcome, treatment involves either a thrombolysis or a thrombectomy. Several prognostic scores have been developed to predict outcome after stroke, such as the ASPECT score and the Collateral score. These scores are derived parameters from CT images of the brain. In this project we will research if the use of the entire CT image can contribute in the outcome prediction. Hereby a convolutional neural network will be trained and evaluated to improve the decision making process.
Joost Wooning, MSc thesis student (TU Delft)
Augmented Reality (AR) is an upcoming technique which can be used to display patient images which are overlaid on the normal vision of the user using Head Mounted Displays (HMDs), these use semi-transparent displays directly in front of the user’s eyes. To display the images in the correct position, the position of the HDM relative to the patient has to be known, previous research used a navigation system to track the position of the HMD. However, in this project we will research the accuracy of using only the internal camera of the HMD to track the position of the patient, AR markers will be attached to the patient to be able to display the image in the correct position.


During intervention in surgical oncology, localization of specific tissues (e.g. tumours, blood vessels) can be difficult.. In this project we look at the possibility of using augmented reality (AR) during surgery to provide more visual information to the surgeon during the intervention. Mixed reality glasses are used to superimpose the 3D model onto reality. A part of this project is the registration (alignment) problem between the pre-operative images and intra-operative position. To align pre-operative images with the patient during the surgery, landmarks visible on both images and reality must be used. Here we want to investigate if RGB-D camera (conventional camera + depth/distance) of the Microsoft Hololens 2 could be a solution to align the pre-operative image with the patient using the surface shape of his/her body. Can such a surface based method be accurate and make the use of additional markers redundant?

Enzo Kerkhof, MSc thesis student (TU Delft | EUR | Leiden Univ.)
Jet Peek, MSc thesis student (TU Delft | EUR | Leiden Univ.)
Double outlet right ventricle (DORV) is a complex congenital heart disease associated with very variable anatomy of the heart. In this patient group, the optimal surgical approach can be difficult to assess on conventional 2D ultrasound (US) and computed tomography (CT) imaging. For additional anatomical understanding physical 3D prints or 3D Virtual Reality (VR) models can be used. A stereoscopic head mounted display is used, using MedicalVR software to visualize CT scans and highlighted segmentations can be visualized in 3D. In this research we compared 3D printing and 3D-VR and 2D US/CT for surgical planning of DORV patients. Participants were enthusiastic, both 3D models were of additional value for cardiac surgeons and cardiologists, and spatial relationships were better visible on 3D-VR and 3D prints.
Myrthe van den Berg, MSc thesis student (TU Eindhoven)
Image-guided interventional techniques such as CT-guided radiofrequency ablation (RFA) are powerful tools in the management secondary liver malignancies. During a RFA procedure, NCECT is commonly used to assess the treatment. In order to localize the tumour on a NCE-CT scan, the interventional radiologist mentally maps the location of the tumour from the diagnostic images to the intra-operative images. Software that accurately localizes the tumour on NCE-CT by means of spatially aligning the diagnostic and intra-operative CT images, would solve the disadvantages of this time-consuming and potentially inaccurate procedure. In this research we are exploring the feasibility of using a Deep Learning Image Registration framework to accelerate this process.
Tessa Kos, MSc thesis student ( TU Delft | EUR | Leiden Univ.)
The preoperative planning of autologous breast reconstructions using abdominal adipocutaneous tissue consists of a visualization of the abdominal wall vasculature, to minimize donor site morbidity and decrease operative time. The current gold standard is a visualizations of the CTAs in 2D planes on a 2D screen. The effectiveness of the planning depends on image quality and a surgeon’s ability to mentally transform the 2D planes into a mental 3D image and translate this to the patient on the table. This project is a qualitative study, where we investigate the benefit of a 3D visualization on a 2D screen and in virtual reality (VR) for preoperative planning of autologous breast reconstructions. For this, 3D models of the anatomical structures are created and their appearance in a 2D view and in VR are assessed by reconstructive plastic surgeons.
Vincent Hellebrekers, MSc thesis student ( TU Delft | EUR )
Digital subtraction angiography (DSA) is an X-ray imaging technique used during endovascular therapy in stroke patients. It allows the surgeon to see whether a vessel is still occluded during the procedure, and to evaluate the status of the patient by giving a TICI score. I am developing a framework to digitally register DSA images of a patient, before and after treatment, to automatically track progression. For this I will explore classical and deep learning based computer vision techniques. This can become a framework to build a more objective, and less variable metric than the TICI score to improve patient prognosis.




Vânia Silva, MSc thesis student (University of Coimbra)

Glaucoma is an insidious and unpredictable ophthalmic disease. However, its damage is preventable, making an early diagnostic and close monitoring crucial. Optical Coherence Tomography (OCT) is a low-coherence interferometry technique, widely used to retrieve relevant glaucoma biomarkers. OCT images are characterized by speckle patterns, resultant from an interference phenomenon. These patterns are known to be a carrier of information, and their analysis has many biomedical applications. In this project, five different methods to study and infer OCT speckle properties were implemented and applied to glaucomatous OCT, in order to assess their potential to aid glaucoma diagnosis. The speckle-related features and other clinically relevant data (age, gender, retinal layers thickness) were used to develop a predictive model based on discriminative Event-Based modelling (DEBM): a data-driven technique to estimate the sequence in which biomarkers for a disease become abnormal. This model aims at not only predicting the presence of glaucoma, but also providing an interpretable insight to the disease and its processes.
Rita Marques, MSc thesis student (University of Coimbra)
Glaucoma is an irreversible but preventable disease, and one of the main causes of blindness worldwide. The Optic Nerve Head (ONH) represents the intraocular section of the optic nerve, which is prone to damage by increases in the Intraocular Pressure (IOP). The advent of Optical Coherence Tomography (OCT) has enabled the evaluation of ONH parameters (biomarkers) but they are mostly extracted through manual segmentation of the ONH tissues, a time-consuming and prone to bias task that limits their usability in clinical practice. Therefore, in this project we developed a deep learning based segmentation model for five structures of the ONH (RNFL, RPE/ BM complex, other retinal layers, choroid and LC) in OCT data using a U-net. Six features (optic disc diameter, BMO-MRW, RNFL thickness, RNFL area, LC depth and LCCI) were automatically obtained from the best model automatic segmentations.
Fenna ten Haaf, MSc thesis student (Erasmus University Rotterdam)
Glaucoma is a progressive eye disease where early detection is critical, as the progression is irreversible and can lead to blindness. Additionally, the staging of glaucoma severity can aid in treatment, but the test for severity is unreliable and time-consuming. In this project, we aim to predict glaucoma severity using deep learning methods by addressing two tasks: the classification of glaucoma versus healthy subjects and the prediction of the visual field mean deviation (VF MD) score. We approach these tasks with the use of different imaging modalities: fundus photographs, optical coherence tomography (OCT), and the relatively unexplored optical coherence tomography angiography (OCTA). Each modality is used to train separate Convolutional Neural Networks, as well as being combined in multi-input models.



PIERRE AMBROSINI, PHD AUGMENTED REALITY IN SURGICAL ONCOLOGY
Post-Doc
Project Funding Convergence for Health and Technology: “Smart instruments and interventions”
Research period September 2020 – August 2021
Email p.ambrosini@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Surgery.
During intervention in surgical oncology, position of specific tissues such as tumors, blood vessels, nerves can be difficult to localize. Generally, in order to visualize the tissues of interest and make a 3d mental representation of their positions, surgeons have to look at pre-operative images acquired before the intervention (e.g. CT, SPECT scan or MRI image). In this project we look at the possibility of using augmented reality during surgery to provide more visual information to the surgeon. Such information would be extracted from pre-operative and intra-operative images/measurements (ultrasound, magnetic proximity sensor). Mixed-reality glasses (optical see-through headset) would be used to superimpose 3d models (e.g. tumor, blood vessels) onto the reality. Thus, surgeons could see both the patient and augmented 3d information. Different augmented reality solutions are possible. One could propose a way to visualize and manipulate the 3d model in order to look at it in any direction and also manually align it onto the patient using hand motion. Another solution is to automatically align
the 3d model with the reality using clear landmarks visible on both the pre-operative image and inside or outside the body of the patient. Skin surface could be detected as well with depth sensor camera and helping with the 3d model alignment. Such visualization could help the surgeon to understand the 3D spatial environment of where the tumor is and how to operate. In our project, we now focus on augmented reality during breast cancer surgery. In order to superimpose the 3d tumor model onto the patient, several tasks need to be tackled:
- Alignment of the 3d pre-operative breast image with the patient
- Deformation of the breast/tumor to fit the current patient position
- Visualization using the mixed-reality glasses with an optimal perception of the tumor shape/depth w.r.t to the patient

Figure 1. Breast skin (left) and thorax with tumors (right) can be visualized in 3D and aligned with the patient by surgeons using mixed-reality smart glasses during the surgery.

MERLIN: MULTI-MODAL, MULTI-SCALE RETINAL
IMAGING
DANILO A JESUS, PHD
Post-Doc
Project Funding European Commission: H2020-ICT-2016-2017 (MERLIN) / Erasmus MC-TKI-LSH 2020: Retinal screening for early detection of Alzheimer Disease (Eye2Brain)
Research period July 2018 – September 2021 / October 2021 – May 2022
Email d.andradedejesus@erasmusmc.nl
When light from a star enters the Earth, atmospheric turbulence can distort and move the image in various ways producing a blurred image. Adaptive Optics (AO) has been broadly used in astrophysics to correct these distortions, using a wavefront sensor to measure and compensate for the optical aberrations the atmosphere has introduced. The wavefront passing through the pupil of the eye may also suffer aberrations reducing the quality of the image formed at the retina. Whereas spectacles and contact lenses can correct loworder aberrations, sufficient for normal vision, it is generally insufficient to achieve microscopic resolution needed for retinal imaging diagnosis.
Hence, the MERLIN project has currently developed a novel medical imaging prototype which integrates Adaptive Optics correction, with conventional imaging techniques such as Scanning Laser Ophthalmoscopy (SLO), Optical Coherence Tomography (OCT), and OCT Angiography (OCT-A). The overall ambition of the project is to improve in-depth diagnosis and therapeutic follow-up of
diseases that impact the eye’s retina by increasing the ability to resolve the microscopic structures with multiscale and multi-modal imaging (see Figure 1).
Quantitative imaging biomarkers which require segmentation of structures visible in the SLO, OCT, OCT-A and AO images will allow relevant quantifications of the retinal microvascular density, photoreceptor morphology (size, shape, number of neighbors), diameter/volume of microdrusen (for early stage AMD), and atrophic lesion progression between visits. To enable these quantifications, new approaches were implemented and adjusted to the structure to segment, and the availability of training data.
In EYE2BRAIN, we are developing algorithms for early detection of Alzheimer’s disease based on retinal OCT imaging and fundus photos. Our findings will enable us to detect patients at risk of Alzheimer‘s disease who are eligible for preventive measures or who are suitable for clinical trials.

Figure 1. Schematics of multimodal and multi-scale imaging including SLO, OCT, OCTA and AO applied to the eye’s retina.

MERLIN: MULTI-MODAL, MULTI-SCALE RETINAL IMAGING
LUISA SÁNCHEZ BREA, PHD
Post-Doc
Project Funding European Commission: H2020-ICT-2016-2017 (Merlin) / Erasmus MC-TKI-LSH 2020: Retinal screening for early detection of Alzheimer Disease (Eye2Brain)
Research period July 2018 – September 2021 / October 2021 – May 2022
Email m.sanchezbrea@erasmusmc.nl
The human eye and, specifically, the retina, is a highly sensitive region of the body, adequate for the early diagnosis of multiple pathologies of varying severity.
Many symptoms for retinal diseases are currently detected through medical imaging acquisition, and visual inspection of the data by a clinician. However, this process is tedious, time-consuming, and highly reliant on the clinicians’ experience. Therefore, automatic approaches are required. The goal of the Erasmus MC in MERLIN, a European project that aims to create a multi-modal, multiscale retinal imaging device through the joint effort of six public and private institutions, is to develop image processing and machine learning algorithms that provide objective, repeatable, clinically-relevant quantifications.

One of the main characteristics of MERLIN is the use of Adaptive Optics (AO), a technology that allows to increase the resolution of the images so that even individual cells (Fig. 1) and small blood vessels (Fig. 2) can be observed and, therefore, automatically quantified. Variation on the number of cells is indicative of pathologies such as inherited retinal diseases, while variations on the capillaries are linked to vascular diseases. This way, the use of image processing techniques and Artificial Intelligence in AO-enhanced imaging modalities enables the analysis of retinal structures much earlier than conventional technologies, hence providing an earlier and wider window of opportunity to tackle the disease.

Figure 1. Left: Adaptive Optics image showing photoreceptor cells. Right: from top to bottom, zoomed-in region of interest, segmentation of the photoreceptor centres, and overlapped image.
Figure 2. Left: Adaptive Optics image showing vessels. Centre: segmentation of the vasculature. Right: overlapped images.

AUGMENTED REALITY NAVIGATION FOR CRANIOMAXILLOFACIAL SURGERY
MOHAMED BENMAHDJOUB, MSC
PhD Student
Advisors Theo van Walsum & Eppo B. Wolvius
Project Funding Erasmus MC
Research period October 2018 – September 2022
Email m.benmahdjoub@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine & Oral and Maxillofacial Surgery.
Navigation systems in the context of surgery assist the surgeon intraoperatively to operate based on plans prepared preoperatively on patient-specific data. These conventional navigation systems present 3 mains issues:
• Only 2D views are being used on a 2D screen.
• It is difficult to relate the relative position of anatomical structures to each other (Depth perception issue).
• It is hard to coordinate between the images visualized on the screen and the hands of a surgeon since the images are on a different plane then where the patient is (Hand-eye coordination issue).
The previous three points oblige the surgeons to:
• Mentally reconstruct the 3D aspect of the anatomical structures of the patient.
• Mentally relate the exact position of the instrument with respect to the patient and thus move it to the next right location.
• Continuously switch focus between the screen and the intervention area

Augmented reality is the process of overlaying data on the real world. This data can be Text, 3D and audio signals. In surgery context, this technology would be helpful into solving the previously mentioned issues for projecting 3D patient-specific data (MRI, CT…) on top of the intervention area or the position of the instruments (drilling, cutting instruments…) in the space even if they are hidden behind patient tissue…
The goals of this project are:
• Develop intraoperative solutions that would help integrate an external augmented reality device into the current workflow of the navigation systems.
• Conduct experiments (Phantom studies or case studies) to investigate the feasibility, usability and accuracy of the solutions in the context of craniomaxillofacial surgery.

Figure 1. Navigation using augmented reality on a spine phantom.
Figure 2. Studying user perception under instrument alignment task (Instrument to holographic planned trajectory) in augmented reality.

QUANTITATIVE IMAGE ANALYSIS FOR STROKE BIOMARKERS
JIAHANG SU, MSC
Advisors Wiro J Niessen , Aad van der Lugt & Theo van Walsum
Project Funding CONTRAST: Collaboration for New Treatments in Acute Stroke
Research period November 2017 – November 2021
Email j.su@erasmusmc.nl
Stroke is the first cause of death in pathological aging disease. Stroke occurs when there is abnormal blood supply in the brain. The blood leaking leads to hemorrhagic stroke and the blood blockage leads to the ischemic stroke. The research focus in CONTRAST project is to improve the treatment outcome of ischemic stroke.
The treatment outcome of ischemic stroke is related to many impact factors. In standard protocol, the patient with ischemic stroke will first take CTA (computed tomography angiography) to determine if the patient will benefit from intervention. Before and after intervention the DSA (digital subtraction angiography) will be taken with the purpose of surgical quality evaluation. Then, a CT will be taken for the follow-up treatment. The problem in such protocol is that each decision making is biased by the clinician or radiologist. Therefore, in this project, we want to find the strong statistic relation between each decision making and the treatment outcome by quantitative analysis of image biomarker.
The development of the imaging biomarkers requires development of effective and fast methods to process 2D+t (DSA sequences) and 3D (CT, CTA) images. For this, we would like to use GPU to develop and validate deep learning models and image processing algorithms that can output quantitative imaging biomarkers for stroke. The focus is on automated methods that can accurately quantify various biomarkers, such as those that can identify early ischemic changes and follow-up infarct core volume, collateral capacity and the effect of interventions. These analysis methods, complemented with other analysis methods identified in the project, will be integrated into image processing pipelines, to facilitate standardized analysis of all imaging data that is being acquired by clinical consortium partners in the CONTRAST project.

The final target of this project is a set of vessel biomarkers from prognosis process that provide clinical diagnostic aid to the treatment of ischemic stroke. To achieve this, we first need to discriminate the arterial structures and venou structures in the critical regions. Thus, we extract the arterial trees with deep reinforcement learning techniques. Fig. 1a shows the proposed network. Fig. 1b and 1c demonstrate one of example full brain vascular network and corresponding anterior tree.
Figure 1. a) The proposed network architecture; b) The full brain annotation; c) The anterior atrial tree.
PhD Student

QUANTITATIVE STROKE IMAGING
RUISHENG SU, MSC
PhD Student
Advisors Theo van Walsum, Wiro Niessen, Aad van der Lugt & Danny Ruijters
Project Funding Q-Maestro TKI project
Research period February 2020 – January 2024
Email r.su@erasmusmc.nl
Stroke is one of the worldwide leading causes of death and permanent disability. Ischemic stroke, which is caused by an occluded artery of the brain, is the most common stroke type, accounting for about 88% of all strokes. Recently, endovascular therapy (EVT) has been proven effective in removing large vessel occlusions (LVO) in the anterior circulation, leading to improved outcome for patients suffering from acute ischemic stroke.
Digital Subtracted Angiography (DSA) is the imaging modality for visual procedural guidance and reperfusion assessment in EVT. Such DSA image series (2D+t) provide information on vasculature, perfusion profile and brain anatomy, which are valuable for treatment quality assessment, therapeutic decision making, as well as outcome prediction.
Several factors limit the efficiency and effectiveness in utilizing DSA image information in clinical practice. Currently such images are mainly visually inspected by interventional neuroradiologist based on coarsely defined grades for treatment assessment, which can be subjective and error-prone. Furthermore, despite rapid research progress


in computational stroke related biomarker extraction from computed tomographic angiography (CTA) and CT Perfusion (CTP) images, the potential value of various computerized DSA image biomarkers is yet to be further discovered considering the data heterogeneity and the complexity in perfusion dynamics.
Therefore, this project aims to assist clinicians to maximize patient benefits in an automated manner leveraging advanced image processing techniques and artificial intelligence. Automatic and quantitative DSA image analysis methods can facilitate accurate and objective treatment assessment and improved outcome prediction. Examples of such algorithms include deep learning-based peri-operative vessel perforation detection (see Figure 1) and automatic artery-vein segmentation in DSA series (see Figure 2). Furthermore, the temporal flow of contrast material in DSA contains valuable information about blood flow and perfusion dynamics, from which numerous biomarkers could be extracted for treatment assessment. Ultimately, the clinical value of these automatic image biomarkers will be underpinned by their association with treatment outcomes.


Figure 1. Two examples of intracranial vessel perforation during endovascular thrombectomy.
Figure 2. Automatic vessel segmentation in DSA images. Left: input image; right: segmented image.

CT-ULTRASOUND FUSION FOR IMAGE GUIDANCE IN LIVER INTERVENTION
YUANYUAN SUN, MSC
Advisors Wiro Niessen, Theo van Walsum & Adriaan Moelker
Project Funding China Scholarship Council (CSC)
Research period February 2017 – 2022
Email y.sun@erasmusmc.nl
Liver cancer is the six most frequent cancer in the world and the second most common cause of death from cancer. Percutaneous ablation procedures (Radiofrequency Ablation, Microwave ablation, Cryoablation, etc.) have been widely adopted in recent years as the treatment for patients with liver cancer, because of the associated benefits: less trauma and quicker recovery.
Image guidance is required in these procedures to visualize target lesions and instruments, and then therefore helps surgeons to operate on the right lesion site. Ultrasound is the preferred imaging modality as it is real-time, movable and relatively safe and cheap. However, tumors are not always visible in the ultrasound images whereas they are generally clearly visible in pre-operational images (CT/MR). Therefore, the idea of integrating CT/MR with ultrasound images has been proposed. To enable the fusion of CT and ultrasound images, the key is to accurately spatially align them. The solution to it is CT-US registration, which is the focus of this project.
Specifically, our focus is on investigating CT-US registration techniques. We have first been investigating on rigid/affine registration. Later, it is necessary to extend to non-rigid case as liver is a soft-tissue organ and may present significant deformations due to respiratory motion or other tissue deformations (such as cardiac motion). Specifically, the project can be divided into the following tasks:
• First, a roughly initial alignment of CT and ultrasound images is required. To achieve this, we first stitch a series of 4D ultrasound images are to produce a panorama, and then register CT image with it using deep learning methods.
• Then a subsequent registration is used to refine the alignment.
Deep learning is a powerful tool which has been proved promising in medical image registration. We have been investigating on using it to solve our problem. For example, using deep learning to synthesize ultrasound image from the corresponding CT image, and therefore to convert a multi-modal problem to a mono-modal case.

The outcomes of the research will first contribute to the improvement of image guidance in liver tumor ablation as the work is primarily set for it. However, registration of 3D ultrasound to CT images can potentially be used in any other interventions where ultrasound is used for guidance, and preoperative CT images are available as well, e.g. in laparoscopy surgery.
Figure 1. An illustration of stitched ultrasound image of a patient liver. The left is the corresponding single ultrasound image.

AUGMENTED REALITY NAVIGATION FOR SURGERY
ABDULLAH THABIT, MSC
Student
Advisors Wiro Niessen, Eppo Wolvius & Theo van Walsum
Project Funding Smart Surgery Lab
Research period November 2020 – October 2024
Email a.thabit@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Oral & Maxillofacial Surgery
Navigation has become standard of care for several surgical areas, such as neurosurgical, orthopedic and spinal procedures. Whereas such navigation enables the use of pre-operative imaging and planning during the procedure, the handeye coordination and the need to switch focus (from the operative field to the navigation screen back-and-forth) are still considered drawbacks. The recent introduction of Augmented Reality (AR) devices permits for more immersive navigation approaches, where the pre-operative information and planning is directly visualized in the fieldof-view of the surgeon. Such approaches have great potential to improve surgical procedures and may both enable better training and deliver safer surgery.
To that end, our project has two main objectives:

1. Sutures locating on phantoms: (a) Skull phantom covered with surgical foam to hide sutures, (b) delineated sutures by the participant, (c) the delineation task, (d) view of the HL2 during the experiment with lambdoid (red) and sagittal (blue) sutures shown.
1) To develop and assess a vision-based AR navigation system, where an AR headset such as Microsoft HoloLens can be used for both tracking and visualization. The project focuses on assessing and developing augmented reality based key components required for navigation, such as: patient-to-model alignment, patient and surgical instruments tracking, and the visualization of the virtual model in the surgical scene.
2) To bring AR solutions to the operating room to help in surgery planning and navigation. For example, in minimally invasive craniosynostosis surgeries. Surgeons plan their surgery by manually locating cranial sutures to identify where to place their incisions. This approach is prone to error could lead to complications
and an increase in the surgery time. We have proposed an AR navigation system that is combined with an Electromagnetic tracking system, which visualizes the cranial sutures aligned on the patient head. This system facilitates for more reliable and accurate planning of the surgery. It has been tested in vitro with two physical phantoms, demonstrating a clinical applicability with less than 5mm error in locating the cranial sutures. The figure below shows the experimental setup of the conducted phantom study.
Figure
Dr. ir. Stefan Klein is Associate Professor in Medical Image Analysis. He is coprincipal developer of a widely used open-source software package for medical image registration, called Elastix (article cited >2500x), was co-organiser of three grand challenges (CADDementia, TADPOLE, KNOAP2020), general chair of the WBIR2018 conference, and is Associate-Editor of the IEEE Transactions in Medical Imaging. His current research interests include machine learning for medical image analysis, image registration, image reconstruction and

quantification, and disease progression modelling, with applications in oncology, ophthalmology, musculoskeletal disorders, and neurodegenerative disease. Besides performing research, Stefan is also active in setting up infrastructures that facilitate research in medical imaging, and he has for instance initiated a national HealthRI research archive for medical imaging data, currently used by numerous multicentre imaging studies in the Netherlands, and is manager of the Health-RI Imaging Community.
s.klein@erasmusmc.nl
IMAGE REGISTRATION
STEFAN KLEIN, PHD
associate professor

Context
Registration is an important technique in medical image processing. It refers to the process of spatially aligning datasets from different modalities, time points, and/or subjects. Image registration enables fusion of multimodal imaging data, e.g. magnetic resonance imaging (MRI) and ultrasound imaging. It allows the retrospective compensation of patient movements that otherwise would lead to image artifacts. It facilitates the assessment of changes in tissue properties over time (e.g. tumour growth). Image registration can even be used to identify corresponding anatomical structures across subjects.
In the Image Registration group we develop and evaluate novel image registration methods and use these in several applications. Besides, an increasing amount of our research is focusing on the development of artificial intelligence (AI) methods that aid image interpretation for improved diagnosis and prediction.
Top Publications 2021
Sabidussi ER, Klein S, …, Poot DHJ. Recurrent inference machines as inverse problem solvers for MR relaxometry. Medical Image Analysis, 2021.
Venkatraghavan V, Klein S, …, Bron EE, & ADNI. Analyzing the effect of APOE on Alzheimer’s disease progression using an event-based model for stratified populations. NeuroImage, 2021.
Marinescu RV, …, Bron EE, …, Venkatraghavan V, …, Klein S, Alexander DC, & ADNI. The Alzheimer’s Disease Prediction Of Longitudinal Evolution (TADPOLE) Challenge: Results after 1 Year Follow-up. Machine Learning for Biomedical Imaging (MELBA), 2021.
Research Projects: Objectives & Achievements
Novel image registration methods
In collaboration with Dr. Van Walsum, BIGR[eye] was formed in 2018, consisting of postdocs Danilo A. Jesus and Luisa Sánchez Brea. In 2020, the team was further strengthened by research software engineer Konstantinos Ntatsis. Their research aimed at the development of fast motion compensation techniques, to be integrated into a novel multi-modal, multi-scale retina imaging device, in collaboration with the European MERLIN consortium. The project has delivered retinal imaging prototypes that are now being tested at two medical centres. These systems combine several technologies, including optical coherence tomography (OCT) and adaptive optics (AO), to visualize the retina at different scales with multiple modalities (see Figure on next page). In 2021 the MERLIN project was completed, and it received a very positive evaluation by the European Commission. More details on the BIGR[eye] group can be found in the section of Dr. Van Walsum (page 102)
Another application where image registration and motion compensation play an important role is the development of quantitative MRI methods. This research is done in close collaboration with the MR Physics group (Dr. Hernández-Tamames, page 42) and became so successful that it was transformed into a separate research theme, led by Dr. Dirk Poot and described on page 66. A highlight of 2021 was the publication of a paper proposing rapid and accurate T1 and T2 mapping using an “Recurrent Inference Machine”, a state-of-the-art deep learning approach that exploits knowledge of the MR physics.
Finally, we have a fruitful collaboration with the Dept. of Obstetrics and Gynecology, aimed at the analysis of 3D ultrasound images of the embryonic brain during pregnancy. In this research, performed by Wietske Bastiaansen, our objective is to build a spatiotemporal atlas of the developing embryonic brain . In 2021, she performed a comprehensive systematic survey on this topic, and finalized a manuscript on automated segmentation and spatial alignment of the embryo in 3D ultrasound images.
Artificial intelligence in Radiology
In this research line, we develop and evaluate machinelearning techniques for early diagnosis, fine-grained disease staging, and accurate patient stratification.
A highly novel method for neurological disease progression modelling was developed by Vikram Venkatraghavan. This method, coined Discriminative Event Based Model (DEBM) , estimates the order in which biomarkers become abnormal during disease progression, purely based on cross-sectional measurements. This method was applied in several studies, all published in high-impact journals. In 2021, Vikram Venkatraghavan successfully defended his PhD thesis cum laude!
In collaboration with the musculoskeletal imaging group (Dr. Oei, page 290), by PostDoc Jukka Hirvasniemi, a grand challenge on prediction of knee osteoarthritis development , called KNOAP2020, was organised. The results of this challenge were announced in 2021, at the International Workshop on Osteoarthritis Imaging (IWOAI). Furthermore, Jukka started working on a new project on imaging-genetics analysis for knee osteoarthritis, in the context of the Convergence program of Delft University of Technology and Erasmus MC.
Major developments took place in the area of oncology. Sebastian van der Voort and Karin van Garderen developed radiomics and deep learning methods for classification and segmentation of brain tumours, described in the section of Prof. Smits, see page 236. In 2021, Sebastian van der Voort received his PhD degree cum laude , for his excellent work in this field. Karin van Garderen published a paper on automated glioma segmentation in the clinic. Martijn Starmans developed a generic software platform for radiomics, called WORC, and applied this to no less than 12 clinical problems, involving collaborations with numerous clinical researchers within the Erasmus MC. Based on his work on soft-tissue tumours, we received a grant from the Hanarth foundation that will enable us to further develop and validate the radiomics methods for soft-tissue tumours in the coming years. In 2021, PhD student Douwe Spaanderman started working on this topic. Finally, we are participating in H2020 EUCanImage, a large-scale international project funded by the European Commission to build a secure and federated imaging platform for next-generation artificial intelligence in oncology.
Expectations & Directions
In the upcoming years, we will further expand both the fundamental research on development of new image analysis algorithms, and the applied research, in which we test the newly developed methods in clinical applications. Areas of interest in the next years will include radiomics, deep learning, and disease progression modelling. Also, we aim to expand our efforts in the domain of ophthalmic imaging.

Figure1. The MERLIN prototype, a novel multi-modal, multi-scale retina imaging device. Our group contributed with motion compensation and image postprocessing methodology.
Funding
Lambin, Philippe (Maastro), Wiro Niessen, Stefan Klein, Jifke Veenland and Ivo Schoots, and consortium partners, Technology Foundation ‘STW’, 2016-2021: “Radiomics STRaTegy- Non-invasive stratification of tissue heterogeneity for personalized medicine”.
Levecq, Xavier (Imagine Eyes), Wiro Niessen, Theo van Walsum, Stefan Klein, and consortium partners: EU Horizon2020, 2018-2021: “MERLIN – Multi-modal, multiscale retinal imaging”.
Sijbers, Jan (Universiteit Antwerpen), Wiro Niessen, Dirk Poot, Stefan Klein, and consortium partners: EU Horizon2020 MSCA-ITN, 2018-2022: “B-Q MINDED –Breakthroughs in Quantitative Magnetic resonance ImagiNg for improved Detection of brain Diseases”.
Rousian, Melek (Dept. Obstetrics & Gynecology), Stefan Klein, Regine Steegers, Wiro Niessen: Erasmus MC Mrace 2018-2022: “Modelling the impact of maternal obesity on longitudinal prenatal human brain development using a 4D spatiotemporal ultrasound atlas”.
Ikram, Arfan, and consortium partners: ZonMW – Memorabel 2018-2022: Netherlands Consortium of Dementia Cohorts.
Lekadir, Karim (Universitat de Barcelona), Aad van der Lugt, Wiro Niessen, Stefan Klein, Daniel Bos, and consortium
partners: EU Horizon2020, 2020-2023: “EUCanImage – A European Cancer Image Platform Linked to Biological and Health Data for Next-Generation Artificial Intelligence and Precision Medicine in Oncology”.
Klein, Stefan, Jan-Jaap Visser, Dirk Grunhagen, Kees Verhoef, Stefan Sleijfer, Wiro Niessen, Arno van Leenders, Martijn Starmans: Hanarth Fonds, 2021-2025: “Automatic grading and phenotyping of soft-tissue tumors through machine learning to guide personalized cancer treatment”.
Gonzalez, Juan (Health Sciences Institute of Aragon), and consortium partners: EU Horizon2020, 2021-2023, HealthyCloud: Health Research & Innovation Cloud.
Invited Lectures
S. Klein, “Imaging Community”, Health-RI Conference 2021, online
M.P.A. Starmans, “Multicentre studies for more robust radiomics signatures”, European Congress of Radiology, online.
J. Hirvasniemi, “Knee OsteoArthritis Prediction (KNOAP 2020) Challenge”, 15th International Workshop on Osteoarthritis Imaging, Rotterdam.
Highlights
Both Vikram Venkatraghavan and Sebastian van der Voort received their PhD degrees cum laude.
A total number of 12 BSc/MSc students were part of our team in 2021! Due to Covid19 restrictions, these students had to work remotely, from home. Despite this challenging situation, we had very fruitful collaborations. We are very grateful for their important contributions to our research.
Additional Personnel
Mathias Polfliet – Associated PhD student
Mahlet Birhanu – Research Software Engineer Konstantinos Ntatsis – Research Software Engineer
Internship students:
Teun Tanis, Li Shen Ho, Coen van Gruijthuijsen, Iris Huele, Amber Heijdra, Anastasis Alexopoulos, Raneim Mohamed, Casper Donkervoort, Netanja Harlianto, Joris Vromans, Gonnie van Erp, Elsemiek Smilde.

DEEP IMAGING-GENETICS FOR OSTEOARTHRITIS
JUKKA HIRVASNIEMI, PHD
Project Funding Convergence Health & Technology Impulse Programme
Research period January 2021 – December 2022
E-mail j.hirvasniemi@erasmusmc.nl
Osteoarthritis is the most common joint disease in the world. It is a leading cause of disability and results in a tremendous burden for patients and society. Therefore, advances in diagnostics, prevention, and treatment of osteoarthritis will have a major effect on patients and society.
Osteoarthritis affects all tissues in the joint, e.g., causing progressive degeneration of articular cartilage and changes in the subchondral bone density and structure. Disappointedly, the etiology of osteoarthritis is still unsolved.
I am currently working on the Deep Imaging-Genetics for Osteoarthritis project that is a collaboration project between Erasmus MC and TU Delft. The aims of the project are 1.) to improve prediction of osteoarthritis incidence and progression by combining imaging and genetics data using deep learning, 2.) to enhance understanding of osteoarthritis disease etiology and estimate effect of interventions by developing and applying causal inference methods for imaging and genetics data, and 3.) to develop machine learning technology that can robustly cope with missing data and distributional mismatch and that can facilitate causal inference in the context of imaging and genetics data.
We organized the KNee OsteoArthritis Prediction (KNOAP2020) challenge (Figure 1) to objectively compare different algorithms for prediction of osteoarthritis incidence using a test set of over 400 knees with blinded ground truth. Usually, such methods are optimized for specific imaging datasets and it is unclear how different methods would perform on previously unseen data. I presented the results of the KNOAP challenge in a webinar in 2021 and in a keynote lecture at the International Workshop on Osteoarthritis Imaging 2021.

Details of the KNOAP challenge can be found here: https:// knoap2020.grand-challenge.org/. The results of the challenge will be published in a scientific paper in 2022.
I am closely collaborating with the ADMIRE group (PI: Edwin Oei) in musculoskeletal image analysis projects. I am also involved in projects of Department of General Practice that utilize segmentations of meniscus and bone from MRI. I have an on-going collaboration with University of Oulu and I for example co-supervised a PhD student (Robel Gebre, University of Oulu, Finland; PhD awarded 2021) in a project that investigated biomechanical and structural risk factors associated with low-energy acetabular fractures using CT.
Figure 1. The KNee OsteoArthritis Prediction (KNOAP) challenge.

4D SPATIOTEMPORAL ATLAS OF THE EMBRYONIC BRAIN
WIETSKE BASTIAANSEN, MSC
PhD Student
Advisors Stefan Klein , Melek Rousian, Anton Koning, Wiro Niessen & Régine Steegers-Theunissen
Project Funding
Erasmus MC Research Grant: “Modelling the impact of maternal obesity on longitudinal prenatal human brain development using a 4-dimensional spatiotemporal ultrasound atlas”
Research period March 2019 – March 2023
Email w.bastiaansen@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine, and Obstetrics & Gynecology.
The Predict study is a large hospital-based cohort study focusing on the relationship between maternal and paternal health and lifestyle factors, and pre- and postnatal development. Currently, 3D ultrasound data of around 1500 pregnancies are available. The current approach for analyzing the growth of the embryonic brain is measuring volumes semiautomatically in 3D ultrasound images using Virtual Reality techniques. This approach is time-consuming, has a low success rate, and lacks an overview. To overcome this, we will develop a comprehensive four-dimensional (4D) spatiotemporal atlas of the human embryonic and fetal brain, describing growth and development in great detail. This will contribute to our ultimate goal to better diagnose, treat and prevent neurodevelopmental disorders in the future.
The crucial first step for the creation of the atlas is the segmentation and spatial alignment of the embryo in the 3D ultrasound. To achieve this, we developed a deep learning algorithm that learns how to align the image to a given standard orientation. Using this we obtained the segmentation of the embryo in the original image (figure 1). We developed and validated our method on data acquired between the 8th and 12th week of pregnancy.
Furthermore, we started the development of the Spatiotemporal atlas. We will take a learning-based approach to learn an atlas that is representative of the population. This atlas can then be used to analyze if the growth and development of the brain during pregnancy is normal. Furthermore, we can analyze the influence on brain development of mode of conception (spontaneous versus IVF), pre-conceptional BMI of the mother, diet, alcohol consumption, and many other factors.

Figure 1. Visualization of ultrasound datasets from the test set, not used during training of the algorithm. From left to right: segmentation of the image, the ground truth segmentation obtained in Virtual Reality and midsagittal plane in standard position. For visualization, 2D slices of 3D datasets were taken. Embryonic volumes (EV) are given in mm3.

Advisors
RADIOMICS IN HEAD-AND-NECK CANCER
THOMAS
PHIL, BSC
PhD Student
Stefan Klein, Wiro Niessen & Eppo Wolvius
Project Funding Dept. Oral & Maxillofacial Surgery, Erasmus MC
Research period January 2019 – December 2022
Email t.phil@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine, Oral & Maxillo-facial Surgery, and Otorhinolaryngology. We also collaborate with the Leiden University Medical Center.
The field of Radiomics makes use of quantitative feature analyses in medical images to uncover disease characteristics. Advanced image analysis methods and artificial intelligence techniques are used in order to extract more information out of medical images than can be seen by the naked eye.
In this project, we focus on applications of radiomics in the head-and-neck region. Specifically, we focus on oral squamous cell carcinoma, vestibular schwannomas, and cervical spine degeneration, the latter two in collaboration with Leiden University Medical Center.
For oral squamous cell carcinoma, we currently focus on tumors in the tongue. Our work revolves around using radiomics features to investigate if recurrence and pathology can be predicted more accurately.
A vestibular schwannoma is a type of tumor growing from the vestibulocochlear nerve. This tumor is usually benign but it must be resected if the tumor starts growing as it pushes against the brain stem. We are currently investigating if radiomics features are potential predictors of tumor growth.
Cervical spine degeneration is associated with chronic neck pain. Degenerative changes in the neck include the decrease in intervertebral disc space and additional bone formation on the vertebra called osteophyte formation. Our aim is to automate the scoring of this degeneration, for which we are evaluating a combination of radiomics and deep learning techniques.

Figure - Example lateral neutral X-ray of the spine with manual segmentation of vertebrae, used as input for the radiomics analysis.

Advisors Stefan
COMPUTER-AIDED DIAGNOSIS OF SOFT-TISSUE TUMORS
DOUWE J SPAANDERMAN, MSC
PhD Student
Klein, Martijn Starmans & Wiro Niessen
Project Funding Hanarth Fonds: “Automatic grading and phenotyping of soft-tissue tumors through machine learning to guide personalized cancer treatment”
Research period May 2021 – May 2025
Email d.spaanderman@erasmusmc.nl
This is a collaborative project between the Departments of Radiology & Nuclear Medicine and Epidemiology.
Soft-tissue tumors (STTs) are a rare and complex group of lesions with a broad range of differentiation. All STT subtypes greatly differ in their clinical behavior, aggressiveness, molecular background, and preferred treatment given. Diagnosis of the correct phenotype, the grade of aggressiveness, and molecular make-up is therefore of utmost importance. Currently, correct diagnosis requires a biopsy, which is invasive, suffers from intra-tumor heterogeneity and is difficult to repeat. On the other hand, Diagnosis of STT by a radiologist on imaging, such as computed tomography (CT) and magnetic resonance imaging (MRI), tends to be subjective and not precise. Therefore, there is a need for a non-invasive, precise diagnostic method for STT in order to guide personalized cancer treatment. To this end, we are developing a machine learning model to distinguish grading and phenotyping for STT based on imaging features.
As previous research has shown that tumor segmentation is required for accurate disease predictions, we have focused on developing automatic and minimally interactive methods for segmentation of STT. These segmentation models can be used in our radiomics model for automatic diagnosis.
Finally, as STT is a rare disease type, we are collaborating with 9 international partners in order to generate a large imaging dataset, which we intend to make publicly available.
Figure 1. Overview of our automatic and minimally interactive segmentation methods for soft tissue tumors.


MARTIJN P A STARMANS, MSC STREAMLINED QUANTITATIVE IMAGING BIOMARKER DEVELOPMENT
Advisors Wiro Niessen, Jan-Jaap Visser & Stefan Klein
Project Funding
PhD Student
NWO-TTW “Non-invasive stratification of tissue heterogeneity for personalized medicine – Radiomics STRaTeGy” & EuCanShare and EuCanImage (European Union’s Horizon 2020 research and innovation programme under grant agreements Nr. 825903 and Nr. 952103, respectively).
Research period October 2016 – October 2023
Email m.starmans@erasmusmc.nl
The field of radiomics concerns extraction of large amounts of quantitative image features from images in order to predict clinical characteristics. It has a wide range of applications, such as predicting therapy response, genetic mutations and tumor phenotyping. Current practices show promising results, but are targeted at specific problems, therefore not being generalizable and requiring manual tuning.
Therefore, there is a need for a generalized radiomics platform. To this end we developed a multimodal radiomics computational platform called Workflow for Optimal Radiomics Classification (WORC), see the figure. Through the use of automated machine learning, our toolbox can
Figure 1. Schematic overview of our open-source radiomics platform applied to twelve different clinical applications, of which six datasets have been publicly released.
automatically optimize construction of the radiomics workflow per application. To test and improve our toolbox, we are collaborating with multiple radiologists and surgeons from various international hospitals on multiple (20+) clinical applications. WORC has been used for example in liver cancer, sarcoma, prostate cancer, gastric cancer, mesenteric fibrosis, melanoma, head and neck cancer, dementia, glioma, and CPRS, using not only MRI and CT, but also video thermography and MEG data, and predicting various clinical applications such as diagnosis, prognosis, genetics, survival, therapy response and phenotyping.
In all but one application (melanoma), WORC has developed a successful prediction model. Future research includes the extension to other applications, validation of our findings, either externally or through prospective trials, and extending the methodology of WORC.


THE SYMPHONY OF CACOPHONY: UNDERSTANDING THE ORDER IN NEURODEGENERATIVE DISEASES
VIKRAM VENKATRAGHAVAN, PHD
PhD Awarded 8 June 2021 *** CUM LAUDE ***
Advisors Wiro J. Niessen, Stefan Klein, & Esther E. Bron
Project Funding EU’s Horizon 2020: “European Progression Of Neurological Disease (EuroPOND)”
Short CV Vikram Venkatraghavan was born on June 10th, 1988 in Srirangam, a town in the southern part of India. He obtained his Master of science degree, at the School of Medical Science and Technology at the Indian Institute of Technology, Kharagpur. Before starting his PhD, he worked for Angiometrix, a start-up company based in Bangalore and San Francisco, where he led a small team in building an image-processing based system for percutaneous coronary intervention assistance. His work led to the filing of two crucial patents for the company. Following his passion for research, Vikram joined the Biomedical Imaging Group Rotterdam (BIGR) in Erasmus MC as a PhD student. He developed methodologically novel algorithms for understanding the progression of dementia. These novel methods led to two oral presentations at the highly selective and prestigious IPMI conference. He participated in an international competition to predict the progression of Alzheimer’s disease which ended up as one of the winning entries in the challenge. Moreover, he forged close collaborations with clinicians, to increase the clinical relevance of these methods which further led to several impactful papers during his PhD.
This is a collaborative project between the Departments of Radiology & Nuclear Medicine and Epidemiology.
Neurodegenerative diseases such as Alzheimer’s disease (AD) are notoriously heterogeneous; pathologically as well as in their clinical presentation in patients. There are differences between different patients in terms of the pathways of progression, the speed of progression, and the effect the progression has on a patient’s cognition. This myriad of differences not only makes clinical diagnosis of these diseases very challenging, but also has implications for the efficacy of drug trials. As heterogeneous as these diseases are, there is an underlying order in their progression. An underlying method to their disruption of homeostasis. An underlying symphony leading to the cacophony.
This thesis is about developing data-driven methods for understanding the orderly progression of neurodegenerative diseases. We developed novel disease progression modeling methods to estimate the progression timeline of neurodegenerative diseases in a data-driven way from in-vivo patient data. In collaboration with

clinical researchers, we used the developed approaches to obtain novel insights into the neurodegenerative disease pathways of four such diseases: AD, frontotemporal dementia (FTD), Creutzfeldt-Jakob disease (CJD), and multiple sclerosis (MS). Lastly, to check the utility of the developed approaches for (pre-)clinical use, their generalizability to cohorts from multiple memory clinics as well as their ability to identify preclinical and prodromal stages in a population-based cohort was validated in this thesis.
http://hdl.handle.net/1765/135552

Esther Bron is Assistant Professor in Medical Image Analysis and is heading the Neuroimage Analysis and Machine Learning research line. She is affiliated with the Biomedical Imaging Group Rotterdam (BIGR, http://www.bigr.nl). In 2011, Esther received her MSc degree in Medical Natural Sciences – specialization Medical Physics, cum laude – at the VU University, Amsterdam/NL. Esther obtained her PhD degree on March 9 2016 with her thesis entitled Advanced MRI Analysis for Computer-Aided Diagnosis of Dementia. She organized the CADDementia challenge at MICCAI 2014 which compared image-based diagnosis algorithms and led to a high-impact

journal publication. In 2015, Esther spend few months in the Progression Of Neurodegenerative Disease (POND) group at University College London. With this group, she is organizing the TADPOLE challenge which seeks the best prediction method for Alzheimer’s disease. She won the Young eScientist Award 2018 by the Netherlands eScience Center and was finalist for the Young Outstanding Researcher Award 2020 by Alzheimer Nederland. Current research interest include neuroimage analysis, machine learning, translation, multi-center studies, and diagnostic and predictive disease modeling. e.bron@erasmusmc.nl
NEUROIMAGE ANALYSIS & MACHINE LEARNING
ESTHER E BRON, PHD
assistant professor

Context
Brain diseases such as dementia impose an enormous burden to the individual and to society. As a consequence, there is an urgent need to develop effective preventive and therapeutic strategies. Early detection and accurate prediction of the progression of at-risk subjects are key in this development. Early detection is important for successful treatment and accurate prediction will play a major role in clinical trials, e.g. for selecting homogenous patient groups to reduce variability in outcome measures.
Neuroimage analysis and machine learning play a herein a crucial role, i.e. for developing robust quantitative brain imaging biomarkers and methods for early detection and accurate prediction. My research interest is to optimally combine brain imaging, clinical data and artificial intelligence techniques to promote an accurate and early diagnosis, and eventually the right treatment, for patients with neurodegenerative disease. My group’s research focuses on development of both novel biomarkers and methods for detection and prediction. While AI is showing great experimental results and large high-quality datasets are available, methods are not yet finding their way into clinical practice. Therefore I aim to develop and collect accurate diagnostic and prediction methodology, to validate those methods on large and clinically representative datasets, to identify and overcome challenges for clinical implementation.
Top Publications 2021
E.E. Bron, S. Klein, J.M. Papma, L.C. Jiskoot, V. Venkatraghavan , J. Linders, P. Aalten, P.P. De Deyn, G.J. Biessels, J.A.H.R. Claassen, H.A.M. Middelkoop, M. Smits, W.J. Niessen , J.C. van Swieten, W.M. van der Flier, I.H.G.B. Ramakers, A. van der Lugt , Cross-Cohort Generalizability of Deep and Conventional Machine Learning for MRI-based Diagnosis and Prediction of Alzheimer’s Disease , NeuroImage: Clinical, 2021
B. Li, W.J. Niessen, S. Klein, M. de Groot, M.A. Ikram, M.W. Vernooij, E.E. Bron, Longitudinal diffusion MRI analysis using Segis-Net: a single-step deep-learning framework for simultaneous segmentation and registration, NeuroImage, 2021
R.V. Marinescu, N.P. Oxtoby, A.L. Young, E.E. Bron , A.W. Toga, M.W. Weiner, F. Barkhof, N.C. Fox, A. Eshaghi, T. Toni, M. Salaterski, V. Lunina, M. Ansart, S. Durrleman, P. Lu, S. Iddi, D. Li, W.K. Thompson, M.C. Donohue, A. Nahon, Y. Levy, D. Halbersberg, M. Cohen, H. Liao, T. Li, K. Yu, H. Zhu, J.G. Tamez-Pena, A. Ismail, T. Wood, H. Corrada Bravo, M. Nguyen, N. Sun, J. Feng, B.T.T. Yeo, G. Chen, K. Qi, S. Chen, D. Qiu, I. Buciuman, A. Kelner, R. Pop, D. Rimocea, M.M. Ghazi, M. Nielsen, S. Ourselin, L. Sorensen, V. Venkatraghavan , …, S. Klein , D.C. Alexander, The Alzheimer's Disease Prediction Of Longitudinal Evolution (TADPOLE) Challenge: Results after 1 Year Follow-up, Machine Learning for Biomedical Imaging (MELBA), 2021
Research Projects: Objectives & Achievements
Neuroimaging biomarkers & multi-center validation
We develop novel imaging biomarkers based on brain imaging. Recently, we focused on imaging biomarkers of small vessel disease and aging, such as automatic detection of lacunar infarcts, segmentation of cortical infarcts, autoencoders for brain changes, and group-wise registration for sensitive longitudinal diffusion biomarkers, all works led by postdoctoral researcher Bo Li, together with several MSc students (Liselot Goris, Claudia Chinea Hammecher).
In addition, two MSc students – Diede Wijnbergen and Dalia Aljawaheri – developed deep-learning based imaging biomarkers specifically for children with craniosynostosis and compared those to conventional methods. We found that this population required dedicated image analysis methods because of the young age (brain tissue segmentation cannot rely on just signal intensity) and the deformation (atlas-based region segmentation needs a registration procedure that can capture large enough deformations.
For the Heart-Brain Connection study, we finished analysis of the baseline dataset (N=566) and the 2-year follow-up (N=349). This included visual analysis by neuroradiologist, manual infarct segmentation, automatic global/lobar/regional volume quantification, ASL analysis, phase-contrast MRA flow analysis, resulting in a total of 1811 brain MRI variables for each participant. This has resulted in several articles novel biomarkers and sex differences in vascular cognitive impairment (Kuipers et al., 2021a, 2021b; Van den Brink et al., 2021).
In 2021, the ‘Imaging Office’ has developed from a departmental working group to a service facility, a central point for all requests regarding image data management and (automatic) image analysis, for both research and clinic. Amongst others, we aim to offer the analysis of imaging data, by automatic pipelines developed in our department, as a service to companies, researchers, and for translation to clinical practice.
Accurate detection and prediction of dementia
In this research line, we develop and evaluate machine learning methods for early detection of dementia onset and accurate prediction of the progression of the disease.
Supervised machine learning methods like support vector machines (SVM) have shown to be very successful in early detection of Alzheimer’s disease, but more recent methods based on for example convolutional neural networks (CNN) are also very promising. A major topic of interest is the generalizability of such methods to other clinical datasets, on which we published an article using data of the Alzheimer’s Disease Neuroimaging Initiative and the Parelsnoer Neurodegenerative Diseases study. We conclude that deep and conventional classifiers performed equally well for AD classification and their performance decreased only slightly when applied to the external cohort (Bron et al., 2021). Several MSc students worked on related topics: Lotte Mulder (comparison framework for prediction algorithms in Alzheimer’s disease), Myrthe van Haaften (vascular effects in classification of dementia).
Figure 1: Disease progression modeling using imaging, fluid and cognitive data in frontotemporal dementia (genetic progranulin subtype). (A) Cascade of biomarker changes along with the uncertainty associated with it showing that language and neurofilament light-chain are among the first biomarkers to become
abnormal, (B) The disease severity obtained from the model separates presymptomatic and symptomatic mutation carriers. This type of information is the first step towards identifying biomarkers useful for detecting disease onset (Panman et al., 2021).

Disease progression: Frontotemporal dementia
PhD student Vikram Venkatraghavan (see page x) developed a highly novel method for disease progression modelling: Discriminative Event Based Model (DEBM). This method estimates the order in which biomarkers become abnormal during disease progression, purely based on cross-sectional measurements. We applied this method to several diseases (Venkatraghavan et al, 2021, Vinke et al, 2021, Panman et al., 2021, Van het Ende et al., 2021, Marinescu et al., 2021, Dekker et al., 2021)
In particular, we have a strong collaboration with the Neurology department (Prof. J.C. van Swieten, Dr. H. Seelaar) to use this model for new insights into frontotemporal dementia. Frontotemporal dementia, which has a typically young onset age, has a major impact on patients, their families and the society. The diverse clinical manifestations with severe behavioural changes over the course of disease are associated with high caregiver burden and hampers care planning. We applied DEBM to progranulin-related frontotemporal dementia, showing that neurofilament light-chain and language are among the first biomarkers to become abnormal (Panman et al., 2021) (Fig. 1) In another publication in Brain, we looked into fluid biomarkers for frontotemporal dementia and were able to draw conclusions on neuropentraxin measurement cerebrospinal fluid (CSF) being the first biomarker to become detectably abnormal, followed by neurofilament light-chain in serum and CSF (Van der Ende et al., 2021). This type of information is the first step towards identifying biomarkers useful for detecting and predicting disease onset.
Expectations & Directions
In the next years, we aim to further expand all research areas. Regrading the development of novel brain imaging biomarkers, focus will be on lacunar infarcts and craniosynostosis. Regarding detection and prediction methods, our focus will be on state-of-the-art methodology, generalization to other datasets, validation and translation to clinical practice.
Funding
EE Bron , WJ Niessen , J Glastra, WM van der Fier, GJ Biessels, Dutch Heart Foundation PPS grant 2018-2021: “Improvas: Improved Prognosis of Vascular cognitive impairment using automatic quantitative imaging biomarker extraction and disease modelling”
M Daemen (AUMC), GJ Biessels (UMCU), WJ Niessen , EE Bron , and consortium partners: CardioVasculair Onderzoek Nederland (CVON) 2019-2024: “HBCx: Heart-Brain Connection Crossroads
WJ Niessen, F Vos, MA van Buchem, EE Bron , JHJM de Bresser: Medical Delta 2019-2024: “Medical Delta Diagnostics 3.0: Dementia and Stroke”
B Li, EE Bron , F Vos, Convergence in Health and Technology Open Mind grant on ‘Neurodegeneration beyond DTI’. 2021-2022
Invited Lectures
E.E. Bron , Imaging biomarkers and machine learning in dementia, Imaging Biomarkers Part II - Deutscher Röntgenkongress, Berlin, Germany (24-09-2021)
Figure 2: Presentation Human-centred AI for Health, Visit Ministry of Health and minister Hugo de Jonge on 7 July 2021 at Convergence Health and Technology Square Erasmus MC

E.E. Bron, Cross-cohort validation of machine learning for dementia diagnosis and prediction, DEMON Network - Combining imaging data using machine learning workshop, Exeter, UK (14-09-2021)
E.E. Bron , F.M. de Vos, Human-centred AI for Health, Visit Ministry of Health at Convergence Square Erasmus MC (07-07-2021, see Fig. 2)
E.E. Bron , Brain MRI and machine learning for predicting progression of Alzheimer's disease, Festival of Neuroscience - British Neuroscience Association (BNA), Brighton, UK (14-04-2021)
E.E. Bron , Analysis of neurodegeneration on brain imaging using machine learning, Anne Klibanski Visiting Lecture Series, Center for Faculty Development, Massachusetts General Hospital/Harvard Medical School, Boston, MA/USA, (09-03-2021)
Highlights
In 2021, Esther Bron started as chair of the special interest group on Reproducibility and Open Science of the Deep Dementia Phenotyping (DEMON Dementia) Network.
Vikram Venkatraghavan was awarded a Out of the Box grant from the DCVA Hearth-Brain Connection Crossroads consortium.
Vikram Venkatraghavan obtained his PhD degree with this thesis entitled “The symphony of cacophony” on June 8 2021 with a cum laude distinction.
Additional Personnel
Mahlet Birhanu, MSc – Research Software Engineer
Alexander Harms, MSc – Research Software Engineer
Myrthe van Haaften, BSc – MSc Student
Claudia Chinea Hammecher, BSc – MSc Student
Karina Hoefnagel – BSc Student
Liselot Goris, BSc – MSc Student
Lotte Mulder, BSc – MSc Student
Diede Wijnbergen, BSc – MSc Student
Dalia Aljawaheri, BSc – MSc Student

NEUROIMAGE ANALYSIS WITH DEEP LEARNING
BO LI, MSC, PHD
Project Funding Medical Delta Diagnostics 3.0: Dementia and Stroke
Research period September 2019 – June 2022
E-mail b.li@erasmusmc.nl
This project is a joint effort among the Erasmus MC, Delft University of Technology, and Leiden University Medical Center.
Subtle changes in the micro- and macrostructure of brain white matter (WM) have been associated with neurodegeneration in normal aging and in disease. The WM consists of axonal fibers that enable communication between brain regions and can be functionally grouped into WM tracts. To improve the understanding of WM tracts and their involvement in the processes of neurodegeneration, it is essential to segment them and quantify their characteristics with high accuracy, reproducibility, and consistency. For this aim, we have developed deep learning approaches for WM tract segmentation (Neuro4Neuro, NeuroImage 2020) and for longitudinal image analysis by simultaneous optimization (MICCAI 2019). In 2021, we extended the success of these approaches to concurrent segmentation of multiple tracts (Segis-Net, NeuroImage 2021) and to unbiased group-wise mean-space registration of brain MRI (SPIE 2021).
In addition, the complex composition of WM makes diffusion measures alone still open to many biological interpretations. For additional insight into the trajectories of neurodegeneration, it is essential to devise a new imaging

biomarker that uses other complementary multimodal imaging techniques beyond DTI. Macromolecule tissue volume (MTV) derived from qMRI is known to be sensitive to the variation of myelin content, and therefore may serve as such a biomarker. Yet, a large dataset of both DWI and qMRI scans of the same individuals does not currently exist. To tackle this problem and to investigate the added value of MTV for studying neurodegeneration, we proposed a pilot study into developing a “bridging” biomarker that is both indicative of MTV and derivable from existing Rotterdam Study data (grant, Convergence Health & Technology Open Mind Call 2021; Figure 1).
Lacunes of presumed vascular origin are important to assess cerebral small vessel disease and cognitive diseases such as dementia. Whereas recent developments in automatic algorithms have shown to make the detection of lacunes faster, they also showed a large number of false positives, which makes them impractical for use in clinical practice or large-scale studies. Thus, we developed a novel framework that, in addition to lacune detection, outputs a categorical burden score. This score could provide a more practical estimate of lacune presence that simplifies and effectively accelerates the imaging assessment of lacunes (submitted, OHBM 2022). Also, to construct neuroimaging endophenotypes for population studies, we proposed a first unsupervised autoencoder for interpretable dimensionality reduction of high-resolution neuroimaging data that preserves local and global brain structure. As proof of principle, we associated the derived endophenotypes with age and gender, and achieved the state-of-the-art efficiency and interpretability at highresolution (submitted, OHBM 2022; Figure 2).

Figure 2. The reconstructed age- and gender-specific modulated grey matter maps, and the observed changes across age and gender.
Figure 1. Water volume fraction, the complement of MTV.


MOLECULAR IMAGING & THERAPY
IN MEMORIAM
In May 2021 our beloved colleague, Marion Hendriks – de Jong, passed away after a tireless battle against cancer. Marion was Professor of Preclinical Nuclear Medicine in our department, and lead the Translational Molecular Imaging and Therapy research group. A true pioneer in radionuclide theranostics, she was leading in the preclinical and translational development of a large number of novel tracers for cancer imaging and treatment. She was principal investigator in several national and international research projects and co-authored over 400 peer-reviewed papers. She also served in multiple international committees and boards.

Marion was a brilliant scientist and an excellent mentor. She always knew how to inspire and encourage her colleagues. She had an enormous network, a lot of them who she became friends with and she always supported wholeheartedly. Her sincere, caring and warm personality made her an easy person to talk to and confide in. For many of us Marion was more than a promotor, supervisor and colleague. She was also a mother-figure and a friend.
She had an impact on all of our lives, great or small. We still miss her every day.
'The world changes from year to year, our lives from day to day, but the love and memory of you, shall never pass away'
JOINT APPOINTMENT MOLECULAR GENETICS
Julie Nonnekens received her MSc in Biotechnology at Wageningen University in 2009. She obtained her PhD in cancer biology with the focus on DNA repair mechanisms at the University of Toulouse (France) in 2013. Following, she was a postdoc at the Hubrecht Institute working on ribosome biogenesis in cancer and longevity. In 2014 Julie joined the Erasmus MC Department of Radiology & Nuclear Medicine with a joint appointment at Molecular Genetics. The research of her group bridges the interests of both departments in the

field of DNA damage repair mechanisms and nuclear medicine to study the radiation biology of targeted radionuclide anticancer treatment in order to ultimately optimize treatment regimens.
Julie has received several (young investigator) awards and is principal investigator on various research grants including the prestigious ERC starting grant. She is board member (secretary) of the Netherlands Society of Radiobiology and co-founder of the European working group on Radiobiology of Molecular Radionuclide Therapy. j.nonnekens@erasmusmc.nl
RADIOBIOLOGY OF RADIONUCLIDE THERAPY
JULIE NONNEKENS, PHD
assistant professor

Context
Targeted radionuclide therapies (TRT) are revolutionizing treatment of patients with metastasized cancers. During TRT, radiolabeled compounds are targeted to the cancer cells via specific tumor binding (e.g. via receptors). Once bound to the tumor cells, the radionuclides will induce DNA damage leading to cancer cell death. Currently, more cancer patients are being treated with TRT than ever before. However, it is clear that some patients are being over-treated (resulting in toxicity) or under-treated (no tumor regression). This indicates the clinical need for therapy improvement. A better understanding of the radiobiology, i.e. of the biological effects of ionizing radiation of TRTs, could contribute to increasing their effectiveness by providing evidence in favor of one treatment method or regimen over another. With better radiobiological understanding, TRT success could be enhanced and might even progress from mostly palliative towards curative.
Top Publications 2021
Feijtel D , Doeswijk GN , Verkaik NS, Haeck JC, Chicco D, Angotti C, Konijnenberg MW , de Jong M , Nonnekens J . Intra-tumoral somatostatin receptor 2 heterogeneity confers differential radionuclide therapy response in preclinical neuroendocrine tumor models. Theranostics 2021; 11(2):491-505.
Geenen L, Nonnekens J, Konijnenberg MW , Baatout S, de Jong M and Aerts A. Overcoming nephrotoxicity in peptide receptor radionuclide therapy using [177Lu]Lu-DOTA-TATE for the treatment of neuroendocrine tumours. Nucl Med Biol. 2021 NovDec;102-103:1-11.
Tamborino G, Nonnekens J , De Saint-Hubert M, Struelens L, Feijtel D, de Jong M and Konijnenberg MW . Dosimetric evaluation of receptor-heterogeneity on the therapeutic efficacy of peptide receptor radionuclide therapy: correlation with DNA damage induction and in vivo survival. J Nucl Med. April 2021, jnumed.121.262122.
Research Projects: Objectives & Achievements
Cellular effects of TRT in tumor and healthy tissues
The major focus of our work lays on targeting compounds labeled with the beta-particle emitter lutetium-177. These compounds are the somatostatin analogue DOTA[Tyr3]octreotate ([177Lu]Lu-DOTA-TATE) for treatment of neuroendocrine tumors (NET) and prostate specific membrane antigen (PSMA) binding compounds ([177Lu]LuPSMA) for treatment of prostate cancer (PCa). Lutetium177’s β-particles will induce DNA damage leading to tumor cell death with limited harm to healthy tissues. Patient treatment strongly increases progression-free survival and life quality. There is nevertheless still room for improvement, and for possible future therapy optimizations, it is essential to have a better understanding of local treatment effects, both in tumor and healthy tissues.
To gain insight in the underlying radiobiological principles, we are characterizing the TRT-induced DNA damage response (DDR) in cell lines, ex vivo cultured human tumor slices and xenografted mice by using live cell microscopy, molecular biological techniques and histology. We have amongst others shown that TRT induces various types of DNA damage in tumor cells as well as in normal tissue cells. Furthermore, we are elucidating other underlying cellular processes that are activated by TRT using RNA expression analysis, drug screenings, and by creation of knockouts using CRISPR-Cas9 genome editing.
Projects:
• Tumor radiobiology of NET TRT [ Danny Feijtel ]
• Normal tissue radiobiology of NET TRT [ Lorain Geenen ]
• Tumor cell radiobiology of PCa TRT [ Eline Ruigrok ]
• Pathway activation analysis of NET TRT [ Stefan Roobol, Thom Reuvers and Nicole Verkaik ]
Radiobiology of different radiation qualities
Besides lutetium-177, other radionuclides are being used in clinical practice or expected to be implemented in the future. These include the beta- and Auger emitter terbium-161 and the alpha emitter actinium-225. Different radionuclides have different cellular effects and these are based mostly on the type of decay, half-life and range. To be able to better predict which radionuclide is suitable for which indication, we are investigating the difference between these radiation qualities using in vitro biological experiments en in silico dose simulations.

Figure 1. Heterogeneous TRT efficacy caused by non-homogeneous receptor expression. A) Heterogeneous cell death induction in a xenografted tumor 5 days after TRT measured by fluorescent staining. B) Heterogeneous targeted receptor expression (left) in the xenografted tumor and corresponding dose heatmap showing heterogeneous dose distribution leading to the heterogeneous cell death induction.
In addition to the investigation of biological effect of different radiation qualities, we are also focusing on development of detailed dosimetric modes. At the moment, there is no accurate method to determine the dose of TRT on various cellular targets and intratumoral heterogeneous regions. Therefore, it is essential to perform dosimetry to understand radiation dose-effects and integrate them into treatment planning systems for TRT. In this context, in collaboration with Dr. Mark Konijnenberg and the SCK-CEN in Belgium, we are creating models to predict biological responses from (micro)dosimetric quantities by exploring several in vitro and in vivo exposure scenarios.
• Live cell imaging of DNA repair dynamics by lutetium-177 and actinium-225 for NET TRT [ Stefan Roobol ]
• Radiobiological comparison of lutetium-177 and actinium-225 for PCa TRT [ Eline Ruigrok ]
• Radiobiological comparison of lutetium-177 and terbium-161 for healthy tissue and NET TRT [ Lorain Geenen ]
• Micro- and macrodosimetry of NET TRT [ Giulia Tamborino ]

Figure 2. Radiobiological measurements and dose calculations of alpha and beta radionuclide therapy. A) DNA damage induction of [225Ac]Ac-PSMA-I&T and [177Lu]Lu-PSMA-I&T as measured by 53BP1 foci immunofluorescent staining (DNA double strand break marker) in cancer cells (nucleus marked in blue). B) Doseresponse curves of [225Ac]Ac-PSMA-I&T and [177Lu]Lu-PSMA-I&T showing an relative biological effectiveness of 4.2 of [225Ac]AcPSMA-I&T over [177Lu]Lu-PSMA-I&T.
Radiosensitization to improve radionuclide therapy outcome
Work by us and others has shown that TRT can be potentiated by combination with radiosensitizing compounds. Especially, various DDR inhibitors can function as radiosensitizers, and differentially impair DNA repair of TRT induced DNA damage and thereby vastly increase cell death, as we have shown in cells, tumor slices and xenografted tumors. On key example is radiosensitization of TRT for NET tumors using the PARP1 inhibitor olaparib. Our preclinical work has led to the start of various clinical trials worldwide and we are now also starting our own clinical phase 1 trial. In addition to PARP-1 inhibitors, we using drug screens to identify other potential synergistic combinatory regimens.
Projects:
• Radiosensitization to improve TRT outcome for NETs [ Thom Reuvers , Nicole Verkaik ]
• Clinical phase 1 trial of NET TRT in combination with PARP inhibitors [ Nina Becx ]
Expectations & Directions
Our research team is integrating state of the art technological and (radio)biological knowledge to allow for clinical implementation of improved therapeutic
approaches. The research will contribute to a better understanding of the radiobiological effects of TRTs of which not much is known until now. Besides gaining more information about mechanistic cellular effects, the outcome of our research will open a whole new field of possible research endeavors as we are now only covering the top of the iceberg of the radiobiology of TRTs. Future research will focus focused on the consequences of physical and biological parameters of the radiolabeled compounds on radiation dose or on the role of the tumor microenvironment and systemic reactions during TRT.
Funding
Julie Nonnekens. Daniel den Hoed Foundation Fellowship 2016. “Towards personalized radionuclide therapy”. 3-year project 2017-2021.
Julie Nonnekens. KWF Young Investigator Grant 2018. “A radiant future: Improving targeted radionuclide therapy through modulation of DNA damage in the tumor”. 4-year project 2019-2023.
Yann Seimbille , Julie Nonnekens , Marion de Jong . KWF Research Project 2019. “Long-Acting sstr2 antagonists and Pretargeted Alpha Therapy: a Blockbuster Combination for a Safer and more Efficient Treatment of Neuroendocrine Tumors”. 3-year project 2020-2022.
Julie Nonnekens. Erasmus MC Fellowship 2019. “RADIANT: cellular RADIAtion exposure effects of molecular radioNuclide Therapies”. 4-year project 20202024.
Roland Kanaar, Julie Nonnekens, Hans Hofland, Ferry Eskens, Wouter de Herder, Tessa Brabander , Astrid van der Veldt , Mark Konijnenberg , Stijn Koolen. Oncode clinical proof of concept study. “Improving Peptide

Figure 3. Combination of TRT (PRRT) with a DNA repair inhibitor shows synergistic cancer cell killing. A) Viability readout of a drug library screen to identify novel drugs to potentiate TRT. B) Clonogenic survival of NET cells with the different treatments. Each spot represents 1 cell that survived the treatment. C) Quantification of the clonogenic survival from B and normalized to 0 uM of inhibitor
Receptor Radionuclide Therapy with PARP inhibitors: the PRRT-PARPi study”. 4-year project 2021-2024.
Julie Nonnekens , Marlies Goorden. Convergence Open mind grant. ‘Scanning Confocal Nuclear Microscope for improved Radiopharmaceutical Imaging’. 5-month project 2021.
Julie Nonnekens . ERC starting grant 2021. ‘RADIOBIO: Deciphering the radiobiology of targeted radionuclide therapy: from subcellular to intra-tumoural analyses’. 5-year project 2022-2027.
Invited Lectures
‘The black box of radiobiology of radionuclide therapy’.
Anne Klibanski Visiting Lecture Series, Massachusetts General Hospital and Harvard University, USA, online lecture. Feb 2021
‘Radiobiology of radionuclide therapy: using molecular insights to improve treatment outcome for cancer patients’.

Virtual BELNUC’21 Symposium, Belgian Society of Nuclear Medicine. April 2021.
‘Radiobiology of radionuclide therapy’. University of Oxford, MRC Oxford Institute for Radiation Oncology, UK, online lecture. June 2021
‘Radiobiology and molecular imaging of targeted radionuclide therapy’. MILabs user meeting, Society for Nuclear Medicine and Molecular Imaging Annual meeting 2021, online seminar. June 2021
‘Radiobiology of targeted radionuclide therapy: necessity and current challenges’. Workshop Adaptation of the tumour and its ecosystem to radiotherapies. Le Bono, France. Sept 2021
‘Radiobiological responses of internal radionuclide exposures’. European Radiation Research Society, annual congress, Cean, France. Nov 2021
Highlights
Julie Nonnekens received an ERC starting grant 2021 of €1.750.000 for the project ‘RADIOBIO: Deciphering the radiobiology of targeted radionuclide therapy: from subcellular to intra-tumoural analyses’.
Thom Reuvers won the best presentation award for his presentation ’Potentiation of PRRT by modulation of the DNA Damage Response by DNA-PKcs inhibitors’ at the Workshop Adaptation of the tumour and its ecosystem to radiotherapies in Le Bono, France. Furthermore, he received a travel grant to attend this workshop fully funded. Sept 2021.
Thom Reuvers won the Klaas Breur Travel award for his presentation ’Potentiation of PRRT by modulation of the DNA Damage Response by DNA-PKcs inhibitors’ at the annual symposium of the Netherlands Society for Radiobiology (NVRB). Nov 2021.
Lorain Geenen won the 2nd price for her presentation at the Scientific contest of the Belgian Nuclear SocietyYoung Generation for her presentation ‘Overcoming nephrotoxicity in peptide receptor radionuclide therapy using [177Lu]Lu-DOTA-TATE for the treatment of neuroendocrine tumours’. Nov 2021.
Stefan Roobol received a Young Investigator Award from the European Society of Radiation Research (ERRS) to at the ERRS annual congress for his abstract ‘Live single cell tracking and deep learning-based analysis of DNA damage induction and repair following beta particle radionuclide therapy’.
Additional Personnel
Nicole Verkaik, MSc
Julie Nonnekens and Thom Reuvers were interviewed by the Dutch television network Omroep Gelderland about the work performed on finding new TRT combination strategies and this was broadcasted in two television programs: ‘Dutch Cancer Foundation special Alpe d’HuZes’ and ‘Linda breekt uit’.
Julie Nonnekens was interviewed for the Dutch newspaper ‘Algemeen Dagblad’ about her research, motivations and future vision. This interview is part of the column ‘Onder Professoren’ (among professors). Julie talks about her passion for radiobiological research with a specific focus on radionuclide therapy and describes that by better understanding the biological effects at a cellular level for both cancer and healthy cells, rationalized novel treatment strategies can be designed.
I am a research technician specialized in molecular biology and ex vivo studies on patient tumor material. I work in the department of Molecular Genetics. Together with Eline Ruigrok, I have worked on a project with has ‘radiosensitization of prostate tumor cells to improve radionuclide therapy outcome’ as the main objective. Furthermore, I support Danny Feijtel, Thom Reuvers and Stefan Roobol with radiobiological experiments.

Bianca Dijkstra, joint PhD with UMCG (Prof. RJM Groen and Prof. FAE Kruyt)
This MD/PhD project is part of a collaboration between the UMC Groningen and the Erasmus MC and focuses on developing molecular fluorescence guided surgery for meningiomas. Meningiomas are the most frequently occurring brain tumors in adults. Safe, complete surgical resection without tumor recurrence provides the optimal treatment modality. In order to increase resection rates, intraoperative detection of meningioma tissue is necessary. This can be accomplished by fluorescently labelling tumor-specific biomarkers. In previous years, we have developed and preclinically tested meningioma-specific tracers called 800CW-TATE and bevacizumab-IRDye800CW. Currently, we are investigating the use of bevacizumab-IRDye800CW in meningioma patients in a clinical trial (LUMINA trial, Dutch trial register NL9721).
Students
Larissa Lobbezoo, 2nd year MSc student Nanobiology, Erasmus University and TU Delft. Sept 2020 – July 2021.
Daily supervisor Stefan Roobol.
Danilo Remmers, 2nd year MSc student Nanobiology, Erasmus University and TU Delft. March 2021 – Feb 2022.
Daily supervisor Thom Reuvers.

Majd Arb, MSc student Medicine, Erasmus University. June 2021 – Dec 2021. Daily supervisor Thom Reuvers.
Emile Nunh, 2nd year MSc student Biology, Leiden University. Sept 2021 – Feb 2022. Daily supervisor Stefan Roobol.

CELLULAR RADIATION EXPOSURE EFFECTS OF MOLECULAR RADIONUCLIDE THERAPIES
STEFAN ROOBOL, PHD
Project Funding Erasmus MC Fellowship: “RADIANT: cellular RADIAtion exposure effects of molecular radioNclide Therapies”
Research period December 2019 – November 2022
Email s.roobol@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Molecular Genetics.
Tageted radionuclide therapies (TRT) are designed as treatment for patients with metastasized cancers. During TRT, radiolabeled compounds ( αand β -particle emitters) are injected systemically to target cancer cells via tumor-specific characteristics. Little detailed information is available about how the radiolabeled compound exert their cell-killing effect, including the radiobiology of TRT. In contrast, radiobiological principles of external beam radiotherapy (EBRT) have been studied extensively which have led to a powerful anticancer treatment modality. Efficacy of EBRT is based on the cytotoxicity of DNA damage. Induction of DNA damage by EBRT activates multiple cellular pathways leading to cell cycle arrest, DNA repair and/or cell death. Currently, the application of TRT is guided by results from EBRT, leading to suboptimal treatment regimens.
In my project the focus mainly lies on understanding the underlying mechanisms of the existing TRT: Peptide receptor radionuclide therapy (PRRT). PRRT is currently a first-line treatment in the Erasmus MC for metastasized neuroendocrine tumors (NET), using β -particle emitters. Recent data of other TRTs showed that the use of α -particle emitters could lead to higher treatment efficacy. In order to understand and apply α -particle PRRT, it is critical to
gain fundamental understanding and compare to results of β -particle PRRT and EBRT.
With the use of a targeted CRISPR/Cas9 screen we aim to identify DNA damage repair pathways which are activated in the different PRRT strategies. Identifying these pathways might provide crucial understanding for improving therapy. In addition, estimations of the relative biological effectiveness (RBE) of PRRT therapy, accurate dose calculations are needed. By attaching a fluorescent entity to the presently used DOTA-octreotate and engineering a fluorescent DNA damage marker, careful correlation of radionuclide uptake, subcellular localization, and level of DNA damage induction could be mapped (Figure 1). Combining these results will lead to accurate calculation of the RBE of α - and β -particle PRRT compared to EBRT.

Figure 1. First observation of an α-particle entry and 53BP1 focal accumulation using 225Ac-DOTATATE. Focal accumulation of this protein is a marker for DNA double stranded breaks. Cancer cells were genetically adjusted to express the protein 53BP1 with a mClover-tag, generating a fluorescent version of 53BP1. Cells were treated with 225Ac-DOTATATE and during treatment, confocal microscopy Z-stack images were obtained. (Left) Representative image of the 53BP1mClover signal before DNA damage induction. (Right) Representative image of a cell nucleus which was just hit by an α-particle (entry at red arrow). Cell nuclei are depicted with the white dashes line and scale is depicted at the lower right of the left image.

IMPROVING
PRRT IN NETS, A COMBINATION OF 177LU-DOTATATE AND THE PARP INHIBITOR OLAPARIB
NINA BECX, MSC
Advisors Julie Nonnekens, Hans Hofland & Roland Kanaar
Project Funding Oncode
Research period September 2021 – September 2025
Email m.becx@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine, Molecular genetics, and Internal Medicine.
Peptide receptor radionuclide therapy (PRRT) with the beta-emitting radiopharmaceutical 177lutetiumDOTA-Tyr3,octreotate (177Lu-DOTATATE) is an effective and safe treatment option for patients with metastatic neuroendocrine tumors (NETs). In advanced NET patients, 177Lu-DOTATATE has been proven to secure longterm survival in several large retrospective series and was superior to high-dose somatostatin analogs in a randomized phase 3 clinical trial, with a 79% decrease in the risk of progression or death. However, objective response rates are limited and fewer than 1% of the patients can achieve complete response following PRRT. Administering a higher cumulative dose than currently applied will induce more toxicity in healthy tissues and probably will be detrimental to patients. Therefore, adaptations to the currently applied PRRT regimen are needed.
The repair of PRRT-induced DNA damage constitutes a viable target to enhance its antitumor effects. In a number of preclinical models, inhibitors of the enzyme poly ADP ribose polymerase (PARP), essential for repair of singlestrand DNA breaks, have been shown to improve the cytotoxic effects of PRRT without signs of added toxicity. Various PARP inhibitors are registered for the treatment of human cancers, such as ovarian cancer, and BRCA - or homologous repair deficiency (HRD)-dependent prostate and pancreatic cancer and are under investigation in several clinical trials as radiosensitizer. Based on preclinical in vitro and in vivo data, we hypothesize that PARP inhibitors can potentiate radiation-induced tumor cell death in patients treated with PRRT. This therapeutic combination has not been studied in humans before.

Therefore, we are going to conduct a phase 1 dose-escalation study to determine the maximum tolerated dose (MTD) of the PARP inhibitor olaparib in combination with PRRT in patients with a well-differentiated advanced NET, progressive after PRRT. Next to that, we will look into the pharmacokinetics and-dynamics of this combination treatment and will perform additional studies to identify PRRT radiation related specific biomarkers.
PhD Student
Fig 1. Set-up of our phase I dose-escalation study

INVESTIGATING THE RADIOBIOLOGY OF RADIONUCLIDE THERAPY
DANNY FEIJTEL, MSC
Advisors Julie Nonnekens, Roland Kanaar & Frederik Verburg
Project Funding Daniel den Hoed fellowship and EUR fellowship
Research period October 2017 – March 2022
Email d.feijtel@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Molecular Genetics.
Patients that suffer from neuroendocrine cancer are often asymptomatic and present themselves with metastasized disease at the time of diagnosis. This limits the possibilities for resection. Therefore, the patients have a poor prognosis.
In the clinic, the site and multiplicity of neuroendocrine tumors (NETs) are visualized by using radiolabeled somatostatin analogues that target the highly expressed somatostatin receptor 2 (SST2) on NET cells. Recently, an adapted compound, radiolabeled with a highly DNA damaging nuclide (lutetium-177), has been performing very well in the clinic and has been FDA and EMA approved as therapy for metastasized NETs.
Even though peptide receptor radionuclide therapy (PRRT) using [177Lu]Lu-DOTA-TATE strongly improves progression free survival and quality of life, the majority of patients still succumb to recurrence of disease. This emphasizes the unmet need for a better understanding- and improvement of PRRT.
Our preclinical experiments, using SST2-expressing cancer cells in xenografted mice, show that therapeutic effects in different tumor models can be influenced by the heterogeneous expression of the target receptor. We find that PRRT in the context of heterogeneous SST2 expression differentially induces DNA damage in the tumor (Fig.1A, B). Here, in cells that express higher levels of SST2 we also observe higher levels of DNA damage. In line with these data, we find that recurrent tumors that heterogeneously expressed SST2 before PRRT lose high expressing cells, while the distribution and expression levels of SST2 remain the same in homogeneous tumors (Fig1. C, D).

Figure 1:w Intertumoral heterogeneity of PRRT response. (A) Differentially induced DNA damage in a heterogeneous SST2 expressing tumor model. (B) Quantification of DNA damage in SST2 high- and low expressing NCI-H69 tumor cells. (C) Representative images of SST2 expression levels in PRRT naïve and recurrent NCI-H69 and CA20948 tumors. (D) Quantification of SST2 levels in PRRT naïve and recurrent NCI-H69 and CA20948 tumors.
In this study we are providing a deeper understanding of the effects of PRRT on its target. These data can be used to extract new methods of potentiation for [177Lu]Lu-DOTATATE treatment in the battle against NETs.

IN VITRO COMPARISON OF 177LU- AND 161TB-LABELED DOTA-TATE
LORAIN GEENEN, MSC
PhD Student
Advisors Julie Nonnekens, Noami Daems, Koen Vermeulen, An Aerts, Frederik Verburg & Sarah Baatout
Project Funding SCK CEN Fellowship
Research period October 2019 – October 2023
Email l.geenen.1@erasmusmc.nl
This project is a collaboration between the Department of Radiology & Nuclear Medicine of Erasmus MC and The Radiobiology Unit of SCK CEN, Belgian Nuclear Research Centre (Mol, Belgium) within the framework of the NURA program.
Radiopharmaceuticals for targeted radionuclide therapy (TRT) consist of a cancer-seeking molecule labeled with an appropriate radionuclide to deliver therapeutic doses of ionizing radiation directly to the cancer sites, both in the primary tumour as well as in metastatic lesions. This new cancer treatment modality holds promise to be more effective and to reduce the detrimental effects on the healthy tissues. However, much of the TRT radiobiology is not fully investigated yet.
A successful example of a TRT radiopharmaceutical is [177Lu]Lu-DOTA-TATE. Tumour control can be achieved in many patients, however complete remissions remain rare. The treatment is generally well tolerated, but bone marrow and kidney toxicity (figure 1) limit the amount of radioactivity that can be safely administered to the patients.
Therefore, the question arises whether alternative radiometals could be used for TRT which would potentially be more powerful than the currentlyemployed lutetium-177, without causing additional sideeffects. For example, terbium-161, which has similar physical decay characteristics to lutetium-177, but releases additional energy in the form of conversion and Auger electrons.
Within this PhD project we aim to obtain a better understanding of the underlying biological mechanisms of TRT. By investigating the effects of effects of 177Luand 161Tb-labeled DOTA-TATE on both tumour and healthy tissue cells, we aim to identify the different molecular mechanisms involved in the radiation responses. This can on its turn help in determining strategies for increasing

Figure 1. Mechanism of renal uptake and retention of [177Lu] Lu-DOTA-TATE. Geenen et al. 2021. “Overcoming Nephrotoxicity in Peptide Receptor Radionuclide Therapy Using [177Lu]Lu-DOTATATE for the Treatment of Neuroendocrine Tumours.” Nuclear Medicine and Biology 102–103:1–11. Created with BioRender. com
therapeutic efficacy and/or decreasing cytotoxic effects on kidneys and other healthy tissues.
In vitro, neuroendocrine tumour cell models as well as human kidney cell models will be incubated with [177Lu] Lu-DOTA-TATE or [161Tb]Tb-DOTA-TATE. The following endpoints will be assessed: cell viability, apoptic cell death, senescence, DNA damage and repair kinetics and levels of inflammatory markers.

POTENTIATING PRRT BY MODULATION OF THE DNA DAMAGE RESPONSE
THOM REUVERS, MSC
PhD Student
Advisors Julie Nonnekens, Roland Kanaar & Frederik Verburg
Project Funding KWF Young Investigator Grant: A radiant future; Improving targeted radionuclide therapy through modulation of DNA damage in the tumor
Research period September 2019 – September 2023
Email t.reuvers@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Molecular Genetics.
Peptide Receptor Radionuclide Therapy (PRRT) is an FDA- and EMA-approved treatment for advanced gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Somatostatin receptor-positive tumors are targeted by the somatostatin analog DOTA-Tyr 3-octreotate, where the attached radionuclide (lutetium-177) locally induces DNA damage leading to tumor growth inhibition and cell death. Although this modality was shown to be effective in the treatment of GEP-NETs, complete cures are rare. Therefore, there is a clear need to improve the therapeutic window.
The cell employs a tightly controlled network of proteins to counteract radiation-induced damage. Modulation of this cellular response with radiosensitizing compounds has been shown to be a promising strategy to potentiate treatments based on ionizing radiation. In this project, we are trying to alter the cellular response in such a way that tumor cells are selectively sensitized to ionizing radiation from lutetium-177, while sparing healthy tissue.
This research question is approached from both topdown and bottom-up perspectives. First, we are developing high-throughput assays to screen various inhibitor libraries for their PRRT-potentiating effect on various tumor cell lines ( Figure 1 ) . Hits from these assays will be validated further. In other experiments we focus on the radiobiological basis of PRRT and try to elucidate the sensitization mechanisms of our most promising compounds. Eventually, lead compounds will be tested in vivo (mice). The large-scale screening efforts, combined with a deeper understanding of the resulting DNA damage radiobiology, improve understanding of antitumor strategies and may lead to novel combination therapies for GEP-NETs.

Figure 1. Scatterplot of the drug screen results of PRRT-potentiating compounds in a NET in vitro model. Blue diagonal line shows compounds with an additive effect with PRRT. Compounds with a synergistic effect (high combination therapy and low monotherapy toxicity) will be selected as hits. Compounds highlighted by blue dots are examples of different inhibitors against the same protein that show a synergistic effect with PRRT.
NETs are a very heterogeneous group of tumors and therefore a certain treatment will not have the same effect in every patient. Biomarkers will be identified to select eligible tumor types for corresponding combination treatments. This allows for optimal patient selection and to a more personalized form of medicine. All combined, our research can lead to improved survival and qualityof-life for NET patients.

PRECLINCIAL PROSPECTS ON IMPROVING TARGETED RADIONUCLIDE THERAPY FOR PROSTATE CANCER
ELINE RUIGROK, MSC
PhD Student
Advisors Wytske van Weerden, Julie Nonnekens & Frederik Verburg
Project Funding KWF grand: “Hitting the prostate cancer cell via PSMA-targeted radiotherapy: safer and better”
Research period July 2017 – September 2021
Email e.ruigrok@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine, Urology and Molecular Genetics.
Each year >10.000 men are diagnosed with prostate cancer (PCa) in the Netherlands, comprising 10% of all cancer cases. The five year survival rate of patients with distant disease is only 28%. Therefore, novel treatment options for metastatic disease are necessary. Prostate Specific Membrane antigen (PSMA) is a transmembrane enzymatic protein, which is overexpressed in 90-100% of all prostate cancers. Furthermore, PSMA overexpression is correlated to disease progression which makes PSMA an ideal target for imaging and therapy of PCa using radiotracers. Numerous clinical studies have shown that radiolabeled small molecule PSMA-inhibitors (PSMA-617, PSMA-I&T) are excellent for tumor visualization and, when labeled with lutetium-177 or actinium-225, for treatment of metastasized prostate cancer.
This preclinical project aims to uncover the full theranostic potential of PSMA-targeted tracers by selecting the most optimal tracer and radionuclide and enhance the tumor radiosensitivity. Earlier, we reported that [177Lu]Lu-PSMA-617 shows more favorable binding characteristics in vitro in PSMA-positive cells as well as in a unique set of healthy human salivary gland and renal tissues, compared to [177Lu]Lu-PSMA-I&T. However when comparing the tumor uptake, [177Lu]Lu-PSMA-617 and [177Lu]Lu-PSMA-I&T showed similar uptake and induced similar DNA-damage in PCa cells.
A potent strategy to increase the amount of DNAdamage with this therapy is to change the beta emitting lutetium-177 with the alpha emitting actinium-225. The Erasmus MC is the first in the Netherlands to start a clinical trial using [225Ac]Ac-PSMA-I&T, and we performed a unique preclinical comparison study so we could establish an in vitro radiobiological effectiveness (RBE).

Dose-response curves of [225Ac]Ac-PSMA-I&T and [177Lu]Lu-PSMAI&T showing an relative biological effectiveness of 4.2 of [225Ac] Ac-PSMA-I&T over [177Lu]Lu-PSMA-I&T.
In our study we show that there is no difference in the binding characteristics between [225Ac]Ac-PSMA-I&T and [177Lu]Lu-PSMA-I&T. However, a similar dose of [225Ac] Ac-PSMA-I&T led to a lower survival compared to [177Lu] Lu-PSMA-I&T as shown in the figure. We could conclude that [225Ac]Ac-PSMA-I&T has a 4.2 times higher in vitro therapeutic efficacy compared to [177Lu]Lu-PSMA-I&T. Moreover, we show that [ 225Ac]Ac-PSMA-I&T induces a higher number, and most likely more complex DNA damage, which could explain this higher RBE. Our results could contribute to rational design of PSMA-RLT regimens when decisions between (dosing of) actinium-225 and lutetium-177 have to be made.

HOW TO CORRELATE DOSIMETRY AND BIOLOGIC EFFECTS FOR IN VITRO TARGETED ALPHA THERAPY
GIULIA TAMBORINO, MSC
Advisors Marijke De Saint-Hubert, Mark Konijnenberg, Julie Nonnekens, Struelens Lara & Frederik Verburg
Project Funding SCK•CEN Fellowship
Research period October 2017 – September 2021
Email g.tamborino@erasmusmc.nl
This project is a collaboration between the Erasmus MC Department of Radiology & Nuclear Medicine and the SCK•CEN Expert group of Radiation Protection Dosimetry and Calibration
Peptide receptor radionuclide therapy, with the radiolabeled somatostatin receptor agonist DOTA-octreotate (177Lu-DOTATATE), has successfully been employed for treatment of metastasized neuroendocrine tumors in the past years. Evaluating the efficacy of a novel radiopharmaceutical intended for therapeutic use, entails the assessment of biological effects (e.g. colony survival analyses, tumor volume reduction), as well dosimetry calculations at cellular scale. Dose-effect relationship establishment is a pre-requisite for targeted radionuclide therapy (TRT) optimization since it allows prediction of its therapeutic efficacy and side effects and allows comparison of different radiopharmaceuticals. However, preclinical research into PRRT has been marked by the scarcity of dosimetric evaluations, sound radiobiological understanding and absorbed dose-effect models that could predict tumor response.

Schematic representation of the methodology adopted to simulate DSBs formation for in vitro 177Lu-DOTATATE treatment which led to find a good absorbed dose-response correlation.
Our work builds a more refined dosimetry model for in vitro cell experiments with 177Lu-DOTATATE and investigates cell-specific DNA damage and repair mechanisms to correctly shape the correlation between the absorbed dose, established with a novel dosimetry model, and tumor cell survival. Moreover, improving the absorbed dose characterization during in vivo experiments we further extend the dose-response correlation to a larger scale.
Therefore, we developed customized cellular models (polygonal mesh structures), including organelles which can play a key role in the re-localization of the radiophar-
maceutical product (i.e. Golgi apparatus) and combined it with detailed uptake kinetics. Absorbed dose rate as a function of time and repair kinetics were analyzed. Our dosimetric model will be applied to different internal exposure scenarios allowing to investigate reliable doseeffect relationships for cell survival, which ultimately can be integrated into treatment planning during TRT.
The purpose of this PhD project is to build a computational model that is able to predict biological response from (micro)dosimetric quantities for several exposure scenarios at different scales.
PhD Student

Advisors
Project Funding
IONIZING RADIATION QUALITY AND DOSE EFFECTS ON DNA DOUBLE STRAND BREAK REPAIR
STEFAN J. ROOBOL, PHD
PhD Awarded 9 March 2021
Marion de Jong , Roland Kanaar, Dik van Gent & Jeroen Essers
Radiolabeled nano-carriers for customized cancer medicine
Short CV Stefan studied Biology at Leiden University with graduate projects focussing on molecular biology, gene editing and small animal imaging. In 2015, Stefan started his PhD research project at the departments of Radiology & Nuclear medicine and Molecular Genetics. During this period, the focus was on understanding the cellular effects after alpha-particle irradiation and the development of novel methods for radionuclide delivery. This research resulted in 5 research publications which he (co) authored.
After his PhD studies, Stefan pursued a postdoctoral position in the lab of Dr. Julie Nonnekens. Currently, his research focus is directed at understanding the cellular effects after Peptide Receptor Radionuclide Therapy and thereby possibly improving current treatment regimens.
This project was a collaboration between the Departments of Radiology & Nuclear Medicine and Molecular Genetics.
Ionizing radiation (IR) can induce a wide array of different types of DNA damage and, in the context of cancer therapy, is used to eradicate tumor cells. The underlying success of DNA damage-inducing radiation treatment is the rationale that tumor cells coordinately respond to DNA damage, thereby inducing a variety of responses that induce cell death or inhibit cellular proliferation. In cancer therapy, tumors are mostly treated from outside the body, using external beam radiation therapy (EBRT). However, irradiation of tumors deep within the body that reside next to healthy tissue can lead to toxicity. The development of radiopharmaceutical therapy (RPT) has improved treatment of cancers that are located deep in the body and of metastasized disease. Internal irradiation provides the possibility to use radiation with a high linear energy transfer (LET), which is not an option for external irradiation due to a low penetration depth. HighLET radiation has a large probability to eradicate tumor cells, due to its potential to inflict a high amount of DSBs to cells in close proximity. However, to deliver the highLET radiation efficiently and what types of DNA damage are inflicted is not yet fully understood. This thesis describes; (1) The cooperation of NHEJ and HR in IR protection in mice and cells; 2) the development of a novel high-LET external irradiation device; (3) the differences in DSB processing after high- and low-LET irradiation and (4) polymersome processing after cellular uptake to assess efficient and safe delivery of high-LET radionuclides.


hdl.handle.net/1765/135291

Simone Dalm has a BSc in Biomedical science and a MSc in Oncology, which she obtained at the VU University in Amsterdam. In 2012 she started her PhD at the department of Nuclear Medicine of the Erasmus MC and in 2017 she graduated cum laude. She then continued with a postdoc at the same department (by that time the dept of Radiology and Nuclear Medicine joined forces). During her postdoc she established her own research line and started her research group: The Radiotracer Interactions Group. In September 2019 she was promoted to assistant professor.
She received multiple personal and industry sponsored grants (e.g. KWF Young Investigator’s Grant and ZonMw Veni) and is involved in several national and international research projects. She also received multiple awards for her achievements such as the Editor’s choice award of the Society of Nuclear Medicine and Molecular Imaging, multiple Alavi Mandell awards and the Research Prize of the Erasmus University. Her scientific interests include molecular biology, targeted therapy, and nuclear imaging and therapy. s.dalm@erasmusmc.nl
RADIOTRACER INTERACTIONS GROUP
SIMONE DALM, PHD
assistant professor

Context
Target-mediated radionuclide imaging and treatment is successfully applied in the clinic for tumor-targeting. For this, radiotracers are applied directed against molecules that are overexpressed on tumor cells. Depending on the radionuclide connected, the same tracer can be used for imaging (ß+ and γ-emitting radionuclides) and treatment (ß- and α-emitting radionuclides). Our studies focus on (but are not limited to) the application of radiotracers targeting the Gastrin Releasing Peptide Receptor (GRPR) (overexpressed on prostate- and breast cancer), Prostate Specific Membrane Antigen (PSMA) (overexpressed on prostate cancer), the somatostatin receptor (SST) (overexpressed on neuroendocrine- and breast cancer) and Fibroblast Activating Protein (FAP, expressed on cancer associated fibroblasts). expressed on cancer associated fibroblasts. The aim of our studies is to develop and evaluate novel radiotracers and application strategies, and to optimize the use of radiotracers in order to achieve more cure, less side effects and a better quality of life for cancer patients. This includes studies to identify patient groups best suited for application of a specific radiotracer, the development and the application of novel strategies to improve tumor-targeting and minimize off-target organ toxicity, and studies to better understand the mechanism of action of radiotracers.
Top Publications 2021
Combination Therapy, a Promising Approach to Enhance the Efficacy of Radionuclide and Targeted Radionuclide Therapy of Prostate and Breast Cancer. Damiana TST, Dalm SU . Pharmaceutics. 2021 May 7;13(5):674.
Comparing the Effect of Multiple Histone Deacetylase Inhibitors on SSTR2 Expression and [111In]In-DOTATATE Uptake in NET Cells. Klomp MJ, Dalm SU , van Koetsveld PM, Dogan F, de Jong M , Hofland LJ.Cancers (Basel). 2021 Sep 29;13(19):490.
Cancer-Associated Fibroblasts as Players in Cancer Development and Progression and Their Role in Targeted Radionuclide Imaging and Therapy. Koustoulidou S, Hoorens MWH, Dalm SU , Mahajan S, Debets R, Seimbille Y, de Jong M .Cancers (Basel). 2021 Mar 4;13(5):1100.
Research Projects: Objectives & Achievements
Personalized treatment
For targeted radionuclide imaging and therapy to be successful the expression level of the target on tumor cells is essential. Next to that, the distribution of the target (heterogeneous vs homogeneous) is also an important factor for the success of these interventions. During cancer development and progression the expression of important biomarkers, including the biomarkers that we target with our interventions, can vary. In addition, different subtypes of a cancer type can have different molecular characteristics. Moreover, it is most likely that novel developed targeted radionuclide treatments will be applied in late stage disease. In this case, patients have often been treated with other types of anti-cancer treatments such as hormone therapy, chemotherapy or a combination of the two. These treatments can influence target expression as well as radiosensitivity of cancer cells. In our studies we evaluate the expression level and distribution of novel targets in healthy tissues and during the development and progression of cancer. In addition, we also study how other anti-cancer drugs can affect target expression and radiosensitivity of cancer cells. Ultimately the goal is to identify specific patient groups, with respect to disease stage, disease subtype and treatment history that can benefit from specific targeted radionuclide imaging and treatment strategies. Ongoing projects include the effect of hormone treatment and chemotherapy on GRPR expression in prostate and breast cancer (Tyrilshall Damiana), GRPR vs PSMA expression in prostate cancer (Marjolein Verhoeven and Eline Ruigrok), and GRPR expression in breast cancer: normal tissue vs DCIS vs invasive tumors.

1. Target expression can vary between different disease stages and as a consequence of prior treatment. Identification of patient groups best suited for specific target-mediated imaging and therapy is essential for the success of novel treatments.
Novel
therapeutic strategies e.g. pretargeting, combination treatment and targeting the tumor stroma
Although targeted radiotracers are successfully applied clinically, complete response in patients is rare. In addition, since healthy organs often also express the respective target and because of the physiological clearance of the radiopharmaceuticals, healthy organs are often also exposed to radiation which can cause side effects. The above indicates that there is a need for novel developments to increase the efficacy and safety of targeted radionuclide treatment. In line with this, our focus is to develop novel therapeutic strategies to improve the safety and efficacy of treatment. So, one of our projects is focused on developing, applying and optimizing a pre-targeting strategy for GRPRmediated radionuclide imaging and treatment ( Marjolein Verhoeven and Lilian van den Brink ). Here we make use of the pharmacokinetic properties of one of our novel GRPRtargeting radiotracers to apply a 2-step approach that will facilitate high uptake of the radiotracer in the tumor, but prevent accumulation of the radiopharmaceutical in healthy organs that express the GRPR, mainly the pancreas, and thereby reduce the risk of side effects caused by radiation damage to this organ.
Another strategy to improve therapeutic efficacy of our targeted treatment is by increasing the level of target expression. We study the use of epigenetic drugs such as histone deacetylase inhibitors (which stimulate the open euchromatin structure of the DNA associated with active gene transcription) to increase SST expression in neuroendocrine tumor cells ( Ilva Klomp ). An increase in target expression will result in an increase in radiation dose to tumor cells and thereby improve therapeutic efficacy. Moreover, neuroendocrine tumors that have no to low SST expression might become suitable for SSTmediated radionuclide treatment after pre-treatment with these epigenetic drugs.
A third project focusses on delivering cytotoxic radioactivity to cancer cells by targeting cells in the tumor stroma. The tumor stroma is part of a unique environment that encases solid tumors, acting as a kind of barrier. Treatments for e.g. pancreatic cancer are often hindered by this barrier and there is clearly an unmet need for effective treatment of patients suffering from this cancer type. We aim to solve this issue by targeting cancer associated fibroblasts (CAFs) present in the tumor stroma using radiotracers directed against the fibroblast activating protein (FAP) present on these CAFs. The hypothesis is that FAP radiotracers that bind to the CAFs can indirectly irradiate the surrounding tumor cells and thereby eliminate them ( Circe van der Heide ).
Figure

Figure 2. Conventional targeting vs the pretargeting strategy. In the pretargeted approach the binding domain and the radioactive part (effector) of the radiotracer are separated. Both entities will be provided with a click domain that can bind to one another via in vivo click chemistry. By first injecting the binding domain+click, and only injecting the radioactive effector when the tumor to background ratio of the binding domain+click is optimal, we will be able to effectively treat the tumor with high doses and minimize off target toxicity.
Other projects on this topic include the combination of radiolabeled GRPR analogs with hormone therapy for treatment of breast and prostate cancer (Tyro Damiana and Lisette de Kreij-de Bruin), the combination of SST and GRPRmediated radionuclide treatment with immune checkpoint inhibitors (Alp Çelebi) and strategic use of a combination of beta- and alpha-emitting radionuclides for elucidating an anti-cancer immune response.

Figure 3. Pancreatic tumor’s resistance to anti-cancer drugs is often caused by stroma surrounding the tumor cells that acts as a protective barrier, and thereby prevents accumulation of effective doses of these drugs in tumor cells. FAP-targeting radiotracers target FAP expressed on CAFs present in the stroma and thus penetration of this tough barrier is not needed. Radionuclides that are coupled to the FAP-targeting radiopharmaceuticals emit radiation that reaches the tumor cells and can thereby destroy them.
Understanding the mechanism of action of radiotracers
In another attempt to improve the efficacy and safety of targeted radionuclide imaging and treatment, we aim to gain more understanding on the mechanisms of action of radiopharmaceuticals. For example for SST targeting, radiotracers with agonistic properties are currently FDA and EMA approved for imaging and treatment of neuroendocrine tumors. However, recent studies with radiolabeled SST antagonists showed superior uptake in cancer cells in preclinical and pilot clinical studies, even though the agonist can be internalized while the antagonist remains at the cell membrane. This superior uptake is a consequence of the ability of the antagonist to bind to the SST independent of its state, while the agonist can only bind to the SST while in activated state. Gaining more understanding on the exact mechanism behind the targets’ activation state in relation to the binding ability of radiopharmaceuticals will provide novel opportunities to positively influence the binding ability (e.g. by manipulating the receptor state of the target), and thereby improve imaging and treatment efficacy for the respective cancer type.

Figure 4. Differences between radiotracers with agonistic and antagonistic properties. More research is needed to unravel the mechanism behind this difference in binding capability between agonists and antagonists. This will provide novel opportunities to positively influence the binding ability of these molecules and thereby improve imaging and treatment efficacy.
Another example is studying the difference in binding and clearance of GRPR radiopharmaceuticals in the tumor vs the healthy pancreas. Studies have demonstrated that next to prostate and breast tumors, there is high uptake of GRPR radioligands in the healthy GRPR-expressing pancreas. However, the uptake is cleared relatively fast from the pancreas, while tumor uptake retains much better. In addition, adapting the amount of radiopharmaceutical used affects the uptake in the pancreas, while no to little effect is observed when it comes to tumor uptake. The differences in interaction between the GRPR radiopharmaceutical and its target on pancreatic cells and tumor cells remains a mystery. Our aim is to unravel these differences, in order to better understand how to best apply our radiopharmaceutical for optimal tumor to healthy organ ratio.
A third project focusses on studying whether epithelial to mesenchymal transition (EMT) takes place after targeted radionuclide therapy and which pathways are involved in this. Previous studies have demonstrated that EMT can take place after external beam radiation therapy. The transition of cancer cells from an epithelial to a mesenchymal state is frequently associated with a more aggressive phenotype and therapy resistance. We aim to study whether EMT takes place after radionuclide therapy and how the transition can be blocked, ultimately with the goal of enhancing therapeutic efficacy of our treatment (Ilva Klomp and Lilian van den Brink).

