TOOLS,TECHNIQUES ANDPROTOCOLS FORMONITORING ENVIRONMENTAL CONTAMINANTS
Editedby
SATINDERKAURBRAR
KRISHNAMOORTHYHEGDE
VINAYAKLAXMANPACHAPUR
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright © 2019ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronicormechanical, includingphotocopying,recording,oranyinformationstorageandretrievalsystem,withoutpermissioninwriting fromthepublisher.Detailsonhowtoseekpermission,furtherinformationaboutthePublisher’spermissionspolicies andourarrangementswithorganizationssuchastheCopyrightClearanceCenterandtheCopyrightLicensingAgency, canbefoundatourwebsite: www.elsevier.com/permissions
ThisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythePublisher(other thanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperiencebroadenour understanding,changesinresearchmethods,professionalpractices,ormedicaltreatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluatingandusingany information,methods,compounds,orexperimentsdescribedherein.Inusingsuchinformationormethodsthey shouldbemindfuloftheirownsafetyandthesafetyofothers,includingpartiesforwhomtheyhaveaprofessional responsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assumeanyliability foranyinjuryand/ordamagetopersonsorpropertyasamatterofproductsliability,negligenceorotherwise, orfromanyuseoroperationofanymethods,products,instructions,orideascontainedinthematerialherein.
LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary ISBN:978-0-12-814679-8
ForinformationonallElsevierpublicationsvisitour websiteat https://www.elsevier.com/books-and-journals
Publisher: JoeHayton
AcquisitionEditor: KostasMarinakis
EditorialProjectManager: ReddingMorse
ProductionProjectManager: OmerMukthar
Designer: MarkRogers
TypesetbySPiGlobal,India
Contributors
BalRamAdhikari
LaboratoryofBiosensorsandNanomachines,DepartmentofChemistry,UniversityofMontreal, Montreal,QC,Canada
ShadabAhmed
InstituteofBioinformaticsandBiotechnology,SavitribaiPhulePuneUniversity(Formerly UniversityofPune),Pune,India
V.Amrutha
ElectronicsandCommunicationEngineering,NationalInstituteofTechnologyRourkela, Rourkela,India
AntonioAvalos-Ramı ´ rez
NationalCenterinEnvironmentalTechnologyandElectrochemistry,Shawinigan,QC,Canada
RajMohanBalakrishnan
DepartmentofChemicalEngineering,NationalInstituteofTechnology,Surathkal,India
FatimaBendourou
INRS-ETE,UniversityofQuebec,Quebec,QC,Canada
SatinderKaurBrar
INRS-ETE,UniversityofQuebec,Quebec,QC,Canada
MonaChaali
INRS-ETE,UniversityofQuebec,Quebec,QC,Canada
JipingChen
CASKeyLaboratoryofSeparationScienceforAnalyticalChemistry,DalianInstituteof ChemicalPhysics,ChineseAcademyofSciences,Dalian,People’sRepublicofChina
AgnieszkaCuprys
INRS-ETE,UniversityofQuebec,Quebec,QC,Canada
AchleshDaverey
SchoolofEnvironmentandNaturalResources,DoonUniversity,Dehradun,India
BeatrizDelgado-Cano
NationalCenterinEnvironmentalTechnologyandElectrochemistry,Shawinigan,QC,Canada
Dhanjai
DepartmentofMathematicalandPhysicalSciences,ConcordiaUniversityofEdmonton; DepartmentofPhysicalSciences,MacEwanUniversity,Edmonton,AB,Canada;CASKey
LaboratoryofSeparationScienceforAnalyticalChemistry,DalianInstituteofChemicalPhysics, ChineseAcademyofSciences,Dalian,People’sRepublicofChina
DhrubaDhar
DepartmentofBio-Engineering,BirlaInstituteofTechnology,Mesra,Ranchi,India
KasturiDutta
DepartmentofBiotechnologyandMedicalEngineering,NationalInstituteofTechnology Rourkela,Rourkela,India
RosaGalvez-Cloutier
UniversiteLaval,DepartmentofCivilEngineeringandWaterEngineering,Quebec,QC, Canada
LauraGatel
INRS-ETE,UniversityofQuebec,Quebec,QC,Canada
NataliGo ´ mez-Falco ´ n
HigherTechnologicalInstituteofTierraBlanca(ITSTB),TierraBlanca,Veracruz,Mexico
KrishnamoorthyHegde
INRS-ETE,UniversityofQuebec,Quebec,QC,Canada
KaLokHong
WilkesUniversity,Wilkes-Barre,PA,UnitedStates
RekhaJain
DepartmentofMicrobiology,MarwadiUniversity,Rajkot,India
GuneetKaur
DepartmentofBiology,HongKongBaptistUniversity,KowloonTong,HongKong
GagandeepKaur
BiosensorTechnologyLaboratory,DepartmentofBiotechnology,PunjabiUniversity,Patiala, India
PratikKumar
INRS-ETE,UniversityofQuebec,Quebec,QC,Canada
XianboLu
CASKeyLaboratoryofSeparationScie nceforAnalyticalChemistry,Dalian InstituteofChemicalPhysics,Chinese AcademyofSciences,Dalian,People’s RepublicofChina
SamratMaratkar
InstituteofBioinformaticsandBiotechnology,SavitribaiPhulePuneUniversity(Formerly UniversityofPune),Pune,India
AraceliDalilaLariosMartı ´ nez
INRS-ETE,UniversityofQuebec,Quebec,QC,Canada
SabaMiri
INRS-ETE,UniversityofQuebec,Quebec,QC,Canada
SamuelM.Mugo
DepartmentofPhysicalSciences,MacEwanUniversity,Edmonton,AB,Canada
VinodKumarNigam
DepartmentofBio-Engineering,BirlaInstituteofTechnology,Mesra,Ranchi,India
CarlosS.Osorio-Gonza ´ lez
INRS-ETE,UniversityofQuebec,Quebec,QC,Canada
PreetikaKuknurPachapur
INRS-ETE,UniversityofQuebec,Quebec,QC,Canada
VinayakLaxmanPachapur
INRS-ETE,UniversityofQuebec;DepartmentofCivilEngineeringandWaterEngineering, LavalUniversity,Quebec,QC,Canada
VishalPandey
InstituteofBioinformaticsandBiotechnology,SavitribaiPhulePuneUniversity(Formerly UniversityofPune),Pune,India
NachiketPathak
InstituteofBioinformaticsandBiotechnology,SavitribaiPhulePuneUniversity(Formerly UniversityofPune),Pune,India
RamaPulicharla
INRS-ETE,UniversityofQuebec,Quebec,QC,Canada
KeyurRaval
DepartmentofChemicalEngineering,NationalInstituteofTechnology,Surathkal,India
RituRaval
DepartmentofBiotechnology,ManipalInstituteofTechnology,MAHE,Manipal,India
ShounakRoy
BioXCentreandSchoolofBasicSciences,IndianInstituteofTechnologyMandi,Himachal Pradesh,India
RahulSaini
INRS-ETE,UniversityofQuebec,Quebec,QC,Canada
AnganaSarkar
DepartmentofBiotechnologyandMedicalEngineering,NationalInstituteofTechnology Rourkela,Rourkela,India
KuntalDebSarkar
ElectronicsandCommunicationEngineering,NationalInstituteofTechnologyRourkela, Rourkela,India
SantanuSasidharan
DepartmentofBiotechnology,NationalInstituteofTechnology,Warangal,India
PrakashSaudagar
DepartmentofBiotechnology,NationalInstituteofTechnology,Warangal,India
NaeemShaikh
InstituteofBioinformaticsandBiotechnology,SavitribaiPhulePuneUniversity(Formerly UniversityofPune),Pune,India
SujataSinha
DepartmentofBiochemicalEngineeringandBiotechnology,IndianInstituteofTechnology Delhi,NewDelhi,India
AnkitaSinha
KeyLaboratoryofIndustrialEcologyandEnvironmentalEngineering(MinistryofEducation, China),SchoolofEnvironmentalScienceandTechnology,DalianUniversityofTechnology, Dalian,People’sRepublicofChina
AkshaySonawane
InstituteofBioinformaticsandBiotechnology,SavitribaiPhulePuneUniversity(Formerly UniversityofPune),Pune,India
NiranjanSuralikerimath
INRS-ETE,UniversityofQuebec,Quebec,QC,Canada
GayatriSuresh
INRS-ETE,UniversityofQuebec,Quebec,QC,Canada
PriyankaUddandarao
DepartmentofChemicalEngineering,NationalInstituteofTechnology,Surathkal,India
NeelamVerma
DivisionofResearchandDevelopment,LovelyProfessionalUniversity,Phagwara;Biosensor TechnologyLaboratory,DepartmentofBiotechnology,PunjabiUniversity,Patiala,India
MausamVerma
CO2SolutionsInc.,Quebec,QC,Canada

Emergingcontaminants(ECs)oremergingpollutants(EPs)orContaminantsofemergingconcern(CEC)aredefinedassyntheticornaturallyoccurringsubstancesorchemicals thatarenotincludedinroutineenvironmentalmonitoringprogramsbuthavethepotentialtoentertheenvironmentandcauseknownorsuspectedadverseecologicaland(or) humanhealtheffects.Suchsubstanceshavenoregulatorystandards(fewcountriesnow have)butmaybecandidateforfuturelegislationdependingontheirecotoxicity,potentialhealtheffects,publicperception,andfrequencyofoccurrenceintheenvironment [1] Occurrenceofthesecandidatesintheenvironmenthasbeeneitherdiscoveredrecently duetotheadvancementsintheanalyticaltoolsandtechniquesortheirenvironmental presenceandsignificanceareonlynowbeingevaluated.ECsincludeawiderangeof chemicals,suchaspersistentorganicpollutants,pharmaceuticalsandpersonalcareproducts(PPCPs),endocrinedisruptingcompounds(EDCs),nanomaterials [1].AsonFebruary2016,Norman [2] hascompiledalistofmorethan1000ECs,whichinclude surfactants,PPCPs,flameretardants,gasolineadditivesandtheirdegradationproducts,
biocides,pesticidesandtheirdegradationproducts,andvariousprovenorsuspected EDCs. Table1.1 presentscommonclassesofECsalongwiththeirexamplesandknown adverseenvironmentaleffects.
