https://ebookmass.com/product/vapor-generation-techniques-
Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
Flow Analysis for Hydrocarbon Pipeline Engineering
Alessandro Terenzi
https://ebookmass.com/product/flow-analysis-for-hydrocarbon-pipelineengineering-alessandro-terenzi/
ebookmass.com
Islamic Ethics: Fundamental Aspects of Human Conduct Abdulaziz Sachedina
https://ebookmass.com/product/islamic-ethics-fundamental-aspects-ofhuman-conduct-abdulaziz-sachedina/
ebookmass.com
Structured Analytic Techniques for Intelligence Analysis
https://ebookmass.com/product/structured-analytic-techniques-forintelligence-analysis/
ebookmass.com
When Love Breaks Us Monica Arya
https://ebookmass.com/product/when-love-breaks-us-monica-arya/
ebookmass.com
Entre el deber y el deseo: Saga: Amores Tormentosos (Spanish Edition) Melanie Pearson
https://ebookmass.com/product/entre-el-deber-y-el-deseo-saga-amorestormentosos-spanish-edition-melanie-pearson/
ebookmass.com
Metal Oxides and Related Solids for Electrocatalytic Water Splitting Qi Junlei (Ed.) https://ebookmass.com/product/metal-oxides-and-related-solids-forelectrocatalytic-water-splitting-qi-junlei-ed/
ebookmass.com
Communicating National Image through Development and Diplomacy 1st ed. Edition James Pamment
https://ebookmass.com/product/communicating-national-image-throughdevelopment-and-diplomacy-1st-ed-edition-james-pamment/
ebookmass.com
Wildlife Trafficking: A Deconstruction of the Crime, Victims and Offenders 2nd Edition Tanya Wyatt https://ebookmass.com/product/wildlife-trafficking-a-deconstructionof-the-crime-victims-and-offenders-2nd-edition-tanya-wyatt/
ebookmass.com
Medieval Eastern Europe, 500-1300 Florin Curta https://ebookmass.com/product/medieval-eastern-europe-500-1300-florincurta/
ebookmass.com
https://ebookmass.com/product/the-palgrave-handbook-of-mediamisinformation-karen-fowler-watt/
ebookmass.com
VAPORGENERATION TECHNIQUESFORTRACE ELEMENTANALYSIS VAPOR GENERATION TECHNIQUESFOR TRACEELEMENT ANALYSIS FUNDAMENTALASPECTS Editedby
ALESSANDRO D’ULIVO
CNR,InstituteofChemistryofOrganometallicCompounds,Pisa,Italy
RALPH E.STURGEON InorganicChemistryGroup,Metrology,NationalResearchCouncilCanada, Ottawa,ON,Canada
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands
TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright©2022ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans, electronicormechanical,includingphotocopying,recording,oranyinformationstorageand retrievalsystem,withoutpermissioninwritingfromthepublisher.Detailsonhowtoseek permission,furtherinformationaboutthePublisher’spermissionspoliciesandourarrangements withorganizationssuchastheCopyrightClearanceCenterandtheCopyrightLicensingAgency, canbefoundatourwebsite: www.elsevier.com/permissions
Thisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythe Publisher(otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperience broadenourunderstanding,changesinresearchmethods,professionalpractices,ormedical treatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgein evaluatingandusinganyinformation,methods,compounds,orexperimentsdescribedherein.In usingsuchinformationormethodstheyshouldbemindfuloftheirownsafetyandthesafetyof others,includingpartiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors, assumeanyliabilityforanyinjuryand/ordamagetopersonsorpropertyasamatterofproducts liability,negligenceorotherwise,orfromanyuseoroperationofanymethods,products, instructions,orideascontainedinthematerialherein.
ISBN:978-0-323-85834-2
ForInformationonallElsevierpublications visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: SusanDennis
AcquisitionsEditor: KathrynEryilmaz
EditorialProjectManager: IvyDawnTorre
ProductionProjectManager: R.VijayBharath
CoverDesigner: MarkRogers
TypesetbyMPSLimited,Chennai,India
Listofcontributors EduardoBolea GroupofAnalyticalSpectroscopyandSensors(GEAS), InstituteofEnvironmentalSciences(IUCA),UniversityofZaragoza, Zaragoza,Spain
SebastianBurhenn ExperimentalPhysicsII,Ruhr-UniversityBochum, Bochum,Germany
AlessandroD’Ulivo CNR,InstituteofChemistryofOrganometallic Compounds,Pisa,Italy
Jir ˇ ı ´ De ˇ dina InstituteofAnalyticalChemistryoftheCzechAcademyof Sciences,Brno,CzechRepublic
AlessandroD’Ulivo CNR,InstituteofChemistryofOrganometallic Compounds,Pisa,Italy
ZuzanaGajdosechova NationalResearchCouncilCanada,Ottawa,ON, Canada
XiandengHou Analytical&TestingCenter,S ichuanUniversity,Chengdu, P.R.China;KeyLabofGreenC hem&TechofMOEatCollegeof Chemistry,SichuanUniversity,Chengdu,P.R.China
JanKratzer CzechAcademyofSciences,InstituteofAnalyticalChemistry, Brno,CzechRepublic
FranciscoLaborda GroupofAnalyticalSpectroscopyandSensors(GEAS), InstituteofEnvironmentalSciences(IUCA),UniversityofZaragoza, Zaragoza,Spain
XingLiu StateKeyLaboratoryofBiogeologyandEnvironmentalGeology, ChinaUniversityofGeosciences(Wuhan),Wuhan,P.R.China
YueLiu CollegeofChemistry,TianjinNormalUniversity,Tianjin,P.R.China
Toma ´ s ˇ Matous ˇ ek InstituteofAnalyticalChemistryoftheCzechAcademyof Sciences,Brno,CzechRepublic
StanislavMusil InstituteofAnalyticalChemistryoftheCzechAcademyof Sciences,Brno,CzechRepublic
EneaPagliano NationalResearchCouncilCanada,Ottawa,ON,Canada
RalphE.Sturgeon InorganicChemistryGroup,NationalResearchCouncil Canada,Metrology,Ottawa,ON,Canada
XiaodongWen CollegeofPharmacy,DaliUniversity,Dali,P.R.China
YafeiZhen Analytical&TestingCenter,SichuanUniversity,Chengdu, P.R.China
ChengbinZheng KeyLabofGreenChem&TechofMOEatCollegeof Chemistry,SichuanUniversity,Chengdu,P.R.China
ZhenliZhu StateKeyLaboratoryofBiogeologyandEnvironmentalGeology, ChinaUniversityofGeosciences(Wuhan),Wuhan,P.R.China
ZhirongZou CollegeofChemistryandMaterialScience,SichuanNormal University,Chengdu,P.R.China;Analytical&TestingCenter,Sichuan University,Chengdu,P.R.China
Listofcontributorsix Prefacexi
1. Introductiontovaporgenerationtechniques1