Expectations & Directions
Our ultimate goal is to develop and improve novel theranostic strategies using targeted radiopharmaceuticals, in a personalized setting to achieve more cure, less side effects and a better quality of life for cancer patients.
We aim to achieve this by introducing novel radiotracers and application strategies for cancer imaging and treatment into the clinic e.g. pre-targeted GRPR-mediated radionuclide therapy and FAP-targeting radiotracers. This includes combination with other anti-cancer treatments and combinations of radionuclides. One important focus point will be the combination of radionuclide therapy with immunotherapy. In addition, we will keep performing studies to better understand the interaction between radiopharmaceuticals and their target, and with this knowledge we will develop and evaluate novel personalized strategies that we aim to bring to the clinic as fast as possible.
Figure 5. Overview of research goals.
Funding
de Jong M and Dalm SU. Erasmus MC Mrace 2020 – 2024. “Breaking the tumour stroma barrier: A new way to hit cancers using a novel universal targeted radionuclide therapy strategy”.
Dalm SU. Veni ZonMw 2019 – 2022. “Better understanding leads to better decisions: Evaluating the effect of antihormone therapy and chemotherapy on GRPR-targeting”.
Dalm SU and de Jong M. Commercial collaboration Advanced Accelerator Applications, a Novartis company. 2019-2022. Preclinical NeoBOMB1 applications.
de Jong M, Seimbille Y, Dalm SU, Konijnenberg M, Essers J, Beekman F, Goorden M, Denkova A, Djanashvili K. Convergence Plan Erasmus MC – TU Delft 2019 – 2022. “Broad Spectrum High Precision Theranostic Cancer Therapy”.
Dalm SU. KWF Young Investigator Grant/Bas Mulder Award 2018 – 2022. “Click on Target: Developing a safe drug with enhanced therapeutic potential for prostate cancer treatment”.
Additional Personnel
Lilian van den Brink, BSc, Research Technician
Dalm SU, de Jong M, van Weerden WM and van Deurzen CHM Erasmus MC Mrace Grant 2018 – 2022. “A “CLICK” towards better and safer radionuclide therapy of prostate cancer”.
Highlights
Simone Dalm was invited to give a lecture at the Annual conference of the European Association of Nuclear Medicine and Molecular Imaging.
Simone Dalm was invited to give a lecture at the PSMA forum NL.
Simone Dalm was guest editor of a special edition of Pharmaceutics titled “Application of Targeted Radiopharmaceuticals for Cancer Management”.

I am a research technician in the Radiotracer Interactions group led by Dr. Simone Dalm. As a technician I set up and optimize experiments, I coordinate lab duties, organize meetings for the group and support the PhD students. In the past year I have mainly supported 2 research projects. In the first project we study whether radionuclide therapy causes epithelial to mesenchymal transition and which pathways are involved in this. Cells in mesenchymal state are frequently associated with a more aggressive phenotype and are more drug resistant than cells in epithelial state. We aim to study whether the epithelial to mesenchymal transition takes place after radionuclide therapy, and if so whether or not this can be prevented to promote cytotoxic efficacy of our treatment. The second project is focused on developing and evaluating a pretargeting strategy directed towards the gastrin releasing peptide receptor (GRPR) overexpressed on prostate cancer cells. The pre-targeting strategy is based on separating the binding domain from the cytotoxic radionuclide and letting the two agents combine inside the body. Unfortunately the GRPR is not only expressed on prostate cancer cells but also on cells of the healthy pancreas, resulting in binding of GRPR radiopharmaceuticals to this organ as well. An advantage is that radiotracer uptake from the pancreas is cleared relatively fast, while tumor uptake is retained much better. The difference in clearance half-live between the pancreas and the tumor allows us to apply a pre-targeting strategy. Using this strategy we aim to effectively treat the tumor with cytotoxic GRPR-targeting radiopharmaceuticals while preventing/lowering radioactivity uptake in the healthy pancreas and hereby preventing pancreatic toxicity. GRPR-targeting molecules for pre-targeting are developed in collaboration with the Radiochemistry group and I extensively evaluate them in vitro in membrane and cell systems.
Alp Çelebi, MSc, Research Technician
I am a research technician specialized in molecular life sciences with an educational background in neurosciences and molecular biology. I am part of the Radiotracer Interactions Group of the department of Radiology & Nuclear Medicine. My research is focused on studying the combination of immune therapies with targeted radionuclide therapies. The main aims are to identify whether radionuclide therapy (at what doses and what kind of radiation) elucidates an anti-tumor immune response, and whether combining radionuclide therapy with immune checkpoint blockade enhances therapeutic efficacy. Next to this, I am also responsible for managing daily laboratory tasks, as well as supporting my teammates in their experiments when needed.
Lisette W.
de
Kreij-de Bruin, MSc, Research Technician


I am the lab manager of the Central Isotope Laboratories (CIL) and a research technician. As the lab manager I oversee the daily affairs of the lab, and as a research technician I support various research projects. Regarding research, my main focus is on a project aimed at unravelling the effect of previous anti-cancer treatments on the success of targeted radionuclide theranostics. Most research on targeted radionuclide theranostics is performed in treatment-naïve models, while in the clinic these interventions are especially applied in advanced disease. Therefore, our study focusses on investigating the effects of previous therapies on the success of gastrin releasing peptide receptor (GRPR)-targeted radionuclide theranostics in breast and prostate cancer. Using in vitro and in vivo experimental strategies we aim to unravel the effects of previous treatments (e.g. chemotherapies and hormone therapies) on GRPR expression and radiosensitivity, so we can identify the correct patient group and timing for GRPR-mediated interventions.
Corrina de Ridder & Debra Stuurman, Biotechnicians

We are the Research Technician A team that gives support to the experimental animal work. We have a joint appointment and work for the department of Radiology & Nuclear medicine and the department of Urology. At the department of Radiology & Nuclear medicine our daily work consists of setting up and conducting the animal experiments. This includes imaging (e.g. SPECT, PET, CT, MRI and fluorescence imaging), biodistribution, efficacy and toxicity studies. Next to this, we also support processing of biological material, such as histology staining and routine immunohistochemistry. In addition, we are responsible for maintenance of the panel of patient-derived xenograft (PDX) models of prostate cancer that grow in immune deficient mice at the department of Urology. These PDX models are being used in various collaborative projects among others with the department of Radiology & Nuclear Medicine. We are therefore mainly involved in all projects that use these PDX models, mostly focused on targeting the prostate specific membrane antigen (PSMA) and the gastrin releasing peptide receptor (GRPR).

THE EFFECT OF PRIOR ANTI-CANCER TREATMENTS ON THE SUCCESS OF GRPRTARGETED RADIONUCLIDE THERAPY
TYRILLSHALL DAMIANA, MSC
PhD Student
Advisors Simone Dalm & Frederik Verburg
Project Funding Veni – Health Research and Development (ZonMw)
Research period January 2020 – January 2024
Email t.damiana@erasmusmc.nl
The gastrin-releasing peptide receptor (GRPR) is overexpressed in several types of cancers, including prostate cancer (PCa) and breast cancer (BC), making it an attractive target for targeted radionuclide therapy (TRT). In line with this, many GRPR-targeting radiolabeled peptides have been developed for radionuclide imaging and therapy of PCa and BC. These radiotracers are often studied in treatmentnaïve models, while in the clinic, they will most likely be applied in patients with advanced disease. Patients with advanced disease often have already been treated with other anti-cancer agents. This can potentially affect the success of GRPR-mediated TRT. It is known that other anti-cancer treatments, such as hormone therapy and chemotherapy, can alter gene expression and radiosensitivity of cancer cells, two major factors determining the success of TRT. Thus, these therapies can potentially affect GRPR expression and thereby the cellular uptake and radiation dose of GRPRmediated TRT. Regarding radiosensitivity, prior treatments might influence the cytotoxic effect of TRT, independent of the level of GRPR expression. Therefore one of our aims is to identify the effect(s) of prior treatment(s) on the success of GRPR-mediated TRT. Our goal is to determine in which patient group and when this novel intervention should best be applied. Our initial results show that doxorubicin (dox), a chemotherapeutic agent, can influence GRPR expression and radiosensitivity in vitro. Pre-treatment with dox resulted in an increased uptake of the GRPR radiotracer [111In]In-NeoB (Fig 1A, B). We also demonstrated that BC cells pretreated with dox were more resistant to external beam radiation therapy compared to non-treated cells (Fig 1C, D). Although very preliminary, these results show that prior treatments may alter GRPR expression and radiosensitivity. Additional experiments are required to confirm the observed effects.

Figure 1. The effect of doxorubicin on GRPR expression and radiosensitivity. Uptake of [111In]-NeoB (A, B) and cell density after external beam radiation (C, D) before and after pre-treatment with doxorubicin in PC3 human PCa cells and T47D human BC cells.
In a second study we compare three of the most promising GRPR radiotracers (NeoB, RM2, and ProBOMB2). NeoB and RM2 are two of the most well-known and successful GRRP radiotracers. These GRPR radiotracers have successfully been evaluated in preclinical and clinical studies. ProBOMB2 is a more recently developed GRPR radiotracer with potential superior cancer cell specificity. These three GRPR radiotracers have never been compared, and it is unclear whether one of the radiotracers has advantages over the others. Therefore, our aim is to compare these three radiotracers to determine which one is the best for safe and effective TRT. We will provide an overview of the differences between NeoB, RM2, and ProBOMB2 concerning specificity, retention time, and cytotoxicity to guide choices for future studies.

TARGETING FAP IN THE TUMOR STROMA FOR PAN-TUMOR RADIONUCLIDE THERANOSTICS
CIRCE D VAN DER HEIDE, MSC
PhD Student
Advisors Simone Dalm & Frederik Verburg
Project Funding
Erasmus MC MRACE grant: “Breaking the tumor stroma barrier: A new way to hit cancers using a novel universal targeted radionuclide therapy strategy”
Research period January 2021 – January 2025
Email c.vanderheide@erasmusmc.nl
Cancer is more than a collection of malignant cells. Host-derived and non-cellular components surrounding the cancer cells together form the tumor microenvironment (TME). The TME is critical in cancer development and tumor progression. It can make up more than half of the total tumor mass, and forms a protective barrier that causes therapy resistance. Hence, the TME is an interesting target for the development of anti-cancer interventions, such as targeted radionuclide imaging and therapy (theranostics).
The most abundant cell type present in the TME is the Cancer Associated Fibroblast (CAF). CAFs can be characterized by high expression of the Fibroblast Activation Protein-α (FAP). FAP is a membrane-bound protease involved in cancer cell proliferation, invasion, and immunosuppression. Its expression is unique for CAFs; it is not or at undetectable levels expressed in healthy tissues. Approximately 90% of epithelial tumors have FAP-expression in the TME, making FAP an attractive target with potential pan-tumor application for targeted radionuclide theranostics. Figure 1A depicts the concept of FAP-targeted radionuclide therapy.
In line with the above, radiolabeled FAP inhibitors (FAPI) have been developed and tested. Clinical PET/CT imaging studies with these radiotracers e.g. [68Ga]Ga-FAPI-46 have shown great potential. These compounds are promising for imaging, but the pharmacokinetics are very unfavorable for radionuclide therapy. Due to the limited retention time of the current FAPI radiotracers, tumor cells receive only a limited radiation dose. FAP radiotracers with improved retention time and good tumor-to-background ratios are necessary for safe and effective treatment, and thus for clinical implementation of a theranostic FAP radiotracer. Therefore the aim of this PhD project is to develop and evaluate novel FAP radiotracers with improved pharmacokinetic properties.

Figure 1. (A) The concept of FAP-targeted radionuclide therapy. (B) Autoradiography on humane pancreatic ductal adenocarcinoma tissue with [111In]In-FAPI-46 and a novel synthesized FAP-tracer. (C) Quantification of radiotracer binding to the tumor tissue.
This project is a collaboration with the Radiochemistry group of the department. Novel FAP radiotracers are being developed to increase tumor retention time. Subsequently, we are testing these newly synthesized compounds in multiple biological systems. An example is depicted in Figure 1B. In addition, we aim to determine the best strategy for FAP-targeted radionuclide therapy. For this, we will perform studies to better understand the biology of CAFs and FAP expression in different cancers. Based on this knowledge we aim to develop a treatment schedule (i.e. dose and type of radionuclide) for optimal and safe therapeutic efficacy.

Advisors
INCREASING SSTR2 EXPRESSION BY MODULATING THE EPIGENETIC MACHINERY
ILVA KLOMP, MSC
PhD Student
Simone Dalm, Leo Hofland & Clemens Löwik
Project Funding Erasmus MC MRace grant: “Epigenetic therapy to increase efficacy and optimize patient outcome of peptide receptor radionuclide therapy”
Research period December 2018 – December 2022
Email m.j.klomp@erasmusmc.nl
This project is a collaboration between the Department of Radiology & Nuclear Medicine and the Department of Internal Medicine, Division of Endocrinology
Neuroendocrine tumors (NETs) are often characterized by overexpression of somatostatin type-2 receptors (SSTR2). This receptor plays a pivotal role as target for anti-cancer therapies, such as peptide receptor radionuclide therapy (PRRT). In PRRT, the radiolabeled somatostatin analogue [177Lu]Lu-[DOTA,Tyr3]octreotate ([177Lu]Lu-DOTATATE) is administered, which binds with high affinity to SSTR2. Upon binding, the receptor-ligand complex is internalized, leading to accumulation of radioactivity in the cell, resulting in DNA damage and eventually cell death. However, complete therapy responses are still rare and thus there is a need to improve PRRT for NET patients.
The aim of this project is to unravel the involvement of the epigenetic machinery in regulating SSTR2 expression in NETs and subsequently upregulate the tumoral expression of the target receptor. Herein our focus is mainly on histone acetylation. A high level of histone acetylation is linked to euchromatin. In this active state of chromatin, the DNA is accessible for proteins and enzymes involved in regulating gene transcription, thereby potentially increasing protein expression levels.
To gain insights in the mechanisms in which the epigenetic machinery, and more specifically histone acetylation, is involved in regulating tumoral SSTR2, we are using multiple approaches, such as evaluating the effect of histone deacetylase inhibitors on SSTR2 expression in in vitro and in vivo studies using different cell lines/ models. In addition to this, RNA sequencing data and human-derived NET tissues are used to further elucidate the involvement of the epigenetic machinery in regulating SSTR2 expression.

Figure 1. Valproic acid treatment upregulated SSTR2 expression levels in NCI-H69 cells after just 24 hours of treatment as demonstrated with uptake studies using [111In]In-DOTATATE (A), RT-qPCR (B) and IHC (C).
Our ultimate aim is to upregulate tumoral SSTR2 expression levels, eventually improving PRRT response rates. In vitro analysis showed that stimulating the euchromatin state by using histone deacetylase inhibitors (e.g. valproic acid) upregulated SSTR2 expression in several NET cell lines (Fig 1). Moreover, an increased radiosensitivity was demonstrated after valproic acid treatment. Currently we are performing studies to further analyze whether our in vitro results are also observed in vivo , to eventually evaluate whether the observed increased SSTR2 expression leads to an increased cytotoxicity.

Project Funding
MARJOLEIN VERHOEVEN, MSC
GRPR-MEDIATED RADIONUCLIDE IMAGING AND THERAPY PhD Student
Advisors Simone Dalm & Frederik Verburg
Erasmus MC Grant: “‘Click’ with better and safer radionuclide therapy of PCa: The application of in vivo click-chemistry to improve GRPR-mediated tumor targeting and minimize off-target toxicity”
Research period December 2018 – December 2022
Email m.verhoeven.1@erasmusmc.nl
Prostate cancer (PCa) is the fifth leading cause of cancer-related death in men worldwide, meaning there is a continuing need for early detection and effective treatment. The gastrin-releasing peptide receptor (GRPR) is aberrantly overexpressed in 63-100% of human PCa, making it an interesting target for molecular nuclear imaging and therapy.

Figure 1. Binding of GRPR- and PSMAmediated radiotracers to tumor regions (encircled on H&E sections) of primary human PCa specimens.
Radiolabeled GRPR antagonists are very promising candidates for PCa imaging and therapy. Preclinical and clinical studies have demonstrated high tumor uptake of GRPR radiotracers, but unfortunately high levels were also observed in the natural GRPR-expressing pancreas. Especially when GRPR-targeting radiotracers are applied for therapy and high therapeutic doses are used, this could result in severe side effects. Fortunately, the radiotracer clears relatively fast from the healthy pancreas, while tumor uptake is retained much better. This provides a window of opportunity for applying a pretargeting strategy. The concept of pretargeting is based on separating the binding domain from the cytotoxic radionuclide and letting the two agents combine inside the body. By first administering the binding domain and waiting till it is largely cleared from the pancreas but still retained in the tumor, before injecting the small radiolabeled molecule, we aim to effectively treat the tumor and prevent pancreatic toxicity. In this project, we adapted the potent GRPR-targeting radiotracer NeoB to contain a pretargeting moiety. Multiple variants of the targeting vector that differ in linker length have been synthesized. The first in vitro studies were successful as a click reaction was demonstrated. Future work will continue the characterization.
To date, one of the most widely used treatment strategies for primary PCa is the surgical removal of cancer tissue. However, tumor delineation is difficult and complete re-
moval of the tumor is challenging. Consequently, in many cases complete resection is not achieved, which drastically increases the risk of recurrence. The use of dual-modality imaging probes containing both a radioisotope for SPECT or PET imaging and a fluorescent dye for fluorescence imaging can offer a solution. Performing a SPECT or PET scan prior to surgery allows the procedure to be carefully planned. Then real-time fluorescence imaging can be used during surgery to illuminate the tumor tissue and thereby clearly indicate the tumor margins. We have evaluated four GRPR-targeting dual-modality imaging probes in vivo, where they showed high binding affinity and clear tumor visualization on nuclear and optical scans. Mass optimization studies were performed with the most promising probe (in collaboration with the Radiochemistry Group).
In addition to the GRPR, the more broadly studied prostate specific membrane antigen (PSMA) is also overexpressed in PCa. PSMA-targeting radiotracers have previously shown to be very successful in detecting tumor lesions. This raises the question when and if patients would benefit from either a GRPR- or PSMA-mediated radiotracer. In a third project, we aim to study the relationship between expression levels of GRPR and PSMA and the different stages of PCa by evaluating human PCa tissue for GRPR and PSMA mRNA expression and radiotracer binding. The differential uptake of a GRPRand PSMA-mediated radiotracer in tumor regions of four primary human PCa samples is shown in Figure 1.
Ultimately, we aim to further improve PCa clinical care (1) by generating a new efficient and safe therapeutic strategy for GRPR-mediated radionuclide therapy, (2) by developing tools to guide surgeons, (3) and by providing information to support the stratification of patients eligible for nuclear imaging and therapy.
2020-present: Professor of Translational Nuclear Medicine and consultant in Nuclear Medicine, Erasmus MC
2016-2020: Professor of Experimental Nuclear Medicine and deputy head of the Department of Nuclear Medicine, Philipps-University Marburg and University Hospital Gießen-Marburg, Marburg, Germany
2010-2016: Consultant in Nuclear Medicine and Assistant Professor of Nuclear Medicine, RWTH Aachen University Hospital, Aachen, Germany

2008: PhD, Utrecht University, Utrecht The Netherlands
2005-2010: Training in nuclear medicine, University Medical Center Utrecht, Utrecht, the Netherlands and University Hospital Würzburg, Würzburg, Germany
1997-2004: Medical studies at the Catholic University Leuven, Leuven, Belgium and Utrecht University, Utrecht, The Netherlands
f.verburg@erasmusmc.nl
TRANSLATIONAL NUCLEAR MEDICINE FREDERIK A VERBURG MD, PHD
full professor

Context
The discipline of nuclear medicine has a rich tradition of successful translational research at the Erasmus MC. Notable examples to come out of this tradition are the commercial imaging product Octreoscan® and more recently the radionuclide therapeutic agent Lutathera®. The preclinical research group of our department is world-renowned.
The convergence with the TU Delft offers exciting new opportunities to integrate technical research into e.g. detector technologies or novel isotopes as well as various developments in medical informatics, such as artificial intelligence.
The integration of nuclear medicine into Radiology and Nuclear Medicine has offered new chances to further develop the theragnostic principle by means better integration of anatomical and physiological imaging. A prime example of the latter is the new PET/MR scanner which entered into service at the Erasmus MC at the end of 2019.
In order to facilitate and optimize the integration of new preclinical, technical and multimodality imaging concepts into clinical research and the transfer of these concepts from research to clinical practice, the Department of Radiology and Nuclear Medicine has newly created the Chair of Translational Nuclear Medicine in 2020. The year 2021 was characterized by many activities aimed at building up the research line while simultaneously increasingly supporting other, related research lines of the department after the untimely passing of Prof. Dr. Marion de Jong.
Top Publications 2021
Veldhuijzen van Zanten SEM , Neggers SJCMM, Valkema R, Verburg FA. Positive [ 18 F]fluoroethyltyrosine PET/MRI in suspected recurrence of growth hormone-producing pituitary adenoma in a paediatric patient. Eur J Nucl Med Mol Imaging. 2021 Dec;49(1):410-411.
van Velsen EFS, Visser WE, Stegenga MT, Mäder U, Reiners C, van Kemenade FJ, van Ginhoven TM, Verburg FA , Peeters RP. Finding the Optimal Age Cutoff for the UICC/AJCC TNM Staging System in Patients with Papillary or Follicular Thyroid Cancer. Thyroid. 2021 Jul;31(7):1041-1049.
Verburg FA, Nonnekens J, Konijnenberg MW, de Jong M . To go where no one has gone before: the necessity of radiobiology studies for exploration beyond the limits of the "Holy Gray" in radionuclide therapy. Eur J Nucl Med Mol Imaging. 2021 Aug;48(9):2680-2682.
Research Projects: Objectives & Achievements
Objectives
- Establish internal and external cooperations between Erasmus MC Academic Centers of Excellence, the TU Delft and clinical and preclinical groups with the Department of Radiology and Nuclear Medicine to further developments in tracer-based medical imaging and radionuclide therapy
- Introduce novel tracers into clinical research and clinical practice for radionuclide imaging and therapy
- Introduce novel radionuclides into clinical research and clinical practice
- Establisment of novel imaging techniques such as PET/MRI or very high resolution SPECT in clinical research and clinical practice
- Establish novel non-vertebrate animal models to further improve the process of translational tracer development
Achievements
The research line clinical nuclear medicine was newly established in 2020. In 2021 grants were applied for and awarded from the Daniel Den Hoed Foundation and the KWF Kankerbestrijding / Alpe d’Huzes Further grants were applied for (decision still pending).
Expectations & Directions
It is expected to further expand the field of translational nuclear medicine in the near future. In 2020, a number of cooperations were started:
- A multi-center consortium for research of the use of fibroblast activating protein targeted imaging in patients. It is expected to re-submit an improved grant application on this topic in 2022, starting with the topic of localization of carcinoma of unknown primary.
- A multi-center consortium for early phase clinical research of F-18-tetrafluoroborate in differentiated thyroid cancer in was initiated and a grant was won. It is expected that the project will start before the middle of 2022..
- In the convergence trajectory a cooperation with the Chair of Radiation Detection and Medical Imaging has been established in order to do translational research on the application of gyro-free, very-high-resolution SPECT imaging in humans. A grant to enable installation of this machine in the Erasmus MC has been applied for (decision pending)
- Pilot studies on the use of PET/MRI in spinal cord injury have been initiated in cooperation with the departments of orthopedic surgery and neurosurgery. It is expected to start patient inclusion in 2022
- Translational cooperation between the Department of Oncology (Dr. Mostert), and the Department of Radiology and Nuclear Medicine (Radiation Biology, Translational Nuclear Medicine) was started to further investigate genetic changes in relation to PRRT. Pilot analyses from pre-existing datasets are expected in 2022 after which it is expected to plan and seek funding for larger-scale projects in this direction.
- An interdisciplinary cooperation with the Academic Center for Excellence in Thyroid Diseases was started; a pilot study on a diagnostic compound for medullary thyroid cancer is expected to start in early 2023.
- The Chair of Translational Nuclear Medicine has driven the process for replacement of existing nuclear medicine cameras. Procurement of a revolutionary new SPECT/CT is expected for 2022 and procurement of a revolutionary new total body PET/CT is expected for 2023. Both machines are expected to generate new projects.
- Research into invertebrate animal models for use in nuclear medicine developments is expected to begin in 2022.
Invited Lectures
Do we need radiology (research)? No vs. Yes. 1st International Workshop on Radiobiology of Molecular Radiotherapy, March 18, 2021
Preliminary experience with Ac-225-PSMA and upcoming novelties, Semi-annual scientific meeting of the Dutch Society of Nuclear Medicine (NVNG), June 11, 2021
Which DTC patients will profit from postoperative RIT in terms of overall and/or cancer-specific survival? Annual conference of the Korean Society of Nuclear Medicine, August 20, 2021.
Nachsorge des differenzierten Schilddrüsenkarzinoms: ist die aktuelle Praxis in Deutschland noch zeitgemäß? Schilddrüsenkongress 2021, October 7, 2021
Biokinetic modelling and ist clinical impact for 131I therapy of thyroid disease. European Association of
Nuclear Medicine annual conference, October 11, 2021.
Highlights
In May of 2020, Frederik Verburg took up the newly created position of Chair of Translational Nuclear Medicine. The appointment procedure as full professor at the Erasmus MC initiated at that time was completed effective per April 1, 2021.
Obtaining of the Daniel Den Hoed Grant 2021, together with prof. dr. G. Jenster (Experimental Urology), for the development of a new theragnostic agent against advanced urothelial carcinoma
Obtaining of a KWF Kankerbestrijding / Alpe d’Huzes grant for a prospective study on the use of F-18-Tetrafluoroborate (together with Dr. B. de Keizer, UMC Utrecht, and Prof. Dr. A.D. Windhorst, Amsterdam UMC).
Frederik A. Verburg joined the dosimetry committee of the European Association of Nuclear Medicine as a member

NUCLEAR MEDICINE DIAGNOSTICS AND THERAPY FOR GASTRIC CANCER AND LIVER CANCER
ERIK VEGT, MD, PHD
Nuclear Medicine Physician
Project Funding ZonMW grant ‘Doelmatigheid van Zorg’
Research period November 2019 – December 2026
Email e.vegt@erasmusmc.nl
These projects are collaborations between the Departments of Radiology & Nuclear Medicine, Surgery, Gastroenterology and Oncology of Erasmus MC and UMC Utrecht, as well as Amsterdam UMC and Leiden UMC.
Gastric cancer and liver cancer are the third and second leading causes of cancer-related death worldwide.
For gastric cancer, the recommended treatment with curative intent is gastrectomy with lymphadenectomy. For detecting metastatic (non-curable) disease, the accuracy of current staging with gastroscopy and CT of thorax and abdomen is limited. As a result, some patients incorrectly undergo treatment with curative intent, exposing them to the risk of complications and mortality of surgery and perioperative chemotherapy. FDG-PET/CT and staging laparoscopy (SL) have been reported to improve detection of distant and peritoneal metastases, respectively, and have recently been added to the Dutch guidelines. In our study “evaluation of FDG-PET/CT and LAparoscopy in STagIng advanced gastriC cancer (PLASTIC): a Dutch multicenter prospective study” we evaluate the value of FDG-PET/CT and SL in addition to initial staging in patients with locally advanced gastric cancer, in terms of impact on treatment, quality of life and cost-effectiveness. First results show that FDG-PET/CT detected distant metastases in 3% of patients, and SL detected peritoneal or locally non-resectable disease in 19% of patients. We concluded that FDG-PET/CT has limited added value, but SL has considerable impact. Data about quality of life and cost-effectiveness are still being collected and analysed.
Hepatocellular carcinoma (HCC), the most common type of liver cancer, often occurs in patients with liver cirrhosis. At diagnosis, surgery is often impossible due to tumour size and/or comorbidities. Transarterial radioembolization (TARE or SIRT) with microspheres loaded with Ho-166 or Y-90 can be an effective treatment for
these patients. However, delivering an adequate radiation dose to the tumour without harming the normal liver tissue remains a challenge. In a retrospective analyses of liver function after Y-90 SIRT in patients with HCC, we found an increased incidence of liver failure after Y-90 compared to sorafenib. In the iHepar study, which is expected to open in 2022, we are going to investigate the safety and efficacy of dosimetry-based individualized treatment planning of Ho-166 radioembolization, hoping to maximize treatment effect, while minimizing toxicity.

Survival of HCC patients after radioembolization (SIRT). Overall survival in patients who developed liver decompensation after SIRT, compared with patients who did not develop liver decompensation at last follow-up.

Clemens Löwik obtained his master of science degree in Biology (cum laude) at Radboud University in Nijmegen and his PhD degree at the Leiden University Medical Center (LUMC). In 2006 he was appointed as full professor in Experimental Endocrinology and Molecular Imaging at LUMC. He was involved in the discovery and clinical translation of new bisphosphonates and sclerostin for the treatment of bone diseases. As PI of the CTMM project MUSIS he was involved in the clinical implementation of fluorescence guided surgery of tumors and sentinel lymph nodes. In May 2015 he joined the Department of Radiology in EMC. He discovered cyanine dyes that specifically bind to dead necrotic cells that can be clinically translated. He is one of the pioneers in the field of whole body optical imaging and one of the cofounders and past president of the European Society for Molecular Imaging (ESMI). He is co-author of >292 peer reviewed papers, H-index 83 and holds 7 patents.
c.lowik@erasmusmc.nl
OPTICAL MOLECULAR IMAGING
CLEMENS WGM LÖWIK, PHD
full professor

Context
Nowadays, whole body fluorescent imaging (FLI) and bioluminescent imaging (BLI) in small animals are widely applied to study biological and molecular processes. For this gene-reporters expressing fluorescent proteins or luciferases in cells or transgenic animals are used. Also, a lot of new tumour-targeted near infrared fluorescent (NIRF) probes, have been developed enabling NIRF imaging to specifically image tumour tissue and to identify sentinel lymph nodes during surgery. Currently, the focus is on development of multi-modality and theranostic probes that can be used for diagnosis and treatment of cancer. In cancer treatment, combination therapy that includes new immune therapeutic approaches is very promising since it cannot only eradicate primary tumours but also distant metastases. Necrotic cell death only occurs under pathological conditions and is involved in e.g., cancer development and treatment, burns, diabetic ulcers, bacterial infections, trauma and ischemic diseases like stroke and myocardial infarction. Therefore, necrosis is a very interesting target for diagnostic imaging and drug delivery.
Top Publications 2021
Lauwerends LJ, van Driel PBAA, Baatenburg de Jong RJ, Hardillo JAU, Koljenovic S, Puppels G, Mezzanotte L, Löwik CWGM, Rosenthal EL, Vahrmeijer AL, Keereweer S. Real-time fluorescence imaging in intraoperative decision making for cancer surgery. Lancet Oncol. 2021 May;22(5):e186-e195.
Stroet MCM, Dijkstra BM, Dulfer SE, Kruijff S, den Dunnen WFA, Kruyt FAE, Groen RJM, Seimbille Y, Panth KM, Mezzanotte L, Lowik CWGM, de Jong M. Necrosis binding of Ac-Lys0(IRDye800CW)-Tyr3-octreotate: a consequence from cyanine-labeling of small molecules. EJNMMI Res. 2021 May 10;11(1):47.
Deng S, Iscaro A, Zambito G, Mijiti Y, Minicucci M, Essand M, Lowik C, Muthana M, Censi R, Mezzanotte L, Di Martino P. Development of a New Hyaluronic Acid Based Redox-Responsive Nanohydrogel for the Encapsulation of Oncolytic Viruses for Cancer Immunotherapy. Nanomaterials (Basel). 2021 Jan 8;11(1):144.
Research Projects: Objectives & Achievements
Research focus:
1. The development and application of new “smart” optical and multi-modality gene-reporters to study i.e. gene expression, tumor progression and metastasis, apoptosis, inflammation, and to follow trafficking, differentiation and fate of cells (i.e. stem-, immune- and tumor cells). Application of “smart” targeted theranostic nanoparticles. The studies are now focussed on tumours and the tumour micro-environment (TME), especially the role of immune cells.
2. Clinical translation of NIRF probes for image guided surgery of tumours and necrosis specific probes for diagnostic imaging in cancer and heart diseases.
3. Implementation of new immune therapies e.g. tumour vaccines, oncolytic viruses, checkpoint blockers in combination with tumour targeted therapies or (tumour targeted) Photo-Dynamic Therapy (PDT).
The development of new “smart” optical and multi-modality gene-reporters and “smart” targeted theranostic nanoparticles.
In last couple of years we have successfully generated and validated new mutated luciferases (Click Beetle green and Firefly red) and new substrates (luciferin based) that generate light of different wavelengths. Using these dualcolour luciferases we have made transgenic T-cell reporter mice in which all T-cells express Click Beetle green luciferase and when activated also express Firefly red luciferase. Be applying specific luciferin substrates and/or spectral unmixing these mice can be used for all kinds of immune studies involving T-cells and their activation. We are using these mice to study T-cell infiltration of tumors and to investigate how we can make immunological “cold” tumors “hot” enabling a better response to immune checkpoint inhibitors. We have also successfully generated a transgenic M2 macrophage luciferase reporter mouse to study Tumor Associated Macrophages (TAMs). In collaboration with Promega Dr. Laura Mezzanotte of our group has successfully generated new reporter systems based on reconstitution of a split-luciferase technology (NanoBit) that can be used to study (oncolytic) virus infections and for cell tracking. As a partner in the LSH-TKI PPP allowance project of Holland Health named “OA-BioDetectChips” we are developing, new bioluminescent tools for read-out (even with an iPhone) to study Osteoarthritis in a micro-fluidic joint-on-a-chip made by the group of Prof Karperien in TU Twente.
In collaboration with our colleagues from Camerino University in Italy, we have successfully developed and validated, a new Hyaluronic Acid based redox-responsive nanohydrogel for the encapsulation of oncolytic viruses for cancer immunotherapy. In addition, we also developed new, fully biocompatible, PLGA or Hyaluronic Acid (HA) based nanoparticles containing 19F for spectroscopic MRI or DTPA-Gd for enhanced MRI, that are targeted towards M2 macrophages in order to study TAMs.
Clinical translation of broad applicable NIRF probes for image guided surgery of tumours and of necrosis specific probes for diagnostic imaging and drug delivery.
NIRF-imaging is a promising technique that can be used to visualize cancer tissue during surgery. From August 2018 till August 2020, I was a visiting professor at CHUV hospital and Ludwig Cancer Center in the lab of Prof George Coukos in Lausanne, Switzerland, who works on new tumour therapies with a focus on immune therapy. I still am a visiting professor at the University of Lausanne (UNIL). There I collaborated with Prof Elena Goun from the chemistry department of EPFL who now is working at the University of Missouri, who developed new (caged-) luciferin substrates for in vivo BLI imaging and probes for intra-operative NIRF imaging. One of these probes (FFA-ICG) is now on its way to be clinically translated in EMC in a new KWF project by Dr. Laura Mezzanotte and the neurosurgeons. We are participating in 3 Marie Curie ITN H2020 projects. In the pHioniC ITN project we are studying and imaging the role of pH and specific ion transporters on the immune tumormicro-environment in pancreatic cancer and also study the effect of PDT. The PAVE ITN project is about developing and testing nano-vaccines for pancreatic cancer where we will use our immune cell reporter mice, nanoparticles and make new gene-reporters. Finally, in CONCRETE therapeutic RNAs have been developed for cancer treatment and we are now testing and imaging the therapeutic RNAs and treatment response.
In a KWF grant from the Dutch Cancer Foundation, we have successfully developed a radio-labelled necrosis targeting probe that can be used to determine tumour aggressiveness and for early detection of anti-cancer therapy efficacy using either 111-Indium SPECT or 68-Gallium PET. The necrosis specific probe was improved by addition of an albumin binding domain that increased in vitro and in vivo necrosis avidity 10-fold. This probe, that specifically binds to necrotic cells, can also be used to image and diagnose necrosis present after myocardial infarcts, stroke and in unstable plaques. The probe also has Photo-acoustic properties. Therefore, we are now also a partner in the LSHTKI PPP allowance project “PICA-Heart” concerning photoacoustic imaging with contrast agents in heart disease.
In collaboration with Prof. de Rijke from Clinical Chemistry I have successfully started serum lipidomics analysis of glioma patients.
Implementation of new immune therapies like tumour vaccines, oncolytic viruses, checkpoint blockers in combination with traditional therapies.
Cancer immunotherapy has shown promising results although a significant proportion of patients responds poorly or relapses at a later stage, therefore more potent combination therapies are required. Tumour ablation by Photodynamic Therapy (PDT) can strongly reduce tumour mass and induce the release of tumour antigens and proinflammatory mediators, therefore being an attractive option for combination with immunotherapy. In preclinical studies we already have shown that immunotherapy using check point blockers can be efficiently combined with PDT, leading to eradication of the PDT treated primary tumour but also distant secondary tumours not treated with PDT. These results suggest combination of checkpoint blockers with tumour ablation by PDT as a feasible novel treatment strategy for advanced cancer. We are also testing a new therapy that specifically kills tumour cells and subsequently also induces a strong immune response against the tumour in combination with immune therapy.
Expectations & Directions
In our research we will further apply our newly developed mutated luciferases and luciferin substrates for improved bioluminescent imaging e.g., to monitor infections of SARSCov2 mutation variants in hamsters, and our multi-modality gene reporters and transgenic animals for imaging immune cells, especially T-cells and M2 macrophages. We will also use them to study responses especially in (oncolytic)viral infections and new cancer treatments involving immunotherapy.
We will continue to clinically translate our new FFA-ICG probe for image guided surgery in glioma. Further studies will be conducted to optimize immune therapy using clinically available anti-tumour vaccines and/or checkpoint blockers in combination with PDT and oncolytic virus therapy with the aim to bring these combination therapies to the clinic. Similarly, we will continue our research on the clinical translation of the necrosis probes for diagnostic imaging and drug delivery and study the possible application of necroptosis and ferroptosis inhibitors to treat ischemia-reperfusion injury and neuro-degenerative diseases. Finally, we will study the role of metabolism and bio-energetics in cancer progression and metastasis and use this knowledge to improve the diagnosis and treatment of glioma and pancreatic cancer.
Funding
C. Lowik, M. Hendriks-de Jong, L .Mezzanotte KWF, Dutch Cancer Foundation: Development of a radio-labelled necrosis-targeted probe for early detection of anti-cancer therapy and anti-cancer treatment: a new theranostic platform. 2018-2021
C. Lowik and L. Mezzanotte. H2020-MSCA-RISE: CANCER: Immunotherapy approaches to improving cancer outcome and quality of life. 2018-2021. 12 month mobility grant for Lowik and 12 month for Mezzanotte
C. Lowik. H2020-MSCA-ITN: pHioniC: pH and Ion Transport in Pancreatic Cancer.2018-2021
C. Lowik and L.Mezzanotte, J.Essers, G.Van Soest. NWO MIddelgroot: In Vivo Optoacoustic Molecular Imaging for applied cancer; aging and cardiovascular research. 20182021.
C. Lowik and L.Mezzanotte. H2020 Marie Curie ITN. CONCRETE: Development of Cancer RNA Therapeutics 20192023
G.Van Soest , J.Essers, C. Lowik. LSH-TKI PPP allowance project “PICA-Heart” concerning photo-acoustic imaging with contrast agents in heart disease. 2020-2022.
C. Lowik and L.Mezzanotte. LSH-TKI PPP allowance project of “OA-BioDetectChips” concerning studies of osteo-arthritis using a joint-on-chip. 2020-2023.
Invited Lectures (selected)
Prof. C. Lowik for Euro-BioImaging The Virtual Pub: Title: “New tools for in-vivo optical imaging and its translation to the clinic”, 19 March 2021.
Prof. C. Lowik for the H20202-MSCA-RISE project cONCReTE ���� - Webinar “Targeted delivery in cancer”, Thu, Apr 22, 2021
Highlights
Publication of our review paper “Impact of real-time fluorescence imaging on intraoperative decision-making in cancer surgery” in Lancet Oncology (IF 33.752).
Additional Personnel
Alan Chan – Visiting Senior Scientist Vincent van Ginneken – Visiting Senior Scientist

TARGETING NECROSIS FOR DIAGNOSIS AND DRUG DELIVERY IN CANCER
KRANTHI PANTH, PHD
Post-Doc
Advisors Clemens Löwik & Laura Mezzanotte
Project Funding KWF grant : Development of radiolabeled necrosis probes for imaging early treatment efficacy and for imaging inherent necrosis as a maker of tumor aggressiveness
Research period June 2018 – June 2021
Email k.panth@erasmusmc.nl
Background: In patients with solid tumors, quantification of necrosis is of great diagnostic value since in many histopathological studies it has been shown that the amount of spontaneous tumor necrosis correlated with aggressive growth and thus disease prognosis. Necrosis can also be caused by external factors including injury caused to tumor tissue by anti-cancer treatments. Effective therapies in most cases will result in primary programmed necrosis (i.e., necroptosis, oncosis, parthanatos, ferroptosis) or secondary necrosis when apoptotic dying cells are not properly and timely engulfed by neighboring cells or professional phagocytes. Therefore, necrosis imaging is not only beneficial in cancer diagnosis but also in determining early treatment efficacy.

Figure 1: A) Increase in necrosis in doxorubicin treated tumors compared to control tumors (saline) visualized by 111Indium labeled ABD-800CW on SPECT images. B) percentage of injected dose quantified from PET/SPECT images C) Ex vivo colocalization of radioactivity and NIR-fluorescence uptake with dead cells (colorimetric TUNEL staining) in adjacent sections
Aim: The aim of the project is to develop radio-labeled necrosis-targeting probes that can be used to determine spontaneous necrosis as a marker of tumor aggressiveness and also to assess the early treatment efficacy of anti-cancer therapies in breast cancer tumor models.
Results and discussion: We have identified non-toxic nearinfrared fluorescent cyanine dyes that possess strong necrosis targeting properties. When one of these dyes, 800CW was coupled to a clinically approved chelate and radiolabeled with 111Indium, it fully retained its necrosis targeting property. The radioactive signal and the intrinsic fluorescence from the labeled compound perfectly overlapped with dead cell staining (TUNEL) on cryosections of these tumors1. Although, the specificity of the probe is high, the clearance was rapid. Therefore, we improved the probe by adding an albumin binding domain (ABD). The
addition of ABD has improved the probe’s affinity towards necrosis (>10x)2 and enhanced tumor uptake. We further determined the treatment efficacy in doxorubicin and saline treated human breast cancer tumor model, MCF-7. Our data suggested an increase in uptake of the compound in doxorubicin treated group compared to saline group (Figure 1)
Ongoing projects: The necrosis targeting probes (800CW) has good photoacoustic (PA) properties. We are currently evaluating PA imaging of these probes in tumors and cardiovascular diseases.
References:
1) Stroet, M.C.M., et al. Mol Imaging Biol 22, 1333–1341 (2020)
2) Stroet, M.C.M.; Cancers, 14, 4-861 (2022)

MOLECULAR IMAGING OF THE MICROENVIRONMENT-TRANSPORTOME INTERPLAY IN PDAC PhD Student
ROISIN MC MORROW, MSC
Advisors Clemens Löwik, Alan Chan & Laura Mezzanotte
Project Funding EU funded project: H2020-MSCA-ITN Acronym-pHioniC
Research period October 2019 – October 2022
Email r.mcmorrow@erasmusmc.nl
This project is a collaboration between the Department of Radiology & Nuclear Medicine and Percuros.
Pancreatic ductal adenocarcinoma (PDAC) is the most common form of pancreatic cancer, with less than 8% of affected individuals surviving more than 5 years. Such low survivorship rates for PDAC are widely attributed to its often late-stage diagnosis and its rapid dissemination to the lymph nodes and distant sites. Indicating that new diagnostic and treatment options are urgently needed.
Whilst the genetic and epidemiology understanding of PDAC is as well understood as other cancers, the current research has not adequately addressed the molecular mechanisms that provoke the rapid progression of PDAC and more targeted research focus is essential.
In this regard, we are primarily interested in exploring the role which the tumor microenvironment (TME) plays in driving the progression of PDAC and most specifically in determining the involvement of dysregulation of the pH and the transportome in PDAC.
Solid tumors such as PDAC are characterized by a highly acidic microenvironment due to altered expression of ion channels and transporters. This microenvironment affects important cellular processes involved in cell proliferation, migration as well as other components surrounding the tumor cells such as immune cells. In terms of specifics, the research project is focused on looking into the affects ion channel activity has on PDAC tumor progression, pH, and T cell infiltration.

In order to answer these questions, we will develop a biosensor for imaging in vitro and in vivo, ion channels endogenous activity using CRISPR technology and molecular imaging technology, such as the IVIS. Due to the heterogeneous TME, simply targeting one aspect is not sufficient to eradicate the cancer cells. Which, is why we wanted to combine therapeutics that take advantage of the acidic TME or can aid in regulating it in combination with immunotherapy agents to lead to a more effective killing response. An important focus is combining PDT with immune therapy to treat PDAC.
An additional research goal is to explore the effects which the microenvironmenttransportome has on T-cell infiltration in PDAC using a dual-color luciferase transgenic T-cell reporter mouse.
Figure 1. Overview of Project (Created with BioRender)

DEVELOPMENT OF NECROSIS AVID CONTRAST AGENTS FOR DETECTION OF THERAPY EFFICIENCY IN SOLID TUMORS
MARC CM STROET, MSC
PhD Student
IAdvisors Clemens Löwik, Laura Mezzanotte, Yann Seimbille & Kranthi Panth
Project Funding KWF grant : “Development of a radiolabeled necrosis-targeting probe for early detection of anti-cancer therapy efficacy and anti-cancer treatment: a new theranostic platform.”
Research period May 2018 – January 2022
Email m.stroet@erasmusmc.nl
ncreased occurrence of necrotic cell death is predominantly found in disease and this makes it an interesting imaging target for many diseases. For instance, aggressively growing tumors have poorly developed and poorly functional blood vessels in their center, leading to necrotic areas in the center of the tumor. Moreover, effective anticancer therapy induces cell death in tumors leading to necrosis. Therefore, the increase in tumor necrosis can function as an indicator for treatment efficiency, which is currently measured by tumor size reduction after several weeks or months of treatment. On the other hand, treatment induced necrosis can be apparent within days. This means that therapy efficacy can be determined in an early stage, avoiding unnecessary treatment of ineffective therapies. Currently there are no tools for adequate detection of necrosis in clinical settings. We found out that cyanine-based dyes, such as 800CW, exhibit a strong affinity for denaturated intracellular proteins, that can only be reached upon loss of cellular membrane integrity due to necrosis.
We conjugated 800CW with DOTA for radiometal labeling and the resulting chemical compound, DOTA-PEG4-800CW, was labeled with indium-111 for SPECT imaging and with Gallium-68 for PET.
We studied the binding of [111In]In-DOTA-PEG4-800CW to dead and alive cells and we observed selective binding of both radioactivity and fluorescence to the dead cells and not to the live cells. We then injected the tracer in mice with tumors that spontaneously develop a necrotic core. We were able to visualize the tumors by SPECT-CT and confirmed the necrosis binding in the tumors with autoradiography, fluorescence imaging, and dead cell staining.
We now improved the in vivo circulation time of the tracer by including an albumin binding domain to the molecular structure. As a result, we observed a significantly higher uptake by dead cells, both in vitro and in vivo. We were able to detect therapy induced tumor necrosis with our multimodal necrosis probe, using its fluorescence and its SPECT signal. We confirmed the necrosis uptake with a decline in BLI and PET signal from the viable tumor tissue within the same mouse, in a single imaging sequence.

Figure 1. Multimodal imaging of Tumor necrosis in mice.
Laura Mezzanotte obtained her MS degree in Pharmaceutical biotechnology and PhD in Pharmaceutical Sciences from University of Bologna in 2007 and 2011, respectively. During her phD she was awarded the Marlene De Luca young investigator prize for outstanding contribution in the field of Bioluminescence and Chemiluminescence. She carried postdoc research at Leiden University Medical Center from 2011 to 2015 applying molecular imaging (optical and magnetic resonance imaging) for cell tracking in cancer, stem cells and immunology related projects. She joined the department as Assistant Professor in May

2015. She has successfully participated and participate as PI in several European projects and in two Dutch Cancer Society (KWF) projects. She is member of the International society of Bioluminescence and Chemiluminescence, the World Molecular Imaging Society and member elect of council of the European Molecular Imaging Society, where she is also program sub-chair and abstract reviewer for the annual conferences. She is founding member of the ESMI study group on Oncoimmunology and Therapy. She is co-author of 60 peer reviewed papers, H index (ISI) 21 and hold two patents.
l.mezzanotte@erasmusmc.nl
GENETIC ENGINEERING FOR MULTIMODALITY IMAGING
LAURA MEZZANOTTE, PHD
associate professor

Context
Gene reporters have a long history in preclinical research but only recently, with the clinical approval of different cells based therapies for cancer treatment or regenerative medicine, they have been used to track cells in patients. Knowing location of cells and their functional status help patient stratification and evaluation of clinical efficacy for clinical studies. My group is the first that will generate novel reporter genes for preclinical and translational imaging research and combine it with different injectable contrast agents to refine experiments and obtain a better picture of the distribution and function of the cells. In particular the group is focused in imaging T cells and macrophages to assess the functional status (Exhaustion and Activation) of TCR or CAR T and CAR M cells and of Tumor associated macrophages (M2 like-Macrophages) target of anticancer combination therapies and to study the biology of T cells and macrophage in healthy and disease state in vivo.
Top Publications 2021
Zambito G, Chawda C, Mezzanotte L. Emerging Tools for Bioluminescence imaging. Curr Opin Chem Biol. 2021 Aug; 63:86-94.
Gaspar N, Walker JR, Zambito G, Marella-Panth K, Lowik C, Kirkland TA, Mezzanotte L. Evaluation of NanoLuc substrates for bioluminescence imaging of transferred cells in mice. J Photochem Photobiol B. 2021 Mar; 216:112128.
Research Projects: Objectives & Achievements
Novel reporter genes and substrates
Development of new reporter genes for imaging comprehends both the creation of mutants that allow enhance detection and fusion reporter for multimodality imaging. In this regards the research focuses on development of new luciferase mutant and modified luciferin substrates for multicolor bioluminescence. In addition luciferase reporter fused to fluorescent proteins or PET reporter genes are under development in the laboratory. The new reporter genes are generally cloned in vectors that allow co-expression of different reporters at the same time in cells and animals.
Molecular Imaging in Immunology
Since the beginning BLI was applied for imaging cells of the immune system and especially in transplantation studies. Nowadays with the increasing interest on cellular and antibody immunotherapies and use of biologic for chronic inflammatory disease, elucidating the role of different immune cells in vivo becomes of extreme importance to design and employ better therapies. There are transgenic mice that allow multimodal imaging of naïve T cells, NK T cells and dendritic cells. However most of the tools allow cell tracking of adoptively transferred cells while strategies to image endogenous immune response and cell function are still lacking. Our group is actively involved in the development of new transgenic mice models and nanoparticle based imaging probes for evaluation of T cells and macrophages.
Immunovirotherapy of Cancer
The use of Oncolytic viruses as therapeutics has been recently clinically translated after many years of incremental scientific evidence of their efficacy. The first oncolytic virus was licensed by FDA in October 2015 to Amgen (Thousand Oaks, CA, USA) for the treatment of advanced melanoma. Many wild-type viruses show an intrinsic selectivity for replication within cancer cells.. However it is not only the cell killing ability that makes oncolytic viruses a powerful anticancer therapeutic but also their ability to stimulate antitumor immune effector cells. This double effect makes correct to refer to the therapy using oncolytic virus as an oncolytic immunovirotherapy. Moreover since oncolytic viruses activate antitumor immune effector cells, either innate and/or adaptive, their use in combination with immune checkpoint inhibitors is attractive to boost developing T-cell responses against
systemic tumor. Antitumor therapy may actually benefit from those immune responses, which contribute to tumor clearance in which case immune checkpoint inhibition may add to, or synergize with, direct oncolytic virotherapy in clearing tumor cells. My specific interest is to develop system to efficiently track oncolitytic viruses. We successfully engineering oncolytic adenoviruses for efficient imaging of the therapy against cancer and as, in a collaboration effort, encapsulated them in a novel nano-hydrogel formulation for future intravenous administrations.
Nanoparticle based contrast agents for MRI and theragnostic applications.
Magnetic resonance imaging (MRI) is a leading clinical diagnostic technique, which is able to provide whole body imaging and when individual cells are imaged in living animals, it can provide new insights into the biology of cell trafficking and migration. For cells to be visualized by MRI, they generally must be labelled to enable their discrimination from surrounding tissue. The development of magnetic resonance imaging (MRI) contrast agents is therefore an active area of research, where the basis for this interest is the expansion of MRI as a high-resolution and non-invasive important preclinical and clinical imaging modality. Moreover, there are now new opportunities to developing smart materials with multifunctional abilities including MRI contrast in-built within biomaterial structures, functionalization with targeting ligands and the carrying of a therapeutic payload for theragnostic applications. My research is focused on development of targeted nanoparticles containing perfluorocarbon for 19F MRI or novel nano-Gadolinium formulations to improve contrast in MRI.
Expectations & Directions
In the focus area of Molecular Imaging and therapy the research line on genetic engineering for multimodal imaging will continue to develop novel reporter genes for multimodality imaging including optical, nuclear, optoacoustic, ultrasound and magnetic resonance imaging in order to go beyond state-of-the-art imaging in organ on a chip and in vivo. In the coming years, beside gene and cell-based therapies, the research will focus on improving in vivo imaging of pathogens (viruses and bacteria). Moreover, the research will expand on clinical translation of theragnostic optical agents for image guided surgery and photodynamic therapy, with special focus on NIR-II (near infrared II) window emission.
Highlights
Obtaining a KWF project as project leader for the clinical translation of a probe for image guided surgery of Glioblastoma. Publishing 7 papers as corresponding author. Successfully co-promoted Giorgia Zambito and Natasa Gaspar, despite the pandemic.
Funding
M. Karpeirin, L.Moreira Teicaira, L. Mezzanotte and C. Lowik Health–Holland-TKI. OA-Biodetects-CHIPs-Towards osteoarthritis fingerprinting – combining imaging biomarkers and multi-organ-on-chip technology for improved in vitro models. 2021-2024
L. Mezzanotte and partners. Marie Curie EU-RISE-PRISAR2- 12 month mobility grant -2020-2024.
C. Lowik and L. Mezzanotte . H2020-MSCA-RISE: CONCRETEImprovement of RNA therapeutics. 2020-2024. 12 month mobility grant for Lowik and 12 months for Mezzanotte.
C. Lowik and L. Mezzanotte . H2020-MSCA-ITN-2019PAVE: A nanovaccine Approach for the treatment of Pancreatic Cancer. 2020-2024.
P.Katsikis, S.Shoenberger, K. Ishii, C. Schliehe L. Mezzanotte. KWF-Dutch Cancer Foundation: Improving Checkpoint Blockade Therapy with Highly Immunogenic Personalized Neoepitope Vaccines. 2020-2024
C. Lowik , M. Hendriks-de Jong , L . Mezzanotte KWF, Dutch Cancer Foundation: Development of a radio-labelled necrosis-targeted probe for early detection of anti-cancer therapy and anti-cancer treatment: a new theranostic platform. 2018-2021
C. Lowik and L. Mezzanotte . H2020-MSCA-RISE: CANCER: Immunotherapy approaches to improving cancer outcome and quality of life. 2018-2022. 12 month mobility grant for Lowik and 12 month for Mezzanotte
C. Lowik and L. Mezzanotte , J.Essers, G.Van Soest. NWO MIddelgroot: In Vivo Optoacoustic Molecular Imaging for applied cancer; aging and cardiovascular research. 20182021
L.Mezzanotte , R. Balvers, C. Dirven. KWF-Dutch Cancer Foundation: First in man assessment of FA-ICG for image guided surgery of Glioblastoma. 2022-2025
Invited Lectures
Mezzanotte L. Multiplexing in In Vivo Optical Imaging: How to Image Multiple Signals Simultaneously in Small Animals. Webinar hosted by Perkin Elmer. 19 May 2021.
Mezzanotte L. Optical probes: invited introductory lecture EMIM 2021- 26 Aug 2021, Goettingen, Germany.
Additional Personnel
Ana-Luiza Cabral De Seitao Oliveira, MSc.
Ana-Luiza joined our lab as part time lab manager from November. She assists phD students in their lab activities and takes care of orders and genotyping. Her background and expertise is in nanopharmeceutical preparations so she contributes also on related projects.

Emanuele Musella, BSc.
Emanuele joined the lab from October 2021 to February 2022 thanks to an Erasmus + Fellowship in collaboration with University Federico II of Naples and the group of Prof. G Condorelli. Emanuele worked partly on his master thesis project about aptamers as theranostic agents for imaging and photodynamic therapy of Glioblastoma.

Jasmine Bordignon, BSc.
Jasmine joined the lab from June 2021 to November 2021 as a secondee from HealthT company, in the context of the EU-H2020-RISE project PRISAR 2. During her secondment Jasmine got introduced to preclinical and clinical imaging modalities and learned about cancer patient journey.

Francesca De Micco, MSc.
Francesca joined the lab from June 2021 to September 2021 as a secondee from Clinica Mediterranea in Naples, in the context of the EU-H2020-RISE project Concrete. During her secondment Francesca learned about preclinical imaging modalities and worked especially on optical imaging for the development of cell based assay to investigate ncRNAs.


MULTI-MODALITY GENE REPORTERS FOR IMAGING EMT AND TUMOR-IMMUNE CELL INTERACTION
CHINTAN CHAWDA
PhD Student
Advisors Clemens Löwik & Laura Mezzanotte
Project Funding EU founded project : H2020-MSCA-ITN-ETN-2019 Acronym -PAVE
Research period August 2020 – August 2024
Email c.chawda@erasmusmc.nl
Pancreatic cancer is an aggressive and malignant tumor with a dismal prognosis. Thus far, no effective treatment strategy has been established for pancreatic cancer patients. Tumor-targeted vaccines have been widely discussed in recent studies and enabled important breakthroughs in the treatment of pancreatic cancer by preventing the escape of tumor cells from immune surveillance and activating the immune system to eliminate cancer cells. T cells can be activated by the stimulation of tumor-targeted vaccines, but to mount an effective immune response, both immune checkpoint inhibitors and positive costimulatory molecules are required.

The aims of PAVE project are: to elicit long-term immunity against PDAC, using multicomponent nanovaccines; to produce adequate preclinical models and assays, which will be more relevant for testing these new immunological approaches; and to track vaccine biodistribution in vivo by incorporation of imaging contrast agents within biodegradable particles.
Our research aims to develop preclinical model to image PDAC carcinogenesis. In particular, a novel optical reporter genes and constructs will be generated to study in vivo the carcinogenesis of PDAC in human and syngeneic mice models. The use of multicolor luciferases will be used to develop sensors for epithelial-mesenchymal transitions (EMT) of pancreatic cancers in vitro and in vivo cancer. Reporter plasmid will be designed consisting of two bioluminescent gene of distinct emission wavelength and substrates activation.
EMT Sensor: PCDH-EF1-MCS as a backbone plasmid. An EMT driven promoter is followed by Cre sequence downstream. Other plasmid PCDH-EF1 would contain CBR2 flanked by loxp followed by CBG2 with a stop codon. Both will be transduced in KPC cells prior to transplantation. Upon activation of EMT promoter, The cells will switch their color.
These will be tagged following elevated expression from epithelial to mesenchymal phenotype. Designed EMT reporter will be initially tested in-vitro for characterization followed by testing EMT in orthotopic mouse model using KPC induced PDAC tumor model followed by imaging in bioluminescent system. Moreover novel optical tri-modality reporter consisting of a bioluminescent, fluorescent and opto-acoustic genes will be exploited to evaluate syngeneic tumor progression and regression due to therapy.

SMART REPORTER GENES FOR CELLULAR MOLECULAR IMAGING IN TUMOR IMMUNOLOGY
NATASA GASPAR, PHD
PhD Awarded 15 September 2021
Advisors Clemens Lowik, Laura Mezzanotte, & Alan Chan
Project Funding EU founded project : H2020-MSCA-ITN 2016 Acronym -ISPIC
Research period Natasa Gaspar was born on April 20th, 1990 in Benkovac, Croatia. She studied tudied Nutrition Science in Zagreb, University of Food Technology and Biotechnology, from 2009 to 2012. Natasa performed her Master studies in Molecular Biotechnology at the same university from 2012 to 2014. During her masters, she participated in the LLP Erasmus exchange program for excellent students, at the Technical University of Kaiserslautern, Germany where she investigated the effect of Photodynamic inactivation in the Escherichia coli.
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Percuros.
Molecular imaging became a part of standard care for cancer. By visualization what is happening in the body at a cellular level, molecular imaging provides unique information in detection, diagnosis and treatment of cancer. During the last century much research has been performed in order to enhance pre- and post- operative imaging for early detection and therapy efficacy. Despite efforts, targeting cancer remains challenging due to intra- and inter- tumor heterogeneity. In order to fully advance cancer diagnosis and management using molecular imaging, one would like to bridge together fundamental advances in imaging modalities that can provide different information enabling to cross-examine the disease from several angles. Within this thesis we performed preclinical studies in different models with the ultimate aim of using molecular imaging for better cancer management.
One focus is development of bioluminescent oncolytic virus to assess efficacy of immunovirotherapy. I have developed strategies to sensitively image oncolytic virus infection of tumor cells in vivo based on split Nanoluc luciferase approach. For example, the small high-affinity peptide tag (HiBiT), which is only 11 amino-acids in length, is engineered into a clinically used oncolytic adenovirus, and the complementary large protein (LgBiT) was constitutively expressed in tumor cells. Infection of the LgBiT expressing cells with the HiBiT oncolytic virus will reconstitute NanoLuc in the cytosol of the cell, providing strong bioluminescence upon treatment with substrate. This new bioluminescent system serves as an early stage
quantitative viral transduction reporter in vitro and also in vivo in mice, for longitudinal monitoring of oncolytic viral persistence in infected tumor cells.
https://epubs.ogc.nl/?epub=n.gaspar



NEW TOOLS FOR MULTIMODAL IMAGING OF IMMUNE CELLS
GIORGIA ZAMBITO, MSC, PHD
Advisors Clemens Löwik & Laura Mezzanotte
PhD Awarded 8 September 2021
Project Funding EU founded project: H2020-MSCA-ITN 2016 -ISPIC &H2020-MSCA-RISE-2020- cONCReTE
Research period Giorgia Zambito was born in Palermo, Italy. She studied Biological Sciences and she carried out her Master of Science (MSc.) in Cell and Molecular Biology both at the University of Palermo. She performed half of the MSc. program at the Giga research center of Liege (Belgium) thanks to the Erasmus European Exchange program scholarship (2013-2014). During that year, she investigated and wrote her report on the alternative splicing of mRNA in breast cancer cells. In 2015, she started an internship on apoptotic effects of natural compounds on melanoma cells at the National Institute of Research in Palermo. In January 2017, she started her Ph.D. project on new tools for multimodal imaging of immune cells in collaboration with EMC and under the daily supervision of Dr. Laura Mezzanotte. She is member of the European Molecular Imaging Society and she is co-founding member of the Dutch young Molecular Imaging Community (DyMIC). After her PhD studies Giorgia, she pursued a postdoctoral position at the EMC in 2021.
This project was a collaboration between the Departments of Radiology & Nuclear Medicine and Medres
The thesis describes mainly two non-invasive imaging techniques used to visualize immune cells in tumor-bearing mouse models. The first imaging modality explored is Bioluminescence (BL). We first evaluated different D-luciferin dependent luciferases and then we selected and optimized a near-infrared click beetle luciferase mutant (CBG2). This luciferase can be combined with another near-infrared CBR2 luciferase and both react with naphthyl luciferin. These innovative luciferase pairs are used for non-invasive and simultaneous dual-color BL imaging of two immune cell populations in vivo. The second imaging modality used for non-invasive cell tracking is the Magnetic Resonance Imaging (MRI).Tumor-associated macrophages (TAMs) were targeted by polymeric nanoparticles encapsulating 19F as MR contrast agent, and monitored in vivo in breast-cancer tumor bearing mice. At the present as post-doctoral researcher, my research is focused on the identification of novel cancer non-coding RNA therapeutics used as prognostic marker and for their clinical applicability.
https://repub.eur.nl/pub/135676/


JOINT APPOINTMENT IN MEDICAL ONCOLOGY
Astrid van der Veldt is a medical oncologist at Erasmus MC. After obtaining her cum laude medical degree, she completed two PhDs in medical oncology and nuclear medicine, resulting in a double dissertation with cum laude degree in 2012 at the VU University Medical Center in Amsterdam. In 2016, she completed her training in internal medicine and medical oncology at the VU University Medical Center and The Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, respectively. Since 2017 she works at the Departments of Medical Oncology and Radiology & Nuclear Medicine at Erasmus MC. In clinical oncology, her main research interests include immunotherapy and the treatment of urothelial cell cancer, renal cell cancer, and melanoma. In addition, she supervises research collaborations between medical oncology and imaging, including radiology and nuclear medicine. a.vanderveldt@erasmusmc.nl

Tessa Brabander is a nuclear medicine physician at the Erasmus MC. In 2011, she graduated in medicine at the Erasmus University. After graduation, she completed training in nuclear medicine in 2016 and started working as a staff member at the Department of Radiology & Nuclear Medicine. During her residency, she started research at the Department of Nuclear Medicine under supervision of prof dr DJ Kwekkeboom. In October 2017, she received her PhD. Currently, she works as a nuclear medicine physician at the department. Her main research interests are imaging, therapy of neuroendocrine tumors, and other radionuclide therapy with alpha emitting radionuclides. t.brabander@erasmusmc.nl
CLINICAL NUCLEAR MEDICINE: IMAGING AND THERAPY IN ONCOLOGY
ASTRID VAN DER VELDT,
MD,
PHD & TESSA BRABANDER,
MD, PHD
medical o ncologist & nuclear medicine physician

Top Publications 2021
Ling SW, de Jong AC, Schoots IG, Nasserinejad K, Busstra MB, van der Veldt AAM, Brabander T. Comparison of 68Ga-labeled Prostate-specific Membrane Antigen Ligand Positron Emission Tomography/Magnetic Resonance Imaging and Positron Emission Tomography/Computed Tomography for Primary Staging of Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol Open Sci. 2021 Sep 28;33:61-71. doi: 10.1016/j.euros.2021.09.006. PMID: 34632423; PMCID: PMC8488242.
Cox CPW, van Assema DME, Verburg FA, Brabander T, Konijnenberg M, Segbers M. A dedicated paediatric [18F]FDG PET/CT dosage regimen. EJNMMI Res. 2021 Jul 19;11(1):65. doi: 10.1186/s13550-021-00812-8. PMID: 34279735; PMCID: PMC8289942.
Hooijman EL, Chalashkan Y, Ling SW, Kahyargil FF, Segbers M, Bruchertseifer F, Morgenstern A, Seimbille Y, Koolen SLW, Brabander T, de Blois E. Development of [225Ac]Ac-PSMA-I&T for Targeted Alpha Therapy According to GMP Guidelines for Treatment of mCRPC. Pharmaceutics. 2021 May 13;13(5):715. doi: 10.3390/ pharmaceutics13050715. PMID: 34068206; PMCID: PMC8153125.
Context
Nuclear medicine is rapidly changing as a result of the ongoing development of new radiopharmaceuticals. A variety of radiopharmaceuticals is currently available for imaging and their number is still increasing. In the last decade, many new tracers were developed for both diagnostics and radionuclide therapy. In clinical practice, nuclear medicine plays a pivotal role in the diagnosis of many diseases, within for example cardiology, neurology, and medical oncology. The concept of “theranostics” refers to compounds that can be used for both imaging and therapy. To this end, different radionuclides are labeled to the same compound. After injection, compounds labeled with a shortlived radionuclide (e.g. 68Gallium) provide the biodistribution of the compound in the body, which enables the prediction of clinical response to the same compound when labeled with a therapeutic radionuclide such as 177Lutetium (177Lu). Peptide receptor radionuclide therapy (PRRT) with [177Lu-DOTA,Tyr3]octreotate (177Lu-DOTATATE) is an example of a very successful theranostic, as this treatment improves progression-free survival in patients with advanced progressive, somatostatin receptor positive neuroendocrine tumors (NETs).
Research Projects: Objectives & Achievements
Diagnostics
18F-FDG is the most well known PET tracer for clinical diagnostics. This tracer is frequently applied for staging in oncology, but it is also evaluated for response evaluation during therapy, which is illustrated by the IMPACT-study in colorectal cancer. As prostate cancer is not 18F-FDG avid, other tracers, like 68Ga-(prostate specific membrane antigen)PSMA, have been introduced
for clinical evaluation of prostate cancer. As 68Ga-PMSA PET provides high image quality, it is increasingly applied for staging of prostate cancer. In 2021, 68Ga-PMSA PET was evaluated to guide bone biopsies in patients with metastatic prostate cancer (see chapter Anouk de Jong). In addition, 68Ga-PMSA PET will also be investigated for response evaluation in patients with metastatic prostate cancer (see chapter Anouk de Jong). In the IMPACT consortium (IMaging PAtients for Cancer drug selecTion), new tracers are evaluated in collaboration with VUmc, Radboudumc, and UMCG. In the IMPACT trial, the impact of PET using 18F-fluoroestradiol (18F-FES) and 89Zr-trastuzumab, is investigated for treatment planning of patients with metastatic breast cancer. In addition, PET using 89Zr-girentuximab, a monoclonal antibody targeting the glycoprotein CAIX, is evaluated during watchful waiting in patients with metastatic renal cell cancer.
Radionuclide therapy
For decades, the Department of Nuclear Medicine has been focused on radionuclide therapy. Together with the NETTER-1 trial, the results of our large phase 2 trial has resulted in FDA and EMA approval of 177Lu-DOTATATE for gastroenteropancreactic neuroendocrine tumors (GEPNETs). Although 177Lu-DOTATATE has proven efficacy in patients with NET, there is a need to further improve tumor response to PRRT. Therefore, we will investigate the combination of 177Lu-DOTATATE with different systemic therapies, e.g. chemotherapy or PARP-inhibitors (see chapter Julie Nonnekens). This may improve tumour response rates and hopefully progression free survival. In addition, epigenetic drugs are under investigation to improve the number of somatostatin receptors in NETs. For therapy of prostate cancer, the peptide of first choice is PSMA.
The department has invested in a new laboratory which is completely focused on the labeling of alpha emitting radionuclides. Recently, this laboratory hasreceived a full GMP licence for the production of radiopharmaceuticals. These features are unique in Europe and will give us the opportunity to perform studies with alpha emitting radionuclides in patients. The first study wil be the 225AcPSMA in a phase-1 clinical trial in patients with metastatic prostate cancer (see chapter Sui Wai Ling), funded by KWF/Maarten van der Weijden foundation. Also, in 2021 a KWF Young Investigator grant was awarded to start with alpha emitting radionuclides in neuroendocrine tumor patients (225Ac-DOTATATE).
Expectations & Directions
To apply imaging from bench to bedside, collaborations with the preclinal imaging group will be further intensified. For diagnostics, future directions include the development and clinical implementation of new tracers. As the PET-MRI has been installed in 2019, more research collaborations between nuclear medicine and radiology (e.g. radiomics) are planned for e.g. prostate cancer (see chapter Sui Wai Ling) and melanoma brain metastases (see chapter Sophie Derks). The integrated PET-MRI combines a 3.0T MRI with the newest PET technology. This state-of-the-art scanner with simultaneous acquisition of PET and MRI creates many opportunities for imaging. The combination of these two techniques enables visualization of cellular changes by PET and localization by MRI. The PET-MRI can be used as a one-stop scan for staging of different tumor types. In the upcoming years, we will focus on new indications for PET-MRI and its additional value compared to PET-CT or MRI alone.


Funding
Tessa Brabander & Astrid van der Veldt: KWF grant (2019-2022) “Phase I dose escalation study to evaluate tolerability and safety of 225Ac-PSMA in patients with metastatic prostate cancer”
Astrid van der Veldt: EMC fellowship (2019-2023) “Reducing toxicity and improving outcomes in immunotherapy treated melanoma patients”
Astrid van der Veldt: DDH Award (2019-2024) “Early detecting and understanding treatment failure in melanoma brain metastases”
Astrid van der Veldt: KWF Young Investigator Grant Bas Mulder Award (2019-2025) “Safe Stop-QoL: impact of early discontinuation of PD-1 blockade on quality of life (QoL) of patients with advanced melanoma”
Astrid van der Veldt: DUOS grant (2018-2022) “Response measurement study in metastatic castration resistant prostate cancer patients to improve early response evaluation and understand radium-223 induced immune response”
Tessa Brabander & Hans Hofland: Advanced Accelerator Applications Grant 2020-2022: “Expanding the indication of Lutathera”
Astrid van der Veldt & Anne-Marie Dingemans: KWF grant (2020) “Dutch Oncology COVID-19 Consortium (DOCC)”
Astrid van der Veldt & VOICE consortium: ZonMW grant (2021-2023) “VOICE trial: Vaccination against cOvid In CancEr”
Astrid van der Veldt & VOICE consortium: ZonMW grant (2021-2023) “Third vaccination VOICE trial”
Astrid van der Veldt: Trustfonds Erasmus (2021-2026) “Genomic landscape and actionable targets as identified by whole genome sequencing in metastases from patients with renal cell carcinoma”
Tessa Brabander: KWF Young Investigator Grant (20222026): Salvage therapy with 225Ac-DOTATATE for patients with metastatic neuroendocrine tumors
Highlights
Tessa Brabander received the KWF Young Investigator Grant in 2021.
PhD student Karlijn de Joode received De Pieter De Mulder Award 2021 for a research fellowship at The Francis Crick Institute in London.
Invited Lectures
Astrid van der Veldt: Online webinar oncology during COVID-19, Hilversum, 2021, Medtalks
Astrid van der Veldt: Online ASCO journal renal cell cancer and bladder cancer, Hilversum, 2021, Medtalks
Astrid van der Veldt: Online webinar neoadjuvant treatment of melanoma , Hilversum, 2021, Medtalks
Astrid van der Veldt: Online ESMO journal renal cell cancer and bladder cancer, Hilversum, 2021, Medtalks
Astrid van der Veldt: Online webinar cardio-oncology, Hilversum, 2021, Medtalks
Astrid van der Veldt: Online webinar treatment of metastatic melanoma, Hilversum 2021, Medtalks
Astrid van der Veldt: Educational webinar, treatment of melanoma, European Medicines Agency, 2021
Astrid van der Veldt: Online Harvard seminar ‘ 68Ga-PSMA PET to guide bone biopsies for molecular profiling in patients with metastatic prostate cancer’, 2021
Tessa Brabander: Peptide Receptor Radionuclide Therapy in neuroendocrine tumor patients. At the NVNG scientific meeting, 2021
Tessa Brabander: PRRT - practical issues & grey areas. ENETS webinar, 2021
Tessa Brabander: Global Webinar on NET management across the world, Novartis, 2021
Tessa Brabander: Peptide receptor radionuclide therapy in neuroendocrine tumors. ESMO OncologyPRO E-Learning programme, 2021
Tessa Brabander: Present and future of radionuclide therapy. At the Sixth International Congress Clinical Needs and Translational Research in Oncology, 2021.
Additional Personnel
Maud Rijnders, PhD student, Department of Medical Oncology
Evalyn Mulder, PhD student, Departments of Surgery and Medical Oncology
Karlijn de Joode, PhD student, Department of Medical Oncology
MD, PHD MOLECULAR IMAGING BRAIN & HEMATOLOGICAL DISEASES
Nuclear Medicine Physician DANIELLE
VAN ASSEMA,

Daniëlle van Assema is currently involved as a researcher in several (finishing) projects at the Department of Radiology and Nuclear Medicine at Erasmus Medical Center. d.vanassema@erasmusmc.nl
Daniëlle is currently still involved in several research projects at the Erasmus MC;
Neurology
Amyloid deposition and neuro-inflammation
Together with Meike Vernooij, Rebecca Steketee and Joyce van Arendonk we are currently working on a large research project (MEMORABEL AMYVASC study) to assess the relationship between vascular brain damage and amyloid pathology in the brains of elderly non-demented people. For this project, we have included over 600 subjects of the Rotterdam cohort study with varying degrees of vascular pathology and we acquired 18F-Florbetaben amyloid PET scans, partially in dynamic scan mode. In 2018, we started the actual PET scanning and up till now, around 630 participants have been scanned. Data analysis including visual and (semi-)quantitative scoring of scans are being performed at the moment.
A collaboration project together with Harro Seelaar of the Dept. of Neurology to assess neuroinflammatoin in the brain by performing PET scans with the activated microglia tracer 18F-DPA-714 in symptomatic and asymptomatic subjects with a genetic mutation who will develop frontotemporal dementia within time and in a control group (FIND-MORE study) has been approved and we started the actual PET-MRI scanning in 2021.
Hematology
Lymphoproliferative diseases
Daniëlle is a member of the HOVON (Hemato-Oncologie Volwassen Nederland) Imaging group and the NVNG Hematology interest group. In 2021 several new HOVON PET studies have been started at EMC and in other centers. Daniëlle is scoring a Phase II study in newly diagnosed MYC+ DLBCL patients who receive 1 cycle R-CHOP followed by 5 cycles DA-EPOCH. PET scans will be made at baseline, interim and end-of-treatment and will be scored afterwards by Daniëlle. When complete metabolic response is achieved (as scored on PET), patients will be treated with the checkpoint inhibitor nivolumab for one year afterwards.
Other topics
Preparing for PET imaging
Tiny Cox, MNW at Nuclear Medicine and PhD student at our department, is performing studies assessing preparation for PET imaging and PET image quality. The first project was a prospective study including 21 patients with neuro-endocrine tumors and/or metastasis from neuro-endocrine origin who went for 68Ga-DOTATATE PET imaging, and has already been published in 2020. A new study has been performed on optimizing pediatric and adult 18F-FDG dose regimen fot PET-CT imaging, which has been published in 2020.
Publications 2021
Interim PET Evaluation in Diffuse Large B-Cell Lymphoma Using Published Recommendations: Comparison of the Deauville 5-Point Scale and the Δ SUV max Method. Rekowski J, Hüttmann A, Schmitz C, Müller SP, Kurch L, Kotzerke J, Franzius C, Weckesser M, Bengel FM, Freesmeyer M, Hertel A, Krohn T, Holzinger J, Brink I, Haberkorn U, Nyuyki F, van Assema DME , Geworski L, Hasenclever D, Jöckel KH, Dührsen U. J Nucl Med. 2021 Jan;62(1):37-42
A dedicated paediatric [18F]FDG PET/CT dosage regimen. Cox CPW, van Assema DME , Verburg FA, Brabander T, Konijnenberg M, Segbers M. EJNMMI Res. 2021 Jul 19;11(1):65.
[18F]Flortaucipir PET Across Various MAPT Mutations in Presymptomatic and Symptomatic Carriers. Wolters EE, Papma JM, Verfaillie SCJ, Visser D, Weltings E, Groot C, van der Ende EL, Giannini LAA, Tuncel H, Timmers T, Boellaard R, Yaqub M, van Assema DME , Kuijper DA, Segbers M, Rozemuller AJM, Barkhof F, Windhorst AD, van der Flier WM, Pijnenburg YAL, Scheltens P, van Berckel BNM, van Swieten JC, Ossenkoppele R, Seelaar H. Neurology. 2021 Sep 7;97(10)
Restoration of rostral cerebrospinal fluid flow to solve treatment failure caused by obstruction in longterm intrathecal baclofen administration. Delhaas EM, Harhangi BS, van Doormaal PJ, Dinkelaar W, van Es ACGM, van Assema DME , Frankema SPG, van der Lugt A, Huygen FJPM. J Spinal Cord Med. 2021 Mar;44(2):312321.
Isotopic Scintigraphy in Intrathecal Drug Delivery Failure: A Single-Institution Case Series. Delhaas EM, van Assema DME , Fröberg AC, Zwezerijnen BGJC, Harhangi BS, Frankema SPG, Huygen FJPM, van der Lugt A. Neuromodulation. 2021 Oct;24(7):1190-1198.

Advisors Aad
Project Funding
TINY COX, BSC PREPARING FOR PET
van der Lugt, Frederik Verburg, Mark Konijnenberg & Marcel Segbers
Research period December 2020 – December 2024
Email c.cox@erasmusmc.nl
PET image quality is of key importance for optimal lesion detectability and interpretation of scans. PET image quality depends, among others, on the quality of the PET camera, the detector type (analogue/digital), the tracer biodistribution, use of medication affecting this biodistribution, the amount of administered tracer activity, the acquisition time used for scanning and the reconstruction parameters. Image quality is also influenced by patient dependent parameters such as size, due to a variable amount of attenuation within patients with different habitus. Furthermore, different acquisition and dosage protocols are necessary for various tracers/isotopes, due to differences in amongst others positron range and half-life time.
The administered activity to patients should be as low as reasonably achievable (the ALARA principle) to minimize possible detriment due to the use of ionizing radiation. However, the dosage should be sufficient to provide acceptable image quality for diagnosis and semi-quantitative analysis within a reasonable amount of acquisition time.
New tracers with their unique biodistribution as well as new technologies such as PET/MRI with digital detectors request expansion of knowledge to optimize PET image quality with adapted acquisition protocols and dose regimen.
The first part of this PhD project is to propose new dosage regimens for [68Ga]Ga-DOTA-TATE PET imaging in adults and 18F-FDG PET imaging in children. This year, the manuscript of the paediatric [18F]FDG study was published in EJNMMI research (Cox, C.P.W., van Assema, D.M.E., Verburg, F.A. et al. A dedicated paediatric [18F]FDG PET/CT dosage regimen. EJNMMI Res 11, 65 (2021)). We found that body weight has the strongest relation with [18F]FDG PET image quality in children and we proposed a nonlinear dosage regimen based on body mass that will provide a constant and clinical sufficient image quality. This dosage regimen will significantly reduce the effective dose compared to the current

PhD Student
Figure 1. Comparison between SNRnorm liver fits that corresponds with the proposed nonlinear dosage regimen (parameter d fixed to 0.46) and a quadratic adult dosage regimen (parameter d fixed to 1) for children (a), adults (b) and both groups (c). The dashed lines represent the 95% confidence intervals of the fits.
guidelines. Furthermore, we found that a dedicated paediatric dosage regimen is necessary, as a universal dosing regimen for paediatric and adult is not feasible (See figure 1).
In the next part of my PhD project we will assess if potential reduction of the administrated activity in [68Ga]Ga-DOTATATE PET/MRI and [18F]FDG PET/MRI is feasible while maintaining at least equal image quality compared to PET/CT imaging. The data analysis of the [68Ga]Ga-DOTA-TATE study is almost completed and the manuscript will be submitted in 2022.
Furthermore, we will investigate the effect of additional caffeine anhydrous pre-administration on myocardial [18F] FDG uptake suppression.

EARLY DETECTION AND UNDERSTANDING TREATMENT FAILURE IN BRAIN METASTASES
SOPHIE DERKS, MD, MSC
PhD Student
Advisors Marion Smits, Martin van den Bent & Astrid van der Veldt
Project Funding Daniel den Hoed Award 2018, EMC Fellowship 2018
Research period February 2020 – February 2024
Email s.derks@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine, Medical Oncology, and Neurology.
The introduction of new treatments, including targeted therapies and immune checkpoint inhibitors, has improved the prospects for patients with brain metastases in a subgroup of patients with melanoma and non-small cell lung cancer. Nevertheless, survival in most patients with brain metastases is still poor (historically, 4-5 months). Brain metastases are often associated with a poor prognosis. Therefore, there is an urgent need to further improve outcome, by improving diagnostic and therapeutic strategies in patients with brain metastases.
The two main aims of this PhD project are 1) improved detection of brain metastases and treatment failure, and 2) improved understanding of treatment failure in brain metastases.
In a first project, we collaborate with MR physicists Y. Wu and E. Warnert (see page 248) to assess a new MR technique called glucose Chemical Exchange Saturation Transfer (glucoCEST). GlucoCEST MRI detects the water signal that indirectly reflects the signal of the smaller pool of glucose molecules. In healthy volunteers, a small (~1%) but clear increase in glucoCEST contrast enhanced (GCE) signal was measured after i.v. bolus infusion of glucose. In the first patients, a signal increase was also detected in brain metastases (Figure 1). By studying more patients, and by comparing glucoCEST MRI to other MR sequences (e.g. perfusion) and to 2-Deoxy-2-[18F]fluoroglucose (18F-FDG)PET on the PET/MRI apparatus, we aim to better understand the glucoCEST signal. Future perspectives are to explore the possibilities of glucoCEST for clinical practice, e.g. in understanding treatment failure.
A second project is the assessment of a large, retrospective database of all patients with brain metastases who were treated at the Erasmus MC over the past years. Patient


Figure 1. Axial view of the T1 post-contrast image (A) and glucoCEST map (B) in a patient with a solitary brain metastasis (red arrow) of non-small cell lung carcinoma. The dynamic glucoCEST signal (C) was higher in the tumour rim (blue line) than in contralateral cerebellum (red line).
characteristics, diagnostic workup, treatment and follow-up are collected to identify prognostic factors and assess the effect of new treatments over the years. Currently, a cohort of 430 patients with melanoma brain metastases has been identified, and data of patients with non-small cell lung carcinoma will also be collected.
In addition, the impact of screening for asymptomatic brain metastases was assessed in resected stage III melanoma. Future projects will potentially also focus on radiomics for melanoma brain metastases and cost-effectiveness of screening.

Advisors Astrid
BIOMARKERS FOR RADIUM-223
ANOUK C DE JONG, MD
PhD Student
van der Veldt , Martijn Lolkema & Ronald de Wit
Project Funding Bayer, Running Stairs for Cancer (www.runningstairs.nl), DUOS
Research period January 2018 – January 2023
Email a.c.dejong@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Medical Oncology.
As traditional parameters, including PSA, fail in (early) response evaluation of metastatic castration resistant prostate cancer (mCRPC) patients, treated with radium-223, other biomarkers are needed to guide treatment planning. Additionally, there are studies suggesting that radium-223 induces an immune response, which suggests that combining radium-223 with immunotherapy may be an effective treatment strategy. To identify biomarkers for early identification of clinical benefit from radium-223 treatment and to better understand immune response during radium-223 treatment in mCRPC patients, the Radium223Insight study has been designed.
In this study, patients will receive radium-223 according to standard of care, while carefully being monitored by obtaining multi-parametric parameters from blood, imaging and tissue. 68Ga-PSMA-PET scans will be performed prior, during and after treatment with radium-223. Based on prior research, bone biopsies will be obtained from 68Ga-PSMA positive lesions in order to optimize the chance to detect tumor tissue.1 In addition, 89Zr-atezolizumab PET will be performed at baseline and after 12 weeks of treatment to evaluate the effect of radium-223 on immune response. Besides imaging and bone biposies, liquid biopsies and immune cells within blood will be collected.
Within the Radium223Insight, 30 patients will be included. As the inclusion is almost finished, we expect the first results of this study in 2022. Dynamics in uptake on 68Ga-PSMA PET/CT, including calculation of total tumor volume over time using Hermes Hybrid 3D software, will be correlated to clinical outcome (see figure 1).
1 De Jong AC et al. J Nucl Med 2020;61 (11):1607-1614. See also chapter Veldt & Brabander.

Figure 1. 68Ga-PSMA PET/CT as a potential biomarker for response to radium-223 in metastatic castration resistant prostate cancer patients. Using Hermes Hybrid 3D software, 68Ga-PSMA positive tumor lesions are selected and dynamics in total tumor volume over time are correlated to clinical outcome.

ACTINIUM-225-PSMA I&T IN PATIENTS WITH METASTATIC PROSTATE CANCER
SUI WAI LING, MD
PhD Student
Advisors Tessa Brabander, Astrid van der Veldt & Gabriel Krestin
Project Funding KWF Kankerbestrijding (www.kwf.nl)
Research period January 2020 – December 2023
Email s.ling@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Medical Oncology.
2021 was a challenging year due to the covid pandemic and its restrictions. However, our team has put in extra effort to progress with the phase I dose escalation study with actinium-225-PSMA I&T.
First achievement to be mentioned is that our production facility has been approved by the Dutch Health Authority for the production of actinium-225-PSMA I&T which is according to cGMP guidelines. Second achievement to be mentioned is that with the approved production license for actinium-225-PSMA I&T, the phase I dose escalation study with actinium-225-PSMA I&T has also been approved by the Medical Ethics Review Committee. The first patient is planned to be treated with actinium-225-PSMA I&T in March 2022.
Lastly, a brief summary of the rationale and design of this phase I, single-center, open label, repeated dose-escalation and expansion study in patients with metastatic castration-resistant prostate cancer (mCRPC), bearing PSMA expression.
PSMA is a type II transmembrane glycoprotein with a domain both intracellular and extracellular and is expressed
on benign prostate epithelium and on prostate cancer cells. The PSMA expression on prostate cancer cells is a thousand-fold higher than expression on normal tissues and therefore a target for both imaging and therapy of prostate cancer. Actinium-225 is an alpha emitting radionuclide with high linear energy transfer (LET) and limited range in tissue The high LET effectively kills tumor cells through deoxyribonucleic acid (DNA) double strand and DNA cluster breaks and the limited range allows selective tumor cell killing while sparing healthy tissue. Preclinical and preliminary clinical studies have shown that actinium-225-PSMA appears to be a promising radiopharmaceutical for therapy of mCRPC. However, there is still a lack of a phase I dose escalation study.
Within this study, all patients (up to 30 patients) with mCRPC will receive 2 doses of 225Ac-PSMA I&T with 8 weeks in between. The first dose-level will not exceed 8 megabecquerel (MBq). The dose-escalation will proceed following an accelerated 3+3 design which will allow to open the next dose-level (DL) cohort in the absence of dose limiting toxicity (DLT) while the previous one is still ongoing. Up to 4 treatment cohorts will be explored including up to 3 dose-escalation cohorts and a last one (expansion cohort) where patients will be dosed at the recommended dose. All patients will be evaluated for safety.

Figure 1. Design of the phase I actinium-225-PSMA I&T study to evaluate tolerability and safety of 225Ac-PSMA I&T in patients with metastatic prostate cancer. DL = dose level, MBq = megabecquerel

CLINICAL
OUTCOMES OF PEPTIDE RECEPTOR RADIONUCLIDE THERAPY
NOÉMIE S MINCZELES, MD
PhD Student
Advisors Tessa Brabander , Hans Hofland & Wouter de Herder
Project Funding
Research period April 2019 – March 2022
Email n.minczeles@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Endocrinology.
Peptide receptor radionuclide therapy (PRRT) is a theranostic that uses somatostatin receptors (SSTR) as target by labeling somatostatin analogues with radioactive peptides. The NETTER-1 trial and the phase 2 trial conducted in Erasmus MC resulted in the approval of EMA and FDA for progressive, advanced gastroenteropancreatic (and foregut in USA) NETs with 177Lu-DOTATATE. Our research will further explore the clinical use and long-term outcomes of PRRT.
We assessed the efficacy of submaximal activities of PRRT in patients who discontinued for disease-unrelated reasons. In total 243 NET patients received 3.7-27.8 GBq. In 130 patients the submaximal activity was diseaseunrelated (e.g. bone marrow and renal toxicity in 48% and maximal renal absorbed dose in 23%) and they were compared to 350 NET patients who received 29.6 GBq. The disease control rate in the reduced activity group was 85% compared to 93% for the control group (p=0.011). The median PFS (95% CI) was 23 (21-26) months for the reduced activity group and 31 (27-35) months for the control group (p=0.001), and the median OS was 34 (28-40) months and 60 (53-67) months, respectively (p<0.0001). With adjustment for relevant confounders in the multivariable Cox regression, cumulative activity was an independent predictor of PFS and OS.
PRRT can cause dose-limiting toxicities of the bone marrow, liver, and kidneys. We analyzed whether women and men are equally at risk for these DLTs. Women more frequently developed grade ≥2 thrombocytopenia compared to men (25% vs. 18%, p=0.004), due to a significant increase in grade ≥3 thrombocytopenia (11% vs. 6%, p=0.008). Furthermore, the incidence of grade ≥3 anaemia was higher in women (7% vs. 3%, p=0.002). In the multivariable regression model, female sex (OR=2.50,

Figure 1. Kaplan Meier curve of the progression-free survival, measured from the first cycle of PRRT.
95% CI=1.67-3.74) was confirmed to be an independent risk factor for grade ≥2 thrombocytopenia.
Other topics of interest are the evaluations of the efficacy of PRRT in other SSTR positive tumors. Approximately 25 patients with a iodine-refractory differentiated thyroid carcinoma were treated with 177Lu-DOTATATE. Half of the tumor subtypes consisted of Hürthle cell carcinomas. Patients had progressive disease at baseline and the majority had distant metastases. Our preliminary results show a median PFS of 10 months and stable disease as best response in half of the patients. Fifteen patients with progressive meningiomas were treated with PRRT, resulting in stable disease in 40% of the patients, median PFS of 8 months and median overall survival (OS) of 13 months. These could be relevant findings, because for these patient groups only limited treatment options are now available.
Yann Seimbille earned his PhD degree in Radiopharmaceutical Sciences at the University of Sherbrooke in the labs of Profs. Johan van Lier and François Bénard. After a postdoctoral fellowship at the University of California Los Angeles (UCLA) in the labs of Profs. Daniel Silverman and Johannes Czernin, he was appointed as Assistant Professor in the department of Molecular & Medical Pharmacology. Then, he joined the division of Nuclear Medicine & Molecular

Imaging at the University of Geneva and worked two years at Canada’s particle accelerator centre (TRIUMF) in Vancouver before joining Erasmus MC in July 2017. His scientific interests are in radiopharmaceutical sciences, theranostic, multimodality imaging, peptide chemistry, chemical biology and bioorganic chemistry. y.seimbille@erasmusmc.nl
RADIOPHARMACEUTICAL CHEMISTRY
YANN SEIMBILLE, PHD associate
professor

Top Publications 2021
Koustoulidou S, M Hoorens, S Dalm, S Mahajan, R Debets, Y Seimbille and M. de Jong. Cancer-associated fibroblasts as player in cancer development and progression and their role in targeted radionuclide imaging and therapy. Cancers 13(5):1100 (2021)
Handula M, K-T Chen and Y Seimbille. IEDDA: an attractive bioorthogonal reaction for biomedical applications. Molecules 26(15):4640 (2021)
Qiu L, K Li, W Dong, Y Seimbille, Q Liu, F Gao and J Lin. Tumor microenvironment responsive “head-to-foot” self-assembly nanoplatform for positron emission tomography imaging in living subjects. ACS Nano 15(11):18250-18259 (2021)
Hooijman E, Y Chalashkan, S Ling, F Kahyargil, M Segbers, F Bruchertseifer, A Morgenstern, Y Seimbille, S Koolen, T Brabander and E de Blois. Development of [225Ac]Ac-PSMA-I&T for targeted alpha therapy according to GMP guidelines for treatment of mCRPC. Pharmaceutics 13(5):715 (2021)
Context
The RadioPharmaceutical Chemistry group is currently working on the development of radiopharmaceuticals for targeted diagnosis and treatment of cancer. Tandem combination of a biovector with a gamma emitting radionuclide allows noninvasive identification of the molecular aberrations of cancer cells, while labeling of the biovector with a beta- or an alpha-emitter transform it into a therapeutic agent. It corresponds to the so-called “theranostic” paradigm. Our research program is a molecular-imaging-based program focused on theranostics and multimodality imaging probes, with an emphasis on developing these novel radiopharmaceuticals for clinical translation (Fig. 1). A brief summary of our current research activities is presented herein.
Research Projects: Objectives & Achievements
Pretargeting vs. direct targeting
A wide range of peptide-based radiopharmaceuticals has been successfully applied to the treatment of prostate cancer (i.e. [177Lu]Lu-NeoB) and neuroendocrine tumors (i.e. [177Lu]Lu-DOTA-TATE). However, a significant limitation of these radiolabeled peptides is their concomitant accumulation in non-targeted tissues, leading to high radiation doses to healthy organs. For instance, [ 177Lu] Lu-NeoB is known to accumulate in the GRPR-expressing pancreas hampering administration of an optimal dose. We are therefore aiming at applying a pretargeting strategy based on click-chemistry to overcome radioactivity accumulation in healthy tissues. Tumor and non-targeted organs have usually different clearance pattern. Thus, in vivo radiolabeling at an optimal time point of a preinjected peptide will warrant higher radioactivity uptake in tumor lesions while minimizing accumulation in healthy organs
Alpha or Auger?
Recent clinical studies have shown that the therapeutic outcome of peptide receptor radionuclide therapy (PRRT) can be significantly improved by replacing the conventional β - emitter (90Y, 177Lu) with an alpha emitting radionuclide (212Pb , 225Ac) or an Auger emitter ( 195mPt) Within this project, somatostatin receptor subtype 2 agonists are labeled with alpha and Auger emitters, while antagonists are solely labeled with alpha emitting radionuclides. Biological studies will determine which of these approaches result in the greatest tumoricidal effect.
Theranostics and multimodality imaging probes
Peptides are extremely attractive vectors for radiopharmaceuticals due to their fast pharmacokinetics, high specificity and affinity for cellular targets. We recently developed a novel bioorthogonal approach to radiolabel peptides and applied this chemical strategy to the synthesis of theranostics targeting the somatostatin receptor subtype 2 (SSTR2), the gastrin-releasing peptide receptor (GRPR), the prostate specific membrane antigen (PSMA), the carbonic anhydrase IX (CAIX), and the fibroblast activation protein alpha (FAP a ). We will soon employ a similar method to design radiopharmaceuticals for the human epidermal growth factor receptor 2 (HER2) and the chemokine receptor type 4 (CXCR4). Addition of a fluorescent dye to these targeting vectors provides
dual-modality imaging probes for preoperative surgical planning and intraoperative surgical guidance, whereas conjugation of a potent antineoplastic drugs yields small-molecule drug conjugates (SMDC) for targeted chemotherapy Preclinical evaluations of our theranostics are underway to identify which lead candidate could potentially be translated to the clinic.
Expectations & Directions
It is expected that the use of radiopharmaceuticals for diagnosis and therapy will significantly rise in the near future due to the pivotal role of radiopharmaceuticals in personalized medicine and drug development . Moreover, radionuclides and radiopharmaceuticals answering unmet medical needs are becoming more easily accessible. Research-wise the demand for new radiopharmaceuticals is increasing to enhance our knowledge of human disease biology and pathophysiology, and our ability to diagnose and treat diseases. This enthusiasm for radiopharmaceuticals fosters our group to develop new theranostics and imaging probes which can have an impact on cancer patient management.
Funding
Cécile Perrio (University of Caen) and Yann Seimbille : Joint PhD Program – Grant 2018-2022: “ Bioorthogonal radioactive probes for in vivo imaging and therapy of tumors ”
Yann Seimbille , Julie Nonnekens and Marion de Jong Dutch Cancer Foundation Grant: 2019-2022. “Longacting sstr2 antagonists and pretargeted alpha therapy: a blockbuster combination for a safer and more efficient treatment of neuroendocrine tumors”
Yann Seimbille , Mark Konijnenberg and Marion de Jong Kansen voor West: 2019-2023. “FIELD-LAB: Advancing Nuclear Medicine”
Yann Seimbille and Marion de Jong . TU Delft/Erasmus MC convergence kick-off grants: 2020-2022. “Broad spectrum, high precision theranostic cancer therapy”
Yann Seimbille , Marion de Jong , Carolien van Deurzen and Agnes Jager. Erasmus MC grants: 2020-2024. “Theranostics hitting the Achilles’ heels of breast cancer: pointing the arrows at HER2 and GRPR”
Antonia Denkova and Sofia Koustoulidou . Convergence Open Mind grant: 2021. “Advancing cancer treatment with CERN technology”

1. Illustration of the research program of the RadioPharmaceutical Chemistry group.
Highlights
Maryana Handula obtained the “Best Poster Award” for her presentation at the European Molecular Imaging Meeting (EMIM 2021). Maryana’s contribution to the work presented by Natasa Gaspar at the World Molecular Imaging Conference (WMIC 2021) was also acknowledge by the “Best Poster Award”.
Our first studies with the alpha emitter Pb-212, provided by NRG (FIELD-LAB project), have been conducted in 2021.
We were granted last November a permit by IGJ to produce Ac-225 PSMA for a clinical Phase I study. Erasmus MC is the first site in the Netherlands to produce GMP grade radiopharmaceuticals labeled with actinium-225.
In 2021, Yann Seimbille became Associate Professor.
Yann Seimbille is a member of the editorial board of the European Journal of Nuclear Medicine and Molecular Imaging Radiopharmacy and Chemistry.
Figure
Additional Personnel
Marc Stroet, MSc
I am a PhD student specializing in the preparation and evaluation of novel radiotracers, for which part of my research is performed in the RadioPharmaceutical Chemistry group. I am a chemist by training, as I obtained a Bachelor in Organic Chemistry and subsequently specialized with a Master in Medicinal Chemistry. During my studies, I did multiple internships in radiochemistry, which set the base for my PhD. Here, I develop a SPECT tracer for necrosis imaging. This interdisciplinary project involves practically all aspects of radiotracer development, ranging from organic synthesis to in vivo imaging. Further detail of my work can be found in the section dedicated to Optical Molecular Imaging.

Sabine Nauta, Intern
I am a Bio-Pharmaceutical Sciences master student at Leiden University. During the first year of my master, I performed a 9-month internship at the Division of Drug Discovery and Safety at the Leiden Academic Centre for Drug Research (LACDR) on the role of the CCR2/ CCL2 axis in breast cancer. In 2021, I did a 5-month internship in the RadioPharmaceutical Chemistry group at the department of Radiology and Nuclear Medicine of Erasmus MC, supervised by Yann Seimbille and Mark Hoorens. In my project, I develop a small library of LY2510924 derivatives, a peptide known to target the chemokine CXC receptor 4 (CXCR4). A chelator was attached to the peptides via a CBT-Cys click reaction. The reference peptide was labeled with indium-111 and the newly synthesized peptides were tested for their affinity in radioligand binding assay.
Evelien Spaan, Intern
I am a BioPharmaceutical Sciences master student at the division of Drug Delivery Technology at Leiden University and currently specializing in BioTherapeutics. During the first year of my master, I performed a 9-month internship based on the organic synthesis of polypept(o)ides. I am currently working on a 5-month internship focused on solid phase peptide synthesis, at the department of Radiology and Nuclear Medicine of Erasmus MC, supervised by Yann Seimbille and Erika Murce. In my project, I develop prostate-specific membrane antigen (PSMA) based small-molecule drug conjugates for prostate cancer theranostics. This approach combines tumor imaging by PET with tumor treatment via targeted chemotherapy and radionuclide therapy.
Savanne Beekman, Intern
I am a Biology and Medical Laboratory Research student at the Avans Hogenschool. I am performing a 9-month internship at Erasmus MC in the department of Radiology and Nuclear Medicine under the supervision of Yann Seimbille. During my internship, I am responsible for the biological validation of newly synthesized SSTR2-targeting radiotracers for the therapy of neuroendocrine tumours (NETs). In this project, a pretargeting strategy is utilized to ensure the efficient delivery of such tracers without affecting healthy tissues.


Carolline Ntihabose, Intern
I am a Drug Discovery and Safety master student at the Vrije Universiteit Amsterdam (VU Amsterdam), with a specialization in Molecular Pharmacology. Currently, I am pursuing my major internship for 9 months at the department of Radiology and Nuclear Medicine and the department of Pharmacology at Erasmus MC under the supervision of Dr. Erik de Blois and Dr. Stijn Koolen. The focus of my internship is the clinical implementation of 177LuPSMA-I&T for metastatic castration-resistant prostate cancer patients. This requires validation techniques such as HPLC and ITLC to ensure GMP quality.

Jordy van de Merbel, Intern
I am a Chemical and Physical secondary vocational student at the Curio Techniek en Technologie Breda, with a specialization in chemical-physical analyst. Currently, I am performing a 10-month internship at the department of Radiology and Nuclear Medicine of the Erasmus MC under the supervision of Dr Erik de Blois. During my internship, my project was the development and validation of the HPLC method of Lu-177 labelled PSMA-I&T. Final goal is that this method can be used for quality control in daily practice to be able to release the product for patient treatment.

Sophie van den Berg, Intern
I am a Chemical-Physical Analyst student at the Techniek College Rotterdam. In 20202021, I am performing a 9 months internship at Erasmus MC in the department of Radiology and Nuclear Medicine under the supervision of Dr. Erik de Blois. For my internship I developed UPLC methods for the new compounds and peptides, which are synthesized by the other members of the group. With these methods the content of the compounds was determined as well as kinetic studies were performed to optimize radiolabeling conditions. Analyzed compounds will be used for further In vitro and In vivo studies.

