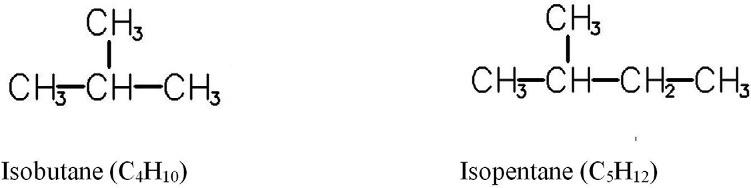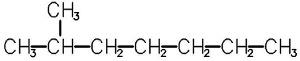ThermophysicalPropertiesofIndividual HydrocarbonsofPetroleumandNaturalGases: Properties,Methods,andLow-CarbonTechnologies BorisA.Grigoriev
https://ebookmass.com/product/thermophysical-properties-ofindividual-hydrocarbons-of-petroleum-and-natural-gasesproperties-methods-and-low-carbon-technologies-boris-a-
grigoriev/
Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
Natural Fiber-Reinforced Composites: Thermal Properties and Applications Senthilkumar Krishnasamy
https://ebookmass.com/product/natural-fiber-reinforced-compositesthermal-properties-and-applications-senthilkumar-krishnasamy/
ebookmass.com
Severe Plastic Deformation: Methods, Processing and Properties Ghader Faraji
https://ebookmass.com/product/severe-plastic-deformation-methodsprocessing-and-properties-ghader-faraji/
ebookmass.com
Calculations and Simulations of Low-Dimensional Materials : Tailoring Properties for Applications 1st Edition Ying Dai
https://ebookmass.com/product/calculations-and-simulations-of-lowdimensional-materials-tailoring-properties-for-applications-1stedition-ying-dai/ ebookmass.com
The Economy and Business Environment of Vietnam 1st ed. Edition Roderick
Macdonald
https://ebookmass.com/product/the-economy-and-business-environment-ofvietnam-1st-ed-edition-roderick-macdonald/
ebookmass.com
Bad Things: The Nature and Normative Role of Harm Neil Feit https://ebookmass.com/product/bad-things-the-nature-and-normativerole-of-harm-neil-feit/
ebookmass.com
Psychology Applied to Modern Life: Adjustment in the 21st Century 12th Edition – Ebook PDF Version
https://ebookmass.com/product/psychology-applied-to-modern-lifeadjustment-in-the-21st-century-12th-edition-ebook-pdf-version/
ebookmass.com
Intimate Empire: The Mansurov Family in Russia and the Orthodox East, 1855-1936 Alexa Von Winning
https://ebookmass.com/product/intimate-empire-the-mansurov-family-inrussia-and-the-orthodox-east-1855-1936-alexa-von-winning/
ebookmass.com
Wideband Microwave Materials Characterization John W. Schultz
https://ebookmass.com/product/wideband-microwave-materialscharacterization-john-w-schultz/ ebookmass.com
The Art of Assembly Language Programming using PIC Technology. Core Fundamentals Theresa Schousek
https://ebookmass.com/product/the-art-of-assembly-languageprogramming-using-pic-technology-core-fundamentals-theresa-schousek-2/
ebookmass.com
Ringworm and Irradiation: The Historical, Medical, and Legal Implications of the Forgotten Epidemic Shifra Shvarts
https://ebookmass.com/product/ringworm-and-irradiation-the-historicalmedical-and-legal-implications-of-the-forgotten-epidemic-shifrashvarts/
ebookmass.com
THERMOPHYSICALPROPERTIESOF INDIVIDUALHYDROCARBONSOF PETROLEUMANDNATURALGASES Properties,Methods,and Low-CarbonTechnologies BORIS A.GRIGORIEV DepartmentoftheStudyofOilandGasReservoirSystems,GubkinRussianStateUniversityofOilandGas, Moscow,Russia
ANATOLY A.GERASIMOV DepartmentofCivilEngineering,KaliningradStateTechnicalUniversity,Kaliningrad,Russia
IGOR S.ALEXANDROV InstituteofMarineTechnology,EnergyandConstruction,KaliningradStateTechnicalUniversity,Kaliningrad,Russia
BORIS V.NEMZER FutureCeuticals,Inc.;UniversityofIllinoisatUrbana-Champaign,Urbana,IL,UnitedStates
GulfProfessionalPublishingisanimprintofElsevier
50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
TheBoulevard,LangfordLane,Kidlington,Oxford,OX51GB,UnitedKingdom
Copyright©2022ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronicor mechanical,includingphotocopying,recording,oranyinformationstorageandretrievalsystem,without permissioninwritingfromthepublisher.Detailsonhowtoseekpermission,furtherinformationaboutthe Publisher’spermissionspoliciesandourarrangementswithorganizationssuchastheCopyrightClearance CenterandtheCopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions. ThisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythePublisher (otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperiencebroadenour understanding,changesinresearchmethods,professionalpractices,ormedicaltreatmentmaybecome necessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluatingandusing anyinformation,methods,compounds,orexperimentsdescribedherein.Inusingsuchinformationormethods theyshouldbemindfuloftheirownsafetyandthesafetyofothers,includingpartiesforwhomtheyhavea professionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assumeanyliability foranyinjuryand/ordamagetopersonsorpropertyasamatterofproductsliability,negligenceorotherwise,or fromanyuseoroperationofanymethods,products,instructions,orideascontainedinthematerialherein.
ISBN:978-0-323-95217-0
ForinformationonallGulfProfessionalpublications visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: CharlotteCockle
SeniorAcquisitionsEditor: KatieHammon
EditorialProjectManager: AliAfzal-Khan
ProductionProjectManager: PremKumarKaliamoorthi
CoverDesigner: GregHarris
TypesetbySTRAIVE,India
Abouttheauthors BorisA.Grigoriev,PhD,ScD,graduated fromtheGroznyStateOilTechnicalUniversityandisamemberoftheRussianAcademy ofSciences.Dr.Grigorieviscurrentlyaprofessoratthedepartmentofthestudyofoiland gasreservoirsystemsatGubkinRussianState UniversityofOilandGas.Hehasauthored morethan400scientificworks,includinga verypopulartextbookonheatandmasstransferand6booksonthermophysicalproperties ofhydrocarbons,oils,petroleumproducts, aqueoussolutions,ordinaryandheavywater, sulfurhexafluoride,andotherenergycarriers. Heisadistinguishedmemberofvarious nationalandinternationalsocietiesandassociationssuchasBureauoftheNationalCommitteeontheThermophysicalPropertiesof Substances,InternationalAssociationforthe PropertiesofWaterandSteam(IAPWS), NationalCommitteeonDataforScienceand Technology(CODATA).
AnatolyA.Gerasimov,PhD,ScD,graduated fromtheGroznyStateOilTechnicalUniversityandiscurrentlyaprofessorinthe departmentofheatandgassupplyandventilationatKaliningradStateTechnical University.Dr.Gerasimovhasauthored
morethan100scientificworksinthefieldof thermophysicalpropertiesofsubstances, including3monographs.
IgorS.Alexandrov,PhD,ScD,graduated fromKaliningradStateTechnicalUniversity andiscurrentlyDirectoroftheInstituteof MarineTechnology,EnergyandConstructionatKaliningradStateTechnicalUniversity.Dr.Alexandrovhasauthoredmore than100scientificworksinthefieldof thermophysicalpropertiesofsubstances.
BorisV.Nemzer,PhD,FRSC,isVicePresidentofR&DandDirectorofResearchand AnalyticalCenteratFutureCeuticals,Inc. (UnitedStates),andaprofessorattheUniversityofIllinoisatUrbana-Champaign (UnitedStates).Dr.Nemzerisanauthorof morethan180scientificpaperspublished inpeer-reviewedjournalsand3booksin theareasofthermophysicalpropertiesof substances,physicalandanalyticalchemistry,nutrition,andfoodsciences.Heisafellowanddistinguishedmemberofvarious nationalandinternationalscientificsocieties andassociates.