AlessandroD’UlivoandRalphE.Sturgeon
1.1Introduction1
1.2Limitationsofcurrentsampleintroductionandatomizationtechniques2
1.3Vaporgenerationtechniques5
1.4FavorablefeaturesandshortcomingsofVGTs8
1.5Overviewofbookstructureandcontent10 References12
PartIChemicalVaporGeneration17 2. Chemicalvaporgenerationbyaqueousboranes19
AlessandroD’Ulivo
2.1Introductionandhistoricalbackground19
2.2Boranereagents,reactionproducts,andapparatus21
2.3Processesandmechanismsofchemicalvaporgeneration32
2.4Factorscontrollingreactivityinchemicalvaporgeneration51
2.5Interferences63
2.6Finalremarks,openquestions,andfuturetrends73 References74
3. Chemicalvaporgenerationoftransitionandnoblemetals91
StanislavMusilandToma´ˇsMatouˇsek
3.1Introductionandbackground91
3.2Experimentalimplementationsofchemicalvaporgeneration92
3.3Efficiencyofchemicalvaporgeneration104
3.4Detaileddiscussionofmechanismsandfundamentalprocessesin chemicalvaporgeneration108
3.5Shortcomingswiththeory,remainingproblems,andlimitations119
3.6Conclusionsandfuturedevelopments120 Acknowledgements122 References122
4. Chemicalvaporgenerationbyaqueousphasealkylation129 ZuzanaGajdosechovaandEneaPagliano
4.1Introduction129
4.2CVGwithtetraalkylborates130
4.3CVGwithtrialkyloxoniumsalts136
4.4MetalspeciationwithGrignardreagents143
4.5Futuretrendsandperspectives144 References145
5. Otherchemicalvaporgenerationtechniques153
AlessandroD’Ulivo,YueLiuandRalphE.Sturgeon
5.1Introduction153
5.2Chelateformation154
5.3Thermalchemicalvaporgeneration163
5.4Generationofvolatileoxides165
5.5Chemicalvaporgenerationofvolatilechlorides169
5.6Chemicalvaporgenerationofvolatilefluorides172
5.7Chemicalvaporgenerationofvolatilebromides173
5.8Chemicalvaporgenerationofvolatilecarbonyls173
5.9Chemicalvaporgenerationofboronesters176
5.10ChemicalvaporgenerationusingSnCl2 179
5.11Concludingremarks179 References180
6. Chemicalvaporgenerationinnonaqueousmedia191 XiaodongWen
6.1Introductionandbackground191
6.2Earlystudiesonchemicalvaporgenerationinnonaqueousmedia193
6.3Experimentalimplementationofthetechnique194
6.4Fundamentalprocesses;theoryandmechanisms200
6.5Remainingproblems,limitations,andshortcomings205
6.6Futuredevelopments205
6.7Conclusions206 References207
PartIINon-ChemicalVaporGeneration211 7. Photo-sono-thermo-chemicalvaporgenerationtechniques213 RalphE.Sturgeon
7.1Generalintroduction213
7.2Photochemicalvaporgeneration214
7.3Sonochemicalvaporgeneration249
7.4Thermochemicalvaporgeneration252
7.5Concludingremarks252 References252
8. Catalystsinphotochemicalvaporgeneration265 ZhirongZou,YafeiZhen,ChengbinZhengandXiandengHou
8.1Introduction265 8.2Heterogeneouscatalysis267 8.3Homogeneouscatalysis271
8.4Conclusions275 Acknowledgments276 References276
9. Plasma-mediatedvaporgenerationtechniques283 XingLiuandZhenliZhu
9.1Generalintroduction283
9.2Sourcesforplasma-mediatedvaporgeneration284
9.3InfluenceofcoexistingionsonPMVG301
9.4AnalyticalperformanceandapplicationsofPMVG305
9.5PossiblemechanismsofPMVG307
9.6Concludingremarksandfuturetrends310 References311
10. Electrochemicalvaporgeneration317 EduardoBoleaandFranciscoLaborda
10.1Introductionandbackgroundtoelectrochemicalvaporgeneration317 10.2FundamentalsandexperimentalimplementationofECVG318 10.3MechanismsofECVG328
10.4Shortcomingsandlimitations:interferencesinECVG330 10.5Finalremarksandfuturedevelopments338 References339
PartIIIAtomizationDevices347
11. Nonplasmadevicesforatomizationanddetectionof volatilemetalspeciesbyatomicabsorptionandfluorescence349 Jiˇrı´ Dedina
11.1Introduction349 11.2Processestakingplaceinonlineatomizers351 11.3Onlineatomization—preliminaryconsiderations352 11.4Onlineatomizers354
11.5In-atomizercollection—preliminaryconsiderations380 11.6Experimentalapproachestoin-atomizercollection382 11.7Conclusionsandfutureperspectives391 Acknowledgments392 Dedication392 References392
12. Dielectricbarrierdischargedevices403 JanKratzerandSebastianBurhenn
12.1Introduction403
12.2DBDconceptanddesigns404
12.3Plasmachemistry:processesandspecies406
12.4Analyticalapplications407
12.5DBDatomizersforAAS409
12.6DBDatomizersforAFS417
12.7DBDexcitationforOES419
12.8Analytepreconcentration427
12.9Speciationanalysis432
12.10Futureperspectives435 Acknowledgment437 References437
Abbreviationsandsymbols443 Index447
Preface Vaporgenerationtechniques(VGTs)coupledwithopticalatomicand massspectrometricdetectionplatformshavebeenemployedforover50 yearstoaugmentanalyticalperformanceforthedeterminationandspeciationofmanyelementsattraceandultratracelevels.Sincetheirintroduction,thepopularityofchemicalVGTshasdramaticallyincreased, andmanyanalyticalprotocolsandregulatedmethodologiesemploy thesederivatizationtechniquesastheysimultaneouslyachieveefficient analyte-matrixseparation.Moreover,despitesignificantrecentadvances inanalyticalatomicspectrometry,mostnotablythoseintheinorganic massspectrometrysector,thepursuitofthebenefitsaccruedbyinterfacingVGtechniquesascomplementaryapproachestoconventional sampleintroductiontoyieldfurtherenhancementsinmeasurement specificity,detectionpower,andminimizationofspectralinterferences undoubtedlypersists.
Some25yearshavepassedsincethemonographbyD ˇ edinaand Tsalev [1] devotedtoVG(primarilyhydridegeneration)fortraceelementanalysiswaspublished,comprehensivelyaddressingtheoretical andexperimentalaspectsinacriticalandin-depthmanner.Inthe interim,newfactsandtheorieshaveemergedsince1995whichcomplement/expandthosetargetedinthattreatise,includingintroductionof severalpromisingnewandrevolutionaryapproachestoVG.Only recently,beginningin2003,dedicatedstudiesofthemechanismsof chemicalVGhaveshednewlightonthisfieldwiththeaimofremoving olderempiricalideasassociatedwiththeiruse.Almostsimultaneously, theintroductionofalternativeVGTs(photochemical,electrochemical, thermochemical,andsonochemical,chelate,andplasmamediated)providedafurtherimpulsetorenewinterestanduseofthesetechniques.