CLINICAL RADIOPHAMACEUTICAL CHEMISTRY
ERIK DE BLOIS, PHD
Clinical Radiochemist
APPOINTMENT IN NUCLEAR MEDICINE

Erik de Blois (1981, Zeist) Curriculum vitae. In June 2005, he received his Bachelor of Science in molecular biology from the University of applied Sciences in Utrecht/ NL. He started a Master in Pharmaceutical Sciences “Drug Innovation” in 2006 and graduated in June 2009.
Erik started to work at the department of Nuclear Medicine in 2002 as a research technician in the radiochemistry group headed by Dr. W.A.P. Breeman. He worked on development of new peptides to visualize tumors using scintigraphy and to treat tumors. The results of these investigations lead to the development of the first registered therapeutic product Lutathera ([177Lu]Lu-DOTA-TATE). In November 2014 he successfully defended his thesis entitled “radiochemical aspects of receptor scintigraphy”. His main focus is the clinical implementation of various non-registered radiopharmaceuticals for Peptide Receptor Radionuclide Scintigraphy and therapy (PRS and PRRT) as Targeted alpha therapy (TAT)
r.deblois@erasmusmc.nl
The clinical radiochemistry group plays an important role in clinical support and implementation, as well as research support of new radiopharmaceuticals and is for now under the umbrella of the radiopharmaceutical chemistry group. An overview of various activities within our focal areas is given below.
In the last two years an alpha lab was realized in our institute in cleanroom environment. Because of health physics regulations, specific equipment needed to be purchased and installed, calibrated and validated. First we are started on the implementation of Ac-225 labelled radiopharmaceuticals. Since alpha particles are very difficult to detect, detection is focused on the first daughter of Ac-225, Fr-221. Fr-221 has a gamma of 218 KeV and has a half-life of only 4.8 min and an equilibrium is reached in ~30 min. To be able to measure the quality of the Ac-225 labelled pharmaceuticals, indirect measurements have to be performed. This is because recoil occurred after first alpha decay, as a result first daughter already leaving its molecule. Also, Ac-225 is ~1000x more toxic then the more commonly used therapeutic nuclide like Lu-177. This results in a much smaller patient dose (8-12 MBq). Additionally, on top of the difficulties to detect also the low amount makes it even more challenging to detect and validate related equipment.
In two years we managed to implement the lab and related equipment and achieved an official license to produce
Ac-225 labelled PSMA fully GMP. With that we are the first institute in Europa and maybe even worldwide who has the GMP license to produce Ac-225 PSMA as a radiopharmaceutical
Main Research Topics
Clinical implementation of [225Ac]Ac-PSMA
Therapy with PSMA labeled with actinium-225 can be very effective in patients with metastatic castration resistant PCa (mCRPC). The use of alpha emitting radionuclides will result in more DNA double strand breaks than treatment with beta emitting radionuclides. A limited number of patients have been treated with [225Ac]Ac-PSMA therapy in Germany, and there is still a lack of a good clinical trial. Therefore, a phase I study is necessary to calculate the recommended dose that is both safe and effective. With the treatment of up to 30 patients, we expect to determine the most appropriate cumulative dose for [225Ac]Ac-PSMA treatment. If the recommended dose can be established, new research can be set up to investigate the effects of this treatment on survival and quality of life
As mentioned earlier detection of Ac-225 is performed by indirect measurement and is based on Fr-221. Therefore this has the consequence for quality control like HPLC. Eluate have to be collected in factions by a fraction collector


Figure 1. Up: Glovebox (Class A), for all open handing of Ac-225. Down: Downflow cabinet with the specific microwave to perform radiolabeling and fumehood to perform quality controls.
and measured in a gammacounter. With this method we are still able to determine radiochemical purity of the Ac-225 labelled radiopharmaceuticals.
Full GMP test runs were successfully performed and first patient treatment is planned Feb 2022.
Clinical implementation of [177Lu]Lu-PSMA
Therapy with [177Lu]Lu-PSMA is proven to be very effective in patients with metastatic castration resistant PCa (Phase III, vision trail). Hopefully soon [177Lu]Lu-PSMA will be available worldwide, till then we will perform in house synthesis of [177Lu]Lu-PSMA to make treatment available for Erasmus MC patients. Labelling will be performed manually and scaled up from 2 patient dose per labelling up to 4 patient dose per labelling to finally be able to treat 8 patients a week.
Before implementation into cleanroom environment, radiolabeling and stability of the Lu-PSMA was optimized.
Reaction conditions and stability was optimized in downscaled form in research labs. Final mixture was formulated and was finally full GMP prepared by Erasmus MC pharmacy (A15). For clinical implementation, related equipment,
methods and quality controls were calibrated and validated. 3 test runs were performed in the cleanroom and showed that product was according all release criteria and was table for 4h. Because of long term experience with PRRT for neuro endocrine tumors infrastructure is already there for more than 15 years, therefore first patient treatment will be treated in Jan 2022.
Invited Lectures
IAEA course: Production and Quality control of theranostic radiopharmaceuticals using alpha and Beta Emitters: Implementation of Ac-225. Online meeting (September 2021)
BelNuc Seminar: Alpha-emitting radionuclides: radio-pharmacy aspects. Brussels/Belgium (September 2021).
Indian Chemical society, 58th annual Convention of chemist and international conference on “recent trends in chemical Sciences: ”Implementation of alpha-emmiting radionuclides: radiopharmacy aspects and measurement of radiochemical purity, Implementation of Ac-225. (December 2021)
Additional personnel
Eline Hooijman (PhD student), Carolline Ntihabose (Master student), Sophie van den Berg, Jordy van der Merbel (MLO-students).
Publications
Ruigrok EAM, van Vliet N, Dalm SU, de Blois E , van Gent DC, Haeck J, de Ridder C, Stuurman D, Konijnenberg MW, van Weerden WM, de Jong M, Nonnekens J. Extensive preclinical evaluation of lutetium-177-labeled PSMA-specific tracers for prostate cancer radionuclide therapy Eur J Nucl Med Mol Imaging. 2021 May.
Hooijman EL, Chalashkan Y, Ling SW, Kahyargil FF, Segbers M, Bruchertseifer F, Morgenstern A, Seimbille Y, Koolen SLW, Brabander T, de Blois E. Development of [225Ac]AcPSMA-I&T for Targeted Alpha Therapy According to GMP Guidelines for Treatment of mCRPC. Pharmaceutics. 2021 May 13.
Bakker IL, Fröberg AC, Busstra MB, Verzijlbergen JF, Konijnenberg M, van Leenders GJLH, Schoots IG, de Blois E , van Weerden WM, Dalm SU, Maina T, Nock BA, de Jong M. GRPr Antagonist 68 Ga-SB3 PET/CT Imaging of Primary Prostate Cancer in Therapy-Naïve Patients Contrast Media Mol Imaging. 2021 Dec 13.

TARGETING CANCER STROMA
MARK W H HOORENS, PHD
Post-doc
Project Funding Convergence Health and Technology
Research period June 2020 – May 2022
Email m.hoorens@erasmusmc.nl
The environment of a tumor has a large effect on the progression of the disease and outcome of therapeutic interventions. One of the main components of the tumor microenvironment are cancer-associated fibroblasts (CAFs). These CAFs support tumor growth, block the immune response and decrease effectiveness of chemotherapeutics. A specific marker for CAFs is the fibroblast activation protein (FAP), a membrane bound protease of which the expression is nearly exclusively detected in CAFs. Therefore, FAP has been recognized as a promising therapeutic target for a wide variety of cancers.
The specific expression of FAP in the tumor microenvironment and recent development of selective and potent inhibitors has resulted in the development of FAP tracers, such as FAPI-04 and FAPI-46. These tracers are increasingly recognized as promising diagnostic tracers: the tracers reach the tumor quickly, give good contrast between tumor and background, and rapidly excreted.
We aim to develop FAP tracers for both diagnostic and therapeutic purposes. This means that we need a tracer with long retention in the tumor. Then, FAP tracers can be labeled with short-lived radionuclides for imaging or with long half-life radionuclides for therapy.
However, developing FAP tracers with long tumor retention is a difficult challenge to tackle. Actually, despite the increasing interest in FAP tracers over the past few years, no predictors have been identified that can correlate the chemical properties of the tracer to tumor retention in vivo
In this project, we design, synthesize and evaluate new FAP tracers. Inspiration is taken from known and newly reported small molecule inhibitors for FAP in which we seek new chemical features for FAP tracers. The project is performed in close collaboration with the group of Dr. Simone Dalm,
in particular with PhD student Circe van der Heide, who performs biological studies in cells and studies in mice for the most potent new tracers.
Due to the specific FAP expression on CAFs and their occurrence in a wide variety of cancers, there is high anticipation in the field of nuclear medicine to use FAP tracers for both diagnosis and therapy. The development of our new FAP tracers can form the starting point for many follow-up studies. In case a FAP tracer with long tumor retention is developed, that will pave the way towards exploring its therapeutic potential. Either way, any new FAP tracer – regardless of their tumor retention – can be used to study the interaction between the protein and the tracers in order to gain deeper understanding of the physiology of FAP and to know how to develop the tools physicians need for their patients.

Figure 1. The tumor microenvironment consists of more than cancer cells. From elsewhere in the body fibroblasts are recruited and co-evolve with the tumor by supporting its growth and protecting it from the immune system. A specific marker for these cancer-associated fibroblasts (CAFs) is the fibroblast activation protein (FAP), a transmembrane enzyme that is involved in tissue remodeling.

Project Funding KWF
LONG-ACTING SSTR2 ANTAGONISTS AND PRETARGETED ALPHA-THERAPY OF NETS
SOFIA KOUSTOULIDOU, PHD
Research period January 2020 – February 2022
Email s.koustoulidou@erasmusmc.nl
Neuroendocrine tumours (NETs) are a heterogeneous and rare group of tumours that originate from cells of the neuroendocrine system. The increasing incidence over the last few decades has raised global attention. NETs are often hard to diagnose, and systemic treatments are therefore necessary since most patients have an already advanced and metastasized disease at the time of diagnosis. Most NETs overexpress the somatostatin receptor type 2 (SSTR2) and various somatostatin analogues (mainly agonists) have been developed for therapy. Despite encouraging clinical results, the overall response rates are still insufficient, implying the need for new approaches. Several reports suggest that somatostatin antagonists can target SSTR2-positive tumours better than conventional agonists in a pre-clinical and clinical setting. By delivering a higher dose to the tumour, the utilization of somatostatin antagonists could prove advantageous.
Somatostatin analogues are usually labelled with a betaemitting radionuclide, such as lutetium-177 (177Lu) or yttrium-90 (90Y). This, however, might be responsible for

Figure 1. Schematic representation of the project aim. The pretargeting strategy will be utilized to avoid accumulation of radionuclide in healthy organs, thus allowing safe and efficient therapy of NETs.
the inadequate response to therapy as the large penetration range of beta-emitters can cause various side effects in healthy organs. For this project, we are planning to use alpha-emitting radionuclides instead (e.g., Ac-225) due to their shorter path length and higher energy transfer. In addition, a pretargeting strategy will be utilized to prevent radiotoxicity of such radiopharmaceuticals in healthy organs (e.g., kidneys). In such an approach, the drug detaches from the radioactivity at injection. In particular, the pharmaceutical, that has been designed to bind both the target antigen and also the radiolabelled tag, is injected first. It is then given time to accumulate at the tumour site but also to clear out from non-targeting tissues. Next, the radiolabeled tag is injected and will either bind to the pharmaceutical at the site of the tumour or will be cleared from the body due to its small size. Taken together, the aim of this project is to combine all the aforementioned strategies in order to optimize SSTR2-targeting radiopharmaceutical for a safe and effective treatment of NETs.

Figure 2. Pretargeting approach in H69 tumour sections. Click reaction between [111In]In-DOTA-PEG11-Tz and the TCO-modified analogue, compound 8, as shown by representative images from ex vivo autoradiography (A) and quantitative analysis expressed as DLU/mm2 (B). JR11 reference analogue was used as a control.

MOLECULAR IMAGING AGENTS TARGETING BRADYKININ B1 RECEPTOR
HANYUE MA, MSC, PHD
Post-doc
Project Funding Convergence Health
and Technology
Research period April 2021 – April 2024
Email h.ma@erasmusmc.nl
Bradykinin (BK: Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg)related peptides are involved in pain regulation and hyperalgesia after tissue injury and inflammation. They stimulate two main transmembrane receptors, B1R and B2R, which belong to the G protein-coupled receptor (GPCR) superfamily. B2R is generally absent throughout the body and involved in vasodilation and inflammatory responses. B1R overexpression is dominated in unhealthy tissues and mediates chronic inflammation, pain, hypotension, trauma, and proliferation of cancer. Therefore, B1R is an attractive cancer imaging biomarker, and there is interest in developing highly potent B1R antagonists.
We focused our attention to design and synthesis a library of peptide derivatives based on the published potent B1R antagonist B9958 (Lys-Lys-Arg-Pro-Hyp-Gly-CpG-Ser-DTic-CpG). The modifications and amino acid substitutions of these sequences are aimed at conferring specificity, increasing stability, and prolonging in vivo pharmacological effects. In addition, the peptides are designed with ability to carry fluorophores and radioactive groups at the N-terminal and act as molecular imaging probes.
Recent studies have shown that techniques used for high-throughput screening (HTS) of GPCRs moved away from traditional radioligand binding techniques toward chemiluminescence and fluorescence. Moreover, fluorescence polarization (FP), using fluorescent ligands, has been reported with significant advances in HTS because of development in dye chemistries, the introduction of sensitive FP plate readers, and the capability of accurate measurements in 384-well plates. We are also interested in designing and developing an FP technique based assay suitable for HTS using in peptide library screening. After the first screening, the top list peptides will be labelled with radionuclides via a DOTA-chelator. The results of radioactivity binding assay and preliminary in vivo studies will guide the optimisation of the next generation of peptide library design.

Figure 1. Structure of B9958 coupled with chelator/fluorescent dye.
Figure 2. Development of novel BK-related peptides for imaging B1R expression.

Advisors
RADIOLABELING OF BIOLOGICS FOR IN VIVO IMAGING AND CANCER THERAPY
JASON
BEAUFREZ, MSC
PhD Student
Cécile Perrio & Yann Seimbille
Project Funding International Joint PhD Fellowship (University of Caen Normandy/Erasmus MC)
Research period September 2018 – February 2022
Email j.beaufrez@erasmusmc.nl
One of the promising strategies in the fight against cancer is targeted radionuclide therapy (TRT). This strategy is based on a vector having high affinity and specificity for the target of interest. Proteins and antibodies are popular vectors, but their exploitation remains limited due to their slow pharmacokinetics often incompatible with short half-life radionuclides resulting in unfavorable dosimetry and increased toxicity to healthy organs.
A popular strategy, to get around the slow pharmacokinetics of large vector, is to first administer a previously functionalized biological vector followed by the injection of the radioisotope. It gives enough time to the biological vector to distribute throughout the body and bind to its target. The radioisotope can then be injected to click quickly to the biological vector avoiding prolonged exposure to ionizing radiation. This strategy is based on an extremely rapid and efficient bioorthogonal reaction, the inverse electron-demand Diels-Alder addition (IEDDA) between a tetrazine (Tz) and a trans -cyclooctene (TCO).
Over the past decade, numerous studies have reported the success of this approach. We therefore aimed at applying this pretargeting strategy to the gastrin-releasing peptide receptors (GRPR). GRPR is a widely targeted receptor in medical imaging and in theragnostic applications, due to its high frequency of expression in human cancers (prostate cancer and mammary carcinoma) and its lack of expression in surrounding healthy tissue. NeoBOMB1 is one of the best GRPR antagonists, with a very good affinity for GRPR. NEOBOMB1 has already been evaluated in (pre)clinical studies at Erasmus MC. Our team then functionalized NeoBOMB1 with a TCO group for pretargeting applications.

One of the major criteria to warrant success of this approach is the accessibility of the TCO click functionality after the formation of the GRPR-NeoBOMB1 complex. To improve the accessibility of the TCO, we introduced different spacer between the trans-cyclooctene and the amino acid sequence responsible for the binding to GRPR. Thus, we selected different length of PEG linkers between the TCO and the heptamer peptide NeoBOMB1. In addition, PEG increases the possible number of hydrogen bonds favorable to an increase in the hydrophilicity of NeoBOMB1. Compounds functionalized with different length of PEGn (n = 4, 8, 12, 24 and 36) were synthesized and tested. Binding affinity results are encouraging and preliminary pretargeting in vitro assays in GRPR+ cell membranes indicated that increasing the size of the PEG chain to PEG 12 improved the efficiency of the IEDDA reaction compared to PEG4 and PEG 8 Further in vitro and in vivo evaluations are required to confirm the potential of these NeoBOMB1 analogs for pretargeted theragnostic applications.
Figure 1. Size linker effects on NeoBOMB1 pre-targeting strategy.

IMPROVEMENT OF PRRT OF NETS BY TARGETED ALPHA/AUGER THERAPY
DYLAN CHAPEAU, MSC
PhD Student
Advisors Frederik Verburg, Mark Konijnenberg & Yann Seimbille
Project Funding Kansen voor West
Research period October 2019 – December 2023
Email d.chapeau@erasmusmc.nl
Neuroendocrine tumors (NETs) are a rare type of tumor. They usually share a common feature which is the overexpression of the somatostatin receptor subtype 2 (sstr2). Peptide receptor radionuclide therapy (PRRT) pairs a peptide-based ligand (e.g., octreotate) with a radionuclide by mean of a chelator. Once the radiopeptide is injected, it binds to the targeted receptors allowing the visualization of the tumors and cancer cells killing. This concept has been successfully applied in patients but the overall response rates after treatment are still insufficient.
To increase lethal effect to cancer cells, we are planning to exchange the conventional β-emitting radionuclide (177Lu) with a highly cytotoxic alpha emitter (212Pb) or Auger emitter (195mPt). Alpha and Auger emitters have a higher linear

energy transfer (LET) and a shorter range than beta particles. It is generally considered that the radionuclide should be at close proximity to the nucleus during decay for Auger particles. Thus, compounds based on the sstr2-agonist octreotate will be preferable labeled with Auger emitters, since this ligand is known to be internalized within cancer cell upon binding to sstr2. However, alpha particles have a range of action which is long enough to impact DNA from the cell surface. Therefore, they can typically be used in conjunction with sstr2-agonists or antagonists (Fig. 1).
After synthetizing our first 4 compounds, they have been labeled with lead-203, an imaging surrogate of the alpha emitter lead-212. Imaging studies in sstr2-positive xenografts were performed with [203Pb]Pb-DO3AM-Amcha-TATE and [203Pb]Pb-DO3AM-Pip-TATE (Fig. 2). Next, our compounds were successfully labeled with lead-212. A second generation, including four sstr2-antagonists, has been synthetized and some preliminary labeling tests have been performed. Meanwhile, few conjugates containing a carboplatin moiety have been prepared, as non-radioactive analogs of the sstr2-agonists that will be labeled with Auger emitter 195mPt.

Figure 1. Mechanism of action of sstr2-mediated PRRT.
Figure 2. microPET/CT imaging of a H69 tumor bearing mice after injection of [203Pb]Pb-DO3AM-Amcha-TATE.

OPTIMISING THE THERAPY OF NETS BY EXTENDING THE BLOOD CIRCULATION OF SSTR2 ANTAGONISTS
MARYANA HANDULA, MSC
PhD Student
Advisors Antonia Denkova, Frederik Verburg & Yann Seimbille
Project Funding KWF
Research period
October 2018 – December 2022
Email m.handula@erasmusmc.nl
The somatostatin receptor subtype 2 (SSTR2) is highly expressed by the neuroendocrine tumors (NETs). Therefore, it has been identified as an ideal target for imaging and therapy. SSTR2 has been a target of choice for targeted radionuclide therapy (TRT) of NETs using agonists (e.g. DOTATATE) or antagonists (e.g. JR11). JR11 showed promising results in several preclinical and clinical studies. However, it has been reported that the peptide is rapidly cleared from the blood circulation, thus compromising optimal therapy of NETs. Our aim was therefore to optimize the pharmacokinetic profile of JR11 to improve its therapeutic efficacy.
For this study, we successfully synthesized and characterized two JR11 analogs carrying an albumin binder, 4-( p -iodophenyl) butanoic acid and 4-( p -methoxyphenyl) butanoic acid, for compound 1 and 2 , respectively. The newly synthesized peptides were successfully radiolabeled with lutetium-177, proved to be hydrophilic and to possess excellent stability for 24 hours in phosphate buffered saline (PBS) and mouse serum at 37 °C. Compound 1 showed higher binding affinity to human albumin in comparison to compound 2
The binding affinity of compounds 1 and 2 towards SSTR2, was 20 to 40-fold lower than the binding affinity of the parent peptide JR11. In vitro studies in a SSTR2-positive cell line showed that both peptides had high cell uptake and conserved their antagonistic properties despite the chemical modifications accomplished.
In vivo SPECT/CT imaging studies of both radioligands and standard JR11 were carried out at 4, 24, 48 and 72 h post-injection of the tracer. High tumor uptake was observed for [177Lu]Lu- 1 and [177Lu]Lu-JR11. However, no significant tumor uptake could be observed for [177Lu]Lu- 2 (Fig. 1). Ex vivo biodistribution studies were performed at the same time points to confirm our previous imaging

Figure 1. SPECT/CT images of [177Lu]Lu-1, [177Lu]Lu-2 and [177Lu] Lu-JR11. Tumors are indicated with a white arrow.
findings. Nevertheless, the results demonstrated that the tumor uptake observed on the SPECT images for [177Lu] Lu- 1 was not completely specific and could be due to the residual activity still present in the blood pool.
We are currently investigating the synthesis of new antagonists that can present a better binding affinity towards SSTR2, as potential candidates for improved therapy of NETs.

CLINICAL IMPLEMENTATION OF RADIOLABELLED THERAPEUTICS
ELINE HOOIJMAN, MSC
PhD Student
Advisors Erik de Blois, Stijn Koolen, Frederik Verburg & Hugo van der Kuy
Project Funding Departments of Radiology and Nuclear Medicine, and Hospital Pharmacy
Research period January 2020 – January 2024
Email e.murcesilva@erasmusmc.nl
Currently, prostate cancer is one of the main cancer diagnoses in men. 10-20% of the prostate cancer develops into metastatic Castration Resistant Prostate Cancer (mCRPC) within 5 years after diagnosis. Patients diagnosed with mCRPC have little to no response to the standardized care of hormone therapy and chemotherapy. Recently, improvements have been made in the field of nuclear medicine towards development of a theragnostic molecule for the treatment of prostate cancer (PC).
T he prostate specific membrane antigen (PSMA) is a protein that is highly expressed in 90-100% of the prostate cancer cells, which makes it an interesting target for treatment and imaging. A PSMA ligand can be labelled with a radioactive isotope, such as Lutetium-177 and Actinium-225. Several studies with [ 177Lu]Lu-PSMA show promising antitumor effects with beta radiation, which can even be improved with the use of Ac-225, which is an alpha particle emitting isotope. The use of an alpha emitter provides advantages over a beta emitter due to the high energy, leading to cell damage, and limited range in tissue thereby selectively killing tumorous cells.
Our study aimed to produce [225Ac]Ac-PSMA-I&T, to be able to perform a clinical phase 1 dose-escalation study. A patient dose is prepared after a lot of research into the optimal conditions. The quality control of the final patient dose is based upon measurement of Fr-221 (218 keV), which is in equilibrium with Ac-225 after approximately six half-lives of Fr-221 (T1 2 = 4.8 min), which can also be seen in figure 1. For determining the radiochemical yield of PSMA with Ac-225, a radio-(i)TLC method is used to separate labelled and unlabelled product. This is crosschecked with the gammacounter and HPGe-detector to obtain measurements at a higher statistical significance and resolution. Furthermore, the radiochemical purity was evaluated by using a HPLC, and separation was performed between the labelled product and other (possible) impurities.
A final patient dose was developed according to the most recent guidelines with a therapeutic activity dose and standardized peptide concentration, which can be produced with a purity of >90% up to three hours after production, enabling patient administration. In 2021 we obtained a manufacturing license to authorize official production according to GMP, which makes us the first lab that is permitted in Europe to produce Ac-225 labelled PSMA, making the start of a phase I dose escalation study in Q1 of 2022 possible.

1.

Figure 2. Decay of an alpha emitter induce a high energie he-lium particle, which can after labelling selectively damage tumor
Figure
Decay scheme of Actinium-225 into Francium-221 and 5 subsequent alpha emissions

A THERANOSTIC PLATFORM BASED ON SMALL MOLECULE DRUG CONJUGATES FOR PROSTATE CANCER
ERIKA MURCE SILVA, MSC
PhD Student
Advisors Frederik Verburg & Yann Seimbille
Project Funding
Research period February 2019 – January 2023
Email e.murcesilva@erasmusmc.nl
Targeted approaches for the diagnosis and treatment of cancer have emerged as promising alternatives to traditional treatments, such as chemotherapy. They allow direct delivery of the cytotoxic agent to the tumor site, thus decreasing toxicity to other organs. Peptide receptor radionuclide therapy (PRRT) utilizes small targeting peptides conjugated to a linker and a chelator, which can be used to incorporate radionuclides for imaging or therapy.
Biovectors based on the Lys-urea-Glu (KuE) motif have been shown to bind strongly to PSMA (prostate-specific membrane antigen), an ideal cellular target for prostate cancer. A small library of KuE derivatives containing different amino acid was synthesized and evaluated for their binding affinity to PSMA in enzymatic assays. The lead candidate was further optimized and a DOTAGA chelator was attached for further radiochemical evaluation through labeling with 111In. The final lead compound, KuE-Ahx-Sta-Phe-Asp-DOTA-GA, was obtained in high radiochemical yield (>95%) and purity (>95%).
Then, the lead candidate served as a platform for further chemical modification (Fig. 1). Dimerization was performed to obtain a homodimer targeting
PSMA. Multivalent ligands bind to receptors with higher avidity, as they present multiple copies of the pharmacophore. This can also lead to an improvement of the pharmacokinetic properties, such as increased tumor uptake and prolonged retention time. The metal chelator can then easily be attached to the dimer via click chemistry. This approach also allows for further modification of the chelator, which can also affect the properties of the compound.
Finally, conjugation of antineoplastic agents (e.g. DM1) to the KuE ligand gives rise to small-molecule drug conjugates (SMDC). Attachment of an imaging probe to the SMDCs will allow in vivo noninvasive identification of the cancerous lesions and visualization of drug circulation and accumulation. Moreover, tandem combination of a therapeutic radionuclide and chemotherapeutic agent will lead to an increased treatment efficacy. If this approach is proven to be successful, it could be applied to different combinations of chemotherapeutic agents and biovectors.
The compounds are currently undergoing in vitro testing in PSMA expressing cell lines for binding affinity, cell uptake and cytotoxicity. The most promising compounds will be selected for SPECT/CT imaging in tumor-bearing mice and therapy efficacy studies.

Figure 1a. Schematic structure of the SMDC and homodimer. 1b. The binding moiety (KuE) of the compounds binds to PSMA, which is overexpressed in prostate cancer cells. The compounds are then internalized, and exert their therapeutic effect by damaging the DNA of the cancerous cells via ionizing radiation or by releasing the cytotoxic agent.

DEVELOPMENT OF NEW GRPRS ANTAGONISTS FOR BREAST CANCER DIAGNOSIS AND TREATMENT
PRICIANA PARAÏSO, PHARMD
PhD Student
Advisors Carolien van Deurzen, Frederik Verburg & Yann Seimbille
Project Funding Erasmus
MC Grant
Research period March 2021 – February 2025
Email p.paraiso@erasmusmc.nl
Breast cancer is the most frequent cancer in women and a major public health problem. It has been predicted that its worldwide incidence will reach 3.2 million new cases in 2050, reflecting the urgent need for new diagnostic tools and treatments. Functional noninvasive nuclear imaging (i.e. SPECT, PET) can provide accurate assessment of receptor status in primary and metastatic lesions and facilitate personalized treatment planning.
In this project, we are aiming to develop a new generation of peptide-based theranostics pointing at one major Achilles’ heels of breast tumors, namely the gastrin releasing peptide receptors (GRPRs). GRPRs are overexpressed and aberrantly activated in a high percentage of breast cancer tissues.
Peptide receptor radionuclide therapy (PRRT) pairs a peptide-based ligand with a radionuclide by mean of a chelator.

Figure 1. Three-dimensional model of the putative binding site of the human GRP receptor. The putative binding pocket is depicted as a dark-gray space-filled structure. Seven amino acids within 5 A˚ of the binding pocket are most important for determining high affinity: D97, L121, I283, Y284, R287, H300, V302 (in yellow).
Once the radiopeptide is injected, it binds to the targeted receptors and its radioactive decay allows visualization of the tumors or cell death due to DNA damage. NeoBOMB1 and ProBOMB1 are proof that this concept has been successfully applied in patients (Fig.2).
However, a concomitant uptake of NeoBOMB1 was observed in the pancreas, which could lead to side effects. ProBOMB1 showed a decreased uptake in this organ. Thereby, optimization of the pharmacokinetic properties of the GRPR ligands can be achieved by slight chemical modifications. A library of 24 analogs has been synthesized by solid-phase peptide synthesis (SPPS). Their affinity for GRPR will be evaluated to give us information regarding their potency as GRPR antagonists and how chemical features could account for it. If successful, it could be applied to rationally design radiopharmaceuticals for targeted diagnosis and treatment of breast cancer.

Figure 2. NeoBOMB1 and ProBOMB1 structures. They are both high affinity GRPR antagonists that present a high uptake in tumors and excellent stability, allowing a very good visualization of cancer lesions.


CLINICAL IMAGING
Aad van der Lugt graduated the Erasmus Medical School in 1988. He was a Junior Doctor in Surgery and Intensive care in Tilburg and Eindhoven, respectively before specializing as Neuroradiologist at Erasmus MC. His PhD degree at Erasmus University Rotterdam (1996) was on “Intravascular Ultrasound”. He has been head of neuroradiological research program since 2002 and became Professor of Neuroradiology and Head & Neck Radiology in 2010. Since 2020 he is

the director of Radiological Research and Training. Prof. Aad van der Lugt is a member of the research committee of the European Society of Radiology (ESR) as well as of the Scientific Executive Committee of BBMRINL. He represents the Netherlands in the EuroBioimaging Board. His research focuses on neurovascular imaging (CTA/MRA), on acute stroke treatment, and on imaging biomarkers in large population-based studies.
a.vanderlugt@erasmusmc.nl
IMAGING IN NEUROVASCULAR DISEASE
AAD VAN DER LUGT, MD, PHD
full professor

Context
This research program is focused on the role of imaging biomarkers in neurovascular diseases with a strong emphasis on ischemic stroke. Imaging in acute stroke aims to support in the diagnosis, assessment of the severity and reversibility of ischemia. Selection of patients for intravenous thrombolysis or intra-arterial thrombectomy is based on imaging biomarkers. Imaging in the sub-acute phase might help in the evaluation of the etiology of ischemic stroke. Recently, more attention is paid to atherosclerosis in other vessel beds than the carotid bifurcation and to the morphology and composition of atherosclerotic disease. A better subdivision of patients according to the presumed etiology of ischemic stroke might improve the selection for optimal secondary preventive measures.
This research program includes technical development and technical evaluation, evaluation of image analysis algorithms, and clinical validation of imaging biomarkers.
Top Publications 2021
Boodt N, Snouckaert van Schauburg PRW, Hund HM, Fereidoonnezhad B, McGarry JP, Akyildiz AC, van Es ACGM, De Meyer SF, Dippel DWJ, Lingsma HF, van Beusekom HMM, van der Lugt A, Gijsen FJH. Mechanical Characterization of Thrombi Retrieved With Endovascular Thrombectomy in Patients With Acute Ischemic Stroke. Stroke. 2021;52:2510-2517.
Bos D, Arshi B, van den Bouwhuijsen QJA, Ikram MK, Selwaness M, Vernooij MW, Kavousi M, van der Lugt A. Atherosclerotic Carotid Plaque Composition and Incident Stroke and Coronary Events. J Am Coll Cardiol. 2021;77:1426-1435.
Luijten SPR, Bos D, Compagne KCJ, Wolff L, Majoie CBLM, Roos YBWEM, van Zwam WH, van Oostenbrugge RJ, Dippel DWJ, van der Lugt A, van Es ACGM; MR CLEAN trial investigators. Association of White Matter Lesions and Outcome After Endovascular Stroke Treatment. Neurology. 2021;96:e333-e342.
Research Projects: Objectives & Achievements
Endovascular treatment in patients with acute stroke: beyond MR CLEAN
Treatment with intravenous (IV) alteplase, aiming at early reperfusion, has proven effective for patients with acute ischemic stroke. In ~25% of patients with acute anterior circulation ischemic stroke, symptoms are caused by a proximal occlusion of a major intracranial artery. Endovascular treatment (EVT) increases the likelihood of recanalization in patients with acute ischemic stroke caused by proximal intracranial arterial occlusion. The MR CLEAN Study demonstrated that intra-arterial treatment administered within 6 hours after stroke onset is effective and safe. After our landmark paper multiple studies have demonstrated the beneficial aspects of EVT. Within the HERMES Collaboration individual patient data have been used to investigate additional research questions focusing on optimal selection of patients for treatment and improving of outcome.
In 2017 three new trials have started 1) to evaluate the effect of peri-procedural medication (MR CLEAN-MED), 2) to evaluate the effects in patients presenting between 6 and 12 hours after the event and (MR CLEAN-LATE) and 3) to evaluate the benefits of direct IAT without prior IVT (MR CLEAN-NoIV). MR CLEAN-MED has been halted due to safety reason. MR CLEAN-Late is still including patients. MR CLEAN NoIV has been finalized, analyzed and reported. The trials have been executed by the CONTRAST-consortium (www.contrast-consortium.nl) in which a biobank infrastructure for data, blood samples, thrombus and imaging has been build. To improve the diagnosis, workflow and prediction of outcome multiple image analysis algorithms have been developed. We are currently building a platform for standardized evaluation of imaging analysis algorithms.
Although recanalization is vital for potential clinical recovery of the patient, about one third of the patients do not show clinical improvement despite successful opening of the occluded vessel. Incomplete microvascular reperfusion (IMR) after successful recanalization is recognized as an important predictor for tissue survival and good clinical outcome. However, no data is available regarding perfusion changes directly after EVT. We aim to perform serial imaging in animals and patients with advance imaging techniques to evaluate early perfusion changes of the ischemic brain after EVT and to identify imaging parameters for the early identification of patients not benefitting from revascularization due to IMR.
With EVT extracted thrombi have become available for histopathologic analysis which has given us the unique opportunity to study the relationship between imaging characteristics of the thrombus and thrombus composition, between thrombus composition and thrombus biomechanical parameters, and between thrombus composition and recanalization. The biobank of MR CLEAN and CONTRAST gives us ample opportunities to investigate the important role of thrombus composition and morphology in the success of EVT.
MRI, CT, and ultrasound of carotid artery atherosclerosis
Ischemic cerebral infarcts are related to the presence of atherosclerotic disease in the carotid artery. Severity of the stenosis is a predictor of clinical symptoms and is used as parameter in the therapeutic decision as to which patients will benefit from carotid intervention. Next to severity of stenosis, plaque morphology is thought to be a major determinant of clinical events. Visualization of atherosclerotic plaque and assessment of vulnerability with non-invasive imaging techniques greatly enhances the understanding of atherosclerotic disease and the cerebrovascular events. The project evaluates different imaging modalities for the visualization of atherosclerotic disease in the carotid bifurcations. Imaging is compared with histologic sections for validation of imaging parameters. Quantification of imaging parameters is validated with manual annotation as gold standard. Serial studies evaluate the progression of atherosclerotic disease and the effect of intervention. The predictive value for plaque imaging parameters for recurrent stroke is evaluate in a multicenter study (ParisK) in which 240 patients have been included. The clinical outcome of the patients in the ParisK study has been analyzed. We concluded that intraplaque hemorrhage and total plaque volume are independent risk factors of recurrent ipsilateral ischemic stroke or TIA in patients with mild-tomoderate carotid stenosis. These plaque characteristics improve also current decision making. Validation studies to implement plaque characteristics in clinical scoring tools are needed.
Expectations & Directions
We will continue to expand the role of imaging in the diagnosis and therapy of neurovascular disease. Personalized medicine requires the stratification of the large group of patients with stroke in subgroups with different prognosis and treatment. Imaging will increase the insight in the pathophysiology of neurovascular diseases, and therefore imaging biomarkers are becoming increasingly important in personalized medicine. We
will continue the collaboration with the neuro and vascular -image analysis groups in which semi-automated algorithms for the extraction of quantitative imaging biomarkers are devolved and validated.
New trials on endovascular treatment have started. The trails will be accompanied by basic studies that focus on optimal per procedural medication to improve the microcirculation. Evaluation of extracted thrombus as well as blood biomarkers will provide insights in cause of lack of clinical improvement after successful recanalisation.
The collaboration with the clinical departments as well with the population imaging group creates a fruitful exchange of ideas and an easy translation of findings in basic research into clinical research questions and vice versa.
Funding
Cisca Wijminga (UMCG), Aad van der Lugt , Gerrit Meijer (NKI), Leon Kenemans (UU), and Gert-Jan van Ommen (LUMC): Netherlands Organization for Scientific Research (NWO) – National Roadmap for Large-Scale Research Facilities 2015-2021: “BBMRI-NL2.0: NL-Biobank Research Facility”
Diederik Dippel, Charles Majoie, Aad van der Lugt , and partners: Dutch Heart Foundation 2016-2022: “CONTRAST, Consortium for New treatments for acute stroke”
Aad van der Lugt , Diederik Dippel and Hester Lingsma: H2020 2017-2022: “INSIST: IN-Silico trials for treatment of acute Ischemic Stroke”
Aad van der Lugt, Wiro Niessen, Stefan Klein, Daniel Bos : H2020 2018-2022: “An EU-Canada joint infrastructure for next-generation multi-Study Heart research (euCanSHare)”
Thomas Hankemeier (LU/EMC), Eline Slagboom (LUMC), Cornelia van Duijn, Arfan Ikram, Simon Mooijaart (LUMC), Aad van der Lugt : Medical Delta 2018-2023: “Metabolomics for clinical advances in the Medical Delta (METABODELTA)”
Nico de Jong, Annemien van den Bosch, Aad van der Lugt : Medical Delta 2018-2023: “Ultrafast Ultrasound for the Heart and Brain (UltraHB)”
Theo van Walsum, Wiro Niessen, Jorrit Glastra (Quantib), Aad van der Lugt : Dutch Heart Foundation 2018-2021: “Automatic CTA image analysis to support treatment selection in acute stroke (ACCurATE)”
Yvo B.W.E.M. Roos (AMC), Edwin van der Pol (AMC), Robert Kuipers (Nico-Lab), Henk Leeuwis (LioniX International B.V.), Frank W. Coumans (Exometry B.V.), Anne Yaël Nossent (LUMC), Jan van Esch (Delft University) Aad van der Lugt: Dutch Heart Foundation 2018-2022: Circulating Nano Traces to Identify the Cause of Stroke (CINTICS)”
Theo van Walsum, Ad van Es: TKI-LSH-PPS 2019-2021: Q-Maestro: Quantitative Microvasculature Assessment in projection angiography of ischemic stroke patients.
Bob Roozenbeek: The Erasmus Initiative “Smarter Choices for Better Health” 2018-2021: Performance feedback as part of Value-Based Health Care: a randomized evaluation of the effect on quality of stroke care.
Bob Roozenbeek: Erasmus MC Efficiency Research grant 2019-2022: Regional implementation of a decision support tool for individualized prehospital triage of suspected stroke patients: a cost-effectiveness study.
Ad van Es: CONTRAST Young Talent Program 2019-2021: Detecting Incomplete Microvascular Reperfusion in Clinical Practice.
Aad van der Lugt, Diederik Dippel: Thrombolytic Science, LLC: DUal thrombolytic therapy with Mutant pro-urokinase (m-pro-urokinase, HisproUK) and low dose Alteplase for ischemic Stroke.
Aad van der Lugt, Wiro Niessen, Stefan Klein, Daniel Bos: H2020 2020-2024: “An European Cancer Image Platform Linked to Biological and Health Data for Next-Generation Artificial Intelligence and Precision Medicine in Oncology (euCanImage)”
Bob Roozenbeek: EUR fellowship 2019-2023. “Reperfusion of the brain after endovascular thrombectomy for ischemic stroke: development of a prognostic framework using clinical and neuroimaging characteristics - the REPERFUSE study”
Invited Lectures
Lugt, Aad van der . The extracranial Vessel Wall: CT, MRI, US? 44th European Society of Neuroradiology Annual Meeting, Geneva/CH (2 Oktober 2021)
Lugt, Aad van der . Imaging of Orbital Disease. Update in Neuro-Imaging (21 Oktober 2021)
Lugt, Aad van der . Pathophysiology and etiology of brain ischemia with a focus on imaging. Update in Neuro-Imaging. (22 Oktober 2021)
Lugt, Aad van der . Current Advances in Stroke Imaging, Clinical Studies and Evidence. Swiss Federation of Clinical Neuro-Societies Imaging Course Neurovascular disease (26 Oktober 2021)
Lugt, Aad van der . CT in Acute Stroke. Galen Course Neuroradiology, European School of Radiology (24 November 2021)
Lugt, Aad van der . European Imaging Biomarkers Alliance. ERSMRMB MRI Together. A global workshop on Open Science and Reproducible MR Research (16 December 2021)

ACUTE MANAGEMENT OF ISCHEMIC STROKE
BOB ROOZENBEEK, MD, PHD
Post-doc & Neurologist
Project Funding Collaboration for New Treatments of Acute Stroke (CONTRAST) WP3B MRCLEAN-MED, BeterKeten Foundation, Theia Foundation, Erasmus MC Efficiency Research, Erasmus Initiatives
Research period August 2017 – August 2021
Email b.roozenbeek@erasmusmc.nl
This project is a collaboration between the Departments Radiology & Nuclear Medicine, Neurology and Public Health.
The acute management of ischemic stroke has changed drastically in the past years. The implementation of endovascular thrombectomy as an effective treatment for patients with ischemic stroke caused by a proximal intracranial arterial occlusion substantially improved patients’ functional outcomes. However, major challenges remain. Our research aims to solve three of the most urgent issues:
1 Preho spital identification of patients eligible for thrombectomy
Since the beneficial effect of thrombectomy is highly time-dependent, treatment needs to be initiated as rapidly as possible. To achieve this, transportation times to specialized intervention hospitals (such as Erasmus MC) need to be minimized. Several prehospital stroke scales were developed to identify patients that are likely to have a large vessel occlusion in the ambulance, which could allow for direct transportation of thrombectomy eligible patients to an intervention hospital. In the PRESTO study, we aimed to prospectively validate these prehospital stroke scales in the field. The main finding was that prehospital stroke scales detect large vessel occlusions with acceptable-to-good accuracy. RACE, G-FAST and CG-FAST were the best performing scales. By the end of 2020, the study report was accepted for publication in The Lancet Neurology. (More info: www.presto-studie.nl.)

2 Optimizing in-ho spital workflow to reduce treatment delays
Another way to minimize treatment delay, is to optimize the in-hospital workflow for acute ischemic stroke. Systematic performance feedback may help intervention centers to improve their workflow. The PERFEQTOS trial is a steppedwedge randomized controlled trial to assess the effect of performance feedback on the quality of stroke care. Thirteen intervention centers in the Netherlands participate. Performance feedback consists of a dashboard with indicators of patient characteristics, processes and outcomes. Local quality improvement teams will use this feedback to implement performance improvement plans. We hypothesize that performance feedback will shorten door-to-groin times and thereby improve quality of stroke care. The study is ongoing. By the end of 2020, seven hospitals were randomized to the intervention group. (More info: www.perfeqtos-trial.nl)

3 Incre asing the benefit of thrombectomy
A considerable proportion of ischemic stroke patients do not recover despite fast and complete recanalization after thrombectomy. It is unknown whether periprocedural antitrombotic or anticoagulant therapy can improve clinical outcome. In the MR CLEAN-MED trial, we assess the effect of intravenous acetylsalicylic acid and unfractionated heparin, alone, or in combination, in patients who undergo thrombectomy. Fiveteen Dutch intervention center participate and five French sites are scheduled to be initiated in 2021. By the end of 2020, >500 patients were included. (More info: www.mrclean-med.nl)


ENDOVASCULAR THROMBECTOMY FOR ACUTE ISCHEMIC STROKE: LESSONS LEARNED FROM THE OCCLUDING THROMBUS
NIKKI BOODT, MSC, MD
Advisors Aad van der Lugt , Diederik Dippel & Hester Lingsma
Project Funding Horizon 2020: IN-Silico trials for treatment of acute Ischemic Stroke (INSIST)
Research period August 2018 – August 2022
Email n.boodt@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine, Neurology and Public Health.
Endovascular thrombectomy (EVT) for patients with acute ischemic stroke due to large vessel occlusion of the anterior circulation is highly effective. Since 2015, the global research focus has changed from a discussion on the efficacy of EVT to how it can be administered more efficiently. In terms of revascularization outcomes, there remains significant room for improvement: In 20-40% of patients in the trials, substantial revascularization could not be achieved. The occluding thrombus might play a crucial role in achieving successful reperfusion. During my PhD program, I have focused on the histological, mechanical and imaging characteristics of stroke thrombi, and their relationship with procedural and clinical outcomes, to help improve EVT efficacy.

tery terminus and permeability (or ‘perviousness’). We have shown that thrombus CT characteristics are associated with thrombus composition (e.g., the amount of red blood cells and fibrin/platelets). Moreover, we were able to show that thrombus CT characteristics are associated with the origin of the thrombus, in line with our hypothesis based on our histological study. This is relevant, as histological analyses can be time consuming, costly and subject to bias.
In general, stroke thrombi consist of four main components: red blood cells, fibrin, platelets and white blood cells. In a MR CLEAN Registry biobank study, we have shown that the amount of red blood cells and fibrin/platelets are related to the origin of the thrombus: red blood cell-rich clots were more likely to be from large artery atherosclerosis strokes, while fibrin/platelet-rich clots were more likely to be cardioembolic. This study confirmed results of earlier studies on the same topic.
In the Netherlands, every stroke patient undergoes non-contrast computed tomography (CT) and computed tomography angiography (CTA) upon arrival at the emergency department. On admission CT imaging, we can assess various characteristics of the occluding thrombus, such as attenuation, length, distance from the internal carotid ar-
In collaboration with the biomechanical department and Technical University Delft, we have performed the first ever study to mechanically characterize human stroke thrombi and assess the association with quantified thrombus composition. Our hypothesis was confirmed: fibrin/platelet-rich thrombi are mechanically stiffer.
A currently much-discussed topic in the field of EVT is which device to use: next to stent retriever (SR), contact aspiration (CA) is rapidly gaining popularity. Many researchers and clinicians suspect that thrombus type might be useful as a guide for selecting first-line EVT device: fibrin/platelet-rich thrombi, which are stiff and thrombectomy-resistant, might benefit more from CA. However, in a MR CLEAN Registry substudy, we have shown that this was not the case.
PhD Student
Mechanical characterization of human stroke thrombi retrieved with EVT.

ENDOVASCULAR TREATMENT FOR ACUTE ISCHEMIC STROKE
VICKY CHALOS-ANDREOU, MD
Advisors Aad van der Lugt , Diederik Dippel, & Bob Roozenbeek , Hester Lingsma
Project Funding Dutch Heart Foundation, Dutch Brain Foundation, Stryker, Medtronic and Cerenovus. Collaboration for New treatments of Acute Stroke (CONTRAST): WP6 Data management.
Research period September 2016 – June 2022
Email v.chalos@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine, Neurology, and Public Health.
For almost two decades, intravenous thrombolysis with alteplase (IVT) had been the standard of care for patients with acute ischemic stroke. Since 2015, endovascular treatment (EVT) has been proven safe and effective for acute ischemic stroke patients with an intracranial large vessel occlusion of the anterior circulation. Since then, EVT has been widely implemented as the standard of care in these patients, also within the Dutch health care system. Yet, a considerable number of these patients does not recover despite successful recanalization after EVT.
The first major aims of my thesis are to identify predictors of functional outcome after EVT, and to develop a clinical tool (MR PREDICTS@24H) that can be applied one day after EVT to predict functional outcome for individual patients at 3 months. Another major aim is to further improve outcome after EVT by 1) discussing considerations in the design of clinical trials, including the selection of a primary outcome measure and type of informed consent procedure, and by 2) optimizing periprocedural antithrombotic management.
For my thesis, I will mainly use data from: (1) The MR CLEAN trial, a randomized controlled trial (RCT) that investigated EVT+ usual care vs usual care alone in the Netherlands; (2) The HERMES collaboration, consisting of 7 pooled RCTs that all investigated EVT + usual care vs usual care alone; (3) The MR CLEAN Registry; a nationwide, prospective, observational study among consecutive patients treated with EVT in the Netherlands.
During my PhD program, I have been the clinical data manager of five ongoing RCTs, conducted within the CONTRAST Consortium. Two of these RCTs focus on improving

Workflow of deferred consent procedure.
the safety and effectiveness of EVT through periprocedural antithrombotic management. The MR CLEAN-NO IV, aims to investigate the added benefit of IVT prior to EVT. The MR CLEAN-MED, of which the research protocol will be included in my thesis, aims to investigate the effect of periprocedural medication (antiplatelet agents, unfractionated heparin, both or neither). In these RCTs patients are included using the deferred consent. procedure, which allows patient inclusion without prior patient or proxy consent. After the study intervention, and when patients or proxies regain their ability to provide informed consent, patients or proxy informed consent must be obtained for trial continuation.

CAROTID ARTERY DISEASE: WALL STRUCTURE AND FLUID MECHANICS
KRISTINE DILBA, MD
PhD Student
Advisors Aad van der Lugt , Ton van der
Steen & Jolanda Wentzel
Project Funding STW-BIOSTRESS project 10813
Research period October 2015 – June 2022
Email k.dilba@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine, Cardiology and Biomedical Engineering.
Atherosclerotic plaque rupture is the main cause of ischemic stroke. Currently, the degree of lumen stenosis is used for risk assessment and treatment strategy. However, clinical events occur even in patients with a low degree of stenosis but these patients are not eligible for revascularization procedure. It motivated researchers to search for other markers of ruptureprone plaques beyond the degree of luminal stenosis. Since plaque rupture (imaging ulcerations) are most often localized proximally to the point of maximum stenosis, where wall shear stress (WSS) is supposedly high, this biomechanical force on the vessel wall could play a role in the development of vulnerable plaques. It was hypothesized that high WSS weakens the fibrous cap through biological pathways which eventually leads to plaque rupture.

Figure. CTA examples (maximum intensity projection views) of different types of dolichoarteriopathy of the extracranial internal carotid artery. ECA, external carotid artery; ICA, internal carotid artery
develop an ulcer. Also, we found that ulcers develop often on the thicker part of the plaque.
The main focus of this research project is the (biomechanical) analysis of the atherosclerotic plaque in carotid arteries from the Plaque At Risk (PARISK) cohort. Patients from the PARISK study underwent non-invasive carotid plaque imaging (US, MDCTA, MRI) at baseline and after 2 years.
In a case-control study we investigated the association between WSS at baseline and newly developed ulcers after 2 years follow-up. I established changes in plaque surface morphology using MDCTA. WSS was calculated using computational fluid dynamics applying MRI-based geometry of the carotid artery. Plaques that developed a new ulcer during the follow-up period had a higher relative minimum WSS at baseline than plaques that did not
Besides atherosclerosis also other (non-atherosclerotic) carotid artery diseases might cause an ischemic stroke. For instance, dolichoarteriopathies, abnormalities in the course and geometry of the extracranial part of the internal carotid artery, are considered to be a cause of stroke and TIA. Its link with cerebrovascular events is most likely related to hemodynamic or thrombo-embolic mechanisms. I assessed the prevalence and variations of ICA dolichoarteriopathies and its associations with cardiovascular risk factors in patients from the PARISK study. We found a prevalence of 69% of any dolichoarteriopathy. The most common type of dolichoarteriopathy was tortuosity (72%), followed by coiling (20%), and kinking (8%). We found that old age and obesity were associated with a higher tortuosity index. In addition, obesity and hypercholesterolemia were independently associated with a more severe type of dolichoarteriopathy.

IMPROVING SAFETY AND CLINICAL OUTCOMES OF REPERFUSION THERAPY FOR ISCHEMIC STROKE
NADINDA VAN DER ENDE, MD
PhD Student
Advisors Aad van der Lugt, Diederik Dippel & Bob Roozenbeek
Project Funding Thrombolytic Science International (TSI)
Research period November 2018 – November 2022
Email n.vanderende@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Neurology.
Teatment with intravenous thrombolysis has been the standard of care for patients with ischemic stroke for more than two decades. Additional treatment with endovascular thrombectomy is possible for patients with an ischemic stroke caused by a proximal intracranial arterial occlusion. This type of occlusion is present in at most 30% of ischemic stroke patients presenting at the emergency department. Patients without a proximal intracranial arterial occlusion can only be treated with intravenous thrombolysis.
Currently, alteplase is the only approved thrombolytic agent. The effectiveness of alteplase for ischemic stroke treatment is limited and the occurrence of intracranial hemorrhage is a major limitation. Dual thrombolytic therapy consisting of a low dose alteplase followed by mutant pro-urokinase, which does not lyse hemostatic fibrin, has a significant potential to be safer and more efficacious.

Expected and actual accrual in the DUMAS trial
In DUal thrombolytic therapy with Mutant pro-urokinase and low dose Alteplase for ischemic Stroke (DUMAS), we aim to assess the safety and efficacy of this dual thrombolytic treatment against usual treatment with alteplase in patients presenting with ischemic stroke. DUMAS is a phase II, randomized controlled trial. We hypothesize that this dual thrombolytic treatment will reduce the occurrence of intracranial hemorrhage in patients with
ischemic stroke compared to patients treated with alteplase alone. We aim to include 200 patients with a discharge diagnosis of ischemic stroke. At the beginning of 2022, a total of 172 patients with a discharge diagnosis of ischemic stroke have been included.
Additional information about DUMAS can be found on https://dumas-trial.nl/

SANNE DEN HARTOG, MD QUALITY OF CARE FOR ISCHEMIC STROKE
PhD Student
Advisors Aad van der Lugt, Diederik Dippel, Bob Roozenbeek & Hester Lingsma
Project Funding Erasmus University: Smarter Choices for Better Health
Research period January 2019 – January 2022
Email s.denhartog@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine, Neurology, and Public Health.
Athough provision of performance feedback to health care professionals is common practice, observational studies of its effect on quality of care have shown mixed results.
We aim to study whether performance feedback to healthcare providers in hospitals providing endovascular thrombectomy (EVT) for ischemic stroke improves care processes and thereby quality of care.
PERFEQTOS is a stepped-wedge cluster randomized trial. Thirteen hospitals in The Netherlands providing EVT participate in this study. Performance feedback consists of 3-monthly reports with indicators on quality of care (structure, process, and outcomes) for patients with ischemic stroke treated with EVT, compared to other hospitals. The performance feedback is provided to local Quality Improvement Teams (QIT), including a neurologist, interventional neuroradiologist, and neurology resident/ nurse. The QIT uses the performance feedback to define target(s) and to develop a Performance Improvement Plan (PIP). The impact of this PIP is evaluated in the next performance report. The control group will not receive structured performance feedback and is not yet required to have a QIT.


Primary outcome is door-to-groin time. Secondary outcomes include door-to-needle time, eTICI score, NIHSS after 24 hours, mRS at 3 months, adjusted for prognostic factors at baseline.
The intervention arm of the study started in July 2020. Every six months 3-4 hospitals will be randomized to cross from control to the intervention group, until all hospitals are crossed over.
We hypothesize that giving feedback to healthcare providers on the performance of their own hospital improves care processes and thereby quality of care.
Study design PERFEQTOS

NOOR SAMUELS, MD ENDOVASCULAR TREATMENT SIMULATIONS
PhD Student
Advisors Aad van der Lugt, Diederik Dippel & Hester Lingsma
Project Funding Horizon 2020: IN-Silico trials for treatment of acute Ischemic STroke (INSIST)
Research period February 2018 – February 2021
Email n.samuels@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine, Neurology and Public Health.
To further improve care for stroke patients, researchers require access to large databases of stroke patients. Realistic synthetic patient cohorts might enable broader access to health care data, while ensuring patients’ privacy. We developed three statistical methods to generate realistic synthetic stroke patients. Finally, we developed a user interface to sample synthetic stroke cohorts based on in- and exclusion criteria of interest. Synthetic stroke patients can be of value to in-silico trials that aim to estimate effectiveness of therapies by modeling different (refinements of) therapeutic interventions in synthetic stroke patient cohorts.

The purpose of INSIST (www.insist-h2020.eu) is to develop an in silico clinical trial for treatment of acute ischemic stroke.
Furthermore, I am identifying risk factors for poor outcome after endovascular treatment (EVT) for ischemic stroke using data of the MR CLEAN Registry and HERMES collaboration. I evaluated the relation between periprocedural hemodynamic and anesthetic management in ischemic stroke patients and outcomes after EVT. There is an ongoing debate on the most optimal anesthetic strategy to increase patient comfort, minimize patient motion, facilitate fast treatment, and reduce the risk of complications. As cerebral autoregulation can be impaired, guided hemodynamic management is needed to optimize cerebral perfusion before and after recanalization. Besides, I am working on a mediation analysis to assess the role of follow-up infarct volume on the effect of EVT on early neurologic deficit using data of the MR CLEAN trial.

Structure of a mediation analysis
Covariate distribution model

MATTHIJS VAN DER SLUIJS, MD QUANTITATIVE ASSESSMENT OF DSA IN STROKE
PhD
Advisors Aad van der Lugt & Theo van Walsum
Project Funding Erasmus MC TKI-LSH: QMAESTRO Quantitative Microvasculature AssEssment in projection angiography of ischemic STROke patients
Research period November 2020 – May 2024
Email p.vandersluijs@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Neurology, and Philips Healthcare.
Over the past decade, endovascular therapy (EVT) has emerged as therapy for patients with acute ischemic stroke caused by an occlusion of proximal intracranial arteries. Due to the increased use of imaging in these patients, imaging biomarkers are being developed for predicting outcome. These imaging biomarkers are primarily based on CT and MR imaging performed before and after the EVT. However, there are currently no accurate imaging biomarkers available in the peri-procedural setting of EVT that could guide the intervention.
The Q-MAESTRO project aims to develop imaging parameters that quantify perfusion restoration after recanalization using Digital Subtraction Angiography (DSA). Currently, extended Thrombolysis In Cerebral Infaction (eTICI), is used to assess reperfusion. This is a six point categorical score based on percentage of reperfusion of the area at risk. With more quantitative approaches more precise reperfusion characteristics, such as microvascular properties, can be identified. This can potentially influence clinical decision making of the neuro-interventionalist performing the EVT, as supplementary locoregional therapeutic action in the intervention stage might still be available.
In my recent work we focused on reproducing automated TICI scoring on a larger dataset including distal occlusion sites. We discovered that the automated scoring method performs equally well, compared to eTICI, for prediction of functional outcome, adjusted for prognostic factors.
Student A

ROC curve of good functional outcome (mRS 90d 0-2), comparing AutoTICI and eTICI in 503 patients of the MR-CLEAN Registry, concerning ICA-M1 and M2 occlusions. P=0.28. Patient selection was based on occlusion locations, and the availability of DSA series in both viewing directions, pre- and postEVT.
Research topics
– Validation of AutoTICI model to automatically quantify microvascular reperfusion in pre- and post-interventional imaging.
– Assessing both the detection and the clinical value of vessel perforation during EVT, observed as contrast leakage into the subarachnoid space, outside of intracranial arteries.

OPTIMIZING ENDOVASCULAR STROKE TREATMENT
WOUTER VAN DER STEEN, MD
PhD Student
Advisors Aad van der Lugt , Diederik Dippel & Bob Roozenbeek
Project Funding Dutch Heart Foundation, Dutch Brain Foundation, Stryker, Medtronic and Cerenovus. Collaboration for New treatments of Acute Stroke (CONTRAST): WP3B MRCLEAN-MED.
Research period Oktober 2019 – June 2022
Email w.vandersteen@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Neurology.
Endovascular treatment (EVT) substantially improves the outcome of patients with ischemic stroke caused by an intracranial large vessel occlusion. However, despite an improvement in outcome, 3-month mortality and functional dependence remains high.
Intracranial hemorrhage (ICH) is a common complication after EVT. An ICH can be classified i) as asymptomatic or symptomatic, and ii) according to imaging characteristics and anatomic description. The association of a symptomatic ICH with worse functional outcome is well known, however, the association of the other classifications of ICH with functional outcome is less understood. We use a pooled dataset of 2 randomized controlled trials to evaluate these associations. Furthermore, a good understanding of why patients develop symptomatic ICH is also lacking. We use the database of a national cohort database (MR CLEAN-Registry), to evaluate the association of baseline characteristics with the occurrence, timing and anatomical location of symptomatic ICH. A better understanding of these determinants could help to improve prevention and management of this serious complication. Eventually this would further improve safety and benefits of EVT for ischemic stroke patients.

Non-contrast CTs of two different patients with an intracranial hemorrhage (ICH) after endovascular treatment. The left ICH is classified as a hemorrhagic infarction (HI), the right as a parenchymal hematoma (PH).
Additionally, interventionists often administer periprocedural antithrombotic agents during EVT to reduce thrombotic complications and to improve angiographic and microvascular reperfusion. However, it has always remained unknown whether these potential benefits outweighed the potentially increased risk of symptomatic ICH. We performed the first randomized controlled trial evaluating the efficacy and safety of periprocedural acetylsalicylic acid or unfractionated heparin during EVT (MR CLEAN-MED). The results of this trial show that the antithrombotic agents are associated with an increased risk of symptomatic ICH, without evidence for a beneficial effect on functional outcome. Therefore, avoiding routine periprocedural treatment with these agents can further increase chances of recovery after EVT.

AUTOMATED IMAGING BIOMARKERS IN ACUTE ISCHEMIC STROKE
LENNARD WOLFF, MD
PhD Student
Advisors Aad van der Lugt & Theo van Walsum
Project Funding Dutch Heart Foundation, the Dutch Brain Foundation, Stryker, Medtronic and Ceronovus. Collaboration for New treatments of Acute Stroke (CONTRAST): WP7 Imaging Biobank.
Research period December 2017 – March 2022
Email l.wolff.1@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Neurology.
Several imaging biomarkers predict outcome in patients with acute ischemic stroke and effects of endovascular treatment (EVT). Most imaging biomarkers are visually assessed by trained observers. Automated analysis tools might decrease the variability in the evaluation of imaging biomarkers with a subsequent improvement of prediction tools. In addition, more relevant imaging biomarkers could be extracted with sophisticated analysis tools such as deep learning algorithms.
In my research project, we validated automated ASPECTS software for detection of early ischemic brain changes on non-contrast CT scans. We concluded that the performance of automated ASPECTS is comparable to expert readers and could support readers in the detection of early ischemic changes.
Besides this, we evaluated an automated software solution for quantification of collateral vessel filling on baseline CTA imaging in patients with acute ischemic stroke. The performance of automated collateral scoring was comparable to expert radiologists.
We also assessed the diagnostic performance of an algorithm for large vessel occlusion detection on CTA. The algorithm provided a high detection rate for proximal occlusions, but performance varied significantly by occlusion location. Detection of distal occlusions needs further improvement.
Finally, deep learning algorithms were implemented in image analysis and evaluated in clinical practice.
During my PhD program, I am responsible for the imaging biobank of the five randomized controlled trials (RCTs), conducted within the CONTRAST Consortium (Collaboration for New Treatments of Acute Stroke). The overarching aim of these RCTs is to improve the safety and effectiveness of new ischemic stroke treatments. In this setting, the acute stroke imaging data of at least 16 hospitals in the Netherlands are currently stored in a central image repository and image data is evaluated by an imaging core-lab. The data will be made available for research.

Visual collateral score grading in patients with an middle cerebral artery occlusion in the M1 segment. 0: absent collaterals, 0% filling of the occluded territory. 1: poor collaterals, >0% and ≤50% filling of the occluded territory. 2: moderate collaterals, >50% and <100% filling of the occluded territory. 3: good collaterals, 100% filling of the occluded territory.

IMAGING AND ENDOVASCULAR TREATMENT OF PATIENTS WITH ACUTE ISCHEMIC STROKE
ROB VAN DE GRAAF, PHD
PhD Awarded 28 September 2021
Advisors Aad van der Lugt, Diederik Dippel & Bob Roozenbeek
Project Funding Dutch Heart Foundation, Dutch Brain Foundation, Stryker, Medtronic and Cerenovus. Collaboration for New treatments of Acute Stroke (CONTRAST): WP3B MRCLEAN-MED.
Short CV
Rob A. van de Graaf was born in 1991 in Zwijndrecht. After finishing his Master of Medicine in 2017, he started as a PhD-candidate at the departments of Radiology & Nuclear Medicine and Neurology of the Erasmus MC, University Medical Center. His main task was the coordination and execution of the MR CLEAN-MED trial. He presented his work at numerous (inter)national conferences. Rob aspires a career as a Radiologist and will start his residency in Radiology in October 2021.
This project was a collaboration between the Departments of Radiology & Nuclear Medicine and Neurology.
Alarge proportion of patients suffering from an acute ischemic stroke caused by a large vessel occlusion in the anterior circulation (most commonly originating from the heart) die or become functionally dependent in daily life when treatment is not offered in time.
In case the right treatment (endovascular treatment) is offered fast and the thrombus can be removed reperfusion can recover and the outcomes are less dramatic (resulting in less patients being functionally dependent or death). Despite the large success of the endovascular treatment (which has only been introduced since 2015) still a large proportion of patients (±50%) does not recover, even when treatment is successful and thrombus has been removed.
In my thesis I evaluated whether outcomes of patients with an ischemic stroke caused by an intracranial large vessel occlusion of the anterior circulation can be improved further by by better reperfusion through modification of periprocedural antithrombotics, anesthesia type and blood pressure.
We found that administration of antithrombotics in the form of aspirin and heparin early after EVT were associated with an increased intracranial hemorrhage risk and should be avoided. Furthermore, we found that local anesthesia at the groin only was associated with better outcomes when compared to patients receiving conscious sedation during EVT. Finally, we concluded that maintaining blood pressure as stable as possible during EVT was associated with better outcomes in comparison to patients in which this was not the case.


Marion Smits is an internationally active Neuroradiologist who combines clinical work with scientific research. She also holds an honorary appointment at the University College London Hospitals NHS Foundation Trust, London/UK.
Marion studied Medicine at Maastricht University and worked as a junior doctor in the United Kingdom before specializing as a Neuroradiologist at Erasmus MC. She obtained her PhD cum laude from Erasmus University Rotterdam in 2008.

Marion is president of the the Dutch Society of Neuroradiology, chair of the Imaging Group as well as the Brain Tumor Group Imaging Subcommittee of the European Organisation for Research and Treatment in Cancer, and active in key national and international organizations. She currently serves on the ESR Executive Council as chair of the publications committee. She is very active within the Medical Delta, being member of its scientific board. marion.smits@erasmusmc.nl
APPLIED PHYSIOLOGICAL NEUROIMAGING
MARION SMITS, MD, PHD
full professor

Context
This research line is focused on the human brain’s function and (micro)structure under physiological and particularly under pathological conditions. Physiological and functional MR neuroimaging techniques are uniquely suited to study the human brain in vivo. These techniques include functional MRI (fMRI), diffusion and perfusion MR imaging. The clinical applicability of these various imaging techniques and their findings are an important aspect of this research line. The research is performed in a continuous interplay between fundamental imaging research and clinical practice, with a primary focus on Neuro-Oncology. This means that there is a close collaboration with clinically as well as technically oriented researchers, in particular within the Erasmus MC Brain Tumor Center and the Medical Delta.
Top Publications 2021
Smits M. MRI biomarkers in neuro-oncology . Nat Rev Neurol 2021 Jun 20. doi: 10.1038/s41582021-00510-y.
Weller M, Van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, Bendszus M, Balana C, Chinot O, Dirven L, French P, Hegi ME, Jakola AS, Platten M, Roth P, Rudà R, Short S, Smits M , Taphoorn MJB, Von Deimling A, Westhphal M, Soffietti R, Reifenberger G, Wick W, for the EANO Task Force on Diffuse Gliomas. European Association of Neuro-Oncology (EANO) guideline on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol 2021;18:170-186.
Van Garderen KA, Van der Voort SR, Versteeg A, Koek M, Gutierrez A, Van Straten M, Rentmeester M, Klein S, Smits M. EASE: Clinical implementation of automated tumor segmentation and volume quantification for adult low-grade glioma . Front Med 2021;8:738425.
Research Projects: Objectives & Achievements
Clinical validation
Physiological neuroimaging techniques are developed by the MRI Physics group led by Prof. Juan Hernandez Tamames (pages 42). Further technical development is achieved through intense collaboration within the Medical Delta, both with the TU Delft (Prof. S. Stallinga) and the MRI physics group at LUMC (Prof. M.P.J. van Osch). Such techniques are explored for their potential to provide imaging markers of disease, within the research line led by Dr. Esther Warnert (page 248). Here, we focus on the transition from these (technical) labs to clinical practice.
One such novel techniques is chemical exchange saturation transfer (CEST). It is still mostly in the preclinical research stage. Together with the King’s College London (Prof. Gareth Barker, Dr. Tobias Wood) we implement amide proton transfer (APT)-CEST in clinical practice and imaging genomics studies. We furthermore validate measurements of APT signal with tissue analyses, using histopathology and proteomics, in collaboration with Prof. Max Kros and Dr. Theo Luider (page 253).
Quantitative physiological MR imaging of the brain is of great interest both for research and clinical applications, and is becoming a realistic possibility with the availability of several imaging sequences with (clinically) acceptable scanning times. Quantitative measures of brain structure and function allow for reference values to distinguish normal from abnormal conditions, followup studies sensitive to subtle changes over time, and the exchange or pooling of data across centers. Arterial spin labeling (ASL) is one such quantitative imaging techniques, having shown to produce robust cerebral blood flow (CBF) measurements in single center group studies. Implementation into multicenter studies seems to be the next step, which may eventually lead to the use of ASL as a clinical biomarker. Other techniques that are being developed and assessed in a clinical environment are other vascular imaging markers (page 252), tissue relaxation measurements and MR fingerprinting approaches.
The clinical implementation and value of existing imaging techniques also requires scientific scrutiny, especially where there is heterogeneity and variation throughout hospitals. For instance, there is a wide variety in the use of perfusion MRI for brain tumor diagnosis and follow-up, even within The Netherlands. In the PERISCOPE project (pages 245 and 283) – a health care evaluation project funded by Leading the Change – we assess the value of perfusion MRI from a clinical and cost-effectiveness perspective in a large multicenter observational study.
Diagnostics
For diagnosis, imaging genomics of brain tumors has gained substantial relevance with the classification of brain tumors by the World Health Organization – updated this year – which relies heavily on tumor genetics. The non-invasive assessment of tumor genotypes is important for treatment decisions and follow-up, and the focus of the iGENE project (pages 246 and 247). We develop and use physiological MR imaging and advanced post-processing techniques for imaging genomics of low grade glioma in a multicenter setting, with advanced image analysis techniques developed by the Biomedical Imaging Group Rotterdam (Prof. W.J. Niessen, Dr. S. Klein). This multidisciplinary project is performed in close collaboration with the departments of Neurology (prof. Martin van den Bent), Neurosurgery (Prof. CD. Dirven, Prof. S. Leenstra, Dr. A.J.P.E. Vincent, Dr. J.J. Schouten), as well as the MC Haaglanden (dr. G. Lycklama a Nijeholt, Prof. M. Taphoorn), University Medical Center Utrecht (Prof. J. Hendrikse, Prof. P. Robe), and Elisabeth-Tweesteden Hospital in Tilburg (Dr. G.J. Rutten, Dr. I. Boukrab).
Tumor genomics are not only relevant for initial diagnosis but also for the changes occurring in virtually all adult glioma, resulting in malignant transformation and treatment resistancy. The longitudinal assessment of tumor genomics in glioma is the focus of the international Glioma Longitudinal ASSessment (GLASS) consortium. The Dutch section (GLASS-NL) of this consortium is led by Prof. P. Wesseling, Pathologist at Amsterdam UMC, and involves the participation of all neuro-oncological centers in the Netherlands. GLASS-NL (page 243), together with Dr. S. Bakas (University of Pennsylvania), is taking the lead in adding imaging to this initiative, working towards the so-called iGLASS section of the consortium.
Surrogate markers
Especially in the context of newly developed treatments, accurate diagnosis and response assessment is of the utmost importance. In patients with brain metastases from melanoma, we investigate novel MRI techniques and PET tracers, exploiting the combined imaging technology the PET-MRI scanner offers (pages 188 and 195). One target of interest is prostate specific membrane antigen (PSMA), which is overexpressed not only in prostate cancer, but also in highly aggressive brain tumours such as glioblastoma and brain metastases. The combined assessment of physiological MR imaging and PSMA on the PET-MRI scanner is expected to provide insights into tumor biology and targets for treatment (pages 244 and 254).
Furthermore, through collaboration with the European Organization for Research and Treatment of Cancer (EORTC) imaging markers of outcome after treatment are investigated.
Image guidance for invasive tumor treatment
Brain tumor treatment through surgery or targeted radiation therapy aims to balance maximal reduction of tumor burden versus minimal damage to eloquent brain structures. Thus, both precise tumor delineation and reliable identification of functionally important tissue at risk is required pre-treatment. Several imaging techniques are developed and evaluated for this purpose.
With functional MRI (fMRI) we aim to gain insight in motor, language, and memory processing both under physiological and pathological conditions. With the department of Neurosurgery we study the effect on language and cognition of brain tumors and tumor surgery (Dr. F. Incekara, Dr. D. Satoer, Dr. A. Vincent, Prof. C. Dirven). Novel, more quantitative and sensitive fMRI techniques through quantitative susceptibility mapping (QSM) will be developed in the context of the Convergence initiative (lead: Prof. J.A. Hernandez Tamames and Dr. S. Weingärtner from TU Delft). A new collaboration with the functional Ultrasound group within CUBE (lead: Dr. P. Kruizinga) opens opportunities to correlate intra-operative findings with pre-operative fMRI in terms of functional imaging characteristics and validity. Additionally tumor vasularization can be assessed in great detail with so-called microDoppler ultrasound, providing important information for MRI based assessemnt of tumor vascularization (page 242).
In a broad collaboration of brain tumor and imaging researchers in the context of proton therapy (HollandPTC) imaging techniques are developed within the RIGEL study (lead: Dr. A. Mendez Romero) to gain insight in radiation induced tissue damage at the micro-architectural level, with the aim to identify differences in tissue sensitivity and provide a proxy measure of long-term cognitive deficits after radiation therapy. As well as from Radiotherapy and HollandPTC, involved Medical Delta researchers are from Leiden University Medical Center (Prof. M.J.P. van Osch) and Delft Technical University (Dr. F. Vos).
Implementation
Through leading roles in European organizations the clinical aspects of imaging protocols and protocol harmonization are assessed and disseminated. Despite the fact that many physiological MR neuroimaging techniques have already been available for decades, some even
being extensively used for fundamental research, their application in patient studies is still relatively limited. Clinical implementation is even less frequent, due to the fact that imaging findings from group studies commonly fail to make the essential transition to the individual patient level. It is the ultimate aim of this research line to provide imaging markers of brain physiology and disease that are directly clinically applicable.
Expectations & Directions
The installation of the hybrid PET-MRI scanner in 2019 has provided a further method for development, validation, and implementation of physiological imaging techniques. Several projects have now been started up (page 244 and 254). The opening of HollandPTC in 2018 has already facilitated collaborations in cancer diagnostics. Further development of Medical Delta projects work towards to non-invasive tumor characterization through imaging is as part of the Cancer Diagnostics 3.0 Program (together with Prof. M.J.P. van Osch and Prof. S. Stallinga) for which funding was obtained from NWO-TTW (page 58).
These multidisciplinary collaborations provide the context for advancing and expanding our current studies, in a continuous interplay between physiological imaging research and highly expert clinical practice. With active position in and connections with the EORTC brain tumor and imaging groups, the ESNR Diagnostic Committee working group on brain tumor imaging, as well the US National Brain Tumor Society, the EU COST Action on Glioma MR imaging (glimr.eu) and the Quantitative Imaging Biomarker Alliance (QIBA) working group on ASL, future efforts are directed at furthering the role of physiological neuroimaging in clinical research on an international level.
Funding
Smits, Marion , Martin van den Bent and Wiro Niessen : Koningin Wilhelmina Fonds 2015-2021: “Non-invasive phenotyping of molecular brain tumour profiles using novel advanced MR imaging and analysis”
Smits, Marion , Anouk van der Hoorn, Jan Willem Dankbaar, Dieta Brandsma, Bas Jasperse, Linda Dirven, Filip de Vos, Myriam Hunink : ZonMW Leading the Change 20182022: “The clinical value of perfusion MRI in primary and secondary brain tumour surveillance”
Smits, Marion, Esther Warnert , Safa Al-Sarraj, Keyoumars Ashkan, Gareth Barker, Martin van den Bent, Thomas Booth, Juan Hernadez-Tamames , Johan (Max) Kros, Theo
Luider, Joost Schouten, Arnaud Vincent, Tobias Wood: The Brain Tumour Charity 2018-2021: “Making the invisible visible: In vivo mapping of molecular biomarkers in adult diffuse glioma with CEST MRI”
Smits, Marion , Thijs van Osch, Sjoerd Stallinga: Medical Delta 2018-2022: “Cancer Diagnostics 3.0: Big data science of in & ex vivo imaging”
Van Osch, Thijs, Frans Vos, Marion Smits, Alejandra Mendez Romero: HollandPTC-Varian 2018-2022: “New methodology for developing biomarkers of radiation-induced toxicity in brain tumour patients based on advanced MR imaging of the microvasculature and white matter microstructure”
Mendez Romero, Alejandra, Martin Taphoorn, Martin van den Bent, Marion Smits , Mischa Hoogeman: HollandPTCVarian 2018-2022: “Improving toxicity modelling, patient selection and clinical outcome of proton therapy in low grade glioma”
Wesseling, Pieter, Johan Kros, Mathilde Kouwenhoven, Marion Smits, Pim French, Mark van der Wiel, Martin van den Bent, Roel Verhaak: Koningin Wilhelmina Fonds 2017-2021: “Glioma Longitudinal AnalySiS in the Netherlands: GLASS-NL”
Warnert, Esther, Radim Jancalek, Lydiane Hirschler, Camille Maumet, Jan Petr, Marion Smits, Patricia Clement, Yelda Özsunar Dayanir. EU COST 2019-2023: “Glioma MR Imaging 2.0: GLiMR2.0.”
Smits, Marion , Thijs van Osch, Dirk Poot, Stefan Klein, Juan Antonio Hernandez Tamames: NWO-TTW Open Technology Programme 2019-2024. “Vascular Signature Mapping of Brain Tumor Genotypes.”
Smits, Marion. NWO Hestia impulse for refugees in science 2021-2023: “The Sound of flow: High-resolution brain tumour vascular signature mapping with mutually informed MRI and intra-operative microDoppler ultrasound”.
Invited Lectures
Van Garderen, K Tumor segmentation workshop . GliMR hybrid networking event, 28 September 2021.
Smits M. Imaging in Neuro-oncology: challenges and opportunities 8 Dec 2021 Martinos Center for Biomedical Imaging, MGH, Charlestown/US, webinar
Smits M. WHO2021 classification for gliomas 29 Nov 2021 RSNA annual meeting, Chicago, IL/US
Smits M. Unknown film interpretation panel 29 Nov 2021 RSNA annual meeting, Chicago, IL/US
Smits M. ASL, DSC, or DCE for brain tumour imaging? 14 Nov 2021 joint ESNR-ESMRMB course on advanced neuroimaging techniques, online
Smits M. Brain tumours: essentials for clinical practice 4 Nov 2021 European School of Radiology (ESOR) ASKLEPIOS, online
Smits M. Clinical assessment of treatment response 11 Oct 2021 IPR 2021, Rome/IT
Smits M. What is the value of radiomics in neuro-oncology practice and in new molecular treatments? 1 Oct 2021 ESNR 2021, Geneva/CH
Smits M. Radiogenomic approaches and advances: towards a virtual biopsy? 1 Oct 2021 ESNR 2021, Geneva/CH
Smits M. Focus on biomarkers in common diseases 19 June 2021 EAN 2021, online
Smits M. Money, money, money May 2021 ISMRM 2021, online
Smits M. Radiomics in glioma grading April 2021 Hot Topics Brain Tumors, online
Smits M. Consensus recommendations, standardized imaging protocol and structured report April 2021 Hot Topics Brain Tumors, online
Smits M. Launching a biomarker successfully into clinical practice 7 March 2021 ECR 2021, online
Smits M. How do I share my trial data 5 March 2021 ECR 2021, online
Smits M. Neuroradiology education: Tumours on demand 2021 ECR 2021, online
Smits M. Radiological imaging 9 Feb 2021 Cancer Drug Development Forum, online
Highlights
Karin van Garderen introduced her tumor segmentation pipeline ‘EASE’ into the clinical routine.
Marion Smits was named ‘Most influential radiology researcher’ by AuntMinnie Europe.
Marion Smits participated in the RSNA unknown film interpretation panel at the 2021 RSNA annual meeting.
Sebastian van der Voort obtained his PhD cum laude.
Sebastian van der Voort won the YOUNG Medical Delta award.
Sebastian van der Voort won a Convergence Health and Technology Open Research Award.
Additional Personnel
C. Tseng, PhD student (TU Delft)
D. van Dorth, PhD student (LUMC)
A. Lavrova, ESR Bracco fellow (St. Petersburg, Russia)
Z.S. Erdal, visiting researcher (Ankara, Turkey)
M. Rosbergen, MSc student (Clinical Technology TU Delft)

THE SOUND OF FLOW: COMBINED MRI & MICRODOPPLER ULTRASOUND OF GLIOMA VASCULATURE
AHMAD ALAFANDI, MD
PhD Student
Advisors Marion Smits & Pieter Kruizinga
Project Funding
Hestia Impulse
Research period September 2021 – September 2023
Email a.alafandi@erasmusmc.nl
This project is a collaboration between the Department of Radiology & Nuclear Medicine and CUBE at Erasmus MC, LUMC and TU Delft.
Primary brain tumor, most commonly glioma, are generally fatal and carry a high burden of disease. Outcome varies widely depending on tumor grade and genetic/molecular profile where distinct tumor molecular profiles are associated with specific vascularization patterns so called ‘vascular signatures’. Our research team at the Medical Delta (collaboration between Erasmus MC and Leiden UMC) focuses on revealing the vascular signature of brain tumors through non-invasive magnetic resonance imaging (MRI) within the ongoing project ‘vascular signature mapping’ (VSM).
The MRI-based VSM technology fits a vascular architecture model to the acquired MRI- signals. This model uses advanced image reconstruction techniques and statistical assumptions about the tumor vascular bed to visualize the small capillaries in order to overcome the insufficient spatial resolution of MRI. These assumptions are
likely to be inaccurate due to the lack of in-vivo information. In my project and thanks to a newly in-house developed technology – high frame rate μDoppler ultrasound – by the Center of Ultrasound and Brain Imaging at Erasmus (CUBE), we are now for the first time able to close the loop between theoretical modelling and in vivo validation of the MRI-VSM models, by incorporating intra-operative in-vivo μDoppler into the MRI-VSM project. The μDoppler images provide unprecedented information about the in-vivo architecture of the tumor vascular bed and expose the brain tumor’s blood flow dynamics in exquisite detail.
Utilizing these ultrasound images, vascular characteristics will be obtained and quantified such as vessel length, diameter, tortuosity, etc. by automatically segmenting and annotating the tumor vessels. These will be implemented as ground truth information to validate and optimize the MRI VSM prototypes in a continual improvement process, eventually resulting in noninvasive MRI based technology to determine the tumor vascularity. This will eventually lead to better insight into the tumor molecular profile and expected biological behavior prior to surgery, necessary for optimally informed, patientcentered decision making.

Figure 1. Combining MRI with intra-operative microDoppler ultrasound (Soloukey et al. Front Neurosci 2019) to obtain vascular signature maps.

LONGITUDINAL ANALYSIS OF LOW-GRADE GLIOMA
KARIN VAN GARDEREN, MSC
Student
Advisors Marion Smits & Stefan Klein
Project Funding KWF EMCR 2017-11026 : Glioma Longitudinal AnalySiS in the Netherlands (GLASS-NL)
Medical Delta Cancer Diagnostics 3.0
Research period July 2018 – September 2022
Email k.vangarderen@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine, Neurology and Pathology and the GLASS-NL consortium.
Much is unknown about the development of lowgrade glioma, whether spontaneously or in relation to therapy. Some tumors remain stable for many years, while others quickly progress to a more malignant type. The latter cases would likely benefit from aggressive treatment while a conservative treatment plan may be better for the former. Despite surgical resection and treatment, all adult low-grade glioma do eventually return in a more malignant form, and generally they become unresponsive to therapy.
The GLASS-NL consortium (led by Prof. Pieter Wesseling, Amsterdam UMC) was initiated to investigate the evolution of low-grade IDH-mutant glioma under pressure of therapy, as a collaboration between all major neuro-oncological centers in the Netherlands. Patients are included that have had at least two tumor resections, so that we can establish the genetic variations that occur over time from tumor tissue obtained at these two time points. The final goal is to establish biomarkers that indicate malignant progression and provide insight to guide (timing of) therapy. Whereas the molecular analysis of material is limited to the moment of resection, imaging is available on a regular basis for all patients. This means that we can monitor the tumor between resections and search for evidence of malignant progression in a noninvasive way.
The main challenge in this project is to deal with the large amount of (imaging) data, and the heterogeneity of multi-center data. We need efficient tools to extract quantitative metrics for longitudinal analysis, and tumor segmentation is the first step towards these metrics. We have now developed a robust pipeline for the sorting, processing and automatic segmentation of longitudinal MR imaging. From a range of segmented and co-registered images, we can then extract valuable information about the local growth and invasion of the tumor.

Figure 1. Examples of glioma lesions and their segmentation, all in sagittal plane. Left: low-grade glioma after resection (T2wFLAIR). Right: typical example of malignant progression, visible as a large enhancing lesion (T1w+contrast). Below: segmentations of non-enhancing parts of the lesion (red) and contrast enhancement (green) that is typical for malignant progression.
PhD

ILANAH J PRUIS, MSC THER ANOSTIC PET-MRI FOR CNS TUMORS
PhD Student
Advisors Marion Smits & Sophie Veldhuijzen van Zanten
Project Funding Stichting Semmy
Research period October 2019 – September 2023
Email i.pruis@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine, Neurology, Neurosurgery and Pathology as well as Amsterdam UMC and the Princess Máxima Center for Pediatric Oncology.
For tumors located in the central nervous system (CNS), non-invasive tools to study cancer biology in vivo are highly appreciated. In recent years, several advanced MRI sequences, as well as nuclear imaging techniques like Positron Emission Tomography (PET), have been developed that enable minimally invasive studies into the molecular biology of tumors. I study the link between advanced MR imaging, molecular diagnosis and possibilities for molecular targeted therapy by application of novel theranostic PET tracers, which conjoin both diagnostic and therapeutic applications.
We first performed an extensive review of the literature and listed all tracers that are currently used for diagnostic and theranostic purposes in CNS tumors. We concluded that PET imaging can serve as a valuable tool to non-invasively study therapeutic efficacy and to ensure greater patient safety of targeted treatment strategies (doi: 10.3390/ijms21031029).
Based on this, we designed a first proof-of-concept PETMRI study for patients with glioma and CNS metastases aimed at non-invasive quantification of the expression of prostate-specific membrane antigen (PSMA), a possible target for therapy located at the tumor vasculature. PSMA is an interesting target for therapy, since pre-clinical immunohistochemistry studies reported expression of PSMA specifically in tumor vasculature of glioma and CNS metastases, and not in healthy tissue. In our study, [68Ga] Ga-PSMA-11 is used to visualize PSMA expression. In addition, we compare the uptake after intravenous versus local intra-arterial injection to determine the optimal route of administration.

Figure 1. Post gadolinium-based contrast agent T1w MR image (A) and [68Ga]Ga-PSMA-11 PET images 240 min after intravenous (B) and selective intra-arterial injection (C) in a recurrent glioblastoma showing a 6-fold increase in PSMA uptake in C compared to B (SUVmax 109 versus 56). Abbreviations: [68Ga]: gallium-68; PSMA: prostate specific membrane antigen; SUV: standardized uptake value.
Patient inclusion is ongoing and our first results look promising (Figure 1).
If PSMA PET-MRI proves to be successful for CNS tumors, meaning sufficient activity concentrations of the tracer at the tumor site and acceptable accumulation at non-target sites, a next step will be to explore the possibility of PETguided radionuclide therapy.
Also, we assessed the added value of the physiological MRI parameters of perfusion and diffusion for non-invasive differentiation of molecular subtypes in low grade glioma. We found perfusion and diffusion are promising measures for prediction of prognosis which could aid clinical decision making in the future. This work is submitted for publication.

Advisors
WOUTER TEUNISSEN, MD, MSC PERISCOPE PROJECT
PhD Student
Marion Smits, Myriam Hunink, Anouk van der Hoorn & Linda Dirven
Project Funding ZonMw Zorgevaluatie Leading the Change
Research period May 2019 – May 2023
Email w.teunissen@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine, Neurology, Radiation Therapy and Epidemiology.
The management of patients with a brain tumor is hampered by several diagnostic challenges, resulting in uncertainty with both patients and health care providers. This may lead to suboptimal treatment decisions and a high surveillance rate with MRI scanning. These challenges are related to two important aspects that guide brain tumor management: 1. the assessment of tumor grade and 2. the distinction between tumor progression and treatment induced abnormalities.
Perfusion MRI (pMRI) is a technique to assess tumor grade (untreated low grade glioma) and to distinguish between tumor progression and treatment induced abnormalities (treated glioma and brain metastasis). Unfortunately there is no consensus on the use of pMRI for brain tumor surveillance in the Netherlands. Therefore we designed a study to obtain the necessary evidence to provide clear guidance on the cost-effective implementation of pMRI for brain tumor surveillance throughout the Netherlands.
We are performing a multicenter (N=15) observational prospective study in which we evaluate the use of pMRI in relation to brain tumor surveillance including treatment decisions and a longitudinal follow-up of quality of life. A retrospective cohort (2008-2018) is added to this group to complement the prospective data.
Our aim is to assess the effect of the use of pMRI in patients with glioma and with brain metastasis in terms of cost-effectiveness and QALYs through decision modelling and to eventually provide clear evidence for implementation in national guidelines. The first preliminary results indicating the impact of pMRI on clinical decision making were presented at the ESNR 2021 in Geneva.

At the end of 2021 we included a total of 300 patients prospectively and over 1,000 retrospectively.
Besides the PERISCOPE project we have started a retrospective study on the correlation between ASL and DSC perfusion MRI in patients with a brain tumor. The first results of this study are submitted for publication.
Figure 1. This figure shows overlapping ROC curves of ASL-CBF, DSC-rCBV (Intellispace Portal) and DSC-rCBV (IB Neuro).

IMAGI NG AND RESECTION OF GLIOBLASTOMA
IN
LIGHT OF MOLECULAR MARKERS
FATIH INCEKARA, MD, PHD
PhD Awarded 10 MARCH 2021
Advisors Marion Smits , Martin van den Bent & Arnaud Vincent
Project Funding KWF project number EMCR 2015-7859
Short CV Fatih studied medicine at the Erasmus MC (2009-2015). In 2016, he started his PhD training and he completed programmes in clinical epidemiology (Master of Science, NIHES) and philosophy (Bachelor of Arts, Erasmus University Rotterdam).
This project is a collaboration between the Departments of Radiology & Nuclear Medicine, Medical Informatics, Neurology, and Neurosurgery
This thesis contributes to our knowledge on the value of imaging and resection of glioma, in particular glioblastoma. We presented promising results on molecular predictions based on preoperative MRI scans using artificial intelligence. I showed in a randomized controlled trial that intraoperative ultrasound enables complete tumor resection more often than standard glioblastoma surgery, without harming patients. We recommend intraoperative ultrasound guided surgery, rather than standard surgery, to achieve complete safe tumor resection. I found that augmented reality was potentially useful to visualize and locate glioblastoma for neurosurgical planning on the operating room.
Complete glioblastoma resection was already associated with improved patient survival. We confirmed this association in molecularly defined IDHwt glioblastoma. We externally validated these results in a multi-center glioblastoma cohort and predicted survival of patients with a glioblastoma with nomograms. In addition, we found that resection of non-contrast enhanced tumor beyond contrast enhancement or supratotal resection showed potential survival benefit for patients with glioblastoma. We encourage further research on the safety of maximizing glioblastoma resection, especially beyond the borders of contrast enhancement.
Finally, we shared future perspectives, in which we expect that new imaging technologies and artificial intelligence will improve how we understand, diagnose and surgically treat patients with brain tumors. We hope that this thesis will be of benefit for future brain tumor research, clinical care, and for patients with a brain tumor.

http://handle.net/1765/134871


EYE TO AI: MR IMAGE ANALYSIS OF GLIOMA USING MACHINE LEARNING
SEBASTIAN R VAN DER VOORT, MSC, PHD
PhD Awarded 26 October 2021 *** CUM LAUDE ***
Advisors Marion Smits, Wiro Niessen, & Stefan Klein
Project Funding KWF project number EMCR 2015-7859
Short CV Sebastian van der Voort obtained his Bachelor of Applied Physics at the Delft University of Technology in 2013. In 2015 he obtained his Master of Applied Physics, cum laude, at the same university. During his PhD he was active as a member of Promeras, the PhD representatives of the Erasmus MC, and YOUNG Medical Delta, an organization that promotes the opportunities of clinical technology to a broader audience. He’s currently continuing his work on the use of machine learning methods for the analysis of glioma as a postdoctoral researcher at the Erasmus MC.
This project is a collaboration between the Departments of Radiology & Nuclear Medicine, Medical Informatics, Neurology and Neurosurgery.
In recent years, automated image analysis and machine learning methods have had a significant impact on biomedical imaging research. At the same time, research on glioma has revealed several genetic features that drive the aggressiveness of these tumors. These two developments have popularized the field of radiomics, where imaging features are extracted from biomedical images and correlated with the clinical characteristics of a tumor. This thesis combines the latest insights from clinical and radiomics research, focusing on the automated image analysis of glioma magnetic resonance imaging (MRI) scans.
In the thesis it was found that using automated image analysis methods it is possible to predict clinically relevant tumor characteristics without the need for a biopsy or resection. For the development of a radiomics method, large, diverse datasets are crucial. To overcome the issue that clinical datasets are often unstructured a method was developed as part of this thesis to predict the type of brain MRI scans and to automatically sort the data based on the predicted type, providing a tremendous speed-up compared to the manual sorting of these datasets. This method was then used to create the largest, most diverse glioma MRI dataset to date. This dataset –the Erasmus Glioma Database (EGD) – has been made publicly available and contains imaging data, genetic and histological features, and tumor segmentations of 774 patients with glioma.

Although there are still hurdles to overcome before automated image analysis and machine learning methods will be commonplace in the clinic, there is a clear potential for these methods to contribute to the diagnosis and treatment of glioma in a significant way.
http://hdl.handle.net /1765/135719

Esther Warnert is a medical engineer and Assistant Professor at the Department of Radiology & Nuclear Medicine. As a full-time researcher she is Principal Investigator of her research line “Benchto-bedside MR Imaging Biomarkers” in which she focusses on development and translation of novel MRI techniques to assess the brain’s physiology for clinical application.
Esther obtained her MSc degree in medical engineering from the Technical University of Eindhoven in 2011. In 2015 she obtained her PhD degree from Cardiff University (Cardiff, United Kingdom) for her research into the development

of non-invasive cerebrovascular MRI measurements.
Esther is chair of “Glioma MR Imaging 2.0”, an international network funded by the European Union’s Cooperation in Science & Technology programme, Junior Fellow of the International Society of Magnetic Resonance in Medicine, where she also is part of the Task Force for Equality, Diversity, and Inclusivity, and board member of the Dutch Neuro-Oncology Society Investigators (LWNO-I) and VENA, the network for women in academia at the Erasmus MC. e.warnert@erasmusmc.nl
BENCH-TO-BEDSIDE
MR IMAGING
BIOMARKERS
ESTHER AH WARNERT, IR, PHD
asisstant professor

Context
This research line focuses on translating advanced physiological MRI techniques from the research domain into clinical practice. This includes development, validation, and application of novel MRI biomarkers that assess function and physiology of healthy and pathological brain tissue. Although all imaging biomarkers under investigation in this research line can be applicable in a range of pathologies, glioma currently is one of my major research interests.
Research Projects: Objectives & Achievements
Development & validation: Oxygen delivery
to the brain
Cerebral hypoxia is a devastating pathophysiological state that can occur in a plethora of diseases, including stroke, chronic hypertension, and brain tumours. The consequences of impaired delivery of oxygen to the brain are severe, from increased resistance to therapy in brain tumours such as gliomas to irreparable tissue damage and cell death in stroke. However, despite these dramatic consequences, there currently is no rapid and non-invasive assessment of hypoxia across the whole brain available in the clinic because of the complex interplay of processes involved in oxygen delivery. Physiological models assessing oxygen delivery
Top Publications 2021
Clement P, Booth T, Borovečki F, Emblem KE, Figueiredo P, Hirschler L, Jančálek R, Keil VC, Maumet C, Özsunar Y, Pernet C, Petr J, Pinto J, Smits M, Warnert EAH, “GliMR: Cross-Border Collaborations to Promote Advanced MRI Biomarkers for Glioma”, J Med Biol Eng 41 (2); 115-125 (2021)
Arzanforoosh, F; Croal, PL; van Garderen, KA; Smits, M; Chappell MA; Warnert, EAH;. “Effect of applying leakage correction on rCBV measurement derived from DSC-MRI in enhancing and nonenhancing glioma”, Frontiers in Oncology; 11; 777 (2021).
Warnert EAH, Wood TC, Incekara F, Barker GJ, Vincent AJP, Schouten J, Kros JM, van den Bent MJ, Smits M, Hernandez Tamames JA. “Mapping tumour heterogeneity with pulsed 3D CEST MRI in nonenhancing glioma at 3 T.” Magnetic Resonance Materials in Physics, Biology and Medicine; Online publication, 1-10. (2021)
to tissue are becoming more and more advanced, including measurements of cerebral blood flow (CBF), oxygen extraction fraction (OEF) and macro- and microvascular structure to encompass the process of oxygen delivery. Additionally, I collaborate with Prof. Dr. Marion Smits, Prof. Dr. Matthias (Thijs) van Osch (LUMC), Prof. Dr. Juan Antonio Herandez Tamames, and Dr. Dirk Poot in the supervision of Krishnapriya Venugopal, PhD candidate, on the development of novel MRI approaches to do cerebrovascular signature mapping.
In collaboration with the Department of Neurosurgery (Prof. Dr. Dirven, Dr. Vincent, Dr. Schouten) a pipeline is now operational in which targeted biopsies, guided by the advanced physiological MR images from my research line, are obtained at the start of resection surgery of patients treated for brain tumours. In collaboration with the Pathology Department (Prof. Dr. Max Kros) hypoxia and vessel size measurements done with MRI are matched to their immunohistochemistry counterparts, which is part of Fatemeh Arzanforoosh PhD research (page 252).
I initiated the Oxygen Axis in 2021, which is a collaboration with Dr. Sebastian Weingartner and Dr. Alina Rwei (both from TU Delft), Dr. Marleen de Mul (Erasmus University) and Dr. Samy Abo Seada (department of Radiology) with the aim to match the MRI-based measurements of oxygen delivery to the brain to the equivalent measured with a wearable device. Ultimately, this may lead to reduced burden for the patient when monitoring oxygen metabolism of the brain is of importance.
Development & validation: Protein measurements in the brain
Malignant transformation occurs in practically all low grade diffuse gliomas. The processes leading up to tumour progression, and ultimately malignant transformation, are invisible with conventional magnetic resonance imaging (MRI) techniques used in routine clinical practice, which are mostly focused on obtaining structural T1- and T2weighted images rather than on accurately mapping in vivo glioma biology. Hence, malignant transformation is usually established at a late stage. As aggressive cell proliferation and migration are underlying tumour growth and vessel formation, biomarkers of these processes can be used for early detection of tumour progression, eventually leading to malignant transformation. Chemical Exchange Saturation Transfer (CEST) imaging is a novel MRI technique with great potential for measuring molecular biomarkers of cell proliferation and migration within gliomas.
In collaboration with King’s College London (Dr. Tobias Wood, Prof. Gareth Barker), CEST MRI was optimized and implemented in 2021. Validation of this measurement with targeted biopsies of glioma is currently on its way in collaboration with the Department of Neurology (Prof. Dr.
Theo Luider and Dr. Lennard Dekker), where biomarkers from CEST MRI are being matched with state-of-the-art proteomics measurements of targeted biopsies of brain tumours.
Development: GlucoCEST on the PET-MRI
The collaboration with Dr. Tobias Wood has progressed into the development of GlucoCEST MRI on the hybrid PET-MRI system. GlucoCEST is a potential substitute for FDG-PET for the assessment of glucose metabolism. A study led by Dr. Astrid van Veldt and in collaboration with Prof. Dr. Marion Smits. In 2021 we developed the required image acquisition protocol and analysis pipeline for dynamic glucoCEST measurements (page 195). Translation of this technique for use in patients diagnosed with brain metastases of melanoma is ongoing.
Application: Perfusion measurements in health and disease
In 2021, the first steps of translating advanced MRI biomarkers of oxygenation and protein content into the treatment planning and follow-up of patients diagnosed with glioblastoma and treated with radiotherapy have been made. In collaboration with Dr. Alejandra Mendez-Romero and Drs. Jaap Jaspers from the Department of Radiotherapy at the Erasmus MC integration of biomarkers resulting from advanced MRI techniques in radiation therapy planning in patients with glioblastoma has been done by Patrick Tang, for his final year MSc thesis in Technical Medicine.
Within this research line there also is focus on translating advanced perfusion techniques into application. In collaboration with Prof. Dr. Meike Vernooij, Dr. Daniel Bos and Dr. Esther Bron, a first analysis was performed on ASLbased perfusion imaging in the Rotterdam Scan Study. In collaboration with Prof. van der Lugt and Sven Luijten, PhD candidate, multi post-label delay ASL is applied to investigate the cerebrovasculature in patients recovering from stroke.
International collaboration: Glioma MR Imaging 2.0
A powerful tool for advancing imaging diagnostics and bringing new MRI biomarkers towards clinical application is connecting researchers and clinicians. The European network “Glioma MR Imaging 2.0” (www.glimr.eu) is doing just that, via hosting virtual, hybrid and onsite meetings and network events. It brings together over 200 researchers and clinicians from 30 countries and is still open to new members.
Expectations & Directions
In 2022, the focus will be on consolidating the validation of advanced MRI biomarkers in patients diagnosed with brain tumours, further development of novel imaging approaches to assess cerebral oxygenation status and advancing the use of novel MRI-based biomarkers of physiology in clinical practice. Key examples are that I am currently working on the design of a multi-centre, multi-vendor clinical trial for the use of CEST MRI for early detection of tumour progression after treatment and that I am focusing on the translation of oxygen metabolism measurements from the brain to the abdomen.
Funding
Esther Warnert, Sebastian Weingartner, Alina Rwei, Samy Abo Seada, Marleen de Mul. Open Mind Call, Convergence Erasmus MC, EUR, TU Delft. “The oxygen axis: breathing air into a collaboration for patient-centred functional monitoring with the O2-Sense”
Esther Warnert, Alejandra Mendez-Romero, Erasmus Trustfonds, “Piloting the use of physiological MRI for precision radiotherapy of IDH-wildtype glioblastoma”
Marion Smits, Esther Warnert, Safa Al-Sarraj, Keyoumars Ashkan, Gareth Barker, Martin van den Bent, Thomas Booth, Juan Hernandez-Tamames, Johan (Max) Kros, Theo Luider, Joost Schouten, Arnaud Vincent, Tobias Wood: The Brain Tumour Charity 2018-2020: “Making the invisible visible: In vivo mapping of molecular biomarkers in adult diffuse glioma with CEST MRI”
Esther Warnert, NWO Veni 016.196.121, 2019-2021:” Food for thought – Oxygen delivery to the brain”
Esther Warnert, Radim Jancalek, Lydiane Hirschler, Camille Maumet, Jan Petr, Marion Smits, Patricia Clement, Yelda Özsunar Dayanir. EU COST Program 2019-2023: “Glioma MR Imaging 2.0: GliMR2.0.”
Invited Lectures
Warnert EAH, “MRI en veiligheid”, Opleiding tot algemeen coördinerend stralingsdeskundige, 15 nov 2021
Warnert EAH, “DSC/DCE: pitfalls, tips & tricks”, European Course on Advanced Imaging Techniques in Neuroradiology, 14 Nov 2021
Warnert EAH, “Can advanced imaging techniques aid early diagnosis of true brain tumour progression after treatment?”, Educational Day of the European Association of Neuro-Oncology, Oct 2021
Warnert EAH, “Going beyond structure: Advancing physiological MRI in glioma diagnostics”, March 2021, Anne Klibanski Visiting Lecture Series, Massachusetts General Hospital, USA https://learn.partners.org/courses/6389/
Highlights
Fatemehsadat Arzanforoosh was invited as a tutor during a Member-Initiated Symposium of the 29th annual meeting of the International Society of Magnetic Resonance in Medicine entitled “Hands-On Analysis of Physiological MRI: DSC MRI II”.
Esther Warnert was committee member of the Equality, Diversity and Inclusivity Task Force of the International Society of Magnetic Resonance in Medicine and VENA (the women in Academia network at the Erasmus MC).
Esther Warnert was elected to be one out of 8 international speakers invited to give a lecture within the Anne Klibanski Visiting Lecture Series of Harvard’s Massachusetts General Hospital.
Esther Warnert, was guest editor of the annual Special Issue of Magnetic Resonance in Medicine, Physics, and Biology entitled “Micro- to Macro-scale magnetic resonance imaging of glioma”
Esther Warnert, together with Patricia Clement (Ghent University, Belgium) developed an information video for patients and caregivers about medical data sharing in accordance with current data privacy regulations. https://glimr.eu/ gdpr-video/
Additional Personnel
Rick Bezemer – BSc student Life Sciences & Chemistry, InHolland Amsterdam, final-year project.
Karina Hoefnagel – BSc student in Psychobiology, University of Amsterdam, final-year project
Mathijs Rosbergen – MSc Student Technical Medicine, TU Delft, final-year project
Patrick Tang – MSc Student Technical Medicine, TU Delft, final-year project
Maaike van der Velden – MSc Student Biomedical Sciences – Neurobiology, University of Amsterdam, final-year project
Krishnapriya Venugopal – PhD candidate, Department of Radiology & Nuclear Medicine, Erasmus MC

A NOVEL MRI FRAMEWORK FOR ASSESSING HYPOXIA
FATEMEHSADAT
ARZANFOROOSH, MSC
Advisors Marion Smits & Esther Warnert
Project Funding NWO Veni 016.196.121: Food for thought – Oxygen delivery to the brain
Research period January 2019 – December 2022
Email f.arzanforoosh@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine, Neurology, Neurosurgery, and Pathology.
The aim of my PhD is to create and validate a new and clinically applicable framework for magnetic resonance imaging (MRI) measuring oxygen delivery to the human brain that can be applied in patients with glioma. One requirement needed to accomplish this goal is to find a reliable technique being able to measure vessel size in glioma. In the previous research I stablished a pipeline for accurate measurement of vessel density and vessel size with VSI (vessel size imaging) technique, considering contrast agent leakage happening in high grade glioma. In this study, I used this pipeline and measured vessel size, vessel density and rCBV (relative cerebral blood volume) to characterize three subtypes of glioma based on the 2021 World Health Organization (WHO) brain tumor classification scheme.
A retrospective dataset consisting of 38 patients with confirmed non-enhancing glioma was used and categorized in three subtypes: Oligo (IDH-mut&1p/19q-codeleted), Astro (IDH-mut), and Glioblastoma (IDH-wt). All patients underwent 3T MRI scanning prior to surgery with a scan protocol including high-resolution structural MRI, three b-values diffusion, and hybrid echo planar imaging (HEPI) to do VSI. In-house code was used for image analysis to obtain vessel size, vessel density and rCBV maps. The average values of these maps across the tumour were determined for each patient separately and then compared across the three glioma subtypes.
The average vessel size for the three subtypes indicated that in Astro tumour vessels were significantly larger compared to Oligo (p=0.02), while in this type of tumour the vessel density was significantly lower compared to both Oligo (p=0.01) and Glioblastoma (p=0.02). Moreover, rCBV appeared to be significantly lower in Astro
compared to Oligo (p=0.001). No significant difference was found between Oligo and Glioblastoma, in any of the three parameters.
The results of this study suggest that each of the vessel features alone could not significantly distinguish between the three WHO 2021 adult glioma subtypes, in this dataset. However, the combination of the vessel parameters provides valuable insight into the microvasculature characteristics of the three glioma subtypes.