Foreword Oneofthemostimportantprioritiesinscientificandtechnologicalprogressinenergy productionistheincreaseintheefficiency ofrecovery,processing,andtransportation ofhydrocarbons.Theproblemsassociated withtheexplorationofcomplex,offshore, gas-hydratefieldsandtheextractionof low-pressuregasfromthefieldsthathave enteredthefinalstagesofoperationrequire thedevelopmentoffundamentalscientific knowledgetocreatenoveltechnologiesfor theproductionandprocessingofhydrocarbons.Therefore,knowledgeofthe thermophysicalpropertiesoffluidsisessentialforthisdevelopment.
Thisreferencemonograph Thermophysical PropertiesofIndividualHydrocarbonsofPetroleumandNaturalandGases compiledbyBoris A.Grigoriev,acorrespondingmemberofthe RussianAcademyofSciences,andhiscoworkerssummarizestheresultsofdecadeslongexperimentalandtheoreticalstudies ofthermophysicalpropertiesofmaingroups ofhydrocarbons,commonlyexistinginoil andgascondensatesunderawiderangeof conditions(fromthetriple-pointtemperaturesto700Kandpressuresupto100MPa) intheliquidandvaporphases,including thecriticalregion.
Themonographpaysmajorattentionto thedescriptionofexperimentalmethods,estimationofmeasurementuncertainties,and reliabilityofexperimentaldata.Theauthors havedecidedtopublishalmostallthe
experimentaldataonthepropertiesofhydrocarbonsobtainedinthethermophysical laboratoryofGroznyStateOilTechnicalUniversity,aswellasthedetailedanalysisof thesedata.
Thisbookalsoexaminesmodernmethods fordevelopingfundamentalandlocalequationsofstateandmethodsfortheprediction ofthermophysicalproperties,whichare usedformodelingtheproduction, processing,andtransportationofhydrocarbons.Themainconceptandprinciplesof hydrogen-basedenergyandtheroleofhydrocarbonsintheprocessoftransitiontohydrogenenergyarediscussed.The significanceofthefundamentalequations ofstateofhydrocarbonsandassociated gasesinsolvingtheproblemsofglobal warmingisemphasized.
Webelievethatthemonographwillbeinterestingandusefultoabroadcommunityof engineersandresearchersworkingin thisfield.
JanV.Sengersa andMikhailA.Anisimovb
aDistinguishedUniversityProfessorEmeritus,Institute ofPhysicalScience&Technology,University ofMaryland,CollegePark,MD,UnitedStates bDistinguishedUniversityProfessor,Department ofChemicalandBiomolecularEngineering, andInstituteofPhysicalScience&Technology, UniversityofMaryland,CollegePark,MD, UnitedStates
DedicatedtothestaffoftheIndustrialThermophysical LaboratoryoftheGroznyStateOilTechnicalUniversity
Preface Oilandnaturalgasarethecoreelements oftheglobaleconomyandpolitics.Today, theproduction,refining,andtransportation ofoilandgasarebecomingobjectsofinternationaldisputesandconflicts.Theyareincreasinglybeingusedastoolstoinfluence countriesandregions.Justafewdecades ago,theeraofoilandgasseemedtobecomingtoanendwiththedepletionoffossilfuel reserves,aswellastheobviousprogressin thedevelopmentofalternativeenergy sources.However,theendofthe20thcenturyandthebeginningofthe21stcentury weremarkedbyanewturninthedevelopmentoftheoilandgasindustry.Large newdepositswerediscoveredintheshelf zonesoftheseasandoceans.Newtechnologiesforthedevelopmentandproductionof hydrocarbonsfromstructurallycomplex fields,suchasshaleoilandgas,havebeen created;oldfieldsoperateddozensandeven hundredsofyearsagohavebeenturnedon duetonewtechnologies,andintensivework isbeingdonetocreatetechnologiesforpilotcommercialproductionofnaturalgasfrom gashydratedeposits.
Atthesametime,duetoclimatechange, today,theissueofdiversifyingtheenergyindustryasoneofthemainanthropogenic “suppliers”ofgreenhousegasesisvery acute.Arationalsolutionmaybethedevelopmentofhydrogenenergy,iftechnological processesandcapacitiesfortheproduction ofhundredsofmillionsoftonsofhydrogen arecreated.Moreover,overthenext 20–30years,themostcompetitivewillbethe processesofhydrogenproductionfrom
naturalgas,oil,coal,biomass,andotherorganicsources.
Ahigh-qualityenergytransitionwillrequirethedevelopmentofefficientprocesses forproducinghydrogenwithalowcarbon footprint(differentlevels:gray,blue,green); capturing,transporting,storing,andefficientlydisposingofcarbondioxide;andreducingman-madeemissionsofnaturaland associatedpetroleumgases.Allthiswill requirefurtherimprovementanddevelopmentoftechnologiesfortheproduction andtransportofcarbonandhydrocarbon rawmaterials,whichareimpossiblewithout theknowledgeofthethermophysicalpropertiesofenergysources,intermediateand finalreactionproducts,reservoirfluids,oilandgas-saturatedreservoirs,etc.
Successesandprospectsforthedevelopmentoftheoilandgasindustryweremade possiblethankstonumerousstudiesofthe propertiesofoil,naturalgas,andtheircomponentsinresearchlaboratoriesindifferent countriesandthesupportofthesestudiesby governmentsandbusinesses.Thekeypoint ofthesestudiesisthestudyofthephysicochemicalandthermophysicalpropertiesof themostimportanthydrocarbonsofoiland naturalgas:alkanes,cycloalkanes,andarenes, aswellasgasesassociatedwiththeextraction ofoilandnaturalgas,suchashydrogen, helium,nitrogen,carbondioxide,hydrogen sulfide,watervapor,andothers.Thedevelopmentofnewtechnologiesforinfluencingthe reservoirandthebottom-holezonetoincrease oilandcondensaterecovery,improving methodsformodelingreservoirsystems,
supercriticaltechnologiesforoilproduction andprocessingrequireknowledgeofthe thermophysicalpropertiesofoil,naturalgas, andtheircomponentsinawiderangeoftemperaturesandpressures.
Systematicstudiesofthephysicochemical propertiesofoilhydrocarbonswerecarried outinthefirsthalfofthe20thcenturyatthe AmericanPetroleumInstitute(API)by F.Rossiniandotheremployees.Thestudyof caloricpropertiesatlowtemperaturesinresearchprojectsNo.6andNo.44was conductedundertheauspicesoftheUSNationalBureauofStandards(NBS).Thework oftheThermodynamicResearchCenterof theTexasA&MUniversitySystemontheformationofadatabankofthethermophysical propertiesofhydrocarbonsandothertechnicallyimportantsubstanceswasnoteworthy. Concurrently,activestudiesofhydrocarbon propertieswerecarriedoutatanumberofuniversitiesinGermany,France,andEngland.
Asignificantcontributiontothestudyof thethermophysicalpropertiesofoilhydrocarbonswasmadebyscientistsfromanumberof researchcentersintheUSSR,suchastheState ServiceofStandardReferenceData(SSSRD), theGroznyStateOilTechnicalUniversity (GSOTU),theAzerbaijanOilandGasInstitute, theMoscowStateUniversity,MoscowPower EngineeringInstitute,OdessaInstituteof MarineFleetEngineers,DagestanScientific CenteroftheRussianAcademyofSciences (RAS),theInstituteofHighTemperaturesof theRAS,InstitutesofThermophysicsofthe UralandSiberianBranchesoftheRAS.
Atthebeginningofthe1960s,large-scale comprehensivestudiesofthethermophysical propertiesofoils,oilproducts,gascondensatesandtheirfractions,aswellasthemain groupsofoilhydrocarbons,werestartedin theIndustrialThermophysicalLaboratory (ITPL)atGSOTU.