Inspiteofnumerousapplications,veryfeweffortshavefocusedon understandingthefundamentalprocessesgoverningthederivatization reactionsassociatedwiththevariousVGTs.Sourcesofinformation, unfortunately,remainmostlyfragmentedinmanypaperspublished acrossaspectrumofscientificjournals.Thisbook, VaporGeneration TechniquesforTraceElementAnalysis:FundamentalAspects,isdevotedto comprehensivecoverageofthefundamentalaspectsofVGTs,encompassingmethodologiesrangingfromtheclassicalchemicalapproaches tothemostrecentfrontiers.Athoroughoverviewandcriticaldiscussion ofthestate-of-the-artofknowledgeofthemechanismsthatcontrolthe
generationofvolatilederivativesandtheiratomization/detectionby atomicspectrometryaswellasvariousmechanismsaccountingfor interferenceeffectsispresentedinsufficientdetailtoencompassathoroughlyexpandedcoverageofthecurrentliteratureinasingle-source collectionofmaterial.Whileservingasanexpertsourceofadvicefor informationrelevanttotheinterpretationofexperimentalresultsand guidanceforimplementationofVGTsinthelaboratory,itisnot intendedtopresentacomprehensivereviewofapplications,withthe exceptionofthosecontributingfurtherbasicknowledgeviaadoptionof leading-edgetechnologyorsheddinglightonsomeessentialattribute(s) ofthechemistryoftheprocessesinvolved.
Withthisobjectiveinmind,theEditorshavejudiciouslyidentified internationallyrenownedexpertsattheforefrontoftheirindividual fieldsofVGandsecuredcontributionswhichservetoachievetheobjectivesofthistreatise.Ahomogeneous,coherentapproachisfollowedin eachchapterwithIUPACterminologybeingusedthroughout,along withaconsistentsetofdefinitionsandrelatedsymbols/abbreviations. Followingabriefintroductiontogenerallyassessthestateofeachtechniquefromabalancedperspectiveofadvantagesandshortcomings,a comprehensivediscussionoffundamentalprocesses,includinginterferencesandopenproblems,ispresented.Relevantconclusionsaresupportedwithprospectsforfuturedirections.Thebookisdividedinto threesections.Part1dealswithchemicalVG(CVG),encompassing CVGbyaqueousboranesandaqueousphasealkylation,CVGin nonaqueousmediaandthatoftransitionandnoblemetals,andother classicalchemicalVGTs.Part2coversnonchemicalVG,highlighting photo-sono-thermo-chemicalprocessesandtheroleplayedbycatalysts insuchprocesses,plasma-mediatedVGandelectrochemicalVG.Part3 isdevotedtoanin-depthdiscussionofavarietyofdedicatednonplasmaatomizationdevicesutilizedforthedetectionofVGspeciesby atomicabsorptionandfluorescenceaswellasdielectricbarrierdischarge(DBD)devices.AlthoughpowerfulICP-basedopticalemission andmassspectrometricinstrumentationarefrequentlycoupledwith VGTstoenhancedetectionpowerormitigatespectralinterferences, thesesources,aswellasclassicalflames,arenottreatedinthisvolume astheyarenotspecificallydedicatedtothedetectionofvolatileVGspeciesinthemannerthatminiaturediffusionflames,quartztubeatomizersandDBDdeviceshaveevolvedandareused.
TheEditorsaregratefulnotonlytothecontributingauthorsforsharingtheirexpertise,amicablediscussions,hardwork,andmaintenance ofadeliveryscheduleduringaperiodofCOVID-19challengesbutalso tothestaffofElsevierfortheirunwaveringsupportofthisprojectand theproductionofthisvolume.
Neophytesaswellasexpertpractitionersshouldfindinterestinthis bookasitservesasacomprehensive,single-sourceoverviewofrecent developments,providingreaderswithanunderstandingofthecorrect implementationandlimitationsunderlyingtheapplicationofVGTsto everydayanalyticalproblemsfacingthetraceelementanalyst.Assuch, thecontentwillappealtothoseinresearchandacademicinstitutions, aswellasprivatesectorlaboratoriesandtheanalyticalinstrumentation industry.
AlessandroD’Ulivo1 andRalphE.Sturgeon2 1CNR,InstituteofChemistryofOrganometallicCompounds,Pisa,Italy, 2InorganicChemistryGroup,Metrology,NationalResearchCouncilCanada, Ottawa,ON,Canada
Reference
[1] J.D ˇ edina,D.L.Tsalev,HydrideGenerationAtomicAbsorptionSpectrometry,John Wiley&Sons,Inc.,Chichester,1995.
Abbreviationsandsymbols AAS atomicabsorptionspectrometry
AB ammoniaborane[BH3NH3]
ABC analyte-boranecomplexintermediates
ac-SEGD alternatingcurrent-drivensolutionelectrodeglowdischarge
AFS atomicfluorescencespectrometry
APDC ammoniumpyrrolidinedithiocarbamate(pyrrolidine-1-carbodithioate) at_sensit sensitivityregisteredbyaparticularatomizer/spectrometersystem(s/g)
BH hydridoboronspecies
BTC 1,3,5-benzenetricarboxylicacid
CAU ChristianAlbrechtsUniversity
cb (CB) conductionband
CBH sodiumcyanotrihydridoborate[NaBH3CN]
CCP capacitivelycoupledplasma
CE capillaryelectrophoresis
CF continuousflow
Che-CVG chelatechemicalvaporgeneration
CPE cloudpointextraction(rapidandtraditional)
CTAB cetyltrimethylammoniumbromide
Cys L-cysteine
CQDs carbonquantumdots
CQTA conventionalquartztubeatomizer
CT cryogenictrap
CTTS chargetransfer-to-solvent
CVG chemicalvaporgeneration
DART directanalysisinrealtime
DBD dielectricbarrierdischarge
DBDTP dibutyldithiophosphate
DCP directcurrentplasma
DDAB didodecyldimethylammoniumbromide
DDTC diethyldithiocarbamate(N,N-diethylcarbamodithioate)
DEDTP O,O0 -diethyldithiophosphate
DLLME dispersiveliquid liquidmicroextraction
DMA dimethylarsenicacid
DMAB dimethylamineborane[BH3NH(Me)2]
DMDTC dimethyldithiocarbamate
DMF dimethylformamide
DF diffusionflame
ECD electroncapturedetector
ECVG electrochemicalvaporgeneration
EKE electrokineticextraction
ELCAD electrolytecathodeatmosphericglowdischarge
ESI electrosprayionization
ESR/EPR electronspin/paramagneticresonance
ETAAS electrothermalatomizationatomicabsorptionspectrometry
EtHg ethylmercury[CH3CH2Hg1]
ETV electrothermalvaporization
FES flameemissionspectroscopy
FEP fluorinatedethylenepropylene
FI flowinjection
FIF flame-in-flame(atomizer)
FIGS flamein-gas-shield(atomizer)
FLA-APGD flowingliquidanodeatmosphericpressureglowdischarge
FTIR Fouriertransforminfraredspectroscopy
GC-MS gaschromatography massspectrometry
GD glowdischarge
GF(A) graphitefurnace(atomizer)
GFAAS graphitefurnaceatomicabsorption
HAC acetylacetone(pentane-2,4-dione)
HFA hexafluoroacetylacetone(1,1,1,5,5,5-hexafluoropentane-2,4-dione)
HG hydridegeneration
HMC hydrido-metal(loid)complex
HPLC highperformanceliquidchromatography
HR highresolution
HRMS high-resolutionmassspectrometry