LimitedinformationisavailableinliteratureonthefateofthesebroadrangesofECs andtheirenvironmentaleffectsatthetracelevels.Thislimitsthepolicymakerstodraft regulationsforthelong-termimpactassessmentduetoexposureofECsatlowlevels.
ThereforeitisimperativetoanalyzeandmonitortheconcentrationsoftheseECsat theemissionsourceaswellaswithinthedifferentenvironmentalmatricesorcompartments(water,air,andsoil)forbetterunderstandingoftheirlong-termimpactassessment [12].AnalysisofECsisnotaneasytaskas [5]:
(a) Environmentalmatricesareverycomplexinnature.
(b) ECsareusuallypresentinverylowlevels(ppttoppb)inenvironmentalsystems.
(c) Multipleisomers/enantiomers/diastereomersoranalogsofECsarepresentinenvironmentalsystems.
(d) ECsare“emerging”innature,thatisrecentlyidentifiedintheenvironmentand lacksanalyticalmethodsforproperidentificationandquantification.
ConventionalanalyticaltechniquesareavailabletodetecttheECsandtheirpossible metabolitesindifferentenvironment.However,suchanalyticaltechniquesaretime
Table1.1 Classificationofemergingpollutantswithtypicalexamplesandassociatedeffects
ClassExample
Endocrine disrupting chemicals
Phthalates(octylphenols, nonylphenols,di(2ethylhexyl)phthalate (DEHP))
BisphenolA;polychlorinated biphenyls(PCBs) Dioxins
PharmaceuticalsAntibiotics(tetracycline, erythromycin);steroidsand hormones;nonsteroidal antiinflammatorydrugs (NSAIDs)
Pesticidesand insecticides
Fipronil;permethrin; fenitrothion; Bacillus thuringiensisisraelensis
Knownenvironmental effectsReferences
• Interfereswithnormalprocessofnaturalbloodborne hormones
• Effectreproductive functions
• Effectcentralnervoussystem [3]
• Antibioticresistance intheenvironment
• Poisoningtobirds andanimals (Diclofenacpoisoningtovultures) [4,5]
• Possiblecarcinogen
• Highlytoxictolizard,bees,gallinaceousbirds
• Endocrine disruption [6,7]
Table1.1 Classificationofemergingpollutantswithtypicalexamplesandassociatedeffects cont’d
ClassExample
Personalcare products
Flame retardants and plasticizers
Fragrances(nitro,polycyclicand macrocyclicmusks, phthalates)
Sunscreenagents (benzophenone, methylbenzylidenecamphor) Insectrepellants(N,Ndiethyl-m-toluamide (DEET)); parahydroxybenzoates
Organophosphateesters (chlorinatedtri(2-chloroethyl) phosphate;and tri(chloropropyl)phosphate; tributylphosphate); polybrominateddiphenyl ethers;tetrabromobisphenol A;bisphenolA
Knownenvironmental effectsReferences
• Bacterialresistance
• Endocrine disruption
• Increasedriskof cancer [8,9]
Industrial additives
BisphenolA;alkylphenols; phthalateesters
Chelatingagents(EDTA), aromaticsulfonates
Hormonesand steroids
Surfactantsand their metabolites
Estradiol,estrone,estriol, diethylstilbestrol(DES)
Alkylphenolethoxylates, 4-nonylphnol 4-Octylphenol,alkylphenol carboxylates;sodiumlauryl sulfates
NanomaterialsCarbonnanotubes;nanowires; TiO2,ZnO,ironoxides, hydroxyapatite,andmetallic nanoparticles
• Endocrine disruption
• Indicationsof increasedriskfor cancer
• Meioticaneuploidy andsynaptic
• Abnormalitiesin animals
• Estrogenicand reproductiveeffects inbirds
• Endocrine disruption
• Canbetoxictoanimals,ecosystems, andhumans
[3,5–7, 10]
[3,5,6]
• Endocrine disruption [9]
Possibleendocrine disruptiveeffect
Possibletoxicityto animalsandaquatic species [6,9]
Ecotoxicityeffectsare atimmaturestate [11]
consuming,monitorpollutantoffline,andrequiresophisticatedandcostlyinstruments. Thereforealotofeffortshavebeenmadetodevelopbiosensor-basedanalyticaltechniques,whicharelessexpensive,quick,andhaveverylowdetectionlimitsforonline monitoringofECsintheenvironment.Thefollowingsectionsdiscussvarioustechniques(conventionalaswellasbiosensorbased)availableforthedetection,identification, andquantificationofECsalongwiththeiradvantagesandlimitations.
2.Conventionaltechniquesforthedetection,identification, andquantificationofECs
2.1Chromatography-basedmethods
Chromatography-basedseparationtechniquessuchasGasChromatography(GC)and LiquidChromatography(LC)coupledwithMassspectrometer(MS)aretheconventionalandmostfrequentlyappliedtoolsforthedetection,identification,andquantificationofECsintheenvironment.Therearevariousfactorswhichdeterminetheuseof eitherGCorLCfortheanalysisofECsindifferentenvironmentalmatrices.GCisadvantageousbecauseofitsfasteranalysisandbetterseparationefficiencythanLC [13]. However,themostimportantcharacteristicsofpollutant(analyte)tobeanalyzedby GCarevolatilityandstabilityathighertemperature.ThereforeGCisthebesttoolto analyzethevolatilepollutant [14]
2.1.1GCandGC-MS
TheapplicationofconventionalGC(one-dimensionalGCor1DGC)islimitedtothe analysisofmixtureshaving50–60pollutants [15].Also,1DGCisnotabletoseparate themixtureofhydrocarbons(>C10) [16].Theseissuesof1DGChavebeenresolved bythedevelopmentofmultidimensionalGCsuchas2DGC(GC GC).In2D GC,twocolumns(ingeneralnonpolarprimarycolumnfollowedbypolarsecondarycolumn)aresequentiallyconnected,whichenhancethepeakcapacityandseparationpower oftheinstrument [16].High-endmultidimensionalGCsystemsuse2DGC(GC GC) coupledwithMSandcanbeusedfortheanalysisofhighlycomplexsamples [13].