Despitethelargenumberofpublications onthepropertiesofoilhydrocarbons,only
forsomeofthemtherewasasetofdataon thermodynamicpropertiesandtransfer coefficients,coveringliquidandgaseous states,phasetransitionlines,andcritical andsupercriticalregionsoverwideranges oftemperaturesandpressures.These substancesincludelighthydrocarbon gases(methane,ethane,propane,andbutane)aswellas n-hexane,cyclohexane, andtoluene.Thesedataarefragmentary formosthydrocarbons,andinformationon themisscatteredovernumerousliterary sources.Therefore,numerousconsumersof thermophysicalinformation—scientificengineersinuniversities,researchanddesign organizations—hadtousetheinformation thatwasnotsufficientlyreliableinsome cases,whichtheyfoundintheliterature, whenconductingthermodynamicand heat-masstransfercalculations.Thesituation wasconstantlycomplicatedbecausemoreand morenewsubstancesandtechnologiesand multicomponentmixtures,forwhichknowledgeofpropertieswasrequired,beganto appear.Inthisregard,new,expensiveexperimentalstudieswerecarriedoutandforecastingcalculationmethodswerecreated.But anincreaseinthevolumeofinformationand itssourcesdoesnotmeananincreaseinits reliability.Atthesametime,inmodern publications(monographs,referencebooks, studyguides)onthedevelopmentofoiland gascondensatefieldsandtransportation andprocessingofhydrocarbons,thesections devotedtothecalculationofthermophysical propertiesarebased,withrareexceptions, onnonsystematicinformation,thereliability ofwhichrequiresadditionalevaluation. Thesameclaimscanbemadeforthe softwareproductsthatwerewidelyusedfor calculatingthethermophysicalpropertiesof hydrocarbons.Thisresultsintheneedfora criticalanalysisofexistingexperimentaldata andmethodsforcalculatingthethermophysicalpropertiesofoilhydrocarbons.Such
ananalysishasbeencarriedoutinthelast 10–15yearsbyseveralinternationalgroups ofscientists:intheUnitedStates(NIST)by Lehmann,Elietal.;inGermany(RuhrUniversity)byW.Wagner;inEngland(Imperial College)byW.Weyhametal.;andinAustralia (UniversityofWesternAustralia)byEricMay, KenMarshetal.InRussia,suchworkswere carriedoutattheKaliningradStateTechnical University,All-RussianInstituteofNatural GasesandGasTechnologies(VNIIGAZ), SSSRD,InstituteofThermophysicsofthe SiberianBranchoftheRAS.Studygroups activelyinteractwitheachotherandexchange informationonbothexperimentaldataand studiesandmethodsforconstructingcalculationequations.
Allavailableexperimentaldataand methodsforcalculatingthethermodynamic propertiesandtransfercoefficientsofconsideredinthisbookhydrocarbonsareanalyzedinthisreferencemonographoffered tothereader.Thereweredifficultieswith thedesignofthebibliographicreferences duetothelargenumberofpublications.It wasconsideredappropriatetolimitreferencestopublicationswithreliableevaluated data.Inaddition,incaseswherewehadthe opportunitytoworkwiththesesandmonographs,referencesweremadetothem,rather thantotheoriginalarticles.Asaresult,the numberofreferenceshasbeenreduced significantly.
Informationonthemaingroupsofhydrocarbonsofoilandgascondensatesandtheirphysicochemicalpropertiesarepresentedinbriefin thefirstchapter.Thereaderisreferredtothe monographsbyF.D.Rossini,A.A.Petrova, andothersforadditionalinformation. Itshouldbenotedourworkinthismonographdifferssignificantlyfromthatofour foreigncolleaguesworkingontheequations ofstate.Ouranalysisanddevelopmentof theequationsofstateandinterpolation dependencesfor n-alkanes,cyclohexane,
andaromatichydrocarbonsarebasedon long-term,comprehensive,wide-rangeexperimentalstudiescarriedoutinITPLatGSOTU (ourownexperimentaldata).Studiesonhydrocarbonpropertiesbydomesticscientists andscientistsfromtheCommonwealthofIndependentStates(CIS)countrieswereusedto themaximumextentintheanalysis.Manyof theseworksareeitherinaccessibletoWestern authorsor,forsomereason,theyhavebeen ignored.Atthesametime,wehavefullyused bothexperimentaldataandmethodological developmentsofourWesterncolleaguesin theanalysis.
Acorrect,objectiveanalysisoftheexperimentaldatainvolvedinthedevelopmentof calculationequationsisimpossiblewithout assessingthereliabilityandaccuracyofthe experimentalmethodsandexperimentalconditionsandconsideringvariouscorrectionsrelatedtodisturbingfactors,thecorrectuseof theappliedmeasurementequipmentand instruments,thepurityofthehydrocarbons understudyandtheirthermalstabilityin thestudiedrangeofstateparameters,etc. Wepaidattentiontoallthesefeatureswhen analyzingandprocessingdatafromstudies byvariousauthors.However,itisnotpossible todescribethemanymethodsandfacilities basedonwhichtheexperimentalmaterial usedinthisworkwasobtained.Atthesame time,weconsideritnecessaryandappropriate toincorporateinthebookdescriptionsoftest facilitiesandexperimentalmethodsformeasuringthermophysicalproperties—density, isobaricheatcapacity,surfacetension,viscosity,andthermalconductivity—developedor improvedinITPLatGSOTU,aswellasplants wherethestudyofisochoricheatcapacity (DagestanScientificCenteroftheRAS)and soundvelocity(KurskStatePedagogical University)werecarriedout.
Inouropinion,theinclusionofthisinformationthesecondchapterisjustifiedbythe factthatthereaderanduserofthisreference
monographwillgetanideaofthecomplexityofathermophysicalexperimentandthe valueofareliableexperimentalsetup,as wellasthevalueofforecastcalculation methodsbasedonreliableexperimentaldata andfundamentaltheory.
Theexpediencycanbeattributedtothe factthatforathermophysicalexperimenta largenumberofdevicesandplantsarecurrentlydevelopedandproducedbyindustry. Theplantsareusuallyautomated,equipped withmicroprocessors,andhaveunifiedmeasuringinstruments,datatransmission,and processingchannels.Thisallowsincreasing theproductivityandreducingthecomplexityofthethermophysicalexperiment.But thereisalsotheflipsideofthecoin—the uniquenessoftestfacilitiesislost,themotivationtodevelopnewresearchmethodsis reduced,andtheexperimentator,whohas obtainedaready-madedeviceinhishands, oftendoesnotknowthemethodologicalsubtleties,anditisdifficultforhimtoassessthe presenceandinfluenceofdisturbingfactors onthestudysubjectandonthemeasurement result.
Therefore,thebookgivesadescriptionof 17originaltestfacilitiesthathavebeensuccessfullyusedtoexperimentallystudythe thermophysicalpropertiesofhydrocarbons andpetroleumproductsfor25yearsupto theknowneventsinGrozny.Theresearch method,itsinherentamendments,thetestfacilitydesign,theexperimentaltechnique, andtheassessmentoftheaccuracyofmeasurementresultsaredescribed.Muchattentionispaidtotheissuesofthermostating, pressurecreationandmeasurement,andaccuracyoftemperaturechanges.Afairlywide rangeofthepropertiesunderstudy,suchas meltingline;saturatedvaporpressure; p-, V-, T-dependence; Cp-, p-, T-dependence; Сv-, ρ-, T-dependence;soundvelocity;surfacetension;thermalconductivity;andviscosity, arecovered.
Thethirdchapteranalyzesthestateofthe researchonthethermodynamicpropertiesof oilhydrocarbonsinthephaseequilibrium lines:crystal-gas,crystal-liquid,andliquidgas.Usinglocalandfundamentalequations ofstate,adatabaseofhydrocarbonpropertiesinthephaseequilibriumlineswas formed;thetemperaturesandenthalpiesof phasetransitionsatthereferencepointswere calculated;andtheindividualandgeneralizedequationsforsaturationpressure,the correspondingdensitiesandheatcapacities oftheliquidandgasphases,aswellasthe surfacetensionwereproposed.
Chapter5devotedtothedevelopmentof multiparameterfundamentalequationsof statewillhaveanindependentinterestfor thereader.Inwritingthischapter,wewere guidedbytheworkofSpan,whichwillbe veryinterestingandusefulforspecialists (SpanR.MultiparameterEquationsofState, 2000),especiallybecausethiswork,in whichmoderneffectivemethodsfor constructingthefundamentalequationsof stateareconsidered,isnotaccessibletoa widecircleofspecialists.Inadditiontothe methodsdescribedinthisspecifiedpublicationandpartiallydescribedbyus,other possiblymoreeffectivemethods,which wehaveexperienceinusing,arealso considered.
Todescribethethermodynamicpropertiesofoilhydrocarbonsintheliquidand gasphases,includingthecriticalstateregion intheparameterrangefromthetriplepoint to700Katpressuresofupto100MPa,we usedmultiparameterfundamentalequations ofstateintheformofthereducedHelmholtz freeenergy.