HTFA trifluoroacetylacetone(1,1,1-trifluoropentane-2,4-dione)
HTHB hydroxytrihydridoborate[BH3OH ]
IAT integratedatomtrap
IC ionchromatography
ICP inductivelycoupledplasma
ICPOES inductivelycoupledplasmaopticalemissionspectrometry
ICPMS inductivelycoupledplasmamassspectrometry
ICP-ToF-MS inductivelycoupledplasmatime-of-flightmassspectrometry
IEP isoelectricpoint
ITEX in-tubeextraction
LC liquidchromatography
LDLS laser-drivenlightsource
LE-DBD liquidelectrodedielectricbarrierdischarge
LEGD liquidelectrodeglowdischarge
LMTC ligand-to-metalchargetransfer
LIF laser-inducedfluorescence
LLE liquid liquidextraction
LMWCA low-molecular-weightcarboxylicacid
LOD limitofdetection
LPME liquid-phasemicroextraction
LSDBD liquidsprayDBD
MC multicollector
MDF miniaturediffusionflame(atomizer)
MECA molecularemissioncavityanalysis
MeHg methylmercury[CH3Hg1]
MIBK methylisobutylketone
MIL MaterialsofInstituteLavoisier
MIP microwave-inducedplasma
MMA monomethylarsonicacid
MMQTA multiplemicroflamequartzT-tube(atomizer)
MWCNT multiwalledcarbonnanotube
MSPD matrix-assistedsolid-phasedispersion
MS massspectrometry
NACVG chemicalvaporgenerationinnonaqueoussolvents
NCI negativechemicalionization
NDAFS nondispersiveatomicfluorescencespectrometry
NFDBD nebulizedfilmDBD
NMR nuclearmagneticresonance
OES opticalemissionspectroscopy
OH observationheight
PCARD photocatalyst-assistedreductiondevice(microfluidic-based)
PCVG photochemicalvaporgeneration
PCN porouscoordinationnetwork
PEVG plasmaelectrochemicalvaporgeneration
PFA perfluoroalkoxy
PN pneumaticnebulization
PMVG plasma-mediatedvaporgeneration
PTFE polytetrafluoroethylene
Py-CVG pyrolysischemicalvaporgeneration
QA quartzatomizer
QTA quartzT-tubeatomizer
RCPE rapidcloudpointextraction
REE rare-earthelement
RNS reactivenitrogenspecies
ROS reactiveoxygenspecies
RTIL roomtemperatureionicliquid
RVC reticulatedvitreouscarbon
SA-DLLME surfactant-assisteddispersiveliquid liquidmicroextraction
SAGD solutionanodeglowdischarge
SC semiconductor
SCGD solutioncathodeglowdischarge
SCVG sonochemicalvaporgeneration
SDBS sodiumdodecylbenzenesulfonate
SD-SEGD single-dropsolutionelectrodeglowdischarge
SeMet selenomethionine
SHE standardhydrogenelectrode
SIFTMS selectedionflowtubemassspectrometry
SN solutionnebulization
spICPMS singleparticlemodeinductivelycoupledplasmamassspectrometry
SPME solid-phasemicroextraction
SPE solidpolymerelectrolytecellwithNafionmembrane supply(t) massofVSanalytedeliveredtoanatomizerperunittime(g/s)
TALIF two-photonabsorptionlaser-inducedfluorescence
TBAB tertbutylamineborane[BH3NH2C(Me)3]
TBT tributyltin(chloride)
TCVG thermochemicalvaporgeneration
TEM transmissionelectronmicroscopy
TFDBD thin-filmDBD
TFDDTC bis-(trifluoroethyl)-dithiocarbamate[bis(2,2,2-trifluoroethyl)carbamodithioate]
THB tetrahydridoborate,[BH4]
TMB trimethylborate
TMAB trimethylamineborane[BH3N(Me)3]
TOC totalorganiccarbon
ToFMS time-of-flightmassspectrometry
UA-DLLME ultrasound-assisteddispersiveliquid liquidmicroextraction
UARS-CPE ultrasound-assistedrapidsynergisticcloudpointextraction
UiO UniversityofOslo
UV VIS ultraviolet visiblespectroscopy
vb (VB) valenceband
VG vaporgeneration
VGT vaporgenerationtechnique
VS volatilespecies
XAT radicalhalogenatomtransfer
XPS X-rayphotoelectronspectroscopy
Symbols αT globalefficiencyoffreeatomproduction(dimensionless)
αi degreeofionization
εext extractionefficiency(inliquid liquidorliquid solidextraction)
εin nebulizationefficiencyinnebulizationsystem(dimensionless)
εd derivatizationefficiencyofaVGprocess(dimensionless)
εs efficiencyoftransferofderivatizedanalytefromtheliquid-to-gasphase (dimensionless)
εt efficiencyoftransferofvolatilederivatizedanalytetothedetector (dimensionless)
εc or βtrap efficiencyofcollectionortrappingaVGspecies(dimensionless)
εr efficiencyofreleaseoftrappedorpreconcentratedVGspecies (dimensionless)
βv vaporizationefficiencyinflamesandplasmas(dimensionless)
βa atomizationefficiency(dimensionless)
βi atomionizationefficiency(dimensionless)
βgen overallstagegenerationefficiency[β gen 5 εd εs εt](dimensionless)
βvol thefractionoftrappedanalytewhichisvolatilized(andtransportedto theobservationvolumeofthedetector,dimensionless)
Na numberoffreeatoms(dimensionless)
Ni numberofions(dimensionless)
Nm numberofmolecules(dimensionless)
NT totalnumberofatoms(dimensionless)
R(t) temporalvalueofameasuredsignal(dimensionless)
Rarea integratedvalueof R(t)(s)
1 Introductiontovaporgeneration techniques AlessandroD’Ulivo1 andRalphE.Sturgeon2 1CNR,InstituteofChemistryofOrganometallicCompounds,Pisa,Italy, 2InorganicChemistryGroup,NationalResearchCouncilCanada, Metrology,Ottawa,ON,Canada
1.1Introduction Analyticalchemistryincreasinglyplaysanimportantroleinthestudy andprotectionofhealthandtheenvironment,qualitycontrolofmaterials, forensicscience,andmanyotherfieldsinwhichelementalanalysisand speciationareneeded,frequentlyachievedwithatomicandmolecular spectroscopicprocedures.Vaporgenerationtechniques(VGTs),coupled withsuchdetectionsystems,wereintroducedmorethan50yearsago [1,2] byinterfacingearlyatomicabsorptioninstrumentationwithwellknownVGchemicalreactions[chemicalvaporgeneration(CVG)],suchas thegenerationofmercuryvaporbyreductionofmercuricionswith SnCl2,andthatofAsH3 frominorganicarsenicusingtheclassic1836 Marshtest [3].SubsequenttothesefirstchemicalapproachestoVG,many otherchemicalreactionsfollowedfortheproductionofvolatilederivatives ofelementalspecies [4],includingintroductionofnovelderivatization methodsbasedonchemicalandphysicalprinciplesdifferentfromCVG.
TheincreaseinpopularityofthedifferentVGTsexpandedoverthe yearsduetotheirfavorableanalyticalcharacteristics,amongthemease ofautomationcombinedwiththepossibilityofachievingunmatched analyticalfiguresofmeritusingsimpleandinexpensiveinstrumentation.Basedontheirapplicationandvalidation,thousandsofresearch papersandmanyregulatedanalyticalmethodsarose.Unfortunately, muchlessattentionhasbeendevotedtotheelucidationoffundamental
aspectsofthemechanismsofgenerationofthevaporspeciesandtheir atomizationprocessesindedicateddevices,aswellasinterference effectstakingplaceatvariousstagesoftheanalyticalprocedures.