Figure: A) Single slice of exemplary MRI image of T2W FLAIR and obtained maps of vessel size, vessel density, rCBV for a patient diagnosed with Oligo. B) Scatter plot of vessel size, vessel density, rCBV; with mean bar for three subtypes of gliomas. * Significantly different, p < 0.05.
PhD Student

YULUN WU, MSC IN VIVO MAPPING OF BIOMARKERS IN ADULT DIFFUSE GLIOMA WITH CEST MRI
Advisors Marion Smits & Esther Warnert
Project Funding The Brain Tumour Charity (GN-000540)
Research period December 2018 – November 2022
Email y.wu@erasmusmc.nl
This project is a collaboration of the Departments of Radiology & Nuclear Medicine and Neurology, Neurosurgery, and Pathology at Erasmus MC and with King’s College London (UK).
Dynamic glucose enhanced (DGE) CEST is being considered for the advanced characterization of brain lesions in which glucose is of interest. Since glucose has off-resonance peaks between 1-4 ppm, the exact off-resonance frequency used for DGE varies. Additionally, the effect size of glucose enhancement, in particular in healthy tissues at 3T is small. To address these issues, we investigated a set-up and analysis pipeline for DGE CEST on a hybrid 3T PET/MR system in which we tested the use of two different offsets (1.2 and 2 ppm) and extended the analysis pipeline to include dynamic B0 correction and principle component analysis (PCA) to improve DGE detection.
This research was under approval of the institutional ethics committee of the Erasmus MC. A 3T PET/MR scanner with a 24 channel head coil (General Electric, Chicago, USA) was used. Static CEST scans were performed using snapshot acquisition. 10 healthy volunteers (M/F=3/7, aged 19-31 y) and one patient (female, 55y) with brain metastases were recruited. Two static scans (baseline and post glucose infusion) were performed, between which DGE scanning was acquired. Dynamic B0 correction was applied, after that, the first 3 components of principle component analysis (PCA) were obtained to reduce the noise of time course. Regions of interest (ROI) were created for white matter (WM), CSF, sagittal sinus (SS) and tumor for human studies. Glucose contrast enhancement (GCE) was calculated. Subsequently, GCE of each time point was averaged in ROIs for further comparison.
In the healthy volunteers, the increase in glucose level in blood was 2-9 mM. Visual inspection of Figure 1 illustrates that the combination of mcflirt and PCA reduced noise due to head motion (orange arrows), and dynamic


Figure 1: The improvement of image quality in steps, in one slice of a single healthy volunteer; axial view of T1 post-contrast image (A), GCE map at 2ppm (B) in one brain slice of the patient that included brain metastasis. The mean GCE in contralateral WM, CSF, SS (2ppm) and in tumor (1.2/2ppm) as a function of time showed in (C).
B0 correction recovered GCE in the frontal lobe (red arrows) in line with previous work. In the brain metastasis located in the left frontal lobe, the detected GCE (2ppm) in tumor region was in correspond to hyper-intensity in T1 post contrast image and was higher than in healthy tissues (2ppm), reaching a difference of approximately ~5% on average during the last 3.3 minutes of dynamic scan. It also showed a larger effect size than in the tumor region under 1.2 ppm. (Figure 2)
Despite small tumor ROI, this works illustrated the potential to detect brain metastases with DGE at 3T. In the future, we will scan more patients to develop DGE CEST including comparison to FDG-PET in detecting brain metastases in patients.
Sophie Veldhuijzen van Zanten graduated as MSc in Medicine and MSc in Epidemiology.
She started her scientific career with laboratory research on molecular neuro-oncology at the Dana-Farber Cancer Institute/ Harvard Medical School. She obtained her PhD on a clinical research project focussing on diffuse intrinsic pontine glioma (DIPG), in which she established the Euro-

pean DIPG registry and became an active member of the (European) Society of Paediatric Oncology.
Currently, Sophie is co-promotor of PhD’s in AUMC, Prinses Máxima Center for Pediatric Oncology and Erasmus MC, and is appointed Assistant Professor of the research line entitled “Theranostics of CNS and H&N tumours”.
s.veldhuijzenvanzanten@erasmusmc.nl
THERANOSTICS OF CNS AND H&N TUMOURS
SOPHIE VELDHUIJZEN VAN ZANTEN, MD, MSC, PHD
assistant professor

Context
This research line focuses on the development of novel biomarkers and (localized) therapies for neurological diseases by advancing the principle of “theranostics”. In theranostics, SPECT and PET tracers are used for both diagnostic and therapeutic purposes in procedures known as “molecular radionuclide imaging” and “image-guided targeted therapy”. These techniques provide the unique opportunity to study the molecular biology of diseases non-invasively and quantify the expression of disease-specific targets, at multiple sites, over various points in time. This allows for in vivo diagnosis, staging and longitudinal monitoring of the disease. Theranostics can also be used to study in vivo behaviour of drugs and quantification of their target binding, for instance behind the bloodbrain barrier. This allows for the selection of the most effective (radio)pharmaceuticals, their optimal dosage and administration route, and for the selection of patients most likely to benefit from treatment.
Research Projects: Objectives & Achievements
Primary brain tumours and Brain metastases
A grant from the Dutch association of parents whose children died of, or suffer from, diffuse intrinsic pontine glioma (DIPG) (Stichting Semmy) enabled a first proof of concept study investigating the expression and binding of prostate-specific membrane antigen (PSMA) in adult patients with glioblastoma or brain metastases.
Top Publications 2021
Veldhuijzen van Zanten SEM, Neggers SJCMM, Valkema R, Verburg FA. Positive [18F]fluoroethyltyrosine PETMRI in suspected recurrence of growth hormoneproducing pituitary adenoma in a paediatric patient. Eur J Nucl Med Mol Imaging 49:410-411 (2021)
Veldhuijzen van Zanten SEM, Bos EM, Verburg FA, van Doormaal PJ. Intracranial hemangiopericytoma showing excellent uptake on arterial injection of [68Ga] DOTATATE. Eur J Nucl Med Mol Imaging 48:1673-1674 (2021)
Veldhuijzen van Zanten SEM, El-Khouly FE, Jansen MHA, Bakker DP, Sanchez Aliaga E, Hendrikse NH, Vandertop WP, van Vuurden DG, Kaspers GJL. A phase I/II study of bevacizumab, irinotecan and erlotinib in children with progressive diffuse intrinsic pontine glioma. J Neurooncol 153:263-271 (2021)
In this study, the potential of PSMA-targeted therapy is investigated by correlating tumour uptake heterogeneity of gallium-68 labelled PSMA with (advanced) MR-imaging biomarkers (i.e., gadolinium enhancement, DSC, ASL, DWI and CEST), as well as with immunohistochemical analyses on tumour material obtained by surgery.
The results of this study created the basis for a first proof of principle study to investigate the therapeutic potential of [177Lu]Lu- or [225Ac]Ac-PSMA in patients with glioma, including DIPG. This first-in-human theranostic study has recently been funded by the Erasmus MC FoundationDaniel den Hoed Fund and is expected to commence in the second half of 2022.
Selective intra-arterial administration of radionuclides for CNS tumours
In all of our studies we aim to optimize compound delivery at the target site while minimizing accumulation at nontarget sites, in order to maximize the effect and minimize the side-effects of future therapeutic studies with alpha or beta particle-emitting radionuclides. With this goal in mind, the application of radionuclides via intra-arterial (IA) delivery was very attractive on theoretical grounds, but had never before been captured through imaging. In a first proof of concept study we showed that selective IA administration of radionuclides indeed results in a significant increase in tracer accumulation at the target site compared to intravenous (IV) administration.
Our first clinical case series consisted of patients receiving palliative care for meningioma (MG), glioblastoma (GBM), and brain metastasis (BM) from lung carcinoma. Upon IA administration of either [68Ga]Ga-DOTA-TATE (in MG) or [68Ga]Ga-PSMA-11 (in GBM/BM) tumour to liver uptake ratios increased with respectively a factor 3 in MG, 10 in BM, and 40 in GBM, resulting in SUVmax values that well exceed the threshold for application of radionuclide therapy. These results warrant IA administration of radiopharmaceuticals in these patients and underline the potential of theranostic strategies for the management of central nervous system (CNS) tumours.
Tumours of the larynx, naso-, oro-, and hypo-pharynx, and cancer of unknown primary origin
In this study we aim to investigate the sensitivity, specificity, positive- and negative predictive value of hybrid FDG PETMR imaging for localizing pathology (T/N/M) in patients with suspected tumours of the head and neck (H&N) area and patients with carcinoma of unknown primary origin. For the latter patient population we also aim to introduce a novel method for detection of pathology by [18F]fluoride-labelled
fibroblast activation protein inhibitor ([18F]FAPI) PET-CT. Compared to [18F]FDG, [18F]F-FAPI should show higher accuracy for localizing pathology, owing to high expression of fibroblast-activated protein on cancer cells and low expression in normal human tissues, resulting in a much higher lesion-to-background signal. This project follows upon a request from patient organization “Missie Tumor Onbekend” and will be executed in a recently established multi-center consortium including seven academic and non-academic centers from all over the Netherlands.
Pituitary adenoma
We recently introduced a novel method for detection of micro-adenoma by O-(2-[18F]-fluoroethyl)-L-tyrosine ([18F]FET) PET-MRI. Pituitary micro-adenoma can cause severely disabling symptoms resulting from hormonal dysregulation. In 40% of patients, diagnostic MRI is inconclusive as micro-adenoma by definition are <10mm and not always sufficiently contrasting to normal pituitary tissue. In a first clinical case series of about 25 patients with Cushing’s disease or acromegaly, but prior negative or inconclusive MRI, we showed that hybrid [18F]FET PETMRI has high accuracy for localizing micro-adenoma. The diagnostic yield of this novel imaging technique even showed to exceed that of the current diagnostic methods (i.e., MRI and petrosal sinus sampling).
In our study, nearly 90% of the patients showed focal uptake of [18F]FET on PET, revealing micro-adenomas as small as 2-3mm in size on MRI. Of these patients, 25% underwent transsphenoidal adenomectomy after which pathology confirmed presence of a micro-adenoma, another third currently awaits surgery, and others stabilized on medication. Of the 10% of patients with a negative PETMRI, two-third proved to be true-negative as pathology did not show a micro-adenoma, and one-third had minimal symptoms that did not required therapy.
Expectations & Directions
In my line of research, we aim to demonstrate that the theranostic approach of disease can aid to improve diagnosis, treatment and follow-up, particularly for lesions in the spine and head & neck area. The advancement of theranostics for diseases of the CNS/ H&N area is pioneering. This research line is also unique in its use of the hybrid PET-MRI technology to produce some of the most highly detailed images of anatomy and molecular (patho)physiology that are currently available.
Our [18F]FET PET-MRI study showed superior accuracy for localizing micro-adenoma over conventional diagnostic approaches. The [68Ga]Ga-PSMA study showed significant

T1 post Gd MR image (A) and fused [68Ga]Ga-DOTA-TATE PET-MRI images 60 min after respectively intravenous (B) and selective intra-arterial injection (C) in a meningioma showing a 3-fold increase in tracer uptake (SUVmax 56 vs. 109). Abbreviations: [68Ga]: gallium-68; Gd: gadolinium; DOTA-TATE: DOTA-DPhe1, Tyr3-octreotate; SUV: standardized uptake value.
and likely therapeutically relevant tracer uptake upon selective-IA administration. And the proposed study of [177Lu]Lu- or [225Ac]Ac-PSMA therapy for patients with malignant glioma will constitute a (world) premiere and may offer a welcome novel therapeutic option that could improve survival and quality of life of patients for whom to date we have nothing else to offer.
The PET-MRI guided theranostic approach of CNS/H&N tumours not only holds great promise for personalized patient care, it is also attractive for pharma companies as it may increase the success rates in (radio)pharmaceutical drug development, shorten the time to market, reduce the number of patients needed in clinical trials, and therefore could lead to more cost-effective care and reduction of health-care system costs.
Funding
Sophie Veldhuijzen van Zanten – Young Scientific Talent Award 2022-2026: “Intra-arterial [177Lu]Lu- or [225Ac]AcPSMA for recurrent / progressive malignant glioma”
Sophie Veldhuijzen van Zanten – Personal Grant 20192023: “Intra-arterial administration of radiolabeled (chemo-)therapeutics for (diffuse intrinsic pontine) glioma, monitored by PET-MRI“
Sophie Veldhuijzen van Zanten and consortium partners of the European DIPG Network – Maintenance Grant 20182022: “European DIPG Registry”
Sophie Veldhuijzen van Zanten – Traveling Scholarship 2015: “Working visit to initiate collaborative research projects with the International DIPG Registry”
Sophie Veldhuijzen van Zanten and consortium partners: European DIPG Network – Maintenance Grant 2015-2018: “European registry for diffuse intrinsic pontine glioma: the SIOPE DIPG Registry”
Sophie Veldhuijzen van Zanten and consortium partners: European DIPG Network – Starting Grant 2012-2014: “European registry for diffuse intrinsic pontine glioma: the SIOPE DIPG Registry”
Invited Lectures
Sophie Veldhuijzen van Zanten. Theranostics for central nervous system tumors guided by hybrid PET-MR imaging. 34th Annual Congress of the European Association of Nuclear Medicine, online, (20-23 October 2021)
Sophie Veldhuijzen van Zanten [18F]FET PET-MRI voor de detectie van het hypofyse micro-adenoom. Wetenschappelijke najaarsbijeekomst NVNG, online (26 november 2021)
Sophie Veldhuijzen van Zanten and consortium partners. Update by the Executive Committee of the SIOPE DIPG Registry. DIPG/DMG Collaborative Symposium, Houston, USA, online (5-6 November 2021)
Highlights
As per 2022, Sophie Veldhuijzen van Zanten was granted the academic rank of Assistant Professor of Radiology & Nuclear Medicine.
Sophie Veldhuijzen van Zanten won the Young Scientific Talent Award of the Erasmus MC Foundation-Daniel den Hoed Fund (Oct. 2021).
Sophie Veldhuijzen van Zanten successfully completed the AIRP Radiologic Pathology Correlation Course held by the American College of Radiology and the ACR Institute for Radiologic Pathology (Sept. - Oct. 2021)
Sophie Veldhuijzen van Zanten obtained the certificate “Stralingsbescherming voor nucleair radiologen” (Eng; Radiation Protection for Nuclear Medicine specialists; Feb. 2021)
Sophie Veldhuijzen van Zanten was selected for the Erasmus MC Female Talent Class 2020-2021.
Additional Personnel
Iris van der Strate – MSc Student
Catarina Simões Padilla – MSc Student
JOINT APPOINTMENT IN CARDIOLOGY
Ricardo Budde obtained both his MD and an MSc in Medical Biology from Utrecht University. His PhD thesis concerned epicardial ultrasound in (minimally invasive) coronary artery bypass surgery. Training as a Radiologist at the University Medical Center Utrecht and registration as a radiologist was completed in 2013. He completed a fellowship in cardiovascular radiology as well as successfully passed the European Diploma in Cardiac Imaging examination in 2014. Subsequently he joined the Erasmus MC as a staff radiologist specializing in cardiovascular and thoracic radiology and is clinical section chief for cardiovascular radiology. He is actively involved in scientific research and has (co)-authored over 200 publications published in peer-reviewed journals, several book chapters and serves as daily supervisor for multiple PhD students. His main research interests include imaging of (prosthetic) heart valves, aortic disease, endocarditis, imaging to optimize cardiac surgery and interventions as well as dose reduction techniques for CT imaging. The Dutch Heart Foundation has awarded him two large research grants as principal investigator including a prestigious Dekker grant. Ricardo is an executive board member of the European Society of Cardiovascular Radiology as well as member of the guidelines committee and Fellow of the Society of Cardiovascular Computed Tomography.
r.budde@erasmusmc.nl

Top Publications 2021
Dilsizian V, Budde RPJ , Chen W, Mankad SV, Lidner JR, Nieman K . Best practices for imaging cardiac device-related infections and endocarditis. A JACC: cardiovascular imaging expert panel statement. JACC Cardiovasc Imaging. 2021 Dec 7:S1936878X(21)00766-X.
Knol WG , Simon J, Harder den A, Bekker M, Suyker W, Heer de LM, Jong de PA, Leiner T, Merkley B, Polos M, Krestin GP , Boersma E, Koudstaal P, Maurovich-Horvat P, Bogers AJJC, Budde RPJ . Effect of routine preoperative screening for aortic calcifications using computed tomography on stroke rate in cardiac surgery: the randomized controlled CRICKET-study. Eur Radiol. 2022 Apr;32(4):26112619. doi: 10.1007/s00330-021-08360-4. Epub 2021 Nov 16.
van der Velde N , Huurman R, Hassing HC, Budde RPJ , van Slegtenhorst MA, Verhagen JMA, Schinkel AFL, Michels M, Hirsch A . Novel Morphological Features on CMR for the Prediction of Pathogenic Sarcomere Gene Variants in Subjects Without Hypertrophic Cardiomyopathy. Front Cardiovasc Med. 2021;8:727405
van der Velde N , Janus CPM, Bowen DJ, Hassing HC, Kardys I, van Leeuwen FE, So-Osman C, Nout RA, Manintveld OC, Hirsch A . Detection of Subclinical Cardiovascular Disease by Cardiovascular Magnetic Resonance in Lymphoma Survivors. JACC Cardio Oncol. 2021 Dec;3(5):695-706
CARDIAC IMAGING
RICARD O BUDDE, MD, PHD & ALEXANDER HIRSCH, MD, PHD
full professor & associate professor

Context
CT and MRI are instrumental techniques for cardiac assessment. Technical developments have occurred with a tremendous pace over the last two decades increasing diagnostic confidence, potential applications and (for CT) a significant decrease in radiation dose. Cardiac CT has its role for anatomical assessment of coronary disease but is now firmly entering the area with functional information as well (e.g. CT derived fractional flow reserve (FFR) and myocardial perfusion). Also, more detailed analysis of coronary plaques and pericoronary fat is gaining ground. Furthermore, its role in guiding cardiovascular interventions is continuous to expand at a rapid pace.
Alongside cardiac CT, Cardiovascular Magnetic Resonance Imaging (CMR) has become an important imaging technique for patients with a wide variety of heart disease. Beside anatomy, quantification of function, and assessment of cardiac fibrosis, there are major breakthroughs in the last decades including flow quantification with 4D flow and tissue characterization using T1-, T2-mapping and assessment of the extracellular volume. These techniques can be useful not only for the diagnostic work-up but also for the assessment of prognosis.
JOINT APPOINTMENT IN CARDIOLOGY
Alexander Hirsch studied Medicine at the University of Amsterdam and received his PhD degree (cum laude) in 2010 from the same University with a dissertation about “Clinical and functional outcomes after revascularization strategies in acute coronary syndromes”. He trained as a cardiologist at the Academic Medical Center in Amsterdam and registered as a cardiologist in 2013. He specialized further in non-invasive imaging with the focus on cardiovascular magnetic resonance imaging (CMR) and obtained his level 3 certification in CMR from the European Society of Cardiology in 2014. He joined the department of Cardiology and Radiology of the Erasmus MC in 2016 and his main focus is CMR.
He is actively involved in scientific research and has (co)-authored over 110 publications published in peerreviewed journals (Hirsch-index 32). His main research interests include CMR in ischemic and non-ischemic cardiomyopathy. He is currently supervising multiple Phd students at the department of cardiology and radiology. a.hirsch@erasmusmc.nl
Cardiac Imaging Group
The cardiac imaging group represents a close collaborative effort by the departments of Radiology/Nuclear Medicine and Cardiology, and consists of staff members, fellows and PhD students from both disciplines. During 2021 we also continued our collaboration with the departments of Thoracic Surgery, Experimental Cardiology, and Pediatric Cardiology on various projects.
Imaging equipment
The year 2021 marked the installation of one of the world’s first photon counting CT (PCCT) scanners in our hospital. The improvements in spatial resolution as well as the abilities of spectral imaging are likely to revolutionize cardiac CT imaging. Especially for cardiac imaging temporal resolution is of the utmost importance. Our PCCT scanner is truly unique since it is a dual source system with all the inherent advantages for temporal resolution and abilities for high-pitch scanning. Important improvements in coronary imaging are expected regarding reducing calcification blooming artefacts, improved ability for quantitative assessment of coronary plaques and better coronary luminal assessment. Also, for prosthetic valve assessment reduction of valve related artefacts and more detailed assessment of valve leaflets, supporting frames and pathological thrombus formation on the valve are expected.
Research Projects: Objectives & Achievements
Coronary Imaging
Traditionally coronary CT angiography (CCTA) imaging has a central role in our cardiac imaging research.
We continue our efforts in assessing CT derived FFR as a tool to add functional information to the anatomical assessment of stenosis severity. We gained initial experience with FFRct by participation in the international multicenter ADVANCE registry using a commercially available algorithm (Heartflow, Redwood, CA/USA). Also, we evaluated on-site software (cFFR, Siemens Medical Solutions, Germany) as part of an international consortium (MACHINE) in various patient populations.
In 2020 we were awarded a “Veelbelovende Zorg” grant to investigate in a multicenter randomized controlled trail (RCT) the clinical use of FFRct in stable chest pain patients that have a >50% - <90%stenosis on CCTA. We named this RCT the “FUSION” study and the first patients were

Figure 1. Three-dimensional volume rendered image of a transcatheter aortic valve prosthesis scanned on the photon counting CT scanner.
included in 2021. Besides the Erasmus MC, the following hospitals in the Netherlands will participate: Admiraal de Ruyter, St. Jansdal, Gelre, Martini, University Medical Center Utrecht, Maastricht University Medical Center and HAGA. More hospitals have expressed a desire to participate and will be onboarded in 2022. The FUSION study will be one of the first RCT’s investigating FFRct in stable chest pain patients with a >50% - <90%stenosis on CCTA. The primary endpoint will be the rate of unnecessary invasive coronary angiographies.
We expanded the use of CCTA and FFRct analyses in a truly unique group of patients in 2021 as well: those after heart transplantation. Transplant patients develop accelerated coronary wall thickening and atherosclerosis (so-called cardiac allograft vasculopathy (CAV)) and are screened at regular intervals. Supported by the team of transplant cardiologists, CCTA is now the preferred test for annual follow-up and we now have patients that are undergoing their 5th annual CCTA. This will provide important insights in the development and progression of CAV. We also used these CT scans to determine epicardial fat volumes, liver fat content and vertebral bone density.
Next to CT derived FFR, dynamic CT perfusion is assessed as another tool to add functional information to anatomical coronary imaging. Patient inclusion for the international multicenter SPECIFIC study (principal investigators Koen Nieman and Fabian Bamberg) that compares dynamic CT perfusion imaging with invasive angiography and FFR was published in 2021.
Coronary CT can be an excellent tool for screening patients at risk for coronary disease and is employed as such in the multicenter CREW-IMAGO study that evaluated patients with a history of pre-eclampsia, polycystic ovary syndrome and primary ovarian insufficiency. In addition, we participate the HARMONY study that looks at coronary calcification in patients with BRCA1/2 gene mutations.
Endocarditis
Endocarditis is a devastating disease. Prosthetic valve endocarditis (PVE) is the most severe complication of valve replacement surgery and has a high mortality rate. Its diagnosis remains difficult as echocardiography is hampered by PHV induced artifacts and blood cultures often being negative despite active infection. Previous research has shown that CT provides valuable information in this setting by demonstrating otherwise undetected aortic root mycotic aneurysms and vegetations that lead to changes in patient management.
Positron emission tomography (PET) combined with CT couples the detailed anatomical information of CT with metabolic information of PET. This powerful combination was added to the 2015 ESC Guidelines on Endocarditis. Together with the University Medical Center Groningen and other Dutch centers we constructed a large database with several hundreds of PVE patients constituting the largest cohort worldwide. After publishing these results in Circulation in 2018 we continued by assessing the role of PET-CT in suspected transcatheter aortic valve endocarditis.
Furthermore, we further expanded the PROSPECTA study by including patients that underwent replacement of the ascending aorta to provide eagerly awaited normal reference values for PET-CT of this specific patient group which we published in 2021. We continued inclusion of patients in the TWISTED study in which we assess the dynamics of FDG uptake around prosthetic heart valves.
We continued to combine forces with our valued colleagues from the University Medical Center in Groningen to collect a large number of PET-CT scans performed in patients with left ventricular assist devices to assess the diagnostic accuracy of PET-CT in these patients as well as other endocarditis related projects.
Our ultimate goal is to incorporate our research findings into clinical care to optimize and improve the care for patients with endocarditis. To that end the multidisciplinary “Endocarditis team” is more active than ever in Erasmus MC. A steady number of patients is referred and discussed twice a week. Given the complexity of the care for patients with (suspected) endocarditis this dedicated team of cardiologists, radiologists, nuclear medicine physicians, infectious disease specialists and thoracic surgeons is perfectly suited to provide optimal diagnosis and treatment advise. Data on the patient characteristics, use of diagnostic techniques and diagnosis was analyzed and submitted for publication.

Figure 2. FFRct analysis of a patient with a stenosis in the proximal LAD on CCTA. The FFRct analysis reveals the FFRct value is normal (0.88) for the LAD lesion so the lesion is not hemodynamically significant.
Aortic and Valve Disease
CT is evolving as a tool to assess both native and prosthetic heart valves. Regarding native valves, analysis of the CT and CMR data generated in the bicuspid aortic valve study a combined study with the University Medical Center Nijmegen and Leiden University Medical Center, has provided a unique insight in the dynamics of the aortic root and growth. In 2019 the 3-year follow-up of this large cohort of patients was completed. All patients underwent echocardiography, CT, and CMR including 4D flow at the same day. In 2021 the first results with regard to wall shear stress (WSS) measurements in relation to aortic growth were published. We showed that increased WSS and especially WSS angle (angle between the magnitude WSS and axial WSS component) predicted aortic growth in bicuspid aortic valve patients. These findings highlight the potential role of WSS measurements to stratify patients at risk for aortic dilatation.
Furthermore, the results of a large multicenter international collaboration project concerning valvular flow quantification with automated valve tracking in 4D flow CMR were published. In this multicenter, multivendor study we showed that independently of locally used CMR scanners and protocols, valvular flow quantification can be performed consistently with automated retrospective valve tracking.
Congenital Heart Disease
Erasmus MC is an expertise center for treatment of patients with congenital heart disease. Imaging plays an ever-increasing role in the diagnosis and follow-up of these patients. Even more so in the decision to re-intervene after initial correction.
Next to bicuspid valve pathology we investigate the role of CT and CMR in planning and follow-up of percutaneously implanted pulmonary valves. In the Cover study we included patients that underwent a percutaneous pulmonary valve implantation. These patients underwent a CMR, cardiac CT and echocardiography on the same day. The inclusion was completed in 2021 and the first results are expected in 2022.
We published a systematic review in combination with our experience in the Erasmus MC about the clinical outcome of patients with anomalous coronary artery with interarterial course. Both revascularization and non-invasive management have good prognosis in adults with anomalous coronary artery with interarterial course during early follow up. We stated that there is need for guidelines and longterm surveillance of this specific patient population.
The Quality of Life study started in 2020 and scanning was finished in 2021. In this study the long term cardiological and psychosocial outcome in adults operated for congenital heart disease in early childhood are studied. Follow-up of this cohort is now more than 40 years and includes patients with a diverse spectrum of congenital heart disease from atrial septal defect to tetralogy of Fallot and transposition of the great arteries. The first results are expected in 2022.
Finally, we finished our exercise CMR study using a pushpull MR-compatible ergometer in patients with bronchopulmonary dysplasia (BPD). In total 60 participants were included: 20 premature born young adults with BPD and 20 premature born young adults without BPD. These were compared with 20 healthy age and gender matched healthy subjects. The aim of the study is to examine cardiorespiratory structure and function during (sub)maximal exercise to reveal dynamic abnormalities that are not apparent on conventional static tests at rest. Inclusion was completed in 2021 and final results are expected in 2022.
Imaging to Optimize Surgery
Iterative reconstruction (IR) algorithms as well as developments of CT scanner hardware have reduced radiation dose substantially. In some cases, CT images may even be acquired at a radiation dose approaching that of a conventional X-ray. As such it may potentially revolutionize radiology by moving from 2D to standard 3D imaging by replacing conventional X-ray images. Whether this additional information will actually lead to earlier diagnosis, better treatment and outcome for patients remains to be established. In the prospective multicenter CRICKET study performed together with University Medical Center Utrecht and Semmelweis Hospital Budapest, we investigated if replacing a routine pre-operative chest X-ray by a low dose chest CT results in better patient outcome after cardiac surgery. Patient inclusion was completed in 2020 and the manuscript detailing the results was published in 2021.
Non-ischemic cardiomyopathy
Several projects were continued and initiated in the field of non-ischemic cardiomyopathy including the value of CMR and or CT in non-compaction cardiomyopathy, hypertrophic cardio-myopathy, cardiac sarcoidosis, and cardio-oncology.
The CMR-substudy of the PROCARBI study was finished and published 2021. This study investigates the late cardiac toxicity induced by radiotherapy alone or combined with anthracycline chemotherapy in patients after Hodgkin lymphoma. In total 80 patients underwent CMR. The
Figure 3. Example of velocity field and wall shear stress (WSS) maps in a patient with bicuspid aortic valve (BAV) and heathy control (figure published by Minderhoud et al. in the Eur Heart J Cardiovasc Imaging 2022; Jan 3 [epub ahead of print]).

study showed that long-term lymphoma survivors are not exempt from cardiovascular disease, which can be detected by changes in left ventricular function and native myocardial T1 with CMR. The follow-up CMR study including a second CMR is expected to be finished in 2022.
We further published a study exploring the role of CMR in patients with a pathogenic sarcomere gene variant without left ventricular hypertrophy (G+/LVH-). In this study, we assessed morphological, volumetric, and functional differences between a cohort of G+/LVH- subjects and healthy controls. The main findings were that the presence of multiple crypts and anterobasal hook only occurred in G+ subjects, and that a simple score system incorporating these and other CMR-derived myocardial morphological features could identify G+/LVH- subjects correctly. A follow-up study studying the use of artificial intelligence in this population is currently performed. Furthermore, the additional role of CMR in comparison to electrocardiogram and echocardiography in this population is explored.
In 2021 also the covid@heart study was started. The objective of the study is to assess the presence and magnitude of myocardial injury, using a combination of transthoracic echocardiography and CMR among individuals with a known baseline cardiovascular health status who are recovered from Covid infection treated at home. In short, participants of the Rotterdam Study who have undergone echocardiography in the past 5 years and had a confirmed diagnosis of Covid-19 are eligible to take part in the CMR substudy. A total of 100 participants will undergo a CMR. The results are expected in 2022.
Expectations & Directions
Coronary CT has shifted from anatomical to functional analysis. CT FFR and perfusion imaging will be further explored, and their role elucidated. The FUSION study is the first study to randomize patients with 50-90% stenosis to FFRct or routine care. It will provide important evidence in a randomized control trial set-up on the effect of using non-invasive FFRct to reduce the number of unnecessary invasive angiographies.
The introduction of PCCT in the clinical arena will lead to important improvements in cardiac CT imaging. After initial phantom experiments, large scale patient studies will define the role of PCCT and the direction this research will take.
The continuous annual follow-up of heart transplant patients with CCTA will provide the temporal results needed to identify the factors that are associated with (accelerated) coronary vasculopathy.
Data from the endocarditis database will shed more light on how to best implement PET/CT and CTA in patients suspected of endocarditis as well as identify important confounding factors. Also, longer term follow-up provides the information needed to evaluate how the diagnostic strategy is translated into quality of care. The results of the PROSPECTA study provided needed insight in the normal healing response after combined valve and ascending aorta replacement. Such data is also needed for other surgical procedures. In 2022 the follow-up results with regard to WSS from our bicuspid aortic valve study are expected. These results are important to explore changes in WSS over time. Furthermore results from several other studies are expected including the cover study, quality of life study, follow-up of procabi study, the exercise CMR study in BPD, covid@heart CMR substudy and CMR study in hypertrophic cardiomyopathy patients.
Hands-on Cardiac CT Course
For many years already, we organize the Hands-on Cardiac CT course in Erasmus MC. Ricardo Budde and Alexander Hirsch serve as course directors and are supported by an enthusiastic and experienced faculty including Marcel Dijkshoorn, CT technician at Erasmus MC.During 5 consecutive days the participants read over 150 CT scans on dedicated workstations fully equipped with the latest post-processing software. The sessions cover the entire spectrum of cardiac imaging form basic application like calcium scoring to advanced applications like valve-invalve TAVI planning as well as FFRct. The course is updated yearly to incorporate the latest developments. This year we moved to a new location (Hilton Hotel Rotterdam) and despite the COVID pandemic were able to organize one course which was completely sold-out with 44 participants. We thank both Siemens and Bayer for their support in organizing this course. Courses are planned also for 2022 and more info can be found on our dedicated course website: www.cardiovascularimaging.nl.

Figure 4. Screen shot of www.cardiovascularimaging.nl the website dedicated to our hands-on CT course.
Funding
Ricardo Budde and Alexander Hirsch and consortium partners: Veelbelovende zorg ZonMW 2020-2025: “Addition of FFRct in the diagnostic pathway of patients with stable chest pain to reduce unnecessary invasive coronaryangiography (FUSION Study)” to evaluate the role fo FFRct in stable chest pain patients”.
Alexander Hirsch and Jolien W. Roos-Hesselink: Erasmus MC Thorax foundation 2018-2021: “Advanced imaging of aortic aneurysms”.
Alexander Hirsch: Nederlandse Hartstichting 2021-2022: “Managing cardiovascular disease and risk in Covid-19 patients in primary care medicine: COVID@HEART CMR substudy”.
Invited Lectures
Ricardo Budde: Coronary Imaging: results of the recent trials. What imaging or therapies should search for. Le Fil Rogue online. 2021.
Ricardo Budde: Pediatric cardiovascular CT: when and how to image. ESCR online. 2021.
Ricardo Budde: Cardiac and aortic emergencies: what to look for. IPR, Rome, Italy. 2021.
Ricardo Budde: CT for infective endocarditis. Vancouver imaging review. Online. 2021.
Ricardo Budde: FFRct and perfusion imaging: read for prime time? Meeting Oostelijk Cardiologen Genootschap, Apeldoorn, the Netherlands, 2021.
Ricardo Budde: Status of FFRct and CT perfusion. Haagse Multimodality Imaging Week, the Hague, the Netherlands, 2021.
Ricardo Budde: Aortic diseases. ESCR webinar, 2021.
Ricardo Budde: CT of the heart: so much more to be discovered. Bayer user meeting online edition, the Netherlands, 2021.
Ricardo Budde: Improving your CT protocol. ICNC-CT virtual edition, 2021.
Ricardo Budde: Fractional flow reserve: an introduction. ECR, virtual edition, 2021.
Ricardo Budde: Acute aortic disease in pregnancy. ECR, virtual edition, 2021.
Ricardo Budde: CT plaque imaging. CVOI course. the Netherlands, 2021.
Alexander Hirsch: 4D flow CMR principles and cardiovascular applications. Saudi Heart Association, hybrid meeting, 2021
Alexander Hirsch: Role of non-invasive imaging for the diagnosis of cardiac amyloidosis. Webinar Cardiac Amyloidosis, 2021.
Alexander Hirsch: Starting up T1 and T2 mapping in your Practice. Netherlands Heart Days, online edition, 2021.
Alexander Hirsch: Myocardial tissue characterization with deep learning reconstruction. Webinar Advantages of Deep Learning in Clinical CMR Practice, 2021.
Alexander Hirsch: Cardiac CT: FFRct and CT perfusion. CVOI imaging course, online edition, 2021.
Highlights
Ricardo Budde was promoted to full professor of “Noninvasive Radiology of the Heart and Great Vessels”.
Alexander Hirsch was promoted to associate professor at the Erasmus MC.
Additional Personnel
Willem A Helbing, MD, PhD
Full Professor, Appointment in Pediatric Cardiology
Dr. Helbing completed his residency in general pediatrics, a fellowship in pediatric cardiology, and a PhD in clinical cardiovascular MRI. He now combines a clinical career as pediatric cardiologist with a research interest in ventricular function in congenital heart disease. He uses MRI to study right and single ventricular function in children and young adults with congenital heart disease and pioneered the use of stress MRI. He is currently is a professor of pediatrics and head of pediatric cardiology at Erasmus MC and Sophia Children’s Hospital, Rotterdam/NL.

Mohamed Attrach, MD
Dr Attrach is a cardiovascular radiologist and contributes to several cardiac CT and MRI research projects, including studies on aortic imaging as well as flow measurements on MRI images. Furthermore, dr. Attrach is a faculty member of the hands-on CT course.

KOEN NIEMAN, MD, PHD CARDIAC CT AND MRI
Associate Professor
JOINT APPOINTMENT IN CARDIOLOGY

Koen Nieman obtained his medical degree at the Radboud University in Nijmegen. In 2000 he joined the cardiac imaging group at the Erasmus MC, and obtained his PhD in 2003 (cum laude). During his cardiology residency at the Erasmus MC, he spent a one-year imaging fellowship at the cardiac CT, MRI, and PET program of the Massachusetts General Hospital (Harvard Medical School, Boston, MA/USA). In 2008 he joined the staff of the department of cardiology, and combined imaging research with clinical responsibilities at the intensive cardiac care unit. He (co-) authored over 200 papers and 20 book chapters. He served as President of the Society of Cardiovascular CT (20202021). He also serves on the editorial board of iJACC. In 2016 he accepted a position at Stanford University. At the Erasmus MC Dr Nieman has an honorary position to continue his scientific collaborations.
koennieman@hotmail.com
Cardiac CT and MRI have developed rapidly over the past decades and now offer various diagnostic opportunities for clinical management and research in cardiovascular disease.
Cardiac CT
Functional CT Applications
Imaging of myocardial perfusion by CT with quantification of myocardial blood flow using dynamic acquisition protocols, allows for assessment of the functional severity of obstructive coronary disease, and represents an alternative for established stress imaging techniques. Over the past years the methodology of dynamic myocardial perfusion imaging has been validated first in animals, and subsequently in humans. These results showed good diagnostic performance, and improvement of the accuracy of cardiac CT to identify patients with hemodynamically significant CAD.
The SPECIFIC trial (PI: Koen Nieman; Fabian Bamberg) is a multicenter study, including sites in Europe, Japan and the US, to validate the diagnostic performance of dynamic stress myocardial perfusion imaging using 3rd generation dual-source CT against invasive fractional flow reserve. The study is financially supported by Siemens Healthineers and Bayer Healthcare. The results of SPECIFIC, which were published in JACC Cardiovascular
Imaging (Nous et al, JACC CVI 2021), showed that the addition of dynamic perfusion imaging to CCTA improved diagnostic performance. This was observed in particular for lesions of intermediate stenotic severity.
Alternatively, functional severity can be determined by developing flow simulations based on CT angiograms using computational fluid dynamics. CT-based fractional flow reserve is an attractive technique for CT-based assessment of the severity of coronary artery disease. In 2016 we started implementation of a commercially available application (HeartFlow, Redwood, CA/USA), offered as a remote service, as part of an international registry (ADVANCE) (Patel, et al 2018; Patel et al, JACC Imaging 2020; Nous, et al, JCCT 2021). In addition, we evaluated a new algorithm (cFFR, Siemens Medical Solutions, Germany) which can performed by physicians on site
The on-site CT-derived FFR algorithm showed good performance in comparison to invasively performed fractional flow reserve (Coenen, Radiology 2015) In collaboration with several other centers around the world, we technically validated a second-generation on-site CTFFR application developed through machine learning (MACHINE consortium), which showed equal accuracy but much faster processing times (Coenen, et al, Circulation CVI, 2018; Nous, et al, Am J Card, 2018). Subsequent studies related to the effect of coronary calcification, gender and co-morbidities on CT-FFR performance were published in 2019 (Nous, et al, AJC 2019; Tesche, et al, JACC Img 2019; Baumann, Eur J Rad 2019).
While perfusion imaging and CT-FFR fulfill similar purposes, we showed they also provide complementary information. We demonstrated that combined, or in sequence, the techniques provide better estimation of hemodynamic coronary disease severity than each separately (Coenen, et al, JACC CVI, 2017)
Clinical Value of Cardiac CT
The diagnostic and prognostic value of CT coronary angiography in comparison to existing methods is an important field of investigation. After publication of the results of the BEACON trial (acute chest pain) and the CRESCENT trial (stable chest pain) in 2016, we recently completed the CRESCENT II trial, which evaluated the clinical performance of a comprehensive cardiac CT protocol that includes stress myocardial perfusion in comparison to standard care for the management of stable coronary artery disease. In the CRESCENT II trial we randomized 268 patients at four Dutch centers, including Maasstad Ziekenhuis, Albert Schweitzer Ziekenhuis and Maastricht University Medical Center. This study was funded by ZonMw, and the Netherlands Heart Foundation, and results were recently published (Lubbers, et al, JACC CVI, 2018). More recently we assessed the complementary value of CT-FFR in the CRESCENT cohorts (Nous, et al, Eur Rad 2020).
Coronary CT Angiography & Contrast Media
The IsoCOR trial is a multicenter study with the Albert Schweitzer Ziekenhuis, Dordrecht and the Maasstad Ziekenhuis in Rotterdam. A total of 300 patient were randomized between iodixanol 270 and iopromide 300 injected at comparable iodine flux. The objective of both studies is to find out whether opacification characteristics and injection parameters are similar. The results of IsoCOR were published in 2017 (Lubbers, et al, Radiology 2017). The CT-CON trial compared the performance of four contrast media for coronary opacification when injected at identical iodine flux (in collaboration with Maasstad Ziekenhuis, Rotterdam; Albert Schweitzer Ziekenhuis, Dordrecht; St Elizabeth Ziekenhuis, Tilburg; Maastricht UMC; and La Sapienza University in Rome), and the results were published in 2019 (Rengo, et al, Eur Rad. 2019).

Figure 1: Angiographic CAD demonstrated by coronary CT angiography (left panel), and functional significance demonstrated using dynamic CT myocardial perfusion imaging. Between brackets the sampled myocardial blood flow in ml/s/100g.

CORONARY CT ANGIOGRAPHY DERIVED FRACTIONAL FLOW RESERVE
ADRIAAN COENEN, MD
Advisors Ricardo Budde, Felix Zijlstra & Koen Nieman
Project Funding
Erasmus MC Radiology and Nuclear Medicine
Research period April 2013 – December 2022
Email a.coenen@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Cardiology.
Coronary CT angiography (CCTA) has developed into an established noninvasive tool for detection of coronary artery disease. The high sensitivity makes it an excellent diagnostic examination to rule out coronary artery disease. However, with a relatively low specificity CCTA has been known to overestimate coronary artery stenosis severity. As these overestimations can result in unnecessary invasive coronary angiography referrals, several strategies are investigated to reduce overestimation.
During the last 2 decades classification of coronary artery stenosis severity has shifted from visual anatomical assessment during invasive coronary angiography towards functional measurement. Fractional flow reserve (FFR) is currently regarded as the gold standard for stenosis severity classification. Fractional flow reserve is an invasive pressure wire guided procedure where the intracoronary blood pressure distal to the stenosis of interest is divided by the blood pressure in the ascending aorta. The examination is performed in pharmacological hyperemia, if the FFR is below 0.80 it is considered a hemodynamically significant coronary artery stenosis.
A relatively new technique is the application of computation fluid dynamics onto the anatomical CT angiography images. By simulating the coronary blood flow, the functional relevance of a coronary artery stenosis can be simulated. Recently, a new on-site approach using machine-learning instead of computational fluid dynamics has been released. Advantages of the machine learning approach are faster computational times, and potential inclusion of other variables outside of computational fluid dynamics (for example plaque composition).
Together with 4 other centers involved in research in onsite CT derived FFR we reanalyzed our data and combined all cases into the MACHINE consortium. This combined analysis showed that machine learning improved diagnostic performance of visual CCTA evaluation. In addition, we set-up an open CT-FFR registry together with EIBIR allowing for new centers to participate in investigating the value of on-site CT-FFR analysis.
In 2021 the MACHINE consortium found CT-FFR improved diagnostic performance over conventional CTA for proximal, middle and distal coronary stenosis (Renker et al. Influence of coronary stenosis location on diagnostic performance of machine learning-based fractional flow reserve from CT angiography. JCCT 2021).

Figure 1: Distribution of machine-learning (ML)–based computed tomography-fractional flow reserve (CT-FFR) accuracy. Coenen et al. Diagnostic Accuracy of a Machine-Learning Approach to Coronary Computed Tomographic Angiography-Based Fractional Flow Reserve: Result From the MACHINE Consortium. Circ Cardiovasc Imaging. 2018
PhD Student

Advisors
Project Funding
IMAGING NATIVE AND PROSTHETIC HEART VALVES USING CT TECHNOLOGIES
MARGUERITE E FAURE, MD
PhD Student
Ricardo Budde & Alexander Hirsch
Erasmus MC
Research period February 2017 – December 2022
Email m.faure@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Cardiology.
This project concerns imaging native and prosthetic heart valves using Computed Tomography (CT) technologies. Approximately 280 000 valve prostheses are implanted worldwide each year, 20% of which are mechanical valves and 80% bioprosthetic valves. In a first study we assessed a novel CT acquisition protocol for comprehensive prosthetic heart valve (PHV) evaluation at limited dose. Bioprostheses are increasingly chosen due to favorable hemodynamic profile and low thrombogenicity. In spite of significant improvements in bioprosthesis design and surgical procedures, the implantation does not necessarily result in a definitive cure, and native valve disease is often replaced by ‘prosthetic valve disease’. Structural valve deterioration (SVD) is the major cause of surgical aortic valve bioprosthesis (bio-sAVR) failure, which increases with time post implantation. Deformation of surgical biological valves may result in incomplete leaflet expansion, and subsequently accelerate valve degeneration. The aim of our second study was to assess the deformity of bio-sAVR after surgical implantation using CT. We found bio-sAVRs to show at least mild deformation in 56% of studied cases and were considered non-circular in 17% of studied valves.
In 2020 we started a study (COVER study) in patients that underwent a percutaneous pulmonary valve implantation (PPVI). This is an effective procedure in patients with congenital heart diseases involving the right ventricular outflow tract. A serious complication that can occur during PPVI is compression of the coronary arteries. To avoid this potentially life-threatening complication, the risk of coronary compression (CC) is assessed beforehand. Recent studies suggest that cardiac CT can detect patients at high and intermediate risk of CC, however little is known about changes after PPVI implantation. As first part of this study we compared the relationship between the coronary arteries and the pulmonary trunk befwore and after PPVI and therefore, give more insight in actual CC risk of PPVI. Our study suggests that there is no relevant change in coronary distance and lumen diameter after PPVI. Conduit expansion does not seem to affect the relationship between the pulmonary trunk and coronary arteries after implantation.
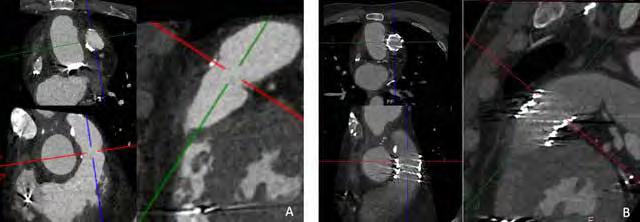
Figure 1: Example of CT scan of a patient before (A) and after (B) percutaneous pulmonary valve implantation (PPVI). CT can evaluate anatomical changes, relationship with coronary arteries and potential complications such as endocarditis.

CT-IMAGING IN CARDIAC SURGERY
WIEBE G KNOL, MD
PhD Student
Advisors Ad Bogers & Ricardo Budde
Project Funding Erasmus MC Dep. of Cardiothoracic surgery and Dep. of Radiology & Nuclear Medicine
Research period January 2018 – December 2022
Email w.knol@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Cardiothoracic surgery.
Computed tomography is a rapidly developing field. The use of techniques like iterative reconstruction and the improvements in both spatial and temporal resolution have opened up new possibilities for implementation of computed tomography in the clinic, also in the field of cardiothoracic surgery. In order to translate these developments into daily clinical practice, we first implement them in a clinical research setting. This will lead to better understanding of how to interpret the images, and how to use computed tomography in the clinical setting.
A good example of this is the expanding role of CT in the diagnosis of coronary artery disease. We have investigated the risks and benefits of using either invasive coronary angiography or coronary CT angiography for the diagnosis of coronary artery disease in a retrospective cohort of patients undergoing cardiac surgery for acute aortic valve endocarditis.
We have also evaluated the role of a non-contrast CT scan of the thoracic aorta as a screening tool for patients prior to cardiac surgery in the randomized controlled CRICKET trial. We observed that preoperative noncontrast CT in cardiac surgery candidates did not influence the surgical approach nor the incidence of perioperative stroke compared with standard of care. Aortic calcification is a frequent finding on the CT-scan in these patients, but results in major surgical alterations to prevent stroke in only few patients. A subanalysis of the CRICKET trial will look into the frequency of incidental findings in these patients, and the consequences that this has on the timing of surgery.


Examples of the standard of care preoperative workup prior to cardiac surgery, a chest X-ray, to evaluate presence of aortic calcification. The addition of CT provides more insight into the presence and location of aortic calcifications (displayed here in sagittal and volume-rendered reconstructions). The CRICKET trial observed that nonetheless, this extra information leads to changes in the surgical approach in only few patients
Following the results of the CRICKET trial, we have set out to evaluate the use of noncontrast CT scans prior to minimally invasive cardiac surgery (MICS), an approach that has been implemented in the Erasmus MC several years ago.

CARDIAC CT FOR COMPREHENSIVE CORONARY ASSESSMENT
FAY NOUS, MD, MSC
PhD Student
Advisors Ricardo Budde, Felix Zijlstra, Koen Nieman
Project Funding Erasmus
MC Radiology & Nuclear Medicine
Research period September 2016 – July 2022
Email f.nous@erasmusmc.nl
This project is collaboration between the Departments of Radiology & Nuclear Medicine and Cardiology.
Coronary computed tomography angiography (CCTA) is a reliable modality for the detection of coronary artery disease (CAD). Current guidelines recommend CCTA in the diagnostic work-up of patients with stable chest pain and suspected CAD. However, many CCTA examinations reveal intermediate coronary artery stenoses, in which case additional functional assessment is required for clinical decision-making. Invasive fractional flow reserve (FFR) is the preferred method for physiological assessment of coronary arteries, but is variably adopted in daily practice due to its invasive nature.
Several cardiac CT techniques, such as CT myocardial perfusion imaging (CT-MPI) and CT derived FFR, have been developed to evaluate the hemodynamic relevance of coronary lesions. Dynamic CT myocardial perfusion imaging (CT-MPI) is able to determine the hemodynamic relevance of the coronary lesions by evaluating the first pass myocardial perfusion in a pharmacological hyperemic state. CT derived fractional flow reserve (CT-FFR) evaluates the ratio of intracoronary blood pressure distal to the lesions and the coronary root based on computational structural and fluid analysis.
We investigated the diagnostic accuracy of CT-MPI and CT-FFR in several populations. An example is the SPECIFIC study, an international multicenter study in which the accuracy of CT-MPI was determined by performing CT-MPI in 125 patients suspected of coronary artery disease prior to invasive coronary angiography and the results are compared with intracoronary FFR. Furthermore, we investigated the (potential) additional value of CT-FFR in the clinical decision making of patients with stable chest pain and heart transplant patients. An example is the ADVANCE registry, an international multicenter registry with the aim of determining the impact of this pathway on decision-making,
downstream invasive coronary angiography, revascularization, and major adverse cardiovascular events. Finally, we assessed the value of CCTA for cardiovascular risk stratification in patients with atrial fibrillation and after heart transplantation.

Figure 1: A case example of a patient with typical angina who underwent (A) coronary angiography and (B) coronary computed tomography angiography, both of which showed an obstructive stenosis in the obtuse marginal artery. (C) Computed tomography-derived fractional flow reserve showed a hemodynamic significant lesion in obtuse marginal artery. (D) Computed tomography myocardial perfusion imaging showed a myocardial perfusion defect in the lateral wall.

FF RCT IN PATIENTS WITH CORONARY ARTERY DISEASE
SIMRAN P SHARMA, MD
PhD Student
Advisors Ricardo Budde, Felix Zijlstra & Alexander Hirsch
Project Funding Regeling Veelbelovende Zorg van Zorginstituut Nederland and ZonMw
Research period October 2020 – October 2024
Email s.sharma@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Cardiology .
Current guidelines focus on the use of coronary computed tomography angiography (CCTA) in patients with low to intermediate risk for coronary artery disease (CAD). Therefore, patients with stable chest pain enter a diagnostic pathway where CCTA is often the first line non-invasive test to detect coronary stenosis. An anatomically significant stenosis on CCTA does however not always cause cardiac ischemia (i.e. hemodynamically significant stenosis). CCTA is often followed by invasive coronary angiography (ICA) to assess the hemodynamic significance of the stenosis which is the key determinant to decide on treatment (revascularization by coronary stenting or surgery). CCTA has a very high negative predictive value but the positive predictive value is moderate. Hence, anatomically significant stenoses on CCTA often turn out not to be hemodynamically significant on ICA. Fractional Flow Reserve derived from coronary computed tomography (FFRct) analysis is a new non-invasive technique that uses the CCTA images as a basis for complex software-based calculations and modelling to provide additional functional information based on the anatomical CCTA images. Thus, FFRct is a totally non-invasive method. Adding the FFRct analysis to the anatomical assessment of CCTA is expected to reduce the number of patients being referred to ICA where no signs of hemodynamically significant stenosis are found on ICA. FFRct is a certified technique and has been adopted already in the United States of America, United Kingdom and Japan.
At this point in time, there are no prospective data on the real-world use of FFRct in the Dutch population of stable chest pain patients with stenosis on CCTA and the effect of FFRct in reducing unnecessary ICA compared to CCTA alone. Evidence in the specific Dutch situation and population is needed to evaluate the impact and cost-effectiveness in the Dutch healthcare system.
To investigate the impact of adding the FFRct analysis in the diagnostic pathway of stable chest pain patients we have set up the FUSION study. FUSION is a national, multicentre, randomised controlled trial. At this point in time, there are 8 centres participating in the FUSION study. This number may increase in the near future.

Figure 1: (A) CCTA with 70% stenosis in the proximal right coronary artery (RCA); (B) FFRct with a value of 0.88 in RCA; (C) ICA with invasive FFR value of 0.89 in RCA.

CMR
IN N ON-ISCHEMIC CARDIOMYOPATHY
NIKKI VAN DER VELDE, MD
PhD Student
Advisors Ricardo Budde , Felix Zijlstra & Alexander Hirsch
Project Funding Erasmus MC
Research period August 2017 – December 2022
Email n.vandervelde.1@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Cardiology.
Cardiomyopathy (CMP) refers to diseases of the heart muscle, which are characterized by structural and functional myocardial abnormalities. CMP can be broadly divided into ischemic and non-ischemic CMP. An example of a specific form of non-ischemic CMP is hypertrophic cardiomyopathy (HCM). CMP can be caused by hereditary disorders or is acquired, for example by cardiac involvement of sarcoidosis or cardiotoxicity due to radiation/ chemotherapy. Cardiovascular magnetic resonance imaging (CMR) plays an important role in the diagnosis and prognosis of these CMPs.
HCM is characterized by left ventricular hypertrophy (LVH) and is the most common hereditary cardiac condition. Carriers of pathogenic DNA variants (G+) can be identified by family screening, even before the manifestation of LVH. G+/LVHsubjects are routinely monitored for phenotypic expression, which, alongside LVH, can include other pre-clinical HCM-related abnormalities like crypts and myocardial fibrosis. CMR has emerged as a valuable technique in diagnosing and followup of HCM. Therefore, we performed two studies to identify pre-clinical HCM-related abnormalities, and investigated the yield of CMR relative to electrocardiogram (ECG) and transthoracic echocardiography (TTE). The first study showed significant morphological differences between the G+/LVH- population and healthy subjects. The second

Figure 1: CMR data in lymphoma survivors and healthy subjects. Figure published in van der Velde et al. JACC CardioOnc 2021;3:695-706
study, we found that an additional CMR examination to the routinely monitoring with ECG and TTE reclassify the HCM diagnosis in a substantial number of patients. In addition, G+ subjects with normal ECG and TTE results, were not diagnosed as HCM on CMR, and were pre-clinical HCM-related abnormalities high in subjects with and without ECG/TTE abnormalities.
Myocardial fibrosis is a common reaction to injury in most CMP, and can be visualized by late gadolinium enhancement (LGE) imaging. Deep learning (DL) methods can be used to enhance image quality. Our study showed an significant increase in LGE image quality by applying a DL based reconstruction algorithm based on noise reduction.
Improvements in treatment of patients with Hodgkin lymphomas (HL) have resulted in an increased survival rate. Long-term survivors are however exposed to late adverse effects of radiation/ chemotherapy, leading to premature cardiovascular morbidity and mortality. These adverse effects can develop even up to decades afterwards treatment initiation. In our study we aimed to identify markers for subclinical cardiovascular disease in asymptomatic HL survivors, using CMR. A reduced ejection fraction, higher T1 value and a lower global strain were found as markers in asymptomatic HL survivors in comparison with healthy subjects.

18F-FDG PET/CT FOR INFECTIVE ENDOCARDITIS
ALI R WAHADAT, MD
Advisors Jolien Roos-Hesselink, Ricardo Budde & Wilco Tanis
Project Funding Departments of Cardiology Haga Hospital and Erasmus MC
Research period March 2018 – December 2022
Email a.wahadat@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Cardiology, Erasmus MC and the Department of Cardiology, Haga Hospital.
Endocarditis is a devastating disease and one with different types of complications. Its diagnosis remains challenging especially in cases of suspected prosthetic valve endocarditis (PVE). The use of 18F-FDG PET/CT and ECG-gated cardiac CTA for the diagnosis of PVE were introduced in the latest European Society of Cardiology (ESC) guidelines for management of infective endocarditis. However specific data about correctly and timely applying these imaging modalities are scarce. In particular the use of 18F-FDG PET/CT is not recommended in cases of patients who underwent their surgery less than 3 months before the scan because of possible false positive results due to inflammation after surgery. However, the exact time when to use this imaging modality has never been described. Together with our colleagues at UMC Utrecht we started a prospective trial to find out more about the physiological FDG uptake around the valve after prosthetic valve implantation. By imaging patients who had a prosthetic valve implantation 1 month, 3 months or 12 months prior to the PET/CT scan we wanted to assess the normal PET/CT imaging findings in the first year after aortic valve implantation. After analysis of the first 37 included patients, we found out that there were no significant differences in visual and quantitative measurements of perivalvular FDG uptake in patients who were 1 month, 3 months or 12 months after aortic valve implantation (included figure). This suggests that the 3 months safety period recommended by the ESC guidelines for infective endocarditis should be reconsidered.
Moreover, little is known about the uptake pattern after a Bentall procedure. As a continuation of our previous study, we investigate the normal 18F-FDG uptake in aortic root and –ascendens prosthesis in patient after a
Bentall procedure. Patients who underwent a Bentall procedure received an 18F-FDG PET/CT 3 months or 1-year after surgery. Visual and quantitative measurements on different part of the operative field showed no significant difference between patients who were scanned 3 months or 1 year after surgery. Furthermore, different uptake patterns were seen on different locations of the prosthesis. This suggests that 18F-FDG uptake remains present in the first year after aortic root and – ascendens prosthesis implantation. The use of 18F-FDG PET/CT for the diagnosis of infection of such prostheses should be done carefully, taking normal variability into account to avoid mistakes.

Figure 1: Examples of 18F-FDG uptake patterns (A: diffuse homogeneous, B: focal, C:diffuse heterogeneous) around the cranial anastomosis.
Ivo Schoots completed his professional radiologic training in 2012 at the Academic Medical Center, Amsterdam after a certified clinical fellowship in abdominal radiology. In 2004 he received his PhD on the ‘crosstalk of coagulation and inflammation in ischemia and reperfusion mechanism’ at the University of Amsterdam (Surgery & Internal Medicine). He performed a postdoctoral research fellowship at Harvard Medical School, Department of Molecular & Vascular Medicine, BIDMC, Boston, MA/USA. His research is now focused on oncologic

abdominal imaging, in particular on developments in prostate cancer MR imaging and imageguided biopsies / interventions, imaging biomarkers, and clinical decision modeling. He participates in international and national working groups on prostate cancer imaging and image-guided targeted biopsies. As PI of the MR PROPER study he is involved in the clinical implementation of prostate MRI in combination with risk stratification. i.schoots@erasmusmc.nl
IVO G SCHO OTS, MD, PHD associate professor ABDOMINAL IMAGING

Context
Our Abdominal Imaging research is focused on several organs, in the lower abdomen as well as in the upper abdomen. Some of our researchers have developed particular skills and interest in advanced MR imaging of the prostate or cervix within the clinical decision-making algorithms, others are more dedicated to liver and colorectal cancer imaging. We are very happy that the collaboration with our clinical partners in Surgery, Gastroenterology, Urology, Gynecology, Medical Oncology, and Radiotherapy are already starting to bear fruit by attracting funding and by creating some promising scientific output. We are convinced that in the coming years some of these areas of research and probably even other topics in abdominal imaging will gain more interest and become increasingly successful.
Top Publications 2021
Castillo T JM, Arif M, Starmans MPA, Niessen WJ, Bangma CH, Schoots IG, Veenland JF. Classification of Clinically Significant Prostate Cancer on MultiParametric MRI: A Validation Study Comparing Deep Learning and Radiomics. Cancers (Basel). 2021 Dec 21;14(1):12.
Starmans MPA, Buisman FE, Renckens M, Willemssen FEJA, van der Voort SR, Groot Koerkamp B, Grünhagen DJ, Niessen WJ, Vermeulen PB, Verhoef C, Visser JJ, Klein S. Distinguishing pure histopathological growth patterns of colorectal liver metastases on CT using deep learning and radiomics: a pilot study. Clin Exp Metastasis. 2021 Oct;38(5):483-494.
Schoots IG, Barentsz JO, Bittencourt LK, Haider MA, Macura KJ, Margolis DJA, Moore CM, Oto A, Panebianco V, Siddiqui MM, Tempany C, Turkbey B, Villeirs GM, Weinreb JC, Padhani AR. PI-RADS Committee Position on MRI Without Contrast Medium in Biopsy-Naive Men With Suspected Prostate Cancer: Narrative Review. AJR Am J Roentgenol. 2021 Jan;216(1):3-19.
277
Research Projects: Objectives & Achievements
Prostate cancer imaging
Prostate cancer is the most common malignancy and leading cause of cancer-related deaths in men. Optimization of diagnosis and treatment of prostate cancer is of great social importance. Prostate cancer, however, is a heterogeneous disease with clinically significant (aggressive) and insignificant (non-aggressive) tumors; only aggressive tumors need to be detected and treated. There is an urgent need for early tumor detection and characterization of tumor aggressiveness.
Imaging and image-guided strategies in diagnosis and treatment of prostate cancer are challenging. However, these strategies may improve diagnostic accuracy, needing less biopsy cores, and may navigate to better therapy choice with subsequent lower morbidity, increased quality of life and at lower costs. Projects on imaging and image-guided strategies of prostate cancer address the need to reduce over-diagnosis, over-biopsy and overtreatment of prostate cancer, due to today’s

less than ideal screening tests. Results of several trials with MRI (Erasmus MC) were published. This have led to influential guideline changes (Fig. 1), and may lead to the renewed question whether to screen for prostate cancer with MRI or not, as prostate cancer screening without MRI shows at least a relative cancer specific mortality reduction of 21% over time, however, with the harms of over-biopsy, over-diagnosis and over-treatment.
Liver imaging
As part of the largest liver center in the Netherlands, continuing efforts are done to improve the possibilities of non-invasive diagnostics of liver tumors.
First, regarding benign liver lesions, our study projects have mainly focused on diagnosis of hepatocellular adenoma (HCA), focal nodular hyperplasia (FNH), and angiomyolipoma on MRI. One of our findings was the superiority of Gadoxetate disodium against Gadobenate dimeglumine for differentiation of HCAs from FNHs. Further research is focused on atypical presentations including imaging of atypical hemangiomata and atypical isointense lesions (AILs) in the hepatobiliary phase. The latter is one of the projects which are set up in close collaboration with all Dutch university medical centers (and is endorsed by the Dutch Benign Liver Tumor Group).
Second, regarding hepatocellular cancer (HCC), our study projects have focused on the use of ultrasound (US) and MRI for the detection of HCC. This tumor is the tenth most common malignancy and sixth cause of cancer-related deaths in men. Our referral center for hepatobiliary diseases is a screening center for HCC in hepatitis and cirrhosis. A review of our own data led to the conclusion that US is inferior to MRI without contrast in HCC detection. Based on these findings, we are currently evaluating a shorter and more (cost) efficient MRI screening protocol.
Third, together with the biomedical imaging group of our department, we developed a robust, multicentric radiomics platform for liver lesion phenotyping using MRI data from different liver centers around the world. This is named by the Liver Artificial Intelligence (LAI) consortium. Efforts are made to find generalizable applications for liver tumor classification and prognosis.
Rectal cancer imaging
Colorectal cancer is the third most common malignancy and third cause of cancer-related deaths in men and women. With the Erasmus MC a few centers in the Netherlands are specialized in treatment of local recurrent rectal cancer. In our institution many of these patients will undergo surgery with curative intention. Dedicated MRI is an integral part of the work-up and important for

clinical decision making. Our research aims to develop and validate modern MR techniques for better selection and follow up of complete responders after preoperative chemo-radiotherapy of (recurrent) rectal cancer. Furthermore, morphological and functional MRI characteristics based on multiparametric MR imaging and post-processing tumor texture analysis may help predict surgical outcome and long-term prognosis (Fig. 2).
Cervical cancer imaging
Cervical cancer is the fourth most common cancer among females, and the fifth cause of cancer-related death in women. In daily practice clinical examination with or without anesthesia is until recently the principal investigation for assessment and therapy planning in cervical cancer. However, since our published systematic review comparing clinical examination vs MRI the new national guideline (Oncoline, under review) have given the latter a more prominent role in primary staging. Based on our findings the international guidelines of ESTRO, together with ESGO and ESP has shifted completely to MRI as the hallmark for diagnostic staging.
Fig 2. Recurrent rectal carcinoma in the perineum: male 68 yrs (p(T2N0M0; R0).3 years after primary surgery, a local recurrence occurred demonstrated on MRI (after 30 Gy radiotherapy on pelvis): (A) T2w-image shows two masses in the perineum (arrows) within scar tissue. Both lesions appear to enhance after gadolinium administration on the DCE image (B). The red-toyellow color fusion image (C) displays an overexposed image that hinders an obvious conclusion, the blue-to-red color scaled fusion image (D) makes clear that the ventral lesion (dashed arrow) does not demonstrate intense enhancement, while the dorsally located lesion (arrow) demonstrates intense enhancement in a dominant rim pattern. Final pathology demonstrated fibrotic tissue without tumor of the ventral lesion, while the dorsal lesion showed active local tumor recurrence.
In an Erasmus MC lead collaborative prospective multicenter study with UZ Leuven and AMC we investigated the value of optimized MR imaging, including diffusion weighted imaging (DWI) in the detection of early tumor residue in the cervix after radiotherapy. The main finding was that DWI forms an irreplaceable part of the protocol since it increases significantly the detection of residual tumor.
Expectations & Directions
Prostate cancer imaging: Means to detect prostate tumors at an early stage and to robustly characterize the aggressiveness are badly needed. MR imaging in prostate cancer is an important research line within the abdominal imaging section. Image-guided biopsy based on multiparametric MR examinations has been established. MRI/ US-fusion guided biopsies has been implemented in clinical trials and in patient care. Association of imaging traits with gene expression profiles of prostate cancer will certainly be an area of interest, in close correlation with the Erasmus MC departments of Urology, Pathology and Biomedical Imaging Group Rotterdam (BIGR) (see p. 72). Developments on nuclear imaging techniques (o.a. PSMA PET CT) will be explored to correlate morphological and functional prostate MR images to PET CT images of prostate metabolism (Fig. 3), most preferably with the new PET MRI scanner. We intend to explore strategies to improve diagnostic accuracy, reduce the number of biopsy cores needed, and improve therapeutic decisionmaking, thereby lowering morbidity, increasing quality of life, and reducing costs.
Hepatic cellular cancer imaging: MR imaging in hepatic cellular cancer (HCC) is expected to become a growing research line within the department, in close collaboration with the Erasmus MC departments of Hepatology/ Gastroenterology and hepatobiliairy Surgery. HCC screening in patients with hepatitis and cirrhosis are becoming centralized in academic centers. Collaborating networks such as the Dutch Hepatic Cancer Group (DHCG) are evolving, in which the Erasmus MC plays a dedicated and central role. Furthermore, interventional therapy plays an important role in minimal invasive liver cancer

Fig 3. [ 68Ga]GaSB3 PET/CT-imaging of a primary prostate cancer patient. A large GS 3+4=7 tumor. A: Maximum intensity projection 60 min post injection (min.p.i.), B: PET imaging below PET/CT imaging, 60 min.p.i., tumor SUVMAX 22.7. C: PET/ CT fusion. D: PET imaging at 210 min.p.i. tumor SUVMAX 20.0. E: corresponding histopathological slides with tumor delineation (red). (ref Bakker, et al. Journal of nuclear medicine (2021) 62, 1517-1523)
treatment. Critical for its success is an accurate target of the lesion. Research is focused on development of real time image fusion techniques to guide interventional treatment and navigation surgery (see page 102) .

(Fig 4) Examples of segmentations of three colorectal liver metas- tases (CRLMs) by the human observers and by the convolutional neural network (CNN) [PhD (dark blue); RAD (light blue); STUD first try (STUD1) (cyan) and second try (STUD2) (magenta); CNN (purple)] on a single axial slice of CT-scans. The bottom row depicts the zoomed in region without the segmentation overlays. The three CRLMs displayed are those with a volume at the 25% percentile (a), 50% percentile (b) and 75% percentile (c) of all metastases in the database.
(Ref Starmans et al, Clinical & Experimental Metastasis (2021) 38:483–494)
Radiomics: MR imaging biomarkers are being explored and validated for personalized decision-making and for prediction of treatment efficacy. A prerequisite for implementation of the multiparametric MR imaging modality is an accurate and fast post-processing method that can translate the complex quantitative information into clinically useful directions. The development and validation of such methods is research in joint cooperation with industrial partners. ‘Radiomics’ entails the extraction of predictive features for diagnostic decision-making and treatment response from standard CT or MR image using mathematical models (Fig 4). We will further expand our imaging expertise and apply novel imaging methods to improve our understanding of the genetic and disease development in abdominal oncology. We will initiate new projects in the field of oncological imaging analy-

sis, with machine learning and deep learning techniques (Fig. 5). We will continue to increase our research efforts into investigating clinically related oncological research questions.
Funding
Schoots, Ivo , Monique Roobol, Gabriel Krestin, Chris Bangma (Urology): ZonMW Health Care Efficiency Research Grant 2017-2020: “Risk Stratification and MRI in addition to Standard Prostate Cancer Detection: An Impact Analysis”
Nieuwenhuyzen-de Boer, Gatske, Heleen Beekhuizen, Ivo Schoots , Wart Hofhuis, Beltman (Gyneacology). ZonMW Health Care Efficiency Research Grant 2017-2020: “Evaluation of effectiveness of the PlasmaJet Surgical device in the treatment of Advanced Stage Ovarian Cancer: a randomized controlled trial in The Netherlands (PlaComOv study)”
Schoots, Ivo, Jifke Veenland, Wiro Niessen Chris Bangma, Monique Roobol (Urology). KWF – STW ‘Technology for Oncology’ Grant 2017-2020. ProstatVision: Visual technology integrating quantitative patient outcomes to support multidisciplinary clinical decision-making.
Schoots, Ivo , and Monique Roobol (Urology): Erasmus MC Health Care Efficiency Research Grant 2015-2019: “Diagnostic and cost effectiveness of the additional use of risk stratification and MRI in standard prostate cancer detection”
Fig. 5 . (1) The magnetic resonance sequences to be used in the model are defined. (2) The lesions from the pathology are copied and registered to the T2w sequence. (3) The diffusion weighted imaging (DWI) and apparent diffusion coefficient (ADC) are resampled and registered to the T2w. (4) Features are extracted from the T2w, DWI and ADC. (5) A radiomics model is created from the features, using an ensemble of the best 50 workflows from 100,000 candidate workflows, where the workflows are different combinations of the different classifiers. (ref Castillo Tovar et al (2021) Diagnostics. 11, 369)
Niessen, Wiro, Ivo Schoots, Jifke Veenland , Chris Bangma (Urology), and Gabriel Krestin : Technology Foundation ‘STW’ – Perspectives for Top Sectors Grant 20162020: “Radiomics: Non-invasive stratification of tissue heterogeneity for personalized medicine (Radiomics STRaTegy)”
Niessen, Wiro, Ivo Schoots, Jifke Veenland , and Chris Bangma (Urology): Erasmus MC-TKI-LSH 2020 – 2023: Personalized Prostate Cancer Management using Multiparametric MRI and Machine Learning (PPCM4)
Bagdi Ulas, Pensky, Wallace, Bolan, Gonda, Hecht, Bruno Marco, Schoots Ivo . NIH grant 2021 - 2024: Deep learning methods for characterization of pancreatic cysts (Cyst-X project)
Schoots Ivo , Uulke Van der Heide (Radiation Oncology). KWF clinical implementation grant: 2021 – 2024. PROCESS study: PROstate Cancer – Expansion of Surveillance Selection criteria with MR imaging.
Van den Bergh Roderick, Rik Somford R (Urology), and Ivo Schoots .: SKMS project/ZonMW 2022 – 2025. Evaluatie en optimalisatie diagnostisch traject prostaatkanker middels MRI.
Dwarkasing Roy, Francois Willemssen , Rob de Man, Bart Takkenberg B, Carine Uyl-De Groot: KWF implementation grant. 2022 – 2025: Validation of a Short and effective MRI Surveillance protocol for hepatocellular carcinoma screening in practice.
Highlights
Daniel Osses defended his thesis “Optimizing prostate cancer screening, detection and active surveillance by risk stratification strategies” (2021) successfully.
Ivo Wagensveld was selected for the EAU Prostate Cancer Abstract Award 2021 for the best abstract published on clinical and experimental studies in prostate cancer “A prospective multicenter comparison study of a riskadapted ultrasound-directed and MRI-directed diagnostic pathway in prostate cancer suspected biopsy naïve men” at the annual congress of the European Association of Urology, virtual 2021.
Ivo Schoots and colleagues within the European Society of Uro-Radiology successfully promoted ‘MRI in prostate cancer diagnosis’ in several European webinars and online courses during the COVID-19 pandemic. Ivo Schoots published the landmark paper of the “PI-RADS Committee Position on MRI Without Contrast Medium in Biopsy-Naive Men With Suspected Prostate Cancer. AJR 2021;216:3-19, as the leading author.
Additional Personnel
Ruben van Waardhuizen, MD radiologist
During his study medicine at the Erasmus MC University Rotterdam, Ruben has started with an research internship entitled: “The Role of MRI in local staging of recurrent rectal cancer; correlation with surgical outcome”, under the supervision of dr. R.S. Dwarkasing. He had submitted a scientific abstracts, that was accepted for podium presentation during the “RadiologenDagen” 2014 and was awarded for best abstract by the The Radiological Society of the Netherlands (NVvR). In 2016 Ruben received a travel grant from the medical school faculty of the Erasmus University in Rotterdam to participate at the Radiological Society of North America, RSNA 2016, in Chicago to present his teaching exhibit on the Role of MRI in local staging of recurrent rectal cancer.

Ruben obtained his medical degree in 2015 at the Erasmus MC University and started in 2020 with a fellowship training in abdominal imaging at the department of Radiology and Nuclear Medicine of the Erasmus MC. He aspires research in collaboration with the department of surgical oncology on the added value of [18F]FDG-PET/MRI to predict tumor response to neo-adjuvant chemo-radiotherapy in patients with locally advanced rectal cancer.
François Willemssen, MD radiologist
François Willemssen obtained his medical degree cum laude in 1999 at the Catholic University of Leuven, Belgium. Afterwards, he completed his residency Radiology also cum laude in 2004 at the Catholic University of Leuven, Belgium. Since 2006 he is a member of the abdominal staff in the Erasmus MC, with main focus on abdominal imaging. His special field of interest is liver imaging, in which he contributes in several research projects. He is responsible for the radiological contribution in the Multidisciplinary Liver Tumor Board (MLTB), and he participates in the research committee of the Dutch Hepatocellular and Cholangiocellular Group (DHCG). He is involved in the revision of two national guidelines, Cholangiocarcinoma and Hepatocellular carcinoma.

IMAGING OF HEPATOBILIARY DISEASES
ROY S DWARKASING, MD, PHD
Radiologist

Roy finished his training as a radiologist at the Erasmus MC in 2002. In 2003 he became staff radiologist with special focus on Abdominal and Pelvic imaging. He finished his thesis on “Dedicated MRI of the lower pelvis” under the supervision of Prof. G.P. Krestin. PhD Awarded 17 February 2016. His research currently focusses on imaging based clinical studies of hepatobiliary diseases.
r.s.dwarkasing@erasmusmc.nl
Imaging Studies for Screening on Hepatocellular Carcinoma (HCC) in High Risk Population
Our center is a screening center for HCC in high risk patients. An internal review of our own data led to the conclusion that ultrasound (US) is inferior to MRI for detection of early HCC. To evaluate the value of MRI as a screening tool for HCC we found that 17.5 % of patients (n=240) developed HCC who underwent annual surveillance with MRI during a follow up period of over 4 years median. For patients with cirrhosis the one year cumulative incidence of HCC was 1%, 10% at three years, and 17% at five years. The study show that MRI has a higher detection rate than ultrasound for early (small) HCC in a screening population. This is supported by recent publications. From our clinical experience we believe that a short MRI screening (SMS) protocol consisting of the following sequences: T2- weighted; T1-weighted in- and out phase imaging and DWI (b 0 – 800 msec), has potential for an effective screening tool for HCC and may offer an improvement over ultrasound screening. To evaluate feasibility of SMS, we included 215 patients for further evaluation: with HCC (n=39, 18%) and HCC- naïve (n= 176). Only SMS sequences were extracted and uploaded (anonymized) to a research server. All SMS data was stored and reviewed in a random order and separately (blinded) by three radiologists with different levels of experience and. Sensitivity ranged from 78-95%, specificity 72-91%, and AUC 0.87-0.92. Intra-class correlation coefficient was 0.89 (95% confidence interval: 0.86-0.91). In addition, liver texture analysis is currently being conducted to assess: 1) discriminating features between livers with and without HCC. 2) probability of HCC development within 2 years on previous MRI in patients with HCC. To reach robust results that can be acknowledged and embraced by the ministry of Health and Health insurance, a project proposal for a prospective multicenter study comparing ultrasound screening with SMS screening was approved for funding by the Dutch Cancer Society (KWF).
Funding is granted for four years with a budget of € 600.000. This project will be conducted by two academic centers (Erasmus MC and Amsterdam MC) and three non-academic hospitals in Rotterdam (Sint Franciscus Gasthuis, Maasstad Ziekenhuis) and Dordrecht (Albert Sweitzer Ziekenhuis). In addition, cost-effectiveness of surveillance with SMS will be assessed.


Figure 1: Ultrasound versus MRI (SMS sequences) for detection of HCC in high risk patients. A) Female patient, 56 years, with history of chronic hepatitis with one focal lesion (2.5 cm, arrow) found on screening US. B) Subsequent MRI (DWI, b value 800 ms) demonstrated a total of three lesions (arrows, respectively 2.5 and 3.0 cm; third lesion not shown) that were confirmed as HCC.


Figure 2: Screening MRI for detection of very early HCC in high risk patients. A) 72-year-old man with chronic hepatitis B. On initial MRI no focal lesions were seen (DWI, b- value 600 ms). B) Follow-up MRI 12 months later revealed a 10 mm sub capsular lesion in segment six with hyper intense signal intensity of DWI (not shown) and hyper vascular arterial phase enhancement (arrow) without wash-out. The lesion was initially diagnosed as a very early HCC (BCLC 0) and successfully treated with local radiofrequency ablation (classified as LIRADS 4).
LIVER MRI AND GYNAECOLOGICAL TUMORS
MAARTEN G THOMEER, MD, PHD
Radiologist

Maarten Thomeer is appointed staff in abdominal radiology, since 2002. He successfully defended his dissertation in 2018 on Abdominal MRI in women’s health: advanced imaging with Myriam Hunink as promotor and Lena van Doorn as co-promotor. He was the cofounder of LAI consortium in 2018, an international benchmark MRI database of pathologically proven liver tumors. He is member of the research committee of the Dutch Benign Liver tumor Group (DBLTG). He was appointed delegate for the national guidelines on cervical and endometrial carcinoma which will be revealed in 2021. Maarten G Thomeer is head responsible for continuing update and quality assessment of the abdominal MRI.
m.thomeer@erasmusmc.nl
We published the results of a Dutch prospective multicenter study evaluating the complications of hepatocellular adenoma (HCA) during pregnancy. Data on the behavior of HCA during pregnancy are still very limited. This study indicates that in well-diagnosed patients, HCAs smaller than 5 cm bear minimal risk for the mother and child. However, close monitoring is still advised. We are now conducting a larger international study to eventually confirm our findings.
Current European guidelines state that HCAs who do not regress to smaller than 5 cm in follow-up should be resected. Recently, we showed that follow-up should eventually be prolonged, since they still may further regress over time after stop of oral contraception. In a new study published in the American Journal of Gastroenterology, we propose a clinical prediction model estimating this probability of further regression over time below 5 cm based on diameter at diagnosis, T0T1 regression and HCA subtype. This chance predicting app is available on hcaprediction.shinyapps.io/calculator.
Other work on HCAs currently are focusing on decisionmaking dilemmas of b-cat HCAs, including b-cat ex7/8 mutation and differentiation of lesions which strongly enhance with gadoxetate disodium (Primovist) in the hepatobiliary phase. The latter nationwide study is endorsed by the institutional members of the Dutch Benign Liver Tumor Group (DBLTG), who are acknowledged for their participation.
The Liver Artificial Intelligence Consortium (LAI-consortium), an Erasmus MC based international collaboration sharing MRI data of liver tumors in a central database
started in 2018. The two principal aims are to create a benchmark dataset for artificial intelligence (AI) applications in primary and secondary liver tumors and secondly, to develop various AI methods to make non-invasive predictions on the nature and prognosis of the liver tumors. We strive to disclose the database to the public in four years. This principle was already used in several other international MRI databases, and is mainly intended for optimization of AI through challenges.
We are currently working on different models for patient specific HCC prognosis. This patient and phenotype specific set-up is mainly intended to optimize therapy (e.g. chemotherapy) and allocation on liver transplantation list. Primary staging and non-invasive response evaluation after therapy are one of the main focusses of our research on gynaecological tumors. Since our publication in 2011 on primary staging of cervical carcinoma, together with more recent data international and national guidelines from clinicians are more and more adopting their primary work-up, putting MRI as the cornerstone of primary staging instead of gynecological examination with our without narcosis. Current research is focusing on response evaluation after radiation therapy, including stereotactic biopsy.

Figure 1. 32 y old female patient with solitary liver tumor with strong uptake of Primovist in the hepatobiliary phase. Our nationwide study on hyper/isointense liver tumors will help find new clues for more reliable differentiation.

MRI PROSTATE WITH PRIOR RISK ASSESSMENT (MR PROPER)
IVO M WAGENSVELD, MD, PHD
Project Funding ZonMw DoelmatigheidsOnderzoek 2017: Risk Assessment and MR imaging in prostate cancer diagnosis: An impact analysis.
Research period October 2020 – present Email l.wagensveld@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Urology.
Prostate cancer is the most common malignancy and leading cause of cancer-related deaths in men. However, prostate cancer has wide spectrum of aggressiveness and a large proportion of prostate cancers will never lead to health problems and mortality. In these clinically insignificant prostate cancers over-diagnosis and over-treatment should be avoided as much as possible, and therefore the diagnostic pathway for prostate cancer should be able to distinguish clinically significant cancers from insignificant cancers.
The MRI-directed diagnostic pathway of prostate cancer is now routinely recommended by many national guidelines over the traditional systematic transrectal ultrasound (TRUS)-directed diagnostic pathway.
The MR PROPER study is a prospective pseudo-randomized multicenter clinical effectiveness study and was conducted in 21 centers in the Netherlands, comparing a TRUS-directed diagnostic pathway with an MRI-directed pathway for prostate cancer. Inclusion for the MR PROPER study was concluded in 2020, and 1944 biopsy-naive men with suspected prostate cancer were included in the study.
The results of the MR PROPER study indicate MRI and TRUS diagnostic pathways preform similarly for detection of prostate cancer, but the MRI-pathway uses fewer biopsies, but costs more and is not as widely available. If prostate MRI is sufficiently available, risk-assessment should preferably be performed with MRI, reducing overdiagnosis and redundant biopsies substantially.

We expect the diagnostic results of the MR PROPER study to be published in the first half of 2022 and are currently working on an economic analysis of the different diagnostic strategies.
Figure 1: Inclusion flowchart of the in the US- and MRI-driven diagnostic pathways for the MR PROPER study

RISK STRATIFICATION AND MP-MRI IN PROSTATE CANCER DETECTION
FRANK-JAN H DROST, MD, MSC
PhD Student
Advisors Monique Roobol, Gabriel Krestin & Ivo Schoots
Project Funding Erasmus MC Grant 2015: “Combined use of Risk calculator and MRI in Prostate Cancer Detection”
Research period October 2015 – November 2018
Email f.drost@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Urology.
His project aimed to improve the detection of clinical significant prostate cancer (PCa) and the follow-up of men with low grade PCa on active surveillance. Refining risk stratification for patient selection and diagnostic tests are therefore of vital importance. He focused on mp-MRI and the ‘Rotterdam Prostate Cancer Risk Calculator’ (RPCRC) for more selectively diagnosing high-grade tumors while reducing “overdiagnosis” of low-grade tumors and costs.
The EAU Guidelines on PCa has incorporated many of the results of his recently published diagnostic test accuracy meta-analysis Cochrane review on the subject of mp-MRI, MRI-targeted biopsies (MRI-TBx) and standard biopsies (SBx). This review showed that the MRI-pathway (MRI with/out MRI-TBx) has a more favorable accuracy SBx for detecting high-grade tumors and avoiding low-grade tumors. The data included 43 pros- and retrospective studies investigating either MRI with MRI-TBx and SBx or MRI with MRI-TBx and Template-guided Bx.
The PRODROME-study (PROstate cancer Detection by additional Risk stratification and MRI Evaluation), performed in the Erasmus MC, has shown that using the RPCRC to select men, with a previous negative SBx, for MRI-TBx can reduce one third of MRI’s, almost half of biopsy procedures, one third of costs and avoids detection of 39% low grade tumors while missing only 9% of high-grade tumors, compared with a strategy in which MRI-TBx and SBx are performed in all men (Figure).
Another study in men with low grade tumors on active surveillance shows that risk stratification with the combination of MRI and PSA density during follow-up may reduce unnecessary MRI-TBx. It also showed that the higher detection
rate by MRI is an opportunity to redefine new risk thresholds for these men on active surveillance.
In another study he showed that transabdominal ultrasound (TAUS)-based risk assessment in primary care may be a cost-effective alternative to transrectal-ultrasound (TRUS)based risk assessment by the urologist to streamline opportunistic PCa screening.
He is finalizing his last paper and will defend his thesis ‘MRI and risk stratification in diagnosing and following prostate cancer patients’ on march 30, 2022.

Figure 1: Absolute prostate volume differences per case between transabdominal (TAUS) and transrectal ultrasound (TRUS) plotted against the total number of ultrasounds performed by the general practitioners.

CRIBRIFORM GROWTH IN PROSTATE CANCER AND ITS RELATION TO MRI CHARACTERISTICS
NESLISAH SEYREK, MD
PhD Student
Advisors Ivo G. Schoots, & G.J.L.H. van Leenders
Project Funding Jaap Schouten Foundation: “Significance of cribriform growth in prostate cancer risk stratification”
Research period January 2020 – July 2022
Email n.seyrek@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Pathology.
Her research aims to find radiological quantifiable features of the cribriform growth in prostate cancer patients with the guidance of multiparametric MR (mpMR) imaging.
Prostate cancer is among the most common cancers in men worldwide. Despite the favorable survival rates of prostate cancer, active surveillance eligibility of Grade group (GG) 2 prostate cancer patients still remain contradictory. Research in last decade showed that invasive cribriform and/or intraductal carcinoma (IC/IDC), especially differs from the rest of its sub-patterns due to its significant association with shorter biochemical recurrence and metastasis-free survival rates as well as higher disease specific deaths. Due to its association with aggressive PCa, identification of these growth patterns plays crucial role in disease control and treatment preference. Although IC/IDC is found more than the half of the RP specimens, almost half of the IC/IDC is being missed in biopsy which might lead to compromised treatment of patients with potentially more aggressive disease. Since mpMRI is rapidly being adopted for PCa detection, information acquired from its quantifiable parameters could also be beneficial for predicting IC/IDC prior to radical prostatectomy.
Literature has proven that tumors with cribriform growth have more aggressive and apparent MR imaging features. Although mpMR imaging is a promising modality for the tumor detection in prostate cancer patients, quantifiable MRI outcomes associated with cribriform architecture presence is little known. To this extent, Neslisah investigates the quantifiable imaging parameters of the tumor beyond the conventional PI-RADS scoring such as: measuring longest diameter of the tumor, capsular contact
length, radius of invasion, lowest ADC value; investigating bulging of the tumor, capsular line irregularity, capsular line erase, capsular breakthrough, tumor shape and neurovascular bundle asymmetry. By this means, she is aiming a meticulous and detailed approach for the lesion to define the imagine parameters associated with cribriform architecture, thus leading to better risk stratification in pre-surgical diagnostic work-up phase for prostate cancer patients.
Furthermore, she aims to extend her exploration of the quantifiable imaging parameters of cribriform growth via radiomics and potential deep learning techniques; especially in the value of ADC sequences in cribriform architecture prediction. By this means, she is aiming to identify an imaging biomarker for cribriform presence.

Figure 1: A demonstration of a) sub-regio segmentation of the prostate, b) index lesion segmentation on T2W and ADC images and c) cribriform suspicious areas.

OPTIMIZING PROSTATE CANCER SCREENING, DETECTION AND ACTIVE SURVEILLANCE BY RISK STRATIFICATION STRATEGIES
DANIËL F OSSES, MSC, MD, PHD
PhD Awarded 3 November 2021
Advisors Gabriel Krestin, Monique Roobol & Ivo Schoots
Project Funding ZonMw DoelmatigheidsOnderzoek 2017: Risk Assessment and MR imaging in prostate cancer diagnosis: An impact analysis.
Short CV From April 2017 until December 2020 Daniël Osses worked on his PhD project at the Erasmus University Medical Center. As part of his Urology traineeship, he is currently working as a resident at the General Surgery department of the Rijnstate Hospital. In the future, he will continue his traineeship at the Urology departments of the Canisius-Wilhelmina Hospital and Radboud University Medical Center.
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Urology.
The main objective of his thesis is to study whether the use of risk stratification strategies at time of prostate cancer detection (Part I) and at initiation and during an Active Surveillance (AS) strategy for low-risk prostate cancer (Part II) could safely reduce the harms of prostate cancer screening without affecting the benefit of screening, and reduce the harms of unnecessary immediate active treatment while having full cancer control.
In Part I it was shown that the use of the ERSPC Rotterdam Prostate Cancer Risk Calculator (RPCRC) in the primary care could safely reduce the rate of men with a prostate-specific antigen (PSA) level ≥3.0 ng/ml referred to the urologist with almost 50%. In addition, Part I provides evidence that the ERSPC RPCRC can be used as upfront risk stratification tool for the selection of biopsy-naïve candidates for an MRI and biopsy procedure in the urology outpatient clinic.
In Part II it is shown that the currently applied one size fits all approach in most AS protocols could result in a lot of unnecessary follow-up testing and related costs. Combining data of clinical predictors, MRI outcome and potentially visible changes on MRI is advisable as risk stratification strategy before deciding to perform a repeat biopsy procedure in men on AS. Doing so, substantial unnecessary follow-up biopsy procedures will be avoided, while maintaining a high detection rate of upgrading to high-risk prostate cancer.

http://hdl.handle.net/1765/136663

JOINT APPOINTMENT AT STANFORD UNIVERSITY
Edwin Oei is a musculoskeletal radiologist, Associate Professor of musculoskeletal imaging, and Section Chief of musculoskeletal radiology in Erasmus MC’s Department of Radiology & Nuclear Medicine. He obtained his medical degree in 2004 and his PhD on MRI for traumatic knee injury in 2009, both from Erasmus University Rotterdam. He also holds an MSc in Clinical Epidemiology from the Netherlands Institute for Health Sciences. Dr Oei is the principal investigator of musculoskeletal imaging research and engages in many academic activities including supervising 12 PhD students, lecturing, board and committee memberships, and refereeing

for various journals. He is the Treasurer in the Executive Board of the European Society for Magnetic Resonance in Medicine and Biology (ESMRMB) and the President of the Musculoskeletal MR Study Group of the International Society for Magnetic Resonance in Medicine. In 2013, Edwin Oei spent a one year research sabbatical as a Visiting Assistant Professor in the Joint and Osteoarthritis Imaging with Novel Techniques (JOINT) lab of the Department of Radiology of Stanford University, CA/USA. Dr. Oei is also the principal coordinator of the Academic Center for Molecular and Cellular Imaging at Erasmus MC. e.oei@erasmusmc.nl oei@stanford.edu
ADVANCED MUSCULOSKELETAL IMAGING RESEARCH ERASMUS MC (ADMIRE)
EDWIN HG OEI, MD, PHD associate professor

Top Publications 2021
Breda SJ, Oei EHG, Zwerver J, Visser E, Waarsing E, Krestin GP , de Vos RJ. Effectiveness of progressive tendon-loading exercise therapy in patients with patellar tendinopathy: a randomised clinical trial. Br J Sports Med. 2021 May;55(9):501-509.
Hirvasniemi J, Klein S, Bierma-Zeinstra S, Vernooij MW , Schiphof D, Oei EHG . A machine learning approach to distinguish between knees without and with osteoarthritis using MRI-based radiomic features from tibial bone. Eur Radiol. 2021 Nov;31(11):85138521.
Van Zadelhoff TA, Okuno J, Bierma-Zeinstra SMA, Bos PK, Moelker A, Oei EHG. Association between Baseline Osteoarthritic Features on MR Imaging and Clinical Outcome after Genicular Artery Embolization for Knee Osteoarthritis. J Vasc Interv Radiol 2021 Apr;32(4):497503.
Context
Our research emphasis is on advanced imaging of musculoskeletal diseases, particularly those with a large burden for patients and society, such as osteoarthritis, osteoporosis, sports injuries, and chronic musculoskeletal pain. Sensitive and accurate imaging biomarkers are currently lacking, yet they are key to detecting these diseases earlier, providing better understanding of their etiology and pathophysiology, and discovery of new therapies. Therefore, we develop, improve, and validate innovative CT, MRI, ultrasound and nuclear imaging techniques, and apply these in clinical studies. Many of these techniques are aimed at visualizing and quantitatively measuring compositional changes in tissues such as cartilage and meniscus, or pathological tissue processes such as inflammation, abnormal perfusion or increased metabolic activity. Another important research focus is on musculoskeletal population imaging, in which we utilize information from MRI and other imaging techniques in the largescale population based Rotterdam Study and the Generation R cohort to address the epidemiology, genetics, and development of musculoskeletal diseases. We also are active in improving image analysis methods, among other using radiomics and artificial intellgence algorithms.
Research Projects: Objectives & Achievements
Development
and validation of
novel quantitative imaging techniques for joint diseases
This effort focuses on improved acquisition and image analysis, assessment of reproducibility, and validation of innovative quantitative MRI, CT and ultrasound techniques for joint diseases. One of the larger efforts is in quantitative MRI and CT methods for cartilage that provide information on cartilage quality. By measuring key biochemical cartilage composites — proteoglycans and collagen — that are affected in early stages and with subtle changes of cartilage diseases such as osteoarthritis, these techniques enable study of cartilage disease at earlier stages and accurate follow-up with numerical outcomes. Our wide range of available quantitative imaging techniques include delayed gadolinium enhanced MRI of cartilage (dGEMRIC), T2-mapping (Figure), T1-rho mapping, quantitative CT arthrography and ultrashort echo time (UTE) MRI, most of which are also useful to study the fibrocartilage of the meniscus.
We have an established track record in validation such techniques acquired in-vivo with feasible clinical protocols against a wide range of ex-vivo laboratory reference tests, for example in patients undergoing total knee replacement to validate compositional cartilage techniques. Similarly, we have validated in-vivo T2-mapping of the meniscus against tissue analysis after meniscectomy. We also validate techniques against clinical outcomes, for example in a recently completed study of T1 and T1rho cartilage mapping conducted in clinical patient care in collaboration with the Joint and Osteoarthritis Imaging with Novel Techniques (JOINT) lab (PI: Garry E Gold, MD) and Body Magnetic Resonance (BMR) Group (PI: Brian Hargreaves, PhD) of the Department of Radiology of Stanford University.
Synovitis, characterized by synovial membrane thickening and joint effusion, is frequently observed in osteoarthritis and is considered a potential target for novel treatment strategies. Also in collaboration with Stanford University, we studied a modified version of the novel three-dimensional (3D) double echo steady state (DESS) sequence to image synovitis without contrast agent by applying a diffusion gradient between the first and second echoes. In this project, we validated the technique against contrast-enhanced MRI, and compared the findings with power-Doppler and contrast-enhanced ultrasound (CEUS), tissue references, and blood markers of inflammation in patients with various degrees of knee osteoarthritis and synovitis. We demonstrated that DESS MRI is suitable to diagnose synovitis, albeit with an undersestimation of severity.
We also have a particular interest in perfusion imaging using dynamic contrast-enhanced MRI (DCE-MRI). The effort is targeted at improving DCE-MRI image acquisition and image analysis including pharmacokinetic modeling, and applying DCE-MRI in clinical studies to quantitatively study abberant perfusion particularly in knee osteoarthritis features such as bone marrow lesions, synovitis and inflammation of Hoffa’s fat pad.
In this research line, we collaborate closely with the MR Physics in Medicine group (Juan Hernandez Tamames, PhD, Gyula Kotek, PhD, and Piotr Wielopolski, PhD), Biomedical Imaging Group Rotterdam (Stefan Klein, PhD and Dirk Poot, PhD), Department of Orthopedic Surgery (Gerjo van Osch, PhD), and General Electric Healthcare (Maggie Fung, PhD, Mika Vogel, PhD).
Application of quantitative MRI techniques in clinical osteoarthritis studies
Quantitative MRI techniques for measuring cartilage composition are applied in many clinical studies conducted by our group or in collaboration with other partners. For example, we performed a study in 20 early-stage knee osteoarthritis patients treated with viscosupplementation with hyaluronic acid in which we applied dGEMRIC before and after the treatment to assess the potential beneficial effect of hyaluronic acid on cartilage composition. The study showed no compositional cartilage changes before and after therapy. In another study, we applied dGEMRIC, T2-, and T1rho-mapping to study the role of cartilage changes in the development or pain perception in patellofemoral pain syndrome. In this project, we also studied applied DCE-MRI to characterize blood perfusion in several joint tissues. T2- and T1rhomapping have also been applied as outcome measures in randomized controlled clinical trials on high tibial osteotomy versus brace treatment, and on conservative versus operative treatment for traumatic meniscal tears. We also apply single-photon emission computed tomography (SPECT-CT) to study bone activity in patients with predominantly unicompartmental osteoarthritis before and after treatment. Advanced quantitative MRI techniques to study cartilage composition and perfusion are also applied in a therapeutic randomized controlled clinical trial on the efficacy of transarticular genicular artery embolization for the treatment of knee osteoarthritis (NEO-study) that led by our group and currently almost completed. In these clinical studies we collaborate closely with the Departments of Orthopedic Surgery (Max Reijman, PhD, PhD, Koen Bos, MD, PhD, Jan Verhaar, MD PhD) and General Practice (Sita BiermaZeinstra, PhD and Marienke van Middelkoop, PhD).
Clinical osteoarthritis studies with morphological imaging
Although the role of advanced quantitative imaging methods for osteoarthritis is increasingly recognized as mentioned above, more traditional morphological imaging techniques remain important. Therefore, we participate in several clinical studies that use morphological MRI as an outcome. These include ongoing studies into the occurrence of early osteoarthritis after anterior cruciate ligament (ACL) rupture, after ACL reconstruction (KNALL study), and after knee trauma in general practice, a study on the preventative effect of weight reduction and glucosamine in overweight women on the incidence of knee osteoarthritis (PROOF study), a study on the effect of menopause on the development of knee osteoarthritis (FOCUM trial), and a study into the effect of a lifestyle intervention on knee ostearthritis development (LITE study)
We typically apply the semi-quantitative MRI Osteoarthritis Knee Score (MOAKS) on the acquired MRI scans and have devised a MOAKS training program for researchers. Using the results of these studies, we are also involved in defining criteria for the diagnosis and progression of osteoarthritis based on morphological features. In the multicenter CHECK cohort of over 1000 participants we assess the incidence of radiographic knee and hip osteoarthritis using a long follow-up of 10 years. In these projects we collaborate with the Departments of Orthopedic Surgery (Max Reijman, PhD; Duncan Meuffels, MD, PhD, Jan Verhaar, MD, PhD) and General Practice (Sita Bierma-Zeinstra, PhD, Jos Runhaar, PhD) of Erasmus MC, and with the Department of Radiology of Maasstad Hospital Rotterdam (Dammis Vroegindeweij, MD PhD). Our expertise with MOAKS is also the focus of several international collaborations e.g. with Stanford University (Feliks Kogan, PhD) with regard to a project on PET-MRI for osteoarthritis, the University of Queensland, Brisbane, Australia (Natalie Collins, PhD) concerning a study on patellofemoral pain, LaTrobe University, Melbourne, Australia (Adam Culvenor, PhD) with regard to a project on knee osteoarthritis after cruciate ligament rupture, and with Lund University, Sweden (Martin Englund, PhD) concerning a knee osteoarthritis study using 7T MRI.

Advanced imaging of sports injuries
We recently completed the first phase of a s tudy funded by the National Basketball Association of the US and GE Healthcare into patellar tendinopathy (jumper’s knee) in jumping athletes. In this randomized controlled trial of two different exercise therapies to treat this debilitating condition, we implemented advanced MRI and ultrasound imaging techniques at three time points before and after therapy. In particular, MRI was performed using a 3D ultrashort echo time (UTE) technique (3D UTE Cones) that allows high resolution imaging and T2* mapping of the inflamed tendon (Figure 1). Ultrasound was performed with shearwave elastrography, which probes the stiffness of tendon tissue as an additional imaging biomarker. Key findings of the study include 1) In patients with patellar tendinopathy, progressive tendon-loading exercises (PTLE) resulted in a significantly better clinical outcome after 24 weeks than eccentric exercise therapy (EET); 2) T2* relaxation times of the patellar tendon decreased significantly in athletes with patellar tendinopathy performing exercise therapy at 24 weeks and this decrease was associated with improved clinical outcome, and 3) Patellar tendinopathy is associated with significantly higher patellar tendon stiffness in SWE and decrease in stiffness during early recovery phase corresponds to better clinical outcome. In 2022, we will start 5-years follow-up measurements including questionnaires and ultrasound, which will make this study the largest RCT into patellar tendinopathy with long-term follow-up/
This is study is conducted in collaboration with the Department of Orthopedics & Sports Medicine (Robert-Jan de Vos, MD, PhD, Denise Eygendaal, MD, PhD) and the University Medical Center Groningen (Hans Zwerver, MD, PhD).
Figure 1. Axial 3D ultrashort echo time (UTE) MRI image of a patient with patellar tendinopathy. An area of increased signal intensity is visible in the patellar tendon (left). Quantitative analysis (right) shows subregions with different pools of water with short, mixed and long T2* relaxation times.