Asalreadynoted,severalstudygroups areworkingonthedevelopmentofthese equationsfortechnicallyimportantsubstances.Equationsofstatehavealreadybeen obtainedforanumberofsubstances.Based onananalysisofpreviouslypublished
equationsofstate,wedecidedtotakeadvantageoftheachievementsofWagner, Lemmonetal.andincludetheequationsof statedevelopedforlighthydrocarbons (gasessuchasCH4,C2H6,C3H8, n-C4H10, isoC4H10)andcyclicliquidhydrocarbons (namelycyclopentane,methylcyclohexane, propylcyclohexane,benzene,andtoluene) inthebook.Wedevelopedourown multiparameterfundamentalequationsof stateforliquid n-alkanesfrom С5Н12 to С13Н28; o-, m-,and p-xylenes;ethylbenzene; andcyclohexaneaswellasgeneralizedequationsofstatefor n-alkanes(from С5Н12 to С50Н102)andcyclichydrocarbons.Itshould benotedthatalmostallpublicationsonthe thermodynamicpropertiesofoilhydrocarbonswereusedduringthedevelopment andverificationoftheseequations.PublicationsbyresearchersfromRussiaandthe countriesoftheformerUSSRwillbeofinteresttoWesternresearchers.
Thethermodynamicpropertiesofhydrocarbonsinthecriticalregionaredescribed bythecrossoverequationsofstate.Thecoefficientsofthecrossoverequationfor Н2О and СО2 (J.Sengersandhisgroup), СН4 and С2Н6 (A.Abbasi),alkanesfrom n-С5Н12 to n-С8Н18,cyclohexane,benzene,andtoluene, aswellasforthegeneralizedcrossover equationofstateareobtained.
Considerableattentionispaidtotheanalysisandselectionofexperimentalandcalculateddataonthetransfercoefficientsofoil hydrocarbons.Chapters8and10consider theoretical,semiempirical,andempirical methodsforcalculatingviscosityandthermalconductivity,respectively.Thebasisof thedatabasetoobtainindividualandgeneralizedinterpolationequationsforcalculating transportcoefficientswasformedbyinhouseresearch,aswellasworkperformed inBaku,atAzizbekovAzineftekhim;in
Dagestan,attheInstituteofPhysicsofthe DagestanScientificCenteroftheRAS;at theInstituteofHighTemperaturesofthe RAS;andatSSSRD.Wehaveproposedindividualandgeneralizedinterpolationequationsforthermalconductivityandviscosity ofthemostimportantoilhydrocarbons.
Inconclusion,wenotethatinthisreference monographofferedtothereader,theinformationontheestimatedaccuracyofthethermodynamicandtransportpropertiesofthe mostimportantoilhydrocarbons[n-alkanes from СН4 to С13Н18;isoalkanes(isobutane, isopentane,neopentane,isohexane);cyclanes (cyclopentane,cyclohexane,methylcyclohexane,propylcyclohexane,benzene,toluene,ethylbenzene,and o-, m-, p-xylenes)]as wellasgasesassociatedwithoilandnatural gasextraction(hydrogen,helium,carbon dioxide,hydrogensulfide,nitrogen,water vapor)isconcentratedintheformof multiparameterfundamentalequationsof stateandinterpolationequations.Thevast majorityoftheseequationsandexperimental datausedtocreatethemwereobtainedby theauthorsofthisbookorwiththeirparticipation.Thematerialconcentratedinthismonographcanbeusedforsoftwaretosimulate reservoiroilandgassystemsandheatand masstransferprocessesinhydrocarbon processing,transportation,andusetechnologies,whiledevelopingforecastingmethods forcalculatingthepropertiesofother homologousseries,aswellashydrocarbon derivatives.
WearegratefultoProfessorJ.Sengersand ProfessorM.AnisimovfromtheUniversity ofMaryland,UnitedStates,aswellasDoctor E.W.LemmonfromtheUSNationalInstituteofStandardsandTechnologyandProfessorW.WagnerfromtheRuhrUniversity ofGermany,fortheircontinuedattention andsupportofourresearch.
Contents Abouttheauthorsvii
Forewordix
Prefacexi
1.Hydrocarbonsofpetroleumand naturalgases
1.1Themaingroupsofpetroleumandnaturalgas hydrocarbons1
1.2Basicphysicalandchemicalpropertiesof hydrocarbons5 References12
2.Experimentalmethods,apparatuses andresultsofexperimental measurementsofthethermodynamic properties
2.1Experimentaluncertainties15
2.2Thermalproperties22
2.3Isobaricheatcapacity124
2.4Isochoricheatcapacity188
2.5Speedofsound203
2.6Surfacetension213
2.7Conclusionsandrecommendations232 References233
3.Thermodynamicpropertiesonthephase equilibriumlines
3.1Sublimationpointline251
3.2Meltingpointline258
3.3Thermalpropertiesonthesaturationline liquidgas261
3.4Surfacetension303
3.5Caloricpropertiesontheliquid-gassaturation curve305
3.6Conclusionsandrecommendations310 References310
4.Thermodynamicfunctionsof hydrocarbonsintheidealgasstate
4.1Methodsfordeterminingthethermodynamic propertiesintheidealgasstate323
4.2Empiricalcorrelationsforcalculatingtheidealgas functions326
4.3Predictivemethodsforcalculatingidealgas functionsofhydrocarbons333 References333
5.Fundamentalequationsofstate ofindividualsubstances
5.1Overviewoffundamentalequationsof state335
5.2Methodsofconstructingfundamental equationsofstatebasedonexperimentaldata ofvarioustypes355
5.3Fundamentalequationsofstateatthecritical point385
5.4Conclusionsandrecommendations390 References391
6.Modernfundamentalequationsofstate forthemostimportanthydrocarbonsofoil, gascondensates,andassociatedgases
6.1Overviewofthepublishedequationsof state397
6.2Criticalregion502
6.3Conclusionsandrecommendations519 References520
7.Experimentalapparatusandresults ofmeasuringtheviscosityof hydrocarbons
7.1Apparatusformeasuringtheviscosityof hydrocarbonsattheatmosphericpressure541
7.2Experimentalsetupformeasuringtheviscosity ofliquidhydrocarbonsatpressuresupto 60MPa544
7.3Experimentalsetupformeasuringtheviscosityof hydrocarbonsintheliquidandgasphases561
7.4Apparatusformeasuringthedynamicviscosity ofliquidsandgasesbythecapillarymethodata constantpressuredrop568
7.5Theresultsoftheexperimentalmeasurement ofthehydrocarbonviscosities589
7.6Conclusionsandrecommendations602 References603
8.Methodsforcalculatingtheviscosity ofhydrocarbons
8.1Dilutegasviscosity607
8.2Methodsforcalculatingviscositiesinawiderange ofstateparameters619
8.3Viscosityinthecriticalregion641
8.4Correlationequationsofviscositiesofoilsand naturalgaseshydrocarbons644
8.5Conclusionsandrecommendations677 References678
9.Experimentalapparatusandresults ofmeasuringthethermalconductivity ofhydrocarbons
9.1Stationaryhotwiremethod688
9.2Stationarymethodofcoaxialcylinders720
9.3Themethodofcoaxialcylindersinthemode ofmonotonousheating743
9.4Resultsoftheexperimentalstudyofthethermal conductivityofhydrocarbons757
9.5Conclusionsandrecommendations813 References814
10.Methodsforcalculatingthethermal conductivityofhydrocarbons
10.1Dilutegasthermalconductivityof substances825
10.2Methodsforcalculatingthermalconductivities inawiderangeofstateparameters833
10.3Thermalconductivityinthecritical region841
10.4Correlationequationsofthermalconductivities ofhydrocarbons847
10.5Conclusionsandrecommendations875 References875
11.Thermophysicalaspectsoflow-carbon technologiesintheenergyandoil andgasindustry
11.1Generaldataaboutglobalclimatechange881 11.2Hydrogenenergy889
11.3Storageanddisposalofcarbondioxide894
11.4Conclusions897 References898
AppendixA901 AppendixB943 AppendixC969 AppendixD973 AppendixE997 Index1051
1 Hydrocarbonsofpetroleum andnaturalgases 1.1Themaingroupsofpetroleumandnaturalgashydrocarbons
Petroleumandnaturalgasesarecomplexmixturesofhydrocarbonsandnonhydrocarbon components—nitrogen,hydrogen,oxygen,sulfur,hydrogensulfide,etc.Withoutgoinginto details,wenotethatthedifferencebetweenapetroleumreservoirandanaturalgasreservoir isonlyinthephasebehaviorwhenthetemperatureandpressureoftheformationchange.The hydrocarboncompositionofpetroleumandnaturalgasesisdiverse.Hundredsofhydrocarbonsofvariousstructuresrepresentingalmostallhomologousseries,withtheexceptionof alkenes,whicharenotcontainedinpetroleumandnaturalgases,werefoundinthem.F.D. Rossinietal.havebeensystematicallystudyingthephysicochemicalandsomethermodynamicpropertiesofpetroleumhydrocarbonsforseveralyears.SufficientlydetailedinformationonthesestudiesiscontainedinRef. [1].