ThisbookisdevotedtoacomprehensivecoverageofthefundamentalaspectsofVGTs,presentingthemostrecentVGapproachesthat havebeenaddedtothisfield,anddiscussingthestate-of-the-artof knowledgeonthemechanismsthatcontrolthegenerationofvolatile derivativesandtheiratomization,aswellasvariousmechanisms accountingforinterferenceeffects.
1.2Limitationsofcurrentsampleintroductionandatomization techniques Problemsrelatingtosampleintroductiontechniquesremainamajorconcernfortraceelementdeterminationandspeciationbyanalyticalatomic spectrometry.Nearly30yearsaftertheinventionofatomicabsorptionspectroscopy(AAS)bySirAlanWalsh [5,6] anditscommercialacceptanceasan analyticaltechnique,BrownerandBoornstillconsideredsampleintroductiontobethe Achilles’Heel ofatomicspectroscopy [7].
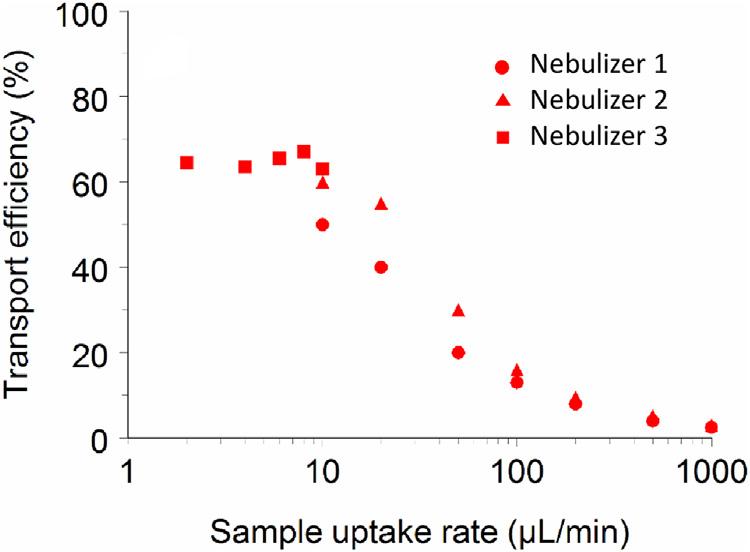
Anidealsampleintroductiontechniqueshouldprovideanefficient andeconomicalmeansofreproduciblyintroducinganalyteintothe atomization/excitation/ionizationsystem(i.e.,detectiondevice)while simultaneouslypreventing/minimizingpossibleinterferencefromother componentsofthesamplematrix.Nebulizers,predominantlyemployed forliquidsampleintroductionintoflamesandplasmas,possesspoor sampleutilizationefficienciesbecausethevolumefractionofsample successfullyintroducedintotheatomizationdevice, εin,isoftenlower than B5%(εin , B0.05) [7 10] whenoperatingatsampleuptake ratesinexcessoftypically100 μLmin 1.Mostoftheanalytesthat shouldhavebeentransferredtothedetectiondeviceareinsteadlostto thewastestream. Fig.1.1 showstheimpactofsampleuptakerateon theefficiencywithwhichtheanalytereachesthedetectiondevice(typicallyaflameorplasma).
Inthecaseofplasmas,deploymentofexpensivelow-flownebulizers operatingbelow10 μLmin 1 canachievesome60%sampleutilization efficiency.OperationofconventionalnebulizersatmLmin 1 uptake ratesresultsingenerationofhighprimarydropletdensitiesinspray chambersandtheirrapidcoalescencetoyieldquitelowtertiaryaerosol outputsontheorderofafewpercent [8].Thetertiarydropletsample fraction,capableofbeingtransportedtotheatomizer,isthendesolvated tosubmicron-sizeddryparticleswhich,iftypicallysmallerthan200nm indiameter,arevaporizedwithhighefficiency(β v B1)inhightemperatureplasmas [10 12].Thisisnotalwaysthecaseinflamesas
1.2Limitationsofcurrentsampleintroductionandatomizationtechniques
FIGURE1.1 Analyteintroduction(transport)efficiency, εin,asafunctionofsample uptakeratewhencoupledtoanunheateddouble-passspraychamber:(1)concentric (MeinhardTR-30-A3);(2)concentric(MeinhardHEN);(3)enhancedparallelpath (BurgenerResearchMiraMist).Source:ReproducedwithpermissionfromJ.W.Olesik, Inductivelycoupledplasmamassspectrometers,in:H.Holland,K.Turekian(Eds.),Treatiseon Geochemistry,vol.15,seconded.,Elsevier,2014,pp.309 336,withpermissionfromElsevier [10].
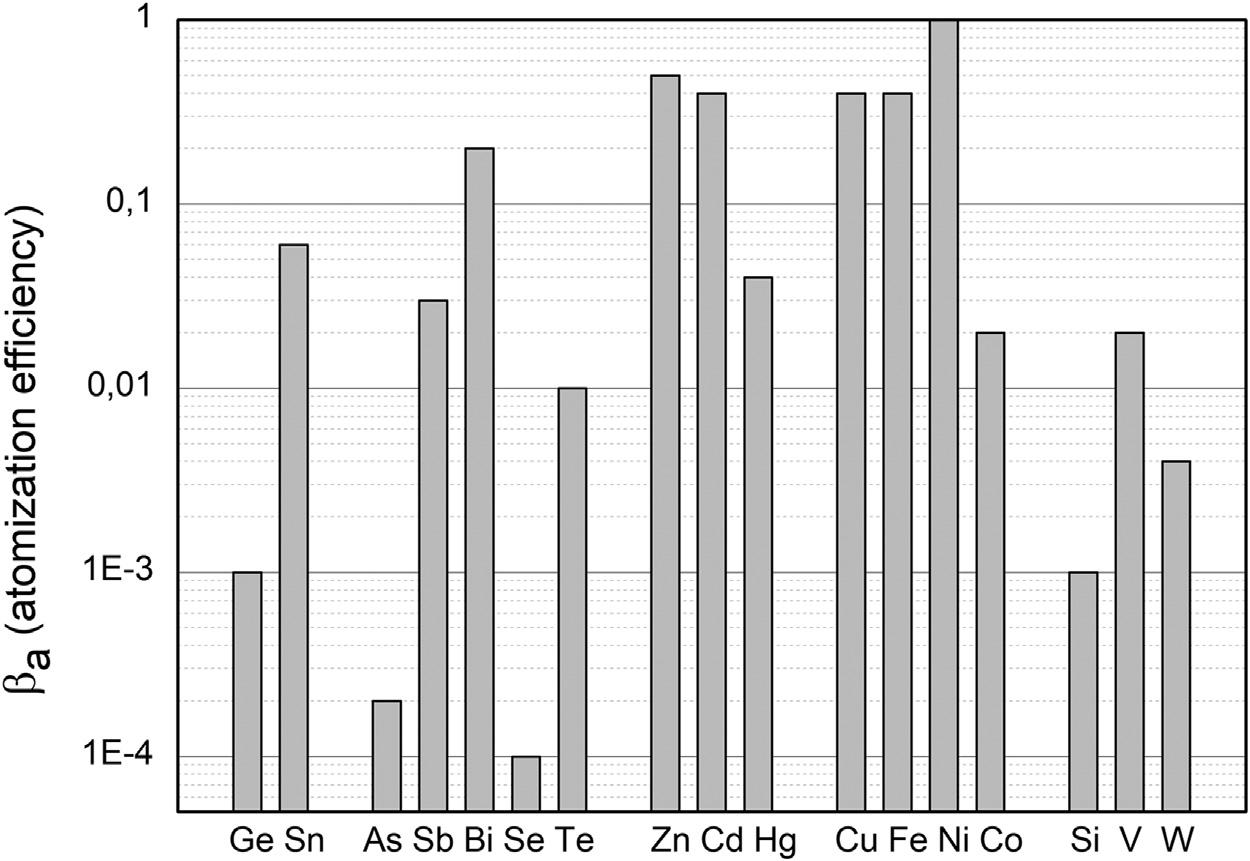
FIGURE1.2 Atomizationefficiency, β a,forsomeselectedelementsinpremixedairacetyleneflames.Source:CompiledfromB.W.Smith,G.E.Parsons,G.E.Bentley,Handbookof FlameSpectroscopy,SpringerScience,NewYork,1975 [13].