PET-MRI to study chronic pain generators
We are currently finalizing the preparations of a new project on PET-MRI to study chronic pain generators, for which inclusion is expected to start in March 2022. In this study, we will apply PET-MRI with the purpose of elucidating pain generators in patients with chronic low back or hip pain. A randomized controlled clinical trial of 200 patients will be performed in which patients are randomized to either undergo PET-MRI or follow usual care (without PET-MRI). A comprehensive whole-body 18F-FDG PET-MRI protocol will be performed, including DCE-MRI and post-contrast acquisitions. We will assess pain symptoms over a 1 year follow-up period in both trial arms, as well as record differential diagnosis, treatment plan, and confidence in the diagnosis with treating physicians. In patients treated for a specific lesion visible on PETMRI, we will repeat PET-MRI after six months. This effort will not only focus on image acquisition, but also on image analysis and structured reporting. For this study we collaborate with nuclear medicine physicians in our department, with the Pain Medicine Center of Erasmus MC (Frank Huygen, MD, PhD, Cecile de Vos, PhD, Joost Jongen, MD, PhD), with Stanford University (Sandip Biswal, MD, Daehyun Yoon, PhD) where this application of PET-MRI was developed, and with GE Healthcare.
Population imaging in the Rotterdam Study: Osteoarthritis, osteoporosis and Scheuermann’s disease
We participate actively in the Rotterdam Study (RS), an open population-based cohort study of nearly 15000 individuals aged ≥ 45, applying knee, hip, hand, and spine radiography in all participants for classic and genetic epidemiological studies on osteoarthritis. Using radiological phenotyping, this has led to the discovery of several (genetic) determinants of osteoarthritis and associations with other diseases. More imaging data is being collected on a continued basis, which will provide long-term follow-up for a large number of participants. Knee MRI was acquired at two time points in a subgroup of 891 women, for which the MRI protocol included quantitative T2-mapping of cartilage, which enhances sensitivity for detection of osteoarthritis onset or progression.
We also study epidemiology and genetics of osteoporosis with radiological phenotyping in RS. For this purpose, we assess digitized lateral spine radiographs acquired in all participants for osteoporotic vertebral fractures. We apply semi-quantitative algorithm-based and computer assisted automated morphometric methods scoring methods for vertebral fractures, and assess differences between them. The data are used for (genetic) epidemiological

analyses and to study associations with other biomarkers including dual-energy X-ray absorptiometry (DXA). In addition, we study the epidemiology of Scheuermann’s disease, a mimicker of vertebral fractures. In RS, we collaborate with the Departments of General Practice (Sita Bierma-Zeinstra, PhD, Dieuwke Schiphof, PhD) and Internal Medicine (André Uitterlinden, PhD, Fernando Rivadeneira, MD PhD, Joyce van Meurs, PhD, Ling Oei, MD, PhD).
Population imaging in the Generation R cohort
In the context of a multidisciplinary collaborative effort of the Departments of Radiology & Nuclear Medicine, Orthopedic Surgery, General Practice, Internal Medicine, and Epidemiology, we apply MR imaging of the hips, knees and spine in the Generation R cohort, a populationbased prospective cohort study from fetal life until young adulthood in a multi-ethnic urban population. The study is designed to identify early environmental and genetic causes of normal and abnormal growth, development and health. Our effort focuses on the determinants of normal and abnormal musculoskeletal growth and development, as well as the risk factors of musculoskeletal diseases. Studying early life determinants of musculoskeletal health will potentially help to identify individuals at risk to develop musculoskeletal diseases later in life and allow implementing interventions to maintain musculoskeletal health and delay the onset of disabling diseases like osteoporosis and osteoarthritis. While follow-up MRI
Figure 2. 3D segmentation of the knee bones from MRI in the Generation R study.

measurements at the age of 17-19 years are currently being performed, our current multidepartmental effort is targeted on analyzing hip, knee (Figure 2) and spine scans acquired at the age of 9 and 13 years, with the help of the Biomedical Imaging Group Rotterdam. On these scans, we aim to identify MR image specific markers of pre-symptomatic diseases, visualize normal and abnormal growth and development patterns. In addition, our group has also performed whole body composition MRI scans at the age of 13 years, which we have recently processed with regard to visceral adipose tissue (VAT). We are currently analyzing relationships between VAT and lung function outcomes and physical activity. In this project we collaborate with the Department of Pediatrics (Vincent Jaddoe, MD, PhD, Liesbeth Duijts, MD, PhD), Public Health (Hein Raat, MD, PhD), and with the German Center for Neurodegenerative Diseases (DZNE) (Santiago Estrada, MSc).
Advanced post-processing and analysis of musculoskeletal imaging data
In collaboration with the Biomedical Imaging Group Rotterdam (BIGR), we developed Software for Postprocessing And Registration of Cartilage of the Knee (SPARCK), which is an advanced post-processing pipeline for quantitative knee MRI data from dGEMRIC, T2-, and T1rho mapping. As an important feature, the pipeline includes automated image registration algorithms which corrects for patient motion and allows for systematic comparison of different MRI techniques on matching
slices and in matching cartilage regions. The software is being improved and expanded on a continuous basis and this process is driven largely by the questions and requirements of specific clinical studies. For example, the SPARCK now includes automated registration capabilities for the patellofemoral joint in addition to the femorotibial joint, and is also able to analyze blood perfusion data from dynamic contrast enhanced MRI (DCE-MRI). Together with BIGR, we are currently evaluating different analysis methods and comparing different pharmacokinetic models for quantitative DCE-MRI of bone.
We also are applying deep learning methods and radiomic analysis for the analysis of MRI and radiography data, particularly for osteoarthritis. Related to MRI in the Generation R study (see above) and other largescale clinical studies, we collaborate closely with BIGR on automated segmentation tools for bone, cartilage, mensicus and synovium (Figure 3). The closest collaborators in BIGR include Stefan Klein, PhD, Dirk Poot, PhD, Jukka Hirvasniemi, PhD, and Wiro Niessen, PhD. We also collaborate with the University of Oulu, Finland (Aleksei Tiulpin, PhD, Simo Saarakkala, PhD) on deep learning in osteoarthritis imaging.
Finally, we are conducting several projects on artificial intelligence for musculoskeletal imaging in the clinic, for instance for the detection of fractures and diagnosis and grading of hip and knee osteoarthritis. These projects are conducted together with the Value-based Imaging Group (Jacob Visser, MD, PhD) and various industrial collaborators.
Miscellaneous musculoskeletal imaging studies
In collaboration with the Departments of General Practice, Clinical Genetics, Rheumatology, Orthopedics and Sports Medicine, and Traumatology, we participate in many other musculoskeletal imaging studies, mostly emphasizing on trauma related, inflammatory and degenerative musculoskeletal conditions. For example, in a cohort study of ankle injured patients in general practice we perform MRI to study determinants for persistent complaints and the occurrence of osteoarthritis, This study is conducted in collaboration with Albert Schweitzer Hospital, Dordrecht (Nienke Katier, MD, PhD). Examples of other studies in which we participate include a clinical study of families with a genetic SMAD3 mutation that predispose to extensive musculoskeletal degenerative abnormalities, projects on advanced statistical shape modeling with radiographic, MRI and CT data to study the relationship of femoro-acetabular impingement of the hip and early osteoarthritis; on advanced MRI with CT-like images for pubic stress injuries; on a rapid non-contrast
Figure 3. Automated segmentation of the synovium from MRI of the knee based on a DCE-MRI sequence.
Dixon MRI sequence of the hands to evaluate clinically suspect arthralgia; and several studies on radiography and MRI of spine degeneration.
Expectations & Directions
The Musculoskeletal Imaging Research Group’s activities will continue to focus on the development, improvement, and validation of innovative imaging methods for common musculoskeletal diseases such as osteoarthritis and sports injuries. While quantitative MRI techniques for a variety of joint tissues (e.g. joint cartilage, meniscus, ligaments) and processes (e.g. synovitis and blood perfusion) will remain an important research focus, there will also be an increased research activity in nuclear imaging and CT techniques
In particular, the ADMIRE group is planning to further expand its activities on the PET-MRI scanner and will conduct several projects on PET-MRI of knee osteoarthritis. Additionally, the installation of a photon counting CT (PCCT) scanner in 2021 has opened up new avenues for musculoskeletal applications that we are currently exploring and will most likely be growing into a separate research line. Promising applications of PCCT include ultra high resolution imaging of fractures and bone structure (to characterize osteoporosis) (Figure 4), improved imaging around metal implants, and improved visualization of soft tissues based on spectral information.
Further, our group will further strenghten collaborations with TU Delft in the context of the Convergence initiative between Erasmus MC and TU Delft. In particular, we will launch new projects linking advanced imaging with data acquired in a novel biomechanics imaging and gait lab that is currently being installed in our department (collaboration with Jaap Harlaar, PhD). We are also embarking on new collaborations with engineers in MR physics and image analysis at TU Delft. Given the trends in artificial intelligence, we expect that this will the topic of more research, specifically related to development of new algorithms, validation, and determining the added value, both in the context of clinical research and clinical musculoskeletal radiology.
In population imaging, particularly Generation R, we will be able to study increasingly available imaging biomarkers with other (clinical, genetics, environmental, behavioral, etc) data collected in the project, while we will continue extracting more imaging markers from the wealth of available MRI scans.

Consequently, it is expected that the ADMIRE group will further strengthen its position within the multidisciplinary research network around osteoarthritis (Rotterdam OsteoArthritis Research; ROAR) and other musculoskeletal diseases in Erasmus MC Rotterdam and in several Academic Centers within Erasmus MC. We also aim to further intensify our strong collaboration with local and international partners, in particular with Stanford University and the University of Wisconsin, as well as with industrial collaborators such as General Electric Healthcare and Siemens Healthineers.
Funding
Oei, Edwin : General Electric Healthcare: “Pinpointing the source of chronic pain and therapy response with wholebody 18F FDG-PET/MRI” (2020-2024)
Oei, Edwin : National Basketball Association / General Electric Healthcare Orthopedics and Sports Medicine Collaboration Tendinopathy Call for Proposals: “Progressive tendon-loading exercise therapy for patellar tendinopathy in jumping athletes: a randomized controlled clinical trial evaluated with advanced 3D ultrashort echo time MRI” (2016-2021)
Oei, Edwin : Coolsingel Foundation: “Embolization therapy as a novel treatment for knee osteoarthritis: a randomized multicenter trial in the Rotterdam region” (20192021)
Oei, Edwin : Dutch Arthritis Association: “Assessment of knee synovitis with novel non-contrast MRI and ultrasound” (2016-2021)
Figure 4. High resolution imaging of the elbow with detailed visualization of trabecular bone structure using photon counting CT.
Oei, Edwin: Erasmus MC Efficiency Research: “Novel transcatheter arterial embolization for treatment of knee osteoarthritis: a randomized sham-controlled clinical trial” (2019-2021)
Van der Heijden, Rianne : Young Reseachers Grant, the European Society of Musculoskeletal Radiology (ESSR) 2018: “Shedding light on infrapatellar fat pad signal abnormalities and blood perfusion using quantitative dynamic contrast enhanced MR” (2018-2021)
Oei, Edwin (co-applicant): Horizon 2020 EIC Accelerator: “AI algorithms in musculoskeletal radiography”. Main applicant: Radiobotics, Copenhagen, Denmark (2020-2023)
Oei, Edwin (co-applicant): Independent Research Fund Denmark: “Investigating pathology and tissue biomarkers of Osgood Schlatter to enhance treatment of children with growth-related pain”. Main applicant: M.Rathleff, University of Aalborg, Denmark (2019-2023)
Oei, Edwin (co-applicant): NWO Zon-MW Open Competition: “Biomechanical precision diagnostics in osteoarthritis.”. Main applicant”. Main applicant: S. Bierma-Zeinstra (General Practice/Orthopedics) (2020-2025)
Oei, Edwin (co-applicant): TU Delft-Erasmus MC Convergence Flagship Themes: “Deep imaging-genetics for osteoarthritis”. Main applicant: S.Klein (Radiology & Nuclear Medicine), M. Loog (TU Delft) (2020-2023)
Oei, Edwin (co-applicant): TU Delft-Erasmus MC Convergence Flagship Themes: “Precision biomechanics diagnostics of cartilage load in knee osteoarthritis.”. Main applicant: S. Bierma-Zeinstra (General Practice), J Harlaar (TU Delft) (2020-2023)
Oei, Edwin (co-applicant): ZonMw Gender en Gezondheid - Algemene onderzoeksronde: “The FOCUM human disease model for development of OA”. Main applicant: S.M.A. Bierma-Zeinstra (General Practice) (2019-2021)
Oei, Edwin (co-applicant): ZonMw Gender en Gezondheid - Algemene onderzoeksronde: “IFEROA: Identification of the female specific etiology and risk groups for osteoarthritis” (2018-2022) Main applicant: S.M.A. Bierma-Zeinstra (General Practice)
Oei, Edwin (co-applicant): ZonMw Gender en Gezondheid - Algemene onderzoeksronde: “Diagnosis, prevalence and associated factors of osteoarthritis in adults with intellectual disabilities.” (2020-2026) Main applicant: D.A.M. Maes-Festen (AVG/General Practice)
Oei, Edwin (co-applicant): Reumafonds (Dutch Arthritis Foundation) Fundamental Research 2017: “A gut feeling about osteoarthritis: the role of the gut microbiome in osteoarthritic pain and progression” (2018-2022) Main applicant: J.B.J. van Meurs (Internal Medicine)
Oei, Edwin (co-applicant): National Health and Medical Research Council, Australia: “SUPER rehabilitation RCT for young people with old knees”. (2018-2022) Main applicant: K. Crossley (La Trobe, Melbourne, Australia)
Oei, Edwin (project team member): FOREUM Foundation for Research in Rheumatology, Preclinical Phases of Rheumatic and Musculoskeletal Diseases: “Novel Treatment Targets in Early-stage Osteoarthritis” (2018-2022) Main applicant: M. Englund (Lund University, Sweden):
Reijman, Max (Orthopedic Surgery), Edwin Oei , and partners: NWO ZonMw Health Care Efficiency Research Grant 2014- 2020: “Should a traumatic meniscal tear be resected?”
Oei, Edwin : Hitachi Medical Systems/RSNA Research Seed Grant: “Quantitative dynamic contrast-enhanced MRI for studying bone perfusion” (2015-2019)
Sita Bierma-Zeinstra (General Practice), Jan Verhaar (Orthopedic Surgery), and Edwin Oei : Netherlands Orthopaedic Association/ Dutch Arthritis Foundation 2013-2017: “Optimal timing for orthopaedic surgery in osteoarthritis” (2013-2019)
Invited Lectures
Oei, Edwin: “From first abstract to being a PI”. International Society for Magnetic Resonance in Medicine (ISMRM) Benelux Chapter Event, Online, 26 March 2021.
Oei, Edwin: “Year in Review 2021: Imaging”. Osteoarthritis Research Society International (OARSI) World Congress on Osteoarthritis, Online, 1 May 2021.
Van der Heijden, Rianne: “Frontiers in MSK MRI: Perfusion and Contrast Kinetics”. 29th Annual Meeting of the International Society for Magnetic Resonance in Medicine (ISMRM), Online, 15-20 May 2021.
Oei, Edwin : “Quantitative MRI Needs and Opportunities: OA Population Studies”. 29th Annual Meeting of the International Society for Magnetic Resonance in Medicine (ISMRM), Online, 15-20 May 2021.
Oei, Edwin : “Imaging of patellar tendinopathy”. Vereniging voor Sportgeneeskunde (VSG). Zeist, Netherlands, 5 October 2021.
Van der Heijden, Rianne : “Role of imaging in spinal pain”. European Pain Federation (EFIC) Virtual Pain Education Summit, 4-6 November 2021.
Hanff, David: Sandwhichcursus MSK: traumatische 0302-2021: “Traumatische knieletsels, wat de orthopedisch chirurg wil weten” .
Hanff, David: Jaarcongres van college van clubartsen en consulenten: 22-05-2021: ”Aanvullende beeldvormende diagnostiek van de lies”.
Hanff, David: British Association of Sport and Exercise Medicine Spring conferense 28-5-2021(2 praatjes):Title: Imaging of the Athletic Groin Pain: The insides of a radiologist ” Imaging of the hip in athletes”.
Highlights
Edwin Oei co-hosted the International Workshop on Osteoarthritis Imaging (IWOAI) in Rotterdam from 30 June to 2 July 2021 which, after a period of lockdown, was attended by 25 in-person and 100 online attendees.
Rianne van der Heijden was awarded a Bracco Fellowship for Translational Research in Advanced MRI which will allow her to spend two years as a Visiting Assisting Professor at the University of Wisconsin, Madison, USA as of April 2022.
Edwin Oei was the Education Officer and subsequently the Treasurer in the Executive Board of the European Society for Magnetic Resonance in Medicine and Biology (ESMRMB).
Additional Personnel
Galied SR Muradin, MD, PhD, Musculoskeletal radiologist
Edwin Oei was the President of the Musculoskeletal MR Study Group of the International Society for Magnetic Resonance in Medicine (ISMRM).
Edwin Oei was a member of the European Imaging Biomarkers Alliance (EIBALL).
Stephan Breda won the best oral presentation award during the ‘Star Paper Session’ of the annual scientific meeting of the Dutch Society for Sports Medicine (VSG) for his presentation entitled “Effectiveness of Progressive Tendon-Loading Exercise Therapy in Patients with Patellar Tendinopathy: A Randomised Clinical Trial.
Edwin Oei joined the editorial board of the new journal “Osteoarthritis Imaging”.
Rianne van der Heijden joined the Open Science Initiative for Perfusion Imaging (OSIPI) working group, part of the part of ISMRM Perfusion Study Group as well as the DCE-MRI and MSK Biomarker Committees of the Quantitative Imaging Biomarkers Alliance (QIBA) of the RSNA. With OSIPI, Rianne co-authored a summa cum laude award-winning poster at the ISMRM annual meeting 2021.
Edwin Oei delivered a prestigious invited lecture on the “Year in Review 2021: Imaging” during the virtual Osteoarthritis Research Society International (OARSI) World Congress on Osteoarthritis on 1 May 2021.
Edwin Oei was a co-guest editor of a special issue on “Cartilage Assessment using Quantitative MRI” in Frontiers in Endocrinology.
Edwin Oei served as a member of Scientific Program Committee Annual Meeting of the Radiological Society of North America (RSNA), the Annual Meeting Program Committee of the International Society for Magnetic Resonance in Medicine (ISMRM), and the Congress Planning Committee of the Annual Meeting of the European Society for Magnetic Resonance in Medicine and Biology (ESMRMB), all held on-line or as hybrid meetings due to the Covid-19 pandemic.
Galied Muradin is a musculoskeletal radiologist in Erasmus MC Rotterdam. He is involved in several multidisciplinary research projects focusing on MRI and ultrasound imaging in early rheumatoid arthritis, high resolution MRI imaging of osteoarthritis in small joints, high resolution MRI imaging of tendon injuries, CT scan protocol evaluation of patients with high energy trauma, CT imaging of cervical spine fractures, MRI imaging of knee injury, and MRI imaging of chondroid bone lesions. These projects are performed in collaboration with the Departments of Trauma Surgery, Neuro-surgery, Rheumatology, Plastic & Reconstructive Surgery, Rehabilitation Medicine, and Pathology.

David Hanff, MD, Musculoskeletal radiologist
David Hanff is a staff musculoskeletal radiologist in Erasmus MC Rotterdam who is involved in several clinical research studies, particularly in the field of sports medicine (e.g. on groin injuries, knee instability after injury, and femaro-acetabular impingement) and bone imaging with MRI. In these studies, Dr. Hanff is responsible for the interpretation of imaging findings on radiography, ultrasound, CT and MRI. Dr. Hanff also has a particular interest in musculoskeletal tumors, on which he collaborates in research projects with Leiden University Medical Center.

Huib Ruitenbeek, MSc, Junior researcher
Huib Ruitenbeek has a background in Technical Medicine is working in the ADMIRE group as a junior researcher. His research focuses on the value of Artificial Intelligence (AI) in Musculoskeletal Radiology and the applicability of existing commercial software suites. He will study several AI algorithms, including those able to detect fractures througout the body on radiographys, fractures of the cervical spine on CT and algorithms that detect and grade knee and hip osteoarthritis. Clinical evaluation of these algorithms and studies describing the effect on radiology workflow are limited. He collbarates with several AIvendors and is also embedded in the Value-based Imaging group.

Eveline Molendijk, MD, Junior researcher
Eveline Molendijk is a junior researcher co-affiliated with the Department of General Practice of Erasmus MC. She is leading the FOCUM (Females discontinuing Oral Contraceptives Use at Menopausal age) study, which is investigating the influence of menopause on knee osteoarthritis. FOCUM is an observational prospective cohort study with two years of follow-up in females between 50-60 years of age. A “sudden menopause” in this study is modeled as a change in hormones due to stopping oral contraceptive (OC) use. Osteoarthritis is characterized on advanced MRI including T2-mapping. In addition, radiographs and DXA scanned are performed. 2 years follow-up of FOCUM will start mid-2022.

Núria Jansen, MSc, PhD stud ent
Núria Jansen is a PhD student affiliated with the Department of General Practice of Erasmus MC, but with strong links to the ADMIRE group. She is leading the Leefstijl Interventie Trial (LITE) study, which is a randomized controlled clinical trial of 234 subjects investigating the influence of a combined lifestyle intervention, compared to regular care, on knee osteoarthritis. Imaging in this study consists of morphological MRI performed at baseline and after 2 years of follow-up. These MRI scans will be assessed for structural osteoarthritis using the MRI Osteoarthritis Knee Score (MOAKS).


RIANNE A VAN DER HEIJDEN, MD, PHD ADVANCED (PET)-MRI IN MUSCULOSKELETAL PAIN
Post-doc
Project Funding Imaging Program of Excellence, Department of Radiology & Nuclear Medicine, Erasmus MC
Research period July 2019 – Present
Email r.a.vanderheijden@erasmusmc.nl
These projects are collaborations with the Departments of Radiology & Nuclear Medicine, General Practice, Neurology, Orthopedics and Anesthesia.
In her post-doctoral project, dr. van der Heijden focuses on elucidating the pathophysiology of musculoskeletal conditions using advanced quantitative (PET)-MRI.
Chronic musculoskeletal pain is an immense burden for patients and society. Adequate treatment is often lacking, mainly because the exact pathophysiology is unknown and the pain generator remains unidentified by conventional imaging methods. Advanced imaging techniques are needed to solve this problem. Dynamic contrast-enhanced magnetic resonance imaging (DCE MRI) measures the blood perfusion of tissues. Increased perfusion occurs when there is an increased demand of oxygen, for instance in tumoral processes or inflammation. Fluorodeoxyglucose positron emission tomography ([18F] FDG PET) MRI measures glucose metabolism. Increased glucose metabolism is known to accompany pain due to the increased demand in case of increased synaptic signaling and/or inflammation.
One of her projects focused on the infrapatellar fat pad (IPFP). The IPFP is a reservoir of inflammatory cytokines. In case of inflammation this would be the first site to show increased perfusion (Figure 1). However, patients with knee pain did not show increased perfusion of the IPFP compared to controls. Moreover, inflammatory and neo-angiogenic biomarker levels were not higher among patients with knee pain. She is also actively involved efforts to accelerate the clinical implementation of quantitative DCE-MRI. She joined the Quantitative Imaging Biomarker Alliance (QIBA) of the Radiologic Society of North America (RSNA), which aims to standardize acquisition, and the Open Science Initiative for Perfusion Imaging (OSIPI) of the International Society for Magnetic Resonance Imaging in Medicine (ISMRM) with the aim to standardize post-processing.
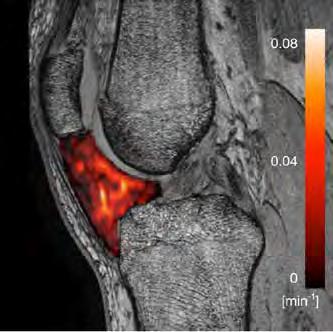
Figure 1. Overlay of a Ktrans perfusion map of the IPFP on the non-fat-saturated fast-spoiled gradient-echo sequence in a patient with knee pain.
Furthermore, dr. van der Heijden co-supervises the new PET-MRI project for chronic low back and hip pain.
She has been awarded a Bracco Fellowship for Translational Research in Advanced MRI by the Department of Radiology, University of Madison, Wisconsin, USA, where she will become a visiting assistant professor for two years after finishing her residency early 2022.

ADVANCED MRI AND ULTRASOUND OF
PATELLAR TENDINOPATHY
STEPHAN J BREDA, MD
PhD Student
Advisors Edwin Oei, Robert-Jan de Vos & Gabriel Krestin
Project Funding National Basketball Association / General Electric Healthcare Orthopedics and Sports Medicine Collaboration Tendinopathy CFP: “Progressive tendon-loading exercise therapy for patellar tendinopathy in jumping athletes: a randomized controlled clinical trial evaluated with advanced 3D ultrashort echo time MRI”
Research period September 2016 – present
Email s.breda@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Orthopedic Surgery
The Jumper Study is a randomized controlled trial in 76 athletes with patellar tendinopathy (Jumper’s knee) aged 18-35 years. Athletes included in this study play tendon-loading sports (e.g. basketball, volleyball) for at least three times a week. Symptoms of the Jumper’s knee include pain at the attachment of the patellar tendon to the inferior patellar border, decreased exercise tolerance and even rupture. Exercise therapy is a key component for the treatment of patellar tendinopathy. Eccentric exercise therapy (EET) is considered as the best available treatment, despite the fact that these exercises are painful to perform and have found to be ineffective when applied in-season. Progressive tendonloading exercises (PTLE), which have been proposed as an alternative therapy, are performed within the limits of pain.
The main purpose of the Jumper Study was to compare the effectiveness of usual care EET and PTLE. Participants were randomly assigned to EET or PTLE, with a follow-up duration of 24 weeks. The main investigator was blinded for the treatment allocation. Primary outcome was the validated VISA-P questionnaire (scale 0-100), where a score of 100 represents no pain, maximum function and unrestricted ability to play sports. Secondary outcomes included strength and flexibility measurements, additional questionnaires and quantitative imaging methods. Shear-wave elastography was performed to assess patellar tendon stiffness. Quantitative assessment of T2* relaxation times was performed using 3D ultrashort echotime (UTE) MRI.

Figure 1. The unadjusted time course of the mean VISA-P score in the PTLE-group (intervention) and EET group (control).
We found that PTLE was superior to usual care EET after 24 weeks follow-up. The adjusted mean between-group difference on the VISA-P scale was 9 points (Figure). There was a trend towards a higher return to sports rate in the PTLE-group (43% vs. 27%). Both quantitative imaging methods demonstrated the patellar tendon’s ability to undergo structural changes in response to exercise therapy. Decreasing patellar tendon stiffness during the course of exercise therapy was associated with improved clinical outcome. Decreasing T2* relaxation times in the degenerative tissue of the patellar tendon were associated with improved clinical outcome. We suggest that PTLE should be regarded as standard initial care for the treatment of patients with PT.

LONG-TERM EFFECT OF EXERCISE FOR PATIENTS WITH PATELLAR
TENDINOPATHY
JIE DENG, MD
PhD Student
Advisors Edwin Oei, Robert-Jan de Vos, Gabriel Krestin & Denise Eygendaal
Project Funding China Scholarship Council (CSC)
Research period September 2021 – September 2025
Email j.deng@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine, Orthopedics and Sports Medicine.
Patellar tendinopathy is a frequent overuse injury that causes pain and impaired performance in jumping athletes. This painful chronic disorder may lead to impaired athletic performance and sports-related activities. It even hampers young individuals to maintain work productivity. Conservative management is considered to be the first line of treatment, in which exercise treatment plays the most important role. In our previous RCT study (JUMPER study), positive outcomes were reported in patients with patellar tendinopathy treated with progressive exercise treatment at 24 weeks.
This project is a continuation of the JUMPER study, in which we will use questionnaires, physical examination and ultrasound to detect the long-term effect of progressive exercise treatment, and determine the prognosis factors for the course of conservative treatment. For this purpose, we are planning to perform 5-years follow-up measurements, consisting of questionnaires, physical examinations and ultrasound (US) in subjects of the JUMPER study.
Specifically, our objectives are to: 1) Study the cross-sectional association between conventional US parameters, shear-wave elastography (SWE) and ultrashort echo time (UTE) MRI and patient-reported outcomes; 2) Evaluate prognostic factors for conventional US associated with patient-reported outcome of PT after conservative treatment; 3) Study the association between exercise-related factors and patient-reported outcomes; 4) Assess persistence of symptoms, participation in sports, change in functional test results, and ultrasonographic appearance of the patellar tendon at long-term (5 years) follow-up.
The figure shows an example of conventional ultrasound and share-wave elastography in patients with patellar tendinopathy.

Figure 1. Conventional ultrasound in Asymptomatic athletes with patellar tendinopathy. (a) Asymptomatic athletes; (b) patellar tendinopathy

GROWING UP: THE INFLUENCE OF LIFESTYLE ON JOINT HEALTH
MARLEEN M VAN DEN HEUVEL, MD
PhD Student
Advisors Marienke van Middelkoop, Edwin Oei, Jeroen Renkens & Sita Bierma-Zeinstra
Project Funding EUR Fellowship 2017
Research period June 2017 – Present
Email m.m.vandenheuvel@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine, General Practice, Orthopedics, and the Generation R Study group.
Low back pain is a common problem and already present in children and adolescents, but the etiology of these complaints is often unknown. Structural spinal abnormalities and shape variations might play a role and have been shown in children on MRI, but research in general populations is scarce.
For this project data from the Generation R Study is used, a population-based prospective cohort study from fetal life onwards. The Generation R Study includes a total of 9749 children and their mothers. Our study population consists of the children who participated in the 9-yearold measurements, including a MRI of the lumbar spine.
For each child the MRI of the spine has been examined and scored for structural abnormalities such as disc degeneration, disc herniation, and transitional vertebrae, by using a semi-quantitative scoring list. Furthermore, all MRIs have been annotated using the ViewR (developed by the BIGR group) in order to obtain quantitative shape and alignment measurements, i.e. pelvic incidence and vertebral shape. Information about weight status and physical activity is available from questionnaires, physical examinations, and accelerometry measurements at several time points during the follow up of the Generation R Study.
The primary outcome for this project is the prevalence of structural spinal abnormalities and shape variations in children aged 9 years. Secondary outcomes are the associations of these spinal abnormalities, spinopelvic alignment, and vertebral shape with the children’s weight status and physical activity level.

Figure 1. T2-weighted image of a participant with a decreased disc height at L5-S1 (A), disc bulging at L2-L3 and L3-L4 (B), and endplate irregularities at L5-S1 (C).

Advisors
ELUCIDATING VERTEBRAL FRACTURE RISK AS A HALLMARK OF OSTEOPOROSIS
FJORDA KOROMANI, MD
This project is a collaboration between the Departments of Radiology & Nuclear Medicine, Internal Medicine and Epidemiology. PhD Student
Ling Oei, Edwin Oei , Fernando Rivadeneira & Gabriel Krestin
Project Funding ERAWEB Fellowship
Research period September 2014 – Present Email f.koromani@erasmusmc.nl
Vertebral fractures are the most common osteoporotic fracture; they are strong predictors of subsequent fracture and fracture associated mortality. Only 30 % of vertebral fractures come to medical attention and there are several reasons that contribute in this underreporting, one of which is the lack of a gold standard definition to diagnose vertebral fractures in radiographic images. Currently, there are several radiologic scoring methods to diagnose and classify vertebral fractures; methods which are based on different diagnosing criteria. In my thesis, I aim to compare the quantitative morphometry (QM) which is based in direct measurement of vertebral body dimensions and shape, and the algorithm based qualitative (ABQ) method which diagnoses fractures based on endplate depression. I compared the QM and ABQ scoring methods to each other and build evidence on which would be the most appropriate to apply in clinical care. Furthermore, I have identified risk factors for vertebral fractures to improve the fracture risk assessment in patients with osteoporosis. Studies in my thesis are embedded in the Rotterdam Study. In most of the projects, I have sought replication of my findings in other cohorts or performed a meta-analysis with data from the Rotterdam Study and previously published data. In “Location of first spinal fracture as determinant of future vertebral fracture risk: a multi-cohort study” I examined if the location and number of prevalent VF affects the risk of subsequent radiographic VF and major osteoporotic fractures. The results showed that in women, a first vertebral fracture located in the thoracolumbar junction (T12-L1) holds an increased risk of subsequent vertebral fracture compared to one located at T4-T5 (fig.1). This suggests that the load distribution in the spine plays a role in the etiology of the fracture cascade commonly observed in the elderly. In “Vertebral Fractures in Individuals With Type 2 Diabetes: More Than Skeletal Complications Alone” we could establish in a meta-analysis

Figure 1. This graph shows subsequent vertebral fracture risk in women, across six cohorts (n = 13663). The data points are odds ratios (OR) and the figure shows that a first vertebral fracture located in the thoracolumbar junction (T12-L1) holds an increased risk of subsequent vertebral fracture compared to one located at T4-T5. This suggests that the load distribution in the spine plays a role in the etiology of the fracture cascade commonly observed in the elderly. X axis-location of first vertebral fracture, y axis- odds ratio 95% CI
comprising data from several cohorts, that individuals with type 2 diabetes are at increased risk of vertebral fractures. These findings have important implications for the clinical management of individuals with type 2 diabetes as they could benefit from systematic assessment for presence of vertebral fractures. In “Osteoporotic Vertebral Fracture Prevalence Varies Widely Between Qualitative and Quantitative Radiological Assessment Methods: The Rotterdam Study” we found that estimates of prevalent and incident QM and ABQ diagnosed vertebral fractures differ largely.

18F-FDG PET/MRI FOR PATIENTS WITH CHRONIC PAIN
MARIJN MOSTERT, MSC
This project is a collaboration between the Departments of Radiology & Nuclear Medicine, Orthopedic Surgery, and the Center for Pain Medicine. PhD Student
Advisors Edwin Oei & Rianne van der Heijden
Project Funding General Electric Healthcare: “Pinpointing the source of chronic pain and therapy response with whole-body 18F FDG-PET/MRI”.
Research period September 2021 – September 2025
Email m.mostert@erasmusmc.nl
Chronic lower back pain and hip pain have an immense burden on both patients and the healthcare system in general. Many patients are treated sub optimally, partly due to failure of conventional imaging methods to accurately identify pain generators. Recently, it has been shown that molecular imaging methods such as 18F-FDG PET/MRI are able to identify and precisely locate sources of pain by visualizing metabolic changes induced by pain. However, further assessment of the added value of 18F-FDG PET/MRI to standard clinical care is needed.
The AMPHiBI Trial aims to evaluate the effectiveness of 18F-FDG PET/MRI in a randomized controlled setting. Patients with chronic pain in the lower back region despite conventional treatment, and patients with chronic hip pain after total hip replacement will be recruited at the outpatient clinics of the Erasmus MC Center for Pain Medicine and the department of Orthopedic Surgery.
In this open randomized controlled trial, half of the participants will undergo whole body 18F-FDG PET/MRI, as well as several dedicated contrast enhanced imaging series of the lower back and hip region. Participants in the control group will undergo standard clinical care. We aim to include a total of 200 patients and the duration of follow-up is 1 year, with measurements at baseline and 1, 3, 6 and 12 months.
The primary outcome is patient-reported pain score at 6 months. Secondary outcomes include change in diagnosis or treatment plan based on 18F-FDG PET/MRI, and evolution of clinical outcomes including quality of life and function. Extensive quantitative image analyses

Figure 1. AMPHiBI Trial design. Participants are randomized in either the control group or the PET/MRI group. The duration of follow-up is 1 year, with pain score measurements at 1, 3, 6 and 12 months.
will also be performed, evaluating change in FDG uptake after treatment, dynamic contrast enhanced MRI, and their correlation with serological inflammatory markers and quantitative sensory testing.

JOOST VERSCHUEREN, MD BRACE VERSUS OSTEOTOMY TRIAL
Advisors Edwin Oei, Max Reijman, Sita Bierma-Zeinstra & Gabriel Krestin
PhD Student
Project Funding Dutch Arthritis Association (Reumafonds) and Netherlands Orthopaedic Association (NOV): “Optimal timing for orthopaedic surgery in osteoarthritis”
Research period November 2013 – October 2022
Email j.verschueren@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Orthopedic Surgery.
Osteoarthritis of the knee is a very common disease, affecting over 300,000 people in the Netherlands. The process can affect the whole joint or only one side. The inner side of the knee, the medial compartment, is most commonly affected which is accompanied by a bowlegged appearance (varus deformity). In case of medial knee osteoarthritis both surgical and non-surgical options are available. So far, the effects of both treatment modalities have never been compared by means of a clinical trial. Autumn 2014 a multicenter randomized controlled clinical trial was started, coordinated by Erasmus MC, to investigate the clinical and structural effects of a surgical and non-surgical treatment in patients with medial knee osteoarthritis. Patients with isolated medial knee osteoarthritis will be recruited in 9 clinics in the Netherlands. We will compare an orthopedic unloader knee brace to a surgical procedure (high tibial osteotomy) aimed a realigning the knee joint. Both treatments reduce the varus deformity and therefore decrease the load on the medial compartment of the knee.
The primary endpoint of the study is knee pain after one year of treatment. As a secondary objective we will look at structural changes in cartilage induced by the treatments using advanced MRI and SPECT/CT techniques. MRI imaging using T2-mapping and T1rho-mapping is performed at the Erasmus MC with a 3 Tesla MRI scanner. These MR sequences are aimed at measuring joint tissue composition. SPECT/CT imaging enables us to visualize and quantify bone metabolism which is an important feature in early osteoarthritis. With these techniques, it is possible to diagnose osteoarthritis at the earliest stages and to follow-up the disease in a sensitive and quantitative manner. The scans are performed before start and

the T2 values. Higher values indicate cartilage of lesser quality.
after one year of treatment. We expect a reduction of the osteoarthritic process in the medial knee compartment, whereas deterioration is expected in the lateral compartment. For image post-processing, in-house developed software is used in collaboration with the Biomedical Imaging Group Rotterdam.
Inclusion was completed in 2020. We have included 51 patients in this clinical trial. Follow-up measurements in the last patient were recently performed. At this moment, we are analyzing the imaging data.
Figure 1. Segmentation of the weight-bearing cartilage on a T2 weighted image of the medial compartment. The colors represent

THE ROLE OF THE MENISCUS IN KNEE OSTEOARTHRITIS STUDIED WITH MRI
JAN A VAN DER VOET, MD
PhD Student
Advisors Sita Bierma-Zeinstra, Gabriel Krestin, Edwin Oei & Jos Runhaar
Project Funding ZonMw, Reumafonds
Research period November 2014 – November 2021
Email j.a.vandervoet@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and General Practice.
Meniscal extrusion is a common finding in knees with osteoarthritis (OA). Since knee OA is a largely irreversible condition, identifying risk factors before onset or in an early stage of the disease is of great importance. Meniscal extrusion might be such an interesting target for early detection. Extrusion is where the meniscus is partially or totally displaced from the tibial cartilage surface. The generally accepted idea is that a displaced meniscus affects the weight-bearing and load distribution capacities within in the knee joint, which leads to loss of cartilage, ultimately resulting in knee OA. Previous studies affirm the association between meniscal extrusion and incident knee OA, however, most of these studies were carried out in cohorts of subjects with established OA. One of the main objectives from this project is to evaluate the role of the meniscus, particularly extrusion, in the pathogenesis of OA. To confirm a causal relationship, we evaluated the association between baseline meniscal extrusion and incident knee OA in a population free of OA at baseline. In our first study, we found an independent relationship between meniscal extrusion and the onset of OA after 30 months follow-up. These findings were validated in another study with two different cohorts with a longer follow-up time, with similar results, providing further evidence that meniscal extrusion is largely independently related to incident knee OA. To search for possible targets to prevent extrusion and thereby OA, we evaluated possible modifiable risk factors associated with absolute and relative longitudinal change in extrusion. The greatest change in extrusion was seen with incident medial meniscal tears, followed by (change in) BMI. These findings might justify surgical repair and/or refixation of a torn meniscus, especially in case of a traumatic radial or root tear. Two other papers focused on baseline and change in meniscal volume and the interplay between meniscus volume and meniscal
extrusion, concluding that a larger baseline volume and decrease in volume over time are significantly associated with knee OA development.
In future studies, we want to assess the influence of postmenopausal status on meniscus extrusion and OA. Furthermore, we want to evaluate the correlation between meniscal morphology and position, cartilage thickness and joint space width (JSW), a parameter used in clinical practice to diagnose knee OA.
Most of our data are derived from the PROOF study, a prospective intervention study in a high-risk population of 407 middle-aged overweight women, free of clinical and radiographic knee OA at baseline. Quantitative and semi-quantitative measurements were performed on 1.5T MRI’s made at baseline and follow-up after +/- 2.5 and 6.6 years.

Figure 1. Coronal PD weighted image showing an example of measurements of meniscal body width, meniscal body extrusion and tibial plateau width.

MUSCULOSKELETAL MRI IN THE GENERATION R STUDY
DESIRÉE K DE VREEDE, MSC, MD
PhD Student
Advisors Aad van der Lugt & Edwin Oei
Project Funding
Research period October 2016 – Februari 2023
Email d.devreede@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine, Medical Informatics and Epidemiology.
This research is embedded in the Generation R Study, a population-based prospective cohort study in Rotterdam that is following children from fetal life until early adulthood. A total of 3231 MRI scans of the hip joints, pelvic skeleton, lower abdomen and lower lumbar spine were collected from children at the age of 10.2 years (mean, range 8.6-12.9 years). The purpose of this study is to define normative values of the hip in 3D (e.g. the neck shaft angle of the proximal femur, the femoral head diameter, the femoral offset and the narrowest neck width) for this age group. With this large dataset we hope to identify genetic and environmental causes of normal and abnormal growth and development of the hip.
Due to the size of the dataset, manual segmentation, although precise, would be very laborious and time consuming and could be subject to inter- and intra-observer variability. Therefore, an automated multi-atlas segmentation with an appearance model is implemented to systematically and accurately gather bone parameters of the hip. The multi-atlas appearance model complements the multi-atlas segmentation model by providing information about the appearance of the structure of interest. Optimization of parameter selection is needed to have a fully-automated segmentation method capable of subtracting the anatomical structure of interest from the MR image. The figure shows an automated segmentation of the proximal femur.
Follow-up of this cohort at the age of 13 years commenced in 2017. At this age, hip, knee and full body MRI scans were collected.

Automated segmentation of the proximal femoral, with the neck shaft angle (NSA) indicated.

BODY COMPOSITION MRI IN GENERATION R
TONG WU, MD
PhD Student
Advisors Edwin Oei, Stefan Klein & Meike Vernooij
Project Funding China Scholarship Council (CSC)
Research period September 2019 – August 2023
Email w.tong.1@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Generation R.
Body composition, especially visceral fat plays vital role in the development of children and adolescents. In this project we will use MRI along with DXA techniques to analyze body composition in participants of the Generation R Study.
Specifically, our objectives are: 1. To optimize and implement a novel MRI analysis method using deep learning for automated quantification of quantitative body fat volumes, in collaboration with the German Center for Neurodegenerative Diseases. Currently, this pipeline has been finished by retraining and we are writing a paper about this work. 2. To correlate those body fat composition parameters to epidemiological data in Generation R Study. We recently published a systematic review about the association between visceral fat and respiratory outcomes in which we found that in children, there are few studies available and in adults, higher visceral fat level is related to higher risk of asthma; We are currently analyzing (1) the association between body fat and respiratory outcomes in a longitudinal design. We found that fat mass has more adverse associations with lung function and asthma from childhood to adolescent; (2) the association of physical activity and screen time with body composition among children at age of 13 years.
The figures show (1) an example of abdominal fat segmentation in children, including the subcutaneous adipose tissue and visceral adipose tissue compartments; (2) after retraining the pipeline of segmentation with a 4-Fold Cross-validation, we generated a full model (orange color) which showed good performance for subcutaneous and visceral adipose tissue.



Figure 2. 4-Fold cross validation models plus a full model were used for retraining the segmentation tool.
Figure 1. Abdominal adipose tissue segmentation on whole body MR scan. Blue represents subcutaneous adipose tissue; green represents visceral adipose tissue.

GENICULAR ARTERY EMBOLIZATION FOR KNEE OSTEOARTHRITIS
TIJMEN A VAN ZADELHOFF, MD
PhD Student
Advisors Adriaan Moelker, Edwin Oei & Gabriel Krestin
Project Funding
Stichting Coolsingel, COOK Medical, MRace
Research period February 2018 – May 2022
Email t.vanzadelhoff@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine, General Practice and Orthopedic surgery.
Osteoarthritis (OA) of the knee is the most common joint disease. The prevalence of osteoarthritis is expected to increase parallel to the aging of the population. Pain in the knee is the most common symptom. Conservative treatment consists of lifestyle changes, physiotherapy and pharmacological treatment. If these treatments fail, a definitive solution is a total knee replacement. The purpose of this project is to test a newly emerging therapy for patients suffering from knee OA resistant to conservative therapy.
A recent insight that angiogenesis around the knee and accompanying nerves are most likely a contributor to pain and inflammation in knee OA forms the basis of this new treatment. Arterial embolization of the neovessels using 75 -100 μm microspheres will restore the normal vasculature around the knee and hypothetically reduce pain and synovitis (see figure).
In order to account for the well-known placebo effect we will conduct a double blind randomized sham controlled clinical trial. The sham group will undergo a procedure mimicking arterial embolization but only an incision in the groin will be made. The projected number of inclusion is 58 patients. The duration of follow-up is 1 year, with measurements including detailed MRI at 1, 4, 8 and 12 months post intervention. Patients will be recruited at the outpatient clinic of the department of Orthopedic surgery of the Erasmus MC.
The primary outcome is pain after 4 months and will be assessed using the KOOS questionnaire. Furthermore extensive semi-quantitative and quantitative MRI analysis will be performed. Additionally, pain pressure threshold test-
ing will be performed in order to evaluate the peripheral and central pain sensitization of the patients.
Patient inclusion has been completed. Data on the primary outcome is expected in May 2022. After completion of this trial we hope to have established a new, effective treatment for a patient group that has limited treatment options.
Figure 1. Sagittal contrast-enhanced high resolution spoiled gradient recalled echo sequence (SPGR) of the knee. Note the extensive suprapatellar enhancement indicating synovitis.


ADVANCED IMAGING OF INFLAMMATION IN KNEE OSTEOARTHRITIS
BAS A DE VRIES, MSC, PHD
PhD Awarded 25 May 2021
Advisors Edwin Oei & Gabriel Krestin
Project Funding Dutch Arthritis Association (Reumafonds): “DISKO: The diagnostic imaging work-up of synovitis in knee osteoarthritis with a novel non-contrast MRI technique or ultrasound”
Short CV Bas de Vries (1991) holds a MSc degree in Technical Medicine from the University of Twente. In 2016 he started his PhD research at the Department of Radiology & Nuclear Medicine at Erasmus MC Rotterdam, which resulted in this thesis. He currently works as a Clinical Support Specialist with Masimo, a global medical technology company that develops and manufactures innovative noninvasive patient monitoring technologies,
This thesis was a collaboration between the Departments of Radiology & Nuclear Medicine and Orthopedic Surgery.
This thesis focused on imaging methods to study the role of inflammation in knee osteoarthritis. The aims of the thesis were I) to evaluate disturbed perfusion patterns in subchondral bone and the infrapatellar fat pad using perfusion MRI, and II) to assess new magnetic resonance and ultrasound imaging methods for diagnosis of synovitis in knee osteoarthritis. Key findings of the these were:
1) Increased perfusion in the epimetaphyseal bone, subchondral bone and bone marrow lesions is observed in unicompartmental knee osteoarthritis.
2) Higher volumes of the infrapatellar fat pad and increased blood perfusion, as a surrogate marker of inflammation, are both not associated with patellofemoral pain.
3) Elevated dynamic contrast-enhanced (DCE) MRI perfusion parameters within T2FS-hyperintense infrapatellar fat pad regions in patients with osteoarthritis suggest an inflammatory pathogenesis in osteoarthritis, but not in patellofemoral pain and healthy subjects
4) Ultrasound has limited overall accuracy in detecting synovitis in knee osteoarthritis compared to contrast-enhanced MRI.
5) qDESS synovitis images can differentiate between the synovial membrane and joint effusion.

https://repub.eur.nl/pub/135413

Dr. Adriaan Moelker earned his MD in 2000 and his PhD in 2004. Following a 4 and half year residency in Radiology, he became a staff radiologist at Erasmus MC. Since 2011, he is head of the Section of Interventional Radiology, the largest interventional radiology practice in the Netherlands with 8 interventional radiologists, one fellow and several residents. Adriaan collaborates intensively with the “Image Guidance in Interventions and Therapy” research theme of the Biomedical Imaging Group Rotterdam. This research focuses on improving image guidance by integrating pre-operative image information in the interventional situation for vascular and soft tissue applications. A project with the Department of Radiotherapy

concerns the comparison of stereotactic irradiation with minimally invasive chemo-embolization, and a joint research line with the Department of Gastroenterology focuses on mesenteric ischemia, particularly on the application of covered stents in atherosclerotic mesenteric arteries. A shamed controlled randomized clinical study on the endovascular treatment of patients with osteoarthritis has been started in collaboration with the musculoskeletal imaging research group and the department of Orthopedics and General Practice. Collaborations with other departments in the Erasmus MC and with University Medical Center Utrecht concern the care of oncology patients. a.moelker@erasmusmc.nl
IMPROVING IMAGE-GUIDED DIAGNOSIS AND TREATMENT IN INTERVENTIONAL RADIOLOGY
ADRIAAN MOELKER, MD, PHD, EBIR
assistant professor

Context
Interventional radiology is a rapidly evolving and changing field in medicine. Specifically, in the oncologic domain, interventional radiology has become the fourth pillar in oncology treatment besides surgery, internal oncology and radiotherapy. New minimally invasively procedures are introduced annually with new tumor targeting devices, further miniaturization of devices such as microcatheters and wires, and new access techniques such as through the (distal) radial artery. However, most of these have not been optimized for use by doctors or have even been scientifically proven beneficial for the patient. Also, new techniques are introduced using equipment previously developed for different purposes. Our research therefore focuses on the assessment of safety and efficacy of novel treatment techniques and on improvement of minimally invasive interventional imaging methods.
Top Publications
2021
Terlouw LG, van Noord D, van Walsum T, Bruno MJ, Moelker A. Mesenteric artery calcium scoring: a potential screening method for chronic mesenteric ischemia. Eur Radiol. 2021 Jun;31(6):4212-4220.
van Zadelhoff TA, Okuno Y, Bos PK, Bierma-Zeinstra SMA, Krestin GP, Moelker A, Oei EHG. Association between Baseline Osteoarthritic Features on MR Imaging and Clinical Outcome after Genicular Artery Embolization for Knee Osteoarthritis. J Vasc Interv Radiol. 2021 Apr;32(4):497-503.
Sun YY, Kekec T, Moelker A, Niessen WJ, Van Walsum T. Transformation optimization and image blending for 3D liver ultrasound series stitching. Proceedings of SPIE: Medical Imaging 2020.
Research Projects: Objectives & Achievements
Image fusion: From offline image data analysis to online image data acquisition in the clinics
Interventional Radiology uses a minimally invasive approach for treating patients with e.g. radiofrequency ablation (RFA), microwave ablation (MWA), chemoembolization (TACE) and transjugular intrahepatic portacaval stent (TIPS) placement. Interventions are performed under real-time image guidance using ultrasound and fluoroscopy mainly. Unfortunately, these imaging modalities are 2-dimensional with limited visualization capabilities making them suboptimal in all instances, presented by suboptimal user interfaces. CT, as a 3-dimensional imaging technique also frequently used in interventional radiology, is hampered by low tissue contrast and the use of contrast agents. In this research line, we are working towards bridging the gap between current technological (imaging) possibilities and medical practice in the field of oncology. Strong collaboration is together with Theo van Walsum. Medical students support this research in smaller projects.
The overall aim of this research line is to transform the interventional suite into an efficient interventional cockpit: the interventional radiologist will be provided with enhanced imaging and decision support, fitted to an optimized workflow making use of new techniques such as novel technical developments including real-time 3D/4D ultrasound imaging or CT/CT fusion for improvement of image guidance by aligning online imaging data, presenting the interventional radiologists’ devices, with preoperative imaging data during image guided interventions. As an example, an automated non-rigid image registration of pre- and post-interventional CT images provides better insight in the ablation success of liver tumors and in a pilot study registration software has been tested in clinical practice by Nagtegaal.
In this pilot, the interventional radiologist is provided information on liver tumour localisation in the intraprocedural CT images after a liver tumor ablation procedure. The tumor localization was derived from pre-interventional diagnostic CT images transferred to the interventional images using computer-based rigid registration and non-rigid registration, developed by Luu et al.. A previous study demonstrated the feasibility of clinical use of the registration software which has previously demonstrated better tumor localization compared to the interventional radiologists’ mental mapping abilities (Boulkhrif et al.). Next step will be the evaluation of the impact of the registration methods on clinical decision making and treatment effect such as local tumor control in a randomized clinical trial. Collaboration with the Leiden University Medical Center (dr. Mark Burgmans, interventional radiologist) has been initiated on a similar study with a rigid registration technique.



How to treat HCC: TACE versus SBRT
Interventional chemoembolization is compared to stereotactic body radiation therapy in patients with hepatocellular carcinoma in a collaborative research line with the department of Radiotherapy (“The TRENDY trial”). This study compares head-to-head the standard treatment, transarterial chemoembolization with drug-eluting beads loaded with doxorubicin, with the experimental arm, stereotactic body radiation therapy in patients with hepatocellular carcinoma (HCC). To the best of our knowledge this study will be the first in the world that will compare both techniques in a randomized clinical trial. Patient eligible for this trial are those with hepatocellular carcinoma, stage A-B (Barcelona Clinic Liver Cancer stage system), with one tumor nodule of ≤ 6cm or cumulative ≤ 6cm with more nodules in the liver
Fig 1. Visualization of the manual (blue) and automatic (red) segmentation of an post-interventional ablation zone.
not eligible for surgery or ablation. Patients may become eligible for future transplantation (so-called bridging or downstaging). Primary endpoint of the study is the time to progression of HCC. Secondary end- points are overall survival, time to local recurrence, response rate (complete and partial response) to treatment, toxicity, quality of life, and treatment-related costs. The study is closed and the data analysed: radiation therapy tends to higher local antitumoral activity than TACE in this small group of HCC patients, and no detrimental effects on time to progression, overall survival, toxicity and quality of life. The study is a multicenter phase II randomized controlled trial in which most academic centers of the Netherlands are participating, and centers in Germany, Belgium, France and Denmark. The study started mid2015 and 30 patients are included.

How to treat HCC: Holmium embolization
In case patients with HCC are not amenable to treatment with ablation or TACE, but with otherwise localized disease without metastases, a viable treatment option to improve survival or to bridge to liver transplantation is radioembolization. To this end, Yttrium 90 (Y90) loaded glass spheres are infused intra-arterially. As a drawback, Y90 treatment effect is difficult to predict. Therefore, the department of interventional radiology of Erasmus MC participates in a study, initiated at the University Medical Center in Utrecht (UMCU). This multi-center, interventional, non- randomized, non-comparative, open label, early phase II study (HEPAR Primary study) investigates the local treatment using 166Ho-radioembolization, which potentially offers an effective treatment with a more personal approach. The study is financially supported by the Dutch Cancer Society (KWF, Koningin Wilhelmina Fonds).
Intra-arterial treatment of neuroendocrine liver metastases
A second collaboration with the department of radiology of UMCU is on the treatment of neuro-endocrine tumor (NET) liver metastases with intra-arterial infusion of somatostatin-bound radionuclides (lutetium-177-dotatate). The majority of NET patients present with metastases, most often including liver metastases. These patients have a poorer prognosis and lower quality of life. Currently, intravenous administered lutetium-177-dotatate have shown to improve tumor response rates and progression free survival (PFS). Despite of the increased tumor response rate and PFS, liver metastases still remain the major cause of morbidity and mortality in these patients. The objective of this collaborative study is to investigate the impact of intra-arterial administration of 177-Lutetium-dotatate on the intrahepatic biodistribution in patients with NET liver metastases.

CoBaGi-trial: Covered versus uncovered stenting in mesenteric ischemia
Severe complaints of postprandial abdominal pain, fear of eating, and consecutive weight loss characterize the disease of chronic mesenteric ischaemia (CMI) caused by stenosis of the mesenteric arteries. Symptomatic CMI is an uncommon, potentially underdiagnosed condition and if left untreated, associated with a high morbidity and mortality. Patients with abdominal complaints often undergo extensive diagnostic testing, including abdominal imaging by CT, before CMI is even considered. Early risk stratification based on standard CT imaging could facilitate the diagnostic trajectory, raise awareness, reduce diagnostic delays in CMI patients, and avoid an extensive and cumbersome diagnostic workup of CMI in patients without CMI. The mesenteric artery calcium score (MACS)
Fig 2: Local control on imaging after radiotherapy and TACE
Fig 3: Post-treatment PET/CT-scan after lutetium-177-dotatate injection in the right hepatic artery.
has been developed as a measure calculated on CT images and enables discrimination of CMI patients from non-CMI patients in a study by Terlouw et al. (published in Eur Rad 2020). A follow-up study designed a MACS based score chart to facilitate the selection of patients with a low probability of CMI, in whom a further diagnostic workup can be omitted, and to validate the CTA-based score chart proposed by van Dijk et al, which guides treatment decisions. Nowadays standard care in significant chronic CMI is the use of bare metal stents, i.e. without any coverage, although the primary patency of these stents is low. According to retrospective data the patency of covered stents is significantly higher compared to bare metal stents. Therefore, this research line in collaboration with the Department of Gastrointestinal Medicine focuses on the comparison of covered versus bare stents in the mesenteric arteries in patients with CMI (the CoBaGi-trial), and in a prospective randomized controlled multicenter trial. Participating centers are currently Maasstad Hospital, Medical Spectrum Twente, Sint Antonius Hospital, Jeroen Bosch Hospital and Bernhoven Hospital. Primary endpoints are the primary and secondary patency rates of covered stents versus bare-metal stents. Secondary objectives are freedom from restenosis, from symptom recurrence and from re-intervention, and clinical outcome in terms of quality of life and costs after 6-, 12and 24-months after stent implantation. Patient accrual has been finalized and early 2021 data collection will be completed. The project is financially supported by an educational grant of Atrium Medical. The last patient has been treated and inclusion has been closed. Currently, the data is analysed and end-points seem to be reached.
NEO-study: neo-vascularization embolization in patients with osteoarthritis
Superselective transcatheter arterial embolization has recently been proposed as an efficacious therapy for therapy-resistant osteoarthritis (OA) of the knee, providing substantial pain reduction at short-term as well as long-term follow-up up till 4 years. A potential working mechanism of treatment effect is that the normalization of the amount of blood vessels and blood flow achieved by embolization reduces inflammation (Fig. 4). The main objective of this double-blinded randomized sham-controlled clinical trial, initiated by dr. Adriaan Moelker and Edwin Oei, is to assess whether transcatheter arterial embolization of neovessels in patients with symptomatic knee OA results in significant pain reduction compared to sham treatment. Secondary objectives are to assess whether reduction of neovessels is related to pain relief and to explore whether decrease of inflammation is a mediating factor between neovessel reduction and pain relief. The study is financially supported by Cook Medi-
cal, Stichting Coolsingel and Medische Research Advies Commisie Erasmus MC, Rotterdam (MRace). A PhD student Tijmen Zadelhoff started early 2018. Y. Okuno, founder of the treatment, is involved as an adviser. The research team visited the Okuno Clinic for educational purposes. Currently, half of required subjects have been included and inclusion is expected to be complete early 2021.


Fig. 4: Angiographic image of lateral margin of the knee joint with a clearly hyperemic region with neo vascularization (A) treated with 75 micron particles resulting in (B) reduction of neovascularization.
TESLA-trial: Direct metal stenting of unresectable malignant bile duct stenosis
Most patients with perihilar cholangiocarcinoma (pCCA) are ineligible for curative-intent resection because of metastatic disease, locally advanced disease, or due to comorbidity. The key to successful palliative treatment is adequate biliary drainage to improve the patient’s wellbeing and to allow for palliative systemic therapy. Endoscopic biliary drainage with plastic stents is the most common technique in the Netherlands. However, the main problem is bacterial colonization of the previously sterile intrahepatic bile ducts, because the stents cross the ampulla. Cholangitis often develops, reflected by a 35% mortality within 3 months after diagnosis. The only method to avoid colonization of the bile ducts is percutaneous placement of uncovered self-expandable metal stents (SEMS) that do not cross the ampulla. This pilot study evaluates whether direct percutaneous SEMS placement for palliative treatment of perihilar cholangiocarcinoma is feasible and safe. Secondary outcome is survival. Until now, all patients have been included. The pilot study is still including patients as an extension cohort. A protocol is written for an international multi-
center randomized clinical trial. This research is a collaborative project with the department of Surgery and Gastro-Enterology.

Fig. 5: Bilateral stents in the common bile duct in a 50 year old patient with hilar cholangiocarcinoma.
Expectations & Directions
In the imaging fusion projects, the three challenges to be addressed are: 1) initial alignment of the pre-operative information with the interventional scene, 2) keeping this alignment up-to-date using real-time imaging, and 3) making these technologies fast enough for deployment in the interventional radiologist’s practice. The focus of the coming years is on implementing these techniques in clinical practice and. The Trendy-trial, CoBaGi-sudy and NEO-study are all disclosure of the data mid-2022.
Funding
Mendez Romero, Alejandra, and Adriaan Moelker : Netherlands Cancer Society Grant 2014-2019: “Transarterial chemoembolization with drug-eluting beads versus stereotactic body radiation therapy for hepatocellular carcinoma: A multicenter randomized phase II trial (The TRENDY trial)”
Adriaan Moelker : Atrium Medical 2013-2017: “Covered versus bare metal stenting in mesenteric arteries in patients with mesenteric ischemia: The COBAGI Study”
Moelker Adriaan , Oei Edwin: Cook Medical 2018-2021: “Novel transcatheter arterial embolization for treatment of knee osteoarthritis: a randomized sham-controlled clinical trial”
Moelker Adriaan , Oei Edwin: Boston-Scientific 20202021: “Novel transcatheter arterial embolization for treatment of knee osteoarthritis: a randomized shamcontrolled clinical trial”
Oei Edwin, Moelker Adriaan : Stichting Coolsingel 2018: “Novel transcatheter arterial embolization for treatment of knee osteoarthritis: a randomized sham-controlled clinical trial”
Oei Edwin, Moelker Adriaan: Medische Research Advies Commissie Erasmus MC, Rotterdam (MRace) 2018: “Novel transcatheter arterial embolization for treatment of knee osteoarthritis: a randomized sham-controlled clinical trial”
Marnix Lam, Adriaan Moelker , PMP Tokkel: Advanced Accelerator Applications (AAA) Stichting Life Sciences Health (Health-Holland) 2018-2022: “Intra-arterial lutetium-177-dotatate for treatment of patients with neuro-endocrine tumor liver metastases: The LUTIA Study”
Luke Terlouw, Desiree Leemreis, Adriaan Moelker , Marco Bruno: United European Gastroenterology Activity Grant programme 2018: “Clinical guidelines for the management of CMI”
Marco Bruno, Desiree van Noord, Adriaan Moelker : Maag Darm Lever Stichting 2021: “Promoting early diagnosis of chronic mesenteric ischemia by a mesenteric artery calcium score based risk stratification and detection of postprandial mucosal ischemia by butyrate/glucose breath testing”
Invited Lectures
Moelker, A. Endovascular treatment of chronic mesenteric ischemia - CIRSE 2021, Online
Moelker, A. Radial access for visceral and renal interventions - CIRSE 2021, Online
Moelker, A. Knee arthritis: overview, imaging, and treatment options – GEST 2021, Online

CHRONIC MESENTERIC ISCHEMIA
AND TREATMENT
IMPROVING DIAGNOSIS
DUYGU HARMANKAYA, MD
PhD Student
Advisors Adriaan Moelker , Marco Bruno & Désirée van Noord
Project Funding MLDS Right on Time 2021. “Promoting early diagnosis of chronic mesenteric ischemia by a mesenteric artery calcium score based risk stratification and detection of postprandial mucosal ischemia by butyrate/glucose breath testing.”
Research period January 2021 – January 2024
Email d.harmankaya@erasmusmc.nl
This project is a collaboration between the Department of Radiology & Nuclear Medicine and the Department of Gastroenterology and Hepatology
Chronic mesenteric ischemia (CMI) is a invalidating disease, defined as insufficient blood supply to the gastro-intestinal tract causing complaints of post-prandial pain, fear of eating and weight loss. CMI is a diagnosis that is difficult to establish as symptoms are highly variable and diagnostic test may be inconclusive. This PhD project focusses on improving the diagnosis and treatment of CMI. The joint projects with the Department of Radiology and Nuclear Medicine and the department of Gastroenterology & Hepatology are described below.
Better diagnostic tools are needed since the exclusion of CMI currently requires a cumbersome complication-prone diagnostic workup and since a definitive diagnosis is mainly established per exclusionem. Quantification of mesenteric arterial calcification on computed tomography (CT) seems suitable for this purpose, which is in line with the coronary artery calcium score. The mesenteric artery calcium score (MACS) is a promising tool that seems to be a sensitive test with a high negative predictive value (NPV). A low MACS rules out CMI, while an increased MACS identifies patients in whom a full workup of CMI is warranted.
MALS is a rare (incidence 1.3 per 100.000) and controversial cause of chronic mesenteric ischemia. Yet, in our experience, asymptomatic stenoses due to CA compression are frequently encountered on computed topographies’, performed for other indications. Suggesting that CA compression is not that rare, but rarely results in MALS. This could be explained by the compensatory capacity of the extensive collateral mesenteric circulation. The CLAMPER study with both inspiration and expiration images of the CA, in an asymptomatic population, could shed light on the

Figure 1. a) Celiac artery compression during maximum inspiration. b) Celiac artery compression during maximum expiration. During expiration, the severity of the stenosis increases.
actual prevalence of CA compression. Knowing the prevalence would be useful in order to educate physicians that a CA stenosis due to CA compression is a common finding, but rarely results in MALS.

CHRONIC MESENTERIC ISCHEMIA
LUKE G TERLOUW, MD
Advisors Adriaan Moelker , Marco Bruno & Désirée van Noord
PhD Student
Project Funding Atrium Medical 2013-2017: “Covered versus bare metal stents in patients with chronic mesenteric ischemia: The CoBaGI Study”
Research period February 2018 – March 2021
Email l.vandijk@erasmusmc.nl
This project is a collaboration between the Department of Radiology & Nuclear Medicine and the Department of Gastroenterology and Hepatology
Chronic Mesenteric lschemia (CMI) causes incapacitating complaints of post-prandial pain, food fear, and weight loss. CMI is the result of insufficient blood supply to the gastro-intestinal tract and is most often caused by ≥1 atherosclerotic mesenteric artery stenosis. Presence of an extensive collateral circulation and the absence of a gold standard diagnostic test causes a diagnostic challenge. A consensus diagnosis of CMI is currently used in clinical practice and is established by an expert team of vascular surgeons, interventional radiologists, and gastroenterologists. Consensus is based on the presenting symptoms, results of imaging, and visible light spectroscopy (VLS). This PhD project focusses on the diagnosis and treatment of CMI. The projects of the Department of Radiology are described below.
Abdominal complaints and mesenteric artery stenosis are both frequent findings, yet few patients have CMI. The Mesenteric Artery Calcium Scoring (MACS) study investigated the ability to identify patients at risk of CMI based on calcium scoring. A score chart using the calcium score and typical symptoms showed to have a 97.9% negative predictive value. This score chart seems able to rule out CMI, providing a simple tool to omit a further (invasive) diagnostic workup in selected patients.
In order to develop a much-needed reliable non-invasive diagnostic test for CMI a prospective cohort study to assess the feasibility of pre-prandial and postprandial MRblood flow measurements in the mesenteric arteries and veins was started in 2017.
Endovascular stenting with Bare-metal stents is the first choice of treatment in occlusive CMI, although retrospec-

tive analysis showed higher patency rates of covered stents in patients with CMI. The CoBaGI study is a multicenter randomized controlled trial designed to assess the patency of covered stents versus bare-metal stents in the mesenteric arteries. The results are expected to be published in 2021. The CoBaGI study is performed by the Dutch Mesenteric lschemia Study group (DMIS).
The Erasmus MC has initiated the development of a European multidisciplinary clinical guideline on the management of CMI. The guideline has been endorsed by UEG, EAGEN, ESGAR, CIRSE, NVMDL, HSG, and DMIS and has been published in the UEG journal.
Fig. 1: Regions of interest for calculation of the mesenteric artery calcium score on computed tomography angiography

Personal mission statement: To identify, validate, and implement innovations to improve the life of patients with lung diseases.
Harm Tiddens received his MD in 1985 and his PhD in 1998. He has been a respiratory specialist in the Sophia Children’s Hospital since 1994, with a joint appointment in Radiology since 2007. He has been the chairman of the Sophia CF-research for 2 decades. He finished his board membership of the European CF Society in 2018. He cofounded the European CF Society Clinical Trial Network (ECFS-CTN). He has extensive international experience, having had visiting professorships at U-Washington Children’s Hospital (Seattle, WA/USA). Currently, and at Princess Margaret Hospital for Children (Perth/AU) he is visiting professor at Ningxia University, (Yinchuan, China). He is founder and director of LungAnalysis an image analysis core laboratory for lung images. Harm Tiddens is (co )author of >220 peerreviewed papers and of >40 book chapters. h.tiddens@erasmusmc.nl
SENSITIVE DETECTION AND MONITORING OF LUNG ABNORMALITIES
HARM AWM TIDDENS, MD, PHD
full professor

Context
Lung function tests have been the cornerstone to detect and monitor lung diseases in clinical practice and studies for over half a century. However, these functional outcome measures are relatively insensitive. Chest CT has superior sensitivity to detect structural changes. Unfortunately, the role of chest CT especially in children has been restricted due to ionizing radiation, the lack of quantitative outcome measures, and the lack of sensitive and accurate image analysis tools.
The LungAnalysis research group works to resolve these issues. LungAnalysis is developing more sensitive low dose chest CT protocols and is implementing standardized low dose chest CT within clinical networks. In 2013 the Erasmus MC LungAnalysis core laboratory (headed by Dr M. Kemner) was set up for the development and validation of image analysis outcomes and for standardization of chest CT and chest MRI imaging protocols. Furthermore, since 2007 LungAnalysis has been running an extensive program developing chest MRI, led by Dr P. Ciet, as a radiation-free alternative imaging modality for CT to study lung development in health and disease.
Top Publications 2021
Tiddens HAWM, Chen Y, Andrinopoulou E, Davis SD, Rosenfeld M, Ratjen F, Kronmal RA, Hinckley Stukovsky KD, Dasiewicz A, Stick A, on behalf of the SHIP-CT Study Group. The effect of inhaled hypertonic saline on lung structure in preschool children with cystic fibrosis (SHIP-CT): a multicentre, randomised, doubleblind, controlled trial. Lancet Respir Med In press
Aliberti S, Goeminne PC, O’Donnell AE, Aksamit TR, AlJahdali H, Barker AF, Blasi F, Boersma WG, Crichton ML, De Soyza A, Dimakou KE, Elborn SJ, Feldman C, Tiddens H, Haworth CS, Hill AT, Loebinger MR, Martinez-Garcia MA, Meerburg JJ, Menendez R, Morgan LC, Murris MS, Polverino E, Ringshausen FC, Shteinberg M, Sverzellati N, Tino G, Torres A, Vandendriessche T, Vendrell M, Welte T, Wilson R, Wong CA, Chalmers JD. Criteria and definitions for the radiological and clinical diagnosis of bronchiectasis in adults for use in clinical trials: international consensus recommendations. Lancet Respir Med. 2021:S2213-2600
Elders B, Ciet P, Tiddens H, van den Bosch W, Wielopolski P, Pullens B. MRI of the upper airways in children and young adults: the MUSIC study.Thorax. 2021 Jan;76(1):44-52.
Research Projects: Objectives & Achievements
Chest CT outcome measures
Bronchiectasis and trapped air have been well validated as outcome measures in cystic fibrosis (CF). However, there were still missing pieces of the validation puzzle to be addressed. In close collaboration with scientists in Perth (PI Prof S. Stick), we developed and validated a sensitive alternative image analysis method (PRAGMA-CF) to score chest CTs in children and adults. PRAGMA-CF has been used in three large studies in young children as outcome measure and is substantially more sensitive to detect early CF lung changes than previously semi-quantitative scoring methods. In 2021 4 more papers were added to the list of 25 PRAGMA-CF validation papers. PRAGMA-CF has been automated by 2 companies (Thirona and Resonance Health) using artificial intelligence strategies.
Standardized chest CT
To use chest CT related outcome measures in multicenter clinical trials and for patient registries standardized image acquisition and image analysis techniques are needed. In 2020 LungAnalysis received a project grant from the European CF Society (ECFS) to standardize in the 2021 and 2022 all 58 sites involved in the ECFS clinical trial network (CTN). To run this SCIFI-II project we make use of the interactive website developed by PhD student J. Meerburg and the LungAnalysis staff as part of the iABC project funded by IMI. In 2021 18 sites have been standardized. The website facilitates multiple clinical studies that required CT standardization. In addition LungAnalysis has been appointed by the ECFSCTN as the CT-expert centre.
Image analysis and bronchiectasis
Abnormal widening (bronchiectasis) and or thickening of airways are an important feature of many lung diseases. To identify abnormal airways on a chest CT, airway dimensions have to be compared to the adjacent artery, which function as reference structure. The manual assessment of all visible airways and arteries on a single chest CT can take up to 5 days. For this reason, PhD student Antonio Garcia-Uceda Juarez (p 98) has continued to work on an algorithm for the automated detection of bronchiectasis in CT scans. This algorithm is developed by the BIGR group of Prof. De Bruijne (p 88) as part of a large international bronchiectasis study (iABC) and tested on various data sets. Furthermore, PhD student Qianting Lv works in close collaboration with the image
analysis company Thirona (Nijmegen) to incorporate automated airway artery (AA) measurements into their certified software LungQTM. In Q4 2021 the algorithm was completed. We showed that AA-LungQ is able to fully automatically analyze chest CTs of CF patients. Validation studies in asthma, bronchiectasis, chronic obstructive pulmonary disease, and ciliary dyskinesia are ongoing.
Chest CTs in patient registries and modeling studies
For rare chest diseases accurate imaging based outcome measures are of key importance not only for clinical studies but also for patient registries. Postdoc D. Caudri who returned from Perth (PI Prof Stick) in 2018 is using imaging related outcomes to answer clinical questions within the AREST-CF registry. A grant was written to incorporate imaging related outcome measures also for the European CF Society Patient Registry. A similar project is ongoing for the bronchiectasis registry (EMBARC) (PhD Pieters p 334).
Chest CT is also used by our group to improve our understanding of aerosol treatment. PhD student J. Meerburg modelled the relation between the unique airway geometry of CF patients and the deposition pattern of inhaled tobramycin dry powder (in close collaboration with FluiDDA (Kontich, Belgium)).
Development of chest MRI
The sensitivity of chest MRI to study lung morphology in CF lung disease is inferior to that of chest CT. However, the sensitivity of our routine clinical protocol has been substantially improved by Assistant Professor Dr P. Ciet and MRI physicist P. Wielopolski. They also worked on MRI sequences to study the dynamic qualities of the lung. These cine MRI sequences were used in several clinical studies by the LungAnalysis group studying the dynamic properties of central airways and diaphragm. In addition, time efficient sequences for chest MRI were successfully used to study 4000 children in the Generation R birth cohort. We collaborate with Dr Morana (Treviso, Italy) on the development of MRI sequences to study lung inflammation. In 2021 we completed a collaborative project with the University of Sheffield (PI Prof J. Wild) and Hannover (PI Prof J. Vogel-Claussen) developing and validating an MRI protocol that will allow us to acquire information on Ventilation, Inflammation, Perfusion and Structure (VIPS-MRI-project).
PhD student Bernadette Elders successfully completed her study investigating the upper airways of children using MRI sequences. These patients had open airway
surgery for a laryngeal stenosis at a young age. The airway geometry and vocal cord function were evaluated using static and dynamic MRI sequences in this follow up study (MUSIC study).
Expectations & Directions
LungAnalysis research group
During the 2020 and 2021 pandemic LungAnalysis managed to continue its activities. Dr Kemner-van de Corput successfully implemented the Digital Research Environment (DRE) into the LA workflow to allow researchers to continue their image analysis work from home. LungAnalysis project manager J. van de Puttelaar did an excellent job making project management more efficient. Together with M. Bonte, they have organized MS Teams meetings with many ECFS-CTN for the SCIFI-II CT standardization project. Badies Manai is the research nurse involved in the practical execution of the MRI studies.
LungAnalysis will continue to develop and validate automated systems to quantify image related biomarkers in close collaboration with image analysis companies such as Thirona (Nijmegen). LungAnalysis works closely together with the radiology department. The goal of 2022 is to integrate the automated PRAGMA-CF and the AA-ratio analysis systems into the radiology workflow so these become available for clinical care within the Erasmus MC.
LungAnalysis will continue to work on the development and validation of the VIPS-MRI platform and on advanced image analysis strategies for the acquired MR images. Validation studies by using cine-MRI for the diagnosis of malacia and diaphragm dysfunction in COPD patients are ongoing (PhD student Y. Wang). In addition, we aim to study airway morphology and the mechanical behavior of lung parenchyma in patients with severe asthma (PhD student Wytse van den Bosch) using both CT and cineMRI.
Sophia Pediatric Chest Center PCC and LungAnalysis
In 2015 Erasmus MC-Sophia started the Sophia Pediatric Chest Center (SPCC) for the treatment of complicated heart and lung diseases. LungAnalysis is an important clinical and research infrastructure for the SPCC, as integrated structure function imaging plays an increasingly important role to determine management and follow-up of patients treated in the SPCC. LungAnalysis continues to participate in national and international observational and intervention studies. CF: SHIP-CT project, RECOVER project (Dr. P. McNally), MODUL-CF (Prof. I. Sermet-Gaud-
elus); bronchiectasis: iABC, IMI project, ASPEN (Insmed); chronic lung disease of prematurity (Prof I. Reiss, Dr L. Duijts); idiopathic scoliosis (PhD student J. Renkens); Non Tuberculous Mycobacteria (Prof C. Wainwright); primary ciliary dyskinesia (Prof S. Davis, Prof M. Rosenfeld); neuro-muscular diseases (Prof P. van Doorn, Prof A. van der Ploeg).
In 2021 the Radiology department acquired a photon counting CT (PCCT). This scanner improves spatial resolution almost five fold and can reduce significantly image noise. This is of major importance for imaging of the chest in preschool children age where visualization of the airways is restricted due to the relative poor resolution in relation to the small airway size at this age. Similarly, it will allow substantial better visualization of congenital and interstitial lung abnormalities in children. In older children it will allow to obtain clinical relevant information at lower radiation cost. We aim to start several studies to optimize the use of PCCT for children..
Funding
Harm Tiddens: Grant by Ningxia University Hospital for visiting Professorship Ningxia University, Yinchuan China”
Harm Tiddens: SSWO Program Grant 2017-2022 “Lung magnetic resonance imaging (MRI) in pediatric lung diseases”
Peter Sly (Brisbane), Steve Stick (Perth), Harm Tiddens, and consortium partners: Cystic Fibrosis Foundation Clinical Research Award 2012-2022: “Multi-center, randomized, placebo-controlled study of azithromycin in the primary prevention of radiologically-defined bronchiectasis in infants with cystic fibrosis”
Stephanie Davis (Indianapolis) Viral Pathogenesis in Early CF. NIH grant. Image analysis by LungAnalysis Erasmus MC-Sophia.
Stephanie Davis (Indianapolis), Margaret Rosenfeld (Seattle) PCD project analyzing a large number of PCDCTs
Claire Wainwright (Brisbane); FAB-study: Clinical and psychosocial changes over late childhood and adolescence and early life determinants of long-term clinical outcomes in cystic fibrosis. NHMRC Australia. Image analysis by LungAnalysis Erasmus MC-Sophia.
Claire Wainwright (Brisbane); CFF grant. Finding the optimal treatment for Mycobacterium abscessus treatment (FORMAT study) Site standardization and Image analysis by LungAnalysis Erasmus MC-Sophia.
Harm Tiddens and Kors van der Ent (Utrecht): Dutch Cystic Fibrosis Foundation 2017-2022: “Standardized follow up for children with CF diagnosed by newborn screening” as part of the NCFS HIT-CF II program.
Harm Tiddens: iABC (IMI grant) 2016–2022 Inhaled antibiotics in bronchiectasis and cystic fibrosis.
Harm Tiddens and Stephen Stick: Cystic Fibrosis Foundation Therapeutics 2016-2022. Saline Hypertonic in Preschoolers with cystic fibrosis and lung structure as measured by computed tomography (CT) (SHIP-CT study).
Harm Tiddens: Unconditional grant by Vectura. Chest CT and magnetic resonance imaging of small airway disease in severe asthma (TARGET-study).
Harm Tiddens and Eva van Rikxoort; PPP grant. Computer assisted diagnosis (CAD) for monitoring CF airway disease (the CAD-CAD project)
Harm Tiddens: Standardized ECFS-CTN Chest Imaging Framework for Intervention and personalized medicine for CF: a follow up study. (SCIFI-2 study). CT standardization of 58 ECFS-CTN sites.
Paul McNally; CFF grand. Real world outcomes with novel modifier therapy combinations in children with CF (RECOVER study). CT standardization of all Irish CF sites and image analysis by LungAnalysis Erasmus MC-Sophia.
Invited Lectures
17-03-2021 The importance of HRCT in modern CF management? RMCH Regional Paediatric Cystic Fibrosis Training day, Royal Manchester Children’s Hospital, online.
03-07-2021 Machine learning CT in Bronchiectasis. 2nd European NTM & Bronchiectasis Workshop, online.
04-10-2021 The importance of HCRT in modern CF management. Meeting pediatric pulmonologists Beatrix Children’s Hospital / University Hospital Groningen.
25-10-2021 Imaging in chronic pediatric lung disease. XXV Congresso Nazionale SIMRI, Verona, Italië.
Highlights: LungAnalysis Core Lab
LungAnalysis (LA), founded in 2013, has registered its participation in over 83 national and international research studies and clinical trials. In these studies chest CT is used to phenotype patients and used as an outcome measure underlining the importance of image analysis for lung research. As an Erasmus MC core lab, LA provides services to investigator initiated research projects and clinical trials but also offers image analysis services to industry initiated studies. Image analysis strategies with novel biomarkers are a continuing development. LA has many (inter-)national collaborations with software developing teams such as the Biomedical Imaging Group Rotterdam of Erasmus MC, Intrasense, Politecnico di Milano, and Thirona. Collaboration with Thirona is an important partner for LA to bring validated image analysis algorithms to the bedside. These algorithms are relevant for a wide range of lung diseases.
LungAnalysis and Thirona are proud to have completed in Q4 2021 the development and internal validation of a fully automated sensitive image analysis algorithm for measuring airway-artery (AA) dimensions of all visible AA-pairs for monitoring airways disease. Extensive external validation in various cohorts and for various airway diseases is ongoing.

Pierluigi Ciet is a former LungAnalysis PhD and current Assistant Professor. He received in 2020 the prestigious VENI grant which will allow him to further develop his research line in chest-MRI into the area of interstitial lung diseases. Pierluigi has been named during the International Pediatric Radiology Conference in Rome (2021) chair of the thoracic section of the cardiothoracic imaging taskforce of the European Society of Pediatric Radiology (ESPR). Within this position, he will act to increase connections and collaborations with European Respiratory Society (ERS) and European Society of Thoracic Imaging (ESTI).
Additional Personnel
Dr. Mariëtte PC Kemner-van de Corput –Head LungAnalysis
Jorien van de Puttelaar – Program Manager LA,
study coordinator SHIP-CT and SCIFI-II
Merlijn Bonte – LA technician, coordinator SCIFI-II
Giulia Colzani – Visiting scientist
Roos Wichertjes – LA student
Job de Ridder - LA student
Lotte L Berns – LA student
Charlotte Bosch – LA student
Muhsen Al Sharad – LA student
Mariette Kemner-van de Corput, PhD
Mariëtte Kemner is the Head of LungAnalysis, an image analysis core laboratory. She is responsible for all LA project coordination management, data management and overall quality management and reporting. Since 2020 Jorien van de Puttelaar is the program manager of LungAnalysis. She does all the LA contracting, project planning and timelines. Merlijn Bonte is the LA image analysis technician who is responsible for LA employee training, image analysis services for research and patient care. In 2021, LungAnalysis has implemented the Research Suite facilities to perform image analysis service by means of cloud computing (DRE).
Since 2018 LungAnalysis is involved to execute image analysis services for patient care. In 2019 a new workflow was implemented to meet the demands for image analysis for clinical care. LungAnalysis-Patient Care is working in collaboration with the Imaging Trial Bureau of the Erasmus MC.

Mariëtte Kemner, Merlijn Bonte, Jennifer Meerburg (P 335) and the medical physicist Marcel van Straten (P 60) developed a website for LungAnalysis. The website has online e-learning modules for site personnel for sites to standardize chest CT procedure for research studies and clinical trials. The website is currently used for clinical studies and for standardization of chest CTs within the ECFS clinical trial network.

REAL-WORLD EVIDENCE ON STRUCTURAL LUNG DAMAGE IN CHRONIC LUNG DISEASES
DAAN CAUDRI, MD, PHD
Post-doc
Project Funding American Cystic Fibrosis Foundation (CFF) out-off-cycle application
Research period January 2017 – December 2024
Email d.caudri@erasmusmc.nl / daan.caudri@telethonkids.org.au
Dr. Caudri has a permanent position as Pediatric Pulmonologist and Somnologist at the Erasmus MC Sophia Children’s Hospital. He is trained as a clinical epidemiologist. He is vice director of the Erasmus MC core laboratory ‘LungAnalysis’ (headed by Research Coordinator Dr. M. Kemner). In October 2022 he will take over the role of Director of LungAnalysis from Prof. Dr. Harm Tiddens.
Dr. Caudri has particular interest and experience in the epidemiological study of early markers of later disease. This can be applied in the context of clinical disease prediction as well as with the aim to find causal and modifiable risk factors. He performed his fellowship in Pediatric Pulmonology at the internationally renowned AREST-CF group of Prof dr. Stephen Stick in Perth, Western Australia at the Telethon Kids Institute, where he remains affiliated as Honorary Research Fellow.
Dr. Caudri has extensive experience in the analysis of realworld evidence using longitudinal clinical data bases and international patient data registries. During his fellowship at the AREST-CF research group he combined data from the National Australian CF patient registry with the AREST-CF database. This approach allowed for sufficient power and level of detail to analyze and find new prognostic or causal association is a rare lung disease such as Cystic Fibrosis. Currently Dr. Caudri is setting up a European network to collect CT data on structural lung disease to be included in the European CF Society Patient Registry (ECFSPR). This is the biggest real-world evidence data base on CF patients worldwide and has decades worth of high-quality clinical data from European CF centers. Currently more than 50,000 CF patients contribute data to this registry.
For this project network is being created to collect large numbers of CT routine clinical CT scans, starting with CF centers linked to the European CF Clinical Trial Network Secondly, as part of this project a framework will be created to allow for the automatic scoring of many thousands of
CT scans. There is close collaborating with the automated medical image analysis company Thirona. Joint efforts by Thirona, LungAnalysis and the Department of Pediatric Pulmonology has led to the recent development of fully automated CT scoring algorithms. This has now created the optimal circumstances to collect and score very large numbers of CT scans to obtain unbiased and accurate structural lung imaging outcomes. The resulting scores can be used for a wide range of epidemiological studies, particularly when combined with the wealth of data already available in the ECFSPR.
Dr. Caudri is co-promotor of Federico Mollica, who in his PhD project focusses on assessing and phenotyping structural lung disease in various lung diseases. By using validated CT scores and he will investigate the effects of a new medication for non-CF bronchiectasis in the ASPEN study. As part of the ASPECT study he will use different CT scores to evaluate the effects of early Aspergillus on structural lung disease in CF patients with the aim to define clinically relevant diagnosis categories.
A second PhD student that Dr. Caudri is supervising is the radiologist in training Angelina Pieters. Her PhD project focusses on the describing and phenotyping structural lung abnormalities from patients with non-CF Bronchiectasis. This study also uses the real-world evidence data from the European non-CF Bronchiectasis (EMBARC) patient registry.
Relevant recent publication
A screening tool to identify risk for bronchiectasis progression in children with cystic fibrosis. Caudri D, Turkovic L, de Klerk NH, et al. Pediatr Pulmonol. 2022 Jan;57(1):122-131.
Aspergillus-related lung disease in people with cystic fibrosis: can imaging help us to diagnose disease? Lv Q, Elders BBLJ, Warris A, Caudri D, Ciet P, Tiddens HAWM. Eur Respir Rev. 2021 Nov 17;30(162)

THORACIC MAGNETIC RESONANCE IMAGING
PIERLUIGI CIET, MD, PHD
Assistant Professor
Project Funding American Cystic Fibrosis Foundation (CFF), ZonMW (VENI 2020)
Research period December 2016-September 2024
Email p.ciet@erasmusmc.nl
These projects are a collaboration between the Departments of Radiology & Nuclear Medicine and (Pediatric) Pulmonology.
Dr. Ciet is assistant professor in pediatric and adult thoracic imaging and co-promotor of 6 PhD students. He is also coordinator of all chest magnetic resonance imaging (MRI) studies within the Erasmus MC core laboratory “LungAnalysis”. His research line focuses on the development of new innovative chest MRI techniques for the diagnosis and monitoring of pediatric and adult pulmonary diseases.
Dr. Ciet finalized his fellowship in pediatric radiology in 2021 at Erasmus MC, he is currently appointed as pediatric and thoracic radiologist (clinical fellowship 2014 at Harvard Medical School). This unique training background helps him in the development of multiple chest MRI protocol for the assessment of structure and function.
Final results of the study on children treated for laryngeal tracheal stenosis showed that MRI findings correlated strongly with the function of the vocal cords, making MRI a perfect clinical tool for this cohort. Imaging derived from this protocol are currently analyzed to simulate surgical procedure in the upper airways and to define the most appropriate anatomical region to correct airflow obstruction.
Dr. Ciet together with the department of Pediatric Surgery worked to the improvement of MRI protocols for children with congenital lung abnormalities (CLA) (See example images). Furthermore, a structured report for an objective and quantitative assessment of CLA was developed, which provides useful information also to predict pathology features of CLA. This structured report will be soon integrated in the PACS system.

Figure 1. Comparison 1 mm isotropic end- inspiratory CT (A) and 1.5 mm free-breathing end-respiratory triggered zero echo time (ZTE-MRI) (B) in patient with congenital lung abnormality. Note cystic lesion in the left lower lobe on CT (arrow) and on the ZTEMRI (arrow).
In 2021, Dr. Ciet collaborated to two projects related to COVID pneumonia. Together with the pulmonology department of the Erasmus MC, he correlated imaging findings of patients after COVID pneumonia with respiratory, physical, and psychological outcomes, showing persistent health problems after six months from hospital discharge. In a retrospective study with the hospital Treviso (Italy), Dr. Ciet contributed to define the most specific radiographic pattern of COVID pneumonia. Dr. Ciet is also about to start the Magnetic resonance Interstitial Lung Disease (M-ILD) study, awarded last year by a VENI grant.
Dr. Ciet was recently appointed chair of the thoracic section of the cardiothoracic imaging taskforce of the European Society of Pediatric Radiology (ESPR). During his appointment Dr. Ciet will be in charge of 1) maintaining contact with other societies that deal with pediatric imaging of the thorax, lungs, heart; 2) organizing joint publications on standards in pediatric cardiothoracic imaging and 3) to promote joint European Multicenter Studies.

IMAGING,
MODELLING AND TREATMENT OF SMALL AIRWAYS DISEASE IN SEVERE ASTHMA
WYTSE VAN DEN BOSCH, MD
PhD Student
Advisors Harm Tiddens, Hettie Janssens & Pierluigi Ciet
Project Funding Vectura Group PLC
Research period December 2018 – December 2022
Email w.b.vandenbosch@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Paediatric Pulmonology
Asthma is one of the most common lung diseases in children. Over the years it was shown that the small airways (<2mm) are involved across almost all asthma severities. In an extensive review we published last year we describe that structural and functional alterations of the epithelium, extracellular matrix and airway smooth muscle are frequently observed in the small airways of patients with asthma. It is therefore likely that these small airways alterations play a pivotal role in asthma pathophysiology. CT-scans are frequently performed in patients with severe asthma to exclude conditions that mimic asthma and other pulmonary abnormalities that could contribute to the symptoms patients are experiencing. On CT, airways with dimensions smaller than <2 mm in diameter cannot be directly evaluated as they are below the resolution of most CT scanners. However, small airways involvement in asthmatics patients can be detected indirectly on expiratory CT scans at the residual volume level as low attenuation regions (LAR), also often described as ‘’trapped air. In the literature bronchial wall thickening and LAR have been described in (severe) asthma patients. However, due to the heterogeneity within the disease of asthma it is unclear what structural and functional alterations characterize severe asthma. I was able to collect 160 spirometer guided chest CT-scans of children with severe asthma that were made in the Sophia Children’s hospital (Fig. 1). Currently, we are analysing these scans to investigate what structural alterations are associated with severe asthma and whether the small airways are also affected. For the analyses of airway dimensions we are using artificial Intelligence based algorithms to automatically measure the airway and accompanying artery dimensions.

Figure 1. Spirometer guided expiratory chest CT in a patient with severe asthma. Large low attenuation regions (Black arrows) are present in the left lung. This is suggestive for small airways disease.
Spirometer guided MRI is another imaging modality that can provide us with knowledge on small airways involvement in patients with asthma. The major advantage of MRI is that it can provide us with structural and functional information regarding the lungs and airways in a single examination without the use of ionizing radiation. The LungAnalysis research group has developed proton MRI protocols that allow us to detect low intensity regions (LIR) reflecting regions that are hypo-perfused and/or contain trapped air. As MRI does not require ionizing radiation it is an attractive option to monitor LIR as an outcome measure in clinical studies. In 2022 we will start including severe asthma patients for a study that will investigate the effects of bronchodilators on LIR and lung dynamics using chest MRI.

CHEST CT ASSESSMENT TO MONITOR PROGRESSION IN YOUNG CHILDREN WITH CYSTIC FIBROSIS
YUXIN CHEN, MSC, MD
PhD Student
Advisors Harm Tiddens, Pierluigi Ciet & Daan Caudri
Project Funding Funded by Cystic Fibrosis Foundation Therapeutics (CFFT)
Research period September 2021 – September 2023
Email y.chen.1@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Pediatric Pulmonology and Allergology.
Ifinished my research master program in clinical research in Netherlands Institute for Health Sciences and obtained a Master of Science degree on September 2nd 2021. For my PhD program I continued working on the project which started during my master program focused on chest CT assessment to monitor disease progression in young children with cystic fibrosis (CF).
CF lung disease is characterized by early impairment of mucociliary clearance, chronic airway infection, and inflammation. Structural lung disease has already developed in a large proportion of preschool children with CF and has a negative impact on prognosis and quality of life. Effective therapies are needed to prevent progression of structural lung disease in early age. The first project of my PhD is to evaluate the effect of inhaled 7% hypertonic saline on structural lung changes as measured by chest CT using the Perth-Rotterdam Annotated Grid Morphometric Analysis for CF (PRAGMA-CF) method in preschool children with CF relative to isotonic saline (SHIP-CT study). The results of this trial show a positive treatment effect of twice daily hypertonic saline inhalation on structural airway changes on chest CT over 48 weeks. The manuscript of this result has been accepted for publication in Lancet Respiratory Medicine.
The second part of my PhD focuses on validating automated image analysis in young children with CF. An automated airway and artery (AA) method was recently developed and validated to detect structural airway changes on chest CT. This automated AA-method is able to detect all visible AA-pairs and quantify diameters of each airway and adjacent artery. In addition, automated PRAGMA-CF method has been developed that allow to replace the manual PRAGMA-CF by quantifying structural lung abnormalities

Figure 1. One annotated inspiratory CT slice by PRAGMA-CF method. For each grid cell, the observer assessed the presence of the sub-scores: healthy airways (green), bronchiectasis (red), mucus plugging (yellow), airway wall thickening (orange, not present in this example), and atelectasis (pink).
on all available CT slices. These automated methods will be clinically validated in multiple data cohorts such as SHIP-CT and COMBAT-CF study in young children with CF.
Another project in my PhD will be about evaluating risk factors of early determinants in later disease progression in late childhood and early adolescence in children with cystic fibrosis (CF-FAB study). This study monitors progression of CF lung disease using both functional and structural outcome measures in children with CF from diagnosis up to the age of 14 years. I scored all CTs using the manual PRAGMA-CF scoring system. In addition we will run the automated PRAGMA-CF and AA-method to compare the sensitivity of these systems to detect disease progression.

MULTICENTER IMAGE QUALITY STANDARDIZATION IN LUNG MRI: THE VIPS STUDY
GIULIA COLZANI, BSC PhD
Advisors Juan Antonio Hernández Tamames, Harm Tiddens & Pierluigi Ciet
Project Funding
Research period January 2020 – April 2021
Email g.colzani@erasmusmc.nl
Student
This project is a collaboration between the Departments of Radiology & Nuclear Medicine, Paediatric Pulmonology and Allergology.
My research fellowship focused on the development of a standardized chest Magnetic Resonance Imaging (MRI) protocol to assess functional and structural outcomes for Cystic Fibrosis (CF) Lung Disease. CF lung disease remains the main cause of morbidity and mortality in CF patients, close monitoring of disease progression is crucial to determine when to start treatment and to monitor the effect of such treatment. Imaging plays an important role, where chest computed Tomography (CT) is the current gold standard. To reduce ionizing radiation burden, chest MRI has been introduced as a radiation free alternative for chest CT, but large variability in protocols and image quality remain between CF centers.
Chest MRI can provide in a single examination information about Ventilation, Inflammation, Perfusion and Structure (VIPS). During my fellowship, I worked on standardization of image quality within the VIPS-MRI consortium, led by the Erasmus MC, and including centers in Hannover (Germany), Sheffield (UK) and Treviso (Italy). The VIPS-MRI consortium aims to provide a standardized high image quality MRI protocol for monitoring CF lung disease that can be used across CF centers that have access to MRI.
I also worked on the development of post-processing tools for the assessment of ventilation and perfusion using Fourier Decomposition (FD) MRI. FD-MRI is a new chest MRI technique that can provide ventilation and perfusion maps without using intravenous or gaseous contrast agents. We aim to use this FD-MRI in studies in patients with CF, bronchopulmonary dysplasia, and congenital lung abnormalities.

Comparison between ventilation colored image obtained with Fourier Decomposition MRI (FD-MRI) and Minimum Intensity Projection (MinIP) end-expiratory CT image. FD-MRI assesses ventilation defects by measuring variations in lung signal intensity (SI) during the breathing cycle. Regions where SI does not change represent regions of air trapping (AT) and are shown as low SI (blue-green areas on image on the left, white arrow). In the corresponding CT figure, regions of AT in the right upper lobe appear as to low density regions (dark areas on the image on the right)

MAGNETIC RESONANCE IMAGINGOF
THE PEDIATRIC
RESPIRATORY
TRACT
BERNADETTE ELDERS, MD PhD Student
Advisors Harm Tiddens & Pierluigi Ciet
Project Funding Vrienden voor Sophia
Research period September 2017– September 2021
Email b.elders@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine, Pediatric Pulmonology and Allergology.
Chest Magnetic Resonance Imaging (MRI) is promising for the imaging of the pediatric respiratory tract. However, it remains a challenging technique, mostly for low spatial resolution, long scanning times and motion artefacts. This PhD program focusses on the development and testing of MRI protocols to image the pediatric respiratory tract and is divided into two parts: part I MR imaging of the upper and part II MR imaging of the lower respiratory tract.
In part I results from the ‘Magnetic resonance imaging of the upper respiratory tract in children and young adults’ (MUSIC) study are presented. In which we have successfully developed and tested a static and dynamic MRI protocol to image the upper airways in children post laryngotracheal stenosis repair. Results from the MUSIC study have been published in two manuscripts, and a third manuscript describing the use of MRI to conduct Computational Fluid Dynamic modelling analyses to predict the outcome of surgery on the upper airway has recently been submitted. In addition, our upper airway MRI protocol is now implemented for clinical practice in our hospital for children with complex upper airway diseases.
Part II of this PhD thesis consists of research on Bronchopulmonary dysplasia (BPD) and Congenital Lung Ab-
normalities (CLA). In the VIBE study we have developed a MRI protocol to image structural and functional lung changes in school age children with BPD. Our findings show that MRI is able to identify the most relevant structural lung changes in this patient cohort. Results from the VIBE study have recently been submitted for publication. In the VINyL study, we have developed a chest MRI protocol to image the lungs of premature born patients in the neonatal age. The first results from this study look very promising and inclusions of the VINyL study will continue throughout 2022.
In the C LAM study, we have developed and tested a noncontrast enhanced chest MRI protocol for the long term follow up of CLA. Our results show that chest MRI can visualize relevant lung abnormalities in this patient cohort, and described the evolution of the relative size of CLA as well as associated parenchymal abnormalities. The figure shows visualization of lesion vascularization on axial contrast-enhanced postnatal Computed Tomography (CT) (A and D), MRA FIESTA (B and E) and T2-w PROPELLER (C and F) in a patient with a bronchopulmonary sequestration. The figure shows venous drainage of the lesion into the hemiazygos vein (thin arrow on A,B,C) and a bronchocèle (thick arrow on A,B,C) and aberrant arterial supply from the aorta descendens (arrow on D,E,F). The manuscript from the CLAM study has recently been submitted for publication.


COMPUTER-AIDED DIAGNOSIS FOR MONITORING CF AIRWAY DISEASE: THE CAD-CAD METHOD
QIANTING LV, MD PhD Student
Advisors Harm Tiddens, Marleen de Bruijne & Pierluigi Ciet
Project Funding Nederlandse Cystic Fibrosis Stichting (NCFS) – Health Holland (PPS)
Research period October 2018 – March 2023
Email l.qianting@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Pediatric Pulmonology, Allergology, BIGR and Thirona
Cystic Fibrosis (CF) lung disease is characterized by progressive structure changes that start in infancy. The most important changes are airway wall thickening, mucus plugging, and bronchiectasis. Manual measurement of all visible airway and artery (AA) pairs on chest Computed Tomography (CT) scans of CF patients has shown to be a sensitive method to detect and monitor airways disease in CF (Kuo et al, 2017). However, this method is extremely time consuming. For this reason we are developing and validating an automated measuring method to assess AA-dimensions in collaboration with Thirona, an image analysis company in Nijmegen.
Artificial Intelligence based algorithms were developed by Thirona to automatically measure the dimensions of airways and their accompanying artery. This automated AA-method allows us to objectively detect airway wall thickening and bronchiectasis.
40 out of 70 CT scans from the Erasmus MC Sophia CFcohort were used as internal training dataset to make the algorithm robust for CF abnormalities. For validation we compared the ability to discriminate between airway dimensions of chest-CTs of 11 CF-patients (median age 11, [7-16] years) and of 12, age matched, normal control chest-CTs. Furthermore, the automatic AA-analysis was compared against a manual AA-method. The automated AA-method showed a high accuracy to detect structural airway changes comparable to manual methods. Next, the AA-method was used to assess the ability to detect disease progression on chest-CT of two external longitudinal CF cohorts. It was shown that the AA-method was able to detect disease progression in a cohort of preschool

1. Color coded map of the bronchial tree of a CF patient using the AA-method: a. Airway wall thickening (in red); b. Airways with bronchiectasis (in red). Figure courtesy by Thirona, Nijmegen: LungQ software®
children and in a cohort of adults. Further validation in several other CF cohorts is ongoing. In addition, the AAmethod is now used to assess its performance to track lung disease in patients with asthma, COPD, primary ciliary dyskinesia, and bronchiectasis patients.
For all these studies the ErasmusMC LungAnalysis core laboratory works closely together with Thirona (Nijmegen, The Netherlands).
Figure

Advisors
Project Funding
PHENOTYPING OF CHEST COMPUTED TOMOGRAPHY SCANS OF PATIENTS WITH BRONCHIECTASIS
FEDERICO MOLLICA, MD
Harm Tiddens, Pierluigi Ciet & Daan Caudri
Research period January 2020 – January 2024
Email f.mollica@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Paediatric Pulmonology.
Chronic lung diseases are characterized by progressive diffuse airway abnormalities such as airway wall thickening and bronchiectasis.
Bronchiectasis (BE) is defined as an abnormal widening of the airways, which can develop as a result of chronic infection and inflammation. BE can best be observed on chest computed tomography (CT) scans. Treatment strategies for BE patients are often derived from cystic fibrosis (CF) treatments, because few clinical studies have been performed in the field of non-CF BE.
Chest CT is the gold standard to diagnose and monitor BE.
To determine efficacy of therapies for BE in clinical studies, there is a need for the development of sensitive outcome measures. Within this PhD project different BE scoring methods for chest CTs will be further validated for CT scans of BE patients.
We use the BE-CT scoring method to measure the extension of BE detected on 2000 Chest CTs for the ASPEN study (ClinicalTrials.gov: NCT04594369). A score between (03) is given for each lobe assessing the extend of BE. The BE-CT score is part of the eligibility evaluation for the ASPEN study investigating the effect Brensocatib on the progression of BE. In addition, for the ASPEN study we will evaluate the efficacy of Brensocatib on various innovative chest CT outcomes in a sub study.
In the FORMAT study we aim to identify typical lung structural abnormalities on chest CTs of patients affected by atypical mycobacteria. This study runs in Australia and Europe. To use chest CT as outcome measure for this study, standardisation of image quality and lung volumes is needed. To accomplish this LungAnalysis has developed

Figure 1. MERAGMA-PCD scoring method of an inspiratory chest CT in a patient with Primary Ciliary Dyskinesia.
Large atelectasis and bronchiectasis (purple color) are present.
a website for centres participating in the FORMAT study (https://lunganalysis.erasmusmc.nl/). Through the website we are able to provide training and certification of centres, give interactive feedback on acquired CT-scans, and support by an online helpdesk.
ASPECT is a pilot study with the goal of finding a fingerprint of structural lung abnormalities in chest CTs of CF patients typical for damage caused by aspergillus fumigatus. To do so we study correlations between the presence of aspergillus in sputum , immunological markers related to aspergillus infection, and chest CTs findings, making use of the Erasmus MC – Sophia Children’s Hospital clinical dataset.
For the Copenhagen perfusion study we study the relation between local structural lung changes and markers of lung perfusion in chest CTs of children with CF. Furthermore for the PCD study we use MERAGMA-PCD and two other scoring methods to evaluate structural lung abnormalities on Chest CTs of patients affected by Primary Ciliary Dyskinesia .