Samplepretreatmentsuchasextractionofpollutantfromenvironmentalmatricesis prerequisiteforthechromatographicidentificationandanalysesofECs.Therearevarioustechniquessuchassolid-phaseextraction(SPE),liquid-liquidmicro-extraction (LLME),andmicrowave-assistedextraction(MAE)fortheextractionofpollutantfrom theenvironmentalmatrices [17].SPEmethodpriortoGC-MSanalysesforthescreening ofECssuchasneutralandacidicpharmaceuticals,bisphenolAandtheirchlorinated derivatives,endocrinedisruptingphenoliccompoundsandsteroidsinwater,andwastewatersampleshasbeenmostextensivelyusedbytheresearchersaroundtheworld [17–20].Kotowskaetal. [21] identified120compoundsincludingdrugremnantssuch asibuprofen,naproxen,andcaffeinefromwastewatersample.Antoniouetal. [22]
developedasolid-phasemicro-extraction(SPME)methodaspretreatmenttechniquefor theextractionofPPCPsandEDCsfromthewastewatertreatmentplanteffluentsand analyzedtheseECsbyGC-MS.Thedevelopedmethodbytheauthorsissimple,solvent free,andlowcost.However,theanalysistime(extractionprocedureandGC-MSanalysis)reportedbytheauthorsisabout2h.Graphene,acarbonnanomaterialduetoitshigh surfaceareahasbeenusedasadsorbentmatrixinSPEfortheanalysisofPPCPsinwastewatersamplesbyGC-MS [23].Upto87.6%recoveryoftheanalytehasbeenreportedby usingthegraphene-basedSPE.Ultrasound-assistedextractionhasalsobeenusedasa low-costmethodfortheextractionofECsfromtheenvironmentalsamples [17,24]
Theextractiontime(5–45min)andsolventconsumptioninultrasound-assistedextractionarelowerthantheclassicaltechniques [24].Recently,ultrasound-assistedextraction iscombinedwithSPMEcoupledwithGC-MSfortheanalysisofPPCPsinriversediments [24].Combinationofultrasound-assistedextractionandSPMEintegratesmultiple steps,thatis,extraction,cleaning,isolation,andenrichmentofanalytesinaminiaturized system,whichrequiredverysmallamountofsample(mL)withlimitsofdetectionand quantificationofPPCPs <0.25ng/gand <0.8ng/g,respectively,inriversediment [24]. PolarECsarelessvolatileandtherefore,derivatizationisnecessarytoanalyzethemby GC.DerivatizationreducesthepolarityandenhancesthevolatilityofpolarECbyconvertingpolargroupsintolesspolarmoieties [25,26].Thisstepenhancestheseparation, selectivity,andsensitivityofthepolarcontaminantbyGC [27].However,thisadditional derivatizationstepintheanalysisofpolarECsbyGC-MSistimeconsuming,tedious, laborious,anduseshighlytoxicandcarcinogenicchemicalssuchasdiazomethanefor derivatizationofchlorinatedphenoxyacidherbicides.Moreover,chancesofsamplecontaminationincreaseduringderivatization [28].Furthermore,temperature-sensitiveECs cannotbeanalyzedbyGC-basedtechniques.
2.1.2LCandLC-MS
LCsystemsarethepreferredoverGC-basedtechniquesfortheanalysisofpolarand temperature-sensitiveECsindifferentenvironmentalmatrices.However,theanalysis ofpollutantsbyLC-basedtechniquesismoretimeconsumingthanGC-basedtechniques.ThereforeLCsystemshavebeenupgradedintherecentpastthroughmodern approachesinordertoachievethefastseparationofECswhilemaintainingthehighresolutionandseparationefficiency.Ultra-high-pressureLC(UHPLC)usescolumns packedwith <2 μmparticles(sub-2 μmparticlespackedcolumns)comparedtoconventionalhigh-pressureLC(HPLC)columnshaving5or3 μmparticles.Advantagesof UHPLCoverconventionalHPLCinclude:(1)lesseramountofsolventsrequiredfor sampleelution,(2)muchnarrowerandconcentratedbandsareobtained,and(3)separationspeedincreasesuptoninefold [29,30].ThebestpartofUHPLC-MSsystemisvery quick(<5min)separationoftargetcompoundwithquantificationrangeof10–50ng/L [31].Duetotheseadvantages,UHPLCisoneofthemostpromisinganalyticaltoolsfor
theanalysisandmultiresiduescreeningoforganicECsinenvironmentalmatrices [32]. Forexample,analysisofantibioticresiduesandtheirmetabolites,drugsofabuseandtheir metabolitesinurbanwastewaterandsurfacewater [33,34] andpesticidesinwastewater havebeendetectedandanalyzedbyUHPLC [35].UHPLCcoupledwithMS(UHPLCMSsystems)isefficientinscreeningofsuspectandnontargetECsinwatersamples [36, 37].However,themaindrawbackofUHPLCsystemisincreaseinbackpressure(upto 27-fold)andtherefore,aspecialhardwareisrequiredtohandlethishighpressure,which increasesthecostofLCsystem [31,38]
Core-shellcolumnsorfused-corecolumnsinplaceofsub-2 μmparticlespackedcolumnsareusedinLCsystemstoovercomethehighbackpressureissueofUHPLC.Coreshellcolumnsusesuperficiallyporousparticles,whichenablehigh-speedanalysiswhile maintainingthehighseparationefficiencyequivalenttoUHPLCwithoutincreasingthe backpressure.ApplicationsofLCsystemsusingfused-corecolumnsforanalysisofEcs suchaspharmaceuticals(illicitdrugs,psychiatricdrugs,andselectedhumanmetabolites, etc.),bisphenolAandtheirmetabolitesinwastewateranddrinkingwater [39–42],naphthenicacidsinsurfacewaters [43],andpolarpesticidesanditsdegradationproductsand somenitro-phenolsinrainwater [44] areavailableinliterature.
DevelopmentofautomatedinstrumentssuchasonlineSPEcoupledtoLCandMS (SPE-LC-MSorSPE-LC-MS/MS)isanotheradvancementinLC.Suchautomated instrumentsintegratethreesteps—extraction,purification,anddetectionandhave user-friendlyadvancedintegratedLC-MScontrolsoftwareandrequiresmallsamplevolume(aslowas1mL) [32,45].Thereforeautomatedinstrumentsarehighlyrecommended whensamplevolumeisverylow.SPE-LC-MSorSPE-LC-MS/MStechniqueshave becomemoreandmorepopularintherecentpastforthedetectionanddetermination ofEC [46,47].Forexample,Wodeetal. [48] usedamultiresidueanalyticalmethod forthesimultaneousdeterminationof72ECs(industrialchemicals,pharmaceuticals, psychoactivesubstances,flameretardants,neutralandacidicpesticides)inwatersamples withUHPLC-MS.Recently,anautomatedonlineSPEcoupledtoLC-tandemmass spectrometry(SPE-LC-MS/MS)hasbeenusedbyAnumolandSnyder [46] fortherapid analysisoftraceorganiccompoundssuchaspharmaceuticals,PCPs,hormones,andpesticidesinwater.Theauthorsusedpolymericreversed-phasecartridgesinSPE-LC-MS/ MS.Gorgaetal. [47,49] analyzedendocrinedisruptersandrelatedcompoundsinvarious environmentalmatrixes(river,sediments,wastewater,andsewagesludge).
Despitetheadvancementsinthetoolsandtechniquesandaccuracyandsensitivity, chromatographicmethods(LC-MSandGC-MS)havethefollowinglimitations [50,51]:
1. Theyarenotsuitableforon-siteandcontinuousanalysisofEC.
2. Thesemethodsarelimitedtocentralizedhigh-endlaboratories.
3. Instrumentationsystemsareverycostly.
4. Overallanalysis(includingsamplepretreatment)ofECsistimeconsuming.
5. Highlyskilledandtrainedpersonnelisneededtousethesemethods.
2.2Immunochemicaltechniques
Immunochemicaltechniquessuchasenzyme-linkedimmunosorbentassays(ELISA)and time-resolvedfluoroimmunoassay(TRFIA)havealsobeendevelopedforthedetection andquantificationofECssuchasEDCfromtheenvironmentalmatrices [52–56].ELISA isbasedontheprincipleofantigen-antibodyinteraction,whileTRFIAisbasedonthe fluorescencepropertiesofthelanthanideions [56].TRFIAisultrasensitivethanELISA butlanthanideionsusedinTRFIAareverycostly,whichdiscourageitsuseoverELISA. Immunochemicaltechniquesareadvantageousoverchromatographictechniquesdueto theirfastanalysistime,selectivity,sensitivity,reliability,simplicity(simpleornosample pretreatment),andlowsamplevolume [56].However,thelimitationsordrawbacksof immunochemicaltechniquesare [57] asfollows:
1. Biologicalmaterials(antibody)usedintheimmunochemicaltechniquesarelessstable.
2. Assayprocedureiscomplicatedandhavemultisteps.
3. Highlyskilledpersonisrequiredforpreparationofantibodyandanalysis.
4. Difficulttouseon-site.
3.Biosensorsforthedetection,identification,andquantificationofECs
Biosensorisdefinedas“adevicethatusesspecificbiochemicalreactionsmediatedbyisolatedenzymes,immunosystems,tissues,organellesorwholecellstodetectchemicalcompoundsusuallybyelectrical,thermaloropticalsignals”bytheInternationalUnionof PureandAppliedChemistry(IUPAC) [57].Inotherwords,biosensorssensethesignals producedbythebio/chemicalreactionsinvolvingtheanalyte.Thesesignalsareproportionaltotheconcentrationoftheanalyteinthereactionmixture [58].Thebiosensorsare gaininglotofattentioninthescreeninganddetectionofECsintheenvironmentalmatricesduetotheirselectivity,reproducibility,stability,sensitivity,portability,miniaturization,on-sitemonitoringandenablepermanentandunattendedoperationinthefield [57–59].ThebiomonitoringofECsbyusingbiosensorsisanotheradvantageover GC-LC-basedsystems.Determinationofeco-effects(cytotoxicity,genotoxicity,endocrinedisruptingeffects,etc.)ofenvironmentalcontaminantsisfeasiblebybiosensors [57]. Justinoetal. [60] summarizedtherecentapplicationsofdifferentbiosensorsinenvironmentalmonitoringofECssuchaspesticides(includingorganophosphorouspesticides), pathogens,andendocrinedisruptingchemicals.Thelimitofdetectionofbiosensorswas reportedtobeuptoppblevels [60].