theirtemperaturesarenothighenoughforthecompletevaporization oftheresultantdryresiduesofmanyrefractoryspecies(Fig.1.2). Ultimately,chemicaland/orthermalprocessesbringaboutdissociation ofvaporizedmoleculestoyieldgaseousfreeatoms,(thedesiredtarget
forAASandatomicfluorescencespectrometricdetection[AFS]),excited stateatomsandions[forinductivelycoupledplasmaopticalemission spectrometricdetection(ICPOES)]andions(formassspectrometric [MS]detection).Itshouldbenotedthatthesequentialprocessesofliquiddroplettransferanddesolvationfollowedbyvaporizationofthe solidresidueanditsatomization,excitation,andionizationarekineticallycontrolled.Alloccurwithinaflowingstreamofgases(combustion productsinflamesandinertsupportgaswithplasmas)havingstructuredtemperaturefieldssuchthatthereisafinitetimeavailablewithin whichthepopulationofeachspeciesattainsasteadystate.Thisgives risetotheoccurrenceofoptimaldetectionregionswithintheatomizer wherelocalmaximaoccurforfreeatoms,excitedstatespecies,and ionizedanalyte.Withintheatomizer,thetotalatompopulation, NT,is distributedamongmolecularspecies, Nm,freeatoms(ingroundand excitedstates), Na,andionizedspecies, Ni.Thus NT 5 Nm 1 Na 1 Ni [14]. Theefficiencyoftheatomizationprocessisdefinedas β a 5 Na/NT [14], andstronglydependsontheelement [9,11,14,15],asdemonstratedby thedatashownin Fig.1.2,wherein Na 1 Ni 5 totalatomicspecies.
Formostnonmetallicelements,theefficiencyofatomizationislower thanmanyofthemetallicelements,sometimesbyordersofmagnitude. Thecombinedeffectsof εin, β v,and β a provideanindicationofthe globalefficiencyoffreeatomproduction, αT 5 εin β v β a.Itisselfevidentthat αT cannotbegreaterthantheintroductionefficiency (αT # εin).Insomecases,particularlynonmetals,evenassuming β v 5 1, the αT valuesareverylow,andinthepartpermillionrange [11,13].
Graphitefurnaceatomizers(GF),mostlyemployedincombination withAAS(GFAAS),possessmuchhigheratomizationefficiencies thanflames [16 18] aswellasmorefavorablesamplevaporization characteristics,thatis, β v approachesunity(duetogreatereffectivetemperaturesandtypically103-foldlongerresidencetimesduringwhichall processescanproceed).Notethatinthiscase,theeffective εin 5 1for discretesampleintroduction.Itfollowsthat αT valuesarealsomuch higherthaninflames,typically0.01 # αT # 1.Asaresult,despitethe smallsamplevolumesused,graphitefurnacesyieldbetterdetection limitsthancomparableAASmeasurementsinflamesandopticalemissionfromplasmas,makingthemsometimescompetitivewithICPMS systems [19,20]
Forgraphitefurnaces,the Achilles’Heel manifestsitselfintheform ofinterferences,whichcanbemuchmoreseriousthanwithflame andplasma-basedtechniques.Manystudieshavebeendevotedto thistopicanditscontrolbysuchmeansasuseofmatrixmodifiers [19],graphitetubesurfacemodifiers [19,21],Zeemanbackgroundcorrection [19,21],andcontinuumsourcehigh-resolutionAASspectrometers [20].
1.3Vaporgenerationtechniques Anumberofthelimitationsandshortcomingsnotedaboveforliquid sampleintroductionintoflames,plasmas,andgraphiteatomizerscan largelybeovercomebytheadoptionofVGTsand,notsurprisingly, Bingsetal.concludedthattheidealsample,evenforanICP,wouldbe gaseous [22].Ingeneral,VGisaprocesswhereinnonvolatile(usually ionic,metallic,ororganometallic)compoundsformvolatileorsemivolatilespeciesthroughchemical,physical,orbiologicalprocessesthat mayresultintheirtransferfromthecondensedphasetothegasphase [23].Inanalyticalchemistry,VGTsarisefromderivatizationofthe analyticalspeciesofinterest[solvatedatomicions,oxyanions,oxycations,andsimpleorganometal(-semimetal)species]tocreatenonpolar orlow-polarvolatilespecies,asforexamplehydrides,alkylatedcompounds,variouscomplexeswithinorganicandorganicligandsand othervolatilespecies,whichremaintobeidentified [23].
VGTscanmostgenerallybecharacterizedbytwoindependentmajor stages:the generation ofvolatileformsoftargetedspecies(performed inageneratororreactor)and atomization/detection ofsuchspecies (achievedinanatomizerintegratedwithanatomicspectrometer). Whereasthesecondstageisspecificforthegivenspectroscopicmethod, thefirstiscommontoallVGapproaches.
Duringthe generation stage,theliquidsample(slurrieshavealsobeen processed [24])canbeintroducedintothereactorviaseveralmeans, includingfromacontinuousorflowinjectionsystem,aseffluentfroma chromatographicdevice(highperformanceliquidchromatography,ion chromatography,capillaryelectrophoresis,gaschromatography[GC], etc.)orbydiscreteinjection.Clearly,theefficiencyofthisprocessis unity(equivalentto εin 5 1)andrequiresnofurtherconsideration.