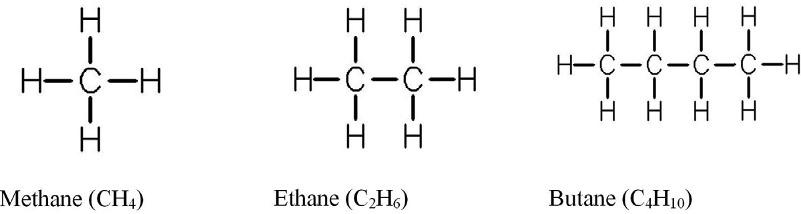
1.1.1Alkanes Alkanesaresaturatedhydrocarbonswithanopenhydrocarbonchain.Innaturalhydrocarbonmixtures,theyarecontainedeitherintheformofnormalstructurealiphatic hydrocarbons—n-alkanes:CH3–⋯ (–CH2–)n-2 ⋯–CH3,orintheformofisomers—isoalkanes. ThealkaneformulahastheformCnH2n+2,where n isthenumberofcarbonatomsinthemolecule.Thefirstrepresentativeofthishomologousrangeismethane(CH4).Asanexample,the structureofthree n-alkanes,methane,ethane,andbutane,isshownin Fig.1.1.TheC C C bondvalenceangleis112°42’andthedistancebetweencarbonatomsis1.53A ˚ innormal alkanes,judgingbymeasurementsbasedonelectrondiffraction.Detailedinformationon thestructureandchemicalpropertiesofalkanesispresentedinRefs. [2,3]
Forsimplicityofillustration,structuresthatareusuallyrepresentedbytheCH2 and CH3 groupsarenotwrittenbutshowonlycarbonbonds.Forexample,thestructurefor n-heptadecaneisshownin Fig.1.2
Thefirstrepresentativeofisoalkanesisisobutane.Someexamplesofisomersareshownin Figs.1.3and1.4.
FIG.1.1 Structureofsomenormalalkanes.
FIG.1.2 Structureof n-heptadecane(C17H36).
FIG.1.3 Structureofisobutaneandisopentane(methylbutane).
FIG.1.4 Structureofisooctane(2-methylheptane).
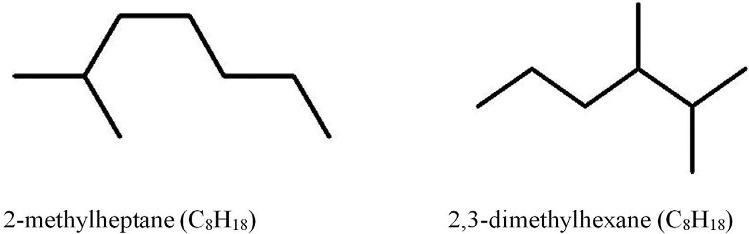
Inthecaseofisooctane,iftheCH3 groupisattachedtoanothercarbonatom,thenwewill getanothersubstance—3-methylheptane.Thereareotheroptionstoattachamethylgroup, forexample,2,3-dimethylhexaneand2-methylheptaneshowninasimplifiedformin Fig.1.5.
Inthenameofthesubstances,thenumbersindicatethecarbonatomtowhichamethyl groupisattached.Accordingly,therearetwoisomersforbutane,3ones—forpentane,5 ones—forhexane,9ones—forheptane,18ones—foroctane,35ones—fornonane,etc.The numberofisomersincreasessignificantlywiththenumberofcarbonatomsinthemolecule. ThenumberofisomersinC5–C12 paraffinsexceeds600,althoughonly200–400ofthemare foundinpetroleummixtures.
Alkanesaregaseous,liquid,orsolidsubstancesundernormalconditions.Gaseouscompoundscontainfrom1to4carbonatoms(C1–C4)inthechainandarepartofassociatedand naturalgases(methane,ethane,propane,butane,isobutane).Compoundscontainingfrom5 to15carbonatoms(C5–C15)areliquidsubstances,andn-alkaneswithcarbonatomsover16 aresolids,whichcanbeinadissolvedorcrystallinestateatthenormaltemperaturein petroleum,gascondensates,andhigh-boilingfractions.
1.1Themaingroupsofpetroleumandnaturalgashydrocarbons
Structureof2-methylheptaneand2,3-dimethylhexane.
Thetotalalkanecontentinpetroleumisgenerally25%–30%(notincludingdissolvedgases). Consideringthedissolved-statehydrocarbons,thealkanecontentrisesto40%–50%,andin somepetroleum—to50%–70%.However,therearepetroleumsinwhichthealkanecontent isonly10%–15%,similartogascondensates.
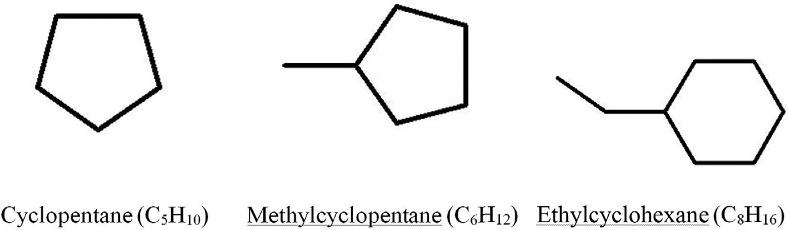
1.1.2Cycloalkanes(naphthenes) Thecycloalkanemoleculeisaclosedchain(ring).ThecycloalkaneseriesbeginwithcyclopropaneC3H6,followedbycyclobutane,cyclopentane,etc.DetailedinformationonthestructureandchemicalpropertiesofcycloalkanesispresentedinRef. [4].Thethreemaintypesof naphthenesareshownin Fig.1.6.
Ifonlyoneparaffinicalkylgroup(i.e.,methyl-,ethyl-,propyl-, n-butyl, )isattachedtoa cyclopentanehydrocarbon,thenthegroupiscalled n-alkylcyclopentanes.Thefirsttwohydrocarbonsshownin Fig.1.6 areintheabovegroup.Thereisalsoahomologousseriesof n-alkylcyclohexanes.Oneoftherepresentativesofthisseries—ethylcyclohexane—isshown in Fig.1.6.Naphthenichydrocarbonshavingonlyoneringarecalledmonocycloparaffinsor mononaphthenes.
Thecycloalkanecontentinpetroleumandnaturalgascondensatesis25%–75%.Cycloalkanes arepresentinallfractions.Theircontentusuallyincreasesasthefractionsbecomeheavier,and decreasesonlyinthehighestboilingpetroleumfractionsduetoanincreaseinthecontentofaromaticstructures.
Five-andsix-memberedringsarethemoststableones.Theyprevailinpetroleumandgas condensates—manyhomologuesofcyclopentaneandcyclohexanehavebeendiscovered. Higherfractionsofpetroleumalsocontainbicyclicandtricyclichydrocarbonsofvarious
FIG.1.6 Maintypesofnaphthenichydrocarbons.
FIG.1.5
structures,mainlywithtwocommoncarbonatoms.Inaddition,hydrocarbons,whicharevariouscombinationsoffive-andsix-memberedrings,oftencontainingaromaticrings—thesocalled hybridhydrocarbons,werefoundinpetroleum.