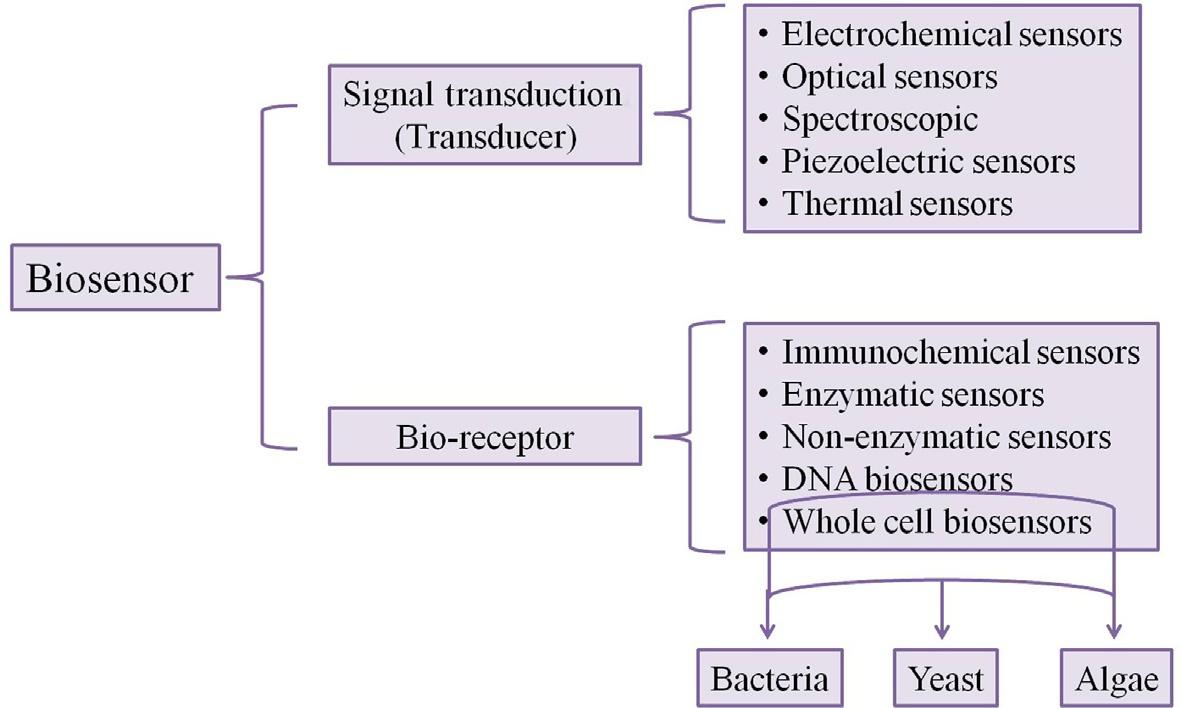
Atypicalbiosensorhasthefollowingmaincomponents:analyte(thepollutant),bioreceptor(recognizetheanalytethroughbiochemicalreactionsandproducessignals), transducer(convertbiochemicalsignalstoelectronicsignals),anddisplay.Biosensors arebroadlyclassifiedintotwogroupsbasedonthetypeoftransducerandbioreceptor type. Fig.1.1 showsthedifferentclassesofbiosensors.
3.1Aptasensorsfordetectionofemergingcontaminants
Aptamersarethesingle-strandedDNAorRNAofknownsequences,havehighaffinity andspecificitytothetargetmoleculestobedetected.Aptamer-basedtechnologieshave gainedtremendousinterestinsensingapotentiallylargenumberofenvironmentalcontaminationinrecenttimeduetotheirhighersensitivity,selectivity,lessexpensiveinvitro system,andenhancedenvironmentalstability.Anaptasensorconsistsofknownoligonucleotidesequence,thatis,aptamerasthesensingelementeithercombinedwithinthe systemorcloselyassociatedwithaphysiochemicaltransducersystem [61].Besidethesignificantapplicationofaptasensorinmedicalfield,thistechniqueisnowmorepopularas thesensorsystemofseveralenvironmentalcontaminantsincludingheavymetals,toxins, pesticides,andotherharmfulsmallmolecules.Mycotoxins,themajorgroupoftoxins thatarepresentinourfoodhavebeendetectedbythisaptasensor [62].Recently,anumberofheavymetalsincludingAs3+,Cu2+,andPb2+ havebeendetectedfromcontaminatedwatersampleatverylowconcentrationbythistechnique [63].Aseriesofaptamers whichbindtowithhighaffinitytodifferentpoisonousorgano-phosphorouspesticides suchasphorate,profenofos,isocarbophos,andomethoateashasalreadybeendeveloped [64].Aptasensorscapableofrapidlydetectingpathogensincludingbothvirusandbacteria withimprovedanalyticalperformancehavebeendesignedwithoutpriorinformationof theirmolecularstructures [65].Intheenvironmentalmonitoring,theapplicationofelectrochemicalaptasensorsisimmense,andthisisoneofthefascinatingareas.Thefuture progressinaptasensorstechnologywillrevealthedetectionofunexploredtargetanalytes relevanttotheenvironmentalcontaminants.
Fig.1.1 Classificationofbiosensors.
3.2Enzymeandwholecellbiosensors
Enzymebiosensoremployedvariousgroupsofenzymessuchaslaccase,tyrosinase,peroxidases,acetylcholinesterase,cytochromeP450,monoamineoxidaseasrecognition element [65–68].Theyhavewideapplicationsinmonitoringenvironmentalcontaminantsincludingphenolsandtheirdegradativeproducts [65,66],pesticides [69],herbicides [70],andpharmaceuticals [67].Acoupleofportableenzyme-basedbiosensors havealsobeenreportedinliteratureforon-sitepesticidedetection [71,72]
Themainadvantagesofusingenzyme-basedbiosensorsincludeshortanalysistime (analysissamplewithinminutesorhours)andnoethicalissuesassociatedwiththem. However,theyarecostlierandcandetectnarrowerrangeofpollutantsthanwholecell biosensors.Enzymebiosensorsdetectagroupofpollutants,forexample,totalpesticides ratherthanindividualpollutant.Ontheotherhand,wholecell(bacteria,yeast,algae) basedbiosensorsareinexpensiveandeasybuthaveethicalissues.Theanalysistimeof wholecell-basedbiosensorsisinhourstodays [59].
Yeastbiosensors(anexampleofwholecellbiosensors)havebeendevelopedforselected endocrinedisruptors(EDC)suchasestrogens [73],glucocorticoids [74],bisphenolA [75], serotonin [76],andsoon,andselectedheavymetals [77,78].Thereforeyeastbiosensorfor otherECsneedstobedevelopedforitswiderandrealworldapplications.Long-termstorageofyeastbiosensorsischallengingandneedsfurtheradvancements.Fewauthorssuggestedandtestedthestorageofyeastbiosensorsatlowtemperature( 18or 20°C)to maintaintheviabilityofcellsforupto10–12months [79,80].However,reactivation ofyeastcellsfromdeepfreezestoragebeforeon-siteanalysismayneedseveralhours [59]
3.3Immunosensors
ImmunosensorsarebasedontheimmunochemicaltechniquessuchasELISAtodetect theECsintheenvironment.Theyutilizetheantibodyorantigenasbioreceptortoproducesignalsandatransducer(electrochemical,optical,piezoelectric,etc.)toconvertthe biosignalsintoreadableform.Immunosensorsarehighlyselectiveandcanbeusedto studythetoxicityofanindividualpollutant [81].Maurizetal. [82] hadcontinuously monitoredthechlorpyrifos,anorganophosphatepesticideatpartpertrillionlevelsinreal watersamples(groundwater,surfacewater,anddrinkingwater)usingaportableimmunosensor.Theportableimmunosensortookonly20minfortheanalysiswithoutsample preparationsuchasextractionorcleanup.