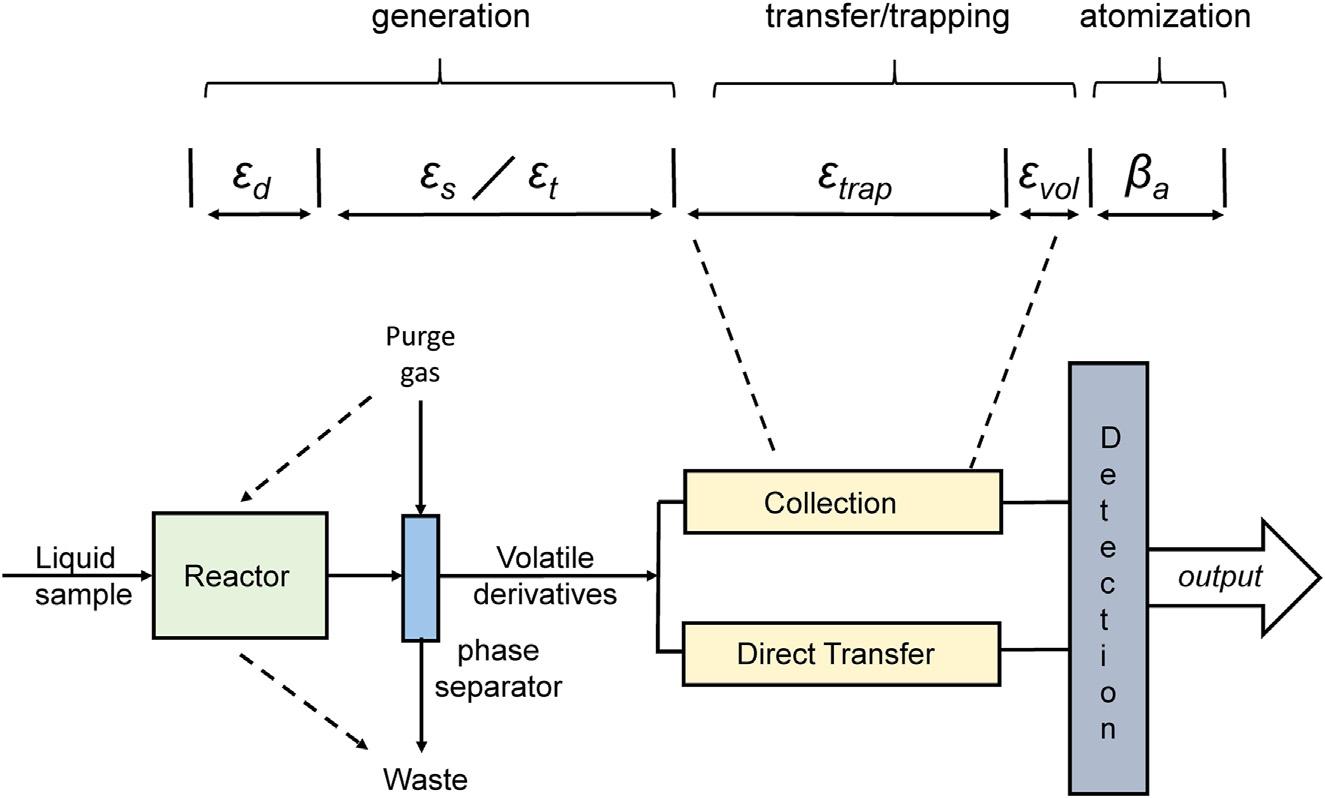
Asillustratedin Fig.1.3,itisoftendesirabletofurtherconsiderthis overallgenerationstageintermsoftwofundamentalprocesses:chemical derivatization/transformationoccurringinthereactor,characterizedby anefficiency εd,andthephasetransferofthevolatilespecieswithina gas liquidseparation(GLS)unitwhichoccurswhentheliquidreaction mixtureisspargedbya(typically)inertgas,characterizedbyaphase separationefficiency εs,anditsconcurrenttransferforpossiblepostprocessingcharacterizedbyanefficiency εt.Anumberofapproachescan beusedfortheconversionofanalytestovolatilespecies,includingchemicalderivatizationbysuitablechemicalreagents(CVGandvolatile chelategeneration)andradical-mediatedderivatizationusingdifferent physicalprinciplesforradicalproduction[photochemicalVG(PCVG), sonochemicalVG(SCVG),andthermochemicalVG(TCVG)],plasmamediatedderivatizationVG(PMVG),andelectrochemicalderivatization VG(ECVG).Asnotedearlier,studiesdevotedtoelucidationofthe
FIGURE1.3 Schematicrepresentationofagenericvaporgenerationtechniquecoupled toanatomicabsorptionspectrometerillustratingtheefficienciescharacterizingallmajor steps,including:derivatization,whereinanalyticalspeciesareconvertedtovolatilederivativeswithagenerationefficiency εd,theirtransferduringspargingfromthesolutionphase intothegasphasewithanefficiency εs,andtransporttothedetectionsystemwithatransfer efficiency εt,eitherdirectlyorafteraconcentration(trapping)step.Ifconcentration/trappingofthevolatilespeciesisconductedfollowedbytheirrapidrelease,theseprocessesare accomplishedwithacollectionefficiency εtrap andsubsequentrelease(volatilization)efficiency εvol.Thevolatilederivativesareeitherconvertedtofreeatoms,withanatomization efficiency β a,orremainasmolecularspecies,beingdetectedwithasuitablespectroscopic technique.Foratomiciondetection,ionizationefficiency β i 5 Ni/NT isconsidered.
mechanismsgoverningthegenerationofvolatilespeciesusingdifferent VGTsarerelativelyscarcecomparedtothosededicatedtotheirapplication.Themechanismsofgenerationarespecificallyhighlightedinthis book.Thus,generallywiththeexceptionofPMVG,theoverallgeneration stageisachievedwiththecombineduseoftwoseparatedevices.
Itremainstonotethatthegenerationstagemaybeoperatedineither oftwomodes:(1)directtransferofthegeneratedspeciestothedetectionsystemand(2)analytecollectionpriortotransporttothedetection system.Thesearebrieflyconsideredbelow.
1. Inthedirecttransfermode,thevolatilespeciesreleasedfromthe reactor-GLSsystemhasanoverallefficiencyof β r 5 εd εs.Asthe volatilespeciesleavingtheGLSisconveyeddirectlytotheatomizer/ detectionsystemwithanefficiency εt,theoverallgenerationstage efficiencyisthentheproduct β gen 5 εd εs εt,representingthe fractionofanalyteintroducedintothegeneratorwhichis successfullyderivatized,releasedfromtheliquidreactionmedium,
andtransportedtoanatomizerfordetection,thelatterhavinga characteristicatomizationefficiency β a.
2. Inthecollectionmode,thereleasedvolatilespeciesisfirsttransferred (withanefficiency β gen)toadevicewhichmaybeanintegralpartof thegeneratorortheatomizer/detectorunit.Traditionally,balloons, cryogenicdevices,orredoxsystemscontainingaliquidreagentwere usedtocollecteitherthegaseouscomponentsordecomposethe speciestoconcentrateitintoasmallvolumeofliquidforsubsequent processing.Moremodernapproachesnowtrapthevolatilespeciesby directingitto,andsubsequentlyimmobilizingiton,asolidsupport [25],suchasbyformationofamercuryamalgamonagoldsubstrate [26] orviathermaldecompositiononaheatedgraphitesurfaceorthin filmofreducednoblemetal [27],aswellasbyinducingdeposition, typicallyasareducedmetaloroxide,onvarioussubstratesby manipulatingthechemistryinthetransportgas [28].Suchapproaches enabletheintegrationofthegeneratedfluxofspecies,permittingthe processingoflargervolumesofsamplesuchastoeffectasignificant enhancementinrelativelimitsofdetectionsince,oncetrapped,the analytecanbesubsequentlyrapidlydesorbedandtransportedto asuitableatomizerinashorttimeinterval(asforexampleinatandem systemsuchasagraphitefurnacelinkedtoanICPMS)toenhance signal-to-noiseratios [29],orthetrappingdevicemaybeanintegral partoftheatomizeritself [28,30].Theseprocessescanbecharacterized byanexperimentallydeterminedefficiencyrepresentingboththatfor thetrappingstep, εtrap,oritsconvolutionwiththesubsequentrelease (volatilization)step(εtrap εvol).