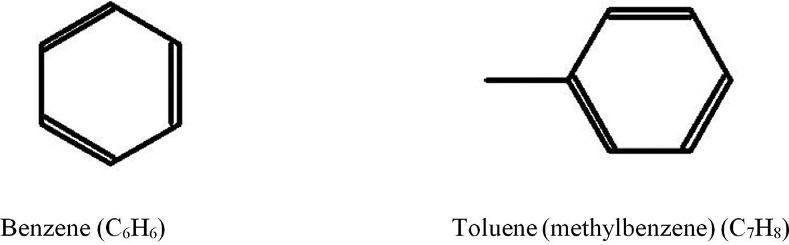
1.1.3Aromatichydrocarbons(arenes) Aseriesofaromatichydrocarbonsareformedbybenzeneanditsderivatives—toluene, xylenes,ethylbenzene,propylbenzene,etc.Inabroadersense,arenesarehydrocarbons, whosemolecularstructureincludesatleastonebenzenering.Detailedinformationonthe structureandchemicalpropertiesofarenesispresentedinRef. [3].Fourdifferentaromatic hydrocarbonsareshownin Figs.1.7and1.8
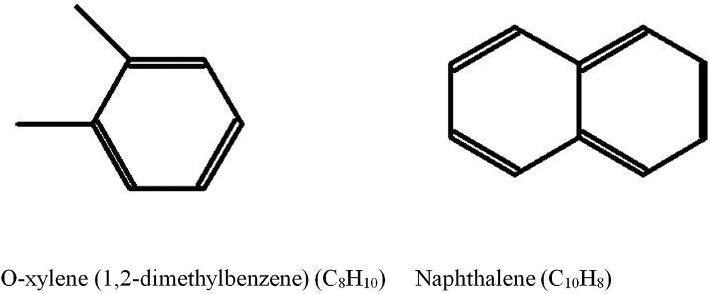
Eachcompoundinthestructuresshownin Figs.1.7and1.8 containsaCHgrouponthe benzenering.Acarbonatomisonlycontainedatthesitesofattachmentofalkylgroups (forexample,intoluene).Althoughbenzenehasthreecarbon–carbondoublebonds,ithas auniqueelectronarrangementthatallowsbenzenetoberelativelyinactive(nonreacting). Thepetroleumcompositionincludesbenzeneanditsderivativeswithattachedmethyl,ethyl, propyl,orhigheralkylgroups.SuchsubstancesarecalledalkylbenzeneswiththegeneralformulaCnH2n-6 (where n 6).Ingeneral,aromaticserieswhereoneringispresentarecalled monoaromatic.Inturn,naphthaleneanditsderivativeshavingtworingsarecalled
FIG.1.7 Structureofbenzeneandtoluene.
FIG.1.8 Structureofo-xyleneandnaphthalene.
diaromatic.Often,heavypetroleumfractionscontainmanybenzeneandnaphthenicrings connectedtoeachother.Suchstructuresarecalledpolyaromatic.
Thearenecontentinthepetroleumofvariousfieldsvarieswidelyandusuallyrangesfrom 15%to50%.Arenesarerepresentedinpetroleumbybenzeneanditshomologues,aswellas byderivativesofbi-andpolycyclichydrocarbons.Thearenehydrocarbonscontentingas condensatesalsovarieswidely—from1.5%to50%.
1.2Basicphysicalandchemicalpropertiesofhydrocarbons Themainphysicochemicalpropertiesofhydrocarbonsincludemolarmass,normalboiling point,relativedensity,refractiveindex,andkinematicviscositycoefficient.Theseproperties areusedintheidentificationofhydrocarbonsandespeciallycomplexhydrocarbonmixtures ofnaturalandprocessorigin.
1.2.1Molarmass M (kg/kmol) Unlikecomplexhydrocarbonmixturesofuncertaincomposition,themolarmassofindividualhydrocarbonsisdeterminedwithhighaccuracybutcanbecalculatedquiteaccurately usingthechemicalformulaofthesubstance,takingthemassofthehydrogenatom mH ¼ 1.0079kg/kmolandthemassofthecarbonatom mC ¼ 12.011kg/kmol.
1.2.2Normalboilingpointtemperature Tb Itisdeterminedexperimentallyatnormalbarometricpressure p ¼ 101,325Pa(760mmHg). Thenormalboilingpointdeterminationaccuracymainlydependsonthetemperature maintainingaccuracyofatestspecimenandthetemperatureandpressuremeasuringaccuracy.Contributingfactors—temperaturerange,thermalstability,andpurityofthetestsubstance—areequallyimportant.Ingeneral,wecanconcludethatthehigherthenormalboiling pointofahydrocarbon,thelessaccuratelyitisdetermined.Foraseriesofcomplexhighmolecularweighthydrocarbons,therearenoexperimentaldataon Tb andcalculationmethods areused.Theseareusuallymethodsofstructural-groupcomponents [5,6] orempiricalcorrelations;forexample,thefollowingsimpleformulaisproposedinRef. [7].
where Cfrac isthefractionofcarbonatomsinthemolecule: Cfrac ¼ nC nC + nH ; nC isthenumberof carbonatomsinthemolecule; nH isthenumberofhydrogenatoms;and M isthemolarmass (massofkilomole)(kg/kmol).
Theaccuracyofthecalculationisdifficulttoevaluate,butitisnothigh,andthesemethods canonlyberecommendedforevaluativecalculations.
1.2.3Relativedensity
Itistheratioofthedensityofasubstanceatatemperature t2 tothedensityofwaterata temperature t1.Indomesticpractice [8,9], t1 ¼ 4 °C, t2 ¼ 20 °Caretakenforsubstanceswitha meltingpointbelow20 °С and t2 ¼ 70 °C—forsubstanceswithahighermeltingpoint.Inthe UnitedStatesandEngland,therelativedensityisdeterminedatatemperatureof60 °F,i.e., t1 ¼ t2 ¼ 15.56 °C.Indomesticliterature,thisdensityisindicatedas ρ15 15.Torecalculatethe values ρ4 t in ρ15 15,theliteraturerecommendsvariousformulasandtables [9,10].Theauthor’s methodology,accordingtowhichtherecalculationiscarriedoutpracticallywithoutlossof accuracy,ispresentedhere [11].
where ρ4 t istherelativedensityatatemperature t2 ¼ t and γ isthetemperaturecorrectionof densitydeterminedbytheformula [11].
where RE isthespecificEijkmanrefraction,whichdoesnotdependontemperatureandis determinedbytheformula [12]
where nD t and ρ4 t aretherefractiveindexandrelativedensityatthe t temperature,respectively. Therelativedensityisgenerallydeterminedbythepycnometricmethod [9] withanerror of 0.02%.Theaccuracyforwell-studiedhydrocarbons,thedensityofwhichwasdetermined byotherinstrumentalmethods,canbeincreasedto 0.01%.
1.2.4Opticalproperties
Inpractice,toidentifyhydrocarbons,opticalpropertiessuchasrefractiveindex,molecular refraction,anddispersionareused.
Itisknownthatthelesstherefractiveindex nD t ofhydrocarbons,thegreatertherelativehydrogencontentinthem.Therefractiveindexofcycliccompoundsisgreaterthanthatofaliphatic ones.Cycloalkanesoccupyanintermediatepositionbetweenarenesandalkanes.Therefractive indexinahomologousseriesincreaseswithchainelongation.Themostnoticeablechangesare observedinthefirstmembersofthehomologousseriesandthenthechangesaregradually smoothedout.However,thereareexceptionstothisrule.Therefractiveindexforcycloalkanes (cyclopentane,cyclohexane,cycloheptane)andarenes(benzeneanditshomologues)isfirstly decreasedandthenincreasedasthelengthornumberofalkylsubstituentsisgrowing.
Alinearrelationshipinthehomologicalseriesofhydrocarbonsisobservedbetweenthe densityandtherefractiveindex.
Inadditiontotherefractiveindex,someofitsderivativesarealsoveryimportantcharacteristics,forexample,specificrefraction:
Gladstone-Dahlformula
Lorentz-Lorenzformula
where ρ isthehydrocarbondensitymeasuredatthesametemperatureastherefractiveindex.
Theproductofthespecificrefractionbythemolarmass M iscalledamolecularrefraction:
Themolecularrefractionisadditiveformixtures.Inaddition,molecularrefractionisequal tothesumofatomicrefractions,i.e.,thegroupcontributionmethodcanbeusedtocalculateit.