3.4Molecularlyimprintedpolymer(MIP)biosensors
MIP-basedsensorshavebeengaininghugeattentioninrecenttimes.Thebasicworking principleofMIPisthecreationofhighlystableligandwithspecificandselectivecratersina 3D-polymericnetwork,complementarytothetargetanalyticsbypolymerizationofa monomerandacross-linker.Thecavitynotonlyiscomplementarywithrespecttotarget
molecule’sshape,size,andconformationbutalsoprovidescovalentandnoncovalentinteractionpointsandacoordinationsphereforproperconjugationofthetemplatemolecule [83].MIPsaresyntheticpolymers,whichcanonlybeusedasaffinitysensorsbutnotasa catalyticbiochemicalenzymemimickingsensors [84].Thistechnologyhasbeendescribed aspromisinganalyticaldevicesindiversefields,includingenvironmental,food,pharmaceutical,andclinicalanalysis [84].MIPtechnologyisadvantageouswithrespecttoits desiredportability,quickresponse,highspecificity,acutesensitivity,andlessprice.For effectivedetection,MIP-basedsensorsshouldbecoupledwithvarioustransducerssystems includingelectrochemical,optical,conductometric,fluorescence,andpiezoelectric [85] MIPsarealongwithotheranalyticaltechniquessuchasLC,capillaryelectrochromatography,SPE,bindingassays,andsoon,alsousedasselectivetoolsforthedetectionofvarious inorganicandorganicenvironmentalpollutants [86].
TheapplicationsofMIPbiosensorshavealreadybeeninsuccessinanalyzingvolatile organicsfromtheirmixtures,studyingdegradationofhydrocarbon,detectingpesticides, pharmaceuticalcompoundsinpollutedenvironmentalsample,inevaluatingoxidationreductionreactions,inbio-analyzingofsignalingmolecules/drugsbytargetingwholecells, viruses,orbacteria [87–91].AcombinationofMIPsandtransducersformasynergistic device.MIPsshouldhavefulfilledcertaincriteriaduringfabrication.Theyshouldhave properselectivity,bindingstrength,regenerationabilityandstabilityintermsofwithstandingextremepH,organicbase,hightemperatureandpressureduringoperationalcondition aswellasduringstorage [87,92].Moreover,thistechnologyisquitesuitableandadvantageousforthedetectionofnonelectroactivemoleculessuchaspesticides,drugs,andsoon. DespitetheplentifuladvantagesofMIPstherateofcommercialactivityisstilllimited.
3.5Nanomaterial-basedbiosensors
Nanomaterial-basedbiosensorsarealsoattractingalotofattentionduetoitsuniquecharacteristicsandadvantagesoverotherbiosensors.Nanomaterialsofferaveryhighsurface area-to-volumeratio,whichenhancesthecatalyticfunctionandsensingresponse [93] Theyalsooffergreatbiochemicalcompatibilitywithverylowdetectablelimits.Silver nanoparticle-basedelectrochemicalbiosensorshavebeendevelopedforthedetection andmonitoringofestrogenicsubstancesinwatersampleswithdetectionlimitsofup to1ng/L [94].Recently,Pdwormlikenanochains/graphiticcarbonnitridenanocompositesandacetylcholinesterase-basedbiosensorsweredevelopedforthedetectionof organophosphatepesticideswithgoodreproducibilityandstability [95].Hassaanetal. [96] developedopticalnano-biosensorsforthedetectionofpharmaceuticalsandother contaminants.Theauthorsusedhorseradishperoxidaseenzymeasabioreceptorfor thedetectionandquantificationofphenol,resorcinol,epinephrine,andacetaminophen. However,applicationofsuchopticalnano-biosensorsforrealenvironmentalmatrices needstobeverified.
4.Conclusion
Despitevariousadvantages(suchasselectivity,portability,fasteranalysis,costeffectiveness.andreal-timemonitoringofpollutants)overconventionalanalyticaltechniques,biosensorsforenvironmentalapplicationsarestillinthedevelopingstageandface variouschallengesduetothecomplexityoftheenvironment.Thepresenceofothercontaminantsintheenvironmentalmatricesinterfereswiththeanalysisoftargetpollutantby biosensorsandaffectsthedetectionlimits [68].MIPandnanomaterial-basedbiosensors wouldbeanalternativeoption.However,applicationsofMIPbiosensorsaremainly focusedonbiomedicalfield.Ontheotherhand,variousdifficultiesrelatedtotheconstructionofbiosensorsbasedonnanomaterialshavebeenpointedoutbyMaduraiveeran andJin [93].Forexample,controlledsynthesisofnanoparticles,optimizationofmultimetallicnanoparticlesforbetterspecificityandstabilityinrealworldsamples,designing ofsuitableinterfaceforimprovedsensitivity,andsoon,arestillchallengingforthedevelopmentofnanomaterial-basedbiosensors.Commercializationofbiosensorsforon-site monitoringofEPsintheenvironmentshouldbethefocusoffutureresearch.
References
[1] V.Geissen,H.Mol,E.Klumpp,G.Umlauf,M.Nadal,M.vanderPloeg,S.E.A.T.M.vanderZee, C.J.Ritsema,Emergingpollutantsintheenvironment:achallengeforwaterresourcemanagement, Int.SoilWaterConserv.Res.3(1)(2015)57–65.
[2]NormanNetwork,Availablefrom: www.norman-network.net.Accessed10January2018.
[3] P.Erkekoglu,B.Kocer-Gumusel,Environmentaleffectsofendocrine-disruptingchemicals:aspecial focusonphthalatesandbisphenolA,in:M.L.Larramendy,S.Soloneski(Eds.),EnvironmentalHealth Risk—HazardousFactorstoLivingSpecies,IntechOpen,London,UK,2016(Chapter6).
[4] C.G.Daughton,Pharmaceuticalsandtheenvironment(PiE):evolutionandimpactofthepublished literaturerevealedbybibliometricanalysis,Sci.TotalEnviron.562(2016)391–426.
[5] Q.Liu,Q.Zhou,G.Jiang,Nanomaterialsforanalysisandmonitoringofemergingchemicalpollutants, TrendsAnal.Chem.58(2014)10–22.
[6] A.Pal,Y.He,M.Jekel,M.Reinhard,K.Y.Gin,Emergingcontaminantsofpublichealthsignificance aswaterqualityindicatorcompoundsintheurbanwatercycle,Environ.Int.71(2014)46–62.
[7] M.Raghav,S.Eden,K.Mitchell,B.Witte,Contaminantsofemergingconcerninwater,Arroyo (2013)1–12.
[8] S.Merel,S.A.Snyder,Criticalassessmentoftheubiquitousoccurrenceandfateoftheinsectrepellent N,N-diethyl-m-toluamideinwater,Environ.Int.96(2016)98–117.
[9] A.I.Stefanakis,J.A.Becker,Areviewofemergingcontaminantsinwater:classification,sourcesand potentialrisks,in:A.E.McKeown,G.Bugyi(Eds.),ImpactofWaterPollutiononHumanHealth andEnvironmentalSustainability,IGIGlobal,Vancouver,2015,pp.57–82.
[10] A.Marklund,B.Andersson,P.Haglund,OrganophosphorusflameretardantsandplasticizersinSwedishsewagetreatmentplants,Environ.Sci.Technol.39(19)(2005)7423–7429.
[11] E.J.E.Stuart,R.G.Compton,Nanoparticles-emergingcontaminants,in:L.Moretto,K.Kalcher (Eds.),EnvironmentalAnalysisbyElectrochemicalSensorsandBiosensors.NanostructureScience andTechnology,Springer,NewYork,NY,2015.
[12] M.Gavrilescu,K.Demnerova,J.Aamand,S.Agathos,F.Fava,Emergingpollutantsintheenvironment:presentandfuturechallengesinbiomonitoring,ecologicalrisksandbioremediation,NewBiotechnol.32(1)(2015)147–156.
[13] M.Lorenzo,Y.Pico,Gaschromatographyandmassspectroscopytechniquesforthedetectionof chemicalcontaminantsandresiduesinfoods,in:D.Schrenk,A.Cartus(Eds.),ChemicalContaminants andResiduesinFood,WoodheadPublishing,Elsevier,UK,2017,pp.17–61.
[14] S.E.Prebihalo,K.L.Berrier,C.E.Freye,H.D.Bahaghighat,N.R.Moore,D.K.Pinkerton, R.E.Synovec,Multidimensionalgaschromatography:advancesininstrumentation,chemometrics, andapplications,Anal.Chem.90(1)(2018)505–532.
[15] P.J.Baugh,Advancesandchangesinthetechniquesofmulti-dimensionalandcomprehensivechromatographyandwhencoupledwithmassspectrometry,J.Anal.Bioanal.Tech.7(2016)4.
[16] M.S.Alam,R.M.Harison,Recentadvancesintheapplicationof2-dimensionalgaschromatography withsoftandhardionisationtime-of-flightmassspectrometryinenvironmentalanalysis,Chem.Sci. 7(2016)3968.
[17] U.Kotowska,J.Kapelewska,J.Sturgulewska,Determinationofphenolsandpharmaceuticalsin municipalwastewatersfrompolishtreatmentplantsbyultrasound-assistedemulsificationmicroextractionfollowedbyGC-MS,Environ.Sci.Pollut.Res.Int.21(1)(2014)660–673.
[18] O.Ballesteros,A.Zafra,A.Navalo ´ n,J.L.Vı´lchez,Sensitivegaschromatographic-massspectrometric methodforthedeterminationofphthalateesters,alkylphenols,bisphenolAandtheirchlorinatedderivativesinwastewatersamples,J.Chromatogr.A1121(2)(2006)154–162.