The atomization/detection stageistypicallybasedonuseofatomic spectrometricmethods.ThemostpopularincludeAASandAFS [31 35],butavarietyofplasmasourcesas(ICPOES,ICPMS,microwaveinducedplasma [36 38],capacitivelycoupledplasma [39,40], directcurrentplasma [41,42],glowdischarge [43 46] anddielectric barrierdischarge[DBD]devices [34,35])havebeenused.Interfacing withmassspectrometrictechniqueshasprimarilybeenlimitedtoICP MS,whereasconventionalGCMS(andotherMStechniques)have essentiallybeenusedforspeciesidentificationanddiagnosticstudies. Interestingly,molecularspectroscopy,suchasfouriertranforminfrared spectroscopy [47 49] andmolecularemissioncavityanalysis [50 52] havealsobeenemployed,forwhichnoanalyteatomizationisrequired. Plasmasourcemethodsinherentlyentailgeneral-purposeatomizers optimalforefficientatomizationofgeneratedvolatilespecies.Bycontrast,thoseforAAS(andpartiallyalsoforAFS)areusuallynotidealfor theatomizationofvolatilespecies.Assuch,dedicatedatomizers,such asquartztubeatomizers(QTA) [33],graphiteandmetalatomizers [33],
miniatureflames [33] andDBDdevices [34,35],havemostcommonly beenemployedincombinationwithAASorAFSdetection.
Asevidentfromearlierdiscussion,theatomization/detectionstage maycompriseeitheroftwomodes(Fig.1.3):(1)directatomizationand (2)in-atomizercollection.
1. Withdirectatomization,thevolatilespeciesareimmediately introducedintothedetectionvolumeofthespectrometerwherein atomizationoccurs.Alloftheabove-mentionedatomizers/detectors arecapableofoperationinthismode.Clearly,theatomization efficiency, β a,isthefractionofanalytetransportedintotheatomizer whichisconvertedtodetectablespecies(freeatoms).Itisevident thatthiswouldbethemostsignificantconsiderationifAAS detectionisused,butotherspectroscopiesnecessitatethe considerationofadditionalsubsequentexcitationandionization efficiencies.
2. Within-atomizercollection,thevolatilespeciesaretrappedinthe atomizer(withanefficiency εtrap)concurrentwithitsgeneration, phaseseparation,andtransfer.Itisthenvolatilized(withan efficiency εvol)andatomized(withanefficiency β a).Thismodecan beperformedonlywithsomededicatedatomizersemployedfor AASorAFSdetection,namelyQTA,graphiteandmetalatomizers, aswellasDBDs,orwithtandemsystemssuchasanelectrothermal vaporizercoupledwithaplasmasourceforOESorMSdetection.
1.4FavorablefeaturesandshortcomingsofVGTs ThereisafundamentaldesiretoutilizeVGTsastheygenerallyconfer severalsignificantadvantagesforanalyses,including:(1)efficientmatrix separation,whichoftenleadstoareductionofinterferencesandbetter detectionlimits;(2)hightransport(introduction)efficiencyofanalyteinto theatomicspectroscopicdetectors;(3)highselectivity(insomecases)to permitdifferentiationofchemicalspeciesofaparticularelement;and(4) enablingtheuseofgasphaseseparationmethods(i.e.,GC)forspeciation ofinorganicaswellasorganometal(-semimetal)speciesofsomeelements. Noteworthyisthatthereisthepossibilityforfurtherpostmanipulationof thegaseousproductsofderivatization,subjectingthemeithertopreconcentrationinacoldtrap,chemicaltrap,chromatographiccolumn,orto in-atomizercollection,asnotedabove.Thismethodologyleadstofavorablyenhancedrelativedetectioncapabilities.
Asconcernsimprovementsinsensitivityanddetectionpowerthat canbeachievedwithrespecttoconventionalspectroscopicmethods employingliquidnebulizationordirectsamplingwithaGF,thesewill 8 1.Introductiontovaporgenerationtechniques
bedependentonthespecificVGTemployedandontheanalyte.Inthis case,thecriticalstepentailsderivatization,forwhichtheconversion efficiencyoftheanalytetoavolatilespeciesmaybemuchlessthat quantitative,despite β v and β a remainingquantitative.Inprinciple, β v doesnothavetobespecificallyaccountedforwithVGTssinceitisfrequentlyagaseousmolecularcompoundthatisintroducedtothedetector(atomizationcell)intheabsenceofanysolvent,thusconsumingno “vaporization”energyfromtheatomizationdevice.Moreover, β a may beenhancedwithVGTssincesimplemoleculesaretypicallyformed havinglowatomizationenergies.Suchconditionsmaygeneratemore favorablevaluesof αT comparedtoconventionalsampleintroduction techniquesusingflames,plasmas,andGFatomizers.
Generally,allVGTspresentsimilarfavorablefeaturesandpotential shortcomings.Theircommonlimitationsremaininterferenceeffects causedbyconcomitantspeciespresentinsamplesolutions.Interferences typicallymanifestthemselvesbyperturbing εd, εs,or β a parameters.A classificationschemeforinterferenceswasfirstpostulatedbyD ˇ edina [53] forhydridegeneration.Ingeneral,suchastrategystillmaintains somevalidityandcanthusalsobeofrelevancetootherVGTs.
Liquidphaseinterferencesaffecttheapparentderivatizationefficiency(εd)ofthevolatilespeciesinthatitmayinitiallybeformedinthe reactionmixturewithlowerefficiency(comparedtothecalibratorsample),orgeneratedmoreslowly(kineticeffect),orquantitativelyformed inthereactionmixturebutthegas liquidphasetransfer(εs)occurs withreducedefficiency.
Gasphaseinterferencesmayalsooccurandcanbefurtherclassified astransportinterferences,collectioninterferences,andatomization interferences.
Transportinterferenceeffectsmayariseduetoalossof(unstable) volatileanalytespeciesalongconduitlinesortoaperturbationoftheir transportkinetics.Collectioninterferencescantakeplaceeitherinthe collectionmodeofgenerationand/orduringin-atomizertrapping modeoftheatomization/detectionstagethroughalterationoftrapping(εtrap )andvolatilization(εvol )efficienciescharacteristicofvarious devices(i.e.,trappinginaGCcolumn,cryogeniccollectiondevice, QTA,graphiteatomizer,DBD).
Atomizationinterferencesaffecting β a encompassthosetypically presentinspecificatomizersdedicatedtotheatomizationofvolatile species(QTA,miniatureflames,andbarrierdischargedevices).Such interferencesarenotproblematicwhenusingplasmasourceatomic spectrometricmethods.
Ingeneral,alltypesofinterferencescanmanifesttheireffectsdirectly duringtheanalyticalrun(directinterferences)aswellasduringsuccessiveruns(memoryeffectinterferences).