BRONCHIECTASIS: QUANTIFICATION, CHARACTERIZATION AND CLINICAL CONSEQUENCES
ANGELINA PIETERS, MD
PhD Student
Advisors Harm Tiddens, Menno van der Eerden, & Pierluigi Ciet
Project Funding Innovative Medicines Initiative, grant agreement n° 115721, FP7/2007-2013 and EFPI
Research period May 2020 – May 2023
Email a.l.p.pieters@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Pediatric Pulmonology and Allergology
Bronchiectasis (BE), an abnormal widening of the airways which can develop as a result of chronic infection and inflammation, is relatively poorly investigated in respiratory medicine. There are no approved therapies for BE and only a few large-scale representative epidemiological studies. To counteract this paucity of evidence, the European Multi-center Bronchiectasis Audit and Research Collaboration (EMBARC) registry was established in 2012. The EMBARC registry is a prospective, pan-European observational study of patients with BE that aims to harmonize existing databases, identify determinants of BE and to raise awareness of BE at an international level. Imaging of the lungs plays an important role in detection and quantification of BE, especially with Computed Tomography (CT). However, quantitative CT data for BE has been so far poorly investigated and standardized and is currently not included in the EMBARC registry. The aim of my PhD is to develop and incorporate relevant chest CT related quantitative outcomes into the EMBARC registry.
The Erasmus MC LungAnalysis core-laboratory has developed a quantitative morphometric scoring system for BE known as BEST-CT, which is based on the PRAGMACF annotation method which is well validated for Cystic Fibrosis (CF). The goal is to develop a fully automated BEST-CT scoring method. At this point, I have manually annotated 500 CTs of patients included in the EMBARC registry. Next, this annotated data set will be used to develop an automated version of BEST-CT using artificial intelligence strategies. In addition, LungAnalysis has developed a website for the standardization of chest CT imaging protocols to be used for BE patients included in the EMBARC registry.

Figure 1. End-inspiratory CT slice scored with the iBEST-CT method. For each grid box, the observer scores the following radiological features in hierarchical order: atelectasis and/or consolidation (pink), bronchiectasis with mucus (purple), bronchiectasis (red), mucus plugging (yellow), airway wall thickening (not present in example), healthy airways (bright green), and healthy parenchyma (dark green).
Another imaging analysis technique, developed by LungAnalysis in collaboration with Thirona (Nijmegen, Netherlands), is the Airway-Artery method. This method will used to fully automatically assess on chest CTs in the EMBARC population airway-artery dimensions of hundreds of airway-artery pairs throughout the bronchial tree.

IMAGING AND TREATMENT OF BRONCHIECTASIS
JENNIFER J MEERBURG, MSC, MD, PHD
PhD Awarded 10 November 2021
Advisors Harm Tiddens & Marcel van Straten
Project Funding Innovative Medicines Initiative (IMI) funded by the European Union, Novartis Pharma AG and Basilea Pharmaceuticals
Short CV
Jennifer Meerburg studied medicines at the Erasmus University, and started her PhD in 2015 at Erasmus MC. She was involved in the iBEST study of the European Inhaled Antibiotics in Bronchiectasis and Cystic fibrosis (iABC) consortium, being responsible for the collection and analysis of computed tomography scans of bronchiectasis patients. Furthermore, she led the TIPTIS study, a Dutch multi-center trial within the field of inhaled antibiotics for cystic fibrosis patients. Besides doing research, she committed herself as board member to Skate4AIR, an annual fund raising event for Cystic Fibrosis. She is currently in training to become a general practitioner.
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Pediatric Pulmonology and Allergology
The thesis “Imaging and Treatment of Bronchiectasis” focuses on the diagnosis and treatment of bronchiectasis. The radiological definition of bronchiectasis was reviewed for which 122 papers were included. This systematic review showed that there are no validated diagnostic criteria for bronchiectasis, and different cut-off values for airway-artery ratios are being used. The results of this review were used in a Delphi process that was performed to come up with a definition for bronchiectasis that can be used until validation studies have been performed (Aliberti et al, Lancet Respiratory).
In two studies, computed tomography (CT) images were analysed using systematic scoring systems.
First, CT scans a European cohort of 138 patients with granulomatous interstitial lung disease were retrospectively collected and analysed using the Hartmann and Baumann scoring method. In this study, Hartmann showed to a more sensitive method to detect changes in bronchiectasis over time.
Secondly, a new CT analysis system for bronchiectasis patients was developed: the BronchiEctasis Scoring Technique for CT (BEST-CT). The BEST-CT is a morphometric scoring system

in which an overlaying grid is placed on CT scan slices, after which the grid boxes are annotated by an observer for the presence of abnormalities. The outcomes of the analysis with BEST-CT were compared with those of the Hartmann method, intra-branch tapering, and clinical parameters.
The BEST-CT scoring method is currently being used to analyse CT 1000 chest CTs of patients participating the European bronchiectasis patient registry (EMBARC).



IMAGING IN HEALTH SCIENCES
JOINT APPOINTMENT IN EPIDEMIOLOGY
Meike Vernooij was trained in Radiology at Erasmus MC, followed by a clinical fellowship in Neuroradiology and Head & Neck Radiology. Meike combined her clinical work with a PhD in Neuroimaging/ Neuroepidemiology – completed cum laude in 2009 – and has been working as a clinician-researcher in the internationally renowned Rotterdam Study since 2005. Her main interest is the study of brain changes on imaging in aging, and in particular imaging markers for stroke and dementia. Ultimate aim is to improve insight into etiology and

prediction of these diseases. She was awarded the Lourens Penning Prize by the Radiological Society of the Netherlands in 2008 and the Lucien Appel Prize in Neuroradiology by the European Society of Neuroradiology in 2014. Since 2010, she is principal investigator of Population Imaging of the Rotterdam Study and as such coleading the Rotterdam Scan Study. In 2017, Meike was appointed as professor of Population Imaging. She has authored over 350 peer-reviewed publications and is currently supervising 9 PhD students and 3 postdocs. m.vernooij@erasmusmc.nl
POPULATION IMAGING
MEIK E W VERNOOIJ, MD, PHD
full professor

Context
Once patients present with symptoms, in many cases irreversible damage is already present. This is true for many diseases that are common in the population, like cardiovascular disease and dementia. Clinical studies are usually limited to studying diagnosis, prognosis and treatment of disease. If we want to understand why disease develops and which factors drive its development, we need to study disease in its earliest forms, when symptoms are not yet present. This is the area in which population-based studies operate. Studies that start out with presumed healthy individuals, assess potential disease determinants and follow participants for occurrence of disease (Figure 1). Over the past decades, imaging is playing an increasingly important role in this study of associations between determinants and disease, by allowing us to non-invasively directly study the tissue at risk. Population Imaging, the large-scale acquisition of medical images in controlled population-based cohorts, allows to investigate structural and functional changes in the human body that may indicate early disease, can be used to identify persons at risk of developing disease, or may aid in disease prediction.

Top Publications 2021
van der Velpen IF, Melis RJF, Perry M, VernooijDassen MJF, Ikram MA and Vernooij MW. Social Health Is Associated With Structural Brain Changes in Older Adults: The Rotterdam Study. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021.
van Arendonk J, Yilmaz P, Steketee R, Zijlmans JL, Lamballais S, Niessen WJ, Neitzel J, Ikram MA and Vernooij MW. Resistance to developing brain pathology due to vascular risk factors: the role of educational attainment . Neurobiol Aging. 2021;106:197-206.
Venkatraghavan V, Vinke EJ, Bron EE, Niessen WJ, Ikram MA, Klein S, Vernooij MW and Alzheimer’s Disease Neuroimaging I. Progression along datadriven disease timelines is predictive of Alzheimer’s disease in a population-based cohort. Neuroimage . 2021;238:118233.
Figure 1. Schematic representation of Population Imaging
Research Projects: Objectives & Achievements
Our Population Imaging studies at Erasmus MC primarily take place within two large cohorts. The Rotterdam Study is a prospective, population-based study aimed at investigating determinants of chronic and disabling diseases among nearly 15,000 persons aged 45 years and over. The Generation R Study is a prospective cohort study among 10,000 children who are followed from fetal life until young adulthood in a multi-ethnic urban population. Whereas the Rotterdam Study focuses at disease at old age, Generation R mainly aims to study child development, both physically and mentally.
Population imaging within the Rotterdam Study currently comprises brain MR imaging (more than 12,000 scans in over 8,000 individuals), CT-assessed arterial calcification (2,500 persons), carotid MR imaging (over 1,500 persons) and musculoskeletal imaging (knee MRI in over 800 subjects). Since 2018, we are also performing brain amyloid PET CT (with a florbetaben tracer) in > 640 Rotterdam Study participants (data inclusion ended in December 2021). In 2020, we started in a subcohort of 200 participants high-field brain MRI (7T) to study cerebral small vessels in more detail.
We apply automated computer algorithms to process all imaging data to extract relevant imaging features (e.g. volumetric assessments, but also more advanced measures such as white matter tractography or structure shape on brain MRI scans; or shear stress measurements on carotid MRI and calcification patterns on vascular CT). In 2020, we started in a subcohort of 200 participants high-field brain MRI (7T) to study cerebral small vessels in more detail.
Primary collaborators of the Department of Radiology within the population imaging research line in the Rotterdam Study are the Department of Epidemiology (department chair professor Arfan Ikram), the Biomedical Imaging Group (PI professor Wiro Niessen), the Intracranial Atherosclerosis research group (PI Dr. Daniel Bos) and the department of Neurology (PI Clinical Neuroepidemiology professor Kamran Ikram). Within Generation R, Dr. Tonya White (joint appointment in Child Psychiatry) and Dr. Steven Kushner (Psychiatry) are primary collaborators.
Cardiovascular disease: MDCT and MRI
Arteriosclerosis is a systemic disease with a predilection for the coronary arteries, the aorta and the carotid arteries. Imaging based measures of arteriosclerosis at these sites improves our understanding of the disease
and may ultimately improve risk prediction of clinical events including stroke and coronary heart disease. See for further details on this research line 362 .
Cerebrovascular and neurodegenerative disease
Since 2005, all participants in the Rotterdam Study undergo MRI of the brain. The imaging protocol includes structural brain imaging for volumetric and shape analysis of various brain structures. This provides for assessing focal structural abnormalities – including brain infarcts and lacunes, white matter lesions, and microbleeds. In addition, diffusion tensor imaging yields quantitative information on the integrity of normal appearing white matter. Furthermore, we are using freely available software, such as Freesurfer, to obtain quantitative information on structural volumes, e.g. cortical thickness. Since 2012, resting-state functional MRI has been added to the imaging protocol, in order to assess measures of functional brain connectivity. Since 2020, the research dedicated MRI scanner was upgraded, allowing us to also acquire arterial spin labeling (ASL).
The main research questions are: How does vascular and degenerative brain pathology affect the development of dementia or ischemic and hemorrhagic stroke? What are the risk factors for cognitive decline and dementia? How can we predict an individual’s risk to develop dementia or stroke? Can we unravel the genetic basis of dementia and stroke by using imaging markers as endophenotypes? Over 6,000 participants have already been scanned and multiple time-point follow-up brain MRI is available in over 4,000 individuals (> 12,000 scans in total). All scans are also read for incidental abnormalities, which has yielded unprecedented information on occurrence and natural course of brain changes and abnormalities in communitydwelling persons.
Examples of results in recent years
Normal brain aging is still only sparsely understood, though it is an essential background to compare several age-related diseases against. We have written recently several landmark papers which provide basic insight into structural and functional brain aging in the general population, demonstrating for example the sequence with which brain changes occur during aging. In this line of research, we are continuously searching for ways to extract most meaningful (quantitative) information from the image data (Figure 2).

Legend Figure 2: In the left panel, manual hippocampal measurement is shown, yielding a simple volume measure. Middle and right panels show more advanced measures of shape (middle) and subfield (right), resulting in potentially more sensitive and powerful quantitative markers.
We have furthermore shown that white matter microstructure has added value over macrostructure in cognitive deterioration and that tract-specific regional deterioration of white matter in aging relates to cognitive performance, to risk of stroke and to mortality. This research is instrumental in changing our way of thinking of white matter as a ‘bulk substance’ into differentiated tracts with specialized functions in aging, and to understand that we need to study tract-specific changes in cognitive deterioriation and dementia.
With respect to cerebral small vessel disease, we have shown that in the general population, cerebral microbleed presence relates to risk of stroke and dementia, further strengthening the view of microbleeds as a ‘missing link’ between vascular disease and neurodegeneration (Figure 3).
Expectations & Directions
Imaging in population-based studies is becoming ever more important in studying determinants of disease and in disease prediction. Non-invasive imaging techniques, such as MRI, enable us to detect increasingly subtle and early pathologic changes in asymptomatic individuals, tremendously enlarging our power and sensitivity to study common diseases, like stroke and dementia. In the coming years, we expect particular progress to be made by exploring the interrelationship between structure and function of bodily tissues. The amyloid PET data that were recently required in > 640 study participants will enable us to study the vascular and amyloid pathways towards dementia in depth, gaining more understanding in how these pathologies alone or in conjunction may lead to neurodegeneration and cognitive decline.

3. Microbleed incidence data resulting from the Rotterdam Study
Legend Figure 3: Data on 4-year microbleed incidence in population imaging, demonstrating a high incidence of (multiple) new microbleeds in persons with microbleeds at baseline scan.
Studies like the 7T small vessel disease study that is executed in collaboration with University Medical Centre Utrecht, will greatly add to this by allowing to directly image and measure microangiopathy, one of the most important contributors to both dementia and stroke. Furthermore, advances in image processing, yielding quantification of more and new markers and data-driven artificial intelligence research techniques (machine
Figure 2. Example of shift in quantitative image measurements.
Figure
learning, deep learning) will bring the field of population imaging forward. Also, combining imaging with other high-dimensional data such as genomics, proteomics and metabolomics, is highly promising in unravelling pathways of disease and better understand disease pathophysiology. Finally, we will focus in the next years even more on the clinical relevance and prognosis of the imaging markers assessed in our cohorts.
Funding
Meike Vernooij, Frank Wolters, Arfan Ikram: A Personalized Medicine Approach for Alzheimer’s Disease (ABOARD); a public-private partnership receiving funding from ZonMW and Health Holland (2021-2025).
Julia Neitzel: DIVERT-AD, a global Marie Curie Fellowship (2021-2024).
Eddy van der Zee, Martien Kas, Meike Vernooij , Arfan Ikram, Henning Tiemeier, Rene Melis, Myrra VernooijDassen, Marieke Perry: Memorabel grant “Social factors in cognitive decline and dementia: towards an early intervention approach”(2017-2021)
Meike Vernooij, Arfan Ikram, Danielle van Assema, Roelf Valkema , Kamran Ikram: Memorabel grant “Amyloid pathology and vascular disease in focus: exploring interaction in two pathways towards neurodegeneration”(2017-2022).
Invited Lectures
Vernooij M. Quantitative Imaging in Neuroradiology; invited presentation for annual conference of ESNR, Geneva, Switz., Oct 2021
Vernooij M. Imaging in small vessel disease; keynote lecture in MICCAI Challenge Sattelite Session; online, Sept 2021
Vernooij M. Microbleeds: Black Sheep in Secondary Stroke Prevention Strategies?; invited lecture for European Stroke Conference; online, Sept 2021.
Vernooij M. Population imaging, lessons learned from neuro-imaging; invited keynote for IWOAI, online, July 2021.
Vernooij M. Imaging in small vessel disease; webinar for the British Society for Neuroradiology, online; May 2021.
Vernooij M. Imaging the Aging Brain: Changes Across the Lifespan; invited lecture for ISMRM, online; May 2021.
Highlights
The AmyVasc study (amyloid PET CT in Rotterdam Study participants) concluded their data collection with 640 scanned participants.
Visual rating of all 640 amyloid PET CT scans was finalized.
The AmyVasc study was featured in a webinar for World Alzheimer’s Day organized by the Alzheimer Center and ERGO.
Julia Neitzel was awarded a Marie Curie Global Fellowship for a collaboration between Harvard T.H. Chan School of Public Health and Erasmus MC on understanding resistance and resilience factors in the asymptomatic stage of dementia.
Meike Vernooij, Frank Wolters and Arfan Ikram joined the ABOARD consortium for better prediction and prevention of Alzheimer’s Disease.
Additional Personnel
Michiel van den Akker – Student Assistant MRI Ommoord
Mehdi Badaoui – Student Assistant MRI Ommoord
Fengli Bottema – Student Assistant MRI Ommoord
Chiara Bruggink – Student Assistant MRI Ommoord
Tristan Calon - Student Assistant MRI Ommoord
Dina El Bojaddaini - Student Assistant MRI Ommoord
Eline van Campen – Student Assistant MRI Ommoord –Team Leader
Rachida Hadouch – Radiology Assistant MRI Ommoord
Mellan Hoek – Student Assistant MRI Ommoord
Eileen Kikkert – Student Assistant MRI Ommoord
Freya Huijsmans – Student Assistant MRI Ommoord
Sevket Kilic – Student Assistant MRI Ommoord
Julia Krijgsman – Student Assistant MRI Ommoord – Team Leader
Philip Lambermon – Student Assistant MRI Ommoord
Julia van Miert - Student Assistant MRI Ommoord
Hoa Nguyen - Student Assistant MRI Ommoord
Laura Oudshoorn – Student Assistant MRI Ommoord
Michiel van Riel – Student Assistant MRI Ommoord
Mariska Riemens – Student Assistant MRI Ommoord
Evelien Rodenburg – Student Assistant MRI Ommoord
Levy Schimmel – Student Assistant MRI Ommoord
Anne-Sterre Schutter – Student Assistant MRI Ommoord
Rosa Stroom – Student Assistant MRI Ommoord
Hafsa Tozkoparan – Student Assistant MRI Ommoord
Anna Smak Gregoor – Student Assistant MRI Ommoord
Celine Tuik – Student Assistant MRI Ommoord
Marco Verweij – Student Assistant MRI Ommoord
Eline van der Walle – Student Assistant MRI Ommoord

RESILIENCE & RISK FACTORS OF DEMENTIA
JULIA NEITZEL, PHD
Project Funding COSTREAM & Marie Curie Individual Fellowship
Research period September 2020 – August 2021 & September 2021– August 2024
Email j.neitzel@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine, Erasmus MC, Rotterdam, NL and Epidemiology, Harvard T.H. Chan School of Public Health, Boston, USA
My overall research focus is on the identification of genetic and lifestyle factors as well as brain characteristics that help an individual to avoid neuropathology (increase resistance) or, if already developed, to cope with neuropathology (increase resilience).
I received a Marie Curie Global Fellowship to work with Prof Albert Hofman and other colleagues at Harvard T.H. Chan School of Public Health on understanding resistance and resilience factors in the asymptomatic stage of dementia. One of the key questions I aim to answer is whether a favorable lifestyle profile can ameliorate the genetic risk of dementia (e.g. ApoEe4). To this end I will study gene-environment interactions on imaging-based markers of neuropathology (amyloid deposition on PET, cerebrovascular lesions and neurodegeneration on MRI) in European and American prospective cohort studies.
Regarding genetic resilience factors, I showed that KlothoVS heterozygosity (KL-VShet), which occurs in about 20% of the population, is associated with lower Alzheimer’s disease (AD) neuropathology (Neitzel et al, Nat Commun, 2021). With rising beta-amyloid (Aβ) plaque levels, KL-VShet carriers experienced lower neurofibrillary tangles measured on Flortaucipir-PET than non-carriers (data from 551 participants of the Alzheimer’s Disease Neuroimaging Initiative). It could be additionally demonstrated that KL-VShet was related to better memory performance and that this effect was mediated by lower tau.
Complementary to resilience factors, I started investigating the effect of vascular risk factors on dementia outcome. Together with colleagues from the ISD (Munich, Germany), I found that particularly hypertension in midlife was associated with a higher risk of developing dementia (mean

1 Comparing the effects of age, APOEe4, diabetes, and hypertension on Aβ burden
Estimated mean difference in FBB-SUVr values across 34 cortical brain regions contrasting groups who are younger vs. older than 70 years, APOEe4 carriers vs. non-carriers, diabetic vs. diabetesfree or hypertensive vs normotensive.
follow-up 9 years) in 229,976 participants from the UK Biobank (Malik, Georgakis, Neitzel et al., Alzheimer Dement, 2021).
In our most recent work (Arendonk, Neitzel, Steketee et al, in preparation), we studied the effect of vascular risk factors on Aβ pathology measured on Florbetaben-PET in 506 participants of the Rotterdam Study. A diagnosis of diabetes seven years prior to PET was associated with a higher risk of a positive Aβ status (OR[95%CI] = 4.07[1.66–9.71], P = 0.002) and higher SUVr values, indicating more severe Aβ pathology (Fig. 1). Hypertension was also related to higher SUVr values, but primarily in ApoEe4 carriers. No effect was found for hypercholesterolemia, obesity, physical inactivity and smoking.
Fig.

PHYSICAL ACTIVITY AND BRAIN
HEALTH
MARÍA RODRIGUEZ-AYLLON, PHD
Post-doc
Project Funding Ramón Areces Fellowship (https://www.fundacionareces.es/fundacionareces/es/)
Research period January 2021 – December 2022
Email m.rodriguez@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Epidemiology.
My overall research focused on understanding the role of physical activity in brain health across the lifespan. Strong evidence demonstrates that physical activity is associated with better cognitive development at the early stages of life and a reduced risk of developing cognitive impairment later in life. However, the role of physical activity in relation to brain structure still remains controversial. In a cross-sectional study, we found that higher levels of physical activity were associated with greater white matter microstructure in children from the Generation R (Rodriguez-Ayllon, NeuroImage, 2019).
Those results were not confirmed by a longitudinal study carried out in elderly people from the Rotterdam Study, where we found that brain structure, in terms of brain volume and white matter microstructure, predicted physical activity levels, but physical activity did not predict brain structure over time (https://papers.ssrn.com/sol3/papers. cfm?abstract_id=389144).
Particularly, using neuroimaging data from 4,365 middle-aged and elderly individuals, we found that a better brain structure (i.e., higher total brain volume, gray matter volume, white matter volume and microstructure) was associated with an increase in levels of physical activity (i.e., walking and sports) over time. However, the inverse association (i.e., physical activity associates with changes in brain structure over time) was not observed in this study.
These findings might guide future policies to effectively develop physical activity interventions in elderly individuals with poorer brain health. Although the causal role of brain structure on physical activity levels could not be determined by this study, the bidirectional longitudinal design gives new insights that (unstructured) physical activity levels may be predicted by emerging neuroimaging features in

Figure 1. Association between total physical activity and tract- specific MD in children from Generation R.
middle-aged and old age, and underscores the importance of repeated measures in elucidating the etiology of brain and behavior.
My short-term purpose is to confirm or contrast these findings using data from the UK Biobank dataset. My long-term scientific-technical interest is to explore the mechanisms linking physical activity with brain health indicators to design more effective physical activity interventions which might improve people´s general health and quality of life.

REBECCA STEKETEE, PHD NEUROIMAGING IN DEMENTIA
Project Funding Theia Foundation: “Diagnosis of dementia with MRI in the greater Rotterdam area: faster and better”; ZonMW Memorabel: “Amyloid imaging in focus”; H2020-EU: “CoSTREAM – Common mechanisms and pathways in Stroke and Alzheimer’s disease.”
Research period January 2016 – April 2022
Email r.steketee@erasmusmc.nl
These projects are collaborations between the Departments of Radiology & Nuclear Medicine, Epidemiology and the Alzheimer Center Erasmus MC.
My work focuses on neuroimaging in dementia, both within the clinical setting of the Alzheimer Center Erasmus MC as well as the population neuroimaging research line of the Rotterdam Study, with the ultimate goal to build a bridge between these fields.
To realize this goal, we investigate how population-based imaging information can facilitate the imaging diagnosis of dementia. To that end, I am studying the implementation of software for automated quantification of structural brain changes (Quantib™ ND) in clinical practice. This software automatically provides quantitative information on brain volumes relative to age- and sex-specific reference data from the Rotterdam Study, enabling comparison of individual patients’ brain volumes to reference data from a healthy aging population. In a prospective study, we are investigating whether such normative quantification affects diagnostic confidence of neuroradiologists, compared to routine visual assessment, within Erasmus MC but also in a multicenter setting in the greater Rotterdam area.
We also explored the differential effects on dementia diagnostics in a clinical context when using Quantib™ ND or QUIBIM Precision® Platform Brain Atrophy Screening, which is another normative quantification software package available at the department. Using retrospective data from the Alzheimer Center Erasmus MC imaging database, we found that despite diagnostic accuracy, sensitivity and specificity not being significantly different between software packages, different packages can produce distinct effects at the level of clinical interpretation. Clinics should not assume that different packages can be used interchangeably, and we suggest to evaluate packages internally before adopting them in clinical practice.
Examples of brain amyloid plaque deposition in subjects from the AmyVasc study: 1: none; 2: moderate; 3: significant. BAPL 2 may define a ‘gray zone’ that characterizes pre-AD dementia levels of amyloid burden. Quantification of this gray zone may help determine who will eventually progress to dementia.
Finally, this year marked the completion of the AmyVasc project, which focuses on how systemic vascular pathology and vascular brain pathology relate to amyloid-ß pathology within the Rotterdam Study. Visual rating of the amyloid PET images is finalized and shows amyloid deposition in 20% of participants. A large subset of that 20% was challenging to rate visually: scans were not unequivocally positive or negative in this healthy cohort (figure). In 2022 we aim to establish this ‘gray zone’ of early amyloid deposition in asymptomatic subjects also quantitatively, when all scans have been processed and amyloid uptake values in a multitude of brain regions have been obtained.

VASCULAR COGNITIVE IMPAIRMENT AND POST-STROKE DEMENTIA
FRANK J WOLTERS, MD, PHD
Post-doc
Project Funding NWO Veni; Cure Alzheimer’s Fund; Erasmus Trust Fonds
Research period
Email f.j.wolters@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine, Epidemiology and Alzheimer Center.
The core of my research aims at improving secondary prevention of vascular cognitive impairment, with a particular focus on epidemiological methods and post-stroke dementia. I combine my experience in epidemiology and clinical neurology to link population and clinical neuroimaging in light of disease outcome. I will highlight three main research interests of mine in more detail below.
Covert brain infarcts –defined as infarcts on MRI that were not preceded by (recognised) clinical stroke symptoms– are seen in 20% of elderly individuals on routine brain MRI, and increase the risk of subsequent cardiovascular disease and cognitive decline. Despite the large potential for secondary prevention (Figure), patients often go untreated, as optimal management is undetermined due in part to uncertainty about which patients are at highest risk. I combine data from various population cohorts to find determinants of high risk, such as size, number and location, and to ultimately further diagnostic and therapeutic management in clinical patients, like those visiting the Alzheimer Centre outpatient clinics.
Dementia is an important complication of stroke, with one third of patients developing dementia in the first year after a major stroke. In the Rotterdam Study, I investigate how prognosis after TIA/stroke varies with event severity and premorbid determinants, including cerebral small-vessel disease, arteriosclerosis, and brain atrophy. By taking these findings to clinical studies, such as MRCLEAN and the Erasmus Stroke Cohort, I aim to identify predictors of poststroke dementia that facilitate patient information and personalised care.
Heart disease is an acknowledged contributor to stroke risk, and emerging evidence indicates heart failure and atrial fibrillation also predispose to dementia. Within the Heart-

Cardiovascular risk factor management is often overlooked in patients with covert brain infarcts on clinical brain MRI. From earlier evidence of the population-based Rotterdam Study, we know that worse control of risk factors is associated with poorer cognitive performance (AAIC 2021). These findings highlight the unfulfilled potential for secondary prevention in this patient population.
Brain Connection research group of the Dutch CardioVascular Alliance, I investigate determinants of vascular brain injury in relation to pathology along the heart-brain axis. By further joining forces with various population cohorts in the international CCC collaboration, I investigate how cardiac dysfunction relates to brain pathology on MRI from midlife to late-life.
Finally, I continuously aim to improve methods that facilitate causal inference from observational research, in neuroimaging and beyond. For example, I examine the added value of automated segmentations over clinical scan ratings, and have validated a reliable means for retrospective grading of stroke severity in medical records.

Advisors
JOYCE VAN ARENDONK, MSC AMYLOID PATHOLOGY AND VASCULAR DISEASE IN NEURODEGENERATION
PhD Student
Meike
Vernooij & Arfan Ikram
Project Funding ZonMW Memorabel: “ Amyloid pathology and vascular disease in focus: exploring interaction in two pathways towards neurodegeneration”
Research period May 2018 - April 2022
Email j.vanarendonk.1@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Epidemiology.
The exact etiology of Alzheimer’s disease (AD) remains unclear to this day, even though the first case arose already in 1902. During the last decade, several causes have been pinpointed to contribute to the development of AD, of which amyloid- β (A β ) and vascular pathology are the most prominent factors in the early stages.
Although the A β and vascular pathways have been studied independently in various settings, it still remains unclear how the two pathologies interact in the early, pre-dementia stages. To elucidate this interaction we initiated the AmyVasc study in 2018. Participants of the Rotterdam Study aged 60 years and older and who have had a previous MRI scan underwent amyloid PET imaging at the Erasmus MC. We finalized the data collection at the end of this year after scanning 640 participants, and also finished all the visual A β assessments. With a pipeline in place for the semi-quantitative analysis, we expect to start analyzing the entire AmyVasc study population soon.

Associations between vascular risk factors and cortical amyloid PET SUVr values (A) and amyloid PET positivity (B). The whisker plots illustrate standardized beta values with 95% CI (panel A), and odds ratios with 95% CI (panel B). All models are adjusted for age, sex, education, APOE4 allele count and the number of years between vascular risk assessment and PET. Abbreviations: APOE4, apolipoprotein E ε4 allele; CI, confidence interval; PET, positron emission tomography; SUVr, standard uptake value ratio
In the meanwhile, we already processed a subset of 506 PET/CT-scans at the beginning of this year, because we had to cease scanning participants again in December 2020 due to the COVID-19 pandemic. With this processed subset of amyloid PET/CT-scans, we studied the association between vascular risk factors, assessed at two previous time points, and A β pathology. Participants’ mean age at time of amyloid PET was 68 years (range: 60-90), 262 participants (51.8%) were women and 158 participants (31.2%) carried at least one APOE4 risk allele. The prevalence of an amyloid-positive PET scan in-
creased with age (12% in those 60-69 years vs. 45.5% in those 80-89 years old) and APOE4 allele count (8.3% in non-carriers vs. 34.8 to 58.8% in carriers of one or two risk allele(s)). Moreover, we observed that diabetes was associated with a higher prevalence and severity of A β pathology in both APOE4 carriers and non-carriers. Hypertension was associated with more severe A β pathology, but in APOE4 carriers only. We did not find an association between high BMI, hypercholesterolemia, physical inactivity or smoking and the prevalence and severity of A β pathology (Fig 1).

ZOOMING IN AT CEREBRAL MICROVASCULAR FUNCTION
HANNA BODDE, MSC
Research Employee
Advisors Meike Vernooij, Arfan Ikram & Geert Jan Biessels
Project Funding ZonMW VICI: “Microvascular function and brain networks: Keys to understanding the vascular burden in dementia”.
Research period April 2021 – December 2022
Email h.bodde@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Epidemiology, Erasmus MC and the Department of Neurology at the UMC Utrecht.
Cerebral small vessel diseases (SVDs) are a major cause of stroke and dementia. Yet, no SVD-specific treatments exist, which is largely due to poor understanding of the underlying pathophysiology. Previous research studying SVDs focused on brain parenchymal lesion-detection with MRI. However, SVD-lesions represent an end stage and are insufficiently specific to understand dynamic disease processes.
Using 7T MRI, researchers from UMCU have developed novel techniques to probe microvascular function at the level of individual small arteries and at brain tissue level. They have examined which aspects of microvascular function are affected in patients with symptomatic SVDs and how microvascular function related to other markers of SVD-related brain injury and cognition. Manifestations of SVDs are also very common in the general population and many people harbor presymptomatic stages of SVDs. For a complete understanding of the aetiology and impact of SVDs, observations in these presymptomatic stages are needed complementary to observations in patients with manifest disease.
In the current study, we are using 7T MRI to assess variation in microvascular function in 200 older individuals from the Rotterdam study with no history of clinical stroke or dementia. The microvascular function will be related to (previous) exposure to vascular risk factors, severity of SVD lesions, cognition, and blood markers relevant to microvascular (dys)function. These results will help to unravel the etiological mechanism underlying cerebral SVDs in humans.
Thus far, 41 participants were scanned. We plan to finish scanning the remaining 159 participants in 2022.

Example 7 Tesla scan

MATHIJS ROSBERGEN, MSC
PERSONALIZED PREDICTION OF ALZHEIMER’S DISEASE PhD Student
Advisors Meike Vernooij, Arfan Ikram & Frank Wolters
Project Funding ZonMW/TKI Health Holland: ABOARD consortium
Research period August 2021 – February 2025
Email m.rosbergen@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine, Epidemiology and Alzheimer Center Erasmus MC
When an individual is diagnosed with Alzheimer’s disease, severe brain damage has already occurred. Treatment in an early stage of Alzheimer’s disease is crucial to prevent brain damage and dementia. To identify the individuals with high risk of Alzheimer’s disease, personalized prediction models are necessary. The aim of my research is to update and improve existing prediction models to identify individuals with high risk of Alzheimer’s disease.
This research takes place within the ABOARD consortium. Project ABOARD is a national collaboration between university medical centers, healthcare organizations, commercial parties and patient organizations. Currently, treatment of Alzheimer’s disease focusses on the stage in which dementia already has occurred, resulting in a suboptimal quality of life. The goal of this collaboration is to stop Alzheimer’s disease before dementia arises by creating a personalized approach regarding diagnosis, prevention and prediction.
This research will be conducted with data primarily from the longitudinal population based Rotterdam Study. The aim of my research within the ABOARD project focusses on a personalized prediction of Alzheimer’s disease. My projects will focus on exploring new predictive markers, primarily based on MR imaging. Machine learning will potentially be used when it is necessary to unravel hidden predictors in imaging data. Also, promising new imaging biomarkers will be evaluated in their potential to improve existing prediction models. These improved models will eventually be externally validated in other cohorts. In this way, we hope to identify individuals with high risk of Alzheimer’s disease and pave the way for treatment and prevention of Alzheimer’s disease before dementia arises.


SOCIAL FACTORS IN COGNITIVE DECLINE AND DEMENTIA
ISABELLE VAN DER VELPEN, MD
PhD Student
Advisors Arfan Ikram, Meike Vernooij, René Melis & Marieke Perry
Project Funding ZonMW Memorabel: “Social factors in cognitive decline and dementia: towards an early intervention approach.”
Research period July 2018 – April 2022
Email i.vandervelpen@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine, Epidemiology, Erasmus MC and the Department of Geriatrics at the Radboud UMC in Nijmegen.
Social health has been linked to the development of dementia, but thus far the underlying pathophysiology has remained unclear. Social health is the relational domain of health and encompasses the interactional competencies of the individual and their immediate social environment. As such, social health can range from having a satisfying social life to loneliness, and includes concepts that relate to social network structure and the experienced quality of social relationships. We previously found that social health was associated with global brain volumes and white matter microstructural integrity in healthy older adults. Conversely, differences in brain health might explain differences in the individual experience of social health. The COVID-19 pandemic illustrated that experienced social health differs widely across individuals, and highlighted the importance of social interactions for our overall health.
To gain insight into which factors affect our social health in the face of a crisis, we studied determinants of social health trajectories during the COVID-19 pandemic in the Rotterdam Study. Using previously-collected Rotterdam Study data and repeated questionnaires from 5,017 older adults from April 22nd to July 31st 2020, we showed that loneliness prevalence initially doubled at COVID-19 baseline compared to previous years and that loneliness and social isolation scores improved during three months of follow-up. Depressive symptoms, female sex, pre-pandemic loneliness, living alone, and not owning a pet were independently associated with lower social connectedness and higher social isolation and loneliness at COVID-19 baseline, but recovery of social health over time was similar for all these determinants. In a subset of 1,720 participants with a brain MRI prior to the pandemic, larger intracranial volume was associated with an increase in social connectedness over time,

Change in social connectedness from April 22nd 2020 to July 31st 2020. Solid lines represent the marginal (group) change in social health over time, dashed lines represent 95% confidence intervals. Dates during which physical distancing restrictions in the Netherlands were lifted are denoted with a (May 11th 2020), b (June 1st 2020) and c (July 1st 2020). Separate trajectories are shown for mean (in orange), larger (+1SD in green), and smaller (-1SD in purple) intracranial volume prior to the pandemic.
while smaller intracranial volume was associated with a decrease in social connectedness (see figure). Although this association was subtle, it suggests that brain reserve may play a role in how individuals cope with a social challenge.
Social health and brain health are highly interconnected. In the search for strategies to prevent dementia, improving social health may be a valuable tool to promote brain health in older age.

MODELING ‘HEALTHY’ BRAIN AGING
ELINE J VINKE, MSC
Advisors Arfan Ikram & Meike Vernooij
Project Funding Horizon 2020, EuroPOND: “Data-driven models for Progression Of Neurological Disease
Research period August 2016 – December 2021
Email e.vinke@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Epidemiology.
The EuroPOND (European Progression of Neurological Disease initiative) initiative develops computational models and uses state-of-the-art data-science techniques to understand a range of neurological disorders. The aim of my research project within EuroPOND is the characterization of normal brain aging, using data from the longitudinal population based Rotterdam Study. My projects are focused on modeling and disentangling the broad spectrum of ‘healthy’ brain aging using longitudinal brain MRI data.

Understanding the aging process is of great importance, since it can help us understand pathology and clinical disease as well as the functioning of biological processes of the human body at a higher age. Looking at the aging brain specifically, there is a large variation in functioning at a high age, and the structural changes that have occurred in the brain. Moreover we know that there are similarities between brain aging and neurodegenerative disease. With this in mind it may not be self-evident that when we talk about brain aging, it is often referred to as ‘normal brain aging’. What do we mean with normal? What is normal and is there one normal? My research is focused on trying to answer these questions.
Correcting for age in longitudinal studies: When you study the relationship between a determinant and an outcome, in which age is an important confounder, it is important to integrate the interaction between age at baseline with follow-up time (scenario C), to correct for this non-linear relationship. In the third image of each scenario the individual estimated trajectories with follow-up time are shown. If the research question is whether for example hearing loss (non-linearly related to age as well) is related to a faster cognitive decline, slope differences in the individual cognition trajectories could be attributed to hearing loss, whereas it would probably be better explained by age at baseline. PhD Student

Advisors
COGNITIVE AND BRAIN RESERVE IN THE MIDDLE-AGED AND ELDERY
JENDÉ ZIJLMANS, MD, MSC
PhD Student
Annemarie
Luik, Meike Vernooij & Arfan Ikram
Project Funding European Union’s Horizon 2020 research and innovation program (M.A. Ikram, 678543, ORACLE)
Research period Jan 2018 – June 2022
Email j.zijlmans@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Epidemiology.
Neuropathological damage may lead to clinical expression of brain diseases in certain individuals, while not in others. These differences in susceptibility to neuropathology may be explained by cognitive and brain reserve, which refer to individual differences in the functionality and structure of the brain. Within the population-based Generation-R and Rotterdam Study we aimed to investigate the associations of cognitive and brain reserve with several health outcomes, and which factors are associated with cognitive reserve. In our studies, we defined cognitive reserve as a latent variable that captures common variance across five cognitive tests, while adjusting for age, sex, education, total brain volume, intracranial volume and white matter hyperintensity volume. We defined brain reserve as the proportion of healthy appearing brain volume over the total intracranial volume.
This year, we finalized the data collection and cleaning for the Origins of Alzheimer’s Disease Across the Life course (ORACLE) Study. We collected data on 2,083 parents of the children of Generation-R study between 2017-2021. The ORACLE study included a dedicated research visit for the parents during which extensive measures on brain health were collected, including neuroimaging, cognitive testing and questionnaires.
Further, we showed in 1,490 participants of the Rotterdam study (mean age 74±5.5 years, follow-up: 5.3±1.4 years) that that a higher cognitive and brain reserve are associated with a lower mortality risk. We also showed that frailty modifies the relationship between cognitive reserve and mortality, such that a higher cognitive reserve is associated with lower mortality particularly in frail participants ( Figure 1 ). Second, in a cross-sectional study of 4,309 participants from the Rotterdam Study (mean

Figure 1. Survival curves in groups stratified based on frailty and median cognitive reserve (CR). The Lancet Healthy Longevity (2021)
age 63.9±10.7) we showed that current smoking, diabetes mellitus, and depressive symptoms are associated with a lower cognitive reserve, whereas more alcohol use was associated with a higher cognitive reserve, but with clear differences between men and women.
In the coming year, we will finalize two other projects within the Rotterdam study that investigate (1) the association of cognitive and brain reserve with late-life depression, and (2) the association between sleep and cognitive reserve. Additionally, we will also finalize a project within the ORACLE study that investigates the long-term cognitive and brain-MRI outcomes after a head injury with loss of consciousness in the past.
JOINT APPOINTMENT IN CLINICAL GENETICS
Dr. Adams is a clinician performing multidisciplinary research. In parallel to medical school he completed two master programs in cell biology and epidemiology. His experimental research was to determine interaction networks of transcription factors in neural stem cells. His epidemiological research was on population genetics and imaging of neurodegeneration. During his doctoral research he developed novel statistical methods and neuroimaging protocols, processed images, and studied genetic associations.

He leads the Precision Epidemiology group, which focuses on improving our fundamental understanding and clinical care of neurodegenerative disorders. The core basis of the group is detailed characterization of large populations. The group currently consists of 5 postdocs, 2 PhD students, and support staff, part of which have affiliations with Radiology. So far he has over 100 publications (h-index = 35), many in leading journals. He has initiated and led multiple consortia, including the UNITED consortium. h.adams @erasmusmc.nl
PRECISION EPIDEMIOLOGY
HIEAB ADAMS, MD, PHD associate
professor

Context
There is tremendous interindividual variation. From conception onwards, no two persons are the same and more differences accumulate during life. This variation has a basis in genetics, the environment, and stochastic events. Importantly, it is the reason why some people develop diseases during their life and other don’t.
The Precision Epidemiology group aims to quantify the variation that exists between individuals that may explain the differences in health outcomes. ‘Precision’ refers to the in-depth characterization of individuals. Within the group, we employ imaging and genetics as the two driving forces of technology to measure variation. On the other axis is ‘Epidemiology’, i.e. on which population to apply this technology. The focus of the group is on complex diseases, with specific interest in studying neurodegenerative disorders and oncology at a global scale. Also, an important aspect of using such innovative and big datasets is the development of novel methodology, including statistics, software, and experimental assays.
Top Publications 2021
Pawlak et al. Neural correlates of orbital telorism. Cortex. (2021)
Knol et al. Genetic architecture of orbital telorism. Human Molecular Genetics. (2021)
Vilor et al. Genetic Influences on Hippocampal Subfields: An Emerging Area of Neuroscience Research. Neurology Genetics. (2021)
Research Projects: Objectives & Achievements
Genetics of brain structure
Within this research line we aim to determine the genetics underlying brain structure. This mostly concerns genome-wide association studies of normal variation in brain structure. Initially this focused on gross MRI phenotypes, such as head size and volumes of specific brain structures, but more recently we have tackled high-dimensional MRI phenotypes as well. These describe the brain in greater detail and provide more insights into the genetic architecture of brain structure. There are several major ongoing GWAS efforts, with group members at prominent positions (first and last/corresponding authors). Important collaborators are the CHARGE and ENIGMA consortia.
We have discovered hundreds of novel genetic loci that determine brain structure. Some of the genetic variants seem to have general effects on the brain, e.g. total brain volume, whereas other have a specific influence on subregions of the brain, e.g. the shape of the amygdala. Interestingly, these variants are sometimes near or inside genes that also cause clinical syndromes with (severely) abnormal brain structure.
Imaging of neurodegeneration
The brain is the primary target of neurodegenerative disorders. Understanding the course of brain changes from health until disease requires non-invasive imaging of these changes. Particularly, subtle brain changes that occur in the earliest phases are important to capture. In this research line we perform MRI studies of neurodegeneration using high-dimensional imaging phenotypes. The core work now revolves around the recently initiated UNITED consortium, which is led by our group and already the largest neuroimaging consortium worldwide, with over 100.000 participants. Ongoing work includes the enrollment of new studies and processing of imaging (and genetic) data.
Strikingly, the majority of the MRI studies performed in the field of neurodegeneration are of persons from European descent. There is an underrepresentation of other ethnic groups, which makes generalization of research findings difficult to all people. Our consortium therefore specifically tries to recruit studies with persons from these unrepresented regions, i.e. Africa, Asia, and South America.
Expectations & Directions
While epidemiological findings can help us understand the mechanisms of diseases, the benefits to patients often remains elusive. The intersection of epidemiology with the clinic holds great potential and is a key interest of the group. This concerns exploring clinical applications of research findings from complex traits, with a major emphasis on imaging and genome-wide association studies. While the clinical yield seems to be limited in the field of neurodegeneration, we are now also starting to explore possibilities within cancer which is more promising. This is done in collaboration with clinicians from the Erasmus MC, other academic medical center in the Netherlands, and internationally.
Two imaging-focused gaps that we will address in our future work are described below.
Neurogenetics: from normal variation to clinical mutation.
Within our GWAS studies of brain structure, we identified common variants influencing normal variation in brain structure at genes causing Mendelian forms of brain abnormalities. For example, we identified common variants (30% carriership) related to head size at PTEN, a gene which causes a severe macrocephaly syndrome when mutated.
For this, we will determine common genetic variation underlying normal variation in novel brain phenotypes such as gyrification, callosal thickness, and skull shape. Next, we will use these findings to overlap with genetic sequencing data from known and unknown clinical cases of related brain phenotypes. This could lead to the identification of causal genes for GWAS loci, and potentially solving clinical cases where variants of unknown significance have been identified.
Neuroimaging: a worldwide study of neurodegeneration.
Neuroimaging studies have been plagued by a lack of replication of results, which is partly due to the use of small sample sizes. We recently set up the world’s largest neuroimaging consortium, which currently contains 100.000 participants, but is growing rapidly. The aim is have half a million participants included by 2025, with a specific focus on underrepresented regions, i.e. Africa, Asia, and South America.
We will perform brain-wide analyses of Alzheimer’s disease, Parkinson’s disease, and frontotemporal dementia using cutting-edge technology within a large and ethnically diverse population. The resulting brain maps are expected to be a valuable global resource for future research and clinical references.
Funding
Hieab Adams & Raymond Poot & Bas van Steensel: NWO Open Competition 2021-2025: “Identifying causal genetic variants for a better understanding and diagnosis of neurodevelopmental disorders”
Tavia Evans: Alzheimer Nederland 2021-2023: “Investigating Geographical Variation in Brain Aging and Neurodegeneration”
Tavia Evans: NWO Women in Science 2021-2023: “Neurodegenerative disease variation across ethnicity- A detailed neuroimaging investigation”
Hieab Adams & Rick van der Vliet & Sirwan Darweesh & Bas Bloem: Parkinson Foundation & Parkinson Vereniging: “ABCD-Parkinson: A Biomarker based on Circulating cell-free DNA for Parkinson’s disease”
Hieab Adams: Erasmus MC Fellowship 2020-2024: “The Uncovering Neurodegenerative Insight Through Ethnic Diversity Consortium“
Mikolaj Pawlak, MD PhD
Hieab Adams: NWO Veni 2018-2022: “SO-BIG: Spatial Overlap Between Imaging and Genetics “
Elizabeth Loehrer, Hieab Adams: Leading Fellows 20192021: “POPSICLE: Population-based Stem Cell Induction for Complex Diseases and Large-scale Experimentation”
Hieab Adams: NWO Rekentijd 2019-2021: “The Uncovering Neurodegenerative Insight Through Ethnic Diversity Consortium”
Highlights
Tavia Evans acquired another personal grant, further supporting her career in neuroimaging. She is well on her way for becoming a leading researcher for analyzing brain differences in diverse populations.
The UNITED consortium organized several webinars, including one in Spanish. This was to be more accessible for potential collaborators in South America, in preparation for a 2022 visit of the region. The webinar identified a lot of clinicians and researchers that we are eager to meet in person.
Additional Personnel
Mikolaj Pawlak – Visiting Scientist
Dr. Pawlak is a visiting neurologist from Poznan, Poland. His work focuses on imaging analyses of neurological disorders, including dementia, multiple sclerosis, and Parkinson’s disease. His expertise is in advanced analyses of MRI images and development of novel acquisition protocols.

Annemieke van Beek, MSc
Drs. Van Beek is Chief of Communications for the UNITED consortium (0.6 FTE). She works on the outreach to underrepresented regions, including Africa, Asia, and South America. She also holds a joint appointment at the Alzheimer’s Center of the Erasmus MC, department of Neurology (0.4 FTE).


UNCOVERING NEURDEGENERATIVE INSIGHTS THROUGH ETHNIC DIVERSITY
TAVIA EVANS, MSC, PHD
Project Funding Alzheimer Nederland Pilot Grant, Women In Science Grant (NWO)
Research period October 2018 – December 2021
Email t.evans@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Clinical Genetics.
Neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease and frontotemporal lobal dementia are leading causes of morbidity and mortality worldwide. Most individuals with these disorders suffer from “complex” forms of these diseases. For example, a combination of many components, potentially millions of genetic and non-genetic factors, results in a person’s brain becoming affected by such a disease. Current research within this area is shockingly lacking in diversity. The majority of research, and findings that are thus applied within clinical practice, are reported on mainly middle-class Caucasian individuals. This creates huge restrictions on not only the generalizability of the research, but also our understanding of neurodegenerative processes across ethnically diverse populations. Taking cue from large genetic consortiums we have begun a large neuroimaging consortium, the UNITED consortium, with an emphasis of including samples from currently underrepresented countries. Given the complex nature of neurodegenerative diseases, researching these requires advanced techniques that can disentangle the multitude of factors underlying neurodegeneration. The current aim is to use cutting-edge MRI methods a that measure subtle changes in the brain’s structure and function at the highest resolution, such as voxel wise- and vertex-based information. Through this we hope to unravel the complexity of neurodegenerative diseases and pave the way for better application within treatment and prevention.
In addition to this we aim to utilize existing clinical data within currently under represented regions and investigate the possibilities and practicality of low-field MRI in research and clinical environments within these regions. Post-doc
Pilot analysis we undertook has shown subtle differences across ethnicities in these relationships. Additional analysis has also shown that heterogeneity within elements of MRI acquisition has some effect on the associations between MRI derived markers and neurodegenerative diseases. Investigations into detailed MRI markers and neurodegenerative diseases such as mild cognitive impairment and Alzheimer’s disease is currently being investigated within the whole UNITED consortium including over 80 collaborative sites.
Figure 1. Pilot Study: Differences in relationship between the shape of subcortical brain structures and Alzheimer’s disease across ethnicities. Top: subcortical shape t values. Bottom: differences in subcortical shape across ethnicities in comparison to European.


Project Funding
NEUROGENETICS: FROM NORMAL VARIATION TO CLINICAL MUTATION
SANDER LAMBALLAIS, PHD
Post-doc
Research period January 2020 – December 2022
Email s.lamballaistessensohn@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Clinical Genetics.
Without a doubt, the brain is the most complex organ in the human body. Brain-related diseases and conditions arise from variation at the micro, meso and macro scale of both the structural and functional connections. Such brain-related differences arise in part due to genetic variation. We aim to develop and apply methods that link genetics and its sequelae to the complexity of the brain.

In our group, we developed a method to assess pleiotropy based on the results of genome-wide association studies (GWAS). Pleiotropy refers to genes or genetic loci that influence multiple traits. Existing methods have varying limitations, ranging from inflated false positives rates to only working on smaller sections of the genome. Our method provides genome-wide results with a low false positive rate in a matter of seconds. The method can be used to identify pleiotropic genetic variants, which in turn can be used to identify shared genetic and functional mechanisms between various traits. We aim to apply the method to understand how the genetics of endophenotypes and brain development are linked.
In terms of imaging, we are focused on high dimensional genetic analyses of brain structure. We have been able to map the heritability structure of various cerebral cortex characteristics at over 300,000 points across the cortex. We are further exploring the genetic mechanisms underlying these characteristics, and we aim to see whether and to what extent these are shared. Finally, we will link these results to the genetic architectures of neurodegenerative diseases, to better understand how disease manifests in the brain.
Figure 1. Example of a GWAS Manhattan plot for the pleiotropic structure across eight psychiatric disorders: ADHD, anorexia nervosa, ASD, BD, MDD, OCD, schizophrenia, and Tourette’s syndrome. Each peak represents a signal across any combination of these eight disorders. The green line represents the adjusted genome-wide significance threshold.

UNCOVERING THE GENETIC AND MOLECULAR CHARACTERIZATION OF CEREBRAL ALZHEIMER’S DISEASE ENDOPHENOTYPES
NATALIA VILOR-TEJEDOR, MSC, PHD
Project Funding Postdoctoral grant, Juan de la Cierva Programme (IJC2020-043216-I), Ministry of Science and Innovation– Spanish State Research Agency / AlzheimerNederland Project (#WE.15-2019-09)
Research period January 2021 – December 2022
Email n.vilortejedor@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Clinical Genetics.
As consequence of the progressive ageing of the population and the increase in life expectancy, the prevalence of Alzheimer’s disease (AD), a progressive, irreversible, and incapacitating neurodegenerative disease, is also in the rise, causing great social-economic burden. Over the past four decades, numerous clinical trials have failed to identify any drugs that could cure dementia or AD, or even substantially modify the disease process. Therefore, prevention in cognitively unimpaired individuals at risk of developing the disease or in its early stages remains to be the key to the success of intervention studies. AD is a complex neurodegenerative disorder in which several epidemiological and molecular factors are at play. Although there is clear evidence on epidemiological AD risk factors, molecular and biological mechanisms and their use for improving disease prediction still require further exploration. For instance, the exact biological mechanisms by which AD risk factors may affect the brain remain largely unknown. In this project, we aim at disentangling the genetic and molecular characterization of cerebral AD endophenotypes (e.g. brain volumes) in stages before symptoms develop (i.e. prevention), and in disease progression (i.e. molecular characterization/understanding of disease progression) paving the way for preventive practices and personalized medicine. In addition, the multivariate nature of the proposed framework will allow, not only to jointly assess the genetic information but also to model the association of the genetic profiles in different cerebral AD endophenotypes at the same time, in both, crosssectional and longitudinal models, and thus, to be more sensitive to assess associations and to define specific molecular mechanisms associated with AD.

Figure 1. Genetic Studies of Hippocampal subfields. Adapted from: Vilor-Tejedor et al. 2021. Neurology Genetics.
JOINT APPOINTMENT IN EPIDEMIOLOGY
Daniel Bos was trained as a medical doctor and epidemiologist at Erasmus MC. He obtained his PhD in the field of vascular imaging and vascular epidemiology in 2013. The main focus of his work is on the elucidation of the etiology and pathophysiology of arteriosclerosis, and the clarification of the contribution of arteriosclerosis to clinical neurovascular and neurodegenerative diseases. For his research he leverages the strengths of

population-based studies and combines this with clinical patient-studies in order to be able to make a direct clinical impact with his work. Over the years, he has been awarded the Best Scientific Paper Award by the Radiological Society of the Netherlands and the Lourens Penning Prize by the Radiological Society of the Netherlands. He holds a position as a visiting scientist and lecturer at the Harvard School of Public Health and a position as guest-professor at KU Leuven. Daniel has authored over 100 peer-reviewed publications. d.bos@erasmusmc.nl
IMAGING OF ARTERIOSCLEROSIS: FROM POPULATION TO CLINICAL PRACTICE
DANIEL BOS, MD, PHD
associate professor

Context
Arteriosclerosis is a highly frequent vascular disease causing stiffening of arteries throughout the whole arterial system. Major clinical consequences of arteriosclerosis include myocardial infarction and stroke, which are top causes of morbidity and mortality in middle-aged and elderly persons worldwide. Due to the aging of the population, the global burden of arteriosclerosis, and thereby of these clinical conditions, will continue to rise in the coming decades.
The research in “Imaging of Arteriosclerosis: from Population to Clinical Practice” is focused on the elucidation of the etiology and pathophysiology of arteriosclerosis, and on the clarification of the contribution of arteriosclerosis to clinical neurovascular and neurodegenerative diseases, with a strong emphasis on the use of state-of-the-art medical imaging. Within this unique research-field on the interface of arteriosclerosis, neurovascular, and neurodegenerative diseases, the research group specifically aims at obtaining relevant imaging-based biomarkers for improved understanding of arteriosclerosis and improved prediction of abovementioned diseases, and at identifying potential targets for intervention strategies.
Top Publications 2021
Bos D, Arshi B, van den Bouwhuijsen Q, Ikram M, Selwaness M, Vernooij M, Kavousi M, van der Lugt A. Atherosclerotic Carotid Plaque Composition and incident Stroke and Coronary Events. JACC. 2021; 77(11):14261435.
Vinke E, Yilmaz P, van der Toorn J, Fakhry R, Frenzen K, Dubost F, Licher S, de Bruijne M, Kavousi M, Ikram M, Vernooij M, Bos D. Intracranial arteriosclerosis is related to cerebral small vessel disease: a prospective cohort study. Neurobiol Aging. 2021;105:16-24.
Luijten S, van der Donk S, Compagne K, Yo L, Sprengers M, Majoie C, Roos Y, van Zwam W, van Oostenbrugge R, Dippel D , van der Lugt A, Roozenbeek B , Bos D. Intracranial carotid artery calcification subtype and collaterals in patients undergoing endovascular thrombectomy. Atherosclerosis. 2021 Oct 13;337:1-6
Research Projects: Objectives & Achievements
Intracranial Arteriosclerosis
This line of research is specifically focused on the causes and consequences of intracranial arteriosclerosis. In this field, important pioneering-work on the identification and quantification of calcification in the intracranial carotid arteries and the vertebrobasilar system has been performed by our research group. By applying non-enhanced computed tomography to participants of the population-based Rotterdam Study, we demonstrated that the presence and amount of intracranial carotid artery calcification is the strongest risk factor for developing a first-ever stroke, and that the amount of calcification at this location even contributes to the development of dementia, including Alzheimer’s Disease. This work still has a considerable global impact and has also led to an extension of the research horizon towards clinical studies on the influence of intracranial arteriosclerosis in stroke patients. In these patients, the amount of intracranial arteriosclerosis at time of admission to the hospital directly influences the prognosis after stroke treatment.
Recent work in the field of pathology and imaging uncovered the existence of two morphological patterns of intracranial arteriosclerotic calcification. Besides intimal calcification as part of classical atherosclerosis, circular calcification of the internal elastic membrane was also commonly observed. These two patterns may contribute differentially to stroke and may influence stroke treatment, given the divergent effects of calcification on the arterial wall structure and the accompanying structural stresses within the artery. This idea is further corroborated by the distinctive effects on functional outcome after endovascular treatment in acute ischemic stroke patients that we found for the two patterns and by our findings that the status of the collateral cerebral circulation differs according to the dominant calcification patters. These findings provide a solid basis for further investigations into these phenotypes, representing an important research focus of this group.
Advanced Atherosclerotic Plaque Imaging
This line of research is devoted to the most common subtype of arteriosclerosis, namely atherosclerosis. Atherosclerosis is characterized by thickening of the intimal layer of the arterial vessel wall due to lipid-accumulation, neo-vascularization, and calcification. Especially when located in the carotid artery bifurcation, atherosclerosis is known to substantially increase the risk of ischemic stroke. The work in this research line is targeted at the optimal identification

Figure 1: Example of presence of atherosclerotic plaque (red arrowhead) in the left internal carotid artery (red square). Using the 3D-T1w-GRE sequence, the presence of intraplaque hemorrhage is readily visualized (hyperintense area).
and evaluation of atherosclerosis in the carotid artery bifurcation. Using advanced, state-of-the-art imaging modalities (CT, MRI, etc.), the aim of the research group is to further understand the development of this disease and the value of assessing plaque composition and plaque morphology for the prediction of neurovascular and neurodegenerative diseases. Recent accomplishments of the research group in this field have shown that the presence of intraplaque hemorrhage (Figure) in carotid artery atherosclerosis is the single most important risk factor for a first-ever stroke and stroke recurrence. Moreover, our work showed that the composition of these plaques substantially differ between men and women, which is one of the topic we aim to further elucidate in coming years.
Optimization of Cardiovascular Image Analysis
This research line revolves around the early detection of vascular disease, by maximally leveraging existing medical imaging examinations. By using novel methods for imageanalysis (e.g. machine-learning algorithms), we aim to obtain more sensitive and more accurate imaging markers of vascular disease. My group performed important pioneering-work on the quantification of epicardial fat as emerging markers of vascular risk. Over the last year, this novel marker of vascular risk has gained rapid attention, which has led to a strong collaboration on its role in hearttransplantation patients with the Dept. of Cardiology. This collaboration was further extended with additional measurements of imaging-based markers of vascular risk (liver density) and overall-health status (bone density), which can all be readily assessed using conventional CT.
Expectations & Directions
The field of research into arteriosclerosis is rapidly evolving and substantial developments at many levels will occur in which the research of this group will play an important role. First, through the contributions of research of this group, intracranial arteriosclerosis is increasingly being recognized as an important risk factor for stroke, but may also play a key role in the development of dementia. In order to advance these insights, detailed visualization of arteriosclerotic disease in the cerebral vessels (not only the large afferent arteries such as the carotids and the vertebral arteries, but particularly in the smaller arteries) using combinations of different advanced imaging modalities is the way forward. An important focus should be on the difference between ‘pure’ atherosclerotic changes in the arteries (intimal thickening, plaque formation) and hardening/stiffening of the arteries through calcifications in the medial layer of the artery. Subsequently, using population-based data, etiological differences between these types of arteriosclerosis can be determined. Similarly, these features of arteriosclerosis may be added to existing risk-prediction models for stroke and even dementia, further contributing to their early identification and to the development of therapeutic and preventative strategies.
Second, rapid developments in the field of imaging (photon-counting CT) and image-post-processing (using machine learning algorithms) now allow for the possibility to investigate arteriosclerotic disease in much more detail. In addition to ‘conventional’ measurements such as the Agatston score, or volumetric assessments of calcification or plaque-size, it is becoming possible to also assess shape characteristics of the disease, make vesselstress calculations, link specific configurations of arteriosclerotic disease (including combinations of shape, volume and other properties) with outcomes. Third, an important achievement of this group was the completion of a large-scale follow-up imaging study (10-15 years of follow-up) targeted at the visualization of change in the amount of vascular calcification across multiple arteries. These data are unique in the world and will provide valuable insight into the natural course of arteriosclerosis.
Funding
Daniel Bos (PI) – Alzheimer’s Association Research Grant: “Trajectories of Vascular Disease in Aging to Predict Dementia.”
Daniel Bos (collaborator) and consortium partners –World Cancer Research Fund: “Coffee, coffee metabolites, hepatic fat accumulation and colorectal cancer outcomes.”
Daniel Bos (PI), Meike Vernooij, Frank Wolters, Julia Neitzel, Geert-Jan Biessels – Cure Alzheimer Fund: “Intracranial Arteriosclerosis and Alzheimer’s pathology.”
Invited Lectures
Bos D. Computed Tomography and DECT: what is the reproducibility and the level of comparison with histology?. Keynote lecture at The fil rouge of Atherosclerosis Annual Meeting, November 2021, Online.
Highlights
Tim van den Beukel was awarded the best MSc-thesis award in Medicine from University Utrecht , for his thesis on the intracranial arteriosclerosis in stroke (supervisor Daniel Bos ).
Daniel Bos was selected for the leadership programme of the Dutch CardioVascular Alliance (DCVA).
Dianne van Dam-Nolen was award the best abstract prize at the European Congress of Radiology , for her abstract entitled: “A Prospective Multicenter Study To Improve Diagnosis Of High-Risk Carotid Plaques.”
Daniel Bos was appointed as Guest-Professor at the department of Cardiovascular Sciences at KU Leuven .

IMAGING OF THE PLAQUE AT RISK
DIANNE VAN DAM-NOLEN, MSC, MD
PhD Student
Advisors Aad van der Lugt, Peter Koudstaal & Daniel Bos
Project Funding The PARISK study is supported by the Center for Translational Molecular Medicine (CTMM) and the Dutch Heart Foundation
Research period June 2017 – June 2022
Email h.nolen@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Neurology.
The PARISK study is a multicenter prospective cohort study aiming to improve identification of patients having mild-to-moderate carotid artery stenosis with a high recurrent stroke risk. Patients with recent neurological symptoms due to ischemia in the territory of the carotid artery and <70% stenosis in the ipsilateral carotid artery were included in this study. We performed carotid and brain imaging (using e.g. ultrasound, CTA and MRI) at baseline and after 2 years follow-up.

Figure 1: Kaplan-Meier curve showing the survival probability for ipsilateral recurrent ischemic stroke or TIA in patients with and without carotid IPH.
Figure 2: T1W FSPGR MR image (A) showing a highintense signal indicative of IPH. CT image (B) showing contrast material reaching into a plaque indicative of plaque ulceration. CT image (C) showing a relatively large bulk of calcifications. T1W pre-contrast TSE MR image (D) showing a delineated carotid plaque to measure total plaque volume.

Last years, we have shown that plaque composition is predictive for recurrent stroke risk (Figure 1). It is important to assess the composition of the carotid plaque besides the traditionally used degree of stenosis, since atherosclerosis can both grow into to lumen as into the vessel wall (inward versus outward remodeling). Figure 2 shows examples of carotid plaque characteristics, including plaque composition.
Moreover, we investigated sex differences in carotid atherosclerosis and found that men have more severe carotid plaques than women. This finding could explain sex differences in stroke epidemiology and suggests that sex-specific management of stroke patients would be helpful to determine which patients would benefit from carotid revascularization.
Currently, we are working on projects focusing on changes in plaque composition and size during follow-up. Do plaques only progress over time or could they also regress? This could give valuable insights in the natural course of atherosclerosis.

IMAGING BIOMARKERS OF PROGNOSIS AND INFARCT EVOLUTION IN ACUTE ISCHEMIC
SVEN PR LUIJTEN, MD
Advisors Aad van der Lugt, Diederik Dippel, Daniel Bos & Bob Roozenbeek
STROKE PhD Student
Project Funding Dutch Heart Foundation, Dutch Brain Foundation, Stryker, Medtronic and Cerenovus. Collaboration for New treatments of Acute Stroke (CONTRAST): WP7 Imaging Biobank & Young Talent Program.
Research period January 2019 – January 2023
Email s.luijten@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Neurology.
Endovascular treatment (EVT) has been proven beneficial across diverse subgroups of patients with acute ischemic stroke. Despite the broad applicability of EVT and recanalization rates of up to 80%, a substantial number of treated patients does not regain functional independence. More in-depth knowledge of additional patient characteristics influencing functional outcome is required to more accurately predict individual patient benefit and aid clinical decision-making regarding eligibility for EVT.
My research will focus on assessing the influence of baseline imaging characteristics on prognosis and expected treatment benefit of EVT. Clinical and imaging data from the Multicenter Randomized Clinical trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands (MR CLEAN) and Highly Effective Reperfusion evaluated in Multiple Endovascular Stroke Trials (HERMES) databases will be used. In contrast to previous studies focusing on stroke related imaging signs such as the Alberta Stroke Program for Early CT Score (ASPECTS) or collateral status, this project will mainly focus on pre-existent imaging features including intracranial carotid artery calcification (ICAC), white matter lesions (WML) and brain atrophy.
Functional outcome and treatment benefit are, however, also influenced by (procedural) factors occurring after the decision has been made to perform EVT. Therefore, my research will also focus on determining the prognostic value of early perfusion changes and infarct evolution directly after successful recanalization. Temporal changes in brain tissue perfusion and infarct evolution directly after successful recanalization will be tracked by means of repeated MRI acquisition including ASL, DSC, DWI and FLAIR imaging.

Figure 1: Example images of a patient presenting with a right M1 middle cerebral artery (MCA) occlusion who was successfully treated with thrombectomy (TICI 2b). (A) Top row includes baseline CT images showing large perfusion deficit in the right MCA territory/ (B) Follow-up diffusion-weighted imaging (DWI) shows infarction in right MCA territory with hyperperfusion mainly in the ischemic core (red)on arterial spin labeling (ASL) MRI.
The overall aims of my project will be:
1) To identify imaging biomarkers useful for evaluating prognosis and effect of EVT in acute ischemic stroke
2) Gain insight into early changes in perfusion and infarct evolution directly after successful recanalization
3) To contribute to the selection of patients eligible for additional (pharmacological) treatments

ARTERIOSCLEROSIS: A POPULATIONBASED APPROACH TO AETIOLOGY AND DI SEASE RISK
JANINE VAN DER TOORN, MSC
PhD Student
Advisors Arfan Ikram, Meike Vernooij, Daniel Bos & Maryam Kavousi
Research period April 2018 – October 2021
Email j.vandertoorn@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Epidemiology.
Arteriosclerosis is a highly prevalent vascular disease considered the most important cause of cardiovascular events worldwide. Many facets regarding the multifactorial origin of arteriosclerosis and its clinical consequences remain incompletely understood. The overall aim of my research was to provide insights into the causes and consequences of arteriosclerosis from a population-based perspective. Particular emphasis has been put on knowledge gaps related to differences in the burden of arteriosclerosis across arteries and between women and men. The investigations in my thesis are based on extensive (imaging) data from the population-based Rotterdam Study.
The first part of my thesis was devoted to questions on the aetiology of arteriosclerosis. We investigated cardiovascular risk profiles underlying the differences in burden of arteriosclerosis across arteries and sex. Moreover, we investigated the development of arteriosclerosis by studying changes in arteriosclerosis over time. We found a considerable increase in volumes of calcification, as proxy for arteriosclerosis, in the coronary arteries, aortic arch, extracranial, and intracranial carotid arteries, covering a 14-year timespan. Cardiovascular risk factors were associated with changes in arterial calcification with sex-specific differential effects across arteries.
The second part of my thesis was focused on consequences of arteriosclerosis. We investigated the role of arteriosclerosis in the aetiology of various disease manifestations as well as its potential for the prediction of disease. We found that distinct morphological subtypes of intracranial arteriosclerosis (intimal calcification, internal elastic laminal calcification, and a mixed subtype) are associated with a higher risk of stroke among middle-aged and elderly people. Furthermore, taking into account recent modifications in cardiovascular disease guidelines, we assessed the added value of arterial calcification in multiple

Figure 1: Cross-section of an artery with arteriosclerotic features 1, tunica intima; 2, tunica media; 3, internal elastic lamina; 4, thickened tunica intima; 5, lumen; 6, calcification in the tunica intima; 7, calcification in the internal elastic lamina; 8, Mönckeberg’s medial sclerosis.
arteries, beyond traditional risk factors, to the prediction of cardiovascular disease. Coronary artery calcification in both sexes, and intracranial calcification in women only contributed to 10-year cardiovascular disease risk prediction. We also assessed the value of information on carotid plaque components to the prediction of cardiovascular disease. Particularly in women, carotid intraplaque haemorrhage was a robust predictor of incident cardiovascular disease, beyond traditional risk factors, plaque size, and stenosis. These findings indicate a potential of carotid intraplaque haemorrhage as marker of plaque vulnerability.
In my thesis I endeavoured to provide novel insights into the multifactorial aetiology and consequences of arteriosclerosis, which hopefully opens avenues for future research and creates opportunities for optimisation of preventive strategies for cardiovascular risk reduction. In 2022, I will defend my thesis.

ATHEROSCLEROTIC PLAQUE MORPHOLOGY OF THE CAROTID ARTERY
TAIHRA ZADI, MSC
PhD Student
Advisors Aad van der Lugt & Daniel Bos
Project Funding GE Healthcare
Research period April 2014 – June 2021
Email t.zadi@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Neurology.
Atherosclerotic plaque composition in the carotid artery has been an important topic of research for the last few decades. Different components harbor different risks of plaque ruputure and a (recurrent) stroke. Particularly the presence of a lipid-rich necrotic core, thin fibrous cap or intraplaque hemorrhage are components that are increasingly established as risk factors for ischemic stroke. Conversely, plaques with more calcification seem to be less prone to rupture. Vessel wall imaging techniques like CTA and MRA are used to identify atherosclerotic plaque components.
My recent work is focused on the natural course of calcification in the carotid atherosclerosis. We quantified the development of calcification in both intra- and extracranial internal carotid artery, over two years. Along with the progression and regression of plaque calcification, this change was compared to the change in other plaque determinants and accordingly in the plaque composition.

Additionally, more attention has been paid to atherosclerotic plaque characteristics as a diagnostic tool. Selection for carotid endarterectomy is currently driven by a luminal narrowing of more than 70%. However, patients with mild-to-moderate stenosis and vulnerable plaque are still missed, due to this selection method. Several guidelines have recommended the use of imaging based selection methods independent of the degree of carotid stenosis. This method has been investigated within The Patient At Risk (PARISK study) and gives more insight into the clinical implication of atherosclerotic plaque characteristic.

Figure 1. Internal carotid artery with increase and decrease of extracranial (ECAC) and intracranial (ICAC) calcification volume.

THE EFFECT OF PLAQUE STRUCTURAL STRESS ON HUMAN CAROTID PLAQUE PROGRESSION
AIKATERINI TZIOTZIOU, MSC
PhD Student
Advisors Ali Cagdas Akyildiz, Daniel Bos, Ton van der Steen & Aad van der Lugt
Project Funding PhD-project Erasmus MC-Grants (MRace) 2019
Research period January 2021 – January 2025
Email a.tziotziou@erasmusmc.nl
This project is a collaboration between the Departments Radiology & Nuclear Medicine and Cardiology.
Crotid artery disease is a major cause of cerebrovascular events. Atherosclerosis in carotid arteries starts with the initial phase of plaque onset and progression to a non-symptomatic, early stage plaque. Subsequently, some plaques further progress into an event-causing, advanced stage plaque. However, features of high-risk carotid plaques that connote clinical events and symptoms remain not well-defined. Biomechanical forces are shown to greatly impact atherosclerosis. Areas of arterial wall exposed to low levels of blood flow-driven shear stress have been demonstrated to be the preferred locations for atherosclerotic plaque initiation. Moreover, plaque rupture locations have been strongly associated with high levels of blood pressure-driven plaque stresses. Levels and distribution of the structural stresses vary among plaques. Although their significant impact in atherosclerotic plaque initiation and rupture has been established, the critical role of blood flow- and pressure-driven plaque stresses in plaque progression and change in composition over time is yet to be explored.
In this project, we hypothesize that carotid plaque stresses play a pivotal role in the course of atherosclerosis, and combined with plaque morphometric measurements they can be instrumental for predictions of plaque progression, composition change over time and future clinical events. The study aims 1.) to unravel the effect of blood pressure- and flow-driven plaque stresses on carotid plaque progression and change in composition, and 2.) to identify morphometric and stress (functional) markers for plaque progression, change in composition, and future clinical events (Figure 1).
Figure 1: Overview of study pipeline.

Jacob Visser is a musculoskeletal radiologist, health economist and epidemiologist. As of July 2020, he is appointed as assistant professor in value-based imaging and working on issues such as structured reporting, decision support software, and integrated diagnostics. Furthermore, he is involved in setting up further collaboration with radiologists at the Radiology department of the Massachusetts General Hospital

(MGH) in Boston, MA, USA, and Stanford University, Stanford, CA, USA. In addition, he is a member of the Quality Committee of the Dutch Society for Radiologists and the RSNA Working Group for Common Data Elements. He has served in the ESR eHealth and Informatics Subcommittee. As of January 1, 2020, he was appointed as Chief Medical Information Officer at the Erasmus MC. j.j.visser@erasmusmc.nl
VALUE-BASED IMAGING
JACOB J VISSER, MD, PHD, MSC
assistant professor

Context
As health care rapidly changes from volume to value-based, there is an urgent need for radiologists to position themselves from a valuebased perspective.
Therefore, the Radiology Department at the Erasmus MC has started the value-based imaging program. This means that all activities in the Radiology Department are evaluated in the light of value-based imaging.
The value-based imaging program is closely related to the IT department. Most topics that are being covered by this program require expertise in the field of information technology.
Top Publications 2021
Brady AP, Bello JA, Derchi LE, Fuchsjäger M, Goergen S, Krestin GP , Lee EJY, Levin DC, Pressacco J, Rao VM, Slavotinek J, Visser JJ , Walker REA, Brink JA. Radiology in the Era of Value-based Healthcare: A Multi-Society Expert Statement from the ACR, CAR, ESR, IS3R, RANZCR, and RSNA. Radiology 2021 Mar; 298(3):486-491.
Starmans MPA, Buisman FE, Renckens M, Willemssen FEJA, van der Voort SR, Groot Koerkamp B, Grünhagen DJ, Niessen WJ, Vermeulen PB, Verhoef C, Visser JJ, Klein S. Distinguishing pure histopathological growth patterns of colorectal liver metastases on CT using deep learning and radiomics: a pilot study. Clin Exp Metastasis. 2021 Oct;38(5):483-494. doi: 10.1007/s10585-021-10119-6.
Angus L, Starmans MPA , Rajicic A, Odink AE , Jalving M, Niessen WJ, Visser JJ , Sleijfer S, Klein S , van der Veldt AAM . The BRAF P.V600E Mutation Status of Melanoma Lung Metastases Cannot Be Discriminated on Computed Tomography by LIDC Criteria nor Radiomics Using Machine Learning. J Pers Med. 2021 Apr 1;11(4):257.
Research Projects: Objectives & Achievements
Artificial intelligence
Currently, several projects are under investigation in the value-based imaging program. Firstly, as good quality of radiology reports is a core element in the era of value-based imaging, strategies are developed to investigate the quality of these reports. As manual review to identify sufficient cases for retrospective analysis is often infeasible, a stepwise natural language processing (NLP)-method for case identification was developed and applied for the identification of critical finding cases.
Quantitative and radiogenomics imaging is becoming more important. It turned out that visual assessment alone was not able to generate all available information. Instead, state-of-the-art computer techniques like machine and deep learning are able to extract quantitative features that are able to play an important role in the diagnostics of musculoskeletal and other diseases. A model was developed and is to be validated that is able to predict the MDM2-amplification in lipoid tumors.
Implementation of artificial intelligence algorithms in the radiology workflow is becoming a hot topic. Although several algorithms have been developed of which some are CEmarked / FDA approved and only a few validated in clinical practice, it is important to make the right decisions about implementation of those algorithms. Depending on the type of the algorithm, automatic measurements, case prioritization, pathology detection, a certain amount of research is needed before usage in the clinic can start.
Data-driven workflow
More than 200,000 exams are being performed in our department annually. This requires good logistics and insights in the workflow. In order to get the information about the processes, it is needed to identify relevant data sources. Modern software packages allow for visual representation of process and quality indicators. The research focuses on how to involve people and use dash boarding tools in order to improve efficiency and quality metrics.
Integrated diagnostics will be one of the key steps forward in the coming decade. In this respect, the work-up for patients with adrenal incidentaloma was evaluated. Furthermore, the diagnostic work-up for cervix carcinoma, carcinoma of unknown primary, and chest pain was evaluated. Currently, a workflow integration of radiology and pathology findings is investigated in lung cancer patients. Also for liver and brain tumors, further integration of radiology and pathology can increase efficiency and improve diagnosis.

Artificial intelligence algorithm automatically detects lungnodule
In addition, it is to foreseen that image oriented specialties like dermatology can be involved in the integrated diagnostics approach.
Expectations & Directions
In the coming years, we aim to further expand our research in the domains of artificial intelligence, data-driven workflow, and integrated diagnostics. An important challenge will be to bring these together and establish reference architectures and pipelines how to structurally evaluate new workflows and technologies.
Funding
Klein S, Starmans MPA, Visser JJ, Verhoef D, Grunhagen D: Hanarth Fonds: 2021-2025: “Automatic grading and phenotyping of soft-tissue tumors through machine learning to guide personalized cancer treatment”
Visser JJ, Klein S et al, Stichting Coolsingel: 2017-2018, “Radiogenomics in desmoid tumoren en goedgedifferentieerde liposarcomen”
Kors JA, Visser JJ: Stichting Kwaliteitsgelden Medisch Specialisten 2016-2019: “Critical findings in radiology”
Invited Lectures
Integrated diagnostics – informatics considerations; RSNA 2021, Chicago, IL, USA

IMPLEMENTATION OF ARTIFICIAL INTELLIGENCE IN RADIOLOGY PRACTICE
LAURENS TOPFF MD
Advisors Jan-Jaap Visser, Erik Ranschaert & Regina Beets-Tan
Project Funding
Research period March 2019 – February 2023
Email l.topff@nki.nl
This project is a collaboration between the Department of Radiology & Nuclear Medicine and Netherlands Cancer Institute –Antoni van Leeuwenhoek
Artificial intelligence (AI) provides opportunities for radiologists to deliver new value to patients. The study investigates the optimization of radiology workflow processes through implementation of AI. The focus includes all components of the imaging chain, starting at the scheduling of imaging examinations, to image analysis, to reporting and communication by the radiologist, and operational aspects such as business analytics.
The main purpose of the research project is to assess the value and cost-effectiveness of AI in radiology practice to improve workflow and patient care, while also considering ethical issues. The approach is to validate existing AI applications into real-world clinical radiology workflows, by testing efficacy and efficiency of the software. The second approach is to develop AI solutions that increase the operational efficiency of the radiology department. The goal is to deliver actionable information that can be implemented into practice.
Background
Advances in artificial intelligence in radiology are occurring at a rapid pace due to the increase in computational capabilities, data availability, and deep learning techniques. The currently available algorithms are considered narrow artificial intelligence, as they focus on very specific tasks. Recent publications have demonstrated that AI applications in radiology can perform at the level of radiologists for narrow tasks.
There is no indication in the literature that AI can replace radiologists in routine clinical practice in the near future. Nevertheless, there is evidence that AI tools can assist and augment radiologists to work more accurately and efficiently. Therefore, AI has great potential to transform
the radiology workflow, as radiologists today are faced with several challenges such as the continuous growth in demand for imaging, increasing data per imaging examination, lowering of reimbursement rates and limited staff and equipment resources.
Most early AI applications focus on lesion detection, segmentation, and classification of images. Nevertheless, there are many more use cases beyond image interpretation. AI is a powerful tool to identify complex patterns in clinical and operational data, and to predict events and behavior. Therefore, AI can benefit many areas in the radiology workflow.

AI-based detection of incidental pulmonary embolism allows prioritization of positive CT scans in the worklist of the radiologist, thereby reducing the time to diagnosis of acute findings. The heatmap highlights a large embolism in the main pulmonary arteries on a follow-up CT of an oncology patient
JOINT APPOINTMENTS IN EPIDEMIOLOGY AND HARVARD
T.H. CHAN SCHOOL OF PUBLIC HEALTH
Dr. Hunink completed a BSc in applied mathematics, an MD degree, and a PhD in health decision sciences. She trained as a radiologist in Amsterdam (VUMC), did sub-specialty training in interventional and cardiovascular radiology in Boston (BWH/Harvard), and did a research fellowship at Harvard (HSPH/BWH/HMS).
She currently directs the Assessment of Radiological Technology (ART) program and the division of Clinical Epidemiology at the Erasmus MC and she is adjunct

professor of Health Decision Science at the Harvard TH Chan School of Public Health, Boston. In addition, she is the Director of the Netherlands Institute for Health Sciences at Erasmus MC. Her research interests include technology assessment of imaging biomarkers and image-guided therapies, computerized decision support for evidence-based use of imaging tests, optimizing the design of RCTs for non-pharmaceutical interventions, and evaluating the effects of lifestyle interventions that foster resilience and wellbeing among healthcare professionals.
m.hunink@erasmusmc.nl
ASSESSMENT OF RADIOLOGICAL TECHNOLOGY (ART)
MG MYRIAM HUNINK, MD, PHD
full professor

Context
Medical imaging tests and imaging biomarkers play an important role in diagnostic and prognostic prediction, which in turn are crucial in making wise therapeutic decisions. Whereas precision medicine aims to provide such predictions at the subgroup level, personalized medicine combines these predictions with individual patientcentered outcomes and values, as well as settingspecific considerations, in order to make smart choices. The continual development of novel imaging technologies for diagnosis, prognosis, and treatment requires comparative effectiveness research and health technology assessment to justify its use. The results from such research lead to value-based imaging.
Top Publications 2021
Fakhry F, Rouwet EV, Spillenaar Bilgen R, van der Laan L, Wever JJ, Teijink JAW, Hoffmann WH, van Petersen A, van Brussel JP, Stultiens GNM, Derom A, den Hoed PT, Ho GH, van Dijk LC, Verhofstad N, Orsini M, Hulst I, van Sambeek M, Rizopoulos D, Moelker A and Hunink MGM. Endovascular Revascularization Plus Supervised Exercise Versus Supervised Exercise Only for Intermittent Claudication: A Cost-Effectiveness Analysis. Circ Cardiovasc Interv. 2021;14:e010703.
Ferket BS, Hunink MGM, Masharani U, Max W, Yeboah J and Fleischmann KE. Long-term Predictions of Incident Coronary Artery Calcium to 85 Years of Age for Asymptomatic Individuals With and Without Type 2 Diabetes. Diabetes Care. 2021;44:1664-1671.
Heath A*, Hunink MGM*, Krijkamp E*, Pechlivanoglou P*. (*all authors contributed equally). Prioritisation and design of clinical trials. Eur J Epidemiol. 2021 Nov; 36(11):1111-1121.
Objectives & Achievements
The main objectives of this research program are to (1) assess the added value of imaging tests and imaging markers for personalized decision making ( Evidencebased and Value-based Radiology) ; (2) develop and assess the value of computerized decision support systems that guide imaging referrals for the appropriate and justified use of imaging tests; (3) assess the (cost-) effectiveness of image-guided therapies in comparison to medical treatment, surgery, and lifestyle interventions; (4) develop methods to optimize study design for the evaluation of pharmaceutical and non-pharmaceutical interventions; and (5) evaluate the effects of lifestyle interventions that foster resilience and wellbeing. The methods we use include systematic reviews, meta-analyses, prediction modeling, randomized controlled trials, computer simulation of randomized trials, comparative effectiveness research, and health technology assessments. Our research focuses on optimizing health care decisions by combining the best-available quantitative evidence on risks and benefits from diverse sources and integrating patient-centered outcome measures, preferences, quality of life, and costs. Ultimately, we intend to incorporate the results in clinical guidelines and computerized decision support systems to facilitate patient-physician shared decision making and guide the optimal use of imaging technology.
Our work has included the design, performance, and supervision of randomized controlled trials (RCTs) and computer simulation models to evaluate both diagnostic imaging tests as well as image-guided therapies. Some of our studies have led to practical web-based prediction models and smartphone tools that can help guide evidence-based and justified use of imaging tests and therapeutic interventions.
Evidence-based and Value-Based Radiology
Imaging is used for screening, early diagnosis, clinical diagnosis, treatment planning, image-guided therapy, and follow-up after treatment. With the advancement of imaging methods, indications for imaging are shifting. This research line encompasses diverse projects in radiology that aim to determine what the optimal imaging algorithm is for particular patients based on the added value to the patient and society. The projects in this line of research cover a wide range of topics, including diagnostic imaging and surveillance strategies in cardiovascular, neurological, musculoskeletal and oncological disease.
We are contributing to the PERISCOPE study which analyzes the value of perfusion MRI in the surveillance after treatment of a brain tumor (see Jeremy Labrecque). The PERISCOPE project is a collaborative effort with the Physiological NeuroImaging group, led by Marion Smits, and we work with Wouter Teunissen on this project. Melvin Lam, master student in our group, played an important role in this project. He investigated the change in decisions made by the neurosurgical team (neuroradiologist, neurologist, neurosurgeon) on the basis of the perfusion MRI.
We are supporting the musculoskeletal imaging group, led by Edwin Oei, with their AMPHiBI randomized controlled trial evaluating the value of PET-MRI in patients with chronic hip pain and patients with lower back pain.
Takahiro Kinoshita completed his analysis on the costeffectiveness of a hybrid emergency room system for severe trauma. Inge-Marie Obdeijn evaluated MRI for breast cancer and investigated the value of different imaging strategies. Andrea Lo completed her analysis on the use of PET for staging early-stage follicular lymphoma. Ongoing projects include the evaluation of imaging surveillance strategies for surgically treated pituitary adenoma (Lisa Caulley), imaging surveillance strategies for patients with an unruptured intracranial aneurysms (Tim Cras), CT angiography vs catheter angiography for chronic limb-threatening ischemia (Zach Feldman), and other cardiovascular imaging as described below.
Optimizing the Imaging Workup for suspected coronary artery dise ase
Stable chest symptoms (chest pain, exertional breathlessness, exertional fatigue, or tiredness) are common symptoms that may indicate the presence of coronary artery disease (CAD). Catheterization with contrast angiography is generally considered the reference test for the workup. However, it has risks for the patient and is costly. Research efforts in cardiac imaging therefore focus on non-invasive, or minimally invasive, diagnostic tests so that catheterization can be avoided. Non-invasive diagnostic tests for coronary artery disease include exercise electrocardiography, stress echocardiography, CT calcium scoring, CT coronary angiography, myocardial perfusion CT, computed fractional flow reserve (FFR), single photon emission computed tomography (SPECT), positron emission tomography (PET), perfusion MRI, stress MRI, and fusion imaging. This line of research compares the non-invasive tests mutually, compares them with contrast angiography, determines the added value of imaging compared with no-testing strategies, and evaluates imaging workup algorithms. This is a collaborative effort with Ricardo Budde and Alexander Hirsch of the Cardiac Imaging group.
Currently we are supporting the FUSION study, an RCT which evaluates the value of CT Fractional Flow Reserve in the workup and treatment planning of patients with chest pain suspected of having coronary artery disease (see contribution Simran Sharma, Cardiac Imaging). Furthermore, we are evaluating the cost-effectiveness of triage strategies for chest pain patients that include pretest probability prediction models, CTCA, CT FFR, and PET (Olivier Clerc, Thom Korthals).
Imaging asymptomatic cardiovascular disease: Screening and prevention
Judicious use of novel imaging tests can potentially have a major impact on cardiovascular disease (CVD) outcomes by identifying those who can benefit from preventive interventions. Guidelines for screening and prevention of CVD typically advocate classification into low-, intermediate-, and high-risk groups. Further workup and preventive interventions can then be tailored to the risk of disease and future events. Initial prognostic risk classification is advocated with risk scores based on demographics and traditional risk factors. Novel imaging markers hold the promise to refine such risk classification. Which imaging procedure to use in which situation and the impact on outcomes must be elucidated before personalized screening and prevention can become a reality. This is of particular importance in defined patient populations at high risk, for example, patients with diabetes mellitus.
In this research line we use decision modeling and computer simulation studies to integrate the best-available evidence in order to assess imaging markers for screening and prevention of CVD. We are working on a modeling study evaluating the role of CT coronary artery calcium to guide preventive therapy in diabetes mellitus type II patients and evaluating novel therapeutic drugs for such

patients. This is a collaborative effort with researchers at UCSF in San Francisco (Kirsten Fleischmann, Umesh Masharani, Wendy Max) and at Mt Sinai in New York (Bart Ferket) and one of our master students (Luuk Avezaat).
Decision support for imaging referrals
To ensure evidence-based choices for the use of imaging technology and evidence-based management of imaging findings in day-to-day clinical practice requires implementation of a computerized decision support system which is available at the point of care and is adaptable to the individual patient. Our vision is to provide the most recent evidence on test performance together with prediction models that are dynamically updated and revised in an easy-to-use format to support decision-making with respect to diagnostic imaging at the point of care.
A step to achieve this ultimate goal is to provide imaging referral guidelines in the form of a computerized decision support system (CDSS). In this line of research, we have initiated a clinical trial to evaluate the effect of introducing a CDSS in the hospital setting that guides imaging requests, the ESR iGuide. The Medical Imaging Decision and Support (MIDAS) study will be performed as a multi-center cluster randomized trial with departments acting as clusters combined with a before-after-revert design. Four hospitals with each 8 participating departments for a total of 32 clusters have been recruited for the study and are collecting data. All departments started recently with the control condition. Subsequently departments will be randomly assigned to the active intervention or the control condition. In the revert condition decision support is removed to evaluate the sustainable educational effect of temporary use of the system. Stijntje Dijk is working on this project (see her contribution). This is a collaborative effort with Thomas Kröncke in Augsburg, Jörg Barkhausen in Lübeck, Olav Janssen in Kiel, Peter Mildenberger in Mainz, and Florian Demuth of the ESR iGuide.

Figure 1. Panel A. The research cycle based on an error-driven approach.
Panel B. The research cycle based on a value-driven approach. We advocate using a value-driven approach.
Evaluating Image-guided therapy
The growth of minimally-invasive image-guided therapies is exponential and such technologies are increasingly applied. However, is rapid implementation of these novel technologies justified? What is the evidence? What are the short- and long-term effects of the new technology compared to its alternatives? Do patient-reported outcome measures improve? Are outcomes generalizable across countries? Are the additional costs of these cuttingedge technologies justified? These questions drive this research line. Our focus is on endovascular interventions for disease of the cerebral arteries, carotid arteries, thoracic aorta, abdominal aorta, mesenterial arteries, and peripheral arteries for obstructive and aneurysmal disease. In this research line we assess, review, and integrate the best-available evidence using systematic reviews, metaanalysis, and decision modeling and we provide methodological expertise for the performance of RCTs.
We recently published the cost-effectiveness analysis of an RCT comparing combined endovascular revascularization with exercise therapy vs exercise therapy alone (the ERASE trial) for peripheral artery disease (Farzin Fakhry). In addition, we have worked on modeling studies of endovascular therapy for acute ischemic stroke.
Methods to optimize study design
The randomized controlled trial (RCT) has been the standard study design for evaluating health-care interventions for decades. Such trials involve millions of patients and cost billions of Euro’s. Furthermore, the trials are timeconsuming which leads to delayed implementation of potentially beneficial interventions. In this research line we aim to reduce the cost, time, number of patients needed, and burden to patients participating in trials evaluating health-care interventions by optimizing the design of such trials.
Rather than designing trials with the focus on minimizing statistical error (Figure 1A), we advocate using a valuedriven approach (Figure 1B). Using decision modeling of health benefits, patient values, and costs, taking into account the uncertainty around the input parameters (Figure 2), we can subsequently determine the uncertainty around the outcomes and calculate the value of reducing this uncertainty by doing further research. These analyses help prioritize research and guides study design (see contribution Eline Krijkamp).
In addition, we are utilizing causal inference methods to analyze observational data using target trial designs, that is, emulating a randomized trial design within the data taking into account confounding factors (see contribu-

Figure 2. Decision modeling using a probabilistic approach: all input parameters are modelled with their uncertainty reflected in distributions which is then propagated through the model to produce the uncertainty around the outcomes.
tion Jeremy Labrecque). Furthermore, we are developing other methods on the intersection between Medical Decision Making and Causal Inference.
In this research line we focus on non-pharmacological interventions including diagnostic and prognostic imaging biomarkers, medical devices for image-guided therapy, lifestyle interventions, and organizational interventions. Nevertheless, during the pandemic we expanded this line of research to also guide trial design for emerging pharmacological therapies for COVID-19 (see Stijntje Dijk). Prioritization of research is of particular interest during a pandemic because performing more trials of an unproven therapy that appears to be beneficial has an opportunity cost: by delaying implementation of the drug many lives could potentially be lost. Since we have the expertise to tackle this policy issue, we felt it was our responsibility to contribute to research efforts related to the pandemic with this analysis. The first paper from this project is now in press.

3. Wordcloud response to a poll at ECR 2021 to the question “what are the stressors in your work / life ?’’
Figure
The effect of lifestyle interventions
Chronic stress and burnout have become an epidemic among health care professionals and form a threat to the sustainability of health care. The COVID pandemic has only worsened the situation and has highlighted the importance of a healthy lifestyle, resilience and well-being. Radiologists (in-training) and radiological technologists are among those at the highest risk due to the push for productivity and efficiency, the increased interaction with computers rather than human beings, the administrative burden, medico-legal issues, 24/7 connectivity, and long hours in dark rooms (Figures 3 and 4). To maintain our professionalism and health and happiness, preventive measures at both the individual and organizational level are necessary.
In this research line we evaluate lifestyle interventions such as physical exercise, yoga, mindfulness-based stress reduction, martial arts, and music therapy (Figure 5) to reduce chronic stress, prevent burnout, and increase resilience and wellbeing among health care professionals and those in training for healthcare professions. We are performing systematic reviews, observational studies, RCTs, and modeling studies of interventions. In a clinical trial among medical students, research master students and PhD students, we evaluate the effects of lifestyle interventions. The study has a hybrid design combining a longitudinal cohort, a nested RCT, a preference design, sequential multiple assignment, and adaptive design. (See www.DESTRESS.info for more information). We plan to implement and evaluate the results not only among students but also among employees in the field of radiology.
Expectations & Directions
Our future projects will integrate various study designs in order to maximize the return on research investment. A typical project would integrate simulation studies, a before-after study, a randomized controlled trial (RCT), a prospective longitudinal observational study and value of information analyses. Ideally, we would simulate the study prior to actually performing it. The results of our research will inform patients, physicians, insurers, industry, and healthcare policy makers and will guide future research.
In the research line concerning professional well-being and resilience we intend to develop and evaluate interventions that reduce experienced stress among radiologists (in-training) and radiological technologists. Our intention is to ensure that our field remains an attractive specialty to pursue and that professionalism will be maintained by ensuring that our employees are happy, healthy, resilient and engaged.

Figure 4. Wordcloud response to a poll at ECR 2021 to the question “how do you feel after one of ‘those’ workdays when everything seems to be going wrong ?’’
Funding
H unink, Myriam. Calcium scoring in primary prevention of cardiovascular disease for individuals with diabetes. With Kirsten Fleischmann (PI), Cardiologist at UCSF, and Bart Ferket, Mt Sinai. American Diabetes Association Grant 20182022
Hunink, Myriam. Decreasing stress through resilience training for students (DESTRESS). Higher Education Quality and Innovation Agenda (“Studie Voorschot Middelen”). 2019 – 2022.
Smits, Marion, Anouk van der Hoorn, Jan Willem Dankbaar, Dieta Brandsma, Bas Jasperse, Linda Dirven, Filip de Vos, Myriam Hunink: “The clinical value of perfusion MRI in primary and secondary brain tumor surveillance”. ZonMW, Leading the Change 2018-2021.
Sardanelli, Francesca (PI), Inge-Marie Obdeijn et.al. Multicenter International Prospective Meta-Analysis (MIPA) of individual woman data (MIPA study). et al. EIBIR-EuroAIM/ EUSOBI and Bayer Pharma, 2016-2021.
Kroencke, Thomas, Myriam Hunink, Stijntje Dijk. Medical Imaging Decision and Support (MIDAS). German Innovation Fund, 2019-2023.
Krijkamp, Eline. SMDM fellowship for young investigators. Gordon and Betty Moore Foundation. 2019-2022.
Hunink, Myriam, Stijntje Dijk, Eline Krijkamp. Emerging Therapies for COVID-19: the value of more clinical trials vs implementation. Gordon and Betty Moore Foundation. SMDM COVID Decision Making Initiative. 2020-2021.
Budde, Ricardo (PI), Alexander Hirsch, Myriam Hunink. Addition of CT Fractional flow reserve in the diagnostic pathway of patients with stable chest pain to reduce unnecessary invasive coronary angiography (FUSION Study). ZonMW Health Care Efficiency Research, 2021-2024.
Ferket, Bart (PI), Kirsten Fleischmann, Umesh Masharani, Wendy Max, Myriam Hunink . Novel antidiabetic medications to reduce cardiovascular events in patients with diabetes mellitus type 2 – a modelling study. RO1, National Institutes of Health, USA, 2021-2024.
Invited Lectures
Dijk, Stijntje. Economic Considerations. Healthcare Professionals In Focus Programme. European Congress of Radiology, Online March 2021.
Hunink, Myriam. The science of mindfulness and meditation. Healthcare Professionals In Focus Programme. European Congress of Radiology, Online March 2021.
Dijk, Stijntje, Myriam Hunink, Eline Krijkamp. Emerging Therapies for COVID-19: the value of more clinical trials vs implementation. Symposium COVID-19 Decision Models: Connecting modelers and decision makers. April 2021.
Highlights
Stijntje Dijk, Eline Krijkamp and Myriam Hunink worked on a decision model about emerging therapies for COVID-19. Goal of the project was to determine the value of performing more clinical trials of promising new therapies compared to immediate implementation.

Myriam Hunink organized and moderated an In Focus Programme for the European Congress of Radiology, Online March 2021, entitled ‘’Healthcare Professionals in Focus’’ about well-being and resilience, consisting of 4 sessions and 12 workshops. (Figures 3, 4 and 5)
Master students
Melvin Lam, Nicole Lageweg, Bo vd Berg, Kelly van Hoof, Sophie Jacobs, Chia-Ping Lu, Aradhana Pandit, Tim Cras, Andrea Lo, Takahiro Kinoshita, Zach Feldman, Olivier Clerc.
External collaborations
Kirsten Fleischmann, UCSF, San Francisco
Umesh Masharani, UCSF, San Francisco
Wendy Max, UCSF, San Francisco
Bart Ferket, Mt Sinai, New York
Thomas Kröncke, Univeristy Hospital Augsburg
Jörg Barkhausen, UKSH, Lübeck
Olav Janssen, UKSH, Kiel
Peter Mildenberger, University of Mainz
Florian Demuth, ESR, Vienna
Wolfgang Kunz, LMU, Munich
Uwe Siebert, UMIT, Hal in Tirol
John Wong, NEMC, Boston
Natalia Kunst, Harvard, Boston
Zach Feldman, MGH, Harvard, Boston
Olivier Clerc, BWH, Harvard, Boston

Figure 5. European Congress of Radiology 2021 ’’Healthcare Professionals in Focus’’: online workshops about well-being and resilience

JEREMY LABRECQUE, MSC, PHD CAUSAL INFERENCE, DECISION-MAKING AND PERFUSION MRI
Project Funding PERISCOPE study; NWO replicatiestudies grant
Research period January 2020 – January 2022
Email j.labrecque@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Epidemiology.
Causal inference provides us with a framework that allows us to be clear about what our research question is, the assumptions required for our analyses to work and to demonstrate potential biases in those analyses. I have used causal inference to improve the quality of research in a number of different contexts.
The main focus of my work over the past few years has been using causal inference to better understand how Mendelian randomization works when exposures change over time finding that this can sometimes cause important bias in these types of studies. My work with the PERISCOPE study has nudged me more in the direction of decision making where, I believe, there is a lot of potential for the incorporation of the many advances that have occurred in causal inference over the past few decades. Decisions, after all, clearly end up having many causal impacts on many aspects of a patient’s longevity, health, quality of life as well as causal impacts on healthcare providers and the health care system itself (See Figure).
A prime example of this is the work of Melvin Lam, a talented Masters student I had the pleasure to work with. His work took causal inference methods and used them in a way to estimate how many treatment decisions in patients with brain tumors might have been different depending on whether or not the neurologists had access to information from a perfusion MRI. Working with a PhD student, Wouter Teunissen, we will work to apply causal inference concepts to evaluate the cost-effectiveness of the perfusion MRI in patients with brain tumors.
The future will take me further into the direction of using causal inference for better decision making as I was awarded a VENI grant to study how to use causal bias analysis to make better decisions. All decision models include parameters that are causal in nature and all of these are estimated
with some risk of bias adding uncertainty to the overall decision. By using causal bias analysis in the decisionmaking model, we can incorporate this type of uncertainty into our decisions. This will help us identify when plausible biases in causal parameters in decision models could potentially change our decision or when even these plausible biases are not enough for us to change our minds.
I believe there is multiple careers’ worth of research to be done on the topic of integrating causal inference and decision models helping to improve medical decision making in radiology and beyond!

Figure 1. A simplified causal graph depicting some of the potential impacts of a treatment decision. Each of these potential impacts represents a causal parameter that must be used in a decision model. Because of the difficulty of estimating causal effects without bias, we must incorporate all this potential for bias into our decision making.