[19] M.J.Go ´ mez,A.Aguera,M.Mezcua,J.Hurtado,F.Mocholı´,A.R.Ferna ´ ndez-Alba,Simultaneous analysisofneutralandacidicpharmaceuticalsaswellasrelatedcompoundsbygaschromatographytandemmassspectrometryinwastewater,Talanta73(2)(2007)314–320.
[20] R.Liu,J.L.Zhou,A.Wilding,Simultaneousdeterminationofendocrinedisruptingphenoliccompoundsandsteroidsinwaterbysolid-phaseextraction-gaschromatography-massspectrometry, J.Chromatogr.A1022(1–2)(2004)179–189.
[21] U.Kotowska,K.Bieganska,V.A.Isidorov,Screeningoftraceorganiccompoundsinmunicipalwastewaterbygaschromatography-massspectrometry,Pol.J.Environ.Stud.21(1)(2012)129–138.
[22] C.V.Antoniou,E.E.Koukouraki,E.Diamadopoulos,Analysisofselectedpharmaceuticalcompounds andendocrinedisruptersinmunicipalwastewaterusingsolid-phasemicroextractionandgaschromatography,WaterEnviron.Res.81(7)(2009)664–669.
[23] Y.Yu,L.Wu,Applicationofgraphenefortheanalysisofpharmaceuticalsandpersonalcareproductsin wastewater,Anal.Bioanal.Chem.405(14)(2013)4913–4919.
[24] A.Dı´az,A.A.Pen ˜ a-Alvarez,Simplemethodforthesimultaneousdeterminationofpharmaceuticalsand personalcareproductsinriversedimentbyultrasound-assistedextractionfollowedbysolid-phase microextractioncoupledwithgaschromatography-massspectrometry,J.Chromatogr.Sci.55(9) (2017)946–953.
[25] R.Aznar,B.Albero,C.Sa ´ nchez-Brunete,E.Miguel,I.Martı´n-Girela,J.L.Tadeo,Simultaneous determinationofmulticlassemergingcontaminantsinaquaticplantsbyultrasound-assistedmatrix solid-phasedispersionandGC-MS,Environ.Sci.Pollut.Res.Int.24(9)(2017)7911–7920.
[26] A.B.Fialkov,U.Steiner,S.J.Lehotay,A.Amirav,SensitivityandnoiseinGC-MS:achievinglow limitsofdetectionfordifficultanalytes,Int.J.MassSpectrom.260(2007)31–48.
[27] A.Jurek,E.Leitner,Comparingdifferentgaschromatographicmethodsforthequantificationof bisphenolA(BPA)tracelevelsinpaperandcardboardproductsfromthemarket,FoodAddit.Contam. PartA32(8)(2015)1331–1342.
[28] M. Petrovi,S.Gonzalez, D. Barcelo ´ ,Analysisandremovalofemergingcontaminantsinwastewaterand drinkingwater,TrACTrendsAnalyt.Chem.22(10)(2003)685–696.
[29] D.T.Nguyen,D.Guillarme,S.Rudaz,J.L.Veuthey,Fastanalysisinliquidchromatographyusingsmall particlesizeandhighpressure,J.Sep.Sci.29(12)(2006)1836–1848.
[30] D.Guillarme,J.Ruta,S.Rudaz,J.L.Veuthey,Newtrendsinfastandhigh-resolutionliquidchromatography:acriticalcomparisonofexistingapproaches,Anal.Bioanal.Chem.397(3)(2010) 1069–1082.
[31] M.Farre,L.Kantiani,M.Petrovic,S.Perez,D.Barcelo ´ ,Achievementsandfuturetrendsintheanalysis ofemergingorganiccontaminantsinenvironmentalsamplesbymassspectrometryandbioanalytical techniques,J.Chromatogr.A1259(2012)86–99.
[32] M. Celic,M.Farre,M.L.deAlda,S.Perez,D.Barcelo ´ ,M.Petrovic,Environmentalanalysis:emerging pollutants,in:LiquidChromatography,seconded.,Elsevier,UnitedStates,2017,pp.451–477.
[33] M.Gros,S.Rodrı´guez-Mozaz,D.Barcelo ´ ,Rapidanalysisofmulticlassantibioticresiduesandsomeof theirmetabolitesinhospital,urbanwastewaterandriverwaterbyultra-high-performanceliquidchromatographycoupledtoquadrupole-lineariontraptandemmassspectrometry,J.Chromatogr.A 1292(2013)173–188.
[34] D.R.Baker,B.Kasprzyk-Hordern,Multi-residueanalysisofdrugsofabuseinwastewaterandsurface waterbysolid-phaseextractionandliquidchromatography-positiveelectrosprayionisationtandem massspectrometry,J.Chromatogr.A1218(12)(2011)620–631.
[35] N.Barco-Bonilla,R.Romero-Gonza ´ lez,P.Plaza-Bolanos,A.G.Frenich,J.L.M.Vidal,Analysisand studyofthedistributionofpolarandnon-polarpesticidesinwastewatereffluentsfrommodernand conventionaltreatments,J.Chromatogr.A1217(50)(2010)7817–7825.
[36] F.Gosetti,E.Mazzucco,M.C.Gennaro,E.Marengo,Contaminantsinwater:non-targetUHPLC/ MSanalysis,Environ.Chem.Lett.14(1)(2016)51–65.
[37] A.Masia ´ ,J.Campo,C.Blasco,Y.Pico ´ ,Ultra-highperformanceliquidchromatographyquadrupole time-of-flightmassspectrometrytoidentifycontaminantsinwater:aninsightonenvironmentalforensics,J.Chromatogr.A1345(2014)86–97.
[38] V.Gonza ´ lez-Ruiz,A.I.Olives,M.A.Martı´n,Core-shellparticlesleadthewaytorenewinghighperformanceliquidchromatography,TrendsAnal.Chem.64(2015)17–28.
[39] V.L.Borova,N.C.Maragou,P.Gago-Ferrero,C.Pistos,N.S.Thomaidis,Highlysensitivedeterminationof68psychoactivepharmaceuticals,illicitdrugs,andrelatedhumanmetabolitesinwastewaterby liquidchromatography-tandemmassspectrometry,Anal.Bioanal.Chem.406(17)(2014)4273–4285.
[40] I.Tarcomnicu,A.L.vanNuijs,W.Simons,L.Bervoets,R.Blust,P.G.Jorens, H.Neels,A.Covaci,Simultaneousdeterminationof15top-prescribedpharmaceuticalsandtheir metabolitesininfluentwastewaterbyreversed-phaseliquidchromatographycoupledtotandemmass spectrometry,Talanta83(3)(2011)795–803.
[41] M.Pedrouzo,F.Borrull,E.Pocurull,R.M.Marce,Drugsofabuseandtheirmetabolitesinwasteand surfacewatersbyliquidchromatography-tandemmassspectrometry,J.Sep.Sci.34(10)(2011) 1091–1101.
[42] H.Gallart-Ayala,E.Moyano,M.T.Galceran,On-linesolidphaseextractionfastliquid chromatography-tandemmassspectrometryfortheanalysisofbisphenolAanditschlorinatedderivativesinwatersamples,J.Chromatogr.A1217(21)(2010)3511–3518.
[43] D.Shang,M.Kim,M.Haberl,A.Legzdins,Developmentofarapidliquidchromatographytandem massspectrometrymethodforscreeningoftracenaphthenicacidsinaqueousenvironments, J.Chromatogr.A1278(2013)98–107.
[44] R.Bossi,K.V.Vejrup,B.B.Mogensen,W.A.Asman,AnalysisofpolarpesticidesinrainwaterinDenmarkbyliquidchromatography-tandemmassspectrometry,J.Chromatogr.A957(1)(2002)27–36.
[45] Z.Kuklenyik,A.M.Calafat,J.R.Barr,J.L.Pirkle,Designofonlinesolidphaseextractionliquid chromatography-tandemmassspectrometry(SPE-LC-MS/MS)hyphenatedsystemsforquantitative analysisofsmallorganiccompoundsinbiologicalmatrices,J.Sep.Sci.34(2011)3606–3618.
[46] T.Anumol,S.A.Snyder,Rapidanalysisoftraceorganiccompoundsinwaterbyautomatedonline solid-phaseextractioncoupledtoliquidchromatography—tandemmassspectrometry,Talanta 132(2015)77.
[47] M.Gorga,M.Petrovic,D.Barcelo ´ ,Multi-residueanalyticalmethodforthedeterminationofendocrinedisruptorsandrelatedcompoundsinriverandwastewaterusingdualcolumnliquidchromatographyswitchingsystemcoupledtomassspectrometry,J.Chromatogr.A1295(2013)57–66.