Therefractiveindexofatestsubstancedependsontheincidentlightwavelength.Therefractiveindexisthemostimportantforlightwithashorterwavelengthandviceversa.The dependencebetweentherefractiveindexoflightanditswavelengthforagivensubstanceis characterizedbythedispersion(scattering)oflight.
Inlaboratorypractice,alightsourcewithayellowsodiumline D (wavelength λ ¼ 589.3nm), aswellashydrogenlines F (λ ¼ 486.1nm)and G (λ ¼ 434.1nm),ismostoftenused.
Thedifference(nF nG)iscalledanaveragedispersion,andtheratio
arelativedispersion.Finally,theratio
iscalledaspecificdispersion.Thespecificdispersionsofarenesaremuchhigherthanthoseof saturatedalkanesandcycloalkanes.
Intheidentificationofhydrocarbons,onemorecharacteristicthatisderivedfromtherefractiveindex—a RI refractometricdifference—iswidelyused.
Thisvalueisalmostconstantforhydrocarbonsofthesamehomologousseries. Therefractiveindexinlaboratoryconditionsisusuallydeterminedonrefractometersfor the D linewithanaccuracyof (2 ∙ 10 4 –1.5 ∙ 10 5)dependingonthetypeofrefractometer.
1.2.5Kinematicviscosity Viscosityisoneofthemostimportantthermophysicalcharacteristicsofasubstance;it variesoverawiderangedependingonthetemperature,pressure,andstructureofhydrocarbons.Thekinematicviscosity ν (m2/s)whichisequaltotheratioofdynamicviscositytoliquiddensityatthesametemperatureandpressure,iswidelyusedasaphysicochemical characteristicofasubstanceintechnologicalpractice.Kinematicviscosityasaphysicochemicalcharacteristicismeasuredunderlaboratoryconditionsatanatmosphericpressureinthe liquidphase.Measurementsareusuallytakenusingcapillaryviscometers.Indomesticpractice,Pinkevichviscometers(GOST33-66)andVPZh-typeviscometers(GOST∗ 10028-62)are used.Themeasurementerrorisgenerally0.5%–1.0%.Moreaccurateresultscanbeobtained inspecialviscositystudiesbyvariousmethods,someofwhichwillbediscussedin Chapter7
Thekinematicviscosityoftheliquidphaseischaracterizedbyastrongtemperaturedependence,especiallynearthemeltingpoint.Variousempiricalformulasareusedtointerpolate thekinematicviscosity,butthereisnogenerallyacceptedformula,sinceviscosity,asalready noted,hasastrongdependencenotonlyontemperaturebutalsoonthestructureofthe hydrocarbon.
1.2.6Criticalproperties Criticalproperties—criticaltemperature Tc ,criticalpressure pc ,andcriticaldensity ρc —areimportantthermophysicalcharacteristicsofasubstance;theyarewidelyusedas parametersforreducingtoadimensionlessfor minvariousequationsofstateandforecast methodsforcalculatingthermophysicalc haracteristicsbasedontheprincipleof correspondingstates.Criticalpropertiesareusuallydeterminedexperimentally,usingboth directandindirectmethodsintheprocessofstudyinganythermodynamicpropertiesinthe criticalregion.Alotofworkspublishedinspecialperiodicalshavebeendevotedtothe methodsforthedeterminationofcriticalprop erties,dataanalysis,andgeneralization (see,forexample,Refs. [13– 16]).Thisbookdoesnotaddresstheseissues.However,given theimportanceofcriticalpropertiesandthefactthattheyareusedinthedevelopmentof equationsandcalculationmethods, Table.1.1 providesthemainphysicochemicalproperties,includingcritical,ofhydrocarbonsofva rioushomologousseries,whichwerestudied intheITPLandarediscussedinthispublication.Thecriticalpropertiesofhydrocarbons,for whichtheauthorsdevelopedfundamentalorinterpolationequations,arealsodiscussedin moredetailin Chapter3
TABLE1.1 Mainphysicochemicalpropertiesofthestudiedhydrocarbons.
Notes: Measured: а) at343 К; в) at358 К; с) at298 К; d) at313 К; е) at346 К; f) at410 К;obtainedbycalculation.TherefractiveindexofallsubstanceswasdeterminedbyanIRF-23 refractometer;therelativedensityisdeterminedbythepycnometricmethod.
References [1] F.D.Rossini,B.J.Meir,A.J.Strafe,HydrocarbonsofOil,Gostoptekhizdat,1957.470p.
[2] A.A.Petrov,ChemistryofAlkanes,Nauka,Moscow,1974.244p.
[3] A.A.Petrov,HydrocarbonsofOil,Nauka,Moscow,1984.237p.
[4] A.A.Petrov,ChemistryofNaphthenes,Nauka,Moscow,1971.388p.
[5] N.Skander,C.E.Chitour,Predictionofphysicalpropertiesofhydrocarbonsandpetroleumfractionsbyanew group-contributionmethod,Petrol.Coal.45(3–4)(2003)168–173.
[6] J.Marrero,R.Gani,Group-contributionbasedestimationofpurecomponentproperties,FluidPhaseEquilib. 183–184(2001)183–208.
[7] I.Al-MalahKamal,Predictionofnormalboilingpointsofhydrocarbonsusingsimplemolecularproperties, J.Adv.Chem.Eng.3(2013).ArticelID235654,9p.
[8] B.M.Rybak,AnalysisofOilandOilProducts,Gostoptekhizdat,Moscow,1962.888p.
[9] GOST3900-85,PetroleumandPetroleumProducts.MethodsforDeterminationofDensity,PublishingHouseof Standards,Moscow,1985.137p.
[10] V.F.Abrosimov,V.K.Bezugliy,N.K.Bolotin,etal.,MethodsforCalculationoftheThermophysicalPropertiesof GasesandLiquids,Chimiya,Moscow,1974.248p.
[11] B.A.Grigoriev,G.F.Bogatov,A.A.Gerasimov,ThermophysicalPropertiesofOil,OilProducts,GasCondensates andTheirFractions,MEIPublishingHouse,Moscow,1999.372p.
[12] K.Van-Nes,K.Van-Vesten,CompositionofOilFractionsofPetroleumandTheirAnalysis,PublishingHouseof ForeignLiterature,Moscow,1954.463p.
[13] K.A.Kobe,R.E.Lynn,Thecriticalconstantsoforganicsubstances,J.Chem.Rev.52(1953)117–236.
[14] A.P.Kudchadker,G.H.Alani,B.J.Zwolinsky,Thecriticalconstantsoforganicsubstances,Chem.Rev.68 (6)(1968)659–735.
[15] D.Ambrose,C.Tsonopoulos,Vapor-liquidcriticalpropertiesofelementsandcompounds.2.Normalalkanes, J.Chem.Eng.Data40(3)(1995)531–546.
[16] I.Owczarck,K.Blazej,Recommendedcriticaltemperatures.Pt.II.Aromaticandcyclichydrocarbons,J.Phys. Chem.Ref.Data33(2)(2004)541–548.
2 Experimentalmethods,apparatuses andresultsofexperimental measurementsofthethermodynamic properties Thedatabaseofexperimentaldataonthethermalandphysicalpropertiesofthemain groupsofoilandgascondensateshydrocarbons,whichunderpinnedthedevelopedhighprecisionequationsforcalculatingthermodynamicandtransportproperties,includestheresultsofmanyyearsofresearchbylocalandforeignscientistswhouseddifferentmethodsand experimentalsetups.
Itshouldbenotedthatinthesecondhalfofthe20thcenturyintheUSSR,inaccordance withtheplansoftheUSSRAcademyofSciencesonthecomplexproblemsofThermophysics andPetrochemistry,theinterdisciplinarycomprehensiveprogramoftheUSSRStateStandard“Datasystemsonthepropertiesofthemostimportantandprospectivematerials,substancesandorganicfuelsfortheenergysector,”full-fledgedstudiesofthethermophysical propertiesofsubstanceswerecarriedoutbyindustrialresearchoftheMinistryofHigherEducationandtheMinistryofOilandGasIndustryoftheUSSRandothers.Thecountryhas advancedtothepositionoftheworldleaderinthissciencediscipline;excellentschoolsof sciencehavebeencreatedinMoscow,Kiev,Baku,Odessa,Novosibirsk,Ekaterinburg, Grozny,andothers.Manyscientificconferences,workshops,andseminarswereheldin thecountry;databases,informationsystemswereestablished;experimentaltechniqueswere improved;andfundamentallynewmethodsandsettingswerecreated.