MRI VERSUS MAMMOGRAPHY FOR BREAST CANCER SCREENING IN WOMEN WITH HIGH FAMILIAL RISK (FAMRISC
STUDY)
A INGE-MARIE OBDEIJN, MD, PHD
Project Funding Funding FaMRIsc studie: Zon MW, KWF kanker bestrijding, Pink Ribbon, Stichting Coolsingel, Sisters Hope
Research period Permanent position
Email a.obdeijn@erasmusmc.nl
Approximately 15% of all breast cancers occur in women with a family history of breast cancer, but for whom no causative hereditary gene mutation has been found. Screening guidelines of women with familial risk of breast cancer differ between countries. We performed a national multicenter randomized controlled trial (FaMRIsc) to compare MRI screening with mammography in women with at least 20 % cumulative life time breast cancer risk because of familial predisposition. Women with a BRCA1/2 gene mutation were excluded.
Participants were randomly allocated (1:1) to the MRIgroup or the Mammography-group. Women in the MRI-group (n=675) received annual MRI and biennial mammography; women in the Mammography-group (n=680) underwent annual mammography according the current Dutch guidelines. More breast cancers were detected in the MRI-group than in the Mammographygroup. We saw that that the invasive cancers in the MRIgroup were smaller than in the mammography-group and less frequent node positive, importantly, also in the incident rounds. We concluded that MRI screening detected cancers at an earlier stage than mammography. The lower number of late-stage cancers identified in incident rounds with MRI screening might decrease breast cancer related mortality. The earlier stage of breast cancers detected with MRI screening might reduce the use of adjuvant chemotherapy and thereby reduce the costs.
We performed cost-effectiveness analyses of several screening protocols with varying ages and intervals, including both FaMRIsc screening protocols to determine the best screening protocol. MRI screening every 18 months between the ages of 35 and 60 years followed by the national screening program was considered optimal and cost-effective (ICER €21380). Annual screening
(4%)2 (1%)0
little trust8 (7%)4 (3%)2 (2%) 2 (1%) Neutral4 (4%)6 (4%)1 (1%) 31 (21%)
Quite some trust 52 (48%)50 (35%)17 (16%)13 (9%)
alternating MRI and mammography between the ages of 35 and 50 years, followed by the national screening program, gave similar outcomes.
By questionnaire we evaluated the experience, expectations and preferences regarding screening MRI and mammography in a subgroup of the included women. Almost all women preferred screening with MRI. The high chance of early cancer detection was considered the most important advantage of MRI screening in the MRI-group (95%) as well as in the Mammography-group (74%),while this was also the main disadvantage of mammographic screening (57% MRI-group; 72% of the Mammographygroup).
The next step will be to implement the results in the screening guidelines.
Table 1. Women’s trust in screening modalities
Do

Advisors
CLINICAL EFFECTIVENESS AND DECISION MAKING IN OTOLARYNGOLOGY
LISA CAULLEY, MD, MPH
PhD Student
Myriam Hunink & Shaun Kilty
Project Funding Canadian Institutes of Health Research Doctoral Award, PSI Foundation Research Trainee Award, University of Ottawa Junior Research Chair
Research period January 2018 – July 2022
Email Lic955@mail.harvard.edu
This project is a collaboration between the Erasmus Departments of Radiology & Nuclear Medicine and Epidemiology and Ottawa Hospital Research Institute Clinical Epidemiology Program.
LLisa Caulley is investigating evidence-based strategies to guide diagnostic and therapeutic decisionmaking in otolaryngology-head and neck surgery. Her doctoral degree is supported by the Canadian Institutes of Health Research (CIHR) Doctoral award and PSI Foundation Research Trainee Award. She was appointed as an Associate Scientist at the Ottawa Hospital Research Institute Clinical Epidemiology and Center for Journalology, and an Assistant Professor at the University of Ottawa Department of Otolaryngology-Head and Neck Surgery in 2020. In 2021, Lisa Caulley published 6 manuscripts (5 first author) in the peer-reviewed literature. Her papers have been published in leading journals including JAMA Otolaryngology-Head and Neck Surgery. She collaborated on the recently published Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement. She was appointed to the editorial board of CMAJ and the Ontario Health Technology Advisory Committee Health Quality Ontario. She received the CIHR COVID (valued at $48,000), and the University of Ottawa Junior Research Chair (valued at $80,000) in support of her doctoral degree.
Lisa supervised or co-supervised 1 graduate and 6 undergraduate student research projects. She has been a teaching assistant at Erasmus University Medical Center Rotterdam for Decision Making in Medicine (EWP02), and Using R for Decision Modeling in Health Technology Assessment (CE16). Lisa collaborated on a COVID-19 research project investigating COVID vaccine perception amongst Black Canadians.

Figure 1

Advisors
OPTIMIZING DECISIONS IN HEALTH(CARE)
STIJNTJE W DIJK, MD,MSC
PhD Student
Myriam Hunink
Project Funding Medical Imaging Decision And Support (German Innovation Fund), SMDM Covid-19 Decision Modeling Initiative (Gordon and Betty Moore Foundation), DESTRESS (Studievoorschotmiddelen)
Research period October 2020 – April 2024
Email s.dijk@erasmusmc.nl
These projects are a collaboration between the Departments of Radiology & Nuclear Medicine and Epidemiology.
Every day in health and healthcare, decisions are made, varying from the individual level (for example, a clinician deciding which diagnostic test to order), to the organizational level (whether a hospital should implement a hospital-wide decision support system), to a societal or global level (whether the value of conducting a new randomized controlled trial into a promising treatment outweighs the potential benefits of immediate implementation). This thesis research will address the generation of evidence for, and optimization of decision making in various areas of health and health care.
In one of our main projects, we will investigate the effect of implementing computerized decision support systems to guide imaging referrals in clinical practice as part of the Medical Imaging Decision and Support study (the MIDAS study). This is a multi-center cluster randomized trial with departments acting as clusters, combined with a before-and-after revert design. Four hospitals across Germany are currently preparing for the launch of the study, in which the ESR iGuide will be implemented in the imaging referral systems of all participating departments as a control condition. Half of all departments will then be randomized to switch to the active intervention in which requesting physicians will receive feedback from the iGuide on their requests and appropriate exams are suggested after which they may decide to change their request. Imaging requests are categorized as appropriate, under certain conditions appropriate and inappropriate. In the revert condition of the study, the decision support tool is then removed to study the sustainable educational effect of using the system. The project has incurred some delays due to the COVID-19 pandemic and implementation challenges, however the iGuide has
been integrated in the ordering system of each site, and we expect to start the collection of control data and perform randomization early 2022.
There are several additional projects that we are currently working on. We submitted our first paper on the value of conducting further RCT’s on promising treatments for hospitalized patients using value of information analysis (VOI) and aims to inform decision makers on the potential (foregone) benefits of early or delayed implementation of such treatments. Secondly, we are working on the analysis of the results and an economic evaluation of mindfulness-based therapies for future health professionals as part of the DESTRESS study. In January 2022 we will collect the last groups’ 1-year follow up data and will start the result analysis. We presented a simplified cost benefit analysis of implementing similar interventions to build resilience and prevent burnout at the ECR online conference in 2021.

Figure 1. VOI: Treatment approval and further research trade-offs

Advisors
OPTIMIZING MEDICAL DECISION MAKING OPEN-SOURCE MODELING AND RESOURCE PRIORITIZATION IN HEALTHCARE
ELINE KRIJKAMP, MSC
PhD Student
Myriam Hunink & Petros Pechlivanoglou
Project Funding Erasmus MC: “Doelmatigheidsproject”, SMDM Fellowship in medical decision making, Gordon and Betty Moore Foundation, SMDM COVID Decision Making Initiative
Research period Mei 2018 – Mei 2022
Email e.krijkamp@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine, Epidemiology and the DARTH workgroup.
Decision makers in healthcare frequently face decisions on the allocation of scarce resources under conditions of uncertainty. Healthcare resources include all materials, personnel, facilities, and funds needed to provide healthcare services. Regardless of decision uncertainty, a decision needs to be made about the use of these resources. This requires decision makers in healthcare to evaluate the consequences of their decisions regarding both health outcomes and resource utilization. To maximize the value that can be achieved from the available resources, healthcare decisions can be supported by decision-analytic models (Figure).
Decision-analytic models can evaluate both clinical and economic aspects of a decision problem. They are considered the most comprehensive and suitable approach to inform decision-making in healthcare. However, building reliable decision-analytic models that are transparent to stakeholders is challenging.
We demonstrate how open-source decision-analytic models can be used in this context. Similar to healthcare resources, resources to fund research are limited as well. Resource funds can be defined as all facilities, materials, human resources, study participants, and funding required to perform a (clinical) study. Cutting-edge methods in decision analysis can be used to prioritize both healthcare and research resources. These methods are called value of information (VOI) analyses. VOI analyses are used to quantify the monetary value of
reducing decision uncertainty via the collection of additional evidence. We say that the additional evidence has value when it has the potential to reduce the costs associated with making the wrong decision.
New techniques to perform the analyses in combination with the use of open-source decision-analytic models facilitate an efficient implementation of VOI methods. Decision makers are still not familiar with these methods leading to the limited application of VOI in the design of clinical studies. We aim to communicate the potential value of VOI methods to a broad audience and have applied it to COVID-19 treatment strategies.
Figure 1. Disability adjusted life years (DALYs) and Years of life lost (YLL) per month of delay of selected surgical procedures during a period of scarcity.


TRAUMATIC BRAIN INJURY
EPIDEMIOLOGY, RISK FACTORS AND DECISION MAKING
CRISPIJN L. VAN DEN BRAND, MSC, MD, PHD
PhD Awarded 24 March 2022
Advisors Myriam Hunink & Korne Jellema
Project Funding St. Jacobus Foundation
Short CV Crispijn Lennart van den Brand was born on March 24th 1975 in Rotterdam. After graduating from Medical School in Leiden he started training in Emergency Medicine. At the end of 2011 he became an Emergency Physician and continued to work in the Haaglanden Medical Center. In 2019 he completed a Master in Health Economics, Policy and Law at the Erasmus University. Between Since 2019 and 2021 he worked as head of the registry and research department at the Dutch Institute for Clinical Auditing (DICA), Leiden. In 2021 he started to work as an Emergency Physician in the Erasmus Medical Center. Crispijn was a member of the board of the Dutch Society of Emergency Physicians from 2009 until 2017 and was president of this society from 2013 until 2016.
This project is a collaboration between the Departments of Radiology & Nuclear Medicine, Emergency Medicine and Neurology.
Each year millions of people die or become disabled as a result of traumatic brain injury (TBI). The incidence of TBI is increasing worldwide, including the Netherlands.
The first part of this thesis focusses on epidemiological trends in TBI during a period of 15 years in the Netherlands. We observed an increase in emergency department (ED) visits and hospitalizations for TBI with 75% and 95% respectively. Remarkable is the shift in age groups. The number of ED visits by elderly (65+ years) for TBI increased almost fivefold during the study period. Meanwhile the number of ‘young’ (<65 years) TBI patients decreased. Expressed in mortality: at the beginning of the study period 34% of the patients that died because of TBI was 65 years or older; 15 years later this figure had increased to 63%. This increase cannot solely be explained by ageing of the population. Other important factors are that the elderly live independent and remain active until a higher age than in the past.
Research presented in this thesis shows that the number of CT-scans for TBI increases rapidly. Research in 10 ED and more than 5000 patients shows that all frequently used existing decision rules for selective CT-scanning in TBI have their shortcomings. Therefore, an existing decision rule was updated to perform better in the current population of TBI patients.

https://repub.eur.nl/pub/135368

APPOINTMENT IN CHILD AND ADOLESCENT PSYCHIATRY
Tonya White received her Bachelors degree (magna cum laude) in Electrical Engineering from the University of Utah and her Masters Degree in Electrical Engineering from the University of Illinois. She obtained a Medical Degree from the University of Illinois. Thereafter she completed a combined residency in Pediatrics, Psychiatry, and Child and Adolescent Psychiatry at the University of Utah and a Research Fellowship in Neuroimaging at the University of Iowa.

She was an assistant professor at the University of Minnesota for 8 years prior to joining the faculty at the Erasmus University Medical Centre in 2009. She started a PhD in the Department of Biomedical Engineering at the University of Minnesota in 2005 and completed her PhD at the Erasmus University in September 2010. She set up and directs the Pediatric Population Neuroimaging group with the Department of Child and Adolescent Psychiatry and the Generation R Study.
t.white@erasmusmc.nl
PEDIATRIC POPULATION NEUROIMAGING
TONYA WHITE, MD, PHD
associate professor

Top Publications 2021
Alemany S, Blok E, Jansen PR, Muetzel RL, White T (2021) Brain morphology, autistic traits, and polygenic risk for autism: A population-based neuroimaging study. Autism Research 14(10): 2085-2099.
Steegers C, Blok E, Lamballais S, Jaddoe VVW, Bernardoni F, Vernooij M, van der Ende J, Hillegers M, Micali N, Ehrlich S, Jansen P, Dieleman G, White T (2021) The association between body mass index and brain morphology in children: a population-based study. Brain Structure and Function 226(3): 787-800.
Lamballais S, Jansen PR, Labrecque JA, Ikram MA, White T (2021) Genetic scores for adult subcortical volumes associate with subcortical volumes during infancy and childhood. Hum Brain Mapp 42(6): 15831593.
Context
Pediatric population neuroimaging lies at the interface between the disciplines of child & adolescent psychiatry, radiology, paediatrics, and epidemiology. While there are challenges in being at the interface of four different disciplines, there are also tremendous opportunities to address specific questions that lie at this interface, including the translation of neuroimaging findings into clinical practice or public health messages. My group works as a team focusing on the different research domains listed below.
Research Projects: Objectives & Achievements
Effects of prenatal and early life exposures on brain development
Prenatal life is a period with the greatest growth and development of the brain. The brain develops from a single cell shortly after conception to a brain that resembles in many respects an adult brain at the time of birth, albeit about a third of the size. Thus, it is very possible that influences during prenatal life could have global effects on brain development. My group is currently exploring the role of prenatal exposures and downstream effects on brain development due to many different exposures, including low maternal folate, maternal immune activation (in collaboration with Veerle Bergink at Mt. Sinai Medical Center) (Figure 1), and environmental exposures such as phthalates and bisphenols (in collaboration with Akhgar Ghassabian at NYU). In addition,my group is exploring environmental-wide factors that can contribute to emerging psychopathology.

Figure 1. Results of a mediation analysis in which the exposure is ‘maternal immune activation during pregnancy’, the mediator ‘gestational age at birth’ and the outcome ‘child cerebellar volume’. Figure A represents the natural direct effect (NDE) and natural indirect effect (NIE) for continuous C-reactive protein (CRP). Figure B represents the NDE and NIE for categorical CRP (>10 mg/L compared to the reference group <1 mg/L).
Psychopathology along a continuum
The traditional view of psychopathology has been one of dichotomous disorders (health versus illness). However, there has been a shift so as to include not only a categorical approaches (i.e., diagnosis versus no diagnosis), but also a dimensional approach to psychiatric symptoms (i.e., continuum of symptoms within the population). This can be seen by the adoption of the term ‘autism spec-
trum disorder,’ which reflects that autistic symptoms can be found along a continuum within the population. If the symptoms can be found along a continuum, then it would be reasonable to assume that the neurobiology underlying the symptoms may also lie along such a continuum. Large population-based studies are well suited to address psychopathology along a continuum, as they contain not only children with clinical diagnoses, but also children with subclinical and minor symptoms. Thus, one major goal of my group is to evaluate whether the underlying neurobiology of psychiatric symptoms are related to the clinical phenotype across the continuum within the pediatric population.

Figure 2. Differences in patterns of brain gyrification in children with higher levels of autistic traits within the general pediatric population. The blue regions have a more minimally adjusted model (age and sex), whereas the purple regions show differences after additionally adjusting for multiple covariates or confounders [Alemany et al. 2021 Autism Research].
Emerging psychopathology
An aim within my group is to evaluate the neurobiology involved in emerging severe psychopathology. There are still many unanswered questions as to the premorbid neurodevelopmental trajectories of children who later develop severe psychopathology. Questions such as:
• Are there changes in the brain that can be seen even before the clinical symptoms present?
• Are brain changes prenent very early and become ‘unmasked’ with later neurodevelopment?
• Or alternatively, does neurodevelopment follow the same pattern of typically developing children, with at some point a deviation in the trajectory at the same time as the illness begins?
• Yet an additional alternative is whether the persistence in specific behavior result in changes in the brain, so that the gross differences that are seen on MRI actually emerge in the brain due to downstream effects of persistent behavior.
Since most studies evaluate children during the prodromal phase or after the onset of their disorder, there is little information regarding the neurodevelopmental trajectories leading up to the disorder. Large populationbases studies provide an optimal source to obtain neuroimaging data prior to the onset of illness in order to address the pre-morbid status of the brain.
Imaging Genetics
Both brain development and emerging psychopathology are largely driven by genetic factors, thus the combination of genetic and neuroimaging research is a natural extension to better understand the brain in illness and in health. In this regard, my group works together with consortium members, such as the ENIGMA Consortium, to evaluate the role of genes in neurodevelopment. We also explore the underlying neurobiology, behavior, and cognition in children who are genetically at-risk. Since many of the major psychiatric disorders are thought to be a caused at least partially by a combination of multiple genes, we have used polygenic risk scores to identify children at-risk (or alliteratively, ‘at-protection’) for psychopathology or behavioral or cognitive traits.
Typical Brain Development / Methodologies
An understanding of deviations in neurodevelopment and neurodevelopmental trajectories associated with psychiatric disorders can only be understood in the context of typical brain development. Thus, within my group we are also evaluating typical structural and functional development of the brain, such as brain differences related to variations in body mass index in the general pediatric population (Figures 3 & 4). We are currently working to develop growth curves of brain development that hopefully will be able to eventually be used in clinical neuroradiological settings. The growth curve models that we apply are those that are also used by the World Health Organization to develop growth curves of height, weight, and head circumference. In addition, to address specific neurodevelopmental questions, we also need at times to also develop new tools to focus on specific questions. Thus, novel image processing methods to address specific neurodevelopmental questions are also a goal within my group. Tools to automatically evaluate the quality of structural images have been recently developed and we found that even small amounts of movement in participants can affect the measurements of specific aspects of cortical morphology. Finally, I have been very interested of late as to the role of stochastic processes in brain development, which offers a level of neurodiversity that may be beneficial in the context of evolutionary biology.

Figure 3. Within the diversity of body mass index in the general population, we found an inverted-U shaped relationship between body mass index (BMI-SDS) and gyrification patterns as measured by the gyrification index (GI) in the general paediatric population [Steegers et al. 2021 Brain Struct Funct].

Figure 4. The relationship between BMI and gyrification evaluating either those with a high BMI (overweight) or a low BMI (possible anorexia-nervosa). The different models reflect different covariates/confounders added to the model. [Steegers et al. 2021 Brain Struct Funct].
Expectations & Directions
In my leading the neuroimaging component of the Generation R Study, our goal is to continue to integrate with the Generation R Study to scan as many of the now late adolescent and young adults as possible. We completed the third wave of scanning which makes our sample the worlds-largest single site neuroimaging study in children. We have started data collection on the fourth wave of 17 to 20 year old youth, which has been delayed and slow to start due to the SARS-Covid-19 pandemic. Our goals for the coming year are to maintain our reputation for producing high quality research in the areas described above. In addition, given the challenges associated with reproducibility in neuroimaging studies, coupled with the large sample size of school age children, we plan to design methods to embed reproducibility into our image processing and statistical analyses.
Highlights
Serves on the Scientific Advisory Board for the Bergen Center for Brain Plasticity in Bergen Norway: Providing recommendations regarding research directed towards their four day treatment program for obsessive compulsive disorder.
Editor-in-Chief of Aperture Neuro: The Journal of the Organization of Human Brain Mapping.
Serving as an official mentor for a Young Investigator Brain and Behavior Research Award awarded to Akhgar Ghassabian by the Brain and Behavior Research Foundation.
Lectures
Invited Presentation, International Academic Exchange Center, Northeast University: December 2020, Virtual Presentation Shenyang, China: The Challenges of Data Sharing in Population-Based Research.
Invited Presentation, National Institutes of Mental Health: November 2020, Virtual Presentation: Bethesda, MD, USA: Pediatric population neuroimaging: Prenatal, Early-life, and childhood studies of Autism Spectrum Disorder… Coloring outside the lines.
Funding
National Institutes of Environmental Health Sciences (NIEHS): R01 ES031069-01A1 (Site PI): Total Grant: $ 2,210,576 Erasmus MC amount $ 619,443. September 17, 2021 – June 30, 2026. Project title: “Brain influences of phthalates and bisphenols in adolescence.”
Sophia Children’s Hospital Research Foundation: #S2265 (Co-Principle Investigator with Gwen Dieleman): € 300,000. January 2022 – December 2025. Project title: “Extracting the Anorexia Brain Network Cascade underlying the distorted body image and fear of weight gain in adolescent girls with anorexia nervosa.”
National Institutes of Mental Health: R01 R01MH124776 (Site PI): $ 685,792. September 1, 2021 – August 31, 2026. Project title: “The impact of prenatal maternal infection and inflammation on human brain development and psychopathology during adolescence.”

BRAVE: ‘BRAIN FUNCTIONING AND ATTENTIONAL PROCESSING IN ADOLESCENT ANOREXIA NERVOSA: PREDICTORS OF ITS DIFFERENTIAL COURSE?’
KATRIEN BRACKÉ, MSC, MD
PhD Student
Advisors Gwen Dieleman, Tonya White & Meike Vernooij
Project Funding SSWO
Research period September 2020 – August 2024
Email k.bracke@erasmusmc.nl
This project is a collaboration between the Departments of Radiology & Nuclear Medicine and Child- and Adolescent Psychiatry.
The BRAVE study is a longitudinal cohort study in female adolescent patients with first-onset anorexia nervosa (AN), with a repeated measures design. We investigate brain functioning and attentional processing in a clinical case-control sample of adolescents with first-onset AN (N=79) compared to gender-, age- and education-matched healthy controls (n=75).
Within this framework the primary aim of the project is to identify predictors of one-year treatment response after initial clinical diagnosis in a large sample of adolescent girls with AN.
We focus on the following four areas of research: (1) neurobiological processes, (2) neuropsychological functions, (3) behavior, and (4) physical health. Second, we investigate the association between clinically significant changes and neurobiological, cognitive and attentional changes during one-year treatment.
The main purposes of the BRAVE study are:
1. To investigate the differences in global and local brain structures between adolescents with first-onset AN compared to healthy controls. Furthermore we aim to investigate if there is an association between these brain volumetrics and AN symptomatology.
2. To investigate the prognostic utility of structural MRI measures on the clinical outcome in patients with AN.
3. To assess brain resting state connectivity in adolescent females with first-onset AN in comparison with healthy controls.
4. To develop clinically relevant growth charts for global and regional brain regions in adolescents with AN, compared to population-based normative growth charts of brain development.

BRIDGE(ING THE) GAP STUDY
MARJOLEIN HG DREMMEN, MD
PhD Student
Advisors Tonya White & Meike Vernooij
Project Funding
Research period January 2018 – Present
Email m.dremmen@erasmusmc.nl
This project is a collaboration between the Erasmus MC Departments of Radiology & Nuclear Medicine, Child and Adolescent Psychiatry, Neurology, and Pediatrics, and the Generation R Study.
BRain development, Imaging trajectories and Deviations in brain morpholoGy in the pEdiatric population; BRIDGing thE gap (BRIDGE gap study)
Reference growth curves are an established concept in many fields of child development. This is extremely beneficial for in depth understanding of disease, treatment, and follow-up in children. However, there currently are no growth charts that can be applied to assess trajectories of neurodevelopment. The Generation R Study has one of the largest population-based samples of children with over 7000 neuroimaging scans of children and adolescents. This is a unique opportunity to test the utility of the comparison of clinical scans with population-based derived normative data.
Within this framework, the primary goal of this project is to develop clinically relevant growth curves from structural MR brain scans for global and regional brain regions in children and adolescents. In addition, we will replicate and test the robustness of these curves by applying our growth models to large, freely available MR pediatric cohorts. To test the applicability of these growth curves in a clinical setting we will use these charts to assess deviations in neurodevelopment in different patient groups.
The second goal is to study variations in brain development to help determine whether a neuroradiological finding is a relevant abnormality in brain development or not.
The Generation R Study cohort will provide a bridge to the application of both reference growth curves and prediction models in pediatric neuroradiology. The main aim of this cohort is to study normal and abnormal development and health of individuals from childhood until young adulthood.
Fig 1. An example of a reference growth curve for age and length of a child. The aim is to develop similar neurodevelopment curves of global and regional brain regions.

The desirable outcome of this research project is:To construct reference growth curves for total and regional brain volumes in children and adolescents. Furthermore, to focus on differences in gender and ethnicity to bridge the gap to understand the diversity of typical and atypical brain development.
- To be able to detect early deviations in brain development in specific patients groups. Age-related changes in brain morphology are crucial to better understanding the neurobiology of these clinical conditions and eventually bridge the gap to possible therapeutic targets.
- To gain knowledge on variations in brain development and evaluate the consequences of incidental findings to bridge the gap between widely accepted consensus and factual evidence-based medicine.

Advisors
Project Funding NIH R01
PhD Student MATERNAL IMMUNE ACTIVATION
Tonya White & Veerle Bergink
Research period September 2021 – present
Email a.suleri@erasmusmc.nl
ANNA SULERI, BSC
This project is a collaboration between the Departments of Radiology, Child & Adolescent Psychiatry and Psychology, and Generation R.
One of the major environmental factors that has been implicated in the pathogenesis of neurodevelopmental disorders is maternal immune activation. Using Generation R, an ongoing large population-based cohort with a follow-up of 16 years, we will investigate the effect of maternal immune activation on the development of psychopathology. Moreover, we will use neuroimaging data to explore potential biological mechanisms underlying the effects.
Now more than ever, understanding these mechanisms is of utmost importance. With the global COVID-19 pandemic affecting millions of people worldwide, pregnant women are at increased risk of severe infection and inflammation, with a potential long-term impact on the health of their offspring. In the proposed study, we aim to bridge this gap in knowledge by using preexisting bio samples and data from mother-child dyads in the Generation R cohort. We will measure inflammatory markers in early and mid-pregnancy in available serum samples of mothers.
Using already collected longitudinal neuroimaging and clinical data of children at age 14 years, will allow us to deepen our understanding of the mechanisms involved. Generation R is a large prospective birth cohort from Rotterdam, The Netherlands, with detailed information starting in the prenatal period, and a follow-up period ranging into adolescence. Rotterdam is the second largest city in the Netherlands.
Aim 1 is to assess maternal immune activation and its association with fetal growth and preterm birth. Aim 2 is to investigate the impact of maternal immune activation on brain structure and connectivity at age 14 years. Aim 3 is to investigate the impact of maternal immune activation on

Fig 1. An example of a reference growth curve for age and length of a child. The aim is to develop similar neurodevelopment curves of global and regional brain regions.
executive function, behavior, and psychopathology at age 14 years. See Figure 1 for an visualization of the aims.
In the past months we have investigated the effect of elevated levels of C-reactive protein during the first eighteen weeks of gestation on child brain morphology. In this project we found a relationship between elevated levels of C-reactive protein and reduced cerebellar volume in the whole sample, and reduced total brain volume in specifically girls.
Moreover, we have assessed the direct effect of prenatal maternal infection on psychopathology at age 14 and the indirect effect via multiple maternal, perinatal and child factors. Here, we observed strong direct effects of prenatal maternal infection on both internalizing and externalizing symptoms of psychopathology. Moreover, we found a moderating role of maternal intoxications, breast feeding and child body mass index. We observed no mediating effect of the placenta.


INPUT & OUTPUT
GRANTS
Personal Grants/ Fellowships
Adams, Hieab: VENI Grant 2018-2022: “SOBIG: Spatial Overlap Between Imaging and Genetics”
Adams, Hieab, Elizabeth Loehrer: Leading Fellowship grant 2019-2021: POPSICLE: Population-based Stem Cell Induction for Complex Diseases and Large-scale Experimentation
Adams, Hieab: Erasmus MC Fellowship 2020-2024: “The Uncovering Neurodegenerative Insight Through Ethnic Diversity Consortium“
Booij, Ronald: Academy Van Leersum grant 2021-2022: “Clinical application of photon counting CT: a quantum leap in medical imaging”
Brabander, Tessa: KWF Young Investigator Grant (2022-2026): Salvage therapy with 225Ac-DOTATATE for patients with metastatic neuroendocrine tumors
Bron, Esther: Young eScientist Award 20182020: “TADPOLE-SHARE: SHaring TADPOLE’s Algorithms for Reuse and Evaluation”
Bruijne, Marleen de: NWO VICI 2019-2024: “Learning imaging biomarkers: Machine learning techniques for data-driven disease prediction”
Bruijne, Marleen de: NWO ASPASIA 2020
Ciet, Pierluigi: VENI Grant 2020-2023: “Effectieve monitoring van interstitiële longziekte (ILD) met beeldvorming: de M-ILD-studie”
Chen, Shuai: China Scholarship Council (CSC) PhD scholarship 2017-2021: “Machine learning in medical image analysis”
Dalm, Simone: VENI ZonMw 2019 – 2022: “Better understanding leads to better decisions: Evaluating the effect of anti-hormone therapy and chemotherapy on GRPR-targeting”
Es, Ad van: CONTRAST Young Talent Program 2019-2020: Detecting Incomplete Microvascular Reperfusion in Clinical Practice
Evans, Tavia: Alzheimer Nederland 20212023: “Investigating Geographical Variation in Brain Aging and Neurodegeneration”
Evans, Tavia: NWO Women in Science 20212023: “Neurodegenerative disease variation across ethnicity - A detailed neuroimaging investigation”
Van der Heijden, Rianne: Young Reseachers Grant 2018-2021, the European Society of Musculoskeletal Radiology (ESSR) 2018: “Shedding light on infrapatellar fat pad signal abnormalities and blood perfusion using quantitative dynamic contrast enhanced MR”
Krijkamp, Eline: SMDM fellowship for young investigators. Gordon and Betty Moore Foundation 2019-2022
Neitzel, Julia: Global Marie Curie Fellowship (2021-2024): DIVERT-AD
Niessen, Wiro: Technology Foundation ‘STW’ – Simon Stevin Master Award 2015-2022: “Medical image analysis”
Nonnekens, Julie: Erasmus MC Fellowship 2020-2024: “RADIANT: cellular RADIAtion exposure effects of molecular radioNuclide Therapies”
Nonnekens, Julie : KWF Young Investigator Grant 4-year project 2019-2023. “ A radiant future: Improving targeted radionuclide therapy through modulation of DNA damage in the tumor ”
Nonnekens, Julie : ERC starting grant 2021 2022-2027: ‘RADIOBIO: Deciphering the radiobiology of targeted radionuclide therapy: from subcellular to intra-tumoural analyses’.
Roozenbeek, Bob : EUR fellowship 20192023: “Reperfusion of the brain after endovascular thrombectomy for ischemic stroke: development of a prognostic framework using clinical and neuroimaging characteristics - the REPERFUSE study”
Veldhuijzen van Zanten, Sophie : Personal Grant 2019-2023: “Intra-arterial administration of radiolabeled (chemo-) therapeutics for (diffuse intrinsic pontine) glioma, monitored by PET-MRI“
Veldhuijzen van Zanten, Sophie : Young Scientific Talent Award 2022-2026: “Intraarterial [177Lu]Lu- or [225Ac]Ac-PSMA for recurrent / progressive malignant glioma”
Veldt, Astrid van der : Erasmus MC fellowship 2019-2022: “Reducing toxicity and improving outcomes in immunotherapy treated melanoma patients”
Veldt, Astrid van der : KWF Young Investigator Grant Bas Mulder Award 2019-2025: “Safe Stop-QoL: impact of early discontinuation of PD-1 blockade on quality of life (QoL) of patients with advanced melanoma”
Warnert, Esther : VENI grant 2019-2021: Food for thought: Oxygen delivery to the brain
Dalm, Simone: KWF Young Investigator Grant/Bas Mulder Award 2018 – 2022: “Click on Target: Developing a safe drug with enhanced therapeutic potential for prostate cancer treatment”
International Grants
Bagdi Ulas, Pensky, Wallace, Bolan, Gonda, Hecht, Bruno Marco, Ivo Schoots : NIH grant 2021 - 2024: Deep learning methods for characterization of pancreatic cysts (Cyst-X project)
Bos, Daniel (collaborator) and consortium partners: World Cancer Research Fund: “Coffee, coffee metabolites, hepatic fat accumulation and colorectal cancer outcomes.”
Chan, Alan (Percuros), Lowik, Clemens and L. Mezzanotte and consortium partners : H2020-MSCA-RISE 2018-2022: CANCER: Immunotherapy approaches to improving cancer outcome and quality of life
Chan, Alan (Percuros), Lowik, Clemens, Mezzanotte, Laura and consortium partners : H2020 Marie Curie ITN. PAVE 20192022: A nanovaccine Approach for the treatment of Pancreatic Cancer
Chan, Alan (Percuros), Mezzanotte, Laura and consortium partners: Marie Curie EU-RISE-PRISAR2, 2019-2024: proactive monitoring of cancer as an alternative to surgery
Condorelli, Gerolama (University of Naples), Lowik, Clemens, Mezzanotte, Laura and consortium partners : H2020 Marie Curie ITN. CONCRETE 2019-2023: Development of Cancer RNA Therapeutics
Elborn, Stuart (QUB), Harm Tiddens , Marleen de Bruijne and consortium partners: Innovative Medicines Initiative (IMI) Grant 2016-2022: “iABC – Inhaled antibiotics in bronchiectasis and cystic fibrosis”
Ferket, Bart (PI), Kirsten Fleischmann, Umesh Masharani, Wendy Max, Myriam Hunink . Novel antidiabetic medications to reduce cardiovascular events in patients with diabetes mellitus type 2 – a modelling
study. RO1, National Institutes of Health, USA, 2021-2024.
Kroencke, Thomas, Myriam Hunink: German Innovation Fund 2019-2022: Medical Imaging Decision and Support (MIDAS)
Lekadir, Karim (University of Barcelona), Aad van der Lugt, Wiro Niessen, Stefan Klein, Daniel Bos and consortium partners: H2020 2018-2022: “An EU-Canada joint infrastructure for next-generation multi-Study Heart research (euCanSHare)”
Levecq, Xavier (Imagine Eyes), Theo van Walsum, Stefan Klein, Wiro Niessen and consortium partners: H2020 Ecsel program IA Grant 2018-2021: MERLIN: Multi-modal, multi-scale retinal imaging
Lowik C. and L. Mezzanotte. H2020-MSCARISE: CONCRETE: Improvement of RNA therapeutics. 2020-2024. 12 month mobility grant for Lowik and 12 months for Mezzanotte.
Lowik C. and L. Mezzanotte. H2020-MSCAITN-2019-PAVE: A nanovaccine Approach for the treatment of Pancreatic Cancer. 20202024.
Majoie, Charles (Amsterdam University Medical Center), Aad van der Lugt, Diederik Dippel, Hester Lingsma and consortium partners: European Union Research & Innovation Action Project 2017-2021: “INSIST: IN-Silico trials for treatment of acute Ischemic Stroke”.
Mezzanotte L. and partners: Marie Curie EU-RISE-PRISAR2- 12 month mobility grant, 2020-2024.
Oei, Edwin (co-applicant): National Health and Medical Research Council 2018-2022, Australia: “SUPER rehabilitation RCT for young people with old knees”. Main applicant: K. Crossley (La Trobe, Melbourne, Australia)
Oei, Edwin (co-applicant): Horizon EIC Accelerator 2020-2023: “AI algorithms in musculoskeletal radiography”. Main applicant: Radiobotics, Copenhagen, Denmark
Oei, Edwin (co-applicant): Independent Research Fund Denmark 2019-2023: “Investigating pathology and tissue biomarkers of Osgood Schlatter to enhance treatment of children with growth-related pain”. Main applicant: M.Rathleff, University of Aalborg, Denmark
Perrio, Cécile (University of Caen) and Yann Seimbille: Joint PhD Program – Grant 20182021: “Bioorthogonal radioactive probes for in vivo imaging and therapy of tumors”
Sijbers, Jan (University Antwerpen), Wiro Niessen, Dirk Poot, Stefan Klein and consortium partners: EU Horizon2020 MSCA-ITN, 2018-2022: “B-Q MINDED - Breakthroughs in Quantitative Magnetic resonance ImagiNg for improved DEtection of brain Diseases”.
Schwab, Albert (University of Munster), Clemens Lowik and consortium partners: H2020-MSCA-ITN 2018-2021: pHioniC: pH and Ion Transport in Pancreatic Cancer.
Tiddens, Harm: European Cystic Fibrosis Society 2020-2022: Standardized ECFS-CTN Chest Imaging Framework for Intervention and personalized medicine for CF: a follow up study. (SCIFI-2 study). CT standardization of 58 ECFS-CTN sites.
Veldhuijzen van Zanten, Sophie and consortium partners: European DIPG Network – Maintenance Grant 2018-2022: “European registry for diffuse intrinsic pontine glioma: the SIOPE DIPG Registry”
Walsum, Theo van, Wiro Niessen, Stefan Klein and consortium partners: H2020 Project Merlin 2018-2021
Warnert, Esther, Radim Jancalek, Lydiane Hirschler, Camille Maumet, Jan Petr, Marion Smits, Patricia Clement, Yelda Özsunar Dayanir: EU COST 2019-2023: “Glioma MR Imaging 2.0: GLiMR2.0.”
Wendelboe Nielsen, Olav (University of Copenhagen), Marleen de Bruijne and consortium partners: RegionH 2019-2022: “BreathCT: Diagnosing Patients Admitted with Breathlessness - Development and Validation of Machine Learning Algorithms based on Images from Computed Tomography”
White, Tonya, Henning Tiemeier, and Benjamin Lahey: National Institutes of Drug Abuse (NIDA) 2017-2021, USA: “Early exposures and neurodevelopmental, behavioral, and health outcomes.”
White, Tonya: National Institutes of Mental Health 2021-2026: “The impact of prenatal maternal infection and inflammation on human brain development and psychopathology during adolescence.”
White, Tonya and consortium partners: National Institutes of Environmental Health Sciences (NIEHS) 2021 - 2026: “Brain influences of phthalates and bisphenols in adolescence.”
Wiesinger, Florian (GE), Steven Petit, Hernández-Tamames Juan A and consortium partners: EU EIT Health 2019-2022: “Deep MR-Only Radiotherapy”
National Grants
Abo Saeda, Samy , Anke van der Eerden, Agnita Bonn, Juan A. Hernandez-Tamames: Dutch Parkinson Vereniging 2021-2023: Advanced MR Protocol for Parkinsonisms
Adams, Hieab, Raymond Poot, Bas van Steensel: NWO Open Competition 20212025: “Identifying causal genetic variants for a better understanding and diagnosis of neurodevelopmental disorders"
Adams, Hieab: NWO Rekentijd 2019-2021: “The Uncovering Neurodegenerative Insight Through Ethnic Diversity Consortium”
Van den Bergh, Roderick, Rik Somford R (Urology), Ivo Schoots: SKMS project/ZonMW 2022 – 2025: Evaluatie en optimalisatie diagnostisch traject prostaatkanker middels MRI
Bierma-Zeinstra, S.M.A., Edwin Oei: ZonMw Gender en Gezondheid - Algemene onderzoeksronde 2018-2022: “IFEROA: Identification of the female specific etiology and risk groups for osteoarthritis”
Budde, Ricardo, Alexander Hirsch, Myriam Hunink and consortium partners: Veelbelovende zorg ZonMW 2020-2025: “Addition of
FFRct in the diagnostic pathway of patients with stable chest pain to reduce unnecessary invasive coronaryangiography (FUSION Study)” to evaluate the role fo FFRct in stable chest pain patients”.
Diest, Prof. Dr. P.J. van, Astrid van der Veldt: ZonMW grant 2019-2022: “Predicting metastastic melanoma to immunotherapy with radiomics and pathomics”
Dobbelsteen, John van den (TU-Delft), Adriaan Moelker: NWO-TTW 2018-2022: “Steerable needle for percutaneous interventions”
Ginneken, Bram van, Marleen de Bruijne and consortium partners: NWO-STW Perspectief Programme grant 2016—2023: DLMedIA: Deep Learning for Medical Image Analysis
Hunink, Myriam: Higher Education Quality and Innovation Agenda (“Studie Voorschot Middelen”) 2019 – 2022: Decreasing stress through resilience training for students (DESTRESS)
Ikram, Arfan, and consortium partners: ZonMW – Memorabel 2018-2022: Netherlands Consortium of Dementia Cohorts
Kanaar, Roland, Julie Nonnekens, Hans Hofland, Ferry Eskens, Wouter de Herder, Tessa Brabander, Astrid van der Veldt, Mark Konijnenberg, Stijn Koolen: Oncode clinical proof of concept study 2021-2024: “Improving Peptide Receptor Radionuclide Therapy with PARP inhibitors: the PRRTPARPi study”
M. Karpeirin, L.Moreira Teicaira, L. Mezzanotte and C. Lowik: Health–Holland-TKI 2020-2024: OA-Biodetects-CHIPs-Towards osteoarthritis fingerprinting – combining imaging biomarkers and multi-organ-on-chip technology for improved in vitro models.
Lambin, Philippe (Maastro), Wiro Niessen, Stefan Klein, Jifke Veenland and Ivo Schoots, and consortium partners: Technology Foundation ‘STW’, 2016-2021: “Radiomics STRaTegy- Non-invasive stratification of tissue heterogeneity for personalized medicine”
Lugt, Aad van der, Wiro Niessen and consortium partners: CVON project CONTRAST 2017-2022
Lowik, Clemens, Laura Mezzanotte, J.Essers, G.Van Soest: NWO MIddelgroot 2018-2021: In Vivo Optoacoustic Molecular Imaging for applied cancer; aging and cardiovascular research.
Niessen, Wiro, Ivo Schoots, Jifke Veenland, Chris Bangma (Urology): Erasmus MC-TKILSH 2020 – 2023: Personalized Prostate Cancer Management using Multi-parametric MRI and Machine Learning (PPCM4)
Niessen, Wiro, Daniel Bos and consortium partners: NWO Big Data Grant, 2019-2023: “MyDigiTwin: Your Digital Twin to improve early detection and promote risk self-management of cardiovascular disease.”
Nieuwenhuyzen-de Boer, Gatske, Heleen Beekhuizen, Ivo Schoots, Wart Hofhuis, Beltman (Gyneacology): ZonMW Health Care Efficiency Research Grant 2017-2021: “Evaluation of effectiveness of the PlasmaJet Surgical device in the treatment of Advanced Stage Ovarian Cancer: A randomized controlled trial in The Netherlands (PlaComOv study)”
Nonnekens, Julie: Daniel den Hoed Foundation Fellowship 2017-2021: “Towards personalized radionuclide therapy”.
Oudkerk, Vliegenthart, Marleen de Bruijne and consortium partners: ZonMW Innovative Medical Devices Initiative - Technology for Sustainable Healthcare 2018—2023: “B3CARE”
Oei, Edwin (co-applicant): ZonMw Gender en Gezondheid - Algemene onderzoeksronde 2019-2021: “The FOCUM human disease model for development of OA”. Main applicant: S.M.A. Bierma-Zeinstra (General Practice)
Oei, Edwin (co-applicant): ZonMw Gender en Gezondheid - Algemene onderzoeksronde 2020-2026: “Diagnosis, prevalence and associated factors of osteoarthritis in adults with intellectual disabilities.” Main applicant: D.A.M. Maes-Festen (AVG/General Practice)
Oei, Edwin (co-applicant): NWO Zon-MW Open Competition 2020-2025: “Biomechanical precision diagnostics in osteoarthritis.”. Main applicant: S. Bierma-Zeinstra (General Practice/Orthopedics)
Seimbille, Yann, Mark Konijnenberg, Marion de Jong: Kansen voor West 20192023: “FIELD-LAB: Advancing Nuclear Medicine”
Schoots, Ivo , Monique Roobol, Gabriel Krestin, Chris Bangma (Urology): ZonMW Health Care Efficiency Research Grant 2017-2021: “Risk Stratification and MRI in addition to Standard Prostate Cancer Detection: An Impact Analysis”
Smits, Marion, Anouk van der Hoorn, Jan Willem Dankbaar, Dieta Brandsma, Bas Jasperse, Linda Dirven, Filip de Vos, Myriam Hunink : ZonMW Leading the Change 2018-2021: “The clinical value of perfusion MRI in primary and secondary brain tumour surveillance”
Smits, Marion, Thijs van Osch, Dirk Poot, Stefan Klein, Juan Antonio Hernandez-Tamames : NWO-TTW Open Technology Programme 2019-2024: “Vascular Signature Mapping of Brain Tumor Genotypes.”
Smits, Marion : NWO Hestia impulse for refugees in science 2021-2023: “The Sound of flow: High-resolution brain tumour vascular signature mapping with mutually informed MRI and intra-operative microDoppler ultrasound”.
Soest, G.Van, J.Essers, C. Lowik. : LSH-TKI PPP allowance project 2020-2022: “PICA-Heart” concerning photo-acoustic imaging with contrast agents in heart disease
van der Veldt, Astrid and VOICE consortium: ZonMW grant 2021-2023: “VOICE trial: Vaccination against cOvid In CancEr”
van der Veldt, Astrid and VOICE consortium: ZonMW grant 2021-2023: “Third vaccination VOICE trial”
Vernooij, Meike, Arfan Ikram , Danielle van Assema, Roelf Valkema, Kamran Ikram: ZonMW Memorabel grant, 2017-2022: “Amyloid pathology and vascular disease in fo-
cus: exploring interaction in two pathways towards neurodegeneration”
Vernooij, Meike, Frank Wolters, Arfan Ikram: A public-private partnership receiving funding from ZonMW and Health Holland (20212025): A Personalized Medicine Approach for Alzheimer’s Disease (ABOARD)
Walsum, Theo van, Ad van Es, Danny Ruijters (Philips Healthcare): Health Holland TKI Call, 2019-2023: Q-Maestro: Quantitative Microvasculature AssEssment in projection angiography of ischemis STROke patients
Wijminga, Cisca (UMCG), Aad van der Lugt, Wiro Niessen, Gerrit Meijer (NKI), Leon Kenemans (UU), and Gert-Jan van Ommen (LUMC): Netherlands Organization for Scientific Research (NWO) – National Roadmap for LargeScale Research Facilities 2015-2021: “BBMRI-NL2.0: NL-Biobank Research Facility”
Niessen, Wiro, Gerrit Meijer, Leone Flikweert, Ruben Kok, Health-RI (national) innovation fund grant for health data infrastructure
Zee, Eddy van der, Martien Kas, Meike Vernooij, Arfan Ikram, Henning Tiemeier, Rene Melis, Myrra Vernooij-Dassen, Marieke Perry: ZonMW Memorabel grant, 2017-2021: “Social factors in cognitive decline and dementia: towards an early intervention approach”
Charitable Organizations
Adams, Hieab, Rick van der Vliet, Sirwan Darweesh & Bas Bloem: Parkinson Foundation & Parkinson Vereniging: “ABCD-Parkinson: A Biomarker based on Circulating cell-free DNA for Parkinson’s disease”
Bos, Daniel: Alzheimer’s Association Research Grant: “Trajectories of Vascular Disease in Aging to Predict Dementia.”
Bos, Daniel, Meike Vernooij, Frank Wolters, Julia Neitzel, Geert-Jan Biessels: Cure Alzheimer Fund: “Intracranial Arteriosclerosis and Alzheimer’s pathology.”
Brabander, Tessa, Astrid van der Veldt: KWF grant (2019-2022) “Phase I dose escalation study to evaluate tolerability and safety of
225Ac-PSMA in patients with metastatic prostate cancer”
Bron, EE, WJ Niessen, J Glastra, WM van der Fier, GJ BiesselsL: KWF PPS grant 2018-2021: “Improvas: Improved Prognosis of Vascular cognitive impairment using automatic quantitative imaging biomarker extraction and disease modelling”
Daemen, Mat (AUMC), GJ Biessels (UMCU), WJ Niessen, EE Bron and consortium partners: CardioVasculair Onderzoek Nederland (CVON) 2019-2024: “HBCx: Heart-Brain Connection Crossroads”
Dalm, Simone: KWF Young Investigator’s Award 2018-2022: “Click on Target: Developing a Safe Drug with Enhanced Therapeutic Potential for Prostate Cancer Treatment”
Dippel, Diederik, Charles Majoie (AMC), Aad van der Lugt and consortium partners: Dutch Heart Foundation/Netherlands Brain Foundation 2017-2022: “CONTRAST: Collaboration for new treatment of acute stroke”
Dwarkasing, Roy, Francois Willemssen, Rob de Man, Bart Takkenberg, Carine Uyl-De Groot: KWF implementation grant 2021 –2025: Validation of a Short and effective MRI Surveillance protocol for hepatocellular carcinoma screening in practice
Oei, Edwin (project team member): FOREUM Foundation for Research in Rheumatology 2018-2022, Preclinical Phases of Rheumatic and Musculoskeletal Diseases: “Novel Treatment Targets in Early-stage Osteoarthritis”. Main applicant: M. Englund (Lund University, Sweden)
Hirsch, Alexander: Nederlandse Hartstichting 2021-2022: “Managing cardiovascular disease and risk in Covid-19 patients in primary care medicine: COVID@HEART CMR substudy”.
Hunink, Myriam: American Diabetes Association Grant 2018-2021: Calcium scoring in primary prevention of cardiovascular disease for individuals with diabetes. With Kirsten Fleischmann (PI), Cardiologist at UCSF, and Bart Ferket, Mt Sinai.
Hunink, Myriam, Stijntje Dijk, Eline Krijkamp: Gordon and Betty Moore Foundation, 20202021: Emerging Therapies for COVID-19: the value of more clinical trials vs implementation. SMDM COVID Decision Making Initiative.
Katsikis, P., S.Shoenberger, K. Ishii, C. Schliehe L. Mezzanotte: KWF grant 2019-2024: “Improving Checkpoint Blockade Therapy with Highly Immunogenic Personalized Neoepitope Vaccines”.
Klein, Stefan, Jan-Jaap Visser, Dirk Grunhagen, Kees Verhoef, Stefan Sleijfer, Wiro Niessen, Arno van Leenders, Martijn Starmans: Hanarth Fonds 2021-2025: “Automatic grading and phenotyping of soft-tissue tumors through machine learning to guide personalized cancer treatment”
Lam, Marnix (Utrecht), Adriaan Moelker, Karel van Erpecum (Utrecht), Hugo Jong (Utrecht): KWF Grant 2017-2021: “Holmium-166 microspheres for radioembolization in HCC patient: New generation microspheres for individualized treatment”
Löwik, Clemens, M. Hendriks-de Jong, L. Mezzanotte KWF grant 2018-2021 : “Development of a radio-labelled necrosis-targeted probe for early detection of anti-cancer therapy and anti-cancer treatment: a new theranostic platform”.
Mezzanotte, L., R. Balvers, C. Dirven. KWFDutch Cancer Foundation: First in man assessment of FA-ICG for image guided surgery of Glioblastoma. 2022-2025
Niessen, Wiro, Aad van der Lugt, Marion Smits, Rebecca Steketee, Meike Vernooij and consortium partners: Alzheimer Netherlands TKI 2018-2020: Imaging biomarkers for early diagnosis (BEYOND)
Oei, Edwin (co-applicant): Reumafonds (Dutch Arthritis Foundation) Fundamental Research 2018-2022: “A gut feeling about osteoarthritis: the role of the gut microbiome in osteoarthritic pain and progression”. Main applicant: J.B.J. van Meurs (Internal Medicine)
Oei, Edwin: Dutch Arthritis Association 2016-2021: “Assessment of knee synovitis with novel non-contrast MRI and ultrasound”
Oei, Edwin, Robert-Jan de Vos (Orthopedics): US National Basketball Association (NBA)/GE Healthcare Orthopedics and Sports Medicine Collaboration 2016-2021: “Progressive tendon-loading exercise therapy for patellar tendinopathy in jumping athletes: A randomized controlled clinical trial evaluated with advanced 3D ultrashort echo time MRI”
Oei Edwin: Coolsingel Foundation 20192021: “Embolization therapy as a novel treatment for knee osteoarthritis: a randomized multicenter trial in the Rotterdam region”
Paulides, Maarten (Radiation Oncology), Van Rhoon Gerard (Radiation Oncology), Franckena Martina (Radiation Oncology), Hernández-Tamames Juan A. (Radiology): KWF Grant 2018-2021: “Multi-coil magnetic resonance guided hyperthermia for precision treatment of advanced head and neck carcinoma”
Petit, Steven, Juan Antonio Hernández-Tamames, Aad van der Lugt , et al.: Dutch Cancer Foundation: COMPLETE Project for Holistic Assessment of Oropharingheal Cancer 2019-2023: Oropharynx Cancer
Roos, Yvo B.W.E.M. (AMC), Edwin van der Pol (AMC), Robert Kuipers (Nico-Lab), Henk Leeuwis (LioniX International B.V.), Frank W. Coumans (Exometry B.V.), Anne Yaël Nossent (LUMC), Jan van Esch (Delft University), Aad van der Lugt: Dutch Heart Foundation 20182022: “Circulating Nano Traces to Identify the Cause of Stroke (CINTICS)”
Schoots, Ivo, Jifke Veenland, Wiro Niessen, Chris Bangma, Monique Roobol (Urology), KWF - STW ‘Technology for Oncology’ Grant 2017-2021: ProstatVision: “Visual technology integrating quantitative patient outcomes to support multidisciplinary clinical decision-making.”
Seimbille, Yann, Julie Nonnekens, Marion de Jong: KWF Research Project 2020-2022: “Long-Acting sstr2 antagonists and Pretargeted Alpha Therapy: a Blockbuster Combination for a Safer and more Efficient Treatment of Neuroendocrine Tumors”
Sly, Peter (Brisbane), Steve Stick (Perth), Harm Tiddens and consortium partners:
Cystic Fibrosis Foundation Clinical Research Award 2012-2022: “Multi-center, randomized, placebo-controlled study of azithromycin in the primary prevention of radiologically-defined bronchiectasis in infants with cystic fibrosis”
Smits, Marion, Martin van den Bent (Neurology), and Wiro Niessen: KWF Grant 2016-2021: “Non-invasive phenotyping of molecular brain tumor profiles using novel advanced MR imaging and analysis”
Smits, Marion, Esther Warnert, Safa Al-Sarraj, Keyoumars Ashkan, Gareth Barker , Martin van den Bent, Thomas Booth, Juan Hernadez-Tamames, Johan (Max) Kros, Theo Luider, Joost Schouten, Arnaud Vincent, Tobias Wood: The Brain Tumour Charity 20182021: “Making the invisible visible: In vivo mapping of molecular biomarkers in adult diffuse glioma with CEST MRI”
Terlouw, Luke, Leemreis, Desiree, Moelker, Adriaan, Bruno, Marco: United European Gastroenterology Activity Grant programme 2018: “Clinical guidelines for the management of CMI”
Tiddens, Harm, Kors van der Ent (UMCU): Dutch Cystic Fibrosis Foundation 2017-2022: “Standardized follow up for children with CF diagnosed by newborn screening” as part of the NCFS-HIT-CT II program.
Schoots Ivo, Uulke Van der Heide (Radiation Oncology): KWF clinical implementation grant: 2021 – 2024: PROCESS study: PROstate Cancer - Expansion of Surveillance Selection criteria with MR imaging.
Tiddens, Harm, Stephen Stick (Perth): Cystic Fibrosis Foundation Therapeutics Grant 2016-2021: “Saline hypertonic in preschoolers with cystic fibrosis and lung structure as measured by computed tomography (SHIP-CT study)”
Tiddens, Harm: Stichting Sophia WO Program Grant 2017-2022 “Lung magnetic resonance imaging (MRI) in pediatric lung diseases”
Tiddens, Harm, Eva van Rikxoort, Marleen de Bruijne: Netherlands CF foundation 20192022: “Computer assisted diagnosis for monitoring CF airway Disease”
Tiddens, Harm, Eva van Rikxoort: PPP grant: Computer assisted diagnosis (CAD) for monitoring CF airway disease (the CAD-CAD project)
Veldt, Astrid van der: DDH Award 20192021: “Early detecting and understanding treatment failure in melanoma brain metastases”
van der Veldt, Astrid: DUOS grant 20182022: “Response measurement study in metastatic castration resistant prostate cancer patients to improve early response evaluation and understand radium-223 induced immune response”
van der Veldt, Astrid, Anne-Marie Dingemans: KWF grant 2020: “Dutch Oncology COVID-19 Consortium (DOCC)”
Walsum, Theo van, Wiro Niessen, Aad van der Lugt, Jorrit Glastra: Dutch Heart Foundation PPS call 2018-2020: Accurate: Automatic CTA image analysis to support treatment selection in acute stroke.
Wesseling, Pieter, Johan Kros, Mathilde Kouwenhoven, Marion Smits , Pim French, Mark van der Wiel, Martin van den Bent, Roel Verhaak: KWF grant 2017-2021: “Glioma Longitudinal AnalySiS in the Netherlands: GLASSNL”
Institutional Grants
Bos, Daniel, M. Kamran Ikram, Ali Akyildiz: Erasmus MC MRACE grant for PhD-project 2019-2023: “Dissecting the role of intracranial arteriosclerosis in stroke.”
Dalm, Simone: MRACE Grant for PhD student 2018-2022: “The application of in vivo click-chemistry to improve GRPR-mediated tumor targeting and minimize off-target toxicity”
Dalm, Simone U, Marion de Jong, WM van Weerden, CHM van Deurzen: Erasmus MC Mrace Grant 2018 – 2022: “A “CLICK” towards better and safer radionuclide therapy of prostate cancer”
Denkova, Antonia, Sofia Koustoulidou: Convergence Open Mind Grant 2021: “Advancing cancer treatment with CERN technology”
Hankemeier, Thomas (LUMC/EMC), Eline Slagboom (LUMC), Cornelia van Duijn, Arfan Ikram, Simon Mooijaart (LUMC), Aad van der Lugt: Medical Delta 2018-2023: “Metabolomics for clinical advances in the Medical Delta (METABODELTA)”
Hernández-Tamames, Juan Antonio, Weingartner, de Bruijne, Marleen and consortium partners: ERASMUS MC - TU DELFT Convergence Program 2019-2022: Quantitative Susceptibility MRI: Deep insights in cardio- and neuro-vasculature
Hernández-Tamames Juan A, Tonya White, Meike Vernooij: MRace project 2019-2021: HARPS Harmonization of Erasmus MC Resonantors for Population Studies.
Hirsch, Alexander and Jolien W. Roos-Hesselink: Erasmus MC Thorax foundation 20182021: “Advanced imaging of aortic aneurysms”.
Hofland, Leo, Marion de Jong: Erasmus MC grant 2018-2022: Epigenetic therapy to increase efficacy and optimize patient outcome of peptide receptor radionuclide therapy.
Jong, Marion de, Yann Seimbille, Freek Beekman: Convergence Project with TU Delft 2019-2022: Targeted Radio-molecules for High Precision Cancer Therapy.
Jong, Marion de, Simone U Dalm: Erasmus MC Mrace 2020 – 2024: “Breaking the tumour stroma barrier: A new way to hit cancers using a novel universal targeted radionuclide therapy strategy”
Jong, Nico de, Annemien van den Bosch, Aad van der Lugt: Medical Delta 2018-2023: “Ultrafast Ultrasound for the Heart and Brain (UltraHB)”
Niessen, Wiro, Mark van Buchem, Frans Vos: Medical Delta 3.0 Dementia & Stroke 2018 –2022
Niessen, Wiro, F Vos, MA van Buchem, EE Bron, JHJM de Bresser: Medical Delta 20192024: “Medical Delta Diagnostics 3.0: Dementia and Stroke”
Nonnekens, Julie, Marlies Goorden: Convergence Open mind grant 2021: ‘Scanning
Confocal Nuclear Microscope for improved Radiopharmaceutical Imaging’.
Oei, Edwin: Erasmus MC Efficiency Research 2019-2021: “Novel transcatheter arterial embolization for treatment of knee osteoarthritis: a randomized sham-controlled clinical trial”
Oei, Edwin (co-applicant): TU Delft-Erasmus
MC Convergence Flagship Themes 20202023: “Deep imaging-genetics for osteoarthritis”. Main applicant: S. Klein (Radiology & Nuclear Medicine), M. Loog (TU Delft)
Oei, Edwin (co-applicant): TU Delft-Erasmus MC Convergence Flagship Themes 20202023: “Precision biomechanics diagnostics of cartilage load in knee osteoarthritis.” Main applicant: S. Bierma-Zeinstra (General Practice), J. Harlaar (TU Delft)
Roozenbeek, Bob: MRACE 2019-2022: Regional implementation of a decision support tool for individualized prehospital triage of suspected stroke patients: a cost-effectiveness study.
Roozenbeek, Bob: The Erasmus Initiative “Smarter Choices for Better Health” 20182021: Performance feedback as part of Value-Based Health Care: a randomized evaluation of the effect on quality of stroke care.
Rousian, Melek (Dept. Obstetrics & Gynecology), Stefan Klein, Regine Steegers, Wiro Niessen: Erasmus MC MRace 2018-2022: “Modelling the impact of maternal obesity on longitudinal prenatal human brain development using a 4D spatiotemporal ultrasound atlas”.
Seimbille, Yann, Marion de Jong, Simone Dalm, Mark Konijnenberg, J Essers, F Beekman, M Goorden, A Denkove, K Djanashvili: TU Delft/Erasmus MC Convergence Kick-off Grants: 2019-2022: “Broad spectrum, high precision theranostic cancer therapy”
Seimbille, Yann, Marion de Jong, Carolien van Deurzen and Agnes Jager : Erasmus MC grants: 2020-2024: “Theranostics hitting the Achilles’ heels of breast cancer: pointing the arrows at HER2 and GRPR”
Smits, Marion, Thijs van Osch, Sjoerd Stallinga: Medical Delta 2018-2022: “Cancer Diagnostics 3.0: Big data science of in & ex vivo imaging”
van der Veldt, Astrid: Trustfonds Erasmus 2021-2026: “Genomic landscape and actionable targets as identified by whole genome sequencing in metastases from patients with renal cell carcinoma”
Walsum, Theo van: TU Delft – Erasmus MC Convergence project 2019-2023: Smart Surgical Knife with AR: Combining Smart Knife with Augmented Reality
White, Tonya, Gwen Dieleman: Sophia Children’s Hospital Research Foundation 20222025: : “Extracting the Anorexia Brain Network Cascade underlying the distorted body image and fear of weight gain in adolescent girls with anorexia nervosa.”
Wolvius, Eppo, Wiro Niessen, Theo van Walsum: Convergence project 2019-2023: Erasmus MC Smart Surgery Lab
Investigator initiated industry sponsored grants
Brabander, Tessa , Hans Hofland: Advanced Accelerator Applications Grant 2019-2021: “Expanding the indication of Lutathera”
Dippel, Diederik (Neurology), Aad van der Lugt on behalf of the CONTRAST Consortium: Stryker Inc Clinical Trial Support 2017-2021: “MR CLEAN-No IV: Comparison of direct intra-arterial treatment and intravenous treatment for acute ischemic stroke caused by a proximal intracranial occlusion”
Hernández-Tamames, Juan Antonio , Gyula Kotek: GE Healthcare WorkStatement 20202022: Signal Evolution Transient Imaging II
Krestin, Gabriel, Marcel van Straten : Siemens Medical Solutions Master Research Agreement 2012-2021: “State-of-the-art CT imaging”
Lam, Marnix, Moelker, Adriaan , Tokkel, PMP: Advanced Accelerator Applications (AAA) Stichting Life Sciences Health
(Health-Holland) 2018-2022: “Intra-arterial lutetium-177-dotatate for treatment of patients with neuro-endocrine tumor liver metastases: The LUTIA Study”
Lugt, Aad van der , Diederik Dippel: Thrombolytic Science LLC 2019-2023: DUal thrombolytic therapy with Mutant prourokinase (m-pro-urokinase, HisproUK) and low dose Alteplase for ischemic Stroke.
Mendez Romero, Alejandra, Martin Taphoorn, Martin van den Bent, Marion Smits , Mischa Hoogeman: HollandPTC-Varian 2018-2022: “Improving toxicity modelling, patient selection and clinical outcome of proton therapy in low grade glioma”
Moelker, Adriaan, Edwin Oei : Cook Medical 2018-2021: “Novel transcatheter arterial embolization for the treatment of knee osteoarthritis: A multi-center randomized sham-controlled clinical trial”
Moelker Adriaan, Oei Edwin : Boston-Scientific 2020-2021: “Novel transcatheter arterial embolization for treatment of knee osteoarthritis: a randomized sham-controlled clinical trial”
Oei Edwin: General Electric Healthcare 2020-2024: “Pinpointing the source of chronic pain and therapy response with whole-body 18F FDG-PET/MRI”
Osch, Thijs van, Frans Vos, Marion Smits , Alejandra Mendez Romero: HollandPTCVarian 2018-2022: “New methodology for developing biomarkers of radiationinduced toxicity in brain tumour patients based on advanced MR imaging of the microvasculature and white matter microstructure”
Petit, Steven, Hernández-Tamames Juan A, Aad van der Lugt et al.: Elekta Research Grant 2018-2022: “Oropharynx Cancer”
Sardanelli, Francesco (PI), Inge-Marie Obdeijn and consortium partners : EIBIR-EuroAIM/EUSOBI and Bayer Pharma 2016-2021: Multicenter International Prospective Meta-Analysis (MIPA) of individual woman data (MIPA study)
Tiddens, Harms : Unconditional grant by Vectura: Chest CT and magnetic resonance imaging of small airway disease in severe asthma (TARGET-study)
CONFERENCE CONTRIBUTIONS 2021 (SELECTION)
This section provides a selection of the 2020 conference contributions from the Erasmus MC Department of Radiology & Nuclear Medicine. Given the large number of conferences to which we contribute, this overview is necessarily incomplete, but it gives some idea of the magnitude of our conference participation.
The Netherlands
Leusden 1
Rotterdam 8
Zeist 1
Amsterdam 1
Groningen 1
Italië
Milan 1
Verona 1
Rome 2
UK
Manchester 1
Switserland
Geneva 3
France
Caen 2
Marseille 2
Le Bono 2
Austria
Vienna 1
PUBLICATIONS 2021
External publication partners for the Department of Radiology & Nuclear Medicine, 2017-2021
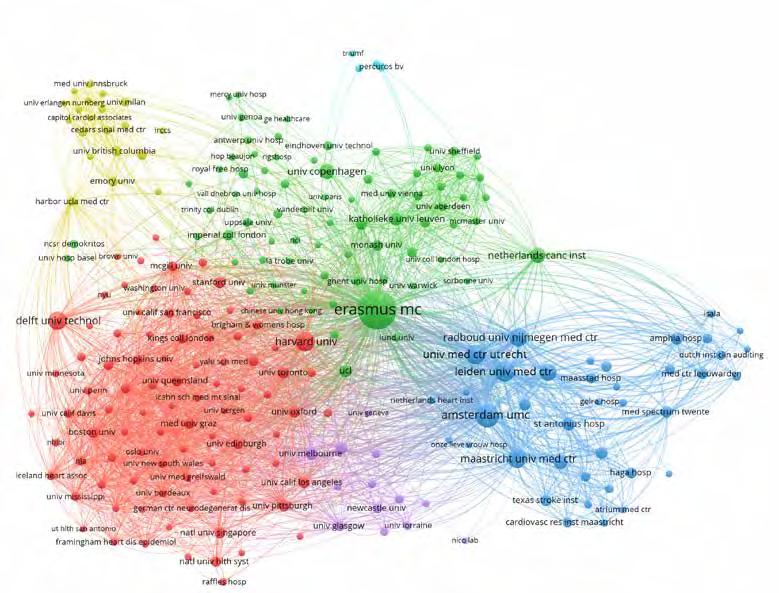
PhD dissertations
1. Booij, R. 2021, February 23. The “Knowledgeable” CT Scanner: Optimization by technological advancements. EUR Prom./ coprom.: Prof. dr. G.P. Krestin.

2. Roobol, S.J. 2021, March 09. Ionizing Radiation Quality and Dose Effects on DNA Double Strand Break Repair. EUR Prom./ coprom.: Prof. dr. M. de Jong.

3. Incekara, F. 2021, March 10 Imaging and resection of glioblastoma. EUR Prom./coprom.: Prof. dr. M. Smits

4. de Vries, B. 2021 May 25. Perfusion imaging of synovitis in knee osteoarthritis. EUR Prom./coprom.: Prof. dr. G.P. Krestin / dr. E. Oei.

5. Venkatraghavan, V. 2021, June 08. The symphony of cacophony: in neurodegenerative diseases. EUR Prom./coprom.: Prof. dr. W.J. Niessen

6. Zambito, G. 2021, September 08. New tools for multimoda; imaging of immune cells. EUR Prom./coprom.: Prof. dr. C. Lowik / dr. L. Mezzanotte

7. Gaspar, N. 2021, September 15. Smart Reporter Genes for Cellular Molecular Imaging in Tumor Immunology. EUR Prom./coprom.: Prof. dr. C. Lowik / dr. L. Mezzanotte

8. Sedghi Gamechi, Z. 2021, September 21. Automatic Quantification of the Aorta and Pulmonary Artery in Chest CT methods and validation in lung screening. EUR Prom./ coprom.: Prof. dr. M de Bruijne & Prof. dr. W.J. Niessen

9. van de Graaf, R. 2021 September 28. Improving outcomes by better reperfusion after endovascular treatment for acute ischemic stroke. EUR Prom./coprom.: Prof. dr. A. van der Lugt.

10. van der Voort, S. 2021, October 26. CUM LAUDE. Eye to AI: MR image analysis of glioma using machine learning. EUR Prom./coprom.: Prof. dr. M. Smits & Prof. dr. W.J. Niessen.

11. Osses, D. 2021, November 03. Optimizing prostate cancer screening, detection and active surveillance by risk stratification strategies. EUR Prom./coprom.: Prof. dr. G.P. Krestin / dr. I.G. Schoots.

12. Meerburg, J. 2021, November 10. Imaging and treatment of bronchiectasis: Chest computed tomography to diagnose and quantify bronchiectasis, and to optimise inhalation therapy . EUR Prom./coprom.: Prof. dr. H.A.W.M. Tiddens / dr. ir. M. van Straten.