[48] F.Wode,C.Reilich,P.vanBaar,U.Dunnbier,M.Jekel, T. Reemtsma,Multiresidueanalytical methodforthesimultaneousdeterminationof72micropollutantsinaqueoussampleswithultrahigh performanceliquidchromatography–highresolutionmassspectrometry,J.Chromatogr.A1270(2012) 118.
[49] M.Gorga,S.Insa,M.Petrovic,D.Barcelo,Analysisofendocrinedisruptersandrelatedcompoundsin sedimentsandsewagesludgeusingon-lineturbulentflowchromatographyliquidchromatographytandemmassspectrometry,J.Chromatogr.A1352(2014)29–37.
[50] V.Scognamiglio,A.Antonacci,L.Patrolecco,M.D.Lambreva,S.C.Litescu, V.Ghuge,G.Rea,Analyticaltoolsmonitoringendocrinedisruptingchemicals,TrendsAnal.Chem. 80(2016)555–567.
[51] B.Chocarro-Ruiz,A.Ferna´ndez-Gavela,S.Herranz,L.M.Lechuga,Nanophotoniclabel-freebiosensorsforenvironmentalmonitoring,Curr.Opin.Biotechnol.45(2017)175–183.
[52] G.R.Marchesini,E.Meulenberg,W.Haasnoot,H.Irth,Biosensorimmunoassaysforthedetectionof bisphenolA,Anal.Chim.Acta528(1)(2005)37–45.
[53] G.R.Marchesini,K.Koopal,E.Meulenberg,W.Haasnoot,H.Irth,Spreeta-basedbiosensorassaysfor endocrinedisruptors,Biosens.Bioelectron.22(9–10)(2007)1908–1915.
[54] S.Rodriguez-Monaz,M.Lope´zdeAlda,D.Barcelo,AnalysisofbisphenolAinnaturalwatersby meansofanopticalimmunosensor,WaterRes.39(20)(2005)5071–5079.
[55] A.Kim,C.R.Li,C.-F.Jin,K.W.Lee,S.-H.Lee,K.-J.Shon,N.G.Park,D.-K.Kim,S.-W.Kang,Y.B.Shim,J.S.Park,AsensitiveandreliablequantificationmethodforbisphenolAbasedonmodified competitiveELISAmethod,Chemosphere68(7)(2007)1204–1209.
[56] Z.Zhang,K.Zeng,J.Liu,Immunochemicaldetectionofemergingorganiccontaminantsinenvironmentalwaters,trendsinanalyticalchemistry,TrACTrendsAnalyt.Chem.87(2017)49–57.
[57] A.N.Bezbaruah,H.Kalita,Sensorsandbiosensorsforendocrinedisruptingchemicals:state-of-the-art andfuturetrends,in:TreatmentofMicropollutantsinWaterandWastewater,IWAPublishing, London,2010.
[58] N.Bhalla,P.Jolly,N.Formisano,P.Estrela,Introductiontobiosensors,EssaysBiochem.60(2016)1–8.
[59] S.Jarque,M.Bittner,L.Blaha,K.Hilscherova,Yeastbiosensorsfordetectionofenvironmentalpollutants:currentstateandlimitations,TrendsBiotechnol.34(5)(2016)408–419.
[60] C.I.L.Justino,A.C.Duarte,T.A.P.Rocha-Santos,Recentprogressinbiosensorsforenvironmental monitoring:areview,Sensors17(12)(2017)2918.
[61] W.Zhang,Q.X.Liu,Z.H.Guo,J.S.Lin,Practicalapplicationofaptamer-basedbiosensorsindetection oflowmolecularweightpollutantsinwatersources,Molecules23(2)(2018)344.
[62] S.Amaya-Gonza ´ lez,N.de-los-Santos-A ´ lvarez,A.J.Miranda-Ordieres,M.J.Lobo-Castano ´ n, Aptamer-basedanalysis:apromisingalternativeforfoodsafetycontrol,Sensors13(12)(2013) 16292–16311.
[63] A.Hayat,J.L.Marty,Aptamerbasedelectrochemicalsensorsforemergingenvironmentalpollutants, Front.Chem.2(2014)41.PMC.Web.11September2018.
[64] C.H.Wang,S.T.Kang,Y.H.Lee,Y.L.Luo,Y.F.Huang,C.K.Yeh,Aptamer-conjugatedanddrugloadedacousticdropletsforultrasoundtheranosis,Biomaterials33(6)(2012)1939–1947.
[65] H.B.Yildiz,J.Castillo,D.A.Guschin,L.Toppare,W.Schuhmann,Phenolbiosensorbasedonelectrochemicallycontrolledintegrationoftyrosinaseinaredoxpolymer,Microchim.Acta159(2007) 27–34.
[66] F.Karim,A.N.M.Fakhruddin,Recentadvancesinthedevelopmentofbiosensorforphenol:areview, Rev.Environ.Sci.Biotechnol.11(2012)261–274.
[67] Y.Mie,M.Suzuki,Y.Komatsu,Electrochemicallydrivendrugmetabolismbymembranescontaining humancytochromeP450,J.Am.Chem.Soc.131(2009)6646–6647.
[68] V.K.Nigam,P.Shukla,Enzymebasedbiosensorsfordetectionofenvironmentalpollutants:areview, J.Microbiol.Biotechnol.25(11)(2015)1773–1781.
[69] G.A.Mostafa,Electrochemicalbiosensorsforthedetectionofpesticides,OpenElectrochem.J. 2(2010)22–42.
[70] A.Sassolas,L.J.Blum,B.D.Leca-Bouvier,Immobilizatonstrategiestodevelopenzymatcbiosensors, Biotechnol.Adv.30(2012)489–511.
[71] V.G. Andreou,Y.D.Clonis, A portablefiber-opticpesticidebiosensorbasedonimmobilizedcholinesteraseandsol-gelentrappedbromocresolpurpleforin-fielduse,Biosens.Bioelectron.17(2002)61–69.
[72] R.T.Andres,R.Narayanaswamy,Fibre-opticpesticidebiosensorbasedoncovalentlyimmobilized acetylcholinesteraseandthymolblue,Talanta44(1997)1335–1352.
[73] J.Sanseverino,R.K.Gupta,A.C.Layton,S.S.Patterson,S.A.Ripp,L.Saidak,M.L.Simpson, T.W.Schultz,G.S.Sayler,UseofSaccharomycescerevisiaeBLYESexpressingbacterialbioluminescenceforrapid,sensitivedetectionofestrogeniccompounds,Appl.Environ.Microbiol.71(2005) 4455–4460.
[74] T.F.H.Bovee,R.J.R.Helsdingen,A.R.M.Hamers,B.A.Brouwer,M.W.F.Nielen,Recombinant cellbioassaysforthedetectionof(gluco)corticosteroidsandendocrine-disruptingpotenciesofseveral environmentalPCBcontaminants,Anal.Bioanal.Chem.401(2011)873–882.
[75] J.Rajasarkka,M.Virta,CharacterizationofabisphenolAspecificyeastbioreporterutilizingthebisphenolA-targetedreceptor,Anal.Chem.85(21)(2013)10067–10074.
[76] Y.Nakamura,J.Ishii,A.Kondo,Applicationsofyeast-basedsignalingsensorforcharacterizationof antagonistandanalysisofsite-directedmutantsofthehumanserotonin1Areceptor,Biotechnol. Bioeng.112(2015)1906–1915.
[77] H.Matsuura,Y.Yamamoto,M.Muraoka,K.Akaishi,Y.Hori,K.Uemura,N.Tsuji, K.Harada,K.Hirata,T.Bamba,H.Miyasaka,K.Kuroda,M.Ueda,Developmentofsurfaceengineeredyeastcellsdisplayingphytochelatinsynthaseandtheirapplicationtocadmiumbiosensors bythecombineduseofpyrene-excimerfluorescence,Biotechnol.Prog.29(2013)1197–1202.
[78] I.Vopa ´ lenska ´ ,L.Va ´ chova ´ ,Z.Palkova ´ ,Newbiosensorfordetectionofcopperionsinwaterbasedon immobilizedgeneticallymodifiedyeastcells,Biosens.Bioelectron.72(2015)160–167.
[79] S.Jarque,M.Bittner,K.Hilscherova ´ ,Freeze-dryingassuitablemethodtoachieveready-to-useyeast biosensorsforandrogenicandestrogeniccompounds,Chemosphere148(2016)204–210.
[80] A.A.Weaver,S.Halweg,M.Joyce,M.Lieberman,H.V.Goodson,Incorporatingyeastbiosensorsinto paper-basedanalyticaltoolsforpharmaceuticalanalysis,Anal.Bioanal.Chem.407(2015)615–619.
[81] N.Verma,A.Bhardwaj,Biosensortechnologyforpesticides:areview,Appl.Biochem.Biotechnol. 175(2015)3093–3119.