Excellentthermophysicalschools,whereoilhydrocarbonresearchwascarriedout,were createdinGrozny(GroznyStateOilTechnicalUniversity),Baku(Azizneftekhimn.a. Azizbekov,AzerbaijanPolytechnicInstitute),Dagestan(InstituteofPhysicsoftheDagestan ScientificCenteroftheAcademyofSciencesoftheUSSR),Odessa(InstituteofMarineEngineers,OdessaTechnologicalInstitutenamedafterLomonosov),Kiev(KievUniversity),Moscow(MSU,MPEI,UnitedInstituteofHighTemperatures,etc.),St.Petersburg(LITMO,
TechnologicalInstituteoftheRefrigerationIndustry),etc.Thedataobtainedinthelaboratoriesofthesescientificschoolswereusedbyusinpreparingthemonograph.
Significantexperimentalstudiesofthehydrocarbonpropertieswereperformedinthe UnitedStates(NationalInstituteofStandardsandTechnologyNIST,AmericanPetroleum InstituteAPI,ColoradoSchoolofMines,UniversityofMaryland,etc.),Germany(RuhrUniversity,TechnicalUniversitiesofMunich,KarlsruheInstituteofTechnologyandothers), France,theUnitedKingdom,andothersTheexperimentaldataobtainedandsetupsdevelopedintheseresearchcenterswerealsothesubjectofthoroughanalysis.
Itisnotpossibletodescribealltheexperimentalsetupsofdifferentscientificschoolsorat leastgiveabriefdescriptionofthembecauseoftheenormousamountofmaterial,variety, andcontinuousimprovementofmeasurementmethods.Meanwhile,thereaderanduser ofthereferencemonograph,inouropinion,shouldhaveanideaofexperimentalsetups andmethodsformeasuringvariousthermophysicalproperties,theircapabilities,measurementaccuracy,thedifficultyofobtaining,andthevalueofeachreliableexperimentalpoint. Computerizationofresearch,alongwiththeenormouspositiveeffectofplanningandacceleratinganexperiment,visualizingit,modeling,andpredictingtheresults,oftenleadstheresearcher,especiallyifhe/sheisnotadeveloperandcreatorofthemeasurementmethodand experimentalsetup,awayfromsuchimportantaspectsastheexperimentsetupmethodology anddefiningrealaccuracyoftheresults.Manyarticlesonthepropertiesofhydrocarbonsoftenfailtoprovideinformationonbothmethodsoftheirmeasurementandaccuracy.Therefore,weconsidereditexpedienttofocusmoreonexperimentalresearchmethods,a descriptionoftheexperimentalsetupsinwhichtheexperimentaldatawereobtained,analysis,andcalculationofmeasurementaccuracy.
Thebasisofthisreviewcomprisesexperimentalsetupsbuiltin1960–90attheIndustrial ThermophysicalLaboratory(ITPL)oftheGroznyStateOilTechnicalUniversity(GSOTU) inwhichuniquecomprehensivestudiesofthethermophysicalpropertiesofthemaingroups ofoilhydrocarbonsandgascondensates,aswellasmorethan500samplesoftheirfractions, productprocessing,fuels,andoilswerecarriedout.
Whenchoosingresearchmethodsandsetupsdesigns,weassumeditnecessarytoensure:
thepossibilityofmeasuringthermalpropertiesinawiderangeoftemperatures(170–700K) andpressures(0.1–150MPa);
highaccuracy,reliability,andsimplicityofmeasurement;
independenceofmeasurementsfromliterarysources,thepossibilityofobtainingresults, asarule,bytwoindependentmethods;
thepossibilityofconductinganexperimentwithsubstancesthatdiffersignificantlyin physicalandchemicalproperties(meltingandboilingpoints,viscosity,density,etc.);
thepossibilitytothoroughlyflushthesetupwithoutdismantlingit,quicklyandreliably fillingitwithhydrocarbonunderinvestigation;and
thepossibilitytocontroltheabsenceofthermaltransformationsofhydrocarbonsin experimentsathightemperatures.
Itisknownthatthermaltransformationsofhydrocarbonsoccurathightemperatures.Due tothisfact,itwasnecessarytodeterminetheupperboundarybytemperatureandlowerby pressureforeachhydrocarbon,beyondwhichdestructivenonequilibriumprocessesoccur. Theparametersoftheseboundariesweredeterminedexperimentallyata p, v, T setup,by
samplingatcertaintemperatureintervalsandanalyzingsamplesofthehydrocarbonunder study.Physicalandchemicalpropertiesweredetermined:relativedensity ρ4 20,refractiveindex nD20,andchromatographicanalysiswasalsocarriedout.Accordingtothemeasurement results,thecorrespondingdependenceswerebuiltandthemaximumtemperaturewasdeterminedatwhichtherewerenochangesinthepropertiesandpurityofthehydrocarbon inexperimentsperformedatsetupsmadeofchromium-nickelsteels.Wewilldemonstrate themagnitudeofthesechangesasexemplifiedwith n-hexaneandcyclohexane.Thus,while for n-hexaneintheexperimentsat623.15Ktherewerenochangesinpropertiesandpurity,in thesampletakenat648.15Kthefollowingproductsofthermaltransformationswerefound: n-propane,propylene, n-butane,butene-1,pentane,andhexene,whichisconsistentwith studies [1].Inthecyclohexanesampletakenat723.15K,thecontentofthemaincomponent wasonly64%,methylcyclohexane28%and8%werevariousclassesofhydrocarbonsand resins(over30substances).Inthesampletakenat698.15K,thecontentofthemaincomponentwasabout99.9%,i.e.,thermalprocesseswereabsentandtheexperimentwasin equilibrium.
Thus,asaresultofspeciallabor-intensiveandsubtleexperiments,thethermalstability parametersofallthehydrocarbonsstudiedbyusinmeasuringcellsmadeofnickelchromiumsteelsweredetermined.Whenanalyzingthedataofotherresearchersathightemperaturesoutsidethethermalstabilityparameters,thefactorofthepossibleinfluenceonthe resultsofthermaldestructionwastakenintoaccount.
BasedontheobjectivesofthestudyandtherequirementsforexperimentalsetupsinITPL, morethan30setupswerecreated.Hereweprovideanabridgeddescriptionof17experimentalsetupsformeasuringdensity,saturatedvaporpressure,meltingpressure,isobaricheat capacity,surfacetension,dynamicviscositycoefficient,thermalconductivitycoefficientof themaingroupsofoilhydrocarbonsandgascondensates.Inaddition,takingintoaccount theimportanceofdataonisochoricheatcapacityandsoundvelocity,setupsdesignedto studythesepropertiesanddevelopedinthecountry’sleadingthermophysicallaboratories, theInstituteofPhysicsoftheDagestanBranchoftheUSSRAcademyofSciences,andinthe thermophysicallaboratoryofKurskStatePedagogicalUniversity,willalsobedescribed.
2.1Experimentaluncertainties Priortoconsideringthedesignsofexperimentalsetupsandmethodsformeasuringthe thermodynamicpropertiesofhydrocarbons,wemakeafewgeneralcommentsontheestimationofmeasurementaccuracyandcorrespondingterminology.
Asalreadynoted,anexperimentalstudyofthermophysicalproperties(TPS)wascarried outatthegroundsofITPLoftheGSOTUorITPLformorethanthreedecades.Duringthis time,therewasanimprovementinbothmeasuringinstrumentsandmethodsforassessing theaccuracyofexperimentalresults.Themainresultsandprovisionsoftheaccuracyestimationtheory,basedontheirstatisticalnature,wereobtainedinthe1960s–70softhelastcentury.However,thedirectapplicationoftheoreticalprinciplestoarealexperimentisalways fraughtwithcertaindifficulties,asarule,duetothelackofstatisticalinformation.Ittook sometimetoadaptthetheoreticalmethodstoarealthermophysicalexperimentandputthem