Publications 2020
Full papers (first author):
1. Angus L, Starmans MPA, Rajicic A, Odink AE, Jalving M, Niessen WJ, Visser JJ, Sleijfer S, Klein S and van der Veldt AAM The BRAF P.V600E Mutation Status of Melanoma Lung Metastases Cannot Be Discriminated on Computed Tomography by LIDC Criteria nor Radiomics Using Machine Learning. J Pers Med . 2021;11:257.
2. Arslan M, Schaap J, Rood PPM, Nieman K Budde RPJ, van Dalen BM, Attrach M Dubois EA and Dedic A. Undetectable High-Sensitivity Troponin T as a Gatekeeper for Coronary Computed Tomography Angiography in Patients Suspected of Acute Coronary Syndrome. Cardiology 2021;146:713-719.
3. Arslan M, Schaap J, Van Gorsel B, Budde RP, Bekkers SC, Van Cauteren YJ, Damman P, Habets J, Dubois EA and Dedic A Coronary CT angiography for improved assessment of patients with acute chest pain and low-range positive high-sensitivity troponins: study protocol for a prospective, observational, multicentre study (COURSE trial). BMJ Open 2021;11:e049349.
4. Arzanforoosh F, Croal PL, van Garderen KA Smits M, Chappell MA and Warnert EAH. Effect of Applying Leakage Correction on rCBV Measurement Derived From DSC-MRI in Enhancing and Nonenhancing Glioma. Front Oncol . 2021;11:648528.
5. Bakker IL, Froberg AC, Busstra MB, Verzijlbergen JF, Konijnenberg M, van Leenders G, Schoots IG, de Blois E, van Weerden WM, Dalm SU, Maina T, Nock BA and de Jong M. GRPr Antagonist (68)GaSB3 PET/CT Imaging of Primary Prostate Cancer in Therapy-Naive Patients. J Nucl Med . 2021;62:1517-1523.
6. Benmahdjoub M, Niessen WJ, Wolvius EB and van Walsum T. Virtual extensions improve perception-based instrument alignment using optical seethrough devices. Ieee T Vis Comput Gr 2021;27:4332-4341.
7. Benmahdjoub M, van Walsum T, van Twisk P and Wolvius EB. Augmented reality in craniomaxillofacial surgery: added value and proposed recommendations through a systematic review of the literature. Int J Oral Maxillofac Surg 2021;50:969-978.
8. Blomjous MSH, Budde RPJ, Bekker MWA, Kauling RM, Cuypers J, van den Bosch AE, Roos-Hesselink JW and Hirsch A. Clinical outcome of anomalous coronary artery with interarterial course in adults: Single-center experience combined with a systematic review. Int J Cardiol 2021;335:32-39.
9. Boccalini S, Bons LR, van den Hoven AT, van den Bosch AE, Krestin GP, Roos-Hesselink J and Budde RPJ. Bicuspid aortic valve annulus: assessment of geometry and size changes during the cardiac cycle as measured with a standardized method to define the annular plane. Eur Radiol . 2021;31:8116-8129.
10. Boodt N, Snouckaert van Schauburg PRW, Hund HM, Fereidoonnezhad B, McGarry JP, Akyildiz AC, van Es ACGM, De Meyer SF, Dippel DWJ, Lingsma HF, van Beusekom HMM, van der Lugt A and Gijsen FJH. Mechanical Characterization of Thrombi Retrieved With Endovascular Thrombectomy in Patients With Acute Ischemic Stroke. Stroke . 2021;52:2510-2517.
11. Booij R, van Straten M, Wimmer A and Budde RPJ. Automated patient positioning in CT using a 3D camera for body contour detection: accuracy in pediatric patients. Eur Radiol . 2021;31:131-138.
12. Bortsova G Bos D Dubost F Vernooij MW, Ikram MK, van Tulder G and de Bruijne M. Automated Segmentation and Volume Measurement of Intracranial Internal Carotid Artery Calcification at Noncontrast CT. Radiol Artif Intell 2021;3:e200226.
13. Bortsova G, Gonzalez-Gonzalo C, Wetstein SC, Dubost F, Katramados I, Hogeweg L, Liefers B, van Ginneken B, Pluim JPW, Veta M, Sanchez CI and de Bruijne M. Adversarial attack vulnerability of medical image analysis systems:
Unexplored factors. Medical Image Analysis . 2021;73:102141.
14. Bos D, Arshi B, van den Bouwhuijsen QJA, Ikram MK, Selwaness M, Vernooij MW, Kavousi M and van der Lugt A. Atherosclerotic Carotid Plaque Composition and Incident Stroke and Coronary Events. J Am Coll Cardiol . 2021;77:1426-1435.
15. Bos D van Dam-Nolen DHK, Gupta A, Saba L, Saloner D, Wasserman BA and van der Lugt A. Advances in Multimodality Carotid Plaque Imaging: AJR Expert Panel Narrative Review. AJR Am J Roentgenol 2021;217:16-26.
16. Breda SJ, de Vos RJ, Poot DHJ, Krestin GP, Hernandez-Tamames JA and Oei EHG Association Between T2 (*) Relaxation Times Derived From Ultrashort Echo Time MRI and Symptoms During Exercise Therapy for Patellar Tendinopathy: A Large Prospective Study. J Magn Reson Imaging . 2021;54:1596-1605.
17. Breda SJ Oei EHG, Zwerver J, Visser E, Waarsing E, Krestin GP and de Vos RJ. Effectiveness of progressive tendon-loading exercise therapy in patients with patellar tendinopathy: a randomised clinical trial. Br J Sports Med . 2021;55:501509.
18. Bron EE, Klein S, Papma JM, Jiskoot LC, Venkatraghavan V, Linders J, Aalten P, De Deyn PP, Biessels GJ, Claassen J, Middelkoop HAM, Smits M, Niessen WJ, van Swieten JC, van der Flier WM, Ramakers I, van der Lugt A, Alzheimer’s Disease Neuroimaging I and Parelsnoer Neurodegenerative Diseases study g. Cross-cohort generalizability of deep and conventional machine learning for MRI-based diagnosis and prediction of Alzheimer’s disease. Neuroimage Clin . 2021;31:102712.
19. Castillo T, Arif M, Starmans MPA, Niessen WJ, Bangma CH, Schoots IG and Veenland JF. Classification of Clinically Significant Prostate Cancer on Multi-Parametric MRI: A Validation Study Comparing Deep Learning and Radiomics. Cancers (Basel) 2021;14:12.
20. Castillo T, Starmans MPA, Arif M, Niessen WJ, Klein S, Bangma CH, Schoots IG and Veenland JF. A Multi-Center, Multi-Vendor Study to Evaluate the Generalizability of a Radiomics Model for Classifying Prostate cancer: High Grade vs. Low Grade. Diagnostics (Basel) . 2021;11:369.
21. Cox CPW, van Assema DME, Verburg FA, Brabander T, Konijnenberg M and Segbers M. A dedicated paediatric [(18)F] FDG PET/CT dosage regimen. EJNMMI Res . 2021;11:65.
22. Croll PH, Boelens M, Vernooij MW, van de Rest O, Zillikens MC, Ikram MA and Voortman T. Associations of vitamin D deficiency with MRI markers of brain health in a community sample. Clin Nutr 2021;40:72-78.
23. Croll PH, Vinke EJ, Armstrong NM, Licher S, Vernooij MW, Baatenburg de Jong RJ, Goedegebure A and Ikram MA. Hearing loss and cognitive decline in the general population: a prospective cohort study. J Neurol . 2021;268:860-871.
24. Damiana TST and Dalm SU. Combination Therapy, a Promising Approach to Enhance the Efficacy of Radionuclide and Targeted Radionuclide Therapy of Prostate and Breast Cancer. Pharmaceutics 2021;13:674.
25. de Vries BA, Breda SJ, Sveinsson B, McWalter EJ, Meuffels DE, Krestin GP, Hargreaves BA, Gold GE and Oei EHG. Detection of knee synovitis using non-contrast-enhanced qDESS compared with contrast-enhanced MRI. Arthritis Res Ther . 2021;23:55.
26. Delhaas EM, Harhangi BS, van Doormaal PJ, Dinkelaar W, van Es A, van Assema DME, Frankema SPG, van der Lugt A and Huygen F. Restoration of rostral cerebrospinal fluid flow to solve treatment failure caused by obstruction in long-term intrathecal baclofen administration. J Spinal Cord Med . 2021;44:312-321.
27. Delhaas EM, van Assema DME, Froberg AC, Zwezerijnen B, Harhangi BS, Frankema SPG, Huygen F and van der Lugt A. Isotopic Scintigraphy in Intrathecal
Drug Delivery Failure: A Single-Institution Case Series. Neuromodulation 2021;24:1190-1198.
28. Derks S, Jongen JLM, van den Bent MJ and van der Veldt AAM. Assessment of imaging biomarkers in the follow-up of brain metastases after SRS. Neuro Oncol 2021;23:1983-1984.
29. Dilba K, van Dam-Nolen DHK, Crombag G, van der Kolk AG, Koudstaal PJ, Nederkoorn PJ, Hendrikse J, Kooi ME, van der Steen AFW, Wentzel JJ, van der Lugt A and Bos D. Dolichoarteriopathies of the extracranial internal carotid artery: The Plaque At RISK study. Eur J Neurol 2021;28:3133-3138.
30. Dilba K, van Dam-Nolen DHK, Korteland SA, van der Kolk AG, Kassem M, Bos D, Koudstaal PJ, Nederkoorn PJ, Hendrikse J, Kooi ME, Gijsen FJH, van der Steen AFW, van der Lugt A and Wentzel JJ. The Association Between Time-Varying Wall Shear Stress and the Development of Plaque Ulcerations in Carotid Arteries From the Plaque at Risk Study. Front Cardiovasc Med . 2021;8:732646.
31. Dilba K, van Dam-Nolen DHK, van Dijk AC, Kassem M, van der Steen AFW, Koudstaal PJ, Nederkoorn PJ, Hendrikse J, Kooi ME, Wentzel JJ and van der Lugt A. Plaque Composition as a Predictor of Plaque Ulceration in Carotid Artery Atherosclerosis: The Plaque At RISK Study. AJNR Am J Neuroradiol . 2021;42:144-151.
32. Dilba K, van Dijk AC, Crombag G, van der Steen AFW, Daemen MJ, Koudstaal PJ, Nederkoorn PJ, Hendrikse J, Kooi ME, van der Lugt A and Wentzel JJ. Association between Intraplaque Hemorrhage and Vascular Remodeling in Carotid Arteries: The Plaque at RISK (PARISK) Study. Cerebrovasc Dis . 2021;50:94-99.
33. Elders B, Ciet P, Tiddens HAWM, van den Bosch W, Wielopolski P and Pullens B. MRI of the upper airways in children and young adults: the MUSIC study. Thorax 2021;76:44-52.
34. Elders B, Hakkesteegt MM, Ciet P, Tiddens HAWM, Wielopolski P and Pullens
B. Structure and Function of the Vocal Cords after Airway Reconstruction on Magnetic Resonance Imaging. Laryngoscope . 2021;131:E2402-E2408.
35. Feijtel D, Doeswijk GN, Verkaik NS, Haeck JC, Chicco D, Angotti C, Konijnenberg MW, de Jong M and Nonnekens J. Inter and intra-tumor somatostatin receptor 2 heterogeneity influences peptide receptor radionuclide therapy response. Theranostics . 2021;11:491-505.
36. Garcia-Uceda A, Selvan R, Saghir Z, Tiddens HAWM and de Bruijne M. Automatic airway segmentation from computed tomography using robust and efficient 3-D convolutional neural networks. Sci RepUk . 2021;11:16001.
37. Gaspar N, Walker JR, Zambito G, Marella-Panth K, Lowik C, Kirkland TA and Mezzanotte L. Evaluation of NanoLuc substrates for bioluminescence imaging of transferred cells in mice. J Photochem Photobiol B . 2021;216:112128.
38. Geenen L, Nonnekens J, Konijnenberg M, Baatout S, De Jong M and Aerts A. Overcoming nephrotoxicity in peptide receptor radionuclide therapy using [(177) Lu]Lu-DOTA-TATE for the treatment of neuroendocrine tumours. Nucl Med Biol 2021;102-103:1-11.
39. Handula M, Chen KT and Seimbille Y IEDDA: An Attractive Bioorthogonal Reaction for Biomedical Applications. Molecules . 2021;26:4640.
40. Hilal S, Doolabi A, Vrooman H, Ikram MK, Ikram MA and Vernooij MW. Clinical Relevance of Cortical Cerebral Microinfarcts on 1.5T Magnetic Resonance Imaging in the Late-Adult Population. Stroke 2021;52:922-930.
41. Hirvasniemi J, Klein S, Bierma-Zeinstra S, Vernooij MW, Schiphof D and Oei EHG A machine learning approach to distinguish between knees without and with osteoarthritis using MRI-based radiomic features from tibial bone. Eur Radiol 2021;31:8513-8521.
42. Hooijman EL, Chalashkan Y, Ling SW, Kahyargil FF, Segbers M, Bruchertseifer F, Morgenstern A, Seimbille Y, Koolen SLW, Brabander T and de Blois E. Development of [(225)Ac]Ac-PSMA-I&T for Targeted Alpha Therapy According to GMP Guidelines for Treatment of mCRPC. Pharmaceutics . 2021;13:715.
43. Huizinga W, Poot DHJ, Vinke EJ, Wenzel F, Bron EE, Toussaint N, Ledig C, Vrooman H, Ikram MA, Niessen WJ, Vernooij MW and Klein S. Differences Between MR Brain Region Segmentation Methods: Impact on Single-Subject Analysis. Front Big Data . 2021;4:577164.
44. Incekara F, Smits M, Dirven L, Bos EM, Balvers RK, Haitsma IK, Schouten JW and Vincent A. Intraoperative B-Mode Ultrasound Guided Surgery and the Extent of Glioblastoma Resection: A Randomized Controlled Trial. Front Oncol 2021;11:649797.
45. Klomp MJ, Dalm SU, de Jong M, Feelders RA, Hofland J and Hofland LJ. Epigenetic regulation of somatostatin and somatostatin receptors in neuroendocrine tumors and other types of cancer. Rev Endocr Metab Disord . 2021;22:495-510.
46. Klomp MJ, Dalm SU, van Koetsveld PM, Dogan F, de Jong M and Hofland LJ. Comparing the Effect of Multiple Histone Deacetylase Inhibitors on SSTR2 Expression and [(111)In]In-DOTATATE Uptake in NET Cells. Cancers (Basel) 2021;13:4905.
47. Knol WG, Budde RPJ, Mahtab EAF, Bekkers JA and Bogers A. Intimal aortic atherosclerosis in cardiac surgery: surgical strategies to prevent embolic stroke. Eur J Cardiothorac Surg . 2021;60:12591267.
48. Knol WG, Oei FB, Budde RPJ and Ter Horst M. A case report of an interrupted inferior vena cava and azygos continuation: implications for preoperative screening in minimally invasive cardiac surgery. Eur Heart J Case Rep . 2021;5:ytab308.
50. Knol WG, Wahadat AR, Roos-Hesselink JW, Van Mieghem NM, Tanis W, Bogers A and Budde RPJ. Screening for coronary artery disease in early surgical treatment of acute aortic valve infective endocarditis. Interact Cardiovasc Thorac Surg 2021;32:522-529.
51. Konijnenberg M, Herrmann K, Kobe C, Verburg F, Hindorf C, Hustinx R and Lassmann M. EANM position paper on article 56 of the Council Directive 2013/59/ Euratom (basic safety standards) for nuclear medicine therapy. Eur J Nucl Med Mol Imaging . 2021;48:67-72.
52. Koromani F, Alonso N, Alves I, Brandi ML, Foessl I, Formosa MM, Morgenstern MF, Karasik D, Kolev M, Makitie O, Ntzani E, Obermayer-Pietsch B, Ohlsson C, Rauner M, Soe K, Soldatovic I, Teti A, Valjevac A and Rivadeneira F. The “GEnomics of Musculo Skeletal Traits TranslatiOnal NEtwork”: Origins, Rationale, Organization, and Prospects. Front Endocrinol 2021;12:709815.
53. Koromani F, Ghatan S, van Hoek M, Zillikens MC, Oei EHG, Rivadeneira F and Oei L. Type 2 Diabetes Mellitus and Vertebral Fracture Risk. Curr Osteoporos Rep 2021;19:50-57.
54. Kotek G, Nunez-Gonzalez L, Vogel MW, Krestin GP, Poot DHJ and Hernandez-Tamames JA. From signal-based to comprehensive magnetic resonance imaging. Sci Rep . 2021;11:17216.
55. Koustoulidou S, Hoorens MWH, Dalm SU, Mahajan S, Debets R, Seimbille Y and de Jong M. Cancer-Associated Fibroblasts as Players in Cancer Development and Progression and Their Role in Targeted Radionuclide Imaging and Therapy. Cancers (Basel) . 2021;13:1100.
56. Li B, Niessen WJ, Klein S, de Groot M, Ikram MA, Vernooij MW and Bron EE
49. Knol WG, Thuijs D, Odink AE, Maurovich-Horvat P, de Jong PA, Krestin GP, Bogers A and Budde RPJ. Preoperative Chest Computed Tomography Screening for Coronavirus Disease 2019 in Asymptomatic Patients Undergoing Cardiac Surgery. Semin Thorac Cardiovasc Surg 2021;33:417-424.
Longitudinal diffusion MRI analysis using Segis-Net: A single-step deep-learning framework for simultaneous segmentation and registration. Neuroimage 2021;235:118004.
57. Ling SW de Jong AC Schoots IG, Nasserinejad K, Busstra MB, van der Veldt AAM and Brabander T. Comparison of (68)Ga-labeled Prostate-specific Membrane Antigen Ligand Positron Emission Tomography/Magnetic Resonance Imaging and Positron Emission Tomography/Computed Tomography for Primary Staging of Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol Open Sci . 2021;33:61-71.
58. Luijten SPR Bos D Compagne KCJ Wolff L, Majoie C, Roos Y, van Zwam WH, van Oostenbrugge RJ, Dippel DWJ, van der Lugt A, van Es A and MR CLEAN Trial Investigators. Association of White Matter Lesions and Outcome After Endovascular Stroke Treatment. Neurology 2021;96:e333-e342.
59. Luijten SPR, van der Donk SC, Compagne KCJ, Yo LSF, Sprengers MES, Majoie C, Roos Y, van Zwam WH, van Oostenbrugge R, Dippel DWJ, van der Lugt A, Roozenbeek B, Bos D and MR CLEAN Registry Investigators. Intracranial carotid artery calcification subtype and collaterals in patients undergoing endovascular thrombectomy. Atherosclerosis 2021;337:1-6.
60. Luijten SPR Wolff L and van der Lugt A The True Potential of Artificial Intelligence for Detection of Large-Vessel Occlusion: The Role of M2 Occlusions. AJNR Am J Neuroradiol . 2021;42:E46.
61. Luu HM, van Walsum T, Franklin D, Pham PC, Vu LD, Moelker A, Staring M, VanHoang X, Niessen W and Trung NL. Efficiently compressing 3D medical images for teleinterventions via CNNs and anisotropic diffusion. Med Phys . 2021;48:28772890.
62. Lv Q, Elders B, Warris A, Caudri D, Ciet P and Tiddens HAWM. Aspergillus-related lung disease in people with cystic fibro-
sis: can imaging help us to diagnose disease? Eur Respir Rev . 2021;30:210103.
63. Meester EJ, de Blois E, Krenning BJ, van der Steen AFW, Norenberg JP, van Gaalen K, Bernsen MR, de Jong M and van der Heiden K. Autoradiographical assessment of inflammation-targeting radioligands for atherosclerosis imaging: potential for plaque phenotype identification. EJNMMI Res . 2021;11:27.
64. Meester EJ, Krenning BJ, de Blois E, de Jong M, van der Steen AFW, Bernsen MR and van der Heiden K. Imaging inflammation in atherosclerotic plaques, targeting SST2 with [(111)In]In-DOTA-JR11. J Nucl Cardiol . 2021;28:2506-2513.
65. Minczeles NS, Hofland J, de Herder WW and Brabander T. Strategies Towards Improving Clinical Outcomes of Peptide Receptor Radionuclide Therapy. Curr Oncol Rep . 2021;23:46.
66. Minderhoud SCS, Wentzel JJ, Roos-Hesselink JW and Hirsch A. Six-year serial follow-up with aortic 4D flow cardiovascular magnetic resonance in a patient with bicuspid aortic valve. Eur Heart J Cardiovasc Imaging . 2021;22:e144.
67. Mostert JM, Romeijn SR, Dibbets-Schneider P, Rietbergen DDD, Pereira Arias-Bouda LM, Gotz C, DiFranco MD, Dimai HP and Grootjans W. Inter-observer agreement of vertebral fracture assessment with dual-energy x-ray absorptiometry equipment. Arch Osteoporos . 2021;17:4.
68. Nous F, Budde RPJ, Fairbairn TA, Akasaka T, Norgaard BL, Berman DS, Raff G, Hurwitz-Koweek LM, Pontone G, Kawasaki T, Sand NPR, Jensen JM, Amano T, Poon M, Ovrehus KA, Sonck J, Rabbat MG, Mullen S, De Bruyne B, Rogers C, Matsuo H, Bax JJ, Leipsic J, Patel MR and Nieman K. Temporal changes in FFRCT-Guided Management of Coronary Artery Disease - Lessons from the ADVANCE Registry. J Cardiovasc Comput Tomogr . 2021;15:48-55.
69. Nous F, Roest S, van Dijkman ED, Attrach M, Caliskan K, Brugts JJ, Nieman K, Hirsch A, Constantinescu AA, Manintveld OC and Budde RPJ. Clinical implemen-
tation of coronary computed tomography angiography for routine detection of cardiac allograft vasculopathy in heart transplant patients. Transpl Int 2021;34:1886-1894.
70. Nunez-Gonzalez L, Kotek G, Gomez PA, Buonincontri G, Vogel M, Krestin GP, Poot DHJ and Hernandez-Tamames JA Accuracy and repeatability of QRAPMASTER and MRF-vFA. Magn Reson Imaging 2021;83:196-207.
71. Oei EHG, van Zadelhoff TA, Eijgenraam SM, Klein S, Hirvasniemi J and van der Heijden RA. 3D MRI in Osteoarthritis. Semin Musculoskelet Radiol . 2021;25:468479.
72. Polfliet M, Hendriks MS, Guyader JM, Ten Hove I, Mast H, Vandemeulebroucke J, van der Lugt A, Wolvius EB and Klein S. Registration of magnetic resonance and computed tomography images in patients with oral squamous cell carcinoma for three-dimensional virtual planning of mandibular resection and reconstruction. Int J Oral Maxillofac Surg 2021;50:1386-1393.
73. Ruigrok EAM, van Vliet N, Dalm SU, de Blois E, van Gent DC, Haeck J, de Ridder C, Stuurman D, Konijnenberg MW, van Weerden WM, de Jong M and Nonnekens J. Extensive preclinical evaluation of lutetium-177-labeled PSMA-specific tracers for prostate cancer radionuclide therapy. Eur J Nucl Med Mol Imaging 2021;48:1339-1350.
74. Sabidussi ER, Klein S, Caan MWA, Bazrafkan S, den Dekker AJ, Sijbers J, Niessen WJ and Poot DHJ. Recurrent inference machines as inverse problem solvers for MR relaxometry. Med Image Anal 2021;74:102220.
75. Samuels N, van de Graaf RA, van den Berg CAL, Nieboer D, Eralp I, Treurniet KM, Emmer BJ, Immink RV, Majoie C, van Zwam WH, Bokkers RPH, Uyttenboogaart M, van Hasselt B, Muhling J, Burke JF, Roozenbeek B, van der Lugt A, Dippel DWJ, Lingsma HF, van Es A and MR CLEAN Registry Investigators. Blood Pressure During Endovascular Treatment Under
Conscious Sedation or Local Anesthesia. Neurology . 2021;96:e171-e181.
76. Samuels N, van de Graaf RA, van den Berg CAL, Uniken Venema SM, Bala K, van Doormaal PJ, van der Steen W, Witvoet E, Boiten J, den Hertog H, Schonewille WJ, Hofmeijer J, Schreuder F, Schreuder T, van der Worp HB, Roos Y, Majoie C, Burke JF, van Es A, van der Lugt A, Roozenbeek B, Lingsma HF, Dippel DWJ and MR CLEAN Registry Investigators. Blood Pressure in the First 6 Hours Following Endovascular Treatment for Ischemic Stroke Is Associated With Outcome. Stroke 2021;52:3514-3522.
77. Santos EMM, Arrarte Terreros N, Kappelhof M, Borst J, Boers AMM, Lingsma HF, Berkhemer OA, Dippel DWJ, Majoie CB, Marquering HA, Niessen WJ and MR CLEAN Investigators. Associations of thrombus perviousness derived from entire thrombus segmentation with functional outcome in patients with acute ischemic stroke. J Biomech 2021;128:110700.
78. Schoots IG, Ahmed HU and Padhani AR. Can Diagnostic Magnetic Resonance Imaging for Suspected Clinically Significant Prostate Cancer Predict Unfavorable Long-term Outcome for Diagnosed Men for Pretreatment Counseling? Eur Urol Oncol . 2021;4:529-531.
79. Schoots IG, Barentsz JO, Bittencourt LK, Haider MA, Macura KJ, Margolis DJA, Moore CM, Oto A, Panebianco V, Siddiqui MM, Tempany C, Turkbey B, Villeirs GM, Weinreb JC and Padhani AR. PI-RADS Committee Position on MRI Without Contrast Medium in Biopsy-Naive Men With Suspected Prostate Cancer: Narrative Review. AJR Am J Roentgenol . 2021;216:319.
80. Schoots IG and Padhani AR. Risk-adapted biopsy decision based on prostate magnetic resonance imaging and prostate-specific antigen density for enhanced biopsy avoidance in first prostate cancer diagnostic evaluation. BJU Int . 2021;127:175-178.
81. Sedghi Gamechi Z, Arias-Lorza AM,
Saghir Z, Bos D and de Bruijne M. Assessment of fully automatic segmentation of pulmonary artery and aorta on noncontrast CT with optimal surface graph cuts. Med Phys . 2021;48:7837-7849.
82. Sijtsema ND, Petit SF, Poot DHJ, Verduijn GM, van der Lugt A, Hoogeman MS and Hernandez-Tamames JA. An optimal acquisition and post-processing pipeline for hybrid IVIM-DKI in head and neck. Magn Reson Med . 2021;85:777-789.
83. Smits M. MRI biomarkers in neuro-oncology. Nat Rev Neurol . 2021;17:486-500.
84. Smits M. Update on neuroimaging in brain tumours. Curr Opin Neurol 2021;34:497-504.
85. Starmans MPA, Buisman FE, Renckens M, Willemssen F, van der Voort SR, Groot Koerkamp B, Grunhagen DJ, Niessen WJ, Vermeulen PB, Verhoef C, Visser JJ and Klein S. Distinguishing pure histopathological growth patterns of colorectal liver metastases on CT using deep learning and radiomics: a pilot study. Clin Exp Metastasis . 2021;38:483-494.
86. Stroet MCM de Blois E Haeck J Seimbille Y, Mezzanotte L, de Jong M, Lowik C and Panth KM. In Vivo Evaluation of Gallium-68-Labeled IRDye800CW as a Necrosis Avid Contrast Agent in Solid Tumors. Contrast Media Mol Imaging 2021:2853522.
87. Stroet MCM, Dijkstra BM, Dulfer SE, Kruijff S, den Dunnen WFA, Kruyt FAE, Groen RJM, Seimbille Y, Panth KM, Mezzanotte L, Lowik C and de Jong M. Necrosis binding of Ac-Lys(0)(IRDye800CW)-Tyr(3)-octreotate: a consequence from cyanine-labeling of small molecules. EJNMMI Res 2021;11:47.
88. Sumser K, Drizdal T, Bellizzi GG, Hernandez-Tamames JA, van Rhoon GC and Paulides MM. Experimental Validation of the MRcollar: An MR Compatible Applicator for Deep Heating in the Head and Neck Region. Cancers (Basel) . 2021;13:5617.
89. Terlouw LG, van Noord D, van Walsum T Bruno MJ and Moelker A. Mesenteric ar-
tery calcium scoring: a potential screening method for chronic mesenteric ischemia. Eur Radiol . 2021;31:4212-4220.
90. Terlouw LG, van Noord D, van Walsum T, van Dijk LJD, Moelker A and Bruno MJ. Early risk stratification of patients with suspected chronic mesenteric ischaemia using a symptom and mesenteric artery calcium score based score chart. United European Gastroenterol J . 2021;9:626634.
91. van Arendonk J, Yilmaz P, Steketee R, Zijlmans JL, Lamballais S, Niessen WJ Neitzel J, Ikram MA and Vernooij MW Resistance to developing brain pathology due to vascular risk factors: the role of educational attainment. Neurobiol Aging 2021;106:197-206.
92. van Dam-Nolen DHK, van Dijk AC, Crombag G, Lucci C, Kooi ME, Hendrikse J, Nederkoorn PJ, Daemen MJ, van der Steen AFW, Koudstaal PJ, Kronenberg F, Roeters van Lennep JE, Mulder MT and van der Lugt A. Lipoprotein(a) levels and atherosclerotic plaque characteristics in the carotid artery: The Plaque at RISK (PARISK) study. Atherosclerosis . 2021;329:22-29.
93. van de Berg NJ, Meeuwsen FC, Doukas M, Kronreif G, Moelker A and van den Dobbelsteen JJ. Steerable needles for radio-frequency ablation in cirrhotic livers. Sci Rep . 2021;11:309.
94. van de Graaf RA, Zinkstok SM, Chalos V, Goldhoorn RB, Majoie CB, van Oostenbrugge RJ, van der Lugt A, Dippel DW, Roos YB, Lingsma HF, van Es AC, Roozenbeek B and MR CLEAN Registry Investigators. Prior antiplatelet therapy in patients undergoing endovascular treatment for acute ischemic stroke: Results from the MR CLEAN Registry. Int J Stroke 2021;16:476-485.
95. van den Bosch WB, James AL and Tiddens H. Structure and function of small airways in asthma patients revisited. Eur Respir Rev . 2021;30:200186.
96. van den Bosch WB, Kloosterman SF, Andrinopoulou ER, Greidanus R, Pijnenburg MWH, Tiddens HAWM and Janssens HM.
Small airways targeted treatment with smart nebulizer technology could improve severe asthma in children: a retrospective analysis. J Asthma . 2021:1-11.
97. van den Heuvel MM, Griffioen NE, Achterberg HC, Oei EHG, Renkens JJM, Bierma-Zeinstra SMA and van Middelkoop M. Spinopelvic alignment and lumbar vertebral shape in children: associations with structural spinal abnormalities and body composition in the generation R study. Eur Spine J . 2021:248-257.
98. van den Heuvel MM, Oei EHG, Renkens JJM, Bierma-Zeinstra SMA and van Middelkoop M. Structural spinal abnormalities on MRI and associations with weight status in a general pediatric population. Spine J . 2021;21:465-476.
99. van der Heijden RA, de Vries BA, Poot DHJ, van Middelkoop M, Bierma-Zeinstra SMA, Krestin GP and Oei EHG
Quantitative volume and dynamic contrast-enhanced MRI derived perfusion of the infrapatellar fat pad in patellofemoral pain. Quant Imaging Med Surg 2021;11:133-142.
100. van der Heijden RA and Oei EHG. Editorial for “Failed Total Hip Arthroplasty: Diagnostic Performance of Locoregional Lymphadenopathy at MRI to Identify Infected Implants”. J Magn Reson Imaging 2021;53:211-212.
101. van der Toorn JE, Bos D, Arshi B, Leening MJG, Vernooij MW, Ikram MA, Ikram MK and Kavousi M. Arterial calcification at different sites and prediction of atherosclerotic cardiovascular disease among women and men. Atherosclerosis 2021;337:27-34.
102. van der Velde N, Hassing HC, Bakker BJ, Wielopolski PA, Lebel RM, Janich MA, Kardys I, Budde RPJ and Hirsch A. Improvement of late gadolinium enhancement image quality using a deep learning-based reconstruction algorithm and its influence on myocardial scar quantification. Eur Radiol . 2021;31:3846-3855.
103. van der Velde N, Huurman R, Hassing HC, Budde RPJ, van Slegtenhorst MA, Verha-
gen JMA, Schinkel AFL, Michels M and Hirsch A. Novel Morphological Features on CMR for the Prediction of Pathogenic Sarcomere Gene Variants in Subjects Without Hypertrophic Cardiomyopathy. Front Cardiovasc Med . 2021;8:727405.
104. van der Velde N, Janus CPM, Bowen DJ, Hassing HC, Kardys I, van Leeuwen FE, So-Osman C, Nout RA, Manintveld OC and Hirsch A. Detection of Subclinical Cardiovascular Disease by Cardiovascular Magnetic Resonance in Lymphoma Survivors. JACC CardioOncol . 2021;3:695-706.
105. van der Veldt AAM, Oosting SF, Dingemans AC, Fehrmann RSN, GeurtsvanKessel C, Jalving M, Rimmelzwaan GF, Kvistborg P, Blank CU, Smit EF, Lemmens V, Hiltermann TJN, Koopmans MPG, Huckriede ALW, Rots NY, van Els C, van Baarle D, Haanen J and de Vries EGE. COVID-19 vaccination: the VOICE for patients with cancer. Nat Med . 2021;27:568-569.
106. van der Voet JA, Wesselius D, Zhang F, Vroegindeweij D, Oei EH, Bierma-Zeinstra SMA, Englund M and Runhaar J. Factors associated with longitudinal change of meniscal extrusion in overweight women without clinical signs of osteoarthritis. Rheumatology (Oxford) 2021;60:5175-5184.
107. van der Voort SR Incekara F, Wijnenga MMJ, Kapsas G, Gahrmann R, Schouten JW, Dubbink HJ, Vincent A, van den Bent MJ, French PJ, Klein S and Smits M. The Erasmus Glioma Database (EGD): Structural MRI scans, WHO 2016 subtypes, and segmentations of 774 patients with glioma. Data Brief . 2021;37:107191.
108. van der Voort SR, Smits M, Klein S and Alzheimer’s Disease Neuroimaging I. DeepDicomSort: An Automatic Sorting Algorithm for Brain Magnetic Resonance Imaging Data. Neuroinformatics 2021;19:159-184.
109. van der Werf NR Booij R, Schmidt B, Flohr TG, Leiner T, de Groen JJ, Bos D, Budde RPJ, Willemink MJ and Greuter MJW. Evaluating a calcium-aware kernel for CT CAC scoring with varying surrounding materials and heart rates:
a dynamic phantom study. Eur Radiol 2021;31:9211-9220.
110. van der Werf NR, van Gent M, Booij R, Bos D, van der Lugt A, Budde RPJ, Greuter MJW and van Straten M. Dose Reduction in Coronary Artery Calcium Scoring Using Mono-Energetic Images from Reduced Tube Voltage Dual-Source Photon-Counting CT Data: A Dynamic Phantom Study. Diagnostics (Basel) . 2021;11:2192.
111. van Garderen KA, van der Voort SR, Versteeg A, Koek M, Gutierrez A, van Straten M Rentmeester M Klein S and Smits M. EASE: Clinical Implementation of Automated Tumor Segmentation and Volume Quantification for Adult LowGrade Glioma. Front Med (Lausanne) 2021;8:738425.
112. van Hilten A, Kushner SA, Kayser M, Ikram MA, Adams HHH, Klaver CCW, Niessen WJ and Roshchupkin GV. GenNet framework: interpretable deep learning for predicting phenotypes from genetic data. Commun Biol . 2021;4:1094.
113. van Tiel S, Maina T, Nock BA, Konijnenberg M, Blois E, Seimbille Y, Bernsen MLE and de Jong M. Albutate-1, a Novel Long-Circulating Radiotracer Targeting the Somatostatin Receptor Subtype 2. Journal of Nuclear Medicine & Radiation Sciences . 2021;2:3-9.
114. van Zadelhoff TA, Okuno Y, Bos PK, Bierma-Zeinstra SMA, Krestin GP, Moelker A and Oei EHG. Association between Baseline Osteoarthritic Features on MR Imaging and Clinical Outcome after Genicular Artery Embolization for Knee Osteoarthritis. J Vasc Interv Radiol . 2021;32:497503.
115. Veldhuijzen van Zanten SEM, Bos EM, Verburg FA and van Doormaal PJ. Intracranial hemangiopericytoma showing excellent uptake on arterial injection of [(68)Ga]DOTATATE. Eur J Nucl Med Mol Imaging . 2021;48:1673-1674.
116. Veldhuijzen van Zanten SEM, Neggers S, Valkema R and Verburg FA. Positive [(18)F]fluoroethyltyrosine PET/MRI in suspected recurrence of growth hor-
mone-producing pituitary adenoma in a paediatric patient. Eur J Nucl Med Mol Imaging . 2021;49:410-411.
117. Venema E, Roozenbeek B, Mulder M, Brown S, Majoie C, Steyerberg EW, Demchuk AM, Muir KW, Davalos A, Mitchell PJ, Bracard S, Berkhemer OA, Lycklama ANGJ, van Oostenbrugge RJ, Roos Y, van Zwam WH, van der Lugt A, Hill MD, White P, Campbell BCV, Guillemin F, Saver JL, Jovin TG, Goyal M, Dippel DWJ, Lingsma HF and the HERMES collaborators and MR CLEAN Registry Investigators. Prediction of Outcome and Endovascular Treatment Benefit: Validation and Update of the MR PREDICTS Decision Tool. Stroke 2021;52:2764-2772.
118. Venkatraghavan V, Klein S, Fani L, Ham LS, Vrooman H, Ikram MK, Niessen WJ, Bron EE and Alzheimer’s Disease Neuroimaging I. Analyzing the effect of APOE on Alzheimer’s disease progression using an event-based model for stratified populations. Neuroimage 2021;227:117646.
119. Venkatraghavan V, Vinke EJ, Bron EE, Niessen WJ, Ikram MA, Klein S, Vernooij MW and Alzheimer’s Disease Neuroimaging I. Progression along data-driven disease timelines is predictive of Alzheimer’s disease in a population-based cohort. Neuroimage . 2021;238:118233.
120. Verburg FA, Amthauer H, Binse I, Brink I, Buck A, Darr A, Dierks C, Koch C, Konig U, Kreissl MC, Luster M, Reuter C, Scheidhauer K, Willenberg HS, Zielke A and Schott M. Questions and Controversies in the Clinical Application of Tyrosine Kinase Inhibitors to Treat Patients with Radioiodine-Refractory Differentiated Thyroid Carcinoma: Expert Perspectives. Horm Metab Res . 2021;53:149-160.
121. Verburg FA, Konijnenberg MW and Nonnekens J. The limits of the “holy gray” in radioembolization and beyond : Beyond the “holy Gray”. Eur J Nucl Med Mol Imaging . 2021;48:4118-4119.
122. Verburg FA, Nonnekens J, Konijnenberg MW and de Jong M. To go where no one has gone before: the necessity of radio-
biology studies for exploration beyond the limits of the “Holy Gray” in radionuclide therapy. Eur J Nucl Med Mol Imaging . 2021;48:2680-2682.
123. Verschueren J, Eijgenraam SM, Klein S, Poot DHJ, Bierma-Zeinstra SMA, Hernandez Tamames JA, Wielopolski PA, Reijman M and Oei EHG. T2 mapping of healthy knee cartilage: multicenter multivendor reproducibility. Quant Imaging Med Surg . 2021;11:1247-1255.
124. Verschueren J, Van Langeveld SJ, Dragoo JL, Bierma-Zeinstra SMA, Reijman M, Gold GE and Oei EHG. T2 relaxation times of knee cartilage in 109 patients with knee pain and its association with disease characteristics. Acta Orthop 2021;92:335-340.
125. Vinke EJ, Yilmaz P, van der Toorn JE, Fakhry R, Frenzen K, Dubost F, Licher S, de Bruijne M, Kavousi M, Ikram MA, Vernooij MW and Bos D. Intracranial arteriosclerosis is related to cerebral small vessel disease: a prospective cohort study. Neurobiol Aging . 2021;105:16-24.
126. Wahadat AR, Tanis W, Scholtens AM, Bekker M, Graven LH, Swart LE, den Harder AM, Lam M, de Heer LM, Roos-Hesselink JW and Budde RPJ. Normal imaging findings after aortic valve implantation on (18)F-Fluorodeoxyglucose positron emission tomography with computed tomography. J Nucl Cardiol . 2021;28:22582268.
127. Wahadat AR, Tanis W, Swart LE, Scholtens A, Krestin GP, van Mieghem NM, Schurink CAM, van der Spoel TIG, van den Brink FS, Vossenberg T, Slart R, Glaudemans A, Roos-Hesselink JW and Budde RPJ Added value of (18)F-FDG-PET/CT and cardiac CTA in suspected transcatheter aortic valve endocarditis. J Nucl Cardiol 2021;28:2072-2082.
128. Warnert EAH, Kasper L, Meltzer CC, Lightfoote JB, Bucknor MD, Haroon H, Duggan G, Gowland P, Wald L, Miller KL, Morris EA and Anazodo UC. Resonate: Reaching Excellence Through Equity, Diversity, and Inclusion in ISMRM. J Magn Reson Imaging . 2021;53:1608-1611.
129. Wolff L, Berkhemer OA, van Es A, van Zwam WH, Dippel DWJ, Majoie C, van Walsum T van der Lugt A and MR CLEAN Investigators. Validation of automated Alberta Stroke Program Early CT Score (ASPECTS) software for detection of early ischemic changes on non-contrast brain CT scans. Neuroradiology 2021;63:491-498.
130. Zambito G Chawda C and Mezzanotte L Emerging tools for bioluminescence imaging. Curr Opin Chem Biol . 2021;63:8694.
131. Zambito G, Deng S, Haeck J, Gaspar N, Himmelreich U, Censi R, Lowik C, Di Martino P and Mezzanotte L. Fluorinated PLGA-PEG-Mannose Nanoparticles for Tumor-Associated Macrophage Detection by Optical Imaging and MRI. Front Med (Lausanne) . 2021;8:712367.
132. Zambito G, Hall MP, Wood MG, Gaspar N, Ridwan Y, Stellari FF, Shi C, Kirkland TA, Encell LP, Lowik C and Mezzanotte L. Red-shifted click beetle luciferase mutant expands the multicolor bioluminescent palette for deep tissue imaging. iScience . 2021;24:101986.
133. Zambito G and Mezzanotte L. Near-infrared bioluminescence imaging of two cell populations in living mice. STAR Protoc 2021;2:100662.
134. Zhang C, Klein S, Cristobal-Huerta A, Hernandez-Tamames JA and Poot DHJ APIR4EMC: Autocalibrated parallel imaging reconstruction for extended multi-contrast imaging. Magn Reson Imaging . 2021;78:80-89.
Full Papers (co-author)
135. Abdel-Alim T, Iping R, Wolvius EB, Mathijssen IMJ, Dirven CMF, Niessen WJ, van Veelen MC and Roshchupkin GV Three-Dimensional Stereophotogrammetry in the Evaluation of Craniosynostosis: Current and Potential Use Cases. J Craniofac Surg . 2021;32:956-963.
136. Abrams-Pompe RS, Fanti S, Schoots IG, Moore CM, Turkbey B, Vickers AJ, Walz
J, Steuber T and Eastham JA. The Role of Magnetic Resonance Imaging and Positron Emission Tomography/Computed Tomography in the Primary Staging of Newly Diagnosed Prostate Cancer: A Systematic Review of the Literature. Eur Urol Oncol . 2021;4:370-395.
137. Aerts A, Eberlein U, Holm S, Hustinx R, Konijnenberg M, Strigari L, van Leeuwen FWB, Glatting G and Lassmann M. EANM position paper on the role of radiobiology in nuclear medicine. Eur J Nucl Med Mol Imaging . 2021;48:3365-3377.
138. Alemany S, Blok E, Jansen PR, Muetzel RL and White T. Brain morphology, autistic traits, and polygenic risk for autism: A population-based neuroimaging study. Autism Res . 2021;14:2085-2099.
139. Alves F, Antunes IF, Cazzola E, Cleeren F, Cornelissen B, Denkova A, Engle J, Faivre-Chauvet A, Gillings N, Hendrikx J, Jalilian AR, van der Meulen NP, Mikolajczak R, Neels OC, Pillai MRA, Reilly R, Rubow S, Seimbille Y, Spreckelmeyer S, Szymanski W and Taddei C. Highlight selection of radiochemistry and radiopharmacy developments by editorial board. EJNMMI Radiopharm Chem . 2021;6:31.
140. Amier RP, Marcks N, Hooghiemstra AM, Nijveldt R, van Buchem MA, de Roos A, Biessels GJ, Kappelle LJ, van Oostenbrugge RJ, van der Geest RJ, Bots ML, Greving JP, Niessen WJ, van Osch MJP, de Bresser J, van de Ven PM, van der Flier WM, Brunner-La Rocca HP, van Rossum AC and Heart-Brain Connection C. Hypertensive Exposure Markers by MRI in Relation to Cerebral Small Vessel Disease and Cognitive Impairment. JACC Cardiovasc Imaging . 2021;14:176-185.
141. Amini M, den Hartog SJ, van Leeuwen N, Eijkenaar F, Kuhrij LS, Stolze LJ, Nederkoorn PJ, Lingsma HF, van Es ACGM, van den Wijngaard IR, van der Lugt A, Dippel DWJ, Roozenbeek B and the PERFEQTOS Investigators. Performance feedback on the quality of care in hospitals performing thrombectomy for ischemic stroke (PERFEQTOS): protocol of a stepped wedge cluster randomized trial. Trials 2021;22:870.9.
142. Arslan M, Schaap J, Moelker A, Rood PPM, Boersma E, Nieman K, Dubois EA and Dedic A. Coronary CT angiography for suspected acute coronary syndrome: sex-associated differences. Neth Heart J 2021;29:518-524.
143. Bangma CH, van Leenders G, Roobol MJ, Schoots IG and Anser Prostate Cancer N. Restricting False-positive Magnetic Resonance Imaging Scans to Reduce Overdiagnosis of Prostate Cancer. Eur Urol 2021;79:30-32.
144. Bates AS, Ayers J, Kostakopoulos N, Lumsden T, Schoots IG, Willemse PM, Yuan Y, van den Bergh RCN, Grummet JP, van der Poel HG, Rouviere O, Moris L, Cumberbatch MG, Lardas M, Liew M, Van den Broeck T, Gandaglia G, Fossati N, Briers E, De Santis M, Fanti S, Gillessen S, Oprea-Lager DE, Ploussard G, Henry AM, Tilki D, van der Kwast TH, Wiegel T, N’Dow J, Mason MD, Cornford P, Mottet N and Lam TBL. A Systematic Review of Focal Ablative Therapy for Clinically Localised Prostate Cancer in Comparison with Standard Management Options: Limitations of the Available Evidence and Recommendations for Clinical Practice and Further Research. Eur Urol Oncol . 2021;4:405-423.
145. Benali F, Hinsenveld WH, van der Leij C, Roozenbeek B, van de Graaf RA, Staals J, Lingsma HF, van der Lugt A, Majoie CBM, van Zwam WH and the MR CLEAN Registry Investigators. Effect of Heparinized Flush Concentration on Safety and Efficacy During Endovascular Thrombectomy for Acute Ischemic Stroke: Results from the MR CLEAN Registry. Cardiovasc Intervent Radiol . 2021;44:750-755.
146. Bernhardt P, Svensson J, Hemmingsson J, van der Meulen NP, Zeevaart JR, Konijnenberg MW, Muller C and Kindblom J. Dosimetric Analysis of the Short-Ranged Particle Emitter (161)Tb for Radionuclide Therapy of Metastatic Prostate Cancer. Cancers (Basel) . 2021;13:2011.
147. Berzenji D, Sewnaik A, Keereweer S, Monserez DA, Verduijn GM, van Meerten E, Mast H, Mureau MAM, van der Lugt A, Koljenovic S, Dronkers EAC, Baatenburg de
Jong RJ and Hardillo JA. Dissemination patterns and chronology of distant metastasis affect survival of patients with head and neck squamous cell carcinoma. Oral Oncol . 2021;119:105356.
148. Biewenga M, van der Kooij MK, Wouters M, Aarts MJB, van den Berkmortel F, de Groot JWB, Boers-Sonderen MJ, Hospers GAP, Piersma D, van Rijn RS, Suijkerbuijk KPM, Ten Tije AJ, van der Veldt AAM Vreugdenhil G, Haanen J, van der Eertwegh AJM, van Hoek B and Kapiteijn E. Checkpoint inhibitor induced hepatitis and the relation with liver metastasis and outcome in advanced melanoma patients. Hepatol Int . 2021;15:510-519.
149. Blazevic A, Brabander T, Zandee WT, Hofland J, Franssen GJH, van Velthuysen MF, Feelders RA and De Herder WW. Evolution of the Mesenteric Mass in Small Intestinal Neuroendocrine Tumours. Cancers (Basel) . 2021;13:443.
150. Blazevic A, Starmans MPA, Brabander T, Dwarkasing RS van Gils RAH, Hofland J, Franssen GJH, Feelders RA, Niessen WJ, Klein S and de Herder WW. Predicting symptomatic mesenteric mass in small intestinal neuroendocrine tumors using radiomics. Endocr Relat Cancer 2021;28:529-539.
151. Boekhout AH, Rogiers A, Jozwiak K, Boers-Sonderen MJ, van den Eertwegh AJ, Hospers GA, de Groot JWB, Aarts MJB, Kapiteijn E, Ten Tije AJ, Piersma D, Vreugdenhil G, van der Veldt AA, Suijkerbuijk KPM, Rozeman EA, Neyns B, Janssen KJ, van de Poll-Franse LV and Blank CU. Health-related quality of life of longterm advanced melanoma survivors treated with anti-CTLA-4 immune checkpoint inhibition compared to matched controls. Acta Oncol . 2021;60:69-77.
152. Boon J, Ploem T, Simpson CS, Hermann I, Akcakaya M, Oei EH, Zadpoor AA, Tumer N, Piscaer TM, Tourais J and Weingartner S. Magnetic Resonance Imaging compatible Elastic Loading Mechanism (MELM): A minimal footprint device for MR imaging under load. Annu Int Conf IEEE Eng Med Biol Soc . 2021:3721-3724.
153. Borowczyk M, Wolinski K, Wieckowska B, Jodlowska-Siewert E, Szczepanek-Parulska E, Verburg FA and Ruchala M. Sonographic Features Differentiating Follicular Thyroid Cancer from Follicular Adenoma-A Meta-Analysis. Cancers (Basel) 2021;13:938.
154. Brady AP, Bello JA, Derchi LE, Fuchsjager M, Goergen S, Krestin GP, Lee EJ, Levin DC, Pressacco J, Rao VM, Slavotinek J, Visser JJ, Walker RE and Brink JA. Radiology in the era of value-based healthcare: A multi-society expert statement from the ACR, CAR, ESR, IS3R, RANZCR and RSNA. J Med Imaging Radiat Oncol 2021;65:60-66.
155. Brady AP, Bello JA, Derchi LE, Fuchsjager M, Goergen S, Krestin GP, Lee EJY, Levin DC, Pressacco J, Rao VM, Slavotinek J, Visser JJ, Walker REA and Brink JA. Radiology in the Era of Value-Based Healthcare: A Multi Society Expert Statement From the ACR, CAR, ESR, IS3R, RANZCR, and RSNA. J Am Coll Radiol 2021;18:877-883.
156. Brady AP, Bello JA, Derchi LE, Fuchsjager M, Goergen S, Krestin GP, Lee EJY, Levin DC, Pressacco J, Rao VM, Slavotinek J, Visser JJ, Walker REA and Brink JA. Radiology in the Era of Value-based Healthcare: A Multi-Society Expert Statement from the ACR, CAR, ESR, IS3R, RANZCR, and RSNA. Radiology . 2021;298:486491.
157. Bressler J, Davies G, Smith AV, Saba Y, Bis JC, Jian X, Hayward C, Yanek L, Smith JA, Mirza SS, Wang R, Adams HHH, Becker D, Boerwinkle E, Campbell A, Cox SR, Eiriksdottir G, Fawns-Ritchie C, Gottesman RF, Grove ML, Guo X, Hofer E, Kardia SLR, Knol MJ, Koini M, Lopez OL, Marioni RE, Nyquist P, Pattie A, Polasek O, Porteous DJ, Rudan I, Satizabal CL, Schmidt H, Schmidt R, Sidney S, Simino J, Smith BH, Turner ST, van der Lee SJ, Ware EB, Whitmer RA, Yaffe K, Yang Q, Zhao W, Gudnason V, Launer LJ, Fitzpatrick AL, Psaty BM, Fornage M, Arfan Ikram M, van Duijn CM, Seshadri S, Mosley TH and Deary IJ. Association of low-frequency and rare coding variants with information processing
speed. Transl Psychiatry . 2021;11:613.
158. Bruggeman AAE, Kappelhof M, Arrarte Terreros N, Tolhuisen ML, Konduri PR, Boodt N, van Beusekom HMM, Hund HM, Taha A, van der Lugt A, Roos Y, van Es ACGM, van Zwam WH, Postma AA, Dippel DWJ, Lingsma HF, Marquering HA, Emmer BJ, Majoie C and the MR CLEAN Registry Investigators. Endovascular treatment for calcified cerebral emboli in patients with acute ischemic stroke. J Neurosurg 2021:1-11.
159. Cahalane R, Boodt N, Akyildiz AC, Giezen JA, Mondeel M, van der Lugt A, Marquering H and Gijsen F. A review on the association of thrombus composition with mechanical and radiological imaging characteristics in acute ischemic stroke. J Biomech . 2021;129:110816.
160. Camarasa R, Bos D, Hendrikse J, Nederkoorn P, Kooi M, van der Lugt A and de Bruijne M. A Quantitative Comparison of Epistemic Uncertainty Maps Applied to Multi-Class Segmentation. Journal of Machine Learning for Biomedical Imaging (MELBA) . 2021;013:1-39.
161. Caulley L, Hunink MG, Randolph GW and Shin JJ. Evidence-Based Medicine in Otolaryngology, Part XI: Modeling and Analysis to Support Decisions. Otolaryngol Head Neck Surg . 2021;164:462-472.
162. Chalian M, Li X, Guermazi A, Obuchowski NA, Carrino JA, Oei EH, Link TM, Committee RQMB and Members SQMBC. The QIBA Profile for MRI-based Compositional Imaging of Knee Cartilage. Radiology 2021;301:423-432.
163. Chappell MA, McConnell FAK, Golay X, Gunther M, Hernandez-Tamames JA, van Osch MJ and Asllani I. Partial volume correction in arterial spin labeling perfusion MRI: A method to disentangle anatomy from physiology or an analysis step too far? Neuroimage . 2021;238:118236.
164. Cheplygina V, Perez-Rovira A, Kuo W, Tiddens HAWM and de Bruijne M. Crowdsourcing airway annotations in chest computed tomography images. Plos One
2021;16:e0249580.
165. Chiesa C, Sjogreen-Gleisner K, Walrand S, Strigari L, Flux G, Gear J, Stokke C, Gabina PM, Bernhardt P and Konijnenberg M. EANM dosimetry committee series on standard operational procedures: a unified methodology for (99m)Tc-MAA pre- and (90)Y peri-therapy dosimetry in liver radioembolization with (90)Y microspheres. EJNMMI Phys . 2021;8:77.
166. Clement P, Booth T, Borovecki F, Emblem KE, Figueiredo P, Hirschler L, Jancalek R, Keil VC, Maumet C, Ozsunar Y, Pernet C, Petr J, Pinto J, Smits M and Warnert EAH GliMR: Cross-Border Collaborations to Promote Advanced MRI Biomarkers for Glioma. J Med Biol Eng . 2021;41:115125.
167. Collins NJ, van der Heijden RA, Macri EM, de Kanter JL, Oei EHG, Crossley KM, Bierma-Zeinstra SMA and van Middelkoop M. Patellofemoral alignment, morphology and structural features are not related to sitting pain in individuals with patellofemoral pain. Knee . 2021;28:104109.
168. Cornelissen B, Terry S, Nonnekens J, Pouget JP and European Working Group on the Radiobiology of Molecular R. First Symposium of the European Working Group on the Radiobiology of Molecular Radiotherapy. J Nucl Med 2021;62:14N-15N.
169. Cornford P, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, Fanti S, Fossati N, Gandaglia G, Gillessen S, Grivas N, Grummet J, Henry AM, der Kwast THV, Lam TB, Lardas M, Liew M, Mason MD, Moris L, Oprea-Lager DE, der Poel HGV, Rouviere O, Schoots IG, Tilki D, Wiegel T, Willemse PM and Mottet N. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur Urol 2021;79:263-282.
170. Cortes Hidalgo AP, Thijssen S, Delaney SW, Vernooij MW, Jansen PW, Bakermans-Kranenburg MJ, van IMH, White
T and Tiemeier H. Harsh Parenting and Child Brain Morphology: A Population-Based Study. Child Maltreat 2021:1077559520986856.
171. Costagliola di Polidoro A, Zambito G, Haeck J, Mezzanotte L, Lamfers M, Netti PA and Torino E. Theranostic Design of Angiopep-2 Conjugated Hyaluronic Acid Nanoparticles (Thera-ANG-cHANPs) for Dual Targeting and Boosted Imaging of Glioma Cells. Cancers (Basel) 2021;13:503.
172. Crowley C, Connor OJO, Ciet P, Tiddens HAWM and Maher MM. The evolving role of radiological imaging in cystic fibrosis. Curr Opin Pulm Med . 2021;27:575-585.
173. Damotte V, van der Lee SJ, Chouraki V, Grenier-Boley B, Simino J, Adams H, Tosto G, White C, Terzikhan N, Cruchaga C, Knol MJ, Li S, Schraen S, Grove ML, Satizabal C, Amin N, Berr C, Younkin S, Alzheimer’s Disease Neuroimaging I, Gottesman RF, Buee L, Beiser A, Knopman DS, Uitterlinden A, DeCarli C, Bressler J, DeStefano A, Dartigues JF, Yang Q, Boerwinkle E, Tzourio C, Fornage M, Ikram MA, Amouyel P, de Jager P, Reitz C, Mosley TH, Lambert JC, Seshadri S and van Duijn CM. Plasma amyloid beta levels are driven by genetic variants near APOE, BACE1, APP, PSEN2: A genome-wide association study in over 12,000 non-demented participants. Alzheimers Dement . 2021;17:1663-1674.
174. de Glas NA, Bastiaannet E, van den Bos F, Mooijaart SP, van der Veldt AAM, Suijkerbuijk KPM, Aarts MJB, van den Berkmortel F, Blank CU, Boers-Sonderen MJ, van den Eertwegh AJM, de Groot JB, Haanen J, Hospers GAP, Jalving H, Piersma D, van Rijn RS, Ten Tije AJ, Vreugdenhil G, Wouters M, Portielje JEA and Kapiteijn EW. Toxicity, Response and Survival in Older Patients with Metastatic Melanoma Treated with Checkpoint Inhibitors. Cancers (Basel) . 2021;13:2826.
175. de Joode K, Dingemans AC and van der Veldt AAM. Remarkable Healthy Cohort of Patients With Cancer. J Clin Oncol 2021;39:1092-1093.
176. de Joode K, Oostvogels AAM, GeurtsvanKessel CH, de Vries RD, Mathijssen RHJ, Debets R and van der Veldt AAM. Case Report: Adequate T and B Cell Responses in a SARS-CoV-2 Infected Patient After Immune Checkpoint Inhibition. Front Immunol . 2021;12:627186.
177. de Kanter JLM, Oei EHG, Schiphof D, van Meer BL, van Middelkoop M, Reijman S, Bierma-Zeinstra SMA and van der Heijden RA. Prevalence of small osteophytes on knee MRI in several large clinical and population-based studies of various age groups and OA risk factors. Osteoarthritis and Cartilage Open . 2021;3:100187.
178. de Leeuw FA, Karamujic-Comic H, Tijms BM, Peeters CFW, Kester MI, Scheltens P, Ahmad S, Vojinovic D, Adams HHH, Hankemeier T, Bos D, van der Lugt A, Vernooij MW, Ikram MA, Amin N, Barkhof F, Teunissen CE, van Duijn CM and van der Flier WM. Circulating metabolites are associated with brain atrophy and white matter hyperintensities. Alzheimers Dement . 2021;17:205-214.
179. de Man FM, van Eerden RAG, van Doorn GM, Oomen-de Hoop E, Koolen SLW, Olieman JF, de Bruijn P, Veraart JN, van Halteren HK, Sandberg Y, Moelker A, JNM IJ, Lolkema MP, van Gelder T, Dolle MET, de Bruin RWF and Mathijssen RHJ. Effects of Protein and Calorie Restriction on the Metabolism and Toxicity Profile of Irinotecan in Cancer Patients. Clin Pharmacol Ther . 2021;109:1304-1313.
180. de Mestier L, Lamarca A, Hernando J, Zandee W, Alonso-Gordoa T, Perrier M, Walenkamp AM, Chakrabarty B, Landolfi S, Van Velthuysen MF, Kats-Ugurlu G, Caminoa A, Ronot M, Manoharan P, Garcia-Alvarez A, Brabander T, Garcia Gomez-Muriel MI, Cadiot G, Couvelard A, Capdevila J, Pavel ME and Cros J. Treatment outcomes of advanced digestive well-differentiated grade 3 NETs. Endocr Relat Cancer . 2021;28:549-561.
181. de Meza MM, Ismail RK, Rauwerdink D, van Not OJ, van Breeschoten J, Blokx WAM, de Boer A, van Dartel M, Hilarius DL, Ellebaek E, Bonenkamp HJ, Blank CU,
Aarts MJB, van Akkooi ACJ, van den Berkmortel F, Boers-Sonderen MJ, de Groot JWB, Haanen JB, Hospers GAP, Kapiteijn EW, Piersma D, van Rijn RS, van der Veldt AAM, Vreugdenhil A, Westgeest HM, van den Eertwegh AJM, Suijkerbuijk KPM and Wouters M. Adjuvant treatment for melanoma in clinical practice - Trial versus reality. Eur J Cancer . 2021;158:234-245.
182. de Mol CL, Bruijstens AL, Jansen PR, Dremmen M, Wong Y, van der Lugt A, White T and Neuteboom RF. Prevalence of radiologically isolated syndrome in a pediatric population-based cohort: A longitudinal description of a rare diagnosis. Mult Scler . 2021;27:1790-1793.
183. de Mol CL, Looman KIM, van Luijn MM, Kreft KL, Jansen PR, van Zelm MC, Smolders J, White TJH, Moll HA and Neuteboom RF. T cell composition and polygenic multiple sclerosis risk: A population-based study in children. Eur J Neurol . 2021;28:3731-3741.
184. de Reijke TM, Schoots IG and van den Berg K. ‘Intelligent case-finding’ in prostate cancer: an alternative to population-based screening. ‘Intelligente opsporing’ van prostaatkanker. Ned Tijdschr Geneeskd . 2021;165:D5997.
185. de With M, Hurkmans DP, Oomen-de Hoop E, Lalouti A, Bins S, El Bouazzaoui S, van Brakel M, Debets R, Aerts J, van Schaik RHN, Mathijssen RHJ and van der Veldt AAM. Germline Variation in PDCD1 Is Associated with Overall Survival in Patients with Metastatic Melanoma Treated with Anti-PD-1 Monotherapy. Cancers (Basel) . 2021;13:1370.
186. DeBoer EM, Kimbell JS, Pickett K, Hatch JE, Akers K, Brinton J, Hall GL, King L, Ramanauskas F, Rosenow T, Stick SM, Tiddens HAWM, Ferkol TW, Ranganathan SC, Davis SD and Arest CF. Lung inflammation and simulated airway resistance in infants with cystic fibrosis. Respir Physiol Neurobiol . 2021;293:103722.
187. Dekker I, Schoonheim MM, Venkatraghavan V, Eijlers AJC, Brouwer I, Bron EE, Klein S, Wattjes MP, Wink AM, Geurts
JJG, Uitdehaag BMJ, Oxtoby NP, Alexander DC, Vrenken H, Killestein J, Barkhof F and Wottschel V. The sequence of structural, functional and cognitive changes in multiple sclerosis. Neuroimage Clin 2021;29:102550.
188. Delaney SW, Cortes Hidalgo AP, White T, Haneuse S, Ressler KJ, Tiemeier H and Kubzansky LD. Are all threats equal? Associations of childhood exposure to physical attack versus threatened violence with preadolescent brain structure. Dev Cogn Neurosci . 2021;52:101033.
189. Deng S, Gigliobianco MR, Mijiti Y, Minicucci M, Cortese M, Campisi B, Voinovich D, Battistelli M, Salucci S, Gobbi P, Lupidi G, Zambito G, Mezzanotte L, Censi R and Di Martino P. Dually Cross-Linked Core-Shell Structure Nanohydrogel with Redox-Responsive Degradability for Intracellular Delivery. Pharmaceutics 2021;13:2048.
190. Deng S, Iscaro A, Zambito G, Mijiti Y, Minicucci M, Essand M, Lowik C, Muthana M, Censi R, Mezzanotte L and Di Martino P. Development of a New Hyaluronic Acid Based Redox-Responsive Nanohydrogel for the Encapsulation of Oncolytic Viruses for Cancer Immunotherapy. Nanomaterials (Basel) . 2021;11:144.
191. Dijkstra BM, de Jong M, Stroet MCM, Andreae F, Dulfer SE, Everts M, Kruijff S, Nonnekens J, den Dunnen WFA, Kruyt FAE and Groen RJM. Evaluation of AcLys(0)(IRDye800CW)Tyr(3)-octreotate as a novel tracer for SSTR2-targeted molecular fluorescence guided surgery in meningioma. J Neurooncol . 2021;153:211222.
192. Dudurych I, Garcia-Uceda A, Saghir Z, Tiddens HAWM, Vliegenthart R and de Bruijne M. Creating a training set for artificial intelligence from initial segmentations of airways. European Radiology Experimental . 2021;5:54.
193. Duvekot MH, Venema E, Lingsma HF, Coutinho JM, van der Worp HB, Hofmeijer J, Bokkers RP, van Es AC, van der Lugt A, Kerkhoff H, Dippel DW, Roozenbeek B and the MR CLEAN Registry Investiga-
tors. Sensitivity of prehospital stroke scales for different intracranial large vessel occlusion locations. Eur Stroke J 2021;6:194-204.
194. Duvekot MHC, Venema E, Rozeman AD, Moudrous W, Vermeij FH, Biekart M, Lingsma HF, Maasland L, Wijnhoud AD, Mulder L, Alblas KCL, van Eijkelenburg RPJ, Buijck BI, Bakker J, Plaisier AS, Hensen JH, Lycklama ANGJ, van Doormaal PJ, van Es AC, van der Lugt A, Kerkhoff H, Dippel DWJ, Roozenbeek B and the PRESTO investigators. Comparison of eight prehospital stroke scales to detect intracranial large-vessel occlusion in suspected stroke (PRESTO): a prospective observational study. Lancet Neurol 2021;20:213-221.
195. Eilsberger F, Tuttle RM, Librizzi D, Pfestroff A, Luster M and Verburg FA Perioperative diagnostics of patients referred for radioiodine therapy of differentiated thyroid carcinoma: referral center experience in an iodine-insufficient country. Endocrine . 2021;72:721-726.
196. Eilsberger F and Verburg FA. Controversies in Radioiodine Treatment of Lowand Intermediate-risk Thyroid Cancer. Clin Oncol (R Coll Radiol) . 2021;33:6874.
197. El Sayed M, Hirsch A, Boekholdt M, van Dussen L, Datema M, Hollak C and Langeveld M. Influence of sex and phenotype on cardiac outcomes in patients with Fabry disease. Heart . 2021;107:18891897.
198. Eyck BM, van der Wilk BJ, Noordman BJ, Wijnhoven BPL, Lagarde SM, Hartgrink HH, Coene P, Dekker JWT, Doukas M, van der Gaast A, Heisterkamp J, Kouwenhoven EA, Nieuwenhuijzen GAP, Pierie JEN, Rosman C, van Sandick JW, van der Sangen MJC, Sosef MN, van der Zaag ES, Spaander MCW, Valkema R, Lingsma HF, Steyerberg EW, van Lanschot JJB and SANO Study group. Updated protocol of the SANO trial: a stepped-wedge cluster randomised trial comparing surgery with active surveillance after neoadjuvant chemoradiotherapy for oesophageal cancer. Trials . 2021;22:345.
199. Fakhry F, Rouwet EV, Spillenaar Bilgen R, van der Laan L, Wever JJ, Teijink JAW, Hoffmann WH, van Petersen A, van Brussel JP, Stultiens GNM, Derom A, den Hoed PT, Ho GH, van Dijk LC, Verhofstad N, Orsini M, Hulst I, van Sambeek M, Rizopoulos D, Moelker A and Hunink MGM. Endovascular Revascularization Plus Supervised Exercise Versus Supervised Exercise Only for Intermittent Claudication: A Cost-Effectiveness Analysis. Circ Cardiovasc Interv . 2021;14:e010703.
200. Fani L, van Dam-Nolen DHK, Vernooij M, Kavousi M, van der Lugt A and Bos D Circulatory markers of immunity and carotid atherosclerotic plaque. Atherosclerosis . 2021;325:69-74.
201. Ferket BS, Hunink MGM, Masharani U, Max W, Yeboah J and Fleischmann KE. Long-term Predictions of Incident Coronary Artery Calcium to 85 Years of Age for Asymptomatic Individuals With and Without Type 2 Diabetes. Diabetes Care 2021;44:1664-1671.
202. Flies CM, Snijders TJ, Van Seeters T, Smits M, De Vos FYF, Hendrikse J and Dankbaar JW. Perfusion imaging with arterial spin labeling (ASL)-MRI predicts malignant progression in lowgrade (WHO grade II) gliomas. Neuroradiology . 2021;63:20232033.
203. Fohner AE, Bartz TM, Tracy RP, Adams HHH, Bis JC, Djousse L, Satizabal CL, Lopez OL, Seshadri S, Mukamal KJ, Kuller LH, Psaty BM and Longstreth WT, Jr. Association of Serum Neurofilament Light Chain Concentration and MRI Findings in Older Adults: The Cardiovascular Health Study. Neurology . 2021:e903-e911.
204. Fournier L, Costaridou L, Bidaut L, Michoux N, Lecouvet FE, de Geus-Oei LF, Boellaard R, Oprea-Lager DE, Obuchowski NA, Caroli A, Kunz WG, Oei EH, O’Connor JPB, Mayerhoefer ME, Franca M, Alberich-Bayarri A, Deroose CM, Loewe C, Manniesing R, Caramella C, Lopci E, Lassau N, Persson A, Achten R, Rosendahl K, Clement O, Kotter E, Golay X, Smits M, Dewey M, Sullivan DC, van der Lugt A, deSouza NM and the European Society of Radiology. Incorporat-
ing radiomics into clinical trials: expert consensus endorsed by the European Society of Radiology on considerations for data-driven compared to biologically driven quantitative biomarkers. Eur Radiol . 2021;31:6001-6012.
205. Fournier L, de Geus-Oei LF, Regge D, Oprea-Lager DE, D’Anastasi M, Bidaut L, Bauerle T, Lopci E, Cappello G, Lecouvet F, Mayerhoefer M, Kunz WG, Verhoeff JJC, Caruso D, Smits M, Hoffmann RT, Gourtsoyianni S, Beets-Tan R, Neri E, deSouza NM, Deroose CM and Caramella C. Twenty Years On: RECIST as a Biomarker of Response in Solid Tumours an EORTC Imaging Group - ESOI Joint Paper. Front Oncol . 2021;11:800547.
206. Franken MG, Leeneman B, Aarts MJB, van Akkooi ACJ, van den Berkmortel F, Boers-Sonderen MJ, van den Eertwegh AJM, de Groot JWB, Hospers GAP, Kapiteijn E, Piersma D, van Rijn RS, Suijkerbuijk KPM, van der Veldt AAM, Westgeest HM, Wouters M, Haanen J and Uyl-de Groot CA. Trends in survival and costs in metastatic melanoma in the era of novel targeted and immunotherapeutic drugs. ESMO Open . 2021;6:100320.
207. Funck KL, Budde RPJ, Viuff MH, Wen J, Jensen JM, Norgaard BL, Bons LR, Duijnhouwer AL, Dey D, Mortensen KH, Andersen NH, Roos-Hesselink JW and Gravholt CH. Coronary plaque burden in Turner syndrome a coronary computed tomography angiography study. Heart Vessels 2021;36:14-23.
208. Garousi J, von Witting E, Borin J, Vorobyeva A, Altai M, Vorontsova O, Konijnenberg MW, Oroujeni M, Orlova A, Tolmachev V and Hober S. Radionuclide therapy using ABD-fused ADAPT scaffold protein: Proof of Principle. Biomaterials 2021;266:120381.
209. Gebre RK, Hirvasniemi J, Lantto I, Saarakkala S, Leppilahti J and Jamsa T. Discrimination of Low-Energy Acetabular Fractures from Controls Using Computed Tomography-Based Bone Characteristics. Ann Biomed Eng . 2021;49:367-381.
210. G iovanella L, Fontana M, Keller F, Verburg FA and Ceriani L. Clinical performance of calcitonin and procalcitonin Elecsys((R)) immunoassays in patients with medullary thyroid carcinoma. Clin Chem Lab Med . 2021;59:743-747.
211. Gong ZQ, Gao P, Jiang C, Xing XX, Dong HM, White T, Castellanos FX, Li HF and Zuo XN. DREAM : A Toolbox to Decode Rhythms of the Brain System. Neuroinformatics . 2021;19:529-545.
212. Graham SE, Clarke SL, Wu KH, ……., de Borst GJ, Kim EK, Adams HHH, Ikram MA, Zhu X, Asselbergs FW, Kraaijeveld AO, Beulens JWJ, Shu XO, Rallidis LS, Pedersen O, Hansen T, …., Hirschhorn JN, Kathiresan S, Program VAMV, Global Lipids Genetics C, Boehnke M, Natarajan P, Peloso GM, Brown CD, Morris AP, Assimes TL, Deloukas P, Sun YV and Willer CJ. The power of genetic diversity in genome-wide association studies of lipids. Nature . 2021;600:675-679.
213. Guglielmi V, Compagne KCJ, Sarrami AH, Sluis WM, van den Berg LA, van der Sluijs PM, Mandell DM, van der Lugt A, Roos Y, Majoie C, Dippel DWJ, Emmer BJ, van Es A, Coutinho JM and the MR CLEAN Trial and the MR CLEAN Registry Investigators. Assessment of Recurrent Stroke Risk in Patients With a Carotid Web. JAMA Neurol . 2021;78:826-833.
214. Harlaar L, Ciet P, van Tulder G, Pittaro A, van Kooten HA, van der Beek NAME, Brusse E, Wielopolski PA de Bruijne M van der Ploeg AT, Tiddens HAWM and van Doorn PA. Chest MRI to diagnose early diaphragmatic weakness in Pompe disease. Orphanet Journal of Rare Diseases 2021;16:21.
215. Heath A, Hunink MG, Krijkamp E and Pechlivanoglou P. Prioritisation and design of clinical trials. Eur J Epidemiol 2021;36:1111-1121.
216. Hehakaya C, Moors EHM, Verkooijen HM, Grobbee DE, Verburg FA and Lam M. (177)Lu-PSMA for advanced prostate cancer: are we ready to play big? Eur J Nucl Med Mol Imaging . 2021;48:23252328.
217. Hermelijn SM, Elders B, Ciet P, Wijnen RMH, Tiddens HAWM and Schnater JM. A clinical guideline for structured assessment of CT-imaging in congenital lung abnormalities. Paediatr Respir Rev 2021;37:80-88.
218. Hoek VT, Edomskis PP, Menon AG, Dwarkasing RS, Kleinrensink GJ, Jeekel J and Lange JF. Calcification of the Aorta-Iliac Trajectory as a Risk Factor for Anastomotic Leakage in Colorectal Surgery: Individual Patient Data Meta-Analysis and Systematic Review. Surg Technol Int 2021;39:155-165.
219. Hokken TW, van Wiechen MP, Ooms JF, El Azzouzi I, de Ronde M, Kardys I, Budde R, Daemen J, de Jaegere PP and Van Mieghem NM. Impact of Interventricular membranous septum length on pacemaker need with different Transcatheter aortic valve implantation systems. Int J Cardiol . 2021;333:152-158.
220. Hoppener DJ, Grunhagen DJ, Eggermont AMM, van der Veldt AAM and Verhoef C. An Overview of Liver Directed Locoregional Therapies. Surg Oncol Clin N Am 2021;30:103-123.
221. Hove DT, Sinha B, Glaudemans A, Gomes A, Swart LE, Tanis W, Budde RPJ and Slart R. (18)F-FDG-Uptake in Mediastinal Lymph Nodes in Suspected Prosthetic Valve Endocarditis: Predictor or Confounder? Front Cardiovasc Med 2021;8:717774.
222. Huang AL, Leipsic JA, Zekry SB, Sellers S, Ahmadi AA, Blanke P, Hadamitzky M, Kim YJ, Conte E, Andreini D, Pontone G, Budoff MJ, Gottlieb I, Lee BK, Chun EJ, Cademartiri F, Maffei E, Marques H, Shin S, Choi JH, Virmani R, Samady H, Stone PH, Berman DS, Narula J, Shaw LJ, Bax JJ and Chang HJ. Effects of chronic kidney disease and declining renal function on coronary atherosclerotic plaque progression: a PARADIGM substudy. Eur Heart J Cardiovasc Imaging . 2021;22:10721082.
223. Hurkmans DP, Sassen SDT, de Joode K, Putter L, Basak EA, Wijkhuijs AJM, Joerger M, Debets R, Koch BCP, Van der Leest
CH, Schreurs MWJ, van der Veldt AAM, Aerts J, Mathijssen RHJ and Koolen SLW. Prospective real-world study on the pharmacokinetics of pembrolizumab in patients with solid tumors. J Immunother Cancer . 2021;9:e002344.
224. Huurman R, Michels M, Bowen DJ, van Slegtenhorst MA, Hirsch A and Schinkel AFL. Left-ventricular outflow tract acceleration time is associated with symptoms in patients with obstructive hypertrophic cardiomyopathy. J Ultrasound 2021;24:279-287.
225. Huurman R, Schinkel AFL, de Jong PL, van Slegtenhorst MA, Hirsch A and Michels M. Impact of sex on timing and clinical outcome of septal myectomy for obstructive hypertrophic cardiomyopathy. Int J Cardiol . 2021;323:133-139.
226. Ismail RK, Sikkes NO, Wouters M, Hilarius DL, Pasmooij AMG, van den Eertwegh AJM, Aarts MJB, van den Berkmortel F, Boers-Sonderen MJ, de Groot JWB, Haanen J, Hospers GAP, Kapiteijn E, Piersma D, van Rijn RS, Suijkerbuijk KPM, Ten Tije BJ, van der Veldt AAM, Vreugdenhil A, van Dartel M and de Boer A. Postapproval trials versus patient registries: comparability of advanced melanoma patients with brain metastases. Melanoma Res . 2021;31:58-66.
227. Jadnanansing R, Blankers M, Dwarkasing R, Etwaroo K, Lumsden V, Dekker J and Bipat R. Prevalence of substance use disorders in an urban and a rural area in Suriname. Trop Med Health . 2021;49:12.
228. Jimenez-Solem E, Petersen TS, Hansen C, Hansen C, Lioma C, Igel C, Boomsma W, Krause O, Lorenzen S, Selvan R, Petersen J, Nyeland ME, Ankarfeldt MZ, Virenfeldt GM, Winther-Jensen M, Linneberg A, Ghazi MM, Detlefsen N, Lauritzen AD, Smith AG, de Bruijne M, Ibragimov B, Petersen J, Lillholm M, Middleton J, Mogensen SH, Thorsen-Meyer HC, Perner A, Helleberg M, Kaas-Hansen BS, Bonde M, Bonde A, Pai A, Nielsen M and Sillesen M. Developing and validating COVID-19 adverse outcome risk prediction models from a bi-national Europe-
an cohort of 5594 patients. Sci Rep-Uk 2021;11:3246.
229. Jochems A, Bastiaannet E, Aarts MJB, van Akkooi ACJ, van den Berkmortel F, Boers-Sonderen MJ, van den Eertwegh AJM, de Glas NG, de Groot JWB, Haanen J, Hospers GAP, van der Hoeven JJM, Piersma D, van Rijn RS, Suijkerbuijk KPM, Ten Tije AJ, van der Veldt AAM, Vreugdenhil G, van Zeijl MCT, Kapiteijn E and Wouters M. Outcomes for systemic therapy in older patients with metastatic melanoma: Results from the Dutch Melanoma Treatment Registry. J Geriatr Oncol 2021;12:1031-1038.
230. Juffermans JF, Minderhoud SCS, Wittgren J, Kilburg A, Ese A, Fidock B, Zheng YC, Zhang JM, Blanken CPS, Lamb HJ, Goeman JJ, Carlsson M, Zhao S, Planken RN, van Ooij P, Zhong L, Chen X, Garg P, Emrich T, Hirsch A, Toger J and Westenberg JJM. Multicenter Consistency Assessment of Valvular Flow Quantification With Automated Valve Tracking in 4D Flow CMR. JACC Cardiovasc Imaging 2021;14:1354-1366.
231. Kaiser Y, Singh SS, Zheng KH, Verbeek R, Kavousi M, Pinto SJ, Vernooij MW, Sijbrands EJG, Boekholdt SM, de Rijke YB, Stroes ESG and Bos D. Lipoprotein(a) is robustly associated with aortic valve calcium. Heart . 2021;107:1422-1428.
232. Kan HE, Heunis S, Taylor S, Wagner A and White T. ESMRMB annual meeting roundtable discussion: “Challenges and solutions in data sharing-an MRI perspective”. MAGMA . 2021;34:483-486.
233. Kappelhof M, Tolhuisen ML, Treurniet KM, Dutra BG, Alves H, Zhang G, Brown S, Muir KW, Davalos A, Roos Y, Saver JL, Demchuk AM, Jovin TG, Bracard S, Campbell BCV, van der Lugt A, Guillemin F, White P, Hill MD, Dippel DWJ, Mitchell PJ, Goyal M, Marquering HA, Majoie C and the HERMES Collaborators. Endovascular Treatment Effect Diminishes With Increasing Thrombus Perviousness: Pooled Data From 7 Trials on Acute Ischemic Stroke. Stroke . 2021;52:3633-3641.
234. Karnik NS, Cortese S, Njoroge WFM, Drury SS, Frazier JA, McCauley E, Henderson SW, White T, Althoff RR and Novins DK. Editorial: Analyzing Treatment and Prescribing in Large Administrative Datasets With a Lens on Equity. J Am Acad Child Adolesc Psychiatry . 2021;60:818-820.
235. Kersten MJ, Driessen J, Zijlstra JM, Plattel WJ, Morschhauser F, Lugtenburg PJ, Brice P, Hutchings M, Gastinne T, Liu R, Burggraaff CN, Nijland M, Tonino SH, Arens AIJ, Valkema R, van Tinteren H, Lopez-Yurda M, Diepstra A, De Jong D and Hagenbeek A. Combining brentuximab vedotin with dexamethasone, high-dose cytarabine and cisplatin as salvage treatment in relapsed or refractory Hodgkin lymphoma: the phase II HOVON/LLPC Transplant BRaVE study. Haematologica 2021;106:1129-1137.
236. Khan MS, Stamp E, Sammon C, Brabander T, de Herder WW and Pavel ME. Matching-adjusted indirect treatment comparison of [(177)Lu]Lu-DOTA-TATE, everolimus and sunitinib in advanced, unresectable gastroenteropancreatic neuroendocrine tumours: Relative effectiveness of [(177)Lu]Lu-DOTA-TATE in gastroenteropancreatic neuroendocrine tumours. EJC Suppl . 2021;16:5-13.
237. Kinoshita T, Moriwaki K, Hanaki N, Kitamura T, Yamakawa K, Fukuda T, Hunink MGM and Fujimi S. Cost-effectiveness of a hybrid emergency room system for severe trauma: a health technology assessment from the perspective of the third-party payer in Japan. World J Emerg Surg . 2021;16:2.
238. Konduri P, van Kranendonk K, Boers A, Treurniet K, Berkhemer O, Yoo AJ, van Zwam W, van Oostenbrugge R, van der Lugt A, Dippel D, Roos Y, Bot J, Majoie C, Marquering H and the MR CLEAN Trial Investigators. The Role of Edema in Subacute Lesion Progression After Treatment of Acute Ischemic Stroke. Front Neurol . 2021;12:705221.
239. Konduri P, van Voorst H, Bucker A, van Kranendonk K, Boers A, Treurniet K, Berkhemer O, Yoo AJ, van Zwam W, van
Oostenbrugge R, van der Lugt A, Dippel D, Roos Y, Bot J, Majoie C and Marquering H. Posttreatment Ischemic Lesion Evolution Is Associated With Reduced Favorable Functional Outcome in Patients With Stroke. Stroke . 2021;52:35233531.
240. Koppelmans V, van der Willik KD, Aleman BMP, van Leeuwen FE, Kavousi M, Arshi B, Vernooij MW, Ikram MA and Schagen SB. Long-term effects of adjuvant treatment for breast cancer on carotid plaques and brain perfusion. Breast Cancer Res Treat 2021;186:167-176.
241. Krenning BJ, der Heiden KV, Duncker DJ, de Jong M and Bernsen MR. Nuclear Imaging of Post-infarction Inflammation in Ischemic Cardiac Diseases - New Radiotracers for Potential Clinical Applications. Curr Radiopharm . 2021;14:184208.
242. Kuiper LM, Ikram MK, Kavousi M, Vernooij MW, Ikram MA and Bos D. C-factor: a summary measure for systemic arterial calcifications. BMC Cardiovasc Disord 2021;21:317.
243. Kuipers S, Biessels GJ, Greving JP, Amier RP, de Bresser J, Bron EE, van der Flier WM, van der Geest RJ, Hooghiemstra AM, van Oostenbrugge RJ, van Osch MJP, Kappelle LJ, Exalto LG and Heart-Brain Connection C. Sex and Cardiovascular Function in Relation to Vascular Brain Injury in Patients with Cognitive Complaints. J Alzheimers Dis . 2021;84:261-271.
244. Labrecque JA, Hunink MMG, Ikram MA and Ikram MK. Do Case-Control Studies Always Estimate Odds Ratios? Am J Epidemiol . 2021;190:318-321.
245. Lamballais S, Adank MC, Hussainali RF, Schalekamp-Timmermans S, Vernooij MW, Luik AI, Steegers EAP and Ikram MA. Design and overview of the Origins of Alzheimer’s Disease Across the Life course (ORACLE) study. Eur J Epidemiol 2021;36:117-127.
246. Lamballais S, Jansen PR, Labrecque JA, Ikram MA and White T. Genetic scores
for adult subcortical volumes associate with subcortical volumes during infancy and childhood. Hum Brain Mapp 2021;42:1583-1593.
247. Langezaal LCM, van der Hoeven E, Mont’Alverne FJA, de Carvalho JJF, Lima FO, Dippel DWJ, van der Lugt A, Lo RTH, Boiten J, Lycklama ANGJ, Staals J, van Zwam WH, Nederkoorn PJ, Majoie C, Gerber JC, Mazighi M, Piotin M, Zini A, Vallone S, Hofmeijer J, Martins SO, Nolte CH, Szabo K, Dias FA, Abud DG, Wermer MJH, Remmers MJM, Schneider H, Rueckert CM, de Laat KF, Yoo AJ, van Doormaal PJ, van Es A, Emmer BJ, Michel P, Puetz V, Audebert HJ, Pontes-Neto OM, Vos JA, Kappelle LJ, Algra A, Schonewille WJ and Group BS. Endovascular Therapy for Stroke Due to Basilar-Artery Occlusion. N Engl J Med . 2021;384:1910-1920.
248. Lassmann M, Eberlein U, Gear J, Konijnenberg M and Kunikowska J. Dosimetry for Radiopharmaceutical Therapy: The European Perspective. J Nucl Med 2021;62:73S-79S.
249. Lauwerends LJ, van Driel P, Baatenburg de Jong RJ, Hardillo JAU, Koljenovic S, Puppels G, Mezzanotte L, Lowik C, Rosenthal EL, Vahrmeijer AL and Keereweer S. Real-time fluorescence imaging in intraoperative decision making for cancer surgery. Lancet Oncol . 2021;22:e186-e195.
250. Le Rhun E, Guckenberger M, Smits M, Dummer R, Bachelot T, Sahm F, Galldiks N, de Azambuja E, Berghoff AS, Metellus P, Peters S, Hong YK, Winkler F, Schadendorf D, van den Bent M, Seoane J, Stahel R, Minniti G, Wesseling P, Weller M, Preusser M, Board EE and clinicalguidelines@esmo.org EGCEa. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with brain metastasis from solid tumours. Ann Oncol . 2021;32:1332-1347.
251. LeCouffe NE, Kappelhof M, Treurniet KM, Rinkel LA, Bruggeman AE, Berkhemer OA, Wolff L, van Voorst H, Tolhuisen ML, Dippel DWJ, van der Lugt A, van Es A, Boiten J, …., Postma AA, van Doormaal PJ, van den Berg R, van der Hoorn
A, Beenen LFM, Nieboer D, Lingsma HF, Emmer BJ, Coutinho JM, Majoie C, Roos Y and the MR CLEAN-NO IV Investigators. A Randomized Trial of Intravenous Alteplase before Endovascular Treatment for Stroke. N Engl J Med . 2021;385:18331844.
252. Levitis E, van Praag CDG, Gau R, ……, Rutherford S, Sharan M, Shaw TB, Syeda WT, Testerman MM, Toro R, Valk SL, Van Den Bossche S, Varoquaux G, Vasa F, Veldsman M, Vohryzek J, Wagner AS, Walsh RJ, White T, Wong FT, Xie X, Yan CG, Yang YF, Yee Y, Zanitti GE, Van Gulick AE, Duff E and Maumet C. Centering inclusivity in the design of online conferences-An OHBM-Open Science perspective. Gigascience . 2021;10:giab051.
253. Lin J, Peng Y, Liu Q, Li K, Lv G, Seimbille Y, Huang G, Gao F and Qiu L. Pharmacological evaluation of imidazole-derived bisphosphonates on receptor activator of nuclear factor-kappaB ligand-induced osteoclast differentiation and function. Chem Biol Drug Des . 2021;97:121-133.
254. Loebinger MR, Polverino E, Chalmers JD, Tiddens HAWM, Goossens H, Tunney M, Ringshausen FC, Hill AT, Pathan R, Angyalosi G, Blasi F, Elborn SJ, Haworth CS and Team i-T. Efficacy and safety of TOBI Podhaler in Pseudomonas aeruginosa-infected bronchiectasis patients: iBEST study. Eur Respir J . 2021;57:2001451.
255. Lopez-Vicente M, Agcaoglu O, Perez-Crespo L, Estevez-Lopez F, Heredia-Genestar JM, Mulder RH, Flournoy JC, van Duijvenvoorde ACK, Guroglu B, White T, Calhoun V, Tiemeier H and Muetzel RL. Developmental Changes in Dynamic Functional Connectivity From Childhood Into Adolescence. Front Syst Neurosci 2021;15:724805.
256. Lopez-Vicente M, Lamballais S, Louwen S, Hillegers M, Tiemeier H, Muetzel RL and White T. White matter microstructure correlates of age, sex, handedness and motor ability in a population-based sample of 3031 school-age children. Neuroimage . 2021;227:117643.
257. Lu Y, Dimitrov L, Chen SH, Bielak LF, Bis JC, Feitosa MF, Lu L, Kavousi M, Raffield LM, Smith AV, Wang L, Weiss S, Yao J, Zhu J, Gudmundsson EF, Gudmundsdottir V, Bos D, Ghanbari M, Ikram MA, Hwang SJ, Taylor KD, Budoff MJ, Gislason GK, O’Donnell CJ, An P, Franceschini N, Freedman BI, Fu YP, Guo X, Heiss G, Kardia SLR, Wilson JG, Langefeld CD, Schminke U, Uitterlinden AG, Lange LA, Peyser PA, Gudnason VG, Psaty BM, Rotter JI, Bowden DW and Ng MCY. Multiethnic Genome-Wide Association Study of Subclinical Atherosclerosis in Individuals With Type 2 Diabetes. Circ Genom Precis Med . 2021;14:e003258.
258. Lubczynska MJ, Muetzel RL, El Marroun H, Hoek G, Kooter IM, Thomson EM, Hillegers M, Vernooij MW, White T, Tiemeier H and Guxens M. Air pollution exposure during pregnancy and childhood and brain morphology in preadolescents. Environ Res . 2021;198:110446.
259. Luraghi G, Bridio S, Rodriguez Matas JF, Dubini G, Boodt N, Gijsen FJH, van der Lugt A, Fereidoonnezhad B, Moerman KM, McGarry P, Konduri PR, Arrarte Terreros N, Marquering HA, Majoie C, Migliavacca F and the INSIST Investigators. The first virtual patient-specific thrombectomy procedure. J Biomech 2021;126:110622.
260. Lutterman SL, Zwaveling-Soonawala N, Verberne HJ, Verburg FA, van Trotsenburg ASP and Mooij CF. The Efficacy and Short- and Long-Term Side Effects of Radioactive Iodine Treatment in Pediatric Graves’ Disease: A Systematic Review. Eur Thyroid J . 2021;10:353-363.
261. Luurtsema G, Pichler V, Bongarzone S, Seimbille Y, Elsinga P, Gee A and Vercouillie J. EANM guideline for harmonisation on molar activity or specific activity of radiopharmaceuticals: impact on safety and imaging quality. EJNMMI Radiopharm Chem . 2021;6:34.
262. Lysen TS, Yilmaz P, Dubost F, Ikram MA, de Bruijne M, Vernooij MW and Luik AI. Sleep and perivascular spaces in the middle-aged and elderly population. J Sleep Res . 2021:e13485.
263. Ma Y, Blacker D, Viswanathan A, van Veluw SJ, Bos D, Vernooij MW, Hyman BT, Tzourio C, Das S and Hofman A. Visit-to-Visit Blood Pressure Variability, Neuropathology, and Cognitive Decline. Neurology . 2021;96:e2812-e2823.
264. Maat APWM, Zuijdendorp MM, van Leenders GJL, Hirsch A and Rothbarth J. An exceptionally large sarcoma of the thoraco-abdominal wall with ingrowth in the pericardium and stomach: a case report. Current Challenges in Thoracic Surgery . 2021;3:1-7.
265. Manfrini E, Smits M, Thust S, Geiger S, Bendella Z, Petr J, Solymosi L and Keil VC. From research to clinical practice: a European neuroradiological survey on quantitative advanced MRI implementation. Eur Radiol . 2021;31:6334-6341.
266. Mansi R, Nock BA, Dalm SU, Busstra MB, van Weerden WM and Maina T. Radiolabeled Bombesin Analogs. Cancers (Basel) . 2021;13:5766.
267. Marinescu R, Oxtoby N, Young A, Bron EE, Toga A, Weiner M, Barkhof F, Fox NC, Eshaghi A, Toni T, Salaterski M, Lunina V, Ansart M, Durrleman S, Lu P, Iddi S, Li D, Thompson W, Donohue M and Alexander DC. The Alzheimer’s Disease Prediction Of Longitudinal Evolution (TADPOLE) Challenge: Results after 1 Year Follow-up. Journal of Machine Learning for Biomedical Imaging (MELBA). 2021;019:1-60.
268. Meijer D, Luiting HB, van Leeuwen PJ, Remmers S, Jansen BHE, Bodar YJL, Witteveen T, Schaake EE, van der Poel HG, Wondergem M, Busstra MB, Nieuwenhuijzen JA, Meijnen P, Brabander T, van Moorselaar RJA, Hendrikse NH, Oprea-Lager DE, Roobol MJ and Vis AN. Prostate Specific Membrane Antigen Positron Emission Tomography/Computerized Tomography in the Evaluation of Initial Response in Candidates Who Underwent Salvage Radiation Therapy after Radical Prostatectomy for Prostate Cancer. J Urol . 2021;205:1100-1109.
269. Moerman AM, Korteland S, Dilba K, van Gaalen K, Poot DHJ, van der Lugt A, Ver-
hagen HJM, Wentzel JJ, van der Steen AFW, Gijsen FJH and van der Heiden K. The Correlation Between Wall Shear Stress and Plaque Composition in Advanced Human Carotid Atherosclerosis. Front Bioeng Biotechnol . 2021;9:828577.
270. Mol MO, Nijmeijer SWR, van Rooij JGJ, van Spaendonk RML, Pijnenburg YAL, van der Lee SJ, van Minkelen R, Donker Kaat L, Rozemuller AJM, Janse van Mantgem MR, van Rheenen W, van Es MA, Veldink JH, Hennekam FAM, Vernooij M, van Swieten JC, Cohn-Hokke PE, Seelaar H and Dopper EGP. Distinctive pattern of temporal atrophy in patients with frontotemporal dementia and the I383V variant in TARDBP. J Neurol Neurosurg Psychiatry . 2021;92:787-789.
271. Mooldijk SS, Licher S, Vinke EJ, Vernooij MW, Ikram MK and Ikram MA. Season of birth and the risk of dementia in the population-based Rotterdam Study. Eur J Epidemiol . 2021;36:497-506.
272. Morey JR, Jiang S, Klein S, Max W, Masharani U, Fleischmann KE, Hunink MGM and Ferket BS. Estimating Long-Term Health Utility Scores and Expenditures for Cardiovascular Disease From the Medical Expenditure Panel Survey. Circ Cardiovasc Qual Outcomes . 2021;14:e006769.
273. Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, Fanti S, Fossati N, Gandaglia G, Gillessen S, Grivas N, Grummet J, Henry AM, van der Kwast TH, Lam TB, Lardas M, Liew M, Mason MD, Moris L, Oprea-Lager DE, van der Poel HG, Rouviere O, Schoots IG, Tilki D, Wiegel T, Willemse PM and Cornford P. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol . 2021;79:243-262.
274. Mulder E, de Joode K, Litiere S, Ten Tije AJ, Suijkerbuijk KPM, Boers-Sonderen MJ, Hospers GAP, de Groot JWB, van den Eertwegh AJM, Aarts MJB, Piersma D, van Rijn RS, Kapiteijn E, Vreugdenhil G, van den Berkmortel F, Hoop EO, Franken MG, Ryll B, Rutkowski P, Sleijfer S, Haanen J and van der Veldt AAM. Early discontinu-
ation of PD-1 blockade upon achieving a complete or partial response in patients with advanced melanoma: the multicentre prospective Safe Stop trial. BMC Cancer . 2021;21:323.
275. Mulder E, Dwarkasing JT, Tempel D, van der Spek A, Bosman L, Verver D, Mooyaart AL, van der Veldt AAM, Verhoef C, Nijsten TEC, Grunhagen DJ and Hollestein LM. Validation of a clinicopathological and gene expression profile model for sentinel lymph node metastasis in primary cutaneous melanoma. Br J Dermatol 2021;184:944-951.
276. Mulder E, Grunhagen DJ, Sleijfer S, Uylde Groot CA and van der Veldt AAM Adjuvant treatment in patients with melanoma: The planning of scanning. Eur J Cancer . 2021;157:306-307.
277. Mulder E, Smit L, Grunhagen DJ, Verhoef C, Sleijfer S, van der Veldt AAM and Uylde Groot CA. Cost-effectiveness of adjuvant systemic therapies for patients with high-risk melanoma in Europe: a model-based economic evaluation. ESMO Open . 2021;6:100303.
278. Mulder E, Stahlie EHA, Verver D, Lemstra C, Been LB, Mooyaart AL, Brabander T, Vegt E, Verburg FA, van der Veldt AAM, Verhoef C, van Akkooi ACJ and Grunhagen DJ. False positive FDG uptake in melanoma patients treated with talimogene laherparepvec (T-VEC). J Surg Oncol 2021;124:1161-1165.
279. Mulder TA, Korevaar TIM, Peeters RP, van Herwaarden AE, de Rijke YB, White T and Tiemeier H. Urinary Iodine Concentrations in Pregnant Women and Offspring Brain Morphology. Thyroid 2021;31:964-972.
280. Mutluer FO, van der Velde N, Voorneveld J, Bosch JG, Roos-Hesselink JW, van der Geest RJ, Hirsch A and van den Bosch A. Evaluation of intraventricular flow by multimodality imaging: a review and meta-analysis. Cardiovasc Ultrasound 2021;19:38.
281. Nevalainen MT, Veikkola O, Thevenot J, Tiulpin A, Hirvasniemi J, Niinimaki J and
Saarakkala SS. Acoustic emissions and kinematic instability of the osteoarthritic knee joint: comparison with radiographic findings. Sci Rep . 2021;11:19558.
282. Novins DK, Stoddard J, Althoff RR, Charach A, Cortese S, Cullen KR, Frazier JA, Glatt SJ, Henderson SW, Herringa RJ, Hulvershorn L, Kieling C, McBride AB, McCauley E, Middeldorp CM, Reiersen AM, Rockhill CM, Sagot AJ, Scahill L, Simonoff E, Stewart SE, Szigethy E, Taylor JH, White T and Zima BT. Editors’ Note and Special Communication: Research Priorities in Child and Adolescent Mental Health Emerging From the COVID-19 Pandemic. J Am Acad Child Adolesc Psychiatry . 2021;60:544-554 e8.
283. Ooms JF, Ceusters J, Hirsch A, Budde RPJ and Van Mieghem NM. Accuracy of three-dimensional computational modeling in prediction of the dynamic neo left ventricular outflow tract with transcatheter mitral valve replacement. Int J Cardiol . 2021;336:93-96.
284. Ooms JF, Hirsch A, Von der Thusen JH, Michels M and Van Mieghem NM. Intracardiac Echocardiography-Guided Biopsy in the Work-Up of an Unexplained Cardiac Mass. JACC Cardiovasc Interv 2021;14:e297-e299.
285. Ooms JF, Wang DD, Rajani R, Redwood S, Little SH, Chuang ML, Popma JJ, Dahle G, Pfeiffer M, Kanda B, Minet M, Hirsch A, Budde RP, De Jaegere PP, Prendergast B, O’Neill W and Van Mieghem NM. Computed Tomography-Derived 3D Modeling to Guide Sizing and Planning of Transcatheter Mitral Valve Interventions. JACC Cardiovasc Imaging . 2021;14:1644-1658.
286. Oosterloo BC, Croll PH, Goedegebure A, Roshchupkin GV, Baatenburg de Jong RJ, Ikram MA and Vernooij MW. Tinnitus and Its Central Correlates: A Neuroimaging Study in a Large Aging Population. Ear Hear . 2021;42:1428-1435.
287. Oosting SF, van der Veldt AAM, GeurtsvanKessel CH, Fehrmann RSN, van Binnendijk RS, Dingemans AC, Smit EF, Hiltermann TJN, den Hartog G, Jalving M, Westphal TT, Bhattacharya A, van der
Heiden M, Rimmelzwaan GF, Kvistborg P, Blank CU, Koopmans MPG, Huckriede ALW, van Els C, Rots NY, van Baarle D, Haanen J and de Vries EGE. mRNA-1273 COVID-19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours: a prospective, multicentre, non-inferiority trial. Lancet Oncol . 2021;22:16811691.
288. Ospel JM, van der Lugt A, Gounis M, Goyal M and Majoie C. A clinical perspective on endovascular stroke treatment biomechanics. J Biomech . 2021;127:110694.
289. Padhani AR, Rouviere O and Schoots IG Magnetic Resonance Imaging for Tailoring the Need to Biopsy During Follow-up for Men on Active Surveillance for Prostate Cancer. Eur Urol . 2021;80:564-566.
290. Padhani AR, Schoots I and Villeirs G. Contrast Medium or No Contrast Medium for Prostate Cancer Diagnosis. That Is the Question. J Magn Reson Imaging 2021;53:13-22.
291. Padhani AR, Schoots IG and Barentsz JO. Fast Magnetic Resonance Imaging as a Viable Method for Directing the Prostate Cancer Diagnostic Pathway. Eur Urol Oncol . 2021;4:863-865.
292. Padhani AR, Schoots IG, Turkbey B, Giannarini G and Barentsz JO. A multifaceted approach to quality in the MRI-directed biopsy pathway for prostate cancer diagnosis. Eur Radiol . 2021;31:43864389.
293. Panebianco V, Villeirs G, Weinreb JC, Turkbey BI, Margolis DJ, Richenberg J, Schoots IG, Moore CM, Futterer J, Macura KJ, Oto A, Bittencourt LK, Haider MA, Salomon G, Tempany CM, Padhani AR and Barentsz JO. Prostate Magnetic Resonance Imaging for Local Recurrence Reporting (PI-RR): International Consensus -based Guidelines on Multiparametric Magnetic Resonance Imaging for Prostate Cancer Recurrence after Radiation Therapy and Radical Prostatectomy. Eur Urol Oncol . 2021;4:868-876.
294. Panman JL, Venkatraghavan V, van der Ende EL, Steketee RME, Jiskoot LC, Poos JM, Dopper EGP, Meeter LHH, Donker Kaat L, Rombouts S, Vernooij MW, Kievit AJA, Premi E, Cosseddu M, Bonomi E, Olives J, Rohrer JD, Sanchez-Valle R, Borroni B, Bron EE, Van Swieten JC, Papma JM, Klein S and the GENFI consortium investigators. Modelling the cascade of biomarker changes in GRN-related frontotemporal dementia. J Neurol Neurosurg Psychiatry . 2021;92:494-501.
295. Paulo A, Vaz PG, Andrade De Jesus D, Sanchez Brea L, Van Eijgen J, Cardoso J, van Walsum T, Klein S, Stalmans I and Barbosa Breda J. Optical Coherence Tomography Imaging of the Lamina Cribrosa: Structural Biomarkers in Nonglaucomatous Diseases. J Ophthalmol 2021:8844614.
296. Pawlak MA, Knol MJ, Vernooij MW, Ikram MA, Adams HHH and Evans TE. Neural correlates of orbital telorism. Cortex 2021;145:315-326.
297. Pemberton HG, Goodkin O, Prados F, Das RK, Vos SB, Moggridge J, Coath W, Gordon E, Barrett R, Schmitt A, Whiteley-Jones H, Burd C, Wattjes MP, Haller S, Vernooij MW, Harper L, Fox NC, Paterson RW, Schott JM, Bisdas S, White M, Ourselin S, Thornton JS, Yousry TA, Cardoso MJ, Barkhof F and Alzheimer’s Disease Neuroimaging I. Automated quantitative MRI volumetry reports support diagnostic interpretation in dementia: a multi-rater, clinical accuracy study. Eur Radiol 2021;31:5312-5323.
298. Pemberton HG, Zaki LAM, Goodkin O, Das RK, Steketee RME, Barkhof F and Vernooij MW. Technical and clinical validation of commercial automated volumetric MRI tools for dementia diagnosis-a systematic review. Neuroradiology 2021;63:1773-1789.
299. Penzkofer T, Padhani AR, Turkbey B, Haider MA, Huisman H, Walz J, Salomon G, Schoots IG, Richenberg J, Villeirs G, Panebianco V, Rouviere O, Logager VB and Barentsz J. ESUR/ESUI position paper: developing artificial intelligence for precision diagnosis of prostate cancer
using magnetic resonance imaging. Eur Radiol . 2021;31:9567-9578.
300. Petranovic Ovcaricek P, Giovanella L, Carrio Gasset I, Hindie E, Huellner MW, Luster M, Piccardo A, Weber T, Talbot JN and Verburg FA. The EANM practice guidelines for parathyroid imaging. Eur J Nucl Med Mol Imaging . 2021;48:2801-2822.
301. Petranovic Ovcaricek P, Verburg FA, Hoffmann M, Iakovou I, Mihailovic J, Vrachimis A, Luster M and Giovanella L. Higher thyroid hormone levels and cancer. Eur J Nucl Med Mol Imaging . 2021;48:808821.
302. Pezzolo E, Mutlu U, Vernooij MW, Dowlatshahi EA, Gisondi P, Girolomoni G, Nijsten T, Ikram MA and Wakkee M. Psoriasis is not associated with cognition, brain imaging markers, and risk for dementia: The Rotterdam Study. J Am Acad Dermatol . 2021;85:671-680.
303. Pirkl CM, Nunez-Gonzalez L, Kofler F, Endt S, Grundl L, Golbabaee M, Gomez PA, Cencini M, Buonincontri G, Schulte RF, Smits M, Wiestler B, Menze BH, Menzel MI and Hernandez-Tamames JA Accelerated 3D whole-brain T1, T2, and proton density mapping: feasibility for clinical glioma MR imaging. Neuroradiology . 2021;63:1831-1851.
304. Pirson FAV, Hinsenveld WH, Goldhoorn RB, Staals J, de Ridder IR, van Zwam WH, van Walderveen MAA, Lycklama ANGJ, Uyttenboogaart M, Schonewille WJ, van der Lugt A, Dippel DWJ, Roos Y, Majoie C, van Oostenbrugge RJ and MR CLEANLATE Investigators. MR CLEAN-LATE, a multicenter randomized clinical trial of endovascular treatment of acute ischemic stroke in The Netherlands for late arrivals: study protocol for a randomized controlled trial. Trials . 2021;22:160.
305. Prasetya H, Ramos LA, Epema T, Treurniet KM, Emmer BJ, van den Wijngaard IR, Zhang G, Kappelhof M, Berkhemer OA Yoo AJ, Roos YB, van Oostenbrugge RJ, Dippel DW, van Zwam WH, van der Lugt A, de Mol BA, Majoie CB, Bavel EV, Marquering HA and the MR CLEAN Registry Investigators. qTICI: Quantitative as-
sessment of brain tissue reperfusion on digital subtraction angiograms of acute ischemic stroke patients. Int J Stroke 2021;16:207-216.
306. Prior JC, Oei EHG, Brown JP, Oei L, Koromani F and Lentle BC. Where’s the break? Critique of radiographic vertebral fracture diagnostic methods. Osteoporos Int 2021;32:2391-2395.
307. Prive BM, Peters SMB, Muselaers CHJ, van Oort IM, Janssen MJR, Sedelaar JPM, Konijnenberg MW, Zamecnik P, Uijen MJM, Schilham MGM, Eek A, Scheenen TWJ, Verzijlbergen JF, Gerritsen WR, Mehra N, Kerkmeijer LGW, Smeenk RJ, Somford DM, van Basten JA, Heskamp S, Barentsz JO, Gotthardt M, Witjes JA and Nagarajah J. Lutetium-177-PSMA-617 in Low-Volume Hormone-Sensitive Metastatic Prostate Cancer: A Prospective Pilot Study. Clin Cancer Res . 2021;27:3595-3601.
308. Reesink DJ, Schilham MGM, van der Hoeven E, Schoots IG, van Melick HHE and van den Bergh RCN. Comparison of risk-calculator and MRI and consecutive pathways as upfront stratification for prostate biopsy. World J Urol 2021;39:2453-2461.
309. Reus LM, Jansen IE, Mol MO, van Ruissen F, van Rooij J, van Schoor NM, Tesi N, Reinders MJT, Huisman MA, Holstege H, Visser PJ, de Boer SCM, Hulsman M, Ahmad S, Amin N, Uitterlinden AG, Ikram A, van Duijn CM, Seelaar H, Ramakers I, Verhey FRJ, van der Lugt A, Claassen J, Jan Biessels G, De Deyn PP, Scheltens P, van der Flier WM, van Swieten JC, Pijnenburg YAL and van der Lee SJ. Genome-wide association study of frontotemporal dementia identifies a C9ORF72 haplotype with a median of 12-G4C2 repeats that predisposes to pathological repeat expansions. Transl Psychiatry . 2021;11:451.
310. Roest S, Brugts JJ, van Kampen JJA, von der Thusen JH, Constantinescu AA, Caliskan K, Hirsch A and Manintveld OC. COVID-19-related myocarditis postheart transplantation. Int J Infect Dis 2021;107:34-36.
311. Roosen J, Klaassen NJM, Westlund Gotby LEL, Overduin CG, Verheij M, Konijnenberg MW and Nijsen JFW. To 1000 Gy and back again: a systematic review on dose-response evaluation in selective internal radiation therapy for primary and secondary liver cancer. Eur J Nucl Med Mol Imaging . 2021;48:3776-3790.
312. Runhaar J, Dam EB, Oei EHG and Bierma-Zeinstra SMA. Medial Cartilage Surface Integrity as a Surrogate Measure for Incident Radiographic Knee Osteoarthritis following Weight Changes. Cartilage 2021;13:424S-427S.
313. Ruskó L, Capala ME, Czipczer V, Kolozsvári B, Deák-Karancsi B, Czabány R, Gyalai B, Tan T, Végváry Z, Borzasi E, Együd Z, Kószó R, Paczona V, Fodor E, Bobb C, Cozzini C, Kaushik S, Darázs B, Verduijn GM, Pearson R, Maxwell R, Mccallum H, Hernandez-Tamames JA, Hideghéty K, Petit SF and Wiesinger F. Deep-Learning-based Segmentation of Organs-at-Risk in the Head for MR-assisted Radiation Therapy Planning. Proceedings of the 14th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2021) . 2021;2:31-43.
314. Sadeghi AH, Wahadat AR, Dereci A, Budde RPJ, Tanis W, Roos-Hesselink JW, Takkenberg H, Taverne Y, Mahtab EAF and Bogers A. Remote multidisciplinary heart team meetings in immersive virtual reality: a first experience during the COVID-19 pandemic. BMJ Innov 2021;7:311-315.
315. Saeedan MB, Wang TKM, Cremer P, Wahadat AR, Budde RPJ, Unai S, Pettersson GB and Bolen MA. Role of Cardiac CT in Infective Endocarditis: Current Evidence, Opportunities, and Challenges. Radiol Cardiothorac Imaging . 2021;3:e200378.
316. Salgado R, El Addouli H and Budde RPJ Transcatheter Aortic Valve Implantation: The Evolving Role of the Radiologist in 2021. Rofo . 2021;193:1411-1425.
317. Sammallahti S, Tiemeier H, Louwen S, Steegers E, Hillegers M, Jaddoe VWV and White T. Fetal-placental blood flow and neurodevelopment in childhood: popu-
lation-based neuroimaging study. Ultrasound Obstet Gynecol . 2021;58:245-253.
318. Sanchez-Margallo JA, Tas L, Moelker A, van den Dobbelsteen JJ, Sanchez-Margallo FM, Lango T, van Walsum T and van de Berg NJ. Block-matching-based registration to evaluate ultrasound visibility of percutaneous needles in liver-mimicking phantoms. Med Phys 2021;48:7602-7612.
319. Scholtes MP, Alberts AR, Ifle IG, Verhagen P, van der Veldt AAM and Zuiverloon TCM. Biomarker-Oriented Therapy in Bladder and Renal Cancer. Int J Mol Sci 2021;22:2832.
320. Schouten WR, Arkenbosch JHC, van der Woude CJ, de Vries AC, Stevens HP, Fuhler GM, Dwarkasing RS, van Ruler O and de Graaf EJR. Efficacy and safety of autologous adipose-derived stromal vascular fraction enriched with platelet-rich plasma in flap repair of transsphincteric cryptoglandular fistulas. Tech Coloproctol . 2021;25:1301-1309.
321. Shoamanesh A, Akoudad S, Himali JJ, Beiser AS, DeCarli C, Seshadri S, Ikram M, Romero JR and Vernooij MW. Cortical superficial siderosis in the general population: The Framingham Heart and Rotterdam studies. Int J Stroke . 2021;16:798808.
322. Silva CCV, El Marroun H, Sammallahti S, Vernooij MW, Muetzel RL, Santos S and Jaddoe VWV. Patterns of Fetal and Infant Growth and Brain Morphology at Age 10 Years. JAMA Netw Open 2021;4:e2138214.
323. Sorensen LB, Rathleff MS, Dean BJF, Oei E, Magnusson SP, Olesen JL and Holden S. A systematic review of imaging findings in patients with Osgood-Schlatter disease. Transl Sports Med . 2021;4:772787.
324. Sosna J, Pyatigorskaya N, Krestin G, Denton E, Stanislav K, Morozov S, Kumamaru KK, Jankharia B, Mildenberger P, Forster B, Schouman-Clayes E, Bradey A, Akata D, Brkljacic B, Grassi R, Plako A, Papanagiotou H, Maksimovic R and Lexa F. Inter-
national survey on residency programs in radiology: similarities and differences among 17 countries. Clin Imaging 2021;79:230-234.
325. Steegers C, Blok E, Lamballais S, Jaddoe V, Bernardoni F, Vernooij M, van der Ende J, Hillegers M, Micali N, Ehrlich S, Jansen P, Dieleman G and White T. The association between body mass index and brain morphology in children: a population-based study. Brain Struct Funct 2021;226:787-800.
326. Steegers C, Dieleman G, Moskalenko V, Santos S, Hillegers M, White T and Jansen PW. The longitudinal relationship between set-shifting at 4 years of age and eating disorder related features at 9 years of age in the general pediatric population. Int J Eat Disord . 2021;54:21802191.
327. Strachinaru M, Hirsch A, Bowen D and Caliskan K. Echocardiography from a transhepatic approach in left ventricular assist device patients with difficult transthoracic imaging. Eur Heart J Case Rep . 2021;5:ytab108.
328. Su R, Cornelissen SAP, van der Sluijs M, van Es A, van Zwam WH, Dippel DWJ, Lycklama G, van Doormaal PJ, Niessen WJ, van der Lugt A and van Walsum T. autoTICI: Automatic Brain Tissue Reperfusion Scoring on 2D DSA Images of Acute Ischemic Stroke Patients. IEEE Trans Med Imaging . 2021;40:2380-2391.
329. Sudol-Szopinska I, Giraudo C, Oei EHG and Jans L. Imaging update in inflammatory arthritis. J Clin Orthop Trauma 2021;20:101491.
330. Teixeira LM and Mezzanotte L. New bioimaging avenues for organs-on-chips by integration of bioluminescence. View-China . 2021;2:1-10.
331. Ten Hove D, Slart R, Sinha B, Glaudemans A and Budde RPJ. (18)F-FDG PET/CT in Infective Endocarditis: Indications and Approaches for Standardization. Curr Cardiol Rep . 2021;23:130.
332. Toemen L, Santos S, Roest AAW, Vernooij MW, Helbing WA, Gaillard R and Jaddoe VWV. Pericardial adipose tissue, cardiac structures, and cardiovascular risk factors in school-age children. Eur Heart J Cardiovasc Imaging . 2021;22:307-313.
333. Tolhuisen ML, Kappelhof M, Dutra BG, Jansen IGH, Guglielmi V, Dippel DWJ, van Zwam WH, van Oostenbrugge RJ, van der Lugt A, Roos Y, Majoie C, Caan MWA, Marquering HA and the MR CLEAN Registry Investigators. Influence of Onset to Imaging Time on Radiological Thrombus Characteristics in Acute Ischemic Stroke. Front Neurol . 2021;12:693427.
334. Treurniet KM, LeCouffe NE, Kappelhof M, Emmer BJ, van Es A, Boiten J, Lycklama GJ, Keizer K, Yo LSF, Lingsma HF, van Zwam WH, de Ridder I, van Oostenbrugge RJ, van der Lugt A, Dippel DWJ, Coutinho JM, Roos Y, Majoie C and the MR CLEANNO IV Investigators. MR CLEAN-NO IV: intravenous treatment followed by endovascular treatment versus direct endovascular treatment for acute ischemic stroke caused by a proximal intracranial occlusion-study protocol for a randomized clinical trial. Trials . 2021;22:141.
335. Tzovara A, Amarreh I, Borghesani V, Chakravarty MM, DuPre E, Grefkes C, Haugg A, Jollans L, Lee HW, Newman SD, Olsen RK, Ratnanather JT, Rippon G, Uddin LQ, Vega MLB, Veldsman M, White T and Badhwar A. Embracing diversity and inclusivity in an academic setting: Insights from the Organization for Human Brain Mapping. Neuroimage 2021;229:117742.
336. Uleman JF, Melis RJF, Quax R, van der Zee EA, Thijssen D, Dresler M, van de Rest O, van der Velpen IF, Adams HHH, Schmand B, de Kok I, de Bresser J, Richard E, Verbeek M, Hoekstra AG, Rouwette E and Olde Rikkert MGM. Mapping the multicausality of Alzheimer’s disease through group model building. Geroscience 2021;43:829-843.
337. Valkema MJ, van der Wilk BJ, Eyck BM, Wijnhoven BPL, Spaander MCW, Doukas M, Lagarde SM, Schreurs WMJ, Roef MJ, van Lanschot JJB and Valkema R. Surveil-
lance of Clinically Complete Responders Using Serial (18)F-FDG PET/CT Scans in Patients with Esophageal Cancer After Neoadjuvant Chemoradiotherapy. J Nucl Med . 2021;62:486-492.
338. van Breeschoten J, van den Eertwegh AJM, de Wreede LC, Hilarius DL, van Zwet EW, Haanen JB, Blank CU, Aarts MJB, van den Berkmortel F, de Groot JWB, Hospers GAP, Kapiteijn E, Piersma D, van Rijn RS, Stevense-den Boer MAM, van der Veldt AAM, Vreugdenhil G, Boers-Sonderen MJ, Suijkerbuijk KPM and Wouters M. Hospital Variation in Cancer Treatments and Survival OutComes of Advanced Melanoma Patients: Nationwide Quality Assurance in The Netherlands. Cancers (Basel) 2021;13:5077.
339. van Breeschoten J, Wouters M, de Wreede LC, Hilarius DH, Haanen JB, Blank CU, Aarts MJB, van den Berkmortel F, de Groot JB, Hospers GAP, Kapiteijn E, Piersma D, van Rijn RS, Suijkerbuijk KPM, Blokx WAM, Ten Tije AJ, van der Veldt AAM, Vreugdenhil G, Boers MJ and van den Eertwegh AJM. Nationwide Outcomes of Advanced Melanoma According to BRAFV600 Status. Am J Clin Oncol 2021;44:82-89.
340. van Breeschoten J, Wouters M, Hilarius DL, Haanen JB, Blank CU, Aarts MJB, van den Berkmortel F, de Groot JB, Hospers GAP, Kapiteijn E, Piersma D, van Rijn RS, Suijkerbuijk KPM, Blokx WAM, Tije BJT, van der Veldt AAM, Vreugdenhil A, Boers-Sonderen MJ and van den Eertwegh AJM. First-line BRAF/MEK inhibitors versus anti-PD-1 monotherapy in BRAF(V600)-mutant advanced melanoma patients: a propensity-matched survival analysis. Br J Cancer . 2021;124:12221230.
341. van de Voort EMF, Struik GM, Birnie E, Moelker A, Verhoef C and Klem T. Thermal Ablation as an Alternative for Surgical Resection of Small (</= 2 cm) Breast Cancers: A Meta-Analysis. Clin Breast Cancer . 2021;21:e715-e730.
342. van de Voort EMF, Struik GM, Koppert LB, Moelker A, Debets R, Yo G, Macco M, Sinke R, Franckena M, Birnie E, Verhoef
C and Klem T. Treatment of early-stage breast cancer with percutaneous thermal ablation, an open-label randomised phase 2 screening trial: rationale and design of the THERMAC trial. BMJ Open 2021;11:e052992.
343. van de Wouw J, Steenhorst JJ, Sorop O, van Drie RWA, Wielopolski PA, Kleinjan A, Hirsch A, Duncker DJ and Merkus D. Impaired pulmonary vasomotor control in exercising swine with multiple comorbidities. Basic Res Cardiol . 2021;116:51.
344. van den Brink H, Ferro DA, Bresser J, Bron EE, Onkenhout LP, Kappelle LJ, Biessels GJ and Heart-Brain Connection C. Cerebral cortical microinfarcts in patients with internal carotid artery occlusion. J Cereb Blood Flow Metab . 2021;41:26902698.
345. van den Broeck T, Oprea-Lager D, Moris L, Kailavasan M, Briers E, Cornford P, De Santis M, Gandaglia G, Gillessen Sommer S, Grummet JP, Grivas N, Lam TBL, Lardas M, Liew M, Mason M, O’Hanlon S, Pecanka J, Ploussard G, Rouviere O, Schoots IG, Tilki D, van den Bergh RCN, van der Poel H, Wiegel T, Willemse PP, Yuan CY and Mottet N. A Systematic Review of the Impact of Surgeon and Hospital Caseload Volume on Oncological and Nononcological Outcomes After Radical Prostatectomy for Nonmetastatic Prostate Cancer. Eur Urol . 2021;80:531-545.
346. van der Kooij MK, Dekkers OM, Aarts MJB, van den Berkmortel F, Boers-Sonderen MJ, de Groot JWB, Hospers GAP, Piersma D, van Rijn RS, Suijkerbuijk KPM, Westgeest HM, van der Veldt AAM, Vreugdenhil G, Wilgenhof S, Wouters M, Haanen JB, van den Eertwegh AJM and Kapiteijn E. Sex-Based Differences in Treatment with Immune Checkpoint Inhibition and Targeted Therapy for Advanced Melanoma: A Nationwide Cohort Study. Cancers (Basel) . 2021;13:4639.
347. van der Kooij MK, Suijkerbuijk KPM, Aarts MJB, van den Berkmortel F, Blank CU, Boers-Sonderen MJ, van Breeschoten J, van den Eertwegh AJM, de Groot JWB, Haanen JB, Hospers GAP, Piersma D, van Rijn RS, Ten Tije AJ, van der Veldt AAM,
Vreugdenhil G, van Zeijl MCT, Wouters M, Dekkers OM and Kapiteijn E. Safety and Efficacy of Checkpoint Inhibition in Patients With Melanoma and Preexisting Autoimmune Disease : A Cohort Study. Ann Intern Med . 2021;174:641-648.
348. van der Linde D, Budde RPJ and Roos-Hesselink JW. Coronary aneurysm in a young patient with Turner syndrome. Cardiol Young . 2021;31:1019-1020.
349. van der Meulen M, Dirven L, Habets EJJ, Bakunina K, Smits M, Achterberg HC, Seute T, Cull G, Schouten H, Zijlstra JM, Brandsma D, Enting RH, Beijert M, Taphoorn MJB, van den Bent MJ, Issa S, Doorduijn JK and Bromberg JEC. Neurocognitive functioning and radiologic changes in primary CNS lymphoma patients: results from the HOVON 105/ALLG NHL 24 randomized controlled trial. Neuro Oncol . 2021;23:1315-1326.
350. van der Meulen M, Postma AA, Smits M, Bakunina K, Minnema MC, Seute T, Cull G, Enting RH, van der Poel M, Stevens WBC, Brandsma D, Beeker A, Doorduijn JK, Issa S, van den Bent MJ and Bromberg JEC. Extent of radiological response does not reflect survival in primary central nervous system lymphoma. Neurooncol Adv 2021;3:vdab007.
351. van der Velde B, White T and Kemner C. The emergence of a theta social brain network during infancy. Neuroimage 2021;240:118298.
352. van Es A, Hunfeld MAW, van den Wijngaard I, Kraemer U, Engelen M, van Hasselt B, Fransen PSS, Dippel DWJ, Majoie C, van der Lugt A, Emmer BJ and the MR CLEAN Registry Investigators. Endovascular Treatment for Acute Ischemic Stroke in Children: Experience From the MR CLEAN Registry. Stroke . 2021;52:781788.
353. van der Wilk BJ, Noordman BJ, Neijenhuis LKA, Nieboer D, Nieuwenhuijzen GAP, Sosef MN, Henegouwen M, Lagarde SM, Spaander MCW, Valkema R, Biermann K, Wijnhoven BPL, van der Gaast A, van Lanschot JJB, Doukas M, Nikkessen S, Luyer M, Schoon EJ, Roef MJ, van Lijnschoten
I, Oostenbrug LE, Riedl RG, Gisbertz SS, Krishnadath KK, Bennink RJ, Meijer SL and Collaborators. Active Surveillance Versus Immediate Surgery in Clinically Complete Responders After Neoadjuvant Chemoradiotherapy for Esophageal Cancer: A Multicenter Propensity Matched Study. Ann Surg . 2021;274:1009-1016.
354. van Doorn DJ, Hendriks P, Burgmans MC, Rietbergen DDD, Coenraad MJ, van Delden OM, Bennink RJ, Labeur TA, Klumpen HJ, Eskens F, Moelker A, Vegt E, Sprengers D, Mostafavi N, Ijzermans J and Takkenberg RB. Liver Decompensation as Late Complication in HCC Patients with Long-Term Response following Selective Internal Radiation Therapy. Cancers (Basel) . 2021;13:5427.
355. van Dorth D, Venugopal K, Poot DHJ, Hirschler L, de Bresser J, Smits M, Hernandez-Tamames JA, Debacker CS and van Osch MJP. Dependency of R2 and R2 * relaxation on Gd-DTPA concentration in arterial blood: Influence of hematocrit and magnetic field strength. NMR Biomed . 2021:e4653.
356. van Genuchten WJ, Toemen L, Roest AAW, Vernooij MW, Gaillard R, Helbing WA and Jaddoe VWV. Ethnic differences in childhood right and left cardiac structure and function assessed by cardiac magnetic resonance imaging. Eur J Pediatr . 2021;180:1257-1266.
357. van Helden DL, den Dulk L, Steijn B and Vernooij MW. Gender, networks and academic leadership: A systematic review. Educ Manag Adm Lead 2021:17411432211034172.
358. van Velsen EFS, Peeters RP, Stegenga MT, van Kemenade FJ, van Ginhoven TM, Verburg FA and Visser WE. The influence of age on disease outcome in 2015 ATA high-risk differentiated thyroid cancer patients. Eur J Endocrinol 2021;185:421-429.
359. van Velsen EFS, Visser WE, Stegenga MT, Mader U, Reiners C, van Kemenade FJ, van Ginhoven TM, Verburg FA and Peeters RP. Finding the Optimal Age Cutoff for the UICC/AJCC TNM Staging System in Pa-
tients with Papillary or Follicular Thyroid Cancer. Thyroid . 2021;31:1041-1049.
360. van Voorst H, Kunz WG, van den Berg LA, Kappelhof M, Pinckaers FME, Goyal M, Hunink MGM, Emmer BJ, Mulder MT, Dippel DWJ, Coutinho JM, Marquering HA, Boogaarts HD, van der Lugt A, van Zwam WH, Roos Y, Buskens E, Dijkgraaf MGW, Majoie C and the MR CLEAN Registry Investigators. Quantified health and cost effects of faster endovascular treatment for large vessel ischemic stroke patients in the Netherlands. J Neurointerv Surg 2021;13:1099-1105.
361. van Waning JI, Caliskan K, Chelu RG, van der Velde N, Pezzato A, Michels M, van Slegtenhorst MA, Boersma E, Nieman K, Majoor-Krakauer D and Hirsch A. Diagnostic Cardiovascular Magnetic Resonance Imaging Criteria in Noncompaction Cardiomyopathy and the Yield of Genetic Testing. Can J Cardiol 2021;37:433-442.
362. van Zeijl MCT, de Wreede LC, van den Eertwegh AJM, Wouters M, Jochems A, Schouwenburg MG, Aarts MJB, van Akkooi ACJ, van den Berkmortel F, de Groot JWB, Hospers GAP, Kapiteijn E, Piersma D, van Rijn RS, Suijkerbuijk KPM, Ten Tije AJ, van der Veldt AAM, Vreugdenhil G, van der Hoeven JJM and Haanen J. Survival outcomes of patients with advanced melanoma from 2013 to 2017: Results of a nationwide population-based registry. Eur J Cancer . 2021;144:242-251.
363. Verbruggen LC, Kok JL, Teepen JC, Janssens GO, de Boer CM, Stalpers LJA, Vernooij MW, van Dulmen-den Broeder E, Loonen JJ, van den Heuvel-Eibrink MM, Tissing WJE, van der Heiden-van der Loo M, Versluys AB, Neggers S, van Leeuwen FE, Hoving EW, Wesseling P, Kremer LCM, Ronckers CM, van der Pal HJH and the Dutch LATER Study group. Clinical characteristics of subsequent histologically confirmed meningiomas in long-term childhood cancer survivors: A Dutch LATER study. Eur J Cancer . 2021;150:240249.
364. Versluis JM, Hendriks AM, Weppler AM, Brown LJ, de Joode K, Suijkerbuijk KPM,
Zimmer L, Kapiteijn EW, Allayous C, Johnson DB, Hepner A, Mangana J, Bhave P, Jansen YJL, Trojaniello C, Atkinson V, Storey L, Lorigan P, Ascierto PA, Neyns B, Haydon A, Menzies AM, Long GV, Lebbe C, van der Veldt AAM, Carlino MS, Sandhu S, van Tinteren H, de Vries EGE, Blank CU and Jalving M. The role of local therapy in the treatment of solitary melanoma progression on immune checkpoint inhibition: A multicentre retrospective analysis. Eur J Cancer . 2021;151:72-83.
365. Verver D, Grunhagen DJ, van Akkooi ACJ, Aarts MJB, van den Berkmortel F, van den Eertwegh AJM, de Groot JWB, Boers-Sonderen MJ, Haanen J, Hospers GAP, Kapiteijn E, Piersma D, van Rijn RS, Suijkerbuijk KPM, Tije AJT, Vreugdenhil G, Verhoef C and van der Veldt AAM
Clinical outcome of patients with metastatic melanoma of unknown primary in the era of novel therapy. Cancer Immunol Immunother . 2021;70:3123-3135.
366. Vilor-Tejedor N, Evans TE, Adams HH, Gonzalez-de-Echavarri JM, Molinuevo JL, Guigo R, Gispert JD and Operto G. Genetic Influences on Hippocampal Subfields: An Emerging Area of Neuroscience Research. Neurol Genet . 2021;7:e591.
367. Vlastra W, van Nieuwkerk AC, Bronzwaer AGT, Versteeg A, Bron EE, Niessen WJ, Mutsaerts H, van der Ster BJP, Majoie C, Biessels GJ, Nederveen AJ, Daemen M, van Osch MJP, Baan J, Piek JJ, Van Lieshout JJ and Delewi R. Cerebral Blood Flow in Patients with Severe Aortic Valve Stenosis Undergoing Transcatheter Aortic Valve Implantation. J Am Geriatr Soc 2021;69:494-499.
368. Vroegindeweij LHP, Wielopolski PA, Boon AJW, Wilson JHP, Verdijk RM, Zheng S, Bonnet S, Bossoni L, van der Weerd L, Hernandez-Tamames JA and Langendonk JG. MR imaging for the quantitative assessment of brain iron in aceruloplasminemia: A postmortem validation study. Neuroimage . 2021;245:118752.
369. Weeland CJ, White T, Vriend C, Muetzel RL, Starreveld J, Hillegers MHJ, Tiemeier H and van den Heuvel OA. Brain Morphology Associated With Obsessive-Compul-
sive Symptoms in 2,551 Children From the General Population. J Am Acad Child Adolesc Psychiatry . 2021;60:470-478.
370. Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, Bendszus M, Balana C, Chinot O, Dirven L, French P, Hegi ME, Jakola AS, Platten M, Roth P, Ruda R, Short S, Smits M, Taphoorn MJB, von Deimling A, Westphal M, Soffietti R, Reifenberger G and Wick W. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol . 2021;18:170-186.
371. Wijnant SRA, Bos D, Brusselle G, Grymonprez M, Rietzschel E, Vernooij MW, Terzikhan N and Lahousse L. Comparison of cerebral blood flow in subjects with and without chronic obstructive pulmonary disease from the population-based Rotterdam Study. BMJ Open 2021;11:e053671.
372. Willemse PM, Davis NF, Grivas N, Zattoni F, Lardas M, Briers E, Cumberbatch MG, De Santis M, Dell’Oglio P, Donaldson JF, Fossati N, Gandaglia G, Gillessen S, Grummet JP, Henry AM, Liew M, MacLennan S, Mason MD, Moris L, Plass K, O’Hanlon S, Omar MI, Oprea-Lager DE, Pang KH, Paterson CC, Ploussard G, Rouviere O, Schoots IG, Tilki D, van den Bergh RCN, Van den Broeck T, van der Kwast TH, van der Poel HG, Wiegel T, Yuan CY, Cornford P, Mottet N and Lam TBL. Systematic Review of Active Surveillance for Clinically Localised Prostate Cancer to Develop Recommendations Regarding Inclusion of Intermediate-risk Disease, Biopsy Characteristics at Inclusion and Monitoring, and Surveillance Repeat Biopsy Strategy. Eur Urol . 2021:02215-6.
373. Wolthuis N, Satoer D, Veenstra W, Smits M, Wagemakers M, Vincent A, Bastiaanse R, Cherian PJ and Bosma I. Resting-State Electroencephalography Functional Connectivity Networks Relate to Pre- and Postoperative Language Functioning in Low-Grade Glioma and Meningioma Patients. Front Neurosci . 2021;15:785969.
374. Wong TY, Zhang H, White T, Xu L and Qiu A. Common functional brain networks between attention deficit and disrup-
tive behaviors in youth. Neuroimage 2021;245:118732.
375. Xerxa Y, Delaney SW, Rescorla LA, Hillegers MHJ, White T, Verhulst FC, Muetzel RL and Tiemeier H. Association of Poor Family Functioning From Pregnancy Onward With Preadolescent Behavior and Subcortical Brain Development. JAMA Psychiatry . 2021;78:29-37.
376. Xu D, van der Voet J, Hansson NM, Klein S, Oei EHG, Wagner F, Bierma-Zeinstra SMA and Runhaar J. Association between meniscal volume and development of knee osteoarthritis. Rheumatology (Oxford) . 2021;60:1392-1399.
377. Xu D van der Voet J, Waarsing JH, Oei EH Klein S, Englund M, Zhang F, Bierma-Zeinstra S and Runhaar J. Are changes in meniscus volume and extrusion associated to knee osteoarthritis development? A structural equation model. Osteoarthritis Cartilage . 2021;29:1426-1431.
378. Yavuzyigitoglu S, Tang MCY, Jansen M, Geul KW, Dwarkasing RS, Vaarwater J, Drabarek W, Verdijk RM, Paridaens D, Naus NC, Brosens E, de Klein A and Kilic E. Radiological Patterns of Uveal Melanoma Liver Metastases in Correlation to Genetic Status. Cancers (Basel) . 2021;13:5316.
379. Zandee WT, Brabander T, Blazevic A, Minczeles NS, Feelders RA, de Herder WW and Hofland J. Peptide Receptor Radionuclide Therapy With 177Lu-DOTATATE for Symptomatic Control of Refractory Carcinoid Syndrome. J Clin Endocrinol Metab . 2021;106:e3665-e3672.
380. Zhang X, Mens MMJ, Abozaid YJ, Bos D, Darwish Murad S, de Knegt RJ, Ikram MA, Pan Q and Ghanbari M. Circulatory microRNAs as potential biomarkers for fatty liver disease: the Rotterdam study. Aliment Pharmacol Ther . 2021;53:432-442.
381. Zhang X, van Rooij JGJ, Wakabayashi Y, Hwang SJ, Yang Y, Ghanbari M, Bos D, Consortium B, Levy D, Johnson AD, van Meurs JBJ, Kavousi M, Zhu J and O’Donnell CJ. Genome-wide transcriptome study using deep RNA sequencing for myocardial infarction and coronary ar-
tery calcification. BMC Med Genomics 2021;14:45.
382. Zhou C, Louwman M, Wakkee M, van der Veldt A, Grunhagen D, Verhoef C, Mooyaart A, Nijsten T and Hollestein L. Primary Melanoma Characteristics of Metastatic Disease: A Nationwide Cancer Registry Study. Cancers (Basel) 2021;13:4431.
383. Zou R, El Marroun H, Cecil C, Jaddoe VWV, Hillegers M, Tiemeier H and White T Maternal folate levels during pregnancy and offspring brain development in late childhood. Clin Nutr . 2021;40:33913400.
384. Zou R, El Marroun H, Voortman T, Hillegers M, White T and Tiemeier H. Maternal polyunsaturated fatty acids during pregnancy and offspring brain development in childhood. Am J Clin Nutr 2021;114:124-133.
Corrections and Responses
385. Bos D, Vernooij MW, Kavousi M and van der Lugt A. Reply: Carotid Intraplaque Hemorrhage and Cardiovascular Events: Are Antithrombotic Therapies the Missing Piece of the Puzzle? J Am Coll Cardiol 2021;78:198-200.
386. Dijkstra BM, de Jong M, Stroet MCM, Andreae F, Dulfer SE, Everts M, Kruijff S, Nonnekens J, den Dunnen WFA, Kruyt FAE and Groen RJM. Correction to: Evaluation of Ac-Lys(0)(IRDye800CW) Tyr(3)-octreotate as a novel tracer for SSTR2-targeted molecular fluorescence guided surgery in meningioma. J Neurooncol . 2021;153:223.
387. Fournier L, Costaridou L, Bidaut L, Michoux N, Lecouvet FE, de Geus-Oei LF, Boellaard R, Oprea-Lager DE, Obuchowski NA, Caroli A, Kunz WG, Oei EH, O’Connor JPB, Mayerhoefer ME, Franca M, Alberich-Bayarri A, Deroose CM, Loewe C, Manniesing R, Caramella C, Lopci E, Lassau N, Persson A, Achten R, Rosendahl K, Clement O, Kotter E, Golay X, Smits M, Dewey M, Sullivan DC, van der Lugt A, deSouza NM and the European Society
of Radiology. Correction to: Incorporating radiomics into clinical trials: expert consensus endorsed by the European Society of Radiology on considerations for data-driven compared to biologically driven quantitative biomarkers. Eur Radiol . 2021;31:6408-6409.
388. Meijer D, Luiting HB, van Leeuwen PJ, Remmers S, Jansen BHE, Bodar YJL, Witteveen T, Schaake EE, van der Poel HG, Wondergem M, Busstra MB, Nieuwenhuijzen JA, Meijnen P, Brabander T, van Moorselaar RJA, Hendrikse NH, Oprea-Lager DE, Roobol MJ and Vis AN. Reply by Authors. J Urol . 2021;205:1108-1109.
389. Pemberton HG, Zaki LAM, Goodkin O, Das RK, Steketee RME, Barkhof F and Vernooij MW. Correction to: Technical and clinical validation of commercial automated volumetric MRI tools for dementia diagnosis-a systematic review. Neuroradiology . 2021;63:1955.
390. Peters SMB, Meyer Viol SL, van der Werf NR, de Jong N, van Velden FHP, Meeuwis A, Konijnenberg MW, Gotthardt M, de Jong H and Segbers M. Correction to: Variability in lutetium-177 SPECT quantification between different state-ofthe-art SPECT/CT systems. EJNMMI Phys 2021;8:59.
391. Petranovic Ovcaricek P, Verburg FA, Hoffmann M, Iakovou I, Mihailovic J, Vrachimis A, Luster M and Giovanella L. Correction to: Higher thyroid hormone levels and cancer. Eur J Nucl Med Mol Imaging 2021;48:951-953.
392. van Breeschoten J, Wouters M, Hilarius DL, Haanen JB, Blank CU, Aarts MJB, van den Berkmortel F, de Groot JB, Hospers GAP, Kapiteijn E, Piersma D, van Rijn RS, Suijkerbuijk KPM, Blokx WAM, Ten Tije BJ, van der Veldt AAM, Vreugdenhil A, Boers-Sonderen MJ and van den Eertwegh AJM. Correction: First-line BRAF/MEK inhibitors versus anti-PD-1 monotherapy in BRAF(V600)-mutant advanced melanoma patients: a propensity-matched survival analysis. Br J Cancer 2021;124:1746.
INDEX













EMIM Conference
Online Christmas Party
Graduation Celebration
Webinar
Nihes Research Master Graduation
Alternative Christmas Party
Graduation Ceremony
Cycling for Buiten Adem!
Drinks in the Park
Group outing @ Mooie Boules
