[82] E.Mauriz,A.Calle,L.M.Lechuga,J.Quintana,A.Montoya,J.J.Manclus,Real-timedetectionof chlorpyrifosatpartpertrillionlevelsinground,surfaceanddrinkingwatersamplesbyaportablesurface plasmonresonanceimmunosensor,Anal.Chim.Acta561(1–2)(2006)40–47.
[83] B.Mattiasson,G.Erturk,Whyusingmolecularlyimprintedpolymersinconnectiontobiosensors? Sensors(Basel)17(2)(2017)246.
[84] L.Uzun,A.P.Turner,Molecularly-imprintedpolymersensors:realisingtheirpotential,Biosens.Bioelectron.76(2016)131–144.
[85] Z.O.Uygun,H.D.E.Uygun,N.Ermiş,E.Canbay,Molecularlyimprintedsensors:newsensingtechnologies,in:T.Rinken(Ed.),Biosensors:MicroandNanoscaleApplications,InTechOpen,London, UK,2015.
[86] V.Pichon,F.Chapuis-Hugon,Roleofmolecularlyimprintedpolymersforselectivedeterminationof environmentalpollutants:areview,Anal.Chim.Acta622(1–2)(2008)48–61.
[87] G.Mustafa,P.A.Lieberzeit,MIPsensorsonthewaytoreal-worldapplications,in:S.A.Piletsky, M.J.Whitcombe(Eds.),DesigningReceptorsfortheNextGenerationofBiosensors,Springer, Berlin,Heidelberg,2012.
[88] G.Ert € urk,D.Berillo,M.Hedstr € om,B.Mattiasson,Microcontact-BSAimprintedcapacitivebiosensor forreal-time,sensitiveandselectivedetectionofBSA,Biotechnol.Rep.3(2014)65–72.
[89] G.Ert€ urk,M.Hedstr € om,M.A.T € umer,A.Denizli,B.Mattiasson,Real-timeprostate-specificantigen detectionwithprostate-specificantigenimprintedcapacitivebiosensors,Anal. Chim.Acta891(2015)120–129.
[90] A.Poma,A.Guerreiro,M.J.Whitcombe,E.V.Piletska,A.P.Turner,S.A.Piletsky,Solid-phasesynthesisofmolecularlyimprintedpolymernanoparticleswithareusabletemplate“plasticantibodies” Adv.Funct.Mater.23(2013)2821–2827.
[91] G.Selvolini,G.Marrazza,MIP-basedsensors:promisingnewtoolsforcancerbiomarkerdetermination,Sensors17(4)(2017)718.
[92] L.M.Kindschy,E.C.Alocilja,Areviewofmolecularlyimprintedpolymersforbiosensordevelopment forfoodandagriculturalapplications,T.ASAE47(4)(2004)1375.
[93] G.Maduraiveeran,W.Jin,Nanomaterialsbasedelectrochemicalsensorandbiosensorplatformsfor environmentalapplications,TrendsEnviron.Analyt.Chem.13(2017)10–23.
[94] W.Xia,Y.Y.Li,Y.J.Wan,T.A.Chen,J.Wei,Y.Lin,S.Q.Xu,ElectrochemicalbiosensorforestrogenicsubstanceusinglipidbilayersmodifiedbyAunanoparticles,Biosens.Bioelectron.25(2010) 2253–2258.
[95] B.Wang,C.Ye,X.Zhong,Y.Chai,S.Chen,R.Yuan,Electrochemicalbiosensorfororganophosphatepesticidesandhuperzine—adetectionbasedonPdwormlikenanochains/graphiticcarbon nitridenanocompositesandacetylcholinesterase,Electroanalysis8(2016)304–311.
[96] A.Hassaan,S.Yaneva,L.Yotova,Designofopticalnanobiosensorsfordetectionoftoxiccompounds andpharmaceuticalproducts,in:Nano-optics:PrinciplesEnablingBasicResearchandApplications, Springer,Dordrecht,2017,pp.453–454.
RajMohanBalakrishnan
Intherecentyearsenvironmentalsecurityhasbeenthreatenedbyawiderangeofenvironmentalpollutantsduetovariousindustrial,agricultural,land,andotheranthropogenicactivities.Thereforeamajorconcernliesinmonitoring/detectingwater,soil, andairpollutants [1].Further,thedetectionofthesepollutantsessentiallyshouldbe inthelevelofassessingthesafetyoftheenvironment.RecentliteraturesreportthedetectionofpollutantsintherangeofmolL 1 tomgL 1 orevenlower [2,3].Althoughthe existinganalyticalmethodssuchashighperformanceliquidchromatography(HPLC), inductivelycoupledplasmaopticalemissionspectrometry(ICP-OES),atomicabsorptionspectrometry(AAS),inductivelycoupledplasmamassspectrometry(ICP-MS), andsoon,areaccurate,thereisaneedforsimple,portable,andrapidmethodsforpollutantdetection [4]
Biosensorsareanalyticalhybriddevicesthatcanprovidespecificqualitativeorsemiquantitativedatabyintegratingbiologicalorbiomimeticdetectionelement.Thedetectionelementisusuallybiologicalinnaturealongwithatransducer.Thetransducer convertsabiochemicalphenomenonintoanelectricalsignal [5,6].Thisapproach exploitsthemolecularbindingeventandalsobringsresearchersfromdifferentareas ofscienceandengineeringtogetheronacommonplatform.Pastdecadehasseentremendousriseindevelopmentofnoveltoolsforefficientenvironmentalmonitoring.Clark andLyonswerepioneersinbiosensorsdevelopmentresearch.Later,UpdikeandHicks developedthefirstenzyme-basedbiosensorintheyear1967,whereanoxygenelectrode hadpolyacrylamideimmobilizedwithenzymeglucoseoxidaseonitssurfacetofacilitate rapidandquantitativedeterminationofglucose [7–9].
2.Classificationofbiosensors
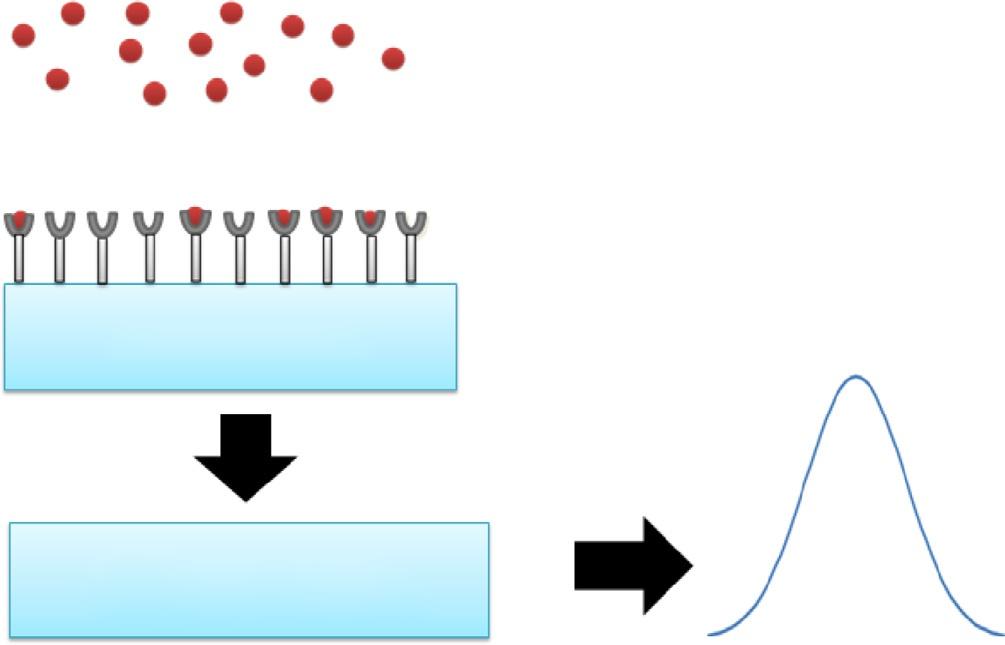
Atypicalbiosensorconsistsofabiologicalsensingcomponent/bioreceptorandaphysical transducer/messenger [10] (Fig.2.1).Thebioreceptoristhekeycomponentinthebiosensor,whichisresponsibleforthespecificity,responsetime,affinity,andlifetimeofthe biosensor.Thesebioreceptorsincludemicroorganisms,enzymes,antibodies,DNA,oligonucleotides,aswellasanimalandplantcellsortissuesthatbindtoaspecificanalyteina givenenvironment [11].Thebioreceptorisintegratedwithorimmobilizedonatransducersurface,whichrespondstothesensingactivityofthereceptor.Thetransducerelementsareworkingontheelectrochemicalandopticalprinciplesthattransforma chemical/physicaleventintoaquantifiablesignal.Theeventcouldbeapotentialdifference,current,conductivity,luminescence,fluorescence,pHchange,andsoon.
Fig.2.1 Schematicrepresentationoftypicalbiosensor.
Bioreceptor Transducer
Sample analyte